User login
SUSTAIN 10: Weight loss, glycemic control better with semaglutide than liraglutide
BARCELONA – Patients with type 2 diabetes who were treated with semaglutide achieved greater reductions in glycated hemoglobin (HbA1c) levels and body weight, compared with those receiving liraglutide, according to results presented at the annual meeting of the European Association for the Study of Diabetes.
In the phase 3b SUSTAIN 10 trial, conducted in 11 European countries, mean glycated hemoglobin at 30 weeks decreased by 1.7% with once-weekly semaglutide and 1.0% for once-daily liraglutide, from the overall baseline level of 8.2%. The estimated treatment difference (ETD) between the two treatments was –0.69 percentage points (95% confidence interval, –0.82 to –0.56; P less than .0001).
Mean body weight decreased during the same period by 5.8 kg with semaglutide and 1.9 kg with liraglutide, from a baseline of 96.9 kg. The ETD was 3.83 kg (95% CI, –4.57 to –3.09; P less than .0001).
The doses of semaglutide and liraglutide used in the study were 1.0 mg and 1.2 mg, respectively, the latter being the dose that is used most commonly in clinical practice, study investigator Matthew Capehorn, MB, CAB, explained in an interview at the meeting.
“We know that at a dose of 1.8 mg, liraglutide is more effective than 1.2 mg, but it’s about whether it is deemed more cost effective,” said Dr. Capehorn, who is clinical manager at Rotherham (England) Institute for Obesity, Clifton Medical Centre. “Certainly, in the United Kingdom, we’re encouraged to use the 1.2-mg dose” according to guidance from the National Institute for Heath and Care Excellence, and “other European countries are the same.”
Dr. Capehorn noted that studies are being done with a higher dose of semaglutide to see if it has potential as a weight loss drug in its own right in patients who do not have type 2 diabetes. “I care as much about obesity and cardiovascular disease as I do about chasing the HbA1c level and getting that reduced, so I would rather pick an agent that covers all three [components], than just looking at the HbA1c,” he said.
In SUSTAIN 10,577 adults with type 2 diabetes and an HbA1c level of between 7.0% and 11.0% who were on stable doses of one to three oral antidiabetic drugs were randomized to receive semaglutide (n = 290) or liraglutide (n = 287) for 30 weeks.
The primary endpoint was the change in HbA1c from baseline to week 30, and the secondary confirmatory endpoint was change in body weight over the same period.
In presenting the findings, which were simultaneously published in Diabetes & Metabolism, Dr. Capehorn noted that the efficacy results were consistent with those of other SUSTAIN trials that compared semaglutide with other glucagonlike peptide–1 receptor antagonists, notably SUSTAIN 3 (with exenatide extended release) and SUSTAIN 7 (with dulaglutide).
Other efficacy findings from SUSTAIN 10 were that semaglutide produced greater mean changes than did liraglutide in both fasting plasma glucose and in a 7-point, self-monitoring of blood glucose profile.
A greater percentage of people treated with semaglutide, compared with liraglutide, also achieved their glycemic targets of less than 7.0% (80% vs. 46%, respectively) and of 6.5% or less (58% vs. 25%), and their weight loss targets of 5% or more (56% vs. 18%) and 10% or more (19% vs. 4%).
In addition, more semaglutide- than liraglutide-treated patients achieved an HbA1c target of less than 7.0% without severe or blood glucose–confirmed symptomatic hypoglycemia, with or without weight gain (76% vs. 37%; P less than .0001). There were also more semaglutide patients who achieved an HbA1c reduction of 1% or more and a weight loss reduction of 10% or more (17% vs. 4% for liraglutide, P less than .0001).
The safety profiles were similar for semaglutide and liraglutide, Dr. Capehorn noted, but gastrointestinal adverse events were more prevalent in patients receiving semaglutide, compared with liraglutide (43.9% vs. 38.3%), and more patients receiving semaglutide discontinued treatment prematurely because of those adverse events (11.4% vs. 6.6% for liraglutide).
Novo Nordisk sponsored the study. Dr. Capehorn reported receiving research funding from, providing advisory board support to, and speaker fees from Novo Nordisk and from several other companies.
SOURCE: Capehorn M et al. EASD 2019, Oral Presentation 53; Capehorn M et al. Diabetes Metab. 2019 Sep 17. doi: 10.1016/j.diabet.2019.101117.
BARCELONA – Patients with type 2 diabetes who were treated with semaglutide achieved greater reductions in glycated hemoglobin (HbA1c) levels and body weight, compared with those receiving liraglutide, according to results presented at the annual meeting of the European Association for the Study of Diabetes.
In the phase 3b SUSTAIN 10 trial, conducted in 11 European countries, mean glycated hemoglobin at 30 weeks decreased by 1.7% with once-weekly semaglutide and 1.0% for once-daily liraglutide, from the overall baseline level of 8.2%. The estimated treatment difference (ETD) between the two treatments was –0.69 percentage points (95% confidence interval, –0.82 to –0.56; P less than .0001).
Mean body weight decreased during the same period by 5.8 kg with semaglutide and 1.9 kg with liraglutide, from a baseline of 96.9 kg. The ETD was 3.83 kg (95% CI, –4.57 to –3.09; P less than .0001).
The doses of semaglutide and liraglutide used in the study were 1.0 mg and 1.2 mg, respectively, the latter being the dose that is used most commonly in clinical practice, study investigator Matthew Capehorn, MB, CAB, explained in an interview at the meeting.
“We know that at a dose of 1.8 mg, liraglutide is more effective than 1.2 mg, but it’s about whether it is deemed more cost effective,” said Dr. Capehorn, who is clinical manager at Rotherham (England) Institute for Obesity, Clifton Medical Centre. “Certainly, in the United Kingdom, we’re encouraged to use the 1.2-mg dose” according to guidance from the National Institute for Heath and Care Excellence, and “other European countries are the same.”
Dr. Capehorn noted that studies are being done with a higher dose of semaglutide to see if it has potential as a weight loss drug in its own right in patients who do not have type 2 diabetes. “I care as much about obesity and cardiovascular disease as I do about chasing the HbA1c level and getting that reduced, so I would rather pick an agent that covers all three [components], than just looking at the HbA1c,” he said.
In SUSTAIN 10,577 adults with type 2 diabetes and an HbA1c level of between 7.0% and 11.0% who were on stable doses of one to three oral antidiabetic drugs were randomized to receive semaglutide (n = 290) or liraglutide (n = 287) for 30 weeks.
The primary endpoint was the change in HbA1c from baseline to week 30, and the secondary confirmatory endpoint was change in body weight over the same period.
In presenting the findings, which were simultaneously published in Diabetes & Metabolism, Dr. Capehorn noted that the efficacy results were consistent with those of other SUSTAIN trials that compared semaglutide with other glucagonlike peptide–1 receptor antagonists, notably SUSTAIN 3 (with exenatide extended release) and SUSTAIN 7 (with dulaglutide).
Other efficacy findings from SUSTAIN 10 were that semaglutide produced greater mean changes than did liraglutide in both fasting plasma glucose and in a 7-point, self-monitoring of blood glucose profile.
A greater percentage of people treated with semaglutide, compared with liraglutide, also achieved their glycemic targets of less than 7.0% (80% vs. 46%, respectively) and of 6.5% or less (58% vs. 25%), and their weight loss targets of 5% or more (56% vs. 18%) and 10% or more (19% vs. 4%).
In addition, more semaglutide- than liraglutide-treated patients achieved an HbA1c target of less than 7.0% without severe or blood glucose–confirmed symptomatic hypoglycemia, with or without weight gain (76% vs. 37%; P less than .0001). There were also more semaglutide patients who achieved an HbA1c reduction of 1% or more and a weight loss reduction of 10% or more (17% vs. 4% for liraglutide, P less than .0001).
The safety profiles were similar for semaglutide and liraglutide, Dr. Capehorn noted, but gastrointestinal adverse events were more prevalent in patients receiving semaglutide, compared with liraglutide (43.9% vs. 38.3%), and more patients receiving semaglutide discontinued treatment prematurely because of those adverse events (11.4% vs. 6.6% for liraglutide).
Novo Nordisk sponsored the study. Dr. Capehorn reported receiving research funding from, providing advisory board support to, and speaker fees from Novo Nordisk and from several other companies.
SOURCE: Capehorn M et al. EASD 2019, Oral Presentation 53; Capehorn M et al. Diabetes Metab. 2019 Sep 17. doi: 10.1016/j.diabet.2019.101117.
BARCELONA – Patients with type 2 diabetes who were treated with semaglutide achieved greater reductions in glycated hemoglobin (HbA1c) levels and body weight, compared with those receiving liraglutide, according to results presented at the annual meeting of the European Association for the Study of Diabetes.
In the phase 3b SUSTAIN 10 trial, conducted in 11 European countries, mean glycated hemoglobin at 30 weeks decreased by 1.7% with once-weekly semaglutide and 1.0% for once-daily liraglutide, from the overall baseline level of 8.2%. The estimated treatment difference (ETD) between the two treatments was –0.69 percentage points (95% confidence interval, –0.82 to –0.56; P less than .0001).
Mean body weight decreased during the same period by 5.8 kg with semaglutide and 1.9 kg with liraglutide, from a baseline of 96.9 kg. The ETD was 3.83 kg (95% CI, –4.57 to –3.09; P less than .0001).
The doses of semaglutide and liraglutide used in the study were 1.0 mg and 1.2 mg, respectively, the latter being the dose that is used most commonly in clinical practice, study investigator Matthew Capehorn, MB, CAB, explained in an interview at the meeting.
“We know that at a dose of 1.8 mg, liraglutide is more effective than 1.2 mg, but it’s about whether it is deemed more cost effective,” said Dr. Capehorn, who is clinical manager at Rotherham (England) Institute for Obesity, Clifton Medical Centre. “Certainly, in the United Kingdom, we’re encouraged to use the 1.2-mg dose” according to guidance from the National Institute for Heath and Care Excellence, and “other European countries are the same.”
Dr. Capehorn noted that studies are being done with a higher dose of semaglutide to see if it has potential as a weight loss drug in its own right in patients who do not have type 2 diabetes. “I care as much about obesity and cardiovascular disease as I do about chasing the HbA1c level and getting that reduced, so I would rather pick an agent that covers all three [components], than just looking at the HbA1c,” he said.
In SUSTAIN 10,577 adults with type 2 diabetes and an HbA1c level of between 7.0% and 11.0% who were on stable doses of one to three oral antidiabetic drugs were randomized to receive semaglutide (n = 290) or liraglutide (n = 287) for 30 weeks.
The primary endpoint was the change in HbA1c from baseline to week 30, and the secondary confirmatory endpoint was change in body weight over the same period.
In presenting the findings, which were simultaneously published in Diabetes & Metabolism, Dr. Capehorn noted that the efficacy results were consistent with those of other SUSTAIN trials that compared semaglutide with other glucagonlike peptide–1 receptor antagonists, notably SUSTAIN 3 (with exenatide extended release) and SUSTAIN 7 (with dulaglutide).
Other efficacy findings from SUSTAIN 10 were that semaglutide produced greater mean changes than did liraglutide in both fasting plasma glucose and in a 7-point, self-monitoring of blood glucose profile.
A greater percentage of people treated with semaglutide, compared with liraglutide, also achieved their glycemic targets of less than 7.0% (80% vs. 46%, respectively) and of 6.5% or less (58% vs. 25%), and their weight loss targets of 5% or more (56% vs. 18%) and 10% or more (19% vs. 4%).
In addition, more semaglutide- than liraglutide-treated patients achieved an HbA1c target of less than 7.0% without severe or blood glucose–confirmed symptomatic hypoglycemia, with or without weight gain (76% vs. 37%; P less than .0001). There were also more semaglutide patients who achieved an HbA1c reduction of 1% or more and a weight loss reduction of 10% or more (17% vs. 4% for liraglutide, P less than .0001).
The safety profiles were similar for semaglutide and liraglutide, Dr. Capehorn noted, but gastrointestinal adverse events were more prevalent in patients receiving semaglutide, compared with liraglutide (43.9% vs. 38.3%), and more patients receiving semaglutide discontinued treatment prematurely because of those adverse events (11.4% vs. 6.6% for liraglutide).
Novo Nordisk sponsored the study. Dr. Capehorn reported receiving research funding from, providing advisory board support to, and speaker fees from Novo Nordisk and from several other companies.
SOURCE: Capehorn M et al. EASD 2019, Oral Presentation 53; Capehorn M et al. Diabetes Metab. 2019 Sep 17. doi: 10.1016/j.diabet.2019.101117.
