User login
Crusted Demodicosis in an Immunocompetent Patient
To the Editor:
Demodicosis is an infection of humans caused by species of the genus of saprophytic mites Demodex (most commonly Demodex brevis and Demodex folliculorum) that feed on the pilosebaceous unit.1Demodex mites are believed to be a commensal species in humans; an increase in mite concentration or mite penetration of the dermis, however, can cause a shift from a commensal to a pathologic form.2 Demodicosis manifests in a variety of forms, including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis. The likelihood of colonization increases with age; the mite rarely is observed in children but is found at a rate approaching 100% in the elderly population.3 It is hypothesized that manifestation of disease might be due to a decrease in immune function or an inherited HLA antigen that causes local immunosuppression.4
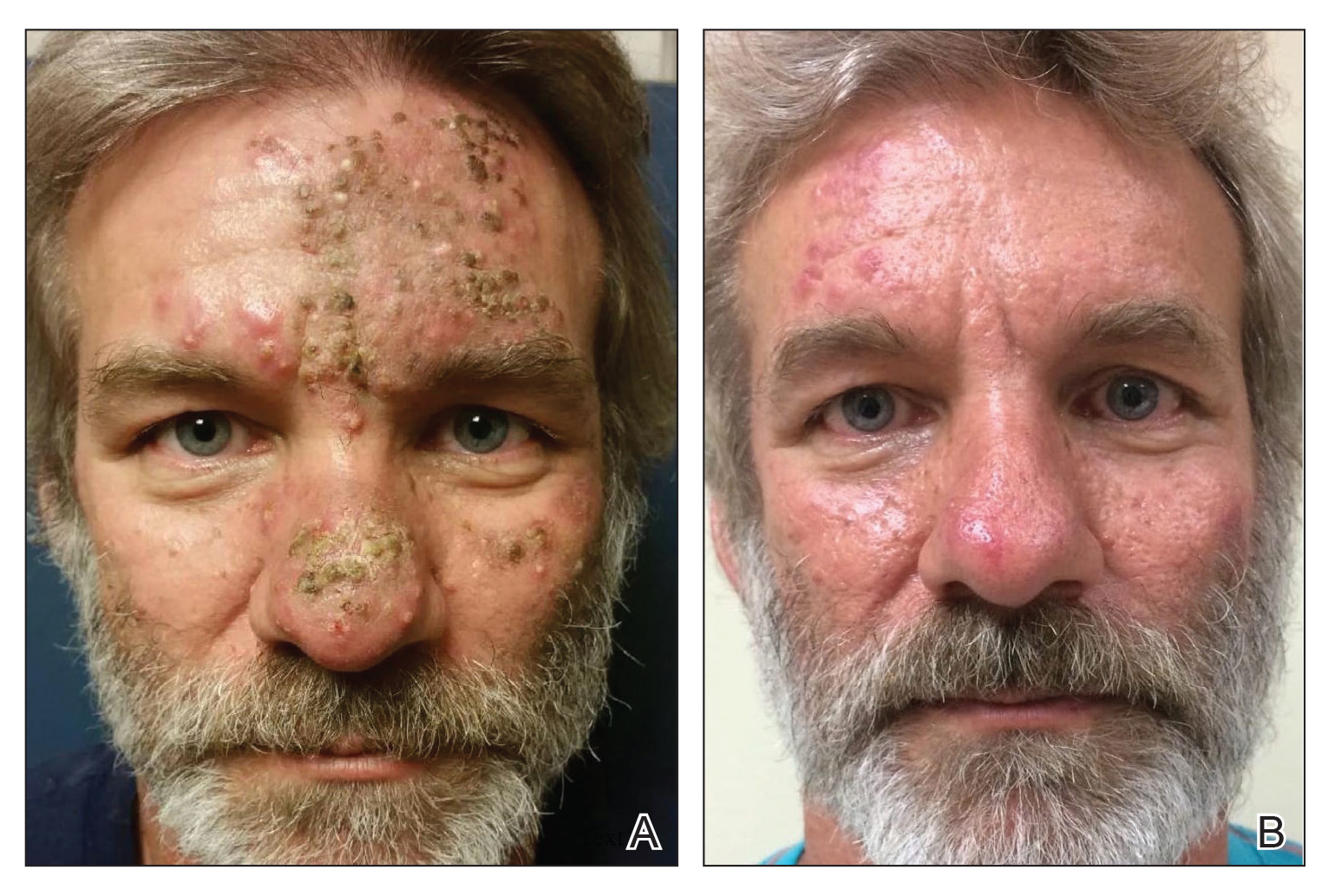
A 51-year-old man who was otherwise healthy presented to our clinic with a crusting rash on the face of 9 weeks’ duration. The rash began a few days after he demolished a rotting wooden shed in his backyard. Lesions began as pustules on the left cheek, which then developed notable crusting over the next 5 to 7 days and spread to involve the forehead, nose, and right cheek (Figure 1A).
The patient had no underlying immunosuppressive disease; a human immunodeficiency virus screen, complete blood cell count, and tests of hepatic function were all unremarkable. He denied a history of frequent or recurrent sinopulmonary infections, skin infections, or infectious diarrheal illnesses. He had been seen by his primary care physician who had treated him for herpes zoster without improvement.
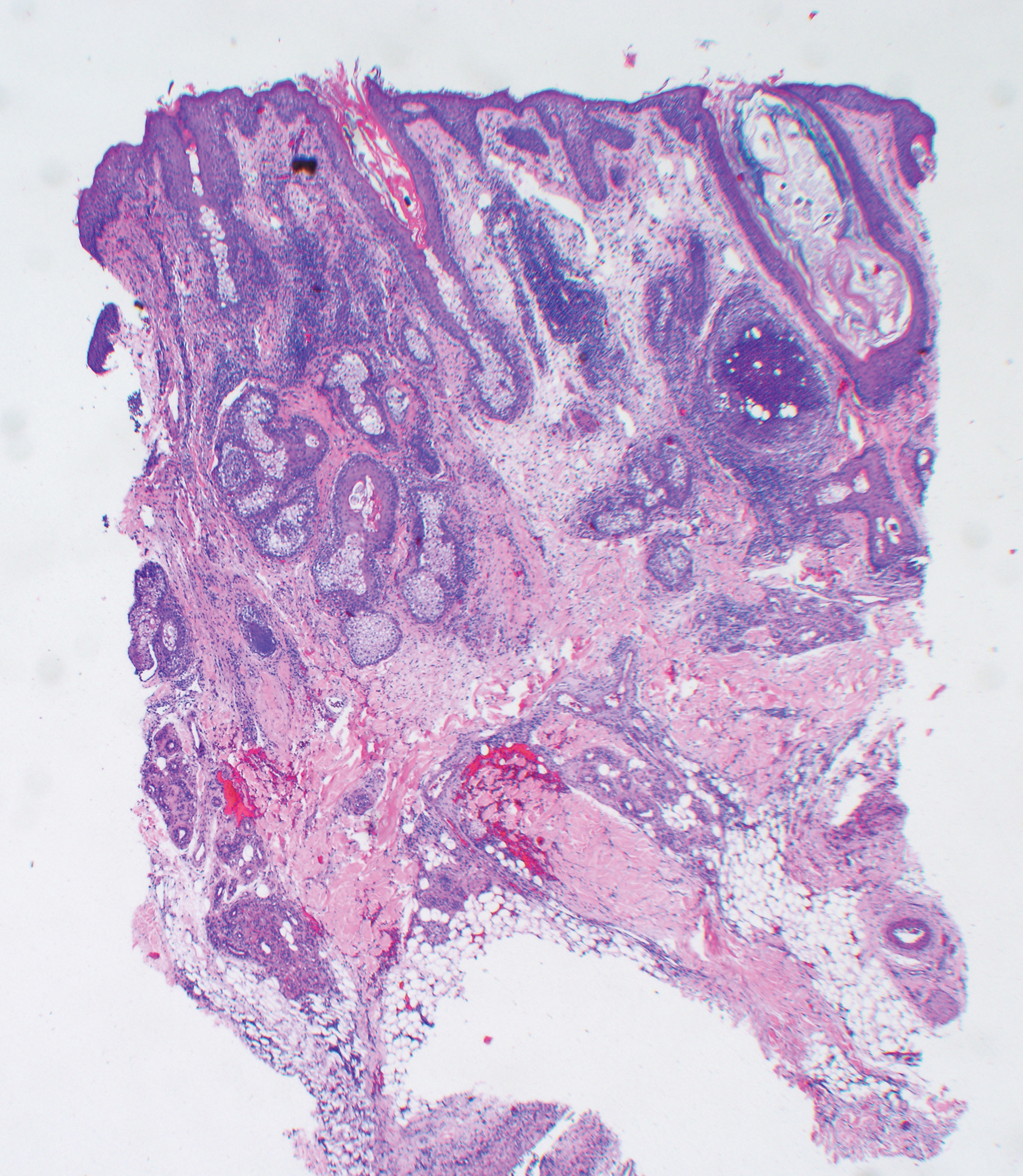
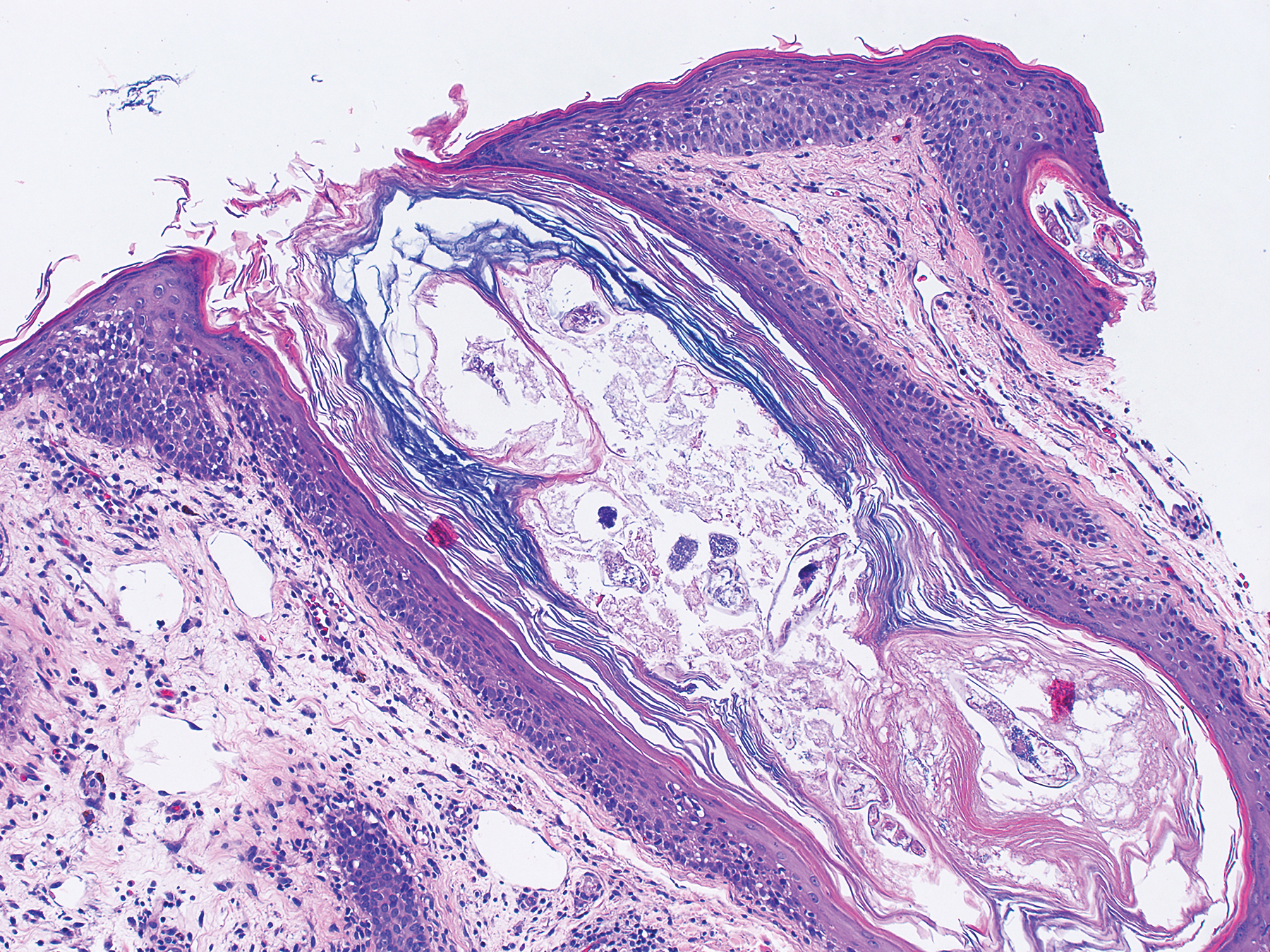
At our initial evaluation, biopsy was performed; specimens were sent for histopathologic analysis and culture. Findings included a dermal neutrophilic inflammation, a dense perivascular and perifollicular lymphoplasmacytic infiltrate with foci of neutrophilic pustules within the follicles (Figure 2), numerous intrafollicular Demodex mites (Figure 3), perifollicular vague noncaseating granuloma, and mild sebaceous hyperplasia. Grocott methenamine-silver stain and acid-fast bacilli stain were negative.
