User login
Effects of psychotropic medications on thyroid function
Ms. L, age 53, presents to an inpatient psychiatric unit with depression, difficulty concentrating, fatigue, cognitive blunting, loss of appetite, increased alcohol intake, and recent suicidal ideation. Her symptoms began 3 months ago and gradually worsened. Her medical and psychiatric history is significant for hypertension, fibromyalgia, and chronic pain (back and neck), major depressive disorder (MDD; recurrent, severe), and generalized anxiety disorder (GAD). Ms. L’s current medication regimen includes lisinopril, 40 mg daily; fluoxetine, 60 mg daily; mirtazapine, 30 mg at bedtime; gabapentin, 300 mg twice daily; alprazolam, 0.5 mg twice daily as needed for anxiety; and oral docusate, 100 mg twice daily as needed. Her blood pressure is 124/85 mm Hg, heart rate is 66 beats per minute, and an electrocardiogram is normal. Laboratory workup reveals a potassium level of 4.4 mEq/L, blood urea nitrogen level of 20 mg/dL, serum creatinine level of 0.8 mg/dL, estimated creatinine clearance of 89.6 mL/min, free triiodothyronine (T3) levels of 2.7 pg/mL, thyroid-stimulating hormone (TSH) level of 7.68 mIU/L, free thyroxine (T4) level of 1.3 ng/dL, and blood ethanol level <10 mg/dL. In addition to the symptoms Ms. L initially described, a review of systems reveals word-finding difficulty, cold intolerance, constipation, hair loss, brittle nails, and dry skin.
To target Ms. L’s MDD, GAD, fibromyalgia, and chronic pain, fluoxetine, 60 mg daily is cross titrated beginning on Day 1 to duloxetine, 60 mg twice daily, over 4 days. Mirtazapine is decreased on Day 3 to 7.5 mg at bedtime to target Ms. L’s sleep and appetite. Due to the presence of several symptoms associated with hypothyroidism and a slightly elevated TSH level, on Day 6 we initiate adjunctive levothyroxine, 50 mcg daily each morning to target symptomatic subclinical hypothyroidism, and to potentially augment the other medications prescribed to address Ms. L’s MDD.
Thyroid hormone function is a complex physiological process controlled through the hypothalamic-pituitary-thyroid (HPT) axis. Psychotropic medications can impact thyroid hormone function and contribute to aberrations in thyroid physiology.1 Because patients with mental illness may require multiple psychotropic medications, it is imperative to understand the potential effects of these agents.
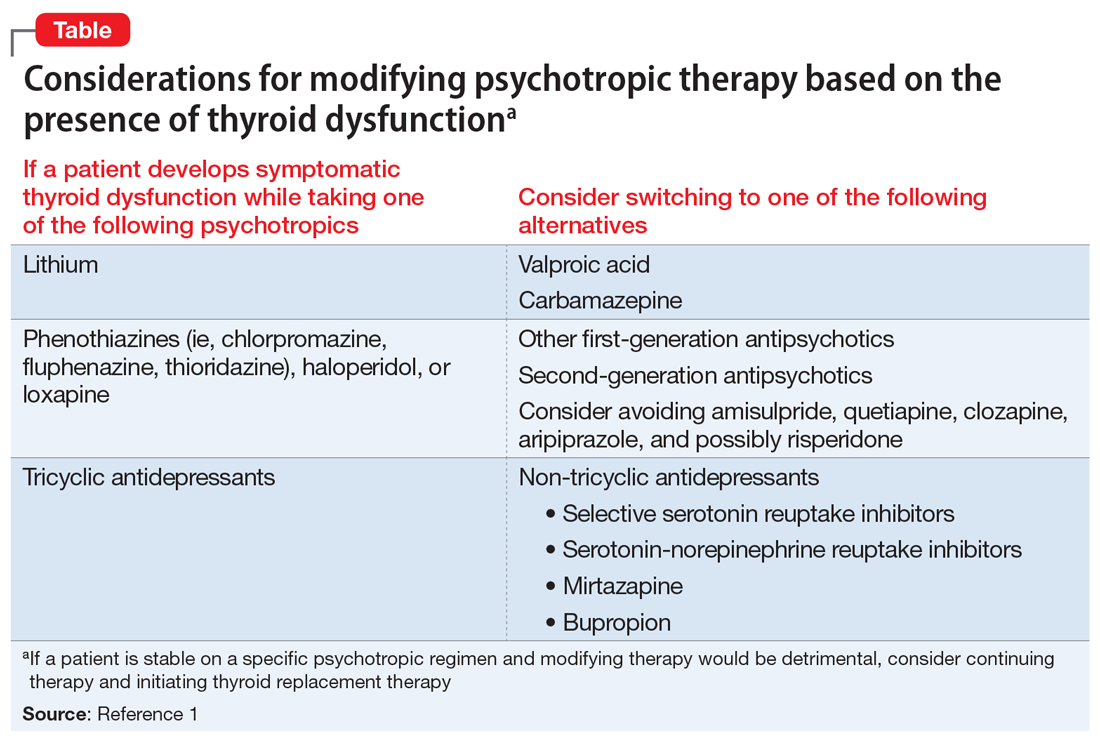
Antidepressants can induce hypothyroidism along multiple points of hormonal synthesis and iodine utilization. Tricyclic antidepressants have been implicated in the development of drug-iodide complexes, thus reducing biologically active iodine.2 Tricyclic antidepressants also can bind thyroid peroxidase, an enzyme necessary in the production of T4 and T3, altering hormonal production, resulting in a hypothyroid state.1 Non-tricyclic antidepressants (ie, selective serotonin reuptake inhibitors [SSRIs] and non-SSRIs [including serotonin-norepinephrine reuptake inhibitors and mirtazapine]) have also been implicated in thyroid dysfunction. Selective serotonin reuptake inhibitors have the propensity to induce hypothyroidism through inhibition of thyroid hormones T4 and T3.1,3 This inhibition is not always seen with concurrent reductions in TSH levels. Conversely, non-SSRIs can influence thyroid hormone levels with great variation, leading to thyroid hormone levels that are increased, decreased, or unchanged.1 Patients with a history of thyroid dysfunction should receive close thyroid function monitoring, especially while taking antidepressants.
Antipsychotics have a proclivity to induce hypothyroidism by means similar to antidepressants via hormonal manipulation and immunogenicity. Phenothiazines impact thyroid function through hormonal activation and degradation, and induction of autoimmunity.1 Autoimmunity may develop by means of antibody production or antigen immunization through the major histocompatibility complex.2 Other first-generation antipsychotics (FGAs) (eg, haloperidol and loxapine) are known to antagonize dopamine receptors in the tuberoinfundibular pathway, resulting in increased prolactin levels. Hyperprolactinemia may result in increased TSH levels through HPT axis activation.1 Additionally, FGAs can induce an immunogenic effect through production of antithyroid antibodies.1 Similar to FGAs, second-generation antipsychotics (SGAs) can increase TSH levels through hyperprolactinemia. Further research focused on SGAs is needed to determine how profound this effect may be.
The Table1 outlines considerations for modifying psychotropic therapy based on the presence of concurrent thyroid dysfunction. Thyroid function should be routinely assessed in patients treated with antipsychotics.
Mood stabilizers are capable of altering thyroid function and inducing a hypothyroid state. Lithium has been implicated in both hypothyroidism and hyperthyroidism due to its inhibition of hormonal secretion, and toxicity to thyroid cells with chronic use, respectively.1,4 Hypothyroidism can develop shortly after initiating lithium; women tend to have a greater predilection for thyroid dysfunction than men.1 Carbamazepine (CBZ) can reduce thyroid hormone levels without having a direct effect on TSH or thyroid dysfunction.1 As with lithium, women tend to be more susceptible to this effect. Valproic acid (VPA) has been shown to either increase, decrease, or have no impact on thyroid hormone levels, with little effect on TSH.1 When VPA is given in combination with CBZ, significant reductions in thyroid levels with a concurrent increase in TSH can occur.1 In patients with preexisting thyroid dysfunction, the combination of VPA and CBZ should be used with caution.
Continue to: CASE
CASE CONTINUED
By Day 8, Ms. L reports less fatigue, clearer thinking, improved concentration, and less pain. She also no longer reports suicidal ideation, and demonstrates improved appetite and mood. She is discharged on Day 9 of her hospitalization.
The treatment team refers Ms. L for outpatient follow-up in 4 weeks, with a goal TSH level <3.0. Unfortunately, the effects of levothyroxine on Ms. L’s TSH level could not be determined during her hospital stay, and she has not returned to the facility since the initial presentation.
Thyroid function and mood
Ms. L’s case illustrates how thyroid function, pain, cognition, and mood may be interconnected. It is important to address all potential underlying comorbidities and establish appropriate outpatient care and follow-up so that patients may experience a more robust recovery. Further, this case highlights the importance of ruling out other potential medical causes of MDD during the initial diagnosis, and during times of recurrence or relapse, especially when a recent stressor, medication changes, or medication nonadherence cannot be identified as potential contributors.
Related Resources
- Cojić M, Cvejanov-Kezunović L. Subclinical hypothyroidism – whether and when to start treatment? Open Access Maced J Med Sci. 2017;5(7):1042-1046.
- Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200-1235.
- Iosifescu DV. ‘Supercharge’ antidepressants by adding thyroid hormones. Current Psychiatry. 2006;5(7):15-20,25.
Drug Brand Names
Alprazolam • Xanax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Gabapentin • Neurontin
Haloperidol • Haldol
Levothyroxine • Synthroid
Lisinopril • Prinivil, Zestril
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Mirtazapine • Remeron
Quetiapine • Seroquel
Risperidone • Risperdal
Thioridazine • Mellaril
Valproic acid • Depakote
1. Bou Khalil R, Richa S. Thyroid adverse effect of psychotropic drugs: a review. Clin Neuropharm. 2001;34(6):248-255.
2. Sauvage MF, Marquet P, Rousseau A, et al. Relationship between psychotropic drugs and thyroid function: a review. Toxicol Appl Pharmacol. 1998;149(2):127-135.
3. Shelton RC, Winn S, Ekhatore N, et al. The effects of antidepressants on the thyroid axis in depression. Biol Psychiatry. 1993;33(2):120-126.
4. Kundra P, Burman KD. The effect of medications on thyroid function tests. Med Clin North Am. 2012;96(2):283-295.
Ms. L, age 53, presents to an inpatient psychiatric unit with depression, difficulty concentrating, fatigue, cognitive blunting, loss of appetite, increased alcohol intake, and recent suicidal ideation. Her symptoms began 3 months ago and gradually worsened. Her medical and psychiatric history is significant for hypertension, fibromyalgia, and chronic pain (back and neck), major depressive disorder (MDD; recurrent, severe), and generalized anxiety disorder (GAD). Ms. L’s current medication regimen includes lisinopril, 40 mg daily; fluoxetine, 60 mg daily; mirtazapine, 30 mg at bedtime; gabapentin, 300 mg twice daily; alprazolam, 0.5 mg twice daily as needed for anxiety; and oral docusate, 100 mg twice daily as needed. Her blood pressure is 124/85 mm Hg, heart rate is 66 beats per minute, and an electrocardiogram is normal. Laboratory workup reveals a potassium level of 4.4 mEq/L, blood urea nitrogen level of 20 mg/dL, serum creatinine level of 0.8 mg/dL, estimated creatinine clearance of 89.6 mL/min, free triiodothyronine (T3) levels of 2.7 pg/mL, thyroid-stimulating hormone (TSH) level of 7.68 mIU/L, free thyroxine (T4) level of 1.3 ng/dL, and blood ethanol level <10 mg/dL. In addition to the symptoms Ms. L initially described, a review of systems reveals word-finding difficulty, cold intolerance, constipation, hair loss, brittle nails, and dry skin.
To target Ms. L’s MDD, GAD, fibromyalgia, and chronic pain, fluoxetine, 60 mg daily is cross titrated beginning on Day 1 to duloxetine, 60 mg twice daily, over 4 days. Mirtazapine is decreased on Day 3 to 7.5 mg at bedtime to target Ms. L’s sleep and appetite. Due to the presence of several symptoms associated with hypothyroidism and a slightly elevated TSH level, on Day 6 we initiate adjunctive levothyroxine, 50 mcg daily each morning to target symptomatic subclinical hypothyroidism, and to potentially augment the other medications prescribed to address Ms. L’s MDD.
Thyroid hormone function is a complex physiological process controlled through the hypothalamic-pituitary-thyroid (HPT) axis. Psychotropic medications can impact thyroid hormone function and contribute to aberrations in thyroid physiology.1 Because patients with mental illness may require multiple psychotropic medications, it is imperative to understand the potential effects of these agents.
Antidepressants can induce hypothyroidism along multiple points of hormonal synthesis and iodine utilization. Tricyclic antidepressants have been implicated in the development of drug-iodide complexes, thus reducing biologically active iodine.2 Tricyclic antidepressants also can bind thyroid peroxidase, an enzyme necessary in the production of T4 and T3, altering hormonal production, resulting in a hypothyroid state.1 Non-tricyclic antidepressants (ie, selective serotonin reuptake inhibitors [SSRIs] and non-SSRIs [including serotonin-norepinephrine reuptake inhibitors and mirtazapine]) have also been implicated in thyroid dysfunction. Selective serotonin reuptake inhibitors have the propensity to induce hypothyroidism through inhibition of thyroid hormones T4 and T3.1,3 This inhibition is not always seen with concurrent reductions in TSH levels. Conversely, non-SSRIs can influence thyroid hormone levels with great variation, leading to thyroid hormone levels that are increased, decreased, or unchanged.1 Patients with a history of thyroid dysfunction should receive close thyroid function monitoring, especially while taking antidepressants.
Antipsychotics have a proclivity to induce hypothyroidism by means similar to antidepressants via hormonal manipulation and immunogenicity. Phenothiazines impact thyroid function through hormonal activation and degradation, and induction of autoimmunity.1 Autoimmunity may develop by means of antibody production or antigen immunization through the major histocompatibility complex.2 Other first-generation antipsychotics (FGAs) (eg, haloperidol and loxapine) are known to antagonize dopamine receptors in the tuberoinfundibular pathway, resulting in increased prolactin levels. Hyperprolactinemia may result in increased TSH levels through HPT axis activation.1 Additionally, FGAs can induce an immunogenic effect through production of antithyroid antibodies.1 Similar to FGAs, second-generation antipsychotics (SGAs) can increase TSH levels through hyperprolactinemia. Further research focused on SGAs is needed to determine how profound this effect may be.
The Table1 outlines considerations for modifying psychotropic therapy based on the presence of concurrent thyroid dysfunction. Thyroid function should be routinely assessed in patients treated with antipsychotics.
Mood stabilizers are capable of altering thyroid function and inducing a hypothyroid state. Lithium has been implicated in both hypothyroidism and hyperthyroidism due to its inhibition of hormonal secretion, and toxicity to thyroid cells with chronic use, respectively.1,4 Hypothyroidism can develop shortly after initiating lithium; women tend to have a greater predilection for thyroid dysfunction than men.1 Carbamazepine (CBZ) can reduce thyroid hormone levels without having a direct effect on TSH or thyroid dysfunction.1 As with lithium, women tend to be more susceptible to this effect. Valproic acid (VPA) has been shown to either increase, decrease, or have no impact on thyroid hormone levels, with little effect on TSH.1 When VPA is given in combination with CBZ, significant reductions in thyroid levels with a concurrent increase in TSH can occur.1 In patients with preexisting thyroid dysfunction, the combination of VPA and CBZ should be used with caution.
Continue to: CASE
CASE CONTINUED
By Day 8, Ms. L reports less fatigue, clearer thinking, improved concentration, and less pain. She also no longer reports suicidal ideation, and demonstrates improved appetite and mood. She is discharged on Day 9 of her hospitalization.
The treatment team refers Ms. L for outpatient follow-up in 4 weeks, with a goal TSH level <3.0. Unfortunately, the effects of levothyroxine on Ms. L’s TSH level could not be determined during her hospital stay, and she has not returned to the facility since the initial presentation.
Thyroid function and mood
Ms. L’s case illustrates how thyroid function, pain, cognition, and mood may be interconnected. It is important to address all potential underlying comorbidities and establish appropriate outpatient care and follow-up so that patients may experience a more robust recovery. Further, this case highlights the importance of ruling out other potential medical causes of MDD during the initial diagnosis, and during times of recurrence or relapse, especially when a recent stressor, medication changes, or medication nonadherence cannot be identified as potential contributors.
Related Resources
- Cojić M, Cvejanov-Kezunović L. Subclinical hypothyroidism – whether and when to start treatment? Open Access Maced J Med Sci. 2017;5(7):1042-1046.
- Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200-1235.
- Iosifescu DV. ‘Supercharge’ antidepressants by adding thyroid hormones. Current Psychiatry. 2006;5(7):15-20,25.
Drug Brand Names
Alprazolam • Xanax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Gabapentin • Neurontin
Haloperidol • Haldol
Levothyroxine • Synthroid
Lisinopril • Prinivil, Zestril
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Mirtazapine • Remeron
Quetiapine • Seroquel
Risperidone • Risperdal
Thioridazine • Mellaril
Valproic acid • Depakote
Ms. L, age 53, presents to an inpatient psychiatric unit with depression, difficulty concentrating, fatigue, cognitive blunting, loss of appetite, increased alcohol intake, and recent suicidal ideation. Her symptoms began 3 months ago and gradually worsened. Her medical and psychiatric history is significant for hypertension, fibromyalgia, and chronic pain (back and neck), major depressive disorder (MDD; recurrent, severe), and generalized anxiety disorder (GAD). Ms. L’s current medication regimen includes lisinopril, 40 mg daily; fluoxetine, 60 mg daily; mirtazapine, 30 mg at bedtime; gabapentin, 300 mg twice daily; alprazolam, 0.5 mg twice daily as needed for anxiety; and oral docusate, 100 mg twice daily as needed. Her blood pressure is 124/85 mm Hg, heart rate is 66 beats per minute, and an electrocardiogram is normal. Laboratory workup reveals a potassium level of 4.4 mEq/L, blood urea nitrogen level of 20 mg/dL, serum creatinine level of 0.8 mg/dL, estimated creatinine clearance of 89.6 mL/min, free triiodothyronine (T3) levels of 2.7 pg/mL, thyroid-stimulating hormone (TSH) level of 7.68 mIU/L, free thyroxine (T4) level of 1.3 ng/dL, and blood ethanol level <10 mg/dL. In addition to the symptoms Ms. L initially described, a review of systems reveals word-finding difficulty, cold intolerance, constipation, hair loss, brittle nails, and dry skin.
To target Ms. L’s MDD, GAD, fibromyalgia, and chronic pain, fluoxetine, 60 mg daily is cross titrated beginning on Day 1 to duloxetine, 60 mg twice daily, over 4 days. Mirtazapine is decreased on Day 3 to 7.5 mg at bedtime to target Ms. L’s sleep and appetite. Due to the presence of several symptoms associated with hypothyroidism and a slightly elevated TSH level, on Day 6 we initiate adjunctive levothyroxine, 50 mcg daily each morning to target symptomatic subclinical hypothyroidism, and to potentially augment the other medications prescribed to address Ms. L’s MDD.
Thyroid hormone function is a complex physiological process controlled through the hypothalamic-pituitary-thyroid (HPT) axis. Psychotropic medications can impact thyroid hormone function and contribute to aberrations in thyroid physiology.1 Because patients with mental illness may require multiple psychotropic medications, it is imperative to understand the potential effects of these agents.
Antidepressants can induce hypothyroidism along multiple points of hormonal synthesis and iodine utilization. Tricyclic antidepressants have been implicated in the development of drug-iodide complexes, thus reducing biologically active iodine.2 Tricyclic antidepressants also can bind thyroid peroxidase, an enzyme necessary in the production of T4 and T3, altering hormonal production, resulting in a hypothyroid state.1 Non-tricyclic antidepressants (ie, selective serotonin reuptake inhibitors [SSRIs] and non-SSRIs [including serotonin-norepinephrine reuptake inhibitors and mirtazapine]) have also been implicated in thyroid dysfunction. Selective serotonin reuptake inhibitors have the propensity to induce hypothyroidism through inhibition of thyroid hormones T4 and T3.1,3 This inhibition is not always seen with concurrent reductions in TSH levels. Conversely, non-SSRIs can influence thyroid hormone levels with great variation, leading to thyroid hormone levels that are increased, decreased, or unchanged.1 Patients with a history of thyroid dysfunction should receive close thyroid function monitoring, especially while taking antidepressants.
Antipsychotics have a proclivity to induce hypothyroidism by means similar to antidepressants via hormonal manipulation and immunogenicity. Phenothiazines impact thyroid function through hormonal activation and degradation, and induction of autoimmunity.1 Autoimmunity may develop by means of antibody production or antigen immunization through the major histocompatibility complex.2 Other first-generation antipsychotics (FGAs) (eg, haloperidol and loxapine) are known to antagonize dopamine receptors in the tuberoinfundibular pathway, resulting in increased prolactin levels. Hyperprolactinemia may result in increased TSH levels through HPT axis activation.1 Additionally, FGAs can induce an immunogenic effect through production of antithyroid antibodies.1 Similar to FGAs, second-generation antipsychotics (SGAs) can increase TSH levels through hyperprolactinemia. Further research focused on SGAs is needed to determine how profound this effect may be.
The Table1 outlines considerations for modifying psychotropic therapy based on the presence of concurrent thyroid dysfunction. Thyroid function should be routinely assessed in patients treated with antipsychotics.
Mood stabilizers are capable of altering thyroid function and inducing a hypothyroid state. Lithium has been implicated in both hypothyroidism and hyperthyroidism due to its inhibition of hormonal secretion, and toxicity to thyroid cells with chronic use, respectively.1,4 Hypothyroidism can develop shortly after initiating lithium; women tend to have a greater predilection for thyroid dysfunction than men.1 Carbamazepine (CBZ) can reduce thyroid hormone levels without having a direct effect on TSH or thyroid dysfunction.1 As with lithium, women tend to be more susceptible to this effect. Valproic acid (VPA) has been shown to either increase, decrease, or have no impact on thyroid hormone levels, with little effect on TSH.1 When VPA is given in combination with CBZ, significant reductions in thyroid levels with a concurrent increase in TSH can occur.1 In patients with preexisting thyroid dysfunction, the combination of VPA and CBZ should be used with caution.
Continue to: CASE
CASE CONTINUED
By Day 8, Ms. L reports less fatigue, clearer thinking, improved concentration, and less pain. She also no longer reports suicidal ideation, and demonstrates improved appetite and mood. She is discharged on Day 9 of her hospitalization.
The treatment team refers Ms. L for outpatient follow-up in 4 weeks, with a goal TSH level <3.0. Unfortunately, the effects of levothyroxine on Ms. L’s TSH level could not be determined during her hospital stay, and she has not returned to the facility since the initial presentation.
Thyroid function and mood
Ms. L’s case illustrates how thyroid function, pain, cognition, and mood may be interconnected. It is important to address all potential underlying comorbidities and establish appropriate outpatient care and follow-up so that patients may experience a more robust recovery. Further, this case highlights the importance of ruling out other potential medical causes of MDD during the initial diagnosis, and during times of recurrence or relapse, especially when a recent stressor, medication changes, or medication nonadherence cannot be identified as potential contributors.
Related Resources
- Cojić M, Cvejanov-Kezunović L. Subclinical hypothyroidism – whether and when to start treatment? Open Access Maced J Med Sci. 2017;5(7):1042-1046.
- Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200-1235.
- Iosifescu DV. ‘Supercharge’ antidepressants by adding thyroid hormones. Current Psychiatry. 2006;5(7):15-20,25.
Drug Brand Names
Alprazolam • Xanax
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Clozapine • Clozaril
Duloxetine • Cymbalta
Fluoxetine • Prozac
Fluphenazine • Prolixin
Gabapentin • Neurontin
Haloperidol • Haldol
Levothyroxine • Synthroid
Lisinopril • Prinivil, Zestril
Lithium • Eskalith, Lithobid
Loxapine • Loxitane
Mirtazapine • Remeron
Quetiapine • Seroquel
Risperidone • Risperdal
Thioridazine • Mellaril
Valproic acid • Depakote
1. Bou Khalil R, Richa S. Thyroid adverse effect of psychotropic drugs: a review. Clin Neuropharm. 2001;34(6):248-255.
2. Sauvage MF, Marquet P, Rousseau A, et al. Relationship between psychotropic drugs and thyroid function: a review. Toxicol Appl Pharmacol. 1998;149(2):127-135.
3. Shelton RC, Winn S, Ekhatore N, et al. The effects of antidepressants on the thyroid axis in depression. Biol Psychiatry. 1993;33(2):120-126.
4. Kundra P, Burman KD. The effect of medications on thyroid function tests. Med Clin North Am. 2012;96(2):283-295.
1. Bou Khalil R, Richa S. Thyroid adverse effect of psychotropic drugs: a review. Clin Neuropharm. 2001;34(6):248-255.
2. Sauvage MF, Marquet P, Rousseau A, et al. Relationship between psychotropic drugs and thyroid function: a review. Toxicol Appl Pharmacol. 1998;149(2):127-135.
3. Shelton RC, Winn S, Ekhatore N, et al. The effects of antidepressants on the thyroid axis in depression. Biol Psychiatry. 1993;33(2):120-126.
4. Kundra P, Burman KD. The effect of medications on thyroid function tests. Med Clin North Am. 2012;96(2):283-295.
Prescribing medications in an emergency situation? Document your rationale
Emergent medication use is indicated in numerous clinical scenarios, including psychotic agitation, physical aggression, or withdrawal from substances. While there is plenty of literature to help clinicians with medical record documentation in various other settings,1-3 there is minimal guidance on how to document your rationale for using psychiatric medications in emergency situations.
I have designed a template for structuring progress notes that has helped me to quickly explain my decision-making for using psychiatric medications during an emergency. When writing a progress note to justify your clinical actions in these situations, ask yourself the following questions:
- What symptoms/behaviors needed to be emergently treated? (Use direct quotes from the patient.)
- Which nonpharmacologic interventions were attempted prior to using a medication?
- Does the patient have any medication allergies? (Document if you were unable to assess for allergies.)
- Why did you select this specific route for medication administration?
- What was your rationale for using the specific medication(s)?
- What was the rationale for the selected dose?
- Who was present during medication administration?
- Which (if any) concurrent interventions did you order during or after medication administration?
- Were any safety follow-up checks ordered after medication administration?
A sample progress note
To help illustrate how these questions could guide a clinician’s writing, the following is a progress note I created using this template:
“Patient woke up at 3:15
1. Gutheil TG. Fundamentals of medical record documentation. Psychiatry (Edgmont). 2004;1(3):26-28.
2. Guth T, Morrissey T. Medical documentation and ED charting. Clerkship Directors in Emergency Medicine. https://saem.org/cdem/education/online-education/m3-curriculum/documentation/documentation-of-em-encounters. Updated 2015. Accessed October 10, 2019.
3. Aftab A, Latorre S, Nagle-Yang S. Effective note-writing: a primer for psychiatry residents. Psychiatric Times. http://www.psychiatrictimes.com/couch-crisis/effective-note-writing-primer-psychiatry-residents. Published January 13, 2017. Accessed October 10, 2019.
Emergent medication use is indicated in numerous clinical scenarios, including psychotic agitation, physical aggression, or withdrawal from substances. While there is plenty of literature to help clinicians with medical record documentation in various other settings,1-3 there is minimal guidance on how to document your rationale for using psychiatric medications in emergency situations.
I have designed a template for structuring progress notes that has helped me to quickly explain my decision-making for using psychiatric medications during an emergency. When writing a progress note to justify your clinical actions in these situations, ask yourself the following questions:
- What symptoms/behaviors needed to be emergently treated? (Use direct quotes from the patient.)
- Which nonpharmacologic interventions were attempted prior to using a medication?
- Does the patient have any medication allergies? (Document if you were unable to assess for allergies.)
- Why did you select this specific route for medication administration?
- What was your rationale for using the specific medication(s)?
- What was the rationale for the selected dose?
- Who was present during medication administration?
- Which (if any) concurrent interventions did you order during or after medication administration?
- Were any safety follow-up checks ordered after medication administration?
A sample progress note
To help illustrate how these questions could guide a clinician’s writing, the following is a progress note I created using this template:
“Patient woke up at 3:15
Emergent medication use is indicated in numerous clinical scenarios, including psychotic agitation, physical aggression, or withdrawal from substances. While there is plenty of literature to help clinicians with medical record documentation in various other settings,1-3 there is minimal guidance on how to document your rationale for using psychiatric medications in emergency situations.
I have designed a template for structuring progress notes that has helped me to quickly explain my decision-making for using psychiatric medications during an emergency. When writing a progress note to justify your clinical actions in these situations, ask yourself the following questions:
- What symptoms/behaviors needed to be emergently treated? (Use direct quotes from the patient.)
- Which nonpharmacologic interventions were attempted prior to using a medication?
- Does the patient have any medication allergies? (Document if you were unable to assess for allergies.)
- Why did you select this specific route for medication administration?
- What was your rationale for using the specific medication(s)?
- What was the rationale for the selected dose?
- Who was present during medication administration?
- Which (if any) concurrent interventions did you order during or after medication administration?
- Were any safety follow-up checks ordered after medication administration?
A sample progress note
To help illustrate how these questions could guide a clinician’s writing, the following is a progress note I created using this template:
“Patient woke up at 3:15
1. Gutheil TG. Fundamentals of medical record documentation. Psychiatry (Edgmont). 2004;1(3):26-28.
2. Guth T, Morrissey T. Medical documentation and ED charting. Clerkship Directors in Emergency Medicine. https://saem.org/cdem/education/online-education/m3-curriculum/documentation/documentation-of-em-encounters. Updated 2015. Accessed October 10, 2019.
3. Aftab A, Latorre S, Nagle-Yang S. Effective note-writing: a primer for psychiatry residents. Psychiatric Times. http://www.psychiatrictimes.com/couch-crisis/effective-note-writing-primer-psychiatry-residents. Published January 13, 2017. Accessed October 10, 2019.
1. Gutheil TG. Fundamentals of medical record documentation. Psychiatry (Edgmont). 2004;1(3):26-28.
2. Guth T, Morrissey T. Medical documentation and ED charting. Clerkship Directors in Emergency Medicine. https://saem.org/cdem/education/online-education/m3-curriculum/documentation/documentation-of-em-encounters. Updated 2015. Accessed October 10, 2019.
3. Aftab A, Latorre S, Nagle-Yang S. Effective note-writing: a primer for psychiatry residents. Psychiatric Times. http://www.psychiatrictimes.com/couch-crisis/effective-note-writing-primer-psychiatry-residents. Published January 13, 2017. Accessed October 10, 2019.
Dealing with deception: How to manage patients who are ‘faking it’
Patients who fabricate or exaggerate psychiatric symptoms for primary or secondary gain may elicit negative responses from health care professionals. As clinicians, we may believe that such patients are wasting our time and taking resources away from other patients who are genuinely struggling with mental illness and are more deserving of assistance. However, patients who are fabricating or exaggerating their symptoms have legitimate clinical needs that we should strive to understand. If we view them as having reasons for their actions without becoming complicit in their deception, we may find it easier to work with them.
Managing patients who are fabricating or exaggerating
Caring for patients who attempt to mislead us is a challenging proposition. The relevant research is scarce, and there are few recommended interventions for managing patients who fabricate or exaggerate symptoms.1 Direct confrontation and accusation are often unproductive and should be used sparingly. Indirect approaches tend to be more effective.
It is important to manage our countertransference at the outset while establishing and maintaining rapport. Although we may become frustrated, we should avoid using sarcasm or overt skepticism; instead, we should validate these patients’ emotions because their emotional turmoil could be driving their fabrication or exaggeration. We should attempt to explore their specific motivations by focusing our questions on detecting the underlying stressors or conditions.2
To assess our patients’ motives, consider asking the following:
- What kind of problems have these symptoms caused you in your day-to-day life?
- What would make life better for you?
- What are you hoping I can do for you today?
We should ask open-ended questions as well as interview patients over a long period of time and on multiple occasions to observe the consistency of their reported symptoms. In addition, we should take good notes and document our observations to compare what our patients tell us during their appointments.
Addressing inconsistencies
While exploring our patients’ motives, when it is appropriate, we can gently confront discrepancies in their report by asking:
- I am confused about your symptoms. Help me understand what is happening. Can you tell me more? (Then ask specific follow-up questions based on their answer.)
- What do you mean when you say you are experiencing this symptom?
- I am not sure if I understand what you said correctly. These symptoms do not typically occur in the way that you described. Could you tell me more?
- The symptoms you described are unusual to me. Is there something else going on that I am not aware of?
- Do you think these symptoms have been coming up because you are under stress?
- Is it possible that you want to (avoid work, avoid jail, be prescribed a specific medication, etc.) and that this is the only way you could think of to get what you need?
- Is it possible that you are describing what you are experiencing so that you can convince others that you are having problems?
Despite our best efforts, some patients may not drop their guard and will continue to fabricate or exaggerate their symptoms. However, establishing and maintaining rapport, exploring our patients’ potential motives to mislead, and gently confronting discrepancies in their report may maximize the chances of successfully engaging them and developing appropriate treatment plans.
1. Brady MC, Scher LM, Newman W. “I just saw Big Bird. He was 100 feet tall!” Malingering in the emergency room. Current Psychiatry. 2013;12(10):33-40.
2. Schnellbacher S, O’Mara H. Identifying and managing malingering and factitious disorder in the military. Curr Psychiatry Rep. 2016;18(11):105.
Patients who fabricate or exaggerate psychiatric symptoms for primary or secondary gain may elicit negative responses from health care professionals. As clinicians, we may believe that such patients are wasting our time and taking resources away from other patients who are genuinely struggling with mental illness and are more deserving of assistance. However, patients who are fabricating or exaggerating their symptoms have legitimate clinical needs that we should strive to understand. If we view them as having reasons for their actions without becoming complicit in their deception, we may find it easier to work with them.
Managing patients who are fabricating or exaggerating
Caring for patients who attempt to mislead us is a challenging proposition. The relevant research is scarce, and there are few recommended interventions for managing patients who fabricate or exaggerate symptoms.1 Direct confrontation and accusation are often unproductive and should be used sparingly. Indirect approaches tend to be more effective.
It is important to manage our countertransference at the outset while establishing and maintaining rapport. Although we may become frustrated, we should avoid using sarcasm or overt skepticism; instead, we should validate these patients’ emotions because their emotional turmoil could be driving their fabrication or exaggeration. We should attempt to explore their specific motivations by focusing our questions on detecting the underlying stressors or conditions.2
To assess our patients’ motives, consider asking the following:
- What kind of problems have these symptoms caused you in your day-to-day life?
- What would make life better for you?
- What are you hoping I can do for you today?
We should ask open-ended questions as well as interview patients over a long period of time and on multiple occasions to observe the consistency of their reported symptoms. In addition, we should take good notes and document our observations to compare what our patients tell us during their appointments.
Addressing inconsistencies
While exploring our patients’ motives, when it is appropriate, we can gently confront discrepancies in their report by asking:
- I am confused about your symptoms. Help me understand what is happening. Can you tell me more? (Then ask specific follow-up questions based on their answer.)
- What do you mean when you say you are experiencing this symptom?
- I am not sure if I understand what you said correctly. These symptoms do not typically occur in the way that you described. Could you tell me more?
- The symptoms you described are unusual to me. Is there something else going on that I am not aware of?
- Do you think these symptoms have been coming up because you are under stress?
- Is it possible that you want to (avoid work, avoid jail, be prescribed a specific medication, etc.) and that this is the only way you could think of to get what you need?
- Is it possible that you are describing what you are experiencing so that you can convince others that you are having problems?
Despite our best efforts, some patients may not drop their guard and will continue to fabricate or exaggerate their symptoms. However, establishing and maintaining rapport, exploring our patients’ potential motives to mislead, and gently confronting discrepancies in their report may maximize the chances of successfully engaging them and developing appropriate treatment plans.
Patients who fabricate or exaggerate psychiatric symptoms for primary or secondary gain may elicit negative responses from health care professionals. As clinicians, we may believe that such patients are wasting our time and taking resources away from other patients who are genuinely struggling with mental illness and are more deserving of assistance. However, patients who are fabricating or exaggerating their symptoms have legitimate clinical needs that we should strive to understand. If we view them as having reasons for their actions without becoming complicit in their deception, we may find it easier to work with them.
Managing patients who are fabricating or exaggerating
Caring for patients who attempt to mislead us is a challenging proposition. The relevant research is scarce, and there are few recommended interventions for managing patients who fabricate or exaggerate symptoms.1 Direct confrontation and accusation are often unproductive and should be used sparingly. Indirect approaches tend to be more effective.
It is important to manage our countertransference at the outset while establishing and maintaining rapport. Although we may become frustrated, we should avoid using sarcasm or overt skepticism; instead, we should validate these patients’ emotions because their emotional turmoil could be driving their fabrication or exaggeration. We should attempt to explore their specific motivations by focusing our questions on detecting the underlying stressors or conditions.2
To assess our patients’ motives, consider asking the following:
- What kind of problems have these symptoms caused you in your day-to-day life?
- What would make life better for you?
- What are you hoping I can do for you today?
We should ask open-ended questions as well as interview patients over a long period of time and on multiple occasions to observe the consistency of their reported symptoms. In addition, we should take good notes and document our observations to compare what our patients tell us during their appointments.
Addressing inconsistencies
While exploring our patients’ motives, when it is appropriate, we can gently confront discrepancies in their report by asking:
- I am confused about your symptoms. Help me understand what is happening. Can you tell me more? (Then ask specific follow-up questions based on their answer.)
- What do you mean when you say you are experiencing this symptom?
- I am not sure if I understand what you said correctly. These symptoms do not typically occur in the way that you described. Could you tell me more?
- The symptoms you described are unusual to me. Is there something else going on that I am not aware of?
- Do you think these symptoms have been coming up because you are under stress?
- Is it possible that you want to (avoid work, avoid jail, be prescribed a specific medication, etc.) and that this is the only way you could think of to get what you need?
- Is it possible that you are describing what you are experiencing so that you can convince others that you are having problems?
Despite our best efforts, some patients may not drop their guard and will continue to fabricate or exaggerate their symptoms. However, establishing and maintaining rapport, exploring our patients’ potential motives to mislead, and gently confronting discrepancies in their report may maximize the chances of successfully engaging them and developing appropriate treatment plans.
1. Brady MC, Scher LM, Newman W. “I just saw Big Bird. He was 100 feet tall!” Malingering in the emergency room. Current Psychiatry. 2013;12(10):33-40.
2. Schnellbacher S, O’Mara H. Identifying and managing malingering and factitious disorder in the military. Curr Psychiatry Rep. 2016;18(11):105.
1. Brady MC, Scher LM, Newman W. “I just saw Big Bird. He was 100 feet tall!” Malingering in the emergency room. Current Psychiatry. 2013;12(10):33-40.
2. Schnellbacher S, O’Mara H. Identifying and managing malingering and factitious disorder in the military. Curr Psychiatry Rep. 2016;18(11):105.
Anathemas of psychiatric practice
The quarterly report of the State Medical Board can be a sobering read. In addition to the usual updates about new regulations or requirements for licensed physicians, there is always the disciplinary actions “blacklist” of dozens of medical practitioners in all specialties whose licenses were revoked or suspended due to a shocking array of serious violations.
Those infractions range from Medicare billing fraud to prescribing narcotics to fictitious patients to engaging in sex with a patient to walking into the operating room drunk. It is truly disheartening to see dozens of physicians destroy their careers by committing a panoply of odious, repugnant, or illegal actions.
The term “anathema” comes to mind when I read about those miscreants. This Greek term is occasionally used in scholarly or religious publications, but rarely in everyday conversations or articles. Anathema refers to something detested, shunned, or denounced. When used by the clergy, it connotes something to condemn, such as a sinful or evil act.
Like all other medical specialists, we psychiatrists have a noble mission of treating and relieving the suffering of those afflicted with brain disorders that manifest as mood, thought, perceptual, behavioral, or cognitive abnormalities. Our main goal is to restore health, wellness, and quality of life to the millions of individuals who buckle under the weight of genetic redispersion, adverse environmental events, or both. So psychiatrists do a lot of “good,” which benefits all those who live with mental illness. However, psychiatric practice may have some pitfalls that occasionally lead to anathemas, no matter how diligently a practitioner tries to avoid them. The code of psychiatric ethics is a shield that can preempt anathemas from contaminating clinical practice, but human error will occur when the ethical compass fails.
Here are some examples of anathemas that may rear their ugly heads if a practitioner is not constantly on the alert. It is likely you, the readers of
- Sexual contact with a patient. This major anathema must not occur under any circumstance. It will have grave professional consequences for the practitioner and serious emotional repercussions for the patient.
- Breach of confidentiality. This is a sacred rule in psychiatric practice that must not be broken under any circumstance. Breaching confidentiality will rupture the therapeutic bond and trust that a patient has with a psychiatrist (or psychiatric nurse practitioner).
- Causing physical or emotional harm. This anathema can have serious legal implications in addition to being an unacceptable professional violation.
- Failure to assess patients for suicidal or homicidal risk. The life of the patient, and others, may be at stake if this critical component is missing in the evaluation of psychiatric patients, even if they appear “stable.”
- Irrational and hazardous polypharmacy. This type of harm must never occur during medical management of psychiatric patients, and may have legal consequences.
- Not seeking collateral information. This may seem like a “minor” anathema, but it can have major repercussions if a gap of clinically important data about the patient leads to erroneous diagnosis or inappropriate treatment. Regrettably, informants are sometimes unavailable.
- Assessing patients from the neck up only. Psychiatrists are, first and foremost, physicians who must evaluate the entire medical status of the patient, not just his/her mind. There are numerous bidirectional effects between the body and the brain that can influence diagnosis, holistic treatment, medical outcomes, and prognosis.
- Treating patients with medication only, without any concomitant psychotherapy. Such a suboptimal practice is an anathema that is not excusable due to a “lack of time.” Every psychiatric patient deserves a biopsychosocial treatment approach.
- Not inquiring about adherence at every visit. It is impossible to assess the effectiveness of treatment if adherence is partial or poor. Patients must be constantly reminded that while their psychiatrists are committed to their care, full adherence is a vital responsibility for them to fulfill to ensure optimal outcome.
- Ignoring the patient’s cues, both verbal and nonverbal. Being rushed by a large workload, a full schedule, or the demands of electronic medical records that distract a psychiatrist from fully attending to what the patient’s words, facial expressions, or body language convey can lead to a failure to meet the patient’s needs. Even worse, it may lead to missing a serious message a patient is consciously or unconsciously trying to relay.
- Lowering expectations. Nothing is more devastating for patients than to feel that the psychiatrist does not believe he/she will ever achieve wellness, or that they are beyond help and will never improve, recover, or overcome disabling psychiatric illness. This will generate profound hopelessness in vulnerable patients, who crave having a normal life free from illness or disability.
- Using the same medication for all patients. This is an anathema because one size does not fit all, and patients deserve to have their psychiatrists customize their pharmacotherapy to match their medical status and tolerability. For example, the 11 FDA-approved second-generation antipsychotics are not all the same, and a psychiatrist must select the member of that class that is most likely to be a good match for each patient based on that patient’s medical history and the safety/tolerability profile of each antipsychotic.
- Not continuously upgrading one’s practice to incorporate new evidence-based findings of more effective therapeutic strategies. It is an anathema to continue practicing what was learned in residency 25 to 30 years ago when there’s new knowledge and many advances permeating psychiatric practice today.
- Using alcohol or recreational drugs during a shift in the clinic or the hospital. No explanation is needed for this anathema!
- Prescribing for patients without a full evaluation. That’s poor clinical practice, and also is illegal.
- Billing for patients who were never examined. That’s fraudulent, and stupid!
In an editorial I wrote last year intended for graduates of psychiatry residency training programs about the “DNA of psychiatric practice,” I described what comprises good psychiatric practice.1 Anathemas can be regarded as “mutations” within the DNA of psychiatric practice. It is always my hope that none of the freshly minted psychiatrists going into practice will ever commit an anathema, and end up on the “list of shame” in their State Medical Board’s quarterly report….
1. Nasrallah HA. The DNA of psychiatric practice: a covenant with our patients. Current Psychiatry. 2018;17(5):20,22.
The quarterly report of the State Medical Board can be a sobering read. In addition to the usual updates about new regulations or requirements for licensed physicians, there is always the disciplinary actions “blacklist” of dozens of medical practitioners in all specialties whose licenses were revoked or suspended due to a shocking array of serious violations.
Those infractions range from Medicare billing fraud to prescribing narcotics to fictitious patients to engaging in sex with a patient to walking into the operating room drunk. It is truly disheartening to see dozens of physicians destroy their careers by committing a panoply of odious, repugnant, or illegal actions.
The term “anathema” comes to mind when I read about those miscreants. This Greek term is occasionally used in scholarly or religious publications, but rarely in everyday conversations or articles. Anathema refers to something detested, shunned, or denounced. When used by the clergy, it connotes something to condemn, such as a sinful or evil act.
Like all other medical specialists, we psychiatrists have a noble mission of treating and relieving the suffering of those afflicted with brain disorders that manifest as mood, thought, perceptual, behavioral, or cognitive abnormalities. Our main goal is to restore health, wellness, and quality of life to the millions of individuals who buckle under the weight of genetic redispersion, adverse environmental events, or both. So psychiatrists do a lot of “good,” which benefits all those who live with mental illness. However, psychiatric practice may have some pitfalls that occasionally lead to anathemas, no matter how diligently a practitioner tries to avoid them. The code of psychiatric ethics is a shield that can preempt anathemas from contaminating clinical practice, but human error will occur when the ethical compass fails.
Here are some examples of anathemas that may rear their ugly heads if a practitioner is not constantly on the alert. It is likely you, the readers of
- Sexual contact with a patient. This major anathema must not occur under any circumstance. It will have grave professional consequences for the practitioner and serious emotional repercussions for the patient.
- Breach of confidentiality. This is a sacred rule in psychiatric practice that must not be broken under any circumstance. Breaching confidentiality will rupture the therapeutic bond and trust that a patient has with a psychiatrist (or psychiatric nurse practitioner).
- Causing physical or emotional harm. This anathema can have serious legal implications in addition to being an unacceptable professional violation.
- Failure to assess patients for suicidal or homicidal risk. The life of the patient, and others, may be at stake if this critical component is missing in the evaluation of psychiatric patients, even if they appear “stable.”
- Irrational and hazardous polypharmacy. This type of harm must never occur during medical management of psychiatric patients, and may have legal consequences.
- Not seeking collateral information. This may seem like a “minor” anathema, but it can have major repercussions if a gap of clinically important data about the patient leads to erroneous diagnosis or inappropriate treatment. Regrettably, informants are sometimes unavailable.
- Assessing patients from the neck up only. Psychiatrists are, first and foremost, physicians who must evaluate the entire medical status of the patient, not just his/her mind. There are numerous bidirectional effects between the body and the brain that can influence diagnosis, holistic treatment, medical outcomes, and prognosis.
- Treating patients with medication only, without any concomitant psychotherapy. Such a suboptimal practice is an anathema that is not excusable due to a “lack of time.” Every psychiatric patient deserves a biopsychosocial treatment approach.
- Not inquiring about adherence at every visit. It is impossible to assess the effectiveness of treatment if adherence is partial or poor. Patients must be constantly reminded that while their psychiatrists are committed to their care, full adherence is a vital responsibility for them to fulfill to ensure optimal outcome.
- Ignoring the patient’s cues, both verbal and nonverbal. Being rushed by a large workload, a full schedule, or the demands of electronic medical records that distract a psychiatrist from fully attending to what the patient’s words, facial expressions, or body language convey can lead to a failure to meet the patient’s needs. Even worse, it may lead to missing a serious message a patient is consciously or unconsciously trying to relay.
- Lowering expectations. Nothing is more devastating for patients than to feel that the psychiatrist does not believe he/she will ever achieve wellness, or that they are beyond help and will never improve, recover, or overcome disabling psychiatric illness. This will generate profound hopelessness in vulnerable patients, who crave having a normal life free from illness or disability.
- Using the same medication for all patients. This is an anathema because one size does not fit all, and patients deserve to have their psychiatrists customize their pharmacotherapy to match their medical status and tolerability. For example, the 11 FDA-approved second-generation antipsychotics are not all the same, and a psychiatrist must select the member of that class that is most likely to be a good match for each patient based on that patient’s medical history and the safety/tolerability profile of each antipsychotic.
- Not continuously upgrading one’s practice to incorporate new evidence-based findings of more effective therapeutic strategies. It is an anathema to continue practicing what was learned in residency 25 to 30 years ago when there’s new knowledge and many advances permeating psychiatric practice today.
- Using alcohol or recreational drugs during a shift in the clinic or the hospital. No explanation is needed for this anathema!
- Prescribing for patients without a full evaluation. That’s poor clinical practice, and also is illegal.
- Billing for patients who were never examined. That’s fraudulent, and stupid!
In an editorial I wrote last year intended for graduates of psychiatry residency training programs about the “DNA of psychiatric practice,” I described what comprises good psychiatric practice.1 Anathemas can be regarded as “mutations” within the DNA of psychiatric practice. It is always my hope that none of the freshly minted psychiatrists going into practice will ever commit an anathema, and end up on the “list of shame” in their State Medical Board’s quarterly report….
The quarterly report of the State Medical Board can be a sobering read. In addition to the usual updates about new regulations or requirements for licensed physicians, there is always the disciplinary actions “blacklist” of dozens of medical practitioners in all specialties whose licenses were revoked or suspended due to a shocking array of serious violations.
Those infractions range from Medicare billing fraud to prescribing narcotics to fictitious patients to engaging in sex with a patient to walking into the operating room drunk. It is truly disheartening to see dozens of physicians destroy their careers by committing a panoply of odious, repugnant, or illegal actions.
The term “anathema” comes to mind when I read about those miscreants. This Greek term is occasionally used in scholarly or religious publications, but rarely in everyday conversations or articles. Anathema refers to something detested, shunned, or denounced. When used by the clergy, it connotes something to condemn, such as a sinful or evil act.
Like all other medical specialists, we psychiatrists have a noble mission of treating and relieving the suffering of those afflicted with brain disorders that manifest as mood, thought, perceptual, behavioral, or cognitive abnormalities. Our main goal is to restore health, wellness, and quality of life to the millions of individuals who buckle under the weight of genetic redispersion, adverse environmental events, or both. So psychiatrists do a lot of “good,” which benefits all those who live with mental illness. However, psychiatric practice may have some pitfalls that occasionally lead to anathemas, no matter how diligently a practitioner tries to avoid them. The code of psychiatric ethics is a shield that can preempt anathemas from contaminating clinical practice, but human error will occur when the ethical compass fails.
Here are some examples of anathemas that may rear their ugly heads if a practitioner is not constantly on the alert. It is likely you, the readers of
- Sexual contact with a patient. This major anathema must not occur under any circumstance. It will have grave professional consequences for the practitioner and serious emotional repercussions for the patient.
- Breach of confidentiality. This is a sacred rule in psychiatric practice that must not be broken under any circumstance. Breaching confidentiality will rupture the therapeutic bond and trust that a patient has with a psychiatrist (or psychiatric nurse practitioner).
- Causing physical or emotional harm. This anathema can have serious legal implications in addition to being an unacceptable professional violation.
- Failure to assess patients for suicidal or homicidal risk. The life of the patient, and others, may be at stake if this critical component is missing in the evaluation of psychiatric patients, even if they appear “stable.”
- Irrational and hazardous polypharmacy. This type of harm must never occur during medical management of psychiatric patients, and may have legal consequences.
- Not seeking collateral information. This may seem like a “minor” anathema, but it can have major repercussions if a gap of clinically important data about the patient leads to erroneous diagnosis or inappropriate treatment. Regrettably, informants are sometimes unavailable.
- Assessing patients from the neck up only. Psychiatrists are, first and foremost, physicians who must evaluate the entire medical status of the patient, not just his/her mind. There are numerous bidirectional effects between the body and the brain that can influence diagnosis, holistic treatment, medical outcomes, and prognosis.
- Treating patients with medication only, without any concomitant psychotherapy. Such a suboptimal practice is an anathema that is not excusable due to a “lack of time.” Every psychiatric patient deserves a biopsychosocial treatment approach.
- Not inquiring about adherence at every visit. It is impossible to assess the effectiveness of treatment if adherence is partial or poor. Patients must be constantly reminded that while their psychiatrists are committed to their care, full adherence is a vital responsibility for them to fulfill to ensure optimal outcome.
- Ignoring the patient’s cues, both verbal and nonverbal. Being rushed by a large workload, a full schedule, or the demands of electronic medical records that distract a psychiatrist from fully attending to what the patient’s words, facial expressions, or body language convey can lead to a failure to meet the patient’s needs. Even worse, it may lead to missing a serious message a patient is consciously or unconsciously trying to relay.
- Lowering expectations. Nothing is more devastating for patients than to feel that the psychiatrist does not believe he/she will ever achieve wellness, or that they are beyond help and will never improve, recover, or overcome disabling psychiatric illness. This will generate profound hopelessness in vulnerable patients, who crave having a normal life free from illness or disability.
- Using the same medication for all patients. This is an anathema because one size does not fit all, and patients deserve to have their psychiatrists customize their pharmacotherapy to match their medical status and tolerability. For example, the 11 FDA-approved second-generation antipsychotics are not all the same, and a psychiatrist must select the member of that class that is most likely to be a good match for each patient based on that patient’s medical history and the safety/tolerability profile of each antipsychotic.
- Not continuously upgrading one’s practice to incorporate new evidence-based findings of more effective therapeutic strategies. It is an anathema to continue practicing what was learned in residency 25 to 30 years ago when there’s new knowledge and many advances permeating psychiatric practice today.
- Using alcohol or recreational drugs during a shift in the clinic or the hospital. No explanation is needed for this anathema!
- Prescribing for patients without a full evaluation. That’s poor clinical practice, and also is illegal.
- Billing for patients who were never examined. That’s fraudulent, and stupid!
In an editorial I wrote last year intended for graduates of psychiatry residency training programs about the “DNA of psychiatric practice,” I described what comprises good psychiatric practice.1 Anathemas can be regarded as “mutations” within the DNA of psychiatric practice. It is always my hope that none of the freshly minted psychiatrists going into practice will ever commit an anathema, and end up on the “list of shame” in their State Medical Board’s quarterly report….
1. Nasrallah HA. The DNA of psychiatric practice: a covenant with our patients. Current Psychiatry. 2018;17(5):20,22.
1. Nasrallah HA. The DNA of psychiatric practice: a covenant with our patients. Current Psychiatry. 2018;17(5):20,22.
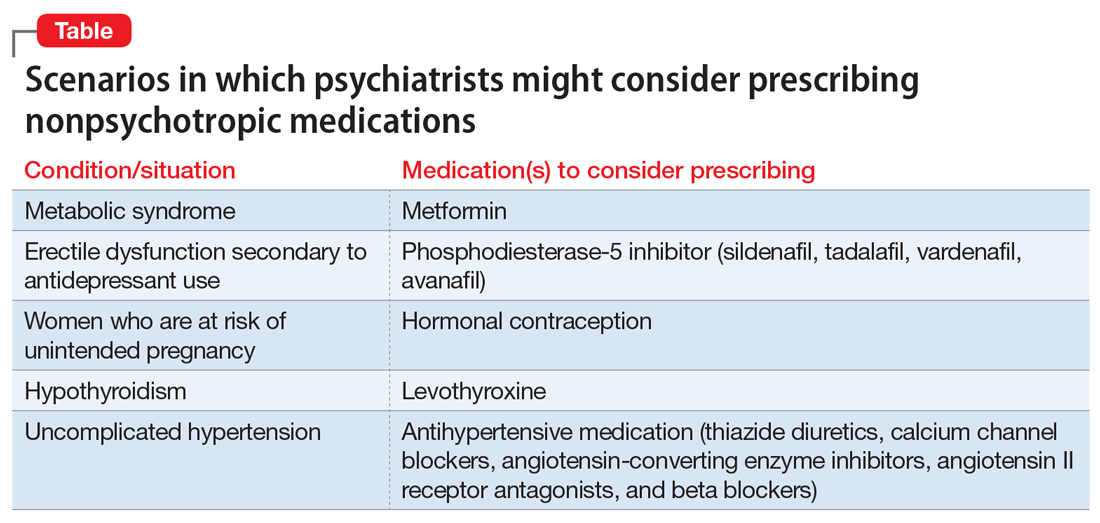
Should psychiatrists prescribe nonpsychotropic medications?
In our experience, most psychiatrists are uncomfortable with prescribing a medication when they feel that doing so would be outside their scope of practice. But there are many situations when prescribing a nonpsychotropic medication would be the correct choice. In this article, we discuss the scope of psychiatric practice, and present 4 case studies that illustrate situations in which psychiatrists should feel comfortable prescribing nonpsychotropic medications.
Defining the scope of practice
What is the scope of a psychiatrist’s practice? Scope of practice usually describes activities that a health care practitioner is allowed to undertake as defined by the terms of his/her license. A license to practice medicine does not include any stipulation restricting practice to a specific medical specialty. However, a local entity may delineate scope of practice within its organization. For instance, local practice standards held by the Detroit Wayne Mental Health Authority (DWMHA) state “Psychiatrists…shall not exceed their scope of practice as per DWMHA credentialing and privileging. For example, a Psychiatrist…who [has] not been appropriately privileged to deliver services to children shall not treat children, excepting crisis situations.”1
Like physicians in other specialties, psychiatrists are not limited to prescribing only a subset of medications commonly associated with their specialty. But for many psychiatrists, prescribing nonpsychotropic medications is complicated by individual and local factors. On one hand, some psychiatrists do not feel it is their role to prescribe nonpsychotropic medications,2 or even some psychotropic medications that may be more complex to prescribe, such as lithium, clozapine, or monoamine oxidase inhibitors.3-5 However, many feel comfortable prescribing complex combinations of psychotropic medications, or prescribing in a way that does not necessarily make sense (eg, prescribing benztropine as prophylaxis for dystonia when starting an antipsychotic).
Reviewing an average day at one urban psychiatric clinic, these questions seem to come up in half of the patient population, especially in patients with chronic mental illness, multiple medical comorbidities, and limited access to health care. When a young patient walks in without an appointment with an acute dystonic reaction secondary to the initiation of antipsychotics a couple of days ago, there is no hesitation to swiftly and appropriately prescribe an IM anticholinergic medication. But why are psychiatrists often hesitant to prescribe nonpsychotropic medications to treat other adverse effects of medications? Lack of knowledge? Lack of training?
Psychiatrists who practice in hospital systems often have immediate access to consultants, and this availability may encourage them to defer to the consultant for treatment of certain adverse effects. We have seen psychiatrists consult Neurology regarding the prescription of donepezil for mild neurocognitive disorder due to Alzheimer’s disease, or Endocrinology regarding prescription of levothyroxine for lithium-induced hypothyroidism.
However, there are numerous scenarios in which psychiatrists should feel comfortable prescribing nonpsychotropic medications or managing medication adverse effects, regardless of whether they consider it to be within or outside their scope of practice. The following case examples illustrate several such situations.
CASE 1
Ms. W, age 30, has been diagnosed with schizophrenia. She requests a refill of quetiapine, 800 mg/d. This medication has been clearly beneficial in alleviating her psychotic symptoms. However, since her last visit 3 months ago, her face appears more round, and she has gained 9 kg. Further evaluation indicates that she has developed metabolic syndrome and pre-diabetes.