REPORTING FROM EASD 2019
Blood test might rival PET scan for detecting brain amyloidosis
ST. LOUIS – according to a report at the annual meeting of the American Neurological Association. The research was also published in Neurology (2019 Oct 22;93[17]:e1647-59).
Investigators at Washington University, St. Louis, found that, among 158 mostly cognitively normal people in their 60s and 70s, the plasma ratio of amyloid-beta 42 peptide to amyloid-beta 40 peptide identified people who were PET positive and PET negative for amyloid with an area under the curve of 0.88 (95% confidence interval, 0.82-0.93) and climbed to 0.94 when combined with age and Apolipoprotein E epsilon 4 status (95% CI, 0.90-0.97), “which is really quite spectacular for a blood test,” said study lead Suzanne Schindler, MD, PhD, who is affiliated with the university.
People who had a positive blood test – a ratio below .1281 – but a negative PET scan were 15 times more likely to convert to a positive scan at an average of 4 years than subjects with a negative test. “The blood test [detected] brain changes of Alzheimer’s disease before the amyloid PET scan,” Dr. Schindler said.
Amyloid-beta 42 – the number refers to how many amino acids are in the peptide chain – is much stickier and more prone to aggregate in plaques than amyloid-beta 40. The ratio of the two falls as the 42 form is sequestered preferentially into amyloid plaques while the level of amyloid-beta 40 remains more constant, she explained at the meeting.
The team concluded that the test accurately “predicts current and future brain amyloidosis” and “could be used in prevention trials to screen for individuals likely to be amyloid PET-positive and at risk for Alzheimer disease dementia.”
“We are really excited about it. I think there’s been recognition for a long time that a blood test would really be a game changer. We still have a little bit more work to do, but I don’t think it’s that far away,” Dr. Schindler said in an interview after her presentation.
The goal of Alzheimer’s research is to slow, reverse, or even prevent brain pathology before symptoms set in, at which point damage is likely irreversible. For that to happen, plaques need to be detected early.
Currently there are two ways to do that, both with difficulties: PET scans, which are expensive, expose people to radiation, and of limited availability, and spinal fluid analysis, which involves a lumbar puncture that “not many people want to undergo.” The problems slow down enrollment for prevention trials, Dr. Schindler said.
The blood test, which the Food and Drug Administration granted breakthrough status in January 2019, could offer a much easier and less expensive way to identify subjects and monitor outcomes. It could “really speed up enrollment and help us get to effective drugs faster,” she said.
Beyond that, clinicians could use it to help figure out what’s going on in older people with cognitive issues. If a drug or some other way is ever found to prevent Alzheimer’s, there’s even the possibility of screening patients for amyloidosis during routine exams. Potentially, “I think the market is huge,” she said.
The test is being developed by a company, C2N diagnostics, founded by Dr. Schindler’s colleagues at the university, and could be available commercially in 2-3 years. It involves high precision immunoprecipitation and liquid chromatography/mass spectrometry, so “it isn’t something your general lab is going to do. It’s probably going to be a couple centers that have this test, and everybody mails their samples in, which we do for a lot of different tests,” she said.
Several companies are working on similar assays.
Dr. Schindler said she has no financial stake in the blood test.
SOURCE: Schindler S et al. Neurology. 2019 Oct 22;93(17):e1647-59.
ST. LOUIS – according to a report at the annual meeting of the American Neurological Association. The research was also published in Neurology (2019 Oct 22;93[17]:e1647-59).
Investigators at Washington University, St. Louis, found that, among 158 mostly cognitively normal people in their 60s and 70s, the plasma ratio of amyloid-beta 42 peptide to amyloid-beta 40 peptide identified people who were PET positive and PET negative for amyloid with an area under the curve of 0.88 (95% confidence interval, 0.82-0.93) and climbed to 0.94 when combined with age and Apolipoprotein E epsilon 4 status (95% CI, 0.90-0.97), “which is really quite spectacular for a blood test,” said study lead Suzanne Schindler, MD, PhD, who is affiliated with the university.
People who had a positive blood test – a ratio below .1281 – but a negative PET scan were 15 times more likely to convert to a positive scan at an average of 4 years than subjects with a negative test. “The blood test [detected] brain changes of Alzheimer’s disease before the amyloid PET scan,” Dr. Schindler said.
Amyloid-beta 42 – the number refers to how many amino acids are in the peptide chain – is much stickier and more prone to aggregate in plaques than amyloid-beta 40. The ratio of the two falls as the 42 form is sequestered preferentially into amyloid plaques while the level of amyloid-beta 40 remains more constant, she explained at the meeting.
The team concluded that the test accurately “predicts current and future brain amyloidosis” and “could be used in prevention trials to screen for individuals likely to be amyloid PET-positive and at risk for Alzheimer disease dementia.”
“We are really excited about it. I think there’s been recognition for a long time that a blood test would really be a game changer. We still have a little bit more work to do, but I don’t think it’s that far away,” Dr. Schindler said in an interview after her presentation.
The goal of Alzheimer’s research is to slow, reverse, or even prevent brain pathology before symptoms set in, at which point damage is likely irreversible. For that to happen, plaques need to be detected early.
Currently there are two ways to do that, both with difficulties: PET scans, which are expensive, expose people to radiation, and of limited availability, and spinal fluid analysis, which involves a lumbar puncture that “not many people want to undergo.” The problems slow down enrollment for prevention trials, Dr. Schindler said.
The blood test, which the Food and Drug Administration granted breakthrough status in January 2019, could offer a much easier and less expensive way to identify subjects and monitor outcomes. It could “really speed up enrollment and help us get to effective drugs faster,” she said.
Beyond that, clinicians could use it to help figure out what’s going on in older people with cognitive issues. If a drug or some other way is ever found to prevent Alzheimer’s, there’s even the possibility of screening patients for amyloidosis during routine exams. Potentially, “I think the market is huge,” she said.
The test is being developed by a company, C2N diagnostics, founded by Dr. Schindler’s colleagues at the university, and could be available commercially in 2-3 years. It involves high precision immunoprecipitation and liquid chromatography/mass spectrometry, so “it isn’t something your general lab is going to do. It’s probably going to be a couple centers that have this test, and everybody mails their samples in, which we do for a lot of different tests,” she said.
Several companies are working on similar assays.
Dr. Schindler said she has no financial stake in the blood test.
SOURCE: Schindler S et al. Neurology. 2019 Oct 22;93(17):e1647-59.
ST. LOUIS – according to a report at the annual meeting of the American Neurological Association. The research was also published in Neurology (2019 Oct 22;93[17]:e1647-59).
Investigators at Washington University, St. Louis, found that, among 158 mostly cognitively normal people in their 60s and 70s, the plasma ratio of amyloid-beta 42 peptide to amyloid-beta 40 peptide identified people who were PET positive and PET negative for amyloid with an area under the curve of 0.88 (95% confidence interval, 0.82-0.93) and climbed to 0.94 when combined with age and Apolipoprotein E epsilon 4 status (95% CI, 0.90-0.97), “which is really quite spectacular for a blood test,” said study lead Suzanne Schindler, MD, PhD, who is affiliated with the university.
People who had a positive blood test – a ratio below .1281 – but a negative PET scan were 15 times more likely to convert to a positive scan at an average of 4 years than subjects with a negative test. “The blood test [detected] brain changes of Alzheimer’s disease before the amyloid PET scan,” Dr. Schindler said.
Amyloid-beta 42 – the number refers to how many amino acids are in the peptide chain – is much stickier and more prone to aggregate in plaques than amyloid-beta 40. The ratio of the two falls as the 42 form is sequestered preferentially into amyloid plaques while the level of amyloid-beta 40 remains more constant, she explained at the meeting.
The team concluded that the test accurately “predicts current and future brain amyloidosis” and “could be used in prevention trials to screen for individuals likely to be amyloid PET-positive and at risk for Alzheimer disease dementia.”
“We are really excited about it. I think there’s been recognition for a long time that a blood test would really be a game changer. We still have a little bit more work to do, but I don’t think it’s that far away,” Dr. Schindler said in an interview after her presentation.
The goal of Alzheimer’s research is to slow, reverse, or even prevent brain pathology before symptoms set in, at which point damage is likely irreversible. For that to happen, plaques need to be detected early.
Currently there are two ways to do that, both with difficulties: PET scans, which are expensive, expose people to radiation, and of limited availability, and spinal fluid analysis, which involves a lumbar puncture that “not many people want to undergo.” The problems slow down enrollment for prevention trials, Dr. Schindler said.
The blood test, which the Food and Drug Administration granted breakthrough status in January 2019, could offer a much easier and less expensive way to identify subjects and monitor outcomes. It could “really speed up enrollment and help us get to effective drugs faster,” she said.
Beyond that, clinicians could use it to help figure out what’s going on in older people with cognitive issues. If a drug or some other way is ever found to prevent Alzheimer’s, there’s even the possibility of screening patients for amyloidosis during routine exams. Potentially, “I think the market is huge,” she said.
The test is being developed by a company, C2N diagnostics, founded by Dr. Schindler’s colleagues at the university, and could be available commercially in 2-3 years. It involves high precision immunoprecipitation and liquid chromatography/mass spectrometry, so “it isn’t something your general lab is going to do. It’s probably going to be a couple centers that have this test, and everybody mails their samples in, which we do for a lot of different tests,” she said.
Several companies are working on similar assays.
Dr. Schindler said she has no financial stake in the blood test.
SOURCE: Schindler S et al. Neurology. 2019 Oct 22;93(17):e1647-59.
REPORTING FROM ANA 2019
MMS linked with better survival in early-stage melanoma
according to a retrospective cohort study.
In the study, which was published in JAMA Dermatology, patients who underwent MMS had a “modest survival advantage” when compared with those who were treated with WME, the approach recommended for treatment of invasive melanoma without nodal or extralymphatic metastases in national guidelines, reported the investigators.
“We sought herein to investigate the association of the type of surgical excision – WME or MMS – with overall survival for cases of American Joint Committee on Cancer Cancer Staging Manual 8th edition (AJCC-8) stage I invasive melanoma,” wrote Shayan Cheraghlou, of Yale University, New Haven, Conn., and colleagues.
The researchers identified a total of 70,319 patients diagnosed with stage I invasive melanoma between Jan. 1, 2004, and Dec. 31, 2014. Data were collected from the National Cancer Database, including 3,234 (4.6%) and 67,085 (95.4%) patients who underwent MMS and WME, respectively. The median age of patients in the cohort was 57 years; 47.7% were female, and almost 97% were white.
In the survival analysis, the team adjusted for clinical and tumor-specific variables and conducted a matched analysis using propensity scores. The primary outcome measured was overall survival.
After analysis, the researchers found that MMS was associated with modestly better overall survival when compared with WME after adjustments (hazard ratio, 0.86; 95% confidence interval, 0.76-0.97). In the propensity score–matched analysis, a similar modest survival advantage was seen for patients who underwent MMS (hazard ratio, 0.82; 95% CI, 0.68-0.98).
“Significant differences in treatment practices based on the treatment facility were noted, with academic facilities more than twice as likely as nonacademic facilities to use MMS,” they wrote.
The researchers acknowledged a key limitation of the study was the use of a convenience sample, as opposed to a population-based sample. As a result, the generalizability of the findings may be limited to certain treatment facilities.
“These data suggest that MMS is an effective approach compared with WME for AJCC-8 stage I invasive melanoma,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Cheraghlou S et al. JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2890.
While controversial historically, evidence showing benefit for Mohs micrographic surgery (MMS) in patients with melanoma has been reported. The findings from the current study add to the body of retrospective data suggesting improved survival for those with early-stage disease.
The survival benefit found by Cheraghlou et al., “although relatively novel,” is not surprising. Previous population-based and database studies have demonstrated a nonsignificant trend toward a survival advantage in patients with early-stage melanoma. In addition, no survival disadvantages have been reported in any other stage of malignancy.
The primary advantage of MMS is the ability of the surgery to allow for full tumor resection. Reducing the likelihood of recurrence and ensuring local control is maximized remain key strategies to ensuring survival in patients with melanoma.
Database studies have limitations, and care should be taken not to overinterpret the results of a study with two groups of patients that are disproportionate in size. As the authors of the study note, their results support the need for prospective studies to compare surgical melanoma treatments. And until those studies can be done, “the weight of existing evidence suggests that MMS is a safe and effective treatment for melanoma.”
These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2622) by Ian Maher, MD, professor and director of dermatologic surgery at the University of Minnesota, Minneapolis. He reported having no conflicts of interest.
While controversial historically, evidence showing benefit for Mohs micrographic surgery (MMS) in patients with melanoma has been reported. The findings from the current study add to the body of retrospective data suggesting improved survival for those with early-stage disease.
