Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
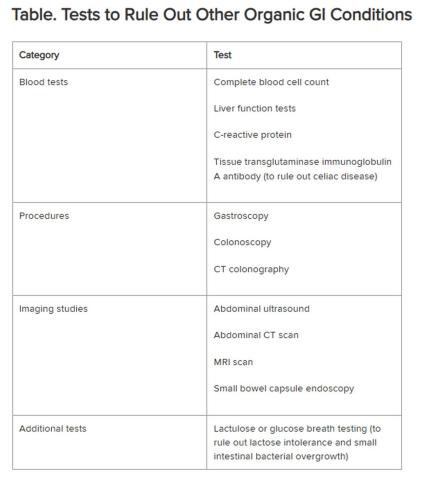
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.