Review of clinical and pathological data yielded a final diagnosis of crusted demodicosis with a background of rosacea. The patient was ultimately treated with a single dose of oral ivermectin 15 mg with a second dose 7 days later in addition to daily application of ivermectin cream 1% to affected areas of his rash. He had notable improvement with this regimen, with complete resolution within 6 weeks (Figure 1B). The patient noted mild recurrence 14 to 21 days after discontinuing topical ivermectin.
The 2 species of Demodex that cause disease in humans each behave distinctively: D folliculorum, with a cigar-shaped body, favors superficial hair follicles; D brevis, a smaller form, burrows deeper into skin where it feeds on the pilosebaceous unit.1 Colonization occurs through direct skin-skin contact that begins as early as infancy and becomes more common with age due to development of sebaceous glands, the main source of nourishment for the mites.2
Demodicosis is classified as primary and secondary. In a prospective study of patients with clinical findings of demodicosis, Akilov et al1 discovered that the 2 forms can be differentiated by skin distribution, seasonality, mite species, and preexisting dermatoses. Primary demodicosis is categorized by sudden onset of symptoms on healthy skin, usually the face. Secondary demodicosis develops progressively in patients with preexisting skin disease, such as rosacea, and can have a broader distribution, involving the face and trunk.2 Clinical manifestations of demodicosis are broad and include pruritic papulopustular, nodulocystic, crusted, and abscesslike lesions.5
Most cases of demodicosis reported in the literature are associated with either local or systemic immunosuppression.6-8 In a case report, an otherwise immunocompetent child developed facial demodicosis after local immunosuppression from chronic use of 2 topical steroid agents.9
Demodex infestation can be diagnosed using a variety of methods, including standardized skin surface biopsy, punch biopsy, and potassium hydroxide analysis. Standardized skin surface biopsy is the preferred method to diagnose demodicosis because it is noninvasive and samples the superficial follicle where Demodex mites typically reside. Diagnosis is made by identifying 5 or more Demodex mites in a low-power field or more than 5 mites per square centimeter in standardized skin surface biopsy.2 Other potential diagnostic tools reported in the literature include dermoscopy and confocal laser scanning microscopy.10,11
There is no standard therapeutic regimen for demodicosis because evidence-based trials regarding the efficacy of treatments are lacking. Oral ivermectin 200 µg/kg in a single dose is considered the preferred treatment; it can be combined with oral erythromycin, topical permethrin, or topical metronidazole.5-7,9
Our case is unique, as crusted demodicosis developed in an immunocompetent adult. Demodicosis usually causes severe eruptions in immunocompromised persons, with only 1 case report detailing a papulopustular rash in an immunocompetent adult.12,13
The pathogenesis of demodicosis remains unclear. Many mechanisms have been hypothesized to play a role in its pathogenesis, including mechanical obstruction of hair follicles, hypersensitivity reaction to Demodex mites, immune dysregulation, and a foreign-body granulomatous reaction to the skeleton of the mite.2,3 Our patient’s particular infestation could have been caused by an exuberant reaction to Demodex; however, it is likely that many factors played a role in his disease process to cause an increase in mite density and subsequent manifestations of disease.
- Akilov OE, Butov YS, Mumcuoglu KY. A clinico-pathological approach to the classification of human demodicosis. J Dtsch Dermatol Ges. 2005;3:607-614.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618-626.
- Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3-6.
- Noy ML, Hughes S, Bunker CB. Another face of demodicosis. Clin Exp Dermatol. 2016;41:958-959.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Morrás PG, Santos SP, Imedio IL, et al. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20:28-30.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42:724-726.
- Clyti E, Nacher M, Sainte-Marie D, et al. Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. Int J Dermatol. 2006;45:1066-1068.
- Guerrero-González GA, Herz-Ruelas ME, Gómez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med. 2014;2014:458046.
- Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35-38.
- Harmelin Y, Delaunay P, Erfan N, et al. Interest of confocal laser scanning microscopy for the diagnosis and treatment monitoring of demodicosis. J Eur Acad Dermatol Venereol. 2014;28:255-257.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Kaur T, Jindal N, Bansal R, et al. Facial demodicidosis: a diagnostic challenge. Indian J Dermatol. 2012;57:72-73.
To the Editor:
Demodicosis is an infection of humans caused by species of the genus of saprophytic mites Demodex (most commonly Demodex brevis and Demodex folliculorum) that feed on the pilosebaceous unit.1Demodex mites are believed to be a commensal species in humans; an increase in mite concentration or mite penetration of the dermis, however, can cause a shift from a commensal to a pathologic form.2 Demodicosis manifests in a variety of forms, including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis. The likelihood of colonization increases with age; the mite rarely is observed in children but is found at a rate approaching 100% in the elderly population.3 It is hypothesized that manifestation of disease might be due to a decrease in immune function or an inherited HLA antigen that causes local immunosuppression.4
A 51-year-old man who was otherwise healthy presented to our clinic with a crusting rash on the face of 9 weeks’ duration. The rash began a few days after he demolished a rotting wooden shed in his backyard. Lesions began as pustules on the left cheek, which then developed notable crusting over the next 5 to 7 days and spread to involve the forehead, nose, and right cheek (Figure 1A).
The patient had no underlying immunosuppressive disease; a human immunodeficiency virus screen, complete blood cell count, and tests of hepatic function were all unremarkable. He denied a history of frequent or recurrent sinopulmonary infections, skin infections, or infectious diarrheal illnesses. He had been seen by his primary care physician who had treated him for herpes zoster without improvement.
At our initial evaluation, biopsy was performed; specimens were sent for histopathologic analysis and culture. Findings included a dermal neutrophilic inflammation, a dense perivascular and perifollicular lymphoplasmacytic infiltrate with foci of neutrophilic pustules within the follicles (Figure 2), numerous intrafollicular Demodex mites (Figure 3), perifollicular vague noncaseating granuloma, and mild sebaceous hyperplasia. Grocott methenamine-silver stain and acid-fast bacilli stain were negative.
Review of clinical and pathological data yielded a final diagnosis of crusted demodicosis with a background of rosacea. The patient was ultimately treated with a single dose of oral ivermectin 15 mg with a second dose 7 days later in addition to daily application of ivermectin cream 1% to affected areas of his rash. He had notable improvement with this regimen, with complete resolution within 6 weeks (Figure 1B). The patient noted mild recurrence 14 to 21 days after discontinuing topical ivermectin.
The 2 species of Demodex that cause disease in humans each behave distinctively: D folliculorum, with a cigar-shaped body, favors superficial hair follicles; D brevis, a smaller form, burrows deeper into skin where it feeds on the pilosebaceous unit.1 Colonization occurs through direct skin-skin contact that begins as early as infancy and becomes more common with age due to development of sebaceous glands, the main source of nourishment for the mites.2
Demodicosis is classified as primary and secondary. In a prospective study of patients with clinical findings of demodicosis, Akilov et al1 discovered that the 2 forms can be differentiated by skin distribution, seasonality, mite species, and preexisting dermatoses. Primary demodicosis is categorized by sudden onset of symptoms on healthy skin, usually the face. Secondary demodicosis develops progressively in patients with preexisting skin disease, such as rosacea, and can have a broader distribution, involving the face and trunk.2 Clinical manifestations of demodicosis are broad and include pruritic papulopustular, nodulocystic, crusted, and abscesslike lesions.5
Most cases of demodicosis reported in the literature are associated with either local or systemic immunosuppression.6-8 In a case report, an otherwise immunocompetent child developed facial demodicosis after local immunosuppression from chronic use of 2 topical steroid agents.9
Demodex infestation can be diagnosed using a variety of methods, including standardized skin surface biopsy, punch biopsy, and potassium hydroxide analysis. Standardized skin surface biopsy is the preferred method to diagnose demodicosis because it is noninvasive and samples the superficial follicle where Demodex mites typically reside. Diagnosis is made by identifying 5 or more Demodex mites in a low-power field or more than 5 mites per square centimeter in standardized skin surface biopsy.2 Other potential diagnostic tools reported in the literature include dermoscopy and confocal laser scanning microscopy.10,11
There is no standard therapeutic regimen for demodicosis because evidence-based trials regarding the efficacy of treatments are lacking. Oral ivermectin 200 µg/kg in a single dose is considered the preferred treatment; it can be combined with oral erythromycin, topical permethrin, or topical metronidazole.5-7,9
Our case is unique, as crusted demodicosis developed in an immunocompetent adult. Demodicosis usually causes severe eruptions in immunocompromised persons, with only 1 case report detailing a papulopustular rash in an immunocompetent adult.12,13
The pathogenesis of demodicosis remains unclear. Many mechanisms have been hypothesized to play a role in its pathogenesis, including mechanical obstruction of hair follicles, hypersensitivity reaction to Demodex mites, immune dysregulation, and a foreign-body granulomatous reaction to the skeleton of the mite.2,3 Our patient’s particular infestation could have been caused by an exuberant reaction to Demodex; however, it is likely that many factors played a role in his disease process to cause an increase in mite density and subsequent manifestations of disease.
To the Editor:
Demodicosis is an infection of humans caused by species of the genus of saprophytic mites Demodex (most commonly Demodex brevis and Demodex folliculorum) that feed on the pilosebaceous unit.1Demodex mites are believed to be a commensal species in humans; an increase in mite concentration or mite penetration of the dermis, however, can cause a shift from a commensal to a pathologic form.2 Demodicosis manifests in a variety of forms, including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis. The likelihood of colonization increases with age; the mite rarely is observed in children but is found at a rate approaching 100% in the elderly population.3 It is hypothesized that manifestation of disease might be due to a decrease in immune function or an inherited HLA antigen that causes local immunosuppression.4
A 51-year-old man who was otherwise healthy presented to our clinic with a crusting rash on the face of 9 weeks’ duration. The rash began a few days after he demolished a rotting wooden shed in his backyard. Lesions began as pustules on the left cheek, which then developed notable crusting over the next 5 to 7 days and spread to involve the forehead, nose, and right cheek (Figure 1A).
The patient had no underlying immunosuppressive disease; a human immunodeficiency virus screen, complete blood cell count, and tests of hepatic function were all unremarkable. He denied a history of frequent or recurrent sinopulmonary infections, skin infections, or infectious diarrheal illnesses. He had been seen by his primary care physician who had treated him for herpes zoster without improvement.
At our initial evaluation, biopsy was performed; specimens were sent for histopathologic analysis and culture. Findings included a dermal neutrophilic inflammation, a dense perivascular and perifollicular lymphoplasmacytic infiltrate with foci of neutrophilic pustules within the follicles (Figure 2), numerous intrafollicular Demodex mites (Figure 3), perifollicular vague noncaseating granuloma, and mild sebaceous hyperplasia. Grocott methenamine-silver stain and acid-fast bacilli stain were negative.
Review of clinical and pathological data yielded a final diagnosis of crusted demodicosis with a background of rosacea. The patient was ultimately treated with a single dose of oral ivermectin 15 mg with a second dose 7 days later in addition to daily application of ivermectin cream 1% to affected areas of his rash. He had notable improvement with this regimen, with complete resolution within 6 weeks (Figure 1B). The patient noted mild recurrence 14 to 21 days after discontinuing topical ivermectin.