The survival benefit found by Cheraghlou et al., “although relatively novel,” is not surprising. Previous population-based and database studies have demonstrated a nonsignificant trend toward a survival advantage in patients with early-stage melanoma. In addition, no survival disadvantages have been reported in any other stage of malignancy.
The primary advantage of MMS is the ability of the surgery to allow for full tumor resection. Reducing the likelihood of recurrence and ensuring local control is maximized remain key strategies to ensuring survival in patients with melanoma.
Database studies have limitations, and care should be taken not to overinterpret the results of a study with two groups of patients that are disproportionate in size. As the authors of the study note, their results support the need for prospective studies to compare surgical melanoma treatments. And until those studies can be done, “the weight of existing evidence suggests that MMS is a safe and effective treatment for melanoma.”
These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2622) by Ian Maher, MD, professor and director of dermatologic surgery at the University of Minnesota, Minneapolis. He reported having no conflicts of interest.
While controversial historically, evidence showing benefit for Mohs micrographic surgery (MMS) in patients with melanoma has been reported. The findings from the current study add to the body of retrospective data suggesting improved survival for those with early-stage disease.
The survival benefit found by Cheraghlou et al., “although relatively novel,” is not surprising. Previous population-based and database studies have demonstrated a nonsignificant trend toward a survival advantage in patients with early-stage melanoma. In addition, no survival disadvantages have been reported in any other stage of malignancy.
The primary advantage of MMS is the ability of the surgery to allow for full tumor resection. Reducing the likelihood of recurrence and ensuring local control is maximized remain key strategies to ensuring survival in patients with melanoma.
Database studies have limitations, and care should be taken not to overinterpret the results of a study with two groups of patients that are disproportionate in size. As the authors of the study note, their results support the need for prospective studies to compare surgical melanoma treatments. And until those studies can be done, “the weight of existing evidence suggests that MMS is a safe and effective treatment for melanoma.”
These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2622) by Ian Maher, MD, professor and director of dermatologic surgery at the University of Minnesota, Minneapolis. He reported having no conflicts of interest.
according to a retrospective cohort study.
In the study, which was published in JAMA Dermatology, patients who underwent MMS had a “modest survival advantage” when compared with those who were treated with WME, the approach recommended for treatment of invasive melanoma without nodal or extralymphatic metastases in national guidelines, reported the investigators.
“We sought herein to investigate the association of the type of surgical excision – WME or MMS – with overall survival for cases of American Joint Committee on Cancer Cancer Staging Manual 8th edition (AJCC-8) stage I invasive melanoma,” wrote Shayan Cheraghlou, of Yale University, New Haven, Conn., and colleagues.
The researchers identified a total of 70,319 patients diagnosed with stage I invasive melanoma between Jan. 1, 2004, and Dec. 31, 2014. Data were collected from the National Cancer Database, including 3,234 (4.6%) and 67,085 (95.4%) patients who underwent MMS and WME, respectively. The median age of patients in the cohort was 57 years; 47.7% were female, and almost 97% were white.
In the survival analysis, the team adjusted for clinical and tumor-specific variables and conducted a matched analysis using propensity scores. The primary outcome measured was overall survival.
After analysis, the researchers found that MMS was associated with modestly better overall survival when compared with WME after adjustments (hazard ratio, 0.86; 95% confidence interval, 0.76-0.97). In the propensity score–matched analysis, a similar modest survival advantage was seen for patients who underwent MMS (hazard ratio, 0.82; 95% CI, 0.68-0.98).
“Significant differences in treatment practices based on the treatment facility were noted, with academic facilities more than twice as likely as nonacademic facilities to use MMS,” they wrote.
The researchers acknowledged a key limitation of the study was the use of a convenience sample, as opposed to a population-based sample. As a result, the generalizability of the findings may be limited to certain treatment facilities.
“These data suggest that MMS is an effective approach compared with WME for AJCC-8 stage I invasive melanoma,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Cheraghlou S et al. JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2890.
according to a retrospective cohort study.
In the study, which was published in JAMA Dermatology, patients who underwent MMS had a “modest survival advantage” when compared with those who were treated with WME, the approach recommended for treatment of invasive melanoma without nodal or extralymphatic metastases in national guidelines, reported the investigators.
“We sought herein to investigate the association of the type of surgical excision – WME or MMS – with overall survival for cases of American Joint Committee on Cancer Cancer Staging Manual 8th edition (AJCC-8) stage I invasive melanoma,” wrote Shayan Cheraghlou, of Yale University, New Haven, Conn., and colleagues.
The researchers identified a total of 70,319 patients diagnosed with stage I invasive melanoma between Jan. 1, 2004, and Dec. 31, 2014. Data were collected from the National Cancer Database, including 3,234 (4.6%) and 67,085 (95.4%) patients who underwent MMS and WME, respectively. The median age of patients in the cohort was 57 years; 47.7% were female, and almost 97% were white.
In the survival analysis, the team adjusted for clinical and tumor-specific variables and conducted a matched analysis using propensity scores. The primary outcome measured was overall survival.
After analysis, the researchers found that MMS was associated with modestly better overall survival when compared with WME after adjustments (hazard ratio, 0.86; 95% confidence interval, 0.76-0.97). In the propensity score–matched analysis, a similar modest survival advantage was seen for patients who underwent MMS (hazard ratio, 0.82; 95% CI, 0.68-0.98).
“Significant differences in treatment practices based on the treatment facility were noted, with academic facilities more than twice as likely as nonacademic facilities to use MMS,” they wrote.
The researchers acknowledged a key limitation of the study was the use of a convenience sample, as opposed to a population-based sample. As a result, the generalizability of the findings may be limited to certain treatment facilities.
“These data suggest that MMS is an effective approach compared with WME for AJCC-8 stage I invasive melanoma,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Cheraghlou S et al. JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2890.
FROM JAMA DERMATOLOGY
Severe hypoglycemia, poor glycemic control fuels fracture risk in older diabetic patients
Patients with type 2 diabetes and poor glycemic control or severe hypoglycemia may be at greater risk for fracture, according to recent research from a Japanese cohort of older men and postmenopausal women.
“The impacts of severe hypoglycemia and poor glycemic control on fractures appeared to be independent,” noted Yuji Komorita, MD, PhD, of the department of medicine and clinical science, Graduate School of Medical Sciences at Kyushu University, and colleagues. “This study suggests that the glycemic target to prevent fractures may be HbA1c <75 mmol/mol, which is far higher than that used to prevent microvascular complications, and higher than that for older adults with type 2 diabetes.”
Dr. Komorita and colleagues performed a prospective analysis of fracture incidence for 2,755 men and 1,951 postmenopausal women with type 2 diabetes in the Fukuoka Diabetes Registry who were mean 66 years old between April 2008 and October 2010. At the start of the study, the researchers assessed patient diabetes duration, previous fracture history, physical activity, alcohol and smoking status, whether patients were treated for diabetic retinopathy with laser photocoagulation, and their history of coronary artery disease or stroke. Patients were followed for a median 5.3 years, during which fractures were assessed through an annual self-administered questionnaire, with the results stratified by glycemic control and hypoglycemia.
Overall, there were 249 men and 413 women who experienced fractures during the study period, with a follow-up rate of 97.6%. In a multivariate analysis, patients with a higher risk of fracture included those with two or more episodes of severe hypoglycemia (hazard ratio, 2.25; 95% confidence interval, 1.57-3.22) and one episode of severe hypoglycemia (HR, 1.57; 95% CI, 1.11-2.20). In patients without severe hypoglycemic episodes, there was an increased risk of fracture in those with baseline hemoglobin A1c (HbA1c) level of 53 to less than 64 mmol/mol (7% to less than 8%; HR, 1.14; 0.94-1.39), 64 to less than 75 mmol/mol (8% to less than 9%; HR, 1.11; 95% CI, 0.86-1.43), and at least 75 mmol/mol (at least 9%; HR, 1.45; 95% CI, 1.06-1.98).
Compared with postmenopausal women, the unadjusted risk of fracture was higher in men with multiple severe hypoglycemic episodes (HR, 3.46; 95% CI, 2.05-5.85) and one episode of hypoglycemia (HR, 2.81; 95% CI, 1.74-4.56). These higher risks in older men persisted after adjustment for age, multivariate factors, and HbA1c.
“The association between severe hypoglycemia, poor glycemic control, and fracture risk at any anatomic site seems to be stronger in men than in postmenopausal women, although the interaction between men and postmenopausal women for fracture risk was not significant,” the researchers said. “The higher incidence rate of fractures in postmenopausal women, compared with men, was attributed to drastic changes in sex hormones after menopause, which may reduce the apparent impacts of hyperglycemia and severe hypoglycemia on postmenopausal women.”
Researchers said they did not consider potential external factors for fracture incidence, nor did they measure incident falls or other markers of bone health, such as bone mineral density and 25-hydroxyvitamin D levels. They also noted among the limitations of the study the self-reported nature of fracture reporting, and the lack of generalizability of the results.
This study was funded in part by grants from The Japan Society for the Promotion of Science KAKENHI from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Junior Scientist Development Grant supported by the Japan Diabetes Society; and the Lilly Research Grant Program for Bone & Mineral Research. The authors reported no relevant conflicts of interest.
SOURCE: Komorita Y et al. Diabet Med. 2019 Sep 25. doi: 10.1111/dme.14142.
Patients with type 2 diabetes and poor glycemic control or severe hypoglycemia may be at greater risk for fracture, according to recent research from a Japanese cohort of older men and postmenopausal women.
“The impacts of severe hypoglycemia and poor glycemic control on fractures appeared to be independent,” noted Yuji Komorita, MD, PhD, of the department of medicine and clinical science, Graduate School of Medical Sciences at Kyushu University, and colleagues. “This study suggests that the glycemic target to prevent fractures may be HbA1c <75 mmol/mol, which is far higher than that used to prevent microvascular complications, and higher than that for older adults with type 2 diabetes.”
Dr. Komorita and colleagues performed a prospective analysis of fracture incidence for 2,755 men and 1,951 postmenopausal women with type 2 diabetes in the Fukuoka Diabetes Registry who were mean 66 years old between April 2008 and October 2010. At the start of the study, the researchers assessed patient diabetes duration, previous fracture history, physical activity, alcohol and smoking status, whether patients were treated for diabetic retinopathy with laser photocoagulation, and their history of coronary artery disease or stroke. Patients were followed for a median 5.3 years, during which fractures were assessed through an annual self-administered questionnaire, with the results stratified by glycemic control and hypoglycemia.
Overall, there were 249 men and 413 women who experienced fractures during the study period, with a follow-up rate of 97.6%. In a multivariate analysis, patients with a higher risk of fracture included those with two or more episodes of severe hypoglycemia (hazard ratio, 2.25; 95% confidence interval, 1.57-3.22) and one episode of severe hypoglycemia (HR, 1.57; 95% CI, 1.11-2.20). In patients without severe hypoglycemic episodes, there was an increased risk of fracture in those with baseline hemoglobin A1c (HbA1c) level of 53 to less than 64 mmol/mol (7% to less than 8%; HR, 1.14; 0.94-1.39), 64 to less than 75 mmol/mol (8% to less than 9%; HR, 1.11; 95% CI, 0.86-1.43), and at least 75 mmol/mol (at least 9%; HR, 1.45; 95% CI, 1.06-1.98).
Compared with postmenopausal women, the unadjusted risk of fracture was higher in men with multiple severe hypoglycemic episodes (HR, 3.46; 95% CI, 2.05-5.85) and one episode of hypoglycemia (HR, 2.81; 95% CI, 1.74-4.56). These higher risks in older men persisted after adjustment for age, multivariate factors, and HbA1c.
“The association between severe hypoglycemia, poor glycemic control, and fracture risk at any anatomic site seems to be stronger in men than in postmenopausal women, although the interaction between men and postmenopausal women for fracture risk was not significant,” the researchers said. “The higher incidence rate of fractures in postmenopausal women, compared with men, was attributed to drastic changes in sex hormones after menopause, which may reduce the apparent impacts of hyperglycemia and severe hypoglycemia on postmenopausal women.”
Researchers said they did not consider potential external factors for fracture incidence, nor did they measure incident falls or other markers of bone health, such as bone mineral density and 25-hydroxyvitamin D levels. They also noted among the limitations of the study the self-reported nature of fracture reporting, and the lack of generalizability of the results.
This study was funded in part by grants from The Japan Society for the Promotion of Science KAKENHI from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Junior Scientist Development Grant supported by the Japan Diabetes Society; and the Lilly Research Grant Program for Bone & Mineral Research. The authors reported no relevant conflicts of interest.
SOURCE: Komorita Y et al. Diabet Med. 2019 Sep 25. doi: 10.1111/dme.14142.
