User login
Efficacy of postvenetoclax therapy may depend on prior agent exposure in CLL
ORLANDO – For a patient with chronic lymphocytic leukemia (CLL) who has discontinued venetoclax, choosing the best next therapy may depend on what novel agents the patient was exposed to and why they discontinued them, according to Anthony R. Mato, MD, with the Center for CLL at Memorial Sloan Kettering Cancer Center in New York.
If the patient is Bruton tyrosine kinase (BTK) inhibitor naive, then use of a BTK inhibitor after venetoclax would be supported, Dr. Mato said, by the high overall response rates and durable remissions that he and his coinvestigators documented in a retrospective, multicenter study designed specifically to address the gap in knowledge regarding what to use after venetoclax.
If the patient is BTK inhibitor exposed, then the reason for discontinuation needs to be considered before going with that venetoclax-to-BTK inhibitor sequence, Dr. Mato said during an oral presentation at the annual meeting of the American Society of Hematology.
“In patients with resistance to a BTK inhibitor, the sequence was not supported – it did not appear to be effective,” he said. “However, in the setting of intolerance, an alternate BTK inhibitor could be considered.”
The study did not support a venetoclax-to-PI3K inhibitor sequence in PI3K-naive patients, he added, noting that remissions did not appear to be durable, suggesting a potential overlap in resistance mechanisms between agents.
All told, the most effective therapies for in the postvenetoclax setting included the use of a BTK inhibitor in BTK inhibitor–naive or previously responsive patients, and allogeneic transplant following double novel-agent exposure.
“These data may provide support for venetoclax’s earlier use in the course of CLL, and may guide clinical practice and aid in the design of future clinical trials to address sequencing of novel agents,” Dr. Mato told attendees.
While prospective and real-world data clearly show that venetoclax is active in ibrutinib- or idelalisib-exposed patients, data are conversely “variable and limited” with regard to outcomes for next therapies following venetoclax.
“Current data addressing this key sequencing question, I feel, is a major limitation in supporting the sequence of venetoclax to a BTK inhibitor,” Dr. Mato said.
Accordingly, Dr. Mato and colleagues at 31 centers internationally planned and conducted this study, which included data on 326 patients treated with venetoclax who then discontinued for any reason.
“I wanted to highlight that 50% of the sites for this trial were recruited by a single tweet,” said Dr. Mato, adding that he and his coauthors received no funding to conduct this study and volunteered their time to complete it.
They found that, in BTK inhibitor–naive patients who discontinued venetoclax, subsequent BTK inhibitor treatment was associated with a high overall response rate and durable remissions, with a median progression-free survival (PFS) of 32 months.
In BTK inhibitor–exposed patients, response to postvenetoclax BTK inhibitor treatment depended on the reason for discontinuation, with a favorable result (PFS not reached with a mean follow-up of 7.7 months) in patients who were intolerant of the prior BTK inhibitor. By contrast, median PFS was only about 4 months for patients who were resistant to the prior BTK inhibitor.
PI3K inhibitors did not produce durable remissions after venetoclax, with a median PFS also of just 4 months, Dr. Mato reported.
However, cellular therapies appeared to be effective after venetoclax. Allogeneic hematopoietic stem cell transplantation was particularly effective, with the median PFS not reached, while chimeric antigen receptor T-cell therapy produced a PFS of 9 months.
Dr. Mato emphasized that the results of the retrospective trial were “hypothesis generating” and noted that patients in the study had received a median of 3, and up to 11, prior therapies. “This population are probably not our patients receiving venetoclax in clinical practice. They’re more heavily pretreated.”
Dr. Mato reported disclosures related to Gilead, AstraZeneca, AbbVie, Sunesis, Johnson & Johnson, TG Therapeutics, Loxo Oncology, DTRM Biopharma, Genentech, Janssen, Acerta Pharma, Pharmacyclics, and Celgene.
SOURCE: Mato AR et al. ASH 2019, Abstract 502.
ORLANDO – For a patient with chronic lymphocytic leukemia (CLL) who has discontinued venetoclax, choosing the best next therapy may depend on what novel agents the patient was exposed to and why they discontinued them, according to Anthony R. Mato, MD, with the Center for CLL at Memorial Sloan Kettering Cancer Center in New York.
If the patient is Bruton tyrosine kinase (BTK) inhibitor naive, then use of a BTK inhibitor after venetoclax would be supported, Dr. Mato said, by the high overall response rates and durable remissions that he and his coinvestigators documented in a retrospective, multicenter study designed specifically to address the gap in knowledge regarding what to use after venetoclax.
If the patient is BTK inhibitor exposed, then the reason for discontinuation needs to be considered before going with that venetoclax-to-BTK inhibitor sequence, Dr. Mato said during an oral presentation at the annual meeting of the American Society of Hematology.
“In patients with resistance to a BTK inhibitor, the sequence was not supported – it did not appear to be effective,” he said. “However, in the setting of intolerance, an alternate BTK inhibitor could be considered.”
The study did not support a venetoclax-to-PI3K inhibitor sequence in PI3K-naive patients, he added, noting that remissions did not appear to be durable, suggesting a potential overlap in resistance mechanisms between agents.
All told, the most effective therapies for in the postvenetoclax setting included the use of a BTK inhibitor in BTK inhibitor–naive or previously responsive patients, and allogeneic transplant following double novel-agent exposure.
“These data may provide support for venetoclax’s earlier use in the course of CLL, and may guide clinical practice and aid in the design of future clinical trials to address sequencing of novel agents,” Dr. Mato told attendees.
While prospective and real-world data clearly show that venetoclax is active in ibrutinib- or idelalisib-exposed patients, data are conversely “variable and limited” with regard to outcomes for next therapies following venetoclax.
“Current data addressing this key sequencing question, I feel, is a major limitation in supporting the sequence of venetoclax to a BTK inhibitor,” Dr. Mato said.
Accordingly, Dr. Mato and colleagues at 31 centers internationally planned and conducted this study, which included data on 326 patients treated with venetoclax who then discontinued for any reason.
“I wanted to highlight that 50% of the sites for this trial were recruited by a single tweet,” said Dr. Mato, adding that he and his coauthors received no funding to conduct this study and volunteered their time to complete it.
They found that, in BTK inhibitor–naive patients who discontinued venetoclax, subsequent BTK inhibitor treatment was associated with a high overall response rate and durable remissions, with a median progression-free survival (PFS) of 32 months.
In BTK inhibitor–exposed patients, response to postvenetoclax BTK inhibitor treatment depended on the reason for discontinuation, with a favorable result (PFS not reached with a mean follow-up of 7.7 months) in patients who were intolerant of the prior BTK inhibitor. By contrast, median PFS was only about 4 months for patients who were resistant to the prior BTK inhibitor.
PI3K inhibitors did not produce durable remissions after venetoclax, with a median PFS also of just 4 months, Dr. Mato reported.
However, cellular therapies appeared to be effective after venetoclax. Allogeneic hematopoietic stem cell transplantation was particularly effective, with the median PFS not reached, while chimeric antigen receptor T-cell therapy produced a PFS of 9 months.
Dr. Mato emphasized that the results of the retrospective trial were “hypothesis generating” and noted that patients in the study had received a median of 3, and up to 11, prior therapies. “This population are probably not our patients receiving venetoclax in clinical practice. They’re more heavily pretreated.”
Dr. Mato reported disclosures related to Gilead, AstraZeneca, AbbVie, Sunesis, Johnson & Johnson, TG Therapeutics, Loxo Oncology, DTRM Biopharma, Genentech, Janssen, Acerta Pharma, Pharmacyclics, and Celgene.
SOURCE: Mato AR et al. ASH 2019, Abstract 502.
ORLANDO – For a patient with chronic lymphocytic leukemia (CLL) who has discontinued venetoclax, choosing the best next therapy may depend on what novel agents the patient was exposed to and why they discontinued them, according to Anthony R. Mato, MD, with the Center for CLL at Memorial Sloan Kettering Cancer Center in New York.
If the patient is Bruton tyrosine kinase (BTK) inhibitor naive, then use of a BTK inhibitor after venetoclax would be supported, Dr. Mato said, by the high overall response rates and durable remissions that he and his coinvestigators documented in a retrospective, multicenter study designed specifically to address the gap in knowledge regarding what to use after venetoclax.
If the patient is BTK inhibitor exposed, then the reason for discontinuation needs to be considered before going with that venetoclax-to-BTK inhibitor sequence, Dr. Mato said during an oral presentation at the annual meeting of the American Society of Hematology.
“In patients with resistance to a BTK inhibitor, the sequence was not supported – it did not appear to be effective,” he said. “However, in the setting of intolerance, an alternate BTK inhibitor could be considered.”
The study did not support a venetoclax-to-PI3K inhibitor sequence in PI3K-naive patients, he added, noting that remissions did not appear to be durable, suggesting a potential overlap in resistance mechanisms between agents.
All told, the most effective therapies for in the postvenetoclax setting included the use of a BTK inhibitor in BTK inhibitor–naive or previously responsive patients, and allogeneic transplant following double novel-agent exposure.
“These data may provide support for venetoclax’s earlier use in the course of CLL, and may guide clinical practice and aid in the design of future clinical trials to address sequencing of novel agents,” Dr. Mato told attendees.
While prospective and real-world data clearly show that venetoclax is active in ibrutinib- or idelalisib-exposed patients, data are conversely “variable and limited” with regard to outcomes for next therapies following venetoclax.
“Current data addressing this key sequencing question, I feel, is a major limitation in supporting the sequence of venetoclax to a BTK inhibitor,” Dr. Mato said.
Accordingly, Dr. Mato and colleagues at 31 centers internationally planned and conducted this study, which included data on 326 patients treated with venetoclax who then discontinued for any reason.
“I wanted to highlight that 50% of the sites for this trial were recruited by a single tweet,” said Dr. Mato, adding that he and his coauthors received no funding to conduct this study and volunteered their time to complete it.
They found that, in BTK inhibitor–naive patients who discontinued venetoclax, subsequent BTK inhibitor treatment was associated with a high overall response rate and durable remissions, with a median progression-free survival (PFS) of 32 months.
In BTK inhibitor–exposed patients, response to postvenetoclax BTK inhibitor treatment depended on the reason for discontinuation, with a favorable result (PFS not reached with a mean follow-up of 7.7 months) in patients who were intolerant of the prior BTK inhibitor. By contrast, median PFS was only about 4 months for patients who were resistant to the prior BTK inhibitor.
PI3K inhibitors did not produce durable remissions after venetoclax, with a median PFS also of just 4 months, Dr. Mato reported.
However, cellular therapies appeared to be effective after venetoclax. Allogeneic hematopoietic stem cell transplantation was particularly effective, with the median PFS not reached, while chimeric antigen receptor T-cell therapy produced a PFS of 9 months.
Dr. Mato emphasized that the results of the retrospective trial were “hypothesis generating” and noted that patients in the study had received a median of 3, and up to 11, prior therapies. “This population are probably not our patients receiving venetoclax in clinical practice. They’re more heavily pretreated.”
Dr. Mato reported disclosures related to Gilead, AstraZeneca, AbbVie, Sunesis, Johnson & Johnson, TG Therapeutics, Loxo Oncology, DTRM Biopharma, Genentech, Janssen, Acerta Pharma, Pharmacyclics, and Celgene.
SOURCE: Mato AR et al. ASH 2019, Abstract 502.
REPORTING FROM ASH 2019
Contacts with health care professionals increased among adults
Adults aged 18-64 years were more likely to see or talk to a health care professional in 2017-2018 than they were in 2012-2013, according to the Centers for Disease Control and Prevention.
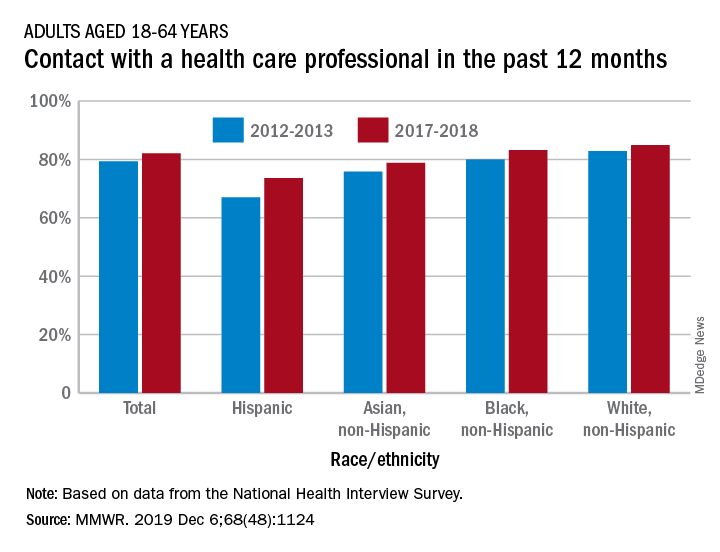
The percentage of American adults who had seen or talked to a health care professional in the past 12 months rose from 79.3% in 2012-2013 to 82.1% in 2017-2018, Michael E. Martinez, MPH, and Tainya C. Clarke, PhD, reported in the Morbidity and Mortality Weekly Report.
Analysis by race/ethnicity showed that Hispanic adults were still the least likely to have seen or talked to a health care professional in 2017-2018, even though they had the largest increase – more than six percentage points – between the two time periods, the CDC investigators reported.
White adults were the most likely to have seen or talked to a health care provider in both 2012-2013 and 2017-2018 but their 2.1-percentage-point increase over the course of the analysis was the smallest of the four groups included, based on data from the National Health Interview Survey.
SOURCE: Martinez ME, Clarke TC. MMWR. 2019 Dec 6;68(48):1124.
Adults aged 18-64 years were more likely to see or talk to a health care professional in 2017-2018 than they were in 2012-2013, according to the Centers for Disease Control and Prevention.

The percentage of American adults who had seen or talked to a health care professional in the past 12 months rose from 79.3% in 2012-2013 to 82.1% in 2017-2018, Michael E. Martinez, MPH, and Tainya C. Clarke, PhD, reported in the Morbidity and Mortality Weekly Report.
Analysis by race/ethnicity showed that Hispanic adults were still the least likely to have seen or talked to a health care professional in 2017-2018, even though they had the largest increase – more than six percentage points – between the two time periods, the CDC investigators reported.
White adults were the most likely to have seen or talked to a health care provider in both 2012-2013 and 2017-2018 but their 2.1-percentage-point increase over the course of the analysis was the smallest of the four groups included, based on data from the National Health Interview Survey.
SOURCE: Martinez ME, Clarke TC. MMWR. 2019 Dec 6;68(48):1124.
Adults aged 18-64 years were more likely to see or talk to a health care professional in 2017-2018 than they were in 2012-2013, according to the Centers for Disease Control and Prevention.

The percentage of American adults who had seen or talked to a health care professional in the past 12 months rose from 79.3% in 2012-2013 to 82.1% in 2017-2018, Michael E. Martinez, MPH, and Tainya C. Clarke, PhD, reported in the Morbidity and Mortality Weekly Report.
Analysis by race/ethnicity showed that Hispanic adults were still the least likely to have seen or talked to a health care professional in 2017-2018, even though they had the largest increase – more than six percentage points – between the two time periods, the CDC investigators reported.
White adults were the most likely to have seen or talked to a health care provider in both 2012-2013 and 2017-2018 but their 2.1-percentage-point increase over the course of the analysis was the smallest of the four groups included, based on data from the National Health Interview Survey.
SOURCE: Martinez ME, Clarke TC. MMWR. 2019 Dec 6;68(48):1124.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Task force advocates selective screening for abdominal aortic aneurysms
Men aged 65-75 years with a history of smoking should undergo one-time screening for abdominal aortic aneurysms (AAA), but clinicians can selectively screen men in this age group who don’t smoke, according to updated recommendations from the U.S. Preventive Services Task Force published in JAMA.
The task force issued a B recommendation for screening men aged 65-75 years with a smoking history and a C recommendation for selectively screening male never smokers in this age group in an update to the previous recommendations issued in 2014.
The task force also recommended against screening for AAA in women with no history of smoking (D recommendation) and cited insufficient evidence to make recommendations about AAA screening for women with a history of smoking or a family history of AAA (I statement).
