User login
Microbleeds After Brain Injury Predict Worse Disability
Traumatic microbleeds (TMBs) may indicate vascular injury and predict worse outcomes after even minor brain injury, according to a study at the National Institute of Neurological Disorders and Stroke.
The study involved 439 adults with head injuries treated in the emergency department. The participants had magnetic resonance imaging (MRI) scans within 48 hours of the injury and again during 4 subsequent visits. They also completed behavioral and outcome questionnaires.
Microbleeds appear as small dark lesions on MRI scans but are usually too small to be seen on computer tomography (CT) scans. Sometimes they appear as dots (punctate), sometimes they are linear. In previous studies, researchers examined TMBs in the acute phase of traumatic brain injury (TBI) and stroke and found linear-appearing TMBs only in patients with TBI, suggesting that at least linear TMBs are consistent with trauma and might be the result of injured vessels. They conjectured that TMBs seen on MRI might be a form of traumatic vascular injury distinct from primary injury to the axons.
In this study, one-third of the patients had TMBs. More than half (58%) of the participants with severe head injury showed microbleeds, as did 27% of patients with mild injuries. In most patients with microbleeds, they appeared as linear streaks or dotted lesions. The study also revealed that the frontal lobes were the region most likely to show microbleeds.
The researchers controlled for variables known to predict poor outcome, such as trauma level and trauma-related injury on CT. Even so, microbleeds significantly predicted worse outcome. Patients with both punctate and linear TMBs were twice as likely to have disability (Glasgow Outcome Scale-Extended ≤6) on follow-up.
One participant’s family donated his brain for further analysis after he died. Imaging with a more powerful MRI scanner and a detailed histologic analysis allowed the researchers to better understand the pathology.
The researchers found that what appeared as a punctate TMB on MRI corresponded to iron-laden macrophages in the perivascular space surrounding a vascular tree that extended over centimeters. That was surprising, the researchers say. They expected to see iron within the parenchyma, but they also found iron inside macrophages outside of the parenchyma between the vessel and neuropil, tracking alongside vessels.
The researchers say that finding signified that the extent of injury was more extensive than indicated on MRI and had consequences to cellular function over a larger area of brain. In fact, they suggest, punctate and linear TMBs may not be distinct entities: The difference in shape may be “an issue of resolution.”
The researchers conclude that TMBs could be biomarkers for vascular injury. They also note that the leakage of blood from damaged blood vessels can trigger an inflammatory response. The damage to vessels, the disruption of normal pathways of blood flow, and the influx of inflammatory cells could result in secondary injury to the brain tissue due to ischemia.
Thus, TMBs may also be useful biomarkers for identifying which patients are candidates for treatments that reduce ischemic damage or improve microvascular cerebral blood flow.
Traumatic microbleeds (TMBs) may indicate vascular injury and predict worse outcomes after even minor brain injury, according to a study at the National Institute of Neurological Disorders and Stroke.
The study involved 439 adults with head injuries treated in the emergency department. The participants had magnetic resonance imaging (MRI) scans within 48 hours of the injury and again during 4 subsequent visits. They also completed behavioral and outcome questionnaires.
Microbleeds appear as small dark lesions on MRI scans but are usually too small to be seen on computer tomography (CT) scans. Sometimes they appear as dots (punctate), sometimes they are linear. In previous studies, researchers examined TMBs in the acute phase of traumatic brain injury (TBI) and stroke and found linear-appearing TMBs only in patients with TBI, suggesting that at least linear TMBs are consistent with trauma and might be the result of injured vessels. They conjectured that TMBs seen on MRI might be a form of traumatic vascular injury distinct from primary injury to the axons.
In this study, one-third of the patients had TMBs. More than half (58%) of the participants with severe head injury showed microbleeds, as did 27% of patients with mild injuries. In most patients with microbleeds, they appeared as linear streaks or dotted lesions. The study also revealed that the frontal lobes were the region most likely to show microbleeds.
The researchers controlled for variables known to predict poor outcome, such as trauma level and trauma-related injury on CT. Even so, microbleeds significantly predicted worse outcome. Patients with both punctate and linear TMBs were twice as likely to have disability (Glasgow Outcome Scale-Extended ≤6) on follow-up.
One participant’s family donated his brain for further analysis after he died. Imaging with a more powerful MRI scanner and a detailed histologic analysis allowed the researchers to better understand the pathology.
The researchers found that what appeared as a punctate TMB on MRI corresponded to iron-laden macrophages in the perivascular space surrounding a vascular tree that extended over centimeters. That was surprising, the researchers say. They expected to see iron within the parenchyma, but they also found iron inside macrophages outside of the parenchyma between the vessel and neuropil, tracking alongside vessels.
The researchers say that finding signified that the extent of injury was more extensive than indicated on MRI and had consequences to cellular function over a larger area of brain. In fact, they suggest, punctate and linear TMBs may not be distinct entities: The difference in shape may be “an issue of resolution.”
The researchers conclude that TMBs could be biomarkers for vascular injury. They also note that the leakage of blood from damaged blood vessels can trigger an inflammatory response. The damage to vessels, the disruption of normal pathways of blood flow, and the influx of inflammatory cells could result in secondary injury to the brain tissue due to ischemia.
Thus, TMBs may also be useful biomarkers for identifying which patients are candidates for treatments that reduce ischemic damage or improve microvascular cerebral blood flow.
Traumatic microbleeds (TMBs) may indicate vascular injury and predict worse outcomes after even minor brain injury, according to a study at the National Institute of Neurological Disorders and Stroke.
The study involved 439 adults with head injuries treated in the emergency department. The participants had magnetic resonance imaging (MRI) scans within 48 hours of the injury and again during 4 subsequent visits. They also completed behavioral and outcome questionnaires.
Microbleeds appear as small dark lesions on MRI scans but are usually too small to be seen on computer tomography (CT) scans. Sometimes they appear as dots (punctate), sometimes they are linear. In previous studies, researchers examined TMBs in the acute phase of traumatic brain injury (TBI) and stroke and found linear-appearing TMBs only in patients with TBI, suggesting that at least linear TMBs are consistent with trauma and might be the result of injured vessels. They conjectured that TMBs seen on MRI might be a form of traumatic vascular injury distinct from primary injury to the axons.
In this study, one-third of the patients had TMBs. More than half (58%) of the participants with severe head injury showed microbleeds, as did 27% of patients with mild injuries. In most patients with microbleeds, they appeared as linear streaks or dotted lesions. The study also revealed that the frontal lobes were the region most likely to show microbleeds.
The researchers controlled for variables known to predict poor outcome, such as trauma level and trauma-related injury on CT. Even so, microbleeds significantly predicted worse outcome. Patients with both punctate and linear TMBs were twice as likely to have disability (Glasgow Outcome Scale-Extended ≤6) on follow-up.
One participant’s family donated his brain for further analysis after he died. Imaging with a more powerful MRI scanner and a detailed histologic analysis allowed the researchers to better understand the pathology.
The researchers found that what appeared as a punctate TMB on MRI corresponded to iron-laden macrophages in the perivascular space surrounding a vascular tree that extended over centimeters. That was surprising, the researchers say. They expected to see iron within the parenchyma, but they also found iron inside macrophages outside of the parenchyma between the vessel and neuropil, tracking alongside vessels.
The researchers say that finding signified that the extent of injury was more extensive than indicated on MRI and had consequences to cellular function over a larger area of brain. In fact, they suggest, punctate and linear TMBs may not be distinct entities: The difference in shape may be “an issue of resolution.”
The researchers conclude that TMBs could be biomarkers for vascular injury. They also note that the leakage of blood from damaged blood vessels can trigger an inflammatory response. The damage to vessels, the disruption of normal pathways of blood flow, and the influx of inflammatory cells could result in secondary injury to the brain tissue due to ischemia.
Thus, TMBs may also be useful biomarkers for identifying which patients are candidates for treatments that reduce ischemic damage or improve microvascular cerebral blood flow.
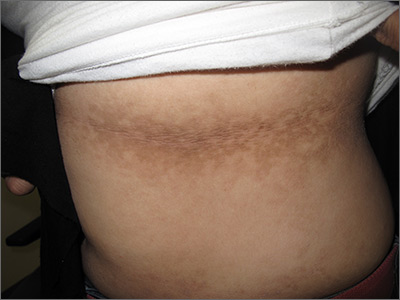
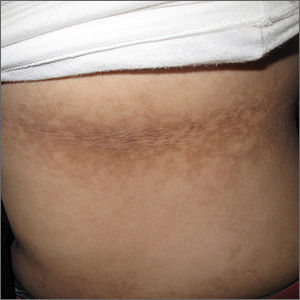
Dark patches around the trunk
The FP noticed a lacy net-like or reticulate appearance and thin brown papules to warty plaques over the trunk and recognized this condition as confluent and reticulated papillomatosis (CARP). A potassium hydroxide (KOH) test of a skin scraping failed to reveal yeast forms or hyphae. The FP determined that a biopsy was not necessary for diagnosis due to the distinct clinical appearance and negative KOH test. However, a biopsy could have distinguished this presentation from similar appearing disorders, including acanthosis nigricans and pityriasis versicolor.
CARP is an uncommon disorder of keratinization that affects adolescents and young adults, and is more common in Caucasians. A classic presentation involves the neck, chest, and abdomen. The differential diagnosis includes acanthosis nigricans and pityriasis versicolor, as well as more rare disorders that include Darier disease and keratosis follicularis.
There appears to be an association between the disorder and weight (specifically, being overweight). In addition, some familial cases have been reported.
Most recently, Dietzia papillomatosis, a gram-positive actinomycete has been implicated as a likely cause, which supports antibiotic therapy as the first-line approach. Minocycline 50 mg bid for 6 weeks clears the papules and plaques for most patients. Azithromycin and clarithromycin are alternatives, with various dosing strategies lasting 6 to 12 weeks. Complete clearance may take months to more than a year. About 15% of patients will experience recurrence.
This patient was treated with minocycline 50 mg bid for 12 weeks, a more common strategy at the time she was diagnosed. This led to complete clearance at 3 months, and she remained clear a year after beginning treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained).
The FP noticed a lacy net-like or reticulate appearance and thin brown papules to warty plaques over the trunk and recognized this condition as confluent and reticulated papillomatosis (CARP). A potassium hydroxide (KOH) test of a skin scraping failed to reveal yeast forms or hyphae. The FP determined that a biopsy was not necessary for diagnosis due to the distinct clinical appearance and negative KOH test. However, a biopsy could have distinguished this presentation from similar appearing disorders, including acanthosis nigricans and pityriasis versicolor.
CARP is an uncommon disorder of keratinization that affects adolescents and young adults, and is more common in Caucasians. A classic presentation involves the neck, chest, and abdomen. The differential diagnosis includes acanthosis nigricans and pityriasis versicolor, as well as more rare disorders that include Darier disease and keratosis follicularis.
There appears to be an association between the disorder and weight (specifically, being overweight). In addition, some familial cases have been reported.
Most recently, Dietzia papillomatosis, a gram-positive actinomycete has been implicated as a likely cause, which supports antibiotic therapy as the first-line approach. Minocycline 50 mg bid for 6 weeks clears the papules and plaques for most patients. Azithromycin and clarithromycin are alternatives, with various dosing strategies lasting 6 to 12 weeks. Complete clearance may take months to more than a year. About 15% of patients will experience recurrence.
This patient was treated with minocycline 50 mg bid for 12 weeks, a more common strategy at the time she was diagnosed. This led to complete clearance at 3 months, and she remained clear a year after beginning treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained).
The FP noticed a lacy net-like or reticulate appearance and thin brown papules to warty plaques over the trunk and recognized this condition as confluent and reticulated papillomatosis (CARP). A potassium hydroxide (KOH) test of a skin scraping failed to reveal yeast forms or hyphae. The FP determined that a biopsy was not necessary for diagnosis due to the distinct clinical appearance and negative KOH test. However, a biopsy could have distinguished this presentation from similar appearing disorders, including acanthosis nigricans and pityriasis versicolor.
CARP is an uncommon disorder of keratinization that affects adolescents and young adults, and is more common in Caucasians. A classic presentation involves the neck, chest, and abdomen. The differential diagnosis includes acanthosis nigricans and pityriasis versicolor, as well as more rare disorders that include Darier disease and keratosis follicularis.
There appears to be an association between the disorder and weight (specifically, being overweight). In addition, some familial cases have been reported.
Most recently, Dietzia papillomatosis, a gram-positive actinomycete has been implicated as a likely cause, which supports antibiotic therapy as the first-line approach. Minocycline 50 mg bid for 6 weeks clears the papules and plaques for most patients. Azithromycin and clarithromycin are alternatives, with various dosing strategies lasting 6 to 12 weeks. Complete clearance may take months to more than a year. About 15% of patients will experience recurrence.
This patient was treated with minocycline 50 mg bid for 12 weeks, a more common strategy at the time she was diagnosed. This led to complete clearance at 3 months, and she remained clear a year after beginning treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained).
FDA advisory committee supports birth control patch approval
Most of the committee members based their decisions on the need for additional contraceptive options for patients. However, most also expressed concerns about its efficacy and offered suggestions for product labeling that called attention to high rates of unintended pregnancies and increased risk of venous thromboembolism (VTE) in obese women.
The agency’s Bone, Reproductive and Urologic Drugs Advisory Committee reviewed safety and efficacy data for AG200-15, a combined hormonal contraceptive patch developed by Agile Therapeutics. The treatment regimen involves application of a patch to the abdomen, buttock, or upper torso, and the patch is changed weekly for 3 weeks, followed by 1 week without a patch.
Elizabeth Garner, MD, consultant and former chief medical officer of Agile, presented study data on safety and effectiveness of the patch. The key study (known as Study 23) considered by the FDA included 1,736 women aged 35 years and younger. The primary efficacy endpoint was the pregnancy rate in the women who used the patch. Women reported sexual activity and back-up contraception use in e-diaries.
A total of 68 pregnancies occurred in the study population after 15,165 evaluable cycles, yielding an overall Pearl Index of 5.83 across all weight and body mass index groups. Historically, a Pearl Index of 5 has been the standard measure for effectiveness in contraceptive products, with lower being better. The index is defined as the number of pregnancies per 100 woman-years of product use. For example, a Pearl Index of 0.1 means that 1 in 1,000 women who use the same contraceptive method for 1 year becomes pregnant.
A subgroup analysis showed reduced efficacy in women with a higher BMI. The Pearl Index for women with a BMI of less than 30 kg/m2 (defined as nonobese) was 4.34, whereas in women with a BMI of 30 kg/m2 and higher (defined as obese), the index was 8.64, nearly double that of nonobese women. No significant differences in the index were noted based on race/ethnicity.
The company described the patch as filling a niche and providing an additional alternative for women seeking a noninvasive method of contraception. It proposed a limitation of use (LOU) as part of the product label that would provide detailed information on efficacy based on the Pearl Index for the different categories of BMI and would suggest that the patch may be less effective for women with obesity. Most of the committee members favored use of a LOU statement on the label, but some noted that it might limit prescriptions to nonobese women.
The committee expressed concern over the Pearl data in the study. The FDA has never approved a contraceptive product with a Pearl Index of greater than 5, said Yun Tang, PhD, a statistical reviewer for the agency’s Office of Translational Sciences, who presented the evaluation of the effectiveness of AG200-15.
Key safety concerns raised in discussion included the risk of venous thromboembolism and the risk of unscheduled bleeding. Both of those issues were significantly more common among obese women, said Nneka McNeal-Jackson, MD, clinical reviewer for the FDA, who presented details on the safety profile and risk-benefit considerations for the patch.
Overall, in Study 23, the incidence rate of VTE was 28/10,000 women-years, with cases in five participants. Four of those were deemed related to the patch, and all occurred in obese women.
Virginia C. “Jennie” Leslie, MD, of Oregon Health and Science University, Portland, voted no to recommending approval of the patch mainly because of efficacy concerns. “My goal is to do no harm, and I have concerns regarding efficacy and giving our patients a false sense of hope,” she said.
Even those members who voted yes expressed concerns about the efficacy data and VTE risk in obese women and recommended postmarketing studies and appropriate labeling to help clinicians in shared decision making with their patients.
Esther Eisenberg, MD, of the National Institutes of Health, noted that the patch fills a need, certainly for women with a BMI less than 30 kg/m2, and suggested that use be limited to women in that lower BMI category.
Other committee members suggested that the product not be restricted based on BMI, but rather that the LOU provide clear explanations of how effectiveness decreases as BMI increases.
David J. Margolis, MD, of the University of Pennsylvania, Philadelphia, opted to abstain from voting, in part based on concerns about the study design and a lack of additional data from the company.
Most of the committee members based their decisions on the need for additional contraceptive options for patients. However, most also expressed concerns about its efficacy and offered suggestions for product labeling that called attention to high rates of unintended pregnancies and increased risk of venous thromboembolism (VTE) in obese women.
The agency’s Bone, Reproductive and Urologic Drugs Advisory Committee reviewed safety and efficacy data for AG200-15, a combined hormonal contraceptive patch developed by Agile Therapeutics. The treatment regimen involves application of a patch to the abdomen, buttock, or upper torso, and the patch is changed weekly for 3 weeks, followed by 1 week without a patch.
