User login
Topical benzyl benzoate–based treatment reduced Demodex in patients with rosacea
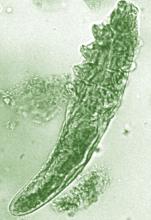
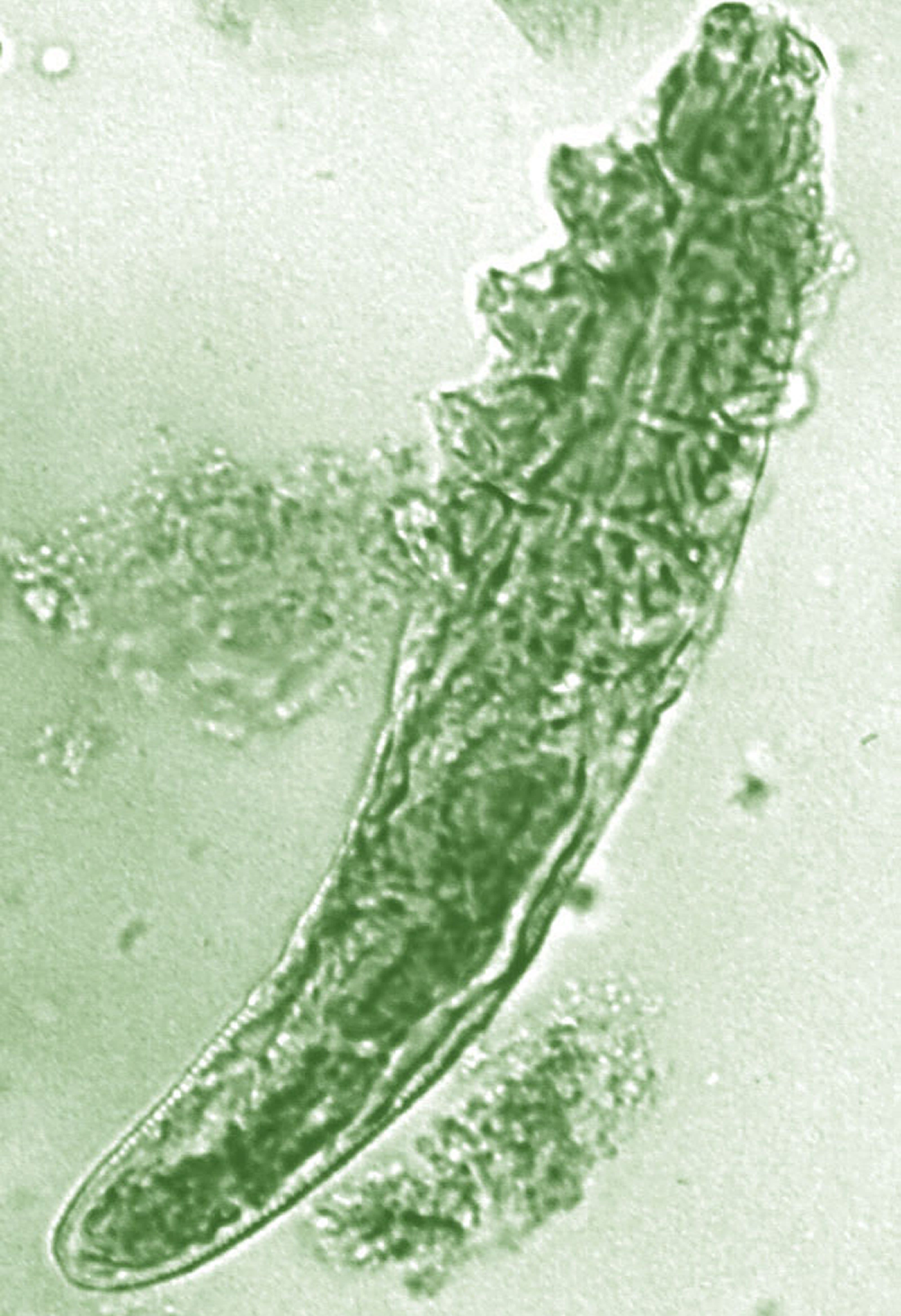
Daily treatment with benzyl benzoate (BB) cream reduced Demodex densities in patients with and without rosacea, and was associated with improvement in clinical signs, according to F.M.N. Forton, MD, of the Dermatology Clinic, Brussels, and his coauthor in the Journal of the European Academy of Dermatology and Venereology.
The retrospective study comprised 394 patients treated between 2002 and 2010; 117 of them had rosacea with papulopustules and the remainder only demodicosis. Their mean age was 49 years; most (278) were women. They had been treated with one of three doses of BB cream with crotamiton 10% cream: crotamiton applied in the morning, and BB 12% plus crotamiton in the evening; BB 12% plus crotamiton applied twice daily; and BB 20%-24% plus crotamiton applied once in the evening. Demodex densities (Dds) were measured with two consecutive standardized skin surface biopsies and deep biopsies at baseline and follow-up. Symptoms were measured with an investigator global assessment (IGA).
The authors said they had previously found that BB had acaricidal effects on Demodex, as did crotamiton “to a lesser extent,” but that the two treatments have not been well studied. They also referred to the increasing evidence that Demodex has a role in papulopustular rosacea, and that ivermectin, which is acaricidal, is recommended for topical treatment of papulopustular rosacea.
In the study, a mean of 2.7 months after starting treatment, mean Dds were significantly lower for the entire cohort, decreasing by 72.4% (plus or minus 2.6%) from baseline. Dds had normalized in 35% of patients, and in 31% of patients, symptoms had cleared.
Treatment was considered effective in 46% of patients and curative in 20%. Men responded slightly better, with clearance in 34% vs. 20% of women. The two regimens using the higher dose of BB were more effective than those using the lower dose and were associated with better compliance. Compliance overall was 77%.
After a mean of nearly 3 months of treatment, “topical application of BB (with crotamiton) was effective at reducing Dds and clearing clinical symptoms, not only in demodicosis but also in rosacea with papulopustules, indirectly supporting a key role of the mite in the pathophysiology of rosacea,” the authors concluded.
Neither of these products are approved in the United States for treating rosacea.
Dr. Forton disclosed that he occasionally works as a consultant for Galderma; the second author had no disclosures. The study had no funding source.
Source: Forton FMN et al. J Eur Acad Dermatol Venereol. 2019 Sep 7. doi: 10.1111/jdv.15938.
Daily treatment with benzyl benzoate (BB) cream reduced Demodex densities in patients with and without rosacea, and was associated with improvement in clinical signs, according to F.M.N. Forton, MD, of the Dermatology Clinic, Brussels, and his coauthor in the Journal of the European Academy of Dermatology and Venereology.
The retrospective study comprised 394 patients treated between 2002 and 2010; 117 of them had rosacea with papulopustules and the remainder only demodicosis. Their mean age was 49 years; most (278) were women. They had been treated with one of three doses of BB cream with crotamiton 10% cream: crotamiton applied in the morning, and BB 12% plus crotamiton in the evening; BB 12% plus crotamiton applied twice daily; and BB 20%-24% plus crotamiton applied once in the evening. Demodex densities (Dds) were measured with two consecutive standardized skin surface biopsies and deep biopsies at baseline and follow-up. Symptoms were measured with an investigator global assessment (IGA).
The authors said they had previously found that BB had acaricidal effects on Demodex, as did crotamiton “to a lesser extent,” but that the two treatments have not been well studied. They also referred to the increasing evidence that Demodex has a role in papulopustular rosacea, and that ivermectin, which is acaricidal, is recommended for topical treatment of papulopustular rosacea.
In the study, a mean of 2.7 months after starting treatment, mean Dds were significantly lower for the entire cohort, decreasing by 72.4% (plus or minus 2.6%) from baseline. Dds had normalized in 35% of patients, and in 31% of patients, symptoms had cleared.
Treatment was considered effective in 46% of patients and curative in 20%. Men responded slightly better, with clearance in 34% vs. 20% of women. The two regimens using the higher dose of BB were more effective than those using the lower dose and were associated with better compliance. Compliance overall was 77%.
After a mean of nearly 3 months of treatment, “topical application of BB (with crotamiton) was effective at reducing Dds and clearing clinical symptoms, not only in demodicosis but also in rosacea with papulopustules, indirectly supporting a key role of the mite in the pathophysiology of rosacea,” the authors concluded.
Neither of these products are approved in the United States for treating rosacea.
Dr. Forton disclosed that he occasionally works as a consultant for Galderma; the second author had no disclosures. The study had no funding source.
Source: Forton FMN et al. J Eur Acad Dermatol Venereol. 2019 Sep 7. doi: 10.1111/jdv.15938.
Daily treatment with benzyl benzoate (BB) cream reduced Demodex densities in patients with and without rosacea, and was associated with improvement in clinical signs, according to F.M.N. Forton, MD, of the Dermatology Clinic, Brussels, and his coauthor in the Journal of the European Academy of Dermatology and Venereology.
The retrospective study comprised 394 patients treated between 2002 and 2010; 117 of them had rosacea with papulopustules and the remainder only demodicosis. Their mean age was 49 years; most (278) were women. They had been treated with one of three doses of BB cream with crotamiton 10% cream: crotamiton applied in the morning, and BB 12% plus crotamiton in the evening; BB 12% plus crotamiton applied twice daily; and BB 20%-24% plus crotamiton applied once in the evening. Demodex densities (Dds) were measured with two consecutive standardized skin surface biopsies and deep biopsies at baseline and follow-up. Symptoms were measured with an investigator global assessment (IGA).
The authors said they had previously found that BB had acaricidal effects on Demodex, as did crotamiton “to a lesser extent,” but that the two treatments have not been well studied. They also referred to the increasing evidence that Demodex has a role in papulopustular rosacea, and that ivermectin, which is acaricidal, is recommended for topical treatment of papulopustular rosacea.
In the study, a mean of 2.7 months after starting treatment, mean Dds were significantly lower for the entire cohort, decreasing by 72.4% (plus or minus 2.6%) from baseline. Dds had normalized in 35% of patients, and in 31% of patients, symptoms had cleared.
Treatment was considered effective in 46% of patients and curative in 20%. Men responded slightly better, with clearance in 34% vs. 20% of women. The two regimens using the higher dose of BB were more effective than those using the lower dose and were associated with better compliance. Compliance overall was 77%.
After a mean of nearly 3 months of treatment, “topical application of BB (with crotamiton) was effective at reducing Dds and clearing clinical symptoms, not only in demodicosis but also in rosacea with papulopustules, indirectly supporting a key role of the mite in the pathophysiology of rosacea,” the authors concluded.
Neither of these products are approved in the United States for treating rosacea.
Dr. Forton disclosed that he occasionally works as a consultant for Galderma; the second author had no disclosures. The study had no funding source.
Source: Forton FMN et al. J Eur Acad Dermatol Venereol. 2019 Sep 7. doi: 10.1111/jdv.15938.
FROM JEADV
Secondary Syphilis Mimicking Molluscum Contagiosum in the Beard Area of an AIDS Patient
To the Editor:
A 46-year-old man with a history of AIDS (viral load, 28,186 copies/mL; CD4 count, 22 cells/μL) presented with a 40-lb weight loss over the last 6 months as well as dysphagia and a new-onset pruritic facial eruption of 1 week’s duration. The facial lesions quickly spread to involve the beard area and the upper neck. His medical history was notable for nicotine dependence, seborrheic dermatitis, molluscum contagiosum (MC), treated neurosyphilis and latent tuberculosis, hypertension, a liver mass suspected to be a hemangioma, and erythrocytosis. He was diagnosed with human immunodeficiency virus infection 19 years prior to presentation and was not compliant with the prescribed highly active antiretroviral therapy.
