User login
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
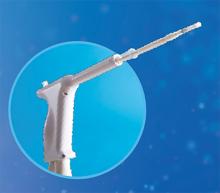
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp