User login
Postoperative voiding dysfunction refers to the acute inability to spontaneously and adequately empty the bladder after surgery. Postoperative voiding dysfunction occurs in 21% to 42% of pelvic reconstructive surgeries, as well as 7% to 21% of benign gynecologic surgeries.1-4 While much of its peril lies in patient discomfort or dissatisfaction with temporary bladder drainage, serious consequences of the disorder include bladder overdistension injury with inadequate drainage and urinary tract infection (UTI) associated with prolonged catheterization.4-6
Although transient postoperative voiding dysfunction is associated with anti-incontinence surgery, tricyclic antidepressant use, diabetes, preoperative voiding dysfunction, and postoperative narcotic use, it also may occur in patients without risk factors.4,7,8 Thus, all gynecologic surgeons should be prepared to assess and manage the patient with postoperative voiding dysfunction.
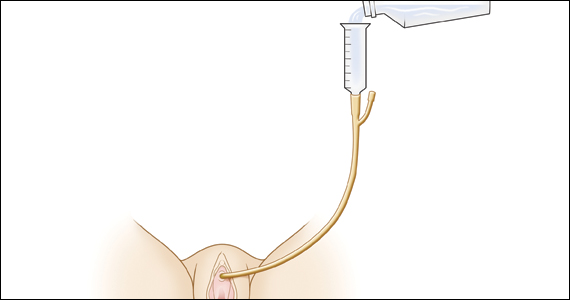
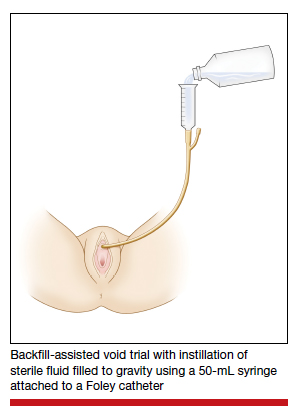
Diagnosis of postoperative voiding dysfunction can be approached in myriad ways, including spontaneous (or natural) bladder filling or bladder backfill followed by spontaneous void. When compared with spontaneous void trials, backfill-assisted void trial is associated with improved accuracy in predicting voiding dysfunction in patients who undergo urogynecologic surgery, leading to widespread adoption of the procedure following pelvic reconstructive surgeries.9,10
Criteria for “passing” a void trial may include the patient’s subjective feeling of having emptied her bladder; having a near-baseline force of stream; or commonly by objective parameters of voided volume and postvoid residual (PVR), assessed via catheterization or bladder scan.3,6,10 Completing a postoperative void trial typically requires significant nursing effort because of the technical demands of backfilling the bladder, obtaining the voided volume and PVR, or assessing subjective emptying.
Management of postoperative voiding dysfunction typically consists of continuous drainage with a transurethral catheter or clean intermittent self-catheterization (CISC). Patients discharged home with a bladder drainage method also may be prescribed various medications, such as antibiotics, anticholinergics, and bladder analgesics, which often depends on provider practice.
Given the minimal universal guidance available for gynecologic surgeons on postoperative voiding dysfunction, we review several articles that contribute new evidence on the assessment and management of this condition.
Continue to: How can we efficiently approach the postoperative void trial for pelvic floor surgery?
How can we efficiently approach the postoperative void trial for pelvic floor surgery?
Chao L, Mansuria S. Postoperative bladder filling after outpatient laparoscopic hysterectomy and time to discharge: a randomized controlled trial. Obstet Gynecol. 2019;133:879-887.
Despite efforts to implement and promote enhanced recovery after surgery pathways, waiting for spontaneous void can be a barrier to efficient same-day discharge. Chao and Mansuria conducted a randomized controlled trial (RCT) to determine whether backfilling the bladder intraoperatively, compared with spontaneous (physiologic) filling, would reduce time to discharge in patients undergoing total laparoscopic hysterectomy (TLH) or supracervical hysterectomy (SCH).
Study details

Women undergoing TLH or laparoscopic SCH for benign indications were randomly assigned to undergo either a backfill-assisted void trial in the operating room with 200 mL of sterile normal saline (n = 75) or Foley catheter removal with spontaneous fill in the postanesthesia care unit (PACU) (n = 78).
For both groups, the maximum time allowed for spontaneous void was 5 hours. A successful void trial was defined as a voided volume of at least 200 mL. If a patient was unable to void at least 200 mL, a bladder scan was performed, and the patient was considered to have failed the void trial if a PVR of 200 mL or greater was noted. If the PVR was less than 200 mL, the patient was given an additional 1 hour to spontaneously void 200 mL by 6 hours after the surgery. Patients who failed the void trial were discharged home with a transurethral catheter.
The primary outcome was time to discharge, and the sample size (153 participants included in the analysis) allowed 80% power to detect a 30-minute difference in time to discharge. Participant baseline characteristics, concomitant procedures, and indication for hysterectomy were similar for both groups.
Results. The mean time to discharge was 273.4 minutes for the backfill-assisted void trial group and 283.2 minutes for the spontaneous fill group, a difference of 9.8 minutes that was not statistically significant (P = .45).
Although it was not a primary outcome, time to spontaneous void was 24.9 minutes shorter in the backfill group (P = .04). Rates of postoperative voiding dysfunction did not differ between the 2 groups (6.7% for the backfill group and 12.8% for the spontaneous fill group; P = .2). There were no significant differences in emergency department visits, UTI rates, or readmissions.
Bladder backfill is safe, simple, and may reduce time to spontaneous void
Strengths of the study included its prospective randomized design, blinded outcome assessors, and diversity in benign gynecologic surgeries performed. Although this study found a reduced time to spontaneous void in the backfill group, it was not powered to assess this difference, limiting ability to draw conclusions from those data. Data on postoperative nausea and pain scores also were not collected, which likely influenced the overall time to discharge.
Void trial completion is one of many criteria to fulfill prior to patient discharge, and a reduced time to first void may not decrease the overall length of PACU stay if other factors, such as nausea or pain, are not controlled. Nonetheless, backfilling the bladder intraoperatively is a safe alternative that may decrease the time to first spontaneous void, and it is a relatively simple alteration in the surgical workflow that could significantly lessen PACU nursing demands.
Backfilling the bladder in the operating room prior to catheter discontinuation can reduce time to first spontaneous void, but not the overall time to discharge.
Continue to: Algorithm assesses need for PVR, although further study required...
Algorithm assesses need for PVR, although further study required
Meekins AR, Siddiqui N, Amundsen CL, et al. Improving postoperative efficiency: an algorithm for expedited void trials after urogynecologic surgery. South Med J. 2017;110:785-790.