Patients with type 2 diabetes and poor glycemic control or severe hypoglycemia may be at greater risk for fracture, according to recent research from a Japanese cohort of older men and postmenopausal women.
“The impacts of severe hypoglycemia and poor glycemic control on fractures appeared to be independent,” noted Yuji Komorita, MD, PhD, of the department of medicine and clinical science, Graduate School of Medical Sciences at Kyushu University, and colleagues. “This study suggests that the glycemic target to prevent fractures may be HbA1c <75 mmol/mol, which is far higher than that used to prevent microvascular complications, and higher than that for older adults with type 2 diabetes.”
Dr. Komorita and colleagues performed a prospective analysis of fracture incidence for 2,755 men and 1,951 postmenopausal women with type 2 diabetes in the Fukuoka Diabetes Registry who were mean 66 years old between April 2008 and October 2010. At the start of the study, the researchers assessed patient diabetes duration, previous fracture history, physical activity, alcohol and smoking status, whether patients were treated for diabetic retinopathy with laser photocoagulation, and their history of coronary artery disease or stroke. Patients were followed for a median 5.3 years, during which fractures were assessed through an annual self-administered questionnaire, with the results stratified by glycemic control and hypoglycemia.
Overall, there were 249 men and 413 women who experienced fractures during the study period, with a follow-up rate of 97.6%. In a multivariate analysis, patients with a higher risk of fracture included those with two or more episodes of severe hypoglycemia (hazard ratio, 2.25; 95% confidence interval, 1.57-3.22) and one episode of severe hypoglycemia (HR, 1.57; 95% CI, 1.11-2.20). In patients without severe hypoglycemic episodes, there was an increased risk of fracture in those with baseline hemoglobin A1c (HbA1c) level of 53 to less than 64 mmol/mol (7% to less than 8%; HR, 1.14; 0.94-1.39), 64 to less than 75 mmol/mol (8% to less than 9%; HR, 1.11; 95% CI, 0.86-1.43), and at least 75 mmol/mol (at least 9%; HR, 1.45; 95% CI, 1.06-1.98).
Compared with postmenopausal women, the unadjusted risk of fracture was higher in men with multiple severe hypoglycemic episodes (HR, 3.46; 95% CI, 2.05-5.85) and one episode of hypoglycemia (HR, 2.81; 95% CI, 1.74-4.56). These higher risks in older men persisted after adjustment for age, multivariate factors, and HbA1c.
“The association between severe hypoglycemia, poor glycemic control, and fracture risk at any anatomic site seems to be stronger in men than in postmenopausal women, although the interaction between men and postmenopausal women for fracture risk was not significant,” the researchers said. “The higher incidence rate of fractures in postmenopausal women, compared with men, was attributed to drastic changes in sex hormones after menopause, which may reduce the apparent impacts of hyperglycemia and severe hypoglycemia on postmenopausal women.”
Researchers said they did not consider potential external factors for fracture incidence, nor did they measure incident falls or other markers of bone health, such as bone mineral density and 25-hydroxyvitamin D levels. They also noted among the limitations of the study the self-reported nature of fracture reporting, and the lack of generalizability of the results.
This study was funded in part by grants from The Japan Society for the Promotion of Science KAKENHI from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Junior Scientist Development Grant supported by the Japan Diabetes Society; and the Lilly Research Grant Program for Bone & Mineral Research. The authors reported no relevant conflicts of interest.
SOURCE: Komorita Y et al. Diabet Med. 2019 Sep 25. doi: 10.1111/dme.14142.
FROM DIABETIC MEDICINE
THC use reported in majority of vaping-related illnesses
(EVALI), according to the Centers for Disease Control and Prevention.
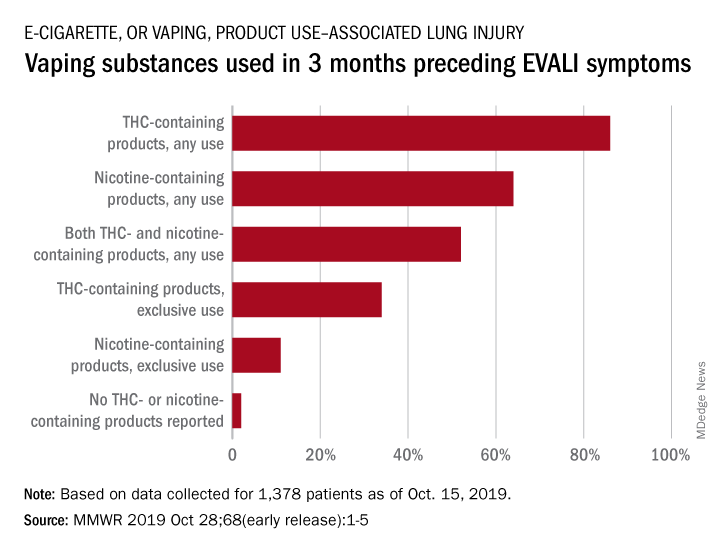
In the largest analysis to date, exclusive use of THC-containing products was reported for 34% of the 1,378 patients with confirmed or probable EVALI as of Oct. 15, 2019. Among those who died, 63% had been using THC exclusively during the 3 months preceding symptom onset, Erin D. Moritz, PhD, and associates said Oct. 28 in the Morbidity and Mortality Weekly Report.
Almost two-thirds (64%) of all EVALI patients had used nicotine-containing products at some time in the 3 months before symptom onset, and nicotine use was exclusive for 11%. Any nicotine use was reported for 37% of EVALI-related deaths, with exclusive use at 16%, the investigators reported.
“The data presented here suggest that THC-containing products are playing an important role in this outbreak,” they wrote, but “to date, no single compound or ingredient has emerged as the cause of EVALI, and there might be more than one cause.”
Dr. Moritz and associates also noted that many “patients likely did not know the content of the e-cigarette, or vaping, products they used,” which may have led to misclassification of substances.
SOURCE: Moritz ED et al. MMWR. Morbidity and mortality weekly report 2019 Oct 28;68(early release):1-4.
(EVALI), according to the Centers for Disease Control and Prevention.

In the largest analysis to date, exclusive use of THC-containing products was reported for 34% of the 1,378 patients with confirmed or probable EVALI as of Oct. 15, 2019. Among those who died, 63% had been using THC exclusively during the 3 months preceding symptom onset, Erin D. Moritz, PhD, and associates said Oct. 28 in the Morbidity and Mortality Weekly Report.
Almost two-thirds (64%) of all EVALI patients had used nicotine-containing products at some time in the 3 months before symptom onset, and nicotine use was exclusive for 11%. Any nicotine use was reported for 37% of EVALI-related deaths, with exclusive use at 16%, the investigators reported.
“The data presented here suggest that THC-containing products are playing an important role in this outbreak,” they wrote, but “to date, no single compound or ingredient has emerged as the cause of EVALI, and there might be more than one cause.”
Dr. Moritz and associates also noted that many “patients likely did not know the content of the e-cigarette, or vaping, products they used,” which may have led to misclassification of substances.
SOURCE: Moritz ED et al. MMWR. Morbidity and mortality weekly report 2019 Oct 28;68(early release):1-4.
(EVALI), according to the Centers for Disease Control and Prevention.

In the largest analysis to date, exclusive use of THC-containing products was reported for 34% of the 1,378 patients with confirmed or probable EVALI as of Oct. 15, 2019. Among those who died, 63% had been using THC exclusively during the 3 months preceding symptom onset, Erin D. Moritz, PhD, and associates said Oct. 28 in the Morbidity and Mortality Weekly Report.
Almost two-thirds (64%) of all EVALI patients had used nicotine-containing products at some time in the 3 months before symptom onset, and nicotine use was exclusive for 11%. Any nicotine use was reported for 37% of EVALI-related deaths, with exclusive use at 16%, the investigators reported.
“The data presented here suggest that THC-containing products are playing an important role in this outbreak,” they wrote, but “to date, no single compound or ingredient has emerged as the cause of EVALI, and there might be more than one cause.”
Dr. Moritz and associates also noted that many “patients likely did not know the content of the e-cigarette, or vaping, products they used,” which may have led to misclassification of substances.
SOURCE: Moritz ED et al. MMWR. Morbidity and mortality weekly report 2019 Oct 28;68(early release):1-4.
FROM MMWR
Sleep problems in pregnancy presage postnatal depression
COPENHAGEN – Tiina Paunio, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“I think it is very important to understand that we need to screen pregnant women for sleep problems, even those without a history of depression, so we can have early treatment of insomnia – and also depression – because postnatal maternal depression is very much a risk for the child during a vulnerable period for development,” said Dr. Paunio, professor of psychiatry at the University of Helsinki.
She was a coinvestigator in a prospective study of the Finnish CHILD-SLEEP longitudinal birth cohort in which 1,398 women completed the Basic Nordic Sleep Questionnaire and the 10-item version of the Center for Epidemiological Studies Depression Scale (CES-D) at about gestational week 32 and again around 3 months following delivery. Postnatal depressiveness as defined by a CES-D score of at least 10 points was present in 10.3% of the mothers. After adjusting for prenatal depressiveness and other potential confounders, the investigators found that tiredness during the day, poor general sleep quality, getting less than 6 hours of sleep, taking longer than 20 minutes to fall asleep, and sleep loss of 2 hours or more per night during pregnancy were each associated with clinically significant postnatal depressive symptoms, with odds ratios of 1.87-2.19.
The full details of the study have been published (Arch Womens Ment Health. 2019 Jun;22[3]:327-37).
The impetus for this study of sleep problems in pregnancy as a predictor of postnatal depressive symptoms was a body of evidence linking insomnia to depression in both men and women. But it turns out that insomnia is a significant predictor of later onset of a wide variety of psychiatric disorders, not only depression, as highlighted in a recent systematic review and meta-analysis conducted by an international team of investigators, Dr. Paunio observed.
Baseline insomnia symptoms were associated with a 183% increased risk of later onset of depression, a 223% increased risk of anxiety, a 35%greater risk of alcohol abuse, and a 28% increased risk of psychosis. However, the insomnia/psychosis link must be viewed as tentative, as it was examined in only a single published study. The investigators rated the overall risk of bias in the studies included in their meta-analysis as moderate (Sleep Med Rev. 2019 Feb;43:96-105).
For Dr. Paunio, these findings suggest that interventional studies of early and effective treatment of insomnia as a potential means of preventing psychiatric disorders are in order.
She reported receiving research funding from the Academy of Finland, the Gyllenberg Foundation, and Finska Lakaresallskapet.
COPENHAGEN – Tiina Paunio, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“I think it is very important to understand that we need to screen pregnant women for sleep problems, even those without a history of depression, so we can have early treatment of insomnia – and also depression – because postnatal maternal depression is very much a risk for the child during a vulnerable period for development,” said Dr. Paunio, professor of psychiatry at the University of Helsinki.
She was a coinvestigator in a prospective study of the Finnish CHILD-SLEEP longitudinal birth cohort in which 1,398 women completed the Basic Nordic Sleep Questionnaire and the 10-item version of the Center for Epidemiological Studies Depression Scale (CES-D) at about gestational week 32 and again around 3 months following delivery. Postnatal depressiveness as defined by a CES-D score of at least 10 points was present in 10.3% of the mothers. After adjusting for prenatal depressiveness and other potential confounders, the investigators found that tiredness during the day, poor general sleep quality, getting less than 6 hours of sleep, taking longer than 20 minutes to fall asleep, and sleep loss of 2 hours or more per night during pregnancy were each associated with clinically significant postnatal depressive symptoms, with odds ratios of 1.87-2.19.
The full details of the study have been published (Arch Womens Ment Health. 2019 Jun;22[3]:327-37).
The impetus for this study of sleep problems in pregnancy as a predictor of postnatal depressive symptoms was a body of evidence linking insomnia to depression in both men and women. But it turns out that insomnia is a significant predictor of later onset of a wide variety of psychiatric disorders, not only depression, as highlighted in a recent systematic review and meta-analysis conducted by an international team of investigators, Dr. Paunio observed.
Baseline insomnia symptoms were associated with a 183% increased risk of later onset of depression, a 223% increased risk of anxiety, a 35%greater risk of alcohol abuse, and a 28% increased risk of psychosis. However, the insomnia/psychosis link must be viewed as tentative, as it was examined in only a single published study. The investigators rated the overall risk of bias in the studies included in their meta-analysis as moderate (Sleep Med Rev. 2019 Feb;43:96-105).
For Dr. Paunio, these findings suggest that interventional studies of early and effective treatment of insomnia as a potential means of preventing psychiatric disorders are in order.
She reported receiving research funding from the Academy of Finland, the Gyllenberg Foundation, and Finska Lakaresallskapet.
