User login
Does withholding an ACE inhibitor or ARB before surgery improve outcomes?
EVIDENCE SUMMARY
An international prospective cohort study analyzed data from 14,687 patients, 4802 of whom were on an ACEI or ARB, to study the effect on 30-day morbidity and mortality of withholding the medications 24 hours before a noncardiac surgery.1 Of the ACEI or ARB users, 26% (1245) withheld their medication and 3557 continued it 24 hours before surgery.
Large study shows benefit in withholding meds
Patients who withheld the ACEI or ARB were less likely to experience the primary composite outcome of all-cause death, stroke, or myocardial injury (150/1245 [12%] vs 459/3557 [12.9%]; adjusted relative risk [RR] = 0.82; 95% confidence interval [CI], 0.70-0.96; P = .01; number needed to treat [NNT] = 116) and intraoperative hypotension (adjusted RR = 0.80; 95% CI, 0.72-0.93; P < .001; NNT = 18). For the NNT calculation, which the investigators didn’t perform, the treatment is the number needed to withhold an ACEI or ARB to show benefit.
Smaller, weaker studies yield different results
A retrospective cohort analysis of propensity-matched ACEI users with ACEI nonusers (9028 in each group) undergoing noncardiac surgery compared intra- and postoperative respiratory complications or mortality.2 The study found no association with either 30-day mortality (odds ratio [OR] = 0.93; 95% CI, 0.73-1.19) or the composite of in-hospital morbidity and mortality (OR = 1.06; 95% CI, 0.97-1.15). Limitations included comparison of users with nonusers as opposed to an intention-to-withhold study, the retrospective nature of the study, and the fact that outcomes were gathered from ICD-9 billing codes rather than obtained prospectively.
A Cochrane review assessed the benefits and harms of perioperative ACEIs or ARBs on mortality and morbidity in adults undergoing any type of surgery.3 Seven RCTs with a total of 571 participants were included in the review. Overall, the review didn’t find evidence to support prevention of mortality, morbidity, and complications by perioperative ACEIs or ARBs because the included studies were of low and very low methodological quality, had a high risk for bias, and lacked power. Moreover, the review didn’t assess the effect of withholding ACEIs or ARBs before surgery.
A random-effects meta-analysis of 5 studies (3 randomized trials and 2 observational studies) totaling 434 patients suggested that patients receiving ACEIs or ARBs immediately before surgery were more likely to develop hypotension requiring vasopressors (RR = 1.50; 95% CI, 1.15-1.96).4 Sufficient data weren’t available to assess other outcomes, and the included studies were relatively small and generally not powered to observe clinically significant consequences nor designed to measure the incidence of patient-important outcomes.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2014 American College of Cardiology/American Heart Association Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery states that continuing ACEIs or ARBs perioperatively is reasonable (class IIa recommendation [moderate benefit of treatment relative to risk]; level of evidence [LOE], B [data from limited populations and single randomized or nonrandomized trials]). 5
The guideline also recommends that if ACEIs or ARBs are held before surgery, it is reasonable to restart them as soon as clinically feasible postoperatively (class IIa recommendation; LOE, C [data from very limited populations and consensus opinion or case studies]).
Editor’s Takeaway
The results of the large prospective cohort contradict those of previous smaller, methodologically weaker studies, and the new findings should be taken seriously.1 Nevertheless, selection bias (why did investigators stop the ACEI?) remains. Until we have a large RCT, the preop question to ask may be why not stop the ACEI?
1. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation prospective cohort. Anesthesiology. 2017;126:16-27.
2. Turan A, You J, Shiba A, et al. Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114:552-560.
3. Zou Z, Yuan HB, Yang B, et al. Perioperative angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults. Cochrane Database Syst Rev. 2016;(1):CD009210.
4. Rosenman DJ, McDonald FS, Ebbert JO, et al. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3:319-325.
5. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;130:e278-e333.
EVIDENCE SUMMARY
An international prospective cohort study analyzed data from 14,687 patients, 4802 of whom were on an ACEI or ARB, to study the effect on 30-day morbidity and mortality of withholding the medications 24 hours before a noncardiac surgery.1 Of the ACEI or ARB users, 26% (1245) withheld their medication and 3557 continued it 24 hours before surgery.
Large study shows benefit in withholding meds
Patients who withheld the ACEI or ARB were less likely to experience the primary composite outcome of all-cause death, stroke, or myocardial injury (150/1245 [12%] vs 459/3557 [12.9%]; adjusted relative risk [RR] = 0.82; 95% confidence interval [CI], 0.70-0.96; P = .01; number needed to treat [NNT] = 116) and intraoperative hypotension (adjusted RR = 0.80; 95% CI, 0.72-0.93; P < .001; NNT = 18). For the NNT calculation, which the investigators didn’t perform, the treatment is the number needed to withhold an ACEI or ARB to show benefit.
Smaller, weaker studies yield different results
A retrospective cohort analysis of propensity-matched ACEI users with ACEI nonusers (9028 in each group) undergoing noncardiac surgery compared intra- and postoperative respiratory complications or mortality.2 The study found no association with either 30-day mortality (odds ratio [OR] = 0.93; 95% CI, 0.73-1.19) or the composite of in-hospital morbidity and mortality (OR = 1.06; 95% CI, 0.97-1.15). Limitations included comparison of users with nonusers as opposed to an intention-to-withhold study, the retrospective nature of the study, and the fact that outcomes were gathered from ICD-9 billing codes rather than obtained prospectively.
A Cochrane review assessed the benefits and harms of perioperative ACEIs or ARBs on mortality and morbidity in adults undergoing any type of surgery.3 Seven RCTs with a total of 571 participants were included in the review. Overall, the review didn’t find evidence to support prevention of mortality, morbidity, and complications by perioperative ACEIs or ARBs because the included studies were of low and very low methodological quality, had a high risk for bias, and lacked power. Moreover, the review didn’t assess the effect of withholding ACEIs or ARBs before surgery.
A random-effects meta-analysis of 5 studies (3 randomized trials and 2 observational studies) totaling 434 patients suggested that patients receiving ACEIs or ARBs immediately before surgery were more likely to develop hypotension requiring vasopressors (RR = 1.50; 95% CI, 1.15-1.96).4 Sufficient data weren’t available to assess other outcomes, and the included studies were relatively small and generally not powered to observe clinically significant consequences nor designed to measure the incidence of patient-important outcomes.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2014 American College of Cardiology/American Heart Association Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery states that continuing ACEIs or ARBs perioperatively is reasonable (class IIa recommendation [moderate benefit of treatment relative to risk]; level of evidence [LOE], B [data from limited populations and single randomized or nonrandomized trials]). 5
The guideline also recommends that if ACEIs or ARBs are held before surgery, it is reasonable to restart them as soon as clinically feasible postoperatively (class IIa recommendation; LOE, C [data from very limited populations and consensus opinion or case studies]).