Continue to: Metabolic adverse effects
Metabolic adverse effects, such as metabolic syndrome, diabetic ketoacidosis, and cardiovascular disease, are well-known risks of prescribing second-generation antipsychotics.6 In such situations, psychiatrists often advise patients to modify their diet, increase physical activity, and follow up with their primary care physician to determine if other medications are needed. However, getting a patient with a serious mental illness to exercise and modify her/his diet is difficult, and many of these patients do not have a primary care physician.
For patients such as Ms. W, a psychiatrist should consider prescribing metformin. Wu et al7 found that in addition to lifestyle modifications, metformin had the greatest effect on antipsychotic-induced weight gain. In this study, metformin alone had more impact on reversing weight gain and increasing insulin sensitivity than lifestyle modifications alone.7 This is crucial because these patients are especially vulnerable to cardiac disease.8 Metformin is well tolerated and has a low risk of causing hypoglycemia. Concerns regarding lactic acidosis have abated to the extent that the estimated glomerular filtration rate (eGFR) limits for using metformin have been lowered significantly. After reviewing the contraindications, the only knowledge needed to prescribe metformin is the patient’s kidney function and a brief understanding of the titration needed to minimize gastrointestinal adverse effects.9 Thus, prescribing metformin would be a fairly logical and easy first step for managing metabolic syndrome, especially in a patient whose motivation for increasing physical activity and modifying his/her diet is doubtful.
CASE 2
Mr. B, age 45, has major depressive disorder that has been well-controlled on paroxetine, 40 mg/d, for the past 2 years. He has no history of physical illness. On his most recent visit, he appears uncomfortable and nervous. After a long discussion, he discloses that his sex life isn’t what it used to be since starting paroxetine. He is bothered by erectile problems and asks whether he can “get some Viagra.”
Sexual adverse effects, such as erectile dysfunction, are frequently associated with the use of selective serotonin reuptake inhibitors.10 Although managing these adverse effects requires careful evaluation, in most cases, psychiatrists should be able to treat them.10 The logical choice in this case would be to prescribe one of the 4 FDA-approved phosphodiesterase-5 inhibitors (sildenafil [Viagra], tadalafil [Cialis], vardenafil [Levitra], and avanafil [Stendra]. However, Balon et al11 found that few psychiatrists prescribe phosphodiesterase-5 inhibitors, although they believed that they should be prescribing to treat their patients’ sexual dysfunction. Managing these adverse effects is important not only for the patient’s quality of life and relationship with his/her partner, but also for the therapeutic alliance. In a systematic review of 23 trials, Taylor et al12 examined >1,800 patients who were prescribed a medication to address sexual dysfunction secondary to antidepressants. They found that for men, adding a phosphodiesterase-5 inhibitor was appropriate and effective, and for women, adding bupropion at high doses should be considered.12 Like many other adverse effects, sexual adverse effects surely play a role in medication compliance. Dording et al13 found that the addition of sildenafil, 50 to 100 mg as needed, resulted in increased treatment satisfaction and overall contentment in 102 patients who complained of sexual dysfunction in the follow-up phase of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) antidepressant trials. In most cases, with proper psychoeducation, prescription of
CASE 3
Ms. G, age 22, was recently discharged from an inpatient psychiatric unit after an episode of mania. She was prescribed carbamazepine, 600 mg/d, and ziprasidone, 40 mg twice a day, and appears to be doing well on this regimen. When asked about what led to her admission, she recalls having an elevated mood, increased energy, hypersexuality, impulsivity, and poor judgment. She reveals that she had several sexual partners during her manic episode, and worries that if such behavior occurs again, she may get pregnant. Yet Ms. G was not prescribed birth control upon discharge.
Continue to: Contraception
Contraception. We believe that psychiatrists have an obligation to protect patients from consequences of mental illness. Much the same way that psychiatrists hope to prevent suicide in a patient who has depression, patients should be protected from risks encountered in the manic phase of bipolar disorder. Another reason to prescribe contraceptives in such patients is the teratogenic effects of mood stabilizers. Pagano et al14 reviewed 6 studies that examined common forms of hormonal birth control to determine their safety in patients with depression or bipolar disorder. They found that overall, use of hormonal contraception was not associated with a worse clinical course of disease.
Many available forms of birth control are available. When prescribing in an outpatient setting, a daily oral medication or a monthly depot injection are convenient options.
CASE 4
Mr. P, age 65, has bipolar I disorder and is stable on risperidone long-acting injection, 37.7 mg bimonthly, and lithium, 1,200 mg/d. He reports that he is doing well but has noticed a recent decrease in energy and weight gain without any change in mood. Laboratory testing conducted prior to this visit revealed a thyroid-stimulating hormone (TSH) level of 4 mU/L (normal range: 0.4 to 4.0 mU/L). Six months ago, Mr. P’s TSH level was 2.8 mU/L. The resident supervisor suggests discussing the case with an endocrinologist.
Thyroid function. The impact of lithium on the thyroid gland is well established; however, psychiatrists’ response to such changes are not.15 Gitlin16 reviewed the many adverse effects of lithium and presented various management strategies to address findings such as Mr. P’s. Two important points are that lithium should not be discontinued in light of hypothyroidism, and synthetic thyroxine (levothyroxine) can be initiated and titrated to return TSH levels to a normal range.16 Levothyroxine can be started at low doses (eg, 25 to 50 mcg/d) and increased every 6 weeks until a normal TSH level is achieved.17 Managing lithium-induced clinical or subclinical hypothyroidism can prevent further pathology and possible relapse to depression.
Incorporating integrated care
In all these cases, the prescription of a medication with which some psychiatrists are not comfortable prescribing would have been the logical, easiest, and preferable choice. Of course, when initiating any medication, boxed warnings, contraindications, and drug–drug interactions should be reviewed. Initial dosages and titration schedules can be found in every medication’s FDA-approved prescribing information document (package insert), as well as in numerous reference books and articles.
Continue to: We acknowledge...
We acknowledge that prescribing a nonpsychotropic medication is not always a psychiatrist’s best choice, and that in patients with multiple medical comorbidities and drug–drug interactions that are not clearly defined, referring to or consulting a specialist is appropriate. We in no way support reckless prescribing, but instead present an opportunity to expand the perception of what should be considered within a psychiatrist’s scope of practice, and call for further education of psychiatrists so that they are more comfortable managing these adverse effects and/or prescribing at least some nonpsychotropic medications.
We exhort integrated medical care during this time of a physician shortage; however, we do not practice this way. Interestingly, physicians in primary care, such as those in family medicine or obstetrics and gynecology, frequently attempt to treat patients with psychiatric conditions in an attempt to provide integrated care. Numerous articles have discussed these efforts.18-20 However, this type of integrated care seems less frequent in psychiatry, even though the practice of modern psychiatry in the United States shows substantial overlap with the practice of physicians in primary care specialties.21 There are few articles or practical guidelines for psychiatrists who wish to treat patients’ physical illnesses, particularly patients with severe mental illness (see Related Resources, page 56). If we practice in an integrated manner to treat one of the simple conditions we described above, we can eliminate the need for a patient to visit a second physician, pay another co-pay, pay another bus fare, and take another day off work. This can be particularly helpful for patients who at times have to decide between paying for groceries or for medications. Having one clinician manage a patient’s medications also can decrease the risk of polypharmacy.
In addition to the case scenarios described in this article, there are more clinical situations and nonpsychotropic medications that psychiatrists could manage. Considering them outside the scope of psychiatric practice and being uncomfortable or ambivalent about them is not an excuse. We hope that psychiatrists can increase their expertise in this area, and can start to practice as the primary care physicians they claim they are, and should be.
Bottom Line
Many psychiatrists are uncomfortable prescribing nonpsychotropic medications, but there are numerous clinical scenarios in which the practice would make sense. This could include cases of metabolic syndrome, sexual dysfunction secondary to antidepressant use, or other adverse effects of commonly prescribed psychotropic medications.
Related Resources
- McCarron RM, Xiong GL, Keenan CR, et al. Preventive medical care in psychiatry. A practical guide for clinicians. Arlington, VA: American Psychiatric Association Publishing; 2015.
- McCarron RM, Xiong GL, Keenan CR, et al. Study guide to preventive medical care in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Washington, DC: American Psychiatric Association Publishing; 2019.
Drug Brand Names
Avanafil • Stendra
Benztropine • Cogentin
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Levothyroxine • Levoxyl, Synthroid
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone long-acting injection • Risperdal Consta
Sildenafil • Viagra
Tadalafil • Cialis
Vardenafil • Levitra
Ziprasidone • Geodon
1. Detroit Wayne Integrated Health Network. DWMHA psychiatric practice standards. http://dwihn.org/files/2015/6451/9628/Psychiatric_Practice_Standards.pdf. Revised June 2018. Accessed October 8, 2019.
2. Seaman JJ, Cornfield RM, Cummings DM, et al. Exploring psychiatric prescribing practices: the relationship between the role of the provider and the appropriateness of prescribing. Gen Hosp Psychiatry. 1987;9(3):220-224.
3. Zivanovic O. Lithium: a classic drug—frequently discussed, but, sadly, seldom prescribed! Aust N Z J Psychiatry. 2017;51(9):886-896.
4. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatric Services. 2014;65(2):186-192.
5. Balon R, Mufti R, Arfken C. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatric Services. 1999;50(7):945-947.
6. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233.
7. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193.
8. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
9. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Internal Med. 2002;137(1):25-33.
10. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504-1509.
11. Balon R, Morreale MK, Segraves RT. Prescribing of phosphodiesterase-5 inhibitors among psychiatrists. J Sex Marital Ther. 2014;40(3):165-169.
12. Taylor MJ, Rudkin L, Bullemor-Day P, et al. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382.
13. Dording CM, LaRocca RA, Hails KA, et al. The effect of sildenafil on quality of life. Ann Clin Psychiatry. 2013;25(1):3-10.
14. Pagano HP, Zapata LB, Berry-Bibee EN, et al. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94(6):641-649.
15. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3.
16. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27.
17. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615.
18. Hackley B, Sharma C, Kedzior A, et al. Managing mental health conditions in primary care settings. J Midwifery Women’s Health. 2010;55(1):9-19.
19. Fitelson E, McGibbon C. Evaluation and management of behavioral health disorders in women: an overview of major depression, bipolar disorder, anxiety disorders, and sleep in the primary care setting. Obstet Gynecol Clin North Am. 2016;43(2):231-246.
20. Colorafi K, Vanselow J, Nelson T. Treating anxiety and depression in primary care: reducing barriers to access. Fam Pract Manag. 2017;24(4):11-16.
21. McCall WV. Defining the unique scope of psychiatric practice in 2015. J ECT. 2015;31(4):203-204.
In our experience, most psychiatrists are uncomfortable with prescribing a medication when they feel that doing so would be outside their scope of practice. But there are many situations when prescribing a nonpsychotropic medication would be the correct choice. In this article, we discuss the scope of psychiatric practice, and present 4 case studies that illustrate situations in which psychiatrists should feel comfortable prescribing nonpsychotropic medications.
Defining the scope of practice
What is the scope of a psychiatrist’s practice? Scope of practice usually describes activities that a health care practitioner is allowed to undertake as defined by the terms of his/her license. A license to practice medicine does not include any stipulation restricting practice to a specific medical specialty. However, a local entity may delineate scope of practice within its organization. For instance, local practice standards held by the Detroit Wayne Mental Health Authority (DWMHA) state “Psychiatrists…shall not exceed their scope of practice as per DWMHA credentialing and privileging. For example, a Psychiatrist…who [has] not been appropriately privileged to deliver services to children shall not treat children, excepting crisis situations.”1
Like physicians in other specialties, psychiatrists are not limited to prescribing only a subset of medications commonly associated with their specialty. But for many psychiatrists, prescribing nonpsychotropic medications is complicated by individual and local factors. On one hand, some psychiatrists do not feel it is their role to prescribe nonpsychotropic medications,2 or even some psychotropic medications that may be more complex to prescribe, such as lithium, clozapine, or monoamine oxidase inhibitors.3-5 However, many feel comfortable prescribing complex combinations of psychotropic medications, or prescribing in a way that does not necessarily make sense (eg, prescribing benztropine as prophylaxis for dystonia when starting an antipsychotic).
Reviewing an average day at one urban psychiatric clinic, these questions seem to come up in half of the patient population, especially in patients with chronic mental illness, multiple medical comorbidities, and limited access to health care. When a young patient walks in without an appointment with an acute dystonic reaction secondary to the initiation of antipsychotics a couple of days ago, there is no hesitation to swiftly and appropriately prescribe an IM anticholinergic medication. But why are psychiatrists often hesitant to prescribe nonpsychotropic medications to treat other adverse effects of medications? Lack of knowledge? Lack of training?
Psychiatrists who practice in hospital systems often have immediate access to consultants, and this availability may encourage them to defer to the consultant for treatment of certain adverse effects. We have seen psychiatrists consult Neurology regarding the prescription of donepezil for mild neurocognitive disorder due to Alzheimer’s disease, or Endocrinology regarding prescription of levothyroxine for lithium-induced hypothyroidism.
However, there are numerous scenarios in which psychiatrists should feel comfortable prescribing nonpsychotropic medications or managing medication adverse effects, regardless of whether they consider it to be within or outside their scope of practice. The following case examples illustrate several such situations.
CASE 1
Ms. W, age 30, has been diagnosed with schizophrenia. She requests a refill of quetiapine, 800 mg/d. This medication has been clearly beneficial in alleviating her psychotic symptoms. However, since her last visit 3 months ago, her face appears more round, and she has gained 9 kg. Further evaluation indicates that she has developed metabolic syndrome and pre-diabetes.
Continue to: Metabolic adverse effects
Metabolic adverse effects, such as metabolic syndrome, diabetic ketoacidosis, and cardiovascular disease, are well-known risks of prescribing second-generation antipsychotics.6 In such situations, psychiatrists often advise patients to modify their diet, increase physical activity, and follow up with their primary care physician to determine if other medications are needed. However, getting a patient with a serious mental illness to exercise and modify her/his diet is difficult, and many of these patients do not have a primary care physician.
For patients such as Ms. W, a psychiatrist should consider prescribing metformin. Wu et al7 found that in addition to lifestyle modifications, metformin had the greatest effect on antipsychotic-induced weight gain. In this study, metformin alone had more impact on reversing weight gain and increasing insulin sensitivity than lifestyle modifications alone.7 This is crucial because these patients are especially vulnerable to cardiac disease.8 Metformin is well tolerated and has a low risk of causing hypoglycemia. Concerns regarding lactic acidosis have abated to the extent that the estimated glomerular filtration rate (eGFR) limits for using metformin have been lowered significantly. After reviewing the contraindications, the only knowledge needed to prescribe metformin is the patient’s kidney function and a brief understanding of the titration needed to minimize gastrointestinal adverse effects.9 Thus, prescribing metformin would be a fairly logical and easy first step for managing metabolic syndrome, especially in a patient whose motivation for increasing physical activity and modifying his/her diet is doubtful.
CASE 2
Mr. B, age 45, has major depressive disorder that has been well-controlled on paroxetine, 40 mg/d, for the past 2 years. He has no history of physical illness. On his most recent visit, he appears uncomfortable and nervous. After a long discussion, he discloses that his sex life isn’t what it used to be since starting paroxetine. He is bothered by erectile problems and asks whether he can “get some Viagra.”
Sexual adverse effects, such as erectile dysfunction, are frequently associated with the use of selective serotonin reuptake inhibitors.10 Although managing these adverse effects requires careful evaluation, in most cases, psychiatrists should be able to treat them.10 The logical choice in this case would be to prescribe one of the 4 FDA-approved phosphodiesterase-5 inhibitors (sildenafil [Viagra], tadalafil [Cialis], vardenafil [Levitra], and avanafil [Stendra]. However, Balon et al11 found that few psychiatrists prescribe phosphodiesterase-5 inhibitors, although they believed that they should be prescribing to treat their patients’ sexual dysfunction. Managing these adverse effects is important not only for the patient’s quality of life and relationship with his/her partner, but also for the therapeutic alliance. In a systematic review of 23 trials, Taylor et al12 examined >1,800 patients who were prescribed a medication to address sexual dysfunction secondary to antidepressants. They found that for men, adding a phosphodiesterase-5 inhibitor was appropriate and effective, and for women, adding bupropion at high doses should be considered.12 Like many other adverse effects, sexual adverse effects surely play a role in medication compliance. Dording et al13 found that the addition of sildenafil, 50 to 100 mg as needed, resulted in increased treatment satisfaction and overall contentment in 102 patients who complained of sexual dysfunction in the follow-up phase of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) antidepressant trials. In most cases, with proper psychoeducation, prescription of
CASE 3
Ms. G, age 22, was recently discharged from an inpatient psychiatric unit after an episode of mania. She was prescribed carbamazepine, 600 mg/d, and ziprasidone, 40 mg twice a day, and appears to be doing well on this regimen. When asked about what led to her admission, she recalls having an elevated mood, increased energy, hypersexuality, impulsivity, and poor judgment. She reveals that she had several sexual partners during her manic episode, and worries that if such behavior occurs again, she may get pregnant. Yet Ms. G was not prescribed birth control upon discharge.
Continue to: Contraception
Contraception. We believe that psychiatrists have an obligation to protect patients from consequences of mental illness. Much the same way that psychiatrists hope to prevent suicide in a patient who has depression, patients should be protected from risks encountered in the manic phase of bipolar disorder. Another reason to prescribe contraceptives in such patients is the teratogenic effects of mood stabilizers. Pagano et al14 reviewed 6 studies that examined common forms of hormonal birth control to determine their safety in patients with depression or bipolar disorder. They found that overall, use of hormonal contraception was not associated with a worse clinical course of disease.
Many available forms of birth control are available. When prescribing in an outpatient setting, a daily oral medication or a monthly depot injection are convenient options.
CASE 4
Mr. P, age 65, has bipolar I disorder and is stable on risperidone long-acting injection, 37.7 mg bimonthly, and lithium, 1,200 mg/d. He reports that he is doing well but has noticed a recent decrease in energy and weight gain without any change in mood. Laboratory testing conducted prior to this visit revealed a thyroid-stimulating hormone (TSH) level of 4 mU/L (normal range: 0.4 to 4.0 mU/L). Six months ago, Mr. P’s TSH level was 2.8 mU/L. The resident supervisor suggests discussing the case with an endocrinologist.
Thyroid function. The impact of lithium on the thyroid gland is well established; however, psychiatrists’ response to such changes are not.15 Gitlin16 reviewed the many adverse effects of lithium and presented various management strategies to address findings such as Mr. P’s. Two important points are that lithium should not be discontinued in light of hypothyroidism, and synthetic thyroxine (levothyroxine) can be initiated and titrated to return TSH levels to a normal range.16 Levothyroxine can be started at low doses (eg, 25 to 50 mcg/d) and increased every 6 weeks until a normal TSH level is achieved.17 Managing lithium-induced clinical or subclinical hypothyroidism can prevent further pathology and possible relapse to depression.
Incorporating integrated care
In all these cases, the prescription of a medication with which some psychiatrists are not comfortable prescribing would have been the logical, easiest, and preferable choice. Of course, when initiating any medication, boxed warnings, contraindications, and drug–drug interactions should be reviewed. Initial dosages and titration schedules can be found in every medication’s FDA-approved prescribing information document (package insert), as well as in numerous reference books and articles.
Continue to: We acknowledge...
We acknowledge that prescribing a nonpsychotropic medication is not always a psychiatrist’s best choice, and that in patients with multiple medical comorbidities and drug–drug interactions that are not clearly defined, referring to or consulting a specialist is appropriate. We in no way support reckless prescribing, but instead present an opportunity to expand the perception of what should be considered within a psychiatrist’s scope of practice, and call for further education of psychiatrists so that they are more comfortable managing these adverse effects and/or prescribing at least some nonpsychotropic medications.
We exhort integrated medical care during this time of a physician shortage; however, we do not practice this way. Interestingly, physicians in primary care, such as those in family medicine or obstetrics and gynecology, frequently attempt to treat patients with psychiatric conditions in an attempt to provide integrated care. Numerous articles have discussed these efforts.18-20 However, this type of integrated care seems less frequent in psychiatry, even though the practice of modern psychiatry in the United States shows substantial overlap with the practice of physicians in primary care specialties.21 There are few articles or practical guidelines for psychiatrists who wish to treat patients’ physical illnesses, particularly patients with severe mental illness (see Related Resources, page 56). If we practice in an integrated manner to treat one of the simple conditions we described above, we can eliminate the need for a patient to visit a second physician, pay another co-pay, pay another bus fare, and take another day off work. This can be particularly helpful for patients who at times have to decide between paying for groceries or for medications. Having one clinician manage a patient’s medications also can decrease the risk of polypharmacy.
In addition to the case scenarios described in this article, there are more clinical situations and nonpsychotropic medications that psychiatrists could manage. Considering them outside the scope of psychiatric practice and being uncomfortable or ambivalent about them is not an excuse. We hope that psychiatrists can increase their expertise in this area, and can start to practice as the primary care physicians they claim they are, and should be.
Bottom Line
Many psychiatrists are uncomfortable prescribing nonpsychotropic medications, but there are numerous clinical scenarios in which the practice would make sense. This could include cases of metabolic syndrome, sexual dysfunction secondary to antidepressant use, or other adverse effects of commonly prescribed psychotropic medications.
Related Resources
- McCarron RM, Xiong GL, Keenan CR, et al. Preventive medical care in psychiatry. A practical guide for clinicians. Arlington, VA: American Psychiatric Association Publishing; 2015.
- McCarron RM, Xiong GL, Keenan CR, et al. Study guide to preventive medical care in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Washington, DC: American Psychiatric Association Publishing; 2019.
Drug Brand Names
Avanafil • Stendra
Benztropine • Cogentin
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Levothyroxine • Levoxyl, Synthroid
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone long-acting injection • Risperdal Consta
Sildenafil • Viagra
Tadalafil • Cialis
Vardenafil • Levitra
Ziprasidone • Geodon
In our experience, most psychiatrists are uncomfortable with prescribing a medication when they feel that doing so would be outside their scope of practice. But there are many situations when prescribing a nonpsychotropic medication would be the correct choice. In this article, we discuss the scope of psychiatric practice, and present 4 case studies that illustrate situations in which psychiatrists should feel comfortable prescribing nonpsychotropic medications.
Defining the scope of practice
What is the scope of a psychiatrist’s practice? Scope of practice usually describes activities that a health care practitioner is allowed to undertake as defined by the terms of his/her license. A license to practice medicine does not include any stipulation restricting practice to a specific medical specialty. However, a local entity may delineate scope of practice within its organization. For instance, local practice standards held by the Detroit Wayne Mental Health Authority (DWMHA) state “Psychiatrists…shall not exceed their scope of practice as per DWMHA credentialing and privileging. For example, a Psychiatrist…who [has] not been appropriately privileged to deliver services to children shall not treat children, excepting crisis situations.”1
Like physicians in other specialties, psychiatrists are not limited to prescribing only a subset of medications commonly associated with their specialty. But for many psychiatrists, prescribing nonpsychotropic medications is complicated by individual and local factors. On one hand, some psychiatrists do not feel it is their role to prescribe nonpsychotropic medications,2 or even some psychotropic medications that may be more complex to prescribe, such as lithium, clozapine, or monoamine oxidase inhibitors.3-5 However, many feel comfortable prescribing complex combinations of psychotropic medications, or prescribing in a way that does not necessarily make sense (eg, prescribing benztropine as prophylaxis for dystonia when starting an antipsychotic).
Reviewing an average day at one urban psychiatric clinic, these questions seem to come up in half of the patient population, especially in patients with chronic mental illness, multiple medical comorbidities, and limited access to health care. When a young patient walks in without an appointment with an acute dystonic reaction secondary to the initiation of antipsychotics a couple of days ago, there is no hesitation to swiftly and appropriately prescribe an IM anticholinergic medication. But why are psychiatrists often hesitant to prescribe nonpsychotropic medications to treat other adverse effects of medications? Lack of knowledge? Lack of training?
Psychiatrists who practice in hospital systems often have immediate access to consultants, and this availability may encourage them to defer to the consultant for treatment of certain adverse effects. We have seen psychiatrists consult Neurology regarding the prescription of donepezil for mild neurocognitive disorder due to Alzheimer’s disease, or Endocrinology regarding prescription of levothyroxine for lithium-induced hypothyroidism.
However, there are numerous scenarios in which psychiatrists should feel comfortable prescribing nonpsychotropic medications or managing medication adverse effects, regardless of whether they consider it to be within or outside their scope of practice. The following case examples illustrate several such situations.
CASE 1
Ms. W, age 30, has been diagnosed with schizophrenia. She requests a refill of quetiapine, 800 mg/d. This medication has been clearly beneficial in alleviating her psychotic symptoms. However, since her last visit 3 months ago, her face appears more round, and she has gained 9 kg. Further evaluation indicates that she has developed metabolic syndrome and pre-diabetes.
Continue to: Metabolic adverse effects
Metabolic adverse effects, such as metabolic syndrome, diabetic ketoacidosis, and cardiovascular disease, are well-known risks of prescribing second-generation antipsychotics.6 In such situations, psychiatrists often advise patients to modify their diet, increase physical activity, and follow up with their primary care physician to determine if other medications are needed. However, getting a patient with a serious mental illness to exercise and modify her/his diet is difficult, and many of these patients do not have a primary care physician.
For patients such as Ms. W, a psychiatrist should consider prescribing metformin. Wu et al7 found that in addition to lifestyle modifications, metformin had the greatest effect on antipsychotic-induced weight gain. In this study, metformin alone had more impact on reversing weight gain and increasing insulin sensitivity than lifestyle modifications alone.7 This is crucial because these patients are especially vulnerable to cardiac disease.8 Metformin is well tolerated and has a low risk of causing hypoglycemia. Concerns regarding lactic acidosis have abated to the extent that the estimated glomerular filtration rate (eGFR) limits for using metformin have been lowered significantly. After reviewing the contraindications, the only knowledge needed to prescribe metformin is the patient’s kidney function and a brief understanding of the titration needed to minimize gastrointestinal adverse effects.9 Thus, prescribing metformin would be a fairly logical and easy first step for managing metabolic syndrome, especially in a patient whose motivation for increasing physical activity and modifying his/her diet is doubtful.
CASE 2
Mr. B, age 45, has major depressive disorder that has been well-controlled on paroxetine, 40 mg/d, for the past 2 years. He has no history of physical illness. On his most recent visit, he appears uncomfortable and nervous. After a long discussion, he discloses that his sex life isn’t what it used to be since starting paroxetine. He is bothered by erectile problems and asks whether he can “get some Viagra.”
Sexual adverse effects, such as erectile dysfunction, are frequently associated with the use of selective serotonin reuptake inhibitors.10 Although managing these adverse effects requires careful evaluation, in most cases, psychiatrists should be able to treat them.10 The logical choice in this case would be to prescribe one of the 4 FDA-approved phosphodiesterase-5 inhibitors (sildenafil [Viagra], tadalafil [Cialis], vardenafil [Levitra], and avanafil [Stendra]. However, Balon et al11 found that few psychiatrists prescribe phosphodiesterase-5 inhibitors, although they believed that they should be prescribing to treat their patients’ sexual dysfunction. Managing these adverse effects is important not only for the patient’s quality of life and relationship with his/her partner, but also for the therapeutic alliance. In a systematic review of 23 trials, Taylor et al12 examined >1,800 patients who were prescribed a medication to address sexual dysfunction secondary to antidepressants. They found that for men, adding a phosphodiesterase-5 inhibitor was appropriate and effective, and for women, adding bupropion at high doses should be considered.12 Like many other adverse effects, sexual adverse effects surely play a role in medication compliance. Dording et al13 found that the addition of sildenafil, 50 to 100 mg as needed, resulted in increased treatment satisfaction and overall contentment in 102 patients who complained of sexual dysfunction in the follow-up phase of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) antidepressant trials. In most cases, with proper psychoeducation, prescription of
CASE 3
Ms. G, age 22, was recently discharged from an inpatient psychiatric unit after an episode of mania. She was prescribed carbamazepine, 600 mg/d, and ziprasidone, 40 mg twice a day, and appears to be doing well on this regimen. When asked about what led to her admission, she recalls having an elevated mood, increased energy, hypersexuality, impulsivity, and poor judgment. She reveals that she had several sexual partners during her manic episode, and worries that if such behavior occurs again, she may get pregnant. Yet Ms. G was not prescribed birth control upon discharge.
Continue to: Contraception
Contraception. We believe that psychiatrists have an obligation to protect patients from consequences of mental illness. Much the same way that psychiatrists hope to prevent suicide in a patient who has depression, patients should be protected from risks encountered in the manic phase of bipolar disorder. Another reason to prescribe contraceptives in such patients is the teratogenic effects of mood stabilizers. Pagano et al14 reviewed 6 studies that examined common forms of hormonal birth control to determine their safety in patients with depression or bipolar disorder. They found that overall, use of hormonal contraception was not associated with a worse clinical course of disease.
Many available forms of birth control are available. When prescribing in an outpatient setting, a daily oral medication or a monthly depot injection are convenient options.
CASE 4
Mr. P, age 65, has bipolar I disorder and is stable on risperidone long-acting injection, 37.7 mg bimonthly, and lithium, 1,200 mg/d. He reports that he is doing well but has noticed a recent decrease in energy and weight gain without any change in mood. Laboratory testing conducted prior to this visit revealed a thyroid-stimulating hormone (TSH) level of 4 mU/L (normal range: 0.4 to 4.0 mU/L). Six months ago, Mr. P’s TSH level was 2.8 mU/L. The resident supervisor suggests discussing the case with an endocrinologist.
