User login
Fertility-sparing surgery is safe for most with epithelial ovarian cancer
Most young women with epithelial ovarian cancer can undergo surgery preserving the unaffected ovary and uterus – and thus their fertility – without compromising their survival, a cohort study of more than 9,000 women in Cancer suggests.
“Loss of reproductive capability and surgical menopause can negatively affect survivorship and quality of life among young women with ovarian cancer,” noted the investigators, who were led by Sarah M. Crafton, MD, division of gynecologic oncology, Allegheny Health Network in Pittsburgh. “ASCO has published guidelines to address the importance of implementing fertility preservation counseling as standard of care for all patients of reproductive age with cancer. However, the safety of such procedures should be thoroughly assessed in ongoing analyses.”
Dr. Crafton and colleagues used the Surveillance, Epidemiology, and End Results (SEER) program database and the National Cancer Database (NCDB) to retrospectively identify women 44 years old or younger with a primary epithelial ovarian cancer. The women were classified as having undergone surgery that spared fertility (unilateral salpingo-oophorectomy with uterine preservation) or surgery that did not (bilateral salpingo-oophorectomy with hysterectomy).
Study results, reported in Cancer, were based on 9,017 women – 3,932 from the SEER database and 5,085 from the NCDB – with epithelial ovarian cancer diagnosed between the ages of 15 and 44 years. Median follow-up was 6.5 years in SEER and 4.6 years in NCDB.
Overall, 26.1% of the SEER cohort and 24.8% of the NCDB cohort had undergone fertility-sparing surgery. In both cohorts, odds of this surgery were higher among younger women, those with a more recent ovarian cancer diagnosis, and those who did not receive adjuvant chemotherapy.
Among women with stage II-IV serous epithelial ovarian cancer in the SEER cohort, those who underwent fertility-sparing surgery had poorer overall survival (hazard ratio for death, 1.61; P = .0008). However, fertility-sparing surgery was not significantly associated with survival in other SEER subgroups defined by stage and grade or by stage and histology or in any NCDB subgroup defined by these parameters.
“In general, our findings regarding survival support the current National Comprehensive Cancer Network recommendation that fertility-sparing surgery can be considered as an alternative for traditional, comprehensive staging for those patients who desire fertility, for whom ovarian retention is technically feasible, and who have early-stage disease,” Dr. Crafton and coinvestigators wrote.
“Our observation of an increased risk of death associated with fertility-sparing surgery among women with advanced-stage, serous epithelial ovarian cancer in the SEER population supports the clinical recommendation that the decision to pursue fertility-sparing surgery should be individualized on the basis of patient/provider counseling and disease characteristics,” they concluded.
Dr. Crafton did not report any disclosures. The study was supported by the National Cancer Institute.
SOURCE: Crafton SM et al. Cancer. 2019 Nov 27. doi: 10.1002/cncr.32620.
Most young women with epithelial ovarian cancer can undergo surgery preserving the unaffected ovary and uterus – and thus their fertility – without compromising their survival, a cohort study of more than 9,000 women in Cancer suggests.
“Loss of reproductive capability and surgical menopause can negatively affect survivorship and quality of life among young women with ovarian cancer,” noted the investigators, who were led by Sarah M. Crafton, MD, division of gynecologic oncology, Allegheny Health Network in Pittsburgh. “ASCO has published guidelines to address the importance of implementing fertility preservation counseling as standard of care for all patients of reproductive age with cancer. However, the safety of such procedures should be thoroughly assessed in ongoing analyses.”
Dr. Crafton and colleagues used the Surveillance, Epidemiology, and End Results (SEER) program database and the National Cancer Database (NCDB) to retrospectively identify women 44 years old or younger with a primary epithelial ovarian cancer. The women were classified as having undergone surgery that spared fertility (unilateral salpingo-oophorectomy with uterine preservation) or surgery that did not (bilateral salpingo-oophorectomy with hysterectomy).
Study results, reported in Cancer, were based on 9,017 women – 3,932 from the SEER database and 5,085 from the NCDB – with epithelial ovarian cancer diagnosed between the ages of 15 and 44 years. Median follow-up was 6.5 years in SEER and 4.6 years in NCDB.
Overall, 26.1% of the SEER cohort and 24.8% of the NCDB cohort had undergone fertility-sparing surgery. In both cohorts, odds of this surgery were higher among younger women, those with a more recent ovarian cancer diagnosis, and those who did not receive adjuvant chemotherapy.
Among women with stage II-IV serous epithelial ovarian cancer in the SEER cohort, those who underwent fertility-sparing surgery had poorer overall survival (hazard ratio for death, 1.61; P = .0008). However, fertility-sparing surgery was not significantly associated with survival in other SEER subgroups defined by stage and grade or by stage and histology or in any NCDB subgroup defined by these parameters.
“In general, our findings regarding survival support the current National Comprehensive Cancer Network recommendation that fertility-sparing surgery can be considered as an alternative for traditional, comprehensive staging for those patients who desire fertility, for whom ovarian retention is technically feasible, and who have early-stage disease,” Dr. Crafton and coinvestigators wrote.
“Our observation of an increased risk of death associated with fertility-sparing surgery among women with advanced-stage, serous epithelial ovarian cancer in the SEER population supports the clinical recommendation that the decision to pursue fertility-sparing surgery should be individualized on the basis of patient/provider counseling and disease characteristics,” they concluded.
Dr. Crafton did not report any disclosures. The study was supported by the National Cancer Institute.
SOURCE: Crafton SM et al. Cancer. 2019 Nov 27. doi: 10.1002/cncr.32620.
Most young women with epithelial ovarian cancer can undergo surgery preserving the unaffected ovary and uterus – and thus their fertility – without compromising their survival, a cohort study of more than 9,000 women in Cancer suggests.
“Loss of reproductive capability and surgical menopause can negatively affect survivorship and quality of life among young women with ovarian cancer,” noted the investigators, who were led by Sarah M. Crafton, MD, division of gynecologic oncology, Allegheny Health Network in Pittsburgh. “ASCO has published guidelines to address the importance of implementing fertility preservation counseling as standard of care for all patients of reproductive age with cancer. However, the safety of such procedures should be thoroughly assessed in ongoing analyses.”
Dr. Crafton and colleagues used the Surveillance, Epidemiology, and End Results (SEER) program database and the National Cancer Database (NCDB) to retrospectively identify women 44 years old or younger with a primary epithelial ovarian cancer. The women were classified as having undergone surgery that spared fertility (unilateral salpingo-oophorectomy with uterine preservation) or surgery that did not (bilateral salpingo-oophorectomy with hysterectomy).
Study results, reported in Cancer, were based on 9,017 women – 3,932 from the SEER database and 5,085 from the NCDB – with epithelial ovarian cancer diagnosed between the ages of 15 and 44 years. Median follow-up was 6.5 years in SEER and 4.6 years in NCDB.
Overall, 26.1% of the SEER cohort and 24.8% of the NCDB cohort had undergone fertility-sparing surgery. In both cohorts, odds of this surgery were higher among younger women, those with a more recent ovarian cancer diagnosis, and those who did not receive adjuvant chemotherapy.
Among women with stage II-IV serous epithelial ovarian cancer in the SEER cohort, those who underwent fertility-sparing surgery had poorer overall survival (hazard ratio for death, 1.61; P = .0008). However, fertility-sparing surgery was not significantly associated with survival in other SEER subgroups defined by stage and grade or by stage and histology or in any NCDB subgroup defined by these parameters.
“In general, our findings regarding survival support the current National Comprehensive Cancer Network recommendation that fertility-sparing surgery can be considered as an alternative for traditional, comprehensive staging for those patients who desire fertility, for whom ovarian retention is technically feasible, and who have early-stage disease,” Dr. Crafton and coinvestigators wrote.
“Our observation of an increased risk of death associated with fertility-sparing surgery among women with advanced-stage, serous epithelial ovarian cancer in the SEER population supports the clinical recommendation that the decision to pursue fertility-sparing surgery should be individualized on the basis of patient/provider counseling and disease characteristics,” they concluded.
Dr. Crafton did not report any disclosures. The study was supported by the National Cancer Institute.
SOURCE: Crafton SM et al. Cancer. 2019 Nov 27. doi: 10.1002/cncr.32620.
FROM CANCER
CTS5 score partially validated for predicting late distant breast cancer recurrences
SAN ANTONIO – The Clinical Treatment Score post 5 years (CTS5) has been validated for the prediction of late distant recurrences in a large contemporary cohort of breast cancer patients drawn from the landmark TAILORx study – but only provided they’re over age 50 at the time of their initial breast cancer diagnosis, Ivana Sestak, PhD, reported at the San Antonio Breast Cancer Symposium.
“The CTS5 was much less prognostic in younger patients, and we did not observe good discrimination for the CTS5 in this cohort,” she said. “Further evaluation in premenopausal cohorts is needed before CTS5 can be applied to younger patients.”
She and her coworkers developed the CTS5 as a simple, expeditious tool to identify women at high risk of late distance recurrence of estrogen receptor–positive breast cancer after successfully completing 5 years of endocrine therapy. It’s designed to serve as an aid to physicians and patients in clinical decision making: Women who are CTS5 high risk are likely to benefit from extended endocrine therapy beyond the 5-year mark, while those at low risk are not.
“Trials so far have shown only a modest risk reduction of around 5% with extended endocrine therapy. This may be partly due to the fact that none of these trials had specifically selected patients who were at high risk of developing a late recurrence. It is therefore crucial that we identify those patients who are at high risk of late recurrence, as they will benefit most from extended endocrine therapy,” explained Dr. Sestak, a medical statistician at Queen Mary University, London.
The CTS5 calculator is freely available online at www.cts5-calculator.com. Clinicians simply plug in readily available information on four specific variables for their patients who have completed 5 years of endocrine therapy free of distant recurrence: age at breast cancer diagnosis, tumor size in millimeters, tumor grade, and number of involved nodes. The calculator promptly spits out a CTS5 score and the associated risk of distant recurrence during years 5-10 after initial diagnosis. That risk is categorized as low if it’s 5% or less in years 5-10, and high if it’s greater than 10%.
The CTS5 was developed and validated using long-term follow-up data on more than 11,000 postmenopausal breast cancer patients in the ATAC and BIG1-98 randomized trials. The CTS5 performed well in those tests. But those studies were completed more than a decade ago and were limited to postmenopausal patients. Dr. Sestak and coinvestigators wanted to assess the tool’s discriminatory powers in a contemporary population of breast cancer patients that included large numbers of premenopausal women. So they tapped into the National Cancer Institute–sponsored TAILORx study, which included 7,353 breast cancer patients who were distant recurrence free after 5 years. All had early-stage, hormone receptor–positive, HER2-negative, and axillary node–negative breast cancer. And all underwent baseline testing using Genomic Health’s Oncotype DX Breast Recurrence Score to assess expression of 21 genes associated with breast cancer recurrence.
