User login
Fecal incontinence (FI), also known as accidental bowel leakage, is the involuntary loss of feces, which includes both liquid and solid stool as defined by the International Continence Society (ICS) and the International Urogynecological Association (IUGA).1,2 Fecal incontinence is common, occurring in 7% to 25% of community-dwelling women, and it increases with age.2-6 The condition is rarely addressed, with only 30% of women seeking care.6-8 This is due to patient embarrassment and the lack of a reliable screening tool. However, FI affects quality of life and mental health, and the associated economic burden likely will rise given the increased prevalence of FI among older women.2,4,7,9
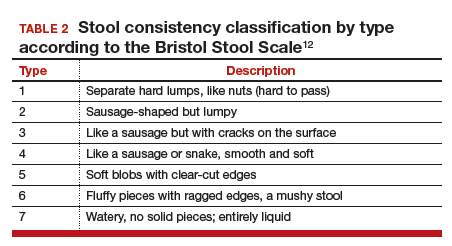
Fecal incontinence occurs due to poor stool consistency, anal and pelvic muscle weakness, reduced rectal compliance, reduced or increased rectal sensation, or bowel inflammation or dysfunction. Many conditions can cause FI (TABLE 1).5,10,11 It is therefore important to elicit a full medical history with a focus on specific bowel symptoms, such as stool consistency type (TABLE 2),12 FI frequency, and duration of symptoms, as well as to perform a complete examination to identify any readily reversible or malignant causes. A colonoscopy is recommended for individuals who meet screening criteria or present with a change in bowel symptoms, such as diarrhea, bleeding, or obstruction.13,14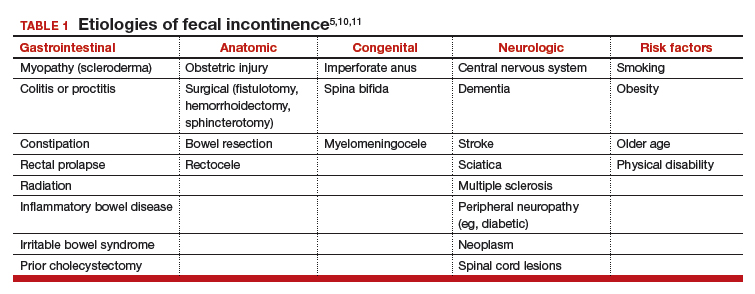
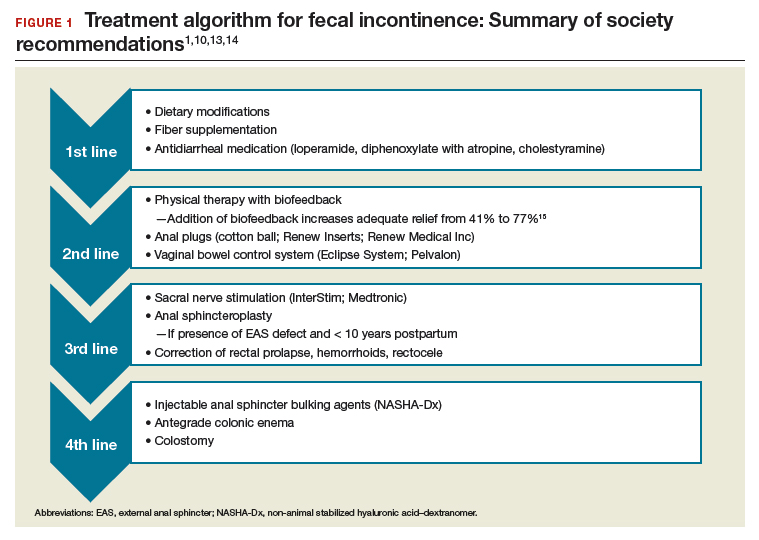
Fecal incontinence treatments include a range of approaches categorized from conservative, or first-line therapy, to fourth-line surgical managements (FIGURE 1).1,10,13,14 In this Update, we review the results of 3 well-designed trials that enrolled women with frequent nonneurogenic FI.
Common first- and second-line treatments produce equivalent improvements in FI symptoms at
6 months
Jelovsek JE, Markland AD, Whitehead WE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Controlling faecal incontinence in women by performing anal exercises with biofeedback or loperamide: a randomized clinical trial. Lancet Gastroenterol Hepatol. 2019;4:698-710.
In a multicenter, randomized trial of first- and second-line treatments for FI, Jelovsek and colleagues evaluated the efficacy of oral placebo, loperamide, pelvic floor physical therapy (PFPT) with biofeedback using anorectal manometry, or combination therapy over a 24-week period.
Continue to: Four treatments compared...
Four treatments compared
Three hundred women with FI occurring monthly for 3 months were included in the trial. Women were excluded if they had a stool classification of type 1 or type 7 on the Bristol Stool Scale, inflammatory bowel disease (IBD), history of rectovaginal fistula or cloacal defect, rectal prolapse, prior bowel diversion, fecal impaction, neurologic disorder leading to incontinence, use of loperamide or diphenoxylate within the last 30 days, childbirth within the last 3 months, need for antiretroviral drugs, hepatic impairment, or chronic abdominal pain without diarrhea.
Baseline characteristics and symptoms severity were similar among participants. The average age of the women was 63 years, with 79% white and 85% postmenopausal. Participants had a mean (SD) of 1.6 (1.8) leaks per day.
Participants were randomly assigned in a 0.5:1:1:1 fashion to receive oral placebo, loperamide, oral placebo with PFPT/biofeedback, or loperamide with PFPT/biofeedback. All participants received a standardized educational pamphlet that outlined dietary and behavioral recommendations.
Women assigned to PFPT/biofeedback received 6 sessions every other week. Loperamide was started at a dosage of 2 mg per day with the possibility of dose maintenance, escalation, reduction, or discontinuation.