To determine ways to further maximize postoperative efficiency, Meekins and colleagues sought to determine whether certain voided volumes during backfill-assisted void trials could obviate the need for PVR assessment.
Void trial results calculated to develop algorithm
The study was a secondary analysis of a previously conducted RCT that assessed antibiotics for the prevention of UTI after urogynecologic surgery. Void trials from the parent RCT were performed via the backfill-assisted method in which the bladder was backfilled in the PACU with 300 mL of normal saline or until the patient reported urgency to void, after which the catheter was removed and the patient was prompted to void immediately.
Postvoid residual levels were assessed via ultrasonography or catheterization. A void trial was considered to be passed when a PVR was less than 100 mL or less than 50% of the total bladder volume, with a minimum voided volume of 200 mL.
In the follow-up study, the authors analyzed the void trial results of 255 women of the original 264 in the parent RCT. A total of 69% of patients passed their void trial. The authors assessed the optimal positive predictive value (PPV) and negative predictive value (NPV) combinations, which were then used to create lower and upper voided volume thresholds that would best predict a failed or passed trial, thus obviating PVR measurement.
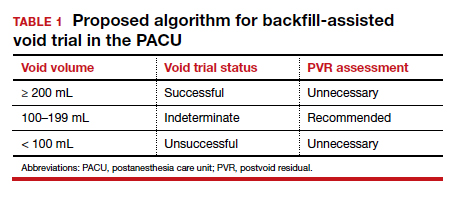
Results. When patients voided less than 100 mL, the NPV was 96.7% (meaning that they had a 96.7% chance of failing the void trial). When patients voided 200 mL or more, the PPV was 97% (meaning that they had a 97% chance of passing the void trial). Receiver operating characteristic analysis confirmed that voided volume alone was an excellent predictor of final void trial results, with area under the curve of 0.97. The authors estimated that applying this algorithm to their study population would have eliminated the need for assessing PVR in 85% of patients. Ultimately, they proposed the algorithm shown in TABLE 1.
A potential alternative for assessing PVR
This study's strengths include the use of prospectively and systematically collected void trial data in a large patient population undergoing various urogynecologic procedures. By contrast, the generalizability of the results is limited regarding other void trial methods, such as spontaneous filling and void, as well as populations outside of the studied institution.
With the algorithm, the authors estimated that the majority of postoperative patients would no longer require a PVR assessment in the PACU. This could have beneficial downstream implications, including decreasing the nursing workload, reducing total time in the PACU, and minimizing patient discomfort with PVR assessment.
While further studies are needed to validate the proposed algorithm in larger populations, this study provides evidence of an efficient alternative to the traditional approach to PVR assessment in the PACU.
Application of the algorithm proposed by the study investigators has the potential to eliminate the need for a PVR assessment in most patients following a backfill-assisted void trial.
Continue to: An alternative to Foley use if a patient does not know CISC...
An alternative to Foley use if a patient does not know CISC
Boyd SS, O'Sullivan DM, Tunitsky-Bitton E. A comparison of two methods of catheter management after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:1037-1045.
The traditional indwelling catheter as a postoperative bladder drainage method has a number of drawbacks, including an increased rate of UTI, patient discomfort, and potential limitations in mobility due to the presence of a drainage bag.5
Boyd and colleagues reported on a variation of traditional transurethral catheterization that hypothetically allows for improved mobility. With this method, the transurethral catheter is occluded with a plastic plug that is intermittently plugged and unplugged (plug-unplug method) for bladder drainage. To test whether activity levels are improved with the plug-unplug method versus the continuous drainage approach, the authors conducted an RCT in women undergoing pelvic reconstructive surgery to compare the plug-unplug method with transurethral catheterization (with a continuous drainage bag) and a reference group of freely voiding women.
Study particulars and outcomes
The trial's primary outcome was the patients' activity score as measured by the Activity Assessment Scale (AAS) at 5 to 7 days postoperatively. Because of the theoretically increased risk of a UTI with opening and closing a closed drainage system, secondary outcomes included the UTI rate, the time to pass an outpatient void trial, postoperative pain, patient satisfaction, and catheter effect. To detect an effect size of 0.33 in the primary outcome between the 3 groups, 90 participants were needed along with a difference in proportions of 0.3 between the catheterized and noncatheterized groups.
The participants were randomly assigned 1:1 preoperatively to the continuous drainage or plug-unplug method. All patients underwent a backfill-assisted void trial prior to hospital discharge; the first 30 randomly assigned patients to pass their void trial comprised the reference group. Patients in the plug-unplug arm were instructed to uncap the plastic plug to drain their bladder when they felt the urge to void or at least every 4 hours. All catheterized patients were provided with a large drainage bag for gravity-based drainage for overnight use.
Participants who were discharged home with a catheter underwent an outpatient void trial between postoperative days 5 and 7. A urinalysis was performed at that time and a urine culture was done if a patient reported UTI symptoms. All patients underwent routine follow-up until they passed the office void trial.
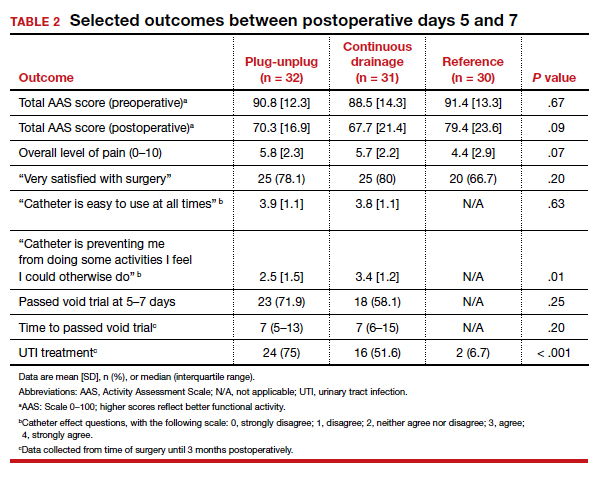
Results. Ninety-three women were included in the primary analysis. There were no differences in baseline characteristics between groups. No difference was detected in activity by AAS scores between all 3 groups (scores: plug-unplug, 70.3; continuous drainage, 67.7; reference arm, 79.4; P = .09). The 2 treatment arms had no overall difference in culture-positive UTI (plug-unplug, 68.8%; continuous drainage, 48.4%; P = .625). No significant difference was found in the percentage of patients who passed their initial outpatient void trial (plug-unplug, 71.9%, vs continuous drainage, 58.1%; P = .25) (TABLE 2).
