User login
This month in the journal CHEST®
Editor’s Picks
Editorials
Pulmonary Embolism Cardiac Arrest: Thrombolysis During Cardiopulmonary Resuscitation and Improved Survival. By Drs. B. W. Bottiger and W. A. Wetsch.
Interstitial Lung Abnormalities: A Word of Caution.
By Drs. V. Tzilas and D. Bouros.
Original Research
Thrombolysis During Resuscitation for Out-of-Hospital Cardiac Arrest Caused by Pulmonary Embolism Increases 30-Day Survival: Findings From the French National Cardiac Arrest Registry.
By Dr. F. Javaudin, et al.Interstitial Lung Abnormalities and Lung Cancer Risk in the National Lung Screening Trial. By Dr. S-A. Whittaker Brown, et al.
Commentary
Publishing a Clinical Research Manuscript: Guidance for Early-Career Researchers With a Focus on Pulmonary and Critical Care Medicine.
By Dr. E. M. Viglianti, et al.
Editor’s Picks
Editor’s Picks
Editorials
Pulmonary Embolism Cardiac Arrest: Thrombolysis During Cardiopulmonary Resuscitation and Improved Survival. By Drs. B. W. Bottiger and W. A. Wetsch.
Interstitial Lung Abnormalities: A Word of Caution.
By Drs. V. Tzilas and D. Bouros.
Original Research
Thrombolysis During Resuscitation for Out-of-Hospital Cardiac Arrest Caused by Pulmonary Embolism Increases 30-Day Survival: Findings From the French National Cardiac Arrest Registry.
By Dr. F. Javaudin, et al.Interstitial Lung Abnormalities and Lung Cancer Risk in the National Lung Screening Trial. By Dr. S-A. Whittaker Brown, et al.
Commentary
Publishing a Clinical Research Manuscript: Guidance for Early-Career Researchers With a Focus on Pulmonary and Critical Care Medicine.
By Dr. E. M. Viglianti, et al.
Editorials
Pulmonary Embolism Cardiac Arrest: Thrombolysis During Cardiopulmonary Resuscitation and Improved Survival. By Drs. B. W. Bottiger and W. A. Wetsch.
Interstitial Lung Abnormalities: A Word of Caution.
By Drs. V. Tzilas and D. Bouros.
Original Research
Thrombolysis During Resuscitation for Out-of-Hospital Cardiac Arrest Caused by Pulmonary Embolism Increases 30-Day Survival: Findings From the French National Cardiac Arrest Registry.
By Dr. F. Javaudin, et al.Interstitial Lung Abnormalities and Lung Cancer Risk in the National Lung Screening Trial. By Dr. S-A. Whittaker Brown, et al.
Commentary
Publishing a Clinical Research Manuscript: Guidance for Early-Career Researchers With a Focus on Pulmonary and Critical Care Medicine.
By Dr. E. M. Viglianti, et al.
Nutrition support during adult critical illness
Many critically ill patients you care for cannot maintain volitional oral intake. Therefore, nutrition support, through enteral or parenteral routes, remains a cornerstone in ensuring our critically ill patients receive substrates like glucose and protein. To understand the supportive role of nutrition during critical illness, let’s identify and contextualize the different phases of critical illness.
Phases of critical illness
The European Society of Parenteral and Enteral Nutrition’s (ESPEN) 2018 critical care nutrition guideline incorporates stages of critical illness in making nutrition recommendations (Singer P et al. Clin Nutr. 2019;38:48-79). The first week of critical illness is the acute phase and hallmarked by catabolism and metabolic and hemodynamic instability. The late phase is thereafter and hallmarked by rehabilitation and anabolism or chronic critical illness. The acute phase is further divided into early (days 1-2) and late acute phase (days 3-7). The time-points are arbitrary and merely serve as placeholders. An objective marker to distinguish phases does not exist and transition periods will be different for each patient.
Acute phase
Critical illness defining conditions like circulatory shock, respiratory failure, and trauma are stressors and lead to two key acute phase perturbations that nutrition may have a role in altering:
The first is hypercatabolism. Critical illness defining conditions activate neuroendocrine, inflammatory/immune, adipokine, and GI tract hormone pathways that increase serum glucagon, cortisol, and catecholamines to promote glycogenolysis, gluconeogenesis, insulin resistance, protein catabolism, and restricted/impaired anabolism.
The second is gut dysfunction. During health, there is cross-talk signaling that occurs between commensal bacteria, epithelium, and the immune system, which maintains gut barrier functions, achieved, for example, by promoting tight junction protein production. Acute critical illness pathophysiology loosens epithelial tight junctions, and the gut barrier is breached, creating an opportunity for downstream migration of pancreatic enzymes and cytokines. Furthermore, the microbiome morphs into a virulent pathobiome, which induces gut-derived inflammation.
When, where, and how much should we feed critically ill patients?
Since the acute phase of critical illness begins a series of events leading to negative energy balance and gut dysfunction, you might find early nutrition provision intuitive. Indeed, the 2016 ASPEN/SCCM and 2018 ESPEN critical care nutrition guidelines recommend early (within 24-48 hours of ICU admission) enteral nutrition (EN), delivered into the stomach, for all critically ill patients unable to maintain volitional intake. Meta-analyses of randomized controlled trials (RCT) conducted between1979 and 2013 show early EN reduces both mortality and infectious complications, compared with no early nutrition (McClave SA et al. JPEN. 2016;40:159-211).
RCT level data do not show superiority of EN over parenteral nutrition (PN). Nonetheless, early EN is recommended over PN because it maintains epithelial barrier function and supports immunity.
What is the optimal nutrition dose? The 2016 ASPEN/SCCM guideline recommends getting to >80% estimated energy goal within 48-72 hours in patients with high nutrition risk while the 2018 ESPEN guideline suggests maintaining a hypocaloric, or not exceeding 70% of prescribed energy goal, during the early acute phase. The recommendation is based on meta-analyses of RCTs conducted between 2011 and 2017, which shows no mortality difference between hypocaloric and isocaloric nutrition.
Biologically plausible rationale for starting hypocaloric, as opposed to full dose nutrition, during the acute phase of critical illness includes: (a) the acute phase represents a period of hemodynamic instability and mitochondrial dysfunction, and full-dose EN may lead to feeding intolerance and lack of substrate utilization, respectively; (b) in those with risk factors (like pre-existing malnutrition), starting full dose nutrition may lead to refeeding syndrome; and (c) endogenous glucose production occurs during the acute phase, and full dose nutrition may worsen hyperglycemia.
Therefore, during the early acute phase of critical illness, hypocaloric feeding using an isosmotic formula, with a slow up-titration to goal rate thereafter, while monitoring for feeding intolerance and refeeding syndrome is a reasonable starting point.
What is the role of parenteral nutrition in critical illness?
PN can be exclusive or supplemental (in a patient receiving EN). Historically, providers may have been reluctant to utilize PN for fear of infectious morbidity; however, contemporary pragmatic-design RCTs demonstrate safety with exclusive PN (Harvey SE et al. N Engl J Med. 2014;371:1673-84). When your patient has a contraindication for EN or does not tolerate it despite a trial of small bowel feeding, meta-analyses have shown a mortality benefit of early exclusive PN in malnourished patients, as compared with no nutrition (Braunschweig C et al. Am J Clin Nutr.2001;74:534-42).
As for supplemental PN (SPN), the 2016 ASPEN/SCCM guideline does not recommend it until day 7 in all critically ill patients, while the 2018 ESPEN guideline recommends its use on a case-by-case basis. Since, two trials inform SPN use. The EAT-ICU trial showed no difference in 6-month physical function between EN group and early-goal-directed nutritiongroup, which included SPN to achieve estimated energy requirement during the first week of critical illness (Allingstrup MJ et al. Intensive Care Med. 2017;43:1637-47). The TOP-UP trial compared EN alone with EN plus SPN in nutritionally high risk patients (ie, those who stand to have more complications as a result of undernutrition) and found those with a BMI < 25 kg/m2 and those with a NUTRIC score >5 who received supplemental PN atop EN had improved 30-day mortality, as compared with EN alone (Wischmeyer P et al. Crit Care. 2017;21:142). Mortality was a secondary outcome, and further study of supplemental PN in nutritionally high-risk patients is warranted. Until further data are available, supplemental PN should probably be restricted during the acute phase of critical illness.
Protein may be the important substrate
Proteolysis is the rule during critical illness, and amino acids are liberated from skeletal muscle breakdown. Using ultrasound, Puthucheary et al found a 17.7% reduction in rectus femoris cross-sectional area in 63 critically ill adults and identified muscle cellular infiltration at ICU day 10, suggesting critical illness leads to quantitative and qualitative muscle defects (Puthucheary Z et al. JAMA. 2013;15:1591-1600).
Since survivorship from critical illness is increasing, acquired loss of muscle mass may contribute to post-ICU physical functioning impairments. Thus, protein may be the most important substrate to deliver during critical illness. The 2016 ASPEN/SCCM guideline recommends 1.2 – 2.0 g/kg actual body weight (ABW)/day in nonobese critically ill patients.
Unfortunately, the optimal protein dose and the timing of intake are unknown. Observational studies suggest benefit with lower and higher doses, which creates equipoise for protein dose. The signal may be lost in heterogeneity, and observational data suggest higher protein dose may benefit patients with high nutritional risk. In terms of timing, one observational study found lower (<0.8 g/kg/d) protein dose before day 3 followed by higher (>0.8 g/kg/d) dose thereafter was associated with mortality benefit (Koekkoek WAC et al. Clin Nutr.2019;38:883-890).
Until stronger data are available to guide optimal protein dose and timing, it is reasonable to observe the 2016 ASPEN/SCCM guideline protein recommendation of at least 1.2 g/kg/day. The 2018 ESPEN guideline recommends a similar dose of 1.3 g/kg/day.
Future research and summary
Many questions remain unanswered and present opportunities for future research. Priorities for critical care nutrition research include studying the impact of combined nutrition and exercise in the acute and late phases of critical illness and identifying best tools to differentiate responses to caloric and protein intake.
In summary, critical illness has acute and late phases. The acute phase is a hypercatabolic state leading to negative energy and nitrogen balance and gut dysfunction. Take-home points for nutrition support in the acute phase of critical illness are:
1. It is reasonable to start early hypocaloric EN with an isosmotic formula with slow up-titration over the first week of critical illness while monitoring for refeeding syndrome and feeding intolerance.
2. Use exclusive PN in ICU patients with pre-existing malnutrition when EN is contraindicated or not tolerated.
3. Supplemental PN should probably be restricted during the acute phase of critical illness.
4. Optimal protein dose and timing are unknown. It is reasonable to start with at least 1.2 g/kg ABW/day in non-obese patients.
Dr. Patel is with the Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin.
Dr. Rice is with the Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Vanderbilt University, Nashville, Tennessee.
Many critically ill patients you care for cannot maintain volitional oral intake. Therefore, nutrition support, through enteral or parenteral routes, remains a cornerstone in ensuring our critically ill patients receive substrates like glucose and protein. To understand the supportive role of nutrition during critical illness, let’s identify and contextualize the different phases of critical illness.
Phases of critical illness
The European Society of Parenteral and Enteral Nutrition’s (ESPEN) 2018 critical care nutrition guideline incorporates stages of critical illness in making nutrition recommendations (Singer P et al. Clin Nutr. 2019;38:48-79). The first week of critical illness is the acute phase and hallmarked by catabolism and metabolic and hemodynamic instability. The late phase is thereafter and hallmarked by rehabilitation and anabolism or chronic critical illness. The acute phase is further divided into early (days 1-2) and late acute phase (days 3-7). The time-points are arbitrary and merely serve as placeholders. An objective marker to distinguish phases does not exist and transition periods will be different for each patient.
Acute phase
Critical illness defining conditions like circulatory shock, respiratory failure, and trauma are stressors and lead to two key acute phase perturbations that nutrition may have a role in altering:
The first is hypercatabolism. Critical illness defining conditions activate neuroendocrine, inflammatory/immune, adipokine, and GI tract hormone pathways that increase serum glucagon, cortisol, and catecholamines to promote glycogenolysis, gluconeogenesis, insulin resistance, protein catabolism, and restricted/impaired anabolism.
The second is gut dysfunction. During health, there is cross-talk signaling that occurs between commensal bacteria, epithelium, and the immune system, which maintains gut barrier functions, achieved, for example, by promoting tight junction protein production. Acute critical illness pathophysiology loosens epithelial tight junctions, and the gut barrier is breached, creating an opportunity for downstream migration of pancreatic enzymes and cytokines. Furthermore, the microbiome morphs into a virulent pathobiome, which induces gut-derived inflammation.
When, where, and how much should we feed critically ill patients?
Since the acute phase of critical illness begins a series of events leading to negative energy balance and gut dysfunction, you might find early nutrition provision intuitive. Indeed, the 2016 ASPEN/SCCM and 2018 ESPEN critical care nutrition guidelines recommend early (within 24-48 hours of ICU admission) enteral nutrition (EN), delivered into the stomach, for all critically ill patients unable to maintain volitional intake. Meta-analyses of randomized controlled trials (RCT) conducted between1979 and 2013 show early EN reduces both mortality and infectious complications, compared with no early nutrition (McClave SA et al. JPEN. 2016;40:159-211).
