User login
No clear-cut evidence of vedolizumab effect in retrospective study of primary sclerosing cholangitis
While initial case reports and series provided preliminarily encouraging results, a larger retrospective study has provided no clear-cut evidence of biochemical response to vedolizumab in patients with primary sclerosing cholangitis (PSC) and inflammatory bowel disease, investigators report.
A subset of patients in the retrospective analysis did experience a substantial drop in alkaline phosphatase (ALP), according to investigators with the International Primary Sclerosing Cholangitis Study Group.
Overall, however, levels of that cholestasis marker rose by a small but statistically significant amount in this study, which included more than 100 patients with PSC and inflammatory bowel disease (IBD).
Responses were more likely in patients with cirrhosis and in those with elevated ALP at baseline, both of which are indicators of more aggressive disease, according to investigator Kate D. Lynch, MD, PhD, of the University of Oxford (England) and her coauthors.
The rate of liver outcomes was in line with the natural history of the disease, according to Dr. Lynch and coinvestigators, who added that most patients had an endoscopic IBD response, as might be expected based on studies of IBD-only patients treated with vedolizumab.
“Despite the disappointment with lack of a uniform response, further evaluation of vedolizumab as a beneficial treatment in PSC may be warranted in a subset of patients via a stratified randomized clinical trial,” Dr. Lynch and coauthors said in their report, which was published in Clinical Gastroenterology and Hepatology.
Vedolizumab, a monoclonal antibody against integrin alpha4beta7, is effective in Crohn’s disease and ulcerative colitis, according to investigators, who added that the “gut-homing pathway” it targets has also been implicated in the pathophysiology of PSC.
“It is possible that vedolizumab may play a role in reducing lymphocyte infiltration into the liver in patients with PSC and thereby in reducing hepatic and biliary inflammation, authors of the retrospective analysis said.
Their analysis included 102 patients with PSC and IBD at 20 centers in Europe and North America. All patients had received at least three doses of vedolizumab for their IBD, given according to the usual dosing schedule. Most of the patients were male (64 patients, or 62.8%) and about 90% had classical large-duct PSC. About one-fifth had cirrhosis, and the majority (about 65%) had ulcerative colitis. Patients were followed until death, liver transplant, or 56 days after the last vedolizumab dose.
The median ALP increased from 1.53 times the upper limit of normal at baseline to 1.64 times the upper limit of normal by the last follow-up, an increase that was statistically significant (P = .018) but not clinically significant, according to investigators. Likewise, they said, statistically significant increases were seen overall in median alanine transaminase and aspartate aminotransferase levels.
However, 21 patients (20.6%) had a drop in ALP of at least 20% from baseline to last follow-up, and another 39 patients (38.2%) had stable ALP over that period, data show, while the remaining 42 (41.2%) had an increase of 20% or more.
Cirrhosis was associated with a near fivefold odds of a 20% or greater ALP drop from baseline to follow-up (odds ratio, 4.70, 95% confidence interval, 1.61-13.76), according to results of univariate analysis, which investigators said were “reproduced” in multivariate analysis.
While no other variables were so clearly linked to a 20% or greater drop in ALP, Dr. Lynch and colleagues said there was a “trend toward an association” in patients with ALP raised at baseline, and in those who had Crohn’s disease or IBD-unspecified instead of ulcerative colitis.
Endoscopic IBD responses were seen in 42 out of 74 patients (56.8%) for whom those data were available, investigators added.
A total of 22 patients (20.9%) had a liver-related outcome over median follow-up of 561 days; however, that outcome may be “slightly overrepresented” by an incidence of cholangitis in 8.8%, which in and of itself is not necessarily an indicator of advanced liver disease, said Dr. Lynch and coauthors in their report.
“This proportion of liver-related outcomes is consistent with the natural history of PSC and does not by itself indicate that vedolizumab treatment is harmful in PSC,” they said, adding that the findings were similar to a study of simtuzumab, a monoclonal antibody directed against lysyl oxidase-like 2, in patients with PSC, of whom 20.1% had a PSC-related event and the incidence of cholangitis was 13.2%.
The retrospective study was supported by the Birmingham National Institute for Health Research (NIHR) Biomedical Research Centre in the United Kingdom. Authors of the report provided disclosures related to Takeda, AbbVie, Dr. Falk Pharma, Intercept, MSD, Janssen, Vifor, Gilead, and Novartis, among others.
SOURCE: Lynch KD et al. Clin Gastroenterol Hepatol. 2019 May 14. doi: 10.1016/j.cgh.2019.05.013.
While initial case reports and series provided preliminarily encouraging results, a larger retrospective study has provided no clear-cut evidence of biochemical response to vedolizumab in patients with primary sclerosing cholangitis (PSC) and inflammatory bowel disease, investigators report.
A subset of patients in the retrospective analysis did experience a substantial drop in alkaline phosphatase (ALP), according to investigators with the International Primary Sclerosing Cholangitis Study Group.
Overall, however, levels of that cholestasis marker rose by a small but statistically significant amount in this study, which included more than 100 patients with PSC and inflammatory bowel disease (IBD).
Responses were more likely in patients with cirrhosis and in those with elevated ALP at baseline, both of which are indicators of more aggressive disease, according to investigator Kate D. Lynch, MD, PhD, of the University of Oxford (England) and her coauthors.
The rate of liver outcomes was in line with the natural history of the disease, according to Dr. Lynch and coinvestigators, who added that most patients had an endoscopic IBD response, as might be expected based on studies of IBD-only patients treated with vedolizumab.
“Despite the disappointment with lack of a uniform response, further evaluation of vedolizumab as a beneficial treatment in PSC may be warranted in a subset of patients via a stratified randomized clinical trial,” Dr. Lynch and coauthors said in their report, which was published in Clinical Gastroenterology and Hepatology.
Vedolizumab, a monoclonal antibody against integrin alpha4beta7, is effective in Crohn’s disease and ulcerative colitis, according to investigators, who added that the “gut-homing pathway” it targets has also been implicated in the pathophysiology of PSC.
“It is possible that vedolizumab may play a role in reducing lymphocyte infiltration into the liver in patients with PSC and thereby in reducing hepatic and biliary inflammation, authors of the retrospective analysis said.
Their analysis included 102 patients with PSC and IBD at 20 centers in Europe and North America. All patients had received at least three doses of vedolizumab for their IBD, given according to the usual dosing schedule. Most of the patients were male (64 patients, or 62.8%) and about 90% had classical large-duct PSC. About one-fifth had cirrhosis, and the majority (about 65%) had ulcerative colitis. Patients were followed until death, liver transplant, or 56 days after the last vedolizumab dose.
The median ALP increased from 1.53 times the upper limit of normal at baseline to 1.64 times the upper limit of normal by the last follow-up, an increase that was statistically significant (P = .018) but not clinically significant, according to investigators. Likewise, they said, statistically significant increases were seen overall in median alanine transaminase and aspartate aminotransferase levels.
However, 21 patients (20.6%) had a drop in ALP of at least 20% from baseline to last follow-up, and another 39 patients (38.2%) had stable ALP over that period, data show, while the remaining 42 (41.2%) had an increase of 20% or more.
Cirrhosis was associated with a near fivefold odds of a 20% or greater ALP drop from baseline to follow-up (odds ratio, 4.70, 95% confidence interval, 1.61-13.76), according to results of univariate analysis, which investigators said were “reproduced” in multivariate analysis.
While no other variables were so clearly linked to a 20% or greater drop in ALP, Dr. Lynch and colleagues said there was a “trend toward an association” in patients with ALP raised at baseline, and in those who had Crohn’s disease or IBD-unspecified instead of ulcerative colitis.
Endoscopic IBD responses were seen in 42 out of 74 patients (56.8%) for whom those data were available, investigators added.
A total of 22 patients (20.9%) had a liver-related outcome over median follow-up of 561 days; however, that outcome may be “slightly overrepresented” by an incidence of cholangitis in 8.8%, which in and of itself is not necessarily an indicator of advanced liver disease, said Dr. Lynch and coauthors in their report.
“This proportion of liver-related outcomes is consistent with the natural history of PSC and does not by itself indicate that vedolizumab treatment is harmful in PSC,” they said, adding that the findings were similar to a study of simtuzumab, a monoclonal antibody directed against lysyl oxidase-like 2, in patients with PSC, of whom 20.1% had a PSC-related event and the incidence of cholangitis was 13.2%.
The retrospective study was supported by the Birmingham National Institute for Health Research (NIHR) Biomedical Research Centre in the United Kingdom. Authors of the report provided disclosures related to Takeda, AbbVie, Dr. Falk Pharma, Intercept, MSD, Janssen, Vifor, Gilead, and Novartis, among others.
SOURCE: Lynch KD et al. Clin Gastroenterol Hepatol. 2019 May 14. doi: 10.1016/j.cgh.2019.05.013.
While initial case reports and series provided preliminarily encouraging results, a larger retrospective study has provided no clear-cut evidence of biochemical response to vedolizumab in patients with primary sclerosing cholangitis (PSC) and inflammatory bowel disease, investigators report.
A subset of patients in the retrospective analysis did experience a substantial drop in alkaline phosphatase (ALP), according to investigators with the International Primary Sclerosing Cholangitis Study Group.
Overall, however, levels of that cholestasis marker rose by a small but statistically significant amount in this study, which included more than 100 patients with PSC and inflammatory bowel disease (IBD).
Responses were more likely in patients with cirrhosis and in those with elevated ALP at baseline, both of which are indicators of more aggressive disease, according to investigator Kate D. Lynch, MD, PhD, of the University of Oxford (England) and her coauthors.
The rate of liver outcomes was in line with the natural history of the disease, according to Dr. Lynch and coinvestigators, who added that most patients had an endoscopic IBD response, as might be expected based on studies of IBD-only patients treated with vedolizumab.
“Despite the disappointment with lack of a uniform response, further evaluation of vedolizumab as a beneficial treatment in PSC may be warranted in a subset of patients via a stratified randomized clinical trial,” Dr. Lynch and coauthors said in their report, which was published in Clinical Gastroenterology and Hepatology.
Vedolizumab, a monoclonal antibody against integrin alpha4beta7, is effective in Crohn’s disease and ulcerative colitis, according to investigators, who added that the “gut-homing pathway” it targets has also been implicated in the pathophysiology of PSC.
“It is possible that vedolizumab may play a role in reducing lymphocyte infiltration into the liver in patients with PSC and thereby in reducing hepatic and biliary inflammation, authors of the retrospective analysis said.
Their analysis included 102 patients with PSC and IBD at 20 centers in Europe and North America. All patients had received at least three doses of vedolizumab for their IBD, given according to the usual dosing schedule. Most of the patients were male (64 patients, or 62.8%) and about 90% had classical large-duct PSC. About one-fifth had cirrhosis, and the majority (about 65%) had ulcerative colitis. Patients were followed until death, liver transplant, or 56 days after the last vedolizumab dose.
The median ALP increased from 1.53 times the upper limit of normal at baseline to 1.64 times the upper limit of normal by the last follow-up, an increase that was statistically significant (P = .018) but not clinically significant, according to investigators. Likewise, they said, statistically significant increases were seen overall in median alanine transaminase and aspartate aminotransferase levels.
However, 21 patients (20.6%) had a drop in ALP of at least 20% from baseline to last follow-up, and another 39 patients (38.2%) had stable ALP over that period, data show, while the remaining 42 (41.2%) had an increase of 20% or more.
Cirrhosis was associated with a near fivefold odds of a 20% or greater ALP drop from baseline to follow-up (odds ratio, 4.70, 95% confidence interval, 1.61-13.76), according to results of univariate analysis, which investigators said were “reproduced” in multivariate analysis.
While no other variables were so clearly linked to a 20% or greater drop in ALP, Dr. Lynch and colleagues said there was a “trend toward an association” in patients with ALP raised at baseline, and in those who had Crohn’s disease or IBD-unspecified instead of ulcerative colitis.
Endoscopic IBD responses were seen in 42 out of 74 patients (56.8%) for whom those data were available, investigators added.
A total of 22 patients (20.9%) had a liver-related outcome over median follow-up of 561 days; however, that outcome may be “slightly overrepresented” by an incidence of cholangitis in 8.8%, which in and of itself is not necessarily an indicator of advanced liver disease, said Dr. Lynch and coauthors in their report.
“This proportion of liver-related outcomes is consistent with the natural history of PSC and does not by itself indicate that vedolizumab treatment is harmful in PSC,” they said, adding that the findings were similar to a study of simtuzumab, a monoclonal antibody directed against lysyl oxidase-like 2, in patients with PSC, of whom 20.1% had a PSC-related event and the incidence of cholangitis was 13.2%.
The retrospective study was supported by the Birmingham National Institute for Health Research (NIHR) Biomedical Research Centre in the United Kingdom. Authors of the report provided disclosures related to Takeda, AbbVie, Dr. Falk Pharma, Intercept, MSD, Janssen, Vifor, Gilead, and Novartis, among others.
SOURCE: Lynch KD et al. Clin Gastroenterol Hepatol. 2019 May 14. doi: 10.1016/j.cgh.2019.05.013.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Claims data improves cancer registry information on treatment
Linking cancer registry data to health insurance claims databases could significantly improve the capture of cancer treatment data, investigators report.
“As the number of new chemotherapy agents and targeted drugs approved for cancer treatment increases, estimating the population-based survival differences related to these treatments is increasingly important,” wrote Mia Hashibe, PhD, from the Utah Cancer Registry, and coauthors in JCO Cancer Clinical Informatics.
In this study, researchers identified 13,533 reportable cancer diagnoses in the Utah Cancer Registry between January 2013 and June 2014, of which 10,759 (79.1%) had health claims data in the Utah all-payer claims database.
Among these 10,759 patients, 24.1% had identifiable claims for chemotherapy. By linking the registry with the health claims database, researchers were able to identify an additional 497 patients in the registry who received chemotherapy, 590 treated with hormone therapy, 326 treated with radiation therapy, and 1,190 treated with immunotherapy.
The addition of the health claims data increased the proportion of patients treated with chemotherapy to 27.6%, the proportion of patients treated hormone therapy increased from 14.1% to 18.8%, immunotherapy increased from 4.3% to 13.2%, and radiation therapy increased from 24.9% to 27.5%.
The health claims data was particularly comprehensive when it came to information about hormone therapy for breast cancer, chemotherapy for lung or colorectal cancer, radiation therapy in patients with lung or prostate cancer, and biologic therapy in patients with melanoma.
The health claims data was also able to provide more information about the chemotherapy agents used and the duration of treatment, the authors reported.
“Even after augmenting with APCD [all-payer claims data], there was an indication of under-reporting of chemotherapy for breast cancer by the cancer registry variable compared with abstraction,” the authors wrote. “A factor contributing to cancers that were coded as treated through the augmented cancer registry variable, but not from abstraction, was when the therapy was determined by the abstractor to not be the first course.”
However, the cancer registry did have some data about patient therapy that was not found in the health claims database.
The authors noted that this was a pilot study to see whether claims data could improve information on cancer treatment and link that data to information in cancer registries.
“Next steps will include routine linkage for additional years of diagnosis and incorporation of APCD information into registry treatment variables.”
The study was supported by the National Cancer Institute, the Centers for Disease Control and Prevention, the Huntsman Cancer Foundation, and the University of Utah, Salt Lake City. One author declared institutional funding from a pharmaceutical company. No other conflicts of interest were declared.
SOURCE: Hashibe M et al. JCO Clin Cancer Inform. 2019 Oct. doi: 10.1200/CCI.19.00027.
Linking cancer registry data to health insurance claims databases could significantly improve the capture of cancer treatment data, investigators report.
“As the number of new chemotherapy agents and targeted drugs approved for cancer treatment increases, estimating the population-based survival differences related to these treatments is increasingly important,” wrote Mia Hashibe, PhD, from the Utah Cancer Registry, and coauthors in JCO Cancer Clinical Informatics.
In this study, researchers identified 13,533 reportable cancer diagnoses in the Utah Cancer Registry between January 2013 and June 2014, of which 10,759 (79.1%) had health claims data in the Utah all-payer claims database.
Among these 10,759 patients, 24.1% had identifiable claims for chemotherapy. By linking the registry with the health claims database, researchers were able to identify an additional 497 patients in the registry who received chemotherapy, 590 treated with hormone therapy, 326 treated with radiation therapy, and 1,190 treated with immunotherapy.
The addition of the health claims data increased the proportion of patients treated with chemotherapy to 27.6%, the proportion of patients treated hormone therapy increased from 14.1% to 18.8%, immunotherapy increased from 4.3% to 13.2%, and radiation therapy increased from 24.9% to 27.5%.
The health claims data was particularly comprehensive when it came to information about hormone therapy for breast cancer, chemotherapy for lung or colorectal cancer, radiation therapy in patients with lung or prostate cancer, and biologic therapy in patients with melanoma.
The health claims data was also able to provide more information about the chemotherapy agents used and the duration of treatment, the authors reported.
“Even after augmenting with APCD [all-payer claims data], there was an indication of under-reporting of chemotherapy for breast cancer by the cancer registry variable compared with abstraction,” the authors wrote. “A factor contributing to cancers that were coded as treated through the augmented cancer registry variable, but not from abstraction, was when the therapy was determined by the abstractor to not be the first course.”
However, the cancer registry did have some data about patient therapy that was not found in the health claims database.
The authors noted that this was a pilot study to see whether claims data could improve information on cancer treatment and link that data to information in cancer registries.
“Next steps will include routine linkage for additional years of diagnosis and incorporation of APCD information into registry treatment variables.”
The study was supported by the National Cancer Institute, the Centers for Disease Control and Prevention, the Huntsman Cancer Foundation, and the University of Utah, Salt Lake City. One author declared institutional funding from a pharmaceutical company. No other conflicts of interest were declared.
SOURCE: Hashibe M et al. JCO Clin Cancer Inform. 2019 Oct. doi: 10.1200/CCI.19.00027.
Linking cancer registry data to health insurance claims databases could significantly improve the capture of cancer treatment data, investigators report.
“As the number of new chemotherapy agents and targeted drugs approved for cancer treatment increases, estimating the population-based survival differences related to these treatments is increasingly important,” wrote Mia Hashibe, PhD, from the Utah Cancer Registry, and coauthors in JCO Cancer Clinical Informatics.
