User login
Policy in Clinical Practice: Medicare Advantage and Observation Hospitalizations
CLINICAL SCENARIO
A 73-year-old man presents to the emergency department with sepsis secondary to community-acquired pneumonia. The patient requires supplemental oxygen and is started on intravenous antibiotics. His admitting physician expects he will need more than two nights of hospital care and suggests that inpatient status, rather than outpatient (observation) status, would be appropriate under Medicare’s “Two-Midnight Rule.” The physician also suspects the patient may need a brief stay in a skilled nursing facility (SNF) following the mentioned hospitalization and notes that the patient has a Medicare Advantage plan (Table) and wonders if the Two-Midnight Rule applies. Further, she questions whether Medicare’s “Three-Midnight Rule” for SNF benefits will factor in the patient’s discharge planning.
BACKGROUND AND HISTORY
Since the 1970s, the Centers for Medicare & Medicaid Services (CMS) has allowed enrollees to receive their Medicare benefits from privately managed health plans through the so-called Medicare Advantage programs. CMS contracts with commercial insurers who, in exchange for a set payment per Medicare enrollee, “accept full responsibility (ie, risk) for the costs of their enrollees’ care.”1 Over the past 20 years the percent of Medicare Advantage enrollees has nearly doubled nationwide, from 18% to 34%, and is projected to grow even further to 42% by 2028.2,3 The reasons beneficiaries choose to enroll in Medicare Advantage over Traditional Medicare have yet to be thoroughly studied; ease of enrollment and plan administration, as well as lower deductibles, copays, and out-of-pocket maximums for in-network services, are thought to be some of the driving factors.
The federal government has asserted two goals for the development of Medicare Advantage: beneficiary choice and economic efficiency.1 Medicare Advantage plans must be actuarially equal to Traditional Medicare but do not have to cover services in precisely the same way. Medicare Advantage plans may achieve cost savings through narrower networks, strict control of access to SNF services and acute care inpatient rehabilitation, and prior authorization requirements, the latter of which has received recent congressional attention.4,5 On the other hand, many Medicare Advantage plans offer dental, fitness, optical, and caregiver benefits that are not included under Traditional Medicare. Beneficiaries can theoretically compare the coverage and costs of Traditional Medicare to Medicare Advantage programs and make informed choices based on their individualized needs. The second stated goal for the Medicare Advantage option assumes that privately managed plans provide care at lower costs compared with CMS; this assumption has yet to be confirmed with solid data. Indeed, a recent analysis comparing the overall costs of Medicare Advantage to those of Traditional Medicare concluded that Medicare Advantage costs CMS more than Traditional Medicare,6 perhaps in part due to risk adjustment practices.7
POLICY IN PRACTICE
There are a number of areas of uncertainty regarding the specifics of how Medicare Advantage plans work, including Medicare Advantage programs’ use of outpatient (observation) stays. CMS has tried to provide guidance to healthcare organizations and clinicians regarding the appropriate use of inpatient hospitalizations for patients with Traditional Medicare, including the implementation of the Two-Midnight Rule in 2013. According to the rule, clinicians should place inpatient admission orders when they reasonably expect a patient’s care to extend across two midnights.8 Such admission decisions are subject to review by Medicare contractors and Quality Improvement Organizations.
In contrast, Medicare Advantage plans which enter into contracts with specific healthcare systems are not required to abide by CMS’ guidelines for the Two-Midnight Rule.9 When Medicare Advantage firms negotiate contracts with individual hospitals and healthcare organizations, CMS has been clear that such contracts are not required to include the Two-Midnight Rule when it comes to making hospitalization status decisions.10 Instead, in these instances, Medicare Advantage plans often use proprietary decision tools containing clinical criteria, such as Milliman Care Guidelines or InterQual, and/or their own plan’s internal criteria as part of the decision-making process to grant inpatient or outpatient (observation) status. More importantly, CMS has stated that for hospitals and healthcare systems that do not contract with Medicare Advantage programs, the Two-Midnight Rule should apply when it comes to making hospitalization status decisions.10
Implications for Patients
Currently, there are no data available to compare between Medicare Advantage enrollees and traditional medicine beneficiaries in terms of the frequency of observation use and out-of-pocket cost for observation stays. As alluded to in the patient’s case, the use of outpatient (observation) status has implications for a patient’s posthospitalization SNF benefit. Under Traditional Medicare, patients must be hospitalized for three consecutive inpatient midnights in order to qualify for the SNF benefit. Time spent under outpatient (observation) status does not count toward this three-day requirement. Interestingly, some Medicare Advantage programs have demonstrated innovation in this area, waiving the three inpatient midnight requirement for their beneficiaries;11 there is evidence, however, that compared with their Traditional Medicare counterparts, Medicare Advantage beneficiaries are admitted to lower quality SNFs.12 The posthospitalization consequences of an inpatient versus outpatient (observation) status determination for a Medicare Advantage beneficiary is thus unclear, further complicating the decision-making process for patients when it comes to choosing a Medicare policy, and for providers when it comes to choosing an admission status.
Implications for Clinicians and Healthcare Systems
After performing an initial history and physical exam, if a healthcare provider determines that a patient requires hospitalization, an order is placed to classify the stay as inpatient or outpatient (observation). For beneficiaries with Traditional Medicare or a Medicare Advantage plan that has not contracted with the hospital, clinicians should follow the Two-Midnight Rule for making this determination. For contracted Medicare Advantage, the rules are variable. Under Medicare’s Conditions of Participation, hospitals and healthcare organizations are required to have utilization management (UM) programs to assist physicians in making appropriate admission decisions. UM reviews can happen at any point during or after a patient’s stay, however, and physicians may have to make decisions using their best judgment at the time of admission without real-time input from UM teams.
Outpatient (observation) care and the challenges surrounding appropriate status orders have complicated the admission decision. In one study of 2014 Traditional Medicare claims, almost half of outpatient (observation) stays contained a status change.13 Based on a recent survey of hospitalist physicians, about two-thirds of hospitalists report at least monthly requests from patients to change their status.14 Hospital medicine physicians report that these requests “can severely damage the therapeutic bond”14 between provider and patient because the provider must assign status based on CMS rules, not patient request.
COMMENTARY AND RECOMMENDATIONS
CMS could improve the current system in one of two ways. First, CMS could require that all Medicare Advantage plans follow the same polices as Traditional Medicare policies regarding the Two- and Three-Midnight Rules. This would eliminate the need for both hospitals and healthcare organizations to dedicate time and resources to negotiating with each Medicare Advantage program and to managing each Medicare Advantage patient admission based on a specific contract. Ideally, CMS could completely eliminate its outpatient (observation) policy so that all hospitalizations are treated exactly the same, classified under the same billing status and with beneficiaries having the same postacute benefit. This would be consistent with the sentiment behind the recent Office of Inspector General’s (OIG) report suggesting that CMS consider counting outpatient midnights toward the three-midnight requirement for postacute SNF care “so that beneficiaries receiving similar hospital care have similar access to these services.”15
WHAT SHOULD I TELL MY PATIENT?
The physician in the example above should tell their patient that they will be admitted as an inpatient given her expectation that the patient will need hospitalization for oxygen support, parenteral antibiotics, and evaluation by physical therapy to determine a medically appropriate discharge plan. The physician should document the medical necessity for the admission, specifically her expectation that the patient will require at least two midnights of medically necessary hospital care. If the patient has Traditional Medicare, this documentation, along with the inpatient status order, will fulfill the requirements for an inpatient stay. If the patient has a Medicare Advantage plan, the physician can advise the patient that the plan administrators will ultimately determine if an inpatient stay will be covered or denied.
CONCLUSIONS
In the proposed clinical scenario, the rules determining the patient’s hospitalization status depend on whether the hospital contracts with the patient’s Medicare Advantage plan, and if so, what the contracted criteria are in determining inpatient and outpatient (observation) status. The physician could consider real-time input from the hospital’s UM team, if available. Regardless of UM input, if the physician hospitalizes the patient as an inpatient, the Medicare Advantage plan administrators will make a determination regarding the appropriateness of the admission status, as well as whether the patient qualifies for posthospitalization Medicare SNF benefits (if requested) and, additionally, which SNFs will be covered. If denied, the hospitalist will have the option of a peer-to-peer discussion with the insurance company to overturn the denial. Given the confusion, complexity, and implications presented by this admission status decision-making process, standardization across Traditional Medicare and Medicare Advantage plans, or a budget-neutral plan to eliminate status distinction altogether, is certainly warranted.
1.McGuire TG, Newhouse JP, Sinaiko AD. An economic history of Medicare Part C. Millbank Q. 2011;89(2):289-332. https://doi.org/10.1111/j.1468-0009.2011.00629.x.
2. Medicare Advantage. Available at: https://www.kff.org/medicare/fact-sheet/medicare-advantage/.
3. Neuman P, Jacobson G. Medicare Advantage checkup. N Engl J Med. 2018;379(22):2163-2172. https://doi.org/10.1056/NEJMhpr1804089.
4. HR 3107: improving seniors’ timely access to Care Act of 2019. Available at: https://www.congress.gov/bill/116th-congress/house-bill/3107/text?q=%7B%22search%22%3A%5B%22prior+authorization%22%5D%7D&r=1&s=1.
5. Gadbois EA, Tyler DA, Shield RR, et al. Medicare Advantage control of postacute costs: perspective from stakeholders. Am J Manag Care. 2018;24(12):e386-e392.
6. Rooke-Ley H, Broome T, Mostashari F, Cavanaugh S. Evaluating Medicare programs against saving taxpayer dollars. Health Affairs, August 16, 2019. Available at: https://www.healthaffairs.org/do/10.1377/hblog20190813.223707/full/.
7. Office of Inspector General. Billions in estimated Medicare Advantage payments from chart reviews raise concerns. December 2019. Available at: https://oig.hhs.gov/oei/reports/oei-03-17-00470.pdf. Accessed December 15, 2019.
8. Fact sheet: two-midnight rule. Available at: https://www.cms.gov/newsroom/fact-sheets/fact-sheet-two-midnight-rule-0.
9. Locke C, Hu E. Medicare’s two-midnight rule: what hospitalists must know. Available at: https://www.the-hospitalist.org/hospitalist/article/194971/medicares-two-midnight-rule.
10. Announcement of calendar year (CY) 2019 Medicare Advantage capitation rates and Medicare Advantage and part D payment policies and final call letter. Page 206. Available at: https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Downloads/Announcement2019.pdf. Accessed November 18, 2019.
11. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trivedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Aff. 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054.
12. Meyers D, Mor V, Rahman M. Medicare Advantage enrollees more likely to enter lower-quality nursing homes compared to fee-for-service enrollees. Health Aff. 2018;37(1):78-85. https://doi.org/10.1377/hlthaff.2017.0714.
13. Sheehy A, Shi F, Kind AJH. Identifying observation stays in Medicare data: Policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
14. The hospital observation care problem: perspectives and solutions from the Society of Hospital Medicine. Available at: https://www.hospitalmedicine.org/globalassets/policy-and-advocacy/advocacy-pdf/shms-observation-white-paper-2017. Accessed November 18, 2019.
15. U.S. Department of Health & Human Services, Office of Inspector General. Solutions to reduce fraud, waste and abuse in HHS programs: OIG’s top recommendations. Available at: https://oig.hhs.gov/reports-and-publications/compendium/. Accessed November 22, 2019.
CLINICAL SCENARIO
A 73-year-old man presents to the emergency department with sepsis secondary to community-acquired pneumonia. The patient requires supplemental oxygen and is started on intravenous antibiotics. His admitting physician expects he will need more than two nights of hospital care and suggests that inpatient status, rather than outpatient (observation) status, would be appropriate under Medicare’s “Two-Midnight Rule.” The physician also suspects the patient may need a brief stay in a skilled nursing facility (SNF) following the mentioned hospitalization and notes that the patient has a Medicare Advantage plan (Table) and wonders if the Two-Midnight Rule applies. Further, she questions whether Medicare’s “Three-Midnight Rule” for SNF benefits will factor in the patient’s discharge planning.
BACKGROUND AND HISTORY
Since the 1970s, the Centers for Medicare & Medicaid Services (CMS) has allowed enrollees to receive their Medicare benefits from privately managed health plans through the so-called Medicare Advantage programs. CMS contracts with commercial insurers who, in exchange for a set payment per Medicare enrollee, “accept full responsibility (ie, risk) for the costs of their enrollees’ care.”1 Over the past 20 years the percent of Medicare Advantage enrollees has nearly doubled nationwide, from 18% to 34%, and is projected to grow even further to 42% by 2028.2,3 The reasons beneficiaries choose to enroll in Medicare Advantage over Traditional Medicare have yet to be thoroughly studied; ease of enrollment and plan administration, as well as lower deductibles, copays, and out-of-pocket maximums for in-network services, are thought to be some of the driving factors.
The federal government has asserted two goals for the development of Medicare Advantage: beneficiary choice and economic efficiency.1 Medicare Advantage plans must be actuarially equal to Traditional Medicare but do not have to cover services in precisely the same way. Medicare Advantage plans may achieve cost savings through narrower networks, strict control of access to SNF services and acute care inpatient rehabilitation, and prior authorization requirements, the latter of which has received recent congressional attention.4,5 On the other hand, many Medicare Advantage plans offer dental, fitness, optical, and caregiver benefits that are not included under Traditional Medicare. Beneficiaries can theoretically compare the coverage and costs of Traditional Medicare to Medicare Advantage programs and make informed choices based on their individualized needs. The second stated goal for the Medicare Advantage option assumes that privately managed plans provide care at lower costs compared with CMS; this assumption has yet to be confirmed with solid data. Indeed, a recent analysis comparing the overall costs of Medicare Advantage to those of Traditional Medicare concluded that Medicare Advantage costs CMS more than Traditional Medicare,6 perhaps in part due to risk adjustment practices.7
POLICY IN PRACTICE
There are a number of areas of uncertainty regarding the specifics of how Medicare Advantage plans work, including Medicare Advantage programs’ use of outpatient (observation) stays. CMS has tried to provide guidance to healthcare organizations and clinicians regarding the appropriate use of inpatient hospitalizations for patients with Traditional Medicare, including the implementation of the Two-Midnight Rule in 2013. According to the rule, clinicians should place inpatient admission orders when they reasonably expect a patient’s care to extend across two midnights.8 Such admission decisions are subject to review by Medicare contractors and Quality Improvement Organizations.
