User login
Current management of Barrett esophagus and esophageal adenocarcinoma
All cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus.1 But most cases of Barrett esophagus go undiagnosed. And Barrett esophagus is seen in 5% to 15% of patients with gastroesophageal reflux disease.2 These facts clearly emphasize the need for screening. Here, we review the rationale and recommendations for screening and surveillance, as well as the range of treatment options.
SCOPE OF THE PROBLEM
The American Cancer Society estimated there were 17,290 new cases of esophageal cancer and 15,850 deaths from it in the United States in 2018.3 Of the 2 main histologic types of esophageal cancer, adenocarcinoma and squamous cell cancer, adenocarcinoma is more common in the United States.
The precursor lesion is Barrett esophagus, defined as an extension of salmon-colored mucosa at least 1 cm into the tubular esophagus proximal to the gastroesophageal junction, with biopsy confirmation of intestinal metaplasia.4
The natural course of progression to dysplasia and cancer in Barrett esophagus is unknown but is thought to be stepwise, from no dysplasia to low-grade dysplasia to high-grade dysplasia and cancer, and the cancer risk depends on the degree of dysplasia: the annual risk is 0.33% if there is no dysplasia, 0.54% with low-grade dysplasia, and 7% with high-grade dysplasia.4
Although all cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus,1 more than 90% of patients with newly diagnosed esophageal adenocarcinoma do not have a prior diagnosis of Barrett esophagus.5 Therefore, there is a substantial unmet need to expand screening for Barrett esophagus in people at risk.
GASTROESOPHAGEAL REFLUX DISEASE IS A RISK FACTOR FOR CANCER
The rationale behind screening is that detecting Barrett esophagus early and intervening in a timely manner in patients at higher risk of developing adenocarcinoma will decrease mortality.
Chronic gastroesophageal reflux disease is a strong risk factor for esophageal adenocarcinoma (odds ratio [OR] 7.7, 95% confidence interval [CI] 5.3–11.4), and the risk increases when symptoms are long-standing (> 20 years) or severe (OR 43.5, 95% CI 18.3–103.5) or occur daily (OR 5.5, 95% CI 3.2–9.3).6
Reflux symptoms are scored as follows:
- Heartburn only, 1 point
- Regurgitation only, 1 point
- Heartburn with regurgitation, 1.5 points
- Nightly symptoms (2 points if yes, 0 if no)
- Symptoms once a week, 0 points; 2 to 6 times a week, 1 point; 7 to 15 times a week, 2 points; more than 15 times a week, 3 points.6
A score of 4.5 or higher indicates severe reflux disease. However, it is worth noting that the annual incidence of esophageal adenocarcinoma in patients with long-term gastroesophageal reflux disease is less than 0.001%.7
RISK FACTORS FOR BARRETT ESOPHAGUS
Risk factors for Barrett esophagus include:
Male sex. Barrett esophagus is more prevalent in men than in women, at a ratio of 2 to 1; but in individuals under age 50, the ratio is 4 to 1.8
Age 50 or older. Barrett esophagus is usually diagnosed in the sixth to seventh decade of life, and the prevalence increases from 2.1% in the third decade to 9.3% in the sixth decade.9
White race. It is more prevalent in whites than in blacks (5.0% vs 1.5%, P < .0001).10
Central obesity. Waist circumference is an independent risk factor: every 5-cm increase carries an OR of 1.14 (95% CI 1.03–1.27, P = .02).11
Cigarette smoking increases the risk of Barrett esophagus (OR 1.42; 95% CI 1.15–1.76).12
A family history of Barrett esophagus or esophageal adenocarcinoma is a strong risk factor (OR 12, 95% CI 3.3–44.8). In 1 study, the risk in first- and second-degree relatives of patients with Barrett esophagus was 24%, compared with 5% in a control population (P < .005).13
SCREENING GUIDELINES AND DRAWBACKS
The standard screening method is esophagogastroduodenoscopy with sedation, with careful visual inspection and 4-quadrant biopsies every 2 cm using the Seattle protocol, ie, including biopsy of any mucosal irregularities in salmon-colored mucosa above the gastroesophageal junction (Figure 1).4
Endoscopic screening is cost-effective, costing $10,440 per quality-adjusted life-year saved, which is well below the accepted threshold of less than $100,000.14 However, it is still expensive, invasive, and not ideal for screening large populations.
Less-invasive methods under study
Less-invasive, less-expensive methods being tested for mass screening include:
Unsedated transnasal endoscopy. Done with only topical anesthesia, it has high diagnostic accuracy and is quicker and more cost-effective than standard esophagogastroduodenoscopy, with fewer adverse effects. However, the procedure has not yet gained widespread acceptance for regular use by gastroenterologists.15
A swallowable sponge. Another promising test is cell collection using the Cytosponge Cell Collection Device (Medtronic, Minneapolis, MN). An encapsulated compressed sponge with a string attached is swallowed; in the stomach, the capsule dissolves, and the sponge expands and is then withdrawn using the attached string. The obtained cytology sample from the lower esophagus is then tested for trefoil factor 3, a protein biomarker for Barrett esophagus.16
A retractable balloon. The EsoCheck Cell Collection Device is a retractable balloon attached to a string. When swallowed, it gathers distal esophageal cells for detecting methylated DNA markers for Barrett esophagus.17
Esophageal capsule endoscopy uses a camera to visualize the esophagus, but lacks the ability to obtain biopsy samples.
Other screening methods are being tested, although data are limited. Liquid biopsy uses a blood sample to detect microRNAs that are dysregulated in cancer. The “electronic nose” is a device that detects exhaled volatile organic compounds altered in Barrett esophagus. Another test involves taking an oral wash sample to study the oral microbiome for a pattern associated with adenocarcinoma.18–21
SURVEILLANCE: WHAT’S INVOLVED, WHAT’S AVAILABLE
Surveillance in Barrett esophagus aims to detect premalignant changes or early-stage adenocarcinoma to provide longer survival and lower cancer-related mortality. Recent evidence suggests that patients with esophageal adenocarcinoma that is diagnosed in a Barrett esophagus surveillance program have an earlier stage of disease and therefore a survival benefit.22
Patient education is essential
Before enrolling a patient in a surveillance program, the clinician should explain the risks, benefits, and limitations, the importance of periodic endoscopy, and the possible eventual need for endoscopic therapy or surgery.
The endoscopic procedure
Surveillance involves examination by high-definition white-light endoscopy, with random 4-quadrant biopsies every 2 cm (or every 1 cm in patients with a history of dysplasia) and biopsy of any mucosal irregularity (nodule, ulcer, or other visible lesion). The degree of dysplasia determines the frequency of follow-up surveillance intervals and the need for endoscopic eradication therapy, as presented in professional society guidelines (Table 1).4,23,24
Advanced methods for detecting dysplasia
Newer endoscopic surveillance techniques include dye-based chromoendoscopy, narrow-band imaging, confocal laser endomicroscopy, volumetric laser endomicroscopy, and wide-area transepithelial sampling with computer-assisted 3-dimensional analysis. All these techniques are used to increase the detection of dysplasia. Of these, dye-based chromoendoscopy, narrow-band imaging, and confocal laser endomicroscopy meet current criteria of the American Society for Gastrointestinal Endoscopy for preservation and incorporation of valuable endoscopic innovations.23
MANAGEMENT OF NONDYSPLASTIC BARRETT ESOPHAGUS
A proton pump inhibitor (PPI) is recommended to control reflux symptoms in patients with nondysplastic Barrett esophagus. But it is important to counsel patients on additional ways to protect against esophageal adenocarcinoma, such as:
- Low to moderate alcohol consumption
- Regular physical activity
- Increased dietary intake of fruits, vegetables, folate, fiber, beta-carotene, and vitamin C
- Weight control
- Smoking cessation.25
Surveillance endoscopy with 4-quadrant biopsies at 2-cm intervals is recommended every 3 to 5 years (Table 1).
DOES CHEMOPREVENTION HAVE A ROLE?
Chemoprevention is an exciting area of research in preventing progression to adenocarcinoma in patients with Barrett esophagus. Various drugs such as aspirin, other nonsteroidal anti-inflammatory drugs (NSAIDs), PPIs, metformin, and statins have been studied.
Aspirin
Aspirin has been shown to prevent development of Barrett esophagus in patients with reflux disease,26 but more studies are needed to validate those findings.
PPIs
Gastroesophageal reflux disease is a primary risk factor for esophageal adenocarcinoma, and gastric acid suppression with PPIs reduces cancer risk. PPI therapy is associated with a 71% decrease in the risk of high-grade dysplasia and adenocarcinoma in patients with Barrett esophagus (OR 0.29, 95% CI 0.12–0.79).27 Long-term therapy (> 2 to 3 years) has a higher protective effect (adjusted OR 0.45, 95% CI 0.19–1.06) than short-term therapy (< 2 to 3 years) (adjusted OR 1.09, 95% CI 0.47–2.56).27
NSAIDs
NSAIDs, including aspirin, have been associated with decreased risk of colon, stomach, lung, breast, and esophageal cancer due to their potential to inhibit cyclooxygenase 2 (COX-2) enzymes.
A meta-analysis demonstrated that aspirin and NSAIDs led to a 32% reduction in the risk of adenocarcinoma (OR 0.68, 95% CI 0.56–0.83). The benefit was even greater if the drug was taken daily or more frequently (OR 0.56, 95% CI 0.43–0.73, P < .001) or was taken for 10 or more years (OR 0.63, 95% CI 0.45–0.90, P = .04).28
PPI plus aspirin
The best evidence for the role of PPIs and aspirin in reducing the risk of dysplasia comes from the Aspirin and Esomeprazole Chemoprevention in Barrett’s Metaplasia Trial.29 This randomized, controlled trial compared 4 regimens consisting of esomeprazole (a PPI) in either a high dose (40 mg twice daily) or a low dose (20 mg once daily) plus either aspirin (300 or 320 mg per day) or no aspirin in 2,557 patients with Barrett esophagus. The composite end point was the time to all-cause mortality, adenocarcinoma, or high-grade dysplasia.
At a median follow-up of 8.9 years, the combination of high-dose esomeprazole plus aspirin had the strongest effect compared with low-dose esomeprazole without aspirin (time ratio 1.59, 95% CI 1.14–2.23, P = .0068). The number needed to treat was 34 for esomeprazole and 43 for aspirin.29
Based on these data, we can conclude that aspirin and PPIs can prevent dysplasia and all-cause mortality in Barrett esophagus.
Metformin: No evidence of benefit
Metformin was studied as a protective agent against obesity-associated cancers including esophageal adenocarcinoma, as it reduces insulin levels.
In a randomized controlled trial30 in 74 patients with Barrett esophagus, metformin (starting at 500 mg daily, increasing to 2,000 mg/day by week 4) was compared with placebo. At 12 weeks, the percent change in esophageal levels of the biomarker pS6K1—an intracellular mediator of insulin and insulin-like growth factor activation in Barrett epithelium—did not differ significantly between the 2 groups (1.4% with metformin vs −14.7% with placebo; 1-sided P = .80). This suggested that metformin did not significantly alter proliferation or apoptosis in Barrett epithelium, despite reducing serum insulin levels and insulin resistance. Thus, metformin did not demonstrate a chemoprotective effect in preventing the progression of Barrett esophagus to adenocarcinoma.
Vitamin D: No evidence of benefit
Vitamin D affects genes regulating proliferation, apoptosis, and differentiation, and has therefore been studied as a potential antineoplastic agent. Its deficiency has also been associated with increased risk of esophageal adenocarcinoma. However, its efficacy in chemoprevention is unclear.31
One study found no association between serum 25-hydroxyvitamin D levels and prevalence of dysplasia in Barrett esophagus (P = .90). An increase in vitamin D levels had no effect on progression to dysplasia or cancer (for every 5-nmol/L increase from baseline, hazard ratio 0.98, P = .62).32
In another study, supplementation with vitamin D3 (cholecalciferol 50,000 IU weekly) plus a PPI for 12 weeks significantly improved the serum 25-hydroxyvitamin D levels without significant changes in gene expression from Barrett epithelium.33 These findings were confirmed in a meta-analysis that showed no consistent association between vitamin D exposure and risk of esophageal neoplasm.34
Thus, there is currently no evidence to support vitamin D for chemoprevention in Barrett esophagus or esophageal adenocarcinoma.
Statins
In addition to lowering cholesterol, statins have antiproliferative, pro-apoptotic, anti-angiogenic, and immunomodulatory effects that prevent cancer, leading to a 41% reduction in the risk of adenocarcinoma in patients with Barrett esophagus in one study (adjusted OR 0.59, 95% CI 0.45–0.78); the number needed to treat with statins to prevent 1 case of adenocarcinoma was 389.35
A meta-analysis also showed that statin use was associated with a lower risk of progression of Barrett esophagus (OR 0.48, 95% CI 0.31–0.73).36
In general, statins appear promising for chemoprevention, but more study is needed.
When is chemoprevention appropriate?
Chemoprevention is not recommended for all patients with Barrett esophagus, given that the condition affects 1% to 2% of the US adult population, and very few patients have progression to esophageal adenocarcinoma. Rather, chemoprevention may be considered in patients with Barrett esophagus and multiple risk factors for adenocarcinoma.
INDEFINITE DYSPLASIA
In Barrett esophagus with indefinite dysplasia, either the epithelial abnormalities are insufficient for a diagnosis of dysplasia, or the nature of the epithelial abnormalities is uncertain due to inflammation or technical difficulties with specimen processing. The risk of high-grade dysplasia or cancer within 1 year of the diagnosis of indefinite dysplasia varies between 1.9% and 15%.37 The recommendation for management is to optimize acid-suppressive therapy for 3 to 6 months and then to repeat esophagogastroduodenoscopy. If indefinite dysplasia is noted again, repeat endoscopy in 12 months is recommended.2
ENDOSCOPIC ERADICATION: AN OVERVIEW
Because dysplasia in Barrett esophagus carries a high risk of progression to cancer, the standard of care is endoscopic mucosal resection of visible lesions, followed by ablation of the flat mucosa, with the aim of achieving complete eradication of intestinal metaplasia.4,38 The initial endoscopic treatment is followed by outpatient sessions every 8 to 10 weeks until the dysplasia is eradicated. A key part of treatment during this time is maximal acid suppression with a PPI twice daily and a histamine-2 blocker at night. In rare cases, fundoplication is required to control reflux refractory to medical therapy.
After eradication is confirmed, continued surveillance is necessary, as recurrences have been reported at a rate of 4.8% per year for intestinal metaplasia, and 2% per year for dysplasia.39
Current endoscopic resection techniques
Endoscopic resection techniques include mucosal resection, submucosal dissection, radiofrequency ablation, cryotherapy, argon plasma coagulation, and photodynamic therapy (Figure 2).
In mucosal resection, the lesion is either suctioned into a band ligator, after which a band is placed around the lesion, or suctioned into a cap fitted at the end of the endoscope, after which the lesion is removed using a snare.
In submucosal dissection, a liquid is injected into the submucosa to lift the lesion, making it easier to remove. The procedure is technically complex and requires additional training.
In radiofrequency ablation, a special catheter is passed through the endoscope to ablate the affected epithelium by thermal injury. Argon plasma coagulation works in a similar way, but uses ionized argon gas to induce thermal coagulation of metaplastic epithelium.
Cryotherapy produces cellular injury by rapid freezing and thawing of tissue using a cryogen such as liquid nitrogen or nitrous oxide.
In photodynamic therapy, a photosensitizer (porfimer sodium) is administered and taken up preferentially by metaplastic epithelium; it is then activated by transmission of red light using the endoscope, leading to destruction of the metaplastic epithelium.
Of the different techniques, radiofrequency ablation has the most evidence for efficacy and hence is the most commonly used.
All of these procedures are generally well tolerated and have favorable side-effect profiles. After radiofrequency ablation with or without mucosal resection, esophageal strictures are noted in 5.6% of patients, and bleeding and perforation occur rarely (1% and 0.6% of patients, respectively).40 Submucosal dissection is associated with a higher rate of complications such as stricture formation (11% of patients) and bleeding or perforation (1.5% of patients).41
LOW-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
Most patients with low-grade dysplasia (73%) are down-staged to nondysplastic Barrett esophagus or to indefinite for dysplasia after review by expert pathologists.42 Patients with confirmed and persistent low-grade dysplasia are at higher risk of progression.43
Once low-grade dysplasia is confirmed by a second gastrointestinal pathologist, the patient should undergo endoscopic ablation. A landmark study by Shaheen et al44 demonstrated the benefit of radiofrequency ablation in achieving complete eradication of dysplasia (90.5% vs 22.7% for a sham procedure) and complete eradication of intestinal metaplasia (77.4% vs 2.3% for a sham procedure). In another trial of 136 patients with low-grade dysplasia followed for 3 years, Phoa et al45 demonstrated that radiofrequency ablation reduced the rate of progression to high-grade dysplasia by 25% and to adenocarcinoma by 7.4% compared with endoscopic surveillance.
Patients with confirmed low-grade dysplasia who do not undergo eradication therapy should have surveillance endoscopy every 6 to 12 months (Table 1).
HIGH-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
As with low-grade dysplasia, the diagnosis of high-grade dysplasia needs to be confirmed by a second pathologist with gastrointestinal expertise. In the past, the treatment was esophagectomy, but due to lower morbidity and equivalent efficacy of radiofrequency ablation,46 the current treatment of choice is endoscopic mucosal resection of raised lesions, followed by radiofrequency ablation of the entire affected segment.
In the study by Shaheen et al,44 42 patients with high-grade dysplasia were randomized to radiofrequency ablation and 21 to a sham procedure, and 81% of ablation patients achieved complete eradication of dysplasia vs 19% with the sham procedure. Eradication of intestinal metaplasia was achieved in 77% of ablation patients vs 2% of patients with the sham therapy. Results of 3-year follow-up from the same cohort showed complete eradication of dysplasia in 98% and of intestinal metaplasia in 91%.47
Endoscopic eradication therapy is recommended for all patients with Barrett esophagus and high-grade dysplasia without a life-limiting comorbidity. Alternatively, surveillance every 3 months is an option if the patient does not wish to undergo eradication therapy. Radiofrequency ablation is more cost-effective than esophagectomy or endoscopic surveillance followed by treatment once patients develop adenocarcinoma.48,49
EARLY ESOPHAGEAL ADENOCARCINOMA: RECOMMENDED MANAGEMENT
Adenocarcinoma limited to the mucosa and without evidence of nodal involvement can be resected endoscopically. In patients with localized cancer, mucosal resection is done not only for therapeutic purposes but also for staging. Ideal management is multidisciplinary, including a gastroenterologist, thoracic surgeon, oncologist, pathologist, and radiation oncologist.
If lesions have features suggesting submucosal invasion or are greater than 1.5 cm in size, or if it is difficult to separate (ie, lift) the mucosa from the submucosal layer with injection of saline, then submucosal dissection is recommended.50 Because of the risk of metachronous lesions, ablation of the remaining Barrett esophagus mucosa is recommended after resection of cancer.
Endoscopic eradication is highly effective and durable for the treatment of intramucosal esophageal adenocarcinoma. In a study of 1,000 patients, 963 patients (96.3%) had achieved a complete response; 12 patients (3.7%) underwent surgery after eradication failed during a follow-up of almost 5 years.51 Metachronous lesions or recurrence of cancer developed during the follow-up period in 140 patients (14.5%) but were successfully treated endoscopically in 115, resulting in a long-term complete remission rate of 93.8%.
POSTABLATION MANAGEMENT
Because of the risk of recurrence of dysplasia after ablation, long-term PPI therapy and surveillance are recommended.
Surveillance endoscopy involves 4-quadrant biopsies taken every 1 cm from the entire length of segment where Barrett esophagus had been seen before ablation.
The timing of surveillance intervals depends on the preablation grade of dysplasia. For low-grade dysplasia, the recommendation is every 6 months for the first year after ablation and, if there is no recurrence of dysplasia, annually after that.2 After treatment of high-grade dysplasia or intramucosal adenocarcinoma, the recommendation is every 3 months for the first year, every 6 months in the second year, and then annually.2
- Mendes de Almeida JC, Chaves P, Pereira AD, Altorki NK. Is Barrett’s esophagus the precursor of most adenocarcinomas of the esophagus and cardia? A biochemical study. Ann Surg 1997; 226(6):725–733. pmid:9409571
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61(2):226–231. pmid:15729230
- National Cancer Institute. Cancer stat facts: esophageal cancer. https://seer.cancer.gov/statfacts/html/esoph.html. Accessed August 6, 2019.
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111(1):30–50. doi:10.1038/ajg.2015.322
- Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002; 122(1):26–33. pmid:11781277
- Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999; 340(11):825–831. doi:10.1056/NEJM199903183401101
- Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. JAMA 2002; 287(15):1972–1981. pmid:11960540
- van Blankenstein M, Looman CW, Johnston BJ, Caygill CP. Age and sex distribution of the prevalence of Barrett’s esophagus found in a primary referral endoscopy center. Am J Gastroenterol 2005; 100(3):568–576.
- Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointest Endosc 2010; 71(1):21–27. doi:10.1016/j.gie.2009.06.035
- Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett’s esophagus among racial groups in a multi-center consortium. Dig Dis Sci 2009; 54(5):964–971. doi:10.1007/s10620-009-0742-3
- Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s esophagus: a pooled analysis from the international BEACON consortium. Gut 2013; 62(12):1684–1691. doi:10.1136/gutjnl-2012-303753
- Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol 2013; 28(8):1258–1273. doi:10.1111/jgh.12230
- Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett’s esophagus, esophageal adenocarcinoma, and esophagogastric junctional adenocarcinoma in Caucasian adults. Gut 2002; 51(3):323–328. pmid:12171951
- Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 2003; 138(3):176–186. pmid:12558356
- Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol 2006; 101(12):2693–2703.
- Ross-Innes CS, Chettouh H, Achilleos A, et al; BEST2 study group. Risk stratification of Barrett’s esophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol 2017; 2(1):23–31. doi:10.1016/S2468-1253(16)30118-2
- Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018; 10(424). pii:eaao5848. doi:10.1126/scitranslmed.aao5848
- Chan DK, Zakko L, Visrodia KH, et al. Breath testing for Barrett’s esophagus using exhaled volatile organic compound profiling with an electronic nose device. Gastroenterology 2017; 152(1):24–26. doi:10.1053/j.gastro.2016.11.001
- Kumar S, Huang J, Abbassi-Ghadi N, et al. Mass spectrometric analysis of exhaled breath for the identification of volatile organic compound biomarkers in esophageal and gastric adenocarcinoma. Ann Surg 2015; 262(6):981–990. doi:10.1097/SLA.0000000000001101
- Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res 2017; 77(23):6777–6787. doi:10.1158/0008-5472.CAN-17-1296
- Mallick R, Patnaik SK, Wani S, Bansal A. A systematic review of esophageal microrna markers for diagnosis and monitoring of Barrett’s esophagus. Dig Dis Sci 2016; 61(4):1039–1050. doi:10.1007/s10620-015-3959-3
- Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology 2018; 154(8):2068–2086.e5. doi:10.1053/j.gastro.2018.02.022
- ASGE Technology Committee; Thosani N, Abu Dayyeh BK, Sharma P, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE preservation and incorporation of valuable endoscopic innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc 2016; 83(4):684–698.e7. doi:10.1016/j.gie.2016.01.007
- Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 2011; 140(3):e18–e52. doi:10.1053/j.gastro.2011.01.031
- Castro C, Peleteiro B, Lunet N. Modifiable factors and esophageal cancer: a systematic review of published meta-analyses. J Gastroenterol 2018; 53(1):37–51. doi:10.1007/s00535-017-1375-5
- Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012; 10(7):722–727. doi:10.1016/j.cgh.2012.02.031
- Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gut 2014; 63(8):1229–1237. doi:10.1136/gutjnl-2013-305997
- Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012; 142(3):442–452.e5. doi:10.1053/j.gastro.2011.11.019
- Jankowski JAZ, de Caestecker J, Love SB, et al; AspECT Trial Team. Esomeprazole and aspirin in Barrett’s esophagus (AspECT): a randomised factorial trial. Lancet 2018; 392(10145):400–408. doi:10.1016/S0140-6736(18)31388-6
- Chak A, Buttar NS, Foster NR, et al; Cancer Prevention Network. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2015; 13(4):665–672.e1–e4. doi:10.1016/j.cgh.2014.08.040
- Rouphael C, Kamal A, Sanaka MR, Thota PN. Vitamin D in esophageal cancer: is there a role for chemoprevention? World J Gastrointest Oncol 2018; 10(1):23–30. doi:10.4251/wjgo.v10.i1.23
- Thota PN, Kistangari G, Singh P, et al. Serum 25-hydroxyvitamin D levels and the risk of dysplasia and esophageal adenocarcinoma in patients with Barrett’s esophagus. Dig Dis Sci 2016; 61(1):247–254. doi:10.1007/s10620-015-3823-5
- Cummings LC, Thota PN, Willis JE, et al. A nonrandomized trial of vitamin D supplementation for Barrett’s esophagus. PLoS One 2017;1 2(9):e0184928. doi:10.1371/journal.pone.0184928
- Zgaga L, O’Sullivan F, Cantwell MM, Murray LJ, Thota PN, Coleman HG. Markers of vitamin D exposure and esophageal cancer risk: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2016; 25(6):877–886. doi:10.1158/1055-9965.EPI-15-1162
- Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11(6):620–629. doi:10.1016/j.cgh.2012.12.036
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors associated with progression of Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 6(7):1046–1055.e8. doi:10.1016/j.cgh.2017.11.044
- Thota PN, Kistangari G, Esnakula AK, Gonzalo DH, Liu XL. Clinical significance and management of Barrett’s esophagus with epithelial changes indefinite for dysplasia. World J Gastrointest Pharmacol Ther 2016; 7(3):406–411. doi:10.4292/wjgpt.v7.i3.406
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012; 143(2):336–346. doi:10.1053/j.gastro.2012.04.032
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017; 85(3):482–495.e4. doi:10.1016/j.gie.2016.09.022
- Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14(8):1086–1095.e6. doi:10.1016/j.cgh.2016.04.001
- Yang D, Zou F, Xiong S, Forde JJ, Wang Y, Draganov PV. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc 2018; 87(6):1383–1393. doi:10.1016/j.gie.2017.09.038
- Duits LC, Phoa KN, Curvers WL, et al. Barrett’s esophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015; 64(5):700–706. doi:10.1136/gutjnl-2014-307278
- Duits LC, van der Wel MJ, Cotton CC, et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology 2017; 152(5):993–1001.e1. doi:10.1053/j.gastro.2016.12.008
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009; 360(22):2277–2288. doi:10.1056/NEJMoa0808145
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014; 311(12):1209–1217. doi:10.1001/jama.2014.2511
- Hu Y, Puri V, Shami VM, Stukenborg GJ, Kozower BD. Comparative effectiveness of esophagectomy versus endoscopic treatment for esophageal high-grade dysplasia. Ann Surg 2016; 263(4):719–726. doi:10.1097/SLA.0000000000001387
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011; 141(2):460–468. doi:10.1053/j.gastro.2011.04.061
- Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology 2012; 143(3):567–575. doi:10.1053/j.gastro.2012.05.010
- Boger PC, Turner D, Roderick P, Patel P. A UK-based cost-utility analysis of radiofrequency ablation or oesophagectomy for the management of high-grade dysplasia in Barrett’s esophagus. Aliment Pharmacol Ther 2010; 32(11-12):1332–1342. doi:10.1111/j.1365-2036.2010.04450.x
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47(9):829–854. doi:10.1055/s-0034-1392882
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014; 146(3):652–660.e1. doi:10.1053/j.gastro.2013.11.006
All cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus.1 But most cases of Barrett esophagus go undiagnosed. And Barrett esophagus is seen in 5% to 15% of patients with gastroesophageal reflux disease.2 These facts clearly emphasize the need for screening. Here, we review the rationale and recommendations for screening and surveillance, as well as the range of treatment options.
SCOPE OF THE PROBLEM
The American Cancer Society estimated there were 17,290 new cases of esophageal cancer and 15,850 deaths from it in the United States in 2018.3 Of the 2 main histologic types of esophageal cancer, adenocarcinoma and squamous cell cancer, adenocarcinoma is more common in the United States.
The precursor lesion is Barrett esophagus, defined as an extension of salmon-colored mucosa at least 1 cm into the tubular esophagus proximal to the gastroesophageal junction, with biopsy confirmation of intestinal metaplasia.4
The natural course of progression to dysplasia and cancer in Barrett esophagus is unknown but is thought to be stepwise, from no dysplasia to low-grade dysplasia to high-grade dysplasia and cancer, and the cancer risk depends on the degree of dysplasia: the annual risk is 0.33% if there is no dysplasia, 0.54% with low-grade dysplasia, and 7% with high-grade dysplasia.4
Although all cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus,1 more than 90% of patients with newly diagnosed esophageal adenocarcinoma do not have a prior diagnosis of Barrett esophagus.5 Therefore, there is a substantial unmet need to expand screening for Barrett esophagus in people at risk.
GASTROESOPHAGEAL REFLUX DISEASE IS A RISK FACTOR FOR CANCER
The rationale behind screening is that detecting Barrett esophagus early and intervening in a timely manner in patients at higher risk of developing adenocarcinoma will decrease mortality.
Chronic gastroesophageal reflux disease is a strong risk factor for esophageal adenocarcinoma (odds ratio [OR] 7.7, 95% confidence interval [CI] 5.3–11.4), and the risk increases when symptoms are long-standing (> 20 years) or severe (OR 43.5, 95% CI 18.3–103.5) or occur daily (OR 5.5, 95% CI 3.2–9.3).6
Reflux symptoms are scored as follows:
- Heartburn only, 1 point
- Regurgitation only, 1 point
- Heartburn with regurgitation, 1.5 points
- Nightly symptoms (2 points if yes, 0 if no)
- Symptoms once a week, 0 points; 2 to 6 times a week, 1 point; 7 to 15 times a week, 2 points; more than 15 times a week, 3 points.6
A score of 4.5 or higher indicates severe reflux disease. However, it is worth noting that the annual incidence of esophageal adenocarcinoma in patients with long-term gastroesophageal reflux disease is less than 0.001%.7
RISK FACTORS FOR BARRETT ESOPHAGUS
Risk factors for Barrett esophagus include:
Male sex. Barrett esophagus is more prevalent in men than in women, at a ratio of 2 to 1; but in individuals under age 50, the ratio is 4 to 1.8
Age 50 or older. Barrett esophagus is usually diagnosed in the sixth to seventh decade of life, and the prevalence increases from 2.1% in the third decade to 9.3% in the sixth decade.9
White race. It is more prevalent in whites than in blacks (5.0% vs 1.5%, P < .0001).10
Central obesity. Waist circumference is an independent risk factor: every 5-cm increase carries an OR of 1.14 (95% CI 1.03–1.27, P = .02).11
Cigarette smoking increases the risk of Barrett esophagus (OR 1.42; 95% CI 1.15–1.76).12
A family history of Barrett esophagus or esophageal adenocarcinoma is a strong risk factor (OR 12, 95% CI 3.3–44.8). In 1 study, the risk in first- and second-degree relatives of patients with Barrett esophagus was 24%, compared with 5% in a control population (P < .005).13
SCREENING GUIDELINES AND DRAWBACKS
The standard screening method is esophagogastroduodenoscopy with sedation, with careful visual inspection and 4-quadrant biopsies every 2 cm using the Seattle protocol, ie, including biopsy of any mucosal irregularities in salmon-colored mucosa above the gastroesophageal junction (Figure 1).4
Endoscopic screening is cost-effective, costing $10,440 per quality-adjusted life-year saved, which is well below the accepted threshold of less than $100,000.14 However, it is still expensive, invasive, and not ideal for screening large populations.
Less-invasive methods under study
Less-invasive, less-expensive methods being tested for mass screening include:
Unsedated transnasal endoscopy. Done with only topical anesthesia, it has high diagnostic accuracy and is quicker and more cost-effective than standard esophagogastroduodenoscopy, with fewer adverse effects. However, the procedure has not yet gained widespread acceptance for regular use by gastroenterologists.15
A swallowable sponge. Another promising test is cell collection using the Cytosponge Cell Collection Device (Medtronic, Minneapolis, MN). An encapsulated compressed sponge with a string attached is swallowed; in the stomach, the capsule dissolves, and the sponge expands and is then withdrawn using the attached string. The obtained cytology sample from the lower esophagus is then tested for trefoil factor 3, a protein biomarker for Barrett esophagus.16
A retractable balloon. The EsoCheck Cell Collection Device is a retractable balloon attached to a string. When swallowed, it gathers distal esophageal cells for detecting methylated DNA markers for Barrett esophagus.17
Esophageal capsule endoscopy uses a camera to visualize the esophagus, but lacks the ability to obtain biopsy samples.
Other screening methods are being tested, although data are limited. Liquid biopsy uses a blood sample to detect microRNAs that are dysregulated in cancer. The “electronic nose” is a device that detects exhaled volatile organic compounds altered in Barrett esophagus. Another test involves taking an oral wash sample to study the oral microbiome for a pattern associated with adenocarcinoma.18–21
SURVEILLANCE: WHAT’S INVOLVED, WHAT’S AVAILABLE
Surveillance in Barrett esophagus aims to detect premalignant changes or early-stage adenocarcinoma to provide longer survival and lower cancer-related mortality. Recent evidence suggests that patients with esophageal adenocarcinoma that is diagnosed in a Barrett esophagus surveillance program have an earlier stage of disease and therefore a survival benefit.22
Patient education is essential
Before enrolling a patient in a surveillance program, the clinician should explain the risks, benefits, and limitations, the importance of periodic endoscopy, and the possible eventual need for endoscopic therapy or surgery.
The endoscopic procedure
Surveillance involves examination by high-definition white-light endoscopy, with random 4-quadrant biopsies every 2 cm (or every 1 cm in patients with a history of dysplasia) and biopsy of any mucosal irregularity (nodule, ulcer, or other visible lesion). The degree of dysplasia determines the frequency of follow-up surveillance intervals and the need for endoscopic eradication therapy, as presented in professional society guidelines (Table 1).4,23,24
Advanced methods for detecting dysplasia
Newer endoscopic surveillance techniques include dye-based chromoendoscopy, narrow-band imaging, confocal laser endomicroscopy, volumetric laser endomicroscopy, and wide-area transepithelial sampling with computer-assisted 3-dimensional analysis. All these techniques are used to increase the detection of dysplasia. Of these, dye-based chromoendoscopy, narrow-band imaging, and confocal laser endomicroscopy meet current criteria of the American Society for Gastrointestinal Endoscopy for preservation and incorporation of valuable endoscopic innovations.23
MANAGEMENT OF NONDYSPLASTIC BARRETT ESOPHAGUS
A proton pump inhibitor (PPI) is recommended to control reflux symptoms in patients with nondysplastic Barrett esophagus. But it is important to counsel patients on additional ways to protect against esophageal adenocarcinoma, such as:
- Low to moderate alcohol consumption
- Regular physical activity
- Increased dietary intake of fruits, vegetables, folate, fiber, beta-carotene, and vitamin C
- Weight control
- Smoking cessation.25
Surveillance endoscopy with 4-quadrant biopsies at 2-cm intervals is recommended every 3 to 5 years (Table 1).
DOES CHEMOPREVENTION HAVE A ROLE?
Chemoprevention is an exciting area of research in preventing progression to adenocarcinoma in patients with Barrett esophagus. Various drugs such as aspirin, other nonsteroidal anti-inflammatory drugs (NSAIDs), PPIs, metformin, and statins have been studied.
Aspirin
Aspirin has been shown to prevent development of Barrett esophagus in patients with reflux disease,26 but more studies are needed to validate those findings.
PPIs
Gastroesophageal reflux disease is a primary risk factor for esophageal adenocarcinoma, and gastric acid suppression with PPIs reduces cancer risk. PPI therapy is associated with a 71% decrease in the risk of high-grade dysplasia and adenocarcinoma in patients with Barrett esophagus (OR 0.29, 95% CI 0.12–0.79).27 Long-term therapy (> 2 to 3 years) has a higher protective effect (adjusted OR 0.45, 95% CI 0.19–1.06) than short-term therapy (< 2 to 3 years) (adjusted OR 1.09, 95% CI 0.47–2.56).27
NSAIDs
NSAIDs, including aspirin, have been associated with decreased risk of colon, stomach, lung, breast, and esophageal cancer due to their potential to inhibit cyclooxygenase 2 (COX-2) enzymes.
A meta-analysis demonstrated that aspirin and NSAIDs led to a 32% reduction in the risk of adenocarcinoma (OR 0.68, 95% CI 0.56–0.83). The benefit was even greater if the drug was taken daily or more frequently (OR 0.56, 95% CI 0.43–0.73, P < .001) or was taken for 10 or more years (OR 0.63, 95% CI 0.45–0.90, P = .04).28
PPI plus aspirin
The best evidence for the role of PPIs and aspirin in reducing the risk of dysplasia comes from the Aspirin and Esomeprazole Chemoprevention in Barrett’s Metaplasia Trial.29 This randomized, controlled trial compared 4 regimens consisting of esomeprazole (a PPI) in either a high dose (40 mg twice daily) or a low dose (20 mg once daily) plus either aspirin (300 or 320 mg per day) or no aspirin in 2,557 patients with Barrett esophagus. The composite end point was the time to all-cause mortality, adenocarcinoma, or high-grade dysplasia.
At a median follow-up of 8.9 years, the combination of high-dose esomeprazole plus aspirin had the strongest effect compared with low-dose esomeprazole without aspirin (time ratio 1.59, 95% CI 1.14–2.23, P = .0068). The number needed to treat was 34 for esomeprazole and 43 for aspirin.29
Based on these data, we can conclude that aspirin and PPIs can prevent dysplasia and all-cause mortality in Barrett esophagus.
Metformin: No evidence of benefit
Metformin was studied as a protective agent against obesity-associated cancers including esophageal adenocarcinoma, as it reduces insulin levels.
In a randomized controlled trial30 in 74 patients with Barrett esophagus, metformin (starting at 500 mg daily, increasing to 2,000 mg/day by week 4) was compared with placebo. At 12 weeks, the percent change in esophageal levels of the biomarker pS6K1—an intracellular mediator of insulin and insulin-like growth factor activation in Barrett epithelium—did not differ significantly between the 2 groups (1.4% with metformin vs −14.7% with placebo; 1-sided P = .80). This suggested that metformin did not significantly alter proliferation or apoptosis in Barrett epithelium, despite reducing serum insulin levels and insulin resistance. Thus, metformin did not demonstrate a chemoprotective effect in preventing the progression of Barrett esophagus to adenocarcinoma.
Vitamin D: No evidence of benefit
Vitamin D affects genes regulating proliferation, apoptosis, and differentiation, and has therefore been studied as a potential antineoplastic agent. Its deficiency has also been associated with increased risk of esophageal adenocarcinoma. However, its efficacy in chemoprevention is unclear.31
One study found no association between serum 25-hydroxyvitamin D levels and prevalence of dysplasia in Barrett esophagus (P = .90). An increase in vitamin D levels had no effect on progression to dysplasia or cancer (for every 5-nmol/L increase from baseline, hazard ratio 0.98, P = .62).32
In another study, supplementation with vitamin D3 (cholecalciferol 50,000 IU weekly) plus a PPI for 12 weeks significantly improved the serum 25-hydroxyvitamin D levels without significant changes in gene expression from Barrett epithelium.33 These findings were confirmed in a meta-analysis that showed no consistent association between vitamin D exposure and risk of esophageal neoplasm.34
Thus, there is currently no evidence to support vitamin D for chemoprevention in Barrett esophagus or esophageal adenocarcinoma.
Statins
In addition to lowering cholesterol, statins have antiproliferative, pro-apoptotic, anti-angiogenic, and immunomodulatory effects that prevent cancer, leading to a 41% reduction in the risk of adenocarcinoma in patients with Barrett esophagus in one study (adjusted OR 0.59, 95% CI 0.45–0.78); the number needed to treat with statins to prevent 1 case of adenocarcinoma was 389.35
A meta-analysis also showed that statin use was associated with a lower risk of progression of Barrett esophagus (OR 0.48, 95% CI 0.31–0.73).36
In general, statins appear promising for chemoprevention, but more study is needed.
When is chemoprevention appropriate?
Chemoprevention is not recommended for all patients with Barrett esophagus, given that the condition affects 1% to 2% of the US adult population, and very few patients have progression to esophageal adenocarcinoma. Rather, chemoprevention may be considered in patients with Barrett esophagus and multiple risk factors for adenocarcinoma.
