User login
American Epilepsy Society (AES): Annual Meeting
Studies highlight benefits and risks of ketogenic diet for refractory epilepsy
WASHINGTON – The ketogenic diet is well established as an effective treatment for patients with refractory epilepsy, and although studies presented at the annual meeting of the American Epilepsy Society confirm its value for both children and adults, others raise important concerns.
Researchers from the University of Missouri – Kansas City (UMKC), for example, reported that adhering to a ketogenic diet (KGD) reduced the number of emergency department visits and hospitalizations, and decreased the length of epilepsy-related hospital stays in children with pharmacologically refractory epilepsy.
In 98 children from the Children’s Mercy Hospital–UMKC database who had complete records available and who remained on the diet for at least 6 months, the number of ED visits decreased by 64%, and the charges associated with those visits decreased by 50% from the 12 months before diet initiation to the 12 months after, Dr. Anastasia Luniova reported in a poster at the meeting.
After an initial increase in the total number of hospitalizations and the number of hospital days, due largely to stays associated with diet initiation, the number of hospitalizations decreased by 61% and the number of hospital days decreased by 66%. Associated charges decreased by 47%.
Children in this retrospective chart study from a level IV pediatric epilepsy center included 62 boys and 36 girls with an average age of 4.4 years at initiation of the ketogenic diet. The average diet duration was 31.7 months.
"This study provides evidence that the KGD has a positive impact in children with pharmacologically refractory epilepsy by reducing the number of ED visits, numbers of hospitalizations, as well as length of hospital stay related to epilepsy, and associated comorbidities. ... Further data analysis is necessary for detailed cost-effectiveness assessment of the KGD," Dr. Luniova wrote.
The KGD was also safe and effective in a study of 10 adults with refractory status epilepticus, Dr. John C. Probasco of Johns Hopkins University, Baltimore, reported in a poster.
While further studies are needed to determine the applicability of a KGD in adult patients, as well as the long-term outcomes, the findings suggest it is safe and feasible, Dr. Probasco said.
The retrospective case study at four medical centers included patients over age 17 years with status epilepticus that continued for at least 24 hours after initiation of general anesthetic medication, or that recurred following weaning from, or discontinuation of, the treatment. The patients, including four men and six women, had a median age of 33 years, and seven had encephalitis. Prior to KGD initiation, the median duration of status epilepticus was 21.5 days, and the median number of antiepileptic drugs (AEDs) used was seven.
Nine of the 10 patients achieved ketosis within a median of 3 days, and all patients had cessation of status epilepticus within 3 days. Furthermore, seven had clinical and/or electrographic seizure resolution within 7 days, and nine had such resolution within 1 month. At discharge, the median number of antiepileptic drugs prescribed was four, Dr. Probasco reported.
Another study demonstrated the beneficial effects of a KGD on immunoglobulin levels and infection frequency.
"In addition to its known side effects, the [KGD] is considered to lead to an increase in infection frequency causing possible neutrophil function impairment and the reason behind this has not yet been explained completely," wrote Dr. Orkide Güzel of Izmir (Turkey) Dr. Behçet Uz Children’s Hospital.
But a review of the records of 36 children with resistant epilepsy, including 17 girls and 19 boys with a mean age of 39.5 months, showed no significant differences with respect to the number and severity of infections before and after KGD initiation, and immunoglobulin levels remained normal for the patients’ ages. At the same time, their number of seizures and AED usage decreased. Epileptic encephalopathy in five patients also went away after KGD treatment.
However, several other studies found that in children, the diet may be linked with decreases in growth and bone health.
Delayed growth is considered a potential side effect of the KGD, and UMKC investigators set out to assess growth related to caloric intake in infants and children being treated with a KGD. In another retrospective chart study of 76 children treated at Children’s Mercy Hospital, Kansas City, mean weight- and height-for-age percentiles and z scores declined over a period of 12 months, though not statistically significantly, Dr. Lindsey Thompson of Children’s Mercy reported in a poster.
For example, the weight percentile declined from 49.48% at baseline to 42.94% at 12 months, and the height percentile decreased from 52.08% to 45.06%. These decreases occurred despite an increase in caloric intake from 999 kcal at baseline to 1,134 kcal at 12 months.
The results require further prospective evaluation, Dr. Thompson said.
Another study found a significant risk of developing osteopenia among patients on a KGD, compared with matched controls.
Of 132 children included in the retrospective matched cohort study, 66 were treated with AEDs and a KGD (initiated at a mean age of 4.3 years), and 66 were treated with AEDs alone.
The KGD group had fractures – often unrelated to trauma – more often than did those on AEDs alone (14% vs. 8%, respectively). The incidence of both osteopenia and fractures increased in tandem with the duration of the KGD, according to the group of investigators from the University of British Columbia, Vancouver, led by Dr. Mary B. Connolly.
There was a history of trauma in one-third of the KGD patients with fracture, compared with 100% of those in the AED-only group. In addition, all patients in the KGD group had osteopenia, compared with 60% of those in the control group.
Nonambulatory status was a risk factor for osteopenia and fractures in both groups, and a longer duration of KGD was associated with greater risk. For instance, among those treated for 1-3 years, 4% had fractures and 27% had osteopenia, and among those treated for 4 or more years, 20% had fractures and 43% had osteopenia.
"Our results suggest that prolonged treatment with the KD may be associated with significant morbidity, including osteopenia and fractures," Dr. Connolly wrote, noting that the incidence of osteopenia in the study was likely underestimated because of the retrospective design.
The findings, which support those of prior long-term studies, suggest that monitoring of bone health and vitamin D and calcium supplementation is important in this population, she said.
WASHINGTON – The ketogenic diet is well established as an effective treatment for patients with refractory epilepsy, and although studies presented at the annual meeting of the American Epilepsy Society confirm its value for both children and adults, others raise important concerns.
Researchers from the University of Missouri – Kansas City (UMKC), for example, reported that adhering to a ketogenic diet (KGD) reduced the number of emergency department visits and hospitalizations, and decreased the length of epilepsy-related hospital stays in children with pharmacologically refractory epilepsy.
In 98 children from the Children’s Mercy Hospital–UMKC database who had complete records available and who remained on the diet for at least 6 months, the number of ED visits decreased by 64%, and the charges associated with those visits decreased by 50% from the 12 months before diet initiation to the 12 months after, Dr. Anastasia Luniova reported in a poster at the meeting.
After an initial increase in the total number of hospitalizations and the number of hospital days, due largely to stays associated with diet initiation, the number of hospitalizations decreased by 61% and the number of hospital days decreased by 66%. Associated charges decreased by 47%.
Children in this retrospective chart study from a level IV pediatric epilepsy center included 62 boys and 36 girls with an average age of 4.4 years at initiation of the ketogenic diet. The average diet duration was 31.7 months.
"This study provides evidence that the KGD has a positive impact in children with pharmacologically refractory epilepsy by reducing the number of ED visits, numbers of hospitalizations, as well as length of hospital stay related to epilepsy, and associated comorbidities. ... Further data analysis is necessary for detailed cost-effectiveness assessment of the KGD," Dr. Luniova wrote.
The KGD was also safe and effective in a study of 10 adults with refractory status epilepticus, Dr. John C. Probasco of Johns Hopkins University, Baltimore, reported in a poster.
While further studies are needed to determine the applicability of a KGD in adult patients, as well as the long-term outcomes, the findings suggest it is safe and feasible, Dr. Probasco said.
The retrospective case study at four medical centers included patients over age 17 years with status epilepticus that continued for at least 24 hours after initiation of general anesthetic medication, or that recurred following weaning from, or discontinuation of, the treatment. The patients, including four men and six women, had a median age of 33 years, and seven had encephalitis. Prior to KGD initiation, the median duration of status epilepticus was 21.5 days, and the median number of antiepileptic drugs (AEDs) used was seven.
Nine of the 10 patients achieved ketosis within a median of 3 days, and all patients had cessation of status epilepticus within 3 days. Furthermore, seven had clinical and/or electrographic seizure resolution within 7 days, and nine had such resolution within 1 month. At discharge, the median number of antiepileptic drugs prescribed was four, Dr. Probasco reported.
Another study demonstrated the beneficial effects of a KGD on immunoglobulin levels and infection frequency.
"In addition to its known side effects, the [KGD] is considered to lead to an increase in infection frequency causing possible neutrophil function impairment and the reason behind this has not yet been explained completely," wrote Dr. Orkide Güzel of Izmir (Turkey) Dr. Behçet Uz Children’s Hospital.
But a review of the records of 36 children with resistant epilepsy, including 17 girls and 19 boys with a mean age of 39.5 months, showed no significant differences with respect to the number and severity of infections before and after KGD initiation, and immunoglobulin levels remained normal for the patients’ ages. At the same time, their number of seizures and AED usage decreased. Epileptic encephalopathy in five patients also went away after KGD treatment.
However, several other studies found that in children, the diet may be linked with decreases in growth and bone health.
Delayed growth is considered a potential side effect of the KGD, and UMKC investigators set out to assess growth related to caloric intake in infants and children being treated with a KGD. In another retrospective chart study of 76 children treated at Children’s Mercy Hospital, Kansas City, mean weight- and height-for-age percentiles and z scores declined over a period of 12 months, though not statistically significantly, Dr. Lindsey Thompson of Children’s Mercy reported in a poster.
For example, the weight percentile declined from 49.48% at baseline to 42.94% at 12 months, and the height percentile decreased from 52.08% to 45.06%. These decreases occurred despite an increase in caloric intake from 999 kcal at baseline to 1,134 kcal at 12 months.
The results require further prospective evaluation, Dr. Thompson said.
Another study found a significant risk of developing osteopenia among patients on a KGD, compared with matched controls.
Of 132 children included in the retrospective matched cohort study, 66 were treated with AEDs and a KGD (initiated at a mean age of 4.3 years), and 66 were treated with AEDs alone.
The KGD group had fractures – often unrelated to trauma – more often than did those on AEDs alone (14% vs. 8%, respectively). The incidence of both osteopenia and fractures increased in tandem with the duration of the KGD, according to the group of investigators from the University of British Columbia, Vancouver, led by Dr. Mary B. Connolly.
There was a history of trauma in one-third of the KGD patients with fracture, compared with 100% of those in the AED-only group. In addition, all patients in the KGD group had osteopenia, compared with 60% of those in the control group.
Nonambulatory status was a risk factor for osteopenia and fractures in both groups, and a longer duration of KGD was associated with greater risk. For instance, among those treated for 1-3 years, 4% had fractures and 27% had osteopenia, and among those treated for 4 or more years, 20% had fractures and 43% had osteopenia.
"Our results suggest that prolonged treatment with the KD may be associated with significant morbidity, including osteopenia and fractures," Dr. Connolly wrote, noting that the incidence of osteopenia in the study was likely underestimated because of the retrospective design.
