User login
Study suggests physicians and patients overestimate asthma control
NEW ORLEANS – according to an observational study.
More than half (53%) of cases physicians rated as controlled were actually uncontrolled according to the Asthma Control Test (ACT), and 30% of patients who considered their asthma controlled actually had uncontrolled asthma according to the ACT.
Reynold A. Panettieri Jr., MD, of Rutgers University in New Brunswick, N.J., presented these findings at the annual meeting of the American College of Chest Physicians.
The findings are from the CHRONICLE study, an ongoing observational study of adults with severe asthma who are being treated by U.S. allergists or pulmonologists. The study enrolled 796 patients from Feb. 2018 to Feb. 2019, and 482 of them were evaluable because they completed the necessary surveys.
The patients’ median age at enrollment was 55 years, and 68% of patients were female. Most were white (82%), 12% were black, 6% were an “other” race, and 7% had Hispanic ethnicity. The median body mass index was 31 kg/m2.
Patients received care from an allergist (49%), a pulmonologist (38%), or both (13%). Patients were treated with biologics (n = 370), maintenance systemic corticosteroids (n = 64), or high-dosage inhaled corticosteroids with additional controllers (n = 90).
At patient enrollment, physicians reported their assessment of patients’ asthma control and completed the 5-point Global Evaluation of Treatment Effectiveness (GETE). The physicians’ assessments of patients were informed by patients’ verbal reports (50%), lung function testing (44%), in-office ACT (41%), and recent exacerbations (39%).
Patients also completed the ACT and GETE online at the time of enrollment. Neither patients nor physicians were privy to the other group’s responses.
Overall, physicians said 279 patients had controlled asthma. However, according to the ACT, 27% of these cases were very poorly controlled, 26% were not well controlled, and 47% were well controlled.
“So [when] we as a provider say the patient’s controlled, we’re wrong half the time,” Dr. Panettieri said.
However, physicians were more accurate when deeming patients’ asthma uncontrolled. Physicians said 201 cases of asthma were uncontrolled, and the ACT said 64% of these cases were very poorly controlled, 22% were not well controlled, and 13% were well controlled.
Compared with the physicians’ results, the patients’ reports were more in line with ACT results. However, the patients still overestimated control.
In all, 222 patients said their asthma was controlled. According to the ACT, 70% of these cases were well controlled, 23% were not well controlled, and 7% were very poorly controlled.
Patients were even more accurate when deeming their asthma uncontrolled. A total of 258 patients said their asthma was uncontrolled. According to the ACT, 74% of these cases were very poorly controlled, 25% were not well controlled, and 1% were well controlled.
“About 99% of the time, when a patient tells you they’re uncontrolled, they’re uncontrolled by the ACT,” Dr. Panettieri said.
Though patients were fairly accurate when assessing asthma control, they were less accurate when gauging treatment effectiveness. A majority of patients overestimated the effectiveness of treatment.
There were 124 patients who did not have any improvement after treatment, according to physicians. Although 23% of the patients concurred with this assessment, 77% said they did experience some improvement.
On the other hand, there were 355 patients who had some improvement after treatment according to physicians, and most of these patients (96%) agreed that they had some improvement.
Dr. Panettieri said these results support use of the ACT and similar tools. When using these tools isn’t feasible, Dr. Panettieri recommends simply asking patients how they are feeling. However, he said, providers should not rely on a patient’s report of treatment effectiveness to assess asthma control.
This study is supported by AstraZeneca. Dr. Panettieri disclosed relationships with AstraZeneca, Sanofi, Regeneron, Genentech, and Novartis.
SOURCE: Panettieri R et al. CHEST 2019. Abstract, doi. 10.1016/j.chest.2019.08.272.
NEW ORLEANS – according to an observational study.
More than half (53%) of cases physicians rated as controlled were actually uncontrolled according to the Asthma Control Test (ACT), and 30% of patients who considered their asthma controlled actually had uncontrolled asthma according to the ACT.
Reynold A. Panettieri Jr., MD, of Rutgers University in New Brunswick, N.J., presented these findings at the annual meeting of the American College of Chest Physicians.
The findings are from the CHRONICLE study, an ongoing observational study of adults with severe asthma who are being treated by U.S. allergists or pulmonologists. The study enrolled 796 patients from Feb. 2018 to Feb. 2019, and 482 of them were evaluable because they completed the necessary surveys.
The patients’ median age at enrollment was 55 years, and 68% of patients were female. Most were white (82%), 12% were black, 6% were an “other” race, and 7% had Hispanic ethnicity. The median body mass index was 31 kg/m2.
Patients received care from an allergist (49%), a pulmonologist (38%), or both (13%). Patients were treated with biologics (n = 370), maintenance systemic corticosteroids (n = 64), or high-dosage inhaled corticosteroids with additional controllers (n = 90).
At patient enrollment, physicians reported their assessment of patients’ asthma control and completed the 5-point Global Evaluation of Treatment Effectiveness (GETE). The physicians’ assessments of patients were informed by patients’ verbal reports (50%), lung function testing (44%), in-office ACT (41%), and recent exacerbations (39%).
Patients also completed the ACT and GETE online at the time of enrollment. Neither patients nor physicians were privy to the other group’s responses.
Overall, physicians said 279 patients had controlled asthma. However, according to the ACT, 27% of these cases were very poorly controlled, 26% were not well controlled, and 47% were well controlled.
“So [when] we as a provider say the patient’s controlled, we’re wrong half the time,” Dr. Panettieri said.
However, physicians were more accurate when deeming patients’ asthma uncontrolled. Physicians said 201 cases of asthma were uncontrolled, and the ACT said 64% of these cases were very poorly controlled, 22% were not well controlled, and 13% were well controlled.
Compared with the physicians’ results, the patients’ reports were more in line with ACT results. However, the patients still overestimated control.
In all, 222 patients said their asthma was controlled. According to the ACT, 70% of these cases were well controlled, 23% were not well controlled, and 7% were very poorly controlled.
Patients were even more accurate when deeming their asthma uncontrolled. A total of 258 patients said their asthma was uncontrolled. According to the ACT, 74% of these cases were very poorly controlled, 25% were not well controlled, and 1% were well controlled.
“About 99% of the time, when a patient tells you they’re uncontrolled, they’re uncontrolled by the ACT,” Dr. Panettieri said.
Though patients were fairly accurate when assessing asthma control, they were less accurate when gauging treatment effectiveness. A majority of patients overestimated the effectiveness of treatment.
There were 124 patients who did not have any improvement after treatment, according to physicians. Although 23% of the patients concurred with this assessment, 77% said they did experience some improvement.
On the other hand, there were 355 patients who had some improvement after treatment according to physicians, and most of these patients (96%) agreed that they had some improvement.
Dr. Panettieri said these results support use of the ACT and similar tools. When using these tools isn’t feasible, Dr. Panettieri recommends simply asking patients how they are feeling. However, he said, providers should not rely on a patient’s report of treatment effectiveness to assess asthma control.
This study is supported by AstraZeneca. Dr. Panettieri disclosed relationships with AstraZeneca, Sanofi, Regeneron, Genentech, and Novartis.
SOURCE: Panettieri R et al. CHEST 2019. Abstract, doi. 10.1016/j.chest.2019.08.272.
NEW ORLEANS – according to an observational study.
More than half (53%) of cases physicians rated as controlled were actually uncontrolled according to the Asthma Control Test (ACT), and 30% of patients who considered their asthma controlled actually had uncontrolled asthma according to the ACT.
Reynold A. Panettieri Jr., MD, of Rutgers University in New Brunswick, N.J., presented these findings at the annual meeting of the American College of Chest Physicians.
The findings are from the CHRONICLE study, an ongoing observational study of adults with severe asthma who are being treated by U.S. allergists or pulmonologists. The study enrolled 796 patients from Feb. 2018 to Feb. 2019, and 482 of them were evaluable because they completed the necessary surveys.
The patients’ median age at enrollment was 55 years, and 68% of patients were female. Most were white (82%), 12% were black, 6% were an “other” race, and 7% had Hispanic ethnicity. The median body mass index was 31 kg/m2.
Patients received care from an allergist (49%), a pulmonologist (38%), or both (13%). Patients were treated with biologics (n = 370), maintenance systemic corticosteroids (n = 64), or high-dosage inhaled corticosteroids with additional controllers (n = 90).
At patient enrollment, physicians reported their assessment of patients’ asthma control and completed the 5-point Global Evaluation of Treatment Effectiveness (GETE). The physicians’ assessments of patients were informed by patients’ verbal reports (50%), lung function testing (44%), in-office ACT (41%), and recent exacerbations (39%).
Patients also completed the ACT and GETE online at the time of enrollment. Neither patients nor physicians were privy to the other group’s responses.
Overall, physicians said 279 patients had controlled asthma. However, according to the ACT, 27% of these cases were very poorly controlled, 26% were not well controlled, and 47% were well controlled.
“So [when] we as a provider say the patient’s controlled, we’re wrong half the time,” Dr. Panettieri said.
However, physicians were more accurate when deeming patients’ asthma uncontrolled. Physicians said 201 cases of asthma were uncontrolled, and the ACT said 64% of these cases were very poorly controlled, 22% were not well controlled, and 13% were well controlled.
Compared with the physicians’ results, the patients’ reports were more in line with ACT results. However, the patients still overestimated control.
In all, 222 patients said their asthma was controlled. According to the ACT, 70% of these cases were well controlled, 23% were not well controlled, and 7% were very poorly controlled.
Patients were even more accurate when deeming their asthma uncontrolled. A total of 258 patients said their asthma was uncontrolled. According to the ACT, 74% of these cases were very poorly controlled, 25% were not well controlled, and 1% were well controlled.
“About 99% of the time, when a patient tells you they’re uncontrolled, they’re uncontrolled by the ACT,” Dr. Panettieri said.
Though patients were fairly accurate when assessing asthma control, they were less accurate when gauging treatment effectiveness. A majority of patients overestimated the effectiveness of treatment.
There were 124 patients who did not have any improvement after treatment, according to physicians. Although 23% of the patients concurred with this assessment, 77% said they did experience some improvement.
On the other hand, there were 355 patients who had some improvement after treatment according to physicians, and most of these patients (96%) agreed that they had some improvement.
Dr. Panettieri said these results support use of the ACT and similar tools. When using these tools isn’t feasible, Dr. Panettieri recommends simply asking patients how they are feeling. However, he said, providers should not rely on a patient’s report of treatment effectiveness to assess asthma control.
This study is supported by AstraZeneca. Dr. Panettieri disclosed relationships with AstraZeneca, Sanofi, Regeneron, Genentech, and Novartis.
SOURCE: Panettieri R et al. CHEST 2019. Abstract, doi. 10.1016/j.chest.2019.08.272.
REPORTING FROM CHEST 2019
FDA approves ustekinumab for ulcerative colitis
. This is the human interleukin-12 and IL-23 antagonist’s fourth indication since it was first approved by the FDA in 2009.
The approval is based on findings from the phase 3 UNIFI clinical trial, which achieved its primary endpoint of clinical remission over the course of both induction and maintenance. The 8-week induction study saw 19% of patients achieve clinical remission and 58% demonstrating clinical response; at the 1 year mark, after 44 weeks of maintenance therapy, 43% of patients were in clinical remission without steroids. The trial also assessed a novel histologic-endoscopic mucosal improvement endpoint that examined cellular improvement through both histologic examination and images observed during colonoscopy; 17% of treated patients achieved this endpoint at week 8, and 44% had by 1 year.
“The FDA approval of Stelara for [ulcerative colitis] represents an exciting milestone, offering patients a new option that has demonstrated improvement of the histology and endoscopic appearance of the intestinal lining, while also offering patients the potential for response and remission without the need for steroids,” said William J. Sandborn, MD, chief of the division of gastroenterology and professor of medicine at University of California, San Diego, and a study investigator.
Because of ustekinumab’s effects on the immune system, it may increase the risk of serious infection; as such, patients and health care professionals should discuss any signs of infection before initiation and continue discussions afterward. There are also concerns about risks of cancers, serious allergic reactions, and lung inflammation.
Full prescribing information is available on the Janssen website, as is the full press release regarding the approval
. This is the human interleukin-12 and IL-23 antagonist’s fourth indication since it was first approved by the FDA in 2009.
The approval is based on findings from the phase 3 UNIFI clinical trial, which achieved its primary endpoint of clinical remission over the course of both induction and maintenance. The 8-week induction study saw 19% of patients achieve clinical remission and 58% demonstrating clinical response; at the 1 year mark, after 44 weeks of maintenance therapy, 43% of patients were in clinical remission without steroids. The trial also assessed a novel histologic-endoscopic mucosal improvement endpoint that examined cellular improvement through both histologic examination and images observed during colonoscopy; 17% of treated patients achieved this endpoint at week 8, and 44% had by 1 year.
“The FDA approval of Stelara for [ulcerative colitis] represents an exciting milestone, offering patients a new option that has demonstrated improvement of the histology and endoscopic appearance of the intestinal lining, while also offering patients the potential for response and remission without the need for steroids,” said William J. Sandborn, MD, chief of the division of gastroenterology and professor of medicine at University of California, San Diego, and a study investigator.
Because of ustekinumab’s effects on the immune system, it may increase the risk of serious infection; as such, patients and health care professionals should discuss any signs of infection before initiation and continue discussions afterward. There are also concerns about risks of cancers, serious allergic reactions, and lung inflammation.
Full prescribing information is available on the Janssen website, as is the full press release regarding the approval
. This is the human interleukin-12 and IL-23 antagonist’s fourth indication since it was first approved by the FDA in 2009.
The approval is based on findings from the phase 3 UNIFI clinical trial, which achieved its primary endpoint of clinical remission over the course of both induction and maintenance. The 8-week induction study saw 19% of patients achieve clinical remission and 58% demonstrating clinical response; at the 1 year mark, after 44 weeks of maintenance therapy, 43% of patients were in clinical remission without steroids. The trial also assessed a novel histologic-endoscopic mucosal improvement endpoint that examined cellular improvement through both histologic examination and images observed during colonoscopy; 17% of treated patients achieved this endpoint at week 8, and 44% had by 1 year.
“The FDA approval of Stelara for [ulcerative colitis] represents an exciting milestone, offering patients a new option that has demonstrated improvement of the histology and endoscopic appearance of the intestinal lining, while also offering patients the potential for response and remission without the need for steroids,” said William J. Sandborn, MD, chief of the division of gastroenterology and professor of medicine at University of California, San Diego, and a study investigator.
Because of ustekinumab’s effects on the immune system, it may increase the risk of serious infection; as such, patients and health care professionals should discuss any signs of infection before initiation and continue discussions afterward. There are also concerns about risks of cancers, serious allergic reactions, and lung inflammation.
Full prescribing information is available on the Janssen website, as is the full press release regarding the approval
Checklist may improve quality measures in the surgical ICU
NEW ORLEANS – A standardized checklist may help reduce errors and improve common quality measures in critically ill patients, results of a recent retrospective analysis of 200 consecutive patients suggest.
Use of the checklist was linked to significantly shorter hospital length of stay and ICU length of stay as well as fewer days on a ventilator in the analysis, which was presented here at the annual meeting of the American College of Chest Physicians. The 10-item standardized checklist covered a variety topics ranging from comfort, prophylaxis, and sedation to infection control and prevention, nutrition, and medication management review.
Although health economics weren’t evaluated in this analysis, changes in those quality measures might also impact the bottom line, according to study coauthor Priscilla Chow, DO, a resident at Suburban Community Hospital in East Norriton, Pa.
“Obviously, if the patient is spending less time in the hospital and fewer days on the ventilator and in the ICU, then we can potentially also be more cost effective in our care,” Dr. Chow said in a podium presentation at the meeting.
The use of checklists to standardize processes and reduce errors is a “relatively simple approach” that was adopted from the airline industry and now has been evaluated in a variety of medical care settings, according to Dr. Chow.
