User login
2019 Update on bone health
Prior to last year, this column was titled “Update on osteoporosis.” My observation, however, is that too many ObGyn providers simply measure bone mass (known as bone mineral density, or BMD), label a patient as normal, osteopenic, or osteoporotic, and then consider pharmacotherapy. The FRAX fracture prediction algorithm, which incorporates age, weight, height, history of any previous fracture, family history of hip fracture, current smoking, use of glucocorticoid medications, and any history of rheumatoid arthritis, has refined the screening process somewhat, if and when it is utilized. As clinicians, we should never lose sight of our goal: to prevent fragility fractures. Having osteoporosis increases that risk, but not having osteoporosis does not eliminate it.
In this Update, I highlight various ways in which work published this past year may help us to improve our patients’ bone health and reduce fragility fractures.
Updated ISCD guidance emphasizes appropriate BMD testing, use of the
Z-score, and terminology
International Society for Clinical Densitometry. 2019 ISCD Official Positions-Adult. June 2019. https://www.iscd.org/official-positions/2019-ISCD-official-positions-adult.
Continue to: Indications for BMD testing...
Indications for BMD testing
The ISCD's indications for BMD testing remain for women age 65 and older. For postmenopausal women younger than age 65, a BMD test is indicated if they have a risk factor for low bone mass, such as 1) low body weight, 2) prior fracture, 3) high-risk medication use, or 4) a disease or condition associated with bone loss. A BMD test also is indicated for women during the menopausal transition with clinical risk factors for fracture, such as low body weight, prior fracture, or high-risk medication use. Interestingly, the ISCD recommendation for men is similar but uses age 70 for this group.
In addition, the ISCD recommends BMD testing in adults with a fragility fracture, with a disease or condition associated with low bone mass, or taking medications associated with low bone mass, as well as for anyone being considered for pharmacologic therapy, being treated (to monitor treatment effect), not receiving therapy in whom evidence of bone loss would lead to treatment, and in women discontinuing estrogen who should be considered for BMD testing according to the indications already mentioned.
Sites to assess for osteoporosis. The World Health Organization international reference standard for osteoporosis diagnosis is a T-score of -2.5 or less at the femoral neck. The reference standard, from which the T-score is calculated, is for white women aged 20 to 29 years of age from the database of the Third National Health and Nutrition Examination Survey. Osteoporosis also may be diagnosed in postmenopausal women if the T-score of the lumbar spine, total hip, or femoral neck is -2.5 or less. In certain circumstances, the 33% radius (also called the one-third radius) may be utilized. Other hip regions of interest, including Ward's area and the greater trochanter, should not be used for diagnosis.
The skeletal sites at which to measure BMD include the anteroposterior of the spine and hip in all patients. In terms of the spine, use L1-L4 for spine BMD measurement. However, exclude vertebrae that are affected by local structural changes or artifact. Use 3 vertebrae if 4 cannot be used, and 2 if 3 cannot be used. BMD-based diagnostic classification should not be made using a single vertebra. Anatomically abnormal vertebrae may be excluded from analysis if they are clearly abnormal and nonassessable within the resolution of the system, or if there is more than a 1.0 T-score difference between the vertebra in question and adjacent vertebrae. When vertebrae are excluded, the BMD of the remaining vertebrae are used to derive the T-score.
For BMD measurement at the hip, the femoral neck or total proximal femur—whichever is lowest—should be used. Either hip may be measured. Data are insufficient on whether mean T-scores for bilateral hip BMD should be used for diagnosis.
Terminology. While the ISCD retains the term osteopenia, the term low bone mass or low bone density is preferred. People with low bone mass or density are not necessarily at high fracture risk.
Concerning BMD reporting in women prior to menopause, Z-scores, not T-scores, are preferred. A Z-score of -2.0 or lower is defined as "below the expected range for age"; a Z-score above -2.0 is "within the expected range for age."
Use of serial BMD testing
Finally, regarding serial BMD measurements, such testing in combination with clinical assessment of fracture risk can be used to determine whether treatment should be initiated in untreated patients. Furthermore, serial BMD testing can monitor a patient's response to therapy by finding an increase or stability of bone density. It should be used to monitor individuals following cessation of osteoporosis drug therapy. Serial BMD testing can detect loss of bone density, indicating the need to assess treatment adherence, evaluate possible secondary causes of osteoporosis, and possibly re-evaluate therapeutic options.
Intervals between BMD testing should be determined according to each patient's clinical status. Typically, 1 year after initiating or changing therapy is appropriate, with longer intervals once therapeutic effect is established.
Patients commonly ask for BMD testing and ObGyn providers commonly order it. Understanding appropriate use of BMD testing in terms of who to scan, what sites to evaluate, when there may be spurious results of vertebrae due to artifacts, avoiding T-scores in premenopausal women in favor of Z-scores, understanding that low bone mass is a preferred term to osteopenia, and knowing how to order and use serial BMD testing will likely improve our role as the frontline providers to improving bone health in our patients.
Continue to: Dyspareunia drug has positive effects on bone...
Dyspareunia drug has positive effects on bone
de Villiers TJ, Altomare C, Particco M, et al. Effects of ospemifene on bone in postmenopausal women. Climacteric. 2019;22:442-447.
Previously, ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.3 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.4
Effects on bone formation/resorption biomarkers
In a recent study, de Villiers and colleagues reported the first phase 3 trial that looked at markers of bone formation and bone resorption.5 A total of 316 women were randomly assigned to receive ospemifene, and 315 received placebo.
Demographic and baseline characteristics were similar between treatment groups. Participants' mean age was approximately 60 years, mean body mass index (BMI) was 27.2 kg/m2, and mean duration of VVA was 8 to 9 years. Serum levels of 9 bone biomarkers were similar between groups at baseline.
At week 12, all 5 markers of bone resorption improved with ospemifene treatment, and 3 of the 5 (NTX, CTX, and TRACP-5b) did so in a statistically significant fashion compared with placebo (P≤.02). In addition, at week 12, all 4 markers of bone formation improved with ospemifene treatment compared with placebo (P≤.008). Furthermore, lower bone resorption markers with ospemifene were observed regardless of time since menopause (≤ 5 years or
> 5 years) or baseline BMD, whether normal, osteopenic, or osteoporotic.
Interpret results cautiously
The authors caution that the data are limited to biochemical markers rather than fracture or BMD. It is known that there is good correlation between biochemical markers for bone turnover and the occurrence of fracture.6
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. The preclinical animal data and human markers of bone turnover all support the antiresorptive action of ospemifene on bones. Thus, one may safely surmise that ospemifene's direction of activity in bone is virtually indisputable. The magnitude of that activity is, however, unstudied. Therefore, when choosing an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit in bone may be appropriate for that particular patient, although one would not use it as a stand-alone agent for bone only.
Continue to: Sarcopenia adds to osteoporotic risk for fractures...
Sarcopenia adds to osteoporotic risk for fractures
Lima RM, de Oliveira RJ, Raposo R, et al. Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women. Arch Osteoporos. 2019;14:38.
In 1989, the term sarcopenia was introduced to refer to the age-related decline in skeletal muscle mass.8 Currently, sarcopenia is defined as a progressive decline in muscle mass, strength, and physical function, thus increasing the risk for various adverse outcomes, including osteoporosis.9 Although muscle and bone tissues differ morphologically, their functioning is closely interconnected.
The sarcopenia-osteoporosis connection
Lima and colleagues sought to investigate the relationship between sarcopenia and osteoporosis.10 They measured women's fat free mass with dual-energy x-ray absorptiometry (DXA) scanning, muscle strength using a dynamometer to measure knee extension torque while participants were seated, and functional performance using the timed "up and go test" in which participants were timed as they got up from a chair, walked 3 meters around a cone, and returned to sit in the chair.10,11
The authors used definitions from the European Working Group on Sarcopenia in Older People (EWGSOP). Participants who had normal results in all 3 domains were considered nonsarcopenic. Presarcopenia was defined as having low fat free mass on DXA scanning but normal strength and function. Participants who had low fat free mass and either low strength or low function were labeled as having sarcopenia. Severe sarcopenia was defined as abnormal results in all 3 domains.
Two hundred thirty-four women (mean age, 68.3 years; range, 60-80) underwent BMD testing and were evaluated according to the 3 domains of possible sarcopenia. All were community dwelling and did not have cognitive impairment or functional dependency.
The rates of osteoporosis were 15.8%, 19.2%, 35.3%, and 46.2% for nonsarcopenia, presarcopenia, sarcopenia, and severe sarcopenia, respectively (P=.002). Whole-body and femoral neck BMD values were significantly lower among all sarcopenia stages when compared with nonsarcopenia (P<.05). The severe sarcopenia group showed the lowest lumbar spine T-scores (P<.05). When clustered, sarcopenia and severe sarcopenia presented a significantly higher risk for osteoporosis (odds ratio, 3.4; 95% confidence interval [CI], 1.5-7.8).
Consider sarcopenia a risk factor
The authors concluded that these "results provide support for the concept that a dose-response relationship exists between sarcopenia stages, BMD, and the presence of osteoporosis. These findings strengthen the clinical significance of the EWGSOP sarcopenia definitions and indicate that severe sarcopenia should be viewed with attention by healthcare professionals."
Osteoporotic fractures are defined as fragility fractures. While "frailty" has been a risk factor for such fractures in the past, increasing evidence now suggests that what we previously called frailty includes a significant component of loss of muscle mass, strength, and function—referred to as sarcopenia. While it is not likely that many ObGyns will perform objective testing for sarcopenia, conducting even a subjective assessment of such status should be considered in addition to BMD determinations in making decisions about pharmacotherapy.
Continue to: Certain characteristics may offset fracture risk in aromatase inhibitor users...
Certain characteristics may offset fracture risk in aromatase inhibitor users
Leslie WD, Morin SN, Lix LM, et al. Fracture risk in women with breast cancer initiating aromatase inhibitor therapy: a registry-based cohort study. Oncologist. 2019;24:1432-1438.
The use of AIs increases bone turnover and induces bone loss at trabecular-rich bone sites at an average rate of 1% to 3% per year, with reports of up to a threefold increased fracture incidence.13 By contrast, a large nationwide population-based cohort study using US Medicare data identified minimal fracture risk from AI use compared with tamoxifen use (11% higher for nonvertebral fractures, not significantly increased for hip fractures).14
An article published previously in this column reported that women on AIs treated with intravenous zoledronic acid had improvements in BMD, while women treated with denosumab had statistically significant fewer fractures compared with those receiving placebo, whether they had normal bone mass, osteopenia, or osteoporosis at
baseline.15-17
Data derived from a population-based BMD registry
In a recent cohort study, Leslie and colleagues offer the opinion that "observations in the clinical trial setting may differ from routine clinical practice."18 The authors examined fracture outcomes using a large clinical registry of BMD results from women in Manitoba, Canada. They identified women at least 40 years of age initiating AI therapy for breast cancer (n = 1,775), women with breast cancer not receiving AI therapy (n = 1,016), and women from the general population without breast cancer (n = 34,205).
Fracture outcomes were assessed after a mean of 6.2 years for the AI users, all of whom had at least 12 months of AI exposure. At baseline, AI users had higher BMI, higher BMD, lower osteoporosis prevalence, and fewer prior fractures than women from the general population or women with breast cancer without AI use (all P<.001). After adjusting for all covariates, AI users were not at significantly greater risk for major osteoporotic fractures (hazard ratio [HR], 1.15; 95% CI, 0.93-1.42), hip fracture (HR, 0.90; 95% CI, 0.56-1.43), or any fracture (HR, 1.06; 95% CI, 0.88-1.28) compared with the general population.
Results challenge prevailing view
Thus, the authors concluded that higher baseline BMI, BMD, and lower prevalence of prior fracture at baseline may offset the adverse effects of AI exposure. Although confirmatory data from large cohort studies are required, the authors stated that their findings challenge the view that all women with breast cancer initiating AI therapy should be considered at high risk for fracture.
It is well known that women with estrogen receptor-positive breast cancers tend to be more obese than noncancer patients and have higher levels of circulating estrogens. The study by Leslie and colleagues shows that such patients will have fewer previous fractures and better baseline bone mass values than the general population. This may prompt us to rethink whether all women initiating AI therapy need to be treated for fracture prevention, as some previous studies have suggested. Clearly, further study is necessary.
- International Society for Clinical Densitometry. 2019 ISCD Official Positions-Adult. June 2019. https://www.iscd.org/official-positions/2019-iscd-official-positions-adult. Accessed November 22, 2019.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18:17-22.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- de Villiers TJ, Altomare C, Particco M, et al. Effects of ospemifene on bone in postmenopausal women. Climacteric. 2019;22:442-447.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int. 2014;25:1439-1443.
- Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1988. Am J Clin Nutr. 1989;50(5 suppl):1121-1235.
- Drey M, Sieber CC, Bertsch T, et al. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899.
- Lima RM, de Oliveira RJ, Raposo R, et al. Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women. Arch Osteoporos. 2019;14:38.
- Mathias S, Nayak U, Isaacs B. Balance in elderly patients: the "get-up and go" test. Arch Phys Med Rehabil. 1986;67:387-389.
- Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255-2269.
- Schmidt N, Jacob L, Coleman R, et al. The impact of treatment compliance on fracture risk in women with breast cancer treated with aromatase inhibitors in the United Kingdom. Breast Cancer Res Treat. 2016;155:151-157.
- Neuner JM, Shi Y, Kong AL, et al. Fractures in a nationwide population-based cohort of users of breast cancer hormonal therapy. J Cancer Surviv. 2018;12:268-275.
- Goldstein SR. 2015 Update on osteoporosis. OBG Manag. 2015;27:31-39.
- Majithia N, Atherton PJ, Lafky JM, et al. Zoledronic acid for treatment of osteopenia and osteoporosis in women with primary breast cancer undergoing adjuvant aromatase inhibitor therapy: a 5-year follow-up. Support Care Cancer. 2016;24:1219-1226.
- Gnant M, Pfeiler G, Dubsky PC, et al; Austrian Breast and Colorectal Cancer Study Group. Adjuvant denosumab in breast cancer (ABCSG-18): a multicenter, randomized, double-blind, placebo-controlled trial. Lancet. 2015;386:433-443.
- Leslie WD, Morin SN, Lix LM, et al. Fracture risk in women with breast cancer initiating aromatase inhibitor therapy: a registry-based cohort study. Oncologist. 2019;24:1432-1438.
Prior to last year, this column was titled “Update on osteoporosis.” My observation, however, is that too many ObGyn providers simply measure bone mass (known as bone mineral density, or BMD), label a patient as normal, osteopenic, or osteoporotic, and then consider pharmacotherapy. The FRAX fracture prediction algorithm, which incorporates age, weight, height, history of any previous fracture, family history of hip fracture, current smoking, use of glucocorticoid medications, and any history of rheumatoid arthritis, has refined the screening process somewhat, if and when it is utilized. As clinicians, we should never lose sight of our goal: to prevent fragility fractures. Having osteoporosis increases that risk, but not having osteoporosis does not eliminate it.
In this Update, I highlight various ways in which work published this past year may help us to improve our patients’ bone health and reduce fragility fractures.
Updated ISCD guidance emphasizes appropriate BMD testing, use of the
Z-score, and terminology
International Society for Clinical Densitometry. 2019 ISCD Official Positions-Adult. June 2019. https://www.iscd.org/official-positions/2019-ISCD-official-positions-adult.
Continue to: Indications for BMD testing...
Indications for BMD testing
The ISCD's indications for BMD testing remain for women age 65 and older. For postmenopausal women younger than age 65, a BMD test is indicated if they have a risk factor for low bone mass, such as 1) low body weight, 2) prior fracture, 3) high-risk medication use, or 4) a disease or condition associated with bone loss. A BMD test also is indicated for women during the menopausal transition with clinical risk factors for fracture, such as low body weight, prior fracture, or high-risk medication use. Interestingly, the ISCD recommendation for men is similar but uses age 70 for this group.
In addition, the ISCD recommends BMD testing in adults with a fragility fracture, with a disease or condition associated with low bone mass, or taking medications associated with low bone mass, as well as for anyone being considered for pharmacologic therapy, being treated (to monitor treatment effect), not receiving therapy in whom evidence of bone loss would lead to treatment, and in women discontinuing estrogen who should be considered for BMD testing according to the indications already mentioned.
Sites to assess for osteoporosis. The World Health Organization international reference standard for osteoporosis diagnosis is a T-score of -2.5 or less at the femoral neck. The reference standard, from which the T-score is calculated, is for white women aged 20 to 29 years of age from the database of the Third National Health and Nutrition Examination Survey. Osteoporosis also may be diagnosed in postmenopausal women if the T-score of the lumbar spine, total hip, or femoral neck is -2.5 or less. In certain circumstances, the 33% radius (also called the one-third radius) may be utilized. Other hip regions of interest, including Ward's area and the greater trochanter, should not be used for diagnosis.
The skeletal sites at which to measure BMD include the anteroposterior of the spine and hip in all patients. In terms of the spine, use L1-L4 for spine BMD measurement. However, exclude vertebrae that are affected by local structural changes or artifact. Use 3 vertebrae if 4 cannot be used, and 2 if 3 cannot be used. BMD-based diagnostic classification should not be made using a single vertebra. Anatomically abnormal vertebrae may be excluded from analysis if they are clearly abnormal and nonassessable within the resolution of the system, or if there is more than a 1.0 T-score difference between the vertebra in question and adjacent vertebrae. When vertebrae are excluded, the BMD of the remaining vertebrae are used to derive the T-score.
For BMD measurement at the hip, the femoral neck or total proximal femur—whichever is lowest—should be used. Either hip may be measured. Data are insufficient on whether mean T-scores for bilateral hip BMD should be used for diagnosis.
Terminology. While the ISCD retains the term osteopenia, the term low bone mass or low bone density is preferred. People with low bone mass or density are not necessarily at high fracture risk.
Concerning BMD reporting in women prior to menopause, Z-scores, not T-scores, are preferred. A Z-score of -2.0 or lower is defined as "below the expected range for age"; a Z-score above -2.0 is "within the expected range for age."
Use of serial BMD testing
Finally, regarding serial BMD measurements, such testing in combination with clinical assessment of fracture risk can be used to determine whether treatment should be initiated in untreated patients. Furthermore, serial BMD testing can monitor a patient's response to therapy by finding an increase or stability of bone density. It should be used to monitor individuals following cessation of osteoporosis drug therapy. Serial BMD testing can detect loss of bone density, indicating the need to assess treatment adherence, evaluate possible secondary causes of osteoporosis, and possibly re-evaluate therapeutic options.
Intervals between BMD testing should be determined according to each patient's clinical status. Typically, 1 year after initiating or changing therapy is appropriate, with longer intervals once therapeutic effect is established.
Patients commonly ask for BMD testing and ObGyn providers commonly order it. Understanding appropriate use of BMD testing in terms of who to scan, what sites to evaluate, when there may be spurious results of vertebrae due to artifacts, avoiding T-scores in premenopausal women in favor of Z-scores, understanding that low bone mass is a preferred term to osteopenia, and knowing how to order and use serial BMD testing will likely improve our role as the frontline providers to improving bone health in our patients.
Continue to: Dyspareunia drug has positive effects on bone...
Dyspareunia drug has positive effects on bone
de Villiers TJ, Altomare C, Particco M, et al. Effects of ospemifene on bone in postmenopausal women. Climacteric. 2019;22:442-447.
Previously, ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.3 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.4
Effects on bone formation/resorption biomarkers
In a recent study, de Villiers and colleagues reported the first phase 3 trial that looked at markers of bone formation and bone resorption.5 A total of 316 women were randomly assigned to receive ospemifene, and 315 received placebo.
Demographic and baseline characteristics were similar between treatment groups. Participants' mean age was approximately 60 years, mean body mass index (BMI) was 27.2 kg/m2, and mean duration of VVA was 8 to 9 years. Serum levels of 9 bone biomarkers were similar between groups at baseline.
At week 12, all 5 markers of bone resorption improved with ospemifene treatment, and 3 of the 5 (NTX, CTX, and TRACP-5b) did so in a statistically significant fashion compared with placebo (P≤.02). In addition, at week 12, all 4 markers of bone formation improved with ospemifene treatment compared with placebo (P≤.008). Furthermore, lower bone resorption markers with ospemifene were observed regardless of time since menopause (≤ 5 years or
> 5 years) or baseline BMD, whether normal, osteopenic, or osteoporotic.
Interpret results cautiously
The authors caution that the data are limited to biochemical markers rather than fracture or BMD. It is known that there is good correlation between biochemical markers for bone turnover and the occurrence of fracture.6
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. The preclinical animal data and human markers of bone turnover all support the antiresorptive action of ospemifene on bones. Thus, one may safely surmise that ospemifene's direction of activity in bone is virtually indisputable. The magnitude of that activity is, however, unstudied. Therefore, when choosing an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit in bone may be appropriate for that particular patient, although one would not use it as a stand-alone agent for bone only.
Continue to: Sarcopenia adds to osteoporotic risk for fractures...
Sarcopenia adds to osteoporotic risk for fractures
Lima RM, de Oliveira RJ, Raposo R, et al. Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women. Arch Osteoporos. 2019;14:38.
In 1989, the term sarcopenia was introduced to refer to the age-related decline in skeletal muscle mass.8 Currently, sarcopenia is defined as a progressive decline in muscle mass, strength, and physical function, thus increasing the risk for various adverse outcomes, including osteoporosis.9 Although muscle and bone tissues differ morphologically, their functioning is closely interconnected.
The sarcopenia-osteoporosis connection
Lima and colleagues sought to investigate the relationship between sarcopenia and osteoporosis.10 They measured women's fat free mass with dual-energy x-ray absorptiometry (DXA) scanning, muscle strength using a dynamometer to measure knee extension torque while participants were seated, and functional performance using the timed "up and go test" in which participants were timed as they got up from a chair, walked 3 meters around a cone, and returned to sit in the chair.10,11
The authors used definitions from the European Working Group on Sarcopenia in Older People (EWGSOP). Participants who had normal results in all 3 domains were considered nonsarcopenic. Presarcopenia was defined as having low fat free mass on DXA scanning but normal strength and function. Participants who had low fat free mass and either low strength or low function were labeled as having sarcopenia. Severe sarcopenia was defined as abnormal results in all 3 domains.
