User login
Risk Factors Associated With Multidrug-Resistant Pneumonia in Nonhospitalized Patients
Successful treatment of pneumonia depends on timely diagnosis and administration of antibiotics. Multidrug-resistant organisms (MDROs) complicate antibiotic therapies by rendering some antibiotic agents ineffective. Inappropriate initial therapy has been associated with a more than 2-fold increase in the risk of mortality.1 Because culture results are not available immediately, clinicians prescribe antibiotics empirically and must rely on guidelines and knowledge of risk factors associated with MDRO infection to make these selections.
Treatment guidelines exist for hospital-acquired and ventilator-associated pneumonia (HAP/VAP) and community-acquired pneumonia (CAP) to assist with empiric antibiotic selection. For HAP/VAP, 2 to 3 antibiotics with a broad-spectrum of activity are used due to increased prevalence of MDROs in hospitals, whereastreatment of CAP involves more narrow coverage because bacteria that cause this infection typically have fewer antibiotic resistances.2,3 The HAP/VAP guidelines stratify the risk of pneumonia due to the presence of a MDRO acquired during a hospitalization. However, neither the CAP nor HAP/VAP guidelines offer risk-stratification guidance for nonhospitalized patients who develop pneumonia but who may have become colonized with a MDRO during a previous hospitalization or from another exposure to a health care facility.
Health care-associated pneumonia (HCAP) was first described in the 2005 American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA) nosocomial pneumonia guidelines and was associated with criteria intended to aid clinician identification of nonhospitalized patients at risk for MDRO pneumonia, which warranted empiric broad-spectrum antibiotic therapy.2 According to these guidelines, patients were classified as having HCAP if they had been hospitalized for at least 48 hours in the past 90 days, admitted from a nursing home, received recent intravenous antibiotics, had hemodialysis in the past 30 days, had a history of home infusion therapy or wound care, received intravenous chemotherapy, or had a family member with MDRO colonization.
Since publication of the 2005 guidelines, HCAP has been criticized as being a poor predictor of MDRO infection. A 2014 meta-analysis of 24 studies investigated the discriminating ability of HCAP and reported that the specificity and sensitivity for MDRO infections was 71.2% and 53.7%, respectively.3 In 2016, the ATS/IDSA guidelines were updated to remove HCAP due to the risk of antibiotic overprescribing.4
Literature Review
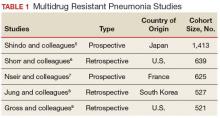
Although criteria previously defining a patient as having HCAP have been shown to be a poor discriminator of MDRO pneumonia as a whole, MDRO infections still pose a threat to nonhospitalized patients who have exposure to the health care system. A literature review was performed to identify independent HCAP risk factors that may increase the risk of MDRO pneumonia infecting a nonhospitalized patient needing empiric broad-spectrum antibiotic therapy. All included studies were prospective or retrospective observational cohort studies that performed logistic regression analyses to assess the association between MDRO isolation and the previously defined HCAP risk factors (Table 1).