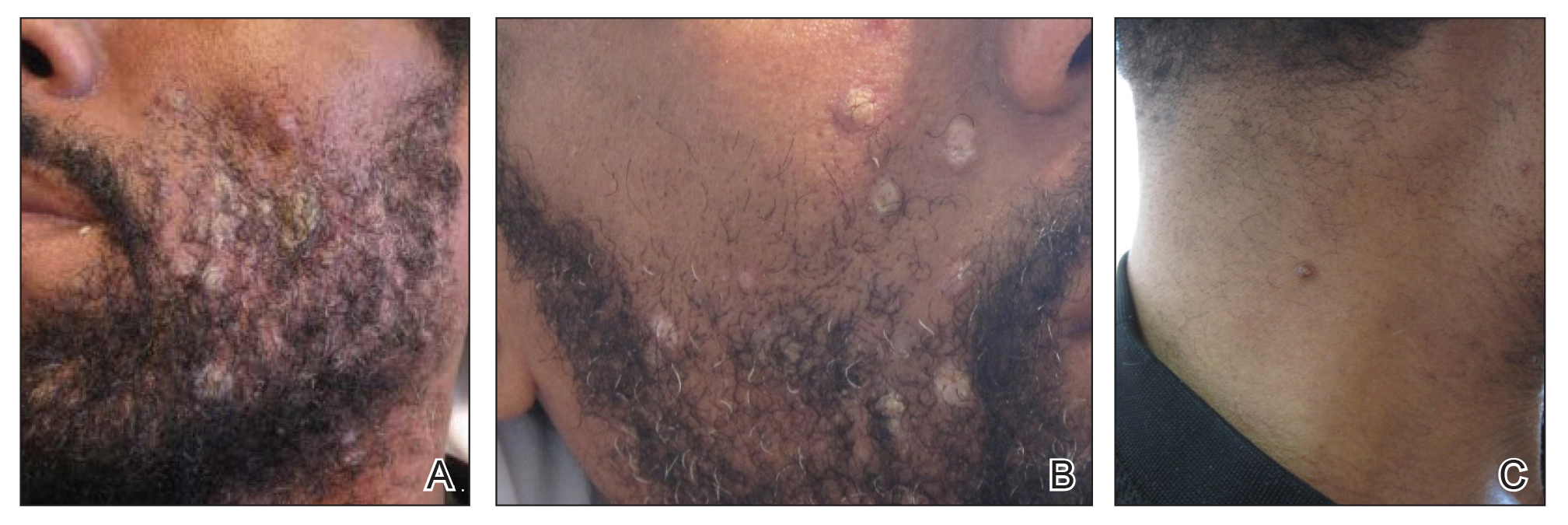
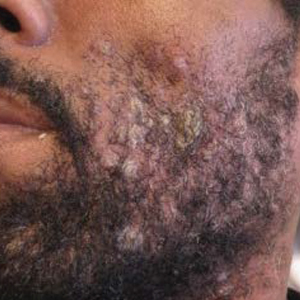
Skin examination revealed multiple discrete and coalescing, 2- to 12-mm, nonumbilicated, hyperkeratotic papules and nodules localized to the left and right beard areas (Figure 1A). A few discrete, 2- to 5-mm, umbilicated papules were noted in the right beard area (Figure 1B), as well as on the right side of the neck (Figure 1C), buttocks, and legs. Mild erythema with yellow-white scale was present in the alar creases. Examination of the oropharyngeal mucosa revealed multiple thick white plaques that were easily scraped off with a tongue depressor. Examination of the palms, soles, and anogenital areas was normal.
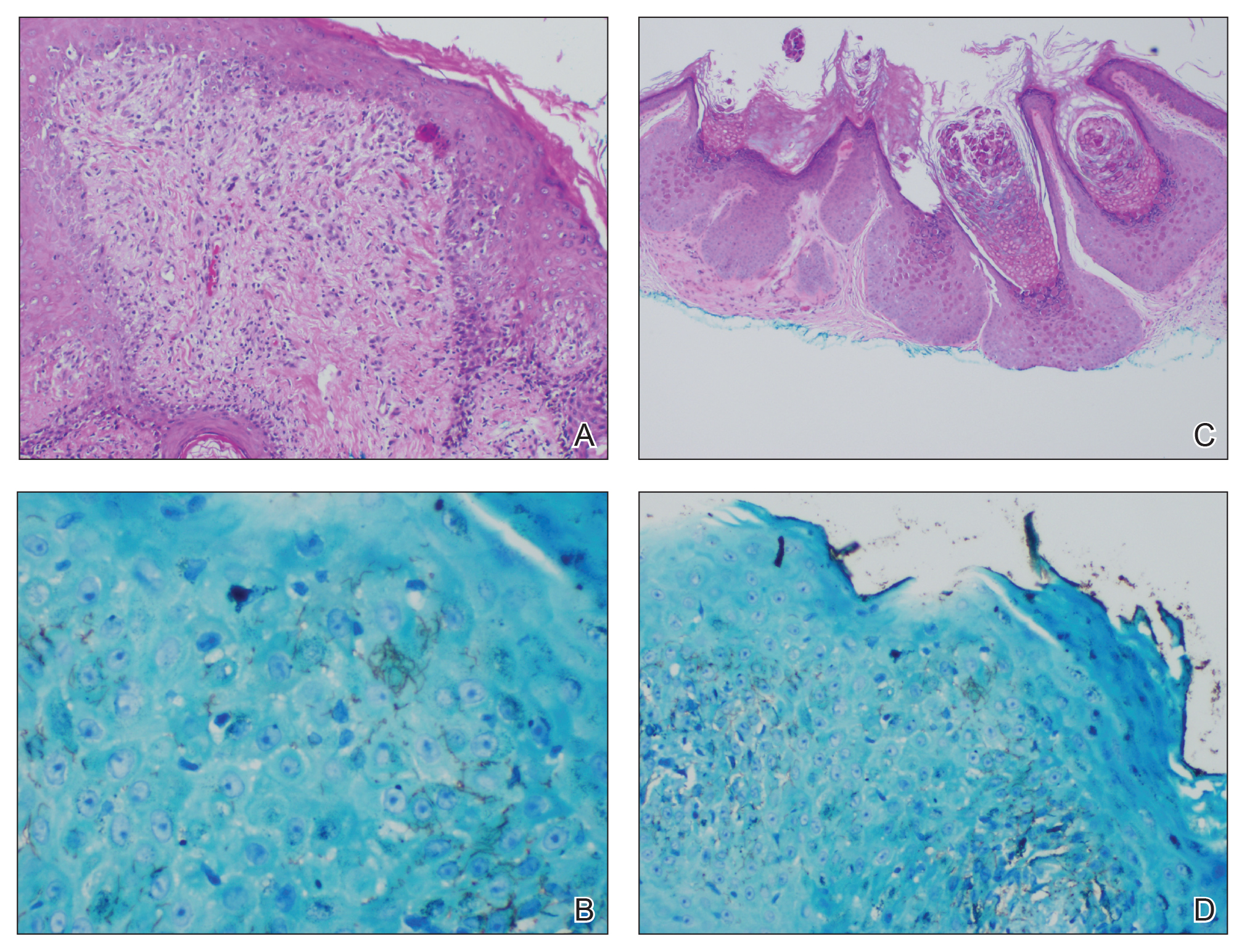
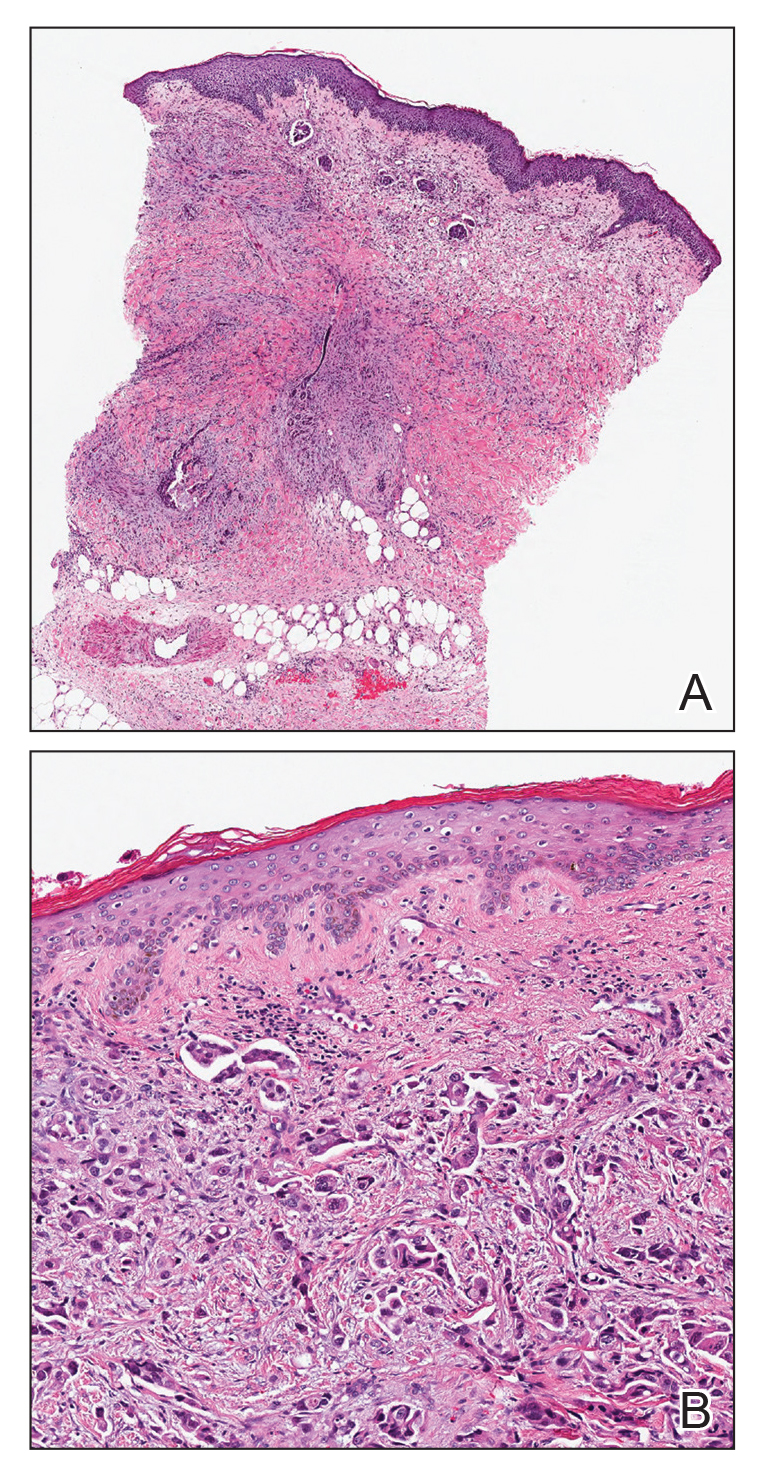
A punch biopsy of a nonumbilicated hyperkeratotic papule from the left beard area demonstrated spongiosis; neutrophilic microabscess formation; plasma cells; and a superficial and deep perivascular, predominantly lymphohistiocytic infiltrate (Figure 2A). Spirochete immunohistochemical staining of tissue highlighted abundant organisms in the dermoepidermal junction and vascular endothelial cells (Figure 2B). Other tissue stains for bacteria, including acid-fast bacilli, and fungi were negative. Bacterial culture of tissue from the lesion in the left beard area grew Staphylococcus aureus. Results of acid-fast and fungal cultures of tissue were negative. Shave biopsy of the umbilicated papule on the right side of the neck demonstrated classic invagination of the epidermis into the dermis and intracytoplasmic viral inclusions consistent with MC (Figure 2C). Spirochete immunohistochemical staining of the same tissue sample was negative (Figure 2D).
Serum rapid plasma reagin was reactive with a titer of 1:128 compared to the last known reactive rapid plasma reagin titer of 1:1 five years prior to presentation. A fluorescent treponemal antibody absorption test and VDRL test of cerebrospinal fluid was nonreactive. Fungal, bacterial, and acid-fast cultures of cerebral spinal fluid and a cryptococcal antigen test were negative. Serum cryptococcal antigen and coccidioides complement fixation tests were negative. Cytomegalovirus plasma polymerase chain reaction and urine histoplasma antigen testing were negative. Computed tomography of the chest revealed a new 1.9×1.6×2.1-cm3 cavitary lesion with distal tree-in-bud opacities in the lingula of the left lung. Acid-fast blood culture was negative, and acid-fast sputum culture was positive for Mycobacterium kansasii.
The cutaneous pathology findings and serologic findings confirmed the diagnoses of cutaneous secondary syphilis (SS) in the beard area and MC on the right side of the neck. Clinical diagnoses of seborrheic dermatitis of the alar creases and esophageal candidiasis also were made. The patient was treated with intramuscular penicillin G 2.4 million U once weekly for 3 weeks. The lesions confined to the beard area rapidly resolved within 7 days after the first dose of antibiotics, which further supported the diagnosis of localized cutaneous SS. Fluconazole 100 mg once daily was prescribed for the esophageal candidiasis, and he also was started on a regimen of rifampin 600 mg once daily, isoniazid 300 mg once daily, ethambutol 1200 mg once daily, and pyrazinamide 1500 mg once daily.
Syphilis is well known as the great masquerader due to its many possible manifestations. Many patients present with typical palmar and plantar dermatoses.1 Other documented SS presentations include eruptions ranging from a few to diffusely disseminated maculopapular lesions with or without scale on the trunk and upper extremities; pustular and nodular lesions of the face; alopecia; grayish white patches on the oral mucosa; and ulcerative, psoriasiform, follicular, and lichenoid lesions.2 Cutaneous SS has not been commonly reported in a localized distribution to the beard area with a clinical appearance mimicking hyperkeratotic MC lesions.3 Secondary syphilis is not known to spread through autoinoculation, presumably from shaving (as in our case), as might occur with other cutaneous infectious processes such as MC, verruca vulgaris, S aureus, and dermatophytosis in the beard area.
The differential diagnosis for hyperkeratotic papules and nodules localized to the beard area in human immunodeficiency virus–infected males includes MC, verruca vulgaris, chronic verrucous varicella-zoster virus, crusted scabies, tuberculosis verrucosa cutis, hypertrophic lichen planus, and disseminated deep fungal infections including cryptococcosis and coccidioidomycosis. In the setting of immunosuppression, the diagnosis of hyperkeratotic MC was favored in our patient given the co-location of classic umbilicated MC lesions with the hyperkeratotic papules and nodules. It is common to see MC autoinoculated in the beard area in men from shaving, as well as for MC to present in an atypical manner, particularly as hyperkeratotic lesions, in patients with AIDS.4 The predominant localized beard distribution and lack of other mucocutaneous manifestations of SS at presentation supported a clinical diagnosis of hyperkeratotic MC in our patient.
