User login
Did You Know? Psoriasis and psoriatic arthritis



Leadership & Professional Development: Ultra-Brief Teaching; It’s Now or Never
“The most valuable of all talents is that of never using two words when one will do.“
—Thomas Jefferson
Attendings, residents, and medical students identify education as a top purpose of team rounds.1 Learners report being dissatisfied with teaching on rounds most of the time.2 Time with learners is a finite resource that has become even more precious with increasing clinical demands and work hour restrictions.3 Attendings report insufficient time to teach on rounds, and often neglect teaching because of time constraints.4 What can we do to in the face of this conflict between time and teaching?
One approach to this problem is what we call “ultra-brief, deliberate teaching sessions.” These sessions, or UBDTs, led by clinicians, create dedicated time for teaching on service. UBDTs ideally occur before team rounds because, in our experience, this is when the team is most unified and focused. Our sessions are time-limited (5 minutes or less) and designed so they are applicable to clinical scenarios the team is actively facing. Other learners can also lead these sessions with faculty coaching. Sessions of germane size and scope include: (1) Focus on a single clinical question from the previous day; (2) Discuss Choosing Wisely® recommendations from a single specialty; (3) Provide a concise cognitive framework for a diagnostic or treatment dilemma (eg, draw a simple algorithm to evaluate causes of hyponatremia); (4) Review one image or electrocardiogram; (5) Present one case-based multiple-choice question; (6) Prime the team with a structured approach to a difficult conversation (eg, opioid discussions, goals of care).
As an example, if our team orders intravenous antihypertensives overnight, a UBDT session on asymptomatic hypertension would occur. The first minute may involve a discussion on the definition of hypertensive emergency versus asymptomatic hypertension. Next, we spend one minute asking learners the common causes of inpatient hypertension (eg, missed medications, pain, anxiety, withdrawal), highlighting that this warrants a bedside assessment. For two minutes, we next discuss the management options for asymptomatic hypertension with an emphasis on the avoidance of intravenous antihypertensives, tying this back to our current patient. Questions are welcomed, and a one-page summary of the major points and references is distributed during or after the talk. A repository of common topics and summaries may be a useful faculty development resource to be shared.
We have found UBDTs to be easy to implement for a variety of clinician educators. Because they are so brief and focused, they are also fun to create and share among teaching faculty. Importantly, these sessions should not delay clinical work. To ensure the avoidance of this trap, don’t select a topic that is too large or involves complex clinical reasoning, exceeds 5 minutes, or lead a UBDT session in a distracting environment or without preparation.
While we have not found a way to slow down time, UBDT sessions prior to the start of rounds can prioritize teaching, ensure the delivery of important content, and engage learners without significantly delaying clinical work. We invite you to try one!
Acknowledgments
The authors thank John Ragsdale, MD, MS for his leadership and support for UBDTs.
Disclosures
We have no relevant conflicts of interest to report. No payment or services from a third party were received for any aspect of this submitted work. We have no financial relationships with entities in the bio-medical arena that could be perceived to influence, or that give the appearance of potentially influencing, what was written in this submitted work.
1. Hulland O, Farnan J, Rabinowitz R, et al. What’s the Purpose of Rounds? A Qualitative Study Examining the Perception of Faculty and Students. J Hosp Med. 2017;12(11):892-897. https://doi.org/10.12788/jhm.2835
2. Merritt FW, Noble MN, Prochazka AV, et al. Attending rounds: What do the all-star teachers do? Med Teach. 2017;39(1):100-104. https://doi.org/10.1080/0142159X.2017.1248914
3. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. https://doi.org/10.1001/jamainternmed.2013.6041.
4. Crumlish CM, Yialamas MA, McMahon GT. Quantification of Bedside Teaching by an Academic Hospitalist Group. J Hosp Med. 2009;4(5);304-307. https://doi.org/10.1002/jhm.540
“The most valuable of all talents is that of never using two words when one will do.“
—Thomas Jefferson
Attendings, residents, and medical students identify education as a top purpose of team rounds.1 Learners report being dissatisfied with teaching on rounds most of the time.2 Time with learners is a finite resource that has become even more precious with increasing clinical demands and work hour restrictions.3 Attendings report insufficient time to teach on rounds, and often neglect teaching because of time constraints.4 What can we do to in the face of this conflict between time and teaching?
One approach to this problem is what we call “ultra-brief, deliberate teaching sessions.” These sessions, or UBDTs, led by clinicians, create dedicated time for teaching on service. UBDTs ideally occur before team rounds because, in our experience, this is when the team is most unified and focused. Our sessions are time-limited (5 minutes or less) and designed so they are applicable to clinical scenarios the team is actively facing. Other learners can also lead these sessions with faculty coaching. Sessions of germane size and scope include: (1) Focus on a single clinical question from the previous day; (2) Discuss Choosing Wisely® recommendations from a single specialty; (3) Provide a concise cognitive framework for a diagnostic or treatment dilemma (eg, draw a simple algorithm to evaluate causes of hyponatremia); (4) Review one image or electrocardiogram; (5) Present one case-based multiple-choice question; (6) Prime the team with a structured approach to a difficult conversation (eg, opioid discussions, goals of care).
As an example, if our team orders intravenous antihypertensives overnight, a UBDT session on asymptomatic hypertension would occur. The first minute may involve a discussion on the definition of hypertensive emergency versus asymptomatic hypertension. Next, we spend one minute asking learners the common causes of inpatient hypertension (eg, missed medications, pain, anxiety, withdrawal), highlighting that this warrants a bedside assessment. For two minutes, we next discuss the management options for asymptomatic hypertension with an emphasis on the avoidance of intravenous antihypertensives, tying this back to our current patient. Questions are welcomed, and a one-page summary of the major points and references is distributed during or after the talk. A repository of common topics and summaries may be a useful faculty development resource to be shared.
We have found UBDTs to be easy to implement for a variety of clinician educators. Because they are so brief and focused, they are also fun to create and share among teaching faculty. Importantly, these sessions should not delay clinical work. To ensure the avoidance of this trap, don’t select a topic that is too large or involves complex clinical reasoning, exceeds 5 minutes, or lead a UBDT session in a distracting environment or without preparation.
While we have not found a way to slow down time, UBDT sessions prior to the start of rounds can prioritize teaching, ensure the delivery of important content, and engage learners without significantly delaying clinical work. We invite you to try one!
Acknowledgments
The authors thank John Ragsdale, MD, MS for his leadership and support for UBDTs.
Disclosures
We have no relevant conflicts of interest to report. No payment or services from a third party were received for any aspect of this submitted work. We have no financial relationships with entities in the bio-medical arena that could be perceived to influence, or that give the appearance of potentially influencing, what was written in this submitted work.
“The most valuable of all talents is that of never using two words when one will do.“
—Thomas Jefferson
Attendings, residents, and medical students identify education as a top purpose of team rounds.1 Learners report being dissatisfied with teaching on rounds most of the time.2 Time with learners is a finite resource that has become even more precious with increasing clinical demands and work hour restrictions.3 Attendings report insufficient time to teach on rounds, and often neglect teaching because of time constraints.4 What can we do to in the face of this conflict between time and teaching?
One approach to this problem is what we call “ultra-brief, deliberate teaching sessions.” These sessions, or UBDTs, led by clinicians, create dedicated time for teaching on service. UBDTs ideally occur before team rounds because, in our experience, this is when the team is most unified and focused. Our sessions are time-limited (5 minutes or less) and designed so they are applicable to clinical scenarios the team is actively facing. Other learners can also lead these sessions with faculty coaching. Sessions of germane size and scope include: (1) Focus on a single clinical question from the previous day; (2) Discuss Choosing Wisely® recommendations from a single specialty; (3) Provide a concise cognitive framework for a diagnostic or treatment dilemma (eg, draw a simple algorithm to evaluate causes of hyponatremia); (4) Review one image or electrocardiogram; (5) Present one case-based multiple-choice question; (6) Prime the team with a structured approach to a difficult conversation (eg, opioid discussions, goals of care).
As an example, if our team orders intravenous antihypertensives overnight, a UBDT session on asymptomatic hypertension would occur. The first minute may involve a discussion on the definition of hypertensive emergency versus asymptomatic hypertension. Next, we spend one minute asking learners the common causes of inpatient hypertension (eg, missed medications, pain, anxiety, withdrawal), highlighting that this warrants a bedside assessment. For two minutes, we next discuss the management options for asymptomatic hypertension with an emphasis on the avoidance of intravenous antihypertensives, tying this back to our current patient. Questions are welcomed, and a one-page summary of the major points and references is distributed during or after the talk. A repository of common topics and summaries may be a useful faculty development resource to be shared.
We have found UBDTs to be easy to implement for a variety of clinician educators. Because they are so brief and focused, they are also fun to create and share among teaching faculty. Importantly, these sessions should not delay clinical work. To ensure the avoidance of this trap, don’t select a topic that is too large or involves complex clinical reasoning, exceeds 5 minutes, or lead a UBDT session in a distracting environment or without preparation.
While we have not found a way to slow down time, UBDT sessions prior to the start of rounds can prioritize teaching, ensure the delivery of important content, and engage learners without significantly delaying clinical work. We invite you to try one!
Acknowledgments
The authors thank John Ragsdale, MD, MS for his leadership and support for UBDTs.
Disclosures
We have no relevant conflicts of interest to report. No payment or services from a third party were received for any aspect of this submitted work. We have no financial relationships with entities in the bio-medical arena that could be perceived to influence, or that give the appearance of potentially influencing, what was written in this submitted work.
1. Hulland O, Farnan J, Rabinowitz R, et al. What’s the Purpose of Rounds? A Qualitative Study Examining the Perception of Faculty and Students. J Hosp Med. 2017;12(11):892-897. https://doi.org/10.12788/jhm.2835
2. Merritt FW, Noble MN, Prochazka AV, et al. Attending rounds: What do the all-star teachers do? Med Teach. 2017;39(1):100-104. https://doi.org/10.1080/0142159X.2017.1248914
3. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. https://doi.org/10.1001/jamainternmed.2013.6041.
4. Crumlish CM, Yialamas MA, McMahon GT. Quantification of Bedside Teaching by an Academic Hospitalist Group. J Hosp Med. 2009;4(5);304-307. https://doi.org/10.1002/jhm.540
1. Hulland O, Farnan J, Rabinowitz R, et al. What’s the Purpose of Rounds? A Qualitative Study Examining the Perception of Faculty and Students. J Hosp Med. 2017;12(11):892-897. https://doi.org/10.12788/jhm.2835
2. Merritt FW, Noble MN, Prochazka AV, et al. Attending rounds: What do the all-star teachers do? Med Teach. 2017;39(1):100-104. https://doi.org/10.1080/0142159X.2017.1248914
3. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. https://doi.org/10.1001/jamainternmed.2013.6041.
4. Crumlish CM, Yialamas MA, McMahon GT. Quantification of Bedside Teaching by an Academic Hospitalist Group. J Hosp Med. 2009;4(5);304-307. https://doi.org/10.1002/jhm.540
©2019 Society of Hospital Medicine
Open Clinical Trials for Diabetes Mellitus Harm Reduction (FULL)
Providing access to clinical trials for native American, veteran, and active-duty military patients can be a challenge, but a significant number of trials are now recruiting from those populations. Many trials explicitly recruit patients from the US Department of Veterans
Affairs (VA), the military, and Indian Health Service. The VA Office of Research and Development alone sponsors more than 480 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of October 24, 2018; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on preventing diabetes mellitus or improving patient care. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials. gov.
Diabetes Prevention Program Outcomes Study (DPPOS)
The Diabetes Prevention Program (DPP) was a multicenter trial examining the ability of an intensive lifestyle or metformin to prevent or delay the development of diabetes in a high risk population due to the presence of impaired glucose tolerance (IGT). The DPP has ended early demonstrating that lifestyle reduced diabetes onset by 58% and metformin reduced diabetes onset by 31%.
ID: NCT00038727
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases
Location: George Washington University, Rockville, Maryland
Efforts to Improve Diabetes Control
The primary objectives of this study are: (1) test the longterm effectiveness of a peer mentor model on improving glucose control, blood pressure, LDL levels, diabetes mellitus quality of life, and depression scores in a mixed race population of poorly controlled diabetic veterans; (2) test the effectiveness of using former peer mentees as peer mentors as a means of creating a self-sustaining program; and (3) test the effects of becoming a mentor on those who were originally mentees given a growing literature that being a mentor is good for your health. Secondary objectives include: (1) in those randomized to being a mentee, explore mentor characteristics associated with improved HbA1c.
ID: NCT01651117
Sponsor: VA Office of Research and Development
Location: Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania
A Patient-Centered Strategy for Improving Diabetes Prevention in Urban American Indians
The goal of the proposed research is to identify effective patient-centered strategies to prevent diabetes in high-risk populations in real world settings. The investigators will accomplish this by conducting a randomized controlled trial comparing an enhanced Diabetes Prevention Program addressing psychosocial stressors to a standard version in a high-risk population of urban American Indian
and Alaskan Native peoples within a primary care setting.
ID: NCT02266576
Sponsor: Stanford University
Locations: Timpany Center of San Jose State University, California; Stanford University School of Medicine, California
Physical Activity and Participation
Physical activity is the cornerstone of good diabetes management, and yet effective physical activity intervention is not available. The investigators developed a lifestyle intervention based on individual’s home activity patterns. The goal of the study is to test the efficacy of this intervention among veterans with diabetes in a randomized-controlled trial. In addition to physical activity, the investigators will also assess if the intervention will improve social participation among veterans.
ID: NCT02268916
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Caring Others Increasing EngageMent in PACT (CO-IMPACT)
This trial will compare two methods of increasing engagement in care and success in diabetes management, among patients with diabetes with high-risk features, who also have family members involved in their care.
ID: NCT02328326
Sponsor: VA Office of Research and Development
Locations: VA Ann Arbor Healthcare System, Michigan;VA Pittsburgh Healthcare System, Pennsylvania
STEP UP to Avert Amputation in Diabetes (STEP UP)
This study will evaluate a comprehensive tailored behavioral intervention aimed to improve foot self-care and self-monitoring (combined with dermal thermometry) to prevent recurrent ulcers in Veterans at highest risk of amputation. This intervention may be a novel strategy for improving self-care and early detection of foot abnormalities in this at-risk population using psychological theories to target multiple health behaviors simultaneously. This could be an efficient and cost-effective approach to improve diabetes-related foot health behavior, and other risk factors in patients who are vulnerable to devastating consequences related to amputation.
ID: NCT02356848
Sponsor: VA Office of Research and Development
Location: Manhattan Campus of the VA NY Harbor Healthcare System
Physical Activity Behavior Change for Older Adults After Dysvascular Amputation (PABC)
This pilot study will use mobile-health technology to deliver an intervention designed for lasting physical activity behavior change. The study will assess the feasibility of using the Physical Activity Behavior Change (PABC) intervention for Veterans with lower limb amputation. This intervention will be delivered using wrist-worn wearable activity sensors and a home-based tablet computer to allow real-time physical activity feedback and video interface between the participants and the therapist.
ID: NCT02738086
Sponsor: VA Office of Research and Development
Location: Rocky Mountain Regional VA Medical Center, Aurora, Colorado
ForgIng New Paths to Prevent DIabeTes (FINDIT)
This study will evaluate the effects of screening for type 2 diabetes mellitus (T2DM) and brief counseling about screening test results on weight and key health behaviors among veterans with risk factors for T2DM. Study participants will be randomly assigned to 1 of 2 study groups: (1) Blood Test Group; or (2) Brochure Group. Participants in the Blood Test Group will complete a blood test called hemoglobin A1c (HbA1c) which measures average blood sugar levels. Participants will receive brief counseling about the results from their primary care provider or someone authorized to speak on their behalf. Participants randomly selected for the Brochure Group will review a handout from the VA National Center for Health Promotion and Disease Prevention (NCP) on recommended screening tests and immunizations. All participants will be asked to complete a survey prior to study group assignment, immediately after a Primary Care appointment, 3 months after enrollment, and 12 months after enrollment.
ID: NCT02747108
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Using Technology to Share Fitness Goals and Results to Improve Diabetes Outcomes
The investigators will recruit DoD beneficiaries, aged 18 years or older and diagnosed with type 2 diabetes. Patients will be randomized into one of two groups. Group 1 will use a fitness tracker but will not be able to see other participants data and group 2 will use a fitness tracker and will be able to see other members daily and weekly results. Outcome measures will be assessed at baseline, 3 months and 6 months to include hemoglobin A1c, weight, body mass index, blood pressure, and number of hours and days fitness tracker is used. The goal is to see if the group randomized into an online community will have improved activity and outcome measurements compared with those who use the pedometer alone.
ID: NCT02761018
Sponsor: Mike O’Callaghan Military Hospital
Location: Mike O’Callaghan Federal Medical Center, Nellis Air Force Base, Nevada
Healthy Living Partnerships to Prevent Diabetes in Veterans Pilot Study (HELP Vets)
Diabetes and obesity are both major public health concerns and the prevalence of diabetes is even higher in the patient population of the VA. This planning project is designed to adapt a successful weight-loss program for delivery through an existing outpatient clinic to reach local veterans at risk for developing diabetes. The information gathered as a part of this project will be used to plan a larger trial designed to improve the health of veterans by offering them a diabetes prevention program through their usual source of healthcare.
ID: NCT02835495
Sponsor: Wake Forest University Health Sciences
Location: Wake Forest School of Medicine
Mindful Stress Reduction in Diabetes Self-Management Education for Veterans (MindSTRIDE)
The purpose of this study is to see if adding Mindfulness training to diabetes education reduces feelings of stress and makes it easier to adhere to healthy behaviors that improve diabetes outcomes (such as hemoglobin A1c).
ID: NCT02928952
Sponsor: VA Office of Research and Development
Location: VA Pittsburgh Healthcare System University Drive Division, Pittsburgh, Pennsylvania
Improving Diabetes Care Through Effective Personalized Patient Portal Interactions
Patient-facing eHealth technologies are those that connect patients and the healthcare system, and include online patient portals. Although many organizations are adopting patient portals, there is limited understanding of how the different portal features help improve health outcomes. This study is designed to develop and test an intervention to improve adoption and use of patient portal features for diabetes management.
ID: NCT02953262
Sponsor: VA Office of Research and Development
Locations: Edith Nourse Rogers Memorial Veterans Hospital, Bedford, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus, Massachusetts.
Home-Based Kidney Care in Native American’s of New Mexico (HBKC)
People reach end stage renal disease (ESRD) due to progressive chronic kidney disease (CKD), which is associated with increased risk for heart disease and death. The burden of chronic kidney disease is increased among minority populations compared to Caucasians. New Mexico American Indians are experiencing an epidemic of chronic kidney disease due primarily to the high rates of obesity and diabetes. The present study entitled Home-Based Kidney Care is designed to delay / reduce rates of ESRD by early interventions in CKD. Investigators propose to assess the safety and efficacy of conducting a full-scale study to determine if home based care delivered
by a collaborative team composed of community health workers, the Albuquerque Area Indian Health Board and University of New Mexico faculty will decrease the risk for the development and the progression of CKD.
ID: NCT03179085
Sponsor: University of New Mexico
Location: University of New Mexico, Albuquerque
INcreasing Veteran EngagemeNT to Prevent Diabetes (INVENT)
This study will evaluate a VA MyHealtheVet Secure Messaging intervention that uses different intervention messaging strategies designed to increase engagement in behaviors to prevent type 2 diabetes (T2DM). After completing a baseline survey, participants will be randomly assigned to receive different novel presentations of information about ways to prevent T2DM through both secure messaging and US mail. The investigators will test the 5 presentations that each: (1) represent an innovative approach from behavioral economics or health psychology with great promise to increase engagement in behaviors to prevent T2DM among patients with prediabetes; and (2) have not been tested in this setting.
ID: NCT03403231
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Self-efficacy, Beliefs and Adherence—Pilot and Feasibility Trial of a Pharmacist-led Intervention
This study uses an intervention mixed methods design. The overall purpose is to improve medication adherence and assess the clinical impact on diabetes outcomes among patients with uncontrolled diabetes. We will examine if usual care combined with a clinic-based health literacy/psychosocial support intervention improves medication adherence compared to usual care alone. A randomized controlled trial will be conducted at William S. Middleton Memorial Veterans Hospital in Madison, targeting individuals with
uncontrolled diabetes. The patient-centered health literacy intervention will focus on enhancing patients’ self-efficacy and addressing patients’ negative beliefs in medicine and illness.
ID: NCT03406923
Sponsor: University of Wisconsin, Madison
Location: William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin
Practical Telemedicine to Improve Control and Engagement for Veterans With Clinic-Refractory Diabetes Mellitus (PRACTICE-DM)
Diabetes generates significant morbidity, mortality, and costs within the Veterans Health Administration (VHA). Veterans with persistently poor diabetes control despite clinic-based care are among the highest-risk diabetes patients in VHA, and contribute disproportionately to VHA’s massive burden of diabetes complications and costs. VHA critically needs effective, practical management alternatives for veterans whose diabetes does not respond to clinic-based management. The proposed study will address this need by leveraging VHA’s unique Home Telehealth capacity to deliver comprehensive telemedicine-based management for veterans with persistently poor diabetes control despite clinic-based care. Because this intensive intervention is delivered using only existing Home Telehealth workforce, infrastructure, and technical resources—which are ubiquitous at VHA centers nationwide—it could represent an effective, practical approach to improving outcomes in veterans with PPDM, potentially translating to a substantial reduction in VHA’s diabetes burden.
ID: NCT03520413
Sponsor: VA Office of Research and Development
Locations: Durham VA Medical Center, North Carolina; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
Cooking for Health
Type 2 diabetes is a leading cause of morbidity and mortality among American Indians in the US. Although healthy diet is a key component of diabetes management programs, many American Indians face contextual barriers to adopting a healthy diet including: difficulty budgeting for food on low-incomes, low literacy and numeracy when purchasing food, and limited cooking skills. The proposed project will develop, implement, and evaluate a culturally-targeted healthy foods budgeting, purchasing, and cooking skills intervention aimed at improving the cardio-metabolic health of American Indians with type 2 diabetes who live in rural areas.
ID: NCT03699709
Sponsor: University of Washington
Location: Missouri Breaks Industries Research, Eagle Butte, South Dakota
Providing access to clinical trials for native American, veteran, and active-duty military patients can be a challenge, but a significant number of trials are now recruiting from those populations. Many trials explicitly recruit patients from the US Department of Veterans
Affairs (VA), the military, and Indian Health Service. The VA Office of Research and Development alone sponsors more than 480 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of October 24, 2018; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on preventing diabetes mellitus or improving patient care. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials. gov.
Diabetes Prevention Program Outcomes Study (DPPOS)
The Diabetes Prevention Program (DPP) was a multicenter trial examining the ability of an intensive lifestyle or metformin to prevent or delay the development of diabetes in a high risk population due to the presence of impaired glucose tolerance (IGT). The DPP has ended early demonstrating that lifestyle reduced diabetes onset by 58% and metformin reduced diabetes onset by 31%.
ID: NCT00038727
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases
Location: George Washington University, Rockville, Maryland
Efforts to Improve Diabetes Control
The primary objectives of this study are: (1) test the longterm effectiveness of a peer mentor model on improving glucose control, blood pressure, LDL levels, diabetes mellitus quality of life, and depression scores in a mixed race population of poorly controlled diabetic veterans; (2) test the effectiveness of using former peer mentees as peer mentors as a means of creating a self-sustaining program; and (3) test the effects of becoming a mentor on those who were originally mentees given a growing literature that being a mentor is good for your health. Secondary objectives include: (1) in those randomized to being a mentee, explore mentor characteristics associated with improved HbA1c.
ID: NCT01651117
Sponsor: VA Office of Research and Development
Location: Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania
A Patient-Centered Strategy for Improving Diabetes Prevention in Urban American Indians
The goal of the proposed research is to identify effective patient-centered strategies to prevent diabetes in high-risk populations in real world settings. The investigators will accomplish this by conducting a randomized controlled trial comparing an enhanced Diabetes Prevention Program addressing psychosocial stressors to a standard version in a high-risk population of urban American Indian
and Alaskan Native peoples within a primary care setting.
ID: NCT02266576
Sponsor: Stanford University
Locations: Timpany Center of San Jose State University, California; Stanford University School of Medicine, California
Physical Activity and Participation
Physical activity is the cornerstone of good diabetes management, and yet effective physical activity intervention is not available. The investigators developed a lifestyle intervention based on individual’s home activity patterns. The goal of the study is to test the efficacy of this intervention among veterans with diabetes in a randomized-controlled trial. In addition to physical activity, the investigators will also assess if the intervention will improve social participation among veterans.
ID: NCT02268916
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Caring Others Increasing EngageMent in PACT (CO-IMPACT)
This trial will compare two methods of increasing engagement in care and success in diabetes management, among patients with diabetes with high-risk features, who also have family members involved in their care.
ID: NCT02328326
Sponsor: VA Office of Research and Development
Locations: VA Ann Arbor Healthcare System, Michigan;VA Pittsburgh Healthcare System, Pennsylvania
STEP UP to Avert Amputation in Diabetes (STEP UP)
This study will evaluate a comprehensive tailored behavioral intervention aimed to improve foot self-care and self-monitoring (combined with dermal thermometry) to prevent recurrent ulcers in Veterans at highest risk of amputation. This intervention may be a novel strategy for improving self-care and early detection of foot abnormalities in this at-risk population using psychological theories to target multiple health behaviors simultaneously. This could be an efficient and cost-effective approach to improve diabetes-related foot health behavior, and other risk factors in patients who are vulnerable to devastating consequences related to amputation.
ID: NCT02356848
Sponsor: VA Office of Research and Development
Location: Manhattan Campus of the VA NY Harbor Healthcare System
Physical Activity Behavior Change for Older Adults After Dysvascular Amputation (PABC)
This pilot study will use mobile-health technology to deliver an intervention designed for lasting physical activity behavior change. The study will assess the feasibility of using the Physical Activity Behavior Change (PABC) intervention for Veterans with lower limb amputation. This intervention will be delivered using wrist-worn wearable activity sensors and a home-based tablet computer to allow real-time physical activity feedback and video interface between the participants and the therapist.
ID: NCT02738086
Sponsor: VA Office of Research and Development
Location: Rocky Mountain Regional VA Medical Center, Aurora, Colorado
ForgIng New Paths to Prevent DIabeTes (FINDIT)
This study will evaluate the effects of screening for type 2 diabetes mellitus (T2DM) and brief counseling about screening test results on weight and key health behaviors among veterans with risk factors for T2DM. Study participants will be randomly assigned to 1 of 2 study groups: (1) Blood Test Group; or (2) Brochure Group. Participants in the Blood Test Group will complete a blood test called hemoglobin A1c (HbA1c) which measures average blood sugar levels. Participants will receive brief counseling about the results from their primary care provider or someone authorized to speak on their behalf. Participants randomly selected for the Brochure Group will review a handout from the VA National Center for Health Promotion and Disease Prevention (NCP) on recommended screening tests and immunizations. All participants will be asked to complete a survey prior to study group assignment, immediately after a Primary Care appointment, 3 months after enrollment, and 12 months after enrollment.
ID: NCT02747108
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Using Technology to Share Fitness Goals and Results to Improve Diabetes Outcomes
The investigators will recruit DoD beneficiaries, aged 18 years or older and diagnosed with type 2 diabetes. Patients will be randomized into one of two groups. Group 1 will use a fitness tracker but will not be able to see other participants data and group 2 will use a fitness tracker and will be able to see other members daily and weekly results. Outcome measures will be assessed at baseline, 3 months and 6 months to include hemoglobin A1c, weight, body mass index, blood pressure, and number of hours and days fitness tracker is used. The goal is to see if the group randomized into an online community will have improved activity and outcome measurements compared with those who use the pedometer alone.
ID: NCT02761018
Sponsor: Mike O’Callaghan Military Hospital
Location: Mike O’Callaghan Federal Medical Center, Nellis Air Force Base, Nevada
Healthy Living Partnerships to Prevent Diabetes in Veterans Pilot Study (HELP Vets)
Diabetes and obesity are both major public health concerns and the prevalence of diabetes is even higher in the patient population of the VA. This planning project is designed to adapt a successful weight-loss program for delivery through an existing outpatient clinic to reach local veterans at risk for developing diabetes. The information gathered as a part of this project will be used to plan a larger trial designed to improve the health of veterans by offering them a diabetes prevention program through their usual source of healthcare.
ID: NCT02835495
Sponsor: Wake Forest University Health Sciences
Location: Wake Forest School of Medicine
Mindful Stress Reduction in Diabetes Self-Management Education for Veterans (MindSTRIDE)
The purpose of this study is to see if adding Mindfulness training to diabetes education reduces feelings of stress and makes it easier to adhere to healthy behaviors that improve diabetes outcomes (such as hemoglobin A1c).
ID: NCT02928952
Sponsor: VA Office of Research and Development
Location: VA Pittsburgh Healthcare System University Drive Division, Pittsburgh, Pennsylvania
Improving Diabetes Care Through Effective Personalized Patient Portal Interactions
Patient-facing eHealth technologies are those that connect patients and the healthcare system, and include online patient portals. Although many organizations are adopting patient portals, there is limited understanding of how the different portal features help improve health outcomes. This study is designed to develop and test an intervention to improve adoption and use of patient portal features for diabetes management.
ID: NCT02953262
Sponsor: VA Office of Research and Development
Locations: Edith Nourse Rogers Memorial Veterans Hospital, Bedford, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus, Massachusetts.
Home-Based Kidney Care in Native American’s of New Mexico (HBKC)
People reach end stage renal disease (ESRD) due to progressive chronic kidney disease (CKD), which is associated with increased risk for heart disease and death. The burden of chronic kidney disease is increased among minority populations compared to Caucasians. New Mexico American Indians are experiencing an epidemic of chronic kidney disease due primarily to the high rates of obesity and diabetes. The present study entitled Home-Based Kidney Care is designed to delay / reduce rates of ESRD by early interventions in CKD. Investigators propose to assess the safety and efficacy of conducting a full-scale study to determine if home based care delivered
by a collaborative team composed of community health workers, the Albuquerque Area Indian Health Board and University of New Mexico faculty will decrease the risk for the development and the progression of CKD.
ID: NCT03179085
Sponsor: University of New Mexico
Location: University of New Mexico, Albuquerque
INcreasing Veteran EngagemeNT to Prevent Diabetes (INVENT)
This study will evaluate a VA MyHealtheVet Secure Messaging intervention that uses different intervention messaging strategies designed to increase engagement in behaviors to prevent type 2 diabetes (T2DM). After completing a baseline survey, participants will be randomly assigned to receive different novel presentations of information about ways to prevent T2DM through both secure messaging and US mail. The investigators will test the 5 presentations that each: (1) represent an innovative approach from behavioral economics or health psychology with great promise to increase engagement in behaviors to prevent T2DM among patients with prediabetes; and (2) have not been tested in this setting.
ID: NCT03403231
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Self-efficacy, Beliefs and Adherence—Pilot and Feasibility Trial of a Pharmacist-led Intervention
This study uses an intervention mixed methods design. The overall purpose is to improve medication adherence and assess the clinical impact on diabetes outcomes among patients with uncontrolled diabetes. We will examine if usual care combined with a clinic-based health literacy/psychosocial support intervention improves medication adherence compared to usual care alone. A randomized controlled trial will be conducted at William S. Middleton Memorial Veterans Hospital in Madison, targeting individuals with
uncontrolled diabetes. The patient-centered health literacy intervention will focus on enhancing patients’ self-efficacy and addressing patients’ negative beliefs in medicine and illness.
ID: NCT03406923
Sponsor: University of Wisconsin, Madison
Location: William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin
Practical Telemedicine to Improve Control and Engagement for Veterans With Clinic-Refractory Diabetes Mellitus (PRACTICE-DM)
Diabetes generates significant morbidity, mortality, and costs within the Veterans Health Administration (VHA). Veterans with persistently poor diabetes control despite clinic-based care are among the highest-risk diabetes patients in VHA, and contribute disproportionately to VHA’s massive burden of diabetes complications and costs. VHA critically needs effective, practical management alternatives for veterans whose diabetes does not respond to clinic-based management. The proposed study will address this need by leveraging VHA’s unique Home Telehealth capacity to deliver comprehensive telemedicine-based management for veterans with persistently poor diabetes control despite clinic-based care. Because this intensive intervention is delivered using only existing Home Telehealth workforce, infrastructure, and technical resources—which are ubiquitous at VHA centers nationwide—it could represent an effective, practical approach to improving outcomes in veterans with PPDM, potentially translating to a substantial reduction in VHA’s diabetes burden.
ID: NCT03520413
Sponsor: VA Office of Research and Development
Locations: Durham VA Medical Center, North Carolina; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
Cooking for Health
Type 2 diabetes is a leading cause of morbidity and mortality among American Indians in the US. Although healthy diet is a key component of diabetes management programs, many American Indians face contextual barriers to adopting a healthy diet including: difficulty budgeting for food on low-incomes, low literacy and numeracy when purchasing food, and limited cooking skills. The proposed project will develop, implement, and evaluate a culturally-targeted healthy foods budgeting, purchasing, and cooking skills intervention aimed at improving the cardio-metabolic health of American Indians with type 2 diabetes who live in rural areas.
ID: NCT03699709
Sponsor: University of Washington
Location: Missouri Breaks Industries Research, Eagle Butte, South Dakota
Providing access to clinical trials for native American, veteran, and active-duty military patients can be a challenge, but a significant number of trials are now recruiting from those populations. Many trials explicitly recruit patients from the US Department of Veterans
Affairs (VA), the military, and Indian Health Service. The VA Office of Research and Development alone sponsors more than 480 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of October 24, 2018; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on preventing diabetes mellitus or improving patient care. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials. gov.
Diabetes Prevention Program Outcomes Study (DPPOS)
The Diabetes Prevention Program (DPP) was a multicenter trial examining the ability of an intensive lifestyle or metformin to prevent or delay the development of diabetes in a high risk population due to the presence of impaired glucose tolerance (IGT). The DPP has ended early demonstrating that lifestyle reduced diabetes onset by 58% and metformin reduced diabetes onset by 31%.
ID: NCT00038727
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases
Location: George Washington University, Rockville, Maryland
Efforts to Improve Diabetes Control
The primary objectives of this study are: (1) test the longterm effectiveness of a peer mentor model on improving glucose control, blood pressure, LDL levels, diabetes mellitus quality of life, and depression scores in a mixed race population of poorly controlled diabetic veterans; (2) test the effectiveness of using former peer mentees as peer mentors as a means of creating a self-sustaining program; and (3) test the effects of becoming a mentor on those who were originally mentees given a growing literature that being a mentor is good for your health. Secondary objectives include: (1) in those randomized to being a mentee, explore mentor characteristics associated with improved HbA1c.
ID: NCT01651117
Sponsor: VA Office of Research and Development
Location: Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania
A Patient-Centered Strategy for Improving Diabetes Prevention in Urban American Indians
The goal of the proposed research is to identify effective patient-centered strategies to prevent diabetes in high-risk populations in real world settings. The investigators will accomplish this by conducting a randomized controlled trial comparing an enhanced Diabetes Prevention Program addressing psychosocial stressors to a standard version in a high-risk population of urban American Indian
and Alaskan Native peoples within a primary care setting.
ID: NCT02266576
Sponsor: Stanford University
Locations: Timpany Center of San Jose State University, California; Stanford University School of Medicine, California
Physical Activity and Participation
Physical activity is the cornerstone of good diabetes management, and yet effective physical activity intervention is not available. The investigators developed a lifestyle intervention based on individual’s home activity patterns. The goal of the study is to test the efficacy of this intervention among veterans with diabetes in a randomized-controlled trial. In addition to physical activity, the investigators will also assess if the intervention will improve social participation among veterans.