The CTS5 proved to be highly prognostic in the overall TAILORx population. But upon drilling down further, Dr. Sestak and coworkers determined that CTS5 had only marginal prognostic value in the 2,259 women age 50 years or younger. Indeed, not a single patient in that age group was categorized as CTS5 high risk, and the actual distant recurrence rates during years 5-9 weren’t significantly different between the low- and intermediate-risk CTS5 groups.
In contrast, CTS5 performed well as a prognosticator in the 2,257 TAILORx participants over age 50 who received both chemotherapy and endocrine therapy during their first 5 years following diagnosis. For a fast and simple test with zero cost, it displayed impressive discriminatory power: The 63.8% of women classified as CTS5 low risk had a 2.6% distant recurrence rate – and thus constituted a group who could reasonably avoid extended endocrine therapy – while the 3.5% who were CTS5 high risk had a 9.5% event rate, and the intermediate-risk group had a 7.3% event rate. The prognostic power of CTS5 in the 2,837 women aged over 50 years who received only hormonal therapy was less robust, albeit still statistically significant.
In women classified as being at low risk of recurrence based upon an Oncotype DX score of 0-10, the CTS5 was not a significant prognosticator for the prediction of late distant recurrences. However, in those who were at intermediate or high risk as determined by a score of 11-100 on the Oncotype test, CTS5 was highly prognostic.
A significant limitation of this CTS5 validation study in the TAILORx population was that only a median 2.86 years of follow-up data after the 5-year mark was available – not sufficient time for a large number of distant recurrences. The rate was 3.1% in women treated with only endocrine therapy who had an Oncotype DX score of 0-25, and 3.8% in those with a score of 11-100 who received both chemotherapy and endocrine therapy.
Dr. Sestak shrugged off the less than stellar performance of the CTS5 in women aged age 50 years or younger in the TAILORx analysis.
“We developed the CTS5 specifically in postmenopausal women, so we’re not really surprised that it’s less prognostic in young women,” she said.
Her group next plans to evaluate the CTS5 in another large premenopausal cohort of breast cancer patients.
“If it’s not prognostic there, then we’ll have to adjust the algorithm and recalibrate it specifically for younger patients,” according to Dr. Sestak.
The TAILORx validation study was supported by Breast Cancer Now, Cancer Research UK, Exact Sciences, and the University of London. Dr. Sestak reported having received honoraria from Myriad Genetics, Nanostring Technology, and Pfizer Oncology.
SOURCE: Sestak I et al. SABCS 2019, Abstract GS4-03.
SAN ANTONIO – The Clinical Treatment Score post 5 years (CTS5) has been validated for the prediction of late distant recurrences in a large contemporary cohort of breast cancer patients drawn from the landmark TAILORx study – but only provided they’re over age 50 at the time of their initial breast cancer diagnosis, Ivana Sestak, PhD, reported at the San Antonio Breast Cancer Symposium.
“The CTS5 was much less prognostic in younger patients, and we did not observe good discrimination for the CTS5 in this cohort,” she said. “Further evaluation in premenopausal cohorts is needed before CTS5 can be applied to younger patients.”
She and her coworkers developed the CTS5 as a simple, expeditious tool to identify women at high risk of late distance recurrence of estrogen receptor–positive breast cancer after successfully completing 5 years of endocrine therapy. It’s designed to serve as an aid to physicians and patients in clinical decision making: Women who are CTS5 high risk are likely to benefit from extended endocrine therapy beyond the 5-year mark, while those at low risk are not.
“Trials so far have shown only a modest risk reduction of around 5% with extended endocrine therapy. This may be partly due to the fact that none of these trials had specifically selected patients who were at high risk of developing a late recurrence. It is therefore crucial that we identify those patients who are at high risk of late recurrence, as they will benefit most from extended endocrine therapy,” explained Dr. Sestak, a medical statistician at Queen Mary University, London.
The CTS5 calculator is freely available online at www.cts5-calculator.com. Clinicians simply plug in readily available information on four specific variables for their patients who have completed 5 years of endocrine therapy free of distant recurrence: age at breast cancer diagnosis, tumor size in millimeters, tumor grade, and number of involved nodes. The calculator promptly spits out a CTS5 score and the associated risk of distant recurrence during years 5-10 after initial diagnosis. That risk is categorized as low if it’s 5% or less in years 5-10, and high if it’s greater than 10%.
The CTS5 was developed and validated using long-term follow-up data on more than 11,000 postmenopausal breast cancer patients in the ATAC and BIG1-98 randomized trials. The CTS5 performed well in those tests. But those studies were completed more than a decade ago and were limited to postmenopausal patients. Dr. Sestak and coinvestigators wanted to assess the tool’s discriminatory powers in a contemporary population of breast cancer patients that included large numbers of premenopausal women. So they tapped into the National Cancer Institute–sponsored TAILORx study, which included 7,353 breast cancer patients who were distant recurrence free after 5 years. All had early-stage, hormone receptor–positive, HER2-negative, and axillary node–negative breast cancer. And all underwent baseline testing using Genomic Health’s Oncotype DX Breast Recurrence Score to assess expression of 21 genes associated with breast cancer recurrence.
The CTS5 proved to be highly prognostic in the overall TAILORx population. But upon drilling down further, Dr. Sestak and coworkers determined that CTS5 had only marginal prognostic value in the 2,259 women age 50 years or younger. Indeed, not a single patient in that age group was categorized as CTS5 high risk, and the actual distant recurrence rates during years 5-9 weren’t significantly different between the low- and intermediate-risk CTS5 groups.
In contrast, CTS5 performed well as a prognosticator in the 2,257 TAILORx participants over age 50 who received both chemotherapy and endocrine therapy during their first 5 years following diagnosis. For a fast and simple test with zero cost, it displayed impressive discriminatory power: The 63.8% of women classified as CTS5 low risk had a 2.6% distant recurrence rate – and thus constituted a group who could reasonably avoid extended endocrine therapy – while the 3.5% who were CTS5 high risk had a 9.5% event rate, and the intermediate-risk group had a 7.3% event rate. The prognostic power of CTS5 in the 2,837 women aged over 50 years who received only hormonal therapy was less robust, albeit still statistically significant.
In women classified as being at low risk of recurrence based upon an Oncotype DX score of 0-10, the CTS5 was not a significant prognosticator for the prediction of late distant recurrences. However, in those who were at intermediate or high risk as determined by a score of 11-100 on the Oncotype test, CTS5 was highly prognostic.
A significant limitation of this CTS5 validation study in the TAILORx population was that only a median 2.86 years of follow-up data after the 5-year mark was available – not sufficient time for a large number of distant recurrences. The rate was 3.1% in women treated with only endocrine therapy who had an Oncotype DX score of 0-25, and 3.8% in those with a score of 11-100 who received both chemotherapy and endocrine therapy.
Dr. Sestak shrugged off the less than stellar performance of the CTS5 in women aged age 50 years or younger in the TAILORx analysis.
“We developed the CTS5 specifically in postmenopausal women, so we’re not really surprised that it’s less prognostic in young women,” she said.
Her group next plans to evaluate the CTS5 in another large premenopausal cohort of breast cancer patients.
“If it’s not prognostic there, then we’ll have to adjust the algorithm and recalibrate it specifically for younger patients,” according to Dr. Sestak.
The TAILORx validation study was supported by Breast Cancer Now, Cancer Research UK, Exact Sciences, and the University of London. Dr. Sestak reported having received honoraria from Myriad Genetics, Nanostring Technology, and Pfizer Oncology.
SOURCE: Sestak I et al. SABCS 2019, Abstract GS4-03.
SAN ANTONIO – The Clinical Treatment Score post 5 years (CTS5) has been validated for the prediction of late distant recurrences in a large contemporary cohort of breast cancer patients drawn from the landmark TAILORx study – but only provided they’re over age 50 at the time of their initial breast cancer diagnosis, Ivana Sestak, PhD, reported at the San Antonio Breast Cancer Symposium.
“The CTS5 was much less prognostic in younger patients, and we did not observe good discrimination for the CTS5 in this cohort,” she said. “Further evaluation in premenopausal cohorts is needed before CTS5 can be applied to younger patients.”
She and her coworkers developed the CTS5 as a simple, expeditious tool to identify women at high risk of late distance recurrence of estrogen receptor–positive breast cancer after successfully completing 5 years of endocrine therapy. It’s designed to serve as an aid to physicians and patients in clinical decision making: Women who are CTS5 high risk are likely to benefit from extended endocrine therapy beyond the 5-year mark, while those at low risk are not.
“Trials so far have shown only a modest risk reduction of around 5% with extended endocrine therapy. This may be partly due to the fact that none of these trials had specifically selected patients who were at high risk of developing a late recurrence. It is therefore crucial that we identify those patients who are at high risk of late recurrence, as they will benefit most from extended endocrine therapy,” explained Dr. Sestak, a medical statistician at Queen Mary University, London.
The CTS5 calculator is freely available online at www.cts5-calculator.com. Clinicians simply plug in readily available information on four specific variables for their patients who have completed 5 years of endocrine therapy free of distant recurrence: age at breast cancer diagnosis, tumor size in millimeters, tumor grade, and number of involved nodes. The calculator promptly spits out a CTS5 score and the associated risk of distant recurrence during years 5-10 after initial diagnosis. That risk is categorized as low if it’s 5% or less in years 5-10, and high if it’s greater than 10%.
The CTS5 was developed and validated using long-term follow-up data on more than 11,000 postmenopausal breast cancer patients in the ATAC and BIG1-98 randomized trials. The CTS5 performed well in those tests. But those studies were completed more than a decade ago and were limited to postmenopausal patients. Dr. Sestak and coinvestigators wanted to assess the tool’s discriminatory powers in a contemporary population of breast cancer patients that included large numbers of premenopausal women. So they tapped into the National Cancer Institute–sponsored TAILORx study, which included 7,353 breast cancer patients who were distant recurrence free after 5 years. All had early-stage, hormone receptor–positive, HER2-negative, and axillary node–negative breast cancer. And all underwent baseline testing using Genomic Health’s Oncotype DX Breast Recurrence Score to assess expression of 21 genes associated with breast cancer recurrence.
The CTS5 proved to be highly prognostic in the overall TAILORx population. But upon drilling down further, Dr. Sestak and coworkers determined that CTS5 had only marginal prognostic value in the 2,259 women age 50 years or younger. Indeed, not a single patient in that age group was categorized as CTS5 high risk, and the actual distant recurrence rates during years 5-9 weren’t significantly different between the low- and intermediate-risk CTS5 groups.
In contrast, CTS5 performed well as a prognosticator in the 2,257 TAILORx participants over age 50 who received both chemotherapy and endocrine therapy during their first 5 years following diagnosis. For a fast and simple test with zero cost, it displayed impressive discriminatory power: The 63.8% of women classified as CTS5 low risk had a 2.6% distant recurrence rate – and thus constituted a group who could reasonably avoid extended endocrine therapy – while the 3.5% who were CTS5 high risk had a 9.5% event rate, and the intermediate-risk group had a 7.3% event rate. The prognostic power of CTS5 in the 2,837 women aged over 50 years who received only hormonal therapy was less robust, albeit still statistically significant.