Study outcomes. The primary outcome was a change from baseline to 24 weeks in the Vaizey FI symptom severity score, which assesses fecal frequency, urgency, and use of pads and medications. Secondary outcomes included assessment of a 7-day bowel diary and other quality-of-life measures. Data at 24 weeks were available for 89% of the women.
All treatment groups experienced improved FI symptoms
Based on changes in Vaizey scores after 24 weeks of treatment, women in all treatment groups had similar improvement in symptoms severity. However, those who received loperamide and PFPT/biofeedback had decreased pad changes per week and more accident-free days compared with women treated with placebo and biofeedback. Quality of life at 24 weeks was not statistically different between treatment groups as improvement was seen in all groups, including those who received oral placebo and patient education.
Adverse events. The proportion of gastrointestinal adverse effects was similar between treatment groups, ranging from 45% to 63%. Constipation was the most common adverse event overall and was more common in those taking loperamide, occurring in 51% of the loperamide plus PFPT/biofeedback group, 38% of those who received loperamide alone, 23% of the biofeedback with placebo group, and 12% of the placebo-alone group.
Strengths and limitations. Strengths of this study include its multisite, large sample size, low dropout rate, and sufficiently powered design to compare various combinations of first- and second-line therapies in women with a mean baseline FI of 1.6 leaks per day. Another strength is the robustness of the PFPT/biofeedback sessions that used anorectal manometry. This may, however, limit the study's external validity given that clinical use of this device is likely rare. Additionally, the population was comprised largely of postmenopausal and white women, which may make the findings less generalizable to other populations.
Women who suffer from frequent FI may require both loperamide and PFPT/biofeedback if they want to increase the likelihood of accident-free days and use of fewer pads. Should they note increased constipation or are not amenable to scheduled PFPT sessions, formalized education about dietary modifications, according to this study, will provide improvement in symptom severity.
Continue to: Novel vaginal bowel control system...
Novel vaginal bowel control system is effective, durable over 12 months for FI treatment
Richter HE, Dunivan G, Brown HW, et al. A 12-month clinical durability of effectiveness and safety evaluation of a vaginal bowel control system for the nonsurgical treatment of fecal incontinence. Female Pelvic Med Reconstr Surg. 2019;25:113-119.
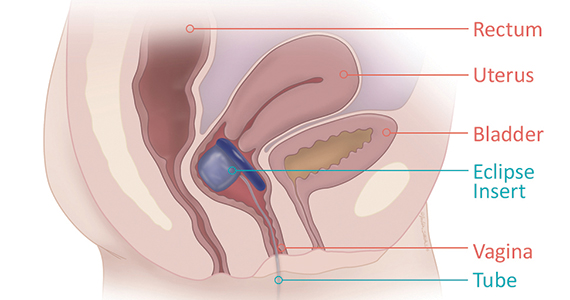
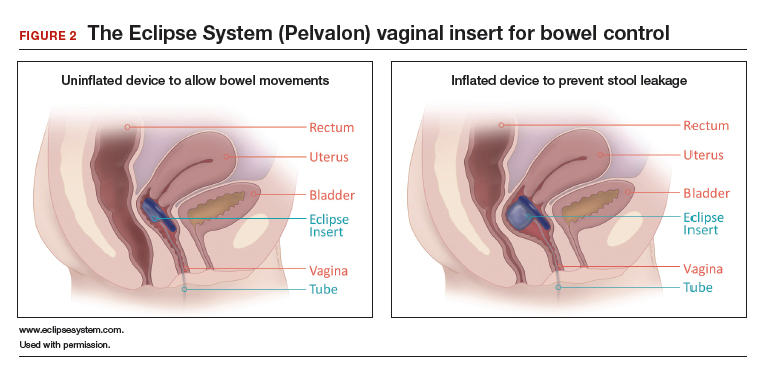
Richter and colleagues characterized clinical success, effect on quality of life, and durability over 12 months of a novel vaginal bowel control device (Eclipse System; Pelvalon) for FI in a prospective cohort study. The device is a silicone-coated vaginal insert with a detachable pump and balloon that deflects the rectovaginal septum posteriorly, thus impeding the passage of stool in the rectum (FIGURE 2).
Study eligibility criteria and treatment protocol
Women were eligible for the study if they had 4 or more episodes of fecal soiling on a 2-week bowel diary and had FI for at least 6 months. Participants were excluded if they had prolapse outside the hymen, rectovaginal fistula, IBD, congenital anorectal malformation, urinary or colorectal infection, chronic pelvic or anorectal pain, pregnancy or planning pregnancy in the next 5 months, unmanaged chronic watery diarrhea, presence of an open wound or tear in the vagina, significant urogenital atrophy, or any psychiatric or neurologic disorder that would hinder the ability to participate.
Participants successfully fitted with the device (3 attempts were allowed) were entered into the study's run-in phase. Those who were successfully fitted and had a 50% or greater reduction in FI continued into the treatment phase with 12 months of follow-up.
Of the 137 women eligible for device fitting, 62% were successfully fitted. The 73 (86%) women who had a 50% or greater reduction in FI during the run-in period comprised the intent-to-treat study population. On average, these women were 61.3 years of age, with 70% white and 82% postmenopausal. At baseline, they had a mean of 14.1 episodes of FI over 2 weeks. (Prior to enrollment, 97.3% of women attempted self-management strategies, 17.8% to 23% failed conservative therapy, and 7.8% to 13.7% failed surgical therapy.) The follow-up rate at 12 months was 74%.
Study outcomes. The primary outcome was treatment success, defined as proportion of subjects with a 50% or greater reduction in FI episodes at 3 months; this outcome also was evaluated at 6 and 12 months. Secondary outcomes were the number of FI episodes and quality-of-life measures at 3, 6, and 12 months.