The 2 species of Demodex that cause disease in humans each behave distinctively: D folliculorum, with a cigar-shaped body, favors superficial hair follicles; D brevis, a smaller form, burrows deeper into skin where it feeds on the pilosebaceous unit.1 Colonization occurs through direct skin-skin contact that begins as early as infancy and becomes more common with age due to development of sebaceous glands, the main source of nourishment for the mites.2
Demodicosis is classified as primary and secondary. In a prospective study of patients with clinical findings of demodicosis, Akilov et al1 discovered that the 2 forms can be differentiated by skin distribution, seasonality, mite species, and preexisting dermatoses. Primary demodicosis is categorized by sudden onset of symptoms on healthy skin, usually the face. Secondary demodicosis develops progressively in patients with preexisting skin disease, such as rosacea, and can have a broader distribution, involving the face and trunk.2 Clinical manifestations of demodicosis are broad and include pruritic papulopustular, nodulocystic, crusted, and abscesslike lesions.5
Most cases of demodicosis reported in the literature are associated with either local or systemic immunosuppression.6-8 In a case report, an otherwise immunocompetent child developed facial demodicosis after local immunosuppression from chronic use of 2 topical steroid agents.9
Demodex infestation can be diagnosed using a variety of methods, including standardized skin surface biopsy, punch biopsy, and potassium hydroxide analysis. Standardized skin surface biopsy is the preferred method to diagnose demodicosis because it is noninvasive and samples the superficial follicle where Demodex mites typically reside. Diagnosis is made by identifying 5 or more Demodex mites in a low-power field or more than 5 mites per square centimeter in standardized skin surface biopsy.2 Other potential diagnostic tools reported in the literature include dermoscopy and confocal laser scanning microscopy.10,11
There is no standard therapeutic regimen for demodicosis because evidence-based trials regarding the efficacy of treatments are lacking. Oral ivermectin 200 µg/kg in a single dose is considered the preferred treatment; it can be combined with oral erythromycin, topical permethrin, or topical metronidazole.5-7,9
Our case is unique, as crusted demodicosis developed in an immunocompetent adult. Demodicosis usually causes severe eruptions in immunocompromised persons, with only 1 case report detailing a papulopustular rash in an immunocompetent adult.12,13
The pathogenesis of demodicosis remains unclear. Many mechanisms have been hypothesized to play a role in its pathogenesis, including mechanical obstruction of hair follicles, hypersensitivity reaction to Demodex mites, immune dysregulation, and a foreign-body granulomatous reaction to the skeleton of the mite.2,3 Our patient’s particular infestation could have been caused by an exuberant reaction to Demodex; however, it is likely that many factors played a role in his disease process to cause an increase in mite density and subsequent manifestations of disease.
- Akilov OE, Butov YS, Mumcuoglu KY. A clinico-pathological approach to the classification of human demodicosis. J Dtsch Dermatol Ges. 2005;3:607-614.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618-626.
- Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3-6.
- Noy ML, Hughes S, Bunker CB. Another face of demodicosis. Clin Exp Dermatol. 2016;41:958-959.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Morrás PG, Santos SP, Imedio IL, et al. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20:28-30.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42:724-726.
- Clyti E, Nacher M, Sainte-Marie D, et al. Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. Int J Dermatol. 2006;45:1066-1068.
- Guerrero-González GA, Herz-Ruelas ME, Gómez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med. 2014;2014:458046.
- Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35-38.
- Harmelin Y, Delaunay P, Erfan N, et al. Interest of confocal laser scanning microscopy for the diagnosis and treatment monitoring of demodicosis. J Eur Acad Dermatol Venereol. 2014;28:255-257.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Kaur T, Jindal N, Bansal R, et al. Facial demodicidosis: a diagnostic challenge. Indian J Dermatol. 2012;57:72-73.
- Akilov OE, Butov YS, Mumcuoglu KY. A clinico-pathological approach to the classification of human demodicosis. J Dtsch Dermatol Ges. 2005;3:607-614.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618-626.
- Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3-6.
- Noy ML, Hughes S, Bunker CB. Another face of demodicosis. Clin Exp Dermatol. 2016;41:958-959.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Morrás PG, Santos SP, Imedio IL, et al. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20:28-30.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42:724-726.
- Clyti E, Nacher M, Sainte-Marie D, et al. Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. Int J Dermatol. 2006;45:1066-1068.
- Guerrero-González GA, Herz-Ruelas ME, Gómez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med. 2014;2014:458046.
- Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35-38.
- Harmelin Y, Delaunay P, Erfan N, et al. Interest of confocal laser scanning microscopy for the diagnosis and treatment monitoring of demodicosis. J Eur Acad Dermatol Venereol. 2014;28:255-257.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Kaur T, Jindal N, Bansal R, et al. Facial demodicidosis: a diagnostic challenge. Indian J Dermatol. 2012;57:72-73.
Practice Points
- The Demodex mite, believed to be a commensal species in humans, has the ability to shift to a pathologic form in immunocompromised patients.
- Demodicosis can manifest in a variety of forms including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis.
Early palliative care consult decreases in-hospital mortality
NEW ORLEANS – When initiated early, meeting certain end-of-life criteria, results of a recent randomized clinical trial suggest.
The rate of in-hospital mortality was lower for critical care patients receiving an early consultation, compared with those who received palliative care initiated according to usual standards in the randomized, controlled trial, described at the annual meeting of the American College of Chest Physicians.
In addition, more health care surrogates were chosen in the hospital when palliative care medicine was involved earlier, according to investigator Scott Helgeson, MD, fellow in pulmonary critical care at the Mayo Clinic in Jacksonville, Fla.
Taken together, Dr. Helgeson said, those findings suggest the importance of getting palliative care involved “very early, while the patient can still make decisions.”
“There are a lot of things that can get in the way of adequate conversations, and that’s when the palliative care team can come in,” Dr. Helgeson said in an interview.
This study is the first reported to date to look at the impact on patient care outcomes specifically within 24 hours of medical ICU admission, according to Dr. Helgeson and coinvestigators
In their randomized study, patients were eligible if they met at least one of several criteria, including advanced age (80 years or older), late-stage dementia, post–cardiac arrest, metastatic cancer, end-stage organ failure, recurrent ICU admissions, an APACHE II score of 14 or higher, a SOFA score of 9 or higher, preexisting functional dependency, or consideration for a tracheostomy or permanent feeding tube.
Of 29 patients randomized, 14 received early palliative care, and 15 received standard palliative care, which was defined as starting “whenever the treating team deems (it) is appropriate,” according to the published abstract.
Hospital mortality occurred in none of the patients in the early palliative care group, versus six in the usual care group (P = .01), Dr. Helgeson and colleagues found. Moreover, seven health care surrogates were chosen in hospital in the early palliative care group, versus none in the usual care group (P less than .01).
Length of stay in the ICU or in hospital did not vary by treatment group, according to the investigators.
About one-fifth of deaths in the United States take place in or around ICU admissions, according to the investigators, who noted that those admissions can result in changing goals from cure to comfort – though sometimes too late.
Dr. Helgeson and coauthors disclosed that they had no relationships relevant to this research presentation.
SOURCE: Helgeson S, et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.803.
NEW ORLEANS – When initiated early, meeting certain end-of-life criteria, results of a recent randomized clinical trial suggest.
The rate of in-hospital mortality was lower for critical care patients receiving an early consultation, compared with those who received palliative care initiated according to usual standards in the randomized, controlled trial, described at the annual meeting of the American College of Chest Physicians.
In addition, more health care surrogates were chosen in the hospital when palliative care medicine was involved earlier, according to investigator Scott Helgeson, MD, fellow in pulmonary critical care at the Mayo Clinic in Jacksonville, Fla.
Taken together, Dr. Helgeson said, those findings suggest the importance of getting palliative care involved “very early, while the patient can still make decisions.”
“There are a lot of things that can get in the way of adequate conversations, and that’s when the palliative care team can come in,” Dr. Helgeson said in an interview.
This study is the first reported to date to look at the impact on patient care outcomes specifically within 24 hours of medical ICU admission, according to Dr. Helgeson and coinvestigators
In their randomized study, patients were eligible if they met at least one of several criteria, including advanced age (80 years or older), late-stage dementia, post–cardiac arrest, metastatic cancer, end-stage organ failure, recurrent ICU admissions, an APACHE II score of 14 or higher, a SOFA score of 9 or higher, preexisting functional dependency, or consideration for a tracheostomy or permanent feeding tube.
Of 29 patients randomized, 14 received early palliative care, and 15 received standard palliative care, which was defined as starting “whenever the treating team deems (it) is appropriate,” according to the published abstract.
Hospital mortality occurred in none of the patients in the early palliative care group, versus six in the usual care group (P = .01), Dr. Helgeson and colleagues found. Moreover, seven health care surrogates were chosen in hospital in the early palliative care group, versus none in the usual care group (P less than .01).
Length of stay in the ICU or in hospital did not vary by treatment group, according to the investigators.
About one-fifth of deaths in the United States take place in or around ICU admissions, according to the investigators, who noted that those admissions can result in changing goals from cure to comfort – though sometimes too late.
Dr. Helgeson and coauthors disclosed that they had no relationships relevant to this research presentation.
SOURCE: Helgeson S, et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.803.
NEW ORLEANS – When initiated early, meeting certain end-of-life criteria, results of a recent randomized clinical trial suggest.
The rate of in-hospital mortality was lower for critical care patients receiving an early consultation, compared with those who received palliative care initiated according to usual standards in the randomized, controlled trial, described at the annual meeting of the American College of Chest Physicians.
In addition, more health care surrogates were chosen in the hospital when palliative care medicine was involved earlier, according to investigator Scott Helgeson, MD, fellow in pulmonary critical care at the Mayo Clinic in Jacksonville, Fla.
Taken together, Dr. Helgeson said, those findings suggest the importance of getting palliative care involved “very early, while the patient can still make decisions.”
“There are a lot of things that can get in the way of adequate conversations, and that’s when the palliative care team can come in,” Dr. Helgeson said in an interview.
This study is the first reported to date to look at the impact on patient care outcomes specifically within 24 hours of medical ICU admission, according to Dr. Helgeson and coinvestigators
In their randomized study, patients were eligible if they met at least one of several criteria, including advanced age (80 years or older), late-stage dementia, post–cardiac arrest, metastatic cancer, end-stage organ failure, recurrent ICU admissions, an APACHE II score of 14 or higher, a SOFA score of 9 or higher, preexisting functional dependency, or consideration for a tracheostomy or permanent feeding tube.
Of 29 patients randomized, 14 received early palliative care, and 15 received standard palliative care, which was defined as starting “whenever the treating team deems (it) is appropriate,” according to the published abstract.
Hospital mortality occurred in none of the patients in the early palliative care group, versus six in the usual care group (P = .01), Dr. Helgeson and colleagues found. Moreover, seven health care surrogates were chosen in hospital in the early palliative care group, versus none in the usual care group (P less than .01).
Length of stay in the ICU or in hospital did not vary by treatment group, according to the investigators.
About one-fifth of deaths in the United States take place in or around ICU admissions, according to the investigators, who noted that those admissions can result in changing goals from cure to comfort – though sometimes too late.
Dr. Helgeson and coauthors disclosed that they had no relationships relevant to this research presentation.
SOURCE: Helgeson S, et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.803.
REPORTING FROM CHEST 2019
Flu vaccine: Larger impact on influenza burden than you thought?
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at pdnews@mdedge.com.
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at pdnews@mdedge.com.
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at pdnews@mdedge.com.
Soccer pros may face increased risk of death from neurodegenerative disease
, findings from a retrospective epidemiologic analysis suggest.
Former professional soccer players included in the analysis also received more dementia-related medication prescriptions than did controls, Daniel F. Mackay, PhD, of the Institute of Health and Wellbeing at the University of Glasgow (Scotland) and his colleagues reported online Oct. 21 in The New England Journal of Medicine.
Overall mortality during a median follow-up of 18 years from study entry at the age of 40 years was 15.4% among 7,676 former players, and 16.5% among 23,028 controls matched based on age, sex, and degree of social deprivation. All-cause mortality was lower among players versus controls before age 70 years, and was higher thereafter, and the mortality rates associated with ischemic heart disease and lung cancer were lower among the players (hazard ratios, 0.80 and 0.53, respectively), the investigators found.