In women classified as being at low risk of recurrence based upon an Oncotype DX score of 0-10, the CTS5 was not a significant prognosticator for the prediction of late distant recurrences. However, in those who were at intermediate or high risk as determined by a score of 11-100 on the Oncotype test, CTS5 was highly prognostic.
A significant limitation of this CTS5 validation study in the TAILORx population was that only a median 2.86 years of follow-up data after the 5-year mark was available – not sufficient time for a large number of distant recurrences. The rate was 3.1% in women treated with only endocrine therapy who had an Oncotype DX score of 0-25, and 3.8% in those with a score of 11-100 who received both chemotherapy and endocrine therapy.
Dr. Sestak shrugged off the less than stellar performance of the CTS5 in women aged age 50 years or younger in the TAILORx analysis.
“We developed the CTS5 specifically in postmenopausal women, so we’re not really surprised that it’s less prognostic in young women,” she said.
Her group next plans to evaluate the CTS5 in another large premenopausal cohort of breast cancer patients.
“If it’s not prognostic there, then we’ll have to adjust the algorithm and recalibrate it specifically for younger patients,” according to Dr. Sestak.
The TAILORx validation study was supported by Breast Cancer Now, Cancer Research UK, Exact Sciences, and the University of London. Dr. Sestak reported having received honoraria from Myriad Genetics, Nanostring Technology, and Pfizer Oncology.
SOURCE: Sestak I et al. SABCS 2019, Abstract GS4-03.
REPORTING FROM SABCS 2019
Being whole
Medicine is a rewarding but demanding field. Part of being a professional is handling the stresses of the job. That ability is as important as tying strong horizontal mattress sutures, choosing correct antibiotics to treat staph, and gently breaking bad news.
The divorce and suicide rate among physicians are evidence that many physicians handle stress poorly. Our rates of depression, burnout, alcoholism, and substance abuse are further evidence of the suffering caused by unmitigated stress. It is endemic and destructive, harming physicians, their loved ones, and their patients. While this situation has long been true, in the past decade its importance has become better recognized. Some scholars have added physician wellness or fulfillment as a fourth aim of medical care to complement the triple aims of patient outcomes, consumer experience, and financial stewardship.
The sources of stress can be either external or internal. Internally, many physicians feel torn between competing professional roles. Recently one fellow in pediatric intensive care wrote an insightful reflective essay about two conflicting roles (“Virtue and Suffering: Where the Personal and Professional Collide,” Lauren Rissman, MD. reflectivemeded.org). One side is the potential benefits of technology and modern medical interventions. The other side is compassion and knowing when to say enough. Finding that balance – or boundary – or mixture is difficult. Even more difficult is helping patients/parents who are struggling with those choices.
Some old models of the doctor-patient relationship insisted on an emotional detachment to promote objectivity. This often is paired with using nondirective counseling. The admonishment for a physician to be nondirective comes up in end-of-life care choices in the ICU. It comes up in genetic counseling, particularly in the prenatal time frame, and when I do ethics consults requiring values clarification and mediation. But I also have found times during shared decision making when the model of a fully informed consumer choice is not valid. There are situations in which a paradigm of emotional detachment impairs the ability to convey empathy, compassion, and presence. Being detached also may prevent the moments of personal connection between doctor and patient that are the intangible rewards of the vocation. A good physician knows how to choose among these idealized models. It requires being genuine when employing a diverse bag of bedside tools.
High technology and highly invasive care pose dilemmas in assessing outcomes, minimizing suffering, and ensuring financial stewardship. When one addresses those different types of dilemmas happening simultaneously, the initial approach can be to separate the different influences into separate vectors. But when one does this on a regular basis, it fractures one’s self-image. To survive and flourish, the physician juggling these competing, conflicting goals must shun the split personality and seek to live as an integrated moral agent. This integration is not achieved by working harder or longer or even smarter. It requires time and effort directed to self-reflection. When pediatric ethicists get together at conferences, I notice that about one-half of them are neonatal ICU docs and one-quarter are pediatric ICU docs. Many view their work in ethics as a survival mechanism. We all are looking for answers to questions we may not be able to fully articulate.
In this short column I will not endeavor to offer a neat package of advice on how to achieve being whole. Dr. Rissman in her essay is just starting her career while I’m nearing the end of mine. It is a lifelong process to integrate oneself rather than exist in turmoil. It truly is a journey, not a destination. After a career dedicated to considering technology, compassion, and costs, I know there are no simple solutions. I also know that it is important to keep seeking better answers.
To encourage group discussion of ethical problems, I have heard facilitators say that there are no right and wrong answers. I strongly disagree. In ethics, there often is more than one correct answer. Ethicists can write books on why one right answer is slightly better than another right answer for a particular individual or population. We live for debates over such minutiae. In the real world of medical ethics, there also are definitely wrong answers.
Professional athletes know the importance of recovery after an intense workout. Muscles have accumulated microscopic tears that must heal. Professional physicians must develop a personal regimen of caring for overexertion of their own emotional and moral/spiritual muscles in order to remain whole.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
Medicine is a rewarding but demanding field. Part of being a professional is handling the stresses of the job. That ability is as important as tying strong horizontal mattress sutures, choosing correct antibiotics to treat staph, and gently breaking bad news.
The divorce and suicide rate among physicians are evidence that many physicians handle stress poorly. Our rates of depression, burnout, alcoholism, and substance abuse are further evidence of the suffering caused by unmitigated stress. It is endemic and destructive, harming physicians, their loved ones, and their patients. While this situation has long been true, in the past decade its importance has become better recognized. Some scholars have added physician wellness or fulfillment as a fourth aim of medical care to complement the triple aims of patient outcomes, consumer experience, and financial stewardship.
The sources of stress can be either external or internal. Internally, many physicians feel torn between competing professional roles. Recently one fellow in pediatric intensive care wrote an insightful reflective essay about two conflicting roles (“Virtue and Suffering: Where the Personal and Professional Collide,” Lauren Rissman, MD. reflectivemeded.org). One side is the potential benefits of technology and modern medical interventions. The other side is compassion and knowing when to say enough. Finding that balance – or boundary – or mixture is difficult. Even more difficult is helping patients/parents who are struggling with those choices.
Some old models of the doctor-patient relationship insisted on an emotional detachment to promote objectivity. This often is paired with using nondirective counseling. The admonishment for a physician to be nondirective comes up in end-of-life care choices in the ICU. It comes up in genetic counseling, particularly in the prenatal time frame, and when I do ethics consults requiring values clarification and mediation. But I also have found times during shared decision making when the model of a fully informed consumer choice is not valid. There are situations in which a paradigm of emotional detachment impairs the ability to convey empathy, compassion, and presence. Being detached also may prevent the moments of personal connection between doctor and patient that are the intangible rewards of the vocation. A good physician knows how to choose among these idealized models. It requires being genuine when employing a diverse bag of bedside tools.
High technology and highly invasive care pose dilemmas in assessing outcomes, minimizing suffering, and ensuring financial stewardship. When one addresses those different types of dilemmas happening simultaneously, the initial approach can be to separate the different influences into separate vectors. But when one does this on a regular basis, it fractures one’s self-image. To survive and flourish, the physician juggling these competing, conflicting goals must shun the split personality and seek to live as an integrated moral agent. This integration is not achieved by working harder or longer or even smarter. It requires time and effort directed to self-reflection. When pediatric ethicists get together at conferences, I notice that about one-half of them are neonatal ICU docs and one-quarter are pediatric ICU docs. Many view their work in ethics as a survival mechanism. We all are looking for answers to questions we may not be able to fully articulate.
In this short column I will not endeavor to offer a neat package of advice on how to achieve being whole. Dr. Rissman in her essay is just starting her career while I’m nearing the end of mine. It is a lifelong process to integrate oneself rather than exist in turmoil. It truly is a journey, not a destination. After a career dedicated to considering technology, compassion, and costs, I know there are no simple solutions. I also know that it is important to keep seeking better answers.
To encourage group discussion of ethical problems, I have heard facilitators say that there are no right and wrong answers. I strongly disagree. In ethics, there often is more than one correct answer. Ethicists can write books on why one right answer is slightly better than another right answer for a particular individual or population. We live for debates over such minutiae. In the real world of medical ethics, there also are definitely wrong answers.
Professional athletes know the importance of recovery after an intense workout. Muscles have accumulated microscopic tears that must heal. Professional physicians must develop a personal regimen of caring for overexertion of their own emotional and moral/spiritual muscles in order to remain whole.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
Medicine is a rewarding but demanding field. Part of being a professional is handling the stresses of the job. That ability is as important as tying strong horizontal mattress sutures, choosing correct antibiotics to treat staph, and gently breaking bad news.
The divorce and suicide rate among physicians are evidence that many physicians handle stress poorly. Our rates of depression, burnout, alcoholism, and substance abuse are further evidence of the suffering caused by unmitigated stress. It is endemic and destructive, harming physicians, their loved ones, and their patients. While this situation has long been true, in the past decade its importance has become better recognized. Some scholars have added physician wellness or fulfillment as a fourth aim of medical care to complement the triple aims of patient outcomes, consumer experience, and financial stewardship.
The sources of stress can be either external or internal. Internally, many physicians feel torn between competing professional roles. Recently one fellow in pediatric intensive care wrote an insightful reflective essay about two conflicting roles (“Virtue and Suffering: Where the Personal and Professional Collide,” Lauren Rissman, MD. reflectivemeded.org). One side is the potential benefits of technology and modern medical interventions. The other side is compassion and knowing when to say enough. Finding that balance – or boundary – or mixture is difficult. Even more difficult is helping patients/parents who are struggling with those choices.
Some old models of the doctor-patient relationship insisted on an emotional detachment to promote objectivity. This often is paired with using nondirective counseling. The admonishment for a physician to be nondirective comes up in end-of-life care choices in the ICU. It comes up in genetic counseling, particularly in the prenatal time frame, and when I do ethics consults requiring values clarification and mediation. But I also have found times during shared decision making when the model of a fully informed consumer choice is not valid. There are situations in which a paradigm of emotional detachment impairs the ability to convey empathy, compassion, and presence. Being detached also may prevent the moments of personal connection between doctor and patient that are the intangible rewards of the vocation. A good physician knows how to choose among these idealized models. It requires being genuine when employing a diverse bag of bedside tools.
High technology and highly invasive care pose dilemmas in assessing outcomes, minimizing suffering, and ensuring financial stewardship. When one addresses those different types of dilemmas happening simultaneously, the initial approach can be to separate the different influences into separate vectors. But when one does this on a regular basis, it fractures one’s self-image. To survive and flourish, the physician juggling these competing, conflicting goals must shun the split personality and seek to live as an integrated moral agent. This integration is not achieved by working harder or longer or even smarter. It requires time and effort directed to self-reflection. When pediatric ethicists get together at conferences, I notice that about one-half of them are neonatal ICU docs and one-quarter are pediatric ICU docs. Many view their work in ethics as a survival mechanism. We all are looking for answers to questions we may not be able to fully articulate.