Treatment success, patient satisfaction high
In the treatment phase, women had sustained improvements in symptom severity and quality-of-life measures over 12 months. Treatment success was 73% at 3 months, 71% at 6 months, and 70% at 12 months. Complete continence was achieved in 46% of participants at 12 months, and major FI episodes (requiring immediate change of undergarments) decreased from 5.0 at baseline to 0.5 at 12 months. Quality-of-life measures were improved at 3 months, and improvement was sustained over 12 months. Satisfaction was 94% at 12 months.
Adverse events. No serious device-related adverse events occurred. Mild device-related adverse events were experienced by 45% of women during the fitting process and by 38% during treatment period. These included vaginal wall injury such as hyperemia and erosion; vaginal or pelvic discomfort; vaginal infection; constipation; and lower urinary tract issues such as urinary tract infection, urinary incontinence, and voiding dysfunction. No adverse events led to treatment discontinuation.
Strengths and limitations. Strengths of this study include that it was conducted at multiple clinical sites, had a large sample size, and had a 1-year follow-up period in a population with daily FI. A limitation was that only women who had a 50% or greater reduction in FI episodes during the run-in period were followed for 12 months; however, this was 86% of the original cohort. The use of a comparative group using other devices, such as anal plugs, would have strengthened this study.
The Eclipse intravaginal bowel control device (approved by the US Food and Drug Administration in 2015) provided a sustained 50% or greater reduction in FI episodes in more than 70% of women wearing the device for 1 year, with high patient satisfaction. Thus, for women who fail conservative treatment methods for FI, clinicians should consider referring them to a urogynecologist or specialist who is knowledgeable in fitting this vaginal bowel control device.
Continue to: Sacroneuromodulation for FI…
Sacral neuromodulation for FI is effective long-term
Hull T, Giese C, Wexner SD, et al; for the SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56:234-245.
In this multicenter, prospective cohort study, Hull and colleagues evaluated the 5-year efficacy of sacral neuromodulation (SNM), also known as sacral nerve stimulation, for treatment of FI. This study followed an earlier investigation by Wexner and colleagues, which reported that 83% of 120 patients treated with SNM had a 50% or greater improvement in FI episodes at 12 months.16
Details of the study
The investigators enrolled 133 participants (92% female) who had more than 2 episodes of FI per week for longer than 6 months (12 months after vaginal delivery). Participants were excluded if they had congenital anorectal malformations, prior rectal surgery within the past 12 months (or 24 months if due to cancer), defects greater than 120° of the external anal sphincter (EAS), IBD, unmanaged chronic watery diarrhea, stool consistency type 6 or type 7 on the Bristol Stool Scale, sequela of pelvic radiation, active anal abscess or fistula, pregnancy, or planned pregnancy.
Eligible participants underwent a 2-stage procedure with the InterStim bowel control device (Medtronic). If participants experienced a 50% or greater reduction in incontinence episodes with a wearable external SNM device in the test stimulation (stage 1), they received the chronic SNM implant device (stage 2).
Participants who underwent device implantation were followed at 1, 3, and 6 months and annually for 5 years or until they exited the study. Bowel diaries and quality of life assessments were completed at baseline and at follow-up.
The primary outcome was therapeutic success, defined as 50% or greater improvement in FI episodes per week.
A total of 120 participants (90%) underwent implantation of the chronic lead and neuromodulator, and 76 (63%) were followed for 5 years. Baseline characteristics available in the initial study of 133 participants showed that the mean age was 60.5 years; 25% had undergone a prior anal sphincteroplasty; and 16.5% and 10.5% had EAS or internal anal sphincter (IAS) defects, respectively, on endoanal ultrasonography.16
Therapeutic success was high at 5 years
At the 5-year follow-up, 89% (64/72) of participants met therapeutic success, with a reduction in weekly FI episodes from 9.1 at baseline to 1.7 at 5 years. The number of incontinence pads required decreased, and more participants wore no pads at 5 years. In the intention-to-treat analysis, carrying forward the baseline FI rate in participants who lacked follow-up data, the therapeutic success rate was 69%. Quality-of-life measures improved at 5 years, both statistically and by minimal clinical difference.
Adverse events. Sixty-eight percent of participants experienced device-related adverse events, including implant site pain, change in sensation of stimulation, change in efficacy, implant site infection, or neurostimulator battery depletion (neurostimulator use commonly expires after 3 to 5 years). Of these events, 80% were successfully treated with medications, reprogramming, or no intervention. The 5-year probability of device revision or replacement was 24.4%, and the 5-year probability of device explant was 19.0%.
Strengths and limitations. Overall, this study was a well-designed, multicenter trial with long-term follow-up that showed significant improvement in FI with the use of SNM. Its strengths include the enrollment of postmenopausal women who had current defects in EAS and/or IAS on endoanal ultrasonography and 25% who had a prior sphincteroplasty. The findings therefore are relevant to the gynecologic population in whom anal sphincteroplasty would not be recommended. The study also accounted for dropouts and reported the adjusted success rate of 69% at 5 years in that group.
The lack of a control arm to rule out the placebo effect is a limitation of this study, although randomized trials comparing the effect of SNM "on" versus "off" showed greater improvement with the device "on."17
Sacral neuromodulation is an excellent therapy for women with daily FI who have failed noninvasive options and desire to proceed to a more durable, long-lasting device therapy. Although adverse events may occur, they are mild and most often resolve with device reprogramming.
- Sultan AH, Monga A, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female anorectal dysfunction. Neurourol Urodyn. 2017;36:10-34.
- Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127-136.
- Bharucha AE, Zinsmeister AR, Locke GR, et al. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004-1008.
- Perry S, Shaw C, McGrother C, et al; Leicestershire MRC Incontinence Study Team. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480-484.
- Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005-2010. Clin Gastroenterol Hepatol. 2014;12:636-643.e1-2.
- Brown HW, Wexner SD, Lukacz ES. Factors associated with care seeking among women with accidental bowel leakage. Female Pelvic Med Reconstr Surg. 2013;19:66-71.
- Norton NJ. The perspective of the patient. Gastroenterology. 2004;126(1 suppl 1):S175-S179.