ID: NCT02268916
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Caring Others Increasing EngageMent in PACT (CO-IMPACT)
This trial will compare two methods of increasing engagement in care and success in diabetes management, among patients with diabetes with high-risk features, who also have family members involved in their care.
ID: NCT02328326
Sponsor: VA Office of Research and Development
Locations: VA Ann Arbor Healthcare System, Michigan;VA Pittsburgh Healthcare System, Pennsylvania
STEP UP to Avert Amputation in Diabetes (STEP UP)
This study will evaluate a comprehensive tailored behavioral intervention aimed to improve foot self-care and self-monitoring (combined with dermal thermometry) to prevent recurrent ulcers in Veterans at highest risk of amputation. This intervention may be a novel strategy for improving self-care and early detection of foot abnormalities in this at-risk population using psychological theories to target multiple health behaviors simultaneously. This could be an efficient and cost-effective approach to improve diabetes-related foot health behavior, and other risk factors in patients who are vulnerable to devastating consequences related to amputation.
ID: NCT02356848
Sponsor: VA Office of Research and Development
Location: Manhattan Campus of the VA NY Harbor Healthcare System
Physical Activity Behavior Change for Older Adults After Dysvascular Amputation (PABC)
This pilot study will use mobile-health technology to deliver an intervention designed for lasting physical activity behavior change. The study will assess the feasibility of using the Physical Activity Behavior Change (PABC) intervention for Veterans with lower limb amputation. This intervention will be delivered using wrist-worn wearable activity sensors and a home-based tablet computer to allow real-time physical activity feedback and video interface between the participants and the therapist.
ID: NCT02738086
Sponsor: VA Office of Research and Development
Location: Rocky Mountain Regional VA Medical Center, Aurora, Colorado
ForgIng New Paths to Prevent DIabeTes (FINDIT)
This study will evaluate the effects of screening for type 2 diabetes mellitus (T2DM) and brief counseling about screening test results on weight and key health behaviors among veterans with risk factors for T2DM. Study participants will be randomly assigned to 1 of 2 study groups: (1) Blood Test Group; or (2) Brochure Group. Participants in the Blood Test Group will complete a blood test called hemoglobin A1c (HbA1c) which measures average blood sugar levels. Participants will receive brief counseling about the results from their primary care provider or someone authorized to speak on their behalf. Participants randomly selected for the Brochure Group will review a handout from the VA National Center for Health Promotion and Disease Prevention (NCP) on recommended screening tests and immunizations. All participants will be asked to complete a survey prior to study group assignment, immediately after a Primary Care appointment, 3 months after enrollment, and 12 months after enrollment.
ID: NCT02747108
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Using Technology to Share Fitness Goals and Results to Improve Diabetes Outcomes
The investigators will recruit DoD beneficiaries, aged 18 years or older and diagnosed with type 2 diabetes. Patients will be randomized into one of two groups. Group 1 will use a fitness tracker but will not be able to see other participants data and group 2 will use a fitness tracker and will be able to see other members daily and weekly results. Outcome measures will be assessed at baseline, 3 months and 6 months to include hemoglobin A1c, weight, body mass index, blood pressure, and number of hours and days fitness tracker is used. The goal is to see if the group randomized into an online community will have improved activity and outcome measurements compared with those who use the pedometer alone.
ID: NCT02761018
Sponsor: Mike O’Callaghan Military Hospital
Location: Mike O’Callaghan Federal Medical Center, Nellis Air Force Base, Nevada
Healthy Living Partnerships to Prevent Diabetes in Veterans Pilot Study (HELP Vets)
Diabetes and obesity are both major public health concerns and the prevalence of diabetes is even higher in the patient population of the VA. This planning project is designed to adapt a successful weight-loss program for delivery through an existing outpatient clinic to reach local veterans at risk for developing diabetes. The information gathered as a part of this project will be used to plan a larger trial designed to improve the health of veterans by offering them a diabetes prevention program through their usual source of healthcare.
ID: NCT02835495
Sponsor: Wake Forest University Health Sciences
Location: Wake Forest School of Medicine
Mindful Stress Reduction in Diabetes Self-Management Education for Veterans (MindSTRIDE)
The purpose of this study is to see if adding Mindfulness training to diabetes education reduces feelings of stress and makes it easier to adhere to healthy behaviors that improve diabetes outcomes (such as hemoglobin A1c).
ID: NCT02928952
Sponsor: VA Office of Research and Development
Location: VA Pittsburgh Healthcare System University Drive Division, Pittsburgh, Pennsylvania
Improving Diabetes Care Through Effective Personalized Patient Portal Interactions
Patient-facing eHealth technologies are those that connect patients and the healthcare system, and include online patient portals. Although many organizations are adopting patient portals, there is limited understanding of how the different portal features help improve health outcomes. This study is designed to develop and test an intervention to improve adoption and use of patient portal features for diabetes management.
ID: NCT02953262
Sponsor: VA Office of Research and Development
Locations: Edith Nourse Rogers Memorial Veterans Hospital, Bedford, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus, Massachusetts.
Home-Based Kidney Care in Native American’s of New Mexico (HBKC)
People reach end stage renal disease (ESRD) due to progressive chronic kidney disease (CKD), which is associated with increased risk for heart disease and death. The burden of chronic kidney disease is increased among minority populations compared to Caucasians. New Mexico American Indians are experiencing an epidemic of chronic kidney disease due primarily to the high rates of obesity and diabetes. The present study entitled Home-Based Kidney Care is designed to delay / reduce rates of ESRD by early interventions in CKD. Investigators propose to assess the safety and efficacy of conducting a full-scale study to determine if home based care delivered
by a collaborative team composed of community health workers, the Albuquerque Area Indian Health Board and University of New Mexico faculty will decrease the risk for the development and the progression of CKD.
ID: NCT03179085
Sponsor: University of New Mexico
Location: University of New Mexico, Albuquerque
INcreasing Veteran EngagemeNT to Prevent Diabetes (INVENT)
This study will evaluate a VA MyHealtheVet Secure Messaging intervention that uses different intervention messaging strategies designed to increase engagement in behaviors to prevent type 2 diabetes (T2DM). After completing a baseline survey, participants will be randomly assigned to receive different novel presentations of information about ways to prevent T2DM through both secure messaging and US mail. The investigators will test the 5 presentations that each: (1) represent an innovative approach from behavioral economics or health psychology with great promise to increase engagement in behaviors to prevent T2DM among patients with prediabetes; and (2) have not been tested in this setting.
ID: NCT03403231
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Self-efficacy, Beliefs and Adherence—Pilot and Feasibility Trial of a Pharmacist-led Intervention
This study uses an intervention mixed methods design. The overall purpose is to improve medication adherence and assess the clinical impact on diabetes outcomes among patients with uncontrolled diabetes. We will examine if usual care combined with a clinic-based health literacy/psychosocial support intervention improves medication adherence compared to usual care alone. A randomized controlled trial will be conducted at William S. Middleton Memorial Veterans Hospital in Madison, targeting individuals with
uncontrolled diabetes. The patient-centered health literacy intervention will focus on enhancing patients’ self-efficacy and addressing patients’ negative beliefs in medicine and illness.
ID: NCT03406923
Sponsor: University of Wisconsin, Madison
Location: William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin
Practical Telemedicine to Improve Control and Engagement for Veterans With Clinic-Refractory Diabetes Mellitus (PRACTICE-DM)
Diabetes generates significant morbidity, mortality, and costs within the Veterans Health Administration (VHA). Veterans with persistently poor diabetes control despite clinic-based care are among the highest-risk diabetes patients in VHA, and contribute disproportionately to VHA’s massive burden of diabetes complications and costs. VHA critically needs effective, practical management alternatives for veterans whose diabetes does not respond to clinic-based management. The proposed study will address this need by leveraging VHA’s unique Home Telehealth capacity to deliver comprehensive telemedicine-based management for veterans with persistently poor diabetes control despite clinic-based care. Because this intensive intervention is delivered using only existing Home Telehealth workforce, infrastructure, and technical resources—which are ubiquitous at VHA centers nationwide—it could represent an effective, practical approach to improving outcomes in veterans with PPDM, potentially translating to a substantial reduction in VHA’s diabetes burden.
ID: NCT03520413
Sponsor: VA Office of Research and Development
Locations: Durham VA Medical Center, North Carolina; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
Cooking for Health
Type 2 diabetes is a leading cause of morbidity and mortality among American Indians in the US. Although healthy diet is a key component of diabetes management programs, many American Indians face contextual barriers to adopting a healthy diet including: difficulty budgeting for food on low-incomes, low literacy and numeracy when purchasing food, and limited cooking skills. The proposed project will develop, implement, and evaluate a culturally-targeted healthy foods budgeting, purchasing, and cooking skills intervention aimed at improving the cardio-metabolic health of American Indians with type 2 diabetes who live in rural areas.
ID: NCT03699709
Sponsor: University of Washington
Location: Missouri Breaks Industries Research, Eagle Butte, South Dakota
Portopulmonary Hypertension: Treatment
Portopulmonary hypertension (POPH) is a form of group 1 pulmonary arterial hypertension. When treating patients with POPH, baseline assessment is necessary so that response to therapy can be measured as the change from baseline. Patients should undergo echocardiography and right heart catheterization, and their exercise capacity and NYHA functional class should be determined. Patients with POPH should be considered for treatment if they are NYHA functional class II or above and/or their mean pulmonary artery pressure (MPAP) is greater than 35 mm Hg in transplant candidates. The goal in the treatment and management of POPH is to improve pulmonary hemodynamics by reducing the obstruction to pulmonary arterial flow and to preserve right ventricular function (Table). This article, the second in a 2-part review of POPH in patients with liver disease, reviews the role of medical therapy and liver transplantation in treatment. Evaluation and diagnosis of POPH are discussed in a separate article.

Medical Therapy
Prostanoids
Although prostacyclin and prostaglandin analogs entered routine clinical practice for POPH in the 1990s, reports of investigational use date back to the 1980s. Prostanoids are potent vasodilators with antiplatelet aggregation and antiproliferative properties. Prostacyclin synthase is reduced in patients with PAH, resulting in decreased concentration of prostacyclin with vasoconstriction and proliferative changes in the pulmonary vasculature.1
Epoprostenol
Epoprostenol is also known as synthetic prostaglandin I2 or prostacyclin. It was the first therapy approved for the treatment of PAH in 1995 by the US Food and Drug Administration (FDA) as a continuous intravenous infusion.2,3 It also inhibits platelet aggregation and may help modulate pulmonary vascular remodeling.4,5 Epoprostenol is derived from the metabolism of arachidonic acid and is a potent pulmonary and systemic vasodilator. One study reported an immediate 11.8% decrease in MPAP, 24% decrease in pulmonary vascular resistance (PVR) and 28% drop in systemic vascular resistance (SVR) during an epoprostenol infusion.6 The authors reported that epoprostenol was a more potent vasodilator than nitric oxide and may have a role in predicting the reversibility of POPH. In a case series of 33 patients with secondary pulmonary hypertension (including 7 patients with POPH) treated with continuous intravenous prostacyclin for approximately 1 year, exercise tolerance, NYHA functional class, and pulmonary hemodynamics improved in each patient compared to baseline.7 Krowka et al studied 14 patients with moderate to severe POPH treated with intravenous epoprostenol.8 No significant side effects were noted and treatment resulted in significant improvements in PVR, MPAP, and cardiac output. In 2007, Fix et al published a large retrospective cohort of patients with moderate to severe POPH.9 Nineteen patients treated with epoprostenol were compared to 17 patients with no treatment. After a median treatment period of 15.4 months, the epoprostenol group showed significant improvement in MPAP, PVR and cardiac output, but survival did not differ between the 2 groups.
Epoprostenol has often been considered a bridge to transplant in patients with POPH. Sussman et al described 8 consecutive patients with POPH who were treated with intravenous epoprostenol (2 to 8 ng/kg/min dose).10 Liver transplant was considered in 7 of the 8 patients when MPAP decreased to less than 35 mm Hg. Six patients were eventually listed for liver transplant, but 2 died waiting on the list. Long-term outcomes in the group of transplanted recipients were excellent. They remained alive and well at least 9 to 18 months post-transplant, and half did not require long-term vasodilator therapy post-orthotopic liver transplant. Similarly, Ashfaq et al published their data on 16 patients with moderate-to-severe POPH who were treated with vasodilator therapy.11 MPAP decreased to acceptable levels in 75% of the treated patients, and 11 went on to liver transplantation. Rates of 1- and 5-year survival in the transplanted patients were 91% and 67% respectively. None of the patients who failed vasodilator therapy survived.
Epoprostenol has a short half-life (3 to 5 minutes) and requires continuous infusion through central access via an infusion pump. Aseptic technique must be maintained to avoid blood stream infections. Pump failure or loss of vascular access can result in rebound pulmonary vasoconstriction that can be life-threatening and requires immediate attention. Side effects associated with epoprostenol include flushing, headache, nausea/vomiting, bradycardia, chest pain, jaw pain, diarrhea, and musculoskeletal pain.
Patients on epoprostenol should be monitored for prostanoid overdose. In the case of patients with chronic liver disease, epoprostenol increases systemic vasodilation in patients with already low systemic vascular tone. As a result, cardiac output may increase to the point of high cardiac output failure. MPAP will remain elevated secondary to high cardiac output rather than high PVR. In these patients, right heart catheterization will show an elevated MPAP in the setting of normal to low PVR/transpulmonary gradient (TPG) values. Lowering the epoprostenol dose will successfully reduce both cardiac output and MPAP.
Treprostinil
Treprostinil is a prostacyclin analog that is available in intravenous, inhalational, and subcutaneous form, although subcutaneous dosing may be limited by pain. Sakai et al published a small case series of 3 patients with PAH and end-stage liver disease treated with intravenous treprostinil.12 Pulmonary hemodynamics improved in all patients, and 2 patients went on to an uneventful liver transplantation. More than 10 years later, data were published on 255 patients with PAH on therapy with bosentan or sildenafil randomized to additional inhaled treprostinil.13 Treprostinil proved to be safe and well tolerated, with improvement in quality of life measures but no improvement in other secondary endpoints.
Iloprost
Inhaled iloprost is another prostacyclin that has a short therapeutic half-life of 20 to 30 minutes and requires frequent administration (6 to 9 times daily). In study in which patients with severe POPH were treated for up to 3 years with inhaled iloprost,14 survival rates at 1, 2, and 3 years were 77%, 62%, and 46%, respectively. A second study published in 2010 was designed to assess the acute effects of inhaled iloprost on pulmonary hemodynamics and evaluate the clinical outcome after 12 months of treatment.15 Iloprost was found to rapidly reduce pulmonary arterial pressure and PVR. In the long-term evaluation, inhaled iloprost increased the 6-minute walk distance (6MWD) and functional class, but no change was noted in the systolic pulmonary artery pressure. The authors concluded that iloprost might provide symptomatic improvement and improvement in exercise capacity.
Selexipag
Selexipag is an oral selective IP prostacyclin receptor agonist that is structurally distinct from other prostacyclins.16 In a phase 3 randomized double blind clinical trial, PAH patients treated with selexipag had lower composite of death or complication of PAH to the end of the study period.17 This effect was consistent across all dose ranges, but POPH patients were excluded from this study. Safety and efficacy of selexipag has not been evaluated in POPH patients.
Endothelin Receptor Antagonists
Endothelin receptor antagonists block the production of endothelin-1 (ET-1), a potent vasoconstrictor and smooth muscle mitogen that may contribute to the development of PAH. Three different receptors have been described: endothelin A, endothelin B, and endothelin B2. Elevated ET-1 levels have been reported in patients with chronic liver disease and may originate from hepatosplanchnic circulation.18
Bosentan
Bosentan is an oral, nonspecific, ET-1A and ET-1B receptor antagonist. Initial use of bosentan in patients with POPH was limited because of concern for hepatotoxicity. Approximately 10% of patients on bosentan were reported to have mild hepatic side effects in the form of elevated aminotransferases, but severe injury has been reported.19 One of the first clinical experiences of bosentan in patients with POPH was published in 2005. Hoeper et al followed 11 patients with Child A cirrhosis and severe POPH.20 All patients included were in NYHA functional class III or IV and were treated with bosentan for over 1 year. Exercise capacity and symptoms improved in all treated patients. The medication was tolerated well and there was no evidence of drug-induced liver injury. A single case report showed the effectiveness of bosentan in a 43-year-old man with alcohol-related liver disease (Child-Pugh A) and right ventricular enlargement and dysfunction secondary to POPH.21 Pulmonary arterial pressure decreased, exercise capacity increased, and improvement was maintained over 2 years.
In a group of 31 patients with Child A or B cirrhosis and severe POPH, bosentan had significantly better effects than inhaled iloprost on exercise capacity, hemodynamics, and survival.14 One, 2, and 3-year survival rates in the bosentan group were 94%, 89%, and 89% (compared to 77%, 62%, and 46% in the iloprost group). Both drugs were considered safe with no reported hepatotoxicity. In 2013, Savale et al published data on 34 patients with POPH, Child-Pugh A and/or B who were treated with bosentan for a median of 43 months.22 The authors reported significant improvements in hemodynamics, NYHA functional class, and 6WMD. Event-free survival rates at 1, 2, and 3 years were 82%, 63%, and 47%, respectively.
Ambrisentan
Ambrisentan is a highly selective ET-1A receptor antagonist with once daily dosing and a lower risk of hepatotoxicity compared to bosentan. Fourteen patients with moderate to severe POPH treated with ambrisentan in 4 German hospitals were retrospectively analyzed.23 Median follow-up was 16 months, and the study demonstrated significant improvement in exercise capacity and clinical symptoms without significant change in liver function tests. Cartin-Ceba et al published their experience of 13 patients with moderate to severe POPH treated with ambrisentan monotherapy.24 Patients were followed for a median of 613 days and on treatment for a median time of 390 days. Significant improvements were shown in pulmonary arterial pressure and PVR without adverse effect on hepatic function. Over 270 patients with PAH (6% with POPH) received ambrisentan from March 2009 through June 2013 at a large United Kingdom portal hypertension referral center.25 Discontinuation due to side effects was higher than previously reported. Discontinuation due to abnormal transaminases was uncommon.
Macitentan
Macitentan is a dual endothelin-receptor antagonist developed by modifying the structure of bosentan to increase efficacy and safety. The SERAPHIN trial compared oral macitentan to placebo in 250 patients with moderate to severe PAH, some of whom were also on a stable dose of oral or inhaled therapy for PAH.26 Over a 2-year period, patients treated with macitentan were less likely to have progression of their disease or die on therapy (38% and 31% versus 46%), regardless of if they were receiving additional oral therapy and more likely to have improvement of their exercise capacity and WHO functional class. Nasopharyngitis and significant anemia were more common in the macitentan group, but there was no difference in the rate of liver function test abnormalities compared to placebo. Trials with macitentan are currently ongoing in patients with POPH.
Phosphodiesterase-5 Inhibitors
Cyclic guanosine monophosphate (cGMP) is the mediator of nitric oxide–induced vasodilation. Phosphodiesterase-5 (PDE-5) inhibitors prolong the vasodilatory effects of cyclic guanosine monophosphate by preventing its hydrolysis, thereby reducing the pulmonary arterial pressure.
Sildenafil
Sildenafil is the most widely accepted PDE-5 inhibitor for POPH. Fourteen patients with moderate to severe POPH were treated with sildenafil (50 mg 3 times per day) in an observational study published by Reichenberger et al in 2006.27 Eight patients were newly started on sildenafil, whereas sildenafil was added to inhaled prostanoids in the remaining 6x patients. Sildenafil significantly decreased 66MWD, MPAP, PVR, and cardiac index alone or in combination with inhaled prostanoids.
Sildenafil has also been used as a bridge to transplant in liver transplant candidates with POPH. Ten patients with POPH treated with sildenafil monotherapy were followed for a 21±16 months.28 Patients improved symptomatically and increased their 6MWD at 1 year by 30 meters or more. Three patients became transplant eligible and another 3 patients were stable, without progression of their liver disease or POPH. Four patients were not considered transplant candidates, 2 because of refractory POPH and 2 for other comorbidities. The authors concluded that sildenafil monotherapy could stabilize or improve pulmonary hemodynamics in patients with POPH and eventually lead to liver transplantation. Gough et al took a similar look at 9 patients with POPH treated with sildenafil.29 All patients had initial and follow-up right heart catheterizations within a period of 3 years. Mean PVR improved in all patients, decreasing from 575 to 375 dynes/s/cm–5. MPAP decreased to ≤ 35 mmHg in 4 patients, 1 of whom went on to receive a liver transplant. Overall sildenafil improved pulmonary hemodynamics in this small cohort of POPH patients.
Tadalafil
Tadalafil is another oral PDE-5 inhibitor but with a longer half-life than sildenafil. Unlike sildenafil, which requires 3 times daily dosing, tadalafil requires once daily administration. A few case reports have demonstrated tadalafil’s effectiveness for POPH in combination with other medical therapy (eg, sildenafil, ambrisentan).30,31
Guanylate Cyclase Stimulator
Riociguat
Riociguat is a first-in-class activator of soluble form of guanylate cyclase that increases levels of cyclic GMP. Two randomized clinical trials, PATENT, a study in PAH patients, and CHEST, a study in patients with chronic thromboembolic pulmonary hypertension showed improvement in 6MWD at 12 weeks (PATENT) or 16 weeks (CHEST), with improvement in secondary endpoints such as PVR, N-terminal pro b-type natriuretic peptide and WHO functional class.32,33 Riociguat may have potential advantages in patients with POPH given that it has a favorable liver safety profile. A subgroup analysis of patients enrolled in the PATENT study showed that 13 had POPH and 11 were randomized to receive riociguat 2.5 mg 3 times daily dose and 2 received placebo.34 Riociguat was well tolerated and improved 6MWD that was maintained over 2 years in the open label extension.
Medications to Avoid
Nonselective beta-blockers are commonly recommended in patients with portal hypertension to help prevent variceal hemorrhage. However, in patients with POPH, beta-blockers have been shown to decrease exercise capacity and worsen pulmonary hemodynamics. A study of 10 patients with moderate to severe POPH who were receiving beta-blockers for variceal bleeding prophylaxis showed that 6MWD improved in almost all of the patients, cardiac output increased by 28%, and PVR decreased by 19% when beta-blockers were discontinued.35 The authors concluded that the use of beta-blockers should be avoided in this patient population.
Calcium channel blockers should not be used in patients with POPH because they can cause significant hypotension due to systemic vasodilatation and decreased right ventricular filling. Patients with portal hypertension and chronic liver disease commonly have low systemic vascular resistance and are particularly susceptible to the deleterious effects of calcium channel blockers.
Transplantation
Liver transplantation is a potential cure for POPH and its role in POPH has evolved over the past 2 decades. In 1997, Ramsay et al published their review of 1205 consecutive liver transplants at Baylor University Medical Center (BUMC) in Texas.36 The incidence of POPH in this group was 8.5%, with the majority of patients having mild POPH. Liver transplant outcomes were not affected by mild and moderate pulmonary hypertension. However, patients with severe POPH (n = 7, systolic pulmonary artery pressure > 60 mm Hg) had a mortality rate of 42% at 9 months post-transplantation and 71% at 36 months post-transplant. The surviving patients continued to deteriorate with progressive right heart failure and no improvement in POPH.
To understand the effect of liver transplantation on POPH, one must understand the hemodynamic changes that occur with POPH and during liver transplant. The right ventricle is able to manage the same volume as the left ventricle under normal circumstances, but is unable to pump against a significant pressure gradient.37 In the setting of POPH, right ventricular hypertrophy occurs and RV output remains stable for some time. With time, pulmonary artery pressure increases secondary to pulmonary arteriolar vasoconstriction, intimal thickening, and progressive occlusion of the pulmonary vascular bed. Right ventricular failure may occur as a result. Cardiac output increases significantly at the time of reperfusion during liver transplant (up to 3-fold in 15 minutes),38 and in the setting of a noncompliant vascular bed, the patient is at risk for right heart failure. This is the likely explanation to such high perioperative mortality rates in patients with uncontrolled POPH. Failure to decrease MPAP to less than 50 mm Hg is considered a complete contraindication to liver transplant at most institutions. Many transplant centers will list patients for liver transplant if MPAP can be decreased to less than 35 mm Hg and PVR < 400 dynes/s/cm–5. These parameters are thought to represent an adequate right ventricular reserve and a compliant pulmonary vascular bed.37 However, even with good pressure control, the anesthesiology and critical care teams must be prepared to deal with acute right heart failure peri-operatively. Intraoperative transesophageal echocardiography has been recommended to closely follow right ventricular function.38 Inhaled or intravenous dilators are the most effective agents in the event of a pulmonary hypertensive crisis.
Review of Outcomes
A retrospective review evaluated 43 patients with untreated POPH who underwent attempted liver transplantation.39 Data were collected from 18 peer-reviewed studies and 7 patients at the authors’ institution. Overall mortality was 35% (15 patients), with almost all of the deaths secondary to cardiac dysfunction. Two deaths occurred intraoperatively and 8 deaths occurred during the transplant hospitalization. The transplant could not be successfully completed in 4 of the patients. MPAP > 50 mm Hg was associated with 100% mortality, whereas patients with MPAP between 35 mm Hg and 50 mm Hg had a 50% mortality. No mortality was noted in patients with MPAP < 35 mm Hg.
Liver transplantation has been shown to be successful in patients with controlled POPH. Sussman et al published their data on 8 patients with severe POPH in 2006. In this prospective study, all patients were treated with sequential epoprostenol infusions and 7 of the 8 patients experienced a significant reduction in MPAP and PVR. Six patients were listed for liver transplant, 4 of who were transplanted successfully and alive up to 5 years later.
The Baylor University Medical Center published their data on POPH patients who received liver transplants in 2007.11 POPH was confirmed by right heart catheterization in 30 patients evaluated for liver transplant. Sixteen patients were considered to be suitable candidates for transplant and MPAP was decreased to less than 35 mmHg in 12 patients with vasodilator therapy. Eleven patients eventually underwent liver transplant and 1- and 5-year survival rates were 91% and 67%.
Compared to medical therapy or liver transplant alone, patients who receive medical therapy followed by liver transplantation have the best survival. The Mayo Clinic retrospectively reviewed 74 POPH patients identified between 1994 and 2007.40 Patients were categorized in 1 of 3 categories: no medical therapy, medical therapy alone for POPH, or medical therapy for POPH followed by liver transplantation. Patients who received no medical therapy for POPH and no liver transplant had the worst outcomes, with a dismal 5-year survival of only 14% with over 50% deceased at 1 year of diagnosis. Five-year survival was 45% in patients who received medical therapy only. Patients who received medical therapy with prostacyclin followed by liver transplantation had the best outcomes, with a 5-year survival of 67% versus 25% in those who were transplanted without prior prostacyclin therapy.
We reported the longest follow-up study for patients undergoing liver transplantation with POPH in 2014.41 Seven patients with moderate to severe POPH received a liver transplant at our institution between June 2004 and January 2011. Mean pulmonary artery pressure was reduced to < 35 mm Hg, with appropriate POPH therapy in all of the patients. Both the graft and patient survival rates were 85.7% after a median follow-up of 7.8 years. The 1 patient who did not survive died from complications related to recurrent hepatitis C and cirrhosis, not from POPH-related issues. Four of the remaining 6 patients continue to require oral vasodilator therapy post-transplant, suggesting irreversible remodeling of the pulmonary vasculature. Two patients (4.4 and 8.5 years post-transplant) have no evidence of pulmonary hypertension post-transplant and therefore do not require medical treatment for pulmonary hypertension. We concluded that POPH responsive to vasodilator therapy is an appropriate indication for liver transplant, with excellent long-term survival.
Hollatz et al published their data on 11 patients with moderate to severe POPH who were successfully treated (mostly with oral sildenafil and subcutaneous treprostinil) as a bridge to liver transplant.42 The mortality rate was 0, with a follow-up duration of 7 to 60 months. Interestingly, 7 of the 11 patients (64%) were off all pulmonary vasodilators post-transplant. Ashfaq et al reported similar results.11 Nine of 11 patients with treated moderate to severe POPH who received liver transplants stopped vasodilator therapy at a median period of 9.2 months post-transplant. Raevens et al described a group of 3 patients with POPH who went on to liver transplant after their pulmonary pressures were decreased with combined oral vasodilator therapy: 1 required continued long-term vasodilator therapy, another was weaned off medications after transplant, and the third patient died during the liver transplant from perioperative complications that induced uncontrolled pulmonary hypertension.43
Patient Selection
In 2006, the United Network for Organ Sharing (UNOS) initiated a policy whereby a higher priority for liver transplantation was granted for highly selected patients in the United States.44 UNOS policy 3.6.4.5.6 upgraded POPH patients to a MELD score of 22, with an increase in MELD every 3 months as long as MPAP remained < 35 mm Hg and PVR remained < 400 dynes/s/cm–5. One hundred fifty-five patients were granted MELD exception points for POPH between 2002 and 2010 and went on to receive liver transplants.45 Goldberg et al collected data from the Organ Procurement and Transplantation Network (OPTN) and compared outcomes of patients with approved POPH MELD exception points versus waitlist candidates with no exception points.46 One hundred fifty-five waitlisted patients received POPH MELD exception points, with only 43.1% meeting OPTN exception requirements. One-third did not fulfill hemodynamic criteria consistent with POPH or had missing data, and 80% went on to receive a liver transplant. Waitlist candidates receiving POPH MELD exception points also had increased waitlist mortality and several early post-transplant deaths. The authors felt these data highlighted the need for OPTN/UNOS to revise their policy for POPH MELD exceptions points, revise how points are rewarded, and continue research to help risk stratify these patients to minimize perioperative complications.
Conclusion
Several effective medical treatment regimens are available, including prostanoids, endothelin receptor antagonists, and PDE-5 inhibitors. Liver transplantation is a potential cure but is only recommended if MPAP can be decreased to ≤ 35 mmHg. Long-term follow-up studies have shown these patients do well several years post-transplant but may continue to require oral therapy for their POPH.
1. Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925-1932.
2. Chin K, Rubin L. Pulmonary arterial hypertension. Am Coll Cardiol. 2008;51:1527-1538.
3. Doran A, Harris S, Goetz B. Advances in prostanoid infusion therapy for pulmonary arterial hypertension. J Infus Nurs. 2008;31:336-345.
4. Chin KM, Channick RN, De Lemos JA, ET AL. Hemodynamics and epoprostenol use are associated with thrombocytopenia in pulmonary arterial hypertension. Chest. 2009;135:130-136.
5. Hoshikawa Y, Voelkel NF, Gesell TL, et al. Prostacyclin receptor-dependent modulation of pulmonary vascular remodeling. Am J Respir Crit Care Med. 2001;164:314-318.
6. Ricci GL, Melgosa MT, Burgos F, et al. Assessment of acute pulmonary vascular reactivity in portopulmonary hypertension. Liver Transplant. 2007;13:1506-1514.
7. McLaughlin V V, Genthner DE, Panella MM, et al. Compassionate use of continuous prostacyclin in the management of secondary pulmonary hypertension: a case series. Ann Intern Med. 1999;130:740-743.
8. Krowka MJ, Frantz RP, McGoon MD, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology. 1999;30:641-648.
9. Fix OK, Bass NM, De Morco T, Merriman RB. Long-term follow-up of portopulmonary hypertension: Effect of treatment with epoprostenol. Liver Transplant. 2007;13:875-885.
10. Sussman N, Kaza V, Barshes N, et al. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant. 2006;6:2177-2182.
11. Ashfaq M, Chinnakotla S, Rogers L, et al. The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant. 2007;7:1258-1264.
12. Sakai T, Planinsic RM, Mathier MA, et al. initial experience using continuous intravenous treprostinil to manage pulmonary arterial hypertension in patients with end-stage liver disease. Transpl Int. 2009;22:554-561.
13. McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: A randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915-1922.
14. Hoeper MM, Seyfarth HJ, Hoeffken G, et al. Experience with inhaled iloprost and bosentan in portopulmonary hypertension. Eur Respir J. 2007;30:1096-1102.
15. Melgosa MT, Ricci GL, Garcia-Pagan JC et al. Acute and long-term effects of inhaled iloprost in portopulmonary hypertension. Liver Transplant. 2010;16:348-356.
16. Simonneau G, Torbicki A, Hoeper MM, et al. Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J. 2012;40:874-880
17. Sitbon O, Channick R, Chin, KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522-2533.
18. Moller S, Gulberg V, Henriksen JH, Gerbes AL. Endothelin-1 and endothelin-3 in cirrhosis: Relations to systemic and splanchnic haemodynamics. J Hepatol. 1995;23:135-144.
19. Eriksson C, Gustavsson A, Kronvall T, Tysk C. Hepatotoxicity by bosentan in a patient with portopulmonary hypertension : a case-report and review of the literature. J Gastrointestin Liver Dis. 2011;20:77-80.
20. Hoeper MM, Halank M, Marx C, et al. Bosentan therapy for portopulmonary hypertension. Eur Respir J. 2005;25:502-508.
21. Stähler G, Von Hunnius P. Successful treatment of portopulmonary hypertension with bosentan: Case report.: Eur J Clin Investig. 2006;36:62-66.
22. Savale L, Magnier R, Le Pavec J, et al. Efficacy, safety and pharmacokinetics of bosentan in portopulmonary hypertension. Eur. 2013;41:96-103.
23. Halank M, Knudsen L, Seyfarth H, et al. Ambrisentan improves exercise capacity and symptoms in patients with portopulmonary hypertension. Z Gastroenterol. 2011;49:1258-1262.
24. Cartin-Ceba R, Swanson K, Iyer V, et al. Safety and efficacy of ambrisentan for the treatment of portopulmonary hypertension. Chest. 2011;139:109-114.
25. Condliffe R, Elliot C, Hurdman J, et al. Ambrisentan therapy in pulmonary hypertension: clinical use and tolerability in a referral centre. Ther Adv Respir Dis. 2014;8:71-77.
26. Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809-818.
27. Reichenberger F, Voswinckel R, Steveling E, et al. Sildenafil treatment for portopulmonary hypertension. Eur Respir J. 2006;28:563-567.
28. Hemnes AR RI. Sildenafil monotherapy in portopulmonary hypertension can facilitate liver transplantation. Liver Transplant. 2009;15:15-19.
29. Gough WR. Sildenafil therapy is associated with improved hemodynamics in liver transplantation candidates with pulmonary arterial hypertension. Liver Transplant. 2009;15:30-36.
30. Yamashita Y. Hemodynamic effects of ambrisentan-tadalafil combination therapy on progressive portopulmonary hypertension. World J Hepatol. 2014;6:825.
31. Bremer HC, Kreisel W, Roecker K, et al. Phosphodiesterase 5 inhibitors lower both portal and pulmonary pressure in portopulmonary hypertension: a case report. J Med Case Rep. 2007;1:46.
32. Ghofrani HA, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013:369;330-340.
33. Ghofrani HA, Galie N, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013:369;319-329
34. Cartin-Ceba R, Halank M, Ghofrani HA, et al. Riociguat treatment for portopulmonary hypertension: a subgroup analysis from the PATENT-1/-2 studies. Pulm Circ. 2018: 8:2045894018769305.
35. Provencher S, Herve P, Jais X, et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. 2006. Gastroenterology. 2006;130:120-126.
