User login
Update: Minimally invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
CASE: Abdominal entry leads to life-threatening injury
A 50-year-old woman with a BMI of 25 kg/m2, a strong family history of breast and ovarian cancer, and a confirmed BRCA mutation was scheduled for prophylactic bilateral salpingo-oophorectomy via robotic laparoscopy on November 26, 2009. At the time of the procedure, the gynecologic surgeon selected a site for the camera trocar that was several centimeters above the umbilicus. After making a transverse incision, he inserted a Veress needle and insufflated the abdomen with CO2 gas until intra-abdominal pressure reached 17 mm Hg. He then thrust an 11-inch disposable trocar through the anterior abdominal wall, attached the camera to the laparoscope, confirmed proper intraperitoneal placement, and inserted two additional trocars under direct vision.
Shortly after these actions, the anesthesiologist reported that the patient’s blood pressure had dropped precipitously, along with end tidal CO2. The surgeon examined the peritoneal cavity and discovered blood in the right paracolic gutter. The anesthesiologist advised the surgeon that he could no longer detect the patient’s blood pressure; electrocardiography revealed pulseless electrical activity.
The surgical team began chest compressions, evacuated the pneumoperitoneum, and removed all trocars. Blood was noted on the camera trocar, and the device was secured by the OR staff. The surgeon performed an emergent laparotomy, making the incision within 4 minutes of the beginning of CPR. Exploration revealed a large retroperitoneal hematoma above the area of the aortic bifurcation and inferior vena cava.
General and vascular surgeons were called. The general surgeon opened the retroperitoneum and found an extreme amount of clotted and unclotted blood. The vascular surgeon described the initial injury as a 1.5-cm laceration of the distal aorta, just above the bifurcation. A cell saver was requested and recorded blood loss of 12,000 mL.
The vascular surgeon clamped the aorta proximally; he also clamped both common iliac arteries. He then repaired the lacerations on the aorta using 5-0 Prolene suture (Ethicon). The aorta was significantly narrowed, however, so the surgeon decided to replace the distal aorta, which he then resected and repaired using a 14-mm Dacron graft (DuPont).
Further inspection revealed continuing retroperitoneal bleeding. The vascular surgeon found and repaired a laceration of the inferior mesenteric vein. He also clipped multiple small veins to stop bleeding.
When a hole in the transverse colon was identified, the general surgeon—who had left the operating table—rescrubbed to repair it. He also discovered an injury to the mesentery of the transverse colon and repaired both wounds, resecting the perforated segment. The divided, stapled colon was dropped back into the abdomen because the bowel was dusky. Despite an epinephrine drip, the patient was hypotensive and coagulopathic. The abdomen was packed and covered with sterile cassette film, with towels covering the open wound.
The patient was taken to the postanesthesia care unit in guarded condition and was subsequently transferred to the ICU, where her blood pressure dropped again. She was returned to the OR, where the packs were removed and a bleeding right common iliac artery was repaired using 5-0 Prolene suture. The next day, she underwent bilateral salpingo-oophorectomy with a transverse colon colostomy.
Because of the colon injury, the vascular surgeon believed that the Dacron graft had been contaminated. On December 1, the graft was taken down, a left femoral-vein autograft was harvested, and a reconstructive conduit was created for the terminal aorta. The patient underwent three additional procedures to place mesh into the abdominal wall. When the mesh became infected, it was removed.
The patient remained in the hospital for 1 month, after which she was transferred to a long-term care facility. She suffered permanent neurologic injuries because of prolonged hypoxia and continues to require supportive care.
How could this catastrophe have been avoided?
Traumatic injury to the great retroperitoneal vessels is an emergent and life-threatening event. During gynecologic laparoscopy, it is most likely to occur during entry into the anterior abdominal wall.
Most laparoscopic procedures require entry into the anterior abdominal wall for placement of a trocar and a sleeve that serves as a portal for insertion of the endoscope. Secondary ports provide entry points for manipulative and operative tools.
The most critical entry point is primary placement of the viewing device. Secondary trocars are always inserted under direct visualization; therefore, they carry a lower risk of inflicting injury to underlying viscera and vessels.
Practice safe entry
In the early days of laparoscopy, only one method of entry existed. Over time, however, several other techniques have been devised.
The initial method—still widely utilized—is known as the closed or blind technique. The surgeon creates a pneumoperitoneum with the use of a needle that is 18 gauge to 2.5 mm in diameter; the needle is placed through a subumbilical incision. Once intraperitoneal placement is confirmed, CO2 gas is infused into the peritoneal cavity until the abdomen is tympanic to percussion (usually at pressures of 14 to 18 mm Hg).
Next, the surgeon aims the trocar toward the uterus at a 45° angle, maintaining the device in the midline. Entry is confirmed by opening the trocar’s trap-door valve and witnessing a rush of CO2 gas.
Another entry technique—the open technique—is used almost universally by general surgeons. The procedure is a type of microlaparotomy. After making the subumbilical incision, the fascia of the abdominal wall is pierced and the peritoneum is grasped and opened bluntly or sharply. Once the edges of the peritoneum are secured, a blunt trocar (Hasson trocar) is inserted. Then the trocar is removed, leaving the sleeve in place to accept the laparoscope.
Another entry variation, called direct entry, employs no pneumoperitoneum. In this approach, the surgeon grabs the anterior abdominal wall, sharply elevating it, and directly thrusts the reusable or disposable trocar into the abdominal cavity.
An extensive review of entry techniques has been published elsewhere.1
Many complications arise from entry techniques and devices
A survey of Australian gynecologists about entry techniques found that 73% of respondents used a Veress needle and pneumoperitoneum for entry and that 83% used a location other than the infraumbilical site when periumbilical adhesions were suspected. Twenty-one percent had experienced a major retroperitoneal vascular injury, but 33% lacked a plan to manage such injuries.2
In their review of entry techniques, Vilos and colleagues asserted that Veress-needle insertion should be accompanied by pneumoperitoneal pressures of 20 to 30 mm Hg rather than a predetermined volume of CO2 gas.1 They also recommended insertion in the left upper quadrant when periumbilical adhesions are suspected or when insufflation at the umbilicus fails three times.
Newer entry devices include the optical-view trocar and the radially expanding trocar. The first consists of a plastic, conically tipped instrument that is optically clear. At least hypothetically, this device permits the surgeon to view each layer of the abdominal wall as he or she thrusts the device under “direct vision” into the abdominal cavity.
The radially expanding trocar is inserted over a Veress needle into the abdominal cavity. Its initial diameter is only 3 mm; once the instrument is in place, however, a blunt plastic trocar and sleeve are pushed into the mesh-like, radially expanding tube until it reaches 11 to 12 mm in diameter. The blunt trocar is then removed, leaving the plastic sheath and mesh material in place to accept the laparoscope. One key advantage of this device is the mesh component, which resists slippage or movement as the laparoscope is moved in and out of the sheath.
