User login
Type 2 Diabetes Workup
Shortness of breath and abdominal pain
On the basis of the patient's history and clinical presentation, the likely diagnosis is ketosis-prone diabetes (KPD) type 2. KPD is widely thought of as an atypical diabetes syndrome, though some groups consider it to be a common clinical presentation in newly diagnosed patients with type 2 diabetes (T2D) rather than a subtype of disease. The condition is more prevalent in males and among Black and Hispanic populations.
Definitive diagnosis of the type of diabetes can present a clinical challenge during acute presentation. Although the majority of diabetic ketoacidosis (DKA) episodes occur in patients previously diagnosed with type 1 diabetes, an estimated 34% occur in patients with T2D. For patients who are obese or have a family history of diabetes, there should be a high index of suspicion for new-onset DKA in type 2 diabetes.
Patients with KPD typically present in a state of ketoacidosis with a brief but acute history of hyperglycemic symptoms. Hyperglycemia, elevated anion gap acidosis, and ketonemia form the expected constellation of DKA symptoms. A key consideration in the differential diagnosis is hyperosmolar hyperglycemic state (HHS). Patients with HHS are much more likely to have altered mental status than are patients with DKA. Metabolic acidosis and ketonemia are absent or mild, and anion gap is either normal or slightly elevated. In HHS, extreme elevations of glucose are seen, with a lack of significant ketoacidosis. Glucose levels tend to be higher in HHS than in DKA; they are almost always > 600 mg/dL, and levels > 1000 mg/dL are not uncommon. In DKA, glucose levels are still markedly high — generally 500-800 mg/dL but rarely exceeding 900 mg/dL. Patients with DKA also usually present with an A1c > 10% and a blood pH < 7.30.
Treatment for KPD is initially acute, beginning with aggressive intravenous fluid and insulin therapy A. Once this state resolves, insulin requirements typically decrease for patients with T2D, and they are able to maintain adequate glycemic control with an oral therapy regimen.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia.
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
On the basis of the patient's history and clinical presentation, the likely diagnosis is ketosis-prone diabetes (KPD) type 2. KPD is widely thought of as an atypical diabetes syndrome, though some groups consider it to be a common clinical presentation in newly diagnosed patients with type 2 diabetes (T2D) rather than a subtype of disease. The condition is more prevalent in males and among Black and Hispanic populations.
Definitive diagnosis of the type of diabetes can present a clinical challenge during acute presentation. Although the majority of diabetic ketoacidosis (DKA) episodes occur in patients previously diagnosed with type 1 diabetes, an estimated 34% occur in patients with T2D. For patients who are obese or have a family history of diabetes, there should be a high index of suspicion for new-onset DKA in type 2 diabetes.
Patients with KPD typically present in a state of ketoacidosis with a brief but acute history of hyperglycemic symptoms. Hyperglycemia, elevated anion gap acidosis, and ketonemia form the expected constellation of DKA symptoms. A key consideration in the differential diagnosis is hyperosmolar hyperglycemic state (HHS). Patients with HHS are much more likely to have altered mental status than are patients with DKA. Metabolic acidosis and ketonemia are absent or mild, and anion gap is either normal or slightly elevated. In HHS, extreme elevations of glucose are seen, with a lack of significant ketoacidosis. Glucose levels tend to be higher in HHS than in DKA; they are almost always > 600 mg/dL, and levels > 1000 mg/dL are not uncommon. In DKA, glucose levels are still markedly high — generally 500-800 mg/dL but rarely exceeding 900 mg/dL. Patients with DKA also usually present with an A1c > 10% and a blood pH < 7.30.
Treatment for KPD is initially acute, beginning with aggressive intravenous fluid and insulin therapy A. Once this state resolves, insulin requirements typically decrease for patients with T2D, and they are able to maintain adequate glycemic control with an oral therapy regimen.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia.
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
On the basis of the patient's history and clinical presentation, the likely diagnosis is ketosis-prone diabetes (KPD) type 2. KPD is widely thought of as an atypical diabetes syndrome, though some groups consider it to be a common clinical presentation in newly diagnosed patients with type 2 diabetes (T2D) rather than a subtype of disease. The condition is more prevalent in males and among Black and Hispanic populations.
Definitive diagnosis of the type of diabetes can present a clinical challenge during acute presentation. Although the majority of diabetic ketoacidosis (DKA) episodes occur in patients previously diagnosed with type 1 diabetes, an estimated 34% occur in patients with T2D. For patients who are obese or have a family history of diabetes, there should be a high index of suspicion for new-onset DKA in type 2 diabetes.