Thyroid function. The impact of lithium on the thyroid gland is well established; however, psychiatrists’ response to such changes are not.15 Gitlin16 reviewed the many adverse effects of lithium and presented various management strategies to address findings such as Mr. P’s. Two important points are that lithium should not be discontinued in light of hypothyroidism, and synthetic thyroxine (levothyroxine) can be initiated and titrated to return TSH levels to a normal range.16 Levothyroxine can be started at low doses (eg, 25 to 50 mcg/d) and increased every 6 weeks until a normal TSH level is achieved.17 Managing lithium-induced clinical or subclinical hypothyroidism can prevent further pathology and possible relapse to depression.
Incorporating integrated care
In all these cases, the prescription of a medication with which some psychiatrists are not comfortable prescribing would have been the logical, easiest, and preferable choice. Of course, when initiating any medication, boxed warnings, contraindications, and drug–drug interactions should be reviewed. Initial dosages and titration schedules can be found in every medication’s FDA-approved prescribing information document (package insert), as well as in numerous reference books and articles.
Continue to: We acknowledge...
We acknowledge that prescribing a nonpsychotropic medication is not always a psychiatrist’s best choice, and that in patients with multiple medical comorbidities and drug–drug interactions that are not clearly defined, referring to or consulting a specialist is appropriate. We in no way support reckless prescribing, but instead present an opportunity to expand the perception of what should be considered within a psychiatrist’s scope of practice, and call for further education of psychiatrists so that they are more comfortable managing these adverse effects and/or prescribing at least some nonpsychotropic medications.
We exhort integrated medical care during this time of a physician shortage; however, we do not practice this way. Interestingly, physicians in primary care, such as those in family medicine or obstetrics and gynecology, frequently attempt to treat patients with psychiatric conditions in an attempt to provide integrated care. Numerous articles have discussed these efforts.18-20 However, this type of integrated care seems less frequent in psychiatry, even though the practice of modern psychiatry in the United States shows substantial overlap with the practice of physicians in primary care specialties.21 There are few articles or practical guidelines for psychiatrists who wish to treat patients’ physical illnesses, particularly patients with severe mental illness (see Related Resources, page 56). If we practice in an integrated manner to treat one of the simple conditions we described above, we can eliminate the need for a patient to visit a second physician, pay another co-pay, pay another bus fare, and take another day off work. This can be particularly helpful for patients who at times have to decide between paying for groceries or for medications. Having one clinician manage a patient’s medications also can decrease the risk of polypharmacy.
In addition to the case scenarios described in this article, there are more clinical situations and nonpsychotropic medications that psychiatrists could manage. Considering them outside the scope of psychiatric practice and being uncomfortable or ambivalent about them is not an excuse. We hope that psychiatrists can increase their expertise in this area, and can start to practice as the primary care physicians they claim they are, and should be.
Bottom Line
Many psychiatrists are uncomfortable prescribing nonpsychotropic medications, but there are numerous clinical scenarios in which the practice would make sense. This could include cases of metabolic syndrome, sexual dysfunction secondary to antidepressant use, or other adverse effects of commonly prescribed psychotropic medications.
Related Resources
- McCarron RM, Xiong GL, Keenan CR, et al. Preventive medical care in psychiatry. A practical guide for clinicians. Arlington, VA: American Psychiatric Association Publishing; 2015.
- McCarron RM, Xiong GL, Keenan CR, et al. Study guide to preventive medical care in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Washington, DC: American Psychiatric Association Publishing; 2019.
Drug Brand Names
Avanafil • Stendra
Benztropine • Cogentin
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Levothyroxine • Levoxyl, Synthroid
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone long-acting injection • Risperdal Consta
Sildenafil • Viagra
Tadalafil • Cialis
Vardenafil • Levitra
Ziprasidone • Geodon
1. Detroit Wayne Integrated Health Network. DWMHA psychiatric practice standards. http://dwihn.org/files/2015/6451/9628/Psychiatric_Practice_Standards.pdf. Revised June 2018. Accessed October 8, 2019.
2. Seaman JJ, Cornfield RM, Cummings DM, et al. Exploring psychiatric prescribing practices: the relationship between the role of the provider and the appropriateness of prescribing. Gen Hosp Psychiatry. 1987;9(3):220-224.
3. Zivanovic O. Lithium: a classic drug—frequently discussed, but, sadly, seldom prescribed! Aust N Z J Psychiatry. 2017;51(9):886-896.
4. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatric Services. 2014;65(2):186-192.
5. Balon R, Mufti R, Arfken C. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatric Services. 1999;50(7):945-947.
6. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233.
7. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193.
8. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
9. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Internal Med. 2002;137(1):25-33.
10. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504-1509.
11. Balon R, Morreale MK, Segraves RT. Prescribing of phosphodiesterase-5 inhibitors among psychiatrists. J Sex Marital Ther. 2014;40(3):165-169.
12. Taylor MJ, Rudkin L, Bullemor-Day P, et al. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382.
13. Dording CM, LaRocca RA, Hails KA, et al. The effect of sildenafil on quality of life. Ann Clin Psychiatry. 2013;25(1):3-10.
14. Pagano HP, Zapata LB, Berry-Bibee EN, et al. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94(6):641-649.
15. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3.
16. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27.
17. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615.
18. Hackley B, Sharma C, Kedzior A, et al. Managing mental health conditions in primary care settings. J Midwifery Women’s Health. 2010;55(1):9-19.
19. Fitelson E, McGibbon C. Evaluation and management of behavioral health disorders in women: an overview of major depression, bipolar disorder, anxiety disorders, and sleep in the primary care setting. Obstet Gynecol Clin North Am. 2016;43(2):231-246.
20. Colorafi K, Vanselow J, Nelson T. Treating anxiety and depression in primary care: reducing barriers to access. Fam Pract Manag. 2017;24(4):11-16.
21. McCall WV. Defining the unique scope of psychiatric practice in 2015. J ECT. 2015;31(4):203-204.
1. Detroit Wayne Integrated Health Network. DWMHA psychiatric practice standards. http://dwihn.org/files/2015/6451/9628/Psychiatric_Practice_Standards.pdf. Revised June 2018. Accessed October 8, 2019.
2. Seaman JJ, Cornfield RM, Cummings DM, et al. Exploring psychiatric prescribing practices: the relationship between the role of the provider and the appropriateness of prescribing. Gen Hosp Psychiatry. 1987;9(3):220-224.
3. Zivanovic O. Lithium: a classic drug—frequently discussed, but, sadly, seldom prescribed! Aust N Z J Psychiatry. 2017;51(9):886-896.
4. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatric Services. 2014;65(2):186-192.
5. Balon R, Mufti R, Arfken C. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatric Services. 1999;50(7):945-947.
6. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233.
7. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193.
8. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
9. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Internal Med. 2002;137(1):25-33.
10. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504-1509.
11. Balon R, Morreale MK, Segraves RT. Prescribing of phosphodiesterase-5 inhibitors among psychiatrists. J Sex Marital Ther. 2014;40(3):165-169.
12. Taylor MJ, Rudkin L, Bullemor-Day P, et al. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382.
13. Dording CM, LaRocca RA, Hails KA, et al. The effect of sildenafil on quality of life. Ann Clin Psychiatry. 2013;25(1):3-10.
14. Pagano HP, Zapata LB, Berry-Bibee EN, et al. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94(6):641-649.
15. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3.
16. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27.
17. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615.
18. Hackley B, Sharma C, Kedzior A, et al. Managing mental health conditions in primary care settings. J Midwifery Women’s Health. 2010;55(1):9-19.
19. Fitelson E, McGibbon C. Evaluation and management of behavioral health disorders in women: an overview of major depression, bipolar disorder, anxiety disorders, and sleep in the primary care setting. Obstet Gynecol Clin North Am. 2016;43(2):231-246.
20. Colorafi K, Vanselow J, Nelson T. Treating anxiety and depression in primary care: reducing barriers to access. Fam Pract Manag. 2017;24(4):11-16.
21. McCall WV. Defining the unique scope of psychiatric practice in 2015. J ECT. 2015;31(4):203-204.
A reflection on Ghana’s mental health system
In recent years, the delivery of mental health services in Ghana has expanded substantially, especially since the passing of the Mental Health Act in 2012. In this article, I reflect on my experience as a visiting psychiatry resident in August 2018 at 2 Ghanaian hospitals located in Accra and Navrongo. Evident strengths of the mental health system were family support for patients and the scope of psychiatrists, while the most prominent weakness was the inadequate funding. As treatment of mental illness expands, more funding, psychiatrists, and mental health workers will be critical for the continued success of Ghana’s mental health system.
Psychiatric treatment in Ghana
Ghana has a population of approximately 28 million people, yet the country has an estimated 18 to 25 psychiatrists, up from 11 psychiatrists in 2011.1-3 Compared with the United States, which has 10.54 psychiatrists per 100,000 people (approximately 1 psychiatrist per 9,500 people), Ghana has .058 psychiatrists per 100,000 people (approximately 1 psychiatrist per 1.7 million people).4 In Ghana, most psychiatric care is delivered by mental health nurses, community mental health officers (CMHOs), and clinical psychiatric officers; supervision by psychiatrists is limited.3 Due to low public awareness, a scarcity of clinicians, and limited access to diagnostic services and medications, individuals with psychiatric illness in Ghana are often stigmatized, undertreated, and mistreated. To address this, in March 2012, Ghana passed Mental Health Act 846, which established a mental health commission and outlined protections for individuals with mental health needs.5 Since then, the number of people seeking treatment and the number of clinicians have expanded, but there are still significant challenges, such as a lack of funding for medications and facilities, and limited clinicians.6
During my last year of psychiatry residency at Mount Sinai Hospital in New York, I spent several weeks in Ghana at 2 institutions, observing and supervising the provision of psychiatric services. This was my first experience with the country’s health care system; therefore, my objectives were to:
- assess the current state of psychiatric services through observation and interviews with clinical staff
- provide instruction to clinicians in areas of need.
Two-thirds of my time was spent at the Accra Psychiatric Hospital, 1 of only 3 psychiatric hospitals in Ghana, all of which are located in the southern region of the country. The remainder of my time was spent at the Navrongo War Memorial Hospital in Ghana’s Northern Region.
The Accra Psychiatric Hospital is a sprawling complex near the center of the capital city. Every morning I walked through a large outdoor waiting area to the examination room, which was filled with at least 30 patients by 9
Navrongo War Memorial Hospital. There are no practicing psychiatrists in the northern region of the country; therefore, all mental health care is delivered by mental health nurses and CMHOs. CMHOs have 1 year of training plus a minimum of 2 years of service. They focus on identifying psychiatric cases in the community and coordinating treatment. Nurses have prescribing rights. A psychiatrist should be scheduled to visit the various districts in the region every 6 months to provide supervision, but this is not always feasible.
When I visited, I was the only psychiatrist who had been to this hospital in more than 1 year. During my time there, I reviewed the treatment protocols and gave lectures on the management of psychiatric emergencies and motivational interviewing, because addiction to alcohol and tramadol are 2 of the most pressing mental health problems in the country.7 I also saw patients with nurses, and supervised them on their assessment and treatment.
Continue to: In Ghana...
In Ghana, psychiatric services are often delivered using the community mental health model, in which many patients are visited in their homes. One morning, we went to a prayer camp to see if there were any individuals who would benefit from psychiatric services. There were no cases that day, but during the visit I sat under a tree where a few years before it was not uncommon to find a person who was psychotic or agitated chained to the tree. Several years of outreach by the local nurses has resulted in the camp leaders better recognizing mental illness early and contacting the nurses, as opposed to locking a person in chains for an extended period.
On one occasion, we answered a crisis call where a person experiencing a psychotic episode had locked himself in his house. The team talked with the individual through a locked screen door for 30 minutes, after which he eventually came out of the home to speak with us. A few days later, the patient accepted fluphenazine decanoate injection at his home. Two weeks later, he came to the outpatient clinic to continue treatment. Four months later, the patient was still in treatment and had started an apprenticeship for repairing cars.
As I was walking out of the hospital on my last day, I was called back to see a woman with a seizure who had been brought to the hospital. Unfortunately, there was no more diazepam in stock with which to treat her. This event highlighted the lack of resources available in this setting.
3 Take-home messages
My experience at both hospitals led me to reflect on 3 important factors impacting the mental health system in Ghana:
Family support. For at least 80% of appointments, patients were accompanied by family members or friends. The family hierarchy is still dominant in the Ghanaian culture, and clinicians often need the buy-in of the family, especially when financial support is required. More often than not, families enhanced patients’ treatment, but in some instances, they were a barrier.
Continue to: The types of cases
The types of cases. Most of the patients coming to both hospitals had diagnoses of bipolar disorder, schizophrenia, substance use disorder, or epilepsy. My impression was that patients or family members sought treatment for disorders that were conspicuous. I saw <5 cases of depression or anxiety. I wonder if this was because:
- patients with these disorders were referred to psychologists
- patients sought out faith-based treatment
- there was a lower incidence of these disorders, or these disorders were detected less frequently.
Inadequate funding. Despite the clinicians’ astute observations and diagnoses, they faced challenges, including a lack of access to medications because pharmacies were out of stock, or the patient or hospital could not afford the medication. At times, these challenges resulted in patients admitted to the hospital not receiving medications. When Mental Health Act 846 was implemented, it was widely purported that mental health care would be available to everyone, but the funding mechanism was not firmly established.8,9 Currently, laboratory workup, mental health treatment, and medications are not covered by health insurance, and government funding for mental health is insufficient. Therefore, in most areas, the entire cost burden of psychiatric care falls on patients and their families, or on hospitals.
Making progress despite barriers
In her inaugural address, former American Psychiatric Association President Altha J. Stewart, MD, named expanding the organization’s work in global mental health as one of her 3 primary goals.10 There are several means by which American psychiatrists can support the work of psychiatrists in Ghana and elsewhere. One way is by helping the mental health commission and other entities within the country petition the government and health insurance companies to expand coverage for mental health services. Teleconferencing, in which psychiatrists in Ghana or other parts of the world provide supervision to mid-level clinicians, has been piloted in other countries such as Liberia and could be implemented to address the critical shortages of psychiatrists in certain regions.11
In the past 7 years, Ghana has made significant strides in destigmatizing mental illness, and as a result more individuals are seeking treatment and more clinicians at all levels are being trained. Despite significant barriers, physicians, nurses, and other mental health workers deliver empathic and evidence-based treatment in a manner that defies the mental health system’s current limitations.
1. Ofori-Atta A, Attafuah J, Jack H, et al. Joining psychiatric care and faith healing in a prayer camp in Ghana: randomised trial. Br J Psychiatry. 2018;212(1):34-41.
2. Ghana has only 18 psychiatrists; experts beg government for more funds. GhanaWeb. https://www.ghanaweb.com/GhanaHomePage/NewsArchive/Ghana-has-only-18-psychiatrists-experts-beg-government-for-more-funds-591732. Published October 17, 2017. Accessed July 24, 2019.
3. Agyapong VIO, Farren C, McAuliffe E. Improving Ghana’s mental healthcare through task-shifting-psychiatrists and health policy directors perceptions about government’s commitment and the role of community mental health workers. Global Health. 2016;12:57.
4. World Health Organization. Global Health Observatory data repository. http://apps.who.int/gho/data/node.main.MHHR?lang=en. Published April 25, 2019. Accessed July 24, 2019.
5. Walker GH, Osei A. Mental health law in Ghana. BJPsych Int. 2017;14(2):38-39.
6. Doku VC, Wusu-Takyi A, Awakame J. Implementing the Mental Health Act in Ghana: any challenges ahead? Ghana Med J. 2012;46(4):241-250.
7. Kissiedu E. High dose Tramadol floods market. Business Day. http://businessdayghana.com/high-dose-tramadol-floods-market/. Published September 25, 2017. Accessed July 24, 2019.
8. Badu E, O’Brien AP, Mitchell R. An integrative review of potential enablers and barriers to accessing mental health services in Ghana. Health Res Policy Syst. 2018;16(1):110.
9. Ghana mental health care delivery risks collapse for lack of funds. News Ghana. https://www.newsghana.com.gh/ghana-mental-health-care-delivery-risks-collapse-for-lack-of-funds/. Published May 29, 2018. Accessed July 24, 2019.
10. Stewart AJ. Response to the Presidential Address. Am J Psychiatry. 2018;175(8):726-727.
11. Katz, CL, Washington FB, Sacco M, et al. A resident-based telepsychiatry supervision pilot program in Liberia. Psychiatr Serv. 2018;70(3):243-246.
In recent years, the delivery of mental health services in Ghana has expanded substantially, especially since the passing of the Mental Health Act in 2012. In this article, I reflect on my experience as a visiting psychiatry resident in August 2018 at 2 Ghanaian hospitals located in Accra and Navrongo. Evident strengths of the mental health system were family support for patients and the scope of psychiatrists, while the most prominent weakness was the inadequate funding. As treatment of mental illness expands, more funding, psychiatrists, and mental health workers will be critical for the continued success of Ghana’s mental health system.
Psychiatric treatment in Ghana
Ghana has a population of approximately 28 million people, yet the country has an estimated 18 to 25 psychiatrists, up from 11 psychiatrists in 2011.1-3 Compared with the United States, which has 10.54 psychiatrists per 100,000 people (approximately 1 psychiatrist per 9,500 people), Ghana has .058 psychiatrists per 100,000 people (approximately 1 psychiatrist per 1.7 million people).4 In Ghana, most psychiatric care is delivered by mental health nurses, community mental health officers (CMHOs), and clinical psychiatric officers; supervision by psychiatrists is limited.3 Due to low public awareness, a scarcity of clinicians, and limited access to diagnostic services and medications, individuals with psychiatric illness in Ghana are often stigmatized, undertreated, and mistreated. To address this, in March 2012, Ghana passed Mental Health Act 846, which established a mental health commission and outlined protections for individuals with mental health needs.5 Since then, the number of people seeking treatment and the number of clinicians have expanded, but there are still significant challenges, such as a lack of funding for medications and facilities, and limited clinicians.6
During my last year of psychiatry residency at Mount Sinai Hospital in New York, I spent several weeks in Ghana at 2 institutions, observing and supervising the provision of psychiatric services. This was my first experience with the country’s health care system; therefore, my objectives were to:
- assess the current state of psychiatric services through observation and interviews with clinical staff
- provide instruction to clinicians in areas of need.
Two-thirds of my time was spent at the Accra Psychiatric Hospital, 1 of only 3 psychiatric hospitals in Ghana, all of which are located in the southern region of the country. The remainder of my time was spent at the Navrongo War Memorial Hospital in Ghana’s Northern Region.
The Accra Psychiatric Hospital is a sprawling complex near the center of the capital city. Every morning I walked through a large outdoor waiting area to the examination room, which was filled with at least 30 patients by 9
Navrongo War Memorial Hospital. There are no practicing psychiatrists in the northern region of the country; therefore, all mental health care is delivered by mental health nurses and CMHOs. CMHOs have 1 year of training plus a minimum of 2 years of service. They focus on identifying psychiatric cases in the community and coordinating treatment. Nurses have prescribing rights. A psychiatrist should be scheduled to visit the various districts in the region every 6 months to provide supervision, but this is not always feasible.
When I visited, I was the only psychiatrist who had been to this hospital in more than 1 year. During my time there, I reviewed the treatment protocols and gave lectures on the management of psychiatric emergencies and motivational interviewing, because addiction to alcohol and tramadol are 2 of the most pressing mental health problems in the country.7 I also saw patients with nurses, and supervised them on their assessment and treatment.
Continue to: In Ghana...
In Ghana, psychiatric services are often delivered using the community mental health model, in which many patients are visited in their homes. One morning, we went to a prayer camp to see if there were any individuals who would benefit from psychiatric services. There were no cases that day, but during the visit I sat under a tree where a few years before it was not uncommon to find a person who was psychotic or agitated chained to the tree. Several years of outreach by the local nurses has resulted in the camp leaders better recognizing mental illness early and contacting the nurses, as opposed to locking a person in chains for an extended period.
On one occasion, we answered a crisis call where a person experiencing a psychotic episode had locked himself in his house. The team talked with the individual through a locked screen door for 30 minutes, after which he eventually came out of the home to speak with us. A few days later, the patient accepted fluphenazine decanoate injection at his home. Two weeks later, he came to the outpatient clinic to continue treatment. Four months later, the patient was still in treatment and had started an apprenticeship for repairing cars.
As I was walking out of the hospital on my last day, I was called back to see a woman with a seizure who had been brought to the hospital. Unfortunately, there was no more diazepam in stock with which to treat her. This event highlighted the lack of resources available in this setting.
3 Take-home messages
My experience at both hospitals led me to reflect on 3 important factors impacting the mental health system in Ghana:
Family support. For at least 80% of appointments, patients were accompanied by family members or friends. The family hierarchy is still dominant in the Ghanaian culture, and clinicians often need the buy-in of the family, especially when financial support is required. More often than not, families enhanced patients’ treatment, but in some instances, they were a barrier.
Continue to: The types of cases
The types of cases. Most of the patients coming to both hospitals had diagnoses of bipolar disorder, schizophrenia, substance use disorder, or epilepsy. My impression was that patients or family members sought treatment for disorders that were conspicuous. I saw <5 cases of depression or anxiety. I wonder if this was because:
- patients with these disorders were referred to psychologists
- patients sought out faith-based treatment
- there was a lower incidence of these disorders, or these disorders were detected less frequently.
Inadequate funding. Despite the clinicians’ astute observations and diagnoses, they faced challenges, including a lack of access to medications because pharmacies were out of stock, or the patient or hospital could not afford the medication. At times, these challenges resulted in patients admitted to the hospital not receiving medications. When Mental Health Act 846 was implemented, it was widely purported that mental health care would be available to everyone, but the funding mechanism was not firmly established.8,9 Currently, laboratory workup, mental health treatment, and medications are not covered by health insurance, and government funding for mental health is insufficient. Therefore, in most areas, the entire cost burden of psychiatric care falls on patients and their families, or on hospitals.
Making progress despite barriers
In her inaugural address, former American Psychiatric Association President Altha J. Stewart, MD, named expanding the organization’s work in global mental health as one of her 3 primary goals.10 There are several means by which American psychiatrists can support the work of psychiatrists in Ghana and elsewhere. One way is by helping the mental health commission and other entities within the country petition the government and health insurance companies to expand coverage for mental health services. Teleconferencing, in which psychiatrists in Ghana or other parts of the world provide supervision to mid-level clinicians, has been piloted in other countries such as Liberia and could be implemented to address the critical shortages of psychiatrists in certain regions.11
In the past 7 years, Ghana has made significant strides in destigmatizing mental illness, and as a result more individuals are seeking treatment and more clinicians at all levels are being trained. Despite significant barriers, physicians, nurses, and other mental health workers deliver empathic and evidence-based treatment in a manner that defies the mental health system’s current limitations.
In recent years, the delivery of mental health services in Ghana has expanded substantially, especially since the passing of the Mental Health Act in 2012. In this article, I reflect on my experience as a visiting psychiatry resident in August 2018 at 2 Ghanaian hospitals located in Accra and Navrongo. Evident strengths of the mental health system were family support for patients and the scope of psychiatrists, while the most prominent weakness was the inadequate funding. As treatment of mental illness expands, more funding, psychiatrists, and mental health workers will be critical for the continued success of Ghana’s mental health system.
Psychiatric treatment in Ghana
Ghana has a population of approximately 28 million people, yet the country has an estimated 18 to 25 psychiatrists, up from 11 psychiatrists in 2011.1-3 Compared with the United States, which has 10.54 psychiatrists per 100,000 people (approximately 1 psychiatrist per 9,500 people), Ghana has .058 psychiatrists per 100,000 people (approximately 1 psychiatrist per 1.7 million people).4 In Ghana, most psychiatric care is delivered by mental health nurses, community mental health officers (CMHOs), and clinical psychiatric officers; supervision by psychiatrists is limited.3 Due to low public awareness, a scarcity of clinicians, and limited access to diagnostic services and medications, individuals with psychiatric illness in Ghana are often stigmatized, undertreated, and mistreated. To address this, in March 2012, Ghana passed Mental Health Act 846, which established a mental health commission and outlined protections for individuals with mental health needs.5 Since then, the number of people seeking treatment and the number of clinicians have expanded, but there are still significant challenges, such as a lack of funding for medications and facilities, and limited clinicians.6
During my last year of psychiatry residency at Mount Sinai Hospital in New York, I spent several weeks in Ghana at 2 institutions, observing and supervising the provision of psychiatric services. This was my first experience with the country’s health care system; therefore, my objectives were to:
- assess the current state of psychiatric services through observation and interviews with clinical staff
- provide instruction to clinicians in areas of need.
Two-thirds of my time was spent at the Accra Psychiatric Hospital, 1 of only 3 psychiatric hospitals in Ghana, all of which are located in the southern region of the country. The remainder of my time was spent at the Navrongo War Memorial Hospital in Ghana’s Northern Region.
The Accra Psychiatric Hospital is a sprawling complex near the center of the capital city. Every morning I walked through a large outdoor waiting area to the examination room, which was filled with at least 30 patients by 9
Navrongo War Memorial Hospital. There are no practicing psychiatrists in the northern region of the country; therefore, all mental health care is delivered by mental health nurses and CMHOs. CMHOs have 1 year of training plus a minimum of 2 years of service. They focus on identifying psychiatric cases in the community and coordinating treatment. Nurses have prescribing rights. A psychiatrist should be scheduled to visit the various districts in the region every 6 months to provide supervision, but this is not always feasible.
When I visited, I was the only psychiatrist who had been to this hospital in more than 1 year. During my time there, I reviewed the treatment protocols and gave lectures on the management of psychiatric emergencies and motivational interviewing, because addiction to alcohol and tramadol are 2 of the most pressing mental health problems in the country.7 I also saw patients with nurses, and supervised them on their assessment and treatment.
Continue to: In Ghana...
In Ghana, psychiatric services are often delivered using the community mental health model, in which many patients are visited in their homes. One morning, we went to a prayer camp to see if there were any individuals who would benefit from psychiatric services. There were no cases that day, but during the visit I sat under a tree where a few years before it was not uncommon to find a person who was psychotic or agitated chained to the tree. Several years of outreach by the local nurses has resulted in the camp leaders better recognizing mental illness early and contacting the nurses, as opposed to locking a person in chains for an extended period.
On one occasion, we answered a crisis call where a person experiencing a psychotic episode had locked himself in his house. The team talked with the individual through a locked screen door for 30 minutes, after which he eventually came out of the home to speak with us. A few days later, the patient accepted fluphenazine decanoate injection at his home. Two weeks later, he came to the outpatient clinic to continue treatment. Four months later, the patient was still in treatment and had started an apprenticeship for repairing cars.
As I was walking out of the hospital on my last day, I was called back to see a woman with a seizure who had been brought to the hospital. Unfortunately, there was no more diazepam in stock with which to treat her. This event highlighted the lack of resources available in this setting.
3 Take-home messages
My experience at both hospitals led me to reflect on 3 important factors impacting the mental health system in Ghana:
Family support. For at least 80% of appointments, patients were accompanied by family members or friends. The family hierarchy is still dominant in the Ghanaian culture, and clinicians often need the buy-in of the family, especially when financial support is required. More often than not, families enhanced patients’ treatment, but in some instances, they were a barrier.
Continue to: The types of cases
The types of cases. Most of the patients coming to both hospitals had diagnoses of bipolar disorder, schizophrenia, substance use disorder, or epilepsy. My impression was that patients or family members sought treatment for disorders that were conspicuous. I saw <5 cases of depression or anxiety. I wonder if this was because:
- patients with these disorders were referred to psychologists
- patients sought out faith-based treatment
- there was a lower incidence of these disorders, or these disorders were detected less frequently.
Inadequate funding. Despite the clinicians’ astute observations and diagnoses, they faced challenges, including a lack of access to medications because pharmacies were out of stock, or the patient or hospital could not afford the medication. At times, these challenges resulted in patients admitted to the hospital not receiving medications. When Mental Health Act 846 was implemented, it was widely purported that mental health care would be available to everyone, but the funding mechanism was not firmly established.8,9 Currently, laboratory workup, mental health treatment, and medications are not covered by health insurance, and government funding for mental health is insufficient. Therefore, in most areas, the entire cost burden of psychiatric care falls on patients and their families, or on hospitals.
Making progress despite barriers
In her inaugural address, former American Psychiatric Association President Altha J. Stewart, MD, named expanding the organization’s work in global mental health as one of her 3 primary goals.10 There are several means by which American psychiatrists can support the work of psychiatrists in Ghana and elsewhere. One way is by helping the mental health commission and other entities within the country petition the government and health insurance companies to expand coverage for mental health services. Teleconferencing, in which psychiatrists in Ghana or other parts of the world provide supervision to mid-level clinicians, has been piloted in other countries such as Liberia and could be implemented to address the critical shortages of psychiatrists in certain regions.11
In the past 7 years, Ghana has made significant strides in destigmatizing mental illness, and as a result more individuals are seeking treatment and more clinicians at all levels are being trained. Despite significant barriers, physicians, nurses, and other mental health workers deliver empathic and evidence-based treatment in a manner that defies the mental health system’s current limitations.
1. Ofori-Atta A, Attafuah J, Jack H, et al. Joining psychiatric care and faith healing in a prayer camp in Ghana: randomised trial. Br J Psychiatry. 2018;212(1):34-41.
2. Ghana has only 18 psychiatrists; experts beg government for more funds. GhanaWeb. https://www.ghanaweb.com/GhanaHomePage/NewsArchive/Ghana-has-only-18-psychiatrists-experts-beg-government-for-more-funds-591732. Published October 17, 2017. Accessed July 24, 2019.
3. Agyapong VIO, Farren C, McAuliffe E. Improving Ghana’s mental healthcare through task-shifting-psychiatrists and health policy directors perceptions about government’s commitment and the role of community mental health workers. Global Health. 2016;12:57.
4. World Health Organization. Global Health Observatory data repository. http://apps.who.int/gho/data/node.main.MHHR?lang=en. Published April 25, 2019. Accessed July 24, 2019.