Vilos and colleagues concluded that open entry was neither superior nor inferior to other entry techniques and that direct entry without pneumoperitoneum may be as safe as Veress-needle techniques and associated with a lower risk of gas embolism. They also reported that shielded trocars are not associated with fewer visceral or vascular injuries and that visual-entry trocars lack superiority, compared with other devices, for the prevention of visceral or vascular injuries.1
Other review articles about entry techniques similarly found no objective evidence that any single technique is superior.3 However, data are conflicting on the safety of the optical trocar, compared with other trocars, with some data showing marginal advantages and others demonstrating no difference.4-6
Follow a few key entry guidelines
In 1990, Yuzpe reported a mail-in survey of 800 practicing ObGyns in Canada on the topic of pneumoperitoneum and trocar injuries.7 Of the 407 physicians who responded, 16.7% reported that the pneumoperitoneum needle caused a visceral or major vessel injury, and 16.5% attributed the injury to the primary trocar. Among 109 vessel injuries, 31 were caused by the pneumoperitoneum needle, and 28 of 104 injuries were caused by the primary trocar.
To be safe, Veress needle and primary trocar entry require critical attention to the angle and direction of the thrust relative to the abdominal cavity (FIGURE, page 24). For example, if the Veress needle or the sharp tip of the trocar deviate to the right or left of the midline during entry into the abdomen, injury to the iliac vessels is a clear risk.
Most laparoscopic surgeons stand on the left-hand side of the patient and face her feet. Trocar deviation for a right-handed person tends to vector to the right, especially when a twisting motion is utilized. Correct alignment of the primary trocar is straight down the middle of the lower abdomen on a virtual or real line drawn from the center of the navel to the center of the symphysis.
An entry angle of 45° to 60° will carry the needle or trocar toward the bladder or uterus and away from the aorta and left common iliac vein. In contrast, a 90° thrust will aim the device dangerously toward the great vessels. A slightly upward and right-sided deviation from the subumbilical entry will place the needle and trocar in the direction of the inferior vena cava and right common iliac vessels. A 90° entry with deviation to the left will position the entry device at the inferior mesenteric vessels and the left common iliac vessels.
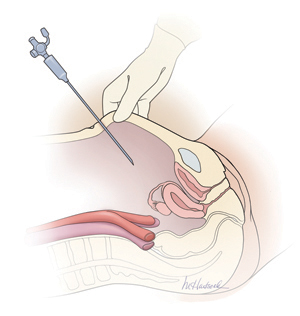
Primary abdominal entry
An entry angle of 45° to 60°, regardless of whether a needle or trocar is used, will carry the device toward the bladder or uterus and away from the aorta and left common iliac vein.In a review of access complications associated with laparoscopy, including major vascular injuries, Philips and Amaral listed variables responsible for large-vessel injury; they also documented the incidence of such injuries associated with laparoscopic cholecystectomy.8 They recommended that the patient be placed in the Trendelenburg position and that the needle or trocar be inserted at a 45° angle that stays within the midline; they also concluded that the trocar should be placed when pneumoperitoneal pressures exceed 20 mm Hg. They advised against direct insertion in patients with a history of pelvic surgery as well as in thin patients.
Place secondary trocars under direct visualization
Secondary trocars should always be placed under direct, visually controlled entry and, at least hypothetically, should never injure any great vessel. Nevertheless, secondary trocars do sometimes cause injury, most often as a result of extreme lateral entry near the inguinal ligament. The vessels at risk are the external iliac artery and vein.
Injuries are also invariably associated with adhesiolysis and anatomic problems. Precise knowledge of pelvic anatomy is not only a requisite for pelvic surgery in general but also for laparoscopic surgery, in which the operative view is less clear than it is in open procedures.
Know the risks associated with operative tools
Suturing and knot tying are not easy maneuvers during laparoscopic procedures and add significant operative time. Although they are performed more easily when robotics is utilized, few gynecologists are skilled practitioners. As a result, accessory instruments have been developed to prevent and control bleeding during laparoscopic operations. These devices include monopolar and bipolar instruments, lasers, ultrasonic tools, and stapling devices.
Avoid monopolar electrosurgery
This modality should be avoided whenever possible because the risk of injury is significantly higher than with bipolar electrosurgery. The key disadvantages of monopolar energy are high-frequency leaks; low-frequency currents; direct, indirect, and capacitative coupling; and return-electrode failures. None of these problems are common with bipolar techniques.
However, all electrosurgical devices carry a risk of thermal injury through direct tissue contact and conduction of heat to neighboring tissues and structures.
A full discussion of the physics and tissue actions of electrosurgical devices may be found elsewhere.9
CO2 is the safest laser
A variety of lasers have been used in laparoscopic surgery. The neodymium–YAG, KTP-532, and CO2 lasers have been used most frequently for gynecologic operations.
Because of its wavelength, the CO2 laser is the safest device for intra-abdominal use. Advantages include precision and control. In addition, the CO2 laser is absorbed by water very effectively. As a result, hydrodissection techniques can facilitate effective backstopping of the laser beam in strategic locations, thereby preventing injury to surrounding structures.
Laser energy is not conducted through tissue in the same way that electrosurgical energy is conducted. Therefore, the laser is ideal for vaporizing endometrial implants and cutting adhesions.
Beware of heat generated by the ultrasonic shears
This device, known more commonly as the Harmonic Scalpel (Ethicon), employs high-frequency sound waves to shear and coagulate tissue and prevent bleeding. It does not require conduction through tissues but does require contact with tissues. Because friction produces heat, these devices can become hot enough to inflict unintended burns on tissues that are inadvertently touched by the hot tip or by heat transmitted from the operative site by thermal conduction.
Stapler may inadvertently involve adjacent structures
This laparoscopic device has the advantage of not requiring or emitting energy other than the mechanical force of the operator’s hand. Disadvantages associated with the stapler center on the inadvertent inclusion of other structures within the jaws of the instrument. In addition, the staplers themselves tend to be large and somewhat unwieldy in close quarters, adding to the risk of stapling nearby viscera.
Further information on the physics and actions of lasers, ultrasonic shears, and staplers is available.10,11
Obesity may increase the risk of major vessel injury
A recent study by Baggish found obesity to be a high-risk circumstance for major vessel injury.12 In the study, 22 of 31 women who sustained injury were overweight or obese, with a BMI ranging from 26 to 30 kg/m2.
Obesity increases the risk of major vessel injury because of the greater elasticity of the anterior abdominal wall. As force secondary to the downward thrust of the trocar is placed on the abdominal wall, it is pushed inward in the direction of the posterior wall. In contrast, thin women have rigid abdominal walls with minimal elasticity, so the force of the trocar thrust does not create significant displacement.