In this study, researchers identified 13,533 reportable cancer diagnoses in the Utah Cancer Registry between January 2013 and June 2014, of which 10,759 (79.1%) had health claims data in the Utah all-payer claims database.
Among these 10,759 patients, 24.1% had identifiable claims for chemotherapy. By linking the registry with the health claims database, researchers were able to identify an additional 497 patients in the registry who received chemotherapy, 590 treated with hormone therapy, 326 treated with radiation therapy, and 1,190 treated with immunotherapy.
The addition of the health claims data increased the proportion of patients treated with chemotherapy to 27.6%, the proportion of patients treated hormone therapy increased from 14.1% to 18.8%, immunotherapy increased from 4.3% to 13.2%, and radiation therapy increased from 24.9% to 27.5%.
The health claims data was particularly comprehensive when it came to information about hormone therapy for breast cancer, chemotherapy for lung or colorectal cancer, radiation therapy in patients with lung or prostate cancer, and biologic therapy in patients with melanoma.
The health claims data was also able to provide more information about the chemotherapy agents used and the duration of treatment, the authors reported.
“Even after augmenting with APCD [all-payer claims data], there was an indication of under-reporting of chemotherapy for breast cancer by the cancer registry variable compared with abstraction,” the authors wrote. “A factor contributing to cancers that were coded as treated through the augmented cancer registry variable, but not from abstraction, was when the therapy was determined by the abstractor to not be the first course.”
However, the cancer registry did have some data about patient therapy that was not found in the health claims database.
The authors noted that this was a pilot study to see whether claims data could improve information on cancer treatment and link that data to information in cancer registries.
“Next steps will include routine linkage for additional years of diagnosis and incorporation of APCD information into registry treatment variables.”
The study was supported by the National Cancer Institute, the Centers for Disease Control and Prevention, the Huntsman Cancer Foundation, and the University of Utah, Salt Lake City. One author declared institutional funding from a pharmaceutical company. No other conflicts of interest were declared.
SOURCE: Hashibe M et al. JCO Clin Cancer Inform. 2019 Oct. doi: 10.1200/CCI.19.00027.
FROM JCO CLINICAL CANCER INFORMATICS
Evidence grows for early axSpA treatment, uveitis flare prevention
The findings from the C-axSpAnd study that Jonathan Kay, MD, and colleagues reported at the annual meeting of the American College of Rheumatology are not surprising. Earlier studies in patients with ankylosing spondylitis showed that short symptom duration is one of the best predictors of good treatment response to TNFi therapy. The highest response rates were obtained in studies conducted in axSpA patients with symptom duration of less than 5 years or even less than 3 years. Since nonradiographic axial spondyloarthritis (nr-axSpA) and r-axSpA are considered as two stages of one disease, it is logical that the same effect is also observed in studies in nr-axSpA. Indeed, in the first study of a tumor necrosis factor inhibitor (TNFi) in nr-axSpA (ABILITY-1), patients with symptom duration less than 5 years responded much better to the TNFi adalimumab than did those with longer symptom duration, and the delta of the response between adalimumab and placebo was much greater. All these results together indicate that early disease stage associated with favorable treatment response in axSpA is better defined by symptom duration than by the presence or absence of structural damage in the sacroiliac joints. Furthermore, these data stress the importance of the early diagnosis in axSpA.
We also know from observational studies and subanalyses from clinical trials that treatment with monoclonal antibodies against TNF is associated with reduction of uveitis flares in axSpA. However, no prospective clinical studies had been conducted with acute anterior uveitis flares as the primary outcome until the C-VIEW study, which was presented by Irene E. van der Horst-Bruinsma, MD, PhD, at ACR 2019. The results of C-VIEW are therefore the first to prospectively address the question of reduction of uveitis flares under TNFi. The main limitation of the study is the lack of a control group, which makes interpretation of the results difficult because it is not clear to what extent the natural course of the disease – which might involve very long flare-free periods lasting from months to years – contributed to the reduction of flares. A randomized, controlled study aimed at label extension is highly desired for patients with acute anterior uveitis, especially for those with a frequently relapsing course resistant to local treatment.
Denis Poddubnyy, MD , is head of the rheumatology department at Charite-Universitätsmedizin Berlin. He disclosed receiving research grants from AbbVie, Lilly, Merck, Novartis, and Pfizer, as well as receiving consultancy or speaker fees from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, and UCB.
The findings from the C-axSpAnd study that Jonathan Kay, MD, and colleagues reported at the annual meeting of the American College of Rheumatology are not surprising. Earlier studies in patients with ankylosing spondylitis showed that short symptom duration is one of the best predictors of good treatment response to TNFi therapy. The highest response rates were obtained in studies conducted in axSpA patients with symptom duration of less than 5 years or even less than 3 years. Since nonradiographic axial spondyloarthritis (nr-axSpA) and r-axSpA are considered as two stages of one disease, it is logical that the same effect is also observed in studies in nr-axSpA. Indeed, in the first study of a tumor necrosis factor inhibitor (TNFi) in nr-axSpA (ABILITY-1), patients with symptom duration less than 5 years responded much better to the TNFi adalimumab than did those with longer symptom duration, and the delta of the response between adalimumab and placebo was much greater. All these results together indicate that early disease stage associated with favorable treatment response in axSpA is better defined by symptom duration than by the presence or absence of structural damage in the sacroiliac joints. Furthermore, these data stress the importance of the early diagnosis in axSpA.
We also know from observational studies and subanalyses from clinical trials that treatment with monoclonal antibodies against TNF is associated with reduction of uveitis flares in axSpA. However, no prospective clinical studies had been conducted with acute anterior uveitis flares as the primary outcome until the C-VIEW study, which was presented by Irene E. van der Horst-Bruinsma, MD, PhD, at ACR 2019. The results of C-VIEW are therefore the first to prospectively address the question of reduction of uveitis flares under TNFi. The main limitation of the study is the lack of a control group, which makes interpretation of the results difficult because it is not clear to what extent the natural course of the disease – which might involve very long flare-free periods lasting from months to years – contributed to the reduction of flares. A randomized, controlled study aimed at label extension is highly desired for patients with acute anterior uveitis, especially for those with a frequently relapsing course resistant to local treatment.
Denis Poddubnyy, MD , is head of the rheumatology department at Charite-Universitätsmedizin Berlin. He disclosed receiving research grants from AbbVie, Lilly, Merck, Novartis, and Pfizer, as well as receiving consultancy or speaker fees from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, and UCB.
The findings from the C-axSpAnd study that Jonathan Kay, MD, and colleagues reported at the annual meeting of the American College of Rheumatology are not surprising. Earlier studies in patients with ankylosing spondylitis showed that short symptom duration is one of the best predictors of good treatment response to TNFi therapy. The highest response rates were obtained in studies conducted in axSpA patients with symptom duration of less than 5 years or even less than 3 years. Since nonradiographic axial spondyloarthritis (nr-axSpA) and r-axSpA are considered as two stages of one disease, it is logical that the same effect is also observed in studies in nr-axSpA. Indeed, in the first study of a tumor necrosis factor inhibitor (TNFi) in nr-axSpA (ABILITY-1), patients with symptom duration less than 5 years responded much better to the TNFi adalimumab than did those with longer symptom duration, and the delta of the response between adalimumab and placebo was much greater. All these results together indicate that early disease stage associated with favorable treatment response in axSpA is better defined by symptom duration than by the presence or absence of structural damage in the sacroiliac joints. Furthermore, these data stress the importance of the early diagnosis in axSpA.
We also know from observational studies and subanalyses from clinical trials that treatment with monoclonal antibodies against TNF is associated with reduction of uveitis flares in axSpA. However, no prospective clinical studies had been conducted with acute anterior uveitis flares as the primary outcome until the C-VIEW study, which was presented by Irene E. van der Horst-Bruinsma, MD, PhD, at ACR 2019. The results of C-VIEW are therefore the first to prospectively address the question of reduction of uveitis flares under TNFi. The main limitation of the study is the lack of a control group, which makes interpretation of the results difficult because it is not clear to what extent the natural course of the disease – which might involve very long flare-free periods lasting from months to years – contributed to the reduction of flares. A randomized, controlled study aimed at label extension is highly desired for patients with acute anterior uveitis, especially for those with a frequently relapsing course resistant to local treatment.
Denis Poddubnyy, MD , is head of the rheumatology department at Charite-Universitätsmedizin Berlin. He disclosed receiving research grants from AbbVie, Lilly, Merck, Novartis, and Pfizer, as well as receiving consultancy or speaker fees from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, and UCB.
Certolizumab may reduce uveitis flares, axSpA disease activity
ATLANTA – Certolizumab pegol, a PEGylated, monoclonal, anti–tumor necrosis factor antibody, reduces recurrent acute anterior uveitis flares and improves disease activity in patients with axial spondyloarthritis, according to findings from the open-label, 96-week, phase 4 C-VIEW study.
When given earlier in the course of disease, the treatment, which is the only Food and Drug Administration–approved tumor necrosis factor inhibitor (TNFi) for the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), also shortens symptom duration, a post hoc analysis of data from the multicenter, phase 3 C-axSpAnd study suggests. The findings from both studies were presented during a session at the annual meeting of the American College of Rheumatology.
C-VIEW
In 85 patients with active axSpA who completed 48 weeks of certolizumab pegol therapy in the C-VIEW study, the acute anterior uveitis (AAU) flare incidence over 48 weeks was a mean of 0.2, compared with 1.5 flares per person in the 48 weeks prior to treatment initiation, reported Irene E. van der Horst-Bruinsma, MD, PhD, of Amsterdam University Medical Center. The comparison was adjusted for possible within-patient correlations, flare period (pre- and post baseline), and axSpA disease duration.
This finding, from a preplanned interim analysis, represented a flare incidence of 18.7 versus 146.6 per 100 patient-years, during treatment versus prior to treatment – an 87% reduction – and the difference was statistically significant (P less than .001), Dr. van der Horst-Bruinsma said.
The percentage of patients experiencing one flare was 12.4% during therapy, compared with 64% prior to therapy, and the percentage experiencing two or more flares was 2.2% versus 24.7%, respectively, she said, adding that, in the 13 patients who experienced flares both before and during treatment, the mean flare duration was reduced during treatment (58.4 vs. 97.4 days). A comparison of radiographic and nr-axSpA patients showed similar reductions in flares during versus prior to treatment, going from 144.5 to 19.0 flares per 100 patient-years with radiographic disease and from 158.9 to 17.2 flares per 100 patient-years in nr-axSpA.
Furthermore, after 48 weeks of treatment, disease activity had improved substantially, with mean Ankylosing Spondylitis Disease Activity Score (ASDAS) improving from 3.5 to 2.0 at week 48, 94.2% of patients reaching ASDAS clinical improvement at week 48, and mean Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score decreasing significantly from 6.5 to 3.3 at week 48.
“ASDAS 20 was reached by 75% of the patients, the ASDAS 40 by 54%, and the ASDAS partial remission criteria were reached by 31% of the patients,” she said.
Study participants were adults with a mean age of 46.5 years and active disease according to Assessment of Spondyloarthritis International Society (ASAS) criteria and a history of recurrent AAU flares (either two or more in total, or one or more in the year prior to study entry). They were HLA-B27 positive, eligible for anti-TNF therapy because they had an inadequate response (or contraindication) to at least two prior NSAIDs, were biologic naive, or had failed to respond to no more than one prior anti-TNF agent. Both radiographic and nr-axSpA patients were included, and of 115 who enrolled, 89 initiated treatment, including 76 with radiographic disease and 13 with nonradiographic disease; 85 completed week 48 of treatment.
Certolizumab pegol was given at a loading dose of 400 mg at weeks 0, 2, and 4, followed by 200 mg every 2 weeks through week 96, and was well tolerated. No new safety signals were identified, Dr. van der Horst-Bruinsma said.
“We know that acute anterior uveitis, an inflammation of ... the uveal tract, is the most common extra-articular manifestation in axial spondyloarthritis,” she said. “It is reported in up to 40% of patients and is associated with significant clinical burden.”
AAU is also strongly associated with the HLA-B27 antigen, therefore patients who do not have ankylosing spondylitis but who are HLA-B27 positive also are at risk, she said, noting that previous studies have shown that TNF inhibitors reduce the incidence of AAU flares in patients with radiographic axSpA (ankylosing spondylitis), but that data in nr-axSpA are scarce.
The aim of C-VIEW was to analyze the impact of certolizumab pegol treatment on AAU flares in patients with active radiographic or nr-axSpA and a recent history of AAU, she said.
“C-VIEW was the first study to examine the impact of certolizumab on the incidence of acute anterior uveitis flares in HLA-B27-positive patients with a recent history of acute anterior uveitis, including patients with nr-axSpA ... and in conclusion we can say that these results indicate that certolizumab is a suitable treatment option for patients with axSpA and a history of recurrent acute anterior uveitis,” she said.
C-axSpAnd
In the pivotal 3-year C-axSpAnd study, which included a 52‑week, double-blind, placebo-controlled period, 159 patients with active nr-axSpA, objective signs of inflammation, and previous failure of at least two NSAIDS were treated with certolizumab pegol, and 158 similar patients received placebo. Both groups received nonbiologic background medication.
The results of the trial, published in Arthritis & Rheumatology in March 2019, showed that adding certolizumab pegol to background medication is superior to adding placebo in patients with active nr-axSpA and led to its FDA approval for axSpA in March 2019, but the effects of symptom duration on outcomes with certolizumab pegol have not been well studied, Jonathan Kay, MD, said at the ACR meeting.
The current post hoc analysis stratified patients based on symptom duration and showed that certolizumab pegol recipients with less than 5 years of symptoms at baseline had improved outcomes at weeks 12 and 52, compared with those who had 5 or more years of symptoms at baseline, said Dr. Kay of UMass Memorial Medical Center and the University of Massachusetts, Worcester.
For example, major improvement in ASDAS at week 52, the primary outcome measure, was achieved by 55% of 80 patients with shorter symptom duration, compared with 39.2% of 79 patients with longer symptom duration, and the ASAS 40 responder rates in the groups, respectively, were 58.5% and 36.7% at 12 weeks and 65% and 48.1% at 52 weeks, he said.
Certolizumab pegol recipients with shorter symptom duration also had greater improvement in BASDAI score, nocturnal spinal pain, fatigue, morning stiffness, and the 36-item Short Form Survey physical component score, he noted.
Using a cutoff of 3 years rather than 5 years, responder rates for major improvement in ASDAS and ASAS 40 were still greater in certolizumab pegol–treated patients with shorter symptom duration: At 52 weeks, 56.4% of 55 patients with less than 3 years of symptoms, compared with 42.3% of 104 with 3 or more years of symptoms, achieved major improvement in ASDAS, and ASAS 40 responder rates were 65.5%, compared with 51.9%, respectively.
Response rates in the placebo arm were low, compared with both certolizumab pegol groups, and no consistent trend in outcomes was observed based on symptom duration in that arm, Dr. Kay noted.
Study subjects were adults with a diagnosis of axSpA, active disease, fulfillment of ASAS classification criteria, and at least 12 months of inflammatory back pain. The trial excluded those with radiographic sacroiliitis meeting the modified New York classification criteria and who had exposure to more than one TNFi prior to baseline or primary failure of any TNFi. As in the C-VIEW study, participants were randomized to receive 400 mg certolizumab pegol at weeks 0, 2, and 4, and then 200 mg every 2 weeks thereafter through week 52.
The findings are notable because patients with axSpA – including radiographic disease and nr-axSpA – often experience delays in diagnosis, which can lead to a delay in treatment and a reduced quality of life because of the back pain, fatigue, and morning stiffness that commonly occur with the disease.
“Women, especially, with axial spondyloarthritis experience a longer delay in diagnosis than do male patients,” Dr. Kay noted.
The findings of this post hoc analysis underscore the risks associated with such a delay. “These results imply that early diagnosis enabling earlier treatment is important for patients with nonradiographic axSpA, as it is for patients with radiographic axSpA,” he concluded.
The C-VIEW and C-axSpAnd studies were funded by UCB. Dr. van der Horst-Bruinsma reported receiving honoraria, consulting fees, and/or research grants from UCB as well as from AbbVie, Bristol-Myers Squibb, Merck, Novartis, and Pfizer. Dr. Kay reported receiving grant/research support from Gilead, Pfizer, and UCB, and consulting fees from AbbVie, Alvotech, Boehringer Ingelheim, Celltrion, Merck, Novartis, Samsung Bioepis, Sandoz, and UCB.
SOURCES: van der Horst-Bruinsma I et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 935; Kay J et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 936.
ATLANTA – Certolizumab pegol, a PEGylated, monoclonal, anti–tumor necrosis factor antibody, reduces recurrent acute anterior uveitis flares and improves disease activity in patients with axial spondyloarthritis, according to findings from the open-label, 96-week, phase 4 C-VIEW study.
When given earlier in the course of disease, the treatment, which is the only Food and Drug Administration–approved tumor necrosis factor inhibitor (TNFi) for the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), also shortens symptom duration, a post hoc analysis of data from the multicenter, phase 3 C-axSpAnd study suggests. The findings from both studies were presented during a session at the annual meeting of the American College of Rheumatology.
C-VIEW
In 85 patients with active axSpA who completed 48 weeks of certolizumab pegol therapy in the C-VIEW study, the acute anterior uveitis (AAU) flare incidence over 48 weeks was a mean of 0.2, compared with 1.5 flares per person in the 48 weeks prior to treatment initiation, reported Irene E. van der Horst-Bruinsma, MD, PhD, of Amsterdam University Medical Center. The comparison was adjusted for possible within-patient correlations, flare period (pre- and post baseline), and axSpA disease duration.
This finding, from a preplanned interim analysis, represented a flare incidence of 18.7 versus 146.6 per 100 patient-years, during treatment versus prior to treatment – an 87% reduction – and the difference was statistically significant (P less than .001), Dr. van der Horst-Bruinsma said.
The percentage of patients experiencing one flare was 12.4% during therapy, compared with 64% prior to therapy, and the percentage experiencing two or more flares was 2.2% versus 24.7%, respectively, she said, adding that, in the 13 patients who experienced flares both before and during treatment, the mean flare duration was reduced during treatment (58.4 vs. 97.4 days). A comparison of radiographic and nr-axSpA patients showed similar reductions in flares during versus prior to treatment, going from 144.5 to 19.0 flares per 100 patient-years with radiographic disease and from 158.9 to 17.2 flares per 100 patient-years in nr-axSpA.