- Guan W, Schmuhl NB, Brown HW. Response re: If we don't ask, they won't tell: screening for urinary and fecal incontinence by primary care providers. J Am Board Fam Med. 2019;32:119.3-120.
- Whitehead WE, Borrud L, Goode PS, et al; Pelvic Floor Disorders Network. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
- Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141-1157.
- Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559-1566.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924.
- Paquette IM, Varma MG, Kaiser AM, et al. The American Society of Colon and Rectal Surgeons' clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum. 2015;58:623-636.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 210: Fecal incontinence. Obstet Gynecol. 2019;133:e260-e273.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52:1730-1737.
- Wexner SD, Coller JA, Devroede G, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010;251:441-449.
- Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg. 2005;242:662-669.
Fecal incontinence (FI), also known as accidental bowel leakage, is the involuntary loss of feces, which includes both liquid and solid stool as defined by the International Continence Society (ICS) and the International Urogynecological Association (IUGA).1,2 Fecal incontinence is common, occurring in 7% to 25% of community-dwelling women, and it increases with age.2-6 The condition is rarely addressed, with only 30% of women seeking care.6-8 This is due to patient embarrassment and the lack of a reliable screening tool. However, FI affects quality of life and mental health, and the associated economic burden likely will rise given the increased prevalence of FI among older women.2,4,7,9
Fecal incontinence occurs due to poor stool consistency, anal and pelvic muscle weakness, reduced rectal compliance, reduced or increased rectal sensation, or bowel inflammation or dysfunction. Many conditions can cause FI (TABLE 1).5,10,11 It is therefore important to elicit a full medical history with a focus on specific bowel symptoms, such as stool consistency type (TABLE 2),12 FI frequency, and duration of symptoms, as well as to perform a complete examination to identify any readily reversible or malignant causes. A colonoscopy is recommended for individuals who meet screening criteria or present with a change in bowel symptoms, such as diarrhea, bleeding, or obstruction.13,14

Fecal incontinence treatments include a range of approaches categorized from conservative, or first-line therapy, to fourth-line surgical managements (FIGURE 1).1,10,13,14 In this Update, we review the results of 3 well-designed trials that enrolled women with frequent nonneurogenic FI.

Common first- and second-line treatments produce equivalent improvements in FI symptoms at
6 months
Jelovsek JE, Markland AD, Whitehead WE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Controlling faecal incontinence in women by performing anal exercises with biofeedback or loperamide: a randomized clinical trial. Lancet Gastroenterol Hepatol. 2019;4:698-710.
In a multicenter, randomized trial of first- and second-line treatments for FI, Jelovsek and colleagues evaluated the efficacy of oral placebo, loperamide, pelvic floor physical therapy (PFPT) with biofeedback using anorectal manometry, or combination therapy over a 24-week period.
Continue to: Four treatments compared...
Four treatments compared
Three hundred women with FI occurring monthly for 3 months were included in the trial. Women were excluded if they had a stool classification of type 1 or type 7 on the Bristol Stool Scale, inflammatory bowel disease (IBD), history of rectovaginal fistula or cloacal defect, rectal prolapse, prior bowel diversion, fecal impaction, neurologic disorder leading to incontinence, use of loperamide or diphenoxylate within the last 30 days, childbirth within the last 3 months, need for antiretroviral drugs, hepatic impairment, or chronic abdominal pain without diarrhea.
Baseline characteristics and symptoms severity were similar among participants. The average age of the women was 63 years, with 79% white and 85% postmenopausal. Participants had a mean (SD) of 1.6 (1.8) leaks per day.
Participants were randomly assigned in a 0.5:1:1:1 fashion to receive oral placebo, loperamide, oral placebo with PFPT/biofeedback, or loperamide with PFPT/biofeedback. All participants received a standardized educational pamphlet that outlined dietary and behavioral recommendations.
Women assigned to PFPT/biofeedback received 6 sessions every other week. Loperamide was started at a dosage of 2 mg per day with the possibility of dose maintenance, escalation, reduction, or discontinuation.
Study outcomes. The primary outcome was a change from baseline to 24 weeks in the Vaizey FI symptom severity score, which assesses fecal frequency, urgency, and use of pads and medications. Secondary outcomes included assessment of a 7-day bowel diary and other quality-of-life measures. Data at 24 weeks were available for 89% of the women.
All treatment groups experienced improved FI symptoms
Based on changes in Vaizey scores after 24 weeks of treatment, women in all treatment groups had similar improvement in symptoms severity. However, those who received loperamide and PFPT/biofeedback had decreased pad changes per week and more accident-free days compared with women treated with placebo and biofeedback. Quality of life at 24 weeks was not statistically different between treatment groups as improvement was seen in all groups, including those who received oral placebo and patient education.
Adverse events. The proportion of gastrointestinal adverse effects was similar between treatment groups, ranging from 45% to 63%. Constipation was the most common adverse event overall and was more common in those taking loperamide, occurring in 51% of the loperamide plus PFPT/biofeedback group, 38% of those who received loperamide alone, 23% of the biofeedback with placebo group, and 12% of the placebo-alone group.
Strengths and limitations. Strengths of this study include its multisite, large sample size, low dropout rate, and sufficiently powered design to compare various combinations of first- and second-line therapies in women with a mean baseline FI of 1.6 leaks per day. Another strength is the robustness of the PFPT/biofeedback sessions that used anorectal manometry. This may, however, limit the study's external validity given that clinical use of this device is likely rare. Additionally, the population was comprised largely of postmenopausal and white women, which may make the findings less generalizable to other populations.
Women who suffer from frequent FI may require both loperamide and PFPT/biofeedback if they want to increase the likelihood of accident-free days and use of fewer pads. Should they note increased constipation or are not amenable to scheduled PFPT sessions, formalized education about dietary modifications, according to this study, will provide improvement in symptom severity.