Patients with KPD typically present in a state of ketoacidosis with a brief but acute history of hyperglycemic symptoms. Hyperglycemia, elevated anion gap acidosis, and ketonemia form the expected constellation of DKA symptoms. A key consideration in the differential diagnosis is hyperosmolar hyperglycemic state (HHS). Patients with HHS are much more likely to have altered mental status than are patients with DKA. Metabolic acidosis and ketonemia are absent or mild, and anion gap is either normal or slightly elevated. In HHS, extreme elevations of glucose are seen, with a lack of significant ketoacidosis. Glucose levels tend to be higher in HHS than in DKA; they are almost always > 600 mg/dL, and levels > 1000 mg/dL are not uncommon. In DKA, glucose levels are still markedly high — generally 500-800 mg/dL but rarely exceeding 900 mg/dL. Patients with DKA also usually present with an A1c > 10% and a blood pH < 7.30.
Treatment for KPD is initially acute, beginning with aggressive intravenous fluid and insulin therapy A. Once this state resolves, insulin requirements typically decrease for patients with T2D, and they are able to maintain adequate glycemic control with an oral therapy regimen.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia.
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
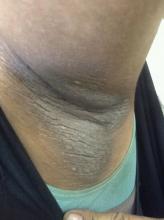
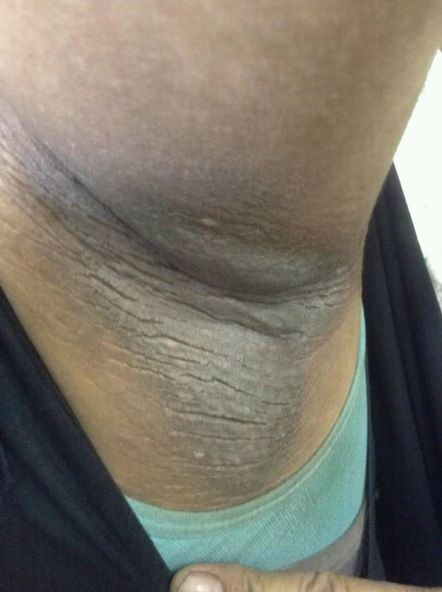
A 21-year-old man presents with shortness of breath and abdominal pain. He has a BMI of 34.6 and explains that he has had asthma for several years, using an inhaler when needed. He reports a few weeks of polydipsia and polyuria. The patient notes that his father has kidney disease. He believes other close relatives are managing chronic metabolic conditions but does not know any further detail regarding their diagnoses. On laboratory testing, blood pH is 6.30 and bicarbonate level is 11.1 mmol/L. A1c is 12.0%. Acanthosis nigricans are noted on the neck and in the axilla bilaterally.
Type 2 Diabetes Presentation
Patient with tachycardia
On the basis of the patient's personal and family history together with his presentation, the likely diagnosis is latent autoimmune diabetes in adults (LADA). LADA is characterized by beta-cell loss and insulin resistance. This slowly evolving form of autoimmune diabetes comprises 2%-12% of all patients with adult-onset diabetes. Patients with LADA present with evidence of autoimmunity and varying C-peptide levels, which decrease more slowly in this subgroup than in patients with type 1 diabetes (T1D). They also have immunogenic markers associated with T1D, primarily anti-glutamic acid decarboxylase (GAD) antibodies.
Patients with LADA are often misdiagnosed as having T2D. The clinical picture of LADA overlaps with that of T2D, with patients being insulin resistant and often overweight. In addition, presenting symptoms of LADA — excessive thirst, blurred vision, and high blood glucose — are also seen in T2D. Although LADA is technically classified as T1D, some groups posit that the condition exists on a spectrum between T1D and T2D. Compared with patients with T2D, those with LADA are generally younger at diagnosis (often in their 30s), have lower BMI, and report a personal or family history of autoimmune diseases, such as the patient in this quiz. Throughout the disease course, individuals with LADA show a reduced frequency of metabolic syndrome compared with those with T2D.
Key to diagnosis is the absence of insulin requirement for at least 6 months. Anti-GAD antibodies are the most sensitive marker for LADA; other autoantibodies that occur less frequently include ICA, IA-2A, ZnT8A, and tetraspanin 7 autoantibodies. With a paucity of large-scale clinical trials in LADA, current treatment strategies are not based on consensus guidelines, though an expert panel has published management recommendations. Category 1 patients (defined as a C-peptide level < 0.3 nmol/L) are treated with intensive insulin therapy. The recommendation for category 2 patients (defined as C-peptide values ≥ 0.3 and ≤ 0.7 nmol/L) is a modified American Diabetes Association/European Association for the Study of Diabetes algorithm for T2D. However, patients with category 2 LADA may need to initiate insulin therapy earlier to combat beta-cell failure (ostensibly because LADA is an autoimmune disease beta-cell function declines much faster than in T2D). For category 3 patients (defined as C-peptide values > 0.7 nmol/L), treatment decisions are made in response to changing C-peptide levels.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia.