Unique presentations of SS have been documented, including nodular lesions of the face, but they typically have been accompanied by other stigmata of SS such as the classic palmoplantar or truncal maculopapular rash.3 One notable difference in our case was the localized beard distribution of the syphilitic cutaneous lesions in a man with AIDS. Our case reinforces the importance of cutaneous biopsies in immunocompromised patients. It is known that SS spreads hematogenously; however, in our case it was suspected that the new lesions may have spread locally through autoinoculation via beard hair removal, as the hyperkeratotic lesions were limited to the beard area. Koebnerization secondary to trauma induced by beard hair removal was considered in this case; however, koebnerization is known to occur in noninfectious dermatologic conditions, such as psoriasis, lichen planus, lichen nitidus, and vitiligo, but not in infections such as syphilis. Our case is pivotal in raising the question of whether SS can be autoinoculated in the beard area.
- Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18:205-216.
- Dourmishev LA, Dourmishev AL. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Cohen SE, Klausner JD, Engelman J, et al. Syphilis in the modern era: an update for physicians. Infect Dis Clin North Am. 2013;27:705-722.
- Filo-Rogulska M, Pindycka-Plaszcznska M, Januszewski K, et al. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol. 2013;30:56-58.
To the Editor:
A 46-year-old man with a history of AIDS (viral load, 28,186 copies/mL; CD4 count, 22 cells/μL) presented with a 40-lb weight loss over the last 6 months as well as dysphagia and a new-onset pruritic facial eruption of 1 week’s duration. The facial lesions quickly spread to involve the beard area and the upper neck. His medical history was notable for nicotine dependence, seborrheic dermatitis, molluscum contagiosum (MC), treated neurosyphilis and latent tuberculosis, hypertension, a liver mass suspected to be a hemangioma, and erythrocytosis. He was diagnosed with human immunodeficiency virus infection 19 years prior to presentation and was not compliant with the prescribed highly active antiretroviral therapy.
Skin examination revealed multiple discrete and coalescing, 2- to 12-mm, nonumbilicated, hyperkeratotic papules and nodules localized to the left and right beard areas (Figure 1A). A few discrete, 2- to 5-mm, umbilicated papules were noted in the right beard area (Figure 1B), as well as on the right side of the neck (Figure 1C), buttocks, and legs. Mild erythema with yellow-white scale was present in the alar creases. Examination of the oropharyngeal mucosa revealed multiple thick white plaques that were easily scraped off with a tongue depressor. Examination of the palms, soles, and anogenital areas was normal.
A punch biopsy of a nonumbilicated hyperkeratotic papule from the left beard area demonstrated spongiosis; neutrophilic microabscess formation; plasma cells; and a superficial and deep perivascular, predominantly lymphohistiocytic infiltrate (Figure 2A). Spirochete immunohistochemical staining of tissue highlighted abundant organisms in the dermoepidermal junction and vascular endothelial cells (Figure 2B). Other tissue stains for bacteria, including acid-fast bacilli, and fungi were negative. Bacterial culture of tissue from the lesion in the left beard area grew Staphylococcus aureus. Results of acid-fast and fungal cultures of tissue were negative. Shave biopsy of the umbilicated papule on the right side of the neck demonstrated classic invagination of the epidermis into the dermis and intracytoplasmic viral inclusions consistent with MC (Figure 2C). Spirochete immunohistochemical staining of the same tissue sample was negative (Figure 2D).
Serum rapid plasma reagin was reactive with a titer of 1:128 compared to the last known reactive rapid plasma reagin titer of 1:1 five years prior to presentation. A fluorescent treponemal antibody absorption test and VDRL test of cerebrospinal fluid was nonreactive. Fungal, bacterial, and acid-fast cultures of cerebral spinal fluid and a cryptococcal antigen test were negative. Serum cryptococcal antigen and coccidioides complement fixation tests were negative. Cytomegalovirus plasma polymerase chain reaction and urine histoplasma antigen testing were negative. Computed tomography of the chest revealed a new 1.9×1.6×2.1-cm3 cavitary lesion with distal tree-in-bud opacities in the lingula of the left lung. Acid-fast blood culture was negative, and acid-fast sputum culture was positive for Mycobacterium kansasii.
The cutaneous pathology findings and serologic findings confirmed the diagnoses of cutaneous secondary syphilis (SS) in the beard area and MC on the right side of the neck. Clinical diagnoses of seborrheic dermatitis of the alar creases and esophageal candidiasis also were made. The patient was treated with intramuscular penicillin G 2.4 million U once weekly for 3 weeks. The lesions confined to the beard area rapidly resolved within 7 days after the first dose of antibiotics, which further supported the diagnosis of localized cutaneous SS. Fluconazole 100 mg once daily was prescribed for the esophageal candidiasis, and he also was started on a regimen of rifampin 600 mg once daily, isoniazid 300 mg once daily, ethambutol 1200 mg once daily, and pyrazinamide 1500 mg once daily.
Syphilis is well known as the great masquerader due to its many possible manifestations. Many patients present with typical palmar and plantar dermatoses.1 Other documented SS presentations include eruptions ranging from a few to diffusely disseminated maculopapular lesions with or without scale on the trunk and upper extremities; pustular and nodular lesions of the face; alopecia; grayish white patches on the oral mucosa; and ulcerative, psoriasiform, follicular, and lichenoid lesions.2 Cutaneous SS has not been commonly reported in a localized distribution to the beard area with a clinical appearance mimicking hyperkeratotic MC lesions.3 Secondary syphilis is not known to spread through autoinoculation, presumably from shaving (as in our case), as might occur with other cutaneous infectious processes such as MC, verruca vulgaris, S aureus, and dermatophytosis in the beard area.
The differential diagnosis for hyperkeratotic papules and nodules localized to the beard area in human immunodeficiency virus–infected males includes MC, verruca vulgaris, chronic verrucous varicella-zoster virus, crusted scabies, tuberculosis verrucosa cutis, hypertrophic lichen planus, and disseminated deep fungal infections including cryptococcosis and coccidioidomycosis. In the setting of immunosuppression, the diagnosis of hyperkeratotic MC was favored in our patient given the co-location of classic umbilicated MC lesions with the hyperkeratotic papules and nodules. It is common to see MC autoinoculated in the beard area in men from shaving, as well as for MC to present in an atypical manner, particularly as hyperkeratotic lesions, in patients with AIDS.4 The predominant localized beard distribution and lack of other mucocutaneous manifestations of SS at presentation supported a clinical diagnosis of hyperkeratotic MC in our patient.
Unique presentations of SS have been documented, including nodular lesions of the face, but they typically have been accompanied by other stigmata of SS such as the classic palmoplantar or truncal maculopapular rash.3 One notable difference in our case was the localized beard distribution of the syphilitic cutaneous lesions in a man with AIDS. Our case reinforces the importance of cutaneous biopsies in immunocompromised patients. It is known that SS spreads hematogenously; however, in our case it was suspected that the new lesions may have spread locally through autoinoculation via beard hair removal, as the hyperkeratotic lesions were limited to the beard area. Koebnerization secondary to trauma induced by beard hair removal was considered in this case; however, koebnerization is known to occur in noninfectious dermatologic conditions, such as psoriasis, lichen planus, lichen nitidus, and vitiligo, but not in infections such as syphilis. Our case is pivotal in raising the question of whether SS can be autoinoculated in the beard area.
To the Editor:
A 46-year-old man with a history of AIDS (viral load, 28,186 copies/mL; CD4 count, 22 cells/μL) presented with a 40-lb weight loss over the last 6 months as well as dysphagia and a new-onset pruritic facial eruption of 1 week’s duration. The facial lesions quickly spread to involve the beard area and the upper neck. His medical history was notable for nicotine dependence, seborrheic dermatitis, molluscum contagiosum (MC), treated neurosyphilis and latent tuberculosis, hypertension, a liver mass suspected to be a hemangioma, and erythrocytosis. He was diagnosed with human immunodeficiency virus infection 19 years prior to presentation and was not compliant with the prescribed highly active antiretroviral therapy.
Skin examination revealed multiple discrete and coalescing, 2- to 12-mm, nonumbilicated, hyperkeratotic papules and nodules localized to the left and right beard areas (Figure 1A). A few discrete, 2- to 5-mm, umbilicated papules were noted in the right beard area (Figure 1B), as well as on the right side of the neck (Figure 1C), buttocks, and legs. Mild erythema with yellow-white scale was present in the alar creases. Examination of the oropharyngeal mucosa revealed multiple thick white plaques that were easily scraped off with a tongue depressor. Examination of the palms, soles, and anogenital areas was normal.
A punch biopsy of a nonumbilicated hyperkeratotic papule from the left beard area demonstrated spongiosis; neutrophilic microabscess formation; plasma cells; and a superficial and deep perivascular, predominantly lymphohistiocytic infiltrate (Figure 2A). Spirochete immunohistochemical staining of tissue highlighted abundant organisms in the dermoepidermal junction and vascular endothelial cells (Figure 2B). Other tissue stains for bacteria, including acid-fast bacilli, and fungi were negative. Bacterial culture of tissue from the lesion in the left beard area grew Staphylococcus aureus. Results of acid-fast and fungal cultures of tissue were negative. Shave biopsy of the umbilicated papule on the right side of the neck demonstrated classic invagination of the epidermis into the dermis and intracytoplasmic viral inclusions consistent with MC (Figure 2C). Spirochete immunohistochemical staining of the same tissue sample was negative (Figure 2D).
Serum rapid plasma reagin was reactive with a titer of 1:128 compared to the last known reactive rapid plasma reagin titer of 1:1 five years prior to presentation. A fluorescent treponemal antibody absorption test and VDRL test of cerebrospinal fluid was nonreactive. Fungal, bacterial, and acid-fast cultures of cerebral spinal fluid and a cryptococcal antigen test were negative. Serum cryptococcal antigen and coccidioides complement fixation tests were negative. Cytomegalovirus plasma polymerase chain reaction and urine histoplasma antigen testing were negative. Computed tomography of the chest revealed a new 1.9×1.6×2.1-cm3 cavitary lesion with distal tree-in-bud opacities in the lingula of the left lung. Acid-fast blood culture was negative, and acid-fast sputum culture was positive for Mycobacterium kansasii.
The cutaneous pathology findings and serologic findings confirmed the diagnoses of cutaneous secondary syphilis (SS) in the beard area and MC on the right side of the neck. Clinical diagnoses of seborrheic dermatitis of the alar creases and esophageal candidiasis also were made. The patient was treated with intramuscular penicillin G 2.4 million U once weekly for 3 weeks. The lesions confined to the beard area rapidly resolved within 7 days after the first dose of antibiotics, which further supported the diagnosis of localized cutaneous SS. Fluconazole 100 mg once daily was prescribed for the esophageal candidiasis, and he also was started on a regimen of rifampin 600 mg once daily, isoniazid 300 mg once daily, ethambutol 1200 mg once daily, and pyrazinamide 1500 mg once daily.
Syphilis is well known as the great masquerader due to its many possible manifestations. Many patients present with typical palmar and plantar dermatoses.1 Other documented SS presentations include eruptions ranging from a few to diffusely disseminated maculopapular lesions with or without scale on the trunk and upper extremities; pustular and nodular lesions of the face; alopecia; grayish white patches on the oral mucosa; and ulcerative, psoriasiform, follicular, and lichenoid lesions.2 Cutaneous SS has not been commonly reported in a localized distribution to the beard area with a clinical appearance mimicking hyperkeratotic MC lesions.3 Secondary syphilis is not known to spread through autoinoculation, presumably from shaving (as in our case), as might occur with other cutaneous infectious processes such as MC, verruca vulgaris, S aureus, and dermatophytosis in the beard area.
The differential diagnosis for hyperkeratotic papules and nodules localized to the beard area in human immunodeficiency virus–infected males includes MC, verruca vulgaris, chronic verrucous varicella-zoster virus, crusted scabies, tuberculosis verrucosa cutis, hypertrophic lichen planus, and disseminated deep fungal infections including cryptococcosis and coccidioidomycosis. In the setting of immunosuppression, the diagnosis of hyperkeratotic MC was favored in our patient given the co-location of classic umbilicated MC lesions with the hyperkeratotic papules and nodules. It is common to see MC autoinoculated in the beard area in men from shaving, as well as for MC to present in an atypical manner, particularly as hyperkeratotic lesions, in patients with AIDS.4 The predominant localized beard distribution and lack of other mucocutaneous manifestations of SS at presentation supported a clinical diagnosis of hyperkeratotic MC in our patient.
Unique presentations of SS have been documented, including nodular lesions of the face, but they typically have been accompanied by other stigmata of SS such as the classic palmoplantar or truncal maculopapular rash.3 One notable difference in our case was the localized beard distribution of the syphilitic cutaneous lesions in a man with AIDS. Our case reinforces the importance of cutaneous biopsies in immunocompromised patients. It is known that SS spreads hematogenously; however, in our case it was suspected that the new lesions may have spread locally through autoinoculation via beard hair removal, as the hyperkeratotic lesions were limited to the beard area. Koebnerization secondary to trauma induced by beard hair removal was considered in this case; however, koebnerization is known to occur in noninfectious dermatologic conditions, such as psoriasis, lichen planus, lichen nitidus, and vitiligo, but not in infections such as syphilis. Our case is pivotal in raising the question of whether SS can be autoinoculated in the beard area.
- Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18:205-216.