Baggish also found that disposable trocars accounted for 90% of major vascular injuries and that use of long trocars accounted for 43% of deaths.12
Injury and death are rare but real risks
In a multicenter study in France over 9 years, investigators reviewed 29,966 diagnostic and operative laparoscopic procedures and found a mortality rate of 3.33 deaths for every 100,000 laparoscopies and an overall complication rate of 4.64 complications for every 1,000 procedures.13 They found the complication rate to be significantly correlated with the complexity of the procedure (P = .0001). One in three complications (34.1%; n = 43) occurred during set-up, and one in four (28.6%) were not identified intraoperatively.13
The risk of great vessel injury associated with laparoscopy most frequently quoted is 0.5 injury for every 1,000 procedures.14 A multicenter study reported the prevalence of this complication to be 1.05 injuries per 1,000 procedures.15
The mortality rate associated with major vessel injury has been reported in several studies to range from 8% to 17%.14-17
Two articles measured the distance from various points on the anterior abdominal wall to the great retroperitoneal vessels during laparoscopic operations; they also measured the force required for the trocar to penetrate the abdominal wall.18,19 They found significant differences in the distance from the site of primary trocar insertion to the aorta and iliac vessels, depending on the BMI of the patient. In women with a BMI below 25 kg/m2, the mean distance to the aorta was 11.21 cm. In women with a BMI of 25 to 30, it was 14.14 cm, and in women with a BMI over 30, it was 15.14 cm. They also found variations in the mean thickness of the abdominal wall, which was 3.48 cm, 3.85 cm, and 5.05 cm in women with a BMI of less than 25, 25–30, and more than 30, respectively.
As for the force required for entry, investigators found that disposable cutting trocars can traverse the anterior abdominal wall with less force and less time, compared with reusable trocars and optical viewing devices.18,19
Another study measured the thickness of the abdominal wall and the distance to the great vessels by magnetic resonance imaging or computed tomography.20 However, this study was not performed during laparoscopy with pneumoperitoneum in place.
As previously mentioned, Baggish reported on 31 cases of major-vessel injury associated with laparoscopic operations involving 49 major-vessel injuries. Twenty-eight injuries occurred as a result of entry techniques: 26 occurred during primary trocar insertion, and two were related to secondary trocar thrusts.12 Four injuries and three deaths were associated with use of an 11-inch disposable trocar.
Of the injuries associated with primary trocar insertion, 10 occurred during direct insertion and 26 after creation of pneumoperitoneum. Open laparoscopy was performed in two cases.12 The TABLE details the number of vessels injured and the sites of injury in this study.
Seven women (23%) died as a direct result of venous injury. Collateral injury to other structures was observed in 16 cases. Blood loss ranged from 1,000 mL to 7,000 mL.12
Sites of major vessel injury in one study of gynecologic laparoscopy
| Site | Number of vascular injuries |
|---|---|
| Right iliac artery | 14 |
| Right iliac vein | 12 |
| Left iliac artery | 3 |
| Left iliac vein | 9 |
| Aorta | 4 |
| Vena cava | 2 |
| Mesenteric | 2 |
| Interior epigastric* | 2 |
| Other | 1 |
| Total injuries | 49 |
| Source: Baggish12 | |
Avoid these common errors
The most common errors in gynecologic laparoscopy include:
- delayed diagnosis
- failure to act on a visible retroperitoneal hematoma
- failure to cross-match adequate supplies of blood and blood products
- failure to adequately transfuse blood and blood products
- clamping the large damaged vessel
- opening the abdomen via Pfannenstiel incision
- failure to call for a vascular surgeon in a timely manner.
When a major vascular injury occurs, a well-informed surgeon will take the following measures:
- call for a vascular surgeon immediately. (Baggish found that there was a substantial delay in getting a vascular surgeon to the operating table in four of 31 cases.12)
- open the abdomen via a midline incision
- use a sponge stick to apply direct pressure to the bleeding vessel
- obtain an emergency type and cross-match and order a minimum of 6 U of blood plus fresh frozen plasma
- obtain a baseline complete blood count, platelet count, fibrinogen level, and test for fibrin-split products
- advise the anesthesiologist to seek additional help
- call for additional OR nursing personnel
- assign one circulator to run stats and record critical data.21-33
Prevention is the best strategy
As the opening case demonstrates, major vessel injury can occur without warning and cause cascading problems that can lead to permanent disability—even death. Because most serious vessel injuries occur during entry into the anterior abdominal wall, careful attention to entry techniques and the patient’s unique circumstances (obesity, presence of adhesions) can go a long way toward averting injury. Vigilance for the possibility of injury is also important throughout the procedure. When injury does occur, it is critical to call for help as soon as possible and to have safeguards in place to manage it.
Tune in again in October 2012 for Part 2 of this series, which offers insight into gastrointestinal and urinary tract injuries during laparoscopy and offers valuable guidance on avoiding and managing related complications.
We want to hear from you! Tell us what you think.
Update: Minimally invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
CASE: Abdominal entry leads to life-threatening injury
A 50-year-old woman with a BMI of 25 kg/m2, a strong family history of breast and ovarian cancer, and a confirmed BRCA mutation was scheduled for prophylactic bilateral salpingo-oophorectomy via robotic laparoscopy on November 26, 2009. At the time of the procedure, the gynecologic surgeon selected a site for the camera trocar that was several centimeters above the umbilicus. After making a transverse incision, he inserted a Veress needle and insufflated the abdomen with CO2 gas until intra-abdominal pressure reached 17 mm Hg. He then thrust an 11-inch disposable trocar through the anterior abdominal wall, attached the camera to the laparoscope, confirmed proper intraperitoneal placement, and inserted two additional trocars under direct vision.
Shortly after these actions, the anesthesiologist reported that the patient’s blood pressure had dropped precipitously, along with end tidal CO2. The surgeon examined the peritoneal cavity and discovered blood in the right paracolic gutter. The anesthesiologist advised the surgeon that he could no longer detect the patient’s blood pressure; electrocardiography revealed pulseless electrical activity.
The surgical team began chest compressions, evacuated the pneumoperitoneum, and removed all trocars. Blood was noted on the camera trocar, and the device was secured by the OR staff. The surgeon performed an emergent laparotomy, making the incision within 4 minutes of the beginning of CPR. Exploration revealed a large retroperitoneal hematoma above the area of the aortic bifurcation and inferior vena cava.
General and vascular surgeons were called. The general surgeon opened the retroperitoneum and found an extreme amount of clotted and unclotted blood. The vascular surgeon described the initial injury as a 1.5-cm laceration of the distal aorta, just above the bifurcation. A cell saver was requested and recorded blood loss of 12,000 mL.
The vascular surgeon clamped the aorta proximally; he also clamped both common iliac arteries. He then repaired the lacerations on the aorta using 5-0 Prolene suture (Ethicon). The aorta was significantly narrowed, however, so the surgeon decided to replace the distal aorta, which he then resected and repaired using a 14-mm Dacron graft (DuPont).