Furthermore, after 48 weeks of treatment, disease activity had improved substantially, with mean Ankylosing Spondylitis Disease Activity Score (ASDAS) improving from 3.5 to 2.0 at week 48, 94.2% of patients reaching ASDAS clinical improvement at week 48, and mean Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score decreasing significantly from 6.5 to 3.3 at week 48.
“ASDAS 20 was reached by 75% of the patients, the ASDAS 40 by 54%, and the ASDAS partial remission criteria were reached by 31% of the patients,” she said.
Study participants were adults with a mean age of 46.5 years and active disease according to Assessment of Spondyloarthritis International Society (ASAS) criteria and a history of recurrent AAU flares (either two or more in total, or one or more in the year prior to study entry). They were HLA-B27 positive, eligible for anti-TNF therapy because they had an inadequate response (or contraindication) to at least two prior NSAIDs, were biologic naive, or had failed to respond to no more than one prior anti-TNF agent. Both radiographic and nr-axSpA patients were included, and of 115 who enrolled, 89 initiated treatment, including 76 with radiographic disease and 13 with nonradiographic disease; 85 completed week 48 of treatment.
Certolizumab pegol was given at a loading dose of 400 mg at weeks 0, 2, and 4, followed by 200 mg every 2 weeks through week 96, and was well tolerated. No new safety signals were identified, Dr. van der Horst-Bruinsma said.
“We know that acute anterior uveitis, an inflammation of ... the uveal tract, is the most common extra-articular manifestation in axial spondyloarthritis,” she said. “It is reported in up to 40% of patients and is associated with significant clinical burden.”
AAU is also strongly associated with the HLA-B27 antigen, therefore patients who do not have ankylosing spondylitis but who are HLA-B27 positive also are at risk, she said, noting that previous studies have shown that TNF inhibitors reduce the incidence of AAU flares in patients with radiographic axSpA (ankylosing spondylitis), but that data in nr-axSpA are scarce.
The aim of C-VIEW was to analyze the impact of certolizumab pegol treatment on AAU flares in patients with active radiographic or nr-axSpA and a recent history of AAU, she said.
“C-VIEW was the first study to examine the impact of certolizumab on the incidence of acute anterior uveitis flares in HLA-B27-positive patients with a recent history of acute anterior uveitis, including patients with nr-axSpA ... and in conclusion we can say that these results indicate that certolizumab is a suitable treatment option for patients with axSpA and a history of recurrent acute anterior uveitis,” she said.
C-axSpAnd
In the pivotal 3-year C-axSpAnd study, which included a 52‑week, double-blind, placebo-controlled period, 159 patients with active nr-axSpA, objective signs of inflammation, and previous failure of at least two NSAIDS were treated with certolizumab pegol, and 158 similar patients received placebo. Both groups received nonbiologic background medication.
The results of the trial, published in Arthritis & Rheumatology in March 2019, showed that adding certolizumab pegol to background medication is superior to adding placebo in patients with active nr-axSpA and led to its FDA approval for axSpA in March 2019, but the effects of symptom duration on outcomes with certolizumab pegol have not been well studied, Jonathan Kay, MD, said at the ACR meeting.
The current post hoc analysis stratified patients based on symptom duration and showed that certolizumab pegol recipients with less than 5 years of symptoms at baseline had improved outcomes at weeks 12 and 52, compared with those who had 5 or more years of symptoms at baseline, said Dr. Kay of UMass Memorial Medical Center and the University of Massachusetts, Worcester.
For example, major improvement in ASDAS at week 52, the primary outcome measure, was achieved by 55% of 80 patients with shorter symptom duration, compared with 39.2% of 79 patients with longer symptom duration, and the ASAS 40 responder rates in the groups, respectively, were 58.5% and 36.7% at 12 weeks and 65% and 48.1% at 52 weeks, he said.
Certolizumab pegol recipients with shorter symptom duration also had greater improvement in BASDAI score, nocturnal spinal pain, fatigue, morning stiffness, and the 36-item Short Form Survey physical component score, he noted.
Using a cutoff of 3 years rather than 5 years, responder rates for major improvement in ASDAS and ASAS 40 were still greater in certolizumab pegol–treated patients with shorter symptom duration: At 52 weeks, 56.4% of 55 patients with less than 3 years of symptoms, compared with 42.3% of 104 with 3 or more years of symptoms, achieved major improvement in ASDAS, and ASAS 40 responder rates were 65.5%, compared with 51.9%, respectively.
Response rates in the placebo arm were low, compared with both certolizumab pegol groups, and no consistent trend in outcomes was observed based on symptom duration in that arm, Dr. Kay noted.
Study subjects were adults with a diagnosis of axSpA, active disease, fulfillment of ASAS classification criteria, and at least 12 months of inflammatory back pain. The trial excluded those with radiographic sacroiliitis meeting the modified New York classification criteria and who had exposure to more than one TNFi prior to baseline or primary failure of any TNFi. As in the C-VIEW study, participants were randomized to receive 400 mg certolizumab pegol at weeks 0, 2, and 4, and then 200 mg every 2 weeks thereafter through week 52.
The findings are notable because patients with axSpA – including radiographic disease and nr-axSpA – often experience delays in diagnosis, which can lead to a delay in treatment and a reduced quality of life because of the back pain, fatigue, and morning stiffness that commonly occur with the disease.
“Women, especially, with axial spondyloarthritis experience a longer delay in diagnosis than do male patients,” Dr. Kay noted.
The findings of this post hoc analysis underscore the risks associated with such a delay. “These results imply that early diagnosis enabling earlier treatment is important for patients with nonradiographic axSpA, as it is for patients with radiographic axSpA,” he concluded.
The C-VIEW and C-axSpAnd studies were funded by UCB. Dr. van der Horst-Bruinsma reported receiving honoraria, consulting fees, and/or research grants from UCB as well as from AbbVie, Bristol-Myers Squibb, Merck, Novartis, and Pfizer. Dr. Kay reported receiving grant/research support from Gilead, Pfizer, and UCB, and consulting fees from AbbVie, Alvotech, Boehringer Ingelheim, Celltrion, Merck, Novartis, Samsung Bioepis, Sandoz, and UCB.
SOURCES: van der Horst-Bruinsma I et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 935; Kay J et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 936.
ATLANTA – Certolizumab pegol, a PEGylated, monoclonal, anti–tumor necrosis factor antibody, reduces recurrent acute anterior uveitis flares and improves disease activity in patients with axial spondyloarthritis, according to findings from the open-label, 96-week, phase 4 C-VIEW study.
When given earlier in the course of disease, the treatment, which is the only Food and Drug Administration–approved tumor necrosis factor inhibitor (TNFi) for the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), also shortens symptom duration, a post hoc analysis of data from the multicenter, phase 3 C-axSpAnd study suggests. The findings from both studies were presented during a session at the annual meeting of the American College of Rheumatology.
C-VIEW
In 85 patients with active axSpA who completed 48 weeks of certolizumab pegol therapy in the C-VIEW study, the acute anterior uveitis (AAU) flare incidence over 48 weeks was a mean of 0.2, compared with 1.5 flares per person in the 48 weeks prior to treatment initiation, reported Irene E. van der Horst-Bruinsma, MD, PhD, of Amsterdam University Medical Center. The comparison was adjusted for possible within-patient correlations, flare period (pre- and post baseline), and axSpA disease duration.
This finding, from a preplanned interim analysis, represented a flare incidence of 18.7 versus 146.6 per 100 patient-years, during treatment versus prior to treatment – an 87% reduction – and the difference was statistically significant (P less than .001), Dr. van der Horst-Bruinsma said.
The percentage of patients experiencing one flare was 12.4% during therapy, compared with 64% prior to therapy, and the percentage experiencing two or more flares was 2.2% versus 24.7%, respectively, she said, adding that, in the 13 patients who experienced flares both before and during treatment, the mean flare duration was reduced during treatment (58.4 vs. 97.4 days). A comparison of radiographic and nr-axSpA patients showed similar reductions in flares during versus prior to treatment, going from 144.5 to 19.0 flares per 100 patient-years with radiographic disease and from 158.9 to 17.2 flares per 100 patient-years in nr-axSpA.
Furthermore, after 48 weeks of treatment, disease activity had improved substantially, with mean Ankylosing Spondylitis Disease Activity Score (ASDAS) improving from 3.5 to 2.0 at week 48, 94.2% of patients reaching ASDAS clinical improvement at week 48, and mean Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score decreasing significantly from 6.5 to 3.3 at week 48.
“ASDAS 20 was reached by 75% of the patients, the ASDAS 40 by 54%, and the ASDAS partial remission criteria were reached by 31% of the patients,” she said.
Study participants were adults with a mean age of 46.5 years and active disease according to Assessment of Spondyloarthritis International Society (ASAS) criteria and a history of recurrent AAU flares (either two or more in total, or one or more in the year prior to study entry). They were HLA-B27 positive, eligible for anti-TNF therapy because they had an inadequate response (or contraindication) to at least two prior NSAIDs, were biologic naive, or had failed to respond to no more than one prior anti-TNF agent. Both radiographic and nr-axSpA patients were included, and of 115 who enrolled, 89 initiated treatment, including 76 with radiographic disease and 13 with nonradiographic disease; 85 completed week 48 of treatment.
Certolizumab pegol was given at a loading dose of 400 mg at weeks 0, 2, and 4, followed by 200 mg every 2 weeks through week 96, and was well tolerated. No new safety signals were identified, Dr. van der Horst-Bruinsma said.
“We know that acute anterior uveitis, an inflammation of ... the uveal tract, is the most common extra-articular manifestation in axial spondyloarthritis,” she said. “It is reported in up to 40% of patients and is associated with significant clinical burden.”
AAU is also strongly associated with the HLA-B27 antigen, therefore patients who do not have ankylosing spondylitis but who are HLA-B27 positive also are at risk, she said, noting that previous studies have shown that TNF inhibitors reduce the incidence of AAU flares in patients with radiographic axSpA (ankylosing spondylitis), but that data in nr-axSpA are scarce.
The aim of C-VIEW was to analyze the impact of certolizumab pegol treatment on AAU flares in patients with active radiographic or nr-axSpA and a recent history of AAU, she said.
“C-VIEW was the first study to examine the impact of certolizumab on the incidence of acute anterior uveitis flares in HLA-B27-positive patients with a recent history of acute anterior uveitis, including patients with nr-axSpA ... and in conclusion we can say that these results indicate that certolizumab is a suitable treatment option for patients with axSpA and a history of recurrent acute anterior uveitis,” she said.
C-axSpAnd
In the pivotal 3-year C-axSpAnd study, which included a 52‑week, double-blind, placebo-controlled period, 159 patients with active nr-axSpA, objective signs of inflammation, and previous failure of at least two NSAIDS were treated with certolizumab pegol, and 158 similar patients received placebo. Both groups received nonbiologic background medication.
The results of the trial, published in Arthritis & Rheumatology in March 2019, showed that adding certolizumab pegol to background medication is superior to adding placebo in patients with active nr-axSpA and led to its FDA approval for axSpA in March 2019, but the effects of symptom duration on outcomes with certolizumab pegol have not been well studied, Jonathan Kay, MD, said at the ACR meeting.
The current post hoc analysis stratified patients based on symptom duration and showed that certolizumab pegol recipients with less than 5 years of symptoms at baseline had improved outcomes at weeks 12 and 52, compared with those who had 5 or more years of symptoms at baseline, said Dr. Kay of UMass Memorial Medical Center and the University of Massachusetts, Worcester.
For example, major improvement in ASDAS at week 52, the primary outcome measure, was achieved by 55% of 80 patients with shorter symptom duration, compared with 39.2% of 79 patients with longer symptom duration, and the ASAS 40 responder rates in the groups, respectively, were 58.5% and 36.7% at 12 weeks and 65% and 48.1% at 52 weeks, he said.
Certolizumab pegol recipients with shorter symptom duration also had greater improvement in BASDAI score, nocturnal spinal pain, fatigue, morning stiffness, and the 36-item Short Form Survey physical component score, he noted.
Using a cutoff of 3 years rather than 5 years, responder rates for major improvement in ASDAS and ASAS 40 were still greater in certolizumab pegol–treated patients with shorter symptom duration: At 52 weeks, 56.4% of 55 patients with less than 3 years of symptoms, compared with 42.3% of 104 with 3 or more years of symptoms, achieved major improvement in ASDAS, and ASAS 40 responder rates were 65.5%, compared with 51.9%, respectively.
Response rates in the placebo arm were low, compared with both certolizumab pegol groups, and no consistent trend in outcomes was observed based on symptom duration in that arm, Dr. Kay noted.
Study subjects were adults with a diagnosis of axSpA, active disease, fulfillment of ASAS classification criteria, and at least 12 months of inflammatory back pain. The trial excluded those with radiographic sacroiliitis meeting the modified New York classification criteria and who had exposure to more than one TNFi prior to baseline or primary failure of any TNFi. As in the C-VIEW study, participants were randomized to receive 400 mg certolizumab pegol at weeks 0, 2, and 4, and then 200 mg every 2 weeks thereafter through week 52.
The findings are notable because patients with axSpA – including radiographic disease and nr-axSpA – often experience delays in diagnosis, which can lead to a delay in treatment and a reduced quality of life because of the back pain, fatigue, and morning stiffness that commonly occur with the disease.
“Women, especially, with axial spondyloarthritis experience a longer delay in diagnosis than do male patients,” Dr. Kay noted.
The findings of this post hoc analysis underscore the risks associated with such a delay. “These results imply that early diagnosis enabling earlier treatment is important for patients with nonradiographic axSpA, as it is for patients with radiographic axSpA,” he concluded.
The C-VIEW and C-axSpAnd studies were funded by UCB. Dr. van der Horst-Bruinsma reported receiving honoraria, consulting fees, and/or research grants from UCB as well as from AbbVie, Bristol-Myers Squibb, Merck, Novartis, and Pfizer. Dr. Kay reported receiving grant/research support from Gilead, Pfizer, and UCB, and consulting fees from AbbVie, Alvotech, Boehringer Ingelheim, Celltrion, Merck, Novartis, Samsung Bioepis, Sandoz, and UCB.
SOURCES: van der Horst-Bruinsma I et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 935; Kay J et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 936.
REPORTING FROM ACR 2019
Pyoderma Gangrenosum Developing After Chest Tube Placement in a Patient With Chronic Lymphocytic Leukemia
Diagnosis of a neutrophilic dermatosis, such as pyoderma gangrenosum (PG), often is challenging at onset because it can be impossible to distinguish clinically and histopathologically from acute infection in an immunosuppressed patient, necessitating a detailed history as well as correlation pathology with microbial tissue cultures. The dermatologist’s ability to distinguish a neutrophilic dermatosis from active infection is of paramount importance because the decision to treat with surgical debridement, in addition to an antibiotic regimen, can have grave consequences in the misdiagnosed patient.
Pyoderma gangrenosum is a neutrophilic dermatosis histologically characterized by a pandermal neutrophilic infiltrate without evidence of an infectious cause or true vasculitis. It is classically associated with inflammatory bowel disease or an underlying hematologic malignancy. Pyoderma gangrenosum in the setting of chronic lymphocytic leukemia (CLL) is rare, with as few as 4 cases having been described in the literature and only 1 case of PG developing after a surgical procedure.1-4 We present a case of PG occurring at a chest tube site in a patient with CLL. We highlight the challenges and therapeutic importance of arriving at the correct diagnosis.
Case Report
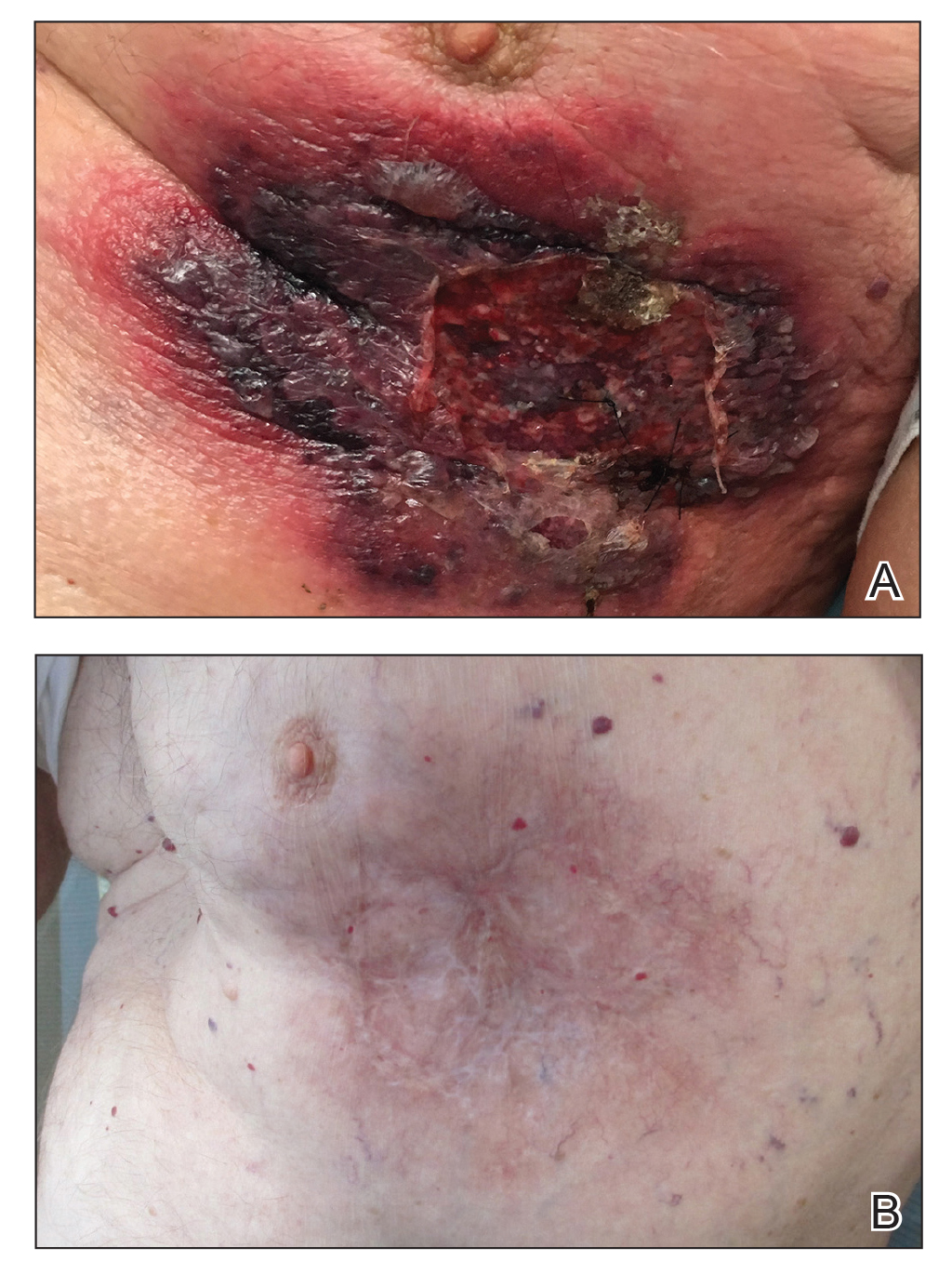
An 87-year-old man with a history of refractory CLL was admitted to the hospital with pneumonia and pleural effusion requiring chest tube placement (left). His most recent therapeutic regimen for CLL was rituximab and bendamustine, which was administered 9 days prior to admission. After removal of the chest tube, an erythematous plaque with central necrosis surrounding the chest tube site developed (Figure 1A). During this time period, the patient had documented intermittent fevers, leukopenia, and neutropenia. Serial blood cultures yielded no growth. Because the patient was on broad-spectrum antibiotic coverage, dermatology was consulted for possible angioinvasive fungal infection.