- Dourmishev LA, Dourmishev AL. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Cohen SE, Klausner JD, Engelman J, et al. Syphilis in the modern era: an update for physicians. Infect Dis Clin North Am. 2013;27:705-722.
- Filo-Rogulska M, Pindycka-Plaszcznska M, Januszewski K, et al. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol. 2013;30:56-58.
- Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18:205-216.
- Dourmishev LA, Dourmishev AL. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Cohen SE, Klausner JD, Engelman J, et al. Syphilis in the modern era: an update for physicians. Infect Dis Clin North Am. 2013;27:705-722.
- Filo-Rogulska M, Pindycka-Plaszcznska M, Januszewski K, et al. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol. 2013;30:56-58.
Practice Points
- Recognize typical and atypical presentations of secondary syphilis (SS).
- This case reinforces the importance of cutaneous biopsies in immunocompromised patients.
- Consider the possibility of autoinoculation in SS.
Readmission for COPD exacerbation upped in-hospital mortality risk
NEW ORLEANS – Reduction of readmission rates among individuals hospitalized for an acute exacerbation of COPD could reduce mortality and health care expenditures, results of a large, retrospective study suggest.
said researcher Anand Muthu Krishnan, MBBS, an from the University of Connecticut, Farmington.
“This is not a small problem,” Dr. Krishnan said in a podium presentation at the annual meeting of the American College of Chest Physicians. “The amount of money that can be saved can be put into primary care for curbing COPD and better patient outcomes, basically, if you’re able to put in checkpoints to stop this problem.”
Bundled care interventions by interdisciplinary teams have thus far proven effective at improving quality of care and improving process measures in this setting, said Dr. Krishnan.
The retrospective cohort study by Dr. Krishnan and colleagues included 530,229 adult patients in the 2016 National Readmission Database who had a principal diagnosis of acute COPD exacerbation. The mean age of the patients was 68 years, and 58% were female.
The rates of readmission at 30 days after discharge were 16.3% for any cause and 5.4% specifically for COPD, the researchers found. Of note, the in-hospital mortality rate increased from 1.1% to 3.8% during readmission (P less than .01), Dr. Krishnan said.
Readmissions were linked to a cumulative length of stay of 458,677 days, with corresponding hospital costs of $0.97 billion and charges of $4.0 billion; the COPD-specific readmissions were associated with cumulative length of stay of 132,026 days, costs of $253 million, and charges of $1 billion, Dr. Krishnan reported.
Dr. Krishnan and coauthors disclosed no relationships relevant to their study.
SOURCE: Krishnan AM et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.229.
NEW ORLEANS – Reduction of readmission rates among individuals hospitalized for an acute exacerbation of COPD could reduce mortality and health care expenditures, results of a large, retrospective study suggest.
said researcher Anand Muthu Krishnan, MBBS, an from the University of Connecticut, Farmington.
“This is not a small problem,” Dr. Krishnan said in a podium presentation at the annual meeting of the American College of Chest Physicians. “The amount of money that can be saved can be put into primary care for curbing COPD and better patient outcomes, basically, if you’re able to put in checkpoints to stop this problem.”
Bundled care interventions by interdisciplinary teams have thus far proven effective at improving quality of care and improving process measures in this setting, said Dr. Krishnan.
The retrospective cohort study by Dr. Krishnan and colleagues included 530,229 adult patients in the 2016 National Readmission Database who had a principal diagnosis of acute COPD exacerbation. The mean age of the patients was 68 years, and 58% were female.
The rates of readmission at 30 days after discharge were 16.3% for any cause and 5.4% specifically for COPD, the researchers found. Of note, the in-hospital mortality rate increased from 1.1% to 3.8% during readmission (P less than .01), Dr. Krishnan said.
Readmissions were linked to a cumulative length of stay of 458,677 days, with corresponding hospital costs of $0.97 billion and charges of $4.0 billion; the COPD-specific readmissions were associated with cumulative length of stay of 132,026 days, costs of $253 million, and charges of $1 billion, Dr. Krishnan reported.
Dr. Krishnan and coauthors disclosed no relationships relevant to their study.
SOURCE: Krishnan AM et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.229.
NEW ORLEANS – Reduction of readmission rates among individuals hospitalized for an acute exacerbation of COPD could reduce mortality and health care expenditures, results of a large, retrospective study suggest.
said researcher Anand Muthu Krishnan, MBBS, an from the University of Connecticut, Farmington.
“This is not a small problem,” Dr. Krishnan said in a podium presentation at the annual meeting of the American College of Chest Physicians. “The amount of money that can be saved can be put into primary care for curbing COPD and better patient outcomes, basically, if you’re able to put in checkpoints to stop this problem.”
Bundled care interventions by interdisciplinary teams have thus far proven effective at improving quality of care and improving process measures in this setting, said Dr. Krishnan.
The retrospective cohort study by Dr. Krishnan and colleagues included 530,229 adult patients in the 2016 National Readmission Database who had a principal diagnosis of acute COPD exacerbation. The mean age of the patients was 68 years, and 58% were female.
The rates of readmission at 30 days after discharge were 16.3% for any cause and 5.4% specifically for COPD, the researchers found. Of note, the in-hospital mortality rate increased from 1.1% to 3.8% during readmission (P less than .01), Dr. Krishnan said.
Readmissions were linked to a cumulative length of stay of 458,677 days, with corresponding hospital costs of $0.97 billion and charges of $4.0 billion; the COPD-specific readmissions were associated with cumulative length of stay of 132,026 days, costs of $253 million, and charges of $1 billion, Dr. Krishnan reported.
Dr. Krishnan and coauthors disclosed no relationships relevant to their study.
SOURCE: Krishnan AM et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.229.
REPORTING FROM CHEST 2019
Erythematous Plaques and Nodules on the Abdomen and Groin
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.
The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.
The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.
The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
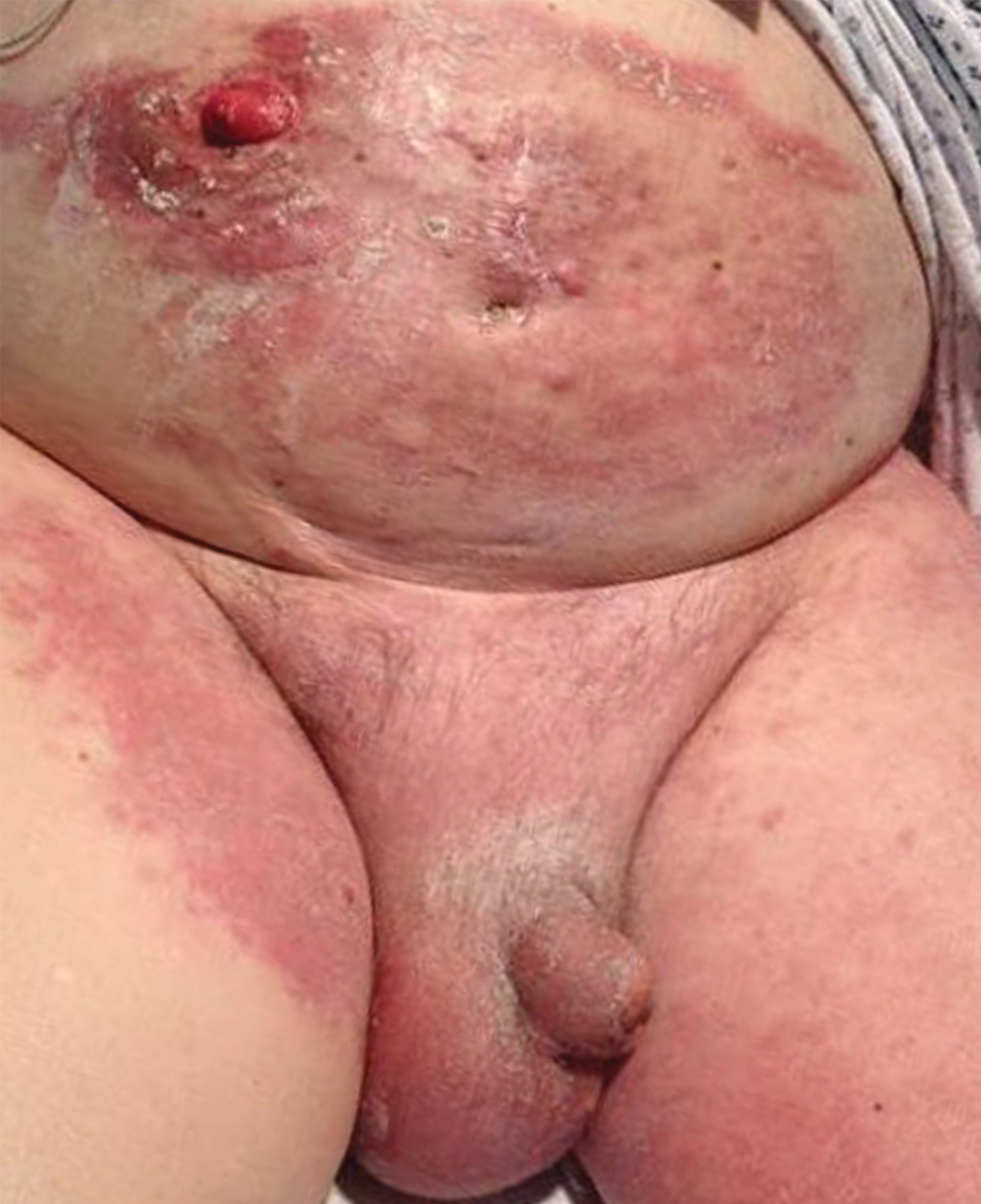
An 82-year-old man presented with acute abdominal pain and distension as well as an abdominal rash of 4 months' duration that was expanding despite treatment with topical miconazole. He had a history of melanoma and bladder cancer treated with cystoprostatectomy. He previously was diagnosed with candidiasis of his urostomy and was taking oral fluconazole. Physical examination revealed a large, well-demarcated, erythematous, smooth plaque covering the entire abdomen, scrotum, penis, inguinal folds, and bilateral upper thighs, with several satellite plaques and firm nodules clustered around the umbilicus. An 8-mm punch biopsy of a periumbilical nodule was performed.
Starting PCSK9 inhibitor in acute-phase ACS under study
PARIS – The first-ever randomized trial of in-hospital initiation of a PCSK9 inhibitor on top of guideline-recommended high-intensity statin therapy in the very-high-risk acute phase of an acute coronary syndrome (ACS) safely resulted in dramatically lower LDL cholesterol levels than with early prescribing of a high-intensity statin alone, Konstantinos C. Koskinas, MD, reported at the annual congress of the European Society of Cardiology.
compared with 11% of patients randomized to high-intensity atorvastatin at 40 mg/day plus placebo injections. Moreover, 96% of patients on atorvastatin 40 mg/day plus evolocumab at 420 mg per subcutaneous injection were below the former target of an LDL cholesterol less than 70 mg/dL, as were 38% of those on the high-intensity statin alone, according to Dr. Koskinas, a cardiologist at the University of Bern (Switzerland).
The seven-center Swiss EVOPACS trial, featuring 308 ACS patients, could be considered a proof-of-concept study, as it lacked the size and duration to be powered to assess clinical outcomes.
“The clinical impact of very early LDL lowering with evolocumab initiated in the acute setting of ACS warrants further investigation in a dedicated cardiovascular outcomes trial,” Dr. Koskinas asserted. “We see this as the natural next step. Discussions are underway about a long-term trial with clinical endpoints, but no decisions have been made.”
The rationale for the EVOPACS trial is based upon current standard practice in ACS management, which includes initiation of a high-intensity statin during the acute phase of ACS, a particularly high-risk period for recurrent events. This practice has a Class IA recommendation in the guidelines based on published evidence that it results in a significantly reduced rate of the composite of death, MI, or rehospitalization for ACS within 30 days, compared with a less aggressive approach to LDL cholesterol lowering.
Yet even though the PCSK9 inhibitors are the 800-lb gorillas of LDL cholesterol lowering, they’ve never been tested in the setting of acute-phase ACS. For example, in the landmark ODYSSEY OUTCOMES trial, alirocumab was initiated on average 2.6 months after ACS, while in FOURIER the lag time between ACS and the start of evolocumab was 3.4 years, the cardiologist noted.
In contrast, all of the 37% of EVOPACS participants with an ST-segment elevation MI were enrolled in the study and on treatment within 24 hours after symptom onset. So were more than one-third of those with non–ST-elevation ACS, with the remainder getting onboard 24-72 hours after symptom onset.
The safety and tolerability of dual LDL cholesterol–lowering therapy were excellent in the brief EVOPACS study. There were no significant between-group differences in adverse events or serious adverse events, nor in prespecified events of special interest, including muscle pain, neurocognitive changes, or elevated liver enzyme levels.
The LDL cholesterol lowering achieved with dual therapy in EVOPACS was jaw dropping: Over the course of 8 weeks, the mean LDL cholesterol went from 132 to 31 mg/dL. In patients on early high-intensity atorvastatin alone, LDL cholesterol went from 139 to 80 mg/dL.
The full details of the EVOPACS trial have been published (J Am Coll Cardiol. 2019 Aug 16. doi: 10.1016/j.jacc.2019.08.010.
The trial was funded by Amgen. Dr. Koskinas reported receiving honoraria from Amgen and Sanofi.