Further inspection revealed continuing retroperitoneal bleeding. The vascular surgeon found and repaired a laceration of the inferior mesenteric vein. He also clipped multiple small veins to stop bleeding.
When a hole in the transverse colon was identified, the general surgeon—who had left the operating table—rescrubbed to repair it. He also discovered an injury to the mesentery of the transverse colon and repaired both wounds, resecting the perforated segment. The divided, stapled colon was dropped back into the abdomen because the bowel was dusky. Despite an epinephrine drip, the patient was hypotensive and coagulopathic. The abdomen was packed and covered with sterile cassette film, with towels covering the open wound.
The patient was taken to the postanesthesia care unit in guarded condition and was subsequently transferred to the ICU, where her blood pressure dropped again. She was returned to the OR, where the packs were removed and a bleeding right common iliac artery was repaired using 5-0 Prolene suture. The next day, she underwent bilateral salpingo-oophorectomy with a transverse colon colostomy.
Because of the colon injury, the vascular surgeon believed that the Dacron graft had been contaminated. On December 1, the graft was taken down, a left femoral-vein autograft was harvested, and a reconstructive conduit was created for the terminal aorta. The patient underwent three additional procedures to place mesh into the abdominal wall. When the mesh became infected, it was removed.
The patient remained in the hospital for 1 month, after which she was transferred to a long-term care facility. She suffered permanent neurologic injuries because of prolonged hypoxia and continues to require supportive care.
How could this catastrophe have been avoided?
Traumatic injury to the great retroperitoneal vessels is an emergent and life-threatening event. During gynecologic laparoscopy, it is most likely to occur during entry into the anterior abdominal wall.
Most laparoscopic procedures require entry into the anterior abdominal wall for placement of a trocar and a sleeve that serves as a portal for insertion of the endoscope. Secondary ports provide entry points for manipulative and operative tools.
The most critical entry point is primary placement of the viewing device. Secondary trocars are always inserted under direct visualization; therefore, they carry a lower risk of inflicting injury to underlying viscera and vessels.
Practice safe entry
In the early days of laparoscopy, only one method of entry existed. Over time, however, several other techniques have been devised.
The initial method—still widely utilized—is known as the closed or blind technique. The surgeon creates a pneumoperitoneum with the use of a needle that is 18 gauge to 2.5 mm in diameter; the needle is placed through a subumbilical incision. Once intraperitoneal placement is confirmed, CO2 gas is infused into the peritoneal cavity until the abdomen is tympanic to percussion (usually at pressures of 14 to 18 mm Hg).
Next, the surgeon aims the trocar toward the uterus at a 45° angle, maintaining the device in the midline. Entry is confirmed by opening the trocar’s trap-door valve and witnessing a rush of CO2 gas.
Another entry technique—the open technique—is used almost universally by general surgeons. The procedure is a type of microlaparotomy. After making the subumbilical incision, the fascia of the abdominal wall is pierced and the peritoneum is grasped and opened bluntly or sharply. Once the edges of the peritoneum are secured, a blunt trocar (Hasson trocar) is inserted. Then the trocar is removed, leaving the sleeve in place to accept the laparoscope.
Another entry variation, called direct entry, employs no pneumoperitoneum. In this approach, the surgeon grabs the anterior abdominal wall, sharply elevating it, and directly thrusts the reusable or disposable trocar into the abdominal cavity.
An extensive review of entry techniques has been published elsewhere.1
Many complications arise from entry techniques and devices
A survey of Australian gynecologists about entry techniques found that 73% of respondents used a Veress needle and pneumoperitoneum for entry and that 83% used a location other than the infraumbilical site when periumbilical adhesions were suspected. Twenty-one percent had experienced a major retroperitoneal vascular injury, but 33% lacked a plan to manage such injuries.2
In their review of entry techniques, Vilos and colleagues asserted that Veress-needle insertion should be accompanied by pneumoperitoneal pressures of 20 to 30 mm Hg rather than a predetermined volume of CO2 gas.1 They also recommended insertion in the left upper quadrant when periumbilical adhesions are suspected or when insufflation at the umbilicus fails three times.
Newer entry devices include the optical-view trocar and the radially expanding trocar. The first consists of a plastic, conically tipped instrument that is optically clear. At least hypothetically, this device permits the surgeon to view each layer of the abdominal wall as he or she thrusts the device under “direct vision” into the abdominal cavity.
The radially expanding trocar is inserted over a Veress needle into the abdominal cavity. Its initial diameter is only 3 mm; once the instrument is in place, however, a blunt plastic trocar and sleeve are pushed into the mesh-like, radially expanding tube until it reaches 11 to 12 mm in diameter. The blunt trocar is then removed, leaving the plastic sheath and mesh material in place to accept the laparoscope. One key advantage of this device is the mesh component, which resists slippage or movement as the laparoscope is moved in and out of the sheath.
Vilos and colleagues concluded that open entry was neither superior nor inferior to other entry techniques and that direct entry without pneumoperitoneum may be as safe as Veress-needle techniques and associated with a lower risk of gas embolism. They also reported that shielded trocars are not associated with fewer visceral or vascular injuries and that visual-entry trocars lack superiority, compared with other devices, for the prevention of visceral or vascular injuries.1
Other review articles about entry techniques similarly found no objective evidence that any single technique is superior.3 However, data are conflicting on the safety of the optical trocar, compared with other trocars, with some data showing marginal advantages and others demonstrating no difference.4-6
Follow a few key entry guidelines
In 1990, Yuzpe reported a mail-in survey of 800 practicing ObGyns in Canada on the topic of pneumoperitoneum and trocar injuries.7 Of the 407 physicians who responded, 16.7% reported that the pneumoperitoneum needle caused a visceral or major vessel injury, and 16.5% attributed the injury to the primary trocar. Among 109 vessel injuries, 31 were caused by the pneumoperitoneum needle, and 28 of 104 injuries were caused by the primary trocar.
To be safe, Veress needle and primary trocar entry require critical attention to the angle and direction of the thrust relative to the abdominal cavity (FIGURE, page 24). For example, if the Veress needle or the sharp tip of the trocar deviate to the right or left of the midline during entry into the abdomen, injury to the iliac vessels is a clear risk.
Most laparoscopic surgeons stand on the left-hand side of the patient and face her feet. Trocar deviation for a right-handed person tends to vector to the right, especially when a twisting motion is utilized. Correct alignment of the primary trocar is straight down the middle of the lower abdomen on a virtual or real line drawn from the center of the navel to the center of the symphysis.
An entry angle of 45° to 60° will carry the needle or trocar toward the bladder or uterus and away from the aorta and left common iliac vein. In contrast, a 90° thrust will aim the device dangerously toward the great vessels. A slightly upward and right-sided deviation from the subumbilical entry will place the needle and trocar in the direction of the inferior vena cava and right common iliac vessels. A 90° entry with deviation to the left will position the entry device at the inferior mesenteric vessels and the left common iliac vessels.