Physical examination revealed an indurated, erythematous-violaceous, targetoid, well-defined, ulcerated plaque with central necrosis on the left side of the chest. Notably, we observed an isolated bulla with an erythematous base within the right antecubital fossa at the site of intravenous placement, suggesting pathergy.
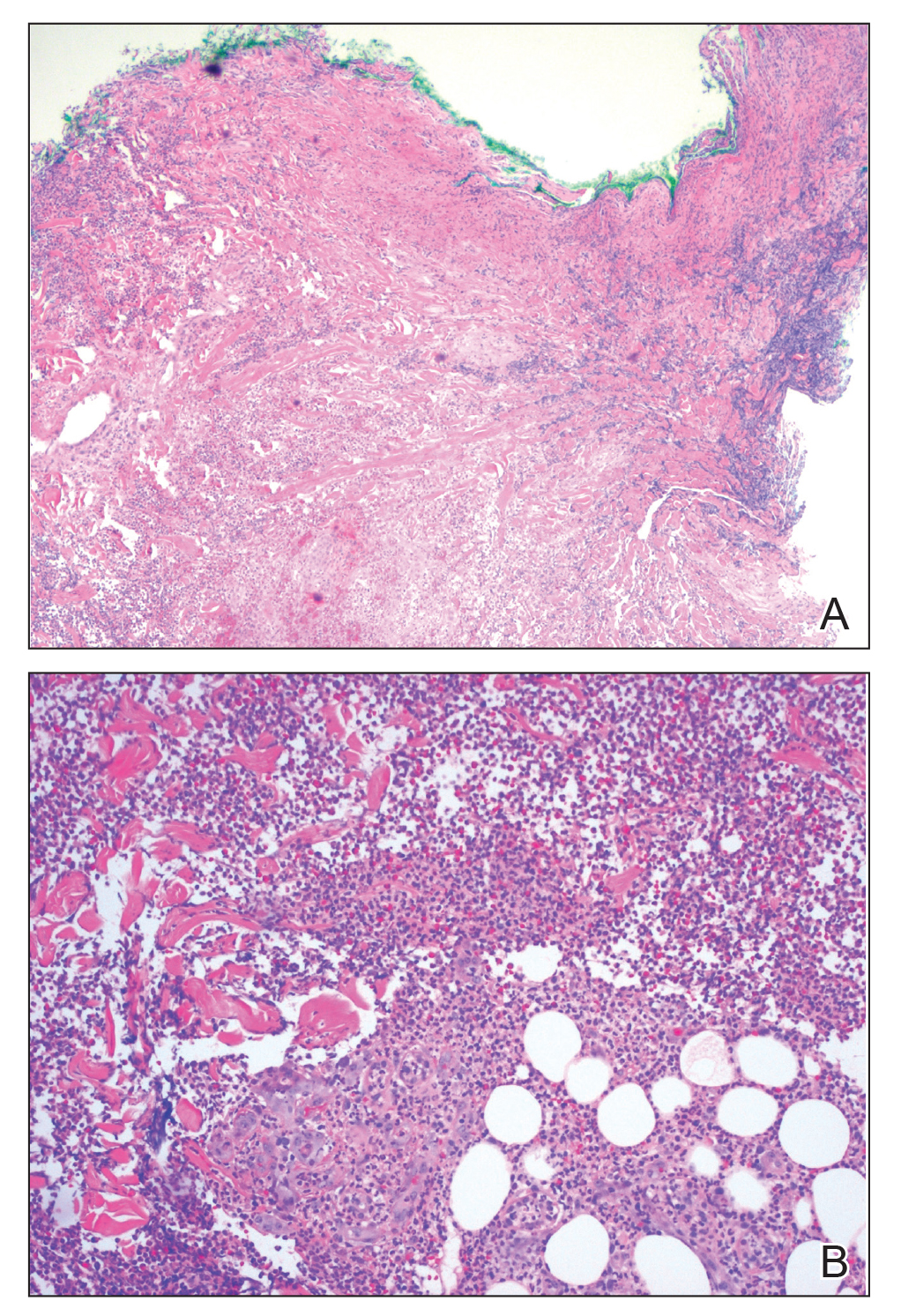
Multiple punch biopsies revealed an ulcer with an underlying dense neutrophilic infiltrate within the dermis and subcutaneous tissues (Figure 2). Grocott-Gomori methenamine-silver, periodic acid–Schiff, and acid-fast bacillus stains were all negative for organisms. Tissue cultures for bacterial, fungal, and acid-fast bacilli revealed no growth. Due to the rapidly expanding nature of the plaque and the possibility of infection despite negative microbial stains and cultures, the patient was scheduled for surgical debridement by the surgical team.
Opportunely, after thoughtful consideration of the clinical history, histopathology, and negative tissue cultures, we made a diagnosis of PG, a condition that would have been further exacerbated by debridement and unimproved with antibiotics. Based on our recommendation, the patient received immunosuppressive treatment with prednisone 60 mg/d and triamcinolone ointment 0.1%. He experienced immediate clinical improvement, allowing him to be discharged to the care of dermatology as an outpatient. He continued to receive a monthly rituximab infusion. We intentionally tapered the patient’s prednisone dosage slowly over 4 months and photodocumented steady improvement with eventual resolution of the PG (Figure 1B).
Comment
Pathogenesis of PG
Pyoderma gangrenosum lies in the spectrum of neutrophilic dermatoses, which are characterized histologically by a pandermal neutrophilic infiltrate without evidence of an infectious cause or true vasculitis. Clinically, PG typically presents as a steadily expanding ulceration with an undermined or slightly raised border, and often is associated with the pathergy phenomenon. Historically, PG is classically linked to inflammatory bowel disease; however, association with underlying malignancy, including acute myelogenous leukemia, chronic myelogenous leukemia, myeloma, and myeloid metaplasia, also has been described.5
Pathogenesis of CLL
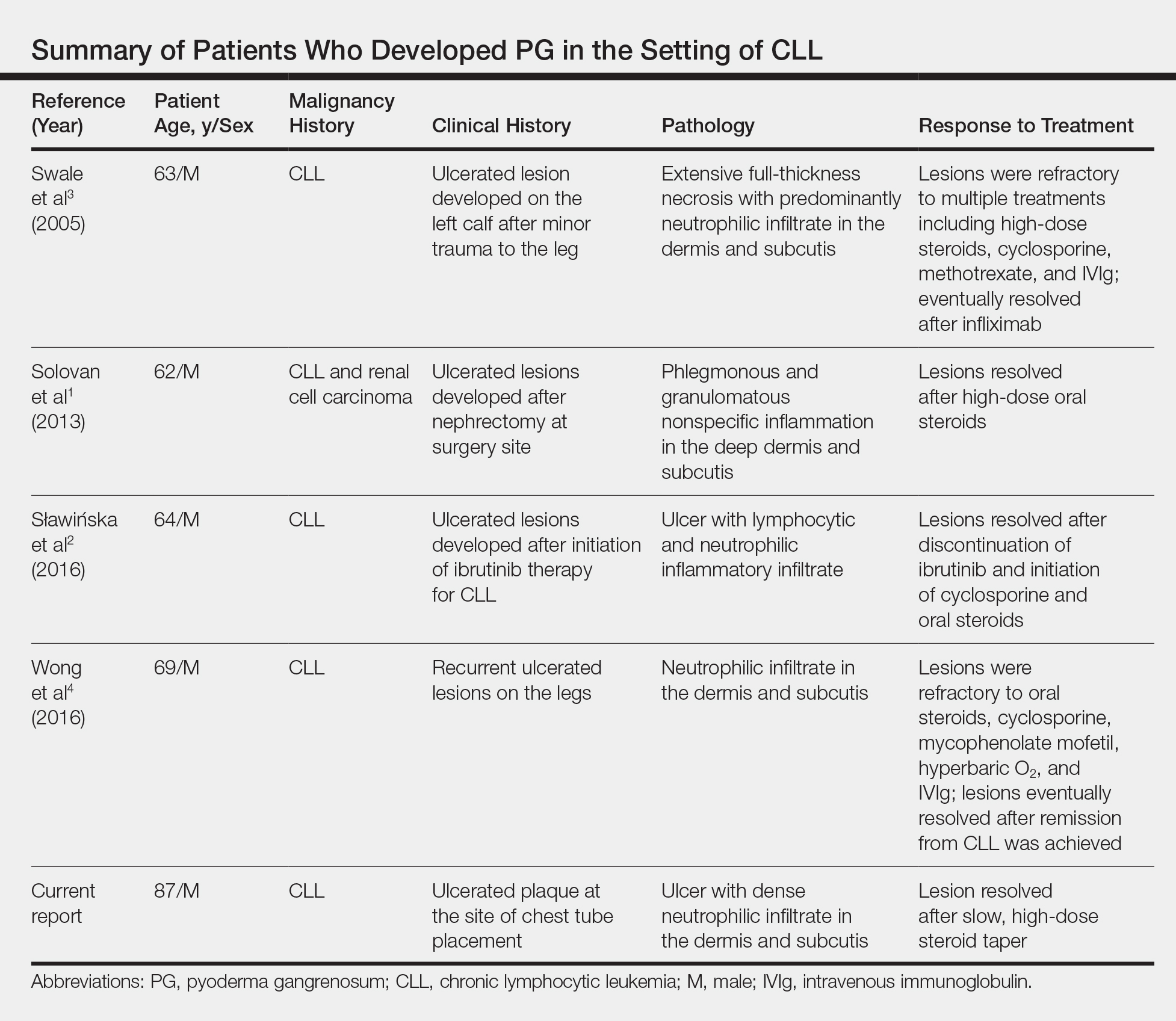
Chronic lymphocytic leukemia represents the most prevalent form of leukemia in US adults, with the second highest annual incidence.6 Cutaneous findings are seen in 25% of patients with CLL, varying from leukemia cutis to secondary findings such as vasculitis, purpura, generalized pruritus, exfoliative erythroderma, paraneoplastic pemphigus, infections, and rarely neutrophilic dermatoses.7 According to a PubMed search of articles indexed for MEDLINE using the term pyoderma gangrenosum in CLL, only 4 cases of PG occurring in the setting of CLL exist in the literature, with 1 case demonstrating development after a surgical procedure, making ours the second such case (Table).1-4
Diagnosis
Making the diagnosis of a neutrophilic dermatosis such as PG or Sweet syndrome (SS) in the hospital setting is not only difficult but also imperative, considering that the counterdiagnosis more often is an infectious process. The distinction between individual neutrophilic dermatoses is less crucial at the onset because the initial treatment is the same.
Sweet syndrome is classically the most challenging entity within the spectrum to differentiate from PG. However, our case outlines several key distinguishing features:
• The lesion in classic PG is a rapidly expanding ulceration with undermined borders, whereas SS is less commonly associated with ulceration and instead classically presents with multiple edematous papules that progress to juicy plaques.8
• The pathergy phenomenon has been reported in SS, though it is more commonly associated with PG.9
• In reported cases of SS that were related to cutaneous trauma, lesions developed outside the area of trauma and there was documented leukocytosis and neutrophilia.10-14
• Although leukocytosis is part of the minor diagnostic criteria for SS, it is not required for the diagnosis of PG. Considering that our patient had ulcerated lesions, lesions only at the site of trauma, and leukopenia with intermittent neutropenia, the diagnosis was consistent with PG.
The primary value of early recognition and diagnosis of PG lies in the physician’s ability to distinguish PG from an infectious process, which can be challenging in an immunosuppressed patient with an underlying hematologic malignancy.
Conclusion
This case report represents our experience in arriving at the correct diagnosis of PG in a febrile neutrophilic patient with CLL. In the case of PG in a complicated patient, it is critical to initiate appropriate treatment and avoid inappropriate therapies. Aggressive surgical debridement could have resulted in a fatal outcome for our patient, highlighting the need for dermatologists to raise physician awareness of this challenging disease.
Acknowledgments
The authors acknowledge the contributions of Sarah Shalin, MD, PhD; Nikhil Meena, MD; and Aditya Chada, MD (all from Little Rock, Arkansas), for excellent patient care.
- Solovan C, Smiszek R, Wickenhauser C, et al. Postoperative pyoderma gangrenosum in association with renal cell carcinoma and chronic lymphocytic leukemia. Infect Dis Ther. 2013;2:75-80.
- Sławińska M, Barańska-Rybak W, Sobjanek M, et al. Ibrutinib-induced pyoderma gangrenosum. Pol Arch Med Wewn. 2016;126:710-711.
- Swale VJ, Saha M, Kapur N, et al. Pyoderma gangrenosum outside the context of inflammatory bowel disease treated successfully with infliximab. Clin Exp Dermatol. 2005;30:134-136.
- Wong SM, McComish J, Douglass J, et al. Rare skin manifestations successfully treated with primary B-cell chronic lymphocytic leukemia treatment. J Cutan Pathol. 2016;43:552-555.
- Jockenhöfer F, Herberger K, Schaller J, et al. Tricenter analysis of cofactors and comorbidity in patients with pyoderma gangrenosum. J Dtsch Dermatol Ges. 2016;14:1023-1030.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. 7.
- Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855-865.
- Beasley JM, Sluzevich JC. A recurrent vesiculobullous eruption on the head, trunk, and extremities. Bullous Sweet’s syndrome. Int J Dermatol. 2016;55:149-150.
- Awan F, Hamadani M, Devine S. Paraneoplastic Sweet’s syndrome and the pathergy phenomenon. Ann Hematol. 2007;86:613-614.
- de Moya MA, Wong JT, Kroshinsky D, et al. Case records of the Massachusetts General Hospital. Case 28-2012. A 30-year-old woman with shock and abdominal-wall necrosis after cesarean section. N Engl J Med. 2012;367:1046-1057.
- Minocha R, Sebaratnam DF, Choi JY. Sweet’s syndrome following surgery: cutaneous trauma as a possible aetiological co-factor in neutrophilic dermatoses. Australas J Dermatol. 2015;56:
e74-e76. - Phua YS, Al-Ani SA, She RB, et al. Sweet’s syndrome triggered by scalding: a case study and review of the literature. Burns. 2010;36:e49-e52.
- Schwarz RE, Quinn MA, Molina A. Acute postoperative dermatosis at the site of the electrocautery pad: sweet diagnosis of a burning issue. Surg Today. 2000;30:207-209.
- Tan AW, Tan HH, Lim PL. Bullous Sweet’s syndrome following influenza vaccination in a HIV-infected patient. Int J Dermatol. 2006;45:1254-1255.
Diagnosis of a neutrophilic dermatosis, such as pyoderma gangrenosum (PG), often is challenging at onset because it can be impossible to distinguish clinically and histopathologically from acute infection in an immunosuppressed patient, necessitating a detailed history as well as correlation pathology with microbial tissue cultures. The dermatologist’s ability to distinguish a neutrophilic dermatosis from active infection is of paramount importance because the decision to treat with surgical debridement, in addition to an antibiotic regimen, can have grave consequences in the misdiagnosed patient.
Pyoderma gangrenosum is a neutrophilic dermatosis histologically characterized by a pandermal neutrophilic infiltrate without evidence of an infectious cause or true vasculitis. It is classically associated with inflammatory bowel disease or an underlying hematologic malignancy. Pyoderma gangrenosum in the setting of chronic lymphocytic leukemia (CLL) is rare, with as few as 4 cases having been described in the literature and only 1 case of PG developing after a surgical procedure.1-4 We present a case of PG occurring at a chest tube site in a patient with CLL. We highlight the challenges and therapeutic importance of arriving at the correct diagnosis.
Case Report
An 87-year-old man with a history of refractory CLL was admitted to the hospital with pneumonia and pleural effusion requiring chest tube placement (left). His most recent therapeutic regimen for CLL was rituximab and bendamustine, which was administered 9 days prior to admission. After removal of the chest tube, an erythematous plaque with central necrosis surrounding the chest tube site developed (Figure 1A). During this time period, the patient had documented intermittent fevers, leukopenia, and neutropenia. Serial blood cultures yielded no growth. Because the patient was on broad-spectrum antibiotic coverage, dermatology was consulted for possible angioinvasive fungal infection.
Physical examination revealed an indurated, erythematous-violaceous, targetoid, well-defined, ulcerated plaque with central necrosis on the left side of the chest. Notably, we observed an isolated bulla with an erythematous base within the right antecubital fossa at the site of intravenous placement, suggesting pathergy.
Multiple punch biopsies revealed an ulcer with an underlying dense neutrophilic infiltrate within the dermis and subcutaneous tissues (Figure 2). Grocott-Gomori methenamine-silver, periodic acid–Schiff, and acid-fast bacillus stains were all negative for organisms. Tissue cultures for bacterial, fungal, and acid-fast bacilli revealed no growth. Due to the rapidly expanding nature of the plaque and the possibility of infection despite negative microbial stains and cultures, the patient was scheduled for surgical debridement by the surgical team.