Mortality rates from stroke or cerebrovascular disease were similar in the players and controls (HR, 0.88), they noted.
However, mortality with neurodegenerative disease listed as the primary cause was 1.7% in players versus 0.5% in controls (HR adjusted for competing risks of death, 3.45), they said. The estimated risk of death with neurodegenerative disease was highest among those with Alzheimer’s disease and lowest for those with Parkinson’s disease (HRs, 5.07 and 2.15, respectively).
Dementia-related medications also were prescribed more frequently for players vs. controls (odds ratio, 4.90).
A subgroup analysis showed no significant difference between goalkeepers and outfielders with respect to mortality with neurodegenerative disease listed as a factor (HR, 0.73), but dementia-related medications were prescribed less often to goalkeepers (OR, 0.41).
Concerns about the risk of neurodegenerative diseases among participants in contact sports have been raised, in part because of the recognition of pathologic changes of chronic traumatic encephalopathy among participants across a range of such sports, the investigators explained, noting that data regarding the risk of neurodegenerative disease among former professional soccer players are limited.
The findings of the current study, in terms of lower all-cause mortality up to the age of 70 years, are similar to those in previous studies involving elite athletes across a range of sports, and “may reflect higher levels of physical activity and lower levels of obesity and smoking in elite athletes than in the general population,” they noted.
“In contrast, mortality from neurodegenerative disease was higher among former soccer players, a finding consistent with studies involving former players in the U.S. National Football League,” they added, concluding that the findings, which “may be valuable to inform the management of risks in the sport,” require confirmation in prospective studies.
This study was supported by the Football Association and Professional Footballers’ Association, and by an NHS Research Scotland Career Researcher Fellowship. Dr. Mackay reported having no relevant financial disclosures.
SOURCE: Mackay D et al. N Engl J Med. 2019 Oct 21. doi: 10.1056/NEJMoa1908483.
The good news from the study by Mackay et al. is that mortality from common nonneurologic diseases is lower among former elite soccer players vs. controls; the bad news is that mortality from neurodegenerative diseases is higher and prescriptions for dementia-related medications more common, Robert A. Stern, PhD, wrote in an editorial.
The findings add to existing evidence that repetitive head impact in contact sports may increase the risk of neurodegenerative disease and dementia, but “should not engender undue fear and panic among soccer players, parents, and coaches,” as the findings cannot be generalized to recreational, amateur, or collegiate-level soccer, Dr. Stern said.
The findings should, however, lead to research and awareness of potential consequences of heading the ball in amateur soccer, he argued, noting that “perhaps ... there is already adequate evidence that repeated blows to the brain from heading in professional soccer is an occupational risk that needs to be addressed.”
Dr. Stern is with the Boston University Chronic Traumatic Encephalopathy Center, Boston University. He disclosed financial relationships (receipt of grants, personal fees, and/or other relationships outside the submitted work) with the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the Concussion Legacy Foundation, Biogen, Eli Lilly, Psychological Assessment Resources, and King Devick Technologies.
The good news from the study by Mackay et al. is that mortality from common nonneurologic diseases is lower among former elite soccer players vs. controls; the bad news is that mortality from neurodegenerative diseases is higher and prescriptions for dementia-related medications more common, Robert A. Stern, PhD, wrote in an editorial.
The findings add to existing evidence that repetitive head impact in contact sports may increase the risk of neurodegenerative disease and dementia, but “should not engender undue fear and panic among soccer players, parents, and coaches,” as the findings cannot be generalized to recreational, amateur, or collegiate-level soccer, Dr. Stern said.
The findings should, however, lead to research and awareness of potential consequences of heading the ball in amateur soccer, he argued, noting that “perhaps ... there is already adequate evidence that repeated blows to the brain from heading in professional soccer is an occupational risk that needs to be addressed.”
Dr. Stern is with the Boston University Chronic Traumatic Encephalopathy Center, Boston University. He disclosed financial relationships (receipt of grants, personal fees, and/or other relationships outside the submitted work) with the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the Concussion Legacy Foundation, Biogen, Eli Lilly, Psychological Assessment Resources, and King Devick Technologies.
The good news from the study by Mackay et al. is that mortality from common nonneurologic diseases is lower among former elite soccer players vs. controls; the bad news is that mortality from neurodegenerative diseases is higher and prescriptions for dementia-related medications more common, Robert A. Stern, PhD, wrote in an editorial.
The findings add to existing evidence that repetitive head impact in contact sports may increase the risk of neurodegenerative disease and dementia, but “should not engender undue fear and panic among soccer players, parents, and coaches,” as the findings cannot be generalized to recreational, amateur, or collegiate-level soccer, Dr. Stern said.
The findings should, however, lead to research and awareness of potential consequences of heading the ball in amateur soccer, he argued, noting that “perhaps ... there is already adequate evidence that repeated blows to the brain from heading in professional soccer is an occupational risk that needs to be addressed.”
Dr. Stern is with the Boston University Chronic Traumatic Encephalopathy Center, Boston University. He disclosed financial relationships (receipt of grants, personal fees, and/or other relationships outside the submitted work) with the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the Concussion Legacy Foundation, Biogen, Eli Lilly, Psychological Assessment Resources, and King Devick Technologies.
, findings from a retrospective epidemiologic analysis suggest.
Former professional soccer players included in the analysis also received more dementia-related medication prescriptions than did controls, Daniel F. Mackay, PhD, of the Institute of Health and Wellbeing at the University of Glasgow (Scotland) and his colleagues reported online Oct. 21 in The New England Journal of Medicine.
Overall mortality during a median follow-up of 18 years from study entry at the age of 40 years was 15.4% among 7,676 former players, and 16.5% among 23,028 controls matched based on age, sex, and degree of social deprivation. All-cause mortality was lower among players versus controls before age 70 years, and was higher thereafter, and the mortality rates associated with ischemic heart disease and lung cancer were lower among the players (hazard ratios, 0.80 and 0.53, respectively), the investigators found.
Mortality rates from stroke or cerebrovascular disease were similar in the players and controls (HR, 0.88), they noted.
However, mortality with neurodegenerative disease listed as the primary cause was 1.7% in players versus 0.5% in controls (HR adjusted for competing risks of death, 3.45), they said. The estimated risk of death with neurodegenerative disease was highest among those with Alzheimer’s disease and lowest for those with Parkinson’s disease (HRs, 5.07 and 2.15, respectively).
Dementia-related medications also were prescribed more frequently for players vs. controls (odds ratio, 4.90).
A subgroup analysis showed no significant difference between goalkeepers and outfielders with respect to mortality with neurodegenerative disease listed as a factor (HR, 0.73), but dementia-related medications were prescribed less often to goalkeepers (OR, 0.41).
Concerns about the risk of neurodegenerative diseases among participants in contact sports have been raised, in part because of the recognition of pathologic changes of chronic traumatic encephalopathy among participants across a range of such sports, the investigators explained, noting that data regarding the risk of neurodegenerative disease among former professional soccer players are limited.
The findings of the current study, in terms of lower all-cause mortality up to the age of 70 years, are similar to those in previous studies involving elite athletes across a range of sports, and “may reflect higher levels of physical activity and lower levels of obesity and smoking in elite athletes than in the general population,” they noted.
“In contrast, mortality from neurodegenerative disease was higher among former soccer players, a finding consistent with studies involving former players in the U.S. National Football League,” they added, concluding that the findings, which “may be valuable to inform the management of risks in the sport,” require confirmation in prospective studies.
This study was supported by the Football Association and Professional Footballers’ Association, and by an NHS Research Scotland Career Researcher Fellowship. Dr. Mackay reported having no relevant financial disclosures.
SOURCE: Mackay D et al. N Engl J Med. 2019 Oct 21. doi: 10.1056/NEJMoa1908483.
, findings from a retrospective epidemiologic analysis suggest.
Former professional soccer players included in the analysis also received more dementia-related medication prescriptions than did controls, Daniel F. Mackay, PhD, of the Institute of Health and Wellbeing at the University of Glasgow (Scotland) and his colleagues reported online Oct. 21 in The New England Journal of Medicine.
Overall mortality during a median follow-up of 18 years from study entry at the age of 40 years was 15.4% among 7,676 former players, and 16.5% among 23,028 controls matched based on age, sex, and degree of social deprivation. All-cause mortality was lower among players versus controls before age 70 years, and was higher thereafter, and the mortality rates associated with ischemic heart disease and lung cancer were lower among the players (hazard ratios, 0.80 and 0.53, respectively), the investigators found.
Mortality rates from stroke or cerebrovascular disease were similar in the players and controls (HR, 0.88), they noted.
However, mortality with neurodegenerative disease listed as the primary cause was 1.7% in players versus 0.5% in controls (HR adjusted for competing risks of death, 3.45), they said. The estimated risk of death with neurodegenerative disease was highest among those with Alzheimer’s disease and lowest for those with Parkinson’s disease (HRs, 5.07 and 2.15, respectively).
Dementia-related medications also were prescribed more frequently for players vs. controls (odds ratio, 4.90).
A subgroup analysis showed no significant difference between goalkeepers and outfielders with respect to mortality with neurodegenerative disease listed as a factor (HR, 0.73), but dementia-related medications were prescribed less often to goalkeepers (OR, 0.41).
Concerns about the risk of neurodegenerative diseases among participants in contact sports have been raised, in part because of the recognition of pathologic changes of chronic traumatic encephalopathy among participants across a range of such sports, the investigators explained, noting that data regarding the risk of neurodegenerative disease among former professional soccer players are limited.
The findings of the current study, in terms of lower all-cause mortality up to the age of 70 years, are similar to those in previous studies involving elite athletes across a range of sports, and “may reflect higher levels of physical activity and lower levels of obesity and smoking in elite athletes than in the general population,” they noted.
“In contrast, mortality from neurodegenerative disease was higher among former soccer players, a finding consistent with studies involving former players in the U.S. National Football League,” they added, concluding that the findings, which “may be valuable to inform the management of risks in the sport,” require confirmation in prospective studies.
This study was supported by the Football Association and Professional Footballers’ Association, and by an NHS Research Scotland Career Researcher Fellowship. Dr. Mackay reported having no relevant financial disclosures.
SOURCE: Mackay D et al. N Engl J Med. 2019 Oct 21. doi: 10.1056/NEJMoa1908483.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Updated international consensus recommendations on management of acute upper GI bleeding
Guidelines on the management of acute upper gastrointestinal bleeding have been updated, including recommendations on managing patients on antiplatelet or anticoagulant therapy and on use of endoscopy and new therapeutic approaches.
Writing in Annals of Internal Medicine, an international group of experts published an update to the 2010 International Consensus Recommendations on the Management of Patients With Nonvariceal Upper Gastrointestinal Bleeding, with a focus on resuscitation and risk assessment; pre-endoscopic, endoscopic, and pharmacologic management; and secondary prophylaxis.
Alan N. Barkun, MDCM, MSc, from McGill University, Montreal, and coauthors first recommended that fluid resuscitation should be initiated in patients with acute upper gastrointestinal bleeding and hemodynamic instability to avoid hemorrhagic shock and restore end-organ perfusion and tissue oxygenation while the bleeding is brought under control.
They acknowledged the uncertainty around whether colloid or crystalloid fluid should be used, but suggested routine use of colloids was not justified because they were more expensive and did not appear to increase survival.
On the question of whether the resuscitation should be aggressive or restrictive in its timing and rate, the group said there was not enough evidence to support a recommendation on this. “The important issue in patients with hemorrhagic shock due to trauma or UGIB [upper gastrointestinal bleeding] is to stop the bleeding while minimizing hemodynamic compromise,” they wrote.
They also advised blood transfusions in patients with a hemoglobin level below 80 g/L who did not have underlying cardiovascular disease, but suggested a higher hemoglobin threshold for those with underlying cardiovascular disease.
The second recommendation was that patients with a Glasgow Blatchford score of 1 or less were at very low risk for rebleeding and mortality, and these patients may therefore not need hospitalization or inpatient endoscopy. They advised against using the AIMS65 prognostic score for this purpose because it was designed to identify patients at high risk of death, not those at low risk for safe discharge.
In regard to endoscopic management, they advocated that all patients with acute upper gastrointestinal bleeding – whether low or high risk – undergo endoscopy within 24 hours of presentation. This was even more urgent in patients being treated with anticoagulants. “Because of the recognized benefits of early endoscopy, coagulopathy should be treated as necessary but endoscopy should not be delayed,” they wrote.