36. Ramsay M a, Simpson BR, Nguyen T, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3:494-500.
37. Safdar Z, Bartolome S, Sussman N. Portopulmonary hypertension : an update. Liver Tranpl. 2012;18:881-891.
38. Ramsay M. Portopulmonary hypertension and right heart failure in patients with cirrhosis. Curr Opin Anaesthesiol. 2010;23:145-150.
39. Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443-450.
40. Swanson KL, Wiesner RH, Nyberg SL, et al. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445-2453.
41. Khaderi S, Khan R, Safdar Z, et al. Long-term follow-up of portopulmonary hypertension patients after liver transplantation. Liver Transplant. 2014;20:724-727.
42. Hollatz TJ, Musat A, Westphal S, et al. Treatment with sildenafil and treprostinil allows successful liver transplantation of patients with moderate to severe portopulmonary hypertension. Liver Transpl. 2012:686-695.
43. Raevens S, De Pauw M, Reyntjens K, et al. Oral vasodilator therapy in patients with moderate to severe portopulmonary hypertension as a bridge to liver transplantation. Eur J Gastroenterol Hepatol. 2012:1-8.
44. Krowka M, Fallon M, Mulligan D. Model for end-stage liver disease (MELD) exception for portopulmonary hypertension. Liver Transplant. 2006;12:S114-S116.
45. Krowka M, Wiesner R, Rosen C. Portopulmonary hypertension outcomes in the era of MELD exception. Liver Transplant. 2012;18:S259.
46. Goldberg DS, Batra S, Sahay S, et al. MELD Exceptions for portopulmonary hypertension: current policy and future implementation. Am J Transplant. 2014;14:2081-2087.
Portopulmonary hypertension (POPH) is a form of group 1 pulmonary arterial hypertension. When treating patients with POPH, baseline assessment is necessary so that response to therapy can be measured as the change from baseline. Patients should undergo echocardiography and right heart catheterization, and their exercise capacity and NYHA functional class should be determined. Patients with POPH should be considered for treatment if they are NYHA functional class II or above and/or their mean pulmonary artery pressure (MPAP) is greater than 35 mm Hg in transplant candidates. The goal in the treatment and management of POPH is to improve pulmonary hemodynamics by reducing the obstruction to pulmonary arterial flow and to preserve right ventricular function (Table). This article, the second in a 2-part review of POPH in patients with liver disease, reviews the role of medical therapy and liver transplantation in treatment. Evaluation and diagnosis of POPH are discussed in a separate article.

Medical Therapy
Prostanoids
Although prostacyclin and prostaglandin analogs entered routine clinical practice for POPH in the 1990s, reports of investigational use date back to the 1980s. Prostanoids are potent vasodilators with antiplatelet aggregation and antiproliferative properties. Prostacyclin synthase is reduced in patients with PAH, resulting in decreased concentration of prostacyclin with vasoconstriction and proliferative changes in the pulmonary vasculature.1
Epoprostenol
Epoprostenol is also known as synthetic prostaglandin I2 or prostacyclin. It was the first therapy approved for the treatment of PAH in 1995 by the US Food and Drug Administration (FDA) as a continuous intravenous infusion.2,3 It also inhibits platelet aggregation and may help modulate pulmonary vascular remodeling.4,5 Epoprostenol is derived from the metabolism of arachidonic acid and is a potent pulmonary and systemic vasodilator. One study reported an immediate 11.8% decrease in MPAP, 24% decrease in pulmonary vascular resistance (PVR) and 28% drop in systemic vascular resistance (SVR) during an epoprostenol infusion.6 The authors reported that epoprostenol was a more potent vasodilator than nitric oxide and may have a role in predicting the reversibility of POPH. In a case series of 33 patients with secondary pulmonary hypertension (including 7 patients with POPH) treated with continuous intravenous prostacyclin for approximately 1 year, exercise tolerance, NYHA functional class, and pulmonary hemodynamics improved in each patient compared to baseline.7 Krowka et al studied 14 patients with moderate to severe POPH treated with intravenous epoprostenol.8 No significant side effects were noted and treatment resulted in significant improvements in PVR, MPAP, and cardiac output. In 2007, Fix et al published a large retrospective cohort of patients with moderate to severe POPH.9 Nineteen patients treated with epoprostenol were compared to 17 patients with no treatment. After a median treatment period of 15.4 months, the epoprostenol group showed significant improvement in MPAP, PVR and cardiac output, but survival did not differ between the 2 groups.
Epoprostenol has often been considered a bridge to transplant in patients with POPH. Sussman et al described 8 consecutive patients with POPH who were treated with intravenous epoprostenol (2 to 8 ng/kg/min dose).10 Liver transplant was considered in 7 of the 8 patients when MPAP decreased to less than 35 mm Hg. Six patients were eventually listed for liver transplant, but 2 died waiting on the list. Long-term outcomes in the group of transplanted recipients were excellent. They remained alive and well at least 9 to 18 months post-transplant, and half did not require long-term vasodilator therapy post-orthotopic liver transplant. Similarly, Ashfaq et al published their data on 16 patients with moderate-to-severe POPH who were treated with vasodilator therapy.11 MPAP decreased to acceptable levels in 75% of the treated patients, and 11 went on to liver transplantation. Rates of 1- and 5-year survival in the transplanted patients were 91% and 67% respectively. None of the patients who failed vasodilator therapy survived.
Epoprostenol has a short half-life (3 to 5 minutes) and requires continuous infusion through central access via an infusion pump. Aseptic technique must be maintained to avoid blood stream infections. Pump failure or loss of vascular access can result in rebound pulmonary vasoconstriction that can be life-threatening and requires immediate attention. Side effects associated with epoprostenol include flushing, headache, nausea/vomiting, bradycardia, chest pain, jaw pain, diarrhea, and musculoskeletal pain.
Patients on epoprostenol should be monitored for prostanoid overdose. In the case of patients with chronic liver disease, epoprostenol increases systemic vasodilation in patients with already low systemic vascular tone. As a result, cardiac output may increase to the point of high cardiac output failure. MPAP will remain elevated secondary to high cardiac output rather than high PVR. In these patients, right heart catheterization will show an elevated MPAP in the setting of normal to low PVR/transpulmonary gradient (TPG) values. Lowering the epoprostenol dose will successfully reduce both cardiac output and MPAP.
Treprostinil
Treprostinil is a prostacyclin analog that is available in intravenous, inhalational, and subcutaneous form, although subcutaneous dosing may be limited by pain. Sakai et al published a small case series of 3 patients with PAH and end-stage liver disease treated with intravenous treprostinil.12 Pulmonary hemodynamics improved in all patients, and 2 patients went on to an uneventful liver transplantation. More than 10 years later, data were published on 255 patients with PAH on therapy with bosentan or sildenafil randomized to additional inhaled treprostinil.13 Treprostinil proved to be safe and well tolerated, with improvement in quality of life measures but no improvement in other secondary endpoints.
Iloprost
Inhaled iloprost is another prostacyclin that has a short therapeutic half-life of 20 to 30 minutes and requires frequent administration (6 to 9 times daily). In study in which patients with severe POPH were treated for up to 3 years with inhaled iloprost,14 survival rates at 1, 2, and 3 years were 77%, 62%, and 46%, respectively. A second study published in 2010 was designed to assess the acute effects of inhaled iloprost on pulmonary hemodynamics and evaluate the clinical outcome after 12 months of treatment.15 Iloprost was found to rapidly reduce pulmonary arterial pressure and PVR. In the long-term evaluation, inhaled iloprost increased the 6-minute walk distance (6MWD) and functional class, but no change was noted in the systolic pulmonary artery pressure. The authors concluded that iloprost might provide symptomatic improvement and improvement in exercise capacity.
Selexipag
Selexipag is an oral selective IP prostacyclin receptor agonist that is structurally distinct from other prostacyclins.16 In a phase 3 randomized double blind clinical trial, PAH patients treated with selexipag had lower composite of death or complication of PAH to the end of the study period.17 This effect was consistent across all dose ranges, but POPH patients were excluded from this study. Safety and efficacy of selexipag has not been evaluated in POPH patients.
Endothelin Receptor Antagonists
Endothelin receptor antagonists block the production of endothelin-1 (ET-1), a potent vasoconstrictor and smooth muscle mitogen that may contribute to the development of PAH. Three different receptors have been described: endothelin A, endothelin B, and endothelin B2. Elevated ET-1 levels have been reported in patients with chronic liver disease and may originate from hepatosplanchnic circulation.18
Bosentan
Bosentan is an oral, nonspecific, ET-1A and ET-1B receptor antagonist. Initial use of bosentan in patients with POPH was limited because of concern for hepatotoxicity. Approximately 10% of patients on bosentan were reported to have mild hepatic side effects in the form of elevated aminotransferases, but severe injury has been reported.19 One of the first clinical experiences of bosentan in patients with POPH was published in 2005. Hoeper et al followed 11 patients with Child A cirrhosis and severe POPH.20 All patients included were in NYHA functional class III or IV and were treated with bosentan for over 1 year. Exercise capacity and symptoms improved in all treated patients. The medication was tolerated well and there was no evidence of drug-induced liver injury. A single case report showed the effectiveness of bosentan in a 43-year-old man with alcohol-related liver disease (Child-Pugh A) and right ventricular enlargement and dysfunction secondary to POPH.21 Pulmonary arterial pressure decreased, exercise capacity increased, and improvement was maintained over 2 years.
In a group of 31 patients with Child A or B cirrhosis and severe POPH, bosentan had significantly better effects than inhaled iloprost on exercise capacity, hemodynamics, and survival.14 One, 2, and 3-year survival rates in the bosentan group were 94%, 89%, and 89% (compared to 77%, 62%, and 46% in the iloprost group). Both drugs were considered safe with no reported hepatotoxicity. In 2013, Savale et al published data on 34 patients with POPH, Child-Pugh A and/or B who were treated with bosentan for a median of 43 months.22 The authors reported significant improvements in hemodynamics, NYHA functional class, and 6WMD. Event-free survival rates at 1, 2, and 3 years were 82%, 63%, and 47%, respectively.
Ambrisentan
Ambrisentan is a highly selective ET-1A receptor antagonist with once daily dosing and a lower risk of hepatotoxicity compared to bosentan. Fourteen patients with moderate to severe POPH treated with ambrisentan in 4 German hospitals were retrospectively analyzed.23 Median follow-up was 16 months, and the study demonstrated significant improvement in exercise capacity and clinical symptoms without significant change in liver function tests. Cartin-Ceba et al published their experience of 13 patients with moderate to severe POPH treated with ambrisentan monotherapy.24 Patients were followed for a median of 613 days and on treatment for a median time of 390 days. Significant improvements were shown in pulmonary arterial pressure and PVR without adverse effect on hepatic function. Over 270 patients with PAH (6% with POPH) received ambrisentan from March 2009 through June 2013 at a large United Kingdom portal hypertension referral center.25 Discontinuation due to side effects was higher than previously reported. Discontinuation due to abnormal transaminases was uncommon.
Macitentan
Macitentan is a dual endothelin-receptor antagonist developed by modifying the structure of bosentan to increase efficacy and safety. The SERAPHIN trial compared oral macitentan to placebo in 250 patients with moderate to severe PAH, some of whom were also on a stable dose of oral or inhaled therapy for PAH.26 Over a 2-year period, patients treated with macitentan were less likely to have progression of their disease or die on therapy (38% and 31% versus 46%), regardless of if they were receiving additional oral therapy and more likely to have improvement of their exercise capacity and WHO functional class. Nasopharyngitis and significant anemia were more common in the macitentan group, but there was no difference in the rate of liver function test abnormalities compared to placebo. Trials with macitentan are currently ongoing in patients with POPH.
Phosphodiesterase-5 Inhibitors
Cyclic guanosine monophosphate (cGMP) is the mediator of nitric oxide–induced vasodilation. Phosphodiesterase-5 (PDE-5) inhibitors prolong the vasodilatory effects of cyclic guanosine monophosphate by preventing its hydrolysis, thereby reducing the pulmonary arterial pressure.
Sildenafil
Sildenafil is the most widely accepted PDE-5 inhibitor for POPH. Fourteen patients with moderate to severe POPH were treated with sildenafil (50 mg 3 times per day) in an observational study published by Reichenberger et al in 2006.27 Eight patients were newly started on sildenafil, whereas sildenafil was added to inhaled prostanoids in the remaining 6x patients. Sildenafil significantly decreased 66MWD, MPAP, PVR, and cardiac index alone or in combination with inhaled prostanoids.
Sildenafil has also been used as a bridge to transplant in liver transplant candidates with POPH. Ten patients with POPH treated with sildenafil monotherapy were followed for a 21±16 months.28 Patients improved symptomatically and increased their 6MWD at 1 year by 30 meters or more. Three patients became transplant eligible and another 3 patients were stable, without progression of their liver disease or POPH. Four patients were not considered transplant candidates, 2 because of refractory POPH and 2 for other comorbidities. The authors concluded that sildenafil monotherapy could stabilize or improve pulmonary hemodynamics in patients with POPH and eventually lead to liver transplantation. Gough et al took a similar look at 9 patients with POPH treated with sildenafil.29 All patients had initial and follow-up right heart catheterizations within a period of 3 years. Mean PVR improved in all patients, decreasing from 575 to 375 dynes/s/cm–5. MPAP decreased to ≤ 35 mmHg in 4 patients, 1 of whom went on to receive a liver transplant. Overall sildenafil improved pulmonary hemodynamics in this small cohort of POPH patients.
Tadalafil
Tadalafil is another oral PDE-5 inhibitor but with a longer half-life than sildenafil. Unlike sildenafil, which requires 3 times daily dosing, tadalafil requires once daily administration. A few case reports have demonstrated tadalafil’s effectiveness for POPH in combination with other medical therapy (eg, sildenafil, ambrisentan).30,31
Guanylate Cyclase Stimulator
Riociguat
Riociguat is a first-in-class activator of soluble form of guanylate cyclase that increases levels of cyclic GMP. Two randomized clinical trials, PATENT, a study in PAH patients, and CHEST, a study in patients with chronic thromboembolic pulmonary hypertension showed improvement in 6MWD at 12 weeks (PATENT) or 16 weeks (CHEST), with improvement in secondary endpoints such as PVR, N-terminal pro b-type natriuretic peptide and WHO functional class.32,33 Riociguat may have potential advantages in patients with POPH given that it has a favorable liver safety profile. A subgroup analysis of patients enrolled in the PATENT study showed that 13 had POPH and 11 were randomized to receive riociguat 2.5 mg 3 times daily dose and 2 received placebo.34 Riociguat was well tolerated and improved 6MWD that was maintained over 2 years in the open label extension.
Medications to Avoid
Nonselective beta-blockers are commonly recommended in patients with portal hypertension to help prevent variceal hemorrhage. However, in patients with POPH, beta-blockers have been shown to decrease exercise capacity and worsen pulmonary hemodynamics. A study of 10 patients with moderate to severe POPH who were receiving beta-blockers for variceal bleeding prophylaxis showed that 6MWD improved in almost all of the patients, cardiac output increased by 28%, and PVR decreased by 19% when beta-blockers were discontinued.35 The authors concluded that the use of beta-blockers should be avoided in this patient population.
Calcium channel blockers should not be used in patients with POPH because they can cause significant hypotension due to systemic vasodilatation and decreased right ventricular filling. Patients with portal hypertension and chronic liver disease commonly have low systemic vascular resistance and are particularly susceptible to the deleterious effects of calcium channel blockers.
Transplantation
Liver transplantation is a potential cure for POPH and its role in POPH has evolved over the past 2 decades. In 1997, Ramsay et al published their review of 1205 consecutive liver transplants at Baylor University Medical Center (BUMC) in Texas.36 The incidence of POPH in this group was 8.5%, with the majority of patients having mild POPH. Liver transplant outcomes were not affected by mild and moderate pulmonary hypertension. However, patients with severe POPH (n = 7, systolic pulmonary artery pressure > 60 mm Hg) had a mortality rate of 42% at 9 months post-transplantation and 71% at 36 months post-transplant. The surviving patients continued to deteriorate with progressive right heart failure and no improvement in POPH.
To understand the effect of liver transplantation on POPH, one must understand the hemodynamic changes that occur with POPH and during liver transplant. The right ventricle is able to manage the same volume as the left ventricle under normal circumstances, but is unable to pump against a significant pressure gradient.37 In the setting of POPH, right ventricular hypertrophy occurs and RV output remains stable for some time. With time, pulmonary artery pressure increases secondary to pulmonary arteriolar vasoconstriction, intimal thickening, and progressive occlusion of the pulmonary vascular bed. Right ventricular failure may occur as a result. Cardiac output increases significantly at the time of reperfusion during liver transplant (up to 3-fold in 15 minutes),38 and in the setting of a noncompliant vascular bed, the patient is at risk for right heart failure. This is the likely explanation to such high perioperative mortality rates in patients with uncontrolled POPH. Failure to decrease MPAP to less than 50 mm Hg is considered a complete contraindication to liver transplant at most institutions. Many transplant centers will list patients for liver transplant if MPAP can be decreased to less than 35 mm Hg and PVR < 400 dynes/s/cm–5. These parameters are thought to represent an adequate right ventricular reserve and a compliant pulmonary vascular bed.37 However, even with good pressure control, the anesthesiology and critical care teams must be prepared to deal with acute right heart failure peri-operatively. Intraoperative transesophageal echocardiography has been recommended to closely follow right ventricular function.38 Inhaled or intravenous dilators are the most effective agents in the event of a pulmonary hypertensive crisis.
Review of Outcomes
A retrospective review evaluated 43 patients with untreated POPH who underwent attempted liver transplantation.39 Data were collected from 18 peer-reviewed studies and 7 patients at the authors’ institution. Overall mortality was 35% (15 patients), with almost all of the deaths secondary to cardiac dysfunction. Two deaths occurred intraoperatively and 8 deaths occurred during the transplant hospitalization. The transplant could not be successfully completed in 4 of the patients. MPAP > 50 mm Hg was associated with 100% mortality, whereas patients with MPAP between 35 mm Hg and 50 mm Hg had a 50% mortality. No mortality was noted in patients with MPAP < 35 mm Hg.
Liver transplantation has been shown to be successful in patients with controlled POPH. Sussman et al published their data on 8 patients with severe POPH in 2006. In this prospective study, all patients were treated with sequential epoprostenol infusions and 7 of the 8 patients experienced a significant reduction in MPAP and PVR. Six patients were listed for liver transplant, 4 of who were transplanted successfully and alive up to 5 years later.
The Baylor University Medical Center published their data on POPH patients who received liver transplants in 2007.11 POPH was confirmed by right heart catheterization in 30 patients evaluated for liver transplant. Sixteen patients were considered to be suitable candidates for transplant and MPAP was decreased to less than 35 mmHg in 12 patients with vasodilator therapy. Eleven patients eventually underwent liver transplant and 1- and 5-year survival rates were 91% and 67%.
Compared to medical therapy or liver transplant alone, patients who receive medical therapy followed by liver transplantation have the best survival. The Mayo Clinic retrospectively reviewed 74 POPH patients identified between 1994 and 2007.40 Patients were categorized in 1 of 3 categories: no medical therapy, medical therapy alone for POPH, or medical therapy for POPH followed by liver transplantation. Patients who received no medical therapy for POPH and no liver transplant had the worst outcomes, with a dismal 5-year survival of only 14% with over 50% deceased at 1 year of diagnosis. Five-year survival was 45% in patients who received medical therapy only. Patients who received medical therapy with prostacyclin followed by liver transplantation had the best outcomes, with a 5-year survival of 67% versus 25% in those who were transplanted without prior prostacyclin therapy.
We reported the longest follow-up study for patients undergoing liver transplantation with POPH in 2014.41 Seven patients with moderate to severe POPH received a liver transplant at our institution between June 2004 and January 2011. Mean pulmonary artery pressure was reduced to < 35 mm Hg, with appropriate POPH therapy in all of the patients. Both the graft and patient survival rates were 85.7% after a median follow-up of 7.8 years. The 1 patient who did not survive died from complications related to recurrent hepatitis C and cirrhosis, not from POPH-related issues. Four of the remaining 6 patients continue to require oral vasodilator therapy post-transplant, suggesting irreversible remodeling of the pulmonary vasculature. Two patients (4.4 and 8.5 years post-transplant) have no evidence of pulmonary hypertension post-transplant and therefore do not require medical treatment for pulmonary hypertension. We concluded that POPH responsive to vasodilator therapy is an appropriate indication for liver transplant, with excellent long-term survival.
Hollatz et al published their data on 11 patients with moderate to severe POPH who were successfully treated (mostly with oral sildenafil and subcutaneous treprostinil) as a bridge to liver transplant.42 The mortality rate was 0, with a follow-up duration of 7 to 60 months. Interestingly, 7 of the 11 patients (64%) were off all pulmonary vasodilators post-transplant. Ashfaq et al reported similar results.11 Nine of 11 patients with treated moderate to severe POPH who received liver transplants stopped vasodilator therapy at a median period of 9.2 months post-transplant. Raevens et al described a group of 3 patients with POPH who went on to liver transplant after their pulmonary pressures were decreased with combined oral vasodilator therapy: 1 required continued long-term vasodilator therapy, another was weaned off medications after transplant, and the third patient died during the liver transplant from perioperative complications that induced uncontrolled pulmonary hypertension.43
Patient Selection
In 2006, the United Network for Organ Sharing (UNOS) initiated a policy whereby a higher priority for liver transplantation was granted for highly selected patients in the United States.44 UNOS policy 3.6.4.5.6 upgraded POPH patients to a MELD score of 22, with an increase in MELD every 3 months as long as MPAP remained < 35 mm Hg and PVR remained < 400 dynes/s/cm–5. One hundred fifty-five patients were granted MELD exception points for POPH between 2002 and 2010 and went on to receive liver transplants.45 Goldberg et al collected data from the Organ Procurement and Transplantation Network (OPTN) and compared outcomes of patients with approved POPH MELD exception points versus waitlist candidates with no exception points.46 One hundred fifty-five waitlisted patients received POPH MELD exception points, with only 43.1% meeting OPTN exception requirements. One-third did not fulfill hemodynamic criteria consistent with POPH or had missing data, and 80% went on to receive a liver transplant. Waitlist candidates receiving POPH MELD exception points also had increased waitlist mortality and several early post-transplant deaths. The authors felt these data highlighted the need for OPTN/UNOS to revise their policy for POPH MELD exceptions points, revise how points are rewarded, and continue research to help risk stratify these patients to minimize perioperative complications.
Conclusion
Several effective medical treatment regimens are available, including prostanoids, endothelin receptor antagonists, and PDE-5 inhibitors. Liver transplantation is a potential cure but is only recommended if MPAP can be decreased to ≤ 35 mmHg. Long-term follow-up studies have shown these patients do well several years post-transplant but may continue to require oral therapy for their POPH.
Portopulmonary hypertension (POPH) is a form of group 1 pulmonary arterial hypertension. When treating patients with POPH, baseline assessment is necessary so that response to therapy can be measured as the change from baseline. Patients should undergo echocardiography and right heart catheterization, and their exercise capacity and NYHA functional class should be determined. Patients with POPH should be considered for treatment if they are NYHA functional class II or above and/or their mean pulmonary artery pressure (MPAP) is greater than 35 mm Hg in transplant candidates. The goal in the treatment and management of POPH is to improve pulmonary hemodynamics by reducing the obstruction to pulmonary arterial flow and to preserve right ventricular function (Table). This article, the second in a 2-part review of POPH in patients with liver disease, reviews the role of medical therapy and liver transplantation in treatment. Evaluation and diagnosis of POPH are discussed in a separate article.

Medical Therapy
Prostanoids
Although prostacyclin and prostaglandin analogs entered routine clinical practice for POPH in the 1990s, reports of investigational use date back to the 1980s. Prostanoids are potent vasodilators with antiplatelet aggregation and antiproliferative properties. Prostacyclin synthase is reduced in patients with PAH, resulting in decreased concentration of prostacyclin with vasoconstriction and proliferative changes in the pulmonary vasculature.1
Epoprostenol
Epoprostenol is also known as synthetic prostaglandin I2 or prostacyclin. It was the first therapy approved for the treatment of PAH in 1995 by the US Food and Drug Administration (FDA) as a continuous intravenous infusion.2,3 It also inhibits platelet aggregation and may help modulate pulmonary vascular remodeling.4,5 Epoprostenol is derived from the metabolism of arachidonic acid and is a potent pulmonary and systemic vasodilator. One study reported an immediate 11.8% decrease in MPAP, 24% decrease in pulmonary vascular resistance (PVR) and 28% drop in systemic vascular resistance (SVR) during an epoprostenol infusion.6 The authors reported that epoprostenol was a more potent vasodilator than nitric oxide and may have a role in predicting the reversibility of POPH. In a case series of 33 patients with secondary pulmonary hypertension (including 7 patients with POPH) treated with continuous intravenous prostacyclin for approximately 1 year, exercise tolerance, NYHA functional class, and pulmonary hemodynamics improved in each patient compared to baseline.7 Krowka et al studied 14 patients with moderate to severe POPH treated with intravenous epoprostenol.8 No significant side effects were noted and treatment resulted in significant improvements in PVR, MPAP, and cardiac output. In 2007, Fix et al published a large retrospective cohort of patients with moderate to severe POPH.9 Nineteen patients treated with epoprostenol were compared to 17 patients with no treatment. After a median treatment period of 15.4 months, the epoprostenol group showed significant improvement in MPAP, PVR and cardiac output, but survival did not differ between the 2 groups.
Epoprostenol has often been considered a bridge to transplant in patients with POPH. Sussman et al described 8 consecutive patients with POPH who were treated with intravenous epoprostenol (2 to 8 ng/kg/min dose).10 Liver transplant was considered in 7 of the 8 patients when MPAP decreased to less than 35 mm Hg. Six patients were eventually listed for liver transplant, but 2 died waiting on the list. Long-term outcomes in the group of transplanted recipients were excellent. They remained alive and well at least 9 to 18 months post-transplant, and half did not require long-term vasodilator therapy post-orthotopic liver transplant. Similarly, Ashfaq et al published their data on 16 patients with moderate-to-severe POPH who were treated with vasodilator therapy.11 MPAP decreased to acceptable levels in 75% of the treated patients, and 11 went on to liver transplantation. Rates of 1- and 5-year survival in the transplanted patients were 91% and 67% respectively. None of the patients who failed vasodilator therapy survived.
Epoprostenol has a short half-life (3 to 5 minutes) and requires continuous infusion through central access via an infusion pump. Aseptic technique must be maintained to avoid blood stream infections. Pump failure or loss of vascular access can result in rebound pulmonary vasoconstriction that can be life-threatening and requires immediate attention. Side effects associated with epoprostenol include flushing, headache, nausea/vomiting, bradycardia, chest pain, jaw pain, diarrhea, and musculoskeletal pain.
Patients on epoprostenol should be monitored for prostanoid overdose. In the case of patients with chronic liver disease, epoprostenol increases systemic vasodilation in patients with already low systemic vascular tone. As a result, cardiac output may increase to the point of high cardiac output failure. MPAP will remain elevated secondary to high cardiac output rather than high PVR. In these patients, right heart catheterization will show an elevated MPAP in the setting of normal to low PVR/transpulmonary gradient (TPG) values. Lowering the epoprostenol dose will successfully reduce both cardiac output and MPAP.
Treprostinil
Treprostinil is a prostacyclin analog that is available in intravenous, inhalational, and subcutaneous form, although subcutaneous dosing may be limited by pain. Sakai et al published a small case series of 3 patients with PAH and end-stage liver disease treated with intravenous treprostinil.12 Pulmonary hemodynamics improved in all patients, and 2 patients went on to an uneventful liver transplantation. More than 10 years later, data were published on 255 patients with PAH on therapy with bosentan or sildenafil randomized to additional inhaled treprostinil.13 Treprostinil proved to be safe and well tolerated, with improvement in quality of life measures but no improvement in other secondary endpoints.
Iloprost
Inhaled iloprost is another prostacyclin that has a short therapeutic half-life of 20 to 30 minutes and requires frequent administration (6 to 9 times daily). In study in which patients with severe POPH were treated for up to 3 years with inhaled iloprost,14 survival rates at 1, 2, and 3 years were 77%, 62%, and 46%, respectively. A second study published in 2010 was designed to assess the acute effects of inhaled iloprost on pulmonary hemodynamics and evaluate the clinical outcome after 12 months of treatment.15 Iloprost was found to rapidly reduce pulmonary arterial pressure and PVR. In the long-term evaluation, inhaled iloprost increased the 6-minute walk distance (6MWD) and functional class, but no change was noted in the systolic pulmonary artery pressure. The authors concluded that iloprost might provide symptomatic improvement and improvement in exercise capacity.
Selexipag
Selexipag is an oral selective IP prostacyclin receptor agonist that is structurally distinct from other prostacyclins.16 In a phase 3 randomized double blind clinical trial, PAH patients treated with selexipag had lower composite of death or complication of PAH to the end of the study period.17 This effect was consistent across all dose ranges, but POPH patients were excluded from this study. Safety and efficacy of selexipag has not been evaluated in POPH patients.
Endothelin Receptor Antagonists
Endothelin receptor antagonists block the production of endothelin-1 (ET-1), a potent vasoconstrictor and smooth muscle mitogen that may contribute to the development of PAH. Three different receptors have been described: endothelin A, endothelin B, and endothelin B2. Elevated ET-1 levels have been reported in patients with chronic liver disease and may originate from hepatosplanchnic circulation.18
Bosentan
Bosentan is an oral, nonspecific, ET-1A and ET-1B receptor antagonist. Initial use of bosentan in patients with POPH was limited because of concern for hepatotoxicity. Approximately 10% of patients on bosentan were reported to have mild hepatic side effects in the form of elevated aminotransferases, but severe injury has been reported.19 One of the first clinical experiences of bosentan in patients with POPH was published in 2005. Hoeper et al followed 11 patients with Child A cirrhosis and severe POPH.20 All patients included were in NYHA functional class III or IV and were treated with bosentan for over 1 year. Exercise capacity and symptoms improved in all treated patients. The medication was tolerated well and there was no evidence of drug-induced liver injury. A single case report showed the effectiveness of bosentan in a 43-year-old man with alcohol-related liver disease (Child-Pugh A) and right ventricular enlargement and dysfunction secondary to POPH.21 Pulmonary arterial pressure decreased, exercise capacity increased, and improvement was maintained over 2 years.
In a group of 31 patients with Child A or B cirrhosis and severe POPH, bosentan had significantly better effects than inhaled iloprost on exercise capacity, hemodynamics, and survival.14 One, 2, and 3-year survival rates in the bosentan group were 94%, 89%, and 89% (compared to 77%, 62%, and 46% in the iloprost group). Both drugs were considered safe with no reported hepatotoxicity. In 2013, Savale et al published data on 34 patients with POPH, Child-Pugh A and/or B who were treated with bosentan for a median of 43 months.22 The authors reported significant improvements in hemodynamics, NYHA functional class, and 6WMD. Event-free survival rates at 1, 2, and 3 years were 82%, 63%, and 47%, respectively.
Ambrisentan
Ambrisentan is a highly selective ET-1A receptor antagonist with once daily dosing and a lower risk of hepatotoxicity compared to bosentan. Fourteen patients with moderate to severe POPH treated with ambrisentan in 4 German hospitals were retrospectively analyzed.23 Median follow-up was 16 months, and the study demonstrated significant improvement in exercise capacity and clinical symptoms without significant change in liver function tests. Cartin-Ceba et al published their experience of 13 patients with moderate to severe POPH treated with ambrisentan monotherapy.24 Patients were followed for a median of 613 days and on treatment for a median time of 390 days. Significant improvements were shown in pulmonary arterial pressure and PVR without adverse effect on hepatic function. Over 270 patients with PAH (6% with POPH) received ambrisentan from March 2009 through June 2013 at a large United Kingdom portal hypertension referral center.25 Discontinuation due to side effects was higher than previously reported. Discontinuation due to abnormal transaminases was uncommon.
Macitentan
Macitentan is a dual endothelin-receptor antagonist developed by modifying the structure of bosentan to increase efficacy and safety. The SERAPHIN trial compared oral macitentan to placebo in 250 patients with moderate to severe PAH, some of whom were also on a stable dose of oral or inhaled therapy for PAH.26 Over a 2-year period, patients treated with macitentan were less likely to have progression of their disease or die on therapy (38% and 31% versus 46%), regardless of if they were receiving additional oral therapy and more likely to have improvement of their exercise capacity and WHO functional class. Nasopharyngitis and significant anemia were more common in the macitentan group, but there was no difference in the rate of liver function test abnormalities compared to placebo. Trials with macitentan are currently ongoing in patients with POPH.
Phosphodiesterase-5 Inhibitors
Cyclic guanosine monophosphate (cGMP) is the mediator of nitric oxide–induced vasodilation. Phosphodiesterase-5 (PDE-5) inhibitors prolong the vasodilatory effects of cyclic guanosine monophosphate by preventing its hydrolysis, thereby reducing the pulmonary arterial pressure.
Sildenafil
Sildenafil is the most widely accepted PDE-5 inhibitor for POPH. Fourteen patients with moderate to severe POPH were treated with sildenafil (50 mg 3 times per day) in an observational study published by Reichenberger et al in 2006.27 Eight patients were newly started on sildenafil, whereas sildenafil was added to inhaled prostanoids in the remaining 6x patients. Sildenafil significantly decreased 66MWD, MPAP, PVR, and cardiac index alone or in combination with inhaled prostanoids.
Sildenafil has also been used as a bridge to transplant in liver transplant candidates with POPH. Ten patients with POPH treated with sildenafil monotherapy were followed for a 21±16 months.28 Patients improved symptomatically and increased their 6MWD at 1 year by 30 meters or more. Three patients became transplant eligible and another 3 patients were stable, without progression of their liver disease or POPH. Four patients were not considered transplant candidates, 2 because of refractory POPH and 2 for other comorbidities. The authors concluded that sildenafil monotherapy could stabilize or improve pulmonary hemodynamics in patients with POPH and eventually lead to liver transplantation. Gough et al took a similar look at 9 patients with POPH treated with sildenafil.29 All patients had initial and follow-up right heart catheterizations within a period of 3 years. Mean PVR improved in all patients, decreasing from 575 to 375 dynes/s/cm–5. MPAP decreased to ≤ 35 mmHg in 4 patients, 1 of whom went on to receive a liver transplant. Overall sildenafil improved pulmonary hemodynamics in this small cohort of POPH patients.
Tadalafil
Tadalafil is another oral PDE-5 inhibitor but with a longer half-life than sildenafil. Unlike sildenafil, which requires 3 times daily dosing, tadalafil requires once daily administration. A few case reports have demonstrated tadalafil’s effectiveness for POPH in combination with other medical therapy (eg, sildenafil, ambrisentan).30,31
Guanylate Cyclase Stimulator
Riociguat
Riociguat is a first-in-class activator of soluble form of guanylate cyclase that increases levels of cyclic GMP. Two randomized clinical trials, PATENT, a study in PAH patients, and CHEST, a study in patients with chronic thromboembolic pulmonary hypertension showed improvement in 6MWD at 12 weeks (PATENT) or 16 weeks (CHEST), with improvement in secondary endpoints such as PVR, N-terminal pro b-type natriuretic peptide and WHO functional class.32,33 Riociguat may have potential advantages in patients with POPH given that it has a favorable liver safety profile. A subgroup analysis of patients enrolled in the PATENT study showed that 13 had POPH and 11 were randomized to receive riociguat 2.5 mg 3 times daily dose and 2 received placebo.34 Riociguat was well tolerated and improved 6MWD that was maintained over 2 years in the open label extension.