Previous studies have demonstrated that checklist-driven care may reduce the incidence of postoperative complications, central line–associated bloodstream infection, ventilator-associated pneumonia, and catheter associated–urinary tract infection.
The present retrospective data analysis by Dr. Chow and colleagues included 200 consecutive patients admitted to the surgical ICU at an urban level 1 trauma center, including 100 patients managed according to the checklist and 100 managed according to standard processes.
Though survival to discharge was comparable between the groups, use of the checklist was associated with a significantly shorter hospital length of stay versus standard care (23.9 vs. 9.5 days). Likewise, the ICU length of stay was shorter in the checklist group (13.0 vs. 6.5 days), and the checklist group had fewer ventilator days (7.7 to 2.8).
Injury Severity Score did not differ between groups, though overall, use of the checklist resulted in more of the underlying topics being addressed in clinical documentation (5.0 vs. 8.7 items).
Dr. Chow and colleagues disclosed that they had no relationships relevant to their study.
SOURCE: Akella K et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.201.
NEW ORLEANS – A standardized checklist may help reduce errors and improve common quality measures in critically ill patients, results of a recent retrospective analysis of 200 consecutive patients suggest.
Use of the checklist was linked to significantly shorter hospital length of stay and ICU length of stay as well as fewer days on a ventilator in the analysis, which was presented here at the annual meeting of the American College of Chest Physicians. The 10-item standardized checklist covered a variety topics ranging from comfort, prophylaxis, and sedation to infection control and prevention, nutrition, and medication management review.
Although health economics weren’t evaluated in this analysis, changes in those quality measures might also impact the bottom line, according to study coauthor Priscilla Chow, DO, a resident at Suburban Community Hospital in East Norriton, Pa.
“Obviously, if the patient is spending less time in the hospital and fewer days on the ventilator and in the ICU, then we can potentially also be more cost effective in our care,” Dr. Chow said in a podium presentation at the meeting.
The use of checklists to standardize processes and reduce errors is a “relatively simple approach” that was adopted from the airline industry and now has been evaluated in a variety of medical care settings, according to Dr. Chow.
Previous studies have demonstrated that checklist-driven care may reduce the incidence of postoperative complications, central line–associated bloodstream infection, ventilator-associated pneumonia, and catheter associated–urinary tract infection.
The present retrospective data analysis by Dr. Chow and colleagues included 200 consecutive patients admitted to the surgical ICU at an urban level 1 trauma center, including 100 patients managed according to the checklist and 100 managed according to standard processes.
Though survival to discharge was comparable between the groups, use of the checklist was associated with a significantly shorter hospital length of stay versus standard care (23.9 vs. 9.5 days). Likewise, the ICU length of stay was shorter in the checklist group (13.0 vs. 6.5 days), and the checklist group had fewer ventilator days (7.7 to 2.8).
Injury Severity Score did not differ between groups, though overall, use of the checklist resulted in more of the underlying topics being addressed in clinical documentation (5.0 vs. 8.7 items).
Dr. Chow and colleagues disclosed that they had no relationships relevant to their study.
SOURCE: Akella K et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.201.
NEW ORLEANS – A standardized checklist may help reduce errors and improve common quality measures in critically ill patients, results of a recent retrospective analysis of 200 consecutive patients suggest.
Use of the checklist was linked to significantly shorter hospital length of stay and ICU length of stay as well as fewer days on a ventilator in the analysis, which was presented here at the annual meeting of the American College of Chest Physicians. The 10-item standardized checklist covered a variety topics ranging from comfort, prophylaxis, and sedation to infection control and prevention, nutrition, and medication management review.
Although health economics weren’t evaluated in this analysis, changes in those quality measures might also impact the bottom line, according to study coauthor Priscilla Chow, DO, a resident at Suburban Community Hospital in East Norriton, Pa.
“Obviously, if the patient is spending less time in the hospital and fewer days on the ventilator and in the ICU, then we can potentially also be more cost effective in our care,” Dr. Chow said in a podium presentation at the meeting.
The use of checklists to standardize processes and reduce errors is a “relatively simple approach” that was adopted from the airline industry and now has been evaluated in a variety of medical care settings, according to Dr. Chow.
Previous studies have demonstrated that checklist-driven care may reduce the incidence of postoperative complications, central line–associated bloodstream infection, ventilator-associated pneumonia, and catheter associated–urinary tract infection.
The present retrospective data analysis by Dr. Chow and colleagues included 200 consecutive patients admitted to the surgical ICU at an urban level 1 trauma center, including 100 patients managed according to the checklist and 100 managed according to standard processes.
Though survival to discharge was comparable between the groups, use of the checklist was associated with a significantly shorter hospital length of stay versus standard care (23.9 vs. 9.5 days). Likewise, the ICU length of stay was shorter in the checklist group (13.0 vs. 6.5 days), and the checklist group had fewer ventilator days (7.7 to 2.8).
Injury Severity Score did not differ between groups, though overall, use of the checklist resulted in more of the underlying topics being addressed in clinical documentation (5.0 vs. 8.7 items).
Dr. Chow and colleagues disclosed that they had no relationships relevant to their study.
SOURCE: Akella K et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.201.
REPORTING FROM CHEST 2019
Certain diabetes drugs may thwart dementia
COPENHAGEN – Selected antidiabetes medications appear to blunt the increased risk of dementia associated with type 2 diabetes, according to a Danish national case control registry study.
This benefit applies to the newer antidiabetic agents – specifically, the dipeptidyl peptidase 4 (DPP4) inhibitors, the glucagon-like peptide 1 (GLP1) analogs, and the sodium-glucose transport protein 2 (SGLT2) inhibitors – and metformin as well, Merete Osler, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
In contrast, neither insulin nor the sulfonylureas showed any signal of a protective effect against development of dementia. In fact, the use of sulfonylureas was associated with a small but statistically significant 7% increased risk, added Dr. Osler, of the University of Copenhagen.
Elsewhere at the meeting, investigators tapped a Swedish national registry to demonstrate that individuals with type 1 diabetes have a sharply reduced risk of developing schizophrenia.
Type 2 diabetes medications and dementia
Dr. Osler and colleagues are among several groups of investigators who have previously shown that patients with type 2 diabetes have an increased risk of dementia.
“This has raised the question of the role of dysregulated glucose metabolism in the development of this neurodegenerative disorder, and the possible effect of antidiabetic medications,” she noted.
To further explore this issue, which links two great ongoing global epidemics, Dr. Osler and coinvestigators conducted a nested case-control study including all 176,250 patients with type 2 diabetes in the comprehensive Danish National Diabetes Register for 1995-2012. The 11,619 patients with type 2 diabetes who received a dementia diagnosis were matched with 46,476 type 2 diabetes patients without dementia. The objective was to determine associations between dementia and ever-use and cumulative dose of antidiabetes drugs, alone and in combination, in logistic regression analyses adjusted for demographics, comorbid conditions, marital status, diabetic complications, and year of dementia diagnosis.
Patients who had ever used metformin had an adjusted 6% reduction in the likelihood of dementia compared with metformin nonusers, a modest but statistically significant difference. Those on a DPP4 inhibitor had a 20% reduction in risk. The GLP1 analogs were associated with a 42% decrease in risk. So were the SGLT2 inhibitors. A dose-response relationship was evident: The higher the cumulative exposure to these agents, the lower the odds of dementia.
Combination therapy is common in type 2 diabetes, so the investigators scrutinized the impact of a variety of multidrug combinations. Combinations including a DPP4 inhibitor or GLP1 analog were also associated with significantly reduced dementia risk.
Records of glycemic control in the form of hemoglobin A1c values were available on only 1,446 type 2 diabetic dementia patients and 4,003 matched controls. An analysis that incorporated this variable showed that the observed anti-dementia effect of selected diabetes drugs was independent of glycemic control, according to Dr. Osler.
The protective effect appeared to extend to both Alzheimer’s disease and vascular dementias, although firm conclusions can’t be drawn on this score because the study was insufficiently powered to address that issue.
Dr. Osler noted that the Danish study confirms a recent Taiwanese study showing an apparent protective effect against dementia for metformin in patients with type 2 diabetes (Aging Dis. 2019 Feb 1;10(1):37-48).
“Ours is the first study on the newer diabetic drugs, so our results need to be confirmed,” she pointed out.
If confirmed, however, it would warrant exploration of these drugs more generally as potential interventions to prevent dementia. That could open a whole new chapter in the remarkable story of the SGLT2 inhibitors, a class of drugs originally developed for treatment of type 2 diabetes but which in major randomized clinical trials later proved to be so effective in the treatment of heart failure that they are now considered cardiology drugs first.
Asked if she thinks these antidiabetes agents have a general neuroprotective effect or, instead, that the observed reduced risk of dementia is a function of patients being treated better early on with modern drugs, the psychiatrist replied, “I think it might be a combination of both, especially because we find different risk estimates between the drugs.”
Dr. Osler reported having no financial conflicts of interest regarding the study, which was funded by the Danish Diabetes Foundation, the Danish Medical Association, and several other foundations.
The full study details were published online shortly before her presentation at ECNP 2019 (Eur J Endocrinol. 2019 Aug 1. pii: EJE-19-0259.R1. doi: 10.1530/EJE-19-0259).
Type 1 diabetes and schizophrenia risk
Kristina Melkersson, MD, PhD, presented a cohort study that utilized Swedish national registries to examine the relationship between type 1 diabetes and schizophrenia. The study comprised 1,745,977 individuals, of whom 10,117 had type 1 diabetes, who were followed for a median of 9.7 and maximum of 18 years from their 13th birthday. During follow-up, 1,280 individuals were diagnosed with schizophrenia and 649 others with schizoaffective disorder. The adjusted risk of schizophrenia was 70% lower in patients with type 1 diabetes. However, there was no difference in the risk of schizoaffective disorder in the type 1 diabetic versus nondiabetic subjects.
The Swedish data confirm the findings of an earlier Finnish national study showing that the risk of schizophrenia is reduced in patients with type 1 diabetes (Arch Gen Psychiatry. 2007 Aug;64(8):894-9). These findings raise the intriguing possibility that autoimmunity somehow figures into the etiology of the psychiatric disorder. Other investigators have previously reported a reduced prevalence of rheumatoid arthritis in patients with schizophrenia, noted Dr. Melkersson of the Karolinska Institute in Stockholm.
She reported having no financial conflicts regarding her study.
SOURCE: Osler M. ECNP Abstract P180. Melkersson K. Abstract 81.
COPENHAGEN – Selected antidiabetes medications appear to blunt the increased risk of dementia associated with type 2 diabetes, according to a Danish national case control registry study.
This benefit applies to the newer antidiabetic agents – specifically, the dipeptidyl peptidase 4 (DPP4) inhibitors, the glucagon-like peptide 1 (GLP1) analogs, and the sodium-glucose transport protein 2 (SGLT2) inhibitors – and metformin as well, Merete Osler, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
In contrast, neither insulin nor the sulfonylureas showed any signal of a protective effect against development of dementia. In fact, the use of sulfonylureas was associated with a small but statistically significant 7% increased risk, added Dr. Osler, of the University of Copenhagen.
Elsewhere at the meeting, investigators tapped a Swedish national registry to demonstrate that individuals with type 1 diabetes have a sharply reduced risk of developing schizophrenia.
Type 2 diabetes medications and dementia
Dr. Osler and colleagues are among several groups of investigators who have previously shown that patients with type 2 diabetes have an increased risk of dementia.
“This has raised the question of the role of dysregulated glucose metabolism in the development of this neurodegenerative disorder, and the possible effect of antidiabetic medications,” she noted.
To further explore this issue, which links two great ongoing global epidemics, Dr. Osler and coinvestigators conducted a nested case-control study including all 176,250 patients with type 2 diabetes in the comprehensive Danish National Diabetes Register for 1995-2012. The 11,619 patients with type 2 diabetes who received a dementia diagnosis were matched with 46,476 type 2 diabetes patients without dementia. The objective was to determine associations between dementia and ever-use and cumulative dose of antidiabetes drugs, alone and in combination, in logistic regression analyses adjusted for demographics, comorbid conditions, marital status, diabetic complications, and year of dementia diagnosis.
Patients who had ever used metformin had an adjusted 6% reduction in the likelihood of dementia compared with metformin nonusers, a modest but statistically significant difference. Those on a DPP4 inhibitor had a 20% reduction in risk. The GLP1 analogs were associated with a 42% decrease in risk. So were the SGLT2 inhibitors. A dose-response relationship was evident: The higher the cumulative exposure to these agents, the lower the odds of dementia.
Combination therapy is common in type 2 diabetes, so the investigators scrutinized the impact of a variety of multidrug combinations. Combinations including a DPP4 inhibitor or GLP1 analog were also associated with significantly reduced dementia risk.
Records of glycemic control in the form of hemoglobin A1c values were available on only 1,446 type 2 diabetic dementia patients and 4,003 matched controls. An analysis that incorporated this variable showed that the observed anti-dementia effect of selected diabetes drugs was independent of glycemic control, according to Dr. Osler.
The protective effect appeared to extend to both Alzheimer’s disease and vascular dementias, although firm conclusions can’t be drawn on this score because the study was insufficiently powered to address that issue.
Dr. Osler noted that the Danish study confirms a recent Taiwanese study showing an apparent protective effect against dementia for metformin in patients with type 2 diabetes (Aging Dis. 2019 Feb 1;10(1):37-48).
“Ours is the first study on the newer diabetic drugs, so our results need to be confirmed,” she pointed out.
If confirmed, however, it would warrant exploration of these drugs more generally as potential interventions to prevent dementia. That could open a whole new chapter in the remarkable story of the SGLT2 inhibitors, a class of drugs originally developed for treatment of type 2 diabetes but which in major randomized clinical trials later proved to be so effective in the treatment of heart failure that they are now considered cardiology drugs first.
Asked if she thinks these antidiabetes agents have a general neuroprotective effect or, instead, that the observed reduced risk of dementia is a function of patients being treated better early on with modern drugs, the psychiatrist replied, “I think it might be a combination of both, especially because we find different risk estimates between the drugs.”
Dr. Osler reported having no financial conflicts of interest regarding the study, which was funded by the Danish Diabetes Foundation, the Danish Medical Association, and several other foundations.
The full study details were published online shortly before her presentation at ECNP 2019 (Eur J Endocrinol. 2019 Aug 1. pii: EJE-19-0259.R1. doi: 10.1530/EJE-19-0259).
Type 1 diabetes and schizophrenia risk
Kristina Melkersson, MD, PhD, presented a cohort study that utilized Swedish national registries to examine the relationship between type 1 diabetes and schizophrenia. The study comprised 1,745,977 individuals, of whom 10,117 had type 1 diabetes, who were followed for a median of 9.7 and maximum of 18 years from their 13th birthday. During follow-up, 1,280 individuals were diagnosed with schizophrenia and 649 others with schizoaffective disorder. The adjusted risk of schizophrenia was 70% lower in patients with type 1 diabetes. However, there was no difference in the risk of schizoaffective disorder in the type 1 diabetic versus nondiabetic subjects.
The Swedish data confirm the findings of an earlier Finnish national study showing that the risk of schizophrenia is reduced in patients with type 1 diabetes (Arch Gen Psychiatry. 2007 Aug;64(8):894-9). These findings raise the intriguing possibility that autoimmunity somehow figures into the etiology of the psychiatric disorder. Other investigators have previously reported a reduced prevalence of rheumatoid arthritis in patients with schizophrenia, noted Dr. Melkersson of the Karolinska Institute in Stockholm.
She reported having no financial conflicts regarding her study.
SOURCE: Osler M. ECNP Abstract P180. Melkersson K. Abstract 81.
COPENHAGEN – Selected antidiabetes medications appear to blunt the increased risk of dementia associated with type 2 diabetes, according to a Danish national case control registry study.