In contrast, Medicare Advantage plans which enter into contracts with specific healthcare systems are not required to abide by CMS’ guidelines for the Two-Midnight Rule.9 When Medicare Advantage firms negotiate contracts with individual hospitals and healthcare organizations, CMS has been clear that such contracts are not required to include the Two-Midnight Rule when it comes to making hospitalization status decisions.10 Instead, in these instances, Medicare Advantage plans often use proprietary decision tools containing clinical criteria, such as Milliman Care Guidelines or InterQual, and/or their own plan’s internal criteria as part of the decision-making process to grant inpatient or outpatient (observation) status. More importantly, CMS has stated that for hospitals and healthcare systems that do not contract with Medicare Advantage programs, the Two-Midnight Rule should apply when it comes to making hospitalization status decisions.10
Implications for Patients
Currently, there are no data available to compare between Medicare Advantage enrollees and traditional medicine beneficiaries in terms of the frequency of observation use and out-of-pocket cost for observation stays. As alluded to in the patient’s case, the use of outpatient (observation) status has implications for a patient’s posthospitalization SNF benefit. Under Traditional Medicare, patients must be hospitalized for three consecutive inpatient midnights in order to qualify for the SNF benefit. Time spent under outpatient (observation) status does not count toward this three-day requirement. Interestingly, some Medicare Advantage programs have demonstrated innovation in this area, waiving the three inpatient midnight requirement for their beneficiaries;11 there is evidence, however, that compared with their Traditional Medicare counterparts, Medicare Advantage beneficiaries are admitted to lower quality SNFs.12 The posthospitalization consequences of an inpatient versus outpatient (observation) status determination for a Medicare Advantage beneficiary is thus unclear, further complicating the decision-making process for patients when it comes to choosing a Medicare policy, and for providers when it comes to choosing an admission status.
Implications for Clinicians and Healthcare Systems
After performing an initial history and physical exam, if a healthcare provider determines that a patient requires hospitalization, an order is placed to classify the stay as inpatient or outpatient (observation). For beneficiaries with Traditional Medicare or a Medicare Advantage plan that has not contracted with the hospital, clinicians should follow the Two-Midnight Rule for making this determination. For contracted Medicare Advantage, the rules are variable. Under Medicare’s Conditions of Participation, hospitals and healthcare organizations are required to have utilization management (UM) programs to assist physicians in making appropriate admission decisions. UM reviews can happen at any point during or after a patient’s stay, however, and physicians may have to make decisions using their best judgment at the time of admission without real-time input from UM teams.
Outpatient (observation) care and the challenges surrounding appropriate status orders have complicated the admission decision. In one study of 2014 Traditional Medicare claims, almost half of outpatient (observation) stays contained a status change.13 Based on a recent survey of hospitalist physicians, about two-thirds of hospitalists report at least monthly requests from patients to change their status.14 Hospital medicine physicians report that these requests “can severely damage the therapeutic bond”14 between provider and patient because the provider must assign status based on CMS rules, not patient request.
COMMENTARY AND RECOMMENDATIONS
CMS could improve the current system in one of two ways. First, CMS could require that all Medicare Advantage plans follow the same polices as Traditional Medicare policies regarding the Two- and Three-Midnight Rules. This would eliminate the need for both hospitals and healthcare organizations to dedicate time and resources to negotiating with each Medicare Advantage program and to managing each Medicare Advantage patient admission based on a specific contract. Ideally, CMS could completely eliminate its outpatient (observation) policy so that all hospitalizations are treated exactly the same, classified under the same billing status and with beneficiaries having the same postacute benefit. This would be consistent with the sentiment behind the recent Office of Inspector General’s (OIG) report suggesting that CMS consider counting outpatient midnights toward the three-midnight requirement for postacute SNF care “so that beneficiaries receiving similar hospital care have similar access to these services.”15
WHAT SHOULD I TELL MY PATIENT?
The physician in the example above should tell their patient that they will be admitted as an inpatient given her expectation that the patient will need hospitalization for oxygen support, parenteral antibiotics, and evaluation by physical therapy to determine a medically appropriate discharge plan. The physician should document the medical necessity for the admission, specifically her expectation that the patient will require at least two midnights of medically necessary hospital care. If the patient has Traditional Medicare, this documentation, along with the inpatient status order, will fulfill the requirements for an inpatient stay. If the patient has a Medicare Advantage plan, the physician can advise the patient that the plan administrators will ultimately determine if an inpatient stay will be covered or denied.
CONCLUSIONS
In the proposed clinical scenario, the rules determining the patient’s hospitalization status depend on whether the hospital contracts with the patient’s Medicare Advantage plan, and if so, what the contracted criteria are in determining inpatient and outpatient (observation) status. The physician could consider real-time input from the hospital’s UM team, if available. Regardless of UM input, if the physician hospitalizes the patient as an inpatient, the Medicare Advantage plan administrators will make a determination regarding the appropriateness of the admission status, as well as whether the patient qualifies for posthospitalization Medicare SNF benefits (if requested) and, additionally, which SNFs will be covered. If denied, the hospitalist will have the option of a peer-to-peer discussion with the insurance company to overturn the denial. Given the confusion, complexity, and implications presented by this admission status decision-making process, standardization across Traditional Medicare and Medicare Advantage plans, or a budget-neutral plan to eliminate status distinction altogether, is certainly warranted.
CLINICAL SCENARIO
A 73-year-old man presents to the emergency department with sepsis secondary to community-acquired pneumonia. The patient requires supplemental oxygen and is started on intravenous antibiotics. His admitting physician expects he will need more than two nights of hospital care and suggests that inpatient status, rather than outpatient (observation) status, would be appropriate under Medicare’s “Two-Midnight Rule.” The physician also suspects the patient may need a brief stay in a skilled nursing facility (SNF) following the mentioned hospitalization and notes that the patient has a Medicare Advantage plan (Table) and wonders if the Two-Midnight Rule applies. Further, she questions whether Medicare’s “Three-Midnight Rule” for SNF benefits will factor in the patient’s discharge planning.
BACKGROUND AND HISTORY
Since the 1970s, the Centers for Medicare & Medicaid Services (CMS) has allowed enrollees to receive their Medicare benefits from privately managed health plans through the so-called Medicare Advantage programs. CMS contracts with commercial insurers who, in exchange for a set payment per Medicare enrollee, “accept full responsibility (ie, risk) for the costs of their enrollees’ care.”1 Over the past 20 years the percent of Medicare Advantage enrollees has nearly doubled nationwide, from 18% to 34%, and is projected to grow even further to 42% by 2028.2,3 The reasons beneficiaries choose to enroll in Medicare Advantage over Traditional Medicare have yet to be thoroughly studied; ease of enrollment and plan administration, as well as lower deductibles, copays, and out-of-pocket maximums for in-network services, are thought to be some of the driving factors.
The federal government has asserted two goals for the development of Medicare Advantage: beneficiary choice and economic efficiency.1 Medicare Advantage plans must be actuarially equal to Traditional Medicare but do not have to cover services in precisely the same way. Medicare Advantage plans may achieve cost savings through narrower networks, strict control of access to SNF services and acute care inpatient rehabilitation, and prior authorization requirements, the latter of which has received recent congressional attention.4,5 On the other hand, many Medicare Advantage plans offer dental, fitness, optical, and caregiver benefits that are not included under Traditional Medicare. Beneficiaries can theoretically compare the coverage and costs of Traditional Medicare to Medicare Advantage programs and make informed choices based on their individualized needs. The second stated goal for the Medicare Advantage option assumes that privately managed plans provide care at lower costs compared with CMS; this assumption has yet to be confirmed with solid data. Indeed, a recent analysis comparing the overall costs of Medicare Advantage to those of Traditional Medicare concluded that Medicare Advantage costs CMS more than Traditional Medicare,6 perhaps in part due to risk adjustment practices.7
POLICY IN PRACTICE
There are a number of areas of uncertainty regarding the specifics of how Medicare Advantage plans work, including Medicare Advantage programs’ use of outpatient (observation) stays. CMS has tried to provide guidance to healthcare organizations and clinicians regarding the appropriate use of inpatient hospitalizations for patients with Traditional Medicare, including the implementation of the Two-Midnight Rule in 2013. According to the rule, clinicians should place inpatient admission orders when they reasonably expect a patient’s care to extend across two midnights.8 Such admission decisions are subject to review by Medicare contractors and Quality Improvement Organizations.
In contrast, Medicare Advantage plans which enter into contracts with specific healthcare systems are not required to abide by CMS’ guidelines for the Two-Midnight Rule.9 When Medicare Advantage firms negotiate contracts with individual hospitals and healthcare organizations, CMS has been clear that such contracts are not required to include the Two-Midnight Rule when it comes to making hospitalization status decisions.10 Instead, in these instances, Medicare Advantage plans often use proprietary decision tools containing clinical criteria, such as Milliman Care Guidelines or InterQual, and/or their own plan’s internal criteria as part of the decision-making process to grant inpatient or outpatient (observation) status. More importantly, CMS has stated that for hospitals and healthcare systems that do not contract with Medicare Advantage programs, the Two-Midnight Rule should apply when it comes to making hospitalization status decisions.10
Implications for Patients
Currently, there are no data available to compare between Medicare Advantage enrollees and traditional medicine beneficiaries in terms of the frequency of observation use and out-of-pocket cost for observation stays. As alluded to in the patient’s case, the use of outpatient (observation) status has implications for a patient’s posthospitalization SNF benefit. Under Traditional Medicare, patients must be hospitalized for three consecutive inpatient midnights in order to qualify for the SNF benefit. Time spent under outpatient (observation) status does not count toward this three-day requirement. Interestingly, some Medicare Advantage programs have demonstrated innovation in this area, waiving the three inpatient midnight requirement for their beneficiaries;11 there is evidence, however, that compared with their Traditional Medicare counterparts, Medicare Advantage beneficiaries are admitted to lower quality SNFs.12 The posthospitalization consequences of an inpatient versus outpatient (observation) status determination for a Medicare Advantage beneficiary is thus unclear, further complicating the decision-making process for patients when it comes to choosing a Medicare policy, and for providers when it comes to choosing an admission status.
Implications for Clinicians and Healthcare Systems
After performing an initial history and physical exam, if a healthcare provider determines that a patient requires hospitalization, an order is placed to classify the stay as inpatient or outpatient (observation). For beneficiaries with Traditional Medicare or a Medicare Advantage plan that has not contracted with the hospital, clinicians should follow the Two-Midnight Rule for making this determination. For contracted Medicare Advantage, the rules are variable. Under Medicare’s Conditions of Participation, hospitals and healthcare organizations are required to have utilization management (UM) programs to assist physicians in making appropriate admission decisions. UM reviews can happen at any point during or after a patient’s stay, however, and physicians may have to make decisions using their best judgment at the time of admission without real-time input from UM teams.
Outpatient (observation) care and the challenges surrounding appropriate status orders have complicated the admission decision. In one study of 2014 Traditional Medicare claims, almost half of outpatient (observation) stays contained a status change.13 Based on a recent survey of hospitalist physicians, about two-thirds of hospitalists report at least monthly requests from patients to change their status.14 Hospital medicine physicians report that these requests “can severely damage the therapeutic bond”14 between provider and patient because the provider must assign status based on CMS rules, not patient request.
COMMENTARY AND RECOMMENDATIONS
CMS could improve the current system in one of two ways. First, CMS could require that all Medicare Advantage plans follow the same polices as Traditional Medicare policies regarding the Two- and Three-Midnight Rules. This would eliminate the need for both hospitals and healthcare organizations to dedicate time and resources to negotiating with each Medicare Advantage program and to managing each Medicare Advantage patient admission based on a specific contract. Ideally, CMS could completely eliminate its outpatient (observation) policy so that all hospitalizations are treated exactly the same, classified under the same billing status and with beneficiaries having the same postacute benefit. This would be consistent with the sentiment behind the recent Office of Inspector General’s (OIG) report suggesting that CMS consider counting outpatient midnights toward the three-midnight requirement for postacute SNF care “so that beneficiaries receiving similar hospital care have similar access to these services.”15
WHAT SHOULD I TELL MY PATIENT?
The physician in the example above should tell their patient that they will be admitted as an inpatient given her expectation that the patient will need hospitalization for oxygen support, parenteral antibiotics, and evaluation by physical therapy to determine a medically appropriate discharge plan. The physician should document the medical necessity for the admission, specifically her expectation that the patient will require at least two midnights of medically necessary hospital care. If the patient has Traditional Medicare, this documentation, along with the inpatient status order, will fulfill the requirements for an inpatient stay. If the patient has a Medicare Advantage plan, the physician can advise the patient that the plan administrators will ultimately determine if an inpatient stay will be covered or denied.
CONCLUSIONS
In the proposed clinical scenario, the rules determining the patient’s hospitalization status depend on whether the hospital contracts with the patient’s Medicare Advantage plan, and if so, what the contracted criteria are in determining inpatient and outpatient (observation) status. The physician could consider real-time input from the hospital’s UM team, if available. Regardless of UM input, if the physician hospitalizes the patient as an inpatient, the Medicare Advantage plan administrators will make a determination regarding the appropriateness of the admission status, as well as whether the patient qualifies for posthospitalization Medicare SNF benefits (if requested) and, additionally, which SNFs will be covered. If denied, the hospitalist will have the option of a peer-to-peer discussion with the insurance company to overturn the denial. Given the confusion, complexity, and implications presented by this admission status decision-making process, standardization across Traditional Medicare and Medicare Advantage plans, or a budget-neutral plan to eliminate status distinction altogether, is certainly warranted.
1.McGuire TG, Newhouse JP, Sinaiko AD. An economic history of Medicare Part C. Millbank Q. 2011;89(2):289-332. https://doi.org/10.1111/j.1468-0009.2011.00629.x.
2. Medicare Advantage. Available at: https://www.kff.org/medicare/fact-sheet/medicare-advantage/.
3. Neuman P, Jacobson G. Medicare Advantage checkup. N Engl J Med. 2018;379(22):2163-2172. https://doi.org/10.1056/NEJMhpr1804089.
4. HR 3107: improving seniors’ timely access to Care Act of 2019. Available at: https://www.congress.gov/bill/116th-congress/house-bill/3107/text?q=%7B%22search%22%3A%5B%22prior+authorization%22%5D%7D&r=1&s=1.
5. Gadbois EA, Tyler DA, Shield RR, et al. Medicare Advantage control of postacute costs: perspective from stakeholders. Am J Manag Care. 2018;24(12):e386-e392.
6. Rooke-Ley H, Broome T, Mostashari F, Cavanaugh S. Evaluating Medicare programs against saving taxpayer dollars. Health Affairs, August 16, 2019. Available at: https://www.healthaffairs.org/do/10.1377/hblog20190813.223707/full/.
7. Office of Inspector General. Billions in estimated Medicare Advantage payments from chart reviews raise concerns. December 2019. Available at: https://oig.hhs.gov/oei/reports/oei-03-17-00470.pdf. Accessed December 15, 2019.
8. Fact sheet: two-midnight rule. Available at: https://www.cms.gov/newsroom/fact-sheets/fact-sheet-two-midnight-rule-0.
9. Locke C, Hu E. Medicare’s two-midnight rule: what hospitalists must know. Available at: https://www.the-hospitalist.org/hospitalist/article/194971/medicares-two-midnight-rule.
10. Announcement of calendar year (CY) 2019 Medicare Advantage capitation rates and Medicare Advantage and part D payment policies and final call letter. Page 206. Available at: https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Downloads/Announcement2019.pdf. Accessed November 18, 2019.
11. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trivedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Aff. 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054.
12. Meyers D, Mor V, Rahman M. Medicare Advantage enrollees more likely to enter lower-quality nursing homes compared to fee-for-service enrollees. Health Aff. 2018;37(1):78-85. https://doi.org/10.1377/hlthaff.2017.0714.
13. Sheehy A, Shi F, Kind AJH. Identifying observation stays in Medicare data: Policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
14. The hospital observation care problem: perspectives and solutions from the Society of Hospital Medicine. Available at: https://www.hospitalmedicine.org/globalassets/policy-and-advocacy/advocacy-pdf/shms-observation-white-paper-2017. Accessed November 18, 2019.