The current prevalence of AAA in the United States is unclear because of the low rate of screening, but data from countries including the United Kingdom, Sweden, Denmark, and New Zealand have shown a decline in AAA among screened men aged 65 years and older, according to the USPSTF report.
Risk factors for AAA include smoking, male gender, older age, and having a first-degree relative with AAA, the task force noted.
In an evidence review accompanying the recommendations, Janelle M. Guirguis-Blake, MD, of the University of Washington, Tacoma, and colleagues analyzed data from 33 studies. They found a significant reduction in AAA-related mortality over 12-15 years’ follow-up among men aged 65 years and older who underwent AAA screening, compared with unscreened controls (odds ratio, 0.65). In addition, the risk of ruptures related to AAA was significantly lower over 12-15 years among men who underwent screening, compared with unscreened controls (OR, 0.62). However, no significant difference was noted in all-cause mortality over 12-15 years between screened and unscreened groups (relative risk, 0.99; 95% confidence interval, 0.98-1.00).
Data from four studies of early surgery to treat small aneurysms showed no significant difference in AAA-related mortality or all-cause mortality.
“Screening for AAA entails a simple, noninvasive, and focused ultrasonography examination that costs roughly $50. The only potential harms are the psychologic burden of knowing of the presence of an aneurysm and the risk of elective surgery,” wrote Marc Schermerhorn, MD, of Beth Israel Deaconess Medical Center, Boston, in an accompanying editorial published in JAMA Surgery (doi: 10.1001/jamasurg.2019.5234).
“The latter can be calculated for each patient, weighed against the risk of rupture, and together with the estimated life expectancy, should be factored into the decision to screen and the decision to operate. We as a country can do better to detect and treat this disease cost effectively for all appropriate patients including women and elderly individuals,” he said.
Dr. Schermerhorn noted that overall the recommendations are reasonable, but he expressed concern for three populations excluded from the guidelines that warrant additional consideration: nonsmokers with equivalent risk factors, patients older than 75 years, and women. “In the meantime, we should work to ensure that patients determined appropriate by the USPSTF are actually screened,” he said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose. Dr. Schermerhorn disclosed relationships with Abbott, Cook Medical, Endologix, Medtronic, and Philips.
SOURCE: Guirguis-Blake JM et al. JAMA. 2019. doi: 10.1001/jama.2019.17021.
Men aged 65-75 years with a history of smoking should undergo one-time screening for abdominal aortic aneurysms (AAA), but clinicians can selectively screen men in this age group who don’t smoke, according to updated recommendations from the U.S. Preventive Services Task Force published in JAMA.
The task force issued a B recommendation for screening men aged 65-75 years with a smoking history and a C recommendation for selectively screening male never smokers in this age group in an update to the previous recommendations issued in 2014.
The task force also recommended against screening for AAA in women with no history of smoking (D recommendation) and cited insufficient evidence to make recommendations about AAA screening for women with a history of smoking or a family history of AAA (I statement).
The current prevalence of AAA in the United States is unclear because of the low rate of screening, but data from countries including the United Kingdom, Sweden, Denmark, and New Zealand have shown a decline in AAA among screened men aged 65 years and older, according to the USPSTF report.
Risk factors for AAA include smoking, male gender, older age, and having a first-degree relative with AAA, the task force noted.
In an evidence review accompanying the recommendations, Janelle M. Guirguis-Blake, MD, of the University of Washington, Tacoma, and colleagues analyzed data from 33 studies. They found a significant reduction in AAA-related mortality over 12-15 years’ follow-up among men aged 65 years and older who underwent AAA screening, compared with unscreened controls (odds ratio, 0.65). In addition, the risk of ruptures related to AAA was significantly lower over 12-15 years among men who underwent screening, compared with unscreened controls (OR, 0.62). However, no significant difference was noted in all-cause mortality over 12-15 years between screened and unscreened groups (relative risk, 0.99; 95% confidence interval, 0.98-1.00).
Data from four studies of early surgery to treat small aneurysms showed no significant difference in AAA-related mortality or all-cause mortality.
“Screening for AAA entails a simple, noninvasive, and focused ultrasonography examination that costs roughly $50. The only potential harms are the psychologic burden of knowing of the presence of an aneurysm and the risk of elective surgery,” wrote Marc Schermerhorn, MD, of Beth Israel Deaconess Medical Center, Boston, in an accompanying editorial published in JAMA Surgery (doi: 10.1001/jamasurg.2019.5234).
“The latter can be calculated for each patient, weighed against the risk of rupture, and together with the estimated life expectancy, should be factored into the decision to screen and the decision to operate. We as a country can do better to detect and treat this disease cost effectively for all appropriate patients including women and elderly individuals,” he said.
Dr. Schermerhorn noted that overall the recommendations are reasonable, but he expressed concern for three populations excluded from the guidelines that warrant additional consideration: nonsmokers with equivalent risk factors, patients older than 75 years, and women. “In the meantime, we should work to ensure that patients determined appropriate by the USPSTF are actually screened,” he said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose. Dr. Schermerhorn disclosed relationships with Abbott, Cook Medical, Endologix, Medtronic, and Philips.
SOURCE: Guirguis-Blake JM et al. JAMA. 2019. doi: 10.1001/jama.2019.17021.
Men aged 65-75 years with a history of smoking should undergo one-time screening for abdominal aortic aneurysms (AAA), but clinicians can selectively screen men in this age group who don’t smoke, according to updated recommendations from the U.S. Preventive Services Task Force published in JAMA.
The task force issued a B recommendation for screening men aged 65-75 years with a smoking history and a C recommendation for selectively screening male never smokers in this age group in an update to the previous recommendations issued in 2014.
The task force also recommended against screening for AAA in women with no history of smoking (D recommendation) and cited insufficient evidence to make recommendations about AAA screening for women with a history of smoking or a family history of AAA (I statement).
The current prevalence of AAA in the United States is unclear because of the low rate of screening, but data from countries including the United Kingdom, Sweden, Denmark, and New Zealand have shown a decline in AAA among screened men aged 65 years and older, according to the USPSTF report.
Risk factors for AAA include smoking, male gender, older age, and having a first-degree relative with AAA, the task force noted.
In an evidence review accompanying the recommendations, Janelle M. Guirguis-Blake, MD, of the University of Washington, Tacoma, and colleagues analyzed data from 33 studies. They found a significant reduction in AAA-related mortality over 12-15 years’ follow-up among men aged 65 years and older who underwent AAA screening, compared with unscreened controls (odds ratio, 0.65). In addition, the risk of ruptures related to AAA was significantly lower over 12-15 years among men who underwent screening, compared with unscreened controls (OR, 0.62). However, no significant difference was noted in all-cause mortality over 12-15 years between screened and unscreened groups (relative risk, 0.99; 95% confidence interval, 0.98-1.00).
Data from four studies of early surgery to treat small aneurysms showed no significant difference in AAA-related mortality or all-cause mortality.
“Screening for AAA entails a simple, noninvasive, and focused ultrasonography examination that costs roughly $50. The only potential harms are the psychologic burden of knowing of the presence of an aneurysm and the risk of elective surgery,” wrote Marc Schermerhorn, MD, of Beth Israel Deaconess Medical Center, Boston, in an accompanying editorial published in JAMA Surgery (doi: 10.1001/jamasurg.2019.5234).
“The latter can be calculated for each patient, weighed against the risk of rupture, and together with the estimated life expectancy, should be factored into the decision to screen and the decision to operate. We as a country can do better to detect and treat this disease cost effectively for all appropriate patients including women and elderly individuals,” he said.
Dr. Schermerhorn noted that overall the recommendations are reasonable, but he expressed concern for three populations excluded from the guidelines that warrant additional consideration: nonsmokers with equivalent risk factors, patients older than 75 years, and women. “In the meantime, we should work to ensure that patients determined appropriate by the USPSTF are actually screened,” he said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose. Dr. Schermerhorn disclosed relationships with Abbott, Cook Medical, Endologix, Medtronic, and Philips.
SOURCE: Guirguis-Blake JM et al. JAMA. 2019. doi: 10.1001/jama.2019.17021.
FROM JAMA
Reduction in convulsive seizure frequency is associated with improved executive function in Dravet syndrome
BALTIMORE – according to data presented at the annual meeting of the American Epilepsy Society. Large reductions in convulsive seizure frequency for prolonged periods may improve everyday deficits in executive function in these patients, according to the investigators.
Dravet syndrome often entails cognitive impairment, including deficits in executive function. The frequency and severity of convulsive seizures are believed to worsen cognitive impairment over time, but few researchers have conducted long-term studies to test this hypothesis. Adjunctive fenfluramine significantly reduced the frequency of convulsive seizures and improved executive function after 14 weeks in a phase 3 study of patients with Dravet syndrome.
An open-label extension of a phase 3 study
In an open-label extension of this study, Joseph Sullivan, MD, director of the pediatric epilepsy center at the University of California, San Francisco, Benioff Children’s Hospital, and colleagues analyzed the relationship between changes in convulsive seizure frequency and executive function. The investigators also examined the effect of reducing convulsive seizure frequency by comparing patients with profound reductions (greater than 75%) versus patients with minimal reductions (less than 25%).
Patients aged 2-18 years entered the open-label study and received adjunctive fenfluramine for 1 year. At the beginning of the open-label phase, the dose was titrated to effect. The dose ranged from 0.2 mg/kg per day to 0.7 mg/kg per day and was administered as 2.5 mg/mL of fenfluramine. The maximum dose was 17 mg with stiripentol or 26 mg without.
The investigators calculated the percent difference in convulsive seizure frequency per 28 days from baseline to the end of the open-label study. They evaluated executive function using the Behavior Rating Inventory of Executive Function (BRIEF), which caregivers completed at baseline and year 1 for patients aged 5-18 years. Scores on the BRIEF were updated to the newer version: BRIEF2. Dr. Sullivan and colleagues calculated Spearman’s rho correlation coefficients to evaluate the association between BRIEF2 Behavior Regulation Index, Emotion Regulation Index, Cognitive Regulation Index, and Global Executive Composite scores. Lower scores on the BRIEF2 indexes and composite indicate better executive functioning. In addition, the researchers compared clinically meaningful change in BRIEF2 indexes and composite scores from baseline to year 1 between patients with minimal and profound reductions in convulsive seizure frequency using Fisher’s exact test. They defined a clinically meaningful change as an improvement in the Reliable Change Index of greater than 95%.
Profound reduction in seizure frequency was common
At the time of analysis, 53 patients had completed at least 1 year of open-label fenfluramine and had baseline and year 1 BRIEF2 data. Patients’ median age was 10 years, and 57% of patients were male. The median reduction from prerandomization baseline in convulsive seizure frequency was 71%. The reduction ranged from 99.7% to 55.0%.
Twenty-four (45%) patients had a reduction in convulsive seizure frequency of greater than 75%, and 11 (21%) had a reduction of less than 25%. Change in convulsive seizure frequency correlated significantly with Emotion Regulation Index and Global Executive Composite. Change in seizure frequency tended to correlate with Cognitive Regulation Index, but the result was not statistically significant. Change in convulsive seizure frequency was not significantly associated with Behavior Regulation Index. A significantly higher percentage of patients in the profound responder group had significant, clinically meaningful improvements on Emotion Regulation Index and Global Executive Composite, compared with minimal responders.
Zogenix, the company that is developing fenfluramine as a treatment for Dravet syndrome, funded the study. Several investigators are employees of Zogenix.
SOURCE: Bishop KI et al. AES 2019, Abstract 2.438.
BALTIMORE – according to data presented at the annual meeting of the American Epilepsy Society. Large reductions in convulsive seizure frequency for prolonged periods may improve everyday deficits in executive function in these patients, according to the investigators.
Dravet syndrome often entails cognitive impairment, including deficits in executive function. The frequency and severity of convulsive seizures are believed to worsen cognitive impairment over time, but few researchers have conducted long-term studies to test this hypothesis. Adjunctive fenfluramine significantly reduced the frequency of convulsive seizures and improved executive function after 14 weeks in a phase 3 study of patients with Dravet syndrome.
An open-label extension of a phase 3 study
In an open-label extension of this study, Joseph Sullivan, MD, director of the pediatric epilepsy center at the University of California, San Francisco, Benioff Children’s Hospital, and colleagues analyzed the relationship between changes in convulsive seizure frequency and executive function. The investigators also examined the effect of reducing convulsive seizure frequency by comparing patients with profound reductions (greater than 75%) versus patients with minimal reductions (less than 25%).
Patients aged 2-18 years entered the open-label study and received adjunctive fenfluramine for 1 year. At the beginning of the open-label phase, the dose was titrated to effect. The dose ranged from 0.2 mg/kg per day to 0.7 mg/kg per day and was administered as 2.5 mg/mL of fenfluramine. The maximum dose was 17 mg with stiripentol or 26 mg without.
The investigators calculated the percent difference in convulsive seizure frequency per 28 days from baseline to the end of the open-label study. They evaluated executive function using the Behavior Rating Inventory of Executive Function (BRIEF), which caregivers completed at baseline and year 1 for patients aged 5-18 years. Scores on the BRIEF were updated to the newer version: BRIEF2. Dr. Sullivan and colleagues calculated Spearman’s rho correlation coefficients to evaluate the association between BRIEF2 Behavior Regulation Index, Emotion Regulation Index, Cognitive Regulation Index, and Global Executive Composite scores. Lower scores on the BRIEF2 indexes and composite indicate better executive functioning. In addition, the researchers compared clinically meaningful change in BRIEF2 indexes and composite scores from baseline to year 1 between patients with minimal and profound reductions in convulsive seizure frequency using Fisher’s exact test. They defined a clinically meaningful change as an improvement in the Reliable Change Index of greater than 95%.
Profound reduction in seizure frequency was common
At the time of analysis, 53 patients had completed at least 1 year of open-label fenfluramine and had baseline and year 1 BRIEF2 data. Patients’ median age was 10 years, and 57% of patients were male. The median reduction from prerandomization baseline in convulsive seizure frequency was 71%. The reduction ranged from 99.7% to 55.0%.
Twenty-four (45%) patients had a reduction in convulsive seizure frequency of greater than 75%, and 11 (21%) had a reduction of less than 25%. Change in convulsive seizure frequency correlated significantly with Emotion Regulation Index and Global Executive Composite. Change in seizure frequency tended to correlate with Cognitive Regulation Index, but the result was not statistically significant. Change in convulsive seizure frequency was not significantly associated with Behavior Regulation Index. A significantly higher percentage of patients in the profound responder group had significant, clinically meaningful improvements on Emotion Regulation Index and Global Executive Composite, compared with minimal responders.
Zogenix, the company that is developing fenfluramine as a treatment for Dravet syndrome, funded the study. Several investigators are employees of Zogenix.
SOURCE: Bishop KI et al. AES 2019, Abstract 2.438.
BALTIMORE – according to data presented at the annual meeting of the American Epilepsy Society. Large reductions in convulsive seizure frequency for prolonged periods may improve everyday deficits in executive function in these patients, according to the investigators.
Dravet syndrome often entails cognitive impairment, including deficits in executive function. The frequency and severity of convulsive seizures are believed to worsen cognitive impairment over time, but few researchers have conducted long-term studies to test this hypothesis. Adjunctive fenfluramine significantly reduced the frequency of convulsive seizures and improved executive function after 14 weeks in a phase 3 study of patients with Dravet syndrome.
An open-label extension of a phase 3 study
In an open-label extension of this study, Joseph Sullivan, MD, director of the pediatric epilepsy center at the University of California, San Francisco, Benioff Children’s Hospital, and colleagues analyzed the relationship between changes in convulsive seizure frequency and executive function. The investigators also examined the effect of reducing convulsive seizure frequency by comparing patients with profound reductions (greater than 75%) versus patients with minimal reductions (less than 25%).
Patients aged 2-18 years entered the open-label study and received adjunctive fenfluramine for 1 year. At the beginning of the open-label phase, the dose was titrated to effect. The dose ranged from 0.2 mg/kg per day to 0.7 mg/kg per day and was administered as 2.5 mg/mL of fenfluramine. The maximum dose was 17 mg with stiripentol or 26 mg without.
