User login
The measles comeback of 2019
Measles made a comeback in 2019.
The Centers for Disease Control and Prevention reported that, as of Dec. 5, 2019, 1,276 individual cases of measles of measles were confirmed in 31 states, the largest number since 1992. This number is a major uptick in cases, compared with previous years since 2000 when the CDC declared measles eliminated from the United States. No deaths have been reported for 2019.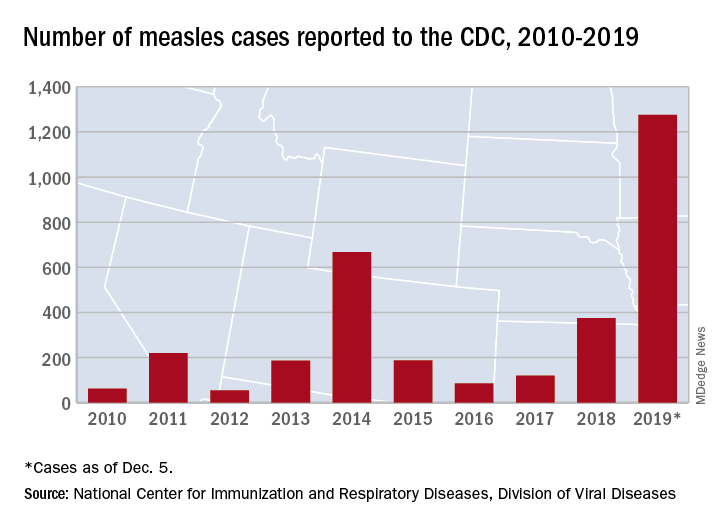
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York and occurred in primarily in underimmunized, close-knit communities and in patients with links to international travel. A total of 124 of the people who got measles this year were hospitalized, and 61 reported having complications, including pneumonia and encephalitis. The overall median patient age was 6 years (31% aged 1-4 years, 27% aged 5-17 years, and 29% aged at least 18 years).
The good news is that most of these cases occurred in unvaccinated patients. The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.7% for two doses of the MMR vaccine, falling just short of the CDC recommended 95% vaccination rate threshold. The CDC reported an approximate 2.5% rate of vaccination exemptions among school-age children.
The bad news is that, despite the high rate of MMR vaccination rates among U.S. children, there are gaps in measles protection in the U.S. population because of factors leaving patients immunocompromised and antivaccination sentiment that has led some parents to defer or refuse the MMR.
In addition, adults who were vaccinated prior to 1968 with either inactivated measles vaccine or measles vaccine of unknown type may have limited immunity. The inactivated measles vaccine, which was available in 1963-1967, did not achieve effective measles protection.
A global measles surge
While antivaccination sentiment contributed to the 2019 measles cases, a more significant factor may be the global surge of measles. More than 140,000 people worldwide died from measles in 2018, according to the World Health Organization and the CDC.
“[Recent data on measles] indicates that during the first 6 months of the year there have been more measles cases reported worldwide than in any year since 2006. From Jan. 1 to July 31, 2019, 182 countries reported 364,808 measles cases to the WHO. This surpasses the 129,239 reported during the same time period in 2018. WHO regions with the biggest increases in cases include the African region (900%), the Western Pacific region (230%), and the European region (150%),” according to a CDC report.
Studies on hospitalization and complications linked to measles in the United States are scarce, but two outbreaks in Minnesota (2011 and 2017) provided some data on what to expect if the measles surge continues into 2020. The investigators found that poor feeding was a primary reason for admission (97%); additional complications included otitis media (42%), pneumonia (30%), and tracheitis (6%). Three-quarters received antibiotics, 30% required oxygen, and 21% received vitamin A. Median length of stay was 3.7 days (range, 1.1-26.2 days) (Pediatr Infect Dis J. 2019 Jun;38[6]:547-52. doi: 10.1097/INF.0000000000002221).
‘Immunological amnesia’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science (2019 Nov 1;366[6465]599-606).
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
Maternal-acquired immunity fades
In another study of measles immunity, maternal antibodies were found to be insufficient to provide immunity to infants after 6 months.
The study of 196 infants showed that maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics (2019 Dec 1. doi 10.1542/peds.2019-0630).
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days), 2 months (61-89 days), 3 months (90-119 days), 4 months, 5 months, 6-9 months, and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay, because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL), and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Huong Q. McLean, PhD, of Marshfield (Wisc.) Clinic Research Institute, and Walter A. Orenstein, MD, of Emory University, Atlanta, noted in an editorial.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Bianca Nogrady and Tara Haelle contributed to this story.
Measles made a comeback in 2019.
The Centers for Disease Control and Prevention reported that, as of Dec. 5, 2019, 1,276 individual cases of measles of measles were confirmed in 31 states, the largest number since 1992. This number is a major uptick in cases, compared with previous years since 2000 when the CDC declared measles eliminated from the United States. No deaths have been reported for 2019.
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York and occurred in primarily in underimmunized, close-knit communities and in patients with links to international travel. A total of 124 of the people who got measles this year were hospitalized, and 61 reported having complications, including pneumonia and encephalitis. The overall median patient age was 6 years (31% aged 1-4 years, 27% aged 5-17 years, and 29% aged at least 18 years).
The good news is that most of these cases occurred in unvaccinated patients. The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.7% for two doses of the MMR vaccine, falling just short of the CDC recommended 95% vaccination rate threshold. The CDC reported an approximate 2.5% rate of vaccination exemptions among school-age children.
The bad news is that, despite the high rate of MMR vaccination rates among U.S. children, there are gaps in measles protection in the U.S. population because of factors leaving patients immunocompromised and antivaccination sentiment that has led some parents to defer or refuse the MMR.
In addition, adults who were vaccinated prior to 1968 with either inactivated measles vaccine or measles vaccine of unknown type may have limited immunity. The inactivated measles vaccine, which was available in 1963-1967, did not achieve effective measles protection.
A global measles surge
While antivaccination sentiment contributed to the 2019 measles cases, a more significant factor may be the global surge of measles. More than 140,000 people worldwide died from measles in 2018, according to the World Health Organization and the CDC.
“[Recent data on measles] indicates that during the first 6 months of the year there have been more measles cases reported worldwide than in any year since 2006. From Jan. 1 to July 31, 2019, 182 countries reported 364,808 measles cases to the WHO. This surpasses the 129,239 reported during the same time period in 2018. WHO regions with the biggest increases in cases include the African region (900%), the Western Pacific region (230%), and the European region (150%),” according to a CDC report.
Studies on hospitalization and complications linked to measles in the United States are scarce, but two outbreaks in Minnesota (2011 and 2017) provided some data on what to expect if the measles surge continues into 2020. The investigators found that poor feeding was a primary reason for admission (97%); additional complications included otitis media (42%), pneumonia (30%), and tracheitis (6%). Three-quarters received antibiotics, 30% required oxygen, and 21% received vitamin A. Median length of stay was 3.7 days (range, 1.1-26.2 days) (Pediatr Infect Dis J. 2019 Jun;38[6]:547-52. doi: 10.1097/INF.0000000000002221).
‘Immunological amnesia’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science (2019 Nov 1;366[6465]599-606).
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
Maternal-acquired immunity fades
In another study of measles immunity, maternal antibodies were found to be insufficient to provide immunity to infants after 6 months.
The study of 196 infants showed that maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics (2019 Dec 1. doi 10.1542/peds.2019-0630).
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days), 2 months (61-89 days), 3 months (90-119 days), 4 months, 5 months, 6-9 months, and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay, because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL), and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Huong Q. McLean, PhD, of Marshfield (Wisc.) Clinic Research Institute, and Walter A. Orenstein, MD, of Emory University, Atlanta, noted in an editorial.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Bianca Nogrady and Tara Haelle contributed to this story.
Measles made a comeback in 2019.
The Centers for Disease Control and Prevention reported that, as of Dec. 5, 2019, 1,276 individual cases of measles of measles were confirmed in 31 states, the largest number since 1992. This number is a major uptick in cases, compared with previous years since 2000 when the CDC declared measles eliminated from the United States. No deaths have been reported for 2019.
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York and occurred in primarily in underimmunized, close-knit communities and in patients with links to international travel. A total of 124 of the people who got measles this year were hospitalized, and 61 reported having complications, including pneumonia and encephalitis. The overall median patient age was 6 years (31% aged 1-4 years, 27% aged 5-17 years, and 29% aged at least 18 years).
The good news is that most of these cases occurred in unvaccinated patients. The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.7% for two doses of the MMR vaccine, falling just short of the CDC recommended 95% vaccination rate threshold. The CDC reported an approximate 2.5% rate of vaccination exemptions among school-age children.
The bad news is that, despite the high rate of MMR vaccination rates among U.S. children, there are gaps in measles protection in the U.S. population because of factors leaving patients immunocompromised and antivaccination sentiment that has led some parents to defer or refuse the MMR.
In addition, adults who were vaccinated prior to 1968 with either inactivated measles vaccine or measles vaccine of unknown type may have limited immunity. The inactivated measles vaccine, which was available in 1963-1967, did not achieve effective measles protection.
A global measles surge
While antivaccination sentiment contributed to the 2019 measles cases, a more significant factor may be the global surge of measles. More than 140,000 people worldwide died from measles in 2018, according to the World Health Organization and the CDC.
“[Recent data on measles] indicates that during the first 6 months of the year there have been more measles cases reported worldwide than in any year since 2006. From Jan. 1 to July 31, 2019, 182 countries reported 364,808 measles cases to the WHO. This surpasses the 129,239 reported during the same time period in 2018. WHO regions with the biggest increases in cases include the African region (900%), the Western Pacific region (230%), and the European region (150%),” according to a CDC report.
Studies on hospitalization and complications linked to measles in the United States are scarce, but two outbreaks in Minnesota (2011 and 2017) provided some data on what to expect if the measles surge continues into 2020. The investigators found that poor feeding was a primary reason for admission (97%); additional complications included otitis media (42%), pneumonia (30%), and tracheitis (6%). Three-quarters received antibiotics, 30% required oxygen, and 21% received vitamin A. Median length of stay was 3.7 days (range, 1.1-26.2 days) (Pediatr Infect Dis J. 2019 Jun;38[6]:547-52. doi: 10.1097/INF.0000000000002221).
‘Immunological amnesia’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science (2019 Nov 1;366[6465]599-606).
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
Maternal-acquired immunity fades
In another study of measles immunity, maternal antibodies were found to be insufficient to provide immunity to infants after 6 months.
The study of 196 infants showed that maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics (2019 Dec 1. doi 10.1542/peds.2019-0630).
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days), 2 months (61-89 days), 3 months (90-119 days), 4 months, 5 months, 6-9 months, and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay, because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL), and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Huong Q. McLean, PhD, of Marshfield (Wisc.) Clinic Research Institute, and Walter A. Orenstein, MD, of Emory University, Atlanta, noted in an editorial.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Bianca Nogrady and Tara Haelle contributed to this story.
FDA okays first generics for Eliquis
The Food and Drug Administration has approved two applications for first generic versions of apixaban (Eliquis, Bristol-Myers Squibb/Pfizer) tablets to reduce the risk for stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
The FDA gave the go-ahead to market generic versions of apixaban to Micro Labs Limited and Mylan Pharmaceuticals.
“Today’s approvals of the first generics of apixaban are an example of how the FDA’s generic drug program improves access to lower-cost, safe, and high-quality medicines,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a statement today. “These approvals mark the first generic approvals of a direct oral anticoagulant.”
It is estimated that between 2.7 and 6.1 million people in the United States have atrial fibrillation. Many of these individuals use anticoagulants or anticlotting drugs to reduce that risk. Direct oral anticoagulants, however, do not require repeated blood testing.
Apixaban was approved by the FDA in December 2012 for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Additional indications in the United States are to treat and prevent the recurrence of deep vein thrombosis (DVT) and pulmonary embolism (PE) and as DVT/PE prophylaxis in adults who have undergone hip or knee replacement surgery.
The FDA reminds providers that, as with brand name apixaban, generic versions must be dispensed with a medication guide that provides important instructions on the drug’s uses and risks. Healthcare professionals should counsel patients on signs and symptoms of possible bleeding.
As with other FDA-approved anticlotting drugs, bleeding, including life-threatening and fatal bleeding, is the most serious risk with apixaban.
Full prescribing information for the drug also warns about the increased risk for stroke in patients who discontinue use of the drug without taking some other form of anticoagulation. Epidural or spinal hematoma, which may cause long-term or permanent paralysis, may occur in patients treated with apixaban who are undergoing spinal epidural anesthesia or spinal puncture.
This story first appeared on Medscape.com.
The Food and Drug Administration has approved two applications for first generic versions of apixaban (Eliquis, Bristol-Myers Squibb/Pfizer) tablets to reduce the risk for stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
The FDA gave the go-ahead to market generic versions of apixaban to Micro Labs Limited and Mylan Pharmaceuticals.
“Today’s approvals of the first generics of apixaban are an example of how the FDA’s generic drug program improves access to lower-cost, safe, and high-quality medicines,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a statement today. “These approvals mark the first generic approvals of a direct oral anticoagulant.”
It is estimated that between 2.7 and 6.1 million people in the United States have atrial fibrillation. Many of these individuals use anticoagulants or anticlotting drugs to reduce that risk. Direct oral anticoagulants, however, do not require repeated blood testing.
Apixaban was approved by the FDA in December 2012 for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Additional indications in the United States are to treat and prevent the recurrence of deep vein thrombosis (DVT) and pulmonary embolism (PE) and as DVT/PE prophylaxis in adults who have undergone hip or knee replacement surgery.