This benefit applies to the newer antidiabetic agents – specifically, the dipeptidyl peptidase 4 (DPP4) inhibitors, the glucagon-like peptide 1 (GLP1) analogs, and the sodium-glucose transport protein 2 (SGLT2) inhibitors – and metformin as well, Merete Osler, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
In contrast, neither insulin nor the sulfonylureas showed any signal of a protective effect against development of dementia. In fact, the use of sulfonylureas was associated with a small but statistically significant 7% increased risk, added Dr. Osler, of the University of Copenhagen.
Elsewhere at the meeting, investigators tapped a Swedish national registry to demonstrate that individuals with type 1 diabetes have a sharply reduced risk of developing schizophrenia.
Type 2 diabetes medications and dementia
Dr. Osler and colleagues are among several groups of investigators who have previously shown that patients with type 2 diabetes have an increased risk of dementia.
“This has raised the question of the role of dysregulated glucose metabolism in the development of this neurodegenerative disorder, and the possible effect of antidiabetic medications,” she noted.
To further explore this issue, which links two great ongoing global epidemics, Dr. Osler and coinvestigators conducted a nested case-control study including all 176,250 patients with type 2 diabetes in the comprehensive Danish National Diabetes Register for 1995-2012. The 11,619 patients with type 2 diabetes who received a dementia diagnosis were matched with 46,476 type 2 diabetes patients without dementia. The objective was to determine associations between dementia and ever-use and cumulative dose of antidiabetes drugs, alone and in combination, in logistic regression analyses adjusted for demographics, comorbid conditions, marital status, diabetic complications, and year of dementia diagnosis.
Patients who had ever used metformin had an adjusted 6% reduction in the likelihood of dementia compared with metformin nonusers, a modest but statistically significant difference. Those on a DPP4 inhibitor had a 20% reduction in risk. The GLP1 analogs were associated with a 42% decrease in risk. So were the SGLT2 inhibitors. A dose-response relationship was evident: The higher the cumulative exposure to these agents, the lower the odds of dementia.
Combination therapy is common in type 2 diabetes, so the investigators scrutinized the impact of a variety of multidrug combinations. Combinations including a DPP4 inhibitor or GLP1 analog were also associated with significantly reduced dementia risk.
Records of glycemic control in the form of hemoglobin A1c values were available on only 1,446 type 2 diabetic dementia patients and 4,003 matched controls. An analysis that incorporated this variable showed that the observed anti-dementia effect of selected diabetes drugs was independent of glycemic control, according to Dr. Osler.
The protective effect appeared to extend to both Alzheimer’s disease and vascular dementias, although firm conclusions can’t be drawn on this score because the study was insufficiently powered to address that issue.
Dr. Osler noted that the Danish study confirms a recent Taiwanese study showing an apparent protective effect against dementia for metformin in patients with type 2 diabetes (Aging Dis. 2019 Feb 1;10(1):37-48).
“Ours is the first study on the newer diabetic drugs, so our results need to be confirmed,” she pointed out.
If confirmed, however, it would warrant exploration of these drugs more generally as potential interventions to prevent dementia. That could open a whole new chapter in the remarkable story of the SGLT2 inhibitors, a class of drugs originally developed for treatment of type 2 diabetes but which in major randomized clinical trials later proved to be so effective in the treatment of heart failure that they are now considered cardiology drugs first.
Asked if she thinks these antidiabetes agents have a general neuroprotective effect or, instead, that the observed reduced risk of dementia is a function of patients being treated better early on with modern drugs, the psychiatrist replied, “I think it might be a combination of both, especially because we find different risk estimates between the drugs.”
Dr. Osler reported having no financial conflicts of interest regarding the study, which was funded by the Danish Diabetes Foundation, the Danish Medical Association, and several other foundations.
The full study details were published online shortly before her presentation at ECNP 2019 (Eur J Endocrinol. 2019 Aug 1. pii: EJE-19-0259.R1. doi: 10.1530/EJE-19-0259).
Type 1 diabetes and schizophrenia risk
Kristina Melkersson, MD, PhD, presented a cohort study that utilized Swedish national registries to examine the relationship between type 1 diabetes and schizophrenia. The study comprised 1,745,977 individuals, of whom 10,117 had type 1 diabetes, who were followed for a median of 9.7 and maximum of 18 years from their 13th birthday. During follow-up, 1,280 individuals were diagnosed with schizophrenia and 649 others with schizoaffective disorder. The adjusted risk of schizophrenia was 70% lower in patients with type 1 diabetes. However, there was no difference in the risk of schizoaffective disorder in the type 1 diabetic versus nondiabetic subjects.
The Swedish data confirm the findings of an earlier Finnish national study showing that the risk of schizophrenia is reduced in patients with type 1 diabetes (Arch Gen Psychiatry. 2007 Aug;64(8):894-9). These findings raise the intriguing possibility that autoimmunity somehow figures into the etiology of the psychiatric disorder. Other investigators have previously reported a reduced prevalence of rheumatoid arthritis in patients with schizophrenia, noted Dr. Melkersson of the Karolinska Institute in Stockholm.
She reported having no financial conflicts regarding her study.
SOURCE: Osler M. ECNP Abstract P180. Melkersson K. Abstract 81.
REPORTING FROM ECNP 2019
Immunotherapy enables nephrectomy with good outcomes in advanced RCC
Some patients with advanced renal cell carcinoma (RCC) treated with immune checkpoint inhibitors (ICIs) can safely undergo nephrectomy and experience favorable surgical outcomes and pathologic responses, a cohort study suggests.
“The introduction of several novel classes of systemic therapies, including targeted therapies and most recently ICI, has revolutionized the management of metastatic RCC over the last decade,” noted the investigators, who were led by Nirmish Singla, MD, from the department of urology at the University of Texas Southwestern Medical Center, Dallas. “With these new therapies, the role of nephrectomy in the treatment paradigm for advanced RCC has continued to evolve.”
The investigators undertook a single-center retrospective cohort study, assessing outcomes of 11 nephrectomies (10 radical, 1 partial) in 10 patients with advanced RCC who had received ICIs. Half had received nivolumab (Opdivo) alone, while the other half had received nivolumab in combination with ipilimumab (Yervoy); in six patients, these therapies were given first line.
Study results reported in Urologic Oncology showed that, at the time of nephrectomy, the patients had a median age of 64 years, with a range from 41 years to 83 years. Surgery was performed laparoscopically in five cases, and four patients had a concomitant thrombectomy.
The median operative time was 180 minutes, and the median estimated blood loss was 100 mL. None of the patients experienced major intraoperative complications. Four experienced postoperative complications; in three, they were addressed with interventional radiology procedures. The median length of stay was 4 days.
Pathology findings showed that one patient achieved a response to immunotherapy in the primary tumor (pT0), and three of four patients who underwent resection of hepatic, pulmonary, or adrenal metastases had no detectable cancer (pM0). All surgical margins were negative.
During a median postoperative follow-up of 180 days, one patient died of progressive disease more than 3 months after surgery, and another died of pulmonary embolism complicated by sepsis. Six patients did not have any complications or readmissions.
“In our experience, nephrectomy following ICI for RCC is both safe and technically feasible. Surgical and postoperative outcomes are encouraging, and pathologic response to ICI is strikingly favorable in both the primary tumor and metastatic sites,” Dr. Singla and coinvestigators wrote. “Biopsies of lesions responding radiographically to ICIs should be considered prior to surgical excision.”
“As multimodal management in the immunotherapy era continues to evolve, the utility and timing of nephrectomy combined with ICI in selected patients warrants further study,” they conclude.
Dr. Singla disclosed that he had no relevant conflicts of interest. The study did not receive any specific funding.
SOURCE: Singla N et al. Urol Oncol. 2019 Sep 12. doi: 10.1016/j.urolonc.2019.08.012.
Some patients with advanced renal cell carcinoma (RCC) treated with immune checkpoint inhibitors (ICIs) can safely undergo nephrectomy and experience favorable surgical outcomes and pathologic responses, a cohort study suggests.
“The introduction of several novel classes of systemic therapies, including targeted therapies and most recently ICI, has revolutionized the management of metastatic RCC over the last decade,” noted the investigators, who were led by Nirmish Singla, MD, from the department of urology at the University of Texas Southwestern Medical Center, Dallas. “With these new therapies, the role of nephrectomy in the treatment paradigm for advanced RCC has continued to evolve.”
The investigators undertook a single-center retrospective cohort study, assessing outcomes of 11 nephrectomies (10 radical, 1 partial) in 10 patients with advanced RCC who had received ICIs. Half had received nivolumab (Opdivo) alone, while the other half had received nivolumab in combination with ipilimumab (Yervoy); in six patients, these therapies were given first line.
Study results reported in Urologic Oncology showed that, at the time of nephrectomy, the patients had a median age of 64 years, with a range from 41 years to 83 years. Surgery was performed laparoscopically in five cases, and four patients had a concomitant thrombectomy.
The median operative time was 180 minutes, and the median estimated blood loss was 100 mL. None of the patients experienced major intraoperative complications. Four experienced postoperative complications; in three, they were addressed with interventional radiology procedures. The median length of stay was 4 days.
Pathology findings showed that one patient achieved a response to immunotherapy in the primary tumor (pT0), and three of four patients who underwent resection of hepatic, pulmonary, or adrenal metastases had no detectable cancer (pM0). All surgical margins were negative.
During a median postoperative follow-up of 180 days, one patient died of progressive disease more than 3 months after surgery, and another died of pulmonary embolism complicated by sepsis. Six patients did not have any complications or readmissions.
“In our experience, nephrectomy following ICI for RCC is both safe and technically feasible. Surgical and postoperative outcomes are encouraging, and pathologic response to ICI is strikingly favorable in both the primary tumor and metastatic sites,” Dr. Singla and coinvestigators wrote. “Biopsies of lesions responding radiographically to ICIs should be considered prior to surgical excision.”
“As multimodal management in the immunotherapy era continues to evolve, the utility and timing of nephrectomy combined with ICI in selected patients warrants further study,” they conclude.
Dr. Singla disclosed that he had no relevant conflicts of interest. The study did not receive any specific funding.
SOURCE: Singla N et al. Urol Oncol. 2019 Sep 12. doi: 10.1016/j.urolonc.2019.08.012.
Some patients with advanced renal cell carcinoma (RCC) treated with immune checkpoint inhibitors (ICIs) can safely undergo nephrectomy and experience favorable surgical outcomes and pathologic responses, a cohort study suggests.
“The introduction of several novel classes of systemic therapies, including targeted therapies and most recently ICI, has revolutionized the management of metastatic RCC over the last decade,” noted the investigators, who were led by Nirmish Singla, MD, from the department of urology at the University of Texas Southwestern Medical Center, Dallas. “With these new therapies, the role of nephrectomy in the treatment paradigm for advanced RCC has continued to evolve.”
The investigators undertook a single-center retrospective cohort study, assessing outcomes of 11 nephrectomies (10 radical, 1 partial) in 10 patients with advanced RCC who had received ICIs. Half had received nivolumab (Opdivo) alone, while the other half had received nivolumab in combination with ipilimumab (Yervoy); in six patients, these therapies were given first line.
Study results reported in Urologic Oncology showed that, at the time of nephrectomy, the patients had a median age of 64 years, with a range from 41 years to 83 years. Surgery was performed laparoscopically in five cases, and four patients had a concomitant thrombectomy.
The median operative time was 180 minutes, and the median estimated blood loss was 100 mL. None of the patients experienced major intraoperative complications. Four experienced postoperative complications; in three, they were addressed with interventional radiology procedures. The median length of stay was 4 days.
Pathology findings showed that one patient achieved a response to immunotherapy in the primary tumor (pT0), and three of four patients who underwent resection of hepatic, pulmonary, or adrenal metastases had no detectable cancer (pM0). All surgical margins were negative.
During a median postoperative follow-up of 180 days, one patient died of progressive disease more than 3 months after surgery, and another died of pulmonary embolism complicated by sepsis. Six patients did not have any complications or readmissions.
“In our experience, nephrectomy following ICI for RCC is both safe and technically feasible. Surgical and postoperative outcomes are encouraging, and pathologic response to ICI is strikingly favorable in both the primary tumor and metastatic sites,” Dr. Singla and coinvestigators wrote. “Biopsies of lesions responding radiographically to ICIs should be considered prior to surgical excision.”
“As multimodal management in the immunotherapy era continues to evolve, the utility and timing of nephrectomy combined with ICI in selected patients warrants further study,” they conclude.
Dr. Singla disclosed that he had no relevant conflicts of interest. The study did not receive any specific funding.
SOURCE: Singla N et al. Urol Oncol. 2019 Sep 12. doi: 10.1016/j.urolonc.2019.08.012.
FROM UROLOGIC ONCOLOGY
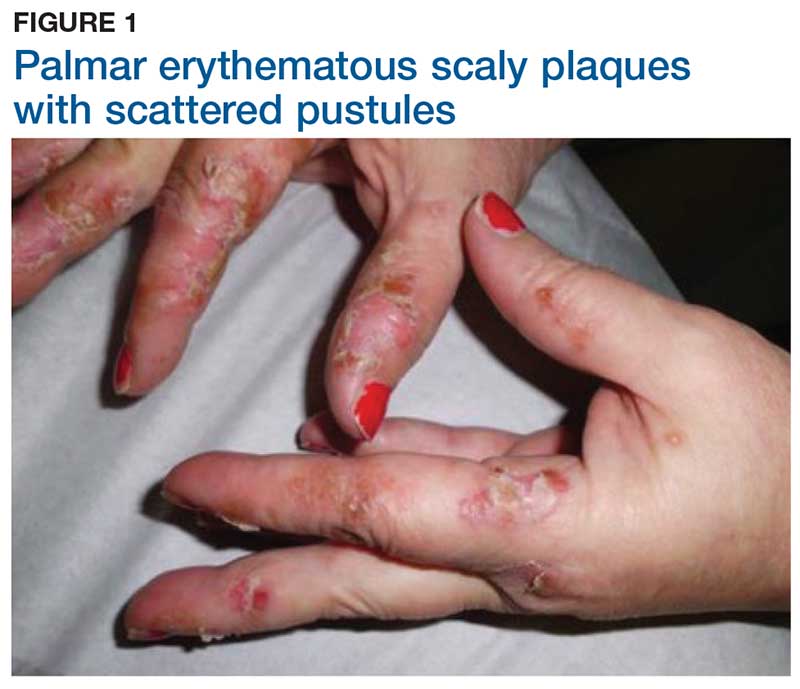
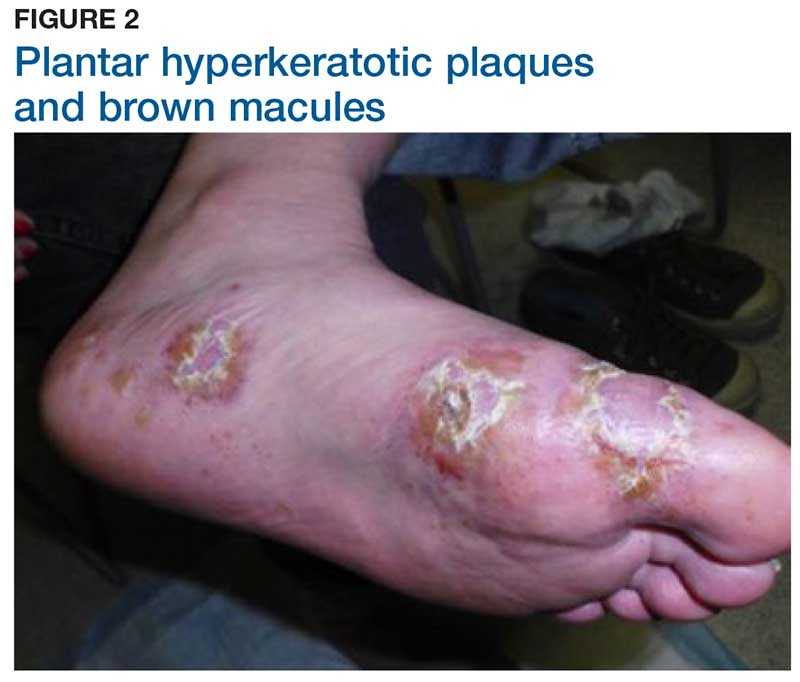
Discoloration and Bullous Lesions on the Hands
The Diagnosis: Irritant Contact Dermatitis and Hyperpigmentation Due to Juglone
Clinical suspicion, resemblance to similar cases, and questioning the patient about his behavior prior to the onset of symptoms led to the diagnosis of irritant contact dermatitis and hyperpigmentation due to juglone in this case. Walnuts belong to the botanical family of Juglandaceae and are the seed of the trees of the genus Juglans, which encompass 24 different species. The nuts from all species included in this genus are edible.1 The most well-known species of walnut is the common walnut (Juglans regia), which is native to the Balkans region in southeast Europe, southwest and central Asia extending to the Himalayas, and southwest China.1
Walnut fruits are rich in phenolic compounds. Thirteen phenolic compounds have been identified in walnut husks including chlorogenic acid, caffeic acid, ferulic acid, sinapic acid, gallic acid, ellagic acid, protocatechuic acid, syringic acid, vanillic acid, catechin, epicatechin, myricetin, and juglone.2 Juglone, also called 5-hydroxy-1,4-napthoquinone, is a yellow naphthoquinone pigment that occurs naturally in the leaves, roots, husks, and bark of plants in the Juglandaceae family, particularly the black walnut (Juglans nigra).3,4
Juglans regia, also known as English or Persian walnut, contains potent chemical constituents and has been used to treat diverse ailments such as diarrhea, hyperglycemia, cancer, infectious diseases, anorexia, asthma, helminthiasis, arthritis, sinusitis, stomachache, and skin disorders (eg, eczema; acne; alopecia; scalp itching, peeling, and dandruff), and as an adjunctive emollient and itch-relieving treatment.5,6
The juice of walnut shells from the J regia tree have been used for centuries to color the skin and hair.7 Irritation and skin hyperpigmentation have been associated with topical walnut use.5 As a naphthoquinone, juglone also is reported to exert some toxic effects on normal tissues including acute irritant contact dermatitis.4 As the active ingredient from the green husk of walnuts, it has been considered a strong sensitizer in guinea pigs,1 but contact sensitivity in humans rarely has been reported.7
Juglone is known to react with the keratin proteins present in the skin to form sclerojuglonic compounds, which have UV protection properties and a red-brown color.8 The resulting reaction gives rise to chromophore groups with a strong pigmenting action that absorbs visible colors (especially violet) and reflects yellow and red, resulting in the coloration ranging from red to deep brown.7 The mechanism of skin pigmentation does not involve the melanocytes. Hyperchromia involving the hands--particularly the palms, fingers, and nails--lasts 1 to 4 weeks depending on the intensity of the pigmentation. Housewives and agricultural workers are the at-risk population.7 Acute irritant contact dermatitis and hyperpigmentation due to juglone mainly has been observed during the early autumn in agricultural workers and housewives who remove the green husk of walnuts.9
Addison disease can present with pigmentary changes in the skin and mucous membranes; it also is accompanied by fatigue, anorexia, weakness, and weight loss, none of which were noted in our patient. A fixed drug eruption tends to have an annular or oval form and is related to the intake of medication (mostly antibiotics) up to 2 weeks prior to the onset of the dermatosis. Our patient did not have any chronic disease or take any medication prior to the dermatosis and lacked the classic clinical morphology of this entity. Hemochromatosis affects not only the skin but also the liver, myocardial fibers, and other internal organs. Our patient did not have any clinical manifestations of liver or heart failure or diabetes mellitus.
