User login
UPDATE ON PELVIC FLOOR DYSFUNCTION
Vulvar Pain Syndromes 3-Part Series
- Making the correct diagnosis
(September 2011) - A bounty of treatments-but not all of them are proven
(October 2011) - Provoked vestibulodynia
(Coming in November 2011)
Chronic pelvic pain: 11 critical questions about causes and care
Fred M. Howard, MD (August 2009)
Vague symptoms. Unexpected flares. Inconsistent manifestations. These characteristics can make diagnosis and treatment of chronic pelvic pain frustrating for both patient and physician. Most patients undergo myriad tests and studies to uncover the source of their pain—but a targeted pelvic exam may be all that is necessary to identify a prevalent but commonly overlooked cause of pelvic pain. Levator myalgia, myofascial pelvic pain syndrome, and pelvic floor spasm are all terms that describe a condition that may affect as many as 78% of women who are given a diagnosis of chronic pelvic pain.1 This syndrome may be represented by an array of symptoms, including pelvic pressure, dyspareunia, rectal discomfort, and irritative urinary symptoms such as spasms, frequency, and urgency. It is characterized by the presence of tight, band-like pelvic muscles that reproduce the patient’s pain when palpated.2
Diagnosis of this syndrome often surprises the patient. Although the concept of a muscle spasm is not foreign, the location is unexpected. Patients and physicians alike may forget that there is a large complex of muscles that completely lines the pelvic girdle. To complicate matters, the patient often associates the onset of her symptoms with an acute event such as a “bad” urinary tract infection or pelvic or vaginal surgery, which may divert attention from the musculature. Although a muscle spasm may be the cause of the patient’s pain, it’s important to realize that an underlying process may have triggered the original spasm. To provide effective treatment of pain, therefore, you must identify the fundamental cause, assuming that it is reversible, rather than focus exclusively on symptoms.
Although there are many therapeutic options for levator myalgia, an appraisal of the extensive literature on these medications is beyond the scope of this article. Rather, we will review alternative treatment modalities and summarize the results of five trials that explored physical therapy, trigger-point or chemodenervation injection, and neuromodulation (TABLE).
Weighing the nonpharmaceutical options for treatment
of myofascial pelvic pain
| Treatment | Pros | Cons |
|---|---|---|
| Physical therapy | Minimally invasive Moderate long-term success | Requires highly specialized therapist |
| Trigger-point injection | Minimally invasive Performed in clinic Immediate short-term success | Optimal injectable agent is unknown Botulinum toxin A lacks FDA approval for this indication Limited information on adverse events and long-term efficacy |
| Percutaneous tibial nerve stimulation | Minimally invasive Performed in clinic | Requires numerous office visits for treatment Lacks FDA approval for this indication Limited information on long-term efficacy |
| Sacral neuromodulation | Moderately invasive Permanent implant | Requires implantation in operating room Lacks FDA approval for this indication Limited information on long-term efficacy |
Pelvic myofascial therapy offers relief—but qualified therapists may be scarce
FitzGerald MP, Anderson RU, Potts J, et al; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urology. 2009;182(2):570–580.
Physical therapy of the pelvic floor—otherwise known as pelvic myofascial therapy—requires a therapist who is highly trained and specialized in this technique. It is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers (FIGURE 1).
This pilot study by the Urological Pelvic Pain Collaborative Research Network evaluated the ability of patients to adhere to pelvic myofascial therapy, the response of their pain to therapy, and adverse events associated with manual therapy. It found that patients were willing to undergo the therapy, despite the invasive nature of the maneuvers, because it was significantly effective.
Details of the study
Patients (both men and women) were randomized to myofascial physical therapy or global therapeutic massage. Myofascial therapy consisted of internal or vaginal manipulation of the trigger-point muscle bundles and tissues of the pelvic floor. It also focused on muscles of the hip girdle and abdomen. The comparison group underwent traditional Western full-body massage. In both groups, treatment lasted 1 hour every week, and participants agreed to 10 full treatments.
Patients were eligible for the study if they experienced pelvic pain, urinary frequency, or bladder discomfort in the previous 6 months. In addition, an examiner must have been able to elicit tenderness upon palpation of the pelvic floor during examination. Patients were excluded if they showed signs of urinary tract infection or dysmenorrhea.
A total of 47 patients were randomized—24 to global massage and 23 to myofascial physical therapy. Overall, the myofascial group experienced a significantly higher rate of improvement in the global response at 12 weeks than did patients in the global-massage group (57% vs 21%; P=.03). Patients were willing to engage in myofascial pelvic therapy, and adverse events were minor.
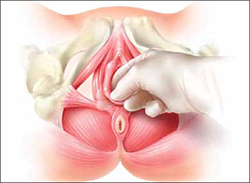
FIGURE 1 Transvaginal myofascial therapy
Physical therapy of the pelvic floor is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers.