Elizabeth Garner, MD, consultant and former chief medical officer of Agile, presented study data on safety and effectiveness of the patch. The key study (known as Study 23) considered by the FDA included 1,736 women aged 35 years and younger. The primary efficacy endpoint was the pregnancy rate in the women who used the patch. Women reported sexual activity and back-up contraception use in e-diaries.
A total of 68 pregnancies occurred in the study population after 15,165 evaluable cycles, yielding an overall Pearl Index of 5.83 across all weight and body mass index groups. Historically, a Pearl Index of 5 has been the standard measure for effectiveness in contraceptive products, with lower being better. The index is defined as the number of pregnancies per 100 woman-years of product use. For example, a Pearl Index of 0.1 means that 1 in 1,000 women who use the same contraceptive method for 1 year becomes pregnant.
A subgroup analysis showed reduced efficacy in women with a higher BMI. The Pearl Index for women with a BMI of less than 30 kg/m2 (defined as nonobese) was 4.34, whereas in women with a BMI of 30 kg/m2 and higher (defined as obese), the index was 8.64, nearly double that of nonobese women. No significant differences in the index were noted based on race/ethnicity.
The company described the patch as filling a niche and providing an additional alternative for women seeking a noninvasive method of contraception. It proposed a limitation of use (LOU) as part of the product label that would provide detailed information on efficacy based on the Pearl Index for the different categories of BMI and would suggest that the patch may be less effective for women with obesity. Most of the committee members favored use of a LOU statement on the label, but some noted that it might limit prescriptions to nonobese women.
The committee expressed concern over the Pearl data in the study. The FDA has never approved a contraceptive product with a Pearl Index of greater than 5, said Yun Tang, PhD, a statistical reviewer for the agency’s Office of Translational Sciences, who presented the evaluation of the effectiveness of AG200-15.
Key safety concerns raised in discussion included the risk of venous thromboembolism and the risk of unscheduled bleeding. Both of those issues were significantly more common among obese women, said Nneka McNeal-Jackson, MD, clinical reviewer for the FDA, who presented details on the safety profile and risk-benefit considerations for the patch.
Overall, in Study 23, the incidence rate of VTE was 28/10,000 women-years, with cases in five participants. Four of those were deemed related to the patch, and all occurred in obese women.
Virginia C. “Jennie” Leslie, MD, of Oregon Health and Science University, Portland, voted no to recommending approval of the patch mainly because of efficacy concerns. “My goal is to do no harm, and I have concerns regarding efficacy and giving our patients a false sense of hope,” she said.
Even those members who voted yes expressed concerns about the efficacy data and VTE risk in obese women and recommended postmarketing studies and appropriate labeling to help clinicians in shared decision making with their patients.
Esther Eisenberg, MD, of the National Institutes of Health, noted that the patch fills a need, certainly for women with a BMI less than 30 kg/m2, and suggested that use be limited to women in that lower BMI category.
Other committee members suggested that the product not be restricted based on BMI, but rather that the LOU provide clear explanations of how effectiveness decreases as BMI increases.
David J. Margolis, MD, of the University of Pennsylvania, Philadelphia, opted to abstain from voting, in part based on concerns about the study design and a lack of additional data from the company.
Most of the committee members based their decisions on the need for additional contraceptive options for patients. However, most also expressed concerns about its efficacy and offered suggestions for product labeling that called attention to high rates of unintended pregnancies and increased risk of venous thromboembolism (VTE) in obese women.
The agency’s Bone, Reproductive and Urologic Drugs Advisory Committee reviewed safety and efficacy data for AG200-15, a combined hormonal contraceptive patch developed by Agile Therapeutics. The treatment regimen involves application of a patch to the abdomen, buttock, or upper torso, and the patch is changed weekly for 3 weeks, followed by 1 week without a patch.
Elizabeth Garner, MD, consultant and former chief medical officer of Agile, presented study data on safety and effectiveness of the patch. The key study (known as Study 23) considered by the FDA included 1,736 women aged 35 years and younger. The primary efficacy endpoint was the pregnancy rate in the women who used the patch. Women reported sexual activity and back-up contraception use in e-diaries.
A total of 68 pregnancies occurred in the study population after 15,165 evaluable cycles, yielding an overall Pearl Index of 5.83 across all weight and body mass index groups. Historically, a Pearl Index of 5 has been the standard measure for effectiveness in contraceptive products, with lower being better. The index is defined as the number of pregnancies per 100 woman-years of product use. For example, a Pearl Index of 0.1 means that 1 in 1,000 women who use the same contraceptive method for 1 year becomes pregnant.
A subgroup analysis showed reduced efficacy in women with a higher BMI. The Pearl Index for women with a BMI of less than 30 kg/m2 (defined as nonobese) was 4.34, whereas in women with a BMI of 30 kg/m2 and higher (defined as obese), the index was 8.64, nearly double that of nonobese women. No significant differences in the index were noted based on race/ethnicity.
The company described the patch as filling a niche and providing an additional alternative for women seeking a noninvasive method of contraception. It proposed a limitation of use (LOU) as part of the product label that would provide detailed information on efficacy based on the Pearl Index for the different categories of BMI and would suggest that the patch may be less effective for women with obesity. Most of the committee members favored use of a LOU statement on the label, but some noted that it might limit prescriptions to nonobese women.
The committee expressed concern over the Pearl data in the study. The FDA has never approved a contraceptive product with a Pearl Index of greater than 5, said Yun Tang, PhD, a statistical reviewer for the agency’s Office of Translational Sciences, who presented the evaluation of the effectiveness of AG200-15.
Key safety concerns raised in discussion included the risk of venous thromboembolism and the risk of unscheduled bleeding. Both of those issues were significantly more common among obese women, said Nneka McNeal-Jackson, MD, clinical reviewer for the FDA, who presented details on the safety profile and risk-benefit considerations for the patch.
Overall, in Study 23, the incidence rate of VTE was 28/10,000 women-years, with cases in five participants. Four of those were deemed related to the patch, and all occurred in obese women.
Virginia C. “Jennie” Leslie, MD, of Oregon Health and Science University, Portland, voted no to recommending approval of the patch mainly because of efficacy concerns. “My goal is to do no harm, and I have concerns regarding efficacy and giving our patients a false sense of hope,” she said.
Even those members who voted yes expressed concerns about the efficacy data and VTE risk in obese women and recommended postmarketing studies and appropriate labeling to help clinicians in shared decision making with their patients.
Esther Eisenberg, MD, of the National Institutes of Health, noted that the patch fills a need, certainly for women with a BMI less than 30 kg/m2, and suggested that use be limited to women in that lower BMI category.
Other committee members suggested that the product not be restricted based on BMI, but rather that the LOU provide clear explanations of how effectiveness decreases as BMI increases.
David J. Margolis, MD, of the University of Pennsylvania, Philadelphia, opted to abstain from voting, in part based on concerns about the study design and a lack of additional data from the company.
FROM THE FDA
Werewolves of Vallejo and a haunted-house doctor’s note
A crappy excuse of a database
Have you ever been so impressed with your bowel movement that you’ve been compelled to record the incident for posterity? No? Just us? Well, you may want to reconsider, because a pair of AI tech companies are looking for a few good poop pictures.
It’s all part of the “Give a S--t” (you can probably guess what we’ve censored out) campaign, a joint venture from Auggi, a gut health start-up, and Seed Health. The companies hope to use photos sent in by regular people to build an app that would help people with chronic gut problems automatically track their own bowel movements. In addition, the photo library could also be used for research into gut-related diseases such as irritable bowl syndrome.
The two companies hope to collect 100,000 photos for their library, which is an absolutely prodigious amount of poop to sort through. But hey, that’s what the AI is for. They already know the AI works, as Auggi created a proof-of-concept library of 36,000 images of faux feces made from blue Play-Doh. The AI was able to recognize consistency according to the Bristol scale basically 100% of the time.
If you’ve been inspired, you can submit your lovely poop pictures here. Seed and Auggi expect contributers to send only one image each, but multiple submissions are welcome. They’ve already received a dozen from LOTME world headquarters. We love a good bowel movement here.
Criminal moon
“The Wolf Man.” “An American Werewolf in London.” “The Howling.” “Teen Wolf.” All terrifying Hollywood tales of bloodthirsty behavior and sanguinary slaughter. (Michael J. Fox as a hirsute homicidal lycan? Okay, maybe not “Teen Wolf.”)
And the propellant igniting all that criminal lycanthropy? The full moon.
Any teacher will swear a full moon portends the kind of student behavior that an entire pot of teachers’ lounge coffee can’t counter. And every cop knows it’s going to be a “Training Day” shift when the lunar light shines brightest.
But is the Thin Blue Line truly stretched to snapping during a full moon? New York University’s BetaGov research team looked at the purported “lunar effect” linking crime and the full moon. A lit review revealed mixed findings for and against a criminal lunar effect. The team then collaborated with the Vallejo, Calif., police department to match the moon’s phases with the city’s crime events. They did the same with departments in Canada and Mexico.
The results? A full moon had no effect on Vallejo’s crime rate, or anywhere else in North America.
While the finding eviscerates the moon-induced mayhem hypothesis, cops walking a full-moonlit beat can at least take comfort in this fact: Unlike London, Vallejo is clearly free of American werewolves.
A doctor’s note … of terror
With Halloween upon us, here’s a veddy scary riddle: When is a sports physical not a sports physical?
When it’s a haunted house physical.
Specifically, when the haunted house is McKamey Manor in Summertown, Tenn. … and in Huntsville, Ala. That’s right, it can be in two places at the same time. Terrifying.
McKamey Manor is considered by many to be the most terrifying haunted house in the United States, and by some to be a “torture chamber under disguise.”
The “Surivial [we think they misspelled it on purpose to make it even scarier] Horror Challenge” is so terrifying that management requires all participants to have a “completed ‘sports physical’ and doctor’s letter stating you are physically and mentally cleared,” as well as proof of medical insurance. Each paying customer also has to “pass a portable drug test on the day of the show,” according to the McKamey Manor website.
The manor also happens to be the subject of a petition, which currently has over 58,000 signatures, asking state officials in Alabama and Tennessee to shut it down because “some people have had to seek professional psychiatric help and medical care for extensive injuries.”
Ironically, we hear that some of the most traumatized customers have been actual physicians who succumbed to the horrors of Prior Approval Asylum, the EHR Torment Room, and the River of the Damned Maintenance of Certification.
A crappy excuse of a database
Have you ever been so impressed with your bowel movement that you’ve been compelled to record the incident for posterity? No? Just us? Well, you may want to reconsider, because a pair of AI tech companies are looking for a few good poop pictures.
It’s all part of the “Give a S--t” (you can probably guess what we’ve censored out) campaign, a joint venture from Auggi, a gut health start-up, and Seed Health. The companies hope to use photos sent in by regular people to build an app that would help people with chronic gut problems automatically track their own bowel movements. In addition, the photo library could also be used for research into gut-related diseases such as irritable bowl syndrome.
The two companies hope to collect 100,000 photos for their library, which is an absolutely prodigious amount of poop to sort through. But hey, that’s what the AI is for. They already know the AI works, as Auggi created a proof-of-concept library of 36,000 images of faux feces made from blue Play-Doh. The AI was able to recognize consistency according to the Bristol scale basically 100% of the time.
If you’ve been inspired, you can submit your lovely poop pictures here. Seed and Auggi expect contributers to send only one image each, but multiple submissions are welcome. They’ve already received a dozen from LOTME world headquarters. We love a good bowel movement here.
Criminal moon
“The Wolf Man.” “An American Werewolf in London.” “The Howling.” “Teen Wolf.” All terrifying Hollywood tales of bloodthirsty behavior and sanguinary slaughter. (Michael J. Fox as a hirsute homicidal lycan? Okay, maybe not “Teen Wolf.”)
And the propellant igniting all that criminal lycanthropy? The full moon.
Any teacher will swear a full moon portends the kind of student behavior that an entire pot of teachers’ lounge coffee can’t counter. And every cop knows it’s going to be a “Training Day” shift when the lunar light shines brightest.
But is the Thin Blue Line truly stretched to snapping during a full moon? New York University’s BetaGov research team looked at the purported “lunar effect” linking crime and the full moon. A lit review revealed mixed findings for and against a criminal lunar effect. The team then collaborated with the Vallejo, Calif., police department to match the moon’s phases with the city’s crime events. They did the same with departments in Canada and Mexico.
The results? A full moon had no effect on Vallejo’s crime rate, or anywhere else in North America.
While the finding eviscerates the moon-induced mayhem hypothesis, cops walking a full-moonlit beat can at least take comfort in this fact: Unlike London, Vallejo is clearly free of American werewolves.
A doctor’s note … of terror
With Halloween upon us, here’s a veddy scary riddle: When is a sports physical not a sports physical?
When it’s a haunted house physical.
Specifically, when the haunted house is McKamey Manor in Summertown, Tenn. … and in Huntsville, Ala. That’s right, it can be in two places at the same time. Terrifying.
McKamey Manor is considered by many to be the most terrifying haunted house in the United States, and by some to be a “torture chamber under disguise.”
The “Surivial [we think they misspelled it on purpose to make it even scarier] Horror Challenge” is so terrifying that management requires all participants to have a “completed ‘sports physical’ and doctor’s letter stating you are physically and mentally cleared,” as well as proof of medical insurance. Each paying customer also has to “pass a portable drug test on the day of the show,” according to the McKamey Manor website.
The manor also happens to be the subject of a petition, which currently has over 58,000 signatures, asking state officials in Alabama and Tennessee to shut it down because “some people have had to seek professional psychiatric help and medical care for extensive injuries.”
Ironically, we hear that some of the most traumatized customers have been actual physicians who succumbed to the horrors of Prior Approval Asylum, the EHR Torment Room, and the River of the Damned Maintenance of Certification.
A crappy excuse of a database
Have you ever been so impressed with your bowel movement that you’ve been compelled to record the incident for posterity? No? Just us? Well, you may want to reconsider, because a pair of AI tech companies are looking for a few good poop pictures.
It’s all part of the “Give a S--t” (you can probably guess what we’ve censored out) campaign, a joint venture from Auggi, a gut health start-up, and Seed Health. The companies hope to use photos sent in by regular people to build an app that would help people with chronic gut problems automatically track their own bowel movements. In addition, the photo library could also be used for research into gut-related diseases such as irritable bowl syndrome.
The two companies hope to collect 100,000 photos for their library, which is an absolutely prodigious amount of poop to sort through. But hey, that’s what the AI is for. They already know the AI works, as Auggi created a proof-of-concept library of 36,000 images of faux feces made from blue Play-Doh. The AI was able to recognize consistency according to the Bristol scale basically 100% of the time.
If you’ve been inspired, you can submit your lovely poop pictures here. Seed and Auggi expect contributers to send only one image each, but multiple submissions are welcome. They’ve already received a dozen from LOTME world headquarters. We love a good bowel movement here.
Criminal moon
“The Wolf Man.” “An American Werewolf in London.” “The Howling.” “Teen Wolf.” All terrifying Hollywood tales of bloodthirsty behavior and sanguinary slaughter. (Michael J. Fox as a hirsute homicidal lycan? Okay, maybe not “Teen Wolf.”)
And the propellant igniting all that criminal lycanthropy? The full moon.
Any teacher will swear a full moon portends the kind of student behavior that an entire pot of teachers’ lounge coffee can’t counter. And every cop knows it’s going to be a “Training Day” shift when the lunar light shines brightest.
But is the Thin Blue Line truly stretched to snapping during a full moon? New York University’s BetaGov research team looked at the purported “lunar effect” linking crime and the full moon. A lit review revealed mixed findings for and against a criminal lunar effect. The team then collaborated with the Vallejo, Calif., police department to match the moon’s phases with the city’s crime events. They did the same with departments in Canada and Mexico.
The results? A full moon had no effect on Vallejo’s crime rate, or anywhere else in North America.
While the finding eviscerates the moon-induced mayhem hypothesis, cops walking a full-moonlit beat can at least take comfort in this fact: Unlike London, Vallejo is clearly free of American werewolves.
A doctor’s note … of terror
With Halloween upon us, here’s a veddy scary riddle: When is a sports physical not a sports physical?
When it’s a haunted house physical.
Specifically, when the haunted house is McKamey Manor in Summertown, Tenn. … and in Huntsville, Ala. That’s right, it can be in two places at the same time. Terrifying.
McKamey Manor is considered by many to be the most terrifying haunted house in the United States, and by some to be a “torture chamber under disguise.”
The “Surivial [we think they misspelled it on purpose to make it even scarier] Horror Challenge” is so terrifying that management requires all participants to have a “completed ‘sports physical’ and doctor’s letter stating you are physically and mentally cleared,” as well as proof of medical insurance. Each paying customer also has to “pass a portable drug test on the day of the show,” according to the McKamey Manor website.
The manor also happens to be the subject of a petition, which currently has over 58,000 signatures, asking state officials in Alabama and Tennessee to shut it down because “some people have had to seek professional psychiatric help and medical care for extensive injuries.”
Ironically, we hear that some of the most traumatized customers have been actual physicians who succumbed to the horrors of Prior Approval Asylum, the EHR Torment Room, and the River of the Damned Maintenance of Certification.