The FDA reminds providers that, as with brand name apixaban, generic versions must be dispensed with a medication guide that provides important instructions on the drug’s uses and risks. Healthcare professionals should counsel patients on signs and symptoms of possible bleeding.
As with other FDA-approved anticlotting drugs, bleeding, including life-threatening and fatal bleeding, is the most serious risk with apixaban.
Full prescribing information for the drug also warns about the increased risk for stroke in patients who discontinue use of the drug without taking some other form of anticoagulation. Epidural or spinal hematoma, which may cause long-term or permanent paralysis, may occur in patients treated with apixaban who are undergoing spinal epidural anesthesia or spinal puncture.
This story first appeared on Medscape.com.
The Food and Drug Administration has approved two applications for first generic versions of apixaban (Eliquis, Bristol-Myers Squibb/Pfizer) tablets to reduce the risk for stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
The FDA gave the go-ahead to market generic versions of apixaban to Micro Labs Limited and Mylan Pharmaceuticals.
“Today’s approvals of the first generics of apixaban are an example of how the FDA’s generic drug program improves access to lower-cost, safe, and high-quality medicines,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a statement today. “These approvals mark the first generic approvals of a direct oral anticoagulant.”
It is estimated that between 2.7 and 6.1 million people in the United States have atrial fibrillation. Many of these individuals use anticoagulants or anticlotting drugs to reduce that risk. Direct oral anticoagulants, however, do not require repeated blood testing.
Apixaban was approved by the FDA in December 2012 for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Additional indications in the United States are to treat and prevent the recurrence of deep vein thrombosis (DVT) and pulmonary embolism (PE) and as DVT/PE prophylaxis in adults who have undergone hip or knee replacement surgery.
The FDA reminds providers that, as with brand name apixaban, generic versions must be dispensed with a medication guide that provides important instructions on the drug’s uses and risks. Healthcare professionals should counsel patients on signs and symptoms of possible bleeding.
As with other FDA-approved anticlotting drugs, bleeding, including life-threatening and fatal bleeding, is the most serious risk with apixaban.
Full prescribing information for the drug also warns about the increased risk for stroke in patients who discontinue use of the drug without taking some other form of anticoagulation. Epidural or spinal hematoma, which may cause long-term or permanent paralysis, may occur in patients treated with apixaban who are undergoing spinal epidural anesthesia or spinal puncture.
This story first appeared on Medscape.com.
ctDNA shows clinical value in advanced breast cancer
SAN ANTONIO – The high accuracy and efficiency of circulating tumor DNA (ctDNA) testing allows for routine clinical use in advanced breast cancer, according to investigators.
The plasmaMATCH trial showed that gene level agreement between ctDNA results measured by digital PCR versus sequencing was as high as 99.4%, reported lead author Nicholas Turner, MA, MRCP, PhD, of The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, London.
Dr. Turner, who presented findings at the San Antonio Breast Cancer Symposium, said that ctDNA testing can detect rare mutations and link patients with targeted therapies that have clinically relevant response rates.
“Multiple somatic mutations are potentially targetable in the treatment of advanced breast cancer,” Dr. Turner said. “In addition, mutations may be acquired [during treatment].”
The diverse and dynamic landscape of mutations in breast cancer creates a need to genotype tumors without repeating biopsies, Dr. Turner said. He noted that ctDNA is one possible means of fulfilling this need, although more prospective research is required to determine clinical utility.
To this end, the investigators conducted the phase II plasmaMATCH trial, a multiple parallel cohort, multicenter study involving 1,044 patients with advanced breast cancer. All patients had ctDNA testing performed prospectively with digital droplet PCR (ddPCR); in addition, ctDNA testing was performed with error-corrected sequencing using Guardant360, either prospectively or retrospectively. If actionable mutations were identified, and consent was provided, then patients entered the treatment cohort, which was composed of 142 participants.
Patients were divided into four parallel treatment cohorts based on ctDNA mutation results and accompanying treatments, as follows:
- (A) ESR1 mutation; extended-dose fulvestrant.
- (B) HER2 mutation; neratinib with or without fulvestrant.
- (C) AKT1 in estrogen receptor–positive disease; capivasertib plus fulvestrant.
- (D) “AKT basket” – AKT1 in estrogen receptor–negative disease or PTEN inactivating mutation; capivasertib.
The primary objective was response rate. For cohort A, at least 13 out of 78 evaluable patients (17%) needed to have a response to infer sufficient efficacy of the matched therapy. For the remaining cohorts, sufficient efficacy was defined by responses in at least 3 out of 16 evaluable patients (19%).
Secondary objectives included frequency of targetable mutations, accuracy of ctDNA testing (to be reported later), and others.
Results showed that ESR1 mutations were most common within the original population (27.7%), followed by AKT1 mutations (4.2%) and HER2 mutations (2.7%). In the treatment cohort, more than half of the patients had a HER2 mutation (58%) and/or an AKT1 mutation (54%), whereas a smaller proportion had an ESR1 mutation (38%). Approximately two-thirds of patients (64%) had hormone receptor–positive, HER2-negative breast cancer; 17% had triple-negative breast cancer; 6% had hormone receptor–positive, HER2-positive disease; 3% had hormone receptor–negative, HER2-positive disease; and 9% had other/unknown phenotypes. Approximately two-thirds of patients (65%) had received at least two lines of prior therapy for advanced disease.
For patients with an ESR1 mutation treated with extended-dose fulvestrant (cohort A) only 8.1% achieved a response, which was below the threshold for inferred efficacy. For patients with a HER2 mutation treated with neratinib with or without fulvestrant (cohort B), 25.0% had a response, thereby demonstrating inferred efficacy. Efficacy was also inferred in patients with an AKT1 mutation treated with capivasertib plus fulvestrant (cohort C), as 22.2% of these patients had a response. In the AKT basket (cohort D), 10.5% of patients had a response, which fell below the efficacy threshold; however, an exploratory analysis of this cohort showed that patients with an AKT1 mutation had a response rate of 33.3% (two out of six patients), which did meet efficacy criteria.
Adverse events were consistent with previous reports. The investigators noted that extended-dose fulvestrant was well tolerated.
“In conclusion, we show that circulating tumor DNA testing offers a simple, efficient and relatively fast method of tumor genotyping,” Dr. Turner said.
The investigators disclosed relationships with Puma Biotechnology, AstraZeneca, Guardant Health, and Bio-Rad.
SOURCE: Turner et al. SABCS. 2019 Dec 12. Abstract GS3-06.
SAN ANTONIO – The high accuracy and efficiency of circulating tumor DNA (ctDNA) testing allows for routine clinical use in advanced breast cancer, according to investigators.
The plasmaMATCH trial showed that gene level agreement between ctDNA results measured by digital PCR versus sequencing was as high as 99.4%, reported lead author Nicholas Turner, MA, MRCP, PhD, of The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, London.
Dr. Turner, who presented findings at the San Antonio Breast Cancer Symposium, said that ctDNA testing can detect rare mutations and link patients with targeted therapies that have clinically relevant response rates.
“Multiple somatic mutations are potentially targetable in the treatment of advanced breast cancer,” Dr. Turner said. “In addition, mutations may be acquired [during treatment].”
The diverse and dynamic landscape of mutations in breast cancer creates a need to genotype tumors without repeating biopsies, Dr. Turner said. He noted that ctDNA is one possible means of fulfilling this need, although more prospective research is required to determine clinical utility.
To this end, the investigators conducted the phase II plasmaMATCH trial, a multiple parallel cohort, multicenter study involving 1,044 patients with advanced breast cancer. All patients had ctDNA testing performed prospectively with digital droplet PCR (ddPCR); in addition, ctDNA testing was performed with error-corrected sequencing using Guardant360, either prospectively or retrospectively. If actionable mutations were identified, and consent was provided, then patients entered the treatment cohort, which was composed of 142 participants.
Patients were divided into four parallel treatment cohorts based on ctDNA mutation results and accompanying treatments, as follows:
- (A) ESR1 mutation; extended-dose fulvestrant.
- (B) HER2 mutation; neratinib with or without fulvestrant.
- (C) AKT1 in estrogen receptor–positive disease; capivasertib plus fulvestrant.
- (D) “AKT basket” – AKT1 in estrogen receptor–negative disease or PTEN inactivating mutation; capivasertib.
The primary objective was response rate. For cohort A, at least 13 out of 78 evaluable patients (17%) needed to have a response to infer sufficient efficacy of the matched therapy. For the remaining cohorts, sufficient efficacy was defined by responses in at least 3 out of 16 evaluable patients (19%).
Secondary objectives included frequency of targetable mutations, accuracy of ctDNA testing (to be reported later), and others.
Results showed that ESR1 mutations were most common within the original population (27.7%), followed by AKT1 mutations (4.2%) and HER2 mutations (2.7%). In the treatment cohort, more than half of the patients had a HER2 mutation (58%) and/or an AKT1 mutation (54%), whereas a smaller proportion had an ESR1 mutation (38%). Approximately two-thirds of patients (64%) had hormone receptor–positive, HER2-negative breast cancer; 17% had triple-negative breast cancer; 6% had hormone receptor–positive, HER2-positive disease; 3% had hormone receptor–negative, HER2-positive disease; and 9% had other/unknown phenotypes. Approximately two-thirds of patients (65%) had received at least two lines of prior therapy for advanced disease.
For patients with an ESR1 mutation treated with extended-dose fulvestrant (cohort A) only 8.1% achieved a response, which was below the threshold for inferred efficacy. For patients with a HER2 mutation treated with neratinib with or without fulvestrant (cohort B), 25.0% had a response, thereby demonstrating inferred efficacy. Efficacy was also inferred in patients with an AKT1 mutation treated with capivasertib plus fulvestrant (cohort C), as 22.2% of these patients had a response. In the AKT basket (cohort D), 10.5% of patients had a response, which fell below the efficacy threshold; however, an exploratory analysis of this cohort showed that patients with an AKT1 mutation had a response rate of 33.3% (two out of six patients), which did meet efficacy criteria.
Adverse events were consistent with previous reports. The investigators noted that extended-dose fulvestrant was well tolerated.
“In conclusion, we show that circulating tumor DNA testing offers a simple, efficient and relatively fast method of tumor genotyping,” Dr. Turner said.
The investigators disclosed relationships with Puma Biotechnology, AstraZeneca, Guardant Health, and Bio-Rad.
SOURCE: Turner et al. SABCS. 2019 Dec 12. Abstract GS3-06.
SAN ANTONIO – The high accuracy and efficiency of circulating tumor DNA (ctDNA) testing allows for routine clinical use in advanced breast cancer, according to investigators.
The plasmaMATCH trial showed that gene level agreement between ctDNA results measured by digital PCR versus sequencing was as high as 99.4%, reported lead author Nicholas Turner, MA, MRCP, PhD, of The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, London.
Dr. Turner, who presented findings at the San Antonio Breast Cancer Symposium, said that ctDNA testing can detect rare mutations and link patients with targeted therapies that have clinically relevant response rates.
“Multiple somatic mutations are potentially targetable in the treatment of advanced breast cancer,” Dr. Turner said. “In addition, mutations may be acquired [during treatment].”
The diverse and dynamic landscape of mutations in breast cancer creates a need to genotype tumors without repeating biopsies, Dr. Turner said. He noted that ctDNA is one possible means of fulfilling this need, although more prospective research is required to determine clinical utility.
To this end, the investigators conducted the phase II plasmaMATCH trial, a multiple parallel cohort, multicenter study involving 1,044 patients with advanced breast cancer. All patients had ctDNA testing performed prospectively with digital droplet PCR (ddPCR); in addition, ctDNA testing was performed with error-corrected sequencing using Guardant360, either prospectively or retrospectively. If actionable mutations were identified, and consent was provided, then patients entered the treatment cohort, which was composed of 142 participants.
Patients were divided into four parallel treatment cohorts based on ctDNA mutation results and accompanying treatments, as follows:
- (A) ESR1 mutation; extended-dose fulvestrant.
- (B) HER2 mutation; neratinib with or without fulvestrant.
- (C) AKT1 in estrogen receptor–positive disease; capivasertib plus fulvestrant.
- (D) “AKT basket” – AKT1 in estrogen receptor–negative disease or PTEN inactivating mutation; capivasertib.
The primary objective was response rate. For cohort A, at least 13 out of 78 evaluable patients (17%) needed to have a response to infer sufficient efficacy of the matched therapy. For the remaining cohorts, sufficient efficacy was defined by responses in at least 3 out of 16 evaluable patients (19%).
Secondary objectives included frequency of targetable mutations, accuracy of ctDNA testing (to be reported later), and others.
Results showed that ESR1 mutations were most common within the original population (27.7%), followed by AKT1 mutations (4.2%) and HER2 mutations (2.7%). In the treatment cohort, more than half of the patients had a HER2 mutation (58%) and/or an AKT1 mutation (54%), whereas a smaller proportion had an ESR1 mutation (38%). Approximately two-thirds of patients (64%) had hormone receptor–positive, HER2-negative breast cancer; 17% had triple-negative breast cancer; 6% had hormone receptor–positive, HER2-positive disease; 3% had hormone receptor–negative, HER2-positive disease; and 9% had other/unknown phenotypes. Approximately two-thirds of patients (65%) had received at least two lines of prior therapy for advanced disease.
