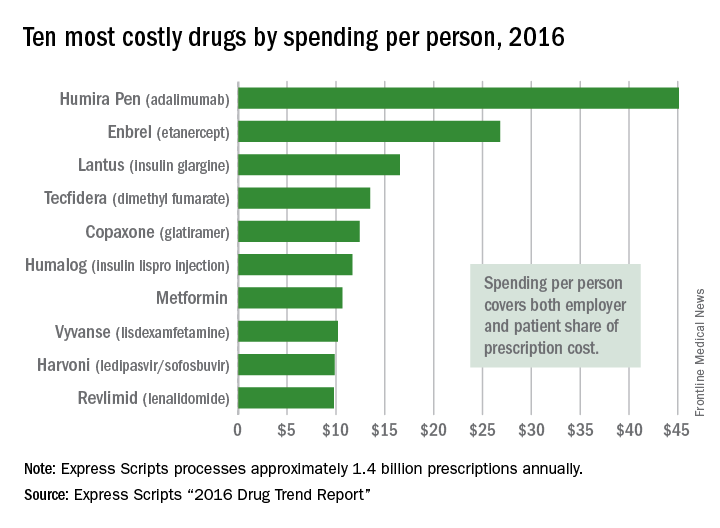
Humira Pen (adalimumab) was the most expensive drug in 2016 when ranked by spending per person, according to pharmacy benefits manager Express Scripts.
Total spending per person with employer-sponsored insurance was $45.11 last year for Humira Pen, which is indicated for rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. Next in spending per person was Enbrel (etanercept) – another drug for arthritis, psoriatic arthritis, ankylosing spondylitis, and psoriasis – at $26.82, followed by the diabetes drug Lantus (insulin glargine) and two multiple sclerosis drugs: Tecfidera (dimethyl fumarate) and Copaxone (glatiramer), Express Scripts said in its “2016 Drug Trend Report.”
The only generic drug in the top 10 was the diabetes drug metformin at number seven, with per-person spending of $10.67 in 2016. Compared with 2015, metformin had the largest increase in spending among the top 10 drugs, with a jump of 160% due mainly to the “launch of a very high-priced generic to Glumetza (metformin extended-release tablets) that’s not interchangeable with any other extended-release metformin,” the company said.Humira Pen had the next-largest increase from 2015 – a mere 28% – while the hepatitis C drug Harvoni (ledipasvir/sofisbuvir) had the largest decrease in per-person spending among the top 10, dropping 54%, the report noted.
Express Scripts processes approximately 1.4 billion prescriptions annually for 85 million insured members from 3,000 client companies.