RCT level data do not show superiority of EN over parenteral nutrition (PN). Nonetheless, early EN is recommended over PN because it maintains epithelial barrier function and supports immunity.
What is the optimal nutrition dose? The 2016 ASPEN/SCCM guideline recommends getting to >80% estimated energy goal within 48-72 hours in patients with high nutrition risk while the 2018 ESPEN guideline suggests maintaining a hypocaloric, or not exceeding 70% of prescribed energy goal, during the early acute phase. The recommendation is based on meta-analyses of RCTs conducted between 2011 and 2017, which shows no mortality difference between hypocaloric and isocaloric nutrition.
Biologically plausible rationale for starting hypocaloric, as opposed to full dose nutrition, during the acute phase of critical illness includes: (a) the acute phase represents a period of hemodynamic instability and mitochondrial dysfunction, and full-dose EN may lead to feeding intolerance and lack of substrate utilization, respectively; (b) in those with risk factors (like pre-existing malnutrition), starting full dose nutrition may lead to refeeding syndrome; and (c) endogenous glucose production occurs during the acute phase, and full dose nutrition may worsen hyperglycemia.
Therefore, during the early acute phase of critical illness, hypocaloric feeding using an isosmotic formula, with a slow up-titration to goal rate thereafter, while monitoring for feeding intolerance and refeeding syndrome is a reasonable starting point.
What is the role of parenteral nutrition in critical illness?
PN can be exclusive or supplemental (in a patient receiving EN). Historically, providers may have been reluctant to utilize PN for fear of infectious morbidity; however, contemporary pragmatic-design RCTs demonstrate safety with exclusive PN (Harvey SE et al. N Engl J Med. 2014;371:1673-84). When your patient has a contraindication for EN or does not tolerate it despite a trial of small bowel feeding, meta-analyses have shown a mortality benefit of early exclusive PN in malnourished patients, as compared with no nutrition (Braunschweig C et al. Am J Clin Nutr.2001;74:534-42).
As for supplemental PN (SPN), the 2016 ASPEN/SCCM guideline does not recommend it until day 7 in all critically ill patients, while the 2018 ESPEN guideline recommends its use on a case-by-case basis. Since, two trials inform SPN use. The EAT-ICU trial showed no difference in 6-month physical function between EN group and early-goal-directed nutritiongroup, which included SPN to achieve estimated energy requirement during the first week of critical illness (Allingstrup MJ et al. Intensive Care Med. 2017;43:1637-47). The TOP-UP trial compared EN alone with EN plus SPN in nutritionally high risk patients (ie, those who stand to have more complications as a result of undernutrition) and found those with a BMI < 25 kg/m2 and those with a NUTRIC score >5 who received supplemental PN atop EN had improved 30-day mortality, as compared with EN alone (Wischmeyer P et al. Crit Care. 2017;21:142). Mortality was a secondary outcome, and further study of supplemental PN in nutritionally high-risk patients is warranted. Until further data are available, supplemental PN should probably be restricted during the acute phase of critical illness.
Protein may be the important substrate
Proteolysis is the rule during critical illness, and amino acids are liberated from skeletal muscle breakdown. Using ultrasound, Puthucheary et al found a 17.7% reduction in rectus femoris cross-sectional area in 63 critically ill adults and identified muscle cellular infiltration at ICU day 10, suggesting critical illness leads to quantitative and qualitative muscle defects (Puthucheary Z et al. JAMA. 2013;15:1591-1600).
Since survivorship from critical illness is increasing, acquired loss of muscle mass may contribute to post-ICU physical functioning impairments. Thus, protein may be the most important substrate to deliver during critical illness. The 2016 ASPEN/SCCM guideline recommends 1.2 – 2.0 g/kg actual body weight (ABW)/day in nonobese critically ill patients.
Unfortunately, the optimal protein dose and the timing of intake are unknown. Observational studies suggest benefit with lower and higher doses, which creates equipoise for protein dose. The signal may be lost in heterogeneity, and observational data suggest higher protein dose may benefit patients with high nutritional risk. In terms of timing, one observational study found lower (<0.8 g/kg/d) protein dose before day 3 followed by higher (>0.8 g/kg/d) dose thereafter was associated with mortality benefit (Koekkoek WAC et al. Clin Nutr.2019;38:883-890).
Until stronger data are available to guide optimal protein dose and timing, it is reasonable to observe the 2016 ASPEN/SCCM guideline protein recommendation of at least 1.2 g/kg/day. The 2018 ESPEN guideline recommends a similar dose of 1.3 g/kg/day.
Future research and summary
Many questions remain unanswered and present opportunities for future research. Priorities for critical care nutrition research include studying the impact of combined nutrition and exercise in the acute and late phases of critical illness and identifying best tools to differentiate responses to caloric and protein intake.
In summary, critical illness has acute and late phases. The acute phase is a hypercatabolic state leading to negative energy and nitrogen balance and gut dysfunction. Take-home points for nutrition support in the acute phase of critical illness are:
1. It is reasonable to start early hypocaloric EN with an isosmotic formula with slow up-titration over the first week of critical illness while monitoring for refeeding syndrome and feeding intolerance.
2. Use exclusive PN in ICU patients with pre-existing malnutrition when EN is contraindicated or not tolerated.
3. Supplemental PN should probably be restricted during the acute phase of critical illness.
4. Optimal protein dose and timing are unknown. It is reasonable to start with at least 1.2 g/kg ABW/day in non-obese patients.
Dr. Patel is with the Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin.
Dr. Rice is with the Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Vanderbilt University, Nashville, Tennessee.
Many critically ill patients you care for cannot maintain volitional oral intake. Therefore, nutrition support, through enteral or parenteral routes, remains a cornerstone in ensuring our critically ill patients receive substrates like glucose and protein. To understand the supportive role of nutrition during critical illness, let’s identify and contextualize the different phases of critical illness.
Phases of critical illness
The European Society of Parenteral and Enteral Nutrition’s (ESPEN) 2018 critical care nutrition guideline incorporates stages of critical illness in making nutrition recommendations (Singer P et al. Clin Nutr. 2019;38:48-79). The first week of critical illness is the acute phase and hallmarked by catabolism and metabolic and hemodynamic instability. The late phase is thereafter and hallmarked by rehabilitation and anabolism or chronic critical illness. The acute phase is further divided into early (days 1-2) and late acute phase (days 3-7). The time-points are arbitrary and merely serve as placeholders. An objective marker to distinguish phases does not exist and transition periods will be different for each patient.
Acute phase
Critical illness defining conditions like circulatory shock, respiratory failure, and trauma are stressors and lead to two key acute phase perturbations that nutrition may have a role in altering:
The first is hypercatabolism. Critical illness defining conditions activate neuroendocrine, inflammatory/immune, adipokine, and GI tract hormone pathways that increase serum glucagon, cortisol, and catecholamines to promote glycogenolysis, gluconeogenesis, insulin resistance, protein catabolism, and restricted/impaired anabolism.
The second is gut dysfunction. During health, there is cross-talk signaling that occurs between commensal bacteria, epithelium, and the immune system, which maintains gut barrier functions, achieved, for example, by promoting tight junction protein production. Acute critical illness pathophysiology loosens epithelial tight junctions, and the gut barrier is breached, creating an opportunity for downstream migration of pancreatic enzymes and cytokines. Furthermore, the microbiome morphs into a virulent pathobiome, which induces gut-derived inflammation.
When, where, and how much should we feed critically ill patients?
Since the acute phase of critical illness begins a series of events leading to negative energy balance and gut dysfunction, you might find early nutrition provision intuitive. Indeed, the 2016 ASPEN/SCCM and 2018 ESPEN critical care nutrition guidelines recommend early (within 24-48 hours of ICU admission) enteral nutrition (EN), delivered into the stomach, for all critically ill patients unable to maintain volitional intake. Meta-analyses of randomized controlled trials (RCT) conducted between1979 and 2013 show early EN reduces both mortality and infectious complications, compared with no early nutrition (McClave SA et al. JPEN. 2016;40:159-211).
RCT level data do not show superiority of EN over parenteral nutrition (PN). Nonetheless, early EN is recommended over PN because it maintains epithelial barrier function and supports immunity.
What is the optimal nutrition dose? The 2016 ASPEN/SCCM guideline recommends getting to >80% estimated energy goal within 48-72 hours in patients with high nutrition risk while the 2018 ESPEN guideline suggests maintaining a hypocaloric, or not exceeding 70% of prescribed energy goal, during the early acute phase. The recommendation is based on meta-analyses of RCTs conducted between 2011 and 2017, which shows no mortality difference between hypocaloric and isocaloric nutrition.
Biologically plausible rationale for starting hypocaloric, as opposed to full dose nutrition, during the acute phase of critical illness includes: (a) the acute phase represents a period of hemodynamic instability and mitochondrial dysfunction, and full-dose EN may lead to feeding intolerance and lack of substrate utilization, respectively; (b) in those with risk factors (like pre-existing malnutrition), starting full dose nutrition may lead to refeeding syndrome; and (c) endogenous glucose production occurs during the acute phase, and full dose nutrition may worsen hyperglycemia.
Therefore, during the early acute phase of critical illness, hypocaloric feeding using an isosmotic formula, with a slow up-titration to goal rate thereafter, while monitoring for feeding intolerance and refeeding syndrome is a reasonable starting point.
What is the role of parenteral nutrition in critical illness?
PN can be exclusive or supplemental (in a patient receiving EN). Historically, providers may have been reluctant to utilize PN for fear of infectious morbidity; however, contemporary pragmatic-design RCTs demonstrate safety with exclusive PN (Harvey SE et al. N Engl J Med. 2014;371:1673-84). When your patient has a contraindication for EN or does not tolerate it despite a trial of small bowel feeding, meta-analyses have shown a mortality benefit of early exclusive PN in malnourished patients, as compared with no nutrition (Braunschweig C et al. Am J Clin Nutr.2001;74:534-42).
As for supplemental PN (SPN), the 2016 ASPEN/SCCM guideline does not recommend it until day 7 in all critically ill patients, while the 2018 ESPEN guideline recommends its use on a case-by-case basis. Since, two trials inform SPN use. The EAT-ICU trial showed no difference in 6-month physical function between EN group and early-goal-directed nutritiongroup, which included SPN to achieve estimated energy requirement during the first week of critical illness (Allingstrup MJ et al. Intensive Care Med. 2017;43:1637-47). The TOP-UP trial compared EN alone with EN plus SPN in nutritionally high risk patients (ie, those who stand to have more complications as a result of undernutrition) and found those with a BMI < 25 kg/m2 and those with a NUTRIC score >5 who received supplemental PN atop EN had improved 30-day mortality, as compared with EN alone (Wischmeyer P et al. Crit Care. 2017;21:142). Mortality was a secondary outcome, and further study of supplemental PN in nutritionally high-risk patients is warranted. Until further data are available, supplemental PN should probably be restricted during the acute phase of critical illness.
Protein may be the important substrate
Proteolysis is the rule during critical illness, and amino acids are liberated from skeletal muscle breakdown. Using ultrasound, Puthucheary et al found a 17.7% reduction in rectus femoris cross-sectional area in 63 critically ill adults and identified muscle cellular infiltration at ICU day 10, suggesting critical illness leads to quantitative and qualitative muscle defects (Puthucheary Z et al. JAMA. 2013;15:1591-1600).
Since survivorship from critical illness is increasing, acquired loss of muscle mass may contribute to post-ICU physical functioning impairments. Thus, protein may be the most important substrate to deliver during critical illness. The 2016 ASPEN/SCCM guideline recommends 1.2 – 2.0 g/kg actual body weight (ABW)/day in nonobese critically ill patients.
Unfortunately, the optimal protein dose and the timing of intake are unknown. Observational studies suggest benefit with lower and higher doses, which creates equipoise for protein dose. The signal may be lost in heterogeneity, and observational data suggest higher protein dose may benefit patients with high nutritional risk. In terms of timing, one observational study found lower (<0.8 g/kg/d) protein dose before day 3 followed by higher (>0.8 g/kg/d) dose thereafter was associated with mortality benefit (Koekkoek WAC et al. Clin Nutr.2019;38:883-890).
Until stronger data are available to guide optimal protein dose and timing, it is reasonable to observe the 2016 ASPEN/SCCM guideline protein recommendation of at least 1.2 g/kg/day. The 2018 ESPEN guideline recommends a similar dose of 1.3 g/kg/day.
Future research and summary
Many questions remain unanswered and present opportunities for future research. Priorities for critical care nutrition research include studying the impact of combined nutrition and exercise in the acute and late phases of critical illness and identifying best tools to differentiate responses to caloric and protein intake.
In summary, critical illness has acute and late phases. The acute phase is a hypercatabolic state leading to negative energy and nitrogen balance and gut dysfunction. Take-home points for nutrition support in the acute phase of critical illness are:
1. It is reasonable to start early hypocaloric EN with an isosmotic formula with slow up-titration over the first week of critical illness while monitoring for refeeding syndrome and feeding intolerance.
