User login
A reliable rubric for evaluating medical apps
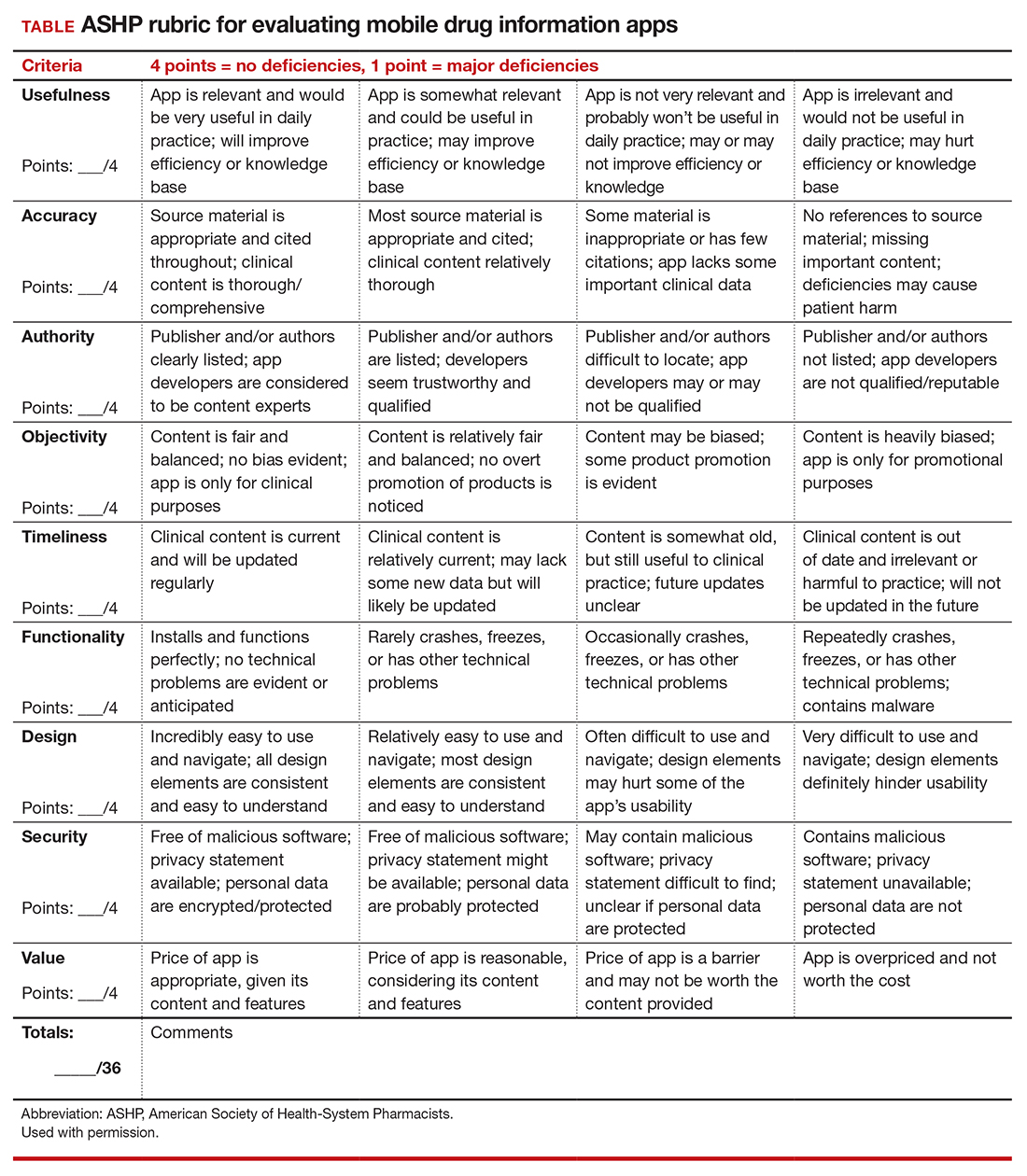
To help ObGyns evaluate mobile apps for use in clinical practice, the American College of Obstetricians and Gynecologists Presidential Task Force of Dr. Eva Chalas recommends a quantitative rubric that was developed by the American Society of Health-System Pharmacists (ASHP) for evaluating drug information apps (TABLE).1 Criteria are graded on a point scale of 1 to 4, with 1 point indicating major deficiencies and 4 points indicating no deficiencies.
The ASHP used the following criteria in evaluating mobile apps:
- Usefulness: the app’s overall usefulness in a particular practice setting
- Accuracy: overall accuracy of the app should be thoroughly examined
- Authority: it is critical to assess authority or authorship to determine that the developers are reputable, qualified, and authoritative enough to create the medical content in question
- Objectivity: to determine if content is fair, balanced, and unbiased
- Timeliness: given that medical information is continually changing, an app must be evaluated based on the timeliness of its content
- Functionality: how the app downloads, deploys, and operates across devices and software platforms (that is, iOS, Android)
- Design: well-designed apps are generally more user friendly and, therefore, useful. They should require minimal or no training and have easily discernible buttons, a clean and uncluttered format, consistent graphics layout, terminology appropriate for the intended audience, streamlined navigation without extraneous steps/gestures, appropriate-sized text, and sufficient white space to improve readability.