In this short column I will not endeavor to offer a neat package of advice on how to achieve being whole. Dr. Rissman in her essay is just starting her career while I’m nearing the end of mine. It is a lifelong process to integrate oneself rather than exist in turmoil. It truly is a journey, not a destination. After a career dedicated to considering technology, compassion, and costs, I know there are no simple solutions. I also know that it is important to keep seeking better answers.
To encourage group discussion of ethical problems, I have heard facilitators say that there are no right and wrong answers. I strongly disagree. In ethics, there often is more than one correct answer. Ethicists can write books on why one right answer is slightly better than another right answer for a particular individual or population. We live for debates over such minutiae. In the real world of medical ethics, there also are definitely wrong answers.
Professional athletes know the importance of recovery after an intense workout. Muscles have accumulated microscopic tears that must heal. Professional physicians must develop a personal regimen of caring for overexertion of their own emotional and moral/spiritual muscles in order to remain whole.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
Choosing Wisely® and its impact on low-value care
Focus energy on ‘low-hanging fruit’
It is a well-known fact that health care expenditure in the United States occupies a large proportion of its gross domestic product. In fact, it was 17.8% in 2016, almost twice what is expended in other advanced countries. However, this expenditure does not necessarily translate into optimal patient outcomes.
In 2012, the Institute of Medicine reported that the U.S. health care system wastes $750 billion per year in spending that does not provide any meaningful outcome to patients or the system; and patients can also suffer a financial impact from the delivery of low-value care.
In 2013, the Pediatrics Committee of the Society of Hospital Medicine published five recommendations through the Choosing Wisely® campaign aimed to decrease the use of low-value interventions. These recommendations were:
1. Do not order chest radiographs (CXR) in children with asthma or bronchiolitis.
2. Do not use systemic corticosteroids in children aged under 2 years with a lower respiratory tract infection.
3. Do not use bronchodilators in children with bronchiolitis.
4. Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy.
5. Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
This publication led to the implementation of quality improvement initiatives across different hospitals and institutions nationally. Eventually, a team of hospitalists developed a report card that could help measure the utilization of these interventions in hospitals that were part of the Children’s Hospital Association (CHA). The data stemming from the report card analysis would allow for benchmarking and comparing performance, as well as determining the secular trend in utilization of these procedures across the different institutions of the CHA.
Reyes et al. recently published the impact of utilization of these scorecards among all hospital members of the CHA in the Journal of Hospital Medicine, noting a positive impact of the SHM Choosing Wisely® recommendation in decreasing the utilization of low-value interventions. The authors compared the performance before and after the publication of the recommendations for a 9-year period (2008-2017). The most relevant impact occurred in children with bronchiolitis, with a decrease of 36% of bronchodilator use and of 31% in CXR utilization. In children with asthma, CXR utilization decreased by 20.8%. The authors found that, although there was a steady decrease in the utilization of low-value services, this was still limited.
What factors could impact the effectiveness of high-value quality initiatives? First of all, quality improvement requires a substantial investment of collective effort and time. It requires a change in culture that often involves changing longstanding paradigms. The Choosing Wisely® recommendations target a very specific, low-clinical-severity population – the focus is on “uncomplicated” disease. This is important as you don’t want to pursue aggressive unnecessary intervention in children and potentially cause harm – for example, unnecessary use of steroids in a child with uncomplicated bronchiolitis who may improve with nasal suctioning alone. There is a need to appraise patients with more complex presentation of these diseases (for example, patients that require escalation of care to ICU), and this is beyond the scope of Choosing Wisely®. Further research is needed to see if higher-value care interventions can be implemented among these higher acuity and severity patients.
In our institution, we have created specific care paths that facilitate following these recommendations. Essentially, we have leveraged the EHR order sets to avoid the inclusion of low-value interventions; all stakeholders (respiratory therapy, nursing, etc.) are aware of the care path and ensure compliance. Even further, as a consequence of the change in culture toward high-value care, we have identified low-value interventions in settings where high-value quality improvement can be implemented – for example, we found that at least 20% of noncritically ill children undergoing an appendectomy receive unnecessary antacid prophylaxis treatment.
Changes always start small; quality improvement requires a lot of effort, and we must focus our energy on “low-hanging fruit,” and also begin tackling higher complexity tasks. In the Choosing Wisely® manuscript cited above, the authors found that there was a change in performance with a tendency toward higher-value care, yet the change was not as substantial as originally thought.
How can we tackle higher complexity tasks if we find it difficult to implement solutions for those of lower complexity? My answer is simple. Maintain a consistent and continuous focus on high value, and ensure the message is iterative and redundant with feedback on performance, decrease in costs, and enhanced patient outcomes.
Dr. Auron is the quality improvement and patient safety officer in the department of hospital medicine at the Cleveland Clinic. He also serves as associate professor of medicine and pediatrics in the staff department of hospital medicine and department of pediatric hospital medicine. This article first appeared on the Hospital Leader, SHM’s official blog, at hospitalleader.org.
Focus energy on ‘low-hanging fruit’
Focus energy on ‘low-hanging fruit’
It is a well-known fact that health care expenditure in the United States occupies a large proportion of its gross domestic product. In fact, it was 17.8% in 2016, almost twice what is expended in other advanced countries. However, this expenditure does not necessarily translate into optimal patient outcomes.
In 2012, the Institute of Medicine reported that the U.S. health care system wastes $750 billion per year in spending that does not provide any meaningful outcome to patients or the system; and patients can also suffer a financial impact from the delivery of low-value care.
In 2013, the Pediatrics Committee of the Society of Hospital Medicine published five recommendations through the Choosing Wisely® campaign aimed to decrease the use of low-value interventions. These recommendations were:
1. Do not order chest radiographs (CXR) in children with asthma or bronchiolitis.
2. Do not use systemic corticosteroids in children aged under 2 years with a lower respiratory tract infection.
3. Do not use bronchodilators in children with bronchiolitis.
4. Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy.
5. Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
This publication led to the implementation of quality improvement initiatives across different hospitals and institutions nationally. Eventually, a team of hospitalists developed a report card that could help measure the utilization of these interventions in hospitals that were part of the Children’s Hospital Association (CHA). The data stemming from the report card analysis would allow for benchmarking and comparing performance, as well as determining the secular trend in utilization of these procedures across the different institutions of the CHA.
Reyes et al. recently published the impact of utilization of these scorecards among all hospital members of the CHA in the Journal of Hospital Medicine, noting a positive impact of the SHM Choosing Wisely® recommendation in decreasing the utilization of low-value interventions. The authors compared the performance before and after the publication of the recommendations for a 9-year period (2008-2017). The most relevant impact occurred in children with bronchiolitis, with a decrease of 36% of bronchodilator use and of 31% in CXR utilization. In children with asthma, CXR utilization decreased by 20.8%. The authors found that, although there was a steady decrease in the utilization of low-value services, this was still limited.
What factors could impact the effectiveness of high-value quality initiatives? First of all, quality improvement requires a substantial investment of collective effort and time. It requires a change in culture that often involves changing longstanding paradigms. The Choosing Wisely® recommendations target a very specific, low-clinical-severity population – the focus is on “uncomplicated” disease. This is important as you don’t want to pursue aggressive unnecessary intervention in children and potentially cause harm – for example, unnecessary use of steroids in a child with uncomplicated bronchiolitis who may improve with nasal suctioning alone. There is a need to appraise patients with more complex presentation of these diseases (for example, patients that require escalation of care to ICU), and this is beyond the scope of Choosing Wisely®. Further research is needed to see if higher-value care interventions can be implemented among these higher acuity and severity patients.
In our institution, we have created specific care paths that facilitate following these recommendations. Essentially, we have leveraged the EHR order sets to avoid the inclusion of low-value interventions; all stakeholders (respiratory therapy, nursing, etc.) are aware of the care path and ensure compliance. Even further, as a consequence of the change in culture toward high-value care, we have identified low-value interventions in settings where high-value quality improvement can be implemented – for example, we found that at least 20% of noncritically ill children undergoing an appendectomy receive unnecessary antacid prophylaxis treatment.
Changes always start small; quality improvement requires a lot of effort, and we must focus our energy on “low-hanging fruit,” and also begin tackling higher complexity tasks. In the Choosing Wisely® manuscript cited above, the authors found that there was a change in performance with a tendency toward higher-value care, yet the change was not as substantial as originally thought.
How can we tackle higher complexity tasks if we find it difficult to implement solutions for those of lower complexity? My answer is simple. Maintain a consistent and continuous focus on high value, and ensure the message is iterative and redundant with feedback on performance, decrease in costs, and enhanced patient outcomes.
Dr. Auron is the quality improvement and patient safety officer in the department of hospital medicine at the Cleveland Clinic. He also serves as associate professor of medicine and pediatrics in the staff department of hospital medicine and department of pediatric hospital medicine. This article first appeared on the Hospital Leader, SHM’s official blog, at hospitalleader.org.
It is a well-known fact that health care expenditure in the United States occupies a large proportion of its gross domestic product. In fact, it was 17.8% in 2016, almost twice what is expended in other advanced countries. However, this expenditure does not necessarily translate into optimal patient outcomes.
In 2012, the Institute of Medicine reported that the U.S. health care system wastes $750 billion per year in spending that does not provide any meaningful outcome to patients or the system; and patients can also suffer a financial impact from the delivery of low-value care.
In 2013, the Pediatrics Committee of the Society of Hospital Medicine published five recommendations through the Choosing Wisely® campaign aimed to decrease the use of low-value interventions. These recommendations were:
1. Do not order chest radiographs (CXR) in children with asthma or bronchiolitis.
2. Do not use systemic corticosteroids in children aged under 2 years with a lower respiratory tract infection.
3. Do not use bronchodilators in children with bronchiolitis.
4. Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy.
5. Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
This publication led to the implementation of quality improvement initiatives across different hospitals and institutions nationally. Eventually, a team of hospitalists developed a report card that could help measure the utilization of these interventions in hospitals that were part of the Children’s Hospital Association (CHA). The data stemming from the report card analysis would allow for benchmarking and comparing performance, as well as determining the secular trend in utilization of these procedures across the different institutions of the CHA.
Reyes et al. recently published the impact of utilization of these scorecards among all hospital members of the CHA in the Journal of Hospital Medicine, noting a positive impact of the SHM Choosing Wisely® recommendation in decreasing the utilization of low-value interventions. The authors compared the performance before and after the publication of the recommendations for a 9-year period (2008-2017). The most relevant impact occurred in children with bronchiolitis, with a decrease of 36% of bronchodilator use and of 31% in CXR utilization. In children with asthma, CXR utilization decreased by 20.8%. The authors found that, although there was a steady decrease in the utilization of low-value services, this was still limited.