For patients with an ESR1 mutation treated with extended-dose fulvestrant (cohort A) only 8.1% achieved a response, which was below the threshold for inferred efficacy. For patients with a HER2 mutation treated with neratinib with or without fulvestrant (cohort B), 25.0% had a response, thereby demonstrating inferred efficacy. Efficacy was also inferred in patients with an AKT1 mutation treated with capivasertib plus fulvestrant (cohort C), as 22.2% of these patients had a response. In the AKT basket (cohort D), 10.5% of patients had a response, which fell below the efficacy threshold; however, an exploratory analysis of this cohort showed that patients with an AKT1 mutation had a response rate of 33.3% (two out of six patients), which did meet efficacy criteria.
Adverse events were consistent with previous reports. The investigators noted that extended-dose fulvestrant was well tolerated.
“In conclusion, we show that circulating tumor DNA testing offers a simple, efficient and relatively fast method of tumor genotyping,” Dr. Turner said.
The investigators disclosed relationships with Puma Biotechnology, AstraZeneca, Guardant Health, and Bio-Rad.
SOURCE: Turner et al. SABCS. 2019 Dec 12. Abstract GS3-06.
REPORTING FROM SABCS 2019
Study Supports Vertigo as “Integral Manifestation” of Migraine, Rather Than Symptom
Key Points:
- The “Migraine and Neck Pain Study” analyzed data from nearly 500 adult participants in an effort to uncover an association between migraine-related episodic vertigo and the phases of migraine.
- The study participants included men and women aged 18 to 65, who had episodic migraine with aura and/or without aura.
- Migraines were divided into 3 time segments for evaluation: (1) Onset of headache, (2) less than 2 hours before the onset of headache, and (3) 2 to 48 hours before the onset of headache.
- 30% of participants reported episodic vertigo at any point during their migraine attack, while 16% reported it at the start of headache, 10% reported it within 2 hours before their headache, and just 3% reported symptoms between 2 and 24 hours beforehand.
- The study concluded that episodic vertigo could be considered more of a “headache phase phenomenon” rather than a prodromal symptom.
Alan M. Rapoport, MD:
Vertigo in a migraineur has long created confusion as to diagnosis and treatment. I myself always wondered how much I had to work up vertigo or even dizziness if a patient had migraine. I also did not know what to do when a patient with migraine had attacks of vertigo without headache. Were they manifestations of migraine and should they be treated that way?
This study examined a 500 adult patient population who had migraine with or without aura. Christian Lampl was interested in seeing how many had headache, and the timing of when vertigo occurred. It was carefully measured to determine if it usually occurred during or before the headache phase. Migraines were divided into 3 time segments for evaluation: (1) Onset of headache, (2) less than 2 hours before the onset of headache, and (3) 2 to 48 hours before the onset of headache, when prodrome occurs.
- The study determined that 30 % of the patients reported vertigo at some point during their migraine attack; 16% reported it at the start of headache, 10% reported it within 2 hours before their headache, and just 3% reported symptoms between 2 and 24 hours beforehand., which would have been in the prodromal phase.
- The study concluded that episodic vertigo could be considered more of a “headache phase phenomenon” rather than a prodromal symptom. This was interesting but it left unanswered one of my questions which is, how many had vertigo unrelated to headache and what is that and how do we treat it.
- Although not addressed in this study, there is consensus that if there is enough vertigo in a migraineur, they should be placed on a migraine preventive therapy. It will be interesting to see what the new monoclonal antibodies to CGRP do to vertigo in a treated migraineur. Some headache specialists will even treat an attack of vertigo without headache with a triptan.
Key Points:
- The “Migraine and Neck Pain Study” analyzed data from nearly 500 adult participants in an effort to uncover an association between migraine-related episodic vertigo and the phases of migraine.
- The study participants included men and women aged 18 to 65, who had episodic migraine with aura and/or without aura.
- Migraines were divided into 3 time segments for evaluation: (1) Onset of headache, (2) less than 2 hours before the onset of headache, and (3) 2 to 48 hours before the onset of headache.
- 30% of participants reported episodic vertigo at any point during their migraine attack, while 16% reported it at the start of headache, 10% reported it within 2 hours before their headache, and just 3% reported symptoms between 2 and 24 hours beforehand.
- The study concluded that episodic vertigo could be considered more of a “headache phase phenomenon” rather than a prodromal symptom.
Alan M. Rapoport, MD:
Vertigo in a migraineur has long created confusion as to diagnosis and treatment. I myself always wondered how much I had to work up vertigo or even dizziness if a patient had migraine. I also did not know what to do when a patient with migraine had attacks of vertigo without headache. Were they manifestations of migraine and should they be treated that way?
This study examined a 500 adult patient population who had migraine with or without aura. Christian Lampl was interested in seeing how many had headache, and the timing of when vertigo occurred. It was carefully measured to determine if it usually occurred during or before the headache phase. Migraines were divided into 3 time segments for evaluation: (1) Onset of headache, (2) less than 2 hours before the onset of headache, and (3) 2 to 48 hours before the onset of headache, when prodrome occurs.
- The study determined that 30 % of the patients reported vertigo at some point during their migraine attack; 16% reported it at the start of headache, 10% reported it within 2 hours before their headache, and just 3% reported symptoms between 2 and 24 hours beforehand., which would have been in the prodromal phase.
- The study concluded that episodic vertigo could be considered more of a “headache phase phenomenon” rather than a prodromal symptom. This was interesting but it left unanswered one of my questions which is, how many had vertigo unrelated to headache and what is that and how do we treat it.
- Although not addressed in this study, there is consensus that if there is enough vertigo in a migraineur, they should be placed on a migraine preventive therapy. It will be interesting to see what the new monoclonal antibodies to CGRP do to vertigo in a treated migraineur. Some headache specialists will even treat an attack of vertigo without headache with a triptan.
Key Points:
- The “Migraine and Neck Pain Study” analyzed data from nearly 500 adult participants in an effort to uncover an association between migraine-related episodic vertigo and the phases of migraine.
- The study participants included men and women aged 18 to 65, who had episodic migraine with aura and/or without aura.
- Migraines were divided into 3 time segments for evaluation: (1) Onset of headache, (2) less than 2 hours before the onset of headache, and (3) 2 to 48 hours before the onset of headache.
- 30% of participants reported episodic vertigo at any point during their migraine attack, while 16% reported it at the start of headache, 10% reported it within 2 hours before their headache, and just 3% reported symptoms between 2 and 24 hours beforehand.
- The study concluded that episodic vertigo could be considered more of a “headache phase phenomenon” rather than a prodromal symptom.
Alan M. Rapoport, MD:
Vertigo in a migraineur has long created confusion as to diagnosis and treatment. I myself always wondered how much I had to work up vertigo or even dizziness if a patient had migraine. I also did not know what to do when a patient with migraine had attacks of vertigo without headache. Were they manifestations of migraine and should they be treated that way?
This study examined a 500 adult patient population who had migraine with or without aura. Christian Lampl was interested in seeing how many had headache, and the timing of when vertigo occurred. It was carefully measured to determine if it usually occurred during or before the headache phase. Migraines were divided into 3 time segments for evaluation: (1) Onset of headache, (2) less than 2 hours before the onset of headache, and (3) 2 to 48 hours before the onset of headache, when prodrome occurs.
- The study determined that 30 % of the patients reported vertigo at some point during their migraine attack; 16% reported it at the start of headache, 10% reported it within 2 hours before their headache, and just 3% reported symptoms between 2 and 24 hours beforehand., which would have been in the prodromal phase.
- The study concluded that episodic vertigo could be considered more of a “headache phase phenomenon” rather than a prodromal symptom. This was interesting but it left unanswered one of my questions which is, how many had vertigo unrelated to headache and what is that and how do we treat it.
- Although not addressed in this study, there is consensus that if there is enough vertigo in a migraineur, they should be placed on a migraine preventive therapy. It will be interesting to see what the new monoclonal antibodies to CGRP do to vertigo in a treated migraineur. Some headache specialists will even treat an attack of vertigo without headache with a triptan.
Urothelial cancer: Less gained from immunotherapy in patients with poor performance scores
Patients with advanced urothelial cancer and a poor performance status may have little to gain from immune checkpoint inhibitor therapy, suggests a multicenter real-world retrospective cohort study.
“The perceived favorable toxicity profile of immune checkpoint inhibitors has led to the selection of these agents for patients otherwise unfit for systemic chemotherapy,” noted the investigators, led by Ali Raza Khaki, MD, a fellow in the division of oncology, department of medicine, University of Washington, Seattle. “However, there is a paucity of data supporting the use of immune checkpoint inhibitors in patients with a poor performance status, who were not very well represented in the clinical trials that led to their approval, with no trial enrolling patients with an ECOG [Eastern Cooperative Oncology Group] performance status greater than or equal to 3 and only 3 trials including patients with an ECOG performance status of 2.”
Dr. Khaki and coinvestigators analyzed data from 499 patients with advanced urothelial cancer treated with immune checkpoint inhibitors at 18 institutions during 2013-2019. Slightly more than one-quarter had an ECOG performance status of 2 or higher.
Study results, reported in Cancer, showed that the overall response rate to immune checkpoint inhibitor therapy was similar regardless of performance status, across patients being treated in different lines of therapy.
However, among patients being treated in the first line, overall survival was better for those with a performance status of 0 to 1 than for those with a performance status of 2 or higher (median, 15.2 vs. 7.2 months; hazard ratio for death, 0.62; P = .01). There was no significant difference in this outcome among patients being treated in subsequent lines (median, 9.8 vs. 8.2 months; hazard ratio, 0.78; P = .27).
Among the 288 patients who died, 10% started immune checkpoint inhibitors in the last 30 days of life and 33% started them in the last 90 days of life. Patients initiating this therapy in the last 30 days of life were almost three time more likely to die in a hospital (odds ratio, 2.89; P = .04).
Notably, among the 11 patients with an ECOG performance status of 3, none had a response to immune checkpoint inhibitors and only two achieved stable disease. Moreover, two patients in this subgroup died within a week of receiving these agents.
“Despite comparable overall response rates, immune checkpoint inhibitors may not overcome the negative prognostic role of a poor performance status, particularly in the first-line setting,” Dr. Khaki and coinvestigators wrote. “Our study underscores the importance of developing prospectively validated predictive biomarkers to aid in identifying those patients most and least likely to benefit from immune checkpoint inhibitors.
“Overall, our data suggest that the decision of immune checkpoint inhibitor initiation near the end of life, akin to the practice for chemotherapy, should be considered carefully, and it should be accompanied by a detailed discussion of the data, rationale, and risks and benefits to minimize unnecessary potential adverse events and the cost and intensity of end-of-life care,” they recommended.
Dr. Khaki did not disclose any conflicts of interest. The study was supported by the National Cancer Institute, the National Center for Advancing Translational Sciences of the National Institutes of Health, the Seattle Translational Tumor Research Program at the Fred Hutchinson Cancer Research Center, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, the Wellcome Trust Strategic Fund, and Merck.
SOURCE: Khaki AR et al. Cancer. 2019 Dec 12. doi: 10.1002/cncr.32645.
Patients with advanced urothelial cancer and a poor performance status may have little to gain from immune checkpoint inhibitor therapy, suggests a multicenter real-world retrospective cohort study.
“The perceived favorable toxicity profile of immune checkpoint inhibitors has led to the selection of these agents for patients otherwise unfit for systemic chemotherapy,” noted the investigators, led by Ali Raza Khaki, MD, a fellow in the division of oncology, department of medicine, University of Washington, Seattle. “However, there is a paucity of data supporting the use of immune checkpoint inhibitors in patients with a poor performance status, who were not very well represented in the clinical trials that led to their approval, with no trial enrolling patients with an ECOG [Eastern Cooperative Oncology Group] performance status greater than or equal to 3 and only 3 trials including patients with an ECOG performance status of 2.”
Dr. Khaki and coinvestigators analyzed data from 499 patients with advanced urothelial cancer treated with immune checkpoint inhibitors at 18 institutions during 2013-2019. Slightly more than one-quarter had an ECOG performance status of 2 or higher.
Study results, reported in Cancer, showed that the overall response rate to immune checkpoint inhibitor therapy was similar regardless of performance status, across patients being treated in different lines of therapy.
However, among patients being treated in the first line, overall survival was better for those with a performance status of 0 to 1 than for those with a performance status of 2 or higher (median, 15.2 vs. 7.2 months; hazard ratio for death, 0.62; P = .01). There was no significant difference in this outcome among patients being treated in subsequent lines (median, 9.8 vs. 8.2 months; hazard ratio, 0.78; P = .27).
Among the 288 patients who died, 10% started immune checkpoint inhibitors in the last 30 days of life and 33% started them in the last 90 days of life. Patients initiating this therapy in the last 30 days of life were almost three time more likely to die in a hospital (odds ratio, 2.89; P = .04).
Notably, among the 11 patients with an ECOG performance status of 3, none had a response to immune checkpoint inhibitors and only two achieved stable disease. Moreover, two patients in this subgroup died within a week of receiving these agents.
“Despite comparable overall response rates, immune checkpoint inhibitors may not overcome the negative prognostic role of a poor performance status, particularly in the first-line setting,” Dr. Khaki and coinvestigators wrote. “Our study underscores the importance of developing prospectively validated predictive biomarkers to aid in identifying those patients most and least likely to benefit from immune checkpoint inhibitors.
“Overall, our data suggest that the decision of immune checkpoint inhibitor initiation near the end of life, akin to the practice for chemotherapy, should be considered carefully, and it should be accompanied by a detailed discussion of the data, rationale, and risks and benefits to minimize unnecessary potential adverse events and the cost and intensity of end-of-life care,” they recommended.