15. U.S. Department of Health & Human Services, Office of Inspector General. Solutions to reduce fraud, waste and abuse in HHS programs: OIG’s top recommendations. Available at: https://oig.hhs.gov/reports-and-publications/compendium/. Accessed November 22, 2019.
1.McGuire TG, Newhouse JP, Sinaiko AD. An economic history of Medicare Part C. Millbank Q. 2011;89(2):289-332. https://doi.org/10.1111/j.1468-0009.2011.00629.x.
2. Medicare Advantage. Available at: https://www.kff.org/medicare/fact-sheet/medicare-advantage/.
3. Neuman P, Jacobson G. Medicare Advantage checkup. N Engl J Med. 2018;379(22):2163-2172. https://doi.org/10.1056/NEJMhpr1804089.
4. HR 3107: improving seniors’ timely access to Care Act of 2019. Available at: https://www.congress.gov/bill/116th-congress/house-bill/3107/text?q=%7B%22search%22%3A%5B%22prior+authorization%22%5D%7D&r=1&s=1.
5. Gadbois EA, Tyler DA, Shield RR, et al. Medicare Advantage control of postacute costs: perspective from stakeholders. Am J Manag Care. 2018;24(12):e386-e392.
6. Rooke-Ley H, Broome T, Mostashari F, Cavanaugh S. Evaluating Medicare programs against saving taxpayer dollars. Health Affairs, August 16, 2019. Available at: https://www.healthaffairs.org/do/10.1377/hblog20190813.223707/full/.
7. Office of Inspector General. Billions in estimated Medicare Advantage payments from chart reviews raise concerns. December 2019. Available at: https://oig.hhs.gov/oei/reports/oei-03-17-00470.pdf. Accessed December 15, 2019.
8. Fact sheet: two-midnight rule. Available at: https://www.cms.gov/newsroom/fact-sheets/fact-sheet-two-midnight-rule-0.
9. Locke C, Hu E. Medicare’s two-midnight rule: what hospitalists must know. Available at: https://www.the-hospitalist.org/hospitalist/article/194971/medicares-two-midnight-rule.
10. Announcement of calendar year (CY) 2019 Medicare Advantage capitation rates and Medicare Advantage and part D payment policies and final call letter. Page 206. Available at: https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Downloads/Announcement2019.pdf. Accessed November 18, 2019.
11. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trivedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Aff. 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054.
12. Meyers D, Mor V, Rahman M. Medicare Advantage enrollees more likely to enter lower-quality nursing homes compared to fee-for-service enrollees. Health Aff. 2018;37(1):78-85. https://doi.org/10.1377/hlthaff.2017.0714.
13. Sheehy A, Shi F, Kind AJH. Identifying observation stays in Medicare data: Policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
14. The hospital observation care problem: perspectives and solutions from the Society of Hospital Medicine. Available at: https://www.hospitalmedicine.org/globalassets/policy-and-advocacy/advocacy-pdf/shms-observation-white-paper-2017. Accessed November 18, 2019.
15. U.S. Department of Health & Human Services, Office of Inspector General. Solutions to reduce fraud, waste and abuse in HHS programs: OIG’s top recommendations. Available at: https://oig.hhs.gov/reports-and-publications/compendium/. Accessed November 22, 2019.
© 2020 Society of Hospital Medicine
Next Steps for Next Steps: The Intersection of Health Policy with Clinical Decision-Making
The Journal of Hospital Medicine introduced the Choosing Wisely®: Next Steps in Improving Healthcare Value series in 20151 as a companion to the popular Choosing Wisely®: Things We Do For No Reason™ series2 that was introduced in October in the same year. Both series were created in partnership with the American Board of Internal Medicine Foundation and were designed in the spirit of the Choosing Wisely® campaign’s mission to “promote conversations between clinicians and patients” in choosing care supported by evidence that minimizes harm, including avoidance of unnecessary treatments and tests.3 The Choosing Wisely®: Next Steps in Improving Healthcare Value series extends these principles as a forum for manuscripts that focus on translating value-based concepts into daily operations, including systems-level care delivery redesign initiatives, payment model innovations, and analyses of relevant policies or practice trends.
INITIAL EXPERIENCE
Since its inception, 16 Choosing Wisely®: Next Steps in Improving Healthcare Value manuscripts have been published, encompassing a wide range of topics such as postacute care transitions,4 the role of hospital medicine practice within accountable care organizations (ACOs),5 and quality and value at end-of-life.6
NEXT STEPS WITH NEXT STEPS
Few physicians receive health policy training.7,8 Hospital medicine practitioners are a core component of the workforce, driving change and value-based improvements at almost every inpatient facility across the country. Regardless of their background or experience, hospital medicine practitioners must interface with legislation, regulation, and other policies every day while providing patient care. Intentional, value-based improvements are more likely to succeed if those providing direct patient care understand health policies, particularly the effects of those policies on transactional, point-of-care decisions.
We are pleased to expand the Choosing Wisely®: Next Steps in Improving Healthcare Value series to include articles exploring health policy implications at the bedside. These articles will use common clinical scenarios to illuminate health policies most germane to hospital medicine practitioners and present applications of the policies as they relate to value at the level of patient–provider interactions. Each article will present a clinical scenario, explain key policy terms, address implications of specific policies in clinical practice, and propose how those policies can be improved (Appendix). Going forward, Choosing Wisely®: Next Steps in Improving Healthcare Value manuscript titles will include either “Policy in Clinical Practice” or “Improving Healthcare Value” to better establish a connection to the series and distinguish between the two article types.
The first Choosing Wisely®: Next Steps in Improving Healthcare Value—Implications of Health Policy on Clinical Decision-Making manuscript appears in this issue of the Journal of Hospital Medicine.9 As is the current practice for Choosing Wisely®: Next Steps in Improving Healthcare Value, authors are requested to send series editors a 500-word precis for review to ensure topic suitability before submission of a full manuscript. The precis, as well as any questions pertaining to the new series, can be directed to nextsteps@hospitalmedicine.org.
The authors thank the American Board of Internal Medicine Foundation for supporting this series.
1. Horwitz L, Masica A, Auerbach A. Introducing choosing wisely: Next steps in improving healthcare value. J Hosp Med. 2015;10(3): 187-189.
2. Feldman L. Choosing wisely: Things we do for no reasons. J Hosp Med. 2015;10(10):696. https://doi.org/10.1002/jhm.2425.
3. Choosing wisely: An initiative of the ABIM Foundation. Available at: http://www.choosingwisely.org/. Accessed July 8, 2019.
4. Conway S, Parekh A, Hughes A, et al. Next steps in improving healthcare value: Postacute care transitions: Developing a skilled nursing facility collaborative within an academic health system. J Hosp Med. 2019;14(3):174-177. https://doi.org/10.12788/jhm.3117.
5. Li J, Williams M. Hospitalist value in an ACO world. J Hosp Med. 2018;13(4):272-276. https://doi.org/10.12788/jhm.2965.
6. Fail R, Meier D. Improving quality of care for seriously ill patients: Opportunities for hospitalists. J Hosp Med. 2018;13(3):194-197. https://doi.org/10.12788/jhm.2896.
7. Fry C, Buntin M, Jain S. Medical schools and health policy: Adapting to the changing health care system. NEJM Catalyst, 2017. Available at: https://catalyst.nejm.org/medical-schools-health-policy-research/. Accessed July 10, 2019.
8. For doctors-in-training, a dose of health policy helps the medicine go down. National Public Radio (NPR), 2016. Available at: https://www.npr.org/sections/health-shots/2016/06/09/481207153/for-doctors-in-training-a-dose-of-health-policy-helps-the-medicine-go-down. Accessed July 10, 2019.
9. Kaiksow FA, Powell WR, Ankuda CK, et al. Policy in clinical practice: Medicare advantage and observation hospitalizations. J Hosp Med. 2020;15(1):6-8. https://doi.org/10.12788/jhm.3364.
The Journal of Hospital Medicine introduced the Choosing Wisely®: Next Steps in Improving Healthcare Value series in 20151 as a companion to the popular Choosing Wisely®: Things We Do For No Reason™ series2 that was introduced in October in the same year. Both series were created in partnership with the American Board of Internal Medicine Foundation and were designed in the spirit of the Choosing Wisely® campaign’s mission to “promote conversations between clinicians and patients” in choosing care supported by evidence that minimizes harm, including avoidance of unnecessary treatments and tests.3 The Choosing Wisely®: Next Steps in Improving Healthcare Value series extends these principles as a forum for manuscripts that focus on translating value-based concepts into daily operations, including systems-level care delivery redesign initiatives, payment model innovations, and analyses of relevant policies or practice trends.
INITIAL EXPERIENCE
Since its inception, 16 Choosing Wisely®: Next Steps in Improving Healthcare Value manuscripts have been published, encompassing a wide range of topics such as postacute care transitions,4 the role of hospital medicine practice within accountable care organizations (ACOs),5 and quality and value at end-of-life.6
NEXT STEPS WITH NEXT STEPS
Few physicians receive health policy training.7,8 Hospital medicine practitioners are a core component of the workforce, driving change and value-based improvements at almost every inpatient facility across the country. Regardless of their background or experience, hospital medicine practitioners must interface with legislation, regulation, and other policies every day while providing patient care. Intentional, value-based improvements are more likely to succeed if those providing direct patient care understand health policies, particularly the effects of those policies on transactional, point-of-care decisions.
We are pleased to expand the Choosing Wisely®: Next Steps in Improving Healthcare Value series to include articles exploring health policy implications at the bedside. These articles will use common clinical scenarios to illuminate health policies most germane to hospital medicine practitioners and present applications of the policies as they relate to value at the level of patient–provider interactions. Each article will present a clinical scenario, explain key policy terms, address implications of specific policies in clinical practice, and propose how those policies can be improved (Appendix). Going forward, Choosing Wisely®: Next Steps in Improving Healthcare Value manuscript titles will include either “Policy in Clinical Practice” or “Improving Healthcare Value” to better establish a connection to the series and distinguish between the two article types.
The first Choosing Wisely®: Next Steps in Improving Healthcare Value—Implications of Health Policy on Clinical Decision-Making manuscript appears in this issue of the Journal of Hospital Medicine.9 As is the current practice for Choosing Wisely®: Next Steps in Improving Healthcare Value, authors are requested to send series editors a 500-word precis for review to ensure topic suitability before submission of a full manuscript. The precis, as well as any questions pertaining to the new series, can be directed to nextsteps@hospitalmedicine.org.
The authors thank the American Board of Internal Medicine Foundation for supporting this series.
The Journal of Hospital Medicine introduced the Choosing Wisely®: Next Steps in Improving Healthcare Value series in 20151 as a companion to the popular Choosing Wisely®: Things We Do For No Reason™ series2 that was introduced in October in the same year. Both series were created in partnership with the American Board of Internal Medicine Foundation and were designed in the spirit of the Choosing Wisely® campaign’s mission to “promote conversations between clinicians and patients” in choosing care supported by evidence that minimizes harm, including avoidance of unnecessary treatments and tests.3 The Choosing Wisely®: Next Steps in Improving Healthcare Value series extends these principles as a forum for manuscripts that focus on translating value-based concepts into daily operations, including systems-level care delivery redesign initiatives, payment model innovations, and analyses of relevant policies or practice trends.
INITIAL EXPERIENCE
Since its inception, 16 Choosing Wisely®: Next Steps in Improving Healthcare Value manuscripts have been published, encompassing a wide range of topics such as postacute care transitions,4 the role of hospital medicine practice within accountable care organizations (ACOs),5 and quality and value at end-of-life.6
NEXT STEPS WITH NEXT STEPS
Few physicians receive health policy training.7,8 Hospital medicine practitioners are a core component of the workforce, driving change and value-based improvements at almost every inpatient facility across the country. Regardless of their background or experience, hospital medicine practitioners must interface with legislation, regulation, and other policies every day while providing patient care. Intentional, value-based improvements are more likely to succeed if those providing direct patient care understand health policies, particularly the effects of those policies on transactional, point-of-care decisions.
We are pleased to expand the Choosing Wisely®: Next Steps in Improving Healthcare Value series to include articles exploring health policy implications at the bedside. These articles will use common clinical scenarios to illuminate health policies most germane to hospital medicine practitioners and present applications of the policies as they relate to value at the level of patient–provider interactions. Each article will present a clinical scenario, explain key policy terms, address implications of specific policies in clinical practice, and propose how those policies can be improved (Appendix). Going forward, Choosing Wisely®: Next Steps in Improving Healthcare Value manuscript titles will include either “Policy in Clinical Practice” or “Improving Healthcare Value” to better establish a connection to the series and distinguish between the two article types.
The first Choosing Wisely®: Next Steps in Improving Healthcare Value—Implications of Health Policy on Clinical Decision-Making manuscript appears in this issue of the Journal of Hospital Medicine.9 As is the current practice for Choosing Wisely®: Next Steps in Improving Healthcare Value, authors are requested to send series editors a 500-word precis for review to ensure topic suitability before submission of a full manuscript. The precis, as well as any questions pertaining to the new series, can be directed to nextsteps@hospitalmedicine.org.
The authors thank the American Board of Internal Medicine Foundation for supporting this series.
1. Horwitz L, Masica A, Auerbach A. Introducing choosing wisely: Next steps in improving healthcare value. J Hosp Med. 2015;10(3): 187-189.
2. Feldman L. Choosing wisely: Things we do for no reasons. J Hosp Med. 2015;10(10):696. https://doi.org/10.1002/jhm.2425.
3. Choosing wisely: An initiative of the ABIM Foundation. Available at: http://www.choosingwisely.org/. Accessed July 8, 2019.
4. Conway S, Parekh A, Hughes A, et al. Next steps in improving healthcare value: Postacute care transitions: Developing a skilled nursing facility collaborative within an academic health system. J Hosp Med. 2019;14(3):174-177. https://doi.org/10.12788/jhm.3117.
5. Li J, Williams M. Hospitalist value in an ACO world. J Hosp Med. 2018;13(4):272-276. https://doi.org/10.12788/jhm.2965.
6. Fail R, Meier D. Improving quality of care for seriously ill patients: Opportunities for hospitalists. J Hosp Med. 2018;13(3):194-197. https://doi.org/10.12788/jhm.2896.
7. Fry C, Buntin M, Jain S. Medical schools and health policy: Adapting to the changing health care system. NEJM Catalyst, 2017. Available at: https://catalyst.nejm.org/medical-schools-health-policy-research/. Accessed July 10, 2019.
8. For doctors-in-training, a dose of health policy helps the medicine go down. National Public Radio (NPR), 2016. Available at: https://www.npr.org/sections/health-shots/2016/06/09/481207153/for-doctors-in-training-a-dose-of-health-policy-helps-the-medicine-go-down. Accessed July 10, 2019.
9. Kaiksow FA, Powell WR, Ankuda CK, et al. Policy in clinical practice: Medicare advantage and observation hospitalizations. J Hosp Med. 2020;15(1):6-8. https://doi.org/10.12788/jhm.3364.
1. Horwitz L, Masica A, Auerbach A. Introducing choosing wisely: Next steps in improving healthcare value. J Hosp Med. 2015;10(3): 187-189.
