User login
Estimates of the annual incidence of sudden cardiac death (SCD) vary from 180,000 to 456,000.1 About 80% to 85% of the cases of SCD are due to a ventricular arrhythmia.2 One type of ventricular arrhythmia, torsades de pointes (TdP), is caused by a prolongation of the QT interval. Because the QT interval is dependent on heart rate, clinicians use the corrected QT (QTc) interval, which has been adjusted for heart rate. The HHS recommends using a gender-independent threshold of 450 msec to define QTc prolongation when conducting research.3 Additionally, a QTc interval > 500 msec is associated with an increased risk for TdP.4 Female gender, hypokalemia, hypomagnesemia, and medical conditions such as congenital long QT syndrome, heart failure, and left ventricular hypertrophy can predispose a person to QTc prolongation.5
Methadone is a synthetic opioid used for chronic pain management or for opioid or heroin addiction. In 2006, the FDA issued a public health advisory, which was followed by the addition of a black box warning to the labeling of methadone regarding cardiac abnormalities that caused serious adverse effects, including QT prolongation, TdP, and death.6 Additional recommendations to evaluate the pharmacokinetic and pharmacodynamic drug interactions were also added to the labeling.
Related: Reducing Opioid Use for Chronic Pain
Several prospective studies, cross-sectional studies, and retrospective reviews have reported QTc prolongation with methadone.7-14 Many cases of QTc prolongation and TdP have been in patients receiving methadone in large doses (> 100 mg/d); however, incidences have also occurred in those receiving typical doses of methadone for addiction treatment.8,15,16 Multiple studies have demonstrated that methadone-induced QTc prolongation is dose-dependent.8,17-20 In an observational study of 90 subjects who were undergoing methadone maintenance treatment, the subjects taking < 60 mg/d of methadone had a 7.7 msec prolongation of the QTc interval, which was significantly less QTc prolongation than in those receiving 60 mg/d to 109 mg/d (15.6 msec, P < .001) and 110 mg/d to 150 mg/d (17.4 msec, P = .001).21
However, in the current literature there are few studies evaluating the QTc prolonging effects of methadone when used in lower doses for pain, such as those used at the Southern Arizona VA Health Care System (SAVAHCS).8 Given the increased risk of cardiac arrhythmias, it is important to understand the effects of methadone on the QTc interval in a veteran patient population using methadone at lower doses for pain. Understanding this risk can help clinicians develop strategies and protocols for the safe use of methadone.
The purpose of this study was to evaluate the effect of methadone on the QTc interval among patients at SAVAHCS. The primary objective was to determine whether methadone prolongs the QTc interval when used for pain. Secondary outcomes included evaluations of the (1) QTc interval when stratified by the QTc interval obtained while prescribed methadone; (2) effects of low, medium, and high doses of methadone on the QTc interval; (3) effects of the concurrent use of QTc prolonging medications on the QTc interval; and (4) effects of the concurrent use of strong inhibitors of methadone clearance on the QTc interval. It was hypothesized that methadone, when used for pain, causes a significant prolongation of the QTc interval, and methadone-induced QTc prolongation is dose-dependent. It was also hypothesized that the QTc interval will be more prolonged when methadone is used concurrently with other medications with a known or conditional risk of TdP or with medications that are strong inhibitors of methadone clearance.
Methods
Full Institutional Review Board approval was obtained prior to initiating this retrospective pre-post study. This study used the electronic medical records (EMRs) of SAVAHCS from July 1, 2004, to July 31, 2012, to compare the QTc interval of patients on stable doses of methadone with the baseline QTc interval. Patients included were aged 18 to 87 years and dispensed a new prescription for methadone between January 1, 2006, and July 31, 2010. Patients must have been adherent to methadone as defined by a medication possession ratio of ≥ 0.8. Patients without a baseline electrocardiogram (ECG) within the 18 months prior to starting methadone, without at least 1 follow-up ECG 7 days to 2 years after the initial prescription, who had a diagnosis of heart failure, or used an implanted cardiac defibrillator or pacemaker as indicated by ICD-9 codes were excluded.
Information collected from the EMR included demographics (age and gender), the QTc interval before starting methadone (pre-QTc), the first QTc interval recorded while prescribed methadone (post-QTc) at a stable dose (defined as ≥ 7 days without a dose change), methadone total daily dose at the time of the post-QTc, concurrent QTc prolonging medications used at the time of each ECG, time elapsed between pre-QTc and post-QTc, time elapsed between the pre-QTc and initiation of methadone, and time elapsed between starting methadone and the post-QTc. All ECGs were recorded using a 12-lead ECG by the MAC 5500 Resting ECG Analysis System (GE Healthcare), which automatically calculates the QTc interval.
For the primary outcome, the mean pre-QTc and post-QTc were compared. These QTc intervals were further analyzed as secondary outcomes. The mean pre-QTc and post-QTc were compared when stratified by post-QTc of < 450 msec, 450 msec to 499 msec, and ≥ 500 msec. To analyze the dose effect of methadone, the mean pre-QTc and post-QTc were compared when stratified by methadone total daily dose: low (≤ 15 mg/d), medium (16-30 mg/d), and high (> 30 mg/d). Dose ranges were based on typical SAVAHCS prescriptions.
An additional secondary outcome was to determine the effect of the concurrent use of QTc prolonging medications on the QTc interval. The medications the subject was taking at the time of each ECG were reviewed to determine whether any of the medications were listed as Drugs with a risk of Torsades de Pointes or Drugs with a conditional risk of Torsades de Pointes on the CredibleMeds website.22,23 Drugs with a conditional risk of TdP are defined as drugs in which there is evidence that the drug prolongs the QTc interval and has a risk of TdP but only under certain conditions, such as high doses or drug interactions. The mean pre-QTc with and without concurrent use of QTc prolonging medications was compared with the mean post-QTc with QTc prolonging medications in addition to methadone.
Because methadone is extensively metabolized by cytochrome P450 3A4 (CYP3A4), another outcome was to determine the effect of the concurrent use of strong CYP3A4 inhibitors on the QTc interval. The medications the subject was taking at the time of each ECG were reviewed to determine whether any of the medications were listed as strong CYP3A4 inhibitors in The Pharmacist’s Letter/The Prescriber’s Letter.24 The mean pre-QTc with and without the concurrent use of strong CYP3A4 inhibitors was compared with the mean post-QTc with strong CYP3A4 inhibitors in addition to methadone.
Related: Pharmacist-Managed Collaborative Practice for Chronic Stable Angina
The primary and secondary outcomes were compared using a paired t test and descriptive statistics; demographics were analyzed using descriptive statistics. Based on the findings of a previous study, a minimum of 8 patients were needed to meet a power of 0.8 with an alpha 0.05 and a medium effect size when comparing the mean pre-QTc and post-QTc for the primary outcome.25
Results
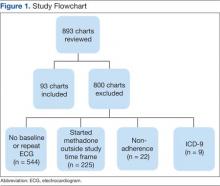
Of 893 EMRs reviewed, 93 met inclusion criteria (Figure 1). The main reason for exclusion was lack of pre-QTc and/or post-QTc (n = 544). The mean age was 58 years (± 10 years), 92% were male, and the mean daily methadone dose at the time of the post-QTc was 29 mg (5 mg-160 mg) (Table 1). Thirty patients were prescribed ≥ 1 QTc prolonging medication at the time of the pre-QTc, 40 patients were prescribed ≥ 1 QTc prolonging medication at the time of the post-QTc, and 0 patients were prescribed a strong CYP3A4 inhibitor at the time of either QTc. There was an average of 151 days (0-554 days) between the pre-QTc and methadone initiation, 161 days (8-664 days) between methadone initiation and the post-QTc, and 312 days (10-1,003 days) between the pre-QTc and post-QTc.
