User login
Understanding Principles of High Reliability Organizations Through the Eyes of VIONE, A Clinical Program to Improve Patient Safety by Deprescribing Potentially Inappropriate Medications and Reducing Polypharmacy
High reliability organizations (HROs) incorporate continuous process improvement through leadership commitment to create a safety culture that works toward creating a zero-harm environment.1 The Veterans Health Administration (VHA) has set transformational goals for becoming an HRO. In this article, we describe VIONE, an expanding medication deprescribing clinical program, which exemplifies the translation of HRO principles into health care system models. Both VIONE and HRO are globally relevant.
Reducing medication errors and related adverse drug events are important for achieving zero harm. Preventable medical errors rank behind heart disease and cancer as the third leading cause of death in the US.2 The simultaneous use of multiple medications can lead to dangerous drug interactions, adverse outcomes, and challenges with adherence. When a person is taking multiple medicines, known as polypharmacy, it is more likely that some are potentially inappropriate medications (PIM). Current literature highlights the prevalence and dangers of polypharmacy, which ranks among the top 10 common causes of death in the US, as well as suggestions to address preventable adverse outcomes from polypharmacy and PIM.3-5
Deprescribing of PIM frequently results in better disease management with improved health outcomes and quality of life.4 Many health care settings lack standardized approaches or set expectations to proactively deprescribe PIM. There has been insufficient emphasis on how to make decisions for deprescribing medications when therapeutic benefits are not clear and/or when the adverse effects may outweigh the therapeutic benefits.5
It is imperative to provide practice guidance for deprescribing nonessential medications along with systems-based infrastructure to enable integrated and effective assessments during opportune moments in the health care continuum. Multimodal approaches that include education, risk stratification, population health management interventions, research and resource allocation can help transform organizational culture in health care facilities toward HRO models of care, aiming at zero harm to patients.
The practical lessons learned from VIONE implementation science experiences on various scales and under diverse circumstances, cumulative wisdom from hindsight, foresight and critical insights gathered during nationwide spread of VIONE over the past 3 years continues to propel us toward the desirable direction and core concepts of an HRO.
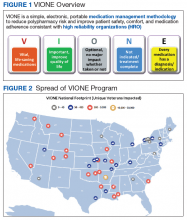
The VIONE program facilitates practical, real-time interventions that could be tailored to various health care settings, organizational needs, and available resources. VIONE implements an electronic Computerized Patient Record System (CPRS) tool to enable planned cessation of nonessential medications that are potentially harmful, inappropriate, not indicated, or not necessary. The VIONE tool supports systematic, individualized assessment and adjustment through 5 filters (Figure 1). It prompts providers to assign 1 of these filters intuitively and objectively. VIONE combines clinical evidence for best practices, an interprofessional team approach, patient engagement, adapted use of existing medical records systems, and HRO principles for effective implementation.
As a tool to support safer prescribing practices, VIONE aligns closely with HRO principles (Table 1) and core pillars (Table 2).6-8 A zero-harm safety culture necessitates that medications be used for correct reasons, over a correct duration of time, and following a correct schedule while monitoring for adverse outcomes. However, reality generally falls significantly short of this for a myriad of reasons, such as compromised health literacy, functional limitations, affordability, communication gaps, patients seen by multiple providers, and an accumulation of prescriptions due to comorbidities, symptom progression, and management of adverse effects. Through a sharpened focus on both precision medicine and competent prescription management, VIONE is a viable opportunity for investing in the zero-harm philosophy that is integral to an HRO.
Design and Implementation
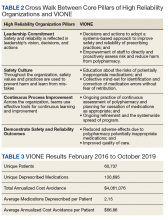
Initially launched in 2016 in a 15-bed inpatient, subacute rehabilitation unit within a VHA tertiary care facility, VIONE has been sustained and gradually expanded to 38 other VHA facility programs (Figure 2). Recognizing the potential value if adopted into widespread use, VIONE was a Gold Status winner in the VHA Under Secretary for Health Shark Tank-style competition in 2017 and was selected by the VHA Diffusion of Excellence as an innovation worthy of scale and spread through national dissemination.9 A toolkit for VIONE implementation, patient and provider brochures, VIONE vignette, and National Dialog template also have been created.10
Implementing VIONE in a new facility requires an actively engaged core team committed to patient safety and reduction of polypharmacy and PIM, interest and availability to lead project implementation strategies, along with meaningful local organizational support. The current structure for VIONE spread is as follows:
- Interested VHA participants review information and contact vavione@va.gov.
- The VIONE team orients implementing champions, mainly pharmacists, physicians, nurse practitioners, and physician assistants at a facility program level, offering guidance and available resources.
- Clinical Application Coordinators at Central Arkansas VA Healthcare System and participating facilities collaborate to add deprescribing menu options in CPRS and install the VIONE Polypharmacy Reminder Dialog template.
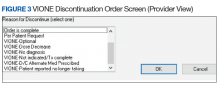
- Through close and ongoing collaborations, medical providers and clinical pharmacists proceed with deprescribing, aiming at planned cessation of nonessential and PIM, using the mnemonic prompt of VIONE. Vital and Important medications are continued and consolidated while a methodical plan is developed to deprescribe any medications that could lead to more harm than benefit and qualify based on the filters of Optional, Not indicated, and Every medicine has a diagnosis/reason. They select the proper discontinuation reasons in the CPRS medication menu (Figure 3) and document the rationale in the progress notes. It is highly encouraged that the collaborating pharmacists and health care providers add each other as cosigners and communicate effectively. Clinical pharmacy specialists also use the VIONE Polypharmacy Reminder Dialog Template (RDT) to document complete medication reviews with veterans to include deprescribing rationale and document shared decision making.
- A VIONE national dashboard captures deprescribing data in real time and automates reporting with daily updates that are readily accessible to all implementing facilities. Minimum data captured include the number of unique veterans impacted, number of medications deprescribed, cumulative cost avoidance to date, and number of prescriptions deprescribed per veteran. The dashboard facilitates real-time use of individual patient data and has also been designed to capture data from VHA administrative data portals and Corporate Data Warehouse.
Results
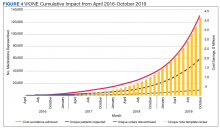
As of October 31, 2019, the assessment of polypharmacy using the VIONE tool across VHA sites has benefited > 60,000 unique veterans, of whom 49.2% were in urban areas, 47.7% in rural areas, and 3.1% in highly rural areas. Elderly male veterans comprised a clear majority. More than 128,000 medications have been deprescribed. The top classes of medications deprescribed are antihypertensives, over-the-counter medications, and antidiabetic medications. An annualized cost avoidance of > $4.0 million has been achieved. Cost avoidance is the cost of medications that otherwise would have continued to be filled and paid for by the VHA if they had not been deprescribed, projected for a maximum of 365 days. The calculation methodology can be summarized as follows:
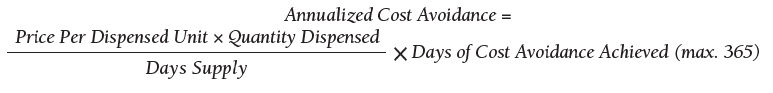
The calculations reported in Table 3 and Figure 4 are conservative and include only chronic outpatient prescriptions and do not account for medications deprescribed in inpatient units, nursing home, community living centers, or domiciliary populations. Data tracked separately from inpatient and community living center patient populations indicated an additional 25,536 deprescribed medications, across 28 VA facilities, impacting 7,076 veterans with an average 2.15 medications deprescribed per veteran. The additional achieved cost avoidance was $370,272 (based on $14.50 average cost per prescription). Medications restarted within 30 days of deprescribing are not included in these calculations.
The cost avoidance calculation further excludes the effects of VIONE implementation on many other types of interventions. These interventions include, but are not limited to, changing from aggressive care to end of life, comfort care when strongly indicated; reduced emergency department visits or invasive diagnostic and therapeutic approaches, when not indicated; medical supplies, antimicrobial preparations; labor costs related to packaging, mailing, and administering prescriptions; reduced/prevented clinical waste; reduced decompensation of systemic illnesses and subsequent health care needs precipitated by iatrogenic disturbances and prolonged convalescence; and overall changes to prescribing practices through purposeful and targeted interactions with colleagues across various disciplines and various hierarchical levels.
Discussion
The VIONE clinical program exemplifies the translation of HRO principles into health care system practices. VIONE offers a systematic approach to improve medication management with an emphasis on deprescribing nonessential medications across various health care settings, facilitating VHA efforts toward zero harm. It demonstrates close alignment with the key building blocks of an HRO. Effective VIONE incorporation into an organizational culture reflects leadership commitment to safety and reliability in their vision and actions. By empowering staff to proactively reduce inappropriate medications and thereby prevent patient harm, VIONE contributes to enhancing an enterprise-wide culture of safety, with fewer errors and greater reliability. As a standardized decision support tool for the ongoing practice of assessment and planned cessation of potentially inappropriate medications, VIONE illustrates how continuous process improvement can be a part of staff-engaged, veteran-centered, highly reliable care. The standardization of the VIONE tool promotes achievement and sustainment of desired HRO principles and practices within health care delivery systems.
Conclusions
The VIONE program was launched not as a cost savings or research program but as a practical, real-time bedside or ambulatory care intervention to improve patient safety. Its value is reflected in the overwhelming response from scholarly and well-engaged colleagues expressing serious interests in expanding collaborations and tailoring efforts to add more depth and breadth to VIONE related efforts.
Acknowledgments
The authors express their gratitude to Central Arkansas VA Healthcare System leadership, Clinical Applications Coordinators, and colleagues for their unconditional support, to the Diffusion of Excellence programs at US Department of Veterans Affairs Central Office for their endorsement, and to the many VHA participants who renew our optimism and energy as we continue this exciting journey. We also thank Bridget B. Kelly for her assistance in writing and editing of the manuscript.
1. Chassin MR, Jerod ML. High-reliability health care: getting there from here. The Joint Commission. Milbank Q. 2013;91(3):459-490.
2. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
3. Quinn KJ, Shah NH. A dataset quantifying polypharmacy in the United States. Sci Data. 2017;4:170167.
4. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
5. Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med. 2016;176(4):482-483.
6. US Department of Veterans Affairs. High reliability. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx. [Nonpublic source, not verified.]
7. Gordon S, Mendenhall P, O’Connor BB. Beyond the Checklist: What Else Health Care Can Learn from Aviation Teamwork and Safety. Ithaca, NY: Cornell University Press; 2013.
8. Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 2000.
9. US Department of Veterans Affairs. Diffusion of Excellence. https://www.va.gov/HEALTHCAREEXCELLENCE/diffusion-of-excellence/. Updated August 10, 2018. Accessed June 26, 2019.
10. US Department of Veterans Affairs. VIONE program toolkit. https://www.vapulse.net/docs/DOC-259375. [Nonpublic source, not verified.]
High reliability organizations (HROs) incorporate continuous process improvement through leadership commitment to create a safety culture that works toward creating a zero-harm environment.1 The Veterans Health Administration (VHA) has set transformational goals for becoming an HRO. In this article, we describe VIONE, an expanding medication deprescribing clinical program, which exemplifies the translation of HRO principles into health care system models. Both VIONE and HRO are globally relevant.
Reducing medication errors and related adverse drug events are important for achieving zero harm. Preventable medical errors rank behind heart disease and cancer as the third leading cause of death in the US.2 The simultaneous use of multiple medications can lead to dangerous drug interactions, adverse outcomes, and challenges with adherence. When a person is taking multiple medicines, known as polypharmacy, it is more likely that some are potentially inappropriate medications (PIM). Current literature highlights the prevalence and dangers of polypharmacy, which ranks among the top 10 common causes of death in the US, as well as suggestions to address preventable adverse outcomes from polypharmacy and PIM.3-5
Deprescribing of PIM frequently results in better disease management with improved health outcomes and quality of life.4 Many health care settings lack standardized approaches or set expectations to proactively deprescribe PIM. There has been insufficient emphasis on how to make decisions for deprescribing medications when therapeutic benefits are not clear and/or when the adverse effects may outweigh the therapeutic benefits.5
It is imperative to provide practice guidance for deprescribing nonessential medications along with systems-based infrastructure to enable integrated and effective assessments during opportune moments in the health care continuum. Multimodal approaches that include education, risk stratification, population health management interventions, research and resource allocation can help transform organizational culture in health care facilities toward HRO models of care, aiming at zero harm to patients.
The practical lessons learned from VIONE implementation science experiences on various scales and under diverse circumstances, cumulative wisdom from hindsight, foresight and critical insights gathered during nationwide spread of VIONE over the past 3 years continues to propel us toward the desirable direction and core concepts of an HRO.
The VIONE program facilitates practical, real-time interventions that could be tailored to various health care settings, organizational needs, and available resources. VIONE implements an electronic Computerized Patient Record System (CPRS) tool to enable planned cessation of nonessential medications that are potentially harmful, inappropriate, not indicated, or not necessary. The VIONE tool supports systematic, individualized assessment and adjustment through 5 filters (Figure 1). It prompts providers to assign 1 of these filters intuitively and objectively. VIONE combines clinical evidence for best practices, an interprofessional team approach, patient engagement, adapted use of existing medical records systems, and HRO principles for effective implementation.
As a tool to support safer prescribing practices, VIONE aligns closely with HRO principles (Table 1) and core pillars (Table 2).6-8 A zero-harm safety culture necessitates that medications be used for correct reasons, over a correct duration of time, and following a correct schedule while monitoring for adverse outcomes. However, reality generally falls significantly short of this for a myriad of reasons, such as compromised health literacy, functional limitations, affordability, communication gaps, patients seen by multiple providers, and an accumulation of prescriptions due to comorbidities, symptom progression, and management of adverse effects. Through a sharpened focus on both precision medicine and competent prescription management, VIONE is a viable opportunity for investing in the zero-harm philosophy that is integral to an HRO.
Design and Implementation
Initially launched in 2016 in a 15-bed inpatient, subacute rehabilitation unit within a VHA tertiary care facility, VIONE has been sustained and gradually expanded to 38 other VHA facility programs (Figure 2). Recognizing the potential value if adopted into widespread use, VIONE was a Gold Status winner in the VHA Under Secretary for Health Shark Tank-style competition in 2017 and was selected by the VHA Diffusion of Excellence as an innovation worthy of scale and spread through national dissemination.9 A toolkit for VIONE implementation, patient and provider brochures, VIONE vignette, and National Dialog template also have been created.10
Implementing VIONE in a new facility requires an actively engaged core team committed to patient safety and reduction of polypharmacy and PIM, interest and availability to lead project implementation strategies, along with meaningful local organizational support. The current structure for VIONE spread is as follows:
- Interested VHA participants review information and contact vavione@va.gov.
- The VIONE team orients implementing champions, mainly pharmacists, physicians, nurse practitioners, and physician assistants at a facility program level, offering guidance and available resources.
- Clinical Application Coordinators at Central Arkansas VA Healthcare System and participating facilities collaborate to add deprescribing menu options in CPRS and install the VIONE Polypharmacy Reminder Dialog template.
- Through close and ongoing collaborations, medical providers and clinical pharmacists proceed with deprescribing, aiming at planned cessation of nonessential and PIM, using the mnemonic prompt of VIONE. Vital and Important medications are continued and consolidated while a methodical plan is developed to deprescribe any medications that could lead to more harm than benefit and qualify based on the filters of Optional, Not indicated, and Every medicine has a diagnosis/reason. They select the proper discontinuation reasons in the CPRS medication menu (Figure 3) and document the rationale in the progress notes. It is highly encouraged that the collaborating pharmacists and health care providers add each other as cosigners and communicate effectively. Clinical pharmacy specialists also use the VIONE Polypharmacy Reminder Dialog Template (RDT) to document complete medication reviews with veterans to include deprescribing rationale and document shared decision making.
- A VIONE national dashboard captures deprescribing data in real time and automates reporting with daily updates that are readily accessible to all implementing facilities. Minimum data captured include the number of unique veterans impacted, number of medications deprescribed, cumulative cost avoidance to date, and number of prescriptions deprescribed per veteran. The dashboard facilitates real-time use of individual patient data and has also been designed to capture data from VHA administrative data portals and Corporate Data Warehouse.
Results
As of October 31, 2019, the assessment of polypharmacy using the VIONE tool across VHA sites has benefited > 60,000 unique veterans, of whom 49.2% were in urban areas, 47.7% in rural areas, and 3.1% in highly rural areas. Elderly male veterans comprised a clear majority. More than 128,000 medications have been deprescribed. The top classes of medications deprescribed are antihypertensives, over-the-counter medications, and antidiabetic medications. An annualized cost avoidance of > $4.0 million has been achieved. Cost avoidance is the cost of medications that otherwise would have continued to be filled and paid for by the VHA if they had not been deprescribed, projected for a maximum of 365 days. The calculation methodology can be summarized as follows:
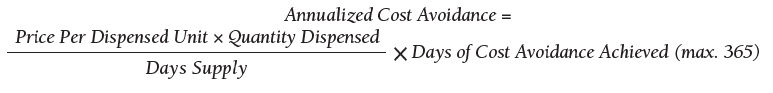
The calculations reported in Table 3 and Figure 4 are conservative and include only chronic outpatient prescriptions and do not account for medications deprescribed in inpatient units, nursing home, community living centers, or domiciliary populations. Data tracked separately from inpatient and community living center patient populations indicated an additional 25,536 deprescribed medications, across 28 VA facilities, impacting 7,076 veterans with an average 2.15 medications deprescribed per veteran. The additional achieved cost avoidance was $370,272 (based on $14.50 average cost per prescription). Medications restarted within 30 days of deprescribing are not included in these calculations.
The cost avoidance calculation further excludes the effects of VIONE implementation on many other types of interventions. These interventions include, but are not limited to, changing from aggressive care to end of life, comfort care when strongly indicated; reduced emergency department visits or invasive diagnostic and therapeutic approaches, when not indicated; medical supplies, antimicrobial preparations; labor costs related to packaging, mailing, and administering prescriptions; reduced/prevented clinical waste; reduced decompensation of systemic illnesses and subsequent health care needs precipitated by iatrogenic disturbances and prolonged convalescence; and overall changes to prescribing practices through purposeful and targeted interactions with colleagues across various disciplines and various hierarchical levels.
Discussion
The VIONE clinical program exemplifies the translation of HRO principles into health care system practices. VIONE offers a systematic approach to improve medication management with an emphasis on deprescribing nonessential medications across various health care settings, facilitating VHA efforts toward zero harm. It demonstrates close alignment with the key building blocks of an HRO. Effective VIONE incorporation into an organizational culture reflects leadership commitment to safety and reliability in their vision and actions. By empowering staff to proactively reduce inappropriate medications and thereby prevent patient harm, VIONE contributes to enhancing an enterprise-wide culture of safety, with fewer errors and greater reliability. As a standardized decision support tool for the ongoing practice of assessment and planned cessation of potentially inappropriate medications, VIONE illustrates how continuous process improvement can be a part of staff-engaged, veteran-centered, highly reliable care. The standardization of the VIONE tool promotes achievement and sustainment of desired HRO principles and practices within health care delivery systems.
Conclusions
The VIONE program was launched not as a cost savings or research program but as a practical, real-time bedside or ambulatory care intervention to improve patient safety. Its value is reflected in the overwhelming response from scholarly and well-engaged colleagues expressing serious interests in expanding collaborations and tailoring efforts to add more depth and breadth to VIONE related efforts.
Acknowledgments
The authors express their gratitude to Central Arkansas VA Healthcare System leadership, Clinical Applications Coordinators, and colleagues for their unconditional support, to the Diffusion of Excellence programs at US Department of Veterans Affairs Central Office for their endorsement, and to the many VHA participants who renew our optimism and energy as we continue this exciting journey. We also thank Bridget B. Kelly for her assistance in writing and editing of the manuscript.
High reliability organizations (HROs) incorporate continuous process improvement through leadership commitment to create a safety culture that works toward creating a zero-harm environment.1 The Veterans Health Administration (VHA) has set transformational goals for becoming an HRO. In this article, we describe VIONE, an expanding medication deprescribing clinical program, which exemplifies the translation of HRO principles into health care system models. Both VIONE and HRO are globally relevant.
Reducing medication errors and related adverse drug events are important for achieving zero harm. Preventable medical errors rank behind heart disease and cancer as the third leading cause of death in the US.2 The simultaneous use of multiple medications can lead to dangerous drug interactions, adverse outcomes, and challenges with adherence. When a person is taking multiple medicines, known as polypharmacy, it is more likely that some are potentially inappropriate medications (PIM). Current literature highlights the prevalence and dangers of polypharmacy, which ranks among the top 10 common causes of death in the US, as well as suggestions to address preventable adverse outcomes from polypharmacy and PIM.3-5
Deprescribing of PIM frequently results in better disease management with improved health outcomes and quality of life.4 Many health care settings lack standardized approaches or set expectations to proactively deprescribe PIM. There has been insufficient emphasis on how to make decisions for deprescribing medications when therapeutic benefits are not clear and/or when the adverse effects may outweigh the therapeutic benefits.5
It is imperative to provide practice guidance for deprescribing nonessential medications along with systems-based infrastructure to enable integrated and effective assessments during opportune moments in the health care continuum. Multimodal approaches that include education, risk stratification, population health management interventions, research and resource allocation can help transform organizational culture in health care facilities toward HRO models of care, aiming at zero harm to patients.
The practical lessons learned from VIONE implementation science experiences on various scales and under diverse circumstances, cumulative wisdom from hindsight, foresight and critical insights gathered during nationwide spread of VIONE over the past 3 years continues to propel us toward the desirable direction and core concepts of an HRO.
The VIONE program facilitates practical, real-time interventions that could be tailored to various health care settings, organizational needs, and available resources. VIONE implements an electronic Computerized Patient Record System (CPRS) tool to enable planned cessation of nonessential medications that are potentially harmful, inappropriate, not indicated, or not necessary. The VIONE tool supports systematic, individualized assessment and adjustment through 5 filters (Figure 1). It prompts providers to assign 1 of these filters intuitively and objectively. VIONE combines clinical evidence for best practices, an interprofessional team approach, patient engagement, adapted use of existing medical records systems, and HRO principles for effective implementation.
As a tool to support safer prescribing practices, VIONE aligns closely with HRO principles (Table 1) and core pillars (Table 2).6-8 A zero-harm safety culture necessitates that medications be used for correct reasons, over a correct duration of time, and following a correct schedule while monitoring for adverse outcomes. However, reality generally falls significantly short of this for a myriad of reasons, such as compromised health literacy, functional limitations, affordability, communication gaps, patients seen by multiple providers, and an accumulation of prescriptions due to comorbidities, symptom progression, and management of adverse effects. Through a sharpened focus on both precision medicine and competent prescription management, VIONE is a viable opportunity for investing in the zero-harm philosophy that is integral to an HRO.
Design and Implementation
Initially launched in 2016 in a 15-bed inpatient, subacute rehabilitation unit within a VHA tertiary care facility, VIONE has been sustained and gradually expanded to 38 other VHA facility programs (Figure 2). Recognizing the potential value if adopted into widespread use, VIONE was a Gold Status winner in the VHA Under Secretary for Health Shark Tank-style competition in 2017 and was selected by the VHA Diffusion of Excellence as an innovation worthy of scale and spread through national dissemination.9 A toolkit for VIONE implementation, patient and provider brochures, VIONE vignette, and National Dialog template also have been created.10
Implementing VIONE in a new facility requires an actively engaged core team committed to patient safety and reduction of polypharmacy and PIM, interest and availability to lead project implementation strategies, along with meaningful local organizational support. The current structure for VIONE spread is as follows:
- Interested VHA participants review information and contact vavione@va.gov.
- The VIONE team orients implementing champions, mainly pharmacists, physicians, nurse practitioners, and physician assistants at a facility program level, offering guidance and available resources.
- Clinical Application Coordinators at Central Arkansas VA Healthcare System and participating facilities collaborate to add deprescribing menu options in CPRS and install the VIONE Polypharmacy Reminder Dialog template.
- Through close and ongoing collaborations, medical providers and clinical pharmacists proceed with deprescribing, aiming at planned cessation of nonessential and PIM, using the mnemonic prompt of VIONE. Vital and Important medications are continued and consolidated while a methodical plan is developed to deprescribe any medications that could lead to more harm than benefit and qualify based on the filters of Optional, Not indicated, and Every medicine has a diagnosis/reason. They select the proper discontinuation reasons in the CPRS medication menu (Figure 3) and document the rationale in the progress notes. It is highly encouraged that the collaborating pharmacists and health care providers add each other as cosigners and communicate effectively. Clinical pharmacy specialists also use the VIONE Polypharmacy Reminder Dialog Template (RDT) to document complete medication reviews with veterans to include deprescribing rationale and document shared decision making.
- A VIONE national dashboard captures deprescribing data in real time and automates reporting with daily updates that are readily accessible to all implementing facilities. Minimum data captured include the number of unique veterans impacted, number of medications deprescribed, cumulative cost avoidance to date, and number of prescriptions deprescribed per veteran. The dashboard facilitates real-time use of individual patient data and has also been designed to capture data from VHA administrative data portals and Corporate Data Warehouse.
Results
As of October 31, 2019, the assessment of polypharmacy using the VIONE tool across VHA sites has benefited > 60,000 unique veterans, of whom 49.2% were in urban areas, 47.7% in rural areas, and 3.1% in highly rural areas. Elderly male veterans comprised a clear majority. More than 128,000 medications have been deprescribed. The top classes of medications deprescribed are antihypertensives, over-the-counter medications, and antidiabetic medications. An annualized cost avoidance of > $4.0 million has been achieved. Cost avoidance is the cost of medications that otherwise would have continued to be filled and paid for by the VHA if they had not been deprescribed, projected for a maximum of 365 days. The calculation methodology can be summarized as follows:
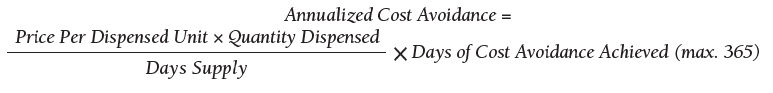
The calculations reported in Table 3 and Figure 4 are conservative and include only chronic outpatient prescriptions and do not account for medications deprescribed in inpatient units, nursing home, community living centers, or domiciliary populations. Data tracked separately from inpatient and community living center patient populations indicated an additional 25,536 deprescribed medications, across 28 VA facilities, impacting 7,076 veterans with an average 2.15 medications deprescribed per veteran. The additional achieved cost avoidance was $370,272 (based on $14.50 average cost per prescription). Medications restarted within 30 days of deprescribing are not included in these calculations.
The cost avoidance calculation further excludes the effects of VIONE implementation on many other types of interventions. These interventions include, but are not limited to, changing from aggressive care to end of life, comfort care when strongly indicated; reduced emergency department visits or invasive diagnostic and therapeutic approaches, when not indicated; medical supplies, antimicrobial preparations; labor costs related to packaging, mailing, and administering prescriptions; reduced/prevented clinical waste; reduced decompensation of systemic illnesses and subsequent health care needs precipitated by iatrogenic disturbances and prolonged convalescence; and overall changes to prescribing practices through purposeful and targeted interactions with colleagues across various disciplines and various hierarchical levels.
Discussion
The VIONE clinical program exemplifies the translation of HRO principles into health care system practices. VIONE offers a systematic approach to improve medication management with an emphasis on deprescribing nonessential medications across various health care settings, facilitating VHA efforts toward zero harm. It demonstrates close alignment with the key building blocks of an HRO. Effective VIONE incorporation into an organizational culture reflects leadership commitment to safety and reliability in their vision and actions. By empowering staff to proactively reduce inappropriate medications and thereby prevent patient harm, VIONE contributes to enhancing an enterprise-wide culture of safety, with fewer errors and greater reliability. As a standardized decision support tool for the ongoing practice of assessment and planned cessation of potentially inappropriate medications, VIONE illustrates how continuous process improvement can be a part of staff-engaged, veteran-centered, highly reliable care. The standardization of the VIONE tool promotes achievement and sustainment of desired HRO principles and practices within health care delivery systems.
Conclusions
The VIONE program was launched not as a cost savings or research program but as a practical, real-time bedside or ambulatory care intervention to improve patient safety. Its value is reflected in the overwhelming response from scholarly and well-engaged colleagues expressing serious interests in expanding collaborations and tailoring efforts to add more depth and breadth to VIONE related efforts.
Acknowledgments
The authors express their gratitude to Central Arkansas VA Healthcare System leadership, Clinical Applications Coordinators, and colleagues for their unconditional support, to the Diffusion of Excellence programs at US Department of Veterans Affairs Central Office for their endorsement, and to the many VHA participants who renew our optimism and energy as we continue this exciting journey. We also thank Bridget B. Kelly for her assistance in writing and editing of the manuscript.
1. Chassin MR, Jerod ML. High-reliability health care: getting there from here. The Joint Commission. Milbank Q. 2013;91(3):459-490.
2. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
3. Quinn KJ, Shah NH. A dataset quantifying polypharmacy in the United States. Sci Data. 2017;4:170167.
4. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
5. Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med. 2016;176(4):482-483.
6. US Department of Veterans Affairs. High reliability. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx. [Nonpublic source, not verified.]
7. Gordon S, Mendenhall P, O’Connor BB. Beyond the Checklist: What Else Health Care Can Learn from Aviation Teamwork and Safety. Ithaca, NY: Cornell University Press; 2013.
8. Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 2000.
9. US Department of Veterans Affairs. Diffusion of Excellence. https://www.va.gov/HEALTHCAREEXCELLENCE/diffusion-of-excellence/. Updated August 10, 2018. Accessed June 26, 2019.
10. US Department of Veterans Affairs. VIONE program toolkit. https://www.vapulse.net/docs/DOC-259375. [Nonpublic source, not verified.]
1. Chassin MR, Jerod ML. High-reliability health care: getting there from here. The Joint Commission. Milbank Q. 2013;91(3):459-490.
2. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
3. Quinn KJ, Shah NH. A dataset quantifying polypharmacy in the United States. Sci Data. 2017;4:170167.
4. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
5. Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med. 2016;176(4):482-483.
6. US Department of Veterans Affairs. High reliability. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx. [Nonpublic source, not verified.]
7. Gordon S, Mendenhall P, O’Connor BB. Beyond the Checklist: What Else Health Care Can Learn from Aviation Teamwork and Safety. Ithaca, NY: Cornell University Press; 2013.
8. Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 2000.
9. US Department of Veterans Affairs. Diffusion of Excellence. https://www.va.gov/HEALTHCAREEXCELLENCE/diffusion-of-excellence/. Updated August 10, 2018. Accessed June 26, 2019.
10. US Department of Veterans Affairs. VIONE program toolkit. https://www.vapulse.net/docs/DOC-259375. [Nonpublic source, not verified.]
Aging and Trauma: Post Traumatic Stress Disorder Among Korean War Veterans
The Korean War lasted from June 25, 1950 through July 27, 1953. Although many veterans of the Korean War experienced traumas during extremely stressful combat conditions. However, they would not have been diagnosed with posttraumatic stress disorder (PTSD) at the time because the latter did not exist as a formal diagnosis until the publication of the third edition of the Diagnostic and Statistical Manual (DSM) in 1980.1 Prior to 1980, psychiatric syndromes resulting from war and combat exposure where known by numerous other terms including shell shock, chronic traumatic war neurosis, and combat fatigue/combat exhaustion.2,3 Military psychiatrists attended to combat fatigue during the course of the Korean War, but as was true of World War I and II, the focus was on returning soldiers to duty. Combat fatigue was generally viewed as a transient condition.4-8
Although now octo- and nonagenarians, in 2019 there are 1.2 million living Korean War veterans in the US, representing 6.7% of all current veterans.9 Understanding their war experiences and the nature of their current and past presentation of PTSD is relevant not only in formal mental health settings, but in primary care settings, including home-based primary care, as well as community living centers, skilled nursing facilities and assisted living facilities. Older adults with PTSD often present with somatic concerns rather than spontaneously reporting mental health symptoms.10 Beyond the short-term clinical management of Korean War veterans with PTSD, consideration of their experiences also has long-term relevance for the appropriate treatment of other veteran cohorts as they age in coming decades.
The purpose of this article is to provide a clinically focused overview of PTSD in Korean War veterans, to help promote understanding of this often-forgotten group of veterans, and to foster optimized personalized care. This overview will include a description of the Korean War veteran population and the Korean War itself, the manifestations and identification of PTSD among Korean War veterans, and treatment approaches using evidence-based psychotherapies and pharmacotherapies. Finally, we provide recommendations for future research to address present empirical gaps in the understanding and treatment of Korean War veterans with PTSD.
Causes and Course of the Korean War
When working with Korean War veterans it is important to consider the special nature of that specific conflict. Space considerations limit our ability to do justice to the complex history and numerous battles of the Korean War, but information in the following summary was gleaned from several excellent histories.11-13
The Korean War has been referred to as The Forgotten War, a concern expressed even during the latter parts of the war.14,15 But the war and its veterans warrant remembering. The root and proximal causes of the Korean War are complex and not fully agreed upon by the main participants.16-19 In part this may reflect the fact that there was no clear victor in the Korean War, so that the different protagonists have developed their own versions of the history of the conflict. Also, US involvement and the public reaction to the war must be viewed within the larger historical context of that time. This context included the recent end of 4 years of US involvement in World War II (1941-1945) and the subsequent rapid rise of Cold War tensions between the US and the Soviet Union. The latter also included a worldwide fear of nuclear war and the US fear of the global spread of communism. These fears were fueled by the Soviet-led Berlin Blockade from June 1948 through May 1949, the Soviet Union’s successful atomic bomb test in August 1949, the founding of the People’s Republic of China in October 1949, and the February 1950 Sino-Soviet Treaty of Friendship and Alliance.13
In the closing days of World War II, the US and Soviet Union agreed to a temporary division of Korea along the 38th parallel to facilitate timely and efficient surrender of Japanese troops. But as Cold War tensions rose, the temporary division became permanent, and Soviet- and US-backed governments of the north and south, respectively, were officially established on the Korean peninsula in 1948. Although by 1949 the Soviets and US had withdrawn most troops from the peninsula, tensions between the north and south continued to mount and hostilities increased. To this day the exact causes of the eruption of war remain disputed, although it is clear that ideological as well as economic factors played a role, and both leaders of North and South Korea were pledging to reunite the peninsula under their respective leadership.16-19 The tension culminated on June 25, 1950, when North Korean troops crossed the 38th parallel and invaded South Korea. On June 27, 1950, President Truman ordered US naval and air forces to support South Korea and then ordered the involvement of ground troops on June 30.16,17,19
Although several other member countries of the United Nations (UN) provided troops, 90% of the troops were from the US. About 5.7 million US military personnel served during the war, including about 1.8 million in Korea itself. The US forces experienced approximately 34,000 battle-related deaths, 103,000 were wounded, and 7,000 were prisoners of war (POWs).11,20-22 The nature and events of the Korean War made it particularly stressful and traumatizing for the soldiers, sailors, and marines involved throughout its entire course. These included near defeat in the early months, a widely alternating war front along the north/south axis during the first year, and subsequently, not only intense constant battles on the fronts, but also a demanding and exhausting guerrilla war in the south, which lasted throughout the remainder of the conflict.11,15 The US troops during the initial months of the war have been described as outnumbered and underprepared, as many in the initial phase were reassigned from peace-time occupation duty in Japan.7
The first year of war was characterized by a repeated north-to-south/south-to-north shifts in control of territory. During the first 3 months, the North Korean forces overwhelmed the South and captured control of all but 2 South Korean cities in the far southeastern region (Pusan, now Busan; and Daegu), and US and UN forces were forced to retreat to the perimeter around Pusan. The intense Battle of Pusan Perimeter lasted from August 4, 1950 to September 18, 1950, and resulted in massive causalities as well as a flood of civilian refugees.
The course of the war began to change in early September 1950 with the landing of amphibious US/UN forces at Inchon, behind North Korean lines, which cut off southern supply routes for the North Korean troops.11 US/UN forces soon crossed to the north of the 38th parallel and captured the North Korean capital, Pyongyang, on October 19, 1950. They continued to push north and approached the Yalu River border with China by late November 1950, but then the Chinese introduced their own troops forcing a southward retreat of US/UN troops during which there were again numerous US/UN casualties. Chinese troops retook Seoul in late December 1950/early January 1951. However, the US/UN forces soon recaptured Seoul and advanced back to the 38th parallel. This back-and-forth across the 38th parallel continued until July 1951 when the front line of battle stabilized there. Although the line stabilized, intense battles and casualties continued for 2 more years. During this period US/UN troops also had to deal with guerrilla warfare behind the front lines due to the actions of communist partisans and isolated North Korean troops. This situation continued until the armistice was signed July 27, 1953.
Trauma and Characteristic Stresses of the War
There were many factors that made the Korean War experience different from previous wars, particularly World War II. For example, in contrast to the strong public support during and after World War II, public support for the Korean War in the US was low, particularly during its final year.23 In public opinion polls from October 1952 through April 1953, only 23% to 39% reported feeling that the war was worth fighting.23 A retrospective 1985 survey also found that 70% of World War II veterans, but only 33% of Korean War veterans reported feeling appreciated by the US public on their return from the war.24
Those fighting in the initial months of the war faced a particularly grim situation. According to LTC Philip Smith, who served as Division Psychiatrist on the Masan Front (Pusan Perimeter) during August and September of 1950, “Fighting was almost continuous and all available troops were on the fighting front… For the most part these soldiers were soft from occupation duty, many had not received adequate combat basic training, no refresher combat training in Korea had as yet been instituted,” he reported.7 “The extremes of climate coupled with the generally rugged mountainous terrain in Korea were physical factors of importance…These men were psychologically unprepared for the horrors and isolation of war.” LTC Smith noted that the change in status from civilian or occupation life to the marked deprivation of the war in Korea had been “too abrupt to allow as yet for a reasonable adjustment to the new setting” and that as a result “the highest rate of wounded and neuropsychiatric casualties in the Korean campaign resulted.”7
Even after this initial period, the nature of the shifting war, the challenging terrain, the high military casualty rate, and the high rate of civilian casualties and displacement continued throughout the war.
PTSD in Korean War Veterans
It is clear that Korean War combat veterans were exposed to traumatic events. It is unknown how many developed PTSD. While notions of psychological distress and disability related to combat trauma exposure have existed for centuries, Korean War and World War II veterans are a remaining link to pre-DSM PTSD mental health in the military. Military/forward psychiatry—psychiatric services near the battle zone rather than requiring evacuation of patients—was present in Korea from the early months of the war, but the focus of forward psychiatry was to reduce psychiatric causalities from combat fatigue and maximize rapid return-to-duty.4-6 With no real conception of PTSD, there were limited treatments available, and evidenced-based trauma-focused treatments for PTSD would not be introduced for at least another 4 decades.27-29
Skinner and Kaplick conducted a historical review of case descriptions of trauma-related conditions from World War I through the Vietnam War and noted the consistent inclusion of hyperarousal and intrusive symptoms, although there also was a greater emphasis on somatic conversion or hysteria symptoms in the earlier descriptions.30 By the Korean War, descriptions of combat fatigue included a number of symptoms that overlap with PTSD, including preoccupation with the traumatic stressor, nightmares, irritability/anger, increased startle, and hyperarousal.31 But following the acute phases, attention to any chronic problems associated with these conditions waned. As was acknowledged by a military psychiatrist in a 1954 talk, studies of the long-term adjustment of those who had “broken down in combat” were sorely needed.6 In a small 1965 study reported by Archibald and Tuddenham, persistent symptoms of combat fatigue among Korean War veterans were definitely present, and there was even a suggestion that the symptoms had increased over the decade since the war.32
Given the stoicism that typified cultural expectations for military men during this period, Korean War veterans may also have been reluctant to seek mental health treatment either at the time or later. In short, it is likely that a nontrivial proportion of Korean War veterans with PTSD were underdiagnosed and received suboptimal or no mental health treatment for decades following their war experiences.33 Although the nature of the war, deployment, and public support were distinct in World War II vs the Korean War, the absence of attention to the long-term effects of disorders related to combat trauma and the cultural expectations for stoicism suggest that PTSD among aging World War II veterans may also have gone underrecognized and undertreated.
Apart from the lack of interest in chronic effects of stressors, another problem that has plagued the limited empirical research on Korean War veterans has been the propensity to combine Korean War with World War II veteran samples in studies. Because World War II veterans have outnumbered Korean War veterans until recently, combined samples tended to have relatively few Korean War veterans. Nevertheless, from those studies that have been reported in which 2 groups were compared, important differences have been revealed. Specifically, although precise estimates of the prevalence of PTSD among Korean War combat veterans have varied depending on sampling and method, studies from the 1990s and early 2000s suggested that the prevalence of PTSD and other mental health concerns as well as the severity of symptoms, suicide risk, and psychosocial adjustment difficulties were worse among Korean War combat veterans relative to those among World War II combat veterans; however, both groups had lower prevalence than did Vietnam War combat veterans.21,34-37 Several authors speculated that these differences in outcome were at least partially due to differences in public support for the respective wars.36,37
Although there has been a paucity of research on psychiatric issues and PTSD in Korean War veterans, POWs who were very likely to have been exposed to extreme psychological traumas have received some attention. There have been comparisons of mortality and morbidity among POWs from the Korean War (PWK), World War II Pacific Theater (PWJ), and Europe (PWE).38 Among measures that were administered to the former POWs, the overall pattern seen from survey data in the mid-1960s revealed significantly worse health and functioning among the PWK and PWJ groups relative to the PWE group, with psychiatric difficulties being the most commonly reported impairments among the former 2 groups. This pattern was found most strongly with regards to objective measures, such as hospitalizations for “psychoneuroses,” and US Department of Veterans Affairs (VA) disability records, as well as based on self-reported psychosocial/recreational difficulties measured using the Cornell Medical Index (CMI).38
Gold and colleagues reported a follow-up study of more than 700 former POWs who were reinterviewed between 1989 and 1992.39 Although there was no scale of PTSD symptoms prior to formulation of the diagnosis in 1980, the CMI was a self-reported checklist that included a large range of both medical as well as behavioral and psychiatric symptoms. Thus, using CMI survey responses from 1965, the authors examined the factor structure (ie, the correlational relationships between multiple scale items and subgroupings of items) of the CMI relative to diagnosis of PTSD in 1989 to 1992 based on results from the Structured Clinical Interview for the DSM-III-R (SCID). The intent was to help discern whether the component domains of PTSD were present and intercorrelated in a pattern similar to that of the contemporary diagnosis. The investigators examined the factor structure of 20 psychological items from the CMI that appeared relevant to PTSD criteria using the 1965 data. Three factors (subgroups of highly intercorrelated items) were found: irritability (31% of variance), fearfulness/anxiousness (9% of the variance), and social withdrawal (7% of the variance). Although these did not directly correspond to, or fully cover, DSM PTSD domains or criteria, there does appear to be a thematic resemblance of the CMI findings with PTSD, including alterations in arousal and mood, vigilance, and startle.
Identification and Treatment of PTSD in Older Veterans
Of the 1.2 million living Korean War veterans in the US, 36.3% use VA provided health care.40 There are a number of complicating factors to consider in the current identification and treatment of PTSD in this cohort, including their advanced age; physical, cognitive, and social changes associated with normal aging; the associated medical and cognitive comorbidities; and the specific social-contextual factors in that age cohort. Any combination of these factors may complicate recognition, diagnosis, and treatment. It is also important to be cognizant of the additional stressors that may have been experienced by ethnic minorites and women serving in Korea, which are poorly documented and studied. Racial integration of the US military began during the Korean War, but the general pattern was for African American soldiers to be assigned to all-white units, rather than the reverse.14,41,42 And although the majority of military personnel serving in Korea were male, there were women serving in health care positions at mobile army surgical hospital (MASH) units, medical air evacuation (Medevac) aircraft, and off-shore hospital ships.
The clinical presentation of PTSD in older adults has varied, which may partially relate to the time elapsed since the index trauma. For example, older veterans in general may show less avoidance behavior as a part of PTSD, but in those who experience trauma later in life there may actually be greater avoidance.43,44 There have also been discrepant reports of intrusion or reexperiencing of symptoms, with these also potentially reduced in older veterans.43,44 However, sleep disturbances seem to be very common among elderly combat veterans, and attention should be paid to the possible presence of sleep apnea, which may be more common in veterans with PTSD in general.43,45,46
PTSD symptoms may reemerge after decades of remission or quiescence during retirement and/or with the emergence of neurocognitive impairment, such as Alzheimer disease or dementia. These individuals may have more difficulty engaging in distracting activities and work and spend more time engaging in reminiscence about the past, which can include increased focus on traumatic memories.45,47 Davison and colleagues have suggested a concept they call later-adulthood trauma reengagement (LATR) where later in life combat veterans may “confront and rework their wartime memories in an effort to find meaning and build coherence.”48 This process can be a double-edged sword, leading at times positively to enhanced personal growth or negatively to increased symptoms; preventive interventions may be able foster a more positive outcome.48
There is some evidence supporting the validity of the Clinician Administered PTSD Scale (CAPS) for the evaluation of PTSD in older adults, although this was based on the DSM-III-revised criteria for PTSD and an earlier version of CAPS.49 Bhattarai and colleagues examined responses to the 35-item Mississippi Scale for Combat-Related PTSD (M-PTSD) using VA clinical data collected between 2008 and 2015 on veterans of each combat era from World War II through the post-9/11.50 Strong internal consistency and test-retest reliability of the M-PTSD was observed within each veteran era sample. However, using chart diagnosis of PTSD as the criterion standard, the cut-scores for optimal balance of sensitivity and specificity of the M-PTSD scores were substantially lower for the older cohorts (World War II and Korean War veterans) relative to those for Vietnam and more recent veteran cohorts. The authors concluded that M-PTSD can be validly used to screen for PTSD in veterans within each of these cohorts but recommended using lower than standard cut-scores for Korean War and World War II veterans.50
This is also consistent with reports that suggest the use of lower cut-scores on self-administered PTSD symptom screens.43,44 For the clinician interested in quantifying the severity of PTSD, the most recent tools available are the CAPS-5 and the PCL-5, which have both been created in accordance with the DSM-5. The CAPS-5 is a rater-administered tool, and the PCL-5 is self-administered by the veteran. Although there has been little research using these newer tools in geriatric populations, they can currently serve as a means of tracking the severity of PTSD while we await measures that are better validated in Korean War and other older veterans.
Beyond specific empirical guidance, VA clinicians must presently rely on clinical observations and experience. Patients from the Korean War cohort often present at the insistence of a family member for changes in sleep, mood, behavior, or cognition. When the veterans themselves present, older adults with PTSD often focus more on somatic concerns (including pain, sleep, and gastrointestinal disturbance) than psychiatric problems per se. The latter tendency may in part be due to the salience of such symptoms for them, but perhaps also due to considerable stigma of mental health care that is still largely present in this group.43,44
Psychotherapy
Current VA treatment guidelines recommend trauma-focused therapies, with the strongest evidence base for prolonged exposure (PE), cognitive processing therapy (CPT), and eye movement desensitization and reprocessing (EMDR) therapies.51
There have been several excellent prior reviews discussing treatment of PTSD in older adults generally.10,43,44,52 These reviews have invariably expressed concern about the lack of sufficient empirical studies, but based on evidence from studies and case reports, there seems to be tentative support that trauma-focused therapies are acceptable and efficacious for use with older adults with PTSD. In their recent scoping review, Pless Kaiser and colleagues made several recommendations for trauma-focused therapy with older adults, including slow/careful pacing and use of compensatory aids for cognitive and sensory deficits.44 When cognitive impairment has exacerbated PTSD symptoms, they suggest therapists consider using an adapted form of CPT completed without a trauma narrative. For PE they recommend extending content across sessions and involving spouse or caregivers to assist with in vivo exposure and homework completion.44
Recent studies suggest that PTSD may be a risk factor for the later development of neurodegenerative disorders, and it is often during assessments for dementia that a revelation of PTSD occurs.10,43,47,55 Cognitive impairment may also be of relevance in deciding on the type of psychotherapy to be implemented, as it may have more adverse effects on the effectiveness of CPT than of exposure-based treatments (PE or EMDR). It may be useful to perform a cognitive assessment prior to initiation of a cognitive-based therapy, although extensive cognitive testing may not be practical or may be contraindicated because of fatigue. A brief screening tool such as the Montreal Cognitive Assessment or the Mini-Mental State Examinationmay be helpful.56, 57
Prolonged exposure has been reported by many clinicians to be effective in older adults with PTSD; however, due consideration should be given to the needs of individuals, as many have functioned for decades by suppressing memories.
Apart from the treatment needs for specific PTSD symptoms, the decades-long effects of poor sleep, irritability, hypervigilance, and dissociation also have social consequences for patients, including marital discord and divorce, and social and family isolation that should be addressed in therapy when appropriate. In addition, many Korean War veterans, like all veterans, sought postmilitary employment in professions that are associated with higher rates of exposure to psychological trauma, such as police or fire departments, and this may have an exacerbating effect on PTSD.58
Pharmacotherapy
There is very little empirical evidence guiding pharmacologic approaches to PTSD in older veterans. This population is at increased risk for many comorbidities, and pharmacologic treatments many require dosage adjustments, as is the case for any geriatric patient. Selective serotonin reuptake inhibitor (SSRI) and serotonin norepinephrine reuptake inhibitor (SNRI) medications have been proposed for some cases of PTSD.59,60 Health care providers may consider the SSRIs escitalopram or sertraline preferentially given their decreased potential for drug-drug interactions, anticholinergic effects, or cardiac toxicity compared with that of other drugs in this class.60,61 As venlafaxine can increase blood pressure, especially at higher doses, prescribers may choose duloxetine as an alternative if a SNRI is indicated.60 For veterans when prazosin is being considered for nightmare control, monitoring for hypotension, orthostasis, and the administration of other antihypertensives or prostatic hypertrophy medications is necessary.61 The use of benzodiazepines, while not recommended for PTSD, should be viewed with even greater trepidation in a geriatric population given enhanced risk of falls and confusion in the geriatric veteran population.60,62
Conclusions
Many of the oldest veterans (aged > 80 years) are from the Korean War era. The harsh and unique nature of the war, as well as the differences in context and support from the US public, and the outcome of the war, may have all contributed to and elevation of “combat fatigue” and PTSD among combat veterans from the Korean War. As the “forgotten war” cohort also has been forgotten by researchers, relatively little is known about posttraumatic stress sequelae of these veterans in the decades following the war.
From available evidence, we can readily surmise that problems were underrecognized and suboptimally diagnosed and treated. There is tentative evidence supporting the use of standard interviews and rating scales, such as the CAPS, M-PTSD, and PCL, but lower cut-scores than applied with Vietnam and later veteran cohorts are generally recommended to avoid excessive false negative errors. In terms of psychotherapy treatment, there is again a stark paucity of systematic research, but the limited evidence from studies of PTSD treatment in older adults from the general population tentatively support the acceptability and potential efficacy of recognized evidence-based trauma-focused psychotherapies for PTSD. Research on medication treatment is similarly lacking, but the general recommendations for the use of SSRI or SNRI medications seem to be valid, at least in our clinical experience, and the general rules for geriatric psychopharmacology definitely apply here—start low, go slow.
There are several important avenues for future research. Most pressing among these are establishing the effectiveness of existing treatments, and the modifications that may be needed in the broader context of the above factors, as well as the physical and cognitive changes associated with advanced age. Further research on the phenomenologic aspects of PTSD among Korean War and subsequent cohorts are also needed, as the information obtained will not only guide more effective personalized treatment of the Korean War veterans who remain with us, but also inform future generations of care in terms of the degree and dimensions of variability that may present between cohorts and within cohorts over the life span.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Arlington VA: American Psychiatric Association; 1980.
2. Friedman MJ, Schnurr PP, McDonagh-Coyle A. Posttraumatic stress disorder in the military veteran. Psychiatr Clin North Am. 1994;17(2):265-277.
3. Salmon TW. The Care and Treatment of Mental Diseases and War Neuroses (“Shell Shock”) in the British Army. New York: War Work Committee of the National Committee for Mental Hygiene, Inc; 1917.
4. Jones E, Wessely S. “Forward psychiatry” in the military: its origins and effectiveness. J Trauma Stress. 2003;16(4):411-419.
5. Newman RA. Combat fatigue: a review to the Korean conflict. Mil Med. 1964;129:921-928.
6. Harris FG. Some comments on the differential diagnosis and treatment of psychiatric breakdowns in Korea. https://history.amedd.army.mil/booksdocs/korea/recad2/ch9-2.html. Published April 30, 1954. Accessed November 8, 2019.
7. Smith PB. Psychiatric experiences during the Korean conflict. Am Pract Dig Treat. 1955;6(2):183-189.
8. Koontz AR. Psychiatry in the Korean War. Military Surg.
1950;107(6):444-445.
9. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Population Tables - Table 2L: VETPOP2016 Living Veterans by period of service, gender, 2015-2045. https://www.va.gov/vetdata/docs/Demographics/New_Vetpop_Model/2L_VetPop2016
_POS_National.xlsx. Accessed November 8, 2019.
10. Cook JM, McCarthy E, Thorp SR. Older adults with PTSD: brief state of research and evidence-based psychotherapy case illustration. Am J Geriatr Psychiatry. 2017;25(5):522-530.
11. Millett AR. Korean War: 1950-1953. Encylopaedia Britannica. https://www.britannica.com/event/Korean-War#accordion-article-history. Updated Nov 7, 2019. Accessed November 8, 2019.
12. Stack L. Korean War, a ‘forgotten’ conflict that shaped the modern world. The New York Times. January 2, 2018. https://www.nytimes.com/2018/01/01/world/asia/korean-war-history.html. Accessed November 8, 2019.
13. Westad OA. The Cold War: A World History. New York: Basic Books; 2018.
14. Young C, Conard PL, Armstrong ML, Lacy D. Older military veteran care: many still believe they are forgotten. J Holist Nurs. 2018;36(3):291-300.
15. Huebner AJ. Kilroy is back, 1950-1953. In: The Warrior Image: Soldiers in American Culture From the Second World War to the Vietnam Era. Chapel Hill, NC: The University of North Carolina Press; 2008:97-131.
16. The annexation of Korea (editorial). Japan Times. https://www.japantimes.co.jp/opinion/2010/08/29/editorials/the-annexation-of-korea/#.XPgvJvlKhhE. Published August 29, 2010. Accessed November 8, 2019.
17. Gupta K. How did the Korean war begin? China Q. 1972;52:699-716.
18. Lin L, Zhao Y, Ogawa M, Hoge J, Kim BY. Whose history? An analysis of the Korean War in history textbooks from the United States, South Korea, Japan, and China. Social Studies. 2009;100(5):222-232.
19. Weathersby K. The Korean War revisited. Wilson Q. 1999;23(3):91.
20. US Department of Veterans Affairs, Office of Program and Data Analyses, Assistant Secretary for Planning and Analysis. Data on veterans of the Korean War. https://www.va.gov/vetdata/docs/SpecialReports/KW2000.pdf. Published June 2000. Accessed November 8, 2019.
21. Brooks MS, Fulton L. Evidence of poorer life-course mental health outcomes among veterans of the Korean War cohort. Aging Ment Health. 2010;14(2):177-183.
22. US Department of Veterans Affairs, Office of Public Affairs. America’s wars. https://www.va.gov/opa/publications/factsheets/fs_americas_wars.pdf. Accessed November 8, 2019.
23. Memorandum on recent polls on Korea. https://www.eisenhowerlibrary.gov/sites/default/files/research/online-documents/korean-war/public-opinion-1953-06-02.pdf. Published June 2, 1953. Accessed November 8, 2019.
24. Elder GH Jr, Clipp EC. Combat experience and emotional health: impairment and resilience in later life. J Pers. 1989;57(2):311-341.
25. US Department of Veterans Affairs. Public health: cold injuries. https://www.publichealth.va.gov/PUBLICHEALTH/exposures/cold-injuries/index.asp. Updated July 31, 2019. Accessed November 8, 2019.
26. US Department of Veterans Affairs. Korean War veterans health issues. https://www.va.gov/health-care/health-needs-conditions/health-issues-related-to-service-era
/korean-war/. Updated June 14, 2019. Accessed November 8, 2019.
27. Shapiro F. Efficacy of the eye movement desensitization procedure in the treatment of traumatic memories. J Trauma Stress. 1989;2(2):199-223.
28. Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. J Consul Clin Psychol. 1992;60(5):748-756.
29. Foa EB, Rothbaum BO. Treating Trauma of Rape: Cognitive-Behavioral Therapy for PTSD. New York: Guilford; 2001.
30. Skinner R, Kaplick PM. Cultural shift in mental illness: a comparison of stress responses in World War I and the Vietnam War. JRSM Open. 2017;8(12):2054270417746061.
31. Kardiner A, Spiegel H. War Stress and Neurotic Illness. New York: Hoeber; 1947.
32. Archibald HC, Tuddenham RD. Persistent stress reaction after combat: a 20-year follow-up. Arch Gen Psychiatry. 1965;12:475-481.
33. Cook JM, Simiola V. Trauma and aging. Curr Psychiatry Rep. 2018;20(10):93.
34. Rosenheck R, Fontana A. Long-term sequelae of combat in World War II, Korea and Vietnam: a comparative study. In: McCaughey BG, Fullerton CS, Ursano RJ, eds. Individual
and Community Responses to Trauma and Disaster: The Structure of Human Chaos. New York: Cambridge University Press; 1994:330-359.
35. Blake DD, Keane TM, Wine PR, Mora C, Taylor KL, Lyons JA. Prevalence of PTSD symptoms in combat veterans seeking medical treatment. J Trauma Stress. 1990;3(1):15-27.
36. McCranie EW, Hyer LA. Posttraumatic stress disorder symptoms in Korean conflict and World War II combat veterans seeking outpatient treatment. J Trauma Stress. 2000;13(3):427-439.
37. Fontana A, Rosenheck R. Traumatic war stressors and psychiatric symptoms among World War II, Korean, and Vietnam War veterans. Psychology Aging. 1994;9(1):27-33.
38. Beebe GW. Follow-up studies of World War II and Korean war prisoners. II. Morbidity, disability, and maladjustments. Am J Epidemiol. 1975;101(5):400-422.
39. Gold PB, Engdahl BE, Eberly RE, Blake RJ, Page WF, Frueh BC. Trauma exposure, resilience, social support, and PTSD construct validity among former prisoners of war. Social Psychiatry Psychiatr Epidemiol. 2000;35(1):36-42.
40. US Department of Veterans Affairs. Key statistics by veteran status and period of service. https://www.va.gov/vetdata/docs/SpecialReports/KeyStats.pdf. Accessed November 11, 2019.
41. Bowers WT, Hammond WM, MacGarrigle GL. Black Soldier, White Army. Washington DC: US Army Center of Military History; 1996.
42. Black HK. Three generations, three wars: African American veterans. Gerontologist. 2016;56(1):33-41.
43. Thorp SR, Sones HM, Cook JM. Posttraumatic stress disorder among older adults. In: Sorocco KH, Lauderdale S, eds. Cognitive Behavior Therapy With Older Adults: Innovations Across Care Settings. New York: Springer; 2011:189-217.
44. Pless Kaiser A, Cook JM, Glick DM, Moye J. Posttraumatic stress disorder in older adults: a conceptual review. Clinical Gerontol. 2019;42(4):359-376.
45. Sadavoy J. Survivors. A review of the late-life effects of prior psychological trauma. Am J Geriatr Psychiatry. 1997;5(4):287-301.
46. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636.
47. Mota N, Tsai J, Kirwin PD, et al. Late-life exacerbation of PTSD symptoms in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2016;77(3):348-354.
48. Davison EH, Kaiser AP, Spiro A 3rd, Moye J, King LA, King DW. From Late-onset stress symptomatology to later-adulthood trauma reengagement in aging combat veterans: taking a broader view. Gerontologist. 2016;56(1):14-21.
49. Hyer L, Summers MN, Boyd S, Litaker M, Boudewyns P. Assessment of older combat veterans with the clinician-administered PTSD scale. J Trauma Stress. 1996;9(3):587-593.
50. Bhattarai JJ, Oehlert ME, Weber DK. Psychometric properties of the Mississippi Scale for combat-related posttraumatic stress disorder based on veterans’ period of service. Psychol Serv. 2018. [Epub ahead of print]
51. US Department of Veterans Affairs, US Department of Defense. VA/DOD Clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf.
Updated 2017. Accessed November 11, 2019.
52. Dinnen S, Simiola V, Cook JM. Post-traumatic stress disorder in older adults: a systematic review of the psychotherapy treatment literature. Aging Ment Health. 2015;19(2):144-150.
53. Jakel RJ. Posttraumatic Stress Disorder in the Elderly. Psychiatr Clin North Am. 2018;41(1):165-175.
54. Thorp SR, Glassman LH, Wells SY, et al. A randomized controlled trial of prolonged exposure therapy versus relaxation training for older veterans with military-related PTSD. J Anxiety Disord. 2019;64:45-54.
55. Kang B, Xu H, McConnell ES. Neurocognitive and psychiatric comorbidities of posttraumatic stress disorder among older veterans: a systematic review. Int J Geriatr Psychiatry. 2019;34(4):522-538.
56. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
57. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
58. Paton D. Traumatic Stress in Police Officers a Career-Length Assessment From Recruitment to Retirement. Springfield, IL: Charles C. Thomas; 2009.
59. Alexander W. Pharmacotherapy for post-traumatic stress disorder in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37(1):32-38.
60. Beck JG, Sloan DM, Friedman MJ. Pharmacotherapy for PTSD. In: The Oxford Handbook of Traumatic Stress Disorders. Oxford University Press; 2012.
61. Waltman SH, Shearer D, Moore BA. Management of posttraumatic nightmares: a review of pharmacologic and nonpharmacologic treatments since 2013. Curr Psychiatry Rep. 2018;20(12):108.
62. Díaz-Gutiérrez MJ, Martínez-Cengotitabengoa M, Sáez de Adana E, et al. Relationship between the use of benzodiazepines and falls in older adults: a systematic review. Maturitas. 2017;101:17-22.
The Korean War lasted from June 25, 1950 through July 27, 1953. Although many veterans of the Korean War experienced traumas during extremely stressful combat conditions. However, they would not have been diagnosed with posttraumatic stress disorder (PTSD) at the time because the latter did not exist as a formal diagnosis until the publication of the third edition of the Diagnostic and Statistical Manual (DSM) in 1980.1 Prior to 1980, psychiatric syndromes resulting from war and combat exposure where known by numerous other terms including shell shock, chronic traumatic war neurosis, and combat fatigue/combat exhaustion.2,3 Military psychiatrists attended to combat fatigue during the course of the Korean War, but as was true of World War I and II, the focus was on returning soldiers to duty. Combat fatigue was generally viewed as a transient condition.4-8
Although now octo- and nonagenarians, in 2019 there are 1.2 million living Korean War veterans in the US, representing 6.7% of all current veterans.9 Understanding their war experiences and the nature of their current and past presentation of PTSD is relevant not only in formal mental health settings, but in primary care settings, including home-based primary care, as well as community living centers, skilled nursing facilities and assisted living facilities. Older adults with PTSD often present with somatic concerns rather than spontaneously reporting mental health symptoms.10 Beyond the short-term clinical management of Korean War veterans with PTSD, consideration of their experiences also has long-term relevance for the appropriate treatment of other veteran cohorts as they age in coming decades.
The purpose of this article is to provide a clinically focused overview of PTSD in Korean War veterans, to help promote understanding of this often-forgotten group of veterans, and to foster optimized personalized care. This overview will include a description of the Korean War veteran population and the Korean War itself, the manifestations and identification of PTSD among Korean War veterans, and treatment approaches using evidence-based psychotherapies and pharmacotherapies. Finally, we provide recommendations for future research to address present empirical gaps in the understanding and treatment of Korean War veterans with PTSD.
Causes and Course of the Korean War
When working with Korean War veterans it is important to consider the special nature of that specific conflict. Space considerations limit our ability to do justice to the complex history and numerous battles of the Korean War, but information in the following summary was gleaned from several excellent histories.11-13
The Korean War has been referred to as The Forgotten War, a concern expressed even during the latter parts of the war.14,15 But the war and its veterans warrant remembering. The root and proximal causes of the Korean War are complex and not fully agreed upon by the main participants.16-19 In part this may reflect the fact that there was no clear victor in the Korean War, so that the different protagonists have developed their own versions of the history of the conflict. Also, US involvement and the public reaction to the war must be viewed within the larger historical context of that time. This context included the recent end of 4 years of US involvement in World War II (1941-1945) and the subsequent rapid rise of Cold War tensions between the US and the Soviet Union. The latter also included a worldwide fear of nuclear war and the US fear of the global spread of communism. These fears were fueled by the Soviet-led Berlin Blockade from June 1948 through May 1949, the Soviet Union’s successful atomic bomb test in August 1949, the founding of the People’s Republic of China in October 1949, and the February 1950 Sino-Soviet Treaty of Friendship and Alliance.13
In the closing days of World War II, the US and Soviet Union agreed to a temporary division of Korea along the 38th parallel to facilitate timely and efficient surrender of Japanese troops. But as Cold War tensions rose, the temporary division became permanent, and Soviet- and US-backed governments of the north and south, respectively, were officially established on the Korean peninsula in 1948. Although by 1949 the Soviets and US had withdrawn most troops from the peninsula, tensions between the north and south continued to mount and hostilities increased. To this day the exact causes of the eruption of war remain disputed, although it is clear that ideological as well as economic factors played a role, and both leaders of North and South Korea were pledging to reunite the peninsula under their respective leadership.16-19 The tension culminated on June 25, 1950, when North Korean troops crossed the 38th parallel and invaded South Korea. On June 27, 1950, President Truman ordered US naval and air forces to support South Korea and then ordered the involvement of ground troops on June 30.16,17,19
Although several other member countries of the United Nations (UN) provided troops, 90% of the troops were from the US. About 5.7 million US military personnel served during the war, including about 1.8 million in Korea itself. The US forces experienced approximately 34,000 battle-related deaths, 103,000 were wounded, and 7,000 were prisoners of war (POWs).11,20-22 The nature and events of the Korean War made it particularly stressful and traumatizing for the soldiers, sailors, and marines involved throughout its entire course. These included near defeat in the early months, a widely alternating war front along the north/south axis during the first year, and subsequently, not only intense constant battles on the fronts, but also a demanding and exhausting guerrilla war in the south, which lasted throughout the remainder of the conflict.11,15 The US troops during the initial months of the war have been described as outnumbered and underprepared, as many in the initial phase were reassigned from peace-time occupation duty in Japan.7
The first year of war was characterized by a repeated north-to-south/south-to-north shifts in control of territory. During the first 3 months, the North Korean forces overwhelmed the South and captured control of all but 2 South Korean cities in the far southeastern region (Pusan, now Busan; and Daegu), and US and UN forces were forced to retreat to the perimeter around Pusan. The intense Battle of Pusan Perimeter lasted from August 4, 1950 to September 18, 1950, and resulted in massive causalities as well as a flood of civilian refugees.
The course of the war began to change in early September 1950 with the landing of amphibious US/UN forces at Inchon, behind North Korean lines, which cut off southern supply routes for the North Korean troops.11 US/UN forces soon crossed to the north of the 38th parallel and captured the North Korean capital, Pyongyang, on October 19, 1950. They continued to push north and approached the Yalu River border with China by late November 1950, but then the Chinese introduced their own troops forcing a southward retreat of US/UN troops during which there were again numerous US/UN casualties. Chinese troops retook Seoul in late December 1950/early January 1951. However, the US/UN forces soon recaptured Seoul and advanced back to the 38th parallel. This back-and-forth across the 38th parallel continued until July 1951 when the front line of battle stabilized there. Although the line stabilized, intense battles and casualties continued for 2 more years. During this period US/UN troops also had to deal with guerrilla warfare behind the front lines due to the actions of communist partisans and isolated North Korean troops. This situation continued until the armistice was signed July 27, 1953.
Trauma and Characteristic Stresses of the War
There were many factors that made the Korean War experience different from previous wars, particularly World War II. For example, in contrast to the strong public support during and after World War II, public support for the Korean War in the US was low, particularly during its final year.23 In public opinion polls from October 1952 through April 1953, only 23% to 39% reported feeling that the war was worth fighting.23 A retrospective 1985 survey also found that 70% of World War II veterans, but only 33% of Korean War veterans reported feeling appreciated by the US public on their return from the war.24
Those fighting in the initial months of the war faced a particularly grim situation. According to LTC Philip Smith, who served as Division Psychiatrist on the Masan Front (Pusan Perimeter) during August and September of 1950, “Fighting was almost continuous and all available troops were on the fighting front… For the most part these soldiers were soft from occupation duty, many had not received adequate combat basic training, no refresher combat training in Korea had as yet been instituted,” he reported.7 “The extremes of climate coupled with the generally rugged mountainous terrain in Korea were physical factors of importance…These men were psychologically unprepared for the horrors and isolation of war.” LTC Smith noted that the change in status from civilian or occupation life to the marked deprivation of the war in Korea had been “too abrupt to allow as yet for a reasonable adjustment to the new setting” and that as a result “the highest rate of wounded and neuropsychiatric casualties in the Korean campaign resulted.”7
Even after this initial period, the nature of the shifting war, the challenging terrain, the high military casualty rate, and the high rate of civilian casualties and displacement continued throughout the war.
PTSD in Korean War Veterans
It is clear that Korean War combat veterans were exposed to traumatic events. It is unknown how many developed PTSD. While notions of psychological distress and disability related to combat trauma exposure have existed for centuries, Korean War and World War II veterans are a remaining link to pre-DSM PTSD mental health in the military. Military/forward psychiatry—psychiatric services near the battle zone rather than requiring evacuation of patients—was present in Korea from the early months of the war, but the focus of forward psychiatry was to reduce psychiatric causalities from combat fatigue and maximize rapid return-to-duty.4-6 With no real conception of PTSD, there were limited treatments available, and evidenced-based trauma-focused treatments for PTSD would not be introduced for at least another 4 decades.27-29
Skinner and Kaplick conducted a historical review of case descriptions of trauma-related conditions from World War I through the Vietnam War and noted the consistent inclusion of hyperarousal and intrusive symptoms, although there also was a greater emphasis on somatic conversion or hysteria symptoms in the earlier descriptions.30 By the Korean War, descriptions of combat fatigue included a number of symptoms that overlap with PTSD, including preoccupation with the traumatic stressor, nightmares, irritability/anger, increased startle, and hyperarousal.31 But following the acute phases, attention to any chronic problems associated with these conditions waned. As was acknowledged by a military psychiatrist in a 1954 talk, studies of the long-term adjustment of those who had “broken down in combat” were sorely needed.6 In a small 1965 study reported by Archibald and Tuddenham, persistent symptoms of combat fatigue among Korean War veterans were definitely present, and there was even a suggestion that the symptoms had increased over the decade since the war.32
Given the stoicism that typified cultural expectations for military men during this period, Korean War veterans may also have been reluctant to seek mental health treatment either at the time or later. In short, it is likely that a nontrivial proportion of Korean War veterans with PTSD were underdiagnosed and received suboptimal or no mental health treatment for decades following their war experiences.33 Although the nature of the war, deployment, and public support were distinct in World War II vs the Korean War, the absence of attention to the long-term effects of disorders related to combat trauma and the cultural expectations for stoicism suggest that PTSD among aging World War II veterans may also have gone underrecognized and undertreated.
Apart from the lack of interest in chronic effects of stressors, another problem that has plagued the limited empirical research on Korean War veterans has been the propensity to combine Korean War with World War II veteran samples in studies. Because World War II veterans have outnumbered Korean War veterans until recently, combined samples tended to have relatively few Korean War veterans. Nevertheless, from those studies that have been reported in which 2 groups were compared, important differences have been revealed. Specifically, although precise estimates of the prevalence of PTSD among Korean War combat veterans have varied depending on sampling and method, studies from the 1990s and early 2000s suggested that the prevalence of PTSD and other mental health concerns as well as the severity of symptoms, suicide risk, and psychosocial adjustment difficulties were worse among Korean War combat veterans relative to those among World War II combat veterans; however, both groups had lower prevalence than did Vietnam War combat veterans.21,34-37 Several authors speculated that these differences in outcome were at least partially due to differences in public support for the respective wars.36,37
Although there has been a paucity of research on psychiatric issues and PTSD in Korean War veterans, POWs who were very likely to have been exposed to extreme psychological traumas have received some attention. There have been comparisons of mortality and morbidity among POWs from the Korean War (PWK), World War II Pacific Theater (PWJ), and Europe (PWE).38 Among measures that were administered to the former POWs, the overall pattern seen from survey data in the mid-1960s revealed significantly worse health and functioning among the PWK and PWJ groups relative to the PWE group, with psychiatric difficulties being the most commonly reported impairments among the former 2 groups. This pattern was found most strongly with regards to objective measures, such as hospitalizations for “psychoneuroses,” and US Department of Veterans Affairs (VA) disability records, as well as based on self-reported psychosocial/recreational difficulties measured using the Cornell Medical Index (CMI).38
Gold and colleagues reported a follow-up study of more than 700 former POWs who were reinterviewed between 1989 and 1992.39 Although there was no scale of PTSD symptoms prior to formulation of the diagnosis in 1980, the CMI was a self-reported checklist that included a large range of both medical as well as behavioral and psychiatric symptoms. Thus, using CMI survey responses from 1965, the authors examined the factor structure (ie, the correlational relationships between multiple scale items and subgroupings of items) of the CMI relative to diagnosis of PTSD in 1989 to 1992 based on results from the Structured Clinical Interview for the DSM-III-R (SCID). The intent was to help discern whether the component domains of PTSD were present and intercorrelated in a pattern similar to that of the contemporary diagnosis. The investigators examined the factor structure of 20 psychological items from the CMI that appeared relevant to PTSD criteria using the 1965 data. Three factors (subgroups of highly intercorrelated items) were found: irritability (31% of variance), fearfulness/anxiousness (9% of the variance), and social withdrawal (7% of the variance). Although these did not directly correspond to, or fully cover, DSM PTSD domains or criteria, there does appear to be a thematic resemblance of the CMI findings with PTSD, including alterations in arousal and mood, vigilance, and startle.
Identification and Treatment of PTSD in Older Veterans
Of the 1.2 million living Korean War veterans in the US, 36.3% use VA provided health care.40 There are a number of complicating factors to consider in the current identification and treatment of PTSD in this cohort, including their advanced age; physical, cognitive, and social changes associated with normal aging; the associated medical and cognitive comorbidities; and the specific social-contextual factors in that age cohort. Any combination of these factors may complicate recognition, diagnosis, and treatment. It is also important to be cognizant of the additional stressors that may have been experienced by ethnic minorites and women serving in Korea, which are poorly documented and studied. Racial integration of the US military began during the Korean War, but the general pattern was for African American soldiers to be assigned to all-white units, rather than the reverse.14,41,42 And although the majority of military personnel serving in Korea were male, there were women serving in health care positions at mobile army surgical hospital (MASH) units, medical air evacuation (Medevac) aircraft, and off-shore hospital ships.
The clinical presentation of PTSD in older adults has varied, which may partially relate to the time elapsed since the index trauma. For example, older veterans in general may show less avoidance behavior as a part of PTSD, but in those who experience trauma later in life there may actually be greater avoidance.43,44 There have also been discrepant reports of intrusion or reexperiencing of symptoms, with these also potentially reduced in older veterans.43,44 However, sleep disturbances seem to be very common among elderly combat veterans, and attention should be paid to the possible presence of sleep apnea, which may be more common in veterans with PTSD in general.43,45,46
PTSD symptoms may reemerge after decades of remission or quiescence during retirement and/or with the emergence of neurocognitive impairment, such as Alzheimer disease or dementia. These individuals may have more difficulty engaging in distracting activities and work and spend more time engaging in reminiscence about the past, which can include increased focus on traumatic memories.45,47 Davison and colleagues have suggested a concept they call later-adulthood trauma reengagement (LATR) where later in life combat veterans may “confront and rework their wartime memories in an effort to find meaning and build coherence.”48 This process can be a double-edged sword, leading at times positively to enhanced personal growth or negatively to increased symptoms; preventive interventions may be able foster a more positive outcome.48
There is some evidence supporting the validity of the Clinician Administered PTSD Scale (CAPS) for the evaluation of PTSD in older adults, although this was based on the DSM-III-revised criteria for PTSD and an earlier version of CAPS.49 Bhattarai and colleagues examined responses to the 35-item Mississippi Scale for Combat-Related PTSD (M-PTSD) using VA clinical data collected between 2008 and 2015 on veterans of each combat era from World War II through the post-9/11.50 Strong internal consistency and test-retest reliability of the M-PTSD was observed within each veteran era sample. However, using chart diagnosis of PTSD as the criterion standard, the cut-scores for optimal balance of sensitivity and specificity of the M-PTSD scores were substantially lower for the older cohorts (World War II and Korean War veterans) relative to those for Vietnam and more recent veteran cohorts. The authors concluded that M-PTSD can be validly used to screen for PTSD in veterans within each of these cohorts but recommended using lower than standard cut-scores for Korean War and World War II veterans.50
This is also consistent with reports that suggest the use of lower cut-scores on self-administered PTSD symptom screens.43,44 For the clinician interested in quantifying the severity of PTSD, the most recent tools available are the CAPS-5 and the PCL-5, which have both been created in accordance with the DSM-5. The CAPS-5 is a rater-administered tool, and the PCL-5 is self-administered by the veteran. Although there has been little research using these newer tools in geriatric populations, they can currently serve as a means of tracking the severity of PTSD while we await measures that are better validated in Korean War and other older veterans.
Beyond specific empirical guidance, VA clinicians must presently rely on clinical observations and experience. Patients from the Korean War cohort often present at the insistence of a family member for changes in sleep, mood, behavior, or cognition. When the veterans themselves present, older adults with PTSD often focus more on somatic concerns (including pain, sleep, and gastrointestinal disturbance) than psychiatric problems per se. The latter tendency may in part be due to the salience of such symptoms for them, but perhaps also due to considerable stigma of mental health care that is still largely present in this group.43,44
Psychotherapy
Current VA treatment guidelines recommend trauma-focused therapies, with the strongest evidence base for prolonged exposure (PE), cognitive processing therapy (CPT), and eye movement desensitization and reprocessing (EMDR) therapies.51
There have been several excellent prior reviews discussing treatment of PTSD in older adults generally.10,43,44,52 These reviews have invariably expressed concern about the lack of sufficient empirical studies, but based on evidence from studies and case reports, there seems to be tentative support that trauma-focused therapies are acceptable and efficacious for use with older adults with PTSD. In their recent scoping review, Pless Kaiser and colleagues made several recommendations for trauma-focused therapy with older adults, including slow/careful pacing and use of compensatory aids for cognitive and sensory deficits.44 When cognitive impairment has exacerbated PTSD symptoms, they suggest therapists consider using an adapted form of CPT completed without a trauma narrative. For PE they recommend extending content across sessions and involving spouse or caregivers to assist with in vivo exposure and homework completion.44
Recent studies suggest that PTSD may be a risk factor for the later development of neurodegenerative disorders, and it is often during assessments for dementia that a revelation of PTSD occurs.10,43,47,55 Cognitive impairment may also be of relevance in deciding on the type of psychotherapy to be implemented, as it may have more adverse effects on the effectiveness of CPT than of exposure-based treatments (PE or EMDR). It may be useful to perform a cognitive assessment prior to initiation of a cognitive-based therapy, although extensive cognitive testing may not be practical or may be contraindicated because of fatigue. A brief screening tool such as the Montreal Cognitive Assessment or the Mini-Mental State Examinationmay be helpful.56, 57
Prolonged exposure has been reported by many clinicians to be effective in older adults with PTSD; however, due consideration should be given to the needs of individuals, as many have functioned for decades by suppressing memories.
Apart from the treatment needs for specific PTSD symptoms, the decades-long effects of poor sleep, irritability, hypervigilance, and dissociation also have social consequences for patients, including marital discord and divorce, and social and family isolation that should be addressed in therapy when appropriate. In addition, many Korean War veterans, like all veterans, sought postmilitary employment in professions that are associated with higher rates of exposure to psychological trauma, such as police or fire departments, and this may have an exacerbating effect on PTSD.58
Pharmacotherapy
There is very little empirical evidence guiding pharmacologic approaches to PTSD in older veterans. This population is at increased risk for many comorbidities, and pharmacologic treatments many require dosage adjustments, as is the case for any geriatric patient. Selective serotonin reuptake inhibitor (SSRI) and serotonin norepinephrine reuptake inhibitor (SNRI) medications have been proposed for some cases of PTSD.59,60 Health care providers may consider the SSRIs escitalopram or sertraline preferentially given their decreased potential for drug-drug interactions, anticholinergic effects, or cardiac toxicity compared with that of other drugs in this class.60,61 As venlafaxine can increase blood pressure, especially at higher doses, prescribers may choose duloxetine as an alternative if a SNRI is indicated.60 For veterans when prazosin is being considered for nightmare control, monitoring for hypotension, orthostasis, and the administration of other antihypertensives or prostatic hypertrophy medications is necessary.61 The use of benzodiazepines, while not recommended for PTSD, should be viewed with even greater trepidation in a geriatric population given enhanced risk of falls and confusion in the geriatric veteran population.60,62
Conclusions
Many of the oldest veterans (aged > 80 years) are from the Korean War era. The harsh and unique nature of the war, as well as the differences in context and support from the US public, and the outcome of the war, may have all contributed to and elevation of “combat fatigue” and PTSD among combat veterans from the Korean War. As the “forgotten war” cohort also has been forgotten by researchers, relatively little is known about posttraumatic stress sequelae of these veterans in the decades following the war.
From available evidence, we can readily surmise that problems were underrecognized and suboptimally diagnosed and treated. There is tentative evidence supporting the use of standard interviews and rating scales, such as the CAPS, M-PTSD, and PCL, but lower cut-scores than applied with Vietnam and later veteran cohorts are generally recommended to avoid excessive false negative errors. In terms of psychotherapy treatment, there is again a stark paucity of systematic research, but the limited evidence from studies of PTSD treatment in older adults from the general population tentatively support the acceptability and potential efficacy of recognized evidence-based trauma-focused psychotherapies for PTSD. Research on medication treatment is similarly lacking, but the general recommendations for the use of SSRI or SNRI medications seem to be valid, at least in our clinical experience, and the general rules for geriatric psychopharmacology definitely apply here—start low, go slow.
There are several important avenues for future research. Most pressing among these are establishing the effectiveness of existing treatments, and the modifications that may be needed in the broader context of the above factors, as well as the physical and cognitive changes associated with advanced age. Further research on the phenomenologic aspects of PTSD among Korean War and subsequent cohorts are also needed, as the information obtained will not only guide more effective personalized treatment of the Korean War veterans who remain with us, but also inform future generations of care in terms of the degree and dimensions of variability that may present between cohorts and within cohorts over the life span.
The Korean War lasted from June 25, 1950 through July 27, 1953. Although many veterans of the Korean War experienced traumas during extremely stressful combat conditions. However, they would not have been diagnosed with posttraumatic stress disorder (PTSD) at the time because the latter did not exist as a formal diagnosis until the publication of the third edition of the Diagnostic and Statistical Manual (DSM) in 1980.1 Prior to 1980, psychiatric syndromes resulting from war and combat exposure where known by numerous other terms including shell shock, chronic traumatic war neurosis, and combat fatigue/combat exhaustion.2,3 Military psychiatrists attended to combat fatigue during the course of the Korean War, but as was true of World War I and II, the focus was on returning soldiers to duty. Combat fatigue was generally viewed as a transient condition.4-8
Although now octo- and nonagenarians, in 2019 there are 1.2 million living Korean War veterans in the US, representing 6.7% of all current veterans.9 Understanding their war experiences and the nature of their current and past presentation of PTSD is relevant not only in formal mental health settings, but in primary care settings, including home-based primary care, as well as community living centers, skilled nursing facilities and assisted living facilities. Older adults with PTSD often present with somatic concerns rather than spontaneously reporting mental health symptoms.10 Beyond the short-term clinical management of Korean War veterans with PTSD, consideration of their experiences also has long-term relevance for the appropriate treatment of other veteran cohorts as they age in coming decades.
The purpose of this article is to provide a clinically focused overview of PTSD in Korean War veterans, to help promote understanding of this often-forgotten group of veterans, and to foster optimized personalized care. This overview will include a description of the Korean War veteran population and the Korean War itself, the manifestations and identification of PTSD among Korean War veterans, and treatment approaches using evidence-based psychotherapies and pharmacotherapies. Finally, we provide recommendations for future research to address present empirical gaps in the understanding and treatment of Korean War veterans with PTSD.
Causes and Course of the Korean War
When working with Korean War veterans it is important to consider the special nature of that specific conflict. Space considerations limit our ability to do justice to the complex history and numerous battles of the Korean War, but information in the following summary was gleaned from several excellent histories.11-13
The Korean War has been referred to as The Forgotten War, a concern expressed even during the latter parts of the war.14,15 But the war and its veterans warrant remembering. The root and proximal causes of the Korean War are complex and not fully agreed upon by the main participants.16-19 In part this may reflect the fact that there was no clear victor in the Korean War, so that the different protagonists have developed their own versions of the history of the conflict. Also, US involvement and the public reaction to the war must be viewed within the larger historical context of that time. This context included the recent end of 4 years of US involvement in World War II (1941-1945) and the subsequent rapid rise of Cold War tensions between the US and the Soviet Union. The latter also included a worldwide fear of nuclear war and the US fear of the global spread of communism. These fears were fueled by the Soviet-led Berlin Blockade from June 1948 through May 1949, the Soviet Union’s successful atomic bomb test in August 1949, the founding of the People’s Republic of China in October 1949, and the February 1950 Sino-Soviet Treaty of Friendship and Alliance.13
In the closing days of World War II, the US and Soviet Union agreed to a temporary division of Korea along the 38th parallel to facilitate timely and efficient surrender of Japanese troops. But as Cold War tensions rose, the temporary division became permanent, and Soviet- and US-backed governments of the north and south, respectively, were officially established on the Korean peninsula in 1948. Although by 1949 the Soviets and US had withdrawn most troops from the peninsula, tensions between the north and south continued to mount and hostilities increased. To this day the exact causes of the eruption of war remain disputed, although it is clear that ideological as well as economic factors played a role, and both leaders of North and South Korea were pledging to reunite the peninsula under their respective leadership.16-19 The tension culminated on June 25, 1950, when North Korean troops crossed the 38th parallel and invaded South Korea. On June 27, 1950, President Truman ordered US naval and air forces to support South Korea and then ordered the involvement of ground troops on June 30.16,17,19
Although several other member countries of the United Nations (UN) provided troops, 90% of the troops were from the US. About 5.7 million US military personnel served during the war, including about 1.8 million in Korea itself. The US forces experienced approximately 34,000 battle-related deaths, 103,000 were wounded, and 7,000 were prisoners of war (POWs).11,20-22 The nature and events of the Korean War made it particularly stressful and traumatizing for the soldiers, sailors, and marines involved throughout its entire course. These included near defeat in the early months, a widely alternating war front along the north/south axis during the first year, and subsequently, not only intense constant battles on the fronts, but also a demanding and exhausting guerrilla war in the south, which lasted throughout the remainder of the conflict.11,15 The US troops during the initial months of the war have been described as outnumbered and underprepared, as many in the initial phase were reassigned from peace-time occupation duty in Japan.7
The first year of war was characterized by a repeated north-to-south/south-to-north shifts in control of territory. During the first 3 months, the North Korean forces overwhelmed the South and captured control of all but 2 South Korean cities in the far southeastern region (Pusan, now Busan; and Daegu), and US and UN forces were forced to retreat to the perimeter around Pusan. The intense Battle of Pusan Perimeter lasted from August 4, 1950 to September 18, 1950, and resulted in massive causalities as well as a flood of civilian refugees.
The course of the war began to change in early September 1950 with the landing of amphibious US/UN forces at Inchon, behind North Korean lines, which cut off southern supply routes for the North Korean troops.11 US/UN forces soon crossed to the north of the 38th parallel and captured the North Korean capital, Pyongyang, on October 19, 1950. They continued to push north and approached the Yalu River border with China by late November 1950, but then the Chinese introduced their own troops forcing a southward retreat of US/UN troops during which there were again numerous US/UN casualties. Chinese troops retook Seoul in late December 1950/early January 1951. However, the US/UN forces soon recaptured Seoul and advanced back to the 38th parallel. This back-and-forth across the 38th parallel continued until July 1951 when the front line of battle stabilized there. Although the line stabilized, intense battles and casualties continued for 2 more years. During this period US/UN troops also had to deal with guerrilla warfare behind the front lines due to the actions of communist partisans and isolated North Korean troops. This situation continued until the armistice was signed July 27, 1953.