The findings, which support those of prior long-term studies, suggest that monitoring of bone health and vitamin D and calcium supplementation is important in this population, she said.
WASHINGTON – The ketogenic diet is well established as an effective treatment for patients with refractory epilepsy, and although studies presented at the annual meeting of the American Epilepsy Society confirm its value for both children and adults, others raise important concerns.
Researchers from the University of Missouri – Kansas City (UMKC), for example, reported that adhering to a ketogenic diet (KGD) reduced the number of emergency department visits and hospitalizations, and decreased the length of epilepsy-related hospital stays in children with pharmacologically refractory epilepsy.
In 98 children from the Children’s Mercy Hospital–UMKC database who had complete records available and who remained on the diet for at least 6 months, the number of ED visits decreased by 64%, and the charges associated with those visits decreased by 50% from the 12 months before diet initiation to the 12 months after, Dr. Anastasia Luniova reported in a poster at the meeting.
After an initial increase in the total number of hospitalizations and the number of hospital days, due largely to stays associated with diet initiation, the number of hospitalizations decreased by 61% and the number of hospital days decreased by 66%. Associated charges decreased by 47%.
Children in this retrospective chart study from a level IV pediatric epilepsy center included 62 boys and 36 girls with an average age of 4.4 years at initiation of the ketogenic diet. The average diet duration was 31.7 months.
"This study provides evidence that the KGD has a positive impact in children with pharmacologically refractory epilepsy by reducing the number of ED visits, numbers of hospitalizations, as well as length of hospital stay related to epilepsy, and associated comorbidities. ... Further data analysis is necessary for detailed cost-effectiveness assessment of the KGD," Dr. Luniova wrote.
The KGD was also safe and effective in a study of 10 adults with refractory status epilepticus, Dr. John C. Probasco of Johns Hopkins University, Baltimore, reported in a poster.
While further studies are needed to determine the applicability of a KGD in adult patients, as well as the long-term outcomes, the findings suggest it is safe and feasible, Dr. Probasco said.
The retrospective case study at four medical centers included patients over age 17 years with status epilepticus that continued for at least 24 hours after initiation of general anesthetic medication, or that recurred following weaning from, or discontinuation of, the treatment. The patients, including four men and six women, had a median age of 33 years, and seven had encephalitis. Prior to KGD initiation, the median duration of status epilepticus was 21.5 days, and the median number of antiepileptic drugs (AEDs) used was seven.
Nine of the 10 patients achieved ketosis within a median of 3 days, and all patients had cessation of status epilepticus within 3 days. Furthermore, seven had clinical and/or electrographic seizure resolution within 7 days, and nine had such resolution within 1 month. At discharge, the median number of antiepileptic drugs prescribed was four, Dr. Probasco reported.
Another study demonstrated the beneficial effects of a KGD on immunoglobulin levels and infection frequency.
"In addition to its known side effects, the [KGD] is considered to lead to an increase in infection frequency causing possible neutrophil function impairment and the reason behind this has not yet been explained completely," wrote Dr. Orkide Güzel of Izmir (Turkey) Dr. Behçet Uz Children’s Hospital.
But a review of the records of 36 children with resistant epilepsy, including 17 girls and 19 boys with a mean age of 39.5 months, showed no significant differences with respect to the number and severity of infections before and after KGD initiation, and immunoglobulin levels remained normal for the patients’ ages. At the same time, their number of seizures and AED usage decreased. Epileptic encephalopathy in five patients also went away after KGD treatment.
However, several other studies found that in children, the diet may be linked with decreases in growth and bone health.
Delayed growth is considered a potential side effect of the KGD, and UMKC investigators set out to assess growth related to caloric intake in infants and children being treated with a KGD. In another retrospective chart study of 76 children treated at Children’s Mercy Hospital, Kansas City, mean weight- and height-for-age percentiles and z scores declined over a period of 12 months, though not statistically significantly, Dr. Lindsey Thompson of Children’s Mercy reported in a poster.
For example, the weight percentile declined from 49.48% at baseline to 42.94% at 12 months, and the height percentile decreased from 52.08% to 45.06%. These decreases occurred despite an increase in caloric intake from 999 kcal at baseline to 1,134 kcal at 12 months.
The results require further prospective evaluation, Dr. Thompson said.
Another study found a significant risk of developing osteopenia among patients on a KGD, compared with matched controls.
Of 132 children included in the retrospective matched cohort study, 66 were treated with AEDs and a KGD (initiated at a mean age of 4.3 years), and 66 were treated with AEDs alone.
The KGD group had fractures – often unrelated to trauma – more often than did those on AEDs alone (14% vs. 8%, respectively). The incidence of both osteopenia and fractures increased in tandem with the duration of the KGD, according to the group of investigators from the University of British Columbia, Vancouver, led by Dr. Mary B. Connolly.
There was a history of trauma in one-third of the KGD patients with fracture, compared with 100% of those in the AED-only group. In addition, all patients in the KGD group had osteopenia, compared with 60% of those in the control group.
Nonambulatory status was a risk factor for osteopenia and fractures in both groups, and a longer duration of KGD was associated with greater risk. For instance, among those treated for 1-3 years, 4% had fractures and 27% had osteopenia, and among those treated for 4 or more years, 20% had fractures and 43% had osteopenia.
"Our results suggest that prolonged treatment with the KD may be associated with significant morbidity, including osteopenia and fractures," Dr. Connolly wrote, noting that the incidence of osteopenia in the study was likely underestimated because of the retrospective design.
The findings, which support those of prior long-term studies, suggest that monitoring of bone health and vitamin D and calcium supplementation is important in this population, she said.
AT AES 2013
Postsurgery antiepileptic drug withdrawal appears safe in seizure-free kids
WASHINGTON – Antiepileptic drug reduction should be considered in children who are seizure free after epilepsy surgery, according to findings from a retrospective chart study.
Patients in the study who withdrew from antiepileptic drug (AED) treatment were not significantly more likely to have seizure recurrence than were those who did not, thereby meeting the common secondary goal of discontinuing or reducing AEDs after epilepsy surgery. Thus, the findings of this study may provide reassurance to neurologists who are hesitant to withdraw AEDs after surgery because of concerns that seizures may recur, Dr. Katherine C. Nickels of the Mayo Clinic, Rochester, Minn., reported in a poster at the annual meeting of the American Epilepsy Society.
Of 79 children who underwent resective surgery for intractable epilepsy between 2008 and 2012 and who were followed for a mean of 33.6 months, 50 were seizure free at 3 months, and 49 of those children were treated with antiepileptic drugs. At last follow-up, 37 (76%) were seizure free, including 24 of 28 (86%) in whom AEDs were reduced, and 13 of 21 (62%) in whom AEDs were not reduced, the researchers found.
The study involved children from birth through age 17 years who underwent surgery at the Mayo Clinic. A medical chart review was conducted to determine whether patients were seizure free 3 months after surgery, and to determine the number of medical and nonmedical therapies tried before and at the time of surgery, when therapies were reduced after surgery (if at all), the number of medications and therapies used at last follow-up, and seizure outcomes based on Engel classification at 3, 6, 12, 24, and 36 months, and at final follow-up after surgery.
Seizure recurrence was not found to be associated with abnormal findings on MRI, type and location of resection, or underlying pathology.
AED reduction was not a significant risk factor for seizure recurrence, regardless of when AED reduction occurred, Dr. Nickels noted. The median time to recurrence was 9 months after surgery, regardless of whether AEDs were reduced.
The AES meeting did not require reports of financial disclosures.
WASHINGTON – Antiepileptic drug reduction should be considered in children who are seizure free after epilepsy surgery, according to findings from a retrospective chart study.
Patients in the study who withdrew from antiepileptic drug (AED) treatment were not significantly more likely to have seizure recurrence than were those who did not, thereby meeting the common secondary goal of discontinuing or reducing AEDs after epilepsy surgery. Thus, the findings of this study may provide reassurance to neurologists who are hesitant to withdraw AEDs after surgery because of concerns that seizures may recur, Dr. Katherine C. Nickels of the Mayo Clinic, Rochester, Minn., reported in a poster at the annual meeting of the American Epilepsy Society.
Of 79 children who underwent resective surgery for intractable epilepsy between 2008 and 2012 and who were followed for a mean of 33.6 months, 50 were seizure free at 3 months, and 49 of those children were treated with antiepileptic drugs. At last follow-up, 37 (76%) were seizure free, including 24 of 28 (86%) in whom AEDs were reduced, and 13 of 21 (62%) in whom AEDs were not reduced, the researchers found.
The study involved children from birth through age 17 years who underwent surgery at the Mayo Clinic. A medical chart review was conducted to determine whether patients were seizure free 3 months after surgery, and to determine the number of medical and nonmedical therapies tried before and at the time of surgery, when therapies were reduced after surgery (if at all), the number of medications and therapies used at last follow-up, and seizure outcomes based on Engel classification at 3, 6, 12, 24, and 36 months, and at final follow-up after surgery.
Seizure recurrence was not found to be associated with abnormal findings on MRI, type and location of resection, or underlying pathology.
AED reduction was not a significant risk factor for seizure recurrence, regardless of when AED reduction occurred, Dr. Nickels noted. The median time to recurrence was 9 months after surgery, regardless of whether AEDs were reduced.
The AES meeting did not require reports of financial disclosures.
WASHINGTON – Antiepileptic drug reduction should be considered in children who are seizure free after epilepsy surgery, according to findings from a retrospective chart study.
Patients in the study who withdrew from antiepileptic drug (AED) treatment were not significantly more likely to have seizure recurrence than were those who did not, thereby meeting the common secondary goal of discontinuing or reducing AEDs after epilepsy surgery. Thus, the findings of this study may provide reassurance to neurologists who are hesitant to withdraw AEDs after surgery because of concerns that seizures may recur, Dr. Katherine C. Nickels of the Mayo Clinic, Rochester, Minn., reported in a poster at the annual meeting of the American Epilepsy Society.
Of 79 children who underwent resective surgery for intractable epilepsy between 2008 and 2012 and who were followed for a mean of 33.6 months, 50 were seizure free at 3 months, and 49 of those children were treated with antiepileptic drugs. At last follow-up, 37 (76%) were seizure free, including 24 of 28 (86%) in whom AEDs were reduced, and 13 of 21 (62%) in whom AEDs were not reduced, the researchers found.
The study involved children from birth through age 17 years who underwent surgery at the Mayo Clinic. A medical chart review was conducted to determine whether patients were seizure free 3 months after surgery, and to determine the number of medical and nonmedical therapies tried before and at the time of surgery, when therapies were reduced after surgery (if at all), the number of medications and therapies used at last follow-up, and seizure outcomes based on Engel classification at 3, 6, 12, 24, and 36 months, and at final follow-up after surgery.
Seizure recurrence was not found to be associated with abnormal findings on MRI, type and location of resection, or underlying pathology.