Our patient was treated with drainage of the blisters. Due to the extent of the dermatosis, prednisone 25 mg/d also was initiated. The patient was instructed to avoid direct contact with the husk of walnuts. At 1-month follow-up, the hyperpigmentation had resolved with no relapse (Figure).
- Costa J, Carrapatoso I, Oliveira MB, et al. Walnut allergens: molecular characterization, detection and clinical relevance. Clin Exp Allergy. 2013;44:319-341.
- Cosmulescu S, Trandafir I, Achim G, et al. Phenolics of green husk in mature walnut fruits. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38:53-56.
- Cosmulescu S, Trandafir I, Achim G, et al. Juglone content in leaf and green husk of five walnut (Juglans regia L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2011;39:237-240.
- Aithal BK, Sunil Kumar MR, Rao BN, et al. Evaluation of pharmacokinetic, biodistribution, pharmacodynamic, and toxicity profile of free juglone and its sterically stabilized liposomes. J Pharm Sci. 2011;100:3517-3528.
- Panth N, Paudel KR, Karki R. Phytochemical profile and biological activity of Juglans regia. J Integr Med. 2016;14:359-373.
- Aburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003;17:987-1000.
- Bonamonte D, Foti C, Angelini G. Hyperpigmentation and contact dermatitis due to Juglans regia. Contact Dermatitis. 2001;44:101-102.
- Dweck AC. Natural ingredients for colouring and styling. Int J Cosmet Sci. 2002;24:287-302.
- Neri I, Bianchi F, Giacomini F, et al. Acute irritant contact dermatitis due to Juglans regia. Contact Dermatitis. 2006;55:62-63.
The Diagnosis: Irritant Contact Dermatitis and Hyperpigmentation Due to Juglone
Clinical suspicion, resemblance to similar cases, and questioning the patient about his behavior prior to the onset of symptoms led to the diagnosis of irritant contact dermatitis and hyperpigmentation due to juglone in this case. Walnuts belong to the botanical family of Juglandaceae and are the seed of the trees of the genus Juglans, which encompass 24 different species. The nuts from all species included in this genus are edible.1 The most well-known species of walnut is the common walnut (Juglans regia), which is native to the Balkans region in southeast Europe, southwest and central Asia extending to the Himalayas, and southwest China.1
Walnut fruits are rich in phenolic compounds. Thirteen phenolic compounds have been identified in walnut husks including chlorogenic acid, caffeic acid, ferulic acid, sinapic acid, gallic acid, ellagic acid, protocatechuic acid, syringic acid, vanillic acid, catechin, epicatechin, myricetin, and juglone.2 Juglone, also called 5-hydroxy-1,4-napthoquinone, is a yellow naphthoquinone pigment that occurs naturally in the leaves, roots, husks, and bark of plants in the Juglandaceae family, particularly the black walnut (Juglans nigra).3,4
Juglans regia, also known as English or Persian walnut, contains potent chemical constituents and has been used to treat diverse ailments such as diarrhea, hyperglycemia, cancer, infectious diseases, anorexia, asthma, helminthiasis, arthritis, sinusitis, stomachache, and skin disorders (eg, eczema; acne; alopecia; scalp itching, peeling, and dandruff), and as an adjunctive emollient and itch-relieving treatment.5,6
The juice of walnut shells from the J regia tree have been used for centuries to color the skin and hair.7 Irritation and skin hyperpigmentation have been associated with topical walnut use.5 As a naphthoquinone, juglone also is reported to exert some toxic effects on normal tissues including acute irritant contact dermatitis.4 As the active ingredient from the green husk of walnuts, it has been considered a strong sensitizer in guinea pigs,1 but contact sensitivity in humans rarely has been reported.7
Juglone is known to react with the keratin proteins present in the skin to form sclerojuglonic compounds, which have UV protection properties and a red-brown color.8 The resulting reaction gives rise to chromophore groups with a strong pigmenting action that absorbs visible colors (especially violet) and reflects yellow and red, resulting in the coloration ranging from red to deep brown.7 The mechanism of skin pigmentation does not involve the melanocytes. Hyperchromia involving the hands--particularly the palms, fingers, and nails--lasts 1 to 4 weeks depending on the intensity of the pigmentation. Housewives and agricultural workers are the at-risk population.7 Acute irritant contact dermatitis and hyperpigmentation due to juglone mainly has been observed during the early autumn in agricultural workers and housewives who remove the green husk of walnuts.9
Addison disease can present with pigmentary changes in the skin and mucous membranes; it also is accompanied by fatigue, anorexia, weakness, and weight loss, none of which were noted in our patient. A fixed drug eruption tends to have an annular or oval form and is related to the intake of medication (mostly antibiotics) up to 2 weeks prior to the onset of the dermatosis. Our patient did not have any chronic disease or take any medication prior to the dermatosis and lacked the classic clinical morphology of this entity. Hemochromatosis affects not only the skin but also the liver, myocardial fibers, and other internal organs. Our patient did not have any clinical manifestations of liver or heart failure or diabetes mellitus.
Our patient was treated with drainage of the blisters. Due to the extent of the dermatosis, prednisone 25 mg/d also was initiated. The patient was instructed to avoid direct contact with the husk of walnuts. At 1-month follow-up, the hyperpigmentation had resolved with no relapse (Figure).
The Diagnosis: Irritant Contact Dermatitis and Hyperpigmentation Due to Juglone
Clinical suspicion, resemblance to similar cases, and questioning the patient about his behavior prior to the onset of symptoms led to the diagnosis of irritant contact dermatitis and hyperpigmentation due to juglone in this case. Walnuts belong to the botanical family of Juglandaceae and are the seed of the trees of the genus Juglans, which encompass 24 different species. The nuts from all species included in this genus are edible.1 The most well-known species of walnut is the common walnut (Juglans regia), which is native to the Balkans region in southeast Europe, southwest and central Asia extending to the Himalayas, and southwest China.1
Walnut fruits are rich in phenolic compounds. Thirteen phenolic compounds have been identified in walnut husks including chlorogenic acid, caffeic acid, ferulic acid, sinapic acid, gallic acid, ellagic acid, protocatechuic acid, syringic acid, vanillic acid, catechin, epicatechin, myricetin, and juglone.2 Juglone, also called 5-hydroxy-1,4-napthoquinone, is a yellow naphthoquinone pigment that occurs naturally in the leaves, roots, husks, and bark of plants in the Juglandaceae family, particularly the black walnut (Juglans nigra).3,4
Juglans regia, also known as English or Persian walnut, contains potent chemical constituents and has been used to treat diverse ailments such as diarrhea, hyperglycemia, cancer, infectious diseases, anorexia, asthma, helminthiasis, arthritis, sinusitis, stomachache, and skin disorders (eg, eczema; acne; alopecia; scalp itching, peeling, and dandruff), and as an adjunctive emollient and itch-relieving treatment.5,6
The juice of walnut shells from the J regia tree have been used for centuries to color the skin and hair.7 Irritation and skin hyperpigmentation have been associated with topical walnut use.5 As a naphthoquinone, juglone also is reported to exert some toxic effects on normal tissues including acute irritant contact dermatitis.4 As the active ingredient from the green husk of walnuts, it has been considered a strong sensitizer in guinea pigs,1 but contact sensitivity in humans rarely has been reported.7
Juglone is known to react with the keratin proteins present in the skin to form sclerojuglonic compounds, which have UV protection properties and a red-brown color.8 The resulting reaction gives rise to chromophore groups with a strong pigmenting action that absorbs visible colors (especially violet) and reflects yellow and red, resulting in the coloration ranging from red to deep brown.7 The mechanism of skin pigmentation does not involve the melanocytes. Hyperchromia involving the hands--particularly the palms, fingers, and nails--lasts 1 to 4 weeks depending on the intensity of the pigmentation. Housewives and agricultural workers are the at-risk population.7 Acute irritant contact dermatitis and hyperpigmentation due to juglone mainly has been observed during the early autumn in agricultural workers and housewives who remove the green husk of walnuts.9
Addison disease can present with pigmentary changes in the skin and mucous membranes; it also is accompanied by fatigue, anorexia, weakness, and weight loss, none of which were noted in our patient. A fixed drug eruption tends to have an annular or oval form and is related to the intake of medication (mostly antibiotics) up to 2 weeks prior to the onset of the dermatosis. Our patient did not have any chronic disease or take any medication prior to the dermatosis and lacked the classic clinical morphology of this entity. Hemochromatosis affects not only the skin but also the liver, myocardial fibers, and other internal organs. Our patient did not have any clinical manifestations of liver or heart failure or diabetes mellitus.
Our patient was treated with drainage of the blisters. Due to the extent of the dermatosis, prednisone 25 mg/d also was initiated. The patient was instructed to avoid direct contact with the husk of walnuts. At 1-month follow-up, the hyperpigmentation had resolved with no relapse (Figure).
- Costa J, Carrapatoso I, Oliveira MB, et al. Walnut allergens: molecular characterization, detection and clinical relevance. Clin Exp Allergy. 2013;44:319-341.
- Cosmulescu S, Trandafir I, Achim G, et al. Phenolics of green husk in mature walnut fruits. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38:53-56.
- Cosmulescu S, Trandafir I, Achim G, et al. Juglone content in leaf and green husk of five walnut (Juglans regia L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2011;39:237-240.
- Aithal BK, Sunil Kumar MR, Rao BN, et al. Evaluation of pharmacokinetic, biodistribution, pharmacodynamic, and toxicity profile of free juglone and its sterically stabilized liposomes. J Pharm Sci. 2011;100:3517-3528.
- Panth N, Paudel KR, Karki R. Phytochemical profile and biological activity of Juglans regia. J Integr Med. 2016;14:359-373.
- Aburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003;17:987-1000.
- Bonamonte D, Foti C, Angelini G. Hyperpigmentation and contact dermatitis due to Juglans regia. Contact Dermatitis. 2001;44:101-102.
- Dweck AC. Natural ingredients for colouring and styling. Int J Cosmet Sci. 2002;24:287-302.
- Neri I, Bianchi F, Giacomini F, et al. Acute irritant contact dermatitis due to Juglans regia. Contact Dermatitis. 2006;55:62-63.
- Costa J, Carrapatoso I, Oliveira MB, et al. Walnut allergens: molecular characterization, detection and clinical relevance. Clin Exp Allergy. 2013;44:319-341.
- Cosmulescu S, Trandafir I, Achim G, et al. Phenolics of green husk in mature walnut fruits. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38:53-56.
- Cosmulescu S, Trandafir I, Achim G, et al. Juglone content in leaf and green husk of five walnut (Juglans regia L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2011;39:237-240.
- Aithal BK, Sunil Kumar MR, Rao BN, et al. Evaluation of pharmacokinetic, biodistribution, pharmacodynamic, and toxicity profile of free juglone and its sterically stabilized liposomes. J Pharm Sci. 2011;100:3517-3528.
- Panth N, Paudel KR, Karki R. Phytochemical profile and biological activity of Juglans regia. J Integr Med. 2016;14:359-373.
- Aburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003;17:987-1000.
- Bonamonte D, Foti C, Angelini G. Hyperpigmentation and contact dermatitis due to Juglans regia. Contact Dermatitis. 2001;44:101-102.
- Dweck AC. Natural ingredients for colouring and styling. Int J Cosmet Sci. 2002;24:287-302.
- Neri I, Bianchi F, Giacomini F, et al. Acute irritant contact dermatitis due to Juglans regia. Contact Dermatitis. 2006;55:62-63.