Two hundred thirty-four women (mean age, 68.3 years; range, 60-80) underwent BMD testing and were evaluated according to the 3 domains of possible sarcopenia. All were community dwelling and did not have cognitive impairment or functional dependency.
The rates of osteoporosis were 15.8%, 19.2%, 35.3%, and 46.2% for nonsarcopenia, presarcopenia, sarcopenia, and severe sarcopenia, respectively (P=.002). Whole-body and femoral neck BMD values were significantly lower among all sarcopenia stages when compared with nonsarcopenia (P<.05). The severe sarcopenia group showed the lowest lumbar spine T-scores (P<.05). When clustered, sarcopenia and severe sarcopenia presented a significantly higher risk for osteoporosis (odds ratio, 3.4; 95% confidence interval [CI], 1.5-7.8).
Consider sarcopenia a risk factor
The authors concluded that these "results provide support for the concept that a dose-response relationship exists between sarcopenia stages, BMD, and the presence of osteoporosis. These findings strengthen the clinical significance of the EWGSOP sarcopenia definitions and indicate that severe sarcopenia should be viewed with attention by healthcare professionals."
Osteoporotic fractures are defined as fragility fractures. While "frailty" has been a risk factor for such fractures in the past, increasing evidence now suggests that what we previously called frailty includes a significant component of loss of muscle mass, strength, and function—referred to as sarcopenia. While it is not likely that many ObGyns will perform objective testing for sarcopenia, conducting even a subjective assessment of such status should be considered in addition to BMD determinations in making decisions about pharmacotherapy.
Continue to: Certain characteristics may offset fracture risk in aromatase inhibitor users...
Certain characteristics may offset fracture risk in aromatase inhibitor users
Leslie WD, Morin SN, Lix LM, et al. Fracture risk in women with breast cancer initiating aromatase inhibitor therapy: a registry-based cohort study. Oncologist. 2019;24:1432-1438.
The use of AIs increases bone turnover and induces bone loss at trabecular-rich bone sites at an average rate of 1% to 3% per year, with reports of up to a threefold increased fracture incidence.13 By contrast, a large nationwide population-based cohort study using US Medicare data identified minimal fracture risk from AI use compared with tamoxifen use (11% higher for nonvertebral fractures, not significantly increased for hip fractures).14
An article published previously in this column reported that women on AIs treated with intravenous zoledronic acid had improvements in BMD, while women treated with denosumab had statistically significant fewer fractures compared with those receiving placebo, whether they had normal bone mass, osteopenia, or osteoporosis at
baseline.15-17
Data derived from a population-based BMD registry
In a recent cohort study, Leslie and colleagues offer the opinion that "observations in the clinical trial setting may differ from routine clinical practice."18 The authors examined fracture outcomes using a large clinical registry of BMD results from women in Manitoba, Canada. They identified women at least 40 years of age initiating AI therapy for breast cancer (n = 1,775), women with breast cancer not receiving AI therapy (n = 1,016), and women from the general population without breast cancer (n = 34,205).
Fracture outcomes were assessed after a mean of 6.2 years for the AI users, all of whom had at least 12 months of AI exposure. At baseline, AI users had higher BMI, higher BMD, lower osteoporosis prevalence, and fewer prior fractures than women from the general population or women with breast cancer without AI use (all P<.001). After adjusting for all covariates, AI users were not at significantly greater risk for major osteoporotic fractures (hazard ratio [HR], 1.15; 95% CI, 0.93-1.42), hip fracture (HR, 0.90; 95% CI, 0.56-1.43), or any fracture (HR, 1.06; 95% CI, 0.88-1.28) compared with the general population.
Results challenge prevailing view
Thus, the authors concluded that higher baseline BMI, BMD, and lower prevalence of prior fracture at baseline may offset the adverse effects of AI exposure. Although confirmatory data from large cohort studies are required, the authors stated that their findings challenge the view that all women with breast cancer initiating AI therapy should be considered at high risk for fracture.
It is well known that women with estrogen receptor-positive breast cancers tend to be more obese than noncancer patients and have higher levels of circulating estrogens. The study by Leslie and colleagues shows that such patients will have fewer previous fractures and better baseline bone mass values than the general population. This may prompt us to rethink whether all women initiating AI therapy need to be treated for fracture prevention, as some previous studies have suggested. Clearly, further study is necessary.
Prior to last year, this column was titled “Update on osteoporosis.” My observation, however, is that too many ObGyn providers simply measure bone mass (known as bone mineral density, or BMD), label a patient as normal, osteopenic, or osteoporotic, and then consider pharmacotherapy. The FRAX fracture prediction algorithm, which incorporates age, weight, height, history of any previous fracture, family history of hip fracture, current smoking, use of glucocorticoid medications, and any history of rheumatoid arthritis, has refined the screening process somewhat, if and when it is utilized. As clinicians, we should never lose sight of our goal: to prevent fragility fractures. Having osteoporosis increases that risk, but not having osteoporosis does not eliminate it.
In this Update, I highlight various ways in which work published this past year may help us to improve our patients’ bone health and reduce fragility fractures.
Updated ISCD guidance emphasizes appropriate BMD testing, use of the
Z-score, and terminology
International Society for Clinical Densitometry. 2019 ISCD Official Positions-Adult. June 2019. https://www.iscd.org/official-positions/2019-ISCD-official-positions-adult.
Continue to: Indications for BMD testing...
Indications for BMD testing
The ISCD's indications for BMD testing remain for women age 65 and older. For postmenopausal women younger than age 65, a BMD test is indicated if they have a risk factor for low bone mass, such as 1) low body weight, 2) prior fracture, 3) high-risk medication use, or 4) a disease or condition associated with bone loss. A BMD test also is indicated for women during the menopausal transition with clinical risk factors for fracture, such as low body weight, prior fracture, or high-risk medication use. Interestingly, the ISCD recommendation for men is similar but uses age 70 for this group.
In addition, the ISCD recommends BMD testing in adults with a fragility fracture, with a disease or condition associated with low bone mass, or taking medications associated with low bone mass, as well as for anyone being considered for pharmacologic therapy, being treated (to monitor treatment effect), not receiving therapy in whom evidence of bone loss would lead to treatment, and in women discontinuing estrogen who should be considered for BMD testing according to the indications already mentioned.
Sites to assess for osteoporosis. The World Health Organization international reference standard for osteoporosis diagnosis is a T-score of -2.5 or less at the femoral neck. The reference standard, from which the T-score is calculated, is for white women aged 20 to 29 years of age from the database of the Third National Health and Nutrition Examination Survey. Osteoporosis also may be diagnosed in postmenopausal women if the T-score of the lumbar spine, total hip, or femoral neck is -2.5 or less. In certain circumstances, the 33% radius (also called the one-third radius) may be utilized. Other hip regions of interest, including Ward's area and the greater trochanter, should not be used for diagnosis.
The skeletal sites at which to measure BMD include the anteroposterior of the spine and hip in all patients. In terms of the spine, use L1-L4 for spine BMD measurement. However, exclude vertebrae that are affected by local structural changes or artifact. Use 3 vertebrae if 4 cannot be used, and 2 if 3 cannot be used. BMD-based diagnostic classification should not be made using a single vertebra. Anatomically abnormal vertebrae may be excluded from analysis if they are clearly abnormal and nonassessable within the resolution of the system, or if there is more than a 1.0 T-score difference between the vertebra in question and adjacent vertebrae. When vertebrae are excluded, the BMD of the remaining vertebrae are used to derive the T-score.
For BMD measurement at the hip, the femoral neck or total proximal femur—whichever is lowest—should be used. Either hip may be measured. Data are insufficient on whether mean T-scores for bilateral hip BMD should be used for diagnosis.
Terminology. While the ISCD retains the term osteopenia, the term low bone mass or low bone density is preferred. People with low bone mass or density are not necessarily at high fracture risk.
Concerning BMD reporting in women prior to menopause, Z-scores, not T-scores, are preferred. A Z-score of -2.0 or lower is defined as "below the expected range for age"; a Z-score above -2.0 is "within the expected range for age."
Use of serial BMD testing
Finally, regarding serial BMD measurements, such testing in combination with clinical assessment of fracture risk can be used to determine whether treatment should be initiated in untreated patients. Furthermore, serial BMD testing can monitor a patient's response to therapy by finding an increase or stability of bone density. It should be used to monitor individuals following cessation of osteoporosis drug therapy. Serial BMD testing can detect loss of bone density, indicating the need to assess treatment adherence, evaluate possible secondary causes of osteoporosis, and possibly re-evaluate therapeutic options.
Intervals between BMD testing should be determined according to each patient's clinical status. Typically, 1 year after initiating or changing therapy is appropriate, with longer intervals once therapeutic effect is established.
Patients commonly ask for BMD testing and ObGyn providers commonly order it. Understanding appropriate use of BMD testing in terms of who to scan, what sites to evaluate, when there may be spurious results of vertebrae due to artifacts, avoiding T-scores in premenopausal women in favor of Z-scores, understanding that low bone mass is a preferred term to osteopenia, and knowing how to order and use serial BMD testing will likely improve our role as the frontline providers to improving bone health in our patients.
Continue to: Dyspareunia drug has positive effects on bone...
Dyspareunia drug has positive effects on bone
de Villiers TJ, Altomare C, Particco M, et al. Effects of ospemifene on bone in postmenopausal women. Climacteric. 2019;22:442-447.
Previously, ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.3 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.4
Effects on bone formation/resorption biomarkers
In a recent study, de Villiers and colleagues reported the first phase 3 trial that looked at markers of bone formation and bone resorption.5 A total of 316 women were randomly assigned to receive ospemifene, and 315 received placebo.
Demographic and baseline characteristics were similar between treatment groups. Participants' mean age was approximately 60 years, mean body mass index (BMI) was 27.2 kg/m2, and mean duration of VVA was 8 to 9 years. Serum levels of 9 bone biomarkers were similar between groups at baseline.
At week 12, all 5 markers of bone resorption improved with ospemifene treatment, and 3 of the 5 (NTX, CTX, and TRACP-5b) did so in a statistically significant fashion compared with placebo (P≤.02). In addition, at week 12, all 4 markers of bone formation improved with ospemifene treatment compared with placebo (P≤.008). Furthermore, lower bone resorption markers with ospemifene were observed regardless of time since menopause (≤ 5 years or
> 5 years) or baseline BMD, whether normal, osteopenic, or osteoporotic.
Interpret results cautiously
The authors caution that the data are limited to biochemical markers rather than fracture or BMD. It is known that there is good correlation between biochemical markers for bone turnover and the occurrence of fracture.6
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. The preclinical animal data and human markers of bone turnover all support the antiresorptive action of ospemifene on bones. Thus, one may safely surmise that ospemifene's direction of activity in bone is virtually indisputable. The magnitude of that activity is, however, unstudied. Therefore, when choosing an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit in bone may be appropriate for that particular patient, although one would not use it as a stand-alone agent for bone only.
Continue to: Sarcopenia adds to osteoporotic risk for fractures...
Sarcopenia adds to osteoporotic risk for fractures
Lima RM, de Oliveira RJ, Raposo R, et al. Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women. Arch Osteoporos. 2019;14:38.
In 1989, the term sarcopenia was introduced to refer to the age-related decline in skeletal muscle mass.8 Currently, sarcopenia is defined as a progressive decline in muscle mass, strength, and physical function, thus increasing the risk for various adverse outcomes, including osteoporosis.9 Although muscle and bone tissues differ morphologically, their functioning is closely interconnected.
The sarcopenia-osteoporosis connection
Lima and colleagues sought to investigate the relationship between sarcopenia and osteoporosis.10 They measured women's fat free mass with dual-energy x-ray absorptiometry (DXA) scanning, muscle strength using a dynamometer to measure knee extension torque while participants were seated, and functional performance using the timed "up and go test" in which participants were timed as they got up from a chair, walked 3 meters around a cone, and returned to sit in the chair.10,11
The authors used definitions from the European Working Group on Sarcopenia in Older People (EWGSOP). Participants who had normal results in all 3 domains were considered nonsarcopenic. Presarcopenia was defined as having low fat free mass on DXA scanning but normal strength and function. Participants who had low fat free mass and either low strength or low function were labeled as having sarcopenia. Severe sarcopenia was defined as abnormal results in all 3 domains.
Two hundred thirty-four women (mean age, 68.3 years; range, 60-80) underwent BMD testing and were evaluated according to the 3 domains of possible sarcopenia. All were community dwelling and did not have cognitive impairment or functional dependency.
The rates of osteoporosis were 15.8%, 19.2%, 35.3%, and 46.2% for nonsarcopenia, presarcopenia, sarcopenia, and severe sarcopenia, respectively (P=.002). Whole-body and femoral neck BMD values were significantly lower among all sarcopenia stages when compared with nonsarcopenia (P<.05). The severe sarcopenia group showed the lowest lumbar spine T-scores (P<.05). When clustered, sarcopenia and severe sarcopenia presented a significantly higher risk for osteoporosis (odds ratio, 3.4; 95% confidence interval [CI], 1.5-7.8).
Consider sarcopenia a risk factor
The authors concluded that these "results provide support for the concept that a dose-response relationship exists between sarcopenia stages, BMD, and the presence of osteoporosis. These findings strengthen the clinical significance of the EWGSOP sarcopenia definitions and indicate that severe sarcopenia should be viewed with attention by healthcare professionals."
Osteoporotic fractures are defined as fragility fractures. While "frailty" has been a risk factor for such fractures in the past, increasing evidence now suggests that what we previously called frailty includes a significant component of loss of muscle mass, strength, and function—referred to as sarcopenia. While it is not likely that many ObGyns will perform objective testing for sarcopenia, conducting even a subjective assessment of such status should be considered in addition to BMD determinations in making decisions about pharmacotherapy.
Continue to: Certain characteristics may offset fracture risk in aromatase inhibitor users...
Certain characteristics may offset fracture risk in aromatase inhibitor users
Leslie WD, Morin SN, Lix LM, et al. Fracture risk in women with breast cancer initiating aromatase inhibitor therapy: a registry-based cohort study. Oncologist. 2019;24:1432-1438.
The use of AIs increases bone turnover and induces bone loss at trabecular-rich bone sites at an average rate of 1% to 3% per year, with reports of up to a threefold increased fracture incidence.13 By contrast, a large nationwide population-based cohort study using US Medicare data identified minimal fracture risk from AI use compared with tamoxifen use (11% higher for nonvertebral fractures, not significantly increased for hip fractures).14
An article published previously in this column reported that women on AIs treated with intravenous zoledronic acid had improvements in BMD, while women treated with denosumab had statistically significant fewer fractures compared with those receiving placebo, whether they had normal bone mass, osteopenia, or osteoporosis at
baseline.15-17
Data derived from a population-based BMD registry
In a recent cohort study, Leslie and colleagues offer the opinion that "observations in the clinical trial setting may differ from routine clinical practice."18 The authors examined fracture outcomes using a large clinical registry of BMD results from women in Manitoba, Canada. They identified women at least 40 years of age initiating AI therapy for breast cancer (n = 1,775), women with breast cancer not receiving AI therapy (n = 1,016), and women from the general population without breast cancer (n = 34,205).
Fracture outcomes were assessed after a mean of 6.2 years for the AI users, all of whom had at least 12 months of AI exposure. At baseline, AI users had higher BMI, higher BMD, lower osteoporosis prevalence, and fewer prior fractures than women from the general population or women with breast cancer without AI use (all P<.001). After adjusting for all covariates, AI users were not at significantly greater risk for major osteoporotic fractures (hazard ratio [HR], 1.15; 95% CI, 0.93-1.42), hip fracture (HR, 0.90; 95% CI, 0.56-1.43), or any fracture (HR, 1.06; 95% CI, 0.88-1.28) compared with the general population.
Results challenge prevailing view
Thus, the authors concluded that higher baseline BMI, BMD, and lower prevalence of prior fracture at baseline may offset the adverse effects of AI exposure. Although confirmatory data from large cohort studies are required, the authors stated that their findings challenge the view that all women with breast cancer initiating AI therapy should be considered at high risk for fracture.
It is well known that women with estrogen receptor-positive breast cancers tend to be more obese than noncancer patients and have higher levels of circulating estrogens. The study by Leslie and colleagues shows that such patients will have fewer previous fractures and better baseline bone mass values than the general population. This may prompt us to rethink whether all women initiating AI therapy need to be treated for fracture prevention, as some previous studies have suggested. Clearly, further study is necessary.
- International Society for Clinical Densitometry. 2019 ISCD Official Positions-Adult. June 2019. https://www.iscd.org/official-positions/2019-iscd-official-positions-adult. Accessed November 22, 2019.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18:17-22.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- de Villiers TJ, Altomare C, Particco M, et al. Effects of ospemifene on bone in postmenopausal women. Climacteric. 2019;22:442-447.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int. 2014;25:1439-1443.
- Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1988. Am J Clin Nutr. 1989;50(5 suppl):1121-1235.
- Drey M, Sieber CC, Bertsch T, et al. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899.
- Lima RM, de Oliveira RJ, Raposo R, et al. Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women. Arch Osteoporos. 2019;14:38.
- Mathias S, Nayak U, Isaacs B. Balance in elderly patients: the "get-up and go" test. Arch Phys Med Rehabil. 1986;67:387-389.
- Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255-2269.
- Schmidt N, Jacob L, Coleman R, et al. The impact of treatment compliance on fracture risk in women with breast cancer treated with aromatase inhibitors in the United Kingdom. Breast Cancer Res Treat. 2016;155:151-157.
- Neuner JM, Shi Y, Kong AL, et al. Fractures in a nationwide population-based cohort of users of breast cancer hormonal therapy. J Cancer Surviv. 2018;12:268-275.
- Goldstein SR. 2015 Update on osteoporosis. OBG Manag. 2015;27:31-39.
- Majithia N, Atherton PJ, Lafky JM, et al. Zoledronic acid for treatment of osteopenia and osteoporosis in women with primary breast cancer undergoing adjuvant aromatase inhibitor therapy: a 5-year follow-up. Support Care Cancer. 2016;24:1219-1226.
- Gnant M, Pfeiler G, Dubsky PC, et al; Austrian Breast and Colorectal Cancer Study Group. Adjuvant denosumab in breast cancer (ABCSG-18): a multicenter, randomized, double-blind, placebo-controlled trial. Lancet. 2015;386:433-443.
- Leslie WD, Morin SN, Lix LM, et al. Fracture risk in women with breast cancer initiating aromatase inhibitor therapy: a registry-based cohort study. Oncologist. 2019;24:1432-1438.
- International Society for Clinical Densitometry. 2019 ISCD Official Positions-Adult. June 2019. https://www.iscd.org/official-positions/2019-iscd-official-positions-adult. Accessed November 22, 2019.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18:17-22.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- de Villiers TJ, Altomare C, Particco M, et al. Effects of ospemifene on bone in postmenopausal women. Climacteric. 2019;22:442-447.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int. 2014;25:1439-1443.
- Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1988. Am J Clin Nutr. 1989;50(5 suppl):1121-1235.
- Drey M, Sieber CC, Bertsch T, et al. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899.
- Lima RM, de Oliveira RJ, Raposo R, et al. Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women. Arch Osteoporos. 2019;14:38.
- Mathias S, Nayak U, Isaacs B. Balance in elderly patients: the "get-up and go" test. Arch Phys Med Rehabil. 1986;67:387-389.
- Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255-2269.
- Schmidt N, Jacob L, Coleman R, et al. The impact of treatment compliance on fracture risk in women with breast cancer treated with aromatase inhibitors in the United Kingdom. Breast Cancer Res Treat. 2016;155:151-157.
- Neuner JM, Shi Y, Kong AL, et al. Fractures in a nationwide population-based cohort of users of breast cancer hormonal therapy. J Cancer Surviv. 2018;12:268-275.
- Goldstein SR. 2015 Update on osteoporosis. OBG Manag. 2015;27:31-39.
- Majithia N, Atherton PJ, Lafky JM, et al. Zoledronic acid for treatment of osteopenia and osteoporosis in women with primary breast cancer undergoing adjuvant aromatase inhibitor therapy: a 5-year follow-up. Support Care Cancer. 2016;24:1219-1226.
- Gnant M, Pfeiler G, Dubsky PC, et al; Austrian Breast and Colorectal Cancer Study Group. Adjuvant denosumab in breast cancer (ABCSG-18): a multicenter, randomized, double-blind, placebo-controlled trial. Lancet. 2015;386:433-443.
- Leslie WD, Morin SN, Lix LM, et al. Fracture risk in women with breast cancer initiating aromatase inhibitor therapy: a registry-based cohort study. Oncologist. 2019;24:1432-1438.
Thanksgiving took a bite out of HealthCare.gov
Health care insurance may have taken a bit of a back seat to turkey and shopping last week as according to the Centers for Medicare & Medicaid Services.
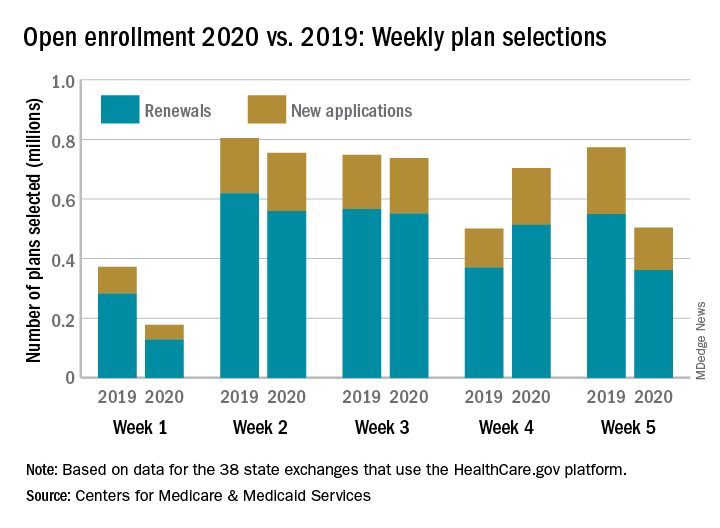
Consumers selected 28% fewer plans during week 5 (Nov. 24-30) of Open Enrollment 2020 than in week 4. A similar drop of 33% occurred last year between week 3 of open enrollment and week 4, which included Thanksgiving and Black Friday, CMS data show.