Trauma and Characteristic Stresses of the War
There were many factors that made the Korean War experience different from previous wars, particularly World War II. For example, in contrast to the strong public support during and after World War II, public support for the Korean War in the US was low, particularly during its final year.23 In public opinion polls from October 1952 through April 1953, only 23% to 39% reported feeling that the war was worth fighting.23 A retrospective 1985 survey also found that 70% of World War II veterans, but only 33% of Korean War veterans reported feeling appreciated by the US public on their return from the war.24
Those fighting in the initial months of the war faced a particularly grim situation. According to LTC Philip Smith, who served as Division Psychiatrist on the Masan Front (Pusan Perimeter) during August and September of 1950, “Fighting was almost continuous and all available troops were on the fighting front… For the most part these soldiers were soft from occupation duty, many had not received adequate combat basic training, no refresher combat training in Korea had as yet been instituted,” he reported.7 “The extremes of climate coupled with the generally rugged mountainous terrain in Korea were physical factors of importance…These men were psychologically unprepared for the horrors and isolation of war.” LTC Smith noted that the change in status from civilian or occupation life to the marked deprivation of the war in Korea had been “too abrupt to allow as yet for a reasonable adjustment to the new setting” and that as a result “the highest rate of wounded and neuropsychiatric casualties in the Korean campaign resulted.”7
Even after this initial period, the nature of the shifting war, the challenging terrain, the high military casualty rate, and the high rate of civilian casualties and displacement continued throughout the war.
PTSD in Korean War Veterans
It is clear that Korean War combat veterans were exposed to traumatic events. It is unknown how many developed PTSD. While notions of psychological distress and disability related to combat trauma exposure have existed for centuries, Korean War and World War II veterans are a remaining link to pre-DSM PTSD mental health in the military. Military/forward psychiatry—psychiatric services near the battle zone rather than requiring evacuation of patients—was present in Korea from the early months of the war, but the focus of forward psychiatry was to reduce psychiatric causalities from combat fatigue and maximize rapid return-to-duty.4-6 With no real conception of PTSD, there were limited treatments available, and evidenced-based trauma-focused treatments for PTSD would not be introduced for at least another 4 decades.27-29
Skinner and Kaplick conducted a historical review of case descriptions of trauma-related conditions from World War I through the Vietnam War and noted the consistent inclusion of hyperarousal and intrusive symptoms, although there also was a greater emphasis on somatic conversion or hysteria symptoms in the earlier descriptions.30 By the Korean War, descriptions of combat fatigue included a number of symptoms that overlap with PTSD, including preoccupation with the traumatic stressor, nightmares, irritability/anger, increased startle, and hyperarousal.31 But following the acute phases, attention to any chronic problems associated with these conditions waned. As was acknowledged by a military psychiatrist in a 1954 talk, studies of the long-term adjustment of those who had “broken down in combat” were sorely needed.6 In a small 1965 study reported by Archibald and Tuddenham, persistent symptoms of combat fatigue among Korean War veterans were definitely present, and there was even a suggestion that the symptoms had increased over the decade since the war.32
Given the stoicism that typified cultural expectations for military men during this period, Korean War veterans may also have been reluctant to seek mental health treatment either at the time or later. In short, it is likely that a nontrivial proportion of Korean War veterans with PTSD were underdiagnosed and received suboptimal or no mental health treatment for decades following their war experiences.33 Although the nature of the war, deployment, and public support were distinct in World War II vs the Korean War, the absence of attention to the long-term effects of disorders related to combat trauma and the cultural expectations for stoicism suggest that PTSD among aging World War II veterans may also have gone underrecognized and undertreated.
Apart from the lack of interest in chronic effects of stressors, another problem that has plagued the limited empirical research on Korean War veterans has been the propensity to combine Korean War with World War II veteran samples in studies. Because World War II veterans have outnumbered Korean War veterans until recently, combined samples tended to have relatively few Korean War veterans. Nevertheless, from those studies that have been reported in which 2 groups were compared, important differences have been revealed. Specifically, although precise estimates of the prevalence of PTSD among Korean War combat veterans have varied depending on sampling and method, studies from the 1990s and early 2000s suggested that the prevalence of PTSD and other mental health concerns as well as the severity of symptoms, suicide risk, and psychosocial adjustment difficulties were worse among Korean War combat veterans relative to those among World War II combat veterans; however, both groups had lower prevalence than did Vietnam War combat veterans.21,34-37 Several authors speculated that these differences in outcome were at least partially due to differences in public support for the respective wars.36,37
Although there has been a paucity of research on psychiatric issues and PTSD in Korean War veterans, POWs who were very likely to have been exposed to extreme psychological traumas have received some attention. There have been comparisons of mortality and morbidity among POWs from the Korean War (PWK), World War II Pacific Theater (PWJ), and Europe (PWE).38 Among measures that were administered to the former POWs, the overall pattern seen from survey data in the mid-1960s revealed significantly worse health and functioning among the PWK and PWJ groups relative to the PWE group, with psychiatric difficulties being the most commonly reported impairments among the former 2 groups. This pattern was found most strongly with regards to objective measures, such as hospitalizations for “psychoneuroses,” and US Department of Veterans Affairs (VA) disability records, as well as based on self-reported psychosocial/recreational difficulties measured using the Cornell Medical Index (CMI).38
Gold and colleagues reported a follow-up study of more than 700 former POWs who were reinterviewed between 1989 and 1992.39 Although there was no scale of PTSD symptoms prior to formulation of the diagnosis in 1980, the CMI was a self-reported checklist that included a large range of both medical as well as behavioral and psychiatric symptoms. Thus, using CMI survey responses from 1965, the authors examined the factor structure (ie, the correlational relationships between multiple scale items and subgroupings of items) of the CMI relative to diagnosis of PTSD in 1989 to 1992 based on results from the Structured Clinical Interview for the DSM-III-R (SCID). The intent was to help discern whether the component domains of PTSD were present and intercorrelated in a pattern similar to that of the contemporary diagnosis. The investigators examined the factor structure of 20 psychological items from the CMI that appeared relevant to PTSD criteria using the 1965 data. Three factors (subgroups of highly intercorrelated items) were found: irritability (31% of variance), fearfulness/anxiousness (9% of the variance), and social withdrawal (7% of the variance). Although these did not directly correspond to, or fully cover, DSM PTSD domains or criteria, there does appear to be a thematic resemblance of the CMI findings with PTSD, including alterations in arousal and mood, vigilance, and startle.
Identification and Treatment of PTSD in Older Veterans
Of the 1.2 million living Korean War veterans in the US, 36.3% use VA provided health care.40 There are a number of complicating factors to consider in the current identification and treatment of PTSD in this cohort, including their advanced age; physical, cognitive, and social changes associated with normal aging; the associated medical and cognitive comorbidities; and the specific social-contextual factors in that age cohort. Any combination of these factors may complicate recognition, diagnosis, and treatment. It is also important to be cognizant of the additional stressors that may have been experienced by ethnic minorites and women serving in Korea, which are poorly documented and studied. Racial integration of the US military began during the Korean War, but the general pattern was for African American soldiers to be assigned to all-white units, rather than the reverse.14,41,42 And although the majority of military personnel serving in Korea were male, there were women serving in health care positions at mobile army surgical hospital (MASH) units, medical air evacuation (Medevac) aircraft, and off-shore hospital ships.
The clinical presentation of PTSD in older adults has varied, which may partially relate to the time elapsed since the index trauma. For example, older veterans in general may show less avoidance behavior as a part of PTSD, but in those who experience trauma later in life there may actually be greater avoidance.43,44 There have also been discrepant reports of intrusion or reexperiencing of symptoms, with these also potentially reduced in older veterans.43,44 However, sleep disturbances seem to be very common among elderly combat veterans, and attention should be paid to the possible presence of sleep apnea, which may be more common in veterans with PTSD in general.43,45,46
PTSD symptoms may reemerge after decades of remission or quiescence during retirement and/or with the emergence of neurocognitive impairment, such as Alzheimer disease or dementia. These individuals may have more difficulty engaging in distracting activities and work and spend more time engaging in reminiscence about the past, which can include increased focus on traumatic memories.45,47 Davison and colleagues have suggested a concept they call later-adulthood trauma reengagement (LATR) where later in life combat veterans may “confront and rework their wartime memories in an effort to find meaning and build coherence.”48 This process can be a double-edged sword, leading at times positively to enhanced personal growth or negatively to increased symptoms; preventive interventions may be able foster a more positive outcome.48
There is some evidence supporting the validity of the Clinician Administered PTSD Scale (CAPS) for the evaluation of PTSD in older adults, although this was based on the DSM-III-revised criteria for PTSD and an earlier version of CAPS.49 Bhattarai and colleagues examined responses to the 35-item Mississippi Scale for Combat-Related PTSD (M-PTSD) using VA clinical data collected between 2008 and 2015 on veterans of each combat era from World War II through the post-9/11.50 Strong internal consistency and test-retest reliability of the M-PTSD was observed within each veteran era sample. However, using chart diagnosis of PTSD as the criterion standard, the cut-scores for optimal balance of sensitivity and specificity of the M-PTSD scores were substantially lower for the older cohorts (World War II and Korean War veterans) relative to those for Vietnam and more recent veteran cohorts. The authors concluded that M-PTSD can be validly used to screen for PTSD in veterans within each of these cohorts but recommended using lower than standard cut-scores for Korean War and World War II veterans.50
This is also consistent with reports that suggest the use of lower cut-scores on self-administered PTSD symptom screens.43,44 For the clinician interested in quantifying the severity of PTSD, the most recent tools available are the CAPS-5 and the PCL-5, which have both been created in accordance with the DSM-5. The CAPS-5 is a rater-administered tool, and the PCL-5 is self-administered by the veteran. Although there has been little research using these newer tools in geriatric populations, they can currently serve as a means of tracking the severity of PTSD while we await measures that are better validated in Korean War and other older veterans.
Beyond specific empirical guidance, VA clinicians must presently rely on clinical observations and experience. Patients from the Korean War cohort often present at the insistence of a family member for changes in sleep, mood, behavior, or cognition. When the veterans themselves present, older adults with PTSD often focus more on somatic concerns (including pain, sleep, and gastrointestinal disturbance) than psychiatric problems per se. The latter tendency may in part be due to the salience of such symptoms for them, but perhaps also due to considerable stigma of mental health care that is still largely present in this group.43,44
Psychotherapy
Current VA treatment guidelines recommend trauma-focused therapies, with the strongest evidence base for prolonged exposure (PE), cognitive processing therapy (CPT), and eye movement desensitization and reprocessing (EMDR) therapies.51
There have been several excellent prior reviews discussing treatment of PTSD in older adults generally.10,43,44,52 These reviews have invariably expressed concern about the lack of sufficient empirical studies, but based on evidence from studies and case reports, there seems to be tentative support that trauma-focused therapies are acceptable and efficacious for use with older adults with PTSD. In their recent scoping review, Pless Kaiser and colleagues made several recommendations for trauma-focused therapy with older adults, including slow/careful pacing and use of compensatory aids for cognitive and sensory deficits.44 When cognitive impairment has exacerbated PTSD symptoms, they suggest therapists consider using an adapted form of CPT completed without a trauma narrative. For PE they recommend extending content across sessions and involving spouse or caregivers to assist with in vivo exposure and homework completion.44
Recent studies suggest that PTSD may be a risk factor for the later development of neurodegenerative disorders, and it is often during assessments for dementia that a revelation of PTSD occurs.10,43,47,55 Cognitive impairment may also be of relevance in deciding on the type of psychotherapy to be implemented, as it may have more adverse effects on the effectiveness of CPT than of exposure-based treatments (PE or EMDR). It may be useful to perform a cognitive assessment prior to initiation of a cognitive-based therapy, although extensive cognitive testing may not be practical or may be contraindicated because of fatigue. A brief screening tool such as the Montreal Cognitive Assessment or the Mini-Mental State Examinationmay be helpful.56, 57
Prolonged exposure has been reported by many clinicians to be effective in older adults with PTSD; however, due consideration should be given to the needs of individuals, as many have functioned for decades by suppressing memories.
Apart from the treatment needs for specific PTSD symptoms, the decades-long effects of poor sleep, irritability, hypervigilance, and dissociation also have social consequences for patients, including marital discord and divorce, and social and family isolation that should be addressed in therapy when appropriate. In addition, many Korean War veterans, like all veterans, sought postmilitary employment in professions that are associated with higher rates of exposure to psychological trauma, such as police or fire departments, and this may have an exacerbating effect on PTSD.58
Pharmacotherapy
There is very little empirical evidence guiding pharmacologic approaches to PTSD in older veterans. This population is at increased risk for many comorbidities, and pharmacologic treatments many require dosage adjustments, as is the case for any geriatric patient. Selective serotonin reuptake inhibitor (SSRI) and serotonin norepinephrine reuptake inhibitor (SNRI) medications have been proposed for some cases of PTSD.59,60 Health care providers may consider the SSRIs escitalopram or sertraline preferentially given their decreased potential for drug-drug interactions, anticholinergic effects, or cardiac toxicity compared with that of other drugs in this class.60,61 As venlafaxine can increase blood pressure, especially at higher doses, prescribers may choose duloxetine as an alternative if a SNRI is indicated.60 For veterans when prazosin is being considered for nightmare control, monitoring for hypotension, orthostasis, and the administration of other antihypertensives or prostatic hypertrophy medications is necessary.61 The use of benzodiazepines, while not recommended for PTSD, should be viewed with even greater trepidation in a geriatric population given enhanced risk of falls and confusion in the geriatric veteran population.60,62
Conclusions
Many of the oldest veterans (aged > 80 years) are from the Korean War era. The harsh and unique nature of the war, as well as the differences in context and support from the US public, and the outcome of the war, may have all contributed to and elevation of “combat fatigue” and PTSD among combat veterans from the Korean War. As the “forgotten war” cohort also has been forgotten by researchers, relatively little is known about posttraumatic stress sequelae of these veterans in the decades following the war.
From available evidence, we can readily surmise that problems were underrecognized and suboptimally diagnosed and treated. There is tentative evidence supporting the use of standard interviews and rating scales, such as the CAPS, M-PTSD, and PCL, but lower cut-scores than applied with Vietnam and later veteran cohorts are generally recommended to avoid excessive false negative errors. In terms of psychotherapy treatment, there is again a stark paucity of systematic research, but the limited evidence from studies of PTSD treatment in older adults from the general population tentatively support the acceptability and potential efficacy of recognized evidence-based trauma-focused psychotherapies for PTSD. Research on medication treatment is similarly lacking, but the general recommendations for the use of SSRI or SNRI medications seem to be valid, at least in our clinical experience, and the general rules for geriatric psychopharmacology definitely apply here—start low, go slow.
There are several important avenues for future research. Most pressing among these are establishing the effectiveness of existing treatments, and the modifications that may be needed in the broader context of the above factors, as well as the physical and cognitive changes associated with advanced age. Further research on the phenomenologic aspects of PTSD among Korean War and subsequent cohorts are also needed, as the information obtained will not only guide more effective personalized treatment of the Korean War veterans who remain with us, but also inform future generations of care in terms of the degree and dimensions of variability that may present between cohorts and within cohorts over the life span.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Arlington VA: American Psychiatric Association; 1980.
2. Friedman MJ, Schnurr PP, McDonagh-Coyle A. Posttraumatic stress disorder in the military veteran. Psychiatr Clin North Am. 1994;17(2):265-277.
3. Salmon TW. The Care and Treatment of Mental Diseases and War Neuroses (“Shell Shock”) in the British Army. New York: War Work Committee of the National Committee for Mental Hygiene, Inc; 1917.
4. Jones E, Wessely S. “Forward psychiatry” in the military: its origins and effectiveness. J Trauma Stress. 2003;16(4):411-419.
5. Newman RA. Combat fatigue: a review to the Korean conflict. Mil Med. 1964;129:921-928.
6. Harris FG. Some comments on the differential diagnosis and treatment of psychiatric breakdowns in Korea. https://history.amedd.army.mil/booksdocs/korea/recad2/ch9-2.html. Published April 30, 1954. Accessed November 8, 2019.
7. Smith PB. Psychiatric experiences during the Korean conflict. Am Pract Dig Treat. 1955;6(2):183-189.
8. Koontz AR. Psychiatry in the Korean War. Military Surg.
1950;107(6):444-445.
9. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Population Tables - Table 2L: VETPOP2016 Living Veterans by period of service, gender, 2015-2045. https://www.va.gov/vetdata/docs/Demographics/New_Vetpop_Model/2L_VetPop2016
_POS_National.xlsx. Accessed November 8, 2019.
10. Cook JM, McCarthy E, Thorp SR. Older adults with PTSD: brief state of research and evidence-based psychotherapy case illustration. Am J Geriatr Psychiatry. 2017;25(5):522-530.
11. Millett AR. Korean War: 1950-1953. Encylopaedia Britannica. https://www.britannica.com/event/Korean-War#accordion-article-history. Updated Nov 7, 2019. Accessed November 8, 2019.
12. Stack L. Korean War, a ‘forgotten’ conflict that shaped the modern world. The New York Times. January 2, 2018. https://www.nytimes.com/2018/01/01/world/asia/korean-war-history.html. Accessed November 8, 2019.
13. Westad OA. The Cold War: A World History. New York: Basic Books; 2018.
14. Young C, Conard PL, Armstrong ML, Lacy D. Older military veteran care: many still believe they are forgotten. J Holist Nurs. 2018;36(3):291-300.
15. Huebner AJ. Kilroy is back, 1950-1953. In: The Warrior Image: Soldiers in American Culture From the Second World War to the Vietnam Era. Chapel Hill, NC: The University of North Carolina Press; 2008:97-131.
16. The annexation of Korea (editorial). Japan Times. https://www.japantimes.co.jp/opinion/2010/08/29/editorials/the-annexation-of-korea/#.XPgvJvlKhhE. Published August 29, 2010. Accessed November 8, 2019.
17. Gupta K. How did the Korean war begin? China Q. 1972;52:699-716.
18. Lin L, Zhao Y, Ogawa M, Hoge J, Kim BY. Whose history? An analysis of the Korean War in history textbooks from the United States, South Korea, Japan, and China. Social Studies. 2009;100(5):222-232.
19. Weathersby K. The Korean War revisited. Wilson Q. 1999;23(3):91.
20. US Department of Veterans Affairs, Office of Program and Data Analyses, Assistant Secretary for Planning and Analysis. Data on veterans of the Korean War. https://www.va.gov/vetdata/docs/SpecialReports/KW2000.pdf. Published June 2000. Accessed November 8, 2019.
21. Brooks MS, Fulton L. Evidence of poorer life-course mental health outcomes among veterans of the Korean War cohort. Aging Ment Health. 2010;14(2):177-183.
22. US Department of Veterans Affairs, Office of Public Affairs. America’s wars. https://www.va.gov/opa/publications/factsheets/fs_americas_wars.pdf. Accessed November 8, 2019.
23. Memorandum on recent polls on Korea. https://www.eisenhowerlibrary.gov/sites/default/files/research/online-documents/korean-war/public-opinion-1953-06-02.pdf. Published June 2, 1953. Accessed November 8, 2019.
24. Elder GH Jr, Clipp EC. Combat experience and emotional health: impairment and resilience in later life. J Pers. 1989;57(2):311-341.
25. US Department of Veterans Affairs. Public health: cold injuries. https://www.publichealth.va.gov/PUBLICHEALTH/exposures/cold-injuries/index.asp. Updated July 31, 2019. Accessed November 8, 2019.
26. US Department of Veterans Affairs. Korean War veterans health issues. https://www.va.gov/health-care/health-needs-conditions/health-issues-related-to-service-era
/korean-war/. Updated June 14, 2019. Accessed November 8, 2019.
27. Shapiro F. Efficacy of the eye movement desensitization procedure in the treatment of traumatic memories. J Trauma Stress. 1989;2(2):199-223.
28. Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. J Consul Clin Psychol. 1992;60(5):748-756.
29. Foa EB, Rothbaum BO. Treating Trauma of Rape: Cognitive-Behavioral Therapy for PTSD. New York: Guilford; 2001.
30. Skinner R, Kaplick PM. Cultural shift in mental illness: a comparison of stress responses in World War I and the Vietnam War. JRSM Open. 2017;8(12):2054270417746061.
31. Kardiner A, Spiegel H. War Stress and Neurotic Illness. New York: Hoeber; 1947.
32. Archibald HC, Tuddenham RD. Persistent stress reaction after combat: a 20-year follow-up. Arch Gen Psychiatry. 1965;12:475-481.
33. Cook JM, Simiola V. Trauma and aging. Curr Psychiatry Rep. 2018;20(10):93.
34. Rosenheck R, Fontana A. Long-term sequelae of combat in World War II, Korea and Vietnam: a comparative study. In: McCaughey BG, Fullerton CS, Ursano RJ, eds. Individual
and Community Responses to Trauma and Disaster: The Structure of Human Chaos. New York: Cambridge University Press; 1994:330-359.
35. Blake DD, Keane TM, Wine PR, Mora C, Taylor KL, Lyons JA. Prevalence of PTSD symptoms in combat veterans seeking medical treatment. J Trauma Stress. 1990;3(1):15-27.
36. McCranie EW, Hyer LA. Posttraumatic stress disorder symptoms in Korean conflict and World War II combat veterans seeking outpatient treatment. J Trauma Stress. 2000;13(3):427-439.
37. Fontana A, Rosenheck R. Traumatic war stressors and psychiatric symptoms among World War II, Korean, and Vietnam War veterans. Psychology Aging. 1994;9(1):27-33.
38. Beebe GW. Follow-up studies of World War II and Korean war prisoners. II. Morbidity, disability, and maladjustments. Am J Epidemiol. 1975;101(5):400-422.
39. Gold PB, Engdahl BE, Eberly RE, Blake RJ, Page WF, Frueh BC. Trauma exposure, resilience, social support, and PTSD construct validity among former prisoners of war. Social Psychiatry Psychiatr Epidemiol. 2000;35(1):36-42.
40. US Department of Veterans Affairs. Key statistics by veteran status and period of service. https://www.va.gov/vetdata/docs/SpecialReports/KeyStats.pdf. Accessed November 11, 2019.
41. Bowers WT, Hammond WM, MacGarrigle GL. Black Soldier, White Army. Washington DC: US Army Center of Military History; 1996.
42. Black HK. Three generations, three wars: African American veterans. Gerontologist. 2016;56(1):33-41.
43. Thorp SR, Sones HM, Cook JM. Posttraumatic stress disorder among older adults. In: Sorocco KH, Lauderdale S, eds. Cognitive Behavior Therapy With Older Adults: Innovations Across Care Settings. New York: Springer; 2011:189-217.
44. Pless Kaiser A, Cook JM, Glick DM, Moye J. Posttraumatic stress disorder in older adults: a conceptual review. Clinical Gerontol. 2019;42(4):359-376.
45. Sadavoy J. Survivors. A review of the late-life effects of prior psychological trauma. Am J Geriatr Psychiatry. 1997;5(4):287-301.
46. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636.
47. Mota N, Tsai J, Kirwin PD, et al. Late-life exacerbation of PTSD symptoms in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2016;77(3):348-354.
48. Davison EH, Kaiser AP, Spiro A 3rd, Moye J, King LA, King DW. From Late-onset stress symptomatology to later-adulthood trauma reengagement in aging combat veterans: taking a broader view. Gerontologist. 2016;56(1):14-21.
49. Hyer L, Summers MN, Boyd S, Litaker M, Boudewyns P. Assessment of older combat veterans with the clinician-administered PTSD scale. J Trauma Stress. 1996;9(3):587-593.
50. Bhattarai JJ, Oehlert ME, Weber DK. Psychometric properties of the Mississippi Scale for combat-related posttraumatic stress disorder based on veterans’ period of service. Psychol Serv. 2018. [Epub ahead of print]
51. US Department of Veterans Affairs, US Department of Defense. VA/DOD Clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf.
Updated 2017. Accessed November 11, 2019.
52. Dinnen S, Simiola V, Cook JM. Post-traumatic stress disorder in older adults: a systematic review of the psychotherapy treatment literature. Aging Ment Health. 2015;19(2):144-150.
53. Jakel RJ. Posttraumatic Stress Disorder in the Elderly. Psychiatr Clin North Am. 2018;41(1):165-175.
54. Thorp SR, Glassman LH, Wells SY, et al. A randomized controlled trial of prolonged exposure therapy versus relaxation training for older veterans with military-related PTSD. J Anxiety Disord. 2019;64:45-54.
55. Kang B, Xu H, McConnell ES. Neurocognitive and psychiatric comorbidities of posttraumatic stress disorder among older veterans: a systematic review. Int J Geriatr Psychiatry. 2019;34(4):522-538.
56. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
57. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
58. Paton D. Traumatic Stress in Police Officers a Career-Length Assessment From Recruitment to Retirement. Springfield, IL: Charles C. Thomas; 2009.
59. Alexander W. Pharmacotherapy for post-traumatic stress disorder in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37(1):32-38.
60. Beck JG, Sloan DM, Friedman MJ. Pharmacotherapy for PTSD. In: The Oxford Handbook of Traumatic Stress Disorders. Oxford University Press; 2012.
61. Waltman SH, Shearer D, Moore BA. Management of posttraumatic nightmares: a review of pharmacologic and nonpharmacologic treatments since 2013. Curr Psychiatry Rep. 2018;20(12):108.
62. Díaz-Gutiérrez MJ, Martínez-Cengotitabengoa M, Sáez de Adana E, et al. Relationship between the use of benzodiazepines and falls in older adults: a systematic review. Maturitas. 2017;101:17-22.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Arlington VA: American Psychiatric Association; 1980.
2. Friedman MJ, Schnurr PP, McDonagh-Coyle A. Posttraumatic stress disorder in the military veteran. Psychiatr Clin North Am. 1994;17(2):265-277.
3. Salmon TW. The Care and Treatment of Mental Diseases and War Neuroses (“Shell Shock”) in the British Army. New York: War Work Committee of the National Committee for Mental Hygiene, Inc; 1917.
4. Jones E, Wessely S. “Forward psychiatry” in the military: its origins and effectiveness. J Trauma Stress. 2003;16(4):411-419.
5. Newman RA. Combat fatigue: a review to the Korean conflict. Mil Med. 1964;129:921-928.
6. Harris FG. Some comments on the differential diagnosis and treatment of psychiatric breakdowns in Korea. https://history.amedd.army.mil/booksdocs/korea/recad2/ch9-2.html. Published April 30, 1954. Accessed November 8, 2019.
7. Smith PB. Psychiatric experiences during the Korean conflict. Am Pract Dig Treat. 1955;6(2):183-189.
8. Koontz AR. Psychiatry in the Korean War. Military Surg.
1950;107(6):444-445.
9. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Population Tables - Table 2L: VETPOP2016 Living Veterans by period of service, gender, 2015-2045. https://www.va.gov/vetdata/docs/Demographics/New_Vetpop_Model/2L_VetPop2016
_POS_National.xlsx. Accessed November 8, 2019.
10. Cook JM, McCarthy E, Thorp SR. Older adults with PTSD: brief state of research and evidence-based psychotherapy case illustration. Am J Geriatr Psychiatry. 2017;25(5):522-530.
11. Millett AR. Korean War: 1950-1953. Encylopaedia Britannica. https://www.britannica.com/event/Korean-War#accordion-article-history. Updated Nov 7, 2019. Accessed November 8, 2019.
12. Stack L. Korean War, a ‘forgotten’ conflict that shaped the modern world. The New York Times. January 2, 2018. https://www.nytimes.com/2018/01/01/world/asia/korean-war-history.html. Accessed November 8, 2019.
13. Westad OA. The Cold War: A World History. New York: Basic Books; 2018.
14. Young C, Conard PL, Armstrong ML, Lacy D. Older military veteran care: many still believe they are forgotten. J Holist Nurs. 2018;36(3):291-300.
15. Huebner AJ. Kilroy is back, 1950-1953. In: The Warrior Image: Soldiers in American Culture From the Second World War to the Vietnam Era. Chapel Hill, NC: The University of North Carolina Press; 2008:97-131.
16. The annexation of Korea (editorial). Japan Times. https://www.japantimes.co.jp/opinion/2010/08/29/editorials/the-annexation-of-korea/#.XPgvJvlKhhE. Published August 29, 2010. Accessed November 8, 2019.
17. Gupta K. How did the Korean war begin? China Q. 1972;52:699-716.
18. Lin L, Zhao Y, Ogawa M, Hoge J, Kim BY. Whose history? An analysis of the Korean War in history textbooks from the United States, South Korea, Japan, and China. Social Studies. 2009;100(5):222-232.
19. Weathersby K. The Korean War revisited. Wilson Q. 1999;23(3):91.
20. US Department of Veterans Affairs, Office of Program and Data Analyses, Assistant Secretary for Planning and Analysis. Data on veterans of the Korean War. https://www.va.gov/vetdata/docs/SpecialReports/KW2000.pdf. Published June 2000. Accessed November 8, 2019.
21. Brooks MS, Fulton L. Evidence of poorer life-course mental health outcomes among veterans of the Korean War cohort. Aging Ment Health. 2010;14(2):177-183.
22. US Department of Veterans Affairs, Office of Public Affairs. America’s wars. https://www.va.gov/opa/publications/factsheets/fs_americas_wars.pdf. Accessed November 8, 2019.
23. Memorandum on recent polls on Korea. https://www.eisenhowerlibrary.gov/sites/default/files/research/online-documents/korean-war/public-opinion-1953-06-02.pdf. Published June 2, 1953. Accessed November 8, 2019.
24. Elder GH Jr, Clipp EC. Combat experience and emotional health: impairment and resilience in later life. J Pers. 1989;57(2):311-341.
25. US Department of Veterans Affairs. Public health: cold injuries. https://www.publichealth.va.gov/PUBLICHEALTH/exposures/cold-injuries/index.asp. Updated July 31, 2019. Accessed November 8, 2019.
26. US Department of Veterans Affairs. Korean War veterans health issues. https://www.va.gov/health-care/health-needs-conditions/health-issues-related-to-service-era
/korean-war/. Updated June 14, 2019. Accessed November 8, 2019.