Continue to: Novel vaginal bowel control system...
Novel vaginal bowel control system is effective, durable over 12 months for FI treatment
Richter HE, Dunivan G, Brown HW, et al. A 12-month clinical durability of effectiveness and safety evaluation of a vaginal bowel control system for the nonsurgical treatment of fecal incontinence. Female Pelvic Med Reconstr Surg. 2019;25:113-119.
Richter and colleagues characterized clinical success, effect on quality of life, and durability over 12 months of a novel vaginal bowel control device (Eclipse System; Pelvalon) for FI in a prospective cohort study. The device is a silicone-coated vaginal insert with a detachable pump and balloon that deflects the rectovaginal septum posteriorly, thus impeding the passage of stool in the rectum (FIGURE 2).

Study eligibility criteria and treatment protocol
Women were eligible for the study if they had 4 or more episodes of fecal soiling on a 2-week bowel diary and had FI for at least 6 months. Participants were excluded if they had prolapse outside the hymen, rectovaginal fistula, IBD, congenital anorectal malformation, urinary or colorectal infection, chronic pelvic or anorectal pain, pregnancy or planning pregnancy in the next 5 months, unmanaged chronic watery diarrhea, presence of an open wound or tear in the vagina, significant urogenital atrophy, or any psychiatric or neurologic disorder that would hinder the ability to participate.
Participants successfully fitted with the device (3 attempts were allowed) were entered into the study's run-in phase. Those who were successfully fitted and had a 50% or greater reduction in FI continued into the treatment phase with 12 months of follow-up.
Of the 137 women eligible for device fitting, 62% were successfully fitted. The 73 (86%) women who had a 50% or greater reduction in FI during the run-in period comprised the intent-to-treat study population. On average, these women were 61.3 years of age, with 70% white and 82% postmenopausal. At baseline, they had a mean of 14.1 episodes of FI over 2 weeks. (Prior to enrollment, 97.3% of women attempted self-management strategies, 17.8% to 23% failed conservative therapy, and 7.8% to 13.7% failed surgical therapy.) The follow-up rate at 12 months was 74%.
Study outcomes. The primary outcome was treatment success, defined as proportion of subjects with a 50% or greater reduction in FI episodes at 3 months; this outcome also was evaluated at 6 and 12 months. Secondary outcomes were the number of FI episodes and quality-of-life measures at 3, 6, and 12 months.
Treatment success, patient satisfaction high
In the treatment phase, women had sustained improvements in symptom severity and quality-of-life measures over 12 months. Treatment success was 73% at 3 months, 71% at 6 months, and 70% at 12 months. Complete continence was achieved in 46% of participants at 12 months, and major FI episodes (requiring immediate change of undergarments) decreased from 5.0 at baseline to 0.5 at 12 months. Quality-of-life measures were improved at 3 months, and improvement was sustained over 12 months. Satisfaction was 94% at 12 months.
Adverse events. No serious device-related adverse events occurred. Mild device-related adverse events were experienced by 45% of women during the fitting process and by 38% during treatment period. These included vaginal wall injury such as hyperemia and erosion; vaginal or pelvic discomfort; vaginal infection; constipation; and lower urinary tract issues such as urinary tract infection, urinary incontinence, and voiding dysfunction. No adverse events led to treatment discontinuation.
Strengths and limitations. Strengths of this study include that it was conducted at multiple clinical sites, had a large sample size, and had a 1-year follow-up period in a population with daily FI. A limitation was that only women who had a 50% or greater reduction in FI episodes during the run-in period were followed for 12 months; however, this was 86% of the original cohort. The use of a comparative group using other devices, such as anal plugs, would have strengthened this study.
The Eclipse intravaginal bowel control device (approved by the US Food and Drug Administration in 2015) provided a sustained 50% or greater reduction in FI episodes in more than 70% of women wearing the device for 1 year, with high patient satisfaction. Thus, for women who fail conservative treatment methods for FI, clinicians should consider referring them to a urogynecologist or specialist who is knowledgeable in fitting this vaginal bowel control device.
Continue to: Sacroneuromodulation for FI…
Sacral neuromodulation for FI is effective long-term
Hull T, Giese C, Wexner SD, et al; for the SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56:234-245.
In this multicenter, prospective cohort study, Hull and colleagues evaluated the 5-year efficacy of sacral neuromodulation (SNM), also known as sacral nerve stimulation, for treatment of FI. This study followed an earlier investigation by Wexner and colleagues, which reported that 83% of 120 patients treated with SNM had a 50% or greater improvement in FI episodes at 12 months.16
Details of the study
The investigators enrolled 133 participants (92% female) who had more than 2 episodes of FI per week for longer than 6 months (12 months after vaginal delivery). Participants were excluded if they had congenital anorectal malformations, prior rectal surgery within the past 12 months (or 24 months if due to cancer), defects greater than 120° of the external anal sphincter (EAS), IBD, unmanaged chronic watery diarrhea, stool consistency type 6 or type 7 on the Bristol Stool Scale, sequela of pelvic radiation, active anal abscess or fistula, pregnancy, or planned pregnancy.
Eligible participants underwent a 2-stage procedure with the InterStim bowel control device (Medtronic). If participants experienced a 50% or greater reduction in incontinence episodes with a wearable external SNM device in the test stimulation (stage 1), they received the chronic SNM implant device (stage 2).
Participants who underwent device implantation were followed at 1, 3, and 6 months and annually for 5 years or until they exited the study. Bowel diaries and quality of life assessments were completed at baseline and at follow-up.
The primary outcome was therapeutic success, defined as 50% or greater improvement in FI episodes per week.