Through week 5, total plans selections for 2020 health insurance coverage came in at almost 2.9 million, which is down about 10% from last year’s 5-week total of 3.2 million for 2019 coverage.
The HealthCare.gov platform is being used by 38 states for the 2020 benefit year, and so far Florida residents have selected the most plans, almost 797,000. Texas is next with just over 400,000 selections, followed by Georgia with 173,000 and North Carolina with 162,000, CMS reported Dec. 4.
Health care insurance may have taken a bit of a back seat to turkey and shopping last week as according to the Centers for Medicare & Medicaid Services.

Consumers selected 28% fewer plans during week 5 (Nov. 24-30) of Open Enrollment 2020 than in week 4. A similar drop of 33% occurred last year between week 3 of open enrollment and week 4, which included Thanksgiving and Black Friday, CMS data show.
Through week 5, total plans selections for 2020 health insurance coverage came in at almost 2.9 million, which is down about 10% from last year’s 5-week total of 3.2 million for 2019 coverage.
The HealthCare.gov platform is being used by 38 states for the 2020 benefit year, and so far Florida residents have selected the most plans, almost 797,000. Texas is next with just over 400,000 selections, followed by Georgia with 173,000 and North Carolina with 162,000, CMS reported Dec. 4.
Health care insurance may have taken a bit of a back seat to turkey and shopping last week as according to the Centers for Medicare & Medicaid Services.

Consumers selected 28% fewer plans during week 5 (Nov. 24-30) of Open Enrollment 2020 than in week 4. A similar drop of 33% occurred last year between week 3 of open enrollment and week 4, which included Thanksgiving and Black Friday, CMS data show.
Through week 5, total plans selections for 2020 health insurance coverage came in at almost 2.9 million, which is down about 10% from last year’s 5-week total of 3.2 million for 2019 coverage.
The HealthCare.gov platform is being used by 38 states for the 2020 benefit year, and so far Florida residents have selected the most plans, almost 797,000. Texas is next with just over 400,000 selections, followed by Georgia with 173,000 and North Carolina with 162,000, CMS reported Dec. 4.
Snow Way to Take Care of Your Heart
ANSWER
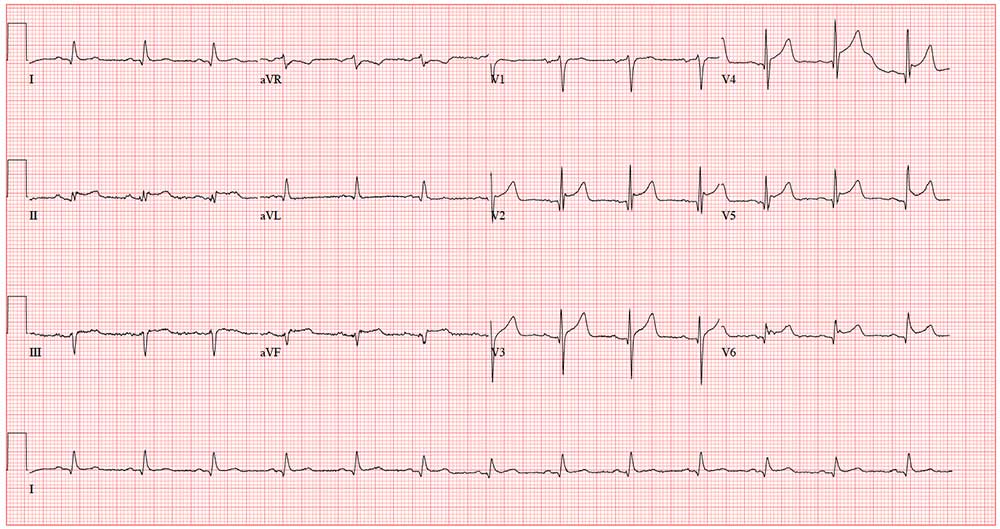
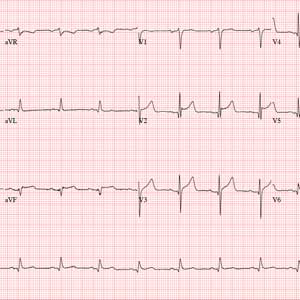
This ECG shows normal sinus rhythm, an anterior myocardial infarction, and inferolateral injury consistent with an acute ST-elevation myocardial infarction (STEMI).
A P wave for every QRS complex and a QRS complex with every P wave, with a consistent PR interval and a rate > 60 and < 100 beats/min, signifies sinus rhythm.
Criteria for an anterior STEMI include new ST elevation (≥ 2 mm [0.2 mV]) at the J point in leads V3 and V4. Inferolateral injury is indicated inferiorly by ST changes in leads II, III, and aVL and laterally by the ST elevation in leads V5 and V6.
Subsequent cardiac catheterization showed an occluded proximal left anterior descending artery and significant diagonal and obtuse marginal disease.
ANSWER
This ECG shows normal sinus rhythm, an anterior myocardial infarction, and inferolateral injury consistent with an acute ST-elevation myocardial infarction (STEMI).
A P wave for every QRS complex and a QRS complex with every P wave, with a consistent PR interval and a rate > 60 and < 100 beats/min, signifies sinus rhythm.
Criteria for an anterior STEMI include new ST elevation (≥ 2 mm [0.2 mV]) at the J point in leads V3 and V4. Inferolateral injury is indicated inferiorly by ST changes in leads II, III, and aVL and laterally by the ST elevation in leads V5 and V6.
Subsequent cardiac catheterization showed an occluded proximal left anterior descending artery and significant diagonal and obtuse marginal disease.
ANSWER
This ECG shows normal sinus rhythm, an anterior myocardial infarction, and inferolateral injury consistent with an acute ST-elevation myocardial infarction (STEMI).
A P wave for every QRS complex and a QRS complex with every P wave, with a consistent PR interval and a rate > 60 and < 100 beats/min, signifies sinus rhythm.
Criteria for an anterior STEMI include new ST elevation (≥ 2 mm [0.2 mV]) at the J point in leads V3 and V4. Inferolateral injury is indicated inferiorly by ST changes in leads II, III, and aVL and laterally by the ST elevation in leads V5 and V6.
Subsequent cardiac catheterization showed an occluded proximal left anterior descending artery and significant diagonal and obtuse marginal disease.
A 58-year-old man is snowmobiling with friends when he develops crushing substernal chest pain. He immediately stops his snowmobile and waves his arms for help—but by the time his friends reach him, he is lying on the ground, clutching his chest.
When asked what happened, he tells his friends that he’s been experiencing chest pain for the past hour but didn’t want to stop or interrupt their fun. He further reveals that he’s had chest “twinges” for the past 2 months, but they were always brief, and he didn’t think they were anything to be concerned about. He acknowledges that the current episode is “far worse” than what he previously experienced.
Because they are in the wilderness, no one in the group is able to establish cellphone service to call 911. The patient is loaded onto the back of another snowmobile for the 30-minute ride to the parking lot, where cellular service is accessible. They call 911, and an ACLS ambulance arrives about 50 minutes later.
An ECG is obtained in the field and transmitted to the receiving hospital, and the catherization lab is notified of an incoming patient. Transport to the hospital takes an hour; during the trip, the patient is administered oxygen, morphine, nitroglycerin, and an aspirin, and he is noted to have several nonsustained episodes of polymorphic ventricular tachycardia. The patient arrives at the hospital about 4 hours after onset of chest pain.
Medical history includes longstanding uncontrolled hypertension, recent onset of type 2 diabetes, and gastric reflux. He has never had shortness of breath, dyspnea on exertion, syncope, or near-syncope.
Current medications include lisinopril and metformin. However, the patient informs you that he hasn’t taken lisinopril in more than 3 months, and although he’s been given a prescription for metformin, he hasn’t filled it. He has no known drug allergies.
The patient is a mechanic at a local auto dealership. He smokes between 1 and 1.5 packs of cigarettes per day and has attempted to quit several times. He also consumes about 1 case of beer per week.
He is divorced, has no children, and lives alone. Both parents died in an automobile accident. The patient knows his father had several heart attacks beginning in his mid-50s and his mother “had thyroid problems.” His grandparents were known to have coronary artery disease and diabetes.
Review of systems is positive for a longstanding smoker’s cough and a healing burn on his right forearm, attributed to a welding injury.
His pretransport vital signs include a blood pressure of 178/88 mm Hg; pulse, 88 beats/min; respiratory rate, 18 breaths/min-1; and temperature, 97.6ºF. His stated weight is 265 lb and his height, 69 in.
Your findings on the physical exam corroborate those called in by the paramedics: an obese white male in obvious distress but alert and cooperative. His lungs reveal diffuse rales and crackles that clear with vigorous coughing. His cardiac exam reveals a regular rhythm at a rate of 80 beats/min with no murmurs or rubs. The abdomen is obese but otherwise normal. There is no peripheral edema. Pulses are strong and equal bilaterally. The neurologic exam is grossly intact. A bandaged second-degree burn is noted on the lower right forearm.
A repeat ECG shows a ventricular rate of 80 beats/min; PR interval, 162 ms; QRS duration, 106 ms; QT/QTc interval, 370/426 ms; P axis, 51°; R axis, –20°; and T axis, 70°. What is your interpretation?
FDA fast-tracks psilocybin for major depressive disorder
Psilocybin, a short-acting compound that is the psychoactive ingredient in “magic mushrooms,” has received a Breakthrough Therapy designation from the Food and Drug Administration for the treatment of adults with major depressive disorder.
The designation was given to the Usona Institute, a nonprofit medical research organization, and comes in the wake of Usona’s launch of a phase 2 clinical trial that will include about 80 participants at seven study sites across the United States, according to a press release. Two sites are currently recruiting patients, and the others are expected to begin recruiting in 2020.
Breakthrough Therapy designation as defined by the FDA means that, based on preliminary research, “the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint.” In this case, Usona is working with the University of Wisconsin’s University Hospital in Madison, and other collaborators, according to a presentation by Malynn Utzinger, MD, director of integrative medicine and cofounder of the organization.
More information on the Usona Institute and Usona’s clinical trials is available at https://usonaclinicaltrials.org/.
Psilocybin, a short-acting compound that is the psychoactive ingredient in “magic mushrooms,” has received a Breakthrough Therapy designation from the Food and Drug Administration for the treatment of adults with major depressive disorder.
The designation was given to the Usona Institute, a nonprofit medical research organization, and comes in the wake of Usona’s launch of a phase 2 clinical trial that will include about 80 participants at seven study sites across the United States, according to a press release. Two sites are currently recruiting patients, and the others are expected to begin recruiting in 2020.
Breakthrough Therapy designation as defined by the FDA means that, based on preliminary research, “the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint.” In this case, Usona is working with the University of Wisconsin’s University Hospital in Madison, and other collaborators, according to a presentation by Malynn Utzinger, MD, director of integrative medicine and cofounder of the organization.
More information on the Usona Institute and Usona’s clinical trials is available at https://usonaclinicaltrials.org/.
Psilocybin, a short-acting compound that is the psychoactive ingredient in “magic mushrooms,” has received a Breakthrough Therapy designation from the Food and Drug Administration for the treatment of adults with major depressive disorder.
The designation was given to the Usona Institute, a nonprofit medical research organization, and comes in the wake of Usona’s launch of a phase 2 clinical trial that will include about 80 participants at seven study sites across the United States, according to a press release. Two sites are currently recruiting patients, and the others are expected to begin recruiting in 2020.
Breakthrough Therapy designation as defined by the FDA means that, based on preliminary research, “the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint.” In this case, Usona is working with the University of Wisconsin’s University Hospital in Madison, and other collaborators, according to a presentation by Malynn Utzinger, MD, director of integrative medicine and cofounder of the organization.
More information on the Usona Institute and Usona’s clinical trials is available at https://usonaclinicaltrials.org/.
Prosody recognition associated with functioning in first-episode schizophrenia
Affective prosody recognition is associated with role and social functioning in patients with a recent first episode of schizophrenia, according to Kelsey A. Bonfils, PhD, and associates.
The investigators conducted an analysis of 49 patients aged between 18 and 45 years with a recent first episode of schizophrenia who were participating in a larger randomized, controlled trial. Symptoms of schizophrenia were assessed using a 24-item version of the Brief Psychiatric Rating Scale (BPRS) and functioning was assessed using the Global Functioning Scale (GFS) and Role Functioning Scale (RFS). Study participants took the Prosody Task, which assessed the ability to recognize happiness, sadness, anger, fear, and disgust, and the Facial Emotion Identification Test (FEIT), which assesses the ability to recognize happiness, sadness, anger, fear, surprise, and disgust, reported Dr. Bonfils of the Veterans Affairs Pittsburgh Healthcare System and the department of psychiatry at the University of Pittsburgh. The study was published in Schizophrenia Research: Cognition.
In the Prosody Task, patients were significantly more likely to recognize anger (45.6% correct) and sadness (43.8%), and significantly less likely to recognize disgust (21.9%). In the FEIT, patients were most likely to recognize happiness (97.5%), followed by surprise (90.0%), anger (85.0%), sadness (77.5%), disgust (73.8%), and fear (55.0%).
Performance in the Prosody Task was associated with GFS role functioning and RFS social functioning, while FEIT performance was not significantly associated with any functioning measure. In terms of symptoms, Prosody Task performance was negatively associated with disorganization in the BPRS, and FEIT performance was associated with disorganization, reality distortion, and positive symptoms.
“These findings are consistent with the view that emotion recognition deficits could be contributing to deficits in the ability of people with first-episode schizophrenia to adequately function in the real world, both in relationships with friends and in normative young adult roles,” the investigators wrote.
Dr. Bonfils reported no conflicts of interest. Three coauthors reported receiving support, research grants, and funding from several pharmaceutical companies.
SOURCE: Bonfils KA et al. Schizophr Res Cogn. 2019. doi: 10.1016/j.scog.2019.100153.
Affective prosody recognition is associated with role and social functioning in patients with a recent first episode of schizophrenia, according to Kelsey A. Bonfils, PhD, and associates.
The investigators conducted an analysis of 49 patients aged between 18 and 45 years with a recent first episode of schizophrenia who were participating in a larger randomized, controlled trial. Symptoms of schizophrenia were assessed using a 24-item version of the Brief Psychiatric Rating Scale (BPRS) and functioning was assessed using the Global Functioning Scale (GFS) and Role Functioning Scale (RFS). Study participants took the Prosody Task, which assessed the ability to recognize happiness, sadness, anger, fear, and disgust, and the Facial Emotion Identification Test (FEIT), which assesses the ability to recognize happiness, sadness, anger, fear, surprise, and disgust, reported Dr. Bonfils of the Veterans Affairs Pittsburgh Healthcare System and the department of psychiatry at the University of Pittsburgh. The study was published in Schizophrenia Research: Cognition.
In the Prosody Task, patients were significantly more likely to recognize anger (45.6% correct) and sadness (43.8%), and significantly less likely to recognize disgust (21.9%). In the FEIT, patients were most likely to recognize happiness (97.5%), followed by surprise (90.0%), anger (85.0%), sadness (77.5%), disgust (73.8%), and fear (55.0%).
Performance in the Prosody Task was associated with GFS role functioning and RFS social functioning, while FEIT performance was not significantly associated with any functioning measure. In terms of symptoms, Prosody Task performance was negatively associated with disorganization in the BPRS, and FEIT performance was associated with disorganization, reality distortion, and positive symptoms.
“These findings are consistent with the view that emotion recognition deficits could be contributing to deficits in the ability of people with first-episode schizophrenia to adequately function in the real world, both in relationships with friends and in normative young adult roles,” the investigators wrote.
Dr. Bonfils reported no conflicts of interest. Three coauthors reported receiving support, research grants, and funding from several pharmaceutical companies.
SOURCE: Bonfils KA et al. Schizophr Res Cogn. 2019. doi: 10.1016/j.scog.2019.100153.
Affective prosody recognition is associated with role and social functioning in patients with a recent first episode of schizophrenia, according to Kelsey A. Bonfils, PhD, and associates.
The investigators conducted an analysis of 49 patients aged between 18 and 45 years with a recent first episode of schizophrenia who were participating in a larger randomized, controlled trial. Symptoms of schizophrenia were assessed using a 24-item version of the Brief Psychiatric Rating Scale (BPRS) and functioning was assessed using the Global Functioning Scale (GFS) and Role Functioning Scale (RFS). Study participants took the Prosody Task, which assessed the ability to recognize happiness, sadness, anger, fear, and disgust, and the Facial Emotion Identification Test (FEIT), which assesses the ability to recognize happiness, sadness, anger, fear, surprise, and disgust, reported Dr. Bonfils of the Veterans Affairs Pittsburgh Healthcare System and the department of psychiatry at the University of Pittsburgh. The study was published in Schizophrenia Research: Cognition.
In the Prosody Task, patients were significantly more likely to recognize anger (45.6% correct) and sadness (43.8%), and significantly less likely to recognize disgust (21.9%). In the FEIT, patients were most likely to recognize happiness (97.5%), followed by surprise (90.0%), anger (85.0%), sadness (77.5%), disgust (73.8%), and fear (55.0%).
Performance in the Prosody Task was associated with GFS role functioning and RFS social functioning, while FEIT performance was not significantly associated with any functioning measure. In terms of symptoms, Prosody Task performance was negatively associated with disorganization in the BPRS, and FEIT performance was associated with disorganization, reality distortion, and positive symptoms.
“These findings are consistent with the view that emotion recognition deficits could be contributing to deficits in the ability of people with first-episode schizophrenia to adequately function in the real world, both in relationships with friends and in normative young adult roles,” the investigators wrote.
Dr. Bonfils reported no conflicts of interest. Three coauthors reported receiving support, research grants, and funding from several pharmaceutical companies.
SOURCE: Bonfils KA et al. Schizophr Res Cogn. 2019. doi: 10.1016/j.scog.2019.100153.
FROM SCHIZOPHRENIA RESEARCH: COGNITION
Intensive BP control reduced dementia but increased brain atrophy and hurt cognition
SAN DIEGO – Intensive blood pressure control over 4 years reduced the overall risk of all-cause dementia by 17%, compared with standard care, but in subanalyses of the Systolic Blood Pressure Intervention Trial (SPRINT) it was also associated with significant decreases in cognitive function and total brain volume, researchers said at the Clinical Trials on Alzheimer’s Disease conference.
Whether these between-group differences were clinically meaningful was the topic of some debate, but they were enough to prompt Mary Sano, PhD, to strongly state her reservations.
“The cardiovascular effects of SPRINT were impressive, but I am concerned about minimizing the potentially negative effect on cognition,” said Dr. Sano, professor of psychiatry and director of the Alzheimer’s Disease Research Center at the Icahn School of Medicine at Mount Sinai, New York. “Do I really want to treat a healthy, nonimpaired patient like this if I have to warn them that their cognition might actually get worse? We just cannot minimize this risk. There is very strong evidence that [intensive treatment of blood pressure] might be a step backward in cognition. Would you lower your own blood pressure at a risk of losing some points on your cognition?”
The subanalyses were conducted as part of the SPRINT Memory and Cognition In Decreased Hypertension (SPRINT MIND) substudy, which looked at cardiovascular and mortality outcomes in 9,361 subjects whose hypertension was managed intensively or by standard care (target systolic blood pressure less than 120 mm Hg vs. less than 140 mm Hg). The trial was stopped early because of a 25% reduction in the primary composite cardiovascular disease endpoint and a 27% reduction in all-cause mortality in the intensive-treatment group.
SPRINT MIND examined the risks of incident probable dementia, mild cognitive impairment (MCI), and a composite outcome of both. Intensive control reduced the risk of MCI by 19% and the combined outcome by 15%.
At the conference, SPRINT MIND investigators presented three long-term subanalyses with a median intervention and follow-up time of about 4 years.
Sarah Gaussoin of Wake Forest University, Winston-Salem, N.C., presented unpublished data detailing the effects of intensive control on several dementia subtypes: nonamnestic single domain, nonamnestic multidomain, amnestic single domain, and amnestic multidomain. There were 640 subjects in this analysis.
After a median of 3.3 years of intervention and 5 years of follow-up, there were no differences in the rate of incident probable dementia between the single- and multidomain nonamnestic groups. “We did see a strong 22% decreased risk in single-domain versus multidomain amnestic MCI, however,” she said.
Nicholas Pajewski, PhD, also of Wake Forest University, discussed more detailed cognitive outcomes in SPRINT MIND among 2,900 subjects who had a full battery of cognitive testing at every assessment over 5 years. The outcomes included memory deficit and processing speed.
Dr. Pajewski reported finding no significant difference between the groups in the rates of memory decline in either outcome. But there was a greater rate of decline in processing speed in the intensively treated group, he added. The difference was small but statistically significant.
The difference was largely driven by results of a single cognitive test – the Trail Making Test Part A. “It corresponded to about a 1.25-second increase over 4 years,” in processing speed on this test, Dr. Pajewski said.
There were no between-group differences in any of the other domains explored, including language, executive function, global cognitive function, or the Montreal Cognitive Assessment.
“Obviously, these results are perplexing,” given the overall positive results of SPRINT MIND, he said. “Intensive blood pressure control is a beneficial thing, and we expected to see an effect on memory, or a blunting of decline, and instead we saw some small decrements going the other way. This led us to speculate about what’s going on.”