27. Shapiro F. Efficacy of the eye movement desensitization procedure in the treatment of traumatic memories. J Trauma Stress. 1989;2(2):199-223.
28. Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. J Consul Clin Psychol. 1992;60(5):748-756.
29. Foa EB, Rothbaum BO. Treating Trauma of Rape: Cognitive-Behavioral Therapy for PTSD. New York: Guilford; 2001.
30. Skinner R, Kaplick PM. Cultural shift in mental illness: a comparison of stress responses in World War I and the Vietnam War. JRSM Open. 2017;8(12):2054270417746061.
31. Kardiner A, Spiegel H. War Stress and Neurotic Illness. New York: Hoeber; 1947.
32. Archibald HC, Tuddenham RD. Persistent stress reaction after combat: a 20-year follow-up. Arch Gen Psychiatry. 1965;12:475-481.
33. Cook JM, Simiola V. Trauma and aging. Curr Psychiatry Rep. 2018;20(10):93.
34. Rosenheck R, Fontana A. Long-term sequelae of combat in World War II, Korea and Vietnam: a comparative study. In: McCaughey BG, Fullerton CS, Ursano RJ, eds. Individual
and Community Responses to Trauma and Disaster: The Structure of Human Chaos. New York: Cambridge University Press; 1994:330-359.
35. Blake DD, Keane TM, Wine PR, Mora C, Taylor KL, Lyons JA. Prevalence of PTSD symptoms in combat veterans seeking medical treatment. J Trauma Stress. 1990;3(1):15-27.
36. McCranie EW, Hyer LA. Posttraumatic stress disorder symptoms in Korean conflict and World War II combat veterans seeking outpatient treatment. J Trauma Stress. 2000;13(3):427-439.
37. Fontana A, Rosenheck R. Traumatic war stressors and psychiatric symptoms among World War II, Korean, and Vietnam War veterans. Psychology Aging. 1994;9(1):27-33.
38. Beebe GW. Follow-up studies of World War II and Korean war prisoners. II. Morbidity, disability, and maladjustments. Am J Epidemiol. 1975;101(5):400-422.
39. Gold PB, Engdahl BE, Eberly RE, Blake RJ, Page WF, Frueh BC. Trauma exposure, resilience, social support, and PTSD construct validity among former prisoners of war. Social Psychiatry Psychiatr Epidemiol. 2000;35(1):36-42.
40. US Department of Veterans Affairs. Key statistics by veteran status and period of service. https://www.va.gov/vetdata/docs/SpecialReports/KeyStats.pdf. Accessed November 11, 2019.
41. Bowers WT, Hammond WM, MacGarrigle GL. Black Soldier, White Army. Washington DC: US Army Center of Military History; 1996.
42. Black HK. Three generations, three wars: African American veterans. Gerontologist. 2016;56(1):33-41.
43. Thorp SR, Sones HM, Cook JM. Posttraumatic stress disorder among older adults. In: Sorocco KH, Lauderdale S, eds. Cognitive Behavior Therapy With Older Adults: Innovations Across Care Settings. New York: Springer; 2011:189-217.
44. Pless Kaiser A, Cook JM, Glick DM, Moye J. Posttraumatic stress disorder in older adults: a conceptual review. Clinical Gerontol. 2019;42(4):359-376.
45. Sadavoy J. Survivors. A review of the late-life effects of prior psychological trauma. Am J Geriatr Psychiatry. 1997;5(4):287-301.
46. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636.
47. Mota N, Tsai J, Kirwin PD, et al. Late-life exacerbation of PTSD symptoms in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2016;77(3):348-354.
48. Davison EH, Kaiser AP, Spiro A 3rd, Moye J, King LA, King DW. From Late-onset stress symptomatology to later-adulthood trauma reengagement in aging combat veterans: taking a broader view. Gerontologist. 2016;56(1):14-21.
49. Hyer L, Summers MN, Boyd S, Litaker M, Boudewyns P. Assessment of older combat veterans with the clinician-administered PTSD scale. J Trauma Stress. 1996;9(3):587-593.
50. Bhattarai JJ, Oehlert ME, Weber DK. Psychometric properties of the Mississippi Scale for combat-related posttraumatic stress disorder based on veterans’ period of service. Psychol Serv. 2018. [Epub ahead of print]
51. US Department of Veterans Affairs, US Department of Defense. VA/DOD Clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf.
Updated 2017. Accessed November 11, 2019.
52. Dinnen S, Simiola V, Cook JM. Post-traumatic stress disorder in older adults: a systematic review of the psychotherapy treatment literature. Aging Ment Health. 2015;19(2):144-150.
53. Jakel RJ. Posttraumatic Stress Disorder in the Elderly. Psychiatr Clin North Am. 2018;41(1):165-175.
54. Thorp SR, Glassman LH, Wells SY, et al. A randomized controlled trial of prolonged exposure therapy versus relaxation training for older veterans with military-related PTSD. J Anxiety Disord. 2019;64:45-54.
55. Kang B, Xu H, McConnell ES. Neurocognitive and psychiatric comorbidities of posttraumatic stress disorder among older veterans: a systematic review. Int J Geriatr Psychiatry. 2019;34(4):522-538.
56. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
57. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
58. Paton D. Traumatic Stress in Police Officers a Career-Length Assessment From Recruitment to Retirement. Springfield, IL: Charles C. Thomas; 2009.
59. Alexander W. Pharmacotherapy for post-traumatic stress disorder in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37(1):32-38.
60. Beck JG, Sloan DM, Friedman MJ. Pharmacotherapy for PTSD. In: The Oxford Handbook of Traumatic Stress Disorders. Oxford University Press; 2012.
61. Waltman SH, Shearer D, Moore BA. Management of posttraumatic nightmares: a review of pharmacologic and nonpharmacologic treatments since 2013. Curr Psychiatry Rep. 2018;20(12):108.
62. Díaz-Gutiérrez MJ, Martínez-Cengotitabengoa M, Sáez de Adana E, et al. Relationship between the use of benzodiazepines and falls in older adults: a systematic review. Maturitas. 2017;101:17-22.
VA Ketamine Controversies
To the Editor: We read with interest the editorial on the clinical use of intranasal esketamine in treatment-resistant depression by Editor-in-Chief Cynthia Geppert in the October 2019 issue of Federal Practitioner.1 A recent case report published in your journal illustrated the success of IV ketamine in alleviating refractory chronic pain caused by a rare disease.2 Ketamine has been well established as an appropriate adjuvant as well as an alternative to opioids in attenuating acute postoperative pain and in certain chronic pain syndromes.3 We write out of concern for the rapidity of adoption of intranasal esketamine without considering the merits of IV ketamine.
When adopting new treatments or extending established drugs for newer indications, clinicians must balance beneficence and nonmaleficence. There is an urgent need for better treatment options for depression, suicidality, posttraumatic stress disorder (PTSD), and chronic pain in the veteran population. However, one must proceed with caution before wide adoption of a treatment that lacks real-world data on sustained or long-term benefits.4 Enthusiasm for this drug must also be tempered by the documented adverse effect (AE) of hepatic injury and the lack of data tracking this AE from repeated, long-term use.5 With these considerations in mind, reliable dosing and predictable pharmacokinetics are of great importance.
In addition to outpatient esketamine, outpatient IV administration of racemic ketamine remains an advantageous option with unique benefits compared with esketamine. Pharmacokinetically, IV ketamine is superior to intranasal esketamine. The bioavailability of intranasal esketamine is likely to be variable. A patient with a poor intranasal application or poor absorption might be falsely labeled an esketamine nonresponder. Increasing intranasal esketamine dosage to avoid false nonresponders may place other patients at risk for overdose and undesired AEs, including dysphoria and hallucinations. The variable bioavailability of intranasal ketamine adds complexity to the examination of its clinical effectiveness. IV ketamine should provide a predictable drug level and more reliable data. One might retort that esketamine is not the same as ketamine. True, esketamine is the S-enantiomer of ketamine, whereas ketamine is a racemic mixture of S- and R-ketamine. However, there is no clear evidence of clinically relevant differences between these formulations.5
Psychomimetic effects and cardiovascular changes are the most common short-term AEs resulting from ketamine.5 An IV infusion allows the treating physician to slowly titrate the administered ketamine to reach an effective concentration at the target site. Unlike an all-or-none intranasal administration, an infusion can be stopped at the first appearance of an AE. Psychomimetic effects, such as hallucinations, visual disturbances, and dysphoria are thought to occur in a dose-dependent fashion and remit once a ketamine infusion is stopped.5 Furthermore, cardiovascular AEs, such as hypertension and tachycardia, are commonly in patients with a body mass index > 30, with IV administration on a mg/kg basis. This suggests that calculated ideal body weight is a safer denominator, and reliable dosing is important to mitigating AEs.6
We urge caution with the widespread adoption of intranasal esketamine and suggest the advantages of the IV route, which offers predictability of AEs and titratability of dose. Questions remain regarding the appropriate dose and formulation of ketamine, rate of infusion, and route of administration for chronic pain and psychiatric indications.5,7 It is our responsibility to further study the long-term safety profile of ketamine and determine an appropriate dose of ketamine. The IV route allows many veterans to be helped in a safe and controllable manner.
Eugene Raggi, MD; and Srikantha L. Rao, MD, MS, FAS
1. Geppert CMA. The VA ketamine controversies. Fed Pract. 2019;36(10):446-447.
2. Eliason AH, Seo Y, Murphy D, Beal C. Adiposis dolorosa pain management. Fed Pract. 2019;36(11):530-533.
3. Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241-254.
4. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci. 2019;44(6):384-385.
5. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
6. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
7. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852-e857.
To the Editor: We read with interest the editorial on the clinical use of intranasal esketamine in treatment-resistant depression by Editor-in-Chief Cynthia Geppert in the October 2019 issue of Federal Practitioner.1 A recent case report published in your journal illustrated the success of IV ketamine in alleviating refractory chronic pain caused by a rare disease.2 Ketamine has been well established as an appropriate adjuvant as well as an alternative to opioids in attenuating acute postoperative pain and in certain chronic pain syndromes.3 We write out of concern for the rapidity of adoption of intranasal esketamine without considering the merits of IV ketamine.
When adopting new treatments or extending established drugs for newer indications, clinicians must balance beneficence and nonmaleficence. There is an urgent need for better treatment options for depression, suicidality, posttraumatic stress disorder (PTSD), and chronic pain in the veteran population. However, one must proceed with caution before wide adoption of a treatment that lacks real-world data on sustained or long-term benefits.4 Enthusiasm for this drug must also be tempered by the documented adverse effect (AE) of hepatic injury and the lack of data tracking this AE from repeated, long-term use.5 With these considerations in mind, reliable dosing and predictable pharmacokinetics are of great importance.
In addition to outpatient esketamine, outpatient IV administration of racemic ketamine remains an advantageous option with unique benefits compared with esketamine. Pharmacokinetically, IV ketamine is superior to intranasal esketamine. The bioavailability of intranasal esketamine is likely to be variable. A patient with a poor intranasal application or poor absorption might be falsely labeled an esketamine nonresponder. Increasing intranasal esketamine dosage to avoid false nonresponders may place other patients at risk for overdose and undesired AEs, including dysphoria and hallucinations. The variable bioavailability of intranasal ketamine adds complexity to the examination of its clinical effectiveness. IV ketamine should provide a predictable drug level and more reliable data. One might retort that esketamine is not the same as ketamine. True, esketamine is the S-enantiomer of ketamine, whereas ketamine is a racemic mixture of S- and R-ketamine. However, there is no clear evidence of clinically relevant differences between these formulations.5
Psychomimetic effects and cardiovascular changes are the most common short-term AEs resulting from ketamine.5 An IV infusion allows the treating physician to slowly titrate the administered ketamine to reach an effective concentration at the target site. Unlike an all-or-none intranasal administration, an infusion can be stopped at the first appearance of an AE. Psychomimetic effects, such as hallucinations, visual disturbances, and dysphoria are thought to occur in a dose-dependent fashion and remit once a ketamine infusion is stopped.5 Furthermore, cardiovascular AEs, such as hypertension and tachycardia, are commonly in patients with a body mass index > 30, with IV administration on a mg/kg basis. This suggests that calculated ideal body weight is a safer denominator, and reliable dosing is important to mitigating AEs.6
We urge caution with the widespread adoption of intranasal esketamine and suggest the advantages of the IV route, which offers predictability of AEs and titratability of dose. Questions remain regarding the appropriate dose and formulation of ketamine, rate of infusion, and route of administration for chronic pain and psychiatric indications.5,7 It is our responsibility to further study the long-term safety profile of ketamine and determine an appropriate dose of ketamine. The IV route allows many veterans to be helped in a safe and controllable manner.
Eugene Raggi, MD; and Srikantha L. Rao, MD, MS, FAS
To the Editor: We read with interest the editorial on the clinical use of intranasal esketamine in treatment-resistant depression by Editor-in-Chief Cynthia Geppert in the October 2019 issue of Federal Practitioner.1 A recent case report published in your journal illustrated the success of IV ketamine in alleviating refractory chronic pain caused by a rare disease.2 Ketamine has been well established as an appropriate adjuvant as well as an alternative to opioids in attenuating acute postoperative pain and in certain chronic pain syndromes.3 We write out of concern for the rapidity of adoption of intranasal esketamine without considering the merits of IV ketamine.
When adopting new treatments or extending established drugs for newer indications, clinicians must balance beneficence and nonmaleficence. There is an urgent need for better treatment options for depression, suicidality, posttraumatic stress disorder (PTSD), and chronic pain in the veteran population. However, one must proceed with caution before wide adoption of a treatment that lacks real-world data on sustained or long-term benefits.4 Enthusiasm for this drug must also be tempered by the documented adverse effect (AE) of hepatic injury and the lack of data tracking this AE from repeated, long-term use.5 With these considerations in mind, reliable dosing and predictable pharmacokinetics are of great importance.
In addition to outpatient esketamine, outpatient IV administration of racemic ketamine remains an advantageous option with unique benefits compared with esketamine. Pharmacokinetically, IV ketamine is superior to intranasal esketamine. The bioavailability of intranasal esketamine is likely to be variable. A patient with a poor intranasal application or poor absorption might be falsely labeled an esketamine nonresponder. Increasing intranasal esketamine dosage to avoid false nonresponders may place other patients at risk for overdose and undesired AEs, including dysphoria and hallucinations. The variable bioavailability of intranasal ketamine adds complexity to the examination of its clinical effectiveness. IV ketamine should provide a predictable drug level and more reliable data. One might retort that esketamine is not the same as ketamine. True, esketamine is the S-enantiomer of ketamine, whereas ketamine is a racemic mixture of S- and R-ketamine. However, there is no clear evidence of clinically relevant differences between these formulations.5
Psychomimetic effects and cardiovascular changes are the most common short-term AEs resulting from ketamine.5 An IV infusion allows the treating physician to slowly titrate the administered ketamine to reach an effective concentration at the target site. Unlike an all-or-none intranasal administration, an infusion can be stopped at the first appearance of an AE. Psychomimetic effects, such as hallucinations, visual disturbances, and dysphoria are thought to occur in a dose-dependent fashion and remit once a ketamine infusion is stopped.5 Furthermore, cardiovascular AEs, such as hypertension and tachycardia, are commonly in patients with a body mass index > 30, with IV administration on a mg/kg basis. This suggests that calculated ideal body weight is a safer denominator, and reliable dosing is important to mitigating AEs.6
We urge caution with the widespread adoption of intranasal esketamine and suggest the advantages of the IV route, which offers predictability of AEs and titratability of dose. Questions remain regarding the appropriate dose and formulation of ketamine, rate of infusion, and route of administration for chronic pain and psychiatric indications.5,7 It is our responsibility to further study the long-term safety profile of ketamine and determine an appropriate dose of ketamine. The IV route allows many veterans to be helped in a safe and controllable manner.
Eugene Raggi, MD; and Srikantha L. Rao, MD, MS, FAS
1. Geppert CMA. The VA ketamine controversies. Fed Pract. 2019;36(10):446-447.
2. Eliason AH, Seo Y, Murphy D, Beal C. Adiposis dolorosa pain management. Fed Pract. 2019;36(11):530-533.
3. Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241-254.
4. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci. 2019;44(6):384-385.
5. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
6. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
7. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852-e857.
1. Geppert CMA. The VA ketamine controversies. Fed Pract. 2019;36(10):446-447.
2. Eliason AH, Seo Y, Murphy D, Beal C. Adiposis dolorosa pain management. Fed Pract. 2019;36(11):530-533.
3. Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241-254.
4. Talbot J, Phillips JL, Blier P. Ketamine for chronic depression: two cautionary tales. J Psychiatry Neurosci. 2019;44(6):384-385.
5. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
6. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
7. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852-e857.
The Worst and the Best of 2019
Readers may recall that at the end of each calendar as opposed to fiscal year—I know it is hard to believe time exists outside the Federal system—Federal Practitioner publishes my ethics-focused version of the familiar year-end roundup. This year I am reversing the typical order of most annual rankings by putting the worst first for 2 morally salient reasons.
The first is that, sadly, it is almost always easier to identify multiple incidents that compete ignominiously for the “worst” of federal health care. Even more disappointing, it is comparatively difficult to find stories for the “best” that are of the same scale and scope as the bad news. This is not to say that every day there are not individual narratives of courage and compassion reported in US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA), and hundreds more unsung heroes.
The second reason is that as human beings our psychology is such that we gravitate toward the worst things more powerfully and persistently than we do the best. This is in part why it is more difficult to find uplifting stories and why the demoralizing ones affect us so strongly. In an exhaustive review of the subject, psychologists Roy Baumeister and colleagues conclude that,
When equal measures of good and bad are present, however, the psychological effects of bad ones outweigh those of the good ones. This may in fact be a general principle or law of psychological phenomena, possibly reflecting the innate predispositions of the psyche or at least reflecting the almost inevitable adaptation of each individual to the exigencies of daily life.2
I am thus saving the best for last in the hope that it will be more memorable and impactful than the worst.
Unique to this year’s look-back, both the negative and the positive accounts come from the domain of end-of-life care. And unlike prior reviews where the lack of administrative vigilance and professional competence affected hundreds of patients, families, and staff, each of this year’s incidents involve a single patient.
An incident that occurred in September 2019 at a VA Community Living Center (CLC) in Georgia stood out in infamy apart from all others. It was the report of a veteran in a VA nursing home who had been bitten more than 100 times by ants crawling all over his room. He died shortly afterward. In a scene out of a horror movie tapping into the most primeval human fears, his daughter Laquana Ross described her father, a Vietnam Air Force veteran with cancer, to media and VA officials in graphic terms. “I understand mistakes happen,” she said. “I’ve had ants. But he was bit by ants two days in a row. They feasted on him.”3
In this new era of holding its senior executive service accountable, the outraged chair of the Senate Veterans Affairs Committee demanded that heads roll, and the VA acted rapidly to comply.4 The VA Central Office placed the network director on administrative leave, reassigned the chief medical officer, and initiated quality and safety reviews as well as an administrative investigative board to scrutinize how the parent Atlanta VA medical center managed the situation. In total, 9 officials connected to the incident were placed on leave. The VA apologized, with VA Secretary Robert Wilke zeroing in on the core values involved in the tragedy, “This is about basic humanity and dignity,” he said. “I don’t care what steps were taken to address the issues. We did not treat a vet with the dignity that he and his family deserved.”5 Yet it was the veteran’s daughter, with unbelievable charity, who asked the most crucial question that must be answered within the framework of a just culture if similar tragedies are not to occur in the future, “I know the staff, without a shadow of doubt, respected my dad and even loved him,” Ross said. “But what’s their ability to assess situations and fix things?”3
To begin to give Ms. Ross the answer she deserves, we must understand that the antithesis of love is not hate but indifference; of compassion, it is not cruelty but coldness. A true just culture reserves individual blame for those who have ill-will and adopts a systems perspective of organizational improvement toward most other types of errors.6 This means that the deplorable conditions in the CLC cannot be charged to the failure of a single staff member to fulfil their obligations but to collective collapse at many levels of the organization. Just culture is ethically laudable and far superior to the history in federal service of capricious punishment or institutional apathy that far too often were the default reactions to media exposures or congressional ire. Justice, though necessary, is not sufficient to achieve virtue. Those who work in health care also must be inspired to offer mercy, kindness, and compassion, especially in our most sacred privilege to provide care of the dying.
The best of 2019 illustrates this distinction movingly. This account also involves a Vietnam veteran, this time a Marine also dying of cancer, which happened just about a month after the earlier report. To be transparent it occurred at my home VA medical center in New Mexico. I was peripherally involved in the case as a consultant but had no role in the wondrous things that transpired. The last wish of a patient dying in the hospice unit on campus was to see his beloved dog who had been taken to the local city animal shelter when he was hospitalized because he had no friends or family to look after the companion animal. A social worker on the palliative care team called the animal shelter and explained the patient did not have much time left but wanted to see his dog before he died. Working together with support from facility leadership, shelter workers brought the dog to visit with the patient for an entire day while hospice staff cried with joy and sadness.7
As the epigraph for this editorial from Dame Cicely Saunders, the founder of the modern hospice movement, says, the difference between unspeakable pain and meaningful suffering can be measured in the depth of compassion caregivers show to the dying. It is this quality of mercy that in one case condemns, and in the other praises, us all as health care and administrative professionals in the service of our country. Baumeister and colleagues suggest that the human tendency to magnify the bad and minimize the good in everyday myopia may in a wider vision actually be a reason for hope:
It may be that humans and animals show heightened awareness of and responded more quickly to negative information because it signals a need for change. Hence, the adaptiveness of self-regulation partly lies in the organism’s ability to detect when response modifications are necessary and when they are unnecessary. Moreover, the lessons learned from bad events should ideally be retained permanently so that the same dangers or costs are not encountered repeatedly. Meanwhile, good events (such as those that provide a feeling of satisfaction and contentment) should ideally wear off so that the organism is motivated to continue searching for more and better outcomes.2
Let us all take this lesson into our work in 2020 so that when it comes time to write this column next year in the chilling cold of late autumn there will be more stories of light than darkness from which to choose.
1. Saunders C. The management of patients in the terminal stage. In: Raven R, ed. Cancer, Vol. 6. London: Butterworth and Company; 1960:403-417.
2. Baumeister RF, Bratslavasky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev General Psychol. 2001;5(4);323-370.
3. Knowles H. ‘They feasted on him’: Ants at VA nursing home bite a veteran 100 times before his death, daughter says. Washington Post. September 17, 2019. https://www.washingtonpost.com/health/2019/09/13/they-feasted-him-ants-va-nursing-home-bit-veteran-times-before-his-death-daughter-says. Accessed November 25, 2019.
4. Axelrod T. GOP senator presses VA after veteran reportedly bitten by ants in nursing home. https://thehill.com/homenews/senate/461196-gop-senator-presses-va-after-veteran-reportedly-bitten-by-ants-at-nursing. Published September 12, 2019. Accessed November 25, 2019.
5. Kime P. Nine VA leaders, staff placed on leave amid anti-bite scandal. https://www.military.com/daily-news/2019/09/17/nine-va-leaders-staff-placed-leave-amid-ant-bite-scandal.html. Published September 17, 2019. Accessed November 22, 2019.
6. Sculli GL, Hemphill R. Culture of safety and just culture. https://www.patientsafety.va.gov/docs/joe/just_culture_2013_tagged.pdf. Accessed November 22, 2019.
7. Hughes M. A Vietnam veteran in hospice care got to see his beloved dog one last time. https://www.cnn.com/2019/10/21/us/veteran-dying-wish-dog-trnd/index.html. Published October 21, 2019. Accessed November 22, 2019.
Readers may recall that at the end of each calendar as opposed to fiscal year—I know it is hard to believe time exists outside the Federal system—Federal Practitioner publishes my ethics-focused version of the familiar year-end roundup. This year I am reversing the typical order of most annual rankings by putting the worst first for 2 morally salient reasons.
The first is that, sadly, it is almost always easier to identify multiple incidents that compete ignominiously for the “worst” of federal health care. Even more disappointing, it is comparatively difficult to find stories for the “best” that are of the same scale and scope as the bad news. This is not to say that every day there are not individual narratives of courage and compassion reported in US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA), and hundreds more unsung heroes.
The second reason is that as human beings our psychology is such that we gravitate toward the worst things more powerfully and persistently than we do the best. This is in part why it is more difficult to find uplifting stories and why the demoralizing ones affect us so strongly. In an exhaustive review of the subject, psychologists Roy Baumeister and colleagues conclude that,
When equal measures of good and bad are present, however, the psychological effects of bad ones outweigh those of the good ones. This may in fact be a general principle or law of psychological phenomena, possibly reflecting the innate predispositions of the psyche or at least reflecting the almost inevitable adaptation of each individual to the exigencies of daily life.2
I am thus saving the best for last in the hope that it will be more memorable and impactful than the worst.
Unique to this year’s look-back, both the negative and the positive accounts come from the domain of end-of-life care. And unlike prior reviews where the lack of administrative vigilance and professional competence affected hundreds of patients, families, and staff, each of this year’s incidents involve a single patient.
An incident that occurred in September 2019 at a VA Community Living Center (CLC) in Georgia stood out in infamy apart from all others. It was the report of a veteran in a VA nursing home who had been bitten more than 100 times by ants crawling all over his room. He died shortly afterward. In a scene out of a horror movie tapping into the most primeval human fears, his daughter Laquana Ross described her father, a Vietnam Air Force veteran with cancer, to media and VA officials in graphic terms. “I understand mistakes happen,” she said. “I’ve had ants. But he was bit by ants two days in a row. They feasted on him.”3
In this new era of holding its senior executive service accountable, the outraged chair of the Senate Veterans Affairs Committee demanded that heads roll, and the VA acted rapidly to comply.4 The VA Central Office placed the network director on administrative leave, reassigned the chief medical officer, and initiated quality and safety reviews as well as an administrative investigative board to scrutinize how the parent Atlanta VA medical center managed the situation. In total, 9 officials connected to the incident were placed on leave. The VA apologized, with VA Secretary Robert Wilke zeroing in on the core values involved in the tragedy, “This is about basic humanity and dignity,” he said. “I don’t care what steps were taken to address the issues. We did not treat a vet with the dignity that he and his family deserved.”5 Yet it was the veteran’s daughter, with unbelievable charity, who asked the most crucial question that must be answered within the framework of a just culture if similar tragedies are not to occur in the future, “I know the staff, without a shadow of doubt, respected my dad and even loved him,” Ross said. “But what’s their ability to assess situations and fix things?”3
To begin to give Ms. Ross the answer she deserves, we must understand that the antithesis of love is not hate but indifference; of compassion, it is not cruelty but coldness. A true just culture reserves individual blame for those who have ill-will and adopts a systems perspective of organizational improvement toward most other types of errors.6 This means that the deplorable conditions in the CLC cannot be charged to the failure of a single staff member to fulfil their obligations but to collective collapse at many levels of the organization. Just culture is ethically laudable and far superior to the history in federal service of capricious punishment or institutional apathy that far too often were the default reactions to media exposures or congressional ire. Justice, though necessary, is not sufficient to achieve virtue. Those who work in health care also must be inspired to offer mercy, kindness, and compassion, especially in our most sacred privilege to provide care of the dying.
The best of 2019 illustrates this distinction movingly. This account also involves a Vietnam veteran, this time a Marine also dying of cancer, which happened just about a month after the earlier report. To be transparent it occurred at my home VA medical center in New Mexico. I was peripherally involved in the case as a consultant but had no role in the wondrous things that transpired. The last wish of a patient dying in the hospice unit on campus was to see his beloved dog who had been taken to the local city animal shelter when he was hospitalized because he had no friends or family to look after the companion animal. A social worker on the palliative care team called the animal shelter and explained the patient did not have much time left but wanted to see his dog before he died. Working together with support from facility leadership, shelter workers brought the dog to visit with the patient for an entire day while hospice staff cried with joy and sadness.7
As the epigraph for this editorial from Dame Cicely Saunders, the founder of the modern hospice movement, says, the difference between unspeakable pain and meaningful suffering can be measured in the depth of compassion caregivers show to the dying. It is this quality of mercy that in one case condemns, and in the other praises, us all as health care and administrative professionals in the service of our country. Baumeister and colleagues suggest that the human tendency to magnify the bad and minimize the good in everyday myopia may in a wider vision actually be a reason for hope:
It may be that humans and animals show heightened awareness of and responded more quickly to negative information because it signals a need for change. Hence, the adaptiveness of self-regulation partly lies in the organism’s ability to detect when response modifications are necessary and when they are unnecessary. Moreover, the lessons learned from bad events should ideally be retained permanently so that the same dangers or costs are not encountered repeatedly. Meanwhile, good events (such as those that provide a feeling of satisfaction and contentment) should ideally wear off so that the organism is motivated to continue searching for more and better outcomes.2
Let us all take this lesson into our work in 2020 so that when it comes time to write this column next year in the chilling cold of late autumn there will be more stories of light than darkness from which to choose.
Readers may recall that at the end of each calendar as opposed to fiscal year—I know it is hard to believe time exists outside the Federal system—Federal Practitioner publishes my ethics-focused version of the familiar year-end roundup. This year I am reversing the typical order of most annual rankings by putting the worst first for 2 morally salient reasons.
The first is that, sadly, it is almost always easier to identify multiple incidents that compete ignominiously for the “worst” of federal health care. Even more disappointing, it is comparatively difficult to find stories for the “best” that are of the same scale and scope as the bad news. This is not to say that every day there are not individual narratives of courage and compassion reported in US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA), and hundreds more unsung heroes.