The investigators calculated the percent difference in convulsive seizure frequency per 28 days from baseline to the end of the open-label study. They evaluated executive function using the Behavior Rating Inventory of Executive Function (BRIEF), which caregivers completed at baseline and year 1 for patients aged 5-18 years. Scores on the BRIEF were updated to the newer version: BRIEF2. Dr. Sullivan and colleagues calculated Spearman’s rho correlation coefficients to evaluate the association between BRIEF2 Behavior Regulation Index, Emotion Regulation Index, Cognitive Regulation Index, and Global Executive Composite scores. Lower scores on the BRIEF2 indexes and composite indicate better executive functioning. In addition, the researchers compared clinically meaningful change in BRIEF2 indexes and composite scores from baseline to year 1 between patients with minimal and profound reductions in convulsive seizure frequency using Fisher’s exact test. They defined a clinically meaningful change as an improvement in the Reliable Change Index of greater than 95%.
Profound reduction in seizure frequency was common
At the time of analysis, 53 patients had completed at least 1 year of open-label fenfluramine and had baseline and year 1 BRIEF2 data. Patients’ median age was 10 years, and 57% of patients were male. The median reduction from prerandomization baseline in convulsive seizure frequency was 71%. The reduction ranged from 99.7% to 55.0%.
Twenty-four (45%) patients had a reduction in convulsive seizure frequency of greater than 75%, and 11 (21%) had a reduction of less than 25%. Change in convulsive seizure frequency correlated significantly with Emotion Regulation Index and Global Executive Composite. Change in seizure frequency tended to correlate with Cognitive Regulation Index, but the result was not statistically significant. Change in convulsive seizure frequency was not significantly associated with Behavior Regulation Index. A significantly higher percentage of patients in the profound responder group had significant, clinically meaningful improvements on Emotion Regulation Index and Global Executive Composite, compared with minimal responders.
Zogenix, the company that is developing fenfluramine as a treatment for Dravet syndrome, funded the study. Several investigators are employees of Zogenix.
SOURCE: Bishop KI et al. AES 2019, Abstract 2.438.
REPORTING FROM AES 2019
Age, gender, and race influence treatment decisions for calcaneus fracture
, according to a new study on the management of calcaneus fractures.
“While our study demonstrated socio-demographic disparities regarding utilization of open reduction and internal fixation of calcaneus fractures, the exact reasons for these disparities remain unclear,” wrote Boris A. Zelle, MD, of UT Health San Antonio and his coauthors. The study was published in the Journal of Orthopaedic Surgery and Research.
To assess how certain variables might impact how patients manage decisions regarding orthopedic trauma surgery, the researchers identified 17,156 patients with closed calcaneus fractures via the National Inpatient Sample (NIS) and analyzed their treatment and demographic data. The statistical analysis included variables such as age, sex, insurance status, race/ethnicity, income, hospital location, and hospital size. A total of 59% of the patients (n = 10,117) underwent nonoperative management of their calcaneus fracture while 41% (n = 7,039) underwent open reduction and internal fixation. A multivariate logistic regression determined that variables like older age, female gender, being on Medicare, being African American or Hispanic, and having a lower estimated income by zip code were all associated with significantly lower use of surgical treatment (P less than .05). Not surprisingly, clinical comorbidities that were also associated with lower surgical rates included diabetes, peripheral vascular disease, a history of drug and alcohol abuse, and psychosis (P less than .05).
The authors acknowledged their study’s potential limitations, including a lack of research data on why these patients and providers chose surgery or otherwise. In addition, all available patient data came from a multicenter database, which can come with data entry issues. Finally, relying on data from only inpatient admissions “introduces a potential selection bias” by overrepresenting patients with “potentially more comorbidities and social issues.”
One author reported receiving consultant fees, speaker fees, and grant support from several organizations and medical corporations. The other authors reported no potential conflicts of interest.
SOURCE: Zelle BA et al. J Orthop Surg Res. 2019 Nov 12. doi: 10.1186/s13018-019-1402-8.
, according to a new study on the management of calcaneus fractures.
“While our study demonstrated socio-demographic disparities regarding utilization of open reduction and internal fixation of calcaneus fractures, the exact reasons for these disparities remain unclear,” wrote Boris A. Zelle, MD, of UT Health San Antonio and his coauthors. The study was published in the Journal of Orthopaedic Surgery and Research.
To assess how certain variables might impact how patients manage decisions regarding orthopedic trauma surgery, the researchers identified 17,156 patients with closed calcaneus fractures via the National Inpatient Sample (NIS) and analyzed their treatment and demographic data. The statistical analysis included variables such as age, sex, insurance status, race/ethnicity, income, hospital location, and hospital size. A total of 59% of the patients (n = 10,117) underwent nonoperative management of their calcaneus fracture while 41% (n = 7,039) underwent open reduction and internal fixation. A multivariate logistic regression determined that variables like older age, female gender, being on Medicare, being African American or Hispanic, and having a lower estimated income by zip code were all associated with significantly lower use of surgical treatment (P less than .05). Not surprisingly, clinical comorbidities that were also associated with lower surgical rates included diabetes, peripheral vascular disease, a history of drug and alcohol abuse, and psychosis (P less than .05).
The authors acknowledged their study’s potential limitations, including a lack of research data on why these patients and providers chose surgery or otherwise. In addition, all available patient data came from a multicenter database, which can come with data entry issues. Finally, relying on data from only inpatient admissions “introduces a potential selection bias” by overrepresenting patients with “potentially more comorbidities and social issues.”
One author reported receiving consultant fees, speaker fees, and grant support from several organizations and medical corporations. The other authors reported no potential conflicts of interest.
SOURCE: Zelle BA et al. J Orthop Surg Res. 2019 Nov 12. doi: 10.1186/s13018-019-1402-8.
, according to a new study on the management of calcaneus fractures.
“While our study demonstrated socio-demographic disparities regarding utilization of open reduction and internal fixation of calcaneus fractures, the exact reasons for these disparities remain unclear,” wrote Boris A. Zelle, MD, of UT Health San Antonio and his coauthors. The study was published in the Journal of Orthopaedic Surgery and Research.
To assess how certain variables might impact how patients manage decisions regarding orthopedic trauma surgery, the researchers identified 17,156 patients with closed calcaneus fractures via the National Inpatient Sample (NIS) and analyzed their treatment and demographic data. The statistical analysis included variables such as age, sex, insurance status, race/ethnicity, income, hospital location, and hospital size. A total of 59% of the patients (n = 10,117) underwent nonoperative management of their calcaneus fracture while 41% (n = 7,039) underwent open reduction and internal fixation. A multivariate logistic regression determined that variables like older age, female gender, being on Medicare, being African American or Hispanic, and having a lower estimated income by zip code were all associated with significantly lower use of surgical treatment (P less than .05). Not surprisingly, clinical comorbidities that were also associated with lower surgical rates included diabetes, peripheral vascular disease, a history of drug and alcohol abuse, and psychosis (P less than .05).
The authors acknowledged their study’s potential limitations, including a lack of research data on why these patients and providers chose surgery or otherwise. In addition, all available patient data came from a multicenter database, which can come with data entry issues. Finally, relying on data from only inpatient admissions “introduces a potential selection bias” by overrepresenting patients with “potentially more comorbidities and social issues.”
One author reported receiving consultant fees, speaker fees, and grant support from several organizations and medical corporations. The other authors reported no potential conflicts of interest.
SOURCE: Zelle BA et al. J Orthop Surg Res. 2019 Nov 12. doi: 10.1186/s13018-019-1402-8.
FROM THE JOURNAL OF ORTHOPAEDIC SURGERY AND RESEARCH
Negligent use of steroids
Question: Mr. M, a car mechanic, was treated with long-term ACTH and Kenalog after he developed severe contact dermatitis from daily exposure to petroleum-based solvents. His subsequent course was complicated by cataracts and osteoporosis. Which of the following is true in case he files a malpractice action?
A. Treatment with steroids was medically indicated for Mr. Mechanic’s dermatologic condition, so the doctor could not have breached the standard of care.
B. Under the “Learned Intermediary” doctrine, both the manufacturer and the prescribing doctor are jointly liable.
C. Corticosteroids are a known cause of osteoporosis and other complications, but not of cataracts, so that part of the malpractice action should be thrown out.
D. The plaintiff would prevail even if he could not find an expert witnesses to testify as to standard of care, since it is “common knowledge” that steroids cause osteoporosis.
E. Lack of informed consent may be his best legal theory of liability, as many jurisdictions now use the patient-centered standard, which does not require expert testimony.
Answer: E. The above hypothetical was modified from an old Montana case1 in which the patient failed in his negligence lawsuit because he did not have expert witnesses to testify as to standard of care and to adequacy of warning label. However, in some jurisdictions under today’s case law, informed consent relies on a subjective, i.e., patient-oriented standard, and expert testimony is unnecessary to prove breach of duty, although still needed to prove causation.
Steroid-related litigation
Steroid-related malpractice litigation is quite prevalent. In a retrospective study of a tertiary medical center from 1996 to 2008, Nash and coworkers identified 83 such cases.2 Steroids were prescribed for pain (23%), asthma or another pulmonary condition (20%), a dermatologic condition (18%), an autoimmune condition (17%), or allergies (6%).
Learned intermediary
“Drug reps” have a responsibility to inform doctors of both benefits and risks of their medications, a process termed “fair balance.” Generally speaking, if a doctor fails to warn the patient of a medication risk, and injury results, the patient may have a claim against the doctor but not the drug manufacturer. This is termed the “learned intermediary” doctrine, which is also applicable to medical devices such as dialysis equipment, breast implants, and blood products.
The justification is that manufacturers can reasonably rely on the treating doctor to warn of adverse effects, which are disclosed to the profession through their sales reps and in the package insert and PDR. The treating doctor, in turn, is expected to use his or her professional judgment to adequately warn the patient. It is simply not feasible for the manufacturer to directly warn every patient without usurping the doctor-patient relationship. However, where known complications were undisclosed to the FDA and the profession, then plaintiff attorneys can file class action lawsuits directed at the manufacturer.
Complications
Complications arising out of the use of steroids are typical examples of medical products liability. This may be on the basis of the doctor having prescribed the medication without a proper indication or where contraindicated, or may have prescribed “the wrong dose for the wrong patient by the wrong route.” In addition, there may have been a lack of informed consent, i.e., failure to explain the underlying condition and the material risks associated with using the drug. Other acts of negligence, e.g., vicarious liability, may also apply.
Corticosteroids such as Prednisone, Decadron, Kenalog, etc., are widely prescribed, and can cause serious complications, especially when used in high doses for extended periods. Examples include suppression of the immune system with supervening infections, steroid osteoporosis and fractures,3 aseptic necrosis, steroid diabetes, hypertension, emotional changes, weight gain, cataracts, neurological complications, and many others. As in all malpractice actions, the plaintiff bears the burden of proof covering the four requisite tort elements, i.e., duty, breach of duty, causation, and damages. Expert testimony is almost always needed in a professional negligence lawsuit.
Aseptic necrosis is a feared complication of steroid therapy.
A recent report4 featured a nurse in her 40s who developed aseptic necrosis of the right shoulder and both hips after taking high dose prednisone for 6 months. She was being treated for idiopathic thrombocytopenic purpura by a hematologist as well as sarcoidosis by a pulmonologist. The plaintiff claimed that both defendants negligently prescribed the medication for an extended period of time without proper monitoring, which caused her severe bone complications requiring a hip and shoulder replacement. The defendants maintained that the steroid medication was necessary to treat the life-threatening conditions from which the plaintiff suffered and that the dosage was carefully monitored and was not excessive. However, in a jury trial, the defendant hematologist and pulmonologist were each found 50% negligent, and the patient was awarded $4.1 million in damages.
In a case5 of steroid-related neurological sequelae, a Colorado jury awarded $14.9 million to a couple against an outpatient surgery center for negligently administering an epidural dose of Kenalog that rendered the patient paraplegic, and for failure to obtain informed consent. The jury awarded the woman, age 57, approximately $1.7 million in past and future medical expenses; $3.2 million in unspecified economic damages; and $6.5 million in past and future noneconomic damages such as pain and suffering. Her husband will receive $3.5 million in past and future noneconomic damages for loss of consortium, according to the verdict. Two years before the injection date of 2013, the drug maker had announced that Kenalog should not be used for epidural procedures because of cord complications including infarction and paraplegia.
Contributory role
The putative offending drug does not have to be the sole cause of injury; if it played a contributory role, the court may find the presence of liability. For example, a Kansas appeals court6 upheld a jury award of $2.88 million in the case of a 40-year-old man who took his life after neurologic complications followed an epidural injection. During one of patient’s visits for chronic low back pain, the defendant-anesthesiologist administered an epidural steroid injection into an area left swollen from a previous injection.
The patient developed neurologic symptoms, and lumbar puncture yielded green pus caused by methicillin-resistant Staphylococcus aureus. He went on to develop arachnoiditis, which left him with impotence, incontinence, and excruciating pain. His lawsuit contended the injection needle had passed through an infected edematous area, causing meningitis and arachnoiditis. Before the case went to trial, the patient took his life because of unremitting pain.
In March 2014, a Johnson County jury found the doctor 75% at fault and the clinic 25% at fault and awarded damages, which were reduced to $1.67 million because Kansas caps noneconomic damages at $250,000. The court rejected the defendants’ argument that the trial judge improperly instructed the jury it could find liability only if negligence “caused” rather than merely “contributed to” the patient’s death, holding that “... one who contributes to a wrongful death is a cause of that death as contemplated by the wrongful death statute.”
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at siang@hawaii.edu .
References
1. Hill v. Squibb Sons, E.R, 592 P.2d 1383 (Mont. 1979).
2. Nash JJ et al, Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011; 144:10-5.
3. Buckley L. et al, Glucocorticoid-Induced Osteoporosis. N Engl J Med 2018; 379:2547-56.
4. Zarin’s Jury Verdict: Review and Analysis. Article ID 40229, Philadelphia County.
5. Robbin Smith et al. v. The Surgery Center at Lone Tree, 2015-CV-30922, Douglas County District Court, Colo. Verdict for plaintiff, March 23, 2017.
6. Burnette v. Kimber L. Eubanks, M.D., & Paincare, P.A., 379 P.3d 372 (Kan. Ct. App. 2016).
Question: Mr. M, a car mechanic, was treated with long-term ACTH and Kenalog after he developed severe contact dermatitis from daily exposure to petroleum-based solvents. His subsequent course was complicated by cataracts and osteoporosis. Which of the following is true in case he files a malpractice action?
A. Treatment with steroids was medically indicated for Mr. Mechanic’s dermatologic condition, so the doctor could not have breached the standard of care.
B. Under the “Learned Intermediary” doctrine, both the manufacturer and the prescribing doctor are jointly liable.
C. Corticosteroids are a known cause of osteoporosis and other complications, but not of cataracts, so that part of the malpractice action should be thrown out.
D. The plaintiff would prevail even if he could not find an expert witnesses to testify as to standard of care, since it is “common knowledge” that steroids cause osteoporosis.
E. Lack of informed consent may be his best legal theory of liability, as many jurisdictions now use the patient-centered standard, which does not require expert testimony.
Answer: E. The above hypothetical was modified from an old Montana case1 in which the patient failed in his negligence lawsuit because he did not have expert witnesses to testify as to standard of care and to adequacy of warning label. However, in some jurisdictions under today’s case law, informed consent relies on a subjective, i.e., patient-oriented standard, and expert testimony is unnecessary to prove breach of duty, although still needed to prove causation.
Steroid-related litigation
Steroid-related malpractice litigation is quite prevalent. In a retrospective study of a tertiary medical center from 1996 to 2008, Nash and coworkers identified 83 such cases.2 Steroids were prescribed for pain (23%), asthma or another pulmonary condition (20%), a dermatologic condition (18%), an autoimmune condition (17%), or allergies (6%).
Learned intermediary
“Drug reps” have a responsibility to inform doctors of both benefits and risks of their medications, a process termed “fair balance.” Generally speaking, if a doctor fails to warn the patient of a medication risk, and injury results, the patient may have a claim against the doctor but not the drug manufacturer. This is termed the “learned intermediary” doctrine, which is also applicable to medical devices such as dialysis equipment, breast implants, and blood products.