5. Walker GH, Osei A. Mental health law in Ghana. BJPsych Int. 2017;14(2):38-39.
6. Doku VC, Wusu-Takyi A, Awakame J. Implementing the Mental Health Act in Ghana: any challenges ahead? Ghana Med J. 2012;46(4):241-250.
7. Kissiedu E. High dose Tramadol floods market. Business Day. http://businessdayghana.com/high-dose-tramadol-floods-market/. Published September 25, 2017. Accessed July 24, 2019.
8. Badu E, O’Brien AP, Mitchell R. An integrative review of potential enablers and barriers to accessing mental health services in Ghana. Health Res Policy Syst. 2018;16(1):110.
9. Ghana mental health care delivery risks collapse for lack of funds. News Ghana. https://www.newsghana.com.gh/ghana-mental-health-care-delivery-risks-collapse-for-lack-of-funds/. Published May 29, 2018. Accessed July 24, 2019.
10. Stewart AJ. Response to the Presidential Address. Am J Psychiatry. 2018;175(8):726-727.
11. Katz, CL, Washington FB, Sacco M, et al. A resident-based telepsychiatry supervision pilot program in Liberia. Psychiatr Serv. 2018;70(3):243-246.
1. Ofori-Atta A, Attafuah J, Jack H, et al. Joining psychiatric care and faith healing in a prayer camp in Ghana: randomised trial. Br J Psychiatry. 2018;212(1):34-41.
2. Ghana has only 18 psychiatrists; experts beg government for more funds. GhanaWeb. https://www.ghanaweb.com/GhanaHomePage/NewsArchive/Ghana-has-only-18-psychiatrists-experts-beg-government-for-more-funds-591732. Published October 17, 2017. Accessed July 24, 2019.
3. Agyapong VIO, Farren C, McAuliffe E. Improving Ghana’s mental healthcare through task-shifting-psychiatrists and health policy directors perceptions about government’s commitment and the role of community mental health workers. Global Health. 2016;12:57.
4. World Health Organization. Global Health Observatory data repository. http://apps.who.int/gho/data/node.main.MHHR?lang=en. Published April 25, 2019. Accessed July 24, 2019.
5. Walker GH, Osei A. Mental health law in Ghana. BJPsych Int. 2017;14(2):38-39.
6. Doku VC, Wusu-Takyi A, Awakame J. Implementing the Mental Health Act in Ghana: any challenges ahead? Ghana Med J. 2012;46(4):241-250.
7. Kissiedu E. High dose Tramadol floods market. Business Day. http://businessdayghana.com/high-dose-tramadol-floods-market/. Published September 25, 2017. Accessed July 24, 2019.
8. Badu E, O’Brien AP, Mitchell R. An integrative review of potential enablers and barriers to accessing mental health services in Ghana. Health Res Policy Syst. 2018;16(1):110.
9. Ghana mental health care delivery risks collapse for lack of funds. News Ghana. https://www.newsghana.com.gh/ghana-mental-health-care-delivery-risks-collapse-for-lack-of-funds/. Published May 29, 2018. Accessed July 24, 2019.
10. Stewart AJ. Response to the Presidential Address. Am J Psychiatry. 2018;175(8):726-727.
11. Katz, CL, Washington FB, Sacco M, et al. A resident-based telepsychiatry supervision pilot program in Liberia. Psychiatr Serv. 2018;70(3):243-246.
Recognizing and treating ketamine abuse
The N-methyl-
Physicians need to be aware, however, that many patients use illicit ketamine, either for recreational purposes or as self-treatment to control depressive symptoms. To help clinicians identify the signs of ketamine abuse, we discuss the adverse effects of illicit use, and suggest treatment approaches.
Ad
Ketamine can be consumed in various ways; snorting it in a powder form is a preferred route for recreational use.2 The primary disadvantage of oral use is that it increases the likelihood of nausea and vomiting.2
While ketamine is generally safe in a supervised clinical setting, approximately 2.5 million individuals use various illicit forms of ketamine—which is known as Special K and by other names—in recreational settings (eg, dance clubs) where it might be used with other substances.3 Alcohol, in particular, compounds the sedative effects of ketamine and can lead to death by overdose.
At a subanesthetic dose, ketamine can induce dissociative and/or transcendental states that are particularly attractive to those intrigued by mystical experiences, pronounced changes in perception, or euphoria.4 High doses of ketamine—relative to a commonly used recreational dose—can produce a unique “K-hole” state in which a user is unable to control his/her body and could lose consciousness.5 A K-hole state may trigger a cycle of delirium that warrants immediate clinical attention.3
Researchers have postulated that NMDA antagonism may negatively impact memory consolidation.3,6 Even more troubling is the potential for systemic injuries because illicit ketamine use may contribute to ulcerative cystitis, severely disturbed kidney function (eg, hydronephrosis), or epigastric pain.3 Chronic abuse tends to result in more systemic sequelae, affecting the bladder, kidneys, and heart. Adverse effects that require emergent care include blood in urine, changes in vision (eg, nystagmus), chest discomfort, labored breathing, agitation, seizures, and/or altered consciousness.6
Treating ketamine abuse
Treatment should be tailored to the patient’s symptoms. If the patient presents with “K-bladder” (ie, ketamine bladder syndrome), he/she may need surgical intervention or a cystectomy.4,7 Therapeutic management of K-bladder entails recognizing bladder symptoms that are specific to ketamine use, such as interstitial or ulcerative cystitis and lower urinary tract symptoms.7 Clinicians should monitor patients for increased voiding episodes during the day, voiding urgency, or a general sense of bladder fullness. Patients with K-bladder also may complain of suprapubic pain or blood in the urine.7
Continue to: Consider referring patients to...
Consider referring patients to an individualized, ketamine-specific rehabilitation program that is modeled after other substance-specific rehabilitation programs. It is critical to address withdrawal symptoms (eg, anorexia, fatigue, tremors, chills, tachycardia, nightmares, etc.). Patients undergoing ketamine withdrawal may develop anxiety and depression, with or without suicidal ideation, that might persist during a 4- to 5-day withdrawal period.8
‘Self-medicating’ ketamine users
Clinicians need to be particularly vigilant for situations in which a patient has used ketamine in an attempt to control his/her depressive symptoms. Some researchers have described ketamine as a revolutionary drug for TRD, and it is reasonable to suspect that some patients with depressive symptoms may have consulted Internet sources to learn how to self-medicate using ketamine. Patients who have consumed smaller doses of ketamine recreationally may have developed a tolerance in which the receptors are no longer responsive to the effects at that dose, and therefore might not respond when given ketamine in a clinical setting. Proper history taking and patient education are essential for these users, and clinicians may need to develop a personalized therapeutic plan for ketamine administration. If, on the other hand, a patient has a history of chronic ketamine use (perhaps at high doses), depression may occur secondary to this type of ketamine abuse. For such patients, clinicians should explore alternative treatment modalities, such as transcranial magnetic stimulation.
1. Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283-290.
2. Davis K. What are the uses of ketamine? Medical News Today. https://www.medicalnewstoday.com/articles/302663.php. Updated October 12, 2017. Published October 11, 2019.
3. Chaverneff F. Ketamine: mechanisms of action, uses in pain medicine, and side effects. Clinical Pain Advisor. https://www.clinicalpainadvisor.com/home/conference-highlights/painweek-2018/ketamine-mechanisms-of-action-uses-in-pain-medicine-and-side-effects/. Published 2018. Accessed October 11, 2019.
4. Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016;37(7):865-872.
5. Orhurhu VJ, Claus LE, Cohen SP. Ketamine toxicity. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK541087. Updated April 11, 2019. Accessed October 18, 2019.
6. Pai A, Heining M. Ketamine. Continuing Education in Anaesthesia Critical Care & Pain. 20071;7(2):59-63.
7. Logan K. Addressing ketamine bladder syndrome. Nursing Times. https://www.nursingtimes.net/clinical-archive/medicine-management/addressing-ketamine-bladder-syndrome-19-06-2011/. Published June 19, 2011. Accessed October 11, 2019.
8. Lin PC, Lane HY, Lin CH. Spontaneous remission of ketamine withdrawal-related depression. Clin Neuropharmacol. 2016;39(1):51-52.
The N-methyl-
Physicians need to be aware, however, that many patients use illicit ketamine, either for recreational purposes or as self-treatment to control depressive symptoms. To help clinicians identify the signs of ketamine abuse, we discuss the adverse effects of illicit use, and suggest treatment approaches.
Ad
Ketamine can be consumed in various ways; snorting it in a powder form is a preferred route for recreational use.2 The primary disadvantage of oral use is that it increases the likelihood of nausea and vomiting.2
While ketamine is generally safe in a supervised clinical setting, approximately 2.5 million individuals use various illicit forms of ketamine—which is known as Special K and by other names—in recreational settings (eg, dance clubs) where it might be used with other substances.3 Alcohol, in particular, compounds the sedative effects of ketamine and can lead to death by overdose.
At a subanesthetic dose, ketamine can induce dissociative and/or transcendental states that are particularly attractive to those intrigued by mystical experiences, pronounced changes in perception, or euphoria.4 High doses of ketamine—relative to a commonly used recreational dose—can produce a unique “K-hole” state in which a user is unable to control his/her body and could lose consciousness.5 A K-hole state may trigger a cycle of delirium that warrants immediate clinical attention.3
Researchers have postulated that NMDA antagonism may negatively impact memory consolidation.3,6 Even more troubling is the potential for systemic injuries because illicit ketamine use may contribute to ulcerative cystitis, severely disturbed kidney function (eg, hydronephrosis), or epigastric pain.3 Chronic abuse tends to result in more systemic sequelae, affecting the bladder, kidneys, and heart. Adverse effects that require emergent care include blood in urine, changes in vision (eg, nystagmus), chest discomfort, labored breathing, agitation, seizures, and/or altered consciousness.6
Treating ketamine abuse
Treatment should be tailored to the patient’s symptoms. If the patient presents with “K-bladder” (ie, ketamine bladder syndrome), he/she may need surgical intervention or a cystectomy.4,7 Therapeutic management of K-bladder entails recognizing bladder symptoms that are specific to ketamine use, such as interstitial or ulcerative cystitis and lower urinary tract symptoms.7 Clinicians should monitor patients for increased voiding episodes during the day, voiding urgency, or a general sense of bladder fullness. Patients with K-bladder also may complain of suprapubic pain or blood in the urine.7
Continue to: Consider referring patients to...
Consider referring patients to an individualized, ketamine-specific rehabilitation program that is modeled after other substance-specific rehabilitation programs. It is critical to address withdrawal symptoms (eg, anorexia, fatigue, tremors, chills, tachycardia, nightmares, etc.). Patients undergoing ketamine withdrawal may develop anxiety and depression, with or without suicidal ideation, that might persist during a 4- to 5-day withdrawal period.8
‘Self-medicating’ ketamine users
Clinicians need to be particularly vigilant for situations in which a patient has used ketamine in an attempt to control his/her depressive symptoms. Some researchers have described ketamine as a revolutionary drug for TRD, and it is reasonable to suspect that some patients with depressive symptoms may have consulted Internet sources to learn how to self-medicate using ketamine. Patients who have consumed smaller doses of ketamine recreationally may have developed a tolerance in which the receptors are no longer responsive to the effects at that dose, and therefore might not respond when given ketamine in a clinical setting. Proper history taking and patient education are essential for these users, and clinicians may need to develop a personalized therapeutic plan for ketamine administration. If, on the other hand, a patient has a history of chronic ketamine use (perhaps at high doses), depression may occur secondary to this type of ketamine abuse. For such patients, clinicians should explore alternative treatment modalities, such as transcranial magnetic stimulation.
The N-methyl-
Physicians need to be aware, however, that many patients use illicit ketamine, either for recreational purposes or as self-treatment to control depressive symptoms. To help clinicians identify the signs of ketamine abuse, we discuss the adverse effects of illicit use, and suggest treatment approaches.
Ad
Ketamine can be consumed in various ways; snorting it in a powder form is a preferred route for recreational use.2 The primary disadvantage of oral use is that it increases the likelihood of nausea and vomiting.2
While ketamine is generally safe in a supervised clinical setting, approximately 2.5 million individuals use various illicit forms of ketamine—which is known as Special K and by other names—in recreational settings (eg, dance clubs) where it might be used with other substances.3 Alcohol, in particular, compounds the sedative effects of ketamine and can lead to death by overdose.
At a subanesthetic dose, ketamine can induce dissociative and/or transcendental states that are particularly attractive to those intrigued by mystical experiences, pronounced changes in perception, or euphoria.4 High doses of ketamine—relative to a commonly used recreational dose—can produce a unique “K-hole” state in which a user is unable to control his/her body and could lose consciousness.5 A K-hole state may trigger a cycle of delirium that warrants immediate clinical attention.3
Researchers have postulated that NMDA antagonism may negatively impact memory consolidation.3,6 Even more troubling is the potential for systemic injuries because illicit ketamine use may contribute to ulcerative cystitis, severely disturbed kidney function (eg, hydronephrosis), or epigastric pain.3 Chronic abuse tends to result in more systemic sequelae, affecting the bladder, kidneys, and heart. Adverse effects that require emergent care include blood in urine, changes in vision (eg, nystagmus), chest discomfort, labored breathing, agitation, seizures, and/or altered consciousness.6
Treating ketamine abuse
Treatment should be tailored to the patient’s symptoms. If the patient presents with “K-bladder” (ie, ketamine bladder syndrome), he/she may need surgical intervention or a cystectomy.4,7 Therapeutic management of K-bladder entails recognizing bladder symptoms that are specific to ketamine use, such as interstitial or ulcerative cystitis and lower urinary tract symptoms.7 Clinicians should monitor patients for increased voiding episodes during the day, voiding urgency, or a general sense of bladder fullness. Patients with K-bladder also may complain of suprapubic pain or blood in the urine.7
Continue to: Consider referring patients to...
Consider referring patients to an individualized, ketamine-specific rehabilitation program that is modeled after other substance-specific rehabilitation programs. It is critical to address withdrawal symptoms (eg, anorexia, fatigue, tremors, chills, tachycardia, nightmares, etc.). Patients undergoing ketamine withdrawal may develop anxiety and depression, with or without suicidal ideation, that might persist during a 4- to 5-day withdrawal period.8
‘Self-medicating’ ketamine users
Clinicians need to be particularly vigilant for situations in which a patient has used ketamine in an attempt to control his/her depressive symptoms. Some researchers have described ketamine as a revolutionary drug for TRD, and it is reasonable to suspect that some patients with depressive symptoms may have consulted Internet sources to learn how to self-medicate using ketamine. Patients who have consumed smaller doses of ketamine recreationally may have developed a tolerance in which the receptors are no longer responsive to the effects at that dose, and therefore might not respond when given ketamine in a clinical setting. Proper history taking and patient education are essential for these users, and clinicians may need to develop a personalized therapeutic plan for ketamine administration. If, on the other hand, a patient has a history of chronic ketamine use (perhaps at high doses), depression may occur secondary to this type of ketamine abuse. For such patients, clinicians should explore alternative treatment modalities, such as transcranial magnetic stimulation.
1. Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283-290.
2. Davis K. What are the uses of ketamine? Medical News Today. https://www.medicalnewstoday.com/articles/302663.php. Updated October 12, 2017. Published October 11, 2019.
3. Chaverneff F. Ketamine: mechanisms of action, uses in pain medicine, and side effects. Clinical Pain Advisor. https://www.clinicalpainadvisor.com/home/conference-highlights/painweek-2018/ketamine-mechanisms-of-action-uses-in-pain-medicine-and-side-effects/. Published 2018. Accessed October 11, 2019.
4. Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016;37(7):865-872.
5. Orhurhu VJ, Claus LE, Cohen SP. Ketamine toxicity. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK541087. Updated April 11, 2019. Accessed October 18, 2019.
6. Pai A, Heining M. Ketamine. Continuing Education in Anaesthesia Critical Care & Pain. 20071;7(2):59-63.
7. Logan K. Addressing ketamine bladder syndrome. Nursing Times. https://www.nursingtimes.net/clinical-archive/medicine-management/addressing-ketamine-bladder-syndrome-19-06-2011/. Published June 19, 2011. Accessed October 11, 2019.
8. Lin PC, Lane HY, Lin CH. Spontaneous remission of ketamine withdrawal-related depression. Clin Neuropharmacol. 2016;39(1):51-52.
1. Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283-290.
2. Davis K. What are the uses of ketamine? Medical News Today. https://www.medicalnewstoday.com/articles/302663.php. Updated October 12, 2017. Published October 11, 2019.
3. Chaverneff F. Ketamine: mechanisms of action, uses in pain medicine, and side effects. Clinical Pain Advisor. https://www.clinicalpainadvisor.com/home/conference-highlights/painweek-2018/ketamine-mechanisms-of-action-uses-in-pain-medicine-and-side-effects/. Published 2018. Accessed October 11, 2019.
4. Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016;37(7):865-872.
5. Orhurhu VJ, Claus LE, Cohen SP. Ketamine toxicity. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK541087. Updated April 11, 2019. Accessed October 18, 2019.
6. Pai A, Heining M. Ketamine. Continuing Education in Anaesthesia Critical Care & Pain. 20071;7(2):59-63.
7. Logan K. Addressing ketamine bladder syndrome. Nursing Times. https://www.nursingtimes.net/clinical-archive/medicine-management/addressing-ketamine-bladder-syndrome-19-06-2011/. Published June 19, 2011. Accessed October 11, 2019.
8. Lin PC, Lane HY, Lin CH. Spontaneous remission of ketamine withdrawal-related depression. Clin Neuropharmacol. 2016;39(1):51-52.
Neuropsychological testing: A useful but underutilized resource
We have all treated a patient for whom you know you had the diagnosis correct, the medication regimen was working, and the patient adhered to treatment, but something was still “off.” There was something cognitively that wasn’t right, and you had identified subtle (and some overt) errors in the standard psychiatric cognitive assessment that didn’t seem amenable to psychotropic medications. Perhaps what was needed was neuropsychological testing, one of the most useful but underutilized resources available to help fine-tune diagnosis and treatment. Finding a neuropsychologist who is sensitive to the unique needs of patients with psychiatric disorders, and knowing what and how to communicate the clinical picture and need for the referral, can be challenging due to the limited availability, time, and cost of a full battery of standardized tests.
This article describes the purpose of neuropsychological testing, why it is an important part of psychiatry, and how to make the best use of it.
What is neuropsychological testing?
Neuropsychological testing is a comprehensive evaluation designed to assess cognitive functioning, such as attention, language, learning, memory, and visuospatial and executive functioning. Neuropsychology has its own vocabulary and lexicon that are important for psychiatric clinicians to understand. Some terms, such as aphasia, working memory, and dementia, are familiar to many clinicians. However, others, such as information processing speed, performance validity testing, and semantic memory, might not be. Common neuropsychological terms are defined in Table 1.
The neuropsychologist’s role
A neuropsychologist is a psychologist with advanced training in brain-behavior relationships who can help determine if cognitive problems are related to neurologic, medical, or psychiatric factors. A neuropsychological evaluation can identify the etiology of a patient’s cognitive difficulties, such as stroke, poorly controlled diabetes, or mental health symptoms, to help guide treatment. It can be difficult to determine if a patient who is experiencing significant cognitive, functional, or behavioral changes has an underlying cognitive disorder (eg, dementia or major neurocognitive disorder) or something else, such as a psychiatric condition. Indeed, many psychiatric conditions, including schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), and major depressive disorder (MDD), can present with significant cognitive difficulties. Thus, when patients report an increase in forgetfulness or changes in their ability to care for themselves, neuropsychological testing can help determine the cause.
How to refer to a neuropsychologist
Developing a referral network with a neuropsychologist should be a component of establishing a psychiatric practice. A neuropsychologist can help identify deficits that may interfere with the patient’s ability to adhere to a treatment plan, monitor medications, or actively participate in treatment and therapy. When making a referral for neuropsychological testing, it is important to be clear about the specific concerns so the neuropsychologist knows how to best evaluate the patient. A psychiatric clinician does not order specific neuropsychological tests, but thoroughly describes the problem so the neuropsychologist can determine the appropriate tests after interviewing the patient. For example, if a patient reports memory problems, it is essential to give the neuropsychologist specific clinical data so he/she can determine if the symptoms are due to a neurodegenerative or psychiatric condition. Then, after interviewing the patient (and, possibly, a family member), the neuropsychologist can construct a battery of tests to best answer the question.
Which neuropsychological tests are available?
There is a large battery of neuropsychological tests that require a licensed psychologist to administer and interpret.1 Those commonly used in research and practice to differentiate neurologically-based cognitive deficits associated with psychiatric disorders include the Wechsler Adult Intelligence Scale-4th edition (WAIS-IV) for assessing intelligence, the California Verbal Learning Test-Third Edition (CVLT-3) for verbal memory and learning, the Brief Visuospatial Memory Test-Revised for visual memory, the Wisconsin Card Sorting Test (WCST) for executive functions, and the Ruff 2&7 Selective Attention Test for sustained attention.2 These and other commonly used tests are described in Table 2.1
Neuropsychological testing vs psychological testing
The neuropsychologist will use psychometric properties (such as the validity and reliability of the test) and available normative data to pick the most appropriate tests. To date, there are no specific tests that clearly delineate psychiatric from nonpsychiatric etiologies, although the Screen for Cognitive Impairment in Psychiatry (SCIP)3 was developed in 2013 to explore cognitive abilities in the functional psychoses; it is beginning to be used in other studies.4,5 The neuropsychologist will consider the patient’s current concerns, the onset and progression of these concerns, and the pattern in testing behavior to help determine if psychiatric conditions are the most likely etiology.
Continue to: In addition to cognitive tests...
In addition to cognitive tests, the neuropsychologist might also administer psychological tests. These might include commonly used screening tools such as the Patient Health Questionnaire-9 (PHQ-9)6 or Geriatric Depression Scale (GDS),7 or more comprehensive objective personality measures, such as the Minnesota Multiphasic Personality Inventory-2-Restructured Format (MMPI-2-RF)8 or Personality Assessment Inventory (PAI).9 These tests, along with a thorough clinical history, can help identify if a psychiatric condition is present. In addition, for the more extensive tests such as the MMPI-2-RF or PAI, there are certain neuropsychological profiles that are consistent with a psychiatric etiology for cognitive difficulties. These profiles are formulated based on specific test scores in combination with complex patient variables.
Understanding the report
While there will be stylistic differences in reports depending on the neuropsychologist’s setting, referral source, and personal preferences, most will include discussion of why the patient was referred for evaluation and a description of the onset and progression of the problem.10 Reports often also include pertinent medical and psychiatric history, substance use history, and family medical history. A section on social history is important to help establish premorbid functioning, and might include information about prenatal/birth complications, developmental milestones, educational history, and occupational history. Information about current psychosocial support or stressors, including marital status or current/past legal issues, can be helpful. In addition to this history, there is often a section on behavioral observations, especially if anything stood out or might have affected the validity of the data.
There are also objective measures of validity that the neuropsychologist might administer to evaluate whether the results are valid. Issues of validity are monitored through the evaluation, and are used to determine if the results are consistent with known neurologic patterns. If the results are deemed not valid, then low scores cannot be reliably interpreted as evidence of impairment. This is akin to an arm moving during an X-ray, thereby blurring the results. If valid, the results of objective testing are include in the neuropsychologist’s report; this can range from providing raw scores, standard scores, and/or percentiles to a general description of how the patient did on testing.
The section that is usually of most interest to psychiatric clinicians is the summary, which explains the results, might offer a diagnosis, and discusses possible etiologies. This might be where the neuropsychologist discusses if the findings are due to a neurologic or psychiatric condition. From this comes the neuropsychologist’s recommendations. When a psychiatric condition is determined to be the underlying etiology, the neuropsychologist might recommend psychotherapy or some other psychiatric treatment.
Why is neuropsychological testing important?
Schizophrenia, MDD, bipolar disorder, and PTSD produce significant neurobiologic changes that often result in deterioration of a patient’s global cognitive function. Increased emphasis and attention in psychiatric research have yielded more clues to the neurobiology of cognition. However, even though many psychiatric clinicians are trained in cognitive assessments, such as the “clock test,” “serial sevens,” “numbers forward and backward,” “proverb,” and “word recall,” and common scenarios to evaluate judgment and insight, such as “mailing a letter” and “smoke in a movie theatre,” most of these components are not completed during a standard psychiatric evaluation. Because the time allotted to completing a psychiatric evaluation continues to be shortened, it is sometimes difficult to complete the “6 bullets” required by the Centers for Medicare & Medicaid Services as part of the mental status exam (Table 311).
Continue to: To date, the best evidence...
To date, the best evidence for neuropsychological deficits exists for patients with schizophrenia, bipolar disorder, MDD, and PTSD.12,13 The Box2,14-24 describes the findings of studies of neuropsychological deficits in patients with schizophrenia and bipolar disorder.
Box
Patients with schizophrenia have been the subjects of neuropsychological testing for decades. The results have shown deficits on many standardized tests, including those of attention, memory, and executive functioning, although some patients might perform within normal limits.15
A federal initiative through the National Institute of Mental Health (NIMH) known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) was developed in the late 1990s to develop consensus on the underlying cognitive deficits in schizophrenia. MATRICS was created with the hopes that it would allow the FDA to approve treatments for those cognitive deficits independent of psychosis because current psychotropic medications have minimal efficacy on cognition.16,17 The MATRICS group identified working memory, attention/vigilance, verbal learning and memory, visual learning and memory, speed of processing, reasoning and problem solving, and social cognition as the key cognitive domains most affected in schizophrenia.14 The initial program has since evolved into 3 distinct NIMH programs: CNTRICS18 (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia), TURNS19 (Treatment Units for Research on Neurocognition in Schizophrenia), and TENETS20 (Treatment and Evaluation Network for Trials in Schizophrenia). The combination of neuropsychological testing and neuroimaging has led to the conceptualization of schizophrenia as a neurodevelopmental disorder.
Individuals at risk for psychosis
As clinicians, we have long heard from parents of children with schizophrenia a standard trajectory of functional decline: early premorbid changes, a fairly measurable prodromal period marked by subtle deterioration in cognitive functioning, followed by the actual illness trajectory. In a recent meta-analysis, researchers compared the results of 60 neuropsychological tests comprising 9 domains in people who were at clinical high risk for psychosis who eventually converted to a psychotic disorder (CHR-P), those at clinical high risk who did not convert to psychosis (CHR-NP), and healthy controls.21 They found that neuropsychological performance deficits were greater in CHR-P individuals than in those in the CHR-NP group, and both had greater deficits than healthy controls.
For many patients with schizophrenia, full cognitive maturation is never reached.22 In general, decreased motivation in schizophrenia has been correlated with neurocognitive deficits.23
Schizophrenia vs bipolar disorder
In a study comparing neuropsychological functioning in patients with schizophrenia and bipolar disorder with psychotic features (BP-P), researchers found greater deficits in schizophrenia, including immediate verbal recall, working memory, processing speed, and verbal fluency.22 Patients with BP-P demonstrated impairment consistent with generalized impairment in verbal learning and memory, working memory, and processing speed.22
Children/adolescents
In a recent study comparing child and adolescent offspring of patients with schizophrenia (n = 41) and bipolar disorder (n = 90), researchers identified neuropsychological deficits in visual memory for both groups, suggesting common genetic linkages. The schizophrenia offspring scored lower in verbal memory and word memory, while bipolar offspring scored lower on the processing speed index and visual memory.2
Information processing
Another study compared the results of neuropsychological testing and the P300 component of auditory event-related potential (an electrophysiological measure) in 30 patients with schizophrenia, siblings without illness, and normal controls.24 The battery of neuropsychological tests included the Digit Symbol Substitution Test, Digit Vigilance Test, Trail Making Test-B, and Stroop test. The P300 is well correlated with information processing. Researchers found decreased P300 amplitude and latency in the patients and normal levels in the controls; siblings scored somewhere in between.24 Scores on the neuropsychological tests were consistently below normal in both patients and their siblings, with patients scoring the lowest.24
Continue to: Neuropsychological testing
Neuropsychological testing: 2 Case studies
The following 2 cases illustrate the pivotal role of neuropsychological testing in formulating an accurate differential diagnosis, and facilitating improved outcomes.
Case 1
A veteran with PTSD and memory complaints
Mr. J, age 70, is a married man who spent his career in the military, including combat service in the Vietnam War. His service in Vietnam included an event in which he couldn’t save platoon members from an ambush and death in a firefight, after which he developed PTSD. He retired after 25 years of service.
Mr. J’s psychiatrist refers him to a neuropsychologist for complaints of memory difficulties, including a fear that he’s developing Alzheimer’s disease (AD). Because of the concern for AD, he undergoes tests of learning and memory, such as the CVLT-3, the Brief Visuospatial Memory Test-Revised, and the Logical Memory subtest from the Wechsler Memory Scale–4th Edition. Other tests include a measure of confrontation naming, verbal fluency (phonemic and semantic fluency), construction, attention, processing speed, and problem solving. In addition, a measure of psychiatric and emotional functioning is also administered (the MMPI-2-RF).
The results determined that Mr. J’s subjective experience of recall deficits is better explained by anxiety resulting from the cumulative impact of day-to-day emotional stress in the setting of chronic PTSD.25 Mr. J was experiencing cognitive sequelae from a complicated emotional dynamic, comprised of situational stress, amplified by coping difficulties that were rooted in older posttraumatic symptoms. These emotions, and the cognitive load they generated, interfered with the normal processes of attention and organization necessary for the encoding of information to be remembered.26 He described being visibly angered by the clutter in his home (the result of multiple people living there, including a young grandchild), having his efforts to get things done interrupted by the needs of others, and a perceived loss of control gradually generalized to even mundane circumstances, as often occurs with traumatic responses. In short, he was chronically overwhelmed and not experiencing the beginnings of dementia.