Dr. Khaki did not disclose any conflicts of interest. The study was supported by the National Cancer Institute, the National Center for Advancing Translational Sciences of the National Institutes of Health, the Seattle Translational Tumor Research Program at the Fred Hutchinson Cancer Research Center, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, the Wellcome Trust Strategic Fund, and Merck.
SOURCE: Khaki AR et al. Cancer. 2019 Dec 12. doi: 10.1002/cncr.32645.
Patients with advanced urothelial cancer and a poor performance status may have little to gain from immune checkpoint inhibitor therapy, suggests a multicenter real-world retrospective cohort study.
“The perceived favorable toxicity profile of immune checkpoint inhibitors has led to the selection of these agents for patients otherwise unfit for systemic chemotherapy,” noted the investigators, led by Ali Raza Khaki, MD, a fellow in the division of oncology, department of medicine, University of Washington, Seattle. “However, there is a paucity of data supporting the use of immune checkpoint inhibitors in patients with a poor performance status, who were not very well represented in the clinical trials that led to their approval, with no trial enrolling patients with an ECOG [Eastern Cooperative Oncology Group] performance status greater than or equal to 3 and only 3 trials including patients with an ECOG performance status of 2.”
Dr. Khaki and coinvestigators analyzed data from 499 patients with advanced urothelial cancer treated with immune checkpoint inhibitors at 18 institutions during 2013-2019. Slightly more than one-quarter had an ECOG performance status of 2 or higher.
Study results, reported in Cancer, showed that the overall response rate to immune checkpoint inhibitor therapy was similar regardless of performance status, across patients being treated in different lines of therapy.
However, among patients being treated in the first line, overall survival was better for those with a performance status of 0 to 1 than for those with a performance status of 2 or higher (median, 15.2 vs. 7.2 months; hazard ratio for death, 0.62; P = .01). There was no significant difference in this outcome among patients being treated in subsequent lines (median, 9.8 vs. 8.2 months; hazard ratio, 0.78; P = .27).
Among the 288 patients who died, 10% started immune checkpoint inhibitors in the last 30 days of life and 33% started them in the last 90 days of life. Patients initiating this therapy in the last 30 days of life were almost three time more likely to die in a hospital (odds ratio, 2.89; P = .04).
Notably, among the 11 patients with an ECOG performance status of 3, none had a response to immune checkpoint inhibitors and only two achieved stable disease. Moreover, two patients in this subgroup died within a week of receiving these agents.
“Despite comparable overall response rates, immune checkpoint inhibitors may not overcome the negative prognostic role of a poor performance status, particularly in the first-line setting,” Dr. Khaki and coinvestigators wrote. “Our study underscores the importance of developing prospectively validated predictive biomarkers to aid in identifying those patients most and least likely to benefit from immune checkpoint inhibitors.
“Overall, our data suggest that the decision of immune checkpoint inhibitor initiation near the end of life, akin to the practice for chemotherapy, should be considered carefully, and it should be accompanied by a detailed discussion of the data, rationale, and risks and benefits to minimize unnecessary potential adverse events and the cost and intensity of end-of-life care,” they recommended.
Dr. Khaki did not disclose any conflicts of interest. The study was supported by the National Cancer Institute, the National Center for Advancing Translational Sciences of the National Institutes of Health, the Seattle Translational Tumor Research Program at the Fred Hutchinson Cancer Research Center, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, the Wellcome Trust Strategic Fund, and Merck.
SOURCE: Khaki AR et al. Cancer. 2019 Dec 12. doi: 10.1002/cncr.32645.
FROM CANCER
North American Blastomycosis in an Immunocompromised Patient
Blastomycosis is a systemic fungal infection that is endemic in the South Central, Midwest, and southeastern regions of the United States, as well as in provinces of Canada bordering the Great Lakes. After inhalation of Blastomyces dermatitidis spores, which are taken up by bronchopulmonary macrophages, there is an approximate 30- to 45-day incubation period. The initial response at the infected site is suppurative, which progresses to granuloma formation. Blastomyces dermatitidis most commonly infects the lungs, followed by the skin, bones, prostate, and central nervous system (CNS). Therapy for blastomycosis is determined by the severity of the clinical presentation and consideration of the toxicities of the antifungal agent.
We present the case of a 38-year-old man with a medical history of human immunodeficiency virus (HIV) infection and AIDS who reported a 3- to 4-week history of respiratory and cutaneous symptoms. Initial clinical impression favored secondary syphilis; however, after laboratory evaluation and lack of response to treatment for syphilis, further investigation revealed a diagnosis of widespread cutaneous North American blastomycosis.
Case Report
A 38-year-old man with a medical history of HIV infection and AIDS presented to the emergency department at a medical center in Minneapolis, Minnesota, with a cough; chest discomfort; and concomitant nonpainful, mildly pruritic papules and plaques of 3 to 4 weeks’ duration that initially appeared on the face and ears and spread to the trunk, arms, palms, legs, and feet. He had a nonpainful ulcer on the glans penis. Symptoms began while he was living in Atlanta, Georgia, before relocating to Minneapolis. A chest radiograph was negative.
The initial clinical impression favored secondary syphilis. Intramuscular penicillin G benzathine (2.4 million U) weekly for 3 weeks was initiated by the primary care team based on clinical suspicion alone without laboratory evidence of a positive rapid plasma reagin or VDRL test. Because laboratory evaluation and lack of response to treatment did not support syphilis, dermatology consultation was requested.
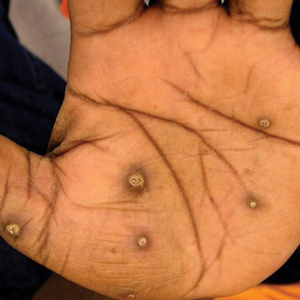
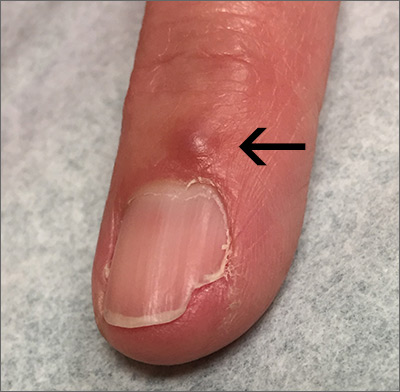
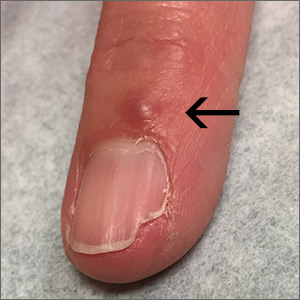
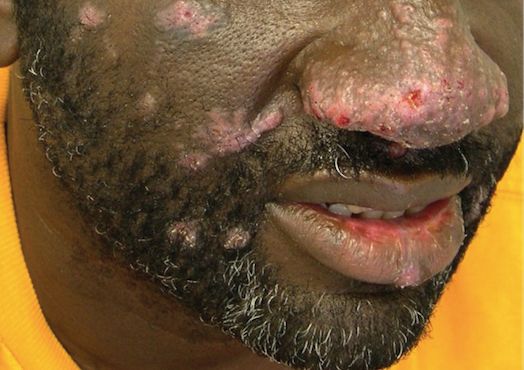
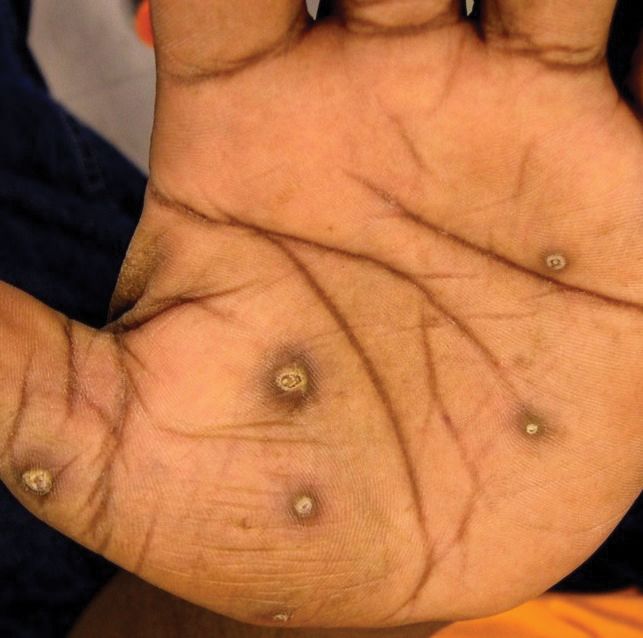
The patient had a history of crack cocaine abuse. He reported sexual activity with a single female partner while living in a halfway house in the Minneapolis–St. Paul area. Physical examination showed an age-appropriate man in no acute distress who was alert and oriented. He had well-demarcated papules and plaques on the forehead, ears, nose, cutaneous and mucosal lips, chest, back, arms, legs, palms, and soles. Many of the facial papules were pink, nonscaly, and concentrated around the nose and mouth; some were umbilicated (Figure 1). Trunk and extensor papules and plaques were well demarcated, oval, and scaly; some had erosions centrally and were excoriated. Palmar papules were round and had peripheral brown hyperpigmentation and central scale (Figure 2). A 1-cm, shallow, nontender, oval ulceration withraised borders was located on the glans penis under the foreskin (Figure 3).
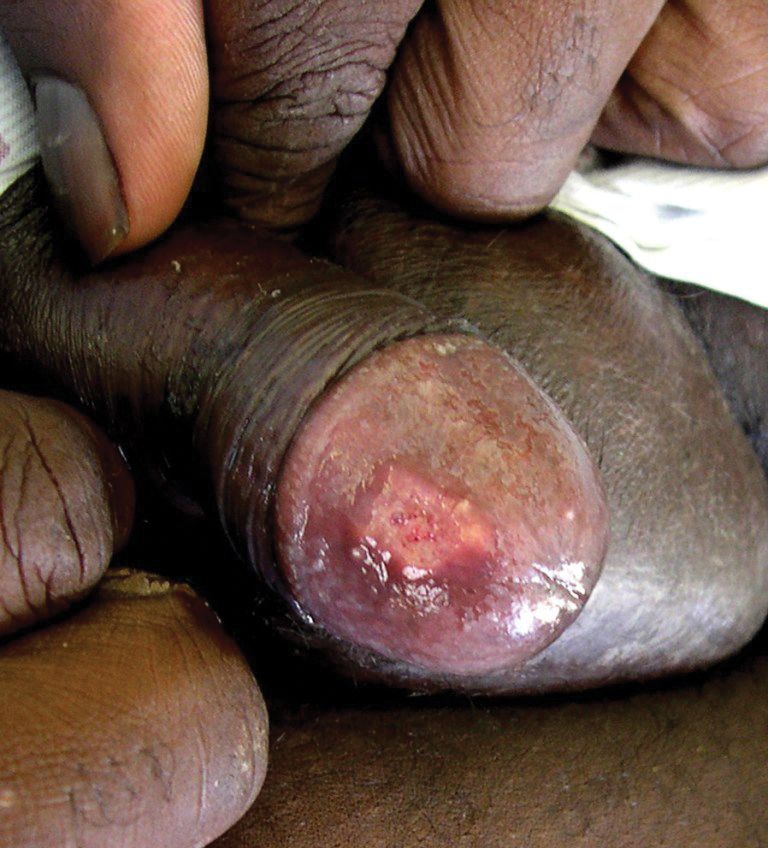
A rapid plasma reagin test was nonreactive; a fluorescent treponemal antibody absorption test was negative. Chest radiograph, magnetic resonance imaging, and electroencephalogram were normal. In addition, spinal fluid drawn from a tap was negative on India ink and Gram stain preparations and was negative for cryptococcal antigen. In addition, spinal fluid was negative for fungal and bacterial growth, as were blood cultures.
Abnormal tests included a positive enzyme-linked immunosorbent assay and Western blot test for HIV, with an absolute CD4 count of 6 cells/mL and a viral load more than 100,000 copies/mL. Urine histoplasmosis antigen was markedly elevated. A potassium hydroxide preparation was performed on the skin of the right forearm, revealing broad-based budding yeast, later confirmed on skin and sputum cultures to be B dermatitidis.