Patients with acutely bleeding ulcers with high-risk stigmata should undergo endoscopic therapy preferably with thermocoagulation or sclerosant injection, or with hemoclips depending on the bleeding location and patient characteristics.
The group also included two conditional recommendations, based on very-low-quality evidence, that patients with actively bleeding ulcers receive TC-325 hemostatic powder as temporizing therapy to stop the bleeding if conventional endoscopic therapies aren’t available or fail. However, they stressed that TC-325 should not be used as a single therapeutic strategy.
Because of a lack of efficacy data and low availability of expertise in the technology, the authors said they could not make a recommendation for or against using a Doppler endoscopic probe (DEP) to assess the need for further endoscopic therapy.
“The group generally agreed that although making a recommendation for or against using DEP to manage UGIB is premature, DEP has the potential to alter the usual approach to visually assessing bleeding lesion risk when evaluating the need for, and adequacy of, endoscopic hemostasis.”
The guidelines also addressed the issue of pharmacologic management of acute upper gastrointestinal bleeding. They strongly recommended that patients with bleeding ulcers and high-risk stigmata who have undergone successful endoscopic therapy should then receive an intravenous loading dose of proton pump inhibitor (PPI) therapy, followed by continuous intravenous infusion.
“Cost-effectiveness studies have suggested that high-dose intravenous PPIs after successful endoscopic hemostasis improve outcomes at a modest cost increase relative to non–high-dose intravenous or oral PPI strategies,” they wrote.
A second conditional recommendation, based on very-low-quality evidence, was that patients with a bleeding ulcer who were at high risk for rebleeding be also treated twice-daily with oral PPIs for 2 weeks, then once-daily. They also recommended patients on cardiovascular prophylaxis with single or dual antiplatelet therapy or anticoagulant therapy be given PPIs.
“The consensus group concluded that, for high-risk patients with an ongoing need for anticoagulants, the evidence suggests that the benefits of secondary prophylaxis outweigh the risks.”
The group was supported by a grant from CIHR Institute of Nutrition, Metabolism and Diabetes and from the Saudi Gastroenterology Association. Nine authors declared grants, personal fees, honoraria and other funding from the pharmaceutical and medical device sector outside the submitted work. No other conflicts of interest were declared.
SOURCE: Barkun A et al. Ann Intern Med 2019, October 22. doi: 10.7326/M19-1795.
These updated consensus guidelines provide a rigorous review of evidence on managing nonvariceal upper gastrointestinal bleeding. The recommendations for patients on anticoagulant or antiplatelet therapy will be particularly helpful because of increasing use of these medications. The advice on proton pump inhibitor therapy in patients on these drugs who have had previous ulcer bleeding can help allay concerns about possible integrations between PPIs and clopidogrel.
While the guidelines recommend using the Glasgow Blatchford scale to guide hospitalization decisions, prognostic scores are not commonly used in the emergency department, and many patients present with a Glasgow Blatchford score greater than 1, so this tool may have little impact on hospitalization rates. More studies are needed to compare clinical judgment with these prognostic scores.
Angel Lanas, MD, is from the University Clinic Hospital at the University of Zaragoza (Spain). These comments are adapted from an accompanying editorial (Ann Intern Med 2019, October 22. doi: 10.7326/M19-2789). Dr. Lanas declared unrelated personal fees from the pharmaceutical sector.
These updated consensus guidelines provide a rigorous review of evidence on managing nonvariceal upper gastrointestinal bleeding. The recommendations for patients on anticoagulant or antiplatelet therapy will be particularly helpful because of increasing use of these medications. The advice on proton pump inhibitor therapy in patients on these drugs who have had previous ulcer bleeding can help allay concerns about possible integrations between PPIs and clopidogrel.
While the guidelines recommend using the Glasgow Blatchford scale to guide hospitalization decisions, prognostic scores are not commonly used in the emergency department, and many patients present with a Glasgow Blatchford score greater than 1, so this tool may have little impact on hospitalization rates. More studies are needed to compare clinical judgment with these prognostic scores.
Angel Lanas, MD, is from the University Clinic Hospital at the University of Zaragoza (Spain). These comments are adapted from an accompanying editorial (Ann Intern Med 2019, October 22. doi: 10.7326/M19-2789). Dr. Lanas declared unrelated personal fees from the pharmaceutical sector.
These updated consensus guidelines provide a rigorous review of evidence on managing nonvariceal upper gastrointestinal bleeding. The recommendations for patients on anticoagulant or antiplatelet therapy will be particularly helpful because of increasing use of these medications. The advice on proton pump inhibitor therapy in patients on these drugs who have had previous ulcer bleeding can help allay concerns about possible integrations between PPIs and clopidogrel.
While the guidelines recommend using the Glasgow Blatchford scale to guide hospitalization decisions, prognostic scores are not commonly used in the emergency department, and many patients present with a Glasgow Blatchford score greater than 1, so this tool may have little impact on hospitalization rates. More studies are needed to compare clinical judgment with these prognostic scores.
Angel Lanas, MD, is from the University Clinic Hospital at the University of Zaragoza (Spain). These comments are adapted from an accompanying editorial (Ann Intern Med 2019, October 22. doi: 10.7326/M19-2789). Dr. Lanas declared unrelated personal fees from the pharmaceutical sector.
Guidelines on the management of acute upper gastrointestinal bleeding have been updated, including recommendations on managing patients on antiplatelet or anticoagulant therapy and on use of endoscopy and new therapeutic approaches.
Writing in Annals of Internal Medicine, an international group of experts published an update to the 2010 International Consensus Recommendations on the Management of Patients With Nonvariceal Upper Gastrointestinal Bleeding, with a focus on resuscitation and risk assessment; pre-endoscopic, endoscopic, and pharmacologic management; and secondary prophylaxis.
Alan N. Barkun, MDCM, MSc, from McGill University, Montreal, and coauthors first recommended that fluid resuscitation should be initiated in patients with acute upper gastrointestinal bleeding and hemodynamic instability to avoid hemorrhagic shock and restore end-organ perfusion and tissue oxygenation while the bleeding is brought under control.
They acknowledged the uncertainty around whether colloid or crystalloid fluid should be used, but suggested routine use of colloids was not justified because they were more expensive and did not appear to increase survival.
On the question of whether the resuscitation should be aggressive or restrictive in its timing and rate, the group said there was not enough evidence to support a recommendation on this. “The important issue in patients with hemorrhagic shock due to trauma or UGIB [upper gastrointestinal bleeding] is to stop the bleeding while minimizing hemodynamic compromise,” they wrote.
They also advised blood transfusions in patients with a hemoglobin level below 80 g/L who did not have underlying cardiovascular disease, but suggested a higher hemoglobin threshold for those with underlying cardiovascular disease.
The second recommendation was that patients with a Glasgow Blatchford score of 1 or less were at very low risk for rebleeding and mortality, and these patients may therefore not need hospitalization or inpatient endoscopy. They advised against using the AIMS65 prognostic score for this purpose because it was designed to identify patients at high risk of death, not those at low risk for safe discharge.
In regard to endoscopic management, they advocated that all patients with acute upper gastrointestinal bleeding – whether low or high risk – undergo endoscopy within 24 hours of presentation. This was even more urgent in patients being treated with anticoagulants. “Because of the recognized benefits of early endoscopy, coagulopathy should be treated as necessary but endoscopy should not be delayed,” they wrote.
Patients with acutely bleeding ulcers with high-risk stigmata should undergo endoscopic therapy preferably with thermocoagulation or sclerosant injection, or with hemoclips depending on the bleeding location and patient characteristics.
The group also included two conditional recommendations, based on very-low-quality evidence, that patients with actively bleeding ulcers receive TC-325 hemostatic powder as temporizing therapy to stop the bleeding if conventional endoscopic therapies aren’t available or fail. However, they stressed that TC-325 should not be used as a single therapeutic strategy.
Because of a lack of efficacy data and low availability of expertise in the technology, the authors said they could not make a recommendation for or against using a Doppler endoscopic probe (DEP) to assess the need for further endoscopic therapy.
“The group generally agreed that although making a recommendation for or against using DEP to manage UGIB is premature, DEP has the potential to alter the usual approach to visually assessing bleeding lesion risk when evaluating the need for, and adequacy of, endoscopic hemostasis.”
The guidelines also addressed the issue of pharmacologic management of acute upper gastrointestinal bleeding. They strongly recommended that patients with bleeding ulcers and high-risk stigmata who have undergone successful endoscopic therapy should then receive an intravenous loading dose of proton pump inhibitor (PPI) therapy, followed by continuous intravenous infusion.
“Cost-effectiveness studies have suggested that high-dose intravenous PPIs after successful endoscopic hemostasis improve outcomes at a modest cost increase relative to non–high-dose intravenous or oral PPI strategies,” they wrote.
A second conditional recommendation, based on very-low-quality evidence, was that patients with a bleeding ulcer who were at high risk for rebleeding be also treated twice-daily with oral PPIs for 2 weeks, then once-daily. They also recommended patients on cardiovascular prophylaxis with single or dual antiplatelet therapy or anticoagulant therapy be given PPIs.
“The consensus group concluded that, for high-risk patients with an ongoing need for anticoagulants, the evidence suggests that the benefits of secondary prophylaxis outweigh the risks.”
The group was supported by a grant from CIHR Institute of Nutrition, Metabolism and Diabetes and from the Saudi Gastroenterology Association. Nine authors declared grants, personal fees, honoraria and other funding from the pharmaceutical and medical device sector outside the submitted work. No other conflicts of interest were declared.
SOURCE: Barkun A et al. Ann Intern Med 2019, October 22. doi: 10.7326/M19-1795.
Guidelines on the management of acute upper gastrointestinal bleeding have been updated, including recommendations on managing patients on antiplatelet or anticoagulant therapy and on use of endoscopy and new therapeutic approaches.
Writing in Annals of Internal Medicine, an international group of experts published an update to the 2010 International Consensus Recommendations on the Management of Patients With Nonvariceal Upper Gastrointestinal Bleeding, with a focus on resuscitation and risk assessment; pre-endoscopic, endoscopic, and pharmacologic management; and secondary prophylaxis.
Alan N. Barkun, MDCM, MSc, from McGill University, Montreal, and coauthors first recommended that fluid resuscitation should be initiated in patients with acute upper gastrointestinal bleeding and hemodynamic instability to avoid hemorrhagic shock and restore end-organ perfusion and tissue oxygenation while the bleeding is brought under control.
They acknowledged the uncertainty around whether colloid or crystalloid fluid should be used, but suggested routine use of colloids was not justified because they were more expensive and did not appear to increase survival.
On the question of whether the resuscitation should be aggressive or restrictive in its timing and rate, the group said there was not enough evidence to support a recommendation on this. “The important issue in patients with hemorrhagic shock due to trauma or UGIB [upper gastrointestinal bleeding] is to stop the bleeding while minimizing hemodynamic compromise,” they wrote.
They also advised blood transfusions in patients with a hemoglobin level below 80 g/L who did not have underlying cardiovascular disease, but suggested a higher hemoglobin threshold for those with underlying cardiovascular disease.
The second recommendation was that patients with a Glasgow Blatchford score of 1 or less were at very low risk for rebleeding and mortality, and these patients may therefore not need hospitalization or inpatient endoscopy. They advised against using the AIMS65 prognostic score for this purpose because it was designed to identify patients at high risk of death, not those at low risk for safe discharge.
In regard to endoscopic management, they advocated that all patients with acute upper gastrointestinal bleeding – whether low or high risk – undergo endoscopy within 24 hours of presentation. This was even more urgent in patients being treated with anticoagulants. “Because of the recognized benefits of early endoscopy, coagulopathy should be treated as necessary but endoscopy should not be delayed,” they wrote.
Patients with acutely bleeding ulcers with high-risk stigmata should undergo endoscopic therapy preferably with thermocoagulation or sclerosant injection, or with hemoclips depending on the bleeding location and patient characteristics.
The group also included two conditional recommendations, based on very-low-quality evidence, that patients with actively bleeding ulcers receive TC-325 hemostatic powder as temporizing therapy to stop the bleeding if conventional endoscopic therapies aren’t available or fail. However, they stressed that TC-325 should not be used as a single therapeutic strategy.