Editor’s Takeaway
The results of the large prospective cohort contradict those of previous smaller, methodologically weaker studies, and the new findings should be taken seriously.1 Nevertheless, selection bias (why did investigators stop the ACEI?) remains. Until we have a large RCT, the preop question to ask may be why not stop the ACEI?
EVIDENCE SUMMARY
An international prospective cohort study analyzed data from 14,687 patients, 4802 of whom were on an ACEI or ARB, to study the effect on 30-day morbidity and mortality of withholding the medications 24 hours before a noncardiac surgery.1 Of the ACEI or ARB users, 26% (1245) withheld their medication and 3557 continued it 24 hours before surgery.
Large study shows benefit in withholding meds
Patients who withheld the ACEI or ARB were less likely to experience the primary composite outcome of all-cause death, stroke, or myocardial injury (150/1245 [12%] vs 459/3557 [12.9%]; adjusted relative risk [RR] = 0.82; 95% confidence interval [CI], 0.70-0.96; P = .01; number needed to treat [NNT] = 116) and intraoperative hypotension (adjusted RR = 0.80; 95% CI, 0.72-0.93; P < .001; NNT = 18). For the NNT calculation, which the investigators didn’t perform, the treatment is the number needed to withhold an ACEI or ARB to show benefit.
Smaller, weaker studies yield different results
A retrospective cohort analysis of propensity-matched ACEI users with ACEI nonusers (9028 in each group) undergoing noncardiac surgery compared intra- and postoperative respiratory complications or mortality.2 The study found no association with either 30-day mortality (odds ratio [OR] = 0.93; 95% CI, 0.73-1.19) or the composite of in-hospital morbidity and mortality (OR = 1.06; 95% CI, 0.97-1.15). Limitations included comparison of users with nonusers as opposed to an intention-to-withhold study, the retrospective nature of the study, and the fact that outcomes were gathered from ICD-9 billing codes rather than obtained prospectively.
A Cochrane review assessed the benefits and harms of perioperative ACEIs or ARBs on mortality and morbidity in adults undergoing any type of surgery.3 Seven RCTs with a total of 571 participants were included in the review. Overall, the review didn’t find evidence to support prevention of mortality, morbidity, and complications by perioperative ACEIs or ARBs because the included studies were of low and very low methodological quality, had a high risk for bias, and lacked power. Moreover, the review didn’t assess the effect of withholding ACEIs or ARBs before surgery.
A random-effects meta-analysis of 5 studies (3 randomized trials and 2 observational studies) totaling 434 patients suggested that patients receiving ACEIs or ARBs immediately before surgery were more likely to develop hypotension requiring vasopressors (RR = 1.50; 95% CI, 1.15-1.96).4 Sufficient data weren’t available to assess other outcomes, and the included studies were relatively small and generally not powered to observe clinically significant consequences nor designed to measure the incidence of patient-important outcomes.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2014 American College of Cardiology/American Heart Association Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery states that continuing ACEIs or ARBs perioperatively is reasonable (class IIa recommendation [moderate benefit of treatment relative to risk]; level of evidence [LOE], B [data from limited populations and single randomized or nonrandomized trials]). 5
The guideline also recommends that if ACEIs or ARBs are held before surgery, it is reasonable to restart them as soon as clinically feasible postoperatively (class IIa recommendation; LOE, C [data from very limited populations and consensus opinion or case studies]).
Editor’s Takeaway
The results of the large prospective cohort contradict those of previous smaller, methodologically weaker studies, and the new findings should be taken seriously.1 Nevertheless, selection bias (why did investigators stop the ACEI?) remains. Until we have a large RCT, the preop question to ask may be why not stop the ACEI?
1. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation prospective cohort. Anesthesiology. 2017;126:16-27.
2. Turan A, You J, Shiba A, et al. Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114:552-560.
3. Zou Z, Yuan HB, Yang B, et al. Perioperative angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults. Cochrane Database Syst Rev. 2016;(1):CD009210.
4. Rosenman DJ, McDonald FS, Ebbert JO, et al. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3:319-325.
5. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;130:e278-e333.
1. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation prospective cohort. Anesthesiology. 2017;126:16-27.
2. Turan A, You J, Shiba A, et al. Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114:552-560.
3. Zou Z, Yuan HB, Yang B, et al. Perioperative angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults. Cochrane Database Syst Rev. 2016;(1):CD009210.
4. Rosenman DJ, McDonald FS, Ebbert JO, et al. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3:319-325.
5. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;130:e278-e333.
EVIDENCE-BASED ANSWER:
A guarded yes, because the evidence of benefit is from observational studies and applies to noncardiac surgery. Withholding angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) 24 hours before noncardiac surgery has been associated with a 30-day lower risk for all-cause death, stroke, myocardial injury, and intraoperative hypotension (18% adjusted relative risk reduction).
The finding is based on 1 international prospective cohort study and, of note, is an association and a likelihood of benefit. Confirmation would require a large randomized trial (RCT; strength of recommendation [SOR]: B, good-quality international prospective cohort study).
Do oral decongestants have a clinically significant effect on BP in patients with hypertension?