The second reason is that as human beings our psychology is such that we gravitate toward the worst things more powerfully and persistently than we do the best. This is in part why it is more difficult to find uplifting stories and why the demoralizing ones affect us so strongly. In an exhaustive review of the subject, psychologists Roy Baumeister and colleagues conclude that,
When equal measures of good and bad are present, however, the psychological effects of bad ones outweigh those of the good ones. This may in fact be a general principle or law of psychological phenomena, possibly reflecting the innate predispositions of the psyche or at least reflecting the almost inevitable adaptation of each individual to the exigencies of daily life.2
I am thus saving the best for last in the hope that it will be more memorable and impactful than the worst.
Unique to this year’s look-back, both the negative and the positive accounts come from the domain of end-of-life care. And unlike prior reviews where the lack of administrative vigilance and professional competence affected hundreds of patients, families, and staff, each of this year’s incidents involve a single patient.
An incident that occurred in September 2019 at a VA Community Living Center (CLC) in Georgia stood out in infamy apart from all others. It was the report of a veteran in a VA nursing home who had been bitten more than 100 times by ants crawling all over his room. He died shortly afterward. In a scene out of a horror movie tapping into the most primeval human fears, his daughter Laquana Ross described her father, a Vietnam Air Force veteran with cancer, to media and VA officials in graphic terms. “I understand mistakes happen,” she said. “I’ve had ants. But he was bit by ants two days in a row. They feasted on him.”3
In this new era of holding its senior executive service accountable, the outraged chair of the Senate Veterans Affairs Committee demanded that heads roll, and the VA acted rapidly to comply.4 The VA Central Office placed the network director on administrative leave, reassigned the chief medical officer, and initiated quality and safety reviews as well as an administrative investigative board to scrutinize how the parent Atlanta VA medical center managed the situation. In total, 9 officials connected to the incident were placed on leave. The VA apologized, with VA Secretary Robert Wilke zeroing in on the core values involved in the tragedy, “This is about basic humanity and dignity,” he said. “I don’t care what steps were taken to address the issues. We did not treat a vet with the dignity that he and his family deserved.”5 Yet it was the veteran’s daughter, with unbelievable charity, who asked the most crucial question that must be answered within the framework of a just culture if similar tragedies are not to occur in the future, “I know the staff, without a shadow of doubt, respected my dad and even loved him,” Ross said. “But what’s their ability to assess situations and fix things?”3
To begin to give Ms. Ross the answer she deserves, we must understand that the antithesis of love is not hate but indifference; of compassion, it is not cruelty but coldness. A true just culture reserves individual blame for those who have ill-will and adopts a systems perspective of organizational improvement toward most other types of errors.6 This means that the deplorable conditions in the CLC cannot be charged to the failure of a single staff member to fulfil their obligations but to collective collapse at many levels of the organization. Just culture is ethically laudable and far superior to the history in federal service of capricious punishment or institutional apathy that far too often were the default reactions to media exposures or congressional ire. Justice, though necessary, is not sufficient to achieve virtue. Those who work in health care also must be inspired to offer mercy, kindness, and compassion, especially in our most sacred privilege to provide care of the dying.
The best of 2019 illustrates this distinction movingly. This account also involves a Vietnam veteran, this time a Marine also dying of cancer, which happened just about a month after the earlier report. To be transparent it occurred at my home VA medical center in New Mexico. I was peripherally involved in the case as a consultant but had no role in the wondrous things that transpired. The last wish of a patient dying in the hospice unit on campus was to see his beloved dog who had been taken to the local city animal shelter when he was hospitalized because he had no friends or family to look after the companion animal. A social worker on the palliative care team called the animal shelter and explained the patient did not have much time left but wanted to see his dog before he died. Working together with support from facility leadership, shelter workers brought the dog to visit with the patient for an entire day while hospice staff cried with joy and sadness.7
As the epigraph for this editorial from Dame Cicely Saunders, the founder of the modern hospice movement, says, the difference between unspeakable pain and meaningful suffering can be measured in the depth of compassion caregivers show to the dying. It is this quality of mercy that in one case condemns, and in the other praises, us all as health care and administrative professionals in the service of our country. Baumeister and colleagues suggest that the human tendency to magnify the bad and minimize the good in everyday myopia may in a wider vision actually be a reason for hope:
It may be that humans and animals show heightened awareness of and responded more quickly to negative information because it signals a need for change. Hence, the adaptiveness of self-regulation partly lies in the organism’s ability to detect when response modifications are necessary and when they are unnecessary. Moreover, the lessons learned from bad events should ideally be retained permanently so that the same dangers or costs are not encountered repeatedly. Meanwhile, good events (such as those that provide a feeling of satisfaction and contentment) should ideally wear off so that the organism is motivated to continue searching for more and better outcomes.2
Let us all take this lesson into our work in 2020 so that when it comes time to write this column next year in the chilling cold of late autumn there will be more stories of light than darkness from which to choose.
1. Saunders C. The management of patients in the terminal stage. In: Raven R, ed. Cancer, Vol. 6. London: Butterworth and Company; 1960:403-417.
2. Baumeister RF, Bratslavasky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev General Psychol. 2001;5(4);323-370.
3. Knowles H. ‘They feasted on him’: Ants at VA nursing home bite a veteran 100 times before his death, daughter says. Washington Post. September 17, 2019. https://www.washingtonpost.com/health/2019/09/13/they-feasted-him-ants-va-nursing-home-bit-veteran-times-before-his-death-daughter-says. Accessed November 25, 2019.
4. Axelrod T. GOP senator presses VA after veteran reportedly bitten by ants in nursing home. https://thehill.com/homenews/senate/461196-gop-senator-presses-va-after-veteran-reportedly-bitten-by-ants-at-nursing. Published September 12, 2019. Accessed November 25, 2019.
5. Kime P. Nine VA leaders, staff placed on leave amid anti-bite scandal. https://www.military.com/daily-news/2019/09/17/nine-va-leaders-staff-placed-leave-amid-ant-bite-scandal.html. Published September 17, 2019. Accessed November 22, 2019.
6. Sculli GL, Hemphill R. Culture of safety and just culture. https://www.patientsafety.va.gov/docs/joe/just_culture_2013_tagged.pdf. Accessed November 22, 2019.
7. Hughes M. A Vietnam veteran in hospice care got to see his beloved dog one last time. https://www.cnn.com/2019/10/21/us/veteran-dying-wish-dog-trnd/index.html. Published October 21, 2019. Accessed November 22, 2019.
1. Saunders C. The management of patients in the terminal stage. In: Raven R, ed. Cancer, Vol. 6. London: Butterworth and Company; 1960:403-417.
2. Baumeister RF, Bratslavasky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev General Psychol. 2001;5(4);323-370.
3. Knowles H. ‘They feasted on him’: Ants at VA nursing home bite a veteran 100 times before his death, daughter says. Washington Post. September 17, 2019. https://www.washingtonpost.com/health/2019/09/13/they-feasted-him-ants-va-nursing-home-bit-veteran-times-before-his-death-daughter-says. Accessed November 25, 2019.
4. Axelrod T. GOP senator presses VA after veteran reportedly bitten by ants in nursing home. https://thehill.com/homenews/senate/461196-gop-senator-presses-va-after-veteran-reportedly-bitten-by-ants-at-nursing. Published September 12, 2019. Accessed November 25, 2019.
5. Kime P. Nine VA leaders, staff placed on leave amid anti-bite scandal. https://www.military.com/daily-news/2019/09/17/nine-va-leaders-staff-placed-leave-amid-ant-bite-scandal.html. Published September 17, 2019. Accessed November 22, 2019.
6. Sculli GL, Hemphill R. Culture of safety and just culture. https://www.patientsafety.va.gov/docs/joe/just_culture_2013_tagged.pdf. Accessed November 22, 2019.
7. Hughes M. A Vietnam veteran in hospice care got to see his beloved dog one last time. https://www.cnn.com/2019/10/21/us/veteran-dying-wish-dog-trnd/index.html. Published October 21, 2019. Accessed November 22, 2019.
Progressive, pruritic eruption of firm, skin-colored papules
Scleromyxedema, or generalized lichen myxedematosus, is a primary cutaneous mucinosis with unknown pathogenesis characterized by generalized firm, skin-colored papules and is commonly associated with an underlying monoclonal gammopathy (usually Ig-gamma paraproteinemia).
Scleromyxedema may have associated internal involvement, including neurologic, gastrointestinal, pulmonary, renal, cardiovascular, ophthalmological, or musculoskeletal. Histopathology demonstrates mucin in the dermis seen with Alcian blue staining, proliferation of fibroblasts, and increased collagen deposition.
The condition is chronic and progressive. Intravenous immunoglobulin is considered first-line treatment. Thalidomide and corticosteroids have been reported to also be efficacious.
It is associated with hematologic disorders, including IgA monoclonal gammopathy, as well as myeloproliferative disorders, leukemia, infections, and inflammatory bowel disease. Although its pathophysiology is not well understood, vascular immune complex deposition, repetitive inflammation, and subsequent fibrosis may play a role. On histology, there is leukocytoclastic vasculitis with polymorphonuclear cell infiltrate and fibrin deposition in the superficial and mid-dermis and onion-skin fibrosis.
EED often self-resolves within 5-10 years, although it can become chronic and recurrent. Dapsone, niacinamide, antimalarials, NSAIDs, tetracyclines, corticosteroids, colchicine, and plasmapheresis are reported treatments. This patient’s EED was recalcitrant to prednisone and responded to colchicine.
Scleromyxedema and EED are both rare, distinct cutaneous entities associated with different underlying paraproteinemias and to the best of our knowledge, have not been previously reported to coexist in a single patient.
This case and the photos were submitted by Rachel Fayne, BA; Yumeng Li, MD, MS; Fabrizio Galimberti, MD, PhD; and Brian Morrison, MD, of the department of dermatology and cutaneous surgery at the University of Miami.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Scleromyxedema, or generalized lichen myxedematosus, is a primary cutaneous mucinosis with unknown pathogenesis characterized by generalized firm, skin-colored papules and is commonly associated with an underlying monoclonal gammopathy (usually Ig-gamma paraproteinemia).
Scleromyxedema may have associated internal involvement, including neurologic, gastrointestinal, pulmonary, renal, cardiovascular, ophthalmological, or musculoskeletal. Histopathology demonstrates mucin in the dermis seen with Alcian blue staining, proliferation of fibroblasts, and increased collagen deposition.
The condition is chronic and progressive. Intravenous immunoglobulin is considered first-line treatment. Thalidomide and corticosteroids have been reported to also be efficacious.
It is associated with hematologic disorders, including IgA monoclonal gammopathy, as well as myeloproliferative disorders, leukemia, infections, and inflammatory bowel disease. Although its pathophysiology is not well understood, vascular immune complex deposition, repetitive inflammation, and subsequent fibrosis may play a role. On histology, there is leukocytoclastic vasculitis with polymorphonuclear cell infiltrate and fibrin deposition in the superficial and mid-dermis and onion-skin fibrosis.
EED often self-resolves within 5-10 years, although it can become chronic and recurrent. Dapsone, niacinamide, antimalarials, NSAIDs, tetracyclines, corticosteroids, colchicine, and plasmapheresis are reported treatments. This patient’s EED was recalcitrant to prednisone and responded to colchicine.
Scleromyxedema and EED are both rare, distinct cutaneous entities associated with different underlying paraproteinemias and to the best of our knowledge, have not been previously reported to coexist in a single patient.
This case and the photos were submitted by Rachel Fayne, BA; Yumeng Li, MD, MS; Fabrizio Galimberti, MD, PhD; and Brian Morrison, MD, of the department of dermatology and cutaneous surgery at the University of Miami.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Scleromyxedema, or generalized lichen myxedematosus, is a primary cutaneous mucinosis with unknown pathogenesis characterized by generalized firm, skin-colored papules and is commonly associated with an underlying monoclonal gammopathy (usually Ig-gamma paraproteinemia).
Scleromyxedema may have associated internal involvement, including neurologic, gastrointestinal, pulmonary, renal, cardiovascular, ophthalmological, or musculoskeletal. Histopathology demonstrates mucin in the dermis seen with Alcian blue staining, proliferation of fibroblasts, and increased collagen deposition.
The condition is chronic and progressive. Intravenous immunoglobulin is considered first-line treatment. Thalidomide and corticosteroids have been reported to also be efficacious.
It is associated with hematologic disorders, including IgA monoclonal gammopathy, as well as myeloproliferative disorders, leukemia, infections, and inflammatory bowel disease. Although its pathophysiology is not well understood, vascular immune complex deposition, repetitive inflammation, and subsequent fibrosis may play a role. On histology, there is leukocytoclastic vasculitis with polymorphonuclear cell infiltrate and fibrin deposition in the superficial and mid-dermis and onion-skin fibrosis.
EED often self-resolves within 5-10 years, although it can become chronic and recurrent. Dapsone, niacinamide, antimalarials, NSAIDs, tetracyclines, corticosteroids, colchicine, and plasmapheresis are reported treatments. This patient’s EED was recalcitrant to prednisone and responded to colchicine.
Scleromyxedema and EED are both rare, distinct cutaneous entities associated with different underlying paraproteinemias and to the best of our knowledge, have not been previously reported to coexist in a single patient.
This case and the photos were submitted by Rachel Fayne, BA; Yumeng Li, MD, MS; Fabrizio Galimberti, MD, PhD; and Brian Morrison, MD, of the department of dermatology and cutaneous surgery at the University of Miami.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.

New opioid recommendations: Pain from most dermatologic procedures should be managed with acetaminophen, ibuprofen
has recommended.
Rotation flaps, interpolation flaps, wedge resections, cartilage alar-batten grafts, and Mustarde flaps were among the 20 procedures that can be managed with up to 10 oral oxycodone 5-mg equivalents, according to the panel. Only the Abbe procedure might warrant dispensing up to 15 oxycodone 5-mg pills, Justin McLawhorn, MD, and colleagues wrote in the Journal of the American Academy of Dermatology. The recommended amount of opioids are in addition to nonopioid analgesics, the guidelines point out.
All the other procedures can – and should – be managed with a combination of acetaminophen and ibuprofen, either alone or in an alternating dose pattern, said Dr. McLawhorn, of the department of dermatology at the University of Oklahoma Health Sciences Center, Oklahoma City, and coauthors.
But limited opioid prescribing is an important part of healing for patients who undergo the most invasive procedures, they wrote. “The management of complications, including adequate pain control, should be tailored to each patient on a case-by-case basis. Moreover, any pain management plan should not strictly adhere to any single guideline, but rather should be formed with consideration of the expected pain from the procedure and/or closure and consider the patient’s expectations for pain control.”
The time is ripe for dermatologists to make a stand in combating the opioid crisis, according to a group email response to questions from Dr. McLawhorn, Thomas Stasko, MD, professor and chair of dermatology at the University of Oklahoma, Oklahoma City, and Lindsey Collins, MD, also of the University of Oklahoma.
“The opioid crisis has reached epidemic proportions. More than 70,000 Americans have died from an opioid overdose in 2017,” they wrote. “Moreover, recent data suggest that nearly 6% of postsurgical, opioid-naive patients become long-term users of opioids. The lack of specific evidence-based recommendations likely contributes to a wide variety in prescribing patterns and a steady supply of unused opioids. Countering the opioid crisis necessitates a restructuring of the opioid prescribing practices that addresses pain in a procedure-specific manner. These recommendations are one tool in the dermatologists’ arsenal that can be used as a reference to help guide opioid management and prevent excessive opioid prescriptions at discharge following dermatologic interventions.”
Unfortunately, they added, dermatologists have inadvertently fueled the opioid abuse fire.
“It is difficult to quantify which providers are responsible for the onslaught of opioids into our communities,” the authors wrote in the email interview. “However, we can deduce, based on recent opioid prescribing patterns, that dermatologists provide approximately 500,000 unused opioid pills to their communities on an annual basis. This is the result of a wide variation in practice patterns and narratives that have been previously circulated in an attempt to mitigate the providers’ perception of the addictive nature of opioid analgesics. Our hope is that by addressing pain in a procedure-specific manner, we can help to limit the excessive number of unused opioid pills that are provided by dermatologists and ultimately decrease the rate of opioid-related complications, including addiction and death.”
Still, patients need and deserve effective pain management after a procedure. In the guidelines, the investigators wrote that a “one-size-fits-all” approach “does not account for the mechanism of pain, the invasiveness of the procedure, or the anatomic structures that are manipulated. As a result, current guidelines cannot accurately predict the quantity of opioids that are necessary to manage postoperative pain.”
The panel brought together experts in general dermatology, dermatologic surgery, cosmetics, and phlebology to develop a consensus on opioid prescribing guidelines for 87 of the most common procedures. Everyone on the panel was a member of the American College of Mohs Surgery, American Academy of Dermatology, or the American Vein and Lymphatic Society. The panel conducted a literature review to determine which procedures might require opioids and which would not. At least 75% of the panel had to agree on a reasonable but effective opioid amount; they were then polled as to whether they might employ that recommendation in their own clinical practice.
The recommendations are aimed at patients who experienced no peri- or postoperative complications.
The panel agreed that acetaminophen and ibuprofen – alone, in combination, or with opioids – were reasonable choices for all the 87 procedures. In such instances, acetaminophen 1 g can be staggered with ibuprofen 400 mg every 4 or 8 hours.
“I think providers will encounter a mixed bag of preconceived notions regarding patients’ expectations for pain control,” Dr. McLawhorn and coauthors wrote in the interview. “The important point for providers to make is to emphasize the noninferiority of acetaminophen and/or ibuprofen in controlling acute pain for patients who are not dependent on opioids for the management of chronic pain. Our experience in caring for many surgical patients has shown that patients are usually receptive to the use of nonopioid analgesics as many are familiar with their addictive potential because of the uptick in the publicity of the opioid-related complications.”
In cases where opioids might be appropriate, the panel unanimously agreed that dose limits be imposed. For 15 of the 87 procedures, the panel recommend a maximum prescription of 10 oxycodone 5-mg equivalents. Only one other – the Abbe flap – might warrant more, with a maximum of 15 oxycodone 5-mg pills at discharge.
Sometimes called a “lip switch,” the Abbe flap is reconstruction for full-thickness lip defects. It is a composite flap that moves skin, muscle, mucosa, and blood supply from the lower lip to reconstruct a defect of the upper lip. This reconstruction attempts to respect the native anatomic landmarks of the lip and allow for a better functional outcome.
“Because of the extensive nature of the repair and the anatomic territories that are manipulated, including the suturing of the lower lip to the upper lip with delayed separation, adequate pain control may require opioid analgesics in the immediate postoperative period,” the team wrote in the interview.
The panel could not agree on pain management strategies for five other procedures: Karapandzic flaps, en bloc nail excisions, facial resurfacing with deep chemical peels, and small- or large-volume liposuction. This was partly because of a lack of personal experience. Only 8 of the 40 panelists performed Karapandzic flaps. The maximum number of 5-mg oxycodone tablets any panelist prescribed for Karapandzic flaps and en bloc nail excisions was 20.
Facial resurfacing was likewise an uncommon procedure for the panel, with just 11 members performing this using deep chemical peels. However, five of those panelists said that opioids were routinely needed for postoperative pain with a maximum of 15 oxycodone 5-mg equivalents. And just four panelists performed liposuction, for which they used a maximum of 15 oxycodone 5-mg equivalents.
“However,” they wrote in the guidelines, “these providers noted that the location where the procedure is performed strongly influences the need for opioid pain management, with small-volume removal in the neck, arms, or flanks being unlikely to require opioids for adequate pain control, whereas large-volume removal in the thighs, knees, and hips may routinely require opioids.”
Addressing patient expectations is a very important part of pain management, the panel noted. “Patients will invariably experience postoperative pain after cutaneous surgeries or other interventions, often peaking within 4 hours after surgery. Wound tension, size and type of repair, anatomical location/nerve innervation, and patient pain tolerance are all factors that contribute to postoperative discomfort and should be considered when developing a postoperative pain management plan.”
Ultimately, according to Dr. McLawhorn and coauthors, the decision to use opioids at discharge for postoperative pain control should be an individual one based on patients’ comorbidities and expectations.
“Admittedly, many of the procedures listed within the recommendations may result in a rather large or complex defect that requires an equally large or complex repair,” they wrote in the interview. “However, proper education of the patient and provider regarding the risks of addiction with the use of opioids even short term should be discussed as part of every preoperative consultation. Furthermore, the patient and the provider must discuss their expectations for postoperative pain interventions for adequate pain control.”
SOURCE: McLawhorn J et al. J Am Acad Dermatol. 2019 Nov 12. doi: 10.1016/j.jaad.2019.09.080.
has recommended.
Rotation flaps, interpolation flaps, wedge resections, cartilage alar-batten grafts, and Mustarde flaps were among the 20 procedures that can be managed with up to 10 oral oxycodone 5-mg equivalents, according to the panel. Only the Abbe procedure might warrant dispensing up to 15 oxycodone 5-mg pills, Justin McLawhorn, MD, and colleagues wrote in the Journal of the American Academy of Dermatology. The recommended amount of opioids are in addition to nonopioid analgesics, the guidelines point out.
All the other procedures can – and should – be managed with a combination of acetaminophen and ibuprofen, either alone or in an alternating dose pattern, said Dr. McLawhorn, of the department of dermatology at the University of Oklahoma Health Sciences Center, Oklahoma City, and coauthors.
But limited opioid prescribing is an important part of healing for patients who undergo the most invasive procedures, they wrote. “The management of complications, including adequate pain control, should be tailored to each patient on a case-by-case basis. Moreover, any pain management plan should not strictly adhere to any single guideline, but rather should be formed with consideration of the expected pain from the procedure and/or closure and consider the patient’s expectations for pain control.”
The time is ripe for dermatologists to make a stand in combating the opioid crisis, according to a group email response to questions from Dr. McLawhorn, Thomas Stasko, MD, professor and chair of dermatology at the University of Oklahoma, Oklahoma City, and Lindsey Collins, MD, also of the University of Oklahoma.
“The opioid crisis has reached epidemic proportions. More than 70,000 Americans have died from an opioid overdose in 2017,” they wrote. “Moreover, recent data suggest that nearly 6% of postsurgical, opioid-naive patients become long-term users of opioids. The lack of specific evidence-based recommendations likely contributes to a wide variety in prescribing patterns and a steady supply of unused opioids. Countering the opioid crisis necessitates a restructuring of the opioid prescribing practices that addresses pain in a procedure-specific manner. These recommendations are one tool in the dermatologists’ arsenal that can be used as a reference to help guide opioid management and prevent excessive opioid prescriptions at discharge following dermatologic interventions.”
Unfortunately, they added, dermatologists have inadvertently fueled the opioid abuse fire.
“It is difficult to quantify which providers are responsible for the onslaught of opioids into our communities,” the authors wrote in the email interview. “However, we can deduce, based on recent opioid prescribing patterns, that dermatologists provide approximately 500,000 unused opioid pills to their communities on an annual basis. This is the result of a wide variation in practice patterns and narratives that have been previously circulated in an attempt to mitigate the providers’ perception of the addictive nature of opioid analgesics. Our hope is that by addressing pain in a procedure-specific manner, we can help to limit the excessive number of unused opioid pills that are provided by dermatologists and ultimately decrease the rate of opioid-related complications, including addiction and death.”
Still, patients need and deserve effective pain management after a procedure. In the guidelines, the investigators wrote that a “one-size-fits-all” approach “does not account for the mechanism of pain, the invasiveness of the procedure, or the anatomic structures that are manipulated. As a result, current guidelines cannot accurately predict the quantity of opioids that are necessary to manage postoperative pain.”
The panel brought together experts in general dermatology, dermatologic surgery, cosmetics, and phlebology to develop a consensus on opioid prescribing guidelines for 87 of the most common procedures. Everyone on the panel was a member of the American College of Mohs Surgery, American Academy of Dermatology, or the American Vein and Lymphatic Society. The panel conducted a literature review to determine which procedures might require opioids and which would not. At least 75% of the panel had to agree on a reasonable but effective opioid amount; they were then polled as to whether they might employ that recommendation in their own clinical practice.
The recommendations are aimed at patients who experienced no peri- or postoperative complications.
The panel agreed that acetaminophen and ibuprofen – alone, in combination, or with opioids – were reasonable choices for all the 87 procedures. In such instances, acetaminophen 1 g can be staggered with ibuprofen 400 mg every 4 or 8 hours.
“I think providers will encounter a mixed bag of preconceived notions regarding patients’ expectations for pain control,” Dr. McLawhorn and coauthors wrote in the interview. “The important point for providers to make is to emphasize the noninferiority of acetaminophen and/or ibuprofen in controlling acute pain for patients who are not dependent on opioids for the management of chronic pain. Our experience in caring for many surgical patients has shown that patients are usually receptive to the use of nonopioid analgesics as many are familiar with their addictive potential because of the uptick in the publicity of the opioid-related complications.”
In cases where opioids might be appropriate, the panel unanimously agreed that dose limits be imposed. For 15 of the 87 procedures, the panel recommend a maximum prescription of 10 oxycodone 5-mg equivalents. Only one other – the Abbe flap – might warrant more, with a maximum of 15 oxycodone 5-mg pills at discharge.
Sometimes called a “lip switch,” the Abbe flap is reconstruction for full-thickness lip defects. It is a composite flap that moves skin, muscle, mucosa, and blood supply from the lower lip to reconstruct a defect of the upper lip. This reconstruction attempts to respect the native anatomic landmarks of the lip and allow for a better functional outcome.
“Because of the extensive nature of the repair and the anatomic territories that are manipulated, including the suturing of the lower lip to the upper lip with delayed separation, adequate pain control may require opioid analgesics in the immediate postoperative period,” the team wrote in the interview.
The panel could not agree on pain management strategies for five other procedures: Karapandzic flaps, en bloc nail excisions, facial resurfacing with deep chemical peels, and small- or large-volume liposuction. This was partly because of a lack of personal experience. Only 8 of the 40 panelists performed Karapandzic flaps. The maximum number of 5-mg oxycodone tablets any panelist prescribed for Karapandzic flaps and en bloc nail excisions was 20.
Facial resurfacing was likewise an uncommon procedure for the panel, with just 11 members performing this using deep chemical peels. However, five of those panelists said that opioids were routinely needed for postoperative pain with a maximum of 15 oxycodone 5-mg equivalents. And just four panelists performed liposuction, for which they used a maximum of 15 oxycodone 5-mg equivalents.
“However,” they wrote in the guidelines, “these providers noted that the location where the procedure is performed strongly influences the need for opioid pain management, with small-volume removal in the neck, arms, or flanks being unlikely to require opioids for adequate pain control, whereas large-volume removal in the thighs, knees, and hips may routinely require opioids.”
Addressing patient expectations is a very important part of pain management, the panel noted. “Patients will invariably experience postoperative pain after cutaneous surgeries or other interventions, often peaking within 4 hours after surgery. Wound tension, size and type of repair, anatomical location/nerve innervation, and patient pain tolerance are all factors that contribute to postoperative discomfort and should be considered when developing a postoperative pain management plan.”
Ultimately, according to Dr. McLawhorn and coauthors, the decision to use opioids at discharge for postoperative pain control should be an individual one based on patients’ comorbidities and expectations.
“Admittedly, many of the procedures listed within the recommendations may result in a rather large or complex defect that requires an equally large or complex repair,” they wrote in the interview. “However, proper education of the patient and provider regarding the risks of addiction with the use of opioids even short term should be discussed as part of every preoperative consultation. Furthermore, the patient and the provider must discuss their expectations for postoperative pain interventions for adequate pain control.”
SOURCE: McLawhorn J et al. J Am Acad Dermatol. 2019 Nov 12. doi: 10.1016/j.jaad.2019.09.080.
has recommended.
Rotation flaps, interpolation flaps, wedge resections, cartilage alar-batten grafts, and Mustarde flaps were among the 20 procedures that can be managed with up to 10 oral oxycodone 5-mg equivalents, according to the panel. Only the Abbe procedure might warrant dispensing up to 15 oxycodone 5-mg pills, Justin McLawhorn, MD, and colleagues wrote in the Journal of the American Academy of Dermatology. The recommended amount of opioids are in addition to nonopioid analgesics, the guidelines point out.
All the other procedures can – and should – be managed with a combination of acetaminophen and ibuprofen, either alone or in an alternating dose pattern, said Dr. McLawhorn, of the department of dermatology at the University of Oklahoma Health Sciences Center, Oklahoma City, and coauthors.
But limited opioid prescribing is an important part of healing for patients who undergo the most invasive procedures, they wrote. “The management of complications, including adequate pain control, should be tailored to each patient on a case-by-case basis. Moreover, any pain management plan should not strictly adhere to any single guideline, but rather should be formed with consideration of the expected pain from the procedure and/or closure and consider the patient’s expectations for pain control.”
The time is ripe for dermatologists to make a stand in combating the opioid crisis, according to a group email response to questions from Dr. McLawhorn, Thomas Stasko, MD, professor and chair of dermatology at the University of Oklahoma, Oklahoma City, and Lindsey Collins, MD, also of the University of Oklahoma.
“The opioid crisis has reached epidemic proportions. More than 70,000 Americans have died from an opioid overdose in 2017,” they wrote. “Moreover, recent data suggest that nearly 6% of postsurgical, opioid-naive patients become long-term users of opioids. The lack of specific evidence-based recommendations likely contributes to a wide variety in prescribing patterns and a steady supply of unused opioids. Countering the opioid crisis necessitates a restructuring of the opioid prescribing practices that addresses pain in a procedure-specific manner. These recommendations are one tool in the dermatologists’ arsenal that can be used as a reference to help guide opioid management and prevent excessive opioid prescriptions at discharge following dermatologic interventions.”
Unfortunately, they added, dermatologists have inadvertently fueled the opioid abuse fire.