Medications to Avoid
Nonselective beta-blockers are commonly recommended in patients with portal hypertension to help prevent variceal hemorrhage. However, in patients with POPH, beta-blockers have been shown to decrease exercise capacity and worsen pulmonary hemodynamics. A study of 10 patients with moderate to severe POPH who were receiving beta-blockers for variceal bleeding prophylaxis showed that 6MWD improved in almost all of the patients, cardiac output increased by 28%, and PVR decreased by 19% when beta-blockers were discontinued.35 The authors concluded that the use of beta-blockers should be avoided in this patient population.
Calcium channel blockers should not be used in patients with POPH because they can cause significant hypotension due to systemic vasodilatation and decreased right ventricular filling. Patients with portal hypertension and chronic liver disease commonly have low systemic vascular resistance and are particularly susceptible to the deleterious effects of calcium channel blockers.
Transplantation
Liver transplantation is a potential cure for POPH and its role in POPH has evolved over the past 2 decades. In 1997, Ramsay et al published their review of 1205 consecutive liver transplants at Baylor University Medical Center (BUMC) in Texas.36 The incidence of POPH in this group was 8.5%, with the majority of patients having mild POPH. Liver transplant outcomes were not affected by mild and moderate pulmonary hypertension. However, patients with severe POPH (n = 7, systolic pulmonary artery pressure > 60 mm Hg) had a mortality rate of 42% at 9 months post-transplantation and 71% at 36 months post-transplant. The surviving patients continued to deteriorate with progressive right heart failure and no improvement in POPH.
To understand the effect of liver transplantation on POPH, one must understand the hemodynamic changes that occur with POPH and during liver transplant. The right ventricle is able to manage the same volume as the left ventricle under normal circumstances, but is unable to pump against a significant pressure gradient.37 In the setting of POPH, right ventricular hypertrophy occurs and RV output remains stable for some time. With time, pulmonary artery pressure increases secondary to pulmonary arteriolar vasoconstriction, intimal thickening, and progressive occlusion of the pulmonary vascular bed. Right ventricular failure may occur as a result. Cardiac output increases significantly at the time of reperfusion during liver transplant (up to 3-fold in 15 minutes),38 and in the setting of a noncompliant vascular bed, the patient is at risk for right heart failure. This is the likely explanation to such high perioperative mortality rates in patients with uncontrolled POPH. Failure to decrease MPAP to less than 50 mm Hg is considered a complete contraindication to liver transplant at most institutions. Many transplant centers will list patients for liver transplant if MPAP can be decreased to less than 35 mm Hg and PVR < 400 dynes/s/cm–5. These parameters are thought to represent an adequate right ventricular reserve and a compliant pulmonary vascular bed.37 However, even with good pressure control, the anesthesiology and critical care teams must be prepared to deal with acute right heart failure peri-operatively. Intraoperative transesophageal echocardiography has been recommended to closely follow right ventricular function.38 Inhaled or intravenous dilators are the most effective agents in the event of a pulmonary hypertensive crisis.
Review of Outcomes
A retrospective review evaluated 43 patients with untreated POPH who underwent attempted liver transplantation.39 Data were collected from 18 peer-reviewed studies and 7 patients at the authors’ institution. Overall mortality was 35% (15 patients), with almost all of the deaths secondary to cardiac dysfunction. Two deaths occurred intraoperatively and 8 deaths occurred during the transplant hospitalization. The transplant could not be successfully completed in 4 of the patients. MPAP > 50 mm Hg was associated with 100% mortality, whereas patients with MPAP between 35 mm Hg and 50 mm Hg had a 50% mortality. No mortality was noted in patients with MPAP < 35 mm Hg.
Liver transplantation has been shown to be successful in patients with controlled POPH. Sussman et al published their data on 8 patients with severe POPH in 2006. In this prospective study, all patients were treated with sequential epoprostenol infusions and 7 of the 8 patients experienced a significant reduction in MPAP and PVR. Six patients were listed for liver transplant, 4 of who were transplanted successfully and alive up to 5 years later.
The Baylor University Medical Center published their data on POPH patients who received liver transplants in 2007.11 POPH was confirmed by right heart catheterization in 30 patients evaluated for liver transplant. Sixteen patients were considered to be suitable candidates for transplant and MPAP was decreased to less than 35 mmHg in 12 patients with vasodilator therapy. Eleven patients eventually underwent liver transplant and 1- and 5-year survival rates were 91% and 67%.
Compared to medical therapy or liver transplant alone, patients who receive medical therapy followed by liver transplantation have the best survival. The Mayo Clinic retrospectively reviewed 74 POPH patients identified between 1994 and 2007.40 Patients were categorized in 1 of 3 categories: no medical therapy, medical therapy alone for POPH, or medical therapy for POPH followed by liver transplantation. Patients who received no medical therapy for POPH and no liver transplant had the worst outcomes, with a dismal 5-year survival of only 14% with over 50% deceased at 1 year of diagnosis. Five-year survival was 45% in patients who received medical therapy only. Patients who received medical therapy with prostacyclin followed by liver transplantation had the best outcomes, with a 5-year survival of 67% versus 25% in those who were transplanted without prior prostacyclin therapy.
We reported the longest follow-up study for patients undergoing liver transplantation with POPH in 2014.41 Seven patients with moderate to severe POPH received a liver transplant at our institution between June 2004 and January 2011. Mean pulmonary artery pressure was reduced to < 35 mm Hg, with appropriate POPH therapy in all of the patients. Both the graft and patient survival rates were 85.7% after a median follow-up of 7.8 years. The 1 patient who did not survive died from complications related to recurrent hepatitis C and cirrhosis, not from POPH-related issues. Four of the remaining 6 patients continue to require oral vasodilator therapy post-transplant, suggesting irreversible remodeling of the pulmonary vasculature. Two patients (4.4 and 8.5 years post-transplant) have no evidence of pulmonary hypertension post-transplant and therefore do not require medical treatment for pulmonary hypertension. We concluded that POPH responsive to vasodilator therapy is an appropriate indication for liver transplant, with excellent long-term survival.
Hollatz et al published their data on 11 patients with moderate to severe POPH who were successfully treated (mostly with oral sildenafil and subcutaneous treprostinil) as a bridge to liver transplant.42 The mortality rate was 0, with a follow-up duration of 7 to 60 months. Interestingly, 7 of the 11 patients (64%) were off all pulmonary vasodilators post-transplant. Ashfaq et al reported similar results.11 Nine of 11 patients with treated moderate to severe POPH who received liver transplants stopped vasodilator therapy at a median period of 9.2 months post-transplant. Raevens et al described a group of 3 patients with POPH who went on to liver transplant after their pulmonary pressures were decreased with combined oral vasodilator therapy: 1 required continued long-term vasodilator therapy, another was weaned off medications after transplant, and the third patient died during the liver transplant from perioperative complications that induced uncontrolled pulmonary hypertension.43
Patient Selection
In 2006, the United Network for Organ Sharing (UNOS) initiated a policy whereby a higher priority for liver transplantation was granted for highly selected patients in the United States.44 UNOS policy 3.6.4.5.6 upgraded POPH patients to a MELD score of 22, with an increase in MELD every 3 months as long as MPAP remained < 35 mm Hg and PVR remained < 400 dynes/s/cm–5. One hundred fifty-five patients were granted MELD exception points for POPH between 2002 and 2010 and went on to receive liver transplants.45 Goldberg et al collected data from the Organ Procurement and Transplantation Network (OPTN) and compared outcomes of patients with approved POPH MELD exception points versus waitlist candidates with no exception points.46 One hundred fifty-five waitlisted patients received POPH MELD exception points, with only 43.1% meeting OPTN exception requirements. One-third did not fulfill hemodynamic criteria consistent with POPH or had missing data, and 80% went on to receive a liver transplant. Waitlist candidates receiving POPH MELD exception points also had increased waitlist mortality and several early post-transplant deaths. The authors felt these data highlighted the need for OPTN/UNOS to revise their policy for POPH MELD exceptions points, revise how points are rewarded, and continue research to help risk stratify these patients to minimize perioperative complications.
Conclusion
Several effective medical treatment regimens are available, including prostanoids, endothelin receptor antagonists, and PDE-5 inhibitors. Liver transplantation is a potential cure but is only recommended if MPAP can be decreased to ≤ 35 mmHg. Long-term follow-up studies have shown these patients do well several years post-transplant but may continue to require oral therapy for their POPH.
1. Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925-1932.
2. Chin K, Rubin L. Pulmonary arterial hypertension. Am Coll Cardiol. 2008;51:1527-1538.
3. Doran A, Harris S, Goetz B. Advances in prostanoid infusion therapy for pulmonary arterial hypertension. J Infus Nurs. 2008;31:336-345.
4. Chin KM, Channick RN, De Lemos JA, ET AL. Hemodynamics and epoprostenol use are associated with thrombocytopenia in pulmonary arterial hypertension. Chest. 2009;135:130-136.
5. Hoshikawa Y, Voelkel NF, Gesell TL, et al. Prostacyclin receptor-dependent modulation of pulmonary vascular remodeling. Am J Respir Crit Care Med. 2001;164:314-318.
6. Ricci GL, Melgosa MT, Burgos F, et al. Assessment of acute pulmonary vascular reactivity in portopulmonary hypertension. Liver Transplant. 2007;13:1506-1514.
7. McLaughlin V V, Genthner DE, Panella MM, et al. Compassionate use of continuous prostacyclin in the management of secondary pulmonary hypertension: a case series. Ann Intern Med. 1999;130:740-743.
8. Krowka MJ, Frantz RP, McGoon MD, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology. 1999;30:641-648.
9. Fix OK, Bass NM, De Morco T, Merriman RB. Long-term follow-up of portopulmonary hypertension: Effect of treatment with epoprostenol. Liver Transplant. 2007;13:875-885.
10. Sussman N, Kaza V, Barshes N, et al. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant. 2006;6:2177-2182.
11. Ashfaq M, Chinnakotla S, Rogers L, et al. The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant. 2007;7:1258-1264.
12. Sakai T, Planinsic RM, Mathier MA, et al. initial experience using continuous intravenous treprostinil to manage pulmonary arterial hypertension in patients with end-stage liver disease. Transpl Int. 2009;22:554-561.
13. McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: A randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915-1922.
14. Hoeper MM, Seyfarth HJ, Hoeffken G, et al. Experience with inhaled iloprost and bosentan in portopulmonary hypertension. Eur Respir J. 2007;30:1096-1102.
15. Melgosa MT, Ricci GL, Garcia-Pagan JC et al. Acute and long-term effects of inhaled iloprost in portopulmonary hypertension. Liver Transplant. 2010;16:348-356.
16. Simonneau G, Torbicki A, Hoeper MM, et al. Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J. 2012;40:874-880
17. Sitbon O, Channick R, Chin, KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522-2533.
18. Moller S, Gulberg V, Henriksen JH, Gerbes AL. Endothelin-1 and endothelin-3 in cirrhosis: Relations to systemic and splanchnic haemodynamics. J Hepatol. 1995;23:135-144.
19. Eriksson C, Gustavsson A, Kronvall T, Tysk C. Hepatotoxicity by bosentan in a patient with portopulmonary hypertension : a case-report and review of the literature. J Gastrointestin Liver Dis. 2011;20:77-80.
20. Hoeper MM, Halank M, Marx C, et al. Bosentan therapy for portopulmonary hypertension. Eur Respir J. 2005;25:502-508.
21. Stähler G, Von Hunnius P. Successful treatment of portopulmonary hypertension with bosentan: Case report.: Eur J Clin Investig. 2006;36:62-66.
22. Savale L, Magnier R, Le Pavec J, et al. Efficacy, safety and pharmacokinetics of bosentan in portopulmonary hypertension. Eur. 2013;41:96-103.
23. Halank M, Knudsen L, Seyfarth H, et al. Ambrisentan improves exercise capacity and symptoms in patients with portopulmonary hypertension. Z Gastroenterol. 2011;49:1258-1262.
24. Cartin-Ceba R, Swanson K, Iyer V, et al. Safety and efficacy of ambrisentan for the treatment of portopulmonary hypertension. Chest. 2011;139:109-114.
25. Condliffe R, Elliot C, Hurdman J, et al. Ambrisentan therapy in pulmonary hypertension: clinical use and tolerability in a referral centre. Ther Adv Respir Dis. 2014;8:71-77.
26. Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809-818.
27. Reichenberger F, Voswinckel R, Steveling E, et al. Sildenafil treatment for portopulmonary hypertension. Eur Respir J. 2006;28:563-567.
28. Hemnes AR RI. Sildenafil monotherapy in portopulmonary hypertension can facilitate liver transplantation. Liver Transplant. 2009;15:15-19.
29. Gough WR. Sildenafil therapy is associated with improved hemodynamics in liver transplantation candidates with pulmonary arterial hypertension. Liver Transplant. 2009;15:30-36.
30. Yamashita Y. Hemodynamic effects of ambrisentan-tadalafil combination therapy on progressive portopulmonary hypertension. World J Hepatol. 2014;6:825.
31. Bremer HC, Kreisel W, Roecker K, et al. Phosphodiesterase 5 inhibitors lower both portal and pulmonary pressure in portopulmonary hypertension: a case report. J Med Case Rep. 2007;1:46.
32. Ghofrani HA, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013:369;330-340.
33. Ghofrani HA, Galie N, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013:369;319-329
34. Cartin-Ceba R, Halank M, Ghofrani HA, et al. Riociguat treatment for portopulmonary hypertension: a subgroup analysis from the PATENT-1/-2 studies. Pulm Circ. 2018: 8:2045894018769305.
35. Provencher S, Herve P, Jais X, et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. 2006. Gastroenterology. 2006;130:120-126.
36. Ramsay M a, Simpson BR, Nguyen T, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3:494-500.
37. Safdar Z, Bartolome S, Sussman N. Portopulmonary hypertension : an update. Liver Tranpl. 2012;18:881-891.
38. Ramsay M. Portopulmonary hypertension and right heart failure in patients with cirrhosis. Curr Opin Anaesthesiol. 2010;23:145-150.
39. Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443-450.
40. Swanson KL, Wiesner RH, Nyberg SL, et al. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445-2453.
41. Khaderi S, Khan R, Safdar Z, et al. Long-term follow-up of portopulmonary hypertension patients after liver transplantation. Liver Transplant. 2014;20:724-727.
42. Hollatz TJ, Musat A, Westphal S, et al. Treatment with sildenafil and treprostinil allows successful liver transplantation of patients with moderate to severe portopulmonary hypertension. Liver Transpl. 2012:686-695.
43. Raevens S, De Pauw M, Reyntjens K, et al. Oral vasodilator therapy in patients with moderate to severe portopulmonary hypertension as a bridge to liver transplantation. Eur J Gastroenterol Hepatol. 2012:1-8.
44. Krowka M, Fallon M, Mulligan D. Model for end-stage liver disease (MELD) exception for portopulmonary hypertension. Liver Transplant. 2006;12:S114-S116.
45. Krowka M, Wiesner R, Rosen C. Portopulmonary hypertension outcomes in the era of MELD exception. Liver Transplant. 2012;18:S259.
46. Goldberg DS, Batra S, Sahay S, et al. MELD Exceptions for portopulmonary hypertension: current policy and future implementation. Am J Transplant. 2014;14:2081-2087.
1. Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925-1932.
2. Chin K, Rubin L. Pulmonary arterial hypertension. Am Coll Cardiol. 2008;51:1527-1538.
3. Doran A, Harris S, Goetz B. Advances in prostanoid infusion therapy for pulmonary arterial hypertension. J Infus Nurs. 2008;31:336-345.
4. Chin KM, Channick RN, De Lemos JA, ET AL. Hemodynamics and epoprostenol use are associated with thrombocytopenia in pulmonary arterial hypertension. Chest. 2009;135:130-136.
5. Hoshikawa Y, Voelkel NF, Gesell TL, et al. Prostacyclin receptor-dependent modulation of pulmonary vascular remodeling. Am J Respir Crit Care Med. 2001;164:314-318.
6. Ricci GL, Melgosa MT, Burgos F, et al. Assessment of acute pulmonary vascular reactivity in portopulmonary hypertension. Liver Transplant. 2007;13:1506-1514.
7. McLaughlin V V, Genthner DE, Panella MM, et al. Compassionate use of continuous prostacyclin in the management of secondary pulmonary hypertension: a case series. Ann Intern Med. 1999;130:740-743.
8. Krowka MJ, Frantz RP, McGoon MD, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology. 1999;30:641-648.
9. Fix OK, Bass NM, De Morco T, Merriman RB. Long-term follow-up of portopulmonary hypertension: Effect of treatment with epoprostenol. Liver Transplant. 2007;13:875-885.
10. Sussman N, Kaza V, Barshes N, et al. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant. 2006;6:2177-2182.
11. Ashfaq M, Chinnakotla S, Rogers L, et al. The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant. 2007;7:1258-1264.
12. Sakai T, Planinsic RM, Mathier MA, et al. initial experience using continuous intravenous treprostinil to manage pulmonary arterial hypertension in patients with end-stage liver disease. Transpl Int. 2009;22:554-561.
13. McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: A randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915-1922.
14. Hoeper MM, Seyfarth HJ, Hoeffken G, et al. Experience with inhaled iloprost and bosentan in portopulmonary hypertension. Eur Respir J. 2007;30:1096-1102.
15. Melgosa MT, Ricci GL, Garcia-Pagan JC et al. Acute and long-term effects of inhaled iloprost in portopulmonary hypertension. Liver Transplant. 2010;16:348-356.
16. Simonneau G, Torbicki A, Hoeper MM, et al. Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J. 2012;40:874-880
17. Sitbon O, Channick R, Chin, KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522-2533.
18. Moller S, Gulberg V, Henriksen JH, Gerbes AL. Endothelin-1 and endothelin-3 in cirrhosis: Relations to systemic and splanchnic haemodynamics. J Hepatol. 1995;23:135-144.
19. Eriksson C, Gustavsson A, Kronvall T, Tysk C. Hepatotoxicity by bosentan in a patient with portopulmonary hypertension : a case-report and review of the literature. J Gastrointestin Liver Dis. 2011;20:77-80.
20. Hoeper MM, Halank M, Marx C, et al. Bosentan therapy for portopulmonary hypertension. Eur Respir J. 2005;25:502-508.
21. Stähler G, Von Hunnius P. Successful treatment of portopulmonary hypertension with bosentan: Case report.: Eur J Clin Investig. 2006;36:62-66.
22. Savale L, Magnier R, Le Pavec J, et al. Efficacy, safety and pharmacokinetics of bosentan in portopulmonary hypertension. Eur. 2013;41:96-103.
23. Halank M, Knudsen L, Seyfarth H, et al. Ambrisentan improves exercise capacity and symptoms in patients with portopulmonary hypertension. Z Gastroenterol. 2011;49:1258-1262.
24. Cartin-Ceba R, Swanson K, Iyer V, et al. Safety and efficacy of ambrisentan for the treatment of portopulmonary hypertension. Chest. 2011;139:109-114.
25. Condliffe R, Elliot C, Hurdman J, et al. Ambrisentan therapy in pulmonary hypertension: clinical use and tolerability in a referral centre. Ther Adv Respir Dis. 2014;8:71-77.
26. Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809-818.
27. Reichenberger F, Voswinckel R, Steveling E, et al. Sildenafil treatment for portopulmonary hypertension. Eur Respir J. 2006;28:563-567.
28. Hemnes AR RI. Sildenafil monotherapy in portopulmonary hypertension can facilitate liver transplantation. Liver Transplant. 2009;15:15-19.
29. Gough WR. Sildenafil therapy is associated with improved hemodynamics in liver transplantation candidates with pulmonary arterial hypertension. Liver Transplant. 2009;15:30-36.
30. Yamashita Y. Hemodynamic effects of ambrisentan-tadalafil combination therapy on progressive portopulmonary hypertension. World J Hepatol. 2014;6:825.
31. Bremer HC, Kreisel W, Roecker K, et al. Phosphodiesterase 5 inhibitors lower both portal and pulmonary pressure in portopulmonary hypertension: a case report. J Med Case Rep. 2007;1:46.
32. Ghofrani HA, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013:369;330-340.
33. Ghofrani HA, Galie N, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013:369;319-329
34. Cartin-Ceba R, Halank M, Ghofrani HA, et al. Riociguat treatment for portopulmonary hypertension: a subgroup analysis from the PATENT-1/-2 studies. Pulm Circ. 2018: 8:2045894018769305.
35. Provencher S, Herve P, Jais X, et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. 2006. Gastroenterology. 2006;130:120-126.
36. Ramsay M a, Simpson BR, Nguyen T, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3:494-500.
37. Safdar Z, Bartolome S, Sussman N. Portopulmonary hypertension : an update. Liver Tranpl. 2012;18:881-891.
38. Ramsay M. Portopulmonary hypertension and right heart failure in patients with cirrhosis. Curr Opin Anaesthesiol. 2010;23:145-150.
39. Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443-450.
40. Swanson KL, Wiesner RH, Nyberg SL, et al. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445-2453.
41. Khaderi S, Khan R, Safdar Z, et al. Long-term follow-up of portopulmonary hypertension patients after liver transplantation. Liver Transplant. 2014;20:724-727.
42. Hollatz TJ, Musat A, Westphal S, et al. Treatment with sildenafil and treprostinil allows successful liver transplantation of patients with moderate to severe portopulmonary hypertension. Liver Transpl. 2012:686-695.
43. Raevens S, De Pauw M, Reyntjens K, et al. Oral vasodilator therapy in patients with moderate to severe portopulmonary hypertension as a bridge to liver transplantation. Eur J Gastroenterol Hepatol. 2012:1-8.
44. Krowka M, Fallon M, Mulligan D. Model for end-stage liver disease (MELD) exception for portopulmonary hypertension. Liver Transplant. 2006;12:S114-S116.
45. Krowka M, Wiesner R, Rosen C. Portopulmonary hypertension outcomes in the era of MELD exception. Liver Transplant. 2012;18:S259.
46. Goldberg DS, Batra S, Sahay S, et al. MELD Exceptions for portopulmonary hypertension: current policy and future implementation. Am J Transplant. 2014;14:2081-2087.
Portopulmonary Hypertension: Evaluation and Diagnosis
Pulmonary arterial hypertension (PAH) is a rare disease that is associated with high mortality and is characterized by pulmonary vascular remodeling. Portopulmonary hypertension (POPH) is a form of PAH that occurs in patients with portal hypertension where no alternative cause of PAH can be identified. POPH is documented in approximately 4.5% to 8.5% of liver transplant candidates,1,2 but there is no relationship between the existence or severity of POPH and the severity of liver dysfunction.3 Mantz and Craig described the first case of POPH in a 53-year-old woman with enlarged pulmonary arteries that exhibited forceful pulsations more characteristic of the aorta than a low-pressure pulmonary trunk.4 Autopsy revealed findings of chronic liver disease including a stenotic portal vein, portocaval shunt, and esophageal varices. In both PAH and POPH, pre-capillary pulmonary arteries have characteristic lesions, such as intimal thickening, endothelial proliferation, and thrombotic changes. This 2-part article reviews the diagnosis and treatment of patients with POPH. Here, we review the epidemiology, prognosis, pathogenesis, and diagnosis of POPH; current treatment options for POPH are reviewed in a separate article.
Definition
The term POPH was first used by Yoshida et al in 1993 to describe the first successful liver transplant in a patient with POPH, a 39-year-old man with chronic hepatitis.5 The World Health Organization (WHO) classifies POPH as a form of Group 1 PAH.6 The criteria that must be met to make a diagnosis of POPH are shown in the Table 1.7
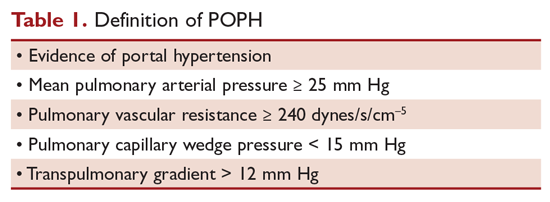
Moderate POPH is defined as a mean pulmonary artery pressure (MPAP) between 35 mm Hg and < 45 mm Hg, whereas severe POPH is MPAP ≥ 45 mm Hg. Moderate and severe POPH are considered contraindications to liver transplant because of high perioperative and postoperative mortality rates.8 In 2000, the Mayo Clinic retrospectively reviewed 43 patients with POPH who underwent attempted liver transplantation.9 The cardiopulmonary-related mortality rate in patients with a MPAP of 35 to < 50 mm Hg was 50% and 100% for those with MPAP > 50 mm Hg. No mortality was noted in patients with a pre-liver transplant MPAP of < 35 mm Hg and transpulmonary gradient (TPG) < 15 mm Hg.
Epidemiology
In 1983, a series of 17,901 autopsied patients showed a primary pulmonary hypertension prevalence of 0.13% and a prevalence of 0.73% in patients with cirrhosis.10 In 1987, Rich et al published data from the National Institutes of Health’s national registry of primary pulmonary hypertension.11 The registry included data from 187 patients from 32 centers. Further analyses by Groves et al concluded that 8.3% of the patients likely had POPH.12 Humbert et al published data on the French pulmonary hypertension registry experience in 2006.13 The French registry included 674 patients from 17 university hospitals; 10.4% of these patients had POPH. The largest prospective study was published by Hadengue et al in 1991.14 In this study, 507 patients hospitalized with portal hypertension but without known pulmonary hypertension underwent cardiac catheterization; 10 patients (2%) had pulmonary hypertension and more than half were clinically asymptomatic. Finally, the Registry to Evaluate Early And Long-term pulmonary arterial hypertension disease management (REVEAL registry) documented a 5.3% frequency of POPH (174 of 3525) in the United States.15
Prognosis
Individuals with POPH have worse outcomes compared to other forms of PAH. Median survival prior to the introduction of vasodilator therapy was a dismal 6 months and mean survival was 15 months.16 The cause of death in patients with POPH is equally distributed between right heart failure from POPH and direct complications of chronic liver disease.1 Le Pavec et al retrospectively analyzed all patients referred to the French Referral Center with POPH between 1984 and 2004 (154 patients).1 Approximately 50% of the patients were Child-Turcotte-Pugh class B or C, and 60% were classified as New York Health Association (NYHA) class III or IV. In these patients, 1-, 3-, and 5-year survival rates were 88%, 75%, and 68%, respectively. Major independent prognostic risk factors were presence and severity of cirrhosis and preservation of right ventricular function. Interestingly, NYHA functional class was not related to survival in this study, although it has clearly been identified as a strong prognostic factor in idiopathic PAH.
Krowka et al evaluated 174 patients with POPH enrolled in the REVEAL Registry,15 a multicenter, observational, US-based study comprised of more than 3500 patients with PAH. Despite having better hemodynamic parameters at diagnosis, patients with POPH had significantly poorer survival and all-cause hospitalization compared with patients with idiopathic PAH (IPAH) or hereditary PAH (HPAH). Two-year survival from enrollment was 67% in POPH versus 85% in those with IPAH/HPAH (P < 0.001). Five-year survival from time of diagnosis was 40% versus 64% (P < 0.001). Additionally, patients with POPH were less likely to be on PAH-specific therapy at enrollment, with only 25% on treatment at the time of entry. These findings were replicated in 2005 when Kawut et al retrospectively compared 13 patients with POPH with 33 patients with IPAH.17 Despite having a higher cardiac index and lower pulmonary vascular resistance than patients with IPAH, patients with POPH had a higher risk of death (hazard ratio, 2.8, P = 0.04), likely reflecting the combination of 2 serious diseases.
In 2008 the Mayo Clinic published their retrospective analysis of patients with POPH to determine the natural history of POPH.18 Patients were categorized into 3 groups: (1) no medical therapy for POPH and no liver transplant; (2) medical therapy for POPH alone; (3) medical therapy for POPH followed by liver transplant. The study included 74 patients between 1994 through 2007; 19 patients who did not receive treatment for POPH or liver transplant truly represented the natural history of POPH. Their 5-year survival was only 14%, and over half were deceased 1 year after diagnosis. The largest group consisted of patients who received therapy for POPH but no liver transplant. This group did remarkably better than those who received no therapy at all, with a 5-year survival of 45%. However, the patients with the overall best survival were those who received a combination of treatment for POPH followed by liver transplant. Their 5-year survival was 67%. Survival at 5 years was only 25% for the small group of patients who received transplant without PAH therapy. Once again, mortality did not correlate with the severity of hepatic dysfunction or baseline hemodynamic data.
Pathogenesis
The pathogenesis of POPH is unclear. Multiple studies have shown that there is minimal, if any, association with pulmonary hypertension and the severity of liver disease or portal hypertension.19,20 Portal hypertension is the result of an increase in intrahepatic resistance and an increase in blood flow into the portal circulation. Collateral vessels develop and blood from the splanchnic circulation is allowed directly into the systemic venous circulation, bypassing the liver. One of the most widely accepted theories is that a humoral substance, that would otherwise be metabolized by the liver, is able to reach the pulmonary circulation through collaterals, resulting in POPH.21 Pelicelli et al evaluated the possible role of endothelin-1, interleukin-6, interleukin 1β, and tumor necrosis factor in the pathogenesis of POPH.22 Plasma concentrations of these cytokines were compared between patients with POPH and patients with cirrhosis but no POPH. Patients with POPH had higher concentrations of endothelin-1 and interleukin-6, suggesting antagonists for these cytokines may have a role in the treatment of POPH. The role of endothelin-1 was further supported by Kamath et al in 200023 when they determined the pulmonary vascular bed is exposed to increased levels of circulating endothelin-1a in the setting of cirrhosis. Endothelin-1 is a potent vasoconstrictor and facilitator of smooth muscle proliferation.
In addition to collateral circulation allowing mediators to reach the pulmonary arterial bed in portal hypertension, high flow may trigger a vasoproliferative process in the pulmonary vascular bed. Patients with advanced liver disease have a low systemic vascular resistance, with a compensatory increase in cardiac output. An increase in cardiac output can lead to shear stress of the pulmonary vascular endothelial layer. Although the resistance of the pulmonary vasculature may decrease rapidly to help normalize pulmonary pressures, persistent circulatory overload could result in irreversible vascular changes. Autopsy and lung explant studies show that POPH is characterized by obstructive and remodeling changes in the pulmonary arterial bed.24 Initially, medial hypertrophy with smooth muscle proliferation is present. As the disease advances, platelet aggregates, in situ thrombosis, and intimal fibrosis develop. Finally, web-like lesions involving the entire pulmonary wall develop with recanalization for the passage of pulmonary arterial flow. These changes are identical to the changes observed in patients with other forms of PAH.
Not all patients with portal hypertension develop POPH, suggesting that genetic predisposition may play a role in POPH development. The Pulmonary Vascular Complications of Liver Study Group published a multicenter case-control study that attempted to identify genetic risk factors for POPH in patients with advanced liver disease.25 More than 1000 common single nucleotide polymorphisms (SNPs) in 93 candidate genes were genotyped in each patient. When compared to controls, multiple SNPs in the genes coding for estrogen receptor 1, aromatase, phosphodiesterase 5, angiopoietin 1, and calcium binding protein A4 were associated with an increased risk of POPH. One year earlier, the same study group concluded that female sex (adjusted odds ratio [OR], 2.90) and autoimmune hepatitis (adjusted OR, 4.02) were associated with a higher risk for POPH, whereas hepatitis C was associated with a decreased risk.20
Clinical Presentation
Clinical presentation is variable in POPH. Patients referred to a pulmonologist will usually present with symptoms similar to patients with other forms of PAH. In a retrospective analysis of patients referred to the French Referral Center for Pulmonary Hypertension, 60% of the patients belonged to NYHA functional class III or IV.1 In a series of 78 patients with POPH, the most common presenting pulmonary symptom was dyspnea on exertion (81%), followed by syncope, chest pain, and fatigue (< 33%).16 Symptoms such as syncope and chest pain are usually markers of severe POPH.3 Stigmata of portal hypertension, such as ascites, spider angiomata, and palmar erythema, may be present on exam. An accentuated pulmonary component of the second heart sound can be seen in 82% of patients and a systolic murmur caused by tricuspid regurgitation in 69% of patients.16 Patients with severe POPH may have jugular vein distention, peripheral edema, and a third heart sound.
Diagnostic Evaluation
Chest x-rays may show prominent pulmonary arteries and cardiomegaly in patients with POPH, whereas electrocardiogram can suggest right ventricular hypertrophy and right axis deviation. The best screening test for POPH in patients with portal hypertension is echocardiography. Routine screening for POPH is recommended during liver transplant evaluation in the practice guidelines from the American Association for the Study of Liver Disease.26 Right-sided cardiac chamber enlargement and right ventricular pressure or volume overload can be assessed on echocardiography. Colle et al followed 165 patients evaluated for liver transplantation who underwent transthoracic Doppler echocardiography and right heart catheterization.27 Seventeen patients met the criteria for POPH on echocardiography (presence of tricuspid regurgitation and calculated systolic pulmonary artery pressure over 30 mm Hg) and right heart catheterization confirmed the diagnosis in 10 patients. Right ventricular systolic pressure (RVSP) estimate of ≤ 30 mm Hg on 2-dimensional echo had a 100% sensitivity and negative predictive value. Positive predictive value was poor at 59%, reiterating the need for right heart catheterization in the diagnosis of POPH. When Kim et al used a RVSP threshold of 50 mm Hg, 72% had at least moderate pulmonary hypertension, including 30% with severe pulmonary hypertension.28 Raevens et al analyzed data from 152 patients who underwent pretransplant echocardiography and catheterization.2 Their data show a RVSP threshold of greater than 38 mm Hg by echocardiography had a specificity of 82% and sensitivity and negative predictive value of 100%. The European Respiratory Society recommendations state that PAH should be considered unlikely if echocardiography estimates a RVSP ≤36 mm Hg and likely if the RVSP is estimated at > 50 mm Hg.29 We recommend repeating echocardiography every 6 to 12 months in patients listed for liver transplantation, as pulmonary hemodynamics may change over time.
Computed tomography (CT) may have a complementary role in the future for the noninvasive detection of POPH. In a study published in 2014, 49 patients referred for liver transplantation were retrospectively reviewed.30 Measured CT signs included the main pulmonary artery/ascending aorta diameter ratio, the mean left and right main pulmonary artery diameter, and the enlargement of the pulmonary artery compared to the ascending aorta. Compared to the transthoracic echocardiography alone, an algorithm incorporating CT and echocardiography improved the detection of POPH (area under curve = 0.8, P < 0.0001).
A diagnosis of POPH can only be confirmed when PAH exists in a patient with portal hypertension, as determined by right heart catheterization, and no other cause of PAH can be identified. MPAP should be 25 mm Hg or greater, PVR of 240 dynes/s/cm–5, wedge pressure of 15 mm Hg or less, and TPG greater than 12 mm Hg. Krowka et al showed the value of right heart catheterization in their 10-year prospective, echocardiography-catheterization algorithm study.19 Of 1235 liver transplant candidates who underwent echocardiography, 104 patients had a RVSP exceeding 50 mm Hg. Almost all of these patients had a right heart catheterization. All cause pulmonary hypertension (MPAP > 25 mm Hg) was confirmed in 90% of the patients, and 35% had a PVR < 240 dynes/s/cm–5 and pulmonary capillary wedge pressure (PCWP) > 15 mm Hg, suggesting fluid overload. Forty-one patients had significant POPH, with a PVR > 400 dynes/s/cm–5, and 24% also had an elevated PCWP. TPG was > 12 mm Hg in all of these patients, confirming POPH. As demonstrated by this study, right heart catheterization is required to confirm the diagnosis of POPH because high flow and fluid overload can lead to elevated pulmonary artery pressures.