INDEFINITE DYSPLASIA
In Barrett esophagus with indefinite dysplasia, either the epithelial abnormalities are insufficient for a diagnosis of dysplasia, or the nature of the epithelial abnormalities is uncertain due to inflammation or technical difficulties with specimen processing. The risk of high-grade dysplasia or cancer within 1 year of the diagnosis of indefinite dysplasia varies between 1.9% and 15%.37 The recommendation for management is to optimize acid-suppressive therapy for 3 to 6 months and then to repeat esophagogastroduodenoscopy. If indefinite dysplasia is noted again, repeat endoscopy in 12 months is recommended.2
ENDOSCOPIC ERADICATION: AN OVERVIEW
Because dysplasia in Barrett esophagus carries a high risk of progression to cancer, the standard of care is endoscopic mucosal resection of visible lesions, followed by ablation of the flat mucosa, with the aim of achieving complete eradication of intestinal metaplasia.4,38 The initial endoscopic treatment is followed by outpatient sessions every 8 to 10 weeks until the dysplasia is eradicated. A key part of treatment during this time is maximal acid suppression with a PPI twice daily and a histamine-2 blocker at night. In rare cases, fundoplication is required to control reflux refractory to medical therapy.
After eradication is confirmed, continued surveillance is necessary, as recurrences have been reported at a rate of 4.8% per year for intestinal metaplasia, and 2% per year for dysplasia.39
Current endoscopic resection techniques
Endoscopic resection techniques include mucosal resection, submucosal dissection, radiofrequency ablation, cryotherapy, argon plasma coagulation, and photodynamic therapy (Figure 2).
In mucosal resection, the lesion is either suctioned into a band ligator, after which a band is placed around the lesion, or suctioned into a cap fitted at the end of the endoscope, after which the lesion is removed using a snare.
In submucosal dissection, a liquid is injected into the submucosa to lift the lesion, making it easier to remove. The procedure is technically complex and requires additional training.
In radiofrequency ablation, a special catheter is passed through the endoscope to ablate the affected epithelium by thermal injury. Argon plasma coagulation works in a similar way, but uses ionized argon gas to induce thermal coagulation of metaplastic epithelium.
Cryotherapy produces cellular injury by rapid freezing and thawing of tissue using a cryogen such as liquid nitrogen or nitrous oxide.
In photodynamic therapy, a photosensitizer (porfimer sodium) is administered and taken up preferentially by metaplastic epithelium; it is then activated by transmission of red light using the endoscope, leading to destruction of the metaplastic epithelium.
Of the different techniques, radiofrequency ablation has the most evidence for efficacy and hence is the most commonly used.
All of these procedures are generally well tolerated and have favorable side-effect profiles. After radiofrequency ablation with or without mucosal resection, esophageal strictures are noted in 5.6% of patients, and bleeding and perforation occur rarely (1% and 0.6% of patients, respectively).40 Submucosal dissection is associated with a higher rate of complications such as stricture formation (11% of patients) and bleeding or perforation (1.5% of patients).41
LOW-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
Most patients with low-grade dysplasia (73%) are down-staged to nondysplastic Barrett esophagus or to indefinite for dysplasia after review by expert pathologists.42 Patients with confirmed and persistent low-grade dysplasia are at higher risk of progression.43
Once low-grade dysplasia is confirmed by a second gastrointestinal pathologist, the patient should undergo endoscopic ablation. A landmark study by Shaheen et al44 demonstrated the benefit of radiofrequency ablation in achieving complete eradication of dysplasia (90.5% vs 22.7% for a sham procedure) and complete eradication of intestinal metaplasia (77.4% vs 2.3% for a sham procedure). In another trial of 136 patients with low-grade dysplasia followed for 3 years, Phoa et al45 demonstrated that radiofrequency ablation reduced the rate of progression to high-grade dysplasia by 25% and to adenocarcinoma by 7.4% compared with endoscopic surveillance.
Patients with confirmed low-grade dysplasia who do not undergo eradication therapy should have surveillance endoscopy every 6 to 12 months (Table 1).
HIGH-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
As with low-grade dysplasia, the diagnosis of high-grade dysplasia needs to be confirmed by a second pathologist with gastrointestinal expertise. In the past, the treatment was esophagectomy, but due to lower morbidity and equivalent efficacy of radiofrequency ablation,46 the current treatment of choice is endoscopic mucosal resection of raised lesions, followed by radiofrequency ablation of the entire affected segment.
In the study by Shaheen et al,44 42 patients with high-grade dysplasia were randomized to radiofrequency ablation and 21 to a sham procedure, and 81% of ablation patients achieved complete eradication of dysplasia vs 19% with the sham procedure. Eradication of intestinal metaplasia was achieved in 77% of ablation patients vs 2% of patients with the sham therapy. Results of 3-year follow-up from the same cohort showed complete eradication of dysplasia in 98% and of intestinal metaplasia in 91%.47
Endoscopic eradication therapy is recommended for all patients with Barrett esophagus and high-grade dysplasia without a life-limiting comorbidity. Alternatively, surveillance every 3 months is an option if the patient does not wish to undergo eradication therapy. Radiofrequency ablation is more cost-effective than esophagectomy or endoscopic surveillance followed by treatment once patients develop adenocarcinoma.48,49
EARLY ESOPHAGEAL ADENOCARCINOMA: RECOMMENDED MANAGEMENT
Adenocarcinoma limited to the mucosa and without evidence of nodal involvement can be resected endoscopically. In patients with localized cancer, mucosal resection is done not only for therapeutic purposes but also for staging. Ideal management is multidisciplinary, including a gastroenterologist, thoracic surgeon, oncologist, pathologist, and radiation oncologist.
If lesions have features suggesting submucosal invasion or are greater than 1.5 cm in size, or if it is difficult to separate (ie, lift) the mucosa from the submucosal layer with injection of saline, then submucosal dissection is recommended.50 Because of the risk of metachronous lesions, ablation of the remaining Barrett esophagus mucosa is recommended after resection of cancer.
Endoscopic eradication is highly effective and durable for the treatment of intramucosal esophageal adenocarcinoma. In a study of 1,000 patients, 963 patients (96.3%) had achieved a complete response; 12 patients (3.7%) underwent surgery after eradication failed during a follow-up of almost 5 years.51 Metachronous lesions or recurrence of cancer developed during the follow-up period in 140 patients (14.5%) but were successfully treated endoscopically in 115, resulting in a long-term complete remission rate of 93.8%.
POSTABLATION MANAGEMENT
Because of the risk of recurrence of dysplasia after ablation, long-term PPI therapy and surveillance are recommended.
Surveillance endoscopy involves 4-quadrant biopsies taken every 1 cm from the entire length of segment where Barrett esophagus had been seen before ablation.
The timing of surveillance intervals depends on the preablation grade of dysplasia. For low-grade dysplasia, the recommendation is every 6 months for the first year after ablation and, if there is no recurrence of dysplasia, annually after that.2 After treatment of high-grade dysplasia or intramucosal adenocarcinoma, the recommendation is every 3 months for the first year, every 6 months in the second year, and then annually.2
All cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus.1 But most cases of Barrett esophagus go undiagnosed. And Barrett esophagus is seen in 5% to 15% of patients with gastroesophageal reflux disease.2 These facts clearly emphasize the need for screening. Here, we review the rationale and recommendations for screening and surveillance, as well as the range of treatment options.
SCOPE OF THE PROBLEM
The American Cancer Society estimated there were 17,290 new cases of esophageal cancer and 15,850 deaths from it in the United States in 2018.3 Of the 2 main histologic types of esophageal cancer, adenocarcinoma and squamous cell cancer, adenocarcinoma is more common in the United States.
The precursor lesion is Barrett esophagus, defined as an extension of salmon-colored mucosa at least 1 cm into the tubular esophagus proximal to the gastroesophageal junction, with biopsy confirmation of intestinal metaplasia.4
The natural course of progression to dysplasia and cancer in Barrett esophagus is unknown but is thought to be stepwise, from no dysplasia to low-grade dysplasia to high-grade dysplasia and cancer, and the cancer risk depends on the degree of dysplasia: the annual risk is 0.33% if there is no dysplasia, 0.54% with low-grade dysplasia, and 7% with high-grade dysplasia.4
Although all cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus,1 more than 90% of patients with newly diagnosed esophageal adenocarcinoma do not have a prior diagnosis of Barrett esophagus.5 Therefore, there is a substantial unmet need to expand screening for Barrett esophagus in people at risk.
GASTROESOPHAGEAL REFLUX DISEASE IS A RISK FACTOR FOR CANCER
The rationale behind screening is that detecting Barrett esophagus early and intervening in a timely manner in patients at higher risk of developing adenocarcinoma will decrease mortality.
Chronic gastroesophageal reflux disease is a strong risk factor for esophageal adenocarcinoma (odds ratio [OR] 7.7, 95% confidence interval [CI] 5.3–11.4), and the risk increases when symptoms are long-standing (> 20 years) or severe (OR 43.5, 95% CI 18.3–103.5) or occur daily (OR 5.5, 95% CI 3.2–9.3).6
Reflux symptoms are scored as follows:
- Heartburn only, 1 point
- Regurgitation only, 1 point
- Heartburn with regurgitation, 1.5 points
- Nightly symptoms (2 points if yes, 0 if no)
- Symptoms once a week, 0 points; 2 to 6 times a week, 1 point; 7 to 15 times a week, 2 points; more than 15 times a week, 3 points.6
A score of 4.5 or higher indicates severe reflux disease. However, it is worth noting that the annual incidence of esophageal adenocarcinoma in patients with long-term gastroesophageal reflux disease is less than 0.001%.7
RISK FACTORS FOR BARRETT ESOPHAGUS
Risk factors for Barrett esophagus include:
Male sex. Barrett esophagus is more prevalent in men than in women, at a ratio of 2 to 1; but in individuals under age 50, the ratio is 4 to 1.8
Age 50 or older. Barrett esophagus is usually diagnosed in the sixth to seventh decade of life, and the prevalence increases from 2.1% in the third decade to 9.3% in the sixth decade.9
White race. It is more prevalent in whites than in blacks (5.0% vs 1.5%, P < .0001).10
Central obesity. Waist circumference is an independent risk factor: every 5-cm increase carries an OR of 1.14 (95% CI 1.03–1.27, P = .02).11
Cigarette smoking increases the risk of Barrett esophagus (OR 1.42; 95% CI 1.15–1.76).12
A family history of Barrett esophagus or esophageal adenocarcinoma is a strong risk factor (OR 12, 95% CI 3.3–44.8). In 1 study, the risk in first- and second-degree relatives of patients with Barrett esophagus was 24%, compared with 5% in a control population (P < .005).13
SCREENING GUIDELINES AND DRAWBACKS
The standard screening method is esophagogastroduodenoscopy with sedation, with careful visual inspection and 4-quadrant biopsies every 2 cm using the Seattle protocol, ie, including biopsy of any mucosal irregularities in salmon-colored mucosa above the gastroesophageal junction (Figure 1).4
Endoscopic screening is cost-effective, costing $10,440 per quality-adjusted life-year saved, which is well below the accepted threshold of less than $100,000.14 However, it is still expensive, invasive, and not ideal for screening large populations.
Less-invasive methods under study
Less-invasive, less-expensive methods being tested for mass screening include:
Unsedated transnasal endoscopy. Done with only topical anesthesia, it has high diagnostic accuracy and is quicker and more cost-effective than standard esophagogastroduodenoscopy, with fewer adverse effects. However, the procedure has not yet gained widespread acceptance for regular use by gastroenterologists.15
A swallowable sponge. Another promising test is cell collection using the Cytosponge Cell Collection Device (Medtronic, Minneapolis, MN). An encapsulated compressed sponge with a string attached is swallowed; in the stomach, the capsule dissolves, and the sponge expands and is then withdrawn using the attached string. The obtained cytology sample from the lower esophagus is then tested for trefoil factor 3, a protein biomarker for Barrett esophagus.16
A retractable balloon. The EsoCheck Cell Collection Device is a retractable balloon attached to a string. When swallowed, it gathers distal esophageal cells for detecting methylated DNA markers for Barrett esophagus.17
Esophageal capsule endoscopy uses a camera to visualize the esophagus, but lacks the ability to obtain biopsy samples.
Other screening methods are being tested, although data are limited. Liquid biopsy uses a blood sample to detect microRNAs that are dysregulated in cancer. The “electronic nose” is a device that detects exhaled volatile organic compounds altered in Barrett esophagus. Another test involves taking an oral wash sample to study the oral microbiome for a pattern associated with adenocarcinoma.18–21
SURVEILLANCE: WHAT’S INVOLVED, WHAT’S AVAILABLE
Surveillance in Barrett esophagus aims to detect premalignant changes or early-stage adenocarcinoma to provide longer survival and lower cancer-related mortality. Recent evidence suggests that patients with esophageal adenocarcinoma that is diagnosed in a Barrett esophagus surveillance program have an earlier stage of disease and therefore a survival benefit.22
Patient education is essential
Before enrolling a patient in a surveillance program, the clinician should explain the risks, benefits, and limitations, the importance of periodic endoscopy, and the possible eventual need for endoscopic therapy or surgery.
The endoscopic procedure
Surveillance involves examination by high-definition white-light endoscopy, with random 4-quadrant biopsies every 2 cm (or every 1 cm in patients with a history of dysplasia) and biopsy of any mucosal irregularity (nodule, ulcer, or other visible lesion). The degree of dysplasia determines the frequency of follow-up surveillance intervals and the need for endoscopic eradication therapy, as presented in professional society guidelines (Table 1).4,23,24
Advanced methods for detecting dysplasia
Newer endoscopic surveillance techniques include dye-based chromoendoscopy, narrow-band imaging, confocal laser endomicroscopy, volumetric laser endomicroscopy, and wide-area transepithelial sampling with computer-assisted 3-dimensional analysis. All these techniques are used to increase the detection of dysplasia. Of these, dye-based chromoendoscopy, narrow-band imaging, and confocal laser endomicroscopy meet current criteria of the American Society for Gastrointestinal Endoscopy for preservation and incorporation of valuable endoscopic innovations.23
MANAGEMENT OF NONDYSPLASTIC BARRETT ESOPHAGUS
A proton pump inhibitor (PPI) is recommended to control reflux symptoms in patients with nondysplastic Barrett esophagus. But it is important to counsel patients on additional ways to protect against esophageal adenocarcinoma, such as:
- Low to moderate alcohol consumption
- Regular physical activity
- Increased dietary intake of fruits, vegetables, folate, fiber, beta-carotene, and vitamin C
- Weight control
- Smoking cessation.25
Surveillance endoscopy with 4-quadrant biopsies at 2-cm intervals is recommended every 3 to 5 years (Table 1).
DOES CHEMOPREVENTION HAVE A ROLE?
Chemoprevention is an exciting area of research in preventing progression to adenocarcinoma in patients with Barrett esophagus. Various drugs such as aspirin, other nonsteroidal anti-inflammatory drugs (NSAIDs), PPIs, metformin, and statins have been studied.
Aspirin
Aspirin has been shown to prevent development of Barrett esophagus in patients with reflux disease,26 but more studies are needed to validate those findings.
PPIs
Gastroesophageal reflux disease is a primary risk factor for esophageal adenocarcinoma, and gastric acid suppression with PPIs reduces cancer risk. PPI therapy is associated with a 71% decrease in the risk of high-grade dysplasia and adenocarcinoma in patients with Barrett esophagus (OR 0.29, 95% CI 0.12–0.79).27 Long-term therapy (> 2 to 3 years) has a higher protective effect (adjusted OR 0.45, 95% CI 0.19–1.06) than short-term therapy (< 2 to 3 years) (adjusted OR 1.09, 95% CI 0.47–2.56).27
NSAIDs
NSAIDs, including aspirin, have been associated with decreased risk of colon, stomach, lung, breast, and esophageal cancer due to their potential to inhibit cyclooxygenase 2 (COX-2) enzymes.
A meta-analysis demonstrated that aspirin and NSAIDs led to a 32% reduction in the risk of adenocarcinoma (OR 0.68, 95% CI 0.56–0.83). The benefit was even greater if the drug was taken daily or more frequently (OR 0.56, 95% CI 0.43–0.73, P < .001) or was taken for 10 or more years (OR 0.63, 95% CI 0.45–0.90, P = .04).28
PPI plus aspirin
The best evidence for the role of PPIs and aspirin in reducing the risk of dysplasia comes from the Aspirin and Esomeprazole Chemoprevention in Barrett’s Metaplasia Trial.29 This randomized, controlled trial compared 4 regimens consisting of esomeprazole (a PPI) in either a high dose (40 mg twice daily) or a low dose (20 mg once daily) plus either aspirin (300 or 320 mg per day) or no aspirin in 2,557 patients with Barrett esophagus. The composite end point was the time to all-cause mortality, adenocarcinoma, or high-grade dysplasia.
At a median follow-up of 8.9 years, the combination of high-dose esomeprazole plus aspirin had the strongest effect compared with low-dose esomeprazole without aspirin (time ratio 1.59, 95% CI 1.14–2.23, P = .0068). The number needed to treat was 34 for esomeprazole and 43 for aspirin.29
Based on these data, we can conclude that aspirin and PPIs can prevent dysplasia and all-cause mortality in Barrett esophagus.
Metformin: No evidence of benefit
Metformin was studied as a protective agent against obesity-associated cancers including esophageal adenocarcinoma, as it reduces insulin levels.
In a randomized controlled trial30 in 74 patients with Barrett esophagus, metformin (starting at 500 mg daily, increasing to 2,000 mg/day by week 4) was compared with placebo. At 12 weeks, the percent change in esophageal levels of the biomarker pS6K1—an intracellular mediator of insulin and insulin-like growth factor activation in Barrett epithelium—did not differ significantly between the 2 groups (1.4% with metformin vs −14.7% with placebo; 1-sided P = .80). This suggested that metformin did not significantly alter proliferation or apoptosis in Barrett epithelium, despite reducing serum insulin levels and insulin resistance. Thus, metformin did not demonstrate a chemoprotective effect in preventing the progression of Barrett esophagus to adenocarcinoma.
Vitamin D: No evidence of benefit
Vitamin D affects genes regulating proliferation, apoptosis, and differentiation, and has therefore been studied as a potential antineoplastic agent. Its deficiency has also been associated with increased risk of esophageal adenocarcinoma. However, its efficacy in chemoprevention is unclear.31
One study found no association between serum 25-hydroxyvitamin D levels and prevalence of dysplasia in Barrett esophagus (P = .90). An increase in vitamin D levels had no effect on progression to dysplasia or cancer (for every 5-nmol/L increase from baseline, hazard ratio 0.98, P = .62).32
In another study, supplementation with vitamin D3 (cholecalciferol 50,000 IU weekly) plus a PPI for 12 weeks significantly improved the serum 25-hydroxyvitamin D levels without significant changes in gene expression from Barrett epithelium.33 These findings were confirmed in a meta-analysis that showed no consistent association between vitamin D exposure and risk of esophageal neoplasm.34
Thus, there is currently no evidence to support vitamin D for chemoprevention in Barrett esophagus or esophageal adenocarcinoma.
Statins
In addition to lowering cholesterol, statins have antiproliferative, pro-apoptotic, anti-angiogenic, and immunomodulatory effects that prevent cancer, leading to a 41% reduction in the risk of adenocarcinoma in patients with Barrett esophagus in one study (adjusted OR 0.59, 95% CI 0.45–0.78); the number needed to treat with statins to prevent 1 case of adenocarcinoma was 389.35
A meta-analysis also showed that statin use was associated with a lower risk of progression of Barrett esophagus (OR 0.48, 95% CI 0.31–0.73).36
In general, statins appear promising for chemoprevention, but more study is needed.
When is chemoprevention appropriate?
Chemoprevention is not recommended for all patients with Barrett esophagus, given that the condition affects 1% to 2% of the US adult population, and very few patients have progression to esophageal adenocarcinoma. Rather, chemoprevention may be considered in patients with Barrett esophagus and multiple risk factors for adenocarcinoma.
INDEFINITE DYSPLASIA
In Barrett esophagus with indefinite dysplasia, either the epithelial abnormalities are insufficient for a diagnosis of dysplasia, or the nature of the epithelial abnormalities is uncertain due to inflammation or technical difficulties with specimen processing. The risk of high-grade dysplasia or cancer within 1 year of the diagnosis of indefinite dysplasia varies between 1.9% and 15%.37 The recommendation for management is to optimize acid-suppressive therapy for 3 to 6 months and then to repeat esophagogastroduodenoscopy. If indefinite dysplasia is noted again, repeat endoscopy in 12 months is recommended.2
ENDOSCOPIC ERADICATION: AN OVERVIEW
Because dysplasia in Barrett esophagus carries a high risk of progression to cancer, the standard of care is endoscopic mucosal resection of visible lesions, followed by ablation of the flat mucosa, with the aim of achieving complete eradication of intestinal metaplasia.4,38 The initial endoscopic treatment is followed by outpatient sessions every 8 to 10 weeks until the dysplasia is eradicated. A key part of treatment during this time is maximal acid suppression with a PPI twice daily and a histamine-2 blocker at night. In rare cases, fundoplication is required to control reflux refractory to medical therapy.
After eradication is confirmed, continued surveillance is necessary, as recurrences have been reported at a rate of 4.8% per year for intestinal metaplasia, and 2% per year for dysplasia.39
Current endoscopic resection techniques
Endoscopic resection techniques include mucosal resection, submucosal dissection, radiofrequency ablation, cryotherapy, argon plasma coagulation, and photodynamic therapy (Figure 2).
In mucosal resection, the lesion is either suctioned into a band ligator, after which a band is placed around the lesion, or suctioned into a cap fitted at the end of the endoscope, after which the lesion is removed using a snare.
In submucosal dissection, a liquid is injected into the submucosa to lift the lesion, making it easier to remove. The procedure is technically complex and requires additional training.
In radiofrequency ablation, a special catheter is passed through the endoscope to ablate the affected epithelium by thermal injury. Argon plasma coagulation works in a similar way, but uses ionized argon gas to induce thermal coagulation of metaplastic epithelium.
Cryotherapy produces cellular injury by rapid freezing and thawing of tissue using a cryogen such as liquid nitrogen or nitrous oxide.
In photodynamic therapy, a photosensitizer (porfimer sodium) is administered and taken up preferentially by metaplastic epithelium; it is then activated by transmission of red light using the endoscope, leading to destruction of the metaplastic epithelium.
Of the different techniques, radiofrequency ablation has the most evidence for efficacy and hence is the most commonly used.
All of these procedures are generally well tolerated and have favorable side-effect profiles. After radiofrequency ablation with or without mucosal resection, esophageal strictures are noted in 5.6% of patients, and bleeding and perforation occur rarely (1% and 0.6% of patients, respectively).40 Submucosal dissection is associated with a higher rate of complications such as stricture formation (11% of patients) and bleeding or perforation (1.5% of patients).41
LOW-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
Most patients with low-grade dysplasia (73%) are down-staged to nondysplastic Barrett esophagus or to indefinite for dysplasia after review by expert pathologists.42 Patients with confirmed and persistent low-grade dysplasia are at higher risk of progression.43
Once low-grade dysplasia is confirmed by a second gastrointestinal pathologist, the patient should undergo endoscopic ablation. A landmark study by Shaheen et al44 demonstrated the benefit of radiofrequency ablation in achieving complete eradication of dysplasia (90.5% vs 22.7% for a sham procedure) and complete eradication of intestinal metaplasia (77.4% vs 2.3% for a sham procedure). In another trial of 136 patients with low-grade dysplasia followed for 3 years, Phoa et al45 demonstrated that radiofrequency ablation reduced the rate of progression to high-grade dysplasia by 25% and to adenocarcinoma by 7.4% compared with endoscopic surveillance.
Patients with confirmed low-grade dysplasia who do not undergo eradication therapy should have surveillance endoscopy every 6 to 12 months (Table 1).
HIGH-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
As with low-grade dysplasia, the diagnosis of high-grade dysplasia needs to be confirmed by a second pathologist with gastrointestinal expertise. In the past, the treatment was esophagectomy, but due to lower morbidity and equivalent efficacy of radiofrequency ablation,46 the current treatment of choice is endoscopic mucosal resection of raised lesions, followed by radiofrequency ablation of the entire affected segment.
In the study by Shaheen et al,44 42 patients with high-grade dysplasia were randomized to radiofrequency ablation and 21 to a sham procedure, and 81% of ablation patients achieved complete eradication of dysplasia vs 19% with the sham procedure. Eradication of intestinal metaplasia was achieved in 77% of ablation patients vs 2% of patients with the sham therapy. Results of 3-year follow-up from the same cohort showed complete eradication of dysplasia in 98% and of intestinal metaplasia in 91%.47
Endoscopic eradication therapy is recommended for all patients with Barrett esophagus and high-grade dysplasia without a life-limiting comorbidity. Alternatively, surveillance every 3 months is an option if the patient does not wish to undergo eradication therapy. Radiofrequency ablation is more cost-effective than esophagectomy or endoscopic surveillance followed by treatment once patients develop adenocarcinoma.48,49
EARLY ESOPHAGEAL ADENOCARCINOMA: RECOMMENDED MANAGEMENT
Adenocarcinoma limited to the mucosa and without evidence of nodal involvement can be resected endoscopically. In patients with localized cancer, mucosal resection is done not only for therapeutic purposes but also for staging. Ideal management is multidisciplinary, including a gastroenterologist, thoracic surgeon, oncologist, pathologist, and radiation oncologist.
If lesions have features suggesting submucosal invasion or are greater than 1.5 cm in size, or if it is difficult to separate (ie, lift) the mucosa from the submucosal layer with injection of saline, then submucosal dissection is recommended.50 Because of the risk of metachronous lesions, ablation of the remaining Barrett esophagus mucosa is recommended after resection of cancer.
Endoscopic eradication is highly effective and durable for the treatment of intramucosal esophageal adenocarcinoma. In a study of 1,000 patients, 963 patients (96.3%) had achieved a complete response; 12 patients (3.7%) underwent surgery after eradication failed during a follow-up of almost 5 years.51 Metachronous lesions or recurrence of cancer developed during the follow-up period in 140 patients (14.5%) but were successfully treated endoscopically in 115, resulting in a long-term complete remission rate of 93.8%.
POSTABLATION MANAGEMENT
Because of the risk of recurrence of dysplasia after ablation, long-term PPI therapy and surveillance are recommended.
Surveillance endoscopy involves 4-quadrant biopsies taken every 1 cm from the entire length of segment where Barrett esophagus had been seen before ablation.
The timing of surveillance intervals depends on the preablation grade of dysplasia. For low-grade dysplasia, the recommendation is every 6 months for the first year after ablation and, if there is no recurrence of dysplasia, annually after that.2 After treatment of high-grade dysplasia or intramucosal adenocarcinoma, the recommendation is every 3 months for the first year, every 6 months in the second year, and then annually.2
- Mendes de Almeida JC, Chaves P, Pereira AD, Altorki NK. Is Barrett’s esophagus the precursor of most adenocarcinomas of the esophagus and cardia? A biochemical study. Ann Surg 1997; 226(6):725–733. pmid:9409571
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61(2):226–231. pmid:15729230
- National Cancer Institute. Cancer stat facts: esophageal cancer. https://seer.cancer.gov/statfacts/html/esoph.html. Accessed August 6, 2019.
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111(1):30–50. doi:10.1038/ajg.2015.322
- Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002; 122(1):26–33. pmid:11781277
- Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999; 340(11):825–831. doi:10.1056/NEJM199903183401101
- Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. JAMA 2002; 287(15):1972–1981. pmid:11960540
- van Blankenstein M, Looman CW, Johnston BJ, Caygill CP. Age and sex distribution of the prevalence of Barrett’s esophagus found in a primary referral endoscopy center. Am J Gastroenterol 2005; 100(3):568–576.
- Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointest Endosc 2010; 71(1):21–27. doi:10.1016/j.gie.2009.06.035
- Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett’s esophagus among racial groups in a multi-center consortium. Dig Dis Sci 2009; 54(5):964–971. doi:10.1007/s10620-009-0742-3
- Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s esophagus: a pooled analysis from the international BEACON consortium. Gut 2013; 62(12):1684–1691. doi:10.1136/gutjnl-2012-303753
- Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol 2013; 28(8):1258–1273. doi:10.1111/jgh.12230
- Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett’s esophagus, esophageal adenocarcinoma, and esophagogastric junctional adenocarcinoma in Caucasian adults. Gut 2002; 51(3):323–328. pmid:12171951
- Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 2003; 138(3):176–186. pmid:12558356
- Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol 2006; 101(12):2693–2703.
- Ross-Innes CS, Chettouh H, Achilleos A, et al; BEST2 study group. Risk stratification of Barrett’s esophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol 2017; 2(1):23–31. doi:10.1016/S2468-1253(16)30118-2
- Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018; 10(424). pii:eaao5848. doi:10.1126/scitranslmed.aao5848
- Chan DK, Zakko L, Visrodia KH, et al. Breath testing for Barrett’s esophagus using exhaled volatile organic compound profiling with an electronic nose device. Gastroenterology 2017; 152(1):24–26. doi:10.1053/j.gastro.2016.11.001
- Kumar S, Huang J, Abbassi-Ghadi N, et al. Mass spectrometric analysis of exhaled breath for the identification of volatile organic compound biomarkers in esophageal and gastric adenocarcinoma. Ann Surg 2015; 262(6):981–990. doi:10.1097/SLA.0000000000001101
- Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res 2017; 77(23):6777–6787. doi:10.1158/0008-5472.CAN-17-1296
- Mallick R, Patnaik SK, Wani S, Bansal A. A systematic review of esophageal microrna markers for diagnosis and monitoring of Barrett’s esophagus. Dig Dis Sci 2016; 61(4):1039–1050. doi:10.1007/s10620-015-3959-3
- Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology 2018; 154(8):2068–2086.e5. doi:10.1053/j.gastro.2018.02.022
- ASGE Technology Committee; Thosani N, Abu Dayyeh BK, Sharma P, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE preservation and incorporation of valuable endoscopic innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc 2016; 83(4):684–698.e7. doi:10.1016/j.gie.2016.01.007
- Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 2011; 140(3):e18–e52. doi:10.1053/j.gastro.2011.01.031
- Castro C, Peleteiro B, Lunet N. Modifiable factors and esophageal cancer: a systematic review of published meta-analyses. J Gastroenterol 2018; 53(1):37–51. doi:10.1007/s00535-017-1375-5
- Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012; 10(7):722–727. doi:10.1016/j.cgh.2012.02.031
- Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gut 2014; 63(8):1229–1237. doi:10.1136/gutjnl-2013-305997
- Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012; 142(3):442–452.e5. doi:10.1053/j.gastro.2011.11.019
- Jankowski JAZ, de Caestecker J, Love SB, et al; AspECT Trial Team. Esomeprazole and aspirin in Barrett’s esophagus (AspECT): a randomised factorial trial. Lancet 2018; 392(10145):400–408. doi:10.1016/S0140-6736(18)31388-6
- Chak A, Buttar NS, Foster NR, et al; Cancer Prevention Network. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2015; 13(4):665–672.e1–e4. doi:10.1016/j.cgh.2014.08.040
- Rouphael C, Kamal A, Sanaka MR, Thota PN. Vitamin D in esophageal cancer: is there a role for chemoprevention? World J Gastrointest Oncol 2018; 10(1):23–30. doi:10.4251/wjgo.v10.i1.23
- Thota PN, Kistangari G, Singh P, et al. Serum 25-hydroxyvitamin D levels and the risk of dysplasia and esophageal adenocarcinoma in patients with Barrett’s esophagus. Dig Dis Sci 2016; 61(1):247–254. doi:10.1007/s10620-015-3823-5
- Cummings LC, Thota PN, Willis JE, et al. A nonrandomized trial of vitamin D supplementation for Barrett’s esophagus. PLoS One 2017;1 2(9):e0184928. doi:10.1371/journal.pone.0184928
- Zgaga L, O’Sullivan F, Cantwell MM, Murray LJ, Thota PN, Coleman HG. Markers of vitamin D exposure and esophageal cancer risk: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2016; 25(6):877–886. doi:10.1158/1055-9965.EPI-15-1162
- Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11(6):620–629. doi:10.1016/j.cgh.2012.12.036
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors associated with progression of Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 6(7):1046–1055.e8. doi:10.1016/j.cgh.2017.11.044
- Thota PN, Kistangari G, Esnakula AK, Gonzalo DH, Liu XL. Clinical significance and management of Barrett’s esophagus with epithelial changes indefinite for dysplasia. World J Gastrointest Pharmacol Ther 2016; 7(3):406–411. doi:10.4292/wjgpt.v7.i3.406
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012; 143(2):336–346. doi:10.1053/j.gastro.2012.04.032
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017; 85(3):482–495.e4. doi:10.1016/j.gie.2016.09.022
- Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14(8):1086–1095.e6. doi:10.1016/j.cgh.2016.04.001
- Yang D, Zou F, Xiong S, Forde JJ, Wang Y, Draganov PV. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc 2018; 87(6):1383–1393. doi:10.1016/j.gie.2017.09.038
- Duits LC, Phoa KN, Curvers WL, et al. Barrett’s esophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015; 64(5):700–706. doi:10.1136/gutjnl-2014-307278
- Duits LC, van der Wel MJ, Cotton CC, et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology 2017; 152(5):993–1001.e1. doi:10.1053/j.gastro.2016.12.008
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009; 360(22):2277–2288. doi:10.1056/NEJMoa0808145
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014; 311(12):1209–1217. doi:10.1001/jama.2014.2511
- Hu Y, Puri V, Shami VM, Stukenborg GJ, Kozower BD. Comparative effectiveness of esophagectomy versus endoscopic treatment for esophageal high-grade dysplasia. Ann Surg 2016; 263(4):719–726. doi:10.1097/SLA.0000000000001387
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011; 141(2):460–468. doi:10.1053/j.gastro.2011.04.061
- Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology 2012; 143(3):567–575. doi:10.1053/j.gastro.2012.05.010
- Boger PC, Turner D, Roderick P, Patel P. A UK-based cost-utility analysis of radiofrequency ablation or oesophagectomy for the management of high-grade dysplasia in Barrett’s esophagus. Aliment Pharmacol Ther 2010; 32(11-12):1332–1342. doi:10.1111/j.1365-2036.2010.04450.x
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47(9):829–854. doi:10.1055/s-0034-1392882
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014; 146(3):652–660.e1. doi:10.1053/j.gastro.2013.11.006
- Mendes de Almeida JC, Chaves P, Pereira AD, Altorki NK. Is Barrett’s esophagus the precursor of most adenocarcinomas of the esophagus and cardia? A biochemical study. Ann Surg 1997; 226(6):725–733. pmid:9409571
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61(2):226–231. pmid:15729230
- National Cancer Institute. Cancer stat facts: esophageal cancer. https://seer.cancer.gov/statfacts/html/esoph.html. Accessed August 6, 2019.
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111(1):30–50. doi:10.1038/ajg.2015.322
- Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002; 122(1):26–33. pmid:11781277
- Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999; 340(11):825–831. doi:10.1056/NEJM199903183401101
- Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. JAMA 2002; 287(15):1972–1981. pmid:11960540
- van Blankenstein M, Looman CW, Johnston BJ, Caygill CP. Age and sex distribution of the prevalence of Barrett’s esophagus found in a primary referral endoscopy center. Am J Gastroenterol 2005; 100(3):568–576.
- Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointest Endosc 2010; 71(1):21–27. doi:10.1016/j.gie.2009.06.035
- Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett’s esophagus among racial groups in a multi-center consortium. Dig Dis Sci 2009; 54(5):964–971. doi:10.1007/s10620-009-0742-3
- Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s esophagus: a pooled analysis from the international BEACON consortium. Gut 2013; 62(12):1684–1691. doi:10.1136/gutjnl-2012-303753
- Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol 2013; 28(8):1258–1273. doi:10.1111/jgh.12230
- Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett’s esophagus, esophageal adenocarcinoma, and esophagogastric junctional adenocarcinoma in Caucasian adults. Gut 2002; 51(3):323–328. pmid:12171951
- Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 2003; 138(3):176–186. pmid:12558356
- Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol 2006; 101(12):2693–2703.
- Ross-Innes CS, Chettouh H, Achilleos A, et al; BEST2 study group. Risk stratification of Barrett’s esophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol 2017; 2(1):23–31. doi:10.1016/S2468-1253(16)30118-2
- Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018; 10(424). pii:eaao5848. doi:10.1126/scitranslmed.aao5848
- Chan DK, Zakko L, Visrodia KH, et al. Breath testing for Barrett’s esophagus using exhaled volatile organic compound profiling with an electronic nose device. Gastroenterology 2017; 152(1):24–26. doi:10.1053/j.gastro.2016.11.001
- Kumar S, Huang J, Abbassi-Ghadi N, et al. Mass spectrometric analysis of exhaled breath for the identification of volatile organic compound biomarkers in esophageal and gastric adenocarcinoma. Ann Surg 2015; 262(6):981–990. doi:10.1097/SLA.0000000000001101
- Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res 2017; 77(23):6777–6787. doi:10.1158/0008-5472.CAN-17-1296
- Mallick R, Patnaik SK, Wani S, Bansal A. A systematic review of esophageal microrna markers for diagnosis and monitoring of Barrett’s esophagus. Dig Dis Sci 2016; 61(4):1039–1050. doi:10.1007/s10620-015-3959-3
- Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology 2018; 154(8):2068–2086.e5. doi:10.1053/j.gastro.2018.02.022
- ASGE Technology Committee; Thosani N, Abu Dayyeh BK, Sharma P, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE preservation and incorporation of valuable endoscopic innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc 2016; 83(4):684–698.e7. doi:10.1016/j.gie.2016.01.007
- Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 2011; 140(3):e18–e52. doi:10.1053/j.gastro.2011.01.031
- Castro C, Peleteiro B, Lunet N. Modifiable factors and esophageal cancer: a systematic review of published meta-analyses. J Gastroenterol 2018; 53(1):37–51. doi:10.1007/s00535-017-1375-5
- Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012; 10(7):722–727. doi:10.1016/j.cgh.2012.02.031
- Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gut 2014; 63(8):1229–1237. doi:10.1136/gutjnl-2013-305997
- Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012; 142(3):442–452.e5. doi:10.1053/j.gastro.2011.11.019
- Jankowski JAZ, de Caestecker J, Love SB, et al; AspECT Trial Team. Esomeprazole and aspirin in Barrett’s esophagus (AspECT): a randomised factorial trial. Lancet 2018; 392(10145):400–408. doi:10.1016/S0140-6736(18)31388-6
- Chak A, Buttar NS, Foster NR, et al; Cancer Prevention Network. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2015; 13(4):665–672.e1–e4. doi:10.1016/j.cgh.2014.08.040
- Rouphael C, Kamal A, Sanaka MR, Thota PN. Vitamin D in esophageal cancer: is there a role for chemoprevention? World J Gastrointest Oncol 2018; 10(1):23–30. doi:10.4251/wjgo.v10.i1.23
- Thota PN, Kistangari G, Singh P, et al. Serum 25-hydroxyvitamin D levels and the risk of dysplasia and esophageal adenocarcinoma in patients with Barrett’s esophagus. Dig Dis Sci 2016; 61(1):247–254. doi:10.1007/s10620-015-3823-5
- Cummings LC, Thota PN, Willis JE, et al. A nonrandomized trial of vitamin D supplementation for Barrett’s esophagus. PLoS One 2017;1 2(9):e0184928. doi:10.1371/journal.pone.0184928
- Zgaga L, O’Sullivan F, Cantwell MM, Murray LJ, Thota PN, Coleman HG. Markers of vitamin D exposure and esophageal cancer risk: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2016; 25(6):877–886. doi:10.1158/1055-9965.EPI-15-1162
- Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11(6):620–629. doi:10.1016/j.cgh.2012.12.036
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors associated with progression of Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 6(7):1046–1055.e8. doi:10.1016/j.cgh.2017.11.044
- Thota PN, Kistangari G, Esnakula AK, Gonzalo DH, Liu XL. Clinical significance and management of Barrett’s esophagus with epithelial changes indefinite for dysplasia. World J Gastrointest Pharmacol Ther 2016; 7(3):406–411. doi:10.4292/wjgpt.v7.i3.406
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012; 143(2):336–346. doi:10.1053/j.gastro.2012.04.032
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017; 85(3):482–495.e4. doi:10.1016/j.gie.2016.09.022
- Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14(8):1086–1095.e6. doi:10.1016/j.cgh.2016.04.001
- Yang D, Zou F, Xiong S, Forde JJ, Wang Y, Draganov PV. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc 2018; 87(6):1383–1393. doi:10.1016/j.gie.2017.09.038
- Duits LC, Phoa KN, Curvers WL, et al. Barrett’s esophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015; 64(5):700–706. doi:10.1136/gutjnl-2014-307278
- Duits LC, van der Wel MJ, Cotton CC, et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology 2017; 152(5):993–1001.e1. doi:10.1053/j.gastro.2016.12.008
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009; 360(22):2277–2288. doi:10.1056/NEJMoa0808145
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014; 311(12):1209–1217. doi:10.1001/jama.2014.2511
- Hu Y, Puri V, Shami VM, Stukenborg GJ, Kozower BD. Comparative effectiveness of esophagectomy versus endoscopic treatment for esophageal high-grade dysplasia. Ann Surg 2016; 263(4):719–726. doi:10.1097/SLA.0000000000001387
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011; 141(2):460–468. doi:10.1053/j.gastro.2011.04.061
- Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology 2012; 143(3):567–575. doi:10.1053/j.gastro.2012.05.010
- Boger PC, Turner D, Roderick P, Patel P. A UK-based cost-utility analysis of radiofrequency ablation or oesophagectomy for the management of high-grade dysplasia in Barrett’s esophagus. Aliment Pharmacol Ther 2010; 32(11-12):1332–1342. doi:10.1111/j.1365-2036.2010.04450.x
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47(9):829–854. doi:10.1055/s-0034-1392882
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014; 146(3):652–660.e1. doi:10.1053/j.gastro.2013.11.006
KEY POINTS
- Screening is recommended for patients with long-standing reflux symptoms (> 5 years) and 1 or more key risk factors: male sex, age over 50, white race, central obesity, and history of smoking.