The justification is that manufacturers can reasonably rely on the treating doctor to warn of adverse effects, which are disclosed to the profession through their sales reps and in the package insert and PDR. The treating doctor, in turn, is expected to use his or her professional judgment to adequately warn the patient. It is simply not feasible for the manufacturer to directly warn every patient without usurping the doctor-patient relationship. However, where known complications were undisclosed to the FDA and the profession, then plaintiff attorneys can file class action lawsuits directed at the manufacturer.
Complications
Complications arising out of the use of steroids are typical examples of medical products liability. This may be on the basis of the doctor having prescribed the medication without a proper indication or where contraindicated, or may have prescribed “the wrong dose for the wrong patient by the wrong route.” In addition, there may have been a lack of informed consent, i.e., failure to explain the underlying condition and the material risks associated with using the drug. Other acts of negligence, e.g., vicarious liability, may also apply.
Corticosteroids such as Prednisone, Decadron, Kenalog, etc., are widely prescribed, and can cause serious complications, especially when used in high doses for extended periods. Examples include suppression of the immune system with supervening infections, steroid osteoporosis and fractures,3 aseptic necrosis, steroid diabetes, hypertension, emotional changes, weight gain, cataracts, neurological complications, and many others. As in all malpractice actions, the plaintiff bears the burden of proof covering the four requisite tort elements, i.e., duty, breach of duty, causation, and damages. Expert testimony is almost always needed in a professional negligence lawsuit.
Aseptic necrosis is a feared complication of steroid therapy.
A recent report4 featured a nurse in her 40s who developed aseptic necrosis of the right shoulder and both hips after taking high dose prednisone for 6 months. She was being treated for idiopathic thrombocytopenic purpura by a hematologist as well as sarcoidosis by a pulmonologist. The plaintiff claimed that both defendants negligently prescribed the medication for an extended period of time without proper monitoring, which caused her severe bone complications requiring a hip and shoulder replacement. The defendants maintained that the steroid medication was necessary to treat the life-threatening conditions from which the plaintiff suffered and that the dosage was carefully monitored and was not excessive. However, in a jury trial, the defendant hematologist and pulmonologist were each found 50% negligent, and the patient was awarded $4.1 million in damages.
In a case5 of steroid-related neurological sequelae, a Colorado jury awarded $14.9 million to a couple against an outpatient surgery center for negligently administering an epidural dose of Kenalog that rendered the patient paraplegic, and for failure to obtain informed consent. The jury awarded the woman, age 57, approximately $1.7 million in past and future medical expenses; $3.2 million in unspecified economic damages; and $6.5 million in past and future noneconomic damages such as pain and suffering. Her husband will receive $3.5 million in past and future noneconomic damages for loss of consortium, according to the verdict. Two years before the injection date of 2013, the drug maker had announced that Kenalog should not be used for epidural procedures because of cord complications including infarction and paraplegia.
Contributory role
The putative offending drug does not have to be the sole cause of injury; if it played a contributory role, the court may find the presence of liability. For example, a Kansas appeals court6 upheld a jury award of $2.88 million in the case of a 40-year-old man who took his life after neurologic complications followed an epidural injection. During one of patient’s visits for chronic low back pain, the defendant-anesthesiologist administered an epidural steroid injection into an area left swollen from a previous injection.
The patient developed neurologic symptoms, and lumbar puncture yielded green pus caused by methicillin-resistant Staphylococcus aureus. He went on to develop arachnoiditis, which left him with impotence, incontinence, and excruciating pain. His lawsuit contended the injection needle had passed through an infected edematous area, causing meningitis and arachnoiditis. Before the case went to trial, the patient took his life because of unremitting pain.
In March 2014, a Johnson County jury found the doctor 75% at fault and the clinic 25% at fault and awarded damages, which were reduced to $1.67 million because Kansas caps noneconomic damages at $250,000. The court rejected the defendants’ argument that the trial judge improperly instructed the jury it could find liability only if negligence “caused” rather than merely “contributed to” the patient’s death, holding that “... one who contributes to a wrongful death is a cause of that death as contemplated by the wrongful death statute.”
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at siang@hawaii.edu .
References
1. Hill v. Squibb Sons, E.R, 592 P.2d 1383 (Mont. 1979).
2. Nash JJ et al, Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011; 144:10-5.
3. Buckley L. et al, Glucocorticoid-Induced Osteoporosis. N Engl J Med 2018; 379:2547-56.
4. Zarin’s Jury Verdict: Review and Analysis. Article ID 40229, Philadelphia County.
5. Robbin Smith et al. v. The Surgery Center at Lone Tree, 2015-CV-30922, Douglas County District Court, Colo. Verdict for plaintiff, March 23, 2017.
6. Burnette v. Kimber L. Eubanks, M.D., & Paincare, P.A., 379 P.3d 372 (Kan. Ct. App. 2016).
Question: Mr. M, a car mechanic, was treated with long-term ACTH and Kenalog after he developed severe contact dermatitis from daily exposure to petroleum-based solvents. His subsequent course was complicated by cataracts and osteoporosis. Which of the following is true in case he files a malpractice action?
A. Treatment with steroids was medically indicated for Mr. Mechanic’s dermatologic condition, so the doctor could not have breached the standard of care.
B. Under the “Learned Intermediary” doctrine, both the manufacturer and the prescribing doctor are jointly liable.
C. Corticosteroids are a known cause of osteoporosis and other complications, but not of cataracts, so that part of the malpractice action should be thrown out.
D. The plaintiff would prevail even if he could not find an expert witnesses to testify as to standard of care, since it is “common knowledge” that steroids cause osteoporosis.
E. Lack of informed consent may be his best legal theory of liability, as many jurisdictions now use the patient-centered standard, which does not require expert testimony.
Answer: E. The above hypothetical was modified from an old Montana case1 in which the patient failed in his negligence lawsuit because he did not have expert witnesses to testify as to standard of care and to adequacy of warning label. However, in some jurisdictions under today’s case law, informed consent relies on a subjective, i.e., patient-oriented standard, and expert testimony is unnecessary to prove breach of duty, although still needed to prove causation.
Steroid-related litigation
Steroid-related malpractice litigation is quite prevalent. In a retrospective study of a tertiary medical center from 1996 to 2008, Nash and coworkers identified 83 such cases.2 Steroids were prescribed for pain (23%), asthma or another pulmonary condition (20%), a dermatologic condition (18%), an autoimmune condition (17%), or allergies (6%).
Learned intermediary
“Drug reps” have a responsibility to inform doctors of both benefits and risks of their medications, a process termed “fair balance.” Generally speaking, if a doctor fails to warn the patient of a medication risk, and injury results, the patient may have a claim against the doctor but not the drug manufacturer. This is termed the “learned intermediary” doctrine, which is also applicable to medical devices such as dialysis equipment, breast implants, and blood products.
The justification is that manufacturers can reasonably rely on the treating doctor to warn of adverse effects, which are disclosed to the profession through their sales reps and in the package insert and PDR. The treating doctor, in turn, is expected to use his or her professional judgment to adequately warn the patient. It is simply not feasible for the manufacturer to directly warn every patient without usurping the doctor-patient relationship. However, where known complications were undisclosed to the FDA and the profession, then plaintiff attorneys can file class action lawsuits directed at the manufacturer.
Complications
Complications arising out of the use of steroids are typical examples of medical products liability. This may be on the basis of the doctor having prescribed the medication without a proper indication or where contraindicated, or may have prescribed “the wrong dose for the wrong patient by the wrong route.” In addition, there may have been a lack of informed consent, i.e., failure to explain the underlying condition and the material risks associated with using the drug. Other acts of negligence, e.g., vicarious liability, may also apply.
Corticosteroids such as Prednisone, Decadron, Kenalog, etc., are widely prescribed, and can cause serious complications, especially when used in high doses for extended periods. Examples include suppression of the immune system with supervening infections, steroid osteoporosis and fractures,3 aseptic necrosis, steroid diabetes, hypertension, emotional changes, weight gain, cataracts, neurological complications, and many others. As in all malpractice actions, the plaintiff bears the burden of proof covering the four requisite tort elements, i.e., duty, breach of duty, causation, and damages. Expert testimony is almost always needed in a professional negligence lawsuit.
Aseptic necrosis is a feared complication of steroid therapy.
A recent report4 featured a nurse in her 40s who developed aseptic necrosis of the right shoulder and both hips after taking high dose prednisone for 6 months. She was being treated for idiopathic thrombocytopenic purpura by a hematologist as well as sarcoidosis by a pulmonologist. The plaintiff claimed that both defendants negligently prescribed the medication for an extended period of time without proper monitoring, which caused her severe bone complications requiring a hip and shoulder replacement. The defendants maintained that the steroid medication was necessary to treat the life-threatening conditions from which the plaintiff suffered and that the dosage was carefully monitored and was not excessive. However, in a jury trial, the defendant hematologist and pulmonologist were each found 50% negligent, and the patient was awarded $4.1 million in damages.
In a case5 of steroid-related neurological sequelae, a Colorado jury awarded $14.9 million to a couple against an outpatient surgery center for negligently administering an epidural dose of Kenalog that rendered the patient paraplegic, and for failure to obtain informed consent. The jury awarded the woman, age 57, approximately $1.7 million in past and future medical expenses; $3.2 million in unspecified economic damages; and $6.5 million in past and future noneconomic damages such as pain and suffering. Her husband will receive $3.5 million in past and future noneconomic damages for loss of consortium, according to the verdict. Two years before the injection date of 2013, the drug maker had announced that Kenalog should not be used for epidural procedures because of cord complications including infarction and paraplegia.
Contributory role
The putative offending drug does not have to be the sole cause of injury; if it played a contributory role, the court may find the presence of liability. For example, a Kansas appeals court6 upheld a jury award of $2.88 million in the case of a 40-year-old man who took his life after neurologic complications followed an epidural injection. During one of patient’s visits for chronic low back pain, the defendant-anesthesiologist administered an epidural steroid injection into an area left swollen from a previous injection.
The patient developed neurologic symptoms, and lumbar puncture yielded green pus caused by methicillin-resistant Staphylococcus aureus. He went on to develop arachnoiditis, which left him with impotence, incontinence, and excruciating pain. His lawsuit contended the injection needle had passed through an infected edematous area, causing meningitis and arachnoiditis. Before the case went to trial, the patient took his life because of unremitting pain.
In March 2014, a Johnson County jury found the doctor 75% at fault and the clinic 25% at fault and awarded damages, which were reduced to $1.67 million because Kansas caps noneconomic damages at $250,000. The court rejected the defendants’ argument that the trial judge improperly instructed the jury it could find liability only if negligence “caused” rather than merely “contributed to” the patient’s death, holding that “... one who contributes to a wrongful death is a cause of that death as contemplated by the wrongful death statute.”
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at siang@hawaii.edu .
References
1. Hill v. Squibb Sons, E.R, 592 P.2d 1383 (Mont. 1979).
2. Nash JJ et al, Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011; 144:10-5.
3. Buckley L. et al, Glucocorticoid-Induced Osteoporosis. N Engl J Med 2018; 379:2547-56.
4. Zarin’s Jury Verdict: Review and Analysis. Article ID 40229, Philadelphia County.
5. Robbin Smith et al. v. The Surgery Center at Lone Tree, 2015-CV-30922, Douglas County District Court, Colo. Verdict for plaintiff, March 23, 2017.
6. Burnette v. Kimber L. Eubanks, M.D., & Paincare, P.A., 379 P.3d 372 (Kan. Ct. App. 2016).
Think twice: Choosing Wisely recommendations on testing to avoid in pediatric hematology
ORLANDO – There’s with some exceptions.
The list, which was produced by an expert panel with representatives from the American Society of Hematology and the American Society of Pediatric Hematology/Oncology (ASPHO), includes five tests or procedures that are considered unnecessary. The recommendations were released at the annual meeting of the American Society of Hematology.
The five recommendations are:
- Don’t perform routine preoperative hemostatic testing in an otherwise healthy child with no prior personal or family history of bleeding.
- Don’t transfuse platelets in a nonbleeding pediatric patient with a platelet count greater than 10,000/mcL, unless other signs of bleeding are present, or if the patient is set to undergo an invasive procedure.
- Don’t order thrombophilia testing on children with venous access-associated thrombosis in the absence of a positive family history.
- Don’t transfuse packed RBCs for iron-deficiency anemia in asymptomatic pediatric patients when there is no evidence of hemodynamic instability or active bleeding.
- Don’t routinely administer granulocyte colony–stimulating factor (G-CSF) for empiric treatment of pediatric patients with asymptomatic autoimmune neutropenia in the absence of recurrent or severe bacterial and/or fungal infections.
This is the third Choosing Wisely list produced by ASH. The group released the first list in 2013 and the second in 2014. But officials at both ASH and ASPHO have received feedback over the years that there should also be a pediatric-focused list in hematology, said Sarah O’Brien, MD, of Nationwide Children’s Hospital in Columbus, Ohio, and cochair of the expert panel that put together the recommendations.
Hemostatic testing
The panel recommended against preoperative hemostatic screening in healthy children with no personal or family history of excessive bleeding because the test does not effectively predict who will have unexpected surgical bleeding. The testing could instead identify artifacts or disorders unrelated to bleeding risk, such as factor XII deficiency or an infection-associated, transient lupus anticoagulant, according to Veronica H. Flood, MD, of the Medical College of Wisconsin, Milwaukee, and a member of the expert panel.
Performing this type of testing also adds cost and stress for families, and often delays surgery.
A look at the current literature reveals that there is little evidence to support coagulation testing in healthy children undergoing surgery. “Despite all this evidence, there remain practitioners who perform such screening on a regular basis,” Dr. Flood said.
For physicians concerned about bleeding risk, Dr. Flood said that existing guidelines support taking a bleeding history in preoperative patients. “This may take a little more time, but in the end will result in better results and less expense.”
Platelet transfusion
The panel recommended against platelet transfusion in nonbleeding pediatric patients with hypoproliferative thrombocytopenia and a platelet count greater than 10,000/mcL. The caveats for this recommendation are that it does not apply if there are other signs or symptoms of bleeding, if the patient is undergoing an invasive procedure, if the patient is aged 1 year or younger, or if the patient has immune-mediated thrombocytopenia, according to Rachel Bercovitz, MD, of the Ann & Robert H. Lurie Children’s Hospital of Chicago and a member of the expert panel.
Previous studies on the platelet transfusions in patients with hematologic malignancies have shown that 10,000/mcL is the appropriate threshold, with no difference in bleeding above that number and increased bleeding below it, Dr. Bercovitz said.
Additionally, while platelet transfusion is a safe procedure, Dr. Bercovitz said, it is not without acute and long-term risks.
Cost is also a factor. “Platelets are a limited and expensive resource,” she said.
Thrombophilia testing
Thrombophilia testing in children with a central venous catheter-associated thrombosis was once common practice but should be avoided, explained Leslie J. Raffini, MD, of the Children’s Hospital of Philadelphia and a member of the expert panel.
Thrombophilia does not influence the initial management of a first episode of provoked venous thrombosis, it does not inform the intensity of duration of anticoagulant therapy, and it does not predict recurrence of venous thrombosis in children, Dr. Raffini said.
In the 2013 Choosing Wisely list, ASH made the same recommendation against testing in adult patients with venous thromboembolism occurring in the setting of major transient risk factors. Thrombophilia testing is also expensive, often has to be repeated, and can be misinterpreted, Dr. Raffini said.
Packed RBC transfusion
The panel recommended against transfusion with packed RBCs for children with iron-deficiency anemia who have no symptoms and no evidence of hemodynamic instability or active bleeding. Transfusion is appropriate if children are symptomatic or are hemodynamically unstable, said Patrick T. McGann, MD, of Cincinnati Children’s Hospital and a member of the expert panel.
Rather than jump to transfusion, Dr. McGann said this group of asymptomatic and hemodynamically stable children should be treated for their iron deficiency through oral or intravenous iron. “This is not about ignoring iron deficiency.”
Both are effective treatments with multiple options available, he said. But sending a child to the hospital for transfusion is a costly option that is stressful for families and only provides a temporary solution to the issue, since treatment of the underlying iron deficiency still needs to be addressed, Dr. McGann said.
G-CSF treatment
The panel also recommended against routine administration of G-CSF in children with asymptomatic autoimmune neutropenia. Peter E. Newburger, MD, of Boston Children’s Hospital and a member of the expert guideline panel, said that there is limited evidence available and no published guidelines in this area, so the panel was guided by expert opinion.