“It is difficult to quantify which providers are responsible for the onslaught of opioids into our communities,” the authors wrote in the email interview. “However, we can deduce, based on recent opioid prescribing patterns, that dermatologists provide approximately 500,000 unused opioid pills to their communities on an annual basis. This is the result of a wide variation in practice patterns and narratives that have been previously circulated in an attempt to mitigate the providers’ perception of the addictive nature of opioid analgesics. Our hope is that by addressing pain in a procedure-specific manner, we can help to limit the excessive number of unused opioid pills that are provided by dermatologists and ultimately decrease the rate of opioid-related complications, including addiction and death.”
Still, patients need and deserve effective pain management after a procedure. In the guidelines, the investigators wrote that a “one-size-fits-all” approach “does not account for the mechanism of pain, the invasiveness of the procedure, or the anatomic structures that are manipulated. As a result, current guidelines cannot accurately predict the quantity of opioids that are necessary to manage postoperative pain.”
The panel brought together experts in general dermatology, dermatologic surgery, cosmetics, and phlebology to develop a consensus on opioid prescribing guidelines for 87 of the most common procedures. Everyone on the panel was a member of the American College of Mohs Surgery, American Academy of Dermatology, or the American Vein and Lymphatic Society. The panel conducted a literature review to determine which procedures might require opioids and which would not. At least 75% of the panel had to agree on a reasonable but effective opioid amount; they were then polled as to whether they might employ that recommendation in their own clinical practice.
The recommendations are aimed at patients who experienced no peri- or postoperative complications.
The panel agreed that acetaminophen and ibuprofen – alone, in combination, or with opioids – were reasonable choices for all the 87 procedures. In such instances, acetaminophen 1 g can be staggered with ibuprofen 400 mg every 4 or 8 hours.
“I think providers will encounter a mixed bag of preconceived notions regarding patients’ expectations for pain control,” Dr. McLawhorn and coauthors wrote in the interview. “The important point for providers to make is to emphasize the noninferiority of acetaminophen and/or ibuprofen in controlling acute pain for patients who are not dependent on opioids for the management of chronic pain. Our experience in caring for many surgical patients has shown that patients are usually receptive to the use of nonopioid analgesics as many are familiar with their addictive potential because of the uptick in the publicity of the opioid-related complications.”
In cases where opioids might be appropriate, the panel unanimously agreed that dose limits be imposed. For 15 of the 87 procedures, the panel recommend a maximum prescription of 10 oxycodone 5-mg equivalents. Only one other – the Abbe flap – might warrant more, with a maximum of 15 oxycodone 5-mg pills at discharge.
Sometimes called a “lip switch,” the Abbe flap is reconstruction for full-thickness lip defects. It is a composite flap that moves skin, muscle, mucosa, and blood supply from the lower lip to reconstruct a defect of the upper lip. This reconstruction attempts to respect the native anatomic landmarks of the lip and allow for a better functional outcome.
“Because of the extensive nature of the repair and the anatomic territories that are manipulated, including the suturing of the lower lip to the upper lip with delayed separation, adequate pain control may require opioid analgesics in the immediate postoperative period,” the team wrote in the interview.
The panel could not agree on pain management strategies for five other procedures: Karapandzic flaps, en bloc nail excisions, facial resurfacing with deep chemical peels, and small- or large-volume liposuction. This was partly because of a lack of personal experience. Only 8 of the 40 panelists performed Karapandzic flaps. The maximum number of 5-mg oxycodone tablets any panelist prescribed for Karapandzic flaps and en bloc nail excisions was 20.
Facial resurfacing was likewise an uncommon procedure for the panel, with just 11 members performing this using deep chemical peels. However, five of those panelists said that opioids were routinely needed for postoperative pain with a maximum of 15 oxycodone 5-mg equivalents. And just four panelists performed liposuction, for which they used a maximum of 15 oxycodone 5-mg equivalents.
“However,” they wrote in the guidelines, “these providers noted that the location where the procedure is performed strongly influences the need for opioid pain management, with small-volume removal in the neck, arms, or flanks being unlikely to require opioids for adequate pain control, whereas large-volume removal in the thighs, knees, and hips may routinely require opioids.”
Addressing patient expectations is a very important part of pain management, the panel noted. “Patients will invariably experience postoperative pain after cutaneous surgeries or other interventions, often peaking within 4 hours after surgery. Wound tension, size and type of repair, anatomical location/nerve innervation, and patient pain tolerance are all factors that contribute to postoperative discomfort and should be considered when developing a postoperative pain management plan.”
Ultimately, according to Dr. McLawhorn and coauthors, the decision to use opioids at discharge for postoperative pain control should be an individual one based on patients’ comorbidities and expectations.
“Admittedly, many of the procedures listed within the recommendations may result in a rather large or complex defect that requires an equally large or complex repair,” they wrote in the interview. “However, proper education of the patient and provider regarding the risks of addiction with the use of opioids even short term should be discussed as part of every preoperative consultation. Furthermore, the patient and the provider must discuss their expectations for postoperative pain interventions for adequate pain control.”
SOURCE: McLawhorn J et al. J Am Acad Dermatol. 2019 Nov 12. doi: 10.1016/j.jaad.2019.09.080.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Experts bring clarity to end of life difficulties
Understanding family perspective is an important factor
A Vietnam veteran steered clear of the health care system for years, then showed up at the hospital with pneumonia and respiratory failure. He was whisked to the intensive care unit, and cancerous masses were found.
The situation – as described by Jeffrey Frank, MD, director of quality and performance at Vituity, a physician group in Emeryville, Calif. – then got worse.
“No one was there for him,” Dr. Frank said. “He’s laying in the ICU, he does not have the capacity to make decisions, let alone communicate. So the care team needs guidance.”
Too often, hospitalists find themselves in confusing situations involving patients near the end of their lives, having to determine how to go about treating a patient or withholding treatment when patients are not in a position to announce their wishes. When family is present, the health care team thinks the most sensible course of treatment is at odds with what the family wants to be done.
At the Society of Hospital Medicine 2019 Annual Conference, hospitalists with palliative care training offered advice on how to go about handling these difficult situations, which can sometimes become more manageable with certain strategies.
For situations in which there is no designated representative to speak for a patient who is unresponsive – the so-called “unbefriended patient” or “unrepresented patient” – any source of information can be valuable. And health care providers should seek out this input, Dr. Frank said.
“When there is a visitor at the bedside, and as long as they know the person, and they can start giving the medical providers some information about what the patient would have wanted, most of us will talk with that person and that’s actually a good habit,” he said.
Thirty-nine states and the District of Columbia have regulations on whom health care providers should talk to when there is no obvious representative, Dr. Frank said, noting that most of these regulations follow a classic family-tree order. But in the discouraging results of many surveys of health care providers on the subject, most clinicians say that they do not know the regulations in their state, Dr. Frank said. But he said such results betray a silver lining because clinicians say that they would be inclined to use a family tree–style hierarchy in deciding with whom they should speak about end of life decisions.
Hospitalists should at least know whether their hospital has a policy on unrepresented patients, Dr. Frank said.
“That’s your road map on how to get through consenting this patient – what am I going to do with Mr. Smith?” he said. “You may ask yourself, ‘Do I just keep treating him and treating him?’ If you have a policy at your hospital, it will protect you from liability, as well as give you a sense of process.”
Conflicts in communication
An even worse situation, perhaps, is one that many hospitalists have seen: A patient is teetering at the edge of life, and a spouse arrives, along with two daughters from out of state who have not seen their father in a year, said Elizabeth Gundersen, MD, director of the ethics curriculum at Florida Atlantic University, Boca Raton.
“The family requests that the medical team do everything, including intubation and attempts at resuscitation if needed,” she said. “The family says he was fine prior to this admission. Another thing I hear a lot is, ‘He was even sicker than this last year, and he got better.’ ”
Meanwhile, “the medical team consensus is that he is not going to survive this illness,” Dr. Gundersen said.
The situation is so common and problematic that it has a name – the “Daughter from California Syndrome.” (According to medical literature says, it’s called the “Daughter from Chicago Syndrome” in California.)
“This is one of the most agonizing things that happens to us in medicine,” Dr. Gundersen said. “It affects us, it affects our nurses, it affects the entire medical team. It’s agonizing when we feel like treatment has somehow turned to torture.”
Dr. Gundersen said the medical staff should avoid using the word “futile,” or similar language, with families.
“Words matter,” she said. “Inappropriate language can inadvertently convey the feeling that, ‘They’re giving up on my dad – they think it’s hopeless.’ That can make families and the medical team dig in their heels further.”
Sometimes it can be hard to define the terms of decision making. Even if the family and the medical team can agree that no “nonbeneficial treatments” should be administered, Dr. Gundersen said, what exactly does that mean? Does it mean less than a 1% chance of working; less than a 5% chance?
If the medical staff thinks a mode of care won’t be effective, but the family still insists, some states have laws that could help the medical team. In Texas, for example, if the medical team thinks the care they’re giving isn’t helping the patient, and the patient is likely going to have a poor outcome, there’s a legal process that the team can go through, Dr. Gundersen said. But even these laws are seen as potentially problematic because of concerns that they put too much power in the hands of a hospital committee.
Dr. Gundersen strongly advised getting at the root causes of a family’s apprehension. They might not have been informed early enough about the dire nature of an illness to feel they can make a decision comfortably. They also may be receiving information in a piecemeal manner or information that is inconsistent. Another common fear expressed by families is a concern over abandonment by the medical team if a decision is made to forgo a certain treatment. Also, sometimes the goals of care might not be properly detailed and discussed, she said.
But better communication can help overcome these snags, Dr. Gundersen said.
She suggested that sometimes it’s helpful to clarify things with the family, for example, what do they mean by “Do everything”?
“Does it mean ‘I want you to do everything to prolong their life even if they suffer,’ or does it mean ‘I want you do to everything that’s reasonable to try to prolong their life but not at the risk of increased suffering,’ or anywhere in between. Really just having these clarifying conversations is helpful.”
She also emphasized the importance of talking about interests, such as not wanting a patient to suffer, instead of taking positions, such as flatly recommending the withdrawal of treatment.
“It’s easy for both sides to kind of dig in their heels and not communicate effectively,” Dr. Gundersen said.
‘Emotional torture’
There are times when, no matter how skillfully the medical team communicates, they stand at an impasse with the family.
“This is emotional torture for us,” Dr. Gundersen said. “It’s moral distress. We kind of dread these situations. In these cases, trying to support yourself and your team emotionally is the most important thing.”
Ami Doshi, MD, director of palliative care inpatient services at Rady Children’s Hospital in San Diego, described the case of a baby girl that touched on the especially painful issues that can arise in pediatric cases. The 2-month-old girl had been born after a pregnancy affected by polyhydramnios and had an abnormal neurological exam and brain MRI, as well as congenital abnormalities. She’d been intubated for respiratory failure and was now on high-flow nasal cannula therapy. The girl was intolerant to feeding and was put on a nasojejunal feeding tube and then a gastrostomy-jejunostomy tube.
But the baby’s vomiting continued, and she had bradycardia and hypoxia so severe she needed bag mask ventilation to recover. The mother started to feel like she was “torturing” the baby.
The family decided to stop respiratory support but to continue artificial nutrition and hydration, which Dr. Doshi said, has an elevated status in the human psyche. Mentioning discontinuing feeding is fraught with complexity, she said.
“The notion of feeding is such a basic instinct, especially with a baby, that tackling the notion of discontinuing any sort of feeds, orally or tube feeds, is fraught with emotion and angst at times,” Dr. Doshi said.
The girl had respiratory events but recovered from them on her own, but the vomiting and retching continued. Eventually the artificial nutrition and hydration was stopped. But after 5 days, the medical staff began feeling uncomfortable, Dr. Doshi said. “We’re starting to hear from nurses, doctors, other people, that something just doesn’t feel right about what’s happening: ‘She seems okay,’ and, ‘Is it really okay for us to be doing this?’ and ‘Gosh, this is taking a long time.’ ”
The medical staff had, in a sense, joined the family on the emotional roller coaster.
Dr. Doshi said it’s important to remember that there is no ethical or moral distinction between withdrawing a medical intervention and withholding one.
“Stopping an intervention once it has started is no different ethically or legally than not starting it in the first place,” she said.
According to Dr. Doshi, there is a general consensus among medical societies that artificial nutrition and hydration is a medical intervention just like any other and that it should be evaluated within the same framework: Is it overly burdensome? Are we doing harm? Is it consistent with the goal of care? In so doing, be sure to respect patient autonomy and obtain informed consent.
As with so much in medicine, careful communication is a must.
“Paint a picture of what the patient’s trajectory is going to look like with and without artificial nutrition and hydration. At the end of the day, having done all of that, we’re going to ultimately respect what the patient or the surrogate decision maker decides,” Dr. Doshi said.
After assessment the data and the chances of success, and still without clarity about how to proceed, a good option might be considering a “time-limited trial” in which the medical team sits with the family and agrees on a time frame for an intervention and chooses predetermined endpoints for assessing success or failure.
“This can be very powerful to help us understand whether it is beneficial, but also – from the family’s perspective – to know everything was tried,” Dr. Doshi said.
Hospitalists should emphasize what is being added to treatment so that families don’t think only of what is being taken away, she said.
“Usually we are adding a lot – symptom management, a lot of psychosocial support. So what are all the other ways that we’re going to continue to care for the patient, even when we are withdrawing or withholding a specific intervention?” Dr. Doshi noted.
Sometimes, the best healer of distress in the midst of end of life decision making is time itself, Dr. Gundersen said.
In a condolence call, she once spoke with a family member involved in an agonizing case in which the medical team and family were at odds. Yet the man told her: “I know that you all were telling us the entire time that this was going to happen, but I guess we just had to go through our own process.”
Understanding family perspective is an important factor
Understanding family perspective is an important factor
A Vietnam veteran steered clear of the health care system for years, then showed up at the hospital with pneumonia and respiratory failure. He was whisked to the intensive care unit, and cancerous masses were found.
The situation – as described by Jeffrey Frank, MD, director of quality and performance at Vituity, a physician group in Emeryville, Calif. – then got worse.
“No one was there for him,” Dr. Frank said. “He’s laying in the ICU, he does not have the capacity to make decisions, let alone communicate. So the care team needs guidance.”
Too often, hospitalists find themselves in confusing situations involving patients near the end of their lives, having to determine how to go about treating a patient or withholding treatment when patients are not in a position to announce their wishes. When family is present, the health care team thinks the most sensible course of treatment is at odds with what the family wants to be done.
At the Society of Hospital Medicine 2019 Annual Conference, hospitalists with palliative care training offered advice on how to go about handling these difficult situations, which can sometimes become more manageable with certain strategies.
For situations in which there is no designated representative to speak for a patient who is unresponsive – the so-called “unbefriended patient” or “unrepresented patient” – any source of information can be valuable. And health care providers should seek out this input, Dr. Frank said.
“When there is a visitor at the bedside, and as long as they know the person, and they can start giving the medical providers some information about what the patient would have wanted, most of us will talk with that person and that’s actually a good habit,” he said.
Thirty-nine states and the District of Columbia have regulations on whom health care providers should talk to when there is no obvious representative, Dr. Frank said, noting that most of these regulations follow a classic family-tree order. But in the discouraging results of many surveys of health care providers on the subject, most clinicians say that they do not know the regulations in their state, Dr. Frank said. But he said such results betray a silver lining because clinicians say that they would be inclined to use a family tree–style hierarchy in deciding with whom they should speak about end of life decisions.
Hospitalists should at least know whether their hospital has a policy on unrepresented patients, Dr. Frank said.
“That’s your road map on how to get through consenting this patient – what am I going to do with Mr. Smith?” he said. “You may ask yourself, ‘Do I just keep treating him and treating him?’ If you have a policy at your hospital, it will protect you from liability, as well as give you a sense of process.”
Conflicts in communication
An even worse situation, perhaps, is one that many hospitalists have seen: A patient is teetering at the edge of life, and a spouse arrives, along with two daughters from out of state who have not seen their father in a year, said Elizabeth Gundersen, MD, director of the ethics curriculum at Florida Atlantic University, Boca Raton.
“The family requests that the medical team do everything, including intubation and attempts at resuscitation if needed,” she said. “The family says he was fine prior to this admission. Another thing I hear a lot is, ‘He was even sicker than this last year, and he got better.’ ”
Meanwhile, “the medical team consensus is that he is not going to survive this illness,” Dr. Gundersen said.
The situation is so common and problematic that it has a name – the “Daughter from California Syndrome.” (According to medical literature says, it’s called the “Daughter from Chicago Syndrome” in California.)
“This is one of the most agonizing things that happens to us in medicine,” Dr. Gundersen said. “It affects us, it affects our nurses, it affects the entire medical team. It’s agonizing when we feel like treatment has somehow turned to torture.”
Dr. Gundersen said the medical staff should avoid using the word “futile,” or similar language, with families.
“Words matter,” she said. “Inappropriate language can inadvertently convey the feeling that, ‘They’re giving up on my dad – they think it’s hopeless.’ That can make families and the medical team dig in their heels further.”
Sometimes it can be hard to define the terms of decision making. Even if the family and the medical team can agree that no “nonbeneficial treatments” should be administered, Dr. Gundersen said, what exactly does that mean? Does it mean less than a 1% chance of working; less than a 5% chance?
If the medical staff thinks a mode of care won’t be effective, but the family still insists, some states have laws that could help the medical team. In Texas, for example, if the medical team thinks the care they’re giving isn’t helping the patient, and the patient is likely going to have a poor outcome, there’s a legal process that the team can go through, Dr. Gundersen said. But even these laws are seen as potentially problematic because of concerns that they put too much power in the hands of a hospital committee.
Dr. Gundersen strongly advised getting at the root causes of a family’s apprehension. They might not have been informed early enough about the dire nature of an illness to feel they can make a decision comfortably. They also may be receiving information in a piecemeal manner or information that is inconsistent. Another common fear expressed by families is a concern over abandonment by the medical team if a decision is made to forgo a certain treatment. Also, sometimes the goals of care might not be properly detailed and discussed, she said.
But better communication can help overcome these snags, Dr. Gundersen said.
She suggested that sometimes it’s helpful to clarify things with the family, for example, what do they mean by “Do everything”?
“Does it mean ‘I want you to do everything to prolong their life even if they suffer,’ or does it mean ‘I want you do to everything that’s reasonable to try to prolong their life but not at the risk of increased suffering,’ or anywhere in between. Really just having these clarifying conversations is helpful.”
She also emphasized the importance of talking about interests, such as not wanting a patient to suffer, instead of taking positions, such as flatly recommending the withdrawal of treatment.
“It’s easy for both sides to kind of dig in their heels and not communicate effectively,” Dr. Gundersen said.
‘Emotional torture’
There are times when, no matter how skillfully the medical team communicates, they stand at an impasse with the family.
“This is emotional torture for us,” Dr. Gundersen said. “It’s moral distress. We kind of dread these situations. In these cases, trying to support yourself and your team emotionally is the most important thing.”
Ami Doshi, MD, director of palliative care inpatient services at Rady Children’s Hospital in San Diego, described the case of a baby girl that touched on the especially painful issues that can arise in pediatric cases. The 2-month-old girl had been born after a pregnancy affected by polyhydramnios and had an abnormal neurological exam and brain MRI, as well as congenital abnormalities. She’d been intubated for respiratory failure and was now on high-flow nasal cannula therapy. The girl was intolerant to feeding and was put on a nasojejunal feeding tube and then a gastrostomy-jejunostomy tube.
But the baby’s vomiting continued, and she had bradycardia and hypoxia so severe she needed bag mask ventilation to recover. The mother started to feel like she was “torturing” the baby.
The family decided to stop respiratory support but to continue artificial nutrition and hydration, which Dr. Doshi said, has an elevated status in the human psyche. Mentioning discontinuing feeding is fraught with complexity, she said.
“The notion of feeding is such a basic instinct, especially with a baby, that tackling the notion of discontinuing any sort of feeds, orally or tube feeds, is fraught with emotion and angst at times,” Dr. Doshi said.
The girl had respiratory events but recovered from them on her own, but the vomiting and retching continued. Eventually the artificial nutrition and hydration was stopped. But after 5 days, the medical staff began feeling uncomfortable, Dr. Doshi said. “We’re starting to hear from nurses, doctors, other people, that something just doesn’t feel right about what’s happening: ‘She seems okay,’ and, ‘Is it really okay for us to be doing this?’ and ‘Gosh, this is taking a long time.’ ”
The medical staff had, in a sense, joined the family on the emotional roller coaster.
Dr. Doshi said it’s important to remember that there is no ethical or moral distinction between withdrawing a medical intervention and withholding one.
“Stopping an intervention once it has started is no different ethically or legally than not starting it in the first place,” she said.
According to Dr. Doshi, there is a general consensus among medical societies that artificial nutrition and hydration is a medical intervention just like any other and that it should be evaluated within the same framework: Is it overly burdensome? Are we doing harm? Is it consistent with the goal of care? In so doing, be sure to respect patient autonomy and obtain informed consent.
As with so much in medicine, careful communication is a must.
“Paint a picture of what the patient’s trajectory is going to look like with and without artificial nutrition and hydration. At the end of the day, having done all of that, we’re going to ultimately respect what the patient or the surrogate decision maker decides,” Dr. Doshi said.
After assessment the data and the chances of success, and still without clarity about how to proceed, a good option might be considering a “time-limited trial” in which the medical team sits with the family and agrees on a time frame for an intervention and chooses predetermined endpoints for assessing success or failure.
“This can be very powerful to help us understand whether it is beneficial, but also – from the family’s perspective – to know everything was tried,” Dr. Doshi said.
Hospitalists should emphasize what is being added to treatment so that families don’t think only of what is being taken away, she said.
“Usually we are adding a lot – symptom management, a lot of psychosocial support. So what are all the other ways that we’re going to continue to care for the patient, even when we are withdrawing or withholding a specific intervention?” Dr. Doshi noted.
Sometimes, the best healer of distress in the midst of end of life decision making is time itself, Dr. Gundersen said.
In a condolence call, she once spoke with a family member involved in an agonizing case in which the medical team and family were at odds. Yet the man told her: “I know that you all were telling us the entire time that this was going to happen, but I guess we just had to go through our own process.”
A Vietnam veteran steered clear of the health care system for years, then showed up at the hospital with pneumonia and respiratory failure. He was whisked to the intensive care unit, and cancerous masses were found.
The situation – as described by Jeffrey Frank, MD, director of quality and performance at Vituity, a physician group in Emeryville, Calif. – then got worse.
“No one was there for him,” Dr. Frank said. “He’s laying in the ICU, he does not have the capacity to make decisions, let alone communicate. So the care team needs guidance.”
Too often, hospitalists find themselves in confusing situations involving patients near the end of their lives, having to determine how to go about treating a patient or withholding treatment when patients are not in a position to announce their wishes. When family is present, the health care team thinks the most sensible course of treatment is at odds with what the family wants to be done.
At the Society of Hospital Medicine 2019 Annual Conference, hospitalists with palliative care training offered advice on how to go about handling these difficult situations, which can sometimes become more manageable with certain strategies.
For situations in which there is no designated representative to speak for a patient who is unresponsive – the so-called “unbefriended patient” or “unrepresented patient” – any source of information can be valuable. And health care providers should seek out this input, Dr. Frank said.
“When there is a visitor at the bedside, and as long as they know the person, and they can start giving the medical providers some information about what the patient would have wanted, most of us will talk with that person and that’s actually a good habit,” he said.
Thirty-nine states and the District of Columbia have regulations on whom health care providers should talk to when there is no obvious representative, Dr. Frank said, noting that most of these regulations follow a classic family-tree order. But in the discouraging results of many surveys of health care providers on the subject, most clinicians say that they do not know the regulations in their state, Dr. Frank said. But he said such results betray a silver lining because clinicians say that they would be inclined to use a family tree–style hierarchy in deciding with whom they should speak about end of life decisions.
Hospitalists should at least know whether their hospital has a policy on unrepresented patients, Dr. Frank said.
“That’s your road map on how to get through consenting this patient – what am I going to do with Mr. Smith?” he said. “You may ask yourself, ‘Do I just keep treating him and treating him?’ If you have a policy at your hospital, it will protect you from liability, as well as give you a sense of process.”
Conflicts in communication
An even worse situation, perhaps, is one that many hospitalists have seen: A patient is teetering at the edge of life, and a spouse arrives, along with two daughters from out of state who have not seen their father in a year, said Elizabeth Gundersen, MD, director of the ethics curriculum at Florida Atlantic University, Boca Raton.
“The family requests that the medical team do everything, including intubation and attempts at resuscitation if needed,” she said. “The family says he was fine prior to this admission. Another thing I hear a lot is, ‘He was even sicker than this last year, and he got better.’ ”
Meanwhile, “the medical team consensus is that he is not going to survive this illness,” Dr. Gundersen said.
The situation is so common and problematic that it has a name – the “Daughter from California Syndrome.” (According to medical literature says, it’s called the “Daughter from Chicago Syndrome” in California.)
“This is one of the most agonizing things that happens to us in medicine,” Dr. Gundersen said. “It affects us, it affects our nurses, it affects the entire medical team. It’s agonizing when we feel like treatment has somehow turned to torture.”
Dr. Gundersen said the medical staff should avoid using the word “futile,” or similar language, with families.
“Words matter,” she said. “Inappropriate language can inadvertently convey the feeling that, ‘They’re giving up on my dad – they think it’s hopeless.’ That can make families and the medical team dig in their heels further.”
Sometimes it can be hard to define the terms of decision making. Even if the family and the medical team can agree that no “nonbeneficial treatments” should be administered, Dr. Gundersen said, what exactly does that mean? Does it mean less than a 1% chance of working; less than a 5% chance?
If the medical staff thinks a mode of care won’t be effective, but the family still insists, some states have laws that could help the medical team. In Texas, for example, if the medical team thinks the care they’re giving isn’t helping the patient, and the patient is likely going to have a poor outcome, there’s a legal process that the team can go through, Dr. Gundersen said. But even these laws are seen as potentially problematic because of concerns that they put too much power in the hands of a hospital committee.
Dr. Gundersen strongly advised getting at the root causes of a family’s apprehension. They might not have been informed early enough about the dire nature of an illness to feel they can make a decision comfortably. They also may be receiving information in a piecemeal manner or information that is inconsistent. Another common fear expressed by families is a concern over abandonment by the medical team if a decision is made to forgo a certain treatment. Also, sometimes the goals of care might not be properly detailed and discussed, she said.
But better communication can help overcome these snags, Dr. Gundersen said.
She suggested that sometimes it’s helpful to clarify things with the family, for example, what do they mean by “Do everything”?
“Does it mean ‘I want you to do everything to prolong their life even if they suffer,’ or does it mean ‘I want you do to everything that’s reasonable to try to prolong their life but not at the risk of increased suffering,’ or anywhere in between. Really just having these clarifying conversations is helpful.”
She also emphasized the importance of talking about interests, such as not wanting a patient to suffer, instead of taking positions, such as flatly recommending the withdrawal of treatment.
“It’s easy for both sides to kind of dig in their heels and not communicate effectively,” Dr. Gundersen said.
‘Emotional torture’
There are times when, no matter how skillfully the medical team communicates, they stand at an impasse with the family.
“This is emotional torture for us,” Dr. Gundersen said. “It’s moral distress. We kind of dread these situations. In these cases, trying to support yourself and your team emotionally is the most important thing.”
Ami Doshi, MD, director of palliative care inpatient services at Rady Children’s Hospital in San Diego, described the case of a baby girl that touched on the especially painful issues that can arise in pediatric cases. The 2-month-old girl had been born after a pregnancy affected by polyhydramnios and had an abnormal neurological exam and brain MRI, as well as congenital abnormalities. She’d been intubated for respiratory failure and was now on high-flow nasal cannula therapy. The girl was intolerant to feeding and was put on a nasojejunal feeding tube and then a gastrostomy-jejunostomy tube.
But the baby’s vomiting continued, and she had bradycardia and hypoxia so severe she needed bag mask ventilation to recover. The mother started to feel like she was “torturing” the baby.
The family decided to stop respiratory support but to continue artificial nutrition and hydration, which Dr. Doshi said, has an elevated status in the human psyche. Mentioning discontinuing feeding is fraught with complexity, she said.
“The notion of feeding is such a basic instinct, especially with a baby, that tackling the notion of discontinuing any sort of feeds, orally or tube feeds, is fraught with emotion and angst at times,” Dr. Doshi said.
The girl had respiratory events but recovered from them on her own, but the vomiting and retching continued. Eventually the artificial nutrition and hydration was stopped. But after 5 days, the medical staff began feeling uncomfortable, Dr. Doshi said. “We’re starting to hear from nurses, doctors, other people, that something just doesn’t feel right about what’s happening: ‘She seems okay,’ and, ‘Is it really okay for us to be doing this?’ and ‘Gosh, this is taking a long time.’ ”
The medical staff had, in a sense, joined the family on the emotional roller coaster.
Dr. Doshi said it’s important to remember that there is no ethical or moral distinction between withdrawing a medical intervention and withholding one.
“Stopping an intervention once it has started is no different ethically or legally than not starting it in the first place,” she said.
According to Dr. Doshi, there is a general consensus among medical societies that artificial nutrition and hydration is a medical intervention just like any other and that it should be evaluated within the same framework: Is it overly burdensome? Are we doing harm? Is it consistent with the goal of care? In so doing, be sure to respect patient autonomy and obtain informed consent.
As with so much in medicine, careful communication is a must.
“Paint a picture of what the patient’s trajectory is going to look like with and without artificial nutrition and hydration. At the end of the day, having done all of that, we’re going to ultimately respect what the patient or the surrogate decision maker decides,” Dr. Doshi said.
After assessment the data and the chances of success, and still without clarity about how to proceed, a good option might be considering a “time-limited trial” in which the medical team sits with the family and agrees on a time frame for an intervention and chooses predetermined endpoints for assessing success or failure.
“This can be very powerful to help us understand whether it is beneficial, but also – from the family’s perspective – to know everything was tried,” Dr. Doshi said.
Hospitalists should emphasize what is being added to treatment so that families don’t think only of what is being taken away, she said.