Need for specialized training may limit number of therapists
The randomized controlled study design renders these findings fairly reliable. Therapists were unmasked and aware of the treatment arms but were trained to make the different therapy sessions appear as similar as possible.
Although investigators were enthusiastic about their initial findings, additional studies are needed to validate the results. Moreover, these findings may be difficult to generalize because women who volunteer to participate in such a study may differ from the general population.
Nevertheless, patients who suffer from chronic pelvic pain may take heart that there is a nonpharmaceutical alternative to manage their symptoms, although availability is likely limited in many areas. Given the nature of the physical therapy required for this particular location of myofascial pain, specialized training is necessary for therapists. Despite motivated patients and well-informed providers, it may be difficult to find specialized therapists within local vicinities. Referrals to centers where this type of therapy is offered may be necessary.
Pelvic myofascial therapy is an effective and acceptable intervention for the treatment of levator myalgia.
The ideal agent for trigger-point injections remains a mystery
Langford CF, Udvari Nagy S, Ghoniem G M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62.
Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923.
Trigger points are discrete, tender areas within a ridge of contracted muscle. These points may cause focal pain or referred pain upon irritation of the muscle.2 Trigger-point injection therapy aims to anesthetize or relax these points by infiltrating the muscle with medications.
These two studies evaluated the value of trigger-point injections in the treatment of pelvic myofascial pain; they found that the injections provide relief, although the mechanism of action and the ideal agent remain to be determined.
Langford et al: Details of the study
In this prospective study, 18 women who had pelvic pain of at least 6 months’ duration and confirmed trigger points on examination underwent transvaginal injection of a solution of bupivacaine, lidocaine, and triamcinolone. They were assessed by questionnaire at baseline and 3 months after injection. Assessment included a visual analog scale for pain severity. Investigators defined success as a decrease in pain of 50% or more and global-satisfaction and global-cure visual scores of 60% or higher.
Thirteen of the 18 women (72.2%) improved after their first injection, with six women reporting a complete absence of pain. Overall, women reported significant decreases in pain and increases in the rates of satisfaction and cure, meeting the definition of success at 3 months after the injection.
Among the theories proposed to explain the mechanism of action of trigger-point injections are:
- disruption of reflex arcs within skeletal muscle
- release of endorphins
- mechanical changes in abnormally contracted muscle fibers.
This last theory highlights one of the limitations of this study—lack of a placebo arm. Could it be possible that the injection of any fluid produces the same effect?
This study was not designed to investigate the causal relationship between the injection of a particular solution and pain relief, but it does highlight the need for studies to clarify the mechanism of action, including use of a placebo. It also prompts questions about the duration of effect after a single injection.
Goal of chemodenervation is blocking of muscle activity
Botulinum toxin type A (Botox) blocks the release of acetylcholine from presynaptic neurons. The release of acetylcholine stimulates muscle contractions; therefore, blockage of its release reduces muscle activity. This type of chemodenervation has found widespread use, and botulinum toxin A now has approval from the Food and Drug Administration (FDA) for treatment of chronic migraine, limb spasticity, cervical dystonia, strabismus, hyperhidrosis, and facial cosmesis.3 Although it is not approved for pelvic floor levator spasm, its success in treating other myotonic disorders suggests that its application may be relevant.
Abbott et al: Details of the study
Abbott and colleagues performed a double-blind, randomized, controlled trial to compare injection of botulinum toxin A with injection of saline. They measured changes in the pain scale, quality of life, and vaginal pressure.
Women were eligible for the study if they had subjectively reported pelvic pain of more than 2 years’ duration and objective evidence of trigger points (on examination) and elevated vaginal resting pressure (by vaginal manometry). Neither the clinical research staff nor the patient knew the contents of the injections, but all women received a total of four—two at sites in the puborectalis muscle and two in the pubococcygeus muscle.
After periodic assessment by questionnaire and examination through 6 months after injection, no differences were found in the pain score or resting vaginal pressure between the group of women who received botulinum toxin A and the group who received placebo. However, each group experienced a significant reduction in pain and vaginal pressure, compared with baseline. And both groups reported improved quality of life, compared with baseline. Neither group reported voiding dysfunction.
These two studies support the use of trigger-point injection into pelvic floor muscles to reduce pelvic myofascial pain. The findings of Abbott and colleagues, in particular, suggest that the substance that is injected may not be as important as the actual needling of the muscle. Larger studies and comparisons between placebo, botulinum toxin A, and anesthetic solutions are needed to elucidate the therapeutic benefit of these particular medications.
Neuromodulation shows promise as treatment for pelvic myofascial pain
van Balken MR, Vandoninck V, Messelink, BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43(2):158–163.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodriguez LV. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–557.