EVIDENCE SUMMARY
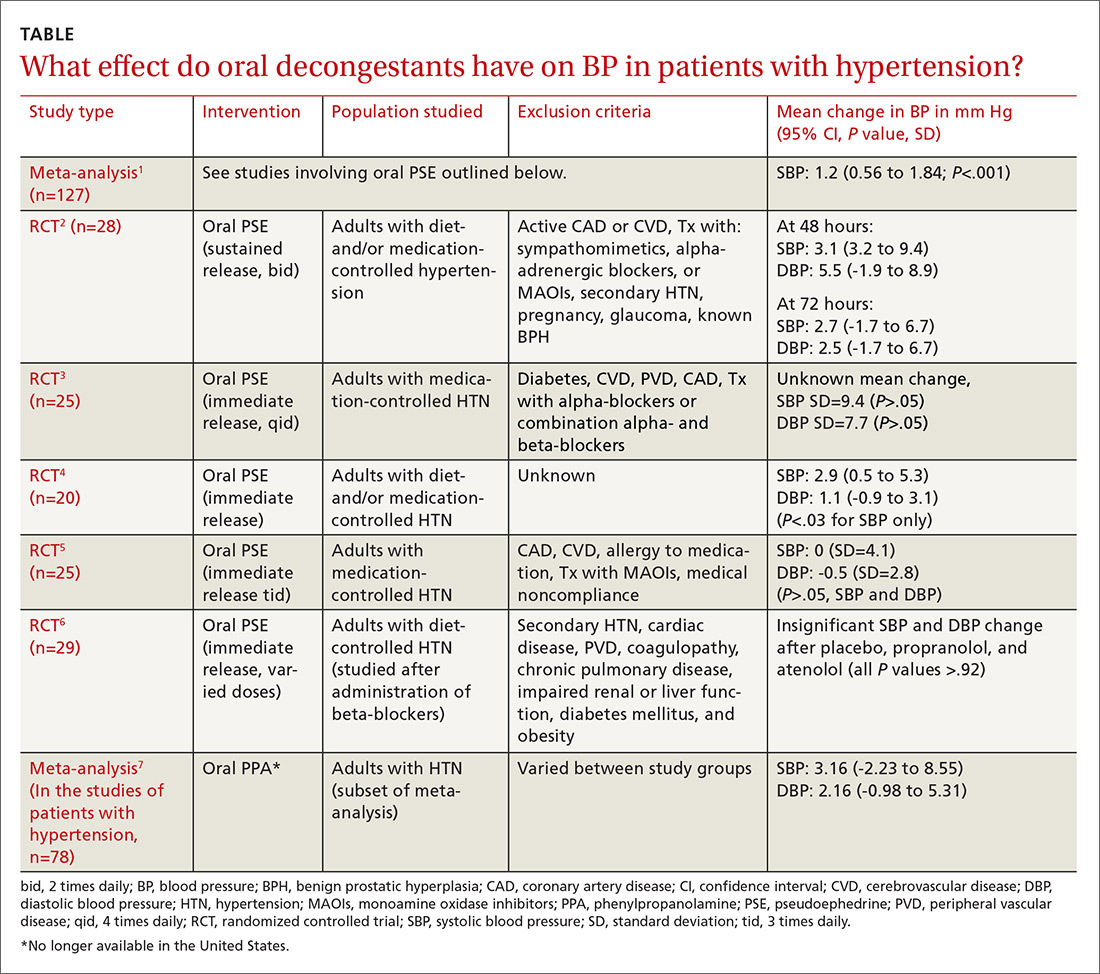
A meta-analysis of 24 RCTs examined the effect of pseudoephedrine on BP and heart rate.1 Just 5 of the 24 studies specifically included hypertensive patients. In the population of patients with hypertension, the meta-analysis showed a small (1.2 mm Hg) rise in systolic BP with pseudoephedrine that was statistically significant (95% confidence interval [CI], 0.56-1.84 mm Hg), but the slight changes in diastolic BP and heart rate were not significant. No patient-oriented outcomes were measured.
The highest quality study within this group was a randomized, double-blind, placebo-controlled crossover study with 28 patients given sustained-release pseudoephedrine 120 mg twice daily for 72 hours, with BP measurements taken at 48 and 72 hours.2 The study was powered to identify an increase in systolic BP of 11 mm Hg, but the results showed just a 3.1 mm Hg rise in systolic BP at 48 hours (see TABLE1-7 for CI and other data).
In another double-blind, placebo-controlled RCT of 29 adults with hypertension (only 25 were included in the data analysis), there was no significant elevation in BP when oral pseudoephedrine was administered over the course of 3 days.3
Across the 5 studies in the meta-analysis, immediate-release and sustained-release forms of pseudoephedrine were included, hypertension was described as controlled but definitions of control were not always specified, and study length varied from 2 hours to 4 weeks.2-6 Patients on antihypertensive medications were included in some of the studies; patients who had active cardiovascular disease, peripheral vascular disease, and/or cerebrovascular disease were excluded.
One study specifically looked at the effects of a single dose of pseudoephedrine on BP in patients treated with 2 different beta-blockers and found no significant change from baseline, but this study was not powered to show differences less than 5 mm Hg.6 The study did show a change of 1 to 2 mm Hg in systolic BP, but this was not statistically significant.
An absence of information on older patients
There is a paucity of literature on treating older adults and medically complex patients (eg, those with uncontrolled or secondary causes of hypertension, cerebrovascular disease, coronary artery disease) with decongestants, as they were excluded in all studies. And the available evidence does not include reports of adverse events other than changes in BP.
1. Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate. Arch Intern Med. 2005;165:1686-1694.
2. Beck RA, Mercado DL, Seguin SM, et al. Cardiovascular effects of pseudoephedrine in medically controlled hypertensive patients. Arch Int Med. 1992;152:1242-1245.
3. Bradley JG, Kallail KJ, Dorsch JN, et al. The effects of pseudoephedrine on blood pressure in patients with controlled, uncomplicated hypertension: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Pract. 1991;4:201-206.
4. Chua SS, Benrimoj SI, Gordon RD, et al. A controlled clinical trial on the cardiovascular effects of single doses of pseudoephedrine in hypertensive patients. Br J Clin Pharmacol. 1989;28:369-372.
5. Coates ML, Rembold CM, Farr BM. Does pseudoephedrine increase blood pressure in patients with controlled hypertension? J Fam Pract. 1995;40:22-26.
6. Mores N, Campia U, Navarra P, et al. No cardiovascular effects of single-dose pseudoephedrine in patients with essential hypertension treated with beta-blockers. Eur J Clin Pharmacol. 1999;55:251-254.
7. Salerno SM, Jackson JL, Berbano EP. The impact of oral phenylpropanolamine on blood pressure: a meta-analysis and review of the literature. J Hum Hypertens. 2005;19:643-652.
EVIDENCE SUMMARY
A meta-analysis of 24 RCTs examined the effect of pseudoephedrine on BP and heart rate.1 Just 5 of the 24 studies specifically included hypertensive patients. In the population of patients with hypertension, the meta-analysis showed a small (1.2 mm Hg) rise in systolic BP with pseudoephedrine that was statistically significant (95% confidence interval [CI], 0.56-1.84 mm Hg), but the slight changes in diastolic BP and heart rate were not significant. No patient-oriented outcomes were measured.
The highest quality study within this group was a randomized, double-blind, placebo-controlled crossover study with 28 patients given sustained-release pseudoephedrine 120 mg twice daily for 72 hours, with BP measurements taken at 48 and 72 hours.2 The study was powered to identify an increase in systolic BP of 11 mm Hg, but the results showed just a 3.1 mm Hg rise in systolic BP at 48 hours (see TABLE1-7 for CI and other data).