Because of a lack of efficacy data and low availability of expertise in the technology, the authors said they could not make a recommendation for or against using a Doppler endoscopic probe (DEP) to assess the need for further endoscopic therapy.
“The group generally agreed that although making a recommendation for or against using DEP to manage UGIB is premature, DEP has the potential to alter the usual approach to visually assessing bleeding lesion risk when evaluating the need for, and adequacy of, endoscopic hemostasis.”
The guidelines also addressed the issue of pharmacologic management of acute upper gastrointestinal bleeding. They strongly recommended that patients with bleeding ulcers and high-risk stigmata who have undergone successful endoscopic therapy should then receive an intravenous loading dose of proton pump inhibitor (PPI) therapy, followed by continuous intravenous infusion.
“Cost-effectiveness studies have suggested that high-dose intravenous PPIs after successful endoscopic hemostasis improve outcomes at a modest cost increase relative to non–high-dose intravenous or oral PPI strategies,” they wrote.
A second conditional recommendation, based on very-low-quality evidence, was that patients with a bleeding ulcer who were at high risk for rebleeding be also treated twice-daily with oral PPIs for 2 weeks, then once-daily. They also recommended patients on cardiovascular prophylaxis with single or dual antiplatelet therapy or anticoagulant therapy be given PPIs.
“The consensus group concluded that, for high-risk patients with an ongoing need for anticoagulants, the evidence suggests that the benefits of secondary prophylaxis outweigh the risks.”
The group was supported by a grant from CIHR Institute of Nutrition, Metabolism and Diabetes and from the Saudi Gastroenterology Association. Nine authors declared grants, personal fees, honoraria and other funding from the pharmaceutical and medical device sector outside the submitted work. No other conflicts of interest were declared.
SOURCE: Barkun A et al. Ann Intern Med 2019, October 22. doi: 10.7326/M19-1795.
FROM ANNALS OF INTERNAL MEDICINE
Race mismatch may affect survival in lung transplant setting
NEW ORLEANS – Race compatibility is a factor that can affect survival and needs to be considered when matching lung transplant candidates to potential donors, results from a large retrospective analysis suggest.
Specifically, whites had significantly worse survival when receiving lungs from African American donors in this registry analysis, according to study investigator Alexis Kofi Okoh, MD.
By contrast, donor-to-recipient race compatibility (DRRC) did not affect posttransplant survival among African American or Hispanic patients, said Dr. Okoh, who is with the lung transplant division at the Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J.
While race mismatch has been shown to affect outcomes in kidney, heart, and liver transplant settings, the data for DRRC in lung transplant prior to this analysis generally has been limited to small, single-center studies, according to Dr. Okoh.
“If you do have the option, [race compatibility] should highly be considered, because it clearly has an impact on outcomes,” Dr. Okoh said in an interview here at the annual meeting of the American College of Chest Physicians.
Considering the race of both donor and recipient is especially important now that the lung transplant population is becoming more ethnically diverse, he added.
The study was based on an analysis of 19,504 lung transplant recipients in the prospectively maintained United Network for Organ Sharing (UNOS) database during 2006-2018. In that cohort, 16,485 recipients were white, 1,787 were African American, and 1,232 were Hispanic.
Race-matched donor organs were used in two-thirds (66.2%) of white recipients, about one-quarter (26.8%) of African American recipients, and one-third (33.0%) of Hispanic recipients.
Overall, survival post–lung transplant was significantly poorer among recipients who did not receive a race-matched organ in Kaplan-Meier survival estimates, Dr. Okoh said, though, that this effect was diminished after they adjusted for patient baseline characteristics (P = 0.2809).
For African American recipients, the unadjusted and adjusted survival estimates were no different regardless of donor race, and likewise, there were no apparent survival differences between Hispanic recipients who received race matched or mismatched organs.
Survival among white recipients, however, was significantly affected by race of the recipient, with decreased survival estimates noted even after adjustment for patient characteristics, according to Dr. Okoh’s presentation.
Results of regression analysis showed that white recipient/African American donor was the only race mismatch to significantly affect survival, Dr. Okoh said in the interview.
The posttransplant survival hazard ratios (and 95% confidence intervals) reported by Dr. Okoh with a no race mismatch serving as reference were 1.15 (1.08-1.23) for whites with African American donors, and 1.09 (1.01-1.18) for whites with Hispanic donors.
Dr. Okoh and coinvestigators reported no relevant conflicts in relation to their study.
SOURCE: Okoh A et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.220.
NEW ORLEANS – Race compatibility is a factor that can affect survival and needs to be considered when matching lung transplant candidates to potential donors, results from a large retrospective analysis suggest.
Specifically, whites had significantly worse survival when receiving lungs from African American donors in this registry analysis, according to study investigator Alexis Kofi Okoh, MD.
By contrast, donor-to-recipient race compatibility (DRRC) did not affect posttransplant survival among African American or Hispanic patients, said Dr. Okoh, who is with the lung transplant division at the Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J.
While race mismatch has been shown to affect outcomes in kidney, heart, and liver transplant settings, the data for DRRC in lung transplant prior to this analysis generally has been limited to small, single-center studies, according to Dr. Okoh.
“If you do have the option, [race compatibility] should highly be considered, because it clearly has an impact on outcomes,” Dr. Okoh said in an interview here at the annual meeting of the American College of Chest Physicians.
Considering the race of both donor and recipient is especially important now that the lung transplant population is becoming more ethnically diverse, he added.
The study was based on an analysis of 19,504 lung transplant recipients in the prospectively maintained United Network for Organ Sharing (UNOS) database during 2006-2018. In that cohort, 16,485 recipients were white, 1,787 were African American, and 1,232 were Hispanic.
Race-matched donor organs were used in two-thirds (66.2%) of white recipients, about one-quarter (26.8%) of African American recipients, and one-third (33.0%) of Hispanic recipients.
Overall, survival post–lung transplant was significantly poorer among recipients who did not receive a race-matched organ in Kaplan-Meier survival estimates, Dr. Okoh said, though, that this effect was diminished after they adjusted for patient baseline characteristics (P = 0.2809).
For African American recipients, the unadjusted and adjusted survival estimates were no different regardless of donor race, and likewise, there were no apparent survival differences between Hispanic recipients who received race matched or mismatched organs.
Survival among white recipients, however, was significantly affected by race of the recipient, with decreased survival estimates noted even after adjustment for patient characteristics, according to Dr. Okoh’s presentation.
Results of regression analysis showed that white recipient/African American donor was the only race mismatch to significantly affect survival, Dr. Okoh said in the interview.
The posttransplant survival hazard ratios (and 95% confidence intervals) reported by Dr. Okoh with a no race mismatch serving as reference were 1.15 (1.08-1.23) for whites with African American donors, and 1.09 (1.01-1.18) for whites with Hispanic donors.
Dr. Okoh and coinvestigators reported no relevant conflicts in relation to their study.
SOURCE: Okoh A et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.220.
NEW ORLEANS – Race compatibility is a factor that can affect survival and needs to be considered when matching lung transplant candidates to potential donors, results from a large retrospective analysis suggest.
Specifically, whites had significantly worse survival when receiving lungs from African American donors in this registry analysis, according to study investigator Alexis Kofi Okoh, MD.
By contrast, donor-to-recipient race compatibility (DRRC) did not affect posttransplant survival among African American or Hispanic patients, said Dr. Okoh, who is with the lung transplant division at the Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J.
While race mismatch has been shown to affect outcomes in kidney, heart, and liver transplant settings, the data for DRRC in lung transplant prior to this analysis generally has been limited to small, single-center studies, according to Dr. Okoh.
“If you do have the option, [race compatibility] should highly be considered, because it clearly has an impact on outcomes,” Dr. Okoh said in an interview here at the annual meeting of the American College of Chest Physicians.
Considering the race of both donor and recipient is especially important now that the lung transplant population is becoming more ethnically diverse, he added.
The study was based on an analysis of 19,504 lung transplant recipients in the prospectively maintained United Network for Organ Sharing (UNOS) database during 2006-2018. In that cohort, 16,485 recipients were white, 1,787 were African American, and 1,232 were Hispanic.
Race-matched donor organs were used in two-thirds (66.2%) of white recipients, about one-quarter (26.8%) of African American recipients, and one-third (33.0%) of Hispanic recipients.
Overall, survival post–lung transplant was significantly poorer among recipients who did not receive a race-matched organ in Kaplan-Meier survival estimates, Dr. Okoh said, though, that this effect was diminished after they adjusted for patient baseline characteristics (P = 0.2809).
For African American recipients, the unadjusted and adjusted survival estimates were no different regardless of donor race, and likewise, there were no apparent survival differences between Hispanic recipients who received race matched or mismatched organs.
Survival among white recipients, however, was significantly affected by race of the recipient, with decreased survival estimates noted even after adjustment for patient characteristics, according to Dr. Okoh’s presentation.
Results of regression analysis showed that white recipient/African American donor was the only race mismatch to significantly affect survival, Dr. Okoh said in the interview.
The posttransplant survival hazard ratios (and 95% confidence intervals) reported by Dr. Okoh with a no race mismatch serving as reference were 1.15 (1.08-1.23) for whites with African American donors, and 1.09 (1.01-1.18) for whites with Hispanic donors.
Dr. Okoh and coinvestigators reported no relevant conflicts in relation to their study.
SOURCE: Okoh A et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.220.
REPORTING FROM CHEST 2019
Dogs steal the show again at CHEST 2019
NEW ORLEANS – Wellness among physicians begins with a focus on reducing stress. Attendees of the CHEST annual meeting were given the opportunity to spend some time with the ultimate stress reliever - puppies. A local animal rescue organization, “Take Paws Rescue,” set up a venue for visiting with (and holding and petting) a litter of adoptable puppies. Several attendees decided on the spot to adopt one of these pups. Others just took the opportunity to cuddle.
Christopher Carroll, MD, a specialist in pediatric critical care with Connecticut Children’s Medical Center in Hartford, knows the value of animals to relieve stress in both patients and doctors. He convinced his medical center to allow dogs into the pediatric ICU, with appropriate cautions, to provide stress relief and joy to both young patients and staff.
The puppies will be in the Exhibit Hall every day at the meeting between 11:30 a.m. and 1 p.m.
NEW ORLEANS – Wellness among physicians begins with a focus on reducing stress. Attendees of the CHEST annual meeting were given the opportunity to spend some time with the ultimate stress reliever - puppies. A local animal rescue organization, “Take Paws Rescue,” set up a venue for visiting with (and holding and petting) a litter of adoptable puppies. Several attendees decided on the spot to adopt one of these pups. Others just took the opportunity to cuddle.
Christopher Carroll, MD, a specialist in pediatric critical care with Connecticut Children’s Medical Center in Hartford, knows the value of animals to relieve stress in both patients and doctors. He convinced his medical center to allow dogs into the pediatric ICU, with appropriate cautions, to provide stress relief and joy to both young patients and staff.
The puppies will be in the Exhibit Hall every day at the meeting between 11:30 a.m. and 1 p.m.
NEW ORLEANS – Wellness among physicians begins with a focus on reducing stress. Attendees of the CHEST annual meeting were given the opportunity to spend some time with the ultimate stress reliever - puppies. A local animal rescue organization, “Take Paws Rescue,” set up a venue for visiting with (and holding and petting) a litter of adoptable puppies. Several attendees decided on the spot to adopt one of these pups. Others just took the opportunity to cuddle.
Christopher Carroll, MD, a specialist in pediatric critical care with Connecticut Children’s Medical Center in Hartford, knows the value of animals to relieve stress in both patients and doctors. He convinced his medical center to allow dogs into the pediatric ICU, with appropriate cautions, to provide stress relief and joy to both young patients and staff.
The puppies will be in the Exhibit Hall every day at the meeting between 11:30 a.m. and 1 p.m.
REPORTING FROM CHEST 2019
Digital inhaler reveals uncontrolled asthma
NEW ORLEANS – Data collected by the ProAir Digihaler suggest .
Researchers studied asthma patients who had experienced exacerbations in the previous year. Patients who also had exacerbations while on study used the ProAir Digihaler about twice a day, on average. Patients without on-study exacerbations used the ProAir Digihaler an average of 1.14 times per day.