AED reduction was not a significant risk factor for seizure recurrence, regardless of when AED reduction occurred, Dr. Nickels noted. The median time to recurrence was 9 months after surgery, regardless of whether AEDs were reduced.
The AES meeting did not require reports of financial disclosures.
AT AES 2013
Poorly controlled seizures linked to high health care use
WASHINGTON – People with epilepsy who were considered to be in intermediate control of their seizures were hospitalized for similar lengths of time and went to the emergency room as often as those with uncontrolled epilepsy in a large retrospective study of commercially insured adults with epilepsy, Dr. Fulton Velez reported at the annual meeting of the American Epilepsy Society.
The results suggest that the use of health care resources may be greater among patients whose seizures are less than optimally controlled, which "may have important health care utilization implications for patients and payers," said Dr. Velez, director of health economics and outcomes research at Sunovion Pharmaceuticals, a manufacturer of antiepileptic drugs (AEDs).
The investigators reviewed the use of health care resources in 26,625 adults with epilepsy who were enrolled in the MarketScan database, a national database that contains data on over 180 million patients since 1995, according to its website. The patients were treated with at least one add-on AED within 60 days of being diagnosed and were followed for at least 1 year (the average follow-up time was 3.25 years).
Dr. Velez and his colleagues fit the patients into three categories:
• Uncontrolled, defined as one emergency room or inpatient stay preceded by two or more changes in drug therapy, either an additional drug or a switch in drugs in the preceding 6 months.
• Well controlled, meaning no change in AED therapy and no epilepsy-related emergency room or inpatient visits.
• Intermediate controlled, which meant that the patient was not classified as uncontrolled or well controlled.
Most (83%) fell into the intermediate-control category, 2% were uncontrolled, and 15% were well controlled. Their mean age was 49 years and almost 60% were women.
As expected, the use of health care resources was markedly higher among the uncontrolled patients, Dr. Velez said. Patients in the uncontrolled category used significantly more AEDs and had significantly more outpatient and neurologist visits than did those in the well-controlled and intermediate-control categories.
The uncontrolled patients used 1.6 times more AEDs than did those who were well controlled and those who were in the intermediate-control category. The rate of outpatient visits was 20% higher among uncontrolled patients than in those who were well controlled. In addition, the rate of neurologist visits among those who were uncontrolled was about 40% higher than in those who were well controlled and 19% higher than those who were in the intermediate-control category, Dr. Velez said.
Not surprisingly, patients with uncontrolled epilepsy visited the emergency room 12 times as often and stayed in the hospital more than 7 times as often as did those with well-controlled epilepsy. Their duration of hospitalizations also was nine times as long.
There were, however, no differences in the rate of emergency room visits or length of hospital stays between the intermediate control and uncontrolled patients, Dr. Velez said. In addition, the rate of inpatient hospitalizations was about 12% higher among the intermediate-control group. "The findings of this study suggest that even small departures from optimal seizure control are associated with a marked increase in health resource utilization among epilepsy patients," he said in a statement issued by the American Epilepsy Society during the meeting.
The study was performed by HERON Evidence Development AB, Stockholm, and the data analysis was funded by Sunovion.
WASHINGTON – People with epilepsy who were considered to be in intermediate control of their seizures were hospitalized for similar lengths of time and went to the emergency room as often as those with uncontrolled epilepsy in a large retrospective study of commercially insured adults with epilepsy, Dr. Fulton Velez reported at the annual meeting of the American Epilepsy Society.
The results suggest that the use of health care resources may be greater among patients whose seizures are less than optimally controlled, which "may have important health care utilization implications for patients and payers," said Dr. Velez, director of health economics and outcomes research at Sunovion Pharmaceuticals, a manufacturer of antiepileptic drugs (AEDs).
The investigators reviewed the use of health care resources in 26,625 adults with epilepsy who were enrolled in the MarketScan database, a national database that contains data on over 180 million patients since 1995, according to its website. The patients were treated with at least one add-on AED within 60 days of being diagnosed and were followed for at least 1 year (the average follow-up time was 3.25 years).
Dr. Velez and his colleagues fit the patients into three categories:
• Uncontrolled, defined as one emergency room or inpatient stay preceded by two or more changes in drug therapy, either an additional drug or a switch in drugs in the preceding 6 months.
• Well controlled, meaning no change in AED therapy and no epilepsy-related emergency room or inpatient visits.
• Intermediate controlled, which meant that the patient was not classified as uncontrolled or well controlled.
Most (83%) fell into the intermediate-control category, 2% were uncontrolled, and 15% were well controlled. Their mean age was 49 years and almost 60% were women.
As expected, the use of health care resources was markedly higher among the uncontrolled patients, Dr. Velez said. Patients in the uncontrolled category used significantly more AEDs and had significantly more outpatient and neurologist visits than did those in the well-controlled and intermediate-control categories.
The uncontrolled patients used 1.6 times more AEDs than did those who were well controlled and those who were in the intermediate-control category. The rate of outpatient visits was 20% higher among uncontrolled patients than in those who were well controlled. In addition, the rate of neurologist visits among those who were uncontrolled was about 40% higher than in those who were well controlled and 19% higher than those who were in the intermediate-control category, Dr. Velez said.
Not surprisingly, patients with uncontrolled epilepsy visited the emergency room 12 times as often and stayed in the hospital more than 7 times as often as did those with well-controlled epilepsy. Their duration of hospitalizations also was nine times as long.
There were, however, no differences in the rate of emergency room visits or length of hospital stays between the intermediate control and uncontrolled patients, Dr. Velez said. In addition, the rate of inpatient hospitalizations was about 12% higher among the intermediate-control group. "The findings of this study suggest that even small departures from optimal seizure control are associated with a marked increase in health resource utilization among epilepsy patients," he said in a statement issued by the American Epilepsy Society during the meeting.
The study was performed by HERON Evidence Development AB, Stockholm, and the data analysis was funded by Sunovion.
WASHINGTON – People with epilepsy who were considered to be in intermediate control of their seizures were hospitalized for similar lengths of time and went to the emergency room as often as those with uncontrolled epilepsy in a large retrospective study of commercially insured adults with epilepsy, Dr. Fulton Velez reported at the annual meeting of the American Epilepsy Society.
The results suggest that the use of health care resources may be greater among patients whose seizures are less than optimally controlled, which "may have important health care utilization implications for patients and payers," said Dr. Velez, director of health economics and outcomes research at Sunovion Pharmaceuticals, a manufacturer of antiepileptic drugs (AEDs).
The investigators reviewed the use of health care resources in 26,625 adults with epilepsy who were enrolled in the MarketScan database, a national database that contains data on over 180 million patients since 1995, according to its website. The patients were treated with at least one add-on AED within 60 days of being diagnosed and were followed for at least 1 year (the average follow-up time was 3.25 years).
Dr. Velez and his colleagues fit the patients into three categories:
• Uncontrolled, defined as one emergency room or inpatient stay preceded by two or more changes in drug therapy, either an additional drug or a switch in drugs in the preceding 6 months.
• Well controlled, meaning no change in AED therapy and no epilepsy-related emergency room or inpatient visits.
• Intermediate controlled, which meant that the patient was not classified as uncontrolled or well controlled.
Most (83%) fell into the intermediate-control category, 2% were uncontrolled, and 15% were well controlled. Their mean age was 49 years and almost 60% were women.
As expected, the use of health care resources was markedly higher among the uncontrolled patients, Dr. Velez said. Patients in the uncontrolled category used significantly more AEDs and had significantly more outpatient and neurologist visits than did those in the well-controlled and intermediate-control categories.
The uncontrolled patients used 1.6 times more AEDs than did those who were well controlled and those who were in the intermediate-control category. The rate of outpatient visits was 20% higher among uncontrolled patients than in those who were well controlled. In addition, the rate of neurologist visits among those who were uncontrolled was about 40% higher than in those who were well controlled and 19% higher than those who were in the intermediate-control category, Dr. Velez said.
Not surprisingly, patients with uncontrolled epilepsy visited the emergency room 12 times as often and stayed in the hospital more than 7 times as often as did those with well-controlled epilepsy. Their duration of hospitalizations also was nine times as long.
There were, however, no differences in the rate of emergency room visits or length of hospital stays between the intermediate control and uncontrolled patients, Dr. Velez said. In addition, the rate of inpatient hospitalizations was about 12% higher among the intermediate-control group. "The findings of this study suggest that even small departures from optimal seizure control are associated with a marked increase in health resource utilization among epilepsy patients," he said in a statement issued by the American Epilepsy Society during the meeting.
The study was performed by HERON Evidence Development AB, Stockholm, and the data analysis was funded by Sunovion.
AT AES 2013
Major finding: There were no differences in the rate of ER visits or length of hospital stays between patients with intermediate control of epilepsy and those with uncontrolled epilepsy, but those with an intermediate level of control had a 12% higher rate of inpatient hospitalization.
Data source: A retrospective study of 26,625 commercially insured adults with epilepsy who were in a national database.
Disclosures: The study was performed by HERON Evidence Development AB, Stockholm. The presenter is an employee of antiepileptic drug manufacturer Sunovion Pharmaceuticals, which funded the data analysis.
Earlier epilepsy surgery may have reproductive benefits for women
WASHINGTON – Younger age at the time of surgery, and receipt of fewer medications prior to surgery were associated with higher rates of pregnancy and birth after surgery in a study of women with epilepsy.
The findings, from a retrospective review of the charts of 113 women who underwent surgery involving cortical resection between 1997 and 2008 for intractable focal epilepsy, suggest that earlier may be better when it comes to surgical intervention for young women with epilepsy who desire pregnancy, Dr. Rachel R. Fabris reported at the annual meeting of the American Epilepsy Society.
For the women included in this analysis, the mean age was 13.3 years at epilepsy onset and the mean age was 30.5 years at time of surgery at the Mayo Clinic, Rochester, Minn. They had an average of 5.57 medication trials, 42% had at least monthly seizures, and 21% had daily seizures. They were followed for a mean of 5.7 years after surgery, and 75% had Engel Class I disease after surgery, said Dr. Fabris of the Mayo Clinic.
Prior to surgery, the women had an average of 0.93 pregnancies each, and an average of 0.73 births; after surgery, a total of 17 women had a total of 35 pregnancies and 25 births, for an average of 1.27 pregnancies and 0.96 births each.
One patient reported infertility after surgery.
Younger patients experienced the greatest increases in the number of pregnancies and births (P = .0036 and .0060, respectively), and those taking fewer medications also experienced a significant change in the number of births after surgery (P = .0362). The former finding is likely not meaningful, because younger women have higher reproductive rates than do older women, in general; the latter finding, however, may suggest a role for earlier surgical intervention in women with epilepsy, Dr. Fabris said.
These preliminary findings provide additional evidence of the importance of early surgery for intractable focal epilepsy, but their significance with respect to reproduction is uncertain given the small number of patients, she said. Dr. Fabris said she believes this to be the first study to evaluate the effect of surgery on reproductive outcomes.