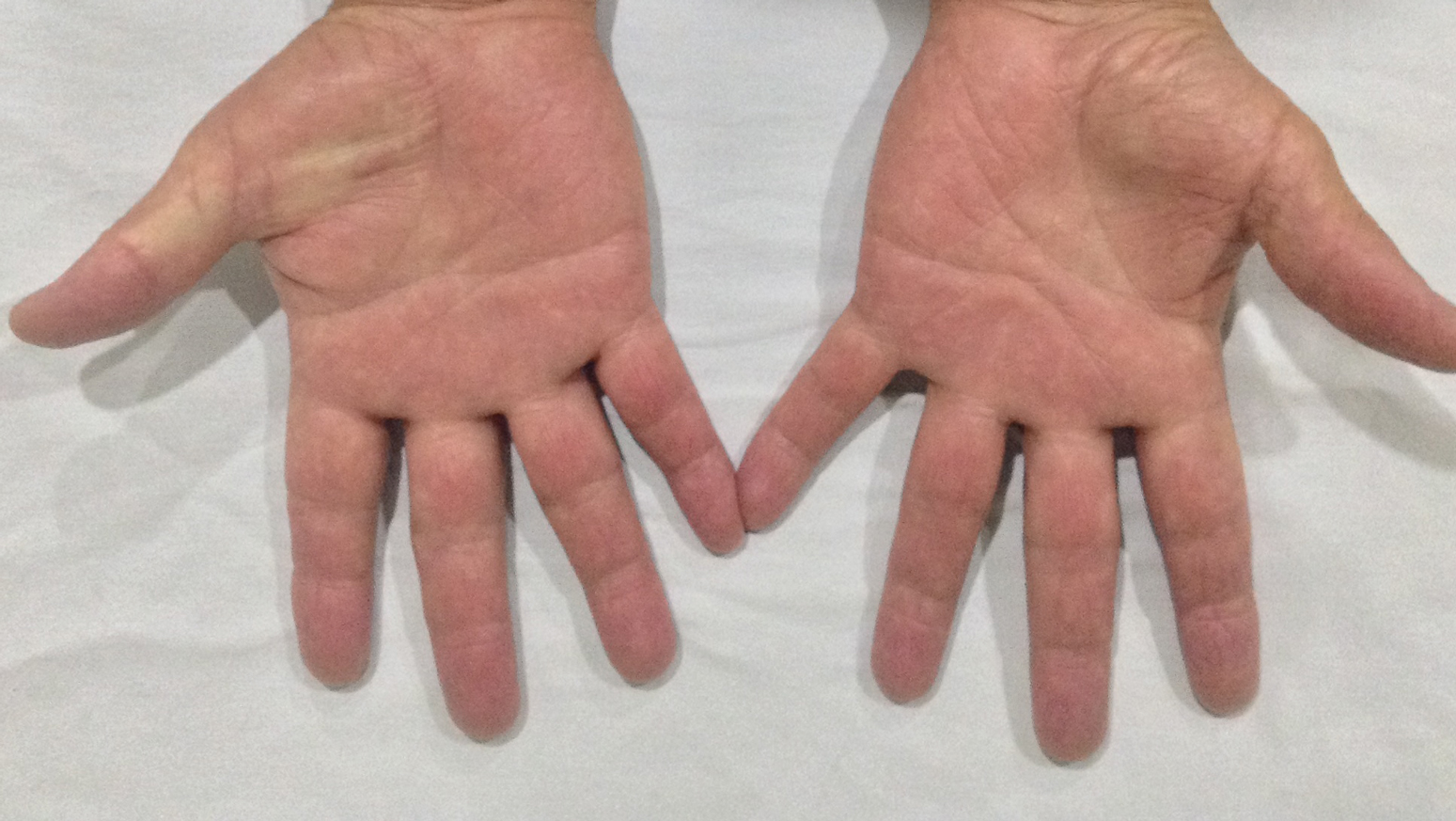
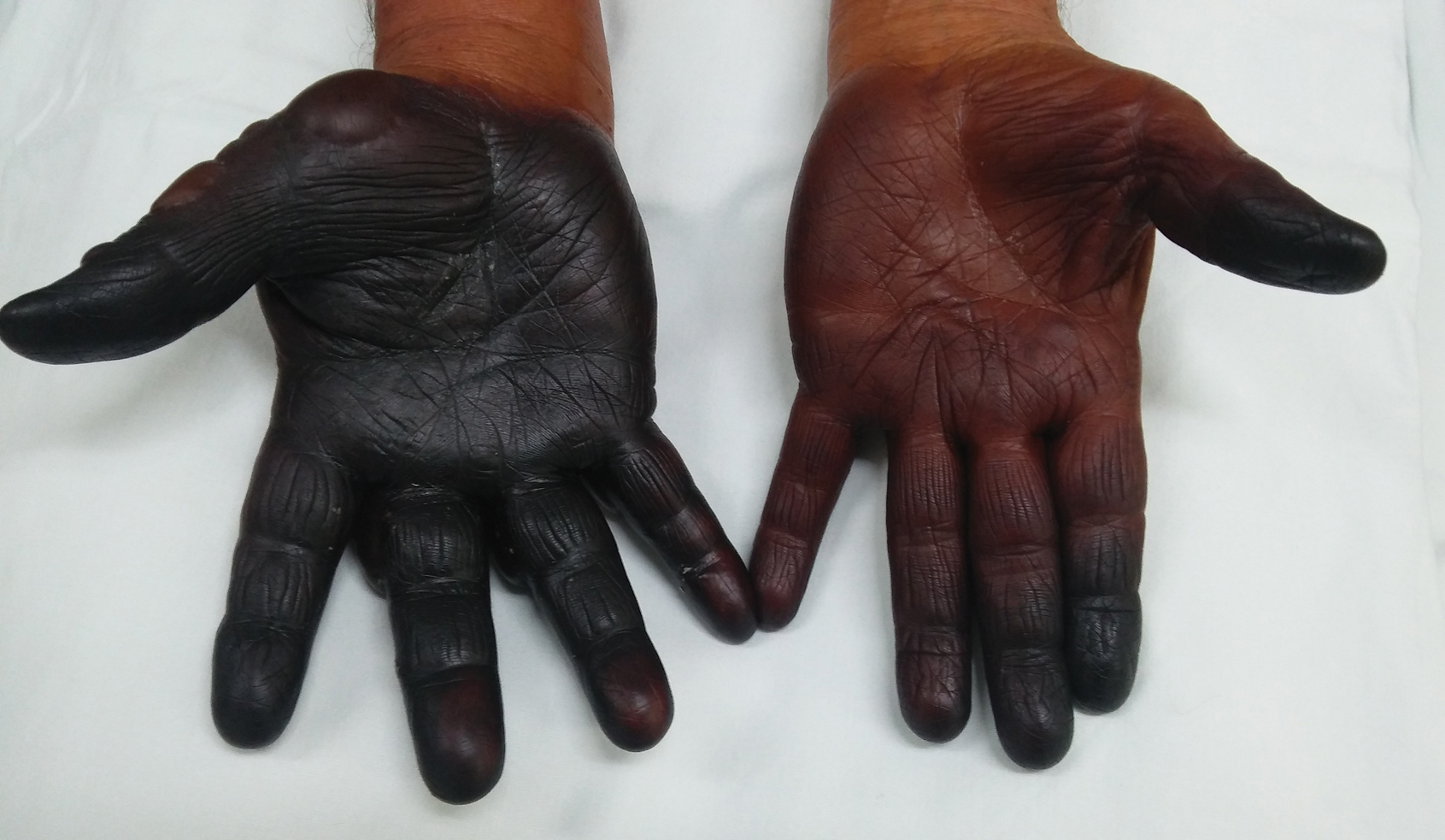
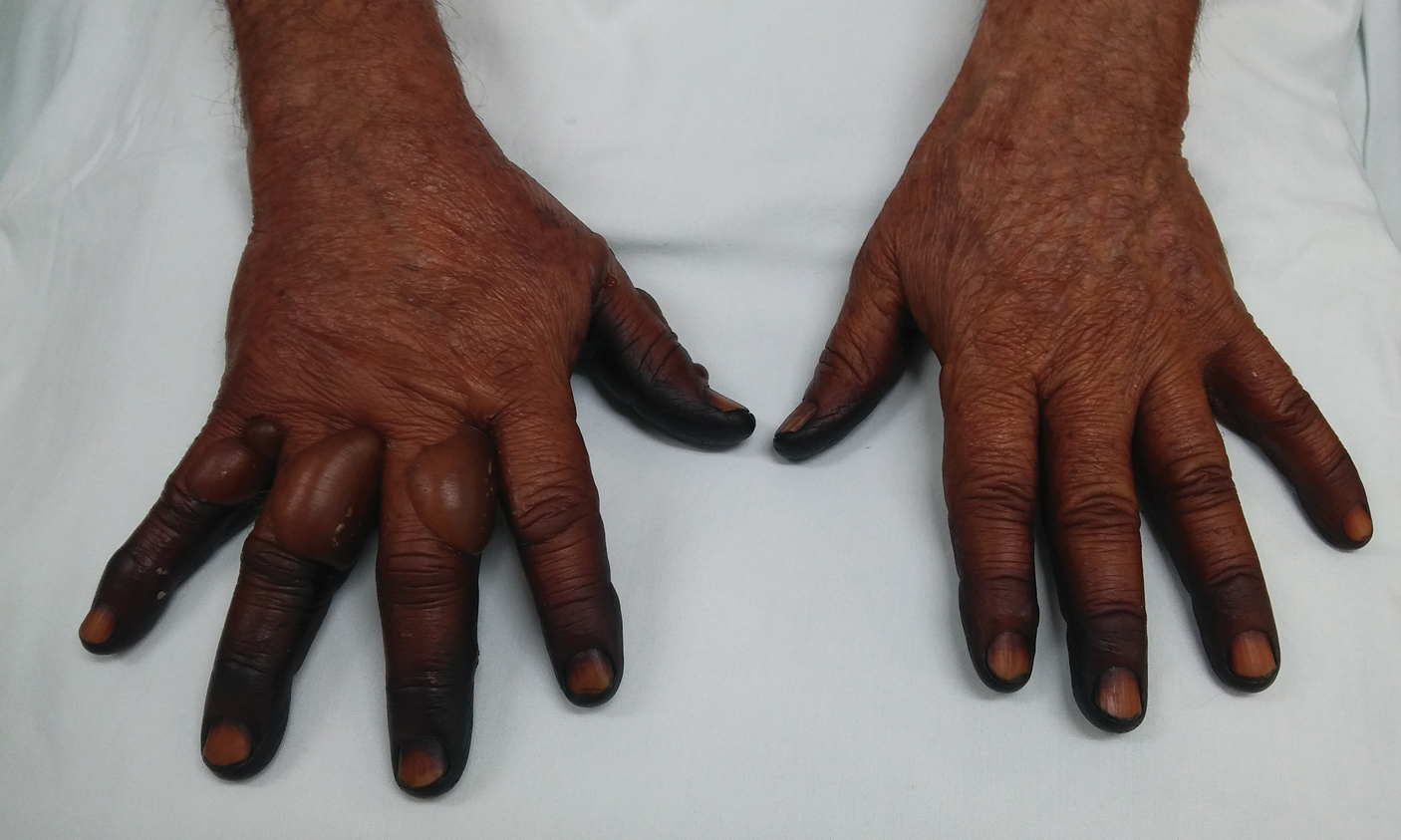
A 71-year-old man presented for evaluation of discoloration and blisters of 1 day's duration on both hands that were more severe on the right hand. The lesions were preceded by a sensation of stinging pain. One hour prior to the onset of symptoms, he had peeled approximately 100 walnuts. He had no relevant medical history. Physical examination revealed dark brown to black discoloration involving both hands (top) extending to the fingernails. Blisters filled with clear fluid also were present on the fingers (bottom).
Couples-based cognitive-behavioral therapy curbs postpartum depression
Postpartum women who participated in a couples-based cognitive-behavioral therapy program showed less postpartum depression than did women in a solo program, according to data from 388 couples.
Previous studies have shown that both men and women experience depression up to 1 year after the birth of a child, but “no studies have compared the relative effectiveness of couple-based and women-alone interventions on parental mental health,” wrote F-W Ngai of the Hong Kong Polytechnic University and colleagues.
In a study published in the BJOG: An International Journal of Obstetrics and Gynaecology, the researchers randomized 134 childbearing Chinese couples to a couples-based cognitive-behavioral intervention (CBI), 124 women to a women-only CBI, and 130 controls who did not receive CBI. The CBI consisted of one 3-hour antenatal group session and two 30-mintue postnatal telephone sessions. Depressive symptoms were assessed during pregnancy as a baseline, and at 6 weeks, 6 months, and 12 months post partum and measured using the Edinburgh Postnatal Depression Scale (EPDS). Demographic characteristics were similar among the groups.
Overall, mothers in the couples-based CBI group showed significant improvement in depressive symptoms at 6 weeks post partum, compared with women in the women-only group or the controls (average differences in scores on the EPDS of 1.46 and 1.71, respectively). In addition, the percentage of women who met criteria for postnatal depression with an EPDS score of at least 10 was significantly lower (17.8% difference) in the couples-based CBI group compared with controls at 6 weeks postpartum. However, the differences between the groups were no longer significant at 6 months and 12 months post partum, and no differences in depression scores were seen among fathers at any time point.
“The findings provide evidence for the effectiveness of the couple-based cognitive behavioral intervention in improving postnatal depression among mothers, but not fathers,” and additional research is needed to find interventions that protect new fathers from depression, the researchers said.
The study findings were limited by several factors including the use only of self-reports for postpartum assessment and the homogeneous nature of the study population (all were educated, first-time Chinese parents), the researchers noted. However, the results support those from previous studies and suggest that couples-based CBI is feasible for use in primary care to promote perinatal health, they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Ngai F-W et al. BJOG. 2019. doi: 10.1111/1471-0528.15862.
Postpartum women who participated in a couples-based cognitive-behavioral therapy program showed less postpartum depression than did women in a solo program, according to data from 388 couples.
Previous studies have shown that both men and women experience depression up to 1 year after the birth of a child, but “no studies have compared the relative effectiveness of couple-based and women-alone interventions on parental mental health,” wrote F-W Ngai of the Hong Kong Polytechnic University and colleagues.
In a study published in the BJOG: An International Journal of Obstetrics and Gynaecology, the researchers randomized 134 childbearing Chinese couples to a couples-based cognitive-behavioral intervention (CBI), 124 women to a women-only CBI, and 130 controls who did not receive CBI. The CBI consisted of one 3-hour antenatal group session and two 30-mintue postnatal telephone sessions. Depressive symptoms were assessed during pregnancy as a baseline, and at 6 weeks, 6 months, and 12 months post partum and measured using the Edinburgh Postnatal Depression Scale (EPDS). Demographic characteristics were similar among the groups.
Overall, mothers in the couples-based CBI group showed significant improvement in depressive symptoms at 6 weeks post partum, compared with women in the women-only group or the controls (average differences in scores on the EPDS of 1.46 and 1.71, respectively). In addition, the percentage of women who met criteria for postnatal depression with an EPDS score of at least 10 was significantly lower (17.8% difference) in the couples-based CBI group compared with controls at 6 weeks postpartum. However, the differences between the groups were no longer significant at 6 months and 12 months post partum, and no differences in depression scores were seen among fathers at any time point.
“The findings provide evidence for the effectiveness of the couple-based cognitive behavioral intervention in improving postnatal depression among mothers, but not fathers,” and additional research is needed to find interventions that protect new fathers from depression, the researchers said.
The study findings were limited by several factors including the use only of self-reports for postpartum assessment and the homogeneous nature of the study population (all were educated, first-time Chinese parents), the researchers noted. However, the results support those from previous studies and suggest that couples-based CBI is feasible for use in primary care to promote perinatal health, they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Ngai F-W et al. BJOG. 2019. doi: 10.1111/1471-0528.15862.
Postpartum women who participated in a couples-based cognitive-behavioral therapy program showed less postpartum depression than did women in a solo program, according to data from 388 couples.
Previous studies have shown that both men and women experience depression up to 1 year after the birth of a child, but “no studies have compared the relative effectiveness of couple-based and women-alone interventions on parental mental health,” wrote F-W Ngai of the Hong Kong Polytechnic University and colleagues.
In a study published in the BJOG: An International Journal of Obstetrics and Gynaecology, the researchers randomized 134 childbearing Chinese couples to a couples-based cognitive-behavioral intervention (CBI), 124 women to a women-only CBI, and 130 controls who did not receive CBI. The CBI consisted of one 3-hour antenatal group session and two 30-mintue postnatal telephone sessions. Depressive symptoms were assessed during pregnancy as a baseline, and at 6 weeks, 6 months, and 12 months post partum and measured using the Edinburgh Postnatal Depression Scale (EPDS). Demographic characteristics were similar among the groups.