- In Barrett esophagus without dysplasia, surveillance endoscopy is recommended every 3 to 5 years to detect dysplasia and early esophageal adenocarcinoma.
- The recommended treatment of dysplasia is endoscopic eradication followed by surveillance endoscopy.
SEEDS for success: Lifestyle management in migraine
Migraine is the second leading cause of years of life lived with a disability globally.1 It affects people of all ages, but particularly during the years associated with the highest productivity in terms of work and family life.
Migraine is a genetic neurologic disease that can be influenced or triggered by environmental factors. However, triggers do not cause migraine. For example, stress does not cause migraine, but it can exacerbate it.
Primary care physicians can help patients reduce the likelihood of a migraine attack, the severity of symptoms, or both by offering lifestyle counseling centered around the mnemonic SEEDS: sleep, exercise, eat, diary, and stress. In this article, each factor is discussed individually for its current support in the literature along with best-practice recommendations.
S IS FOR SLEEP
Before optimizing sleep hygiene, screen for sleep apnea, especially in those who have chronic daily headache upon awakening. An excellent tool is the STOP-Bang screening questionnaire5 (www.stopbang.ca/osa/screening.php). Patients respond “yes” or “no” to the following questions:
- Snoring: Do you snore loudly (louder than talking or loud enough to be heard through closed doors)?
- Tired: Do you often feel tired, fatigued, or sleepy during the daytime?
- Observed: Has anyone observed you stop breathing during your sleep?
- Pressure: Do you have or are you being treated for high blood pressure?
- Body mass index greater than 35 kg/m2?
- Age over 50?
- Neck circumference larger than 40 cm (females) or 42 cm (males)?
- Gender—male?
Each “yes” answer is scored as 1 point. A score less than 3 indicates low risk of obstructive sleep apnea; 3 to 4 indicates moderate risk; and 5 or more indicates high risk. Optimization of sleep apnea with continuous positive airway pressure therapy can improve sleep apnea headache.6 The improved sleep from reduced arousals may also mitigate migraine symptoms.
Behavioral modification for sleep hygiene can convert chronic migraine to episodic migraine.7 One such program is stimulus control therapy, which focuses on using cues to initiate sleep (Table 1). Patients are encouraged to keep the bedroom quiet, dark, and cool, and to go to sleep at the same time every night. Importantly, the bed should be associated only with sleep. If patients are unable to fall asleep within 20 to 30 minutes, they should leave the room so they do not associate the bed with frustration and anxiety. Use of phones, tablets, and television in the bedroom is discouraged as these devices may make it more difficult to fall asleep.8
The next option is sleep restriction, which is useful for comorbid insomnia. Patients keep a sleep diary to better understand their sleep-wake cycle. The goal is 90% sleep efficiency, meaning that 90% of the time in bed (TIB) is spent asleep. For example, if the patient is in bed 8 hours but asleep only 4 hours, sleep efficiency is 50%. The goal is to reduce TIB to match the time asleep and to agree on a prescribed daily wake-up time. When the patient is consistently sleeping 90% of the TIB, add 30-minute increments until he or she is appropriately sleeping 7 to 8 hours at night.9 Naps are not recommended.
Let patients know that their migraine may worsen until a new routine sleep pattern emerges. This method is not recommended for patients with untreated sleep apnea.
E IS FOR EXERCISE
Exercise is broadly recommended for a healthy lifestyle; some evidence suggests that it can also be useful in the management of migraine.10 Low levels of physical activity and a sedentary lifestyle are associated with migraine.11 It is unclear if patients with migraine are less likely to exercise because they want to avoid triggering a migraine or if a sedentary lifestyle increases their risk.
Exercise has been studied for its prophylactic benefits in migraine, and one hypothesis relates to beta-endorphins. Levels of beta-endorphins are reduced in the cerebrospinal fluid of patients with migraine.12 Exercise programs may increase levels while reducing headache frequency and duration.13 One study showed that pain thresholds do not change with exercise programs, suggesting that it is avoidance behavior that is positively altered rather than the underlying pain pathways.14
A systematic review and meta-analysis based on 5 randomized controlled trials and 1 nonrandomized controlled clinical trial showed that exercise reduced monthly migraine days by only 0.6 (± 0.3) days, but the data also suggested that as the exercise intensity increased, so did the positive effects.10
Some data suggest that exercise may also reduce migraine duration and severity as well as the need for abortive medication.10 Two studies in this systematic review15,16 showed that exercise benefits were equivalent to those of migraine preventives such as amitriptyline and topiramate; the combination of amitriptyline and exercise was more beneficial than exercise alone. Multiple types of exercise were beneficial, including walking, jogging, cross-training, and cycling when done for least 6 weeks and for 30 to 50 minutes 3 to 5 times a week.
These findings are in line with the current recommendations for general health from the American College of Sports Medicine, ie, moderate to vigorous cardiorespiratory exercise for 30 to 60 minutes 3 to 5 times a week (or 150 minutes per week). The daily exercise can be continuous or done in intervals of less than 20 minutes. For those with a sedentary lifestyle, as is seen in a significant proportion of the migraine population, light to moderate exercise for less than 20 minutes is still beneficial.17
Based on this evidence, the best current recommendation for patients with migraine is to engage in graded moderate cardiorespiratory exercise, although any exercise is better than none. If a patient is sedentary or has poor exercise tolerance, or both, exercising once a week for shorter time periods may be a manageable place to start.
Some patients may identify exercise as a trigger or exacerbating factor in migraine. These patients may need appropriate prophylactic and abortive therapies before starting an exercise regimen.
THE SECOND E IS FOR EAT (FOOD AND DRINK)
Many patients believe that some foods trigger migraine attacks, but further study is needed. The most consistent food triggers appear to be red wine and caffeine (withdrawal).18,19 Interestingly, patients with migraine report low levels of alcohol consumption,20 but it is unclear if that is because alcohol has a protective effect or if patients avoid it.
Some patients may crave certain foods in the prodromal phase of an attack, eat the food, experience the attack, and falsely conclude that the food caused the attack.21 Premonitory symptoms include fatigue, cognitive changes, homeostatic changes, sensory hyperresponsiveness, and food cravings.21 It is difficult to distinguish between premonitory phase food cravings and true triggers because premonitory symptoms can precede headache by 48 to 72 hours, and the timing for a trigger to be considered causal is not known.22
Chocolate is often thought to be a migraine trigger, but the evidence argues against this and even suggests that sweet cravings are a part of the premonitory phase.23 Monosodium glutamate is often identified as a trigger as well, but the literature is inconsistent and does not support a causal relationship.24 Identifying true food triggers in migraine is difficult, and patients with migraine may have poor quality diets, with some foods acting as true triggers for certain patients.25 These possibilities have led to the development of many “migraine diets,” including elimination diets.
Elimination diets
Elimination diets involve avoiding specific food items over a period of time and then adding them back in one at a time to gauge whether they cause a reaction in the body. A number of these diets have been studied for their effects on headache and migraine:
Gluten-free diets restrict foods that contain wheat, rye, and barley. A systematic review of gluten-free diets in patients with celiac disease found that headache or migraine frequency decreased by 51.6% to 100% based on multiple cohort studies (N = 42,388).26 There are no studies on the use of a gluten-free diet for migraine in patients without celiac disease.
Immunoglobulin G-elimination diets restrict foods that serve as antigens for IgG. However, data supporting these diets are inconsistent. Two small randomized controlled trials found that the diets improved migraine symptoms, but a larger study found no improvement in the number of migraine days at 12 weeks, although there was an initially significant effect at 4 weeks.27–29
Antihistamine diets restrict foods that have high levels of histamines, including fermented dairy, vegetables, soy products, wine, beer, alcohol, and those that cause histamine release regardless of IgE testing results. A prospective single-arm study of antihistamine diets in patients with chronic headache reported symptom improvement, which could be applied to certain comorbidities such as mast cell activation syndrome.30 Another prospective nonrandomized controlled study eliminated foods based on positive IgE skin-prick testing for allergy in patients with recurrent migraine and found that it reduced headache frequency.31
Tyramine-free diets are often recommended due to the presumption that tyramine-containing foods (eg, aged cheese, cured or smoked meats and fish, and beer) are triggers. However, multiple studies have reviewed this theory with inconsistent results,32 and the only study of a tyramine-free diet was negative.33 In addition, commonly purported high-tyramine foods have lower tyramine levels than previously thought.34
Low-fat diets in migraine are supported by 2 small randomized controlled trials and a prospective study showing a decrease in symptom severity; the results for frequency are inconsistent.35–37
Low-glycemic index diets are supported in migraine by 1 randomized controlled trial that showed improvement in migraine frequency in a diet group and in a control group of patients who took a standard migraine-preventive medication to manage their symptoms.38
Other migraine diets
Diets high in certain foods or ingredient ratios, as opposed to elimination diets, have also been studied in patients with migraine. One promising diet containing high levels of omega-3 fatty acids and low levels of omega-6 fatty acids was shown in a systematic review to reduce the duration of migraine but not the frequency or severity.39 A more recent randomized controlled trial of this diet in chronic migraine also showed that it decreased migraine frequency.40
The ketogenic diet (high fat, low carbohydrate) had promising results in a randomized controlled trial in overweight women with migraine and in a prospective study.41,42 However, a prospective study of the Atkins diet in teenagers with chronic daily headaches showed no benefit.43 The ketogenic diet is difficult to follow and may work in part due to weight loss alone, although ketogenesis itself may also play a role.41,44
Sodium levels have been shown to be higher in the cerebrospinal fluid of patients with migraine than in controls, particularly during an attack.45 For a prehypertensive population or an elderly population, a low-sodium diet may be beneficial based on 2 prospective trials.46,47 However, a younger female population without hypertension and low-to-normal body mass index had a reduced probability of migraine while consuming a high-sodium diet.48
Counseling about sodium intake should be tailored to specific patient populations. For example, a diet low in sodium may be appropriate for patients with vascular risk factors such as hypertension, whereas a high-sodium diet may be appropriate in patients with comorbidities like postural tachycardia syndrome or in those with a propensity for low blood pressure or low body mass index.
Encourage routine meals and hydration
The standard advice for patients with migraine is to consume regular meals. Headaches have been associated with fasting, and those with migraine are predisposed to attacks in the setting of fasting.49,50 Migraine is more common when meals are skipped, particularly breakfast.51
It is unclear how fasting lowers the migraine threshold. Nutritional studies show that skipping meals, particularly breakfast, increases low-grade inflammation and impairs glucose metabolism by affecting insulin and fat oxidation metabolism.52 However, hypoglycemia itself is not a consistent cause of headache or migraine attacks.53 As described above, a randomized controlled trial of a low-glycemic index diet actually decreased migraine frequency and severity.38 Skipping meals also reduces energy and is associated with reduced physical activity, perhaps leading to multiple compounding triggers that further lower the migraine threshold.54,55
When counseling patients about the need to eat breakfast, consider what they normally consume (eg, is breakfast just a cup of coffee?). Replacing simple carbohydrates with protein, fats, and fiber may be beneficial for general health, but the effects on migraine are not known, nor is the optimal composition of breakfast foods.55
The optimal timing of breakfast relative to awakening is also unclear, but in general, it should be eaten within 30 to 60 minutes of rising. Also consider patients’ work hours—delayed-phase or shift workers have altered sleep cycles.
Recommendations vary in regard to hydration. Headache is associated with fluid restriction and dehydration,56,57 but only a few studies suggest that rehydration and increased hydration status can improve migraine.58 In fact, a single post hoc analysis of a metoclopramide study showed that intravenous fluid alone for patients with migraine in the emergency room did not improve pain outcomes.59
The amount of water patients should drink daily in the setting of migraine is also unknown, but a study showed benefit with 4 L, which equates to a daily intake of 16 eight-ounce glasses.60 One review on general health that could be extrapolated given the low risk of the intervention indicated that 1.8 L daily (7 to 8 eight-ounce glasses) promoted a euhydration status in most people, although many factors contribute to hydration status.61
Caffeine intake is also a major consideration. Caffeine is a nonspecific adenosine receptor antagonist that modulates adenosine receptors like the pronociceptive 2A receptor, leading to changes integral to the neuropathophysiology of migraine.62 Caffeine has analgesic properties at doses greater than 65 to 200 mg and augments the effects of analgesics such as acetaminophen and aspirin. Chronic caffeine use can lead to withdrawal symptoms when intake is stopped abruptly; this is thought to be due to upregulation of adenosine receptors, but the effect varies based on genetic predisposition.19
The risk of chronic daily headache may relate to high use of caffeine preceding the onset of chronification, and caffeine abstinence may improve response to acute migraine treatment.19,63 There is a dose-dependent risk of headache.64,65 Current recommendations suggest limiting caffeine consumption to less than 200 mg per day or stopping caffeine consumption altogether based on the quantity required for caffeine-withdrawal headache.66 Varying the caffeine dose from day to day may also trigger headache due to the high sensitivity to caffeine withdrawal.
While many diets have shown potential benefit in patients with migraine, more studies are needed before any one “migraine diet” can be recommended. Caution should be taken, as there is risk of adverse effects from nutrient deficiencies or excess levels, especially if the patient is not under the care of a healthcare professional who is familiar with the diet.
Whether it is beneficial to avoid specific food triggers at this time is unclear and still controversial even within the migraine community because some of these foods may be misattributed as triggers instead of premonitory cravings driven by the hypothalamus. It is important to counsel patients with migraine to eat a healthy diet with consistent meals, to maintain adequate hydration, and to keep their caffeine intake low or at least consistent, although these teachings are predominantly based on limited studies with extrapolation from nutrition research.
D IS FOR DIARY
A headache diary is a recommended part of headache management and may enhance the accuracy of diagnosis and assist in treatment modifications. Paper and electronic diaries have been used. Electronic diaries may be more accurate for real-time use, but patients may be more likely to complete a paper one.67 Patients prefer electronic diaries over long paper forms,68 but a practical issue to consider is easy electronic access.
Patients can start keeping a headache diary before the initial consultation to assist with diagnosis, or early in their management. A first-appointment diary mailed with instructions is a feasible option.69 These types of diaries ask detailed questions to help diagnose all major primary headache types including menstrual migraine and to identify concomitant medication-overuse headache. Physicians and patients generally report improved communication with use of a diary.70
Some providers distinguish between a headache diary and a calendar. In standard practice, a headache diary is the general term referring to both, but the literature differentiates between the two. Both should at least include headache frequency, with possible inclusion of other factors such as headache duration, headache intensity, analgesic use, headache impact on function, and absenteeism. Potential triggers including menses can also be tracked. The calendar version can fit on a single page and can be used for simple tracking of headache frequency and analgesia use.
One of the simplest calendars to use is the “stoplight” calendar. Red days are when a patient is completely debilitated in bed. On a yellow day, function at work, school, or daily activities is significantly reduced by migraine, but the patient is not bedbound. A green day is when headache is present but function is not affected. No color is placed if the patient is 100% headache-free.
Acute treatment use can be written in or, to improve compliance, a checkmark can be placed on days of treatment. Patients who are tracking menses circle the days of menstruation. The calendar-diary should be brought to every appointment to track treatment response and medication use.
THE SECOND S IS FOR STRESS
Behavioral management such as cognitive behavioral therapy in migraine has been shown to decrease catastrophizing, migraine disability, and headache severity and frequency.74 Both depression and anxiety can improve along with migraine.75 Cognitive behavioral therapy can be provided in individualized sessions or group sessions, either in person or online.74,76,77 The effects become more prominent about 5 weeks into treatment.78
Biofeedback, which uses behavioral techniques paired with physiologic autonomic measures, has been extensively studied, and shows benefit in migraine, including in meta-analysis.79 The types of biofeedback measurements used include electromyography, electroencephalography, temperature, sweat sensors, heart rate, blood volume pulse feedback, and respiration bands. While biofeedback is generally done under the guidance of a therapist, it can still be useful with minimal therapist contact and supplemental audio.80
Mindfulness, or the awareness of thoughts, feelings, and sensations in the present moment without judgment, is a behavioral technique that can be done alone or paired with another technique. It is often taught through a mindfulness-based stress-reduction program, which relies on a standardized approach. A meta-analysis showed that mindfulness improves pain intensity, headache frequency, disability, self-efficacy, and quality of life.81 It may work by encouraging pain acceptance.82
Relaxation techniques are also employed in migraine management, either alone or in conjunction with techniques mentioned above, such as mindfulness. They include progressive muscle relaxation and deep breathing. Relaxation has been shown to be effective when done by professional trainers as well as lay trainers in both individual and group settings.83,84
In patients with intractable headache, more-intensive inpatient and outpatient programs have been tried. Inpatient admissions with multidisciplinary programs that include a focus on behavioral techniques often paired with lifestyle education and sometimes pharmacologic management can be beneficial.85,86 These programs have also been successfully conducted as multiple outpatient sessions.86–88
Stress management is an important aspect of migraine management. These treatments often involve homework and require active participation.
LIFESTYLE FOR ALL
All patients with migraine should initiate lifestyle modifications (see Advice to patients with migraine: SEEDS for success). Modifications with the highest level of evidence, specifically behavioral techniques, have had the most reproducible results. A headache diary is an essential tool to identify patterns and needs for optimization of acute or preventive treatment regimens. The strongest evidence is for the behavioral management techniques for stress reduction.
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390(10100):1211–1259. doi:10.1016/S0140-6736(17)32154-2
- Vgontzas A, Pavlovic JM. Sleep diorders and migraine: review of literature and potential pathophysiology mechanisms. Headache 2018; 58(7):1030–1039. doi:10.1111/head.13358
- Lund N, Westergaard ML, Barloese M, Glumer C, Jensen RH. Epidemiology of concurrent headache and sleep problems in Denmark. Cephalalgia 2014; 34(10):833–845. doi:10.1177/0333102414543332
- Woldeamanuel YW, Cowan RP. The impact of regular lifestyle behavior in migraine: a prevalence case-referent study. J Neurol 2016; 263(4):669–676. doi:10.1007/s00415-016-8031-5
- Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016; 149(3):631–638. doi:10.1378/chest.15-0903
- Johnson KG, Ziemba AM, Garb JL. Improvement in headaches with continuous positive airway pressure for obstructive sleep apnea: a retrospective analysis. Headache 2013; 53(2):333–343. doi:10.1111/j.1526-4610.2012.02251.x
- Calhoun AH, Ford S. Behavioral sleep modification may revert transformed migraine to episodic migraine. Headache 2007; 47(8):1178–1183. doi:10.1111/j.1526-4610.2007.00780.x
- Calhoun AH, Ford S, Finkel AG, Kahn KA, Mann JD. The prevalence and spectrum of sleep problems in women with transformed migraine. Headache 2006; 46(4):604–610. doi:10.1111/j.1526-4610.2006.00410.x
- Rains JC. Optimizing circadian cycles and behavioral insomnia treatment in migraine. Curr Pain Headache Rep 2008; 12(3):213–219. pmid:18796272
- Lemmens J, De Pauw J, Van Soom T, et al. The effect of aerobic exercise on the number of migraine days, duration and pain intensity in migraine: a systematic literature review and meta-analysis. J Headache Pain 2019; 20(1):16. doi:10.1186/s10194-019-0961-8
- Amin FM, Aristeidou S, Baraldi C, et al; European Headache Federation School of Advanced Studies (EHF-SAS). The association between migraine and physical exercise. J Headache Pain 2018; 19(1):83. doi:10.1186/s10194-018-0902-y
- Genazzani AR, Nappi G, Facchinetti F, et al. Progressive impairment of CSF beta-EP levels in migraine sufferers. Pain 1984; 18:127-133. pmid:6324056
- Hindiyeh NA, Krusz JC, Cowan RP. Does exercise make migraines worse and tension type headaches better? Curr Pain Headache Rep 2013;17:380. pmid:24234818
- Kroll LS, Sjodahl Hammarlund C, Gard G, Jensen RH, Bendtsen L. Has aerobic exercise effect on pain perception in persons with migraine and coexisting tension-type headache and neck pain? A randomized, controlled, clinical trial. Eur J Pain 2018; 10:10. pmid:29635806
- Santiago MD, Carvalho Dde S, Gabbai AA, Pinto MM, Moutran AR, Villa TR. Amitriptyline and aerobic exercise or amitriptyline alone in the treatment of chronic migraine: a randomized comparative study. Arq Neuropsiquiatr 2014; 72(11):851-855. pmid:25410451
- Varkey E, Cider A, Carlsson J, Linde M. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia 2011; 31(14):1428-1438. pmid:21890526
- Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43(7):1334-1359. pmid:21694556
- Guarnieri P, Radnitz CL, Blanchard EB. Assessment of dietary risk factors in chronic headache. Biofeedback Self Regul 1990; 15(1):15–25. pmid:2361144
- Shapiro RE. Caffeine and headaches. Curr Pain Headache Rep 2008; 12(4):311–315. pmid:18625110
- Yokoyama M, Yokoyama T, Funazu K, et al. Associations between headache and stress, alcohol drinking, exercise, sleep, and comorbid health conditions in a Japanese population. J Headache Pain 2009; 10(3):177–185. doi:10.1007/s10194-009-0113-7
- Karsan N, Bose P, Goadsby PJ. The migraine premonitory phase. Continuum (Minneap Minn) 2018; 24(4, Headache):996–1008. doi:10.1212/CON.0000000000000624
- Pavlovic JM, Buse DC, Sollars CM, Haut S, Lipton RB. Trigger factors and premonitory features of migraine attacks: summary of studies. Headache 2014; 54(10):1670–1679. doi:10.1111/head.12468
- Marcus DA, Scharff L, Turk D, Gourley LM. A double-blind provocative study of chocolate as a trigger of headache. Cephalalgia 1997; 17(8):855–862. doi:10.1046/j.1468-2982.1997.1708855.x
- Obayashi Y, Nagamura Y. Does monosodium glutamate really cause headache? A systematic review of human studies. J Headache Pain 2016; 17:54. doi:10.1186/s10194-016-0639-4
- Evans EW, Lipton RB, Peterlin BL, et al. Dietary intake patterns and diet quality in a nationally representative sample of women with and without severe headache or migraine. Headache 2015; 55(4):550–561. doi:10.1111/head.12527
- Zis P, Julian T, Hadjivassiliou M. Headache associated with coeliac disease: a systematic review and meta-analysis. Nutrients 2018; 10(10). doi:10.3390/nu10101445
- Alpay K, Ertas M, Orhan EK, Ustay DK, Lieners C, Baykan B. Diet restriction in migraine, based on IgG against foods: a clinical double-blind, randomised, cross-over trial. Cephalalgia 2010; 30(7):829–837. doi:10.1177/0333102410361404
- Aydinlar EI, Dikmen PY, Tiftikci A, et al. IgG-based elimination diet in migraine plus irritable bowel syndrome. Headache 2013; 53(3):514–525. doi:10.1111/j.1526-4610.2012.02296.x
- Mitchell N, Hewitt CE, Jayakody S, et al. Randomised controlled trial of food elimination diet based on IgG antibodies for the prevention of migraine like headaches. Nutr J 2011; 10:85. doi:10.1186/1475-2891-10-85
- Wantke F, Gotz M, Jarisch R. Histamine-free diet: treatment of choice for histamine-induced food intolerance and supporting treatment for chronic headaches. Clin Exp Allergy 1993; 23(12):982–985. pmid:10779289
- Mansfield LE, Vaughan TR, Waller SF, Haverly RW, Ting S. Food allergy and adult migraine: double-blind and mediator confirmation of an allergic etiology. Ann Allergy 1985; 55(2):126–129. pmid:4025956
- Kohlenberg RJ. Tyramine sensitivity in dietary migraine: a critical review. Headache 1982; 22(1):30–34. pmid:17152742
- Medina JL, Diamond S. The role of diet in migraine. Headache 1978; 18(1):31–34. pmid:649377
- Mosnaim AD, Freitag F, Ignacio R, et al. Apparent lack of correlation between tyramine and phenylethylamine content and the occurrence of food-precipitated migraine. Reexamination of a variety of food products frequently consumed in the United States and commonly restricted in tyramine-free diets. Headache Quarterly. Current Treatment and Research 1996; 7(3):239–249.
- Ferrara LA, Pacioni D, Di Fronzo V, et al. Low-lipid diet reduces frequency and severity of acute migraine attacks. Nutr Metab Cardiovasc Dis 2015; 25(4):370–375. doi:10.1016/j.numecd.2014.12.006
- Bic Z, Blix GG, Hopp HP, Leslie FM, Schell MJ. The influence of a low-fat diet on incidence and severity of migraine headaches. J Womens Health Gend Based Med 1999; 8(5):623–630. doi:10.1089/jwh.1.1999.8.623
- Bunner AE, Agarwal U, Gonzales JF, Valente F, Barnard ND. Nutrition intervention for migraine: a randomized crossover trial. J Headache Pain 2014; 15:69. doi:10.1186/1129-2377-15-69
- Evcili G, Utku U, Ogun MN, Ozdemir G. Early and long period follow-up results of low glycemic index diet for migraine prophylaxis. Agri 2018; 30(1):8–11. doi:10.5505/agri.2017.62443
- Maghsoumi-Norouzabad L, Mansoori A, Abed R, Shishehbor F. Effects of omega-3 fatty acids on the frequency, severity, and duration of migraine attacks: a systematic review and meta-analysis of randomized controlled trials. Nutr Neurosci 2018; 21(9):614–623. doi:10.1080/1028415X.2017.1344371
- Soares AA, Loucana PMC, Nasi EP, Sousa KMH, Sa OMS, Silva-Neto RP. A double- blind, randomized, and placebo-controlled clinical trial with omega-3 polyunsaturated fatty acids (OPFA Ω-3) for the prevention of migraine in chronic migraine patients using amitriptyline. Nutr Neurosci 2018; 21(3):219–223. doi:10.1080/1028415X.2016.1266133
- Di Lorenzo C, Coppola G, Sirianni G, et al. Migraine improvement during short lasting ketogenesis: a proof-of-concept study. Eur J Neurol 2015; 22(1):170–177. doi:10.1111/ene.12550
- Di Lorenzo C, Coppola G, Bracaglia M, et al. Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: a multimodal evoked potentials study. J Headache Pain 2016; 17:58. doi:10.1186/s10194-016-0650-9
- Kossoff EH, Huffman J, Turner Z, Gladstein J. Use of the modified Atkins diet for adolescents with chronic daily headache. Cephalalgia 2010; 30(8):1014–1016. https://journals.sagepub.com/doi/full/10.1111/j.1468-2982.2009.02016.x
- Slavin M, Ailani J. A clinical approach to addressing diet with migraine patients. Curr Neurol Neurosci Rep 2017; 17(2):17. doi:10.1007/s11910-017-0721-6
- Amer M, Woodward M, Appel LJ. Effects of dietary sodium and the DASH diet on the occurrence of headaches: results from randomised multicentre DASH-sodium clinical trial. BMJ Open 2014; 4(12):e006671. doi:10.1136/bmjopen-2014-006671
- Chen L, Zhang Z, Chen W, Whelton PK, Appel LJ. Lower sodium intake and risk of headaches: results from the trial of nonpharmacologic interventions in the elderly. Am J Public Health 2016; 106(7):1270–1275. doi:10.2105/AJPH.2016.303143
- Pogoda JM, Gross NB, Arakaki X, Fonteh AN, Cowan RP, Harrington MG. Severe headache or migraine history is inversely correlated with dietary sodium intake: NHANES 1999–2004. Headache 2016; 56(4):688–698. doi:10.1111/head.12792
- Awada A, al Jumah M. The first-of-Ramadan headache. Headache 1999; 39(7):490–493. pmid:11279933
- Abu-Salameh I, Plakht Y, Ifergane G. Migraine exacerbation during Ramadan fasting. J Headache Pain 2010; 11(6):513–517. doi:10.1007/s10194-010-0242-z
- Nazari F, Safavi M, Mahmudi M. Migraine and its relation with lifestyle in women. Pain Pract 2010; 10(3):228–234. doi:10.1111/j.1533-2500.2009.00343.x
- Nas A, Mirza N, Hagele F, et al. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am J Clin Nutr 2017; 105(6):1351–1361. doi:10.3945/ajcn.116.151332
- Torelli P, Manzoni GC. Fasting headache. Curr Pain Headache Rep 2010; 14(4):284–291. doi:10.1007/s11916-010-0119-5
- Yoshimura E, Hatamoto Y, Yonekura S, Tanaka H. Skipping breakfast reduces energy intake and physical activity in healthy women who are habitual breakfast eaters: a randomized crossover trial. Physiol Behav 2017; 174:89–94. doi:10.1016/j.physbeh.2017.03.008
- Pendergast FJ, Livingstone KM, Worsley A, McNaughton SA. Correlates of meal skipping in young adults: a systematic review. Int J Behav Nutr Phys Act 2016; 13(1):125. doi:10.1186/s12966-016-0451-1
- Maki KC, Phillips-Eakley AK, Smith KN. The effects of breakfast consumption and composition on metabolic wellness with a focus on carbohydrate metabolism. Adv Nutr 2016; 7(3):613S–621S. doi:10.3945/an.115.010314
- Shirreffs SM, Merson SJ, Fraser SM, Archer DT. The effects of fluid restriction on hydration status and subjective feelings in man. Br J Nutr 2004; 91(6):951–958. doi:10.1079/BJN20041149
- Blau JN. Water deprivation: a new migraine precipitant. Headache 2005; 45(6):757–759. doi:10.1111/j.1526-4610.2005.05143_3.x
- Price A, Burls A. Increased water intake to reduce headache: learning from a critical appraisal. J Eval Clin Pract 2015; 21(6):1212–1218. doi:10.1111/jep.12413
- Balbin JE, Nerenberg R, Baratloo A, Friedman BW. Intravenous fluids for migraine: a post hoc analysis of clinical trial data. Am J Emerg Med 2016; 34(4):713–716. doi:10.1016/j.ajem.2015.12.080
- Spigt M, Weerkamp N, Troost J, van Schayck CP, Knottnerus JA. A randomized trial on the effects of regular water intake in patients with recurrent headaches. Fam Pract 2012; 29(4):370–375. doi:10.1093/fampra/cmr112
- Armstrong LE, Johnson EC. Water intake, water balance, and the elusive daily water requirement. Nutrients 2018; 10(12). doi:10.3390/nu10121928
- Fried NT, Elliott MB, Oshinsky ML. The role of adenosine signaling in headache: a review. Brain Sci 2017; 7(3). doi:10.3390/brainsci7030030
- Lee MJ, Choi HA, Choi H, Chung CS. Caffeine discontinuation improves acute migraine treatment: a prospective clinic-based study. J Headache Pain 2016; 17(1):71. doi:10.1186/s10194-016-0662-5
- Shirlow MJ, Mathers CD. A study of caffeine consumption and symptoms; indigestion, palpitations, tremor, headache and insomnia. Int J Epidemiol 1985; 14(2):239–248. doi:10.1093/ije/14.2.239
- Silverman K, Evans SM, Strain EC, Griffiths RR. Withdrawal syndrome after the double-blind cessation of caffeine consumption. N Engl J Med 1992; 327(16):1109–1114. doi:10.1056/NEJM199210153271601
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38(1):1–211. doi:10.1177/0333102417738202
- Krogh AB, Larsson B, Salvesen O, Linde M. A comparison between prospective Internet-based and paper diary recordings of headache among adolescents in the general population. Cephalalgia 2016; 36(4):335–345. doi:10.1177/0333102415591506
- Bandarian-Balooch S, Martin PR, McNally B, Brunelli A, Mackenzie S. Electronic-diary for recording headaches, triggers, and medication use: development and evaluation. Headache 2017; 57(10):1551–1569. doi:10.1111/head.13184
- Tassorelli C, Sances G, Allena M, et al. The usefulness and applicability of a basic headache diary before first consultation: results of a pilot study conducted in two centres. Cephalalgia 2008; 28(10):1023–1030. doi:10.1111/j.1468-2982.2008.01639.x
- Baos V, Ester F, Castellanos A, et al. Use of a structured migraine diary improves patient and physician communication about migraine disability and treatment outcomes. Int J Clin Pract 2005; 59(3):281–286. doi:10.1111/j.1742-1241.2005.00469.x
- Martin PR, MacLeod C. Behavioral management of headache triggers: avoidance of triggers is an inadequate strategy. Clin Psychol Rev 2009; 29(6):483–495. doi:10.1016/j.cpr.2009.05.002
- Giannini G, Zanigni S, Grimaldi D, et al. Cephalalgiaphobia as a feature of high-frequency migraine: a pilot study. J Headache Pain 2013; 14:49. doi:10.1186/1129-2377-14-49
- Westergaard ML, Glumer C, Hansen EH, Jensen RH. Medication overuse, healthy lifestyle behaviour and stress in chronic headache: results from a population-based representative survey. Cephalalgia 2016; 36(1):15–28. doi:10.1177/0333102415578430
- Christiansen S, Jurgens TP, Klinger R. Outpatient combined group and individual cognitive-behavioral treatment for patients with migraine and tension-type headache in a routine clinical setting. Headache 2015; 55(8):1072–1091. doi:10.1111/head.12626
- Martin PR, Aiello R, Gilson K, Meadows G, Milgrom J, Reece J. Cognitive behavior therapy for comorbid migraine and/or tension-type headache and major depressive disorder: an exploratory randomized controlled trial. Behav Res Ther 2015; 73:8–18. doi:10.1016/j.brat.2015.07.005
- Nash JM, Park ER, Walker BB, Gordon N, Nicholson RA. Cognitive-behavioral group treatment for disabling headache. Pain Med 2004; 5(2):178–186. doi:10.1111/j.1526-4637.2004.04031.x
- Sorbi MJ, Balk Y, Kleiboer AM, Couturier EG. Follow-up over 20 months confirms gains of online behavioural training in frequent episodic migraine. Cephalalgia 2017; 37(3):236–250. doi:10.1177/0333102416657145
- Thorn BE, Pence LB, Ward LC, et al. A randomized clinical trial of targeted cognitive behavioral treatment to reduce catastrophizing in chronic headache sufferers. J Pain 2007; 8(12):938–949. doi:10.1016/j.jpain.2007.06.010
- Nestoriuc Y, Martin A. Efficacy of biofeedback for migraine: a meta-analysis. Pain 2007; 128(1–2):111–127. doi:10.1016/j.pain.2006.09.007
- Blanchard EB, Appelbaum KA, Nicholson NL, et al. A controlled evaluation of the addition of cognitive therapy to a home-based biofeedback and relaxation treatment of vascular headache. Headache 1990; 30(6):371–376. pmid:2196240
- Gu Q, Hou JC, Fang XM. Mindfulness meditation for primary headache pain: a meta-analysis. Chin Med J (Engl) 2018; 131(7):829–838. doi:10.4103/0366-6999.228242
- Day MA, Thorn BE. The mediating role of pain acceptance during mindfulness-based cognitive therapy for headache. Complement Ther Med 2016; 25:51–54. doi:10.1016/j.ctim.2016.01.002
- Williamson DA, Monguillot JE, Jarrell MP, Cohen RA, Pratt JM, Blouin DC. Relaxation for the treatment of headache. Controlled evaluation of two group programs. Behav Modif 1984; 8(3):407–424. doi:10.1177/01454455840083007
- Merelle SY, Sorbi MJ, Duivenvoorden HJ, Passchier J. Qualities and health of lay trainers with migraine for behavioral attack prevention. Headache 2010; 50(4):613–625. doi:10.1111/j.1526-4610.2008.01241.x
- Gaul C, van Doorn C, Webering N, et al. Clinical outcome of a headache-specific multidisciplinary treatment program and adherence to treatment recommendations in a tertiary headache center: an observational study. J Headache Pain 2011; 12(4):475–483. doi:10.1007/s10194-011-0348-y
- Wallasch TM, Kropp P. Multidisciplinary integrated headache care: a prospective 12-month follow-up observational study. J Headache Pain 2012; 13(7):521–529. doi:10.1007/s10194-012-0469-y
- Lemstra M, Stewart B, Olszynski WP. Effectiveness of multidisciplinary intervention in the treatment of migraine: a randomized clinical trial. Headache 2002; 42(9):845–854. pmid:12390609
- Krause SJ, Stillman MJ, Tepper DE, Zajac D. A prospective cohort study of outpatient interdisciplinary rehabilitation of chronic headache patients. Headache 2017; 57(3):428–440. doi:10.1111/head.13020
Migraine is the second leading cause of years of life lived with a disability globally.1 It affects people of all ages, but particularly during the years associated with the highest productivity in terms of work and family life.
Migraine is a genetic neurologic disease that can be influenced or triggered by environmental factors. However, triggers do not cause migraine. For example, stress does not cause migraine, but it can exacerbate it.
Primary care physicians can help patients reduce the likelihood of a migraine attack, the severity of symptoms, or both by offering lifestyle counseling centered around the mnemonic SEEDS: sleep, exercise, eat, diary, and stress. In this article, each factor is discussed individually for its current support in the literature along with best-practice recommendations.
S IS FOR SLEEP
Before optimizing sleep hygiene, screen for sleep apnea, especially in those who have chronic daily headache upon awakening. An excellent tool is the STOP-Bang screening questionnaire5 (www.stopbang.ca/osa/screening.php). Patients respond “yes” or “no” to the following questions:
- Snoring: Do you snore loudly (louder than talking or loud enough to be heard through closed doors)?
- Tired: Do you often feel tired, fatigued, or sleepy during the daytime?
- Observed: Has anyone observed you stop breathing during your sleep?
- Pressure: Do you have or are you being treated for high blood pressure?
- Body mass index greater than 35 kg/m2?
- Age over 50?
- Neck circumference larger than 40 cm (females) or 42 cm (males)?
- Gender—male?
Each “yes” answer is scored as 1 point. A score less than 3 indicates low risk of obstructive sleep apnea; 3 to 4 indicates moderate risk; and 5 or more indicates high risk. Optimization of sleep apnea with continuous positive airway pressure therapy can improve sleep apnea headache.6 The improved sleep from reduced arousals may also mitigate migraine symptoms.
Behavioral modification for sleep hygiene can convert chronic migraine to episodic migraine.7 One such program is stimulus control therapy, which focuses on using cues to initiate sleep (Table 1). Patients are encouraged to keep the bedroom quiet, dark, and cool, and to go to sleep at the same time every night. Importantly, the bed should be associated only with sleep. If patients are unable to fall asleep within 20 to 30 minutes, they should leave the room so they do not associate the bed with frustration and anxiety. Use of phones, tablets, and television in the bedroom is discouraged as these devices may make it more difficult to fall asleep.8
The next option is sleep restriction, which is useful for comorbid insomnia. Patients keep a sleep diary to better understand their sleep-wake cycle. The goal is 90% sleep efficiency, meaning that 90% of the time in bed (TIB) is spent asleep. For example, if the patient is in bed 8 hours but asleep only 4 hours, sleep efficiency is 50%. The goal is to reduce TIB to match the time asleep and to agree on a prescribed daily wake-up time. When the patient is consistently sleeping 90% of the TIB, add 30-minute increments until he or she is appropriately sleeping 7 to 8 hours at night.9 Naps are not recommended.
Let patients know that their migraine may worsen until a new routine sleep pattern emerges. This method is not recommended for patients with untreated sleep apnea.