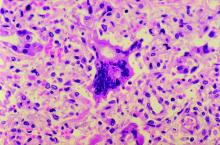
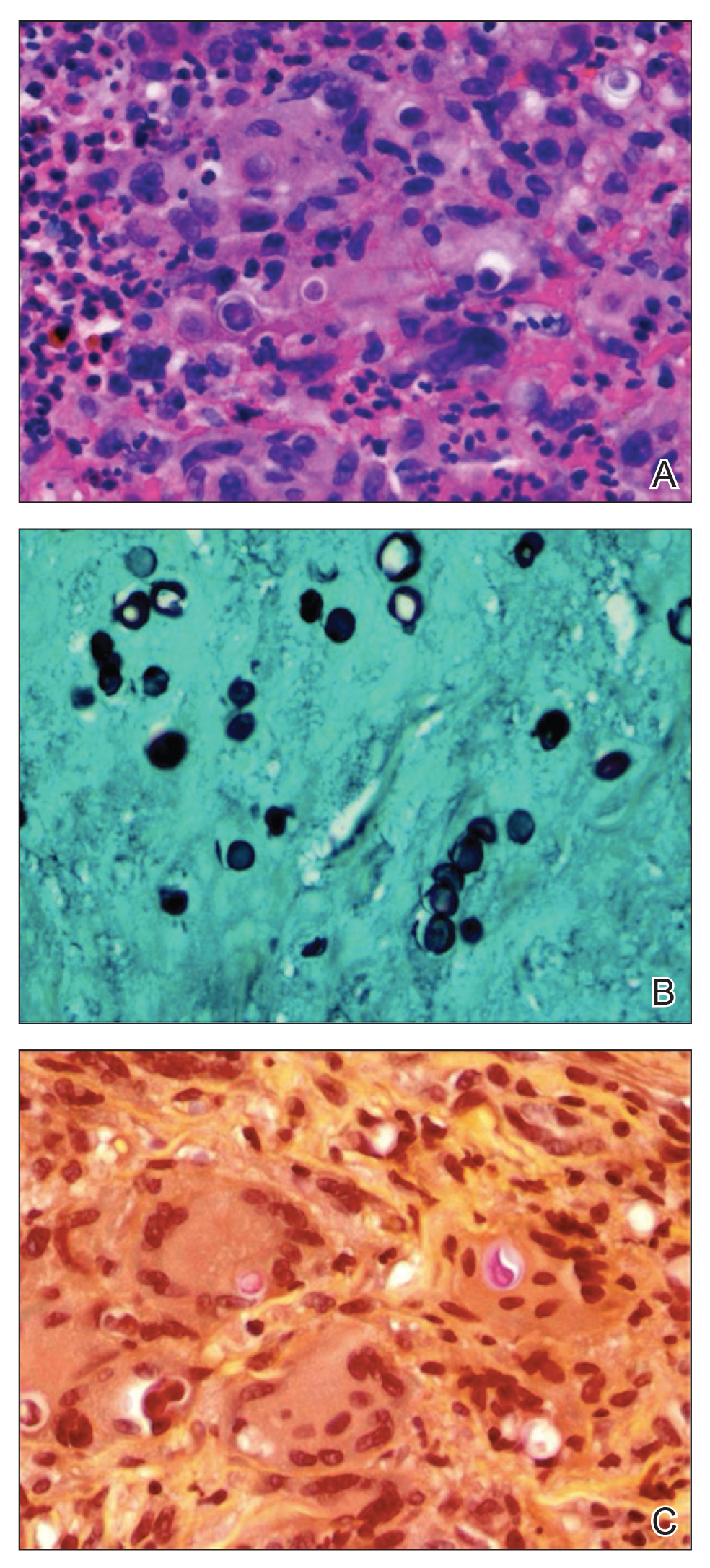
Punch biopsy from the upper back revealed a mixed acute and granulomatous infiltrate with numerous yeast forms (Figure 4A) that were highlighted by Grocott-Gomori methenamine-silver (Figure 4B) and periodic acid–Schiff (Figure 4C) stains.
The patient was treated with intravenous amphotericin with improvement in skin lesions. A healing ointment and occlusive dressing were used on eroded skin lesions. The patient was discharged on oral itraconazole 200 mg twice daily for 6 months (for blastomycosis); oral sulfamethoxazole-trimethoprim 15 mg/kg/d every 8 hours for 21 days (for Pneumocystis carinii pneumonia prophylaxis); oral azithromycin 500 mg daily (for Mycobacterium avium-intracellulare prophylaxis); oral levetiracetam 500 mg every 12 hours (as an antiseizure agent); albuterol 90 µg per actuation; and healing ointment. He continues his chemical dependency program and is being followed by the neurology seizure clinic as well as the outpatient HIV infectious disease clinic for planned reinitiation of highly active antiretroviral therapy.
Comment
Diagnosis
Our patient had an interesting and dramatic presentation of widespread cutaneous North American blastomycosis that was initially considered to be secondary syphilis because of involvement of the palms and soles and the presence of the painless penile ulcer. In addition, the initial skin biopsy finding was considered morphologically consistent with Cryptococcus neoformans based on positive Grocott-Gomori methenamine-silver and periodic acid–Schiff stains and an equivocal mucicarmine stain. However, the potassium hydroxide preparation of skin and positive urine histoplasmosis antigen strongly suggested blastomycosis, which was confirmed by culture of B dermatitidis. The urine histoplasmosis antigen can cross-react with B dermatitidis and other mycoses (eg, Paracoccidioides brasiliensis and Penicillium marneffei); however, because the treatment of either of these mycoses is similar, the value of the test remains high.1
Skin tests and serologic markers are useful epidemiologic tools but are of inadequate sensitivity and specificity to be diagnostic for B dermatitidis. Diagnosis depends on direct examination of tissue or isolation of the fungus in culture.2
Source of Infection
The probable occult source of cutaneous infection was the lungs, given the natural history of disseminated blastomycosis; the history of cough and chest discomfort; the widespread nature of skin lesions; and the ultimate growth of rare yeast forms in sputum. Cutaneous infection generally is from disseminated disease and rarely from direct inoculation.
Unlike many other systemic dimorphic mycoses, blastomycosis usually occurs in healthy hosts and is frequently associated with point-source outbreak. Immunosuppressed patients typically develop infection following exposure to the organism, but reactivation also can occur. Blastomycosis is uncommon among HIV-infected individuals and is not recognized as an AIDS-defining illness.
In a review from Canada of 133 patients with blastomycosis, nearly half had an underlying medical condition but not one typically associated with marked immunosuppression.3 Only 2 of 133 patients had HIV infection. Overall mortality was 6.3%, and the average duration of symptoms before diagnosis was less in those who died vs those who survived the disease.3 In the setting of AIDS or other marked immunosuppression, disease usually is more severe, with multiple-system involvement, including the CNS, and can progress rapidly to death.2
Treatment
Therapy for blastomycosis is determined by the severity of the clinical presentation and consideration of the toxicities of the antifungal agent. There are no randomized, blinded trials comparing antifungal agents, and data on the treatment of blastomycosis in patients infected with HIV are limited. Amphotericin B 3 mg/kg every 24 hours is recommended in life-threatening systemic disease and CNS disease as well as in patients with immune suppression, including AIDS.4 In a retrospective study of 326 patients with blastomycosis, those receiving amphotericin B had a cure rate of 86.5% with a relapse rate of 3.9%; patients receiving ketoconazole had a cure rate of 81.7% with a relapse rate of 14%.4 Although data are limited, chronic suppressive therapy generally is recommended in patients with HIV who have been treated for blastomycosis. Fluconazole, itraconazole, and ketoconazole are all used as chronic suppressive therapy; however, given the higher relapse rate observed with ketoconazole, itraconazole is preferred. Because neither ketoconazole nor itraconazole penetrates the blood-brain barrier, these drugs are not recommended in cases of CNS involvement. Patients with CNS disease or intolerance to itraconazole should be treated with fluconazole for chronic suppression.3
- Wheat J, Wheat H, Connolly P, et al. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis. 1997;24:1169-1171.
- Pappas PG, Pottage JC, Powderly WG, et al. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1992;116:847-853.
- Crampton TL, Light RB, Berg GM, et al. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis. 2002;34:1310-1316. Cited by: Aberg JA. Blastomycosis and HIV. HIV In Site Knowledge Base Chapter. http://hivinsite.ucsf.edu/InSite?page=kb-05-02-09#SIX. Published April 2003. Updated January 2006. Accessed December 16, 2019.
- Chapman SW, Bradsher RW Jr, Campbell GD Jr, et al. Practice guidelines for the management of patients with blastomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:679-683.
Blastomycosis is a systemic fungal infection that is endemic in the South Central, Midwest, and southeastern regions of the United States, as well as in provinces of Canada bordering the Great Lakes. After inhalation of Blastomyces dermatitidis spores, which are taken up by bronchopulmonary macrophages, there is an approximate 30- to 45-day incubation period. The initial response at the infected site is suppurative, which progresses to granuloma formation. Blastomyces dermatitidis most commonly infects the lungs, followed by the skin, bones, prostate, and central nervous system (CNS). Therapy for blastomycosis is determined by the severity of the clinical presentation and consideration of the toxicities of the antifungal agent.
We present the case of a 38-year-old man with a medical history of human immunodeficiency virus (HIV) infection and AIDS who reported a 3- to 4-week history of respiratory and cutaneous symptoms. Initial clinical impression favored secondary syphilis; however, after laboratory evaluation and lack of response to treatment for syphilis, further investigation revealed a diagnosis of widespread cutaneous North American blastomycosis.
Case Report
A 38-year-old man with a medical history of HIV infection and AIDS presented to the emergency department at a medical center in Minneapolis, Minnesota, with a cough; chest discomfort; and concomitant nonpainful, mildly pruritic papules and plaques of 3 to 4 weeks’ duration that initially appeared on the face and ears and spread to the trunk, arms, palms, legs, and feet. He had a nonpainful ulcer on the glans penis. Symptoms began while he was living in Atlanta, Georgia, before relocating to Minneapolis. A chest radiograph was negative.
The initial clinical impression favored secondary syphilis. Intramuscular penicillin G benzathine (2.4 million U) weekly for 3 weeks was initiated by the primary care team based on clinical suspicion alone without laboratory evidence of a positive rapid plasma reagin or VDRL test. Because laboratory evaluation and lack of response to treatment did not support syphilis, dermatology consultation was requested.
The patient had a history of crack cocaine abuse. He reported sexual activity with a single female partner while living in a halfway house in the Minneapolis–St. Paul area. Physical examination showed an age-appropriate man in no acute distress who was alert and oriented. He had well-demarcated papules and plaques on the forehead, ears, nose, cutaneous and mucosal lips, chest, back, arms, legs, palms, and soles. Many of the facial papules were pink, nonscaly, and concentrated around the nose and mouth; some were umbilicated (Figure 1). Trunk and extensor papules and plaques were well demarcated, oval, and scaly; some had erosions centrally and were excoriated. Palmar papules were round and had peripheral brown hyperpigmentation and central scale (Figure 2). A 1-cm, shallow, nontender, oval ulceration withraised borders was located on the glans penis under the foreskin (Figure 3).



A rapid plasma reagin test was nonreactive; a fluorescent treponemal antibody absorption test was negative. Chest radiograph, magnetic resonance imaging, and electroencephalogram were normal. In addition, spinal fluid drawn from a tap was negative on India ink and Gram stain preparations and was negative for cryptococcal antigen. In addition, spinal fluid was negative for fungal and bacterial growth, as were blood cultures.
Abnormal tests included a positive enzyme-linked immunosorbent assay and Western blot test for HIV, with an absolute CD4 count of 6 cells/mL and a viral load more than 100,000 copies/mL. Urine histoplasmosis antigen was markedly elevated. A potassium hydroxide preparation was performed on the skin of the right forearm, revealing broad-based budding yeast, later confirmed on skin and sputum cultures to be B dermatitidis.
Punch biopsy from the upper back revealed a mixed acute and granulomatous infiltrate with numerous yeast forms (Figure 4A) that were highlighted by Grocott-Gomori methenamine-silver (Figure 4B) and periodic acid–Schiff (Figure 4C) stains.
The patient was treated with intravenous amphotericin with improvement in skin lesions. A healing ointment and occlusive dressing were used on eroded skin lesions. The patient was discharged on oral itraconazole 200 mg twice daily for 6 months (for blastomycosis); oral sulfamethoxazole-trimethoprim 15 mg/kg/d every 8 hours for 21 days (for Pneumocystis carinii pneumonia prophylaxis); oral azithromycin 500 mg daily (for Mycobacterium avium-intracellulare prophylaxis); oral levetiracetam 500 mg every 12 hours (as an antiseizure agent); albuterol 90 µg per actuation; and healing ointment. He continues his chemical dependency program and is being followed by the neurology seizure clinic as well as the outpatient HIV infectious disease clinic for planned reinitiation of highly active antiretroviral therapy.
Comment
Diagnosis
Our patient had an interesting and dramatic presentation of widespread cutaneous North American blastomycosis that was initially considered to be secondary syphilis because of involvement of the palms and soles and the presence of the painless penile ulcer. In addition, the initial skin biopsy finding was considered morphologically consistent with Cryptococcus neoformans based on positive Grocott-Gomori methenamine-silver and periodic acid–Schiff stains and an equivocal mucicarmine stain. However, the potassium hydroxide preparation of skin and positive urine histoplasmosis antigen strongly suggested blastomycosis, which was confirmed by culture of B dermatitidis. The urine histoplasmosis antigen can cross-react with B dermatitidis and other mycoses (eg, Paracoccidioides brasiliensis and Penicillium marneffei); however, because the treatment of either of these mycoses is similar, the value of the test remains high.1
Skin tests and serologic markers are useful epidemiologic tools but are of inadequate sensitivity and specificity to be diagnostic for B dermatitidis. Diagnosis depends on direct examination of tissue or isolation of the fungus in culture.2
Source of Infection
The probable occult source of cutaneous infection was the lungs, given the natural history of disseminated blastomycosis; the history of cough and chest discomfort; the widespread nature of skin lesions; and the ultimate growth of rare yeast forms in sputum. Cutaneous infection generally is from disseminated disease and rarely from direct inoculation.
Unlike many other systemic dimorphic mycoses, blastomycosis usually occurs in healthy hosts and is frequently associated with point-source outbreak. Immunosuppressed patients typically develop infection following exposure to the organism, but reactivation also can occur. Blastomycosis is uncommon among HIV-infected individuals and is not recognized as an AIDS-defining illness.
