User login
Findings support endovascular-first approach for ruptured VAAs
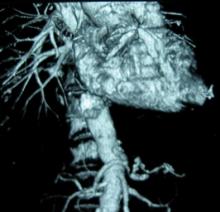
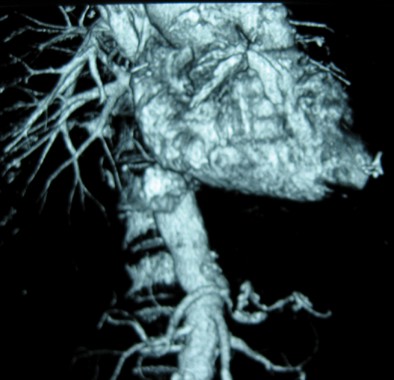
BOSTON – Endovascular interventions for ruptured visceral artery aneurysms are associated with reduced morbidity and mortality, compared with open interventions, according to findings from a retrospective chart review.
Both endovascular and open repairs are safe and durable for intact visceral artery aneurysms, Dr. Ankur J. Shukla reported at a the 2014 Vascular Annual Meeting.
Of 261 patients who presented with visceral artery aneurysms (VAAs), 174 underwent repair: 74 who presented with ruptured VAA and 100 who presented with intact VAA. The majority – 73% of ruptured VAA and 62% of intact VAA – were repaired with an endovascular approach.
Among those with ruptured VAA, 30-day mortality was 7.4% following endovascular repair, compared with 28.6% following open repair, a significant difference, said Dr. Shukla of the University of Pittsburgh Medical Center.
Survival at 3 years of ruptured VAA was about 70% vs. 46.4% in the endovascular and open repair groups, respectively, he said.
About 65% of patients with ruptured VAA presented with pain, and about 30% presented in hemodynamic shock. The most commonly identified etiology was "inflammatory/pancreatitis inflammatory," and 80% of the aneurysms were pseudoaneurysmal in nature.
A large proportion of the aneurysms were in the splenic and arterial beds, but 26% were located in the pancreaticoduodenal arcade, and those had a mean size of 12.7 mm. Most (95%) were pseudoaneurysms.
The outcomes with ruptured VAA were quite good, Dr. Shukla said, noting that the technical success rate was 98.7%.
Although the 30-day reintervention rate with endovascular repair was higher, the difference between the groups was not statistically significant, and there was a trend toward a lower rate of major complications with endovascular repair.
Factors found to be predictors of mortality risk were older age and steroid use, while endovascular repair was found to be protective.
As for the patients with intact VAA, most presented without symptoms, and the most common etiology was atherosclerosis.
"When we looked at the distribution, this was very consistent with what has been reported in the literature, with the splenic and arterial beds really making up the lion’s share of this group. Notable is the fact that 6.7% of our patients had intact aneurysms in the pancreaticoduodenal arcade," he said.
Outcomes in those with intact aneurysms were good. A slightly higher 30-day reintervention rate in those who underwent endovascular repair did not reach statistical significance, and both the endovascular and open repair groups had low rates of major complications.
Survival at 3 years for intact aneurysms did not differ in the endovascular and open repair groups. This was partly due to a 0% 30-day mortality, and – despite the fact that the overall mortality in those with intact aneurysms was 10% – there was zero overall aneurysm-related mortality, he said.
Patients in the study were treated at a single institution between 2003 and 2013. Most were in their mid to late 50s, and there were more men and more individuals on immunosuppressive therapy in the ruptured VAA group. However, comorbidities were similar in the ruptured and intact VAA groups.
Visceral artery aneurysms occur only rarely, affecting 0.1% to 2% of the general population, but because of the increasing use of noninvasive imaging, more of these aneurysms are being detected incidentally.
When they are not found incidentally, they often go undetected and present when they rupture, Dr. Shukla said.
"Because of the increasing utilization and improvement of endovascular technology, we now have a lot of options to fix these aneurysms. But the outcomes are not well defined. Even less well defined is the outcome of ruptured visceral artery aneurysms," he said, noting that most studies have a small sample size or look only at endovascular or open repairs.
Overall, the current study showed that there is "an acute and sharp drop-off in survival with open repair," related, most likely, to operative mortality, he said.
"Based on the findings, we recommend aggressive treatment of pseudoaneurysms and true aneurysms in the pancreaticoduodenal arcade, and advocate for an endovascular-first approach to treating ruptured visceral artery aneurysms, acknowledging that success in this is really predicated on good planning based on advanced imaging and endovascular set-up," he concluded.
Dr. Shukla reported having no disclosures
Visceral Artery Aneurysms (VAA) can be challenging to treat from an open approach especially when the aneurysm is adherent to the surrounding pancreatic, visceral or retroperitoneal tissue. In most areas of vascular disease endovascular therapy is easier. But, endovascular therapy for VAA can be as challenging as the open surgical repair because of vessel tortuosity and access, imaging challenges, and the few options available for durable aneurysm treatment. Dr. Shukla and colleagues reviewed their outcomes in treating VAA over a 10-year period and report high rates of technical success, low morbidity and mortality using endovascular means for both intact and ruptured VAA. The results for endovascular therapy for ruptured VAA were particularly promising. These results indicate that coils and other ablative maneuvers may suffice in the setting of hemorrhage and be preferable to open surgical repair or ligation. This study provides important information on a rare problem and reassures us that the mid-term durability of ablative techniques for VAA is acceptable.
Dr. Vikram Kashyap is professor of surgery, Case Western Reserve University and chief, Division of Vascular Surgery and Endovascular Therapy, and co-director, Harrington Heart & Vascular Institute, University Hospitals Case Medical Center,Cleveland.
Visceral Artery Aneurysms (VAA) can be challenging to treat from an open approach especially when the aneurysm is adherent to the surrounding pancreatic, visceral or retroperitoneal tissue. In most areas of vascular disease endovascular therapy is easier. But, endovascular therapy for VAA can be as challenging as the open surgical repair because of vessel tortuosity and access, imaging challenges, and the few options available for durable aneurysm treatment. Dr. Shukla and colleagues reviewed their outcomes in treating VAA over a 10-year period and report high rates of technical success, low morbidity and mortality using endovascular means for both intact and ruptured VAA. The results for endovascular therapy for ruptured VAA were particularly promising. These results indicate that coils and other ablative maneuvers may suffice in the setting of hemorrhage and be preferable to open surgical repair or ligation. This study provides important information on a rare problem and reassures us that the mid-term durability of ablative techniques for VAA is acceptable.
Dr. Vikram Kashyap is professor of surgery, Case Western Reserve University and chief, Division of Vascular Surgery and Endovascular Therapy, and co-director, Harrington Heart & Vascular Institute, University Hospitals Case Medical Center,Cleveland.
Visceral Artery Aneurysms (VAA) can be challenging to treat from an open approach especially when the aneurysm is adherent to the surrounding pancreatic, visceral or retroperitoneal tissue. In most areas of vascular disease endovascular therapy is easier. But, endovascular therapy for VAA can be as challenging as the open surgical repair because of vessel tortuosity and access, imaging challenges, and the few options available for durable aneurysm treatment. Dr. Shukla and colleagues reviewed their outcomes in treating VAA over a 10-year period and report high rates of technical success, low morbidity and mortality using endovascular means for both intact and ruptured VAA. The results for endovascular therapy for ruptured VAA were particularly promising. These results indicate that coils and other ablative maneuvers may suffice in the setting of hemorrhage and be preferable to open surgical repair or ligation. This study provides important information on a rare problem and reassures us that the mid-term durability of ablative techniques for VAA is acceptable.
Dr. Vikram Kashyap is professor of surgery, Case Western Reserve University and chief, Division of Vascular Surgery and Endovascular Therapy, and co-director, Harrington Heart & Vascular Institute, University Hospitals Case Medical Center,Cleveland.
BOSTON – Endovascular interventions for ruptured visceral artery aneurysms are associated with reduced morbidity and mortality, compared with open interventions, according to findings from a retrospective chart review.
Both endovascular and open repairs are safe and durable for intact visceral artery aneurysms, Dr. Ankur J. Shukla reported at a the 2014 Vascular Annual Meeting.
Of 261 patients who presented with visceral artery aneurysms (VAAs), 174 underwent repair: 74 who presented with ruptured VAA and 100 who presented with intact VAA. The majority – 73% of ruptured VAA and 62% of intact VAA – were repaired with an endovascular approach.
Among those with ruptured VAA, 30-day mortality was 7.4% following endovascular repair, compared with 28.6% following open repair, a significant difference, said Dr. Shukla of the University of Pittsburgh Medical Center.
Survival at 3 years of ruptured VAA was about 70% vs. 46.4% in the endovascular and open repair groups, respectively, he said.
About 65% of patients with ruptured VAA presented with pain, and about 30% presented in hemodynamic shock. The most commonly identified etiology was "inflammatory/pancreatitis inflammatory," and 80% of the aneurysms were pseudoaneurysmal in nature.
A large proportion of the aneurysms were in the splenic and arterial beds, but 26% were located in the pancreaticoduodenal arcade, and those had a mean size of 12.7 mm. Most (95%) were pseudoaneurysms.
The outcomes with ruptured VAA were quite good, Dr. Shukla said, noting that the technical success rate was 98.7%.
Although the 30-day reintervention rate with endovascular repair was higher, the difference between the groups was not statistically significant, and there was a trend toward a lower rate of major complications with endovascular repair.
Factors found to be predictors of mortality risk were older age and steroid use, while endovascular repair was found to be protective.
As for the patients with intact VAA, most presented without symptoms, and the most common etiology was atherosclerosis.
"When we looked at the distribution, this was very consistent with what has been reported in the literature, with the splenic and arterial beds really making up the lion’s share of this group. Notable is the fact that 6.7% of our patients had intact aneurysms in the pancreaticoduodenal arcade," he said.
Outcomes in those with intact aneurysms were good. A slightly higher 30-day reintervention rate in those who underwent endovascular repair did not reach statistical significance, and both the endovascular and open repair groups had low rates of major complications.
Survival at 3 years for intact aneurysms did not differ in the endovascular and open repair groups. This was partly due to a 0% 30-day mortality, and – despite the fact that the overall mortality in those with intact aneurysms was 10% – there was zero overall aneurysm-related mortality, he said.
Patients in the study were treated at a single institution between 2003 and 2013. Most were in their mid to late 50s, and there were more men and more individuals on immunosuppressive therapy in the ruptured VAA group. However, comorbidities were similar in the ruptured and intact VAA groups.
Visceral artery aneurysms occur only rarely, affecting 0.1% to 2% of the general population, but because of the increasing use of noninvasive imaging, more of these aneurysms are being detected incidentally.
When they are not found incidentally, they often go undetected and present when they rupture, Dr. Shukla said.
"Because of the increasing utilization and improvement of endovascular technology, we now have a lot of options to fix these aneurysms. But the outcomes are not well defined. Even less well defined is the outcome of ruptured visceral artery aneurysms," he said, noting that most studies have a small sample size or look only at endovascular or open repairs.
Overall, the current study showed that there is "an acute and sharp drop-off in survival with open repair," related, most likely, to operative mortality, he said.
"Based on the findings, we recommend aggressive treatment of pseudoaneurysms and true aneurysms in the pancreaticoduodenal arcade, and advocate for an endovascular-first approach to treating ruptured visceral artery aneurysms, acknowledging that success in this is really predicated on good planning based on advanced imaging and endovascular set-up," he concluded.
Dr. Shukla reported having no disclosures
BOSTON – Endovascular interventions for ruptured visceral artery aneurysms are associated with reduced morbidity and mortality, compared with open interventions, according to findings from a retrospective chart review.
Both endovascular and open repairs are safe and durable for intact visceral artery aneurysms, Dr. Ankur J. Shukla reported at a the 2014 Vascular Annual Meeting.
Of 261 patients who presented with visceral artery aneurysms (VAAs), 174 underwent repair: 74 who presented with ruptured VAA and 100 who presented with intact VAA. The majority – 73% of ruptured VAA and 62% of intact VAA – were repaired with an endovascular approach.
Among those with ruptured VAA, 30-day mortality was 7.4% following endovascular repair, compared with 28.6% following open repair, a significant difference, said Dr. Shukla of the University of Pittsburgh Medical Center.
Survival at 3 years of ruptured VAA was about 70% vs. 46.4% in the endovascular and open repair groups, respectively, he said.
About 65% of patients with ruptured VAA presented with pain, and about 30% presented in hemodynamic shock. The most commonly identified etiology was "inflammatory/pancreatitis inflammatory," and 80% of the aneurysms were pseudoaneurysmal in nature.
A large proportion of the aneurysms were in the splenic and arterial beds, but 26% were located in the pancreaticoduodenal arcade, and those had a mean size of 12.7 mm. Most (95%) were pseudoaneurysms.
The outcomes with ruptured VAA were quite good, Dr. Shukla said, noting that the technical success rate was 98.7%.
Although the 30-day reintervention rate with endovascular repair was higher, the difference between the groups was not statistically significant, and there was a trend toward a lower rate of major complications with endovascular repair.
Factors found to be predictors of mortality risk were older age and steroid use, while endovascular repair was found to be protective.
As for the patients with intact VAA, most presented without symptoms, and the most common etiology was atherosclerosis.
"When we looked at the distribution, this was very consistent with what has been reported in the literature, with the splenic and arterial beds really making up the lion’s share of this group. Notable is the fact that 6.7% of our patients had intact aneurysms in the pancreaticoduodenal arcade," he said.
Outcomes in those with intact aneurysms were good. A slightly higher 30-day reintervention rate in those who underwent endovascular repair did not reach statistical significance, and both the endovascular and open repair groups had low rates of major complications.
Survival at 3 years for intact aneurysms did not differ in the endovascular and open repair groups. This was partly due to a 0% 30-day mortality, and – despite the fact that the overall mortality in those with intact aneurysms was 10% – there was zero overall aneurysm-related mortality, he said.
Patients in the study were treated at a single institution between 2003 and 2013. Most were in their mid to late 50s, and there were more men and more individuals on immunosuppressive therapy in the ruptured VAA group. However, comorbidities were similar in the ruptured and intact VAA groups.
Visceral artery aneurysms occur only rarely, affecting 0.1% to 2% of the general population, but because of the increasing use of noninvasive imaging, more of these aneurysms are being detected incidentally.
When they are not found incidentally, they often go undetected and present when they rupture, Dr. Shukla said.
"Because of the increasing utilization and improvement of endovascular technology, we now have a lot of options to fix these aneurysms. But the outcomes are not well defined. Even less well defined is the outcome of ruptured visceral artery aneurysms," he said, noting that most studies have a small sample size or look only at endovascular or open repairs.
Overall, the current study showed that there is "an acute and sharp drop-off in survival with open repair," related, most likely, to operative mortality, he said.
"Based on the findings, we recommend aggressive treatment of pseudoaneurysms and true aneurysms in the pancreaticoduodenal arcade, and advocate for an endovascular-first approach to treating ruptured visceral artery aneurysms, acknowledging that success in this is really predicated on good planning based on advanced imaging and endovascular set-up," he concluded.