Opportunely, after thoughtful consideration of the clinical history, histopathology, and negative tissue cultures, we made a diagnosis of PG, a condition that would have been further exacerbated by debridement and unimproved with antibiotics. Based on our recommendation, the patient received immunosuppressive treatment with prednisone 60 mg/d and triamcinolone ointment 0.1%. He experienced immediate clinical improvement, allowing him to be discharged to the care of dermatology as an outpatient. He continued to receive a monthly rituximab infusion. We intentionally tapered the patient’s prednisone dosage slowly over 4 months and photodocumented steady improvement with eventual resolution of the PG (Figure 1B).
Comment
Pathogenesis of PG
Pyoderma gangrenosum lies in the spectrum of neutrophilic dermatoses, which are characterized histologically by a pandermal neutrophilic infiltrate without evidence of an infectious cause or true vasculitis. Clinically, PG typically presents as a steadily expanding ulceration with an undermined or slightly raised border, and often is associated with the pathergy phenomenon. Historically, PG is classically linked to inflammatory bowel disease; however, association with underlying malignancy, including acute myelogenous leukemia, chronic myelogenous leukemia, myeloma, and myeloid metaplasia, also has been described.5
Pathogenesis of CLL
Chronic lymphocytic leukemia represents the most prevalent form of leukemia in US adults, with the second highest annual incidence.6 Cutaneous findings are seen in 25% of patients with CLL, varying from leukemia cutis to secondary findings such as vasculitis, purpura, generalized pruritus, exfoliative erythroderma, paraneoplastic pemphigus, infections, and rarely neutrophilic dermatoses.7 According to a PubMed search of articles indexed for MEDLINE using the term pyoderma gangrenosum in CLL, only 4 cases of PG occurring in the setting of CLL exist in the literature, with 1 case demonstrating development after a surgical procedure, making ours the second such case (Table).1-4
Diagnosis
Making the diagnosis of a neutrophilic dermatosis such as PG or Sweet syndrome (SS) in the hospital setting is not only difficult but also imperative, considering that the counterdiagnosis more often is an infectious process. The distinction between individual neutrophilic dermatoses is less crucial at the onset because the initial treatment is the same.
Sweet syndrome is classically the most challenging entity within the spectrum to differentiate from PG. However, our case outlines several key distinguishing features:
• The lesion in classic PG is a rapidly expanding ulceration with undermined borders, whereas SS is less commonly associated with ulceration and instead classically presents with multiple edematous papules that progress to juicy plaques.8
• The pathergy phenomenon has been reported in SS, though it is more commonly associated with PG.9
• In reported cases of SS that were related to cutaneous trauma, lesions developed outside the area of trauma and there was documented leukocytosis and neutrophilia.10-14
• Although leukocytosis is part of the minor diagnostic criteria for SS, it is not required for the diagnosis of PG. Considering that our patient had ulcerated lesions, lesions only at the site of trauma, and leukopenia with intermittent neutropenia, the diagnosis was consistent with PG.
The primary value of early recognition and diagnosis of PG lies in the physician’s ability to distinguish PG from an infectious process, which can be challenging in an immunosuppressed patient with an underlying hematologic malignancy.
Conclusion
This case report represents our experience in arriving at the correct diagnosis of PG in a febrile neutrophilic patient with CLL. In the case of PG in a complicated patient, it is critical to initiate appropriate treatment and avoid inappropriate therapies. Aggressive surgical debridement could have resulted in a fatal outcome for our patient, highlighting the need for dermatologists to raise physician awareness of this challenging disease.
Acknowledgments
The authors acknowledge the contributions of Sarah Shalin, MD, PhD; Nikhil Meena, MD; and Aditya Chada, MD (all from Little Rock, Arkansas), for excellent patient care.
Diagnosis of a neutrophilic dermatosis, such as pyoderma gangrenosum (PG), often is challenging at onset because it can be impossible to distinguish clinically and histopathologically from acute infection in an immunosuppressed patient, necessitating a detailed history as well as correlation pathology with microbial tissue cultures. The dermatologist’s ability to distinguish a neutrophilic dermatosis from active infection is of paramount importance because the decision to treat with surgical debridement, in addition to an antibiotic regimen, can have grave consequences in the misdiagnosed patient.
Pyoderma gangrenosum is a neutrophilic dermatosis histologically characterized by a pandermal neutrophilic infiltrate without evidence of an infectious cause or true vasculitis. It is classically associated with inflammatory bowel disease or an underlying hematologic malignancy. Pyoderma gangrenosum in the setting of chronic lymphocytic leukemia (CLL) is rare, with as few as 4 cases having been described in the literature and only 1 case of PG developing after a surgical procedure.1-4 We present a case of PG occurring at a chest tube site in a patient with CLL. We highlight the challenges and therapeutic importance of arriving at the correct diagnosis.
Case Report
An 87-year-old man with a history of refractory CLL was admitted to the hospital with pneumonia and pleural effusion requiring chest tube placement (left). His most recent therapeutic regimen for CLL was rituximab and bendamustine, which was administered 9 days prior to admission. After removal of the chest tube, an erythematous plaque with central necrosis surrounding the chest tube site developed (Figure 1A). During this time period, the patient had documented intermittent fevers, leukopenia, and neutropenia. Serial blood cultures yielded no growth. Because the patient was on broad-spectrum antibiotic coverage, dermatology was consulted for possible angioinvasive fungal infection.
Physical examination revealed an indurated, erythematous-violaceous, targetoid, well-defined, ulcerated plaque with central necrosis on the left side of the chest. Notably, we observed an isolated bulla with an erythematous base within the right antecubital fossa at the site of intravenous placement, suggesting pathergy.
Multiple punch biopsies revealed an ulcer with an underlying dense neutrophilic infiltrate within the dermis and subcutaneous tissues (Figure 2). Grocott-Gomori methenamine-silver, periodic acid–Schiff, and acid-fast bacillus stains were all negative for organisms. Tissue cultures for bacterial, fungal, and acid-fast bacilli revealed no growth. Due to the rapidly expanding nature of the plaque and the possibility of infection despite negative microbial stains and cultures, the patient was scheduled for surgical debridement by the surgical team.
Opportunely, after thoughtful consideration of the clinical history, histopathology, and negative tissue cultures, we made a diagnosis of PG, a condition that would have been further exacerbated by debridement and unimproved with antibiotics. Based on our recommendation, the patient received immunosuppressive treatment with prednisone 60 mg/d and triamcinolone ointment 0.1%. He experienced immediate clinical improvement, allowing him to be discharged to the care of dermatology as an outpatient. He continued to receive a monthly rituximab infusion. We intentionally tapered the patient’s prednisone dosage slowly over 4 months and photodocumented steady improvement with eventual resolution of the PG (Figure 1B).
Comment
Pathogenesis of PG
Pyoderma gangrenosum lies in the spectrum of neutrophilic dermatoses, which are characterized histologically by a pandermal neutrophilic infiltrate without evidence of an infectious cause or true vasculitis. Clinically, PG typically presents as a steadily expanding ulceration with an undermined or slightly raised border, and often is associated with the pathergy phenomenon. Historically, PG is classically linked to inflammatory bowel disease; however, association with underlying malignancy, including acute myelogenous leukemia, chronic myelogenous leukemia, myeloma, and myeloid metaplasia, also has been described.5
Pathogenesis of CLL
Chronic lymphocytic leukemia represents the most prevalent form of leukemia in US adults, with the second highest annual incidence.6 Cutaneous findings are seen in 25% of patients with CLL, varying from leukemia cutis to secondary findings such as vasculitis, purpura, generalized pruritus, exfoliative erythroderma, paraneoplastic pemphigus, infections, and rarely neutrophilic dermatoses.7 According to a PubMed search of articles indexed for MEDLINE using the term pyoderma gangrenosum in CLL, only 4 cases of PG occurring in the setting of CLL exist in the literature, with 1 case demonstrating development after a surgical procedure, making ours the second such case (Table).1-4
Diagnosis
Making the diagnosis of a neutrophilic dermatosis such as PG or Sweet syndrome (SS) in the hospital setting is not only difficult but also imperative, considering that the counterdiagnosis more often is an infectious process. The distinction between individual neutrophilic dermatoses is less crucial at the onset because the initial treatment is the same.
Sweet syndrome is classically the most challenging entity within the spectrum to differentiate from PG. However, our case outlines several key distinguishing features:
• The lesion in classic PG is a rapidly expanding ulceration with undermined borders, whereas SS is less commonly associated with ulceration and instead classically presents with multiple edematous papules that progress to juicy plaques.8
• The pathergy phenomenon has been reported in SS, though it is more commonly associated with PG.9
• In reported cases of SS that were related to cutaneous trauma, lesions developed outside the area of trauma and there was documented leukocytosis and neutrophilia.10-14
• Although leukocytosis is part of the minor diagnostic criteria for SS, it is not required for the diagnosis of PG. Considering that our patient had ulcerated lesions, lesions only at the site of trauma, and leukopenia with intermittent neutropenia, the diagnosis was consistent with PG.
The primary value of early recognition and diagnosis of PG lies in the physician’s ability to distinguish PG from an infectious process, which can be challenging in an immunosuppressed patient with an underlying hematologic malignancy.
Conclusion
This case report represents our experience in arriving at the correct diagnosis of PG in a febrile neutrophilic patient with CLL. In the case of PG in a complicated patient, it is critical to initiate appropriate treatment and avoid inappropriate therapies. Aggressive surgical debridement could have resulted in a fatal outcome for our patient, highlighting the need for dermatologists to raise physician awareness of this challenging disease.
Acknowledgments
The authors acknowledge the contributions of Sarah Shalin, MD, PhD; Nikhil Meena, MD; and Aditya Chada, MD (all from Little Rock, Arkansas), for excellent patient care.
- Solovan C, Smiszek R, Wickenhauser C, et al. Postoperative pyoderma gangrenosum in association with renal cell carcinoma and chronic lymphocytic leukemia. Infect Dis Ther. 2013;2:75-80.
- Sławińska M, Barańska-Rybak W, Sobjanek M, et al. Ibrutinib-induced pyoderma gangrenosum. Pol Arch Med Wewn. 2016;126:710-711.
- Swale VJ, Saha M, Kapur N, et al. Pyoderma gangrenosum outside the context of inflammatory bowel disease treated successfully with infliximab. Clin Exp Dermatol. 2005;30:134-136.
- Wong SM, McComish J, Douglass J, et al. Rare skin manifestations successfully treated with primary B-cell chronic lymphocytic leukemia treatment. J Cutan Pathol. 2016;43:552-555.
- Jockenhöfer F, Herberger K, Schaller J, et al. Tricenter analysis of cofactors and comorbidity in patients with pyoderma gangrenosum. J Dtsch Dermatol Ges. 2016;14:1023-1030.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. 7.
- Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855-865.
- Beasley JM, Sluzevich JC. A recurrent vesiculobullous eruption on the head, trunk, and extremities. Bullous Sweet’s syndrome. Int J Dermatol. 2016;55:149-150.
- Awan F, Hamadani M, Devine S. Paraneoplastic Sweet’s syndrome and the pathergy phenomenon. Ann Hematol. 2007;86:613-614.
- de Moya MA, Wong JT, Kroshinsky D, et al. Case records of the Massachusetts General Hospital. Case 28-2012. A 30-year-old woman with shock and abdominal-wall necrosis after cesarean section. N Engl J Med. 2012;367:1046-1057.
- Minocha R, Sebaratnam DF, Choi JY. Sweet’s syndrome following surgery: cutaneous trauma as a possible aetiological co-factor in neutrophilic dermatoses. Australas J Dermatol. 2015;56:
e74-e76. - Phua YS, Al-Ani SA, She RB, et al. Sweet’s syndrome triggered by scalding: a case study and review of the literature. Burns. 2010;36:e49-e52.
- Schwarz RE, Quinn MA, Molina A. Acute postoperative dermatosis at the site of the electrocautery pad: sweet diagnosis of a burning issue. Surg Today. 2000;30:207-209.
- Tan AW, Tan HH, Lim PL. Bullous Sweet’s syndrome following influenza vaccination in a HIV-infected patient. Int J Dermatol. 2006;45:1254-1255.
- Solovan C, Smiszek R, Wickenhauser C, et al. Postoperative pyoderma gangrenosum in association with renal cell carcinoma and chronic lymphocytic leukemia. Infect Dis Ther. 2013;2:75-80.
- Sławińska M, Barańska-Rybak W, Sobjanek M, et al. Ibrutinib-induced pyoderma gangrenosum. Pol Arch Med Wewn. 2016;126:710-711.
- Swale VJ, Saha M, Kapur N, et al. Pyoderma gangrenosum outside the context of inflammatory bowel disease treated successfully with infliximab. Clin Exp Dermatol. 2005;30:134-136.
- Wong SM, McComish J, Douglass J, et al. Rare skin manifestations successfully treated with primary B-cell chronic lymphocytic leukemia treatment. J Cutan Pathol. 2016;43:552-555.
- Jockenhöfer F, Herberger K, Schaller J, et al. Tricenter analysis of cofactors and comorbidity in patients with pyoderma gangrenosum. J Dtsch Dermatol Ges. 2016;14:1023-1030.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. 7.
- Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855-865.
- Beasley JM, Sluzevich JC. A recurrent vesiculobullous eruption on the head, trunk, and extremities. Bullous Sweet’s syndrome. Int J Dermatol. 2016;55:149-150.
- Awan F, Hamadani M, Devine S. Paraneoplastic Sweet’s syndrome and the pathergy phenomenon. Ann Hematol. 2007;86:613-614.
- de Moya MA, Wong JT, Kroshinsky D, et al. Case records of the Massachusetts General Hospital. Case 28-2012. A 30-year-old woman with shock and abdominal-wall necrosis after cesarean section. N Engl J Med. 2012;367:1046-1057.
- Minocha R, Sebaratnam DF, Choi JY. Sweet’s syndrome following surgery: cutaneous trauma as a possible aetiological co-factor in neutrophilic dermatoses. Australas J Dermatol. 2015;56:
e74-e76. - Phua YS, Al-Ani SA, She RB, et al. Sweet’s syndrome triggered by scalding: a case study and review of the literature. Burns. 2010;36:e49-e52.
- Schwarz RE, Quinn MA, Molina A. Acute postoperative dermatosis at the site of the electrocautery pad: sweet diagnosis of a burning issue. Surg Today. 2000;30:207-209.
- Tan AW, Tan HH, Lim PL. Bullous Sweet’s syndrome following influenza vaccination in a HIV-infected patient. Int J Dermatol. 2006;45:1254-1255.
Practice Points
- The primary value of early recognition and diagnosis of pyoderma gangrenosum (PG) lies in the physician’s ability to distinguish PG from an infectious process.
- Surgical debridement would further exacerbate PG, making proper diagnosis of a neutrophilic dermatosis of paramount importance to avoid treatments that could have grave consequences in the misdiagnosed patient.
- Cutaneous findings are seen in one-quarter of patients with chronic lymphocytic leukemia.
- Pyoderma gangrenosum is commonly associated with inflammatory bowel disease but also can be seen in many hematologic malignancies. Physicians should be aware of this association to ensure these patients are diagnosed properly.
Benefits and drawbacks found for risk-based lung cancer screening tools
Risk-based lung cancer screening tools can prevent significantly more lung cancer deaths than the current United States Preventive Services Task Force recommendations, but life-year gains were negligible or reduced and patients would experience greater overdiagnosis, according to new research.
“Current guidelines propose screening eligibility using age and smoking-related criteria, through combinations of accumulated pack-years and years since smoking cessation,” Kevin ten Haaf, PhD, of the department of public health at Erasmus Medical Center in Rotterdam, the Netherlands, and associates wrote in the Journal of the National Cancer Institute. “The USPSTF recommends annual screening between the ages of 55 and 80 years for current and former smokers (quit less than 15 years) who smoked 30 or more pack-years.”
Individual risk assessment utilizing established lung cancer risk–prediction models may have some superiority over pack-years in identifying those most likely to benefit from screening, they wrote, because the models incorporate smoking history in greater detail and consider risk factors such as chronic obstructive pulmonary disease.
Three risk-assessment models were used for the study, in addition to the USPSTF guidelines: the Bach, PLCOm2012, and Lung Cancer Death Risk Assessment Tool (LCDRAT). The study population was a simulated 1950 U.S. cohort from the Smoking History Generator aged between 55 years and 80 years; each simulated smoking history consists of whether and when the person initiates and ceases smoking, average number of cigarettes smoked per day by age, and the age of death from non–lung cancer causes.
The number of lung cancer deaths averted was significantly higher in the risk-based models, compared with the USPSTF recommendations (Bach, 693 per 100,000 population; PLCOm2012, 698 per 100,000 population; LCDRAT, 696 per 100,000 population; USPSTF, 613 per 100,000 population).
However, life-years gained was only modestly higher in the models, compared with the guideline (Bach, 8,660 per 100,000 life-years; PLCOm2012, 8,862 per 100,000 life-years; LCDRAT, 8,631 per 100,000 life-years; USPSTF, 8,590 per 100,000 life-years). In addition, life-years gained for every lung cancer death prevented was greater in the guideline (14.0 years) than in the risk-based models (12.1-12.4 years).
Overdiagnosis was also more common using risk-based tools (Bach, 149 per 100,000; PLCOm2012, 147 per 100,000; LCDRAT, 150 per 100,000; USPSTF, 115 per 100,000). This was mainly because of eligibility for risk-based screening tools increasing with age, the investigators noted.
According to a sensitivity analysis, risk-based models would retain the life-years gained by the USPSTF model if individuals with limited life expectancies (less than 5 years) were excluded. This would also reduce overdiagnosis by 65.3%.
“Future studies should investigate the cost-effectiveness of risk-based screening and the potential for reducing overdiagnosis in high-risk individuals,” the investigators concluded.
One coauthor developed the PLCOm2012 model, but the model is available free to noncommercial users, and the investigator has received no money from its usage. No other conflicts of interest were reported.
SOURCE: ten Haaf K et al. J Natl Cancer Inst. 2019 Nov 29. doi: 10.1093/jnci/djz164.
Risk-based lung cancer screening tools can prevent significantly more lung cancer deaths than the current United States Preventive Services Task Force recommendations, but life-year gains were negligible or reduced and patients would experience greater overdiagnosis, according to new research.