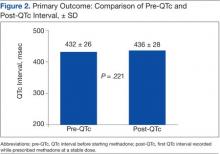
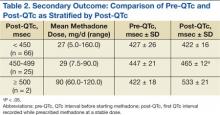
For the primary outcome, there was no significant increase in the QTc interval when comparing the pre-QTc and post-QTc (432 ± 26 msec vs 436 ± 28 msec, P = .221) (Figure 2). When stratified by post QTc, the group of patients with a post-QTc < 450 msec (n = 66) and the group with a post-QTc of ≥ 500 msec (n = 2) had no significant increase in the QTc interval (427 ± 26 msec vs 422 ± 16 msec and 422 ± 18 msec vs 533 ± 21 msec, respectively; P > .05) (Table 2). For the group of patients with a post-QTc of 450 msec to 499 msec (n = 25), methadone significantly prolonged the QTc interval (447 ± 21 msec vs 465 ± 12 msec, P < .001).
When stratified by methadone daily dose of ≤ 15 mg (n = 45), 16 mg to 30 mg (n = 27), and > 30 mg (n = 21), methadone did not significantly prolong the QTc interval in any group (428 ± 29 msec vs 430 ± 25 msec, 436 ± 16 msec vs 439 ± 21 msec, 437 ± 29 msec vs 446 ± 39 msec, respectively; P > .05) (Table 3). For the group of patients using ≥ 1 QTc prolonging medication at the time of the post-QTc and no QTc prolonging medications at the time of the pre-QTc, the addition of methadone did not significantly increase the QTc interval when compared with the pre-QTc (n = 15; 425 ± 23 msec vs 437 ± 31 msec, respectively; P > .05) (Table 4). For the group of patients prescribed ≥ 1 QTc prolonging medication at the pre-QTc and post-QTc, methadone did not significantly prolong the QTc interval (n = 25; 437 ± 32 msec vs 441 ± 33 msec, respectively; P > .05) (Table 5). No subjects were using strong CYP3A4 inhibitors; therefore, the effect of strong CYP3A4 inhibitors could not be assessed.
Discussion
The results of this study suggest that methadone-induced QTc interval prolongation may not be clearly evident at lower doses when used for pain. There was no significant increase in the QTc interval in the low-, medium-, and high-dose methadone groups, nor when analyzing the drug interactions. However, this study was powered based on the primary outcome, and it is possible that the study was underpowered to detect a difference in these secondary outcomes. When stratified by post-QTc, a significant increase in the QTc interval was noted for the group of patients with a post-QTc of 450 msec to 499 msec. The absolute mean differences between the pre-QTc and post-QTc for most of the secondary outcomes are unlikely to be clinically relevant, with the exception of the high-dose methadone group and the group stratified by post-QTc interval of 450 msec to 499 msec.
These results are supported by a prospective pilot study of 64 subjects with advanced cancer, which evaluated the QTc prolonging effects of methadone when used at lower doses (range 3-90 mg/d, median 23 mg/d).26 Only 1 of 64 subjects developed clinically significant QTc interval prolongation (QTc ≥ 500 msec) at the end of the second week of therapy. The mean QTc interval measured at baseline was 427 msec, which increased to a mean of 430 msec after 2 weeks of methadone use (mean dose 23 mg/d) and decreased thereafter (375 msec at 4 weeks with a mean dose of 15 mg/d and 373 msec at 8 weeks with a mean dose 28 mg/d; no P values reported). Additionally, no significant association was found between methadone dose and the QTc interval (P > .05).
This study evaluated the surrogate endpoint of QTc prolongation and found that 2 patients with a pre-QTc < 500 msec (434 msec and 409 msec) had a post-QTc > 500 msec (518 msec and 547 msec). These subjects were both in the high-dose methadone group receiving 120 mg/d and 60 mg/d of methadone, respectively. It is unclear what confounders were present at the time of the post-QTc.
Related: Using Dashboard Technology to Monitor Overdose
The study did not evaluate clinically relevant outcomes such as TdP or SCD; however, there is evidence that methadone when used within a therapeutic dose range is associated with SCD.27 In a prospective evaluation of SCD, 22 subjects using methadone found with therapeutic blood levels were compared with 106 subjects not using methadone. Most subjects were using methadone for pain control or opioid withdrawal. In 5 subjects (23%) in the methadone group, a cardiac abnormality (eg, coronary artery disease) that could have caused SCD was identified compared with 64 subjects (60%) in the group not using methadone (P = .002).
Limitations
There are several limitations of this study. This retrospective study does not allow for conclusions to be direct cause and effect, and the results relied on the EMR and methadone prescription fill dates to determine adherence to methadone, when methadone was initiated, and methadone daily dose. The exclusion criteria for the diagnosis of heart failure and the use of an implanted pacemaker and/or cardioverter defibrillator depended on the accuracy of the ICD-9 codes.
Also, many factors that affect the QTc interval were not assessed, such as potassium and magnesium levels, alcohol, cocaine, and amphetamine use. In addition, over-the-counter medications and medications obtained outside of SAVAHCS were not assessed. It is possible that any of those factors could be confounding variables. Furthermore, a majority of the subjects were male, and subjects with heart failure and those using an implanted pacemaker and/or cardioverter defibrillator were excluded from the study. In clinical practice, the results of the study cannot be generalized to those excluded patient populations. Additionally, the effect size of QTc prolongation observed was lower than was expected. Therefore, this study may not have been powered adequately to detect smaller differences in QTc prolongation.
Another limitation of the study is the high exclusion rate: about 90%. A majority of the patients were excluded due to the lack of ECG monitoring. The reason for obtaining an ECG was not assessed, and many subjects likely had an ECG obtained incidentally. Due to the high exclusion rate, selection bias may have been introduced into the study. Therefore, the 10% of subjects included in the study may not be representative of veterans using methadone for pain.
Very few studies of the effects of methadone on QTc prolongation in veterans have been published. A retrospective chart review by Fareed and colleagues sought to identify whether patients are at high risk for cardiac arrhythmias by adding an onsite ECG screening at baseline and annually for patients using methadone as part of a methadone maintenance program at the Atlanta VAMC.11 The patients in the study were an average age of 56 years, and 93% were male. The mean daily methadone dose was 90 ± 48 mg/d, and the mean duration of treatment was 38 ± 31 months. The mean QTc interval was significantly longer at the most recent QTc interval while using methadone compared with the baseline QTc interval (442 ± 25 msec vs 417 ± 30 msec, respectively; P < .001). Six percent of patients had a significant prolongation of the QTc interval from baseline to > 500 msec, and 27% had a significant prolongation of the QTc interval from baseline to 450 msec to 500 msec (P < .05).
This study and the study by Fareed and colleagues are similar in that both are retrospective, compare baseline QTc intervals and QTc intervals while using methadone, and include subjects of a similar age and gender. However, the subjects in the Fareed and colleagues study were using methadone for maintenance treatment at higher doses than in the present study. Additionally, the results of the studies differ. Fareed and colleagues found a prolongation of the QTc interval with methadone use. Although the present study has many limitations, it adds additional information to the medical literature regarding the QTc interval of veterans using methadone in lower doses at an average of 29 mg/d.
Conclusion
In this study of veterans using methadone for pain, methadone did not significantly increase the QTc interval. Two patients had a prolonged QTc interval of ≥ 500 msec while taking methadone. The QTc interval did not vary by methadone daily dose, and the concurrent use of QTc prolonging medications together with methadone did not increase the QTc interval. The concurrent use of strong CYP3A4 inhibitors could not be assessed, because none of the patients were on these medications.