In another double-blind, placebo-controlled RCT of 29 adults with hypertension (only 25 were included in the data analysis), there was no significant elevation in BP when oral pseudoephedrine was administered over the course of 3 days.3
Across the 5 studies in the meta-analysis, immediate-release and sustained-release forms of pseudoephedrine were included, hypertension was described as controlled but definitions of control were not always specified, and study length varied from 2 hours to 4 weeks.2-6 Patients on antihypertensive medications were included in some of the studies; patients who had active cardiovascular disease, peripheral vascular disease, and/or cerebrovascular disease were excluded.
One study specifically looked at the effects of a single dose of pseudoephedrine on BP in patients treated with 2 different beta-blockers and found no significant change from baseline, but this study was not powered to show differences less than 5 mm Hg.6 The study did show a change of 1 to 2 mm Hg in systolic BP, but this was not statistically significant.
An absence of information on older patients
There is a paucity of literature on treating older adults and medically complex patients (eg, those with uncontrolled or secondary causes of hypertension, cerebrovascular disease, coronary artery disease) with decongestants, as they were excluded in all studies. And the available evidence does not include reports of adverse events other than changes in BP.
EVIDENCE SUMMARY
A meta-analysis of 24 RCTs examined the effect of pseudoephedrine on BP and heart rate.1 Just 5 of the 24 studies specifically included hypertensive patients. In the population of patients with hypertension, the meta-analysis showed a small (1.2 mm Hg) rise in systolic BP with pseudoephedrine that was statistically significant (95% confidence interval [CI], 0.56-1.84 mm Hg), but the slight changes in diastolic BP and heart rate were not significant. No patient-oriented outcomes were measured.
The highest quality study within this group was a randomized, double-blind, placebo-controlled crossover study with 28 patients given sustained-release pseudoephedrine 120 mg twice daily for 72 hours, with BP measurements taken at 48 and 72 hours.2 The study was powered to identify an increase in systolic BP of 11 mm Hg, but the results showed just a 3.1 mm Hg rise in systolic BP at 48 hours (see TABLE1-7 for CI and other data).
In another double-blind, placebo-controlled RCT of 29 adults with hypertension (only 25 were included in the data analysis), there was no significant elevation in BP when oral pseudoephedrine was administered over the course of 3 days.3
Across the 5 studies in the meta-analysis, immediate-release and sustained-release forms of pseudoephedrine were included, hypertension was described as controlled but definitions of control were not always specified, and study length varied from 2 hours to 4 weeks.2-6 Patients on antihypertensive medications were included in some of the studies; patients who had active cardiovascular disease, peripheral vascular disease, and/or cerebrovascular disease were excluded.
One study specifically looked at the effects of a single dose of pseudoephedrine on BP in patients treated with 2 different beta-blockers and found no significant change from baseline, but this study was not powered to show differences less than 5 mm Hg.6 The study did show a change of 1 to 2 mm Hg in systolic BP, but this was not statistically significant.
An absence of information on older patients
There is a paucity of literature on treating older adults and medically complex patients (eg, those with uncontrolled or secondary causes of hypertension, cerebrovascular disease, coronary artery disease) with decongestants, as they were excluded in all studies. And the available evidence does not include reports of adverse events other than changes in BP.
1. Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate. Arch Intern Med. 2005;165:1686-1694.
2. Beck RA, Mercado DL, Seguin SM, et al. Cardiovascular effects of pseudoephedrine in medically controlled hypertensive patients. Arch Int Med. 1992;152:1242-1245.
3. Bradley JG, Kallail KJ, Dorsch JN, et al. The effects of pseudoephedrine on blood pressure in patients with controlled, uncomplicated hypertension: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Pract. 1991;4:201-206.
4. Chua SS, Benrimoj SI, Gordon RD, et al. A controlled clinical trial on the cardiovascular effects of single doses of pseudoephedrine in hypertensive patients. Br J Clin Pharmacol. 1989;28:369-372.
5. Coates ML, Rembold CM, Farr BM. Does pseudoephedrine increase blood pressure in patients with controlled hypertension? J Fam Pract. 1995;40:22-26.
6. Mores N, Campia U, Navarra P, et al. No cardiovascular effects of single-dose pseudoephedrine in patients with essential hypertension treated with beta-blockers. Eur J Clin Pharmacol. 1999;55:251-254.
7. Salerno SM, Jackson JL, Berbano EP. The impact of oral phenylpropanolamine on blood pressure: a meta-analysis and review of the literature. J Hum Hypertens. 2005;19:643-652.
1. Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate. Arch Intern Med. 2005;165:1686-1694.
2. Beck RA, Mercado DL, Seguin SM, et al. Cardiovascular effects of pseudoephedrine in medically controlled hypertensive patients. Arch Int Med. 1992;152:1242-1245.
3. Bradley JG, Kallail KJ, Dorsch JN, et al. The effects of pseudoephedrine on blood pressure in patients with controlled, uncomplicated hypertension: a randomized, double-blind, placebo-controlled trial. J Am Board Fam Pract. 1991;4:201-206.
4. Chua SS, Benrimoj SI, Gordon RD, et al. A controlled clinical trial on the cardiovascular effects of single doses of pseudoephedrine in hypertensive patients. Br J Clin Pharmacol. 1989;28:369-372.
5. Coates ML, Rembold CM, Farr BM. Does pseudoephedrine increase blood pressure in patients with controlled hypertension? J Fam Pract. 1995;40:22-26.
6. Mores N, Campia U, Navarra P, et al. No cardiovascular effects of single-dose pseudoephedrine in patients with essential hypertension treated with beta-blockers. Eur J Clin Pharmacol. 1999;55:251-254.
7. Salerno SM, Jackson JL, Berbano EP. The impact of oral phenylpropanolamine on blood pressure: a meta-analysis and review of the literature. J Hum Hypertens. 2005;19:643-652.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
It is unclear. Pseudoephedrine causes an average increase of 1.2 mm Hg in systolic blood pressure (BP) in patients with controlled hypertension. However, the studies are not adequately powered to provide evidence about whether this rise in systolic BP is linked to patient-oriented outcomes (strength of recommendation [SOR]: C, multiple randomized controlled trials [RCTs] supporting disease-oriented evidence). Significant variations in BP are defined differently among studies (TABLE1-7). In addition, we do not have data on chronic use of oral decongestants; the longest time on medication in these trials was 4 weeks.