The trial relied on a narrow definition of MCI that might have affected the outcomes. There was also a very broad range of ages in the study, ranging from 53 to 86 years. More importantly, he said, the original SPRINT study didn’t collect cognitive data at baseline, so there was no way to know how many subjects already might have had MCI when they entered the trial.
Ilya Nasrallah, MD, PhD, of the University of Pennsylvania, Philadelphia, presented MRI data on white-matter lesions, hippocampal volume fractional anisotropy in the cingulum, and cerebral blood flow. The median time between scans was 4 years, with a median treatment time of 3.4 years.
The standard-care group showed a significantly greater increase in white-matter lesion volume at the follow-up scan than did the intensive-treatment group (1.45 cm3 vs. 0.92 cm3). But the intensively treated group had significantly more brain atrophy, losing a median of 30.6 cm3, compared with a loss of 26.9 cm3 in the standard-treatment group.
“It was a very small difference amounting to less than 1% of the total brain volume, but it was still statistically significant,” Dr. Nasrallah said.
Loss of gray-matter volume drove about two-thirds of the difference in the intensively treated group. There was a corresponding increase in cerebrospinal fluid volume that was driven by differences in the ventricles and the subarachnoid space.
However, there were no significant differences in right, left, or total hippocampal volume. There also were no differences in cingulate bundle anisotropy or cerebral blood flow.
SPRINT was funded by the National Institutes of Health. None of the investigators reported having financial conflicts of interest.
SAN DIEGO – Intensive blood pressure control over 4 years reduced the overall risk of all-cause dementia by 17%, compared with standard care, but in subanalyses of the Systolic Blood Pressure Intervention Trial (SPRINT) it was also associated with significant decreases in cognitive function and total brain volume, researchers said at the Clinical Trials on Alzheimer’s Disease conference.
Whether these between-group differences were clinically meaningful was the topic of some debate, but they were enough to prompt Mary Sano, PhD, to strongly state her reservations.
“The cardiovascular effects of SPRINT were impressive, but I am concerned about minimizing the potentially negative effect on cognition,” said Dr. Sano, professor of psychiatry and director of the Alzheimer’s Disease Research Center at the Icahn School of Medicine at Mount Sinai, New York. “Do I really want to treat a healthy, nonimpaired patient like this if I have to warn them that their cognition might actually get worse? We just cannot minimize this risk. There is very strong evidence that [intensive treatment of blood pressure] might be a step backward in cognition. Would you lower your own blood pressure at a risk of losing some points on your cognition?”
The subanalyses were conducted as part of the SPRINT Memory and Cognition In Decreased Hypertension (SPRINT MIND) substudy, which looked at cardiovascular and mortality outcomes in 9,361 subjects whose hypertension was managed intensively or by standard care (target systolic blood pressure less than 120 mm Hg vs. less than 140 mm Hg). The trial was stopped early because of a 25% reduction in the primary composite cardiovascular disease endpoint and a 27% reduction in all-cause mortality in the intensive-treatment group.
SPRINT MIND examined the risks of incident probable dementia, mild cognitive impairment (MCI), and a composite outcome of both. Intensive control reduced the risk of MCI by 19% and the combined outcome by 15%.
At the conference, SPRINT MIND investigators presented three long-term subanalyses with a median intervention and follow-up time of about 4 years.
Sarah Gaussoin of Wake Forest University, Winston-Salem, N.C., presented unpublished data detailing the effects of intensive control on several dementia subtypes: nonamnestic single domain, nonamnestic multidomain, amnestic single domain, and amnestic multidomain. There were 640 subjects in this analysis.
After a median of 3.3 years of intervention and 5 years of follow-up, there were no differences in the rate of incident probable dementia between the single- and multidomain nonamnestic groups. “We did see a strong 22% decreased risk in single-domain versus multidomain amnestic MCI, however,” she said.
Nicholas Pajewski, PhD, also of Wake Forest University, discussed more detailed cognitive outcomes in SPRINT MIND among 2,900 subjects who had a full battery of cognitive testing at every assessment over 5 years. The outcomes included memory deficit and processing speed.
Dr. Pajewski reported finding no significant difference between the groups in the rates of memory decline in either outcome. But there was a greater rate of decline in processing speed in the intensively treated group, he added. The difference was small but statistically significant.
The difference was largely driven by results of a single cognitive test – the Trail Making Test Part A. “It corresponded to about a 1.25-second increase over 4 years,” in processing speed on this test, Dr. Pajewski said.
There were no between-group differences in any of the other domains explored, including language, executive function, global cognitive function, or the Montreal Cognitive Assessment.
“Obviously, these results are perplexing,” given the overall positive results of SPRINT MIND, he said. “Intensive blood pressure control is a beneficial thing, and we expected to see an effect on memory, or a blunting of decline, and instead we saw some small decrements going the other way. This led us to speculate about what’s going on.”
The trial relied on a narrow definition of MCI that might have affected the outcomes. There was also a very broad range of ages in the study, ranging from 53 to 86 years. More importantly, he said, the original SPRINT study didn’t collect cognitive data at baseline, so there was no way to know how many subjects already might have had MCI when they entered the trial.
Ilya Nasrallah, MD, PhD, of the University of Pennsylvania, Philadelphia, presented MRI data on white-matter lesions, hippocampal volume fractional anisotropy in the cingulum, and cerebral blood flow. The median time between scans was 4 years, with a median treatment time of 3.4 years.
The standard-care group showed a significantly greater increase in white-matter lesion volume at the follow-up scan than did the intensive-treatment group (1.45 cm3 vs. 0.92 cm3). But the intensively treated group had significantly more brain atrophy, losing a median of 30.6 cm3, compared with a loss of 26.9 cm3 in the standard-treatment group.
“It was a very small difference amounting to less than 1% of the total brain volume, but it was still statistically significant,” Dr. Nasrallah said.
Loss of gray-matter volume drove about two-thirds of the difference in the intensively treated group. There was a corresponding increase in cerebrospinal fluid volume that was driven by differences in the ventricles and the subarachnoid space.
However, there were no significant differences in right, left, or total hippocampal volume. There also were no differences in cingulate bundle anisotropy or cerebral blood flow.
SPRINT was funded by the National Institutes of Health. None of the investigators reported having financial conflicts of interest.
SAN DIEGO – Intensive blood pressure control over 4 years reduced the overall risk of all-cause dementia by 17%, compared with standard care, but in subanalyses of the Systolic Blood Pressure Intervention Trial (SPRINT) it was also associated with significant decreases in cognitive function and total brain volume, researchers said at the Clinical Trials on Alzheimer’s Disease conference.
Whether these between-group differences were clinically meaningful was the topic of some debate, but they were enough to prompt Mary Sano, PhD, to strongly state her reservations.
“The cardiovascular effects of SPRINT were impressive, but I am concerned about minimizing the potentially negative effect on cognition,” said Dr. Sano, professor of psychiatry and director of the Alzheimer’s Disease Research Center at the Icahn School of Medicine at Mount Sinai, New York. “Do I really want to treat a healthy, nonimpaired patient like this if I have to warn them that their cognition might actually get worse? We just cannot minimize this risk. There is very strong evidence that [intensive treatment of blood pressure] might be a step backward in cognition. Would you lower your own blood pressure at a risk of losing some points on your cognition?”
The subanalyses were conducted as part of the SPRINT Memory and Cognition In Decreased Hypertension (SPRINT MIND) substudy, which looked at cardiovascular and mortality outcomes in 9,361 subjects whose hypertension was managed intensively or by standard care (target systolic blood pressure less than 120 mm Hg vs. less than 140 mm Hg). The trial was stopped early because of a 25% reduction in the primary composite cardiovascular disease endpoint and a 27% reduction in all-cause mortality in the intensive-treatment group.
SPRINT MIND examined the risks of incident probable dementia, mild cognitive impairment (MCI), and a composite outcome of both. Intensive control reduced the risk of MCI by 19% and the combined outcome by 15%.
At the conference, SPRINT MIND investigators presented three long-term subanalyses with a median intervention and follow-up time of about 4 years.
Sarah Gaussoin of Wake Forest University, Winston-Salem, N.C., presented unpublished data detailing the effects of intensive control on several dementia subtypes: nonamnestic single domain, nonamnestic multidomain, amnestic single domain, and amnestic multidomain. There were 640 subjects in this analysis.
After a median of 3.3 years of intervention and 5 years of follow-up, there were no differences in the rate of incident probable dementia between the single- and multidomain nonamnestic groups. “We did see a strong 22% decreased risk in single-domain versus multidomain amnestic MCI, however,” she said.
Nicholas Pajewski, PhD, also of Wake Forest University, discussed more detailed cognitive outcomes in SPRINT MIND among 2,900 subjects who had a full battery of cognitive testing at every assessment over 5 years. The outcomes included memory deficit and processing speed.
Dr. Pajewski reported finding no significant difference between the groups in the rates of memory decline in either outcome. But there was a greater rate of decline in processing speed in the intensively treated group, he added. The difference was small but statistically significant.
The difference was largely driven by results of a single cognitive test – the Trail Making Test Part A. “It corresponded to about a 1.25-second increase over 4 years,” in processing speed on this test, Dr. Pajewski said.
There were no between-group differences in any of the other domains explored, including language, executive function, global cognitive function, or the Montreal Cognitive Assessment.
“Obviously, these results are perplexing,” given the overall positive results of SPRINT MIND, he said. “Intensive blood pressure control is a beneficial thing, and we expected to see an effect on memory, or a blunting of decline, and instead we saw some small decrements going the other way. This led us to speculate about what’s going on.”
The trial relied on a narrow definition of MCI that might have affected the outcomes. There was also a very broad range of ages in the study, ranging from 53 to 86 years. More importantly, he said, the original SPRINT study didn’t collect cognitive data at baseline, so there was no way to know how many subjects already might have had MCI when they entered the trial.
Ilya Nasrallah, MD, PhD, of the University of Pennsylvania, Philadelphia, presented MRI data on white-matter lesions, hippocampal volume fractional anisotropy in the cingulum, and cerebral blood flow. The median time between scans was 4 years, with a median treatment time of 3.4 years.
The standard-care group showed a significantly greater increase in white-matter lesion volume at the follow-up scan than did the intensive-treatment group (1.45 cm3 vs. 0.92 cm3). But the intensively treated group had significantly more brain atrophy, losing a median of 30.6 cm3, compared with a loss of 26.9 cm3 in the standard-treatment group.
“It was a very small difference amounting to less than 1% of the total brain volume, but it was still statistically significant,” Dr. Nasrallah said.
Loss of gray-matter volume drove about two-thirds of the difference in the intensively treated group. There was a corresponding increase in cerebrospinal fluid volume that was driven by differences in the ventricles and the subarachnoid space.
However, there were no significant differences in right, left, or total hippocampal volume. There also were no differences in cingulate bundle anisotropy or cerebral blood flow.
SPRINT was funded by the National Institutes of Health. None of the investigators reported having financial conflicts of interest.
REPORTING FROM CTAD 2019
Gastroenterology practice evaluations: Can patients get satisfaction?
Although largely untouched by the first and second industrial revolutions in the 18th and 20th centuries, the practice of medicine in the 21st century is increasingly susceptible to the vast transformative power of the third – and rapidly approaching fourth – industrial revolutions. New technological advances and their associated distribution of knowledge and connectedness have allowed patients unprecedented access to health care information. The salutary effects of this change is manifest in a diversity of areas, including registries that facilitate participation in state of the art research such as ClinicalTrials.gov and the ability to track nascent trends in infectious diseases with Google searches.1
Although the stakes may seem lower when patients go online to choose a practitioner, the reality demonstrates just how important those search results can be. With parallels of similar trends in other sectors, there is an increasing emphasis on ranking health care facilities, practitioners, and medical experiences. This phenomenon extends beyond private Internet sites into government scorecards, which has significant implications. But even with widespread access to information, there is frequently a lack of context for interpreting these data. Consequently, it is worth exploring why measuring satisfaction can be important, how patients can rate practitioners, and what to do with the available information to improve care delivery.
The idea to measure patient satisfaction of delivered health care began in earnest during the 1980s with Irwin Press and Rodney Ganey collaborating to create formal processes for collecting data on the “salient aspects of ... health care experience, [involving] the interaction of expectations, preferences, and satisfaction with medical care.”2,3 The enthusiasm for collecting these data has grown greatly since that time. More recently, the federal government began obtaining data in 2002 when the Centers for Medicaid & Medicare Services and the Agency for Healthcare Research and Quality (AHRQ) collaborated to develop a standardized questionnaire for hospitalized patients known as the Hospital Consumer Assessment of Healthcare Providers and Systems, or HCAHPS.4 Subsequently, standardized survey instruments have been developed for nearly every phase of care, including outpatient care (CG-CAHPS), emergency care (ED-CAHPS), and ambulatory surgery care (OAS-CAHPS). These instruments are particularly relevant to gastroenterologists, with questions querying patients about preprocedure instructions, surgery center check-in processes, comfort of procedure and waiting rooms, friendliness of providers, and quality of postprocedure information.
The focus on rating satisfaction intensified in 2010 after the passage of the Affordable Care Act (ACA). Around this time, patient satisfaction and health outcomes became more deeply integrated concepts in health care quality. As part of a broader emphasis in this area, CMS initiated the hospital value-based purchasing (VBP) program, which tied incentive payments for Medicare beneficiaries to hospital-based health care quality and patient satisfaction. Within this schema, 25% of performance, and its associated economic stakes, is measured by HCAHPS scores.5 Other value programs such as the Merit-Based Incentive Payment Program (MIPS) include CAHPS instruments as optional assessments of quality.
Given the financial risks linked to satisfaction rankings and their online visibility, many argue that patient satisfaction is prioritized in organizations above more clinically meaningful metrics. Studies have shown, however, that high levels of patient satisfaction can lead to increased patient loyalty, treatment adherence, patient retention, staff morale, and personal and professional satisfaction.6,7 In fact, not surprisingly, there is an inverse correlation between patient satisfaction and the rates of malpractice lawsuits.7-10
Despite the growing relevance of patient perceptions to clinical practice, measuring satisfaction remains a challenge. While current metrics are particular to an individual patient’s experiences, underlying health conditions influence opinions of these episodes of care. Specifically, patients with depression and anxiety are, in general, less satisfied with the care they receive.11,12 Similarly, patients with chronic diseases on multiple medications and those with more severe symptoms are commonly less satisfied with their care than are patients with acute issues2 and with milder symptoms.3 As gastroenterologists, seeing sicker patients with chronic conditions is not uncommon, and this could serve as a disadvantage when compared with peers in other specialties because scores are not typically adjusted.
Since patient-centered metrics are likely to remain relevant in the future, and with the unique challenges this can present to practicing gastroenterologists, achieving higher degrees of patient satisfaction remains both aspirational and difficult. We will be asked to reconcile and manage not only clinical conundrums but also seemingly conflicting realities of patient preferences. For example, it has been shown that, among patients with irritable bowel syndrome (IBS), more testing led to higher satisfaction only until that testing was performed within the context of a gastroenterologist’s care.13 In contrast, within the endoscopy setting, a preprocedure diagnosis of IBS did not increase the risk for procedure-related dissatisfaction, provided patients were not prescribed chronic psychotropic medication, nervous prior to the procedure, distressed or in pain during the procedure, or had unmet physical or emotional needs during the procedure.14 Furthermore, there is poor correlation between endoscopic quality measures with strong evidence – such as adenoma detection rate, withdrawal time, and cecal intubation rate – and patient satisfaction.15
So, when considering these conflicting findings and evidence that patients’ global rating of their health care is not reliably associated with the quality of the care they receive,16 should we emphasize experience over outcome? As clinicians practicing in an increasingly transparent and value-based health care environment, we are subject to many priorities contending for our attention. We strive to provide care that is at once patient centric, evidence based, and low cost; however, achieving these goals often requires different strategies. At the end of the day, our primary aim is to provide consistently excellent patient care. We believe that quality and experience are not competing principles. Patient satisfaction is relevant and important, but it should not preclude adherence to our primary responsibility of providing high-quality care.
When trying to make clinical decisions that may compromise one of these goals for another, it can be helpful to recall the “me and my family” rule: What kind of care would I want for myself or my loved ones in this situation?
Acknowledgement
We thank Dr. Ziad Gellad (Duke University, Durham, N.C.) for his assistance in reviewing and providing feedback on this manuscript.
1. Proc Natl Acad Sci U S A. 2015;112(47):14473-8. 2. Am J Manag Care. 1997;3(4):579-94.
3. Gut. 2004;53(SUPPL. 4):40-4.
4. Virtual Mentor. 2013;15(11):982-7.
5. J Hosp Med. 2013;8(5):271-7.
6. Int J Health Care Qual Assur. 2011;24(4):266-73.
7. J Cutan Aesthet Surg. 2010;3(3):151-5.
8. Am J Med. 2005;118(10):1126-33.
9. JAMA. 2002;287(22):2951-7. 10. JAMA. 1994;272(20):1583-7.
11. J Diabetes Metab. 2012;3(7):1000210.
12. Am Heart J. 2000;140(1):105-10.
13. J Clin Gastroenterol. 2018;52(7):614-21.
14. Dig Dis Sci. 2005;50(10):1860-71.15. Am J Gastroenterol. 2014;109(7):1089-91.
16. Ann Intern Med. 2006;144(9):665-72.
Dr. Finn is a gastroenterologist with the Palo Alto Medical Foundation, Mountain View, Calif.; Dr. Leiman is assistant professor of medicine, director of esophageal research and quality in the division of gastroenterology, Duke University, Duke Clinical Research Institute, and chair-elect of the AGA Quality Committee.
Although largely untouched by the first and second industrial revolutions in the 18th and 20th centuries, the practice of medicine in the 21st century is increasingly susceptible to the vast transformative power of the third – and rapidly approaching fourth – industrial revolutions. New technological advances and their associated distribution of knowledge and connectedness have allowed patients unprecedented access to health care information. The salutary effects of this change is manifest in a diversity of areas, including registries that facilitate participation in state of the art research such as ClinicalTrials.gov and the ability to track nascent trends in infectious diseases with Google searches.1
Although the stakes may seem lower when patients go online to choose a practitioner, the reality demonstrates just how important those search results can be. With parallels of similar trends in other sectors, there is an increasing emphasis on ranking health care facilities, practitioners, and medical experiences. This phenomenon extends beyond private Internet sites into government scorecards, which has significant implications. But even with widespread access to information, there is frequently a lack of context for interpreting these data. Consequently, it is worth exploring why measuring satisfaction can be important, how patients can rate practitioners, and what to do with the available information to improve care delivery.
The idea to measure patient satisfaction of delivered health care began in earnest during the 1980s with Irwin Press and Rodney Ganey collaborating to create formal processes for collecting data on the “salient aspects of ... health care experience, [involving] the interaction of expectations, preferences, and satisfaction with medical care.”2,3 The enthusiasm for collecting these data has grown greatly since that time. More recently, the federal government began obtaining data in 2002 when the Centers for Medicaid & Medicare Services and the Agency for Healthcare Research and Quality (AHRQ) collaborated to develop a standardized questionnaire for hospitalized patients known as the Hospital Consumer Assessment of Healthcare Providers and Systems, or HCAHPS.4 Subsequently, standardized survey instruments have been developed for nearly every phase of care, including outpatient care (CG-CAHPS), emergency care (ED-CAHPS), and ambulatory surgery care (OAS-CAHPS). These instruments are particularly relevant to gastroenterologists, with questions querying patients about preprocedure instructions, surgery center check-in processes, comfort of procedure and waiting rooms, friendliness of providers, and quality of postprocedure information.
The focus on rating satisfaction intensified in 2010 after the passage of the Affordable Care Act (ACA). Around this time, patient satisfaction and health outcomes became more deeply integrated concepts in health care quality. As part of a broader emphasis in this area, CMS initiated the hospital value-based purchasing (VBP) program, which tied incentive payments for Medicare beneficiaries to hospital-based health care quality and patient satisfaction. Within this schema, 25% of performance, and its associated economic stakes, is measured by HCAHPS scores.5 Other value programs such as the Merit-Based Incentive Payment Program (MIPS) include CAHPS instruments as optional assessments of quality.
Given the financial risks linked to satisfaction rankings and their online visibility, many argue that patient satisfaction is prioritized in organizations above more clinically meaningful metrics. Studies have shown, however, that high levels of patient satisfaction can lead to increased patient loyalty, treatment adherence, patient retention, staff morale, and personal and professional satisfaction.6,7 In fact, not surprisingly, there is an inverse correlation between patient satisfaction and the rates of malpractice lawsuits.7-10
Despite the growing relevance of patient perceptions to clinical practice, measuring satisfaction remains a challenge. While current metrics are particular to an individual patient’s experiences, underlying health conditions influence opinions of these episodes of care. Specifically, patients with depression and anxiety are, in general, less satisfied with the care they receive.11,12 Similarly, patients with chronic diseases on multiple medications and those with more severe symptoms are commonly less satisfied with their care than are patients with acute issues2 and with milder symptoms.3 As gastroenterologists, seeing sicker patients with chronic conditions is not uncommon, and this could serve as a disadvantage when compared with peers in other specialties because scores are not typically adjusted.