- Security: Many apps collect a wide array of personal and device data. Collected data has the potential for being sold to third parties for marketing and advertising purposes. Apps should disclose their privacy policy and provide an explanation as to why personal data are being collected. If personal identifiable information (PII) is collected, then the app should be encrypted. If protected health information (PHI) is collected, the app must follow compliance with HIPAA/HITECH (Health Insurance Portability and Accountability Act/Health Information Technology for Economic and Clinical Health Act). Additionally, apps should not compromise the security or functionality of the mobile device being used.
- Value: appropriateness of an app's cost. ●
- Hanrahan C, Aungst TD, Cole S. Evaluating mobile medical applications. American Society of Health-System Pharmacists eReports. https://www.ashp .org/-/media/store-files/mobile-medical-apps. ashx. Accessed January 22, 2021.
To help ObGyns evaluate mobile apps for use in clinical practice, the American College of Obstetricians and Gynecologists Presidential Task Force of Dr. Eva Chalas recommends a quantitative rubric that was developed by the American Society of Health-System Pharmacists (ASHP) for evaluating drug information apps (TABLE).1 Criteria are graded on a point scale of 1 to 4, with 1 point indicating major deficiencies and 4 points indicating no deficiencies.
The ASHP used the following criteria in evaluating mobile apps:
- Usefulness: the app’s overall usefulness in a particular practice setting
- Accuracy: overall accuracy of the app should be thoroughly examined
- Authority: it is critical to assess authority or authorship to determine that the developers are reputable, qualified, and authoritative enough to create the medical content in question
- Objectivity: to determine if content is fair, balanced, and unbiased
- Timeliness: given that medical information is continually changing, an app must be evaluated based on the timeliness of its content
- Functionality: how the app downloads, deploys, and operates across devices and software platforms (that is, iOS, Android)
- Design: well-designed apps are generally more user friendly and, therefore, useful. They should require minimal or no training and have easily discernible buttons, a clean and uncluttered format, consistent graphics layout, terminology appropriate for the intended audience, streamlined navigation without extraneous steps/gestures, appropriate-sized text, and sufficient white space to improve readability.
- Security: Many apps collect a wide array of personal and device data. Collected data has the potential for being sold to third parties for marketing and advertising purposes. Apps should disclose their privacy policy and provide an explanation as to why personal data are being collected. If personal identifiable information (PII) is collected, then the app should be encrypted. If protected health information (PHI) is collected, the app must follow compliance with HIPAA/HITECH (Health Insurance Portability and Accountability Act/Health Information Technology for Economic and Clinical Health Act). Additionally, apps should not compromise the security or functionality of the mobile device being used.
- Value: appropriateness of an app's cost. ●

To help ObGyns evaluate mobile apps for use in clinical practice, the American College of Obstetricians and Gynecologists Presidential Task Force of Dr. Eva Chalas recommends a quantitative rubric that was developed by the American Society of Health-System Pharmacists (ASHP) for evaluating drug information apps (TABLE).1 Criteria are graded on a point scale of 1 to 4, with 1 point indicating major deficiencies and 4 points indicating no deficiencies.
The ASHP used the following criteria in evaluating mobile apps:
- Usefulness: the app’s overall usefulness in a particular practice setting
- Accuracy: overall accuracy of the app should be thoroughly examined
- Authority: it is critical to assess authority or authorship to determine that the developers are reputable, qualified, and authoritative enough to create the medical content in question
- Objectivity: to determine if content is fair, balanced, and unbiased
- Timeliness: given that medical information is continually changing, an app must be evaluated based on the timeliness of its content
- Functionality: how the app downloads, deploys, and operates across devices and software platforms (that is, iOS, Android)
- Design: well-designed apps are generally more user friendly and, therefore, useful. They should require minimal or no training and have easily discernible buttons, a clean and uncluttered format, consistent graphics layout, terminology appropriate for the intended audience, streamlined navigation without extraneous steps/gestures, appropriate-sized text, and sufficient white space to improve readability.
- Security: Many apps collect a wide array of personal and device data. Collected data has the potential for being sold to third parties for marketing and advertising purposes. Apps should disclose their privacy policy and provide an explanation as to why personal data are being collected. If personal identifiable information (PII) is collected, then the app should be encrypted. If protected health information (PHI) is collected, the app must follow compliance with HIPAA/HITECH (Health Insurance Portability and Accountability Act/Health Information Technology for Economic and Clinical Health Act). Additionally, apps should not compromise the security or functionality of the mobile device being used.
- Value: appropriateness of an app's cost. ●

- Hanrahan C, Aungst TD, Cole S. Evaluating mobile medical applications. American Society of Health-System Pharmacists eReports. https://www.ashp .org/-/media/store-files/mobile-medical-apps. ashx. Accessed January 22, 2021.
- Hanrahan C, Aungst TD, Cole S. Evaluating mobile medical applications. American Society of Health-System Pharmacists eReports. https://www.ashp .org/-/media/store-files/mobile-medical-apps. ashx. Accessed January 22, 2021.