2. Use exclusive PN in ICU patients with pre-existing malnutrition when EN is contraindicated or not tolerated.
3. Supplemental PN should probably be restricted during the acute phase of critical illness.
4. Optimal protein dose and timing are unknown. It is reasonable to start with at least 1.2 g/kg ABW/day in non-obese patients.
Dr. Patel is with the Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin.
Dr. Rice is with the Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Vanderbilt University, Nashville, Tennessee.
Environmental Scan: Drivers of philanthropy
Philanthropy is a driving force supporting and promoting pioneering research and programs in many fields of medicine. Charitable giving, foundation support, and grants touch the lives of millions of patients and also have an impact across all fields of practice of medical practice. But philanthropy is being transformed by changing technology, interests of the giving public, and demands for accountability and transparency. Understanding where these trends are going will give physicians insights into what they can expect from philanthropy and what it might mean for their own institutions and interests.
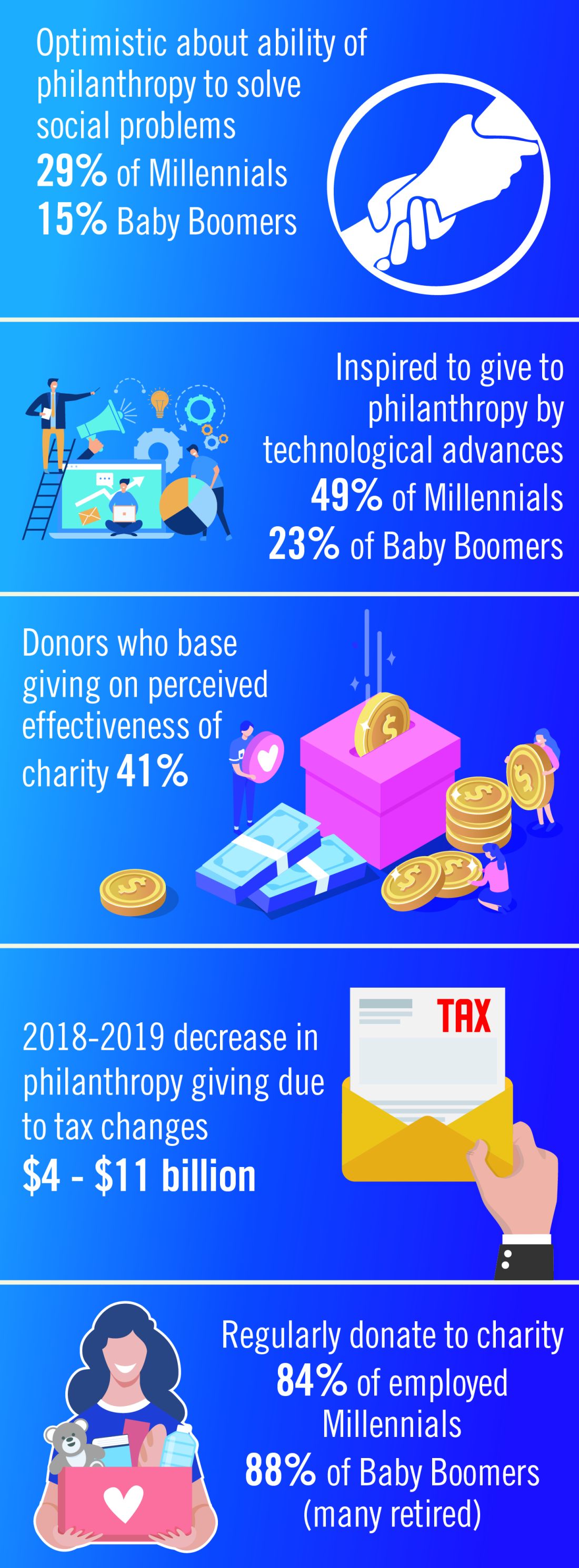
In 2019, Charity Navigator reported total giving to charitable organizations was $427.1 billion, 0.7% measured in current dollars over the revised total of $424.74 billion contributed in 2017. Yet adjusted for inflation, overall giving declined 1.7%, primarily because individual giving declined. Foundation giving increased by an estimated 7.3% over 2017, to $75.86 billion in 2018 (an increase of 4.7%, adjusted for inflation). Giving by corporations is estimated to have increased by 5.4% in 2018, totaling $20.05 billion (an increase of 2.9%, adjusted for inflation).1
Impact investing, transparency, and trust
The demand for increased accountability in philanthropy is growing. Today’s donors want to know their contributions will have a real impact in causes they believe in. As donors become more focused on results, organizations will need to demonstrate their ability to achieve short-term goals that bring them closer to accomplishing their mission and vision. This sentiment may be strongest among Millennials. Nonprofit organizations should expect an increased level of due diligence and a higher level of personal involvement by donors. At least 41% of donors have changed their giving because of increased knowledge about nonprofit effectiveness. Foundations and corporations donate to medical centers and research institutions, but recipients are have an expectation of close involvement of donors, the need for detailed accounts of how funds are spent, and a responsibility to show progress or measurable outcomes.2
Health care–related issues
Two of the top three issues identified by donors as a challenge to be addressed are related to health care, according to Fidelity Charitable. Thirty-nine percent identified “developing treatment or cures for a disease” and 33% cited “access to basic health services” as priority issues. A study by Giving USA estimated that charitable giving to health care organizations rose a strong 7.3% (5.5% adjusted for inflation) in 2017, but giving that year was fueled by a booming stock market and a favorable tax environment. Charitable donations to hospitals tend to reflect the economic health of the community in which the institution is located. Donations to rural hospitals in depressed communities are likely to be far less than to urban institutions in economically strong areas.3
Tax reform
The Tax Cuts and Jobs Act of 2017 will likely affect donations to charitable organizations in 2019. Specifically, the 2017 Tax Act doubled the standard tax deduction, thereby reducing the number of households having to itemize their deductions and eliminating many tax benefits for charitable donations. Middle-class families are expected to opt for the standard deduction while wealthier taxpayers will likely continue itemizing their deductions. As a result, some predict that donors may switch from giving annually to giving every third year so they can itemize in their giving years to get the deduction. Estimates that charitable donations from individuals may decrease as much as $4 billion to $11 billion because of the increase in standard deductions and $0.9 billion to $2.1 billion because of the decrease in the marginal tax rate.4
Technology and peer-to-peer giving
Technological advances that make researching and giving easier and more convenient are likely to have a significant impact on many charitable organizations in 2019. Online donations are likely to increase as organizations make it simple to donate from mobile devices, social media platforms and their websites. Although charitable organizations will continue to directly ask individuals for a donation, many are expanding their efforts to include online social campaigns that leverage peer-to-peer giving. Other technological advancements likely to affect donations in the future include the ability for organizations to incorporate contactless payment programs and blockchain technology. Online giving grew by 12.1% over 2018-2019 with monthly automatic giving increasing by 40% over 2016 to 2017.5
Generational differences in giving
Although the trends identified above are likely to affect the decision to give in 2019, there are some meaningful differences in how different generations embrace these changes. Technological advances, the rise of alternative forms of giving, and increased opportunities to connect with peers about giving influence Millennials significantly more than Baby Boomers. Millennials are more likely to say that they give to make a meaningful difference while Boomers are likely to say that giving is part of their values. Millennials also are more likely to say their giving is more spontaneous while Boomers say their giving is more planned. As many as 49% of Millennials cite technological advances influencing their giving, compared with only 23% of Baby Boomers. This trend continues for the rise of alternative forms of giving (32% of Millennials, compared with 14% of Boomers) and increased opportunities to connect with peers about giving (30%, compared with 11%).
Twenty-nine percent of Millennials are very optimistic about philanthropy’s ability to solve the issues most important to them, compared with only 15% of Baby Boomers.2
One thing they have in common is their priorities. Both generations prioritize challenges related to health, hunger, and the environment.6
Today, foundations need to focus on impact, not just education programs or scholarships. New tech-driven trends in giving, such as the emergence of digital peer-to-peer giving and crowdfunding campaigns, make it possible to tap into high-volume, small-amount donations. To recruit new donors, organizations will need to target their messages based on the audience segment.
References:
1. Giving USA 2019: Annual report for philanthropy for 2018. Accessed Nov. 14, 2019.
2. Fidelity Charitable (2019) Future of philanthropy. Accessed Nov. 10, 2019.3. Betbeze, Philip (2018) Charitable giving to health giving to health organizations rose 7.3% last year. Health Leaders. July 11.
4. Martis & Landy/Indiana University Lilly Family School of Philanthropy (2018) The Philanthropy Outlook 2018 & 2019.
5. M&R Benchmarks 2019.
6. Nonprofit Source (2019) The ultimate list of charitable giving statistics for 2018. Accessed Nov. 10, 2019.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Philanthropy is a driving force supporting and promoting pioneering research and programs in many fields of medicine. Charitable giving, foundation support, and grants touch the lives of millions of patients and also have an impact across all fields of practice of medical practice. But philanthropy is being transformed by changing technology, interests of the giving public, and demands for accountability and transparency. Understanding where these trends are going will give physicians insights into what they can expect from philanthropy and what it might mean for their own institutions and interests.
In 2019, Charity Navigator reported total giving to charitable organizations was $427.1 billion, 0.7% measured in current dollars over the revised total of $424.74 billion contributed in 2017. Yet adjusted for inflation, overall giving declined 1.7%, primarily because individual giving declined. Foundation giving increased by an estimated 7.3% over 2017, to $75.86 billion in 2018 (an increase of 4.7%, adjusted for inflation). Giving by corporations is estimated to have increased by 5.4% in 2018, totaling $20.05 billion (an increase of 2.9%, adjusted for inflation).1
Impact investing, transparency, and trust
The demand for increased accountability in philanthropy is growing. Today’s donors want to know their contributions will have a real impact in causes they believe in. As donors become more focused on results, organizations will need to demonstrate their ability to achieve short-term goals that bring them closer to accomplishing their mission and vision. This sentiment may be strongest among Millennials. Nonprofit organizations should expect an increased level of due diligence and a higher level of personal involvement by donors. At least 41% of donors have changed their giving because of increased knowledge about nonprofit effectiveness. Foundations and corporations donate to medical centers and research institutions, but recipients are have an expectation of close involvement of donors, the need for detailed accounts of how funds are spent, and a responsibility to show progress or measurable outcomes.2
Health care–related issues
Two of the top three issues identified by donors as a challenge to be addressed are related to health care, according to Fidelity Charitable. Thirty-nine percent identified “developing treatment or cures for a disease” and 33% cited “access to basic health services” as priority issues. A study by Giving USA estimated that charitable giving to health care organizations rose a strong 7.3% (5.5% adjusted for inflation) in 2017, but giving that year was fueled by a booming stock market and a favorable tax environment. Charitable donations to hospitals tend to reflect the economic health of the community in which the institution is located. Donations to rural hospitals in depressed communities are likely to be far less than to urban institutions in economically strong areas.3
Tax reform
The Tax Cuts and Jobs Act of 2017 will likely affect donations to charitable organizations in 2019. Specifically, the 2017 Tax Act doubled the standard tax deduction, thereby reducing the number of households having to itemize their deductions and eliminating many tax benefits for charitable donations. Middle-class families are expected to opt for the standard deduction while wealthier taxpayers will likely continue itemizing their deductions. As a result, some predict that donors may switch from giving annually to giving every third year so they can itemize in their giving years to get the deduction. Estimates that charitable donations from individuals may decrease as much as $4 billion to $11 billion because of the increase in standard deductions and $0.9 billion to $2.1 billion because of the decrease in the marginal tax rate.4
Technology and peer-to-peer giving
Technological advances that make researching and giving easier and more convenient are likely to have a significant impact on many charitable organizations in 2019. Online donations are likely to increase as organizations make it simple to donate from mobile devices, social media platforms and their websites. Although charitable organizations will continue to directly ask individuals for a donation, many are expanding their efforts to include online social campaigns that leverage peer-to-peer giving. Other technological advancements likely to affect donations in the future include the ability for organizations to incorporate contactless payment programs and blockchain technology. Online giving grew by 12.1% over 2018-2019 with monthly automatic giving increasing by 40% over 2016 to 2017.5
Generational differences in giving
Although the trends identified above are likely to affect the decision to give in 2019, there are some meaningful differences in how different generations embrace these changes. Technological advances, the rise of alternative forms of giving, and increased opportunities to connect with peers about giving influence Millennials significantly more than Baby Boomers. Millennials are more likely to say that they give to make a meaningful difference while Boomers are likely to say that giving is part of their values. Millennials also are more likely to say their giving is more spontaneous while Boomers say their giving is more planned. As many as 49% of Millennials cite technological advances influencing their giving, compared with only 23% of Baby Boomers. This trend continues for the rise of alternative forms of giving (32% of Millennials, compared with 14% of Boomers) and increased opportunities to connect with peers about giving (30%, compared with 11%).