How well do antivirals shorten genital herpes pain duration?
Oral and intravenous (IV) acyclovir each shorten the duration of pain for a first primary outbreak of herpes by about 50%; topical acyclovir shortens it by about 25% (strength of recommendation [SOR]: B, small randomized controlled trials [RCTs] with some methodological flaws).
Oral valacyclovir and famciclovir are equivalent to oral acyclovir. Adding topical acyclovir to oral acyclovir doesn’t produce additional benefit (SOR: B, RCTs).
Patients with severe disease may require IV acyclovir (SOR: C, expert opinion).
General treatment measures that may improve patient comfort include keeping lesions clean and dry, avoiding tight clothing, taking analgesics, and using ice packs or taking warm baths (SOR: C, expert opinion).
Evidence for using complementary and alternative medicine to treat genital herpes is lacking or conflicting [SOR: C, narrative review of clinical trials].
EVIDENCE SUMMARY
A review of 3 double-blind, placebo-controlled RCTs compared topical, oral, and IV acyclovir in patients with a first episode of genital herpes.1 Researchers recruited a total of 138 patients and randomized them to receive either placebo or one of the following: oral acyclovir (200 mg 5 times daily for 10 days), IV acyclovir (5 mg/kg dose, 3 times daily for 5 days), or 5% topical acyclovir in polyethylene glycol (4 times daily for 6 days).
All treatments shortened duration of pain compared with placebo: oral (3 days vs 7 days, P<.01), IV (5 days vs 9 days, P<.05), and topical (5 days vs 7 days, P<.05).
A subsequent RCT with 50 patients found that adding topical acyclovir to oral acyclovir was no more effective than oral acyclovir alone.2
Oral acyclovir, valacyclovir, and famciclovir work equally well
Head-to-head trials comparing acyclovir with valacyclovir or famciclovir show no difference in decreased duration of pain caused by primary genital herpes. An RCT of 643 adults found valacyclovir (1000 mg twice daily for 10 days) to be as effective and well-tolerated as acyclovir (200 mg 5 times daily for 10 days).3 An RCT of 951 adults demonstrated that famciclovir (250 mg 3 times daily for either 5 or 10 days) worked as well as acyclovir (200 mg 5 times daily for 10 days).4
General treatment measures
Expert opinion recommends the following general treatment measures for genital herpes lesions: keeping the affected area clean and dry, wearing dry, loose-fitting clothing and cotton underwear, and not touching the lesions. Additional symptomatic treatments for local pain include ice packs, baking soda compresses, warm baths, oral analgesics, topical anesthetics, and drying the affected area with cool air.5-8
CAM approaches lack evidence of efficacy
A 2005 nonsystematic review of available scientific data on complementary and alternative medicine found a lack of evidence or conflicting evidence concerning the use of aloe vera, echinacea, L-lysine, bee products (honey pollen), zinc, and eleuthero for the treatment of pain in genital herpes.9
RECOMMENDATIONS
Clinical practice guidelines recommend prescribing oral antiviral therapy for patients with a first episode of genital herpes because patients with mild clinical findings at onset may develop severe or prolonged symptoms. Choices include a 7- to 10-day course of valacyclovir 1 g twice a day, famciclovir 250 mg 3 times a day, acyclovir 400 mg 3 times a day, or acyclovir 400 mg 5 times a day.5,10
The guidelines recommend treating patients with severe disease (such as disseminated infection, pneumonitis, hepatitis, or meningoencephalitis) with IV acyclovir (5-10 mg/kg every 8 hours for 2-7 days or until clinical improvement), followed by oral acyclovir for at least 10 days.
1. Corey L, Benedetti J, Critchlow C, et al. Treatment of primary first-episode genital herpes simplex virus infections with acyclovir: results of topical, intravenous and oral therapy. J Antimicrob Chemother. 1983;12(suppl B):79-88.
2. Kinghorn GR, Abeywickreme I, Jeavons M, et al. Efficacy of combined treatment with oral and topical acyclovir in first episode genital herpes. Genitourin Med. 1986;62:186-188.
3. Fife KH, Barabarash RA, Rudolph T, et al. Valaciclovir versus acyclovir in the treatment of first-episode genital herpes infection. Results of an international, multicenter, double-blind, randomized clinic trial. The Valaciclovir International Herpes Simplex Virus Study Group. Sex Transm Dis. 1997;24:481-486.
4. Loveless M, Sacks SL, Harris JRW. Famciclovir in the management of first-episode genital herpes. Infect Dis Clin Prac. 1997;6(suppl 1):S12-S16.
5. ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin: Clinical management guidelines for obstetrician-gynecologists, number 57, November 2004. Gynecologic herpes simplex virus infections. Obstet Gynecol. 2004;104(5 pt 1):1111-1118.
6. Beauman JG. Genital herpes: a review. Am Fam Physician. 2005;72:1527-1534.
7. Patel R. Progress in meeting today’s demands in genital herpes: an overview of current management. J Infect Dis. 2002;186(suppl 1):S847-S856.
8. Stanberry LR, Rosenthal SL. Genital herpes simplex virus infection in the adolescent: special considerations for management. Pediatr Drugs. 2002;4:291-297.
9. Perfect MM, Bourne N, Ebel C, et al. Use of complementary and alternative medicine for the treatment of genital herpes. Herpes. 2005;12:38-41.
10. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1-110.
Oral and intravenous (IV) acyclovir each shorten the duration of pain for a first primary outbreak of herpes by about 50%; topical acyclovir shortens it by about 25% (strength of recommendation [SOR]: B, small randomized controlled trials [RCTs] with some methodological flaws).
Oral valacyclovir and famciclovir are equivalent to oral acyclovir. Adding topical acyclovir to oral acyclovir doesn’t produce additional benefit (SOR: B, RCTs).
Patients with severe disease may require IV acyclovir (SOR: C, expert opinion).
General treatment measures that may improve patient comfort include keeping lesions clean and dry, avoiding tight clothing, taking analgesics, and using ice packs or taking warm baths (SOR: C, expert opinion).
Evidence for using complementary and alternative medicine to treat genital herpes is lacking or conflicting [SOR: C, narrative review of clinical trials].
EVIDENCE SUMMARY
A review of 3 double-blind, placebo-controlled RCTs compared topical, oral, and IV acyclovir in patients with a first episode of genital herpes.1 Researchers recruited a total of 138 patients and randomized them to receive either placebo or one of the following: oral acyclovir (200 mg 5 times daily for 10 days), IV acyclovir (5 mg/kg dose, 3 times daily for 5 days), or 5% topical acyclovir in polyethylene glycol (4 times daily for 6 days).