For Mr. J, neuropsychological testing helped define the focus and course of therapy. If he had been diagnosed with a major neurocognitive disorder, therapy might have taken a more acceptance and grief-based approach, to help him adjust to a chronic, potentially life-limiting condition. Because this diagnosis was ruled out, and his cognitive complaints were determined to be secondary to a core diagnosis of PTSD, therapy instead focused on treating PTSD.
Continue to: Case 2
Case 2
A 55-year-old with bipolar I disorder
Mr. S, age 55, is taken to the emergency department (ED) because of his complaints of a severe headache. While undergoing brain MRI, Mr. S becomes highly agitated and aggressive to the radiology staff and is transferred to the psychiatric inpatient unit. He has a history of bipolar disorder that was treated with lithium approximately 20 years ago. Due to continued agitation, he is transferred to the state hospital and prescribed multiple medications, including an unspecified first-generation antipsychotic (FGA) that results in drooling and causes him to stoop and shuffle.
Mr. S’s wife contacts a community psychiatrist after becoming frustrated by her inability to communicate with the staff at the state hospital. During a 1-hour consult, she reveals that Mr. S was a competitive speedboat racer and had suffered numerous concussions due to accidents; at least 3 of these concussions that occurred when he was in his 20s and 30s had included a loss of consciousness. Mr. S had always been treated in the ED, and never required hospitalization. He had a previous marriage, was estranged from his ex-wife and 3 children, and has a history of alcohol abuse.
The MRI taken in the ED reveals numerous patches of scar tissue throughout the cortex, most notably in the striatum areas. The psychiatrist suspects that Mr. S’s agitation and irritation were related to focal seizure activity. He encourages Mr. S’s wife to speak with the attending psychiatrist at the state hospital and ask for him to be discharged home under her care.
Eventually, Mr. S is referred for a neurologic consult and neuropsychological testing. The testing included measures of attention and working, learning and memory, and executive functioning. The results reveal numerous deficits that Mr. S had been able to compensate for when he was younger, including problems with recall of newly learned information and difficulty modifying his behavior according to feedback. Mr. S is weaned from high doses of the FGA and is stabilized on 2 antiepileptic agents, sertraline, and low-dose olanzapine. A rehabilitation plan is developed, and Mr. S remains out of the hospital.
A team-based approach
Psychiatric clinicians need to recognize the subtle as well as overt cognitive deficits present in patients with many of the illnesses that we treat on a daily basis. In this era of performance- and value-based care, it is important to understand the common neuropsychological tests available to assist in providing patient-centered care tailored to specific cognitive deficits. Including a neuropsychologist is essential to implementing a team-based approach.
Continue to: Bottom Line
Bottom Line
Neuropsychological testing can help pinpoint key cognitive deficits that interfere with the potential for optimal patient outcomes. Psychiatric clinicians need to be knowledgeable about the common tests used and how to incorporate the results into their diagnosis and treatment plans.
Related Resources
- The American Academy of Clinical Neuropsychology. www.theaacn.org.
- Schwarz L. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
Drug Brand Names
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Sertraline • Zoloft
1. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing: how to understand it. Practical Neurology. 2018;18(3):227-237.
2. de la Serna E, Sugranyes G, Sanchez-Gistau V, et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110-115.
3. Gómez-Benito J, Guilera G, Pino Ó, et al. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry. 2013;13:127.
4. Fuente-Tomas L, Arranz B, Safont G, et al. Classification of patients with bipolar disorder using k-means clustering. PLoS One. 2019;14(1):e0210314.
5. Kronbichler L, Stelzig-Schöler R, Pearce BG, et al. Schizophrenia and category-selectivity in the brain: Normal for faces but abnormal for houses. Front Psychiatry. 2018;9:47.
6. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
7. Yesavage A, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37-49.
8. Ben-Porath YS, Tellegen A. Minnesota multi-phasic personality inventory-2 restructured form: MMPI-2-RF. San Antonio, TX: NCS Pearson; 2008.
9. Morey LC. Personality assessment inventory. Odessa, FL: Psychological Assessment Resources; 1991.
10. Donder J, ed. Neuropsychological report writing. New York, NY: The Guilford Press; 2016.
11. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Evaluation and management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/eval-mgmt-serv-guide-ICN006764.pdf. Published August 2017. Accessed October 10, 2019.
12. Hunt S, Root JC, Bascetta BL. Effort testing in schizophrenia and schizoaffective disorder: validity indicator profile and test of memory malingering performance characteristics. Arch Clin Neuropsychol. 2014;29(2):164-172.
13. Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res. 2015;225(3):407-412.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22(11):1019-1031.
15. Urfer-Parnas, A, Mortensen EL, Parnas J. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300-311.
16. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biolog Psych. 2004;56(5):301-307.
17. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1-3.
18. Kern RS, Green MF, Nuechterlein KH, et al. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72(1):11-19.
19. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131-1137.
20. Geyer M. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psych Sci. 2010;19(4):264-269.
21. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psych. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197.
22. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413-419.
23. Najas-Garcia A, Gomez-Benito J, Hueda-Medina T. The relationship of motivation and neurocognition with functionality in schizophrenia: a meta-analytic review. Community Ment Health J. 2018;54(7):1019-1049.
24. Raghavan DV, Shanmugiah A, Bharathi P, et al. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J Psychiatry. 2016;58(4):454-458.
25. Mozzambani A, Fuso S, Malta S, et al. Long-term follow-up of attentional and executive functions of PTSD patients. Psychol Neurosci. 2017;10(2):215-224.
26. Woon F, Farrer T, Braman C, et al A meta-analysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn Neuropsychiatry. 2017;22(1):1-16.
We have all treated a patient for whom you know you had the diagnosis correct, the medication regimen was working, and the patient adhered to treatment, but something was still “off.” There was something cognitively that wasn’t right, and you had identified subtle (and some overt) errors in the standard psychiatric cognitive assessment that didn’t seem amenable to psychotropic medications. Perhaps what was needed was neuropsychological testing, one of the most useful but underutilized resources available to help fine-tune diagnosis and treatment. Finding a neuropsychologist who is sensitive to the unique needs of patients with psychiatric disorders, and knowing what and how to communicate the clinical picture and need for the referral, can be challenging due to the limited availability, time, and cost of a full battery of standardized tests.
This article describes the purpose of neuropsychological testing, why it is an important part of psychiatry, and how to make the best use of it.
What is neuropsychological testing?
Neuropsychological testing is a comprehensive evaluation designed to assess cognitive functioning, such as attention, language, learning, memory, and visuospatial and executive functioning. Neuropsychology has its own vocabulary and lexicon that are important for psychiatric clinicians to understand. Some terms, such as aphasia, working memory, and dementia, are familiar to many clinicians. However, others, such as information processing speed, performance validity testing, and semantic memory, might not be. Common neuropsychological terms are defined in Table 1.
The neuropsychologist’s role
A neuropsychologist is a psychologist with advanced training in brain-behavior relationships who can help determine if cognitive problems are related to neurologic, medical, or psychiatric factors. A neuropsychological evaluation can identify the etiology of a patient’s cognitive difficulties, such as stroke, poorly controlled diabetes, or mental health symptoms, to help guide treatment. It can be difficult to determine if a patient who is experiencing significant cognitive, functional, or behavioral changes has an underlying cognitive disorder (eg, dementia or major neurocognitive disorder) or something else, such as a psychiatric condition. Indeed, many psychiatric conditions, including schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), and major depressive disorder (MDD), can present with significant cognitive difficulties. Thus, when patients report an increase in forgetfulness or changes in their ability to care for themselves, neuropsychological testing can help determine the cause.
How to refer to a neuropsychologist
Developing a referral network with a neuropsychologist should be a component of establishing a psychiatric practice. A neuropsychologist can help identify deficits that may interfere with the patient’s ability to adhere to a treatment plan, monitor medications, or actively participate in treatment and therapy. When making a referral for neuropsychological testing, it is important to be clear about the specific concerns so the neuropsychologist knows how to best evaluate the patient. A psychiatric clinician does not order specific neuropsychological tests, but thoroughly describes the problem so the neuropsychologist can determine the appropriate tests after interviewing the patient. For example, if a patient reports memory problems, it is essential to give the neuropsychologist specific clinical data so he/she can determine if the symptoms are due to a neurodegenerative or psychiatric condition. Then, after interviewing the patient (and, possibly, a family member), the neuropsychologist can construct a battery of tests to best answer the question.
Which neuropsychological tests are available?
There is a large battery of neuropsychological tests that require a licensed psychologist to administer and interpret.1 Those commonly used in research and practice to differentiate neurologically-based cognitive deficits associated with psychiatric disorders include the Wechsler Adult Intelligence Scale-4th edition (WAIS-IV) for assessing intelligence, the California Verbal Learning Test-Third Edition (CVLT-3) for verbal memory and learning, the Brief Visuospatial Memory Test-Revised for visual memory, the Wisconsin Card Sorting Test (WCST) for executive functions, and the Ruff 2&7 Selective Attention Test for sustained attention.2 These and other commonly used tests are described in Table 2.1
Neuropsychological testing vs psychological testing
The neuropsychologist will use psychometric properties (such as the validity and reliability of the test) and available normative data to pick the most appropriate tests. To date, there are no specific tests that clearly delineate psychiatric from nonpsychiatric etiologies, although the Screen for Cognitive Impairment in Psychiatry (SCIP)3 was developed in 2013 to explore cognitive abilities in the functional psychoses; it is beginning to be used in other studies.4,5 The neuropsychologist will consider the patient’s current concerns, the onset and progression of these concerns, and the pattern in testing behavior to help determine if psychiatric conditions are the most likely etiology.
Continue to: In addition to cognitive tests...
In addition to cognitive tests, the neuropsychologist might also administer psychological tests. These might include commonly used screening tools such as the Patient Health Questionnaire-9 (PHQ-9)6 or Geriatric Depression Scale (GDS),7 or more comprehensive objective personality measures, such as the Minnesota Multiphasic Personality Inventory-2-Restructured Format (MMPI-2-RF)8 or Personality Assessment Inventory (PAI).9 These tests, along with a thorough clinical history, can help identify if a psychiatric condition is present. In addition, for the more extensive tests such as the MMPI-2-RF or PAI, there are certain neuropsychological profiles that are consistent with a psychiatric etiology for cognitive difficulties. These profiles are formulated based on specific test scores in combination with complex patient variables.
Understanding the report
While there will be stylistic differences in reports depending on the neuropsychologist’s setting, referral source, and personal preferences, most will include discussion of why the patient was referred for evaluation and a description of the onset and progression of the problem.10 Reports often also include pertinent medical and psychiatric history, substance use history, and family medical history. A section on social history is important to help establish premorbid functioning, and might include information about prenatal/birth complications, developmental milestones, educational history, and occupational history. Information about current psychosocial support or stressors, including marital status or current/past legal issues, can be helpful. In addition to this history, there is often a section on behavioral observations, especially if anything stood out or might have affected the validity of the data.
There are also objective measures of validity that the neuropsychologist might administer to evaluate whether the results are valid. Issues of validity are monitored through the evaluation, and are used to determine if the results are consistent with known neurologic patterns. If the results are deemed not valid, then low scores cannot be reliably interpreted as evidence of impairment. This is akin to an arm moving during an X-ray, thereby blurring the results. If valid, the results of objective testing are include in the neuropsychologist’s report; this can range from providing raw scores, standard scores, and/or percentiles to a general description of how the patient did on testing.
The section that is usually of most interest to psychiatric clinicians is the summary, which explains the results, might offer a diagnosis, and discusses possible etiologies. This might be where the neuropsychologist discusses if the findings are due to a neurologic or psychiatric condition. From this comes the neuropsychologist’s recommendations. When a psychiatric condition is determined to be the underlying etiology, the neuropsychologist might recommend psychotherapy or some other psychiatric treatment.
Why is neuropsychological testing important?
Schizophrenia, MDD, bipolar disorder, and PTSD produce significant neurobiologic changes that often result in deterioration of a patient’s global cognitive function. Increased emphasis and attention in psychiatric research have yielded more clues to the neurobiology of cognition. However, even though many psychiatric clinicians are trained in cognitive assessments, such as the “clock test,” “serial sevens,” “numbers forward and backward,” “proverb,” and “word recall,” and common scenarios to evaluate judgment and insight, such as “mailing a letter” and “smoke in a movie theatre,” most of these components are not completed during a standard psychiatric evaluation. Because the time allotted to completing a psychiatric evaluation continues to be shortened, it is sometimes difficult to complete the “6 bullets” required by the Centers for Medicare & Medicaid Services as part of the mental status exam (Table 311).
Continue to: To date, the best evidence...
To date, the best evidence for neuropsychological deficits exists for patients with schizophrenia, bipolar disorder, MDD, and PTSD.12,13 The Box2,14-24 describes the findings of studies of neuropsychological deficits in patients with schizophrenia and bipolar disorder.
Box
Patients with schizophrenia have been the subjects of neuropsychological testing for decades. The results have shown deficits on many standardized tests, including those of attention, memory, and executive functioning, although some patients might perform within normal limits.15
A federal initiative through the National Institute of Mental Health (NIMH) known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) was developed in the late 1990s to develop consensus on the underlying cognitive deficits in schizophrenia. MATRICS was created with the hopes that it would allow the FDA to approve treatments for those cognitive deficits independent of psychosis because current psychotropic medications have minimal efficacy on cognition.16,17 The MATRICS group identified working memory, attention/vigilance, verbal learning and memory, visual learning and memory, speed of processing, reasoning and problem solving, and social cognition as the key cognitive domains most affected in schizophrenia.14 The initial program has since evolved into 3 distinct NIMH programs: CNTRICS18 (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia), TURNS19 (Treatment Units for Research on Neurocognition in Schizophrenia), and TENETS20 (Treatment and Evaluation Network for Trials in Schizophrenia). The combination of neuropsychological testing and neuroimaging has led to the conceptualization of schizophrenia as a neurodevelopmental disorder.
Individuals at risk for psychosis
As clinicians, we have long heard from parents of children with schizophrenia a standard trajectory of functional decline: early premorbid changes, a fairly measurable prodromal period marked by subtle deterioration in cognitive functioning, followed by the actual illness trajectory. In a recent meta-analysis, researchers compared the results of 60 neuropsychological tests comprising 9 domains in people who were at clinical high risk for psychosis who eventually converted to a psychotic disorder (CHR-P), those at clinical high risk who did not convert to psychosis (CHR-NP), and healthy controls.21 They found that neuropsychological performance deficits were greater in CHR-P individuals than in those in the CHR-NP group, and both had greater deficits than healthy controls.
For many patients with schizophrenia, full cognitive maturation is never reached.22 In general, decreased motivation in schizophrenia has been correlated with neurocognitive deficits.23
Schizophrenia vs bipolar disorder
In a study comparing neuropsychological functioning in patients with schizophrenia and bipolar disorder with psychotic features (BP-P), researchers found greater deficits in schizophrenia, including immediate verbal recall, working memory, processing speed, and verbal fluency.22 Patients with BP-P demonstrated impairment consistent with generalized impairment in verbal learning and memory, working memory, and processing speed.22
Children/adolescents
In a recent study comparing child and adolescent offspring of patients with schizophrenia (n = 41) and bipolar disorder (n = 90), researchers identified neuropsychological deficits in visual memory for both groups, suggesting common genetic linkages. The schizophrenia offspring scored lower in verbal memory and word memory, while bipolar offspring scored lower on the processing speed index and visual memory.2
Information processing
Another study compared the results of neuropsychological testing and the P300 component of auditory event-related potential (an electrophysiological measure) in 30 patients with schizophrenia, siblings without illness, and normal controls.24 The battery of neuropsychological tests included the Digit Symbol Substitution Test, Digit Vigilance Test, Trail Making Test-B, and Stroop test. The P300 is well correlated with information processing. Researchers found decreased P300 amplitude and latency in the patients and normal levels in the controls; siblings scored somewhere in between.24 Scores on the neuropsychological tests were consistently below normal in both patients and their siblings, with patients scoring the lowest.24
Continue to: Neuropsychological testing
Neuropsychological testing: 2 Case studies
The following 2 cases illustrate the pivotal role of neuropsychological testing in formulating an accurate differential diagnosis, and facilitating improved outcomes.
Case 1
A veteran with PTSD and memory complaints
Mr. J, age 70, is a married man who spent his career in the military, including combat service in the Vietnam War. His service in Vietnam included an event in which he couldn’t save platoon members from an ambush and death in a firefight, after which he developed PTSD. He retired after 25 years of service.
Mr. J’s psychiatrist refers him to a neuropsychologist for complaints of memory difficulties, including a fear that he’s developing Alzheimer’s disease (AD). Because of the concern for AD, he undergoes tests of learning and memory, such as the CVLT-3, the Brief Visuospatial Memory Test-Revised, and the Logical Memory subtest from the Wechsler Memory Scale–4th Edition. Other tests include a measure of confrontation naming, verbal fluency (phonemic and semantic fluency), construction, attention, processing speed, and problem solving. In addition, a measure of psychiatric and emotional functioning is also administered (the MMPI-2-RF).
The results determined that Mr. J’s subjective experience of recall deficits is better explained by anxiety resulting from the cumulative impact of day-to-day emotional stress in the setting of chronic PTSD.25 Mr. J was experiencing cognitive sequelae from a complicated emotional dynamic, comprised of situational stress, amplified by coping difficulties that were rooted in older posttraumatic symptoms. These emotions, and the cognitive load they generated, interfered with the normal processes of attention and organization necessary for the encoding of information to be remembered.26 He described being visibly angered by the clutter in his home (the result of multiple people living there, including a young grandchild), having his efforts to get things done interrupted by the needs of others, and a perceived loss of control gradually generalized to even mundane circumstances, as often occurs with traumatic responses. In short, he was chronically overwhelmed and not experiencing the beginnings of dementia.
For Mr. J, neuropsychological testing helped define the focus and course of therapy. If he had been diagnosed with a major neurocognitive disorder, therapy might have taken a more acceptance and grief-based approach, to help him adjust to a chronic, potentially life-limiting condition. Because this diagnosis was ruled out, and his cognitive complaints were determined to be secondary to a core diagnosis of PTSD, therapy instead focused on treating PTSD.
Continue to: Case 2
Case 2
A 55-year-old with bipolar I disorder
Mr. S, age 55, is taken to the emergency department (ED) because of his complaints of a severe headache. While undergoing brain MRI, Mr. S becomes highly agitated and aggressive to the radiology staff and is transferred to the psychiatric inpatient unit. He has a history of bipolar disorder that was treated with lithium approximately 20 years ago. Due to continued agitation, he is transferred to the state hospital and prescribed multiple medications, including an unspecified first-generation antipsychotic (FGA) that results in drooling and causes him to stoop and shuffle.
Mr. S’s wife contacts a community psychiatrist after becoming frustrated by her inability to communicate with the staff at the state hospital. During a 1-hour consult, she reveals that Mr. S was a competitive speedboat racer and had suffered numerous concussions due to accidents; at least 3 of these concussions that occurred when he was in his 20s and 30s had included a loss of consciousness. Mr. S had always been treated in the ED, and never required hospitalization. He had a previous marriage, was estranged from his ex-wife and 3 children, and has a history of alcohol abuse.
The MRI taken in the ED reveals numerous patches of scar tissue throughout the cortex, most notably in the striatum areas. The psychiatrist suspects that Mr. S’s agitation and irritation were related to focal seizure activity. He encourages Mr. S’s wife to speak with the attending psychiatrist at the state hospital and ask for him to be discharged home under her care.
Eventually, Mr. S is referred for a neurologic consult and neuropsychological testing. The testing included measures of attention and working, learning and memory, and executive functioning. The results reveal numerous deficits that Mr. S had been able to compensate for when he was younger, including problems with recall of newly learned information and difficulty modifying his behavior according to feedback. Mr. S is weaned from high doses of the FGA and is stabilized on 2 antiepileptic agents, sertraline, and low-dose olanzapine. A rehabilitation plan is developed, and Mr. S remains out of the hospital.
A team-based approach
Psychiatric clinicians need to recognize the subtle as well as overt cognitive deficits present in patients with many of the illnesses that we treat on a daily basis. In this era of performance- and value-based care, it is important to understand the common neuropsychological tests available to assist in providing patient-centered care tailored to specific cognitive deficits. Including a neuropsychologist is essential to implementing a team-based approach.
Continue to: Bottom Line
Bottom Line
Neuropsychological testing can help pinpoint key cognitive deficits that interfere with the potential for optimal patient outcomes. Psychiatric clinicians need to be knowledgeable about the common tests used and how to incorporate the results into their diagnosis and treatment plans.
Related Resources
- The American Academy of Clinical Neuropsychology. www.theaacn.org.
- Schwarz L. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
Drug Brand Names
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Sertraline • Zoloft
We have all treated a patient for whom you know you had the diagnosis correct, the medication regimen was working, and the patient adhered to treatment, but something was still “off.” There was something cognitively that wasn’t right, and you had identified subtle (and some overt) errors in the standard psychiatric cognitive assessment that didn’t seem amenable to psychotropic medications. Perhaps what was needed was neuropsychological testing, one of the most useful but underutilized resources available to help fine-tune diagnosis and treatment. Finding a neuropsychologist who is sensitive to the unique needs of patients with psychiatric disorders, and knowing what and how to communicate the clinical picture and need for the referral, can be challenging due to the limited availability, time, and cost of a full battery of standardized tests.
This article describes the purpose of neuropsychological testing, why it is an important part of psychiatry, and how to make the best use of it.
What is neuropsychological testing?
Neuropsychological testing is a comprehensive evaluation designed to assess cognitive functioning, such as attention, language, learning, memory, and visuospatial and executive functioning. Neuropsychology has its own vocabulary and lexicon that are important for psychiatric clinicians to understand. Some terms, such as aphasia, working memory, and dementia, are familiar to many clinicians. However, others, such as information processing speed, performance validity testing, and semantic memory, might not be. Common neuropsychological terms are defined in Table 1.
The neuropsychologist’s role
A neuropsychologist is a psychologist with advanced training in brain-behavior relationships who can help determine if cognitive problems are related to neurologic, medical, or psychiatric factors. A neuropsychological evaluation can identify the etiology of a patient’s cognitive difficulties, such as stroke, poorly controlled diabetes, or mental health symptoms, to help guide treatment. It can be difficult to determine if a patient who is experiencing significant cognitive, functional, or behavioral changes has an underlying cognitive disorder (eg, dementia or major neurocognitive disorder) or something else, such as a psychiatric condition. Indeed, many psychiatric conditions, including schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), and major depressive disorder (MDD), can present with significant cognitive difficulties. Thus, when patients report an increase in forgetfulness or changes in their ability to care for themselves, neuropsychological testing can help determine the cause.
How to refer to a neuropsychologist
Developing a referral network with a neuropsychologist should be a component of establishing a psychiatric practice. A neuropsychologist can help identify deficits that may interfere with the patient’s ability to adhere to a treatment plan, monitor medications, or actively participate in treatment and therapy. When making a referral for neuropsychological testing, it is important to be clear about the specific concerns so the neuropsychologist knows how to best evaluate the patient. A psychiatric clinician does not order specific neuropsychological tests, but thoroughly describes the problem so the neuropsychologist can determine the appropriate tests after interviewing the patient. For example, if a patient reports memory problems, it is essential to give the neuropsychologist specific clinical data so he/she can determine if the symptoms are due to a neurodegenerative or psychiatric condition. Then, after interviewing the patient (and, possibly, a family member), the neuropsychologist can construct a battery of tests to best answer the question.
Which neuropsychological tests are available?
There is a large battery of neuropsychological tests that require a licensed psychologist to administer and interpret.1 Those commonly used in research and practice to differentiate neurologically-based cognitive deficits associated with psychiatric disorders include the Wechsler Adult Intelligence Scale-4th edition (WAIS-IV) for assessing intelligence, the California Verbal Learning Test-Third Edition (CVLT-3) for verbal memory and learning, the Brief Visuospatial Memory Test-Revised for visual memory, the Wisconsin Card Sorting Test (WCST) for executive functions, and the Ruff 2&7 Selective Attention Test for sustained attention.2 These and other commonly used tests are described in Table 2.1
Neuropsychological testing vs psychological testing
The neuropsychologist will use psychometric properties (such as the validity and reliability of the test) and available normative data to pick the most appropriate tests. To date, there are no specific tests that clearly delineate psychiatric from nonpsychiatric etiologies, although the Screen for Cognitive Impairment in Psychiatry (SCIP)3 was developed in 2013 to explore cognitive abilities in the functional psychoses; it is beginning to be used in other studies.4,5 The neuropsychologist will consider the patient’s current concerns, the onset and progression of these concerns, and the pattern in testing behavior to help determine if psychiatric conditions are the most likely etiology.
Continue to: In addition to cognitive tests...
In addition to cognitive tests, the neuropsychologist might also administer psychological tests. These might include commonly used screening tools such as the Patient Health Questionnaire-9 (PHQ-9)6 or Geriatric Depression Scale (GDS),7 or more comprehensive objective personality measures, such as the Minnesota Multiphasic Personality Inventory-2-Restructured Format (MMPI-2-RF)8 or Personality Assessment Inventory (PAI).9 These tests, along with a thorough clinical history, can help identify if a psychiatric condition is present. In addition, for the more extensive tests such as the MMPI-2-RF or PAI, there are certain neuropsychological profiles that are consistent with a psychiatric etiology for cognitive difficulties. These profiles are formulated based on specific test scores in combination with complex patient variables.
Understanding the report
While there will be stylistic differences in reports depending on the neuropsychologist’s setting, referral source, and personal preferences, most will include discussion of why the patient was referred for evaluation and a description of the onset and progression of the problem.10 Reports often also include pertinent medical and psychiatric history, substance use history, and family medical history. A section on social history is important to help establish premorbid functioning, and might include information about prenatal/birth complications, developmental milestones, educational history, and occupational history. Information about current psychosocial support or stressors, including marital status or current/past legal issues, can be helpful. In addition to this history, there is often a section on behavioral observations, especially if anything stood out or might have affected the validity of the data.
There are also objective measures of validity that the neuropsychologist might administer to evaluate whether the results are valid. Issues of validity are monitored through the evaluation, and are used to determine if the results are consistent with known neurologic patterns. If the results are deemed not valid, then low scores cannot be reliably interpreted as evidence of impairment. This is akin to an arm moving during an X-ray, thereby blurring the results. If valid, the results of objective testing are include in the neuropsychologist’s report; this can range from providing raw scores, standard scores, and/or percentiles to a general description of how the patient did on testing.
The section that is usually of most interest to psychiatric clinicians is the summary, which explains the results, might offer a diagnosis, and discusses possible etiologies. This might be where the neuropsychologist discusses if the findings are due to a neurologic or psychiatric condition. From this comes the neuropsychologist’s recommendations. When a psychiatric condition is determined to be the underlying etiology, the neuropsychologist might recommend psychotherapy or some other psychiatric treatment.
Why is neuropsychological testing important?
Schizophrenia, MDD, bipolar disorder, and PTSD produce significant neurobiologic changes that often result in deterioration of a patient’s global cognitive function. Increased emphasis and attention in psychiatric research have yielded more clues to the neurobiology of cognition. However, even though many psychiatric clinicians are trained in cognitive assessments, such as the “clock test,” “serial sevens,” “numbers forward and backward,” “proverb,” and “word recall,” and common scenarios to evaluate judgment and insight, such as “mailing a letter” and “smoke in a movie theatre,” most of these components are not completed during a standard psychiatric evaluation. Because the time allotted to completing a psychiatric evaluation continues to be shortened, it is sometimes difficult to complete the “6 bullets” required by the Centers for Medicare & Medicaid Services as part of the mental status exam (Table 311).
Continue to: To date, the best evidence...
To date, the best evidence for neuropsychological deficits exists for patients with schizophrenia, bipolar disorder, MDD, and PTSD.12,13 The Box2,14-24 describes the findings of studies of neuropsychological deficits in patients with schizophrenia and bipolar disorder.
Box
Patients with schizophrenia have been the subjects of neuropsychological testing for decades. The results have shown deficits on many standardized tests, including those of attention, memory, and executive functioning, although some patients might perform within normal limits.15
A federal initiative through the National Institute of Mental Health (NIMH) known as MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) was developed in the late 1990s to develop consensus on the underlying cognitive deficits in schizophrenia. MATRICS was created with the hopes that it would allow the FDA to approve treatments for those cognitive deficits independent of psychosis because current psychotropic medications have minimal efficacy on cognition.16,17 The MATRICS group identified working memory, attention/vigilance, verbal learning and memory, visual learning and memory, speed of processing, reasoning and problem solving, and social cognition as the key cognitive domains most affected in schizophrenia.14 The initial program has since evolved into 3 distinct NIMH programs: CNTRICS18 (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia), TURNS19 (Treatment Units for Research on Neurocognition in Schizophrenia), and TENETS20 (Treatment and Evaluation Network for Trials in Schizophrenia). The combination of neuropsychological testing and neuroimaging has led to the conceptualization of schizophrenia as a neurodevelopmental disorder.
Individuals at risk for psychosis
As clinicians, we have long heard from parents of children with schizophrenia a standard trajectory of functional decline: early premorbid changes, a fairly measurable prodromal period marked by subtle deterioration in cognitive functioning, followed by the actual illness trajectory. In a recent meta-analysis, researchers compared the results of 60 neuropsychological tests comprising 9 domains in people who were at clinical high risk for psychosis who eventually converted to a psychotic disorder (CHR-P), those at clinical high risk who did not convert to psychosis (CHR-NP), and healthy controls.21 They found that neuropsychological performance deficits were greater in CHR-P individuals than in those in the CHR-NP group, and both had greater deficits than healthy controls.