Overall, mothers in the couples-based CBI group showed significant improvement in depressive symptoms at 6 weeks post partum, compared with women in the women-only group or the controls (average differences in scores on the EPDS of 1.46 and 1.71, respectively). In addition, the percentage of women who met criteria for postnatal depression with an EPDS score of at least 10 was significantly lower (17.8% difference) in the couples-based CBI group compared with controls at 6 weeks postpartum. However, the differences between the groups were no longer significant at 6 months and 12 months post partum, and no differences in depression scores were seen among fathers at any time point.
“The findings provide evidence for the effectiveness of the couple-based cognitive behavioral intervention in improving postnatal depression among mothers, but not fathers,” and additional research is needed to find interventions that protect new fathers from depression, the researchers said.
The study findings were limited by several factors including the use only of self-reports for postpartum assessment and the homogeneous nature of the study population (all were educated, first-time Chinese parents), the researchers noted. However, the results support those from previous studies and suggest that couples-based CBI is feasible for use in primary care to promote perinatal health, they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Ngai F-W et al. BJOG. 2019. doi: 10.1111/1471-0528.15862.
FROM THE BJOG: AN INTERNATIONAL JOURNAL OF OBSTETRICS AND GYNAECOLOGY
EEG asymmetry predicts poor pediatric ECMO outcomes
ST. LOUIS – Children who have background EEG asymmetry while on extracorporeal membrane oxygenation (ECMO) have worse outcomes even after adjustment for recent cardiac arrest and EEG suppression, according to a review of 41 children treated at Washington University, St. Louis.
ECMO is a last-ditch heart/lung bypass for patients near death, be it from infection, trauma, cardiac abnormalities, or any other issue. Children can be on it for days or weeks while problems are addressed and the body attempts to recover. Sometimes ECMO works, and children make a remarkable recovery, but other times they die or are left with severe disabilities, and no one really knows why.
Because of this, the investigators in this review sought to identify predictors of poor outcomes with an eye toward identifying modifiable risk factors, said senior investigator Kristin Guilliams, MD, an assistant professor of pediatric critical care medicine.
“We are trying to figure out why some kids do fantastically, and others don’t. We were looking at whether EEG can give us any clues and new ways to think about modifiable risk factors so that every kid rescued by ECMO can go back to their normal life,” she said at the American Neurological Association annual meeting.
The 41 children had an EEG within a day or 2 of starting ECMO; 22 did well, but 19 had bad outcomes, defined in the study as either dying in the hospital or being discharged with a Functional Status Score above 12, meaning mild dysfunction across six domains or more severe disability in particular ones.
The finding that all four children with EEG suppression – overall low brain activity – did poorly was not surprising, but the fact that EEG background asymmetry – one side of the brain being much less active than the other or giving different signals – in five children predicted poor outcomes, even after adjustment for cardiac arrest and overall suppression, was “a big surprise,” Dr. Guilliams said (odds ratio, 29.3; 95% confidence interval, 2.2-398.3; P = .003).
“The asymmetry tells me that we need to look more closely into brain blood flow patterns on ECMO,” she said. There might be a way to change delivery that could help, but “it’s not obvious right now.” The issue warrants further investigation, Dr. Guilliams said.
Twelve children had ECMO during chest compressions for cardiac arrest, which as expected, also predicted poor outcomes (OR, 9.5; 95% CI 1.6-58.2; P = .008).
Neuroimaging was available for 34 children. Abnormalities (n = 13; P = .2), including ischemia (n = 8; P = .1), hemorrhage (n = 8; P = .06), and seizures (n = 4; P = .2) did not predict poor outcomes, nor did sex, age, and mode of ECMO delivery (veno-arterial versus veno-venous).
As of about a year ago, EEGs at the university are now standard for children on ECMO, with special software to pick out asymmetries. “We are paying more attention to” EEGs, Dr. Guilliams said.
Children were a median of about 10 years old, and subjects were at least 1 year old. There were about equal numbers of boys and girls; 25 children were alive at discharge.
There was no external funding, and Dr. Guilliams didn’t have any disclosures.
ST. LOUIS – Children who have background EEG asymmetry while on extracorporeal membrane oxygenation (ECMO) have worse outcomes even after adjustment for recent cardiac arrest and EEG suppression, according to a review of 41 children treated at Washington University, St. Louis.
ECMO is a last-ditch heart/lung bypass for patients near death, be it from infection, trauma, cardiac abnormalities, or any other issue. Children can be on it for days or weeks while problems are addressed and the body attempts to recover. Sometimes ECMO works, and children make a remarkable recovery, but other times they die or are left with severe disabilities, and no one really knows why.
Because of this, the investigators in this review sought to identify predictors of poor outcomes with an eye toward identifying modifiable risk factors, said senior investigator Kristin Guilliams, MD, an assistant professor of pediatric critical care medicine.
“We are trying to figure out why some kids do fantastically, and others don’t. We were looking at whether EEG can give us any clues and new ways to think about modifiable risk factors so that every kid rescued by ECMO can go back to their normal life,” she said at the American Neurological Association annual meeting.
The 41 children had an EEG within a day or 2 of starting ECMO; 22 did well, but 19 had bad outcomes, defined in the study as either dying in the hospital or being discharged with a Functional Status Score above 12, meaning mild dysfunction across six domains or more severe disability in particular ones.
The finding that all four children with EEG suppression – overall low brain activity – did poorly was not surprising, but the fact that EEG background asymmetry – one side of the brain being much less active than the other or giving different signals – in five children predicted poor outcomes, even after adjustment for cardiac arrest and overall suppression, was “a big surprise,” Dr. Guilliams said (odds ratio, 29.3; 95% confidence interval, 2.2-398.3; P = .003).
“The asymmetry tells me that we need to look more closely into brain blood flow patterns on ECMO,” she said. There might be a way to change delivery that could help, but “it’s not obvious right now.” The issue warrants further investigation, Dr. Guilliams said.
Twelve children had ECMO during chest compressions for cardiac arrest, which as expected, also predicted poor outcomes (OR, 9.5; 95% CI 1.6-58.2; P = .008).
Neuroimaging was available for 34 children. Abnormalities (n = 13; P = .2), including ischemia (n = 8; P = .1), hemorrhage (n = 8; P = .06), and seizures (n = 4; P = .2) did not predict poor outcomes, nor did sex, age, and mode of ECMO delivery (veno-arterial versus veno-venous).
As of about a year ago, EEGs at the university are now standard for children on ECMO, with special software to pick out asymmetries. “We are paying more attention to” EEGs, Dr. Guilliams said.
Children were a median of about 10 years old, and subjects were at least 1 year old. There were about equal numbers of boys and girls; 25 children were alive at discharge.
There was no external funding, and Dr. Guilliams didn’t have any disclosures.
ST. LOUIS – Children who have background EEG asymmetry while on extracorporeal membrane oxygenation (ECMO) have worse outcomes even after adjustment for recent cardiac arrest and EEG suppression, according to a review of 41 children treated at Washington University, St. Louis.
ECMO is a last-ditch heart/lung bypass for patients near death, be it from infection, trauma, cardiac abnormalities, or any other issue. Children can be on it for days or weeks while problems are addressed and the body attempts to recover. Sometimes ECMO works, and children make a remarkable recovery, but other times they die or are left with severe disabilities, and no one really knows why.
Because of this, the investigators in this review sought to identify predictors of poor outcomes with an eye toward identifying modifiable risk factors, said senior investigator Kristin Guilliams, MD, an assistant professor of pediatric critical care medicine.
“We are trying to figure out why some kids do fantastically, and others don’t. We were looking at whether EEG can give us any clues and new ways to think about modifiable risk factors so that every kid rescued by ECMO can go back to their normal life,” she said at the American Neurological Association annual meeting.
The 41 children had an EEG within a day or 2 of starting ECMO; 22 did well, but 19 had bad outcomes, defined in the study as either dying in the hospital or being discharged with a Functional Status Score above 12, meaning mild dysfunction across six domains or more severe disability in particular ones.
The finding that all four children with EEG suppression – overall low brain activity – did poorly was not surprising, but the fact that EEG background asymmetry – one side of the brain being much less active than the other or giving different signals – in five children predicted poor outcomes, even after adjustment for cardiac arrest and overall suppression, was “a big surprise,” Dr. Guilliams said (odds ratio, 29.3; 95% confidence interval, 2.2-398.3; P = .003).
“The asymmetry tells me that we need to look more closely into brain blood flow patterns on ECMO,” she said. There might be a way to change delivery that could help, but “it’s not obvious right now.” The issue warrants further investigation, Dr. Guilliams said.
Twelve children had ECMO during chest compressions for cardiac arrest, which as expected, also predicted poor outcomes (OR, 9.5; 95% CI 1.6-58.2; P = .008).
Neuroimaging was available for 34 children. Abnormalities (n = 13; P = .2), including ischemia (n = 8; P = .1), hemorrhage (n = 8; P = .06), and seizures (n = 4; P = .2) did not predict poor outcomes, nor did sex, age, and mode of ECMO delivery (veno-arterial versus veno-venous).
As of about a year ago, EEGs at the university are now standard for children on ECMO, with special software to pick out asymmetries. “We are paying more attention to” EEGs, Dr. Guilliams said.
Children were a median of about 10 years old, and subjects were at least 1 year old. There were about equal numbers of boys and girls; 25 children were alive at discharge.
There was no external funding, and Dr. Guilliams didn’t have any disclosures.
REPORTING FROM ANA 2019
Acute Palmar and Plantar Rash in a 52-Year-Old Woman
A 52-year-old woman presented to a primary care clinic with a 3-week history of rash on her feet that had spread to her hands in the previous week. She described the rash as painful, burning, and itching with no drainage. She denied any recent illness, fever, chills, medication changes, or environmental exposures. Home treatments included Epsom salt baths and lotion with no improvement.
Past medical history included hypertension. She was a smoker with a 30-pack-year history and drank alcohol on a daily basis. Her medications included losartan and atorvastatin.
On examination, multiple papular and scabbed lesions were present with mild scaling. Additional review of systems and physical exam were benign. A KOH prep showed hyphae. The patient was diagnosed with tinea pedis and prescribed fluconazole (150-mg tablet once per week for 2 weeks).
Two weeks later, after completing the antifungal therapy, the patient returned with pain limiting her ability to bear weight or grasp objects. Clinical examination showed well-demarcated erythematous scaly and hyperkeratotic plaques with scattered papular and pustular lesions on bilateral palmar and medial aspects of plantar surfaces (see Figures 1 and 2). A repeat KOH was not completed. The patient was diagnosed with palmoplantar pustulosis (PPP).
DISCUSSION
PPP is a chronic, relapsing, inflammatory skin condition that results in painful lesions on the palms and the soles.1,2 There is debate as to whether PPP is a variant of psoriasis or a separate condition; depending on physical manifestations, one can be diagnosed with palmoplantar plaque psoriasis, PPP, or a combination of the two.3,4
The exact cause of PPP is unknown; however, increased levels of inflammatory cytokines interleukin (IL)-17 and IL-22 may be involved in the pathogenesis of the disease.5 Additional genetic and environmental factors, most significantly smoking, play an important role in its development.2,6
Clinical presentation
Inflammation associated with PPP typically manifests in the classic features of pustules that coalesce and resolve over several days, resulting in brown macules, hyperkeratosis, fissures, and debilitating pain.4,7 Some patients may have co-occurring onycholysis resulting from nail dystrophy and destruction or plaque psoriasis elsewhere on their body.8 PPP often persists for years with periods of exacerbation and remission, and it significantly affects the patient’s ability to perform activities of daily living without pain.8,9 It is exceedingly rare and most commonly affects middle-aged women with a smoking history or current smoking status.7
Continue to: Laboratory diagnosis
Laboratory diagnosis
The diagnosis of PPP is based on clinical presentation and physical exam. Laboratory testing, such as KOH prep, may assist in ruling out dermatophyte infection; a complete blood count may assist in eliminating a bacterial infection as the cause. Skin biopsy is not necessary unless diagnosis is uncertain or prolonged treatment has not produced a response.
Differential diagnosis
The differential diagnosis of PPP includes skin conditions that involve the palms and/or the soles and may have fungal, allergic, or bacterial origins.
Fungal. Tinea manuum (palms) and tinea pedis (soles) result from dermatophyte infection and manifest with erythema and/or scaling and pruritis. A positive KOH examination can confirm diagnosis. On examination, fungal infections are commonly unilateral and asymmetric.8 Treatment with an antifungal agent should result in resolution of symptoms.
Allergic. Contact with an allergen can result in skin erythema, pruritis, and pain at the exposed area. Contact dermatitis can result from an inflammatory response to an allergen or irritant, and it is often localized and well demarcated. This is an acute condition that resolves over time with antihistamines and avoidance of irritants.
Dyshidrotic eczema results in small, pruritic blisters on the palms and the soles and can be recurrent and related to seasonal allergen exposure. Diagnosis is made from history and physical exam. Treatment often consists of emollients and occasionally topical steroids, depending on the severity.
Continue to: Bacterial
Bacterial. A primary bacterial cause of bilateral skin lesions on the palms and the soles is uncommon. However, any open skin lesion can result in secondary bacterial infection. The pustules of PPP are often sterile and do not require bacterial culture; however, additional symptoms of fever, purulence, warmth, and worsening of symptoms may prompt further evaluation for a bacterial origin or complication.
Management
Due to limited quality data on treatment recommendations, the treatment options for PPP vary greatly. Most studies recommend topical versus systemic therapy for initial management.1-2,8,10-11 Firstline therapy often consists of topical corticosteroids and occlusive dressings, followed by oral retinoids (acitretin, alitretinoin) or photochemotherapy.1,8 Third-line therapy can include immunosuppressants (ciclosporin, methotrexate) or biologics (secukinumab).1,12 Recent data have shown positive results with vitamin D3 analogs (maxacalcitol, betamethasone butyrate propionate) as monotherapy or in combination with corticosteroids.10-11 Duration of therapy ranges from 4 to 8 weeks throughout the literature, depending on severity; however, many patients see improvement in the first few weeks.
Conservative measures to maintain remission include smoking cessation, skin emollients, and avoidance of irritants. It is important to educate patients about the chronicity of the disease and early treatment to prevent secondary infection or significant impact on quality of life.
OUTCOME FOR THE CASE PATIENT
The patient was prescribed triamcinolone acetonide (0.5% ointment applied bid), to be used until symptoms improved. After 1 week of treatment, she confirmed (verbally) that symptoms had resolved. She declined a follow-up visit or referral to dermatology.
CONCLUSION
Although PPP is fairly uncommon, it is important for clinicians to consider this diagnosis in patients presenting with localized rash on their palms and soles. This debilitating condition greatly affects a patient’s quality of life and, although it is chronic in nature, available treatments described in the literature have shown success in both acute resolution and ongoing remission of the disease.
1. Sevrain M, Richard M-A, Barnetche T, et al. Treatment for palmoplantar pustular psoriasis: systematic literature review, evidence-based recommendations and expert opinion. J Eur Acad Dermatol Venereol. 2014;28(suppl 5):13-16.
2. Olazagasti JM, Ma JE, Wetter DA. Clinical features, etiological factors, associated disorders, and treatment of palmoplantar pustulosis: the Mayo Clinic experience, 1996-2013. Mayo Clin Proc. 2017;92(9):1351-1358.
3. Bissonnette R, Suárez-Fariñas M, Li X, et al. Based on molecular profiling of gene expression, palmoplantar pustulosis and palmoplantar pustular psoriasis are highly related diseases that appear to be distinct from psoriasis vulgaris. PLoS One. 2016;11(5):1-11.
4. Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349-358.
5. Bissonnette R, Fuentes-Duculan J, Mashiko S, et al. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J Dermatol Sci. 2017;85(1):20-26.
6. Misiak-Galazka M, Wolska H, Rudnicka L. What do we know about palmoplantar pustulosis? J Eur Acad Dermatol Venereol. 2017;31(1):38-44.