Patients with POPH have a unique clinical profile with characteristics common to patients with primary pulmonary hypertension and chronic liver disease. In a retrospective review that compared 30 patients with PAH, 30 patients with chronic liver disease only, and 30 patients with catheterization-proved POPH,31 patients with POPH had elevated MPAP similar to those with primary PAH, but they also had reduced SVR and elevated cardiac index similar to those with chronic liver disease alone.
Besides POPH, 2 other common causes can lead to increased pulmonary arterial blood flow in patients with portal hypertension. First is a high-flow condition caused by increased cardiac output but with a normal PVR and PCWP. Fluid overload can also lead to pulmonary venous hypertension with increased PCWP, normal cardiac output, and normal PVR. Up to 25% of patients with POPH may present with marked excess volume caused by fluid retention.3 There can be an increase in both PCWP and PVR depending on the presence and the degree of fluid retention. TPG (MPAP – PCWP) > 12 mm Hg was introduced to make such patients less confusing and to help correct for increased PCWP secondary to fluid overload. Obstruction to pulmonary arterial flow is manifest by an increased TPG (Table 2).
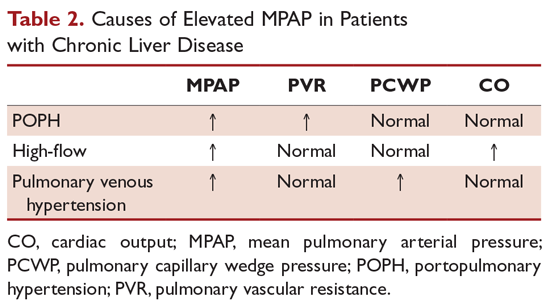
POPH should be distinguished from hepatopulmonary syndrome (HPS), which is another pulmonary vascular consequence of liver disease. Unlike POPH, HPS is characterized by a defect in arterial oxygenation induced by pulmonary vascular dilation.32 Similar to other patients with liver disease, patients with HPS have a normal PVR and increased cardiac output secondary to a high-flow state. HPS is diagnosed by confirmation of an intrapulmonary shunt by echocardiogram. Injection of agitated saline results in saline bubbles being visualized in the left atrium 3 or more cardiac cycles after they appear in the right atrium. Currently, there is no effective medical treatment for HPS and liver transplantation is the only successful treatment.
Conclusion
POPH is an uncommon complication of chronic liver disease. It is defined as PAH in a patient with portal hypertension excluding other causes of PAH. The following criteria must be met to make a diagnosis of POPH: (1) evidence of portal hypertension; (2) MPAP ≥ 35 mm Hg; (3) PVR ≥ 240 dynes/s/cm5; (4) pulmonary capillary wedge pressure ≤ 15 mm Hg; and (5) TPG > 12 mm Hg. Individuals with POPH have worse outcomes compared to other forms of PAH, with a median survival of 6 months without medical therapy. The pathogenesis of POPH is unclear but may be related to a genetic predisposition since not all patients with portal hypertension develop POPH. Echocardiography is an excellent screening test for POPH, but a right heart catheterization must be performed to confirm the diagnosis.
1. Le Pavec J, Souza R, Herve P, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med. 2008;178:637-643.
2. Raevens S, Colle I, Reyntjens K, et al. Echocardiography for the detection of portopulmonary hypertension in liver transplant candidates: An analysis of cutoff values. Liver Transplant. 2013;19:602-610.
3. Krowka MJ. Portopulmonary hypertension. Semin Respir Crit Care Med. 2012;33:17-25.
4. Mantz F. Portal axis thrombosis with spontaneous portocaval shunt and resultant cor pulmonale. AMA Arch Pathol. 1951;52:91-97.
5. Yoshida EM, Erb SR, Pflugfelder PW, et al. Single-lung versus liver transplantation for the treatment of portopulmonary hypertension--a comparison of two patients. Transplantation. 1993;55:688-690.
6. Badesch DB, Champion HC, Gomez Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54 54(1 Suppl):S55-66.
7. Cartin-Ceba R, Krowka MJ. Portopulmonary hypertension. Clin Liver Dis. 2014;18:421-438.
8. Ramsay M, Simpson BR, Nguyen T, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3:494-500.
9. Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443-450.
10. McDonnell P, Toye P, Hutchins G. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis. 1983;127:437-441.
11. Rich S, Dantzker D, Ayres S, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216-223.
12. Groves B. Pulmonary Hypertension Associated with Cirrhosis. Philadelphia: University of Pennsylvania Press; 1990.
13. Habib G, Gressin V, Yaici A, et al. Pulmonary arterial hypertension in France results from a national registry. Am J Respir Crit Care Med. 2006;173:1023-1030.
14. Hadengue A, Benhayoun M, Lebrec D, Benhamou J. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology. 1991;100:520-528.
15. Krowka MJ, Miller DP, Barst RJ, et al. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest. 2012;141:906-915.
16. Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol. 1991;17:492-498.
17. Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transpl. 2005;11:1107-1111.
18. Swanson KL, Wiesner RH, Nyberg SL, et al. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445-2453.
19. Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology. 2006;44:1502-1510.
20. Kawut SM, Krowka MJ, Trotter JF, et al. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196-203.
21. Lebrec D, Capron JP, Dhumeaux D, Benhamou JP. pulmonary hypertension complicating portal hypertension. Am J Rev Resp Dis. 1979;120:849-856.
22. Pellicelli AM, Barbaro G, Puoti C, et al. Plasma cytokines and portopulmonary hypertension in patients with cirrhosis waiting for orthotopic liver transplantation. Angiology. 2010;61:802-806.
23. Kamath PS, Carpenter HA, Lloyd RV, et al. Hepatic localization of endothelin-1 in patients with idiopathic portal hypertension and cirrhosis of the liver. Liver Transpl. 2000;6:596-602.
24. Krowka MJ, Edwards WD. A spectrum of pulmonary vascular pathology in portopulmonary hypertension. Liver Transpl. 2000;6:241-242.
25. Roberts KE, Fallon MB, Krowka MJ, et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med. 2009;179:835-842.
26. Murray KF, Carithers RL. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-1432.
27. Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology. 2003;37:401-409.
28. Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transpl. 2000;6:453-458.
29. Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263.
30. Devaraj A, Loveridge R, Bosanac D, et al. Portopulmonary hypertension: Improved detection using CT and echocardiography in combination. Eur Radiol. 2014;24:2385-2393.
31. Kuo P, Plotkin J, Johnson L, et al. Distinctive clinical features of portopulmonary hypertension. Chest. 1997;112:980-986.
32. Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378-2387.
Pulmonary arterial hypertension (PAH) is a rare disease that is associated with high mortality and is characterized by pulmonary vascular remodeling. Portopulmonary hypertension (POPH) is a form of PAH that occurs in patients with portal hypertension where no alternative cause of PAH can be identified. POPH is documented in approximately 4.5% to 8.5% of liver transplant candidates,1,2 but there is no relationship between the existence or severity of POPH and the severity of liver dysfunction.3 Mantz and Craig described the first case of POPH in a 53-year-old woman with enlarged pulmonary arteries that exhibited forceful pulsations more characteristic of the aorta than a low-pressure pulmonary trunk.4 Autopsy revealed findings of chronic liver disease including a stenotic portal vein, portocaval shunt, and esophageal varices. In both PAH and POPH, pre-capillary pulmonary arteries have characteristic lesions, such as intimal thickening, endothelial proliferation, and thrombotic changes. This 2-part article reviews the diagnosis and treatment of patients with POPH. Here, we review the epidemiology, prognosis, pathogenesis, and diagnosis of POPH; current treatment options for POPH are reviewed in a separate article.
Definition
The term POPH was first used by Yoshida et al in 1993 to describe the first successful liver transplant in a patient with POPH, a 39-year-old man with chronic hepatitis.5 The World Health Organization (WHO) classifies POPH as a form of Group 1 PAH.6 The criteria that must be met to make a diagnosis of POPH are shown in the Table 1.7

Moderate POPH is defined as a mean pulmonary artery pressure (MPAP) between 35 mm Hg and < 45 mm Hg, whereas severe POPH is MPAP ≥ 45 mm Hg. Moderate and severe POPH are considered contraindications to liver transplant because of high perioperative and postoperative mortality rates.8 In 2000, the Mayo Clinic retrospectively reviewed 43 patients with POPH who underwent attempted liver transplantation.9 The cardiopulmonary-related mortality rate in patients with a MPAP of 35 to < 50 mm Hg was 50% and 100% for those with MPAP > 50 mm Hg. No mortality was noted in patients with a pre-liver transplant MPAP of < 35 mm Hg and transpulmonary gradient (TPG) < 15 mm Hg.
Epidemiology
In 1983, a series of 17,901 autopsied patients showed a primary pulmonary hypertension prevalence of 0.13% and a prevalence of 0.73% in patients with cirrhosis.10 In 1987, Rich et al published data from the National Institutes of Health’s national registry of primary pulmonary hypertension.11 The registry included data from 187 patients from 32 centers. Further analyses by Groves et al concluded that 8.3% of the patients likely had POPH.12 Humbert et al published data on the French pulmonary hypertension registry experience in 2006.13 The French registry included 674 patients from 17 university hospitals; 10.4% of these patients had POPH. The largest prospective study was published by Hadengue et al in 1991.14 In this study, 507 patients hospitalized with portal hypertension but without known pulmonary hypertension underwent cardiac catheterization; 10 patients (2%) had pulmonary hypertension and more than half were clinically asymptomatic. Finally, the Registry to Evaluate Early And Long-term pulmonary arterial hypertension disease management (REVEAL registry) documented a 5.3% frequency of POPH (174 of 3525) in the United States.15
Prognosis
Individuals with POPH have worse outcomes compared to other forms of PAH. Median survival prior to the introduction of vasodilator therapy was a dismal 6 months and mean survival was 15 months.16 The cause of death in patients with POPH is equally distributed between right heart failure from POPH and direct complications of chronic liver disease.1 Le Pavec et al retrospectively analyzed all patients referred to the French Referral Center with POPH between 1984 and 2004 (154 patients).1 Approximately 50% of the patients were Child-Turcotte-Pugh class B or C, and 60% were classified as New York Health Association (NYHA) class III or IV. In these patients, 1-, 3-, and 5-year survival rates were 88%, 75%, and 68%, respectively. Major independent prognostic risk factors were presence and severity of cirrhosis and preservation of right ventricular function. Interestingly, NYHA functional class was not related to survival in this study, although it has clearly been identified as a strong prognostic factor in idiopathic PAH.
Krowka et al evaluated 174 patients with POPH enrolled in the REVEAL Registry,15 a multicenter, observational, US-based study comprised of more than 3500 patients with PAH. Despite having better hemodynamic parameters at diagnosis, patients with POPH had significantly poorer survival and all-cause hospitalization compared with patients with idiopathic PAH (IPAH) or hereditary PAH (HPAH). Two-year survival from enrollment was 67% in POPH versus 85% in those with IPAH/HPAH (P < 0.001). Five-year survival from time of diagnosis was 40% versus 64% (P < 0.001). Additionally, patients with POPH were less likely to be on PAH-specific therapy at enrollment, with only 25% on treatment at the time of entry. These findings were replicated in 2005 when Kawut et al retrospectively compared 13 patients with POPH with 33 patients with IPAH.17 Despite having a higher cardiac index and lower pulmonary vascular resistance than patients with IPAH, patients with POPH had a higher risk of death (hazard ratio, 2.8, P = 0.04), likely reflecting the combination of 2 serious diseases.
In 2008 the Mayo Clinic published their retrospective analysis of patients with POPH to determine the natural history of POPH.18 Patients were categorized into 3 groups: (1) no medical therapy for POPH and no liver transplant; (2) medical therapy for POPH alone; (3) medical therapy for POPH followed by liver transplant. The study included 74 patients between 1994 through 2007; 19 patients who did not receive treatment for POPH or liver transplant truly represented the natural history of POPH. Their 5-year survival was only 14%, and over half were deceased 1 year after diagnosis. The largest group consisted of patients who received therapy for POPH but no liver transplant. This group did remarkably better than those who received no therapy at all, with a 5-year survival of 45%. However, the patients with the overall best survival were those who received a combination of treatment for POPH followed by liver transplant. Their 5-year survival was 67%. Survival at 5 years was only 25% for the small group of patients who received transplant without PAH therapy. Once again, mortality did not correlate with the severity of hepatic dysfunction or baseline hemodynamic data.
Pathogenesis
The pathogenesis of POPH is unclear. Multiple studies have shown that there is minimal, if any, association with pulmonary hypertension and the severity of liver disease or portal hypertension.19,20 Portal hypertension is the result of an increase in intrahepatic resistance and an increase in blood flow into the portal circulation. Collateral vessels develop and blood from the splanchnic circulation is allowed directly into the systemic venous circulation, bypassing the liver. One of the most widely accepted theories is that a humoral substance, that would otherwise be metabolized by the liver, is able to reach the pulmonary circulation through collaterals, resulting in POPH.21 Pelicelli et al evaluated the possible role of endothelin-1, interleukin-6, interleukin 1β, and tumor necrosis factor in the pathogenesis of POPH.22 Plasma concentrations of these cytokines were compared between patients with POPH and patients with cirrhosis but no POPH. Patients with POPH had higher concentrations of endothelin-1 and interleukin-6, suggesting antagonists for these cytokines may have a role in the treatment of POPH. The role of endothelin-1 was further supported by Kamath et al in 200023 when they determined the pulmonary vascular bed is exposed to increased levels of circulating endothelin-1a in the setting of cirrhosis. Endothelin-1 is a potent vasoconstrictor and facilitator of smooth muscle proliferation.
In addition to collateral circulation allowing mediators to reach the pulmonary arterial bed in portal hypertension, high flow may trigger a vasoproliferative process in the pulmonary vascular bed. Patients with advanced liver disease have a low systemic vascular resistance, with a compensatory increase in cardiac output. An increase in cardiac output can lead to shear stress of the pulmonary vascular endothelial layer. Although the resistance of the pulmonary vasculature may decrease rapidly to help normalize pulmonary pressures, persistent circulatory overload could result in irreversible vascular changes. Autopsy and lung explant studies show that POPH is characterized by obstructive and remodeling changes in the pulmonary arterial bed.24 Initially, medial hypertrophy with smooth muscle proliferation is present. As the disease advances, platelet aggregates, in situ thrombosis, and intimal fibrosis develop. Finally, web-like lesions involving the entire pulmonary wall develop with recanalization for the passage of pulmonary arterial flow. These changes are identical to the changes observed in patients with other forms of PAH.
Not all patients with portal hypertension develop POPH, suggesting that genetic predisposition may play a role in POPH development. The Pulmonary Vascular Complications of Liver Study Group published a multicenter case-control study that attempted to identify genetic risk factors for POPH in patients with advanced liver disease.25 More than 1000 common single nucleotide polymorphisms (SNPs) in 93 candidate genes were genotyped in each patient. When compared to controls, multiple SNPs in the genes coding for estrogen receptor 1, aromatase, phosphodiesterase 5, angiopoietin 1, and calcium binding protein A4 were associated with an increased risk of POPH. One year earlier, the same study group concluded that female sex (adjusted odds ratio [OR], 2.90) and autoimmune hepatitis (adjusted OR, 4.02) were associated with a higher risk for POPH, whereas hepatitis C was associated with a decreased risk.20
Clinical Presentation
Clinical presentation is variable in POPH. Patients referred to a pulmonologist will usually present with symptoms similar to patients with other forms of PAH. In a retrospective analysis of patients referred to the French Referral Center for Pulmonary Hypertension, 60% of the patients belonged to NYHA functional class III or IV.1 In a series of 78 patients with POPH, the most common presenting pulmonary symptom was dyspnea on exertion (81%), followed by syncope, chest pain, and fatigue (< 33%).16 Symptoms such as syncope and chest pain are usually markers of severe POPH.3 Stigmata of portal hypertension, such as ascites, spider angiomata, and palmar erythema, may be present on exam. An accentuated pulmonary component of the second heart sound can be seen in 82% of patients and a systolic murmur caused by tricuspid regurgitation in 69% of patients.16 Patients with severe POPH may have jugular vein distention, peripheral edema, and a third heart sound.
Diagnostic Evaluation
Chest x-rays may show prominent pulmonary arteries and cardiomegaly in patients with POPH, whereas electrocardiogram can suggest right ventricular hypertrophy and right axis deviation. The best screening test for POPH in patients with portal hypertension is echocardiography. Routine screening for POPH is recommended during liver transplant evaluation in the practice guidelines from the American Association for the Study of Liver Disease.26 Right-sided cardiac chamber enlargement and right ventricular pressure or volume overload can be assessed on echocardiography. Colle et al followed 165 patients evaluated for liver transplantation who underwent transthoracic Doppler echocardiography and right heart catheterization.27 Seventeen patients met the criteria for POPH on echocardiography (presence of tricuspid regurgitation and calculated systolic pulmonary artery pressure over 30 mm Hg) and right heart catheterization confirmed the diagnosis in 10 patients. Right ventricular systolic pressure (RVSP) estimate of ≤ 30 mm Hg on 2-dimensional echo had a 100% sensitivity and negative predictive value. Positive predictive value was poor at 59%, reiterating the need for right heart catheterization in the diagnosis of POPH. When Kim et al used a RVSP threshold of 50 mm Hg, 72% had at least moderate pulmonary hypertension, including 30% with severe pulmonary hypertension.28 Raevens et al analyzed data from 152 patients who underwent pretransplant echocardiography and catheterization.2 Their data show a RVSP threshold of greater than 38 mm Hg by echocardiography had a specificity of 82% and sensitivity and negative predictive value of 100%. The European Respiratory Society recommendations state that PAH should be considered unlikely if echocardiography estimates a RVSP ≤36 mm Hg and likely if the RVSP is estimated at > 50 mm Hg.29 We recommend repeating echocardiography every 6 to 12 months in patients listed for liver transplantation, as pulmonary hemodynamics may change over time.
Computed tomography (CT) may have a complementary role in the future for the noninvasive detection of POPH. In a study published in 2014, 49 patients referred for liver transplantation were retrospectively reviewed.30 Measured CT signs included the main pulmonary artery/ascending aorta diameter ratio, the mean left and right main pulmonary artery diameter, and the enlargement of the pulmonary artery compared to the ascending aorta. Compared to the transthoracic echocardiography alone, an algorithm incorporating CT and echocardiography improved the detection of POPH (area under curve = 0.8, P < 0.0001).
A diagnosis of POPH can only be confirmed when PAH exists in a patient with portal hypertension, as determined by right heart catheterization, and no other cause of PAH can be identified. MPAP should be 25 mm Hg or greater, PVR of 240 dynes/s/cm–5, wedge pressure of 15 mm Hg or less, and TPG greater than 12 mm Hg. Krowka et al showed the value of right heart catheterization in their 10-year prospective, echocardiography-catheterization algorithm study.19 Of 1235 liver transplant candidates who underwent echocardiography, 104 patients had a RVSP exceeding 50 mm Hg. Almost all of these patients had a right heart catheterization. All cause pulmonary hypertension (MPAP > 25 mm Hg) was confirmed in 90% of the patients, and 35% had a PVR < 240 dynes/s/cm–5 and pulmonary capillary wedge pressure (PCWP) > 15 mm Hg, suggesting fluid overload. Forty-one patients had significant POPH, with a PVR > 400 dynes/s/cm–5, and 24% also had an elevated PCWP. TPG was > 12 mm Hg in all of these patients, confirming POPH. As demonstrated by this study, right heart catheterization is required to confirm the diagnosis of POPH because high flow and fluid overload can lead to elevated pulmonary artery pressures.
Patients with POPH have a unique clinical profile with characteristics common to patients with primary pulmonary hypertension and chronic liver disease. In a retrospective review that compared 30 patients with PAH, 30 patients with chronic liver disease only, and 30 patients with catheterization-proved POPH,31 patients with POPH had elevated MPAP similar to those with primary PAH, but they also had reduced SVR and elevated cardiac index similar to those with chronic liver disease alone.
Besides POPH, 2 other common causes can lead to increased pulmonary arterial blood flow in patients with portal hypertension. First is a high-flow condition caused by increased cardiac output but with a normal PVR and PCWP. Fluid overload can also lead to pulmonary venous hypertension with increased PCWP, normal cardiac output, and normal PVR. Up to 25% of patients with POPH may present with marked excess volume caused by fluid retention.3 There can be an increase in both PCWP and PVR depending on the presence and the degree of fluid retention. TPG (MPAP – PCWP) > 12 mm Hg was introduced to make such patients less confusing and to help correct for increased PCWP secondary to fluid overload. Obstruction to pulmonary arterial flow is manifest by an increased TPG (Table 2).

POPH should be distinguished from hepatopulmonary syndrome (HPS), which is another pulmonary vascular consequence of liver disease. Unlike POPH, HPS is characterized by a defect in arterial oxygenation induced by pulmonary vascular dilation.32 Similar to other patients with liver disease, patients with HPS have a normal PVR and increased cardiac output secondary to a high-flow state. HPS is diagnosed by confirmation of an intrapulmonary shunt by echocardiogram. Injection of agitated saline results in saline bubbles being visualized in the left atrium 3 or more cardiac cycles after they appear in the right atrium. Currently, there is no effective medical treatment for HPS and liver transplantation is the only successful treatment.
Conclusion
POPH is an uncommon complication of chronic liver disease. It is defined as PAH in a patient with portal hypertension excluding other causes of PAH. The following criteria must be met to make a diagnosis of POPH: (1) evidence of portal hypertension; (2) MPAP ≥ 35 mm Hg; (3) PVR ≥ 240 dynes/s/cm5; (4) pulmonary capillary wedge pressure ≤ 15 mm Hg; and (5) TPG > 12 mm Hg. Individuals with POPH have worse outcomes compared to other forms of PAH, with a median survival of 6 months without medical therapy. The pathogenesis of POPH is unclear but may be related to a genetic predisposition since not all patients with portal hypertension develop POPH. Echocardiography is an excellent screening test for POPH, but a right heart catheterization must be performed to confirm the diagnosis.
Pulmonary arterial hypertension (PAH) is a rare disease that is associated with high mortality and is characterized by pulmonary vascular remodeling. Portopulmonary hypertension (POPH) is a form of PAH that occurs in patients with portal hypertension where no alternative cause of PAH can be identified. POPH is documented in approximately 4.5% to 8.5% of liver transplant candidates,1,2 but there is no relationship between the existence or severity of POPH and the severity of liver dysfunction.3 Mantz and Craig described the first case of POPH in a 53-year-old woman with enlarged pulmonary arteries that exhibited forceful pulsations more characteristic of the aorta than a low-pressure pulmonary trunk.4 Autopsy revealed findings of chronic liver disease including a stenotic portal vein, portocaval shunt, and esophageal varices. In both PAH and POPH, pre-capillary pulmonary arteries have characteristic lesions, such as intimal thickening, endothelial proliferation, and thrombotic changes. This 2-part article reviews the diagnosis and treatment of patients with POPH. Here, we review the epidemiology, prognosis, pathogenesis, and diagnosis of POPH; current treatment options for POPH are reviewed in a separate article.
Definition
The term POPH was first used by Yoshida et al in 1993 to describe the first successful liver transplant in a patient with POPH, a 39-year-old man with chronic hepatitis.5 The World Health Organization (WHO) classifies POPH as a form of Group 1 PAH.6 The criteria that must be met to make a diagnosis of POPH are shown in the Table 1.7

Moderate POPH is defined as a mean pulmonary artery pressure (MPAP) between 35 mm Hg and < 45 mm Hg, whereas severe POPH is MPAP ≥ 45 mm Hg. Moderate and severe POPH are considered contraindications to liver transplant because of high perioperative and postoperative mortality rates.8 In 2000, the Mayo Clinic retrospectively reviewed 43 patients with POPH who underwent attempted liver transplantation.9 The cardiopulmonary-related mortality rate in patients with a MPAP of 35 to < 50 mm Hg was 50% and 100% for those with MPAP > 50 mm Hg. No mortality was noted in patients with a pre-liver transplant MPAP of < 35 mm Hg and transpulmonary gradient (TPG) < 15 mm Hg.
Epidemiology
In 1983, a series of 17,901 autopsied patients showed a primary pulmonary hypertension prevalence of 0.13% and a prevalence of 0.73% in patients with cirrhosis.10 In 1987, Rich et al published data from the National Institutes of Health’s national registry of primary pulmonary hypertension.11 The registry included data from 187 patients from 32 centers. Further analyses by Groves et al concluded that 8.3% of the patients likely had POPH.12 Humbert et al published data on the French pulmonary hypertension registry experience in 2006.13 The French registry included 674 patients from 17 university hospitals; 10.4% of these patients had POPH. The largest prospective study was published by Hadengue et al in 1991.14 In this study, 507 patients hospitalized with portal hypertension but without known pulmonary hypertension underwent cardiac catheterization; 10 patients (2%) had pulmonary hypertension and more than half were clinically asymptomatic. Finally, the Registry to Evaluate Early And Long-term pulmonary arterial hypertension disease management (REVEAL registry) documented a 5.3% frequency of POPH (174 of 3525) in the United States.15
Prognosis
Individuals with POPH have worse outcomes compared to other forms of PAH. Median survival prior to the introduction of vasodilator therapy was a dismal 6 months and mean survival was 15 months.16 The cause of death in patients with POPH is equally distributed between right heart failure from POPH and direct complications of chronic liver disease.1 Le Pavec et al retrospectively analyzed all patients referred to the French Referral Center with POPH between 1984 and 2004 (154 patients).1 Approximately 50% of the patients were Child-Turcotte-Pugh class B or C, and 60% were classified as New York Health Association (NYHA) class III or IV. In these patients, 1-, 3-, and 5-year survival rates were 88%, 75%, and 68%, respectively. Major independent prognostic risk factors were presence and severity of cirrhosis and preservation of right ventricular function. Interestingly, NYHA functional class was not related to survival in this study, although it has clearly been identified as a strong prognostic factor in idiopathic PAH.
Krowka et al evaluated 174 patients with POPH enrolled in the REVEAL Registry,15 a multicenter, observational, US-based study comprised of more than 3500 patients with PAH. Despite having better hemodynamic parameters at diagnosis, patients with POPH had significantly poorer survival and all-cause hospitalization compared with patients with idiopathic PAH (IPAH) or hereditary PAH (HPAH). Two-year survival from enrollment was 67% in POPH versus 85% in those with IPAH/HPAH (P < 0.001). Five-year survival from time of diagnosis was 40% versus 64% (P < 0.001). Additionally, patients with POPH were less likely to be on PAH-specific therapy at enrollment, with only 25% on treatment at the time of entry. These findings were replicated in 2005 when Kawut et al retrospectively compared 13 patients with POPH with 33 patients with IPAH.17 Despite having a higher cardiac index and lower pulmonary vascular resistance than patients with IPAH, patients with POPH had a higher risk of death (hazard ratio, 2.8, P = 0.04), likely reflecting the combination of 2 serious diseases.
In 2008 the Mayo Clinic published their retrospective analysis of patients with POPH to determine the natural history of POPH.18 Patients were categorized into 3 groups: (1) no medical therapy for POPH and no liver transplant; (2) medical therapy for POPH alone; (3) medical therapy for POPH followed by liver transplant. The study included 74 patients between 1994 through 2007; 19 patients who did not receive treatment for POPH or liver transplant truly represented the natural history of POPH. Their 5-year survival was only 14%, and over half were deceased 1 year after diagnosis. The largest group consisted of patients who received therapy for POPH but no liver transplant. This group did remarkably better than those who received no therapy at all, with a 5-year survival of 45%. However, the patients with the overall best survival were those who received a combination of treatment for POPH followed by liver transplant. Their 5-year survival was 67%. Survival at 5 years was only 25% for the small group of patients who received transplant without PAH therapy. Once again, mortality did not correlate with the severity of hepatic dysfunction or baseline hemodynamic data.
Pathogenesis
The pathogenesis of POPH is unclear. Multiple studies have shown that there is minimal, if any, association with pulmonary hypertension and the severity of liver disease or portal hypertension.19,20 Portal hypertension is the result of an increase in intrahepatic resistance and an increase in blood flow into the portal circulation. Collateral vessels develop and blood from the splanchnic circulation is allowed directly into the systemic venous circulation, bypassing the liver. One of the most widely accepted theories is that a humoral substance, that would otherwise be metabolized by the liver, is able to reach the pulmonary circulation through collaterals, resulting in POPH.21 Pelicelli et al evaluated the possible role of endothelin-1, interleukin-6, interleukin 1β, and tumor necrosis factor in the pathogenesis of POPH.22 Plasma concentrations of these cytokines were compared between patients with POPH and patients with cirrhosis but no POPH. Patients with POPH had higher concentrations of endothelin-1 and interleukin-6, suggesting antagonists for these cytokines may have a role in the treatment of POPH. The role of endothelin-1 was further supported by Kamath et al in 200023 when they determined the pulmonary vascular bed is exposed to increased levels of circulating endothelin-1a in the setting of cirrhosis. Endothelin-1 is a potent vasoconstrictor and facilitator of smooth muscle proliferation.
In addition to collateral circulation allowing mediators to reach the pulmonary arterial bed in portal hypertension, high flow may trigger a vasoproliferative process in the pulmonary vascular bed. Patients with advanced liver disease have a low systemic vascular resistance, with a compensatory increase in cardiac output. An increase in cardiac output can lead to shear stress of the pulmonary vascular endothelial layer. Although the resistance of the pulmonary vasculature may decrease rapidly to help normalize pulmonary pressures, persistent circulatory overload could result in irreversible vascular changes. Autopsy and lung explant studies show that POPH is characterized by obstructive and remodeling changes in the pulmonary arterial bed.24 Initially, medial hypertrophy with smooth muscle proliferation is present. As the disease advances, platelet aggregates, in situ thrombosis, and intimal fibrosis develop. Finally, web-like lesions involving the entire pulmonary wall develop with recanalization for the passage of pulmonary arterial flow. These changes are identical to the changes observed in patients with other forms of PAH.
Not all patients with portal hypertension develop POPH, suggesting that genetic predisposition may play a role in POPH development. The Pulmonary Vascular Complications of Liver Study Group published a multicenter case-control study that attempted to identify genetic risk factors for POPH in patients with advanced liver disease.25 More than 1000 common single nucleotide polymorphisms (SNPs) in 93 candidate genes were genotyped in each patient. When compared to controls, multiple SNPs in the genes coding for estrogen receptor 1, aromatase, phosphodiesterase 5, angiopoietin 1, and calcium binding protein A4 were associated with an increased risk of POPH. One year earlier, the same study group concluded that female sex (adjusted odds ratio [OR], 2.90) and autoimmune hepatitis (adjusted OR, 4.02) were associated with a higher risk for POPH, whereas hepatitis C was associated with a decreased risk.20
Clinical Presentation
Clinical presentation is variable in POPH. Patients referred to a pulmonologist will usually present with symptoms similar to patients with other forms of PAH. In a retrospective analysis of patients referred to the French Referral Center for Pulmonary Hypertension, 60% of the patients belonged to NYHA functional class III or IV.1 In a series of 78 patients with POPH, the most common presenting pulmonary symptom was dyspnea on exertion (81%), followed by syncope, chest pain, and fatigue (< 33%).16 Symptoms such as syncope and chest pain are usually markers of severe POPH.3 Stigmata of portal hypertension, such as ascites, spider angiomata, and palmar erythema, may be present on exam. An accentuated pulmonary component of the second heart sound can be seen in 82% of patients and a systolic murmur caused by tricuspid regurgitation in 69% of patients.16 Patients with severe POPH may have jugular vein distention, peripheral edema, and a third heart sound.
Diagnostic Evaluation
Chest x-rays may show prominent pulmonary arteries and cardiomegaly in patients with POPH, whereas electrocardiogram can suggest right ventricular hypertrophy and right axis deviation. The best screening test for POPH in patients with portal hypertension is echocardiography. Routine screening for POPH is recommended during liver transplant evaluation in the practice guidelines from the American Association for the Study of Liver Disease.26 Right-sided cardiac chamber enlargement and right ventricular pressure or volume overload can be assessed on echocardiography. Colle et al followed 165 patients evaluated for liver transplantation who underwent transthoracic Doppler echocardiography and right heart catheterization.27 Seventeen patients met the criteria for POPH on echocardiography (presence of tricuspid regurgitation and calculated systolic pulmonary artery pressure over 30 mm Hg) and right heart catheterization confirmed the diagnosis in 10 patients. Right ventricular systolic pressure (RVSP) estimate of ≤ 30 mm Hg on 2-dimensional echo had a 100% sensitivity and negative predictive value. Positive predictive value was poor at 59%, reiterating the need for right heart catheterization in the diagnosis of POPH. When Kim et al used a RVSP threshold of 50 mm Hg, 72% had at least moderate pulmonary hypertension, including 30% with severe pulmonary hypertension.28 Raevens et al analyzed data from 152 patients who underwent pretransplant echocardiography and catheterization.2 Their data show a RVSP threshold of greater than 38 mm Hg by echocardiography had a specificity of 82% and sensitivity and negative predictive value of 100%. The European Respiratory Society recommendations state that PAH should be considered unlikely if echocardiography estimates a RVSP ≤36 mm Hg and likely if the RVSP is estimated at > 50 mm Hg.29 We recommend repeating echocardiography every 6 to 12 months in patients listed for liver transplantation, as pulmonary hemodynamics may change over time.
Computed tomography (CT) may have a complementary role in the future for the noninvasive detection of POPH. In a study published in 2014, 49 patients referred for liver transplantation were retrospectively reviewed.30 Measured CT signs included the main pulmonary artery/ascending aorta diameter ratio, the mean left and right main pulmonary artery diameter, and the enlargement of the pulmonary artery compared to the ascending aorta. Compared to the transthoracic echocardiography alone, an algorithm incorporating CT and echocardiography improved the detection of POPH (area under curve = 0.8, P < 0.0001).
A diagnosis of POPH can only be confirmed when PAH exists in a patient with portal hypertension, as determined by right heart catheterization, and no other cause of PAH can be identified. MPAP should be 25 mm Hg or greater, PVR of 240 dynes/s/cm–5, wedge pressure of 15 mm Hg or less, and TPG greater than 12 mm Hg. Krowka et al showed the value of right heart catheterization in their 10-year prospective, echocardiography-catheterization algorithm study.19 Of 1235 liver transplant candidates who underwent echocardiography, 104 patients had a RVSP exceeding 50 mm Hg. Almost all of these patients had a right heart catheterization. All cause pulmonary hypertension (MPAP > 25 mm Hg) was confirmed in 90% of the patients, and 35% had a PVR < 240 dynes/s/cm–5 and pulmonary capillary wedge pressure (PCWP) > 15 mm Hg, suggesting fluid overload. Forty-one patients had significant POPH, with a PVR > 400 dynes/s/cm–5, and 24% also had an elevated PCWP. TPG was > 12 mm Hg in all of these patients, confirming POPH. As demonstrated by this study, right heart catheterization is required to confirm the diagnosis of POPH because high flow and fluid overload can lead to elevated pulmonary artery pressures.
Patients with POPH have a unique clinical profile with characteristics common to patients with primary pulmonary hypertension and chronic liver disease. In a retrospective review that compared 30 patients with PAH, 30 patients with chronic liver disease only, and 30 patients with catheterization-proved POPH,31 patients with POPH had elevated MPAP similar to those with primary PAH, but they also had reduced SVR and elevated cardiac index similar to those with chronic liver disease alone.