PARIS – The first-ever randomized trial of in-hospital initiation of a PCSK9 inhibitor on top of guideline-recommended high-intensity statin therapy in the very-high-risk acute phase of an acute coronary syndrome (ACS) safely resulted in dramatically lower LDL cholesterol levels than with early prescribing of a high-intensity statin alone, Konstantinos C. Koskinas, MD, reported at the annual congress of the European Society of Cardiology.
compared with 11% of patients randomized to high-intensity atorvastatin at 40 mg/day plus placebo injections. Moreover, 96% of patients on atorvastatin 40 mg/day plus evolocumab at 420 mg per subcutaneous injection were below the former target of an LDL cholesterol less than 70 mg/dL, as were 38% of those on the high-intensity statin alone, according to Dr. Koskinas, a cardiologist at the University of Bern (Switzerland).
The seven-center Swiss EVOPACS trial, featuring 308 ACS patients, could be considered a proof-of-concept study, as it lacked the size and duration to be powered to assess clinical outcomes.
“The clinical impact of very early LDL lowering with evolocumab initiated in the acute setting of ACS warrants further investigation in a dedicated cardiovascular outcomes trial,” Dr. Koskinas asserted. “We see this as the natural next step. Discussions are underway about a long-term trial with clinical endpoints, but no decisions have been made.”
The rationale for the EVOPACS trial is based upon current standard practice in ACS management, which includes initiation of a high-intensity statin during the acute phase of ACS, a particularly high-risk period for recurrent events. This practice has a Class IA recommendation in the guidelines based on published evidence that it results in a significantly reduced rate of the composite of death, MI, or rehospitalization for ACS within 30 days, compared with a less aggressive approach to LDL cholesterol lowering.
Yet even though the PCSK9 inhibitors are the 800-lb gorillas of LDL cholesterol lowering, they’ve never been tested in the setting of acute-phase ACS. For example, in the landmark ODYSSEY OUTCOMES trial, alirocumab was initiated on average 2.6 months after ACS, while in FOURIER the lag time between ACS and the start of evolocumab was 3.4 years, the cardiologist noted.
In contrast, all of the 37% of EVOPACS participants with an ST-segment elevation MI were enrolled in the study and on treatment within 24 hours after symptom onset. So were more than one-third of those with non–ST-elevation ACS, with the remainder getting onboard 24-72 hours after symptom onset.
The safety and tolerability of dual LDL cholesterol–lowering therapy were excellent in the brief EVOPACS study. There were no significant between-group differences in adverse events or serious adverse events, nor in prespecified events of special interest, including muscle pain, neurocognitive changes, or elevated liver enzyme levels.
The LDL cholesterol lowering achieved with dual therapy in EVOPACS was jaw dropping: Over the course of 8 weeks, the mean LDL cholesterol went from 132 to 31 mg/dL. In patients on early high-intensity atorvastatin alone, LDL cholesterol went from 139 to 80 mg/dL.
The full details of the EVOPACS trial have been published (J Am Coll Cardiol. 2019 Aug 16. doi: 10.1016/j.jacc.2019.08.010.
The trial was funded by Amgen. Dr. Koskinas reported receiving honoraria from Amgen and Sanofi.
PARIS – The first-ever randomized trial of in-hospital initiation of a PCSK9 inhibitor on top of guideline-recommended high-intensity statin therapy in the very-high-risk acute phase of an acute coronary syndrome (ACS) safely resulted in dramatically lower LDL cholesterol levels than with early prescribing of a high-intensity statin alone, Konstantinos C. Koskinas, MD, reported at the annual congress of the European Society of Cardiology.
compared with 11% of patients randomized to high-intensity atorvastatin at 40 mg/day plus placebo injections. Moreover, 96% of patients on atorvastatin 40 mg/day plus evolocumab at 420 mg per subcutaneous injection were below the former target of an LDL cholesterol less than 70 mg/dL, as were 38% of those on the high-intensity statin alone, according to Dr. Koskinas, a cardiologist at the University of Bern (Switzerland).
The seven-center Swiss EVOPACS trial, featuring 308 ACS patients, could be considered a proof-of-concept study, as it lacked the size and duration to be powered to assess clinical outcomes.
“The clinical impact of very early LDL lowering with evolocumab initiated in the acute setting of ACS warrants further investigation in a dedicated cardiovascular outcomes trial,” Dr. Koskinas asserted. “We see this as the natural next step. Discussions are underway about a long-term trial with clinical endpoints, but no decisions have been made.”
The rationale for the EVOPACS trial is based upon current standard practice in ACS management, which includes initiation of a high-intensity statin during the acute phase of ACS, a particularly high-risk period for recurrent events. This practice has a Class IA recommendation in the guidelines based on published evidence that it results in a significantly reduced rate of the composite of death, MI, or rehospitalization for ACS within 30 days, compared with a less aggressive approach to LDL cholesterol lowering.
Yet even though the PCSK9 inhibitors are the 800-lb gorillas of LDL cholesterol lowering, they’ve never been tested in the setting of acute-phase ACS. For example, in the landmark ODYSSEY OUTCOMES trial, alirocumab was initiated on average 2.6 months after ACS, while in FOURIER the lag time between ACS and the start of evolocumab was 3.4 years, the cardiologist noted.
In contrast, all of the 37% of EVOPACS participants with an ST-segment elevation MI were enrolled in the study and on treatment within 24 hours after symptom onset. So were more than one-third of those with non–ST-elevation ACS, with the remainder getting onboard 24-72 hours after symptom onset.
The safety and tolerability of dual LDL cholesterol–lowering therapy were excellent in the brief EVOPACS study. There were no significant between-group differences in adverse events or serious adverse events, nor in prespecified events of special interest, including muscle pain, neurocognitive changes, or elevated liver enzyme levels.
The LDL cholesterol lowering achieved with dual therapy in EVOPACS was jaw dropping: Over the course of 8 weeks, the mean LDL cholesterol went from 132 to 31 mg/dL. In patients on early high-intensity atorvastatin alone, LDL cholesterol went from 139 to 80 mg/dL.
The full details of the EVOPACS trial have been published (J Am Coll Cardiol. 2019 Aug 16. doi: 10.1016/j.jacc.2019.08.010.
The trial was funded by Amgen. Dr. Koskinas reported receiving honoraria from Amgen and Sanofi.
REPORTING FROM THE ESC CONGRESS 2019
Preconception marijuana use by male partner raises spontaneous abortion risk
PHILADELPHIA – compared with infrequent use or no use of marijuana by the male partner, Alyssa F. Harlow, MPH, reported at the annual meeting of the American Society for Reproductive Medicine.
The male partner’s use of marijuana “one or more times per week in the past 2 months during the preconception period in our study was associated with an increased risk of spontaneous abortion,” said Ms. Harlow, a PhD candidate at Boston University. “The association attenuated for later pregnancy losses, and persisted for those with shorter [pregnancy] attempt time at [study] entry.”
Ms. Harlow and colleagues prospectively collected data from 1,535 couples in the Pregnancy Study Online (PRESTO) study, a preconception cohort study examining risk factors for adverse pregnancy outcomes. PRESTO enrolled women aged 21-45 years and their male partners aged 21 years or older who were attempting to conceive without the use of fertility treatment.
The researchers administered a screening and baseline questionnaire to the women, who then included their male partners in the study. The male partners completed their own baseline questionnaire that asked about demographics, medical history, and lifestyle or behavioral factors including marijuana use. The questions centering around marijuana use asked whether the partner had used marijuana within the past 2 months, and the frequency of marijuana use during that period.
Women in PRESTO were followed every 8 weeks until a pregnancy occurred, or up to 12 months if no pregnancy occurred. If they became pregnant, the women were asked additional questions at less than 12 weeks’ gestation and then again at 32 weeks’ gestation, including questions about any miscarriages, and how long a pregnancy lasted if a miscarriage did occur.
At baseline, 1,267 couples (83%) reported no marijuana use by male partners, 140 couples (9%) reported use less than 1 time per week, and 128 couples (8%) reported marijuana use at least 1 time per week. Men at baseline were similar in age and body mass index among groups, but men who used marijuana were more likely to be cigarette smokers (24% vs. 4%), were more likely to have partners who were cigarette smokers (11% vs. 2%), and were more likely to have partners who use marijuana (43% vs. 3%), compared with couples where the male partners did not use marijuana. Male partners who used marijuana also were less likely to be taking a daily multivitamin (25% vs. 37%), and were more likely to have been diagnosed with anxiety (14% vs. 7%) or depression (20% vs. 9%) compared with male partners who did not use marijuana.
Overall, 269 spontaneous abortions (17.5%) occurred during the study period, and couples where male partners used marijuana one or more times per week had approximately twice the rate of spontaneous abortions, compared with no marijuana use (hazard ratio, 1.99; 95% confidence interval).
Couples in which men who used marijuana less than 1 time per week had a slightly increased risk of spontaneous abortion, but this did not reach statistical significance.
When the results were adjusted for female nonusers of marijuana, the results were “essentially identical,” said Ms. Harlow.
Couples who were trying to conceive for three or fewer cycles at baseline (1,045 couples) had a lower rate of spontaneous abortion than that of couples trying for three or more cycles (490 couples). When the results were stratified by gestational age at loss, the results persisted for couples with a pregnancy loss at less than 8 weeks (1,533 couples), but the effect of marijuana use was reduced for couples with a loss at 8 weeks or more (1,113 couples).
Ms. Harlow noted several limitations to the study, including lack of data on time-varying marijuana use, potential selection bias, and residual confounding. There also is likely misclassification of exposure among some participants because marijuana use was self-reported, she added.
Ms. Harlow reported no relevant conflicts of interest.
SOURCE: Harlow AF et al. ASRM 2019. Abstract O-4.
PHILADELPHIA – compared with infrequent use or no use of marijuana by the male partner, Alyssa F. Harlow, MPH, reported at the annual meeting of the American Society for Reproductive Medicine.
The male partner’s use of marijuana “one or more times per week in the past 2 months during the preconception period in our study was associated with an increased risk of spontaneous abortion,” said Ms. Harlow, a PhD candidate at Boston University. “The association attenuated for later pregnancy losses, and persisted for those with shorter [pregnancy] attempt time at [study] entry.”
Ms. Harlow and colleagues prospectively collected data from 1,535 couples in the Pregnancy Study Online (PRESTO) study, a preconception cohort study examining risk factors for adverse pregnancy outcomes. PRESTO enrolled women aged 21-45 years and their male partners aged 21 years or older who were attempting to conceive without the use of fertility treatment.
The researchers administered a screening and baseline questionnaire to the women, who then included their male partners in the study. The male partners completed their own baseline questionnaire that asked about demographics, medical history, and lifestyle or behavioral factors including marijuana use. The questions centering around marijuana use asked whether the partner had used marijuana within the past 2 months, and the frequency of marijuana use during that period.
Women in PRESTO were followed every 8 weeks until a pregnancy occurred, or up to 12 months if no pregnancy occurred. If they became pregnant, the women were asked additional questions at less than 12 weeks’ gestation and then again at 32 weeks’ gestation, including questions about any miscarriages, and how long a pregnancy lasted if a miscarriage did occur.
At baseline, 1,267 couples (83%) reported no marijuana use by male partners, 140 couples (9%) reported use less than 1 time per week, and 128 couples (8%) reported marijuana use at least 1 time per week. Men at baseline were similar in age and body mass index among groups, but men who used marijuana were more likely to be cigarette smokers (24% vs. 4%), were more likely to have partners who were cigarette smokers (11% vs. 2%), and were more likely to have partners who use marijuana (43% vs. 3%), compared with couples where the male partners did not use marijuana. Male partners who used marijuana also were less likely to be taking a daily multivitamin (25% vs. 37%), and were more likely to have been diagnosed with anxiety (14% vs. 7%) or depression (20% vs. 9%) compared with male partners who did not use marijuana.
Overall, 269 spontaneous abortions (17.5%) occurred during the study period, and couples where male partners used marijuana one or more times per week had approximately twice the rate of spontaneous abortions, compared with no marijuana use (hazard ratio, 1.99; 95% confidence interval).
Couples in which men who used marijuana less than 1 time per week had a slightly increased risk of spontaneous abortion, but this did not reach statistical significance.
When the results were adjusted for female nonusers of marijuana, the results were “essentially identical,” said Ms. Harlow.
Couples who were trying to conceive for three or fewer cycles at baseline (1,045 couples) had a lower rate of spontaneous abortion than that of couples trying for three or more cycles (490 couples). When the results were stratified by gestational age at loss, the results persisted for couples with a pregnancy loss at less than 8 weeks (1,533 couples), but the effect of marijuana use was reduced for couples with a loss at 8 weeks or more (1,113 couples).
Ms. Harlow noted several limitations to the study, including lack of data on time-varying marijuana use, potential selection bias, and residual confounding. There also is likely misclassification of exposure among some participants because marijuana use was self-reported, she added.
Ms. Harlow reported no relevant conflicts of interest.
SOURCE: Harlow AF et al. ASRM 2019. Abstract O-4.
PHILADELPHIA – compared with infrequent use or no use of marijuana by the male partner, Alyssa F. Harlow, MPH, reported at the annual meeting of the American Society for Reproductive Medicine.
The male partner’s use of marijuana “one or more times per week in the past 2 months during the preconception period in our study was associated with an increased risk of spontaneous abortion,” said Ms. Harlow, a PhD candidate at Boston University. “The association attenuated for later pregnancy losses, and persisted for those with shorter [pregnancy] attempt time at [study] entry.”