Catheter impact on postoperative activity considered
Strengths of the study include the prospective randomized design, the inclusion of a noncatheterized reference arm, and use of a validated questionnaire to assess activity. The study was limited, however, by the inability to blind patients to treatment and the lack of power to assess other important outcomes, such as UTI rates.
Although the authors did not find a difference in activity scores between the 2 catheterization methods, no significant difference was found between the catheterized and noncatheterized groups, which suggests that catheters in general may not significantly impact postoperative activity. The theoretical concern that opening and closing a transurethral drainage system would increase UTI rates was not substantiated, although the study was not powered specifically for this outcome.
Ultimately, the plug-unplug method may be a safe alternative for patients who desire to avoid attachment to a drainage bag postoperatively.
Based on the results of an RCT that compared 2 methods of catheter management after pelvic reconstructive surgery, the plug-unplug catheterization method may be an acceptable alternative to traditional catheterization.
- Bladder backfill in the operating room followed by spontaneous void in the postanesthesia care unit (PACU) is a safe and efficient way to assess for postoperative voiding dysfunction.
- Voids of 200 mL or more (following a 300-mL backfill) may not require a PACU postvoid residual assessment.
- Postoperative activity does not appear to be impacted by the presence of an indwelling catheter.
Continue to: Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Lavelle ES, Alam P, Meister M, et al. Antibiotic prophylaxis during catheter-managed postoperative urinary retention after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:727-735.
Limited high-quality evidence supports the use of prophylactic antibiotics during catheterization following prolapse or incontinence surgery, and the Infectious Disease Society of America cautions against routine antibiotic prophylaxis for those requiring catheterization.11
Lavelle and colleagues conducted a multicenter RCT to determine whether nitrofurantoin is more effective than placebo in decreasing UTIs among patients with postoperative voiding dysfunction following surgery for prolapse or incontinence.
Focus of the study
The investigators conducted a double-blind RCT at 5 academic sites that included women with postoperative voiding dysfunction who required catheter management (transurethral indwelling catheter or CISC). Voiding dysfunction was diagnosed by backfill or spontaneous fill void trial and was defined as a PVR of greater than 100 mL. Women were randomly assigned 1:1 to nitrofurantoin 100 mg or placebo taken daily during catheter use. Catheter use was discontinued once an outpatient void trial confirmed efficient voiding.
The primary outcome was symptomatic culture-confirmed UTI within 6 weeks of surgery. Secondary outcomes included frequency of urine cultures with nitrofurantoin-resistant or intermediate-sensitivity isolates and adverse symptoms possibly related to nitrofurantoin. The authors calculated that 154 participants would provide 80% power to detect a decrease in UTI incidence from 33% to 13%, allowing for 10% dropout.
A total of 151 women were randomly assigned and included in the intention-to-treat analysis. There were no differences in baseline characteristics. The median duration of catheter use was 4 days (interquartile range, 3-7).
Results. Overall, 13 women in the nitrofurantoin group and 13 in the placebo group experienced the primary outcome of UTI within 6 weeks postoperatively (17.3% nitrofurantoin vs 17.1% placebo; P = .97; relative risk [RR], 1.01; 95% confidence interval [CI], 0.50-2.04). The number needed to treat with nitrofurantoin to prevent 1 UTI was 500. A subanalysis found no difference in UTI incidence stratified by CISC versus indwelling catheter.
Urine cultures were obtained for 94.5% of all patients reporting UTI symptoms. Four isolates of the 13 cultures in the nitrofurantoin group (30.8%) and 3 in the placebo group (21.4%) showed nitrofurantoin resistance (P = .58). The rate of endorsing at least 1 adverse symptom attributable to nitrofurantoin was similar between groups (68.0% vs 60.5%, respectively; P = .34).
Study strong points and limitations
This study's randomized, placebo-controlled design and multicenter recruitment increase the generalizability of the results. An additional strength is that the authors chose a clinically relevant definition of UTI. The study was likely underpowered, however, to detect differences in secondary outcomes, such as nitrofurantoin resistance. We cannot conclude on the role of antibiotics for patients who require more prolonged catheterization.
Notably, a similar RCT by Dieter and colleagues of 159 patients undergoing daily nitrofurantoin versus placebo during CISC or transurethral catheterization failed to detect a difference in the rate of UTI treatment up to 3 weeks postoperatively with nitrofurantoin prophylaxis.12
Ultimately, the study by Lavelle and colleagues contributes to a growing body of evidence that supports the avoidance of antibiotic prophylaxis during short-term postoperative catheterization.
Nitrofurantoin prophylaxis did not reduce the incidence of postoperative UTI in patients with catheter-managed postoperative voiding dysfunction.
- Prophylactic antibiotics are not necessary for short-term catheterization in postoperative patients.
- Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24:1843-1852.
- Yune JJ, Cheng JW, Wagner H, et al. Postoperative urinary retention after pelvic organ prolapse repair: vaginal versus robotic transabdominal approach. Neurourol Urodyn. 2018;37:1794-1800.
- Ghezzi F, Cromi A, Uccella S, et al. Immediate Foley removal after laparoscopic and vaginal hysterectomy: determinants of postoperative urinary retention. J Minim Invasive Gynecol. 2007;14:706-711.
- Smorgick N, DeLancey J, Patzkowsky K, et al. Risk factors for postoperative urinary retention after laparoscopic and robotic hysterectomy for benign indications. Obstet Gynecol. 2012;120:581-586.
- Dieter AA, Amundsen CL, Visco AG, et al. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18:175-178.
- Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597.e1-e9.
- Kandadai P, Saini J, Patterson D, et al. Urinary retention after hysterectomy and postoperative analgesic use. Female Pelvic Med Reconstr Surg. 2015;21:257-262.
- Liang CC, Lee CL, Chang TC, et al. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy--a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:295-300.
- Foster RT Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627.e1-e4.
- Geller EJ, Hankins KJ, Parnell BA, et al. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118:637-642.
Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Disease Society of America. Clin Infect Dis. 2010;50:625-663.
Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96-103.
Postoperative voiding dysfunction refers to the acute inability to spontaneously and adequately empty the bladder after surgery. Postoperative voiding dysfunction occurs in 21% to 42% of pelvic reconstructive surgeries, as well as 7% to 21% of benign gynecologic surgeries.1-4 While much of its peril lies in patient discomfort or dissatisfaction with temporary bladder drainage, serious consequences of the disorder include bladder overdistension injury with inadequate drainage and urinary tract infection (UTI) associated with prolonged catheterization.4-6
Although transient postoperative voiding dysfunction is associated with anti-incontinence surgery, tricyclic antidepressant use, diabetes, preoperative voiding dysfunction, and postoperative narcotic use, it also may occur in patients without risk factors.4,7,8 Thus, all gynecologic surgeons should be prepared to assess and manage the patient with postoperative voiding dysfunction.