A sepsis death linked to fecal microbiota transplantation
Two cases of bacteremia have been described in two patients who received fecal microbiota transplants from the same donor.
Writing in the New England Journal of Medicine, researchers reported the two case studies of extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli bacteremia, one of which ended in the death of the patient. These cases were previously announced by the Food and Drug Administration in a June 2019 safety alert.
Zachariah DeFilipp, MD, from Massachusetts General Hospital at Harvard Medical School, Boston, and coauthors wrote that fecal microbiota transplantation is rarely associated with complications. Placebo-controlled trials and a systematic review have found similar rates of complications in immunocompromised and immunocompetent recipients. Only four cases of gram-negative bacteremia previously have been reported, and in three of these, there was a plausible alternative explanation for the bacteremia.
In this paper, both patients received fecal microbiota transplantation via frozen oral capsules containing donor stool. These capsules were prepared prior to the implementation of screening for ESBL-producing organisms at the institution, and were not retrospectively tested since this expanded donor screening.
The first patient was a 69-year-old man with liver cirrhosis attributed to hepatitis C infection who was enrolled in a trial of fecal microbiota transplantation via oral capsules to treat hepatic encephalopathy. The first sign of the adverse event was a fever and cough, which developed 17 days after the final dose of 15 capsules. He was treated for pneumonia but failed to improve after 2 days, at which time gram-negative rods were discovered in blood cultures taken at the initial presentation.
After admission and further treatment, blood cultures were found to have ESBL-producing E. coli, and after further treatment, the patient was clinically stable. A stool sample taken after treatment was negative for ESBL-producing E. coli.
The second case study was a 73-year-old man with therapy-related myelodysplastic syndrome who was undergoing allogeneic hematopoietic stem cell transplantation and was receiving fecal microbiota transplantation via oral capsule as part of a phase 2 trial.
Eight days after the last dose of oral capsules, and 5 days after the stem-cell infusion, the man developed a fever, chills, febrile neutropenia and showed altered mental status. He was treated with cefepime but developed hypoxia and labored breathing later that evening, which prompted clinicians to intubate and begin mechanical ventilation.
His blood culture results showed gram-negative rods, and meropenem was added to his antibiotic regimen. However, the patient’s condition worsened, and he died of severe sepsis 2 days later with blood cultures confirmed as positive for ESBL-producing E. coli.
A follow-up investigation revealed that both patients received stool from the same donor. Each lot of three capsules from that donor was found to contain ESBL-producing E. coli with a resistance pattern similar to that seen in the two recipients.
Twenty-two patients had received capsules from this donor. Researchers contacted all the recipients and offered them stool screening for ESBL-producing E. coli. Twelve underwent testing, which found that five had samples that grew on ESBL-producing E. coli–selective medium.
The remaining seven patients who had follow-up testing were receiving treatment for recurrent or refractory Clostridioides difficile infection, and four of these grew samples on the selective medium.
“When FMT is successful, the recipient’s metagenomic burden of antimicrobial resistance genes mimics that of the donor,” the authors wrote. “Although we cannot conclusively attribute positive screening results for ESBL-producing organisms in other asymptomatic recipients to FMT, the rates of positive tests are, in our opinion, unexpectedly high and probably represent transmission through FMT.”
The authors said the donor had no risk factors for carriage of multidrug-resistant organism and had previously donated fecal material before the introduction of routine screening for ESBL-producing organisms.
However, they noted that both patients had risk factors for bacteremia, namely advanced cirrhosis and allogeneic hematopoietic stem cell transplantation and they also received oral antibiotics around the time of the fecal microbiota transplantation.
“Despite the infectious complications reported here, the benefits of FMT should be balanced with the associated risks when considering treatment options for patients with recurrent or refractory C. difficile infection,” the authors wrote. “Ongoing assessment of the risks and benefit of FMT research is needed, as are continuing efforts to improve donor screening to limit transmission of microorganisms that could lead to adverse infectious events.”
The American Gastroenterological Association FMT National Registry is a critical effort to track short- and long-term patient outcomes and potential risks associated with FMT. The registry's goal is to track 4,000 patients for 10 years. If you perform FMT, please contribute to this important initiative. Learn more at www.gastro.org/FMTRegistry.
The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two were attached to a diagnostics company involved in the study.
SOURCE: DeFilipp Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
* This story was updated on Oct. 31, 2019.
Fecal microbiota transplantation could have therapeutic utility in a range of conditions in which primary dysbiosis is suspected, but this study shows the procedure may carry risks that only become apparent after treatment. Improved screening of donors and fecal material could reduce the risks of infections by known agents. However, new pathogens may not be recognized until after they have been transplanted into a new host.
The benefits and risks of fecal microbiota transplantation must be balanced, but up to now the complications have been infrequent and the benefits have clearly outweighed the risks.
Martin J. Blaser, MD, is from Rutgers University in New Brunswick, N.J. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMe1913807). Dr. Blaser declared personal fees and stock options from the medical sector unrelated to the work.
Fecal microbiota transplantation could have therapeutic utility in a range of conditions in which primary dysbiosis is suspected, but this study shows the procedure may carry risks that only become apparent after treatment. Improved screening of donors and fecal material could reduce the risks of infections by known agents. However, new pathogens may not be recognized until after they have been transplanted into a new host.
The benefits and risks of fecal microbiota transplantation must be balanced, but up to now the complications have been infrequent and the benefits have clearly outweighed the risks.
Martin J. Blaser, MD, is from Rutgers University in New Brunswick, N.J. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMe1913807). Dr. Blaser declared personal fees and stock options from the medical sector unrelated to the work.
Fecal microbiota transplantation could have therapeutic utility in a range of conditions in which primary dysbiosis is suspected, but this study shows the procedure may carry risks that only become apparent after treatment. Improved screening of donors and fecal material could reduce the risks of infections by known agents. However, new pathogens may not be recognized until after they have been transplanted into a new host.
The benefits and risks of fecal microbiota transplantation must be balanced, but up to now the complications have been infrequent and the benefits have clearly outweighed the risks.
Martin J. Blaser, MD, is from Rutgers University in New Brunswick, N.J. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMe1913807). Dr. Blaser declared personal fees and stock options from the medical sector unrelated to the work.
Two cases of bacteremia have been described in two patients who received fecal microbiota transplants from the same donor.
Writing in the New England Journal of Medicine, researchers reported the two case studies of extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli bacteremia, one of which ended in the death of the patient. These cases were previously announced by the Food and Drug Administration in a June 2019 safety alert.
Zachariah DeFilipp, MD, from Massachusetts General Hospital at Harvard Medical School, Boston, and coauthors wrote that fecal microbiota transplantation is rarely associated with complications. Placebo-controlled trials and a systematic review have found similar rates of complications in immunocompromised and immunocompetent recipients. Only four cases of gram-negative bacteremia previously have been reported, and in three of these, there was a plausible alternative explanation for the bacteremia.
In this paper, both patients received fecal microbiota transplantation via frozen oral capsules containing donor stool. These capsules were prepared prior to the implementation of screening for ESBL-producing organisms at the institution, and were not retrospectively tested since this expanded donor screening.
The first patient was a 69-year-old man with liver cirrhosis attributed to hepatitis C infection who was enrolled in a trial of fecal microbiota transplantation via oral capsules to treat hepatic encephalopathy. The first sign of the adverse event was a fever and cough, which developed 17 days after the final dose of 15 capsules. He was treated for pneumonia but failed to improve after 2 days, at which time gram-negative rods were discovered in blood cultures taken at the initial presentation.
After admission and further treatment, blood cultures were found to have ESBL-producing E. coli, and after further treatment, the patient was clinically stable. A stool sample taken after treatment was negative for ESBL-producing E. coli.
The second case study was a 73-year-old man with therapy-related myelodysplastic syndrome who was undergoing allogeneic hematopoietic stem cell transplantation and was receiving fecal microbiota transplantation via oral capsule as part of a phase 2 trial.
Eight days after the last dose of oral capsules, and 5 days after the stem-cell infusion, the man developed a fever, chills, febrile neutropenia and showed altered mental status. He was treated with cefepime but developed hypoxia and labored breathing later that evening, which prompted clinicians to intubate and begin mechanical ventilation.
His blood culture results showed gram-negative rods, and meropenem was added to his antibiotic regimen. However, the patient’s condition worsened, and he died of severe sepsis 2 days later with blood cultures confirmed as positive for ESBL-producing E. coli.
A follow-up investigation revealed that both patients received stool from the same donor. Each lot of three capsules from that donor was found to contain ESBL-producing E. coli with a resistance pattern similar to that seen in the two recipients.
Twenty-two patients had received capsules from this donor. Researchers contacted all the recipients and offered them stool screening for ESBL-producing E. coli. Twelve underwent testing, which found that five had samples that grew on ESBL-producing E. coli–selective medium.
The remaining seven patients who had follow-up testing were receiving treatment for recurrent or refractory Clostridioides difficile infection, and four of these grew samples on the selective medium.
“When FMT is successful, the recipient’s metagenomic burden of antimicrobial resistance genes mimics that of the donor,” the authors wrote. “Although we cannot conclusively attribute positive screening results for ESBL-producing organisms in other asymptomatic recipients to FMT, the rates of positive tests are, in our opinion, unexpectedly high and probably represent transmission through FMT.”
The authors said the donor had no risk factors for carriage of multidrug-resistant organism and had previously donated fecal material before the introduction of routine screening for ESBL-producing organisms.
However, they noted that both patients had risk factors for bacteremia, namely advanced cirrhosis and allogeneic hematopoietic stem cell transplantation and they also received oral antibiotics around the time of the fecal microbiota transplantation.
“Despite the infectious complications reported here, the benefits of FMT should be balanced with the associated risks when considering treatment options for patients with recurrent or refractory C. difficile infection,” the authors wrote. “Ongoing assessment of the risks and benefit of FMT research is needed, as are continuing efforts to improve donor screening to limit transmission of microorganisms that could lead to adverse infectious events.”
The American Gastroenterological Association FMT National Registry is a critical effort to track short- and long-term patient outcomes and potential risks associated with FMT. The registry's goal is to track 4,000 patients for 10 years. If you perform FMT, please contribute to this important initiative. Learn more at www.gastro.org/FMTRegistry.
The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two were attached to a diagnostics company involved in the study.
SOURCE: DeFilipp Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
* This story was updated on Oct. 31, 2019.
Two cases of bacteremia have been described in two patients who received fecal microbiota transplants from the same donor.
Writing in the New England Journal of Medicine, researchers reported the two case studies of extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli bacteremia, one of which ended in the death of the patient. These cases were previously announced by the Food and Drug Administration in a June 2019 safety alert.
Zachariah DeFilipp, MD, from Massachusetts General Hospital at Harvard Medical School, Boston, and coauthors wrote that fecal microbiota transplantation is rarely associated with complications. Placebo-controlled trials and a systematic review have found similar rates of complications in immunocompromised and immunocompetent recipients. Only four cases of gram-negative bacteremia previously have been reported, and in three of these, there was a plausible alternative explanation for the bacteremia.
In this paper, both patients received fecal microbiota transplantation via frozen oral capsules containing donor stool. These capsules were prepared prior to the implementation of screening for ESBL-producing organisms at the institution, and were not retrospectively tested since this expanded donor screening.
The first patient was a 69-year-old man with liver cirrhosis attributed to hepatitis C infection who was enrolled in a trial of fecal microbiota transplantation via oral capsules to treat hepatic encephalopathy. The first sign of the adverse event was a fever and cough, which developed 17 days after the final dose of 15 capsules. He was treated for pneumonia but failed to improve after 2 days, at which time gram-negative rods were discovered in blood cultures taken at the initial presentation.
After admission and further treatment, blood cultures were found to have ESBL-producing E. coli, and after further treatment, the patient was clinically stable. A stool sample taken after treatment was negative for ESBL-producing E. coli.
The second case study was a 73-year-old man with therapy-related myelodysplastic syndrome who was undergoing allogeneic hematopoietic stem cell transplantation and was receiving fecal microbiota transplantation via oral capsule as part of a phase 2 trial.
Eight days after the last dose of oral capsules, and 5 days after the stem-cell infusion, the man developed a fever, chills, febrile neutropenia and showed altered mental status. He was treated with cefepime but developed hypoxia and labored breathing later that evening, which prompted clinicians to intubate and begin mechanical ventilation.
His blood culture results showed gram-negative rods, and meropenem was added to his antibiotic regimen. However, the patient’s condition worsened, and he died of severe sepsis 2 days later with blood cultures confirmed as positive for ESBL-producing E. coli.
A follow-up investigation revealed that both patients received stool from the same donor. Each lot of three capsules from that donor was found to contain ESBL-producing E. coli with a resistance pattern similar to that seen in the two recipients.
Twenty-two patients had received capsules from this donor. Researchers contacted all the recipients and offered them stool screening for ESBL-producing E. coli. Twelve underwent testing, which found that five had samples that grew on ESBL-producing E. coli–selective medium.
The remaining seven patients who had follow-up testing were receiving treatment for recurrent or refractory Clostridioides difficile infection, and four of these grew samples on the selective medium.
“When FMT is successful, the recipient’s metagenomic burden of antimicrobial resistance genes mimics that of the donor,” the authors wrote. “Although we cannot conclusively attribute positive screening results for ESBL-producing organisms in other asymptomatic recipients to FMT, the rates of positive tests are, in our opinion, unexpectedly high and probably represent transmission through FMT.”
The authors said the donor had no risk factors for carriage of multidrug-resistant organism and had previously donated fecal material before the introduction of routine screening for ESBL-producing organisms.
However, they noted that both patients had risk factors for bacteremia, namely advanced cirrhosis and allogeneic hematopoietic stem cell transplantation and they also received oral antibiotics around the time of the fecal microbiota transplantation.
“Despite the infectious complications reported here, the benefits of FMT should be balanced with the associated risks when considering treatment options for patients with recurrent or refractory C. difficile infection,” the authors wrote. “Ongoing assessment of the risks and benefit of FMT research is needed, as are continuing efforts to improve donor screening to limit transmission of microorganisms that could lead to adverse infectious events.”
The American Gastroenterological Association FMT National Registry is a critical effort to track short- and long-term patient outcomes and potential risks associated with FMT. The registry's goal is to track 4,000 patients for 10 years. If you perform FMT, please contribute to this important initiative. Learn more at www.gastro.org/FMTRegistry.
The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two were attached to a diagnostics company involved in the study.
SOURCE: DeFilipp Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
* This story was updated on Oct. 31, 2019.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Two cases of bacteremia – one fatal – have been linked to a fecal microbiota transplant.
Major finding: Two patients developed bacteremia after receiving a fecal microbiota transplant from the same donor.
Study details: Case studies.
Disclosures: The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two authors were attached to a diagnostics company involved in the study.
Source: DeFillip Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
Interview with Clyde E. Markowitz, MD on switching therapies during MS treatment
Clyde E. Markowitz, MD, is the director of the Multiple Sclerosis Center at Penn Neuroscience Center and an Associate Professor of Neurology at the Perelman School of Medicine at the University of Pennsylvania. We sat down with Dr. Markowitz to talk about different multiple sclerosis (MS) therapies and how to determine when it might be time to switch a patient’s current regimen.
Why would an MS specialist switch a patient from one drug therapy to another?
The main reason we switch a patient from one treatment to another is usually related to an inadequate response to their current treatment. This can be seen when a patient is having new clinical symptoms suggestive of a relapse. Additional situations which would cause us to consider a switch in treatment include if the patient has had a new abnormalities seen on MRI scans, such as new T2 lesions or gadolinium- enhancing lesions. We might also switch a patient due to intolerance towards the medication they are on. For example, if they are experiencing flu-like symptoms or having Gastrointestinal issues.
In addition, the expectation that the treatment should slow the rate of progression may not be adequately demonstrating the desired effect. In that setting, we may consider a switch to a drug with a different mechanism of action to hopefully better control disease progression.
Laboratory abnormalities while on treatment might also be a consideration for a switch in therapy. Elevated LFTs, or low WBCs can occur on DMTs and may require a change in treatment. Patients on Natalizumab, require JC virus antibody testing. If the patient’s JCV Ab status changes from negative to positive or a rising index may require a change in therapy to avoid the development of PML.
What are some special considerations for patients during a switch in therapy?
We need to take into consideration the patient’s comorbidities. Does the patient have a history of diabetes, hypertension, cardiac concerns or a risk for infectious complications? What is the patient’s age? As individuals age the immune system becomes less robust at fighting infections or surveillance for malignancies. Some of the medications are immunosuppressive and might increase the risk of developing opportunistic infections or cancers.
Family planning should be taken into consideration during the discussion of which medications might be appropriate. Is the patient planning to have a pregnancy in the near future? Some medications might not be appropriate in that case.
Route of administration could be a factor to consider, since there are several medications that are administered as an infusion in a medical office or hospital setting. This could create issues for some patients who are employed and may have to miss work during these infusions. This could be as frequent as monthly or 2-3 times per year. Some patients just starting a new job, may feel uncomfortable taking time off or disclosing that they have MS leading to concerns for job security.