Despite these findings, the study had several limitations, and there is a black box warning included in the labeling of methadone regarding QTc interval prolongation, TdP, and death. Therefore, it is advisable to monitor the QTc interval in patients using methadone, even at low doses. In those patients with a prolonged QTc interval and/or risk factors for QTc prolongation, methadone should either be avoided or used cautiously with close monitoring of the QTc interval.
Acknowledgments
This manuscript was prepared and research was conducted with resources and the use of facilities at the Southern Arizona VA Health Care System in Tucson, Arizona.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Kong MH, Fonarow GC, Peterson ED, et al. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57(7): 794-801.
2. Josephson M, Wellens HJJ. Implantable defibrillators and sudden cardiac death. Circulation. 2004;109(22):2685-2691.
3. International Conference on Harmonisation-Quality. Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Rockville, MD: U.S. Food and Drug Administration; 2005.
4. Committee for Proprietary Medicinal Products. Points to Consider: The Assessment of the Potential for QT Interval Prolongation by Non-Cardiovascular Medicinal Products. London, UK: The European Agency for the Evaluation of Medicinal Products; 1997.
5. Stringer J, Welsh C, Tommasello A. Methadone-associated Q-T interval prolongation and torsades de pointes. Am J Health Syst Pharm. 2009;66(9):825-833.
6. Information for healthcare professionals: Methadone hydrochloride. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm142841.htm. Published November 2006. Updated August 23, 2013. Accessed January 9, 2015.
7. Anchersen K, Clausen T, Gossop M, Hansteen V, Waal H. Prevalence and clinical relevance of corrected QT interval prolongation during methadone and buprenorphine treatment: A mortality assessment study. Addiction. 2009;104(6):993-999.
8. Chang KC, Huang CL, Liang HY, et al. Gender-specific differences in susceptibility to low-dose methadone-associated QTc prolongation in patients with heroin dependence. J Cardiovasc Electrophysiol. 2012;23(5):527-533.
9. Cruciani RA, Sekine R, Homel P, et al. Measurement of QTc in patients receiving chronic methadone therapy. J Pain Symptom Manage. 2005;29(4):385-391.
10. Ehret GB, Voide C, Gex-Fabry M, et al. Drug-induced long QT syndrome in injection drug users receiving methadone: High frequency in hospitalized patients and risk factors. Arch Intern Med. 2006;166(12):1280-1287.
11. Fareed A, Vayalapalli S, Byrd-Sellers J, et al. Onsite QTc interval screening for patients in methadone maintenance treatment. J Addict Dis. 2010;29(1):15-22.
12. Fanoe S, Hvidt C, Ege P, Jensen GB. Syncope and QT prolongation among patients treated with methadone for heroin dependence in the city of Copenhagen. Heart. 2007;93(9):1051-1055.
13. Peles E, Bodner G, Kreek MJ, Rados V, Adelson M. Corrected-QT intervals as related to methadone dose and serum level in methadone maintenance treatment (MMT) patients: A cross-sectional study. Addiction. 2007;102(2):289-300.
14. Wedam EF, Bigelow GE, Johnson RE, Nuzzo PA, Haigney MC. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007;167(22):2469-2475.
15. DOLOPHINE [package insert]. Columbus, OH: Roxane Laboratories, Inc; 2012.
16. Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Ann Intern Med. 2009;150(6):387-395.
17. Krantz MJ, Kutinsky IB, Robertson AD, Mehler PS. Dose-related effects of methadone on QT prolongation in a series of patients with torsade de pointes. Pharmacotherapy. 2003;23(6):802-805.
18. Castro VM, Clements CC, Murphy SN, et al. QT interval and antidepressant use: A cross sectional study of electronic health records. BMJ. 2013;346:f288.
19. Florian J, Garnett CE, Nallani SC, Rappaport BA, Throckmorton DC. A modeling and simulation approach to characterize methadone QT prolongation using pooled data from five clinical trials in MMT patients. Clin Pharmacol Ther. 2012;91(4):666-672.
20. Fonseca F, Marti-Almor J, Pastor A, et al. Prevalence of long QTc interval in methadone maintenance patients. Drug Alcohol Depend. 2009;99(1-3):327-332.
21. Gheshlaghi F, Izadi-Mood N, Mardani A, Piri-Ardekani MR. Dose-dependent effects of methadone on the QT interval in patients under methadone maintenance treatment. Asia Pacific J Med Toxicol. 2013;2(1):6-9.
22. Drugs with a known risk of Torsades de Pointes. CredibleMeds Website. https://www.crediblemeds.org/new-drug-list. Updated January 14, 2015. Accessed January 14, 2015.
23. Drugs with a conditional risk of Torsades de Pointes. CredibleMeds Website. https://www.crediblemeds.org/new-drug-list. Updated January 14, 2015. Accessed January 14, 2015.
24. Cytochrome P450 drug interactions [full update October 2009]. Pharmacist’s Letter/Prescribers Letter. 2006;22(2):220233.
25. Huh B, Park CH. Retrospective analysis of low-dose methadone and QTc prolongation in chronic pain patients. Korean J Anesthesiol. 2010;58(4):338-343.
26. Reddy S, Hui D, El Osta B, et al. The effect of oral methadone on the QTc interval in advanced cancer patients: A prospective pilot study. J Palliat Med. 2010;13(1):33-38.
27. Chugh SS, Socoteanu C, Reinier K, Waltz J, Jui J, Gunson K. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121(1):66-71.
Estimates of the annual incidence of sudden cardiac death (SCD) vary from 180,000 to 456,000.1 About 80% to 85% of the cases of SCD are due to a ventricular arrhythmia.2 One type of ventricular arrhythmia, torsades de pointes (TdP), is caused by a prolongation of the QT interval. Because the QT interval is dependent on heart rate, clinicians use the corrected QT (QTc) interval, which has been adjusted for heart rate. The HHS recommends using a gender-independent threshold of 450 msec to define QTc prolongation when conducting research.3 Additionally, a QTc interval > 500 msec is associated with an increased risk for TdP.4 Female gender, hypokalemia, hypomagnesemia, and medical conditions such as congenital long QT syndrome, heart failure, and left ventricular hypertrophy can predispose a person to QTc prolongation.5
Methadone is a synthetic opioid used for chronic pain management or for opioid or heroin addiction. In 2006, the FDA issued a public health advisory, which was followed by the addition of a black box warning to the labeling of methadone regarding cardiac abnormalities that caused serious adverse effects, including QT prolongation, TdP, and death.6 Additional recommendations to evaluate the pharmacokinetic and pharmacodynamic drug interactions were also added to the labeling.
Related: Reducing Opioid Use for Chronic Pain
Several prospective studies, cross-sectional studies, and retrospective reviews have reported QTc prolongation with methadone.7-14 Many cases of QTc prolongation and TdP have been in patients receiving methadone in large doses (> 100 mg/d); however, incidences have also occurred in those receiving typical doses of methadone for addiction treatment.8,15,16 Multiple studies have demonstrated that methadone-induced QTc prolongation is dose-dependent.8,17-20 In an observational study of 90 subjects who were undergoing methadone maintenance treatment, the subjects taking < 60 mg/d of methadone had a 7.7 msec prolongation of the QTc interval, which was significantly less QTc prolongation than in those receiving 60 mg/d to 109 mg/d (15.6 msec, P < .001) and 110 mg/d to 150 mg/d (17.4 msec, P = .001).21
However, in the current literature there are few studies evaluating the QTc prolonging effects of methadone when used in lower doses for pain, such as those used at the Southern Arizona VA Health Care System (SAVAHCS).8 Given the increased risk of cardiac arrhythmias, it is important to understand the effects of methadone on the QTc interval in a veteran patient population using methadone at lower doses for pain. Understanding this risk can help clinicians develop strategies and protocols for the safe use of methadone.