Diagnosis of postoperative voiding dysfunction can be approached in myriad ways, including spontaneous (or natural) bladder filling or bladder backfill followed by spontaneous void. When compared with spontaneous void trials, backfill-assisted void trial is associated with improved accuracy in predicting voiding dysfunction in patients who undergo urogynecologic surgery, leading to widespread adoption of the procedure following pelvic reconstructive surgeries.9,10
Criteria for “passing” a void trial may include the patient’s subjective feeling of having emptied her bladder; having a near-baseline force of stream; or commonly by objective parameters of voided volume and postvoid residual (PVR), assessed via catheterization or bladder scan.3,6,10 Completing a postoperative void trial typically requires significant nursing effort because of the technical demands of backfilling the bladder, obtaining the voided volume and PVR, or assessing subjective emptying.
Management of postoperative voiding dysfunction typically consists of continuous drainage with a transurethral catheter or clean intermittent self-catheterization (CISC). Patients discharged home with a bladder drainage method also may be prescribed various medications, such as antibiotics, anticholinergics, and bladder analgesics, which often depends on provider practice.
Given the minimal universal guidance available for gynecologic surgeons on postoperative voiding dysfunction, we review several articles that contribute new evidence on the assessment and management of this condition.
Continue to: How can we efficiently approach the postoperative void trial for pelvic floor surgery?
How can we efficiently approach the postoperative void trial for pelvic floor surgery?
Chao L, Mansuria S. Postoperative bladder filling after outpatient laparoscopic hysterectomy and time to discharge: a randomized controlled trial. Obstet Gynecol. 2019;133:879-887.
Despite efforts to implement and promote enhanced recovery after surgery pathways, waiting for spontaneous void can be a barrier to efficient same-day discharge. Chao and Mansuria conducted a randomized controlled trial (RCT) to determine whether backfilling the bladder intraoperatively, compared with spontaneous (physiologic) filling, would reduce time to discharge in patients undergoing total laparoscopic hysterectomy (TLH) or supracervical hysterectomy (SCH).

Study details

Women undergoing TLH or laparoscopic SCH for benign indications were randomly assigned to undergo either a backfill-assisted void trial in the operating room with 200 mL of sterile normal saline (n = 75) or Foley catheter removal with spontaneous fill in the postanesthesia care unit (PACU) (n = 78).
For both groups, the maximum time allowed for spontaneous void was 5 hours. A successful void trial was defined as a voided volume of at least 200 mL. If a patient was unable to void at least 200 mL, a bladder scan was performed, and the patient was considered to have failed the void trial if a PVR of 200 mL or greater was noted. If the PVR was less than 200 mL, the patient was given an additional 1 hour to spontaneously void 200 mL by 6 hours after the surgery. Patients who failed the void trial were discharged home with a transurethral catheter.
The primary outcome was time to discharge, and the sample size (153 participants included in the analysis) allowed 80% power to detect a 30-minute difference in time to discharge. Participant baseline characteristics, concomitant procedures, and indication for hysterectomy were similar for both groups.
Results. The mean time to discharge was 273.4 minutes for the backfill-assisted void trial group and 283.2 minutes for the spontaneous fill group, a difference of 9.8 minutes that was not statistically significant (P = .45).
Although it was not a primary outcome, time to spontaneous void was 24.9 minutes shorter in the backfill group (P = .04). Rates of postoperative voiding dysfunction did not differ between the 2 groups (6.7% for the backfill group and 12.8% for the spontaneous fill group; P = .2). There were no significant differences in emergency department visits, UTI rates, or readmissions.
Bladder backfill is safe, simple, and may reduce time to spontaneous void
Strengths of the study included its prospective randomized design, blinded outcome assessors, and diversity in benign gynecologic surgeries performed. Although this study found a reduced time to spontaneous void in the backfill group, it was not powered to assess this difference, limiting ability to draw conclusions from those data. Data on postoperative nausea and pain scores also were not collected, which likely influenced the overall time to discharge.
Void trial completion is one of many criteria to fulfill prior to patient discharge, and a reduced time to first void may not decrease the overall length of PACU stay if other factors, such as nausea or pain, are not controlled. Nonetheless, backfilling the bladder intraoperatively is a safe alternative that may decrease the time to first spontaneous void, and it is a relatively simple alteration in the surgical workflow that could significantly lessen PACU nursing demands.
Backfilling the bladder in the operating room prior to catheter discontinuation can reduce time to first spontaneous void, but not the overall time to discharge.
Continue to: Algorithm assesses need for PVR, although further study required...
Algorithm assesses need for PVR, although further study required
Meekins AR, Siddiqui N, Amundsen CL, et al. Improving postoperative efficiency: an algorithm for expedited void trials after urogynecologic surgery. South Med J. 2017;110:785-790.
To determine ways to further maximize postoperative efficiency, Meekins and colleagues sought to determine whether certain voided volumes during backfill-assisted void trials could obviate the need for PVR assessment.
Void trial results calculated to develop algorithm
The study was a secondary analysis of a previously conducted RCT that assessed antibiotics for the prevention of UTI after urogynecologic surgery. Void trials from the parent RCT were performed via the backfill-assisted method in which the bladder was backfilled in the PACU with 300 mL of normal saline or until the patient reported urgency to void, after which the catheter was removed and the patient was prompted to void immediately.
Postvoid residual levels were assessed via ultrasonography or catheterization. A void trial was considered to be passed when a PVR was less than 100 mL or less than 50% of the total bladder volume, with a minimum voided volume of 200 mL.
In the follow-up study, the authors analyzed the void trial results of 255 women of the original 264 in the parent RCT. A total of 69% of patients passed their void trial. The authors assessed the optimal positive predictive value (PPV) and negative predictive value (NPV) combinations, which were then used to create lower and upper voided volume thresholds that would best predict a failed or passed trial, thus obviating PVR measurement.
Results. When patients voided less than 100 mL, the NPV was 96.7% (meaning that they had a 96.7% chance of failing the void trial). When patients voided 200 mL or more, the PPV was 97% (meaning that they had a 97% chance of passing the void trial). Receiver operating characteristic analysis confirmed that voided volume alone was an excellent predictor of final void trial results, with area under the curve of 0.97. The authors estimated that applying this algorithm to their study population would have eliminated the need for assessing PVR in 85% of patients. Ultimately, they proposed the algorithm shown in TABLE 1.