"We already know from former studies that women with epilepsy have lower birth rates than women in the general population, and multiple studies have shown this to be a multifactorial process that involves an interplay of endocrine as well as societal factors," she said during a press briefing at the conference.
Since antiepileptic medications also are known to have adverse effects on fertility, it may be that this effect is contributing to the current findings, she noted, concluding that additional study with more patients is needed to provide more meaningful results. There were no relevant financial disclosures.
WASHINGTON – Younger age at the time of surgery, and receipt of fewer medications prior to surgery were associated with higher rates of pregnancy and birth after surgery in a study of women with epilepsy.
The findings, from a retrospective review of the charts of 113 women who underwent surgery involving cortical resection between 1997 and 2008 for intractable focal epilepsy, suggest that earlier may be better when it comes to surgical intervention for young women with epilepsy who desire pregnancy, Dr. Rachel R. Fabris reported at the annual meeting of the American Epilepsy Society.
For the women included in this analysis, the mean age was 13.3 years at epilepsy onset and the mean age was 30.5 years at time of surgery at the Mayo Clinic, Rochester, Minn. They had an average of 5.57 medication trials, 42% had at least monthly seizures, and 21% had daily seizures. They were followed for a mean of 5.7 years after surgery, and 75% had Engel Class I disease after surgery, said Dr. Fabris of the Mayo Clinic.
Prior to surgery, the women had an average of 0.93 pregnancies each, and an average of 0.73 births; after surgery, a total of 17 women had a total of 35 pregnancies and 25 births, for an average of 1.27 pregnancies and 0.96 births each.
One patient reported infertility after surgery.
Younger patients experienced the greatest increases in the number of pregnancies and births (P = .0036 and .0060, respectively), and those taking fewer medications also experienced a significant change in the number of births after surgery (P = .0362). The former finding is likely not meaningful, because younger women have higher reproductive rates than do older women, in general; the latter finding, however, may suggest a role for earlier surgical intervention in women with epilepsy, Dr. Fabris said.
These preliminary findings provide additional evidence of the importance of early surgery for intractable focal epilepsy, but their significance with respect to reproduction is uncertain given the small number of patients, she said. Dr. Fabris said she believes this to be the first study to evaluate the effect of surgery on reproductive outcomes.
"We already know from former studies that women with epilepsy have lower birth rates than women in the general population, and multiple studies have shown this to be a multifactorial process that involves an interplay of endocrine as well as societal factors," she said during a press briefing at the conference.
Since antiepileptic medications also are known to have adverse effects on fertility, it may be that this effect is contributing to the current findings, she noted, concluding that additional study with more patients is needed to provide more meaningful results. There were no relevant financial disclosures.
WASHINGTON – Younger age at the time of surgery, and receipt of fewer medications prior to surgery were associated with higher rates of pregnancy and birth after surgery in a study of women with epilepsy.
The findings, from a retrospective review of the charts of 113 women who underwent surgery involving cortical resection between 1997 and 2008 for intractable focal epilepsy, suggest that earlier may be better when it comes to surgical intervention for young women with epilepsy who desire pregnancy, Dr. Rachel R. Fabris reported at the annual meeting of the American Epilepsy Society.
For the women included in this analysis, the mean age was 13.3 years at epilepsy onset and the mean age was 30.5 years at time of surgery at the Mayo Clinic, Rochester, Minn. They had an average of 5.57 medication trials, 42% had at least monthly seizures, and 21% had daily seizures. They were followed for a mean of 5.7 years after surgery, and 75% had Engel Class I disease after surgery, said Dr. Fabris of the Mayo Clinic.
Prior to surgery, the women had an average of 0.93 pregnancies each, and an average of 0.73 births; after surgery, a total of 17 women had a total of 35 pregnancies and 25 births, for an average of 1.27 pregnancies and 0.96 births each.
One patient reported infertility after surgery.
Younger patients experienced the greatest increases in the number of pregnancies and births (P = .0036 and .0060, respectively), and those taking fewer medications also experienced a significant change in the number of births after surgery (P = .0362). The former finding is likely not meaningful, because younger women have higher reproductive rates than do older women, in general; the latter finding, however, may suggest a role for earlier surgical intervention in women with epilepsy, Dr. Fabris said.
These preliminary findings provide additional evidence of the importance of early surgery for intractable focal epilepsy, but their significance with respect to reproduction is uncertain given the small number of patients, she said. Dr. Fabris said she believes this to be the first study to evaluate the effect of surgery on reproductive outcomes.
"We already know from former studies that women with epilepsy have lower birth rates than women in the general population, and multiple studies have shown this to be a multifactorial process that involves an interplay of endocrine as well as societal factors," she said during a press briefing at the conference.
Since antiepileptic medications also are known to have adverse effects on fertility, it may be that this effect is contributing to the current findings, she noted, concluding that additional study with more patients is needed to provide more meaningful results. There were no relevant financial disclosures.
AT AES 2013
Major finding: Prior to surgery, the women had an average of 0.93 pregnancies each, and an average of 0.73 births; after surgery, a total of 17 women had a total of 35 pregnancies and 25 births, for an average of 1.27 pregnancies and 0.96 births each.
Data source: A chart review involving 113 patients.
Disclosures: There were no relevant financial disclosures.
Multiple antiepileptic drugs may pose risk of aseptic meningitis
WASHINGTON – Three commonly prescribed antiepileptic drugs – gabapentin, levetiracetam, and topiramate – were associated with greater risk of aseptic meningitis than was lamotrigine in a large cohort of new users of antiepileptic drugs.
Although a black box warning regarding the risk of aseptic meningitis was added to lamotrigine in 2010, no such warning is included for these three antiepileptic drugs (AEDs); the findings of this retrospective study have important implications for weighing treatment options in patients with seizures, Alexis Parente reported at the annual meeting of the American Epilepsy Society.
Among 719,749 AED users, including 60,011 children, the hazard ratios for aseptic meningitis were 1.80, 10.22, and 2.65, respectively, for gabapentin, levetiracetam, and topiramate, compared with lamotrigine, said Ms. Parente of Inovalon, Washington.
The median time to development of aseptic meningitis during a follow-up time of up to 12 months was shorter for children, compared with adults (44 days vs. 77 days), and for patients treated with levetiracetam, compared with those treated with lamotrigine (29.5 days vs. 83 days, respectively). There was no difference in time to aseptic meningitis between gabapentin and topiramate, compared with lamotrigine.
The study cohort included patients aged 2 years and older from a large, nationally representative administrative claims database who began taking an AED between 2006 and 2011. The patients were Medicaid-, Medicare-, and privately insured patients who were continuously enrolled with both medical and pharmacy benefits for at least a year prior to the index AED monotherapy prescription was filled. They did not use any other AED in the 90 days prior to the index prescription.
The findings are important, because long-term adherence to AEDs is a critical factor for the successful treatment of epilepsy, psychotic disorders, and pain, and ideal AEDs should have both high potency and tolerability over the long-term, Ms. Parente said. While some small studies have suggested that some second-generation AEDs may be safer than lamotrigine, she noted, their comparative risks have remained largely unclear.
Adverse effects are the leading cause of treatment failures and are associated with up to 25% of treatment discontinuations in these patients, leading to decreased quality of life and increased health care costs, she added.
WASHINGTON – Three commonly prescribed antiepileptic drugs – gabapentin, levetiracetam, and topiramate – were associated with greater risk of aseptic meningitis than was lamotrigine in a large cohort of new users of antiepileptic drugs.
Although a black box warning regarding the risk of aseptic meningitis was added to lamotrigine in 2010, no such warning is included for these three antiepileptic drugs (AEDs); the findings of this retrospective study have important implications for weighing treatment options in patients with seizures, Alexis Parente reported at the annual meeting of the American Epilepsy Society.
Among 719,749 AED users, including 60,011 children, the hazard ratios for aseptic meningitis were 1.80, 10.22, and 2.65, respectively, for gabapentin, levetiracetam, and topiramate, compared with lamotrigine, said Ms. Parente of Inovalon, Washington.
The median time to development of aseptic meningitis during a follow-up time of up to 12 months was shorter for children, compared with adults (44 days vs. 77 days), and for patients treated with levetiracetam, compared with those treated with lamotrigine (29.5 days vs. 83 days, respectively). There was no difference in time to aseptic meningitis between gabapentin and topiramate, compared with lamotrigine.
The study cohort included patients aged 2 years and older from a large, nationally representative administrative claims database who began taking an AED between 2006 and 2011. The patients were Medicaid-, Medicare-, and privately insured patients who were continuously enrolled with both medical and pharmacy benefits for at least a year prior to the index AED monotherapy prescription was filled. They did not use any other AED in the 90 days prior to the index prescription.
The findings are important, because long-term adherence to AEDs is a critical factor for the successful treatment of epilepsy, psychotic disorders, and pain, and ideal AEDs should have both high potency and tolerability over the long-term, Ms. Parente said. While some small studies have suggested that some second-generation AEDs may be safer than lamotrigine, she noted, their comparative risks have remained largely unclear.
Adverse effects are the leading cause of treatment failures and are associated with up to 25% of treatment discontinuations in these patients, leading to decreased quality of life and increased health care costs, she added.
WASHINGTON – Three commonly prescribed antiepileptic drugs – gabapentin, levetiracetam, and topiramate – were associated with greater risk of aseptic meningitis than was lamotrigine in a large cohort of new users of antiepileptic drugs.
Although a black box warning regarding the risk of aseptic meningitis was added to lamotrigine in 2010, no such warning is included for these three antiepileptic drugs (AEDs); the findings of this retrospective study have important implications for weighing treatment options in patients with seizures, Alexis Parente reported at the annual meeting of the American Epilepsy Society.
Among 719,749 AED users, including 60,011 children, the hazard ratios for aseptic meningitis were 1.80, 10.22, and 2.65, respectively, for gabapentin, levetiracetam, and topiramate, compared with lamotrigine, said Ms. Parente of Inovalon, Washington.
The median time to development of aseptic meningitis during a follow-up time of up to 12 months was shorter for children, compared with adults (44 days vs. 77 days), and for patients treated with levetiracetam, compared with those treated with lamotrigine (29.5 days vs. 83 days, respectively). There was no difference in time to aseptic meningitis between gabapentin and topiramate, compared with lamotrigine.
The study cohort included patients aged 2 years and older from a large, nationally representative administrative claims database who began taking an AED between 2006 and 2011. The patients were Medicaid-, Medicare-, and privately insured patients who were continuously enrolled with both medical and pharmacy benefits for at least a year prior to the index AED monotherapy prescription was filled. They did not use any other AED in the 90 days prior to the index prescription.
The findings are important, because long-term adherence to AEDs is a critical factor for the successful treatment of epilepsy, psychotic disorders, and pain, and ideal AEDs should have both high potency and tolerability over the long-term, Ms. Parente said. While some small studies have suggested that some second-generation AEDs may be safer than lamotrigine, she noted, their comparative risks have remained largely unclear.