Twenty-nine percent of Millennials are very optimistic about philanthropy’s ability to solve the issues most important to them, compared with only 15% of Baby Boomers.2
One thing they have in common is their priorities. Both generations prioritize challenges related to health, hunger, and the environment.6
Today, foundations need to focus on impact, not just education programs or scholarships. New tech-driven trends in giving, such as the emergence of digital peer-to-peer giving and crowdfunding campaigns, make it possible to tap into high-volume, small-amount donations. To recruit new donors, organizations will need to target their messages based on the audience segment.
References:
1. Giving USA 2019: Annual report for philanthropy for 2018. Accessed Nov. 14, 2019.
2. Fidelity Charitable (2019) Future of philanthropy. Accessed Nov. 10, 2019.3. Betbeze, Philip (2018) Charitable giving to health giving to health organizations rose 7.3% last year. Health Leaders. July 11.
4. Martis & Landy/Indiana University Lilly Family School of Philanthropy (2018) The Philanthropy Outlook 2018 & 2019.
5. M&R Benchmarks 2019.
6. Nonprofit Source (2019) The ultimate list of charitable giving statistics for 2018. Accessed Nov. 10, 2019.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Philanthropy is a driving force supporting and promoting pioneering research and programs in many fields of medicine. Charitable giving, foundation support, and grants touch the lives of millions of patients and also have an impact across all fields of practice of medical practice. But philanthropy is being transformed by changing technology, interests of the giving public, and demands for accountability and transparency. Understanding where these trends are going will give physicians insights into what they can expect from philanthropy and what it might mean for their own institutions and interests.
In 2019, Charity Navigator reported total giving to charitable organizations was $427.1 billion, 0.7% measured in current dollars over the revised total of $424.74 billion contributed in 2017. Yet adjusted for inflation, overall giving declined 1.7%, primarily because individual giving declined. Foundation giving increased by an estimated 7.3% over 2017, to $75.86 billion in 2018 (an increase of 4.7%, adjusted for inflation). Giving by corporations is estimated to have increased by 5.4% in 2018, totaling $20.05 billion (an increase of 2.9%, adjusted for inflation).1
Impact investing, transparency, and trust
The demand for increased accountability in philanthropy is growing. Today’s donors want to know their contributions will have a real impact in causes they believe in. As donors become more focused on results, organizations will need to demonstrate their ability to achieve short-term goals that bring them closer to accomplishing their mission and vision. This sentiment may be strongest among Millennials. Nonprofit organizations should expect an increased level of due diligence and a higher level of personal involvement by donors. At least 41% of donors have changed their giving because of increased knowledge about nonprofit effectiveness. Foundations and corporations donate to medical centers and research institutions, but recipients are have an expectation of close involvement of donors, the need for detailed accounts of how funds are spent, and a responsibility to show progress or measurable outcomes.2
Health care–related issues
Two of the top three issues identified by donors as a challenge to be addressed are related to health care, according to Fidelity Charitable. Thirty-nine percent identified “developing treatment or cures for a disease” and 33% cited “access to basic health services” as priority issues. A study by Giving USA estimated that charitable giving to health care organizations rose a strong 7.3% (5.5% adjusted for inflation) in 2017, but giving that year was fueled by a booming stock market and a favorable tax environment. Charitable donations to hospitals tend to reflect the economic health of the community in which the institution is located. Donations to rural hospitals in depressed communities are likely to be far less than to urban institutions in economically strong areas.3
Tax reform
The Tax Cuts and Jobs Act of 2017 will likely affect donations to charitable organizations in 2019. Specifically, the 2017 Tax Act doubled the standard tax deduction, thereby reducing the number of households having to itemize their deductions and eliminating many tax benefits for charitable donations. Middle-class families are expected to opt for the standard deduction while wealthier taxpayers will likely continue itemizing their deductions. As a result, some predict that donors may switch from giving annually to giving every third year so they can itemize in their giving years to get the deduction. Estimates that charitable donations from individuals may decrease as much as $4 billion to $11 billion because of the increase in standard deductions and $0.9 billion to $2.1 billion because of the decrease in the marginal tax rate.4
Technology and peer-to-peer giving
Technological advances that make researching and giving easier and more convenient are likely to have a significant impact on many charitable organizations in 2019. Online donations are likely to increase as organizations make it simple to donate from mobile devices, social media platforms and their websites. Although charitable organizations will continue to directly ask individuals for a donation, many are expanding their efforts to include online social campaigns that leverage peer-to-peer giving. Other technological advancements likely to affect donations in the future include the ability for organizations to incorporate contactless payment programs and blockchain technology. Online giving grew by 12.1% over 2018-2019 with monthly automatic giving increasing by 40% over 2016 to 2017.5
Generational differences in giving
Although the trends identified above are likely to affect the decision to give in 2019, there are some meaningful differences in how different generations embrace these changes. Technological advances, the rise of alternative forms of giving, and increased opportunities to connect with peers about giving influence Millennials significantly more than Baby Boomers. Millennials are more likely to say that they give to make a meaningful difference while Boomers are likely to say that giving is part of their values. Millennials also are more likely to say their giving is more spontaneous while Boomers say their giving is more planned. As many as 49% of Millennials cite technological advances influencing their giving, compared with only 23% of Baby Boomers. This trend continues for the rise of alternative forms of giving (32% of Millennials, compared with 14% of Boomers) and increased opportunities to connect with peers about giving (30%, compared with 11%).
Twenty-nine percent of Millennials are very optimistic about philanthropy’s ability to solve the issues most important to them, compared with only 15% of Baby Boomers.2
One thing they have in common is their priorities. Both generations prioritize challenges related to health, hunger, and the environment.6
Today, foundations need to focus on impact, not just education programs or scholarships. New tech-driven trends in giving, such as the emergence of digital peer-to-peer giving and crowdfunding campaigns, make it possible to tap into high-volume, small-amount donations. To recruit new donors, organizations will need to target their messages based on the audience segment.
References:
1. Giving USA 2019: Annual report for philanthropy for 2018. Accessed Nov. 14, 2019.
2. Fidelity Charitable (2019) Future of philanthropy. Accessed Nov. 10, 2019.3. Betbeze, Philip (2018) Charitable giving to health giving to health organizations rose 7.3% last year. Health Leaders. July 11.
4. Martis & Landy/Indiana University Lilly Family School of Philanthropy (2018) The Philanthropy Outlook 2018 & 2019.
5. M&R Benchmarks 2019.
6. Nonprofit Source (2019) The ultimate list of charitable giving statistics for 2018. Accessed Nov. 10, 2019.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Outcomes of epilepsy surgery at 1 year may be better among older patients
BALTIMORE – Older patients may have better outcomes at 1 year after resective surgery for epilepsy than the general population does, according to research presented at the annual meeting of the American Epilepsy Society. A tendency toward greater prevalence of lesional epilepsy and temporal lobe epilepsy (TLE) in the older patients in the study population could explain this difference in outcomes. Although surgery might entail greater risks in older patients, the decision to operate should be based on the patient’s inherent risk, and not on his or her age, said Juan S. Bottan, MD, neurosurgery resident at Hospital Pedro De Elizalde in Buenos Aires, and colleagues.
Epilepsy surgery as a treatment for elderly patients is controversial. These patients generally are not considered to be surgical candidates because of concerns about long disease duration and increased surgical risk. Recent literature, however, suggests that elderly patients can benefit from surgery. Lang et al. found that epilepsy surgery success rates can be higher in selected older patients than in younger patients, although older patients may be at greater risk for postoperative hygroma and memory deficits.
Dr. Bottan and colleagues sought to analyze the role of resective surgery in patients older than age 60 years by evaluating surgical outcomes and safety. The investigators retrospectively analyzed 595 patients who underwent resective epilepsy surgery at Western University in London, Ontario, during 1999-2019. Eligible participants had drug-resistant epilepsy that had failed the best medical management. The researchers identified 31 patients aged 60 years or older and randomly selected 60 patients aged 59 years or younger as a control group. Dr. Bottan and colleagues analyzed the population’s characteristics, presurgical evaluations, postoperative outcome, and complications.
The investigators found no significant differences between groups in terms of hemisphere dominance, side of surgery, the ratio of patients with lesional epilepsy to patients with nonlesional epilepsy, and incidence of TLE over extratemporal epilepsy.
Nevertheless, extratemporal epilepsy was more frequent in older patients. Age and duration of epilepsy were significantly greater in older patients, and invasive recording was significantly more common in younger patients.
The most common pathology results in older patients were mesial temporal sclerosis (39%), gliosis (19%), and other (19%). Among younger patients, the most common pathology results were mesial temporal sclerosis (25%), gliosis (25%), and focal cortical dysplasia (15%).
The rates of Engel Class I outcome at 6 months, 1 year, and 2 years were 92.9%, 88.5%, and 94.7% among older patients and 75%, 63.5%, and 75.8% among younger patients, respectively. The difference between groups in Engel Class I outcome at 1 year was statistically significant. Patients with TLE had a better seizure outcome, regardless of age group, but the rate of good outcome was higher among older patients. The rate of complications was higher among older patients, but the difference was not statistically significant.
The study was not supported by external funding, and the investigators had no disclosures.
SOURCE: Bottan JS et al. AES 2019, Abstract 1.343.
BALTIMORE – Older patients may have better outcomes at 1 year after resective surgery for epilepsy than the general population does, according to research presented at the annual meeting of the American Epilepsy Society. A tendency toward greater prevalence of lesional epilepsy and temporal lobe epilepsy (TLE) in the older patients in the study population could explain this difference in outcomes. Although surgery might entail greater risks in older patients, the decision to operate should be based on the patient’s inherent risk, and not on his or her age, said Juan S. Bottan, MD, neurosurgery resident at Hospital Pedro De Elizalde in Buenos Aires, and colleagues.
Epilepsy surgery as a treatment for elderly patients is controversial. These patients generally are not considered to be surgical candidates because of concerns about long disease duration and increased surgical risk. Recent literature, however, suggests that elderly patients can benefit from surgery. Lang et al. found that epilepsy surgery success rates can be higher in selected older patients than in younger patients, although older patients may be at greater risk for postoperative hygroma and memory deficits.
Dr. Bottan and colleagues sought to analyze the role of resective surgery in patients older than age 60 years by evaluating surgical outcomes and safety. The investigators retrospectively analyzed 595 patients who underwent resective epilepsy surgery at Western University in London, Ontario, during 1999-2019. Eligible participants had drug-resistant epilepsy that had failed the best medical management. The researchers identified 31 patients aged 60 years or older and randomly selected 60 patients aged 59 years or younger as a control group. Dr. Bottan and colleagues analyzed the population’s characteristics, presurgical evaluations, postoperative outcome, and complications.
The investigators found no significant differences between groups in terms of hemisphere dominance, side of surgery, the ratio of patients with lesional epilepsy to patients with nonlesional epilepsy, and incidence of TLE over extratemporal epilepsy.
Nevertheless, extratemporal epilepsy was more frequent in older patients. Age and duration of epilepsy were significantly greater in older patients, and invasive recording was significantly more common in younger patients.
The most common pathology results in older patients were mesial temporal sclerosis (39%), gliosis (19%), and other (19%). Among younger patients, the most common pathology results were mesial temporal sclerosis (25%), gliosis (25%), and focal cortical dysplasia (15%).
The rates of Engel Class I outcome at 6 months, 1 year, and 2 years were 92.9%, 88.5%, and 94.7% among older patients and 75%, 63.5%, and 75.8% among younger patients, respectively. The difference between groups in Engel Class I outcome at 1 year was statistically significant. Patients with TLE had a better seizure outcome, regardless of age group, but the rate of good outcome was higher among older patients. The rate of complications was higher among older patients, but the difference was not statistically significant.
The study was not supported by external funding, and the investigators had no disclosures.
SOURCE: Bottan JS et al. AES 2019, Abstract 1.343.
BALTIMORE – Older patients may have better outcomes at 1 year after resective surgery for epilepsy than the general population does, according to research presented at the annual meeting of the American Epilepsy Society. A tendency toward greater prevalence of lesional epilepsy and temporal lobe epilepsy (TLE) in the older patients in the study population could explain this difference in outcomes. Although surgery might entail greater risks in older patients, the decision to operate should be based on the patient’s inherent risk, and not on his or her age, said Juan S. Bottan, MD, neurosurgery resident at Hospital Pedro De Elizalde in Buenos Aires, and colleagues.
Epilepsy surgery as a treatment for elderly patients is controversial. These patients generally are not considered to be surgical candidates because of concerns about long disease duration and increased surgical risk. Recent literature, however, suggests that elderly patients can benefit from surgery. Lang et al. found that epilepsy surgery success rates can be higher in selected older patients than in younger patients, although older patients may be at greater risk for postoperative hygroma and memory deficits.
Dr. Bottan and colleagues sought to analyze the role of resective surgery in patients older than age 60 years by evaluating surgical outcomes and safety. The investigators retrospectively analyzed 595 patients who underwent resective epilepsy surgery at Western University in London, Ontario, during 1999-2019. Eligible participants had drug-resistant epilepsy that had failed the best medical management. The researchers identified 31 patients aged 60 years or older and randomly selected 60 patients aged 59 years or younger as a control group. Dr. Bottan and colleagues analyzed the population’s characteristics, presurgical evaluations, postoperative outcome, and complications.