A potential alternative for assessing PVR
This study's strengths include the use of prospectively and systematically collected void trial data in a large patient population undergoing various urogynecologic procedures. By contrast, the generalizability of the results is limited regarding other void trial methods, such as spontaneous filling and void, as well as populations outside of the studied institution.
With the algorithm, the authors estimated that the majority of postoperative patients would no longer require a PVR assessment in the PACU. This could have beneficial downstream implications, including decreasing the nursing workload, reducing total time in the PACU, and minimizing patient discomfort with PVR assessment.
While further studies are needed to validate the proposed algorithm in larger populations, this study provides evidence of an efficient alternative to the traditional approach to PVR assessment in the PACU.
Application of the algorithm proposed by the study investigators has the potential to eliminate the need for a PVR assessment in most patients following a backfill-assisted void trial.
Continue to: An alternative to Foley use if a patient does not know CISC...
An alternative to Foley use if a patient does not know CISC
Boyd SS, O'Sullivan DM, Tunitsky-Bitton E. A comparison of two methods of catheter management after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:1037-1045.
The traditional indwelling catheter as a postoperative bladder drainage method has a number of drawbacks, including an increased rate of UTI, patient discomfort, and potential limitations in mobility due to the presence of a drainage bag.5
Boyd and colleagues reported on a variation of traditional transurethral catheterization that hypothetically allows for improved mobility. With this method, the transurethral catheter is occluded with a plastic plug that is intermittently plugged and unplugged (plug-unplug method) for bladder drainage. To test whether activity levels are improved with the plug-unplug method versus the continuous drainage approach, the authors conducted an RCT in women undergoing pelvic reconstructive surgery to compare the plug-unplug method with transurethral catheterization (with a continuous drainage bag) and a reference group of freely voiding women.
Study particulars and outcomes
The trial's primary outcome was the patients' activity score as measured by the Activity Assessment Scale (AAS) at 5 to 7 days postoperatively. Because of the theoretically increased risk of a UTI with opening and closing a closed drainage system, secondary outcomes included the UTI rate, the time to pass an outpatient void trial, postoperative pain, patient satisfaction, and catheter effect. To detect an effect size of 0.33 in the primary outcome between the 3 groups, 90 participants were needed along with a difference in proportions of 0.3 between the catheterized and noncatheterized groups.
The participants were randomly assigned 1:1 preoperatively to the continuous drainage or plug-unplug method. All patients underwent a backfill-assisted void trial prior to hospital discharge; the first 30 randomly assigned patients to pass their void trial comprised the reference group. Patients in the plug-unplug arm were instructed to uncap the plastic plug to drain their bladder when they felt the urge to void or at least every 4 hours. All catheterized patients were provided with a large drainage bag for gravity-based drainage for overnight use.
Participants who were discharged home with a catheter underwent an outpatient void trial between postoperative days 5 and 7. A urinalysis was performed at that time and a urine culture was done if a patient reported UTI symptoms. All patients underwent routine follow-up until they passed the office void trial.
Results. Ninety-three women were included in the primary analysis. There were no differences in baseline characteristics between groups. No difference was detected in activity by AAS scores between all 3 groups (scores: plug-unplug, 70.3; continuous drainage, 67.7; reference arm, 79.4; P = .09). The 2 treatment arms had no overall difference in culture-positive UTI (plug-unplug, 68.8%; continuous drainage, 48.4%; P = .625). No significant difference was found in the percentage of patients who passed their initial outpatient void trial (plug-unplug, 71.9%, vs continuous drainage, 58.1%; P = .25) (TABLE 2).

Catheter impact on postoperative activity considered
Strengths of the study include the prospective randomized design, the inclusion of a noncatheterized reference arm, and use of a validated questionnaire to assess activity. The study was limited, however, by the inability to blind patients to treatment and the lack of power to assess other important outcomes, such as UTI rates.
Although the authors did not find a difference in activity scores between the 2 catheterization methods, no significant difference was found between the catheterized and noncatheterized groups, which suggests that catheters in general may not significantly impact postoperative activity. The theoretical concern that opening and closing a transurethral drainage system would increase UTI rates was not substantiated, although the study was not powered specifically for this outcome.
Ultimately, the plug-unplug method may be a safe alternative for patients who desire to avoid attachment to a drainage bag postoperatively.
Based on the results of an RCT that compared 2 methods of catheter management after pelvic reconstructive surgery, the plug-unplug catheterization method may be an acceptable alternative to traditional catheterization.
- Bladder backfill in the operating room followed by spontaneous void in the postanesthesia care unit (PACU) is a safe and efficient way to assess for postoperative voiding dysfunction.
- Voids of 200 mL or more (following a 300-mL backfill) may not require a PACU postvoid residual assessment.
- Postoperative activity does not appear to be impacted by the presence of an indwelling catheter.
Continue to: Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Lavelle ES, Alam P, Meister M, et al. Antibiotic prophylaxis during catheter-managed postoperative urinary retention after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:727-735.
Limited high-quality evidence supports the use of prophylactic antibiotics during catheterization following prolapse or incontinence surgery, and the Infectious Disease Society of America cautions against routine antibiotic prophylaxis for those requiring catheterization.11
Lavelle and colleagues conducted a multicenter RCT to determine whether nitrofurantoin is more effective than placebo in decreasing UTIs among patients with postoperative voiding dysfunction following surgery for prolapse or incontinence.
Focus of the study
The investigators conducted a double-blind RCT at 5 academic sites that included women with postoperative voiding dysfunction who required catheter management (transurethral indwelling catheter or CISC). Voiding dysfunction was diagnosed by backfill or spontaneous fill void trial and was defined as a PVR of greater than 100 mL. Women were randomly assigned 1:1 to nitrofurantoin 100 mg or placebo taken daily during catheter use. Catheter use was discontinued once an outpatient void trial confirmed efficient voiding.
The primary outcome was symptomatic culture-confirmed UTI within 6 weeks of surgery. Secondary outcomes included frequency of urine cultures with nitrofurantoin-resistant or intermediate-sensitivity isolates and adverse symptoms possibly related to nitrofurantoin. The authors calculated that 154 participants would provide 80% power to detect a decrease in UTI incidence from 33% to 13%, allowing for 10% dropout.
A total of 151 women were randomly assigned and included in the intention-to-treat analysis. There were no differences in baseline characteristics. The median duration of catheter use was 4 days (interquartile range, 3-7).