Ms. Harlow and colleagues prospectively collected data from 1,535 couples in the Pregnancy Study Online (PRESTO) study, a preconception cohort study examining risk factors for adverse pregnancy outcomes. PRESTO enrolled women aged 21-45 years and their male partners aged 21 years or older who were attempting to conceive without the use of fertility treatment.
The researchers administered a screening and baseline questionnaire to the women, who then included their male partners in the study. The male partners completed their own baseline questionnaire that asked about demographics, medical history, and lifestyle or behavioral factors including marijuana use. The questions centering around marijuana use asked whether the partner had used marijuana within the past 2 months, and the frequency of marijuana use during that period.
Women in PRESTO were followed every 8 weeks until a pregnancy occurred, or up to 12 months if no pregnancy occurred. If they became pregnant, the women were asked additional questions at less than 12 weeks’ gestation and then again at 32 weeks’ gestation, including questions about any miscarriages, and how long a pregnancy lasted if a miscarriage did occur.
At baseline, 1,267 couples (83%) reported no marijuana use by male partners, 140 couples (9%) reported use less than 1 time per week, and 128 couples (8%) reported marijuana use at least 1 time per week. Men at baseline were similar in age and body mass index among groups, but men who used marijuana were more likely to be cigarette smokers (24% vs. 4%), were more likely to have partners who were cigarette smokers (11% vs. 2%), and were more likely to have partners who use marijuana (43% vs. 3%), compared with couples where the male partners did not use marijuana. Male partners who used marijuana also were less likely to be taking a daily multivitamin (25% vs. 37%), and were more likely to have been diagnosed with anxiety (14% vs. 7%) or depression (20% vs. 9%) compared with male partners who did not use marijuana.
Overall, 269 spontaneous abortions (17.5%) occurred during the study period, and couples where male partners used marijuana one or more times per week had approximately twice the rate of spontaneous abortions, compared with no marijuana use (hazard ratio, 1.99; 95% confidence interval).
Couples in which men who used marijuana less than 1 time per week had a slightly increased risk of spontaneous abortion, but this did not reach statistical significance.
When the results were adjusted for female nonusers of marijuana, the results were “essentially identical,” said Ms. Harlow.
Couples who were trying to conceive for three or fewer cycles at baseline (1,045 couples) had a lower rate of spontaneous abortion than that of couples trying for three or more cycles (490 couples). When the results were stratified by gestational age at loss, the results persisted for couples with a pregnancy loss at less than 8 weeks (1,533 couples), but the effect of marijuana use was reduced for couples with a loss at 8 weeks or more (1,113 couples).
Ms. Harlow noted several limitations to the study, including lack of data on time-varying marijuana use, potential selection bias, and residual confounding. There also is likely misclassification of exposure among some participants because marijuana use was self-reported, she added.
Ms. Harlow reported no relevant conflicts of interest.
SOURCE: Harlow AF et al. ASRM 2019. Abstract O-4.
REPORTING FROM ASRM 2019
Lifestyle program improves chance of spontaneous conception for women with obesity
PHILADELPHIA – Women with obesity who underwent a lifestyle program targeting healthy eating and physical activity were significantly more likely to achieve pregnancy or become spontaneously pregnant, Jean-Patrice Baillargeon, MD, MSc, reported at the annual meeting of the American Society for Reproductive Medicine.
However, women with polycystic ovary syndrome (PCOS) in the study appeared to benefit more than did women without PCOS who participated in the lifestyle program, said Dr. Baillargeon, from the University of Sherbrooke (Que.).
“ Women with PCOS seemed to benefit more from such a program,” said Dr. Baillargeon.
“These benefits occur along with small changes in weight, but important improvements in lifestyle, so lifestyle seems to be more important than weight change here,” he added.
The researchers randomized 130 women to receive the Fit-For-Fertility lifestyle program or usual care for infertility. The lifestyle program consisted of a low-intensity weekly intervention for 6 weeks in which patients met individually with a kinesiologist and nutritionist every week and also attended group sessions each week. Women in the intervention did not receive fertility treatment for the first 6 months while on the lifestyle program, and if they did not conceive during that time, they continued the program in combination with fertility treatments.
Patients were included if they were aged 18-40 years and had either infertility and a body mass index of 30 kg/m2 or greater or PCOS and a BMI of 27 kg/m2 or greater. Researchers excluded women planning to undergo bariatric surgery, women who were already undergoing another lifestyle intervention, and women with severe infertility or who had a male partner with severe infertility for whom in vitro fertilization was their only option for conceiving. Researchers collected data from patients at baseline and every 6 months up to 18 months, with additional visits for pregnant women scheduled at the beginning of pregnancy and at 26 weeks’ gestation. They collected baseline data on age, BMI, waist circumference, fat mass percentage, daily energy expenditure, and food frequency using the Healthy Eating Index (HEI).
Overall, 46 women in the intervention group and 52 women in the control group had a research visit at 6 months or pregnancy research visit at less than 6 months; of these, 33 women in the intervention group (65%) and 35 women in the control group (61%) had PCOS. At baseline, both PCOS and non-PCOS groups were similar; however, women in the PCOS intervention group had a lower BMI than did women without PCOS in the intervention group (37 kg/m2 vs. 41 kg/m2; P less than .05), while women without PCOS in the intervention group had a higher fat mass percentage than did women with PCOS in the intervention group (46% vs. 49%; P less than .05).
With regard to weight loss, there was a 2.4% reduction in weight among all patients in the intervention group, compared with the control group (P = .003), with a 2.7% reduction in weight for the PCOS group (P = .015) and a 1.8% reduction in the non-PCOS group (P = .139). However, there were no significant differences between PCOS status and the lifestyle intervention, said Dr. Baillargeon.
At 6 months, the quality of women’s diets in the combined PCOS and non-PCOS group that participated in the lifestyle program showed significant improvement, compared with control groups (HEI, 18% vs. 5%; P less than .001). The PCOS group on its own showed significant improvement with the intervention (20% vs. 4%; P less than .001), whereas women without PCOS showed a nonsignificant improvement with the intervention (14% vs. 6%; P = .055). Daily energy expenditure improved in all groups that received the intervention, compared with the control groups, but there were no significant between-group differences in energy expenditure.
When analyzing fertility outcomes at 18 months, the pregnancy rate for all patients who received lifestyle interventions was 61%, compared with 39% in the control group (P = .02; number needed to treat, 4.5). In women with PCOS, those who underwent the lifestyle intervention had a pregnancy rate of 58%, compared with 34% in the control group (P = .05; NNT, 4.3); although women without PCOS who participated in the lifestyle program had an improved pregnancy rate over women in the control group, the results were not significant (67% vs. 46%; P = .18; NNT, 4.7).
The researchers also looked at the spontaneous pregnancy rate and found women who received the intervention had nearly three times the rate of spontaneous pregnancy, compared with women in the control group (33% vs. 12%; P = .01), while women with PCOS in the lifestyle program had nearly five times the rate of spontaneous pregnancy, compared with the control group (27% vs. 6%; P = .02). Women without PCOS in the lifestyle program had nearly twice the increased likelihood of spontaneous pregnancy, but the results were not significant (44% vs. 23%; P = .15).
Women with PCOS in the lifestyle program also had a higher live birth rate, compared with women in the control group (55% vs. 31%; P = .05; NNT, 4.3). Although women without PCOS in the lifestyle program (67% vs. 46%; P = .18; NNT, 4.7) and women in the study overall experienced higher live birth rates (51% vs. 37%; P = .14; NNT, 7.0), compared with the control group, these results were not significant, said Dr. Baillargeon.
“Such lifestyle interventions in women with obesity could significantly lower costs of fertility treatments, which is important,” concluded Dr. Baillargeon.
The Fit-For-Fertility program was funded by an unrestricted grant from Ferring.
SOURCE: Baillargeon J-P, et al. ASRM 2019. Abstract O-95.
PHILADELPHIA – Women with obesity who underwent a lifestyle program targeting healthy eating and physical activity were significantly more likely to achieve pregnancy or become spontaneously pregnant, Jean-Patrice Baillargeon, MD, MSc, reported at the annual meeting of the American Society for Reproductive Medicine.
However, women with polycystic ovary syndrome (PCOS) in the study appeared to benefit more than did women without PCOS who participated in the lifestyle program, said Dr. Baillargeon, from the University of Sherbrooke (Que.).
“ Women with PCOS seemed to benefit more from such a program,” said Dr. Baillargeon.
“These benefits occur along with small changes in weight, but important improvements in lifestyle, so lifestyle seems to be more important than weight change here,” he added.
The researchers randomized 130 women to receive the Fit-For-Fertility lifestyle program or usual care for infertility. The lifestyle program consisted of a low-intensity weekly intervention for 6 weeks in which patients met individually with a kinesiologist and nutritionist every week and also attended group sessions each week. Women in the intervention did not receive fertility treatment for the first 6 months while on the lifestyle program, and if they did not conceive during that time, they continued the program in combination with fertility treatments.
Patients were included if they were aged 18-40 years and had either infertility and a body mass index of 30 kg/m2 or greater or PCOS and a BMI of 27 kg/m2 or greater. Researchers excluded women planning to undergo bariatric surgery, women who were already undergoing another lifestyle intervention, and women with severe infertility or who had a male partner with severe infertility for whom in vitro fertilization was their only option for conceiving. Researchers collected data from patients at baseline and every 6 months up to 18 months, with additional visits for pregnant women scheduled at the beginning of pregnancy and at 26 weeks’ gestation. They collected baseline data on age, BMI, waist circumference, fat mass percentage, daily energy expenditure, and food frequency using the Healthy Eating Index (HEI).
Overall, 46 women in the intervention group and 52 women in the control group had a research visit at 6 months or pregnancy research visit at less than 6 months; of these, 33 women in the intervention group (65%) and 35 women in the control group (61%) had PCOS. At baseline, both PCOS and non-PCOS groups were similar; however, women in the PCOS intervention group had a lower BMI than did women without PCOS in the intervention group (37 kg/m2 vs. 41 kg/m2; P less than .05), while women without PCOS in the intervention group had a higher fat mass percentage than did women with PCOS in the intervention group (46% vs. 49%; P less than .05).
With regard to weight loss, there was a 2.4% reduction in weight among all patients in the intervention group, compared with the control group (P = .003), with a 2.7% reduction in weight for the PCOS group (P = .015) and a 1.8% reduction in the non-PCOS group (P = .139). However, there were no significant differences between PCOS status and the lifestyle intervention, said Dr. Baillargeon.
At 6 months, the quality of women’s diets in the combined PCOS and non-PCOS group that participated in the lifestyle program showed significant improvement, compared with control groups (HEI, 18% vs. 5%; P less than .001). The PCOS group on its own showed significant improvement with the intervention (20% vs. 4%; P less than .001), whereas women without PCOS showed a nonsignificant improvement with the intervention (14% vs. 6%; P = .055). Daily energy expenditure improved in all groups that received the intervention, compared with the control groups, but there were no significant between-group differences in energy expenditure.
When analyzing fertility outcomes at 18 months, the pregnancy rate for all patients who received lifestyle interventions was 61%, compared with 39% in the control group (P = .02; number needed to treat, 4.5). In women with PCOS, those who underwent the lifestyle intervention had a pregnancy rate of 58%, compared with 34% in the control group (P = .05; NNT, 4.3); although women without PCOS who participated in the lifestyle program had an improved pregnancy rate over women in the control group, the results were not significant (67% vs. 46%; P = .18; NNT, 4.7).
The researchers also looked at the spontaneous pregnancy rate and found women who received the intervention had nearly three times the rate of spontaneous pregnancy, compared with women in the control group (33% vs. 12%; P = .01), while women with PCOS in the lifestyle program had nearly five times the rate of spontaneous pregnancy, compared with the control group (27% vs. 6%; P = .02). Women without PCOS in the lifestyle program had nearly twice the increased likelihood of spontaneous pregnancy, but the results were not significant (44% vs. 23%; P = .15).
Women with PCOS in the lifestyle program also had a higher live birth rate, compared with women in the control group (55% vs. 31%; P = .05; NNT, 4.3). Although women without PCOS in the lifestyle program (67% vs. 46%; P = .18; NNT, 4.7) and women in the study overall experienced higher live birth rates (51% vs. 37%; P = .14; NNT, 7.0), compared with the control group, these results were not significant, said Dr. Baillargeon.
“Such lifestyle interventions in women with obesity could significantly lower costs of fertility treatments, which is important,” concluded Dr. Baillargeon.
The Fit-For-Fertility program was funded by an unrestricted grant from Ferring.
SOURCE: Baillargeon J-P, et al. ASRM 2019. Abstract O-95.
PHILADELPHIA – Women with obesity who underwent a lifestyle program targeting healthy eating and physical activity were significantly more likely to achieve pregnancy or become spontaneously pregnant, Jean-Patrice Baillargeon, MD, MSc, reported at the annual meeting of the American Society for Reproductive Medicine.
However, women with polycystic ovary syndrome (PCOS) in the study appeared to benefit more than did women without PCOS who participated in the lifestyle program, said Dr. Baillargeon, from the University of Sherbrooke (Que.).
“ Women with PCOS seemed to benefit more from such a program,” said Dr. Baillargeon.