All treatments shortened duration of pain compared with placebo: oral (3 days vs 7 days, P<.01), IV (5 days vs 9 days, P<.05), and topical (5 days vs 7 days, P<.05).
A subsequent RCT with 50 patients found that adding topical acyclovir to oral acyclovir was no more effective than oral acyclovir alone.2
Oral acyclovir, valacyclovir, and famciclovir work equally well
Head-to-head trials comparing acyclovir with valacyclovir or famciclovir show no difference in decreased duration of pain caused by primary genital herpes. An RCT of 643 adults found valacyclovir (1000 mg twice daily for 10 days) to be as effective and well-tolerated as acyclovir (200 mg 5 times daily for 10 days).3 An RCT of 951 adults demonstrated that famciclovir (250 mg 3 times daily for either 5 or 10 days) worked as well as acyclovir (200 mg 5 times daily for 10 days).4
General treatment measures
Expert opinion recommends the following general treatment measures for genital herpes lesions: keeping the affected area clean and dry, wearing dry, loose-fitting clothing and cotton underwear, and not touching the lesions. Additional symptomatic treatments for local pain include ice packs, baking soda compresses, warm baths, oral analgesics, topical anesthetics, and drying the affected area with cool air.5-8
CAM approaches lack evidence of efficacy
A 2005 nonsystematic review of available scientific data on complementary and alternative medicine found a lack of evidence or conflicting evidence concerning the use of aloe vera, echinacea, L-lysine, bee products (honey pollen), zinc, and eleuthero for the treatment of pain in genital herpes.9
RECOMMENDATIONS
Clinical practice guidelines recommend prescribing oral antiviral therapy for patients with a first episode of genital herpes because patients with mild clinical findings at onset may develop severe or prolonged symptoms. Choices include a 7- to 10-day course of valacyclovir 1 g twice a day, famciclovir 250 mg 3 times a day, acyclovir 400 mg 3 times a day, or acyclovir 400 mg 5 times a day.5,10
The guidelines recommend treating patients with severe disease (such as disseminated infection, pneumonitis, hepatitis, or meningoencephalitis) with IV acyclovir (5-10 mg/kg every 8 hours for 2-7 days or until clinical improvement), followed by oral acyclovir for at least 10 days.
Oral and intravenous (IV) acyclovir each shorten the duration of pain for a first primary outbreak of herpes by about 50%; topical acyclovir shortens it by about 25% (strength of recommendation [SOR]: B, small randomized controlled trials [RCTs] with some methodological flaws).
Oral valacyclovir and famciclovir are equivalent to oral acyclovir. Adding topical acyclovir to oral acyclovir doesn’t produce additional benefit (SOR: B, RCTs).
Patients with severe disease may require IV acyclovir (SOR: C, expert opinion).
General treatment measures that may improve patient comfort include keeping lesions clean and dry, avoiding tight clothing, taking analgesics, and using ice packs or taking warm baths (SOR: C, expert opinion).
Evidence for using complementary and alternative medicine to treat genital herpes is lacking or conflicting [SOR: C, narrative review of clinical trials].
EVIDENCE SUMMARY
A review of 3 double-blind, placebo-controlled RCTs compared topical, oral, and IV acyclovir in patients with a first episode of genital herpes.1 Researchers recruited a total of 138 patients and randomized them to receive either placebo or one of the following: oral acyclovir (200 mg 5 times daily for 10 days), IV acyclovir (5 mg/kg dose, 3 times daily for 5 days), or 5% topical acyclovir in polyethylene glycol (4 times daily for 6 days).
All treatments shortened duration of pain compared with placebo: oral (3 days vs 7 days, P<.01), IV (5 days vs 9 days, P<.05), and topical (5 days vs 7 days, P<.05).
A subsequent RCT with 50 patients found that adding topical acyclovir to oral acyclovir was no more effective than oral acyclovir alone.2
Oral acyclovir, valacyclovir, and famciclovir work equally well
Head-to-head trials comparing acyclovir with valacyclovir or famciclovir show no difference in decreased duration of pain caused by primary genital herpes. An RCT of 643 adults found valacyclovir (1000 mg twice daily for 10 days) to be as effective and well-tolerated as acyclovir (200 mg 5 times daily for 10 days).3 An RCT of 951 adults demonstrated that famciclovir (250 mg 3 times daily for either 5 or 10 days) worked as well as acyclovir (200 mg 5 times daily for 10 days).4
General treatment measures
Expert opinion recommends the following general treatment measures for genital herpes lesions: keeping the affected area clean and dry, wearing dry, loose-fitting clothing and cotton underwear, and not touching the lesions. Additional symptomatic treatments for local pain include ice packs, baking soda compresses, warm baths, oral analgesics, topical anesthetics, and drying the affected area with cool air.5-8
CAM approaches lack evidence of efficacy
A 2005 nonsystematic review of available scientific data on complementary and alternative medicine found a lack of evidence or conflicting evidence concerning the use of aloe vera, echinacea, L-lysine, bee products (honey pollen), zinc, and eleuthero for the treatment of pain in genital herpes.9
RECOMMENDATIONS
Clinical practice guidelines recommend prescribing oral antiviral therapy for patients with a first episode of genital herpes because patients with mild clinical findings at onset may develop severe or prolonged symptoms. Choices include a 7- to 10-day course of valacyclovir 1 g twice a day, famciclovir 250 mg 3 times a day, acyclovir 400 mg 3 times a day, or acyclovir 400 mg 5 times a day.5,10
The guidelines recommend treating patients with severe disease (such as disseminated infection, pneumonitis, hepatitis, or meningoencephalitis) with IV acyclovir (5-10 mg/kg every 8 hours for 2-7 days or until clinical improvement), followed by oral acyclovir for at least 10 days.
1. Corey L, Benedetti J, Critchlow C, et al. Treatment of primary first-episode genital herpes simplex virus infections with acyclovir: results of topical, intravenous and oral therapy. J Antimicrob Chemother. 1983;12(suppl B):79-88.
2. Kinghorn GR, Abeywickreme I, Jeavons M, et al. Efficacy of combined treatment with oral and topical acyclovir in first episode genital herpes. Genitourin Med. 1986;62:186-188.
3. Fife KH, Barabarash RA, Rudolph T, et al. Valaciclovir versus acyclovir in the treatment of first-episode genital herpes infection. Results of an international, multicenter, double-blind, randomized clinic trial. The Valaciclovir International Herpes Simplex Virus Study Group. Sex Transm Dis. 1997;24:481-486.
