The FDA has made it easier and faster for health care professionals (HCPs) to get up-to-date drug safety information for the more than 18,000 approved drugs via its Drug Safety Labeling Changes (SLCs) database. The FDA Center for Drug Evaluation and Research recently launched a new searchable and downloadable database for SLCs information (http://www.fda.gov/slc). In most cases, the improved website provides supplemental labeling information within days of a safety label change. Now when a physician or other HCP prescribes a medicine using an e-prescribing system, the updated drug safety information displays much faster than it did with the previous safety labeling changes system. Here’s how.
Shortly after FDA approval of the new drug safety information for an existing drug, the information is entered into the safety labeling changes database. Health information technology (IT) vendors that provide clinical and drug information support for hospitals and pharmacies are then alerted to integrate the updated data into their systems as well. Instead of waiting weeks for the monthly release of all safety labeling updates, this information now is accessible within days.
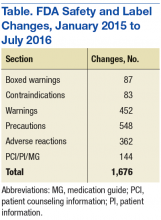
Although SLCs have been available online for many years, previously they were aggregated and posted only monthly. This time frame meant that if a new safety concern was reflected in an approved labeling change early in a month, then the information was not publicly posted until the following month—4 to 5 weeks later. The FDA recognized the need to apply new digital functionalities to shorten the time between an SLC approval and the public availability of the safety information. Between January 2015 and July 2016, FDA made more than 1,500 SLCs (Table).
As health care professionals know, the “labeling” of a medicine includes detailed information provided in the package insert that accompanies the drug whether it’s on the box, inside the product box, or folded and glued to the lid of a bottle. The product labeling includes a summary for the safe and effective use of the drug and is generally intended for use by prescribers and pharmacists.
However, when a drug is approved, not every safety concern or risk potential can be identified or known. Safety information can change multiple times over the lifetime of a drug as the FDA learns about new risks, interactions with other medications, and adverse effects.
After the FDA becomes aware of new safety information, changes to the product labeling may be required. That’s why postmarketing safety oversight is essential to learn more about the effects of medicines when they are used by a large number of people over a long period. If new safety concerns emerge after a medicine is used in a real-world setting, the FDA may require a “Safety Labeling Change.” The FDA’s new, faster connection between updated safety information and safety alerts on the pharmacy computer system can help build improved confidence into each drug prescription.
The new SLCs website contains a database of changed safety information from all sections of the label that addresses a drug’s safety, including:
- Boxed warning
- Contraindications
- Warnings and precautions
- Adverse reactions
- Drug interactions
- Use in specific populations
- Patient counseling information/patient information/medication guide
Health care providers, health IT vendors, and the public now have access to critical safety data that can impact the health of a patient faster than before.
Providing drug safety labeling changes quickly to health care vendors facilitates having the data further integrated into systems frequently accessed by HCPs. It also carries SLC data downstream for integration into drug information systems and other electronic venues, such as social media, news feeds, and websites, with vast reach among health care professionals, patients, and consumers. Some of these include WebMD, Medscape, American Society of Health-System Pharmacists, PDR.net, Epocrates, First Databank, and Yahoo Health.
The data files are downloadable in a comma-separated values format—a feature that allows information to be gathered faster. There also are hyperlinks to the labeling revisions at Drugs@FDA, and notifications are sent to subscribers via an RSS feed.
The FDA continues to pursue and provide innovative ways to rapidly access important information that protects and advances public health and will work to better identify class labeling changes. The FDA’s primary goal for the redesigned SLC Internet interface is to deliver drug safety labeling changes as quickly and efficiently as possible, to help create and promote better patient health.