7. Brunasso AMG, Puntoni M, Aberer W, et al. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168(6):1243-1251.
8. Engin B, As¸kın Ö, Tüzün Y. Palmoplantar psoriasis. Clin Dermatol. 2017; 35(1):19-27.
9. Chung J, Callas Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared to moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623-632.
10. Umezawa Y, Nakagawa H, Tamaki K. Phase III clinical study of maxacalcitol ointment in patients with palmoplantar pustulosis: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2016;43(3):288-293.
11. Muro M, Kawakami H, Matsumoto Y, et al. Topical combination therapy with vitamin D3 and corticosteroid ointment for palmoplantar pustulosis: a prospective, randomized, left-right comparison study. J Dermatolog Treat. 2016;27(1):51-53.
12. Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70-80.
A 52-year-old woman presented to a primary care clinic with a 3-week history of rash on her feet that had spread to her hands in the previous week. She described the rash as painful, burning, and itching with no drainage. She denied any recent illness, fever, chills, medication changes, or environmental exposures. Home treatments included Epsom salt baths and lotion with no improvement.
Past medical history included hypertension. She was a smoker with a 30-pack-year history and drank alcohol on a daily basis. Her medications included losartan and atorvastatin.
On examination, multiple papular and scabbed lesions were present with mild scaling. Additional review of systems and physical exam were benign. A KOH prep showed hyphae. The patient was diagnosed with tinea pedis and prescribed fluconazole (150-mg tablet once per week for 2 weeks).
Two weeks later, after completing the antifungal therapy, the patient returned with pain limiting her ability to bear weight or grasp objects. Clinical examination showed well-demarcated erythematous scaly and hyperkeratotic plaques with scattered papular and pustular lesions on bilateral palmar and medial aspects of plantar surfaces (see Figures 1 and 2). A repeat KOH was not completed. The patient was diagnosed with palmoplantar pustulosis (PPP).
DISCUSSION
PPP is a chronic, relapsing, inflammatory skin condition that results in painful lesions on the palms and the soles.1,2 There is debate as to whether PPP is a variant of psoriasis or a separate condition; depending on physical manifestations, one can be diagnosed with palmoplantar plaque psoriasis, PPP, or a combination of the two.3,4
The exact cause of PPP is unknown; however, increased levels of inflammatory cytokines interleukin (IL)-17 and IL-22 may be involved in the pathogenesis of the disease.5 Additional genetic and environmental factors, most significantly smoking, play an important role in its development.2,6
Clinical presentation
Inflammation associated with PPP typically manifests in the classic features of pustules that coalesce and resolve over several days, resulting in brown macules, hyperkeratosis, fissures, and debilitating pain.4,7 Some patients may have co-occurring onycholysis resulting from nail dystrophy and destruction or plaque psoriasis elsewhere on their body.8 PPP often persists for years with periods of exacerbation and remission, and it significantly affects the patient’s ability to perform activities of daily living without pain.8,9 It is exceedingly rare and most commonly affects middle-aged women with a smoking history or current smoking status.7
Continue to: Laboratory diagnosis
Laboratory diagnosis
The diagnosis of PPP is based on clinical presentation and physical exam. Laboratory testing, such as KOH prep, may assist in ruling out dermatophyte infection; a complete blood count may assist in eliminating a bacterial infection as the cause. Skin biopsy is not necessary unless diagnosis is uncertain or prolonged treatment has not produced a response.
Differential diagnosis
The differential diagnosis of PPP includes skin conditions that involve the palms and/or the soles and may have fungal, allergic, or bacterial origins.
Fungal. Tinea manuum (palms) and tinea pedis (soles) result from dermatophyte infection and manifest with erythema and/or scaling and pruritis. A positive KOH examination can confirm diagnosis. On examination, fungal infections are commonly unilateral and asymmetric.8 Treatment with an antifungal agent should result in resolution of symptoms.
Allergic. Contact with an allergen can result in skin erythema, pruritis, and pain at the exposed area. Contact dermatitis can result from an inflammatory response to an allergen or irritant, and it is often localized and well demarcated. This is an acute condition that resolves over time with antihistamines and avoidance of irritants.
Dyshidrotic eczema results in small, pruritic blisters on the palms and the soles and can be recurrent and related to seasonal allergen exposure. Diagnosis is made from history and physical exam. Treatment often consists of emollients and occasionally topical steroids, depending on the severity.
Continue to: Bacterial
Bacterial. A primary bacterial cause of bilateral skin lesions on the palms and the soles is uncommon. However, any open skin lesion can result in secondary bacterial infection. The pustules of PPP are often sterile and do not require bacterial culture; however, additional symptoms of fever, purulence, warmth, and worsening of symptoms may prompt further evaluation for a bacterial origin or complication.
Management
Due to limited quality data on treatment recommendations, the treatment options for PPP vary greatly. Most studies recommend topical versus systemic therapy for initial management.1-2,8,10-11 Firstline therapy often consists of topical corticosteroids and occlusive dressings, followed by oral retinoids (acitretin, alitretinoin) or photochemotherapy.1,8 Third-line therapy can include immunosuppressants (ciclosporin, methotrexate) or biologics (secukinumab).1,12 Recent data have shown positive results with vitamin D3 analogs (maxacalcitol, betamethasone butyrate propionate) as monotherapy or in combination with corticosteroids.10-11 Duration of therapy ranges from 4 to 8 weeks throughout the literature, depending on severity; however, many patients see improvement in the first few weeks.
Conservative measures to maintain remission include smoking cessation, skin emollients, and avoidance of irritants. It is important to educate patients about the chronicity of the disease and early treatment to prevent secondary infection or significant impact on quality of life.
OUTCOME FOR THE CASE PATIENT
The patient was prescribed triamcinolone acetonide (0.5% ointment applied bid), to be used until symptoms improved. After 1 week of treatment, she confirmed (verbally) that symptoms had resolved. She declined a follow-up visit or referral to dermatology.
CONCLUSION
Although PPP is fairly uncommon, it is important for clinicians to consider this diagnosis in patients presenting with localized rash on their palms and soles. This debilitating condition greatly affects a patient’s quality of life and, although it is chronic in nature, available treatments described in the literature have shown success in both acute resolution and ongoing remission of the disease.
A 52-year-old woman presented to a primary care clinic with a 3-week history of rash on her feet that had spread to her hands in the previous week. She described the rash as painful, burning, and itching with no drainage. She denied any recent illness, fever, chills, medication changes, or environmental exposures. Home treatments included Epsom salt baths and lotion with no improvement.
Past medical history included hypertension. She was a smoker with a 30-pack-year history and drank alcohol on a daily basis. Her medications included losartan and atorvastatin.
On examination, multiple papular and scabbed lesions were present with mild scaling. Additional review of systems and physical exam were benign. A KOH prep showed hyphae. The patient was diagnosed with tinea pedis and prescribed fluconazole (150-mg tablet once per week for 2 weeks).
Two weeks later, after completing the antifungal therapy, the patient returned with pain limiting her ability to bear weight or grasp objects. Clinical examination showed well-demarcated erythematous scaly and hyperkeratotic plaques with scattered papular and pustular lesions on bilateral palmar and medial aspects of plantar surfaces (see Figures 1 and 2). A repeat KOH was not completed. The patient was diagnosed with palmoplantar pustulosis (PPP).
DISCUSSION
PPP is a chronic, relapsing, inflammatory skin condition that results in painful lesions on the palms and the soles.1,2 There is debate as to whether PPP is a variant of psoriasis or a separate condition; depending on physical manifestations, one can be diagnosed with palmoplantar plaque psoriasis, PPP, or a combination of the two.3,4
The exact cause of PPP is unknown; however, increased levels of inflammatory cytokines interleukin (IL)-17 and IL-22 may be involved in the pathogenesis of the disease.5 Additional genetic and environmental factors, most significantly smoking, play an important role in its development.2,6
Clinical presentation
Inflammation associated with PPP typically manifests in the classic features of pustules that coalesce and resolve over several days, resulting in brown macules, hyperkeratosis, fissures, and debilitating pain.4,7 Some patients may have co-occurring onycholysis resulting from nail dystrophy and destruction or plaque psoriasis elsewhere on their body.8 PPP often persists for years with periods of exacerbation and remission, and it significantly affects the patient’s ability to perform activities of daily living without pain.8,9 It is exceedingly rare and most commonly affects middle-aged women with a smoking history or current smoking status.7
Continue to: Laboratory diagnosis
Laboratory diagnosis
The diagnosis of PPP is based on clinical presentation and physical exam. Laboratory testing, such as KOH prep, may assist in ruling out dermatophyte infection; a complete blood count may assist in eliminating a bacterial infection as the cause. Skin biopsy is not necessary unless diagnosis is uncertain or prolonged treatment has not produced a response.
Differential diagnosis
The differential diagnosis of PPP includes skin conditions that involve the palms and/or the soles and may have fungal, allergic, or bacterial origins.
Fungal. Tinea manuum (palms) and tinea pedis (soles) result from dermatophyte infection and manifest with erythema and/or scaling and pruritis. A positive KOH examination can confirm diagnosis. On examination, fungal infections are commonly unilateral and asymmetric.8 Treatment with an antifungal agent should result in resolution of symptoms.
Allergic. Contact with an allergen can result in skin erythema, pruritis, and pain at the exposed area. Contact dermatitis can result from an inflammatory response to an allergen or irritant, and it is often localized and well demarcated. This is an acute condition that resolves over time with antihistamines and avoidance of irritants.
Dyshidrotic eczema results in small, pruritic blisters on the palms and the soles and can be recurrent and related to seasonal allergen exposure. Diagnosis is made from history and physical exam. Treatment often consists of emollients and occasionally topical steroids, depending on the severity.
Continue to: Bacterial
Bacterial. A primary bacterial cause of bilateral skin lesions on the palms and the soles is uncommon. However, any open skin lesion can result in secondary bacterial infection. The pustules of PPP are often sterile and do not require bacterial culture; however, additional symptoms of fever, purulence, warmth, and worsening of symptoms may prompt further evaluation for a bacterial origin or complication.
Management
Due to limited quality data on treatment recommendations, the treatment options for PPP vary greatly. Most studies recommend topical versus systemic therapy for initial management.1-2,8,10-11 Firstline therapy often consists of topical corticosteroids and occlusive dressings, followed by oral retinoids (acitretin, alitretinoin) or photochemotherapy.1,8 Third-line therapy can include immunosuppressants (ciclosporin, methotrexate) or biologics (secukinumab).1,12 Recent data have shown positive results with vitamin D3 analogs (maxacalcitol, betamethasone butyrate propionate) as monotherapy or in combination with corticosteroids.10-11 Duration of therapy ranges from 4 to 8 weeks throughout the literature, depending on severity; however, many patients see improvement in the first few weeks.
Conservative measures to maintain remission include smoking cessation, skin emollients, and avoidance of irritants. It is important to educate patients about the chronicity of the disease and early treatment to prevent secondary infection or significant impact on quality of life.
OUTCOME FOR THE CASE PATIENT
The patient was prescribed triamcinolone acetonide (0.5% ointment applied bid), to be used until symptoms improved. After 1 week of treatment, she confirmed (verbally) that symptoms had resolved. She declined a follow-up visit or referral to dermatology.
CONCLUSION
Although PPP is fairly uncommon, it is important for clinicians to consider this diagnosis in patients presenting with localized rash on their palms and soles. This debilitating condition greatly affects a patient’s quality of life and, although it is chronic in nature, available treatments described in the literature have shown success in both acute resolution and ongoing remission of the disease.
1. Sevrain M, Richard M-A, Barnetche T, et al. Treatment for palmoplantar pustular psoriasis: systematic literature review, evidence-based recommendations and expert opinion. J Eur Acad Dermatol Venereol. 2014;28(suppl 5):13-16.
2. Olazagasti JM, Ma JE, Wetter DA. Clinical features, etiological factors, associated disorders, and treatment of palmoplantar pustulosis: the Mayo Clinic experience, 1996-2013. Mayo Clin Proc. 2017;92(9):1351-1358.
3. Bissonnette R, Suárez-Fariñas M, Li X, et al. Based on molecular profiling of gene expression, palmoplantar pustulosis and palmoplantar pustular psoriasis are highly related diseases that appear to be distinct from psoriasis vulgaris. PLoS One. 2016;11(5):1-11.
4. Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349-358.
5. Bissonnette R, Fuentes-Duculan J, Mashiko S, et al. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J Dermatol Sci. 2017;85(1):20-26.
6. Misiak-Galazka M, Wolska H, Rudnicka L. What do we know about palmoplantar pustulosis? J Eur Acad Dermatol Venereol. 2017;31(1):38-44.
7. Brunasso AMG, Puntoni M, Aberer W, et al. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168(6):1243-1251.
8. Engin B, As¸kın Ö, Tüzün Y. Palmoplantar psoriasis. Clin Dermatol. 2017; 35(1):19-27.
9. Chung J, Callas Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared to moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623-632.
10. Umezawa Y, Nakagawa H, Tamaki K. Phase III clinical study of maxacalcitol ointment in patients with palmoplantar pustulosis: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2016;43(3):288-293.
11. Muro M, Kawakami H, Matsumoto Y, et al. Topical combination therapy with vitamin D3 and corticosteroid ointment for palmoplantar pustulosis: a prospective, randomized, left-right comparison study. J Dermatolog Treat. 2016;27(1):51-53.
12. Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70-80.
1. Sevrain M, Richard M-A, Barnetche T, et al. Treatment for palmoplantar pustular psoriasis: systematic literature review, evidence-based recommendations and expert opinion. J Eur Acad Dermatol Venereol. 2014;28(suppl 5):13-16.
2. Olazagasti JM, Ma JE, Wetter DA. Clinical features, etiological factors, associated disorders, and treatment of palmoplantar pustulosis: the Mayo Clinic experience, 1996-2013. Mayo Clin Proc. 2017;92(9):1351-1358.
3. Bissonnette R, Suárez-Fariñas M, Li X, et al. Based on molecular profiling of gene expression, palmoplantar pustulosis and palmoplantar pustular psoriasis are highly related diseases that appear to be distinct from psoriasis vulgaris. PLoS One. 2016;11(5):1-11.
4. Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349-358.
5. Bissonnette R, Fuentes-Duculan J, Mashiko S, et al. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J Dermatol Sci. 2017;85(1):20-26.
6. Misiak-Galazka M, Wolska H, Rudnicka L. What do we know about palmoplantar pustulosis? J Eur Acad Dermatol Venereol. 2017;31(1):38-44.
7. Brunasso AMG, Puntoni M, Aberer W, et al. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168(6):1243-1251.
8. Engin B, As¸kın Ö, Tüzün Y. Palmoplantar psoriasis. Clin Dermatol. 2017; 35(1):19-27.
9. Chung J, Callas Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared to moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623-632.
10. Umezawa Y, Nakagawa H, Tamaki K. Phase III clinical study of maxacalcitol ointment in patients with palmoplantar pustulosis: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2016;43(3):288-293.
11. Muro M, Kawakami H, Matsumoto Y, et al. Topical combination therapy with vitamin D3 and corticosteroid ointment for palmoplantar pustulosis: a prospective, randomized, left-right comparison study. J Dermatolog Treat. 2016;27(1):51-53.
12. Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70-80.
Adding veliparib to chemotherapy improves PFS in BRCA-mutated breast cancer
BARCELONA – Adding veliparib to chemotherapy improved progression-free survival and provided a more durable benefit than did chemotherapy alone for HER2-negative advanced germline BRCA-associated breast cancer in the randomized, placebo-controlled, phase 3 BROCADE3 study.
Investigator-assessed median progression-free survival (PFS), the primary study endpoint, was 14.5 vs. 12.6 months in 337 patients randomized to receive the poly (ADP-ribose) polymerase inhibitor (PARPi) veliparib along with carboplatin/paclitaxel (CP) and 172 who received placebo and CP (hazard ratio, 0.71), Veronique C. Diéras, MD, reported at the European Society for Medical Oncology Congress.
