Emotional stress can induce different responses in the body, particularly in the cardiovascular system. Apical ballooning syndrome (ABS), also known as takotsubo cardiomyopathy and broken heart syndrome, is a transient cardiomyopathy that mimics an acute myocardial infarction (AMI). Dote and colleagues first described this transient entity in Japan in the early 1990s.1 A case review series reported that 57.2% of patients were Asian, 40% were white.2 Mean patient age was 67 years, although cases of ABS have occurred in children and young adults.3,4
The term tako-tsubo means “octopus trap,” which is the morphology that the left ventricle resembles during systole in patients with this syndrome.5 The pathophysiology of ABS is thought to be mediated by a catecholamine surge. The presentation of ABS is indistinguishable from an AMI. The majority of patients present with angina-like chest pain, ischemic changes on an electrocardiogram (ECG), pulmonary edema, and elevation of cardiac enzymes. Apical ballooning syndrome is accompanied by reversible left ventricular apical ballooning in the absence of angiographically significant coronary artery disease.
Typically, echocardiographic findings show a left ventricle with preserved function in the basal segments, moderate-to-severe dysfunction in the mid portion of the left ventricle, and hypokinesis, akinesis, or dyskinesis in the apex. A unique but not exclusive feature of this syndrome is the occurrence of a preceding emotional trigger, usually sudden or unexpected. Most patients are initially treated for an AMI until angiography can rule out coronary obstruction. After several weeks, the left ventricular systolic function usually returns to normal.
Case Presentation
A 49-year-old woman with a history of arterial hypertension, fibromyalgia, peptic ulcer disease, and major depressive disorder with multiple admissions to the psychiatric ward (last admission was 4 weeks prior to the current presentation) presented to the emergency department, reporting severe retrosternal, oppressive chest pain with 9/10 intensity and 3 hours’ duration. The pain was associated with nausea, vomiting, diaphoresis, and palpitations. She reported no previous episodes of exertional angina, fever, illicit drug use, recent illness, or travel. She also reported no prodromal symptoms.
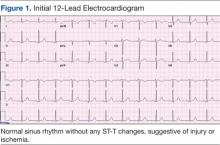
Her initial vital signs were essentially unremarkable, except for mild hypertension (148/84 mm Hg). The physical examination showed an anxious patient in acute distress due to chest pain. A cardiovascular examination revealed a regular heart rate and rhythm, no audible murmurs or gallops, no jugular vein distention, clear breath sounds, and no peripheral edema. The rest of the examination was otherwise unremarkable. An initial 12-lead ECG showed a normal sinus rhythm without any ST-T changes (Figure 1).
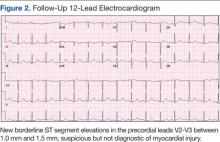
The initial cardiac markers were elevated (troponin T 0.36 ng/mL, CK-MB 4.51 ng/mL), as were NT-proBNP levels (1,057 pg/mL). The rest of the laboratory results were essentially unremarkable. The patient was started on aspirin, clopidogrel, enoxaparin, eptifibatide, and IV nitrates. She was admitted to the coronary care unit with a diagnostic impression of non-ST elevation MI. Despite medical management, the patient’s chest pain persisted for several hours from her initial presentation. A repeated 12-lead ECG revealed new borderline (1-1.5 mm) ST segment elevation in V2-V3, suggestive of possible myocardial injury (Figure 2).
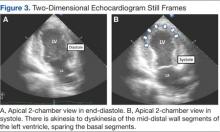
A bedside echocardiogram revealed severe wall motion abnor malities, ranging from hypokinesia to dyskinesia of all mid-to-distal left ventricular wall segments with sparing of the basal segment (Figure 3). The estimated left ventricular ejection fraction was 40% to 45%.
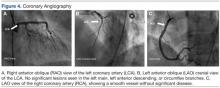
In view of these findings, the patient was taken to the catheterization laboratory for emergent coronary angiography, which ruled out significant obstructive coronary disease (Figure 4).
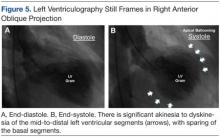
Left ventriculography in right and left anterior oblique projections revealed significant wall motion abnormalities of the mid-to-distal anterolateral and inferior wall segments, sparing the basal and apical segments, giving the appearance of ballooning in systole (Figure 5). The diagnosis of ABS involving the mid ventricular walls was explored.
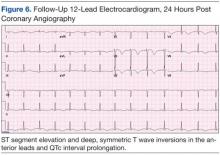
Subsequent sets of cardiac enzymes at 4 and 8 hours after arrival remained elevated, with a maximum troponin T 0.55 and CK-MB of 11.19. Repeated 12-lead ECG 24 hours post coronary angiography revealed anterolateral T wave inversion (Figure 6).
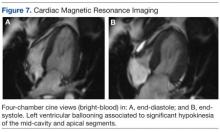
Noncontrast enhanced cardiac magnetic resonance imaging (MRI) (Figure 7) performed 5 days later revealed wall motion abnormalities highly suggestive of ABS, supporting previous echocardiographic and ventriculography findings. Unfortunately, contrast-enhanced phase for evaluation of delayed enhancement could not be completed, because the patient did not continue the study.
Toxicology tests were negative for sympathomimetic drugs. Metanephrine levels were within the normal range. Viral titers for cytomegalovirus and coxsackie virus also were negative. Inflammatory markers were mildly elevated (erythrocyte sedimentation rate, 22 mm/h; C-reactive protein, 4.2 mg/L).