Adverse effects are the leading cause of treatment failures and are associated with up to 25% of treatment discontinuations in these patients, leading to decreased quality of life and increased health care costs, she added.
AT AES 2013
Major finding: Hazard ratios for aseptic meningitis were 1.80, 10.22, and 2.65, respectively, for gabapentin, levetiracetam, and topiramate, compared with lamotrigine.
Data source: A retrospective cohort study involving nearly 720,000 AED users.
Disclosures: No disclosures were provided.
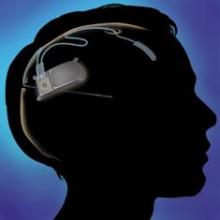
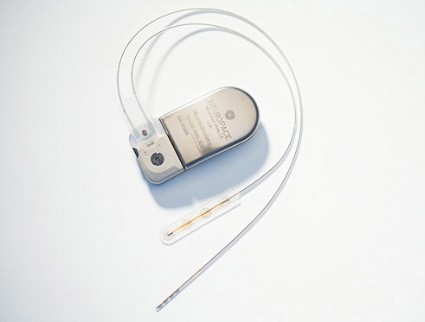
Cortical stimulation device provides safe, sustained seizure reduction
WASHINGTON – A recently approved device that provides responsive cortical stimulation was safe and provided sustained seizure reduction for a substantial number of patients with medically intractable partial onset seizures in a long-term efficacy and safety study.
The device – the RNS system (NeuroPace Inc.), which received Food and Drug Administration approval in November for use in adults – was associated with a median reduction in seizure frequency of 38.9% at 1 year and 51.1% at 2 years in 256 adult patients; 20% of patients were free of seizures for at least 6 months. No safety issues arose over a median of 4.5 years of follow-up (1,200 patient implant-years) as of Nov. 1, 2012, Dr. Gregory K. Bergey reported at the annual meeting of the American Epilepsy Society.
Study subjects had a mean age of 34 years and a mean epilepsy duration of 19.6 years. They were taking a mean of 2.9 antiepileptic drugs. Their seizures were localized to one or two seizure foci, and their median seizure frequency was 10.2 per 28 days, said Dr. Bergey, director of the Johns Hopkins Epilepsy Center in Baltimore.
The study population was extremely refractory, experiencing at least three debilitating seizures monthly and having no months with fewer than two such seizures, he noted.
The first 65 patients were implanted during an earlier 2-year feasibility study, and 191 additional patients were implanted during a 2-year, multicenter, double-blind, randomized, sham-controlled, pivotal study in which the RNS system was also found to be safe and effective as an adjunct treatment for adults with partial-onset seizures.
In the current long-term efficacy and safety study, no unanticipated device-related serious adverse events occurred, and the overall rate of device-related adverse events declined over time. The serious adverse event rate was 0.010 per implant-year for hemorrhage-related events, and 0.023 per implant-year for infection-related events. Most of these events occurred in the first year after implant.
Of 11 deaths that occurred during the study period, 2 involved suicide, 1 occurred because of complications associated with status epilepticus, 1 was because of lymphoma, and 7 were attributed to sudden unexpected death in epilepsy (including 2 among patients not being treated with responsive stimulation) for a rate of 2.5/1,000 patient stimulation-years.
"This new treatment is an option for patients with intractable partial seizures," Dr. Bergey concluded, adding that the current findings, which show progressive improvement over time, support the long-term safety and efficacy of the RNS system.
A 7-year, open-label phase of the study is ongoing to accumulate 9 years of prospective data for those implanted during the feasibility and pivotal studies.
Dr. Bergey reported having no disclosures.
WASHINGTON – A recently approved device that provides responsive cortical stimulation was safe and provided sustained seizure reduction for a substantial number of patients with medically intractable partial onset seizures in a long-term efficacy and safety study.
The device – the RNS system (NeuroPace Inc.), which received Food and Drug Administration approval in November for use in adults – was associated with a median reduction in seizure frequency of 38.9% at 1 year and 51.1% at 2 years in 256 adult patients; 20% of patients were free of seizures for at least 6 months. No safety issues arose over a median of 4.5 years of follow-up (1,200 patient implant-years) as of Nov. 1, 2012, Dr. Gregory K. Bergey reported at the annual meeting of the American Epilepsy Society.
Study subjects had a mean age of 34 years and a mean epilepsy duration of 19.6 years. They were taking a mean of 2.9 antiepileptic drugs. Their seizures were localized to one or two seizure foci, and their median seizure frequency was 10.2 per 28 days, said Dr. Bergey, director of the Johns Hopkins Epilepsy Center in Baltimore.
The study population was extremely refractory, experiencing at least three debilitating seizures monthly and having no months with fewer than two such seizures, he noted.
The first 65 patients were implanted during an earlier 2-year feasibility study, and 191 additional patients were implanted during a 2-year, multicenter, double-blind, randomized, sham-controlled, pivotal study in which the RNS system was also found to be safe and effective as an adjunct treatment for adults with partial-onset seizures.
In the current long-term efficacy and safety study, no unanticipated device-related serious adverse events occurred, and the overall rate of device-related adverse events declined over time. The serious adverse event rate was 0.010 per implant-year for hemorrhage-related events, and 0.023 per implant-year for infection-related events. Most of these events occurred in the first year after implant.
Of 11 deaths that occurred during the study period, 2 involved suicide, 1 occurred because of complications associated with status epilepticus, 1 was because of lymphoma, and 7 were attributed to sudden unexpected death in epilepsy (including 2 among patients not being treated with responsive stimulation) for a rate of 2.5/1,000 patient stimulation-years.
"This new treatment is an option for patients with intractable partial seizures," Dr. Bergey concluded, adding that the current findings, which show progressive improvement over time, support the long-term safety and efficacy of the RNS system.
A 7-year, open-label phase of the study is ongoing to accumulate 9 years of prospective data for those implanted during the feasibility and pivotal studies.
Dr. Bergey reported having no disclosures.
WASHINGTON – A recently approved device that provides responsive cortical stimulation was safe and provided sustained seizure reduction for a substantial number of patients with medically intractable partial onset seizures in a long-term efficacy and safety study.
The device – the RNS system (NeuroPace Inc.), which received Food and Drug Administration approval in November for use in adults – was associated with a median reduction in seizure frequency of 38.9% at 1 year and 51.1% at 2 years in 256 adult patients; 20% of patients were free of seizures for at least 6 months. No safety issues arose over a median of 4.5 years of follow-up (1,200 patient implant-years) as of Nov. 1, 2012, Dr. Gregory K. Bergey reported at the annual meeting of the American Epilepsy Society.
Study subjects had a mean age of 34 years and a mean epilepsy duration of 19.6 years. They were taking a mean of 2.9 antiepileptic drugs. Their seizures were localized to one or two seizure foci, and their median seizure frequency was 10.2 per 28 days, said Dr. Bergey, director of the Johns Hopkins Epilepsy Center in Baltimore.
The study population was extremely refractory, experiencing at least three debilitating seizures monthly and having no months with fewer than two such seizures, he noted.
The first 65 patients were implanted during an earlier 2-year feasibility study, and 191 additional patients were implanted during a 2-year, multicenter, double-blind, randomized, sham-controlled, pivotal study in which the RNS system was also found to be safe and effective as an adjunct treatment for adults with partial-onset seizures.
In the current long-term efficacy and safety study, no unanticipated device-related serious adverse events occurred, and the overall rate of device-related adverse events declined over time. The serious adverse event rate was 0.010 per implant-year for hemorrhage-related events, and 0.023 per implant-year for infection-related events. Most of these events occurred in the first year after implant.
Of 11 deaths that occurred during the study period, 2 involved suicide, 1 occurred because of complications associated with status epilepticus, 1 was because of lymphoma, and 7 were attributed to sudden unexpected death in epilepsy (including 2 among patients not being treated with responsive stimulation) for a rate of 2.5/1,000 patient stimulation-years.
"This new treatment is an option for patients with intractable partial seizures," Dr. Bergey concluded, adding that the current findings, which show progressive improvement over time, support the long-term safety and efficacy of the RNS system.
A 7-year, open-label phase of the study is ongoing to accumulate 9 years of prospective data for those implanted during the feasibility and pivotal studies.
Dr. Bergey reported having no disclosures.
AT AES 2013
Major finding: The median reduction in seizure frequency was 38.9% at 1 year and 51.1% at 2 years; 20% of patients were free of seizures for at least 6 months.
Data source: A study involving 256 patients with medically intractable partial onset seizures who participated in an early feasibility study and a double-blind, randomized, sham-controlled study.
Disclosures: Dr. Bergey reported having no disclosures.
Folic acid supplementation low among women with epilepsy
WASHINGTON – Only about 43% of reproductive-age women with epilepsy who completed a Web-based survey were taking a folic acid supplement, according to interim findings from the Epilepsy Birth Control Registry.
Furthermore, only 46% of those considered to be at risk for becoming pregnant (for example, those who were sexually active and who had no history of infertility) reported taking a folic acid supplement, and among respondents who were taking antiepileptic drugs (AEDs), the lowest rate of folic acid supplement use (25%) was in those taking valproate, a folic acid antagonist, Dr. Andrew G. Herzog reported at the annual meeting of the American Epilepsy Society.
Folic acid deficiency is known to be associated with fetal loss and risk of neural tube defects, and valproate is the AED associated with the highest risk of neural tube malformations, said Dr. Herzog of Harvard Medical School, Boston, and Beth Israel Deaconess Medical Center, Wellesley, Mass.
However, respondents taking valproate in this study were the least likely to have a college degree, and having a college degree was found to be associated with greater likelihood of taking a folic acid supplement. In fact, having an associate college degree or higher was the only significant demographic predictor of folic acid use; 50% of those with a college degree were taking folic acid, compared with 39% of those without a college degree, and those with an advanced degree were twice as likely as those with only a high school education to be taking folic acid, but the numbers were insufficient for determining whether AED type or education level predicted folic acid use, Dr. Herzog noted.
Study subjects were women with epilepsy aged 18 to 47 years. The first 650 to complete the survey at the registry site were included in the analysis.
Prior findings from the registry showed that this is largely a population at high risk for pregnancy; about 60% of pregnancies among respondents were unintended, compared with about 47% in the general population, he said.
Yet, the only category of respondents for which folic acid use was more than 50%, including, for example, those at risk for pregnancy, those not at risk for pregnancy, those using birth control, those not using birth control, and those trying to become pregnant, was the latter – those women actively trying to become pregnant, of whom 70% were taking folic acid, he said.
Factors including age, seizure type, and insurance status were not associated with folic acid use.
Importantly, seeing a health care professional within the year prior to the survey also was not associated with increased folic acid use.