The investigators found no significant differences between groups in terms of hemisphere dominance, side of surgery, the ratio of patients with lesional epilepsy to patients with nonlesional epilepsy, and incidence of TLE over extratemporal epilepsy.
Nevertheless, extratemporal epilepsy was more frequent in older patients. Age and duration of epilepsy were significantly greater in older patients, and invasive recording was significantly more common in younger patients.
The most common pathology results in older patients were mesial temporal sclerosis (39%), gliosis (19%), and other (19%). Among younger patients, the most common pathology results were mesial temporal sclerosis (25%), gliosis (25%), and focal cortical dysplasia (15%).
The rates of Engel Class I outcome at 6 months, 1 year, and 2 years were 92.9%, 88.5%, and 94.7% among older patients and 75%, 63.5%, and 75.8% among younger patients, respectively. The difference between groups in Engel Class I outcome at 1 year was statistically significant. Patients with TLE had a better seizure outcome, regardless of age group, but the rate of good outcome was higher among older patients. The rate of complications was higher among older patients, but the difference was not statistically significant.
The study was not supported by external funding, and the investigators had no disclosures.
SOURCE: Bottan JS et al. AES 2019, Abstract 1.343.
REPORTING FROM AES 2019
Fragmentation of sickle cell disease care starts in young adulthood
ORLANDO – While over time, results of a retrospective study suggest.
Nearly 60% of children between aged10-17 years were seen at just one facility over the course of 7 years in the analysis, which was based on analysis of data for nearly 7,000 patients seen in California during 1991-2016.
That contrasted sharply with young adults, aged 18-25 years, only about 20% of whom were admitted to one facility, said senior study author Anjlee Mahajan, MD, of the University of California, Davis, adding that another 20% were seen at five or more centers over a 7-year follow-up period.
Fragmentation of care didn’t increase the risk of death in this study, as investigators hypothesized it might. However, the outcomes and the quality of care among young adults with SCD who received inpatient care at multiple facilities nevertheless was likely to be affected, Dr. Mahajan said at the annual meeting of the American Society of Hematology.
“Imagine what that would be like to have a chronic, debilitating illness and to have to go to multiple different hospitals, during this vulnerable time period in your life, and being seen by different care providers who may not know you and may not have all of your records as well,” she said in a press conference at the meeting.
Providers and the health care system need to work harder to ensure young adults receive comprehensive and coordinated care, especially at a time when therapeutic advances are improving the treatment of this disease, according to the investigator.
“When you’re seen at one center, you can have a specific pain plan, and maybe when you are going into the emergency room and being admitted, your sickle cell care provider might come and visit you in the hospital or at least be in contact with your team,” Dr. Mahajan said in an interview. “That may not happen if you’re going to be seen at five different hospitals in 7 years.”
Encouraging the concept of “medical home” for SCD may be help ease transition from pediatric to adult care, thereby reducing fragmentation of care for young adults, according to Julie A. Panepinto, MD, professor of pediatric hematology and the director of the center for clinical effectiveness research at the Children’s Research Institute, Medical College of Wisconsin, Milwaukee.
“That 18- to 30-year-old age group historically and repeatedly over time is shown to be the age that relies on the emergency department and that has a higher mortality as they transition,” Dr. Panepinto said in an interview. “So ideally, you would have a pediatric program that’s comprehensive and that can transition an adult patient to a very similar setting with knowledgeable providers in SCD across the spectrum, from the emergency department to the hospital to the outpatient clinic.”
Dr. Mahajan reported no disclosures related to her group’s study. Coauthors provided disclosures related to Pfizer and Janssen.
SOURCE: Shatola A et al. ASH 2019. Abstract 4667.
ORLANDO – While over time, results of a retrospective study suggest.
Nearly 60% of children between aged10-17 years were seen at just one facility over the course of 7 years in the analysis, which was based on analysis of data for nearly 7,000 patients seen in California during 1991-2016.
That contrasted sharply with young adults, aged 18-25 years, only about 20% of whom were admitted to one facility, said senior study author Anjlee Mahajan, MD, of the University of California, Davis, adding that another 20% were seen at five or more centers over a 7-year follow-up period.
Fragmentation of care didn’t increase the risk of death in this study, as investigators hypothesized it might. However, the outcomes and the quality of care among young adults with SCD who received inpatient care at multiple facilities nevertheless was likely to be affected, Dr. Mahajan said at the annual meeting of the American Society of Hematology.
“Imagine what that would be like to have a chronic, debilitating illness and to have to go to multiple different hospitals, during this vulnerable time period in your life, and being seen by different care providers who may not know you and may not have all of your records as well,” she said in a press conference at the meeting.
Providers and the health care system need to work harder to ensure young adults receive comprehensive and coordinated care, especially at a time when therapeutic advances are improving the treatment of this disease, according to the investigator.
“When you’re seen at one center, you can have a specific pain plan, and maybe when you are going into the emergency room and being admitted, your sickle cell care provider might come and visit you in the hospital or at least be in contact with your team,” Dr. Mahajan said in an interview. “That may not happen if you’re going to be seen at five different hospitals in 7 years.”
Encouraging the concept of “medical home” for SCD may be help ease transition from pediatric to adult care, thereby reducing fragmentation of care for young adults, according to Julie A. Panepinto, MD, professor of pediatric hematology and the director of the center for clinical effectiveness research at the Children’s Research Institute, Medical College of Wisconsin, Milwaukee.
“That 18- to 30-year-old age group historically and repeatedly over time is shown to be the age that relies on the emergency department and that has a higher mortality as they transition,” Dr. Panepinto said in an interview. “So ideally, you would have a pediatric program that’s comprehensive and that can transition an adult patient to a very similar setting with knowledgeable providers in SCD across the spectrum, from the emergency department to the hospital to the outpatient clinic.”
Dr. Mahajan reported no disclosures related to her group’s study. Coauthors provided disclosures related to Pfizer and Janssen.
SOURCE: Shatola A et al. ASH 2019. Abstract 4667.
ORLANDO – While over time, results of a retrospective study suggest.
Nearly 60% of children between aged10-17 years were seen at just one facility over the course of 7 years in the analysis, which was based on analysis of data for nearly 7,000 patients seen in California during 1991-2016.
That contrasted sharply with young adults, aged 18-25 years, only about 20% of whom were admitted to one facility, said senior study author Anjlee Mahajan, MD, of the University of California, Davis, adding that another 20% were seen at five or more centers over a 7-year follow-up period.
Fragmentation of care didn’t increase the risk of death in this study, as investigators hypothesized it might. However, the outcomes and the quality of care among young adults with SCD who received inpatient care at multiple facilities nevertheless was likely to be affected, Dr. Mahajan said at the annual meeting of the American Society of Hematology.
“Imagine what that would be like to have a chronic, debilitating illness and to have to go to multiple different hospitals, during this vulnerable time period in your life, and being seen by different care providers who may not know you and may not have all of your records as well,” she said in a press conference at the meeting.
Providers and the health care system need to work harder to ensure young adults receive comprehensive and coordinated care, especially at a time when therapeutic advances are improving the treatment of this disease, according to the investigator.
“When you’re seen at one center, you can have a specific pain plan, and maybe when you are going into the emergency room and being admitted, your sickle cell care provider might come and visit you in the hospital or at least be in contact with your team,” Dr. Mahajan said in an interview. “That may not happen if you’re going to be seen at five different hospitals in 7 years.”
Encouraging the concept of “medical home” for SCD may be help ease transition from pediatric to adult care, thereby reducing fragmentation of care for young adults, according to Julie A. Panepinto, MD, professor of pediatric hematology and the director of the center for clinical effectiveness research at the Children’s Research Institute, Medical College of Wisconsin, Milwaukee.
“That 18- to 30-year-old age group historically and repeatedly over time is shown to be the age that relies on the emergency department and that has a higher mortality as they transition,” Dr. Panepinto said in an interview. “So ideally, you would have a pediatric program that’s comprehensive and that can transition an adult patient to a very similar setting with knowledgeable providers in SCD across the spectrum, from the emergency department to the hospital to the outpatient clinic.”
Dr. Mahajan reported no disclosures related to her group’s study. Coauthors provided disclosures related to Pfizer and Janssen.
SOURCE: Shatola A et al. ASH 2019. Abstract 4667.
REPORTING FROM ASH 2019
Researchers mine free-text diary entries for seizure cluster insights
BALTIMORE – Free-text diary entries by patients with epilepsy are a “largely untapped” source of information about the frequency and treatment of seizure clusters, researchers said at the annual meeting of the American Epilepsy Society. In addition, patients may describe other clinically relevant concerns such as tiredness, depression, head injury, or seizures while driving, researchers said.
To examine how seizure clusters are reflected in the electronic diaries of patients with epilepsy, Joyce A. Cramer, a clinical research consultant and colleagues examined data from EpiDiary, a set of mobile and Web-based apps designed to help patients with epilepsy manage their medications and record their symptoms. EpiDiary prompts patients to indicate whether they were seizure free, had a seizure, or had a seizure cluster on a given day. Patients also have the ability to enter free-text notes.
“This was the first-ever review of the unstructured, free-text notes,” Ms. Cramer said.
Investigators used lexical analysis to identify free-text comments that potentially were about seizure clusters, based on the use of words such as “lots,” “many,” or “repeat.” Researchers reviewed every flagged comment to confirm whether it pertained to a seizure cluster. They defined a cluster as two or more seizures on a calendar day.
An algorithm flagged 5,955 entries by 1,839 users. Clinician review confirmed that 2,645 of the flagged comments (44.4%) pertained to seizure clusters. Of the confirmed clusters, 512 (19.4%) were found only through the free-text notes and had not been documented through structured data elements such as seizure cluster check-boxes or seizure counts.
“Extra medicine was taken for clusters by 553 users on 3,818 days,” the researchers reported. “This was 30.1% of all users and 56.5% of those commenting on clusters.” In some instances, patients named specific medications, including lorazepam, clonazepam, midazolam, clobazam, rectal diazepam, other diazepam, and clorazepate.
Free-text diary entries could help researchers study various topics. The authors highlighted examples of entries that “contained other clinically relevant information,” including the following:
- Massive ongoing cluster with about 20% apneic events.
- My constant question seems to be: HOW can I function in life when just small outings bring about this incessant tiredness?
- Started feeling like I was having an aura and pulled over.
- Thought about suicide for the first time in a while.
Interpretations of the seizure cluster data are limited, the researchers noted. The algorithm might have missed some free-text comments that were about seizure clusters. And in some instances, researchers used words such as “puffs” to identify seizures when a connection to seizures was not entirely clear. In addition, patients may have used a definition of cluster that was different from the definition used by the investigators.
UCB Pharma and Irody, the company that owns EpiDiary, funded the study. Irody’s founder and president was a coauthor, and another author holds stock or options in Irody. Ms. Cramer consults for Irody, UCB, and other pharmaceutical companies.
SOURCE: Fisher RS et al. AES 2019. Abstract 1.424.
BALTIMORE – Free-text diary entries by patients with epilepsy are a “largely untapped” source of information about the frequency and treatment of seizure clusters, researchers said at the annual meeting of the American Epilepsy Society. In addition, patients may describe other clinically relevant concerns such as tiredness, depression, head injury, or seizures while driving, researchers said.
To examine how seizure clusters are reflected in the electronic diaries of patients with epilepsy, Joyce A. Cramer, a clinical research consultant and colleagues examined data from EpiDiary, a set of mobile and Web-based apps designed to help patients with epilepsy manage their medications and record their symptoms. EpiDiary prompts patients to indicate whether they were seizure free, had a seizure, or had a seizure cluster on a given day. Patients also have the ability to enter free-text notes.
“This was the first-ever review of the unstructured, free-text notes,” Ms. Cramer said.
Investigators used lexical analysis to identify free-text comments that potentially were about seizure clusters, based on the use of words such as “lots,” “many,” or “repeat.” Researchers reviewed every flagged comment to confirm whether it pertained to a seizure cluster. They defined a cluster as two or more seizures on a calendar day.
An algorithm flagged 5,955 entries by 1,839 users. Clinician review confirmed that 2,645 of the flagged comments (44.4%) pertained to seizure clusters. Of the confirmed clusters, 512 (19.4%) were found only through the free-text notes and had not been documented through structured data elements such as seizure cluster check-boxes or seizure counts.
“Extra medicine was taken for clusters by 553 users on 3,818 days,” the researchers reported. “This was 30.1% of all users and 56.5% of those commenting on clusters.” In some instances, patients named specific medications, including lorazepam, clonazepam, midazolam, clobazam, rectal diazepam, other diazepam, and clorazepate.
Free-text diary entries could help researchers study various topics. The authors highlighted examples of entries that “contained other clinically relevant information,” including the following:
- Massive ongoing cluster with about 20% apneic events.
- My constant question seems to be: HOW can I function in life when just small outings bring about this incessant tiredness?
- Started feeling like I was having an aura and pulled over.
- Thought about suicide for the first time in a while.