COPENHAGEN – Tiina Paunio, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“I think it is very important to understand that we need to screen pregnant women for sleep problems, even those without a history of depression, so we can have early treatment of insomnia – and also depression – because postnatal maternal depression is very much a risk for the child during a vulnerable period for development,” said Dr. Paunio, professor of psychiatry at the University of Helsinki.
She was a coinvestigator in a prospective study of the Finnish CHILD-SLEEP longitudinal birth cohort in which 1,398 women completed the Basic Nordic Sleep Questionnaire and the 10-item version of the Center for Epidemiological Studies Depression Scale (CES-D) at about gestational week 32 and again around 3 months following delivery. Postnatal depressiveness as defined by a CES-D score of at least 10 points was present in 10.3% of the mothers. After adjusting for prenatal depressiveness and other potential confounders, the investigators found that tiredness during the day, poor general sleep quality, getting less than 6 hours of sleep, taking longer than 20 minutes to fall asleep, and sleep loss of 2 hours or more per night during pregnancy were each associated with clinically significant postnatal depressive symptoms, with odds ratios of 1.87-2.19.
The full details of the study have been published (Arch Womens Ment Health. 2019 Jun;22[3]:327-37).
The impetus for this study of sleep problems in pregnancy as a predictor of postnatal depressive symptoms was a body of evidence linking insomnia to depression in both men and women. But it turns out that insomnia is a significant predictor of later onset of a wide variety of psychiatric disorders, not only depression, as highlighted in a recent systematic review and meta-analysis conducted by an international team of investigators, Dr. Paunio observed.
Baseline insomnia symptoms were associated with a 183% increased risk of later onset of depression, a 223% increased risk of anxiety, a 35%greater risk of alcohol abuse, and a 28% increased risk of psychosis. However, the insomnia/psychosis link must be viewed as tentative, as it was examined in only a single published study. The investigators rated the overall risk of bias in the studies included in their meta-analysis as moderate (Sleep Med Rev. 2019 Feb;43:96-105).
For Dr. Paunio, these findings suggest that interventional studies of early and effective treatment of insomnia as a potential means of preventing psychiatric disorders are in order.
She reported receiving research funding from the Academy of Finland, the Gyllenberg Foundation, and Finska Lakaresallskapet.
REPORTING FROM ECNP 2019
Baricitinib may benefit patients with Aicardi-Goutières syndrome
CHARLOTTE, N.C. – Scores on a novel AGS scale improved, and skin and liver complications resolved in children with AGS who received treatment with baricitinib, according to results presented at the annual meeting of the Child Neurology Society.
AGS is caused by various heritable disorders of the innate immunity that result in excessive interferon production. AGS characteristically manifests as an early-onset encephalopathy that causes intellectual and physical disability, but patients may have a wide range of clinical phenotypes. The disease may involve the skin, liver, lungs, heart, and other organs, as well as the brain.
A multisystem disorder
“The neurologic features, while they are the most compelling for us, are really only the tip of the iceberg,” said Adeline Vanderver, MD, program director of the leukodystrophy center, and the Jacob A. Kamens Endowed Chair in Neurologic Disorders and Translational Neurotherapeutics at Children’s Hospital of Philadelphia. “Nearly every single organ system in the body is affected, from either direct interferon injury or from a secondary vasculopathy related to the interferonopathy.”
Dr. Vanderver presented results from the compassionate use study, which assessed whether the JAK inhibitor baricitinib (Olumiant) may decrease interferon signaling in AGS and limit the morbidity of the disease.
The phase 1, open-label trial “included compassionate use of baricitinib in AGS under the argument that these children did not have time to wait for approval of the drug,” said Dr. Vanderver. In 2018, the Food and Drug Administration approved baricitinib for moderate to severe rheumatoid arthritis in adults with an inadequate response to methotrexate.
The phase 1 trial in AGS included 35 patients with mutation-defined AGS and evidence of inflammatory disease that could be targeted by JAK inhibition. The trial population was 36% female. The average age of disease onset was 0.8 years, and patients’ average age at treatment was 6.1 years. The investigators assessed safety and laboratory data every 3 months and conducted clinical assessments every 6 months.
The heterogeneity of AGS phenotypes within families and across genotypes makes treatment trials in this disorder a challenge, Dr. Vanderver said. Outcome measures may have ceiling or floor effects that fail to capture the range of severity of AGS symptoms. Dr. Vanderver and colleagues developed a novel AGS scale to capture the scope of neurologic function in patients with AGS
.
When the researchers applied the AGS scale to a historical cohort of patients, most had stable scores about 6 months after disease onset. “After the first 6 months of the disease, the disease tends to be much more static, as the children have sustained significant neurologic injury,” Dr. Vanderver said.
They applied the novel AGS scale post hoc as an exploratory endpoint in the phase 1 trial. In addition, parents recorded information in a diary about skin involvement, irritability, seizures, and fever. “Over time, we see a reduction, although not always a statistically significant reduction, in symptom burden,” Dr. Vanderver said. The AGS clinical diary scores reflect “what the parents were telling us – that they felt like their children were feeling better during treatment,” she said.
Several patients had skin conditions that improved with treatment. One patient with dermatitis or eczema had the skin abnormality resolve within 3 days. A patient with full-body panniculitis began healing for the first time after about a month of treatment. Seasonal variations and dose adjustments led to fluctuations in some of the skin conditions. Nevertheless, the results suggested significant improvement in skin manifestations in patients with AGS, Dr. Vanderver said.
Patients generally had stable AGS scale scores in the year before treatment, although a couple of patients who were closer to disease onset had precipitous decline in neurologic function, she said. “We had a statistically significant increase in that scale of neurologic function in our patients during the period of the study, even in patients who had sometimes had years of disease duration,” said Dr. Vanderver.
Dr. Vanderver cautioned that she does not want to overstate the changes in function. Patients with AGS may have less potential for recuperation, compared with patients with other conditions. “A child with significant disruptive CNS disease may not recuperate normal functioning,” Dr. Vanderver said, “but it can be clinically meaningful to families if children start having better head control, smile, communicate, even if they might not regain all their motor milestones.”
In addition, a small subset of patients who had potentially life threatening liver complications from the disease experienced rapid normalization and improvement of liver function. “This blockade can be important not just for neurologic function but also to maintain normal physiologic homeostasis of other organs that are affected by the interferonopathy,” Dr. Vanderver said.
Interferon signaling scores decreased in the days after starting treatment and subsequently leveled out.
Serious adverse events that occurred during the trial, such as hospitalizations, were attributable to AGS. One child died from unrecognized pulmonary hypertension, which is now known to be a complication of AGS but was not at the time.
Harnessing a side effect
The most significant and recurrent laboratory abnormality was thrombocytosis. “That is a known complication of this family of drugs that in many cases allowed us to improve previous treatment-resistant thrombocytopenia, so we kind of like that side effect in most cases, but in two cases it did ... result in dose adjustments, although we never had to stop the medication for that.”
The study offers proof of principle that AGS is treatable, Dr. Vanderver said. A phase 2 trial is enrolling patients closer to disease onset. Early treatment of AGS may remain a challenge until there is newborn screening for the disease, she said.
Dr. Vanderver receives grant and in-kind support for translational research without personal compensation from Eli Lilly, Takeda, Illumina, Biogen, Homology, and Ionis. In addition, Dr. Vanderver serves on the scientific advisory boards of the European Leukodystrophy Association and the United Leukodystrophy Foundation, as well as in an unpaid capacity for Takeda, Ionis, Biogen, and Illumina.
Eli Lilly provided support for the phase 1 study. In addition, the study received support from the AGS Association Americas Family Foundation, National Human Genome Research Institute, National Institute of Neurological Disorders and Stroke, and the Children’s Hospital of Philadelphia Research Institute.
SOURCE: Vanderver A et al. CNS 2019. Abstract PL1-6.
CHARLOTTE, N.C. – Scores on a novel AGS scale improved, and skin and liver complications resolved in children with AGS who received treatment with baricitinib, according to results presented at the annual meeting of the Child Neurology Society.
AGS is caused by various heritable disorders of the innate immunity that result in excessive interferon production. AGS characteristically manifests as an early-onset encephalopathy that causes intellectual and physical disability, but patients may have a wide range of clinical phenotypes. The disease may involve the skin, liver, lungs, heart, and other organs, as well as the brain.
A multisystem disorder
“The neurologic features, while they are the most compelling for us, are really only the tip of the iceberg,” said Adeline Vanderver, MD, program director of the leukodystrophy center, and the Jacob A. Kamens Endowed Chair in Neurologic Disorders and Translational Neurotherapeutics at Children’s Hospital of Philadelphia. “Nearly every single organ system in the body is affected, from either direct interferon injury or from a secondary vasculopathy related to the interferonopathy.”
Dr. Vanderver presented results from the compassionate use study, which assessed whether the JAK inhibitor baricitinib (Olumiant) may decrease interferon signaling in AGS and limit the morbidity of the disease.
The phase 1, open-label trial “included compassionate use of baricitinib in AGS under the argument that these children did not have time to wait for approval of the drug,” said Dr. Vanderver. In 2018, the Food and Drug Administration approved baricitinib for moderate to severe rheumatoid arthritis in adults with an inadequate response to methotrexate.
The phase 1 trial in AGS included 35 patients with mutation-defined AGS and evidence of inflammatory disease that could be targeted by JAK inhibition. The trial population was 36% female. The average age of disease onset was 0.8 years, and patients’ average age at treatment was 6.1 years. The investigators assessed safety and laboratory data every 3 months and conducted clinical assessments every 6 months.
The heterogeneity of AGS phenotypes within families and across genotypes makes treatment trials in this disorder a challenge, Dr. Vanderver said. Outcome measures may have ceiling or floor effects that fail to capture the range of severity of AGS symptoms. Dr. Vanderver and colleagues developed a novel AGS scale to capture the scope of neurologic function in patients with AGS
.
When the researchers applied the AGS scale to a historical cohort of patients, most had stable scores about 6 months after disease onset. “After the first 6 months of the disease, the disease tends to be much more static, as the children have sustained significant neurologic injury,” Dr. Vanderver said.
They applied the novel AGS scale post hoc as an exploratory endpoint in the phase 1 trial. In addition, parents recorded information in a diary about skin involvement, irritability, seizures, and fever. “Over time, we see a reduction, although not always a statistically significant reduction, in symptom burden,” Dr. Vanderver said. The AGS clinical diary scores reflect “what the parents were telling us – that they felt like their children were feeling better during treatment,” she said.
Several patients had skin conditions that improved with treatment. One patient with dermatitis or eczema had the skin abnormality resolve within 3 days. A patient with full-body panniculitis began healing for the first time after about a month of treatment. Seasonal variations and dose adjustments led to fluctuations in some of the skin conditions. Nevertheless, the results suggested significant improvement in skin manifestations in patients with AGS, Dr. Vanderver said.
Patients generally had stable AGS scale scores in the year before treatment, although a couple of patients who were closer to disease onset had precipitous decline in neurologic function, she said. “We had a statistically significant increase in that scale of neurologic function in our patients during the period of the study, even in patients who had sometimes had years of disease duration,” said Dr. Vanderver.
Dr. Vanderver cautioned that she does not want to overstate the changes in function. Patients with AGS may have less potential for recuperation, compared with patients with other conditions. “A child with significant disruptive CNS disease may not recuperate normal functioning,” Dr. Vanderver said, “but it can be clinically meaningful to families if children start having better head control, smile, communicate, even if they might not regain all their motor milestones.”
In addition, a small subset of patients who had potentially life threatening liver complications from the disease experienced rapid normalization and improvement of liver function. “This blockade can be important not just for neurologic function but also to maintain normal physiologic homeostasis of other organs that are affected by the interferonopathy,” Dr. Vanderver said.
Interferon signaling scores decreased in the days after starting treatment and subsequently leveled out.
Serious adverse events that occurred during the trial, such as hospitalizations, were attributable to AGS. One child died from unrecognized pulmonary hypertension, which is now known to be a complication of AGS but was not at the time.
Harnessing a side effect
The most significant and recurrent laboratory abnormality was thrombocytosis. “That is a known complication of this family of drugs that in many cases allowed us to improve previous treatment-resistant thrombocytopenia, so we kind of like that side effect in most cases, but in two cases it did ... result in dose adjustments, although we never had to stop the medication for that.”
The study offers proof of principle that AGS is treatable, Dr. Vanderver said. A phase 2 trial is enrolling patients closer to disease onset. Early treatment of AGS may remain a challenge until there is newborn screening for the disease, she said.