We also consider the side effects of the new treatment. What side effects and safety monitoring are required for a particular medication? Are there frequent blood tests, cardiac monitoring, dermatologic and ophthalmologic monitoring? How will this impact the patient’s quality of life?
In the end, it comes down to the level of monitoring required for a particular treatment, where the patient is in his or her life, and where he or she is in the disease course.
What are some potential complications when switching therapies?
When switching therapies, one of the bigger concerns is how quickly can we get the patient on the new therapy. Some medications when stopped can lead to return of disease activity or possibly lead to a rebound phenomenon with significant inflammatory activity. We focus on transitioning a patient quickly to a new drug that has a rapid mechanism of action thus limiting the amount of time that a patient is without a treatment. However, based on the mechanism of action of the drug you must consider if a wash out is necessary. The question is how quickly can the patient start the new drug thus preventing a rebound phenomenon. Ideally, no wash out would protect the patient best but might have safety concerns depending on the switch drug profile. If the switch was related to concerns for high JC virus antibody titer going off of natalizumab, there may be a need to make sure the patient does not have PML before making the switch. This may require MRIs and CSF analysis prior to switching.
Ultimately, we consider whether the drug we are switching the patient to is going to be more efficacious than the drug that the patient was previously on. We consider the safety and side effect profile of the new medication. We balance the risk of the disease with the risk of the medication. We must factor in the patient’s tolerance for risk as well and make the best decision with all the available factors considered.
Clyde E. Markowitz, MD, is the director of the Multiple Sclerosis Center at Penn Neuroscience Center and an Associate Professor of Neurology at the Perelman School of Medicine at the University of Pennsylvania. We sat down with Dr. Markowitz to talk about different multiple sclerosis (MS) therapies and how to determine when it might be time to switch a patient’s current regimen.
Why would an MS specialist switch a patient from one drug therapy to another?
The main reason we switch a patient from one treatment to another is usually related to an inadequate response to their current treatment. This can be seen when a patient is having new clinical symptoms suggestive of a relapse. Additional situations which would cause us to consider a switch in treatment include if the patient has had a new abnormalities seen on MRI scans, such as new T2 lesions or gadolinium- enhancing lesions. We might also switch a patient due to intolerance towards the medication they are on. For example, if they are experiencing flu-like symptoms or having Gastrointestinal issues.
In addition, the expectation that the treatment should slow the rate of progression may not be adequately demonstrating the desired effect. In that setting, we may consider a switch to a drug with a different mechanism of action to hopefully better control disease progression.
Laboratory abnormalities while on treatment might also be a consideration for a switch in therapy. Elevated LFTs, or low WBCs can occur on DMTs and may require a change in treatment. Patients on Natalizumab, require JC virus antibody testing. If the patient’s JCV Ab status changes from negative to positive or a rising index may require a change in therapy to avoid the development of PML.
What are some special considerations for patients during a switch in therapy?
We need to take into consideration the patient’s comorbidities. Does the patient have a history of diabetes, hypertension, cardiac concerns or a risk for infectious complications? What is the patient’s age? As individuals age the immune system becomes less robust at fighting infections or surveillance for malignancies. Some of the medications are immunosuppressive and might increase the risk of developing opportunistic infections or cancers.
Family planning should be taken into consideration during the discussion of which medications might be appropriate. Is the patient planning to have a pregnancy in the near future? Some medications might not be appropriate in that case.
Route of administration could be a factor to consider, since there are several medications that are administered as an infusion in a medical office or hospital setting. This could create issues for some patients who are employed and may have to miss work during these infusions. This could be as frequent as monthly or 2-3 times per year. Some patients just starting a new job, may feel uncomfortable taking time off or disclosing that they have MS leading to concerns for job security.
We also consider the side effects of the new treatment. What side effects and safety monitoring are required for a particular medication? Are there frequent blood tests, cardiac monitoring, dermatologic and ophthalmologic monitoring? How will this impact the patient’s quality of life?
In the end, it comes down to the level of monitoring required for a particular treatment, where the patient is in his or her life, and where he or she is in the disease course.
What are some potential complications when switching therapies?
When switching therapies, one of the bigger concerns is how quickly can we get the patient on the new therapy. Some medications when stopped can lead to return of disease activity or possibly lead to a rebound phenomenon with significant inflammatory activity. We focus on transitioning a patient quickly to a new drug that has a rapid mechanism of action thus limiting the amount of time that a patient is without a treatment. However, based on the mechanism of action of the drug you must consider if a wash out is necessary. The question is how quickly can the patient start the new drug thus preventing a rebound phenomenon. Ideally, no wash out would protect the patient best but might have safety concerns depending on the switch drug profile. If the switch was related to concerns for high JC virus antibody titer going off of natalizumab, there may be a need to make sure the patient does not have PML before making the switch. This may require MRIs and CSF analysis prior to switching.
Ultimately, we consider whether the drug we are switching the patient to is going to be more efficacious than the drug that the patient was previously on. We consider the safety and side effect profile of the new medication. We balance the risk of the disease with the risk of the medication. We must factor in the patient’s tolerance for risk as well and make the best decision with all the available factors considered.
Clyde E. Markowitz, MD, is the director of the Multiple Sclerosis Center at Penn Neuroscience Center and an Associate Professor of Neurology at the Perelman School of Medicine at the University of Pennsylvania. We sat down with Dr. Markowitz to talk about different multiple sclerosis (MS) therapies and how to determine when it might be time to switch a patient’s current regimen.
Why would an MS specialist switch a patient from one drug therapy to another?
The main reason we switch a patient from one treatment to another is usually related to an inadequate response to their current treatment. This can be seen when a patient is having new clinical symptoms suggestive of a relapse. Additional situations which would cause us to consider a switch in treatment include if the patient has had a new abnormalities seen on MRI scans, such as new T2 lesions or gadolinium- enhancing lesions. We might also switch a patient due to intolerance towards the medication they are on. For example, if they are experiencing flu-like symptoms or having Gastrointestinal issues.
In addition, the expectation that the treatment should slow the rate of progression may not be adequately demonstrating the desired effect. In that setting, we may consider a switch to a drug with a different mechanism of action to hopefully better control disease progression.
Laboratory abnormalities while on treatment might also be a consideration for a switch in therapy. Elevated LFTs, or low WBCs can occur on DMTs and may require a change in treatment. Patients on Natalizumab, require JC virus antibody testing. If the patient’s JCV Ab status changes from negative to positive or a rising index may require a change in therapy to avoid the development of PML.
What are some special considerations for patients during a switch in therapy?
We need to take into consideration the patient’s comorbidities. Does the patient have a history of diabetes, hypertension, cardiac concerns or a risk for infectious complications? What is the patient’s age? As individuals age the immune system becomes less robust at fighting infections or surveillance for malignancies. Some of the medications are immunosuppressive and might increase the risk of developing opportunistic infections or cancers.
Family planning should be taken into consideration during the discussion of which medications might be appropriate. Is the patient planning to have a pregnancy in the near future? Some medications might not be appropriate in that case.
Route of administration could be a factor to consider, since there are several medications that are administered as an infusion in a medical office or hospital setting. This could create issues for some patients who are employed and may have to miss work during these infusions. This could be as frequent as monthly or 2-3 times per year. Some patients just starting a new job, may feel uncomfortable taking time off or disclosing that they have MS leading to concerns for job security.
We also consider the side effects of the new treatment. What side effects and safety monitoring are required for a particular medication? Are there frequent blood tests, cardiac monitoring, dermatologic and ophthalmologic monitoring? How will this impact the patient’s quality of life?
In the end, it comes down to the level of monitoring required for a particular treatment, where the patient is in his or her life, and where he or she is in the disease course.
What are some potential complications when switching therapies?
When switching therapies, one of the bigger concerns is how quickly can we get the patient on the new therapy. Some medications when stopped can lead to return of disease activity or possibly lead to a rebound phenomenon with significant inflammatory activity. We focus on transitioning a patient quickly to a new drug that has a rapid mechanism of action thus limiting the amount of time that a patient is without a treatment. However, based on the mechanism of action of the drug you must consider if a wash out is necessary. The question is how quickly can the patient start the new drug thus preventing a rebound phenomenon. Ideally, no wash out would protect the patient best but might have safety concerns depending on the switch drug profile. If the switch was related to concerns for high JC virus antibody titer going off of natalizumab, there may be a need to make sure the patient does not have PML before making the switch. This may require MRIs and CSF analysis prior to switching.
Ultimately, we consider whether the drug we are switching the patient to is going to be more efficacious than the drug that the patient was previously on. We consider the safety and side effect profile of the new medication. We balance the risk of the disease with the risk of the medication. We must factor in the patient’s tolerance for risk as well and make the best decision with all the available factors considered.
Migraine therapy efficacy leaves ambiguities
NATIONAL HARBOR, MD. –
“There’s very little consistency in study design, making it difficult to make real-world comparisons,” said Carly Rodriguez, PharmD, FAMP, pharmacy director at Moda Health. Dr. Rodriguez presented data on the efficacy and pharmacoeconomic factors of migraine therapy at the annual meeting of the Academy of Managed Care Pharmacy.
The paucity of translatable evidence makes comparing and evaluating newer migraine therapies – such as botulinum toxins and calcitonin gene-related peptide (CGRP) inhibitors – particularly difficult.
These two injectable drug classes are not first-line treatments for migraine; they are currently reserved for patients who are refractory to at least one prophylactic treatment, but they offer important alternatives and additions to therapy.
“OnabotulinumtoxinA makes a good case because it costs less than a single ER visit, but there’s not enough supporting data,” Dr. Rodriguez said. According to a report from the Institute for Clinical and Economic Review (ICER) that evaluated the clinical efficacy and economic impact associated with onabotulinumtoxinA, administering the drug saved $157/headache day averted for 20 baseline headaches per month and $223/headache day avoided for 15 baseline headaches per month.
OnabotulinumtoxinA administration showed a moderate yet significant health benefit in preventing chronic migraines by reducing the number of headache days patients experienced by more than 50%. No benefit for episodic migraines was observed.
Several single- and multicenter studies found that onabotulinumtoxinA produced positive outcomes such as a decreased number of visits to urgent care centers, a lower average number of migraines patients experienced, and improved quality of life.
An ICER report investigating CGRP inhibitors found that the cost of anti-CGRP therapy may not produce viable clinical benefits.
Both botulinum toxins and CGRP inhibitors require prior authorization, and their injectable dosage forms restrict the settings in which they are administered and dispensed. Because botulinum toxins must be administered by a health care professional, the vast majority of these drugs are restricted to medical settings, with brand-to-generic substitution often varying among health plans. For this reason, botulinum toxins rarely appear on formularies. Several health plans consider botulinum toxins interchangeable and may give prescribers options to select the botulinum toxin product of their choice.
According to Dr. Rodriguez, there is some variability as to whether CGRP therapies are available in community pharmacy settings or are restricted to specialty pharmacies. Additionally, some plans consider all CGRP inhibitors to be interchangeable, while others take a more conservative approach.
Overall, generic drugs continue to dominate migraine drug therapy, with triptans leading the way. Generics that are heavily prescribed include beta-blockers, antidepressants, and antiepileptics.
More than 37 million people living in the United States suffer from migraines – approximately 8% of the overall population. Women are four times as likely to have migraines than men.
NATIONAL HARBOR, MD. –
“There’s very little consistency in study design, making it difficult to make real-world comparisons,” said Carly Rodriguez, PharmD, FAMP, pharmacy director at Moda Health. Dr. Rodriguez presented data on the efficacy and pharmacoeconomic factors of migraine therapy at the annual meeting of the Academy of Managed Care Pharmacy.
The paucity of translatable evidence makes comparing and evaluating newer migraine therapies – such as botulinum toxins and calcitonin gene-related peptide (CGRP) inhibitors – particularly difficult.
These two injectable drug classes are not first-line treatments for migraine; they are currently reserved for patients who are refractory to at least one prophylactic treatment, but they offer important alternatives and additions to therapy.
“OnabotulinumtoxinA makes a good case because it costs less than a single ER visit, but there’s not enough supporting data,” Dr. Rodriguez said. According to a report from the Institute for Clinical and Economic Review (ICER) that evaluated the clinical efficacy and economic impact associated with onabotulinumtoxinA, administering the drug saved $157/headache day averted for 20 baseline headaches per month and $223/headache day avoided for 15 baseline headaches per month.
OnabotulinumtoxinA administration showed a moderate yet significant health benefit in preventing chronic migraines by reducing the number of headache days patients experienced by more than 50%. No benefit for episodic migraines was observed.
Several single- and multicenter studies found that onabotulinumtoxinA produced positive outcomes such as a decreased number of visits to urgent care centers, a lower average number of migraines patients experienced, and improved quality of life.
An ICER report investigating CGRP inhibitors found that the cost of anti-CGRP therapy may not produce viable clinical benefits.
Both botulinum toxins and CGRP inhibitors require prior authorization, and their injectable dosage forms restrict the settings in which they are administered and dispensed. Because botulinum toxins must be administered by a health care professional, the vast majority of these drugs are restricted to medical settings, with brand-to-generic substitution often varying among health plans. For this reason, botulinum toxins rarely appear on formularies. Several health plans consider botulinum toxins interchangeable and may give prescribers options to select the botulinum toxin product of their choice.
According to Dr. Rodriguez, there is some variability as to whether CGRP therapies are available in community pharmacy settings or are restricted to specialty pharmacies. Additionally, some plans consider all CGRP inhibitors to be interchangeable, while others take a more conservative approach.
Overall, generic drugs continue to dominate migraine drug therapy, with triptans leading the way. Generics that are heavily prescribed include beta-blockers, antidepressants, and antiepileptics.
More than 37 million people living in the United States suffer from migraines – approximately 8% of the overall population. Women are four times as likely to have migraines than men.
NATIONAL HARBOR, MD. –
“There’s very little consistency in study design, making it difficult to make real-world comparisons,” said Carly Rodriguez, PharmD, FAMP, pharmacy director at Moda Health. Dr. Rodriguez presented data on the efficacy and pharmacoeconomic factors of migraine therapy at the annual meeting of the Academy of Managed Care Pharmacy.
The paucity of translatable evidence makes comparing and evaluating newer migraine therapies – such as botulinum toxins and calcitonin gene-related peptide (CGRP) inhibitors – particularly difficult.
These two injectable drug classes are not first-line treatments for migraine; they are currently reserved for patients who are refractory to at least one prophylactic treatment, but they offer important alternatives and additions to therapy.
“OnabotulinumtoxinA makes a good case because it costs less than a single ER visit, but there’s not enough supporting data,” Dr. Rodriguez said. According to a report from the Institute for Clinical and Economic Review (ICER) that evaluated the clinical efficacy and economic impact associated with onabotulinumtoxinA, administering the drug saved $157/headache day averted for 20 baseline headaches per month and $223/headache day avoided for 15 baseline headaches per month.
OnabotulinumtoxinA administration showed a moderate yet significant health benefit in preventing chronic migraines by reducing the number of headache days patients experienced by more than 50%. No benefit for episodic migraines was observed.
Several single- and multicenter studies found that onabotulinumtoxinA produced positive outcomes such as a decreased number of visits to urgent care centers, a lower average number of migraines patients experienced, and improved quality of life.
An ICER report investigating CGRP inhibitors found that the cost of anti-CGRP therapy may not produce viable clinical benefits.
Both botulinum toxins and CGRP inhibitors require prior authorization, and their injectable dosage forms restrict the settings in which they are administered and dispensed. Because botulinum toxins must be administered by a health care professional, the vast majority of these drugs are restricted to medical settings, with brand-to-generic substitution often varying among health plans. For this reason, botulinum toxins rarely appear on formularies. Several health plans consider botulinum toxins interchangeable and may give prescribers options to select the botulinum toxin product of their choice.
According to Dr. Rodriguez, there is some variability as to whether CGRP therapies are available in community pharmacy settings or are restricted to specialty pharmacies. Additionally, some plans consider all CGRP inhibitors to be interchangeable, while others take a more conservative approach.
Overall, generic drugs continue to dominate migraine drug therapy, with triptans leading the way. Generics that are heavily prescribed include beta-blockers, antidepressants, and antiepileptics.
More than 37 million people living in the United States suffer from migraines – approximately 8% of the overall population. Women are four times as likely to have migraines than men.
REPORTING FROM AMCP NEXUS 2019
Expect some congressional action on drug prices, but not major reform
NATIONAL HARBOR, MD – Congress is considering two separate, comprehensive proposals to address the escalating cost of drug prices, but neither is expected to make it to the President’s desk.
The more likely scenario is that parts of these bills aimed at reforming the Medicare Part D prescription drug program could be added on to a must-pass budget bill before the end of 2019, according to Ross Margulies, senior associate in the Washington office of the law firm Foley Hoag.
A bill championed by House Speaker Nancy Pelosi (D-Calif.), H.R. 3, is considered dead on arrival in the Senate since Senate Majority Leader Mitch McConnell (R-Ky.) has stated that the upper chamber will not take it up, Mr. Margulies said at the annual meeting of the Academy of Managed Care Pharmacy.
Despite this, the House is expected to move on H.R. 3 in mid-November. The bill has gone through three committee markups, and each have amended its language. The final bill language has not been released yet.