2. Feldman L. Choosing wisely: Things we do for no reasons. J Hosp Med. 2015;10(10):696. https://doi.org/10.1002/jhm.2425.
3. Choosing wisely: An initiative of the ABIM Foundation. Available at: http://www.choosingwisely.org/. Accessed July 8, 2019.
4. Conway S, Parekh A, Hughes A, et al. Next steps in improving healthcare value: Postacute care transitions: Developing a skilled nursing facility collaborative within an academic health system. J Hosp Med. 2019;14(3):174-177. https://doi.org/10.12788/jhm.3117.
5. Li J, Williams M. Hospitalist value in an ACO world. J Hosp Med. 2018;13(4):272-276. https://doi.org/10.12788/jhm.2965.
6. Fail R, Meier D. Improving quality of care for seriously ill patients: Opportunities for hospitalists. J Hosp Med. 2018;13(3):194-197. https://doi.org/10.12788/jhm.2896.
7. Fry C, Buntin M, Jain S. Medical schools and health policy: Adapting to the changing health care system. NEJM Catalyst, 2017. Available at: https://catalyst.nejm.org/medical-schools-health-policy-research/. Accessed July 10, 2019.
8. For doctors-in-training, a dose of health policy helps the medicine go down. National Public Radio (NPR), 2016. Available at: https://www.npr.org/sections/health-shots/2016/06/09/481207153/for-doctors-in-training-a-dose-of-health-policy-helps-the-medicine-go-down. Accessed July 10, 2019.
9. Kaiksow FA, Powell WR, Ankuda CK, et al. Policy in clinical practice: Medicare advantage and observation hospitalizations. J Hosp Med. 2020;15(1):6-8. https://doi.org/10.12788/jhm.3364.
© 2020 Society of Hospital Medicine
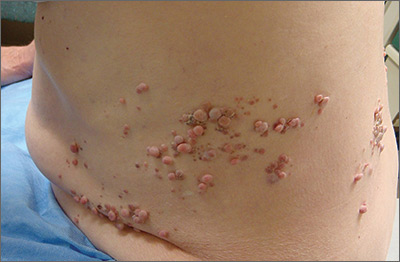
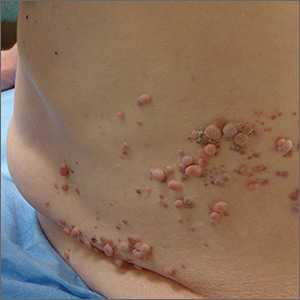
Papules on trunk
Dermatopathologic evaluation of the tissue sample indicated that the lesion was a neurofibroma, and clinical correlation fine-tuned the diagnosis to segmental neurofibromatosis (NF). The diagnosis of segmental NF is clinical, with biopsy to confirm that the lesions are neurofibromas. Segmental NF is a mosaic form of neurofibromatosis type 1 (NF1) that results from a postzygotic mutation of the NF1 gene. While NF1 is a relatively common neurocutaneous disorder that occurs with a frequency of 1 in 3000, segmental NF is uncommon, with an estimated prevalence of 1 in 40,000.
NF1 often follows an autosomal dominant inheritance pattern, although up to 50% of patients with NF1 arise de novo from spontaneous mutations. NF1 is characterized by multiple café-au-lait macules, axillary freckling, neurofibromas, and Lisch nodules (pigmented iris hamartomas). Systemic findings associated with NF1 include malignant peripheral nerve sheath tumors, optic gliomas, and vasculopathy. While patients with segmental NF may exhibit some of these same findings, the distribution of the neurofibromas is often limited to a single dermatome. Additionally, patients with segmental NF typically do not exhibit extracutaneous lesions, systemic involvement, or a family history of NF. Segmental NF treatment typically focuses on symptomatic management or cosmetic concerns.
This patient did not have any of the systemic complications that occasionally occur with segmental NF, so no medical treatment was required. The patient was advised that cutaneous and subcutaneous neurofibromas do not require removal unless there is pain, bleeding, disfigurement, or signs of malignant transformation. The patient was not interested in removal of the nodules for cosmetic reasons, so he was encouraged to seek follow-up care, as needed.
This case was adapted from: Laurent KJ, Beachkofsky TM, Loyd A, et al. Segmental distribution of nodules on trunk. J Fam Pract. 2017;66: 765-767.
Dermatopathologic evaluation of the tissue sample indicated that the lesion was a neurofibroma, and clinical correlation fine-tuned the diagnosis to segmental neurofibromatosis (NF). The diagnosis of segmental NF is clinical, with biopsy to confirm that the lesions are neurofibromas. Segmental NF is a mosaic form of neurofibromatosis type 1 (NF1) that results from a postzygotic mutation of the NF1 gene. While NF1 is a relatively common neurocutaneous disorder that occurs with a frequency of 1 in 3000, segmental NF is uncommon, with an estimated prevalence of 1 in 40,000.
NF1 often follows an autosomal dominant inheritance pattern, although up to 50% of patients with NF1 arise de novo from spontaneous mutations. NF1 is characterized by multiple café-au-lait macules, axillary freckling, neurofibromas, and Lisch nodules (pigmented iris hamartomas). Systemic findings associated with NF1 include malignant peripheral nerve sheath tumors, optic gliomas, and vasculopathy. While patients with segmental NF may exhibit some of these same findings, the distribution of the neurofibromas is often limited to a single dermatome. Additionally, patients with segmental NF typically do not exhibit extracutaneous lesions, systemic involvement, or a family history of NF. Segmental NF treatment typically focuses on symptomatic management or cosmetic concerns.
This patient did not have any of the systemic complications that occasionally occur with segmental NF, so no medical treatment was required. The patient was advised that cutaneous and subcutaneous neurofibromas do not require removal unless there is pain, bleeding, disfigurement, or signs of malignant transformation. The patient was not interested in removal of the nodules for cosmetic reasons, so he was encouraged to seek follow-up care, as needed.
This case was adapted from: Laurent KJ, Beachkofsky TM, Loyd A, et al. Segmental distribution of nodules on trunk. J Fam Pract. 2017;66: 765-767.
Dermatopathologic evaluation of the tissue sample indicated that the lesion was a neurofibroma, and clinical correlation fine-tuned the diagnosis to segmental neurofibromatosis (NF). The diagnosis of segmental NF is clinical, with biopsy to confirm that the lesions are neurofibromas. Segmental NF is a mosaic form of neurofibromatosis type 1 (NF1) that results from a postzygotic mutation of the NF1 gene. While NF1 is a relatively common neurocutaneous disorder that occurs with a frequency of 1 in 3000, segmental NF is uncommon, with an estimated prevalence of 1 in 40,000.
NF1 often follows an autosomal dominant inheritance pattern, although up to 50% of patients with NF1 arise de novo from spontaneous mutations. NF1 is characterized by multiple café-au-lait macules, axillary freckling, neurofibromas, and Lisch nodules (pigmented iris hamartomas). Systemic findings associated with NF1 include malignant peripheral nerve sheath tumors, optic gliomas, and vasculopathy. While patients with segmental NF may exhibit some of these same findings, the distribution of the neurofibromas is often limited to a single dermatome. Additionally, patients with segmental NF typically do not exhibit extracutaneous lesions, systemic involvement, or a family history of NF. Segmental NF treatment typically focuses on symptomatic management or cosmetic concerns.
This patient did not have any of the systemic complications that occasionally occur with segmental NF, so no medical treatment was required. The patient was advised that cutaneous and subcutaneous neurofibromas do not require removal unless there is pain, bleeding, disfigurement, or signs of malignant transformation. The patient was not interested in removal of the nodules for cosmetic reasons, so he was encouraged to seek follow-up care, as needed.
This case was adapted from: Laurent KJ, Beachkofsky TM, Loyd A, et al. Segmental distribution of nodules on trunk. J Fam Pract. 2017;66: 765-767.
SABCS research changes practice
In this edition of “How I Will Treat My Next Patient,” I highlight two presentations from the recent San Antonio Breast Cancer Symposium (SABCS) that will likely change practice for some breast cancer patients – even before the ball drops in Times Square on New Year’s Eve.
Residual cancer burden
Researchers reported a multi-institutional analysis of individual patient-level data on 5,160 patients who had received neoadjuvant chemotherapy (NAC) for localized breast cancer at 11 different centers. They found that residual cancer burden (RCB) was significantly associated with event-free (EFS) and distant recurrence-free survival. The value of calculating RCB was seen across all breast cancer tumor phenotypes (SABCS 2019, Abstract GS5-01).
RCB is calculated by analyzing the residual disease after NAC for the primary tumor bed, the number of positive axillary nodes, and the size of largest node metastasis. It is graded from RCB-0 (pCR) to RCB-III (extensive residual disease).
Both EFS and distant recurrence-free survival were strongly associated with RCB for the overall population and for each breast cancer subtype. For hormone receptor–positive/HER2-negative disease there was a slight hiccup in that RCB-0 was associated with a 10-year EFS of 81%, but EFS was 86% for RCB-I. Fortunately, the prognostic reliability of RCB was clear for RCB-II (69%) and RCB-III (52%). The presenter, W. Fraser Symmans, MD, commented that RCB is most prognostic when higher levels of residual disease are present. RCB remained prognostic in multivariate models adjusting for age, grade, and clinical T and N stage at diagnosis.
How these results influence practice
After neoadjuvant chemotherapy in patients with localized breast cancer, we struggle when patients ask: “So, doctor, how am I likely to do?” We piece together a complicated – and inconsistent – answer, based on breast cancer subtype, original stage of disease, whether pCR was attained, and other factors.
For patients with triple-negative breast cancer, we have the option of adding capecitabine and/or participation in ongoing clinical trials, for patients with residual disease after NAC. Among the HER2-positive patients, we have data from the KATHERINE, ExtaNET, and APHINITY trials that provide options for additional treatment in those patients, as well. For patients with potentially hormonally responsive, HER2-negative disease, we can emphasize the importance of postoperative adjuvant endocrine therapy, the mandate for continued adherence to oral therapy, and the lower likelihood of (and prognostic value of) pCR in that breast cancer subtype.
Where we previously had a complicated, nuanced discussion, we now have data from a multi-institutional, meticulous analysis to guide further treatment, candidacy for clinical trials, and our expectations of what would be meaningful results from additional therapy. This meets my definition of “practice-changing” research.
APHINITY follow-up
APHINITY was a randomized, multicenter, double-blind, placebo-controlled trial that previously demonstrated that pertuzumab added to standard chemotherapy plus 1 year of trastuzumab in operable HER2-positive breast cancer was associated with modest, but statistically significant, improvement in invasive disease–free survival (IDFS), compared with placebo and chemotherapy plus trastuzumab (hazard ratio, 0.81; P = .04). When the initial results – with a median follow-up of 45.4 months – were published, the authors promised an update at 6 years. Martine Piccart, MD, PhD, provided that update at the 2019 SABCS (Abstract GS1-04).
In the updated analysis, overall survival was 94.8% among patients on the pertuzumab arm and 93.9% among patients taking placebo (HR, 0.85). IDFS rates were 90.6% versus 87.8% in the intent-to-treat population. The investigator commented that this difference was caused mainly by a reduction in distant and loco-regional recurrences.
Central nervous system metastases, contralateral invasive breast cancers, and death without a prior event were no different between the two treatment groups.
Improvement in IDFS (the primary endpoint) from pertuzumab was most impressive in the node-positive cohort, 87.9% versus 83.4% for placebo (HR, 0.72). There was no benefit in the node-negative population (95.0% vs. 94.9%; HR, 1.02).
In contrast to the 3-year analysis, the benefits in IDFS were seen regardless of hormone receptor status. Additionally, no new safety concerns emerged. The rate of severe cardiac events was below 1% in both groups.
How these results influence practice
“Patience is a virtue” appeared for the first time in the English language around 1360, in William Langland’s poem “Piers Plowman.” They are true words, indeed.
When the initial results of APHINITY were published in 2017, they led to the approval of pertuzumab as an addition to chemotherapy plus trastuzumab for high-risk, early, HER2-positive breast cancer patients. Still, the difference in IDFS curves was visually unimpressive (absolute difference 1.7% in the intent-to-treat and 3.2% in the node-positive patients) and pertuzumab was associated with more grade 3 toxicities (primarily diarrhea) and treatment discontinuations.
An optimist would have observed that the IDFS curves in 2017 did not start to diverge until 24 months and would have noted that the divergence increased with time. That virtuously patient person would have expected that the second interim analysis would show larger absolute benefits, and that the hormone receptor–positive patients (64% of the total) would not yet have relapsed by the first interim analysis and that pertuzumab’s benefit in that group would emerge late.
That appears to be what is happening. It provides hope that an overall survival benefit will become more evident with time. If the target P value for an overall survival difference (.0012) is met by the time of the third interim analysis, our patience (and the FDA’s decision to grant approval for pertuzumab for this indication) will have been amply rewarded. For selected HER2-posiitve patients with high RCB, especially those who tolerated neoadjuvant trastuzumab plus pertuzumab regimens, postoperative adjuvant pertuzumab may be an appealing option.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two presentations from the recent San Antonio Breast Cancer Symposium (SABCS) that will likely change practice for some breast cancer patients – even before the ball drops in Times Square on New Year’s Eve.
Residual cancer burden
Researchers reported a multi-institutional analysis of individual patient-level data on 5,160 patients who had received neoadjuvant chemotherapy (NAC) for localized breast cancer at 11 different centers. They found that residual cancer burden (RCB) was significantly associated with event-free (EFS) and distant recurrence-free survival. The value of calculating RCB was seen across all breast cancer tumor phenotypes (SABCS 2019, Abstract GS5-01).
RCB is calculated by analyzing the residual disease after NAC for the primary tumor bed, the number of positive axillary nodes, and the size of largest node metastasis. It is graded from RCB-0 (pCR) to RCB-III (extensive residual disease).
Both EFS and distant recurrence-free survival were strongly associated with RCB for the overall population and for each breast cancer subtype. For hormone receptor–positive/HER2-negative disease there was a slight hiccup in that RCB-0 was associated with a 10-year EFS of 81%, but EFS was 86% for RCB-I. Fortunately, the prognostic reliability of RCB was clear for RCB-II (69%) and RCB-III (52%). The presenter, W. Fraser Symmans, MD, commented that RCB is most prognostic when higher levels of residual disease are present. RCB remained prognostic in multivariate models adjusting for age, grade, and clinical T and N stage at diagnosis.
How these results influence practice
After neoadjuvant chemotherapy in patients with localized breast cancer, we struggle when patients ask: “So, doctor, how am I likely to do?” We piece together a complicated – and inconsistent – answer, based on breast cancer subtype, original stage of disease, whether pCR was attained, and other factors.
For patients with triple-negative breast cancer, we have the option of adding capecitabine and/or participation in ongoing clinical trials, for patients with residual disease after NAC. Among the HER2-positive patients, we have data from the KATHERINE, ExtaNET, and APHINITY trials that provide options for additional treatment in those patients, as well. For patients with potentially hormonally responsive, HER2-negative disease, we can emphasize the importance of postoperative adjuvant endocrine therapy, the mandate for continued adherence to oral therapy, and the lower likelihood of (and prognostic value of) pCR in that breast cancer subtype.