The purpose of this study was to evaluate the effect of methadone on the QTc interval among patients at SAVAHCS. The primary objective was to determine whether methadone prolongs the QTc interval when used for pain. Secondary outcomes included evaluations of the (1) QTc interval when stratified by the QTc interval obtained while prescribed methadone; (2) effects of low, medium, and high doses of methadone on the QTc interval; (3) effects of the concurrent use of QTc prolonging medications on the QTc interval; and (4) effects of the concurrent use of strong inhibitors of methadone clearance on the QTc interval. It was hypothesized that methadone, when used for pain, causes a significant prolongation of the QTc interval, and methadone-induced QTc prolongation is dose-dependent. It was also hypothesized that the QTc interval will be more prolonged when methadone is used concurrently with other medications with a known or conditional risk of TdP or with medications that are strong inhibitors of methadone clearance.
Methods
Full Institutional Review Board approval was obtained prior to initiating this retrospective pre-post study. This study used the electronic medical records (EMRs) of SAVAHCS from July 1, 2004, to July 31, 2012, to compare the QTc interval of patients on stable doses of methadone with the baseline QTc interval. Patients included were aged 18 to 87 years and dispensed a new prescription for methadone between January 1, 2006, and July 31, 2010. Patients must have been adherent to methadone as defined by a medication possession ratio of ≥ 0.8. Patients without a baseline electrocardiogram (ECG) within the 18 months prior to starting methadone, without at least 1 follow-up ECG 7 days to 2 years after the initial prescription, who had a diagnosis of heart failure, or used an implanted cardiac defibrillator or pacemaker as indicated by ICD-9 codes were excluded.
Information collected from the EMR included demographics (age and gender), the QTc interval before starting methadone (pre-QTc), the first QTc interval recorded while prescribed methadone (post-QTc) at a stable dose (defined as ≥ 7 days without a dose change), methadone total daily dose at the time of the post-QTc, concurrent QTc prolonging medications used at the time of each ECG, time elapsed between pre-QTc and post-QTc, time elapsed between the pre-QTc and initiation of methadone, and time elapsed between starting methadone and the post-QTc. All ECGs were recorded using a 12-lead ECG by the MAC 5500 Resting ECG Analysis System (GE Healthcare), which automatically calculates the QTc interval.
For the primary outcome, the mean pre-QTc and post-QTc were compared. These QTc intervals were further analyzed as secondary outcomes. The mean pre-QTc and post-QTc were compared when stratified by post-QTc of < 450 msec, 450 msec to 499 msec, and ≥ 500 msec. To analyze the dose effect of methadone, the mean pre-QTc and post-QTc were compared when stratified by methadone total daily dose: low (≤ 15 mg/d), medium (16-30 mg/d), and high (> 30 mg/d). Dose ranges were based on typical SAVAHCS prescriptions.
An additional secondary outcome was to determine the effect of the concurrent use of QTc prolonging medications on the QTc interval. The medications the subject was taking at the time of each ECG were reviewed to determine whether any of the medications were listed as Drugs with a risk of Torsades de Pointes or Drugs with a conditional risk of Torsades de Pointes on the CredibleMeds website.22,23 Drugs with a conditional risk of TdP are defined as drugs in which there is evidence that the drug prolongs the QTc interval and has a risk of TdP but only under certain conditions, such as high doses or drug interactions. The mean pre-QTc with and without concurrent use of QTc prolonging medications was compared with the mean post-QTc with QTc prolonging medications in addition to methadone.
Because methadone is extensively metabolized by cytochrome P450 3A4 (CYP3A4), another outcome was to determine the effect of the concurrent use of strong CYP3A4 inhibitors on the QTc interval. The medications the subject was taking at the time of each ECG were reviewed to determine whether any of the medications were listed as strong CYP3A4 inhibitors in The Pharmacist’s Letter/The Prescriber’s Letter.24 The mean pre-QTc with and without the concurrent use of strong CYP3A4 inhibitors was compared with the mean post-QTc with strong CYP3A4 inhibitors in addition to methadone.
Related: Pharmacist-Managed Collaborative Practice for Chronic Stable Angina
The primary and secondary outcomes were compared using a paired t test and descriptive statistics; demographics were analyzed using descriptive statistics. Based on the findings of a previous study, a minimum of 8 patients were needed to meet a power of 0.8 with an alpha 0.05 and a medium effect size when comparing the mean pre-QTc and post-QTc for the primary outcome.25
Results
Of 893 EMRs reviewed, 93 met inclusion criteria (Figure 1). The main reason for exclusion was lack of pre-QTc and/or post-QTc (n = 544). The mean age was 58 years (± 10 years), 92% were male, and the mean daily methadone dose at the time of the post-QTc was 29 mg (5 mg-160 mg) (Table 1). Thirty patients were prescribed ≥ 1 QTc prolonging medication at the time of the pre-QTc, 40 patients were prescribed ≥ 1 QTc prolonging medication at the time of the post-QTc, and 0 patients were prescribed a strong CYP3A4 inhibitor at the time of either QTc. There was an average of 151 days (0-554 days) between the pre-QTc and methadone initiation, 161 days (8-664 days) between methadone initiation and the post-QTc, and 312 days (10-1,003 days) between the pre-QTc and post-QTc.
For the primary outcome, there was no significant increase in the QTc interval when comparing the pre-QTc and post-QTc (432 ± 26 msec vs 436 ± 28 msec, P = .221) (Figure 2). When stratified by post QTc, the group of patients with a post-QTc < 450 msec (n = 66) and the group with a post-QTc of ≥ 500 msec (n = 2) had no significant increase in the QTc interval (427 ± 26 msec vs 422 ± 16 msec and 422 ± 18 msec vs 533 ± 21 msec, respectively; P > .05) (Table 2). For the group of patients with a post-QTc of 450 msec to 499 msec (n = 25), methadone significantly prolonged the QTc interval (447 ± 21 msec vs 465 ± 12 msec, P < .001).
When stratified by methadone daily dose of ≤ 15 mg (n = 45), 16 mg to 30 mg (n = 27), and > 30 mg (n = 21), methadone did not significantly prolong the QTc interval in any group (428 ± 29 msec vs 430 ± 25 msec, 436 ± 16 msec vs 439 ± 21 msec, 437 ± 29 msec vs 446 ± 39 msec, respectively; P > .05) (Table 3). For the group of patients using ≥ 1 QTc prolonging medication at the time of the post-QTc and no QTc prolonging medications at the time of the pre-QTc, the addition of methadone did not significantly increase the QTc interval when compared with the pre-QTc (n = 15; 425 ± 23 msec vs 437 ± 31 msec, respectively; P > .05) (Table 4). For the group of patients prescribed ≥ 1 QTc prolonging medication at the pre-QTc and post-QTc, methadone did not significantly prolong the QTc interval (n = 25; 437 ± 32 msec vs 441 ± 33 msec, respectively; P > .05) (Table 5). No subjects were using strong CYP3A4 inhibitors; therefore, the effect of strong CYP3A4 inhibitors could not be assessed.
Discussion
The results of this study suggest that methadone-induced QTc interval prolongation may not be clearly evident at lower doses when used for pain. There was no significant increase in the QTc interval in the low-, medium-, and high-dose methadone groups, nor when analyzing the drug interactions. However, this study was powered based on the primary outcome, and it is possible that the study was underpowered to detect a difference in these secondary outcomes. When stratified by post-QTc, a significant increase in the QTc interval was noted for the group of patients with a post-QTc of 450 msec to 499 msec. The absolute mean differences between the pre-QTc and post-QTc for most of the secondary outcomes are unlikely to be clinically relevant, with the exception of the high-dose methadone group and the group stratified by post-QTc interval of 450 msec to 499 msec.