Interpretations of the seizure cluster data are limited, the researchers noted. The algorithm might have missed some free-text comments that were about seizure clusters. And in some instances, researchers used words such as “puffs” to identify seizures when a connection to seizures was not entirely clear. In addition, patients may have used a definition of cluster that was different from the definition used by the investigators.
UCB Pharma and Irody, the company that owns EpiDiary, funded the study. Irody’s founder and president was a coauthor, and another author holds stock or options in Irody. Ms. Cramer consults for Irody, UCB, and other pharmaceutical companies.
SOURCE: Fisher RS et al. AES 2019. Abstract 1.424.
BALTIMORE – Free-text diary entries by patients with epilepsy are a “largely untapped” source of information about the frequency and treatment of seizure clusters, researchers said at the annual meeting of the American Epilepsy Society. In addition, patients may describe other clinically relevant concerns such as tiredness, depression, head injury, or seizures while driving, researchers said.
To examine how seizure clusters are reflected in the electronic diaries of patients with epilepsy, Joyce A. Cramer, a clinical research consultant and colleagues examined data from EpiDiary, a set of mobile and Web-based apps designed to help patients with epilepsy manage their medications and record their symptoms. EpiDiary prompts patients to indicate whether they were seizure free, had a seizure, or had a seizure cluster on a given day. Patients also have the ability to enter free-text notes.
“This was the first-ever review of the unstructured, free-text notes,” Ms. Cramer said.
Investigators used lexical analysis to identify free-text comments that potentially were about seizure clusters, based on the use of words such as “lots,” “many,” or “repeat.” Researchers reviewed every flagged comment to confirm whether it pertained to a seizure cluster. They defined a cluster as two or more seizures on a calendar day.
An algorithm flagged 5,955 entries by 1,839 users. Clinician review confirmed that 2,645 of the flagged comments (44.4%) pertained to seizure clusters. Of the confirmed clusters, 512 (19.4%) were found only through the free-text notes and had not been documented through structured data elements such as seizure cluster check-boxes or seizure counts.
“Extra medicine was taken for clusters by 553 users on 3,818 days,” the researchers reported. “This was 30.1% of all users and 56.5% of those commenting on clusters.” In some instances, patients named specific medications, including lorazepam, clonazepam, midazolam, clobazam, rectal diazepam, other diazepam, and clorazepate.
Free-text diary entries could help researchers study various topics. The authors highlighted examples of entries that “contained other clinically relevant information,” including the following:
- Massive ongoing cluster with about 20% apneic events.
- My constant question seems to be: HOW can I function in life when just small outings bring about this incessant tiredness?
- Started feeling like I was having an aura and pulled over.
- Thought about suicide for the first time in a while.
Interpretations of the seizure cluster data are limited, the researchers noted. The algorithm might have missed some free-text comments that were about seizure clusters. And in some instances, researchers used words such as “puffs” to identify seizures when a connection to seizures was not entirely clear. In addition, patients may have used a definition of cluster that was different from the definition used by the investigators.
UCB Pharma and Irody, the company that owns EpiDiary, funded the study. Irody’s founder and president was a coauthor, and another author holds stock or options in Irody. Ms. Cramer consults for Irody, UCB, and other pharmaceutical companies.
SOURCE: Fisher RS et al. AES 2019. Abstract 1.424.
REPORTING FROM AES 2019
Skip CTs for breakthrough seizures in chronic epilepsy
BALTIMORE – Head CTs for breakthrough seizures in chronic epilepsy are useful for known structural triggers such as brain tumors, but they don’t change management for most patients, according to a review from the SUNY Upstate Medical University, Syracuse, N.Y., emergency department.
“Nonselective use of ED neuroimaging in patients with no new neurological findings” and no known structural problem, is “very low yield, and increases the use of hospital resources and radiation exposure without impacting the immediate care,” concluded investigators led by Shahram Izadyar, MD, an epileptologist and associate professor of neurology at the university.
In short, CTs for breakthrough seizures – routine in many EDs – usually are a waste of time and money. Absent a known structural cause, “there really isn’t a reason to do imaging,” he said at the American Epilepsy Society annual meeting.
Dr. Izadyar wanted to look into the issue after noticing how common CTs were among his breakthrough patients. He and his team reviewed 90 adults with an established diagnosis of epilepsy and on at least one antiepileptic who presented to the university ED for breakthrough seizures during 2017-2018; 39 (43.3%) had head CTs, 51 (56.7%) did not.
CT changed management in three of the four patients (4.4%) who had a known brain tumor, leading, for instance, to steroids for increased tumor edema. The rest of the patients had nonfocal exams, and imaging had no impact on management.
There was no rhyme or reason why some people got CTs and others didn’t; it seemed to be dependent on the provider. Defensive medicine probably had something to do with it, as well as saving time by ordering a CT instead of doing a neurologic exam, Dr. Izadyar said.
People aren’t going to stop doing defensive medicine, but even a small reduction in unnecessary CTs would “be a positive change.” There’s the cost issue, but also the radiation exposure, which is considerable when people end up in the ED every few months for breakthrough seizures, he said.
There were no differences between the CT and no-CT groups in the suspected causes of breakthroughs (P = .93). About half the cases were probably because of noncompliance, about a quarter from sleep deprivation, and the rest from a change in seizure medication or some other issue.
Dr. Izadyar said the next step is taking the findings to his ED colleagues, and perhaps calculating how much money the university would save by skipping CTs in chronic epilepsy patients with no known structural problem.
There were slightly more men than women in the study, and the mean age was 38 years.
There was no industry funding, and the investigators didn’t have any relevant disclosures.
SOURCE: Ali S et al. AES 2019. Abstract 1.213.
BALTIMORE – Head CTs for breakthrough seizures in chronic epilepsy are useful for known structural triggers such as brain tumors, but they don’t change management for most patients, according to a review from the SUNY Upstate Medical University, Syracuse, N.Y., emergency department.
“Nonselective use of ED neuroimaging in patients with no new neurological findings” and no known structural problem, is “very low yield, and increases the use of hospital resources and radiation exposure without impacting the immediate care,” concluded investigators led by Shahram Izadyar, MD, an epileptologist and associate professor of neurology at the university.
In short, CTs for breakthrough seizures – routine in many EDs – usually are a waste of time and money. Absent a known structural cause, “there really isn’t a reason to do imaging,” he said at the American Epilepsy Society annual meeting.
Dr. Izadyar wanted to look into the issue after noticing how common CTs were among his breakthrough patients. He and his team reviewed 90 adults with an established diagnosis of epilepsy and on at least one antiepileptic who presented to the university ED for breakthrough seizures during 2017-2018; 39 (43.3%) had head CTs, 51 (56.7%) did not.
CT changed management in three of the four patients (4.4%) who had a known brain tumor, leading, for instance, to steroids for increased tumor edema. The rest of the patients had nonfocal exams, and imaging had no impact on management.
There was no rhyme or reason why some people got CTs and others didn’t; it seemed to be dependent on the provider. Defensive medicine probably had something to do with it, as well as saving time by ordering a CT instead of doing a neurologic exam, Dr. Izadyar said.
People aren’t going to stop doing defensive medicine, but even a small reduction in unnecessary CTs would “be a positive change.” There’s the cost issue, but also the radiation exposure, which is considerable when people end up in the ED every few months for breakthrough seizures, he said.
There were no differences between the CT and no-CT groups in the suspected causes of breakthroughs (P = .93). About half the cases were probably because of noncompliance, about a quarter from sleep deprivation, and the rest from a change in seizure medication or some other issue.
Dr. Izadyar said the next step is taking the findings to his ED colleagues, and perhaps calculating how much money the university would save by skipping CTs in chronic epilepsy patients with no known structural problem.
There were slightly more men than women in the study, and the mean age was 38 years.
There was no industry funding, and the investigators didn’t have any relevant disclosures.
SOURCE: Ali S et al. AES 2019. Abstract 1.213.
BALTIMORE – Head CTs for breakthrough seizures in chronic epilepsy are useful for known structural triggers such as brain tumors, but they don’t change management for most patients, according to a review from the SUNY Upstate Medical University, Syracuse, N.Y., emergency department.
“Nonselective use of ED neuroimaging in patients with no new neurological findings” and no known structural problem, is “very low yield, and increases the use of hospital resources and radiation exposure without impacting the immediate care,” concluded investigators led by Shahram Izadyar, MD, an epileptologist and associate professor of neurology at the university.
In short, CTs for breakthrough seizures – routine in many EDs – usually are a waste of time and money. Absent a known structural cause, “there really isn’t a reason to do imaging,” he said at the American Epilepsy Society annual meeting.
Dr. Izadyar wanted to look into the issue after noticing how common CTs were among his breakthrough patients. He and his team reviewed 90 adults with an established diagnosis of epilepsy and on at least one antiepileptic who presented to the university ED for breakthrough seizures during 2017-2018; 39 (43.3%) had head CTs, 51 (56.7%) did not.
CT changed management in three of the four patients (4.4%) who had a known brain tumor, leading, for instance, to steroids for increased tumor edema. The rest of the patients had nonfocal exams, and imaging had no impact on management.
There was no rhyme or reason why some people got CTs and others didn’t; it seemed to be dependent on the provider. Defensive medicine probably had something to do with it, as well as saving time by ordering a CT instead of doing a neurologic exam, Dr. Izadyar said.
People aren’t going to stop doing defensive medicine, but even a small reduction in unnecessary CTs would “be a positive change.” There’s the cost issue, but also the radiation exposure, which is considerable when people end up in the ED every few months for breakthrough seizures, he said.
There were no differences between the CT and no-CT groups in the suspected causes of breakthroughs (P = .93). About half the cases were probably because of noncompliance, about a quarter from sleep deprivation, and the rest from a change in seizure medication or some other issue.
Dr. Izadyar said the next step is taking the findings to his ED colleagues, and perhaps calculating how much money the university would save by skipping CTs in chronic epilepsy patients with no known structural problem.
There were slightly more men than women in the study, and the mean age was 38 years.
There was no industry funding, and the investigators didn’t have any relevant disclosures.
SOURCE: Ali S et al. AES 2019. Abstract 1.213.
REPORTING FROM AES 2019
Care coordination, equity can eliminate disparities for nonwhite patients with DLBCL
ORLANDO – Patients with diffuse large B-cell lymphoma (DLBCL) who are members of an ethnic or racial minority do not have worse outcomes than whites when they receive appropriate treatment and institutional support, a study on disparities in cancer care shows.
Although previous studies have shown that minorities with DLBCL have worse outcomes than do whites, results of a study comparing outcomes from 155 patients of white heritage with those of 41 patients from black, Hispanic, or other minority backgrounds found no significant differences in either progression-free survival (PFS) or overall survival in 2 years over follow-up, reported Nilanjan Ghosh, MD, PhD, from the Levine Cancer Institute, Atrium Health, in Charlotte, N.C.
He attributes the results to his center’s robust nurse navigation program, equal access among all patients – regardless of ability to pay – to standard treatments, and to the availability of clinical trial participation and stem cell transplantation.
“I think a key message is that if you are able to offer the same treatment and clinical trials to people irrespective of their race or socioeconomic status and can provide support, you can get equal outcomes as long as the biology is the same in both groups,” he said at a briefing prior to presentation of data in an oral abstract session at the annual meeting of the American Society of Hematology.
Dr. Ghosh pointed to four separate studies that showed that minority populations with DLBCL have worse outcomes than did whites, and noted that both uninsured and Medicaid-insured patients have also been shown to have poorer results, suggesting a role of socioeconomic factors in determining who gets optimum care and who does not.
The investigators compared PFS and OS among white and nonwhite patients with DLBCL treated in their institution, which has a safety-net cancer center. They also looked at the frequencies of clinical trial participation and stem cell transplantation between the groups.
The study included all patients with de novo DLBCL who presented to their center during January 2016–January 2019. They used patient-reported descriptors of race/ethnicity to create one of two cohorts: either self-identified whites (155 patients) or nonwhites (41), a group that included black patients, Hispanic patients, Asian Americans, and Native Americans.
The authors collected data on demographics, disease characteristics (including revised International Prognostic Index and double-hit status), insurance data, treatment, trial enrollment, progression, and death.
They found that nonwhites were significantly younger at diagnosis (median 56 vs. 64 years; P = .007), with an even distribution between the sexes in each group.
Two-thirds of both white and nonwhite patients had government insurance (Medicare or Medicaid). Of the remaining patients, 33% of white had private insurance, compared with 27% of nonwhites. No whites were uninsured, but 3 of the 41 nonwhites (7%) had no insurance.
Of the 155 white patients, 121 (86%) received nurse navigation services, as did 33 of 41 (81%) of nonwhites. The services include lodging assistance for homeless patients, transportation services for patients without cars, and care coordination among primary care physicians, oncologists, and other specialists. The services are part of the center’s standard practice, with excess costs, if any, folded into the budget, Dr. Ghosh said.
Looking at disease characteristics and treatment, the investigators found that risk profiles were similar between the groups. A higher percentage of whites had double-hit lymphoma (11% vs. 7%), but this difference was not statistically significant.