Results. Overall, 13 women in the nitrofurantoin group and 13 in the placebo group experienced the primary outcome of UTI within 6 weeks postoperatively (17.3% nitrofurantoin vs 17.1% placebo; P = .97; relative risk [RR], 1.01; 95% confidence interval [CI], 0.50-2.04). The number needed to treat with nitrofurantoin to prevent 1 UTI was 500. A subanalysis found no difference in UTI incidence stratified by CISC versus indwelling catheter.
Urine cultures were obtained for 94.5% of all patients reporting UTI symptoms. Four isolates of the 13 cultures in the nitrofurantoin group (30.8%) and 3 in the placebo group (21.4%) showed nitrofurantoin resistance (P = .58). The rate of endorsing at least 1 adverse symptom attributable to nitrofurantoin was similar between groups (68.0% vs 60.5%, respectively; P = .34).
Study strong points and limitations
This study's randomized, placebo-controlled design and multicenter recruitment increase the generalizability of the results. An additional strength is that the authors chose a clinically relevant definition of UTI. The study was likely underpowered, however, to detect differences in secondary outcomes, such as nitrofurantoin resistance. We cannot conclude on the role of antibiotics for patients who require more prolonged catheterization.
Notably, a similar RCT by Dieter and colleagues of 159 patients undergoing daily nitrofurantoin versus placebo during CISC or transurethral catheterization failed to detect a difference in the rate of UTI treatment up to 3 weeks postoperatively with nitrofurantoin prophylaxis.12
Ultimately, the study by Lavelle and colleagues contributes to a growing body of evidence that supports the avoidance of antibiotic prophylaxis during short-term postoperative catheterization.
Nitrofurantoin prophylaxis did not reduce the incidence of postoperative UTI in patients with catheter-managed postoperative voiding dysfunction.
- Prophylactic antibiotics are not necessary for short-term catheterization in postoperative patients.
Postoperative voiding dysfunction refers to the acute inability to spontaneously and adequately empty the bladder after surgery. Postoperative voiding dysfunction occurs in 21% to 42% of pelvic reconstructive surgeries, as well as 7% to 21% of benign gynecologic surgeries.1-4 While much of its peril lies in patient discomfort or dissatisfaction with temporary bladder drainage, serious consequences of the disorder include bladder overdistension injury with inadequate drainage and urinary tract infection (UTI) associated with prolonged catheterization.4-6
Although transient postoperative voiding dysfunction is associated with anti-incontinence surgery, tricyclic antidepressant use, diabetes, preoperative voiding dysfunction, and postoperative narcotic use, it also may occur in patients without risk factors.4,7,8 Thus, all gynecologic surgeons should be prepared to assess and manage the patient with postoperative voiding dysfunction.
Diagnosis of postoperative voiding dysfunction can be approached in myriad ways, including spontaneous (or natural) bladder filling or bladder backfill followed by spontaneous void. When compared with spontaneous void trials, backfill-assisted void trial is associated with improved accuracy in predicting voiding dysfunction in patients who undergo urogynecologic surgery, leading to widespread adoption of the procedure following pelvic reconstructive surgeries.9,10
Criteria for “passing” a void trial may include the patient’s subjective feeling of having emptied her bladder; having a near-baseline force of stream; or commonly by objective parameters of voided volume and postvoid residual (PVR), assessed via catheterization or bladder scan.3,6,10 Completing a postoperative void trial typically requires significant nursing effort because of the technical demands of backfilling the bladder, obtaining the voided volume and PVR, or assessing subjective emptying.
Management of postoperative voiding dysfunction typically consists of continuous drainage with a transurethral catheter or clean intermittent self-catheterization (CISC). Patients discharged home with a bladder drainage method also may be prescribed various medications, such as antibiotics, anticholinergics, and bladder analgesics, which often depends on provider practice.
Given the minimal universal guidance available for gynecologic surgeons on postoperative voiding dysfunction, we review several articles that contribute new evidence on the assessment and management of this condition.
Continue to: How can we efficiently approach the postoperative void trial for pelvic floor surgery?
How can we efficiently approach the postoperative void trial for pelvic floor surgery?
Chao L, Mansuria S. Postoperative bladder filling after outpatient laparoscopic hysterectomy and time to discharge: a randomized controlled trial. Obstet Gynecol. 2019;133:879-887.
Despite efforts to implement and promote enhanced recovery after surgery pathways, waiting for spontaneous void can be a barrier to efficient same-day discharge. Chao and Mansuria conducted a randomized controlled trial (RCT) to determine whether backfilling the bladder intraoperatively, compared with spontaneous (physiologic) filling, would reduce time to discharge in patients undergoing total laparoscopic hysterectomy (TLH) or supracervical hysterectomy (SCH).

Study details

Women undergoing TLH or laparoscopic SCH for benign indications were randomly assigned to undergo either a backfill-assisted void trial in the operating room with 200 mL of sterile normal saline (n = 75) or Foley catheter removal with spontaneous fill in the postanesthesia care unit (PACU) (n = 78).
For both groups, the maximum time allowed for spontaneous void was 5 hours. A successful void trial was defined as a voided volume of at least 200 mL. If a patient was unable to void at least 200 mL, a bladder scan was performed, and the patient was considered to have failed the void trial if a PVR of 200 mL or greater was noted. If the PVR was less than 200 mL, the patient was given an additional 1 hour to spontaneously void 200 mL by 6 hours after the surgery. Patients who failed the void trial were discharged home with a transurethral catheter.
The primary outcome was time to discharge, and the sample size (153 participants included in the analysis) allowed 80% power to detect a 30-minute difference in time to discharge. Participant baseline characteristics, concomitant procedures, and indication for hysterectomy were similar for both groups.
Results. The mean time to discharge was 273.4 minutes for the backfill-assisted void trial group and 283.2 minutes for the spontaneous fill group, a difference of 9.8 minutes that was not statistically significant (P = .45).
Although it was not a primary outcome, time to spontaneous void was 24.9 minutes shorter in the backfill group (P = .04). Rates of postoperative voiding dysfunction did not differ between the 2 groups (6.7% for the backfill group and 12.8% for the spontaneous fill group; P = .2). There were no significant differences in emergency department visits, UTI rates, or readmissions.
Bladder backfill is safe, simple, and may reduce time to spontaneous void
Strengths of the study included its prospective randomized design, blinded outcome assessors, and diversity in benign gynecologic surgeries performed. Although this study found a reduced time to spontaneous void in the backfill group, it was not powered to assess this difference, limiting ability to draw conclusions from those data. Data on postoperative nausea and pain scores also were not collected, which likely influenced the overall time to discharge.