Besides POPH, 2 other common causes can lead to increased pulmonary arterial blood flow in patients with portal hypertension. First is a high-flow condition caused by increased cardiac output but with a normal PVR and PCWP. Fluid overload can also lead to pulmonary venous hypertension with increased PCWP, normal cardiac output, and normal PVR. Up to 25% of patients with POPH may present with marked excess volume caused by fluid retention.3 There can be an increase in both PCWP and PVR depending on the presence and the degree of fluid retention. TPG (MPAP – PCWP) > 12 mm Hg was introduced to make such patients less confusing and to help correct for increased PCWP secondary to fluid overload. Obstruction to pulmonary arterial flow is manifest by an increased TPG (Table 2).

POPH should be distinguished from hepatopulmonary syndrome (HPS), which is another pulmonary vascular consequence of liver disease. Unlike POPH, HPS is characterized by a defect in arterial oxygenation induced by pulmonary vascular dilation.32 Similar to other patients with liver disease, patients with HPS have a normal PVR and increased cardiac output secondary to a high-flow state. HPS is diagnosed by confirmation of an intrapulmonary shunt by echocardiogram. Injection of agitated saline results in saline bubbles being visualized in the left atrium 3 or more cardiac cycles after they appear in the right atrium. Currently, there is no effective medical treatment for HPS and liver transplantation is the only successful treatment.
Conclusion
POPH is an uncommon complication of chronic liver disease. It is defined as PAH in a patient with portal hypertension excluding other causes of PAH. The following criteria must be met to make a diagnosis of POPH: (1) evidence of portal hypertension; (2) MPAP ≥ 35 mm Hg; (3) PVR ≥ 240 dynes/s/cm5; (4) pulmonary capillary wedge pressure ≤ 15 mm Hg; and (5) TPG > 12 mm Hg. Individuals with POPH have worse outcomes compared to other forms of PAH, with a median survival of 6 months without medical therapy. The pathogenesis of POPH is unclear but may be related to a genetic predisposition since not all patients with portal hypertension develop POPH. Echocardiography is an excellent screening test for POPH, but a right heart catheterization must be performed to confirm the diagnosis.
1. Le Pavec J, Souza R, Herve P, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med. 2008;178:637-643.
2. Raevens S, Colle I, Reyntjens K, et al. Echocardiography for the detection of portopulmonary hypertension in liver transplant candidates: An analysis of cutoff values. Liver Transplant. 2013;19:602-610.
3. Krowka MJ. Portopulmonary hypertension. Semin Respir Crit Care Med. 2012;33:17-25.
4. Mantz F. Portal axis thrombosis with spontaneous portocaval shunt and resultant cor pulmonale. AMA Arch Pathol. 1951;52:91-97.
5. Yoshida EM, Erb SR, Pflugfelder PW, et al. Single-lung versus liver transplantation for the treatment of portopulmonary hypertension--a comparison of two patients. Transplantation. 1993;55:688-690.
6. Badesch DB, Champion HC, Gomez Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54 54(1 Suppl):S55-66.
7. Cartin-Ceba R, Krowka MJ. Portopulmonary hypertension. Clin Liver Dis. 2014;18:421-438.
8. Ramsay M, Simpson BR, Nguyen T, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3:494-500.
9. Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443-450.
10. McDonnell P, Toye P, Hutchins G. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis. 1983;127:437-441.
11. Rich S, Dantzker D, Ayres S, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216-223.
12. Groves B. Pulmonary Hypertension Associated with Cirrhosis. Philadelphia: University of Pennsylvania Press; 1990.
13. Habib G, Gressin V, Yaici A, et al. Pulmonary arterial hypertension in France results from a national registry. Am J Respir Crit Care Med. 2006;173:1023-1030.
14. Hadengue A, Benhayoun M, Lebrec D, Benhamou J. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology. 1991;100:520-528.
15. Krowka MJ, Miller DP, Barst RJ, et al. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest. 2012;141:906-915.
16. Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol. 1991;17:492-498.
17. Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transpl. 2005;11:1107-1111.
18. Swanson KL, Wiesner RH, Nyberg SL, et al. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445-2453.
19. Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology. 2006;44:1502-1510.
20. Kawut SM, Krowka MJ, Trotter JF, et al. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196-203.
21. Lebrec D, Capron JP, Dhumeaux D, Benhamou JP. pulmonary hypertension complicating portal hypertension. Am J Rev Resp Dis. 1979;120:849-856.
22. Pellicelli AM, Barbaro G, Puoti C, et al. Plasma cytokines and portopulmonary hypertension in patients with cirrhosis waiting for orthotopic liver transplantation. Angiology. 2010;61:802-806.
23. Kamath PS, Carpenter HA, Lloyd RV, et al. Hepatic localization of endothelin-1 in patients with idiopathic portal hypertension and cirrhosis of the liver. Liver Transpl. 2000;6:596-602.
24. Krowka MJ, Edwards WD. A spectrum of pulmonary vascular pathology in portopulmonary hypertension. Liver Transpl. 2000;6:241-242.
25. Roberts KE, Fallon MB, Krowka MJ, et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med. 2009;179:835-842.
26. Murray KF, Carithers RL. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-1432.
27. Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology. 2003;37:401-409.
28. Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transpl. 2000;6:453-458.
29. Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263.
30. Devaraj A, Loveridge R, Bosanac D, et al. Portopulmonary hypertension: Improved detection using CT and echocardiography in combination. Eur Radiol. 2014;24:2385-2393.
31. Kuo P, Plotkin J, Johnson L, et al. Distinctive clinical features of portopulmonary hypertension. Chest. 1997;112:980-986.
32. Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378-2387.
1. Le Pavec J, Souza R, Herve P, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med. 2008;178:637-643.
2. Raevens S, Colle I, Reyntjens K, et al. Echocardiography for the detection of portopulmonary hypertension in liver transplant candidates: An analysis of cutoff values. Liver Transplant. 2013;19:602-610.
3. Krowka MJ. Portopulmonary hypertension. Semin Respir Crit Care Med. 2012;33:17-25.
4. Mantz F. Portal axis thrombosis with spontaneous portocaval shunt and resultant cor pulmonale. AMA Arch Pathol. 1951;52:91-97.
5. Yoshida EM, Erb SR, Pflugfelder PW, et al. Single-lung versus liver transplantation for the treatment of portopulmonary hypertension--a comparison of two patients. Transplantation. 1993;55:688-690.
6. Badesch DB, Champion HC, Gomez Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54 54(1 Suppl):S55-66.
7. Cartin-Ceba R, Krowka MJ. Portopulmonary hypertension. Clin Liver Dis. 2014;18:421-438.
8. Ramsay M, Simpson BR, Nguyen T, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3:494-500.
9. Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443-450.
10. McDonnell P, Toye P, Hutchins G. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis. 1983;127:437-441.
11. Rich S, Dantzker D, Ayres S, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216-223.
12. Groves B. Pulmonary Hypertension Associated with Cirrhosis. Philadelphia: University of Pennsylvania Press; 1990.
13. Habib G, Gressin V, Yaici A, et al. Pulmonary arterial hypertension in France results from a national registry. Am J Respir Crit Care Med. 2006;173:1023-1030.
14. Hadengue A, Benhayoun M, Lebrec D, Benhamou J. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology. 1991;100:520-528.
15. Krowka MJ, Miller DP, Barst RJ, et al. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest. 2012;141:906-915.
16. Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol. 1991;17:492-498.
17. Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transpl. 2005;11:1107-1111.
18. Swanson KL, Wiesner RH, Nyberg SL, et al. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445-2453.
19. Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology. 2006;44:1502-1510.
20. Kawut SM, Krowka MJ, Trotter JF, et al. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196-203.
21. Lebrec D, Capron JP, Dhumeaux D, Benhamou JP. pulmonary hypertension complicating portal hypertension. Am J Rev Resp Dis. 1979;120:849-856.
22. Pellicelli AM, Barbaro G, Puoti C, et al. Plasma cytokines and portopulmonary hypertension in patients with cirrhosis waiting for orthotopic liver transplantation. Angiology. 2010;61:802-806.
23. Kamath PS, Carpenter HA, Lloyd RV, et al. Hepatic localization of endothelin-1 in patients with idiopathic portal hypertension and cirrhosis of the liver. Liver Transpl. 2000;6:596-602.
24. Krowka MJ, Edwards WD. A spectrum of pulmonary vascular pathology in portopulmonary hypertension. Liver Transpl. 2000;6:241-242.
25. Roberts KE, Fallon MB, Krowka MJ, et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med. 2009;179:835-842.
26. Murray KF, Carithers RL. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-1432.
27. Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology. 2003;37:401-409.
28. Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transpl. 2000;6:453-458.
29. Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263.
30. Devaraj A, Loveridge R, Bosanac D, et al. Portopulmonary hypertension: Improved detection using CT and echocardiography in combination. Eur Radiol. 2014;24:2385-2393.
31. Kuo P, Plotkin J, Johnson L, et al. Distinctive clinical features of portopulmonary hypertension. Chest. 1997;112:980-986.
32. Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378-2387.
Taking the editorial torch
Dear colleagues,
I am excited to introduce the November issue of The New Gastroenterologist – which is also my first issue as the new Editor in Chief! First, I am incredibly grateful for this opportunity to be a part of the only existing publication tailored toward trainees and early-career gastroenterologists. Bryson Katona has done a remarkable job for the last 5 years as the publication’s inaugural EIC, as he has laid a great deal of groundwork and really set the standard going forward. Each issue has been a multifaceted compilation of salient clinical topics paired with brief but high-yield articles to help guide personal and professional growth; I hope to continue to do the same and maintain a high level of interest in our newsletter.
In this issue, the In Focus article, brought to you by Adeeti Chiplunker and Christina Ha (Cedars Sinai), discusses inpatient management of acute severe ulcerative colitis. It is an excellent review of the diagnostic workup and therapeutic options, and an important one, as therapies are quickly evolving in inflammatory bowel disease. We also have Manol Jovani (Johns Hopkins) help us navigate the daunting world of statistics, specifically focusing on the interpretation of the P value.
For those interested in or already pursuing careers in private practice but would not like to relinquish their research interests, Chris Fourment (Texas Digestive Disease Consultants) provides a series of helpful tips on how to be effective in conducting clinical research endeavors. In the realm of basic science, Melinda Engevik (Baylor College of Medicine) gives an informative breakdown on how to choose a lab that is the right fit for you.
Also in this issue, Sadeea Abbasi (Cedars Sinai) provides an array of tangible ways for gastroenterologists to become involved in health policy advocacy. Byron Cryer (UT Southwestern), Jesus Rivera-Nieves (UCSD), and Celena NuQuay (AGA) describe how the AGA has been promoting workforce diversity in academic gastroenterology via the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
Finally, as the submission deadline for DDW® 2020 approaches, abstract reviewers for the fellow-directed quality improvement (QI) projects from this past year share helpful tips on crafting memorable QI abstracts (Mohammad Bilal, UT-Galveston; Chung Sang Tse, Brown University; Manol Jovani, Johns Hopkins; and Mer Mietzelfeld, AGA).
If you are interested in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Sincerely,
Vijaya L. Rao, MD
Editor in Chief
Dr. Rao is assistant professor of medicine, University of Chicago, section of gastroenterology, hepatology & nutrition.
Dear colleagues,
I am excited to introduce the November issue of The New Gastroenterologist – which is also my first issue as the new Editor in Chief! First, I am incredibly grateful for this opportunity to be a part of the only existing publication tailored toward trainees and early-career gastroenterologists. Bryson Katona has done a remarkable job for the last 5 years as the publication’s inaugural EIC, as he has laid a great deal of groundwork and really set the standard going forward. Each issue has been a multifaceted compilation of salient clinical topics paired with brief but high-yield articles to help guide personal and professional growth; I hope to continue to do the same and maintain a high level of interest in our newsletter.
In this issue, the In Focus article, brought to you by Adeeti Chiplunker and Christina Ha (Cedars Sinai), discusses inpatient management of acute severe ulcerative colitis. It is an excellent review of the diagnostic workup and therapeutic options, and an important one, as therapies are quickly evolving in inflammatory bowel disease. We also have Manol Jovani (Johns Hopkins) help us navigate the daunting world of statistics, specifically focusing on the interpretation of the P value.
For those interested in or already pursuing careers in private practice but would not like to relinquish their research interests, Chris Fourment (Texas Digestive Disease Consultants) provides a series of helpful tips on how to be effective in conducting clinical research endeavors. In the realm of basic science, Melinda Engevik (Baylor College of Medicine) gives an informative breakdown on how to choose a lab that is the right fit for you.
Also in this issue, Sadeea Abbasi (Cedars Sinai) provides an array of tangible ways for gastroenterologists to become involved in health policy advocacy. Byron Cryer (UT Southwestern), Jesus Rivera-Nieves (UCSD), and Celena NuQuay (AGA) describe how the AGA has been promoting workforce diversity in academic gastroenterology via the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
Finally, as the submission deadline for DDW® 2020 approaches, abstract reviewers for the fellow-directed quality improvement (QI) projects from this past year share helpful tips on crafting memorable QI abstracts (Mohammad Bilal, UT-Galveston; Chung Sang Tse, Brown University; Manol Jovani, Johns Hopkins; and Mer Mietzelfeld, AGA).
If you are interested in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Sincerely,
Vijaya L. Rao, MD
Editor in Chief
Dr. Rao is assistant professor of medicine, University of Chicago, section of gastroenterology, hepatology & nutrition.
Dear colleagues,
I am excited to introduce the November issue of The New Gastroenterologist – which is also my first issue as the new Editor in Chief! First, I am incredibly grateful for this opportunity to be a part of the only existing publication tailored toward trainees and early-career gastroenterologists. Bryson Katona has done a remarkable job for the last 5 years as the publication’s inaugural EIC, as he has laid a great deal of groundwork and really set the standard going forward. Each issue has been a multifaceted compilation of salient clinical topics paired with brief but high-yield articles to help guide personal and professional growth; I hope to continue to do the same and maintain a high level of interest in our newsletter.
In this issue, the In Focus article, brought to you by Adeeti Chiplunker and Christina Ha (Cedars Sinai), discusses inpatient management of acute severe ulcerative colitis. It is an excellent review of the diagnostic workup and therapeutic options, and an important one, as therapies are quickly evolving in inflammatory bowel disease. We also have Manol Jovani (Johns Hopkins) help us navigate the daunting world of statistics, specifically focusing on the interpretation of the P value.
For those interested in or already pursuing careers in private practice but would not like to relinquish their research interests, Chris Fourment (Texas Digestive Disease Consultants) provides a series of helpful tips on how to be effective in conducting clinical research endeavors. In the realm of basic science, Melinda Engevik (Baylor College of Medicine) gives an informative breakdown on how to choose a lab that is the right fit for you.
Also in this issue, Sadeea Abbasi (Cedars Sinai) provides an array of tangible ways for gastroenterologists to become involved in health policy advocacy. Byron Cryer (UT Southwestern), Jesus Rivera-Nieves (UCSD), and Celena NuQuay (AGA) describe how the AGA has been promoting workforce diversity in academic gastroenterology via the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
Finally, as the submission deadline for DDW® 2020 approaches, abstract reviewers for the fellow-directed quality improvement (QI) projects from this past year share helpful tips on crafting memorable QI abstracts (Mohammad Bilal, UT-Galveston; Chung Sang Tse, Brown University; Manol Jovani, Johns Hopkins; and Mer Mietzelfeld, AGA).
If you are interested in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Sincerely,
Vijaya L. Rao, MD
Editor in Chief
Dr. Rao is assistant professor of medicine, University of Chicago, section of gastroenterology, hepatology & nutrition.
Management of the hospitalized ulcerative colitis patient: A primer for the initial approach to care for the practicing gastroenterologist
Introduction
Inpatient management of acute ulcerative colitis (UC) flares can be challenging because of the multiple patient and disease-related factors influencing therapeutic decision making. The clinical course during the first 24-72 hours of the hospitalization will likely guide the decision between rescue medical and surgical therapy. Using available evidence from clinical practice guidelines, we present a day-by-day guide to managing most hospitalized UC patients.
Day 0 – The emergency department (ED)
When an UC patient presents to the ED for evaluation, the initial assessments should focus on the acuity and severity of the flare. Key clinical features of disease severity include the presence of fever, tachycardia, hypotension, or weight loss in addition to worsened gastrointestinal symptoms of stool frequency relative to baseline, rectal bleeding, and abdominal pain. Acute severe ulcerative colitis (ASUC) is often defined using the modified Truelove and Witts criteria.1 A patient meets criteria for ASUC if they have at least six bloody stools per day and at least one sign of systemic toxicity, such as heart rate greater than 90 bpm, temperature at or above 37.8° C, hemoglobin level below 10.5 g/dL, or elevated inflammatory markers.
Initial laboratory assessments should include complete blood counts to identify anemia, potential superimposed infection, or toxicity and a comprehensive metabolic profile to evaluate for dehydration, electrolyte abnormalities, hepatic injury or hypoalbuminemia (an important predictor of surgery), as well as assessment of response to treatment and readmission.2,3 An evaluation at admission of C-reactive protein (CRP) is crucial because changes from the initial value will determine steroid response and predict need for surgical intervention or rescue therapy. A baseline fecal calprotectin can serve as a noninvasive marker that can be followed after discharge to monitor response to therapy.
Clostridioides difficile infection (CDI) must be ruled out in all patients presenting with ASUC regardless of history of antibiotic use or prior negative testing. Concomitant UC and CDI are associated with a four- to sixfold increased risk of in-hospital mortality and a two- to sixfold increased risk of bowel surgery.4-6 Immunoassay testing is inexpensive and fast with a high specificity but has low sensitivity; nucleic acid amplification testing with polymerase chain reaction has a high sensitivity and specificity.7 Knowing which testing algorithm the hospital lab uses helps guide interpretation of results.
For patients meeting criteria for ASUC, obtaining at least an abdominal x-ray is important to assess for colonic dilation to further stratify the patient by risk. Colonic dilation, defined as a transverse colon diameter greater than 5.5 cm, places the patient in the category of fulminant colitis and colorectal surgical consultation should be obtained.8 A CT scan is often ordered first because it can provide a rapid assessment of intra-abdominal processes but is not routinely needed unless hemodynamic instability, an acute abdomen, or markedly abnormal laboratory testing (specifically white blood cell count with bandemia) is present as these can be indicators of toxic megacolon or perforation.8-10
Day 1 – Assess disease severity and assemble the team
Obtaining a thorough clinical history is essential to classify disease severity and identify potential triggers for the acute exacerbation. Potential triggers may include infections, new medications, recent antibiotic use, recent travel, sick contacts, or cessation of treatments. Standard questions include asking about the timing of onset of symptoms, bowel movements during a 24-hour period, and particularly the presence of nocturnal bowel movements. If patients report bloody stools, inquire how often they see blood relative to the total number of bowel movements. The presence and nature of abdominal pain should be elicited, particularly changes in abdominal pain and comparison with previous disease flares. These clinical parameters are used to assess response to treatment; therefore, ask patients to keep a log of their stool frequency, consistency, rectal urgency, and bleeding each day to report to the team during daily rounds.
For patients with ASUC, a full colonoscopy is rarely indicated in the inpatient setting because it is unlikely to change management and poses a risk of perforation.11 However, a sigmoidoscopy within the first 24 hours of admission will provide useful information about the endoscopic disease activity, particularly if features such as deep or well-like ulcers, large mucosal abrasions, or extensive loss of the mucosal layer are present because these are predictors of colectomy.8 Tissue biopsies can exclude cytomegalovirus (CMV) infection, an important consideration for patients on immunosuppression including corticosteroids.12-16
Venous thromboembolism (VTE) prophylaxis is extremely important for hospitalized inflammatory bowel disease (IBD) patients. At baseline, IBD patients have a threefold higher risk of VTE than do non-IBD patients, which increases to approximately sixfold during flares.17 Pharmacologic VTE prophylaxis is recommended for all hospitalized IBD patients, even those with rectal bleeding. This may seem counterintuitive in the setting of “GI bleeding,” so it is important to counsel both patients and team members regarding VTE risks and the role of the prophylactic regimen to ensure adherence. Mechanical VTE prophylaxis can be used in patients with severe bleeding and hemodynamic instability until pharmacologic VTE prophylaxis can be safely initiated.17
Narcotics should be used sparingly for hospitalized IBD patients. Narcotic use is associated with greater likelihood of subsequent IBD hospitalizations, ED visits, and higher costs of health care for patients with IBD.18 Heavy use of opiates, defined as continuous use for more than 30 days at a dose exceeding 50 mg morphine per day or equivalent, was strongly associated with an increased overall mortality in IBD patients.19 Opiates also slow bowel motility and precipitate toxic megacolon, along with any other agent that slows bowel motility, such as anticholinergic medications.8 These agents may also mask bowel frequency symptoms that would otherwise indicate a failure of medical therapy. Similarly, use of NSAIDS should also be avoided because these have been associated with disease relapse and escalating intestinal inflammation.20
Once disease severity has been determined, intravenous corticosteroid therapy may be initiated, ideally once CDI and CMV have been excluded. The recommended dosing of intravenous corticosteroids is methylprednisolone 20 mg IV every 8 hours or equivalent. There is no evidence to support additional benefit for doses exceeding these amounts.8 Prior to starting parenteral corticosteroids, it is important to keep in mind the possible need for rescue therapy during the admission. Recommended testing includes hepatitis B surface antigen and antibody, hepatitis B core antibody and tuberculosis testing if there is no documented negative testing within the past 6-12 months. These labs should be drawn prior to steroid treatment to avoid delays in care and indeterminate results. Finally, a lipid profile is recommended for patients who may be cyclosporine candidates pending response to intravenous corticosteroids. Unless the patient has been admitted with a bowel obstruction, which should raise the suspicion that the diagnosis is actually Crohn’s disease, enteral feeding is preferred for UC patients even if they may have significant food aversion. The early involvement of a registered dietitian is valuable to guide dietary choices and recommend appropriate enteral nutrition supplements. During acute flares, patients may find a low-residue diet to be less stimulating to their gut while their acute flare is being treated. Electrolyte abnormalities should be repleted and consistently monitored during the hospitalization. Providing parenteral intravenous iron for anemic patients will expedite correction of the anemia alongside treatment of the underlying UC.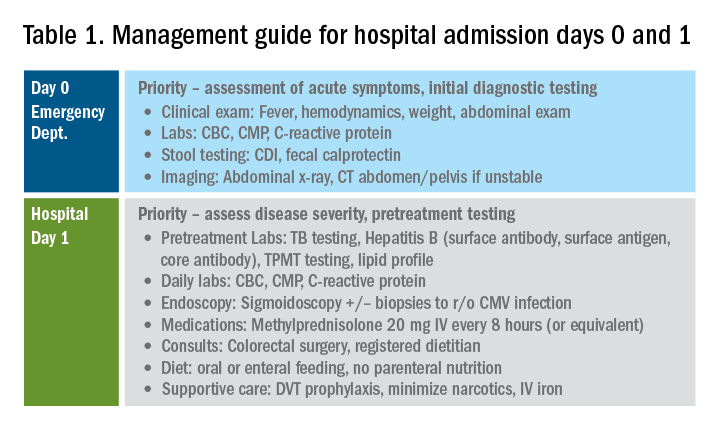
Most UC patients admitted to the hospital will require a multidisciplinary approach with gastroenterologists, surgeons, radiologists, dietitians, and case coordinators/social workers, among others. It is essential to assemble the team, especially the surgeons, earlier during the hospitalization rather than later. It is especially important to discuss the role of the surgeon in the management of UC and explain why the surgeon is being consulted in the context of the patient’s acute presentation. Being transparent about the parameters the GI team are monitoring to determine if and when surgery is the most appropriate and safe approach will improve patients’ acceptance of the surgical team’s role in their care. Specific indications for surgery in ASUC include toxic megacolon, colonic perforation, severe refractory hemorrhage, and failure to respond to medical therapy (Table 1).8
Day 3 – Assessing response to corticosteroids
In addition to daily symptom assessments, a careful abdominal exam should be performed every day with the understanding that steroids (and also narcotics) may mask perforation or pain. Any abrupt decrease or cessation of bowel movements, increasing abdominal distention, or a sudden increase in abdominal pain or tenderness may require abdominal imaging to ensure no interim perforation or severe colonic dilation has occurred while receiving steroid therapy. In these circumstances, the addition of broad spectrum intravenous antibiotics should be considered, particularly if hemodynamic instability (such as tachycardia) is present.
Patients should be assessed for response to intravenous steroid therapy after 3 days of treatment. A meaningful response to corticosteroids is present if the patient has had more than 50% improvement in symptoms, particularly rectal bleeding and stool frequency. A more than 75% improvement in CRP should also be noted from admission to day 3 with an overall trend of improvement.2,21 Additionally, patients should be afebrile, require minimal to no narcotic usage, tolerating oral intake, and be ambulatory. If the patient has met all these parameters, it is reasonable to transition to oral corticosteroids, such as prednisone 40-60 mg daily after a course of 3-5 days of intravenous corticosteroids. Ideally, patients should be observed for 24-48 hours in the hospital after transitioning to oral corticosteroids to make sure that symptoms do not worsen with the switch.
Patients with more than eight bowel movements per day, CRP greater than 4.5 g/dL, deep ulcers on endoscopy, or albumin less than 3.0 g/dL have a higher likelihood of failing intravenous corticosteroid therapy, and these patients should be prepared for rescue therapy.2,21 A patient has failed intravenous corticosteroids by day 3 if they have sustained fever in the absence of an infection, continued CRP elevation or lack of CRP decrease, or ongoing high stool frequency, bleeding, and pain with less than 50% improvement from baseline on admission.8 In the setting of nonresponse to intravenous corticosteroids, it is prudent to involve colorectal surgery to discuss colectomy as an option of equal merit to medical salvage therapies such as infliximab or cyclosporine.
Infliximab is the most readily available rescue therapy for steroid-refractory patients and has been shown to increase colectomy-free survival in patients with ASUC.8 However, patients with the same predictors for intravenous steroid failures (low albumin, high CRP, and/or deep ulcers on endoscopy) are also at the highest risk for infliximab nonresponse. These factors are important to discuss with the patients and colorectal surgery teams when providing the options of treatment strategy, particularly with medication dosing. ASUC with more severe disease biochemically (low albumin, elevated CRP, possibly bandemia) benefit from a higher dose of infliximab at 10 mg/kg, given the likelihood of increased drug clearance in this situation.22,23
From a practical standpoint, it is important to confirm the patient’s insurance status prior to medication administration to make sure therapy can be continued after hospital discharge. Early involvement of the social workers and case coordinators is key to ensuring timely administration of the next dose of treatment. Patients who receive infliximab rescue therapy should be monitored for an additional 1-2 days after administration to ensure they are responding to this therapy with continued monitoring of CRP and symptoms during this period. If there is no response at this point, an additional dose of infliximab may be considered but surgery should not be delayed if there is no meaningful response after the first dose.
Another option for intravenous corticosteroid nonresponders is intravenous cyclosporine because treatment failure rates for cyclosporine and infliximab were similar in head-to-head studies.24 However, patient selection is key to successful utilization of this agent. Unlike infliximab, cyclosporine is primarily an induction agent for steroid nonresponders rather than a maintenance strategy. Therefore, in patients in whom cyclosporine is being considered, thiopurines or vedolizumab are potential options for maintenance therapy. If the patient has poor renal function, low cholesterol, advanced age, significant comorbidities, or a history of nonadherence to therapy, cyclosporine should not be given. Additionally, clinical experience with intravenous cyclosporine administration and monitoring both during inpatient and outpatient care settings should be factored into the decision making for infliximab versus cyclosporine.8
Day 5 and beyond – Discharge planning
Patients who have responded to the initial intravenous steroid course by hospital day 5 should have successfully transitioned to oral steroids with plans to start an appropriate steroid-sparing therapy shortly after discharge. Treatment planning should commence prior to discharge and should be communicated with the outpatient GI team to ensure a smooth transition to the ambulatory care setting, primarily to begin insurance authorizations as soon as possible. If the patient has had a meaningful response to infliximab rescue therapy (improvement by more than 50% in bowel frequency, amount of blood, abdominal pain), discharge planning needs to prioritize obtaining authorization for the second dose within 2 weeks of the initial infusion. These patients are high risk for readmission, and close outpatient follow-up by the ambulatory GI care team is necessary to help direct the tapering of steroids and monitor response to treatment.
If the patient has not responded to intravenous steroid therapy, infliximab, or cyclosporine by day 5-7, then surgery should be strongly considered. Delaying surgery may worsen outcomes as patients become more malnourished, anemic, and continue to receive intravenous steroids. Additional preoperative optimization may be required depending on the patient’s course up to this point (Table 2).
Summary
The cornerstones of inpatient UC management center on a thorough initial evaluation including imaging and endoscopy as appropriate, establishment of baseline parameters, and daily assessment of response to therapy through a combination of patient-reported outcomes and biomarkers of inflammation. With this strategy in mind, practitioners and care teams can manage these complex patients using a consistent strategy focusing on multidisciplinary, evidence-based care.
References
1. Truelove SC et al. Br Med J. 1955 Oct 23;2(4947):1041-8.
2. Ho GT et al. Aliment Pharmacol Ther. 2004 May 15;19(10):1079-87.
3. Tinsley A et al. Scand J Gastroenterol. 2015;50(9):1103-9.
4. Issa M et al. Clin Gastroenterol Hepatol. 2007 Mar;5(3):345-51.
5. Ananthakrishnan AN et al. Gut. 2008 Feb;57(2):205-10.
6. Negron ME et al. Am J Gastroenterol. 2016 May;111(5):691-704.
7. Taylor KN et al. Gynecol Oncol. 2017 Feb;144(2):428-37.
8. Rubin DT et al. Am J Gastroenterol. 2019 Mar;114(3):384-413.
9. Jalan KN et al. Gastroenterology. 1969 Jul;57(1):68-82.
10. Gan SI et al. Am J Gastroenterol. 2003 Nov;98(11):2363-71.
11. Makkar R et al. Gastroenterol Hepatol (N Y). 2013 Sep;9(9):573-83.
12. Hindryckx P et al. Nat Rev Gastroenterol Hepatol. 2016 Nov;13(11):654-64.
13. Yerushalmy-Feler A et al. Curr Infect Dis Rep. 2019 Feb 15;21(2):5.
14. Shukla T et al. J Clin Gastroenterol. 2017 May/Jun;51(5):394-401.
15. McCurdy JD et al. Clin Gastroenterol Hepatol. 2015 Jan;13(1):131-7; quiz e7.
16. Cottone M et al. Am J Gastroenterol. 2001 Mar;96(3):773-5.
17. Nguyen GC et al. Gastroenterology. 2014 Mar;146(3):835-48 e6.
18. Limsrivilai J et al. Clin Gastroenterol Hepatol. 2017 Mar;15(3):385-92 e2.
19. Targownik LE et al. Am J Gastroenterol. 2014 Oct;109(10):1613-20.
20. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
21. Travis SP et al. Gut. 1996 Jun;38(6):905-10.
22. Syal G et al. Mo1891 - Gastroenterology. 2018;154:S841.
23. Ungar B et al. Aliment Pharmacol Ther. 2016 Jun;43(12):1293-9.
24. Laharie D et al. Lancet 2012 Dec 1;380(9857):1909-15.
Dr. Chiplunker is an advanced inflammatory bowel disease fellow; Dr. Ha is associate professor of medicine at the Inflammatory Bowel Disease Center at Cedars-Sinai Medical Center, Los Angeles.
Introduction
Inpatient management of acute ulcerative colitis (UC) flares can be challenging because of the multiple patient and disease-related factors influencing therapeutic decision making. The clinical course during the first 24-72 hours of the hospitalization will likely guide the decision between rescue medical and surgical therapy. Using available evidence from clinical practice guidelines, we present a day-by-day guide to managing most hospitalized UC patients.
Day 0 – The emergency department (ED)
When an UC patient presents to the ED for evaluation, the initial assessments should focus on the acuity and severity of the flare. Key clinical features of disease severity include the presence of fever, tachycardia, hypotension, or weight loss in addition to worsened gastrointestinal symptoms of stool frequency relative to baseline, rectal bleeding, and abdominal pain. Acute severe ulcerative colitis (ASUC) is often defined using the modified Truelove and Witts criteria.1 A patient meets criteria for ASUC if they have at least six bloody stools per day and at least one sign of systemic toxicity, such as heart rate greater than 90 bpm, temperature at or above 37.8° C, hemoglobin level below 10.5 g/dL, or elevated inflammatory markers.
Initial laboratory assessments should include complete blood counts to identify anemia, potential superimposed infection, or toxicity and a comprehensive metabolic profile to evaluate for dehydration, electrolyte abnormalities, hepatic injury or hypoalbuminemia (an important predictor of surgery), as well as assessment of response to treatment and readmission.2,3 An evaluation at admission of C-reactive protein (CRP) is crucial because changes from the initial value will determine steroid response and predict need for surgical intervention or rescue therapy. A baseline fecal calprotectin can serve as a noninvasive marker that can be followed after discharge to monitor response to therapy.
Clostridioides difficile infection (CDI) must be ruled out in all patients presenting with ASUC regardless of history of antibiotic use or prior negative testing. Concomitant UC and CDI are associated with a four- to sixfold increased risk of in-hospital mortality and a two- to sixfold increased risk of bowel surgery.4-6 Immunoassay testing is inexpensive and fast with a high specificity but has low sensitivity; nucleic acid amplification testing with polymerase chain reaction has a high sensitivity and specificity.7 Knowing which testing algorithm the hospital lab uses helps guide interpretation of results.
For patients meeting criteria for ASUC, obtaining at least an abdominal x-ray is important to assess for colonic dilation to further stratify the patient by risk. Colonic dilation, defined as a transverse colon diameter greater than 5.5 cm, places the patient in the category of fulminant colitis and colorectal surgical consultation should be obtained.8 A CT scan is often ordered first because it can provide a rapid assessment of intra-abdominal processes but is not routinely needed unless hemodynamic instability, an acute abdomen, or markedly abnormal laboratory testing (specifically white blood cell count with bandemia) is present as these can be indicators of toxic megacolon or perforation.8-10
Day 1 – Assess disease severity and assemble the team
Obtaining a thorough clinical history is essential to classify disease severity and identify potential triggers for the acute exacerbation. Potential triggers may include infections, new medications, recent antibiotic use, recent travel, sick contacts, or cessation of treatments. Standard questions include asking about the timing of onset of symptoms, bowel movements during a 24-hour period, and particularly the presence of nocturnal bowel movements. If patients report bloody stools, inquire how often they see blood relative to the total number of bowel movements. The presence and nature of abdominal pain should be elicited, particularly changes in abdominal pain and comparison with previous disease flares. These clinical parameters are used to assess response to treatment; therefore, ask patients to keep a log of their stool frequency, consistency, rectal urgency, and bleeding each day to report to the team during daily rounds.