The investigators also found that in their program race was not a barrier to optimum therapy, with 96% of whites and 98% of nonwhites receiving frontline therapy with an anthracycline and rituximab-based regimen, and 4% and 2%, respectively received a non–anthracycline based regimen.
In each group, 39% of patients had disease that either relapsed or was refractory to frontline therapy.
In all, 11% of whites and 12% of nonwhites enrolled in clinical trials, 11% and 19%, respectively, underwent stem cell transplantation.
For patients with relapsed/refractory disease, the 2-year PFS rates were 60% for whites, and 63% for nonwhites, and the 2-year OS rates were 74% and 81%, respectively.
Dr. Ghosh and colleagues concluded that “our safety net cancer center, with extensive nurse navigator support and access to standard treatments, stem cell transplants, and cutting-edge clinical trials may abrogate the inferior outcomes in minority populations that have been previously reported.”
The study was internally funded. Dr. Ghosh reported consulting fees, research funding, speakers bureau activity, and/or honoraria from multiple companies.
SOURCE: Hu B et al. ASH 2019. Abstract 425.
ORLANDO – Patients with diffuse large B-cell lymphoma (DLBCL) who are members of an ethnic or racial minority do not have worse outcomes than whites when they receive appropriate treatment and institutional support, a study on disparities in cancer care shows.
Although previous studies have shown that minorities with DLBCL have worse outcomes than do whites, results of a study comparing outcomes from 155 patients of white heritage with those of 41 patients from black, Hispanic, or other minority backgrounds found no significant differences in either progression-free survival (PFS) or overall survival in 2 years over follow-up, reported Nilanjan Ghosh, MD, PhD, from the Levine Cancer Institute, Atrium Health, in Charlotte, N.C.
He attributes the results to his center’s robust nurse navigation program, equal access among all patients – regardless of ability to pay – to standard treatments, and to the availability of clinical trial participation and stem cell transplantation.
“I think a key message is that if you are able to offer the same treatment and clinical trials to people irrespective of their race or socioeconomic status and can provide support, you can get equal outcomes as long as the biology is the same in both groups,” he said at a briefing prior to presentation of data in an oral abstract session at the annual meeting of the American Society of Hematology.
Dr. Ghosh pointed to four separate studies that showed that minority populations with DLBCL have worse outcomes than did whites, and noted that both uninsured and Medicaid-insured patients have also been shown to have poorer results, suggesting a role of socioeconomic factors in determining who gets optimum care and who does not.
The investigators compared PFS and OS among white and nonwhite patients with DLBCL treated in their institution, which has a safety-net cancer center. They also looked at the frequencies of clinical trial participation and stem cell transplantation between the groups.
The study included all patients with de novo DLBCL who presented to their center during January 2016–January 2019. They used patient-reported descriptors of race/ethnicity to create one of two cohorts: either self-identified whites (155 patients) or nonwhites (41), a group that included black patients, Hispanic patients, Asian Americans, and Native Americans.
The authors collected data on demographics, disease characteristics (including revised International Prognostic Index and double-hit status), insurance data, treatment, trial enrollment, progression, and death.
They found that nonwhites were significantly younger at diagnosis (median 56 vs. 64 years; P = .007), with an even distribution between the sexes in each group.
Two-thirds of both white and nonwhite patients had government insurance (Medicare or Medicaid). Of the remaining patients, 33% of white had private insurance, compared with 27% of nonwhites. No whites were uninsured, but 3 of the 41 nonwhites (7%) had no insurance.
Of the 155 white patients, 121 (86%) received nurse navigation services, as did 33 of 41 (81%) of nonwhites. The services include lodging assistance for homeless patients, transportation services for patients without cars, and care coordination among primary care physicians, oncologists, and other specialists. The services are part of the center’s standard practice, with excess costs, if any, folded into the budget, Dr. Ghosh said.
Looking at disease characteristics and treatment, the investigators found that risk profiles were similar between the groups. A higher percentage of whites had double-hit lymphoma (11% vs. 7%), but this difference was not statistically significant.
The investigators also found that in their program race was not a barrier to optimum therapy, with 96% of whites and 98% of nonwhites receiving frontline therapy with an anthracycline and rituximab-based regimen, and 4% and 2%, respectively received a non–anthracycline based regimen.
In each group, 39% of patients had disease that either relapsed or was refractory to frontline therapy.
In all, 11% of whites and 12% of nonwhites enrolled in clinical trials, 11% and 19%, respectively, underwent stem cell transplantation.
For patients with relapsed/refractory disease, the 2-year PFS rates were 60% for whites, and 63% for nonwhites, and the 2-year OS rates were 74% and 81%, respectively.
Dr. Ghosh and colleagues concluded that “our safety net cancer center, with extensive nurse navigator support and access to standard treatments, stem cell transplants, and cutting-edge clinical trials may abrogate the inferior outcomes in minority populations that have been previously reported.”
The study was internally funded. Dr. Ghosh reported consulting fees, research funding, speakers bureau activity, and/or honoraria from multiple companies.
SOURCE: Hu B et al. ASH 2019. Abstract 425.
ORLANDO – Patients with diffuse large B-cell lymphoma (DLBCL) who are members of an ethnic or racial minority do not have worse outcomes than whites when they receive appropriate treatment and institutional support, a study on disparities in cancer care shows.
Although previous studies have shown that minorities with DLBCL have worse outcomes than do whites, results of a study comparing outcomes from 155 patients of white heritage with those of 41 patients from black, Hispanic, or other minority backgrounds found no significant differences in either progression-free survival (PFS) or overall survival in 2 years over follow-up, reported Nilanjan Ghosh, MD, PhD, from the Levine Cancer Institute, Atrium Health, in Charlotte, N.C.
He attributes the results to his center’s robust nurse navigation program, equal access among all patients – regardless of ability to pay – to standard treatments, and to the availability of clinical trial participation and stem cell transplantation.
“I think a key message is that if you are able to offer the same treatment and clinical trials to people irrespective of their race or socioeconomic status and can provide support, you can get equal outcomes as long as the biology is the same in both groups,” he said at a briefing prior to presentation of data in an oral abstract session at the annual meeting of the American Society of Hematology.
Dr. Ghosh pointed to four separate studies that showed that minority populations with DLBCL have worse outcomes than did whites, and noted that both uninsured and Medicaid-insured patients have also been shown to have poorer results, suggesting a role of socioeconomic factors in determining who gets optimum care and who does not.
The investigators compared PFS and OS among white and nonwhite patients with DLBCL treated in their institution, which has a safety-net cancer center. They also looked at the frequencies of clinical trial participation and stem cell transplantation between the groups.
The study included all patients with de novo DLBCL who presented to their center during January 2016–January 2019. They used patient-reported descriptors of race/ethnicity to create one of two cohorts: either self-identified whites (155 patients) or nonwhites (41), a group that included black patients, Hispanic patients, Asian Americans, and Native Americans.
The authors collected data on demographics, disease characteristics (including revised International Prognostic Index and double-hit status), insurance data, treatment, trial enrollment, progression, and death.
They found that nonwhites were significantly younger at diagnosis (median 56 vs. 64 years; P = .007), with an even distribution between the sexes in each group.
Two-thirds of both white and nonwhite patients had government insurance (Medicare or Medicaid). Of the remaining patients, 33% of white had private insurance, compared with 27% of nonwhites. No whites were uninsured, but 3 of the 41 nonwhites (7%) had no insurance.
Of the 155 white patients, 121 (86%) received nurse navigation services, as did 33 of 41 (81%) of nonwhites. The services include lodging assistance for homeless patients, transportation services for patients without cars, and care coordination among primary care physicians, oncologists, and other specialists. The services are part of the center’s standard practice, with excess costs, if any, folded into the budget, Dr. Ghosh said.
Looking at disease characteristics and treatment, the investigators found that risk profiles were similar between the groups. A higher percentage of whites had double-hit lymphoma (11% vs. 7%), but this difference was not statistically significant.
The investigators also found that in their program race was not a barrier to optimum therapy, with 96% of whites and 98% of nonwhites receiving frontline therapy with an anthracycline and rituximab-based regimen, and 4% and 2%, respectively received a non–anthracycline based regimen.
In each group, 39% of patients had disease that either relapsed or was refractory to frontline therapy.
In all, 11% of whites and 12% of nonwhites enrolled in clinical trials, 11% and 19%, respectively, underwent stem cell transplantation.
For patients with relapsed/refractory disease, the 2-year PFS rates were 60% for whites, and 63% for nonwhites, and the 2-year OS rates were 74% and 81%, respectively.
Dr. Ghosh and colleagues concluded that “our safety net cancer center, with extensive nurse navigator support and access to standard treatments, stem cell transplants, and cutting-edge clinical trials may abrogate the inferior outcomes in minority populations that have been previously reported.”
The study was internally funded. Dr. Ghosh reported consulting fees, research funding, speakers bureau activity, and/or honoraria from multiple companies.
SOURCE: Hu B et al. ASH 2019. Abstract 425.
REPORTING FROM ASH 2019
Aspirin plus a DOAC may do more harm than good in some
ORLANDO – in a large registry-based cohort.
The study, which involved a cohort of 2,045 patients who were followed at 6 anticoagulation clinics in Michigan during January 2009–June 2019, also found no apparent improvement in thrombosis incidence with the addition of aspirin, Jordan K. Schaefer, MD, reported during a press briefing at the annual meeting of the American Society of Hematology.
Of the cohort patients, 639 adults who received a DOAC plus aspirin after VTE or for NVAF without a clear indication were compared with 639 propensity-matched controls. The bleeding event rate per 100 patient years was 39.50 vs. 32.32 at an average of 15.2 months of follow-up in the combination therapy and DOAC monotherapy groups, respectively, said Dr. Schaefer of the division of hematology/oncology, department of internal medicine, University of Michigan, Ann Arbor.
“This result was statistically significant for clinically relevant non-major bleeding, with an 18.7 rate per 100 patient years, compared with 13.5 for DOAC monotherapy,” (P = .02), he said. “We also saw a significant increase in non-major bleeding with combination therapy, compared with direct oral anticoagulant monotherapy” (rate, 32.82 vs. 25.88; P =.04).
No significant difference was seen overall (P =.07) or for other specific types of bleeding, he noted.
The observed rates of thrombosis in the groups, respectively, were 2.35 and 2.23 per 100 patient years (P =.95), he said, noting that patients on combination therapy also had more emergency department visits and hospitalizations, but those differences were not statistically significant.
“Direct-acting oral anticoagulants, which include apixaban, dabigatran, edoxaban, and rivaroxaban, are increasingly used in clinical practice for indications that include the prevention of strokes for patients with nonvalvular atrial fibrillation, and the treatment and secondary prevention of venous thromboembolic disease,” Dr. Schaefer said.
Aspirin is commonly used in clinical practice for various indications, including primary prevention of heart attacks, strokes, and colorectal cancer, as well as for thromboprophylaxis in patients with certain blood disorders or with certain cardiac devices, he added.
“Aspirin is used for the secondary prevention of thrombosis for patients with known coronary artery disease, peripheral artery disease, or carotid artery disease,” he said. “And while adding aspirin to a DOAC is often appropriate after acute coronary syndromes or percutaneous coronary intervention, many patients receive the combination therapy without a clear indication, he said, noting that increasing evidence in recent years, largely from patients treated with warfarin and aspirin, suggest that the approach may do more harm than good for certain patients.
Specifically, there’s a question of whether aspirin is increasing the rates of bleeding without protecting patients from adverse thrombotic outcomes.
“This has specifically been a concern for patients who are on full-dose anticoagulation,” he said.
In the current study, patient demographics, comorbidities, and concurrent medications were well balanced in the treatment and control groups after propensity score matching, he said, noting that patients with a history of heart valve replacement, recent MI, or less than 3 months of follow-up were excluded.
“These findings need to be confirmed in larger studies, but until such data [are] available, clinicians and patients should continue to balance the relative risks and benefits of adding aspirin to their direct oral anticoagulant therapy,” Dr. Schaefer said. “Further research needs to evaluate key subgroups to see if any particular population may benefit from combination therapy compared to DOAC therapy alone.”
Dr. Schaefer reported having no disclosures.
SOURCE: Schaeffer J et al. ASH 2019. Abstract 787.
ORLANDO – in a large registry-based cohort.
The study, which involved a cohort of 2,045 patients who were followed at 6 anticoagulation clinics in Michigan during January 2009–June 2019, also found no apparent improvement in thrombosis incidence with the addition of aspirin, Jordan K. Schaefer, MD, reported during a press briefing at the annual meeting of the American Society of Hematology.
Of the cohort patients, 639 adults who received a DOAC plus aspirin after VTE or for NVAF without a clear indication were compared with 639 propensity-matched controls. The bleeding event rate per 100 patient years was 39.50 vs. 32.32 at an average of 15.2 months of follow-up in the combination therapy and DOAC monotherapy groups, respectively, said Dr. Schaefer of the division of hematology/oncology, department of internal medicine, University of Michigan, Ann Arbor.