Primary abdominal entry
An entry angle of 45° to 60°, regardless of whether a needle or trocar is used, will carry the device toward the bladder or uterus and away from the aorta and left common iliac vein.In a review of access complications associated with laparoscopy, including major vascular injuries, Philips and Amaral listed variables responsible for large-vessel injury; they also documented the incidence of such injuries associated with laparoscopic cholecystectomy.8 They recommended that the patient be placed in the Trendelenburg position and that the needle or trocar be inserted at a 45° angle that stays within the midline; they also concluded that the trocar should be placed when pneumoperitoneal pressures exceed 20 mm Hg. They advised against direct insertion in patients with a history of pelvic surgery as well as in thin patients.
Place secondary trocars under direct visualization
Secondary trocars should always be placed under direct, visually controlled entry and, at least hypothetically, should never injure any great vessel. Nevertheless, secondary trocars do sometimes cause injury, most often as a result of extreme lateral entry near the inguinal ligament. The vessels at risk are the external iliac artery and vein.
Injuries are also invariably associated with adhesiolysis and anatomic problems. Precise knowledge of pelvic anatomy is not only a requisite for pelvic surgery in general but also for laparoscopic surgery, in which the operative view is less clear than it is in open procedures.
Know the risks associated with operative tools
Suturing and knot tying are not easy maneuvers during laparoscopic procedures and add significant operative time. Although they are performed more easily when robotics is utilized, few gynecologists are skilled practitioners. As a result, accessory instruments have been developed to prevent and control bleeding during laparoscopic operations. These devices include monopolar and bipolar instruments, lasers, ultrasonic tools, and stapling devices.
Avoid monopolar electrosurgery
This modality should be avoided whenever possible because the risk of injury is significantly higher than with bipolar electrosurgery. The key disadvantages of monopolar energy are high-frequency leaks; low-frequency currents; direct, indirect, and capacitative coupling; and return-electrode failures. None of these problems are common with bipolar techniques.
However, all electrosurgical devices carry a risk of thermal injury through direct tissue contact and conduction of heat to neighboring tissues and structures.
A full discussion of the physics and tissue actions of electrosurgical devices may be found elsewhere.9
CO2 is the safest laser
A variety of lasers have been used in laparoscopic surgery. The neodymium–YAG, KTP-532, and CO2 lasers have been used most frequently for gynecologic operations.
Because of its wavelength, the CO2 laser is the safest device for intra-abdominal use. Advantages include precision and control. In addition, the CO2 laser is absorbed by water very effectively. As a result, hydrodissection techniques can facilitate effective backstopping of the laser beam in strategic locations, thereby preventing injury to surrounding structures.
Laser energy is not conducted through tissue in the same way that electrosurgical energy is conducted. Therefore, the laser is ideal for vaporizing endometrial implants and cutting adhesions.
Beware of heat generated by the ultrasonic shears
This device, known more commonly as the Harmonic Scalpel (Ethicon), employs high-frequency sound waves to shear and coagulate tissue and prevent bleeding. It does not require conduction through tissues but does require contact with tissues. Because friction produces heat, these devices can become hot enough to inflict unintended burns on tissues that are inadvertently touched by the hot tip or by heat transmitted from the operative site by thermal conduction.
Stapler may inadvertently involve adjacent structures
This laparoscopic device has the advantage of not requiring or emitting energy other than the mechanical force of the operator’s hand. Disadvantages associated with the stapler center on the inadvertent inclusion of other structures within the jaws of the instrument. In addition, the staplers themselves tend to be large and somewhat unwieldy in close quarters, adding to the risk of stapling nearby viscera.
Further information on the physics and actions of lasers, ultrasonic shears, and staplers is available.10,11
Obesity may increase the risk of major vessel injury
A recent study by Baggish found obesity to be a high-risk circumstance for major vessel injury.12 In the study, 22 of 31 women who sustained injury were overweight or obese, with a BMI ranging from 26 to 30 kg/m2.
Obesity increases the risk of major vessel injury because of the greater elasticity of the anterior abdominal wall. As force secondary to the downward thrust of the trocar is placed on the abdominal wall, it is pushed inward in the direction of the posterior wall. In contrast, thin women have rigid abdominal walls with minimal elasticity, so the force of the trocar thrust does not create significant displacement.
Baggish also found that disposable trocars accounted for 90% of major vascular injuries and that use of long trocars accounted for 43% of deaths.12
Injury and death are rare but real risks
In a multicenter study in France over 9 years, investigators reviewed 29,966 diagnostic and operative laparoscopic procedures and found a mortality rate of 3.33 deaths for every 100,000 laparoscopies and an overall complication rate of 4.64 complications for every 1,000 procedures.13 They found the complication rate to be significantly correlated with the complexity of the procedure (P = .0001). One in three complications (34.1%; n = 43) occurred during set-up, and one in four (28.6%) were not identified intraoperatively.13
The risk of great vessel injury associated with laparoscopy most frequently quoted is 0.5 injury for every 1,000 procedures.14 A multicenter study reported the prevalence of this complication to be 1.05 injuries per 1,000 procedures.15
The mortality rate associated with major vessel injury has been reported in several studies to range from 8% to 17%.14-17
Two articles measured the distance from various points on the anterior abdominal wall to the great retroperitoneal vessels during laparoscopic operations; they also measured the force required for the trocar to penetrate the abdominal wall.18,19 They found significant differences in the distance from the site of primary trocar insertion to the aorta and iliac vessels, depending on the BMI of the patient. In women with a BMI below 25 kg/m2, the mean distance to the aorta was 11.21 cm. In women with a BMI of 25 to 30, it was 14.14 cm, and in women with a BMI over 30, it was 15.14 cm. They also found variations in the mean thickness of the abdominal wall, which was 3.48 cm, 3.85 cm, and 5.05 cm in women with a BMI of less than 25, 25–30, and more than 30, respectively.
As for the force required for entry, investigators found that disposable cutting trocars can traverse the anterior abdominal wall with less force and less time, compared with reusable trocars and optical viewing devices.18,19
Another study measured the thickness of the abdominal wall and the distance to the great vessels by magnetic resonance imaging or computed tomography.20 However, this study was not performed during laparoscopy with pneumoperitoneum in place.
As previously mentioned, Baggish reported on 31 cases of major-vessel injury associated with laparoscopic operations involving 49 major-vessel injuries. Twenty-eight injuries occurred as a result of entry techniques: 26 occurred during primary trocar insertion, and two were related to secondary trocar thrusts.12 Four injuries and three deaths were associated with use of an 11-inch disposable trocar.
Of the injuries associated with primary trocar insertion, 10 occurred during direct insertion and 26 after creation of pneumoperitoneum. Open laparoscopy was performed in two cases.12 The TABLE details the number of vessels injured and the sites of injury in this study.