Dr. Vanderver receives grant and in-kind support for translational research without personal compensation from Eli Lilly, Takeda, Illumina, Biogen, Homology, and Ionis. In addition, Dr. Vanderver serves on the scientific advisory boards of the European Leukodystrophy Association and the United Leukodystrophy Foundation, as well as in an unpaid capacity for Takeda, Ionis, Biogen, and Illumina.
Eli Lilly provided support for the phase 1 study. In addition, the study received support from the AGS Association Americas Family Foundation, National Human Genome Research Institute, National Institute of Neurological Disorders and Stroke, and the Children’s Hospital of Philadelphia Research Institute.
SOURCE: Vanderver A et al. CNS 2019. Abstract PL1-6.
CHARLOTTE, N.C. – Scores on a novel AGS scale improved, and skin and liver complications resolved in children with AGS who received treatment with baricitinib, according to results presented at the annual meeting of the Child Neurology Society.
AGS is caused by various heritable disorders of the innate immunity that result in excessive interferon production. AGS characteristically manifests as an early-onset encephalopathy that causes intellectual and physical disability, but patients may have a wide range of clinical phenotypes. The disease may involve the skin, liver, lungs, heart, and other organs, as well as the brain.
A multisystem disorder
“The neurologic features, while they are the most compelling for us, are really only the tip of the iceberg,” said Adeline Vanderver, MD, program director of the leukodystrophy center, and the Jacob A. Kamens Endowed Chair in Neurologic Disorders and Translational Neurotherapeutics at Children’s Hospital of Philadelphia. “Nearly every single organ system in the body is affected, from either direct interferon injury or from a secondary vasculopathy related to the interferonopathy.”
Dr. Vanderver presented results from the compassionate use study, which assessed whether the JAK inhibitor baricitinib (Olumiant) may decrease interferon signaling in AGS and limit the morbidity of the disease.
The phase 1, open-label trial “included compassionate use of baricitinib in AGS under the argument that these children did not have time to wait for approval of the drug,” said Dr. Vanderver. In 2018, the Food and Drug Administration approved baricitinib for moderate to severe rheumatoid arthritis in adults with an inadequate response to methotrexate.
The phase 1 trial in AGS included 35 patients with mutation-defined AGS and evidence of inflammatory disease that could be targeted by JAK inhibition. The trial population was 36% female. The average age of disease onset was 0.8 years, and patients’ average age at treatment was 6.1 years. The investigators assessed safety and laboratory data every 3 months and conducted clinical assessments every 6 months.
The heterogeneity of AGS phenotypes within families and across genotypes makes treatment trials in this disorder a challenge, Dr. Vanderver said. Outcome measures may have ceiling or floor effects that fail to capture the range of severity of AGS symptoms. Dr. Vanderver and colleagues developed a novel AGS scale to capture the scope of neurologic function in patients with AGS
.
When the researchers applied the AGS scale to a historical cohort of patients, most had stable scores about 6 months after disease onset. “After the first 6 months of the disease, the disease tends to be much more static, as the children have sustained significant neurologic injury,” Dr. Vanderver said.
They applied the novel AGS scale post hoc as an exploratory endpoint in the phase 1 trial. In addition, parents recorded information in a diary about skin involvement, irritability, seizures, and fever. “Over time, we see a reduction, although not always a statistically significant reduction, in symptom burden,” Dr. Vanderver said. The AGS clinical diary scores reflect “what the parents were telling us – that they felt like their children were feeling better during treatment,” she said.
Several patients had skin conditions that improved with treatment. One patient with dermatitis or eczema had the skin abnormality resolve within 3 days. A patient with full-body panniculitis began healing for the first time after about a month of treatment. Seasonal variations and dose adjustments led to fluctuations in some of the skin conditions. Nevertheless, the results suggested significant improvement in skin manifestations in patients with AGS, Dr. Vanderver said.
Patients generally had stable AGS scale scores in the year before treatment, although a couple of patients who were closer to disease onset had precipitous decline in neurologic function, she said. “We had a statistically significant increase in that scale of neurologic function in our patients during the period of the study, even in patients who had sometimes had years of disease duration,” said Dr. Vanderver.
Dr. Vanderver cautioned that she does not want to overstate the changes in function. Patients with AGS may have less potential for recuperation, compared with patients with other conditions. “A child with significant disruptive CNS disease may not recuperate normal functioning,” Dr. Vanderver said, “but it can be clinically meaningful to families if children start having better head control, smile, communicate, even if they might not regain all their motor milestones.”
In addition, a small subset of patients who had potentially life threatening liver complications from the disease experienced rapid normalization and improvement of liver function. “This blockade can be important not just for neurologic function but also to maintain normal physiologic homeostasis of other organs that are affected by the interferonopathy,” Dr. Vanderver said.
Interferon signaling scores decreased in the days after starting treatment and subsequently leveled out.
Serious adverse events that occurred during the trial, such as hospitalizations, were attributable to AGS. One child died from unrecognized pulmonary hypertension, which is now known to be a complication of AGS but was not at the time.
Harnessing a side effect
The most significant and recurrent laboratory abnormality was thrombocytosis. “That is a known complication of this family of drugs that in many cases allowed us to improve previous treatment-resistant thrombocytopenia, so we kind of like that side effect in most cases, but in two cases it did ... result in dose adjustments, although we never had to stop the medication for that.”
The study offers proof of principle that AGS is treatable, Dr. Vanderver said. A phase 2 trial is enrolling patients closer to disease onset. Early treatment of AGS may remain a challenge until there is newborn screening for the disease, she said.
Dr. Vanderver receives grant and in-kind support for translational research without personal compensation from Eli Lilly, Takeda, Illumina, Biogen, Homology, and Ionis. In addition, Dr. Vanderver serves on the scientific advisory boards of the European Leukodystrophy Association and the United Leukodystrophy Foundation, as well as in an unpaid capacity for Takeda, Ionis, Biogen, and Illumina.
Eli Lilly provided support for the phase 1 study. In addition, the study received support from the AGS Association Americas Family Foundation, National Human Genome Research Institute, National Institute of Neurological Disorders and Stroke, and the Children’s Hospital of Philadelphia Research Institute.
SOURCE: Vanderver A et al. CNS 2019. Abstract PL1-6.
REPORTING FROM CNS 2019
The SHM Fellow designation: Class of 2020
Society invites applicants in multiple membership categories
In an industry brimming with opportunity and ongoing transformation, it is easy to feel indecisive about your next professional step when ample career paths exist in hospital medicine.
Yingkei Hui, MD, FHM, is an academic hospitalist at St. Vincent Indianapolis, and a Society of Hospital Medicine member since 2015. Seeking to set herself apart as an aspiring patient safety and quality improvement leader while continuing her professional development, she looked to SHM’s Fellow designation as the next piece of her career puzzle.
With more than 14 years of experience in the health care industry, Dr. Hui fell in love with the specialty because of its flexibility and patient-centric focus.
“I have a broad interest in medicine and want to learn everything under the larger umbrella of medicine,” she said. “I also find myself deeply in love with hospital medicine because it provides me with the opportunity to participate in various hospital committees and allows me to enjoy my practice from a macroscopic view of U.S. health care transformation – especially given the popular value-based patient care approach from recent years.”
Dr. Hui’s breadth of experience has allowed her to gain a unique set of perspectives and experiences from international and domestic standpoints. From attending medical school at the Chinese University of Hong Kong to completing her residency on the east coast at Pennsylvania Hospital in Philadelphia – part of the University of Pennsylvania Health System – Dr. Hui has held active medical licenses in New Jersey and currently, Indiana.
“SHM’s Fellow designation allows me to challenge myself in setting my career goal as a patient safety and quality improvement leader in my program,” she said. “It means a lot to me as it is a stand-out recognition of my participation in and contribution to patient care in my institution.”
When asked about the most rewarding aspect of being a part of the hospital medicine community, Dr. Hui identified “satisfaction in the teaching role.” She said she is “motivated by the holistic care for the patients, the integration of medical knowledge and coordination of care, and also the opportunity to conduct quality improvement projects.”
Motivated by her colleagues, Dr. Hui credits SHM with providing her with the inspiration and tools to push herself and advance her career in hospital medicine.
“I enjoy immersing myself in SHM’s patient safety and quality improvement resources; they are perfect for frontline hospitalists and also provide CME [continuing medical education],” she noted. “My previous medical directors were all Senior Fellows; they are my role models and continue to inspire me throughout my career.”
Dr. Hui also said that networking within the SHM community has been encouraging. “I’ve met talented Fellows at a number of hospital medicine annual conferences who have inspired me in the areas of patient care, education, and health promotion,” she explained. “Some of them have extensive publications; they are truly amazing physicians. SHM’s Annual Conference provides great opportunities for networking.”
As Dr. Hui continues to progress her career in hospital medicine, she believes that communication is a key pillar in her success. “Be a true listener and fill your heart with compassion, empathy, and courage,” she said. “Recognize your role as the enabler for the patients to improve their health.”
Completing her Master’s degree in population health management at Johns Hopkins University and expecting to graduate in May 2020, Dr. Hui is the designer of system safety (comprising patient safety, second victim safety, quality improvement, and just culture) in the academic setting of her residency program. She is also chairing a pioneer project for the St. Vincent IM residency program.
Dr. Hui plans to apply for a Senior Fellow designation with SHM in the future.
If you would like to join Dr. Hui and other like-minded hospital medicine leaders in taking your career to the next level, SHM is currently recruiting for the Fellows and Senior Fellows: Class of 2020. Applications are open until Nov. 29 of this year. These designations are available across a variety of membership categories, including physicians, nurse practitioners, physician assistants, and qualified practice administrators. Dedicated to promoting excellence, innovation, and improving the quality of patient care, Fellows designations provide members with a distinguishing credential as established pioneers in the industry.
For more information and to review your eligibility, visit hospitalmedicine.org/fellows.
Ms. Cowan is a marketing communications specialist at the Society of Hospital Medicine.
Society invites applicants in multiple membership categories
Society invites applicants in multiple membership categories
In an industry brimming with opportunity and ongoing transformation, it is easy to feel indecisive about your next professional step when ample career paths exist in hospital medicine.
Yingkei Hui, MD, FHM, is an academic hospitalist at St. Vincent Indianapolis, and a Society of Hospital Medicine member since 2015. Seeking to set herself apart as an aspiring patient safety and quality improvement leader while continuing her professional development, she looked to SHM’s Fellow designation as the next piece of her career puzzle.
With more than 14 years of experience in the health care industry, Dr. Hui fell in love with the specialty because of its flexibility and patient-centric focus.
“I have a broad interest in medicine and want to learn everything under the larger umbrella of medicine,” she said. “I also find myself deeply in love with hospital medicine because it provides me with the opportunity to participate in various hospital committees and allows me to enjoy my practice from a macroscopic view of U.S. health care transformation – especially given the popular value-based patient care approach from recent years.”
Dr. Hui’s breadth of experience has allowed her to gain a unique set of perspectives and experiences from international and domestic standpoints. From attending medical school at the Chinese University of Hong Kong to completing her residency on the east coast at Pennsylvania Hospital in Philadelphia – part of the University of Pennsylvania Health System – Dr. Hui has held active medical licenses in New Jersey and currently, Indiana.
“SHM’s Fellow designation allows me to challenge myself in setting my career goal as a patient safety and quality improvement leader in my program,” she said. “It means a lot to me as it is a stand-out recognition of my participation in and contribution to patient care in my institution.”
When asked about the most rewarding aspect of being a part of the hospital medicine community, Dr. Hui identified “satisfaction in the teaching role.” She said she is “motivated by the holistic care for the patients, the integration of medical knowledge and coordination of care, and also the opportunity to conduct quality improvement projects.”
Motivated by her colleagues, Dr. Hui credits SHM with providing her with the inspiration and tools to push herself and advance her career in hospital medicine.
“I enjoy immersing myself in SHM’s patient safety and quality improvement resources; they are perfect for frontline hospitalists and also provide CME [continuing medical education],” she noted. “My previous medical directors were all Senior Fellows; they are my role models and continue to inspire me throughout my career.”
Dr. Hui also said that networking within the SHM community has been encouraging. “I’ve met talented Fellows at a number of hospital medicine annual conferences who have inspired me in the areas of patient care, education, and health promotion,” she explained. “Some of them have extensive publications; they are truly amazing physicians. SHM’s Annual Conference provides great opportunities for networking.”
As Dr. Hui continues to progress her career in hospital medicine, she believes that communication is a key pillar in her success. “Be a true listener and fill your heart with compassion, empathy, and courage,” she said. “Recognize your role as the enabler for the patients to improve their health.”
Completing her Master’s degree in population health management at Johns Hopkins University and expecting to graduate in May 2020, Dr. Hui is the designer of system safety (comprising patient safety, second victim safety, quality improvement, and just culture) in the academic setting of her residency program. She is also chairing a pioneer project for the St. Vincent IM residency program.