These results are supported by a prospective pilot study of 64 subjects with advanced cancer, which evaluated the QTc prolonging effects of methadone when used at lower doses (range 3-90 mg/d, median 23 mg/d).26 Only 1 of 64 subjects developed clinically significant QTc interval prolongation (QTc ≥ 500 msec) at the end of the second week of therapy. The mean QTc interval measured at baseline was 427 msec, which increased to a mean of 430 msec after 2 weeks of methadone use (mean dose 23 mg/d) and decreased thereafter (375 msec at 4 weeks with a mean dose of 15 mg/d and 373 msec at 8 weeks with a mean dose 28 mg/d; no P values reported). Additionally, no significant association was found between methadone dose and the QTc interval (P > .05).
This study evaluated the surrogate endpoint of QTc prolongation and found that 2 patients with a pre-QTc < 500 msec (434 msec and 409 msec) had a post-QTc > 500 msec (518 msec and 547 msec). These subjects were both in the high-dose methadone group receiving 120 mg/d and 60 mg/d of methadone, respectively. It is unclear what confounders were present at the time of the post-QTc.
Related: Using Dashboard Technology to Monitor Overdose
The study did not evaluate clinically relevant outcomes such as TdP or SCD; however, there is evidence that methadone when used within a therapeutic dose range is associated with SCD.27 In a prospective evaluation of SCD, 22 subjects using methadone found with therapeutic blood levels were compared with 106 subjects not using methadone. Most subjects were using methadone for pain control or opioid withdrawal. In 5 subjects (23%) in the methadone group, a cardiac abnormality (eg, coronary artery disease) that could have caused SCD was identified compared with 64 subjects (60%) in the group not using methadone (P = .002).
Limitations
There are several limitations of this study. This retrospective study does not allow for conclusions to be direct cause and effect, and the results relied on the EMR and methadone prescription fill dates to determine adherence to methadone, when methadone was initiated, and methadone daily dose. The exclusion criteria for the diagnosis of heart failure and the use of an implanted pacemaker and/or cardioverter defibrillator depended on the accuracy of the ICD-9 codes.
Also, many factors that affect the QTc interval were not assessed, such as potassium and magnesium levels, alcohol, cocaine, and amphetamine use. In addition, over-the-counter medications and medications obtained outside of SAVAHCS were not assessed. It is possible that any of those factors could be confounding variables. Furthermore, a majority of the subjects were male, and subjects with heart failure and those using an implanted pacemaker and/or cardioverter defibrillator were excluded from the study. In clinical practice, the results of the study cannot be generalized to those excluded patient populations. Additionally, the effect size of QTc prolongation observed was lower than was expected. Therefore, this study may not have been powered adequately to detect smaller differences in QTc prolongation.
Another limitation of the study is the high exclusion rate: about 90%. A majority of the patients were excluded due to the lack of ECG monitoring. The reason for obtaining an ECG was not assessed, and many subjects likely had an ECG obtained incidentally. Due to the high exclusion rate, selection bias may have been introduced into the study. Therefore, the 10% of subjects included in the study may not be representative of veterans using methadone for pain.
Very few studies of the effects of methadone on QTc prolongation in veterans have been published. A retrospective chart review by Fareed and colleagues sought to identify whether patients are at high risk for cardiac arrhythmias by adding an onsite ECG screening at baseline and annually for patients using methadone as part of a methadone maintenance program at the Atlanta VAMC.11 The patients in the study were an average age of 56 years, and 93% were male. The mean daily methadone dose was 90 ± 48 mg/d, and the mean duration of treatment was 38 ± 31 months. The mean QTc interval was significantly longer at the most recent QTc interval while using methadone compared with the baseline QTc interval (442 ± 25 msec vs 417 ± 30 msec, respectively; P < .001). Six percent of patients had a significant prolongation of the QTc interval from baseline to > 500 msec, and 27% had a significant prolongation of the QTc interval from baseline to 450 msec to 500 msec (P < .05).
This study and the study by Fareed and colleagues are similar in that both are retrospective, compare baseline QTc intervals and QTc intervals while using methadone, and include subjects of a similar age and gender. However, the subjects in the Fareed and colleagues study were using methadone for maintenance treatment at higher doses than in the present study. Additionally, the results of the studies differ. Fareed and colleagues found a prolongation of the QTc interval with methadone use. Although the present study has many limitations, it adds additional information to the medical literature regarding the QTc interval of veterans using methadone in lower doses at an average of 29 mg/d.
Conclusion
In this study of veterans using methadone for pain, methadone did not significantly increase the QTc interval. Two patients had a prolonged QTc interval of ≥ 500 msec while taking methadone. The QTc interval did not vary by methadone daily dose, and the concurrent use of QTc prolonging medications together with methadone did not increase the QTc interval. The concurrent use of strong CYP3A4 inhibitors could not be assessed, because none of the patients were on these medications.
Despite these findings, the study had several limitations, and there is a black box warning included in the labeling of methadone regarding QTc interval prolongation, TdP, and death. Therefore, it is advisable to monitor the QTc interval in patients using methadone, even at low doses. In those patients with a prolonged QTc interval and/or risk factors for QTc prolongation, methadone should either be avoided or used cautiously with close monitoring of the QTc interval.
Acknowledgments
This manuscript was prepared and research was conducted with resources and the use of facilities at the Southern Arizona VA Health Care System in Tucson, Arizona.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Estimates of the annual incidence of sudden cardiac death (SCD) vary from 180,000 to 456,000.1 About 80% to 85% of the cases of SCD are due to a ventricular arrhythmia.2 One type of ventricular arrhythmia, torsades de pointes (TdP), is caused by a prolongation of the QT interval. Because the QT interval is dependent on heart rate, clinicians use the corrected QT (QTc) interval, which has been adjusted for heart rate. The HHS recommends using a gender-independent threshold of 450 msec to define QTc prolongation when conducting research.3 Additionally, a QTc interval > 500 msec is associated with an increased risk for TdP.4 Female gender, hypokalemia, hypomagnesemia, and medical conditions such as congenital long QT syndrome, heart failure, and left ventricular hypertrophy can predispose a person to QTc prolongation.5
Methadone is a synthetic opioid used for chronic pain management or for opioid or heroin addiction. In 2006, the FDA issued a public health advisory, which was followed by the addition of a black box warning to the labeling of methadone regarding cardiac abnormalities that caused serious adverse effects, including QT prolongation, TdP, and death.6 Additional recommendations to evaluate the pharmacokinetic and pharmacodynamic drug interactions were also added to the labeling.
Related: Reducing Opioid Use for Chronic Pain
Several prospective studies, cross-sectional studies, and retrospective reviews have reported QTc prolongation with methadone.7-14 Many cases of QTc prolongation and TdP have been in patients receiving methadone in large doses (> 100 mg/d); however, incidences have also occurred in those receiving typical doses of methadone for addiction treatment.8,15,16 Multiple studies have demonstrated that methadone-induced QTc prolongation is dose-dependent.8,17-20 In an observational study of 90 subjects who were undergoing methadone maintenance treatment, the subjects taking < 60 mg/d of methadone had a 7.7 msec prolongation of the QTc interval, which was significantly less QTc prolongation than in those receiving 60 mg/d to 109 mg/d (15.6 msec, P < .001) and 110 mg/d to 150 mg/d (17.4 msec, P = .001).21
However, in the current literature there are few studies evaluating the QTc prolonging effects of methadone when used in lower doses for pain, such as those used at the Southern Arizona VA Health Care System (SAVAHCS).8 Given the increased risk of cardiac arrhythmias, it is important to understand the effects of methadone on the QTc interval in a veteran patient population using methadone at lower doses for pain. Understanding this risk can help clinicians develop strategies and protocols for the safe use of methadone.