Dr. Shukla reported having no disclosures
AT THE 2014 VASCULAR ANNUAL MEETING
Key clinical point: The researchers recommend aggressive treatment of visceral artery pseudoaneurysms and true aneurysms, with an endovascular-first approach to treating ruptured aneurysms.
Major finding: Thirty-day mortality was 7.4% vs. 26% with endovascular vs. open repair of ruptured VAAs.
Data source: A retrospective chart review involving 174 cases.
Disclosures: Dr. Shukla reported having no disclosures.
Meds fall short for type B dissection
BOSTON – Medical therapy for acute uncomplicated type B aortic dissections was effective in the short term, but was associated with low 6-year intervention-free survival in a review of 298 cases.
Furthermore, patients who received medical therapy without operative intervention had increased mortality at 6 years, compared with those who received intervention, Dr. Christopher A. Durham reported at the Vascular Annual Meeting.
During a mean follow-up of nearly 4.3 years, medical therapy failed in almost 60% of the patients; 114 died after an average of 2.7 years, and 87 required aortic intervention.
Aneurysmal degeneration was the indication for intervention in 65% of patients requiring intervention, and six of these patients experienced a ruptured aneurysm. "Only six of these patients underwent stent placement, with the remainder receiving open aortic replacement," said Dr. Durham of Massachusetts General Hospital, Boston.
The average time to operation in this subset of patients was 2.3 years. Visceral malperfusion was the indication for intervention in 18 patients (21%), and most underwent an endovascular intervention including either stenting or endovascular fenestration. A less common indication for intervention was retrograde type A dissection development in two patients. These patients underwent open replacement.
The average time to intervention in the subset of patients whose indication was not aneurysmal degeneration was 24 days. Early treatment failure – within 15 days of presentation – occurred in 37 patients (12%) and included 12 deaths and 25 interventions.
"In this group of patients who ultimately required an intervention within the acute period, aneurysmal degeneration was the indication in 25% of patients, all of whom were treated with an open approach," Dr. Durham said. Visceral malperfusion was the indication in half of the early interventions.
The 30-day mortality rate among patients with early intervention after initial medical therapy was 12%.
Freedom from intervention was 74% at 6 years, with most interventions occurring within the first 12 months. Intervention-free survival was 55% at 3 years and 41% at 6 years. Only end-stage renal disease was found to be predictive of failure, and age over 70 years was protective against failure (hazard ratio, 0.97), Dr. Durham said, adding that no variables associated with progression to intervention were identified.
Notably, although survival was similar during the first 3 years in those who remained on medical management and those who required intervention (73% and 78%, respectively), survival at 6 years was 58% and 76% in the groups, respectively.
"These data join emerging data demonstrating a survival benefit in patients undergoing intervention when compared to those who are treated with medical therapy alone," he said.
Study subjects were all patients who were initially managed medically for acute uncomplicated type B aortic dissection between March 1999 and March 2011 in a health care system. The patients had a mean age of 66 years at presentation, about 62% were men, and most were white. Nearly 75% had hypertension, and most of those were on therapy. About 5% had end-stage renal disease.
Failure of medical therapy was defined as any death or aortic-related intervention. Early failure was defined as failure within 15 days of presentation.
"Aortic dissection is the most common catastrophic event affecting the aorta, with an incidence exceeding that of ruptured abdominal aortic aneurysm.
"The majority of patients with type B aortic dissections, where the entry tear originates distal to the left subclavian artery, are treated with medical therapy," he said. In fact, medical management aimed at lowering the systolic blood pressure and pulse remains the standard of care, and a number of studies have demonstrated a favorable 1-year survival – ranging from 70% to 90% – with medical therapy alone in this population.
"However, at what cost?" Dr. Durham asked.
"The principal late complication of aortic dissection is aneurysmal degeneration of the outer wall of the false lumen, which has been reported to occur in up to 40% of medically treated patients," he said, adding that, because of a paucity of contemporary data regarding the natural history of medically treated patients, it has been unclear whether the natural history has been altered with current medical therapy.
The current findings suggest that operative intervention is associated with a survival benefit.
As Food and Drug Administration "approval has just been granted for thoracic stent grafts to be used in aortic dissection, it is clear that endovascular coverage of proximal aortic entry tears will become more common in the acute phase. As such, further study is needed to determine which patients presenting with type B dissections will benefit from early intervention," he said.
Dr. Durham reported having no disclosures.
Editor’s Note: The treatment of type B dissection is a controversial subject and this controversy will be addressed in an upcoming Point/Counterpoint article in Vascular Specialist.
BOSTON – Medical therapy for acute uncomplicated type B aortic dissections was effective in the short term, but was associated with low 6-year intervention-free survival in a review of 298 cases.
Furthermore, patients who received medical therapy without operative intervention had increased mortality at 6 years, compared with those who received intervention, Dr. Christopher A. Durham reported at the Vascular Annual Meeting.
During a mean follow-up of nearly 4.3 years, medical therapy failed in almost 60% of the patients; 114 died after an average of 2.7 years, and 87 required aortic intervention.
Aneurysmal degeneration was the indication for intervention in 65% of patients requiring intervention, and six of these patients experienced a ruptured aneurysm. "Only six of these patients underwent stent placement, with the remainder receiving open aortic replacement," said Dr. Durham of Massachusetts General Hospital, Boston.
The average time to operation in this subset of patients was 2.3 years. Visceral malperfusion was the indication for intervention in 18 patients (21%), and most underwent an endovascular intervention including either stenting or endovascular fenestration. A less common indication for intervention was retrograde type A dissection development in two patients. These patients underwent open replacement.
The average time to intervention in the subset of patients whose indication was not aneurysmal degeneration was 24 days. Early treatment failure – within 15 days of presentation – occurred in 37 patients (12%) and included 12 deaths and 25 interventions.
"In this group of patients who ultimately required an intervention within the acute period, aneurysmal degeneration was the indication in 25% of patients, all of whom were treated with an open approach," Dr. Durham said. Visceral malperfusion was the indication in half of the early interventions.
The 30-day mortality rate among patients with early intervention after initial medical therapy was 12%.
Freedom from intervention was 74% at 6 years, with most interventions occurring within the first 12 months. Intervention-free survival was 55% at 3 years and 41% at 6 years. Only end-stage renal disease was found to be predictive of failure, and age over 70 years was protective against failure (hazard ratio, 0.97), Dr. Durham said, adding that no variables associated with progression to intervention were identified.
Notably, although survival was similar during the first 3 years in those who remained on medical management and those who required intervention (73% and 78%, respectively), survival at 6 years was 58% and 76% in the groups, respectively.
"These data join emerging data demonstrating a survival benefit in patients undergoing intervention when compared to those who are treated with medical therapy alone," he said.
Study subjects were all patients who were initially managed medically for acute uncomplicated type B aortic dissection between March 1999 and March 2011 in a health care system. The patients had a mean age of 66 years at presentation, about 62% were men, and most were white. Nearly 75% had hypertension, and most of those were on therapy. About 5% had end-stage renal disease.
Failure of medical therapy was defined as any death or aortic-related intervention. Early failure was defined as failure within 15 days of presentation.
"Aortic dissection is the most common catastrophic event affecting the aorta, with an incidence exceeding that of ruptured abdominal aortic aneurysm.
"The majority of patients with type B aortic dissections, where the entry tear originates distal to the left subclavian artery, are treated with medical therapy," he said. In fact, medical management aimed at lowering the systolic blood pressure and pulse remains the standard of care, and a number of studies have demonstrated a favorable 1-year survival – ranging from 70% to 90% – with medical therapy alone in this population.
"However, at what cost?" Dr. Durham asked.
"The principal late complication of aortic dissection is aneurysmal degeneration of the outer wall of the false lumen, which has been reported to occur in up to 40% of medically treated patients," he said, adding that, because of a paucity of contemporary data regarding the natural history of medically treated patients, it has been unclear whether the natural history has been altered with current medical therapy.
The current findings suggest that operative intervention is associated with a survival benefit.
As Food and Drug Administration "approval has just been granted for thoracic stent grafts to be used in aortic dissection, it is clear that endovascular coverage of proximal aortic entry tears will become more common in the acute phase. As such, further study is needed to determine which patients presenting with type B dissections will benefit from early intervention," he said.
Dr. Durham reported having no disclosures.
Editor’s Note: The treatment of type B dissection is a controversial subject and this controversy will be addressed in an upcoming Point/Counterpoint article in Vascular Specialist.
BOSTON – Medical therapy for acute uncomplicated type B aortic dissections was effective in the short term, but was associated with low 6-year intervention-free survival in a review of 298 cases.
Furthermore, patients who received medical therapy without operative intervention had increased mortality at 6 years, compared with those who received intervention, Dr. Christopher A. Durham reported at the Vascular Annual Meeting.
During a mean follow-up of nearly 4.3 years, medical therapy failed in almost 60% of the patients; 114 died after an average of 2.7 years, and 87 required aortic intervention.
Aneurysmal degeneration was the indication for intervention in 65% of patients requiring intervention, and six of these patients experienced a ruptured aneurysm. "Only six of these patients underwent stent placement, with the remainder receiving open aortic replacement," said Dr. Durham of Massachusetts General Hospital, Boston.
The average time to operation in this subset of patients was 2.3 years. Visceral malperfusion was the indication for intervention in 18 patients (21%), and most underwent an endovascular intervention including either stenting or endovascular fenestration. A less common indication for intervention was retrograde type A dissection development in two patients. These patients underwent open replacement.
The average time to intervention in the subset of patients whose indication was not aneurysmal degeneration was 24 days. Early treatment failure – within 15 days of presentation – occurred in 37 patients (12%) and included 12 deaths and 25 interventions.
"In this group of patients who ultimately required an intervention within the acute period, aneurysmal degeneration was the indication in 25% of patients, all of whom were treated with an open approach," Dr. Durham said. Visceral malperfusion was the indication in half of the early interventions.
The 30-day mortality rate among patients with early intervention after initial medical therapy was 12%.
Freedom from intervention was 74% at 6 years, with most interventions occurring within the first 12 months. Intervention-free survival was 55% at 3 years and 41% at 6 years. Only end-stage renal disease was found to be predictive of failure, and age over 70 years was protective against failure (hazard ratio, 0.97), Dr. Durham said, adding that no variables associated with progression to intervention were identified.
Notably, although survival was similar during the first 3 years in those who remained on medical management and those who required intervention (73% and 78%, respectively), survival at 6 years was 58% and 76% in the groups, respectively.
"These data join emerging data demonstrating a survival benefit in patients undergoing intervention when compared to those who are treated with medical therapy alone," he said.
Study subjects were all patients who were initially managed medically for acute uncomplicated type B aortic dissection between March 1999 and March 2011 in a health care system. The patients had a mean age of 66 years at presentation, about 62% were men, and most were white. Nearly 75% had hypertension, and most of those were on therapy. About 5% had end-stage renal disease.
Failure of medical therapy was defined as any death or aortic-related intervention. Early failure was defined as failure within 15 days of presentation.
"Aortic dissection is the most common catastrophic event affecting the aorta, with an incidence exceeding that of ruptured abdominal aortic aneurysm.
"The majority of patients with type B aortic dissections, where the entry tear originates distal to the left subclavian artery, are treated with medical therapy," he said. In fact, medical management aimed at lowering the systolic blood pressure and pulse remains the standard of care, and a number of studies have demonstrated a favorable 1-year survival – ranging from 70% to 90% – with medical therapy alone in this population.
"However, at what cost?" Dr. Durham asked.
"The principal late complication of aortic dissection is aneurysmal degeneration of the outer wall of the false lumen, which has been reported to occur in up to 40% of medically treated patients," he said, adding that, because of a paucity of contemporary data regarding the natural history of medically treated patients, it has been unclear whether the natural history has been altered with current medical therapy.
The current findings suggest that operative intervention is associated with a survival benefit.
As Food and Drug Administration "approval has just been granted for thoracic stent grafts to be used in aortic dissection, it is clear that endovascular coverage of proximal aortic entry tears will become more common in the acute phase. As such, further study is needed to determine which patients presenting with type B dissections will benefit from early intervention," he said.
Dr. Durham reported having no disclosures.
Editor’s Note: The treatment of type B dissection is a controversial subject and this controversy will be addressed in an upcoming Point/Counterpoint article in Vascular Specialist.
Major finding: Medical therapy failed in nearly 60% of patients during 4.3 years of follow-up.
Data source: A series of 298 cases.
Disclosures: Dr. Durham reported having no disclosures.
USPSTF: Screen older women smokers for AAA
The U.S. Preventive Services Task Force says that women between ages 65 and 75 years who have smoked 100 or more cigarettes in their lives could benefit from one-time ultrasonography screening for abdominal aortic aneurysm (AAA).
The AAA guidelines replace those published by USPSTF in 2005, which had recommended against screening women regardless of their smoking history.
The new guidelines, published online June 23 in Annals of Internal Medicine (doi:10.7326/M14-1204), do not recommend screening women who have never smoked, citing the very low prevalence of AAA in this group.
Nevertheless, the task force’s systematic review, led by current chair Dr. Michael L. LeFevre of the University of Missouri in Columbia, revealed that screening women aged 65-75 years who have smoked or currently smoke – a group for which AAA prevalence is between 0.8% and 2% – could potentially be beneficial, though current evidence remains insufficient to recommend it.
"Prevalence of AAA in women who currently smoke approaches that of men who have never smoked," Dr. LeFevre and his colleagues wrote in the guidelines.
"As such, a small net benefit might exist for this population and appropriate, high-quality research designs should be used to address this question," they added.
The task force continues to recommend that men between the ages of 65 and 75 years who have ever smoked be offered one-time screening with ultrasonography for AAA. Men in this age group who have never smoked may be offered screening if they have certain risk factors, such as advanced age or a family history of AAA.
AAA – a dilation in the wall of the abdominal section of the aorta of 3 cm or larger – is seen in 4% and 7% of men and about 1% of women over the age of 50, USPSTF said. Most AAAs remain asymptomatic until they rupture, in which case the mortality risk has been shown to be higher than 75%.
Women who develop AAA tend to do so at a later age than do men, the task force noted, with most ruptures occurring past age 80 years.
The task force is a voluntary advisory body independent of the U.S. government but supported by the Agency for Healthcare Research and Quality. One of the study’s co-authors, Dr. Douglas Owens of Stanford (Calif.) University disclosed travel support from the agency during the course of the review.
The other task force members declared no conflicts of interest.
The U.S. Preventive Services Task Force says that women between ages 65 and 75 years who have smoked 100 or more cigarettes in their lives could benefit from one-time ultrasonography screening for abdominal aortic aneurysm (AAA).
The AAA guidelines replace those published by USPSTF in 2005, which had recommended against screening women regardless of their smoking history.
The new guidelines, published online June 23 in Annals of Internal Medicine (doi:10.7326/M14-1204), do not recommend screening women who have never smoked, citing the very low prevalence of AAA in this group.
Nevertheless, the task force’s systematic review, led by current chair Dr. Michael L. LeFevre of the University of Missouri in Columbia, revealed that screening women aged 65-75 years who have smoked or currently smoke – a group for which AAA prevalence is between 0.8% and 2% – could potentially be beneficial, though current evidence remains insufficient to recommend it.