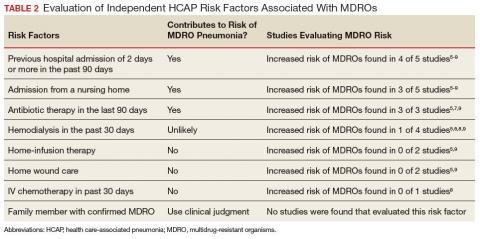
Five studies examined the risk of MDRO infection in patients with a previous hospital admission of 2 days or more in the past 90 days. Shindo and colleagues found a significant increase in MDRO infections by about 2-fold (adjusted odds ratio [AOR], 2.1; 95% confidence interval [CI], 1.2-3.4).5 Shorr and colleagues found a 4-fold increase in likelihood of identifying a MDRO in HCAP (AOR, 4.2; 95% CI, 2.9-6.3).6 Nseir and colleagues and Jung and colleagues found similar results (AOR 3.9, 95% CI 1.7-8.8; AOR 2.7, 95% CI 1.3-5.5, respectively).7,8 Conflicting results were reported by Gross and colleagues who did not find a significant relationship between previous hospitalization and MDRO isolation (AOR 1.2, 95% CI, 0.5-3.2).9
In patients with pneumonia admitted from a nursing home, MDRO infection risk also was evaluated in these 5 studies. Shorr and colleagues, Nseir and colleagues, and Gross and colleagues found significant AORs of 2.7 (95% CI 1.7-4.3), 2.0 (95% CI 1.1-3.7), and 4.2 (95% CI 1.6-11.3), respectively.6,7,9 Shindo and colleagues (AOR 1.1; 95% CI, 0.6-2.0) and Jung and colleagues (AOR 1.9, 95% CI, 0.5-6.9) found this risk factor not significant.5
Receipt of antibiotics within the previous 90 days was assessed in 3 studies. Shindo and colleagues, Nseir and colleagues, and Gross and colleagues all found significant AORs of 2.5 (95% CI 1.2-4.0), 2.3 (95% CI 1.2-4.3), and 2.9 (95% CI 1.1-7.5), respectively.5,7,9 Antibiotic therapy within the previous 90 days is an established risk factor for MDRO pneumonia, and the 2016 ATS/IDSA guidelines consider this a risk factor for HAP and VAP, including pneumonia caused by methicillin resistant Staphylococcus aureus and Pseudomonas aeruginosa.4
The impact of hemodialysis in the previous month on acquisition of MDRO pneumonia was investigated in 4 studies. Shindo and colleagues, Jung and colleagues, and Gross and colleagues concluded that this risk factor was not significantly related to MDRO infection, reporting AORs of 2.2 (95% CI 0.5-9.7), 2.8 (95% CI 0.9-9.2) and 0.7 (95% CI 0.1-5.1), respectively.5,8,9 Shorr and colleagues, however, found a significant AOR of 2.1 (95% CI 1.0-4.3).6
Shindo and colleagues investigated the impact of home infusion therapy on acquisition of pneumonia due to a MDRO and reported a nonsignificant AOR of 0.8 (95% CI 0.4-1.8).5 Gross and colleagues also found a nonsignificant AOR of 0 (P = .1).9 In the Shindo and colleagues study, resistance was found in 107 of 679 patients who did not receive infusion therapy, and 12 of 55 patients who were receiving infusion therapy.5 Gross and colleagues reported that home-infusion therapy was received by 0 of 20 patients with MDRO infection and 4 of the 501 patients without MDRO infection.9
Shindo and colleagues reported that home wound care was not found to be significantly related to MDRO pneumonia as well as did Gross and colleagues: AORs of 3.8 (0.8-18.4) and 1.4 (95% CI 0.5-4.4), respectively.5,9 Jung and colleagues examined IV chemotherapy in the past 30 days, and found this to not significantly impact the odds of MDRO isolation (AOR = 0.62, 95% CI 0.2-1.8).8 No data were available reflecting the risk of a family member with a MDRO.
Limitations
The variables on which logistic regression were performed differed among the studies. Therefore, results cannot be averaged or compared quantitatively, as AORs varied, depending on the variables included. In addition, data were drawn from multiple geographic locations that may impact MDRO prevalence within each patient population. Finally, this review examines the utility of the risk factors formerly included in HCAP. However, other risk factors for MDRO pneumonia outlined by the ATS/IDSA guidelines still should be considered when evaluating patient risk. The 2016 guidelines recommend local incidence of resistant strains be considered when initiating empiric therapy. Review of medical records for previous positive cultures and duration of current hospitalization also should be considered. Although the 2016 ATS/IDSA HAP guidelines are not intended for immunosuppressed patients, this risk factor also may be taken into account.
Conclusion
Review and synthesis of published literature found previous hospital admission (of ≥ 2 days in the past 90 days), admission from a nursing home, and IV antibiotic therapy in the last 90 days to be independent risk factors for identification of MDRO pneumonia in previously nonhospitalized patients (Table 2). Additionally, although no data were found to support this risk factor, existence of an in-home (close contact) source of MDROs would provide ample opportunity for transmission, so evaluation of known exposure to MDROs from contacts should be considered. When choosing empiric antibiotic therapy for patients admitted to the hospital for treatment of pneumonia, consideration of patient history and risk factors that may contribute to infection with a MDRO are recommended.
1. Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462-474.
2. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
3. Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(3):330-339.
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
5. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995.
6. Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care–associated pneumonia. Arch Intern Med. 2008;168(20):2205-2210.
7. Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect. 2010;16(7):902-908.
8. Jung JY, Park MS, Kim YS, et al. Healthcare-associated pneumonia among hospitalized patients in a Korean tertiary hospital. BMC Infectious Diseases. 2011;11:61.
9. Gross AE, Van Schooneveld TC, Olsen KM, et al. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother. 2014;58(9):5262-5268.
Successful treatment of pneumonia depends on timely diagnosis and administration of antibiotics. Multidrug-resistant organisms (MDROs) complicate antibiotic therapies by rendering some antibiotic agents ineffective. Inappropriate initial therapy has been associated with a more than 2-fold increase in the risk of mortality.1 Because culture results are not available immediately, clinicians prescribe antibiotics empirically and must rely on guidelines and knowledge of risk factors associated with MDRO infection to make these selections.
Treatment guidelines exist for hospital-acquired and ventilator-associated pneumonia (HAP/VAP) and community-acquired pneumonia (CAP) to assist with empiric antibiotic selection. For HAP/VAP, 2 to 3 antibiotics with a broad-spectrum of activity are used due to increased prevalence of MDROs in hospitals, whereastreatment of CAP involves more narrow coverage because bacteria that cause this infection typically have fewer antibiotic resistances.2,3 The HAP/VAP guidelines stratify the risk of pneumonia due to the presence of a MDRO acquired during a hospitalization. However, neither the CAP nor HAP/VAP guidelines offer risk-stratification guidance for nonhospitalized patients who develop pneumonia but who may have become colonized with a MDRO during a previous hospitalization or from another exposure to a health care facility.
Health care-associated pneumonia (HCAP) was first described in the 2005 American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA) nosocomial pneumonia guidelines and was associated with criteria intended to aid clinician identification of nonhospitalized patients at risk for MDRO pneumonia, which warranted empiric broad-spectrum antibiotic therapy.2 According to these guidelines, patients were classified as having HCAP if they had been hospitalized for at least 48 hours in the past 90 days, admitted from a nursing home, received recent intravenous antibiotics, had hemodialysis in the past 30 days, had a history of home infusion therapy or wound care, received intravenous chemotherapy, or had a family member with MDRO colonization.
Since publication of the 2005 guidelines, HCAP has been criticized as being a poor predictor of MDRO infection. A 2014 meta-analysis of 24 studies investigated the discriminating ability of HCAP and reported that the specificity and sensitivity for MDRO infections was 71.2% and 53.7%, respectively.3 In 2016, the ATS/IDSA guidelines were updated to remove HCAP due to the risk of antibiotic overprescribing.4
Literature Review
Although criteria previously defining a patient as having HCAP have been shown to be a poor discriminator of MDRO pneumonia as a whole, MDRO infections still pose a threat to nonhospitalized patients who have exposure to the health care system. A literature review was performed to identify independent HCAP risk factors that may increase the risk of MDRO pneumonia infecting a nonhospitalized patient needing empiric broad-spectrum antibiotic therapy. All included studies were prospective or retrospective observational cohort studies that performed logistic regression analyses to assess the association between MDRO isolation and the previously defined HCAP risk factors (Table 1).