Dr. Hui plans to apply for a Senior Fellow designation with SHM in the future.
If you would like to join Dr. Hui and other like-minded hospital medicine leaders in taking your career to the next level, SHM is currently recruiting for the Fellows and Senior Fellows: Class of 2020. Applications are open until Nov. 29 of this year. These designations are available across a variety of membership categories, including physicians, nurse practitioners, physician assistants, and qualified practice administrators. Dedicated to promoting excellence, innovation, and improving the quality of patient care, Fellows designations provide members with a distinguishing credential as established pioneers in the industry.
For more information and to review your eligibility, visit hospitalmedicine.org/fellows.
Ms. Cowan is a marketing communications specialist at the Society of Hospital Medicine.
In an industry brimming with opportunity and ongoing transformation, it is easy to feel indecisive about your next professional step when ample career paths exist in hospital medicine.
Yingkei Hui, MD, FHM, is an academic hospitalist at St. Vincent Indianapolis, and a Society of Hospital Medicine member since 2015. Seeking to set herself apart as an aspiring patient safety and quality improvement leader while continuing her professional development, she looked to SHM’s Fellow designation as the next piece of her career puzzle.
With more than 14 years of experience in the health care industry, Dr. Hui fell in love with the specialty because of its flexibility and patient-centric focus.
“I have a broad interest in medicine and want to learn everything under the larger umbrella of medicine,” she said. “I also find myself deeply in love with hospital medicine because it provides me with the opportunity to participate in various hospital committees and allows me to enjoy my practice from a macroscopic view of U.S. health care transformation – especially given the popular value-based patient care approach from recent years.”
Dr. Hui’s breadth of experience has allowed her to gain a unique set of perspectives and experiences from international and domestic standpoints. From attending medical school at the Chinese University of Hong Kong to completing her residency on the east coast at Pennsylvania Hospital in Philadelphia – part of the University of Pennsylvania Health System – Dr. Hui has held active medical licenses in New Jersey and currently, Indiana.
“SHM’s Fellow designation allows me to challenge myself in setting my career goal as a patient safety and quality improvement leader in my program,” she said. “It means a lot to me as it is a stand-out recognition of my participation in and contribution to patient care in my institution.”
When asked about the most rewarding aspect of being a part of the hospital medicine community, Dr. Hui identified “satisfaction in the teaching role.” She said she is “motivated by the holistic care for the patients, the integration of medical knowledge and coordination of care, and also the opportunity to conduct quality improvement projects.”
Motivated by her colleagues, Dr. Hui credits SHM with providing her with the inspiration and tools to push herself and advance her career in hospital medicine.
“I enjoy immersing myself in SHM’s patient safety and quality improvement resources; they are perfect for frontline hospitalists and also provide CME [continuing medical education],” she noted. “My previous medical directors were all Senior Fellows; they are my role models and continue to inspire me throughout my career.”
Dr. Hui also said that networking within the SHM community has been encouraging. “I’ve met talented Fellows at a number of hospital medicine annual conferences who have inspired me in the areas of patient care, education, and health promotion,” she explained. “Some of them have extensive publications; they are truly amazing physicians. SHM’s Annual Conference provides great opportunities for networking.”
As Dr. Hui continues to progress her career in hospital medicine, she believes that communication is a key pillar in her success. “Be a true listener and fill your heart with compassion, empathy, and courage,” she said. “Recognize your role as the enabler for the patients to improve their health.”
Completing her Master’s degree in population health management at Johns Hopkins University and expecting to graduate in May 2020, Dr. Hui is the designer of system safety (comprising patient safety, second victim safety, quality improvement, and just culture) in the academic setting of her residency program. She is also chairing a pioneer project for the St. Vincent IM residency program.
Dr. Hui plans to apply for a Senior Fellow designation with SHM in the future.
If you would like to join Dr. Hui and other like-minded hospital medicine leaders in taking your career to the next level, SHM is currently recruiting for the Fellows and Senior Fellows: Class of 2020. Applications are open until Nov. 29 of this year. These designations are available across a variety of membership categories, including physicians, nurse practitioners, physician assistants, and qualified practice administrators. Dedicated to promoting excellence, innovation, and improving the quality of patient care, Fellows designations provide members with a distinguishing credential as established pioneers in the industry.
For more information and to review your eligibility, visit hospitalmedicine.org/fellows.
Ms. Cowan is a marketing communications specialist at the Society of Hospital Medicine.
USPSTF recommendations on risk assessment, genetic counseling, and genetic testing for BRCA-related cancer
Breast cancer screening recommendations have evolved over the past decade. BRCA1/2 genes are tumor-suppressor genes. Mutations in these genes place women at an increased risk for developing breast, ovarian, fallopian tube, and peritoneal cancer. Detection of BRCA1/2 mutations through genetic screening can provide patients with more information about their cancer risk and can lead to discussion of prophylactic therapies. This includes increased screening frequency, medical therapy, and surgical interventions.
New USPSTF recommendations address who is at an increased risk for BRCA1/2 mutations. They recommend using screening tools focusing on family history that primary care physicians can utilize to determine who should be referred for genetic counseling to discuss the risks and benefits of genetic screening. The following are the task force’s two primary recommendations:
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing. (B recommendation)
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. (D recommendation)
Breast cancer is the second leading cause of cancer and cancer death for women in the United States. Ovarian cancer ranks fifth in cancer deaths for women in the U.S. By age 70, women with BRCA1/2 mutations have a 45%-65% cumulative lifetime risk of developing breast cancer.
Mutations in BRCA1, specifically, are associated with a 39% lifetime risk for ovarian, fallopian tube, and peritoneal cancer. In contrast, mutations in BRCA2 are associated with a 10%-17% lifetime risk.
The USPSTF also underscores the increased prevalence of BRCA1/2 mutations in the Ashkenazi Jewish population. Three out of seven familial risk assessment tools inquire about Jewish ancestry. This is because the Ashkenazi Jewish population have a higher prevalence of three founder mutations in the BRCA1/2 gene. A member of this population has a 1 in 40 chance of carrying one of these three mutations, whereas the general population has a 1 in 300 chance.
The USPSTF recommends a multistep process of screening. The first step is taking a family history of cancer. For women who have a family history of breast, ovarian, tubal, or peritoneal cancer or a personal history of these cancers, a brief familial risk assessment tool should be used to determine the need for referral for in-depth genetic counseling to determine the need for genetic testing.
It is important to recognize that the validated tools recommended by the USPSTF are specific for genetic risk assessment. General breast cancer risk assessment tools, including the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model, are not recommended.
The sensitivity of the tools recommended by the USPSTF range between 77% and 100%. A number of tools are given as an option with no one tool being better than the other. Perhaps the easiest to implement of the validated tools recommended is the Pedigree Assessment Tool. For this tool, points are assigned for every family member with breast or ovarian cancer as indicated in the table below.
A positive result on a screening tool will lead primary care physicians to appropriately refer patients for genetic counseling. Genetic testing for BRCA1/2 mutations should be limited to those individuals whose personal and/or family history reflects an increased risk for gene mutations after detailed genetic assessment and counseling. The results of the genetic screening should assist a patient in their decision making.
Prophylactic treatment for BRCA1/2 mutation carriers are outside the scope of this recommendation. However the guidelines briefly review risk-reducing therapies including screening, medical, and surgical options. Medical therapy for patients who have BRCA1/2 mutations include the use of tamoxifen, raloxifene, and aromatase inhibitors. Surgical interventions include bilateral mastectomy and salpingo-oopherectomy.
Screening options include earlier and more frequent mammograms and breast MRIs. Screening is largely based on family history and the USPSTF acknowledges their uncertainty when screening women with an unknown family history. Male breast cancer, pancreatic cancer, prostate cancer, and melanoma are also associated with BRCA1/2 mutations. They are not included in this recommendation.
The bottom line
USPSTF recommended that primary care physicians should use familial risk assessment tools to screen women for BRCA1/2 mutations. This includes women with a personal and/or family history of breast, ovarian, tubal, or peritoneal cancer or women with a family history of BRCA1/2 gene mutations. Patients who test positive through one of the suggested screening tools should be referred for genetic counseling. This could lead to genetic testing and subsequent prophylactic therapies and/or increased screenings if the patient so desires. It is of importance to note the USPSTF recommends against routine screening of BRCA1/2 gene mutations for women who do not meet the above requirements.
Reference
USPSTF Recommendation: Assessment, counseling, and testing for BRCA-related cancer. JAMA. 2019;322(7):652-65. doi: 10.1001/jama.2019.10987.
Dr. Style is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Breast cancer screening recommendations have evolved over the past decade. BRCA1/2 genes are tumor-suppressor genes. Mutations in these genes place women at an increased risk for developing breast, ovarian, fallopian tube, and peritoneal cancer. Detection of BRCA1/2 mutations through genetic screening can provide patients with more information about their cancer risk and can lead to discussion of prophylactic therapies. This includes increased screening frequency, medical therapy, and surgical interventions.
New USPSTF recommendations address who is at an increased risk for BRCA1/2 mutations. They recommend using screening tools focusing on family history that primary care physicians can utilize to determine who should be referred for genetic counseling to discuss the risks and benefits of genetic screening. The following are the task force’s two primary recommendations:
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing. (B recommendation)
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. (D recommendation)
Breast cancer is the second leading cause of cancer and cancer death for women in the United States. Ovarian cancer ranks fifth in cancer deaths for women in the U.S. By age 70, women with BRCA1/2 mutations have a 45%-65% cumulative lifetime risk of developing breast cancer.
Mutations in BRCA1, specifically, are associated with a 39% lifetime risk for ovarian, fallopian tube, and peritoneal cancer. In contrast, mutations in BRCA2 are associated with a 10%-17% lifetime risk.
The USPSTF also underscores the increased prevalence of BRCA1/2 mutations in the Ashkenazi Jewish population. Three out of seven familial risk assessment tools inquire about Jewish ancestry. This is because the Ashkenazi Jewish population have a higher prevalence of three founder mutations in the BRCA1/2 gene. A member of this population has a 1 in 40 chance of carrying one of these three mutations, whereas the general population has a 1 in 300 chance.
The USPSTF recommends a multistep process of screening. The first step is taking a family history of cancer. For women who have a family history of breast, ovarian, tubal, or peritoneal cancer or a personal history of these cancers, a brief familial risk assessment tool should be used to determine the need for referral for in-depth genetic counseling to determine the need for genetic testing.
It is important to recognize that the validated tools recommended by the USPSTF are specific for genetic risk assessment. General breast cancer risk assessment tools, including the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model, are not recommended.
The sensitivity of the tools recommended by the USPSTF range between 77% and 100%. A number of tools are given as an option with no one tool being better than the other. Perhaps the easiest to implement of the validated tools recommended is the Pedigree Assessment Tool. For this tool, points are assigned for every family member with breast or ovarian cancer as indicated in the table below.

A positive result on a screening tool will lead primary care physicians to appropriately refer patients for genetic counseling. Genetic testing for BRCA1/2 mutations should be limited to those individuals whose personal and/or family history reflects an increased risk for gene mutations after detailed genetic assessment and counseling. The results of the genetic screening should assist a patient in their decision making.
Prophylactic treatment for BRCA1/2 mutation carriers are outside the scope of this recommendation. However the guidelines briefly review risk-reducing therapies including screening, medical, and surgical options. Medical therapy for patients who have BRCA1/2 mutations include the use of tamoxifen, raloxifene, and aromatase inhibitors. Surgical interventions include bilateral mastectomy and salpingo-oopherectomy.
Screening options include earlier and more frequent mammograms and breast MRIs. Screening is largely based on family history and the USPSTF acknowledges their uncertainty when screening women with an unknown family history. Male breast cancer, pancreatic cancer, prostate cancer, and melanoma are also associated with BRCA1/2 mutations. They are not included in this recommendation.
The bottom line
USPSTF recommended that primary care physicians should use familial risk assessment tools to screen women for BRCA1/2 mutations. This includes women with a personal and/or family history of breast, ovarian, tubal, or peritoneal cancer or women with a family history of BRCA1/2 gene mutations. Patients who test positive through one of the suggested screening tools should be referred for genetic counseling. This could lead to genetic testing and subsequent prophylactic therapies and/or increased screenings if the patient so desires. It is of importance to note the USPSTF recommends against routine screening of BRCA1/2 gene mutations for women who do not meet the above requirements.
Reference
USPSTF Recommendation: Assessment, counseling, and testing for BRCA-related cancer. JAMA. 2019;322(7):652-65. doi: 10.1001/jama.2019.10987.