Since patient-centered metrics are likely to remain relevant in the future, and with the unique challenges this can present to practicing gastroenterologists, achieving higher degrees of patient satisfaction remains both aspirational and difficult. We will be asked to reconcile and manage not only clinical conundrums but also seemingly conflicting realities of patient preferences. For example, it has been shown that, among patients with irritable bowel syndrome (IBS), more testing led to higher satisfaction only until that testing was performed within the context of a gastroenterologist’s care.13 In contrast, within the endoscopy setting, a preprocedure diagnosis of IBS did not increase the risk for procedure-related dissatisfaction, provided patients were not prescribed chronic psychotropic medication, nervous prior to the procedure, distressed or in pain during the procedure, or had unmet physical or emotional needs during the procedure.14 Furthermore, there is poor correlation between endoscopic quality measures with strong evidence – such as adenoma detection rate, withdrawal time, and cecal intubation rate – and patient satisfaction.15
So, when considering these conflicting findings and evidence that patients’ global rating of their health care is not reliably associated with the quality of the care they receive,16 should we emphasize experience over outcome? As clinicians practicing in an increasingly transparent and value-based health care environment, we are subject to many priorities contending for our attention. We strive to provide care that is at once patient centric, evidence based, and low cost; however, achieving these goals often requires different strategies. At the end of the day, our primary aim is to provide consistently excellent patient care. We believe that quality and experience are not competing principles. Patient satisfaction is relevant and important, but it should not preclude adherence to our primary responsibility of providing high-quality care.
When trying to make clinical decisions that may compromise one of these goals for another, it can be helpful to recall the “me and my family” rule: What kind of care would I want for myself or my loved ones in this situation?
Acknowledgement
We thank Dr. Ziad Gellad (Duke University, Durham, N.C.) for his assistance in reviewing and providing feedback on this manuscript.
1. Proc Natl Acad Sci U S A. 2015;112(47):14473-8. 2. Am J Manag Care. 1997;3(4):579-94.
3. Gut. 2004;53(SUPPL. 4):40-4.
4. Virtual Mentor. 2013;15(11):982-7.
5. J Hosp Med. 2013;8(5):271-7.
6. Int J Health Care Qual Assur. 2011;24(4):266-73.
7. J Cutan Aesthet Surg. 2010;3(3):151-5.
8. Am J Med. 2005;118(10):1126-33.
9. JAMA. 2002;287(22):2951-7. 10. JAMA. 1994;272(20):1583-7.
11. J Diabetes Metab. 2012;3(7):1000210.
12. Am Heart J. 2000;140(1):105-10.
13. J Clin Gastroenterol. 2018;52(7):614-21.
14. Dig Dis Sci. 2005;50(10):1860-71.15. Am J Gastroenterol. 2014;109(7):1089-91.
16. Ann Intern Med. 2006;144(9):665-72.
Dr. Finn is a gastroenterologist with the Palo Alto Medical Foundation, Mountain View, Calif.; Dr. Leiman is assistant professor of medicine, director of esophageal research and quality in the division of gastroenterology, Duke University, Duke Clinical Research Institute, and chair-elect of the AGA Quality Committee.
Although largely untouched by the first and second industrial revolutions in the 18th and 20th centuries, the practice of medicine in the 21st century is increasingly susceptible to the vast transformative power of the third – and rapidly approaching fourth – industrial revolutions. New technological advances and their associated distribution of knowledge and connectedness have allowed patients unprecedented access to health care information. The salutary effects of this change is manifest in a diversity of areas, including registries that facilitate participation in state of the art research such as ClinicalTrials.gov and the ability to track nascent trends in infectious diseases with Google searches.1
Although the stakes may seem lower when patients go online to choose a practitioner, the reality demonstrates just how important those search results can be. With parallels of similar trends in other sectors, there is an increasing emphasis on ranking health care facilities, practitioners, and medical experiences. This phenomenon extends beyond private Internet sites into government scorecards, which has significant implications. But even with widespread access to information, there is frequently a lack of context for interpreting these data. Consequently, it is worth exploring why measuring satisfaction can be important, how patients can rate practitioners, and what to do with the available information to improve care delivery.
The idea to measure patient satisfaction of delivered health care began in earnest during the 1980s with Irwin Press and Rodney Ganey collaborating to create formal processes for collecting data on the “salient aspects of ... health care experience, [involving] the interaction of expectations, preferences, and satisfaction with medical care.”2,3 The enthusiasm for collecting these data has grown greatly since that time. More recently, the federal government began obtaining data in 2002 when the Centers for Medicaid & Medicare Services and the Agency for Healthcare Research and Quality (AHRQ) collaborated to develop a standardized questionnaire for hospitalized patients known as the Hospital Consumer Assessment of Healthcare Providers and Systems, or HCAHPS.4 Subsequently, standardized survey instruments have been developed for nearly every phase of care, including outpatient care (CG-CAHPS), emergency care (ED-CAHPS), and ambulatory surgery care (OAS-CAHPS). These instruments are particularly relevant to gastroenterologists, with questions querying patients about preprocedure instructions, surgery center check-in processes, comfort of procedure and waiting rooms, friendliness of providers, and quality of postprocedure information.
The focus on rating satisfaction intensified in 2010 after the passage of the Affordable Care Act (ACA). Around this time, patient satisfaction and health outcomes became more deeply integrated concepts in health care quality. As part of a broader emphasis in this area, CMS initiated the hospital value-based purchasing (VBP) program, which tied incentive payments for Medicare beneficiaries to hospital-based health care quality and patient satisfaction. Within this schema, 25% of performance, and its associated economic stakes, is measured by HCAHPS scores.5 Other value programs such as the Merit-Based Incentive Payment Program (MIPS) include CAHPS instruments as optional assessments of quality.
Given the financial risks linked to satisfaction rankings and their online visibility, many argue that patient satisfaction is prioritized in organizations above more clinically meaningful metrics. Studies have shown, however, that high levels of patient satisfaction can lead to increased patient loyalty, treatment adherence, patient retention, staff morale, and personal and professional satisfaction.6,7 In fact, not surprisingly, there is an inverse correlation between patient satisfaction and the rates of malpractice lawsuits.7-10
Despite the growing relevance of patient perceptions to clinical practice, measuring satisfaction remains a challenge. While current metrics are particular to an individual patient’s experiences, underlying health conditions influence opinions of these episodes of care. Specifically, patients with depression and anxiety are, in general, less satisfied with the care they receive.11,12 Similarly, patients with chronic diseases on multiple medications and those with more severe symptoms are commonly less satisfied with their care than are patients with acute issues2 and with milder symptoms.3 As gastroenterologists, seeing sicker patients with chronic conditions is not uncommon, and this could serve as a disadvantage when compared with peers in other specialties because scores are not typically adjusted.
Since patient-centered metrics are likely to remain relevant in the future, and with the unique challenges this can present to practicing gastroenterologists, achieving higher degrees of patient satisfaction remains both aspirational and difficult. We will be asked to reconcile and manage not only clinical conundrums but also seemingly conflicting realities of patient preferences. For example, it has been shown that, among patients with irritable bowel syndrome (IBS), more testing led to higher satisfaction only until that testing was performed within the context of a gastroenterologist’s care.13 In contrast, within the endoscopy setting, a preprocedure diagnosis of IBS did not increase the risk for procedure-related dissatisfaction, provided patients were not prescribed chronic psychotropic medication, nervous prior to the procedure, distressed or in pain during the procedure, or had unmet physical or emotional needs during the procedure.14 Furthermore, there is poor correlation between endoscopic quality measures with strong evidence – such as adenoma detection rate, withdrawal time, and cecal intubation rate – and patient satisfaction.15
So, when considering these conflicting findings and evidence that patients’ global rating of their health care is not reliably associated with the quality of the care they receive,16 should we emphasize experience over outcome? As clinicians practicing in an increasingly transparent and value-based health care environment, we are subject to many priorities contending for our attention. We strive to provide care that is at once patient centric, evidence based, and low cost; however, achieving these goals often requires different strategies. At the end of the day, our primary aim is to provide consistently excellent patient care. We believe that quality and experience are not competing principles. Patient satisfaction is relevant and important, but it should not preclude adherence to our primary responsibility of providing high-quality care.
When trying to make clinical decisions that may compromise one of these goals for another, it can be helpful to recall the “me and my family” rule: What kind of care would I want for myself or my loved ones in this situation?
Acknowledgement
We thank Dr. Ziad Gellad (Duke University, Durham, N.C.) for his assistance in reviewing and providing feedback on this manuscript.
1. Proc Natl Acad Sci U S A. 2015;112(47):14473-8. 2. Am J Manag Care. 1997;3(4):579-94.
3. Gut. 2004;53(SUPPL. 4):40-4.
4. Virtual Mentor. 2013;15(11):982-7.
5. J Hosp Med. 2013;8(5):271-7.
6. Int J Health Care Qual Assur. 2011;24(4):266-73.
7. J Cutan Aesthet Surg. 2010;3(3):151-5.
8. Am J Med. 2005;118(10):1126-33.
9. JAMA. 2002;287(22):2951-7. 10. JAMA. 1994;272(20):1583-7.
11. J Diabetes Metab. 2012;3(7):1000210.
12. Am Heart J. 2000;140(1):105-10.
13. J Clin Gastroenterol. 2018;52(7):614-21.
14. Dig Dis Sci. 2005;50(10):1860-71.15. Am J Gastroenterol. 2014;109(7):1089-91.
16. Ann Intern Med. 2006;144(9):665-72.
Dr. Finn is a gastroenterologist with the Palo Alto Medical Foundation, Mountain View, Calif.; Dr. Leiman is assistant professor of medicine, director of esophageal research and quality in the division of gastroenterology, Duke University, Duke Clinical Research Institute, and chair-elect of the AGA Quality Committee.
Recurrent Angiotensin-Converting Enzyme Inhibitor-Induced Angioedema Refractory to Fresh Frozen Plasma
Angioedema induced by angiotensin-converting enzyme inhibitors (ACEIs) is present in from 0.1% to 0.7% of treated patients and more often involves the head, neck, face, lips, tongue, and larynx.1 ACEI-induced angioedema results from inhibition of angiotensin-converting enzyme (ACE), which results in reduced degradation and resultant accumulation of bradykinin, a potent inflammatory mediator.2
The treatment of choice is discontinuing all ACEIs; however, the patient may be at increased risk of a subsequent angioedema attack for many weeks.3 Antihistamines (H1 and H2 receptor blockade), epinephrine, and glucocorticoids are effective in allergic/histaminergic angioedema but are usually ineffective for hereditary angioedema or ACEI angioedema and are not recommended for acute therapy.4 Kallikrein-bradykinin pathway targeted therapies are now approved by the Food and Drug Administration (FDA) for hereditary angioedema attacks and have been studied for ACEI-induced angioedema. Ecallantide and icatibant inhibit conversion of precursors to bradykinin. Multiple randomized trials of ecallantide have not shown any advantage over traditional therapies.5 On the other hand, icatibant has shown resolution of angioedema in several case reports and in a randomized trial.6 Icatibant for ACEI-induced angioedema continues to be off-label because the data are conflicting.
Case Presentation
A 67-year-old man presented with a medical history of arterial hypertension (diagnosed 17 years previously), hypercholesterolemia, type 2 diabetes mellitus, alcohol dependence, and obesity. His outpatient medications included simvastatin, aripiprazole, losartan/hydrochlorothiazide, and amlodipine. He was voluntarily admitted for inpatient detoxification. After evaluation by the internist, medication reconciliation was done, and the therapy was adjusted according to medication availability. He reported having no drug allergies, and the losartan was changed for lisinopril. About 24 hours after the first dose of lisinopril, the patient developed swelling of the lips. Antihistamine and IV steroids were administered, and the ACEI was discontinued. His baseline vital signs were temperature 98° F, heart rate 83 beats per minute, respiratory rate 19 breaths per minute, blood pressure 150/94, and oxygen saturation 98% by pulse oximeter.
During the night shift the patient’s symptoms worsened, developing difficulty swallowing and shortness of breath. He was transferred to the medicine intensive care unit (MICU), intubated, and placed on mechanical ventilation to protect his airway. Laryngoscopic examination was notable for edematous tongue, uvula, and larynx. Also, the patient had mild stridor. His laboratory test results showed normal levels of complement, tryptase, and C1 esterase. On the fourth day after admission to MICU (Figure 1), the patient extubated himself. At that time, he did not present stridor or respiratory distress and remained at the MICU for 24 hours for close monitoring.
Thirty-six hours after self-extubation the patient developed stridor and shortness of breath at the general medicine ward. In view of his clinical presentation of recurrent ACEI-induced angioedema, the Anesthesiology Service was consulted. Direct visualization of the airways showed edema of the epiglottis and vocal cords, requiring nasotracheal intubation. Two units of fresh frozen plasma (FFP) were administered. Complete resolution of angioedema took at least 72 hours even after the administration of FFP. As part of the ventilator-associated pneumonia prevention bundle, the patient continued with daily spontaneous breathing trials. On the fourth day, he was he was extubated after a cuff-leak test was positive and his rapid shallow breathing index was adequate.
The cuff-leak test is usually done to predict postextubation stridor. It consists of deflating the endotracheal tube cuff to verify if gas can pass around the tube. Absence of cuff leak is suggestive of airway edema, a risk factor for postextubation stridor and failure of extubation. For example, if the patient has an endotracheal tube that is too large in relation to the patient’s airway, the leak test can result in a false negative. In this case, fiber optic visualization of the airway can confirm the endotracheal tube occluding all the airway even with the cuff deflated and without evidence of swelling of the vocal cords. The rapid shallow breathing index is a ratio of respiratory rate over tidal volume in liters and is used to predict successful extubation. Values < 105 have a high sensitivity for successful extubation.
The patient remained under observation for 24 hours in the MICU and then was transferred to the general medicine ward. Unfortunately, 36 hours after, the patient had a new episode of angioedema requiring endotracheal intubation and placement on mechanical ventilation. This was his third episode of angioedema; he had a difficult airway classified as a Cormack-Lehane grade 3, requiring intubation with fiber-optic laryngoscope. In view of the recurrent events, a tracheostomy was done several days later. Figure 2 shows posttracheostomy X-ray with adequate position of the tracheostomy tube.
The patient was transferred to the Respiratory Care Unit and weaned off mechanical ventilation. He completed an intensive physical rehabilitation program and was discharged home. On discharge, he was followed by the Otorhinolaryngology Service and was decannulated about 5 months after. After tracheostomy decannulation, he developed asymptomatic stridor. A neck computer tomography scan revealed soft tissue thickening at the anterior and lateral aspects of the proximal tracheal likely representing granulation tissue/scarring. The findings were consistent with proximal tracheal stenosis sequelae of tracheostomy and intubation. In Figure 3, the upper portion of the curve represents the expiratory limb of the forced vital capacity and the lower portion represents inspiration. The flow-volume loop graph showed flattening of the inspiratory limb. There was a plateau in the inspiratory limb, suggestive of limitation of inspiratory flow as seen in variable extrathoracic lesions, such as glotticstricture, tumors, and vocal cord paralysis.7 The findings on the flow-volume loop were consistent with the subglottic stenosis identified by laryngoscopic examination. The patient was reluctant to undergo further interventions.
Discussion
The standard therapy for ACEI-inducedangioedema continues to be airway management and discontinuation of medication. However, life-threatening progression of symptoms have led to the use of off-label therapies, including FFP and bradykinin receptor antagonists, such as icatibant, which has been approved by the FDA for the treatment of hereditary angioedema. Icatibant is expensive and most hospitals do not have access to it. When considering the bradykinin pathway for therapy, FFP is commonly used. The cases described in the literature that have reported success with the use of FFP have used up to 2 units. There is no reported benefit of its use beyond 2 units. The initial randomized trials of icatibant for ACEI angioedema showed decreased time of resolution of angioedema.6 However, repeated trials showed conflicting results. At Veterans Affairs Caribbean Healthcare System, this medication was not available, and we decided to use FFP to improve the patient’s symptoms.
The administration of 2 units of FFP has been documented on case reports as a method to decrease the time of resolution of angioedema and the risk of recurrence. The mechanism of action thought to be involved includes the degradation of bradykinin by the enzyme ACE into inactive peptides and by supplying C1 inhibitor.8 No randomized clinical trial has investigated the use of FFP for the treatment of ACEI-induced angioedema. However, a retrospective cohort study report compared patients who presented with acute (nonhereditary) angioedema and airway compromise and received FFP with patients who were not treated with FFP.9 The study suggested a shorter ICU stay in the group treated with FFP, but the findings did not present statistical outcomes.
Nevertheless, our patient had recurrent ACEI-induced angioedema refractory to FFP. In addition to ACE or kininase II, FFP contains high-molecular weight-kininogen and kallikrein, the substrates that form bradykinin, which explained the mechanism of worsening angioedema.10 No randomized trials have investigated the use of FFP for the treatment of bradykinin-induced angioedema nor the appropriate dose.
Conclusion
In view of the emerging case reports of the effectiveness of FFP, this case of refractory angioedema raises concern for its true effectiveness and other possible factors involved in the mechanism of recurrence. Probably it would be unwise to conduct randomized studies in clinical situations such as the ones outlined. A collection of case series where FFP administration was done may be a more reasonable source of conclusions to be analyzed by a panel of experts.
1. Sánchez-Borges M, González-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2(3):195-198.
2. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103-113.
3. Moellman JJ, Bernstein JA, Lindsell C, et al; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469-484.
4. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725-735.
5. van den Elzen M, Go MFLC, Knulst AC, Blankestijn MA, van Os-Medendorp H, Otten HG. Efficacy of treatment of non-hereditary angioedema. Clinic Rev Allerg Immunol. 2018;54(3):412-431.
6. Bas M, Greve J, Stelter S, et al. A randomized trial of icatibant in ace-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418-425.
7. Diaz J, Casal J, Rodriguez W. Flow-volume loops: clinical correlation. PR Health Sci J. 2008;27(2):181-182.
8. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep. 2012;2012:pii:bcr2012006849.
9. Saeb A, Hagglund KH, Cigolle CT. Using fresh frozen plasma for acute airway angioedema to prevent intubation in the emergency department: a retrospective cohort study. Emerg Med Int. 2016;2016:6091510.
10. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377-1382.
Angioedema induced by angiotensin-converting enzyme inhibitors (ACEIs) is present in from 0.1% to 0.7% of treated patients and more often involves the head, neck, face, lips, tongue, and larynx.1 ACEI-induced angioedema results from inhibition of angiotensin-converting enzyme (ACE), which results in reduced degradation and resultant accumulation of bradykinin, a potent inflammatory mediator.2
The treatment of choice is discontinuing all ACEIs; however, the patient may be at increased risk of a subsequent angioedema attack for many weeks.3 Antihistamines (H1 and H2 receptor blockade), epinephrine, and glucocorticoids are effective in allergic/histaminergic angioedema but are usually ineffective for hereditary angioedema or ACEI angioedema and are not recommended for acute therapy.4 Kallikrein-bradykinin pathway targeted therapies are now approved by the Food and Drug Administration (FDA) for hereditary angioedema attacks and have been studied for ACEI-induced angioedema. Ecallantide and icatibant inhibit conversion of precursors to bradykinin. Multiple randomized trials of ecallantide have not shown any advantage over traditional therapies.5 On the other hand, icatibant has shown resolution of angioedema in several case reports and in a randomized trial.6 Icatibant for ACEI-induced angioedema continues to be off-label because the data are conflicting.
Case Presentation
A 67-year-old man presented with a medical history of arterial hypertension (diagnosed 17 years previously), hypercholesterolemia, type 2 diabetes mellitus, alcohol dependence, and obesity. His outpatient medications included simvastatin, aripiprazole, losartan/hydrochlorothiazide, and amlodipine. He was voluntarily admitted for inpatient detoxification. After evaluation by the internist, medication reconciliation was done, and the therapy was adjusted according to medication availability. He reported having no drug allergies, and the losartan was changed for lisinopril. About 24 hours after the first dose of lisinopril, the patient developed swelling of the lips. Antihistamine and IV steroids were administered, and the ACEI was discontinued. His baseline vital signs were temperature 98° F, heart rate 83 beats per minute, respiratory rate 19 breaths per minute, blood pressure 150/94, and oxygen saturation 98% by pulse oximeter.
During the night shift the patient’s symptoms worsened, developing difficulty swallowing and shortness of breath. He was transferred to the medicine intensive care unit (MICU), intubated, and placed on mechanical ventilation to protect his airway. Laryngoscopic examination was notable for edematous tongue, uvula, and larynx. Also, the patient had mild stridor. His laboratory test results showed normal levels of complement, tryptase, and C1 esterase. On the fourth day after admission to MICU (Figure 1), the patient extubated himself. At that time, he did not present stridor or respiratory distress and remained at the MICU for 24 hours for close monitoring.
Thirty-six hours after self-extubation the patient developed stridor and shortness of breath at the general medicine ward. In view of his clinical presentation of recurrent ACEI-induced angioedema, the Anesthesiology Service was consulted. Direct visualization of the airways showed edema of the epiglottis and vocal cords, requiring nasotracheal intubation. Two units of fresh frozen plasma (FFP) were administered. Complete resolution of angioedema took at least 72 hours even after the administration of FFP. As part of the ventilator-associated pneumonia prevention bundle, the patient continued with daily spontaneous breathing trials. On the fourth day, he was he was extubated after a cuff-leak test was positive and his rapid shallow breathing index was adequate.
The cuff-leak test is usually done to predict postextubation stridor. It consists of deflating the endotracheal tube cuff to verify if gas can pass around the tube. Absence of cuff leak is suggestive of airway edema, a risk factor for postextubation stridor and failure of extubation. For example, if the patient has an endotracheal tube that is too large in relation to the patient’s airway, the leak test can result in a false negative. In this case, fiber optic visualization of the airway can confirm the endotracheal tube occluding all the airway even with the cuff deflated and without evidence of swelling of the vocal cords. The rapid shallow breathing index is a ratio of respiratory rate over tidal volume in liters and is used to predict successful extubation. Values < 105 have a high sensitivity for successful extubation.
The patient remained under observation for 24 hours in the MICU and then was transferred to the general medicine ward. Unfortunately, 36 hours after, the patient had a new episode of angioedema requiring endotracheal intubation and placement on mechanical ventilation. This was his third episode of angioedema; he had a difficult airway classified as a Cormack-Lehane grade 3, requiring intubation with fiber-optic laryngoscope. In view of the recurrent events, a tracheostomy was done several days later. Figure 2 shows posttracheostomy X-ray with adequate position of the tracheostomy tube.