In a review from Canada of 133 patients with blastomycosis, nearly half had an underlying medical condition but not one typically associated with marked immunosuppression.3 Only 2 of 133 patients had HIV infection. Overall mortality was 6.3%, and the average duration of symptoms before diagnosis was less in those who died vs those who survived the disease.3 In the setting of AIDS or other marked immunosuppression, disease usually is more severe, with multiple-system involvement, including the CNS, and can progress rapidly to death.2
Treatment
Therapy for blastomycosis is determined by the severity of the clinical presentation and consideration of the toxicities of the antifungal agent. There are no randomized, blinded trials comparing antifungal agents, and data on the treatment of blastomycosis in patients infected with HIV are limited. Amphotericin B 3 mg/kg every 24 hours is recommended in life-threatening systemic disease and CNS disease as well as in patients with immune suppression, including AIDS.4 In a retrospective study of 326 patients with blastomycosis, those receiving amphotericin B had a cure rate of 86.5% with a relapse rate of 3.9%; patients receiving ketoconazole had a cure rate of 81.7% with a relapse rate of 14%.4 Although data are limited, chronic suppressive therapy generally is recommended in patients with HIV who have been treated for blastomycosis. Fluconazole, itraconazole, and ketoconazole are all used as chronic suppressive therapy; however, given the higher relapse rate observed with ketoconazole, itraconazole is preferred. Because neither ketoconazole nor itraconazole penetrates the blood-brain barrier, these drugs are not recommended in cases of CNS involvement. Patients with CNS disease or intolerance to itraconazole should be treated with fluconazole for chronic suppression.3
Blastomycosis is a systemic fungal infection that is endemic in the South Central, Midwest, and southeastern regions of the United States, as well as in provinces of Canada bordering the Great Lakes. After inhalation of Blastomyces dermatitidis spores, which are taken up by bronchopulmonary macrophages, there is an approximate 30- to 45-day incubation period. The initial response at the infected site is suppurative, which progresses to granuloma formation. Blastomyces dermatitidis most commonly infects the lungs, followed by the skin, bones, prostate, and central nervous system (CNS). Therapy for blastomycosis is determined by the severity of the clinical presentation and consideration of the toxicities of the antifungal agent.
We present the case of a 38-year-old man with a medical history of human immunodeficiency virus (HIV) infection and AIDS who reported a 3- to 4-week history of respiratory and cutaneous symptoms. Initial clinical impression favored secondary syphilis; however, after laboratory evaluation and lack of response to treatment for syphilis, further investigation revealed a diagnosis of widespread cutaneous North American blastomycosis.
Case Report
A 38-year-old man with a medical history of HIV infection and AIDS presented to the emergency department at a medical center in Minneapolis, Minnesota, with a cough; chest discomfort; and concomitant nonpainful, mildly pruritic papules and plaques of 3 to 4 weeks’ duration that initially appeared on the face and ears and spread to the trunk, arms, palms, legs, and feet. He had a nonpainful ulcer on the glans penis. Symptoms began while he was living in Atlanta, Georgia, before relocating to Minneapolis. A chest radiograph was negative.
The initial clinical impression favored secondary syphilis. Intramuscular penicillin G benzathine (2.4 million U) weekly for 3 weeks was initiated by the primary care team based on clinical suspicion alone without laboratory evidence of a positive rapid plasma reagin or VDRL test. Because laboratory evaluation and lack of response to treatment did not support syphilis, dermatology consultation was requested.
The patient had a history of crack cocaine abuse. He reported sexual activity with a single female partner while living in a halfway house in the Minneapolis–St. Paul area. Physical examination showed an age-appropriate man in no acute distress who was alert and oriented. He had well-demarcated papules and plaques on the forehead, ears, nose, cutaneous and mucosal lips, chest, back, arms, legs, palms, and soles. Many of the facial papules were pink, nonscaly, and concentrated around the nose and mouth; some were umbilicated (Figure 1). Trunk and extensor papules and plaques were well demarcated, oval, and scaly; some had erosions centrally and were excoriated. Palmar papules were round and had peripheral brown hyperpigmentation and central scale (Figure 2). A 1-cm, shallow, nontender, oval ulceration withraised borders was located on the glans penis under the foreskin (Figure 3).



A rapid plasma reagin test was nonreactive; a fluorescent treponemal antibody absorption test was negative. Chest radiograph, magnetic resonance imaging, and electroencephalogram were normal. In addition, spinal fluid drawn from a tap was negative on India ink and Gram stain preparations and was negative for cryptococcal antigen. In addition, spinal fluid was negative for fungal and bacterial growth, as were blood cultures.
Abnormal tests included a positive enzyme-linked immunosorbent assay and Western blot test for HIV, with an absolute CD4 count of 6 cells/mL and a viral load more than 100,000 copies/mL. Urine histoplasmosis antigen was markedly elevated. A potassium hydroxide preparation was performed on the skin of the right forearm, revealing broad-based budding yeast, later confirmed on skin and sputum cultures to be B dermatitidis.
Punch biopsy from the upper back revealed a mixed acute and granulomatous infiltrate with numerous yeast forms (Figure 4A) that were highlighted by Grocott-Gomori methenamine-silver (Figure 4B) and periodic acid–Schiff (Figure 4C) stains.
The patient was treated with intravenous amphotericin with improvement in skin lesions. A healing ointment and occlusive dressing were used on eroded skin lesions. The patient was discharged on oral itraconazole 200 mg twice daily for 6 months (for blastomycosis); oral sulfamethoxazole-trimethoprim 15 mg/kg/d every 8 hours for 21 days (for Pneumocystis carinii pneumonia prophylaxis); oral azithromycin 500 mg daily (for Mycobacterium avium-intracellulare prophylaxis); oral levetiracetam 500 mg every 12 hours (as an antiseizure agent); albuterol 90 µg per actuation; and healing ointment. He continues his chemical dependency program and is being followed by the neurology seizure clinic as well as the outpatient HIV infectious disease clinic for planned reinitiation of highly active antiretroviral therapy.
Comment
Diagnosis
Our patient had an interesting and dramatic presentation of widespread cutaneous North American blastomycosis that was initially considered to be secondary syphilis because of involvement of the palms and soles and the presence of the painless penile ulcer. In addition, the initial skin biopsy finding was considered morphologically consistent with Cryptococcus neoformans based on positive Grocott-Gomori methenamine-silver and periodic acid–Schiff stains and an equivocal mucicarmine stain. However, the potassium hydroxide preparation of skin and positive urine histoplasmosis antigen strongly suggested blastomycosis, which was confirmed by culture of B dermatitidis. The urine histoplasmosis antigen can cross-react with B dermatitidis and other mycoses (eg, Paracoccidioides brasiliensis and Penicillium marneffei); however, because the treatment of either of these mycoses is similar, the value of the test remains high.1
Skin tests and serologic markers are useful epidemiologic tools but are of inadequate sensitivity and specificity to be diagnostic for B dermatitidis. Diagnosis depends on direct examination of tissue or isolation of the fungus in culture.2
Source of Infection
The probable occult source of cutaneous infection was the lungs, given the natural history of disseminated blastomycosis; the history of cough and chest discomfort; the widespread nature of skin lesions; and the ultimate growth of rare yeast forms in sputum. Cutaneous infection generally is from disseminated disease and rarely from direct inoculation.
Unlike many other systemic dimorphic mycoses, blastomycosis usually occurs in healthy hosts and is frequently associated with point-source outbreak. Immunosuppressed patients typically develop infection following exposure to the organism, but reactivation also can occur. Blastomycosis is uncommon among HIV-infected individuals and is not recognized as an AIDS-defining illness.
In a review from Canada of 133 patients with blastomycosis, nearly half had an underlying medical condition but not one typically associated with marked immunosuppression.3 Only 2 of 133 patients had HIV infection. Overall mortality was 6.3%, and the average duration of symptoms before diagnosis was less in those who died vs those who survived the disease.3 In the setting of AIDS or other marked immunosuppression, disease usually is more severe, with multiple-system involvement, including the CNS, and can progress rapidly to death.2
Treatment
Therapy for blastomycosis is determined by the severity of the clinical presentation and consideration of the toxicities of the antifungal agent. There are no randomized, blinded trials comparing antifungal agents, and data on the treatment of blastomycosis in patients infected with HIV are limited. Amphotericin B 3 mg/kg every 24 hours is recommended in life-threatening systemic disease and CNS disease as well as in patients with immune suppression, including AIDS.4 In a retrospective study of 326 patients with blastomycosis, those receiving amphotericin B had a cure rate of 86.5% with a relapse rate of 3.9%; patients receiving ketoconazole had a cure rate of 81.7% with a relapse rate of 14%.4 Although data are limited, chronic suppressive therapy generally is recommended in patients with HIV who have been treated for blastomycosis. Fluconazole, itraconazole, and ketoconazole are all used as chronic suppressive therapy; however, given the higher relapse rate observed with ketoconazole, itraconazole is preferred. Because neither ketoconazole nor itraconazole penetrates the blood-brain barrier, these drugs are not recommended in cases of CNS involvement. Patients with CNS disease or intolerance to itraconazole should be treated with fluconazole for chronic suppression.3
- Wheat J, Wheat H, Connolly P, et al. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis. 1997;24:1169-1171.
- Pappas PG, Pottage JC, Powderly WG, et al. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1992;116:847-853.
- Crampton TL, Light RB, Berg GM, et al. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis. 2002;34:1310-1316. Cited by: Aberg JA. Blastomycosis and HIV. HIV In Site Knowledge Base Chapter. http://hivinsite.ucsf.edu/InSite?page=kb-05-02-09#SIX. Published April 2003. Updated January 2006. Accessed December 16, 2019.
- Chapman SW, Bradsher RW Jr, Campbell GD Jr, et al. Practice guidelines for the management of patients with blastomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:679-683.
- Wheat J, Wheat H, Connolly P, et al. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis. 1997;24:1169-1171.
- Pappas PG, Pottage JC, Powderly WG, et al. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1992;116:847-853.
- Crampton TL, Light RB, Berg GM, et al. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis. 2002;34:1310-1316. Cited by: Aberg JA. Blastomycosis and HIV. HIV In Site Knowledge Base Chapter. http://hivinsite.ucsf.edu/InSite?page=kb-05-02-09#SIX. Published April 2003. Updated January 2006. Accessed December 16, 2019.
- Chapman SW, Bradsher RW Jr, Campbell GD Jr, et al. Practice guidelines for the management of patients with blastomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:679-683.
Practice Points
- Blastomycosis generally produces a pulmonary form of the disease and, to a lesser extent, extrapulmonary forms, such as cutaneous, osteoarticular, and genitourinary.
- Blastomycosis can be diagnosed by culture, direct visualization of the yeast in affected tissue, antigen testing, or a combination of these methods.
- After inhalation of Blastomyces dermatitidis spores, which are taken up by bronchopulmonary macrophages, there is an approximate 30- to 45-day incubation period.
Standard-dose RT with concurrent chemo found superior for unresectable NSCLC
More is not better when it comes to radiation therapy administered with concurrent chemotherapy for unresectable stage III non–small cell lung cancer (NSCLC), suggests a long-term update of the RTOG 0617 trial.
Initial results of the phase 3 randomized controlled trial, at a median follow-up of 1.9 years, showed that median overall survival was about 8 months longer with the 60-Gy standard dose of radiation compared with a 74-Gy high dose, each given along with paclitaxel and carboplatin (Lancet Oncol. 2015;16:187-99).
The update, now at a median follow-up of 5.1 years and reported in the Journal of Clinical Oncology, recapitulates that finding, again showing a roughly 8-month longer overall survival with the standard dose of radiation. Results continue to show no benefit of adding the anti-EGFR antibody cetuximab (Erbitux) to treatment.
“The 5-year overall survival estimate for the standard-dose radiation arm of RTOG 0617, regardless of cetuximab delivery, was 32.1%. This is among the highest overall survival results of any phase III trial for patients with stage III NSCLC,” noted the investigators, led by Jeffrey D. Bradley, MD, department of radiation oncology, Emory University, Atlanta.
“These results argue strongly that the current standard-of-care radiation dose should be 60 Gy given in 2-Gy daily fractions to a target volume directed at tumor plus margin on the basis of CT and PET/CT, excluding elective nodal irradiation.”
The RTOG 0617 trial was conducted among 496 patients with unresectable stage III NSCLC in the United States and Canada. They were randomized to receive standard-dose or high-dose radiation in addition to concurrent chemotherapy, and randomized again to receive cetuximab or not.
Median overall survival as of the update was 28.7 months with standard-dose radiation therapy, compared with 20.3 months with high-dose radiation therapy (P = .0072), Dr. Bradley and coinvestigators reported. This survival benefit stood up in multivariate analysis (hazard ratio, 1.30; P = .0315).
The standard dose of radiation also yielded better 5-year overall survival (32.1% vs. 23%; P = .007) and 5-year progression-free survival (18.3% vs. 13%; P = .055). Further analyses suggested that these differences were not due to differential radiation therapy compliance.
The high-dose radiation group had more grade 5 adverse events (nine vs. three events), as well as higher rates of treatment-related grade 3 or worse dysphagia (12.1% vs. 3.2%; P = .0005) and esophagitis (17.4% vs. 5.0%; P less than .0001). Pulmonary toxicity was statistically indistinguishable between groups.
Since the RTOG 0617 results were first reported, the standard of care for unresectable stage III NSCLC has changed, as the PACIFIC trial showed an overall survival advantage of adding the immune checkpoint inhibitor durvalumab (Imfinzi) as maintenance therapy after concurrent chemoradiotherapy (N Engl J Med. 2018;379:2342-50).
However, the 2-year overall survival rate with chemoradiation using the standard radiation dose in the former trial (59.6%) is fairly close to that seen with chemoradiation plus maintenance durvalumab in the latter trial (66.3%), the investigators noted.
Dr. Bradley disclosed having a consulting or advisory role with AstraZeneca. The trial was supported by the National Cancer Institute and Eli Lilly.