Neuromodulation is the science of using electrical impulses to alter neuronal activities. The exact mechanisms of action are unclear, but the technology has been utilized to control symptoms of overactive bladder and urinary retention caused by poor relaxation of the urethral and pelvic floor muscles. While studying the effects of sacral nerve root neuromodulation on the bladder, investigators noted improvements in other symptoms, such as pelvic pain.
Neuromodulation of the sacral nerve roots may be achieved by direct conduction of electrical impulses from a lead implanted in the sacrum (sacral neuromodulation) or by the retrograde conduction of these impulses through the posterior tibial nerve (percutaneous tibial nerve stimulation, or PTNS) (FIGURE 2). The tibial nerve arises from sacral nerves L5 to S3 and is one of the larger branches of the sciatic nerve.
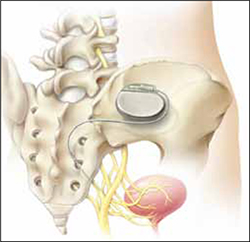
FIGURE 2 InterStim therapy
Stimulation of the sacral nerve has been used successfully to manage overactive bladder and urinary retention and may prove useful in the treatment of pelvic myofascial pain.
Van Balken et al: Details of the study
In this prospective observational study, 33 patients (both male and female) who had chronic pelvic pain by history and examination were treated with weekly, 30-minute outpatient sessions of PTNS for 12 weeks. Participants were asked to provide baseline pain scores and keep a diary of their pain. Quality-of-life questionnaires were also administered at baseline and at 12 weeks.
Investigators considered both subjective and objective success in their outcomes. If a patient elected to continue therapy, he or she was classified as a subjective success. Objective success required a decrease of at least 50% in the pain score. At the end of 12 weeks, although 33 patients (42%) wanted to continue therapy, only seven (21%) met the definition for objective success. Of those seven, six elected to continue therapy.
This study sheds light on a treatment modality that has not been studied adequately for the indication of pelvic pain but that may be promising in patients who have levator myalgia. Limitations of this study include the lack of a placebo arm, short-term outcome, and lack of localization of pain. Furthermore, although PTNS has FDA approval for treatment of urinary urgency, frequency, and urge incontinence, it is not approved for the treatment of pelvic pain. These preliminary findings demonstrate potential but, as with any new indication, long-term comparative studies are needed.
Zabihi et al: Details of the study
Patients in this retrospective study had a diagnosis of interstitial cystitis or chronic pelvic pain. Pelvic myofascial pain and trigger points were not required for eligibility. Thirty patients (21 women and nine men) had temporary placement of a lead containing four small electrodes along the S2 to S4 sacral nerve roots on both sides of the sacrum. They were then followed for a trial period of 2 to 4 weeks. To qualify for the final stage of the study, in which the leads were connected internally to a generator implanted in the buttocks, patients had to report improvement of at least 50% in their symptoms. If their improvement did not meet that threshold, the leads were removed.
Twenty-three patients (77%) met the criteria for permanent implantation. Of these patients, 42% reported improvement of more than 50% at 6 postoperative months. Quality-of-life scores also improved significantly.
Sacral neuromodulation is not FDA-approved for the treatment of chronic pelvic pain; further studies are needed before it can be recommended for this indication.
Neither of these studies required objective evidence of myofascial pain for inclusion. Therefore, although the benefits they demonstrated may be theorized to extend to the relief of myofascial pain, this fact cannot be corroborated.
We want to hear from you! Tell us what you think.
1. Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413-418.
2. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653-660.
3. Allergan, Inc. Medication Guide: BOTOX. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM176360.pdf. Published October 2010. Accessed August 30, 2011.
Vulvar Pain Syndromes 3-Part Series
- Making the correct diagnosis
(September 2011) - A bounty of treatments-but not all of them are proven
(October 2011) - Provoked vestibulodynia
(Coming in November 2011)
Chronic pelvic pain: 11 critical questions about causes and care
Fred M. Howard, MD (August 2009)
Vague symptoms. Unexpected flares. Inconsistent manifestations. These characteristics can make diagnosis and treatment of chronic pelvic pain frustrating for both patient and physician. Most patients undergo myriad tests and studies to uncover the source of their pain—but a targeted pelvic exam may be all that is necessary to identify a prevalent but commonly overlooked cause of pelvic pain. Levator myalgia, myofascial pelvic pain syndrome, and pelvic floor spasm are all terms that describe a condition that may affect as many as 78% of women who are given a diagnosis of chronic pelvic pain.1 This syndrome may be represented by an array of symptoms, including pelvic pressure, dyspareunia, rectal discomfort, and irritative urinary symptoms such as spasms, frequency, and urgency. It is characterized by the presence of tight, band-like pelvic muscles that reproduce the patient’s pain when palpated.2
Diagnosis of this syndrome often surprises the patient. Although the concept of a muscle spasm is not foreign, the location is unexpected. Patients and physicians alike may forget that there is a large complex of muscles that completely lines the pelvic girdle. To complicate matters, the patient often associates the onset of her symptoms with an acute event such as a “bad” urinary tract infection or pelvic or vaginal surgery, which may divert attention from the musculature. Although a muscle spasm may be the cause of the patient’s pain, it’s important to realize that an underlying process may have triggered the original spasm. To provide effective treatment of pain, therefore, you must identify the fundamental cause, assuming that it is reversible, rather than focus exclusively on symptoms.