What factors could impact the effectiveness of high-value quality initiatives? First of all, quality improvement requires a substantial investment of collective effort and time. It requires a change in culture that often involves changing longstanding paradigms. The Choosing Wisely® recommendations target a very specific, low-clinical-severity population – the focus is on “uncomplicated” disease. This is important as you don’t want to pursue aggressive unnecessary intervention in children and potentially cause harm – for example, unnecessary use of steroids in a child with uncomplicated bronchiolitis who may improve with nasal suctioning alone. There is a need to appraise patients with more complex presentation of these diseases (for example, patients that require escalation of care to ICU), and this is beyond the scope of Choosing Wisely®. Further research is needed to see if higher-value care interventions can be implemented among these higher acuity and severity patients.
In our institution, we have created specific care paths that facilitate following these recommendations. Essentially, we have leveraged the EHR order sets to avoid the inclusion of low-value interventions; all stakeholders (respiratory therapy, nursing, etc.) are aware of the care path and ensure compliance. Even further, as a consequence of the change in culture toward high-value care, we have identified low-value interventions in settings where high-value quality improvement can be implemented – for example, we found that at least 20% of noncritically ill children undergoing an appendectomy receive unnecessary antacid prophylaxis treatment.
Changes always start small; quality improvement requires a lot of effort, and we must focus our energy on “low-hanging fruit,” and also begin tackling higher complexity tasks. In the Choosing Wisely® manuscript cited above, the authors found that there was a change in performance with a tendency toward higher-value care, yet the change was not as substantial as originally thought.
How can we tackle higher complexity tasks if we find it difficult to implement solutions for those of lower complexity? My answer is simple. Maintain a consistent and continuous focus on high value, and ensure the message is iterative and redundant with feedback on performance, decrease in costs, and enhanced patient outcomes.
Dr. Auron is the quality improvement and patient safety officer in the department of hospital medicine at the Cleveland Clinic. He also serves as associate professor of medicine and pediatrics in the staff department of hospital medicine and department of pediatric hospital medicine. This article first appeared on the Hospital Leader, SHM’s official blog, at hospitalleader.org.
Consider a Donation to the SVS Foundation
The SVS Foundation is a fundamental part of the Society for Vascular Surgery, entrusted with supporting programs that advance our knowledge of vascular disease and improve the care we provide our patients and communities. A little while back the SVS Foundation published its 2019 Annual Report. This year, the report focuses on how past award recipients have used their grants to impact and improve patient care. More than $13 million in grants over the past three decades have given recipients the support they need to impact the lives of patients and those who provide care. Consider a donation today.
The SVS Foundation is a fundamental part of the Society for Vascular Surgery, entrusted with supporting programs that advance our knowledge of vascular disease and improve the care we provide our patients and communities. A little while back the SVS Foundation published its 2019 Annual Report. This year, the report focuses on how past award recipients have used their grants to impact and improve patient care. More than $13 million in grants over the past three decades have given recipients the support they need to impact the lives of patients and those who provide care. Consider a donation today.
The SVS Foundation is a fundamental part of the Society for Vascular Surgery, entrusted with supporting programs that advance our knowledge of vascular disease and improve the care we provide our patients and communities. A little while back the SVS Foundation published its 2019 Annual Report. This year, the report focuses on how past award recipients have used their grants to impact and improve patient care. More than $13 million in grants over the past three decades have given recipients the support they need to impact the lives of patients and those who provide care. Consider a donation today.
Zanubrutinib achieved high response rate in del(17p) CLL cohort
ORLANDO – Zanubrutinib has produced a high overall response rate in one the largest cohorts of patients with treatment-naive 17p-deletion chronic lymphocytic leukemia (CLL) studied to date.
An overall response rate of nearly 93% was seen in this 109-patient, high-risk cohort, enrolled as part of the phase 3 SEQUOIA study (BGB-3111-304), said Constantine S. Tam, MBBS, MD, of St. Vincent’s Hospital and Peter MacCallum Cancer Centre in Melbourne.
Tolerability of zanubrutinib was essentially consistent with previous reports of the agent as used in other B-cell malignancies, Dr. Tam said in an oral presentation of the results at the annual meeting of the American Society of Hematology.
Deletion of chromosome 17p13.1, or del(17p), is a marker of poor prognosis and poor response to chemotherapy in patients with CLL or small lymphocytic lymphoma (SLL). For patients with del(17p) CLL, the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become a standard of care, Dr. Tam said.
Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK occupancy and minimize off-target inhibition of TEC and epidermal growth factor receptor kinases. “What this effectively means is that we are able to dose this drug at levels much higher than that achievable with ibrutinib, and not get intolerable side effects,” Dr. Tam said.
Zanubrutinib has been approved in the United States for previously treated mantle cell lymphoma, and generated durable responses among CLL/SLL patients with or without del(17p) in a phase 1/2 study, according to Dr. Tam.
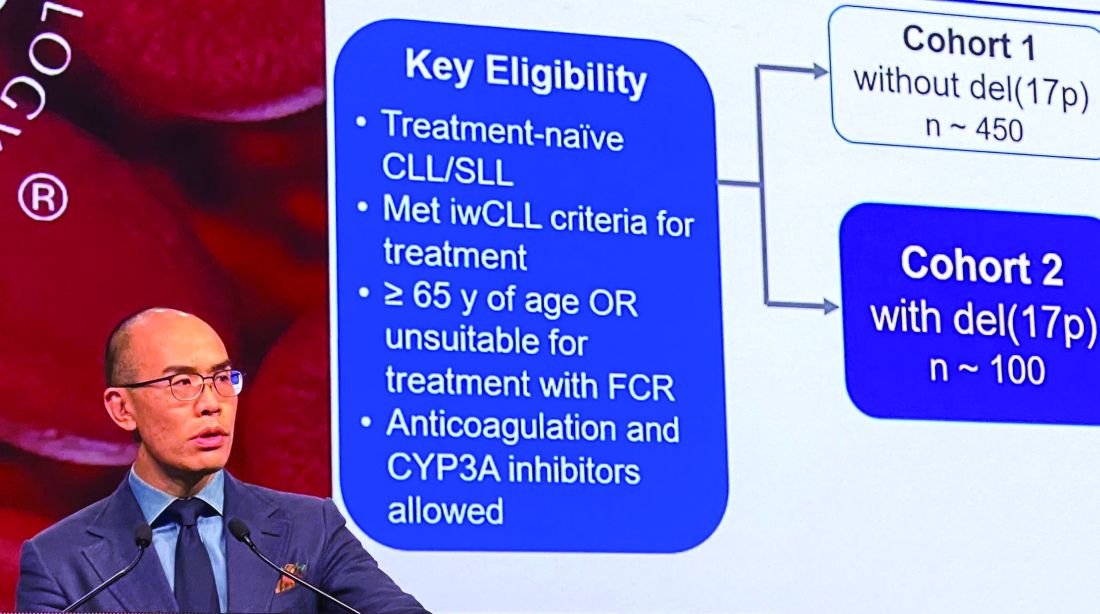
In the present study, which exclusively enrolled patients with del(17p) CLL/SLL, patients received 160 mg twice daily of zanubrutinib, Dr. Tam said. Out of 109 patients enrolled, 10 (9.2%) had SLL. All patients were aged at least 65 years or were deemed unsuitable for treatment with the combination of fludarabine, cyclophosphamide, and rituximab.
Of 109 patients enrolled, 104 received on-study treatment. The median age was 70 years, Dr. Tam reported, and a number of patients had other high-risk markers beyond del(17p), including unmutated IgVH status in 61.5% of patients.
With a median follow-up of 10 months, the overall response rate was 92.7%, including 1.9% complete responses and 78.9% partial responses. “Only one patient had primary progressive disease after starting this drug,” Dr. Tam said.
Time to response was rapid, according to the investigator, at about 2.8 months; after 6 months, 95% of responders remained in response.
Further analysis showed that the response rate was consistent across subgroups. “There was not a single group that did not respond with a high response rate, including poor prognostic groups,” Dr. Tam said.
Most adverse events were grade 1-2 in severity, and the most common events included confusion and upper respiratory tract infection. The only common grade 3 event, according to Dr. Tam, was neutropenia. Rates of grade 3 major bleeding were low, he said, and the rate of grade 3 atrial fibrillation was 0.9%. One patient died due to pneumonia.
The ongoing SEQUOIA study, designed to compare zanubrutinib to the combination of bendamustine and rituximab in patients with previously untreated CLL or SLL, is sponsored by BeiGene. Dr. Tam reported disclosures related to Novartis, Pharmacyclics, AbbVie, BeiGene, Janssen, and Roche.
SOURCE: Tam C et al. ASH 2019, Abstract 499.
ORLANDO – Zanubrutinib has produced a high overall response rate in one the largest cohorts of patients with treatment-naive 17p-deletion chronic lymphocytic leukemia (CLL) studied to date.
An overall response rate of nearly 93% was seen in this 109-patient, high-risk cohort, enrolled as part of the phase 3 SEQUOIA study (BGB-3111-304), said Constantine S. Tam, MBBS, MD, of St. Vincent’s Hospital and Peter MacCallum Cancer Centre in Melbourne.
Tolerability of zanubrutinib was essentially consistent with previous reports of the agent as used in other B-cell malignancies, Dr. Tam said in an oral presentation of the results at the annual meeting of the American Society of Hematology.
Deletion of chromosome 17p13.1, or del(17p), is a marker of poor prognosis and poor response to chemotherapy in patients with CLL or small lymphocytic lymphoma (SLL). For patients with del(17p) CLL, the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become a standard of care, Dr. Tam said.
Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK occupancy and minimize off-target inhibition of TEC and epidermal growth factor receptor kinases. “What this effectively means is that we are able to dose this drug at levels much higher than that achievable with ibrutinib, and not get intolerable side effects,” Dr. Tam said.
Zanubrutinib has been approved in the United States for previously treated mantle cell lymphoma, and generated durable responses among CLL/SLL patients with or without del(17p) in a phase 1/2 study, according to Dr. Tam.
In the present study, which exclusively enrolled patients with del(17p) CLL/SLL, patients received 160 mg twice daily of zanubrutinib, Dr. Tam said. Out of 109 patients enrolled, 10 (9.2%) had SLL. All patients were aged at least 65 years or were deemed unsuitable for treatment with the combination of fludarabine, cyclophosphamide, and rituximab.
Of 109 patients enrolled, 104 received on-study treatment. The median age was 70 years, Dr. Tam reported, and a number of patients had other high-risk markers beyond del(17p), including unmutated IgVH status in 61.5% of patients.
With a median follow-up of 10 months, the overall response rate was 92.7%, including 1.9% complete responses and 78.9% partial responses. “Only one patient had primary progressive disease after starting this drug,” Dr. Tam said.
Time to response was rapid, according to the investigator, at about 2.8 months; after 6 months, 95% of responders remained in response.