This is concerning, given that it has been known for three decades that folic acid deficiency is associated with serious consequences, Dr. Herzog said, suggesting that education about the importance of supplementation may get pushed aside by busy physicians focused more on seizure activity and epilepsy treatment.
Dr. Herzog reported having no disclosures.
WASHINGTON – Only about 43% of reproductive-age women with epilepsy who completed a Web-based survey were taking a folic acid supplement, according to interim findings from the Epilepsy Birth Control Registry.
Furthermore, only 46% of those considered to be at risk for becoming pregnant (for example, those who were sexually active and who had no history of infertility) reported taking a folic acid supplement, and among respondents who were taking antiepileptic drugs (AEDs), the lowest rate of folic acid supplement use (25%) was in those taking valproate, a folic acid antagonist, Dr. Andrew G. Herzog reported at the annual meeting of the American Epilepsy Society.
Folic acid deficiency is known to be associated with fetal loss and risk of neural tube defects, and valproate is the AED associated with the highest risk of neural tube malformations, said Dr. Herzog of Harvard Medical School, Boston, and Beth Israel Deaconess Medical Center, Wellesley, Mass.
However, respondents taking valproate in this study were the least likely to have a college degree, and having a college degree was found to be associated with greater likelihood of taking a folic acid supplement. In fact, having an associate college degree or higher was the only significant demographic predictor of folic acid use; 50% of those with a college degree were taking folic acid, compared with 39% of those without a college degree, and those with an advanced degree were twice as likely as those with only a high school education to be taking folic acid, but the numbers were insufficient for determining whether AED type or education level predicted folic acid use, Dr. Herzog noted.
Study subjects were women with epilepsy aged 18 to 47 years. The first 650 to complete the survey at the registry site were included in the analysis.
Prior findings from the registry showed that this is largely a population at high risk for pregnancy; about 60% of pregnancies among respondents were unintended, compared with about 47% in the general population, he said.
Yet, the only category of respondents for which folic acid use was more than 50%, including, for example, those at risk for pregnancy, those not at risk for pregnancy, those using birth control, those not using birth control, and those trying to become pregnant, was the latter – those women actively trying to become pregnant, of whom 70% were taking folic acid, he said.
Factors including age, seizure type, and insurance status were not associated with folic acid use.
Importantly, seeing a health care professional within the year prior to the survey also was not associated with increased folic acid use.
This is concerning, given that it has been known for three decades that folic acid deficiency is associated with serious consequences, Dr. Herzog said, suggesting that education about the importance of supplementation may get pushed aside by busy physicians focused more on seizure activity and epilepsy treatment.
Dr. Herzog reported having no disclosures.
WASHINGTON – Only about 43% of reproductive-age women with epilepsy who completed a Web-based survey were taking a folic acid supplement, according to interim findings from the Epilepsy Birth Control Registry.
Furthermore, only 46% of those considered to be at risk for becoming pregnant (for example, those who were sexually active and who had no history of infertility) reported taking a folic acid supplement, and among respondents who were taking antiepileptic drugs (AEDs), the lowest rate of folic acid supplement use (25%) was in those taking valproate, a folic acid antagonist, Dr. Andrew G. Herzog reported at the annual meeting of the American Epilepsy Society.
Folic acid deficiency is known to be associated with fetal loss and risk of neural tube defects, and valproate is the AED associated with the highest risk of neural tube malformations, said Dr. Herzog of Harvard Medical School, Boston, and Beth Israel Deaconess Medical Center, Wellesley, Mass.
However, respondents taking valproate in this study were the least likely to have a college degree, and having a college degree was found to be associated with greater likelihood of taking a folic acid supplement. In fact, having an associate college degree or higher was the only significant demographic predictor of folic acid use; 50% of those with a college degree were taking folic acid, compared with 39% of those without a college degree, and those with an advanced degree were twice as likely as those with only a high school education to be taking folic acid, but the numbers were insufficient for determining whether AED type or education level predicted folic acid use, Dr. Herzog noted.
Study subjects were women with epilepsy aged 18 to 47 years. The first 650 to complete the survey at the registry site were included in the analysis.
Prior findings from the registry showed that this is largely a population at high risk for pregnancy; about 60% of pregnancies among respondents were unintended, compared with about 47% in the general population, he said.
Yet, the only category of respondents for which folic acid use was more than 50%, including, for example, those at risk for pregnancy, those not at risk for pregnancy, those using birth control, those not using birth control, and those trying to become pregnant, was the latter – those women actively trying to become pregnant, of whom 70% were taking folic acid, he said.
Factors including age, seizure type, and insurance status were not associated with folic acid use.
Importantly, seeing a health care professional within the year prior to the survey also was not associated with increased folic acid use.
This is concerning, given that it has been known for three decades that folic acid deficiency is associated with serious consequences, Dr. Herzog said, suggesting that education about the importance of supplementation may get pushed aside by busy physicians focused more on seizure activity and epilepsy treatment.
Dr. Herzog reported having no disclosures.
AT AES 2013
Major finding: Only 46% of reproductive-age women with epilepsy who were considered to be at risk for becoming pregnant were taking a folic acid supplement.
Data source: A Web-based survey involving 650 women.
Disclosures: Dr. Herzog reported having no disclosures.
Nursing while taking antiepileptic drugs appears safe
WASHINGTON – Exposure to antiepileptic drugs via breast milk during infancy was not associated with adverse neurodevelopmental effects at age 6 years in the prospective, observational Neurodevelopmental Effects of Antiepileptic Drugs study.
In fact, overall, the children in the study who were exposed to antiepileptic drugs (AEDs) via breast milk exhibited higher IQ and Verbal Index scores than did those who did not breastfeed (adjusted mean IQ scores of 108 vs. 104, and adjusted mean Verbal Index scores of 105 vs. 102, respectively), Dr. Kimford J. Meador of Stanford (Calif.) University reported at the annual meeting of the American Epilepsy Society.
Of 181 children in the multicenter study, 43% were breastfed for an average duration of 7.2 months. AED exposures included carbamazepine, lamotrigine, valproate, or phenytoin. IQ scores were higher for breastfed vs. nonbreastfed children for each of these drug groups except phenytoin (108 vs. 105; 114 vs. 110; 105 vs. 107; and 105 vs. 94, respectively). Significant independent variables predicting age 6 IQ included maternal IQ, AED group, AED dose, periconceptional folate, and breastfeeding.
Scores on cognitive domains other than the Verbal Index, including Nonverbal Index, Memory Index, and Executive Index, also were slightly higher for breastfed vs. nonbreastfed infants, but the differences did not reach statistical significance, Dr. Meador said.
The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, conducted in the United States and the United Kingdom, included pregnant women with epilepsy who were on AED monotherapy. The women were enrolled between 1999 and 2004, and they continued taking AEDs after delivery. Their children were assessed at age 6 years via the Differential Abilities Scales (DAS) and other measures of cognitive ability. Mean scores were adjusted for maternal IQ, AED group, AED dose, and periconceptional folate.
NEAD follows an earlier study that looked at outcomes in the children at age 3 years (Neurology 2010;75:1954-60). That report also demonstrated that children exposed to AEDs via breast milk experienced no adverse neurodevelopmental effects, but outcomes at age 6 years are considered more predictive of school performance and adult ability, Dr. Meador explained during a press briefing at the meeting.
The findings are among the first to evaluate neurodevelopmental outcomes following AED exposure via breast milk, and although they don’t provide a definitive answer about the safety of AED exposure via breast milk, they do provide reassurance for concerned mothers weighing the well-known benefits of breastfeeding with the potential risks of infant AED exposure, he said.
Dr. Meador said that he – along with the NEAD study group – recommends breastfeeding for women who were already taking AEDs during pregnancy, but noted that the findings do not address those who begin taking AEDs after delivery.
"Our recommendation is based on the known positive effects of breastfeeding, the results of our study, and unsubstantiated theoretical risk, and theoretical reasons why breastfeeding on AEDs would not offer additional risk," he concluded.
NEAD was supported by grants from the National Institutes of Health to Dr. Meador and to his coauthor, Dr. N. Browning, and from the U.K. Epilepsy Research Foundation to coauthor Dr. G. A. Baker.
WASHINGTON – Exposure to antiepileptic drugs via breast milk during infancy was not associated with adverse neurodevelopmental effects at age 6 years in the prospective, observational Neurodevelopmental Effects of Antiepileptic Drugs study.
In fact, overall, the children in the study who were exposed to antiepileptic drugs (AEDs) via breast milk exhibited higher IQ and Verbal Index scores than did those who did not breastfeed (adjusted mean IQ scores of 108 vs. 104, and adjusted mean Verbal Index scores of 105 vs. 102, respectively), Dr. Kimford J. Meador of Stanford (Calif.) University reported at the annual meeting of the American Epilepsy Society.
Of 181 children in the multicenter study, 43% were breastfed for an average duration of 7.2 months. AED exposures included carbamazepine, lamotrigine, valproate, or phenytoin. IQ scores were higher for breastfed vs. nonbreastfed children for each of these drug groups except phenytoin (108 vs. 105; 114 vs. 110; 105 vs. 107; and 105 vs. 94, respectively). Significant independent variables predicting age 6 IQ included maternal IQ, AED group, AED dose, periconceptional folate, and breastfeeding.
Scores on cognitive domains other than the Verbal Index, including Nonverbal Index, Memory Index, and Executive Index, also were slightly higher for breastfed vs. nonbreastfed infants, but the differences did not reach statistical significance, Dr. Meador said.
The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, conducted in the United States and the United Kingdom, included pregnant women with epilepsy who were on AED monotherapy. The women were enrolled between 1999 and 2004, and they continued taking AEDs after delivery. Their children were assessed at age 6 years via the Differential Abilities Scales (DAS) and other measures of cognitive ability. Mean scores were adjusted for maternal IQ, AED group, AED dose, and periconceptional folate.
NEAD follows an earlier study that looked at outcomes in the children at age 3 years (Neurology 2010;75:1954-60). That report also demonstrated that children exposed to AEDs via breast milk experienced no adverse neurodevelopmental effects, but outcomes at age 6 years are considered more predictive of school performance and adult ability, Dr. Meador explained during a press briefing at the meeting.
The findings are among the first to evaluate neurodevelopmental outcomes following AED exposure via breast milk, and although they don’t provide a definitive answer about the safety of AED exposure via breast milk, they do provide reassurance for concerned mothers weighing the well-known benefits of breastfeeding with the potential risks of infant AED exposure, he said.
Dr. Meador said that he – along with the NEAD study group – recommends breastfeeding for women who were already taking AEDs during pregnancy, but noted that the findings do not address those who begin taking AEDs after delivery.
"Our recommendation is based on the known positive effects of breastfeeding, the results of our study, and unsubstantiated theoretical risk, and theoretical reasons why breastfeeding on AEDs would not offer additional risk," he concluded.
NEAD was supported by grants from the National Institutes of Health to Dr. Meador and to his coauthor, Dr. N. Browning, and from the U.K. Epilepsy Research Foundation to coauthor Dr. G. A. Baker.