Although there are many therapeutic options for levator myalgia, an appraisal of the extensive literature on these medications is beyond the scope of this article. Rather, we will review alternative treatment modalities and summarize the results of five trials that explored physical therapy, trigger-point or chemodenervation injection, and neuromodulation (TABLE).
Weighing the nonpharmaceutical options for treatment
of myofascial pelvic pain
| Treatment | Pros | Cons |
|---|---|---|
| Physical therapy | Minimally invasive Moderate long-term success | Requires highly specialized therapist |
| Trigger-point injection | Minimally invasive Performed in clinic Immediate short-term success | Optimal injectable agent is unknown Botulinum toxin A lacks FDA approval for this indication Limited information on adverse events and long-term efficacy |
| Percutaneous tibial nerve stimulation | Minimally invasive Performed in clinic | Requires numerous office visits for treatment Lacks FDA approval for this indication Limited information on long-term efficacy |
| Sacral neuromodulation | Moderately invasive Permanent implant | Requires implantation in operating room Lacks FDA approval for this indication Limited information on long-term efficacy |
Pelvic myofascial therapy offers relief—but qualified therapists may be scarce
FitzGerald MP, Anderson RU, Potts J, et al; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urology. 2009;182(2):570–580.
Physical therapy of the pelvic floor—otherwise known as pelvic myofascial therapy—requires a therapist who is highly trained and specialized in this technique. It is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers (FIGURE 1).
This pilot study by the Urological Pelvic Pain Collaborative Research Network evaluated the ability of patients to adhere to pelvic myofascial therapy, the response of their pain to therapy, and adverse events associated with manual therapy. It found that patients were willing to undergo the therapy, despite the invasive nature of the maneuvers, because it was significantly effective.
Details of the study
Patients (both men and women) were randomized to myofascial physical therapy or global therapeutic massage. Myofascial therapy consisted of internal or vaginal manipulation of the trigger-point muscle bundles and tissues of the pelvic floor. It also focused on muscles of the hip girdle and abdomen. The comparison group underwent traditional Western full-body massage. In both groups, treatment lasted 1 hour every week, and participants agreed to 10 full treatments.
Patients were eligible for the study if they experienced pelvic pain, urinary frequency, or bladder discomfort in the previous 6 months. In addition, an examiner must have been able to elicit tenderness upon palpation of the pelvic floor during examination. Patients were excluded if they showed signs of urinary tract infection or dysmenorrhea.
A total of 47 patients were randomized—24 to global massage and 23 to myofascial physical therapy. Overall, the myofascial group experienced a significantly higher rate of improvement in the global response at 12 weeks than did patients in the global-massage group (57% vs 21%; P=.03). Patients were willing to engage in myofascial pelvic therapy, and adverse events were minor.

FIGURE 1 Transvaginal myofascial therapy
Physical therapy of the pelvic floor is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers.
Need for specialized training may limit number of therapists
The randomized controlled study design renders these findings fairly reliable. Therapists were unmasked and aware of the treatment arms but were trained to make the different therapy sessions appear as similar as possible.
Although investigators were enthusiastic about their initial findings, additional studies are needed to validate the results. Moreover, these findings may be difficult to generalize because women who volunteer to participate in such a study may differ from the general population.
Nevertheless, patients who suffer from chronic pelvic pain may take heart that there is a nonpharmaceutical alternative to manage their symptoms, although availability is likely limited in many areas. Given the nature of the physical therapy required for this particular location of myofascial pain, specialized training is necessary for therapists. Despite motivated patients and well-informed providers, it may be difficult to find specialized therapists within local vicinities. Referrals to centers where this type of therapy is offered may be necessary.
Pelvic myofascial therapy is an effective and acceptable intervention for the treatment of levator myalgia.
The ideal agent for trigger-point injections remains a mystery
Langford CF, Udvari Nagy S, Ghoniem G M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62.
Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923.
Trigger points are discrete, tender areas within a ridge of contracted muscle. These points may cause focal pain or referred pain upon irritation of the muscle.2 Trigger-point injection therapy aims to anesthetize or relax these points by infiltrating the muscle with medications.
These two studies evaluated the value of trigger-point injections in the treatment of pelvic myofascial pain; they found that the injections provide relief, although the mechanism of action and the ideal agent remain to be determined.