Further analysis showed that the response rate was consistent across subgroups. “There was not a single group that did not respond with a high response rate, including poor prognostic groups,” Dr. Tam said.
Most adverse events were grade 1-2 in severity, and the most common events included confusion and upper respiratory tract infection. The only common grade 3 event, according to Dr. Tam, was neutropenia. Rates of grade 3 major bleeding were low, he said, and the rate of grade 3 atrial fibrillation was 0.9%. One patient died due to pneumonia.
The ongoing SEQUOIA study, designed to compare zanubrutinib to the combination of bendamustine and rituximab in patients with previously untreated CLL or SLL, is sponsored by BeiGene. Dr. Tam reported disclosures related to Novartis, Pharmacyclics, AbbVie, BeiGene, Janssen, and Roche.
SOURCE: Tam C et al. ASH 2019, Abstract 499.
ORLANDO – Zanubrutinib has produced a high overall response rate in one the largest cohorts of patients with treatment-naive 17p-deletion chronic lymphocytic leukemia (CLL) studied to date.
An overall response rate of nearly 93% was seen in this 109-patient, high-risk cohort, enrolled as part of the phase 3 SEQUOIA study (BGB-3111-304), said Constantine S. Tam, MBBS, MD, of St. Vincent’s Hospital and Peter MacCallum Cancer Centre in Melbourne.
Tolerability of zanubrutinib was essentially consistent with previous reports of the agent as used in other B-cell malignancies, Dr. Tam said in an oral presentation of the results at the annual meeting of the American Society of Hematology.
Deletion of chromosome 17p13.1, or del(17p), is a marker of poor prognosis and poor response to chemotherapy in patients with CLL or small lymphocytic lymphoma (SLL). For patients with del(17p) CLL, the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become a standard of care, Dr. Tam said.
Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK occupancy and minimize off-target inhibition of TEC and epidermal growth factor receptor kinases. “What this effectively means is that we are able to dose this drug at levels much higher than that achievable with ibrutinib, and not get intolerable side effects,” Dr. Tam said.
Zanubrutinib has been approved in the United States for previously treated mantle cell lymphoma, and generated durable responses among CLL/SLL patients with or without del(17p) in a phase 1/2 study, according to Dr. Tam.
In the present study, which exclusively enrolled patients with del(17p) CLL/SLL, patients received 160 mg twice daily of zanubrutinib, Dr. Tam said. Out of 109 patients enrolled, 10 (9.2%) had SLL. All patients were aged at least 65 years or were deemed unsuitable for treatment with the combination of fludarabine, cyclophosphamide, and rituximab.
Of 109 patients enrolled, 104 received on-study treatment. The median age was 70 years, Dr. Tam reported, and a number of patients had other high-risk markers beyond del(17p), including unmutated IgVH status in 61.5% of patients.
With a median follow-up of 10 months, the overall response rate was 92.7%, including 1.9% complete responses and 78.9% partial responses. “Only one patient had primary progressive disease after starting this drug,” Dr. Tam said.
Time to response was rapid, according to the investigator, at about 2.8 months; after 6 months, 95% of responders remained in response.
Further analysis showed that the response rate was consistent across subgroups. “There was not a single group that did not respond with a high response rate, including poor prognostic groups,” Dr. Tam said.
Most adverse events were grade 1-2 in severity, and the most common events included confusion and upper respiratory tract infection. The only common grade 3 event, according to Dr. Tam, was neutropenia. Rates of grade 3 major bleeding were low, he said, and the rate of grade 3 atrial fibrillation was 0.9%. One patient died due to pneumonia.
The ongoing SEQUOIA study, designed to compare zanubrutinib to the combination of bendamustine and rituximab in patients with previously untreated CLL or SLL, is sponsored by BeiGene. Dr. Tam reported disclosures related to Novartis, Pharmacyclics, AbbVie, BeiGene, Janssen, and Roche.
SOURCE: Tam C et al. ASH 2019, Abstract 499.
REPORTING FROM ASH 2019
Cancer researchers win grants from DOD, GO2 Foundation
The GO2 Foundation for Lung Cancer has granted the 2019 Young Innovators Team Awards to two groups of investigators studying non-small cell lung cancer (NSCLC). Each group received $250,000 to support their work.
Yanxiang (Jessie) Guo, PhD, of Rutgers Cancer Institute of New Jersey, and Shawn Davidson, PhD, of Princeton University in New Jersey, won the award for their research on tumor metabolism and immunotherapy in KRAS-mutant NSCLC.
Dr. Guo and Dr. Davidson aim to prove that cancer cell metabolism affects the tumor microenvironment and leads to an impaired antitumor immune response. The pair’s ultimate goal is to overcome resistance to immunotherapy in KRAS-mutant NSCLC.
Matthew Bott, MD, of Memorial Sloan Kettering Cancer Center in New York, and Tuomas Tammela, MD, PhD, of Weill-Cornell Medical College in New York, won the award for their research on age-related differences in NSCLC.
Dr. Bott and Dr. Tammela aim to characterize differences in natural history and treatment response between younger and older patients with NSCLC. The main goal is to determine if different age groups require different approaches to treatment.
Two other researchers, both from Fox Chase Cancer Center in Philadelphia, received grants from the U.S. Department of Defense.
Jeffrey Peterson, PhD, was awarded a $1.4 million grant from the Department of Defense for his research on triple-negative breast cancer. Dr. Peterson will work with postdoctoral research fellows Alexander Beatty, PhD, and Tanu Singh, PhD, to determine if polyunsaturated fatty acids can be used to induce programmed cell death in triple-negative breast cancer.
Dr. Peterson theorizes that metastatic cells may be susceptible to preferential uptake of conjugated linoleic acid, which will trigger ferroptosis and destroy cancer cells without affecting normal cells. His ultimate goal is to set the stage for clinical trials of more effective, less toxic targeted therapies for triple-negative breast cancer.
Phillip Abbosh, MD, PhD, was awarded a $658,800 grant from the Department of Defense to investigate the role of the immune system and certain bacteria in mediating responses to therapy in bladder cancers.
Dr. Abbosh theorizes that a better understanding of the interaction between the immune system and bladder tumors could aid the development of new targeted therapies, and perhaps bacteria found in cancer patients’ bladders could be used to enhance treatment.
The GO2 Foundation for Lung Cancer has granted the 2019 Young Innovators Team Awards to two groups of investigators studying non-small cell lung cancer (NSCLC). Each group received $250,000 to support their work.
Yanxiang (Jessie) Guo, PhD, of Rutgers Cancer Institute of New Jersey, and Shawn Davidson, PhD, of Princeton University in New Jersey, won the award for their research on tumor metabolism and immunotherapy in KRAS-mutant NSCLC.
Dr. Guo and Dr. Davidson aim to prove that cancer cell metabolism affects the tumor microenvironment and leads to an impaired antitumor immune response. The pair’s ultimate goal is to overcome resistance to immunotherapy in KRAS-mutant NSCLC.
Matthew Bott, MD, of Memorial Sloan Kettering Cancer Center in New York, and Tuomas Tammela, MD, PhD, of Weill-Cornell Medical College in New York, won the award for their research on age-related differences in NSCLC.
Dr. Bott and Dr. Tammela aim to characterize differences in natural history and treatment response between younger and older patients with NSCLC. The main goal is to determine if different age groups require different approaches to treatment.
Two other researchers, both from Fox Chase Cancer Center in Philadelphia, received grants from the U.S. Department of Defense.
Jeffrey Peterson, PhD, was awarded a $1.4 million grant from the Department of Defense for his research on triple-negative breast cancer. Dr. Peterson will work with postdoctoral research fellows Alexander Beatty, PhD, and Tanu Singh, PhD, to determine if polyunsaturated fatty acids can be used to induce programmed cell death in triple-negative breast cancer.
Dr. Peterson theorizes that metastatic cells may be susceptible to preferential uptake of conjugated linoleic acid, which will trigger ferroptosis and destroy cancer cells without affecting normal cells. His ultimate goal is to set the stage for clinical trials of more effective, less toxic targeted therapies for triple-negative breast cancer.
Phillip Abbosh, MD, PhD, was awarded a $658,800 grant from the Department of Defense to investigate the role of the immune system and certain bacteria in mediating responses to therapy in bladder cancers.
Dr. Abbosh theorizes that a better understanding of the interaction between the immune system and bladder tumors could aid the development of new targeted therapies, and perhaps bacteria found in cancer patients’ bladders could be used to enhance treatment.
The GO2 Foundation for Lung Cancer has granted the 2019 Young Innovators Team Awards to two groups of investigators studying non-small cell lung cancer (NSCLC). Each group received $250,000 to support their work.
Yanxiang (Jessie) Guo, PhD, of Rutgers Cancer Institute of New Jersey, and Shawn Davidson, PhD, of Princeton University in New Jersey, won the award for their research on tumor metabolism and immunotherapy in KRAS-mutant NSCLC.
Dr. Guo and Dr. Davidson aim to prove that cancer cell metabolism affects the tumor microenvironment and leads to an impaired antitumor immune response. The pair’s ultimate goal is to overcome resistance to immunotherapy in KRAS-mutant NSCLC.
Matthew Bott, MD, of Memorial Sloan Kettering Cancer Center in New York, and Tuomas Tammela, MD, PhD, of Weill-Cornell Medical College in New York, won the award for their research on age-related differences in NSCLC.
Dr. Bott and Dr. Tammela aim to characterize differences in natural history and treatment response between younger and older patients with NSCLC. The main goal is to determine if different age groups require different approaches to treatment.
Two other researchers, both from Fox Chase Cancer Center in Philadelphia, received grants from the U.S. Department of Defense.
Jeffrey Peterson, PhD, was awarded a $1.4 million grant from the Department of Defense for his research on triple-negative breast cancer. Dr. Peterson will work with postdoctoral research fellows Alexander Beatty, PhD, and Tanu Singh, PhD, to determine if polyunsaturated fatty acids can be used to induce programmed cell death in triple-negative breast cancer.
Dr. Peterson theorizes that metastatic cells may be susceptible to preferential uptake of conjugated linoleic acid, which will trigger ferroptosis and destroy cancer cells without affecting normal cells. His ultimate goal is to set the stage for clinical trials of more effective, less toxic targeted therapies for triple-negative breast cancer.
Phillip Abbosh, MD, PhD, was awarded a $658,800 grant from the Department of Defense to investigate the role of the immune system and certain bacteria in mediating responses to therapy in bladder cancers.
Dr. Abbosh theorizes that a better understanding of the interaction between the immune system and bladder tumors could aid the development of new targeted therapies, and perhaps bacteria found in cancer patients’ bladders could be used to enhance treatment.
More than half of pediatric residents feel burned out
More than half of all pediatric residents report feeling burned out, in a trend that has now reached 3 years.