4. Loveless M, Sacks SL, Harris JRW. Famciclovir in the management of first-episode genital herpes. Infect Dis Clin Prac. 1997;6(suppl 1):S12-S16.
5. ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin: Clinical management guidelines for obstetrician-gynecologists, number 57, November 2004. Gynecologic herpes simplex virus infections. Obstet Gynecol. 2004;104(5 pt 1):1111-1118.
6. Beauman JG. Genital herpes: a review. Am Fam Physician. 2005;72:1527-1534.
7. Patel R. Progress in meeting today’s demands in genital herpes: an overview of current management. J Infect Dis. 2002;186(suppl 1):S847-S856.
8. Stanberry LR, Rosenthal SL. Genital herpes simplex virus infection in the adolescent: special considerations for management. Pediatr Drugs. 2002;4:291-297.
9. Perfect MM, Bourne N, Ebel C, et al. Use of complementary and alternative medicine for the treatment of genital herpes. Herpes. 2005;12:38-41.
10. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1-110.
1. Corey L, Benedetti J, Critchlow C, et al. Treatment of primary first-episode genital herpes simplex virus infections with acyclovir: results of topical, intravenous and oral therapy. J Antimicrob Chemother. 1983;12(suppl B):79-88.
2. Kinghorn GR, Abeywickreme I, Jeavons M, et al. Efficacy of combined treatment with oral and topical acyclovir in first episode genital herpes. Genitourin Med. 1986;62:186-188.
3. Fife KH, Barabarash RA, Rudolph T, et al. Valaciclovir versus acyclovir in the treatment of first-episode genital herpes infection. Results of an international, multicenter, double-blind, randomized clinic trial. The Valaciclovir International Herpes Simplex Virus Study Group. Sex Transm Dis. 1997;24:481-486.
4. Loveless M, Sacks SL, Harris JRW. Famciclovir in the management of first-episode genital herpes. Infect Dis Clin Prac. 1997;6(suppl 1):S12-S16.
5. ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin: Clinical management guidelines for obstetrician-gynecologists, number 57, November 2004. Gynecologic herpes simplex virus infections. Obstet Gynecol. 2004;104(5 pt 1):1111-1118.
6. Beauman JG. Genital herpes: a review. Am Fam Physician. 2005;72:1527-1534.
7. Patel R. Progress in meeting today’s demands in genital herpes: an overview of current management. J Infect Dis. 2002;186(suppl 1):S847-S856.
8. Stanberry LR, Rosenthal SL. Genital herpes simplex virus infection in the adolescent: special considerations for management. Pediatr Drugs. 2002;4:291-297.
9. Perfect MM, Bourne N, Ebel C, et al. Use of complementary and alternative medicine for the treatment of genital herpes. Herpes. 2005;12:38-41.
10. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1-110.
Evidence-based answers from the Family Physicians Inquiries Network
Does a low-fat diet help prevent breast cancer?
No. Studies show no evidence that reducing dietary fat decreases a woman’s risk of developing postmenopausal breast cancer within the subsequent 14 years (strength of recommendation [SOR]: B, based on large heterogeneous prospective cohort studies and appropriate meta-analyses of these studies). Overall, evidence is insufficient to recommend for or against reduction in dietary fat to reduce risk of breast cancer for women, although recommendations for prudent fat intake may be justified on other grounds.
Losing weight is still a good strategy
Kathryn Kolasa, PhD, RD, LDN
East Carolina University, Greenville, NC
Women at risk for breast cancer—and cancer survivors—want to know about lifestyle changes that can reduce their risks for cancer or recurrence. There is growing evidence that obesity plays a role in cancer development and promotion.
A low-fat diet has been demonstrated as a successful strategy for weight loss. However, for most women, making these changes can be difficult without extensive instruction, support, and motivation. Limiting sweetened beverages, increasing consumption of fruits and vegetables, and limiting fat intake are 3 strategies women can use to achieve a healthy weight. If this turns out to reduce their risk of breast cancer, so much the better!
Evidence summary
Our Medline search retrieved 1114 English-language studies published from 1960 through October 2006. We limited this set to randomized controlled trials and cohort studies, leaving 212 articles. We then excluded articles that had small sample sizes, did not follow subjects for at least 5 years, did not include original data, included men, did not give prevalence or incidence rate of breast cancer in the subjects, or did not discuss diet assessment tools. Of the remaining articles, we selected the 11 best studies to include in the review.
Early studies evaluating national average dietary fat intake and breast cancer incidence rates showed an almost linear relationship between increased dietary fat and increased breast cancer incidence.1 However, increased fat intake occurs primarily in industrialized nations, providing multiple possible confounders for increased rates of breast cancer, such as pollutants and increased consumption of preservatives, pesticides, and other chemicals.
Case-control studies have shown some minimally increased risk related to dietary fat consumption, but there is concern about recall bias in these studies.2 Since the late 1970s, 7 large, well-designed prospective cohort studies have examined the possible relationship between dietary fat and breast cancer.1 The findings have been somewhat contradictory, with some studies showing statistically significant associations toward increased risk with higher fat intake.3-5
Since the late 1990s, several meta-analyses, a systematic review of these cohort studies, and the Women’s Health Initiative Randomized Controlled Diet Initiative have largely concluded that there is no difference in breast cancer incidence between women with a low-fat diet (<20% of total calories from fat) and women with average or high-fat diets (>40% total calories from fat).1,3,6,7
The meta-analysis performed by Boyd et al did find a statistically significant difference, with relative risks ranging from 1.11 for overall to 1.19 for high-saturated-fat diets.8 The upper limit of all confidence intervals was no higher than 1.35, however, suggesting a lack of clinical significance. The best-designed studies also evaluated dietary composition with regard to key types of fat (saturated, mono- and poly-unsaturated; animal vs vegetable vs marine) and found no significant differences based on type of fat consumed.1
Preliminary evidence indicates that lowering dietary fat consumption may help with secondary prevention of breast cancer, but no large studies have been performed to date.9 Recently, a nested study within the Women’s Intervention Nutrition Study did show that women with breast cancer who decreased their fat intake to a median of 33 g/day had a hazard ratio of 0.76 for relapse over 60 months (compared with controls who ate a median of 51 g/day).10
Recommendations from others
There are no evidence-based or specific recommendations for the primary prevention of postmenopausal breast cancer for women through dietary fat reduction. In particular, neither the American Academy of Family Physicians, American College of Surgeons, National Institutes of Health, American College of Obstetricians and Gynecologists, American College of Physicians, US Preventive Services Task Force, or the Centers for Disease Control and Prevention provide any guidelines on dietary fat restriction for primary prevention of postmenopausal breast cancer.