The daily use among patients without exacerbations suggests their asthma is “still quite uncontrolled,” and, according to guidelines, they may require additional therapy, said Roy Pleasants, PharmD, of the University of North Carolina at Chapel Hill.
Dr. Pleasants presented these findings at the annual meeting of the American College of Chest Physicians.
He and his colleagues conducted a phase 3 study (NCT02969408) of ProAir Digihaler use in adults who had at least one severe clinical asthma exacerbation in the previous 12 months. They had an Asthma Control Questionnaire score of 1.5 or greater, were on moderate-dose inhaled corticosteroids (with or without a long-acting beta-agonist), and had stable asthma controller dosing for at least 3 months.
For this study, the ProAir Digihaler replaced patients’ other rescue medications. The ProAir Digihaler is a digital inhaler that delivers 90 mcg of albuterol per dose, detects the date and time a dose was prepared, and records the inhalation profile. Over a 12-week period, the ProAir Digihaler recorded each use, which was defined as consecutive inhalations within 60 seconds.
Of the 381 patients enrolled in the study, 360 (94.5%) made at least one valid inhalation. The mean age of these patients was 50 years, and 80.6% were female. Of the 360 patients, 64 experienced 78 exacerbations while on study.
Most episodes of inhaler use consisted of a single inhalation (58.9%), although 35.8% consisted of two inhalations, 3.5% consisted of three inhalations, and 1.8% consisted of four or more inhalations.
The mean peak inspiratory flow was 73.18 L/min (standard deviation [SD] 20.33) in patients without exacerbations. Among patients with exacerbations, the mean peak inspiratory flow was 71.36 (SD 23.80) during exacerbation and 74.71 L/min (SD 22.46) outside the exacerbation window, which was 14 days before and after the exacerbation peak.
The mean inhalation volume was 1.45 L (SD 0.75) among patients without exacerbations, 1.44 L (SD 0.66) outside the exacerbation window, and 1.44 L (SD 0.76) during exacerbation. The mean inhalation duration was 1.62 sec (SD 0.88), 1.59 sec (SD 0.77), and 1.61 sec (SD 0.82), respectively.
“If you look at the inhalation volume in the 64 patients who exacerbated, it really didn’t change during exacerbation,” Dr. Pleasants noted. “Essentially, you can say the same thing about inhalation duration.”
Patients without exacerbations used the ProAir Digihaler an average of 1.14 (SD 2.35) times per day. Patients who had at least one exacerbation used the ProAir Digihaler an average of 1.87 (SD 2.78) times per day outside the exacerbation window and 2.43 (SD 3.67) times during exacerbation.
“As you would predict, those exacerbating patients were using more albuterol than patients who were not exacerbating,” Dr. Pleasants said. “Even when they weren’t exacerbating, that frequency of daily albuterol use is pretty much indicating these patients were not so well controlled.”
Dr. Pleasants went on to say that data from the ProAir Digihaler could help identify, in real time, patients with poor asthma control and those with impending exacerbations.
This study was sponsored by Teva, makers of the ProAir Digihaler. Dr. Pleasants disclosed relationships with Teva, Grifols, Sunovion, and Boehringer Ingelheim.
SOURCE: Pleasants R et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.273.
NEW ORLEANS – Data collected by the ProAir Digihaler suggest .
Researchers studied asthma patients who had experienced exacerbations in the previous year. Patients who also had exacerbations while on study used the ProAir Digihaler about twice a day, on average. Patients without on-study exacerbations used the ProAir Digihaler an average of 1.14 times per day.
The daily use among patients without exacerbations suggests their asthma is “still quite uncontrolled,” and, according to guidelines, they may require additional therapy, said Roy Pleasants, PharmD, of the University of North Carolina at Chapel Hill.
Dr. Pleasants presented these findings at the annual meeting of the American College of Chest Physicians.
He and his colleagues conducted a phase 3 study (NCT02969408) of ProAir Digihaler use in adults who had at least one severe clinical asthma exacerbation in the previous 12 months. They had an Asthma Control Questionnaire score of 1.5 or greater, were on moderate-dose inhaled corticosteroids (with or without a long-acting beta-agonist), and had stable asthma controller dosing for at least 3 months.
For this study, the ProAir Digihaler replaced patients’ other rescue medications. The ProAir Digihaler is a digital inhaler that delivers 90 mcg of albuterol per dose, detects the date and time a dose was prepared, and records the inhalation profile. Over a 12-week period, the ProAir Digihaler recorded each use, which was defined as consecutive inhalations within 60 seconds.
Of the 381 patients enrolled in the study, 360 (94.5%) made at least one valid inhalation. The mean age of these patients was 50 years, and 80.6% were female. Of the 360 patients, 64 experienced 78 exacerbations while on study.
Most episodes of inhaler use consisted of a single inhalation (58.9%), although 35.8% consisted of two inhalations, 3.5% consisted of three inhalations, and 1.8% consisted of four or more inhalations.
The mean peak inspiratory flow was 73.18 L/min (standard deviation [SD] 20.33) in patients without exacerbations. Among patients with exacerbations, the mean peak inspiratory flow was 71.36 (SD 23.80) during exacerbation and 74.71 L/min (SD 22.46) outside the exacerbation window, which was 14 days before and after the exacerbation peak.
The mean inhalation volume was 1.45 L (SD 0.75) among patients without exacerbations, 1.44 L (SD 0.66) outside the exacerbation window, and 1.44 L (SD 0.76) during exacerbation. The mean inhalation duration was 1.62 sec (SD 0.88), 1.59 sec (SD 0.77), and 1.61 sec (SD 0.82), respectively.
“If you look at the inhalation volume in the 64 patients who exacerbated, it really didn’t change during exacerbation,” Dr. Pleasants noted. “Essentially, you can say the same thing about inhalation duration.”
Patients without exacerbations used the ProAir Digihaler an average of 1.14 (SD 2.35) times per day. Patients who had at least one exacerbation used the ProAir Digihaler an average of 1.87 (SD 2.78) times per day outside the exacerbation window and 2.43 (SD 3.67) times during exacerbation.
“As you would predict, those exacerbating patients were using more albuterol than patients who were not exacerbating,” Dr. Pleasants said. “Even when they weren’t exacerbating, that frequency of daily albuterol use is pretty much indicating these patients were not so well controlled.”
Dr. Pleasants went on to say that data from the ProAir Digihaler could help identify, in real time, patients with poor asthma control and those with impending exacerbations.
This study was sponsored by Teva, makers of the ProAir Digihaler. Dr. Pleasants disclosed relationships with Teva, Grifols, Sunovion, and Boehringer Ingelheim.
SOURCE: Pleasants R et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.273.
NEW ORLEANS – Data collected by the ProAir Digihaler suggest .
Researchers studied asthma patients who had experienced exacerbations in the previous year. Patients who also had exacerbations while on study used the ProAir Digihaler about twice a day, on average. Patients without on-study exacerbations used the ProAir Digihaler an average of 1.14 times per day.
The daily use among patients without exacerbations suggests their asthma is “still quite uncontrolled,” and, according to guidelines, they may require additional therapy, said Roy Pleasants, PharmD, of the University of North Carolina at Chapel Hill.
Dr. Pleasants presented these findings at the annual meeting of the American College of Chest Physicians.
He and his colleagues conducted a phase 3 study (NCT02969408) of ProAir Digihaler use in adults who had at least one severe clinical asthma exacerbation in the previous 12 months. They had an Asthma Control Questionnaire score of 1.5 or greater, were on moderate-dose inhaled corticosteroids (with or without a long-acting beta-agonist), and had stable asthma controller dosing for at least 3 months.
For this study, the ProAir Digihaler replaced patients’ other rescue medications. The ProAir Digihaler is a digital inhaler that delivers 90 mcg of albuterol per dose, detects the date and time a dose was prepared, and records the inhalation profile. Over a 12-week period, the ProAir Digihaler recorded each use, which was defined as consecutive inhalations within 60 seconds.
Of the 381 patients enrolled in the study, 360 (94.5%) made at least one valid inhalation. The mean age of these patients was 50 years, and 80.6% were female. Of the 360 patients, 64 experienced 78 exacerbations while on study.
Most episodes of inhaler use consisted of a single inhalation (58.9%), although 35.8% consisted of two inhalations, 3.5% consisted of three inhalations, and 1.8% consisted of four or more inhalations.
The mean peak inspiratory flow was 73.18 L/min (standard deviation [SD] 20.33) in patients without exacerbations. Among patients with exacerbations, the mean peak inspiratory flow was 71.36 (SD 23.80) during exacerbation and 74.71 L/min (SD 22.46) outside the exacerbation window, which was 14 days before and after the exacerbation peak.
The mean inhalation volume was 1.45 L (SD 0.75) among patients without exacerbations, 1.44 L (SD 0.66) outside the exacerbation window, and 1.44 L (SD 0.76) during exacerbation. The mean inhalation duration was 1.62 sec (SD 0.88), 1.59 sec (SD 0.77), and 1.61 sec (SD 0.82), respectively.
“If you look at the inhalation volume in the 64 patients who exacerbated, it really didn’t change during exacerbation,” Dr. Pleasants noted. “Essentially, you can say the same thing about inhalation duration.”
Patients without exacerbations used the ProAir Digihaler an average of 1.14 (SD 2.35) times per day. Patients who had at least one exacerbation used the ProAir Digihaler an average of 1.87 (SD 2.78) times per day outside the exacerbation window and 2.43 (SD 3.67) times during exacerbation.
“As you would predict, those exacerbating patients were using more albuterol than patients who were not exacerbating,” Dr. Pleasants said. “Even when they weren’t exacerbating, that frequency of daily albuterol use is pretty much indicating these patients were not so well controlled.”
Dr. Pleasants went on to say that data from the ProAir Digihaler could help identify, in real time, patients with poor asthma control and those with impending exacerbations.
This study was sponsored by Teva, makers of the ProAir Digihaler. Dr. Pleasants disclosed relationships with Teva, Grifols, Sunovion, and Boehringer Ingelheim.
SOURCE: Pleasants R et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.273.
REPORTING FROM CHEST 2019
Patiromer allows more CKD patients to continue on spironolactone
PHILADELPHIA – Among patients with chronic kidney disease with resistant hypertension, coadministration of patiromer enables more patients to stay on spironolactone, Bryan Williams, MD, of University College London, said at the scientific meeting of the Heart Failure Society of America.
Having the potassium-binding polymer on board allowed for more persistent use of spironolactone, both in the subgroup of patients with heart failure, and those without, he said in a late-breaking clinical trials session.
In the international, phase 2 AMBER (Spironolactone With Patiromer in the Treatment of Resistant Hypertension in Chronic Kidney Disease) trial, 295 patients with chronic kidney disease (estimated glomerular filtration rate from 25 to 45 mL/min per 1.73 m2) were randomly assigned to treatment with spironolactone either placebo (148) or patiromer (147).
, for a significant between-group difference of 19.5% (P less than .0001). In addition, blood pressure lowering was significantly greater in the patiromer (–11.7 mm Hg) than in the placebo (–10.8 mm Hg) group. Results of the AMBER trial were published concurrently with Dr. Williams’ presentation (Lancet 2019 Sep 15; doi: 10.1016/S0140-6736(19)32135-X).
While spironolactone is a “highly effective” drug, studies supporting guideline recommendations for its use in resistant hypertension have largely excluded patients with advanced chronic kidney disease because of increased risk of developing spironolactone-induced hyperkalemia, Dr. Williams told attendees. It remains unclear, however, whether coadministration of patiromer will improve long-term outcomes. Also, many placebo-treated patients in AMBER were able to continue on spironolactone without the help of patiromer, prompting one attendee to question whether there was a smarter way to target the drug, rather than treating all patients up front.
“I don’t think it’s going to be easy to say, ‘this patient’s going to respond, and this patient’s not going to respond,’ ” Dr. Williams said in response, “but at least we have an opportunity to try now in a group of patients who simply may be denied treatment because of a perception that it is difficult to use spironolactone in them.”
That perception is actually not unreasonable, he added, given that 66% of patients in the placebo group in AMBER developed hyperkalemia, suggesting that spironolactone is “not an easy drug to use” in chronic kidney disease patients.
John Teerlink, MD, of the San Francisco VA Medical Center, said the AMBER study is “another building block” in a series of developments of enabling therapies.