“Current guidelines propose screening eligibility using age and smoking-related criteria, through combinations of accumulated pack-years and years since smoking cessation,” Kevin ten Haaf, PhD, of the department of public health at Erasmus Medical Center in Rotterdam, the Netherlands, and associates wrote in the Journal of the National Cancer Institute. “The USPSTF recommends annual screening between the ages of 55 and 80 years for current and former smokers (quit less than 15 years) who smoked 30 or more pack-years.”
Individual risk assessment utilizing established lung cancer risk–prediction models may have some superiority over pack-years in identifying those most likely to benefit from screening, they wrote, because the models incorporate smoking history in greater detail and consider risk factors such as chronic obstructive pulmonary disease.
Three risk-assessment models were used for the study, in addition to the USPSTF guidelines: the Bach, PLCOm2012, and Lung Cancer Death Risk Assessment Tool (LCDRAT). The study population was a simulated 1950 U.S. cohort from the Smoking History Generator aged between 55 years and 80 years; each simulated smoking history consists of whether and when the person initiates and ceases smoking, average number of cigarettes smoked per day by age, and the age of death from non–lung cancer causes.
The number of lung cancer deaths averted was significantly higher in the risk-based models, compared with the USPSTF recommendations (Bach, 693 per 100,000 population; PLCOm2012, 698 per 100,000 population; LCDRAT, 696 per 100,000 population; USPSTF, 613 per 100,000 population).
However, life-years gained was only modestly higher in the models, compared with the guideline (Bach, 8,660 per 100,000 life-years; PLCOm2012, 8,862 per 100,000 life-years; LCDRAT, 8,631 per 100,000 life-years; USPSTF, 8,590 per 100,000 life-years). In addition, life-years gained for every lung cancer death prevented was greater in the guideline (14.0 years) than in the risk-based models (12.1-12.4 years).
Overdiagnosis was also more common using risk-based tools (Bach, 149 per 100,000; PLCOm2012, 147 per 100,000; LCDRAT, 150 per 100,000; USPSTF, 115 per 100,000). This was mainly because of eligibility for risk-based screening tools increasing with age, the investigators noted.
According to a sensitivity analysis, risk-based models would retain the life-years gained by the USPSTF model if individuals with limited life expectancies (less than 5 years) were excluded. This would also reduce overdiagnosis by 65.3%.
“Future studies should investigate the cost-effectiveness of risk-based screening and the potential for reducing overdiagnosis in high-risk individuals,” the investigators concluded.
One coauthor developed the PLCOm2012 model, but the model is available free to noncommercial users, and the investigator has received no money from its usage. No other conflicts of interest were reported.
SOURCE: ten Haaf K et al. J Natl Cancer Inst. 2019 Nov 29. doi: 10.1093/jnci/djz164.
Risk-based lung cancer screening tools can prevent significantly more lung cancer deaths than the current United States Preventive Services Task Force recommendations, but life-year gains were negligible or reduced and patients would experience greater overdiagnosis, according to new research.
“Current guidelines propose screening eligibility using age and smoking-related criteria, through combinations of accumulated pack-years and years since smoking cessation,” Kevin ten Haaf, PhD, of the department of public health at Erasmus Medical Center in Rotterdam, the Netherlands, and associates wrote in the Journal of the National Cancer Institute. “The USPSTF recommends annual screening between the ages of 55 and 80 years for current and former smokers (quit less than 15 years) who smoked 30 or more pack-years.”
Individual risk assessment utilizing established lung cancer risk–prediction models may have some superiority over pack-years in identifying those most likely to benefit from screening, they wrote, because the models incorporate smoking history in greater detail and consider risk factors such as chronic obstructive pulmonary disease.
Three risk-assessment models were used for the study, in addition to the USPSTF guidelines: the Bach, PLCOm2012, and Lung Cancer Death Risk Assessment Tool (LCDRAT). The study population was a simulated 1950 U.S. cohort from the Smoking History Generator aged between 55 years and 80 years; each simulated smoking history consists of whether and when the person initiates and ceases smoking, average number of cigarettes smoked per day by age, and the age of death from non–lung cancer causes.
The number of lung cancer deaths averted was significantly higher in the risk-based models, compared with the USPSTF recommendations (Bach, 693 per 100,000 population; PLCOm2012, 698 per 100,000 population; LCDRAT, 696 per 100,000 population; USPSTF, 613 per 100,000 population).
However, life-years gained was only modestly higher in the models, compared with the guideline (Bach, 8,660 per 100,000 life-years; PLCOm2012, 8,862 per 100,000 life-years; LCDRAT, 8,631 per 100,000 life-years; USPSTF, 8,590 per 100,000 life-years). In addition, life-years gained for every lung cancer death prevented was greater in the guideline (14.0 years) than in the risk-based models (12.1-12.4 years).
Overdiagnosis was also more common using risk-based tools (Bach, 149 per 100,000; PLCOm2012, 147 per 100,000; LCDRAT, 150 per 100,000; USPSTF, 115 per 100,000). This was mainly because of eligibility for risk-based screening tools increasing with age, the investigators noted.
According to a sensitivity analysis, risk-based models would retain the life-years gained by the USPSTF model if individuals with limited life expectancies (less than 5 years) were excluded. This would also reduce overdiagnosis by 65.3%.
“Future studies should investigate the cost-effectiveness of risk-based screening and the potential for reducing overdiagnosis in high-risk individuals,” the investigators concluded.
One coauthor developed the PLCOm2012 model, but the model is available free to noncommercial users, and the investigator has received no money from its usage. No other conflicts of interest were reported.
SOURCE: ten Haaf K et al. J Natl Cancer Inst. 2019 Nov 29. doi: 10.1093/jnci/djz164.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Managing preterm birth in those at risk: Expert strategies
Obstetricians face the potential practice dilemma of having withdrawn from the market the only drug approved by the US Food and Drug Administration (FDA) for the prevention of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. In the recently published PROLONG (Progestin's Role in Optimizing Neonatal Gestation) study by Blackwell and colleagues, the trial results revealed that there were no significant differences in preterm birth between women treated with 17 α-hydroxyprogesterone caproate (17P; Makena) and those who received placebo.1 For study details and comments, see "Progesterone supplementation does not PROLONG pregnancy in women at risk for preterm birth: What do we do now?" by Michael House, MD, and Errol Norwitz, MD, PhD, MBA. Subsequently, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to recommend pursuit of approval withdrawal for 17P.
To assess how experienced obstetricians would manage women with previous preterm birth if 17P became unavailable, OBG Management conducted an informal survey. Here, 4 experts respond to the question, "What are you going to do in your practice for women with a history of a previous preterm birth if 17P is no longer an option?"
Not ready to leave behind 17P for recurrent preterm delivery
Patrick Duff, MD
Preterm delivery is arguably the most important problem in perinatal medicine. It occurs in 10% to 12% of all obstetric patients in the United States, and complications of prematurity account for the majority of neonatal deaths. A major risk factor for recurrent preterm delivery is a prior history of spontaneous preterm delivery, with or without preterm premature rupture of membranes. Clearly, prevention of recurrence is of paramount importance.
In the Maternal-Fetal Medicine Units (MFMU) Network trial, Meis and colleagues demonstrated a 34% reduction (relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81) in the risk of recurrent preterm delivery in women who received weekly 250-mg injections of 17P (also called 17-OHPC). After publication of that trial, use of 17P became accepted practice in the United States.2
The PROLONG study by Blackwell and colleagues questions the value of 17P.1 In that international trial, which included 1,708 women from 41 centers in the United States and 52 outside the United States, the authors were unable to show any significant difference in the frequency of preterm delivery < 35 weeks (11.0% in the women receiving 17P and 11.5% in women receiving placebo; RR, 0.95; 95% CI, 0.71-1.26). Even when they examined the subset of women treated at US medical centers, they could not demonstrate any significant difference in treatment outcome.
At least 2 major explanations account for the discrepancy between the MFMU and the Blackwell studies. First, the participants in the PROLONG trial were clearly not at the same increased risk for recurrent preterm delivery as those in the MFMU trial. Second, in the PROLONG trial only the minority of participants were from the United States. In fact, given the relatively low rate of recurrent preterm delivery in the PROLONG trial, the study was underpowered to detect meaningful differences in maternal outcome. Therefore, I am not ready to abandon the use of progesterone supplementation in women at risk for recurrent preterm delivery.
Continue to: If the FDA removes 17P from the market...
If the FDA removes 17P from the market, my approach with at-risk patients will be as follows:
- I will encourage all at-risk women to eliminate obvious risk factors, such as smoking, illicit drug use, and excessive physical activity.
- I will encourage optimal nutrition and appropriate weight gain.
- I will test all patients for chlamydia, gonorrhea, and bacterial vaginosis and treat women who are infected.
- After the patient completes the first trimester, I will treat her with micronized progesterone, 200 mg daily, intravaginally. I will continue this medication until 36 to 37 weeks.
- I will perform an assessment of cervical length at 16, 20, and 24 weeks' gestation. In patients with demonstrable cervical shortening, I will perform a cerclage.
Rational management options for reducing risk of preterm delivery
Alex C. Vidaeff, MD, MPH
Most women who experience a spontaneous preterm delivery (sPTD) do not deliver prematurely in subsequent pregnancies.3 Two recent systematic reviews, in 2014 and 2017, found an overall risk of recurrent sPTD of 20.2% and 30%, respectively.4,5 These numbers are closer to the background event rate of 21.9% in the PROLONG trial, while only a few women have a recurrence risk of more than 50%, as in the Meis MFMU trial.1,2 A public health recommendation cannot be made for an intervention that is expected to work only in rare cases and fail in a majority of cases. Therefore, 17P is no longer a viable option for preventing recurrence in pregnant women with a history of sPTD, with only rare possible exceptions.
What evidence-based alternatives can be offered to pregnant women who had a previous sPTD?
Ultrasound assessment of cervical length has emerged as an effective prognosticator for recurrence in women with a prior sPTD, being able to predict 65.4% of sPTDs at a false-positive rate of 5%.6,7 Furthermore, sonographic cervical length measurements identify high-risk women who may not need any intervention. It has been shown that, among women with prior sPTD who maintain a normal cervical length up to 24 weeks, more than 90% will deliver at 35 weeks or after without intervention.8
In the United States, interventions to reduce sPTD, once a short cervix has been identified, include vaginal progesterone supplementation and cerclage. The benefit from vaginal progesterone has been documented by an individual patient data meta-analysis, while the benefit of cerclage has been highlighted in a Cochrane Review.9,10 The results of an adjusted indirect comparison meta-analysis suggest that both interventions are equally effective.11 Therefore, the decision on how best to minimize the risk of recurrent sPTD must be individualized based on historical and clinical circumstances, as well as the woman's informed choice.
Based on current data, the following approach appears rational to me:
- Cervical ultrasound surveillance between 16 and 24 weeks' gestation to identify the subgroup of women at significantly increased risk of sPTD recurrence.
- With cervical length ≤ 25 mm, vaginal progesterone supplementation may be considered. Preferential consideration for progesterone may be given when lower genital tract inflammation is suspected, given the possible anti-inflammatory action of progesterone.12,13
- If cervical shortening progresses to 15 to 20 mm, cerclage may be considered. Waiting for a cervix < 15 mm may be unadvisable. In conditions of a very short cervix, frequently dilated, with exposure of the fetal membranes, ascending subclinical intra-amniotic infection already may be present, reducing the efficacy of cerclage. Preferential consideration for cerclage also may be given with 2 sPTDs or mid-trimester losses or with a history of a successful cerclage.
Continue to: Screen cervical length early, and use cerclage or vaginal progesterone as appropriate...
Screen cervical length early, and use cerclage or vaginal progesterone as appropriate
Michael G. Ross, MD, MPH
In patients with a history of a previous preterm birth, if 17P is no longer an option, I would revert to screening for short cervix with transvaginal ultrasound.
Screen all high-risk patients at the first prenatal visit, so as not to miss a short cervix before 16 weeks' gestation. Then, beginning at 16 weeks, screen every 2 weeks until approximately 24 weeks.
If the cervix shortens to 25 mm or less, offer cerclage or vaginal progesterone. If the cervix shortens to 20 mm or less, I would strongly support cerclage or vaginal progesterone.
Use of 17P is still an option, for now
Errol R. Norwitz, MD, PhD, MBA
The way in which 17P was handled by the FDA is exactly the way the system is designed to work; this should be seen as a success, not a failure.
Given the urgent need for an intervention to prevent preterm birth, the lack of any alternative, and a single, well-designed randomized controlled trial that confirmed safety and suggested some benefit, the FDA approved 17P supplementation in February 2011 for a limited indication only—one or more prior unexplained sPTD—using the expedited review mechanism.2 Under this mechanism, a follow-up clinical trial is required to confirm efficacy. This was the PROLONG trial, which failed to show any significant benefit of 17P supplementation in terms of either preterm birth prevention or neonatal outcome.1
In October 2019, an FDA advisory committee met again to review these and other data. After presentations from a range of stakeholders and a robust discussion, the advisory committee voted to pursue approval withdrawal of 17P due to the lack of consistent evidence of benefit (it is important to note that this was not because of safety concerns). This is exactly the way the process is designed to work.
Where does this leave physicians and patients? It is clear that progesterone supplementation is not a panacea for preterm birth prevention and is not indicated for all women at high risk, even those with one or more prior unexplained sPTDs. Given that preterm birth is a syndrome and not a single diagnosis, it is still possible that there is a subgroup of women who may benefit from this intervention. For this reason—and because there is no clear alternative and no known downside to the administration of this drug (other than cost)—physicians still may choose to discuss this option with their patients and, after counseling, patients still may choose to accept it. If in doubt, engage the "shared decision-making model"; talk to your patients.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Iams JD, Goldenberg RL, Mercer BM, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units. The Preterm Prediction Study: recurrence of spontaneous preterm birth. Am J Obstet Gynecol. 1998;178:1035-1040.
- Kazemier BM, Buijs PE, Mignini L, et al; EBM CONNECT. Impact of obstetric history on the risk of spontaneous preterm birth in singleton and multiple pregnancies: a systematic review. BJOG. 2014;121:1197-1208.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Owen J, Yost N, Berghella V, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340-1348.
- To MS, Skentou CA, Royston P, et al. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362-367.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Sakai M, Shiozaki A, Tabata M, et al. Evaluation of effectiveness of prophylactic cerclage of a short cervix according to interleukin-8 in cervical mucus. Am J Obstet Gynecol. 2006;194:14-19.
- Vidaeff AC, Ramin SM, Gilstrap LC, et al. Impact of progesterone on cytokine-stimulated nuclear factor-kappa B signaling in HeLa cells. J Matern Fetal Neonatal Med. 2007;20:23-28.
Obstetricians face the potential practice dilemma of having withdrawn from the market the only drug approved by the US Food and Drug Administration (FDA) for the prevention of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. In the recently published PROLONG (Progestin's Role in Optimizing Neonatal Gestation) study by Blackwell and colleagues, the trial results revealed that there were no significant differences in preterm birth between women treated with 17 α-hydroxyprogesterone caproate (17P; Makena) and those who received placebo.1 For study details and comments, see "Progesterone supplementation does not PROLONG pregnancy in women at risk for preterm birth: What do we do now?" by Michael House, MD, and Errol Norwitz, MD, PhD, MBA. Subsequently, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to recommend pursuit of approval withdrawal for 17P.
To assess how experienced obstetricians would manage women with previous preterm birth if 17P became unavailable, OBG Management conducted an informal survey. Here, 4 experts respond to the question, "What are you going to do in your practice for women with a history of a previous preterm birth if 17P is no longer an option?"
Not ready to leave behind 17P for recurrent preterm delivery
Patrick Duff, MD
Preterm delivery is arguably the most important problem in perinatal medicine. It occurs in 10% to 12% of all obstetric patients in the United States, and complications of prematurity account for the majority of neonatal deaths. A major risk factor for recurrent preterm delivery is a prior history of spontaneous preterm delivery, with or without preterm premature rupture of membranes. Clearly, prevention of recurrence is of paramount importance.
In the Maternal-Fetal Medicine Units (MFMU) Network trial, Meis and colleagues demonstrated a 34% reduction (relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81) in the risk of recurrent preterm delivery in women who received weekly 250-mg injections of 17P (also called 17-OHPC). After publication of that trial, use of 17P became accepted practice in the United States.2
The PROLONG study by Blackwell and colleagues questions the value of 17P.1 In that international trial, which included 1,708 women from 41 centers in the United States and 52 outside the United States, the authors were unable to show any significant difference in the frequency of preterm delivery < 35 weeks (11.0% in the women receiving 17P and 11.5% in women receiving placebo; RR, 0.95; 95% CI, 0.71-1.26). Even when they examined the subset of women treated at US medical centers, they could not demonstrate any significant difference in treatment outcome.
At least 2 major explanations account for the discrepancy between the MFMU and the Blackwell studies. First, the participants in the PROLONG trial were clearly not at the same increased risk for recurrent preterm delivery as those in the MFMU trial. Second, in the PROLONG trial only the minority of participants were from the United States. In fact, given the relatively low rate of recurrent preterm delivery in the PROLONG trial, the study was underpowered to detect meaningful differences in maternal outcome. Therefore, I am not ready to abandon the use of progesterone supplementation in women at risk for recurrent preterm delivery.
Continue to: If the FDA removes 17P from the market...
If the FDA removes 17P from the market, my approach with at-risk patients will be as follows:
- I will encourage all at-risk women to eliminate obvious risk factors, such as smoking, illicit drug use, and excessive physical activity.
- I will encourage optimal nutrition and appropriate weight gain.
- I will test all patients for chlamydia, gonorrhea, and bacterial vaginosis and treat women who are infected.
- After the patient completes the first trimester, I will treat her with micronized progesterone, 200 mg daily, intravaginally. I will continue this medication until 36 to 37 weeks.
- I will perform an assessment of cervical length at 16, 20, and 24 weeks' gestation. In patients with demonstrable cervical shortening, I will perform a cerclage.
Rational management options for reducing risk of preterm delivery
Alex C. Vidaeff, MD, MPH
Most women who experience a spontaneous preterm delivery (sPTD) do not deliver prematurely in subsequent pregnancies.3 Two recent systematic reviews, in 2014 and 2017, found an overall risk of recurrent sPTD of 20.2% and 30%, respectively.4,5 These numbers are closer to the background event rate of 21.9% in the PROLONG trial, while only a few women have a recurrence risk of more than 50%, as in the Meis MFMU trial.1,2 A public health recommendation cannot be made for an intervention that is expected to work only in rare cases and fail in a majority of cases. Therefore, 17P is no longer a viable option for preventing recurrence in pregnant women with a history of sPTD, with only rare possible exceptions.