WASHINGTON – Exposure to antiepileptic drugs via breast milk during infancy was not associated with adverse neurodevelopmental effects at age 6 years in the prospective, observational Neurodevelopmental Effects of Antiepileptic Drugs study.
In fact, overall, the children in the study who were exposed to antiepileptic drugs (AEDs) via breast milk exhibited higher IQ and Verbal Index scores than did those who did not breastfeed (adjusted mean IQ scores of 108 vs. 104, and adjusted mean Verbal Index scores of 105 vs. 102, respectively), Dr. Kimford J. Meador of Stanford (Calif.) University reported at the annual meeting of the American Epilepsy Society.
Of 181 children in the multicenter study, 43% were breastfed for an average duration of 7.2 months. AED exposures included carbamazepine, lamotrigine, valproate, or phenytoin. IQ scores were higher for breastfed vs. nonbreastfed children for each of these drug groups except phenytoin (108 vs. 105; 114 vs. 110; 105 vs. 107; and 105 vs. 94, respectively). Significant independent variables predicting age 6 IQ included maternal IQ, AED group, AED dose, periconceptional folate, and breastfeeding.
Scores on cognitive domains other than the Verbal Index, including Nonverbal Index, Memory Index, and Executive Index, also were slightly higher for breastfed vs. nonbreastfed infants, but the differences did not reach statistical significance, Dr. Meador said.
The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, conducted in the United States and the United Kingdom, included pregnant women with epilepsy who were on AED monotherapy. The women were enrolled between 1999 and 2004, and they continued taking AEDs after delivery. Their children were assessed at age 6 years via the Differential Abilities Scales (DAS) and other measures of cognitive ability. Mean scores were adjusted for maternal IQ, AED group, AED dose, and periconceptional folate.
NEAD follows an earlier study that looked at outcomes in the children at age 3 years (Neurology 2010;75:1954-60). That report also demonstrated that children exposed to AEDs via breast milk experienced no adverse neurodevelopmental effects, but outcomes at age 6 years are considered more predictive of school performance and adult ability, Dr. Meador explained during a press briefing at the meeting.
The findings are among the first to evaluate neurodevelopmental outcomes following AED exposure via breast milk, and although they don’t provide a definitive answer about the safety of AED exposure via breast milk, they do provide reassurance for concerned mothers weighing the well-known benefits of breastfeeding with the potential risks of infant AED exposure, he said.
Dr. Meador said that he – along with the NEAD study group – recommends breastfeeding for women who were already taking AEDs during pregnancy, but noted that the findings do not address those who begin taking AEDs after delivery.
"Our recommendation is based on the known positive effects of breastfeeding, the results of our study, and unsubstantiated theoretical risk, and theoretical reasons why breastfeeding on AEDs would not offer additional risk," he concluded.
NEAD was supported by grants from the National Institutes of Health to Dr. Meador and to his coauthor, Dr. N. Browning, and from the U.K. Epilepsy Research Foundation to coauthor Dr. G. A. Baker.
AT AES 2013
Major finding: IQ and Verbal Index scores were higher among children exposed to AEDs via breast milk vs. those not breastfed (adjusted mean IQ scores of 108 vs. 104, and adjusted mean Verbal Index scores of 105 vs. 102, respectively).
Data source: A prospective, observational study involving 181 children either exposed or unexposed to antiepileptic drugs via breast milk.
Disclosures: NEAD was supported by grants from the National Institutes of Health to Dr. Meador and to his coauthor, Dr. N. Browning, and from the UK Epilepsy Research Foundation to coauthor Dr. G. A. Baker.
Anesthetic drugs for status epilepticus linked with death, infection risk
WASHINGTON – The use of anesthetic drugs for the treatment of refractory status epilepticus was associated with a high risk of infection and death among patients treated over a 6-year period at a tertiary academic medical center.
Although prospective, randomized controlled trials are needed to confirm the findings of this retrospective cohort study, the data should heighten awareness of the potential adverse effects of anesthetic drugs, Dr. Peter W. Kaplan said at the annual meeting of the American Epilepsy Society.
Of 171 adults who presented to University Hospital Basel, Switzerland, with status epilepticus between January 2005 and January 2011, 63 (37%) were treated with intravenous anesthetic drugs (IVADs), including thiopental, midazolam, propofol, and/or high-dose phenobarbital, and 18% of the patients died. After adjustment for status epilepticus duration and severity (based on status epilepticus severity score) and for critical medical conditions, those treated with IVADs had a significantly increased risk of death, compared with those who did not receive IVADs (relative risk = 3.16), said Dr. Kaplan of Johns Hopkins University, Baltimore.
Those who received IVADs also had a significantly higher rate of infectious complications (43% vs. 11%). The infections were diagnosed during the course of treatment, and 25 of 27 infections were respiratory infections. The remaining two cases were urinary tract infections.
No significant difference was seen between the groups with respect to seizure control, but ICU stay, a secondary outcome of the study, was significantly longer in those who received IVADs (13.5 days vs. 4.5 days), he said.
Severe hypotension also occurred more often in the patients who received IVADs.
Patients included in the study were adults with a mean age of 64 years. Those who received continuously administered IVADs were refractory to first- and second-line antiepileptic drugs and were treated according to a standard protocol; 29 received midazolam only, 22 received midazolam followed by propofol, and 12 received barbiturates after midazolam. The use of nonanesthetic antiepileptic drugs was similar in the two groups.
The findings, published online Dec. 6 in Neurology ahead of their presentation at the AES meeting, are important because data regarding the risks and benefits of anesthetic drugs for status epilepticus are lacking (Neurology 2013 Dec. 6 [doi:10.1212/WNL.0000000000000009]).
In the Neurology report, principal investigator Dr. Raoul Sutter of University Hospital Basel and his colleagues – including Dr. Kaplan – noted that "most opinion leaders recommend IVADs ... for refractory status epilepticus to induce total seizure suppression, an EEG burst-suppression pattern, or an isoelectric EEG," but explained that "the Neurocritical Care Society outlines the role of IVADs, but notes the lack of supporting data, while the European Federation of Neurological Societies points to the need for further study.
"In our cohort, the relation between the use of IVADs and death was not modified by different grades of status epilepticus severity, suggesting that the association of IVADs with an increased risk of death did not depend on to whom or when, but on the fact that IVADs were used," they wrote.
However, while the findings provide "class 3 evidence that patients with status epilepticus receiving IVADs have a higher proportion of infections and an increased risk of death, compared with those who did not receive IVADs," the findings should be considered preliminary until the association between IVADs and these outcomes are confirmed in prospective randomized trials, Dr. Kaplan said.
Dr. Kaplan reported having no disclosures. Dr. Sutter is supported by the Research Fund of University Basel, the Scientific Society Basel, and the Gottfried Julia Bangerter-Rhyner Foundation. He has held stock from Novartis and Roche since 2005.
WASHINGTON – The use of anesthetic drugs for the treatment of refractory status epilepticus was associated with a high risk of infection and death among patients treated over a 6-year period at a tertiary academic medical center.
Although prospective, randomized controlled trials are needed to confirm the findings of this retrospective cohort study, the data should heighten awareness of the potential adverse effects of anesthetic drugs, Dr. Peter W. Kaplan said at the annual meeting of the American Epilepsy Society.
Of 171 adults who presented to University Hospital Basel, Switzerland, with status epilepticus between January 2005 and January 2011, 63 (37%) were treated with intravenous anesthetic drugs (IVADs), including thiopental, midazolam, propofol, and/or high-dose phenobarbital, and 18% of the patients died. After adjustment for status epilepticus duration and severity (based on status epilepticus severity score) and for critical medical conditions, those treated with IVADs had a significantly increased risk of death, compared with those who did not receive IVADs (relative risk = 3.16), said Dr. Kaplan of Johns Hopkins University, Baltimore.
Those who received IVADs also had a significantly higher rate of infectious complications (43% vs. 11%). The infections were diagnosed during the course of treatment, and 25 of 27 infections were respiratory infections. The remaining two cases were urinary tract infections.
No significant difference was seen between the groups with respect to seizure control, but ICU stay, a secondary outcome of the study, was significantly longer in those who received IVADs (13.5 days vs. 4.5 days), he said.
Severe hypotension also occurred more often in the patients who received IVADs.
Patients included in the study were adults with a mean age of 64 years. Those who received continuously administered IVADs were refractory to first- and second-line antiepileptic drugs and were treated according to a standard protocol; 29 received midazolam only, 22 received midazolam followed by propofol, and 12 received barbiturates after midazolam. The use of nonanesthetic antiepileptic drugs was similar in the two groups.
The findings, published online Dec. 6 in Neurology ahead of their presentation at the AES meeting, are important because data regarding the risks and benefits of anesthetic drugs for status epilepticus are lacking (Neurology 2013 Dec. 6 [doi:10.1212/WNL.0000000000000009]).
In the Neurology report, principal investigator Dr. Raoul Sutter of University Hospital Basel and his colleagues – including Dr. Kaplan – noted that "most opinion leaders recommend IVADs ... for refractory status epilepticus to induce total seizure suppression, an EEG burst-suppression pattern, or an isoelectric EEG," but explained that "the Neurocritical Care Society outlines the role of IVADs, but notes the lack of supporting data, while the European Federation of Neurological Societies points to the need for further study.
"In our cohort, the relation between the use of IVADs and death was not modified by different grades of status epilepticus severity, suggesting that the association of IVADs with an increased risk of death did not depend on to whom or when, but on the fact that IVADs were used," they wrote.
However, while the findings provide "class 3 evidence that patients with status epilepticus receiving IVADs have a higher proportion of infections and an increased risk of death, compared with those who did not receive IVADs," the findings should be considered preliminary until the association between IVADs and these outcomes are confirmed in prospective randomized trials, Dr. Kaplan said.
Dr. Kaplan reported having no disclosures. Dr. Sutter is supported by the Research Fund of University Basel, the Scientific Society Basel, and the Gottfried Julia Bangerter-Rhyner Foundation. He has held stock from Novartis and Roche since 2005.
WASHINGTON – The use of anesthetic drugs for the treatment of refractory status epilepticus was associated with a high risk of infection and death among patients treated over a 6-year period at a tertiary academic medical center.
Although prospective, randomized controlled trials are needed to confirm the findings of this retrospective cohort study, the data should heighten awareness of the potential adverse effects of anesthetic drugs, Dr. Peter W. Kaplan said at the annual meeting of the American Epilepsy Society.
Of 171 adults who presented to University Hospital Basel, Switzerland, with status epilepticus between January 2005 and January 2011, 63 (37%) were treated with intravenous anesthetic drugs (IVADs), including thiopental, midazolam, propofol, and/or high-dose phenobarbital, and 18% of the patients died. After adjustment for status epilepticus duration and severity (based on status epilepticus severity score) and for critical medical conditions, those treated with IVADs had a significantly increased risk of death, compared with those who did not receive IVADs (relative risk = 3.16), said Dr. Kaplan of Johns Hopkins University, Baltimore.