The purpose of this study was to evaluate the effect of methadone on the QTc interval among patients at SAVAHCS. The primary objective was to determine whether methadone prolongs the QTc interval when used for pain. Secondary outcomes included evaluations of the (1) QTc interval when stratified by the QTc interval obtained while prescribed methadone; (2) effects of low, medium, and high doses of methadone on the QTc interval; (3) effects of the concurrent use of QTc prolonging medications on the QTc interval; and (4) effects of the concurrent use of strong inhibitors of methadone clearance on the QTc interval. It was hypothesized that methadone, when used for pain, causes a significant prolongation of the QTc interval, and methadone-induced QTc prolongation is dose-dependent. It was also hypothesized that the QTc interval will be more prolonged when methadone is used concurrently with other medications with a known or conditional risk of TdP or with medications that are strong inhibitors of methadone clearance.
Methods
Full Institutional Review Board approval was obtained prior to initiating this retrospective pre-post study. This study used the electronic medical records (EMRs) of SAVAHCS from July 1, 2004, to July 31, 2012, to compare the QTc interval of patients on stable doses of methadone with the baseline QTc interval. Patients included were aged 18 to 87 years and dispensed a new prescription for methadone between January 1, 2006, and July 31, 2010. Patients must have been adherent to methadone as defined by a medication possession ratio of ≥ 0.8. Patients without a baseline electrocardiogram (ECG) within the 18 months prior to starting methadone, without at least 1 follow-up ECG 7 days to 2 years after the initial prescription, who had a diagnosis of heart failure, or used an implanted cardiac defibrillator or pacemaker as indicated by ICD-9 codes were excluded.
Information collected from the EMR included demographics (age and gender), the QTc interval before starting methadone (pre-QTc), the first QTc interval recorded while prescribed methadone (post-QTc) at a stable dose (defined as ≥ 7 days without a dose change), methadone total daily dose at the time of the post-QTc, concurrent QTc prolonging medications used at the time of each ECG, time elapsed between pre-QTc and post-QTc, time elapsed between the pre-QTc and initiation of methadone, and time elapsed between starting methadone and the post-QTc. All ECGs were recorded using a 12-lead ECG by the MAC 5500 Resting ECG Analysis System (GE Healthcare), which automatically calculates the QTc interval.
For the primary outcome, the mean pre-QTc and post-QTc were compared. These QTc intervals were further analyzed as secondary outcomes. The mean pre-QTc and post-QTc were compared when stratified by post-QTc of < 450 msec, 450 msec to 499 msec, and ≥ 500 msec. To analyze the dose effect of methadone, the mean pre-QTc and post-QTc were compared when stratified by methadone total daily dose: low (≤ 15 mg/d), medium (16-30 mg/d), and high (> 30 mg/d). Dose ranges were based on typical SAVAHCS prescriptions.
An additional secondary outcome was to determine the effect of the concurrent use of QTc prolonging medications on the QTc interval. The medications the subject was taking at the time of each ECG were reviewed to determine whether any of the medications were listed as Drugs with a risk of Torsades de Pointes or Drugs with a conditional risk of Torsades de Pointes on the CredibleMeds website.22,23 Drugs with a conditional risk of TdP are defined as drugs in which there is evidence that the drug prolongs the QTc interval and has a risk of TdP but only under certain conditions, such as high doses or drug interactions. The mean pre-QTc with and without concurrent use of QTc prolonging medications was compared with the mean post-QTc with QTc prolonging medications in addition to methadone.
Because methadone is extensively metabolized by cytochrome P450 3A4 (CYP3A4), another outcome was to determine the effect of the concurrent use of strong CYP3A4 inhibitors on the QTc interval. The medications the subject was taking at the time of each ECG were reviewed to determine whether any of the medications were listed as strong CYP3A4 inhibitors in The Pharmacist’s Letter/The Prescriber’s Letter.24 The mean pre-QTc with and without the concurrent use of strong CYP3A4 inhibitors was compared with the mean post-QTc with strong CYP3A4 inhibitors in addition to methadone.
Related: Pharmacist-Managed Collaborative Practice for Chronic Stable Angina
The primary and secondary outcomes were compared using a paired t test and descriptive statistics; demographics were analyzed using descriptive statistics. Based on the findings of a previous study, a minimum of 8 patients were needed to meet a power of 0.8 with an alpha 0.05 and a medium effect size when comparing the mean pre-QTc and post-QTc for the primary outcome.25
Results
Of 893 EMRs reviewed, 93 met inclusion criteria (Figure 1). The main reason for exclusion was lack of pre-QTc and/or post-QTc (n = 544). The mean age was 58 years (± 10 years), 92% were male, and the mean daily methadone dose at the time of the post-QTc was 29 mg (5 mg-160 mg) (Table 1). Thirty patients were prescribed ≥ 1 QTc prolonging medication at the time of the pre-QTc, 40 patients were prescribed ≥ 1 QTc prolonging medication at the time of the post-QTc, and 0 patients were prescribed a strong CYP3A4 inhibitor at the time of either QTc. There was an average of 151 days (0-554 days) between the pre-QTc and methadone initiation, 161 days (8-664 days) between methadone initiation and the post-QTc, and 312 days (10-1,003 days) between the pre-QTc and post-QTc.
For the primary outcome, there was no significant increase in the QTc interval when comparing the pre-QTc and post-QTc (432 ± 26 msec vs 436 ± 28 msec, P = .221) (Figure 2). When stratified by post QTc, the group of patients with a post-QTc < 450 msec (n = 66) and the group with a post-QTc of ≥ 500 msec (n = 2) had no significant increase in the QTc interval (427 ± 26 msec vs 422 ± 16 msec and 422 ± 18 msec vs 533 ± 21 msec, respectively; P > .05) (Table 2). For the group of patients with a post-QTc of 450 msec to 499 msec (n = 25), methadone significantly prolonged the QTc interval (447 ± 21 msec vs 465 ± 12 msec, P < .001).
When stratified by methadone daily dose of ≤ 15 mg (n = 45), 16 mg to 30 mg (n = 27), and > 30 mg (n = 21), methadone did not significantly prolong the QTc interval in any group (428 ± 29 msec vs 430 ± 25 msec, 436 ± 16 msec vs 439 ± 21 msec, 437 ± 29 msec vs 446 ± 39 msec, respectively; P > .05) (Table 3). For the group of patients using ≥ 1 QTc prolonging medication at the time of the post-QTc and no QTc prolonging medications at the time of the pre-QTc, the addition of methadone did not significantly increase the QTc interval when compared with the pre-QTc (n = 15; 425 ± 23 msec vs 437 ± 31 msec, respectively; P > .05) (Table 4). For the group of patients prescribed ≥ 1 QTc prolonging medication at the pre-QTc and post-QTc, methadone did not significantly prolong the QTc interval (n = 25; 437 ± 32 msec vs 441 ± 33 msec, respectively; P > .05) (Table 5). No subjects were using strong CYP3A4 inhibitors; therefore, the effect of strong CYP3A4 inhibitors could not be assessed.
Discussion
The results of this study suggest that methadone-induced QTc interval prolongation may not be clearly evident at lower doses when used for pain. There was no significant increase in the QTc interval in the low-, medium-, and high-dose methadone groups, nor when analyzing the drug interactions. However, this study was powered based on the primary outcome, and it is possible that the study was underpowered to detect a difference in these secondary outcomes. When stratified by post-QTc, a significant increase in the QTc interval was noted for the group of patients with a post-QTc of 450 msec to 499 msec. The absolute mean differences between the pre-QTc and post-QTc for most of the secondary outcomes are unlikely to be clinically relevant, with the exception of the high-dose methadone group and the group stratified by post-QTc interval of 450 msec to 499 msec.