The patient was transferred to the Respiratory Care Unit and weaned off mechanical ventilation. He completed an intensive physical rehabilitation program and was discharged home. On discharge, he was followed by the Otorhinolaryngology Service and was decannulated about 5 months after. After tracheostomy decannulation, he developed asymptomatic stridor. A neck computer tomography scan revealed soft tissue thickening at the anterior and lateral aspects of the proximal tracheal likely representing granulation tissue/scarring. The findings were consistent with proximal tracheal stenosis sequelae of tracheostomy and intubation. In Figure 3, the upper portion of the curve represents the expiratory limb of the forced vital capacity and the lower portion represents inspiration. The flow-volume loop graph showed flattening of the inspiratory limb. There was a plateau in the inspiratory limb, suggestive of limitation of inspiratory flow as seen in variable extrathoracic lesions, such as glotticstricture, tumors, and vocal cord paralysis.7 The findings on the flow-volume loop were consistent with the subglottic stenosis identified by laryngoscopic examination. The patient was reluctant to undergo further interventions.
Discussion
The standard therapy for ACEI-inducedangioedema continues to be airway management and discontinuation of medication. However, life-threatening progression of symptoms have led to the use of off-label therapies, including FFP and bradykinin receptor antagonists, such as icatibant, which has been approved by the FDA for the treatment of hereditary angioedema. Icatibant is expensive and most hospitals do not have access to it. When considering the bradykinin pathway for therapy, FFP is commonly used. The cases described in the literature that have reported success with the use of FFP have used up to 2 units. There is no reported benefit of its use beyond 2 units. The initial randomized trials of icatibant for ACEI angioedema showed decreased time of resolution of angioedema.6 However, repeated trials showed conflicting results. At Veterans Affairs Caribbean Healthcare System, this medication was not available, and we decided to use FFP to improve the patient’s symptoms.
The administration of 2 units of FFP has been documented on case reports as a method to decrease the time of resolution of angioedema and the risk of recurrence. The mechanism of action thought to be involved includes the degradation of bradykinin by the enzyme ACE into inactive peptides and by supplying C1 inhibitor.8 No randomized clinical trial has investigated the use of FFP for the treatment of ACEI-induced angioedema. However, a retrospective cohort study report compared patients who presented with acute (nonhereditary) angioedema and airway compromise and received FFP with patients who were not treated with FFP.9 The study suggested a shorter ICU stay in the group treated with FFP, but the findings did not present statistical outcomes.
Nevertheless, our patient had recurrent ACEI-induced angioedema refractory to FFP. In addition to ACE or kininase II, FFP contains high-molecular weight-kininogen and kallikrein, the substrates that form bradykinin, which explained the mechanism of worsening angioedema.10 No randomized trials have investigated the use of FFP for the treatment of bradykinin-induced angioedema nor the appropriate dose.
Conclusion
In view of the emerging case reports of the effectiveness of FFP, this case of refractory angioedema raises concern for its true effectiveness and other possible factors involved in the mechanism of recurrence. Probably it would be unwise to conduct randomized studies in clinical situations such as the ones outlined. A collection of case series where FFP administration was done may be a more reasonable source of conclusions to be analyzed by a panel of experts.
Angioedema induced by angiotensin-converting enzyme inhibitors (ACEIs) is present in from 0.1% to 0.7% of treated patients and more often involves the head, neck, face, lips, tongue, and larynx.1 ACEI-induced angioedema results from inhibition of angiotensin-converting enzyme (ACE), which results in reduced degradation and resultant accumulation of bradykinin, a potent inflammatory mediator.2
The treatment of choice is discontinuing all ACEIs; however, the patient may be at increased risk of a subsequent angioedema attack for many weeks.3 Antihistamines (H1 and H2 receptor blockade), epinephrine, and glucocorticoids are effective in allergic/histaminergic angioedema but are usually ineffective for hereditary angioedema or ACEI angioedema and are not recommended for acute therapy.4 Kallikrein-bradykinin pathway targeted therapies are now approved by the Food and Drug Administration (FDA) for hereditary angioedema attacks and have been studied for ACEI-induced angioedema. Ecallantide and icatibant inhibit conversion of precursors to bradykinin. Multiple randomized trials of ecallantide have not shown any advantage over traditional therapies.5 On the other hand, icatibant has shown resolution of angioedema in several case reports and in a randomized trial.6 Icatibant for ACEI-induced angioedema continues to be off-label because the data are conflicting.
Case Presentation
A 67-year-old man presented with a medical history of arterial hypertension (diagnosed 17 years previously), hypercholesterolemia, type 2 diabetes mellitus, alcohol dependence, and obesity. His outpatient medications included simvastatin, aripiprazole, losartan/hydrochlorothiazide, and amlodipine. He was voluntarily admitted for inpatient detoxification. After evaluation by the internist, medication reconciliation was done, and the therapy was adjusted according to medication availability. He reported having no drug allergies, and the losartan was changed for lisinopril. About 24 hours after the first dose of lisinopril, the patient developed swelling of the lips. Antihistamine and IV steroids were administered, and the ACEI was discontinued. His baseline vital signs were temperature 98° F, heart rate 83 beats per minute, respiratory rate 19 breaths per minute, blood pressure 150/94, and oxygen saturation 98% by pulse oximeter.
During the night shift the patient’s symptoms worsened, developing difficulty swallowing and shortness of breath. He was transferred to the medicine intensive care unit (MICU), intubated, and placed on mechanical ventilation to protect his airway. Laryngoscopic examination was notable for edematous tongue, uvula, and larynx. Also, the patient had mild stridor. His laboratory test results showed normal levels of complement, tryptase, and C1 esterase. On the fourth day after admission to MICU (Figure 1), the patient extubated himself. At that time, he did not present stridor or respiratory distress and remained at the MICU for 24 hours for close monitoring.
Thirty-six hours after self-extubation the patient developed stridor and shortness of breath at the general medicine ward. In view of his clinical presentation of recurrent ACEI-induced angioedema, the Anesthesiology Service was consulted. Direct visualization of the airways showed edema of the epiglottis and vocal cords, requiring nasotracheal intubation. Two units of fresh frozen plasma (FFP) were administered. Complete resolution of angioedema took at least 72 hours even after the administration of FFP. As part of the ventilator-associated pneumonia prevention bundle, the patient continued with daily spontaneous breathing trials. On the fourth day, he was he was extubated after a cuff-leak test was positive and his rapid shallow breathing index was adequate.
The cuff-leak test is usually done to predict postextubation stridor. It consists of deflating the endotracheal tube cuff to verify if gas can pass around the tube. Absence of cuff leak is suggestive of airway edema, a risk factor for postextubation stridor and failure of extubation. For example, if the patient has an endotracheal tube that is too large in relation to the patient’s airway, the leak test can result in a false negative. In this case, fiber optic visualization of the airway can confirm the endotracheal tube occluding all the airway even with the cuff deflated and without evidence of swelling of the vocal cords. The rapid shallow breathing index is a ratio of respiratory rate over tidal volume in liters and is used to predict successful extubation. Values < 105 have a high sensitivity for successful extubation.
The patient remained under observation for 24 hours in the MICU and then was transferred to the general medicine ward. Unfortunately, 36 hours after, the patient had a new episode of angioedema requiring endotracheal intubation and placement on mechanical ventilation. This was his third episode of angioedema; he had a difficult airway classified as a Cormack-Lehane grade 3, requiring intubation with fiber-optic laryngoscope. In view of the recurrent events, a tracheostomy was done several days later. Figure 2 shows posttracheostomy X-ray with adequate position of the tracheostomy tube.
The patient was transferred to the Respiratory Care Unit and weaned off mechanical ventilation. He completed an intensive physical rehabilitation program and was discharged home. On discharge, he was followed by the Otorhinolaryngology Service and was decannulated about 5 months after. After tracheostomy decannulation, he developed asymptomatic stridor. A neck computer tomography scan revealed soft tissue thickening at the anterior and lateral aspects of the proximal tracheal likely representing granulation tissue/scarring. The findings were consistent with proximal tracheal stenosis sequelae of tracheostomy and intubation. In Figure 3, the upper portion of the curve represents the expiratory limb of the forced vital capacity and the lower portion represents inspiration. The flow-volume loop graph showed flattening of the inspiratory limb. There was a plateau in the inspiratory limb, suggestive of limitation of inspiratory flow as seen in variable extrathoracic lesions, such as glotticstricture, tumors, and vocal cord paralysis.7 The findings on the flow-volume loop were consistent with the subglottic stenosis identified by laryngoscopic examination. The patient was reluctant to undergo further interventions.
Discussion
The standard therapy for ACEI-inducedangioedema continues to be airway management and discontinuation of medication. However, life-threatening progression of symptoms have led to the use of off-label therapies, including FFP and bradykinin receptor antagonists, such as icatibant, which has been approved by the FDA for the treatment of hereditary angioedema. Icatibant is expensive and most hospitals do not have access to it. When considering the bradykinin pathway for therapy, FFP is commonly used. The cases described in the literature that have reported success with the use of FFP have used up to 2 units. There is no reported benefit of its use beyond 2 units. The initial randomized trials of icatibant for ACEI angioedema showed decreased time of resolution of angioedema.6 However, repeated trials showed conflicting results. At Veterans Affairs Caribbean Healthcare System, this medication was not available, and we decided to use FFP to improve the patient’s symptoms.
The administration of 2 units of FFP has been documented on case reports as a method to decrease the time of resolution of angioedema and the risk of recurrence. The mechanism of action thought to be involved includes the degradation of bradykinin by the enzyme ACE into inactive peptides and by supplying C1 inhibitor.8 No randomized clinical trial has investigated the use of FFP for the treatment of ACEI-induced angioedema. However, a retrospective cohort study report compared patients who presented with acute (nonhereditary) angioedema and airway compromise and received FFP with patients who were not treated with FFP.9 The study suggested a shorter ICU stay in the group treated with FFP, but the findings did not present statistical outcomes.
Nevertheless, our patient had recurrent ACEI-induced angioedema refractory to FFP. In addition to ACE or kininase II, FFP contains high-molecular weight-kininogen and kallikrein, the substrates that form bradykinin, which explained the mechanism of worsening angioedema.10 No randomized trials have investigated the use of FFP for the treatment of bradykinin-induced angioedema nor the appropriate dose.
Conclusion
In view of the emerging case reports of the effectiveness of FFP, this case of refractory angioedema raises concern for its true effectiveness and other possible factors involved in the mechanism of recurrence. Probably it would be unwise to conduct randomized studies in clinical situations such as the ones outlined. A collection of case series where FFP administration was done may be a more reasonable source of conclusions to be analyzed by a panel of experts.
1. Sánchez-Borges M, González-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2(3):195-198.
2. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103-113.
3. Moellman JJ, Bernstein JA, Lindsell C, et al; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469-484.
4. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725-735.
5. van den Elzen M, Go MFLC, Knulst AC, Blankestijn MA, van Os-Medendorp H, Otten HG. Efficacy of treatment of non-hereditary angioedema. Clinic Rev Allerg Immunol. 2018;54(3):412-431.
6. Bas M, Greve J, Stelter S, et al. A randomized trial of icatibant in ace-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418-425.
7. Diaz J, Casal J, Rodriguez W. Flow-volume loops: clinical correlation. PR Health Sci J. 2008;27(2):181-182.
8. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep. 2012;2012:pii:bcr2012006849.
9. Saeb A, Hagglund KH, Cigolle CT. Using fresh frozen plasma for acute airway angioedema to prevent intubation in the emergency department: a retrospective cohort study. Emerg Med Int. 2016;2016:6091510.
10. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377-1382.
1. Sánchez-Borges M, González-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2(3):195-198.
2. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103-113.
3. Moellman JJ, Bernstein JA, Lindsell C, et al; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469-484.
4. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725-735.
5. van den Elzen M, Go MFLC, Knulst AC, Blankestijn MA, van Os-Medendorp H, Otten HG. Efficacy of treatment of non-hereditary angioedema. Clinic Rev Allerg Immunol. 2018;54(3):412-431.
6. Bas M, Greve J, Stelter S, et al. A randomized trial of icatibant in ace-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418-425.
7. Diaz J, Casal J, Rodriguez W. Flow-volume loops: clinical correlation. PR Health Sci J. 2008;27(2):181-182.
8. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep. 2012;2012:pii:bcr2012006849.
9. Saeb A, Hagglund KH, Cigolle CT. Using fresh frozen plasma for acute airway angioedema to prevent intubation in the emergency department: a retrospective cohort study. Emerg Med Int. 2016;2016:6091510.
10. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377-1382.
Women experience more chemoradiotherapy toxicity in rectal cancer
Women are more likely to experience acute toxic effects from chemoradiotherapy for rectal cancer than men, but this does not appear to negatively impact treatment adherence or outcomes, research suggests.
In a research letter published in JAMA Oncology, Markus Diefenhardt, MD, from the University of Frankfurt and coauthors wrote that, while the risk of toxic chemotherapy effects was known to be greater in women for a number of cancers, this association was relatively unexplored for rectal cancer.
The researchers performed a pooled analysis of data from two phase 3, randomized clinical trials, involving 1,016 patients with rectal cancer – 28.6% of whom were female – treated with fluorouracil-based chemoradiotherapy followed by surgery and adjuvant fluorouracil.
They found that women experienced significantly higher rates of leukopenia and diarrhea than men. Grade 3-4 leukopenia was experienced by 28.6% of women, compared with 20.5% of men, and grades 3-4 diarrhea was experienced by 17.2% of women, compared with 8.1% of men.
Despite this, the study found similar rates of adherence to treatment between men and women both for neoadjuvant and adjuvant chemoradiotherapy. Women also had similar rates of disease-free survival and overall survival as men, and there were no significant differences in local recurrence or distant metastases.
“Although to our knowledge no data support using different chemotherapy regimens for men and women with rectal cancer, increased awareness of a higher risk of toxic effects among women may facilitate refinement of fluorouracil-based chemoradiotherapy and adjuvant chemotherapy, such as tailored patient education, closer monitoring of adverse effects, and earlier introduction of supportive measures,” the authors wrote.
The authors proposed several possible explanations for the higher rate of toxic effects in women. For example, women may have lower levels of the enzyme dihydropyridine dehydrogenase, which catabolizes fluorouracil, which could result in overdosing of fluorouracil. Similarly, sex-specific body fat composition could also contribute to fluorouracil overdosing in women.
The study also saw fewer postoperative complications in women, which the authors suggested could be related to the lower rate of abdominoperineal resections in women.
The two clinical trials included in the study were funded by German Cancer Aid. One author declared funding from German Cancer Aid, another declared a range of honoraria, research fees and institutional funding from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Diefendhardt M et al. JAMA Oncol. 2019 Dec 5. doi: 10.1001/jamaoncol.2019.5102.
Women are more likely to experience acute toxic effects from chemoradiotherapy for rectal cancer than men, but this does not appear to negatively impact treatment adherence or outcomes, research suggests.
In a research letter published in JAMA Oncology, Markus Diefenhardt, MD, from the University of Frankfurt and coauthors wrote that, while the risk of toxic chemotherapy effects was known to be greater in women for a number of cancers, this association was relatively unexplored for rectal cancer.
The researchers performed a pooled analysis of data from two phase 3, randomized clinical trials, involving 1,016 patients with rectal cancer – 28.6% of whom were female – treated with fluorouracil-based chemoradiotherapy followed by surgery and adjuvant fluorouracil.
They found that women experienced significantly higher rates of leukopenia and diarrhea than men. Grade 3-4 leukopenia was experienced by 28.6% of women, compared with 20.5% of men, and grades 3-4 diarrhea was experienced by 17.2% of women, compared with 8.1% of men.
Despite this, the study found similar rates of adherence to treatment between men and women both for neoadjuvant and adjuvant chemoradiotherapy. Women also had similar rates of disease-free survival and overall survival as men, and there were no significant differences in local recurrence or distant metastases.
“Although to our knowledge no data support using different chemotherapy regimens for men and women with rectal cancer, increased awareness of a higher risk of toxic effects among women may facilitate refinement of fluorouracil-based chemoradiotherapy and adjuvant chemotherapy, such as tailored patient education, closer monitoring of adverse effects, and earlier introduction of supportive measures,” the authors wrote.
The authors proposed several possible explanations for the higher rate of toxic effects in women. For example, women may have lower levels of the enzyme dihydropyridine dehydrogenase, which catabolizes fluorouracil, which could result in overdosing of fluorouracil. Similarly, sex-specific body fat composition could also contribute to fluorouracil overdosing in women.
The study also saw fewer postoperative complications in women, which the authors suggested could be related to the lower rate of abdominoperineal resections in women.
The two clinical trials included in the study were funded by German Cancer Aid. One author declared funding from German Cancer Aid, another declared a range of honoraria, research fees and institutional funding from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Diefendhardt M et al. JAMA Oncol. 2019 Dec 5. doi: 10.1001/jamaoncol.2019.5102.
Women are more likely to experience acute toxic effects from chemoradiotherapy for rectal cancer than men, but this does not appear to negatively impact treatment adherence or outcomes, research suggests.
In a research letter published in JAMA Oncology, Markus Diefenhardt, MD, from the University of Frankfurt and coauthors wrote that, while the risk of toxic chemotherapy effects was known to be greater in women for a number of cancers, this association was relatively unexplored for rectal cancer.
The researchers performed a pooled analysis of data from two phase 3, randomized clinical trials, involving 1,016 patients with rectal cancer – 28.6% of whom were female – treated with fluorouracil-based chemoradiotherapy followed by surgery and adjuvant fluorouracil.
They found that women experienced significantly higher rates of leukopenia and diarrhea than men. Grade 3-4 leukopenia was experienced by 28.6% of women, compared with 20.5% of men, and grades 3-4 diarrhea was experienced by 17.2% of women, compared with 8.1% of men.
Despite this, the study found similar rates of adherence to treatment between men and women both for neoadjuvant and adjuvant chemoradiotherapy. Women also had similar rates of disease-free survival and overall survival as men, and there were no significant differences in local recurrence or distant metastases.
“Although to our knowledge no data support using different chemotherapy regimens for men and women with rectal cancer, increased awareness of a higher risk of toxic effects among women may facilitate refinement of fluorouracil-based chemoradiotherapy and adjuvant chemotherapy, such as tailored patient education, closer monitoring of adverse effects, and earlier introduction of supportive measures,” the authors wrote.
The authors proposed several possible explanations for the higher rate of toxic effects in women. For example, women may have lower levels of the enzyme dihydropyridine dehydrogenase, which catabolizes fluorouracil, which could result in overdosing of fluorouracil. Similarly, sex-specific body fat composition could also contribute to fluorouracil overdosing in women.
The study also saw fewer postoperative complications in women, which the authors suggested could be related to the lower rate of abdominoperineal resections in women.
The two clinical trials included in the study were funded by German Cancer Aid. One author declared funding from German Cancer Aid, another declared a range of honoraria, research fees and institutional funding from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Diefendhardt M et al. JAMA Oncol. 2019 Dec 5. doi: 10.1001/jamaoncol.2019.5102.
FROM JAMA ONCOLOGY
Key clinical point: Women show significantly higher rates of toxic effects from rectal cancer chemoradiotherapy than men.
Major finding: Women experience significantly higher rates of leukopenia and diarrhea from rectal cancer chemoradiotherapy.
Study details: A pooled analysis of data from two phase 3, randomized, controlled trials in 1,016 patients.
Disclosures: The two clinical trials included in the study were funded by German Cancer Aid. One author declared funding from German Cancer Aid, another declared a range of honoraria, research fees and institutional funding from the pharmaceutical sector. No other conflicts of interest were declared.
Source: Diefendhardt M et al. JAMA Oncol. 2019 Dec 5. doi: 10.1001/jamaoncol.2019.5102.
Millennials in Medicine: Cross-Trained Physicians Not Valued in Medical Marketplace
Millennials, defined as those born between 1981 and 1996, currently comprise 15% of all active physicians in the US.1,2 A recent survey found that nearly 4 of 5 US millennial physicians have a desire for cross-sectional work in areas beyond patient care, such as academic research, health care consulting, entrepreneurship, and health care administration.3
For employers and educators, a better understanding of these preferences, through consideration of the unique education and skill set of the millennial physician workforce, may lead to more effective recruitment of young physicians and improved health systems, avoiding a mismatch between health care provider skills and available jobs that can be costly for both employers and employees.4
This article describes how US millennial physicians are choosing to cross-train (obtaining multiple degrees and/or completing combined medical residency training) throughout undergraduate, medical, and graduate medical education. We also outline ways in which the current physician marketplace may not match the skills of this population and suggest some ways that health care organizations could capitalize on this trend toward more cross-trained personnel in order to effectively recruit and retain the next generation of physicians.