Langford et al: Details of the study
In this prospective study, 18 women who had pelvic pain of at least 6 months’ duration and confirmed trigger points on examination underwent transvaginal injection of a solution of bupivacaine, lidocaine, and triamcinolone. They were assessed by questionnaire at baseline and 3 months after injection. Assessment included a visual analog scale for pain severity. Investigators defined success as a decrease in pain of 50% or more and global-satisfaction and global-cure visual scores of 60% or higher.
Thirteen of the 18 women (72.2%) improved after their first injection, with six women reporting a complete absence of pain. Overall, women reported significant decreases in pain and increases in the rates of satisfaction and cure, meeting the definition of success at 3 months after the injection.
Among the theories proposed to explain the mechanism of action of trigger-point injections are:
- disruption of reflex arcs within skeletal muscle
- release of endorphins
- mechanical changes in abnormally contracted muscle fibers.
This last theory highlights one of the limitations of this study—lack of a placebo arm. Could it be possible that the injection of any fluid produces the same effect?
This study was not designed to investigate the causal relationship between the injection of a particular solution and pain relief, but it does highlight the need for studies to clarify the mechanism of action, including use of a placebo. It also prompts questions about the duration of effect after a single injection.
Goal of chemodenervation is blocking of muscle activity
Botulinum toxin type A (Botox) blocks the release of acetylcholine from presynaptic neurons. The release of acetylcholine stimulates muscle contractions; therefore, blockage of its release reduces muscle activity. This type of chemodenervation has found widespread use, and botulinum toxin A now has approval from the Food and Drug Administration (FDA) for treatment of chronic migraine, limb spasticity, cervical dystonia, strabismus, hyperhidrosis, and facial cosmesis.3 Although it is not approved for pelvic floor levator spasm, its success in treating other myotonic disorders suggests that its application may be relevant.
Abbott et al: Details of the study
Abbott and colleagues performed a double-blind, randomized, controlled trial to compare injection of botulinum toxin A with injection of saline. They measured changes in the pain scale, quality of life, and vaginal pressure.
Women were eligible for the study if they had subjectively reported pelvic pain of more than 2 years’ duration and objective evidence of trigger points (on examination) and elevated vaginal resting pressure (by vaginal manometry). Neither the clinical research staff nor the patient knew the contents of the injections, but all women received a total of four—two at sites in the puborectalis muscle and two in the pubococcygeus muscle.
After periodic assessment by questionnaire and examination through 6 months after injection, no differences were found in the pain score or resting vaginal pressure between the group of women who received botulinum toxin A and the group who received placebo. However, each group experienced a significant reduction in pain and vaginal pressure, compared with baseline. And both groups reported improved quality of life, compared with baseline. Neither group reported voiding dysfunction.
These two studies support the use of trigger-point injection into pelvic floor muscles to reduce pelvic myofascial pain. The findings of Abbott and colleagues, in particular, suggest that the substance that is injected may not be as important as the actual needling of the muscle. Larger studies and comparisons between placebo, botulinum toxin A, and anesthetic solutions are needed to elucidate the therapeutic benefit of these particular medications.
Neuromodulation shows promise as treatment for pelvic myofascial pain
van Balken MR, Vandoninck V, Messelink, BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43(2):158–163.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodriguez LV. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–557.
Neuromodulation is the science of using electrical impulses to alter neuronal activities. The exact mechanisms of action are unclear, but the technology has been utilized to control symptoms of overactive bladder and urinary retention caused by poor relaxation of the urethral and pelvic floor muscles. While studying the effects of sacral nerve root neuromodulation on the bladder, investigators noted improvements in other symptoms, such as pelvic pain.
Neuromodulation of the sacral nerve roots may be achieved by direct conduction of electrical impulses from a lead implanted in the sacrum (sacral neuromodulation) or by the retrograde conduction of these impulses through the posterior tibial nerve (percutaneous tibial nerve stimulation, or PTNS) (FIGURE 2). The tibial nerve arises from sacral nerves L5 to S3 and is one of the larger branches of the sciatic nerve.

FIGURE 2 InterStim therapy
Stimulation of the sacral nerve has been used successfully to manage overactive bladder and urinary retention and may prove useful in the treatment of pelvic myofascial pain.
Van Balken et al: Details of the study
In this prospective observational study, 33 patients (both male and female) who had chronic pelvic pain by history and examination were treated with weekly, 30-minute outpatient sessions of PTNS for 12 weeks. Participants were asked to provide baseline pain scores and keep a diary of their pain. Quality-of-life questionnaires were also administered at baseline and at 12 weeks.
Investigators considered both subjective and objective success in their outcomes. If a patient elected to continue therapy, he or she was classified as a subjective success. Objective success required a decrease of at least 50% in the pain score. At the end of 12 weeks, although 33 patients (42%) wanted to continue therapy, only seven (21%) met the definition for objective success. Of those seven, six elected to continue therapy.