Five studies examined the risk of MDRO infection in patients with a previous hospital admission of 2 days or more in the past 90 days. Shindo and colleagues found a significant increase in MDRO infections by about 2-fold (adjusted odds ratio [AOR], 2.1; 95% confidence interval [CI], 1.2-3.4).5 Shorr and colleagues found a 4-fold increase in likelihood of identifying a MDRO in HCAP (AOR, 4.2; 95% CI, 2.9-6.3).6 Nseir and colleagues and Jung and colleagues found similar results (AOR 3.9, 95% CI 1.7-8.8; AOR 2.7, 95% CI 1.3-5.5, respectively).7,8 Conflicting results were reported by Gross and colleagues who did not find a significant relationship between previous hospitalization and MDRO isolation (AOR 1.2, 95% CI, 0.5-3.2).9
In patients with pneumonia admitted from a nursing home, MDRO infection risk also was evaluated in these 5 studies. Shorr and colleagues, Nseir and colleagues, and Gross and colleagues found significant AORs of 2.7 (95% CI 1.7-4.3), 2.0 (95% CI 1.1-3.7), and 4.2 (95% CI 1.6-11.3), respectively.6,7,9 Shindo and colleagues (AOR 1.1; 95% CI, 0.6-2.0) and Jung and colleagues (AOR 1.9, 95% CI, 0.5-6.9) found this risk factor not significant.5
Receipt of antibiotics within the previous 90 days was assessed in 3 studies. Shindo and colleagues, Nseir and colleagues, and Gross and colleagues all found significant AORs of 2.5 (95% CI 1.2-4.0), 2.3 (95% CI 1.2-4.3), and 2.9 (95% CI 1.1-7.5), respectively.5,7,9 Antibiotic therapy within the previous 90 days is an established risk factor for MDRO pneumonia, and the 2016 ATS/IDSA guidelines consider this a risk factor for HAP and VAP, including pneumonia caused by methicillin resistant Staphylococcus aureus and Pseudomonas aeruginosa.4
The impact of hemodialysis in the previous month on acquisition of MDRO pneumonia was investigated in 4 studies. Shindo and colleagues, Jung and colleagues, and Gross and colleagues concluded that this risk factor was not significantly related to MDRO infection, reporting AORs of 2.2 (95% CI 0.5-9.7), 2.8 (95% CI 0.9-9.2) and 0.7 (95% CI 0.1-5.1), respectively.5,8,9 Shorr and colleagues, however, found a significant AOR of 2.1 (95% CI 1.0-4.3).6
Shindo and colleagues investigated the impact of home infusion therapy on acquisition of pneumonia due to a MDRO and reported a nonsignificant AOR of 0.8 (95% CI 0.4-1.8).5 Gross and colleagues also found a nonsignificant AOR of 0 (P = .1).9 In the Shindo and colleagues study, resistance was found in 107 of 679 patients who did not receive infusion therapy, and 12 of 55 patients who were receiving infusion therapy.5 Gross and colleagues reported that home-infusion therapy was received by 0 of 20 patients with MDRO infection and 4 of the 501 patients without MDRO infection.9
Shindo and colleagues reported that home wound care was not found to be significantly related to MDRO pneumonia as well as did Gross and colleagues: AORs of 3.8 (0.8-18.4) and 1.4 (95% CI 0.5-4.4), respectively.5,9 Jung and colleagues examined IV chemotherapy in the past 30 days, and found this to not significantly impact the odds of MDRO isolation (AOR = 0.62, 95% CI 0.2-1.8).8 No data were available reflecting the risk of a family member with a MDRO.
Limitations
The variables on which logistic regression were performed differed among the studies. Therefore, results cannot be averaged or compared quantitatively, as AORs varied, depending on the variables included. In addition, data were drawn from multiple geographic locations that may impact MDRO prevalence within each patient population. Finally, this review examines the utility of the risk factors formerly included in HCAP. However, other risk factors for MDRO pneumonia outlined by the ATS/IDSA guidelines still should be considered when evaluating patient risk. The 2016 guidelines recommend local incidence of resistant strains be considered when initiating empiric therapy. Review of medical records for previous positive cultures and duration of current hospitalization also should be considered. Although the 2016 ATS/IDSA HAP guidelines are not intended for immunosuppressed patients, this risk factor also may be taken into account.
Conclusion
Review and synthesis of published literature found previous hospital admission (of ≥ 2 days in the past 90 days), admission from a nursing home, and IV antibiotic therapy in the last 90 days to be independent risk factors for identification of MDRO pneumonia in previously nonhospitalized patients (Table 2). Additionally, although no data were found to support this risk factor, existence of an in-home (close contact) source of MDROs would provide ample opportunity for transmission, so evaluation of known exposure to MDROs from contacts should be considered. When choosing empiric antibiotic therapy for patients admitted to the hospital for treatment of pneumonia, consideration of patient history and risk factors that may contribute to infection with a MDRO are recommended.
Successful treatment of pneumonia depends on timely diagnosis and administration of antibiotics. Multidrug-resistant organisms (MDROs) complicate antibiotic therapies by rendering some antibiotic agents ineffective. Inappropriate initial therapy has been associated with a more than 2-fold increase in the risk of mortality.1 Because culture results are not available immediately, clinicians prescribe antibiotics empirically and must rely on guidelines and knowledge of risk factors associated with MDRO infection to make these selections.