"Prevalence of AAA in women who currently smoke approaches that of men who have never smoked," Dr. LeFevre and his colleagues wrote in the guidelines.
"As such, a small net benefit might exist for this population and appropriate, high-quality research designs should be used to address this question," they added.
The task force continues to recommend that men between the ages of 65 and 75 years who have ever smoked be offered one-time screening with ultrasonography for AAA. Men in this age group who have never smoked may be offered screening if they have certain risk factors, such as advanced age or a family history of AAA.
AAA – a dilation in the wall of the abdominal section of the aorta of 3 cm or larger – is seen in 4% and 7% of men and about 1% of women over the age of 50, USPSTF said. Most AAAs remain asymptomatic until they rupture, in which case the mortality risk has been shown to be higher than 75%.
Women who develop AAA tend to do so at a later age than do men, the task force noted, with most ruptures occurring past age 80 years.
The task force is a voluntary advisory body independent of the U.S. government but supported by the Agency for Healthcare Research and Quality. One of the study’s co-authors, Dr. Douglas Owens of Stanford (Calif.) University disclosed travel support from the agency during the course of the review.
The other task force members declared no conflicts of interest.
The U.S. Preventive Services Task Force says that women between ages 65 and 75 years who have smoked 100 or more cigarettes in their lives could benefit from one-time ultrasonography screening for abdominal aortic aneurysm (AAA).
The AAA guidelines replace those published by USPSTF in 2005, which had recommended against screening women regardless of their smoking history.
The new guidelines, published online June 23 in Annals of Internal Medicine (doi:10.7326/M14-1204), do not recommend screening women who have never smoked, citing the very low prevalence of AAA in this group.
Nevertheless, the task force’s systematic review, led by current chair Dr. Michael L. LeFevre of the University of Missouri in Columbia, revealed that screening women aged 65-75 years who have smoked or currently smoke – a group for which AAA prevalence is between 0.8% and 2% – could potentially be beneficial, though current evidence remains insufficient to recommend it.
"Prevalence of AAA in women who currently smoke approaches that of men who have never smoked," Dr. LeFevre and his colleagues wrote in the guidelines.
"As such, a small net benefit might exist for this population and appropriate, high-quality research designs should be used to address this question," they added.
The task force continues to recommend that men between the ages of 65 and 75 years who have ever smoked be offered one-time screening with ultrasonography for AAA. Men in this age group who have never smoked may be offered screening if they have certain risk factors, such as advanced age or a family history of AAA.
AAA – a dilation in the wall of the abdominal section of the aorta of 3 cm or larger – is seen in 4% and 7% of men and about 1% of women over the age of 50, USPSTF said. Most AAAs remain asymptomatic until they rupture, in which case the mortality risk has been shown to be higher than 75%.
Women who develop AAA tend to do so at a later age than do men, the task force noted, with most ruptures occurring past age 80 years.
The task force is a voluntary advisory body independent of the U.S. government but supported by the Agency for Healthcare Research and Quality. One of the study’s co-authors, Dr. Douglas Owens of Stanford (Calif.) University disclosed travel support from the agency during the course of the review.
The other task force members declared no conflicts of interest.
Key clinical point: Women aged 65-75 years who have smoked more than 100 cigarettes ever could benefit from one-time ultrasonography screening for AAA.
Major finding: Screening in women aged 65-75 years who have smoked or currently smoke – a group for which AAA prevalence is between 0.8% and 2% – could potentially be beneficial.
Data source: The USPSTF commissioned a systematic review that assessed the evidence on the benefits and harms of screening for AAA and strategies for managing small (3.0-5.4 cm) screen-detected AAAs.
Disclosures: Dr. Douglas Owens of the Stanford (Calif.) University, disclosed travel support from the agency during the course of the review.
Open thoracoabdominal aortic aneurysm repair in octogenarians: Special considerations
NEW YORK – Outcomes of thoracoabdominal aortic aneurysm (TAAA) repair in octogenarians vary considerably with the extent of repair. Those who undergo Extent II TAAA repair have significantly higher risks of morbidity and mortality, while Extent I, III, and IV repairs can be performed with relatively good outcomes, according to Dr. Muhammad Aftab, who presented the findings at the meeting sponsored by the American Association for Thoracic Surgery.
"Extensive TAAA repair should be performed with caution in octogenarians," says Dr. Aftab, a Fellow in cardiothoracic surgery at the Baylor College of Medicine–Texas Heart Institute, Houston. He recommends that a thorough preoperative discussion to assess the risks and benefits with the patient and his family is necessary before proceeding with surgery.
In this retrospective review of patients seen between January 2005 and September 2013, octogenarians with thoracoabdominal aortic aneurysms (TAAAs) (n = 88) were compared with a younger cohort (n = 1,179 patients, aged 70 years). Dr. Aftab found that octogenarians were threefold more likely to present with aneurysm rupture (13.6% vs. 4.6%; P less than .001) but less likely to present with aortic dissections (12.5% vs. 43.9%; P less than .001) than did the younger patients.
During surgery, the use of other types of adjunctive interventions, such as left heart bypass, cerebrospinal fluid drainage, cold renal perfusion, and visceral perfusion differed significantly among the octogenarians based on the extent of repair and clinical condition (all P less than .001). Because the octogenarians had a greater atherosclerotic burden and higher incidence of renal and mesenteric occlusive disease, they were also more likely to require renal/visceral endarterectomy, stenting, or both (57.9% vs. 33.6%; P less than.001).
Overall, octogenarians had higher rates of operative mortality (26.1% vs. 6.9%), in-hospital deaths (25% vs. 6.4%), 30-day deaths (13.6% vs. 4.8%), and adverse outcomes (36.4% vs. 15.7%; P less than .001) than did the younger group, all significant differences. The outcomes included significantly higher rates of permanent renal failure, cardiac complications, and pulmonary complications. The octogenarians had longer recovery times, as suggested by longer postoperative ICU and hospital stays. Spinal cord deficits and paraplegia were higher in the older group, but the difference was not significant.
Poor outcomes differed according to the extent of surgery, and seemed to be exacerbated for those who underwent repair of Extent II aneurysms (according to the Crawford Classification, these involve the subclavian artery and extend to the bifurcation of the aorta in the pelvis). For instance, the Extent II group had the highest risk of operative mortality (61.5%) vs. Extent I (31.6%), III (21.4%), and IV (10.7%), a significant difference. The Extent II group also had much higher rates of in-hospital and 30-day death rates. The most common causes of deaths for the Extent II octogenarians were multisystem organ failure and cardiac problems.
Adverse outcomes were also significantly much higher for the Extent II group (76.9%) than for the other groups (42.1%, 28.6%, and 21.4%). Similar patterns were found for permanent paraplegia, renal failure requiring permanent dialysis, stroke, and days spent in the ICU. Almost 85% of those who required Extent II repair needed renal/visceral endarterectomy, stenting, or both as a part of the surgical procedure.
Extent II TAAA repair was identified as an independent predictor of perioperative mortality by multivariate analysis, conferring an 11-fold increased risk of death. Aneurysm rupture and dissection were also identified as predictors of perioperative mortality while only Extent II TAAA and dissection were independent predictors of adverse outcomes.
While these problems were exacerbated in those with Extent II repairs, Extent I, III, and IV TAAA repairs may be performed with relatively low risk, according to Dr. Aftab. The results suggest that while octogenarians present more challenges than younger individuals, outcomes vary greatly according to the type of aneurysm repair.
Dr. Aftab had no relevant disclosures.
NEW YORK – Outcomes of thoracoabdominal aortic aneurysm (TAAA) repair in octogenarians vary considerably with the extent of repair. Those who undergo Extent II TAAA repair have significantly higher risks of morbidity and mortality, while Extent I, III, and IV repairs can be performed with relatively good outcomes, according to Dr. Muhammad Aftab, who presented the findings at the meeting sponsored by the American Association for Thoracic Surgery.
"Extensive TAAA repair should be performed with caution in octogenarians," says Dr. Aftab, a Fellow in cardiothoracic surgery at the Baylor College of Medicine–Texas Heart Institute, Houston. He recommends that a thorough preoperative discussion to assess the risks and benefits with the patient and his family is necessary before proceeding with surgery.
In this retrospective review of patients seen between January 2005 and September 2013, octogenarians with thoracoabdominal aortic aneurysms (TAAAs) (n = 88) were compared with a younger cohort (n = 1,179 patients, aged 70 years). Dr. Aftab found that octogenarians were threefold more likely to present with aneurysm rupture (13.6% vs. 4.6%; P less than .001) but less likely to present with aortic dissections (12.5% vs. 43.9%; P less than .001) than did the younger patients.
During surgery, the use of other types of adjunctive interventions, such as left heart bypass, cerebrospinal fluid drainage, cold renal perfusion, and visceral perfusion differed significantly among the octogenarians based on the extent of repair and clinical condition (all P less than .001). Because the octogenarians had a greater atherosclerotic burden and higher incidence of renal and mesenteric occlusive disease, they were also more likely to require renal/visceral endarterectomy, stenting, or both (57.9% vs. 33.6%; P less than.001).
Overall, octogenarians had higher rates of operative mortality (26.1% vs. 6.9%), in-hospital deaths (25% vs. 6.4%), 30-day deaths (13.6% vs. 4.8%), and adverse outcomes (36.4% vs. 15.7%; P less than .001) than did the younger group, all significant differences. The outcomes included significantly higher rates of permanent renal failure, cardiac complications, and pulmonary complications. The octogenarians had longer recovery times, as suggested by longer postoperative ICU and hospital stays. Spinal cord deficits and paraplegia were higher in the older group, but the difference was not significant.
Poor outcomes differed according to the extent of surgery, and seemed to be exacerbated for those who underwent repair of Extent II aneurysms (according to the Crawford Classification, these involve the subclavian artery and extend to the bifurcation of the aorta in the pelvis). For instance, the Extent II group had the highest risk of operative mortality (61.5%) vs. Extent I (31.6%), III (21.4%), and IV (10.7%), a significant difference. The Extent II group also had much higher rates of in-hospital and 30-day death rates. The most common causes of deaths for the Extent II octogenarians were multisystem organ failure and cardiac problems.
Adverse outcomes were also significantly much higher for the Extent II group (76.9%) than for the other groups (42.1%, 28.6%, and 21.4%). Similar patterns were found for permanent paraplegia, renal failure requiring permanent dialysis, stroke, and days spent in the ICU. Almost 85% of those who required Extent II repair needed renal/visceral endarterectomy, stenting, or both as a part of the surgical procedure.
Extent II TAAA repair was identified as an independent predictor of perioperative mortality by multivariate analysis, conferring an 11-fold increased risk of death. Aneurysm rupture and dissection were also identified as predictors of perioperative mortality while only Extent II TAAA and dissection were independent predictors of adverse outcomes.
While these problems were exacerbated in those with Extent II repairs, Extent I, III, and IV TAAA repairs may be performed with relatively low risk, according to Dr. Aftab. The results suggest that while octogenarians present more challenges than younger individuals, outcomes vary greatly according to the type of aneurysm repair.
Dr. Aftab had no relevant disclosures.
NEW YORK – Outcomes of thoracoabdominal aortic aneurysm (TAAA) repair in octogenarians vary considerably with the extent of repair. Those who undergo Extent II TAAA repair have significantly higher risks of morbidity and mortality, while Extent I, III, and IV repairs can be performed with relatively good outcomes, according to Dr. Muhammad Aftab, who presented the findings at the meeting sponsored by the American Association for Thoracic Surgery.
"Extensive TAAA repair should be performed with caution in octogenarians," says Dr. Aftab, a Fellow in cardiothoracic surgery at the Baylor College of Medicine–Texas Heart Institute, Houston. He recommends that a thorough preoperative discussion to assess the risks and benefits with the patient and his family is necessary before proceeding with surgery.
In this retrospective review of patients seen between January 2005 and September 2013, octogenarians with thoracoabdominal aortic aneurysms (TAAAs) (n = 88) were compared with a younger cohort (n = 1,179 patients, aged 70 years). Dr. Aftab found that octogenarians were threefold more likely to present with aneurysm rupture (13.6% vs. 4.6%; P less than .001) but less likely to present with aortic dissections (12.5% vs. 43.9%; P less than .001) than did the younger patients.
During surgery, the use of other types of adjunctive interventions, such as left heart bypass, cerebrospinal fluid drainage, cold renal perfusion, and visceral perfusion differed significantly among the octogenarians based on the extent of repair and clinical condition (all P less than .001). Because the octogenarians had a greater atherosclerotic burden and higher incidence of renal and mesenteric occlusive disease, they were also more likely to require renal/visceral endarterectomy, stenting, or both (57.9% vs. 33.6%; P less than.001).
Overall, octogenarians had higher rates of operative mortality (26.1% vs. 6.9%), in-hospital deaths (25% vs. 6.4%), 30-day deaths (13.6% vs. 4.8%), and adverse outcomes (36.4% vs. 15.7%; P less than .001) than did the younger group, all significant differences. The outcomes included significantly higher rates of permanent renal failure, cardiac complications, and pulmonary complications. The octogenarians had longer recovery times, as suggested by longer postoperative ICU and hospital stays. Spinal cord deficits and paraplegia were higher in the older group, but the difference was not significant.
Poor outcomes differed according to the extent of surgery, and seemed to be exacerbated for those who underwent repair of Extent II aneurysms (according to the Crawford Classification, these involve the subclavian artery and extend to the bifurcation of the aorta in the pelvis). For instance, the Extent II group had the highest risk of operative mortality (61.5%) vs. Extent I (31.6%), III (21.4%), and IV (10.7%), a significant difference. The Extent II group also had much higher rates of in-hospital and 30-day death rates. The most common causes of deaths for the Extent II octogenarians were multisystem organ failure and cardiac problems.
Adverse outcomes were also significantly much higher for the Extent II group (76.9%) than for the other groups (42.1%, 28.6%, and 21.4%). Similar patterns were found for permanent paraplegia, renal failure requiring permanent dialysis, stroke, and days spent in the ICU. Almost 85% of those who required Extent II repair needed renal/visceral endarterectomy, stenting, or both as a part of the surgical procedure.
Extent II TAAA repair was identified as an independent predictor of perioperative mortality by multivariate analysis, conferring an 11-fold increased risk of death. Aneurysm rupture and dissection were also identified as predictors of perioperative mortality while only Extent II TAAA and dissection were independent predictors of adverse outcomes.
While these problems were exacerbated in those with Extent II repairs, Extent I, III, and IV TAAA repairs may be performed with relatively low risk, according to Dr. Aftab. The results suggest that while octogenarians present more challenges than younger individuals, outcomes vary greatly according to the type of aneurysm repair.
Dr. Aftab had no relevant disclosures.
AT AATS AORTIC SYMPOSIUM 2014
Key clinical point: Octogenarians with TAAAs present more challenges than younger individuals and their outcomes vary greatly according to the type of aneurysm repair.