For many patients with schizophrenia, full cognitive maturation is never reached.22 In general, decreased motivation in schizophrenia has been correlated with neurocognitive deficits.23
Schizophrenia vs bipolar disorder
In a study comparing neuropsychological functioning in patients with schizophrenia and bipolar disorder with psychotic features (BP-P), researchers found greater deficits in schizophrenia, including immediate verbal recall, working memory, processing speed, and verbal fluency.22 Patients with BP-P demonstrated impairment consistent with generalized impairment in verbal learning and memory, working memory, and processing speed.22
Children/adolescents
In a recent study comparing child and adolescent offspring of patients with schizophrenia (n = 41) and bipolar disorder (n = 90), researchers identified neuropsychological deficits in visual memory for both groups, suggesting common genetic linkages. The schizophrenia offspring scored lower in verbal memory and word memory, while bipolar offspring scored lower on the processing speed index and visual memory.2
Information processing
Another study compared the results of neuropsychological testing and the P300 component of auditory event-related potential (an electrophysiological measure) in 30 patients with schizophrenia, siblings without illness, and normal controls.24 The battery of neuropsychological tests included the Digit Symbol Substitution Test, Digit Vigilance Test, Trail Making Test-B, and Stroop test. The P300 is well correlated with information processing. Researchers found decreased P300 amplitude and latency in the patients and normal levels in the controls; siblings scored somewhere in between.24 Scores on the neuropsychological tests were consistently below normal in both patients and their siblings, with patients scoring the lowest.24
Continue to: Neuropsychological testing
Neuropsychological testing: 2 Case studies
The following 2 cases illustrate the pivotal role of neuropsychological testing in formulating an accurate differential diagnosis, and facilitating improved outcomes.
Case 1
A veteran with PTSD and memory complaints
Mr. J, age 70, is a married man who spent his career in the military, including combat service in the Vietnam War. His service in Vietnam included an event in which he couldn’t save platoon members from an ambush and death in a firefight, after which he developed PTSD. He retired after 25 years of service.
Mr. J’s psychiatrist refers him to a neuropsychologist for complaints of memory difficulties, including a fear that he’s developing Alzheimer’s disease (AD). Because of the concern for AD, he undergoes tests of learning and memory, such as the CVLT-3, the Brief Visuospatial Memory Test-Revised, and the Logical Memory subtest from the Wechsler Memory Scale–4th Edition. Other tests include a measure of confrontation naming, verbal fluency (phonemic and semantic fluency), construction, attention, processing speed, and problem solving. In addition, a measure of psychiatric and emotional functioning is also administered (the MMPI-2-RF).
The results determined that Mr. J’s subjective experience of recall deficits is better explained by anxiety resulting from the cumulative impact of day-to-day emotional stress in the setting of chronic PTSD.25 Mr. J was experiencing cognitive sequelae from a complicated emotional dynamic, comprised of situational stress, amplified by coping difficulties that were rooted in older posttraumatic symptoms. These emotions, and the cognitive load they generated, interfered with the normal processes of attention and organization necessary for the encoding of information to be remembered.26 He described being visibly angered by the clutter in his home (the result of multiple people living there, including a young grandchild), having his efforts to get things done interrupted by the needs of others, and a perceived loss of control gradually generalized to even mundane circumstances, as often occurs with traumatic responses. In short, he was chronically overwhelmed and not experiencing the beginnings of dementia.
For Mr. J, neuropsychological testing helped define the focus and course of therapy. If he had been diagnosed with a major neurocognitive disorder, therapy might have taken a more acceptance and grief-based approach, to help him adjust to a chronic, potentially life-limiting condition. Because this diagnosis was ruled out, and his cognitive complaints were determined to be secondary to a core diagnosis of PTSD, therapy instead focused on treating PTSD.
Continue to: Case 2
Case 2
A 55-year-old with bipolar I disorder
Mr. S, age 55, is taken to the emergency department (ED) because of his complaints of a severe headache. While undergoing brain MRI, Mr. S becomes highly agitated and aggressive to the radiology staff and is transferred to the psychiatric inpatient unit. He has a history of bipolar disorder that was treated with lithium approximately 20 years ago. Due to continued agitation, he is transferred to the state hospital and prescribed multiple medications, including an unspecified first-generation antipsychotic (FGA) that results in drooling and causes him to stoop and shuffle.
Mr. S’s wife contacts a community psychiatrist after becoming frustrated by her inability to communicate with the staff at the state hospital. During a 1-hour consult, she reveals that Mr. S was a competitive speedboat racer and had suffered numerous concussions due to accidents; at least 3 of these concussions that occurred when he was in his 20s and 30s had included a loss of consciousness. Mr. S had always been treated in the ED, and never required hospitalization. He had a previous marriage, was estranged from his ex-wife and 3 children, and has a history of alcohol abuse.
The MRI taken in the ED reveals numerous patches of scar tissue throughout the cortex, most notably in the striatum areas. The psychiatrist suspects that Mr. S’s agitation and irritation were related to focal seizure activity. He encourages Mr. S’s wife to speak with the attending psychiatrist at the state hospital and ask for him to be discharged home under her care.
Eventually, Mr. S is referred for a neurologic consult and neuropsychological testing. The testing included measures of attention and working, learning and memory, and executive functioning. The results reveal numerous deficits that Mr. S had been able to compensate for when he was younger, including problems with recall of newly learned information and difficulty modifying his behavior according to feedback. Mr. S is weaned from high doses of the FGA and is stabilized on 2 antiepileptic agents, sertraline, and low-dose olanzapine. A rehabilitation plan is developed, and Mr. S remains out of the hospital.
A team-based approach
Psychiatric clinicians need to recognize the subtle as well as overt cognitive deficits present in patients with many of the illnesses that we treat on a daily basis. In this era of performance- and value-based care, it is important to understand the common neuropsychological tests available to assist in providing patient-centered care tailored to specific cognitive deficits. Including a neuropsychologist is essential to implementing a team-based approach.
Continue to: Bottom Line
Bottom Line
Neuropsychological testing can help pinpoint key cognitive deficits that interfere with the potential for optimal patient outcomes. Psychiatric clinicians need to be knowledgeable about the common tests used and how to incorporate the results into their diagnosis and treatment plans.
Related Resources
- The American Academy of Clinical Neuropsychology. www.theaacn.org.
- Schwarz L. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
Drug Brand Names
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Sertraline • Zoloft
1. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing: how to understand it. Practical Neurology. 2018;18(3):227-237.
2. de la Serna E, Sugranyes G, Sanchez-Gistau V, et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110-115.
3. Gómez-Benito J, Guilera G, Pino Ó, et al. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry. 2013;13:127.
4. Fuente-Tomas L, Arranz B, Safont G, et al. Classification of patients with bipolar disorder using k-means clustering. PLoS One. 2019;14(1):e0210314.
5. Kronbichler L, Stelzig-Schöler R, Pearce BG, et al. Schizophrenia and category-selectivity in the brain: Normal for faces but abnormal for houses. Front Psychiatry. 2018;9:47.
6. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
7. Yesavage A, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37-49.
8. Ben-Porath YS, Tellegen A. Minnesota multi-phasic personality inventory-2 restructured form: MMPI-2-RF. San Antonio, TX: NCS Pearson; 2008.
9. Morey LC. Personality assessment inventory. Odessa, FL: Psychological Assessment Resources; 1991.
10. Donder J, ed. Neuropsychological report writing. New York, NY: The Guilford Press; 2016.
11. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Evaluation and management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/eval-mgmt-serv-guide-ICN006764.pdf. Published August 2017. Accessed October 10, 2019.
12. Hunt S, Root JC, Bascetta BL. Effort testing in schizophrenia and schizoaffective disorder: validity indicator profile and test of memory malingering performance characteristics. Arch Clin Neuropsychol. 2014;29(2):164-172.
13. Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res. 2015;225(3):407-412.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22(11):1019-1031.
15. Urfer-Parnas, A, Mortensen EL, Parnas J. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300-311.
16. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biolog Psych. 2004;56(5):301-307.
17. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1-3.
18. Kern RS, Green MF, Nuechterlein KH, et al. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72(1):11-19.
19. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131-1137.
20. Geyer M. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psych Sci. 2010;19(4):264-269.
21. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psych. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197.
22. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413-419.
23. Najas-Garcia A, Gomez-Benito J, Hueda-Medina T. The relationship of motivation and neurocognition with functionality in schizophrenia: a meta-analytic review. Community Ment Health J. 2018;54(7):1019-1049.
24. Raghavan DV, Shanmugiah A, Bharathi P, et al. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J Psychiatry. 2016;58(4):454-458.
25. Mozzambani A, Fuso S, Malta S, et al. Long-term follow-up of attentional and executive functions of PTSD patients. Psychol Neurosci. 2017;10(2):215-224.
26. Woon F, Farrer T, Braman C, et al A meta-analysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn Neuropsychiatry. 2017;22(1):1-16.
1. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing: how to understand it. Practical Neurology. 2018;18(3):227-237.
2. de la Serna E, Sugranyes G, Sanchez-Gistau V, et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110-115.
3. Gómez-Benito J, Guilera G, Pino Ó, et al. The screen for cognitive impairment in psychiatry: diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry. 2013;13:127.
4. Fuente-Tomas L, Arranz B, Safont G, et al. Classification of patients with bipolar disorder using k-means clustering. PLoS One. 2019;14(1):e0210314.
5. Kronbichler L, Stelzig-Schöler R, Pearce BG, et al. Schizophrenia and category-selectivity in the brain: Normal for faces but abnormal for houses. Front Psychiatry. 2018;9:47.
6. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
7. Yesavage A, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37-49.
8. Ben-Porath YS, Tellegen A. Minnesota multi-phasic personality inventory-2 restructured form: MMPI-2-RF. San Antonio, TX: NCS Pearson; 2008.
9. Morey LC. Personality assessment inventory. Odessa, FL: Psychological Assessment Resources; 1991.
10. Donder J, ed. Neuropsychological report writing. New York, NY: The Guilford Press; 2016.
11. Department of Health and Human Services, Centers for Medicare and Medicaid Services. Evaluation and management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/eval-mgmt-serv-guide-ICN006764.pdf. Published August 2017. Accessed October 10, 2019.
12. Hunt S, Root JC, Bascetta BL. Effort testing in schizophrenia and schizoaffective disorder: validity indicator profile and test of memory malingering performance characteristics. Arch Clin Neuropsychol. 2014;29(2):164-172.
13. Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res. 2015;225(3):407-412.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22(11):1019-1031.
15. Urfer-Parnas, A, Mortensen EL, Parnas J. Core of schizophrenia: estrangement, dementia or neurocognitive disorder? Psychopathology. 2010;43(5):300-311.
16. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biolog Psych. 2004;56(5):301-307.
17. Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1-3.
18. Kern RS, Green MF, Nuechterlein KH, et al. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72(1):11-19.
19. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131-1137.
20. Geyer M. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psych Sci. 2010;19(4):264-269.
21. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psych. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197.
22. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413-419.
23. Najas-Garcia A, Gomez-Benito J, Hueda-Medina T. The relationship of motivation and neurocognition with functionality in schizophrenia: a meta-analytic review. Community Ment Health J. 2018;54(7):1019-1049.
24. Raghavan DV, Shanmugiah A, Bharathi P, et al. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J Psychiatry. 2016;58(4):454-458.
25. Mozzambani A, Fuso S, Malta S, et al. Long-term follow-up of attentional and executive functions of PTSD patients. Psychol Neurosci. 2017;10(2):215-224.
26. Woon F, Farrer T, Braman C, et al A meta-analysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn Neuropsychiatry. 2017;22(1):1-16.
Bipolar disorder or borderline personality disorder?
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9
Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.
Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).
Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9
Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.
Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).
Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9
Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.
Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).
Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
Losing a patient to suicide: Navigating the aftermath
At some point during their career, many mental health professionals will lose a patient to suicide, but few will be prepared for the experience and its aftermath. As I described in Part 1 of this article (
A chance for growth
Traumatic experiences such as a suicide loss can paradoxically present a multitude of opportunities for new growth and profound personal transformation.1 Such transformation is primarily fostered by social support in the aftermath of the trauma.2
Virtually all of the models of the clinician’s suicide grief trajectory I described in Part 1 not only assume the eventual resolution of the distressing reactions accompanying the original loss, but also suggest that mastery of these reactions can be a catalyst for both personal and professional growth. Clearly, not everyone who experiences such a loss will experience subsequent growth; there are many reports of clinicians leaving the field3 or becoming “burned out” after this occurs. Yet most clinicians who have described this loss in the literature and in discussion groups (including those I’ve conducted) have reported more positive eventual outcomes. It is difficult to establish whether this is due to a cohort effect—clinicians who are most likely to write about their experiences, be interviewed for research studies, and/or to seek out and participate in discussion/support groups may be more prone to find benefits in this experience, either by virtue of their nature or through the subsequent process of sharing these experiences in a supportive atmosphere.
The literature on patient suicide loss, as well as anecdotal reports, confirms that clinicians who experience optimal support are able to identify many retrospective benefits of their experience.4-6 Clinicians generally report that they are better able to identify potential risk and protective factors for suicide, and are more knowledgeable about optimal interventions with individuals who are suicidal. They also describe an increased sensitivity towards patients who are suicidal and those bereaved by suicide. In addition, clinicians report a reduction in therapeutic grandiosity/omnipotence, and more realistic appraisals and expectations in relation to their clinical competence. In their effort to understand the “whys” of their patient’s suicide, they are likely to retrospectively identify errors in treatment, “missed cues,” or things they might subsequently do differently,7 and to learn from these mistakes. Optimally, clinicians become more aware of their own therapeutic limitations, both in the short- and the long-term, and can use this knowledge to better determine how they will continue their clinical work. They also become much more aware of the issues involved in the aftermath of a patient suicide, including perceived gaps in the clinical and institutional systems that could optimally offer support to families and clinicians.
In addition to the positive changes related to knowledge and clinical skills, many clinicians also note deeper personal changes subsequent to their patient’s suicide, consistent with the literature on posttraumatic growth.1 Munson8 explored internal changes in clinicians following a patient suicide and found that in the aftermath, clinicians experienced both posttraumatic growth and compassion fatigue. He also found that the amount of time that elapsed since the patient’s suicide predicted posttraumatic growth, and the seemingly counterintuitive result that the number of years of clinical experience prior to the suicide was negatively correlated with posttraumatic growth.
Huhra et al4 described some of the existential issues that a clinician is likely to confront following a patient suicide. A clinician’s attempt to find a way to meaningfully understand the circumstances around this loss often prompts reflection on mortality, freedom, choice and personal autonomy, and the scope and limits of one’s responsibility toward others. The suicide challenges one’s previous conceptions and expectations around these professional issues, and the clinician must construct new paradigms that serve to integrate these new experiences and perspectives in a coherent way.
One of the most notable sequelae of this (and to other traumatic) experience is a subsequent desire to make use of the learning inherent in these experiences and to “give back.” Once they feel that they have resolved their own grief process, many clinicians express the desire to support others with similar experiences. Even when their experiences have been quite distressing, many clinicians are able to view the suicide as an opportunity to learn about ongoing limitations in the systems of support, and to work toward changing these in a way that ensures that future clinician-survivors will have more supportive experiences. Many view these new perspectives, and their consequent ability to be more helpful, as “unexpected gifts.” They often express gratitude toward the people and resources that have allowed them to make these transformations. Jones5 noted “the tragedy of patient suicide can also be an opportunity for us as therapists to grow in our skills at assessing and intervening in a suicidal crisis, to broaden and deepen the support we give and receive, to grow in our appreciation of the precious gift that life is, and to help each other live it more fully.”
Continue to: Guidelines for postvention
Guidelines for postvention
When a patient suicide occurs in the context of an agency setting, Grad9 recommends prompt responses on 4 levels:
- administrative
- institutional
- educational
- emotional.
Numerous authors5,10-21 have developed suggestions, guidelines, and detailed postvention protocols to help agencies and clinicians in various mental health settings navigate the often-complicated sequelae to a patient’s suicide. The Table highlights a few of these. Most emphasize that information about suicide loss, including both its statistical likelihood and its potential aftermath, should integrated into clinicians’ general education and training. They also suggest that suicide postvention policies and protocols be in place from the outset, and that such information be incorporated into institutional policy and procedure manuals. In addition, they stress that legal, institutional, and administrative needs be balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.
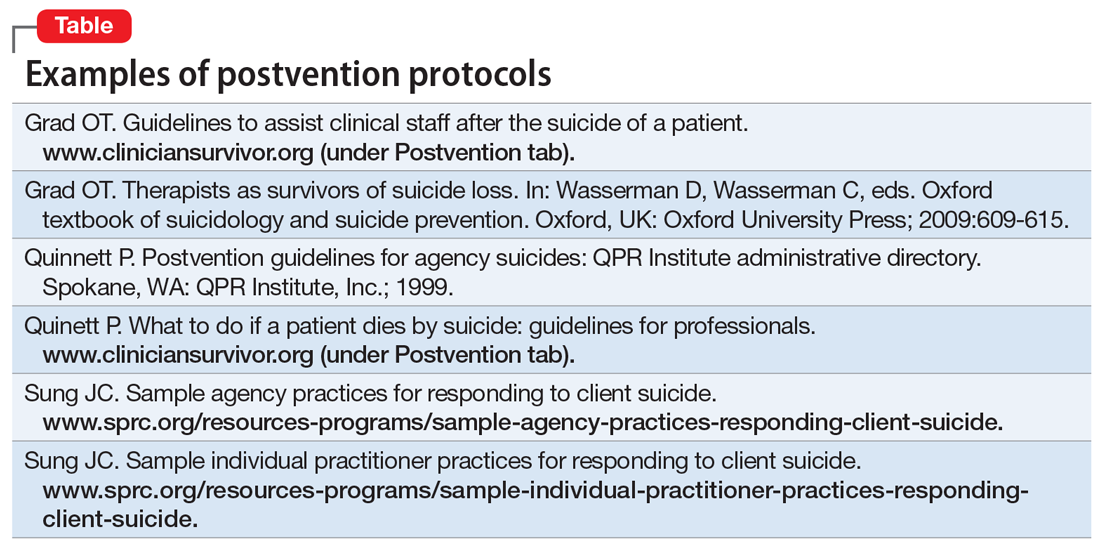
Institutional and administrative procedures
The following are some of the recommended procedures that should take place following a suicide loss. The postvention protocols listed in the Table provide more detailed recommendations.
Legal/ethical. It is essential to consult with a legal representative/risk management specialist associated with the affected agency (ideally, one with specific expertise in suicide litigation.). It is also crucial to clarify who holds privilege after a patient’s death (varies by state), what may and may not be shared under the restrictions of confidentiality and Health Insurance Portability and Accountability Act (HIPAA) laws, and to clarify procedures for chart completion and review. It is also important to clarify the specific information to be shared both within and outside of the agency, and how to address the needs of current patients in the agency settings.
Case review. The optimal purpose of the case review (also known as a psychological autopsy) is to facilitate learning, identify gaps in agency procedures and training, improve pre- and postvention procedures, and help clinicians cope with the loss.22 Again, the legal and administrative needs of the agency need to be balanced with the attention to the emotional impact on the treating clinician.17 Ellis and Patel18 recommend delaying this procedure until the treating clinician is no longer in the “shock” phase of the loss, and is able to think and process events more objectively.
Continue to: Family contact
Family contact. Most authors have recommended that clinicians and/or agencies reach out to surviving families. Although some legal representatives will advise against this, experts in the field of suicide litigation have noted that compassionate family contact reduces liability and facilitates healing for both parties. In a personal communication (May 2008), Eric Harris, of the American Psychological Association Trust, recommended “compassion over caution” when considering these issues. Again, it is important to clarify who holds privilege after a patient’s death in determining when and with whom the patient’s confidential information may be shared. When confidentiality may be broken, clinical judgment should be used to determine how best to present the information to grieving family members.
Even if surviving family members do not hold privilege, there are many things that clinicians can do to be helpful.23 Inevitably, families will want any information that will help them make sense of the loss, and general psychoeducation about mental illness and suicide can be helpful in this regard. In addition, providing information about “Survivors After Suicide” support groups, reading materials, etc., can be helpful. Both support groups and survivor-related bibliographies are available on the web sites of the American Association of Suicidology (www.suicidology.org) and The American Foundation for Suicide Prevention (www.afsp.org).
In addition, clinicians should ask the family if it would be helpful if they were to attend the funeral/memorial services, and how to introduce themselves if asked by other attendees.
Patients in clinics/hospitals. When a patient suicide occurs in a clinic or hospital setting, it is likely to impact other patients in that setting to the extent that they have heard, about the event, even from outside sources.According to Hodgkinson,24 in addition to being overwhelmed with intense feelings about the suicide loss (particularly if they had known the patient), affected patients are likely to be at increased risk for suicidal behaviors. This is consistent with the considerable literature on suicide contagion.
Thus, it is important to clarify information to be shared with patients; however, avoid describing details of the method, because this can foster contagion and “copycat” suicides. In addition, Kaye and Soreff22 noted that these patients may now be concerned about the staff’s ability to be helpful to them, because they were unable to help the deceased. In light of this, take extra care to attend to the impact of the suicide on current patients, and to monitor both pre-existing and new suicidality.
Continue to: Helping affected clinicians
Helping affected clinicians
Suggestions for optimally supporting affected clinicians include:
- clear communication about the nature of upcoming administrative procedures (including chart and institutional reviews)
- consultation from supervisors and/or colleagues that is supportive and reassuring, rather than blaming
- opportunities for the clinician to talk openly about the experience of the loss, either individually or in group settings, without fear of judgment or censure
- recognition that the loss is likely to impact clinical work, support in monitoring this impact, and the provision of medical leaves and/or modified caseloads (ie, fewer high-risk patients) as necessary.
Box 1
Frank Jones and Judy Meade founded the Clinical Survivor Task Force (CSTF) of the American Association of Suicidology (AAS) in 1987. As Jones noted, “clinicians who have lost patients to suicide need a place to acknowledge and carry forward their personal loss…to benefit both personally and professionally from the opportunity to talk with other therapists who have survived the loss of a patient through suicide.”7 Nina Gutin, PhD, and Vanessa McGann, PhD, have co-chaired the CSTF since 2003. It supports clinicians who have lost patients and/or loved ones, with the recognition that both types of losses carry implications within clinical and professional domains. The CSTF provides a listserve, opportunities to participate in video support groups, and a web site (www.cliniciansurvivor.org) that provides information about the clinician-survivor experience, the opportunity to read and post narratives about one’s experience with suicide loss, an updated bibliography maintained by John McIntosh, PhD, a list of clinical contacts, and links to several excellent postvention protocols. In addition, Drs. Gutin and McGann conduct clinician-survivor support activities at the annual AAS conference, and in their respective geographic areas.
Both researchers and clinician-survivors in my practice and support groups have noted that speaking with other clinicians who have experienced suicide loss can be particularly reassuring and validating. If none are available on staff, the listserve and online support groups of the American Association of Suicidology’s Clinician Survivor Task Force may be helpful (Box 17). In addition, the film “Collateral Damages: The Impact of Patient Suicide on the Physician” features physicians describing their experience of losing a patient to suicide (Box 2).
Box 2
“Collateral Damages: The Impact of Patient Suicide on the Physician” is a film that features several physicians speaking about their experience of losing a patient to suicide, as well as a group discussion. Psychiatrists in this educational film include Drs. Glen Gabbard, Sidney Zisook, and Jim Lomax. This resource can be used to facilitate an educational session for physicians, psychologists, residents, or other trainees. Please contact education@afsp.org to request a DVD of this film and a copy of a related article, Prabhakar D, Anzia JM, Balon R, et al. “Collateral damages”: preparing residents for coping with patient suicide. Acad Psychiatry. 2013;37(6):429-430.
Schultz14 offered suggestions for staff in supervisory positions, noting that they may bear at least some clinical and legal responsibility for the treatments that they supervise. She encouraged supervisors to take an active stance in advocating for trainees, to encourage colleagues to express their support, and to discourage rumors and other stigmatizing reactions. Schultz also urges supervisors to14:
- allow extra time for the clinician to engage in the normative exploration of the “whys” that are unique to suicide survivors
- use education about suicide to help the clinician gain a more realistic perspective on their relative culpability
- become aware of and provide education about normative grief reactions following a suicide.
Continue to: Because a suicide loss...
Because a suicide loss is likely to affect a clinician’s subsequent clinical activity, Schultz encourages supervisors to help clinicians monitor this impact on their work.14
A supportive environment is key
Losing a patient to suicide is a complicated, potentially traumatic process that many mental health clinicians will face. Yet with comprehensive and supportive postvention policies in place, clinicians who are impacted are more likely to experience healing and posttraumatic growth in both personal and professional domains.
Bottom Line
Although often traumatic, losing a patient to suicide presents clinicians with an opportunity for personal and professional growth. Following established postvention protocols can help ensure that legal, institutional, and administrative needs are balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.
Related Resources
- American Association of Suicidology Clinician Survivor Task Force. www.cliniciansurvivor.org.
- Dotinga R. Coping when a patient commits suicide. July 21, 2017. www.mdedge.com/psychiatry/article/142975/depression/coping-when-patient-commits-suicide.
- Gutin N. Losing a patent to suicide: What we know. Part 1. 2019;18(10):14-16,19-22,30-32.
1. Tedeschi RG, Calhoun LG. Beyond the concept of recovery: Growth and the experience of loss. Death Stud. 2008;32(1):27-39.
2. Fuentes MA, Cruz D. Posttraumatic growth: positive psychological changes after trauma. Mental Health News. 2009;11(1):31,37.
3. Gitlin M. Aftermath of a tragedy: reaction of psychiatrists to patient suicides. Psychiatr Ann. 2007;37(10):684-687.
4. Huhra R, Hunka N, Rogers J, et al. Finding meaning: theoretical perspectives on patient suicide. Paper presented at: 2004 Annual Conference of the American Association of Suicidology; April 2004; Miami, FL.
5. Jones FA Jr. Therapists as survivors of patient suicide. In: Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton; 1987;126-141.
6. Gutin N, McGann VM, Jordan JR. The impact of suicide on professional caregivers. In: Jordan J, McIntosh J, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:93-111.
7. Hendin H, Lipschitz A, Maltsberger JT, et al. Therapists’ reactions to patients’ suicides. Am J Psychiatry. 2000;157(12):2022-2027.
8. Munson JS. Impact of client suicide on practitioner posttraumatic growth [dissertation]. Gainesville, Florida: University of Florida; 2009.
9. Grad OT. Therapists as survivors of suicide loss. In: Wasserman D, Wasserman C, eds. Oxford textbook of suicidology and suicide prevention. Oxford, UK: Oxford University Press; 2009:609-615.
10. Douglas J, Brown HN. Suicide: understanding and responding: Harvard Medical School perspectives. Madison, CT: International Universities Press; 1989.
11. Farberow NL. The mental health professional as suicide survivor. Clin Neuropsychiatry. 2005;2(1):13-20.
12. Plakun EM, Tillman JG. Responding to clinicians after loss of a patient to suicide. Dir Psychiatry. 2005;25:301-310.
13. Quinnett P. QPR: for suicide prevention. QPR Institute, Inc. www.cliniciansurvivor.org (under Postvention tab). Published September 21, 2009. Accessed August 26, 2019.
14. Schultz, D. Suggestions for supervisors when a therapist experiences a client’s suicide. Women Ther. 2005;28(1):59-69.
15. Spiegelman JS Jr, Werth JL Jr. Don’t forget about me: the experiences of therapists-in-training after a patient has attempted or died by suicide. Women Ther. 2005;28(1):35-57.
16. American Association of Suicidology. Clinician Survivor Task Force. Clinicians as survivors of suicide: postvention information. http://cliniciansurvivor.org. Published May 16, 2016. Accessed January 13, 2019.
17. Whitmore CA, Cook J, Salg L. Supporting residents in the wake of patient suicide. The American Journal of Psychiatry Residents’ Journal. 2017;12(1):5-7.
18. Ellis TE, Patel AB. Client suicide: what now? Cogn Behav Pract. 2012;19(2):277-287.
19. Figueroa S, Dalack GW. Exploring the impact of suicide on clinicians: a multidisciplinary retreat model. J Psychiatr Pract. 2013;19(1):72-77.
20. Lerner U, Brooks, K, McNeil DE, et al. Coping with a patient’s suicide: a curriculum for psychiatry residency training programs. Acad Psychiatry. 2012;36(1):29-33.
21. Prabhakar D, Balon R, Anzia J, et al. Helping psychiatry residents cope with patient suicide. Acad Psychiatry. 2014;38(5):593-597.
22. Kaye NS, Soreff SM. The psychiatrist’s role, responses, and responsibilities when a patient commits suicide. Am J Psychiatry. 1991;148(6):739-743.
23. McGann VL, Gutin N, Jordan JR. Guidelines for postvention care with survivor families after the suicide of a client. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:133-155.
24. Hodgkinson PE. Responding to in-patient suicide. Br J Med Psychol. 1987;60(4):387-392.
At some point during their career, many mental health professionals will lose a patient to suicide, but few will be prepared for the experience and its aftermath. As I described in Part 1 of this article (
A chance for growth
Traumatic experiences such as a suicide loss can paradoxically present a multitude of opportunities for new growth and profound personal transformation.1 Such transformation is primarily fostered by social support in the aftermath of the trauma.2
Virtually all of the models of the clinician’s suicide grief trajectory I described in Part 1 not only assume the eventual resolution of the distressing reactions accompanying the original loss, but also suggest that mastery of these reactions can be a catalyst for both personal and professional growth. Clearly, not everyone who experiences such a loss will experience subsequent growth; there are many reports of clinicians leaving the field3 or becoming “burned out” after this occurs. Yet most clinicians who have described this loss in the literature and in discussion groups (including those I’ve conducted) have reported more positive eventual outcomes. It is difficult to establish whether this is due to a cohort effect—clinicians who are most likely to write about their experiences, be interviewed for research studies, and/or to seek out and participate in discussion/support groups may be more prone to find benefits in this experience, either by virtue of their nature or through the subsequent process of sharing these experiences in a supportive atmosphere.