A total of 120 participants (90%) underwent implantation of the chronic lead and neuromodulator, and 76 (63%) were followed for 5 years. Baseline characteristics available in the initial study of 133 participants showed that the mean age was 60.5 years; 25% had undergone a prior anal sphincteroplasty; and 16.5% and 10.5% had EAS or internal anal sphincter (IAS) defects, respectively, on endoanal ultrasonography.16
Therapeutic success was high at 5 years
At the 5-year follow-up, 89% (64/72) of participants met therapeutic success, with a reduction in weekly FI episodes from 9.1 at baseline to 1.7 at 5 years. The number of incontinence pads required decreased, and more participants wore no pads at 5 years. In the intention-to-treat analysis, carrying forward the baseline FI rate in participants who lacked follow-up data, the therapeutic success rate was 69%. Quality-of-life measures improved at 5 years, both statistically and by minimal clinical difference.
Adverse events. Sixty-eight percent of participants experienced device-related adverse events, including implant site pain, change in sensation of stimulation, change in efficacy, implant site infection, or neurostimulator battery depletion (neurostimulator use commonly expires after 3 to 5 years). Of these events, 80% were successfully treated with medications, reprogramming, or no intervention. The 5-year probability of device revision or replacement was 24.4%, and the 5-year probability of device explant was 19.0%.
Strengths and limitations. Overall, this study was a well-designed, multicenter trial with long-term follow-up that showed significant improvement in FI with the use of SNM. Its strengths include the enrollment of postmenopausal women who had current defects in EAS and/or IAS on endoanal ultrasonography and 25% who had a prior sphincteroplasty. The findings therefore are relevant to the gynecologic population in whom anal sphincteroplasty would not be recommended. The study also accounted for dropouts and reported the adjusted success rate of 69% at 5 years in that group.
The lack of a control arm to rule out the placebo effect is a limitation of this study, although randomized trials comparing the effect of SNM "on" versus "off" showed greater improvement with the device "on."17
Sacral neuromodulation is an excellent therapy for women with daily FI who have failed noninvasive options and desire to proceed to a more durable, long-lasting device therapy. Although adverse events may occur, they are mild and most often resolve with device reprogramming.
Fecal incontinence (FI), also known as accidental bowel leakage, is the involuntary loss of feces, which includes both liquid and solid stool as defined by the International Continence Society (ICS) and the International Urogynecological Association (IUGA).1,2 Fecal incontinence is common, occurring in 7% to 25% of community-dwelling women, and it increases with age.2-6 The condition is rarely addressed, with only 30% of women seeking care.6-8 This is due to patient embarrassment and the lack of a reliable screening tool. However, FI affects quality of life and mental health, and the associated economic burden likely will rise given the increased prevalence of FI among older women.2,4,7,9
Fecal incontinence occurs due to poor stool consistency, anal and pelvic muscle weakness, reduced rectal compliance, reduced or increased rectal sensation, or bowel inflammation or dysfunction. Many conditions can cause FI (TABLE 1).5,10,11 It is therefore important to elicit a full medical history with a focus on specific bowel symptoms, such as stool consistency type (TABLE 2),12 FI frequency, and duration of symptoms, as well as to perform a complete examination to identify any readily reversible or malignant causes. A colonoscopy is recommended for individuals who meet screening criteria or present with a change in bowel symptoms, such as diarrhea, bleeding, or obstruction.13,14

Fecal incontinence treatments include a range of approaches categorized from conservative, or first-line therapy, to fourth-line surgical managements (FIGURE 1).1,10,13,14 In this Update, we review the results of 3 well-designed trials that enrolled women with frequent nonneurogenic FI.

Common first- and second-line treatments produce equivalent improvements in FI symptoms at
6 months
Jelovsek JE, Markland AD, Whitehead WE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Controlling faecal incontinence in women by performing anal exercises with biofeedback or loperamide: a randomized clinical trial. Lancet Gastroenterol Hepatol. 2019;4:698-710.
In a multicenter, randomized trial of first- and second-line treatments for FI, Jelovsek and colleagues evaluated the efficacy of oral placebo, loperamide, pelvic floor physical therapy (PFPT) with biofeedback using anorectal manometry, or combination therapy over a 24-week period.
Continue to: Four treatments compared...
Four treatments compared
Three hundred women with FI occurring monthly for 3 months were included in the trial. Women were excluded if they had a stool classification of type 1 or type 7 on the Bristol Stool Scale, inflammatory bowel disease (IBD), history of rectovaginal fistula or cloacal defect, rectal prolapse, prior bowel diversion, fecal impaction, neurologic disorder leading to incontinence, use of loperamide or diphenoxylate within the last 30 days, childbirth within the last 3 months, need for antiretroviral drugs, hepatic impairment, or chronic abdominal pain without diarrhea.
Baseline characteristics and symptoms severity were similar among participants. The average age of the women was 63 years, with 79% white and 85% postmenopausal. Participants had a mean (SD) of 1.6 (1.8) leaks per day.
Participants were randomly assigned in a 0.5:1:1:1 fashion to receive oral placebo, loperamide, oral placebo with PFPT/biofeedback, or loperamide with PFPT/biofeedback. All participants received a standardized educational pamphlet that outlined dietary and behavioral recommendations.
Women assigned to PFPT/biofeedback received 6 sessions every other week. Loperamide was started at a dosage of 2 mg per day with the possibility of dose maintenance, escalation, reduction, or discontinuation.
Study outcomes. The primary outcome was a change from baseline to 24 weeks in the Vaizey FI symptom severity score, which assesses fecal frequency, urgency, and use of pads and medications. Secondary outcomes included assessment of a 7-day bowel diary and other quality-of-life measures. Data at 24 weeks were available for 89% of the women.
All treatment groups experienced improved FI symptoms
Based on changes in Vaizey scores after 24 weeks of treatment, women in all treatment groups had similar improvement in symptoms severity. However, those who received loperamide and PFPT/biofeedback had decreased pad changes per week and more accident-free days compared with women treated with placebo and biofeedback. Quality of life at 24 weeks was not statistically different between treatment groups as improvement was seen in all groups, including those who received oral placebo and patient education.