In most cases, G-CSF is not necessary because autoimmune neutropenia resolves spontaneously by age 4-5 years and the risk of serious infection is extremely low. Appropriate management includes antibiotics for acute bacterial infection, good dental hygiene, and continued immunizations, Dr. Newburger said.
G-CSF may be appropriate in limited cases to improve quality of life, but it should be started at a low dose of 1-2 mcg/kg.
In cases of serious infection, Dr. Newburger said physicians should consider alternative diagnoses, such as congenital neutropenia or myelodysplastic syndromes.
ORLANDO – There’s with some exceptions.
The list, which was produced by an expert panel with representatives from the American Society of Hematology and the American Society of Pediatric Hematology/Oncology (ASPHO), includes five tests or procedures that are considered unnecessary. The recommendations were released at the annual meeting of the American Society of Hematology.
The five recommendations are:
- Don’t perform routine preoperative hemostatic testing in an otherwise healthy child with no prior personal or family history of bleeding.
- Don’t transfuse platelets in a nonbleeding pediatric patient with a platelet count greater than 10,000/mcL, unless other signs of bleeding are present, or if the patient is set to undergo an invasive procedure.
- Don’t order thrombophilia testing on children with venous access-associated thrombosis in the absence of a positive family history.
- Don’t transfuse packed RBCs for iron-deficiency anemia in asymptomatic pediatric patients when there is no evidence of hemodynamic instability or active bleeding.
- Don’t routinely administer granulocyte colony–stimulating factor (G-CSF) for empiric treatment of pediatric patients with asymptomatic autoimmune neutropenia in the absence of recurrent or severe bacterial and/or fungal infections.
This is the third Choosing Wisely list produced by ASH. The group released the first list in 2013 and the second in 2014. But officials at both ASH and ASPHO have received feedback over the years that there should also be a pediatric-focused list in hematology, said Sarah O’Brien, MD, of Nationwide Children’s Hospital in Columbus, Ohio, and cochair of the expert panel that put together the recommendations.
Hemostatic testing
The panel recommended against preoperative hemostatic screening in healthy children with no personal or family history of excessive bleeding because the test does not effectively predict who will have unexpected surgical bleeding. The testing could instead identify artifacts or disorders unrelated to bleeding risk, such as factor XII deficiency or an infection-associated, transient lupus anticoagulant, according to Veronica H. Flood, MD, of the Medical College of Wisconsin, Milwaukee, and a member of the expert panel.
Performing this type of testing also adds cost and stress for families, and often delays surgery.
A look at the current literature reveals that there is little evidence to support coagulation testing in healthy children undergoing surgery. “Despite all this evidence, there remain practitioners who perform such screening on a regular basis,” Dr. Flood said.
For physicians concerned about bleeding risk, Dr. Flood said that existing guidelines support taking a bleeding history in preoperative patients. “This may take a little more time, but in the end will result in better results and less expense.”
Platelet transfusion
The panel recommended against platelet transfusion in nonbleeding pediatric patients with hypoproliferative thrombocytopenia and a platelet count greater than 10,000/mcL. The caveats for this recommendation are that it does not apply if there are other signs or symptoms of bleeding, if the patient is undergoing an invasive procedure, if the patient is aged 1 year or younger, or if the patient has immune-mediated thrombocytopenia, according to Rachel Bercovitz, MD, of the Ann & Robert H. Lurie Children’s Hospital of Chicago and a member of the expert panel.
Previous studies on the platelet transfusions in patients with hematologic malignancies have shown that 10,000/mcL is the appropriate threshold, with no difference in bleeding above that number and increased bleeding below it, Dr. Bercovitz said.
Additionally, while platelet transfusion is a safe procedure, Dr. Bercovitz said, it is not without acute and long-term risks.
Cost is also a factor. “Platelets are a limited and expensive resource,” she said.
Thrombophilia testing
Thrombophilia testing in children with a central venous catheter-associated thrombosis was once common practice but should be avoided, explained Leslie J. Raffini, MD, of the Children’s Hospital of Philadelphia and a member of the expert panel.
Thrombophilia does not influence the initial management of a first episode of provoked venous thrombosis, it does not inform the intensity of duration of anticoagulant therapy, and it does not predict recurrence of venous thrombosis in children, Dr. Raffini said.
In the 2013 Choosing Wisely list, ASH made the same recommendation against testing in adult patients with venous thromboembolism occurring in the setting of major transient risk factors. Thrombophilia testing is also expensive, often has to be repeated, and can be misinterpreted, Dr. Raffini said.
Packed RBC transfusion
The panel recommended against transfusion with packed RBCs for children with iron-deficiency anemia who have no symptoms and no evidence of hemodynamic instability or active bleeding. Transfusion is appropriate if children are symptomatic or are hemodynamically unstable, said Patrick T. McGann, MD, of Cincinnati Children’s Hospital and a member of the expert panel.
Rather than jump to transfusion, Dr. McGann said this group of asymptomatic and hemodynamically stable children should be treated for their iron deficiency through oral or intravenous iron. “This is not about ignoring iron deficiency.”
Both are effective treatments with multiple options available, he said. But sending a child to the hospital for transfusion is a costly option that is stressful for families and only provides a temporary solution to the issue, since treatment of the underlying iron deficiency still needs to be addressed, Dr. McGann said.
G-CSF treatment
The panel also recommended against routine administration of G-CSF in children with asymptomatic autoimmune neutropenia. Peter E. Newburger, MD, of Boston Children’s Hospital and a member of the expert guideline panel, said that there is limited evidence available and no published guidelines in this area, so the panel was guided by expert opinion.
In most cases, G-CSF is not necessary because autoimmune neutropenia resolves spontaneously by age 4-5 years and the risk of serious infection is extremely low. Appropriate management includes antibiotics for acute bacterial infection, good dental hygiene, and continued immunizations, Dr. Newburger said.
G-CSF may be appropriate in limited cases to improve quality of life, but it should be started at a low dose of 1-2 mcg/kg.
In cases of serious infection, Dr. Newburger said physicians should consider alternative diagnoses, such as congenital neutropenia or myelodysplastic syndromes.
ORLANDO – There’s with some exceptions.
The list, which was produced by an expert panel with representatives from the American Society of Hematology and the American Society of Pediatric Hematology/Oncology (ASPHO), includes five tests or procedures that are considered unnecessary. The recommendations were released at the annual meeting of the American Society of Hematology.
The five recommendations are:
- Don’t perform routine preoperative hemostatic testing in an otherwise healthy child with no prior personal or family history of bleeding.
- Don’t transfuse platelets in a nonbleeding pediatric patient with a platelet count greater than 10,000/mcL, unless other signs of bleeding are present, or if the patient is set to undergo an invasive procedure.
- Don’t order thrombophilia testing on children with venous access-associated thrombosis in the absence of a positive family history.
- Don’t transfuse packed RBCs for iron-deficiency anemia in asymptomatic pediatric patients when there is no evidence of hemodynamic instability or active bleeding.
- Don’t routinely administer granulocyte colony–stimulating factor (G-CSF) for empiric treatment of pediatric patients with asymptomatic autoimmune neutropenia in the absence of recurrent or severe bacterial and/or fungal infections.
This is the third Choosing Wisely list produced by ASH. The group released the first list in 2013 and the second in 2014. But officials at both ASH and ASPHO have received feedback over the years that there should also be a pediatric-focused list in hematology, said Sarah O’Brien, MD, of Nationwide Children’s Hospital in Columbus, Ohio, and cochair of the expert panel that put together the recommendations.
Hemostatic testing
The panel recommended against preoperative hemostatic screening in healthy children with no personal or family history of excessive bleeding because the test does not effectively predict who will have unexpected surgical bleeding. The testing could instead identify artifacts or disorders unrelated to bleeding risk, such as factor XII deficiency or an infection-associated, transient lupus anticoagulant, according to Veronica H. Flood, MD, of the Medical College of Wisconsin, Milwaukee, and a member of the expert panel.
Performing this type of testing also adds cost and stress for families, and often delays surgery.
A look at the current literature reveals that there is little evidence to support coagulation testing in healthy children undergoing surgery. “Despite all this evidence, there remain practitioners who perform such screening on a regular basis,” Dr. Flood said.
For physicians concerned about bleeding risk, Dr. Flood said that existing guidelines support taking a bleeding history in preoperative patients. “This may take a little more time, but in the end will result in better results and less expense.”
Platelet transfusion
The panel recommended against platelet transfusion in nonbleeding pediatric patients with hypoproliferative thrombocytopenia and a platelet count greater than 10,000/mcL. The caveats for this recommendation are that it does not apply if there are other signs or symptoms of bleeding, if the patient is undergoing an invasive procedure, if the patient is aged 1 year or younger, or if the patient has immune-mediated thrombocytopenia, according to Rachel Bercovitz, MD, of the Ann & Robert H. Lurie Children’s Hospital of Chicago and a member of the expert panel.
Previous studies on the platelet transfusions in patients with hematologic malignancies have shown that 10,000/mcL is the appropriate threshold, with no difference in bleeding above that number and increased bleeding below it, Dr. Bercovitz said.
Additionally, while platelet transfusion is a safe procedure, Dr. Bercovitz said, it is not without acute and long-term risks.
Cost is also a factor. “Platelets are a limited and expensive resource,” she said.
Thrombophilia testing
Thrombophilia testing in children with a central venous catheter-associated thrombosis was once common practice but should be avoided, explained Leslie J. Raffini, MD, of the Children’s Hospital of Philadelphia and a member of the expert panel.
Thrombophilia does not influence the initial management of a first episode of provoked venous thrombosis, it does not inform the intensity of duration of anticoagulant therapy, and it does not predict recurrence of venous thrombosis in children, Dr. Raffini said.
In the 2013 Choosing Wisely list, ASH made the same recommendation against testing in adult patients with venous thromboembolism occurring in the setting of major transient risk factors. Thrombophilia testing is also expensive, often has to be repeated, and can be misinterpreted, Dr. Raffini said.
Packed RBC transfusion
The panel recommended against transfusion with packed RBCs for children with iron-deficiency anemia who have no symptoms and no evidence of hemodynamic instability or active bleeding. Transfusion is appropriate if children are symptomatic or are hemodynamically unstable, said Patrick T. McGann, MD, of Cincinnati Children’s Hospital and a member of the expert panel.
Rather than jump to transfusion, Dr. McGann said this group of asymptomatic and hemodynamically stable children should be treated for their iron deficiency through oral or intravenous iron. “This is not about ignoring iron deficiency.”
Both are effective treatments with multiple options available, he said. But sending a child to the hospital for transfusion is a costly option that is stressful for families and only provides a temporary solution to the issue, since treatment of the underlying iron deficiency still needs to be addressed, Dr. McGann said.
G-CSF treatment
The panel also recommended against routine administration of G-CSF in children with asymptomatic autoimmune neutropenia. Peter E. Newburger, MD, of Boston Children’s Hospital and a member of the expert guideline panel, said that there is limited evidence available and no published guidelines in this area, so the panel was guided by expert opinion.
In most cases, G-CSF is not necessary because autoimmune neutropenia resolves spontaneously by age 4-5 years and the risk of serious infection is extremely low. Appropriate management includes antibiotics for acute bacterial infection, good dental hygiene, and continued immunizations, Dr. Newburger said.
G-CSF may be appropriate in limited cases to improve quality of life, but it should be started at a low dose of 1-2 mcg/kg.
In cases of serious infection, Dr. Newburger said physicians should consider alternative diagnoses, such as congenital neutropenia or myelodysplastic syndromes.
REPORTING FROM ASH 2019
Novel approaches to treating NASH in diabetes
BARCELONA – The investigational oral agent cenicriviroc showed positive effects on liver fibrosis in adults with nonalcoholic steatohepatitis (NASH), many of whom had type 2 diabetes, in a phase 2b trial reported at the annual meeting of the European Association for the Study of Diabetes.
Other data released at the meeting, which showed potential positive effects of novel or existing diabetes treatments on nonalcoholic fatty liver disease (NAFLD), included post hoc analyses of a phase 2b study with tirzepatide and a phase 3 study that combined exenatide and dapagliflozin.
Currently, no medications for NAFLD or NASH have been approved in the United States.
CENTAUR with cenicriviroc
Results of the previously reported CENTAUR trial showed that the antifibrotic effects of cenicriviroc, a dual chemokine receptor antagonist, were greatest in patients with more-severe liver disease (Hepatology. 2018;67[5]:1754-67). At the meeting, Henrik Landgren, PhD, of Allergan, presented data from the 2-year trial overall, and specifically in patients with advanced, stage 3 fibrosis.
CENTAUR was a randomized, double-blind, placebo-controlled, multinational study with 289 adults who had biopsy-confirmed NASH, an NAFLD Activity Score (NAS; range, 0-8; score of 5 or more diagnostic of NASH) of 4 or more, and stages 1-3 liver fibrosis as determined by the NASH clinical research network system (Contemp Clin Trials. 2016;47:356-65). The mean age of the patients enrolled at baseline was 54 years, the mean body mass index was 33.9 kg/m2, and just more than half the patients (52%) had type 2 diabetes.
The patients were randomized to three treatment arms: cenicriviroc 150 mg for 2 years; placebo for 1 year, then cenicriviroc 150 mg for 1 year; or placebo for 2 years. The primary endpoint was histologic improvement (reduction of 2 or more points in overall NAS, with reduction of 1 or more points in more than one category of the NAS without worsening of fibrosis at the end of year 1. The key secondary endpoint was complete NASH resolution without worsening of fibrosis at year 2.
Dr. Landgren reported that, at year 1, of the total number of patients, 28.6% of those receiving cenicriviroc achieved an improvement in fibrosis of one or more stages, compared with 19.0% of those receiving placebo. Of the 97 patients who had advanced fibrosis at baseline, 38.3% of those on cenicriviroc and 28.0% of those on placebo achieved the same endpoint.
Those effects were sustained at year 2, Dr. Landgren emphasized, with twice as many cenicriviroc- than placebo-treated patients achieving one or more stage improvement in fibrosis and no worsening of NASH at year 2 (60% and 30%, respectively), with more pronounced improvements in those who had advanced fibrosis at baseline (86% and 60%).
In addition, analyses of biomarkers suggested that cenicriviroc had systematic anti-inflammatory activity, with reductions observed in high-sensitivity C-reactive protein; fibrinogen; and levels of interleukin-6, IL-8, and IL-1-beta.
Dr. Landgren and colleagues noted that cenicriviroc provided antifibrotic benefit in patients with NASH and fibrosis. Those benefits were sustained through year 2 and were more pronounced in patients who had advanced fibrosis at baseline.
The safety of cenicriviroc was “comparable with placebo,” he said, suggesting that the data supported the phase 3 AURORA study that is currently recruiting.
Tirzepatide for NASH
Another approach worth exploring for the treatment of NASH, is the use of tirzepatide, a dual agonist of glucose-dependent insulinotropic polypeptide and the glucagonlike peptide–1 (GLP-1) receptor, according to Axel Haupt, MD, PhD, of Eli Lilly.
Tirzepatide (LY3298176) is currently under investigation for the treatment of type 2 diabetes, and Dr. Haupt reported data from a post hoc analysis of a double-blind, placebo-controlled, phase 2b study showing that “exploratory” serum markers of apoptosis and fibrosis – keratin-18 (K-18) and Pro-C3, respectively – were decreased from baseline to a greater extent in patients treated with tirzepatide than with placebo, while total adiponectin was increased. The latter is “thought to protect the liver from inflammation and fibrosis,” Dr. Haupt observed.
The main results of the trial were published last year (Lancet. 2018;392:2180-93) and showed that, after 26 weeks, there was a dose-dependent decrease in both glycated hemoglobin (HbA1c) and body weight with tirzepatide 10 mg and 15 mg, compared with placebo and an active comparator, dulaglutide 1.5 mg.
The study population was typical of type 2 diabetes: baseline HbA1c was 8.1%; the average body mass index was 32 kg/m2, with a diabetes duration of 5 years; and the main treatment (90%) had been metformin.