“The benefit looks durable; more patients in the veliparib arm were still progression free at 2 years (34% vs. 20%), and at 3 years (26% vs. 11%),” said Dr. Diéras of Institut Curie, Paris, and Centre Eugene Marquis, Rennes, France.
The PFS benefit was confirmed by independent central review, and was apparent in all subgroups analyzed, except perhaps in patients with prior brain metastases, who comprised a very small group, she noted.
Among the secondary study endpoints were median overall survival (33.5 vs. 28.2 months at an interim analysis; HR, 0.95), clinical benefit rate (90.7% and 93.2% at 24 weeks), and objective response rate (75.8% and 74.1%).
“This is very important to note,” she said, referring to the high percentage of patients who benefited from CP alone. “But, again, if we look at the duration of response, in the veliparib arm the median duration of response was 14.7 months, whereas it was 11 months in the placebo arm.”
Participants in the double-blind trial were adults with a median age of 47 years who were randomized 2:1 to CP with veliparib or placebo for the treatment of germline BRCA1- or BRCA2-mutated metastatic breast cancer and had received no more than two prior lines of cytotoxic therapy; 48% were estrogen receptor– and/or progesterone receptor–negative, 8% had prior platinum therapy, 4% had a history of central nervous system metastases, and 19% had prior chemotherapy for metastatic disease.
They received placebo or veliparib at an oral dose of 120 mg twice daily on days −2 to 5 with carboplatin (area under the curve 6 on day 1) and weekly paclitaxel (80 mg/m2 on days 1, 8, and 15) in 21-day cycles until disease progression, and as allowed per study protocol, 44% crossed over from the placebo to veliparib group, Dr. Diéras said.
Common adverse events in the veliparib and placebo groups, respectively, included neutropenia (in 89% and 91% of patients), thrombocytopenia (81% and 71%), anemia (80% and 70%), and nausea (73% and 64%), she said, adding that the most common grade 3 adverse events were anemia (42% and 40%), neutropenia (81% and 84%), and thrombocytopenia (40% and 28%).
However, less than 10% of patients discontinued the study drug because of adverse events, she noted.
“In fact, the addition of veliparib to cytotoxic chemotherapy didn’t impair the administration of cytotoxic chemotherapy,” she said, adding that the mean number of CP cycles was 11 in both arms.
Select adverse events of special interest in the veliparib and placebo groups, respectively, included infection within 14 days of neutropenia (any grade, 37% and 36%; grade 3+, 5.4% and 1.8%), hemorrhage within 14 days of thrombocytopenia (any grade, 10% and 7%; grade 3+, 0.3% and 0%), and myelodysplastic syndromes (0.3% in both groups, with 0 grade 3+ events), she said.
“We know that germline BRCA-mutated breast cancers have increased sensitivity to platinum agents. Moreover, according to the concept of synthetic lethality, we do know also that these mutated tumors are very good candidates for PARP inhibition, so there is a strong rationale to combine a PARP inhibitor with cytotoxic chemotherapy with platinum,” she said.
Early studies of such combinations have been challenging because of exacerbation of myelosuppression, which may be the result of “the PARP trapping activity of some compounds,” but veliparib potently inhibits PARP with minimal PARP trapping, and thus may be better tolerated in combination with CP, she explained.
“In fact, in a phase 2 randomized trial – BROCADE2 – we did observe numerical increases in PFS and overall survival with [veliparib+CP],” she said.
In BROCADE3, the addition to veliparib to CP provided “a statistically significant and clinically meaningful benefit in patients with HER-negative advanced breast cancer and a germline BRCA mutation” without substantially altering the toxicity profile of C/P, she said.
“Considering these results, in my opinion, patients harboring BRCA mutations with advanced breast cancer [who are] candidates for chemotherapy should be offered this treatment option,” she concluded.
Invited discussant Sherene Loi, MBBS, PhD, head of the Translational Breast Cancer Genomics and Therapeutics Laboratory at Peter MacCallum Cancer Center, Victoria, Australia, said the investigators should be commended for conducting this phase 3 trial, and agreed that the approach is “reasonable to consider in a patient who does need chemotherapy.”
However, Dr. Loi said, it remains unclear if the PFS benefit seen in BROCADE3 is “due to the combination upfront and/or the monotherapy,” and suggested waiting for additional data before considering veliparib + CP as the new standard of care for germline BRCA1- and BRCA2-mutated advanced breast cancer.
“Current ESMO guidelines advise that single-agent chemotherapy be given sequentially in the absence of visceral crisis, therefore I think it’s important to await further for mature OS data and patient-reported outcomes,” she said, adding that it is also important to wait for the correlative analyses to “try to understand the rate of BRCA reversions and other resistance mechanisms in plasma.”
BROCADE3 was funded by AbbVie. Dr. Diéras reported advisory/consultancy roles for several pharmaceutical companies including AbbVie. Dr. Loi reported relationships with numerous pharmaceutical companies.
SOURCE: Diéras V et al. ESMO 2019, Abstract LBA9.
BARCELONA – Adding veliparib to chemotherapy improved progression-free survival and provided a more durable benefit than did chemotherapy alone for HER2-negative advanced germline BRCA-associated breast cancer in the randomized, placebo-controlled, phase 3 BROCADE3 study.
Investigator-assessed median progression-free survival (PFS), the primary study endpoint, was 14.5 vs. 12.6 months in 337 patients randomized to receive the poly (ADP-ribose) polymerase inhibitor (PARPi) veliparib along with carboplatin/paclitaxel (CP) and 172 who received placebo and CP (hazard ratio, 0.71), Veronique C. Diéras, MD, reported at the European Society for Medical Oncology Congress.
“The benefit looks durable; more patients in the veliparib arm were still progression free at 2 years (34% vs. 20%), and at 3 years (26% vs. 11%),” said Dr. Diéras of Institut Curie, Paris, and Centre Eugene Marquis, Rennes, France.
The PFS benefit was confirmed by independent central review, and was apparent in all subgroups analyzed, except perhaps in patients with prior brain metastases, who comprised a very small group, she noted.
Among the secondary study endpoints were median overall survival (33.5 vs. 28.2 months at an interim analysis; HR, 0.95), clinical benefit rate (90.7% and 93.2% at 24 weeks), and objective response rate (75.8% and 74.1%).
“This is very important to note,” she said, referring to the high percentage of patients who benefited from CP alone. “But, again, if we look at the duration of response, in the veliparib arm the median duration of response was 14.7 months, whereas it was 11 months in the placebo arm.”
Participants in the double-blind trial were adults with a median age of 47 years who were randomized 2:1 to CP with veliparib or placebo for the treatment of germline BRCA1- or BRCA2-mutated metastatic breast cancer and had received no more than two prior lines of cytotoxic therapy; 48% were estrogen receptor– and/or progesterone receptor–negative, 8% had prior platinum therapy, 4% had a history of central nervous system metastases, and 19% had prior chemotherapy for metastatic disease.
They received placebo or veliparib at an oral dose of 120 mg twice daily on days −2 to 5 with carboplatin (area under the curve 6 on day 1) and weekly paclitaxel (80 mg/m2 on days 1, 8, and 15) in 21-day cycles until disease progression, and as allowed per study protocol, 44% crossed over from the placebo to veliparib group, Dr. Diéras said.
Common adverse events in the veliparib and placebo groups, respectively, included neutropenia (in 89% and 91% of patients), thrombocytopenia (81% and 71%), anemia (80% and 70%), and nausea (73% and 64%), she said, adding that the most common grade 3 adverse events were anemia (42% and 40%), neutropenia (81% and 84%), and thrombocytopenia (40% and 28%).
However, less than 10% of patients discontinued the study drug because of adverse events, she noted.
“In fact, the addition of veliparib to cytotoxic chemotherapy didn’t impair the administration of cytotoxic chemotherapy,” she said, adding that the mean number of CP cycles was 11 in both arms.
Select adverse events of special interest in the veliparib and placebo groups, respectively, included infection within 14 days of neutropenia (any grade, 37% and 36%; grade 3+, 5.4% and 1.8%), hemorrhage within 14 days of thrombocytopenia (any grade, 10% and 7%; grade 3+, 0.3% and 0%), and myelodysplastic syndromes (0.3% in both groups, with 0 grade 3+ events), she said.
“We know that germline BRCA-mutated breast cancers have increased sensitivity to platinum agents. Moreover, according to the concept of synthetic lethality, we do know also that these mutated tumors are very good candidates for PARP inhibition, so there is a strong rationale to combine a PARP inhibitor with cytotoxic chemotherapy with platinum,” she said.
Early studies of such combinations have been challenging because of exacerbation of myelosuppression, which may be the result of “the PARP trapping activity of some compounds,” but veliparib potently inhibits PARP with minimal PARP trapping, and thus may be better tolerated in combination with CP, she explained.
“In fact, in a phase 2 randomized trial – BROCADE2 – we did observe numerical increases in PFS and overall survival with [veliparib+CP],” she said.
In BROCADE3, the addition to veliparib to CP provided “a statistically significant and clinically meaningful benefit in patients with HER-negative advanced breast cancer and a germline BRCA mutation” without substantially altering the toxicity profile of C/P, she said.
“Considering these results, in my opinion, patients harboring BRCA mutations with advanced breast cancer [who are] candidates for chemotherapy should be offered this treatment option,” she concluded.
Invited discussant Sherene Loi, MBBS, PhD, head of the Translational Breast Cancer Genomics and Therapeutics Laboratory at Peter MacCallum Cancer Center, Victoria, Australia, said the investigators should be commended for conducting this phase 3 trial, and agreed that the approach is “reasonable to consider in a patient who does need chemotherapy.”
However, Dr. Loi said, it remains unclear if the PFS benefit seen in BROCADE3 is “due to the combination upfront and/or the monotherapy,” and suggested waiting for additional data before considering veliparib + CP as the new standard of care for germline BRCA1- and BRCA2-mutated advanced breast cancer.
“Current ESMO guidelines advise that single-agent chemotherapy be given sequentially in the absence of visceral crisis, therefore I think it’s important to await further for mature OS data and patient-reported outcomes,” she said, adding that it is also important to wait for the correlative analyses to “try to understand the rate of BRCA reversions and other resistance mechanisms in plasma.”
BROCADE3 was funded by AbbVie. Dr. Diéras reported advisory/consultancy roles for several pharmaceutical companies including AbbVie. Dr. Loi reported relationships with numerous pharmaceutical companies.
SOURCE: Diéras V et al. ESMO 2019, Abstract LBA9.
BARCELONA – Adding veliparib to chemotherapy improved progression-free survival and provided a more durable benefit than did chemotherapy alone for HER2-negative advanced germline BRCA-associated breast cancer in the randomized, placebo-controlled, phase 3 BROCADE3 study.
Investigator-assessed median progression-free survival (PFS), the primary study endpoint, was 14.5 vs. 12.6 months in 337 patients randomized to receive the poly (ADP-ribose) polymerase inhibitor (PARPi) veliparib along with carboplatin/paclitaxel (CP) and 172 who received placebo and CP (hazard ratio, 0.71), Veronique C. Diéras, MD, reported at the European Society for Medical Oncology Congress.
“The benefit looks durable; more patients in the veliparib arm were still progression free at 2 years (34% vs. 20%), and at 3 years (26% vs. 11%),” said Dr. Diéras of Institut Curie, Paris, and Centre Eugene Marquis, Rennes, France.
The PFS benefit was confirmed by independent central review, and was apparent in all subgroups analyzed, except perhaps in patients with prior brain metastases, who comprised a very small group, she noted.
Among the secondary study endpoints were median overall survival (33.5 vs. 28.2 months at an interim analysis; HR, 0.95), clinical benefit rate (90.7% and 93.2% at 24 weeks), and objective response rate (75.8% and 74.1%).
“This is very important to note,” she said, referring to the high percentage of patients who benefited from CP alone. “But, again, if we look at the duration of response, in the veliparib arm the median duration of response was 14.7 months, whereas it was 11 months in the placebo arm.”
Participants in the double-blind trial were adults with a median age of 47 years who were randomized 2:1 to CP with veliparib or placebo for the treatment of germline BRCA1- or BRCA2-mutated metastatic breast cancer and had received no more than two prior lines of cytotoxic therapy; 48% were estrogen receptor– and/or progesterone receptor–negative, 8% had prior platinum therapy, 4% had a history of central nervous system metastases, and 19% had prior chemotherapy for metastatic disease.
They received placebo or veliparib at an oral dose of 120 mg twice daily on days −2 to 5 with carboplatin (area under the curve 6 on day 1) and weekly paclitaxel (80 mg/m2 on days 1, 8, and 15) in 21-day cycles until disease progression, and as allowed per study protocol, 44% crossed over from the placebo to veliparib group, Dr. Diéras said.
Common adverse events in the veliparib and placebo groups, respectively, included neutropenia (in 89% and 91% of patients), thrombocytopenia (81% and 71%), anemia (80% and 70%), and nausea (73% and 64%), she said, adding that the most common grade 3 adverse events were anemia (42% and 40%), neutropenia (81% and 84%), and thrombocytopenia (40% and 28%).
However, less than 10% of patients discontinued the study drug because of adverse events, she noted.
“In fact, the addition of veliparib to cytotoxic chemotherapy didn’t impair the administration of cytotoxic chemotherapy,” she said, adding that the mean number of CP cycles was 11 in both arms.
Select adverse events of special interest in the veliparib and placebo groups, respectively, included infection within 14 days of neutropenia (any grade, 37% and 36%; grade 3+, 5.4% and 1.8%), hemorrhage within 14 days of thrombocytopenia (any grade, 10% and 7%; grade 3+, 0.3% and 0%), and myelodysplastic syndromes (0.3% in both groups, with 0 grade 3+ events), she said.
“We know that germline BRCA-mutated breast cancers have increased sensitivity to platinum agents. Moreover, according to the concept of synthetic lethality, we do know also that these mutated tumors are very good candidates for PARP inhibition, so there is a strong rationale to combine a PARP inhibitor with cytotoxic chemotherapy with platinum,” she said.
Early studies of such combinations have been challenging because of exacerbation of myelosuppression, which may be the result of “the PARP trapping activity of some compounds,” but veliparib potently inhibits PARP with minimal PARP trapping, and thus may be better tolerated in combination with CP, she explained.
“In fact, in a phase 2 randomized trial – BROCADE2 – we did observe numerical increases in PFS and overall survival with [veliparib+CP],” she said.
In BROCADE3, the addition to veliparib to CP provided “a statistically significant and clinically meaningful benefit in patients with HER-negative advanced breast cancer and a germline BRCA mutation” without substantially altering the toxicity profile of C/P, she said.
“Considering these results, in my opinion, patients harboring BRCA mutations with advanced breast cancer [who are] candidates for chemotherapy should be offered this treatment option,” she concluded.
Invited discussant Sherene Loi, MBBS, PhD, head of the Translational Breast Cancer Genomics and Therapeutics Laboratory at Peter MacCallum Cancer Center, Victoria, Australia, said the investigators should be commended for conducting this phase 3 trial, and agreed that the approach is “reasonable to consider in a patient who does need chemotherapy.”
However, Dr. Loi said, it remains unclear if the PFS benefit seen in BROCADE3 is “due to the combination upfront and/or the monotherapy,” and suggested waiting for additional data before considering veliparib + CP as the new standard of care for germline BRCA1- and BRCA2-mutated advanced breast cancer.
“Current ESMO guidelines advise that single-agent chemotherapy be given sequentially in the absence of visceral crisis, therefore I think it’s important to await further for mature OS data and patient-reported outcomes,” she said, adding that it is also important to wait for the correlative analyses to “try to understand the rate of BRCA reversions and other resistance mechanisms in plasma.”
BROCADE3 was funded by AbbVie. Dr. Diéras reported advisory/consultancy roles for several pharmaceutical companies including AbbVie. Dr. Loi reported relationships with numerous pharmaceutical companies.
SOURCE: Diéras V et al. ESMO 2019, Abstract LBA9.
REPORTING FROM ESMO 2019