Where we previously had a complicated, nuanced discussion, we now have data from a multi-institutional, meticulous analysis to guide further treatment, candidacy for clinical trials, and our expectations of what would be meaningful results from additional therapy. This meets my definition of “practice-changing” research.
APHINITY follow-up
APHINITY was a randomized, multicenter, double-blind, placebo-controlled trial that previously demonstrated that pertuzumab added to standard chemotherapy plus 1 year of trastuzumab in operable HER2-positive breast cancer was associated with modest, but statistically significant, improvement in invasive disease–free survival (IDFS), compared with placebo and chemotherapy plus trastuzumab (hazard ratio, 0.81; P = .04). When the initial results – with a median follow-up of 45.4 months – were published, the authors promised an update at 6 years. Martine Piccart, MD, PhD, provided that update at the 2019 SABCS (Abstract GS1-04).
In the updated analysis, overall survival was 94.8% among patients on the pertuzumab arm and 93.9% among patients taking placebo (HR, 0.85). IDFS rates were 90.6% versus 87.8% in the intent-to-treat population. The investigator commented that this difference was caused mainly by a reduction in distant and loco-regional recurrences.
Central nervous system metastases, contralateral invasive breast cancers, and death without a prior event were no different between the two treatment groups.
Improvement in IDFS (the primary endpoint) from pertuzumab was most impressive in the node-positive cohort, 87.9% versus 83.4% for placebo (HR, 0.72). There was no benefit in the node-negative population (95.0% vs. 94.9%; HR, 1.02).
In contrast to the 3-year analysis, the benefits in IDFS were seen regardless of hormone receptor status. Additionally, no new safety concerns emerged. The rate of severe cardiac events was below 1% in both groups.
How these results influence practice
“Patience is a virtue” appeared for the first time in the English language around 1360, in William Langland’s poem “Piers Plowman.” They are true words, indeed.
When the initial results of APHINITY were published in 2017, they led to the approval of pertuzumab as an addition to chemotherapy plus trastuzumab for high-risk, early, HER2-positive breast cancer patients. Still, the difference in IDFS curves was visually unimpressive (absolute difference 1.7% in the intent-to-treat and 3.2% in the node-positive patients) and pertuzumab was associated with more grade 3 toxicities (primarily diarrhea) and treatment discontinuations.
An optimist would have observed that the IDFS curves in 2017 did not start to diverge until 24 months and would have noted that the divergence increased with time. That virtuously patient person would have expected that the second interim analysis would show larger absolute benefits, and that the hormone receptor–positive patients (64% of the total) would not yet have relapsed by the first interim analysis and that pertuzumab’s benefit in that group would emerge late.
That appears to be what is happening. It provides hope that an overall survival benefit will become more evident with time. If the target P value for an overall survival difference (.0012) is met by the time of the third interim analysis, our patience (and the FDA’s decision to grant approval for pertuzumab for this indication) will have been amply rewarded. For selected HER2-posiitve patients with high RCB, especially those who tolerated neoadjuvant trastuzumab plus pertuzumab regimens, postoperative adjuvant pertuzumab may be an appealing option.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two presentations from the recent San Antonio Breast Cancer Symposium (SABCS) that will likely change practice for some breast cancer patients – even before the ball drops in Times Square on New Year’s Eve.
Residual cancer burden
Researchers reported a multi-institutional analysis of individual patient-level data on 5,160 patients who had received neoadjuvant chemotherapy (NAC) for localized breast cancer at 11 different centers. They found that residual cancer burden (RCB) was significantly associated with event-free (EFS) and distant recurrence-free survival. The value of calculating RCB was seen across all breast cancer tumor phenotypes (SABCS 2019, Abstract GS5-01).
RCB is calculated by analyzing the residual disease after NAC for the primary tumor bed, the number of positive axillary nodes, and the size of largest node metastasis. It is graded from RCB-0 (pCR) to RCB-III (extensive residual disease).
Both EFS and distant recurrence-free survival were strongly associated with RCB for the overall population and for each breast cancer subtype. For hormone receptor–positive/HER2-negative disease there was a slight hiccup in that RCB-0 was associated with a 10-year EFS of 81%, but EFS was 86% for RCB-I. Fortunately, the prognostic reliability of RCB was clear for RCB-II (69%) and RCB-III (52%). The presenter, W. Fraser Symmans, MD, commented that RCB is most prognostic when higher levels of residual disease are present. RCB remained prognostic in multivariate models adjusting for age, grade, and clinical T and N stage at diagnosis.
How these results influence practice
After neoadjuvant chemotherapy in patients with localized breast cancer, we struggle when patients ask: “So, doctor, how am I likely to do?” We piece together a complicated – and inconsistent – answer, based on breast cancer subtype, original stage of disease, whether pCR was attained, and other factors.
For patients with triple-negative breast cancer, we have the option of adding capecitabine and/or participation in ongoing clinical trials, for patients with residual disease after NAC. Among the HER2-positive patients, we have data from the KATHERINE, ExtaNET, and APHINITY trials that provide options for additional treatment in those patients, as well. For patients with potentially hormonally responsive, HER2-negative disease, we can emphasize the importance of postoperative adjuvant endocrine therapy, the mandate for continued adherence to oral therapy, and the lower likelihood of (and prognostic value of) pCR in that breast cancer subtype.
Where we previously had a complicated, nuanced discussion, we now have data from a multi-institutional, meticulous analysis to guide further treatment, candidacy for clinical trials, and our expectations of what would be meaningful results from additional therapy. This meets my definition of “practice-changing” research.
APHINITY follow-up
APHINITY was a randomized, multicenter, double-blind, placebo-controlled trial that previously demonstrated that pertuzumab added to standard chemotherapy plus 1 year of trastuzumab in operable HER2-positive breast cancer was associated with modest, but statistically significant, improvement in invasive disease–free survival (IDFS), compared with placebo and chemotherapy plus trastuzumab (hazard ratio, 0.81; P = .04). When the initial results – with a median follow-up of 45.4 months – were published, the authors promised an update at 6 years. Martine Piccart, MD, PhD, provided that update at the 2019 SABCS (Abstract GS1-04).
In the updated analysis, overall survival was 94.8% among patients on the pertuzumab arm and 93.9% among patients taking placebo (HR, 0.85). IDFS rates were 90.6% versus 87.8% in the intent-to-treat population. The investigator commented that this difference was caused mainly by a reduction in distant and loco-regional recurrences.
Central nervous system metastases, contralateral invasive breast cancers, and death without a prior event were no different between the two treatment groups.
Improvement in IDFS (the primary endpoint) from pertuzumab was most impressive in the node-positive cohort, 87.9% versus 83.4% for placebo (HR, 0.72). There was no benefit in the node-negative population (95.0% vs. 94.9%; HR, 1.02).
In contrast to the 3-year analysis, the benefits in IDFS were seen regardless of hormone receptor status. Additionally, no new safety concerns emerged. The rate of severe cardiac events was below 1% in both groups.
How these results influence practice
“Patience is a virtue” appeared for the first time in the English language around 1360, in William Langland’s poem “Piers Plowman.” They are true words, indeed.
When the initial results of APHINITY were published in 2017, they led to the approval of pertuzumab as an addition to chemotherapy plus trastuzumab for high-risk, early, HER2-positive breast cancer patients. Still, the difference in IDFS curves was visually unimpressive (absolute difference 1.7% in the intent-to-treat and 3.2% in the node-positive patients) and pertuzumab was associated with more grade 3 toxicities (primarily diarrhea) and treatment discontinuations.
An optimist would have observed that the IDFS curves in 2017 did not start to diverge until 24 months and would have noted that the divergence increased with time. That virtuously patient person would have expected that the second interim analysis would show larger absolute benefits, and that the hormone receptor–positive patients (64% of the total) would not yet have relapsed by the first interim analysis and that pertuzumab’s benefit in that group would emerge late.
That appears to be what is happening. It provides hope that an overall survival benefit will become more evident with time. If the target P value for an overall survival difference (.0012) is met by the time of the third interim analysis, our patience (and the FDA’s decision to grant approval for pertuzumab for this indication) will have been amply rewarded. For selected HER2-posiitve patients with high RCB, especially those who tolerated neoadjuvant trastuzumab plus pertuzumab regimens, postoperative adjuvant pertuzumab may be an appealing option.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Adult atopic dermatitis brings increased osteoporosis risk
MADRID – – even if they’ve never taken systemic corticosteroids, according to a large observational Danish national registry study.
A key study finding was that these elevated risks were concentrated in the patients who used potent or superpotent topical corticosteroids. Adult AD patients who used mild- or moderate-potency topical steroids were not at significantly increased risk. Neither were patients on topical calcineurin inhibitors, Jacob P. Thyssen, MD, PhD, reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“The absolute risk is low, but it’s real,” commented Dr. Thyssen, professor of dermatology at the University of Copenhagen.
His advice to colleagues: “Dermatologists should consider alternative treatments in the chronic excessive users of topical corticosteroids, or use them in combination with prophylactic treatment to preserve bone homeostasis in such patients.”
He presented the results of a retrospective case-control study of 10,636 Danish adults with AD and 87,989 matched controls. At baseline in this study, which featured a maximum of 20 years of follow-up starting in 1997, participants had no history of osteoporosis.
Dr. Thyssen expressed the absolute risk of being diagnosed with osteoporosis in the study as follows: If 10,000 adult AD patients were followed for 1 year, on average 23.5 of them would be diagnosed with osteoporosis, a rate more than double the 10.3 per 10,000 in the general population. Moreover, on average, 42.6 out of 10,000 adult AD patients would incur a major osteoporotic fracture during a year of follow-up, compared with 32.3 individuals in the general population.
In the subgroup of patients who never used systemic corticosteroids, the risk of being diagnosed with osteoporosis was 12.8 per 10,000 per year, significantly higher than the 7.4 per 10,000 rate in the general population. Similarly, the 1-year rate of major osteoporotic fractures was 33.1 per 10,000 among the AD group and 29.6 in matched controls.
In a Cox regression analysis adjusted for age, sex, socioeconomic status, body mass index, asthma, and the use of a variety of medications thought to potentially have a negative effect upon bone metabolism, the risk of osteoporosis in the entire group of 10,636 adult AD patients was 51% greater than in matched controls, and their risk of major osteoporotic fractures was 18% greater. In the subgroup of AD patients who never used systemic steroids, the risks of osteoporosis and major osteoporotic fractures were 82% and 14% greater than in controls. The medications adjusted for in the regression analysis included proton pump inhibitors, thiazide diuretics, H2 receptor blockers, statins, cyclosporine, hormone therapy, contraceptives, and psychotropic medications.
Scoring Atopic Dermatitis (SCORAD) ratings were available on roughly 4,000 of the adult AD patients. In an analysis of this large subgroup, disease severity as reflected in SCORAD scores did not explain the increased osteoporosis and fracture risks. However, the use of potent or superpotent topical corticosteroids did. Patients who used potent topical steroids had a statistically significant 16% increased risk of being diagnosed with osteoporosis than nonusers, as well as a 7% increased risk of major osteoporotic fractures. Patients who applied superpotent topical steroids had 42% and 18% increased risks of those two adverse outcomes.
In contrast, neither the use of topical calcineurin inhibitors nor mild- or mid-potency topical steroids was associated with increased risk of bone events in a Cox regression analysis adjusted for potential confounders.
A relationship between the use of high-potency topical corticosteroids and adverse bone events is biologically plausible, according to Dr. Thyssen. He and his coinvestigators have previously documented a 100%-400% increased rate of chemical penetration through atopic skin, which is notoriously barrier damaged.
“We find it very likely that, if you put topical steroids on atopic skin in high amounts and for a very long time, you may have systemic effects,” he said.
A great many adult AD patients do exactly that. When Dr. Thyssen and coworkers analyzed Danish national prescription drug registry data for their patient cohort, they found that roughly one-third of the elderly subgroup had filled prescriptions totaling greater than 2 kg of mometasone or other similar-potency steroids over the previous 10 years.
“So we know that a significant proportion of our atopic dermatitis patients are really high users of topical corticosteroids,” the dermatologist noted.
Dr. Thyssen’s national osteoporosis and fracture study was funded with a government research grant. He reported serving as an advisor to and/or recipient of research grants from AbbVie, Pfizer, Leo Pharma, Eli Lilly, Regeneron, Sanofi Genzyme, and Union Therapeutics.
MADRID – – even if they’ve never taken systemic corticosteroids, according to a large observational Danish national registry study.
A key study finding was that these elevated risks were concentrated in the patients who used potent or superpotent topical corticosteroids. Adult AD patients who used mild- or moderate-potency topical steroids were not at significantly increased risk. Neither were patients on topical calcineurin inhibitors, Jacob P. Thyssen, MD, PhD, reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“The absolute risk is low, but it’s real,” commented Dr. Thyssen, professor of dermatology at the University of Copenhagen.
His advice to colleagues: “Dermatologists should consider alternative treatments in the chronic excessive users of topical corticosteroids, or use them in combination with prophylactic treatment to preserve bone homeostasis in such patients.”
He presented the results of a retrospective case-control study of 10,636 Danish adults with AD and 87,989 matched controls. At baseline in this study, which featured a maximum of 20 years of follow-up starting in 1997, participants had no history of osteoporosis.
Dr. Thyssen expressed the absolute risk of being diagnosed with osteoporosis in the study as follows: If 10,000 adult AD patients were followed for 1 year, on average 23.5 of them would be diagnosed with osteoporosis, a rate more than double the 10.3 per 10,000 in the general population. Moreover, on average, 42.6 out of 10,000 adult AD patients would incur a major osteoporotic fracture during a year of follow-up, compared with 32.3 individuals in the general population.
In the subgroup of patients who never used systemic corticosteroids, the risk of being diagnosed with osteoporosis was 12.8 per 10,000 per year, significantly higher than the 7.4 per 10,000 rate in the general population. Similarly, the 1-year rate of major osteoporotic fractures was 33.1 per 10,000 among the AD group and 29.6 in matched controls.
In a Cox regression analysis adjusted for age, sex, socioeconomic status, body mass index, asthma, and the use of a variety of medications thought to potentially have a negative effect upon bone metabolism, the risk of osteoporosis in the entire group of 10,636 adult AD patients was 51% greater than in matched controls, and their risk of major osteoporotic fractures was 18% greater. In the subgroup of AD patients who never used systemic steroids, the risks of osteoporosis and major osteoporotic fractures were 82% and 14% greater than in controls. The medications adjusted for in the regression analysis included proton pump inhibitors, thiazide diuretics, H2 receptor blockers, statins, cyclosporine, hormone therapy, contraceptives, and psychotropic medications.
Scoring Atopic Dermatitis (SCORAD) ratings were available on roughly 4,000 of the adult AD patients. In an analysis of this large subgroup, disease severity as reflected in SCORAD scores did not explain the increased osteoporosis and fracture risks. However, the use of potent or superpotent topical corticosteroids did. Patients who used potent topical steroids had a statistically significant 16% increased risk of being diagnosed with osteoporosis than nonusers, as well as a 7% increased risk of major osteoporotic fractures. Patients who applied superpotent topical steroids had 42% and 18% increased risks of those two adverse outcomes.
In contrast, neither the use of topical calcineurin inhibitors nor mild- or mid-potency topical steroids was associated with increased risk of bone events in a Cox regression analysis adjusted for potential confounders.