The rationale for the NASH-related biomarker analysis was that type 2 diabetes and NAFLD were known to be overlapping conditions, and weight loss had been shown to be an effective means of resolving NASH, Dr. Haupt said. In addition, a small “proof-of-concept” study with the GLP-1 receptor agonist liraglutide had suggested that these drugs may be effective in NASH.
Tirzepatide, at doses of 5, 10, and 15 mg, was associated with significant decreases in K-18 from baseline to week 26 and compared with placebo and the 1-mg tirzepatide dose. Mean baseline concentrations of K-18 were 394.4 U/L in the placebo group and reduced by 22.6 U/L by week 26. Corresponding baseline values for tirzepatide 5 mg were 375.8 U/L (change, –87.6 U/L); for 10 mg, 409.9 U/L (–157.8 U/L); and for 15 mg, 376.2 U/L (–110.6 U/L).
Dr. Haupt noted that a K-18 value of 250 U/L was considered a cutoff for a diagnosis of NASH. “So we really think that we have some NASH patients in this population,” he observed.
At week 26, Pro-C3 levels significantly decreased by 1.2 ng/mL from a baseline of 8.6 ng/mL with tirzepatide 15 mg, compared with an increase of 0.9 ng/mL from a baseline of 9.3 ng/mL for placebo (P less than .05). However, values of between 15-20 ng/mL would be expected for advanced fibrosis, Dr. Haupt said, “so we think we [don’t] have a lot of patients with advanced fibrosis, we have a lower grade of fibrosis or no fibrosis in our patient population.”
By week 26, adiponectin levels significantly increased by 0.9 mg/L from baseline, both with tirzepatide 10 mg (P less than .05) and 15 mg (P less than .05), compared with placebo (–0.1 mg/L; both P less than .05).
“This study was really designed as a type 2 diabetes efficacy study, so the NASH biomarker work is exploratory and only hypothesis generating,” Dr. Haupt noted. “We think there is overlap in type 2 diabetes and NASH, but it is not an ideal population to look into those biomarkers.” There are also other limitations, such as the baseline values across treatment groups not being matched, so there is likely to be some inconsistency in these data, he added.
That said, Dr. Haupt concluded that, “along with the weight-loss findings,” these exploratory biomarker findings supported the further evaluation of tirzepatide in patients with NASH.”
DURATION-8: Exenatide plus dapagliflozin
In another hypothesis-generating post hoc analysis, this time of the phase 3 DURATION-8 clinical trial, a combination of exenatide and dapagliflozin was found to have a beneficial effect on markers of hepatic steatosis and fibrosis in patients with type 2 diabetes.
“We have some good evidence that both GLP-1 receptor agonists and SGLT2 [sodium-glucose cotransporter 2] inhibitors may have benefits in reducing steatosis and even steatohepatitis in [patients with] type 2 diabetes. So the association of two diabetes drugs might provide an advantage. However, this had not previously been tested in a randomized, controlled trial,” observed Cristian Guja, MD, PhD, of Carol Davila University of Medicine and Pharmacy in Bucharest, Romania.
The main aims of the DURATION-8 clinical trial, which ran for 104 weeks, was to compare the efficacy and safety of combining exenatide (2 mg, once a week) and dapagliflozin (10 mg, daily) with either exenatide 2 mg with placebo or dapagliflozin 10 mg with placebo. Results showed greater improved glycemic control and reductions in body weight and systolic BP with the exenatide-dapagliflozin combination.
A total of 685 patients were included in the post hoc analysis, of whom 228 had been treated with the combination, 228 with exenatide plus placebo, and 230 with dapagliflozin plus placebo. At baseline, levels of the markers of NAFLD and fibrosis that were assessed were similar between the groups. Between 81% and 93% of study participants had fatty liver or steatosis as defined by a Fatty Liver Index (FLI) of 60 or more or an overall NAFLD Liver Fat Score (NLFS) of –0.64 or higher. Between 9% and 13% of patients had liver fibrosis, as defined as an NAFLD Fibrosis Score (NFS) above 0.676, a Fibrosis-4 score (FIB-4) of 1.46 or more, or both.
At 28 weeks, the proportion of patients with biomarker scores suggestive of fatty liver disease or steatosis was significantly reduced from baseline with the exenatide-dapagliflozin combination (–10.5% for FLI of 60 or more; –6.5% for NLFS of –0.640 or more), Dr. Guja said, and biomarker scores suggestive of advanced fibrosis (NFS greater than 0.676; FIB-4 of 1.46 or more) were reduced by 4.1% and 3.6%, respectively.
At 28 and 52 weeks, the combination therapy showed stronger effects than exenatide and dapagliflozin alone in improving markers of hepatic steatosis (FLI: 28 weeks, –6.81, –3.90, –4.04; and 52 weeks, –6.23, –3.00, –4.58). The combination therapy also showed improvement for advanced fibrosis biomarkers at both time points (FIB-4: 28 weeks, –0.06, –0.03, –0.04; and 52 weeks, –0.05, –0.02, –0,04).
Dr. Guja noted that, although the study was not powered to assess the effect of on fatty liver, making all these data exploratory, this was the first analysis to describe improvements in biomarkers of fatty liver or steatosis and fibrosis from a large trial. “Some specific, dedicated, prospective trials are needed in the future to validate these findings.”
The CENTAUR study was funded by Allergan, of which Dr. Landgren is an employee. The phase 2b study with tirzepatide was supported by Eli Lilly. Dr. Haupt disclosed being an employee and also holding stocks in the company. The DURATION-8 study was sponsored by AstraZeneca. Dr. Guja disclosed that he had participated in scientific advisory boards and received consulting fees from AstraZeneca and other companies.
SOURCES: Landgren H et al. EASD 2019, Oral Presentation 179; Haupt A et al. EASD 2019, Oral Presentation 177; Guja C et al. EASD 2019, Oral Presentation 178.
BARCELONA – The investigational oral agent cenicriviroc showed positive effects on liver fibrosis in adults with nonalcoholic steatohepatitis (NASH), many of whom had type 2 diabetes, in a phase 2b trial reported at the annual meeting of the European Association for the Study of Diabetes.
Other data released at the meeting, which showed potential positive effects of novel or existing diabetes treatments on nonalcoholic fatty liver disease (NAFLD), included post hoc analyses of a phase 2b study with tirzepatide and a phase 3 study that combined exenatide and dapagliflozin.
Currently, no medications for NAFLD or NASH have been approved in the United States.
CENTAUR with cenicriviroc
Results of the previously reported CENTAUR trial showed that the antifibrotic effects of cenicriviroc, a dual chemokine receptor antagonist, were greatest in patients with more-severe liver disease (Hepatology. 2018;67[5]:1754-67). At the meeting, Henrik Landgren, PhD, of Allergan, presented data from the 2-year trial overall, and specifically in patients with advanced, stage 3 fibrosis.
CENTAUR was a randomized, double-blind, placebo-controlled, multinational study with 289 adults who had biopsy-confirmed NASH, an NAFLD Activity Score (NAS; range, 0-8; score of 5 or more diagnostic of NASH) of 4 or more, and stages 1-3 liver fibrosis as determined by the NASH clinical research network system (Contemp Clin Trials. 2016;47:356-65). The mean age of the patients enrolled at baseline was 54 years, the mean body mass index was 33.9 kg/m2, and just more than half the patients (52%) had type 2 diabetes.
The patients were randomized to three treatment arms: cenicriviroc 150 mg for 2 years; placebo for 1 year, then cenicriviroc 150 mg for 1 year; or placebo for 2 years. The primary endpoint was histologic improvement (reduction of 2 or more points in overall NAS, with reduction of 1 or more points in more than one category of the NAS without worsening of fibrosis at the end of year 1. The key secondary endpoint was complete NASH resolution without worsening of fibrosis at year 2.
Dr. Landgren reported that, at year 1, of the total number of patients, 28.6% of those receiving cenicriviroc achieved an improvement in fibrosis of one or more stages, compared with 19.0% of those receiving placebo. Of the 97 patients who had advanced fibrosis at baseline, 38.3% of those on cenicriviroc and 28.0% of those on placebo achieved the same endpoint.
Those effects were sustained at year 2, Dr. Landgren emphasized, with twice as many cenicriviroc- than placebo-treated patients achieving one or more stage improvement in fibrosis and no worsening of NASH at year 2 (60% and 30%, respectively), with more pronounced improvements in those who had advanced fibrosis at baseline (86% and 60%).
In addition, analyses of biomarkers suggested that cenicriviroc had systematic anti-inflammatory activity, with reductions observed in high-sensitivity C-reactive protein; fibrinogen; and levels of interleukin-6, IL-8, and IL-1-beta.
Dr. Landgren and colleagues noted that cenicriviroc provided antifibrotic benefit in patients with NASH and fibrosis. Those benefits were sustained through year 2 and were more pronounced in patients who had advanced fibrosis at baseline.
The safety of cenicriviroc was “comparable with placebo,” he said, suggesting that the data supported the phase 3 AURORA study that is currently recruiting.
Tirzepatide for NASH
Another approach worth exploring for the treatment of NASH, is the use of tirzepatide, a dual agonist of glucose-dependent insulinotropic polypeptide and the glucagonlike peptide–1 (GLP-1) receptor, according to Axel Haupt, MD, PhD, of Eli Lilly.
Tirzepatide (LY3298176) is currently under investigation for the treatment of type 2 diabetes, and Dr. Haupt reported data from a post hoc analysis of a double-blind, placebo-controlled, phase 2b study showing that “exploratory” serum markers of apoptosis and fibrosis – keratin-18 (K-18) and Pro-C3, respectively – were decreased from baseline to a greater extent in patients treated with tirzepatide than with placebo, while total adiponectin was increased. The latter is “thought to protect the liver from inflammation and fibrosis,” Dr. Haupt observed.
The main results of the trial were published last year (Lancet. 2018;392:2180-93) and showed that, after 26 weeks, there was a dose-dependent decrease in both glycated hemoglobin (HbA1c) and body weight with tirzepatide 10 mg and 15 mg, compared with placebo and an active comparator, dulaglutide 1.5 mg.
The study population was typical of type 2 diabetes: baseline HbA1c was 8.1%; the average body mass index was 32 kg/m2, with a diabetes duration of 5 years; and the main treatment (90%) had been metformin.
The rationale for the NASH-related biomarker analysis was that type 2 diabetes and NAFLD were known to be overlapping conditions, and weight loss had been shown to be an effective means of resolving NASH, Dr. Haupt said. In addition, a small “proof-of-concept” study with the GLP-1 receptor agonist liraglutide had suggested that these drugs may be effective in NASH.
Tirzepatide, at doses of 5, 10, and 15 mg, was associated with significant decreases in K-18 from baseline to week 26 and compared with placebo and the 1-mg tirzepatide dose. Mean baseline concentrations of K-18 were 394.4 U/L in the placebo group and reduced by 22.6 U/L by week 26. Corresponding baseline values for tirzepatide 5 mg were 375.8 U/L (change, –87.6 U/L); for 10 mg, 409.9 U/L (–157.8 U/L); and for 15 mg, 376.2 U/L (–110.6 U/L).
Dr. Haupt noted that a K-18 value of 250 U/L was considered a cutoff for a diagnosis of NASH. “So we really think that we have some NASH patients in this population,” he observed.
At week 26, Pro-C3 levels significantly decreased by 1.2 ng/mL from a baseline of 8.6 ng/mL with tirzepatide 15 mg, compared with an increase of 0.9 ng/mL from a baseline of 9.3 ng/mL for placebo (P less than .05). However, values of between 15-20 ng/mL would be expected for advanced fibrosis, Dr. Haupt said, “so we think we [don’t] have a lot of patients with advanced fibrosis, we have a lower grade of fibrosis or no fibrosis in our patient population.”
By week 26, adiponectin levels significantly increased by 0.9 mg/L from baseline, both with tirzepatide 10 mg (P less than .05) and 15 mg (P less than .05), compared with placebo (–0.1 mg/L; both P less than .05).
“This study was really designed as a type 2 diabetes efficacy study, so the NASH biomarker work is exploratory and only hypothesis generating,” Dr. Haupt noted. “We think there is overlap in type 2 diabetes and NASH, but it is not an ideal population to look into those biomarkers.” There are also other limitations, such as the baseline values across treatment groups not being matched, so there is likely to be some inconsistency in these data, he added.
That said, Dr. Haupt concluded that, “along with the weight-loss findings,” these exploratory biomarker findings supported the further evaluation of tirzepatide in patients with NASH.”
DURATION-8: Exenatide plus dapagliflozin
In another hypothesis-generating post hoc analysis, this time of the phase 3 DURATION-8 clinical trial, a combination of exenatide and dapagliflozin was found to have a beneficial effect on markers of hepatic steatosis and fibrosis in patients with type 2 diabetes.
“We have some good evidence that both GLP-1 receptor agonists and SGLT2 [sodium-glucose cotransporter 2] inhibitors may have benefits in reducing steatosis and even steatohepatitis in [patients with] type 2 diabetes. So the association of two diabetes drugs might provide an advantage. However, this had not previously been tested in a randomized, controlled trial,” observed Cristian Guja, MD, PhD, of Carol Davila University of Medicine and Pharmacy in Bucharest, Romania.
The main aims of the DURATION-8 clinical trial, which ran for 104 weeks, was to compare the efficacy and safety of combining exenatide (2 mg, once a week) and dapagliflozin (10 mg, daily) with either exenatide 2 mg with placebo or dapagliflozin 10 mg with placebo. Results showed greater improved glycemic control and reductions in body weight and systolic BP with the exenatide-dapagliflozin combination.
A total of 685 patients were included in the post hoc analysis, of whom 228 had been treated with the combination, 228 with exenatide plus placebo, and 230 with dapagliflozin plus placebo. At baseline, levels of the markers of NAFLD and fibrosis that were assessed were similar between the groups. Between 81% and 93% of study participants had fatty liver or steatosis as defined by a Fatty Liver Index (FLI) of 60 or more or an overall NAFLD Liver Fat Score (NLFS) of –0.64 or higher. Between 9% and 13% of patients had liver fibrosis, as defined as an NAFLD Fibrosis Score (NFS) above 0.676, a Fibrosis-4 score (FIB-4) of 1.46 or more, or both.
At 28 weeks, the proportion of patients with biomarker scores suggestive of fatty liver disease or steatosis was significantly reduced from baseline with the exenatide-dapagliflozin combination (–10.5% for FLI of 60 or more; –6.5% for NLFS of –0.640 or more), Dr. Guja said, and biomarker scores suggestive of advanced fibrosis (NFS greater than 0.676; FIB-4 of 1.46 or more) were reduced by 4.1% and 3.6%, respectively.
At 28 and 52 weeks, the combination therapy showed stronger effects than exenatide and dapagliflozin alone in improving markers of hepatic steatosis (FLI: 28 weeks, –6.81, –3.90, –4.04; and 52 weeks, –6.23, –3.00, –4.58). The combination therapy also showed improvement for advanced fibrosis biomarkers at both time points (FIB-4: 28 weeks, –0.06, –0.03, –0.04; and 52 weeks, –0.05, –0.02, –0,04).
Dr. Guja noted that, although the study was not powered to assess the effect of on fatty liver, making all these data exploratory, this was the first analysis to describe improvements in biomarkers of fatty liver or steatosis and fibrosis from a large trial. “Some specific, dedicated, prospective trials are needed in the future to validate these findings.”
The CENTAUR study was funded by Allergan, of which Dr. Landgren is an employee. The phase 2b study with tirzepatide was supported by Eli Lilly. Dr. Haupt disclosed being an employee and also holding stocks in the company. The DURATION-8 study was sponsored by AstraZeneca. Dr. Guja disclosed that he had participated in scientific advisory boards and received consulting fees from AstraZeneca and other companies.
SOURCES: Landgren H et al. EASD 2019, Oral Presentation 179; Haupt A et al. EASD 2019, Oral Presentation 177; Guja C et al. EASD 2019, Oral Presentation 178.
BARCELONA – The investigational oral agent cenicriviroc showed positive effects on liver fibrosis in adults with nonalcoholic steatohepatitis (NASH), many of whom had type 2 diabetes, in a phase 2b trial reported at the annual meeting of the European Association for the Study of Diabetes.
Other data released at the meeting, which showed potential positive effects of novel or existing diabetes treatments on nonalcoholic fatty liver disease (NAFLD), included post hoc analyses of a phase 2b study with tirzepatide and a phase 3 study that combined exenatide and dapagliflozin.