This study sheds light on a treatment modality that has not been studied adequately for the indication of pelvic pain but that may be promising in patients who have levator myalgia. Limitations of this study include the lack of a placebo arm, short-term outcome, and lack of localization of pain. Furthermore, although PTNS has FDA approval for treatment of urinary urgency, frequency, and urge incontinence, it is not approved for the treatment of pelvic pain. These preliminary findings demonstrate potential but, as with any new indication, long-term comparative studies are needed.
Zabihi et al: Details of the study
Patients in this retrospective study had a diagnosis of interstitial cystitis or chronic pelvic pain. Pelvic myofascial pain and trigger points were not required for eligibility. Thirty patients (21 women and nine men) had temporary placement of a lead containing four small electrodes along the S2 to S4 sacral nerve roots on both sides of the sacrum. They were then followed for a trial period of 2 to 4 weeks. To qualify for the final stage of the study, in which the leads were connected internally to a generator implanted in the buttocks, patients had to report improvement of at least 50% in their symptoms. If their improvement did not meet that threshold, the leads were removed.
Twenty-three patients (77%) met the criteria for permanent implantation. Of these patients, 42% reported improvement of more than 50% at 6 postoperative months. Quality-of-life scores also improved significantly.
Sacral neuromodulation is not FDA-approved for the treatment of chronic pelvic pain; further studies are needed before it can be recommended for this indication.
Neither of these studies required objective evidence of myofascial pain for inclusion. Therefore, although the benefits they demonstrated may be theorized to extend to the relief of myofascial pain, this fact cannot be corroborated.
We want to hear from you! Tell us what you think.
Vulvar Pain Syndromes 3-Part Series
- Making the correct diagnosis
(September 2011) - A bounty of treatments-but not all of them are proven
(October 2011) - Provoked vestibulodynia
(Coming in November 2011)
Chronic pelvic pain: 11 critical questions about causes and care
Fred M. Howard, MD (August 2009)
Vague symptoms. Unexpected flares. Inconsistent manifestations. These characteristics can make diagnosis and treatment of chronic pelvic pain frustrating for both patient and physician. Most patients undergo myriad tests and studies to uncover the source of their pain—but a targeted pelvic exam may be all that is necessary to identify a prevalent but commonly overlooked cause of pelvic pain. Levator myalgia, myofascial pelvic pain syndrome, and pelvic floor spasm are all terms that describe a condition that may affect as many as 78% of women who are given a diagnosis of chronic pelvic pain.1 This syndrome may be represented by an array of symptoms, including pelvic pressure, dyspareunia, rectal discomfort, and irritative urinary symptoms such as spasms, frequency, and urgency. It is characterized by the presence of tight, band-like pelvic muscles that reproduce the patient’s pain when palpated.2
Diagnosis of this syndrome often surprises the patient. Although the concept of a muscle spasm is not foreign, the location is unexpected. Patients and physicians alike may forget that there is a large complex of muscles that completely lines the pelvic girdle. To complicate matters, the patient often associates the onset of her symptoms with an acute event such as a “bad” urinary tract infection or pelvic or vaginal surgery, which may divert attention from the musculature. Although a muscle spasm may be the cause of the patient’s pain, it’s important to realize that an underlying process may have triggered the original spasm. To provide effective treatment of pain, therefore, you must identify the fundamental cause, assuming that it is reversible, rather than focus exclusively on symptoms.
Although there are many therapeutic options for levator myalgia, an appraisal of the extensive literature on these medications is beyond the scope of this article. Rather, we will review alternative treatment modalities and summarize the results of five trials that explored physical therapy, trigger-point or chemodenervation injection, and neuromodulation (TABLE).
Weighing the nonpharmaceutical options for treatment
of myofascial pelvic pain
| Treatment | Pros | Cons |
|---|---|---|
| Physical therapy | Minimally invasive Moderate long-term success | Requires highly specialized therapist |
| Trigger-point injection | Minimally invasive Performed in clinic Immediate short-term success | Optimal injectable agent is unknown Botulinum toxin A lacks FDA approval for this indication Limited information on adverse events and long-term efficacy |
| Percutaneous tibial nerve stimulation | Minimally invasive Performed in clinic | Requires numerous office visits for treatment Lacks FDA approval for this indication Limited information on long-term efficacy |
| Sacral neuromodulation | Moderately invasive Permanent implant | Requires implantation in operating room Lacks FDA approval for this indication Limited information on long-term efficacy |
Pelvic myofascial therapy offers relief—but qualified therapists may be scarce
FitzGerald MP, Anderson RU, Potts J, et al; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urology. 2009;182(2):570–580.
Physical therapy of the pelvic floor—otherwise known as pelvic myofascial therapy—requires a therapist who is highly trained and specialized in this technique. It is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers (FIGURE 1).