Major finding: A study that compared octogenarians with thoracoabdominal aortic aneurysms (TAAAs) to a younger cohort found that octogenarians were more at risk for aneurysm rupture, were more likely to need visceral-branch endarterectomy/stenting, had more adverse postoperative outcomes, and higher rates of operative mortality and longer postoperative ICU and hospital stays. While these problems were exacerbated in those with Extent II repairs, Extent I, III, and IV TAAA repairs can be performed with relatively low risk. Younger patients were more likely than octogenarians to present with aortic dissections.
Data source: Retrospective review.
Disclosures: Dr. Aftab had no relevant disclosures.
For AAA repair, EVAR not costlier than open procedure
PALM BEACH, FLA. – Costs of three different endovascular repair systems for abdominal aortic aneurysms were slightly lower than that of open repair, but the implants still took up a substantial portion of the initial hospitalization costs, according to a study from a Veterans Affairs database.
“Device performance doesn’t appear to result in statistically different downstream fiscal performance. That should not be a factor in your procurement committees, I believe,” said Dr. Jon Matsumura, chairman of Division of Vascular Surgery at the University of Wisconsin School of Medicine, Madison.
He presented the new set of findings from the 881-patient Open Versus Endovascular Repair Veterans Affairs Cooperative Study, or OVER, at the Southern Association for Vascular Surgery annual meeting.
The cost-effectiveness of endovascular repair of abdominal aortic aneurysm (AAA) has been the subject of several studies, and the findings have changed since the method arrived in the market more than a decade ago.
In 2010, long-term follow-up of the United Kingdom EVAR Trial 1 cohort showed that “Endovascular repair was associated with increased rates of graft-related complications and reinterventions,” and that it was more costly. But the study also showed that there were no differences in total mortality or aneurysm-related mortality in the long term when comparing the two methods.
Meanwhile, in 2012, results of the randomized multicenter trial OVER showed that the method was a cost-effective alternative to open surgery, at least for the first two years after the procedure.
In the latest analysis of the OVER trial, Dr. Matsumura reported that 437 patients were randomized to open repair and 444 to endovascular repair (EVR). Open repair controls were matched to each device cohort, which included Zenith, Excluder, and AneuRx systems.
At the VA, the device takes up to 38% of the cost of EVR, said Dr. Matsumura, so it was important to find out what were the benefits of having one system versus another, or if all the systems were needed.
Although the statistical analysis showed that there were no significant differences between the cost of each device and open repair – in fact, the mean cost of EVR was less than open repair with each system – there were noticeable dollar differences between the systems:
- The Zenith system’s mean total 2-year cost was $78,200, compared with $82,000 in the open repair cohort, leading to a difference of $3,800.
- The Excluder system’s mean cost was $73,400, compared with $82,000 for open repair, leading to a difference of $8,300.
- The AneuRx system’s mean cost was $72,400, compared with $75,600 for open repair, with a cost difference $3,100.
However, device costs didn’t vary much.
The analysis also showed that the total health care costs – the final bill – were not statistically significant different between the two methods.
Dr. Matsumura said understanding the cost-effectiveness of different systems and procedures is imperative in light of the Affordable Care Act, which is shifting the focus of health care delivery from volume toward value.
Dr. Matsumura has received several research grants through his university, and not personally.
nmiller@frontlinemedcom.com<[lb]>
On Twitter @NaseemMiller
PALM BEACH, FLA. – Costs of three different endovascular repair systems for abdominal aortic aneurysms were slightly lower than that of open repair, but the implants still took up a substantial portion of the initial hospitalization costs, according to a study from a Veterans Affairs database.
“Device performance doesn’t appear to result in statistically different downstream fiscal performance. That should not be a factor in your procurement committees, I believe,” said Dr. Jon Matsumura, chairman of Division of Vascular Surgery at the University of Wisconsin School of Medicine, Madison.
He presented the new set of findings from the 881-patient Open Versus Endovascular Repair Veterans Affairs Cooperative Study, or OVER, at the Southern Association for Vascular Surgery annual meeting.
The cost-effectiveness of endovascular repair of abdominal aortic aneurysm (AAA) has been the subject of several studies, and the findings have changed since the method arrived in the market more than a decade ago.
In 2010, long-term follow-up of the United Kingdom EVAR Trial 1 cohort showed that “Endovascular repair was associated with increased rates of graft-related complications and reinterventions,” and that it was more costly. But the study also showed that there were no differences in total mortality or aneurysm-related mortality in the long term when comparing the two methods.
Meanwhile, in 2012, results of the randomized multicenter trial OVER showed that the method was a cost-effective alternative to open surgery, at least for the first two years after the procedure.
In the latest analysis of the OVER trial, Dr. Matsumura reported that 437 patients were randomized to open repair and 444 to endovascular repair (EVR). Open repair controls were matched to each device cohort, which included Zenith, Excluder, and AneuRx systems.
At the VA, the device takes up to 38% of the cost of EVR, said Dr. Matsumura, so it was important to find out what were the benefits of having one system versus another, or if all the systems were needed.
Although the statistical analysis showed that there were no significant differences between the cost of each device and open repair – in fact, the mean cost of EVR was less than open repair with each system – there were noticeable dollar differences between the systems:
- The Zenith system’s mean total 2-year cost was $78,200, compared with $82,000 in the open repair cohort, leading to a difference of $3,800.
- The Excluder system’s mean cost was $73,400, compared with $82,000 for open repair, leading to a difference of $8,300.
- The AneuRx system’s mean cost was $72,400, compared with $75,600 for open repair, with a cost difference $3,100.
However, device costs didn’t vary much.
The analysis also showed that the total health care costs – the final bill – were not statistically significant different between the two methods.
Dr. Matsumura said understanding the cost-effectiveness of different systems and procedures is imperative in light of the Affordable Care Act, which is shifting the focus of health care delivery from volume toward value.
Dr. Matsumura has received several research grants through his university, and not personally.
nmiller@frontlinemedcom.com<[lb]>
On Twitter @NaseemMiller
PALM BEACH, FLA. – Costs of three different endovascular repair systems for abdominal aortic aneurysms were slightly lower than that of open repair, but the implants still took up a substantial portion of the initial hospitalization costs, according to a study from a Veterans Affairs database.
“Device performance doesn’t appear to result in statistically different downstream fiscal performance. That should not be a factor in your procurement committees, I believe,” said Dr. Jon Matsumura, chairman of Division of Vascular Surgery at the University of Wisconsin School of Medicine, Madison.
He presented the new set of findings from the 881-patient Open Versus Endovascular Repair Veterans Affairs Cooperative Study, or OVER, at the Southern Association for Vascular Surgery annual meeting.
The cost-effectiveness of endovascular repair of abdominal aortic aneurysm (AAA) has been the subject of several studies, and the findings have changed since the method arrived in the market more than a decade ago.
In 2010, long-term follow-up of the United Kingdom EVAR Trial 1 cohort showed that “Endovascular repair was associated with increased rates of graft-related complications and reinterventions,” and that it was more costly. But the study also showed that there were no differences in total mortality or aneurysm-related mortality in the long term when comparing the two methods.
Meanwhile, in 2012, results of the randomized multicenter trial OVER showed that the method was a cost-effective alternative to open surgery, at least for the first two years after the procedure.
In the latest analysis of the OVER trial, Dr. Matsumura reported that 437 patients were randomized to open repair and 444 to endovascular repair (EVR). Open repair controls were matched to each device cohort, which included Zenith, Excluder, and AneuRx systems.
At the VA, the device takes up to 38% of the cost of EVR, said Dr. Matsumura, so it was important to find out what were the benefits of having one system versus another, or if all the systems were needed.
Although the statistical analysis showed that there were no significant differences between the cost of each device and open repair – in fact, the mean cost of EVR was less than open repair with each system – there were noticeable dollar differences between the systems:
- The Zenith system’s mean total 2-year cost was $78,200, compared with $82,000 in the open repair cohort, leading to a difference of $3,800.
- The Excluder system’s mean cost was $73,400, compared with $82,000 for open repair, leading to a difference of $8,300.
- The AneuRx system’s mean cost was $72,400, compared with $75,600 for open repair, with a cost difference $3,100.
However, device costs didn’t vary much.
The analysis also showed that the total health care costs – the final bill – were not statistically significant different between the two methods.
Dr. Matsumura said understanding the cost-effectiveness of different systems and procedures is imperative in light of the Affordable Care Act, which is shifting the focus of health care delivery from volume toward value.
Dr. Matsumura has received several research grants through his university, and not personally.
nmiller@frontlinemedcom.com<[lb]>
On Twitter @NaseemMiller
Failure to rescue drives AAA repair mortality
CHICAGO -- Failure to rescue patients from major complications drives much of the variation in hospital mortality for abdominal aortic aneurysm repair, an award-winning study suggests.
"Careful attention to early recognition and management of postoperative complications could be the key to improving mortality," Dr. Seth Waits reported at the annual meeting of the Midwestern Vascular Surgical Society.
Failure to rescue (FTR), or death after a complication, is increasing being recognized as a source of differences in hospital mortality. A recent study reported that women who experienced a major complication after ovarian cancer treatment at a low-volume hospital were 48% more likely to die than were their counterparts at a high-volume hospital. Complication rates, long thought to be the culprit behind higher hospital mortality, were similar at the hospitals, while FTR rates were almost double at the low-volume hospitals (J. Clin. Oncol. 2012;30:3976-82).
For the current analysis, Dr. Waits and his colleagues at the University of Michigan Frankel Cardiovascular Center in Ann Arbor, calculated risk-adjusted mortality rates for 3,215 patients who underwent open or endovascular abdominal aortic aneurysm (AAA) repair at 40 hospitals participating in the Michigan Surgical Quality Collaborative between 2007 and 2012.
For 2,440 patients undergoing endovascular repair, hospital mortality ranged from a low of 0.07% to a high of 6.14%.
Though low- and high-mortality hospitals had similar major complication rates (11.6% vs. 10.6%), FTR rates were 45 times greater in high-mortality hospitals (0.83% vs. 37.5%), Dr. Waits reported.
For 775 patients who underwent open AAA repair, hospital mortality ranged from 4.5% to 16.4%.
Once again, despite low- and high-mortality hospitals having nearly identical complication rates (45.1% vs. 45.8%), but FTR rates were three times higher at the high-mortality hospitals (10.3% vs. 33%), according to Dr. Waits.
An average of 2.85 and 2.66 severe complications occurred per FTR event for open and endovascular repair, respectively.
Transfusion was the most common postoperative complication leading to a FTR event for endovascular and open repair (5.8% and 29.8%, respectively), followed by prolonged intubation (2.4%; 18.2%) and reintubation (9.2%; 2%), according to the meeting’s best poster presentation.
No significant difference was seen in rupture/emergent repair between low- and high-mortality hospitals.
Dr. Waits called for preoperative identification of high-risk patients and use of FTR countermeasures, such as improved ICU admission, anesthesia alerts, and nurse/physician awareness to improve AAA mortality.
"Understanding the mechanisms that underlie failure to rescue offers the opportunity to move from a reactive to proactive approach in our management of complications following abdominal aortic aneurysm repair," he said in an interview.
Dr. Waits and his coauthors reported that they had no financial disclosures.
CHICAGO -- Failure to rescue patients from major complications drives much of the variation in hospital mortality for abdominal aortic aneurysm repair, an award-winning study suggests.
"Careful attention to early recognition and management of postoperative complications could be the key to improving mortality," Dr. Seth Waits reported at the annual meeting of the Midwestern Vascular Surgical Society.
Failure to rescue (FTR), or death after a complication, is increasing being recognized as a source of differences in hospital mortality. A recent study reported that women who experienced a major complication after ovarian cancer treatment at a low-volume hospital were 48% more likely to die than were their counterparts at a high-volume hospital. Complication rates, long thought to be the culprit behind higher hospital mortality, were similar at the hospitals, while FTR rates were almost double at the low-volume hospitals (J. Clin. Oncol. 2012;30:3976-82).
For the current analysis, Dr. Waits and his colleagues at the University of Michigan Frankel Cardiovascular Center in Ann Arbor, calculated risk-adjusted mortality rates for 3,215 patients who underwent open or endovascular abdominal aortic aneurysm (AAA) repair at 40 hospitals participating in the Michigan Surgical Quality Collaborative between 2007 and 2012.
For 2,440 patients undergoing endovascular repair, hospital mortality ranged from a low of 0.07% to a high of 6.14%.
Though low- and high-mortality hospitals had similar major complication rates (11.6% vs. 10.6%), FTR rates were 45 times greater in high-mortality hospitals (0.83% vs. 37.5%), Dr. Waits reported.
For 775 patients who underwent open AAA repair, hospital mortality ranged from 4.5% to 16.4%.
Once again, despite low- and high-mortality hospitals having nearly identical complication rates (45.1% vs. 45.8%), but FTR rates were three times higher at the high-mortality hospitals (10.3% vs. 33%), according to Dr. Waits.
An average of 2.85 and 2.66 severe complications occurred per FTR event for open and endovascular repair, respectively.
Transfusion was the most common postoperative complication leading to a FTR event for endovascular and open repair (5.8% and 29.8%, respectively), followed by prolonged intubation (2.4%; 18.2%) and reintubation (9.2%; 2%), according to the meeting’s best poster presentation.
No significant difference was seen in rupture/emergent repair between low- and high-mortality hospitals.
Dr. Waits called for preoperative identification of high-risk patients and use of FTR countermeasures, such as improved ICU admission, anesthesia alerts, and nurse/physician awareness to improve AAA mortality.
"Understanding the mechanisms that underlie failure to rescue offers the opportunity to move from a reactive to proactive approach in our management of complications following abdominal aortic aneurysm repair," he said in an interview.
Dr. Waits and his coauthors reported that they had no financial disclosures.
CHICAGO -- Failure to rescue patients from major complications drives much of the variation in hospital mortality for abdominal aortic aneurysm repair, an award-winning study suggests.
"Careful attention to early recognition and management of postoperative complications could be the key to improving mortality," Dr. Seth Waits reported at the annual meeting of the Midwestern Vascular Surgical Society.
Failure to rescue (FTR), or death after a complication, is increasing being recognized as a source of differences in hospital mortality. A recent study reported that women who experienced a major complication after ovarian cancer treatment at a low-volume hospital were 48% more likely to die than were their counterparts at a high-volume hospital. Complication rates, long thought to be the culprit behind higher hospital mortality, were similar at the hospitals, while FTR rates were almost double at the low-volume hospitals (J. Clin. Oncol. 2012;30:3976-82).
For the current analysis, Dr. Waits and his colleagues at the University of Michigan Frankel Cardiovascular Center in Ann Arbor, calculated risk-adjusted mortality rates for 3,215 patients who underwent open or endovascular abdominal aortic aneurysm (AAA) repair at 40 hospitals participating in the Michigan Surgical Quality Collaborative between 2007 and 2012.
For 2,440 patients undergoing endovascular repair, hospital mortality ranged from a low of 0.07% to a high of 6.14%.
Though low- and high-mortality hospitals had similar major complication rates (11.6% vs. 10.6%), FTR rates were 45 times greater in high-mortality hospitals (0.83% vs. 37.5%), Dr. Waits reported.