Adverse events. The proportion of gastrointestinal adverse effects was similar between treatment groups, ranging from 45% to 63%. Constipation was the most common adverse event overall and was more common in those taking loperamide, occurring in 51% of the loperamide plus PFPT/biofeedback group, 38% of those who received loperamide alone, 23% of the biofeedback with placebo group, and 12% of the placebo-alone group.
Strengths and limitations. Strengths of this study include its multisite, large sample size, low dropout rate, and sufficiently powered design to compare various combinations of first- and second-line therapies in women with a mean baseline FI of 1.6 leaks per day. Another strength is the robustness of the PFPT/biofeedback sessions that used anorectal manometry. This may, however, limit the study's external validity given that clinical use of this device is likely rare. Additionally, the population was comprised largely of postmenopausal and white women, which may make the findings less generalizable to other populations.
Women who suffer from frequent FI may require both loperamide and PFPT/biofeedback if they want to increase the likelihood of accident-free days and use of fewer pads. Should they note increased constipation or are not amenable to scheduled PFPT sessions, formalized education about dietary modifications, according to this study, will provide improvement in symptom severity.
Continue to: Novel vaginal bowel control system...
Novel vaginal bowel control system is effective, durable over 12 months for FI treatment
Richter HE, Dunivan G, Brown HW, et al. A 12-month clinical durability of effectiveness and safety evaluation of a vaginal bowel control system for the nonsurgical treatment of fecal incontinence. Female Pelvic Med Reconstr Surg. 2019;25:113-119.
Richter and colleagues characterized clinical success, effect on quality of life, and durability over 12 months of a novel vaginal bowel control device (Eclipse System; Pelvalon) for FI in a prospective cohort study. The device is a silicone-coated vaginal insert with a detachable pump and balloon that deflects the rectovaginal septum posteriorly, thus impeding the passage of stool in the rectum (FIGURE 2).

Study eligibility criteria and treatment protocol
Women were eligible for the study if they had 4 or more episodes of fecal soiling on a 2-week bowel diary and had FI for at least 6 months. Participants were excluded if they had prolapse outside the hymen, rectovaginal fistula, IBD, congenital anorectal malformation, urinary or colorectal infection, chronic pelvic or anorectal pain, pregnancy or planning pregnancy in the next 5 months, unmanaged chronic watery diarrhea, presence of an open wound or tear in the vagina, significant urogenital atrophy, or any psychiatric or neurologic disorder that would hinder the ability to participate.
Participants successfully fitted with the device (3 attempts were allowed) were entered into the study's run-in phase. Those who were successfully fitted and had a 50% or greater reduction in FI continued into the treatment phase with 12 months of follow-up.
Of the 137 women eligible for device fitting, 62% were successfully fitted. The 73 (86%) women who had a 50% or greater reduction in FI during the run-in period comprised the intent-to-treat study population. On average, these women were 61.3 years of age, with 70% white and 82% postmenopausal. At baseline, they had a mean of 14.1 episodes of FI over 2 weeks. (Prior to enrollment, 97.3% of women attempted self-management strategies, 17.8% to 23% failed conservative therapy, and 7.8% to 13.7% failed surgical therapy.) The follow-up rate at 12 months was 74%.
Study outcomes. The primary outcome was treatment success, defined as proportion of subjects with a 50% or greater reduction in FI episodes at 3 months; this outcome also was evaluated at 6 and 12 months. Secondary outcomes were the number of FI episodes and quality-of-life measures at 3, 6, and 12 months.
Treatment success, patient satisfaction high
In the treatment phase, women had sustained improvements in symptom severity and quality-of-life measures over 12 months. Treatment success was 73% at 3 months, 71% at 6 months, and 70% at 12 months. Complete continence was achieved in 46% of participants at 12 months, and major FI episodes (requiring immediate change of undergarments) decreased from 5.0 at baseline to 0.5 at 12 months. Quality-of-life measures were improved at 3 months, and improvement was sustained over 12 months. Satisfaction was 94% at 12 months.
Adverse events. No serious device-related adverse events occurred. Mild device-related adverse events were experienced by 45% of women during the fitting process and by 38% during treatment period. These included vaginal wall injury such as hyperemia and erosion; vaginal or pelvic discomfort; vaginal infection; constipation; and lower urinary tract issues such as urinary tract infection, urinary incontinence, and voiding dysfunction. No adverse events led to treatment discontinuation.
Strengths and limitations. Strengths of this study include that it was conducted at multiple clinical sites, had a large sample size, and had a 1-year follow-up period in a population with daily FI. A limitation was that only women who had a 50% or greater reduction in FI episodes during the run-in period were followed for 12 months; however, this was 86% of the original cohort. The use of a comparative group using other devices, such as anal plugs, would have strengthened this study.
The Eclipse intravaginal bowel control device (approved by the US Food and Drug Administration in 2015) provided a sustained 50% or greater reduction in FI episodes in more than 70% of women wearing the device for 1 year, with high patient satisfaction. Thus, for women who fail conservative treatment methods for FI, clinicians should consider referring them to a urogynecologist or specialist who is knowledgeable in fitting this vaginal bowel control device.
Continue to: Sacroneuromodulation for FI…
Sacral neuromodulation for FI is effective long-term
Hull T, Giese C, Wexner SD, et al; for the SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56:234-245.
In this multicenter, prospective cohort study, Hull and colleagues evaluated the 5-year efficacy of sacral neuromodulation (SNM), also known as sacral nerve stimulation, for treatment of FI. This study followed an earlier investigation by Wexner and colleagues, which reported that 83% of 120 patients treated with SNM had a 50% or greater improvement in FI episodes at 12 months.16
Details of the study
The investigators enrolled 133 participants (92% female) who had more than 2 episodes of FI per week for longer than 6 months (12 months after vaginal delivery). Participants were excluded if they had congenital anorectal malformations, prior rectal surgery within the past 12 months (or 24 months if due to cancer), defects greater than 120° of the external anal sphincter (EAS), IBD, unmanaged chronic watery diarrhea, stool consistency type 6 or type 7 on the Bristol Stool Scale, sequela of pelvic radiation, active anal abscess or fistula, pregnancy, or planned pregnancy.