The literature on patient suicide loss, as well as anecdotal reports, confirms that clinicians who experience optimal support are able to identify many retrospective benefits of their experience.4-6 Clinicians generally report that they are better able to identify potential risk and protective factors for suicide, and are more knowledgeable about optimal interventions with individuals who are suicidal. They also describe an increased sensitivity towards patients who are suicidal and those bereaved by suicide. In addition, clinicians report a reduction in therapeutic grandiosity/omnipotence, and more realistic appraisals and expectations in relation to their clinical competence. In their effort to understand the “whys” of their patient’s suicide, they are likely to retrospectively identify errors in treatment, “missed cues,” or things they might subsequently do differently,7 and to learn from these mistakes. Optimally, clinicians become more aware of their own therapeutic limitations, both in the short- and the long-term, and can use this knowledge to better determine how they will continue their clinical work. They also become much more aware of the issues involved in the aftermath of a patient suicide, including perceived gaps in the clinical and institutional systems that could optimally offer support to families and clinicians.
In addition to the positive changes related to knowledge and clinical skills, many clinicians also note deeper personal changes subsequent to their patient’s suicide, consistent with the literature on posttraumatic growth.1 Munson8 explored internal changes in clinicians following a patient suicide and found that in the aftermath, clinicians experienced both posttraumatic growth and compassion fatigue. He also found that the amount of time that elapsed since the patient’s suicide predicted posttraumatic growth, and the seemingly counterintuitive result that the number of years of clinical experience prior to the suicide was negatively correlated with posttraumatic growth.
Huhra et al4 described some of the existential issues that a clinician is likely to confront following a patient suicide. A clinician’s attempt to find a way to meaningfully understand the circumstances around this loss often prompts reflection on mortality, freedom, choice and personal autonomy, and the scope and limits of one’s responsibility toward others. The suicide challenges one’s previous conceptions and expectations around these professional issues, and the clinician must construct new paradigms that serve to integrate these new experiences and perspectives in a coherent way.
One of the most notable sequelae of this (and to other traumatic) experience is a subsequent desire to make use of the learning inherent in these experiences and to “give back.” Once they feel that they have resolved their own grief process, many clinicians express the desire to support others with similar experiences. Even when their experiences have been quite distressing, many clinicians are able to view the suicide as an opportunity to learn about ongoing limitations in the systems of support, and to work toward changing these in a way that ensures that future clinician-survivors will have more supportive experiences. Many view these new perspectives, and their consequent ability to be more helpful, as “unexpected gifts.” They often express gratitude toward the people and resources that have allowed them to make these transformations. Jones5 noted “the tragedy of patient suicide can also be an opportunity for us as therapists to grow in our skills at assessing and intervening in a suicidal crisis, to broaden and deepen the support we give and receive, to grow in our appreciation of the precious gift that life is, and to help each other live it more fully.”
Continue to: Guidelines for postvention
Guidelines for postvention
When a patient suicide occurs in the context of an agency setting, Grad9 recommends prompt responses on 4 levels:
- administrative
- institutional
- educational
- emotional.
Numerous authors5,10-21 have developed suggestions, guidelines, and detailed postvention protocols to help agencies and clinicians in various mental health settings navigate the often-complicated sequelae to a patient’s suicide. The Table highlights a few of these. Most emphasize that information about suicide loss, including both its statistical likelihood and its potential aftermath, should integrated into clinicians’ general education and training. They also suggest that suicide postvention policies and protocols be in place from the outset, and that such information be incorporated into institutional policy and procedure manuals. In addition, they stress that legal, institutional, and administrative needs be balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.

Institutional and administrative procedures
The following are some of the recommended procedures that should take place following a suicide loss. The postvention protocols listed in the Table provide more detailed recommendations.
Legal/ethical. It is essential to consult with a legal representative/risk management specialist associated with the affected agency (ideally, one with specific expertise in suicide litigation.). It is also crucial to clarify who holds privilege after a patient’s death (varies by state), what may and may not be shared under the restrictions of confidentiality and Health Insurance Portability and Accountability Act (HIPAA) laws, and to clarify procedures for chart completion and review. It is also important to clarify the specific information to be shared both within and outside of the agency, and how to address the needs of current patients in the agency settings.
Case review. The optimal purpose of the case review (also known as a psychological autopsy) is to facilitate learning, identify gaps in agency procedures and training, improve pre- and postvention procedures, and help clinicians cope with the loss.22 Again, the legal and administrative needs of the agency need to be balanced with the attention to the emotional impact on the treating clinician.17 Ellis and Patel18 recommend delaying this procedure until the treating clinician is no longer in the “shock” phase of the loss, and is able to think and process events more objectively.
Continue to: Family contact
Family contact. Most authors have recommended that clinicians and/or agencies reach out to surviving families. Although some legal representatives will advise against this, experts in the field of suicide litigation have noted that compassionate family contact reduces liability and facilitates healing for both parties. In a personal communication (May 2008), Eric Harris, of the American Psychological Association Trust, recommended “compassion over caution” when considering these issues. Again, it is important to clarify who holds privilege after a patient’s death in determining when and with whom the patient’s confidential information may be shared. When confidentiality may be broken, clinical judgment should be used to determine how best to present the information to grieving family members.
Even if surviving family members do not hold privilege, there are many things that clinicians can do to be helpful.23 Inevitably, families will want any information that will help them make sense of the loss, and general psychoeducation about mental illness and suicide can be helpful in this regard. In addition, providing information about “Survivors After Suicide” support groups, reading materials, etc., can be helpful. Both support groups and survivor-related bibliographies are available on the web sites of the American Association of Suicidology (www.suicidology.org) and The American Foundation for Suicide Prevention (www.afsp.org).
In addition, clinicians should ask the family if it would be helpful if they were to attend the funeral/memorial services, and how to introduce themselves if asked by other attendees.
Patients in clinics/hospitals. When a patient suicide occurs in a clinic or hospital setting, it is likely to impact other patients in that setting to the extent that they have heard, about the event, even from outside sources.According to Hodgkinson,24 in addition to being overwhelmed with intense feelings about the suicide loss (particularly if they had known the patient), affected patients are likely to be at increased risk for suicidal behaviors. This is consistent with the considerable literature on suicide contagion.
Thus, it is important to clarify information to be shared with patients; however, avoid describing details of the method, because this can foster contagion and “copycat” suicides. In addition, Kaye and Soreff22 noted that these patients may now be concerned about the staff’s ability to be helpful to them, because they were unable to help the deceased. In light of this, take extra care to attend to the impact of the suicide on current patients, and to monitor both pre-existing and new suicidality.
Continue to: Helping affected clinicians
Helping affected clinicians
Suggestions for optimally supporting affected clinicians include:
- clear communication about the nature of upcoming administrative procedures (including chart and institutional reviews)
- consultation from supervisors and/or colleagues that is supportive and reassuring, rather than blaming
- opportunities for the clinician to talk openly about the experience of the loss, either individually or in group settings, without fear of judgment or censure
- recognition that the loss is likely to impact clinical work, support in monitoring this impact, and the provision of medical leaves and/or modified caseloads (ie, fewer high-risk patients) as necessary.
Box 1
Frank Jones and Judy Meade founded the Clinical Survivor Task Force (CSTF) of the American Association of Suicidology (AAS) in 1987. As Jones noted, “clinicians who have lost patients to suicide need a place to acknowledge and carry forward their personal loss…to benefit both personally and professionally from the opportunity to talk with other therapists who have survived the loss of a patient through suicide.”7 Nina Gutin, PhD, and Vanessa McGann, PhD, have co-chaired the CSTF since 2003. It supports clinicians who have lost patients and/or loved ones, with the recognition that both types of losses carry implications within clinical and professional domains. The CSTF provides a listserve, opportunities to participate in video support groups, and a web site (www.cliniciansurvivor.org) that provides information about the clinician-survivor experience, the opportunity to read and post narratives about one’s experience with suicide loss, an updated bibliography maintained by John McIntosh, PhD, a list of clinical contacts, and links to several excellent postvention protocols. In addition, Drs. Gutin and McGann conduct clinician-survivor support activities at the annual AAS conference, and in their respective geographic areas.
Both researchers and clinician-survivors in my practice and support groups have noted that speaking with other clinicians who have experienced suicide loss can be particularly reassuring and validating. If none are available on staff, the listserve and online support groups of the American Association of Suicidology’s Clinician Survivor Task Force may be helpful (Box 17). In addition, the film “Collateral Damages: The Impact of Patient Suicide on the Physician” features physicians describing their experience of losing a patient to suicide (Box 2).
Box 2
“Collateral Damages: The Impact of Patient Suicide on the Physician” is a film that features several physicians speaking about their experience of losing a patient to suicide, as well as a group discussion. Psychiatrists in this educational film include Drs. Glen Gabbard, Sidney Zisook, and Jim Lomax. This resource can be used to facilitate an educational session for physicians, psychologists, residents, or other trainees. Please contact education@afsp.org to request a DVD of this film and a copy of a related article, Prabhakar D, Anzia JM, Balon R, et al. “Collateral damages”: preparing residents for coping with patient suicide. Acad Psychiatry. 2013;37(6):429-430.
Schultz14 offered suggestions for staff in supervisory positions, noting that they may bear at least some clinical and legal responsibility for the treatments that they supervise. She encouraged supervisors to take an active stance in advocating for trainees, to encourage colleagues to express their support, and to discourage rumors and other stigmatizing reactions. Schultz also urges supervisors to14:
- allow extra time for the clinician to engage in the normative exploration of the “whys” that are unique to suicide survivors
- use education about suicide to help the clinician gain a more realistic perspective on their relative culpability
- become aware of and provide education about normative grief reactions following a suicide.
Continue to: Because a suicide loss...
Because a suicide loss is likely to affect a clinician’s subsequent clinical activity, Schultz encourages supervisors to help clinicians monitor this impact on their work.14
A supportive environment is key
Losing a patient to suicide is a complicated, potentially traumatic process that many mental health clinicians will face. Yet with comprehensive and supportive postvention policies in place, clinicians who are impacted are more likely to experience healing and posttraumatic growth in both personal and professional domains.
Bottom Line
Although often traumatic, losing a patient to suicide presents clinicians with an opportunity for personal and professional growth. Following established postvention protocols can help ensure that legal, institutional, and administrative needs are balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.
Related Resources
- American Association of Suicidology Clinician Survivor Task Force. www.cliniciansurvivor.org.
- Dotinga R. Coping when a patient commits suicide. July 21, 2017. www.mdedge.com/psychiatry/article/142975/depression/coping-when-patient-commits-suicide.
- Gutin N. Losing a patent to suicide: What we know. Part 1. 2019;18(10):14-16,19-22,30-32.
At some point during their career, many mental health professionals will lose a patient to suicide, but few will be prepared for the experience and its aftermath. As I described in Part 1 of this article (
A chance for growth
Traumatic experiences such as a suicide loss can paradoxically present a multitude of opportunities for new growth and profound personal transformation.1 Such transformation is primarily fostered by social support in the aftermath of the trauma.2
Virtually all of the models of the clinician’s suicide grief trajectory I described in Part 1 not only assume the eventual resolution of the distressing reactions accompanying the original loss, but also suggest that mastery of these reactions can be a catalyst for both personal and professional growth. Clearly, not everyone who experiences such a loss will experience subsequent growth; there are many reports of clinicians leaving the field3 or becoming “burned out” after this occurs. Yet most clinicians who have described this loss in the literature and in discussion groups (including those I’ve conducted) have reported more positive eventual outcomes. It is difficult to establish whether this is due to a cohort effect—clinicians who are most likely to write about their experiences, be interviewed for research studies, and/or to seek out and participate in discussion/support groups may be more prone to find benefits in this experience, either by virtue of their nature or through the subsequent process of sharing these experiences in a supportive atmosphere.
The literature on patient suicide loss, as well as anecdotal reports, confirms that clinicians who experience optimal support are able to identify many retrospective benefits of their experience.4-6 Clinicians generally report that they are better able to identify potential risk and protective factors for suicide, and are more knowledgeable about optimal interventions with individuals who are suicidal. They also describe an increased sensitivity towards patients who are suicidal and those bereaved by suicide. In addition, clinicians report a reduction in therapeutic grandiosity/omnipotence, and more realistic appraisals and expectations in relation to their clinical competence. In their effort to understand the “whys” of their patient’s suicide, they are likely to retrospectively identify errors in treatment, “missed cues,” or things they might subsequently do differently,7 and to learn from these mistakes. Optimally, clinicians become more aware of their own therapeutic limitations, both in the short- and the long-term, and can use this knowledge to better determine how they will continue their clinical work. They also become much more aware of the issues involved in the aftermath of a patient suicide, including perceived gaps in the clinical and institutional systems that could optimally offer support to families and clinicians.
In addition to the positive changes related to knowledge and clinical skills, many clinicians also note deeper personal changes subsequent to their patient’s suicide, consistent with the literature on posttraumatic growth.1 Munson8 explored internal changes in clinicians following a patient suicide and found that in the aftermath, clinicians experienced both posttraumatic growth and compassion fatigue. He also found that the amount of time that elapsed since the patient’s suicide predicted posttraumatic growth, and the seemingly counterintuitive result that the number of years of clinical experience prior to the suicide was negatively correlated with posttraumatic growth.
Huhra et al4 described some of the existential issues that a clinician is likely to confront following a patient suicide. A clinician’s attempt to find a way to meaningfully understand the circumstances around this loss often prompts reflection on mortality, freedom, choice and personal autonomy, and the scope and limits of one’s responsibility toward others. The suicide challenges one’s previous conceptions and expectations around these professional issues, and the clinician must construct new paradigms that serve to integrate these new experiences and perspectives in a coherent way.
One of the most notable sequelae of this (and to other traumatic) experience is a subsequent desire to make use of the learning inherent in these experiences and to “give back.” Once they feel that they have resolved their own grief process, many clinicians express the desire to support others with similar experiences. Even when their experiences have been quite distressing, many clinicians are able to view the suicide as an opportunity to learn about ongoing limitations in the systems of support, and to work toward changing these in a way that ensures that future clinician-survivors will have more supportive experiences. Many view these new perspectives, and their consequent ability to be more helpful, as “unexpected gifts.” They often express gratitude toward the people and resources that have allowed them to make these transformations. Jones5 noted “the tragedy of patient suicide can also be an opportunity for us as therapists to grow in our skills at assessing and intervening in a suicidal crisis, to broaden and deepen the support we give and receive, to grow in our appreciation of the precious gift that life is, and to help each other live it more fully.”
Continue to: Guidelines for postvention
Guidelines for postvention
When a patient suicide occurs in the context of an agency setting, Grad9 recommends prompt responses on 4 levels:
- administrative
- institutional
- educational
- emotional.
Numerous authors5,10-21 have developed suggestions, guidelines, and detailed postvention protocols to help agencies and clinicians in various mental health settings navigate the often-complicated sequelae to a patient’s suicide. The Table highlights a few of these. Most emphasize that information about suicide loss, including both its statistical likelihood and its potential aftermath, should integrated into clinicians’ general education and training. They also suggest that suicide postvention policies and protocols be in place from the outset, and that such information be incorporated into institutional policy and procedure manuals. In addition, they stress that legal, institutional, and administrative needs be balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.

Institutional and administrative procedures
The following are some of the recommended procedures that should take place following a suicide loss. The postvention protocols listed in the Table provide more detailed recommendations.
Legal/ethical. It is essential to consult with a legal representative/risk management specialist associated with the affected agency (ideally, one with specific expertise in suicide litigation.). It is also crucial to clarify who holds privilege after a patient’s death (varies by state), what may and may not be shared under the restrictions of confidentiality and Health Insurance Portability and Accountability Act (HIPAA) laws, and to clarify procedures for chart completion and review. It is also important to clarify the specific information to be shared both within and outside of the agency, and how to address the needs of current patients in the agency settings.
Case review. The optimal purpose of the case review (also known as a psychological autopsy) is to facilitate learning, identify gaps in agency procedures and training, improve pre- and postvention procedures, and help clinicians cope with the loss.22 Again, the legal and administrative needs of the agency need to be balanced with the attention to the emotional impact on the treating clinician.17 Ellis and Patel18 recommend delaying this procedure until the treating clinician is no longer in the “shock” phase of the loss, and is able to think and process events more objectively.
Continue to: Family contact
Family contact. Most authors have recommended that clinicians and/or agencies reach out to surviving families. Although some legal representatives will advise against this, experts in the field of suicide litigation have noted that compassionate family contact reduces liability and facilitates healing for both parties. In a personal communication (May 2008), Eric Harris, of the American Psychological Association Trust, recommended “compassion over caution” when considering these issues. Again, it is important to clarify who holds privilege after a patient’s death in determining when and with whom the patient’s confidential information may be shared. When confidentiality may be broken, clinical judgment should be used to determine how best to present the information to grieving family members.
Even if surviving family members do not hold privilege, there are many things that clinicians can do to be helpful.23 Inevitably, families will want any information that will help them make sense of the loss, and general psychoeducation about mental illness and suicide can be helpful in this regard. In addition, providing information about “Survivors After Suicide” support groups, reading materials, etc., can be helpful. Both support groups and survivor-related bibliographies are available on the web sites of the American Association of Suicidology (www.suicidology.org) and The American Foundation for Suicide Prevention (www.afsp.org).
In addition, clinicians should ask the family if it would be helpful if they were to attend the funeral/memorial services, and how to introduce themselves if asked by other attendees.
Patients in clinics/hospitals. When a patient suicide occurs in a clinic or hospital setting, it is likely to impact other patients in that setting to the extent that they have heard, about the event, even from outside sources.According to Hodgkinson,24 in addition to being overwhelmed with intense feelings about the suicide loss (particularly if they had known the patient), affected patients are likely to be at increased risk for suicidal behaviors. This is consistent with the considerable literature on suicide contagion.
Thus, it is important to clarify information to be shared with patients; however, avoid describing details of the method, because this can foster contagion and “copycat” suicides. In addition, Kaye and Soreff22 noted that these patients may now be concerned about the staff’s ability to be helpful to them, because they were unable to help the deceased. In light of this, take extra care to attend to the impact of the suicide on current patients, and to monitor both pre-existing and new suicidality.
Continue to: Helping affected clinicians
Helping affected clinicians
Suggestions for optimally supporting affected clinicians include:
- clear communication about the nature of upcoming administrative procedures (including chart and institutional reviews)
- consultation from supervisors and/or colleagues that is supportive and reassuring, rather than blaming
- opportunities for the clinician to talk openly about the experience of the loss, either individually or in group settings, without fear of judgment or censure
- recognition that the loss is likely to impact clinical work, support in monitoring this impact, and the provision of medical leaves and/or modified caseloads (ie, fewer high-risk patients) as necessary.
Box 1
Frank Jones and Judy Meade founded the Clinical Survivor Task Force (CSTF) of the American Association of Suicidology (AAS) in 1987. As Jones noted, “clinicians who have lost patients to suicide need a place to acknowledge and carry forward their personal loss…to benefit both personally and professionally from the opportunity to talk with other therapists who have survived the loss of a patient through suicide.”7 Nina Gutin, PhD, and Vanessa McGann, PhD, have co-chaired the CSTF since 2003. It supports clinicians who have lost patients and/or loved ones, with the recognition that both types of losses carry implications within clinical and professional domains. The CSTF provides a listserve, opportunities to participate in video support groups, and a web site (www.cliniciansurvivor.org) that provides information about the clinician-survivor experience, the opportunity to read and post narratives about one’s experience with suicide loss, an updated bibliography maintained by John McIntosh, PhD, a list of clinical contacts, and links to several excellent postvention protocols. In addition, Drs. Gutin and McGann conduct clinician-survivor support activities at the annual AAS conference, and in their respective geographic areas.
Both researchers and clinician-survivors in my practice and support groups have noted that speaking with other clinicians who have experienced suicide loss can be particularly reassuring and validating. If none are available on staff, the listserve and online support groups of the American Association of Suicidology’s Clinician Survivor Task Force may be helpful (Box 17). In addition, the film “Collateral Damages: The Impact of Patient Suicide on the Physician” features physicians describing their experience of losing a patient to suicide (Box 2).
Box 2
“Collateral Damages: The Impact of Patient Suicide on the Physician” is a film that features several physicians speaking about their experience of losing a patient to suicide, as well as a group discussion. Psychiatrists in this educational film include Drs. Glen Gabbard, Sidney Zisook, and Jim Lomax. This resource can be used to facilitate an educational session for physicians, psychologists, residents, or other trainees. Please contact education@afsp.org to request a DVD of this film and a copy of a related article, Prabhakar D, Anzia JM, Balon R, et al. “Collateral damages”: preparing residents for coping with patient suicide. Acad Psychiatry. 2013;37(6):429-430.
Schultz14 offered suggestions for staff in supervisory positions, noting that they may bear at least some clinical and legal responsibility for the treatments that they supervise. She encouraged supervisors to take an active stance in advocating for trainees, to encourage colleagues to express their support, and to discourage rumors and other stigmatizing reactions. Schultz also urges supervisors to14:
- allow extra time for the clinician to engage in the normative exploration of the “whys” that are unique to suicide survivors
- use education about suicide to help the clinician gain a more realistic perspective on their relative culpability
- become aware of and provide education about normative grief reactions following a suicide.
Continue to: Because a suicide loss...
Because a suicide loss is likely to affect a clinician’s subsequent clinical activity, Schultz encourages supervisors to help clinicians monitor this impact on their work.14
A supportive environment is key
Losing a patient to suicide is a complicated, potentially traumatic process that many mental health clinicians will face. Yet with comprehensive and supportive postvention policies in place, clinicians who are impacted are more likely to experience healing and posttraumatic growth in both personal and professional domains.
Bottom Line
Although often traumatic, losing a patient to suicide presents clinicians with an opportunity for personal and professional growth. Following established postvention protocols can help ensure that legal, institutional, and administrative needs are balanced with the emotional needs of affected clinicians and staff, as well as those of the surviving family.
Related Resources
- American Association of Suicidology Clinician Survivor Task Force. www.cliniciansurvivor.org.
- Dotinga R. Coping when a patient commits suicide. July 21, 2017. www.mdedge.com/psychiatry/article/142975/depression/coping-when-patient-commits-suicide.
- Gutin N. Losing a patent to suicide: What we know. Part 1. 2019;18(10):14-16,19-22,30-32.
1. Tedeschi RG, Calhoun LG. Beyond the concept of recovery: Growth and the experience of loss. Death Stud. 2008;32(1):27-39.
2. Fuentes MA, Cruz D. Posttraumatic growth: positive psychological changes after trauma. Mental Health News. 2009;11(1):31,37.
3. Gitlin M. Aftermath of a tragedy: reaction of psychiatrists to patient suicides. Psychiatr Ann. 2007;37(10):684-687.
4. Huhra R, Hunka N, Rogers J, et al. Finding meaning: theoretical perspectives on patient suicide. Paper presented at: 2004 Annual Conference of the American Association of Suicidology; April 2004; Miami, FL.
5. Jones FA Jr. Therapists as survivors of patient suicide. In: Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton; 1987;126-141.
6. Gutin N, McGann VM, Jordan JR. The impact of suicide on professional caregivers. In: Jordan J, McIntosh J, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:93-111.
7. Hendin H, Lipschitz A, Maltsberger JT, et al. Therapists’ reactions to patients’ suicides. Am J Psychiatry. 2000;157(12):2022-2027.
8. Munson JS. Impact of client suicide on practitioner posttraumatic growth [dissertation]. Gainesville, Florida: University of Florida; 2009.
9. Grad OT. Therapists as survivors of suicide loss. In: Wasserman D, Wasserman C, eds. Oxford textbook of suicidology and suicide prevention. Oxford, UK: Oxford University Press; 2009:609-615.
10. Douglas J, Brown HN. Suicide: understanding and responding: Harvard Medical School perspectives. Madison, CT: International Universities Press; 1989.
11. Farberow NL. The mental health professional as suicide survivor. Clin Neuropsychiatry. 2005;2(1):13-20.
12. Plakun EM, Tillman JG. Responding to clinicians after loss of a patient to suicide. Dir Psychiatry. 2005;25:301-310.
13. Quinnett P. QPR: for suicide prevention. QPR Institute, Inc. www.cliniciansurvivor.org (under Postvention tab). Published September 21, 2009. Accessed August 26, 2019.
14. Schultz, D. Suggestions for supervisors when a therapist experiences a client’s suicide. Women Ther. 2005;28(1):59-69.
15. Spiegelman JS Jr, Werth JL Jr. Don’t forget about me: the experiences of therapists-in-training after a patient has attempted or died by suicide. Women Ther. 2005;28(1):35-57.
16. American Association of Suicidology. Clinician Survivor Task Force. Clinicians as survivors of suicide: postvention information. http://cliniciansurvivor.org. Published May 16, 2016. Accessed January 13, 2019.
17. Whitmore CA, Cook J, Salg L. Supporting residents in the wake of patient suicide. The American Journal of Psychiatry Residents’ Journal. 2017;12(1):5-7.
18. Ellis TE, Patel AB. Client suicide: what now? Cogn Behav Pract. 2012;19(2):277-287.
19. Figueroa S, Dalack GW. Exploring the impact of suicide on clinicians: a multidisciplinary retreat model. J Psychiatr Pract. 2013;19(1):72-77.
20. Lerner U, Brooks, K, McNeil DE, et al. Coping with a patient’s suicide: a curriculum for psychiatry residency training programs. Acad Psychiatry. 2012;36(1):29-33.
21. Prabhakar D, Balon R, Anzia J, et al. Helping psychiatry residents cope with patient suicide. Acad Psychiatry. 2014;38(5):593-597.
22. Kaye NS, Soreff SM. The psychiatrist’s role, responses, and responsibilities when a patient commits suicide. Am J Psychiatry. 1991;148(6):739-743.
23. McGann VL, Gutin N, Jordan JR. Guidelines for postvention care with survivor families after the suicide of a client. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:133-155.
24. Hodgkinson PE. Responding to in-patient suicide. Br J Med Psychol. 1987;60(4):387-392.
1. Tedeschi RG, Calhoun LG. Beyond the concept of recovery: Growth and the experience of loss. Death Stud. 2008;32(1):27-39.
2. Fuentes MA, Cruz D. Posttraumatic growth: positive psychological changes after trauma. Mental Health News. 2009;11(1):31,37.
3. Gitlin M. Aftermath of a tragedy: reaction of psychiatrists to patient suicides. Psychiatr Ann. 2007;37(10):684-687.
4. Huhra R, Hunka N, Rogers J, et al. Finding meaning: theoretical perspectives on patient suicide. Paper presented at: 2004 Annual Conference of the American Association of Suicidology; April 2004; Miami, FL.
5. Jones FA Jr. Therapists as survivors of patient suicide. In: Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton; 1987;126-141.
6. Gutin N, McGann VM, Jordan JR. The impact of suicide on professional caregivers. In: Jordan J, McIntosh J, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:93-111.
7. Hendin H, Lipschitz A, Maltsberger JT, et al. Therapists’ reactions to patients’ suicides. Am J Psychiatry. 2000;157(12):2022-2027.
8. Munson JS. Impact of client suicide on practitioner posttraumatic growth [dissertation]. Gainesville, Florida: University of Florida; 2009.
9. Grad OT. Therapists as survivors of suicide loss. In: Wasserman D, Wasserman C, eds. Oxford textbook of suicidology and suicide prevention. Oxford, UK: Oxford University Press; 2009:609-615.
10. Douglas J, Brown HN. Suicide: understanding and responding: Harvard Medical School perspectives. Madison, CT: International Universities Press; 1989.
11. Farberow NL. The mental health professional as suicide survivor. Clin Neuropsychiatry. 2005;2(1):13-20.
12. Plakun EM, Tillman JG. Responding to clinicians after loss of a patient to suicide. Dir Psychiatry. 2005;25:301-310.
13. Quinnett P. QPR: for suicide prevention. QPR Institute, Inc. www.cliniciansurvivor.org (under Postvention tab). Published September 21, 2009. Accessed August 26, 2019.
14. Schultz, D. Suggestions for supervisors when a therapist experiences a client’s suicide. Women Ther. 2005;28(1):59-69.
15. Spiegelman JS Jr, Werth JL Jr. Don’t forget about me: the experiences of therapists-in-training after a patient has attempted or died by suicide. Women Ther. 2005;28(1):35-57.
16. American Association of Suicidology. Clinician Survivor Task Force. Clinicians as survivors of suicide: postvention information. http://cliniciansurvivor.org. Published May 16, 2016. Accessed January 13, 2019.
17. Whitmore CA, Cook J, Salg L. Supporting residents in the wake of patient suicide. The American Journal of Psychiatry Residents’ Journal. 2017;12(1):5-7.
18. Ellis TE, Patel AB. Client suicide: what now? Cogn Behav Pract. 2012;19(2):277-287.
19. Figueroa S, Dalack GW. Exploring the impact of suicide on clinicians: a multidisciplinary retreat model. J Psychiatr Pract. 2013;19(1):72-77.
20. Lerner U, Brooks, K, McNeil DE, et al. Coping with a patient’s suicide: a curriculum for psychiatry residency training programs. Acad Psychiatry. 2012;36(1):29-33.
21. Prabhakar D, Balon R, Anzia J, et al. Helping psychiatry residents cope with patient suicide. Acad Psychiatry. 2014;38(5):593-597.
22. Kaye NS, Soreff SM. The psychiatrist’s role, responses, and responsibilities when a patient commits suicide. Am J Psychiatry. 1991;148(6):739-743.
23. McGann VL, Gutin N, Jordan JR. Guidelines for postvention care with survivor families after the suicide of a client. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge; 2011:133-155.
24. Hodgkinson PE. Responding to in-patient suicide. Br J Med Psychol. 1987;60(4):387-392.