For 775 patients who underwent open AAA repair, hospital mortality ranged from 4.5% to 16.4%.
Once again, despite low- and high-mortality hospitals having nearly identical complication rates (45.1% vs. 45.8%), but FTR rates were three times higher at the high-mortality hospitals (10.3% vs. 33%), according to Dr. Waits.
An average of 2.85 and 2.66 severe complications occurred per FTR event for open and endovascular repair, respectively.
Transfusion was the most common postoperative complication leading to a FTR event for endovascular and open repair (5.8% and 29.8%, respectively), followed by prolonged intubation (2.4%; 18.2%) and reintubation (9.2%; 2%), according to the meeting’s best poster presentation.
No significant difference was seen in rupture/emergent repair between low- and high-mortality hospitals.
Dr. Waits called for preoperative identification of high-risk patients and use of FTR countermeasures, such as improved ICU admission, anesthesia alerts, and nurse/physician awareness to improve AAA mortality.
"Understanding the mechanisms that underlie failure to rescue offers the opportunity to move from a reactive to proactive approach in our management of complications following abdominal aortic aneurysm repair," he said in an interview.
Dr. Waits and his coauthors reported that they had no financial disclosures.
AT MIDWESTERN VASCULAR 2013
Major finding: FTR rates were 45 times higher in high AAA-mortality hospitals vs. low AAA-mortality hospitals (0.83% vs. 37.5%).
Data source: Retrospective study of 3,215 patients with abdominal aortic aneurysm repair.
Disclosures: Dr. Waits and his coauthors reported having no financial disclosures.
Anatomy dictates endo PAA repair
CHICAGO - Endovascular repair of popliteal artery aneurysms is a relatively safe and viable off-label alternative to open repair, but appropriate anatomy is essential. This includes a landing zone of at least 2 cm above and below the aneurysm, minimal discrepancy in size between the proximal and distal landing zones, and lack of extensive vessel tortuosity due to potential kinking of the endograft, Dr. Neal S. Cayne said at a symposium on vascular surgery sponsored by Northwestern University.
The risk of kinking and graft thrombosis also excludes patients who frequently flex their knee more than 90 degrees, such as carpenters and gardeners.
Patients with a contraindication to antiplatelet medication are also off limits, as clopidogrel (Plavix) has been shown to be a predictor of success, said Dr. Cayne, director of endovascular surgery at New York University Langone Medical Center.
In 2012, his team reported technical success in 25 of 26 endovascular popliteal artery aneurysm (PAA) repairs performed in 21 consecutive patients between January 2004 and January 2011, with the one technical failure due to stent graft infolding (J. Vasc. Surg. 2012;55:1647-53).
Primary and secondary patency rates were both 91.2% at 1 year, and were 85.5% and 91.2%, respectively, at 2 years. All patients were maintained on aspirin or clopidogrel.
No limb loss was reported, but three occlusions occurred during follow-up at 4, 14, and 26 months. One patient required a tibial artery bypass for a nonhealing wound, and two were successfully repaired with open thrombectomy. All three occlusion patients had single-vessel runoff.
Based on our data, in general, I will not stent someone "with single-vessel outflow, and I also will not stent someone who, for some reason, can't take antiplatelet agents," Dr. Cayne said.
Even when patients present urgently and the PAA has thrombosed, endovascular repair is not an option if there is only one outflow vessel. "I would do a bypass; there's nothing wrong with open surgery," he said.
A recent unpublished review of 79 PAAs treated at Langone from 1998 to 2012 with both approaches found 5-year primary patencies of 67% for open repair and 80% for endovascular repair (P less than .05). Secondary patency was 90% in both groups.
One amputation occurred in the open group, but occlusion rates were higher using endovascular repair with one-vessel runoff (P = .003), Dr. Cayne said.
As expected, length of stay was shorter with endovascular repair (1.9 vs. 6.4 days; P less than .001).
Follow-up was longer for the 36 open PAAs than the 43 endovascular PAAs (75 vs. 34 months), but patients in both groups were similar with respect to age, comorbidities, PAA size, runoff, and symptoms, he said.
During a discussion following the presentation, some attendees said they still prefer to use bypass for all patients with PAA, and asked how candidates are selected for stent placement.
"Number one and most important is the anatomy," Dr. Cayne said. "The one advantage of endovascular repair is that if you have a patient too sick to get general or even regional anesthesia, you can do it under local [anesthesia] almost all the time with a small cutdown or puncture. But you do have that long discussion with the patient that this is a non-FDA-approved, off-label use. We provide them with the data, but some patients in New York will come in with pages and pages of literature and say, 'Nope, I want a stent, I want this particular stent, and this is the way I want you to do it.'"
The stent of choice at Langone has been Gore's Viabahn covered stent graft, which is FDA approved for treating occlusive disease rather than PAA. The device is usually oversized by 10%-15%, but no more than that, because of the risk of graft infolding, Dr. Cayne said.
If more than one graft is needed, a maximum of no more than 1-mm size differential between grafts is suggested. A minimum overlap of 2-3 cm between grafts is also preferred.
Last year, surgeons reported a dismal 50% occlusion rate within just 6 weeks in the endovascular treatment of six PAAs using a novel, multilayer stent (Cardiatis's Multilayer Aneurysm Repair System) (J. Endovasc. Ther. 2013;20:381-8), he observed.
Dr. Cayne reported receiving honoraria as a consultant from Cook Medical.
CHICAGO - Endovascular repair of popliteal artery aneurysms is a relatively safe and viable off-label alternative to open repair, but appropriate anatomy is essential. This includes a landing zone of at least 2 cm above and below the aneurysm, minimal discrepancy in size between the proximal and distal landing zones, and lack of extensive vessel tortuosity due to potential kinking of the endograft, Dr. Neal S. Cayne said at a symposium on vascular surgery sponsored by Northwestern University.
The risk of kinking and graft thrombosis also excludes patients who frequently flex their knee more than 90 degrees, such as carpenters and gardeners.
Patients with a contraindication to antiplatelet medication are also off limits, as clopidogrel (Plavix) has been shown to be a predictor of success, said Dr. Cayne, director of endovascular surgery at New York University Langone Medical Center.
In 2012, his team reported technical success in 25 of 26 endovascular popliteal artery aneurysm (PAA) repairs performed in 21 consecutive patients between January 2004 and January 2011, with the one technical failure due to stent graft infolding (J. Vasc. Surg. 2012;55:1647-53).
Primary and secondary patency rates were both 91.2% at 1 year, and were 85.5% and 91.2%, respectively, at 2 years. All patients were maintained on aspirin or clopidogrel.
No limb loss was reported, but three occlusions occurred during follow-up at 4, 14, and 26 months. One patient required a tibial artery bypass for a nonhealing wound, and two were successfully repaired with open thrombectomy. All three occlusion patients had single-vessel runoff.
Based on our data, in general, I will not stent someone "with single-vessel outflow, and I also will not stent someone who, for some reason, can't take antiplatelet agents," Dr. Cayne said.
Even when patients present urgently and the PAA has thrombosed, endovascular repair is not an option if there is only one outflow vessel. "I would do a bypass; there's nothing wrong with open surgery," he said.
A recent unpublished review of 79 PAAs treated at Langone from 1998 to 2012 with both approaches found 5-year primary patencies of 67% for open repair and 80% for endovascular repair (P less than .05). Secondary patency was 90% in both groups.
One amputation occurred in the open group, but occlusion rates were higher using endovascular repair with one-vessel runoff (P = .003), Dr. Cayne said.
As expected, length of stay was shorter with endovascular repair (1.9 vs. 6.4 days; P less than .001).
Follow-up was longer for the 36 open PAAs than the 43 endovascular PAAs (75 vs. 34 months), but patients in both groups were similar with respect to age, comorbidities, PAA size, runoff, and symptoms, he said.
During a discussion following the presentation, some attendees said they still prefer to use bypass for all patients with PAA, and asked how candidates are selected for stent placement.
"Number one and most important is the anatomy," Dr. Cayne said. "The one advantage of endovascular repair is that if you have a patient too sick to get general or even regional anesthesia, you can do it under local [anesthesia] almost all the time with a small cutdown or puncture. But you do have that long discussion with the patient that this is a non-FDA-approved, off-label use. We provide them with the data, but some patients in New York will come in with pages and pages of literature and say, 'Nope, I want a stent, I want this particular stent, and this is the way I want you to do it.'"
The stent of choice at Langone has been Gore's Viabahn covered stent graft, which is FDA approved for treating occlusive disease rather than PAA. The device is usually oversized by 10%-15%, but no more than that, because of the risk of graft infolding, Dr. Cayne said.
If more than one graft is needed, a maximum of no more than 1-mm size differential between grafts is suggested. A minimum overlap of 2-3 cm between grafts is also preferred.
Last year, surgeons reported a dismal 50% occlusion rate within just 6 weeks in the endovascular treatment of six PAAs using a novel, multilayer stent (Cardiatis's Multilayer Aneurysm Repair System) (J. Endovasc. Ther. 2013;20:381-8), he observed.
Dr. Cayne reported receiving honoraria as a consultant from Cook Medical.
CHICAGO - Endovascular repair of popliteal artery aneurysms is a relatively safe and viable off-label alternative to open repair, but appropriate anatomy is essential. This includes a landing zone of at least 2 cm above and below the aneurysm, minimal discrepancy in size between the proximal and distal landing zones, and lack of extensive vessel tortuosity due to potential kinking of the endograft, Dr. Neal S. Cayne said at a symposium on vascular surgery sponsored by Northwestern University.
The risk of kinking and graft thrombosis also excludes patients who frequently flex their knee more than 90 degrees, such as carpenters and gardeners.
Patients with a contraindication to antiplatelet medication are also off limits, as clopidogrel (Plavix) has been shown to be a predictor of success, said Dr. Cayne, director of endovascular surgery at New York University Langone Medical Center.
In 2012, his team reported technical success in 25 of 26 endovascular popliteal artery aneurysm (PAA) repairs performed in 21 consecutive patients between January 2004 and January 2011, with the one technical failure due to stent graft infolding (J. Vasc. Surg. 2012;55:1647-53).
Primary and secondary patency rates were both 91.2% at 1 year, and were 85.5% and 91.2%, respectively, at 2 years. All patients were maintained on aspirin or clopidogrel.
No limb loss was reported, but three occlusions occurred during follow-up at 4, 14, and 26 months. One patient required a tibial artery bypass for a nonhealing wound, and two were successfully repaired with open thrombectomy. All three occlusion patients had single-vessel runoff.
Based on our data, in general, I will not stent someone "with single-vessel outflow, and I also will not stent someone who, for some reason, can't take antiplatelet agents," Dr. Cayne said.
Even when patients present urgently and the PAA has thrombosed, endovascular repair is not an option if there is only one outflow vessel. "I would do a bypass; there's nothing wrong with open surgery," he said.
A recent unpublished review of 79 PAAs treated at Langone from 1998 to 2012 with both approaches found 5-year primary patencies of 67% for open repair and 80% for endovascular repair (P less than .05). Secondary patency was 90% in both groups.
One amputation occurred in the open group, but occlusion rates were higher using endovascular repair with one-vessel runoff (P = .003), Dr. Cayne said.
As expected, length of stay was shorter with endovascular repair (1.9 vs. 6.4 days; P less than .001).
Follow-up was longer for the 36 open PAAs than the 43 endovascular PAAs (75 vs. 34 months), but patients in both groups were similar with respect to age, comorbidities, PAA size, runoff, and symptoms, he said.
During a discussion following the presentation, some attendees said they still prefer to use bypass for all patients with PAA, and asked how candidates are selected for stent placement.
"Number one and most important is the anatomy," Dr. Cayne said. "The one advantage of endovascular repair is that if you have a patient too sick to get general or even regional anesthesia, you can do it under local [anesthesia] almost all the time with a small cutdown or puncture. But you do have that long discussion with the patient that this is a non-FDA-approved, off-label use. We provide them with the data, but some patients in New York will come in with pages and pages of literature and say, 'Nope, I want a stent, I want this particular stent, and this is the way I want you to do it.'"
The stent of choice at Langone has been Gore's Viabahn covered stent graft, which is FDA approved for treating occlusive disease rather than PAA. The device is usually oversized by 10%-15%, but no more than that, because of the risk of graft infolding, Dr. Cayne said.
If more than one graft is needed, a maximum of no more than 1-mm size differential between grafts is suggested. A minimum overlap of 2-3 cm between grafts is also preferred.
Last year, surgeons reported a dismal 50% occlusion rate within just 6 weeks in the endovascular treatment of six PAAs using a novel, multilayer stent (Cardiatis's Multilayer Aneurysm Repair System) (J. Endovasc. Ther. 2013;20:381-8), he observed.
Dr. Cayne reported receiving honoraria as a consultant from Cook Medical.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Major finding: Five-year primary patency was 67% for open repair and 80% for endovascular repair (P less than .05).
Data source: Expert opinion and retrospective study of 79 popliteal artery aneurysm repairs.
Disclosures: Dr. Cayne reported receiving honoraria as a consultant from Cook Medical.
Small AAA: To treat or not to treat
Once again our authorities choose to agree rather than disagree. Although there may be some minor differences in opinion, it appears that both suggest that careful observation is the preferred management for small abdominal aortic aneurysms. However, there may still be some controversy since I believe the data they use to support observation is confounded by including patients whose aneurysms were less than 5 cm. I think almost everyone would agree that it is safe to monitor the <5 cm AAA. But what about the 5.2 cm in a small woman or a patient with chronic obstructive pulmonary disease or a strong family history of rupture or, for that matter, in any patient? If you have an opinion one way or another I invite you to send your comments to info@vascularspecialistonline.com for inclusion in a future edition of Vascular Specialist. In the meantime if you go to www.Vascularspecialistonline.com you can respond to our "Online Question of the Month" about the treatment of these small AAA.
--Dr. Russell Samson is the medical editor of Vascular Specialist.
Procedural risks remain an issue.
By Kenneth Ouriel, M.D.
Abdominal aortic aneurysms (AAA) are treated to prevent death from aneurysm rupture. Depending on the patient’s baseline medical status, however, the risks of the procedure itself may outweigh the risks of leaving the aneurysm untreated.1 Endovascular aneurysm repair (EVAR), originally developed as a less invasive alternative to traditional open surgery,2 has incompletely addressed this issue. While prospective randomized clinical trials demonstrated reduction in early morbidity and mortality with EVAR, early benefits have not translated into long-term survival benefit over open surgical repair.3,4
Several studies have demonstrated improved results with EVAR when performed in patients with smaller aneurysms.5,6 This observation may relate to the frequency of more challenging anatomy in larger aneurysms, with a higher frequency of shorter, larger diameter, angulated, and conical proximal aortic necks. While the potential benefit of repair in patients with larger aneurysms is greatest with respect to the prevention of rupture, some of this benefit may be offset by poorer long-term outcome. These results are only in part a function of complications related to the device, such as proximal endoleaks and migration. In addition, patients with larger aneurysms are slightly older and sicker than those with smaller aneurysms, accounting for an increased frequency of non-aneurysm related events.