“This result was statistically significant for clinically relevant non-major bleeding, with an 18.7 rate per 100 patient years, compared with 13.5 for DOAC monotherapy,” (P = .02), he said. “We also saw a significant increase in non-major bleeding with combination therapy, compared with direct oral anticoagulant monotherapy” (rate, 32.82 vs. 25.88; P =.04).
No significant difference was seen overall (P =.07) or for other specific types of bleeding, he noted.
The observed rates of thrombosis in the groups, respectively, were 2.35 and 2.23 per 100 patient years (P =.95), he said, noting that patients on combination therapy also had more emergency department visits and hospitalizations, but those differences were not statistically significant.
“Direct-acting oral anticoagulants, which include apixaban, dabigatran, edoxaban, and rivaroxaban, are increasingly used in clinical practice for indications that include the prevention of strokes for patients with nonvalvular atrial fibrillation, and the treatment and secondary prevention of venous thromboembolic disease,” Dr. Schaefer said.
Aspirin is commonly used in clinical practice for various indications, including primary prevention of heart attacks, strokes, and colorectal cancer, as well as for thromboprophylaxis in patients with certain blood disorders or with certain cardiac devices, he added.
“Aspirin is used for the secondary prevention of thrombosis for patients with known coronary artery disease, peripheral artery disease, or carotid artery disease,” he said. “And while adding aspirin to a DOAC is often appropriate after acute coronary syndromes or percutaneous coronary intervention, many patients receive the combination therapy without a clear indication, he said, noting that increasing evidence in recent years, largely from patients treated with warfarin and aspirin, suggest that the approach may do more harm than good for certain patients.
Specifically, there’s a question of whether aspirin is increasing the rates of bleeding without protecting patients from adverse thrombotic outcomes.
“This has specifically been a concern for patients who are on full-dose anticoagulation,” he said.
In the current study, patient demographics, comorbidities, and concurrent medications were well balanced in the treatment and control groups after propensity score matching, he said, noting that patients with a history of heart valve replacement, recent MI, or less than 3 months of follow-up were excluded.
“These findings need to be confirmed in larger studies, but until such data [are] available, clinicians and patients should continue to balance the relative risks and benefits of adding aspirin to their direct oral anticoagulant therapy,” Dr. Schaefer said. “Further research needs to evaluate key subgroups to see if any particular population may benefit from combination therapy compared to DOAC therapy alone.”
Dr. Schaefer reported having no disclosures.
SOURCE: Schaeffer J et al. ASH 2019. Abstract 787.
ORLANDO – in a large registry-based cohort.
The study, which involved a cohort of 2,045 patients who were followed at 6 anticoagulation clinics in Michigan during January 2009–June 2019, also found no apparent improvement in thrombosis incidence with the addition of aspirin, Jordan K. Schaefer, MD, reported during a press briefing at the annual meeting of the American Society of Hematology.
Of the cohort patients, 639 adults who received a DOAC plus aspirin after VTE or for NVAF without a clear indication were compared with 639 propensity-matched controls. The bleeding event rate per 100 patient years was 39.50 vs. 32.32 at an average of 15.2 months of follow-up in the combination therapy and DOAC monotherapy groups, respectively, said Dr. Schaefer of the division of hematology/oncology, department of internal medicine, University of Michigan, Ann Arbor.
“This result was statistically significant for clinically relevant non-major bleeding, with an 18.7 rate per 100 patient years, compared with 13.5 for DOAC monotherapy,” (P = .02), he said. “We also saw a significant increase in non-major bleeding with combination therapy, compared with direct oral anticoagulant monotherapy” (rate, 32.82 vs. 25.88; P =.04).
No significant difference was seen overall (P =.07) or for other specific types of bleeding, he noted.
The observed rates of thrombosis in the groups, respectively, were 2.35 and 2.23 per 100 patient years (P =.95), he said, noting that patients on combination therapy also had more emergency department visits and hospitalizations, but those differences were not statistically significant.
“Direct-acting oral anticoagulants, which include apixaban, dabigatran, edoxaban, and rivaroxaban, are increasingly used in clinical practice for indications that include the prevention of strokes for patients with nonvalvular atrial fibrillation, and the treatment and secondary prevention of venous thromboembolic disease,” Dr. Schaefer said.
Aspirin is commonly used in clinical practice for various indications, including primary prevention of heart attacks, strokes, and colorectal cancer, as well as for thromboprophylaxis in patients with certain blood disorders or with certain cardiac devices, he added.
“Aspirin is used for the secondary prevention of thrombosis for patients with known coronary artery disease, peripheral artery disease, or carotid artery disease,” he said. “And while adding aspirin to a DOAC is often appropriate after acute coronary syndromes or percutaneous coronary intervention, many patients receive the combination therapy without a clear indication, he said, noting that increasing evidence in recent years, largely from patients treated with warfarin and aspirin, suggest that the approach may do more harm than good for certain patients.
Specifically, there’s a question of whether aspirin is increasing the rates of bleeding without protecting patients from adverse thrombotic outcomes.
“This has specifically been a concern for patients who are on full-dose anticoagulation,” he said.
In the current study, patient demographics, comorbidities, and concurrent medications were well balanced in the treatment and control groups after propensity score matching, he said, noting that patients with a history of heart valve replacement, recent MI, or less than 3 months of follow-up were excluded.
“These findings need to be confirmed in larger studies, but until such data [are] available, clinicians and patients should continue to balance the relative risks and benefits of adding aspirin to their direct oral anticoagulant therapy,” Dr. Schaefer said. “Further research needs to evaluate key subgroups to see if any particular population may benefit from combination therapy compared to DOAC therapy alone.”
Dr. Schaefer reported having no disclosures.
SOURCE: Schaeffer J et al. ASH 2019. Abstract 787.
REPORTING FROM ASH 2019
Off-the-shelf cellular therapy shows promise in the lab
ORLANDO – A cellular therapy called FT596 is active against B-cell malignancies and, when combined with rituximab, can be more effective than traditional chimeric antigen receptor (CAR) T cells, preclinical research findings suggest.
FT596 is a universal, anti-CD19 CAR natural killer (NK) cell therapy derived from a master induced pluripotent stem cell (iPSC) line.
FT596 reduced tumor growth in mouse models of leukemia and lymphoma. When combined with rituximab, FT596 was able to overcome CD19 antigen escape.
Jode P. Goodridge, PhD, of Fate Therapeutics in San Diego, presented these results at the annual meeting of the American Society of Hematology.
Dr. Goodridge explained that FT596 begins with a source material, such as a fibroblast, that is reprogrammed into an iPSC progenitor cell. That cell is sorted and expanded into a renewable, homogeneous, pluripotent master iPSC line. The iPSCs are differentiated into CD34 cells, which are differentiated into NK cells. The iPSC-derived NK cells are then modified with the following:
- An anti-CD19 CAR that is optimized for NK-cell biology and contains an NKG2D transmembrane domain, a 2B4 costimulatory domain, and a CD3-zeta signaling domain.
- An interleukin-15 receptor fusion that promotes cell survival and reduces the need for cytokine support.
- A high-affinity 158V, noncleavable CD16 Fc receptor that enhances antibody-dependent cellular cytotoxicity when FT596 is combined with a monoclonal antibody such as rituximab.
Dr. Goodridge presented results with FT596, both alone and in combination with rituximab, in vitro and in vivo.
When compared with no treatment, three doses of FT596 monotherapy reduced tumor growth in a mouse model of leukemia (Nalm6). FT596 plus rituximab reduced tumor growth in a mouse model of lymphoma (Raji), when compared with no treatment or rituximab alone.
Three doses of FT596 proved more effective than a single dose of CD19 CAR T-cell therapy in a mouse model of lymphoma (Raji). FT596 both reduced tumor growth and prolonged survival in the mice.
Lastly, in vitro experiments in Raji cells showed that FT596 plus rituximab can produce deeper responses than primary CAR-T cells, and the combination can prevent antigen escape.
Dr. Goodridge said these results support the phase 1 study of FT596, given as monotherapy or in combination with rituximab or obinutuzumab, in patients with relapsed/refractory B-cell lymphomas or chronic lymphocytic leukemia.
Dr. Goodridge is employed by Fate Therapeutics, the company developing FT596.
SOURCE: Goodridge JP et al. ASH 2019. Abstract 301.
ORLANDO – A cellular therapy called FT596 is active against B-cell malignancies and, when combined with rituximab, can be more effective than traditional chimeric antigen receptor (CAR) T cells, preclinical research findings suggest.
FT596 is a universal, anti-CD19 CAR natural killer (NK) cell therapy derived from a master induced pluripotent stem cell (iPSC) line.
FT596 reduced tumor growth in mouse models of leukemia and lymphoma. When combined with rituximab, FT596 was able to overcome CD19 antigen escape.
Jode P. Goodridge, PhD, of Fate Therapeutics in San Diego, presented these results at the annual meeting of the American Society of Hematology.
Dr. Goodridge explained that FT596 begins with a source material, such as a fibroblast, that is reprogrammed into an iPSC progenitor cell. That cell is sorted and expanded into a renewable, homogeneous, pluripotent master iPSC line. The iPSCs are differentiated into CD34 cells, which are differentiated into NK cells. The iPSC-derived NK cells are then modified with the following:
- An anti-CD19 CAR that is optimized for NK-cell biology and contains an NKG2D transmembrane domain, a 2B4 costimulatory domain, and a CD3-zeta signaling domain.
- An interleukin-15 receptor fusion that promotes cell survival and reduces the need for cytokine support.
- A high-affinity 158V, noncleavable CD16 Fc receptor that enhances antibody-dependent cellular cytotoxicity when FT596 is combined with a monoclonal antibody such as rituximab.
Dr. Goodridge presented results with FT596, both alone and in combination with rituximab, in vitro and in vivo.
When compared with no treatment, three doses of FT596 monotherapy reduced tumor growth in a mouse model of leukemia (Nalm6). FT596 plus rituximab reduced tumor growth in a mouse model of lymphoma (Raji), when compared with no treatment or rituximab alone.
Three doses of FT596 proved more effective than a single dose of CD19 CAR T-cell therapy in a mouse model of lymphoma (Raji). FT596 both reduced tumor growth and prolonged survival in the mice.
Lastly, in vitro experiments in Raji cells showed that FT596 plus rituximab can produce deeper responses than primary CAR-T cells, and the combination can prevent antigen escape.
Dr. Goodridge said these results support the phase 1 study of FT596, given as monotherapy or in combination with rituximab or obinutuzumab, in patients with relapsed/refractory B-cell lymphomas or chronic lymphocytic leukemia.
Dr. Goodridge is employed by Fate Therapeutics, the company developing FT596.
SOURCE: Goodridge JP et al. ASH 2019. Abstract 301.
ORLANDO – A cellular therapy called FT596 is active against B-cell malignancies and, when combined with rituximab, can be more effective than traditional chimeric antigen receptor (CAR) T cells, preclinical research findings suggest.
FT596 is a universal, anti-CD19 CAR natural killer (NK) cell therapy derived from a master induced pluripotent stem cell (iPSC) line.
FT596 reduced tumor growth in mouse models of leukemia and lymphoma. When combined with rituximab, FT596 was able to overcome CD19 antigen escape.
Jode P. Goodridge, PhD, of Fate Therapeutics in San Diego, presented these results at the annual meeting of the American Society of Hematology.
Dr. Goodridge explained that FT596 begins with a source material, such as a fibroblast, that is reprogrammed into an iPSC progenitor cell. That cell is sorted and expanded into a renewable, homogeneous, pluripotent master iPSC line. The iPSCs are differentiated into CD34 cells, which are differentiated into NK cells. The iPSC-derived NK cells are then modified with the following:
- An anti-CD19 CAR that is optimized for NK-cell biology and contains an NKG2D transmembrane domain, a 2B4 costimulatory domain, and a CD3-zeta signaling domain.
- An interleukin-15 receptor fusion that promotes cell survival and reduces the need for cytokine support.
- A high-affinity 158V, noncleavable CD16 Fc receptor that enhances antibody-dependent cellular cytotoxicity when FT596 is combined with a monoclonal antibody such as rituximab.
Dr. Goodridge presented results with FT596, both alone and in combination with rituximab, in vitro and in vivo.
When compared with no treatment, three doses of FT596 monotherapy reduced tumor growth in a mouse model of leukemia (Nalm6). FT596 plus rituximab reduced tumor growth in a mouse model of lymphoma (Raji), when compared with no treatment or rituximab alone.
Three doses of FT596 proved more effective than a single dose of CD19 CAR T-cell therapy in a mouse model of lymphoma (Raji). FT596 both reduced tumor growth and prolonged survival in the mice.
Lastly, in vitro experiments in Raji cells showed that FT596 plus rituximab can produce deeper responses than primary CAR-T cells, and the combination can prevent antigen escape.
Dr. Goodridge said these results support the phase 1 study of FT596, given as monotherapy or in combination with rituximab or obinutuzumab, in patients with relapsed/refractory B-cell lymphomas or chronic lymphocytic leukemia.
Dr. Goodridge is employed by Fate Therapeutics, the company developing FT596.
SOURCE: Goodridge JP et al. ASH 2019. Abstract 301.
REPORTING FROM ASH 2019