This pilot study by the Urological Pelvic Pain Collaborative Research Network evaluated the ability of patients to adhere to pelvic myofascial therapy, the response of their pain to therapy, and adverse events associated with manual therapy. It found that patients were willing to undergo the therapy, despite the invasive nature of the maneuvers, because it was significantly effective.
Details of the study
Patients (both men and women) were randomized to myofascial physical therapy or global therapeutic massage. Myofascial therapy consisted of internal or vaginal manipulation of the trigger-point muscle bundles and tissues of the pelvic floor. It also focused on muscles of the hip girdle and abdomen. The comparison group underwent traditional Western full-body massage. In both groups, treatment lasted 1 hour every week, and participants agreed to 10 full treatments.
Patients were eligible for the study if they experienced pelvic pain, urinary frequency, or bladder discomfort in the previous 6 months. In addition, an examiner must have been able to elicit tenderness upon palpation of the pelvic floor during examination. Patients were excluded if they showed signs of urinary tract infection or dysmenorrhea.
A total of 47 patients were randomized—24 to global massage and 23 to myofascial physical therapy. Overall, the myofascial group experienced a significantly higher rate of improvement in the global response at 12 weeks than did patients in the global-massage group (57% vs 21%; P=.03). Patients were willing to engage in myofascial pelvic therapy, and adverse events were minor.

FIGURE 1 Transvaginal myofascial therapy
Physical therapy of the pelvic floor is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers.
Need for specialized training may limit number of therapists
The randomized controlled study design renders these findings fairly reliable. Therapists were unmasked and aware of the treatment arms but were trained to make the different therapy sessions appear as similar as possible.
Although investigators were enthusiastic about their initial findings, additional studies are needed to validate the results. Moreover, these findings may be difficult to generalize because women who volunteer to participate in such a study may differ from the general population.
Nevertheless, patients who suffer from chronic pelvic pain may take heart that there is a nonpharmaceutical alternative to manage their symptoms, although availability is likely limited in many areas. Given the nature of the physical therapy required for this particular location of myofascial pain, specialized training is necessary for therapists. Despite motivated patients and well-informed providers, it may be difficult to find specialized therapists within local vicinities. Referrals to centers where this type of therapy is offered may be necessary.
Pelvic myofascial therapy is an effective and acceptable intervention for the treatment of levator myalgia.
The ideal agent for trigger-point injections remains a mystery
Langford CF, Udvari Nagy S, Ghoniem G M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62.
Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923.
Trigger points are discrete, tender areas within a ridge of contracted muscle. These points may cause focal pain or referred pain upon irritation of the muscle.2 Trigger-point injection therapy aims to anesthetize or relax these points by infiltrating the muscle with medications.
These two studies evaluated the value of trigger-point injections in the treatment of pelvic myofascial pain; they found that the injections provide relief, although the mechanism of action and the ideal agent remain to be determined.
Langford et al: Details of the study
In this prospective study, 18 women who had pelvic pain of at least 6 months’ duration and confirmed trigger points on examination underwent transvaginal injection of a solution of bupivacaine, lidocaine, and triamcinolone. They were assessed by questionnaire at baseline and 3 months after injection. Assessment included a visual analog scale for pain severity. Investigators defined success as a decrease in pain of 50% or more and global-satisfaction and global-cure visual scores of 60% or higher.
Thirteen of the 18 women (72.2%) improved after their first injection, with six women reporting a complete absence of pain. Overall, women reported significant decreases in pain and increases in the rates of satisfaction and cure, meeting the definition of success at 3 months after the injection.
Among the theories proposed to explain the mechanism of action of trigger-point injections are:
- disruption of reflex arcs within skeletal muscle
- release of endorphins
- mechanical changes in abnormally contracted muscle fibers.
This last theory highlights one of the limitations of this study—lack of a placebo arm. Could it be possible that the injection of any fluid produces the same effect?
This study was not designed to investigate the causal relationship between the injection of a particular solution and pain relief, but it does highlight the need for studies to clarify the mechanism of action, including use of a placebo. It also prompts questions about the duration of effect after a single injection.
Goal of chemodenervation is blocking of muscle activity
Botulinum toxin type A (Botox) blocks the release of acetylcholine from presynaptic neurons. The release of acetylcholine stimulates muscle contractions; therefore, blockage of its release reduces muscle activity. This type of chemodenervation has found widespread use, and botulinum toxin A now has approval from the Food and Drug Administration (FDA) for treatment of chronic migraine, limb spasticity, cervical dystonia, strabismus, hyperhidrosis, and facial cosmesis.3 Although it is not approved for pelvic floor levator spasm, its success in treating other myotonic disorders suggests that its application may be relevant.
Abbott et al: Details of the study
Abbott and colleagues performed a double-blind, randomized, controlled trial to compare injection of botulinum toxin A with injection of saline. They measured changes in the pain scale, quality of life, and vaginal pressure.