Noting the less challenging aortic anatomy and younger, healthier characteristics of the subpopulation with smaller AAA, two randomized studies were organized to compare the results of early endovascular repair versus ultrasound or computed tomographic (CT) surveillance.7 The PIVOTAL trial enrolled 728 subjects with AAA between 4 and 5 cm in diameter, randomizing to either early EVAR with the AneuRx or Talent endografts or to ultrasound/CT imaging studies every 6 months. The perioperative mortality rate was 0.6% in the early EVAR group. Over a mean follow-up period of 20 months, there were no differences in all-cause mortality between the two groups, each with 15 deaths (4.1%). The primary endpoint of rupture or aneurysm-related death was similar in the two groups, with a hazard ratio of 0.99 in the early EVAR group. However, at 36 months almost 50% of the surveillance group underwent aneurysm repair for sac enlargement, the development of aneurysm-related symptoms, or patient choice. Interestingly, a follow-up economic substudy documented similar health care costs in the two treatment groups at 48 months of follow-up, even though 36.3% of the surveyed patients did not undergo repair.
A second study, the CEASAR trial, randomized 360 patients with AAA 4.1-5.4 cm in diameter to early EVAR with the Cook Zenith device or to serial ultrasound surveillance. After 4.5 years of follow-up, no significant difference was detected in the primary endpoint of all-cause mortality. The Kaplan-Meier estimates of all-cause mortality was 14.5% in the early repair group versus 10.1% in the surveillance group. Aneurysm-related mortality, aneurysm rupture, and major morbidity rates were similar. Like the PIVOTAL trial, the majority of subjects underwent delayed repair, with a frequency of 60% at 3-years and 85% at 4.5 years.
The findings of these two randomized studies suggest that there is no survival advantage to early EVAR in patients with smaller AAA. With the increasing use of statins, ACE inhibitors, and better overall medical management in patients with AAA, the rate of aneurysm enlargement and risk of rupture are quite low in patients with small AAA.8 While survival benefits have not been demonstrated, however, there appear to be few quantifiable disadvantages to early repair. Moreover, the long-term financial impact to the healthcare system appears to be similar when patients are treated early compared to surveillance with serial imaging studies,9 and patient quality of life may be improved with early EVAR.10 These observations suggest that the repair of smaller AAA should be individualized, based upon the particular clinical presentation and the wishes of a patient. The summary of available data suggests that either approach protects a patient from rupture and surveillance culminates in the eventual repair of the aneurysm in the majority of patients.
Dr. Ouriel is a vascular surgeon and president and CEO of Syntactx.
References
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. J Vasc Interv Radiol, 2012. 23(7): p. 866-72; quiz 872.
3. J Endovasc Ther, 2012. 19(2): p. 182-92.
4. J Vasc Surg, 2012. 55(1): p. 33-40.
5. Ann Vasc Surg, 2012. 26(6): p. 860 e1-7.
6. J Vasc Interv Radiol, 2013. 24(1): p. 49-55
7. J Vasc Surg, 2012. 56(3): p. 630-6.
8. J Cardiovasc Surg (Torino), 2013. 1. Br J Surg 2007;94:702-8.
2. Semin Interv Cardiol 2000;5:3-6.
3. NEnglJMed 2010;362:1863-71.
4. JAMA 2009;302:1535-42.
5.. J Vasc Surg 2003;37:1206-12.
6. J Vasc Surg 2006;44:920-29; discussion 9-31.
7. J Vasc Surg 2010;51:1081-7.
8. EurJVascEndovascSurg 2011;41:2-10.
9. J Vasc Surg 2013;58:302-10.
10. Eur J Vasc Endovasc Surg 2011;41:324-31.
Small aneurysms should be left alone.
By Karl A. Illig, M.D.
The currently accepted recommendation to delay repair of abdominal aortic aneurysms until they reach 5-5.5 cm is based on the historical mortality and morbidity of open repair.1 Endovascular aneurysm repair (EVAR) has clearly been shown to reduce the risk of operation, perhaps by as much as two-thirds. Should we now change the threshold for aneurysm repair, especially if the patient is a candidate for EVAR?
There are many arguments to repair small aneurysms. However, the answer really depends upon empirical data. At least three major papers address this question. In the U.K. Small Aneurysm Trial, 1,090 patients with abdominal aortic aneurysms measuring 4.0-5.5 cm in diameter were randomized to undergo early elective open surgery versus ultrasonic surveillance.2 There was an early survival disadvantage for those undergoing open surgery, as expected, but the curves evened out at 3 years or so, and no survival advantage occurred in either group, obviously favoring observation alone. Similarly, the ADAM group, pursuing the same protocol in 1,136 U.S. veterans, found the same thing, despite a low operative mortality of 2.7%. No advantage to early open repair could be seen.3
What of the situation after endovascular repair? The PIVOTAL trial, the lead author of which wrote the accompanying commentary, randomly assigned 728 patients with aneurysms measuring 4.0-5.0 cm to early endovascular repair with the Medtronic device versus ultrasonic surveillance.4 Aneurysm rupture or aneurysm-related death occurred in only two patients in each group (0.6%). The authors appropriately concluded that surveillance alone was equally as efficacious as early endovascular repair for patients with small aneurysms.
Finally, what of the arguments that, by waiting until the aneurysm is larger, you lose the window of opportunity for endovascular repair? We recently explored this in a cohort of 221 patients undergoing preoperative CT scanning for aneurysms of all sizes.5 With receiver operator curve analysis, a cutoff of 5.7 cm best differentiated those who were endovascular candidates from those who were not. Put another way, the rate of endovascular suitability hovered right around 80% until the aneurysm reached 6 cm, at which point it dropped off. In other words, waiting until the aneurysm reaches 5.5 cm doesn’t reduce the chance that a patient will be a candidate for EVAR.
There are many arguments for early intervention in small aneurysms. The concept that EVAR is safer than the procedure originally used to make the recommendation to wait until 5.5 cm is a valid point. However, empiric data still do not show any benefit for either open or endovascular repair as opposed to surveillance at sizes smaller than this. The operative mortality difference is only a couple of percentage points or so, and long-term survival appears to be identical after the initial risk has passed. This 2% or 3% early survival benefit does not seem to impart any long-term advantage, and long-term survival does not seem to be impaired by being a bit conservative. Until data come along that definitively show benefit for early repair, current guidelines remain valid.
Dr. Illig is the director of vascular surgery at USF Health, Tampa.
References:
1. J Vasc Surg 2009;50(8S):1S-49S.
2. Lancet 1998; 352:1649-55.
3. NEJM 2002; 346 (19): 1437-44.
4. J Vasc Surg 2010; 51:1081-7.
5. J Vasc Surg 2010;52:873-7.
Once again our authorities choose to agree rather than disagree. Although there may be some minor differences in opinion, it appears that both suggest that careful observation is the preferred management for small abdominal aortic aneurysms. However, there may still be some controversy since I believe the data they use to support observation is confounded by including patients whose aneurysms were less than 5 cm. I think almost everyone would agree that it is safe to monitor the <5 cm AAA. But what about the 5.2 cm in a small woman or a patient with chronic obstructive pulmonary disease or a strong family history of rupture or, for that matter, in any patient? If you have an opinion one way or another I invite you to send your comments to info@vascularspecialistonline.com for inclusion in a future edition of Vascular Specialist. In the meantime if you go to www.Vascularspecialistonline.com you can respond to our "Online Question of the Month" about the treatment of these small AAA.
--Dr. Russell Samson is the medical editor of Vascular Specialist.
Procedural risks remain an issue.
By Kenneth Ouriel, M.D.
Abdominal aortic aneurysms (AAA) are treated to prevent death from aneurysm rupture. Depending on the patient’s baseline medical status, however, the risks of the procedure itself may outweigh the risks of leaving the aneurysm untreated.1 Endovascular aneurysm repair (EVAR), originally developed as a less invasive alternative to traditional open surgery,2 has incompletely addressed this issue. While prospective randomized clinical trials demonstrated reduction in early morbidity and mortality with EVAR, early benefits have not translated into long-term survival benefit over open surgical repair.3,4
Several studies have demonstrated improved results with EVAR when performed in patients with smaller aneurysms.5,6 This observation may relate to the frequency of more challenging anatomy in larger aneurysms, with a higher frequency of shorter, larger diameter, angulated, and conical proximal aortic necks. While the potential benefit of repair in patients with larger aneurysms is greatest with respect to the prevention of rupture, some of this benefit may be offset by poorer long-term outcome. These results are only in part a function of complications related to the device, such as proximal endoleaks and migration. In addition, patients with larger aneurysms are slightly older and sicker than those with smaller aneurysms, accounting for an increased frequency of non-aneurysm related events.
Noting the less challenging aortic anatomy and younger, healthier characteristics of the subpopulation with smaller AAA, two randomized studies were organized to compare the results of early endovascular repair versus ultrasound or computed tomographic (CT) surveillance.7 The PIVOTAL trial enrolled 728 subjects with AAA between 4 and 5 cm in diameter, randomizing to either early EVAR with the AneuRx or Talent endografts or to ultrasound/CT imaging studies every 6 months. The perioperative mortality rate was 0.6% in the early EVAR group. Over a mean follow-up period of 20 months, there were no differences in all-cause mortality between the two groups, each with 15 deaths (4.1%). The primary endpoint of rupture or aneurysm-related death was similar in the two groups, with a hazard ratio of 0.99 in the early EVAR group. However, at 36 months almost 50% of the surveillance group underwent aneurysm repair for sac enlargement, the development of aneurysm-related symptoms, or patient choice. Interestingly, a follow-up economic substudy documented similar health care costs in the two treatment groups at 48 months of follow-up, even though 36.3% of the surveyed patients did not undergo repair.
A second study, the CEASAR trial, randomized 360 patients with AAA 4.1-5.4 cm in diameter to early EVAR with the Cook Zenith device or to serial ultrasound surveillance. After 4.5 years of follow-up, no significant difference was detected in the primary endpoint of all-cause mortality. The Kaplan-Meier estimates of all-cause mortality was 14.5% in the early repair group versus 10.1% in the surveillance group. Aneurysm-related mortality, aneurysm rupture, and major morbidity rates were similar. Like the PIVOTAL trial, the majority of subjects underwent delayed repair, with a frequency of 60% at 3-years and 85% at 4.5 years.
The findings of these two randomized studies suggest that there is no survival advantage to early EVAR in patients with smaller AAA. With the increasing use of statins, ACE inhibitors, and better overall medical management in patients with AAA, the rate of aneurysm enlargement and risk of rupture are quite low in patients with small AAA.8 While survival benefits have not been demonstrated, however, there appear to be few quantifiable disadvantages to early repair. Moreover, the long-term financial impact to the healthcare system appears to be similar when patients are treated early compared to surveillance with serial imaging studies,9 and patient quality of life may be improved with early EVAR.10 These observations suggest that the repair of smaller AAA should be individualized, based upon the particular clinical presentation and the wishes of a patient. The summary of available data suggests that either approach protects a patient from rupture and surveillance culminates in the eventual repair of the aneurysm in the majority of patients.
Dr. Ouriel is a vascular surgeon and president and CEO of Syntactx.
References
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. J Vasc Interv Radiol, 2012. 23(7): p. 866-72; quiz 872.
3. J Endovasc Ther, 2012. 19(2): p. 182-92.
4. J Vasc Surg, 2012. 55(1): p. 33-40.
5. Ann Vasc Surg, 2012. 26(6): p. 860 e1-7.
6. J Vasc Interv Radiol, 2013. 24(1): p. 49-55
7. J Vasc Surg, 2012. 56(3): p. 630-6.
8. J Cardiovasc Surg (Torino), 2013. 1. Br J Surg 2007;94:702-8.
2. Semin Interv Cardiol 2000;5:3-6.
3. NEnglJMed 2010;362:1863-71.
4. JAMA 2009;302:1535-42.
5.. J Vasc Surg 2003;37:1206-12.
6. J Vasc Surg 2006;44:920-29; discussion 9-31.
7. J Vasc Surg 2010;51:1081-7.
8. EurJVascEndovascSurg 2011;41:2-10.
9. J Vasc Surg 2013;58:302-10.
10. Eur J Vasc Endovasc Surg 2011;41:324-31.
Small aneurysms should be left alone.
By Karl A. Illig, M.D.
The currently accepted recommendation to delay repair of abdominal aortic aneurysms until they reach 5-5.5 cm is based on the historical mortality and morbidity of open repair.1 Endovascular aneurysm repair (EVAR) has clearly been shown to reduce the risk of operation, perhaps by as much as two-thirds. Should we now change the threshold for aneurysm repair, especially if the patient is a candidate for EVAR?
There are many arguments to repair small aneurysms. However, the answer really depends upon empirical data. At least three major papers address this question. In the U.K. Small Aneurysm Trial, 1,090 patients with abdominal aortic aneurysms measuring 4.0-5.5 cm in diameter were randomized to undergo early elective open surgery versus ultrasonic surveillance.2 There was an early survival disadvantage for those undergoing open surgery, as expected, but the curves evened out at 3 years or so, and no survival advantage occurred in either group, obviously favoring observation alone. Similarly, the ADAM group, pursuing the same protocol in 1,136 U.S. veterans, found the same thing, despite a low operative mortality of 2.7%. No advantage to early open repair could be seen.3
What of the situation after endovascular repair? The PIVOTAL trial, the lead author of which wrote the accompanying commentary, randomly assigned 728 patients with aneurysms measuring 4.0-5.0 cm to early endovascular repair with the Medtronic device versus ultrasonic surveillance.4 Aneurysm rupture or aneurysm-related death occurred in only two patients in each group (0.6%). The authors appropriately concluded that surveillance alone was equally as efficacious as early endovascular repair for patients with small aneurysms.
Finally, what of the arguments that, by waiting until the aneurysm is larger, you lose the window of opportunity for endovascular repair? We recently explored this in a cohort of 221 patients undergoing preoperative CT scanning for aneurysms of all sizes.5 With receiver operator curve analysis, a cutoff of 5.7 cm best differentiated those who were endovascular candidates from those who were not. Put another way, the rate of endovascular suitability hovered right around 80% until the aneurysm reached 6 cm, at which point it dropped off. In other words, waiting until the aneurysm reaches 5.5 cm doesn’t reduce the chance that a patient will be a candidate for EVAR.
There are many arguments for early intervention in small aneurysms. The concept that EVAR is safer than the procedure originally used to make the recommendation to wait until 5.5 cm is a valid point. However, empiric data still do not show any benefit for either open or endovascular repair as opposed to surveillance at sizes smaller than this. The operative mortality difference is only a couple of percentage points or so, and long-term survival appears to be identical after the initial risk has passed. This 2% or 3% early survival benefit does not seem to impart any long-term advantage, and long-term survival does not seem to be impaired by being a bit conservative. Until data come along that definitively show benefit for early repair, current guidelines remain valid.
Dr. Illig is the director of vascular surgery at USF Health, Tampa.