Treatment guidelines exist for hospital-acquired and ventilator-associated pneumonia (HAP/VAP) and community-acquired pneumonia (CAP) to assist with empiric antibiotic selection. For HAP/VAP, 2 to 3 antibiotics with a broad-spectrum of activity are used due to increased prevalence of MDROs in hospitals, whereastreatment of CAP involves more narrow coverage because bacteria that cause this infection typically have fewer antibiotic resistances.2,3 The HAP/VAP guidelines stratify the risk of pneumonia due to the presence of a MDRO acquired during a hospitalization. However, neither the CAP nor HAP/VAP guidelines offer risk-stratification guidance for nonhospitalized patients who develop pneumonia but who may have become colonized with a MDRO during a previous hospitalization or from another exposure to a health care facility.
Health care-associated pneumonia (HCAP) was first described in the 2005 American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA) nosocomial pneumonia guidelines and was associated with criteria intended to aid clinician identification of nonhospitalized patients at risk for MDRO pneumonia, which warranted empiric broad-spectrum antibiotic therapy.2 According to these guidelines, patients were classified as having HCAP if they had been hospitalized for at least 48 hours in the past 90 days, admitted from a nursing home, received recent intravenous antibiotics, had hemodialysis in the past 30 days, had a history of home infusion therapy or wound care, received intravenous chemotherapy, or had a family member with MDRO colonization.
Since publication of the 2005 guidelines, HCAP has been criticized as being a poor predictor of MDRO infection. A 2014 meta-analysis of 24 studies investigated the discriminating ability of HCAP and reported that the specificity and sensitivity for MDRO infections was 71.2% and 53.7%, respectively.3 In 2016, the ATS/IDSA guidelines were updated to remove HCAP due to the risk of antibiotic overprescribing.4
Literature Review
Although criteria previously defining a patient as having HCAP have been shown to be a poor discriminator of MDRO pneumonia as a whole, MDRO infections still pose a threat to nonhospitalized patients who have exposure to the health care system. A literature review was performed to identify independent HCAP risk factors that may increase the risk of MDRO pneumonia infecting a nonhospitalized patient needing empiric broad-spectrum antibiotic therapy. All included studies were prospective or retrospective observational cohort studies that performed logistic regression analyses to assess the association between MDRO isolation and the previously defined HCAP risk factors (Table 1).
Five studies examined the risk of MDRO infection in patients with a previous hospital admission of 2 days or more in the past 90 days. Shindo and colleagues found a significant increase in MDRO infections by about 2-fold (adjusted odds ratio [AOR], 2.1; 95% confidence interval [CI], 1.2-3.4).5 Shorr and colleagues found a 4-fold increase in likelihood of identifying a MDRO in HCAP (AOR, 4.2; 95% CI, 2.9-6.3).6 Nseir and colleagues and Jung and colleagues found similar results (AOR 3.9, 95% CI 1.7-8.8; AOR 2.7, 95% CI 1.3-5.5, respectively).7,8 Conflicting results were reported by Gross and colleagues who did not find a significant relationship between previous hospitalization and MDRO isolation (AOR 1.2, 95% CI, 0.5-3.2).9
In patients with pneumonia admitted from a nursing home, MDRO infection risk also was evaluated in these 5 studies. Shorr and colleagues, Nseir and colleagues, and Gross and colleagues found significant AORs of 2.7 (95% CI 1.7-4.3), 2.0 (95% CI 1.1-3.7), and 4.2 (95% CI 1.6-11.3), respectively.6,7,9 Shindo and colleagues (AOR 1.1; 95% CI, 0.6-2.0) and Jung and colleagues (AOR 1.9, 95% CI, 0.5-6.9) found this risk factor not significant.5
Receipt of antibiotics within the previous 90 days was assessed in 3 studies. Shindo and colleagues, Nseir and colleagues, and Gross and colleagues all found significant AORs of 2.5 (95% CI 1.2-4.0), 2.3 (95% CI 1.2-4.3), and 2.9 (95% CI 1.1-7.5), respectively.5,7,9 Antibiotic therapy within the previous 90 days is an established risk factor for MDRO pneumonia, and the 2016 ATS/IDSA guidelines consider this a risk factor for HAP and VAP, including pneumonia caused by methicillin resistant Staphylococcus aureus and Pseudomonas aeruginosa.4