“Usually we are adding a lot – symptom management, a lot of psychosocial support. So what are all the other ways that we’re going to continue to care for the patient, even when we are withdrawing or withholding a specific intervention?” Dr. Doshi noted.
Sometimes, the best healer of distress in the midst of end of life decision making is time itself, Dr. Gundersen said.
In a condolence call, she once spoke with a family member involved in an agonizing case in which the medical team and family were at odds. Yet the man told her: “I know that you all were telling us the entire time that this was going to happen, but I guess we just had to go through our own process.”
Mole on back
The FP used a dermatoscope to get a better look at the lesion and recognized this as a halo nevus, which is characterized by a central nevus with a halo of depigmentation around it (arrow) and sometimes depigmentation within the nevus itself.
The halo is caused when, occasionally, the body develops an immune reaction to a nevus and its melanocytes. As cytotoxic T cells target those melanocytes, there is loss of pigment to the tissue surrounding the nevus.1
The appearance of the central nevus, rather than the hypopigmentation, determines management strategies. A globular or homogeneous pattern seen on dermoscopy is typically indicative of a benign lesion.2 An atypical pigment network or other melanoma specific structures should raise your suspicions for melanoma or atypical nevus and prompt a deep shave excision sent for pathology to rule out melanoma. A nevus without suspicious features, other than the surrounding hypopigmentation, can be managed conservatively with self-monitoring and re-evaluation.
In this case, the lesion displayed a homogeneous pattern, and the FP advised the patient to self-monitor.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque, NM.
1. Bayer-Garner IB, Ivan D, Schwartz MR, et al. The immunopathology of regression in benign lichenoid keratosis, keratoacanthoma and halo nevus. Clin Med Res. 2004;2:89-97.
2. Porto AC, Blumetti TP, de Paula Ramos Castro R, et al. Recurrent halo nevus: dermoscopy and confocal microscopy features. JAAD Case Rep. 2017;3:256-258.
The FP used a dermatoscope to get a better look at the lesion and recognized this as a halo nevus, which is characterized by a central nevus with a halo of depigmentation around it (arrow) and sometimes depigmentation within the nevus itself.
The halo is caused when, occasionally, the body develops an immune reaction to a nevus and its melanocytes. As cytotoxic T cells target those melanocytes, there is loss of pigment to the tissue surrounding the nevus.1
The appearance of the central nevus, rather than the hypopigmentation, determines management strategies. A globular or homogeneous pattern seen on dermoscopy is typically indicative of a benign lesion.2 An atypical pigment network or other melanoma specific structures should raise your suspicions for melanoma or atypical nevus and prompt a deep shave excision sent for pathology to rule out melanoma. A nevus without suspicious features, other than the surrounding hypopigmentation, can be managed conservatively with self-monitoring and re-evaluation.
In this case, the lesion displayed a homogeneous pattern, and the FP advised the patient to self-monitor.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque, NM.
The FP used a dermatoscope to get a better look at the lesion and recognized this as a halo nevus, which is characterized by a central nevus with a halo of depigmentation around it (arrow) and sometimes depigmentation within the nevus itself.
The halo is caused when, occasionally, the body develops an immune reaction to a nevus and its melanocytes. As cytotoxic T cells target those melanocytes, there is loss of pigment to the tissue surrounding the nevus.1
The appearance of the central nevus, rather than the hypopigmentation, determines management strategies. A globular or homogeneous pattern seen on dermoscopy is typically indicative of a benign lesion.2 An atypical pigment network or other melanoma specific structures should raise your suspicions for melanoma or atypical nevus and prompt a deep shave excision sent for pathology to rule out melanoma. A nevus without suspicious features, other than the surrounding hypopigmentation, can be managed conservatively with self-monitoring and re-evaluation.
In this case, the lesion displayed a homogeneous pattern, and the FP advised the patient to self-monitor.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque, NM.
1. Bayer-Garner IB, Ivan D, Schwartz MR, et al. The immunopathology of regression in benign lichenoid keratosis, keratoacanthoma and halo nevus. Clin Med Res. 2004;2:89-97.
2. Porto AC, Blumetti TP, de Paula Ramos Castro R, et al. Recurrent halo nevus: dermoscopy and confocal microscopy features. JAAD Case Rep. 2017;3:256-258.
1. Bayer-Garner IB, Ivan D, Schwartz MR, et al. The immunopathology of regression in benign lichenoid keratosis, keratoacanthoma and halo nevus. Clin Med Res. 2004;2:89-97.
2. Porto AC, Blumetti TP, de Paula Ramos Castro R, et al. Recurrent halo nevus: dermoscopy and confocal microscopy features. JAAD Case Rep. 2017;3:256-258.
More phase 3 ubrogepant data published as FDA decision nears
published Dec. 4 in the New England Journal of Medicine. In addition, about 38% of patients who receive ubrogepant no longer have their most bothersome migraine-associated symptom, such as photophobia, phonophobia, or nausea, at 2 hours, compared with 28% of patients who receive placebo, said David W. Dodick, MD, and colleagues.
Dr. Dodick, professor of neurology at the Mayo Clinic in Phoenix, and his coauthors described efficacy and safety results from the ACHIEVE I trial. Another phase 3 study of ubrogepant, ACHIEVE II, was published in JAMA in November. That trial evaluated 25- and 50-mg doses of ubrogepant versus placebo and found rates of pain freedom and absence of the most bothersome symptom in the placebo and active treatment arms that were similar to those in ACHIEVE I.
Assessing a gepant for acute migraine treatment
Ubrogepant is an oral calcitonin gene–related peptide (CGRP) receptor antagonist. Allergan, the company developing the drug, has said it expects the Food and Drug Administration to decide in December whether to approve the drug.
To compare ubrogepant 50 mg, ubrogepant 100 mg, and placebo for the acute treatment of migraine, investigators conducted the randomized ACHIEVE I trial. Researchers enrolled 1,672 adults with migraine with or without aura. They excluded patients with clinically significant cardiovascular or cerebrovascular disease. During the trial, patients treated a single migraine attack, and they had the option to take a second dose. In all, 1,436 participants took an initial dose. Patients had an average age of 40.5 years, about 88% were women, and 82% were white.
In ACHIEVE I, the most common adverse events within 48 hours of treatment were nausea, somnolence, and dry mouth, and these events occurred more frequently in the 100-mg–dose group, Dr. Dodick and colleagues reported. Among patients who received ubrogepant, serious adverse events more than 48 hours after treatment but within 30 days of treatment included appendicitis, spontaneous abortion, pericardial effusion, and seizure. No serious adverse events occurred in the placebo group.
The authors noted that, “there was no active comparator and no evaluation of consistency of effect across multiple migraine attacks; therefore it is not possible to determine whether the drug is more or less effective than standard therapies or consistently effective with repeated use.” In addition, “safety and side-effect data from this trial were based on evaluation of a single attack, and therefore safety after repeated use cannot be inferred; an extension trial has assessed the long-term safety of ubrogepant,” they said.
The present trial was performed well, commented Alan M. Rapoport, MD. “The coprimary endpoints of pain freedom and most bothersome symptom freedom, both at 2 hours after dosing, were statistically superior for both doses of ubrogepant versus placebo,” he said. “Some of the secondary endpoints, such as pain relief at 2 hours post dose and sustained pain relief from 2 to 24 hours, were statistically better than placebo.”
“Based on this data, I suspect that the FDA would approve this gepant after appropriate safety data,” said Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles and editor-in-chief of Neurology Reviews. “Many more patients need to take this drug before we can be sure it is safe and effective.”
The CGRP therapeutic landscape
“Other gepants have been shown to be effective, although some have caused a degree of liver toxicity,” said Dr. Rapoport. “Blocking the effect of CGRP on the migraine peripheral nervous system, in this case by preventing the ligand from docking at its receptor by administering an oral CGRP receptor blocker, appears to be effective.” Researchers are studying another oral gepant for similar approval, he added.
Ubrogepant stands to join other treatments targeting CGRP.
“There are currently three, and soon to be four, injectable monoclonal antibodies against CGRP functionality, which are preventive, not acute-care drugs,” Dr. Rapoport said. “The first released was a subcutaneous injection of a CGRP receptor blocker, and the other two are subcutaneous injections of CGRP ligand blockers. The last drug will be an intravenous infusion of a ligand blocker. These recently approved migraine treatments have greatly improved the lives of many of our patients, even when other preventives have failed. I expect ubrogepant and other gepants will do the same for the acute care of migraine.”
Allergan funded the trials of ubrogepant, and some of the authors are Allergan employees and stockholders. Dr. Dodick reported consulting fees and advisory board fees from Allergan and various pharmaceutical companies.
SOURCE: Dodick DW et al. N Engl J Med. 2019;381(23):2230-41. doi: 10.1056/NEJMoa1813049.
published Dec. 4 in the New England Journal of Medicine. In addition, about 38% of patients who receive ubrogepant no longer have their most bothersome migraine-associated symptom, such as photophobia, phonophobia, or nausea, at 2 hours, compared with 28% of patients who receive placebo, said David W. Dodick, MD, and colleagues.
Dr. Dodick, professor of neurology at the Mayo Clinic in Phoenix, and his coauthors described efficacy and safety results from the ACHIEVE I trial. Another phase 3 study of ubrogepant, ACHIEVE II, was published in JAMA in November. That trial evaluated 25- and 50-mg doses of ubrogepant versus placebo and found rates of pain freedom and absence of the most bothersome symptom in the placebo and active treatment arms that were similar to those in ACHIEVE I.
Assessing a gepant for acute migraine treatment
Ubrogepant is an oral calcitonin gene–related peptide (CGRP) receptor antagonist. Allergan, the company developing the drug, has said it expects the Food and Drug Administration to decide in December whether to approve the drug.
To compare ubrogepant 50 mg, ubrogepant 100 mg, and placebo for the acute treatment of migraine, investigators conducted the randomized ACHIEVE I trial. Researchers enrolled 1,672 adults with migraine with or without aura. They excluded patients with clinically significant cardiovascular or cerebrovascular disease. During the trial, patients treated a single migraine attack, and they had the option to take a second dose. In all, 1,436 participants took an initial dose. Patients had an average age of 40.5 years, about 88% were women, and 82% were white.
In ACHIEVE I, the most common adverse events within 48 hours of treatment were nausea, somnolence, and dry mouth, and these events occurred more frequently in the 100-mg–dose group, Dr. Dodick and colleagues reported. Among patients who received ubrogepant, serious adverse events more than 48 hours after treatment but within 30 days of treatment included appendicitis, spontaneous abortion, pericardial effusion, and seizure. No serious adverse events occurred in the placebo group.
The authors noted that, “there was no active comparator and no evaluation of consistency of effect across multiple migraine attacks; therefore it is not possible to determine whether the drug is more or less effective than standard therapies or consistently effective with repeated use.” In addition, “safety and side-effect data from this trial were based on evaluation of a single attack, and therefore safety after repeated use cannot be inferred; an extension trial has assessed the long-term safety of ubrogepant,” they said.
The present trial was performed well, commented Alan M. Rapoport, MD. “The coprimary endpoints of pain freedom and most bothersome symptom freedom, both at 2 hours after dosing, were statistically superior for both doses of ubrogepant versus placebo,” he said. “Some of the secondary endpoints, such as pain relief at 2 hours post dose and sustained pain relief from 2 to 24 hours, were statistically better than placebo.”
“Based on this data, I suspect that the FDA would approve this gepant after appropriate safety data,” said Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles and editor-in-chief of Neurology Reviews. “Many more patients need to take this drug before we can be sure it is safe and effective.”
The CGRP therapeutic landscape
“Other gepants have been shown to be effective, although some have caused a degree of liver toxicity,” said Dr. Rapoport. “Blocking the effect of CGRP on the migraine peripheral nervous system, in this case by preventing the ligand from docking at its receptor by administering an oral CGRP receptor blocker, appears to be effective.” Researchers are studying another oral gepant for similar approval, he added.
Ubrogepant stands to join other treatments targeting CGRP.
“There are currently three, and soon to be four, injectable monoclonal antibodies against CGRP functionality, which are preventive, not acute-care drugs,” Dr. Rapoport said. “The first released was a subcutaneous injection of a CGRP receptor blocker, and the other two are subcutaneous injections of CGRP ligand blockers. The last drug will be an intravenous infusion of a ligand blocker. These recently approved migraine treatments have greatly improved the lives of many of our patients, even when other preventives have failed. I expect ubrogepant and other gepants will do the same for the acute care of migraine.”
Allergan funded the trials of ubrogepant, and some of the authors are Allergan employees and stockholders. Dr. Dodick reported consulting fees and advisory board fees from Allergan and various pharmaceutical companies.
SOURCE: Dodick DW et al. N Engl J Med. 2019;381(23):2230-41. doi: 10.1056/NEJMoa1813049.
published Dec. 4 in the New England Journal of Medicine. In addition, about 38% of patients who receive ubrogepant no longer have their most bothersome migraine-associated symptom, such as photophobia, phonophobia, or nausea, at 2 hours, compared with 28% of patients who receive placebo, said David W. Dodick, MD, and colleagues.
Dr. Dodick, professor of neurology at the Mayo Clinic in Phoenix, and his coauthors described efficacy and safety results from the ACHIEVE I trial. Another phase 3 study of ubrogepant, ACHIEVE II, was published in JAMA in November. That trial evaluated 25- and 50-mg doses of ubrogepant versus placebo and found rates of pain freedom and absence of the most bothersome symptom in the placebo and active treatment arms that were similar to those in ACHIEVE I.
Assessing a gepant for acute migraine treatment
Ubrogepant is an oral calcitonin gene–related peptide (CGRP) receptor antagonist. Allergan, the company developing the drug, has said it expects the Food and Drug Administration to decide in December whether to approve the drug.
To compare ubrogepant 50 mg, ubrogepant 100 mg, and placebo for the acute treatment of migraine, investigators conducted the randomized ACHIEVE I trial. Researchers enrolled 1,672 adults with migraine with or without aura. They excluded patients with clinically significant cardiovascular or cerebrovascular disease. During the trial, patients treated a single migraine attack, and they had the option to take a second dose. In all, 1,436 participants took an initial dose. Patients had an average age of 40.5 years, about 88% were women, and 82% were white.
In ACHIEVE I, the most common adverse events within 48 hours of treatment were nausea, somnolence, and dry mouth, and these events occurred more frequently in the 100-mg–dose group, Dr. Dodick and colleagues reported. Among patients who received ubrogepant, serious adverse events more than 48 hours after treatment but within 30 days of treatment included appendicitis, spontaneous abortion, pericardial effusion, and seizure. No serious adverse events occurred in the placebo group.
The authors noted that, “there was no active comparator and no evaluation of consistency of effect across multiple migraine attacks; therefore it is not possible to determine whether the drug is more or less effective than standard therapies or consistently effective with repeated use.” In addition, “safety and side-effect data from this trial were based on evaluation of a single attack, and therefore safety after repeated use cannot be inferred; an extension trial has assessed the long-term safety of ubrogepant,” they said.
The present trial was performed well, commented Alan M. Rapoport, MD. “The coprimary endpoints of pain freedom and most bothersome symptom freedom, both at 2 hours after dosing, were statistically superior for both doses of ubrogepant versus placebo,” he said. “Some of the secondary endpoints, such as pain relief at 2 hours post dose and sustained pain relief from 2 to 24 hours, were statistically better than placebo.”
“Based on this data, I suspect that the FDA would approve this gepant after appropriate safety data,” said Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles and editor-in-chief of Neurology Reviews. “Many more patients need to take this drug before we can be sure it is safe and effective.”
The CGRP therapeutic landscape
“Other gepants have been shown to be effective, although some have caused a degree of liver toxicity,” said Dr. Rapoport. “Blocking the effect of CGRP on the migraine peripheral nervous system, in this case by preventing the ligand from docking at its receptor by administering an oral CGRP receptor blocker, appears to be effective.” Researchers are studying another oral gepant for similar approval, he added.
Ubrogepant stands to join other treatments targeting CGRP.
“There are currently three, and soon to be four, injectable monoclonal antibodies against CGRP functionality, which are preventive, not acute-care drugs,” Dr. Rapoport said. “The first released was a subcutaneous injection of a CGRP receptor blocker, and the other two are subcutaneous injections of CGRP ligand blockers. The last drug will be an intravenous infusion of a ligand blocker. These recently approved migraine treatments have greatly improved the lives of many of our patients, even when other preventives have failed. I expect ubrogepant and other gepants will do the same for the acute care of migraine.”
Allergan funded the trials of ubrogepant, and some of the authors are Allergan employees and stockholders. Dr. Dodick reported consulting fees and advisory board fees from Allergan and various pharmaceutical companies.
SOURCE: Dodick DW et al. N Engl J Med. 2019;381(23):2230-41. doi: 10.1056/NEJMoa1813049.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Compared with placebo, ubrogepant tablets result in higher rates of pain freedom and freedom from the most bothersome migraine-associated symptom at 2 hours following treatment.
Major finding: About 20% of patients who receive tablets containing 50 mg or 100 mg of ubrogepant for the acute treatment of migraine are pain free 2 hours later, compared with 12% of patients who receive placebo. In addition, about 38% of patients who receive ubrogepant no longer have their most bothersome migraine-associated symptom, such as photophobia, phonophobia, or nausea, at 2 hours, compared with 28% of patients who receive placebo.
Study details: A randomized trial that enrolled 1,672 adults with migraine with or without aura. Participants treated a single migraine attack.
Disclosures: Allergan funded the trial, and some of the authors are Allergan employees and stockholders. Dr. Dodick reported consulting fees and advisory board fees from Allergan and various pharmaceutical companies.
Source: Dodick DW et al. N Engl J Med. 2019;381(23):2230-41. doi: 10.1056/NEJMoa1813049.
Childproofing your protocols, and what’s the deal with airline water?
Childproof your study protocols
Childproof caps on medicine bottles. Like a bank vault lock to third-rate thieves, they’re an impregnable line of defense between inquisitive little hands and heavy-duty pharmaceuticals.
So, you can imagine the delight with which parents learned that their children’s education at an Illinois elementary school included a test of their ability to spring drugs from their fortress-like bottling.
In a quest to advance scientific understanding, a junior high student recruited some of the school’s kindergartners and first graders as participants in a science fair experiment. The hypothesis: “Childproof” medicine bottles were anything but.
The junior high scientist collected data on how quickly the study subjects could defeat assorted bottles. And, being a kindly soul, the adolescent researcher demonstrated techniques for achieving a state of, ahem, pharmaceutical openness.
While teachers were present during the experiment, parental permission slips were not. As any principal or institutional review board knows, informed consent is kind of a big deal.
Surprised parents learned of the experiment only when their kids deftly demonstrated their newfound pill skills. One shocked parent even shared a video of his daughter defeating a childproof lid.
Pharmacies, of course, would remind us all that their bottles are at best “child resistant,” not childproof. It’s a distinction lost on us here at the Bureau of LOTME, however. We’re desperately viewing that how-to video for tips on liberating our statins from the “child resistant” bottle our millennial pharmacist clearly superglued the cap onto.
What’s the deal with airline food?
You probably thought Jerry Seinfeld’s immortal query was rhetorical. No one actually knows what the deal is with airline food, right?
Enter Charles Platkin, PhD, JD, MPH, executive director of the Hunter College NYC Food Policy Center, and editor of DietDetective.com. Not only does he know the deal, he publishes a study detailing the healthiness of the food offered by 11 airlines every year, based on criteria such as healthy nutrients and calorie levels of meals, level of transparency, improvement and maintenance of healthy offerings, and water health.
For the 2018-2019 edition, there’s a tie at the top between Alaska Airlines and Air Canada, both scoring 4 out of 5 on Dr. Platkin’s “Health Score,” both well ahead of Delta and JetBlue in second place with a score of 2.9.
At the bottom? Southwest Airlines, with a paltry 1.7, edging out Spirit and Hawaiian Airlines.
Our personal favorite tidbit from the study has to be the poor scores in water health. Spirit managed to score a measly 1 out of 5 in the water health subscore, and several airlines were below 2. An actual quote from the study: “The quality of drinking water varies by airline, and many have provided passengers with unhealthy water. In general, it’s probably best to avoid drinking coffee and tea on board since they are made with galley water.”
While Hawaiian can take some comfort because they didn’t finish dead last, Dr. Platkin did award them his special “Shame on You” award for not providing all their nutritional information. You can try to hide, Hawaiian Airlines, but Dr. Platkin will find you.
Use the Force, Jack Nicklaus
We here at LOTME usually strive to provide an insightful and up-to-date wrap-up of the latest news on cutting-edge research, clinical breakthroughs, and impolite bodily functions. But right now, we want to talk about something really important: your golf game.
There’s an old saying in golf: Drive for show, putt for dough. That cliché may need a bit of updating, though, now that investigators in Ireland (where they are pretty serious about their golf) have brought science to the party.
Their research indicates that it may be possible for golfers to improve their putting without practicing. Without physically practicing, that is. Without going to the golf course. Just think about it: Golfers can putt better by, you know … just thinking about it.
Imagining the feel of an action without actually performing it is known as kinesthetic imagery ability, and it just might have an effect on putting, researchers from the physical education and sports sciences department at the University of Limerick and Lero, the Science Foundation Ireland Research Centre for Software, reported in Psychology of Sport and Exercise.
In a group of 44 skilled golfers, half watched video of an expert golfer putting in a lab environment “while listening to a motor imagery script consisting of short sentences describing key visual and kinesthetic feelings associated with performing the putting.” Then all the golfers took 40 putts from 15 feet in the lab.
The good kinesthetic imagers in the bunch improved their putting consistency more than subjects with poorer kinesthetic imagery ability and subjects from the nonintervention group.
So, here’s the new and improved old saying for golfers: Drive for show, use your kinesthetic imagery ability for dough. Yup, that’ll definitely catch on.

Childproof your study protocols
Childproof caps on medicine bottles. Like a bank vault lock to third-rate thieves, they’re an impregnable line of defense between inquisitive little hands and heavy-duty pharmaceuticals.
So, you can imagine the delight with which parents learned that their children’s education at an Illinois elementary school included a test of their ability to spring drugs from their fortress-like bottling.
In a quest to advance scientific understanding, a junior high student recruited some of the school’s kindergartners and first graders as participants in a science fair experiment. The hypothesis: “Childproof” medicine bottles were anything but.
The junior high scientist collected data on how quickly the study subjects could defeat assorted bottles. And, being a kindly soul, the adolescent researcher demonstrated techniques for achieving a state of, ahem, pharmaceutical openness.
While teachers were present during the experiment, parental permission slips were not. As any principal or institutional review board knows, informed consent is kind of a big deal.
Surprised parents learned of the experiment only when their kids deftly demonstrated their newfound pill skills. One shocked parent even shared a video of his daughter defeating a childproof lid.
Pharmacies, of course, would remind us all that their bottles are at best “child resistant,” not childproof. It’s a distinction lost on us here at the Bureau of LOTME, however. We’re desperately viewing that how-to video for tips on liberating our statins from the “child resistant” bottle our millennial pharmacist clearly superglued the cap onto.
What’s the deal with airline food?
You probably thought Jerry Seinfeld’s immortal query was rhetorical. No one actually knows what the deal is with airline food, right?
Enter Charles Platkin, PhD, JD, MPH, executive director of the Hunter College NYC Food Policy Center, and editor of DietDetective.com. Not only does he know the deal, he publishes a study detailing the healthiness of the food offered by 11 airlines every year, based on criteria such as healthy nutrients and calorie levels of meals, level of transparency, improvement and maintenance of healthy offerings, and water health.
For the 2018-2019 edition, there’s a tie at the top between Alaska Airlines and Air Canada, both scoring 4 out of 5 on Dr. Platkin’s “Health Score,” both well ahead of Delta and JetBlue in second place with a score of 2.9.
At the bottom? Southwest Airlines, with a paltry 1.7, edging out Spirit and Hawaiian Airlines.
Our personal favorite tidbit from the study has to be the poor scores in water health. Spirit managed to score a measly 1 out of 5 in the water health subscore, and several airlines were below 2. An actual quote from the study: “The quality of drinking water varies by airline, and many have provided passengers with unhealthy water. In general, it’s probably best to avoid drinking coffee and tea on board since they are made with galley water.”
While Hawaiian can take some comfort because they didn’t finish dead last, Dr. Platkin did award them his special “Shame on You” award for not providing all their nutritional information. You can try to hide, Hawaiian Airlines, but Dr. Platkin will find you.
Use the Force, Jack Nicklaus
We here at LOTME usually strive to provide an insightful and up-to-date wrap-up of the latest news on cutting-edge research, clinical breakthroughs, and impolite bodily functions. But right now, we want to talk about something really important: your golf game.
There’s an old saying in golf: Drive for show, putt for dough. That cliché may need a bit of updating, though, now that investigators in Ireland (where they are pretty serious about their golf) have brought science to the party.
Their research indicates that it may be possible for golfers to improve their putting without practicing. Without physically practicing, that is. Without going to the golf course. Just think about it: Golfers can putt better by, you know … just thinking about it.
Imagining the feel of an action without actually performing it is known as kinesthetic imagery ability, and it just might have an effect on putting, researchers from the physical education and sports sciences department at the University of Limerick and Lero, the Science Foundation Ireland Research Centre for Software, reported in Psychology of Sport and Exercise.
In a group of 44 skilled golfers, half watched video of an expert golfer putting in a lab environment “while listening to a motor imagery script consisting of short sentences describing key visual and kinesthetic feelings associated with performing the putting.” Then all the golfers took 40 putts from 15 feet in the lab.
The good kinesthetic imagers in the bunch improved their putting consistency more than subjects with poorer kinesthetic imagery ability and subjects from the nonintervention group.
So, here’s the new and improved old saying for golfers: Drive for show, use your kinesthetic imagery ability for dough. Yup, that’ll definitely catch on.

Childproof your study protocols
Childproof caps on medicine bottles. Like a bank vault lock to third-rate thieves, they’re an impregnable line of defense between inquisitive little hands and heavy-duty pharmaceuticals.
So, you can imagine the delight with which parents learned that their children’s education at an Illinois elementary school included a test of their ability to spring drugs from their fortress-like bottling.
In a quest to advance scientific understanding, a junior high student recruited some of the school’s kindergartners and first graders as participants in a science fair experiment. The hypothesis: “Childproof” medicine bottles were anything but.
The junior high scientist collected data on how quickly the study subjects could defeat assorted bottles. And, being a kindly soul, the adolescent researcher demonstrated techniques for achieving a state of, ahem, pharmaceutical openness.
While teachers were present during the experiment, parental permission slips were not. As any principal or institutional review board knows, informed consent is kind of a big deal.
Surprised parents learned of the experiment only when their kids deftly demonstrated their newfound pill skills. One shocked parent even shared a video of his daughter defeating a childproof lid.
Pharmacies, of course, would remind us all that their bottles are at best “child resistant,” not childproof. It’s a distinction lost on us here at the Bureau of LOTME, however. We’re desperately viewing that how-to video for tips on liberating our statins from the “child resistant” bottle our millennial pharmacist clearly superglued the cap onto.
What’s the deal with airline food?
You probably thought Jerry Seinfeld’s immortal query was rhetorical. No one actually knows what the deal is with airline food, right?
Enter Charles Platkin, PhD, JD, MPH, executive director of the Hunter College NYC Food Policy Center, and editor of DietDetective.com. Not only does he know the deal, he publishes a study detailing the healthiness of the food offered by 11 airlines every year, based on criteria such as healthy nutrients and calorie levels of meals, level of transparency, improvement and maintenance of healthy offerings, and water health.
For the 2018-2019 edition, there’s a tie at the top between Alaska Airlines and Air Canada, both scoring 4 out of 5 on Dr. Platkin’s “Health Score,” both well ahead of Delta and JetBlue in second place with a score of 2.9.
At the bottom? Southwest Airlines, with a paltry 1.7, edging out Spirit and Hawaiian Airlines.
Our personal favorite tidbit from the study has to be the poor scores in water health. Spirit managed to score a measly 1 out of 5 in the water health subscore, and several airlines were below 2. An actual quote from the study: “The quality of drinking water varies by airline, and many have provided passengers with unhealthy water. In general, it’s probably best to avoid drinking coffee and tea on board since they are made with galley water.”
While Hawaiian can take some comfort because they didn’t finish dead last, Dr. Platkin did award them his special “Shame on You” award for not providing all their nutritional information. You can try to hide, Hawaiian Airlines, but Dr. Platkin will find you.
Use the Force, Jack Nicklaus
We here at LOTME usually strive to provide an insightful and up-to-date wrap-up of the latest news on cutting-edge research, clinical breakthroughs, and impolite bodily functions. But right now, we want to talk about something really important: your golf game.
There’s an old saying in golf: Drive for show, putt for dough. That cliché may need a bit of updating, though, now that investigators in Ireland (where they are pretty serious about their golf) have brought science to the party.
Their research indicates that it may be possible for golfers to improve their putting without practicing. Without physically practicing, that is. Without going to the golf course. Just think about it: Golfers can putt better by, you know … just thinking about it.
Imagining the feel of an action without actually performing it is known as kinesthetic imagery ability, and it just might have an effect on putting, researchers from the physical education and sports sciences department at the University of Limerick and Lero, the Science Foundation Ireland Research Centre for Software, reported in Psychology of Sport and Exercise.
In a group of 44 skilled golfers, half watched video of an expert golfer putting in a lab environment “while listening to a motor imagery script consisting of short sentences describing key visual and kinesthetic feelings associated with performing the putting.” Then all the golfers took 40 putts from 15 feet in the lab.
The good kinesthetic imagers in the bunch improved their putting consistency more than subjects with poorer kinesthetic imagery ability and subjects from the nonintervention group.
So, here’s the new and improved old saying for golfers: Drive for show, use your kinesthetic imagery ability for dough. Yup, that’ll definitely catch on.