“The stable 54%-56% rate of burnout found during 2016-2018 in this study is similar to burnout rates previously reported in U.S. studies of pediatric residents,” Kathi Kemper, MD, Ohio State University, Columbus, and colleagues wrote (Pediatrics. 2019 Dec. 16. doi: 10.1542/peds.2019-1030).
The “alarming rate of physician burnout is a call to action on behalf of current trainees and future generations of doctors,” Jeanine Ronan, MD, of Children’s Hospital of Philadelphia, said in an accompanying editorial (Pediatrics. 2019 Dec. 16. doi: 10.1542/peds.2019-3210), adding that “although this concern was identified more than a decade ago, little progress has been made.”
The findings were based on the Pediatric Resident Burnout and Resilience Study Consortium surveys completed at 34 residency programs in 2016 (1,664 residents participated in the survey), 43 in 2017 (2,153 participated), and 49 in 2018 (2,241 participated). The 22-item Maslach Burnout Inventory Human Services Survey was used to assess burnout.
However, researchers noted that there were “no consistent significant differences between residents meeting criteria for burnout and those not meeting criteria for burnout in terms of any demographic characteristic,” the authors added.
For example, residency year and program size were associated with burnout in some years but not others.
There “were significant differences in burnout rates among postgraduate year 1 (PGY1), postgraduate year 2 (PGY2), and postgraduate year 3 (PGY3) in 2016 and 2017, but not in 2018,” the authors stated.
For 2016, burnout rates in PGY1, PGY2 and PGY3 were 36%, 34%, and 30%, respectively. In 2017, the rates changed to 33%, 36%, and 31%, respectively, while in 2018, the rates were reported to be 35% in the first postgraduate year and 33% each for the second and third postgraduate years.
Other findings from the survey revealed that residents who met the criteria for burnout reported worse mental health, more sleepiness, and greater stress, as well as lower mindfulness and self-compassion scores, less confidence in providing compassionate care, and lower levels of empathy and resilience.
“Residents who met the criteria for burnout were more likely to be on high-acuity rotations,” the Dr. Kemper and colleagues observed. “They were approximately twice as likely to report recently having made a medical error, more likely to report a work-life conflict, less likely to have had a vacation within the past month, and less likely to have had a recent weekend off than those who did not meet the criteria for burnout.
Additionally, residents reporting burnout “consistently reported significantly less satisfaction with support from family, spouse, friends, faculty, and colleagues,” the report states. “They also reported significantly lower quality of life, less satisfaction with their choice to go into pediatrics, and less satisfaction with their balance between personal and professional life.”
Dr. Ronan also noted that the survey “showed the negative impact of the environment on trainee performance and ability to cope with stress. Residency training programs should evaluate different scheduling and staffing models to determine if there are opportunities to enhance the learning environment.”
She also noted that given the link between electronic health record usage and administrative tasks and burnout, lessening administrative task burden on trainees may help decrease the rate of burnout.
Dr. Ronan added that mental health services also must be made readily available to trainees.
“The high burnout rate among pediatric residents must be addressed,” she said. “A comprehensive approach would include developing evidence-based training in mindfulness and coping techniques for the individual resident, an institutional approach to improve resident engagement and increase joy in the workplace, and the availability of mental health services when needed to address both urgent and chronic concerns.”
SOURCE: Kemper K et al. Pediatrics 2019 Dec 16. doi: 10.1542/peds.2019-1030.
More than half of all pediatric residents report feeling burned out, in a trend that has now reached 3 years.
“The stable 54%-56% rate of burnout found during 2016-2018 in this study is similar to burnout rates previously reported in U.S. studies of pediatric residents,” Kathi Kemper, MD, Ohio State University, Columbus, and colleagues wrote (Pediatrics. 2019 Dec. 16. doi: 10.1542/peds.2019-1030).
The “alarming rate of physician burnout is a call to action on behalf of current trainees and future generations of doctors,” Jeanine Ronan, MD, of Children’s Hospital of Philadelphia, said in an accompanying editorial (Pediatrics. 2019 Dec. 16. doi: 10.1542/peds.2019-3210), adding that “although this concern was identified more than a decade ago, little progress has been made.”
The findings were based on the Pediatric Resident Burnout and Resilience Study Consortium surveys completed at 34 residency programs in 2016 (1,664 residents participated in the survey), 43 in 2017 (2,153 participated), and 49 in 2018 (2,241 participated). The 22-item Maslach Burnout Inventory Human Services Survey was used to assess burnout.
However, researchers noted that there were “no consistent significant differences between residents meeting criteria for burnout and those not meeting criteria for burnout in terms of any demographic characteristic,” the authors added.
For example, residency year and program size were associated with burnout in some years but not others.
There “were significant differences in burnout rates among postgraduate year 1 (PGY1), postgraduate year 2 (PGY2), and postgraduate year 3 (PGY3) in 2016 and 2017, but not in 2018,” the authors stated.
For 2016, burnout rates in PGY1, PGY2 and PGY3 were 36%, 34%, and 30%, respectively. In 2017, the rates changed to 33%, 36%, and 31%, respectively, while in 2018, the rates were reported to be 35% in the first postgraduate year and 33% each for the second and third postgraduate years.
Other findings from the survey revealed that residents who met the criteria for burnout reported worse mental health, more sleepiness, and greater stress, as well as lower mindfulness and self-compassion scores, less confidence in providing compassionate care, and lower levels of empathy and resilience.
“Residents who met the criteria for burnout were more likely to be on high-acuity rotations,” the Dr. Kemper and colleagues observed. “They were approximately twice as likely to report recently having made a medical error, more likely to report a work-life conflict, less likely to have had a vacation within the past month, and less likely to have had a recent weekend off than those who did not meet the criteria for burnout.
Additionally, residents reporting burnout “consistently reported significantly less satisfaction with support from family, spouse, friends, faculty, and colleagues,” the report states. “They also reported significantly lower quality of life, less satisfaction with their choice to go into pediatrics, and less satisfaction with their balance between personal and professional life.”
Dr. Ronan also noted that the survey “showed the negative impact of the environment on trainee performance and ability to cope with stress. Residency training programs should evaluate different scheduling and staffing models to determine if there are opportunities to enhance the learning environment.”
She also noted that given the link between electronic health record usage and administrative tasks and burnout, lessening administrative task burden on trainees may help decrease the rate of burnout.
Dr. Ronan added that mental health services also must be made readily available to trainees.
“The high burnout rate among pediatric residents must be addressed,” she said. “A comprehensive approach would include developing evidence-based training in mindfulness and coping techniques for the individual resident, an institutional approach to improve resident engagement and increase joy in the workplace, and the availability of mental health services when needed to address both urgent and chronic concerns.”
SOURCE: Kemper K et al. Pediatrics 2019 Dec 16. doi: 10.1542/peds.2019-1030.
More than half of all pediatric residents report feeling burned out, in a trend that has now reached 3 years.
“The stable 54%-56% rate of burnout found during 2016-2018 in this study is similar to burnout rates previously reported in U.S. studies of pediatric residents,” Kathi Kemper, MD, Ohio State University, Columbus, and colleagues wrote (Pediatrics. 2019 Dec. 16. doi: 10.1542/peds.2019-1030).
The “alarming rate of physician burnout is a call to action on behalf of current trainees and future generations of doctors,” Jeanine Ronan, MD, of Children’s Hospital of Philadelphia, said in an accompanying editorial (Pediatrics. 2019 Dec. 16. doi: 10.1542/peds.2019-3210), adding that “although this concern was identified more than a decade ago, little progress has been made.”
The findings were based on the Pediatric Resident Burnout and Resilience Study Consortium surveys completed at 34 residency programs in 2016 (1,664 residents participated in the survey), 43 in 2017 (2,153 participated), and 49 in 2018 (2,241 participated). The 22-item Maslach Burnout Inventory Human Services Survey was used to assess burnout.
However, researchers noted that there were “no consistent significant differences between residents meeting criteria for burnout and those not meeting criteria for burnout in terms of any demographic characteristic,” the authors added.
For example, residency year and program size were associated with burnout in some years but not others.
There “were significant differences in burnout rates among postgraduate year 1 (PGY1), postgraduate year 2 (PGY2), and postgraduate year 3 (PGY3) in 2016 and 2017, but not in 2018,” the authors stated.
For 2016, burnout rates in PGY1, PGY2 and PGY3 were 36%, 34%, and 30%, respectively. In 2017, the rates changed to 33%, 36%, and 31%, respectively, while in 2018, the rates were reported to be 35% in the first postgraduate year and 33% each for the second and third postgraduate years.
Other findings from the survey revealed that residents who met the criteria for burnout reported worse mental health, more sleepiness, and greater stress, as well as lower mindfulness and self-compassion scores, less confidence in providing compassionate care, and lower levels of empathy and resilience.
“Residents who met the criteria for burnout were more likely to be on high-acuity rotations,” the Dr. Kemper and colleagues observed. “They were approximately twice as likely to report recently having made a medical error, more likely to report a work-life conflict, less likely to have had a vacation within the past month, and less likely to have had a recent weekend off than those who did not meet the criteria for burnout.
Additionally, residents reporting burnout “consistently reported significantly less satisfaction with support from family, spouse, friends, faculty, and colleagues,” the report states. “They also reported significantly lower quality of life, less satisfaction with their choice to go into pediatrics, and less satisfaction with their balance between personal and professional life.”
Dr. Ronan also noted that the survey “showed the negative impact of the environment on trainee performance and ability to cope with stress. Residency training programs should evaluate different scheduling and staffing models to determine if there are opportunities to enhance the learning environment.”
She also noted that given the link between electronic health record usage and administrative tasks and burnout, lessening administrative task burden on trainees may help decrease the rate of burnout.
Dr. Ronan added that mental health services also must be made readily available to trainees.
“The high burnout rate among pediatric residents must be addressed,” she said. “A comprehensive approach would include developing evidence-based training in mindfulness and coping techniques for the individual resident, an institutional approach to improve resident engagement and increase joy in the workplace, and the availability of mental health services when needed to address both urgent and chronic concerns.”
SOURCE: Kemper K et al. Pediatrics 2019 Dec 16. doi: 10.1542/peds.2019-1030.
FROM PEDIATRICS
Intersubject correlation analyses can be used to pinpoint neural patterns in ADHD
Functional MRI-based intersubject correlations (ISCs) hold promise for studying the neural bases of ADHD’s heterogeneous symptoms in situations that reflect real-world difficulties, new research shows.
“The present results provide the first evidence of a connection between symptom scales and brain activity recorded when the participants have been involved in a situation that is similar to the ones where their difficulties typically occur,” wrote Juha Salmi, of the department of neuroscience and biomedical engineering at Aalto University in Espoo, Finland, and associates. The study was published in NeuroImage.
Many imaging studies in ADHD are too narrow and fail to reflect real-world situations and distractions, the investigators wrote. For the current study, the investigators conducted fMRI scans of participants in “cocktail party” situations. During fMRI, the participants viewed excerpts from the 2008 Finnish film “Three Wise Men,” in which three men were talking.