These results are supported by a prospective pilot study of 64 subjects with advanced cancer, which evaluated the QTc prolonging effects of methadone when used at lower doses (range 3-90 mg/d, median 23 mg/d).26 Only 1 of 64 subjects developed clinically significant QTc interval prolongation (QTc ≥ 500 msec) at the end of the second week of therapy. The mean QTc interval measured at baseline was 427 msec, which increased to a mean of 430 msec after 2 weeks of methadone use (mean dose 23 mg/d) and decreased thereafter (375 msec at 4 weeks with a mean dose of 15 mg/d and 373 msec at 8 weeks with a mean dose 28 mg/d; no P values reported). Additionally, no significant association was found between methadone dose and the QTc interval (P > .05).
This study evaluated the surrogate endpoint of QTc prolongation and found that 2 patients with a pre-QTc < 500 msec (434 msec and 409 msec) had a post-QTc > 500 msec (518 msec and 547 msec). These subjects were both in the high-dose methadone group receiving 120 mg/d and 60 mg/d of methadone, respectively. It is unclear what confounders were present at the time of the post-QTc.
Related: Using Dashboard Technology to Monitor Overdose
The study did not evaluate clinically relevant outcomes such as TdP or SCD; however, there is evidence that methadone when used within a therapeutic dose range is associated with SCD.27 In a prospective evaluation of SCD, 22 subjects using methadone found with therapeutic blood levels were compared with 106 subjects not using methadone. Most subjects were using methadone for pain control or opioid withdrawal. In 5 subjects (23%) in the methadone group, a cardiac abnormality (eg, coronary artery disease) that could have caused SCD was identified compared with 64 subjects (60%) in the group not using methadone (P = .002).
Limitations
There are several limitations of this study. This retrospective study does not allow for conclusions to be direct cause and effect, and the results relied on the EMR and methadone prescription fill dates to determine adherence to methadone, when methadone was initiated, and methadone daily dose. The exclusion criteria for the diagnosis of heart failure and the use of an implanted pacemaker and/or cardioverter defibrillator depended on the accuracy of the ICD-9 codes.
Also, many factors that affect the QTc interval were not assessed, such as potassium and magnesium levels, alcohol, cocaine, and amphetamine use. In addition, over-the-counter medications and medications obtained outside of SAVAHCS were not assessed. It is possible that any of those factors could be confounding variables. Furthermore, a majority of the subjects were male, and subjects with heart failure and those using an implanted pacemaker and/or cardioverter defibrillator were excluded from the study. In clinical practice, the results of the study cannot be generalized to those excluded patient populations. Additionally, the effect size of QTc prolongation observed was lower than was expected. Therefore, this study may not have been powered adequately to detect smaller differences in QTc prolongation.
Another limitation of the study is the high exclusion rate: about 90%. A majority of the patients were excluded due to the lack of ECG monitoring. The reason for obtaining an ECG was not assessed, and many subjects likely had an ECG obtained incidentally. Due to the high exclusion rate, selection bias may have been introduced into the study. Therefore, the 10% of subjects included in the study may not be representative of veterans using methadone for pain.
Very few studies of the effects of methadone on QTc prolongation in veterans have been published. A retrospective chart review by Fareed and colleagues sought to identify whether patients are at high risk for cardiac arrhythmias by adding an onsite ECG screening at baseline and annually for patients using methadone as part of a methadone maintenance program at the Atlanta VAMC.11 The patients in the study were an average age of 56 years, and 93% were male. The mean daily methadone dose was 90 ± 48 mg/d, and the mean duration of treatment was 38 ± 31 months. The mean QTc interval was significantly longer at the most recent QTc interval while using methadone compared with the baseline QTc interval (442 ± 25 msec vs 417 ± 30 msec, respectively; P < .001). Six percent of patients had a significant prolongation of the QTc interval from baseline to > 500 msec, and 27% had a significant prolongation of the QTc interval from baseline to 450 msec to 500 msec (P < .05).
This study and the study by Fareed and colleagues are similar in that both are retrospective, compare baseline QTc intervals and QTc intervals while using methadone, and include subjects of a similar age and gender. However, the subjects in the Fareed and colleagues study were using methadone for maintenance treatment at higher doses than in the present study. Additionally, the results of the studies differ. Fareed and colleagues found a prolongation of the QTc interval with methadone use. Although the present study has many limitations, it adds additional information to the medical literature regarding the QTc interval of veterans using methadone in lower doses at an average of 29 mg/d.
Conclusion
In this study of veterans using methadone for pain, methadone did not significantly increase the QTc interval. Two patients had a prolonged QTc interval of ≥ 500 msec while taking methadone. The QTc interval did not vary by methadone daily dose, and the concurrent use of QTc prolonging medications together with methadone did not increase the QTc interval. The concurrent use of strong CYP3A4 inhibitors could not be assessed, because none of the patients were on these medications.
Despite these findings, the study had several limitations, and there is a black box warning included in the labeling of methadone regarding QTc interval prolongation, TdP, and death. Therefore, it is advisable to monitor the QTc interval in patients using methadone, even at low doses. In those patients with a prolonged QTc interval and/or risk factors for QTc prolongation, methadone should either be avoided or used cautiously with close monitoring of the QTc interval.
Acknowledgments
This manuscript was prepared and research was conducted with resources and the use of facilities at the Southern Arizona VA Health Care System in Tucson, Arizona.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Kong MH, Fonarow GC, Peterson ED, et al. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57(7): 794-801.
2. Josephson M, Wellens HJJ. Implantable defibrillators and sudden cardiac death. Circulation. 2004;109(22):2685-2691.
3. International Conference on Harmonisation-Quality. Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Rockville, MD: U.S. Food and Drug Administration; 2005.
4. Committee for Proprietary Medicinal Products. Points to Consider: The Assessment of the Potential for QT Interval Prolongation by Non-Cardiovascular Medicinal Products. London, UK: The European Agency for the Evaluation of Medicinal Products; 1997.
5. Stringer J, Welsh C, Tommasello A. Methadone-associated Q-T interval prolongation and torsades de pointes. Am J Health Syst Pharm. 2009;66(9):825-833.
6. Information for healthcare professionals: Methadone hydrochloride. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm142841.htm. Published November 2006. Updated August 23, 2013. Accessed January 9, 2015.
7. Anchersen K, Clausen T, Gossop M, Hansteen V, Waal H. Prevalence and clinical relevance of corrected QT interval prolongation during methadone and buprenorphine treatment: A mortality assessment study. Addiction. 2009;104(6):993-999.
8. Chang KC, Huang CL, Liang HY, et al. Gender-specific differences in susceptibility to low-dose methadone-associated QTc prolongation in patients with heroin dependence. J Cardiovasc Electrophysiol. 2012;23(5):527-533.
9. Cruciani RA, Sekine R, Homel P, et al. Measurement of QTc in patients receiving chronic methadone therapy. J Pain Symptom Manage. 2005;29(4):385-391.
10. Ehret GB, Voide C, Gex-Fabry M, et al. Drug-induced long QT syndrome in injection drug users receiving methadone: High frequency in hospitalized patients and risk factors. Arch Intern Med. 2006;166(12):1280-1287.
11. Fareed A, Vayalapalli S, Byrd-Sellers J, et al. Onsite QTc interval screening for patients in methadone maintenance treatment. J Addict Dis. 2010;29(1):15-22.