“These benefits occur along with small changes in weight, but important improvements in lifestyle, so lifestyle seems to be more important than weight change here,” he added.
The researchers randomized 130 women to receive the Fit-For-Fertility lifestyle program or usual care for infertility. The lifestyle program consisted of a low-intensity weekly intervention for 6 weeks in which patients met individually with a kinesiologist and nutritionist every week and also attended group sessions each week. Women in the intervention did not receive fertility treatment for the first 6 months while on the lifestyle program, and if they did not conceive during that time, they continued the program in combination with fertility treatments.
Patients were included if they were aged 18-40 years and had either infertility and a body mass index of 30 kg/m2 or greater or PCOS and a BMI of 27 kg/m2 or greater. Researchers excluded women planning to undergo bariatric surgery, women who were already undergoing another lifestyle intervention, and women with severe infertility or who had a male partner with severe infertility for whom in vitro fertilization was their only option for conceiving. Researchers collected data from patients at baseline and every 6 months up to 18 months, with additional visits for pregnant women scheduled at the beginning of pregnancy and at 26 weeks’ gestation. They collected baseline data on age, BMI, waist circumference, fat mass percentage, daily energy expenditure, and food frequency using the Healthy Eating Index (HEI).
Overall, 46 women in the intervention group and 52 women in the control group had a research visit at 6 months or pregnancy research visit at less than 6 months; of these, 33 women in the intervention group (65%) and 35 women in the control group (61%) had PCOS. At baseline, both PCOS and non-PCOS groups were similar; however, women in the PCOS intervention group had a lower BMI than did women without PCOS in the intervention group (37 kg/m2 vs. 41 kg/m2; P less than .05), while women without PCOS in the intervention group had a higher fat mass percentage than did women with PCOS in the intervention group (46% vs. 49%; P less than .05).
With regard to weight loss, there was a 2.4% reduction in weight among all patients in the intervention group, compared with the control group (P = .003), with a 2.7% reduction in weight for the PCOS group (P = .015) and a 1.8% reduction in the non-PCOS group (P = .139). However, there were no significant differences between PCOS status and the lifestyle intervention, said Dr. Baillargeon.
At 6 months, the quality of women’s diets in the combined PCOS and non-PCOS group that participated in the lifestyle program showed significant improvement, compared with control groups (HEI, 18% vs. 5%; P less than .001). The PCOS group on its own showed significant improvement with the intervention (20% vs. 4%; P less than .001), whereas women without PCOS showed a nonsignificant improvement with the intervention (14% vs. 6%; P = .055). Daily energy expenditure improved in all groups that received the intervention, compared with the control groups, but there were no significant between-group differences in energy expenditure.
When analyzing fertility outcomes at 18 months, the pregnancy rate for all patients who received lifestyle interventions was 61%, compared with 39% in the control group (P = .02; number needed to treat, 4.5). In women with PCOS, those who underwent the lifestyle intervention had a pregnancy rate of 58%, compared with 34% in the control group (P = .05; NNT, 4.3); although women without PCOS who participated in the lifestyle program had an improved pregnancy rate over women in the control group, the results were not significant (67% vs. 46%; P = .18; NNT, 4.7).
The researchers also looked at the spontaneous pregnancy rate and found women who received the intervention had nearly three times the rate of spontaneous pregnancy, compared with women in the control group (33% vs. 12%; P = .01), while women with PCOS in the lifestyle program had nearly five times the rate of spontaneous pregnancy, compared with the control group (27% vs. 6%; P = .02). Women without PCOS in the lifestyle program had nearly twice the increased likelihood of spontaneous pregnancy, but the results were not significant (44% vs. 23%; P = .15).
Women with PCOS in the lifestyle program also had a higher live birth rate, compared with women in the control group (55% vs. 31%; P = .05; NNT, 4.3). Although women without PCOS in the lifestyle program (67% vs. 46%; P = .18; NNT, 4.7) and women in the study overall experienced higher live birth rates (51% vs. 37%; P = .14; NNT, 7.0), compared with the control group, these results were not significant, said Dr. Baillargeon.
“Such lifestyle interventions in women with obesity could significantly lower costs of fertility treatments, which is important,” concluded Dr. Baillargeon.
The Fit-For-Fertility program was funded by an unrestricted grant from Ferring.
SOURCE: Baillargeon J-P, et al. ASRM 2019. Abstract O-95.
REPORTING FROM ASRM 2019
Clean cuts: Tips and tricks for laparoscopic colpotomy
Three psychiatrists join the Clinical Psychiatry News board
Clinical Psychiatry News is pleased to announce the addition of three physicians to its editorial advisory board: Constance E. Dunlap, MD; Eva Ritvo, MD; and Linda L.M. Worley, MD.
Dr. Dunlap, a psychiatrist and psychoanalyst, is a Washington Psychiatric Society representative to the American Psychiatric Association (APA) Assembly, a past president of the Washington Psychiatric Society, and clinical professor of psychiatry and behavioral sciences at George Washington University, Washington.
She is interested in the role that “difference” – race, culture, and ethnicity – plays in interpersonal relationship and group dynamics. Dr. Dunlap practices in Washington.
Dr. Ritvo is a psychiatrist, author, television and radio personality, and founder of an international initiative – Bekindr – that seeks “to bring more kindness to the world.” She is a Distinguished Life Fellow of the APA and a member of the American College of Psychiatrists.
Dr. Ritvo is former chair of psychiatry and behavioral medicine at Mount Sinai Medical Center in Miami, and former vice chair of psychiatry and behavioral sciences at the University of Miami. She practices in Miami Beach, Fla.
Dr. Worley is regional associate dean of the Northwest Arkansas Campus of the University of Arkansas Medical Sciences (UAMS) College of Medicine in Fayetteville. She is a professor psychiatry, and obstetrics and gynecology at UAMS and an adjunct professor of medicine at the Vanderbilt School of Medicine in Nashville, Tenn.*
There, she serves as teaching faculty in the Vanderbilt Center for Professional Health’s distressed physicians course. Dr. Worley has served as president of the Association for Academic Psychiatry, president of the Academy of Consultation Liaison Psychiatry, and in numerous roles for the APA. She considers herself a hybrid, having been born and raised in the Pacific Northwest then migrating as an adult to the U.S. heartland. Her career has been fueled by a determination to prevent and relieve suffering by creating innovative and life-changing programs.
*This story was updated 2/26/2020.
Clinical Psychiatry News is pleased to announce the addition of three physicians to its editorial advisory board: Constance E. Dunlap, MD; Eva Ritvo, MD; and Linda L.M. Worley, MD.
Dr. Dunlap, a psychiatrist and psychoanalyst, is a Washington Psychiatric Society representative to the American Psychiatric Association (APA) Assembly, a past president of the Washington Psychiatric Society, and clinical professor of psychiatry and behavioral sciences at George Washington University, Washington.
She is interested in the role that “difference” – race, culture, and ethnicity – plays in interpersonal relationship and group dynamics. Dr. Dunlap practices in Washington.
Dr. Ritvo is a psychiatrist, author, television and radio personality, and founder of an international initiative – Bekindr – that seeks “to bring more kindness to the world.” She is a Distinguished Life Fellow of the APA and a member of the American College of Psychiatrists.
Dr. Ritvo is former chair of psychiatry and behavioral medicine at Mount Sinai Medical Center in Miami, and former vice chair of psychiatry and behavioral sciences at the University of Miami. She practices in Miami Beach, Fla.
Dr. Worley is regional associate dean of the Northwest Arkansas Campus of the University of Arkansas Medical Sciences (UAMS) College of Medicine in Fayetteville. She is a professor psychiatry, and obstetrics and gynecology at UAMS and an adjunct professor of medicine at the Vanderbilt School of Medicine in Nashville, Tenn.*
There, she serves as teaching faculty in the Vanderbilt Center for Professional Health’s distressed physicians course. Dr. Worley has served as president of the Association for Academic Psychiatry, president of the Academy of Consultation Liaison Psychiatry, and in numerous roles for the APA. She considers herself a hybrid, having been born and raised in the Pacific Northwest then migrating as an adult to the U.S. heartland. Her career has been fueled by a determination to prevent and relieve suffering by creating innovative and life-changing programs.
*This story was updated 2/26/2020.
Clinical Psychiatry News is pleased to announce the addition of three physicians to its editorial advisory board: Constance E. Dunlap, MD; Eva Ritvo, MD; and Linda L.M. Worley, MD.
Dr. Dunlap, a psychiatrist and psychoanalyst, is a Washington Psychiatric Society representative to the American Psychiatric Association (APA) Assembly, a past president of the Washington Psychiatric Society, and clinical professor of psychiatry and behavioral sciences at George Washington University, Washington.
She is interested in the role that “difference” – race, culture, and ethnicity – plays in interpersonal relationship and group dynamics. Dr. Dunlap practices in Washington.
Dr. Ritvo is a psychiatrist, author, television and radio personality, and founder of an international initiative – Bekindr – that seeks “to bring more kindness to the world.” She is a Distinguished Life Fellow of the APA and a member of the American College of Psychiatrists.
Dr. Ritvo is former chair of psychiatry and behavioral medicine at Mount Sinai Medical Center in Miami, and former vice chair of psychiatry and behavioral sciences at the University of Miami. She practices in Miami Beach, Fla.
Dr. Worley is regional associate dean of the Northwest Arkansas Campus of the University of Arkansas Medical Sciences (UAMS) College of Medicine in Fayetteville. She is a professor psychiatry, and obstetrics and gynecology at UAMS and an adjunct professor of medicine at the Vanderbilt School of Medicine in Nashville, Tenn.*
There, she serves as teaching faculty in the Vanderbilt Center for Professional Health’s distressed physicians course. Dr. Worley has served as president of the Association for Academic Psychiatry, president of the Academy of Consultation Liaison Psychiatry, and in numerous roles for the APA. She considers herself a hybrid, having been born and raised in the Pacific Northwest then migrating as an adult to the U.S. heartland. Her career has been fueled by a determination to prevent and relieve suffering by creating innovative and life-changing programs.
*This story was updated 2/26/2020.
Is it time to expand the use of PARP inhibitors?
In this edition of “How I will treat my next patient,” I review two recent presentations at the European Society of Medical Oncology Congress regarding the expanded use of poly (adenosine diphosphate-ribose) polymerase inhibitors (PARPi) in patients with advanced solid tumors, potentially broadening the indications for this important class of agents.
Metastatic CRPC
Perhaps 25% of prostate cancer patients have loss-of-function mutations – BRCA1, BRCA2, and ATM – or alterations in homologous recombinant repair (HRR) genes. In the PROfound trial, men with metastatic castration-resistant prostate cancer (mCRPC) who had progressed on either abiraterone or enzalutamide and who had DNA-repair mutations were randomized to either olaparib (300 mg b.i.d.) or treatment of physician’s choice (TPC) with either abiraterone or enzalutamide plus prednisone (Hussain M et al. ESMO 2019, Abstract LBA-12).
Two cohorts were enrolled. Cohort A included 245 men with BRCA1, BRCA2, or ATM mutations, and cohort B included 142 men with other alterations (BARD1, BIRP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD15B, RAD15C, RAD15D, or RAD54L). After disease progression, patients could cross over to receive PARPi, which more than 80% of patients eventually did.
Median radiographic progression-free survival (PFS) in cohort A was 7.39 months with PARPi, compared with 3.55 months with TPC, for a hazard ratio for progression on PARPi of 0.34 (P less than .0001). A significant benefit was seen for PARPi in the overall population (both cohorts), with a median radiographic PFS of 5.82 months va. 3.52 months, respectively (HR, 0.49; P less than .0001).
Among patients in cohort A, the objective response rate (ORR) was 33.3% with PARPi, compared with 2.3% for TPC, resulting in an odds ratio for ORR of 20.86 (P less than .0001).
PARPi demonstrated a longer time to pain progression in cohort A, with the median not reached, compared with 9.92 months with TPC (HR, 0.44; P = .0192). Perhaps because of the high proportion of TPC patients who eventually received PARPi, no statistically significant differences in overall survival have yet been seen.
What this means in practice
During my fellowship, a mentor taught that “because quality of life is generally better before progression than afterwards, PFS is a worthy endpoint in its own right.” For that reason, although I would have liked to see the data for cohort B alone, it appears worthwhile for physicians to make every effort to obtain PARPi. The difference in ORR, pain progression, and PFS at 12 months is clinically dramatic.
Of equal significance, however, is that PROfound is the first positive phase 3 biomarker-selected study evaluating a targeted treatment in patients with mCRPC. For prostate cancer – as for breast, ovarian, pancreatic, and several other cancers – the molecular biology and genetic background of our patients dictates the other tumors for which they and their family members are at risk, and expands the treatment armamentarium for them.
For those clinicians who needed to be convinced that “precision medicine” for prostate cancer patients was worthwhile, the PROfound trial should have a profound impact.
Advanced ovarian cancer
The randomized, double-blind, placebo-controlled, phase 3 PAOLA-1/ENGOT-ov25 trial studied patients with stage III-IV ovarian, fallopian tube, or primary peritoneal cancer who had surgery, platinum-taxane chemotherapy, and at least 3 months of bevacizumab. Patients were randomized to maintenance treatment with an additional 12 months of bevacizumab plus 24 months of PARPi with olaparib or placebo. Germline BRCA mutations were not required (Ray-Coquard I et al. ESMO 2019, Abstract LBA2).