Void trial completion is one of many criteria to fulfill prior to patient discharge, and a reduced time to first void may not decrease the overall length of PACU stay if other factors, such as nausea or pain, are not controlled. Nonetheless, backfilling the bladder intraoperatively is a safe alternative that may decrease the time to first spontaneous void, and it is a relatively simple alteration in the surgical workflow that could significantly lessen PACU nursing demands.
Backfilling the bladder in the operating room prior to catheter discontinuation can reduce time to first spontaneous void, but not the overall time to discharge.
Continue to: Algorithm assesses need for PVR, although further study required...
Algorithm assesses need for PVR, although further study required
Meekins AR, Siddiqui N, Amundsen CL, et al. Improving postoperative efficiency: an algorithm for expedited void trials after urogynecologic surgery. South Med J. 2017;110:785-790.
To determine ways to further maximize postoperative efficiency, Meekins and colleagues sought to determine whether certain voided volumes during backfill-assisted void trials could obviate the need for PVR assessment.
Void trial results calculated to develop algorithm
The study was a secondary analysis of a previously conducted RCT that assessed antibiotics for the prevention of UTI after urogynecologic surgery. Void trials from the parent RCT were performed via the backfill-assisted method in which the bladder was backfilled in the PACU with 300 mL of normal saline or until the patient reported urgency to void, after which the catheter was removed and the patient was prompted to void immediately.
Postvoid residual levels were assessed via ultrasonography or catheterization. A void trial was considered to be passed when a PVR was less than 100 mL or less than 50% of the total bladder volume, with a minimum voided volume of 200 mL.
In the follow-up study, the authors analyzed the void trial results of 255 women of the original 264 in the parent RCT. A total of 69% of patients passed their void trial. The authors assessed the optimal positive predictive value (PPV) and negative predictive value (NPV) combinations, which were then used to create lower and upper voided volume thresholds that would best predict a failed or passed trial, thus obviating PVR measurement.
Results. When patients voided less than 100 mL, the NPV was 96.7% (meaning that they had a 96.7% chance of failing the void trial). When patients voided 200 mL or more, the PPV was 97% (meaning that they had a 97% chance of passing the void trial). Receiver operating characteristic analysis confirmed that voided volume alone was an excellent predictor of final void trial results, with area under the curve of 0.97. The authors estimated that applying this algorithm to their study population would have eliminated the need for assessing PVR in 85% of patients. Ultimately, they proposed the algorithm shown in TABLE 1.

A potential alternative for assessing PVR
This study's strengths include the use of prospectively and systematically collected void trial data in a large patient population undergoing various urogynecologic procedures. By contrast, the generalizability of the results is limited regarding other void trial methods, such as spontaneous filling and void, as well as populations outside of the studied institution.
With the algorithm, the authors estimated that the majority of postoperative patients would no longer require a PVR assessment in the PACU. This could have beneficial downstream implications, including decreasing the nursing workload, reducing total time in the PACU, and minimizing patient discomfort with PVR assessment.
While further studies are needed to validate the proposed algorithm in larger populations, this study provides evidence of an efficient alternative to the traditional approach to PVR assessment in the PACU.
Application of the algorithm proposed by the study investigators has the potential to eliminate the need for a PVR assessment in most patients following a backfill-assisted void trial.
Continue to: An alternative to Foley use if a patient does not know CISC...
An alternative to Foley use if a patient does not know CISC
Boyd SS, O'Sullivan DM, Tunitsky-Bitton E. A comparison of two methods of catheter management after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:1037-1045.
The traditional indwelling catheter as a postoperative bladder drainage method has a number of drawbacks, including an increased rate of UTI, patient discomfort, and potential limitations in mobility due to the presence of a drainage bag.5
Boyd and colleagues reported on a variation of traditional transurethral catheterization that hypothetically allows for improved mobility. With this method, the transurethral catheter is occluded with a plastic plug that is intermittently plugged and unplugged (plug-unplug method) for bladder drainage. To test whether activity levels are improved with the plug-unplug method versus the continuous drainage approach, the authors conducted an RCT in women undergoing pelvic reconstructive surgery to compare the plug-unplug method with transurethral catheterization (with a continuous drainage bag) and a reference group of freely voiding women.
Study particulars and outcomes
The trial's primary outcome was the patients' activity score as measured by the Activity Assessment Scale (AAS) at 5 to 7 days postoperatively. Because of the theoretically increased risk of a UTI with opening and closing a closed drainage system, secondary outcomes included the UTI rate, the time to pass an outpatient void trial, postoperative pain, patient satisfaction, and catheter effect. To detect an effect size of 0.33 in the primary outcome between the 3 groups, 90 participants were needed along with a difference in proportions of 0.3 between the catheterized and noncatheterized groups.
The participants were randomly assigned 1:1 preoperatively to the continuous drainage or plug-unplug method. All patients underwent a backfill-assisted void trial prior to hospital discharge; the first 30 randomly assigned patients to pass their void trial comprised the reference group. Patients in the plug-unplug arm were instructed to uncap the plastic plug to drain their bladder when they felt the urge to void or at least every 4 hours. All catheterized patients were provided with a large drainage bag for gravity-based drainage for overnight use.
Participants who were discharged home with a catheter underwent an outpatient void trial between postoperative days 5 and 7. A urinalysis was performed at that time and a urine culture was done if a patient reported UTI symptoms. All patients underwent routine follow-up until they passed the office void trial.
Results. Ninety-three women were included in the primary analysis. There were no differences in baseline characteristics between groups. No difference was detected in activity by AAS scores between all 3 groups (scores: plug-unplug, 70.3; continuous drainage, 67.7; reference arm, 79.4; P = .09). The 2 treatment arms had no overall difference in culture-positive UTI (plug-unplug, 68.8%; continuous drainage, 48.4%; P = .625). No significant difference was found in the percentage of patients who passed their initial outpatient void trial (plug-unplug, 71.9%, vs continuous drainage, 58.1%; P = .25) (TABLE 2).

Catheter impact on postoperative activity considered
Strengths of the study include the prospective randomized design, the inclusion of a noncatheterized reference arm, and use of a validated questionnaire to assess activity. The study was limited, however, by the inability to blind patients to treatment and the lack of power to assess other important outcomes, such as UTI rates.
Although the authors did not find a difference in activity scores between the 2 catheterization methods, no significant difference was found between the catheterized and noncatheterized groups, which suggests that catheters in general may not significantly impact postoperative activity. The theoretical concern that opening and closing a transurethral drainage system would increase UTI rates was not substantiated, although the study was not powered specifically for this outcome.
Ultimately, the plug-unplug method may be a safe alternative for patients who desire to avoid attachment to a drainage bag postoperatively.