Meanwhile in the Senate, the Prescription Drug Pricing Reduction Act (S. 2543) enjoys some bipartisan support, but Mr. Margulies questioned whether there was enough to pass it.
But there are some provisions in both pieces of legislation that have bipartisan support and could ultimately be passed though other legislative vehicles, he said.
One proposal that is common to both bills is a cap on out-of-pocket spending by Part D beneficiaries, though the bills differ on how high to set the cap. The House bill caps annual beneficiary spending at $2,000, while the Senate proposal caps it at $3,600.
The lack of a cap “has increasingly raised some issues over the years as we have seen more and more specialty drugs come on the market that push individuals into the catastrophic phase in that first or second phase of that first or second refill,” Mr. Margulies said.
Another area that is garnering bipartisan support is reforming the structure of Medicare Part D.
Mr. Margulies noted that generally the Part D program has enjoyed bipartisan and consumer support and there “hasn’t been a major restructuring of the Part D benefit since its creation more than a decade ago.”
“I really think this is an area where Congress is in a bipartisan way very focused,” he added.
The redesign proposals in the two bills would fundamentally change how the catastrophic phase is covered. The out-of-pocket limits would eliminate beneficiary cost sharing in this phase, currently set at 5% of list price with no cap on spending, and dramatically reduce the government’s financial exposure during this phase.
Currently, the federal government covers 80% of the cost of drugs for beneficiaries in catastrophic coverage, and the plan sponsors cover the remaining 15%. The House and Senate plans both reduce government coverage to 20%. Under the House proposal, drug plans would be responsible for 50% while manufacturers would cover the remaining 30%. The Senate bill proposes a split of 60% for plans and 20% for manufacturers.
“Under either of these proposals, manufacturers with the highest-priced specialty drugs are probably going to fare the worst because you have that new open-ended liability in the catastrophic phase,” Mr. Margulies said.
On the plan side, “plans will face increased pressure to control costs/utilization,” he added.
These proposals could encourage manufacturers to reduce list prices and drug plans to more aggressively negotiate rebates and discounts.
One element of H.R. 3 that is not a part of the Senate bill is the requirement that the secretary of the Department of Health & Human Services negotiate drug prices for a certain number of high-cost drugs each year. Those negotiations would be backstopped by an international pricing index, with the aim of bringing the prices paid in the United States much closer to the lower prices paid internationally.
H.R. 3 also includes a hefty excise tax for manufacturers who either don’t participate in the negotiations or fail to offer price reductions that are within a specified percentage of the international pricing index.
Mr. Margulies noted that Speaker Pelosi was hoping to get White House endorsement on the drug negotiation provision, since it is similar to regulations proposed by HHS earlier this year, but impeachment proceedings have derailed any chance of getting that endorsement.
Mr. Margulies made no financial disclosures related to his presentation.
NATIONAL HARBOR, MD – Congress is considering two separate, comprehensive proposals to address the escalating cost of drug prices, but neither is expected to make it to the President’s desk.
The more likely scenario is that parts of these bills aimed at reforming the Medicare Part D prescription drug program could be added on to a must-pass budget bill before the end of 2019, according to Ross Margulies, senior associate in the Washington office of the law firm Foley Hoag.
A bill championed by House Speaker Nancy Pelosi (D-Calif.), H.R. 3, is considered dead on arrival in the Senate since Senate Majority Leader Mitch McConnell (R-Ky.) has stated that the upper chamber will not take it up, Mr. Margulies said at the annual meeting of the Academy of Managed Care Pharmacy.
Despite this, the House is expected to move on H.R. 3 in mid-November. The bill has gone through three committee markups, and each have amended its language. The final bill language has not been released yet.
Meanwhile in the Senate, the Prescription Drug Pricing Reduction Act (S. 2543) enjoys some bipartisan support, but Mr. Margulies questioned whether there was enough to pass it.
But there are some provisions in both pieces of legislation that have bipartisan support and could ultimately be passed though other legislative vehicles, he said.
One proposal that is common to both bills is a cap on out-of-pocket spending by Part D beneficiaries, though the bills differ on how high to set the cap. The House bill caps annual beneficiary spending at $2,000, while the Senate proposal caps it at $3,600.
The lack of a cap “has increasingly raised some issues over the years as we have seen more and more specialty drugs come on the market that push individuals into the catastrophic phase in that first or second phase of that first or second refill,” Mr. Margulies said.
Another area that is garnering bipartisan support is reforming the structure of Medicare Part D.
Mr. Margulies noted that generally the Part D program has enjoyed bipartisan and consumer support and there “hasn’t been a major restructuring of the Part D benefit since its creation more than a decade ago.”
“I really think this is an area where Congress is in a bipartisan way very focused,” he added.
The redesign proposals in the two bills would fundamentally change how the catastrophic phase is covered. The out-of-pocket limits would eliminate beneficiary cost sharing in this phase, currently set at 5% of list price with no cap on spending, and dramatically reduce the government’s financial exposure during this phase.
Currently, the federal government covers 80% of the cost of drugs for beneficiaries in catastrophic coverage, and the plan sponsors cover the remaining 15%. The House and Senate plans both reduce government coverage to 20%. Under the House proposal, drug plans would be responsible for 50% while manufacturers would cover the remaining 30%. The Senate bill proposes a split of 60% for plans and 20% for manufacturers.
“Under either of these proposals, manufacturers with the highest-priced specialty drugs are probably going to fare the worst because you have that new open-ended liability in the catastrophic phase,” Mr. Margulies said.
On the plan side, “plans will face increased pressure to control costs/utilization,” he added.
These proposals could encourage manufacturers to reduce list prices and drug plans to more aggressively negotiate rebates and discounts.
One element of H.R. 3 that is not a part of the Senate bill is the requirement that the secretary of the Department of Health & Human Services negotiate drug prices for a certain number of high-cost drugs each year. Those negotiations would be backstopped by an international pricing index, with the aim of bringing the prices paid in the United States much closer to the lower prices paid internationally.
H.R. 3 also includes a hefty excise tax for manufacturers who either don’t participate in the negotiations or fail to offer price reductions that are within a specified percentage of the international pricing index.
Mr. Margulies noted that Speaker Pelosi was hoping to get White House endorsement on the drug negotiation provision, since it is similar to regulations proposed by HHS earlier this year, but impeachment proceedings have derailed any chance of getting that endorsement.
Mr. Margulies made no financial disclosures related to his presentation.
NATIONAL HARBOR, MD – Congress is considering two separate, comprehensive proposals to address the escalating cost of drug prices, but neither is expected to make it to the President’s desk.
The more likely scenario is that parts of these bills aimed at reforming the Medicare Part D prescription drug program could be added on to a must-pass budget bill before the end of 2019, according to Ross Margulies, senior associate in the Washington office of the law firm Foley Hoag.
A bill championed by House Speaker Nancy Pelosi (D-Calif.), H.R. 3, is considered dead on arrival in the Senate since Senate Majority Leader Mitch McConnell (R-Ky.) has stated that the upper chamber will not take it up, Mr. Margulies said at the annual meeting of the Academy of Managed Care Pharmacy.
Despite this, the House is expected to move on H.R. 3 in mid-November. The bill has gone through three committee markups, and each have amended its language. The final bill language has not been released yet.
Meanwhile in the Senate, the Prescription Drug Pricing Reduction Act (S. 2543) enjoys some bipartisan support, but Mr. Margulies questioned whether there was enough to pass it.
But there are some provisions in both pieces of legislation that have bipartisan support and could ultimately be passed though other legislative vehicles, he said.
One proposal that is common to both bills is a cap on out-of-pocket spending by Part D beneficiaries, though the bills differ on how high to set the cap. The House bill caps annual beneficiary spending at $2,000, while the Senate proposal caps it at $3,600.
The lack of a cap “has increasingly raised some issues over the years as we have seen more and more specialty drugs come on the market that push individuals into the catastrophic phase in that first or second phase of that first or second refill,” Mr. Margulies said.
Another area that is garnering bipartisan support is reforming the structure of Medicare Part D.
Mr. Margulies noted that generally the Part D program has enjoyed bipartisan and consumer support and there “hasn’t been a major restructuring of the Part D benefit since its creation more than a decade ago.”
“I really think this is an area where Congress is in a bipartisan way very focused,” he added.
The redesign proposals in the two bills would fundamentally change how the catastrophic phase is covered. The out-of-pocket limits would eliminate beneficiary cost sharing in this phase, currently set at 5% of list price with no cap on spending, and dramatically reduce the government’s financial exposure during this phase.
Currently, the federal government covers 80% of the cost of drugs for beneficiaries in catastrophic coverage, and the plan sponsors cover the remaining 15%. The House and Senate plans both reduce government coverage to 20%. Under the House proposal, drug plans would be responsible for 50% while manufacturers would cover the remaining 30%. The Senate bill proposes a split of 60% for plans and 20% for manufacturers.
“Under either of these proposals, manufacturers with the highest-priced specialty drugs are probably going to fare the worst because you have that new open-ended liability in the catastrophic phase,” Mr. Margulies said.
On the plan side, “plans will face increased pressure to control costs/utilization,” he added.
These proposals could encourage manufacturers to reduce list prices and drug plans to more aggressively negotiate rebates and discounts.
One element of H.R. 3 that is not a part of the Senate bill is the requirement that the secretary of the Department of Health & Human Services negotiate drug prices for a certain number of high-cost drugs each year. Those negotiations would be backstopped by an international pricing index, with the aim of bringing the prices paid in the United States much closer to the lower prices paid internationally.
H.R. 3 also includes a hefty excise tax for manufacturers who either don’t participate in the negotiations or fail to offer price reductions that are within a specified percentage of the international pricing index.
Mr. Margulies noted that Speaker Pelosi was hoping to get White House endorsement on the drug negotiation provision, since it is similar to regulations proposed by HHS earlier this year, but impeachment proceedings have derailed any chance of getting that endorsement.
Mr. Margulies made no financial disclosures related to his presentation.
REPORTING FROM AMCP NEXUS 2019
What every ObGyn should know about Supreme Court rulings in the recent term
The most recently concluded term of the US Supreme Court, which began on October 1, 2018, yielded a number of decisions of interest to health care professionals and to ObGyns in particular. Although the term was viewed by some observers as less consequential than other recent terms, a review of the cases decided paints a picture of a more important term than some commentators expected.
When the term began, the Court had only 8 justices—1 short of a full bench: Judge Brett Kavanaugh had not yet been confirmed by the Senate. He was confirmed on October 6, by a 50-48 vote, and Justice Kavanaugh immediately joined the Court and began to hear and decide cases.
Increasingly, important decisions affect medical practice
From the nature of practice (abortion), to payment for service (Medicare reimbursement), resolution of disputes (arbitration), and fraud and abuse (the federal False Claims Act), the decisions of the Court will have an impact on many areas of medical practice. Organized medicine increasingly has recognized the significance of the work of the Court; nowhere has this been more clearly demonstrated than with amicus curiae (friend of the court) briefs filed by medical organizations.
Amicus curiae briefs. These briefs are filed by persons or organizations not a party to a case the Court is hearing. Their legitimate purpose is to inform the Court of 1) special information within the expertise of the amicus (or amici, plural) or 2) consequences of the decision that might not be apparent from arguments made by the parties to the case. Sometimes, the Court cites amicus briefs for having provided important information about the case.
Filing amicus briefs is time-consuming and expensive; organizations do not file them for trivial reasons. Organizations frequently join together to file a joint brief, to share expenses and express to the Court a stronger position.
Three categories of health professionals file amicus briefs in ObGyn-related cases:
- Major national organizations, often representing broad interests of health care professions or institutions (the American Medical Association [AMA], the Association of American Medical Colleges, and the American Hospital Association [AHA]), have filed a number of amicus briefs over the years.
- Specialty boards increasingly file amicus briefs. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine have filed briefs related to abortion issues.
- In reproductive issues, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians, and the Christian Medical & Dental Associations have been active amicus filers—frequently taking positions different than, even inconsistent with, amicus briefs filed by major specialty boards.
Amicus briefs filed by medical associations provide strong clues to what is important to clinicians. We have looked at such briefs to help us identify topics and cases from the just-concluded term that can be of particular interest to you.
Continue to: Surveying the shadow docket...
Surveying the shadow docket. As part of our review of the past term, we also looked at the so-called shadow docket, which includes decisions regarding writs of certiorari (which cases it agrees to hear); stays (usually delaying implementation of a law); or denials of stays. (Persuading the Court to hear a case is not easy: It hears approximately 70 cases per year out of as many as 7,000 applications to be heard.)
Abortion ruling
At stake. A number of states recently enacted a variety of provisions that might make an abortion more difficult to obtain. Some of the cases challenging these restrictions are making their way through lower courts, and one day might be argued before the Supreme Court. However, the Court has not (yet) agreed to hear the substance of many new abortion-related provisions.
Box v Planned Parenthood of Indiana and Kentucky, Inc.
The Court decided only 1 abortion restriction case this term.1 The Indiana law in question included 2 provisions that the Court considered:
Disposal of remains. The law regulated the manner in which abortion providers can dispose of fetal remains (ie, they cannot be treated as “infectious and pathologic waste”).
Motivation for seeking abortion. The Indiana law makes it illegal for an abortion provider to perform an abortion when the provider knows that the mother is seeking that abortion “solely” because of the fetus’s race, sex, diagnosis of Down syndrome, disability, or related characteristics.
Final rulings. The Court held that the disposal-of-remains provision is constitutional. The provision is “rationally related to the state’s interest in proper disposal of fetal remains.”2 Planned Parenthood had not raised the issue of whether the law might impose an undue burden on a woman’s right to obtain an abortion, so the Court did not decide that issue.
The Court did not consider the constitutionality of the part of the law proscribing certain reasons for seeking an otherwise legal abortion; instead, it awaits lower courts’ review of the issue. Justice Clarence Thomas wrote an extensive concurring opinion suggesting that this law is intended to avoid abortion to achieve eugenic goals.3
Key developments from the shadow docket
The Court issued a stay preventing a Louisiana statute that requires physicians who perform abortions to have admitting privileges at a nearby hospital from going into effect, pending the outcome of litigation about that law.4 Four dissenters noted that all 4 physicians who perform abortions in Louisiana have such privileges. Chief Justice Roberts was the fifth vote to grant the stay. This case likely will make its way back to the Court, as will a number of other state laws being adopted. The issue may be back as soon as the term just starting.
The Court is also considering whether to take another Indiana case, Box v Planned Parenthood of Indiana and Kentucky, Inc. (Box II). This case involves an Indiana ultrasonography viewing option as part of the abortion consent process.5
The Court declined to hear cases from Louisiana and Kansas in which the states had cut off Medicaid funding to Planned Parenthood. Lower courts had stopped the implementation of those laws.6 The legal issue was whether private parties, as opposed to the federal government, had standing to bring the case. For now, the decision of the lower courts to stop implementation of the funding cutoff is in effect. There is a split in the Circuit Courts on the issue, however, making it likely that the Supreme Court will have to resolve it sooner or later.
Health care organizations have filed a number of amicus briefs in these and other cases involving new abortion regulations. ACOG and others filed a brief opposing a Louisiana law that requires abortion providers to have admitting privileges at a nearby facility,7 and a brief opposing a similar Oklahoma law.8 The Association of Pro-Life Obstetricians and Gynecologists and others filed amicus curiae briefs in Box II9 and in an Alabama case involving so-called dismemberment abortion.10
Continue to: Medicare payments...
Medicare payments
Azar, Secretary of Health and Human Services v Allina Health Services, et al11
This case drew interest—and many amicus briefs—from health care providers, including the AMA and the AHA.12,13 There was good reason for their interest: First, the case involved more than $3 billion in reimbursements; second, it represented a potentially important precedent about the rights of providers and patients to comment on Medicare reimbursement changes. The question involved the technical calculation of additional payments made to institutions that serve a disproportionate number of low-income patients (known as Medicare Fractions).
At stake. The issue was a statutory requirement for a 60-day public notice and comment period for rules that “change a substantive legal standard” governing the scope of benefits, eligibility, or payment for services.14 In 2014, the Secretary of the Department of Health and Human Services (HHS) in the Obama administration posted a spreadsheet announcing Medicare fractions rates for hospitals (for 2012)—without formal notice or comment regarding the formula used. (The spreadsheet listed what each qualifying institution would receive, but it was based on a formula that, as noted, had not been subject to public notice and comment.) The AMA and AHA briefs emphasized the importance of a notice and comment period, especially when Medicare reimbursement is involved.
Final ruling. The Court held that the HHS process violated the notice and comment provision, thereby invalidating the policy underlying the so-called spreadsheet reimbursement. The decision was significant: This was a careful statutory interpretation of the 60-day notice and comment period, not the reimbursement policy itself. Presumably, had the HHS Secretary provided for sufficient notice and comment, the formula used would have met the requirements for issuing reimbursement formulas.
Key points. Hospitals will collectively receive $3 or $4 billion as a consequence of the ruling. Perhaps more importantly, the decision signals that HHS is going to have to take seriously the requirement that it publish Medicare-related reimbursement policies for the 60-day period.