Millennial Education
Undergraduates
The number of interdisciplinary undergraduate majors increased by almost 250% from 1975 to 2000.5 In 2010, nearly 20% of US college students graduated with 2 majors, representing a 70% increase in double majors between 2001 and 2011.6,7 One emerging category of interdisciplinary majors in US colleges is health humanities programs, which have quadrupled since 2000.8
Medical school applicants and matriculants reflect this trend. Whereas in 1994, only 19% of applicants to medical school held nonscience degrees, about one-third of applicants now hold such degrees.9,10 We have found no aggregated data on double majors entering US medical schools, but public class profiles suggest that medical school matriculants mirror their undergraduate counterparts in their tendency to hold double majors. In 2016, for example, 15% of the incoming class at the University of Michigan Medical School was composed of double majors, increasing to over 25% in 2017.11
Medical Students
Early dual-degree programs in undergraduate medical training were reserved for MD/PhD programs.12 Most US MD/PhD programs (90 out of 151) now offer doctorates in social sciences, humanities, or other nontraditional fields of graduate medical study, reflecting a shift in interests of those seeking dual-degree training in undergraduate medical education.13 While only 3 MD/PhD programs in the 1970s included trainees in the social sciences, 17 such programs exist today.14
Interest in dual-degree programs offering master’s level study has also increased over the past decade. In 2017, 87 medical schools offered programs for students to pursue a master of public health (MPH) and 41 offered master of science degrees in various fields, up from 52 and 37 institutions, respectively in 2006.15 The number of schools offering combined training in nonscience fields has also grown, with 63 institutions now offering a master of business administration (MBA), nearly double the number offered in 2006.15 At some institutions more than 20% of students are earning a master’s degree or doctorate in addition to their MD degree.16
Residents
The authors found no documentation of US residency training programs, outside of those in the specialty of preventive medicine, providing trainees with formal opportunities to obtain an MBA or MPH prior to 2001.17 However, of the 510 internal medicine residency programs listed on the American Medical Association residency and fellowship database (freida.ama-assn.org), 45 identified as having established a pathway for residents to pursue an MBA, MPH, or PhD during residency.18
Over the past 20 years, combined residency programs have increased 49% (from 128 to 191), which is triple the 16% rate (1,350 to 1,562) of increase in programs in internal medicine, pediatrics, family medicine, psychiatry, and emergency medicine.19,20 A 2009 moratorium on the creation of new combined residency programs in psychiatry and neurology was lifted in 2016and is likely to increase the rate of total combined programs.21
The Table shows the number of categorical and combined residency programs available in 1996 and in 2016. Over 2 decades, 17 new specialty combinations became available for residency training. While there were no combined training programs within these 17 new combinations in 1996,there were 66 programs with these combinations in 2016.19,20
Although surgical specialties are notably absent from the list of combined residency options, likely due to the duration of surgical training, some surgical training programs do offer pathways that culminate in combined degrees,22 and a high number of surgery program directors agree that residents should receive formal training in business and practice management.23
The Medical Job Market
Although today’s young physicians are cross-trained in multiple disciplines, the current job market may not directly match these skill sets. Of the 7,235 jobs listed by the New England Journal of Medicine (NEJM) career center (www.nejmcareercenter.org/jobs), only 54 were targeted at those with combined training, the majority of which were aimed at those trained in internal medicine/pediatrics. Of the combined specialties in the Table, formal positions were listed for only 6.24 A search of nearly 1,500 federal medical positions on USAJOBS (www.usajobs.gov) found only 4 jobs that combined specialties, all restricted to internal medicine/pediatrics.25 When searching for jobs containing the terms MBA, MPH, and public health there were only 8 such positions on NEJM and 7 on USAJOBS.24,25 Although the totality of the medical marketplace may not be best encompassed by these sources, the authors believe NEJM and USAJOBS are somewhat representative of the opportunities for physicians in the US.
Medical jobs tailored to cross-trained physicians do not appear to have kept pace with the numbers of such specialists currently in medical school and residency training. Though millennials are cross-training in increasing numbers, we surmise that they are not doing so as a direct result of the job market.
Future Medicine
Regardless of the mismatch between cross-trained physicians and the current job market, millennials may be well suited for future health systems. In 2001, the National Academies of Sciences, Engineering and Medicine (NASEM) called for increasing interdisciplinary training and improving cross-functional team performance as a major goal for health care providers in twenty-first century health systems.26 NASEM also recommended that academic medical centers develop medical leaders who can manage systems changes required to enhance health, a proposal supported by the fact that hospitals with medically trained CEOs outperform others.27,28
Public Health 3.0, a federal initiative to improve and integrate public health efforts, also emphasizes cross-disciplinary teams and cross-sector partnerships,29 while the Centers for Medicare and Medicaid Services (CMS) has incentivized the development of interprofessional health care teams.30 While cross-training does not automatically connote interdisciplinary training, we believe that cross-training may reveal or develop an interdisciplinary mind-set that may support and embrace interdisciplinary performance. Finally, the US Department of Health and Human Services’ (HHS) Strategic Goals emphasize integrated care for vulnerable populations, something that cross-trained physicians may be especially poised to accomplish.31
A Path Forward
The education, training, and priorities of young physicians demonstrates career interests that diverge from mainstream, traditional options. Data provided herein describe the increasing rates at which millennial physicians are cross-training and have suggested that the current marketplace may not match the interests of this population. The ultimate question is where such cross-trained physicians fit into today’s (or tomorrow’s) health system?
It may be easiest to deploy cross-trained physicians in their respective clinical departments (eg, having a physician trained in internal medicine and pediatrics perform clinical duties in both a medicine department and a pediatrics department). But < 40% of dual-boarded physicians practice both specialties in which they’re trained, so other opportunities should be pursued.32,33 One strategy may be to embrace the promise of interdisciplinary care, as supported by Public Health 3.0 and NASEM.26,29 Our evidence may demonstrate that the interdisciplinary mind-set may be more readily evident in the millennial generation, and that this mind-set may improve interdisciplinary care.
As health is impacted both by direct clinical care as well as programs designed to address population health, cross-trained physicians may be better equipped to integrate aspects of clinical care spanning a variety of clinical fields as well as orchestrating programs designed to improve health at the population level. This mind-set may be best captured by organizations willing to adapt their medical positions to emphasize multidisciplinary training, skills, and capabilities. For example, a physician trained in internal medicine and psychiatry may have the unique training and skill-set to establish an integrated behavioral health clinic that crosses boundaries between traditional departments, emphasizing the whole health of the clinic’s population and not simply focusing on providing services of a particular specialty. Hiring cross-trained physicians throughout such a clinic may benefit the operations of the clinic and improve not only the services provided, but ultimately, the health of that clinic’s patients. By embracing cross-trained physicians, health care organizations and educators may better meet the needs of their employees, likely resulting in a more cost-effective investment for employers, employees, and the health system as a whole.4 Additionally, patient health may also improve.
There is evidence that cross-trained physicians are already likely to hold leadership positions compared with their categorically-trained counterparts, and this may reflect the benefits of an interdisciplinary mind-set.33 Perhaps a cross-trained physician is more likely to see beyond standard, specialty-based institutional barriers and develop processes and programs designed for overall patient benefit. Leadership is a skill that many millennials clearly wish to enhance throughout their career.34 Recruiting cross-trained physicians for leadership positions may reveal synergies between such training and an ability to lead health care organizations into the future.
Many millennial physicians are bringing a new set of skills into the medical marketplace. Health organizations should identify ways to recruit for these skills and deploy them within their systems in order to have more dedicated, engaged employees, more effective health systems, and ultimately, healthier patients.
Acknowledgments
Data from this analysis were presented at the 10th Consortium of Universities for Global Health conference in 2019.35
1. Dimock M. Defining generations: where millennials end and generation Z begins. http://www.pewresearch.org/fact-tank/2018/03/01/defining-generations-where-millennials-end-and-post-millennials-begin/. Published January 17, 2019. Accessed November 7, 2019.
2. IHS Inc. The complexities of physician supply and demand: projections from 2014 to 2025. Final report. https://www.modernhealthcare.com/assets/pdf/CH10888123.pdf. Published April 5, 2016. Accessed November 7, 2019.
3. Miller RN. Millennial physicians sound off on state of medicine today. https://wire.ama-assn.org/life-career/millennial-physicians-sound-state-medicine-today. Published March 27, 2017. Accessed November 7, 2019.
4. World Economic Forum. Matching skills and labour market needs: building social partnerships for better skills and better jobs. http://www3.weforum.org/docs/GAC/2014/WEF_GAC_Employment_MatchingSkillsLabourMarket_Report_2014.pdf. Published January 2014. Accessed November 7, 2019.
5. Brint SG, Turk-Bicakci L, Proctor K, Murphy SP. Expanding the social frame of knowledge: interdisciplinary, degree-granting fields in American Colleges and Universities, 1975–2000. Rev High Ed. 2009;32(2):155-183.
6. National Science Foundation. National survey of college graduates. https://www.nsf.gov/statistics/srvygrads. Updated February 2019. Accessed November 7, 2019.
7. Simon CC. Major decisions. New York Times. November 2, 2012. http://www.nytimes.com/2012/11/04/education/edlife/choosing-one-college-major-out-of-hundreds.html. Accessed November 7, 2019.
8. Berry SL, Erin GL, Therese J. Health humanities baccalaureate programs in the United States. http://www.hiram.edu/wp-content/uploads/2017/09/HHBP2017.pdf. Published September 2017. Accessed November 7, 2019.
9. Sorensen NE, Jackson JR. Science majors and nonscience majors entering medical school: acceptance rates and academic performance. NACADA J. 1997;17(1):32-41.
10. Association of American Medical Colleges. Table A-17: MCAT and GPAs for applicants and matriculants to U.S. medical schools by primary undergraduate major, 2019-2020. https://www.aamc.org/download/321496/data/factstablea17.pdf. Published October 16, 2019. Accessed November 7, 2019.
11. University of Michigan Medical School. Many paths, one destination: medical school welcomes its 170th class of medical students. https://medicine.umich.edu/medschool/news/many-paths-one-destination-medical-school-welcomes-its-170th-class-medical-students. Updated July 29, 2016. Accessed November 7, 2019.
12. Harding CV, Akabas MH, Andersen OS. History and outcomes of 50 years of physician-scientist training in medical scientist training programs. Acad Med. 2017; 92(10):1390-1398.
13. Association of American Medical Colleges. MD-PhD in “social sciences or humanities” and “other non-traditional fields of graduate study” - by school. https://students-residents.aamc.org/choosing-medical-career/careers-medical-research/md-phd-dual-degree-training/non-basic-science-phd-training-school/. Accessed November 8, 2019.
14. Holmes SM, Karlin J, Stonington SD, Gottheil DL. The first nationwide survey of MD-PhDs in the social sciences and humanities: training patterns and career choices. BMC Med Educ. 2017;17(1):60.
15. Association of American Medical Colleges Combined degrees and early acceptance programs. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/combined-degrees-and-early-acceptance-programs. Accessed November 8, 2019.
16. Tufts University School of Medicine. 2023 class profile. http://medicine.tufts.edu/Education/MD-Programs/Doctor-of-Medicine/Class-Profile. Published 2015. Accessed November 8, 2019.
17. Zweifler J, Evan R. Development of a residency/MPH program. Family Med. 2001;33(6):453-458.
18. American Medical Association. The AMA residency and fellowship database. http://freida.ama-assn.org/Freida. Accessed November 7, 2019.
19. National Resident Matching Program. NRMP data. http://www.nrmp.org/wp-content/uploads/2013/08/resultsanddata1996.pdf. Published March 1996. Accessed November 7, 2019.
20. Brotherton SE, Etzel SI. Graduate medical education, 2016-2017. JAMA. 2017;318(23):2368-2387.
21. American Board of Psychiatry and Neurology. Update for psychiatry GME programs on combined training program accreditation/approval February 2012. https://www.umassmed.edu/globalassets/neuropsychiatry/files/combined-program-letter.pdf. Accessed November 7, 2019.
22. Massachusetts General Hospital. Surgical residency program. https://www.massgeneral.org/surgery/education/residency.aspx?id=77. Accessed November 7, 2019.
23. Lusco VC, Martinez SA, Polk HC Jr. Program directors in surgery agree that residents should be formally trained in business and practice management. Am J Surg. 2005;189(1):11-13.
24. New England Journal of Medicine. NEJM CareerCenter. http://www.nejmcareercenter.org. Accessed November 7, 2019.
25. US Office of Personnel Management. USAJOBS. https://www.usajobs.gov. Accessed November 7, 2019.
26. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Published March 2001. Accessed November 7, 2019.
27. Kohn LT, ed; Committee on the Roles of Academic Health Centers in the 21st Century; Institute of Medicine of the National Academies. Academic Health Centers: Leading Change in the 21st Century. National Academy Press: Washington, DC; 2004.
28. Goodall AH. Physician-leaders and hospital performance: is there an association? http://ftp.iza.org/dp5830.pdf. Published July 2011. Accessed November 7, 2019.
29. US Department of Health and Human Services, Office of the Assistant Secretary for Health. Public health 3.0: a call to action to create a 21st century public health infrastructure. https://www.healthypeople.gov/sites/default/files/Public-Health-3.0-White-Paper.pdf. Accessed November 7, 2019.
30. Centers for Medicare and Medicaid Services. Health care innovation awards round one project profiles. http://innovation.cms.gov/files/x/hcia-project-profiles.pdf. Updated December 2013. Accessed November 7, 2019.
31. US Department of Health and Human Services. Strategic Objective 1.3: Improve Americans’ access to healthcare and expand choices of care and service options. https://www.hhs.gov/about/strategic-plan/strategic-goal-1/index.html#obj_1_3. Updated March 18, 2019. Accessed November 7, 2019.
32. Kessler CS, Stallings LA, Gonzalez AA, Templeman TA. Combined residency training in emergency medicine and internal medicine: an update on career outcomes and job satisfaction. Acad Emerg Med. 2009;16(9):894-899.
33. Summergrad P, Silberman E, Price LL. Practice and career outcomes of double-boarded psychiatrists. Psychosomatics. 2011;52(6):537-543.
34. Rigoni B, Adkins A. What millennials want from a new job. Harvard Business Rev. May 11, 2016. https://hbr.org/2016/05/what-millennials-want-from-a-new-job. Accessed November 7, 2019.
35. Jung P, Smith C. Medical millennials: a mismatch between training preferences and employment opportunities. Lancet Glob Health. 2019;7(suppl 1):S38.
Millennials, defined as those born between 1981 and 1996, currently comprise 15% of all active physicians in the US.1,2 A recent survey found that nearly 4 of 5 US millennial physicians have a desire for cross-sectional work in areas beyond patient care, such as academic research, health care consulting, entrepreneurship, and health care administration.3
For employers and educators, a better understanding of these preferences, through consideration of the unique education and skill set of the millennial physician workforce, may lead to more effective recruitment of young physicians and improved health systems, avoiding a mismatch between health care provider skills and available jobs that can be costly for both employers and employees.4
This article describes how US millennial physicians are choosing to cross-train (obtaining multiple degrees and/or completing combined medical residency training) throughout undergraduate, medical, and graduate medical education. We also outline ways in which the current physician marketplace may not match the skills of this population and suggest some ways that health care organizations could capitalize on this trend toward more cross-trained personnel in order to effectively recruit and retain the next generation of physicians.
Millennial Education
Undergraduates
The number of interdisciplinary undergraduate majors increased by almost 250% from 1975 to 2000.5 In 2010, nearly 20% of US college students graduated with 2 majors, representing a 70% increase in double majors between 2001 and 2011.6,7 One emerging category of interdisciplinary majors in US colleges is health humanities programs, which have quadrupled since 2000.8
Medical school applicants and matriculants reflect this trend. Whereas in 1994, only 19% of applicants to medical school held nonscience degrees, about one-third of applicants now hold such degrees.9,10 We have found no aggregated data on double majors entering US medical schools, but public class profiles suggest that medical school matriculants mirror their undergraduate counterparts in their tendency to hold double majors. In 2016, for example, 15% of the incoming class at the University of Michigan Medical School was composed of double majors, increasing to over 25% in 2017.11
Medical Students
Early dual-degree programs in undergraduate medical training were reserved for MD/PhD programs.12 Most US MD/PhD programs (90 out of 151) now offer doctorates in social sciences, humanities, or other nontraditional fields of graduate medical study, reflecting a shift in interests of those seeking dual-degree training in undergraduate medical education.13 While only 3 MD/PhD programs in the 1970s included trainees in the social sciences, 17 such programs exist today.14
Interest in dual-degree programs offering master’s level study has also increased over the past decade. In 2017, 87 medical schools offered programs for students to pursue a master of public health (MPH) and 41 offered master of science degrees in various fields, up from 52 and 37 institutions, respectively in 2006.15 The number of schools offering combined training in nonscience fields has also grown, with 63 institutions now offering a master of business administration (MBA), nearly double the number offered in 2006.15 At some institutions more than 20% of students are earning a master’s degree or doctorate in addition to their MD degree.16
Residents
The authors found no documentation of US residency training programs, outside of those in the specialty of preventive medicine, providing trainees with formal opportunities to obtain an MBA or MPH prior to 2001.17 However, of the 510 internal medicine residency programs listed on the American Medical Association residency and fellowship database (freida.ama-assn.org), 45 identified as having established a pathway for residents to pursue an MBA, MPH, or PhD during residency.18
Over the past 20 years, combined residency programs have increased 49% (from 128 to 191), which is triple the 16% rate (1,350 to 1,562) of increase in programs in internal medicine, pediatrics, family medicine, psychiatry, and emergency medicine.19,20 A 2009 moratorium on the creation of new combined residency programs in psychiatry and neurology was lifted in 2016and is likely to increase the rate of total combined programs.21
The Table shows the number of categorical and combined residency programs available in 1996 and in 2016. Over 2 decades, 17 new specialty combinations became available for residency training. While there were no combined training programs within these 17 new combinations in 1996,there were 66 programs with these combinations in 2016.19,20
Although surgical specialties are notably absent from the list of combined residency options, likely due to the duration of surgical training, some surgical training programs do offer pathways that culminate in combined degrees,22 and a high number of surgery program directors agree that residents should receive formal training in business and practice management.23
The Medical Job Market
Although today’s young physicians are cross-trained in multiple disciplines, the current job market may not directly match these skill sets. Of the 7,235 jobs listed by the New England Journal of Medicine (NEJM) career center (www.nejmcareercenter.org/jobs), only 54 were targeted at those with combined training, the majority of which were aimed at those trained in internal medicine/pediatrics. Of the combined specialties in the Table, formal positions were listed for only 6.24 A search of nearly 1,500 federal medical positions on USAJOBS (www.usajobs.gov) found only 4 jobs that combined specialties, all restricted to internal medicine/pediatrics.25 When searching for jobs containing the terms MBA, MPH, and public health there were only 8 such positions on NEJM and 7 on USAJOBS.24,25 Although the totality of the medical marketplace may not be best encompassed by these sources, the authors believe NEJM and USAJOBS are somewhat representative of the opportunities for physicians in the US.
Medical jobs tailored to cross-trained physicians do not appear to have kept pace with the numbers of such specialists currently in medical school and residency training. Though millennials are cross-training in increasing numbers, we surmise that they are not doing so as a direct result of the job market.
Future Medicine
Regardless of the mismatch between cross-trained physicians and the current job market, millennials may be well suited for future health systems. In 2001, the National Academies of Sciences, Engineering and Medicine (NASEM) called for increasing interdisciplinary training and improving cross-functional team performance as a major goal for health care providers in twenty-first century health systems.26 NASEM also recommended that academic medical centers develop medical leaders who can manage systems changes required to enhance health, a proposal supported by the fact that hospitals with medically trained CEOs outperform others.27,28
Public Health 3.0, a federal initiative to improve and integrate public health efforts, also emphasizes cross-disciplinary teams and cross-sector partnerships,29 while the Centers for Medicare and Medicaid Services (CMS) has incentivized the development of interprofessional health care teams.30 While cross-training does not automatically connote interdisciplinary training, we believe that cross-training may reveal or develop an interdisciplinary mind-set that may support and embrace interdisciplinary performance. Finally, the US Department of Health and Human Services’ (HHS) Strategic Goals emphasize integrated care for vulnerable populations, something that cross-trained physicians may be especially poised to accomplish.31
A Path Forward
The education, training, and priorities of young physicians demonstrates career interests that diverge from mainstream, traditional options. Data provided herein describe the increasing rates at which millennial physicians are cross-training and have suggested that the current marketplace may not match the interests of this population. The ultimate question is where such cross-trained physicians fit into today’s (or tomorrow’s) health system?
It may be easiest to deploy cross-trained physicians in their respective clinical departments (eg, having a physician trained in internal medicine and pediatrics perform clinical duties in both a medicine department and a pediatrics department). But < 40% of dual-boarded physicians practice both specialties in which they’re trained, so other opportunities should be pursued.32,33 One strategy may be to embrace the promise of interdisciplinary care, as supported by Public Health 3.0 and NASEM.26,29 Our evidence may demonstrate that the interdisciplinary mind-set may be more readily evident in the millennial generation, and that this mind-set may improve interdisciplinary care.
As health is impacted both by direct clinical care as well as programs designed to address population health, cross-trained physicians may be better equipped to integrate aspects of clinical care spanning a variety of clinical fields as well as orchestrating programs designed to improve health at the population level. This mind-set may be best captured by organizations willing to adapt their medical positions to emphasize multidisciplinary training, skills, and capabilities. For example, a physician trained in internal medicine and psychiatry may have the unique training and skill-set to establish an integrated behavioral health clinic that crosses boundaries between traditional departments, emphasizing the whole health of the clinic’s population and not simply focusing on providing services of a particular specialty. Hiring cross-trained physicians throughout such a clinic may benefit the operations of the clinic and improve not only the services provided, but ultimately, the health of that clinic’s patients. By embracing cross-trained physicians, health care organizations and educators may better meet the needs of their employees, likely resulting in a more cost-effective investment for employers, employees, and the health system as a whole.4 Additionally, patient health may also improve.
There is evidence that cross-trained physicians are already likely to hold leadership positions compared with their categorically-trained counterparts, and this may reflect the benefits of an interdisciplinary mind-set.33 Perhaps a cross-trained physician is more likely to see beyond standard, specialty-based institutional barriers and develop processes and programs designed for overall patient benefit. Leadership is a skill that many millennials clearly wish to enhance throughout their career.34 Recruiting cross-trained physicians for leadership positions may reveal synergies between such training and an ability to lead health care organizations into the future.
Many millennial physicians are bringing a new set of skills into the medical marketplace. Health organizations should identify ways to recruit for these skills and deploy them within their systems in order to have more dedicated, engaged employees, more effective health systems, and ultimately, healthier patients.