SOURCE: Bradley JD et al. J Clin Oncol. 2019 Dec 16. doi: 10.1200/JCO.19.01162.
More is not better when it comes to radiation therapy administered with concurrent chemotherapy for unresectable stage III non–small cell lung cancer (NSCLC), suggests a long-term update of the RTOG 0617 trial.
Initial results of the phase 3 randomized controlled trial, at a median follow-up of 1.9 years, showed that median overall survival was about 8 months longer with the 60-Gy standard dose of radiation compared with a 74-Gy high dose, each given along with paclitaxel and carboplatin (Lancet Oncol. 2015;16:187-99).
The update, now at a median follow-up of 5.1 years and reported in the Journal of Clinical Oncology, recapitulates that finding, again showing a roughly 8-month longer overall survival with the standard dose of radiation. Results continue to show no benefit of adding the anti-EGFR antibody cetuximab (Erbitux) to treatment.
“The 5-year overall survival estimate for the standard-dose radiation arm of RTOG 0617, regardless of cetuximab delivery, was 32.1%. This is among the highest overall survival results of any phase III trial for patients with stage III NSCLC,” noted the investigators, led by Jeffrey D. Bradley, MD, department of radiation oncology, Emory University, Atlanta.
“These results argue strongly that the current standard-of-care radiation dose should be 60 Gy given in 2-Gy daily fractions to a target volume directed at tumor plus margin on the basis of CT and PET/CT, excluding elective nodal irradiation.”
The RTOG 0617 trial was conducted among 496 patients with unresectable stage III NSCLC in the United States and Canada. They were randomized to receive standard-dose or high-dose radiation in addition to concurrent chemotherapy, and randomized again to receive cetuximab or not.
Median overall survival as of the update was 28.7 months with standard-dose radiation therapy, compared with 20.3 months with high-dose radiation therapy (P = .0072), Dr. Bradley and coinvestigators reported. This survival benefit stood up in multivariate analysis (hazard ratio, 1.30; P = .0315).
The standard dose of radiation also yielded better 5-year overall survival (32.1% vs. 23%; P = .007) and 5-year progression-free survival (18.3% vs. 13%; P = .055). Further analyses suggested that these differences were not due to differential radiation therapy compliance.
The high-dose radiation group had more grade 5 adverse events (nine vs. three events), as well as higher rates of treatment-related grade 3 or worse dysphagia (12.1% vs. 3.2%; P = .0005) and esophagitis (17.4% vs. 5.0%; P less than .0001). Pulmonary toxicity was statistically indistinguishable between groups.
Since the RTOG 0617 results were first reported, the standard of care for unresectable stage III NSCLC has changed, as the PACIFIC trial showed an overall survival advantage of adding the immune checkpoint inhibitor durvalumab (Imfinzi) as maintenance therapy after concurrent chemoradiotherapy (N Engl J Med. 2018;379:2342-50).
However, the 2-year overall survival rate with chemoradiation using the standard radiation dose in the former trial (59.6%) is fairly close to that seen with chemoradiation plus maintenance durvalumab in the latter trial (66.3%), the investigators noted.
Dr. Bradley disclosed having a consulting or advisory role with AstraZeneca. The trial was supported by the National Cancer Institute and Eli Lilly.
SOURCE: Bradley JD et al. J Clin Oncol. 2019 Dec 16. doi: 10.1200/JCO.19.01162.
More is not better when it comes to radiation therapy administered with concurrent chemotherapy for unresectable stage III non–small cell lung cancer (NSCLC), suggests a long-term update of the RTOG 0617 trial.
Initial results of the phase 3 randomized controlled trial, at a median follow-up of 1.9 years, showed that median overall survival was about 8 months longer with the 60-Gy standard dose of radiation compared with a 74-Gy high dose, each given along with paclitaxel and carboplatin (Lancet Oncol. 2015;16:187-99).
The update, now at a median follow-up of 5.1 years and reported in the Journal of Clinical Oncology, recapitulates that finding, again showing a roughly 8-month longer overall survival with the standard dose of radiation. Results continue to show no benefit of adding the anti-EGFR antibody cetuximab (Erbitux) to treatment.
“The 5-year overall survival estimate for the standard-dose radiation arm of RTOG 0617, regardless of cetuximab delivery, was 32.1%. This is among the highest overall survival results of any phase III trial for patients with stage III NSCLC,” noted the investigators, led by Jeffrey D. Bradley, MD, department of radiation oncology, Emory University, Atlanta.
“These results argue strongly that the current standard-of-care radiation dose should be 60 Gy given in 2-Gy daily fractions to a target volume directed at tumor plus margin on the basis of CT and PET/CT, excluding elective nodal irradiation.”
The RTOG 0617 trial was conducted among 496 patients with unresectable stage III NSCLC in the United States and Canada. They were randomized to receive standard-dose or high-dose radiation in addition to concurrent chemotherapy, and randomized again to receive cetuximab or not.
Median overall survival as of the update was 28.7 months with standard-dose radiation therapy, compared with 20.3 months with high-dose radiation therapy (P = .0072), Dr. Bradley and coinvestigators reported. This survival benefit stood up in multivariate analysis (hazard ratio, 1.30; P = .0315).
The standard dose of radiation also yielded better 5-year overall survival (32.1% vs. 23%; P = .007) and 5-year progression-free survival (18.3% vs. 13%; P = .055). Further analyses suggested that these differences were not due to differential radiation therapy compliance.
The high-dose radiation group had more grade 5 adverse events (nine vs. three events), as well as higher rates of treatment-related grade 3 or worse dysphagia (12.1% vs. 3.2%; P = .0005) and esophagitis (17.4% vs. 5.0%; P less than .0001). Pulmonary toxicity was statistically indistinguishable between groups.
Since the RTOG 0617 results were first reported, the standard of care for unresectable stage III NSCLC has changed, as the PACIFIC trial showed an overall survival advantage of adding the immune checkpoint inhibitor durvalumab (Imfinzi) as maintenance therapy after concurrent chemoradiotherapy (N Engl J Med. 2018;379:2342-50).
However, the 2-year overall survival rate with chemoradiation using the standard radiation dose in the former trial (59.6%) is fairly close to that seen with chemoradiation plus maintenance durvalumab in the latter trial (66.3%), the investigators noted.
Dr. Bradley disclosed having a consulting or advisory role with AstraZeneca. The trial was supported by the National Cancer Institute and Eli Lilly.
SOURCE: Bradley JD et al. J Clin Oncol. 2019 Dec 16. doi: 10.1200/JCO.19.01162.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
CAR T-cell therapy advances in CLL
ORLANDO – Lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T-cell therapy, has demonstrated manageable toxicity and promising clinical activity in the phase 1 portion of a trial enrolling heavily pretreated patients with chronic lymphocytic leukemia/small lymphocytic lymphoma, according to an investigator.
The overall response rate exceeded 80%, and most patients in response at 6 months had maintained that response at the 9-month mark, said Tanya Siddiqi, MD, of City of Hope National Medical Center, Duarte, Calif.
“Clinical responses were rapid, improved with time, and were deep and durable,” Dr. Siddiqi said at the annual meeting of the American Society of Hematology.
These findings have provided justification for conducting the phase 2 portion of the study, which is currently enrolling at the higher of two dose levels evaluated in phase 1, she added.
Dr. Siddiqi reported on a total of 23 patients enrolled in the study, known as TRANSCEND CLL 004. All patients had relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and had received at least two prior therapies, including ibrutinib, while about one-third had failed venetoclax as well.
The median patient age was 66 years, and 83% had high-risk features, according to Dr. Siddiqi, who said patients had received a median of five prior lines of therapy.
Nine patients were treated at dose level 1, or 50 x 106 CAR+ T cells, while 14 were treated at dose level 2, or 100 x 106 CAR+ T cells. Two patients experienced grade 3 or 4 dose-limiting toxicities at the second level, including hypertension in one patient, and encephalopathy, muscle weakness, and tumor lysis syndrome (TLS) in the other.
Cytokine release syndrome (CRS) occurred in 17 patients, though only two cases reached grade 3. Neurologic adverse events were seen in nine patients, of which five were grade 3 or 4.
Partial or complete responses were noted in 81.5%, or 18 of 22 evaluable patients, including 10 (45.5%) who had complete remission. In the subset of nine patients who had failed both ibrutinib and venetoclax, that overall response rate was a “very impressive” 89% (eight of nine patients), said Dr. Siddiqi, including 67% complete remissions (six patients).
Undetectable minimal residual disease (MRD) was reported in 65% and 75% of patients, depending on the method used to evaluate it.
About two-thirds of the patients had responses by day 30 evaluation, and responses deepened over time in about one-quarter, according to Dr. Siddiqi. Of 12 patients with a response at 6 months, 10 (83%) were still in response at 9 months, and 8 patients have been in response for 12 months or longer, she reported.
Neurologic adverse events seen in the CLL/SLL patients in this study were associated with higher lymph node tumor burden, and increased levels of interleukin(IL)-16 or tumor necrosis factor (TNF), according to further analysis presented by Dr. Siddiqi.
That raises the possibility that IL-16 or TNF may be a “good predictive biomarker” for neurotoxicity, which seems to be driven at least in part by lymphadenopathy. “If there was a way that we could combine the CAR T-cell with something like a novel agent that can shrink the tumor burden quickly, then maybe we can have even less toxicities with these CAR T cells,” Dr. Siddiqi said.
Dr. Siddiqi reported disclosures related to Kite, TG Therapeutics, Celgene, Janssen, Seattle Genetics, AstraZeneca, PCYC, Juno Therapeutics, and BeiGene.
SOURCE: Siddiqi T et al. ASH 2019, Abstract 503.
ORLANDO – Lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T-cell therapy, has demonstrated manageable toxicity and promising clinical activity in the phase 1 portion of a trial enrolling heavily pretreated patients with chronic lymphocytic leukemia/small lymphocytic lymphoma, according to an investigator.
The overall response rate exceeded 80%, and most patients in response at 6 months had maintained that response at the 9-month mark, said Tanya Siddiqi, MD, of City of Hope National Medical Center, Duarte, Calif.
“Clinical responses were rapid, improved with time, and were deep and durable,” Dr. Siddiqi said at the annual meeting of the American Society of Hematology.
These findings have provided justification for conducting the phase 2 portion of the study, which is currently enrolling at the higher of two dose levels evaluated in phase 1, she added.
Dr. Siddiqi reported on a total of 23 patients enrolled in the study, known as TRANSCEND CLL 004. All patients had relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and had received at least two prior therapies, including ibrutinib, while about one-third had failed venetoclax as well.
The median patient age was 66 years, and 83% had high-risk features, according to Dr. Siddiqi, who said patients had received a median of five prior lines of therapy.
Nine patients were treated at dose level 1, or 50 x 106 CAR+ T cells, while 14 were treated at dose level 2, or 100 x 106 CAR+ T cells. Two patients experienced grade 3 or 4 dose-limiting toxicities at the second level, including hypertension in one patient, and encephalopathy, muscle weakness, and tumor lysis syndrome (TLS) in the other.
Cytokine release syndrome (CRS) occurred in 17 patients, though only two cases reached grade 3. Neurologic adverse events were seen in nine patients, of which five were grade 3 or 4.
Partial or complete responses were noted in 81.5%, or 18 of 22 evaluable patients, including 10 (45.5%) who had complete remission. In the subset of nine patients who had failed both ibrutinib and venetoclax, that overall response rate was a “very impressive” 89% (eight of nine patients), said Dr. Siddiqi, including 67% complete remissions (six patients).
Undetectable minimal residual disease (MRD) was reported in 65% and 75% of patients, depending on the method used to evaluate it.
About two-thirds of the patients had responses by day 30 evaluation, and responses deepened over time in about one-quarter, according to Dr. Siddiqi. Of 12 patients with a response at 6 months, 10 (83%) were still in response at 9 months, and 8 patients have been in response for 12 months or longer, she reported.
Neurologic adverse events seen in the CLL/SLL patients in this study were associated with higher lymph node tumor burden, and increased levels of interleukin(IL)-16 or tumor necrosis factor (TNF), according to further analysis presented by Dr. Siddiqi.
That raises the possibility that IL-16 or TNF may be a “good predictive biomarker” for neurotoxicity, which seems to be driven at least in part by lymphadenopathy. “If there was a way that we could combine the CAR T-cell with something like a novel agent that can shrink the tumor burden quickly, then maybe we can have even less toxicities with these CAR T cells,” Dr. Siddiqi said.
Dr. Siddiqi reported disclosures related to Kite, TG Therapeutics, Celgene, Janssen, Seattle Genetics, AstraZeneca, PCYC, Juno Therapeutics, and BeiGene.
SOURCE: Siddiqi T et al. ASH 2019, Abstract 503.
ORLANDO – Lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T-cell therapy, has demonstrated manageable toxicity and promising clinical activity in the phase 1 portion of a trial enrolling heavily pretreated patients with chronic lymphocytic leukemia/small lymphocytic lymphoma, according to an investigator.
The overall response rate exceeded 80%, and most patients in response at 6 months had maintained that response at the 9-month mark, said Tanya Siddiqi, MD, of City of Hope National Medical Center, Duarte, Calif.
“Clinical responses were rapid, improved with time, and were deep and durable,” Dr. Siddiqi said at the annual meeting of the American Society of Hematology.
These findings have provided justification for conducting the phase 2 portion of the study, which is currently enrolling at the higher of two dose levels evaluated in phase 1, she added.