Those who received IVADs also had a significantly higher rate of infectious complications (43% vs. 11%). The infections were diagnosed during the course of treatment, and 25 of 27 infections were respiratory infections. The remaining two cases were urinary tract infections.
No significant difference was seen between the groups with respect to seizure control, but ICU stay, a secondary outcome of the study, was significantly longer in those who received IVADs (13.5 days vs. 4.5 days), he said.
Severe hypotension also occurred more often in the patients who received IVADs.
Patients included in the study were adults with a mean age of 64 years. Those who received continuously administered IVADs were refractory to first- and second-line antiepileptic drugs and were treated according to a standard protocol; 29 received midazolam only, 22 received midazolam followed by propofol, and 12 received barbiturates after midazolam. The use of nonanesthetic antiepileptic drugs was similar in the two groups.
The findings, published online Dec. 6 in Neurology ahead of their presentation at the AES meeting, are important because data regarding the risks and benefits of anesthetic drugs for status epilepticus are lacking (Neurology 2013 Dec. 6 [doi:10.1212/WNL.0000000000000009]).
In the Neurology report, principal investigator Dr. Raoul Sutter of University Hospital Basel and his colleagues – including Dr. Kaplan – noted that "most opinion leaders recommend IVADs ... for refractory status epilepticus to induce total seizure suppression, an EEG burst-suppression pattern, or an isoelectric EEG," but explained that "the Neurocritical Care Society outlines the role of IVADs, but notes the lack of supporting data, while the European Federation of Neurological Societies points to the need for further study.
"In our cohort, the relation between the use of IVADs and death was not modified by different grades of status epilepticus severity, suggesting that the association of IVADs with an increased risk of death did not depend on to whom or when, but on the fact that IVADs were used," they wrote.
However, while the findings provide "class 3 evidence that patients with status epilepticus receiving IVADs have a higher proportion of infections and an increased risk of death, compared with those who did not receive IVADs," the findings should be considered preliminary until the association between IVADs and these outcomes are confirmed in prospective randomized trials, Dr. Kaplan said.
Dr. Kaplan reported having no disclosures. Dr. Sutter is supported by the Research Fund of University Basel, the Scientific Society Basel, and the Gottfried Julia Bangerter-Rhyner Foundation. He has held stock from Novartis and Roche since 2005.
AT AES 2013
Major finding: Patients treated with IVADs vs. those not treated with IVADs had an increased risk of death (relative risk = 3.16) and higher infection rate (43% vs. 11%).
Data source: A retrospective cohort study involving 171 patients.
Disclosures: Dr. Kaplan reported having no disclosures. Dr. Sutter is supported by the Research Fund of the University Basel, the Scientific Society Basel, and the Gottfried Julia Bangerter-Rhyner Foundation. He has held stock from Novartis and Roche since 2005.
Persistent electrographic seizures occur in one-third of convulsive status epilepticus patients
WASHINGTON – Electrographic seizures persisted following control of convulsive status epilepticus in about a third of children treated in pediatric intensive care units at 11 hospitals, according to a retrospective study.
Of 98 children aged 1 month to 21 years (median, 5 years) who underwent continuous electroencephalographic monitoring (typically for 12-48 hours) after presenting with convulsive status epilepticus (CSE), 32 (33%) had electrographic seizures following control of CSE, including 11 (34%) who had electroencephalographic-only seizures, and 17 (53%) who had electroclinical seizures. In four patients (13%), data regarding clinical correlates were unavailable, Dr. Iván Sánchez Fernández reported at the annual meeting of the American Epilepsy Society.
Factors significantly associated with persistent electrographic seizures were prior diagnosis of epilepsy (P = .029) and the presence of interictal epileptiform discharges (P less than .0005), but about a third of children with persistent electrographic seizures had no clinical correlates associated with the seizure, said Dr. Sánchez Fernández of the department of neurology at Boston Children’s Hospital and Harvard Medical School, Boston.
Few data looking at the rate of electrographic seizures after CSE are available. The current findings are among the first to look at this and to characterize persistent electrographic seizures after CSE, he said.
Of the 32 patients who had electrographic seizures, 15 (47%) had electrographic status epilepticus, including 6 patients (40%) with continuous status epilepticus and 9 (60%) with intermittent status epilepticus. A comparison of patients with electrographic seizures who had status epilepticus and patients with electrographic seizures who did not have status epilepticus, as well as with those who had no electrographic seizures, showed that the presence of an abnormal initial background category on continuous electroencephalogram and the presence of sporadic interictal epileptiform discharges were significantly associated with electrographic status epilepticus, he said.
Slightly more than half (53) of the children included in this study were boys. The typical duration of electrographic seizures was less than 1 minute in 34% of cases, 1-5 minutes in 31% of cases, 6-30 minutes in 22% of cases, and more than 30 minutes in 13% of cases. Seizure onset localization was focal in 41% of cases, multifocal in 20.5% of cases, generalized in 30.8% of cases, and unknown in 7.7% of cases. Maximal spread localization was focal-unilateral in 38% of cases, bilateral in 50% of cases, and unknown in 12% of cases.
Patients with electrographic seizures also stayed in the ICU longer than did those without such seizures. The median ICU stay was 9.5 days for those with electrographic seizures, compared with only 2 days for those without electrographic seizures, but it is unclear from these data whether the seizures were the cause of the prolonged stay, Dr. Sánchez Fernández said.
No disclosures were available at press time.
WASHINGTON – Electrographic seizures persisted following control of convulsive status epilepticus in about a third of children treated in pediatric intensive care units at 11 hospitals, according to a retrospective study.
Of 98 children aged 1 month to 21 years (median, 5 years) who underwent continuous electroencephalographic monitoring (typically for 12-48 hours) after presenting with convulsive status epilepticus (CSE), 32 (33%) had electrographic seizures following control of CSE, including 11 (34%) who had electroencephalographic-only seizures, and 17 (53%) who had electroclinical seizures. In four patients (13%), data regarding clinical correlates were unavailable, Dr. Iván Sánchez Fernández reported at the annual meeting of the American Epilepsy Society.
Factors significantly associated with persistent electrographic seizures were prior diagnosis of epilepsy (P = .029) and the presence of interictal epileptiform discharges (P less than .0005), but about a third of children with persistent electrographic seizures had no clinical correlates associated with the seizure, said Dr. Sánchez Fernández of the department of neurology at Boston Children’s Hospital and Harvard Medical School, Boston.
Few data looking at the rate of electrographic seizures after CSE are available. The current findings are among the first to look at this and to characterize persistent electrographic seizures after CSE, he said.
Of the 32 patients who had electrographic seizures, 15 (47%) had electrographic status epilepticus, including 6 patients (40%) with continuous status epilepticus and 9 (60%) with intermittent status epilepticus. A comparison of patients with electrographic seizures who had status epilepticus and patients with electrographic seizures who did not have status epilepticus, as well as with those who had no electrographic seizures, showed that the presence of an abnormal initial background category on continuous electroencephalogram and the presence of sporadic interictal epileptiform discharges were significantly associated with electrographic status epilepticus, he said.
Slightly more than half (53) of the children included in this study were boys. The typical duration of electrographic seizures was less than 1 minute in 34% of cases, 1-5 minutes in 31% of cases, 6-30 minutes in 22% of cases, and more than 30 minutes in 13% of cases. Seizure onset localization was focal in 41% of cases, multifocal in 20.5% of cases, generalized in 30.8% of cases, and unknown in 7.7% of cases. Maximal spread localization was focal-unilateral in 38% of cases, bilateral in 50% of cases, and unknown in 12% of cases.
Patients with electrographic seizures also stayed in the ICU longer than did those without such seizures. The median ICU stay was 9.5 days for those with electrographic seizures, compared with only 2 days for those without electrographic seizures, but it is unclear from these data whether the seizures were the cause of the prolonged stay, Dr. Sánchez Fernández said.
No disclosures were available at press time.
WASHINGTON – Electrographic seizures persisted following control of convulsive status epilepticus in about a third of children treated in pediatric intensive care units at 11 hospitals, according to a retrospective study.
Of 98 children aged 1 month to 21 years (median, 5 years) who underwent continuous electroencephalographic monitoring (typically for 12-48 hours) after presenting with convulsive status epilepticus (CSE), 32 (33%) had electrographic seizures following control of CSE, including 11 (34%) who had electroencephalographic-only seizures, and 17 (53%) who had electroclinical seizures. In four patients (13%), data regarding clinical correlates were unavailable, Dr. Iván Sánchez Fernández reported at the annual meeting of the American Epilepsy Society.
Factors significantly associated with persistent electrographic seizures were prior diagnosis of epilepsy (P = .029) and the presence of interictal epileptiform discharges (P less than .0005), but about a third of children with persistent electrographic seizures had no clinical correlates associated with the seizure, said Dr. Sánchez Fernández of the department of neurology at Boston Children’s Hospital and Harvard Medical School, Boston.
Few data looking at the rate of electrographic seizures after CSE are available. The current findings are among the first to look at this and to characterize persistent electrographic seizures after CSE, he said.
Of the 32 patients who had electrographic seizures, 15 (47%) had electrographic status epilepticus, including 6 patients (40%) with continuous status epilepticus and 9 (60%) with intermittent status epilepticus. A comparison of patients with electrographic seizures who had status epilepticus and patients with electrographic seizures who did not have status epilepticus, as well as with those who had no electrographic seizures, showed that the presence of an abnormal initial background category on continuous electroencephalogram and the presence of sporadic interictal epileptiform discharges were significantly associated with electrographic status epilepticus, he said.
Slightly more than half (53) of the children included in this study were boys. The typical duration of electrographic seizures was less than 1 minute in 34% of cases, 1-5 minutes in 31% of cases, 6-30 minutes in 22% of cases, and more than 30 minutes in 13% of cases. Seizure onset localization was focal in 41% of cases, multifocal in 20.5% of cases, generalized in 30.8% of cases, and unknown in 7.7% of cases. Maximal spread localization was focal-unilateral in 38% of cases, bilateral in 50% of cases, and unknown in 12% of cases.
Patients with electrographic seizures also stayed in the ICU longer than did those without such seizures. The median ICU stay was 9.5 days for those with electrographic seizures, compared with only 2 days for those without electrographic seizures, but it is unclear from these data whether the seizures were the cause of the prolonged stay, Dr. Sánchez Fernández said.
No disclosures were available at press time.
AT AES 2013
Major finding: Factors significantly associated with persistent electrographic seizures were prior diagnosis of epilepsy (P = .029) and the presence of interictal epileptiform discharges (P less than .0005).
Data source: A retrospective, multicenter study of 98 children with convulsive status epilepticus.
Disclosures: No disclosures were available at press time.