E IS FOR EXERCISE
Exercise is broadly recommended for a healthy lifestyle; some evidence suggests that it can also be useful in the management of migraine.10 Low levels of physical activity and a sedentary lifestyle are associated with migraine.11 It is unclear if patients with migraine are less likely to exercise because they want to avoid triggering a migraine or if a sedentary lifestyle increases their risk.
Exercise has been studied for its prophylactic benefits in migraine, and one hypothesis relates to beta-endorphins. Levels of beta-endorphins are reduced in the cerebrospinal fluid of patients with migraine.12 Exercise programs may increase levels while reducing headache frequency and duration.13 One study showed that pain thresholds do not change with exercise programs, suggesting that it is avoidance behavior that is positively altered rather than the underlying pain pathways.14
A systematic review and meta-analysis based on 5 randomized controlled trials and 1 nonrandomized controlled clinical trial showed that exercise reduced monthly migraine days by only 0.6 (± 0.3) days, but the data also suggested that as the exercise intensity increased, so did the positive effects.10
Some data suggest that exercise may also reduce migraine duration and severity as well as the need for abortive medication.10 Two studies in this systematic review15,16 showed that exercise benefits were equivalent to those of migraine preventives such as amitriptyline and topiramate; the combination of amitriptyline and exercise was more beneficial than exercise alone. Multiple types of exercise were beneficial, including walking, jogging, cross-training, and cycling when done for least 6 weeks and for 30 to 50 minutes 3 to 5 times a week.
These findings are in line with the current recommendations for general health from the American College of Sports Medicine, ie, moderate to vigorous cardiorespiratory exercise for 30 to 60 minutes 3 to 5 times a week (or 150 minutes per week). The daily exercise can be continuous or done in intervals of less than 20 minutes. For those with a sedentary lifestyle, as is seen in a significant proportion of the migraine population, light to moderate exercise for less than 20 minutes is still beneficial.17
Based on this evidence, the best current recommendation for patients with migraine is to engage in graded moderate cardiorespiratory exercise, although any exercise is better than none. If a patient is sedentary or has poor exercise tolerance, or both, exercising once a week for shorter time periods may be a manageable place to start.
Some patients may identify exercise as a trigger or exacerbating factor in migraine. These patients may need appropriate prophylactic and abortive therapies before starting an exercise regimen.
THE SECOND E IS FOR EAT (FOOD AND DRINK)
Many patients believe that some foods trigger migraine attacks, but further study is needed. The most consistent food triggers appear to be red wine and caffeine (withdrawal).18,19 Interestingly, patients with migraine report low levels of alcohol consumption,20 but it is unclear if that is because alcohol has a protective effect or if patients avoid it.
Some patients may crave certain foods in the prodromal phase of an attack, eat the food, experience the attack, and falsely conclude that the food caused the attack.21 Premonitory symptoms include fatigue, cognitive changes, homeostatic changes, sensory hyperresponsiveness, and food cravings.21 It is difficult to distinguish between premonitory phase food cravings and true triggers because premonitory symptoms can precede headache by 48 to 72 hours, and the timing for a trigger to be considered causal is not known.22
Chocolate is often thought to be a migraine trigger, but the evidence argues against this and even suggests that sweet cravings are a part of the premonitory phase.23 Monosodium glutamate is often identified as a trigger as well, but the literature is inconsistent and does not support a causal relationship.24 Identifying true food triggers in migraine is difficult, and patients with migraine may have poor quality diets, with some foods acting as true triggers for certain patients.25 These possibilities have led to the development of many “migraine diets,” including elimination diets.
Elimination diets
Elimination diets involve avoiding specific food items over a period of time and then adding them back in one at a time to gauge whether they cause a reaction in the body. A number of these diets have been studied for their effects on headache and migraine:
Gluten-free diets restrict foods that contain wheat, rye, and barley. A systematic review of gluten-free diets in patients with celiac disease found that headache or migraine frequency decreased by 51.6% to 100% based on multiple cohort studies (N = 42,388).26 There are no studies on the use of a gluten-free diet for migraine in patients without celiac disease.
Immunoglobulin G-elimination diets restrict foods that serve as antigens for IgG. However, data supporting these diets are inconsistent. Two small randomized controlled trials found that the diets improved migraine symptoms, but a larger study found no improvement in the number of migraine days at 12 weeks, although there was an initially significant effect at 4 weeks.27–29
Antihistamine diets restrict foods that have high levels of histamines, including fermented dairy, vegetables, soy products, wine, beer, alcohol, and those that cause histamine release regardless of IgE testing results. A prospective single-arm study of antihistamine diets in patients with chronic headache reported symptom improvement, which could be applied to certain comorbidities such as mast cell activation syndrome.30 Another prospective nonrandomized controlled study eliminated foods based on positive IgE skin-prick testing for allergy in patients with recurrent migraine and found that it reduced headache frequency.31
Tyramine-free diets are often recommended due to the presumption that tyramine-containing foods (eg, aged cheese, cured or smoked meats and fish, and beer) are triggers. However, multiple studies have reviewed this theory with inconsistent results,32 and the only study of a tyramine-free diet was negative.33 In addition, commonly purported high-tyramine foods have lower tyramine levels than previously thought.34
Low-fat diets in migraine are supported by 2 small randomized controlled trials and a prospective study showing a decrease in symptom severity; the results for frequency are inconsistent.35–37
Low-glycemic index diets are supported in migraine by 1 randomized controlled trial that showed improvement in migraine frequency in a diet group and in a control group of patients who took a standard migraine-preventive medication to manage their symptoms.38
Other migraine diets
Diets high in certain foods or ingredient ratios, as opposed to elimination diets, have also been studied in patients with migraine. One promising diet containing high levels of omega-3 fatty acids and low levels of omega-6 fatty acids was shown in a systematic review to reduce the duration of migraine but not the frequency or severity.39 A more recent randomized controlled trial of this diet in chronic migraine also showed that it decreased migraine frequency.40
The ketogenic diet (high fat, low carbohydrate) had promising results in a randomized controlled trial in overweight women with migraine and in a prospective study.41,42 However, a prospective study of the Atkins diet in teenagers with chronic daily headaches showed no benefit.43 The ketogenic diet is difficult to follow and may work in part due to weight loss alone, although ketogenesis itself may also play a role.41,44
Sodium levels have been shown to be higher in the cerebrospinal fluid of patients with migraine than in controls, particularly during an attack.45 For a prehypertensive population or an elderly population, a low-sodium diet may be beneficial based on 2 prospective trials.46,47 However, a younger female population without hypertension and low-to-normal body mass index had a reduced probability of migraine while consuming a high-sodium diet.48
Counseling about sodium intake should be tailored to specific patient populations. For example, a diet low in sodium may be appropriate for patients with vascular risk factors such as hypertension, whereas a high-sodium diet may be appropriate in patients with comorbidities like postural tachycardia syndrome or in those with a propensity for low blood pressure or low body mass index.
Encourage routine meals and hydration
The standard advice for patients with migraine is to consume regular meals. Headaches have been associated with fasting, and those with migraine are predisposed to attacks in the setting of fasting.49,50 Migraine is more common when meals are skipped, particularly breakfast.51
It is unclear how fasting lowers the migraine threshold. Nutritional studies show that skipping meals, particularly breakfast, increases low-grade inflammation and impairs glucose metabolism by affecting insulin and fat oxidation metabolism.52 However, hypoglycemia itself is not a consistent cause of headache or migraine attacks.53 As described above, a randomized controlled trial of a low-glycemic index diet actually decreased migraine frequency and severity.38 Skipping meals also reduces energy and is associated with reduced physical activity, perhaps leading to multiple compounding triggers that further lower the migraine threshold.54,55
When counseling patients about the need to eat breakfast, consider what they normally consume (eg, is breakfast just a cup of coffee?). Replacing simple carbohydrates with protein, fats, and fiber may be beneficial for general health, but the effects on migraine are not known, nor is the optimal composition of breakfast foods.55
The optimal timing of breakfast relative to awakening is also unclear, but in general, it should be eaten within 30 to 60 minutes of rising. Also consider patients’ work hours—delayed-phase or shift workers have altered sleep cycles.
Recommendations vary in regard to hydration. Headache is associated with fluid restriction and dehydration,56,57 but only a few studies suggest that rehydration and increased hydration status can improve migraine.58 In fact, a single post hoc analysis of a metoclopramide study showed that intravenous fluid alone for patients with migraine in the emergency room did not improve pain outcomes.59
The amount of water patients should drink daily in the setting of migraine is also unknown, but a study showed benefit with 4 L, which equates to a daily intake of 16 eight-ounce glasses.60 One review on general health that could be extrapolated given the low risk of the intervention indicated that 1.8 L daily (7 to 8 eight-ounce glasses) promoted a euhydration status in most people, although many factors contribute to hydration status.61
Caffeine intake is also a major consideration. Caffeine is a nonspecific adenosine receptor antagonist that modulates adenosine receptors like the pronociceptive 2A receptor, leading to changes integral to the neuropathophysiology of migraine.62 Caffeine has analgesic properties at doses greater than 65 to 200 mg and augments the effects of analgesics such as acetaminophen and aspirin. Chronic caffeine use can lead to withdrawal symptoms when intake is stopped abruptly; this is thought to be due to upregulation of adenosine receptors, but the effect varies based on genetic predisposition.19
The risk of chronic daily headache may relate to high use of caffeine preceding the onset of chronification, and caffeine abstinence may improve response to acute migraine treatment.19,63 There is a dose-dependent risk of headache.64,65 Current recommendations suggest limiting caffeine consumption to less than 200 mg per day or stopping caffeine consumption altogether based on the quantity required for caffeine-withdrawal headache.66 Varying the caffeine dose from day to day may also trigger headache due to the high sensitivity to caffeine withdrawal.
While many diets have shown potential benefit in patients with migraine, more studies are needed before any one “migraine diet” can be recommended. Caution should be taken, as there is risk of adverse effects from nutrient deficiencies or excess levels, especially if the patient is not under the care of a healthcare professional who is familiar with the diet.
Whether it is beneficial to avoid specific food triggers at this time is unclear and still controversial even within the migraine community because some of these foods may be misattributed as triggers instead of premonitory cravings driven by the hypothalamus. It is important to counsel patients with migraine to eat a healthy diet with consistent meals, to maintain adequate hydration, and to keep their caffeine intake low or at least consistent, although these teachings are predominantly based on limited studies with extrapolation from nutrition research.
D IS FOR DIARY
A headache diary is a recommended part of headache management and may enhance the accuracy of diagnosis and assist in treatment modifications. Paper and electronic diaries have been used. Electronic diaries may be more accurate for real-time use, but patients may be more likely to complete a paper one.67 Patients prefer electronic diaries over long paper forms,68 but a practical issue to consider is easy electronic access.
Patients can start keeping a headache diary before the initial consultation to assist with diagnosis, or early in their management. A first-appointment diary mailed with instructions is a feasible option.69 These types of diaries ask detailed questions to help diagnose all major primary headache types including menstrual migraine and to identify concomitant medication-overuse headache. Physicians and patients generally report improved communication with use of a diary.70
Some providers distinguish between a headache diary and a calendar. In standard practice, a headache diary is the general term referring to both, but the literature differentiates between the two. Both should at least include headache frequency, with possible inclusion of other factors such as headache duration, headache intensity, analgesic use, headache impact on function, and absenteeism. Potential triggers including menses can also be tracked. The calendar version can fit on a single page and can be used for simple tracking of headache frequency and analgesia use.
One of the simplest calendars to use is the “stoplight” calendar. Red days are when a patient is completely debilitated in bed. On a yellow day, function at work, school, or daily activities is significantly reduced by migraine, but the patient is not bedbound. A green day is when headache is present but function is not affected. No color is placed if the patient is 100% headache-free.
Acute treatment use can be written in or, to improve compliance, a checkmark can be placed on days of treatment. Patients who are tracking menses circle the days of menstruation. The calendar-diary should be brought to every appointment to track treatment response and medication use.
THE SECOND S IS FOR STRESS
Behavioral management such as cognitive behavioral therapy in migraine has been shown to decrease catastrophizing, migraine disability, and headache severity and frequency.74 Both depression and anxiety can improve along with migraine.75 Cognitive behavioral therapy can be provided in individualized sessions or group sessions, either in person or online.74,76,77 The effects become more prominent about 5 weeks into treatment.78
Biofeedback, which uses behavioral techniques paired with physiologic autonomic measures, has been extensively studied, and shows benefit in migraine, including in meta-analysis.79 The types of biofeedback measurements used include electromyography, electroencephalography, temperature, sweat sensors, heart rate, blood volume pulse feedback, and respiration bands. While biofeedback is generally done under the guidance of a therapist, it can still be useful with minimal therapist contact and supplemental audio.80
Mindfulness, or the awareness of thoughts, feelings, and sensations in the present moment without judgment, is a behavioral technique that can be done alone or paired with another technique. It is often taught through a mindfulness-based stress-reduction program, which relies on a standardized approach. A meta-analysis showed that mindfulness improves pain intensity, headache frequency, disability, self-efficacy, and quality of life.81 It may work by encouraging pain acceptance.82
Relaxation techniques are also employed in migraine management, either alone or in conjunction with techniques mentioned above, such as mindfulness. They include progressive muscle relaxation and deep breathing. Relaxation has been shown to be effective when done by professional trainers as well as lay trainers in both individual and group settings.83,84
In patients with intractable headache, more-intensive inpatient and outpatient programs have been tried. Inpatient admissions with multidisciplinary programs that include a focus on behavioral techniques often paired with lifestyle education and sometimes pharmacologic management can be beneficial.85,86 These programs have also been successfully conducted as multiple outpatient sessions.86–88
Stress management is an important aspect of migraine management. These treatments often involve homework and require active participation.
LIFESTYLE FOR ALL
All patients with migraine should initiate lifestyle modifications (see Advice to patients with migraine: SEEDS for success). Modifications with the highest level of evidence, specifically behavioral techniques, have had the most reproducible results. A headache diary is an essential tool to identify patterns and needs for optimization of acute or preventive treatment regimens. The strongest evidence is for the behavioral management techniques for stress reduction.
Migraine is the second leading cause of years of life lived with a disability globally.1 It affects people of all ages, but particularly during the years associated with the highest productivity in terms of work and family life.
Migraine is a genetic neurologic disease that can be influenced or triggered by environmental factors. However, triggers do not cause migraine. For example, stress does not cause migraine, but it can exacerbate it.
Primary care physicians can help patients reduce the likelihood of a migraine attack, the severity of symptoms, or both by offering lifestyle counseling centered around the mnemonic SEEDS: sleep, exercise, eat, diary, and stress. In this article, each factor is discussed individually for its current support in the literature along with best-practice recommendations.
S IS FOR SLEEP
Before optimizing sleep hygiene, screen for sleep apnea, especially in those who have chronic daily headache upon awakening. An excellent tool is the STOP-Bang screening questionnaire5 (www.stopbang.ca/osa/screening.php). Patients respond “yes” or “no” to the following questions:
- Snoring: Do you snore loudly (louder than talking or loud enough to be heard through closed doors)?
- Tired: Do you often feel tired, fatigued, or sleepy during the daytime?
- Observed: Has anyone observed you stop breathing during your sleep?
- Pressure: Do you have or are you being treated for high blood pressure?
- Body mass index greater than 35 kg/m2?
- Age over 50?
- Neck circumference larger than 40 cm (females) or 42 cm (males)?
- Gender—male?
Each “yes” answer is scored as 1 point. A score less than 3 indicates low risk of obstructive sleep apnea; 3 to 4 indicates moderate risk; and 5 or more indicates high risk. Optimization of sleep apnea with continuous positive airway pressure therapy can improve sleep apnea headache.6 The improved sleep from reduced arousals may also mitigate migraine symptoms.
Behavioral modification for sleep hygiene can convert chronic migraine to episodic migraine.7 One such program is stimulus control therapy, which focuses on using cues to initiate sleep (Table 1). Patients are encouraged to keep the bedroom quiet, dark, and cool, and to go to sleep at the same time every night. Importantly, the bed should be associated only with sleep. If patients are unable to fall asleep within 20 to 30 minutes, they should leave the room so they do not associate the bed with frustration and anxiety. Use of phones, tablets, and television in the bedroom is discouraged as these devices may make it more difficult to fall asleep.8
The next option is sleep restriction, which is useful for comorbid insomnia. Patients keep a sleep diary to better understand their sleep-wake cycle. The goal is 90% sleep efficiency, meaning that 90% of the time in bed (TIB) is spent asleep. For example, if the patient is in bed 8 hours but asleep only 4 hours, sleep efficiency is 50%. The goal is to reduce TIB to match the time asleep and to agree on a prescribed daily wake-up time. When the patient is consistently sleeping 90% of the TIB, add 30-minute increments until he or she is appropriately sleeping 7 to 8 hours at night.9 Naps are not recommended.
Let patients know that their migraine may worsen until a new routine sleep pattern emerges. This method is not recommended for patients with untreated sleep apnea.
E IS FOR EXERCISE
Exercise is broadly recommended for a healthy lifestyle; some evidence suggests that it can also be useful in the management of migraine.10 Low levels of physical activity and a sedentary lifestyle are associated with migraine.11 It is unclear if patients with migraine are less likely to exercise because they want to avoid triggering a migraine or if a sedentary lifestyle increases their risk.
Exercise has been studied for its prophylactic benefits in migraine, and one hypothesis relates to beta-endorphins. Levels of beta-endorphins are reduced in the cerebrospinal fluid of patients with migraine.12 Exercise programs may increase levels while reducing headache frequency and duration.13 One study showed that pain thresholds do not change with exercise programs, suggesting that it is avoidance behavior that is positively altered rather than the underlying pain pathways.14
A systematic review and meta-analysis based on 5 randomized controlled trials and 1 nonrandomized controlled clinical trial showed that exercise reduced monthly migraine days by only 0.6 (± 0.3) days, but the data also suggested that as the exercise intensity increased, so did the positive effects.10
Some data suggest that exercise may also reduce migraine duration and severity as well as the need for abortive medication.10 Two studies in this systematic review15,16 showed that exercise benefits were equivalent to those of migraine preventives such as amitriptyline and topiramate; the combination of amitriptyline and exercise was more beneficial than exercise alone. Multiple types of exercise were beneficial, including walking, jogging, cross-training, and cycling when done for least 6 weeks and for 30 to 50 minutes 3 to 5 times a week.
These findings are in line with the current recommendations for general health from the American College of Sports Medicine, ie, moderate to vigorous cardiorespiratory exercise for 30 to 60 minutes 3 to 5 times a week (or 150 minutes per week). The daily exercise can be continuous or done in intervals of less than 20 minutes. For those with a sedentary lifestyle, as is seen in a significant proportion of the migraine population, light to moderate exercise for less than 20 minutes is still beneficial.17
Based on this evidence, the best current recommendation for patients with migraine is to engage in graded moderate cardiorespiratory exercise, although any exercise is better than none. If a patient is sedentary or has poor exercise tolerance, or both, exercising once a week for shorter time periods may be a manageable place to start.
Some patients may identify exercise as a trigger or exacerbating factor in migraine. These patients may need appropriate prophylactic and abortive therapies before starting an exercise regimen.
THE SECOND E IS FOR EAT (FOOD AND DRINK)
Many patients believe that some foods trigger migraine attacks, but further study is needed. The most consistent food triggers appear to be red wine and caffeine (withdrawal).18,19 Interestingly, patients with migraine report low levels of alcohol consumption,20 but it is unclear if that is because alcohol has a protective effect or if patients avoid it.
Some patients may crave certain foods in the prodromal phase of an attack, eat the food, experience the attack, and falsely conclude that the food caused the attack.21 Premonitory symptoms include fatigue, cognitive changes, homeostatic changes, sensory hyperresponsiveness, and food cravings.21 It is difficult to distinguish between premonitory phase food cravings and true triggers because premonitory symptoms can precede headache by 48 to 72 hours, and the timing for a trigger to be considered causal is not known.22
Chocolate is often thought to be a migraine trigger, but the evidence argues against this and even suggests that sweet cravings are a part of the premonitory phase.23 Monosodium glutamate is often identified as a trigger as well, but the literature is inconsistent and does not support a causal relationship.24 Identifying true food triggers in migraine is difficult, and patients with migraine may have poor quality diets, with some foods acting as true triggers for certain patients.25 These possibilities have led to the development of many “migraine diets,” including elimination diets.
Elimination diets
Elimination diets involve avoiding specific food items over a period of time and then adding them back in one at a time to gauge whether they cause a reaction in the body. A number of these diets have been studied for their effects on headache and migraine:
Gluten-free diets restrict foods that contain wheat, rye, and barley. A systematic review of gluten-free diets in patients with celiac disease found that headache or migraine frequency decreased by 51.6% to 100% based on multiple cohort studies (N = 42,388).26 There are no studies on the use of a gluten-free diet for migraine in patients without celiac disease.
Immunoglobulin G-elimination diets restrict foods that serve as antigens for IgG. However, data supporting these diets are inconsistent. Two small randomized controlled trials found that the diets improved migraine symptoms, but a larger study found no improvement in the number of migraine days at 12 weeks, although there was an initially significant effect at 4 weeks.27–29
Antihistamine diets restrict foods that have high levels of histamines, including fermented dairy, vegetables, soy products, wine, beer, alcohol, and those that cause histamine release regardless of IgE testing results. A prospective single-arm study of antihistamine diets in patients with chronic headache reported symptom improvement, which could be applied to certain comorbidities such as mast cell activation syndrome.30 Another prospective nonrandomized controlled study eliminated foods based on positive IgE skin-prick testing for allergy in patients with recurrent migraine and found that it reduced headache frequency.31
Tyramine-free diets are often recommended due to the presumption that tyramine-containing foods (eg, aged cheese, cured or smoked meats and fish, and beer) are triggers. However, multiple studies have reviewed this theory with inconsistent results,32 and the only study of a tyramine-free diet was negative.33 In addition, commonly purported high-tyramine foods have lower tyramine levels than previously thought.34
Low-fat diets in migraine are supported by 2 small randomized controlled trials and a prospective study showing a decrease in symptom severity; the results for frequency are inconsistent.35–37
Low-glycemic index diets are supported in migraine by 1 randomized controlled trial that showed improvement in migraine frequency in a diet group and in a control group of patients who took a standard migraine-preventive medication to manage their symptoms.38
Other migraine diets
Diets high in certain foods or ingredient ratios, as opposed to elimination diets, have also been studied in patients with migraine. One promising diet containing high levels of omega-3 fatty acids and low levels of omega-6 fatty acids was shown in a systematic review to reduce the duration of migraine but not the frequency or severity.39 A more recent randomized controlled trial of this diet in chronic migraine also showed that it decreased migraine frequency.40
The ketogenic diet (high fat, low carbohydrate) had promising results in a randomized controlled trial in overweight women with migraine and in a prospective study.41,42 However, a prospective study of the Atkins diet in teenagers with chronic daily headaches showed no benefit.43 The ketogenic diet is difficult to follow and may work in part due to weight loss alone, although ketogenesis itself may also play a role.41,44
Sodium levels have been shown to be higher in the cerebrospinal fluid of patients with migraine than in controls, particularly during an attack.45 For a prehypertensive population or an elderly population, a low-sodium diet may be beneficial based on 2 prospective trials.46,47 However, a younger female population without hypertension and low-to-normal body mass index had a reduced probability of migraine while consuming a high-sodium diet.48
Counseling about sodium intake should be tailored to specific patient populations. For example, a diet low in sodium may be appropriate for patients with vascular risk factors such as hypertension, whereas a high-sodium diet may be appropriate in patients with comorbidities like postural tachycardia syndrome or in those with a propensity for low blood pressure or low body mass index.
Encourage routine meals and hydration
The standard advice for patients with migraine is to consume regular meals. Headaches have been associated with fasting, and those with migraine are predisposed to attacks in the setting of fasting.49,50 Migraine is more common when meals are skipped, particularly breakfast.51
It is unclear how fasting lowers the migraine threshold. Nutritional studies show that skipping meals, particularly breakfast, increases low-grade inflammation and impairs glucose metabolism by affecting insulin and fat oxidation metabolism.52 However, hypoglycemia itself is not a consistent cause of headache or migraine attacks.53 As described above, a randomized controlled trial of a low-glycemic index diet actually decreased migraine frequency and severity.38 Skipping meals also reduces energy and is associated with reduced physical activity, perhaps leading to multiple compounding triggers that further lower the migraine threshold.54,55
When counseling patients about the need to eat breakfast, consider what they normally consume (eg, is breakfast just a cup of coffee?). Replacing simple carbohydrates with protein, fats, and fiber may be beneficial for general health, but the effects on migraine are not known, nor is the optimal composition of breakfast foods.55
The optimal timing of breakfast relative to awakening is also unclear, but in general, it should be eaten within 30 to 60 minutes of rising. Also consider patients’ work hours—delayed-phase or shift workers have altered sleep cycles.
Recommendations vary in regard to hydration. Headache is associated with fluid restriction and dehydration,56,57 but only a few studies suggest that rehydration and increased hydration status can improve migraine.58 In fact, a single post hoc analysis of a metoclopramide study showed that intravenous fluid alone for patients with migraine in the emergency room did not improve pain outcomes.59
The amount of water patients should drink daily in the setting of migraine is also unknown, but a study showed benefit with 4 L, which equates to a daily intake of 16 eight-ounce glasses.60 One review on general health that could be extrapolated given the low risk of the intervention indicated that 1.8 L daily (7 to 8 eight-ounce glasses) promoted a euhydration status in most people, although many factors contribute to hydration status.61
Caffeine intake is also a major consideration. Caffeine is a nonspecific adenosine receptor antagonist that modulates adenosine receptors like the pronociceptive 2A receptor, leading to changes integral to the neuropathophysiology of migraine.62 Caffeine has analgesic properties at doses greater than 65 to 200 mg and augments the effects of analgesics such as acetaminophen and aspirin. Chronic caffeine use can lead to withdrawal symptoms when intake is stopped abruptly; this is thought to be due to upregulation of adenosine receptors, but the effect varies based on genetic predisposition.19
The risk of chronic daily headache may relate to high use of caffeine preceding the onset of chronification, and caffeine abstinence may improve response to acute migraine treatment.19,63 There is a dose-dependent risk of headache.64,65 Current recommendations suggest limiting caffeine consumption to less than 200 mg per day or stopping caffeine consumption altogether based on the quantity required for caffeine-withdrawal headache.66 Varying the caffeine dose from day to day may also trigger headache due to the high sensitivity to caffeine withdrawal.
While many diets have shown potential benefit in patients with migraine, more studies are needed before any one “migraine diet” can be recommended. Caution should be taken, as there is risk of adverse effects from nutrient deficiencies or excess levels, especially if the patient is not under the care of a healthcare professional who is familiar with the diet.
Whether it is beneficial to avoid specific food triggers at this time is unclear and still controversial even within the migraine community because some of these foods may be misattributed as triggers instead of premonitory cravings driven by the hypothalamus. It is important to counsel patients with migraine to eat a healthy diet with consistent meals, to maintain adequate hydration, and to keep their caffeine intake low or at least consistent, although these teachings are predominantly based on limited studies with extrapolation from nutrition research.
D IS FOR DIARY
A headache diary is a recommended part of headache management and may enhance the accuracy of diagnosis and assist in treatment modifications. Paper and electronic diaries have been used. Electronic diaries may be more accurate for real-time use, but patients may be more likely to complete a paper one.67 Patients prefer electronic diaries over long paper forms,68 but a practical issue to consider is easy electronic access.
Patients can start keeping a headache diary before the initial consultation to assist with diagnosis, or early in their management. A first-appointment diary mailed with instructions is a feasible option.69 These types of diaries ask detailed questions to help diagnose all major primary headache types including menstrual migraine and to identify concomitant medication-overuse headache. Physicians and patients generally report improved communication with use of a diary.70
Some providers distinguish between a headache diary and a calendar. In standard practice, a headache diary is the general term referring to both, but the literature differentiates between the two. Both should at least include headache frequency, with possible inclusion of other factors such as headache duration, headache intensity, analgesic use, headache impact on function, and absenteeism. Potential triggers including menses can also be tracked. The calendar version can fit on a single page and can be used for simple tracking of headache frequency and analgesia use.
One of the simplest calendars to use is the “stoplight” calendar. Red days are when a patient is completely debilitated in bed. On a yellow day, function at work, school, or daily activities is significantly reduced by migraine, but the patient is not bedbound. A green day is when headache is present but function is not affected. No color is placed if the patient is 100% headache-free.
Acute treatment use can be written in or, to improve compliance, a checkmark can be placed on days of treatment. Patients who are tracking menses circle the days of menstruation. The calendar-diary should be brought to every appointment to track treatment response and medication use.
THE SECOND S IS FOR STRESS
Behavioral management such as cognitive behavioral therapy in migraine has been shown to decrease catastrophizing, migraine disability, and headache severity and frequency.74 Both depression and anxiety can improve along with migraine.75 Cognitive behavioral therapy can be provided in individualized sessions or group sessions, either in person or online.74,76,77 The effects become more prominent about 5 weeks into treatment.78
Biofeedback, which uses behavioral techniques paired with physiologic autonomic measures, has been extensively studied, and shows benefit in migraine, including in meta-analysis.79 The types of biofeedback measurements used include electromyography, electroencephalography, temperature, sweat sensors, heart rate, blood volume pulse feedback, and respiration bands. While biofeedback is generally done under the guidance of a therapist, it can still be useful with minimal therapist contact and supplemental audio.80
Mindfulness, or the awareness of thoughts, feelings, and sensations in the present moment without judgment, is a behavioral technique that can be done alone or paired with another technique. It is often taught through a mindfulness-based stress-reduction program, which relies on a standardized approach. A meta-analysis showed that mindfulness improves pain intensity, headache frequency, disability, self-efficacy, and quality of life.81 It may work by encouraging pain acceptance.82
Relaxation techniques are also employed in migraine management, either alone or in conjunction with techniques mentioned above, such as mindfulness. They include progressive muscle relaxation and deep breathing. Relaxation has been shown to be effective when done by professional trainers as well as lay trainers in both individual and group settings.83,84
In patients with intractable headache, more-intensive inpatient and outpatient programs have been tried. Inpatient admissions with multidisciplinary programs that include a focus on behavioral techniques often paired with lifestyle education and sometimes pharmacologic management can be beneficial.85,86 These programs have also been successfully conducted as multiple outpatient sessions.86–88
Stress management is an important aspect of migraine management. These treatments often involve homework and require active participation.
LIFESTYLE FOR ALL
All patients with migraine should initiate lifestyle modifications (see Advice to patients with migraine: SEEDS for success). Modifications with the highest level of evidence, specifically behavioral techniques, have had the most reproducible results. A headache diary is an essential tool to identify patterns and needs for optimization of acute or preventive treatment regimens. The strongest evidence is for the behavioral management techniques for stress reduction.
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390(10100):1211–1259. doi:10.1016/S0140-6736(17)32154-2
- Vgontzas A, Pavlovic JM. Sleep diorders and migraine: review of literature and potential pathophysiology mechanisms. Headache 2018; 58(7):1030–1039. doi:10.1111/head.13358
- Lund N, Westergaard ML, Barloese M, Glumer C, Jensen RH. Epidemiology of concurrent headache and sleep problems in Denmark. Cephalalgia 2014; 34(10):833–845. doi:10.1177/0333102414543332
- Woldeamanuel YW, Cowan RP. The impact of regular lifestyle behavior in migraine: a prevalence case-referent study. J Neurol 2016; 263(4):669–676. doi:10.1007/s00415-016-8031-5
- Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016; 149(3):631–638. doi:10.1378/chest.15-0903
- Johnson KG, Ziemba AM, Garb JL. Improvement in headaches with continuous positive airway pressure for obstructive sleep apnea: a retrospective analysis. Headache 2013; 53(2):333–343. doi:10.1111/j.1526-4610.2012.02251.x
- Calhoun AH, Ford S. Behavioral sleep modification may revert transformed migraine to episodic migraine. Headache 2007; 47(8):1178–1183. doi:10.1111/j.1526-4610.2007.00780.x
- Calhoun AH, Ford S, Finkel AG, Kahn KA, Mann JD. The prevalence and spectrum of sleep problems in women with transformed migraine. Headache 2006; 46(4):604–610. doi:10.1111/j.1526-4610.2006.00410.x
- Rains JC. Optimizing circadian cycles and behavioral insomnia treatment in migraine. Curr Pain Headache Rep 2008; 12(3):213–219. pmid:18796272
- Lemmens J, De Pauw J, Van Soom T, et al. The effect of aerobic exercise on the number of migraine days, duration and pain intensity in migraine: a systematic literature review and meta-analysis. J Headache Pain 2019; 20(1):16. doi:10.1186/s10194-019-0961-8
- Amin FM, Aristeidou S, Baraldi C, et al; European Headache Federation School of Advanced Studies (EHF-SAS). The association between migraine and physical exercise. J Headache Pain 2018; 19(1):83. doi:10.1186/s10194-018-0902-y
- Genazzani AR, Nappi G, Facchinetti F, et al. Progressive impairment of CSF beta-EP levels in migraine sufferers. Pain 1984; 18:127-133. pmid:6324056
- Hindiyeh NA, Krusz JC, Cowan RP. Does exercise make migraines worse and tension type headaches better? Curr Pain Headache Rep 2013;17:380. pmid:24234818
- Kroll LS, Sjodahl Hammarlund C, Gard G, Jensen RH, Bendtsen L. Has aerobic exercise effect on pain perception in persons with migraine and coexisting tension-type headache and neck pain? A randomized, controlled, clinical trial. Eur J Pain 2018; 10:10. pmid:29635806
- Santiago MD, Carvalho Dde S, Gabbai AA, Pinto MM, Moutran AR, Villa TR. Amitriptyline and aerobic exercise or amitriptyline alone in the treatment of chronic migraine: a randomized comparative study. Arq Neuropsiquiatr 2014; 72(11):851-855. pmid:25410451
- Varkey E, Cider A, Carlsson J, Linde M. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia 2011; 31(14):1428-1438. pmid:21890526
- Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43(7):1334-1359. pmid:21694556
- Guarnieri P, Radnitz CL, Blanchard EB. Assessment of dietary risk factors in chronic headache. Biofeedback Self Regul 1990; 15(1):15–25. pmid:2361144
- Shapiro RE. Caffeine and headaches. Curr Pain Headache Rep 2008; 12(4):311–315. pmid:18625110
- Yokoyama M, Yokoyama T, Funazu K, et al. Associations between headache and stress, alcohol drinking, exercise, sleep, and comorbid health conditions in a Japanese population. J Headache Pain 2009; 10(3):177–185. doi:10.1007/s10194-009-0113-7
- Karsan N, Bose P, Goadsby PJ. The migraine premonitory phase. Continuum (Minneap Minn) 2018; 24(4, Headache):996–1008. doi:10.1212/CON.0000000000000624
- Pavlovic JM, Buse DC, Sollars CM, Haut S, Lipton RB. Trigger factors and premonitory features of migraine attacks: summary of studies. Headache 2014; 54(10):1670–1679. doi:10.1111/head.12468
- Marcus DA, Scharff L, Turk D, Gourley LM. A double-blind provocative study of chocolate as a trigger of headache. Cephalalgia 1997; 17(8):855–862. doi:10.1046/j.1468-2982.1997.1708855.x
- Obayashi Y, Nagamura Y. Does monosodium glutamate really cause headache? A systematic review of human studies. J Headache Pain 2016; 17:54. doi:10.1186/s10194-016-0639-4
- Evans EW, Lipton RB, Peterlin BL, et al. Dietary intake patterns and diet quality in a nationally representative sample of women with and without severe headache or migraine. Headache 2015; 55(4):550–561. doi:10.1111/head.12527
- Zis P, Julian T, Hadjivassiliou M. Headache associated with coeliac disease: a systematic review and meta-analysis. Nutrients 2018; 10(10). doi:10.3390/nu10101445
- Alpay K, Ertas M, Orhan EK, Ustay DK, Lieners C, Baykan B. Diet restriction in migraine, based on IgG against foods: a clinical double-blind, randomised, cross-over trial. Cephalalgia 2010; 30(7):829–837. doi:10.1177/0333102410361404
- Aydinlar EI, Dikmen PY, Tiftikci A, et al. IgG-based elimination diet in migraine plus irritable bowel syndrome. Headache 2013; 53(3):514–525. doi:10.1111/j.1526-4610.2012.02296.x
- Mitchell N, Hewitt CE, Jayakody S, et al. Randomised controlled trial of food elimination diet based on IgG antibodies for the prevention of migraine like headaches. Nutr J 2011; 10:85. doi:10.1186/1475-2891-10-85
- Wantke F, Gotz M, Jarisch R. Histamine-free diet: treatment of choice for histamine-induced food intolerance and supporting treatment for chronic headaches. Clin Exp Allergy 1993; 23(12):982–985. pmid:10779289
- Mansfield LE, Vaughan TR, Waller SF, Haverly RW, Ting S. Food allergy and adult migraine: double-blind and mediator confirmation of an allergic etiology. Ann Allergy 1985; 55(2):126–129. pmid:4025956
- Kohlenberg RJ. Tyramine sensitivity in dietary migraine: a critical review. Headache 1982; 22(1):30–34. pmid:17152742
- Medina JL, Diamond S. The role of diet in migraine. Headache 1978; 18(1):31–34. pmid:649377
- Mosnaim AD, Freitag F, Ignacio R, et al. Apparent lack of correlation between tyramine and phenylethylamine content and the occurrence of food-precipitated migraine. Reexamination of a variety of food products frequently consumed in the United States and commonly restricted in tyramine-free diets. Headache Quarterly. Current Treatment and Research 1996; 7(3):239–249.