Based on the results of an RCT that compared 2 methods of catheter management after pelvic reconstructive surgery, the plug-unplug catheterization method may be an acceptable alternative to traditional catheterization.
- Bladder backfill in the operating room followed by spontaneous void in the postanesthesia care unit (PACU) is a safe and efficient way to assess for postoperative voiding dysfunction.
- Voids of 200 mL or more (following a 300-mL backfill) may not require a PACU postvoid residual assessment.
- Postoperative activity does not appear to be impacted by the presence of an indwelling catheter.
Continue to: Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Lavelle ES, Alam P, Meister M, et al. Antibiotic prophylaxis during catheter-managed postoperative urinary retention after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:727-735.
Limited high-quality evidence supports the use of prophylactic antibiotics during catheterization following prolapse or incontinence surgery, and the Infectious Disease Society of America cautions against routine antibiotic prophylaxis for those requiring catheterization.11
Lavelle and colleagues conducted a multicenter RCT to determine whether nitrofurantoin is more effective than placebo in decreasing UTIs among patients with postoperative voiding dysfunction following surgery for prolapse or incontinence.
Focus of the study
The investigators conducted a double-blind RCT at 5 academic sites that included women with postoperative voiding dysfunction who required catheter management (transurethral indwelling catheter or CISC). Voiding dysfunction was diagnosed by backfill or spontaneous fill void trial and was defined as a PVR of greater than 100 mL. Women were randomly assigned 1:1 to nitrofurantoin 100 mg or placebo taken daily during catheter use. Catheter use was discontinued once an outpatient void trial confirmed efficient voiding.
The primary outcome was symptomatic culture-confirmed UTI within 6 weeks of surgery. Secondary outcomes included frequency of urine cultures with nitrofurantoin-resistant or intermediate-sensitivity isolates and adverse symptoms possibly related to nitrofurantoin. The authors calculated that 154 participants would provide 80% power to detect a decrease in UTI incidence from 33% to 13%, allowing for 10% dropout.
A total of 151 women were randomly assigned and included in the intention-to-treat analysis. There were no differences in baseline characteristics. The median duration of catheter use was 4 days (interquartile range, 3-7).
Results. Overall, 13 women in the nitrofurantoin group and 13 in the placebo group experienced the primary outcome of UTI within 6 weeks postoperatively (17.3% nitrofurantoin vs 17.1% placebo; P = .97; relative risk [RR], 1.01; 95% confidence interval [CI], 0.50-2.04). The number needed to treat with nitrofurantoin to prevent 1 UTI was 500. A subanalysis found no difference in UTI incidence stratified by CISC versus indwelling catheter.
Urine cultures were obtained for 94.5% of all patients reporting UTI symptoms. Four isolates of the 13 cultures in the nitrofurantoin group (30.8%) and 3 in the placebo group (21.4%) showed nitrofurantoin resistance (P = .58). The rate of endorsing at least 1 adverse symptom attributable to nitrofurantoin was similar between groups (68.0% vs 60.5%, respectively; P = .34).
Study strong points and limitations
This study's randomized, placebo-controlled design and multicenter recruitment increase the generalizability of the results. An additional strength is that the authors chose a clinically relevant definition of UTI. The study was likely underpowered, however, to detect differences in secondary outcomes, such as nitrofurantoin resistance. We cannot conclude on the role of antibiotics for patients who require more prolonged catheterization.
Notably, a similar RCT by Dieter and colleagues of 159 patients undergoing daily nitrofurantoin versus placebo during CISC or transurethral catheterization failed to detect a difference in the rate of UTI treatment up to 3 weeks postoperatively with nitrofurantoin prophylaxis.12
Ultimately, the study by Lavelle and colleagues contributes to a growing body of evidence that supports the avoidance of antibiotic prophylaxis during short-term postoperative catheterization.
Nitrofurantoin prophylaxis did not reduce the incidence of postoperative UTI in patients with catheter-managed postoperative voiding dysfunction.
- Prophylactic antibiotics are not necessary for short-term catheterization in postoperative patients.
- Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24:1843-1852.
- Yune JJ, Cheng JW, Wagner H, et al. Postoperative urinary retention after pelvic organ prolapse repair: vaginal versus robotic transabdominal approach. Neurourol Urodyn. 2018;37:1794-1800.
- Ghezzi F, Cromi A, Uccella S, et al. Immediate Foley removal after laparoscopic and vaginal hysterectomy: determinants of postoperative urinary retention. J Minim Invasive Gynecol. 2007;14:706-711.
- Smorgick N, DeLancey J, Patzkowsky K, et al. Risk factors for postoperative urinary retention after laparoscopic and robotic hysterectomy for benign indications. Obstet Gynecol. 2012;120:581-586.
- Dieter AA, Amundsen CL, Visco AG, et al. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18:175-178.
- Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597.e1-e9.
- Kandadai P, Saini J, Patterson D, et al. Urinary retention after hysterectomy and postoperative analgesic use. Female Pelvic Med Reconstr Surg. 2015;21:257-262.
- Liang CC, Lee CL, Chang TC, et al. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy--a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:295-300.
- Foster RT Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627.e1-e4.
- Geller EJ, Hankins KJ, Parnell BA, et al. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118:637-642.
Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Disease Society of America. Clin Infect Dis. 2010;50:625-663.
Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96-103.
- Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24:1843-1852.
- Yune JJ, Cheng JW, Wagner H, et al. Postoperative urinary retention after pelvic organ prolapse repair: vaginal versus robotic transabdominal approach. Neurourol Urodyn. 2018;37:1794-1800.
- Ghezzi F, Cromi A, Uccella S, et al. Immediate Foley removal after laparoscopic and vaginal hysterectomy: determinants of postoperative urinary retention. J Minim Invasive Gynecol. 2007;14:706-711.
- Smorgick N, DeLancey J, Patzkowsky K, et al. Risk factors for postoperative urinary retention after laparoscopic and robotic hysterectomy for benign indications. Obstet Gynecol. 2012;120:581-586.
- Dieter AA, Amundsen CL, Visco AG, et al. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18:175-178.
- Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597.e1-e9.
- Kandadai P, Saini J, Patterson D, et al. Urinary retention after hysterectomy and postoperative analgesic use. Female Pelvic Med Reconstr Surg. 2015;21:257-262.
- Liang CC, Lee CL, Chang TC, et al. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy--a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:295-300.
- Foster RT Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627.e1-e4.
- Geller EJ, Hankins KJ, Parnell BA, et al. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118:637-642.
Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Disease Society of America. Clin Infect Dis. 2010;50:625-663.
Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96-103.