As reported at ESMO, adding PARPi to bevacizumab maintenance provided a clinically meaningful PFS benefit of 22.1 months, in comparison with 16.6 months for bevacizumab alone. The difference was statistically significant.
For patients with tumor BRCA mutations (tBRCAm), PFS was 37.2 months with olaparib vs. 21.7 months for placebo (HR, 0.31). The PFS benefit was even more impressive for homologous recombination deficient (HRD)–positive patients, inclusive of those with tBRCAm (PFS 37.2 months for PARPi vs. 17.7 months for placebo; and in the 152 HRD-positive patients without tBRCAm, (median PFS 28.1 months vs. 16.6 months; HR, 0.43).
The improved PFS in patients with tBRCAm is similar to that reported in the SOLO1 trial of olaparib monotherapy vs. chemotherapy in newly diagnosed advanced ovarian cancer (N Engl J Med. 2018; 379:2495-2505), but the PFS in the control arm was longer in PAOLA-1 than in SOLO1, perhaps because of the use of bevacizumab in PAOLA-1. PARPi did not affect tolerance to bevacizumab.
In PAOLA-1, the HRD-positive patients who lacked tBRCAm and, by extension, lacked germline BRCA mutations – a new population of patients – was identified who benefited substantially from maintenance PARPi in the first-line setting.
What this means in practice
PAOLA-1 demonstrates that PARPi can improve outcomes in first-line treatment – and in patients beyond those with germline BRCA mutations. As a result, PAOLA-1 potentially changes the standard of care for initial treatment of the respectable fraction of patients with previously untreated, advanced müllerian cancers who have either tBRCAm or HRD positive tumors.
Importantly, PAOLA-1 is one of many published trials that stimulates the discussion of cost vs. value for combinations of biologics. The incremental benefit from the second biologic (in this case PARPi) is almost never completely additive or supra-additive to the benefit associated with the first biologic (in this case, bevacizumab). In that regard, despite the fact that PARPi showed a PFS benefit in the intent-to-treat population overall, precisely defining the patient population that has the greatest benefit will facilitate the goal of getting the treatments of greatest “value for cost” to our patients in the most responsible way.
Additional research will hopefully define the relative contribution of bevacizumab to PARPi in patients who benefited so dramatically from PARPi in PAOLA-1.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I review two recent presentations at the European Society of Medical Oncology Congress regarding the expanded use of poly (adenosine diphosphate-ribose) polymerase inhibitors (PARPi) in patients with advanced solid tumors, potentially broadening the indications for this important class of agents.
Metastatic CRPC
Perhaps 25% of prostate cancer patients have loss-of-function mutations – BRCA1, BRCA2, and ATM – or alterations in homologous recombinant repair (HRR) genes. In the PROfound trial, men with metastatic castration-resistant prostate cancer (mCRPC) who had progressed on either abiraterone or enzalutamide and who had DNA-repair mutations were randomized to either olaparib (300 mg b.i.d.) or treatment of physician’s choice (TPC) with either abiraterone or enzalutamide plus prednisone (Hussain M et al. ESMO 2019, Abstract LBA-12).
Two cohorts were enrolled. Cohort A included 245 men with BRCA1, BRCA2, or ATM mutations, and cohort B included 142 men with other alterations (BARD1, BIRP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD15B, RAD15C, RAD15D, or RAD54L). After disease progression, patients could cross over to receive PARPi, which more than 80% of patients eventually did.
Median radiographic progression-free survival (PFS) in cohort A was 7.39 months with PARPi, compared with 3.55 months with TPC, for a hazard ratio for progression on PARPi of 0.34 (P less than .0001). A significant benefit was seen for PARPi in the overall population (both cohorts), with a median radiographic PFS of 5.82 months va. 3.52 months, respectively (HR, 0.49; P less than .0001).
Among patients in cohort A, the objective response rate (ORR) was 33.3% with PARPi, compared with 2.3% for TPC, resulting in an odds ratio for ORR of 20.86 (P less than .0001).
PARPi demonstrated a longer time to pain progression in cohort A, with the median not reached, compared with 9.92 months with TPC (HR, 0.44; P = .0192). Perhaps because of the high proportion of TPC patients who eventually received PARPi, no statistically significant differences in overall survival have yet been seen.
What this means in practice
During my fellowship, a mentor taught that “because quality of life is generally better before progression than afterwards, PFS is a worthy endpoint in its own right.” For that reason, although I would have liked to see the data for cohort B alone, it appears worthwhile for physicians to make every effort to obtain PARPi. The difference in ORR, pain progression, and PFS at 12 months is clinically dramatic.
Of equal significance, however, is that PROfound is the first positive phase 3 biomarker-selected study evaluating a targeted treatment in patients with mCRPC. For prostate cancer – as for breast, ovarian, pancreatic, and several other cancers – the molecular biology and genetic background of our patients dictates the other tumors for which they and their family members are at risk, and expands the treatment armamentarium for them.
For those clinicians who needed to be convinced that “precision medicine” for prostate cancer patients was worthwhile, the PROfound trial should have a profound impact.
Advanced ovarian cancer
The randomized, double-blind, placebo-controlled, phase 3 PAOLA-1/ENGOT-ov25 trial studied patients with stage III-IV ovarian, fallopian tube, or primary peritoneal cancer who had surgery, platinum-taxane chemotherapy, and at least 3 months of bevacizumab. Patients were randomized to maintenance treatment with an additional 12 months of bevacizumab plus 24 months of PARPi with olaparib or placebo. Germline BRCA mutations were not required (Ray-Coquard I et al. ESMO 2019, Abstract LBA2).
As reported at ESMO, adding PARPi to bevacizumab maintenance provided a clinically meaningful PFS benefit of 22.1 months, in comparison with 16.6 months for bevacizumab alone. The difference was statistically significant.
For patients with tumor BRCA mutations (tBRCAm), PFS was 37.2 months with olaparib vs. 21.7 months for placebo (HR, 0.31). The PFS benefit was even more impressive for homologous recombination deficient (HRD)–positive patients, inclusive of those with tBRCAm (PFS 37.2 months for PARPi vs. 17.7 months for placebo; and in the 152 HRD-positive patients without tBRCAm, (median PFS 28.1 months vs. 16.6 months; HR, 0.43).
The improved PFS in patients with tBRCAm is similar to that reported in the SOLO1 trial of olaparib monotherapy vs. chemotherapy in newly diagnosed advanced ovarian cancer (N Engl J Med. 2018; 379:2495-2505), but the PFS in the control arm was longer in PAOLA-1 than in SOLO1, perhaps because of the use of bevacizumab in PAOLA-1. PARPi did not affect tolerance to bevacizumab.
In PAOLA-1, the HRD-positive patients who lacked tBRCAm and, by extension, lacked germline BRCA mutations – a new population of patients – was identified who benefited substantially from maintenance PARPi in the first-line setting.
What this means in practice
PAOLA-1 demonstrates that PARPi can improve outcomes in first-line treatment – and in patients beyond those with germline BRCA mutations. As a result, PAOLA-1 potentially changes the standard of care for initial treatment of the respectable fraction of patients with previously untreated, advanced müllerian cancers who have either tBRCAm or HRD positive tumors.
Importantly, PAOLA-1 is one of many published trials that stimulates the discussion of cost vs. value for combinations of biologics. The incremental benefit from the second biologic (in this case PARPi) is almost never completely additive or supra-additive to the benefit associated with the first biologic (in this case, bevacizumab). In that regard, despite the fact that PARPi showed a PFS benefit in the intent-to-treat population overall, precisely defining the patient population that has the greatest benefit will facilitate the goal of getting the treatments of greatest “value for cost” to our patients in the most responsible way.
Additional research will hopefully define the relative contribution of bevacizumab to PARPi in patients who benefited so dramatically from PARPi in PAOLA-1.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I review two recent presentations at the European Society of Medical Oncology Congress regarding the expanded use of poly (adenosine diphosphate-ribose) polymerase inhibitors (PARPi) in patients with advanced solid tumors, potentially broadening the indications for this important class of agents.
Metastatic CRPC
Perhaps 25% of prostate cancer patients have loss-of-function mutations – BRCA1, BRCA2, and ATM – or alterations in homologous recombinant repair (HRR) genes. In the PROfound trial, men with metastatic castration-resistant prostate cancer (mCRPC) who had progressed on either abiraterone or enzalutamide and who had DNA-repair mutations were randomized to either olaparib (300 mg b.i.d.) or treatment of physician’s choice (TPC) with either abiraterone or enzalutamide plus prednisone (Hussain M et al. ESMO 2019, Abstract LBA-12).
Two cohorts were enrolled. Cohort A included 245 men with BRCA1, BRCA2, or ATM mutations, and cohort B included 142 men with other alterations (BARD1, BIRP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD15B, RAD15C, RAD15D, or RAD54L). After disease progression, patients could cross over to receive PARPi, which more than 80% of patients eventually did.
Median radiographic progression-free survival (PFS) in cohort A was 7.39 months with PARPi, compared with 3.55 months with TPC, for a hazard ratio for progression on PARPi of 0.34 (P less than .0001). A significant benefit was seen for PARPi in the overall population (both cohorts), with a median radiographic PFS of 5.82 months va. 3.52 months, respectively (HR, 0.49; P less than .0001).
Among patients in cohort A, the objective response rate (ORR) was 33.3% with PARPi, compared with 2.3% for TPC, resulting in an odds ratio for ORR of 20.86 (P less than .0001).
PARPi demonstrated a longer time to pain progression in cohort A, with the median not reached, compared with 9.92 months with TPC (HR, 0.44; P = .0192). Perhaps because of the high proportion of TPC patients who eventually received PARPi, no statistically significant differences in overall survival have yet been seen.
What this means in practice
During my fellowship, a mentor taught that “because quality of life is generally better before progression than afterwards, PFS is a worthy endpoint in its own right.” For that reason, although I would have liked to see the data for cohort B alone, it appears worthwhile for physicians to make every effort to obtain PARPi. The difference in ORR, pain progression, and PFS at 12 months is clinically dramatic.
Of equal significance, however, is that PROfound is the first positive phase 3 biomarker-selected study evaluating a targeted treatment in patients with mCRPC. For prostate cancer – as for breast, ovarian, pancreatic, and several other cancers – the molecular biology and genetic background of our patients dictates the other tumors for which they and their family members are at risk, and expands the treatment armamentarium for them.
For those clinicians who needed to be convinced that “precision medicine” for prostate cancer patients was worthwhile, the PROfound trial should have a profound impact.
Advanced ovarian cancer
The randomized, double-blind, placebo-controlled, phase 3 PAOLA-1/ENGOT-ov25 trial studied patients with stage III-IV ovarian, fallopian tube, or primary peritoneal cancer who had surgery, platinum-taxane chemotherapy, and at least 3 months of bevacizumab. Patients were randomized to maintenance treatment with an additional 12 months of bevacizumab plus 24 months of PARPi with olaparib or placebo. Germline BRCA mutations were not required (Ray-Coquard I et al. ESMO 2019, Abstract LBA2).
As reported at ESMO, adding PARPi to bevacizumab maintenance provided a clinically meaningful PFS benefit of 22.1 months, in comparison with 16.6 months for bevacizumab alone. The difference was statistically significant.
For patients with tumor BRCA mutations (tBRCAm), PFS was 37.2 months with olaparib vs. 21.7 months for placebo (HR, 0.31). The PFS benefit was even more impressive for homologous recombination deficient (HRD)–positive patients, inclusive of those with tBRCAm (PFS 37.2 months for PARPi vs. 17.7 months for placebo; and in the 152 HRD-positive patients without tBRCAm, (median PFS 28.1 months vs. 16.6 months; HR, 0.43).
The improved PFS in patients with tBRCAm is similar to that reported in the SOLO1 trial of olaparib monotherapy vs. chemotherapy in newly diagnosed advanced ovarian cancer (N Engl J Med. 2018; 379:2495-2505), but the PFS in the control arm was longer in PAOLA-1 than in SOLO1, perhaps because of the use of bevacizumab in PAOLA-1. PARPi did not affect tolerance to bevacizumab.
In PAOLA-1, the HRD-positive patients who lacked tBRCAm and, by extension, lacked germline BRCA mutations – a new population of patients – was identified who benefited substantially from maintenance PARPi in the first-line setting.
What this means in practice
PAOLA-1 demonstrates that PARPi can improve outcomes in first-line treatment – and in patients beyond those with germline BRCA mutations. As a result, PAOLA-1 potentially changes the standard of care for initial treatment of the respectable fraction of patients with previously untreated, advanced müllerian cancers who have either tBRCAm or HRD positive tumors.
Importantly, PAOLA-1 is one of many published trials that stimulates the discussion of cost vs. value for combinations of biologics. The incremental benefit from the second biologic (in this case PARPi) is almost never completely additive or supra-additive to the benefit associated with the first biologic (in this case, bevacizumab). In that regard, despite the fact that PARPi showed a PFS benefit in the intent-to-treat population overall, precisely defining the patient population that has the greatest benefit will facilitate the goal of getting the treatments of greatest “value for cost” to our patients in the most responsible way.
Additional research will hopefully define the relative contribution of bevacizumab to PARPi in patients who benefited so dramatically from PARPi in PAOLA-1.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.