Dr. Siddiqi reported on a total of 23 patients enrolled in the study, known as TRANSCEND CLL 004. All patients had relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and had received at least two prior therapies, including ibrutinib, while about one-third had failed venetoclax as well.
The median patient age was 66 years, and 83% had high-risk features, according to Dr. Siddiqi, who said patients had received a median of five prior lines of therapy.
Nine patients were treated at dose level 1, or 50 x 106 CAR+ T cells, while 14 were treated at dose level 2, or 100 x 106 CAR+ T cells. Two patients experienced grade 3 or 4 dose-limiting toxicities at the second level, including hypertension in one patient, and encephalopathy, muscle weakness, and tumor lysis syndrome (TLS) in the other.
Cytokine release syndrome (CRS) occurred in 17 patients, though only two cases reached grade 3. Neurologic adverse events were seen in nine patients, of which five were grade 3 or 4.
Partial or complete responses were noted in 81.5%, or 18 of 22 evaluable patients, including 10 (45.5%) who had complete remission. In the subset of nine patients who had failed both ibrutinib and venetoclax, that overall response rate was a “very impressive” 89% (eight of nine patients), said Dr. Siddiqi, including 67% complete remissions (six patients).
Undetectable minimal residual disease (MRD) was reported in 65% and 75% of patients, depending on the method used to evaluate it.
About two-thirds of the patients had responses by day 30 evaluation, and responses deepened over time in about one-quarter, according to Dr. Siddiqi. Of 12 patients with a response at 6 months, 10 (83%) were still in response at 9 months, and 8 patients have been in response for 12 months or longer, she reported.
Neurologic adverse events seen in the CLL/SLL patients in this study were associated with higher lymph node tumor burden, and increased levels of interleukin(IL)-16 or tumor necrosis factor (TNF), according to further analysis presented by Dr. Siddiqi.
That raises the possibility that IL-16 or TNF may be a “good predictive biomarker” for neurotoxicity, which seems to be driven at least in part by lymphadenopathy. “If there was a way that we could combine the CAR T-cell with something like a novel agent that can shrink the tumor burden quickly, then maybe we can have even less toxicities with these CAR T cells,” Dr. Siddiqi said.
Dr. Siddiqi reported disclosures related to Kite, TG Therapeutics, Celgene, Janssen, Seattle Genetics, AstraZeneca, PCYC, Juno Therapeutics, and BeiGene.
SOURCE: Siddiqi T et al. ASH 2019, Abstract 503.
REPORTING FROM ASH 2019
Fingernail deformity
The groove in the patient’s nail, and associated splitting, was due to a digital mucous cyst seen in the photo proximal to the cuticle.
In spite of the name, these cysts do not contain mucus. Instead, they contain synovial fluid and represent an outpouching of the distal interphalangeal joint of the index finger. This same process is called a ganglion cyst when it is seen at the wrist and a Baker’s cyst when it is behind the knee. These cysts typically form from an increase in synovial fluid, often due to underlying osteoarthritis, injury, or degenerative changes of the joint capsule. If the patient is asymptomatic, no treatment is required. Half of the lesions spontaneously resolve. If the patient is symptomatic, treatment options include a steroid injection; aspirating the cyst and then using a compressive cryosurgery tip to freeze the superficial and deep aspects of the cyst to scar it down; and surgical resection of the cyst.
In this case, the patient was in pain because the abnormally shaped nail was breaking. She elected to have a steroid injection into the cyst—0.25 ml of triamcinolone 20 mg/ml. The family physician (FP) advised her that this would reduce the inflammation in the distal interphalangeal joint, hopefully reducing the size of the digital mucous cyst. The FP also indicated that she might require several injections to achieve a positive outcome and that it would take 6 months for her fingernail to return to normal.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
The groove in the patient’s nail, and associated splitting, was due to a digital mucous cyst seen in the photo proximal to the cuticle.
In spite of the name, these cysts do not contain mucus. Instead, they contain synovial fluid and represent an outpouching of the distal interphalangeal joint of the index finger. This same process is called a ganglion cyst when it is seen at the wrist and a Baker’s cyst when it is behind the knee. These cysts typically form from an increase in synovial fluid, often due to underlying osteoarthritis, injury, or degenerative changes of the joint capsule. If the patient is asymptomatic, no treatment is required. Half of the lesions spontaneously resolve. If the patient is symptomatic, treatment options include a steroid injection; aspirating the cyst and then using a compressive cryosurgery tip to freeze the superficial and deep aspects of the cyst to scar it down; and surgical resection of the cyst.
In this case, the patient was in pain because the abnormally shaped nail was breaking. She elected to have a steroid injection into the cyst—0.25 ml of triamcinolone 20 mg/ml. The family physician (FP) advised her that this would reduce the inflammation in the distal interphalangeal joint, hopefully reducing the size of the digital mucous cyst. The FP also indicated that she might require several injections to achieve a positive outcome and that it would take 6 months for her fingernail to return to normal.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
The groove in the patient’s nail, and associated splitting, was due to a digital mucous cyst seen in the photo proximal to the cuticle.
In spite of the name, these cysts do not contain mucus. Instead, they contain synovial fluid and represent an outpouching of the distal interphalangeal joint of the index finger. This same process is called a ganglion cyst when it is seen at the wrist and a Baker’s cyst when it is behind the knee. These cysts typically form from an increase in synovial fluid, often due to underlying osteoarthritis, injury, or degenerative changes of the joint capsule. If the patient is asymptomatic, no treatment is required. Half of the lesions spontaneously resolve. If the patient is symptomatic, treatment options include a steroid injection; aspirating the cyst and then using a compressive cryosurgery tip to freeze the superficial and deep aspects of the cyst to scar it down; and surgical resection of the cyst.
In this case, the patient was in pain because the abnormally shaped nail was breaking. She elected to have a steroid injection into the cyst—0.25 ml of triamcinolone 20 mg/ml. The family physician (FP) advised her that this would reduce the inflammation in the distal interphalangeal joint, hopefully reducing the size of the digital mucous cyst. The FP also indicated that she might require several injections to achieve a positive outcome and that it would take 6 months for her fingernail to return to normal.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Which children are at greatest risk for atopic dermatitis?
MADRID – A parental history of asthma or allergic rhinitis significantly increases the risk that a child will develop atopic dermatitis, and that risk doubles if a parent has a history of atopic dermatitis rather than another atopic disease, Nina H. Ravn reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
She presented a comprehensive meta-analysis of 149 published studies addressing the risk of developing atopic dermatitis according to parental history of atopic disease. The studies included more than 656,000 participants. The picture that emerged from the meta-analysis was one of a stepwise increase in the risk of pediatric atopic dermatitis according to the type and number of parental atopic diseases present.
“This is something that hopefully can be useful when you talk with parents or parents-to-be with atopic diseases and they want to know how their disease might affect their child,” explained Ms. Ravn of the University of Copenhagen.
It’s also information that clinicians will find helpful in appropriately targeting primary prevention interventions if and when methods of proven efficacy become available. That’s a likely prospect, as this is now an extremely active field of research, she noted.
The meta-analysis showed that a parental history of atopic dermatitis was associated with a 3.3-fold greater risk of atopic dermatitis in the offspring than in families without a parental history of atopy. A parental history of asthma was associated with a 1.56-fold increased risk, while allergic rhinitis in a parent was linked to a 1.68-fold increased risk.
“It does matter what type of atopic disease the parents have,” she observed. “Those with a parental history of asthma or allergic rhinitis can be considered as being at more of an intermediate risk level, while those with a parental history of atopic dermatitis are a particularly high risk group.”
Of note, the risk of pediatric atopic dermatitis was the same regardless of whether the father or mother was the one with a history of atopic disease. If one parent had a history of an atopic disease, the pediatric risk was increased 1.3-fold compared to when the parental history was negative. If both parents had a history of atopic illness, the risk jumped to 2.08-fold. And if one parent had a history of more than one form of atopic disease, the pediatric risk of atopic dermatitis was increased 2.32-fold.
“An interesting result that was new to me what that fathers’ and mothers’ contribution to risk is equal,” said session cochair Andreas Wollenberg, MD, professor of dermatology at Ludwig Maximilian University of Munich. “For the past 2 decades we were always taught that the mother would have a greater impact on that risk.”
“I was also surprised by our findings,” Ms. Ravn replied. “But when we pooled all the data there really was no difference, nor in any of our subanalyses.”
She reported having no financial conflicts regarding her study.
SOURCE: Ravn NH. THE EADV CONGRESS.
MADRID – A parental history of asthma or allergic rhinitis significantly increases the risk that a child will develop atopic dermatitis, and that risk doubles if a parent has a history of atopic dermatitis rather than another atopic disease, Nina H. Ravn reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
She presented a comprehensive meta-analysis of 149 published studies addressing the risk of developing atopic dermatitis according to parental history of atopic disease. The studies included more than 656,000 participants. The picture that emerged from the meta-analysis was one of a stepwise increase in the risk of pediatric atopic dermatitis according to the type and number of parental atopic diseases present.
“This is something that hopefully can be useful when you talk with parents or parents-to-be with atopic diseases and they want to know how their disease might affect their child,” explained Ms. Ravn of the University of Copenhagen.
It’s also information that clinicians will find helpful in appropriately targeting primary prevention interventions if and when methods of proven efficacy become available. That’s a likely prospect, as this is now an extremely active field of research, she noted.
The meta-analysis showed that a parental history of atopic dermatitis was associated with a 3.3-fold greater risk of atopic dermatitis in the offspring than in families without a parental history of atopy. A parental history of asthma was associated with a 1.56-fold increased risk, while allergic rhinitis in a parent was linked to a 1.68-fold increased risk.
“It does matter what type of atopic disease the parents have,” she observed. “Those with a parental history of asthma or allergic rhinitis can be considered as being at more of an intermediate risk level, while those with a parental history of atopic dermatitis are a particularly high risk group.”
Of note, the risk of pediatric atopic dermatitis was the same regardless of whether the father or mother was the one with a history of atopic disease. If one parent had a history of an atopic disease, the pediatric risk was increased 1.3-fold compared to when the parental history was negative. If both parents had a history of atopic illness, the risk jumped to 2.08-fold. And if one parent had a history of more than one form of atopic disease, the pediatric risk of atopic dermatitis was increased 2.32-fold.
“An interesting result that was new to me what that fathers’ and mothers’ contribution to risk is equal,” said session cochair Andreas Wollenberg, MD, professor of dermatology at Ludwig Maximilian University of Munich. “For the past 2 decades we were always taught that the mother would have a greater impact on that risk.”
“I was also surprised by our findings,” Ms. Ravn replied. “But when we pooled all the data there really was no difference, nor in any of our subanalyses.”
She reported having no financial conflicts regarding her study.
SOURCE: Ravn NH. THE EADV CONGRESS.
MADRID – A parental history of asthma or allergic rhinitis significantly increases the risk that a child will develop atopic dermatitis, and that risk doubles if a parent has a history of atopic dermatitis rather than another atopic disease, Nina H. Ravn reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
She presented a comprehensive meta-analysis of 149 published studies addressing the risk of developing atopic dermatitis according to parental history of atopic disease. The studies included more than 656,000 participants. The picture that emerged from the meta-analysis was one of a stepwise increase in the risk of pediatric atopic dermatitis according to the type and number of parental atopic diseases present.
“This is something that hopefully can be useful when you talk with parents or parents-to-be with atopic diseases and they want to know how their disease might affect their child,” explained Ms. Ravn of the University of Copenhagen.
It’s also information that clinicians will find helpful in appropriately targeting primary prevention interventions if and when methods of proven efficacy become available. That’s a likely prospect, as this is now an extremely active field of research, she noted.
The meta-analysis showed that a parental history of atopic dermatitis was associated with a 3.3-fold greater risk of atopic dermatitis in the offspring than in families without a parental history of atopy. A parental history of asthma was associated with a 1.56-fold increased risk, while allergic rhinitis in a parent was linked to a 1.68-fold increased risk.
“It does matter what type of atopic disease the parents have,” she observed. “Those with a parental history of asthma or allergic rhinitis can be considered as being at more of an intermediate risk level, while those with a parental history of atopic dermatitis are a particularly high risk group.”
Of note, the risk of pediatric atopic dermatitis was the same regardless of whether the father or mother was the one with a history of atopic disease. If one parent had a history of an atopic disease, the pediatric risk was increased 1.3-fold compared to when the parental history was negative. If both parents had a history of atopic illness, the risk jumped to 2.08-fold. And if one parent had a history of more than one form of atopic disease, the pediatric risk of atopic dermatitis was increased 2.32-fold.
“An interesting result that was new to me what that fathers’ and mothers’ contribution to risk is equal,” said session cochair Andreas Wollenberg, MD, professor of dermatology at Ludwig Maximilian University of Munich. “For the past 2 decades we were always taught that the mother would have a greater impact on that risk.”
“I was also surprised by our findings,” Ms. Ravn replied. “But when we pooled all the data there really was no difference, nor in any of our subanalyses.”
She reported having no financial conflicts regarding her study.
SOURCE: Ravn NH. THE EADV CONGRESS.
REPORTING FROM The EADV CONGRESS
Key clinical point: Pediatric atopic dermatitis risk varies according to type of parental history of atopic disease.
Major finding: A parental history of atopic dermatitis is associated with a 3.3-fold increased risk of atopic dermatitis in the child, twice the risk associated with parental asthma or allergic rhinitis.
Study details: This was a systematic review and meta-analysis of 149 published studies with 656,711 participants.
Disclosures: The presenter reported having no financial conflicts regarding the study, conducted free of commercial support.
Source: Ravn NH. The EADV Congress.