Seven women (23%) died as a direct result of venous injury. Collateral injury to other structures was observed in 16 cases. Blood loss ranged from 1,000 mL to 7,000 mL.12
Sites of major vessel injury in one study of gynecologic laparoscopy
| Site | Number of vascular injuries |
|---|---|
| Right iliac artery | 14 |
| Right iliac vein | 12 |
| Left iliac artery | 3 |
| Left iliac vein | 9 |
| Aorta | 4 |
| Vena cava | 2 |
| Mesenteric | 2 |
| Interior epigastric* | 2 |
| Other | 1 |
| Total injuries | 49 |
| Source: Baggish12 | |
Avoid these common errors
The most common errors in gynecologic laparoscopy include:
- delayed diagnosis
- failure to act on a visible retroperitoneal hematoma
- failure to cross-match adequate supplies of blood and blood products
- failure to adequately transfuse blood and blood products
- clamping the large damaged vessel
- opening the abdomen via Pfannenstiel incision
- failure to call for a vascular surgeon in a timely manner.
When a major vascular injury occurs, a well-informed surgeon will take the following measures:
- call for a vascular surgeon immediately. (Baggish found that there was a substantial delay in getting a vascular surgeon to the operating table in four of 31 cases.12)
- open the abdomen via a midline incision
- use a sponge stick to apply direct pressure to the bleeding vessel
- obtain an emergency type and cross-match and order a minimum of 6 U of blood plus fresh frozen plasma
- obtain a baseline complete blood count, platelet count, fibrinogen level, and test for fibrin-split products
- advise the anesthesiologist to seek additional help
- call for additional OR nursing personnel
- assign one circulator to run stats and record critical data.21-33
Prevention is the best strategy
As the opening case demonstrates, major vessel injury can occur without warning and cause cascading problems that can lead to permanent disability—even death. Because most serious vessel injuries occur during entry into the anterior abdominal wall, careful attention to entry techniques and the patient’s unique circumstances (obesity, presence of adhesions) can go a long way toward averting injury. Vigilance for the possibility of injury is also important throughout the procedure. When injury does occur, it is critical to call for help as soon as possible and to have safeguards in place to manage it.
Tune in again in October 2012 for Part 2 of this series, which offers insight into gastrointestinal and urinary tract injuries during laparoscopy and offers valuable guidance on avoiding and managing related complications.
We want to hear from you! Tell us what you think.
Update: Minimally invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
CASE: Abdominal entry leads to life-threatening injury
A 50-year-old woman with a BMI of 25 kg/m2, a strong family history of breast and ovarian cancer, and a confirmed BRCA mutation was scheduled for prophylactic bilateral salpingo-oophorectomy via robotic laparoscopy on November 26, 2009. At the time of the procedure, the gynecologic surgeon selected a site for the camera trocar that was several centimeters above the umbilicus. After making a transverse incision, he inserted a Veress needle and insufflated the abdomen with CO2 gas until intra-abdominal pressure reached 17 mm Hg. He then thrust an 11-inch disposable trocar through the anterior abdominal wall, attached the camera to the laparoscope, confirmed proper intraperitoneal placement, and inserted two additional trocars under direct vision.
Shortly after these actions, the anesthesiologist reported that the patient’s blood pressure had dropped precipitously, along with end tidal CO2. The surgeon examined the peritoneal cavity and discovered blood in the right paracolic gutter. The anesthesiologist advised the surgeon that he could no longer detect the patient’s blood pressure; electrocardiography revealed pulseless electrical activity.
The surgical team began chest compressions, evacuated the pneumoperitoneum, and removed all trocars. Blood was noted on the camera trocar, and the device was secured by the OR staff. The surgeon performed an emergent laparotomy, making the incision within 4 minutes of the beginning of CPR. Exploration revealed a large retroperitoneal hematoma above the area of the aortic bifurcation and inferior vena cava.
General and vascular surgeons were called. The general surgeon opened the retroperitoneum and found an extreme amount of clotted and unclotted blood. The vascular surgeon described the initial injury as a 1.5-cm laceration of the distal aorta, just above the bifurcation. A cell saver was requested and recorded blood loss of 12,000 mL.
The vascular surgeon clamped the aorta proximally; he also clamped both common iliac arteries. He then repaired the lacerations on the aorta using 5-0 Prolene suture (Ethicon). The aorta was significantly narrowed, however, so the surgeon decided to replace the distal aorta, which he then resected and repaired using a 14-mm Dacron graft (DuPont).
Further inspection revealed continuing retroperitoneal bleeding. The vascular surgeon found and repaired a laceration of the inferior mesenteric vein. He also clipped multiple small veins to stop bleeding.
When a hole in the transverse colon was identified, the general surgeon—who had left the operating table—rescrubbed to repair it. He also discovered an injury to the mesentery of the transverse colon and repaired both wounds, resecting the perforated segment. The divided, stapled colon was dropped back into the abdomen because the bowel was dusky. Despite an epinephrine drip, the patient was hypotensive and coagulopathic. The abdomen was packed and covered with sterile cassette film, with towels covering the open wound.
The patient was taken to the postanesthesia care unit in guarded condition and was subsequently transferred to the ICU, where her blood pressure dropped again. She was returned to the OR, where the packs were removed and a bleeding right common iliac artery was repaired using 5-0 Prolene suture. The next day, she underwent bilateral salpingo-oophorectomy with a transverse colon colostomy.
Because of the colon injury, the vascular surgeon believed that the Dacron graft had been contaminated. On December 1, the graft was taken down, a left femoral-vein autograft was harvested, and a reconstructive conduit was created for the terminal aorta. The patient underwent three additional procedures to place mesh into the abdominal wall. When the mesh became infected, it was removed.
The patient remained in the hospital for 1 month, after which she was transferred to a long-term care facility. She suffered permanent neurologic injuries because of prolonged hypoxia and continues to require supportive care.
How could this catastrophe have been avoided?
Traumatic injury to the great retroperitoneal vessels is an emergent and life-threatening event. During gynecologic laparoscopy, it is most likely to occur during entry into the anterior abdominal wall.
Most laparoscopic procedures require entry into the anterior abdominal wall for placement of a trocar and a sleeve that serves as a portal for insertion of the endoscope. Secondary ports provide entry points for manipulative and operative tools.
The most critical entry point is primary placement of the viewing device. Secondary trocars are always inserted under direct visualization; therefore, they carry a lower risk of inflicting injury to underlying viscera and vessels.
Practice safe entry
In the early days of laparoscopy, only one method of entry existed. Over time, however, several other techniques have been devised.
The initial method—still widely utilized—is known as the closed or blind technique. The surgeon creates a pneumoperitoneum with the use of a needle that is 18 gauge to 2.5 mm in diameter; the needle is placed through a subumbilical incision. Once intraperitoneal placement is confirmed, CO2 gas is infused into the peritoneal cavity until the abdomen is tympanic to percussion (usually at pressures of 14 to 18 mm Hg).