What evidence-based alternatives can be offered to pregnant women who had a previous sPTD?
Ultrasound assessment of cervical length has emerged as an effective prognosticator for recurrence in women with a prior sPTD, being able to predict 65.4% of sPTDs at a false-positive rate of 5%.6,7 Furthermore, sonographic cervical length measurements identify high-risk women who may not need any intervention. It has been shown that, among women with prior sPTD who maintain a normal cervical length up to 24 weeks, more than 90% will deliver at 35 weeks or after without intervention.8
In the United States, interventions to reduce sPTD, once a short cervix has been identified, include vaginal progesterone supplementation and cerclage. The benefit from vaginal progesterone has been documented by an individual patient data meta-analysis, while the benefit of cerclage has been highlighted in a Cochrane Review.9,10 The results of an adjusted indirect comparison meta-analysis suggest that both interventions are equally effective.11 Therefore, the decision on how best to minimize the risk of recurrent sPTD must be individualized based on historical and clinical circumstances, as well as the woman's informed choice.
Based on current data, the following approach appears rational to me:
- Cervical ultrasound surveillance between 16 and 24 weeks' gestation to identify the subgroup of women at significantly increased risk of sPTD recurrence.
- With cervical length ≤ 25 mm, vaginal progesterone supplementation may be considered. Preferential consideration for progesterone may be given when lower genital tract inflammation is suspected, given the possible anti-inflammatory action of progesterone.12,13
- If cervical shortening progresses to 15 to 20 mm, cerclage may be considered. Waiting for a cervix < 15 mm may be unadvisable. In conditions of a very short cervix, frequently dilated, with exposure of the fetal membranes, ascending subclinical intra-amniotic infection already may be present, reducing the efficacy of cerclage. Preferential consideration for cerclage also may be given with 2 sPTDs or mid-trimester losses or with a history of a successful cerclage.
Continue to: Screen cervical length early, and use cerclage or vaginal progesterone as appropriate...
Screen cervical length early, and use cerclage or vaginal progesterone as appropriate
Michael G. Ross, MD, MPH
In patients with a history of a previous preterm birth, if 17P is no longer an option, I would revert to screening for short cervix with transvaginal ultrasound.
Screen all high-risk patients at the first prenatal visit, so as not to miss a short cervix before 16 weeks' gestation. Then, beginning at 16 weeks, screen every 2 weeks until approximately 24 weeks.
If the cervix shortens to 25 mm or less, offer cerclage or vaginal progesterone. If the cervix shortens to 20 mm or less, I would strongly support cerclage or vaginal progesterone.
Use of 17P is still an option, for now
Errol R. Norwitz, MD, PhD, MBA
The way in which 17P was handled by the FDA is exactly the way the system is designed to work; this should be seen as a success, not a failure.
Given the urgent need for an intervention to prevent preterm birth, the lack of any alternative, and a single, well-designed randomized controlled trial that confirmed safety and suggested some benefit, the FDA approved 17P supplementation in February 2011 for a limited indication only—one or more prior unexplained sPTD—using the expedited review mechanism.2 Under this mechanism, a follow-up clinical trial is required to confirm efficacy. This was the PROLONG trial, which failed to show any significant benefit of 17P supplementation in terms of either preterm birth prevention or neonatal outcome.1
In October 2019, an FDA advisory committee met again to review these and other data. After presentations from a range of stakeholders and a robust discussion, the advisory committee voted to pursue approval withdrawal of 17P due to the lack of consistent evidence of benefit (it is important to note that this was not because of safety concerns). This is exactly the way the process is designed to work.
Where does this leave physicians and patients? It is clear that progesterone supplementation is not a panacea for preterm birth prevention and is not indicated for all women at high risk, even those with one or more prior unexplained sPTDs. Given that preterm birth is a syndrome and not a single diagnosis, it is still possible that there is a subgroup of women who may benefit from this intervention. For this reason—and because there is no clear alternative and no known downside to the administration of this drug (other than cost)—physicians still may choose to discuss this option with their patients and, after counseling, patients still may choose to accept it. If in doubt, engage the "shared decision-making model"; talk to your patients.
Obstetricians face the potential practice dilemma of having withdrawn from the market the only drug approved by the US Food and Drug Administration (FDA) for the prevention of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. In the recently published PROLONG (Progestin's Role in Optimizing Neonatal Gestation) study by Blackwell and colleagues, the trial results revealed that there were no significant differences in preterm birth between women treated with 17 α-hydroxyprogesterone caproate (17P; Makena) and those who received placebo.1 For study details and comments, see "Progesterone supplementation does not PROLONG pregnancy in women at risk for preterm birth: What do we do now?" by Michael House, MD, and Errol Norwitz, MD, PhD, MBA. Subsequently, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to recommend pursuit of approval withdrawal for 17P.
To assess how experienced obstetricians would manage women with previous preterm birth if 17P became unavailable, OBG Management conducted an informal survey. Here, 4 experts respond to the question, "What are you going to do in your practice for women with a history of a previous preterm birth if 17P is no longer an option?"
Not ready to leave behind 17P for recurrent preterm delivery
Patrick Duff, MD
Preterm delivery is arguably the most important problem in perinatal medicine. It occurs in 10% to 12% of all obstetric patients in the United States, and complications of prematurity account for the majority of neonatal deaths. A major risk factor for recurrent preterm delivery is a prior history of spontaneous preterm delivery, with or without preterm premature rupture of membranes. Clearly, prevention of recurrence is of paramount importance.
In the Maternal-Fetal Medicine Units (MFMU) Network trial, Meis and colleagues demonstrated a 34% reduction (relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81) in the risk of recurrent preterm delivery in women who received weekly 250-mg injections of 17P (also called 17-OHPC). After publication of that trial, use of 17P became accepted practice in the United States.2
The PROLONG study by Blackwell and colleagues questions the value of 17P.1 In that international trial, which included 1,708 women from 41 centers in the United States and 52 outside the United States, the authors were unable to show any significant difference in the frequency of preterm delivery < 35 weeks (11.0% in the women receiving 17P and 11.5% in women receiving placebo; RR, 0.95; 95% CI, 0.71-1.26). Even when they examined the subset of women treated at US medical centers, they could not demonstrate any significant difference in treatment outcome.
At least 2 major explanations account for the discrepancy between the MFMU and the Blackwell studies. First, the participants in the PROLONG trial were clearly not at the same increased risk for recurrent preterm delivery as those in the MFMU trial. Second, in the PROLONG trial only the minority of participants were from the United States. In fact, given the relatively low rate of recurrent preterm delivery in the PROLONG trial, the study was underpowered to detect meaningful differences in maternal outcome. Therefore, I am not ready to abandon the use of progesterone supplementation in women at risk for recurrent preterm delivery.
Continue to: If the FDA removes 17P from the market...
If the FDA removes 17P from the market, my approach with at-risk patients will be as follows:
- I will encourage all at-risk women to eliminate obvious risk factors, such as smoking, illicit drug use, and excessive physical activity.
- I will encourage optimal nutrition and appropriate weight gain.
- I will test all patients for chlamydia, gonorrhea, and bacterial vaginosis and treat women who are infected.
- After the patient completes the first trimester, I will treat her with micronized progesterone, 200 mg daily, intravaginally. I will continue this medication until 36 to 37 weeks.
- I will perform an assessment of cervical length at 16, 20, and 24 weeks' gestation. In patients with demonstrable cervical shortening, I will perform a cerclage.
Rational management options for reducing risk of preterm delivery
Alex C. Vidaeff, MD, MPH
Most women who experience a spontaneous preterm delivery (sPTD) do not deliver prematurely in subsequent pregnancies.3 Two recent systematic reviews, in 2014 and 2017, found an overall risk of recurrent sPTD of 20.2% and 30%, respectively.4,5 These numbers are closer to the background event rate of 21.9% in the PROLONG trial, while only a few women have a recurrence risk of more than 50%, as in the Meis MFMU trial.1,2 A public health recommendation cannot be made for an intervention that is expected to work only in rare cases and fail in a majority of cases. Therefore, 17P is no longer a viable option for preventing recurrence in pregnant women with a history of sPTD, with only rare possible exceptions.
What evidence-based alternatives can be offered to pregnant women who had a previous sPTD?
Ultrasound assessment of cervical length has emerged as an effective prognosticator for recurrence in women with a prior sPTD, being able to predict 65.4% of sPTDs at a false-positive rate of 5%.6,7 Furthermore, sonographic cervical length measurements identify high-risk women who may not need any intervention. It has been shown that, among women with prior sPTD who maintain a normal cervical length up to 24 weeks, more than 90% will deliver at 35 weeks or after without intervention.8
In the United States, interventions to reduce sPTD, once a short cervix has been identified, include vaginal progesterone supplementation and cerclage. The benefit from vaginal progesterone has been documented by an individual patient data meta-analysis, while the benefit of cerclage has been highlighted in a Cochrane Review.9,10 The results of an adjusted indirect comparison meta-analysis suggest that both interventions are equally effective.11 Therefore, the decision on how best to minimize the risk of recurrent sPTD must be individualized based on historical and clinical circumstances, as well as the woman's informed choice.
Based on current data, the following approach appears rational to me:
- Cervical ultrasound surveillance between 16 and 24 weeks' gestation to identify the subgroup of women at significantly increased risk of sPTD recurrence.
- With cervical length ≤ 25 mm, vaginal progesterone supplementation may be considered. Preferential consideration for progesterone may be given when lower genital tract inflammation is suspected, given the possible anti-inflammatory action of progesterone.12,13
- If cervical shortening progresses to 15 to 20 mm, cerclage may be considered. Waiting for a cervix < 15 mm may be unadvisable. In conditions of a very short cervix, frequently dilated, with exposure of the fetal membranes, ascending subclinical intra-amniotic infection already may be present, reducing the efficacy of cerclage. Preferential consideration for cerclage also may be given with 2 sPTDs or mid-trimester losses or with a history of a successful cerclage.
Continue to: Screen cervical length early, and use cerclage or vaginal progesterone as appropriate...
Screen cervical length early, and use cerclage or vaginal progesterone as appropriate
Michael G. Ross, MD, MPH
In patients with a history of a previous preterm birth, if 17P is no longer an option, I would revert to screening for short cervix with transvaginal ultrasound.
Screen all high-risk patients at the first prenatal visit, so as not to miss a short cervix before 16 weeks' gestation. Then, beginning at 16 weeks, screen every 2 weeks until approximately 24 weeks.
If the cervix shortens to 25 mm or less, offer cerclage or vaginal progesterone. If the cervix shortens to 20 mm or less, I would strongly support cerclage or vaginal progesterone.
Use of 17P is still an option, for now
Errol R. Norwitz, MD, PhD, MBA
The way in which 17P was handled by the FDA is exactly the way the system is designed to work; this should be seen as a success, not a failure.
Given the urgent need for an intervention to prevent preterm birth, the lack of any alternative, and a single, well-designed randomized controlled trial that confirmed safety and suggested some benefit, the FDA approved 17P supplementation in February 2011 for a limited indication only—one or more prior unexplained sPTD—using the expedited review mechanism.2 Under this mechanism, a follow-up clinical trial is required to confirm efficacy. This was the PROLONG trial, which failed to show any significant benefit of 17P supplementation in terms of either preterm birth prevention or neonatal outcome.1
In October 2019, an FDA advisory committee met again to review these and other data. After presentations from a range of stakeholders and a robust discussion, the advisory committee voted to pursue approval withdrawal of 17P due to the lack of consistent evidence of benefit (it is important to note that this was not because of safety concerns). This is exactly the way the process is designed to work.
Where does this leave physicians and patients? It is clear that progesterone supplementation is not a panacea for preterm birth prevention and is not indicated for all women at high risk, even those with one or more prior unexplained sPTDs. Given that preterm birth is a syndrome and not a single diagnosis, it is still possible that there is a subgroup of women who may benefit from this intervention. For this reason—and because there is no clear alternative and no known downside to the administration of this drug (other than cost)—physicians still may choose to discuss this option with their patients and, after counseling, patients still may choose to accept it. If in doubt, engage the "shared decision-making model"; talk to your patients.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Iams JD, Goldenberg RL, Mercer BM, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units. The Preterm Prediction Study: recurrence of spontaneous preterm birth. Am J Obstet Gynecol. 1998;178:1035-1040.
- Kazemier BM, Buijs PE, Mignini L, et al; EBM CONNECT. Impact of obstetric history on the risk of spontaneous preterm birth in singleton and multiple pregnancies: a systematic review. BJOG. 2014;121:1197-1208.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Owen J, Yost N, Berghella V, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340-1348.
- To MS, Skentou CA, Royston P, et al. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362-367.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Sakai M, Shiozaki A, Tabata M, et al. Evaluation of effectiveness of prophylactic cerclage of a short cervix according to interleukin-8 in cervical mucus. Am J Obstet Gynecol. 2006;194:14-19.
- Vidaeff AC, Ramin SM, Gilstrap LC, et al. Impact of progesterone on cytokine-stimulated nuclear factor-kappa B signaling in HeLa cells. J Matern Fetal Neonatal Med. 2007;20:23-28.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Iams JD, Goldenberg RL, Mercer BM, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units. The Preterm Prediction Study: recurrence of spontaneous preterm birth. Am J Obstet Gynecol. 1998;178:1035-1040.
- Kazemier BM, Buijs PE, Mignini L, et al; EBM CONNECT. Impact of obstetric history on the risk of spontaneous preterm birth in singleton and multiple pregnancies: a systematic review. BJOG. 2014;121:1197-1208.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Owen J, Yost N, Berghella V, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340-1348.
- To MS, Skentou CA, Royston P, et al. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362-367.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Sakai M, Shiozaki A, Tabata M, et al. Evaluation of effectiveness of prophylactic cerclage of a short cervix according to interleukin-8 in cervical mucus. Am J Obstet Gynecol. 2006;194:14-19.
- Vidaeff AC, Ramin SM, Gilstrap LC, et al. Impact of progesterone on cytokine-stimulated nuclear factor-kappa B signaling in HeLa cells. J Matern Fetal Neonatal Med. 2007;20:23-28.
DoD Undertakes New Efforts to Measure and Address Tobacco, Substance Use Disorders
NATIONAL HARBOR, MD—Tobacco, alcohol and prescription drug misuses, and illicit drug use cost the US Department of Defense (DoD) billions in direct health care expenditures and have additional impact as they inhibit force readiness, according to researchers from the Defense Health Agency. To date the DoD has struggled to understand the scope of the problems, although in most cases the researchers believe it is less than that of the general population.
Tobacco use and its related health problems have been linked to higher drop-out rates in basic training, poorer visual acuity, and a higher rate of absenteeism. Financially, tobacco use also extracts a significant cost: an estimated $1.7 billion in additional medical costs to the DoD, including $1 billion for prime beneficiaries and up to $700 million for standard beneficiaries. About $80 million is the result of secondhand smoke, and $5 million is derived from smokeless forms of tobacco. Also the DoD estimated that the cost in loss of productivity and fire injuries for service members is about $63 million.
Alcohol misuse also represents a significant challenge to military health and readiness, costing the DoD $1.3 billion in a 2014 estimate that included $73 million in loss of productivity. In the case of alcohol, the social impact was likely even higher. In fiscal year 2017, the DoD estimated that alcohol was a factor in one-third of attempted suicides. According to the DoD’s 2018 Workforce and Gender Relations survey of active-duty service members, alcohol also was a factor in 62% sexual assaults of women and 49% of sexual assaults of men.
Prescription drug misuse and Illicit drug use are less common, with lower rates than those found in the general population. Cocaine and marijuana are the most common drugs, while pain relievers are the most common prescription drugs being misused.
Service members face a number of risk factors for substance use disorders (SUDs), including service-related injuries, demands of active duty, poor relationships with commanders, separation from family members, psychiatric distress, and boredom. A particular challenge for the Military Health System is getting service members to seek help for SUDs. Many service members may be concerned over actual and perceived consequences for seeking treatment for a SUD, the stigma associated with SUDs, and negative associations with seeking treatment.
In response, the DHA has developed online health education campaigns to complement ongoing service-level programs and policy initiatives. Both You Can Quit 2 for tobacco and Own Your Limits for alcohol include live chats, educational materials, quizzes, and other interactive elements and allow service members to access the resources anonymously. The DoD plans to study their effectiveness.
NATIONAL HARBOR, MD—Tobacco, alcohol and prescription drug misuses, and illicit drug use cost the US Department of Defense (DoD) billions in direct health care expenditures and have additional impact as they inhibit force readiness, according to researchers from the Defense Health Agency. To date the DoD has struggled to understand the scope of the problems, although in most cases the researchers believe it is less than that of the general population.
Tobacco use and its related health problems have been linked to higher drop-out rates in basic training, poorer visual acuity, and a higher rate of absenteeism. Financially, tobacco use also extracts a significant cost: an estimated $1.7 billion in additional medical costs to the DoD, including $1 billion for prime beneficiaries and up to $700 million for standard beneficiaries. About $80 million is the result of secondhand smoke, and $5 million is derived from smokeless forms of tobacco. Also the DoD estimated that the cost in loss of productivity and fire injuries for service members is about $63 million.
Alcohol misuse also represents a significant challenge to military health and readiness, costing the DoD $1.3 billion in a 2014 estimate that included $73 million in loss of productivity. In the case of alcohol, the social impact was likely even higher. In fiscal year 2017, the DoD estimated that alcohol was a factor in one-third of attempted suicides. According to the DoD’s 2018 Workforce and Gender Relations survey of active-duty service members, alcohol also was a factor in 62% sexual assaults of women and 49% of sexual assaults of men.
Prescription drug misuse and Illicit drug use are less common, with lower rates than those found in the general population. Cocaine and marijuana are the most common drugs, while pain relievers are the most common prescription drugs being misused.
Service members face a number of risk factors for substance use disorders (SUDs), including service-related injuries, demands of active duty, poor relationships with commanders, separation from family members, psychiatric distress, and boredom. A particular challenge for the Military Health System is getting service members to seek help for SUDs. Many service members may be concerned over actual and perceived consequences for seeking treatment for a SUD, the stigma associated with SUDs, and negative associations with seeking treatment.