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
On the basis of the patient's personal and family history together with his presentation, the likely diagnosis is latent autoimmune diabetes in adults (LADA). LADA is characterized by beta-cell loss and insulin resistance. This slowly evolving form of autoimmune diabetes comprises 2%-12% of all patients with adult-onset diabetes. Patients with LADA present with evidence of autoimmunity and varying C-peptide levels, which decrease more slowly in this subgroup than in patients with type 1 diabetes (T1D). They also have immunogenic markers associated with T1D, primarily anti-glutamic acid decarboxylase (GAD) antibodies.
Patients with LADA are often misdiagnosed as having T2D. The clinical picture of LADA overlaps with that of T2D, with patients being insulin resistant and often overweight. In addition, presenting symptoms of LADA — excessive thirst, blurred vision, and high blood glucose — are also seen in T2D. Although LADA is technically classified as T1D, some groups posit that the condition exists on a spectrum between T1D and T2D. Compared with patients with T2D, those with LADA are generally younger at diagnosis (often in their 30s), have lower BMI, and report a personal or family history of autoimmune diseases, such as the patient in this quiz. Throughout the disease course, individuals with LADA show a reduced frequency of metabolic syndrome compared with those with T2D.
Key to diagnosis is the absence of insulin requirement for at least 6 months. Anti-GAD antibodies are the most sensitive marker for LADA; other autoantibodies that occur less frequently include ICA, IA-2A, ZnT8A, and tetraspanin 7 autoantibodies. With a paucity of large-scale clinical trials in LADA, current treatment strategies are not based on consensus guidelines, though an expert panel has published management recommendations. Category 1 patients (defined as a C-peptide level < 0.3 nmol/L) are treated with intensive insulin therapy. The recommendation for category 2 patients (defined as C-peptide values ≥ 0.3 and ≤ 0.7 nmol/L) is a modified American Diabetes Association/European Association for the Study of Diabetes algorithm for T2D. However, patients with category 2 LADA may need to initiate insulin therapy earlier to combat beta-cell failure (ostensibly because LADA is an autoimmune disease beta-cell function declines much faster than in T2D). For category 3 patients (defined as C-peptide values > 0.7 nmol/L), treatment decisions are made in response to changing C-peptide levels.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia.
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
On the basis of the patient's personal and family history together with his presentation, the likely diagnosis is latent autoimmune diabetes in adults (LADA). LADA is characterized by beta-cell loss and insulin resistance. This slowly evolving form of autoimmune diabetes comprises 2%-12% of all patients with adult-onset diabetes. Patients with LADA present with evidence of autoimmunity and varying C-peptide levels, which decrease more slowly in this subgroup than in patients with type 1 diabetes (T1D). They also have immunogenic markers associated with T1D, primarily anti-glutamic acid decarboxylase (GAD) antibodies.
Patients with LADA are often misdiagnosed as having T2D. The clinical picture of LADA overlaps with that of T2D, with patients being insulin resistant and often overweight. In addition, presenting symptoms of LADA — excessive thirst, blurred vision, and high blood glucose — are also seen in T2D. Although LADA is technically classified as T1D, some groups posit that the condition exists on a spectrum between T1D and T2D. Compared with patients with T2D, those with LADA are generally younger at diagnosis (often in their 30s), have lower BMI, and report a personal or family history of autoimmune diseases, such as the patient in this quiz. Throughout the disease course, individuals with LADA show a reduced frequency of metabolic syndrome compared with those with T2D.
Key to diagnosis is the absence of insulin requirement for at least 6 months. Anti-GAD antibodies are the most sensitive marker for LADA; other autoantibodies that occur less frequently include ICA, IA-2A, ZnT8A, and tetraspanin 7 autoantibodies. With a paucity of large-scale clinical trials in LADA, current treatment strategies are not based on consensus guidelines, though an expert panel has published management recommendations. Category 1 patients (defined as a C-peptide level < 0.3 nmol/L) are treated with intensive insulin therapy. The recommendation for category 2 patients (defined as C-peptide values ≥ 0.3 and ≤ 0.7 nmol/L) is a modified American Diabetes Association/European Association for the Study of Diabetes algorithm for T2D. However, patients with category 2 LADA may need to initiate insulin therapy earlier to combat beta-cell failure (ostensibly because LADA is an autoimmune disease beta-cell function declines much faster than in T2D). For category 3 patients (defined as C-peptide values > 0.7 nmol/L), treatment decisions are made in response to changing C-peptide levels.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia.
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
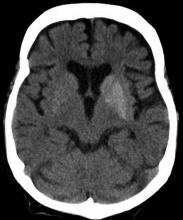
A 33-year-old man presents with blurred vision and tachycardia. Physical examination is remarkable for a BMI of 27 kg/m2. The patient explains that he feels he has lost weight. However, he attributes this change to a new exercise regimen he undertook when he was diagnosed with type 2 diabetes (T2D) about 8 months ago. The patient also notes polydipsia over a series of weeks. He reports that his first cousin may have lupus, though her diagnosis is still uncertain. Axial noncontrast CT demonstrates hyperattenuation that is more pronounced on the left side and involves the lentiform and caudate nuclei bilaterally.