A relationship between the use of high-potency topical corticosteroids and adverse bone events is biologically plausible, according to Dr. Thyssen. He and his coinvestigators have previously documented a 100%-400% increased rate of chemical penetration through atopic skin, which is notoriously barrier damaged.
“We find it very likely that, if you put topical steroids on atopic skin in high amounts and for a very long time, you may have systemic effects,” he said.
A great many adult AD patients do exactly that. When Dr. Thyssen and coworkers analyzed Danish national prescription drug registry data for their patient cohort, they found that roughly one-third of the elderly subgroup had filled prescriptions totaling greater than 2 kg of mometasone or other similar-potency steroids over the previous 10 years.
“So we know that a significant proportion of our atopic dermatitis patients are really high users of topical corticosteroids,” the dermatologist noted.
Dr. Thyssen’s national osteoporosis and fracture study was funded with a government research grant. He reported serving as an advisor to and/or recipient of research grants from AbbVie, Pfizer, Leo Pharma, Eli Lilly, Regeneron, Sanofi Genzyme, and Union Therapeutics.
MADRID – – even if they’ve never taken systemic corticosteroids, according to a large observational Danish national registry study.
A key study finding was that these elevated risks were concentrated in the patients who used potent or superpotent topical corticosteroids. Adult AD patients who used mild- or moderate-potency topical steroids were not at significantly increased risk. Neither were patients on topical calcineurin inhibitors, Jacob P. Thyssen, MD, PhD, reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“The absolute risk is low, but it’s real,” commented Dr. Thyssen, professor of dermatology at the University of Copenhagen.
His advice to colleagues: “Dermatologists should consider alternative treatments in the chronic excessive users of topical corticosteroids, or use them in combination with prophylactic treatment to preserve bone homeostasis in such patients.”
He presented the results of a retrospective case-control study of 10,636 Danish adults with AD and 87,989 matched controls. At baseline in this study, which featured a maximum of 20 years of follow-up starting in 1997, participants had no history of osteoporosis.
Dr. Thyssen expressed the absolute risk of being diagnosed with osteoporosis in the study as follows: If 10,000 adult AD patients were followed for 1 year, on average 23.5 of them would be diagnosed with osteoporosis, a rate more than double the 10.3 per 10,000 in the general population. Moreover, on average, 42.6 out of 10,000 adult AD patients would incur a major osteoporotic fracture during a year of follow-up, compared with 32.3 individuals in the general population.
In the subgroup of patients who never used systemic corticosteroids, the risk of being diagnosed with osteoporosis was 12.8 per 10,000 per year, significantly higher than the 7.4 per 10,000 rate in the general population. Similarly, the 1-year rate of major osteoporotic fractures was 33.1 per 10,000 among the AD group and 29.6 in matched controls.
In a Cox regression analysis adjusted for age, sex, socioeconomic status, body mass index, asthma, and the use of a variety of medications thought to potentially have a negative effect upon bone metabolism, the risk of osteoporosis in the entire group of 10,636 adult AD patients was 51% greater than in matched controls, and their risk of major osteoporotic fractures was 18% greater. In the subgroup of AD patients who never used systemic steroids, the risks of osteoporosis and major osteoporotic fractures were 82% and 14% greater than in controls. The medications adjusted for in the regression analysis included proton pump inhibitors, thiazide diuretics, H2 receptor blockers, statins, cyclosporine, hormone therapy, contraceptives, and psychotropic medications.
Scoring Atopic Dermatitis (SCORAD) ratings were available on roughly 4,000 of the adult AD patients. In an analysis of this large subgroup, disease severity as reflected in SCORAD scores did not explain the increased osteoporosis and fracture risks. However, the use of potent or superpotent topical corticosteroids did. Patients who used potent topical steroids had a statistically significant 16% increased risk of being diagnosed with osteoporosis than nonusers, as well as a 7% increased risk of major osteoporotic fractures. Patients who applied superpotent topical steroids had 42% and 18% increased risks of those two adverse outcomes.
In contrast, neither the use of topical calcineurin inhibitors nor mild- or mid-potency topical steroids was associated with increased risk of bone events in a Cox regression analysis adjusted for potential confounders.
A relationship between the use of high-potency topical corticosteroids and adverse bone events is biologically plausible, according to Dr. Thyssen. He and his coinvestigators have previously documented a 100%-400% increased rate of chemical penetration through atopic skin, which is notoriously barrier damaged.
“We find it very likely that, if you put topical steroids on atopic skin in high amounts and for a very long time, you may have systemic effects,” he said.
A great many adult AD patients do exactly that. When Dr. Thyssen and coworkers analyzed Danish national prescription drug registry data for their patient cohort, they found that roughly one-third of the elderly subgroup had filled prescriptions totaling greater than 2 kg of mometasone or other similar-potency steroids over the previous 10 years.
“So we know that a significant proportion of our atopic dermatitis patients are really high users of topical corticosteroids,” the dermatologist noted.
Dr. Thyssen’s national osteoporosis and fracture study was funded with a government research grant. He reported serving as an advisor to and/or recipient of research grants from AbbVie, Pfizer, Leo Pharma, Eli Lilly, Regeneron, Sanofi Genzyme, and Union Therapeutics.
REPORTING FROM EADV 2019
What is your diagnosis?
Natural course of primary hepatic angiosarcoma with rupture
The pathology demonstrated slitlike vascular channels lined by spindle-shaped endothelial cells with large and hyperchromatic nuclei (Figure C). Primary hepatic angiosarcoma (PHA) was diagnosed. The patient declined operation because of poor prognosis and body performance. Ascites tapping was performed, and the fluid was bloody. The patient received a blood transfusion and died 2 days later.
PHA is an aggressive malignant tumor that originates from the endothelium of liver blood vessels. It is a rare condition and accounts for 0.6%-2.0% of all primary liver tumors and less than 5% of all angiosarcomas.1
Most PHAs exhibit no obvious symptoms and signs, particularly when they are small. As the disease progresses, symptoms and signs including abdominal pain, weakness, fatigue, weight loss, hepatomegaly, and ascites occur. The spontaneous rupture of PHA was reported in 15%-27% of patients in a previous study.2 As in our case, no obvious symptoms were noted in the early stage, and spontaneous rupture was observed in the later stage.
A CT scan of PHA reveals multiple nodules or a dominant mass and a diffusely infiltrating lesion. The tumor is composed of low-density lesions with small heterogeneous hypervascular foci.3 In our patient, a CT scan revealed a hepatic tumor that had a low density when it was small and multiple heterogeneous hypervascular foci when it grew large.
PHAs are very aggressive tumors, and most cases are diagnosed at an advanced stage. The median survival was reported to be 6 months without treatment. Complete resection with clear margins is the choice of treatment; however, the prognosis is poor even after complete resection.2 In our case, operation after diagnosis was declined because of the patient’s poor prognosis and body performance. She lived for 18 months after the diagnosis. The entire natural course of PHA from initial diagnosis to rupture was well presented in our case.
References
1. Zheng YW, Zhang XW, Zhang JL, et al. Primary hepatic angiosarcoma and potential treatment options. J Gastroenterol Hepatol. 2014;29:906–11.
2. Cawich SO, Ramjit C. Herald bleeding from a ruptured primary hepatic angiosarcoma: a case report. Mol Clin Oncol. 2015;3:1063–6.
3. Huang IH, Wu YY, Huang TC, et al. Statistics and outlook of primary hepatic angiosarcoma based on clinical stage. Oncol Lett. 2016;11:3218–22.
ginews@gastro.org
Natural course of primary hepatic angiosarcoma with rupture
The pathology demonstrated slitlike vascular channels lined by spindle-shaped endothelial cells with large and hyperchromatic nuclei (Figure C). Primary hepatic angiosarcoma (PHA) was diagnosed. The patient declined operation because of poor prognosis and body performance. Ascites tapping was performed, and the fluid was bloody. The patient received a blood transfusion and died 2 days later.
PHA is an aggressive malignant tumor that originates from the endothelium of liver blood vessels. It is a rare condition and accounts for 0.6%-2.0% of all primary liver tumors and less than 5% of all angiosarcomas.1
Most PHAs exhibit no obvious symptoms and signs, particularly when they are small. As the disease progresses, symptoms and signs including abdominal pain, weakness, fatigue, weight loss, hepatomegaly, and ascites occur. The spontaneous rupture of PHA was reported in 15%-27% of patients in a previous study.2 As in our case, no obvious symptoms were noted in the early stage, and spontaneous rupture was observed in the later stage.
A CT scan of PHA reveals multiple nodules or a dominant mass and a diffusely infiltrating lesion. The tumor is composed of low-density lesions with small heterogeneous hypervascular foci.3 In our patient, a CT scan revealed a hepatic tumor that had a low density when it was small and multiple heterogeneous hypervascular foci when it grew large.
PHAs are very aggressive tumors, and most cases are diagnosed at an advanced stage. The median survival was reported to be 6 months without treatment. Complete resection with clear margins is the choice of treatment; however, the prognosis is poor even after complete resection.2 In our case, operation after diagnosis was declined because of the patient’s poor prognosis and body performance. She lived for 18 months after the diagnosis. The entire natural course of PHA from initial diagnosis to rupture was well presented in our case.
References
1. Zheng YW, Zhang XW, Zhang JL, et al. Primary hepatic angiosarcoma and potential treatment options. J Gastroenterol Hepatol. 2014;29:906–11.
2. Cawich SO, Ramjit C. Herald bleeding from a ruptured primary hepatic angiosarcoma: a case report. Mol Clin Oncol. 2015;3:1063–6.
3. Huang IH, Wu YY, Huang TC, et al. Statistics and outlook of primary hepatic angiosarcoma based on clinical stage. Oncol Lett. 2016;11:3218–22.
ginews@gastro.org
Natural course of primary hepatic angiosarcoma with rupture
The pathology demonstrated slitlike vascular channels lined by spindle-shaped endothelial cells with large and hyperchromatic nuclei (Figure C). Primary hepatic angiosarcoma (PHA) was diagnosed. The patient declined operation because of poor prognosis and body performance. Ascites tapping was performed, and the fluid was bloody. The patient received a blood transfusion and died 2 days later.
PHA is an aggressive malignant tumor that originates from the endothelium of liver blood vessels. It is a rare condition and accounts for 0.6%-2.0% of all primary liver tumors and less than 5% of all angiosarcomas.1
Most PHAs exhibit no obvious symptoms and signs, particularly when they are small. As the disease progresses, symptoms and signs including abdominal pain, weakness, fatigue, weight loss, hepatomegaly, and ascites occur. The spontaneous rupture of PHA was reported in 15%-27% of patients in a previous study.2 As in our case, no obvious symptoms were noted in the early stage, and spontaneous rupture was observed in the later stage.
A CT scan of PHA reveals multiple nodules or a dominant mass and a diffusely infiltrating lesion. The tumor is composed of low-density lesions with small heterogeneous hypervascular foci.3 In our patient, a CT scan revealed a hepatic tumor that had a low density when it was small and multiple heterogeneous hypervascular foci when it grew large.
PHAs are very aggressive tumors, and most cases are diagnosed at an advanced stage. The median survival was reported to be 6 months without treatment. Complete resection with clear margins is the choice of treatment; however, the prognosis is poor even after complete resection.2 In our case, operation after diagnosis was declined because of the patient’s poor prognosis and body performance. She lived for 18 months after the diagnosis. The entire natural course of PHA from initial diagnosis to rupture was well presented in our case.
References
1. Zheng YW, Zhang XW, Zhang JL, et al. Primary hepatic angiosarcoma and potential treatment options. J Gastroenterol Hepatol. 2014;29:906–11.
2. Cawich SO, Ramjit C. Herald bleeding from a ruptured primary hepatic angiosarcoma: a case report. Mol Clin Oncol. 2015;3:1063–6.
3. Huang IH, Wu YY, Huang TC, et al. Statistics and outlook of primary hepatic angiosarcoma based on clinical stage. Oncol Lett. 2016;11:3218–22.
ginews@gastro.org
What was the diagnosis?
Data point to bidirectional link between sleep disorders and ADHD
in a large longitudinal study of adolescents in China.
Investigators twice assessed 7,072 middle and high school students participating in the larger longitudinal Shandong Adolescent Behavior & Health Cohort – in 2015 and 1 year later in 2016 – for sleep, mental health, psychosocial factors (using the self-administered Adolescent Health Questionnaire, or AHQ), and for ADHD symptoms (using the Youth Self-Report, or YSR, of the Achenbach Child Behavior Checklist).
At baseline, ADHD symptoms were reported by 7.6% of adolescents and were significantly correlated, after adjusting for adolescent and family covariates, with all the sleep variables studied: sleep duration of 7 hours or less per night, insomnia symptoms, poor sleep quality, RLS symptoms, frequent snorting, and hypnotic use, reported Xianchen Liu, MD, PhD, of Shandong (China) University, and coinvestigators. They noted a dose-response relationship between sleep duration and the odds of having ADHD symptoms.
At 1-year follow-up, 4.5% of the 6,531 participants who did not have ADHD symptoms at baseline now reported them. After adjustments for covariates, any insomnia (odds ratio, 1.48), difficulty initiating sleep (one of the insomnia symptoms) (OR, 2.09), RLS (OR, 1.47), and frequent snoring (OR, 2.30) at baseline were each significantly associated with development of incident ADHD symptoms and with ADHD severity at 1 year, they reported in Sleep.
“Given the fact that sleep disorders in adolescents are often underdiagnosed and untreated primarily in the primary care setting, our findings highlight that clinicians should assess and manage short sleep duration and sleep problems for effective treatment of ADHD in adolescents,” as well as for prevention, they wrote.
The AHQ includes questions that assess nocturnal sleep duration and sleep problems during the past month. The adolescent and family variables that were selected as covariates and controlled for include cigarette smoking, alcohol drinking, use of mental health services, chronic physical diseases, and parental education and occupation. Depression was also a covariate but was assessed through a different scale.
The YSR measures eight ADHD symptoms during the past 6 months on a 3-point scale (not true, somewhat or sometimes true, and very true or often true). The adolescent participants of this study were in grades 7, 8, and 10 at baseline. Their mean age at baseline was 15 years; half were male. They were part of the larger Shandong Adolescent and Behavioral Cohort, a longitudinal study of almost 12,000 adolescents.
Growing evidence has demonstrated a bidirectional relationship between sleep problems and ADHD symptoms in pediatric populations, the investigators wrote, and further research is needed to examine the “mediators, moderators, and biological mechanisms of the sleep-ADHD link [in adolescents].”
While there are multiple potential pathways for this link, sleep problems may sometimes result in a cluster of behavioral and cognitive symptoms that are not true ADHD but that mimic the disorder, they noted.
The investigators also noted that approximately 67% of adolescents who had clinically relevant ADHD symptoms at baseline no longer had these symptoms at 1-year follow-up – a finding that “supports the [idea]” that ADHD symptoms with onset in adolescence may be transient or episodic rather than persistent.
The study was funded in part by the National Natural Science Foundation of China. The authors reported that they have no conflicts of interest.
SOURCE: Liu X et al. Sleep. 2019 Dec 2. doi: 10.1093/sleep/zsz294.
in a large longitudinal study of adolescents in China.