In response, the DHA has developed online health education campaigns to complement ongoing service-level programs and policy initiatives. Both You Can Quit 2 for tobacco and Own Your Limits for alcohol include live chats, educational materials, quizzes, and other interactive elements and allow service members to access the resources anonymously. The DoD plans to study their effectiveness.
NATIONAL HARBOR, MD—Tobacco, alcohol and prescription drug misuses, and illicit drug use cost the US Department of Defense (DoD) billions in direct health care expenditures and have additional impact as they inhibit force readiness, according to researchers from the Defense Health Agency. To date the DoD has struggled to understand the scope of the problems, although in most cases the researchers believe it is less than that of the general population.
Tobacco use and its related health problems have been linked to higher drop-out rates in basic training, poorer visual acuity, and a higher rate of absenteeism. Financially, tobacco use also extracts a significant cost: an estimated $1.7 billion in additional medical costs to the DoD, including $1 billion for prime beneficiaries and up to $700 million for standard beneficiaries. About $80 million is the result of secondhand smoke, and $5 million is derived from smokeless forms of tobacco. Also the DoD estimated that the cost in loss of productivity and fire injuries for service members is about $63 million.
Alcohol misuse also represents a significant challenge to military health and readiness, costing the DoD $1.3 billion in a 2014 estimate that included $73 million in loss of productivity. In the case of alcohol, the social impact was likely even higher. In fiscal year 2017, the DoD estimated that alcohol was a factor in one-third of attempted suicides. According to the DoD’s 2018 Workforce and Gender Relations survey of active-duty service members, alcohol also was a factor in 62% sexual assaults of women and 49% of sexual assaults of men.
Prescription drug misuse and Illicit drug use are less common, with lower rates than those found in the general population. Cocaine and marijuana are the most common drugs, while pain relievers are the most common prescription drugs being misused.
Service members face a number of risk factors for substance use disorders (SUDs), including service-related injuries, demands of active duty, poor relationships with commanders, separation from family members, psychiatric distress, and boredom. A particular challenge for the Military Health System is getting service members to seek help for SUDs. Many service members may be concerned over actual and perceived consequences for seeking treatment for a SUD, the stigma associated with SUDs, and negative associations with seeking treatment.
In response, the DHA has developed online health education campaigns to complement ongoing service-level programs and policy initiatives. Both You Can Quit 2 for tobacco and Own Your Limits for alcohol include live chats, educational materials, quizzes, and other interactive elements and allow service members to access the resources anonymously. The DoD plans to study their effectiveness.
PHS Message to Military Health Providers: Join Us
NATIONAL HARBOR, MD—As the Military Health System is undergoing significant structural and eventually manpower changes, ADM Brett P. Giroir, MD, the Assistant Secretary for Health in the US Department of Health and Human Services (HHS) and the US Food and Drug Administration acting commissioner, had one message: Come and join us. “Recruitment and retention are our top priorities,” ADM Giroir told the largely military health care provider audience, “If there is downsizing of any of the military health [system], we want you. If you touch health in any way…we need great people who are committed to our national goals in the Commissioned Corps.”
Not long ago, the US Public Health Service (PHS) was facing its own pressures to either reduce its workforce or to eliminate the PHS Commissioned Corps altogether. “The Corps’ mission assignments and functions have not evolved in step with the public health needs of the nation,” argued the fiscal year 2019 Office of Management and Budget, Budget of The U.S. Government. “It is time for that to change. HHS is committed to providing the best public health services and emergency response at the lowest cost and is undertaking a comprehensive look at how the Corps is structured.”
In response, PHS has undertaken a top-to-bottom audit and reevaluation of its mission, ADM Giroir noted, with the goal of defining the role for the PHS in the 21st century and beyond. As a result, the PHS recently completed the development of a modernization plan. The plan entails specifically managing the force to meet mission requirements, developing and training a Ready Reserve force, enhancing training and professional development for the Commissioned Corps, and updating and improving PHS systems and processes.
As a part of the modernization plan, ADM Giroir outlined projected growth plans for the Corps: an increase from the 6,400 regular Corps officers in FY 2018 to 7,725 by FY 2024 with an additional 2,500 Ready Reserve officers, to “minimally meet the mission requirements as we understand it,” ADM Giroir noted.
According to ADM Giroir, the goals for the Ready Reserve are an essential component in the PHS mission to meet any regional, national, or global public health emergency. The Ready Reserve would be a well-trained public health force that would be ready to deploy quickly. Whereas in the past, PHS officer deployments and specialties were tailored to the needs of the agencies in which they are embedded, this force would be more aligned with the needs for a rapid public health emergency response and would include specialized providers. In that context health care providers with military rapid response training would be highly valued.
Although the PHS has outlined its modernization plan, no budget has been allocated for it. Moreover, as ADM Giroir, has noted, PHS still remains dependent on the budgets of the embedding agencies to pay for the Commissioned Corps. “Right now our force structure is really determined by what federal agencies need,” he noted.
Currently, there are bills pending in both the House of Representatives and the Senate to codify the modernization effort.
NATIONAL HARBOR, MD—As the Military Health System is undergoing significant structural and eventually manpower changes, ADM Brett P. Giroir, MD, the Assistant Secretary for Health in the US Department of Health and Human Services (HHS) and the US Food and Drug Administration acting commissioner, had one message: Come and join us. “Recruitment and retention are our top priorities,” ADM Giroir told the largely military health care provider audience, “If there is downsizing of any of the military health [system], we want you. If you touch health in any way…we need great people who are committed to our national goals in the Commissioned Corps.”
Not long ago, the US Public Health Service (PHS) was facing its own pressures to either reduce its workforce or to eliminate the PHS Commissioned Corps altogether. “The Corps’ mission assignments and functions have not evolved in step with the public health needs of the nation,” argued the fiscal year 2019 Office of Management and Budget, Budget of The U.S. Government. “It is time for that to change. HHS is committed to providing the best public health services and emergency response at the lowest cost and is undertaking a comprehensive look at how the Corps is structured.”
In response, PHS has undertaken a top-to-bottom audit and reevaluation of its mission, ADM Giroir noted, with the goal of defining the role for the PHS in the 21st century and beyond. As a result, the PHS recently completed the development of a modernization plan. The plan entails specifically managing the force to meet mission requirements, developing and training a Ready Reserve force, enhancing training and professional development for the Commissioned Corps, and updating and improving PHS systems and processes.
As a part of the modernization plan, ADM Giroir outlined projected growth plans for the Corps: an increase from the 6,400 regular Corps officers in FY 2018 to 7,725 by FY 2024 with an additional 2,500 Ready Reserve officers, to “minimally meet the mission requirements as we understand it,” ADM Giroir noted.
According to ADM Giroir, the goals for the Ready Reserve are an essential component in the PHS mission to meet any regional, national, or global public health emergency. The Ready Reserve would be a well-trained public health force that would be ready to deploy quickly. Whereas in the past, PHS officer deployments and specialties were tailored to the needs of the agencies in which they are embedded, this force would be more aligned with the needs for a rapid public health emergency response and would include specialized providers. In that context health care providers with military rapid response training would be highly valued.
Although the PHS has outlined its modernization plan, no budget has been allocated for it. Moreover, as ADM Giroir, has noted, PHS still remains dependent on the budgets of the embedding agencies to pay for the Commissioned Corps. “Right now our force structure is really determined by what federal agencies need,” he noted.
Currently, there are bills pending in both the House of Representatives and the Senate to codify the modernization effort.
NATIONAL HARBOR, MD—As the Military Health System is undergoing significant structural and eventually manpower changes, ADM Brett P. Giroir, MD, the Assistant Secretary for Health in the US Department of Health and Human Services (HHS) and the US Food and Drug Administration acting commissioner, had one message: Come and join us. “Recruitment and retention are our top priorities,” ADM Giroir told the largely military health care provider audience, “If there is downsizing of any of the military health [system], we want you. If you touch health in any way…we need great people who are committed to our national goals in the Commissioned Corps.”
Not long ago, the US Public Health Service (PHS) was facing its own pressures to either reduce its workforce or to eliminate the PHS Commissioned Corps altogether. “The Corps’ mission assignments and functions have not evolved in step with the public health needs of the nation,” argued the fiscal year 2019 Office of Management and Budget, Budget of The U.S. Government. “It is time for that to change. HHS is committed to providing the best public health services and emergency response at the lowest cost and is undertaking a comprehensive look at how the Corps is structured.”
In response, PHS has undertaken a top-to-bottom audit and reevaluation of its mission, ADM Giroir noted, with the goal of defining the role for the PHS in the 21st century and beyond. As a result, the PHS recently completed the development of a modernization plan. The plan entails specifically managing the force to meet mission requirements, developing and training a Ready Reserve force, enhancing training and professional development for the Commissioned Corps, and updating and improving PHS systems and processes.
As a part of the modernization plan, ADM Giroir outlined projected growth plans for the Corps: an increase from the 6,400 regular Corps officers in FY 2018 to 7,725 by FY 2024 with an additional 2,500 Ready Reserve officers, to “minimally meet the mission requirements as we understand it,” ADM Giroir noted.
According to ADM Giroir, the goals for the Ready Reserve are an essential component in the PHS mission to meet any regional, national, or global public health emergency. The Ready Reserve would be a well-trained public health force that would be ready to deploy quickly. Whereas in the past, PHS officer deployments and specialties were tailored to the needs of the agencies in which they are embedded, this force would be more aligned with the needs for a rapid public health emergency response and would include specialized providers. In that context health care providers with military rapid response training would be highly valued.
Although the PHS has outlined its modernization plan, no budget has been allocated for it. Moreover, as ADM Giroir, has noted, PHS still remains dependent on the budgets of the embedding agencies to pay for the Commissioned Corps. “Right now our force structure is really determined by what federal agencies need,” he noted.
Currently, there are bills pending in both the House of Representatives and the Senate to codify the modernization effort.
The QI pipeline supported by SHM’s Student Scholar Grant Program
As fall arrives, new interns are rapidly gaining clinical confidence, and residency recruitment season is ramping up. It’s also time to announce the opening of the SHM Student Hospitalist Scholar Grant Program applications; we are now recruiting our sixth group of scholars for the summer and longitudinal programs.
Since its creation in 2015, the grant has supported 23 students in this incredible opportunity to allow trainees to engage in scholarly work with guidance from a mentor to better understand the practice of hospital medicine and to further grow our robust pipeline.
The 2018-2019 cohort of scholars, Matthew Fallon, Philip Huang, and Erin Rainosek, just concluded their projects and are currently preparing their abstracts for submission for Hospital Medicine 2020, where there is a track for Early-Career Hospitalists. The projects targeted a diverse set of domains, including improving upon the patient experience, readmission quality metrics, geographic cohorting, and clinical documentation integrity – all highly relevant topics for a practicing hospitalist.
Matthew Fallon collaborated with his mentor, Dr. Venkata Andukuri, at Creighton University, to reduce the rate of hospital readmission for patients with heart failure, by analyzing retrospective data in a root cause analysis to identify factors that influence readmission rate, then targeting those directly. They also integrated the patient experience by seeking out patient input as to the challenges they face in the management of their heart failure.
Philip Huang worked with his mentor, Dr. Ethan Kuperman, at the Carver College of Medicine, University of Iowa, to improve geographic localization for hospitalized patients to improve care efficiency. They worked closely with an industrial engineering team to create a workflow model integrated into the hospital EHR to designate patient location and were able to better understand the role that other professions play in improving the health care delivery.
Finally, Erin Rainosek teamed up with her mentor, Dr. Luci Leykum, at the University of Texas Health Science Center at San Antonio, to apply a design thinking strategy to redesign the health care experience for hospitalized patients. She engaged in over 120 hours of patient interviews and ultimately identified key themes that impact the experience of care, which will serve as target areas moving forward.
The student scholars in this cohort gained significant insight into the patient experience and quality issues relevant to the field of hospital medicine. We are proud of their accomplishments and look forward to their future successes and careers in hospital medicine. If you would like to learn more about the experience of our scholars this past summer, they have posted full write-ups on the Future Hospitalist RoundUp blog in HMX, SHM’s online community.
For students interested in becoming scholars, SHM offers two options to eligible medical students – the Summer Program and the Longitudinal Program. Both programs allow students to participate in projects related to quality improvement, patient safety, clinical research or hospital operations, in order to learn more about career paths in hospital medicine. Students will have the opportunity to conduct scholarly work with a mentor in these domains, with the option of participating over the summer during a 6-10-week period or longitudinally throughout the course of a year.
Discover additional benefits and how to apply on the SHM website. Applications will close in late January 2020.
Dr. Gottenborg is director of the Hospitalist Training Program within the Internal Medicine Residency Program at the University of Colorado. Dr. Duckett is assistant professor of medicine at the Medical University of South Carolina.
As fall arrives, new interns are rapidly gaining clinical confidence, and residency recruitment season is ramping up. It’s also time to announce the opening of the SHM Student Hospitalist Scholar Grant Program applications; we are now recruiting our sixth group of scholars for the summer and longitudinal programs.
Since its creation in 2015, the grant has supported 23 students in this incredible opportunity to allow trainees to engage in scholarly work with guidance from a mentor to better understand the practice of hospital medicine and to further grow our robust pipeline.
The 2018-2019 cohort of scholars, Matthew Fallon, Philip Huang, and Erin Rainosek, just concluded their projects and are currently preparing their abstracts for submission for Hospital Medicine 2020, where there is a track for Early-Career Hospitalists. The projects targeted a diverse set of domains, including improving upon the patient experience, readmission quality metrics, geographic cohorting, and clinical documentation integrity – all highly relevant topics for a practicing hospitalist.
Matthew Fallon collaborated with his mentor, Dr. Venkata Andukuri, at Creighton University, to reduce the rate of hospital readmission for patients with heart failure, by analyzing retrospective data in a root cause analysis to identify factors that influence readmission rate, then targeting those directly. They also integrated the patient experience by seeking out patient input as to the challenges they face in the management of their heart failure.
Philip Huang worked with his mentor, Dr. Ethan Kuperman, at the Carver College of Medicine, University of Iowa, to improve geographic localization for hospitalized patients to improve care efficiency. They worked closely with an industrial engineering team to create a workflow model integrated into the hospital EHR to designate patient location and were able to better understand the role that other professions play in improving the health care delivery.
Finally, Erin Rainosek teamed up with her mentor, Dr. Luci Leykum, at the University of Texas Health Science Center at San Antonio, to apply a design thinking strategy to redesign the health care experience for hospitalized patients. She engaged in over 120 hours of patient interviews and ultimately identified key themes that impact the experience of care, which will serve as target areas moving forward.
The student scholars in this cohort gained significant insight into the patient experience and quality issues relevant to the field of hospital medicine. We are proud of their accomplishments and look forward to their future successes and careers in hospital medicine. If you would like to learn more about the experience of our scholars this past summer, they have posted full write-ups on the Future Hospitalist RoundUp blog in HMX, SHM’s online community.
For students interested in becoming scholars, SHM offers two options to eligible medical students – the Summer Program and the Longitudinal Program. Both programs allow students to participate in projects related to quality improvement, patient safety, clinical research or hospital operations, in order to learn more about career paths in hospital medicine. Students will have the opportunity to conduct scholarly work with a mentor in these domains, with the option of participating over the summer during a 6-10-week period or longitudinally throughout the course of a year.
Discover additional benefits and how to apply on the SHM website. Applications will close in late January 2020.
Dr. Gottenborg is director of the Hospitalist Training Program within the Internal Medicine Residency Program at the University of Colorado. Dr. Duckett is assistant professor of medicine at the Medical University of South Carolina.
As fall arrives, new interns are rapidly gaining clinical confidence, and residency recruitment season is ramping up. It’s also time to announce the opening of the SHM Student Hospitalist Scholar Grant Program applications; we are now recruiting our sixth group of scholars for the summer and longitudinal programs.
Since its creation in 2015, the grant has supported 23 students in this incredible opportunity to allow trainees to engage in scholarly work with guidance from a mentor to better understand the practice of hospital medicine and to further grow our robust pipeline.
The 2018-2019 cohort of scholars, Matthew Fallon, Philip Huang, and Erin Rainosek, just concluded their projects and are currently preparing their abstracts for submission for Hospital Medicine 2020, where there is a track for Early-Career Hospitalists. The projects targeted a diverse set of domains, including improving upon the patient experience, readmission quality metrics, geographic cohorting, and clinical documentation integrity – all highly relevant topics for a practicing hospitalist.
Matthew Fallon collaborated with his mentor, Dr. Venkata Andukuri, at Creighton University, to reduce the rate of hospital readmission for patients with heart failure, by analyzing retrospective data in a root cause analysis to identify factors that influence readmission rate, then targeting those directly. They also integrated the patient experience by seeking out patient input as to the challenges they face in the management of their heart failure.
Philip Huang worked with his mentor, Dr. Ethan Kuperman, at the Carver College of Medicine, University of Iowa, to improve geographic localization for hospitalized patients to improve care efficiency. They worked closely with an industrial engineering team to create a workflow model integrated into the hospital EHR to designate patient location and were able to better understand the role that other professions play in improving the health care delivery.
Finally, Erin Rainosek teamed up with her mentor, Dr. Luci Leykum, at the University of Texas Health Science Center at San Antonio, to apply a design thinking strategy to redesign the health care experience for hospitalized patients. She engaged in over 120 hours of patient interviews and ultimately identified key themes that impact the experience of care, which will serve as target areas moving forward.
The student scholars in this cohort gained significant insight into the patient experience and quality issues relevant to the field of hospital medicine. We are proud of their accomplishments and look forward to their future successes and careers in hospital medicine. If you would like to learn more about the experience of our scholars this past summer, they have posted full write-ups on the Future Hospitalist RoundUp blog in HMX, SHM’s online community.
For students interested in becoming scholars, SHM offers two options to eligible medical students – the Summer Program and the Longitudinal Program. Both programs allow students to participate in projects related to quality improvement, patient safety, clinical research or hospital operations, in order to learn more about career paths in hospital medicine. Students will have the opportunity to conduct scholarly work with a mentor in these domains, with the option of participating over the summer during a 6-10-week period or longitudinally throughout the course of a year.
Discover additional benefits and how to apply on the SHM website. Applications will close in late January 2020.
Dr. Gottenborg is director of the Hospitalist Training Program within the Internal Medicine Residency Program at the University of Colorado. Dr. Duckett is assistant professor of medicine at the Medical University of South Carolina.