A number of diverse cases ruled on by the Supreme Court are worth mentioning. The Court:
- allowed the President to move various funds from the US Department of Defense into accounts from which the money could be used to build a portion of a wall along the southern US border.1
- essentially killed the "citizenship question" on the census form. Technically, the Court sent the issue back to the Commerce Department for better justification for including the question (the announced reasons appeared to be pretextual).2
- changed, perhaps substantially, the deference that courts give to federal agencies in interpreting regulations.3
- upheld, in 2 cases, treaty rights of Native Americans to special treatment on Indian Lands4,5; the Court held that treaties ordinarily should be interpreted as the tribe understood them at the time they were signed. (These were 5 to 4 decisions; the split in the Court leaves many unanswered questions.)
- made it easier for landowners to file suit in federal court when they claim that the state has "taken" their property without just compensation.6
- held that a refusal of the US Patent and Trademark Office to register "immoral" or "scandalous" trademarks infringes on the First Amendment. (The petitioner sought to register "FUCT" as a trademark for a line of clothing.)7
- allowed an antitrust case by iPhone users against Apple to go forward. At issue: the claim that Apple monopolizes the retail market for apps by requiring buyers to obtain apps from Apple.8
- held that, if a drunk-driving suspect who has been taken into custody is, or becomes, unconscious, the "reasonable search" provision of the Fourth Amendment generally does not prevent a state from taking a blood specimen without a warrant. (Wisconsin had a specific "implied consent" law, by which someone receiving a driving license consents to a blood draw.9)
- decided numerous capital punishment cases. In many ways, this term seemed to be a "capital term." Issues involved in these cases have split the Court; it is reasonable to expect that the divide will endure through upcoming terms.
References
- Donald J. Trump, President of the United States, et al. v Sierra Club, et al. 588 US 19A60 (2019).
- Department of Commerce et al. v New York et al. 18 996 (2018).
- Kisor v Wilkie, Secretary of Veterans Affairs. 18 15 (2018).
- Washington State Department of Licensing v Cougar Den, Inc. 16 1498 (2018).
- Herrera v Wyoming. 17 532 (2018).
- Knick v Township of Scott, Pennsylvania, et al. 17 647 (2018).
- Iancu, Under Secretary of Commerce for Intellectual Property and Director, Patent and Trademark Office v Brunetti. 18 302 (2018).
- Apple Inc. v Pepper et al. 17 204 (2018).
- Mitchell v Wisconsin. 18 6210 (2018).
Liability under the False Claims Act
The False Claims Act (FCA) protects the federal government from fraudulent claims for payment and for shoddy goods and services. It incentivizes (by a percentage of recovery) private parties to bring cases to enforce the law.15 (Of course, the federal government also enforces the Act.)
At stake. The FCA has been of considerable concern to the AHA, the Association of American Medical Colleges, and other health care organizations—understandably so.16 As the AHA informed the Court in an amicus brief, “The prevalence of [FCA] cases has ballooned over the past three decades.... These suits disproportionately target healthcare entities.... Of the 767 new FCA cases filed in 2018, for example, 506 involved healthcare defendants.”17
Final ruling. The Court considered an ambiguity in the statute of limitations for these actions and the Court unanimously ruled to permit an extended time in which qui tam actions (private actions under the law) can be filed.18
Key points. As long a period as 10 years can pass between the time an FCA violation occurs and an action is brought. This decision is likely to increase the number of FCA actions against health care providers because the case can be filed many years after the conduct that gave rise to the complaint.
Continue to: Registering sex offenders...
Registering sex offenders
The Court upheld the constitutionality of the federal Sex Offender Registration and Notification Act (SORNA).19 Sex offenders must register and periodically report, in person, to law enforcement in every state in which the offender works, studies, or resides.
At stake. The case involved the applicability of SORNA registration obligations to those convicted of sex offenses before SORNA was adopted (pre-Act offenders).20 The court upheld registration requirements for pre-Act offenders.
Former Justice Stevens, the longest-living and third-longest-serving Supreme Court justice, died in July 2019 at 99 years of age. He was appointed to the Court in 1975 by President Ford and served until his retirement in 2010, when he was 90. Stevens had recently published a memoir, The Making of a Justice: Reflections on My First 94 Years.
Stevens's judicial philosophy generally is described as having changed over the course of his 35 years of service: He was viewed as becoming more liberal. He was a justice of enduring kindness and integrity. It is possible to find people who disagree with him, but almost impossible to find anyone who disliked him. He was continuously committed to the law and justice in the United States.
Arbitration
The Court continued its practice of deciding at least one case each term that emphasizes that federal law requires that courts rather strictly enforce agreements to arbitrate (instead of to litigate) future disputes.21 In another case, the Court ruled that there can be “class” or “joint” arbitration only if the agreement to arbitrate a dispute clearly permits such class arbitration.22
Pharma’s liability regarding product risk
The Court somewhat limited the liability of pharmaceutical companies for failing to provide adequate warning about the risk that their products pose. The case against Merck involved 500 patients who took denosumab (Fosamax) and suffered atypical femoral fractures.23
At stake. Because prescribing information (in which warnings are provided) must be approved by the US Food and Drug Administration (FDA), the legal test is: Would the FDA have refused to approve a change in the warning if Merck had “fully informed the FDA of the justifications for the warning” required by state law to avoid liability?24,25 Lower-court judges (not juries) will be expected to apply this test in the future.
The doctor and the death penalty
The Court has established a rule that, when a prisoner facing capital punishment objects to a form of execution because it is too painful, he has to propose an alternative that is reasonably available. In one case,26 a physician, an expert witness for the prisoner, did not answer some essential relative-pain questions (ie, would one procedure be more painful than another?).
At stake. The AMA filed an amicus brief in this case, indicating that it is unethical for physicians to participate in an execution. The brief noted that “testimony used to determine which method of execution would reduce physical suffering would constitute physician participation in capital punishment and would be unethical.”27
The expert witness’s failure to answer the question on relative pain had the unfortunate result of reducing the likelihood that the prisoner would prevail in his request for an alternative method of execution.
Analysis
Despite obvious disagreements about big issues (notably, abortion and the death penalty) the Court maintained a courteous and civil demeanor—something not always seen nowadays in other branches of government. Here are facts about the Court’s term just concluded:
- The Court issued 72 merits opinions (about average).
- Only 39% of decisions were unanimous (compared with the average of 49% in recent terms).
- On the other hand, 26% of decisions were split 5 to 4 (compared with a 10% recent average).
- In those 5 to 4 decisions, Justices were in the majority as follows28: Justice Gorsuch, 65%; Justice Kavanaugh, 61%; Justice Thomas, 60%; Chief Justice Roberts and Justices Ginsburg and Alito, each 55%; Justice Breyer, 50%; and Justices Sotomayor and Kagan each at 45%.
- There were 57 dissenting opinions—up from 48 in the previous term.
- What is referred to as “the liberal-conservative split” might seem more profound than it really is: “Every conservative member of the court at some point voted to form a majority with the liberal justices. And every liberal at least once left behind all of his or her usual voting partners to join the conservatives.”29
Continue to: Last, it was a year of personal health issues for...
Last, it was a year of personal health issues for the Court: Justice Ginsburg had a diagnosis of lung cancer and was absent, following surgery, in January. Of retired Justices, Sandra Day O’Connor suffers from dementia and former Justice John Paul Stevens died.
In closing
The Court has accepted approximately 50 cases for the current term, which began on October 7. The first 2 days of the term were spent on arguments about, first, whether a state can abolish the insanity defense and, second, whether nondiscrimination laws (“based on sex”) prohibit discrimination based on sexual orientation or transgender status. Cases also will deal with Patient Protection and Affordable Care Act payments to providers; the Deferred Action for Childhood Arrivals, or DACA; the death penalty; and international child custody disputes. The Court will be accepting more cases for several months. It promises to be a very interesting term.
- Box v Planned Parenthood of Indiana and Kentucky, Inc. 587 US 18 483 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc., at 2.
- Box v Planned Parenthood of Indiana and Kentucky, Inc., Justice Thomas concurring.
- June Medical Services, LLC, et al. v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. 586 US 18A774 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc. Docket 18-1019.
- Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals v Planned Parenthood of Gulf Coast, Inc., et al. 586 US 17 1492 (2018).
- June Medical Services L.L.C., et al., Petitioners, v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. No. 18-1323. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Society for Reproductive Medicine, National Association of Nurse Practitioners in Women's Health, North American Society for Pediatric and Adolescent Gynecology, and Society For Maternal-Fetal Medicine, Amicus Curiae in Support of Petitioners. May 2019.
- Planned Parenthood of Kansas & Eastern Oklahoma, et al., Petitioners, v Larry Jegley, et al., Respondents. No. 17-935. Brief Amici Curiae of American College of Obstetricians and Gynecologists and American Public Health Association as Amici Curiae in Support of Petitioners. February 1, 2018.
- Box v Planned Parenthood of Indiana & Kentucky. No. 18-1019. Brief Amici Curiae of American Association of Pro-Life Obstetricians & Gynecologists, American College of Pediatricians, Care Net, Christian Medical Association, Heartbeat International, Inc., and National Institute Of Family & Life Advocates in Support of Petitioners. March 6, 2019.
- Steven T. Marshall, et al., Petitioners, v West Alabama Women's Center, et al., Respondents. No. 18-837. Brief of Amici Curiae American Association of Pro-Life Obstetricians & Gynecologists and American College of Pediatricians, in Support of Petitioners. January 18, 2019.
- Azar, Secretary of Health and Human Services v Allina Health Services, et al. 17 1484 (2018).
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges as Amici Curiae in Support of Respondents. December 2018.
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of Amici Curiae American Medical Association and Medical Society of the District of Columbia Amici Curiae in Support of Respondents. December 2018.
- 42 U. S. C. §1395hh. https://uscode.house.gov/view.xhtml?req=(title:42%20section:1395hh%20edition:prelim). Accessed October 22, 2019.
- The False Claims Act: a primer. Washington DC: US Department of Justice. www.justice.gov/sites/default/files/civil/legacy/2011/04/22/C-FRAUDS_FCA_Primer.pdf. Accessed October 18, 2019.
- Universal Health Services, Inc., v United States and Commonwealth of Massachusetts ex rel. Julio Escobar and Carmen Correa. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges Amici Curiae in Support of Petitioner. No. 15-7. January 2016.
- Intermountain Health Care, Inc., et al., Petitioners, v United States ex rel. Gerald Polukoff, et al., Respondents. No. 18-911. Brief of the American Hospital Association and Federation of American Hospitals as Amici Curiae in Support of Petitioners. February 13, 2019.
- Cochise Consultancy, Inc., et al., v United States ex rel. Hunt. 18 315 (2018).
- 34 U.S.C. §20901 et seq. [Chapter 209--Child Protection and Safety.] https://uscode.house.gov/view.xhtml?path=/prelim@title34/subtitle2/chapter209&edition=prelim. Accessed October 17, 2019.
- Gundy v United States. 17 6086 (2018).
- Henry Schein, Inc., et al., v Archer & White Sales, Inc. 17 1272 (2018).
- Lamps Plus, Inc., et al., v Varela. 17 988 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018) at 13-14.
- Wyeth v Levine, 555 US 555, 571 (2009).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018). American Medical Association, Amicus Curiae Brief, in Support of Neither Party. July 23, 2018.
- Final stat pack for October term 2018. SCOTUSblog.com. June 28, 2019. https://www.scotusblog.com/wp-content/uploads/2019/07/StatPack_OT18-7_8_19.pdf. Accessed October 17, 2019.
- Barnes R. They're not 'wonder twins': Gorsuch, Kavanaugh shift the Supreme Court, but their differences are striking. Washington Post, June 28, 2019.
The most recently concluded term of the US Supreme Court, which began on October 1, 2018, yielded a number of decisions of interest to health care professionals and to ObGyns in particular. Although the term was viewed by some observers as less consequential than other recent terms, a review of the cases decided paints a picture of a more important term than some commentators expected.
When the term began, the Court had only 8 justices—1 short of a full bench: Judge Brett Kavanaugh had not yet been confirmed by the Senate. He was confirmed on October 6, by a 50-48 vote, and Justice Kavanaugh immediately joined the Court and began to hear and decide cases.
Increasingly, important decisions affect medical practice
From the nature of practice (abortion), to payment for service (Medicare reimbursement), resolution of disputes (arbitration), and fraud and abuse (the federal False Claims Act), the decisions of the Court will have an impact on many areas of medical practice. Organized medicine increasingly has recognized the significance of the work of the Court; nowhere has this been more clearly demonstrated than with amicus curiae (friend of the court) briefs filed by medical organizations.
Amicus curiae briefs. These briefs are filed by persons or organizations not a party to a case the Court is hearing. Their legitimate purpose is to inform the Court of 1) special information within the expertise of the amicus (or amici, plural) or 2) consequences of the decision that might not be apparent from arguments made by the parties to the case. Sometimes, the Court cites amicus briefs for having provided important information about the case.
Filing amicus briefs is time-consuming and expensive; organizations do not file them for trivial reasons. Organizations frequently join together to file a joint brief, to share expenses and express to the Court a stronger position.
Three categories of health professionals file amicus briefs in ObGyn-related cases:
- Major national organizations, often representing broad interests of health care professions or institutions (the American Medical Association [AMA], the Association of American Medical Colleges, and the American Hospital Association [AHA]), have filed a number of amicus briefs over the years.
- Specialty boards increasingly file amicus briefs. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine have filed briefs related to abortion issues.
- In reproductive issues, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians, and the Christian Medical & Dental Associations have been active amicus filers—frequently taking positions different than, even inconsistent with, amicus briefs filed by major specialty boards.
Amicus briefs filed by medical associations provide strong clues to what is important to clinicians. We have looked at such briefs to help us identify topics and cases from the just-concluded term that can be of particular interest to you.
Continue to: Surveying the shadow docket...
Surveying the shadow docket. As part of our review of the past term, we also looked at the so-called shadow docket, which includes decisions regarding writs of certiorari (which cases it agrees to hear); stays (usually delaying implementation of a law); or denials of stays. (Persuading the Court to hear a case is not easy: It hears approximately 70 cases per year out of as many as 7,000 applications to be heard.)
Abortion ruling
At stake. A number of states recently enacted a variety of provisions that might make an abortion more difficult to obtain. Some of the cases challenging these restrictions are making their way through lower courts, and one day might be argued before the Supreme Court. However, the Court has not (yet) agreed to hear the substance of many new abortion-related provisions.
Box v Planned Parenthood of Indiana and Kentucky, Inc.
The Court decided only 1 abortion restriction case this term.1 The Indiana law in question included 2 provisions that the Court considered:
Disposal of remains. The law regulated the manner in which abortion providers can dispose of fetal remains (ie, they cannot be treated as “infectious and pathologic waste”).
Motivation for seeking abortion. The Indiana law makes it illegal for an abortion provider to perform an abortion when the provider knows that the mother is seeking that abortion “solely” because of the fetus’s race, sex, diagnosis of Down syndrome, disability, or related characteristics.
Final rulings. The Court held that the disposal-of-remains provision is constitutional. The provision is “rationally related to the state’s interest in proper disposal of fetal remains.”2 Planned Parenthood had not raised the issue of whether the law might impose an undue burden on a woman’s right to obtain an abortion, so the Court did not decide that issue.
The Court did not consider the constitutionality of the part of the law proscribing certain reasons for seeking an otherwise legal abortion; instead, it awaits lower courts’ review of the issue. Justice Clarence Thomas wrote an extensive concurring opinion suggesting that this law is intended to avoid abortion to achieve eugenic goals.3
Key developments from the shadow docket
The Court issued a stay preventing a Louisiana statute that requires physicians who perform abortions to have admitting privileges at a nearby hospital from going into effect, pending the outcome of litigation about that law.4 Four dissenters noted that all 4 physicians who perform abortions in Louisiana have such privileges. Chief Justice Roberts was the fifth vote to grant the stay. This case likely will make its way back to the Court, as will a number of other state laws being adopted. The issue may be back as soon as the term just starting.
The Court is also considering whether to take another Indiana case, Box v Planned Parenthood of Indiana and Kentucky, Inc. (Box II). This case involves an Indiana ultrasonography viewing option as part of the abortion consent process.5
The Court declined to hear cases from Louisiana and Kansas in which the states had cut off Medicaid funding to Planned Parenthood. Lower courts had stopped the implementation of those laws.6 The legal issue was whether private parties, as opposed to the federal government, had standing to bring the case. For now, the decision of the lower courts to stop implementation of the funding cutoff is in effect. There is a split in the Circuit Courts on the issue, however, making it likely that the Supreme Court will have to resolve it sooner or later.
Health care organizations have filed a number of amicus briefs in these and other cases involving new abortion regulations. ACOG and others filed a brief opposing a Louisiana law that requires abortion providers to have admitting privileges at a nearby facility,7 and a brief opposing a similar Oklahoma law.8 The Association of Pro-Life Obstetricians and Gynecologists and others filed amicus curiae briefs in Box II9 and in an Alabama case involving so-called dismemberment abortion.10
Continue to: Medicare payments...