Investigators twice assessed 7,072 middle and high school students participating in the larger longitudinal Shandong Adolescent Behavior & Health Cohort – in 2015 and 1 year later in 2016 – for sleep, mental health, psychosocial factors (using the self-administered Adolescent Health Questionnaire, or AHQ), and for ADHD symptoms (using the Youth Self-Report, or YSR, of the Achenbach Child Behavior Checklist).
At baseline, ADHD symptoms were reported by 7.6% of adolescents and were significantly correlated, after adjusting for adolescent and family covariates, with all the sleep variables studied: sleep duration of 7 hours or less per night, insomnia symptoms, poor sleep quality, RLS symptoms, frequent snorting, and hypnotic use, reported Xianchen Liu, MD, PhD, of Shandong (China) University, and coinvestigators. They noted a dose-response relationship between sleep duration and the odds of having ADHD symptoms.
At 1-year follow-up, 4.5% of the 6,531 participants who did not have ADHD symptoms at baseline now reported them. After adjustments for covariates, any insomnia (odds ratio, 1.48), difficulty initiating sleep (one of the insomnia symptoms) (OR, 2.09), RLS (OR, 1.47), and frequent snoring (OR, 2.30) at baseline were each significantly associated with development of incident ADHD symptoms and with ADHD severity at 1 year, they reported in Sleep.
“Given the fact that sleep disorders in adolescents are often underdiagnosed and untreated primarily in the primary care setting, our findings highlight that clinicians should assess and manage short sleep duration and sleep problems for effective treatment of ADHD in adolescents,” as well as for prevention, they wrote.
The AHQ includes questions that assess nocturnal sleep duration and sleep problems during the past month. The adolescent and family variables that were selected as covariates and controlled for include cigarette smoking, alcohol drinking, use of mental health services, chronic physical diseases, and parental education and occupation. Depression was also a covariate but was assessed through a different scale.
The YSR measures eight ADHD symptoms during the past 6 months on a 3-point scale (not true, somewhat or sometimes true, and very true or often true). The adolescent participants of this study were in grades 7, 8, and 10 at baseline. Their mean age at baseline was 15 years; half were male. They were part of the larger Shandong Adolescent and Behavioral Cohort, a longitudinal study of almost 12,000 adolescents.
Growing evidence has demonstrated a bidirectional relationship between sleep problems and ADHD symptoms in pediatric populations, the investigators wrote, and further research is needed to examine the “mediators, moderators, and biological mechanisms of the sleep-ADHD link [in adolescents].”
While there are multiple potential pathways for this link, sleep problems may sometimes result in a cluster of behavioral and cognitive symptoms that are not true ADHD but that mimic the disorder, they noted.
The investigators also noted that approximately 67% of adolescents who had clinically relevant ADHD symptoms at baseline no longer had these symptoms at 1-year follow-up – a finding that “supports the [idea]” that ADHD symptoms with onset in adolescence may be transient or episodic rather than persistent.
The study was funded in part by the National Natural Science Foundation of China. The authors reported that they have no conflicts of interest.
SOURCE: Liu X et al. Sleep. 2019 Dec 2. doi: 10.1093/sleep/zsz294.
in a large longitudinal study of adolescents in China.
Investigators twice assessed 7,072 middle and high school students participating in the larger longitudinal Shandong Adolescent Behavior & Health Cohort – in 2015 and 1 year later in 2016 – for sleep, mental health, psychosocial factors (using the self-administered Adolescent Health Questionnaire, or AHQ), and for ADHD symptoms (using the Youth Self-Report, or YSR, of the Achenbach Child Behavior Checklist).
At baseline, ADHD symptoms were reported by 7.6% of adolescents and were significantly correlated, after adjusting for adolescent and family covariates, with all the sleep variables studied: sleep duration of 7 hours or less per night, insomnia symptoms, poor sleep quality, RLS symptoms, frequent snorting, and hypnotic use, reported Xianchen Liu, MD, PhD, of Shandong (China) University, and coinvestigators. They noted a dose-response relationship between sleep duration and the odds of having ADHD symptoms.
At 1-year follow-up, 4.5% of the 6,531 participants who did not have ADHD symptoms at baseline now reported them. After adjustments for covariates, any insomnia (odds ratio, 1.48), difficulty initiating sleep (one of the insomnia symptoms) (OR, 2.09), RLS (OR, 1.47), and frequent snoring (OR, 2.30) at baseline were each significantly associated with development of incident ADHD symptoms and with ADHD severity at 1 year, they reported in Sleep.
“Given the fact that sleep disorders in adolescents are often underdiagnosed and untreated primarily in the primary care setting, our findings highlight that clinicians should assess and manage short sleep duration and sleep problems for effective treatment of ADHD in adolescents,” as well as for prevention, they wrote.
The AHQ includes questions that assess nocturnal sleep duration and sleep problems during the past month. The adolescent and family variables that were selected as covariates and controlled for include cigarette smoking, alcohol drinking, use of mental health services, chronic physical diseases, and parental education and occupation. Depression was also a covariate but was assessed through a different scale.
The YSR measures eight ADHD symptoms during the past 6 months on a 3-point scale (not true, somewhat or sometimes true, and very true or often true). The adolescent participants of this study were in grades 7, 8, and 10 at baseline. Their mean age at baseline was 15 years; half were male. They were part of the larger Shandong Adolescent and Behavioral Cohort, a longitudinal study of almost 12,000 adolescents.
Growing evidence has demonstrated a bidirectional relationship between sleep problems and ADHD symptoms in pediatric populations, the investigators wrote, and further research is needed to examine the “mediators, moderators, and biological mechanisms of the sleep-ADHD link [in adolescents].”
While there are multiple potential pathways for this link, sleep problems may sometimes result in a cluster of behavioral and cognitive symptoms that are not true ADHD but that mimic the disorder, they noted.
The investigators also noted that approximately 67% of adolescents who had clinically relevant ADHD symptoms at baseline no longer had these symptoms at 1-year follow-up – a finding that “supports the [idea]” that ADHD symptoms with onset in adolescence may be transient or episodic rather than persistent.
The study was funded in part by the National Natural Science Foundation of China. The authors reported that they have no conflicts of interest.
SOURCE: Liu X et al. Sleep. 2019 Dec 2. doi: 10.1093/sleep/zsz294.
FROM SLEEP
SGLT2 inhibitors for diabetes safe, effective in older adults
BUSAN, SOUTH KOREA – new research suggests.
Findings from a real-world observational study of 50 older adults with type 2 diabetes were recently presented at the International Diabetes Federation congress by Carlos Trescoli-Serrano, MD, of Hospital Universitario de la Ribera, Valencia, Spain.
“The results are quite similar to those of younger people. … In a selected population they are safe. It doesn’t matter if patients are older or younger. You have to review the patients for complications,” Dr. Trescoli-Serrano said in an interview.
Asked to comment, session moderator Samuel Dagogo-Jack, MD, said: “We need more and more studies in older people for reassurance. The elderly have impaired kidneys, and these drugs depend on kidney filtration. It’s good to have data on elderly patients in the real world.”
Most had comorbidities
The 50 adults were a mean age of 67 years, and the oldest was 81 years. They had a mean diabetes duration of 12.5 years, and 40% were women. They had all been taking SGLT2 inhibitors for more than 3 years (mean 43.9 months) during 2015-2019.
At baseline, most (75%) were also taking metformin, 45% also took sulfonylureas, 37% dipeptidyl peptidase-4 (DPP-4) inhibitors, and 36% insulin.
Most (81%) also had hypertension, half (51%) had hypercholesterolemia, a third (33%) had obesity, and 32% had a previous cardiovascular event.
With SGLT2-inhibitor treatment, the average hemoglobin A1c level dropped from 8.5% to 7.3% (P less than .01), body weight was reduced from 91.0 kg to 84.7 kg (P less than .01), systolic blood pressure from 134.5 mm Hg to 130.4 mm Hg (P = .02), and diastolic blood pressure from 76.2 mm Hg to 73.3 mm Hg (P = .04).
This effect on blood pressure “should be considered” because it means that “antihypertensive treatment might need to be reviewed,” Dr. Trescoli-Serrano commented.
There were no significant changes in estimated glomerular filtration rate (eGFR), microalbuminuria, lipid profile, hematocrit, or heart rate, although there were positive trends in both renal function and lipid profiles.
No patient had a fall, volume depletion symptoms, or diabetic ketoacidosis. However, 38% of patients were treated for urinary-genital infections, and 8% had to stop taking the SGLT2 inhibitor because of such infections.
That percentage seemed unusually high and was “an outlier, not the general experience,” commented Dr. Dagogo-Jack, chief of the Division of Endocrinology, Diabetes and Metabolism at the University of Tennessee Health Science Center, Memphis. He noted that infections with SGLT2 inhibitors are more often genital fungal than urinary tract infections, but the latter are more common in people with diabetes overall, and the study wasn’t randomized, so this might explain the finding.
New nonfatal cardiovascular events occurred in 22% of the patients, mainly cerebrovascular disease during the treatment period. Of those, 66% had had a previous cardiovascular event prior to starting on SGLT2 inhibitors.
Severe hypoglycemia was recorded in one patient, who was also taking insulin. Overall 10% died, mainly because of neoplastic causes.
“Long-term treatment with SGLT2 inhibitors are safe and effective when added to not-well-controlled elderly type 2 diabetes patients in a real-world experience,” Dr. Trescoli-Serrano concluded.
Dr. Dagogo-Jack is a consultant for Merck, Janssen, and Sanofi and owns stock in Dance Pharma and Jana Care.
A version of this story originally appeared on Medscape.com.
BUSAN, SOUTH KOREA – new research suggests.
Findings from a real-world observational study of 50 older adults with type 2 diabetes were recently presented at the International Diabetes Federation congress by Carlos Trescoli-Serrano, MD, of Hospital Universitario de la Ribera, Valencia, Spain.
“The results are quite similar to those of younger people. … In a selected population they are safe. It doesn’t matter if patients are older or younger. You have to review the patients for complications,” Dr. Trescoli-Serrano said in an interview.
Asked to comment, session moderator Samuel Dagogo-Jack, MD, said: “We need more and more studies in older people for reassurance. The elderly have impaired kidneys, and these drugs depend on kidney filtration. It’s good to have data on elderly patients in the real world.”
Most had comorbidities
The 50 adults were a mean age of 67 years, and the oldest was 81 years. They had a mean diabetes duration of 12.5 years, and 40% were women. They had all been taking SGLT2 inhibitors for more than 3 years (mean 43.9 months) during 2015-2019.
At baseline, most (75%) were also taking metformin, 45% also took sulfonylureas, 37% dipeptidyl peptidase-4 (DPP-4) inhibitors, and 36% insulin.
Most (81%) also had hypertension, half (51%) had hypercholesterolemia, a third (33%) had obesity, and 32% had a previous cardiovascular event.
With SGLT2-inhibitor treatment, the average hemoglobin A1c level dropped from 8.5% to 7.3% (P less than .01), body weight was reduced from 91.0 kg to 84.7 kg (P less than .01), systolic blood pressure from 134.5 mm Hg to 130.4 mm Hg (P = .02), and diastolic blood pressure from 76.2 mm Hg to 73.3 mm Hg (P = .04).
This effect on blood pressure “should be considered” because it means that “antihypertensive treatment might need to be reviewed,” Dr. Trescoli-Serrano commented.
There were no significant changes in estimated glomerular filtration rate (eGFR), microalbuminuria, lipid profile, hematocrit, or heart rate, although there were positive trends in both renal function and lipid profiles.
No patient had a fall, volume depletion symptoms, or diabetic ketoacidosis. However, 38% of patients were treated for urinary-genital infections, and 8% had to stop taking the SGLT2 inhibitor because of such infections.
That percentage seemed unusually high and was “an outlier, not the general experience,” commented Dr. Dagogo-Jack, chief of the Division of Endocrinology, Diabetes and Metabolism at the University of Tennessee Health Science Center, Memphis. He noted that infections with SGLT2 inhibitors are more often genital fungal than urinary tract infections, but the latter are more common in people with diabetes overall, and the study wasn’t randomized, so this might explain the finding.
New nonfatal cardiovascular events occurred in 22% of the patients, mainly cerebrovascular disease during the treatment period. Of those, 66% had had a previous cardiovascular event prior to starting on SGLT2 inhibitors.
Severe hypoglycemia was recorded in one patient, who was also taking insulin. Overall 10% died, mainly because of neoplastic causes.
“Long-term treatment with SGLT2 inhibitors are safe and effective when added to not-well-controlled elderly type 2 diabetes patients in a real-world experience,” Dr. Trescoli-Serrano concluded.
Dr. Dagogo-Jack is a consultant for Merck, Janssen, and Sanofi and owns stock in Dance Pharma and Jana Care.
A version of this story originally appeared on Medscape.com.
BUSAN, SOUTH KOREA – new research suggests.
Findings from a real-world observational study of 50 older adults with type 2 diabetes were recently presented at the International Diabetes Federation congress by Carlos Trescoli-Serrano, MD, of Hospital Universitario de la Ribera, Valencia, Spain.
“The results are quite similar to those of younger people. … In a selected population they are safe. It doesn’t matter if patients are older or younger. You have to review the patients for complications,” Dr. Trescoli-Serrano said in an interview.
Asked to comment, session moderator Samuel Dagogo-Jack, MD, said: “We need more and more studies in older people for reassurance. The elderly have impaired kidneys, and these drugs depend on kidney filtration. It’s good to have data on elderly patients in the real world.”
Most had comorbidities
The 50 adults were a mean age of 67 years, and the oldest was 81 years. They had a mean diabetes duration of 12.5 years, and 40% were women. They had all been taking SGLT2 inhibitors for more than 3 years (mean 43.9 months) during 2015-2019.
At baseline, most (75%) were also taking metformin, 45% also took sulfonylureas, 37% dipeptidyl peptidase-4 (DPP-4) inhibitors, and 36% insulin.
Most (81%) also had hypertension, half (51%) had hypercholesterolemia, a third (33%) had obesity, and 32% had a previous cardiovascular event.
With SGLT2-inhibitor treatment, the average hemoglobin A1c level dropped from 8.5% to 7.3% (P less than .01), body weight was reduced from 91.0 kg to 84.7 kg (P less than .01), systolic blood pressure from 134.5 mm Hg to 130.4 mm Hg (P = .02), and diastolic blood pressure from 76.2 mm Hg to 73.3 mm Hg (P = .04).
This effect on blood pressure “should be considered” because it means that “antihypertensive treatment might need to be reviewed,” Dr. Trescoli-Serrano commented.
There were no significant changes in estimated glomerular filtration rate (eGFR), microalbuminuria, lipid profile, hematocrit, or heart rate, although there were positive trends in both renal function and lipid profiles.
No patient had a fall, volume depletion symptoms, or diabetic ketoacidosis. However, 38% of patients were treated for urinary-genital infections, and 8% had to stop taking the SGLT2 inhibitor because of such infections.
That percentage seemed unusually high and was “an outlier, not the general experience,” commented Dr. Dagogo-Jack, chief of the Division of Endocrinology, Diabetes and Metabolism at the University of Tennessee Health Science Center, Memphis. He noted that infections with SGLT2 inhibitors are more often genital fungal than urinary tract infections, but the latter are more common in people with diabetes overall, and the study wasn’t randomized, so this might explain the finding.