- Ferrara LA, Pacioni D, Di Fronzo V, et al. Low-lipid diet reduces frequency and severity of acute migraine attacks. Nutr Metab Cardiovasc Dis 2015; 25(4):370–375. doi:10.1016/j.numecd.2014.12.006
- Bic Z, Blix GG, Hopp HP, Leslie FM, Schell MJ. The influence of a low-fat diet on incidence and severity of migraine headaches. J Womens Health Gend Based Med 1999; 8(5):623–630. doi:10.1089/jwh.1.1999.8.623
- Bunner AE, Agarwal U, Gonzales JF, Valente F, Barnard ND. Nutrition intervention for migraine: a randomized crossover trial. J Headache Pain 2014; 15:69. doi:10.1186/1129-2377-15-69
- Evcili G, Utku U, Ogun MN, Ozdemir G. Early and long period follow-up results of low glycemic index diet for migraine prophylaxis. Agri 2018; 30(1):8–11. doi:10.5505/agri.2017.62443
- Maghsoumi-Norouzabad L, Mansoori A, Abed R, Shishehbor F. Effects of omega-3 fatty acids on the frequency, severity, and duration of migraine attacks: a systematic review and meta-analysis of randomized controlled trials. Nutr Neurosci 2018; 21(9):614–623. doi:10.1080/1028415X.2017.1344371
- Soares AA, Loucana PMC, Nasi EP, Sousa KMH, Sa OMS, Silva-Neto RP. A double- blind, randomized, and placebo-controlled clinical trial with omega-3 polyunsaturated fatty acids (OPFA Ω-3) for the prevention of migraine in chronic migraine patients using amitriptyline. Nutr Neurosci 2018; 21(3):219–223. doi:10.1080/1028415X.2016.1266133
- Di Lorenzo C, Coppola G, Sirianni G, et al. Migraine improvement during short lasting ketogenesis: a proof-of-concept study. Eur J Neurol 2015; 22(1):170–177. doi:10.1111/ene.12550
- Di Lorenzo C, Coppola G, Bracaglia M, et al. Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: a multimodal evoked potentials study. J Headache Pain 2016; 17:58. doi:10.1186/s10194-016-0650-9
- Kossoff EH, Huffman J, Turner Z, Gladstein J. Use of the modified Atkins diet for adolescents with chronic daily headache. Cephalalgia 2010; 30(8):1014–1016. https://journals.sagepub.com/doi/full/10.1111/j.1468-2982.2009.02016.x
- Slavin M, Ailani J. A clinical approach to addressing diet with migraine patients. Curr Neurol Neurosci Rep 2017; 17(2):17. doi:10.1007/s11910-017-0721-6
- Amer M, Woodward M, Appel LJ. Effects of dietary sodium and the DASH diet on the occurrence of headaches: results from randomised multicentre DASH-sodium clinical trial. BMJ Open 2014; 4(12):e006671. doi:10.1136/bmjopen-2014-006671
- Chen L, Zhang Z, Chen W, Whelton PK, Appel LJ. Lower sodium intake and risk of headaches: results from the trial of nonpharmacologic interventions in the elderly. Am J Public Health 2016; 106(7):1270–1275. doi:10.2105/AJPH.2016.303143
- Pogoda JM, Gross NB, Arakaki X, Fonteh AN, Cowan RP, Harrington MG. Severe headache or migraine history is inversely correlated with dietary sodium intake: NHANES 1999–2004. Headache 2016; 56(4):688–698. doi:10.1111/head.12792
- Awada A, al Jumah M. The first-of-Ramadan headache. Headache 1999; 39(7):490–493. pmid:11279933
- Abu-Salameh I, Plakht Y, Ifergane G. Migraine exacerbation during Ramadan fasting. J Headache Pain 2010; 11(6):513–517. doi:10.1007/s10194-010-0242-z
- Nazari F, Safavi M, Mahmudi M. Migraine and its relation with lifestyle in women. Pain Pract 2010; 10(3):228–234. doi:10.1111/j.1533-2500.2009.00343.x
- Nas A, Mirza N, Hagele F, et al. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am J Clin Nutr 2017; 105(6):1351–1361. doi:10.3945/ajcn.116.151332
- Torelli P, Manzoni GC. Fasting headache. Curr Pain Headache Rep 2010; 14(4):284–291. doi:10.1007/s11916-010-0119-5
- Yoshimura E, Hatamoto Y, Yonekura S, Tanaka H. Skipping breakfast reduces energy intake and physical activity in healthy women who are habitual breakfast eaters: a randomized crossover trial. Physiol Behav 2017; 174:89–94. doi:10.1016/j.physbeh.2017.03.008
- Pendergast FJ, Livingstone KM, Worsley A, McNaughton SA. Correlates of meal skipping in young adults: a systematic review. Int J Behav Nutr Phys Act 2016; 13(1):125. doi:10.1186/s12966-016-0451-1
- Maki KC, Phillips-Eakley AK, Smith KN. The effects of breakfast consumption and composition on metabolic wellness with a focus on carbohydrate metabolism. Adv Nutr 2016; 7(3):613S–621S. doi:10.3945/an.115.010314
- Shirreffs SM, Merson SJ, Fraser SM, Archer DT. The effects of fluid restriction on hydration status and subjective feelings in man. Br J Nutr 2004; 91(6):951–958. doi:10.1079/BJN20041149
- Blau JN. Water deprivation: a new migraine precipitant. Headache 2005; 45(6):757–759. doi:10.1111/j.1526-4610.2005.05143_3.x
- Price A, Burls A. Increased water intake to reduce headache: learning from a critical appraisal. J Eval Clin Pract 2015; 21(6):1212–1218. doi:10.1111/jep.12413
- Balbin JE, Nerenberg R, Baratloo A, Friedman BW. Intravenous fluids for migraine: a post hoc analysis of clinical trial data. Am J Emerg Med 2016; 34(4):713–716. doi:10.1016/j.ajem.2015.12.080
- Spigt M, Weerkamp N, Troost J, van Schayck CP, Knottnerus JA. A randomized trial on the effects of regular water intake in patients with recurrent headaches. Fam Pract 2012; 29(4):370–375. doi:10.1093/fampra/cmr112
- Armstrong LE, Johnson EC. Water intake, water balance, and the elusive daily water requirement. Nutrients 2018; 10(12). doi:10.3390/nu10121928
- Fried NT, Elliott MB, Oshinsky ML. The role of adenosine signaling in headache: a review. Brain Sci 2017; 7(3). doi:10.3390/brainsci7030030
- Lee MJ, Choi HA, Choi H, Chung CS. Caffeine discontinuation improves acute migraine treatment: a prospective clinic-based study. J Headache Pain 2016; 17(1):71. doi:10.1186/s10194-016-0662-5
- Shirlow MJ, Mathers CD. A study of caffeine consumption and symptoms; indigestion, palpitations, tremor, headache and insomnia. Int J Epidemiol 1985; 14(2):239–248. doi:10.1093/ije/14.2.239
- Silverman K, Evans SM, Strain EC, Griffiths RR. Withdrawal syndrome after the double-blind cessation of caffeine consumption. N Engl J Med 1992; 327(16):1109–1114. doi:10.1056/NEJM199210153271601
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38(1):1–211. doi:10.1177/0333102417738202
- Krogh AB, Larsson B, Salvesen O, Linde M. A comparison between prospective Internet-based and paper diary recordings of headache among adolescents in the general population. Cephalalgia 2016; 36(4):335–345. doi:10.1177/0333102415591506
- Bandarian-Balooch S, Martin PR, McNally B, Brunelli A, Mackenzie S. Electronic-diary for recording headaches, triggers, and medication use: development and evaluation. Headache 2017; 57(10):1551–1569. doi:10.1111/head.13184
- Tassorelli C, Sances G, Allena M, et al. The usefulness and applicability of a basic headache diary before first consultation: results of a pilot study conducted in two centres. Cephalalgia 2008; 28(10):1023–1030. doi:10.1111/j.1468-2982.2008.01639.x
- Baos V, Ester F, Castellanos A, et al. Use of a structured migraine diary improves patient and physician communication about migraine disability and treatment outcomes. Int J Clin Pract 2005; 59(3):281–286. doi:10.1111/j.1742-1241.2005.00469.x
- Martin PR, MacLeod C. Behavioral management of headache triggers: avoidance of triggers is an inadequate strategy. Clin Psychol Rev 2009; 29(6):483–495. doi:10.1016/j.cpr.2009.05.002
- Giannini G, Zanigni S, Grimaldi D, et al. Cephalalgiaphobia as a feature of high-frequency migraine: a pilot study. J Headache Pain 2013; 14:49. doi:10.1186/1129-2377-14-49
- Westergaard ML, Glumer C, Hansen EH, Jensen RH. Medication overuse, healthy lifestyle behaviour and stress in chronic headache: results from a population-based representative survey. Cephalalgia 2016; 36(1):15–28. doi:10.1177/0333102415578430
- Christiansen S, Jurgens TP, Klinger R. Outpatient combined group and individual cognitive-behavioral treatment for patients with migraine and tension-type headache in a routine clinical setting. Headache 2015; 55(8):1072–1091. doi:10.1111/head.12626
- Martin PR, Aiello R, Gilson K, Meadows G, Milgrom J, Reece J. Cognitive behavior therapy for comorbid migraine and/or tension-type headache and major depressive disorder: an exploratory randomized controlled trial. Behav Res Ther 2015; 73:8–18. doi:10.1016/j.brat.2015.07.005
- Nash JM, Park ER, Walker BB, Gordon N, Nicholson RA. Cognitive-behavioral group treatment for disabling headache. Pain Med 2004; 5(2):178–186. doi:10.1111/j.1526-4637.2004.04031.x
- Sorbi MJ, Balk Y, Kleiboer AM, Couturier EG. Follow-up over 20 months confirms gains of online behavioural training in frequent episodic migraine. Cephalalgia 2017; 37(3):236–250. doi:10.1177/0333102416657145
- Thorn BE, Pence LB, Ward LC, et al. A randomized clinical trial of targeted cognitive behavioral treatment to reduce catastrophizing in chronic headache sufferers. J Pain 2007; 8(12):938–949. doi:10.1016/j.jpain.2007.06.010
- Nestoriuc Y, Martin A. Efficacy of biofeedback for migraine: a meta-analysis. Pain 2007; 128(1–2):111–127. doi:10.1016/j.pain.2006.09.007
- Blanchard EB, Appelbaum KA, Nicholson NL, et al. A controlled evaluation of the addition of cognitive therapy to a home-based biofeedback and relaxation treatment of vascular headache. Headache 1990; 30(6):371–376. pmid:2196240
- Gu Q, Hou JC, Fang XM. Mindfulness meditation for primary headache pain: a meta-analysis. Chin Med J (Engl) 2018; 131(7):829–838. doi:10.4103/0366-6999.228242
- Day MA, Thorn BE. The mediating role of pain acceptance during mindfulness-based cognitive therapy for headache. Complement Ther Med 2016; 25:51–54. doi:10.1016/j.ctim.2016.01.002
- Williamson DA, Monguillot JE, Jarrell MP, Cohen RA, Pratt JM, Blouin DC. Relaxation for the treatment of headache. Controlled evaluation of two group programs. Behav Modif 1984; 8(3):407–424. doi:10.1177/01454455840083007
- Merelle SY, Sorbi MJ, Duivenvoorden HJ, Passchier J. Qualities and health of lay trainers with migraine for behavioral attack prevention. Headache 2010; 50(4):613–625. doi:10.1111/j.1526-4610.2008.01241.x
- Gaul C, van Doorn C, Webering N, et al. Clinical outcome of a headache-specific multidisciplinary treatment program and adherence to treatment recommendations in a tertiary headache center: an observational study. J Headache Pain 2011; 12(4):475–483. doi:10.1007/s10194-011-0348-y
- Wallasch TM, Kropp P. Multidisciplinary integrated headache care: a prospective 12-month follow-up observational study. J Headache Pain 2012; 13(7):521–529. doi:10.1007/s10194-012-0469-y
- Lemstra M, Stewart B, Olszynski WP. Effectiveness of multidisciplinary intervention in the treatment of migraine: a randomized clinical trial. Headache 2002; 42(9):845–854. pmid:12390609
- Krause SJ, Stillman MJ, Tepper DE, Zajac D. A prospective cohort study of outpatient interdisciplinary rehabilitation of chronic headache patients. Headache 2017; 57(3):428–440. doi:10.1111/head.13020
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390(10100):1211–1259. doi:10.1016/S0140-6736(17)32154-2
- Vgontzas A, Pavlovic JM. Sleep diorders and migraine: review of literature and potential pathophysiology mechanisms. Headache 2018; 58(7):1030–1039. doi:10.1111/head.13358
- Lund N, Westergaard ML, Barloese M, Glumer C, Jensen RH. Epidemiology of concurrent headache and sleep problems in Denmark. Cephalalgia 2014; 34(10):833–845. doi:10.1177/0333102414543332
- Woldeamanuel YW, Cowan RP. The impact of regular lifestyle behavior in migraine: a prevalence case-referent study. J Neurol 2016; 263(4):669–676. doi:10.1007/s00415-016-8031-5
- Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016; 149(3):631–638. doi:10.1378/chest.15-0903
- Johnson KG, Ziemba AM, Garb JL. Improvement in headaches with continuous positive airway pressure for obstructive sleep apnea: a retrospective analysis. Headache 2013; 53(2):333–343. doi:10.1111/j.1526-4610.2012.02251.x
- Calhoun AH, Ford S. Behavioral sleep modification may revert transformed migraine to episodic migraine. Headache 2007; 47(8):1178–1183. doi:10.1111/j.1526-4610.2007.00780.x
- Calhoun AH, Ford S, Finkel AG, Kahn KA, Mann JD. The prevalence and spectrum of sleep problems in women with transformed migraine. Headache 2006; 46(4):604–610. doi:10.1111/j.1526-4610.2006.00410.x
- Rains JC. Optimizing circadian cycles and behavioral insomnia treatment in migraine. Curr Pain Headache Rep 2008; 12(3):213–219. pmid:18796272
- Lemmens J, De Pauw J, Van Soom T, et al. The effect of aerobic exercise on the number of migraine days, duration and pain intensity in migraine: a systematic literature review and meta-analysis. J Headache Pain 2019; 20(1):16. doi:10.1186/s10194-019-0961-8
- Amin FM, Aristeidou S, Baraldi C, et al; European Headache Federation School of Advanced Studies (EHF-SAS). The association between migraine and physical exercise. J Headache Pain 2018; 19(1):83. doi:10.1186/s10194-018-0902-y
- Genazzani AR, Nappi G, Facchinetti F, et al. Progressive impairment of CSF beta-EP levels in migraine sufferers. Pain 1984; 18:127-133. pmid:6324056
- Hindiyeh NA, Krusz JC, Cowan RP. Does exercise make migraines worse and tension type headaches better? Curr Pain Headache Rep 2013;17:380. pmid:24234818
- Kroll LS, Sjodahl Hammarlund C, Gard G, Jensen RH, Bendtsen L. Has aerobic exercise effect on pain perception in persons with migraine and coexisting tension-type headache and neck pain? A randomized, controlled, clinical trial. Eur J Pain 2018; 10:10. pmid:29635806
- Santiago MD, Carvalho Dde S, Gabbai AA, Pinto MM, Moutran AR, Villa TR. Amitriptyline and aerobic exercise or amitriptyline alone in the treatment of chronic migraine: a randomized comparative study. Arq Neuropsiquiatr 2014; 72(11):851-855. pmid:25410451
- Varkey E, Cider A, Carlsson J, Linde M. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia 2011; 31(14):1428-1438. pmid:21890526
- Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43(7):1334-1359. pmid:21694556
- Guarnieri P, Radnitz CL, Blanchard EB. Assessment of dietary risk factors in chronic headache. Biofeedback Self Regul 1990; 15(1):15–25. pmid:2361144
- Shapiro RE. Caffeine and headaches. Curr Pain Headache Rep 2008; 12(4):311–315. pmid:18625110
- Yokoyama M, Yokoyama T, Funazu K, et al. Associations between headache and stress, alcohol drinking, exercise, sleep, and comorbid health conditions in a Japanese population. J Headache Pain 2009; 10(3):177–185. doi:10.1007/s10194-009-0113-7
- Karsan N, Bose P, Goadsby PJ. The migraine premonitory phase. Continuum (Minneap Minn) 2018; 24(4, Headache):996–1008. doi:10.1212/CON.0000000000000624
- Pavlovic JM, Buse DC, Sollars CM, Haut S, Lipton RB. Trigger factors and premonitory features of migraine attacks: summary of studies. Headache 2014; 54(10):1670–1679. doi:10.1111/head.12468
- Marcus DA, Scharff L, Turk D, Gourley LM. A double-blind provocative study of chocolate as a trigger of headache. Cephalalgia 1997; 17(8):855–862. doi:10.1046/j.1468-2982.1997.1708855.x
- Obayashi Y, Nagamura Y. Does monosodium glutamate really cause headache? A systematic review of human studies. J Headache Pain 2016; 17:54. doi:10.1186/s10194-016-0639-4
- Evans EW, Lipton RB, Peterlin BL, et al. Dietary intake patterns and diet quality in a nationally representative sample of women with and without severe headache or migraine. Headache 2015; 55(4):550–561. doi:10.1111/head.12527
- Zis P, Julian T, Hadjivassiliou M. Headache associated with coeliac disease: a systematic review and meta-analysis. Nutrients 2018; 10(10). doi:10.3390/nu10101445
- Alpay K, Ertas M, Orhan EK, Ustay DK, Lieners C, Baykan B. Diet restriction in migraine, based on IgG against foods: a clinical double-blind, randomised, cross-over trial. Cephalalgia 2010; 30(7):829–837. doi:10.1177/0333102410361404
- Aydinlar EI, Dikmen PY, Tiftikci A, et al. IgG-based elimination diet in migraine plus irritable bowel syndrome. Headache 2013; 53(3):514–525. doi:10.1111/j.1526-4610.2012.02296.x
- Mitchell N, Hewitt CE, Jayakody S, et al. Randomised controlled trial of food elimination diet based on IgG antibodies for the prevention of migraine like headaches. Nutr J 2011; 10:85. doi:10.1186/1475-2891-10-85
- Wantke F, Gotz M, Jarisch R. Histamine-free diet: treatment of choice for histamine-induced food intolerance and supporting treatment for chronic headaches. Clin Exp Allergy 1993; 23(12):982–985. pmid:10779289
- Mansfield LE, Vaughan TR, Waller SF, Haverly RW, Ting S. Food allergy and adult migraine: double-blind and mediator confirmation of an allergic etiology. Ann Allergy 1985; 55(2):126–129. pmid:4025956
- Kohlenberg RJ. Tyramine sensitivity in dietary migraine: a critical review. Headache 1982; 22(1):30–34. pmid:17152742
- Medina JL, Diamond S. The role of diet in migraine. Headache 1978; 18(1):31–34. pmid:649377
- Mosnaim AD, Freitag F, Ignacio R, et al. Apparent lack of correlation between tyramine and phenylethylamine content and the occurrence of food-precipitated migraine. Reexamination of a variety of food products frequently consumed in the United States and commonly restricted in tyramine-free diets. Headache Quarterly. Current Treatment and Research 1996; 7(3):239–249.
- Ferrara LA, Pacioni D, Di Fronzo V, et al. Low-lipid diet reduces frequency and severity of acute migraine attacks. Nutr Metab Cardiovasc Dis 2015; 25(4):370–375. doi:10.1016/j.numecd.2014.12.006
- Bic Z, Blix GG, Hopp HP, Leslie FM, Schell MJ. The influence of a low-fat diet on incidence and severity of migraine headaches. J Womens Health Gend Based Med 1999; 8(5):623–630. doi:10.1089/jwh.1.1999.8.623
- Bunner AE, Agarwal U, Gonzales JF, Valente F, Barnard ND. Nutrition intervention for migraine: a randomized crossover trial. J Headache Pain 2014; 15:69. doi:10.1186/1129-2377-15-69
- Evcili G, Utku U, Ogun MN, Ozdemir G. Early and long period follow-up results of low glycemic index diet for migraine prophylaxis. Agri 2018; 30(1):8–11. doi:10.5505/agri.2017.62443
- Maghsoumi-Norouzabad L, Mansoori A, Abed R, Shishehbor F. Effects of omega-3 fatty acids on the frequency, severity, and duration of migraine attacks: a systematic review and meta-analysis of randomized controlled trials. Nutr Neurosci 2018; 21(9):614–623. doi:10.1080/1028415X.2017.1344371
- Soares AA, Loucana PMC, Nasi EP, Sousa KMH, Sa OMS, Silva-Neto RP. A double- blind, randomized, and placebo-controlled clinical trial with omega-3 polyunsaturated fatty acids (OPFA Ω-3) for the prevention of migraine in chronic migraine patients using amitriptyline. Nutr Neurosci 2018; 21(3):219–223. doi:10.1080/1028415X.2016.1266133
- Di Lorenzo C, Coppola G, Sirianni G, et al. Migraine improvement during short lasting ketogenesis: a proof-of-concept study. Eur J Neurol 2015; 22(1):170–177. doi:10.1111/ene.12550
- Di Lorenzo C, Coppola G, Bracaglia M, et al. Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: a multimodal evoked potentials study. J Headache Pain 2016; 17:58. doi:10.1186/s10194-016-0650-9
- Kossoff EH, Huffman J, Turner Z, Gladstein J. Use of the modified Atkins diet for adolescents with chronic daily headache. Cephalalgia 2010; 30(8):1014–1016. https://journals.sagepub.com/doi/full/10.1111/j.1468-2982.2009.02016.x
- Slavin M, Ailani J. A clinical approach to addressing diet with migraine patients. Curr Neurol Neurosci Rep 2017; 17(2):17. doi:10.1007/s11910-017-0721-6
- Amer M, Woodward M, Appel LJ. Effects of dietary sodium and the DASH diet on the occurrence of headaches: results from randomised multicentre DASH-sodium clinical trial. BMJ Open 2014; 4(12):e006671. doi:10.1136/bmjopen-2014-006671
- Chen L, Zhang Z, Chen W, Whelton PK, Appel LJ. Lower sodium intake and risk of headaches: results from the trial of nonpharmacologic interventions in the elderly. Am J Public Health 2016; 106(7):1270–1275. doi:10.2105/AJPH.2016.303143
- Pogoda JM, Gross NB, Arakaki X, Fonteh AN, Cowan RP, Harrington MG. Severe headache or migraine history is inversely correlated with dietary sodium intake: NHANES 1999–2004. Headache 2016; 56(4):688–698. doi:10.1111/head.12792
- Awada A, al Jumah M. The first-of-Ramadan headache. Headache 1999; 39(7):490–493. pmid:11279933
- Abu-Salameh I, Plakht Y, Ifergane G. Migraine exacerbation during Ramadan fasting. J Headache Pain 2010; 11(6):513–517. doi:10.1007/s10194-010-0242-z
- Nazari F, Safavi M, Mahmudi M. Migraine and its relation with lifestyle in women. Pain Pract 2010; 10(3):228–234. doi:10.1111/j.1533-2500.2009.00343.x
- Nas A, Mirza N, Hagele F, et al. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am J Clin Nutr 2017; 105(6):1351–1361. doi:10.3945/ajcn.116.151332
- Torelli P, Manzoni GC. Fasting headache. Curr Pain Headache Rep 2010; 14(4):284–291. doi:10.1007/s11916-010-0119-5
- Yoshimura E, Hatamoto Y, Yonekura S, Tanaka H. Skipping breakfast reduces energy intake and physical activity in healthy women who are habitual breakfast eaters: a randomized crossover trial. Physiol Behav 2017; 174:89–94. doi:10.1016/j.physbeh.2017.03.008
- Pendergast FJ, Livingstone KM, Worsley A, McNaughton SA. Correlates of meal skipping in young adults: a systematic review. Int J Behav Nutr Phys Act 2016; 13(1):125. doi:10.1186/s12966-016-0451-1
- Maki KC, Phillips-Eakley AK, Smith KN. The effects of breakfast consumption and composition on metabolic wellness with a focus on carbohydrate metabolism. Adv Nutr 2016; 7(3):613S–621S. doi:10.3945/an.115.010314
- Shirreffs SM, Merson SJ, Fraser SM, Archer DT. The effects of fluid restriction on hydration status and subjective feelings in man. Br J Nutr 2004; 91(6):951–958. doi:10.1079/BJN20041149
- Blau JN. Water deprivation: a new migraine precipitant. Headache 2005; 45(6):757–759. doi:10.1111/j.1526-4610.2005.05143_3.x
- Price A, Burls A. Increased water intake to reduce headache: learning from a critical appraisal. J Eval Clin Pract 2015; 21(6):1212–1218. doi:10.1111/jep.12413
- Balbin JE, Nerenberg R, Baratloo A, Friedman BW. Intravenous fluids for migraine: a post hoc analysis of clinical trial data. Am J Emerg Med 2016; 34(4):713–716. doi:10.1016/j.ajem.2015.12.080
- Spigt M, Weerkamp N, Troost J, van Schayck CP, Knottnerus JA. A randomized trial on the effects of regular water intake in patients with recurrent headaches. Fam Pract 2012; 29(4):370–375. doi:10.1093/fampra/cmr112
- Armstrong LE, Johnson EC. Water intake, water balance, and the elusive daily water requirement. Nutrients 2018; 10(12). doi:10.3390/nu10121928
- Fried NT, Elliott MB, Oshinsky ML. The role of adenosine signaling in headache: a review. Brain Sci 2017; 7(3). doi:10.3390/brainsci7030030
- Lee MJ, Choi HA, Choi H, Chung CS. Caffeine discontinuation improves acute migraine treatment: a prospective clinic-based study. J Headache Pain 2016; 17(1):71. doi:10.1186/s10194-016-0662-5
- Shirlow MJ, Mathers CD. A study of caffeine consumption and symptoms; indigestion, palpitations, tremor, headache and insomnia. Int J Epidemiol 1985; 14(2):239–248. doi:10.1093/ije/14.2.239
- Silverman K, Evans SM, Strain EC, Griffiths RR. Withdrawal syndrome after the double-blind cessation of caffeine consumption. N Engl J Med 1992; 327(16):1109–1114. doi:10.1056/NEJM199210153271601
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38(1):1–211. doi:10.1177/0333102417738202
- Krogh AB, Larsson B, Salvesen O, Linde M. A comparison between prospective Internet-based and paper diary recordings of headache among adolescents in the general population. Cephalalgia 2016; 36(4):335–345. doi:10.1177/0333102415591506
- Bandarian-Balooch S, Martin PR, McNally B, Brunelli A, Mackenzie S. Electronic-diary for recording headaches, triggers, and medication use: development and evaluation. Headache 2017; 57(10):1551–1569. doi:10.1111/head.13184
- Tassorelli C, Sances G, Allena M, et al. The usefulness and applicability of a basic headache diary before first consultation: results of a pilot study conducted in two centres. Cephalalgia 2008; 28(10):1023–1030. doi:10.1111/j.1468-2982.2008.01639.x
- Baos V, Ester F, Castellanos A, et al. Use of a structured migraine diary improves patient and physician communication about migraine disability and treatment outcomes. Int J Clin Pract 2005; 59(3):281–286. doi:10.1111/j.1742-1241.2005.00469.x
- Martin PR, MacLeod C. Behavioral management of headache triggers: avoidance of triggers is an inadequate strategy. Clin Psychol Rev 2009; 29(6):483–495. doi:10.1016/j.cpr.2009.05.002
- Giannini G, Zanigni S, Grimaldi D, et al. Cephalalgiaphobia as a feature of high-frequency migraine: a pilot study. J Headache Pain 2013; 14:49. doi:10.1186/1129-2377-14-49
- Westergaard ML, Glumer C, Hansen EH, Jensen RH. Medication overuse, healthy lifestyle behaviour and stress in chronic headache: results from a population-based representative survey. Cephalalgia 2016; 36(1):15–28. doi:10.1177/0333102415578430
- Christiansen S, Jurgens TP, Klinger R. Outpatient combined group and individual cognitive-behavioral treatment for patients with migraine and tension-type headache in a routine clinical setting. Headache 2015; 55(8):1072–1091. doi:10.1111/head.12626
- Martin PR, Aiello R, Gilson K, Meadows G, Milgrom J, Reece J. Cognitive behavior therapy for comorbid migraine and/or tension-type headache and major depressive disorder: an exploratory randomized controlled trial. Behav Res Ther 2015; 73:8–18. doi:10.1016/j.brat.2015.07.005
- Nash JM, Park ER, Walker BB, Gordon N, Nicholson RA. Cognitive-behavioral group treatment for disabling headache. Pain Med 2004; 5(2):178–186. doi:10.1111/j.1526-4637.2004.04031.x
- Sorbi MJ, Balk Y, Kleiboer AM, Couturier EG. Follow-up over 20 months confirms gains of online behavioural training in frequent episodic migraine. Cephalalgia 2017; 37(3):236–250. doi:10.1177/0333102416657145
- Thorn BE, Pence LB, Ward LC, et al. A randomized clinical trial of targeted cognitive behavioral treatment to reduce catastrophizing in chronic headache sufferers. J Pain 2007; 8(12):938–949. doi:10.1016/j.jpain.2007.06.010
- Nestoriuc Y, Martin A. Efficacy of biofeedback for migraine: a meta-analysis. Pain 2007; 128(1–2):111–127. doi:10.1016/j.pain.2006.09.007
- Blanchard EB, Appelbaum KA, Nicholson NL, et al. A controlled evaluation of the addition of cognitive therapy to a home-based biofeedback and relaxation treatment of vascular headache. Headache 1990; 30(6):371–376. pmid:2196240
- Gu Q, Hou JC, Fang XM. Mindfulness meditation for primary headache pain: a meta-analysis. Chin Med J (Engl) 2018; 131(7):829–838. doi:10.4103/0366-6999.228242
- Day MA, Thorn BE. The mediating role of pain acceptance during mindfulness-based cognitive therapy for headache. Complement Ther Med 2016; 25:51–54. doi:10.1016/j.ctim.2016.01.002
- Williamson DA, Monguillot JE, Jarrell MP, Cohen RA, Pratt JM, Blouin DC. Relaxation for the treatment of headache. Controlled evaluation of two group programs. Behav Modif 1984; 8(3):407–424. doi:10.1177/01454455840083007
- Merelle SY, Sorbi MJ, Duivenvoorden HJ, Passchier J. Qualities and health of lay trainers with migraine for behavioral attack prevention. Headache 2010; 50(4):613–625. doi:10.1111/j.1526-4610.2008.01241.x
- Gaul C, van Doorn C, Webering N, et al. Clinical outcome of a headache-specific multidisciplinary treatment program and adherence to treatment recommendations in a tertiary headache center: an observational study. J Headache Pain 2011; 12(4):475–483. doi:10.1007/s10194-011-0348-y
- Wallasch TM, Kropp P. Multidisciplinary integrated headache care: a prospective 12-month follow-up observational study. J Headache Pain 2012; 13(7):521–529. doi:10.1007/s10194-012-0469-y
- Lemstra M, Stewart B, Olszynski WP. Effectiveness of multidisciplinary intervention in the treatment of migraine: a randomized clinical trial. Headache 2002; 42(9):845–854. pmid:12390609
- Krause SJ, Stillman MJ, Tepper DE, Zajac D. A prospective cohort study of outpatient interdisciplinary rehabilitation of chronic headache patients. Headache 2017; 57(3):428–440. doi:10.1111/head.13020
KEY POINTS
- Sleep: Standard sleep hygiene recommendations to maximize sleep quantity and quality.
- Exercise: 30 to 60 minutes 3 to 5 times a week.
- Eat: Regular healthy meals, adequate hydration, and low or stable caffeine intake.
- Diary: Establish a baseline pattern, assess response to treatment, and monitor analgesia to improve accuracy of migraine diagnosis.
- Stress: Cognitive behavioral therapy, mindfulness, relaxation, biofeedback, and provider-patient trust to minimize anxiety.
Appropriate laboratory testing in Lyme disease
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
KEY POINTS
- Lyme disease, the most common tick-borne infection in North America, is a complex multisystem bacterial disease caused by Borrelia burgdorferi.
- Lyme disease preferably affects the skin, joints, and nervous system and presents with typical and atypical features. Certain clinical features are diagnostic. Its diagnosis is mainly clinical and epidemiologic and, when doubtful, is supported by serologic testing.
- Standard 2-tiered testing is the diagnostic testing method of choice—enzyme-linked immunoassay followed by Western blot. Interpretation of the bands depends on the duration of infection.
- When interpreting the test results, be aware of false-positives and the reasons for them.
A link between A-fib and sleep apnea is no surprise, but why?
Is the relationship between A-fib and sleep apnea more than a coincidence stemming from the number of shared associated comorbidities? Significantly, the treatment of obstructive sleep apnea with continuous positive airway pressure (CPAP) has been shown to decrease the recurrence of A-fib after pharmacologic or electrical conversion and after interventional pulmonary vein interruption.1 This suggests that at least in some cases, sleep apnea plays an active role in initiating and possibly also maintaining A-fib. The immediate culprit mediators that come to mind are hypoxia and hypercapnea; both are at least partially ameliorated by the successful use of CPAP, and both are reasonable physiologic candidates for induction of A-fib. Hypoxia is supported by clinical observation, and hypercapnea by experimental modeling.2
It is easy for clinicians to conceptualize the organ effects of hypoxia and hypercapnea. We are accustomed to seeing clinical ramifications of these in the emergency department and intensive care unit, particularly those affecting the brain and heart, organs critically dependent on transmembrane ion flow. We may recall from biochemistry classes the effects of hypoxia on intracellular metabolism and the implications on energy stores, mitochondrial function, and ion translocation. Recent work on the cellular effects of hypoxia, including research that resulted in a Nobel prize, has drawn major attention to patterned cellular responses to intermittent and persistent hypoxia. This includes recognition of epigenetic changes resulting in localized cardiac remodeling and fibrosis,3 factors that clearly affect the expression of arrhythmias, including A-fib.
But the interrelationship between A-fib and sleep apnea may be even more convoluted and intriguing. It now seems that most things cardiac are associated with inflammation in some guise, and the A-fib connection with sleep apnea may not be an exception. Almost 20 years ago, it was recognized that A-fib is associated with an elevation in circulating C-reactive protein (CRP),4 a biomarker of “inflammation,” although not necessarily an active participant. Recent reviews of this connection have been published,5 and successful anti-inflammatory approaches to preventing A-fib using colchicine have been described.6 So how does this tie in with sleep apnea?
A number of papers have now demonstrated that sleep apnea is also associated with an elevation in CRP,7 perhaps due to increases in tumor necrosis factor (TNF)-alpha in response to the intermittent hypoxia of sleep apnea. TNF can drive the inflammatory response through increased expression of genes regulated by nuclear factor kappa-B.8 While it certainly warrants consideration that the elevated biomarkers of inflammation in patients with sleep apnea actually reflect the presence of the frequent comorbidities, including visceral obesity, treating sleep apnea with CPAP (comparable to what I noted above in patients with A-fib) has been shown to reduce circulating CRP levels.9
As our understanding of the biologic underpinnings of A-fib and sleep apnea continue to grow, the practical clinical implications of the relationship between them, as described by Ayache et al, may achieve greater clarity. The two conditions commonly coexist, and treating the sleep apnea results in better rhythm-directed outcomes in the A-fib.
Stay tuned, there is certainly more to learn about this.
- Shukla A, Aizer A, Holmes D, et al. Effect of sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electropysiol 2015; 1(1–2):41–51. doi:10.1016/j.jacep.2015.02.014
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnea but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Zhang W, Song M, Qu J, Liu G. Epigenetic modifications in cardiovascular aging and diseases. Circ Res 2018; 123(7):773–786. doi:10.1161/CIRCRESAHA.118.312497
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001; 104(24):2886–2891. doi:10.1161/hc4901.101760
- Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012; 60(22):2263–2270. doi:10.1016/j.jacc.2012.04.063
- Lee JZ, Singh N, Howe CL, et al. Colchicine for prevention of post-operative atrial fibrillation: a meta-analysis. JACC Clin Electrophysiol 2016; 2(1):78–85. doi:10.1016/j.jacep.2015.09.016
- Van der Touw T, Andronicos NM, Smart N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019; 24(5):429–435. doi:10.1080/1354750X.2019.1600025
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnea syndrome? Thorax 2009; 64(7):631–636. doi:10.1136/thx.2008.105577
- Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest 2009; 136(1):125–129. doi:10.1378/chest.08-1431
Is the relationship between A-fib and sleep apnea more than a coincidence stemming from the number of shared associated comorbidities? Significantly, the treatment of obstructive sleep apnea with continuous positive airway pressure (CPAP) has been shown to decrease the recurrence of A-fib after pharmacologic or electrical conversion and after interventional pulmonary vein interruption.1 This suggests that at least in some cases, sleep apnea plays an active role in initiating and possibly also maintaining A-fib. The immediate culprit mediators that come to mind are hypoxia and hypercapnea; both are at least partially ameliorated by the successful use of CPAP, and both are reasonable physiologic candidates for induction of A-fib. Hypoxia is supported by clinical observation, and hypercapnea by experimental modeling.2
It is easy for clinicians to conceptualize the organ effects of hypoxia and hypercapnea. We are accustomed to seeing clinical ramifications of these in the emergency department and intensive care unit, particularly those affecting the brain and heart, organs critically dependent on transmembrane ion flow. We may recall from biochemistry classes the effects of hypoxia on intracellular metabolism and the implications on energy stores, mitochondrial function, and ion translocation. Recent work on the cellular effects of hypoxia, including research that resulted in a Nobel prize, has drawn major attention to patterned cellular responses to intermittent and persistent hypoxia. This includes recognition of epigenetic changes resulting in localized cardiac remodeling and fibrosis,3 factors that clearly affect the expression of arrhythmias, including A-fib.
But the interrelationship between A-fib and sleep apnea may be even more convoluted and intriguing. It now seems that most things cardiac are associated with inflammation in some guise, and the A-fib connection with sleep apnea may not be an exception. Almost 20 years ago, it was recognized that A-fib is associated with an elevation in circulating C-reactive protein (CRP),4 a biomarker of “inflammation,” although not necessarily an active participant. Recent reviews of this connection have been published,5 and successful anti-inflammatory approaches to preventing A-fib using colchicine have been described.6 So how does this tie in with sleep apnea?
A number of papers have now demonstrated that sleep apnea is also associated with an elevation in CRP,7 perhaps due to increases in tumor necrosis factor (TNF)-alpha in response to the intermittent hypoxia of sleep apnea. TNF can drive the inflammatory response through increased expression of genes regulated by nuclear factor kappa-B.8 While it certainly warrants consideration that the elevated biomarkers of inflammation in patients with sleep apnea actually reflect the presence of the frequent comorbidities, including visceral obesity, treating sleep apnea with CPAP (comparable to what I noted above in patients with A-fib) has been shown to reduce circulating CRP levels.9
As our understanding of the biologic underpinnings of A-fib and sleep apnea continue to grow, the practical clinical implications of the relationship between them, as described by Ayache et al, may achieve greater clarity. The two conditions commonly coexist, and treating the sleep apnea results in better rhythm-directed outcomes in the A-fib.
Stay tuned, there is certainly more to learn about this.
Is the relationship between A-fib and sleep apnea more than a coincidence stemming from the number of shared associated comorbidities? Significantly, the treatment of obstructive sleep apnea with continuous positive airway pressure (CPAP) has been shown to decrease the recurrence of A-fib after pharmacologic or electrical conversion and after interventional pulmonary vein interruption.1 This suggests that at least in some cases, sleep apnea plays an active role in initiating and possibly also maintaining A-fib. The immediate culprit mediators that come to mind are hypoxia and hypercapnea; both are at least partially ameliorated by the successful use of CPAP, and both are reasonable physiologic candidates for induction of A-fib. Hypoxia is supported by clinical observation, and hypercapnea by experimental modeling.2
It is easy for clinicians to conceptualize the organ effects of hypoxia and hypercapnea. We are accustomed to seeing clinical ramifications of these in the emergency department and intensive care unit, particularly those affecting the brain and heart, organs critically dependent on transmembrane ion flow. We may recall from biochemistry classes the effects of hypoxia on intracellular metabolism and the implications on energy stores, mitochondrial function, and ion translocation. Recent work on the cellular effects of hypoxia, including research that resulted in a Nobel prize, has drawn major attention to patterned cellular responses to intermittent and persistent hypoxia. This includes recognition of epigenetic changes resulting in localized cardiac remodeling and fibrosis,3 factors that clearly affect the expression of arrhythmias, including A-fib.
But the interrelationship between A-fib and sleep apnea may be even more convoluted and intriguing. It now seems that most things cardiac are associated with inflammation in some guise, and the A-fib connection with sleep apnea may not be an exception. Almost 20 years ago, it was recognized that A-fib is associated with an elevation in circulating C-reactive protein (CRP),4 a biomarker of “inflammation,” although not necessarily an active participant. Recent reviews of this connection have been published,5 and successful anti-inflammatory approaches to preventing A-fib using colchicine have been described.6 So how does this tie in with sleep apnea?
A number of papers have now demonstrated that sleep apnea is also associated with an elevation in CRP,7 perhaps due to increases in tumor necrosis factor (TNF)-alpha in response to the intermittent hypoxia of sleep apnea. TNF can drive the inflammatory response through increased expression of genes regulated by nuclear factor kappa-B.8 While it certainly warrants consideration that the elevated biomarkers of inflammation in patients with sleep apnea actually reflect the presence of the frequent comorbidities, including visceral obesity, treating sleep apnea with CPAP (comparable to what I noted above in patients with A-fib) has been shown to reduce circulating CRP levels.9
As our understanding of the biologic underpinnings of A-fib and sleep apnea continue to grow, the practical clinical implications of the relationship between them, as described by Ayache et al, may achieve greater clarity. The two conditions commonly coexist, and treating the sleep apnea results in better rhythm-directed outcomes in the A-fib.
Stay tuned, there is certainly more to learn about this.
- Shukla A, Aizer A, Holmes D, et al. Effect of sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electropysiol 2015; 1(1–2):41–51. doi:10.1016/j.jacep.2015.02.014
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnea but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Zhang W, Song M, Qu J, Liu G. Epigenetic modifications in cardiovascular aging and diseases. Circ Res 2018; 123(7):773–786. doi:10.1161/CIRCRESAHA.118.312497
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001; 104(24):2886–2891. doi:10.1161/hc4901.101760
- Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012; 60(22):2263–2270. doi:10.1016/j.jacc.2012.04.063
- Lee JZ, Singh N, Howe CL, et al. Colchicine for prevention of post-operative atrial fibrillation: a meta-analysis. JACC Clin Electrophysiol 2016; 2(1):78–85. doi:10.1016/j.jacep.2015.09.016
- Van der Touw T, Andronicos NM, Smart N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019; 24(5):429–435. doi:10.1080/1354750X.2019.1600025
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnea syndrome? Thorax 2009; 64(7):631–636. doi:10.1136/thx.2008.105577
- Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest 2009; 136(1):125–129. doi:10.1378/chest.08-1431
- Shukla A, Aizer A, Holmes D, et al. Effect of sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electropysiol 2015; 1(1–2):41–51. doi:10.1016/j.jacep.2015.02.014
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnea but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Zhang W, Song M, Qu J, Liu G. Epigenetic modifications in cardiovascular aging and diseases. Circ Res 2018; 123(7):773–786. doi:10.1161/CIRCRESAHA.118.312497
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001; 104(24):2886–2891. doi:10.1161/hc4901.101760
- Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012; 60(22):2263–2270. doi:10.1016/j.jacc.2012.04.063
- Lee JZ, Singh N, Howe CL, et al. Colchicine for prevention of post-operative atrial fibrillation: a meta-analysis. JACC Clin Electrophysiol 2016; 2(1):78–85. doi:10.1016/j.jacep.2015.09.016
- Van der Touw T, Andronicos NM, Smart N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019; 24(5):429–435. doi:10.1080/1354750X.2019.1600025
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnea syndrome? Thorax 2009; 64(7):631–636. doi:10.1136/thx.2008.105577
- Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest 2009; 136(1):125–129. doi:10.1378/chest.08-1431
Fissured tongue
A 43-year-old man presented with a 3-week history of halitosis. He was also concerned about the irregular appearance of his tongue, which he had noticed over the past 3 years. He had no history of wearing dentures or of any skin disorder.