Acknowledgments
Data from this analysis were presented at the 10th Consortium of Universities for Global Health conference in 2019.35
Millennials, defined as those born between 1981 and 1996, currently comprise 15% of all active physicians in the US.1,2 A recent survey found that nearly 4 of 5 US millennial physicians have a desire for cross-sectional work in areas beyond patient care, such as academic research, health care consulting, entrepreneurship, and health care administration.3
For employers and educators, a better understanding of these preferences, through consideration of the unique education and skill set of the millennial physician workforce, may lead to more effective recruitment of young physicians and improved health systems, avoiding a mismatch between health care provider skills and available jobs that can be costly for both employers and employees.4
This article describes how US millennial physicians are choosing to cross-train (obtaining multiple degrees and/or completing combined medical residency training) throughout undergraduate, medical, and graduate medical education. We also outline ways in which the current physician marketplace may not match the skills of this population and suggest some ways that health care organizations could capitalize on this trend toward more cross-trained personnel in order to effectively recruit and retain the next generation of physicians.
Millennial Education
Undergraduates
The number of interdisciplinary undergraduate majors increased by almost 250% from 1975 to 2000.5 In 2010, nearly 20% of US college students graduated with 2 majors, representing a 70% increase in double majors between 2001 and 2011.6,7 One emerging category of interdisciplinary majors in US colleges is health humanities programs, which have quadrupled since 2000.8
Medical school applicants and matriculants reflect this trend. Whereas in 1994, only 19% of applicants to medical school held nonscience degrees, about one-third of applicants now hold such degrees.9,10 We have found no aggregated data on double majors entering US medical schools, but public class profiles suggest that medical school matriculants mirror their undergraduate counterparts in their tendency to hold double majors. In 2016, for example, 15% of the incoming class at the University of Michigan Medical School was composed of double majors, increasing to over 25% in 2017.11
Medical Students
Early dual-degree programs in undergraduate medical training were reserved for MD/PhD programs.12 Most US MD/PhD programs (90 out of 151) now offer doctorates in social sciences, humanities, or other nontraditional fields of graduate medical study, reflecting a shift in interests of those seeking dual-degree training in undergraduate medical education.13 While only 3 MD/PhD programs in the 1970s included trainees in the social sciences, 17 such programs exist today.14
Interest in dual-degree programs offering master’s level study has also increased over the past decade. In 2017, 87 medical schools offered programs for students to pursue a master of public health (MPH) and 41 offered master of science degrees in various fields, up from 52 and 37 institutions, respectively in 2006.15 The number of schools offering combined training in nonscience fields has also grown, with 63 institutions now offering a master of business administration (MBA), nearly double the number offered in 2006.15 At some institutions more than 20% of students are earning a master’s degree or doctorate in addition to their MD degree.16
Residents
The authors found no documentation of US residency training programs, outside of those in the specialty of preventive medicine, providing trainees with formal opportunities to obtain an MBA or MPH prior to 2001.17 However, of the 510 internal medicine residency programs listed on the American Medical Association residency and fellowship database (freida.ama-assn.org), 45 identified as having established a pathway for residents to pursue an MBA, MPH, or PhD during residency.18
Over the past 20 years, combined residency programs have increased 49% (from 128 to 191), which is triple the 16% rate (1,350 to 1,562) of increase in programs in internal medicine, pediatrics, family medicine, psychiatry, and emergency medicine.19,20 A 2009 moratorium on the creation of new combined residency programs in psychiatry and neurology was lifted in 2016and is likely to increase the rate of total combined programs.21
The Table shows the number of categorical and combined residency programs available in 1996 and in 2016. Over 2 decades, 17 new specialty combinations became available for residency training. While there were no combined training programs within these 17 new combinations in 1996,there were 66 programs with these combinations in 2016.19,20
Although surgical specialties are notably absent from the list of combined residency options, likely due to the duration of surgical training, some surgical training programs do offer pathways that culminate in combined degrees,22 and a high number of surgery program directors agree that residents should receive formal training in business and practice management.23
The Medical Job Market
Although today’s young physicians are cross-trained in multiple disciplines, the current job market may not directly match these skill sets. Of the 7,235 jobs listed by the New England Journal of Medicine (NEJM) career center (www.nejmcareercenter.org/jobs), only 54 were targeted at those with combined training, the majority of which were aimed at those trained in internal medicine/pediatrics. Of the combined specialties in the Table, formal positions were listed for only 6.24 A search of nearly 1,500 federal medical positions on USAJOBS (www.usajobs.gov) found only 4 jobs that combined specialties, all restricted to internal medicine/pediatrics.25 When searching for jobs containing the terms MBA, MPH, and public health there were only 8 such positions on NEJM and 7 on USAJOBS.24,25 Although the totality of the medical marketplace may not be best encompassed by these sources, the authors believe NEJM and USAJOBS are somewhat representative of the opportunities for physicians in the US.
Medical jobs tailored to cross-trained physicians do not appear to have kept pace with the numbers of such specialists currently in medical school and residency training. Though millennials are cross-training in increasing numbers, we surmise that they are not doing so as a direct result of the job market.
Future Medicine
Regardless of the mismatch between cross-trained physicians and the current job market, millennials may be well suited for future health systems. In 2001, the National Academies of Sciences, Engineering and Medicine (NASEM) called for increasing interdisciplinary training and improving cross-functional team performance as a major goal for health care providers in twenty-first century health systems.26 NASEM also recommended that academic medical centers develop medical leaders who can manage systems changes required to enhance health, a proposal supported by the fact that hospitals with medically trained CEOs outperform others.27,28
Public Health 3.0, a federal initiative to improve and integrate public health efforts, also emphasizes cross-disciplinary teams and cross-sector partnerships,29 while the Centers for Medicare and Medicaid Services (CMS) has incentivized the development of interprofessional health care teams.30 While cross-training does not automatically connote interdisciplinary training, we believe that cross-training may reveal or develop an interdisciplinary mind-set that may support and embrace interdisciplinary performance. Finally, the US Department of Health and Human Services’ (HHS) Strategic Goals emphasize integrated care for vulnerable populations, something that cross-trained physicians may be especially poised to accomplish.31
A Path Forward
The education, training, and priorities of young physicians demonstrates career interests that diverge from mainstream, traditional options. Data provided herein describe the increasing rates at which millennial physicians are cross-training and have suggested that the current marketplace may not match the interests of this population. The ultimate question is where such cross-trained physicians fit into today’s (or tomorrow’s) health system?
It may be easiest to deploy cross-trained physicians in their respective clinical departments (eg, having a physician trained in internal medicine and pediatrics perform clinical duties in both a medicine department and a pediatrics department). But < 40% of dual-boarded physicians practice both specialties in which they’re trained, so other opportunities should be pursued.32,33 One strategy may be to embrace the promise of interdisciplinary care, as supported by Public Health 3.0 and NASEM.26,29 Our evidence may demonstrate that the interdisciplinary mind-set may be more readily evident in the millennial generation, and that this mind-set may improve interdisciplinary care.
As health is impacted both by direct clinical care as well as programs designed to address population health, cross-trained physicians may be better equipped to integrate aspects of clinical care spanning a variety of clinical fields as well as orchestrating programs designed to improve health at the population level. This mind-set may be best captured by organizations willing to adapt their medical positions to emphasize multidisciplinary training, skills, and capabilities. For example, a physician trained in internal medicine and psychiatry may have the unique training and skill-set to establish an integrated behavioral health clinic that crosses boundaries between traditional departments, emphasizing the whole health of the clinic’s population and not simply focusing on providing services of a particular specialty. Hiring cross-trained physicians throughout such a clinic may benefit the operations of the clinic and improve not only the services provided, but ultimately, the health of that clinic’s patients. By embracing cross-trained physicians, health care organizations and educators may better meet the needs of their employees, likely resulting in a more cost-effective investment for employers, employees, and the health system as a whole.4 Additionally, patient health may also improve.
There is evidence that cross-trained physicians are already likely to hold leadership positions compared with their categorically-trained counterparts, and this may reflect the benefits of an interdisciplinary mind-set.33 Perhaps a cross-trained physician is more likely to see beyond standard, specialty-based institutional barriers and develop processes and programs designed for overall patient benefit. Leadership is a skill that many millennials clearly wish to enhance throughout their career.34 Recruiting cross-trained physicians for leadership positions may reveal synergies between such training and an ability to lead health care organizations into the future.
Many millennial physicians are bringing a new set of skills into the medical marketplace. Health organizations should identify ways to recruit for these skills and deploy them within their systems in order to have more dedicated, engaged employees, more effective health systems, and ultimately, healthier patients.
Acknowledgments
Data from this analysis were presented at the 10th Consortium of Universities for Global Health conference in 2019.35
1. Dimock M. Defining generations: where millennials end and generation Z begins. http://www.pewresearch.org/fact-tank/2018/03/01/defining-generations-where-millennials-end-and-post-millennials-begin/. Published January 17, 2019. Accessed November 7, 2019.
2. IHS Inc. The complexities of physician supply and demand: projections from 2014 to 2025. Final report. https://www.modernhealthcare.com/assets/pdf/CH10888123.pdf. Published April 5, 2016. Accessed November 7, 2019.
3. Miller RN. Millennial physicians sound off on state of medicine today. https://wire.ama-assn.org/life-career/millennial-physicians-sound-state-medicine-today. Published March 27, 2017. Accessed November 7, 2019.
4. World Economic Forum. Matching skills and labour market needs: building social partnerships for better skills and better jobs. http://www3.weforum.org/docs/GAC/2014/WEF_GAC_Employment_MatchingSkillsLabourMarket_Report_2014.pdf. Published January 2014. Accessed November 7, 2019.
5. Brint SG, Turk-Bicakci L, Proctor K, Murphy SP. Expanding the social frame of knowledge: interdisciplinary, degree-granting fields in American Colleges and Universities, 1975–2000. Rev High Ed. 2009;32(2):155-183.
6. National Science Foundation. National survey of college graduates. https://www.nsf.gov/statistics/srvygrads. Updated February 2019. Accessed November 7, 2019.
7. Simon CC. Major decisions. New York Times. November 2, 2012. http://www.nytimes.com/2012/11/04/education/edlife/choosing-one-college-major-out-of-hundreds.html. Accessed November 7, 2019.
8. Berry SL, Erin GL, Therese J. Health humanities baccalaureate programs in the United States. http://www.hiram.edu/wp-content/uploads/2017/09/HHBP2017.pdf. Published September 2017. Accessed November 7, 2019.
9. Sorensen NE, Jackson JR. Science majors and nonscience majors entering medical school: acceptance rates and academic performance. NACADA J. 1997;17(1):32-41.
10. Association of American Medical Colleges. Table A-17: MCAT and GPAs for applicants and matriculants to U.S. medical schools by primary undergraduate major, 2019-2020. https://www.aamc.org/download/321496/data/factstablea17.pdf. Published October 16, 2019. Accessed November 7, 2019.
11. University of Michigan Medical School. Many paths, one destination: medical school welcomes its 170th class of medical students. https://medicine.umich.edu/medschool/news/many-paths-one-destination-medical-school-welcomes-its-170th-class-medical-students. Updated July 29, 2016. Accessed November 7, 2019.
12. Harding CV, Akabas MH, Andersen OS. History and outcomes of 50 years of physician-scientist training in medical scientist training programs. Acad Med. 2017; 92(10):1390-1398.
13. Association of American Medical Colleges. MD-PhD in “social sciences or humanities” and “other non-traditional fields of graduate study” - by school. https://students-residents.aamc.org/choosing-medical-career/careers-medical-research/md-phd-dual-degree-training/non-basic-science-phd-training-school/. Accessed November 8, 2019.
14. Holmes SM, Karlin J, Stonington SD, Gottheil DL. The first nationwide survey of MD-PhDs in the social sciences and humanities: training patterns and career choices. BMC Med Educ. 2017;17(1):60.
15. Association of American Medical Colleges Combined degrees and early acceptance programs. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/combined-degrees-and-early-acceptance-programs. Accessed November 8, 2019.
16. Tufts University School of Medicine. 2023 class profile. http://medicine.tufts.edu/Education/MD-Programs/Doctor-of-Medicine/Class-Profile. Published 2015. Accessed November 8, 2019.
17. Zweifler J, Evan R. Development of a residency/MPH program. Family Med. 2001;33(6):453-458.
18. American Medical Association. The AMA residency and fellowship database. http://freida.ama-assn.org/Freida. Accessed November 7, 2019.
19. National Resident Matching Program. NRMP data. http://www.nrmp.org/wp-content/uploads/2013/08/resultsanddata1996.pdf. Published March 1996. Accessed November 7, 2019.
20. Brotherton SE, Etzel SI. Graduate medical education, 2016-2017. JAMA. 2017;318(23):2368-2387.
21. American Board of Psychiatry and Neurology. Update for psychiatry GME programs on combined training program accreditation/approval February 2012. https://www.umassmed.edu/globalassets/neuropsychiatry/files/combined-program-letter.pdf. Accessed November 7, 2019.
22. Massachusetts General Hospital. Surgical residency program. https://www.massgeneral.org/surgery/education/residency.aspx?id=77. Accessed November 7, 2019.
23. Lusco VC, Martinez SA, Polk HC Jr. Program directors in surgery agree that residents should be formally trained in business and practice management. Am J Surg. 2005;189(1):11-13.
24. New England Journal of Medicine. NEJM CareerCenter. http://www.nejmcareercenter.org. Accessed November 7, 2019.
25. US Office of Personnel Management. USAJOBS. https://www.usajobs.gov. Accessed November 7, 2019.
26. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Published March 2001. Accessed November 7, 2019.
27. Kohn LT, ed; Committee on the Roles of Academic Health Centers in the 21st Century; Institute of Medicine of the National Academies. Academic Health Centers: Leading Change in the 21st Century. National Academy Press: Washington, DC; 2004.
28. Goodall AH. Physician-leaders and hospital performance: is there an association? http://ftp.iza.org/dp5830.pdf. Published July 2011. Accessed November 7, 2019.
29. US Department of Health and Human Services, Office of the Assistant Secretary for Health. Public health 3.0: a call to action to create a 21st century public health infrastructure. https://www.healthypeople.gov/sites/default/files/Public-Health-3.0-White-Paper.pdf. Accessed November 7, 2019.
30. Centers for Medicare and Medicaid Services. Health care innovation awards round one project profiles. http://innovation.cms.gov/files/x/hcia-project-profiles.pdf. Updated December 2013. Accessed November 7, 2019.
31. US Department of Health and Human Services. Strategic Objective 1.3: Improve Americans’ access to healthcare and expand choices of care and service options. https://www.hhs.gov/about/strategic-plan/strategic-goal-1/index.html#obj_1_3. Updated March 18, 2019. Accessed November 7, 2019.
32. Kessler CS, Stallings LA, Gonzalez AA, Templeman TA. Combined residency training in emergency medicine and internal medicine: an update on career outcomes and job satisfaction. Acad Emerg Med. 2009;16(9):894-899.
33. Summergrad P, Silberman E, Price LL. Practice and career outcomes of double-boarded psychiatrists. Psychosomatics. 2011;52(6):537-543.
34. Rigoni B, Adkins A. What millennials want from a new job. Harvard Business Rev. May 11, 2016. https://hbr.org/2016/05/what-millennials-want-from-a-new-job. Accessed November 7, 2019.
35. Jung P, Smith C. Medical millennials: a mismatch between training preferences and employment opportunities. Lancet Glob Health. 2019;7(suppl 1):S38.
1. Dimock M. Defining generations: where millennials end and generation Z begins. http://www.pewresearch.org/fact-tank/2018/03/01/defining-generations-where-millennials-end-and-post-millennials-begin/. Published January 17, 2019. Accessed November 7, 2019.
2. IHS Inc. The complexities of physician supply and demand: projections from 2014 to 2025. Final report. https://www.modernhealthcare.com/assets/pdf/CH10888123.pdf. Published April 5, 2016. Accessed November 7, 2019.
3. Miller RN. Millennial physicians sound off on state of medicine today. https://wire.ama-assn.org/life-career/millennial-physicians-sound-state-medicine-today. Published March 27, 2017. Accessed November 7, 2019.
4. World Economic Forum. Matching skills and labour market needs: building social partnerships for better skills and better jobs. http://www3.weforum.org/docs/GAC/2014/WEF_GAC_Employment_MatchingSkillsLabourMarket_Report_2014.pdf. Published January 2014. Accessed November 7, 2019.
5. Brint SG, Turk-Bicakci L, Proctor K, Murphy SP. Expanding the social frame of knowledge: interdisciplinary, degree-granting fields in American Colleges and Universities, 1975–2000. Rev High Ed. 2009;32(2):155-183.
6. National Science Foundation. National survey of college graduates. https://www.nsf.gov/statistics/srvygrads. Updated February 2019. Accessed November 7, 2019.
7. Simon CC. Major decisions. New York Times. November 2, 2012. http://www.nytimes.com/2012/11/04/education/edlife/choosing-one-college-major-out-of-hundreds.html. Accessed November 7, 2019.
8. Berry SL, Erin GL, Therese J. Health humanities baccalaureate programs in the United States. http://www.hiram.edu/wp-content/uploads/2017/09/HHBP2017.pdf. Published September 2017. Accessed November 7, 2019.
9. Sorensen NE, Jackson JR. Science majors and nonscience majors entering medical school: acceptance rates and academic performance. NACADA J. 1997;17(1):32-41.
10. Association of American Medical Colleges. Table A-17: MCAT and GPAs for applicants and matriculants to U.S. medical schools by primary undergraduate major, 2019-2020. https://www.aamc.org/download/321496/data/factstablea17.pdf. Published October 16, 2019. Accessed November 7, 2019.
11. University of Michigan Medical School. Many paths, one destination: medical school welcomes its 170th class of medical students. https://medicine.umich.edu/medschool/news/many-paths-one-destination-medical-school-welcomes-its-170th-class-medical-students. Updated July 29, 2016. Accessed November 7, 2019.
12. Harding CV, Akabas MH, Andersen OS. History and outcomes of 50 years of physician-scientist training in medical scientist training programs. Acad Med. 2017; 92(10):1390-1398.
13. Association of American Medical Colleges. MD-PhD in “social sciences or humanities” and “other non-traditional fields of graduate study” - by school. https://students-residents.aamc.org/choosing-medical-career/careers-medical-research/md-phd-dual-degree-training/non-basic-science-phd-training-school/. Accessed November 8, 2019.
14. Holmes SM, Karlin J, Stonington SD, Gottheil DL. The first nationwide survey of MD-PhDs in the social sciences and humanities: training patterns and career choices. BMC Med Educ. 2017;17(1):60.
15. Association of American Medical Colleges Combined degrees and early acceptance programs. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/combined-degrees-and-early-acceptance-programs. Accessed November 8, 2019.
16. Tufts University School of Medicine. 2023 class profile. http://medicine.tufts.edu/Education/MD-Programs/Doctor-of-Medicine/Class-Profile. Published 2015. Accessed November 8, 2019.
17. Zweifler J, Evan R. Development of a residency/MPH program. Family Med. 2001;33(6):453-458.
18. American Medical Association. The AMA residency and fellowship database. http://freida.ama-assn.org/Freida. Accessed November 7, 2019.
19. National Resident Matching Program. NRMP data. http://www.nrmp.org/wp-content/uploads/2013/08/resultsanddata1996.pdf. Published March 1996. Accessed November 7, 2019.
20. Brotherton SE, Etzel SI. Graduate medical education, 2016-2017. JAMA. 2017;318(23):2368-2387.
21. American Board of Psychiatry and Neurology. Update for psychiatry GME programs on combined training program accreditation/approval February 2012. https://www.umassmed.edu/globalassets/neuropsychiatry/files/combined-program-letter.pdf. Accessed November 7, 2019.
22. Massachusetts General Hospital. Surgical residency program. https://www.massgeneral.org/surgery/education/residency.aspx?id=77. Accessed November 7, 2019.
23. Lusco VC, Martinez SA, Polk HC Jr. Program directors in surgery agree that residents should be formally trained in business and practice management. Am J Surg. 2005;189(1):11-13.
24. New England Journal of Medicine. NEJM CareerCenter. http://www.nejmcareercenter.org. Accessed November 7, 2019.
25. US Office of Personnel Management. USAJOBS. https://www.usajobs.gov. Accessed November 7, 2019.
26. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Published March 2001. Accessed November 7, 2019.
27. Kohn LT, ed; Committee on the Roles of Academic Health Centers in the 21st Century; Institute of Medicine of the National Academies. Academic Health Centers: Leading Change in the 21st Century. National Academy Press: Washington, DC; 2004.
28. Goodall AH. Physician-leaders and hospital performance: is there an association? http://ftp.iza.org/dp5830.pdf. Published July 2011. Accessed November 7, 2019.
29. US Department of Health and Human Services, Office of the Assistant Secretary for Health. Public health 3.0: a call to action to create a 21st century public health infrastructure. https://www.healthypeople.gov/sites/default/files/Public-Health-3.0-White-Paper.pdf. Accessed November 7, 2019.
30. Centers for Medicare and Medicaid Services. Health care innovation awards round one project profiles. http://innovation.cms.gov/files/x/hcia-project-profiles.pdf. Updated December 2013. Accessed November 7, 2019.
31. US Department of Health and Human Services. Strategic Objective 1.3: Improve Americans’ access to healthcare and expand choices of care and service options. https://www.hhs.gov/about/strategic-plan/strategic-goal-1/index.html#obj_1_3. Updated March 18, 2019. Accessed November 7, 2019.
32. Kessler CS, Stallings LA, Gonzalez AA, Templeman TA. Combined residency training in emergency medicine and internal medicine: an update on career outcomes and job satisfaction. Acad Emerg Med. 2009;16(9):894-899.
33. Summergrad P, Silberman E, Price LL. Practice and career outcomes of double-boarded psychiatrists. Psychosomatics. 2011;52(6):537-543.
34. Rigoni B, Adkins A. What millennials want from a new job. Harvard Business Rev. May 11, 2016. https://hbr.org/2016/05/what-millennials-want-from-a-new-job. Accessed November 7, 2019.
35. Jung P, Smith C. Medical millennials: a mismatch between training preferences and employment opportunities. Lancet Glob Health. 2019;7(suppl 1):S38.