New nonfatal cardiovascular events occurred in 22% of the patients, mainly cerebrovascular disease during the treatment period. Of those, 66% had had a previous cardiovascular event prior to starting on SGLT2 inhibitors.
Severe hypoglycemia was recorded in one patient, who was also taking insulin. Overall 10% died, mainly because of neoplastic causes.
“Long-term treatment with SGLT2 inhibitors are safe and effective when added to not-well-controlled elderly type 2 diabetes patients in a real-world experience,” Dr. Trescoli-Serrano concluded.
Dr. Dagogo-Jack is a consultant for Merck, Janssen, and Sanofi and owns stock in Dance Pharma and Jana Care.
A version of this story originally appeared on Medscape.com.
IORT boosts local control, survival of glioblastoma
Adding intraoperative radiotherapy to the standard of care for patients with newly diagnosed glioblastoma appear to offer improved local control and survival, compared with those of historical controls without major adverse events, according to authors of a pooled analysis.
Among 51 patients who underwent tumor resection followed by intraoperative radiotherapy (IORT), standard adjuvant chemoradiotherapy, and chemotherapy maintenance, the estimated 2-year overall survival (OS) rate was 38.7%, compared with 26.5% at 2 years for patients who had received external beam radiotherapy (EBRT) and concomitant plus adjuvant temozolomide in a multinational phase 3 trial, reported Gustavo Sarria, MD, from University Medical Center Mannheim (Germany) and colleagues.
The median local progression-free survival (L-PFS) at a median follow-up of 18 months was 16 months, and the median distant PFS (D-PFS) was 30 months, indicating effective delay of tumor recurrence in a substantial proportion of patients.
“Intraoperative radiotherapy does not require extra radiation to travel through healthy tissue as compared to EBRT, thus allowing for local dose escalation at the site of most likely recurrence,” they wrote in Radiotherapy and Oncology.
The investigators performed a retrospective analysis of data on a total of 51 patients with a median age of 55 years who were treated with IORT and standard of care at five centers in Germany, Perum, and China. The patients all underwent brain surgery followed by a single IORT application at doses ranging from 10 to 40 Gy, with low-energy (50-kV) x-rays.
Following surgery, all patients received 60-Gy intensity-modulated or volumetric-modulated arc (IMRT-VMAT) EBRT and concomitant temozolomide chemotherapy followed by maintenance temozolomide.
At a median follow-up of 18 months the median OS was 18 months, median overall PFS was 11.4 months, median L-PFS (new lesions 1 cm or less from the tumor cavity border) was 16 months, and median D-PFS (new lesions more than 1 cm from the tumor cavity border) was 30 months.
The estimated Kaplan-Meier 1-, 2-, and 3-year OS rates were 79.5%, 38.7%, and 25.6%, respectively. The estimated median PFS over the same time points was 46.2%, 29.4%, and 5.9. The 1-, 2-, and 3-year estimated L-PFS was 60.9%, 37.9%, and 12.6%, and D-FPS rates were 76.7%, 65.0%, and 39.0% respectively.
In slightly more than one-third of the cases (35.3%), the first progression occurred locally. Grade 1 radionecrosis occurred in 7.8% of patients, and 17.6% had grade 3 radionecrosis. There were no grade 4 toxicities reported, and no treatment-related deaths.
The investigators noted that IORT added to standard of care is being tested against standard care alone in a multinational phase 3 randomized trial (NCT02685605).
The authors did not receive outside funding for the study. Dr. Sarria reported Grants from Carl Zeiss Meditec outside the submitted work.
SOURCE: Sarria G et al. J Rad Oncol. 2019 Oct 16. doi: 10.1016/j.radonc.2019.09.023.
Adding intraoperative radiotherapy to the standard of care for patients with newly diagnosed glioblastoma appear to offer improved local control and survival, compared with those of historical controls without major adverse events, according to authors of a pooled analysis.
Among 51 patients who underwent tumor resection followed by intraoperative radiotherapy (IORT), standard adjuvant chemoradiotherapy, and chemotherapy maintenance, the estimated 2-year overall survival (OS) rate was 38.7%, compared with 26.5% at 2 years for patients who had received external beam radiotherapy (EBRT) and concomitant plus adjuvant temozolomide in a multinational phase 3 trial, reported Gustavo Sarria, MD, from University Medical Center Mannheim (Germany) and colleagues.
The median local progression-free survival (L-PFS) at a median follow-up of 18 months was 16 months, and the median distant PFS (D-PFS) was 30 months, indicating effective delay of tumor recurrence in a substantial proportion of patients.
“Intraoperative radiotherapy does not require extra radiation to travel through healthy tissue as compared to EBRT, thus allowing for local dose escalation at the site of most likely recurrence,” they wrote in Radiotherapy and Oncology.
The investigators performed a retrospective analysis of data on a total of 51 patients with a median age of 55 years who were treated with IORT and standard of care at five centers in Germany, Perum, and China. The patients all underwent brain surgery followed by a single IORT application at doses ranging from 10 to 40 Gy, with low-energy (50-kV) x-rays.
Following surgery, all patients received 60-Gy intensity-modulated or volumetric-modulated arc (IMRT-VMAT) EBRT and concomitant temozolomide chemotherapy followed by maintenance temozolomide.
At a median follow-up of 18 months the median OS was 18 months, median overall PFS was 11.4 months, median L-PFS (new lesions 1 cm or less from the tumor cavity border) was 16 months, and median D-PFS (new lesions more than 1 cm from the tumor cavity border) was 30 months.
The estimated Kaplan-Meier 1-, 2-, and 3-year OS rates were 79.5%, 38.7%, and 25.6%, respectively. The estimated median PFS over the same time points was 46.2%, 29.4%, and 5.9. The 1-, 2-, and 3-year estimated L-PFS was 60.9%, 37.9%, and 12.6%, and D-FPS rates were 76.7%, 65.0%, and 39.0% respectively.
In slightly more than one-third of the cases (35.3%), the first progression occurred locally. Grade 1 radionecrosis occurred in 7.8% of patients, and 17.6% had grade 3 radionecrosis. There were no grade 4 toxicities reported, and no treatment-related deaths.
The investigators noted that IORT added to standard of care is being tested against standard care alone in a multinational phase 3 randomized trial (NCT02685605).
The authors did not receive outside funding for the study. Dr. Sarria reported Grants from Carl Zeiss Meditec outside the submitted work.
SOURCE: Sarria G et al. J Rad Oncol. 2019 Oct 16. doi: 10.1016/j.radonc.2019.09.023.
Adding intraoperative radiotherapy to the standard of care for patients with newly diagnosed glioblastoma appear to offer improved local control and survival, compared with those of historical controls without major adverse events, according to authors of a pooled analysis.
Among 51 patients who underwent tumor resection followed by intraoperative radiotherapy (IORT), standard adjuvant chemoradiotherapy, and chemotherapy maintenance, the estimated 2-year overall survival (OS) rate was 38.7%, compared with 26.5% at 2 years for patients who had received external beam radiotherapy (EBRT) and concomitant plus adjuvant temozolomide in a multinational phase 3 trial, reported Gustavo Sarria, MD, from University Medical Center Mannheim (Germany) and colleagues.
The median local progression-free survival (L-PFS) at a median follow-up of 18 months was 16 months, and the median distant PFS (D-PFS) was 30 months, indicating effective delay of tumor recurrence in a substantial proportion of patients.
“Intraoperative radiotherapy does not require extra radiation to travel through healthy tissue as compared to EBRT, thus allowing for local dose escalation at the site of most likely recurrence,” they wrote in Radiotherapy and Oncology.
The investigators performed a retrospective analysis of data on a total of 51 patients with a median age of 55 years who were treated with IORT and standard of care at five centers in Germany, Perum, and China. The patients all underwent brain surgery followed by a single IORT application at doses ranging from 10 to 40 Gy, with low-energy (50-kV) x-rays.
Following surgery, all patients received 60-Gy intensity-modulated or volumetric-modulated arc (IMRT-VMAT) EBRT and concomitant temozolomide chemotherapy followed by maintenance temozolomide.
At a median follow-up of 18 months the median OS was 18 months, median overall PFS was 11.4 months, median L-PFS (new lesions 1 cm or less from the tumor cavity border) was 16 months, and median D-PFS (new lesions more than 1 cm from the tumor cavity border) was 30 months.
The estimated Kaplan-Meier 1-, 2-, and 3-year OS rates were 79.5%, 38.7%, and 25.6%, respectively. The estimated median PFS over the same time points was 46.2%, 29.4%, and 5.9. The 1-, 2-, and 3-year estimated L-PFS was 60.9%, 37.9%, and 12.6%, and D-FPS rates were 76.7%, 65.0%, and 39.0% respectively.
In slightly more than one-third of the cases (35.3%), the first progression occurred locally. Grade 1 radionecrosis occurred in 7.8% of patients, and 17.6% had grade 3 radionecrosis. There were no grade 4 toxicities reported, and no treatment-related deaths.
The investigators noted that IORT added to standard of care is being tested against standard care alone in a multinational phase 3 randomized trial (NCT02685605).
The authors did not receive outside funding for the study. Dr. Sarria reported Grants from Carl Zeiss Meditec outside the submitted work.
SOURCE: Sarria G et al. J Rad Oncol. 2019 Oct 16. doi: 10.1016/j.radonc.2019.09.023.
FROM RADIOTHERAPY AND ONCOLOGY
Vermont tops America’s Health Rankings for 2019
The award for healthiest state goes to Vermont in 2019, marking the fifth time the Green Mountain State has taken the top spot in the 30-year span of America’s Health Rankings.

The New England states took 3 of the top 5 spots and 4 of the top 10, while last year’s winner, Hawaii, dropped to third and missed out on the title for only the second time in the last 8 years, according to the America’s Heath Rankings annual report.
Another rankings tradition lived on, however, as Mississippi and Louisiana continued their battle to be the state with the “greatest opportunity for improvement.” In 2019, Mississippi managed to take that dishonor away from Louisiana, which had finished 50th in 2018. The two states have occupied the 49th and 50th spots in the rankings for the last 5 years, with Mississippi ahead 3-2 on 50th-place finishes, based on data from the AHR website.
Alaska (2019 rank, 27th), Virginia (15th), and Wyoming (19th) made the largest improvements, each moving up five spots since 2018, while Maine dropped from 16th to 21st for the largest decline among the states. A look back to the original rankings from 1990 puts New York on top of the list of improvers with a +29 over 30 years and shows Kansas to be the largest decliner with a change of –21, the report said.
At the national level, the report noted some key long-term health improvements and challenges:
- Smoking among adults is down 45% since 1990.
- Infant mortality declined by 43% and decreased in all 50 states.
- Diabetes prevalence has risen by 166% in adults since 1996.
- Obesity has increased by 166% since 1990.
The model used by AHR ranks states using 35 measures of public health in five broad categories: behaviors (Utah, 1st; La., 50th), community and environment (N.H., 1st; La. 50th), policy (Mass., 1st; Tex., 50th), clinical care (Mass., 1st; Miss. 50th), and health outcomes (Hawaii, 1st; Ala., 50th). Health measures include rates of excessive drinking, occupational fatalities, uninsured, preventable hospitalizations, and infant mortality.
America’s Health Rankings are produced by the American Public Health Association and the private, not-for-profit United Health Foundation, which was founded by UnitedHealth Group, operator of UnitedHealthcare.
The award for healthiest state goes to Vermont in 2019, marking the fifth time the Green Mountain State has taken the top spot in the 30-year span of America’s Health Rankings.

The New England states took 3 of the top 5 spots and 4 of the top 10, while last year’s winner, Hawaii, dropped to third and missed out on the title for only the second time in the last 8 years, according to the America’s Heath Rankings annual report.
Another rankings tradition lived on, however, as Mississippi and Louisiana continued their battle to be the state with the “greatest opportunity for improvement.” In 2019, Mississippi managed to take that dishonor away from Louisiana, which had finished 50th in 2018. The two states have occupied the 49th and 50th spots in the rankings for the last 5 years, with Mississippi ahead 3-2 on 50th-place finishes, based on data from the AHR website.
Alaska (2019 rank, 27th), Virginia (15th), and Wyoming (19th) made the largest improvements, each moving up five spots since 2018, while Maine dropped from 16th to 21st for the largest decline among the states. A look back to the original rankings from 1990 puts New York on top of the list of improvers with a +29 over 30 years and shows Kansas to be the largest decliner with a change of –21, the report said.
At the national level, the report noted some key long-term health improvements and challenges:
- Smoking among adults is down 45% since 1990.
- Infant mortality declined by 43% and decreased in all 50 states.
- Diabetes prevalence has risen by 166% in adults since 1996.
- Obesity has increased by 166% since 1990.
The model used by AHR ranks states using 35 measures of public health in five broad categories: behaviors (Utah, 1st; La., 50th), community and environment (N.H., 1st; La. 50th), policy (Mass., 1st; Tex., 50th), clinical care (Mass., 1st; Miss. 50th), and health outcomes (Hawaii, 1st; Ala., 50th). Health measures include rates of excessive drinking, occupational fatalities, uninsured, preventable hospitalizations, and infant mortality.
America’s Health Rankings are produced by the American Public Health Association and the private, not-for-profit United Health Foundation, which was founded by UnitedHealth Group, operator of UnitedHealthcare.
The award for healthiest state goes to Vermont in 2019, marking the fifth time the Green Mountain State has taken the top spot in the 30-year span of America’s Health Rankings.

The New England states took 3 of the top 5 spots and 4 of the top 10, while last year’s winner, Hawaii, dropped to third and missed out on the title for only the second time in the last 8 years, according to the America’s Heath Rankings annual report.
Another rankings tradition lived on, however, as Mississippi and Louisiana continued their battle to be the state with the “greatest opportunity for improvement.” In 2019, Mississippi managed to take that dishonor away from Louisiana, which had finished 50th in 2018. The two states have occupied the 49th and 50th spots in the rankings for the last 5 years, with Mississippi ahead 3-2 on 50th-place finishes, based on data from the AHR website.
Alaska (2019 rank, 27th), Virginia (15th), and Wyoming (19th) made the largest improvements, each moving up five spots since 2018, while Maine dropped from 16th to 21st for the largest decline among the states. A look back to the original rankings from 1990 puts New York on top of the list of improvers with a +29 over 30 years and shows Kansas to be the largest decliner with a change of –21, the report said.
At the national level, the report noted some key long-term health improvements and challenges:
- Smoking among adults is down 45% since 1990.
- Infant mortality declined by 43% and decreased in all 50 states.
- Diabetes prevalence has risen by 166% in adults since 1996.
- Obesity has increased by 166% since 1990.
The model used by AHR ranks states using 35 measures of public health in five broad categories: behaviors (Utah, 1st; La., 50th), community and environment (N.H., 1st; La. 50th), policy (Mass., 1st; Tex., 50th), clinical care (Mass., 1st; Miss. 50th), and health outcomes (Hawaii, 1st; Ala., 50th). Health measures include rates of excessive drinking, occupational fatalities, uninsured, preventable hospitalizations, and infant mortality.
America’s Health Rankings are produced by the American Public Health Association and the private, not-for-profit United Health Foundation, which was founded by UnitedHealth Group, operator of UnitedHealthcare.