A BROAD DIFFERENTIAL DIAGNOSIS
Fissured tongue (scrotal tongue, plicated tongue, lingua plicata) is a common normal variant of the tongue surface with a male preponderance and a reported prevalence of 10% to 20% in the general population, and the incidence increases strikingly with age.1
The cause is not known, but familial clustering is seen, and a polygenic or autosomal dominant hereditary component is presumed.1
The condition may be associated with removable dentures, geographic tongue, pernicious anemia, Sjögren syndrome, psoriasis, acromegaly, macroglossia, oral-facial-digital syndrome type I, Pierre Robin syndrome, Down syndrome, and Melkersson Rosenthal syndrome.2 It is usually asymptomatic, but if the fissures are deep, food may become lodged in them, resulting in tongue inflammation, burning sensation, and halitosis.1
Typically, fissures of varying depth extending to the margin are apparent on the dorsal surface of the tongue. The condition is confined to the anterior two-thirds of the tongue, which is of ectodermal origin. Histologically, the epithelium, lamina propria, and musculature are all involved in the formation of the fissures.3 The deeper fissures may lack filliform papillae due to bacterial inflammation.3 The diagnosis is clinical, and treatment includes reassurance, advice on good oral hygiene, and tongue cleansing.1
- Feil ND, Filippi A. Frequency of fissured tongue (lingua plicata) as a function of age. Swiss Dent J 2016; 126(10):886–897. German. pmid:27808348
- Mangold AR, Torgerson RR, Rogers RS 3rd. Diseases of the tongue. Clin Dermatol 2016; 34(4):458–469. doi:10.1016/j.clindermatol.2016.02.018
- Kullaa-Mikkonen A, Sorvari T. Lingua fissurata: a clinical, stereomicroscopic and histopathological study. Int J Oral Maxillofac Surg 1986; 15(5):525–533. pmid:3097176
A 43-year-old man presented with a 3-week history of halitosis. He was also concerned about the irregular appearance of his tongue, which he had noticed over the past 3 years. He had no history of wearing dentures or of any skin disorder.
A BROAD DIFFERENTIAL DIAGNOSIS
Fissured tongue (scrotal tongue, plicated tongue, lingua plicata) is a common normal variant of the tongue surface with a male preponderance and a reported prevalence of 10% to 20% in the general population, and the incidence increases strikingly with age.1
The cause is not known, but familial clustering is seen, and a polygenic or autosomal dominant hereditary component is presumed.1
The condition may be associated with removable dentures, geographic tongue, pernicious anemia, Sjögren syndrome, psoriasis, acromegaly, macroglossia, oral-facial-digital syndrome type I, Pierre Robin syndrome, Down syndrome, and Melkersson Rosenthal syndrome.2 It is usually asymptomatic, but if the fissures are deep, food may become lodged in them, resulting in tongue inflammation, burning sensation, and halitosis.1
Typically, fissures of varying depth extending to the margin are apparent on the dorsal surface of the tongue. The condition is confined to the anterior two-thirds of the tongue, which is of ectodermal origin. Histologically, the epithelium, lamina propria, and musculature are all involved in the formation of the fissures.3 The deeper fissures may lack filliform papillae due to bacterial inflammation.3 The diagnosis is clinical, and treatment includes reassurance, advice on good oral hygiene, and tongue cleansing.1
A 43-year-old man presented with a 3-week history of halitosis. He was also concerned about the irregular appearance of his tongue, which he had noticed over the past 3 years. He had no history of wearing dentures or of any skin disorder.
A BROAD DIFFERENTIAL DIAGNOSIS
Fissured tongue (scrotal tongue, plicated tongue, lingua plicata) is a common normal variant of the tongue surface with a male preponderance and a reported prevalence of 10% to 20% in the general population, and the incidence increases strikingly with age.1
The cause is not known, but familial clustering is seen, and a polygenic or autosomal dominant hereditary component is presumed.1
The condition may be associated with removable dentures, geographic tongue, pernicious anemia, Sjögren syndrome, psoriasis, acromegaly, macroglossia, oral-facial-digital syndrome type I, Pierre Robin syndrome, Down syndrome, and Melkersson Rosenthal syndrome.2 It is usually asymptomatic, but if the fissures are deep, food may become lodged in them, resulting in tongue inflammation, burning sensation, and halitosis.1
Typically, fissures of varying depth extending to the margin are apparent on the dorsal surface of the tongue. The condition is confined to the anterior two-thirds of the tongue, which is of ectodermal origin. Histologically, the epithelium, lamina propria, and musculature are all involved in the formation of the fissures.3 The deeper fissures may lack filliform papillae due to bacterial inflammation.3 The diagnosis is clinical, and treatment includes reassurance, advice on good oral hygiene, and tongue cleansing.1
- Feil ND, Filippi A. Frequency of fissured tongue (lingua plicata) as a function of age. Swiss Dent J 2016; 126(10):886–897. German. pmid:27808348
- Mangold AR, Torgerson RR, Rogers RS 3rd. Diseases of the tongue. Clin Dermatol 2016; 34(4):458–469. doi:10.1016/j.clindermatol.2016.02.018
- Kullaa-Mikkonen A, Sorvari T. Lingua fissurata: a clinical, stereomicroscopic and histopathological study. Int J Oral Maxillofac Surg 1986; 15(5):525–533. pmid:3097176
- Feil ND, Filippi A. Frequency of fissured tongue (lingua plicata) as a function of age. Swiss Dent J 2016; 126(10):886–897. German. pmid:27808348
- Mangold AR, Torgerson RR, Rogers RS 3rd. Diseases of the tongue. Clin Dermatol 2016; 34(4):458–469. doi:10.1016/j.clindermatol.2016.02.018
- Kullaa-Mikkonen A, Sorvari T. Lingua fissurata: a clinical, stereomicroscopic and histopathological study. Int J Oral Maxillofac Surg 1986; 15(5):525–533. pmid:3097176
Atraumatic splenic rupture in acute myeloid leukemia
A 50-year-old man with acute myeloid leukemia (AML) with a complex karyotype was admitted to the hospital with several days of dull, left-sided abdominal pain. His most recent bone marrow biopsy showed 30% blasts, and immunophenotyping was suggestive of persistent AML (CD13+, CD34+, CD117+, CD33+, CD7+, MPO–). He was on treatment with venetoclax and cytarabine after induction therapy had failed.
On admission, his heart rate was 101 beats per minute and his blood pressure was 122/85 mm Hg. Abdominal examination revealed mild distention, hepatomegaly, and previously known massive splenomegaly, with the splenic tip extending to the umbilicus, and mild tenderness.
Results of laboratory testing revealed persistent pancytopenia:
- Hemoglobin level 6.8 g/dL (reference range 13.0–17.0)
- Total white blood cell count 0.8 × 109/L (4.5–11.0)
- Platelet count 8 × 109/L (150–400).
The next day, he developed severe, acute-onset left-sided abdominal pain. A check of vital signs showed worsening sinus tachycardia at 132 beats per minute and a drop in blood pressure to 90/56 mm Hg. He had worsening diffuse abdominal tenderness with sluggish bowel sounds. His hemoglobin concentration was 6.4 g/dL and platelet count 12 × 109/L.
He received supportive transfusions of blood products. Surgical exploration was deemed risky, given his overall condition and severe thrombocytopenia. Splenic angiography showed no evidence of pseudoaneurysm or focal contrast extravasation. He underwent empiric embolization of the midsplenic artery, after which his hemodynamic status stabilized. He died 4 weeks later of acute respiratory failure from pneumonia.
SPLENIC RUPTURE IN AML
Atraumatic splenic rupture is rare but potentially life-threatening, especially if the diagnosis is delayed. Conditions that can cause splenomegaly and predispose to rupture include infection (infectious mononucleosis, malaria), malignant hematologic disorders (leukemia, lymphoma), other neoplasms, and amyloidosis.1
The literature includes a few reports of splenic rupture in patients with AML.2–4 The proposed mechanisms include bleeding from infarction sites or tumor foci, dysregulated hemostasis, and leukostasis.
The classic presentation of splenic rupture is acute-onset left-sided abdominal pain associated with hypotension and decreasing hemoglobin levels. CT of the abdomen is confirmatory, and resuscitation with crystalloids and blood products is a vital initial step in management. Choice of treatment depends on the patient’s surgical risk and hemodynamic status; options include conservative medical management, splenic artery embolization, and exploratory laparotomy.
In patients with AML and splenomegaly presenting with acute abdominal pain, clinicians need to be aware of this potential hematologic emergency.
- Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg 2009; 96(10):1114–1121. doi:10.1002/bjs.6737
- Gardner JA, Bao L, Ornstein DL. Spontaneous splenic rupture in acute myeloid leukemia with mixed-lineage leukemia gene rearrangement. Med Rep Case Stud 2016; 1:119. doi:10.4172/2572-5130.1000119
- Zeidan AM, Mitchell M, Khatri R, et al. Spontaneous splenic rupture during induction chemotherapy for acute myeloid leukemia. Leuk Lymphoma 2014; 55(1):209–212. doi:10.3109/10428194.2013.796060
- Fahmi Y, Elabbasi T, Khaiz D, et al. Splenic spontaneous rupture associated with acute myeloïd leukemia: report of a case and literature review. Surgery Curr Res 2014; 4:170. doi:10.4172/2161-1076.1000170
A 50-year-old man with acute myeloid leukemia (AML) with a complex karyotype was admitted to the hospital with several days of dull, left-sided abdominal pain. His most recent bone marrow biopsy showed 30% blasts, and immunophenotyping was suggestive of persistent AML (CD13+, CD34+, CD117+, CD33+, CD7+, MPO–). He was on treatment with venetoclax and cytarabine after induction therapy had failed.
On admission, his heart rate was 101 beats per minute and his blood pressure was 122/85 mm Hg. Abdominal examination revealed mild distention, hepatomegaly, and previously known massive splenomegaly, with the splenic tip extending to the umbilicus, and mild tenderness.
Results of laboratory testing revealed persistent pancytopenia:
- Hemoglobin level 6.8 g/dL (reference range 13.0–17.0)
- Total white blood cell count 0.8 × 109/L (4.5–11.0)
- Platelet count 8 × 109/L (150–400).
The next day, he developed severe, acute-onset left-sided abdominal pain. A check of vital signs showed worsening sinus tachycardia at 132 beats per minute and a drop in blood pressure to 90/56 mm Hg. He had worsening diffuse abdominal tenderness with sluggish bowel sounds. His hemoglobin concentration was 6.4 g/dL and platelet count 12 × 109/L.
He received supportive transfusions of blood products. Surgical exploration was deemed risky, given his overall condition and severe thrombocytopenia. Splenic angiography showed no evidence of pseudoaneurysm or focal contrast extravasation. He underwent empiric embolization of the midsplenic artery, after which his hemodynamic status stabilized. He died 4 weeks later of acute respiratory failure from pneumonia.
SPLENIC RUPTURE IN AML
Atraumatic splenic rupture is rare but potentially life-threatening, especially if the diagnosis is delayed. Conditions that can cause splenomegaly and predispose to rupture include infection (infectious mononucleosis, malaria), malignant hematologic disorders (leukemia, lymphoma), other neoplasms, and amyloidosis.1
The literature includes a few reports of splenic rupture in patients with AML.2–4 The proposed mechanisms include bleeding from infarction sites or tumor foci, dysregulated hemostasis, and leukostasis.
The classic presentation of splenic rupture is acute-onset left-sided abdominal pain associated with hypotension and decreasing hemoglobin levels. CT of the abdomen is confirmatory, and resuscitation with crystalloids and blood products is a vital initial step in management. Choice of treatment depends on the patient’s surgical risk and hemodynamic status; options include conservative medical management, splenic artery embolization, and exploratory laparotomy.
In patients with AML and splenomegaly presenting with acute abdominal pain, clinicians need to be aware of this potential hematologic emergency.
A 50-year-old man with acute myeloid leukemia (AML) with a complex karyotype was admitted to the hospital with several days of dull, left-sided abdominal pain. His most recent bone marrow biopsy showed 30% blasts, and immunophenotyping was suggestive of persistent AML (CD13+, CD34+, CD117+, CD33+, CD7+, MPO–). He was on treatment with venetoclax and cytarabine after induction therapy had failed.
On admission, his heart rate was 101 beats per minute and his blood pressure was 122/85 mm Hg. Abdominal examination revealed mild distention, hepatomegaly, and previously known massive splenomegaly, with the splenic tip extending to the umbilicus, and mild tenderness.
Results of laboratory testing revealed persistent pancytopenia:
- Hemoglobin level 6.8 g/dL (reference range 13.0–17.0)
- Total white blood cell count 0.8 × 109/L (4.5–11.0)
- Platelet count 8 × 109/L (150–400).
The next day, he developed severe, acute-onset left-sided abdominal pain. A check of vital signs showed worsening sinus tachycardia at 132 beats per minute and a drop in blood pressure to 90/56 mm Hg. He had worsening diffuse abdominal tenderness with sluggish bowel sounds. His hemoglobin concentration was 6.4 g/dL and platelet count 12 × 109/L.
He received supportive transfusions of blood products. Surgical exploration was deemed risky, given his overall condition and severe thrombocytopenia. Splenic angiography showed no evidence of pseudoaneurysm or focal contrast extravasation. He underwent empiric embolization of the midsplenic artery, after which his hemodynamic status stabilized. He died 4 weeks later of acute respiratory failure from pneumonia.
SPLENIC RUPTURE IN AML
Atraumatic splenic rupture is rare but potentially life-threatening, especially if the diagnosis is delayed. Conditions that can cause splenomegaly and predispose to rupture include infection (infectious mononucleosis, malaria), malignant hematologic disorders (leukemia, lymphoma), other neoplasms, and amyloidosis.1
The literature includes a few reports of splenic rupture in patients with AML.2–4 The proposed mechanisms include bleeding from infarction sites or tumor foci, dysregulated hemostasis, and leukostasis.
The classic presentation of splenic rupture is acute-onset left-sided abdominal pain associated with hypotension and decreasing hemoglobin levels. CT of the abdomen is confirmatory, and resuscitation with crystalloids and blood products is a vital initial step in management. Choice of treatment depends on the patient’s surgical risk and hemodynamic status; options include conservative medical management, splenic artery embolization, and exploratory laparotomy.
In patients with AML and splenomegaly presenting with acute abdominal pain, clinicians need to be aware of this potential hematologic emergency.
- Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg 2009; 96(10):1114–1121. doi:10.1002/bjs.6737
- Gardner JA, Bao L, Ornstein DL. Spontaneous splenic rupture in acute myeloid leukemia with mixed-lineage leukemia gene rearrangement. Med Rep Case Stud 2016; 1:119. doi:10.4172/2572-5130.1000119
- Zeidan AM, Mitchell M, Khatri R, et al. Spontaneous splenic rupture during induction chemotherapy for acute myeloid leukemia. Leuk Lymphoma 2014; 55(1):209–212. doi:10.3109/10428194.2013.796060
- Fahmi Y, Elabbasi T, Khaiz D, et al. Splenic spontaneous rupture associated with acute myeloïd leukemia: report of a case and literature review. Surgery Curr Res 2014; 4:170. doi:10.4172/2161-1076.1000170
- Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg 2009; 96(10):1114–1121. doi:10.1002/bjs.6737
- Gardner JA, Bao L, Ornstein DL. Spontaneous splenic rupture in acute myeloid leukemia with mixed-lineage leukemia gene rearrangement. Med Rep Case Stud 2016; 1:119. doi:10.4172/2572-5130.1000119
- Zeidan AM, Mitchell M, Khatri R, et al. Spontaneous splenic rupture during induction chemotherapy for acute myeloid leukemia. Leuk Lymphoma 2014; 55(1):209–212. doi:10.3109/10428194.2013.796060
- Fahmi Y, Elabbasi T, Khaiz D, et al. Splenic spontaneous rupture associated with acute myeloïd leukemia: report of a case and literature review. Surgery Curr Res 2014; 4:170. doi:10.4172/2161-1076.1000170
Should I evaluate my patient with atrial fibrillation for sleep apnea?
Yes. The prevalence of sleep apnea is exceedingly high in patients with atrial fibrillation—50% to 80% compared with 30% to 60% in respective control groups.1–3 Conversely, atrial fibrillation is more prevalent in those with sleep-disordered breathing than in those without (4.8% vs 0.9%).4
Sleep-disordered breathing comprises obstructive sleep apnea and central sleep apnea. Obstructive sleep apnea, characterized by repetitive upper-airway obstruction during sleep, is accompanied by intermittent hypoxia, rises in carbon dioxide, autonomic nervous system fluctuations, and intrathoracic pressure alterations.5 Central sleep apnea may be neurally mediated and, in the setting of cardiac disease, is characterized by alterations in chemosensitivity and chemoresponsiveness, leading to a state of high loop gain—ie, a hypersensitive ventilatory control system leading to ventilatory drive oscillations.6
Both obstructive and central sleep apnea have been associated with atrial fibrillation. Experimental data implicate obstructive sleep apnea as a trigger of atrial arrhythmogenesis,7,8 and epidemiologic studies support an association between central sleep apnea, Cheyne-Stokes respiration, and incident atrial fibrillation.9
HOW SLEEP APNEA COULD LEAD TO ATRIAL FIBRILLATION
In experiments in animals, intermittent upper-airway obstruction led to forced inspiration, substantial negative intrathoracic pressure, subsequent left atrial distention, and increased susceptibility to atrial fibrillation.10 The autonomic nervous system may be a mediator of apnea-induced atrial fibrillation, as apnea-induced atrial fibrillation is suppressed with autonomic blockade.10
Emerging data also support the hypothesis that intermittent hypoxia7 and resolution of hypercapnia,8 as observed in obstructive sleep apnea, exert atrial electrophysiologic changes that increase vulnerability to atrial arrhythmogenesis.
In a case-crossover study,11 the odds of paroxysmal atrial fibrillation occurring after a respiratory disturbance were 17.9 times higher than after normal breathing (95% confidence interval [CI] 2.2–144.2), though the absolute rate of overall arrhythmia events (including both atrial fibrillation and nonsustained ventricular tachycardia) associated with respiratory disturbances was low (1 excess arrhythmia event per 40,000 respiratory disturbances).
EFFECT OF SLEEP APNEA ON ATRIAL FIBRILLATION MANAGEMENT
Sleep apnea also seems to affect the efficacy of a rhythm-control strategy for atrial fibrillation. For example, patients with obstructive sleep apnea have a higher risk of recurrent atrial fibrillation after cardioversion (82% vs 42% in controls)12 and up to a 25% greater risk of recurrence after catheter ablation compared with those without obstructive sleep apnea (risk ratio 1.25, 95% CI 1.08–1.45).13
Several observational studies showed a higher rate of atrial fibrillation after pulmonary vein isolation in obstructive sleep apnea patients who do not use continuous positive airway pressure (CPAP) than in those who do.14–17 CPAP therapy appears to exert beneficial effects on cardiac structural remodeling; cardiac magnetic resonance imaging shows that patients with sleep apnea who received less than 4 hours of CPAP per night had larger left atrial dimensions and increased left ventricular mass compared with those who received more than 4 hours of CPAP at night.17 However, a need remains for high-quality, large randomized controlled trials to eliminate potential unmeasured biases due to differences that may exist between CPAP users and non-users, such as general adherence to medical therapy and healthcare interventions.
An additional consideration is that the overall utility and value of obtaining a diagnosis of obstructive sleep apnea strictly as it pertains to atrial fibrillation management is affected by whether a rhythm- or rate-control strategy is pursued. In other words, if a patient is deemed to be in permanent atrial fibrillation and a rhythm-control strategy is therefore not pursued, the potential effect of untreated obstructive sleep apnea on atrial fibrillation recurrence could be less important. In this case, however, the other beneficial cardiovascular and systemic effects of diagnosing and treating underlying obstructive sleep apnea would remain.
POPULATION STUDIES
Epidemiologic and clinic-based studies have supported an association between sleep apnea (mostly central, but also obstructive) and atrial fibrillation.4,18
Community-based studies such as the Sleep Heart Health Study4 and the Outcomes of Sleep Disorders in Older Men Study (MrOS Sleep),18 involving thousands of participants, have found the strongest cross-sectional associations of both obstructive and central sleep apnea with nocturnal atrial fibrillation. The findings included a 2 to 5 times higher odds of nocturnal atrial fibrillation, particularly in those with a moderate to severe degree of sleep-disordered breathing—even after adjusting for confounding influences (eg, obesity) and self-reported cardiac disease such as heart failure.
In MrOS Sleep, in an older male cohort, both obstructive and central sleep apnea were associated with nocturnal atrial fibrillation, though central sleep apnea and Cheyne-Stokes respirations had a stronger magnitude of association.18
Further insights can be drawn specifically from patients with heart failure. Sin et al,19 in a 1999 study, found that in 450 patients with systolic heart failure (85% men), the prevalence of sleep-disordered breathing was 25% to 33% (depending on the apnea-hypopnea index cutoff used) for central sleep apnea, and similarly 27% to 38% for obstructive sleep apnea. The prevalence of atrial fibrillation in this group was 10% in women and 15% in men. Atrial fibrillation was reported as a significant risk factor for central sleep apnea, but not for obstructive sleep apnea (for which only male sex and increasing body mass index were significant risk factors). Directionality was not clearly reported in this retrospective study in terms of timing of sleep studies and other assessments: ie, the report did not clearly state which came first, the atrial fibrillation or the sleep apnea. Therefore, the possibility that central sleep apnea is a predictor of atrial fibrillation cannot be excluded.
Yumino et al,20 in a study published in 2009, evaluated 218 patients with heart failure (with a left ventricular ejection fraction of ≤ 45%) and reported a prevalence of moderate to severe sleep apnea of 21% for central sleep apnea and 26% for obstructive sleep apnea. In multivariate analysis, atrial fibrillation was independently associated with central sleep apnea but not obstructive sleep apnea.
In recent cohort studies, central sleep apnea was associated with 2 to 3 times higher odds of developing atrial fibrillation, while obstructive sleep apnea was not a predictor of incident atrial fibrillation.9,21
Although most available studies associate sleep apnea with atrial fibrillation, findings of a case-control study22 did not support a difference in the prevalence of sleep apnea syndrome (defined as apnea index ≥ 5 and apnea-hypopnea index ≥ 15, and the presence of sleep symptoms) in patients with lone atrial fibrillation (no evident cardiovascular disease) compared with controls matched for age, sex, and cardiovascular morbidity.
But observational studies are limited by the potential for residual unmeasured confounding factors and lack of objective cardiac structural data, such as left ventricular ejection fraction and atrial enlargement. Moreover, there can be significant differences in sleep apnea definitions among studies, thus limiting the ability to reach a definitive conclusion about the relationship between sleep apnea and atrial fibrillation.
SCREENING AND DIAGNOSIS
The 2014 joint guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society for the management of atrial fibrillation state that a sleep study may be useful if sleep apnea is suspected.23 The 2019 focused update of the 2014 guidelines24 state that for overweight and obese patients with atrial fibrillation, weight loss combined with risk-factor modification is recommended (class I recommendation, level of evidence B-R, ie, data derived from 1 or more randomized trials or meta-analysis of such studies). Risk-factor modification in this case includes assessment and treatment of underlying sleep apnea, hypertension, hyperlipidemia, glucose intolerance, and alcohol and tobacco use.
Laboratory polysomnography has long been considered the gold standard for sleep apnea diagnosis. In one study,13 obstructive sleep apnea was a greater predictor of atrial fibrillation when diagnosed by polysomnography (risk ratio 1.40, 95% CI 1.16–1.68) compared with identification by screening using the Berlin questionnaire (risk ratio 1.07, 95% CI 0.91–1.27). However, a laboratory sleep study is associated with increased patient burden and limited availability.
Home sleep apnea testing is being increasingly used in the diagnostic evaluation of obstructive sleep apnea and may be a less costly, more available alternative. However, since a home sleep apnea test is less sensitive than polysomnography in detecting obstructive sleep apnea, the American Academy of Sleep Medicine guidelines28 state that if a single home sleep apnea test is negative or inconclusive, polysomnography should be done if there is clinical suspicion of sleep apnea. Moreover, current guidelines from this group recommend that patients with significant cardiorespiratory disease should be tested with polysomnography rather than home sleep apnea testing.22
Further study is needed to determine the optimal screening method for sleep apnea in patients with atrial fibrillation and to clarify the role of home sleep apnea testing. While keeping in mind the limitations of a screening questionnaire in this population, as a general approach it is reasonable to use a screening questionnaire for sleep apnea. And if the screen is positive, further evaluation with a sleep study is merited, whether by laboratory polysomnography, a home sleep apnea test, or referral to a sleep specialist.
MULTIDISCIPLINARY CARE MAY BE IDEAL
Overall, given the high prevalence of sleep apnea in patients with atrial fibrillation, the deleterious effects of sleep apnea in general, the influence of sleep apnea on atrial fibrillation, and the cardiovascular and other beneficial effects of adequate treatment of sleep apnea, patients with atrial fibrillation should be assessed for sleep apnea.
While the optimal strategy in evaluating for sleep apnea in these patients needs to be further defined, a multidisciplinary approach to care involving a primary care provider, cardiologist, and sleep specialist may be ideal.
- Braga B, Poyares D, Cintra F, et al. Sleep-disordered breathing and chronic atrial fibrillation. Sleep Med 2009; 10(2):212–216. doi:10.1016/j.sleep.2007.12.007
- Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004; 110(4):364–367. doi:10.1161/01.CIR.0000136587.68725.8E
- Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J 2008; 29(13):1662–1669. doi:10.1093/eurheartj/ehn214
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916. doi:10.1164/rccm.200509-1442OC
- Cooper VL, Bowker CM, Pearson SB, Elliott MW, Hainsworth R. Effects of simulated obstructive sleep apnoea on the human carotid baroreceptor-vascular resistance reflex. J Physiol 2004; 557(pt 3):1055–1065. doi:10.1113/jphysiol.2004.062513
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607. doi:10.1378/chest.06.2287
- Lévy P, Pépin JL, Arnaud C, et al. Intermittent hypoxia and sleep-disordered breathing: current concepts and perspectives. Eur Respir J 2008; 32(4):1082–1095. doi:10.1183/09031936.00013308
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Tung P, Levitzky YS, Wang R, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc 2017; 6(7). doi:10.1161/JAHA.116.004500
- Iwasaki YK, Shi Y, Benito B, et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm 2012; 9(9):1409–1416.e1. doi:10.1016/j.hrthm.2012.03.024
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804. doi:10.1016/j.jacc.2009.06.038
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107(20):2589–2594. doi:10.1161/01.CIR.0000068337.25994.21
- Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol 2011; 108(1):47–51. doi:10.1016/j.amjcard.2011.02.343
- Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013; 10(3):331–337. doi:10.1016/j.hrthm.2012.11.015
- Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013; 62(4):300–305. doi:10.1016/j.jacc.2013.03.052
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3(5):445–451. doi:10.1161/CIRCEP.109.858381
- Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013; 2(6):e000421. doi:10.1161/JAHA.113.000421
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155. doi:10.1001/archinternmed.2009.138
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail 2009; 15(4):279–285. doi:10.1016/j.cardfail.2008.11.015
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med 2016; 193(7):783–791. doi:10.1164/rccm.201508-1523OC
- Porthan KM, Melin JH, Kupila JT, Venho KK, Partinen MM. Prevalence of sleep apnea syndrome in lone atrial fibrillation: a case-control study. Chest 2004; 125(3):879–885. doi:10.1378/chest.125.3.879
- January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130(23):e199–e267. doi:10.1161/CIR.0000000000000041
- Writing Group Members; January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019; 16(8):e66–e93. doi:10.1016/j.hrthm.2019.01.024
- Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999; 131(7):485–491. doi:10.7326/0003-4819-131-7-199910050-00002
- Chung F, Abdullah HR, Liao P. STOP-bang questionnaire a practical approach to screen for obstructive sleep apnea. Chest 2016; 149(3):631–638. doi:10.1378/chest.15-0903
- Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med 2016; 4(9):742–748. doi:10.1016/S2213-2600(16)30075-3
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13(3):479–504. doi:10.5664/jcsm.6506
Yes. The prevalence of sleep apnea is exceedingly high in patients with atrial fibrillation—50% to 80% compared with 30% to 60% in respective control groups.1–3 Conversely, atrial fibrillation is more prevalent in those with sleep-disordered breathing than in those without (4.8% vs 0.9%).4
Sleep-disordered breathing comprises obstructive sleep apnea and central sleep apnea. Obstructive sleep apnea, characterized by repetitive upper-airway obstruction during sleep, is accompanied by intermittent hypoxia, rises in carbon dioxide, autonomic nervous system fluctuations, and intrathoracic pressure alterations.5 Central sleep apnea may be neurally mediated and, in the setting of cardiac disease, is characterized by alterations in chemosensitivity and chemoresponsiveness, leading to a state of high loop gain—ie, a hypersensitive ventilatory control system leading to ventilatory drive oscillations.6
Both obstructive and central sleep apnea have been associated with atrial fibrillation. Experimental data implicate obstructive sleep apnea as a trigger of atrial arrhythmogenesis,7,8 and epidemiologic studies support an association between central sleep apnea, Cheyne-Stokes respiration, and incident atrial fibrillation.9
HOW SLEEP APNEA COULD LEAD TO ATRIAL FIBRILLATION
In experiments in animals, intermittent upper-airway obstruction led to forced inspiration, substantial negative intrathoracic pressure, subsequent left atrial distention, and increased susceptibility to atrial fibrillation.10 The autonomic nervous system may be a mediator of apnea-induced atrial fibrillation, as apnea-induced atrial fibrillation is suppressed with autonomic blockade.10
Emerging data also support the hypothesis that intermittent hypoxia7 and resolution of hypercapnia,8 as observed in obstructive sleep apnea, exert atrial electrophysiologic changes that increase vulnerability to atrial arrhythmogenesis.
In a case-crossover study,11 the odds of paroxysmal atrial fibrillation occurring after a respiratory disturbance were 17.9 times higher than after normal breathing (95% confidence interval [CI] 2.2–144.2), though the absolute rate of overall arrhythmia events (including both atrial fibrillation and nonsustained ventricular tachycardia) associated with respiratory disturbances was low (1 excess arrhythmia event per 40,000 respiratory disturbances).
EFFECT OF SLEEP APNEA ON ATRIAL FIBRILLATION MANAGEMENT
Sleep apnea also seems to affect the efficacy of a rhythm-control strategy for atrial fibrillation. For example, patients with obstructive sleep apnea have a higher risk of recurrent atrial fibrillation after cardioversion (82% vs 42% in controls)12 and up to a 25% greater risk of recurrence after catheter ablation compared with those without obstructive sleep apnea (risk ratio 1.25, 95% CI 1.08–1.45).13
Several observational studies showed a higher rate of atrial fibrillation after pulmonary vein isolation in obstructive sleep apnea patients who do not use continuous positive airway pressure (CPAP) than in those who do.14–17 CPAP therapy appears to exert beneficial effects on cardiac structural remodeling; cardiac magnetic resonance imaging shows that patients with sleep apnea who received less than 4 hours of CPAP per night had larger left atrial dimensions and increased left ventricular mass compared with those who received more than 4 hours of CPAP at night.17 However, a need remains for high-quality, large randomized controlled trials to eliminate potential unmeasured biases due to differences that may exist between CPAP users and non-users, such as general adherence to medical therapy and healthcare interventions.
An additional consideration is that the overall utility and value of obtaining a diagnosis of obstructive sleep apnea strictly as it pertains to atrial fibrillation management is affected by whether a rhythm- or rate-control strategy is pursued. In other words, if a patient is deemed to be in permanent atrial fibrillation and a rhythm-control strategy is therefore not pursued, the potential effect of untreated obstructive sleep apnea on atrial fibrillation recurrence could be less important. In this case, however, the other beneficial cardiovascular and systemic effects of diagnosing and treating underlying obstructive sleep apnea would remain.
POPULATION STUDIES
Epidemiologic and clinic-based studies have supported an association between sleep apnea (mostly central, but also obstructive) and atrial fibrillation.4,18
Community-based studies such as the Sleep Heart Health Study4 and the Outcomes of Sleep Disorders in Older Men Study (MrOS Sleep),18 involving thousands of participants, have found the strongest cross-sectional associations of both obstructive and central sleep apnea with nocturnal atrial fibrillation. The findings included a 2 to 5 times higher odds of nocturnal atrial fibrillation, particularly in those with a moderate to severe degree of sleep-disordered breathing—even after adjusting for confounding influences (eg, obesity) and self-reported cardiac disease such as heart failure.
In MrOS Sleep, in an older male cohort, both obstructive and central sleep apnea were associated with nocturnal atrial fibrillation, though central sleep apnea and Cheyne-Stokes respirations had a stronger magnitude of association.18
Further insights can be drawn specifically from patients with heart failure. Sin et al,19 in a 1999 study, found that in 450 patients with systolic heart failure (85% men), the prevalence of sleep-disordered breathing was 25% to 33% (depending on the apnea-hypopnea index cutoff used) for central sleep apnea, and similarly 27% to 38% for obstructive sleep apnea. The prevalence of atrial fibrillation in this group was 10% in women and 15% in men. Atrial fibrillation was reported as a significant risk factor for central sleep apnea, but not for obstructive sleep apnea (for which only male sex and increasing body mass index were significant risk factors). Directionality was not clearly reported in this retrospective study in terms of timing of sleep studies and other assessments: ie, the report did not clearly state which came first, the atrial fibrillation or the sleep apnea. Therefore, the possibility that central sleep apnea is a predictor of atrial fibrillation cannot be excluded.
Yumino et al,20 in a study published in 2009, evaluated 218 patients with heart failure (with a left ventricular ejection fraction of ≤ 45%) and reported a prevalence of moderate to severe sleep apnea of 21% for central sleep apnea and 26% for obstructive sleep apnea. In multivariate analysis, atrial fibrillation was independently associated with central sleep apnea but not obstructive sleep apnea.
In recent cohort studies, central sleep apnea was associated with 2 to 3 times higher odds of developing atrial fibrillation, while obstructive sleep apnea was not a predictor of incident atrial fibrillation.9,21
Although most available studies associate sleep apnea with atrial fibrillation, findings of a case-control study22 did not support a difference in the prevalence of sleep apnea syndrome (defined as apnea index ≥ 5 and apnea-hypopnea index ≥ 15, and the presence of sleep symptoms) in patients with lone atrial fibrillation (no evident cardiovascular disease) compared with controls matched for age, sex, and cardiovascular morbidity.
But observational studies are limited by the potential for residual unmeasured confounding factors and lack of objective cardiac structural data, such as left ventricular ejection fraction and atrial enlargement. Moreover, there can be significant differences in sleep apnea definitions among studies, thus limiting the ability to reach a definitive conclusion about the relationship between sleep apnea and atrial fibrillation.
SCREENING AND DIAGNOSIS
The 2014 joint guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society for the management of atrial fibrillation state that a sleep study may be useful if sleep apnea is suspected.23 The 2019 focused update of the 2014 guidelines24 state that for overweight and obese patients with atrial fibrillation, weight loss combined with risk-factor modification is recommended (class I recommendation, level of evidence B-R, ie, data derived from 1 or more randomized trials or meta-analysis of such studies). Risk-factor modification in this case includes assessment and treatment of underlying sleep apnea, hypertension, hyperlipidemia, glucose intolerance, and alcohol and tobacco use.
Laboratory polysomnography has long been considered the gold standard for sleep apnea diagnosis. In one study,13 obstructive sleep apnea was a greater predictor of atrial fibrillation when diagnosed by polysomnography (risk ratio 1.40, 95% CI 1.16–1.68) compared with identification by screening using the Berlin questionnaire (risk ratio 1.07, 95% CI 0.91–1.27). However, a laboratory sleep study is associated with increased patient burden and limited availability.
Home sleep apnea testing is being increasingly used in the diagnostic evaluation of obstructive sleep apnea and may be a less costly, more available alternative. However, since a home sleep apnea test is less sensitive than polysomnography in detecting obstructive sleep apnea, the American Academy of Sleep Medicine guidelines28 state that if a single home sleep apnea test is negative or inconclusive, polysomnography should be done if there is clinical suspicion of sleep apnea. Moreover, current guidelines from this group recommend that patients with significant cardiorespiratory disease should be tested with polysomnography rather than home sleep apnea testing.22
Further study is needed to determine the optimal screening method for sleep apnea in patients with atrial fibrillation and to clarify the role of home sleep apnea testing. While keeping in mind the limitations of a screening questionnaire in this population, as a general approach it is reasonable to use a screening questionnaire for sleep apnea. And if the screen is positive, further evaluation with a sleep study is merited, whether by laboratory polysomnography, a home sleep apnea test, or referral to a sleep specialist.
MULTIDISCIPLINARY CARE MAY BE IDEAL
Overall, given the high prevalence of sleep apnea in patients with atrial fibrillation, the deleterious effects of sleep apnea in general, the influence of sleep apnea on atrial fibrillation, and the cardiovascular and other beneficial effects of adequate treatment of sleep apnea, patients with atrial fibrillation should be assessed for sleep apnea.
While the optimal strategy in evaluating for sleep apnea in these patients needs to be further defined, a multidisciplinary approach to care involving a primary care provider, cardiologist, and sleep specialist may be ideal.
Yes. The prevalence of sleep apnea is exceedingly high in patients with atrial fibrillation—50% to 80% compared with 30% to 60% in respective control groups.1–3 Conversely, atrial fibrillation is more prevalent in those with sleep-disordered breathing than in those without (4.8% vs 0.9%).4
Sleep-disordered breathing comprises obstructive sleep apnea and central sleep apnea. Obstructive sleep apnea, characterized by repetitive upper-airway obstruction during sleep, is accompanied by intermittent hypoxia, rises in carbon dioxide, autonomic nervous system fluctuations, and intrathoracic pressure alterations.5 Central sleep apnea may be neurally mediated and, in the setting of cardiac disease, is characterized by alterations in chemosensitivity and chemoresponsiveness, leading to a state of high loop gain—ie, a hypersensitive ventilatory control system leading to ventilatory drive oscillations.6
Both obstructive and central sleep apnea have been associated with atrial fibrillation. Experimental data implicate obstructive sleep apnea as a trigger of atrial arrhythmogenesis,7,8 and epidemiologic studies support an association between central sleep apnea, Cheyne-Stokes respiration, and incident atrial fibrillation.9
HOW SLEEP APNEA COULD LEAD TO ATRIAL FIBRILLATION
In experiments in animals, intermittent upper-airway obstruction led to forced inspiration, substantial negative intrathoracic pressure, subsequent left atrial distention, and increased susceptibility to atrial fibrillation.10 The autonomic nervous system may be a mediator of apnea-induced atrial fibrillation, as apnea-induced atrial fibrillation is suppressed with autonomic blockade.10
Emerging data also support the hypothesis that intermittent hypoxia7 and resolution of hypercapnia,8 as observed in obstructive sleep apnea, exert atrial electrophysiologic changes that increase vulnerability to atrial arrhythmogenesis.
In a case-crossover study,11 the odds of paroxysmal atrial fibrillation occurring after a respiratory disturbance were 17.9 times higher than after normal breathing (95% confidence interval [CI] 2.2–144.2), though the absolute rate of overall arrhythmia events (including both atrial fibrillation and nonsustained ventricular tachycardia) associated with respiratory disturbances was low (1 excess arrhythmia event per 40,000 respiratory disturbances).