Women were eligible for the study if they had subjectively reported pelvic pain of more than 2 years’ duration and objective evidence of trigger points (on examination) and elevated vaginal resting pressure (by vaginal manometry). Neither the clinical research staff nor the patient knew the contents of the injections, but all women received a total of four—two at sites in the puborectalis muscle and two in the pubococcygeus muscle.
After periodic assessment by questionnaire and examination through 6 months after injection, no differences were found in the pain score or resting vaginal pressure between the group of women who received botulinum toxin A and the group who received placebo. However, each group experienced a significant reduction in pain and vaginal pressure, compared with baseline. And both groups reported improved quality of life, compared with baseline. Neither group reported voiding dysfunction.
These two studies support the use of trigger-point injection into pelvic floor muscles to reduce pelvic myofascial pain. The findings of Abbott and colleagues, in particular, suggest that the substance that is injected may not be as important as the actual needling of the muscle. Larger studies and comparisons between placebo, botulinum toxin A, and anesthetic solutions are needed to elucidate the therapeutic benefit of these particular medications.
Neuromodulation shows promise as treatment for pelvic myofascial pain
van Balken MR, Vandoninck V, Messelink, BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43(2):158–163.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodriguez LV. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–557.
Neuromodulation is the science of using electrical impulses to alter neuronal activities. The exact mechanisms of action are unclear, but the technology has been utilized to control symptoms of overactive bladder and urinary retention caused by poor relaxation of the urethral and pelvic floor muscles. While studying the effects of sacral nerve root neuromodulation on the bladder, investigators noted improvements in other symptoms, such as pelvic pain.
Neuromodulation of the sacral nerve roots may be achieved by direct conduction of electrical impulses from a lead implanted in the sacrum (sacral neuromodulation) or by the retrograde conduction of these impulses through the posterior tibial nerve (percutaneous tibial nerve stimulation, or PTNS) (FIGURE 2). The tibial nerve arises from sacral nerves L5 to S3 and is one of the larger branches of the sciatic nerve.

FIGURE 2 InterStim therapy
Stimulation of the sacral nerve has been used successfully to manage overactive bladder and urinary retention and may prove useful in the treatment of pelvic myofascial pain.
Van Balken et al: Details of the study
In this prospective observational study, 33 patients (both male and female) who had chronic pelvic pain by history and examination were treated with weekly, 30-minute outpatient sessions of PTNS for 12 weeks. Participants were asked to provide baseline pain scores and keep a diary of their pain. Quality-of-life questionnaires were also administered at baseline and at 12 weeks.
Investigators considered both subjective and objective success in their outcomes. If a patient elected to continue therapy, he or she was classified as a subjective success. Objective success required a decrease of at least 50% in the pain score. At the end of 12 weeks, although 33 patients (42%) wanted to continue therapy, only seven (21%) met the definition for objective success. Of those seven, six elected to continue therapy.
This study sheds light on a treatment modality that has not been studied adequately for the indication of pelvic pain but that may be promising in patients who have levator myalgia. Limitations of this study include the lack of a placebo arm, short-term outcome, and lack of localization of pain. Furthermore, although PTNS has FDA approval for treatment of urinary urgency, frequency, and urge incontinence, it is not approved for the treatment of pelvic pain. These preliminary findings demonstrate potential but, as with any new indication, long-term comparative studies are needed.
Zabihi et al: Details of the study
Patients in this retrospective study had a diagnosis of interstitial cystitis or chronic pelvic pain. Pelvic myofascial pain and trigger points were not required for eligibility. Thirty patients (21 women and nine men) had temporary placement of a lead containing four small electrodes along the S2 to S4 sacral nerve roots on both sides of the sacrum. They were then followed for a trial period of 2 to 4 weeks. To qualify for the final stage of the study, in which the leads were connected internally to a generator implanted in the buttocks, patients had to report improvement of at least 50% in their symptoms. If their improvement did not meet that threshold, the leads were removed.
Twenty-three patients (77%) met the criteria for permanent implantation. Of these patients, 42% reported improvement of more than 50% at 6 postoperative months. Quality-of-life scores also improved significantly.
Sacral neuromodulation is not FDA-approved for the treatment of chronic pelvic pain; further studies are needed before it can be recommended for this indication.
Neither of these studies required objective evidence of myofascial pain for inclusion. Therefore, although the benefits they demonstrated may be theorized to extend to the relief of myofascial pain, this fact cannot be corroborated.
We want to hear from you! Tell us what you think.
1. Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413-418.
2. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653-660.
3. Allergan, Inc. Medication Guide: BOTOX. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM176360.pdf. Published October 2010. Accessed August 30, 2011.
1. Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413-418.
2. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653-660.
3. Allergan, Inc. Medication Guide: BOTOX. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM176360.pdf. Published October 2010. Accessed August 30, 2011.