References:
1. J Vasc Surg 2009;50(8S):1S-49S.
2. Lancet 1998; 352:1649-55.
3. NEJM 2002; 346 (19): 1437-44.
4. J Vasc Surg 2010; 51:1081-7.
5. J Vasc Surg 2010;52:873-7.
Once again our authorities choose to agree rather than disagree. Although there may be some minor differences in opinion, it appears that both suggest that careful observation is the preferred management for small abdominal aortic aneurysms. However, there may still be some controversy since I believe the data they use to support observation is confounded by including patients whose aneurysms were less than 5 cm. I think almost everyone would agree that it is safe to monitor the <5 cm AAA. But what about the 5.2 cm in a small woman or a patient with chronic obstructive pulmonary disease or a strong family history of rupture or, for that matter, in any patient? If you have an opinion one way or another I invite you to send your comments to info@vascularspecialistonline.com for inclusion in a future edition of Vascular Specialist. In the meantime if you go to www.Vascularspecialistonline.com you can respond to our "Online Question of the Month" about the treatment of these small AAA.
--Dr. Russell Samson is the medical editor of Vascular Specialist.
Procedural risks remain an issue.
By Kenneth Ouriel, M.D.
Abdominal aortic aneurysms (AAA) are treated to prevent death from aneurysm rupture. Depending on the patient’s baseline medical status, however, the risks of the procedure itself may outweigh the risks of leaving the aneurysm untreated.1 Endovascular aneurysm repair (EVAR), originally developed as a less invasive alternative to traditional open surgery,2 has incompletely addressed this issue. While prospective randomized clinical trials demonstrated reduction in early morbidity and mortality with EVAR, early benefits have not translated into long-term survival benefit over open surgical repair.3,4
Several studies have demonstrated improved results with EVAR when performed in patients with smaller aneurysms.5,6 This observation may relate to the frequency of more challenging anatomy in larger aneurysms, with a higher frequency of shorter, larger diameter, angulated, and conical proximal aortic necks. While the potential benefit of repair in patients with larger aneurysms is greatest with respect to the prevention of rupture, some of this benefit may be offset by poorer long-term outcome. These results are only in part a function of complications related to the device, such as proximal endoleaks and migration. In addition, patients with larger aneurysms are slightly older and sicker than those with smaller aneurysms, accounting for an increased frequency of non-aneurysm related events.
Noting the less challenging aortic anatomy and younger, healthier characteristics of the subpopulation with smaller AAA, two randomized studies were organized to compare the results of early endovascular repair versus ultrasound or computed tomographic (CT) surveillance.7 The PIVOTAL trial enrolled 728 subjects with AAA between 4 and 5 cm in diameter, randomizing to either early EVAR with the AneuRx or Talent endografts or to ultrasound/CT imaging studies every 6 months. The perioperative mortality rate was 0.6% in the early EVAR group. Over a mean follow-up period of 20 months, there were no differences in all-cause mortality between the two groups, each with 15 deaths (4.1%). The primary endpoint of rupture or aneurysm-related death was similar in the two groups, with a hazard ratio of 0.99 in the early EVAR group. However, at 36 months almost 50% of the surveillance group underwent aneurysm repair for sac enlargement, the development of aneurysm-related symptoms, or patient choice. Interestingly, a follow-up economic substudy documented similar health care costs in the two treatment groups at 48 months of follow-up, even though 36.3% of the surveyed patients did not undergo repair.
A second study, the CEASAR trial, randomized 360 patients with AAA 4.1-5.4 cm in diameter to early EVAR with the Cook Zenith device or to serial ultrasound surveillance. After 4.5 years of follow-up, no significant difference was detected in the primary endpoint of all-cause mortality. The Kaplan-Meier estimates of all-cause mortality was 14.5% in the early repair group versus 10.1% in the surveillance group. Aneurysm-related mortality, aneurysm rupture, and major morbidity rates were similar. Like the PIVOTAL trial, the majority of subjects underwent delayed repair, with a frequency of 60% at 3-years and 85% at 4.5 years.
The findings of these two randomized studies suggest that there is no survival advantage to early EVAR in patients with smaller AAA. With the increasing use of statins, ACE inhibitors, and better overall medical management in patients with AAA, the rate of aneurysm enlargement and risk of rupture are quite low in patients with small AAA.8 While survival benefits have not been demonstrated, however, there appear to be few quantifiable disadvantages to early repair. Moreover, the long-term financial impact to the healthcare system appears to be similar when patients are treated early compared to surveillance with serial imaging studies,9 and patient quality of life may be improved with early EVAR.10 These observations suggest that the repair of smaller AAA should be individualized, based upon the particular clinical presentation and the wishes of a patient. The summary of available data suggests that either approach protects a patient from rupture and surveillance culminates in the eventual repair of the aneurysm in the majority of patients.
Dr. Ouriel is a vascular surgeon and president and CEO of Syntactx.
References
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. J Vasc Interv Radiol, 2012. 23(7): p. 866-72; quiz 872.
3. J Endovasc Ther, 2012. 19(2): p. 182-92.
4. J Vasc Surg, 2012. 55(1): p. 33-40.
5. Ann Vasc Surg, 2012. 26(6): p. 860 e1-7.
6. J Vasc Interv Radiol, 2013. 24(1): p. 49-55
7. J Vasc Surg, 2012. 56(3): p. 630-6.
8. J Cardiovasc Surg (Torino), 2013. 1. Br J Surg 2007;94:702-8.
2. Semin Interv Cardiol 2000;5:3-6.
3. NEnglJMed 2010;362:1863-71.
4. JAMA 2009;302:1535-42.
5.. J Vasc Surg 2003;37:1206-12.
6. J Vasc Surg 2006;44:920-29; discussion 9-31.
7. J Vasc Surg 2010;51:1081-7.
8. EurJVascEndovascSurg 2011;41:2-10.
9. J Vasc Surg 2013;58:302-10.
10. Eur J Vasc Endovasc Surg 2011;41:324-31.
Small aneurysms should be left alone.
By Karl A. Illig, M.D.
The currently accepted recommendation to delay repair of abdominal aortic aneurysms until they reach 5-5.5 cm is based on the historical mortality and morbidity of open repair.1 Endovascular aneurysm repair (EVAR) has clearly been shown to reduce the risk of operation, perhaps by as much as two-thirds. Should we now change the threshold for aneurysm repair, especially if the patient is a candidate for EVAR?
There are many arguments to repair small aneurysms. However, the answer really depends upon empirical data. At least three major papers address this question. In the U.K. Small Aneurysm Trial, 1,090 patients with abdominal aortic aneurysms measuring 4.0-5.5 cm in diameter were randomized to undergo early elective open surgery versus ultrasonic surveillance.2 There was an early survival disadvantage for those undergoing open surgery, as expected, but the curves evened out at 3 years or so, and no survival advantage occurred in either group, obviously favoring observation alone. Similarly, the ADAM group, pursuing the same protocol in 1,136 U.S. veterans, found the same thing, despite a low operative mortality of 2.7%. No advantage to early open repair could be seen.3
What of the situation after endovascular repair? The PIVOTAL trial, the lead author of which wrote the accompanying commentary, randomly assigned 728 patients with aneurysms measuring 4.0-5.0 cm to early endovascular repair with the Medtronic device versus ultrasonic surveillance.4 Aneurysm rupture or aneurysm-related death occurred in only two patients in each group (0.6%). The authors appropriately concluded that surveillance alone was equally as efficacious as early endovascular repair for patients with small aneurysms.
Finally, what of the arguments that, by waiting until the aneurysm is larger, you lose the window of opportunity for endovascular repair? We recently explored this in a cohort of 221 patients undergoing preoperative CT scanning for aneurysms of all sizes.5 With receiver operator curve analysis, a cutoff of 5.7 cm best differentiated those who were endovascular candidates from those who were not. Put another way, the rate of endovascular suitability hovered right around 80% until the aneurysm reached 6 cm, at which point it dropped off. In other words, waiting until the aneurysm reaches 5.5 cm doesn’t reduce the chance that a patient will be a candidate for EVAR.
There are many arguments for early intervention in small aneurysms. The concept that EVAR is safer than the procedure originally used to make the recommendation to wait until 5.5 cm is a valid point. However, empiric data still do not show any benefit for either open or endovascular repair as opposed to surveillance at sizes smaller than this. The operative mortality difference is only a couple of percentage points or so, and long-term survival appears to be identical after the initial risk has passed. This 2% or 3% early survival benefit does not seem to impart any long-term advantage, and long-term survival does not seem to be impaired by being a bit conservative. Until data come along that definitively show benefit for early repair, current guidelines remain valid.
Dr. Illig is the director of vascular surgery at USF Health, Tampa.
References:
1. J Vasc Surg 2009;50(8S):1S-49S.
2. Lancet 1998; 352:1649-55.
3. NEJM 2002; 346 (19): 1437-44.
4. J Vasc Surg 2010; 51:1081-7.
5. J Vasc Surg 2010;52:873-7.
Online Question of the Month
Point-Counterpoint: Type II Endoleaks - To treat or not to treat
The management of Type II endoleaks remains controversial. At the Charing Cross meeting this year Jean- Pierre Becquemin and Hence Vehagen debated whether Type II endoleaks should be treated. Dr. Verhagen suggested that results of endoleak treatment are so poor and the natural history of Type II endoleaks so benign that perhaps we shouldn’t even try to treat them. Dr. Becquemin felt that an attempt at treatment for some endoleaks was still necessary. This prompted our editors to suggest a U.S. version of the debate. As you will see both Dr. Geraghty and Dr. Kwolek feel that there is merit in pursuing treatment but with certain caveats. We appreciate their insight into this important subject.
– Dr. Russell Samson is the medical editor of Vascular Specialist.
A slow start can be salvaged
Our group recently published the midterm outcomes of our series of percutaneous glue and /or coil embolizations to treat type II endoleaks (T2EL) accompanied by continued aneurysm sac growth.1 Painstaking retrospective data collection, augmented by blinded review of all follow-up CT scans, demonstrated that at 23 months post intervention, T2EL had persisted or recurred in 72% of patients. Furthermore, the rate of aneurysm sac growth was not altered by the percutaneous intervention.
Other institutions have also reported significant rates of T2EL persistence/recurrence following embolization, and in the Cleveland Clinic experience, continued sac growth in excess of 5 mm in diameter was documented in 56% of patients who had undergone an attempt at T2EL ablation.2-4 In light of this data, one might logically question why I continue to support intervention in this setting.
The midterm reappraisals of T2EL interventions are certainly sobering. We can no longer accept apparent procedural success as a guarantee that these endoleaks will remain clinically and radiographically dormant. Yet these studies were not without their bright spots. In our series, diagnostic angiography (via transfemoral and translumbar approaches) detected occult type I or type III endoleaks in 21% of patients, prompting definitive repair by additional endograft placement. Similar angiographic elucidation of type I and III endoleaks was confirmed by the University of Chicago experience, with a 16% detection rate.2 The diagnosis and treatment of these highly pressurized leaks likely contributed to the low incidence of aneurysm rupture and aneurysm-related mortality seen in most of these series.
Bright spots aside, current midterm results for T2EL intervention leave much to be desired. I would contend, however, that these interventions should be refined, not abandoned. We now appreciate that T2EL mimic the behavior of complex arteriovenous malformations in several ways: the presence of multiple feeding and draining branches, the finding that solitary branch vessel embolization often leads to inflow recruitment from less prominent branches, and the presence of a nidus whose ablation is critical. In retrospect, then, it is not surprising that treatment approaches consisting of single-vessel coiling, or simple embolization of the nidus without concomitant treatment of all feeding branches, failed to produce the desired result. The aggressiveness of early T2EL embolization procedures may have been tempered by the potential for visceral and spinal cord complications.5,6 However, current midterm outcomes data suggest that predictable and durable success in T2EL treatment will require complete nidus ablation and occlusion of all arterial branches of the sac.
How can we pursue this more aggressive goal while maintaining an appropriate safety profile? In several instances, we have pursued this objective via staged interventions: initial angiographic coil/glue deployment, followed by laparoscopic clipping of persistent feeding vessels. The use of Onyx® (EV3-Covidien), as championed by Abularrage and Bosiers,7,8 offers the potential to achieve full treatment with a single intervention.
Advantages of Onyx include predictable polymerization times and lack of adhesion to the delivery catheter. These features permit deliberate and complete obliteration of the nidus, with intentional extension of the polymerizing mixture into the feeding vessels.
T2EL intervention has thus completed its first iteration. The value of routine angiographic examination is clear, particularly as regards the detection of occult type I and III endoleaks. Failure analysis of first-generation T2EL interventions suggests that incomplete obliteration of the endoleak complex (nidus and all feeding branches) sets the stage for vessel recruitment and reestablishment of sac flow. Promising alternative therapies have been identified, and we await their thorough evaluation.
Dr. Geraghty is an associate professor, surgery and radiology, in the division of general surgery, vascular surgery section at Washington University School of Medicine, St. Louis, Mo., and co-director of the Washington University Limb Preservation Center.
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. J Vasc Interv Radiol, 2012. 23(7): p. 866-72; quiz 872.
3. J Endovasc Ther, 2012. 19(2): p. 182-92.
4. J Vasc Surg, 2012. 55(1): p. 33-40.
5. Ann Vasc Surg, 2012. 26(6): p. 860 e1-7.
6. J Vasc Interv Radiol, 2013. 24(1): p. 49-55.
7. J Vasc Surg, 2012. 56(3): p. 630-6.
8. J Cardiovasc Surg (Torino), 2013.
A call for a prospective randomized trial
While I agree with Dr. Geraghty’s comments concerning the initial poor results reported for the percutaneous treatment of Type II endoleaks,1 I disagree with comments made in April 2013 at the Charing Cross meeting by Dr. Hence Verhagen that there is "no need to worry about Type II endoleaks since they are low pressure."2
Early on in our experience we began measuring sac pressures after trans- lumbar catheterization and have found that some aneurysm sacs may in fact have systolic arterial pressures of 8-90 mm HG from large lumbar or IMA collaterals. Furthermore, we and others have described the treatment of patients with ruptured AAA after EVAR who upon open exploration where found to have large patent lumbar arteries as the sole etiology of the ruptured AAA.
Dr. Geraghty and Dr. Sarac at Cleveland Clinic have also described the important observation that these Type II endoleaks may be associated with a Type I or III endoleaks and thus put patients at even higher risk of rupture.3 The extent of these leaks may not be completely elucidated until the time of angiography, again emphasizing the importance of a more aggressive approach to diagnosis and treatment in patients with an enlarging aneurysm sac.
These authors have also appropriately pointed out that overall treatment success rates are low with persistence of type II endoleaks on long–term follow-up. While we initially described an overall success rate of 30%, our approach has changed over time to include direct access to the endoleak nidus with the injection of a flow directed glue (Onyx) to obliterate the flow channel and feeding vessels.
We have found this approach to be more successful than attempting to cannulate each of the feeding lumbar vessels or coil embolization of the aneurysm sac, and can be performed via either a translumbar or transarterial approach using SMA to IMA collaterals. While our success rate using this technique has improved to nearly 80%, several words of caution should be noted. 1) Onyx glue is approved for use in the treatment of intracerebral vascular malformations and does not have a peripheral indication at this time. 2) It is critically important that the interventionalist obtains access into the nidus/flow channel to allow the embolic agent to flow. Otherwise you are injecting glue directly into the sac thrombus and wasting time and an expensive embolic agent. 3) There are often multiple levels of lumbar vessels involved and we have found that we often have to go back several times at different levels to treat extensive Type II endoleaks. All of these observations emphasize the need to further study the treatment of Type II endoleaks and the need for a prospective randomized trial to better identify the best treatment options.