Currently, no medications for NAFLD or NASH have been approved in the United States.
CENTAUR with cenicriviroc
Results of the previously reported CENTAUR trial showed that the antifibrotic effects of cenicriviroc, a dual chemokine receptor antagonist, were greatest in patients with more-severe liver disease (Hepatology. 2018;67[5]:1754-67). At the meeting, Henrik Landgren, PhD, of Allergan, presented data from the 2-year trial overall, and specifically in patients with advanced, stage 3 fibrosis.
CENTAUR was a randomized, double-blind, placebo-controlled, multinational study with 289 adults who had biopsy-confirmed NASH, an NAFLD Activity Score (NAS; range, 0-8; score of 5 or more diagnostic of NASH) of 4 or more, and stages 1-3 liver fibrosis as determined by the NASH clinical research network system (Contemp Clin Trials. 2016;47:356-65). The mean age of the patients enrolled at baseline was 54 years, the mean body mass index was 33.9 kg/m2, and just more than half the patients (52%) had type 2 diabetes.
The patients were randomized to three treatment arms: cenicriviroc 150 mg for 2 years; placebo for 1 year, then cenicriviroc 150 mg for 1 year; or placebo for 2 years. The primary endpoint was histologic improvement (reduction of 2 or more points in overall NAS, with reduction of 1 or more points in more than one category of the NAS without worsening of fibrosis at the end of year 1. The key secondary endpoint was complete NASH resolution without worsening of fibrosis at year 2.
Dr. Landgren reported that, at year 1, of the total number of patients, 28.6% of those receiving cenicriviroc achieved an improvement in fibrosis of one or more stages, compared with 19.0% of those receiving placebo. Of the 97 patients who had advanced fibrosis at baseline, 38.3% of those on cenicriviroc and 28.0% of those on placebo achieved the same endpoint.
Those effects were sustained at year 2, Dr. Landgren emphasized, with twice as many cenicriviroc- than placebo-treated patients achieving one or more stage improvement in fibrosis and no worsening of NASH at year 2 (60% and 30%, respectively), with more pronounced improvements in those who had advanced fibrosis at baseline (86% and 60%).
In addition, analyses of biomarkers suggested that cenicriviroc had systematic anti-inflammatory activity, with reductions observed in high-sensitivity C-reactive protein; fibrinogen; and levels of interleukin-6, IL-8, and IL-1-beta.
Dr. Landgren and colleagues noted that cenicriviroc provided antifibrotic benefit in patients with NASH and fibrosis. Those benefits were sustained through year 2 and were more pronounced in patients who had advanced fibrosis at baseline.
The safety of cenicriviroc was “comparable with placebo,” he said, suggesting that the data supported the phase 3 AURORA study that is currently recruiting.
Tirzepatide for NASH
Another approach worth exploring for the treatment of NASH, is the use of tirzepatide, a dual agonist of glucose-dependent insulinotropic polypeptide and the glucagonlike peptide–1 (GLP-1) receptor, according to Axel Haupt, MD, PhD, of Eli Lilly.
Tirzepatide (LY3298176) is currently under investigation for the treatment of type 2 diabetes, and Dr. Haupt reported data from a post hoc analysis of a double-blind, placebo-controlled, phase 2b study showing that “exploratory” serum markers of apoptosis and fibrosis – keratin-18 (K-18) and Pro-C3, respectively – were decreased from baseline to a greater extent in patients treated with tirzepatide than with placebo, while total adiponectin was increased. The latter is “thought to protect the liver from inflammation and fibrosis,” Dr. Haupt observed.
The main results of the trial were published last year (Lancet. 2018;392:2180-93) and showed that, after 26 weeks, there was a dose-dependent decrease in both glycated hemoglobin (HbA1c) and body weight with tirzepatide 10 mg and 15 mg, compared with placebo and an active comparator, dulaglutide 1.5 mg.
The study population was typical of type 2 diabetes: baseline HbA1c was 8.1%; the average body mass index was 32 kg/m2, with a diabetes duration of 5 years; and the main treatment (90%) had been metformin.
The rationale for the NASH-related biomarker analysis was that type 2 diabetes and NAFLD were known to be overlapping conditions, and weight loss had been shown to be an effective means of resolving NASH, Dr. Haupt said. In addition, a small “proof-of-concept” study with the GLP-1 receptor agonist liraglutide had suggested that these drugs may be effective in NASH.
Tirzepatide, at doses of 5, 10, and 15 mg, was associated with significant decreases in K-18 from baseline to week 26 and compared with placebo and the 1-mg tirzepatide dose. Mean baseline concentrations of K-18 were 394.4 U/L in the placebo group and reduced by 22.6 U/L by week 26. Corresponding baseline values for tirzepatide 5 mg were 375.8 U/L (change, –87.6 U/L); for 10 mg, 409.9 U/L (–157.8 U/L); and for 15 mg, 376.2 U/L (–110.6 U/L).
Dr. Haupt noted that a K-18 value of 250 U/L was considered a cutoff for a diagnosis of NASH. “So we really think that we have some NASH patients in this population,” he observed.
At week 26, Pro-C3 levels significantly decreased by 1.2 ng/mL from a baseline of 8.6 ng/mL with tirzepatide 15 mg, compared with an increase of 0.9 ng/mL from a baseline of 9.3 ng/mL for placebo (P less than .05). However, values of between 15-20 ng/mL would be expected for advanced fibrosis, Dr. Haupt said, “so we think we [don’t] have a lot of patients with advanced fibrosis, we have a lower grade of fibrosis or no fibrosis in our patient population.”
By week 26, adiponectin levels significantly increased by 0.9 mg/L from baseline, both with tirzepatide 10 mg (P less than .05) and 15 mg (P less than .05), compared with placebo (–0.1 mg/L; both P less than .05).
“This study was really designed as a type 2 diabetes efficacy study, so the NASH biomarker work is exploratory and only hypothesis generating,” Dr. Haupt noted. “We think there is overlap in type 2 diabetes and NASH, but it is not an ideal population to look into those biomarkers.” There are also other limitations, such as the baseline values across treatment groups not being matched, so there is likely to be some inconsistency in these data, he added.
That said, Dr. Haupt concluded that, “along with the weight-loss findings,” these exploratory biomarker findings supported the further evaluation of tirzepatide in patients with NASH.”
DURATION-8: Exenatide plus dapagliflozin
In another hypothesis-generating post hoc analysis, this time of the phase 3 DURATION-8 clinical trial, a combination of exenatide and dapagliflozin was found to have a beneficial effect on markers of hepatic steatosis and fibrosis in patients with type 2 diabetes.
“We have some good evidence that both GLP-1 receptor agonists and SGLT2 [sodium-glucose cotransporter 2] inhibitors may have benefits in reducing steatosis and even steatohepatitis in [patients with] type 2 diabetes. So the association of two diabetes drugs might provide an advantage. However, this had not previously been tested in a randomized, controlled trial,” observed Cristian Guja, MD, PhD, of Carol Davila University of Medicine and Pharmacy in Bucharest, Romania.
The main aims of the DURATION-8 clinical trial, which ran for 104 weeks, was to compare the efficacy and safety of combining exenatide (2 mg, once a week) and dapagliflozin (10 mg, daily) with either exenatide 2 mg with placebo or dapagliflozin 10 mg with placebo. Results showed greater improved glycemic control and reductions in body weight and systolic BP with the exenatide-dapagliflozin combination.
A total of 685 patients were included in the post hoc analysis, of whom 228 had been treated with the combination, 228 with exenatide plus placebo, and 230 with dapagliflozin plus placebo. At baseline, levels of the markers of NAFLD and fibrosis that were assessed were similar between the groups. Between 81% and 93% of study participants had fatty liver or steatosis as defined by a Fatty Liver Index (FLI) of 60 or more or an overall NAFLD Liver Fat Score (NLFS) of –0.64 or higher. Between 9% and 13% of patients had liver fibrosis, as defined as an NAFLD Fibrosis Score (NFS) above 0.676, a Fibrosis-4 score (FIB-4) of 1.46 or more, or both.
At 28 weeks, the proportion of patients with biomarker scores suggestive of fatty liver disease or steatosis was significantly reduced from baseline with the exenatide-dapagliflozin combination (–10.5% for FLI of 60 or more; –6.5% for NLFS of –0.640 or more), Dr. Guja said, and biomarker scores suggestive of advanced fibrosis (NFS greater than 0.676; FIB-4 of 1.46 or more) were reduced by 4.1% and 3.6%, respectively.
At 28 and 52 weeks, the combination therapy showed stronger effects than exenatide and dapagliflozin alone in improving markers of hepatic steatosis (FLI: 28 weeks, –6.81, –3.90, –4.04; and 52 weeks, –6.23, –3.00, –4.58). The combination therapy also showed improvement for advanced fibrosis biomarkers at both time points (FIB-4: 28 weeks, –0.06, –0.03, –0.04; and 52 weeks, –0.05, –0.02, –0,04).
Dr. Guja noted that, although the study was not powered to assess the effect of on fatty liver, making all these data exploratory, this was the first analysis to describe improvements in biomarkers of fatty liver or steatosis and fibrosis from a large trial. “Some specific, dedicated, prospective trials are needed in the future to validate these findings.”
The CENTAUR study was funded by Allergan, of which Dr. Landgren is an employee. The phase 2b study with tirzepatide was supported by Eli Lilly. Dr. Haupt disclosed being an employee and also holding stocks in the company. The DURATION-8 study was sponsored by AstraZeneca. Dr. Guja disclosed that he had participated in scientific advisory boards and received consulting fees from AstraZeneca and other companies.
SOURCES: Landgren H et al. EASD 2019, Oral Presentation 179; Haupt A et al. EASD 2019, Oral Presentation 177; Guja C et al. EASD 2019, Oral Presentation 178.
REPORTING FROM EASD 2019
Unit-based rounding in the real world
Balance and flexibility are essential
Many hospitalists agree that their most productive and also sometimes least productive work can happen in the setting of interdisciplinary rounds. How can this paradox be true?
Most hospitals strive to assemble the health care team every day for a brief discussion of each patient’s needs as well as barriers to a safe/successful discharge. On most floors this requires a well-choreographed “dance” of nurses, case managers, social workers, physicians, and advanced practice providers coming together at agreed-upon times. All team members commit to efficient synchronized swimming through the most high-yield details for each patient in order to benefit the patients and families being served.
Of course, there are always challenges to this process in the unpredictable world of patients with acute needs. One variable that is at least partially controllable and tends to promote a more cohesive interdisciplinary experience is that of hospitalist unit-based rounding.
The 2018 State of Hospital Medicine (SoHM) survey reveals that 68% of hospital medicine groups serving adults with greater than 30 physicians employ some degree of unit-based rounding; this trend decreases with smaller group size. About 54% of academic hospital medicine groups use some amount of unit-based rounding. Not surprisingly, smaller hospitals are less likely to have this routine, likely because of fewer total nursing units.
One of the most obvious benefits to unit-based rounding is that the physician or advanced practice provider is more reliably able to participate in the interdisciplinary discussions that day. When more of the team members are at the table each day, patients and families have the best chance of hearing a consistent message around the treatment and discharge plans.
There are challenges to unit-based rounding as well. If patients transfer to different floors for any variety of reasons, strict unit-based rounding may increase handoffs in care. If a hospital has times when it isn’t completely full and nursing units have a varying percentage of being occupied, strict unit-based rounding can cause significant workload inequities among physicians on different units, depending on numbers of patients on each unit.
If there is no attempt at unit-based rounding in larger hospitals, some physicians may be running among five or more units. They work to find different care managers, nurses, and pharmacists – not to mention the challenges of catching patients in their rooms between their departures for diagnostic studies and procedures.
It is often good to balance the benefit of promoting unit-based rounds with the reality of everyday patient care. Some groups maintain that the physician/patient relationship trumps the idea of perfect unit-based rounding. In other words, if a physician establishes a relationship with a patient while they are in the ED being admitted or boarding from overnight, that physician will continue seeing the patient regardless of the patient being assigned to a different unit. It can help for groups to agree that the pursuit of unit-based rounding may create some inequity in the numbers of patients seen each day because of these issues.
In a larger hospital, certain units are often dedicated to specialty care such as cardiac or stroke care. While most hospitalists want to maintain general medical knowledge, there are some who may enjoy having portions of their practice devoted to perioperative medicine or cardiac care, for instance. This promotes familiarity among hospitalists and groups of consultant physicians and nurse practitioners/physician assistants. Over time this allows for enhanced teamwork among those physicians, the nursing team, and the specialty physicians.
Depending on the group’s schedule, patients can be reassigned coinciding with the primary change of service day. This resets the physicians’ patients in the most ideal unit-based way on the evening prior to the first day of rounding for that week or group of shifts.
No matter how you do it, the goal of unit-based rounding is time efficiency for the care team and care coordination benefits for patients and families. If you have other suggestions or questions, go online to SHM HMX to join the discussion.
Take-home message: Unit-based rounding likely has its benefits. Don’t let the inability to achieve perfection in patient distribution to the physicians each day lead to abandonment of attempting these processes.
Dr. McNeal is the division director of inpatient medicine at Baylor Scott & White Medical Center in Temple, Tex.
Balance and flexibility are essential
Balance and flexibility are essential
Many hospitalists agree that their most productive and also sometimes least productive work can happen in the setting of interdisciplinary rounds. How can this paradox be true?
Most hospitals strive to assemble the health care team every day for a brief discussion of each patient’s needs as well as barriers to a safe/successful discharge. On most floors this requires a well-choreographed “dance” of nurses, case managers, social workers, physicians, and advanced practice providers coming together at agreed-upon times. All team members commit to efficient synchronized swimming through the most high-yield details for each patient in order to benefit the patients and families being served.
Of course, there are always challenges to this process in the unpredictable world of patients with acute needs. One variable that is at least partially controllable and tends to promote a more cohesive interdisciplinary experience is that of hospitalist unit-based rounding.
The 2018 State of Hospital Medicine (SoHM) survey reveals that 68% of hospital medicine groups serving adults with greater than 30 physicians employ some degree of unit-based rounding; this trend decreases with smaller group size. About 54% of academic hospital medicine groups use some amount of unit-based rounding. Not surprisingly, smaller hospitals are less likely to have this routine, likely because of fewer total nursing units.
One of the most obvious benefits to unit-based rounding is that the physician or advanced practice provider is more reliably able to participate in the interdisciplinary discussions that day. When more of the team members are at the table each day, patients and families have the best chance of hearing a consistent message around the treatment and discharge plans.
There are challenges to unit-based rounding as well. If patients transfer to different floors for any variety of reasons, strict unit-based rounding may increase handoffs in care. If a hospital has times when it isn’t completely full and nursing units have a varying percentage of being occupied, strict unit-based rounding can cause significant workload inequities among physicians on different units, depending on numbers of patients on each unit.
If there is no attempt at unit-based rounding in larger hospitals, some physicians may be running among five or more units. They work to find different care managers, nurses, and pharmacists – not to mention the challenges of catching patients in their rooms between their departures for diagnostic studies and procedures.
It is often good to balance the benefit of promoting unit-based rounds with the reality of everyday patient care. Some groups maintain that the physician/patient relationship trumps the idea of perfect unit-based rounding. In other words, if a physician establishes a relationship with a patient while they are in the ED being admitted or boarding from overnight, that physician will continue seeing the patient regardless of the patient being assigned to a different unit. It can help for groups to agree that the pursuit of unit-based rounding may create some inequity in the numbers of patients seen each day because of these issues.
In a larger hospital, certain units are often dedicated to specialty care such as cardiac or stroke care. While most hospitalists want to maintain general medical knowledge, there are some who may enjoy having portions of their practice devoted to perioperative medicine or cardiac care, for instance. This promotes familiarity among hospitalists and groups of consultant physicians and nurse practitioners/physician assistants. Over time this allows for enhanced teamwork among those physicians, the nursing team, and the specialty physicians.
Depending on the group’s schedule, patients can be reassigned coinciding with the primary change of service day. This resets the physicians’ patients in the most ideal unit-based way on the evening prior to the first day of rounding for that week or group of shifts.