For patients with ASUC, a full colonoscopy is rarely indicated in the inpatient setting because it is unlikely to change management and poses a risk of perforation.11 However, a sigmoidoscopy within the first 24 hours of admission will provide useful information about the endoscopic disease activity, particularly if features such as deep or well-like ulcers, large mucosal abrasions, or extensive loss of the mucosal layer are present because these are predictors of colectomy.8 Tissue biopsies can exclude cytomegalovirus (CMV) infection, an important consideration for patients on immunosuppression including corticosteroids.12-16
Venous thromboembolism (VTE) prophylaxis is extremely important for hospitalized inflammatory bowel disease (IBD) patients. At baseline, IBD patients have a threefold higher risk of VTE than do non-IBD patients, which increases to approximately sixfold during flares.17 Pharmacologic VTE prophylaxis is recommended for all hospitalized IBD patients, even those with rectal bleeding. This may seem counterintuitive in the setting of “GI bleeding,” so it is important to counsel both patients and team members regarding VTE risks and the role of the prophylactic regimen to ensure adherence. Mechanical VTE prophylaxis can be used in patients with severe bleeding and hemodynamic instability until pharmacologic VTE prophylaxis can be safely initiated.17
Narcotics should be used sparingly for hospitalized IBD patients. Narcotic use is associated with greater likelihood of subsequent IBD hospitalizations, ED visits, and higher costs of health care for patients with IBD.18 Heavy use of opiates, defined as continuous use for more than 30 days at a dose exceeding 50 mg morphine per day or equivalent, was strongly associated with an increased overall mortality in IBD patients.19 Opiates also slow bowel motility and precipitate toxic megacolon, along with any other agent that slows bowel motility, such as anticholinergic medications.8 These agents may also mask bowel frequency symptoms that would otherwise indicate a failure of medical therapy. Similarly, use of NSAIDS should also be avoided because these have been associated with disease relapse and escalating intestinal inflammation.20
Once disease severity has been determined, intravenous corticosteroid therapy may be initiated, ideally once CDI and CMV have been excluded. The recommended dosing of intravenous corticosteroids is methylprednisolone 20 mg IV every 8 hours or equivalent. There is no evidence to support additional benefit for doses exceeding these amounts.8 Prior to starting parenteral corticosteroids, it is important to keep in mind the possible need for rescue therapy during the admission. Recommended testing includes hepatitis B surface antigen and antibody, hepatitis B core antibody and tuberculosis testing if there is no documented negative testing within the past 6-12 months. These labs should be drawn prior to steroid treatment to avoid delays in care and indeterminate results. Finally, a lipid profile is recommended for patients who may be cyclosporine candidates pending response to intravenous corticosteroids. Unless the patient has been admitted with a bowel obstruction, which should raise the suspicion that the diagnosis is actually Crohn’s disease, enteral feeding is preferred for UC patients even if they may have significant food aversion. The early involvement of a registered dietitian is valuable to guide dietary choices and recommend appropriate enteral nutrition supplements. During acute flares, patients may find a low-residue diet to be less stimulating to their gut while their acute flare is being treated. Electrolyte abnormalities should be repleted and consistently monitored during the hospitalization. Providing parenteral intravenous iron for anemic patients will expedite correction of the anemia alongside treatment of the underlying UC.
Most UC patients admitted to the hospital will require a multidisciplinary approach with gastroenterologists, surgeons, radiologists, dietitians, and case coordinators/social workers, among others. It is essential to assemble the team, especially the surgeons, earlier during the hospitalization rather than later. It is especially important to discuss the role of the surgeon in the management of UC and explain why the surgeon is being consulted in the context of the patient’s acute presentation. Being transparent about the parameters the GI team are monitoring to determine if and when surgery is the most appropriate and safe approach will improve patients’ acceptance of the surgical team’s role in their care. Specific indications for surgery in ASUC include toxic megacolon, colonic perforation, severe refractory hemorrhage, and failure to respond to medical therapy (Table 1).8
Day 3 – Assessing response to corticosteroids
In addition to daily symptom assessments, a careful abdominal exam should be performed every day with the understanding that steroids (and also narcotics) may mask perforation or pain. Any abrupt decrease or cessation of bowel movements, increasing abdominal distention, or a sudden increase in abdominal pain or tenderness may require abdominal imaging to ensure no interim perforation or severe colonic dilation has occurred while receiving steroid therapy. In these circumstances, the addition of broad spectrum intravenous antibiotics should be considered, particularly if hemodynamic instability (such as tachycardia) is present.
Patients should be assessed for response to intravenous steroid therapy after 3 days of treatment. A meaningful response to corticosteroids is present if the patient has had more than 50% improvement in symptoms, particularly rectal bleeding and stool frequency. A more than 75% improvement in CRP should also be noted from admission to day 3 with an overall trend of improvement.2,21 Additionally, patients should be afebrile, require minimal to no narcotic usage, tolerating oral intake, and be ambulatory. If the patient has met all these parameters, it is reasonable to transition to oral corticosteroids, such as prednisone 40-60 mg daily after a course of 3-5 days of intravenous corticosteroids. Ideally, patients should be observed for 24-48 hours in the hospital after transitioning to oral corticosteroids to make sure that symptoms do not worsen with the switch.
Patients with more than eight bowel movements per day, CRP greater than 4.5 g/dL, deep ulcers on endoscopy, or albumin less than 3.0 g/dL have a higher likelihood of failing intravenous corticosteroid therapy, and these patients should be prepared for rescue therapy.2,21 A patient has failed intravenous corticosteroids by day 3 if they have sustained fever in the absence of an infection, continued CRP elevation or lack of CRP decrease, or ongoing high stool frequency, bleeding, and pain with less than 50% improvement from baseline on admission.8 In the setting of nonresponse to intravenous corticosteroids, it is prudent to involve colorectal surgery to discuss colectomy as an option of equal merit to medical salvage therapies such as infliximab or cyclosporine.
Infliximab is the most readily available rescue therapy for steroid-refractory patients and has been shown to increase colectomy-free survival in patients with ASUC.8 However, patients with the same predictors for intravenous steroid failures (low albumin, high CRP, and/or deep ulcers on endoscopy) are also at the highest risk for infliximab nonresponse. These factors are important to discuss with the patients and colorectal surgery teams when providing the options of treatment strategy, particularly with medication dosing. ASUC with more severe disease biochemically (low albumin, elevated CRP, possibly bandemia) benefit from a higher dose of infliximab at 10 mg/kg, given the likelihood of increased drug clearance in this situation.22,23
From a practical standpoint, it is important to confirm the patient’s insurance status prior to medication administration to make sure therapy can be continued after hospital discharge. Early involvement of the social workers and case coordinators is key to ensuring timely administration of the next dose of treatment. Patients who receive infliximab rescue therapy should be monitored for an additional 1-2 days after administration to ensure they are responding to this therapy with continued monitoring of CRP and symptoms during this period. If there is no response at this point, an additional dose of infliximab may be considered but surgery should not be delayed if there is no meaningful response after the first dose.
Another option for intravenous corticosteroid nonresponders is intravenous cyclosporine because treatment failure rates for cyclosporine and infliximab were similar in head-to-head studies.24 However, patient selection is key to successful utilization of this agent. Unlike infliximab, cyclosporine is primarily an induction agent for steroid nonresponders rather than a maintenance strategy. Therefore, in patients in whom cyclosporine is being considered, thiopurines or vedolizumab are potential options for maintenance therapy. If the patient has poor renal function, low cholesterol, advanced age, significant comorbidities, or a history of nonadherence to therapy, cyclosporine should not be given. Additionally, clinical experience with intravenous cyclosporine administration and monitoring both during inpatient and outpatient care settings should be factored into the decision making for infliximab versus cyclosporine.8
Day 5 and beyond – Discharge planning
Patients who have responded to the initial intravenous steroid course by hospital day 5 should have successfully transitioned to oral steroids with plans to start an appropriate steroid-sparing therapy shortly after discharge. Treatment planning should commence prior to discharge and should be communicated with the outpatient GI team to ensure a smooth transition to the ambulatory care setting, primarily to begin insurance authorizations as soon as possible. If the patient has had a meaningful response to infliximab rescue therapy (improvement by more than 50% in bowel frequency, amount of blood, abdominal pain), discharge planning needs to prioritize obtaining authorization for the second dose within 2 weeks of the initial infusion. These patients are high risk for readmission, and close outpatient follow-up by the ambulatory GI care team is necessary to help direct the tapering of steroids and monitor response to treatment.

If the patient has not responded to intravenous steroid therapy, infliximab, or cyclosporine by day 5-7, then surgery should be strongly considered. Delaying surgery may worsen outcomes as patients become more malnourished, anemic, and continue to receive intravenous steroids. Additional preoperative optimization may be required depending on the patient’s course up to this point (Table 2).
Summary
The cornerstones of inpatient UC management center on a thorough initial evaluation including imaging and endoscopy as appropriate, establishment of baseline parameters, and daily assessment of response to therapy through a combination of patient-reported outcomes and biomarkers of inflammation. With this strategy in mind, practitioners and care teams can manage these complex patients using a consistent strategy focusing on multidisciplinary, evidence-based care.
References
1. Truelove SC et al. Br Med J. 1955 Oct 23;2(4947):1041-8.
2. Ho GT et al. Aliment Pharmacol Ther. 2004 May 15;19(10):1079-87.
3. Tinsley A et al. Scand J Gastroenterol. 2015;50(9):1103-9.
4. Issa M et al. Clin Gastroenterol Hepatol. 2007 Mar;5(3):345-51.
5. Ananthakrishnan AN et al. Gut. 2008 Feb;57(2):205-10.
6. Negron ME et al. Am J Gastroenterol. 2016 May;111(5):691-704.
7. Taylor KN et al. Gynecol Oncol. 2017 Feb;144(2):428-37.
8. Rubin DT et al. Am J Gastroenterol. 2019 Mar;114(3):384-413.
9. Jalan KN et al. Gastroenterology. 1969 Jul;57(1):68-82.
10. Gan SI et al. Am J Gastroenterol. 2003 Nov;98(11):2363-71.
11. Makkar R et al. Gastroenterol Hepatol (N Y). 2013 Sep;9(9):573-83.
12. Hindryckx P et al. Nat Rev Gastroenterol Hepatol. 2016 Nov;13(11):654-64.
13. Yerushalmy-Feler A et al. Curr Infect Dis Rep. 2019 Feb 15;21(2):5.
14. Shukla T et al. J Clin Gastroenterol. 2017 May/Jun;51(5):394-401.
15. McCurdy JD et al. Clin Gastroenterol Hepatol. 2015 Jan;13(1):131-7; quiz e7.
16. Cottone M et al. Am J Gastroenterol. 2001 Mar;96(3):773-5.
17. Nguyen GC et al. Gastroenterology. 2014 Mar;146(3):835-48 e6.
18. Limsrivilai J et al. Clin Gastroenterol Hepatol. 2017 Mar;15(3):385-92 e2.
19. Targownik LE et al. Am J Gastroenterol. 2014 Oct;109(10):1613-20.
20. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
21. Travis SP et al. Gut. 1996 Jun;38(6):905-10.
22. Syal G et al. Mo1891 - Gastroenterology. 2018;154:S841.
23. Ungar B et al. Aliment Pharmacol Ther. 2016 Jun;43(12):1293-9.
24. Laharie D et al. Lancet 2012 Dec 1;380(9857):1909-15.
Dr. Chiplunker is an advanced inflammatory bowel disease fellow; Dr. Ha is associate professor of medicine at the Inflammatory Bowel Disease Center at Cedars-Sinai Medical Center, Los Angeles.
Introduction
Inpatient management of acute ulcerative colitis (UC) flares can be challenging because of the multiple patient and disease-related factors influencing therapeutic decision making. The clinical course during the first 24-72 hours of the hospitalization will likely guide the decision between rescue medical and surgical therapy. Using available evidence from clinical practice guidelines, we present a day-by-day guide to managing most hospitalized UC patients.
Day 0 – The emergency department (ED)
When an UC patient presents to the ED for evaluation, the initial assessments should focus on the acuity and severity of the flare. Key clinical features of disease severity include the presence of fever, tachycardia, hypotension, or weight loss in addition to worsened gastrointestinal symptoms of stool frequency relative to baseline, rectal bleeding, and abdominal pain. Acute severe ulcerative colitis (ASUC) is often defined using the modified Truelove and Witts criteria.1 A patient meets criteria for ASUC if they have at least six bloody stools per day and at least one sign of systemic toxicity, such as heart rate greater than 90 bpm, temperature at or above 37.8° C, hemoglobin level below 10.5 g/dL, or elevated inflammatory markers.
Initial laboratory assessments should include complete blood counts to identify anemia, potential superimposed infection, or toxicity and a comprehensive metabolic profile to evaluate for dehydration, electrolyte abnormalities, hepatic injury or hypoalbuminemia (an important predictor of surgery), as well as assessment of response to treatment and readmission.2,3 An evaluation at admission of C-reactive protein (CRP) is crucial because changes from the initial value will determine steroid response and predict need for surgical intervention or rescue therapy. A baseline fecal calprotectin can serve as a noninvasive marker that can be followed after discharge to monitor response to therapy.
Clostridioides difficile infection (CDI) must be ruled out in all patients presenting with ASUC regardless of history of antibiotic use or prior negative testing. Concomitant UC and CDI are associated with a four- to sixfold increased risk of in-hospital mortality and a two- to sixfold increased risk of bowel surgery.4-6 Immunoassay testing is inexpensive and fast with a high specificity but has low sensitivity; nucleic acid amplification testing with polymerase chain reaction has a high sensitivity and specificity.7 Knowing which testing algorithm the hospital lab uses helps guide interpretation of results.
For patients meeting criteria for ASUC, obtaining at least an abdominal x-ray is important to assess for colonic dilation to further stratify the patient by risk. Colonic dilation, defined as a transverse colon diameter greater than 5.5 cm, places the patient in the category of fulminant colitis and colorectal surgical consultation should be obtained.8 A CT scan is often ordered first because it can provide a rapid assessment of intra-abdominal processes but is not routinely needed unless hemodynamic instability, an acute abdomen, or markedly abnormal laboratory testing (specifically white blood cell count with bandemia) is present as these can be indicators of toxic megacolon or perforation.8-10
Day 1 – Assess disease severity and assemble the team
Obtaining a thorough clinical history is essential to classify disease severity and identify potential triggers for the acute exacerbation. Potential triggers may include infections, new medications, recent antibiotic use, recent travel, sick contacts, or cessation of treatments. Standard questions include asking about the timing of onset of symptoms, bowel movements during a 24-hour period, and particularly the presence of nocturnal bowel movements. If patients report bloody stools, inquire how often they see blood relative to the total number of bowel movements. The presence and nature of abdominal pain should be elicited, particularly changes in abdominal pain and comparison with previous disease flares. These clinical parameters are used to assess response to treatment; therefore, ask patients to keep a log of their stool frequency, consistency, rectal urgency, and bleeding each day to report to the team during daily rounds.
For patients with ASUC, a full colonoscopy is rarely indicated in the inpatient setting because it is unlikely to change management and poses a risk of perforation.11 However, a sigmoidoscopy within the first 24 hours of admission will provide useful information about the endoscopic disease activity, particularly if features such as deep or well-like ulcers, large mucosal abrasions, or extensive loss of the mucosal layer are present because these are predictors of colectomy.8 Tissue biopsies can exclude cytomegalovirus (CMV) infection, an important consideration for patients on immunosuppression including corticosteroids.12-16
Venous thromboembolism (VTE) prophylaxis is extremely important for hospitalized inflammatory bowel disease (IBD) patients. At baseline, IBD patients have a threefold higher risk of VTE than do non-IBD patients, which increases to approximately sixfold during flares.17 Pharmacologic VTE prophylaxis is recommended for all hospitalized IBD patients, even those with rectal bleeding. This may seem counterintuitive in the setting of “GI bleeding,” so it is important to counsel both patients and team members regarding VTE risks and the role of the prophylactic regimen to ensure adherence. Mechanical VTE prophylaxis can be used in patients with severe bleeding and hemodynamic instability until pharmacologic VTE prophylaxis can be safely initiated.17
Narcotics should be used sparingly for hospitalized IBD patients. Narcotic use is associated with greater likelihood of subsequent IBD hospitalizations, ED visits, and higher costs of health care for patients with IBD.18 Heavy use of opiates, defined as continuous use for more than 30 days at a dose exceeding 50 mg morphine per day or equivalent, was strongly associated with an increased overall mortality in IBD patients.19 Opiates also slow bowel motility and precipitate toxic megacolon, along with any other agent that slows bowel motility, such as anticholinergic medications.8 These agents may also mask bowel frequency symptoms that would otherwise indicate a failure of medical therapy. Similarly, use of NSAIDS should also be avoided because these have been associated with disease relapse and escalating intestinal inflammation.20
Once disease severity has been determined, intravenous corticosteroid therapy may be initiated, ideally once CDI and CMV have been excluded. The recommended dosing of intravenous corticosteroids is methylprednisolone 20 mg IV every 8 hours or equivalent. There is no evidence to support additional benefit for doses exceeding these amounts.8 Prior to starting parenteral corticosteroids, it is important to keep in mind the possible need for rescue therapy during the admission. Recommended testing includes hepatitis B surface antigen and antibody, hepatitis B core antibody and tuberculosis testing if there is no documented negative testing within the past 6-12 months. These labs should be drawn prior to steroid treatment to avoid delays in care and indeterminate results. Finally, a lipid profile is recommended for patients who may be cyclosporine candidates pending response to intravenous corticosteroids. Unless the patient has been admitted with a bowel obstruction, which should raise the suspicion that the diagnosis is actually Crohn’s disease, enteral feeding is preferred for UC patients even if they may have significant food aversion. The early involvement of a registered dietitian is valuable to guide dietary choices and recommend appropriate enteral nutrition supplements. During acute flares, patients may find a low-residue diet to be less stimulating to their gut while their acute flare is being treated. Electrolyte abnormalities should be repleted and consistently monitored during the hospitalization. Providing parenteral intravenous iron for anemic patients will expedite correction of the anemia alongside treatment of the underlying UC.
Most UC patients admitted to the hospital will require a multidisciplinary approach with gastroenterologists, surgeons, radiologists, dietitians, and case coordinators/social workers, among others. It is essential to assemble the team, especially the surgeons, earlier during the hospitalization rather than later. It is especially important to discuss the role of the surgeon in the management of UC and explain why the surgeon is being consulted in the context of the patient’s acute presentation. Being transparent about the parameters the GI team are monitoring to determine if and when surgery is the most appropriate and safe approach will improve patients’ acceptance of the surgical team’s role in their care. Specific indications for surgery in ASUC include toxic megacolon, colonic perforation, severe refractory hemorrhage, and failure to respond to medical therapy (Table 1).8
Day 3 – Assessing response to corticosteroids
In addition to daily symptom assessments, a careful abdominal exam should be performed every day with the understanding that steroids (and also narcotics) may mask perforation or pain. Any abrupt decrease or cessation of bowel movements, increasing abdominal distention, or a sudden increase in abdominal pain or tenderness may require abdominal imaging to ensure no interim perforation or severe colonic dilation has occurred while receiving steroid therapy. In these circumstances, the addition of broad spectrum intravenous antibiotics should be considered, particularly if hemodynamic instability (such as tachycardia) is present.
Patients should be assessed for response to intravenous steroid therapy after 3 days of treatment. A meaningful response to corticosteroids is present if the patient has had more than 50% improvement in symptoms, particularly rectal bleeding and stool frequency. A more than 75% improvement in CRP should also be noted from admission to day 3 with an overall trend of improvement.2,21 Additionally, patients should be afebrile, require minimal to no narcotic usage, tolerating oral intake, and be ambulatory. If the patient has met all these parameters, it is reasonable to transition to oral corticosteroids, such as prednisone 40-60 mg daily after a course of 3-5 days of intravenous corticosteroids. Ideally, patients should be observed for 24-48 hours in the hospital after transitioning to oral corticosteroids to make sure that symptoms do not worsen with the switch.
Patients with more than eight bowel movements per day, CRP greater than 4.5 g/dL, deep ulcers on endoscopy, or albumin less than 3.0 g/dL have a higher likelihood of failing intravenous corticosteroid therapy, and these patients should be prepared for rescue therapy.2,21 A patient has failed intravenous corticosteroids by day 3 if they have sustained fever in the absence of an infection, continued CRP elevation or lack of CRP decrease, or ongoing high stool frequency, bleeding, and pain with less than 50% improvement from baseline on admission.8 In the setting of nonresponse to intravenous corticosteroids, it is prudent to involve colorectal surgery to discuss colectomy as an option of equal merit to medical salvage therapies such as infliximab or cyclosporine.
Infliximab is the most readily available rescue therapy for steroid-refractory patients and has been shown to increase colectomy-free survival in patients with ASUC.8 However, patients with the same predictors for intravenous steroid failures (low albumin, high CRP, and/or deep ulcers on endoscopy) are also at the highest risk for infliximab nonresponse. These factors are important to discuss with the patients and colorectal surgery teams when providing the options of treatment strategy, particularly with medication dosing. ASUC with more severe disease biochemically (low albumin, elevated CRP, possibly bandemia) benefit from a higher dose of infliximab at 10 mg/kg, given the likelihood of increased drug clearance in this situation.22,23
From a practical standpoint, it is important to confirm the patient’s insurance status prior to medication administration to make sure therapy can be continued after hospital discharge. Early involvement of the social workers and case coordinators is key to ensuring timely administration of the next dose of treatment. Patients who receive infliximab rescue therapy should be monitored for an additional 1-2 days after administration to ensure they are responding to this therapy with continued monitoring of CRP and symptoms during this period. If there is no response at this point, an additional dose of infliximab may be considered but surgery should not be delayed if there is no meaningful response after the first dose.
Another option for intravenous corticosteroid nonresponders is intravenous cyclosporine because treatment failure rates for cyclosporine and infliximab were similar in head-to-head studies.24 However, patient selection is key to successful utilization of this agent. Unlike infliximab, cyclosporine is primarily an induction agent for steroid nonresponders rather than a maintenance strategy. Therefore, in patients in whom cyclosporine is being considered, thiopurines or vedolizumab are potential options for maintenance therapy. If the patient has poor renal function, low cholesterol, advanced age, significant comorbidities, or a history of nonadherence to therapy, cyclosporine should not be given. Additionally, clinical experience with intravenous cyclosporine administration and monitoring both during inpatient and outpatient care settings should be factored into the decision making for infliximab versus cyclosporine.8
Day 5 and beyond – Discharge planning
Patients who have responded to the initial intravenous steroid course by hospital day 5 should have successfully transitioned to oral steroids with plans to start an appropriate steroid-sparing therapy shortly after discharge. Treatment planning should commence prior to discharge and should be communicated with the outpatient GI team to ensure a smooth transition to the ambulatory care setting, primarily to begin insurance authorizations as soon as possible. If the patient has had a meaningful response to infliximab rescue therapy (improvement by more than 50% in bowel frequency, amount of blood, abdominal pain), discharge planning needs to prioritize obtaining authorization for the second dose within 2 weeks of the initial infusion. These patients are high risk for readmission, and close outpatient follow-up by the ambulatory GI care team is necessary to help direct the tapering of steroids and monitor response to treatment.

If the patient has not responded to intravenous steroid therapy, infliximab, or cyclosporine by day 5-7, then surgery should be strongly considered. Delaying surgery may worsen outcomes as patients become more malnourished, anemic, and continue to receive intravenous steroids. Additional preoperative optimization may be required depending on the patient’s course up to this point (Table 2).
Summary
The cornerstones of inpatient UC management center on a thorough initial evaluation including imaging and endoscopy as appropriate, establishment of baseline parameters, and daily assessment of response to therapy through a combination of patient-reported outcomes and biomarkers of inflammation. With this strategy in mind, practitioners and care teams can manage these complex patients using a consistent strategy focusing on multidisciplinary, evidence-based care.
References
1. Truelove SC et al. Br Med J. 1955 Oct 23;2(4947):1041-8.
2. Ho GT et al. Aliment Pharmacol Ther. 2004 May 15;19(10):1079-87.
3. Tinsley A et al. Scand J Gastroenterol. 2015;50(9):1103-9.
4. Issa M et al. Clin Gastroenterol Hepatol. 2007 Mar;5(3):345-51.
5. Ananthakrishnan AN et al. Gut. 2008 Feb;57(2):205-10.
6. Negron ME et al. Am J Gastroenterol. 2016 May;111(5):691-704.
7. Taylor KN et al. Gynecol Oncol. 2017 Feb;144(2):428-37.
8. Rubin DT et al. Am J Gastroenterol. 2019 Mar;114(3):384-413.
9. Jalan KN et al. Gastroenterology. 1969 Jul;57(1):68-82.
10. Gan SI et al. Am J Gastroenterol. 2003 Nov;98(11):2363-71.
11. Makkar R et al. Gastroenterol Hepatol (N Y). 2013 Sep;9(9):573-83.
12. Hindryckx P et al. Nat Rev Gastroenterol Hepatol. 2016 Nov;13(11):654-64.
13. Yerushalmy-Feler A et al. Curr Infect Dis Rep. 2019 Feb 15;21(2):5.
14. Shukla T et al. J Clin Gastroenterol. 2017 May/Jun;51(5):394-401.
15. McCurdy JD et al. Clin Gastroenterol Hepatol. 2015 Jan;13(1):131-7; quiz e7.
16. Cottone M et al. Am J Gastroenterol. 2001 Mar;96(3):773-5.
17. Nguyen GC et al. Gastroenterology. 2014 Mar;146(3):835-48 e6.
18. Limsrivilai J et al. Clin Gastroenterol Hepatol. 2017 Mar;15(3):385-92 e2.
19. Targownik LE et al. Am J Gastroenterol. 2014 Oct;109(10):1613-20.
20. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
21. Travis SP et al. Gut. 1996 Jun;38(6):905-10.
22. Syal G et al. Mo1891 - Gastroenterology. 2018;154:S841.
23. Ungar B et al. Aliment Pharmacol Ther. 2016 Jun;43(12):1293-9.
24. Laharie D et al. Lancet 2012 Dec 1;380(9857):1909-15.
Dr. Chiplunker is an advanced inflammatory bowel disease fellow; Dr. Ha is associate professor of medicine at the Inflammatory Bowel Disease Center at Cedars-Sinai Medical Center, Los Angeles.
Digital disruption
One of our lead articles stems from the annual Partners in Value meeting, which was developed by the AGA in partnership with the Digestive Health Physicians Association (Chicago, Oct. 4, 2019). This is an annual meeting about innovations and “what’s next” for GI practices. Anton Decker, MD, an expert in the business of GI and Chair of the Practice Management and Economics Committee, discussed “digital disruption.”
When we discuss digital innovations in health care, most think of telehealth, social media, self-care apps, and remote patient monitoring. As a health system executive, my viewpoint about digital technology has been expanded by other critical needs. At the University of Michigan, we are space constrained (land locked without sufficient parking) and are living with shrinking clinical margins. We see digital technology as a solution to both. As we consolidate our call centers from 27 sites to 1, we plan for 30% of our staff to work from home. Setting up a home work station costs $3,000, compared with office space costs (about $5,000/year). A new clinical site might cost $20 million to build, but that is a fraction of the true life-cycle cost of the building. We have a widely distributed patient base (imagine traveling from Michigan’s Upper Penisula to Ann Arbor for a 20-minute clinic visit).
Many people appreciate “seeing” their doctor from the comfort of their living room. We plan to convert at least 15% of patient visits to telehealth over the next few years although reimbursement rules are still limiting. This year, more than 80% of postsurgical visits (90-day bundled payment) were conducted virtually – mostly by NPs or PAs. Our GI psychologist converted 1,500 patient visit hours to virtual visits last year. In 2019, we completed over 4,000 evisits (management of simple conditions initiated by a patient – essential during flu season) and an increasing number of econsults (primary consultations to specialists). Project ECHO (N Engl J Med. 2011;364:2199) remains the star example of how digital health can improve access, especially for underserved communities.
Virtual care, telehealth, remote patient monitoring, telecommuting and other digital innovations are becoming standards for health systems. Now is the time to think of “face-to-face” visits as option B.
John I. Allen, MD, MBD, AGAF
Editor in Chief
One of our lead articles stems from the annual Partners in Value meeting, which was developed by the AGA in partnership with the Digestive Health Physicians Association (Chicago, Oct. 4, 2019). This is an annual meeting about innovations and “what’s next” for GI practices. Anton Decker, MD, an expert in the business of GI and Chair of the Practice Management and Economics Committee, discussed “digital disruption.”
When we discuss digital innovations in health care, most think of telehealth, social media, self-care apps, and remote patient monitoring. As a health system executive, my viewpoint about digital technology has been expanded by other critical needs. At the University of Michigan, we are space constrained (land locked without sufficient parking) and are living with shrinking clinical margins. We see digital technology as a solution to both. As we consolidate our call centers from 27 sites to 1, we plan for 30% of our staff to work from home. Setting up a home work station costs $3,000, compared with office space costs (about $5,000/year). A new clinical site might cost $20 million to build, but that is a fraction of the true life-cycle cost of the building. We have a widely distributed patient base (imagine traveling from Michigan’s Upper Penisula to Ann Arbor for a 20-minute clinic visit).
Many people appreciate “seeing” their doctor from the comfort of their living room. We plan to convert at least 15% of patient visits to telehealth over the next few years although reimbursement rules are still limiting. This year, more than 80% of postsurgical visits (90-day bundled payment) were conducted virtually – mostly by NPs or PAs. Our GI psychologist converted 1,500 patient visit hours to virtual visits last year. In 2019, we completed over 4,000 evisits (management of simple conditions initiated by a patient – essential during flu season) and an increasing number of econsults (primary consultations to specialists). Project ECHO (N Engl J Med. 2011;364:2199) remains the star example of how digital health can improve access, especially for underserved communities.
Virtual care, telehealth, remote patient monitoring, telecommuting and other digital innovations are becoming standards for health systems. Now is the time to think of “face-to-face” visits as option B.
John I. Allen, MD, MBD, AGAF
Editor in Chief
One of our lead articles stems from the annual Partners in Value meeting, which was developed by the AGA in partnership with the Digestive Health Physicians Association (Chicago, Oct. 4, 2019). This is an annual meeting about innovations and “what’s next” for GI practices. Anton Decker, MD, an expert in the business of GI and Chair of the Practice Management and Economics Committee, discussed “digital disruption.”
When we discuss digital innovations in health care, most think of telehealth, social media, self-care apps, and remote patient monitoring. As a health system executive, my viewpoint about digital technology has been expanded by other critical needs. At the University of Michigan, we are space constrained (land locked without sufficient parking) and are living with shrinking clinical margins. We see digital technology as a solution to both. As we consolidate our call centers from 27 sites to 1, we plan for 30% of our staff to work from home. Setting up a home work station costs $3,000, compared with office space costs (about $5,000/year). A new clinical site might cost $20 million to build, but that is a fraction of the true life-cycle cost of the building. We have a widely distributed patient base (imagine traveling from Michigan’s Upper Penisula to Ann Arbor for a 20-minute clinic visit).
Many people appreciate “seeing” their doctor from the comfort of their living room. We plan to convert at least 15% of patient visits to telehealth over the next few years although reimbursement rules are still limiting. This year, more than 80% of postsurgical visits (90-day bundled payment) were conducted virtually – mostly by NPs or PAs. Our GI psychologist converted 1,500 patient visit hours to virtual visits last year. In 2019, we completed over 4,000 evisits (management of simple conditions initiated by a patient – essential during flu season) and an increasing number of econsults (primary consultations to specialists). Project ECHO (N Engl J Med. 2011;364:2199) remains the star example of how digital health can improve access, especially for underserved communities.
Virtual care, telehealth, remote patient monitoring, telecommuting and other digital innovations are becoming standards for health systems. Now is the time to think of “face-to-face” visits as option B.
John I. Allen, MD, MBD, AGAF
Editor in Chief
AI and machine learning
Recent advances in neuroscience and genetics are providing a new view of brain function in health and disease.1 As discussed in Drs. Hripsime Kalanderian and Henry Nasrallah’s article “Artificial intelligence in psychiatry” (Evidence-Based Reviews,
In a 2016 article, Dr. Arshya Vahabzadeh2 wrote, “In the near term, humans will continue to make the majority of psychiatric diagnoses and provide treatment.” He predicted that data science—specifically machine learning—will help revolutionize how we diagnose, treat, and monitor depression. He foresees a future where AI machines or learning machines will more accurately diagnose and treat depression.
These predictions remind us of the promises made with the introduction of psychoactive drugs to the practice of psychiatry. In the 1970s, the increased emphasis on neurotransmitters led to new biologic models of mental illness and the expansion of the Diagnostic and Statistical Manual of Mental Disorders. The increased use of psychoactive medications relegated the practice of psychotherapy to other mental health professionals. Psychiatry became a “drug-intensive” specialty, and psychiatrists saw themselves as psychopharmacologists.3 During this time, psychiatry became progressively dominated by the pharmaceutical industry. The deregulation of the markets and the for-profit ideology of the pharmaceutical industry resulted in an economically and mutually beneficial alliance between pharmaceutical companies, academic faculty, and individual psychiatrists.
Using the same for-profit ideology and ethics of the pharmaceutical industry, technology-based corporations are making massive investments in the application of these technologies in neuroscience research and clinical practice. The “promise” that AI will diagnose and treat depression more accurately than clinicians will again radically change the psychiatrist’s role. Most of a psychiatrist’s clinical work eventually will be replicated by machine learning and technicians. The human-to-human encounter that is at core of the profession will be replaced by the machine-to-human encounter.4
From the social economic perspective, the American health care system is designed to increase profit and maximize the earnings of the industries.5 Unless social policy changes, expensive new technologies will increase the cost and limit accessibility to health care, benefiting the few at the expense of the majority of people. These technologies will produce a robust return on investment, but the wealth they create will benefit fewer and fewer people.
Several writers have called attention to the social, economic, and ethical consequences of these advances and recommended that the academics and technologists who support AI and machine learning in medicine must “receive sufficient training in ethics” and gain exposure to social and economic issues.6 Darcy et al7 warns of the risks of introducing these advanced technologies in medicine: “As machine learning enters the state-of-the-art clinical practice, medicine thus has the immense obligation to ensure that this technology is harnessed for societal and individual good, fulfilling the ethical basis of the profession.… Ethical design thinking is essential at every stage of development and application of machine learning in advancing health. Toward this
Marco T. Carpio, MD
Psychiatrist, Private Practice
Lynbrook, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Bargeman CI. How the new neuroscience will advance medicine. JAMA. 2015;314(3):221-222.
2. Vahabzadeh A. Can machine learning decode depression? Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2016.4a3. Published April 11, 2016. Accessed October 16, 2019.
3. Angell M. The illusions of psychiatry. The New York Review. https://www.nybooks.com/articles/2011/07/14/illusions-of-psychiatry/. Published July 14, 2011. Accessed October 16, 2019.
4. Heath I, Nessa J. Objectification of physicians and loss of therapeutic power. Lancet. 2007;369(9565):886-888.
5. Wang D. Health care, American style: how did we arrive? Where will we go? Psychiatric Annals. 2014;44(7):342-348.
6. Nourbakhsh IR. The coming robot dystopia. Foreign Affairs. https://www.foreignaffairs.com/articles/2015-06-16/coming-robot-dystopia. Published July 2015. Accessed October 16, 2019.
7. Darcy AM, Louie AK, Roberts LW. Machine learning and the profession of medicine. JAMA. 2016;315(6):551-552.
Recent advances in neuroscience and genetics are providing a new view of brain function in health and disease.1 As discussed in Drs. Hripsime Kalanderian and Henry Nasrallah’s article “Artificial intelligence in psychiatry” (Evidence-Based Reviews,
In a 2016 article, Dr. Arshya Vahabzadeh2 wrote, “In the near term, humans will continue to make the majority of psychiatric diagnoses and provide treatment.” He predicted that data science—specifically machine learning—will help revolutionize how we diagnose, treat, and monitor depression. He foresees a future where AI machines or learning machines will more accurately diagnose and treat depression.