Medicare payments
Azar, Secretary of Health and Human Services v Allina Health Services, et al11
This case drew interest—and many amicus briefs—from health care providers, including the AMA and the AHA.12,13 There was good reason for their interest: First, the case involved more than $3 billion in reimbursements; second, it represented a potentially important precedent about the rights of providers and patients to comment on Medicare reimbursement changes. The question involved the technical calculation of additional payments made to institutions that serve a disproportionate number of low-income patients (known as Medicare Fractions).
At stake. The issue was a statutory requirement for a 60-day public notice and comment period for rules that “change a substantive legal standard” governing the scope of benefits, eligibility, or payment for services.14 In 2014, the Secretary of the Department of Health and Human Services (HHS) in the Obama administration posted a spreadsheet announcing Medicare fractions rates for hospitals (for 2012)—without formal notice or comment regarding the formula used. (The spreadsheet listed what each qualifying institution would receive, but it was based on a formula that, as noted, had not been subject to public notice and comment.) The AMA and AHA briefs emphasized the importance of a notice and comment period, especially when Medicare reimbursement is involved.
Final ruling. The Court held that the HHS process violated the notice and comment provision, thereby invalidating the policy underlying the so-called spreadsheet reimbursement. The decision was significant: This was a careful statutory interpretation of the 60-day notice and comment period, not the reimbursement policy itself. Presumably, had the HHS Secretary provided for sufficient notice and comment, the formula used would have met the requirements for issuing reimbursement formulas.
Key points. Hospitals will collectively receive $3 or $4 billion as a consequence of the ruling. Perhaps more importantly, the decision signals that HHS is going to have to take seriously the requirement that it publish Medicare-related reimbursement policies for the 60-day period.
A number of diverse cases ruled on by the Supreme Court are worth mentioning. The Court:
- allowed the President to move various funds from the US Department of Defense into accounts from which the money could be used to build a portion of a wall along the southern US border.1
- essentially killed the "citizenship question" on the census form. Technically, the Court sent the issue back to the Commerce Department for better justification for including the question (the announced reasons appeared to be pretextual).2
- changed, perhaps substantially, the deference that courts give to federal agencies in interpreting regulations.3
- upheld, in 2 cases, treaty rights of Native Americans to special treatment on Indian Lands4,5; the Court held that treaties ordinarily should be interpreted as the tribe understood them at the time they were signed. (These were 5 to 4 decisions; the split in the Court leaves many unanswered questions.)
- made it easier for landowners to file suit in federal court when they claim that the state has "taken" their property without just compensation.6
- held that a refusal of the US Patent and Trademark Office to register "immoral" or "scandalous" trademarks infringes on the First Amendment. (The petitioner sought to register "FUCT" as a trademark for a line of clothing.)7
- allowed an antitrust case by iPhone users against Apple to go forward. At issue: the claim that Apple monopolizes the retail market for apps by requiring buyers to obtain apps from Apple.8
- held that, if a drunk-driving suspect who has been taken into custody is, or becomes, unconscious, the "reasonable search" provision of the Fourth Amendment generally does not prevent a state from taking a blood specimen without a warrant. (Wisconsin had a specific "implied consent" law, by which someone receiving a driving license consents to a blood draw.9)
- decided numerous capital punishment cases. In many ways, this term seemed to be a "capital term." Issues involved in these cases have split the Court; it is reasonable to expect that the divide will endure through upcoming terms.
References
- Donald J. Trump, President of the United States, et al. v Sierra Club, et al. 588 US 19A60 (2019).
- Department of Commerce et al. v New York et al. 18 996 (2018).
- Kisor v Wilkie, Secretary of Veterans Affairs. 18 15 (2018).
- Washington State Department of Licensing v Cougar Den, Inc. 16 1498 (2018).
- Herrera v Wyoming. 17 532 (2018).
- Knick v Township of Scott, Pennsylvania, et al. 17 647 (2018).
- Iancu, Under Secretary of Commerce for Intellectual Property and Director, Patent and Trademark Office v Brunetti. 18 302 (2018).
- Apple Inc. v Pepper et al. 17 204 (2018).
- Mitchell v Wisconsin. 18 6210 (2018).
Liability under the False Claims Act
The False Claims Act (FCA) protects the federal government from fraudulent claims for payment and for shoddy goods and services. It incentivizes (by a percentage of recovery) private parties to bring cases to enforce the law.15 (Of course, the federal government also enforces the Act.)
At stake. The FCA has been of considerable concern to the AHA, the Association of American Medical Colleges, and other health care organizations—understandably so.16 As the AHA informed the Court in an amicus brief, “The prevalence of [FCA] cases has ballooned over the past three decades.... These suits disproportionately target healthcare entities.... Of the 767 new FCA cases filed in 2018, for example, 506 involved healthcare defendants.”17
Final ruling. The Court considered an ambiguity in the statute of limitations for these actions and the Court unanimously ruled to permit an extended time in which qui tam actions (private actions under the law) can be filed.18
Key points. As long a period as 10 years can pass between the time an FCA violation occurs and an action is brought. This decision is likely to increase the number of FCA actions against health care providers because the case can be filed many years after the conduct that gave rise to the complaint.
Continue to: Registering sex offenders...
Registering sex offenders
The Court upheld the constitutionality of the federal Sex Offender Registration and Notification Act (SORNA).19 Sex offenders must register and periodically report, in person, to law enforcement in every state in which the offender works, studies, or resides.
At stake. The case involved the applicability of SORNA registration obligations to those convicted of sex offenses before SORNA was adopted (pre-Act offenders).20 The court upheld registration requirements for pre-Act offenders.
Former Justice Stevens, the longest-living and third-longest-serving Supreme Court justice, died in July 2019 at 99 years of age. He was appointed to the Court in 1975 by President Ford and served until his retirement in 2010, when he was 90. Stevens had recently published a memoir, The Making of a Justice: Reflections on My First 94 Years.
Stevens's judicial philosophy generally is described as having changed over the course of his 35 years of service: He was viewed as becoming more liberal. He was a justice of enduring kindness and integrity. It is possible to find people who disagree with him, but almost impossible to find anyone who disliked him. He was continuously committed to the law and justice in the United States.
Arbitration
The Court continued its practice of deciding at least one case each term that emphasizes that federal law requires that courts rather strictly enforce agreements to arbitrate (instead of to litigate) future disputes.21 In another case, the Court ruled that there can be “class” or “joint” arbitration only if the agreement to arbitrate a dispute clearly permits such class arbitration.22
Pharma’s liability regarding product risk
The Court somewhat limited the liability of pharmaceutical companies for failing to provide adequate warning about the risk that their products pose. The case against Merck involved 500 patients who took denosumab (Fosamax) and suffered atypical femoral fractures.23
At stake. Because prescribing information (in which warnings are provided) must be approved by the US Food and Drug Administration (FDA), the legal test is: Would the FDA have refused to approve a change in the warning if Merck had “fully informed the FDA of the justifications for the warning” required by state law to avoid liability?24,25 Lower-court judges (not juries) will be expected to apply this test in the future.
The doctor and the death penalty
The Court has established a rule that, when a prisoner facing capital punishment objects to a form of execution because it is too painful, he has to propose an alternative that is reasonably available. In one case,26 a physician, an expert witness for the prisoner, did not answer some essential relative-pain questions (ie, would one procedure be more painful than another?).
At stake. The AMA filed an amicus brief in this case, indicating that it is unethical for physicians to participate in an execution. The brief noted that “testimony used to determine which method of execution would reduce physical suffering would constitute physician participation in capital punishment and would be unethical.”27
The expert witness’s failure to answer the question on relative pain had the unfortunate result of reducing the likelihood that the prisoner would prevail in his request for an alternative method of execution.
Analysis
Despite obvious disagreements about big issues (notably, abortion and the death penalty) the Court maintained a courteous and civil demeanor—something not always seen nowadays in other branches of government. Here are facts about the Court’s term just concluded:
- The Court issued 72 merits opinions (about average).
- Only 39% of decisions were unanimous (compared with the average of 49% in recent terms).
- On the other hand, 26% of decisions were split 5 to 4 (compared with a 10% recent average).
- In those 5 to 4 decisions, Justices were in the majority as follows28: Justice Gorsuch, 65%; Justice Kavanaugh, 61%; Justice Thomas, 60%; Chief Justice Roberts and Justices Ginsburg and Alito, each 55%; Justice Breyer, 50%; and Justices Sotomayor and Kagan each at 45%.
- There were 57 dissenting opinions—up from 48 in the previous term.
- What is referred to as “the liberal-conservative split” might seem more profound than it really is: “Every conservative member of the court at some point voted to form a majority with the liberal justices. And every liberal at least once left behind all of his or her usual voting partners to join the conservatives.”29
Continue to: Last, it was a year of personal health issues for...
Last, it was a year of personal health issues for the Court: Justice Ginsburg had a diagnosis of lung cancer and was absent, following surgery, in January. Of retired Justices, Sandra Day O’Connor suffers from dementia and former Justice John Paul Stevens died.
In closing
The Court has accepted approximately 50 cases for the current term, which began on October 7. The first 2 days of the term were spent on arguments about, first, whether a state can abolish the insanity defense and, second, whether nondiscrimination laws (“based on sex”) prohibit discrimination based on sexual orientation or transgender status. Cases also will deal with Patient Protection and Affordable Care Act payments to providers; the Deferred Action for Childhood Arrivals, or DACA; the death penalty; and international child custody disputes. The Court will be accepting more cases for several months. It promises to be a very interesting term.
The most recently concluded term of the US Supreme Court, which began on October 1, 2018, yielded a number of decisions of interest to health care professionals and to ObGyns in particular. Although the term was viewed by some observers as less consequential than other recent terms, a review of the cases decided paints a picture of a more important term than some commentators expected.
When the term began, the Court had only 8 justices—1 short of a full bench: Judge Brett Kavanaugh had not yet been confirmed by the Senate. He was confirmed on October 6, by a 50-48 vote, and Justice Kavanaugh immediately joined the Court and began to hear and decide cases.
Increasingly, important decisions affect medical practice
From the nature of practice (abortion), to payment for service (Medicare reimbursement), resolution of disputes (arbitration), and fraud and abuse (the federal False Claims Act), the decisions of the Court will have an impact on many areas of medical practice. Organized medicine increasingly has recognized the significance of the work of the Court; nowhere has this been more clearly demonstrated than with amicus curiae (friend of the court) briefs filed by medical organizations.
Amicus curiae briefs. These briefs are filed by persons or organizations not a party to a case the Court is hearing. Their legitimate purpose is to inform the Court of 1) special information within the expertise of the amicus (or amici, plural) or 2) consequences of the decision that might not be apparent from arguments made by the parties to the case. Sometimes, the Court cites amicus briefs for having provided important information about the case.
Filing amicus briefs is time-consuming and expensive; organizations do not file them for trivial reasons. Organizations frequently join together to file a joint brief, to share expenses and express to the Court a stronger position.
Three categories of health professionals file amicus briefs in ObGyn-related cases:
- Major national organizations, often representing broad interests of health care professions or institutions (the American Medical Association [AMA], the Association of American Medical Colleges, and the American Hospital Association [AHA]), have filed a number of amicus briefs over the years.
- Specialty boards increasingly file amicus briefs. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine have filed briefs related to abortion issues.
- In reproductive issues, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians, and the Christian Medical & Dental Associations have been active amicus filers—frequently taking positions different than, even inconsistent with, amicus briefs filed by major specialty boards.
Amicus briefs filed by medical associations provide strong clues to what is important to clinicians. We have looked at such briefs to help us identify topics and cases from the just-concluded term that can be of particular interest to you.
Continue to: Surveying the shadow docket...
Surveying the shadow docket. As part of our review of the past term, we also looked at the so-called shadow docket, which includes decisions regarding writs of certiorari (which cases it agrees to hear); stays (usually delaying implementation of a law); or denials of stays. (Persuading the Court to hear a case is not easy: It hears approximately 70 cases per year out of as many as 7,000 applications to be heard.)
Abortion ruling
At stake. A number of states recently enacted a variety of provisions that might make an abortion more difficult to obtain. Some of the cases challenging these restrictions are making their way through lower courts, and one day might be argued before the Supreme Court. However, the Court has not (yet) agreed to hear the substance of many new abortion-related provisions.
Box v Planned Parenthood of Indiana and Kentucky, Inc.
The Court decided only 1 abortion restriction case this term.1 The Indiana law in question included 2 provisions that the Court considered:
Disposal of remains. The law regulated the manner in which abortion providers can dispose of fetal remains (ie, they cannot be treated as “infectious and pathologic waste”).
Motivation for seeking abortion. The Indiana law makes it illegal for an abortion provider to perform an abortion when the provider knows that the mother is seeking that abortion “solely” because of the fetus’s race, sex, diagnosis of Down syndrome, disability, or related characteristics.
Final rulings. The Court held that the disposal-of-remains provision is constitutional. The provision is “rationally related to the state’s interest in proper disposal of fetal remains.”2 Planned Parenthood had not raised the issue of whether the law might impose an undue burden on a woman’s right to obtain an abortion, so the Court did not decide that issue.
The Court did not consider the constitutionality of the part of the law proscribing certain reasons for seeking an otherwise legal abortion; instead, it awaits lower courts’ review of the issue. Justice Clarence Thomas wrote an extensive concurring opinion suggesting that this law is intended to avoid abortion to achieve eugenic goals.3
Key developments from the shadow docket
The Court issued a stay preventing a Louisiana statute that requires physicians who perform abortions to have admitting privileges at a nearby hospital from going into effect, pending the outcome of litigation about that law.4 Four dissenters noted that all 4 physicians who perform abortions in Louisiana have such privileges. Chief Justice Roberts was the fifth vote to grant the stay. This case likely will make its way back to the Court, as will a number of other state laws being adopted. The issue may be back as soon as the term just starting.
The Court is also considering whether to take another Indiana case, Box v Planned Parenthood of Indiana and Kentucky, Inc. (Box II). This case involves an Indiana ultrasonography viewing option as part of the abortion consent process.5
The Court declined to hear cases from Louisiana and Kansas in which the states had cut off Medicaid funding to Planned Parenthood. Lower courts had stopped the implementation of those laws.6 The legal issue was whether private parties, as opposed to the federal government, had standing to bring the case. For now, the decision of the lower courts to stop implementation of the funding cutoff is in effect. There is a split in the Circuit Courts on the issue, however, making it likely that the Supreme Court will have to resolve it sooner or later.
Health care organizations have filed a number of amicus briefs in these and other cases involving new abortion regulations. ACOG and others filed a brief opposing a Louisiana law that requires abortion providers to have admitting privileges at a nearby facility,7 and a brief opposing a similar Oklahoma law.8 The Association of Pro-Life Obstetricians and Gynecologists and others filed amicus curiae briefs in Box II9 and in an Alabama case involving so-called dismemberment abortion.10
Continue to: Medicare payments...
Medicare payments
Azar, Secretary of Health and Human Services v Allina Health Services, et al11
This case drew interest—and many amicus briefs—from health care providers, including the AMA and the AHA.12,13 There was good reason for their interest: First, the case involved more than $3 billion in reimbursements; second, it represented a potentially important precedent about the rights of providers and patients to comment on Medicare reimbursement changes. The question involved the technical calculation of additional payments made to institutions that serve a disproportionate number of low-income patients (known as Medicare Fractions).
At stake. The issue was a statutory requirement for a 60-day public notice and comment period for rules that “change a substantive legal standard” governing the scope of benefits, eligibility, or payment for services.14 In 2014, the Secretary of the Department of Health and Human Services (HHS) in the Obama administration posted a spreadsheet announcing Medicare fractions rates for hospitals (for 2012)—without formal notice or comment regarding the formula used. (The spreadsheet listed what each qualifying institution would receive, but it was based on a formula that, as noted, had not been subject to public notice and comment.) The AMA and AHA briefs emphasized the importance of a notice and comment period, especially when Medicare reimbursement is involved.
Final ruling. The Court held that the HHS process violated the notice and comment provision, thereby invalidating the policy underlying the so-called spreadsheet reimbursement. The decision was significant: This was a careful statutory interpretation of the 60-day notice and comment period, not the reimbursement policy itself. Presumably, had the HHS Secretary provided for sufficient notice and comment, the formula used would have met the requirements for issuing reimbursement formulas.
Key points. Hospitals will collectively receive $3 or $4 billion as a consequence of the ruling. Perhaps more importantly, the decision signals that HHS is going to have to take seriously the requirement that it publish Medicare-related reimbursement policies for the 60-day period.
A number of diverse cases ruled on by the Supreme Court are worth mentioning. The Court:
- allowed the President to move various funds from the US Department of Defense into accounts from which the money could be used to build a portion of a wall along the southern US border.1
- essentially killed the "citizenship question" on the census form. Technically, the Court sent the issue back to the Commerce Department for better justification for including the question (the announced reasons appeared to be pretextual).2
- changed, perhaps substantially, the deference that courts give to federal agencies in interpreting regulations.3
- upheld, in 2 cases, treaty rights of Native Americans to special treatment on Indian Lands4,5; the Court held that treaties ordinarily should be interpreted as the tribe understood them at the time they were signed. (These were 5 to 4 decisions; the split in the Court leaves many unanswered questions.)