EFFECT OF SLEEP APNEA ON ATRIAL FIBRILLATION MANAGEMENT
Sleep apnea also seems to affect the efficacy of a rhythm-control strategy for atrial fibrillation. For example, patients with obstructive sleep apnea have a higher risk of recurrent atrial fibrillation after cardioversion (82% vs 42% in controls)12 and up to a 25% greater risk of recurrence after catheter ablation compared with those without obstructive sleep apnea (risk ratio 1.25, 95% CI 1.08–1.45).13
Several observational studies showed a higher rate of atrial fibrillation after pulmonary vein isolation in obstructive sleep apnea patients who do not use continuous positive airway pressure (CPAP) than in those who do.14–17 CPAP therapy appears to exert beneficial effects on cardiac structural remodeling; cardiac magnetic resonance imaging shows that patients with sleep apnea who received less than 4 hours of CPAP per night had larger left atrial dimensions and increased left ventricular mass compared with those who received more than 4 hours of CPAP at night.17 However, a need remains for high-quality, large randomized controlled trials to eliminate potential unmeasured biases due to differences that may exist between CPAP users and non-users, such as general adherence to medical therapy and healthcare interventions.
An additional consideration is that the overall utility and value of obtaining a diagnosis of obstructive sleep apnea strictly as it pertains to atrial fibrillation management is affected by whether a rhythm- or rate-control strategy is pursued. In other words, if a patient is deemed to be in permanent atrial fibrillation and a rhythm-control strategy is therefore not pursued, the potential effect of untreated obstructive sleep apnea on atrial fibrillation recurrence could be less important. In this case, however, the other beneficial cardiovascular and systemic effects of diagnosing and treating underlying obstructive sleep apnea would remain.
POPULATION STUDIES
Epidemiologic and clinic-based studies have supported an association between sleep apnea (mostly central, but also obstructive) and atrial fibrillation.4,18
Community-based studies such as the Sleep Heart Health Study4 and the Outcomes of Sleep Disorders in Older Men Study (MrOS Sleep),18 involving thousands of participants, have found the strongest cross-sectional associations of both obstructive and central sleep apnea with nocturnal atrial fibrillation. The findings included a 2 to 5 times higher odds of nocturnal atrial fibrillation, particularly in those with a moderate to severe degree of sleep-disordered breathing—even after adjusting for confounding influences (eg, obesity) and self-reported cardiac disease such as heart failure.
In MrOS Sleep, in an older male cohort, both obstructive and central sleep apnea were associated with nocturnal atrial fibrillation, though central sleep apnea and Cheyne-Stokes respirations had a stronger magnitude of association.18
Further insights can be drawn specifically from patients with heart failure. Sin et al,19 in a 1999 study, found that in 450 patients with systolic heart failure (85% men), the prevalence of sleep-disordered breathing was 25% to 33% (depending on the apnea-hypopnea index cutoff used) for central sleep apnea, and similarly 27% to 38% for obstructive sleep apnea. The prevalence of atrial fibrillation in this group was 10% in women and 15% in men. Atrial fibrillation was reported as a significant risk factor for central sleep apnea, but not for obstructive sleep apnea (for which only male sex and increasing body mass index were significant risk factors). Directionality was not clearly reported in this retrospective study in terms of timing of sleep studies and other assessments: ie, the report did not clearly state which came first, the atrial fibrillation or the sleep apnea. Therefore, the possibility that central sleep apnea is a predictor of atrial fibrillation cannot be excluded.
Yumino et al,20 in a study published in 2009, evaluated 218 patients with heart failure (with a left ventricular ejection fraction of ≤ 45%) and reported a prevalence of moderate to severe sleep apnea of 21% for central sleep apnea and 26% for obstructive sleep apnea. In multivariate analysis, atrial fibrillation was independently associated with central sleep apnea but not obstructive sleep apnea.
In recent cohort studies, central sleep apnea was associated with 2 to 3 times higher odds of developing atrial fibrillation, while obstructive sleep apnea was not a predictor of incident atrial fibrillation.9,21
Although most available studies associate sleep apnea with atrial fibrillation, findings of a case-control study22 did not support a difference in the prevalence of sleep apnea syndrome (defined as apnea index ≥ 5 and apnea-hypopnea index ≥ 15, and the presence of sleep symptoms) in patients with lone atrial fibrillation (no evident cardiovascular disease) compared with controls matched for age, sex, and cardiovascular morbidity.
But observational studies are limited by the potential for residual unmeasured confounding factors and lack of objective cardiac structural data, such as left ventricular ejection fraction and atrial enlargement. Moreover, there can be significant differences in sleep apnea definitions among studies, thus limiting the ability to reach a definitive conclusion about the relationship between sleep apnea and atrial fibrillation.
SCREENING AND DIAGNOSIS
The 2014 joint guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society for the management of atrial fibrillation state that a sleep study may be useful if sleep apnea is suspected.23 The 2019 focused update of the 2014 guidelines24 state that for overweight and obese patients with atrial fibrillation, weight loss combined with risk-factor modification is recommended (class I recommendation, level of evidence B-R, ie, data derived from 1 or more randomized trials or meta-analysis of such studies). Risk-factor modification in this case includes assessment and treatment of underlying sleep apnea, hypertension, hyperlipidemia, glucose intolerance, and alcohol and tobacco use.
Laboratory polysomnography has long been considered the gold standard for sleep apnea diagnosis. In one study,13 obstructive sleep apnea was a greater predictor of atrial fibrillation when diagnosed by polysomnography (risk ratio 1.40, 95% CI 1.16–1.68) compared with identification by screening using the Berlin questionnaire (risk ratio 1.07, 95% CI 0.91–1.27). However, a laboratory sleep study is associated with increased patient burden and limited availability.
Home sleep apnea testing is being increasingly used in the diagnostic evaluation of obstructive sleep apnea and may be a less costly, more available alternative. However, since a home sleep apnea test is less sensitive than polysomnography in detecting obstructive sleep apnea, the American Academy of Sleep Medicine guidelines28 state that if a single home sleep apnea test is negative or inconclusive, polysomnography should be done if there is clinical suspicion of sleep apnea. Moreover, current guidelines from this group recommend that patients with significant cardiorespiratory disease should be tested with polysomnography rather than home sleep apnea testing.22
Further study is needed to determine the optimal screening method for sleep apnea in patients with atrial fibrillation and to clarify the role of home sleep apnea testing. While keeping in mind the limitations of a screening questionnaire in this population, as a general approach it is reasonable to use a screening questionnaire for sleep apnea. And if the screen is positive, further evaluation with a sleep study is merited, whether by laboratory polysomnography, a home sleep apnea test, or referral to a sleep specialist.
MULTIDISCIPLINARY CARE MAY BE IDEAL
Overall, given the high prevalence of sleep apnea in patients with atrial fibrillation, the deleterious effects of sleep apnea in general, the influence of sleep apnea on atrial fibrillation, and the cardiovascular and other beneficial effects of adequate treatment of sleep apnea, patients with atrial fibrillation should be assessed for sleep apnea.
While the optimal strategy in evaluating for sleep apnea in these patients needs to be further defined, a multidisciplinary approach to care involving a primary care provider, cardiologist, and sleep specialist may be ideal.
- Braga B, Poyares D, Cintra F, et al. Sleep-disordered breathing and chronic atrial fibrillation. Sleep Med 2009; 10(2):212–216. doi:10.1016/j.sleep.2007.12.007
- Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004; 110(4):364–367. doi:10.1161/01.CIR.0000136587.68725.8E
- Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J 2008; 29(13):1662–1669. doi:10.1093/eurheartj/ehn214
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916. doi:10.1164/rccm.200509-1442OC
- Cooper VL, Bowker CM, Pearson SB, Elliott MW, Hainsworth R. Effects of simulated obstructive sleep apnoea on the human carotid baroreceptor-vascular resistance reflex. J Physiol 2004; 557(pt 3):1055–1065. doi:10.1113/jphysiol.2004.062513
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607. doi:10.1378/chest.06.2287
- Lévy P, Pépin JL, Arnaud C, et al. Intermittent hypoxia and sleep-disordered breathing: current concepts and perspectives. Eur Respir J 2008; 32(4):1082–1095. doi:10.1183/09031936.00013308
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Tung P, Levitzky YS, Wang R, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc 2017; 6(7). doi:10.1161/JAHA.116.004500
- Iwasaki YK, Shi Y, Benito B, et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm 2012; 9(9):1409–1416.e1. doi:10.1016/j.hrthm.2012.03.024
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804. doi:10.1016/j.jacc.2009.06.038
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107(20):2589–2594. doi:10.1161/01.CIR.0000068337.25994.21
- Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol 2011; 108(1):47–51. doi:10.1016/j.amjcard.2011.02.343
- Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013; 10(3):331–337. doi:10.1016/j.hrthm.2012.11.015
- Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013; 62(4):300–305. doi:10.1016/j.jacc.2013.03.052
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3(5):445–451. doi:10.1161/CIRCEP.109.858381
- Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013; 2(6):e000421. doi:10.1161/JAHA.113.000421
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155. doi:10.1001/archinternmed.2009.138
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail 2009; 15(4):279–285. doi:10.1016/j.cardfail.2008.11.015
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med 2016; 193(7):783–791. doi:10.1164/rccm.201508-1523OC
- Porthan KM, Melin JH, Kupila JT, Venho KK, Partinen MM. Prevalence of sleep apnea syndrome in lone atrial fibrillation: a case-control study. Chest 2004; 125(3):879–885. doi:10.1378/chest.125.3.879
- January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130(23):e199–e267. doi:10.1161/CIR.0000000000000041
- Writing Group Members; January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019; 16(8):e66–e93. doi:10.1016/j.hrthm.2019.01.024
- Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999; 131(7):485–491. doi:10.7326/0003-4819-131-7-199910050-00002
- Chung F, Abdullah HR, Liao P. STOP-bang questionnaire a practical approach to screen for obstructive sleep apnea. Chest 2016; 149(3):631–638. doi:10.1378/chest.15-0903
- Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med 2016; 4(9):742–748. doi:10.1016/S2213-2600(16)30075-3
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13(3):479–504. doi:10.5664/jcsm.6506
- Braga B, Poyares D, Cintra F, et al. Sleep-disordered breathing and chronic atrial fibrillation. Sleep Med 2009; 10(2):212–216. doi:10.1016/j.sleep.2007.12.007
- Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004; 110(4):364–367. doi:10.1161/01.CIR.0000136587.68725.8E
- Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J 2008; 29(13):1662–1669. doi:10.1093/eurheartj/ehn214
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916. doi:10.1164/rccm.200509-1442OC
- Cooper VL, Bowker CM, Pearson SB, Elliott MW, Hainsworth R. Effects of simulated obstructive sleep apnoea on the human carotid baroreceptor-vascular resistance reflex. J Physiol 2004; 557(pt 3):1055–1065. doi:10.1113/jphysiol.2004.062513
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607. doi:10.1378/chest.06.2287
- Lévy P, Pépin JL, Arnaud C, et al. Intermittent hypoxia and sleep-disordered breathing: current concepts and perspectives. Eur Respir J 2008; 32(4):1082–1095. doi:10.1183/09031936.00013308
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Tung P, Levitzky YS, Wang R, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc 2017; 6(7). doi:10.1161/JAHA.116.004500
- Iwasaki YK, Shi Y, Benito B, et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart Rhythm 2012; 9(9):1409–1416.e1. doi:10.1016/j.hrthm.2012.03.024
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804. doi:10.1016/j.jacc.2009.06.038
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107(20):2589–2594. doi:10.1161/01.CIR.0000068337.25994.21
- Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol 2011; 108(1):47–51. doi:10.1016/j.amjcard.2011.02.343
- Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013; 10(3):331–337. doi:10.1016/j.hrthm.2012.11.015
- Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013; 62(4):300–305. doi:10.1016/j.jacc.2013.03.052
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3(5):445–451. doi:10.1161/CIRCEP.109.858381
- Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013; 2(6):e000421. doi:10.1161/JAHA.113.000421
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155. doi:10.1001/archinternmed.2009.138
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail 2009; 15(4):279–285. doi:10.1016/j.cardfail.2008.11.015
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med 2016; 193(7):783–791. doi:10.1164/rccm.201508-1523OC
- Porthan KM, Melin JH, Kupila JT, Venho KK, Partinen MM. Prevalence of sleep apnea syndrome in lone atrial fibrillation: a case-control study. Chest 2004; 125(3):879–885. doi:10.1378/chest.125.3.879
- January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130(23):e199–e267. doi:10.1161/CIR.0000000000000041
- Writing Group Members; January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019; 16(8):e66–e93. doi:10.1016/j.hrthm.2019.01.024
- Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999; 131(7):485–491. doi:10.7326/0003-4819-131-7-199910050-00002
- Chung F, Abdullah HR, Liao P. STOP-bang questionnaire a practical approach to screen for obstructive sleep apnea. Chest 2016; 149(3):631–638. doi:10.1378/chest.15-0903
- Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med 2016; 4(9):742–748. doi:10.1016/S2213-2600(16)30075-3
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13(3):479–504. doi:10.5664/jcsm.6506
Severe hypercalcemia in a 54-year-old woman
A morbidly obese 54-year-old woman presented to the emergency department after experiencing generalized abdominal pain for 3 days. She rated the pain as 5 on a scale of 10 and described it as dull, cramping, waxing and waning, not radiating, and not relieved with changes of position—in fact, not alleviated by anything she had tried. Her pain was associated with nausea and 1 episode of vomiting. She also experienced constipation before the onset of pain.
She denied recent trauma, recent travel, diarrhea, fevers, weakness, shortness of breath, chest pain, other muscle pains, or recent changes in diet. She also denied having this pain in the past. She said she had unintentionally lost some weight but was not certain how much. She denied tobacco, alcohol, or illicit drug use. She had no history of surgery.
Her medical history included hypertension, anemia, and uterine fibroids. Her current medications included losartan, hydrochlorothiazide, and albuterol. She had no family history of significant disease.
INITIAL EVALUATION AND MANAGEMENT
On admission, her temperature was 97.8°F (36.6°C), heart rate 100 beats per minute, blood pressure 136/64 mm Hg, respiratory rate 18 breaths per minute, oxygen saturation 97% on room air, weight 130.6 kg, and body mass index 35 kg/m2.
She was alert and oriented to person, place, and time. She was in mild discomfort but no distress. Her lungs were clear to auscultation, with no wheezing or crackles. Heart rate and rhythm were regular, with no extra heart sounds or murmurs. Bowel sounds were normal in all 4 quadrants, with tenderness to palpation of the epigastric area, but with no guarding or rebound tenderness.
Laboratory test results
Notable results of blood testing at presentation were as follows:
- Hemoglobin 8.2 g/dL (reference range 12.3–15.3)
- Hematocrit 26% (41–50)
- Mean corpuscular volume 107 fL (80–100)
- Blood urea nitrogen 33 mg/dL (8–21); 6 months earlier it was 16
- Serum creatinine 3.6 mg/dL (0.58–0.96); 6 months earlier, it was 0.75
- Albumin 3.3 g/dL (3.5–5)
- Calcium 18.4 mg/dL (8.4–10.2); 6 months earlier, it was 9.6
- Corrected calcium 19 mg/dL.
Findings on imaging, electrocardiography
Chest radiography showed no acute cardiopulmonary abnormalities. Abdominal computed tomography without contrast showed no abnormalities within the pancreas and no evidence of inflammation or obstruction. Electrocardiography showed sinus tachycardia.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s symptoms?
- Primary hyperparathyroidism
- Malignancy
- Her drug therapy
- Familial hypercalcemic hypocalciuria
In total, her laboratory results were consistent with macrocytic anemia, severe hypercalcemia, and acute kidney injury, and she had generalized symptoms.
Primary hyperparathyroidism
A main cause of hypercalcemia is primary hyperparathyroidism, and this needs to be ruled out. Benign adenomas are the most common cause of primary hyperparathyroidism, and a risk factor for benign adenoma is exposure to therapeutic levels of radiation.3
In hyperparathyroidism, there is an increased secretion of parathyroid hormone (PTH), which has multiple effects including increased reabsorption of calcium from the urine, increased excretion of phosphate, and increased expression of 1,25-hydroxyvitamin D hydroxylase to activate vitamin D. PTH also stimulates osteoclasts to increase their expression of receptor activator of nuclear factor kappa B ligand (RANKL), which has a downstream effect on osteoclast precursors to cause bone reabsorption.3
Inherited primary hyperparathyroidism tends to present at a younger age, with multiple overactive parathyroid glands.3 Given our patient’s age, inherited primary hyparathyroidism is thus less likely.
Malignancy
The probability that malignancy is causing the hypercalcemia increases with calcium levels greater than 13 mg/dL. Epidemiologically, in hospitalized patients with hypercalcemia, the source tends to be malignancy.4 Typically, patients who develop hypercalcemia from malignancy have a worse prognosis.5
Solid tumors and leukemias can cause hypercalcemia. The mechanisms include humoral factors secreted by the malignancy, local osteolysis due to tumor invasion of bone, and excessive absorption of calcium due to excess vitamin D produced by malignancies.5 The cancers that most frequently cause an increase in calcium resorption are lung cancer, renal cancer, breast cancer, and multiple myeloma.1
Solid tumors with no bone metastasis and non-Hodgkin lymphoma that release PTH-related protein (PTHrP) cause humoral hypercalcemia in malignancy. The patient is typically in an advanced stage of disease. PTHrP increases serum calcium levels by decreasing the kidney’s ability to excrete calcium and by increasing bone turnover. It has no effect on intestinal absorption because of its inability to stimulate activated vitamin D3. Thus, the increase in systemic calcium comes directly from breakdown of bone and inability to excrete the excess.
PTHrP has a unique role in breast cancer: it is released locally in areas where cancer cells have metastasized to bone, but it does not cause a systemic effect. Bone resorption occurs in areas of metastasis and results from an increase in expression of RANKL and RANK in osteoclasts in response to the effects of PTHrP, leading to an increase in the production of osteoclastic cells.1
Tamoxifen, an endocrine therapy often used in breast cancer, also causes a release of bone-reabsorbing factors from tumor cells, which can partially contribute to hypercalcemia.5
Myeloma cells secrete RANKL, which stimulates osteoclastic activity, and they also release interleukin 6 (IL-6) and activating macrophage inflammatory protein alpha. Serum testing usually shows low or normal intact PTH, PTHrP, and 1,25-dihydroxyvitamin D.1
Patients with multiple myeloma have a worse prognosis if they have a high red blood cell distribution width, a condition shown to correlate with malnutrition, leading to deficiencies in vitamin B12 and to poor response to treatment.6 Up to 14% of patients with multiple myeloma have vitamin B12 deficiency.7
Our patient’s recent weight loss and severe hypercalcemia raise suspicion of malignancy. Further, her obesity makes proper routine breast examination difficult and thus increases the chance of undiagnosed breast cancer.8 Her decrease in renal function and her anemia complicated by hypercalcemia also raise suspicion of multiple myeloma.
Hypercalcemia due to drug therapy
Thiazide diuretics, lithium, teriparatide, and vitamin A in excessive amounts can raise the serum calcium concentration.5 Our patient was taking a thiazide for hypertension, but her extremely high calcium level places drug-induced hypercalcemia as the sole cause lower on the differential list.
Familial hypercalcemic hypocalciuria
Familial hypercalcemic hypocalciuria is a rare autosomal-dominant cause of hypercalcemia in which the ability of the body (and especially the kidneys) to sense levels of calcium is impaired, leading to a decrease in excretion of calcium in the urine.3 Very high calcium levels are rare in hypercalcemic hypocalciuria.3 In our patient with a corrected calcium concentration of nearly 19 mg/dL, familial hypercalcemic hypocalciuria is very unlikely to be the cause of the hypercalcemia.
WHAT ARE THE NEXT STEPS IN THE WORKUP?
As hypercalcemia has been confirmed, the intact PTH level should be checked to determine whether the patient’s condition is PTH-mediated. If the PTH level is in the upper range of normal or is minimally elevated, primary hyperparathyroidism is likely. Elevated PTH confirms primary hyperparathyroidism. A low-normal or low intact PTH confirms a non-PTH-mediated process, and once this is confirmed, PTHrP levels should be checked. An elevated PTHrP suggests humoral hypercalcemia of malignancy. Serum protein electrophoresis, urine protein electrophoresis, and a serum light chain assay should be performed to rule out multiple myeloma.
Vitamin D toxicity is associated with high concentrations of 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D metabolites. These levels should be checked in this patient.
Other disorders that cause hypercalcemia are vitamin A toxicity and hyperthyroidism, so vitamin A and thyroid-stimulating hormone levels should also be checked.5
CASE CONTINUED
After further questioning, the patient said that she had had lower back pain about 1 to 2 weeks before coming to the emergency room; her primary care doctor had said the pain was likely from muscle strain. The pain had almost resolved but was still present.
The results of further laboratory testing were as follows:
- Serum PTH 11 pg/mL (15–65)
- PTHrP 3.4 pmol/L (< 2.0)
- Protein electrophoresis showed a monoclonal (M) spike of 0.2 g/dL (0)
- Activated vitamin D < 5 ng/mL (19.9–79.3)
- Vitamin A 7.2 mg/dL (33.1–100)
- Vitamin B12 194 pg/mL (239–931)
- Thyroid-stimulating hormone 1.21 mIU/ L (0.47–4.68
- Free thyroxine 1.27 ng/dL (0.78–2.19)
- Iron 103 µg/dL (37–170)
- Total iron-binding capacity 335 µg/dL (265–497)
- Transferrin 248 mg/dL (206–381)
- Ferritin 66 ng/mL (11.1–264)
- Urine protein (random) 100 mg/dL (0–20)
- Urine microalbumin (random) 5.9 mg/dL (0–1.6)
- Urine creatinine clearance 88.5 mL/min (88–128)
- Urine albumin-creatinine ratio 66.66 mg/g (< 30).
Imaging reports
A nuclear bone scan showed increased bone uptake in the hip and both shoulders, consistent with arthritis, and increased activity in 2 of the lower left ribs, associated with rib fractures secondary to lytic lesions. A skeletal survey at a later date showed multiple well-circumscribed “punched-out” lytic lesions in both forearms and both femurs.
2. What should be the next step in this patient’s management?
- Intravenous (IV) fluids
- Calcitonin
- Bisphosphonate treatment
- Denosumab
- Hemodialysis
Initial treatment of severe hypercalcemia includes the following:
Start IV isotonic fluids at a rate of 150 mL/h (if the patient is making urine) to maintain urine output at more than 100 mL/h. Closely monitor urine output.
Give calcitonin 4 IU/kg in combination with IV fluids to reduce calcium levels within the first 12 to 48 hours of treatment.
Give a bisphosphonate, eg, zoledronic acid 4 mg over 15 minutes, or pamidronate 60 to 90 mg over 2 hours. Zoledronic acid is preferred in malignancy-induced hypercalcemia because it is more potent. Doses should be adjusted in patients with renal failure.
Give denosumab if hypercalcemia is refractory to bisphosphonates, or when bisphosphonates cannot be used in renal failure.9
Hemodialysis is performed in patients who have significant neurologic symptoms irrespective of acute renal insufficiency.
Our patient was started on 0.9% sodium chloride at a rate of 150 mL/h for severe hypercalcemia. Zoledronic acid 4 mg IV was given once. These measures lowered her calcium level and lessened her acute kidney injury.
ADDITIONAL FINDINGS
Urine testing was positive for Bence Jones protein. Immune electrophoresis, performed because of suspicion of multiple myeloma, showed an elevated level of kappa light chains at 806.7 mg/dL (0.33–1.94) and normal lambda light chains at 0.62 mg/dL (0.57–2.63). The immunoglobulin G level was low at 496 mg/dL (610–1,660). In patients with severe hypercalcemia, these results point to a diagnosis of malignancy. Bone marrow aspiration study showed greater than 10% plasma cells, confirming multiple myeloma.
MULTIPLE MYELOMA
The diagnosis of multiple myeloma is based in part on the presence of 10% or more of clonal bone marrow plasma cells10 and of specific end-organ damage (anemia, hypercalcemia, renal insufficiency, or bone lesions).9
Bone marrow clonality can be shown by the ratio of kappa to lambda light chains as detected with immunohistochemistry, immunofluorescence, or flow cytometry.11 The normal ratio is 0.26 to 1.65 for a patient with normal kidney function. In this patient, however, the ratio was 1,301.08 (806.67 kappa to 0.62 lambda), which was extremely out of range. The patient’s bone marrow biopsy results revealed the presence of 15% clonal bone marrow plasma cells.
Multiple myeloma causes osteolytic lesions through increased activation of osteoclast activating factor that stimulates the growth of osteoclast precursors. At the same time, it inhibits osteoblast formation via multiple pathways, including the action of sclerostin.11 Our patient had lytic lesions in 2 left lower ribs and in both forearms and femurs.
Hypercalcemia in multiple myeloma is attributed to 2 main factors: bone breakdown and macrophage overactivation. Multiple myeloma cells increase the release of macrophage inflammatory protein 1-alpha and tumor necrosis factor, which are inflammatory proteins that cause an increase in macrophages, which cause an increase in calcitriol.11 As noted, our patient’s calcium level at presentation was 18.4 mg/dL uncorrected and 18.96 mg/dL corrected.
Cast nephropathy can occur in the distal tubules from the increased free light chains circulating and combining with Tamm-Horsfall protein, which in turn causes obstruction and local inflammation,12 leading to a rise in creatinine levels and resulting in acute kidney injury,12 as in our patient.
TREATMENT CONSIDERATIONS IN MULTIPLE MYELOMA
Our patient was referred to an oncologist for management.
In the management of multiple myeloma, the patient’s quality of life needs to be considered. With the development of new agents to combat the damages of the osteolytic effects, there is hope for improving quality of life.13,14 New agents under study include anabolic agents such as antisclerostin and anti-Dickkopf-1, which promote osteoblastogenesis, leading to bone formation, with the possibility of repairing existing damage.15
TAKE-HOME POINTS
- If hypercalcemia is mild to moderate, consider primary hyperparathyroidism.
- Identify patients with severe symptoms of hypercalcemia such as volume depletion, acute kidney injury, arrhythmia, or seizures.
- Confirm severe cases of hypercalcemia and treat severe cases effectively.
- Severe hypercalcemia may need further investigation into a potential underlying malignancy.
- Sternlicht H, Glezerman IG. Hypercalcemia of malignancy and new treatment options. Ther Clin Risk Manag 2015; 11:1779–1788. doi:10.2147/TCRM.S83681
- Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcemia. Clin Cardiol 1988; 11(6):395–400. doi:10.1002/clc.4960110607
- Bilezikian JP, Cusano NE, Khan AA, Liu JM, Marcocci C, Bandeira F. Primary hyperparathyroidism. Nat Rev Dis Primers 2016; 2:16033. doi:10.1038/nrdp.2016.33
- Kuchay MS, Kaur P, Mishra SK, Mithal A. The changing profile of hypercalcemia in a tertiary care setting in North India: an 18-month retrospective study. Clin Cases Miner Bone Metab 2017; 14(2):131–135. doi:10.11138/ccmbm/2017.14.1.131
- Rosner MH, Dalkin AC. Onco-nephrology: the pathophysiology and treatment of malignancy-associated hypercalcemia. Clin J Am Soc Nephrol 2012; 7(10):1722–1729. doi:10.2215/CJN.02470312
- Ai L, Mu S, Hu Y. Prognostic role of RDW in hematological malignancies: a systematic review and meta-analysis. Cancer Cell Int 2018; 18:61. doi:10.1186/s12935-018-0558-3
- Baz R, Alemany C, Green R, Hussein MA. Prevalence of vitamin B12 deficiency in patients with plasma cell dyscrasias: a retrospective review. Cancer 2004; 101(4):790–795. doi:10.1002/cncr.20441
- Elmore JG, Carney PA, Abraham LA, et al. The association between obesity and screening mammography accuracy. Arch Intern Med 2004; 164(10):1140–1147. doi:10.1001/archinte.164.10.1140
- Gerecke C, Fuhrmann S, Strifler S, Schmidt-Hieber M, Einsele H, Knop S. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int 2016; 113(27–28):470–476. doi:10.3238/arztebl.2016.0470
- Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91(7):719–734. doi:10.1002/ajh.24402
- Silbermann R, Roodman GD. Myeloma bone disease: pathophysiology and management. J Bone Oncol 2013; 2(2):59–69. doi:10.1016/j.jbo.2013.04.001
- Doshi M, Lahoti A, Danesh FR, Batuman V, Sanders PW; American Society of Nephrology Onco-Nephrology Forum. Paraprotein-related kidney disease: kidney injury from paraproteins—what determines the site of injury? Clin J Am Soc Nephrol 2016; 11(12):2288–2294. doi:10.2215/CJN.02560316
- Reece D. Update on the initial therapy of multiple myeloma. Am Soc Clin Oncol Educ Book 2013. doi:10.1200/EdBook_AM.2013.33.e307
- Nishida H. Bone-targeted agents in multiple myeloma. Hematol Rep 2018; 10(1):7401. doi:10.4081/hr.2018.7401
- Ring ES, Lawson MA, Snowden JA, Jolley I, Chantry AD. New agents in the treatment of myeloma bone disease. Calcif Tissue Int 2018; 102(2):196–209. doi:10.1007/s00223-017-0351-7
A morbidly obese 54-year-old woman presented to the emergency department after experiencing generalized abdominal pain for 3 days. She rated the pain as 5 on a scale of 10 and described it as dull, cramping, waxing and waning, not radiating, and not relieved with changes of position—in fact, not alleviated by anything she had tried. Her pain was associated with nausea and 1 episode of vomiting. She also experienced constipation before the onset of pain.
She denied recent trauma, recent travel, diarrhea, fevers, weakness, shortness of breath, chest pain, other muscle pains, or recent changes in diet. She also denied having this pain in the past. She said she had unintentionally lost some weight but was not certain how much. She denied tobacco, alcohol, or illicit drug use. She had no history of surgery.
Her medical history included hypertension, anemia, and uterine fibroids. Her current medications included losartan, hydrochlorothiazide, and albuterol. She had no family history of significant disease.
INITIAL EVALUATION AND MANAGEMENT
On admission, her temperature was 97.8°F (36.6°C), heart rate 100 beats per minute, blood pressure 136/64 mm Hg, respiratory rate 18 breaths per minute, oxygen saturation 97% on room air, weight 130.6 kg, and body mass index 35 kg/m2.
She was alert and oriented to person, place, and time. She was in mild discomfort but no distress. Her lungs were clear to auscultation, with no wheezing or crackles. Heart rate and rhythm were regular, with no extra heart sounds or murmurs. Bowel sounds were normal in all 4 quadrants, with tenderness to palpation of the epigastric area, but with no guarding or rebound tenderness.
Laboratory test results
Notable results of blood testing at presentation were as follows:
- Hemoglobin 8.2 g/dL (reference range 12.3–15.3)
- Hematocrit 26% (41–50)
- Mean corpuscular volume 107 fL (80–100)
- Blood urea nitrogen 33 mg/dL (8–21); 6 months earlier it was 16
- Serum creatinine 3.6 mg/dL (0.58–0.96); 6 months earlier, it was 0.75
- Albumin 3.3 g/dL (3.5–5)
- Calcium 18.4 mg/dL (8.4–10.2); 6 months earlier, it was 9.6
- Corrected calcium 19 mg/dL.
Findings on imaging, electrocardiography
Chest radiography showed no acute cardiopulmonary abnormalities. Abdominal computed tomography without contrast showed no abnormalities within the pancreas and no evidence of inflammation or obstruction. Electrocardiography showed sinus tachycardia.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s symptoms?
- Primary hyperparathyroidism
- Malignancy
- Her drug therapy
- Familial hypercalcemic hypocalciuria
In total, her laboratory results were consistent with macrocytic anemia, severe hypercalcemia, and acute kidney injury, and she had generalized symptoms.
Primary hyperparathyroidism
A main cause of hypercalcemia is primary hyperparathyroidism, and this needs to be ruled out. Benign adenomas are the most common cause of primary hyperparathyroidism, and a risk factor for benign adenoma is exposure to therapeutic levels of radiation.3
In hyperparathyroidism, there is an increased secretion of parathyroid hormone (PTH), which has multiple effects including increased reabsorption of calcium from the urine, increased excretion of phosphate, and increased expression of 1,25-hydroxyvitamin D hydroxylase to activate vitamin D. PTH also stimulates osteoclasts to increase their expression of receptor activator of nuclear factor kappa B ligand (RANKL), which has a downstream effect on osteoclast precursors to cause bone reabsorption.3
Inherited primary hyperparathyroidism tends to present at a younger age, with multiple overactive parathyroid glands.3 Given our patient’s age, inherited primary hyparathyroidism is thus less likely.
Malignancy
The probability that malignancy is causing the hypercalcemia increases with calcium levels greater than 13 mg/dL. Epidemiologically, in hospitalized patients with hypercalcemia, the source tends to be malignancy.4 Typically, patients who develop hypercalcemia from malignancy have a worse prognosis.5
Solid tumors and leukemias can cause hypercalcemia. The mechanisms include humoral factors secreted by the malignancy, local osteolysis due to tumor invasion of bone, and excessive absorption of calcium due to excess vitamin D produced by malignancies.5 The cancers that most frequently cause an increase in calcium resorption are lung cancer, renal cancer, breast cancer, and multiple myeloma.1
Solid tumors with no bone metastasis and non-Hodgkin lymphoma that release PTH-related protein (PTHrP) cause humoral hypercalcemia in malignancy. The patient is typically in an advanced stage of disease. PTHrP increases serum calcium levels by decreasing the kidney’s ability to excrete calcium and by increasing bone turnover. It has no effect on intestinal absorption because of its inability to stimulate activated vitamin D3. Thus, the increase in systemic calcium comes directly from breakdown of bone and inability to excrete the excess.
PTHrP has a unique role in breast cancer: it is released locally in areas where cancer cells have metastasized to bone, but it does not cause a systemic effect. Bone resorption occurs in areas of metastasis and results from an increase in expression of RANKL and RANK in osteoclasts in response to the effects of PTHrP, leading to an increase in the production of osteoclastic cells.1
Tamoxifen, an endocrine therapy often used in breast cancer, also causes a release of bone-reabsorbing factors from tumor cells, which can partially contribute to hypercalcemia.5
Myeloma cells secrete RANKL, which stimulates osteoclastic activity, and they also release interleukin 6 (IL-6) and activating macrophage inflammatory protein alpha. Serum testing usually shows low or normal intact PTH, PTHrP, and 1,25-dihydroxyvitamin D.1
Patients with multiple myeloma have a worse prognosis if they have a high red blood cell distribution width, a condition shown to correlate with malnutrition, leading to deficiencies in vitamin B12 and to poor response to treatment.6 Up to 14% of patients with multiple myeloma have vitamin B12 deficiency.7
Our patient’s recent weight loss and severe hypercalcemia raise suspicion of malignancy. Further, her obesity makes proper routine breast examination difficult and thus increases the chance of undiagnosed breast cancer.8 Her decrease in renal function and her anemia complicated by hypercalcemia also raise suspicion of multiple myeloma.
Hypercalcemia due to drug therapy
Thiazide diuretics, lithium, teriparatide, and vitamin A in excessive amounts can raise the serum calcium concentration.5 Our patient was taking a thiazide for hypertension, but her extremely high calcium level places drug-induced hypercalcemia as the sole cause lower on the differential list.
Familial hypercalcemic hypocalciuria
Familial hypercalcemic hypocalciuria is a rare autosomal-dominant cause of hypercalcemia in which the ability of the body (and especially the kidneys) to sense levels of calcium is impaired, leading to a decrease in excretion of calcium in the urine.3 Very high calcium levels are rare in hypercalcemic hypocalciuria.3 In our patient with a corrected calcium concentration of nearly 19 mg/dL, familial hypercalcemic hypocalciuria is very unlikely to be the cause of the hypercalcemia.
WHAT ARE THE NEXT STEPS IN THE WORKUP?
As hypercalcemia has been confirmed, the intact PTH level should be checked to determine whether the patient’s condition is PTH-mediated. If the PTH level is in the upper range of normal or is minimally elevated, primary hyperparathyroidism is likely. Elevated PTH confirms primary hyperparathyroidism. A low-normal or low intact PTH confirms a non-PTH-mediated process, and once this is confirmed, PTHrP levels should be checked. An elevated PTHrP suggests humoral hypercalcemia of malignancy. Serum protein electrophoresis, urine protein electrophoresis, and a serum light chain assay should be performed to rule out multiple myeloma.
Vitamin D toxicity is associated with high concentrations of 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D metabolites. These levels should be checked in this patient.
Other disorders that cause hypercalcemia are vitamin A toxicity and hyperthyroidism, so vitamin A and thyroid-stimulating hormone levels should also be checked.5
CASE CONTINUED
After further questioning, the patient said that she had had lower back pain about 1 to 2 weeks before coming to the emergency room; her primary care doctor had said the pain was likely from muscle strain. The pain had almost resolved but was still present.
The results of further laboratory testing were as follows:
- Serum PTH 11 pg/mL (15–65)
- PTHrP 3.4 pmol/L (< 2.0)
- Protein electrophoresis showed a monoclonal (M) spike of 0.2 g/dL (0)
- Activated vitamin D < 5 ng/mL (19.9–79.3)
- Vitamin A 7.2 mg/dL (33.1–100)
- Vitamin B12 194 pg/mL (239–931)
- Thyroid-stimulating hormone 1.21 mIU/ L (0.47–4.68
- Free thyroxine 1.27 ng/dL (0.78–2.19)
- Iron 103 µg/dL (37–170)
- Total iron-binding capacity 335 µg/dL (265–497)
- Transferrin 248 mg/dL (206–381)
- Ferritin 66 ng/mL (11.1–264)
- Urine protein (random) 100 mg/dL (0–20)
- Urine microalbumin (random) 5.9 mg/dL (0–1.6)
- Urine creatinine clearance 88.5 mL/min (88–128)
- Urine albumin-creatinine ratio 66.66 mg/g (< 30).
Imaging reports
A nuclear bone scan showed increased bone uptake in the hip and both shoulders, consistent with arthritis, and increased activity in 2 of the lower left ribs, associated with rib fractures secondary to lytic lesions. A skeletal survey at a later date showed multiple well-circumscribed “punched-out” lytic lesions in both forearms and both femurs.
2. What should be the next step in this patient’s management?
- Intravenous (IV) fluids
- Calcitonin
- Bisphosphonate treatment
- Denosumab
- Hemodialysis
Initial treatment of severe hypercalcemia includes the following:
Start IV isotonic fluids at a rate of 150 mL/h (if the patient is making urine) to maintain urine output at more than 100 mL/h. Closely monitor urine output.
Give calcitonin 4 IU/kg in combination with IV fluids to reduce calcium levels within the first 12 to 48 hours of treatment.
Give a bisphosphonate, eg, zoledronic acid 4 mg over 15 minutes, or pamidronate 60 to 90 mg over 2 hours. Zoledronic acid is preferred in malignancy-induced hypercalcemia because it is more potent. Doses should be adjusted in patients with renal failure.
Give denosumab if hypercalcemia is refractory to bisphosphonates, or when bisphosphonates cannot be used in renal failure.9
Hemodialysis is performed in patients who have significant neurologic symptoms irrespective of acute renal insufficiency.
Our patient was started on 0.9% sodium chloride at a rate of 150 mL/h for severe hypercalcemia. Zoledronic acid 4 mg IV was given once. These measures lowered her calcium level and lessened her acute kidney injury.
ADDITIONAL FINDINGS
Urine testing was positive for Bence Jones protein. Immune electrophoresis, performed because of suspicion of multiple myeloma, showed an elevated level of kappa light chains at 806.7 mg/dL (0.33–1.94) and normal lambda light chains at 0.62 mg/dL (0.57–2.63). The immunoglobulin G level was low at 496 mg/dL (610–1,660). In patients with severe hypercalcemia, these results point to a diagnosis of malignancy. Bone marrow aspiration study showed greater than 10% plasma cells, confirming multiple myeloma.
MULTIPLE MYELOMA
The diagnosis of multiple myeloma is based in part on the presence of 10% or more of clonal bone marrow plasma cells10 and of specific end-organ damage (anemia, hypercalcemia, renal insufficiency, or bone lesions).9
Bone marrow clonality can be shown by the ratio of kappa to lambda light chains as detected with immunohistochemistry, immunofluorescence, or flow cytometry.11 The normal ratio is 0.26 to 1.65 for a patient with normal kidney function. In this patient, however, the ratio was 1,301.08 (806.67 kappa to 0.62 lambda), which was extremely out of range. The patient’s bone marrow biopsy results revealed the presence of 15% clonal bone marrow plasma cells.
Multiple myeloma causes osteolytic lesions through increased activation of osteoclast activating factor that stimulates the growth of osteoclast precursors. At the same time, it inhibits osteoblast formation via multiple pathways, including the action of sclerostin.11 Our patient had lytic lesions in 2 left lower ribs and in both forearms and femurs.