Next, the surgeon aims the trocar toward the uterus at a 45° angle, maintaining the device in the midline. Entry is confirmed by opening the trocar’s trap-door valve and witnessing a rush of CO2 gas.
Another entry technique—the open technique—is used almost universally by general surgeons. The procedure is a type of microlaparotomy. After making the subumbilical incision, the fascia of the abdominal wall is pierced and the peritoneum is grasped and opened bluntly or sharply. Once the edges of the peritoneum are secured, a blunt trocar (Hasson trocar) is inserted. Then the trocar is removed, leaving the sleeve in place to accept the laparoscope.
Another entry variation, called direct entry, employs no pneumoperitoneum. In this approach, the surgeon grabs the anterior abdominal wall, sharply elevating it, and directly thrusts the reusable or disposable trocar into the abdominal cavity.
An extensive review of entry techniques has been published elsewhere.1
Many complications arise from entry techniques and devices
A survey of Australian gynecologists about entry techniques found that 73% of respondents used a Veress needle and pneumoperitoneum for entry and that 83% used a location other than the infraumbilical site when periumbilical adhesions were suspected. Twenty-one percent had experienced a major retroperitoneal vascular injury, but 33% lacked a plan to manage such injuries.2
In their review of entry techniques, Vilos and colleagues asserted that Veress-needle insertion should be accompanied by pneumoperitoneal pressures of 20 to 30 mm Hg rather than a predetermined volume of CO2 gas.1 They also recommended insertion in the left upper quadrant when periumbilical adhesions are suspected or when insufflation at the umbilicus fails three times.
Newer entry devices include the optical-view trocar and the radially expanding trocar. The first consists of a plastic, conically tipped instrument that is optically clear. At least hypothetically, this device permits the surgeon to view each layer of the abdominal wall as he or she thrusts the device under “direct vision” into the abdominal cavity.
The radially expanding trocar is inserted over a Veress needle into the abdominal cavity. Its initial diameter is only 3 mm; once the instrument is in place, however, a blunt plastic trocar and sleeve are pushed into the mesh-like, radially expanding tube until it reaches 11 to 12 mm in diameter. The blunt trocar is then removed, leaving the plastic sheath and mesh material in place to accept the laparoscope. One key advantage of this device is the mesh component, which resists slippage or movement as the laparoscope is moved in and out of the sheath.
Vilos and colleagues concluded that open entry was neither superior nor inferior to other entry techniques and that direct entry without pneumoperitoneum may be as safe as Veress-needle techniques and associated with a lower risk of gas embolism. They also reported that shielded trocars are not associated with fewer visceral or vascular injuries and that visual-entry trocars lack superiority, compared with other devices, for the prevention of visceral or vascular injuries.1
Other review articles about entry techniques similarly found no objective evidence that any single technique is superior.3 However, data are conflicting on the safety of the optical trocar, compared with other trocars, with some data showing marginal advantages and others demonstrating no difference.4-6
Follow a few key entry guidelines
In 1990, Yuzpe reported a mail-in survey of 800 practicing ObGyns in Canada on the topic of pneumoperitoneum and trocar injuries.7 Of the 407 physicians who responded, 16.7% reported that the pneumoperitoneum needle caused a visceral or major vessel injury, and 16.5% attributed the injury to the primary trocar. Among 109 vessel injuries, 31 were caused by the pneumoperitoneum needle, and 28 of 104 injuries were caused by the primary trocar.
To be safe, Veress needle and primary trocar entry require critical attention to the angle and direction of the thrust relative to the abdominal cavity (FIGURE, page 24). For example, if the Veress needle or the sharp tip of the trocar deviate to the right or left of the midline during entry into the abdomen, injury to the iliac vessels is a clear risk.
Most laparoscopic surgeons stand on the left-hand side of the patient and face her feet. Trocar deviation for a right-handed person tends to vector to the right, especially when a twisting motion is utilized. Correct alignment of the primary trocar is straight down the middle of the lower abdomen on a virtual or real line drawn from the center of the navel to the center of the symphysis.
An entry angle of 45° to 60° will carry the needle or trocar toward the bladder or uterus and away from the aorta and left common iliac vein. In contrast, a 90° thrust will aim the device dangerously toward the great vessels. A slightly upward and right-sided deviation from the subumbilical entry will place the needle and trocar in the direction of the inferior vena cava and right common iliac vessels. A 90° entry with deviation to the left will position the entry device at the inferior mesenteric vessels and the left common iliac vessels.

Primary abdominal entry
An entry angle of 45° to 60°, regardless of whether a needle or trocar is used, will carry the device toward the bladder or uterus and away from the aorta and left common iliac vein.In a review of access complications associated with laparoscopy, including major vascular injuries, Philips and Amaral listed variables responsible for large-vessel injury; they also documented the incidence of such injuries associated with laparoscopic cholecystectomy.8 They recommended that the patient be placed in the Trendelenburg position and that the needle or trocar be inserted at a 45° angle that stays within the midline; they also concluded that the trocar should be placed when pneumoperitoneal pressures exceed 20 mm Hg. They advised against direct insertion in patients with a history of pelvic surgery as well as in thin patients.
Place secondary trocars under direct visualization
Secondary trocars should always be placed under direct, visually controlled entry and, at least hypothetically, should never injure any great vessel. Nevertheless, secondary trocars do sometimes cause injury, most often as a result of extreme lateral entry near the inguinal ligament. The vessels at risk are the external iliac artery and vein.
Injuries are also invariably associated with adhesiolysis and anatomic problems. Precise knowledge of pelvic anatomy is not only a requisite for pelvic surgery in general but also for laparoscopic surgery, in which the operative view is less clear than it is in open procedures.
Know the risks associated with operative tools
Suturing and knot tying are not easy maneuvers during laparoscopic procedures and add significant operative time. Although they are performed more easily when robotics is utilized, few gynecologists are skilled practitioners. As a result, accessory instruments have been developed to prevent and control bleeding during laparoscopic operations. These devices include monopolar and bipolar instruments, lasers, ultrasonic tools, and stapling devices.
Avoid monopolar electrosurgery
This modality should be avoided whenever possible because the risk of injury is significantly higher than with bipolar electrosurgery. The key disadvantages of monopolar energy are high-frequency leaks; low-frequency currents; direct, indirect, and capacitative coupling; and return-electrode failures. None of these problems are common with bipolar techniques.
However, all electrosurgical devices carry a risk of thermal injury through direct tissue contact and conduction of heat to neighboring tissues and structures.
A full discussion of the physics and tissue actions of electrosurgical devices may be found elsewhere.9
CO2 is the safest laser
A variety of lasers have been used in laparoscopic surgery. The neodymium–YAG, KTP-532, and CO2 lasers have been used most frequently for gynecologic operations.