“I think it’s a great message for all of us to begin thinking about other therapies we can use to help modify our use of these potential life-saving therapies,” he said in a panel discussion of the results.
Patiromer’s impact on longer-term outcomes is the focus of DIAMOND, a phase 3, randomized, placebo controlled trial that is currently recruiting. DIAMOND will determine whether giving patiromer to patients who developed hyperkalemia on renin-angiotensin-aldosterone system (RAAS) inhibitors decreases cardiovascular deaths and hospitalizations, by virtue of enabling continued RAAS inhibitor use.
Funding for AMBER came from Relypsa, which markets patiromer (Veltassa). Dr. Williams reported consulting for Relypsa during the conduct of the study, along with disclosures outside the scope of the AMBER study (Daiichi Sankyo, Pfizer, Novartis, Servier, Boehringer Ingelheim, and Vascular Dynamics).
SOURCE: Williams B. HFSA 2019.
PHILADELPHIA – Among patients with chronic kidney disease with resistant hypertension, coadministration of patiromer enables more patients to stay on spironolactone, Bryan Williams, MD, of University College London, said at the scientific meeting of the Heart Failure Society of America.
Having the potassium-binding polymer on board allowed for more persistent use of spironolactone, both in the subgroup of patients with heart failure, and those without, he said in a late-breaking clinical trials session.
In the international, phase 2 AMBER (Spironolactone With Patiromer in the Treatment of Resistant Hypertension in Chronic Kidney Disease) trial, 295 patients with chronic kidney disease (estimated glomerular filtration rate from 25 to 45 mL/min per 1.73 m2) were randomly assigned to treatment with spironolactone either placebo (148) or patiromer (147).
, for a significant between-group difference of 19.5% (P less than .0001). In addition, blood pressure lowering was significantly greater in the patiromer (–11.7 mm Hg) than in the placebo (–10.8 mm Hg) group. Results of the AMBER trial were published concurrently with Dr. Williams’ presentation (Lancet 2019 Sep 15; doi: 10.1016/S0140-6736(19)32135-X).
While spironolactone is a “highly effective” drug, studies supporting guideline recommendations for its use in resistant hypertension have largely excluded patients with advanced chronic kidney disease because of increased risk of developing spironolactone-induced hyperkalemia, Dr. Williams told attendees. It remains unclear, however, whether coadministration of patiromer will improve long-term outcomes. Also, many placebo-treated patients in AMBER were able to continue on spironolactone without the help of patiromer, prompting one attendee to question whether there was a smarter way to target the drug, rather than treating all patients up front.
“I don’t think it’s going to be easy to say, ‘this patient’s going to respond, and this patient’s not going to respond,’ ” Dr. Williams said in response, “but at least we have an opportunity to try now in a group of patients who simply may be denied treatment because of a perception that it is difficult to use spironolactone in them.”
That perception is actually not unreasonable, he added, given that 66% of patients in the placebo group in AMBER developed hyperkalemia, suggesting that spironolactone is “not an easy drug to use” in chronic kidney disease patients.
John Teerlink, MD, of the San Francisco VA Medical Center, said the AMBER study is “another building block” in a series of developments of enabling therapies.
“I think it’s a great message for all of us to begin thinking about other therapies we can use to help modify our use of these potential life-saving therapies,” he said in a panel discussion of the results.
Patiromer’s impact on longer-term outcomes is the focus of DIAMOND, a phase 3, randomized, placebo controlled trial that is currently recruiting. DIAMOND will determine whether giving patiromer to patients who developed hyperkalemia on renin-angiotensin-aldosterone system (RAAS) inhibitors decreases cardiovascular deaths and hospitalizations, by virtue of enabling continued RAAS inhibitor use.
Funding for AMBER came from Relypsa, which markets patiromer (Veltassa). Dr. Williams reported consulting for Relypsa during the conduct of the study, along with disclosures outside the scope of the AMBER study (Daiichi Sankyo, Pfizer, Novartis, Servier, Boehringer Ingelheim, and Vascular Dynamics).
SOURCE: Williams B. HFSA 2019.
PHILADELPHIA – Among patients with chronic kidney disease with resistant hypertension, coadministration of patiromer enables more patients to stay on spironolactone, Bryan Williams, MD, of University College London, said at the scientific meeting of the Heart Failure Society of America.
Having the potassium-binding polymer on board allowed for more persistent use of spironolactone, both in the subgroup of patients with heart failure, and those without, he said in a late-breaking clinical trials session.
In the international, phase 2 AMBER (Spironolactone With Patiromer in the Treatment of Resistant Hypertension in Chronic Kidney Disease) trial, 295 patients with chronic kidney disease (estimated glomerular filtration rate from 25 to 45 mL/min per 1.73 m2) were randomly assigned to treatment with spironolactone either placebo (148) or patiromer (147).
, for a significant between-group difference of 19.5% (P less than .0001). In addition, blood pressure lowering was significantly greater in the patiromer (–11.7 mm Hg) than in the placebo (–10.8 mm Hg) group. Results of the AMBER trial were published concurrently with Dr. Williams’ presentation (Lancet 2019 Sep 15; doi: 10.1016/S0140-6736(19)32135-X).
While spironolactone is a “highly effective” drug, studies supporting guideline recommendations for its use in resistant hypertension have largely excluded patients with advanced chronic kidney disease because of increased risk of developing spironolactone-induced hyperkalemia, Dr. Williams told attendees. It remains unclear, however, whether coadministration of patiromer will improve long-term outcomes. Also, many placebo-treated patients in AMBER were able to continue on spironolactone without the help of patiromer, prompting one attendee to question whether there was a smarter way to target the drug, rather than treating all patients up front.
“I don’t think it’s going to be easy to say, ‘this patient’s going to respond, and this patient’s not going to respond,’ ” Dr. Williams said in response, “but at least we have an opportunity to try now in a group of patients who simply may be denied treatment because of a perception that it is difficult to use spironolactone in them.”
That perception is actually not unreasonable, he added, given that 66% of patients in the placebo group in AMBER developed hyperkalemia, suggesting that spironolactone is “not an easy drug to use” in chronic kidney disease patients.
John Teerlink, MD, of the San Francisco VA Medical Center, said the AMBER study is “another building block” in a series of developments of enabling therapies.
“I think it’s a great message for all of us to begin thinking about other therapies we can use to help modify our use of these potential life-saving therapies,” he said in a panel discussion of the results.
Patiromer’s impact on longer-term outcomes is the focus of DIAMOND, a phase 3, randomized, placebo controlled trial that is currently recruiting. DIAMOND will determine whether giving patiromer to patients who developed hyperkalemia on renin-angiotensin-aldosterone system (RAAS) inhibitors decreases cardiovascular deaths and hospitalizations, by virtue of enabling continued RAAS inhibitor use.
Funding for AMBER came from Relypsa, which markets patiromer (Veltassa). Dr. Williams reported consulting for Relypsa during the conduct of the study, along with disclosures outside the scope of the AMBER study (Daiichi Sankyo, Pfizer, Novartis, Servier, Boehringer Ingelheim, and Vascular Dynamics).
SOURCE: Williams B. HFSA 2019.
REPORTING FROM HFSA 2019
Dapagliflozin approved for reducing HF hospitalization in diabetes
The Food And Drug Administration has approved the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) for reducing the risk of hospitalization for heart failure in adults with type 2 diabetes and established cardiovascular disease or multiple cardiovascular risk factors, according to a statement from AstraZeneca.
The approval was based on results from the DECLARE-TIMI 58 cardiovascular outcomes trial, which evaluated dapagliflozin in more than 17,000 patients with type 2 diabetes and cardiovascular risk factors or cardiovascular disease. They showed that dapagliflozin significantly reduced the risk of the primary composite endpoint of hospitalization for heart failure by 27%, compared with placebo (2.5% vs. 3.3%; HR, 0.73; 95% confidence interval, 0.61-0.88).
The drug is an oral, once-daily SGLT2 inhibitor initially approved as a monotherapy or combination therapy for glycemic control in adults with type 2 diabetes. It has additional benefits of weight loss and reduction in blood pressure in concert with diet and exercise in the same population.
“,” Ruud Dobber, PhD, executive vice president of the company’s biopharmaceuticals business unit, said in the statement. “This is promising news for the 30 million people living with type 2 diabetes in the U.S., as heart failure is one of the earliest cardiovascular complications for them, before heart attack or stroke. [Dapagliflozin] now offers the opportunity for physicians to act sooner and reduce the risk of hospitalization for heart failure.”
In September, the agency granted dapagliflozin a Fast Track designation to reduce the risk of cardiovascular death, or the worsening of heart failure in adults with heart failure with reduced ejection fraction or preserved ejection fraction, based on the phase 3 DAPA-HF and DELIVER trials. It also gave the drug Fast Track designation to delay the progression of renal failure and prevent CV and renal death in patients with chronic kidney disease based on the phase 3 DAPA-CKD trial, the statement noted.
The Food And Drug Administration has approved the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) for reducing the risk of hospitalization for heart failure in adults with type 2 diabetes and established cardiovascular disease or multiple cardiovascular risk factors, according to a statement from AstraZeneca.
The approval was based on results from the DECLARE-TIMI 58 cardiovascular outcomes trial, which evaluated dapagliflozin in more than 17,000 patients with type 2 diabetes and cardiovascular risk factors or cardiovascular disease. They showed that dapagliflozin significantly reduced the risk of the primary composite endpoint of hospitalization for heart failure by 27%, compared with placebo (2.5% vs. 3.3%; HR, 0.73; 95% confidence interval, 0.61-0.88).
The drug is an oral, once-daily SGLT2 inhibitor initially approved as a monotherapy or combination therapy for glycemic control in adults with type 2 diabetes. It has additional benefits of weight loss and reduction in blood pressure in concert with diet and exercise in the same population.
“,” Ruud Dobber, PhD, executive vice president of the company’s biopharmaceuticals business unit, said in the statement. “This is promising news for the 30 million people living with type 2 diabetes in the U.S., as heart failure is one of the earliest cardiovascular complications for them, before heart attack or stroke. [Dapagliflozin] now offers the opportunity for physicians to act sooner and reduce the risk of hospitalization for heart failure.”
In September, the agency granted dapagliflozin a Fast Track designation to reduce the risk of cardiovascular death, or the worsening of heart failure in adults with heart failure with reduced ejection fraction or preserved ejection fraction, based on the phase 3 DAPA-HF and DELIVER trials. It also gave the drug Fast Track designation to delay the progression of renal failure and prevent CV and renal death in patients with chronic kidney disease based on the phase 3 DAPA-CKD trial, the statement noted.
The Food And Drug Administration has approved the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) for reducing the risk of hospitalization for heart failure in adults with type 2 diabetes and established cardiovascular disease or multiple cardiovascular risk factors, according to a statement from AstraZeneca.
The approval was based on results from the DECLARE-TIMI 58 cardiovascular outcomes trial, which evaluated dapagliflozin in more than 17,000 patients with type 2 diabetes and cardiovascular risk factors or cardiovascular disease. They showed that dapagliflozin significantly reduced the risk of the primary composite endpoint of hospitalization for heart failure by 27%, compared with placebo (2.5% vs. 3.3%; HR, 0.73; 95% confidence interval, 0.61-0.88).
The drug is an oral, once-daily SGLT2 inhibitor initially approved as a monotherapy or combination therapy for glycemic control in adults with type 2 diabetes. It has additional benefits of weight loss and reduction in blood pressure in concert with diet and exercise in the same population.
“,” Ruud Dobber, PhD, executive vice president of the company’s biopharmaceuticals business unit, said in the statement. “This is promising news for the 30 million people living with type 2 diabetes in the U.S., as heart failure is one of the earliest cardiovascular complications for them, before heart attack or stroke. [Dapagliflozin] now offers the opportunity for physicians to act sooner and reduce the risk of hospitalization for heart failure.”
In September, the agency granted dapagliflozin a Fast Track designation to reduce the risk of cardiovascular death, or the worsening of heart failure in adults with heart failure with reduced ejection fraction or preserved ejection fraction, based on the phase 3 DAPA-HF and DELIVER trials. It also gave the drug Fast Track designation to delay the progression of renal failure and prevent CV and renal death in patients with chronic kidney disease based on the phase 3 DAPA-CKD trial, the statement noted.