- made it easier for landowners to file suit in federal court when they claim that the state has "taken" their property without just compensation.6
- held that a refusal of the US Patent and Trademark Office to register "immoral" or "scandalous" trademarks infringes on the First Amendment. (The petitioner sought to register "FUCT" as a trademark for a line of clothing.)7
- allowed an antitrust case by iPhone users against Apple to go forward. At issue: the claim that Apple monopolizes the retail market for apps by requiring buyers to obtain apps from Apple.8
- held that, if a drunk-driving suspect who has been taken into custody is, or becomes, unconscious, the "reasonable search" provision of the Fourth Amendment generally does not prevent a state from taking a blood specimen without a warrant. (Wisconsin had a specific "implied consent" law, by which someone receiving a driving license consents to a blood draw.9)
- decided numerous capital punishment cases. In many ways, this term seemed to be a "capital term." Issues involved in these cases have split the Court; it is reasonable to expect that the divide will endure through upcoming terms.
References
- Donald J. Trump, President of the United States, et al. v Sierra Club, et al. 588 US 19A60 (2019).
- Department of Commerce et al. v New York et al. 18 996 (2018).
- Kisor v Wilkie, Secretary of Veterans Affairs. 18 15 (2018).
- Washington State Department of Licensing v Cougar Den, Inc. 16 1498 (2018).
- Herrera v Wyoming. 17 532 (2018).
- Knick v Township of Scott, Pennsylvania, et al. 17 647 (2018).
- Iancu, Under Secretary of Commerce for Intellectual Property and Director, Patent and Trademark Office v Brunetti. 18 302 (2018).
- Apple Inc. v Pepper et al. 17 204 (2018).
- Mitchell v Wisconsin. 18 6210 (2018).
Liability under the False Claims Act
The False Claims Act (FCA) protects the federal government from fraudulent claims for payment and for shoddy goods and services. It incentivizes (by a percentage of recovery) private parties to bring cases to enforce the law.15 (Of course, the federal government also enforces the Act.)
At stake. The FCA has been of considerable concern to the AHA, the Association of American Medical Colleges, and other health care organizations—understandably so.16 As the AHA informed the Court in an amicus brief, “The prevalence of [FCA] cases has ballooned over the past three decades.... These suits disproportionately target healthcare entities.... Of the 767 new FCA cases filed in 2018, for example, 506 involved healthcare defendants.”17
Final ruling. The Court considered an ambiguity in the statute of limitations for these actions and the Court unanimously ruled to permit an extended time in which qui tam actions (private actions under the law) can be filed.18
Key points. As long a period as 10 years can pass between the time an FCA violation occurs and an action is brought. This decision is likely to increase the number of FCA actions against health care providers because the case can be filed many years after the conduct that gave rise to the complaint.
Continue to: Registering sex offenders...
Registering sex offenders
The Court upheld the constitutionality of the federal Sex Offender Registration and Notification Act (SORNA).19 Sex offenders must register and periodically report, in person, to law enforcement in every state in which the offender works, studies, or resides.
At stake. The case involved the applicability of SORNA registration obligations to those convicted of sex offenses before SORNA was adopted (pre-Act offenders).20 The court upheld registration requirements for pre-Act offenders.
Former Justice Stevens, the longest-living and third-longest-serving Supreme Court justice, died in July 2019 at 99 years of age. He was appointed to the Court in 1975 by President Ford and served until his retirement in 2010, when he was 90. Stevens had recently published a memoir, The Making of a Justice: Reflections on My First 94 Years.
Stevens's judicial philosophy generally is described as having changed over the course of his 35 years of service: He was viewed as becoming more liberal. He was a justice of enduring kindness and integrity. It is possible to find people who disagree with him, but almost impossible to find anyone who disliked him. He was continuously committed to the law and justice in the United States.
Arbitration
The Court continued its practice of deciding at least one case each term that emphasizes that federal law requires that courts rather strictly enforce agreements to arbitrate (instead of to litigate) future disputes.21 In another case, the Court ruled that there can be “class” or “joint” arbitration only if the agreement to arbitrate a dispute clearly permits such class arbitration.22
Pharma’s liability regarding product risk
The Court somewhat limited the liability of pharmaceutical companies for failing to provide adequate warning about the risk that their products pose. The case against Merck involved 500 patients who took denosumab (Fosamax) and suffered atypical femoral fractures.23
At stake. Because prescribing information (in which warnings are provided) must be approved by the US Food and Drug Administration (FDA), the legal test is: Would the FDA have refused to approve a change in the warning if Merck had “fully informed the FDA of the justifications for the warning” required by state law to avoid liability?24,25 Lower-court judges (not juries) will be expected to apply this test in the future.
The doctor and the death penalty
The Court has established a rule that, when a prisoner facing capital punishment objects to a form of execution because it is too painful, he has to propose an alternative that is reasonably available. In one case,26 a physician, an expert witness for the prisoner, did not answer some essential relative-pain questions (ie, would one procedure be more painful than another?).
At stake. The AMA filed an amicus brief in this case, indicating that it is unethical for physicians to participate in an execution. The brief noted that “testimony used to determine which method of execution would reduce physical suffering would constitute physician participation in capital punishment and would be unethical.”27
The expert witness’s failure to answer the question on relative pain had the unfortunate result of reducing the likelihood that the prisoner would prevail in his request for an alternative method of execution.
Analysis
Despite obvious disagreements about big issues (notably, abortion and the death penalty) the Court maintained a courteous and civil demeanor—something not always seen nowadays in other branches of government. Here are facts about the Court’s term just concluded:
- The Court issued 72 merits opinions (about average).
- Only 39% of decisions were unanimous (compared with the average of 49% in recent terms).
- On the other hand, 26% of decisions were split 5 to 4 (compared with a 10% recent average).
- In those 5 to 4 decisions, Justices were in the majority as follows28: Justice Gorsuch, 65%; Justice Kavanaugh, 61%; Justice Thomas, 60%; Chief Justice Roberts and Justices Ginsburg and Alito, each 55%; Justice Breyer, 50%; and Justices Sotomayor and Kagan each at 45%.
- There were 57 dissenting opinions—up from 48 in the previous term.
- What is referred to as “the liberal-conservative split” might seem more profound than it really is: “Every conservative member of the court at some point voted to form a majority with the liberal justices. And every liberal at least once left behind all of his or her usual voting partners to join the conservatives.”29
Continue to: Last, it was a year of personal health issues for...
Last, it was a year of personal health issues for the Court: Justice Ginsburg had a diagnosis of lung cancer and was absent, following surgery, in January. Of retired Justices, Sandra Day O’Connor suffers from dementia and former Justice John Paul Stevens died.
In closing
The Court has accepted approximately 50 cases for the current term, which began on October 7. The first 2 days of the term were spent on arguments about, first, whether a state can abolish the insanity defense and, second, whether nondiscrimination laws (“based on sex”) prohibit discrimination based on sexual orientation or transgender status. Cases also will deal with Patient Protection and Affordable Care Act payments to providers; the Deferred Action for Childhood Arrivals, or DACA; the death penalty; and international child custody disputes. The Court will be accepting more cases for several months. It promises to be a very interesting term.
- Box v Planned Parenthood of Indiana and Kentucky, Inc. 587 US 18 483 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc., at 2.
- Box v Planned Parenthood of Indiana and Kentucky, Inc., Justice Thomas concurring.
- June Medical Services, LLC, et al. v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. 586 US 18A774 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc. Docket 18-1019.
- Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals v Planned Parenthood of Gulf Coast, Inc., et al. 586 US 17 1492 (2018).
- June Medical Services L.L.C., et al., Petitioners, v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. No. 18-1323. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Society for Reproductive Medicine, National Association of Nurse Practitioners in Women's Health, North American Society for Pediatric and Adolescent Gynecology, and Society For Maternal-Fetal Medicine, Amicus Curiae in Support of Petitioners. May 2019.
- Planned Parenthood of Kansas & Eastern Oklahoma, et al., Petitioners, v Larry Jegley, et al., Respondents. No. 17-935. Brief Amici Curiae of American College of Obstetricians and Gynecologists and American Public Health Association as Amici Curiae in Support of Petitioners. February 1, 2018.
- Box v Planned Parenthood of Indiana & Kentucky. No. 18-1019. Brief Amici Curiae of American Association of Pro-Life Obstetricians & Gynecologists, American College of Pediatricians, Care Net, Christian Medical Association, Heartbeat International, Inc., and National Institute Of Family & Life Advocates in Support of Petitioners. March 6, 2019.
- Steven T. Marshall, et al., Petitioners, v West Alabama Women's Center, et al., Respondents. No. 18-837. Brief of Amici Curiae American Association of Pro-Life Obstetricians & Gynecologists and American College of Pediatricians, in Support of Petitioners. January 18, 2019.
- Azar, Secretary of Health and Human Services v Allina Health Services, et al. 17 1484 (2018).
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges as Amici Curiae in Support of Respondents. December 2018.
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of Amici Curiae American Medical Association and Medical Society of the District of Columbia Amici Curiae in Support of Respondents. December 2018.
- 42 U. S. C. §1395hh. https://uscode.house.gov/view.xhtml?req=(title:42%20section:1395hh%20edition:prelim). Accessed October 22, 2019.
- The False Claims Act: a primer. Washington DC: US Department of Justice. www.justice.gov/sites/default/files/civil/legacy/2011/04/22/C-FRAUDS_FCA_Primer.pdf. Accessed October 18, 2019.
- Universal Health Services, Inc., v United States and Commonwealth of Massachusetts ex rel. Julio Escobar and Carmen Correa. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges Amici Curiae in Support of Petitioner. No. 15-7. January 2016.
- Intermountain Health Care, Inc., et al., Petitioners, v United States ex rel. Gerald Polukoff, et al., Respondents. No. 18-911. Brief of the American Hospital Association and Federation of American Hospitals as Amici Curiae in Support of Petitioners. February 13, 2019.
- Cochise Consultancy, Inc., et al., v United States ex rel. Hunt. 18 315 (2018).
- 34 U.S.C. §20901 et seq. [Chapter 209--Child Protection and Safety.] https://uscode.house.gov/view.xhtml?path=/prelim@title34/subtitle2/chapter209&edition=prelim. Accessed October 17, 2019.
- Gundy v United States. 17 6086 (2018).
- Henry Schein, Inc., et al., v Archer & White Sales, Inc. 17 1272 (2018).
- Lamps Plus, Inc., et al., v Varela. 17 988 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018) at 13-14.
- Wyeth v Levine, 555 US 555, 571 (2009).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018). American Medical Association, Amicus Curiae Brief, in Support of Neither Party. July 23, 2018.
- Final stat pack for October term 2018. SCOTUSblog.com. June 28, 2019. https://www.scotusblog.com/wp-content/uploads/2019/07/StatPack_OT18-7_8_19.pdf. Accessed October 17, 2019.
- Barnes R. They're not 'wonder twins': Gorsuch, Kavanaugh shift the Supreme Court, but their differences are striking. Washington Post, June 28, 2019.
- Box v Planned Parenthood of Indiana and Kentucky, Inc. 587 US 18 483 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc., at 2.
- Box v Planned Parenthood of Indiana and Kentucky, Inc., Justice Thomas concurring.
- June Medical Services, LLC, et al. v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. 586 US 18A774 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc. Docket 18-1019.
- Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals v Planned Parenthood of Gulf Coast, Inc., et al. 586 US 17 1492 (2018).
- June Medical Services L.L.C., et al., Petitioners, v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. No. 18-1323. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Society for Reproductive Medicine, National Association of Nurse Practitioners in Women's Health, North American Society for Pediatric and Adolescent Gynecology, and Society For Maternal-Fetal Medicine, Amicus Curiae in Support of Petitioners. May 2019.
- Planned Parenthood of Kansas & Eastern Oklahoma, et al., Petitioners, v Larry Jegley, et al., Respondents. No. 17-935. Brief Amici Curiae of American College of Obstetricians and Gynecologists and American Public Health Association as Amici Curiae in Support of Petitioners. February 1, 2018.
- Box v Planned Parenthood of Indiana & Kentucky. No. 18-1019. Brief Amici Curiae of American Association of Pro-Life Obstetricians & Gynecologists, American College of Pediatricians, Care Net, Christian Medical Association, Heartbeat International, Inc., and National Institute Of Family & Life Advocates in Support of Petitioners. March 6, 2019.
- Steven T. Marshall, et al., Petitioners, v West Alabama Women's Center, et al., Respondents. No. 18-837. Brief of Amici Curiae American Association of Pro-Life Obstetricians & Gynecologists and American College of Pediatricians, in Support of Petitioners. January 18, 2019.
- Azar, Secretary of Health and Human Services v Allina Health Services, et al. 17 1484 (2018).
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges as Amici Curiae in Support of Respondents. December 2018.
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of Amici Curiae American Medical Association and Medical Society of the District of Columbia Amici Curiae in Support of Respondents. December 2018.
- 42 U. S. C. §1395hh. https://uscode.house.gov/view.xhtml?req=(title:42%20section:1395hh%20edition:prelim). Accessed October 22, 2019.
- The False Claims Act: a primer. Washington DC: US Department of Justice. www.justice.gov/sites/default/files/civil/legacy/2011/04/22/C-FRAUDS_FCA_Primer.pdf. Accessed October 18, 2019.
- Universal Health Services, Inc., v United States and Commonwealth of Massachusetts ex rel. Julio Escobar and Carmen Correa. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges Amici Curiae in Support of Petitioner. No. 15-7. January 2016.
- Intermountain Health Care, Inc., et al., Petitioners, v United States ex rel. Gerald Polukoff, et al., Respondents. No. 18-911. Brief of the American Hospital Association and Federation of American Hospitals as Amici Curiae in Support of Petitioners. February 13, 2019.
- Cochise Consultancy, Inc., et al., v United States ex rel. Hunt. 18 315 (2018).
- 34 U.S.C. §20901 et seq. [Chapter 209--Child Protection and Safety.] https://uscode.house.gov/view.xhtml?path=/prelim@title34/subtitle2/chapter209&edition=prelim. Accessed October 17, 2019.
- Gundy v United States. 17 6086 (2018).
- Henry Schein, Inc., et al., v Archer & White Sales, Inc. 17 1272 (2018).
- Lamps Plus, Inc., et al., v Varela. 17 988 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018) at 13-14.
- Wyeth v Levine, 555 US 555, 571 (2009).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018). American Medical Association, Amicus Curiae Brief, in Support of Neither Party. July 23, 2018.
- Final stat pack for October term 2018. SCOTUSblog.com. June 28, 2019. https://www.scotusblog.com/wp-content/uploads/2019/07/StatPack_OT18-7_8_19.pdf. Accessed October 17, 2019.
- Barnes R. They're not 'wonder twins': Gorsuch, Kavanaugh shift the Supreme Court, but their differences are striking. Washington Post, June 28, 2019.
Medical management of abnormal uterine bleeding in reproductive-age women
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).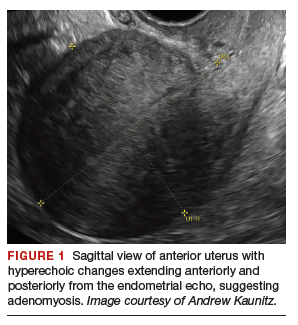
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).
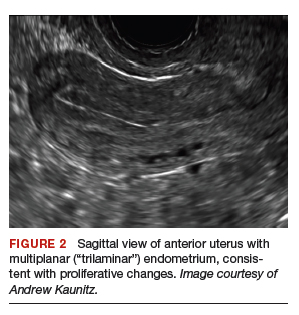

After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6
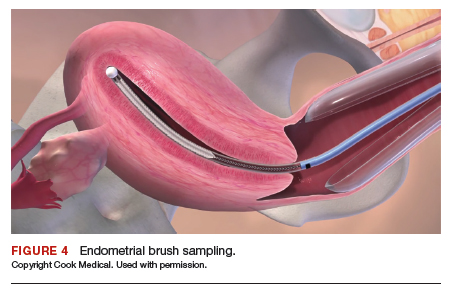
Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.
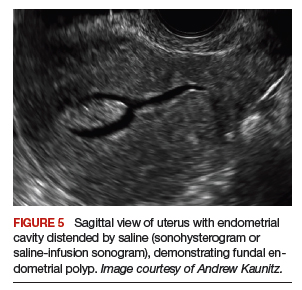
Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16
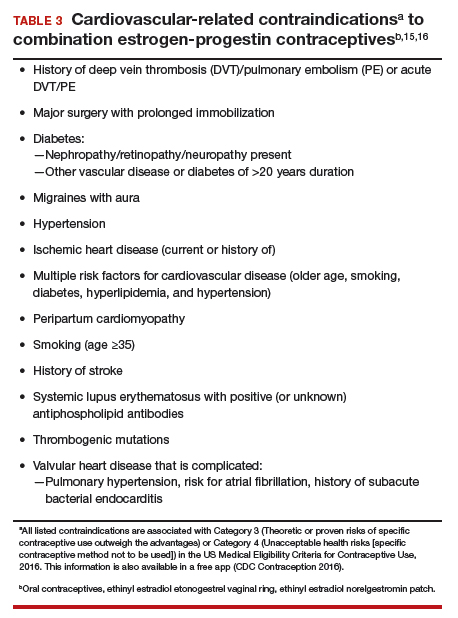
Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.