“There were periods when no additional distractors were presented and periods when the film was embedded with irrelevant distractors that the participants were told to ignore. The three different distractors (white noise, green; jazz music, red; speech, magenta) and nondistracted periods (blue) were presented in a pseudo-randomized order so that all other distractor types had to occur before the same distractor type was presented again,” the authors wrote. Each distractor lasted for 15 seconds.
The ISC approach sought to gauge how much neural networks in each group, 51 with ADHD and 29 without, “ticked together” – that is, synchronized and coordinated in a feature-specific manner.
As expected, across the film, ISCs of those in the ADHD group were weaker than were those in the control group in multiple brain areas, including the left precuneus, bilateral medial occipital cortices, left lateral occipital cortex, left temporoparietal junction, and medial and posterior parts of the left superior temporal cortex. Likewise, when the other distractors occurred – with the exception of constant white noise – weaker ISCs were found among the ADHD group. In fact, there were no brain regions in which healthy controls had weaker ISCs than did those with ADHD.
“ the investigators wrote. “At least theoretically, this approach could be used to identify neural patterns reflecting specific symptoms in complex, dynamic situations.”
One limitation of naturalistic studies is that the inferences are by their nature more general than they might be in more conventional, discrete experiments. Bridging the gap between naturalistic studies such as this one and other more conventional designs are needed, the investigators wrote, because dove-tailing their findings could provide significant insights.
The study was supported by the Academy of Finland and the Åbo Akademi University Endowment for the BrainTrain project. None of the authors have any biomedical financial interests or potential conflicts of interest.
SOURCE: Salmi J et al. Neuroimage. 2019 Nov 12. doi: 10.1016/j.neuroimage.2019.116352.
Functional MRI-based intersubject correlations (ISCs) hold promise for studying the neural bases of ADHD’s heterogeneous symptoms in situations that reflect real-world difficulties, new research shows.
“The present results provide the first evidence of a connection between symptom scales and brain activity recorded when the participants have been involved in a situation that is similar to the ones where their difficulties typically occur,” wrote Juha Salmi, of the department of neuroscience and biomedical engineering at Aalto University in Espoo, Finland, and associates. The study was published in NeuroImage.
Many imaging studies in ADHD are too narrow and fail to reflect real-world situations and distractions, the investigators wrote. For the current study, the investigators conducted fMRI scans of participants in “cocktail party” situations. During fMRI, the participants viewed excerpts from the 2008 Finnish film “Three Wise Men,” in which three men were talking.
“There were periods when no additional distractors were presented and periods when the film was embedded with irrelevant distractors that the participants were told to ignore. The three different distractors (white noise, green; jazz music, red; speech, magenta) and nondistracted periods (blue) were presented in a pseudo-randomized order so that all other distractor types had to occur before the same distractor type was presented again,” the authors wrote. Each distractor lasted for 15 seconds.
The ISC approach sought to gauge how much neural networks in each group, 51 with ADHD and 29 without, “ticked together” – that is, synchronized and coordinated in a feature-specific manner.
As expected, across the film, ISCs of those in the ADHD group were weaker than were those in the control group in multiple brain areas, including the left precuneus, bilateral medial occipital cortices, left lateral occipital cortex, left temporoparietal junction, and medial and posterior parts of the left superior temporal cortex. Likewise, when the other distractors occurred – with the exception of constant white noise – weaker ISCs were found among the ADHD group. In fact, there were no brain regions in which healthy controls had weaker ISCs than did those with ADHD.
“ the investigators wrote. “At least theoretically, this approach could be used to identify neural patterns reflecting specific symptoms in complex, dynamic situations.”
One limitation of naturalistic studies is that the inferences are by their nature more general than they might be in more conventional, discrete experiments. Bridging the gap between naturalistic studies such as this one and other more conventional designs are needed, the investigators wrote, because dove-tailing their findings could provide significant insights.
The study was supported by the Academy of Finland and the Åbo Akademi University Endowment for the BrainTrain project. None of the authors have any biomedical financial interests or potential conflicts of interest.
SOURCE: Salmi J et al. Neuroimage. 2019 Nov 12. doi: 10.1016/j.neuroimage.2019.116352.
Functional MRI-based intersubject correlations (ISCs) hold promise for studying the neural bases of ADHD’s heterogeneous symptoms in situations that reflect real-world difficulties, new research shows.
“The present results provide the first evidence of a connection between symptom scales and brain activity recorded when the participants have been involved in a situation that is similar to the ones where their difficulties typically occur,” wrote Juha Salmi, of the department of neuroscience and biomedical engineering at Aalto University in Espoo, Finland, and associates. The study was published in NeuroImage.
Many imaging studies in ADHD are too narrow and fail to reflect real-world situations and distractions, the investigators wrote. For the current study, the investigators conducted fMRI scans of participants in “cocktail party” situations. During fMRI, the participants viewed excerpts from the 2008 Finnish film “Three Wise Men,” in which three men were talking.
“There were periods when no additional distractors were presented and periods when the film was embedded with irrelevant distractors that the participants were told to ignore. The three different distractors (white noise, green; jazz music, red; speech, magenta) and nondistracted periods (blue) were presented in a pseudo-randomized order so that all other distractor types had to occur before the same distractor type was presented again,” the authors wrote. Each distractor lasted for 15 seconds.
The ISC approach sought to gauge how much neural networks in each group, 51 with ADHD and 29 without, “ticked together” – that is, synchronized and coordinated in a feature-specific manner.
As expected, across the film, ISCs of those in the ADHD group were weaker than were those in the control group in multiple brain areas, including the left precuneus, bilateral medial occipital cortices, left lateral occipital cortex, left temporoparietal junction, and medial and posterior parts of the left superior temporal cortex. Likewise, when the other distractors occurred – with the exception of constant white noise – weaker ISCs were found among the ADHD group. In fact, there were no brain regions in which healthy controls had weaker ISCs than did those with ADHD.
“ the investigators wrote. “At least theoretically, this approach could be used to identify neural patterns reflecting specific symptoms in complex, dynamic situations.”
One limitation of naturalistic studies is that the inferences are by their nature more general than they might be in more conventional, discrete experiments. Bridging the gap between naturalistic studies such as this one and other more conventional designs are needed, the investigators wrote, because dove-tailing their findings could provide significant insights.
The study was supported by the Academy of Finland and the Åbo Akademi University Endowment for the BrainTrain project. None of the authors have any biomedical financial interests or potential conflicts of interest.
SOURCE: Salmi J et al. Neuroimage. 2019 Nov 12. doi: 10.1016/j.neuroimage.2019.116352.
FROM NEUROIMAGE
Heart rate changes during sleep may be diagnostic tool for depression
A heart rate–profiling algorithm shows promise at distinguishing differences in heart rate patterns during sleep between people with depression and healthy controls, research shows.
The algorithm was modeled using machine learning based on 1,203 polysomnograms from either people with depression or healthy controls, according to Mysa Saad, of the sleep research unit of the Royal’s Institute of Mental Health Research in Ottawa, and associates. That final algorithm was then tested on a new sample of 174 individuals (87 controls, 87 with depression) to categorize each person as either depressed or not depressed. This result was compared with medical record diagnoses. The study was published in BMC Psychiatry.
Compared with the control group, and in overall time. The algorithm incorrectly identified 15 patients with depression as being in the control group, and incorrectly identified 20 controls as having depression. The overall accuracy was 79.9%, with a sensitivity of 82.8% and a specificity of 77%.
“In addition to providing an improved biological underpinning for the diagnosis of depression, this [tool] could possibly offer supplemental information to psychiatric clinical assessment, and objective measures for early screening. Moreover, the use of distinct physiological variables as biomarkers of depression may help emphasize the interactions between mental and physical health. This may contribute to reducing the stigma associated with depression, lifting some social barriers to accessing psychiatric treatment, and allowing for more holistic patient care,” the investigators concluded.
Medibio provided partial funding for the salaries of research assistants; no other conflicts of interest were reported.
SOURCE: Saad M et al. BMC Psychiatry. 2019 Jun 7. doi: 10.1186/s12888-019-2152-1.
A heart rate–profiling algorithm shows promise at distinguishing differences in heart rate patterns during sleep between people with depression and healthy controls, research shows.
The algorithm was modeled using machine learning based on 1,203 polysomnograms from either people with depression or healthy controls, according to Mysa Saad, of the sleep research unit of the Royal’s Institute of Mental Health Research in Ottawa, and associates. That final algorithm was then tested on a new sample of 174 individuals (87 controls, 87 with depression) to categorize each person as either depressed or not depressed. This result was compared with medical record diagnoses. The study was published in BMC Psychiatry.
Compared with the control group, and in overall time. The algorithm incorrectly identified 15 patients with depression as being in the control group, and incorrectly identified 20 controls as having depression. The overall accuracy was 79.9%, with a sensitivity of 82.8% and a specificity of 77%.
“In addition to providing an improved biological underpinning for the diagnosis of depression, this [tool] could possibly offer supplemental information to psychiatric clinical assessment, and objective measures for early screening. Moreover, the use of distinct physiological variables as biomarkers of depression may help emphasize the interactions between mental and physical health. This may contribute to reducing the stigma associated with depression, lifting some social barriers to accessing psychiatric treatment, and allowing for more holistic patient care,” the investigators concluded.
Medibio provided partial funding for the salaries of research assistants; no other conflicts of interest were reported.
SOURCE: Saad M et al. BMC Psychiatry. 2019 Jun 7. doi: 10.1186/s12888-019-2152-1.
A heart rate–profiling algorithm shows promise at distinguishing differences in heart rate patterns during sleep between people with depression and healthy controls, research shows.
The algorithm was modeled using machine learning based on 1,203 polysomnograms from either people with depression or healthy controls, according to Mysa Saad, of the sleep research unit of the Royal’s Institute of Mental Health Research in Ottawa, and associates. That final algorithm was then tested on a new sample of 174 individuals (87 controls, 87 with depression) to categorize each person as either depressed or not depressed. This result was compared with medical record diagnoses. The study was published in BMC Psychiatry.
Compared with the control group, and in overall time. The algorithm incorrectly identified 15 patients with depression as being in the control group, and incorrectly identified 20 controls as having depression. The overall accuracy was 79.9%, with a sensitivity of 82.8% and a specificity of 77%.
“In addition to providing an improved biological underpinning for the diagnosis of depression, this [tool] could possibly offer supplemental information to psychiatric clinical assessment, and objective measures for early screening. Moreover, the use of distinct physiological variables as biomarkers of depression may help emphasize the interactions between mental and physical health. This may contribute to reducing the stigma associated with depression, lifting some social barriers to accessing psychiatric treatment, and allowing for more holistic patient care,” the investigators concluded.
Medibio provided partial funding for the salaries of research assistants; no other conflicts of interest were reported.
SOURCE: Saad M et al. BMC Psychiatry. 2019 Jun 7. doi: 10.1186/s12888-019-2152-1.
FROM BMC PSYCHIATRY