Because of its wavelength, the CO2 laser is the safest device for intra-abdominal use. Advantages include precision and control. In addition, the CO2 laser is absorbed by water very effectively. As a result, hydrodissection techniques can facilitate effective backstopping of the laser beam in strategic locations, thereby preventing injury to surrounding structures.
Laser energy is not conducted through tissue in the same way that electrosurgical energy is conducted. Therefore, the laser is ideal for vaporizing endometrial implants and cutting adhesions.
Beware of heat generated by the ultrasonic shears
This device, known more commonly as the Harmonic Scalpel (Ethicon), employs high-frequency sound waves to shear and coagulate tissue and prevent bleeding. It does not require conduction through tissues but does require contact with tissues. Because friction produces heat, these devices can become hot enough to inflict unintended burns on tissues that are inadvertently touched by the hot tip or by heat transmitted from the operative site by thermal conduction.
Stapler may inadvertently involve adjacent structures
This laparoscopic device has the advantage of not requiring or emitting energy other than the mechanical force of the operator’s hand. Disadvantages associated with the stapler center on the inadvertent inclusion of other structures within the jaws of the instrument. In addition, the staplers themselves tend to be large and somewhat unwieldy in close quarters, adding to the risk of stapling nearby viscera.
Further information on the physics and actions of lasers, ultrasonic shears, and staplers is available.10,11
Obesity may increase the risk of major vessel injury
A recent study by Baggish found obesity to be a high-risk circumstance for major vessel injury.12 In the study, 22 of 31 women who sustained injury were overweight or obese, with a BMI ranging from 26 to 30 kg/m2.
Obesity increases the risk of major vessel injury because of the greater elasticity of the anterior abdominal wall. As force secondary to the downward thrust of the trocar is placed on the abdominal wall, it is pushed inward in the direction of the posterior wall. In contrast, thin women have rigid abdominal walls with minimal elasticity, so the force of the trocar thrust does not create significant displacement.
Baggish also found that disposable trocars accounted for 90% of major vascular injuries and that use of long trocars accounted for 43% of deaths.12
Injury and death are rare but real risks
In a multicenter study in France over 9 years, investigators reviewed 29,966 diagnostic and operative laparoscopic procedures and found a mortality rate of 3.33 deaths for every 100,000 laparoscopies and an overall complication rate of 4.64 complications for every 1,000 procedures.13 They found the complication rate to be significantly correlated with the complexity of the procedure (P = .0001). One in three complications (34.1%; n = 43) occurred during set-up, and one in four (28.6%) were not identified intraoperatively.13
The risk of great vessel injury associated with laparoscopy most frequently quoted is 0.5 injury for every 1,000 procedures.14 A multicenter study reported the prevalence of this complication to be 1.05 injuries per 1,000 procedures.15
The mortality rate associated with major vessel injury has been reported in several studies to range from 8% to 17%.14-17
Two articles measured the distance from various points on the anterior abdominal wall to the great retroperitoneal vessels during laparoscopic operations; they also measured the force required for the trocar to penetrate the abdominal wall.18,19 They found significant differences in the distance from the site of primary trocar insertion to the aorta and iliac vessels, depending on the BMI of the patient. In women with a BMI below 25 kg/m2, the mean distance to the aorta was 11.21 cm. In women with a BMI of 25 to 30, it was 14.14 cm, and in women with a BMI over 30, it was 15.14 cm. They also found variations in the mean thickness of the abdominal wall, which was 3.48 cm, 3.85 cm, and 5.05 cm in women with a BMI of less than 25, 25–30, and more than 30, respectively.
As for the force required for entry, investigators found that disposable cutting trocars can traverse the anterior abdominal wall with less force and less time, compared with reusable trocars and optical viewing devices.18,19
Another study measured the thickness of the abdominal wall and the distance to the great vessels by magnetic resonance imaging or computed tomography.20 However, this study was not performed during laparoscopy with pneumoperitoneum in place.
As previously mentioned, Baggish reported on 31 cases of major-vessel injury associated with laparoscopic operations involving 49 major-vessel injuries. Twenty-eight injuries occurred as a result of entry techniques: 26 occurred during primary trocar insertion, and two were related to secondary trocar thrusts.12 Four injuries and three deaths were associated with use of an 11-inch disposable trocar.
Of the injuries associated with primary trocar insertion, 10 occurred during direct insertion and 26 after creation of pneumoperitoneum. Open laparoscopy was performed in two cases.12 The TABLE details the number of vessels injured and the sites of injury in this study.
Seven women (23%) died as a direct result of venous injury. Collateral injury to other structures was observed in 16 cases. Blood loss ranged from 1,000 mL to 7,000 mL.12
Sites of major vessel injury in one study of gynecologic laparoscopy
| Site | Number of vascular injuries |
|---|---|
| Right iliac artery | 14 |
| Right iliac vein | 12 |
| Left iliac artery | 3 |
| Left iliac vein | 9 |
| Aorta | 4 |
| Vena cava | 2 |
| Mesenteric | 2 |
| Interior epigastric* | 2 |
| Other | 1 |
| Total injuries | 49 |
| Source: Baggish12 | |
Avoid these common errors
The most common errors in gynecologic laparoscopy include:
- delayed diagnosis
- failure to act on a visible retroperitoneal hematoma
- failure to cross-match adequate supplies of blood and blood products
- failure to adequately transfuse blood and blood products
- clamping the large damaged vessel
- opening the abdomen via Pfannenstiel incision
- failure to call for a vascular surgeon in a timely manner.
When a major vascular injury occurs, a well-informed surgeon will take the following measures:
- call for a vascular surgeon immediately. (Baggish found that there was a substantial delay in getting a vascular surgeon to the operating table in four of 31 cases.12)
- open the abdomen via a midline incision
- use a sponge stick to apply direct pressure to the bleeding vessel
- obtain an emergency type and cross-match and order a minimum of 6 U of blood plus fresh frozen plasma
- obtain a baseline complete blood count, platelet count, fibrinogen level, and test for fibrin-split products
- advise the anesthesiologist to seek additional help
- call for additional OR nursing personnel
- assign one circulator to run stats and record critical data.21-33
Prevention is the best strategy
As the opening case demonstrates, major vessel injury can occur without warning and cause cascading problems that can lead to permanent disability—even death. Because most serious vessel injuries occur during entry into the anterior abdominal wall, careful attention to entry techniques and the patient’s unique circumstances (obesity, presence of adhesions) can go a long way toward averting injury. Vigilance for the possibility of injury is also important throughout the procedure. When injury does occur, it is critical to call for help as soon as possible and to have safeguards in place to manage it.
Tune in again in October 2012 for Part 2 of this series, which offers insight into gastrointestinal and urinary tract injuries during laparoscopy and offers valuable guidance on avoiding and managing related complications.
We want to hear from you! Tell us what you think.