Dr. Kwolek is the director of the vascular and endovascular training program at Massachusetts General Hospital, Boston, and the chief of vascular surgery at Newton Wellesley Hospital.
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. Management of Type II endoleaks divides the experts at CX 35. Vascular News International,58, May 2012, BIBA publishing.
3. J Vasc Surg, 2012. 55(1): p. 33-40.
4. J Vasc Surg, 2012. 56(3): p. 630-6.
The management of Type II endoleaks remains controversial. At the Charing Cross meeting this year Jean- Pierre Becquemin and Hence Vehagen debated whether Type II endoleaks should be treated. Dr. Verhagen suggested that results of endoleak treatment are so poor and the natural history of Type II endoleaks so benign that perhaps we shouldn’t even try to treat them. Dr. Becquemin felt that an attempt at treatment for some endoleaks was still necessary. This prompted our editors to suggest a U.S. version of the debate. As you will see both Dr. Geraghty and Dr. Kwolek feel that there is merit in pursuing treatment but with certain caveats. We appreciate their insight into this important subject.
– Dr. Russell Samson is the medical editor of Vascular Specialist.
A slow start can be salvaged
Our group recently published the midterm outcomes of our series of percutaneous glue and /or coil embolizations to treat type II endoleaks (T2EL) accompanied by continued aneurysm sac growth.1 Painstaking retrospective data collection, augmented by blinded review of all follow-up CT scans, demonstrated that at 23 months post intervention, T2EL had persisted or recurred in 72% of patients. Furthermore, the rate of aneurysm sac growth was not altered by the percutaneous intervention.
Other institutions have also reported significant rates of T2EL persistence/recurrence following embolization, and in the Cleveland Clinic experience, continued sac growth in excess of 5 mm in diameter was documented in 56% of patients who had undergone an attempt at T2EL ablation.2-4 In light of this data, one might logically question why I continue to support intervention in this setting.
The midterm reappraisals of T2EL interventions are certainly sobering. We can no longer accept apparent procedural success as a guarantee that these endoleaks will remain clinically and radiographically dormant. Yet these studies were not without their bright spots. In our series, diagnostic angiography (via transfemoral and translumbar approaches) detected occult type I or type III endoleaks in 21% of patients, prompting definitive repair by additional endograft placement. Similar angiographic elucidation of type I and III endoleaks was confirmed by the University of Chicago experience, with a 16% detection rate.2 The diagnosis and treatment of these highly pressurized leaks likely contributed to the low incidence of aneurysm rupture and aneurysm-related mortality seen in most of these series.
Bright spots aside, current midterm results for T2EL intervention leave much to be desired. I would contend, however, that these interventions should be refined, not abandoned. We now appreciate that T2EL mimic the behavior of complex arteriovenous malformations in several ways: the presence of multiple feeding and draining branches, the finding that solitary branch vessel embolization often leads to inflow recruitment from less prominent branches, and the presence of a nidus whose ablation is critical. In retrospect, then, it is not surprising that treatment approaches consisting of single-vessel coiling, or simple embolization of the nidus without concomitant treatment of all feeding branches, failed to produce the desired result. The aggressiveness of early T2EL embolization procedures may have been tempered by the potential for visceral and spinal cord complications.5,6 However, current midterm outcomes data suggest that predictable and durable success in T2EL treatment will require complete nidus ablation and occlusion of all arterial branches of the sac.
How can we pursue this more aggressive goal while maintaining an appropriate safety profile? In several instances, we have pursued this objective via staged interventions: initial angiographic coil/glue deployment, followed by laparoscopic clipping of persistent feeding vessels. The use of Onyx® (EV3-Covidien), as championed by Abularrage and Bosiers,7,8 offers the potential to achieve full treatment with a single intervention.
Advantages of Onyx include predictable polymerization times and lack of adhesion to the delivery catheter. These features permit deliberate and complete obliteration of the nidus, with intentional extension of the polymerizing mixture into the feeding vessels.
T2EL intervention has thus completed its first iteration. The value of routine angiographic examination is clear, particularly as regards the detection of occult type I and III endoleaks. Failure analysis of first-generation T2EL interventions suggests that incomplete obliteration of the endoleak complex (nidus and all feeding branches) sets the stage for vessel recruitment and reestablishment of sac flow. Promising alternative therapies have been identified, and we await their thorough evaluation.
Dr. Geraghty is an associate professor, surgery and radiology, in the division of general surgery, vascular surgery section at Washington University School of Medicine, St. Louis, Mo., and co-director of the Washington University Limb Preservation Center.
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. J Vasc Interv Radiol, 2012. 23(7): p. 866-72; quiz 872.
3. J Endovasc Ther, 2012. 19(2): p. 182-92.
4. J Vasc Surg, 2012. 55(1): p. 33-40.
5. Ann Vasc Surg, 2012. 26(6): p. 860 e1-7.
6. J Vasc Interv Radiol, 2013. 24(1): p. 49-55.
7. J Vasc Surg, 2012. 56(3): p. 630-6.
8. J Cardiovasc Surg (Torino), 2013.
A call for a prospective randomized trial
While I agree with Dr. Geraghty’s comments concerning the initial poor results reported for the percutaneous treatment of Type II endoleaks,1 I disagree with comments made in April 2013 at the Charing Cross meeting by Dr. Hence Verhagen that there is "no need to worry about Type II endoleaks since they are low pressure."2
Early on in our experience we began measuring sac pressures after trans- lumbar catheterization and have found that some aneurysm sacs may in fact have systolic arterial pressures of 8-90 mm HG from large lumbar or IMA collaterals. Furthermore, we and others have described the treatment of patients with ruptured AAA after EVAR who upon open exploration where found to have large patent lumbar arteries as the sole etiology of the ruptured AAA.
Dr. Geraghty and Dr. Sarac at Cleveland Clinic have also described the important observation that these Type II endoleaks may be associated with a Type I or III endoleaks and thus put patients at even higher risk of rupture.3 The extent of these leaks may not be completely elucidated until the time of angiography, again emphasizing the importance of a more aggressive approach to diagnosis and treatment in patients with an enlarging aneurysm sac.
These authors have also appropriately pointed out that overall treatment success rates are low with persistence of type II endoleaks on long–term follow-up. While we initially described an overall success rate of 30%, our approach has changed over time to include direct access to the endoleak nidus with the injection of a flow directed glue (Onyx) to obliterate the flow channel and feeding vessels.
We have found this approach to be more successful than attempting to cannulate each of the feeding lumbar vessels or coil embolization of the aneurysm sac, and can be performed via either a translumbar or transarterial approach using SMA to IMA collaterals. While our success rate using this technique has improved to nearly 80%, several words of caution should be noted. 1) Onyx glue is approved for use in the treatment of intracerebral vascular malformations and does not have a peripheral indication at this time. 2) It is critically important that the interventionalist obtains access into the nidus/flow channel to allow the embolic agent to flow. Otherwise you are injecting glue directly into the sac thrombus and wasting time and an expensive embolic agent. 3) There are often multiple levels of lumbar vessels involved and we have found that we often have to go back several times at different levels to treat extensive Type II endoleaks. All of these observations emphasize the need to further study the treatment of Type II endoleaks and the need for a prospective randomized trial to better identify the best treatment options.
Dr. Kwolek is the director of the vascular and endovascular training program at Massachusetts General Hospital, Boston, and the chief of vascular surgery at Newton Wellesley Hospital.
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. Management of Type II endoleaks divides the experts at CX 35. Vascular News International,58, May 2012, BIBA publishing.
3. J Vasc Surg, 2012. 55(1): p. 33-40.
4. J Vasc Surg, 2012. 56(3): p. 630-6.
The management of Type II endoleaks remains controversial. At the Charing Cross meeting this year Jean- Pierre Becquemin and Hence Vehagen debated whether Type II endoleaks should be treated. Dr. Verhagen suggested that results of endoleak treatment are so poor and the natural history of Type II endoleaks so benign that perhaps we shouldn’t even try to treat them. Dr. Becquemin felt that an attempt at treatment for some endoleaks was still necessary. This prompted our editors to suggest a U.S. version of the debate. As you will see both Dr. Geraghty and Dr. Kwolek feel that there is merit in pursuing treatment but with certain caveats. We appreciate their insight into this important subject.
– Dr. Russell Samson is the medical editor of Vascular Specialist.
A slow start can be salvaged
Our group recently published the midterm outcomes of our series of percutaneous glue and /or coil embolizations to treat type II endoleaks (T2EL) accompanied by continued aneurysm sac growth.1 Painstaking retrospective data collection, augmented by blinded review of all follow-up CT scans, demonstrated that at 23 months post intervention, T2EL had persisted or recurred in 72% of patients. Furthermore, the rate of aneurysm sac growth was not altered by the percutaneous intervention.
Other institutions have also reported significant rates of T2EL persistence/recurrence following embolization, and in the Cleveland Clinic experience, continued sac growth in excess of 5 mm in diameter was documented in 56% of patients who had undergone an attempt at T2EL ablation.2-4 In light of this data, one might logically question why I continue to support intervention in this setting.
The midterm reappraisals of T2EL interventions are certainly sobering. We can no longer accept apparent procedural success as a guarantee that these endoleaks will remain clinically and radiographically dormant. Yet these studies were not without their bright spots. In our series, diagnostic angiography (via transfemoral and translumbar approaches) detected occult type I or type III endoleaks in 21% of patients, prompting definitive repair by additional endograft placement. Similar angiographic elucidation of type I and III endoleaks was confirmed by the University of Chicago experience, with a 16% detection rate.2 The diagnosis and treatment of these highly pressurized leaks likely contributed to the low incidence of aneurysm rupture and aneurysm-related mortality seen in most of these series.
Bright spots aside, current midterm results for T2EL intervention leave much to be desired. I would contend, however, that these interventions should be refined, not abandoned. We now appreciate that T2EL mimic the behavior of complex arteriovenous malformations in several ways: the presence of multiple feeding and draining branches, the finding that solitary branch vessel embolization often leads to inflow recruitment from less prominent branches, and the presence of a nidus whose ablation is critical. In retrospect, then, it is not surprising that treatment approaches consisting of single-vessel coiling, or simple embolization of the nidus without concomitant treatment of all feeding branches, failed to produce the desired result. The aggressiveness of early T2EL embolization procedures may have been tempered by the potential for visceral and spinal cord complications.5,6 However, current midterm outcomes data suggest that predictable and durable success in T2EL treatment will require complete nidus ablation and occlusion of all arterial branches of the sac.
How can we pursue this more aggressive goal while maintaining an appropriate safety profile? In several instances, we have pursued this objective via staged interventions: initial angiographic coil/glue deployment, followed by laparoscopic clipping of persistent feeding vessels. The use of Onyx® (EV3-Covidien), as championed by Abularrage and Bosiers,7,8 offers the potential to achieve full treatment with a single intervention.
Advantages of Onyx include predictable polymerization times and lack of adhesion to the delivery catheter. These features permit deliberate and complete obliteration of the nidus, with intentional extension of the polymerizing mixture into the feeding vessels.
T2EL intervention has thus completed its first iteration. The value of routine angiographic examination is clear, particularly as regards the detection of occult type I and III endoleaks. Failure analysis of first-generation T2EL interventions suggests that incomplete obliteration of the endoleak complex (nidus and all feeding branches) sets the stage for vessel recruitment and reestablishment of sac flow. Promising alternative therapies have been identified, and we await their thorough evaluation.
Dr. Geraghty is an associate professor, surgery and radiology, in the division of general surgery, vascular surgery section at Washington University School of Medicine, St. Louis, Mo., and co-director of the Washington University Limb Preservation Center.
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. J Vasc Interv Radiol, 2012. 23(7): p. 866-72; quiz 872.
3. J Endovasc Ther, 2012. 19(2): p. 182-92.
4. J Vasc Surg, 2012. 55(1): p. 33-40.
5. Ann Vasc Surg, 2012. 26(6): p. 860 e1-7.
6. J Vasc Interv Radiol, 2013. 24(1): p. 49-55.
7. J Vasc Surg, 2012. 56(3): p. 630-6.
8. J Cardiovasc Surg (Torino), 2013.
A call for a prospective randomized trial
While I agree with Dr. Geraghty’s comments concerning the initial poor results reported for the percutaneous treatment of Type II endoleaks,1 I disagree with comments made in April 2013 at the Charing Cross meeting by Dr. Hence Verhagen that there is "no need to worry about Type II endoleaks since they are low pressure."2
Early on in our experience we began measuring sac pressures after trans- lumbar catheterization and have found that some aneurysm sacs may in fact have systolic arterial pressures of 8-90 mm HG from large lumbar or IMA collaterals. Furthermore, we and others have described the treatment of patients with ruptured AAA after EVAR who upon open exploration where found to have large patent lumbar arteries as the sole etiology of the ruptured AAA.
Dr. Geraghty and Dr. Sarac at Cleveland Clinic have also described the important observation that these Type II endoleaks may be associated with a Type I or III endoleaks and thus put patients at even higher risk of rupture.3 The extent of these leaks may not be completely elucidated until the time of angiography, again emphasizing the importance of a more aggressive approach to diagnosis and treatment in patients with an enlarging aneurysm sac.
These authors have also appropriately pointed out that overall treatment success rates are low with persistence of type II endoleaks on long–term follow-up. While we initially described an overall success rate of 30%, our approach has changed over time to include direct access to the endoleak nidus with the injection of a flow directed glue (Onyx) to obliterate the flow channel and feeding vessels.
We have found this approach to be more successful than attempting to cannulate each of the feeding lumbar vessels or coil embolization of the aneurysm sac, and can be performed via either a translumbar or transarterial approach using SMA to IMA collaterals. While our success rate using this technique has improved to nearly 80%, several words of caution should be noted. 1) Onyx glue is approved for use in the treatment of intracerebral vascular malformations and does not have a peripheral indication at this time. 2) It is critically important that the interventionalist obtains access into the nidus/flow channel to allow the embolic agent to flow. Otherwise you are injecting glue directly into the sac thrombus and wasting time and an expensive embolic agent. 3) There are often multiple levels of lumbar vessels involved and we have found that we often have to go back several times at different levels to treat extensive Type II endoleaks. All of these observations emphasize the need to further study the treatment of Type II endoleaks and the need for a prospective randomized trial to better identify the best treatment options.
Dr. Kwolek is the director of the vascular and endovascular training program at Massachusetts General Hospital, Boston, and the chief of vascular surgery at Newton Wellesley Hospital.
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. Management of Type II endoleaks divides the experts at CX 35. Vascular News International,58, May 2012, BIBA publishing.
3. J Vasc Surg, 2012. 55(1): p. 33-40.
4. J Vasc Surg, 2012. 56(3): p. 630-6.