These predictions remind us of the promises made with the introduction of psychoactive drugs to the practice of psychiatry. In the 1970s, the increased emphasis on neurotransmitters led to new biologic models of mental illness and the expansion of the Diagnostic and Statistical Manual of Mental Disorders. The increased use of psychoactive medications relegated the practice of psychotherapy to other mental health professionals. Psychiatry became a “drug-intensive” specialty, and psychiatrists saw themselves as psychopharmacologists.3 During this time, psychiatry became progressively dominated by the pharmaceutical industry. The deregulation of the markets and the for-profit ideology of the pharmaceutical industry resulted in an economically and mutually beneficial alliance between pharmaceutical companies, academic faculty, and individual psychiatrists.
Using the same for-profit ideology and ethics of the pharmaceutical industry, technology-based corporations are making massive investments in the application of these technologies in neuroscience research and clinical practice. The “promise” that AI will diagnose and treat depression more accurately than clinicians will again radically change the psychiatrist’s role. Most of a psychiatrist’s clinical work eventually will be replicated by machine learning and technicians. The human-to-human encounter that is at core of the profession will be replaced by the machine-to-human encounter.4
From the social economic perspective, the American health care system is designed to increase profit and maximize the earnings of the industries.5 Unless social policy changes, expensive new technologies will increase the cost and limit accessibility to health care, benefiting the few at the expense of the majority of people. These technologies will produce a robust return on investment, but the wealth they create will benefit fewer and fewer people.
Several writers have called attention to the social, economic, and ethical consequences of these advances and recommended that the academics and technologists who support AI and machine learning in medicine must “receive sufficient training in ethics” and gain exposure to social and economic issues.6 Darcy et al7 warns of the risks of introducing these advanced technologies in medicine: “As machine learning enters the state-of-the-art clinical practice, medicine thus has the immense obligation to ensure that this technology is harnessed for societal and individual good, fulfilling the ethical basis of the profession.… Ethical design thinking is essential at every stage of development and application of machine learning in advancing health. Toward this
Marco T. Carpio, MD
Psychiatrist, Private Practice
Lynbrook, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Recent advances in neuroscience and genetics are providing a new view of brain function in health and disease.1 As discussed in Drs. Hripsime Kalanderian and Henry Nasrallah’s article “Artificial intelligence in psychiatry” (Evidence-Based Reviews,
In a 2016 article, Dr. Arshya Vahabzadeh2 wrote, “In the near term, humans will continue to make the majority of psychiatric diagnoses and provide treatment.” He predicted that data science—specifically machine learning—will help revolutionize how we diagnose, treat, and monitor depression. He foresees a future where AI machines or learning machines will more accurately diagnose and treat depression.
These predictions remind us of the promises made with the introduction of psychoactive drugs to the practice of psychiatry. In the 1970s, the increased emphasis on neurotransmitters led to new biologic models of mental illness and the expansion of the Diagnostic and Statistical Manual of Mental Disorders. The increased use of psychoactive medications relegated the practice of psychotherapy to other mental health professionals. Psychiatry became a “drug-intensive” specialty, and psychiatrists saw themselves as psychopharmacologists.3 During this time, psychiatry became progressively dominated by the pharmaceutical industry. The deregulation of the markets and the for-profit ideology of the pharmaceutical industry resulted in an economically and mutually beneficial alliance between pharmaceutical companies, academic faculty, and individual psychiatrists.
Using the same for-profit ideology and ethics of the pharmaceutical industry, technology-based corporations are making massive investments in the application of these technologies in neuroscience research and clinical practice. The “promise” that AI will diagnose and treat depression more accurately than clinicians will again radically change the psychiatrist’s role. Most of a psychiatrist’s clinical work eventually will be replicated by machine learning and technicians. The human-to-human encounter that is at core of the profession will be replaced by the machine-to-human encounter.4
From the social economic perspective, the American health care system is designed to increase profit and maximize the earnings of the industries.5 Unless social policy changes, expensive new technologies will increase the cost and limit accessibility to health care, benefiting the few at the expense of the majority of people. These technologies will produce a robust return on investment, but the wealth they create will benefit fewer and fewer people.
Several writers have called attention to the social, economic, and ethical consequences of these advances and recommended that the academics and technologists who support AI and machine learning in medicine must “receive sufficient training in ethics” and gain exposure to social and economic issues.6 Darcy et al7 warns of the risks of introducing these advanced technologies in medicine: “As machine learning enters the state-of-the-art clinical practice, medicine thus has the immense obligation to ensure that this technology is harnessed for societal and individual good, fulfilling the ethical basis of the profession.… Ethical design thinking is essential at every stage of development and application of machine learning in advancing health. Toward this
Marco T. Carpio, MD
Psychiatrist, Private Practice
Lynbrook, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Bargeman CI. How the new neuroscience will advance medicine. JAMA. 2015;314(3):221-222.
2. Vahabzadeh A. Can machine learning decode depression? Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2016.4a3. Published April 11, 2016. Accessed October 16, 2019.
3. Angell M. The illusions of psychiatry. The New York Review. https://www.nybooks.com/articles/2011/07/14/illusions-of-psychiatry/. Published July 14, 2011. Accessed October 16, 2019.
4. Heath I, Nessa J. Objectification of physicians and loss of therapeutic power. Lancet. 2007;369(9565):886-888.
5. Wang D. Health care, American style: how did we arrive? Where will we go? Psychiatric Annals. 2014;44(7):342-348.
6. Nourbakhsh IR. The coming robot dystopia. Foreign Affairs. https://www.foreignaffairs.com/articles/2015-06-16/coming-robot-dystopia. Published July 2015. Accessed October 16, 2019.
7. Darcy AM, Louie AK, Roberts LW. Machine learning and the profession of medicine. JAMA. 2016;315(6):551-552.
1. Bargeman CI. How the new neuroscience will advance medicine. JAMA. 2015;314(3):221-222.
2. Vahabzadeh A. Can machine learning decode depression? Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2016.4a3. Published April 11, 2016. Accessed October 16, 2019.
3. Angell M. The illusions of psychiatry. The New York Review. https://www.nybooks.com/articles/2011/07/14/illusions-of-psychiatry/. Published July 14, 2011. Accessed October 16, 2019.
4. Heath I, Nessa J. Objectification of physicians and loss of therapeutic power. Lancet. 2007;369(9565):886-888.
5. Wang D. Health care, American style: how did we arrive? Where will we go? Psychiatric Annals. 2014;44(7):342-348.
6. Nourbakhsh IR. The coming robot dystopia. Foreign Affairs. https://www.foreignaffairs.com/articles/2015-06-16/coming-robot-dystopia. Published July 2015. Accessed October 16, 2019.
7. Darcy AM, Louie AK, Roberts LW. Machine learning and the profession of medicine. JAMA. 2016;315(6):551-552.
The woman who couldn’t stop eating
CASE Uncontrollable eating and weight gain
Ms. C, age 33, presents to an outpatient clinic with complaints of weight gain and “uncontrollable eating.” Ms. C says she’s gained >50 lb over the last year. She describes progressively frequent episodes of overeating during which she feels that she has no control over the amount of food she consumes. She reports eating as often as 10 times a day, and overeating to the point of physical discomfort during most meals. She gives an example of having recently consumed a large pizza, several portions of Chinese food, approximately 20 chicken wings, and half a chocolate cake for dinner. Ms. C admits that on several occasions she has vomited after meals due to feeling extremely full; however, she denies having done so intentionally. She also denies restricting her food intake, misusing laxatives or diuretics, or exercising excessively.
Ms. C expresses frustration and embarrassment with her eating and resulting weight gain. She says she has poor self-esteem, low energy and motivation, and poor concentration. She feels that her condition has significantly impacted her social life, romantic relationships, and family life. She admits she’s been avoiding dating and seeing friends due to her weight gain, and has been irritable with her teenage daughter.
During her initial evaluation, Ms. C is alert and oriented, with a linear and goal-directed thought process. She is somewhat irritable and guarded, wearing large sunglasses that cover most of her face, but is not overtly paranoid. Although she appears frustrated when discussing her condition, she denies feeling hopeless or helpless.
HISTORY Thyroid cancer and mood swings
Ms. C, who is single and unemployed, lives in an apartment with her teenage daughter, with whom she describes having a good relationship. She has been receiving disability benefits for the past 2 years after a motor vehicle accident resulted in multiple fractures of her arm and elbow, and subsequent chronic pain. Ms. C reports a distant history of “problems with alcohol,” but denies drinking any alcohol since being charged with driving under the influence several years ago. She has a 10 pack-year history of smoking and denies any history of illicit drug use.
Two years ago, Ms. C was diagnosed with thyroid carcinoma, and treated with surgical resection and a course of radiation. She has regular visits with her endocrinologist and has been prescribed oral levothyroxine, 150 mcg/d.
Ms. C reports a history of “mood swings” characterized by “snapping at people” and becoming irritable in response to stressful situations, but denies any past symptoms consistent with a manic or hypomanic episode. Ms. C has not been admitted to a psychiatric hospital, nor has she received any prior psychiatric treatment. She reluctantly discloses that approximately 3 years ago she had a less severe episode of uncontrollable eating and weight gain (20 to 30 lb). At that time, she was able to regain her desired physical appearance by going on the “Subway diet” and undergoing liposuction and plastic surgery.
At her current outpatient clinic visit, Ms. C expresses an interest in exploring bariatric surgery as a potential solution to her weight gain.
[polldaddy:10446186]
Continue to: EVALUATION Obese; stable thyroid function
EVALUATION Obese; stable thyroid function
We refer Ms. C for a physical examination and routine blood analysis to rule out any medical contributors to her condition. Her physical examination is reported as normal, with no signs of skin changes, goiter, or exophthalmos. Ms. C is noted to be obese, with a body mass index of 37.2 kg/m2, and an abdominal circumference of 38.5 in.
A blood analysis shows that Ms. C has elevated triglyceride levels (202 mg/dL) and elevated cholesterol levels (210 mg/dL). Her thyroid function tests are within normal limits based on the dose of levothyroxine she’s been receiving. A pregnancy test is negative.
Ms. C gives the team at the clinic permission to contact her endocrinologist, who reports that he does not suspect that Ms. C’s drastic weight gain and abnormal eating patterns are attributable to her history of thyroid carcinoma because her thyroid function tests have been stable on her current regimen.
The authors’ observations
Based on Ms. C’s initial presentation, we strongly suspected a diagnosis of binge eating disorder (BED). Several differential diagnoses were considered and carefully ruled out; Ms. C’s medical workup did not suggest that her weight gain was due to an active medical condition, and she did not meet DSM-5 criteria for a mood or psychotic disorder or anorexia nervosa or bulimia nervosa.
With an estimated lifetime prevalence in the United States of 2.6%, BED is the most prevalent eating disorder (compared with 0.6% for anorexia nervosa and 1% for bulimia nervosa).1 BED is more prevalent in women than in men, and the mean age of onset is mid-20s.
Continue to: BED may be difficult...
BED may be difficult to detect because patients may feel ashamed or guilty and are often hesitant to disclose and discuss their symptoms. Furthermore, they are frequently frustrated by the subjective loss of control over their behaviors. Patients with BED often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics.
Screening for eating disorders
Several screening instruments have been developed to help clinicians identify patients who may need further evaluation for possible diagnosis of an eating disorder, including anorexia nervosa, bulimia nervosa, and BED.2 The SCOFF questionnaire is composed of 5 brief clinician-administered questions to screen for eating disorders.2 The 7-item Binge Eating Disorder Screener (BED-7) is a screening instrument specific for BED that examines a patient’s eating patterns and behaviors during the past 3 months.3
In general, suspect BED in patients who have significant weight dissatisfaction, fluctuation in weight, and depressive symptoms. The DSM-5 criteria for binge eating disorder are shown in Table 14.
BED and comorbid psychiatric disorders
Patients with BED are more likely than the general population to have comorbid psychiatric disorders, including mood and anxiety disorders, attention-deficit/hyperactivity disorder, posttraumatic stress disorder, and substance use disorders. Swanson et al5 found that 83.5% of adolescents who met criteria for BED also met criteria for at least 1 other psychiatric disorder, and 37% endorsed >3 concurrent psychiatric conditions. Once BED is confirmed, it is important to screen for other psychiatric and medical comorbidities that are often present in individuals with BED (Table 21,6).
The rates of diagnosis and treatment of BED remain low. This is likely due to patient factors such as shame and fear of stigma and clinician factors such as lack of awareness, ineffective communication, hesitation to discuss the sensitive topic, or insufficient knowledge about treatment options once BED is diagnosed.
[polldaddy:10446187]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
Ms. C is ambivalent about her BED diagnosis, and becomes angry about it when the proposed treatments do not involve bariatric surgery or cosmetic procedures. Ms. C is enrolled in weekly individual psychotherapy, where she receives a combination of CBT and psychodynamic therapy; however, her attendance is inconsistent. Ms. C is offered a trial of fluoxetine, but adamantly refuses, citing a relative who experienced adverse effects while receiving this type of antidepressant. Ms. C also refuses a trial of topiramate due to concerns of feeling sedated. Finally, she is offered a trial of lisdexamfetamine, 30 mg/d, which was FDA-approved in 2015 to treat moderate to severe BED. We discuss the risks, benefits, and adverse effects of lisdexamfetamine with Ms. C; however, she is hesitant to start this medication and expresses increasing interest in obtaining a consultation for bariatric surgery. Ms. C is provided with extensive education about the risks and dangers of surgery before addressing her eating patterns, and the clinician provides validation, verbal support, and counseling. Ms. C eventually agrees to a trial of lisdexamfetamine, but her insurance denies coverage of this medication.
The authors’ observations
When developing an individualized treatment plan for a patient with BED, the patient’s psychiatric and medical comorbidities should be considered. Treatment goals for patients with BED include:
- abstinence from binge eating
- sustainable weight loss and metabolic health
- reduction in symptoms associated with comorbid conditions
- improvement in self-esteem and overall quality of life.
A 2015 comparative effectiveness review of management and outcomes for patients with BED evaluated pharmacologic, psychologic, behavioral, and combined approaches for treating patients with BED.7 The results suggested that second-generation antidepressants, topiramate, and lisdexamfetamine were superior to placebo in reducing binge-eating episodes and achieving abstinence from binge-eating. Weight reduction was also achieved with topiramate and lisdexamfetamine, and antidepressants helped relieve symptoms of comorbid depression.
Various formats of CBT, including therapist-led and guided self-help, were also superior to placebo in reducing the frequency of binge-eating and promoting abstinence; however, they were generally not effective in treating depression or reducing patients’ weight.7
OUTCOME Fixated on surgery
We appeal the decision of Ms. C’s insurance company; however, during the appeals process, Ms. C becomes increasingly irritable and informs us that she has changed her mind and, with the reported support of her medical doctors, wishes to undergo bariatric surgery. Although we made multiple attempts to engage Ms. C in further treatment, she is lost to follow-up.
Continue to: Bottom Line
Bottom Line
Diagnosing and managing patients with binge eating disorder (BED) can be challenging because patients may hesitate to seek help, and/or have psychiatric and medical comorbidities. They often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics. Once BED is confirmed, screen for other psychiatric and medical comorbidities. A combination of pharmacologic and psychotherapeutic interventions can benefit some patients with BED, but treatment should be individualized.
Related Resources
- National Eating Disorders Association. NEDA. www.nationaleatingdisorders.org/.
- Safer D, Telch C, Chen EY. Dialectical behavior therapy for binge eating and bulimia. New York, NY: Guilford Press; 2017.
Drug Brand Names
Fluoxetine • Prozac
Levothyroxine • Synthroid
Lisdexamfetamine • Vyvanse
Topiramate • Topamax
1. Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358.
2. Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319(7223):1467-1468.
3. Herman BK, Deal LS, DiBenedetti DB, et al. Development of the 7-Item Binge-Eating Disorder screener (BEDS-7). Prim Care Companion CNS Disord. 2016;18(2):10.4088/PCC.15m01896. doi:10.4088/PCC.15m01896.
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Swanson SA, Crow SJ, Le Grange D, et al. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714.
6. Guerdjikova AI, Mori N, Casuto LS, et al. Binge eating disorder. Psychiatric Clinics of North America. 2017;40(2):255-266.
7. Berkman ND, Brownley KA, Peat CM, et al. Management and outcomes of binge-eating disorder. Comparative Effectiveness Reviews, No. 160. Agency for Healthcare Research and Quality (US). https://www.ncbi.nlm.nih.gov/books/NBK338312/. Published December 2015. Accessed July 29, 2019.
CASE Uncontrollable eating and weight gain
Ms. C, age 33, presents to an outpatient clinic with complaints of weight gain and “uncontrollable eating.” Ms. C says she’s gained >50 lb over the last year. She describes progressively frequent episodes of overeating during which she feels that she has no control over the amount of food she consumes. She reports eating as often as 10 times a day, and overeating to the point of physical discomfort during most meals. She gives an example of having recently consumed a large pizza, several portions of Chinese food, approximately 20 chicken wings, and half a chocolate cake for dinner. Ms. C admits that on several occasions she has vomited after meals due to feeling extremely full; however, she denies having done so intentionally. She also denies restricting her food intake, misusing laxatives or diuretics, or exercising excessively.
Ms. C expresses frustration and embarrassment with her eating and resulting weight gain. She says she has poor self-esteem, low energy and motivation, and poor concentration. She feels that her condition has significantly impacted her social life, romantic relationships, and family life. She admits she’s been avoiding dating and seeing friends due to her weight gain, and has been irritable with her teenage daughter.
During her initial evaluation, Ms. C is alert and oriented, with a linear and goal-directed thought process. She is somewhat irritable and guarded, wearing large sunglasses that cover most of her face, but is not overtly paranoid. Although she appears frustrated when discussing her condition, she denies feeling hopeless or helpless.
HISTORY Thyroid cancer and mood swings
Ms. C, who is single and unemployed, lives in an apartment with her teenage daughter, with whom she describes having a good relationship. She has been receiving disability benefits for the past 2 years after a motor vehicle accident resulted in multiple fractures of her arm and elbow, and subsequent chronic pain. Ms. C reports a distant history of “problems with alcohol,” but denies drinking any alcohol since being charged with driving under the influence several years ago. She has a 10 pack-year history of smoking and denies any history of illicit drug use.
Two years ago, Ms. C was diagnosed with thyroid carcinoma, and treated with surgical resection and a course of radiation. She has regular visits with her endocrinologist and has been prescribed oral levothyroxine, 150 mcg/d.
Ms. C reports a history of “mood swings” characterized by “snapping at people” and becoming irritable in response to stressful situations, but denies any past symptoms consistent with a manic or hypomanic episode. Ms. C has not been admitted to a psychiatric hospital, nor has she received any prior psychiatric treatment. She reluctantly discloses that approximately 3 years ago she had a less severe episode of uncontrollable eating and weight gain (20 to 30 lb). At that time, she was able to regain her desired physical appearance by going on the “Subway diet” and undergoing liposuction and plastic surgery.
At her current outpatient clinic visit, Ms. C expresses an interest in exploring bariatric surgery as a potential solution to her weight gain.
[polldaddy:10446186]
Continue to: EVALUATION Obese; stable thyroid function
EVALUATION Obese; stable thyroid function
We refer Ms. C for a physical examination and routine blood analysis to rule out any medical contributors to her condition. Her physical examination is reported as normal, with no signs of skin changes, goiter, or exophthalmos. Ms. C is noted to be obese, with a body mass index of 37.2 kg/m2, and an abdominal circumference of 38.5 in.
A blood analysis shows that Ms. C has elevated triglyceride levels (202 mg/dL) and elevated cholesterol levels (210 mg/dL). Her thyroid function tests are within normal limits based on the dose of levothyroxine she’s been receiving. A pregnancy test is negative.
Ms. C gives the team at the clinic permission to contact her endocrinologist, who reports that he does not suspect that Ms. C’s drastic weight gain and abnormal eating patterns are attributable to her history of thyroid carcinoma because her thyroid function tests have been stable on her current regimen.
The authors’ observations
Based on Ms. C’s initial presentation, we strongly suspected a diagnosis of binge eating disorder (BED). Several differential diagnoses were considered and carefully ruled out; Ms. C’s medical workup did not suggest that her weight gain was due to an active medical condition, and she did not meet DSM-5 criteria for a mood or psychotic disorder or anorexia nervosa or bulimia nervosa.
With an estimated lifetime prevalence in the United States of 2.6%, BED is the most prevalent eating disorder (compared with 0.6% for anorexia nervosa and 1% for bulimia nervosa).1 BED is more prevalent in women than in men, and the mean age of onset is mid-20s.
Continue to: BED may be difficult...
BED may be difficult to detect because patients may feel ashamed or guilty and are often hesitant to disclose and discuss their symptoms. Furthermore, they are frequently frustrated by the subjective loss of control over their behaviors. Patients with BED often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics.
Screening for eating disorders
Several screening instruments have been developed to help clinicians identify patients who may need further evaluation for possible diagnosis of an eating disorder, including anorexia nervosa, bulimia nervosa, and BED.2 The SCOFF questionnaire is composed of 5 brief clinician-administered questions to screen for eating disorders.2 The 7-item Binge Eating Disorder Screener (BED-7) is a screening instrument specific for BED that examines a patient’s eating patterns and behaviors during the past 3 months.3
In general, suspect BED in patients who have significant weight dissatisfaction, fluctuation in weight, and depressive symptoms. The DSM-5 criteria for binge eating disorder are shown in Table 14.
BED and comorbid psychiatric disorders
Patients with BED are more likely than the general population to have comorbid psychiatric disorders, including mood and anxiety disorders, attention-deficit/hyperactivity disorder, posttraumatic stress disorder, and substance use disorders. Swanson et al5 found that 83.5% of adolescents who met criteria for BED also met criteria for at least 1 other psychiatric disorder, and 37% endorsed >3 concurrent psychiatric conditions. Once BED is confirmed, it is important to screen for other psychiatric and medical comorbidities that are often present in individuals with BED (Table 21,6).
The rates of diagnosis and treatment of BED remain low. This is likely due to patient factors such as shame and fear of stigma and clinician factors such as lack of awareness, ineffective communication, hesitation to discuss the sensitive topic, or insufficient knowledge about treatment options once BED is diagnosed.
[polldaddy:10446187]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
Ms. C is ambivalent about her BED diagnosis, and becomes angry about it when the proposed treatments do not involve bariatric surgery or cosmetic procedures. Ms. C is enrolled in weekly individual psychotherapy, where she receives a combination of CBT and psychodynamic therapy; however, her attendance is inconsistent. Ms. C is offered a trial of fluoxetine, but adamantly refuses, citing a relative who experienced adverse effects while receiving this type of antidepressant. Ms. C also refuses a trial of topiramate due to concerns of feeling sedated. Finally, she is offered a trial of lisdexamfetamine, 30 mg/d, which was FDA-approved in 2015 to treat moderate to severe BED. We discuss the risks, benefits, and adverse effects of lisdexamfetamine with Ms. C; however, she is hesitant to start this medication and expresses increasing interest in obtaining a consultation for bariatric surgery. Ms. C is provided with extensive education about the risks and dangers of surgery before addressing her eating patterns, and the clinician provides validation, verbal support, and counseling. Ms. C eventually agrees to a trial of lisdexamfetamine, but her insurance denies coverage of this medication.
The authors’ observations
When developing an individualized treatment plan for a patient with BED, the patient’s psychiatric and medical comorbidities should be considered. Treatment goals for patients with BED include:
- abstinence from binge eating
- sustainable weight loss and metabolic health
- reduction in symptoms associated with comorbid conditions
- improvement in self-esteem and overall quality of life.
A 2015 comparative effectiveness review of management and outcomes for patients with BED evaluated pharmacologic, psychologic, behavioral, and combined approaches for treating patients with BED.7 The results suggested that second-generation antidepressants, topiramate, and lisdexamfetamine were superior to placebo in reducing binge-eating episodes and achieving abstinence from binge-eating. Weight reduction was also achieved with topiramate and lisdexamfetamine, and antidepressants helped relieve symptoms of comorbid depression.
Various formats of CBT, including therapist-led and guided self-help, were also superior to placebo in reducing the frequency of binge-eating and promoting abstinence; however, they were generally not effective in treating depression or reducing patients’ weight.7
OUTCOME Fixated on surgery
We appeal the decision of Ms. C’s insurance company; however, during the appeals process, Ms. C becomes increasingly irritable and informs us that she has changed her mind and, with the reported support of her medical doctors, wishes to undergo bariatric surgery. Although we made multiple attempts to engage Ms. C in further treatment, she is lost to follow-up.
Continue to: Bottom Line
Bottom Line
Diagnosing and managing patients with binge eating disorder (BED) can be challenging because patients may hesitate to seek help, and/or have psychiatric and medical comorbidities. They often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics. Once BED is confirmed, screen for other psychiatric and medical comorbidities. A combination of pharmacologic and psychotherapeutic interventions can benefit some patients with BED, but treatment should be individualized.
Related Resources
- National Eating Disorders Association. NEDA. www.nationaleatingdisorders.org/.
- Safer D, Telch C, Chen EY. Dialectical behavior therapy for binge eating and bulimia. New York, NY: Guilford Press; 2017.
Drug Brand Names
Fluoxetine • Prozac
Levothyroxine • Synthroid
Lisdexamfetamine • Vyvanse
Topiramate • Topamax
CASE Uncontrollable eating and weight gain
Ms. C, age 33, presents to an outpatient clinic with complaints of weight gain and “uncontrollable eating.” Ms. C says she’s gained >50 lb over the last year. She describes progressively frequent episodes of overeating during which she feels that she has no control over the amount of food she consumes. She reports eating as often as 10 times a day, and overeating to the point of physical discomfort during most meals. She gives an example of having recently consumed a large pizza, several portions of Chinese food, approximately 20 chicken wings, and half a chocolate cake for dinner. Ms. C admits that on several occasions she has vomited after meals due to feeling extremely full; however, she denies having done so intentionally. She also denies restricting her food intake, misusing laxatives or diuretics, or exercising excessively.
Ms. C expresses frustration and embarrassment with her eating and resulting weight gain. She says she has poor self-esteem, low energy and motivation, and poor concentration. She feels that her condition has significantly impacted her social life, romantic relationships, and family life. She admits she’s been avoiding dating and seeing friends due to her weight gain, and has been irritable with her teenage daughter.
During her initial evaluation, Ms. C is alert and oriented, with a linear and goal-directed thought process. She is somewhat irritable and guarded, wearing large sunglasses that cover most of her face, but is not overtly paranoid. Although she appears frustrated when discussing her condition, she denies feeling hopeless or helpless.
HISTORY Thyroid cancer and mood swings
Ms. C, who is single and unemployed, lives in an apartment with her teenage daughter, with whom she describes having a good relationship. She has been receiving disability benefits for the past 2 years after a motor vehicle accident resulted in multiple fractures of her arm and elbow, and subsequent chronic pain. Ms. C reports a distant history of “problems with alcohol,” but denies drinking any alcohol since being charged with driving under the influence several years ago. She has a 10 pack-year history of smoking and denies any history of illicit drug use.
Two years ago, Ms. C was diagnosed with thyroid carcinoma, and treated with surgical resection and a course of radiation. She has regular visits with her endocrinologist and has been prescribed oral levothyroxine, 150 mcg/d.
Ms. C reports a history of “mood swings” characterized by “snapping at people” and becoming irritable in response to stressful situations, but denies any past symptoms consistent with a manic or hypomanic episode. Ms. C has not been admitted to a psychiatric hospital, nor has she received any prior psychiatric treatment. She reluctantly discloses that approximately 3 years ago she had a less severe episode of uncontrollable eating and weight gain (20 to 30 lb). At that time, she was able to regain her desired physical appearance by going on the “Subway diet” and undergoing liposuction and plastic surgery.
At her current outpatient clinic visit, Ms. C expresses an interest in exploring bariatric surgery as a potential solution to her weight gain.
[polldaddy:10446186]
Continue to: EVALUATION Obese; stable thyroid function
EVALUATION Obese; stable thyroid function
We refer Ms. C for a physical examination and routine blood analysis to rule out any medical contributors to her condition. Her physical examination is reported as normal, with no signs of skin changes, goiter, or exophthalmos. Ms. C is noted to be obese, with a body mass index of 37.2 kg/m2, and an abdominal circumference of 38.5 in.
A blood analysis shows that Ms. C has elevated triglyceride levels (202 mg/dL) and elevated cholesterol levels (210 mg/dL). Her thyroid function tests are within normal limits based on the dose of levothyroxine she’s been receiving. A pregnancy test is negative.
Ms. C gives the team at the clinic permission to contact her endocrinologist, who reports that he does not suspect that Ms. C’s drastic weight gain and abnormal eating patterns are attributable to her history of thyroid carcinoma because her thyroid function tests have been stable on her current regimen.
The authors’ observations
Based on Ms. C’s initial presentation, we strongly suspected a diagnosis of binge eating disorder (BED). Several differential diagnoses were considered and carefully ruled out; Ms. C’s medical workup did not suggest that her weight gain was due to an active medical condition, and she did not meet DSM-5 criteria for a mood or psychotic disorder or anorexia nervosa or bulimia nervosa.
With an estimated lifetime prevalence in the United States of 2.6%, BED is the most prevalent eating disorder (compared with 0.6% for anorexia nervosa and 1% for bulimia nervosa).1 BED is more prevalent in women than in men, and the mean age of onset is mid-20s.
Continue to: BED may be difficult...
BED may be difficult to detect because patients may feel ashamed or guilty and are often hesitant to disclose and discuss their symptoms. Furthermore, they are frequently frustrated by the subjective loss of control over their behaviors. Patients with BED often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics.
Screening for eating disorders
Several screening instruments have been developed to help clinicians identify patients who may need further evaluation for possible diagnosis of an eating disorder, including anorexia nervosa, bulimia nervosa, and BED.2 The SCOFF questionnaire is composed of 5 brief clinician-administered questions to screen for eating disorders.2 The 7-item Binge Eating Disorder Screener (BED-7) is a screening instrument specific for BED that examines a patient’s eating patterns and behaviors during the past 3 months.3
In general, suspect BED in patients who have significant weight dissatisfaction, fluctuation in weight, and depressive symptoms. The DSM-5 criteria for binge eating disorder are shown in Table 14.
BED and comorbid psychiatric disorders
Patients with BED are more likely than the general population to have comorbid psychiatric disorders, including mood and anxiety disorders, attention-deficit/hyperactivity disorder, posttraumatic stress disorder, and substance use disorders. Swanson et al5 found that 83.5% of adolescents who met criteria for BED also met criteria for at least 1 other psychiatric disorder, and 37% endorsed >3 concurrent psychiatric conditions. Once BED is confirmed, it is important to screen for other psychiatric and medical comorbidities that are often present in individuals with BED (Table 21,6).
The rates of diagnosis and treatment of BED remain low. This is likely due to patient factors such as shame and fear of stigma and clinician factors such as lack of awareness, ineffective communication, hesitation to discuss the sensitive topic, or insufficient knowledge about treatment options once BED is diagnosed.
[polldaddy:10446187]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
Ms. C is ambivalent about her BED diagnosis, and becomes angry about it when the proposed treatments do not involve bariatric surgery or cosmetic procedures. Ms. C is enrolled in weekly individual psychotherapy, where she receives a combination of CBT and psychodynamic therapy; however, her attendance is inconsistent. Ms. C is offered a trial of fluoxetine, but adamantly refuses, citing a relative who experienced adverse effects while receiving this type of antidepressant. Ms. C also refuses a trial of topiramate due to concerns of feeling sedated. Finally, she is offered a trial of lisdexamfetamine, 30 mg/d, which was FDA-approved in 2015 to treat moderate to severe BED. We discuss the risks, benefits, and adverse effects of lisdexamfetamine with Ms. C; however, she is hesitant to start this medication and expresses increasing interest in obtaining a consultation for bariatric surgery. Ms. C is provided with extensive education about the risks and dangers of surgery before addressing her eating patterns, and the clinician provides validation, verbal support, and counseling. Ms. C eventually agrees to a trial of lisdexamfetamine, but her insurance denies coverage of this medication.
The authors’ observations
When developing an individualized treatment plan for a patient with BED, the patient’s psychiatric and medical comorbidities should be considered. Treatment goals for patients with BED include:
- abstinence from binge eating
- sustainable weight loss and metabolic health
- reduction in symptoms associated with comorbid conditions
- improvement in self-esteem and overall quality of life.
A 2015 comparative effectiveness review of management and outcomes for patients with BED evaluated pharmacologic, psychologic, behavioral, and combined approaches for treating patients with BED.7 The results suggested that second-generation antidepressants, topiramate, and lisdexamfetamine were superior to placebo in reducing binge-eating episodes and achieving abstinence from binge-eating. Weight reduction was also achieved with topiramate and lisdexamfetamine, and antidepressants helped relieve symptoms of comorbid depression.
Various formats of CBT, including therapist-led and guided self-help, were also superior to placebo in reducing the frequency of binge-eating and promoting abstinence; however, they were generally not effective in treating depression or reducing patients’ weight.7
OUTCOME Fixated on surgery
We appeal the decision of Ms. C’s insurance company; however, during the appeals process, Ms. C becomes increasingly irritable and informs us that she has changed her mind and, with the reported support of her medical doctors, wishes to undergo bariatric surgery. Although we made multiple attempts to engage Ms. C in further treatment, she is lost to follow-up.
Continue to: Bottom Line
Bottom Line
Diagnosing and managing patients with binge eating disorder (BED) can be challenging because patients may hesitate to seek help, and/or have psychiatric and medical comorbidities. They often present to medical facilities seeking weight loss solutions rather than to psychiatric clinics. Once BED is confirmed, screen for other psychiatric and medical comorbidities. A combination of pharmacologic and psychotherapeutic interventions can benefit some patients with BED, but treatment should be individualized.
Related Resources
- National Eating Disorders Association. NEDA. www.nationaleatingdisorders.org/.
- Safer D, Telch C, Chen EY. Dialectical behavior therapy for binge eating and bulimia. New York, NY: Guilford Press; 2017.
Drug Brand Names
Fluoxetine • Prozac
Levothyroxine • Synthroid
Lisdexamfetamine • Vyvanse
Topiramate • Topamax
1. Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358.
2. Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319(7223):1467-1468.
3. Herman BK, Deal LS, DiBenedetti DB, et al. Development of the 7-Item Binge-Eating Disorder screener (BEDS-7). Prim Care Companion CNS Disord. 2016;18(2):10.4088/PCC.15m01896. doi:10.4088/PCC.15m01896.
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Swanson SA, Crow SJ, Le Grange D, et al. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714.
6. Guerdjikova AI, Mori N, Casuto LS, et al. Binge eating disorder. Psychiatric Clinics of North America. 2017;40(2):255-266.
7. Berkman ND, Brownley KA, Peat CM, et al. Management and outcomes of binge-eating disorder. Comparative Effectiveness Reviews, No. 160. Agency for Healthcare Research and Quality (US). https://www.ncbi.nlm.nih.gov/books/NBK338312/. Published December 2015. Accessed July 29, 2019.
1. Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358.
2. Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319(7223):1467-1468.
3. Herman BK, Deal LS, DiBenedetti DB, et al. Development of the 7-Item Binge-Eating Disorder screener (BEDS-7). Prim Care Companion CNS Disord. 2016;18(2):10.4088/PCC.15m01896. doi:10.4088/PCC.15m01896.
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Swanson SA, Crow SJ, Le Grange D, et al. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714.
6. Guerdjikova AI, Mori N, Casuto LS, et al. Binge eating disorder. Psychiatric Clinics of North America. 2017;40(2):255-266.
7. Berkman ND, Brownley KA, Peat CM, et al. Management and outcomes of binge-eating disorder. Comparative Effectiveness Reviews, No. 160. Agency for Healthcare Research and Quality (US). https://www.ncbi.nlm.nih.gov/books/NBK338312/. Published December 2015. Accessed July 29, 2019.