The impact of hemodialysis in the previous month on acquisition of MDRO pneumonia was investigated in 4 studies. Shindo and colleagues, Jung and colleagues, and Gross and colleagues concluded that this risk factor was not significantly related to MDRO infection, reporting AORs of 2.2 (95% CI 0.5-9.7), 2.8 (95% CI 0.9-9.2) and 0.7 (95% CI 0.1-5.1), respectively.5,8,9 Shorr and colleagues, however, found a significant AOR of 2.1 (95% CI 1.0-4.3).6
Shindo and colleagues investigated the impact of home infusion therapy on acquisition of pneumonia due to a MDRO and reported a nonsignificant AOR of 0.8 (95% CI 0.4-1.8).5 Gross and colleagues also found a nonsignificant AOR of 0 (P = .1).9 In the Shindo and colleagues study, resistance was found in 107 of 679 patients who did not receive infusion therapy, and 12 of 55 patients who were receiving infusion therapy.5 Gross and colleagues reported that home-infusion therapy was received by 0 of 20 patients with MDRO infection and 4 of the 501 patients without MDRO infection.9
Shindo and colleagues reported that home wound care was not found to be significantly related to MDRO pneumonia as well as did Gross and colleagues: AORs of 3.8 (0.8-18.4) and 1.4 (95% CI 0.5-4.4), respectively.5,9 Jung and colleagues examined IV chemotherapy in the past 30 days, and found this to not significantly impact the odds of MDRO isolation (AOR = 0.62, 95% CI 0.2-1.8).8 No data were available reflecting the risk of a family member with a MDRO.
Limitations
The variables on which logistic regression were performed differed among the studies. Therefore, results cannot be averaged or compared quantitatively, as AORs varied, depending on the variables included. In addition, data were drawn from multiple geographic locations that may impact MDRO prevalence within each patient population. Finally, this review examines the utility of the risk factors formerly included in HCAP. However, other risk factors for MDRO pneumonia outlined by the ATS/IDSA guidelines still should be considered when evaluating patient risk. The 2016 guidelines recommend local incidence of resistant strains be considered when initiating empiric therapy. Review of medical records for previous positive cultures and duration of current hospitalization also should be considered. Although the 2016 ATS/IDSA HAP guidelines are not intended for immunosuppressed patients, this risk factor also may be taken into account.
Conclusion
Review and synthesis of published literature found previous hospital admission (of ≥ 2 days in the past 90 days), admission from a nursing home, and IV antibiotic therapy in the last 90 days to be independent risk factors for identification of MDRO pneumonia in previously nonhospitalized patients (Table 2). Additionally, although no data were found to support this risk factor, existence of an in-home (close contact) source of MDROs would provide ample opportunity for transmission, so evaluation of known exposure to MDROs from contacts should be considered. When choosing empiric antibiotic therapy for patients admitted to the hospital for treatment of pneumonia, consideration of patient history and risk factors that may contribute to infection with a MDRO are recommended.
1. Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462-474.
2. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
3. Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(3):330-339.
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
5. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995.
6. Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care–associated pneumonia. Arch Intern Med. 2008;168(20):2205-2210.
7. Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect. 2010;16(7):902-908.
8. Jung JY, Park MS, Kim YS, et al. Healthcare-associated pneumonia among hospitalized patients in a Korean tertiary hospital. BMC Infectious Diseases. 2011;11:61.
9. Gross AE, Van Schooneveld TC, Olsen KM, et al. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother. 2014;58(9):5262-5268.
1. Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462-474.
2. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
3. Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(3):330-339.
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
5. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995.
6. Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care–associated pneumonia. Arch Intern Med. 2008;168(20):2205-2210.
7. Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect. 2010;16(7):902-908.
8. Jung JY, Park MS, Kim YS, et al. Healthcare-associated pneumonia among hospitalized patients in a Korean tertiary hospital. BMC Infectious Diseases. 2011;11:61.
9. Gross AE, Van Schooneveld TC, Olsen KM, et al. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother. 2014;58(9):5262-5268.