Eligible participants underwent a 2-stage procedure with the InterStim bowel control device (Medtronic). If participants experienced a 50% or greater reduction in incontinence episodes with a wearable external SNM device in the test stimulation (stage 1), they received the chronic SNM implant device (stage 2).
Participants who underwent device implantation were followed at 1, 3, and 6 months and annually for 5 years or until they exited the study. Bowel diaries and quality of life assessments were completed at baseline and at follow-up.
The primary outcome was therapeutic success, defined as 50% or greater improvement in FI episodes per week.
A total of 120 participants (90%) underwent implantation of the chronic lead and neuromodulator, and 76 (63%) were followed for 5 years. Baseline characteristics available in the initial study of 133 participants showed that the mean age was 60.5 years; 25% had undergone a prior anal sphincteroplasty; and 16.5% and 10.5% had EAS or internal anal sphincter (IAS) defects, respectively, on endoanal ultrasonography.16
Therapeutic success was high at 5 years
At the 5-year follow-up, 89% (64/72) of participants met therapeutic success, with a reduction in weekly FI episodes from 9.1 at baseline to 1.7 at 5 years. The number of incontinence pads required decreased, and more participants wore no pads at 5 years. In the intention-to-treat analysis, carrying forward the baseline FI rate in participants who lacked follow-up data, the therapeutic success rate was 69%. Quality-of-life measures improved at 5 years, both statistically and by minimal clinical difference.
Adverse events. Sixty-eight percent of participants experienced device-related adverse events, including implant site pain, change in sensation of stimulation, change in efficacy, implant site infection, or neurostimulator battery depletion (neurostimulator use commonly expires after 3 to 5 years). Of these events, 80% were successfully treated with medications, reprogramming, or no intervention. The 5-year probability of device revision or replacement was 24.4%, and the 5-year probability of device explant was 19.0%.
Strengths and limitations. Overall, this study was a well-designed, multicenter trial with long-term follow-up that showed significant improvement in FI with the use of SNM. Its strengths include the enrollment of postmenopausal women who had current defects in EAS and/or IAS on endoanal ultrasonography and 25% who had a prior sphincteroplasty. The findings therefore are relevant to the gynecologic population in whom anal sphincteroplasty would not be recommended. The study also accounted for dropouts and reported the adjusted success rate of 69% at 5 years in that group.
The lack of a control arm to rule out the placebo effect is a limitation of this study, although randomized trials comparing the effect of SNM "on" versus "off" showed greater improvement with the device "on."17
Sacral neuromodulation is an excellent therapy for women with daily FI who have failed noninvasive options and desire to proceed to a more durable, long-lasting device therapy. Although adverse events may occur, they are mild and most often resolve with device reprogramming.
- Sultan AH, Monga A, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female anorectal dysfunction. Neurourol Urodyn. 2017;36:10-34.
- Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127-136.
- Bharucha AE, Zinsmeister AR, Locke GR, et al. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004-1008.
- Perry S, Shaw C, McGrother C, et al; Leicestershire MRC Incontinence Study Team. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480-484.
- Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005-2010. Clin Gastroenterol Hepatol. 2014;12:636-643.e1-2.
- Brown HW, Wexner SD, Lukacz ES. Factors associated with care seeking among women with accidental bowel leakage. Female Pelvic Med Reconstr Surg. 2013;19:66-71.
- Norton NJ. The perspective of the patient. Gastroenterology. 2004;126(1 suppl 1):S175-S179.
- Guan W, Schmuhl NB, Brown HW. Response re: If we don't ask, they won't tell: screening for urinary and fecal incontinence by primary care providers. J Am Board Fam Med. 2019;32:119.3-120.
- Whitehead WE, Borrud L, Goode PS, et al; Pelvic Floor Disorders Network. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
- Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141-1157.
- Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559-1566.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924.
- Paquette IM, Varma MG, Kaiser AM, et al. The American Society of Colon and Rectal Surgeons' clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum. 2015;58:623-636.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 210: Fecal incontinence. Obstet Gynecol. 2019;133:e260-e273.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52:1730-1737.
- Wexner SD, Coller JA, Devroede G, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010;251:441-449.
- Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg. 2005;242:662-669.
- Sultan AH, Monga A, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female anorectal dysfunction. Neurourol Urodyn. 2017;36:10-34.
- Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127-136.
- Bharucha AE, Zinsmeister AR, Locke GR, et al. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004-1008.
- Perry S, Shaw C, McGrother C, et al; Leicestershire MRC Incontinence Study Team. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480-484.
- Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005-2010. Clin Gastroenterol Hepatol. 2014;12:636-643.e1-2.
- Brown HW, Wexner SD, Lukacz ES. Factors associated with care seeking among women with accidental bowel leakage. Female Pelvic Med Reconstr Surg. 2013;19:66-71.
- Norton NJ. The perspective of the patient. Gastroenterology. 2004;126(1 suppl 1):S175-S179.
- Guan W, Schmuhl NB, Brown HW. Response re: If we don't ask, they won't tell: screening for urinary and fecal incontinence by primary care providers. J Am Board Fam Med. 2019;32:119.3-120.
- Whitehead WE, Borrud L, Goode PS, et al; Pelvic Floor Disorders Network. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
- Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141-1157.
- Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559-1566.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924.
- Paquette IM, Varma MG, Kaiser AM, et al. The American Society of Colon and Rectal Surgeons' clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum. 2015;58:623-636.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 210: Fecal incontinence. Obstet Gynecol. 2019;133:e260-e273.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52:1730-1737.
- Wexner SD, Coller JA, Devroede G, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010;251:441-449.
- Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg. 2005;242:662-669.