12. Fanoe S, Hvidt C, Ege P, Jensen GB. Syncope and QT prolongation among patients treated with methadone for heroin dependence in the city of Copenhagen. Heart. 2007;93(9):1051-1055.
13. Peles E, Bodner G, Kreek MJ, Rados V, Adelson M. Corrected-QT intervals as related to methadone dose and serum level in methadone maintenance treatment (MMT) patients: A cross-sectional study. Addiction. 2007;102(2):289-300.
14. Wedam EF, Bigelow GE, Johnson RE, Nuzzo PA, Haigney MC. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007;167(22):2469-2475.
15. DOLOPHINE [package insert]. Columbus, OH: Roxane Laboratories, Inc; 2012.
16. Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Ann Intern Med. 2009;150(6):387-395.
17. Krantz MJ, Kutinsky IB, Robertson AD, Mehler PS. Dose-related effects of methadone on QT prolongation in a series of patients with torsade de pointes. Pharmacotherapy. 2003;23(6):802-805.
18. Castro VM, Clements CC, Murphy SN, et al. QT interval and antidepressant use: A cross sectional study of electronic health records. BMJ. 2013;346:f288.
19. Florian J, Garnett CE, Nallani SC, Rappaport BA, Throckmorton DC. A modeling and simulation approach to characterize methadone QT prolongation using pooled data from five clinical trials in MMT patients. Clin Pharmacol Ther. 2012;91(4):666-672.
20. Fonseca F, Marti-Almor J, Pastor A, et al. Prevalence of long QTc interval in methadone maintenance patients. Drug Alcohol Depend. 2009;99(1-3):327-332.
21. Gheshlaghi F, Izadi-Mood N, Mardani A, Piri-Ardekani MR. Dose-dependent effects of methadone on the QT interval in patients under methadone maintenance treatment. Asia Pacific J Med Toxicol. 2013;2(1):6-9.
22. Drugs with a known risk of Torsades de Pointes. CredibleMeds Website. https://www.crediblemeds.org/new-drug-list. Updated January 14, 2015. Accessed January 14, 2015.
23. Drugs with a conditional risk of Torsades de Pointes. CredibleMeds Website. https://www.crediblemeds.org/new-drug-list. Updated January 14, 2015. Accessed January 14, 2015.
24. Cytochrome P450 drug interactions [full update October 2009]. Pharmacist’s Letter/Prescribers Letter. 2006;22(2):220233.
25. Huh B, Park CH. Retrospective analysis of low-dose methadone and QTc prolongation in chronic pain patients. Korean J Anesthesiol. 2010;58(4):338-343.
26. Reddy S, Hui D, El Osta B, et al. The effect of oral methadone on the QTc interval in advanced cancer patients: A prospective pilot study. J Palliat Med. 2010;13(1):33-38.
27. Chugh SS, Socoteanu C, Reinier K, Waltz J, Jui J, Gunson K. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121(1):66-71.
1. Kong MH, Fonarow GC, Peterson ED, et al. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57(7): 794-801.
2. Josephson M, Wellens HJJ. Implantable defibrillators and sudden cardiac death. Circulation. 2004;109(22):2685-2691.
3. International Conference on Harmonisation-Quality. Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Rockville, MD: U.S. Food and Drug Administration; 2005.
4. Committee for Proprietary Medicinal Products. Points to Consider: The Assessment of the Potential for QT Interval Prolongation by Non-Cardiovascular Medicinal Products. London, UK: The European Agency for the Evaluation of Medicinal Products; 1997.
5. Stringer J, Welsh C, Tommasello A. Methadone-associated Q-T interval prolongation and torsades de pointes. Am J Health Syst Pharm. 2009;66(9):825-833.
6. Information for healthcare professionals: Methadone hydrochloride. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm142841.htm. Published November 2006. Updated August 23, 2013. Accessed January 9, 2015.
7. Anchersen K, Clausen T, Gossop M, Hansteen V, Waal H. Prevalence and clinical relevance of corrected QT interval prolongation during methadone and buprenorphine treatment: A mortality assessment study. Addiction. 2009;104(6):993-999.
8. Chang KC, Huang CL, Liang HY, et al. Gender-specific differences in susceptibility to low-dose methadone-associated QTc prolongation in patients with heroin dependence. J Cardiovasc Electrophysiol. 2012;23(5):527-533.
9. Cruciani RA, Sekine R, Homel P, et al. Measurement of QTc in patients receiving chronic methadone therapy. J Pain Symptom Manage. 2005;29(4):385-391.
10. Ehret GB, Voide C, Gex-Fabry M, et al. Drug-induced long QT syndrome in injection drug users receiving methadone: High frequency in hospitalized patients and risk factors. Arch Intern Med. 2006;166(12):1280-1287.
11. Fareed A, Vayalapalli S, Byrd-Sellers J, et al. Onsite QTc interval screening for patients in methadone maintenance treatment. J Addict Dis. 2010;29(1):15-22.
12. Fanoe S, Hvidt C, Ege P, Jensen GB. Syncope and QT prolongation among patients treated with methadone for heroin dependence in the city of Copenhagen. Heart. 2007;93(9):1051-1055.
13. Peles E, Bodner G, Kreek MJ, Rados V, Adelson M. Corrected-QT intervals as related to methadone dose and serum level in methadone maintenance treatment (MMT) patients: A cross-sectional study. Addiction. 2007;102(2):289-300.
14. Wedam EF, Bigelow GE, Johnson RE, Nuzzo PA, Haigney MC. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007;167(22):2469-2475.
15. DOLOPHINE [package insert]. Columbus, OH: Roxane Laboratories, Inc; 2012.
16. Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Ann Intern Med. 2009;150(6):387-395.
17. Krantz MJ, Kutinsky IB, Robertson AD, Mehler PS. Dose-related effects of methadone on QT prolongation in a series of patients with torsade de pointes. Pharmacotherapy. 2003;23(6):802-805.
18. Castro VM, Clements CC, Murphy SN, et al. QT interval and antidepressant use: A cross sectional study of electronic health records. BMJ. 2013;346:f288.
19. Florian J, Garnett CE, Nallani SC, Rappaport BA, Throckmorton DC. A modeling and simulation approach to characterize methadone QT prolongation using pooled data from five clinical trials in MMT patients. Clin Pharmacol Ther. 2012;91(4):666-672.
20. Fonseca F, Marti-Almor J, Pastor A, et al. Prevalence of long QTc interval in methadone maintenance patients. Drug Alcohol Depend. 2009;99(1-3):327-332.
21. Gheshlaghi F, Izadi-Mood N, Mardani A, Piri-Ardekani MR. Dose-dependent effects of methadone on the QT interval in patients under methadone maintenance treatment. Asia Pacific J Med Toxicol. 2013;2(1):6-9.
22. Drugs with a known risk of Torsades de Pointes. CredibleMeds Website. https://www.crediblemeds.org/new-drug-list. Updated January 14, 2015. Accessed January 14, 2015.
23. Drugs with a conditional risk of Torsades de Pointes. CredibleMeds Website. https://www.crediblemeds.org/new-drug-list. Updated January 14, 2015. Accessed January 14, 2015.
24. Cytochrome P450 drug interactions [full update October 2009]. Pharmacist’s Letter/Prescribers Letter. 2006;22(2):220233.
25. Huh B, Park CH. Retrospective analysis of low-dose methadone and QTc prolongation in chronic pain patients. Korean J Anesthesiol. 2010;58(4):338-343.
26. Reddy S, Hui D, El Osta B, et al. The effect of oral methadone on the QTc interval in advanced cancer patients: A prospective pilot study. J Palliat Med. 2010;13(1):33-38.
27. Chugh SS, Socoteanu C, Reinier K, Waltz J, Jui J, Gunson K. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121(1):66-71.