No matter how you do it, the goal of unit-based rounding is time efficiency for the care team and care coordination benefits for patients and families. If you have other suggestions or questions, go online to SHM HMX to join the discussion.
Take-home message: Unit-based rounding likely has its benefits. Don’t let the inability to achieve perfection in patient distribution to the physicians each day lead to abandonment of attempting these processes.
Dr. McNeal is the division director of inpatient medicine at Baylor Scott & White Medical Center in Temple, Tex.
Many hospitalists agree that their most productive and also sometimes least productive work can happen in the setting of interdisciplinary rounds. How can this paradox be true?
Most hospitals strive to assemble the health care team every day for a brief discussion of each patient’s needs as well as barriers to a safe/successful discharge. On most floors this requires a well-choreographed “dance” of nurses, case managers, social workers, physicians, and advanced practice providers coming together at agreed-upon times. All team members commit to efficient synchronized swimming through the most high-yield details for each patient in order to benefit the patients and families being served.
Of course, there are always challenges to this process in the unpredictable world of patients with acute needs. One variable that is at least partially controllable and tends to promote a more cohesive interdisciplinary experience is that of hospitalist unit-based rounding.
The 2018 State of Hospital Medicine (SoHM) survey reveals that 68% of hospital medicine groups serving adults with greater than 30 physicians employ some degree of unit-based rounding; this trend decreases with smaller group size. About 54% of academic hospital medicine groups use some amount of unit-based rounding. Not surprisingly, smaller hospitals are less likely to have this routine, likely because of fewer total nursing units.
One of the most obvious benefits to unit-based rounding is that the physician or advanced practice provider is more reliably able to participate in the interdisciplinary discussions that day. When more of the team members are at the table each day, patients and families have the best chance of hearing a consistent message around the treatment and discharge plans.
There are challenges to unit-based rounding as well. If patients transfer to different floors for any variety of reasons, strict unit-based rounding may increase handoffs in care. If a hospital has times when it isn’t completely full and nursing units have a varying percentage of being occupied, strict unit-based rounding can cause significant workload inequities among physicians on different units, depending on numbers of patients on each unit.
If there is no attempt at unit-based rounding in larger hospitals, some physicians may be running among five or more units. They work to find different care managers, nurses, and pharmacists – not to mention the challenges of catching patients in their rooms between their departures for diagnostic studies and procedures.
It is often good to balance the benefit of promoting unit-based rounds with the reality of everyday patient care. Some groups maintain that the physician/patient relationship trumps the idea of perfect unit-based rounding. In other words, if a physician establishes a relationship with a patient while they are in the ED being admitted or boarding from overnight, that physician will continue seeing the patient regardless of the patient being assigned to a different unit. It can help for groups to agree that the pursuit of unit-based rounding may create some inequity in the numbers of patients seen each day because of these issues.
In a larger hospital, certain units are often dedicated to specialty care such as cardiac or stroke care. While most hospitalists want to maintain general medical knowledge, there are some who may enjoy having portions of their practice devoted to perioperative medicine or cardiac care, for instance. This promotes familiarity among hospitalists and groups of consultant physicians and nurse practitioners/physician assistants. Over time this allows for enhanced teamwork among those physicians, the nursing team, and the specialty physicians.
Depending on the group’s schedule, patients can be reassigned coinciding with the primary change of service day. This resets the physicians’ patients in the most ideal unit-based way on the evening prior to the first day of rounding for that week or group of shifts.
No matter how you do it, the goal of unit-based rounding is time efficiency for the care team and care coordination benefits for patients and families. If you have other suggestions or questions, go online to SHM HMX to join the discussion.
Take-home message: Unit-based rounding likely has its benefits. Don’t let the inability to achieve perfection in patient distribution to the physicians each day lead to abandonment of attempting these processes.
Dr. McNeal is the division director of inpatient medicine at Baylor Scott & White Medical Center in Temple, Tex.
Repeat LTBI testing best in patients taking biologics with new risk factors
ATLANTA – Patients taking biologics who received latent tuberculosis testing on an annual basis were unlikely to convert from a negative QuantiFERON test to a positive result, which suggests that the test may be unnecessary for patients without new tuberculosis risk factors, according to research presented at the annual meeting of the American College of Rheumatology.
In addition, nearly all of the cost of repeat testing for latent tuberculosis infection (LTBI) went to patients who were not diagnosed with or treated for LTBI, noted Urmi Khanna, MD, a dermatologist with the Cleveland Clinic.
“All in all, about $1.4 million U.S. dollars was spent just on additional QuantiFERON testing, and only 1% of this additional cost was actually spent on testing patients who were diagnosed with and treated for latent tuberculosis,” Dr. Khanna said in her presentation at the meeting.
“Based on this study, we would like to propose that, in low incidence TB regions such as the United States, repeat LTBI testing in patients on biologic therapies should be focused on patients who have new risk factors for TB infection since their last screening,” she said.
The National Psoriasis Foundation has recommended patients be screened annually for LTBI, and the Centers for Disease Control and Prevention and the ACR have recommended patients taking biologics be screened annually for LTBI if they have new risk factors for TB, such as coming into contact with immigrants, a person infected with TB, immunosuppressed individuals, or persons working in areas where TB might be present. Annual screening was also recently added to the Medicare Merit-Based Incentive Payment System (MIPS), which will affect physician reimbursement. “Based on [the addition of this quality outcome measure], we expect that more and more physicians will adopt this practice of annual LTBI screening in all patients on biologics,” Dr. Khanna said.
She and her colleagues examined QuantiFERON tuberculosis test (QFT) results of 10,914 patients from the Cleveland Clinic Foundation between August 2007 and March 2019 where patients were receiving systemic biologic therapy for inflammatory or autoimmune conditions, including nearly 32% with inflammatory bowel disease, 29% with rheumatoid arthritis, and 25% with psoriatic disease. Overall, 5,212 patients were included in the final analysis, and patients had a median of three QFT results. Patients had a median age of 41 years, had taken an average of 1.80 biologics during follow-up, and had a median biologic therapy duration of about 49 months. The most common biologics used were adalimumab (33%), etanercept (17%), and infliximab (17%).
Of these patients, 4,561 patients had negative QFTs (88%), 172 patients had one or more positive QFTs (3%), and 479 patients had one or more indeterminate QFTs (9%). For patients who converted from a negative QFT to a positive QFT, the most common risk factors were exposure to someone with TB (26%), immigrating or traveling to an endemic area (26%), and occupational exposure (16%).
Within the group with one or more positive QFTs, there were 108 patients with baseline positive QFTs prior to starting biologic therapy (2.1%), 61 patients who converted from a baseline negative QFT to a positive QFT (1.2%), and 3 patients where a positive result overlapped with a negative result (0.1%). The majority of patients who converted to a positive QFT result had borderline positive results (70.5%), defined as 0.35 to 1 IU/mL, compared with 29.5% of converters who had a positive QFT result of more than 1.0 IU/mL.
Among the 61 patients who converted to a positive QFT result, 28 patients with LTBI (46%) and 1 patient with an active case of TB (2%) were diagnosed and treated. The active TB case was a 29-year-old patient with inflammatory bowel disease and ankylosing spondylitis receiving adalimumab who had recently traveled to India.
The researchers also examined the cost of additional QFTs in each group. Among negative QFTs, the cost of an additional 9,611 tests was $1,201,375. The cost of additional tests for indeterminate QFTs was $136,200, but Dr. Khanna noted that 99.99% of additional tests in this group were for patients never diagnosed with or treated for LTBI. Additional tests for positive QFTs cost another $47,700, and 26.1% of patients in this group were diagnosed and received treatment for LTBI, compared with 73.9% who did not receive an LTBI diagnosis or treatment.
In the discussion session following the presentation, Dr. Khanna emphasized that discontinuing annual screening in low-risk patients was not standard of care at the Cleveland Clinic, and this study was conducted to raise awareness of focusing testing on patients with new TB risk factors.
Dr. Khanna reported no relevant financial disclosures. A few of her coauthors reported financial relationships with pharmaceutical companies.
SOURCE: Khanna U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1802.
ATLANTA – Patients taking biologics who received latent tuberculosis testing on an annual basis were unlikely to convert from a negative QuantiFERON test to a positive result, which suggests that the test may be unnecessary for patients without new tuberculosis risk factors, according to research presented at the annual meeting of the American College of Rheumatology.
In addition, nearly all of the cost of repeat testing for latent tuberculosis infection (LTBI) went to patients who were not diagnosed with or treated for LTBI, noted Urmi Khanna, MD, a dermatologist with the Cleveland Clinic.
“All in all, about $1.4 million U.S. dollars was spent just on additional QuantiFERON testing, and only 1% of this additional cost was actually spent on testing patients who were diagnosed with and treated for latent tuberculosis,” Dr. Khanna said in her presentation at the meeting.
“Based on this study, we would like to propose that, in low incidence TB regions such as the United States, repeat LTBI testing in patients on biologic therapies should be focused on patients who have new risk factors for TB infection since their last screening,” she said.
The National Psoriasis Foundation has recommended patients be screened annually for LTBI, and the Centers for Disease Control and Prevention and the ACR have recommended patients taking biologics be screened annually for LTBI if they have new risk factors for TB, such as coming into contact with immigrants, a person infected with TB, immunosuppressed individuals, or persons working in areas where TB might be present. Annual screening was also recently added to the Medicare Merit-Based Incentive Payment System (MIPS), which will affect physician reimbursement. “Based on [the addition of this quality outcome measure], we expect that more and more physicians will adopt this practice of annual LTBI screening in all patients on biologics,” Dr. Khanna said.
She and her colleagues examined QuantiFERON tuberculosis test (QFT) results of 10,914 patients from the Cleveland Clinic Foundation between August 2007 and March 2019 where patients were receiving systemic biologic therapy for inflammatory or autoimmune conditions, including nearly 32% with inflammatory bowel disease, 29% with rheumatoid arthritis, and 25% with psoriatic disease. Overall, 5,212 patients were included in the final analysis, and patients had a median of three QFT results. Patients had a median age of 41 years, had taken an average of 1.80 biologics during follow-up, and had a median biologic therapy duration of about 49 months. The most common biologics used were adalimumab (33%), etanercept (17%), and infliximab (17%).
Of these patients, 4,561 patients had negative QFTs (88%), 172 patients had one or more positive QFTs (3%), and 479 patients had one or more indeterminate QFTs (9%). For patients who converted from a negative QFT to a positive QFT, the most common risk factors were exposure to someone with TB (26%), immigrating or traveling to an endemic area (26%), and occupational exposure (16%).
Within the group with one or more positive QFTs, there were 108 patients with baseline positive QFTs prior to starting biologic therapy (2.1%), 61 patients who converted from a baseline negative QFT to a positive QFT (1.2%), and 3 patients where a positive result overlapped with a negative result (0.1%). The majority of patients who converted to a positive QFT result had borderline positive results (70.5%), defined as 0.35 to 1 IU/mL, compared with 29.5% of converters who had a positive QFT result of more than 1.0 IU/mL.
Among the 61 patients who converted to a positive QFT result, 28 patients with LTBI (46%) and 1 patient with an active case of TB (2%) were diagnosed and treated. The active TB case was a 29-year-old patient with inflammatory bowel disease and ankylosing spondylitis receiving adalimumab who had recently traveled to India.
The researchers also examined the cost of additional QFTs in each group. Among negative QFTs, the cost of an additional 9,611 tests was $1,201,375. The cost of additional tests for indeterminate QFTs was $136,200, but Dr. Khanna noted that 99.99% of additional tests in this group were for patients never diagnosed with or treated for LTBI. Additional tests for positive QFTs cost another $47,700, and 26.1% of patients in this group were diagnosed and received treatment for LTBI, compared with 73.9% who did not receive an LTBI diagnosis or treatment.
In the discussion session following the presentation, Dr. Khanna emphasized that discontinuing annual screening in low-risk patients was not standard of care at the Cleveland Clinic, and this study was conducted to raise awareness of focusing testing on patients with new TB risk factors.
Dr. Khanna reported no relevant financial disclosures. A few of her coauthors reported financial relationships with pharmaceutical companies.
SOURCE: Khanna U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1802.
ATLANTA – Patients taking biologics who received latent tuberculosis testing on an annual basis were unlikely to convert from a negative QuantiFERON test to a positive result, which suggests that the test may be unnecessary for patients without new tuberculosis risk factors, according to research presented at the annual meeting of the American College of Rheumatology.
In addition, nearly all of the cost of repeat testing for latent tuberculosis infection (LTBI) went to patients who were not diagnosed with or treated for LTBI, noted Urmi Khanna, MD, a dermatologist with the Cleveland Clinic.
“All in all, about $1.4 million U.S. dollars was spent just on additional QuantiFERON testing, and only 1% of this additional cost was actually spent on testing patients who were diagnosed with and treated for latent tuberculosis,” Dr. Khanna said in her presentation at the meeting.
“Based on this study, we would like to propose that, in low incidence TB regions such as the United States, repeat LTBI testing in patients on biologic therapies should be focused on patients who have new risk factors for TB infection since their last screening,” she said.
The National Psoriasis Foundation has recommended patients be screened annually for LTBI, and the Centers for Disease Control and Prevention and the ACR have recommended patients taking biologics be screened annually for LTBI if they have new risk factors for TB, such as coming into contact with immigrants, a person infected with TB, immunosuppressed individuals, or persons working in areas where TB might be present. Annual screening was also recently added to the Medicare Merit-Based Incentive Payment System (MIPS), which will affect physician reimbursement. “Based on [the addition of this quality outcome measure], we expect that more and more physicians will adopt this practice of annual LTBI screening in all patients on biologics,” Dr. Khanna said.
She and her colleagues examined QuantiFERON tuberculosis test (QFT) results of 10,914 patients from the Cleveland Clinic Foundation between August 2007 and March 2019 where patients were receiving systemic biologic therapy for inflammatory or autoimmune conditions, including nearly 32% with inflammatory bowel disease, 29% with rheumatoid arthritis, and 25% with psoriatic disease. Overall, 5,212 patients were included in the final analysis, and patients had a median of three QFT results. Patients had a median age of 41 years, had taken an average of 1.80 biologics during follow-up, and had a median biologic therapy duration of about 49 months. The most common biologics used were adalimumab (33%), etanercept (17%), and infliximab (17%).
Of these patients, 4,561 patients had negative QFTs (88%), 172 patients had one or more positive QFTs (3%), and 479 patients had one or more indeterminate QFTs (9%). For patients who converted from a negative QFT to a positive QFT, the most common risk factors were exposure to someone with TB (26%), immigrating or traveling to an endemic area (26%), and occupational exposure (16%).
Within the group with one or more positive QFTs, there were 108 patients with baseline positive QFTs prior to starting biologic therapy (2.1%), 61 patients who converted from a baseline negative QFT to a positive QFT (1.2%), and 3 patients where a positive result overlapped with a negative result (0.1%). The majority of patients who converted to a positive QFT result had borderline positive results (70.5%), defined as 0.35 to 1 IU/mL, compared with 29.5% of converters who had a positive QFT result of more than 1.0 IU/mL.
Among the 61 patients who converted to a positive QFT result, 28 patients with LTBI (46%) and 1 patient with an active case of TB (2%) were diagnosed and treated. The active TB case was a 29-year-old patient with inflammatory bowel disease and ankylosing spondylitis receiving adalimumab who had recently traveled to India.
The researchers also examined the cost of additional QFTs in each group. Among negative QFTs, the cost of an additional 9,611 tests was $1,201,375. The cost of additional tests for indeterminate QFTs was $136,200, but Dr. Khanna noted that 99.99% of additional tests in this group were for patients never diagnosed with or treated for LTBI. Additional tests for positive QFTs cost another $47,700, and 26.1% of patients in this group were diagnosed and received treatment for LTBI, compared with 73.9% who did not receive an LTBI diagnosis or treatment.
In the discussion session following the presentation, Dr. Khanna emphasized that discontinuing annual screening in low-risk patients was not standard of care at the Cleveland Clinic, and this study was conducted to raise awareness of focusing testing on patients with new TB risk factors.
Dr. Khanna reported no relevant financial disclosures. A few of her coauthors reported financial relationships with pharmaceutical companies.
SOURCE: Khanna U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1802.
REPORTING FROM ACR 2019