User login
Updated option for breast biopsy
Hologic announces updates to its Brevera® Breast Biopsy System with CorLumina® Imaging Technology. The Brevera system is designed for use with the manufacturer’s Affirm® Prone biopsy guidance system.
For more information, visit https://www.hologic.com.
“Mini-sponge” device shows potential to treat PPH
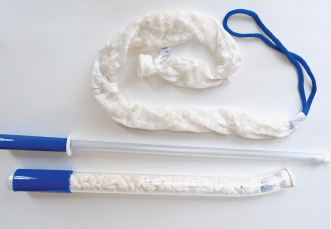
During a pilot study, reports Obstetrx, 9 patients, treated at the University Teaching Hospital in Lusaka, Zambia, did not respond to conventional PPH management options after vaginal birth but did respond, with bleeding resolved in 60 seconds and no adverse events, to the XSTAT device. The device was left in place for a mean time of 1 hour, and none of the patients required further surgical procedures or blood transfusions. The initial placement time of XSTAT (mean time to placement, 62 seconds) was faster than times reported for balloon uterine tamponade devices. The pilot study results were published in Obstetrics & Gynecology.
XSTAT is US Food and Drug Administration–approved to treat high-flow arterial bleeding in prehospital trauma settings, and Obstetrx is planning to submit for 510k clearance in 2022, after the conclusion of a follow-up PPH trial in 2021.
For more information, visit: https://www.obstetrx.com/.
Continue to: AI and ovulation prediction...
AI and ovulation prediction
A woman’s fertility window is typically the 5 days leading up to ovulation, with peak fertility in the 2 to 3 days before ovulation. There are other options for measuring that fertile window, including luteinizing hormone (LH) tests; however, Prima-Temp reports that Priya predicts the fertile window an average of 2.6 days before tests for LH. Utilizing continuous core body temperature measurement, Priya detects subtle changes in temperature patterns that occur prior to ovulation. The app portion of the technology stores and analyzes the temperature measurements, for a high-tech fertility alert system that also offers clinical diagnostic support. Potential users of the Priya system are able to sign up to receive it through the product’s website.
For more information, visit: https://www.priyafertility.com.
Updated option for breast biopsy
Hologic announces updates to its Brevera® Breast Biopsy System with CorLumina® Imaging Technology. The Brevera system is designed for use with the manufacturer’s Affirm® Prone biopsy guidance system.
For more information, visit https://www.hologic.com.
“Mini-sponge” device shows potential to treat PPH
During a pilot study, reports Obstetrx, 9 patients, treated at the University Teaching Hospital in Lusaka, Zambia, did not respond to conventional PPH management options after vaginal birth but did respond, with bleeding resolved in 60 seconds and no adverse events, to the XSTAT device. The device was left in place for a mean time of 1 hour, and none of the patients required further surgical procedures or blood transfusions. The initial placement time of XSTAT (mean time to placement, 62 seconds) was faster than times reported for balloon uterine tamponade devices. The pilot study results were published in Obstetrics & Gynecology.
XSTAT is US Food and Drug Administration–approved to treat high-flow arterial bleeding in prehospital trauma settings, and Obstetrx is planning to submit for 510k clearance in 2022, after the conclusion of a follow-up PPH trial in 2021.
For more information, visit: https://www.obstetrx.com/.
Continue to: AI and ovulation prediction...
AI and ovulation prediction
A woman’s fertility window is typically the 5 days leading up to ovulation, with peak fertility in the 2 to 3 days before ovulation. There are other options for measuring that fertile window, including luteinizing hormone (LH) tests; however, Prima-Temp reports that Priya predicts the fertile window an average of 2.6 days before tests for LH. Utilizing continuous core body temperature measurement, Priya detects subtle changes in temperature patterns that occur prior to ovulation. The app portion of the technology stores and analyzes the temperature measurements, for a high-tech fertility alert system that also offers clinical diagnostic support. Potential users of the Priya system are able to sign up to receive it through the product’s website.
For more information, visit: https://www.priyafertility.com.
Updated option for breast biopsy
Hologic announces updates to its Brevera® Breast Biopsy System with CorLumina® Imaging Technology. The Brevera system is designed for use with the manufacturer’s Affirm® Prone biopsy guidance system.
For more information, visit https://www.hologic.com.
“Mini-sponge” device shows potential to treat PPH
During a pilot study, reports Obstetrx, 9 patients, treated at the University Teaching Hospital in Lusaka, Zambia, did not respond to conventional PPH management options after vaginal birth but did respond, with bleeding resolved in 60 seconds and no adverse events, to the XSTAT device. The device was left in place for a mean time of 1 hour, and none of the patients required further surgical procedures or blood transfusions. The initial placement time of XSTAT (mean time to placement, 62 seconds) was faster than times reported for balloon uterine tamponade devices. The pilot study results were published in Obstetrics & Gynecology.
XSTAT is US Food and Drug Administration–approved to treat high-flow arterial bleeding in prehospital trauma settings, and Obstetrx is planning to submit for 510k clearance in 2022, after the conclusion of a follow-up PPH trial in 2021.
For more information, visit: https://www.obstetrx.com/.
Continue to: AI and ovulation prediction...
AI and ovulation prediction
A woman’s fertility window is typically the 5 days leading up to ovulation, with peak fertility in the 2 to 3 days before ovulation. There are other options for measuring that fertile window, including luteinizing hormone (LH) tests; however, Prima-Temp reports that Priya predicts the fertile window an average of 2.6 days before tests for LH. Utilizing continuous core body temperature measurement, Priya detects subtle changes in temperature patterns that occur prior to ovulation. The app portion of the technology stores and analyzes the temperature measurements, for a high-tech fertility alert system that also offers clinical diagnostic support. Potential users of the Priya system are able to sign up to receive it through the product’s website.
For more information, visit: https://www.priyafertility.com.