Hypercalcemia in multiple myeloma is attributed to 2 main factors: bone breakdown and macrophage overactivation. Multiple myeloma cells increase the release of macrophage inflammatory protein 1-alpha and tumor necrosis factor, which are inflammatory proteins that cause an increase in macrophages, which cause an increase in calcitriol.11 As noted, our patient’s calcium level at presentation was 18.4 mg/dL uncorrected and 18.96 mg/dL corrected.
Cast nephropathy can occur in the distal tubules from the increased free light chains circulating and combining with Tamm-Horsfall protein, which in turn causes obstruction and local inflammation,12 leading to a rise in creatinine levels and resulting in acute kidney injury,12 as in our patient.
TREATMENT CONSIDERATIONS IN MULTIPLE MYELOMA
Our patient was referred to an oncologist for management.
In the management of multiple myeloma, the patient’s quality of life needs to be considered. With the development of new agents to combat the damages of the osteolytic effects, there is hope for improving quality of life.13,14 New agents under study include anabolic agents such as antisclerostin and anti-Dickkopf-1, which promote osteoblastogenesis, leading to bone formation, with the possibility of repairing existing damage.15
TAKE-HOME POINTS
- If hypercalcemia is mild to moderate, consider primary hyperparathyroidism.
- Identify patients with severe symptoms of hypercalcemia such as volume depletion, acute kidney injury, arrhythmia, or seizures.
- Confirm severe cases of hypercalcemia and treat severe cases effectively.
- Severe hypercalcemia may need further investigation into a potential underlying malignancy.
A morbidly obese 54-year-old woman presented to the emergency department after experiencing generalized abdominal pain for 3 days. She rated the pain as 5 on a scale of 10 and described it as dull, cramping, waxing and waning, not radiating, and not relieved with changes of position—in fact, not alleviated by anything she had tried. Her pain was associated with nausea and 1 episode of vomiting. She also experienced constipation before the onset of pain.
She denied recent trauma, recent travel, diarrhea, fevers, weakness, shortness of breath, chest pain, other muscle pains, or recent changes in diet. She also denied having this pain in the past. She said she had unintentionally lost some weight but was not certain how much. She denied tobacco, alcohol, or illicit drug use. She had no history of surgery.
Her medical history included hypertension, anemia, and uterine fibroids. Her current medications included losartan, hydrochlorothiazide, and albuterol. She had no family history of significant disease.
INITIAL EVALUATION AND MANAGEMENT
On admission, her temperature was 97.8°F (36.6°C), heart rate 100 beats per minute, blood pressure 136/64 mm Hg, respiratory rate 18 breaths per minute, oxygen saturation 97% on room air, weight 130.6 kg, and body mass index 35 kg/m2.
She was alert and oriented to person, place, and time. She was in mild discomfort but no distress. Her lungs were clear to auscultation, with no wheezing or crackles. Heart rate and rhythm were regular, with no extra heart sounds or murmurs. Bowel sounds were normal in all 4 quadrants, with tenderness to palpation of the epigastric area, but with no guarding or rebound tenderness.
Laboratory test results
Notable results of blood testing at presentation were as follows:
- Hemoglobin 8.2 g/dL (reference range 12.3–15.3)
- Hematocrit 26% (41–50)
- Mean corpuscular volume 107 fL (80–100)
- Blood urea nitrogen 33 mg/dL (8–21); 6 months earlier it was 16
- Serum creatinine 3.6 mg/dL (0.58–0.96); 6 months earlier, it was 0.75
- Albumin 3.3 g/dL (3.5–5)
- Calcium 18.4 mg/dL (8.4–10.2); 6 months earlier, it was 9.6
- Corrected calcium 19 mg/dL.
Findings on imaging, electrocardiography
Chest radiography showed no acute cardiopulmonary abnormalities. Abdominal computed tomography without contrast showed no abnormalities within the pancreas and no evidence of inflammation or obstruction. Electrocardiography showed sinus tachycardia.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s symptoms?
- Primary hyperparathyroidism
- Malignancy
- Her drug therapy
- Familial hypercalcemic hypocalciuria
In total, her laboratory results were consistent with macrocytic anemia, severe hypercalcemia, and acute kidney injury, and she had generalized symptoms.
Primary hyperparathyroidism
A main cause of hypercalcemia is primary hyperparathyroidism, and this needs to be ruled out. Benign adenomas are the most common cause of primary hyperparathyroidism, and a risk factor for benign adenoma is exposure to therapeutic levels of radiation.3
In hyperparathyroidism, there is an increased secretion of parathyroid hormone (PTH), which has multiple effects including increased reabsorption of calcium from the urine, increased excretion of phosphate, and increased expression of 1,25-hydroxyvitamin D hydroxylase to activate vitamin D. PTH also stimulates osteoclasts to increase their expression of receptor activator of nuclear factor kappa B ligand (RANKL), which has a downstream effect on osteoclast precursors to cause bone reabsorption.3
Inherited primary hyperparathyroidism tends to present at a younger age, with multiple overactive parathyroid glands.3 Given our patient’s age, inherited primary hyparathyroidism is thus less likely.
Malignancy
The probability that malignancy is causing the hypercalcemia increases with calcium levels greater than 13 mg/dL. Epidemiologically, in hospitalized patients with hypercalcemia, the source tends to be malignancy.4 Typically, patients who develop hypercalcemia from malignancy have a worse prognosis.5
Solid tumors and leukemias can cause hypercalcemia. The mechanisms include humoral factors secreted by the malignancy, local osteolysis due to tumor invasion of bone, and excessive absorption of calcium due to excess vitamin D produced by malignancies.5 The cancers that most frequently cause an increase in calcium resorption are lung cancer, renal cancer, breast cancer, and multiple myeloma.1
Solid tumors with no bone metastasis and non-Hodgkin lymphoma that release PTH-related protein (PTHrP) cause humoral hypercalcemia in malignancy. The patient is typically in an advanced stage of disease. PTHrP increases serum calcium levels by decreasing the kidney’s ability to excrete calcium and by increasing bone turnover. It has no effect on intestinal absorption because of its inability to stimulate activated vitamin D3. Thus, the increase in systemic calcium comes directly from breakdown of bone and inability to excrete the excess.
PTHrP has a unique role in breast cancer: it is released locally in areas where cancer cells have metastasized to bone, but it does not cause a systemic effect. Bone resorption occurs in areas of metastasis and results from an increase in expression of RANKL and RANK in osteoclasts in response to the effects of PTHrP, leading to an increase in the production of osteoclastic cells.1
Tamoxifen, an endocrine therapy often used in breast cancer, also causes a release of bone-reabsorbing factors from tumor cells, which can partially contribute to hypercalcemia.5
Myeloma cells secrete RANKL, which stimulates osteoclastic activity, and they also release interleukin 6 (IL-6) and activating macrophage inflammatory protein alpha. Serum testing usually shows low or normal intact PTH, PTHrP, and 1,25-dihydroxyvitamin D.1
Patients with multiple myeloma have a worse prognosis if they have a high red blood cell distribution width, a condition shown to correlate with malnutrition, leading to deficiencies in vitamin B12 and to poor response to treatment.6 Up to 14% of patients with multiple myeloma have vitamin B12 deficiency.7
Our patient’s recent weight loss and severe hypercalcemia raise suspicion of malignancy. Further, her obesity makes proper routine breast examination difficult and thus increases the chance of undiagnosed breast cancer.8 Her decrease in renal function and her anemia complicated by hypercalcemia also raise suspicion of multiple myeloma.
Hypercalcemia due to drug therapy
Thiazide diuretics, lithium, teriparatide, and vitamin A in excessive amounts can raise the serum calcium concentration.5 Our patient was taking a thiazide for hypertension, but her extremely high calcium level places drug-induced hypercalcemia as the sole cause lower on the differential list.
Familial hypercalcemic hypocalciuria
Familial hypercalcemic hypocalciuria is a rare autosomal-dominant cause of hypercalcemia in which the ability of the body (and especially the kidneys) to sense levels of calcium is impaired, leading to a decrease in excretion of calcium in the urine.3 Very high calcium levels are rare in hypercalcemic hypocalciuria.3 In our patient with a corrected calcium concentration of nearly 19 mg/dL, familial hypercalcemic hypocalciuria is very unlikely to be the cause of the hypercalcemia.
WHAT ARE THE NEXT STEPS IN THE WORKUP?
As hypercalcemia has been confirmed, the intact PTH level should be checked to determine whether the patient’s condition is PTH-mediated. If the PTH level is in the upper range of normal or is minimally elevated, primary hyperparathyroidism is likely. Elevated PTH confirms primary hyperparathyroidism. A low-normal or low intact PTH confirms a non-PTH-mediated process, and once this is confirmed, PTHrP levels should be checked. An elevated PTHrP suggests humoral hypercalcemia of malignancy. Serum protein electrophoresis, urine protein electrophoresis, and a serum light chain assay should be performed to rule out multiple myeloma.
Vitamin D toxicity is associated with high concentrations of 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D metabolites. These levels should be checked in this patient.
Other disorders that cause hypercalcemia are vitamin A toxicity and hyperthyroidism, so vitamin A and thyroid-stimulating hormone levels should also be checked.5
CASE CONTINUED
After further questioning, the patient said that she had had lower back pain about 1 to 2 weeks before coming to the emergency room; her primary care doctor had said the pain was likely from muscle strain. The pain had almost resolved but was still present.
The results of further laboratory testing were as follows:
- Serum PTH 11 pg/mL (15–65)
- PTHrP 3.4 pmol/L (< 2.0)
- Protein electrophoresis showed a monoclonal (M) spike of 0.2 g/dL (0)
- Activated vitamin D < 5 ng/mL (19.9–79.3)
- Vitamin A 7.2 mg/dL (33.1–100)
- Vitamin B12 194 pg/mL (239–931)
- Thyroid-stimulating hormone 1.21 mIU/ L (0.47–4.68
- Free thyroxine 1.27 ng/dL (0.78–2.19)
- Iron 103 µg/dL (37–170)
- Total iron-binding capacity 335 µg/dL (265–497)
- Transferrin 248 mg/dL (206–381)
- Ferritin 66 ng/mL (11.1–264)
- Urine protein (random) 100 mg/dL (0–20)
- Urine microalbumin (random) 5.9 mg/dL (0–1.6)
- Urine creatinine clearance 88.5 mL/min (88–128)
- Urine albumin-creatinine ratio 66.66 mg/g (< 30).
Imaging reports
A nuclear bone scan showed increased bone uptake in the hip and both shoulders, consistent with arthritis, and increased activity in 2 of the lower left ribs, associated with rib fractures secondary to lytic lesions. A skeletal survey at a later date showed multiple well-circumscribed “punched-out” lytic lesions in both forearms and both femurs.
2. What should be the next step in this patient’s management?
- Intravenous (IV) fluids
- Calcitonin
- Bisphosphonate treatment
- Denosumab
- Hemodialysis
Initial treatment of severe hypercalcemia includes the following:
Start IV isotonic fluids at a rate of 150 mL/h (if the patient is making urine) to maintain urine output at more than 100 mL/h. Closely monitor urine output.
Give calcitonin 4 IU/kg in combination with IV fluids to reduce calcium levels within the first 12 to 48 hours of treatment.
Give a bisphosphonate, eg, zoledronic acid 4 mg over 15 minutes, or pamidronate 60 to 90 mg over 2 hours. Zoledronic acid is preferred in malignancy-induced hypercalcemia because it is more potent. Doses should be adjusted in patients with renal failure.
Give denosumab if hypercalcemia is refractory to bisphosphonates, or when bisphosphonates cannot be used in renal failure.9
Hemodialysis is performed in patients who have significant neurologic symptoms irrespective of acute renal insufficiency.
Our patient was started on 0.9% sodium chloride at a rate of 150 mL/h for severe hypercalcemia. Zoledronic acid 4 mg IV was given once. These measures lowered her calcium level and lessened her acute kidney injury.
ADDITIONAL FINDINGS
Urine testing was positive for Bence Jones protein. Immune electrophoresis, performed because of suspicion of multiple myeloma, showed an elevated level of kappa light chains at 806.7 mg/dL (0.33–1.94) and normal lambda light chains at 0.62 mg/dL (0.57–2.63). The immunoglobulin G level was low at 496 mg/dL (610–1,660). In patients with severe hypercalcemia, these results point to a diagnosis of malignancy. Bone marrow aspiration study showed greater than 10% plasma cells, confirming multiple myeloma.
MULTIPLE MYELOMA
The diagnosis of multiple myeloma is based in part on the presence of 10% or more of clonal bone marrow plasma cells10 and of specific end-organ damage (anemia, hypercalcemia, renal insufficiency, or bone lesions).9
Bone marrow clonality can be shown by the ratio of kappa to lambda light chains as detected with immunohistochemistry, immunofluorescence, or flow cytometry.11 The normal ratio is 0.26 to 1.65 for a patient with normal kidney function. In this patient, however, the ratio was 1,301.08 (806.67 kappa to 0.62 lambda), which was extremely out of range. The patient’s bone marrow biopsy results revealed the presence of 15% clonal bone marrow plasma cells.
Multiple myeloma causes osteolytic lesions through increased activation of osteoclast activating factor that stimulates the growth of osteoclast precursors. At the same time, it inhibits osteoblast formation via multiple pathways, including the action of sclerostin.11 Our patient had lytic lesions in 2 left lower ribs and in both forearms and femurs.
Hypercalcemia in multiple myeloma is attributed to 2 main factors: bone breakdown and macrophage overactivation. Multiple myeloma cells increase the release of macrophage inflammatory protein 1-alpha and tumor necrosis factor, which are inflammatory proteins that cause an increase in macrophages, which cause an increase in calcitriol.11 As noted, our patient’s calcium level at presentation was 18.4 mg/dL uncorrected and 18.96 mg/dL corrected.
Cast nephropathy can occur in the distal tubules from the increased free light chains circulating and combining with Tamm-Horsfall protein, which in turn causes obstruction and local inflammation,12 leading to a rise in creatinine levels and resulting in acute kidney injury,12 as in our patient.
TREATMENT CONSIDERATIONS IN MULTIPLE MYELOMA
Our patient was referred to an oncologist for management.
In the management of multiple myeloma, the patient’s quality of life needs to be considered. With the development of new agents to combat the damages of the osteolytic effects, there is hope for improving quality of life.13,14 New agents under study include anabolic agents such as antisclerostin and anti-Dickkopf-1, which promote osteoblastogenesis, leading to bone formation, with the possibility of repairing existing damage.15
TAKE-HOME POINTS
- If hypercalcemia is mild to moderate, consider primary hyperparathyroidism.
- Identify patients with severe symptoms of hypercalcemia such as volume depletion, acute kidney injury, arrhythmia, or seizures.
- Confirm severe cases of hypercalcemia and treat severe cases effectively.
- Severe hypercalcemia may need further investigation into a potential underlying malignancy.
- Sternlicht H, Glezerman IG. Hypercalcemia of malignancy and new treatment options. Ther Clin Risk Manag 2015; 11:1779–1788. doi:10.2147/TCRM.S83681
- Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcemia. Clin Cardiol 1988; 11(6):395–400. doi:10.1002/clc.4960110607
- Bilezikian JP, Cusano NE, Khan AA, Liu JM, Marcocci C, Bandeira F. Primary hyperparathyroidism. Nat Rev Dis Primers 2016; 2:16033. doi:10.1038/nrdp.2016.33
- Kuchay MS, Kaur P, Mishra SK, Mithal A. The changing profile of hypercalcemia in a tertiary care setting in North India: an 18-month retrospective study. Clin Cases Miner Bone Metab 2017; 14(2):131–135. doi:10.11138/ccmbm/2017.14.1.131
- Rosner MH, Dalkin AC. Onco-nephrology: the pathophysiology and treatment of malignancy-associated hypercalcemia. Clin J Am Soc Nephrol 2012; 7(10):1722–1729. doi:10.2215/CJN.02470312
- Ai L, Mu S, Hu Y. Prognostic role of RDW in hematological malignancies: a systematic review and meta-analysis. Cancer Cell Int 2018; 18:61. doi:10.1186/s12935-018-0558-3
- Baz R, Alemany C, Green R, Hussein MA. Prevalence of vitamin B12 deficiency in patients with plasma cell dyscrasias: a retrospective review. Cancer 2004; 101(4):790–795. doi:10.1002/cncr.20441
- Elmore JG, Carney PA, Abraham LA, et al. The association between obesity and screening mammography accuracy. Arch Intern Med 2004; 164(10):1140–1147. doi:10.1001/archinte.164.10.1140
- Gerecke C, Fuhrmann S, Strifler S, Schmidt-Hieber M, Einsele H, Knop S. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int 2016; 113(27–28):470–476. doi:10.3238/arztebl.2016.0470
- Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91(7):719–734. doi:10.1002/ajh.24402
- Silbermann R, Roodman GD. Myeloma bone disease: pathophysiology and management. J Bone Oncol 2013; 2(2):59–69. doi:10.1016/j.jbo.2013.04.001
- Doshi M, Lahoti A, Danesh FR, Batuman V, Sanders PW; American Society of Nephrology Onco-Nephrology Forum. Paraprotein-related kidney disease: kidney injury from paraproteins—what determines the site of injury? Clin J Am Soc Nephrol 2016; 11(12):2288–2294. doi:10.2215/CJN.02560316
- Reece D. Update on the initial therapy of multiple myeloma. Am Soc Clin Oncol Educ Book 2013. doi:10.1200/EdBook_AM.2013.33.e307
- Nishida H. Bone-targeted agents in multiple myeloma. Hematol Rep 2018; 10(1):7401. doi:10.4081/hr.2018.7401
- Ring ES, Lawson MA, Snowden JA, Jolley I, Chantry AD. New agents in the treatment of myeloma bone disease. Calcif Tissue Int 2018; 102(2):196–209. doi:10.1007/s00223-017-0351-7
- Sternlicht H, Glezerman IG. Hypercalcemia of malignancy and new treatment options. Ther Clin Risk Manag 2015; 11:1779–1788. doi:10.2147/TCRM.S83681
- Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcemia. Clin Cardiol 1988; 11(6):395–400. doi:10.1002/clc.4960110607
- Bilezikian JP, Cusano NE, Khan AA, Liu JM, Marcocci C, Bandeira F. Primary hyperparathyroidism. Nat Rev Dis Primers 2016; 2:16033. doi:10.1038/nrdp.2016.33
- Kuchay MS, Kaur P, Mishra SK, Mithal A. The changing profile of hypercalcemia in a tertiary care setting in North India: an 18-month retrospective study. Clin Cases Miner Bone Metab 2017; 14(2):131–135. doi:10.11138/ccmbm/2017.14.1.131
- Rosner MH, Dalkin AC. Onco-nephrology: the pathophysiology and treatment of malignancy-associated hypercalcemia. Clin J Am Soc Nephrol 2012; 7(10):1722–1729. doi:10.2215/CJN.02470312
- Ai L, Mu S, Hu Y. Prognostic role of RDW in hematological malignancies: a systematic review and meta-analysis. Cancer Cell Int 2018; 18:61. doi:10.1186/s12935-018-0558-3
- Baz R, Alemany C, Green R, Hussein MA. Prevalence of vitamin B12 deficiency in patients with plasma cell dyscrasias: a retrospective review. Cancer 2004; 101(4):790–795. doi:10.1002/cncr.20441
- Elmore JG, Carney PA, Abraham LA, et al. The association between obesity and screening mammography accuracy. Arch Intern Med 2004; 164(10):1140–1147. doi:10.1001/archinte.164.10.1140
- Gerecke C, Fuhrmann S, Strifler S, Schmidt-Hieber M, Einsele H, Knop S. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int 2016; 113(27–28):470–476. doi:10.3238/arztebl.2016.0470
- Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91(7):719–734. doi:10.1002/ajh.24402
- Silbermann R, Roodman GD. Myeloma bone disease: pathophysiology and management. J Bone Oncol 2013; 2(2):59–69. doi:10.1016/j.jbo.2013.04.001
- Doshi M, Lahoti A, Danesh FR, Batuman V, Sanders PW; American Society of Nephrology Onco-Nephrology Forum. Paraprotein-related kidney disease: kidney injury from paraproteins—what determines the site of injury? Clin J Am Soc Nephrol 2016; 11(12):2288–2294. doi:10.2215/CJN.02560316
- Reece D. Update on the initial therapy of multiple myeloma. Am Soc Clin Oncol Educ Book 2013. doi:10.1200/EdBook_AM.2013.33.e307
- Nishida H. Bone-targeted agents in multiple myeloma. Hematol Rep 2018; 10(1):7401. doi:10.4081/hr.2018.7401
- Ring ES, Lawson MA, Snowden JA, Jolley I, Chantry AD. New agents in the treatment of myeloma bone disease. Calcif Tissue Int 2018; 102(2):196–209. doi:10.1007/s00223-017-0351-7
Low-dose steroids for acute exacerbations of COPD in a non-ICU setting: Worth consideration
Despite guidelines recommending low-dose oral glucocorticoids over high-dose intravenous (IV) glucocorticoids for inpatient management of acute exacerbations of chronic obstructive pulmonary disease (COPD), we have observed that most patients still receive high-dose IV therapy before being transitioned to low-dose oral therapy at discharge. Clinical inertia undoubtedly plays a significant role in the slow adoption of new recommendations, but in this era of evidence-based practice, the unfortunate lack of data supporting low over high steroid doses for acute exacerbations of COPD also contributes to hesitancy of physicians.
A SIGNIFICANT AND GROWING BURDEN
COPD is one of the most common pulmonary conditions managed by hospitalists today, and by the year 2030, it is predicted to become the third leading cause of death worldwide.1
COPD is also a significant economic burden, costing $50 billion to manage in the United States, most of that from the cost of lengthy hospital stays.2 COPD patients have 1 to 2 exacerbations per year.3 Bacterial and viral infections are responsible for most exacerbations, and 15% to 20% are from air pollution and other environmental causes of airway inflammation.3
CHALLENGES TO CHANGING PRACTICE
Glucocorticoids are the gold standard for treatment of acute exacerbations of COPD. It is well-documented that compared with placebo, glucocorticoids reduce mortality risk, length of hospital stay, and exacerbation recurrence after 1 month.4 And while high-dose IV steroid therapy has been the standard approach, oral administration has been found to be noninferior to IV administration with regard to treatment and length of hospital stay.5
While adverse effects are more common at higher doses, the optimal dose and duration of systemic glucocorticoid therapy for acute exacerbations of COPD are still largely at the discretion of the physician. The 2019 report of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends low doses (40 mg) for no more than 5 to 7 days for exacerbations, based on reports that showed no worse outcomes with low-dose oral than with high-dose IV therapy.6,7 (In the 2010 study by Lindenauer et al,7 92% of nearly 80,000 patients received high-dose IV steroids, reflecting standard practice at that time.) However, the GOLD guidelines do not address mortality rates, length of stay, or readmission rates for either approach, as they are devised to direct treatment in patients with stable mild to advanced COPD, not exacerbations.
THE EVIDENCE FOR LOW-DOSE STEROIDS
Mortality rates
Aksoy et al8 established that, compared with placebo, low-dose steroids improved mortality rates in a subset of patients with acute exacerbations, specifically those with eosinophilic exacerbations. This study followed the 2013 Reduction in the Use of Corticosteroids in Exacerbated COPD (REDUCE) trial, which showed mortality rates were not lower with 14 days of low-dose prednisone treatment than with 5 days.9
Length of hospital stay
With regard to length of hospital stay, in 2011 Wang et al10 found no statistically significant difference between high- and low-dose steroid treatment.However, the REDUCE trial found that low-dose steroids shortened the median length of stay by 1 day compared with placebo.9
Hospital readmission rates
The REDUCE trial found no statistically significant difference in readmission rates when comparing 5 days of low-dose treatment vs 14 days.9 However, Aksoy et al8 found that readmission rates were significantly lower with low-dose treatment than with placebo.No study has yet examined readmission rates with high-dose vs low-dose steroid treatment.
What does the evidence tell us?
Low-dose oral glucocorticoid treatment shows definitive benefits in terms of lower mortality rates, shorter hospital length of stay, and lower readmission rates vs placebo in the treatment of acute exacerbations of COPD. Furthermore, a 14-day course is no better than 5 days in terms of mortality rates. And low-dose glucocorticoid treatment shows reduced mortality rates in addition to similar hospital length of stay when compared to high-dose glucocorticoid treatment.
Together, these findings lend credibility to the current GOLD recommendations. However, we have observed that in sharp contrast to the leading clinical guidelines, most patients hospitalized for acute exacerbations of COPD are still treated initially with high-dose IV corticosteroids. Why?
Obstacles that perpetuate the use of high-dose over low-dose treatment include lack of knowledge of glucocorticoid pharmacokinetics among clinicians, use of outdated order sets, and the reflex notion that more of a drug is more efficacious in its desired effect. In addition, administrative obstacles include using high-dose IV steroids to justify an inpatient stay or continued hospitalization.
COUNTERING THE OBSTACLES: THE HOSPITALIST’S ROLE
To counter these obstacles, we propose standardization of inpatient treatment of acute exacerbations of COPD to include initial low-dose steroid treatment in accordance with the most recent GOLD guidelines.6 This would benefit the patient by reducing undesirable effects of high-dose steroids, and at the same time reduce the economic burden of managing COPD exacerbations. Considering the large number of hospitalizations for COPD exacerbation each year, hospitalists can play a large role in this effort by routinely incorporating the low-dose steroid recommendation into their clinical practice.
- World Health Organization. Chronic respiratory diseases: burden of COPD. www.who.int/respiratory/copd/burden/en. Accessed October 16, 2019.
- Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res 2013; 5:235–245. doi:10.2147/CEOR.S34321
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359(22):2355–2365. doi:10.1056/NEJMra0800353
- Walters JA, Tan DJ, White CJ, Gibson PG, Wood-Baker R, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; (9):CD001288. doi:10.1002/14651858.CD001288.pub4
- de Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, van den Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double-blind study. Chest 2007; 132(6):1741–1747. doi:10.1378/chest.07-0208
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2019 report. www.goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed October 16, 2019.
- Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA 2010; 303(23):2359–2367. doi:10.1001/jama.2010.796
- Aksoy E, Güngör S, Agca MÇ, et al. A revised treatment approach for hospitalized patients with eosinophilic and neutrophilic exacerbations of chronic obstructive pulmonary disease. Turk Thorac J 2018; 19(4):193–200. doi:10.5152/TurkThoracJ.2018.18004
- Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013; 309(21):2223–2231. doi:10.1001/jama.2013.5023
- Wang PH, Cheng SL, Wang HC, et al. Systemic steroids in acute exacerbation of COPD—from guidelines to bedside. Int J Clin Pharmacol Ther 2011; 49(11):705–708. doi:10.5414/cp201588
Despite guidelines recommending low-dose oral glucocorticoids over high-dose intravenous (IV) glucocorticoids for inpatient management of acute exacerbations of chronic obstructive pulmonary disease (COPD), we have observed that most patients still receive high-dose IV therapy before being transitioned to low-dose oral therapy at discharge. Clinical inertia undoubtedly plays a significant role in the slow adoption of new recommendations, but in this era of evidence-based practice, the unfortunate lack of data supporting low over high steroid doses for acute exacerbations of COPD also contributes to hesitancy of physicians.
A SIGNIFICANT AND GROWING BURDEN
COPD is one of the most common pulmonary conditions managed by hospitalists today, and by the year 2030, it is predicted to become the third leading cause of death worldwide.1
COPD is also a significant economic burden, costing $50 billion to manage in the United States, most of that from the cost of lengthy hospital stays.2 COPD patients have 1 to 2 exacerbations per year.3 Bacterial and viral infections are responsible for most exacerbations, and 15% to 20% are from air pollution and other environmental causes of airway inflammation.3
CHALLENGES TO CHANGING PRACTICE
Glucocorticoids are the gold standard for treatment of acute exacerbations of COPD. It is well-documented that compared with placebo, glucocorticoids reduce mortality risk, length of hospital stay, and exacerbation recurrence after 1 month.4 And while high-dose IV steroid therapy has been the standard approach, oral administration has been found to be noninferior to IV administration with regard to treatment and length of hospital stay.5
While adverse effects are more common at higher doses, the optimal dose and duration of systemic glucocorticoid therapy for acute exacerbations of COPD are still largely at the discretion of the physician. The 2019 report of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends low doses (40 mg) for no more than 5 to 7 days for exacerbations, based on reports that showed no worse outcomes with low-dose oral than with high-dose IV therapy.6,7 (In the 2010 study by Lindenauer et al,7 92% of nearly 80,000 patients received high-dose IV steroids, reflecting standard practice at that time.) However, the GOLD guidelines do not address mortality rates, length of stay, or readmission rates for either approach, as they are devised to direct treatment in patients with stable mild to advanced COPD, not exacerbations.
THE EVIDENCE FOR LOW-DOSE STEROIDS
Mortality rates
Aksoy et al8 established that, compared with placebo, low-dose steroids improved mortality rates in a subset of patients with acute exacerbations, specifically those with eosinophilic exacerbations. This study followed the 2013 Reduction in the Use of Corticosteroids in Exacerbated COPD (REDUCE) trial, which showed mortality rates were not lower with 14 days of low-dose prednisone treatment than with 5 days.9
Length of hospital stay
With regard to length of hospital stay, in 2011 Wang et al10 found no statistically significant difference between high- and low-dose steroid treatment.However, the REDUCE trial found that low-dose steroids shortened the median length of stay by 1 day compared with placebo.9
Hospital readmission rates
The REDUCE trial found no statistically significant difference in readmission rates when comparing 5 days of low-dose treatment vs 14 days.9 However, Aksoy et al8 found that readmission rates were significantly lower with low-dose treatment than with placebo.No study has yet examined readmission rates with high-dose vs low-dose steroid treatment.
What does the evidence tell us?
Low-dose oral glucocorticoid treatment shows definitive benefits in terms of lower mortality rates, shorter hospital length of stay, and lower readmission rates vs placebo in the treatment of acute exacerbations of COPD. Furthermore, a 14-day course is no better than 5 days in terms of mortality rates. And low-dose glucocorticoid treatment shows reduced mortality rates in addition to similar hospital length of stay when compared to high-dose glucocorticoid treatment.
Together, these findings lend credibility to the current GOLD recommendations. However, we have observed that in sharp contrast to the leading clinical guidelines, most patients hospitalized for acute exacerbations of COPD are still treated initially with high-dose IV corticosteroids. Why?
Obstacles that perpetuate the use of high-dose over low-dose treatment include lack of knowledge of glucocorticoid pharmacokinetics among clinicians, use of outdated order sets, and the reflex notion that more of a drug is more efficacious in its desired effect. In addition, administrative obstacles include using high-dose IV steroids to justify an inpatient stay or continued hospitalization.
COUNTERING THE OBSTACLES: THE HOSPITALIST’S ROLE
To counter these obstacles, we propose standardization of inpatient treatment of acute exacerbations of COPD to include initial low-dose steroid treatment in accordance with the most recent GOLD guidelines.6 This would benefit the patient by reducing undesirable effects of high-dose steroids, and at the same time reduce the economic burden of managing COPD exacerbations. Considering the large number of hospitalizations for COPD exacerbation each year, hospitalists can play a large role in this effort by routinely incorporating the low-dose steroid recommendation into their clinical practice.
Despite guidelines recommending low-dose oral glucocorticoids over high-dose intravenous (IV) glucocorticoids for inpatient management of acute exacerbations of chronic obstructive pulmonary disease (COPD), we have observed that most patients still receive high-dose IV therapy before being transitioned to low-dose oral therapy at discharge. Clinical inertia undoubtedly plays a significant role in the slow adoption of new recommendations, but in this era of evidence-based practice, the unfortunate lack of data supporting low over high steroid doses for acute exacerbations of COPD also contributes to hesitancy of physicians.
A SIGNIFICANT AND GROWING BURDEN
COPD is one of the most common pulmonary conditions managed by hospitalists today, and by the year 2030, it is predicted to become the third leading cause of death worldwide.1
COPD is also a significant economic burden, costing $50 billion to manage in the United States, most of that from the cost of lengthy hospital stays.2 COPD patients have 1 to 2 exacerbations per year.3 Bacterial and viral infections are responsible for most exacerbations, and 15% to 20% are from air pollution and other environmental causes of airway inflammation.3
CHALLENGES TO CHANGING PRACTICE
Glucocorticoids are the gold standard for treatment of acute exacerbations of COPD. It is well-documented that compared with placebo, glucocorticoids reduce mortality risk, length of hospital stay, and exacerbation recurrence after 1 month.4 And while high-dose IV steroid therapy has been the standard approach, oral administration has been found to be noninferior to IV administration with regard to treatment and length of hospital stay.5
While adverse effects are more common at higher doses, the optimal dose and duration of systemic glucocorticoid therapy for acute exacerbations of COPD are still largely at the discretion of the physician. The 2019 report of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends low doses (40 mg) for no more than 5 to 7 days for exacerbations, based on reports that showed no worse outcomes with low-dose oral than with high-dose IV therapy.6,7 (In the 2010 study by Lindenauer et al,7 92% of nearly 80,000 patients received high-dose IV steroids, reflecting standard practice at that time.) However, the GOLD guidelines do not address mortality rates, length of stay, or readmission rates for either approach, as they are devised to direct treatment in patients with stable mild to advanced COPD, not exacerbations.
THE EVIDENCE FOR LOW-DOSE STEROIDS
Mortality rates
Aksoy et al8 established that, compared with placebo, low-dose steroids improved mortality rates in a subset of patients with acute exacerbations, specifically those with eosinophilic exacerbations. This study followed the 2013 Reduction in the Use of Corticosteroids in Exacerbated COPD (REDUCE) trial, which showed mortality rates were not lower with 14 days of low-dose prednisone treatment than with 5 days.9
Length of hospital stay
With regard to length of hospital stay, in 2011 Wang et al10 found no statistically significant difference between high- and low-dose steroid treatment.However, the REDUCE trial found that low-dose steroids shortened the median length of stay by 1 day compared with placebo.9
Hospital readmission rates
The REDUCE trial found no statistically significant difference in readmission rates when comparing 5 days of low-dose treatment vs 14 days.9 However, Aksoy et al8 found that readmission rates were significantly lower with low-dose treatment than with placebo.No study has yet examined readmission rates with high-dose vs low-dose steroid treatment.
What does the evidence tell us?
Low-dose oral glucocorticoid treatment shows definitive benefits in terms of lower mortality rates, shorter hospital length of stay, and lower readmission rates vs placebo in the treatment of acute exacerbations of COPD. Furthermore, a 14-day course is no better than 5 days in terms of mortality rates. And low-dose glucocorticoid treatment shows reduced mortality rates in addition to similar hospital length of stay when compared to high-dose glucocorticoid treatment.
Together, these findings lend credibility to the current GOLD recommendations. However, we have observed that in sharp contrast to the leading clinical guidelines, most patients hospitalized for acute exacerbations of COPD are still treated initially with high-dose IV corticosteroids. Why?
Obstacles that perpetuate the use of high-dose over low-dose treatment include lack of knowledge of glucocorticoid pharmacokinetics among clinicians, use of outdated order sets, and the reflex notion that more of a drug is more efficacious in its desired effect. In addition, administrative obstacles include using high-dose IV steroids to justify an inpatient stay or continued hospitalization.
COUNTERING THE OBSTACLES: THE HOSPITALIST’S ROLE
To counter these obstacles, we propose standardization of inpatient treatment of acute exacerbations of COPD to include initial low-dose steroid treatment in accordance with the most recent GOLD guidelines.6 This would benefit the patient by reducing undesirable effects of high-dose steroids, and at the same time reduce the economic burden of managing COPD exacerbations. Considering the large number of hospitalizations for COPD exacerbation each year, hospitalists can play a large role in this effort by routinely incorporating the low-dose steroid recommendation into their clinical practice.
- World Health Organization. Chronic respiratory diseases: burden of COPD. www.who.int/respiratory/copd/burden/en. Accessed October 16, 2019.
- Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res 2013; 5:235–245. doi:10.2147/CEOR.S34321
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359(22):2355–2365. doi:10.1056/NEJMra0800353
- Walters JA, Tan DJ, White CJ, Gibson PG, Wood-Baker R, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; (9):CD001288. doi:10.1002/14651858.CD001288.pub4
- de Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, van den Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double-blind study. Chest 2007; 132(6):1741–1747. doi:10.1378/chest.07-0208
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2019 report. www.goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed October 16, 2019.
- Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA 2010; 303(23):2359–2367. doi:10.1001/jama.2010.796
- Aksoy E, Güngör S, Agca MÇ, et al. A revised treatment approach for hospitalized patients with eosinophilic and neutrophilic exacerbations of chronic obstructive pulmonary disease. Turk Thorac J 2018; 19(4):193–200. doi:10.5152/TurkThoracJ.2018.18004
- Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013; 309(21):2223–2231. doi:10.1001/jama.2013.5023
- Wang PH, Cheng SL, Wang HC, et al. Systemic steroids in acute exacerbation of COPD—from guidelines to bedside. Int J Clin Pharmacol Ther 2011; 49(11):705–708. doi:10.5414/cp201588
- World Health Organization. Chronic respiratory diseases: burden of COPD. www.who.int/respiratory/copd/burden/en. Accessed October 16, 2019.
- Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res 2013; 5:235–245. doi:10.2147/CEOR.S34321
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359(22):2355–2365. doi:10.1056/NEJMra0800353
- Walters JA, Tan DJ, White CJ, Gibson PG, Wood-Baker R, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; (9):CD001288. doi:10.1002/14651858.CD001288.pub4
- de Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, van den Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double-blind study. Chest 2007; 132(6):1741–1747. doi:10.1378/chest.07-0208
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2019 report. www.goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed October 16, 2019.
- Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA 2010; 303(23):2359–2367. doi:10.1001/jama.2010.796
- Aksoy E, Güngör S, Agca MÇ, et al. A revised treatment approach for hospitalized patients with eosinophilic and neutrophilic exacerbations of chronic obstructive pulmonary disease. Turk Thorac J 2018; 19(4):193–200. doi:10.5152/TurkThoracJ.2018.18004
- Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013; 309(21):2223–2231. doi:10.1001/jama.2013.5023
- Wang PH, Cheng SL, Wang HC, et al. Systemic steroids in acute exacerbation of COPD—from guidelines to bedside. Int J Clin Pharmacol Ther 2011; 49(11):705–708. doi:10.5414/cp201588
Correction: Diabetes management
Information was omitted from Table 1 on page 596 of the article, Makin V, Lansang MC. Diabetes management: beyond hemoglobin A1c (Cleve Clin J Med 2019; 86[9]:595–600, doi:10.3949/ccjm.86a.18031).
The sodium-glucose cotransporter 2 (SGLT2) inhibitors pose a low risk of hypoglyemia, and that should have been noted in the table. The corrected table appears below and online.
Information was omitted from Table 1 on page 596 of the article, Makin V, Lansang MC. Diabetes management: beyond hemoglobin A1c (Cleve Clin J Med 2019; 86[9]:595–600, doi:10.3949/ccjm.86a.18031).
The sodium-glucose cotransporter 2 (SGLT2) inhibitors pose a low risk of hypoglyemia, and that should have been noted in the table. The corrected table appears below and online.
Information was omitted from Table 1 on page 596 of the article, Makin V, Lansang MC. Diabetes management: beyond hemoglobin A1c (Cleve Clin J Med 2019; 86[9]:595–600, doi:10.3949/ccjm.86a.18031).
The sodium-glucose cotransporter 2 (SGLT2) inhibitors pose a low risk of hypoglyemia, and that should have been noted in the table. The corrected table appears below and online.
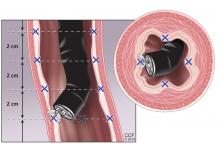

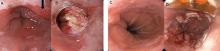
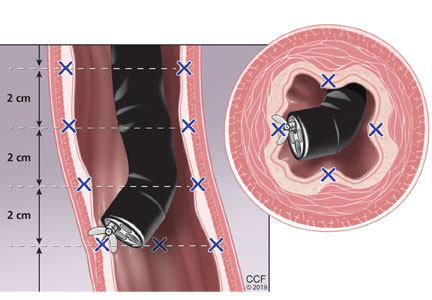







![Positive Western blot test (Borrelia B31 ViraStripe [Viramed Diagnostics]) in a patient who presented with rash and arthritis. This test uses purified specific antigens of strain B31 of Borrelia burgdorferi sensu stricto. Positive Western blot test (Borrelia B31 ViraStripe [Viramed Diagnostics]) in a patient who presented with rash and arthritis. This test uses purified specific antigens of strain B31 of Borrelia burgdorferi sensu stricto.](https://cdn.mdedge.com/files/s3fs-public/styles/medium/public/756fig2.jpg?itok=fMD7ruHJ)