Dr. Style is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Breast cancer screening recommendations have evolved over the past decade. BRCA1/2 genes are tumor-suppressor genes. Mutations in these genes place women at an increased risk for developing breast, ovarian, fallopian tube, and peritoneal cancer. Detection of BRCA1/2 mutations through genetic screening can provide patients with more information about their cancer risk and can lead to discussion of prophylactic therapies. This includes increased screening frequency, medical therapy, and surgical interventions.
New USPSTF recommendations address who is at an increased risk for BRCA1/2 mutations. They recommend using screening tools focusing on family history that primary care physicians can utilize to determine who should be referred for genetic counseling to discuss the risks and benefits of genetic screening. The following are the task force’s two primary recommendations:
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing. (B recommendation)
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. (D recommendation)
Breast cancer is the second leading cause of cancer and cancer death for women in the United States. Ovarian cancer ranks fifth in cancer deaths for women in the U.S. By age 70, women with BRCA1/2 mutations have a 45%-65% cumulative lifetime risk of developing breast cancer.
Mutations in BRCA1, specifically, are associated with a 39% lifetime risk for ovarian, fallopian tube, and peritoneal cancer. In contrast, mutations in BRCA2 are associated with a 10%-17% lifetime risk.
The USPSTF also underscores the increased prevalence of BRCA1/2 mutations in the Ashkenazi Jewish population. Three out of seven familial risk assessment tools inquire about Jewish ancestry. This is because the Ashkenazi Jewish population have a higher prevalence of three founder mutations in the BRCA1/2 gene. A member of this population has a 1 in 40 chance of carrying one of these three mutations, whereas the general population has a 1 in 300 chance.
The USPSTF recommends a multistep process of screening. The first step is taking a family history of cancer. For women who have a family history of breast, ovarian, tubal, or peritoneal cancer or a personal history of these cancers, a brief familial risk assessment tool should be used to determine the need for referral for in-depth genetic counseling to determine the need for genetic testing.
It is important to recognize that the validated tools recommended by the USPSTF are specific for genetic risk assessment. General breast cancer risk assessment tools, including the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model, are not recommended.
The sensitivity of the tools recommended by the USPSTF range between 77% and 100%. A number of tools are given as an option with no one tool being better than the other. Perhaps the easiest to implement of the validated tools recommended is the Pedigree Assessment Tool. For this tool, points are assigned for every family member with breast or ovarian cancer as indicated in the table below.

A positive result on a screening tool will lead primary care physicians to appropriately refer patients for genetic counseling. Genetic testing for BRCA1/2 mutations should be limited to those individuals whose personal and/or family history reflects an increased risk for gene mutations after detailed genetic assessment and counseling. The results of the genetic screening should assist a patient in their decision making.
Prophylactic treatment for BRCA1/2 mutation carriers are outside the scope of this recommendation. However the guidelines briefly review risk-reducing therapies including screening, medical, and surgical options. Medical therapy for patients who have BRCA1/2 mutations include the use of tamoxifen, raloxifene, and aromatase inhibitors. Surgical interventions include bilateral mastectomy and salpingo-oopherectomy.
Screening options include earlier and more frequent mammograms and breast MRIs. Screening is largely based on family history and the USPSTF acknowledges their uncertainty when screening women with an unknown family history. Male breast cancer, pancreatic cancer, prostate cancer, and melanoma are also associated with BRCA1/2 mutations. They are not included in this recommendation.
The bottom line
USPSTF recommended that primary care physicians should use familial risk assessment tools to screen women for BRCA1/2 mutations. This includes women with a personal and/or family history of breast, ovarian, tubal, or peritoneal cancer or women with a family history of BRCA1/2 gene mutations. Patients who test positive through one of the suggested screening tools should be referred for genetic counseling. This could lead to genetic testing and subsequent prophylactic therapies and/or increased screenings if the patient so desires. It is of importance to note the USPSTF recommends against routine screening of BRCA1/2 gene mutations for women who do not meet the above requirements.
Reference
USPSTF Recommendation: Assessment, counseling, and testing for BRCA-related cancer. JAMA. 2019;322(7):652-65. doi: 10.1001/jama.2019.10987.
Dr. Style is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Celiac disease may underlie seizures
CHARLOTTE, N.C. – , according to a retrospective chart review presented at the annual meeting of the Child Neurology Society. Associations between celiac disease and seizures may have implications for screening and treatment, said study author Shanna Swartwood, MD, a fellow in the department of pediatric neurology at University of Utah in Salt Lake City.
“Screening for [celiac disease] early in patients with epilepsy, specifically with temporal EEG findings and intractable epilepsy, is warranted given the improvement of seizure burden that may result from exclusion of gluten from the diet,” said Dr. Swartwood and colleagues.
About 10% of patients with celiac disease have clinical neurologic manifestations, such as seizures. To characterize features of epilepsy in a pediatric population with celiac disease and to examine the effect of a gluten-free diet on seizure burden, Dr. Swartwood and colleagues reviewed patients treated at Primary Children’s Hospital in Salt Lake City since 2002. They identified patients with ICD-10 codes for seizures or epilepsy and celiac disease and reviewed 187 charts in all.
In all, 40 patients with seizures had biopsy-proven celiac disease, and 22 had a diagnosis of celiac disease based on the presence of antibodies. Among those with biopsy-proven celiac disease, 43% had intractable seizures. Among those with antibody-positive celiac disease, 31% had intractable seizures.
Among patients with intractable epilepsy, seizure onset preceded the diagnosis of celiac disease by an average of 5 years. For patients with nonintractable epilepsy, the first seizure occurred 1 year before the celiac disease diagnosis on average, but some patients received a celiac disease diagnosis first.
Focal seizures with secondary generalization and generalized tonic clonic seizures were the most common seizure types in this cohort. Epileptiform activity most often was seen in the temporal lobe. While other studies in patients with celiac disease have found occipital epileptiform activity to be the most common, only one patient in this cohort had activity in that location, Dr. Swartwood noted.
Patients with intractable seizures who adhered to a gluten-free diet “had a fairly robust response in terms of seizure improvement,” she said. Seizure improvement, including a decrease in seizure frequency or a decrease in antiepileptic medication dosage, occurred in seven of nine patients in the biopsy-proven group and in two of three patients in the antibody-positive group who adhered to a gluten-free diet and had intractable seizures. One patient was able to stop antiepileptic medication, and one patient had a complete resolution of seizure activity.
The researchers plan to further study the relationship between celiac disease and epilepsy, including whether various HLA subtypes of celiac disease correlate with seizures, said coinvestigator Cristina Trandafir, MD, PhD, assistant professor of pediatric neurology at University of Utah.
The chart review included relatively few patients with limited data. Nevertheless, the results suggest that there may be “substantial lag time” from first seizure to celiac disease diagnosis and that “earlier diagnosis and earlier placement on a gluten-free diet may be beneficial,” Dr. Swartwood said. Celiac disease may be asymptomatic, and screening for celiac disease with a blood test may make sense for patients with intractable seizures, she said.
The researchers had no relevant disclosures.
CHARLOTTE, N.C. – , according to a retrospective chart review presented at the annual meeting of the Child Neurology Society. Associations between celiac disease and seizures may have implications for screening and treatment, said study author Shanna Swartwood, MD, a fellow in the department of pediatric neurology at University of Utah in Salt Lake City.
“Screening for [celiac disease] early in patients with epilepsy, specifically with temporal EEG findings and intractable epilepsy, is warranted given the improvement of seizure burden that may result from exclusion of gluten from the diet,” said Dr. Swartwood and colleagues.
About 10% of patients with celiac disease have clinical neurologic manifestations, such as seizures. To characterize features of epilepsy in a pediatric population with celiac disease and to examine the effect of a gluten-free diet on seizure burden, Dr. Swartwood and colleagues reviewed patients treated at Primary Children’s Hospital in Salt Lake City since 2002. They identified patients with ICD-10 codes for seizures or epilepsy and celiac disease and reviewed 187 charts in all.
In all, 40 patients with seizures had biopsy-proven celiac disease, and 22 had a diagnosis of celiac disease based on the presence of antibodies. Among those with biopsy-proven celiac disease, 43% had intractable seizures. Among those with antibody-positive celiac disease, 31% had intractable seizures.
Among patients with intractable epilepsy, seizure onset preceded the diagnosis of celiac disease by an average of 5 years. For patients with nonintractable epilepsy, the first seizure occurred 1 year before the celiac disease diagnosis on average, but some patients received a celiac disease diagnosis first.
Focal seizures with secondary generalization and generalized tonic clonic seizures were the most common seizure types in this cohort. Epileptiform activity most often was seen in the temporal lobe. While other studies in patients with celiac disease have found occipital epileptiform activity to be the most common, only one patient in this cohort had activity in that location, Dr. Swartwood noted.
Patients with intractable seizures who adhered to a gluten-free diet “had a fairly robust response in terms of seizure improvement,” she said. Seizure improvement, including a decrease in seizure frequency or a decrease in antiepileptic medication dosage, occurred in seven of nine patients in the biopsy-proven group and in two of three patients in the antibody-positive group who adhered to a gluten-free diet and had intractable seizures. One patient was able to stop antiepileptic medication, and one patient had a complete resolution of seizure activity.
The researchers plan to further study the relationship between celiac disease and epilepsy, including whether various HLA subtypes of celiac disease correlate with seizures, said coinvestigator Cristina Trandafir, MD, PhD, assistant professor of pediatric neurology at University of Utah.
The chart review included relatively few patients with limited data. Nevertheless, the results suggest that there may be “substantial lag time” from first seizure to celiac disease diagnosis and that “earlier diagnosis and earlier placement on a gluten-free diet may be beneficial,” Dr. Swartwood said. Celiac disease may be asymptomatic, and screening for celiac disease with a blood test may make sense for patients with intractable seizures, she said.
The researchers had no relevant disclosures.
CHARLOTTE, N.C. – , according to a retrospective chart review presented at the annual meeting of the Child Neurology Society. Associations between celiac disease and seizures may have implications for screening and treatment, said study author Shanna Swartwood, MD, a fellow in the department of pediatric neurology at University of Utah in Salt Lake City.
“Screening for [celiac disease] early in patients with epilepsy, specifically with temporal EEG findings and intractable epilepsy, is warranted given the improvement of seizure burden that may result from exclusion of gluten from the diet,” said Dr. Swartwood and colleagues.
About 10% of patients with celiac disease have clinical neurologic manifestations, such as seizures. To characterize features of epilepsy in a pediatric population with celiac disease and to examine the effect of a gluten-free diet on seizure burden, Dr. Swartwood and colleagues reviewed patients treated at Primary Children’s Hospital in Salt Lake City since 2002. They identified patients with ICD-10 codes for seizures or epilepsy and celiac disease and reviewed 187 charts in all.
In all, 40 patients with seizures had biopsy-proven celiac disease, and 22 had a diagnosis of celiac disease based on the presence of antibodies. Among those with biopsy-proven celiac disease, 43% had intractable seizures. Among those with antibody-positive celiac disease, 31% had intractable seizures.
Among patients with intractable epilepsy, seizure onset preceded the diagnosis of celiac disease by an average of 5 years. For patients with nonintractable epilepsy, the first seizure occurred 1 year before the celiac disease diagnosis on average, but some patients received a celiac disease diagnosis first.
Focal seizures with secondary generalization and generalized tonic clonic seizures were the most common seizure types in this cohort. Epileptiform activity most often was seen in the temporal lobe. While other studies in patients with celiac disease have found occipital epileptiform activity to be the most common, only one patient in this cohort had activity in that location, Dr. Swartwood noted.
Patients with intractable seizures who adhered to a gluten-free diet “had a fairly robust response in terms of seizure improvement,” she said. Seizure improvement, including a decrease in seizure frequency or a decrease in antiepileptic medication dosage, occurred in seven of nine patients in the biopsy-proven group and in two of three patients in the antibody-positive group who adhered to a gluten-free diet and had intractable seizures. One patient was able to stop antiepileptic medication, and one patient had a complete resolution of seizure activity.
The researchers plan to further study the relationship between celiac disease and epilepsy, including whether various HLA subtypes of celiac disease correlate with seizures, said coinvestigator Cristina Trandafir, MD, PhD, assistant professor of pediatric neurology at University of Utah.
The chart review included relatively few patients with limited data. Nevertheless, the results suggest that there may be “substantial lag time” from first seizure to celiac disease diagnosis and that “earlier diagnosis and earlier placement on a gluten-free diet may be beneficial,” Dr. Swartwood said. Celiac disease may be asymptomatic, and screening for celiac disease with a blood test may make sense for patients with intractable seizures, she said.
The researchers had no relevant disclosures.
REPORTING FROM CNS 2019