The American Heart Association does have guidelines for coronary artery disease prevention for women, which include a low-fat diet.11 The USPSTF has no specific guidelines regarding dietary fat consumption for the general population.
1. Willett WC. Diet and breast cancer. J Intern Med 2001;249:395-411.
2. Bingham SA, Luben R, Welch A, Wareham N, Khaw KT, Day N. Are imprecise methods obscuring a relation between fat and breast cancer?. Lancet 2003;362:212-214.
3. Mattisson I, Wirfalt E, Wallstrom P, Gullberg B, Olsson H, Berglund G. High fat and alcohol intakes are risk factors of postmenopausal breast cancer: a prospective study from the Malmo diet and cancer cohort. Int J Cancer 2004;110:589-597.
4. Sieri S, Krogh V, Muti P, et al. Fat and Protein Intake and subsequent Breast Cancer risk in Postmenopausal Women. Nutr Cancer 2004;42:10-17.
5. Velie E, Kulldorff M, Schairer C, Block G, Albanes D, Schatzkin A. Dietary fat, fat subtypes, and breast cancer in postmenopausal women: a prospective cohort study. J Natl Cancer Inst 2000;92:833-839.
6. Holmes MD, Hunter DJ, Colditz GA, et al. Association of dietary intake of fat and fatty acids with risk of breast cancer. JAMA 1999;281:914-920.
7. Low-Fat Dietary Pattern and risk of Breast Cancer, Colorectal Cancer, and Cardiovascular Disease: The Women’s Health Initiative randomized Controlled Dietary Modification Trial. Available at: www.whi.org/findings/dm/dm.php. Accessed on June 14, 2007.
8. Boyd NF, Stone J, Vogt KN, Connelly BS, Martin LJ, Minkin S. Dietary fat and breast cancer risk revisited: a meta-analysis of the published literature. Br J Cancer 2003;89:1672-1685.
9. Rock CL. Diet and breast cancer: can dietary factors influence survival? J Mammary Gland Biol Neoplasia 2003;8:119-132.
10. Rowan T, Chlebowski GL, Blackburn CA, et al. Dietary Fat Reduction and Breast Cancer Outcome: Interim Efficacy Results From the Women’s Intervention Nutrition Study. J Natl Cancer Inst 2006;98:1767-1776.
11. Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation 2004;109:672-693.
No. Studies show no evidence that reducing dietary fat decreases a woman’s risk of developing postmenopausal breast cancer within the subsequent 14 years (strength of recommendation [SOR]: B, based on large heterogeneous prospective cohort studies and appropriate meta-analyses of these studies). Overall, evidence is insufficient to recommend for or against reduction in dietary fat to reduce risk of breast cancer for women, although recommendations for prudent fat intake may be justified on other grounds.
Losing weight is still a good strategy
Kathryn Kolasa, PhD, RD, LDN
East Carolina University, Greenville, NC
Women at risk for breast cancer—and cancer survivors—want to know about lifestyle changes that can reduce their risks for cancer or recurrence. There is growing evidence that obesity plays a role in cancer development and promotion.
A low-fat diet has been demonstrated as a successful strategy for weight loss. However, for most women, making these changes can be difficult without extensive instruction, support, and motivation. Limiting sweetened beverages, increasing consumption of fruits and vegetables, and limiting fat intake are 3 strategies women can use to achieve a healthy weight. If this turns out to reduce their risk of breast cancer, so much the better!
Evidence summary
Our Medline search retrieved 1114 English-language studies published from 1960 through October 2006. We limited this set to randomized controlled trials and cohort studies, leaving 212 articles. We then excluded articles that had small sample sizes, did not follow subjects for at least 5 years, did not include original data, included men, did not give prevalence or incidence rate of breast cancer in the subjects, or did not discuss diet assessment tools. Of the remaining articles, we selected the 11 best studies to include in the review.
Early studies evaluating national average dietary fat intake and breast cancer incidence rates showed an almost linear relationship between increased dietary fat and increased breast cancer incidence.1 However, increased fat intake occurs primarily in industrialized nations, providing multiple possible confounders for increased rates of breast cancer, such as pollutants and increased consumption of preservatives, pesticides, and other chemicals.
Case-control studies have shown some minimally increased risk related to dietary fat consumption, but there is concern about recall bias in these studies.2 Since the late 1970s, 7 large, well-designed prospective cohort studies have examined the possible relationship between dietary fat and breast cancer.1 The findings have been somewhat contradictory, with some studies showing statistically significant associations toward increased risk with higher fat intake.3-5
Since the late 1990s, several meta-analyses, a systematic review of these cohort studies, and the Women’s Health Initiative Randomized Controlled Diet Initiative have largely concluded that there is no difference in breast cancer incidence between women with a low-fat diet (<20% of total calories from fat) and women with average or high-fat diets (>40% total calories from fat).1,3,6,7
The meta-analysis performed by Boyd et al did find a statistically significant difference, with relative risks ranging from 1.11 for overall to 1.19 for high-saturated-fat diets.8 The upper limit of all confidence intervals was no higher than 1.35, however, suggesting a lack of clinical significance. The best-designed studies also evaluated dietary composition with regard to key types of fat (saturated, mono- and poly-unsaturated; animal vs vegetable vs marine) and found no significant differences based on type of fat consumed.1
Preliminary evidence indicates that lowering dietary fat consumption may help with secondary prevention of breast cancer, but no large studies have been performed to date.9 Recently, a nested study within the Women’s Intervention Nutrition Study did show that women with breast cancer who decreased their fat intake to a median of 33 g/day had a hazard ratio of 0.76 for relapse over 60 months (compared with controls who ate a median of 51 g/day).10
Recommendations from others
There are no evidence-based or specific recommendations for the primary prevention of postmenopausal breast cancer for women through dietary fat reduction. In particular, neither the American Academy of Family Physicians, American College of Surgeons, National Institutes of Health, American College of Obstetricians and Gynecologists, American College of Physicians, US Preventive Services Task Force, or the Centers for Disease Control and Prevention provide any guidelines on dietary fat restriction for primary prevention of postmenopausal breast cancer.
The American Heart Association does have guidelines for coronary artery disease prevention for women, which include a low-fat diet.11 The USPSTF has no specific guidelines regarding dietary fat consumption for the general population.
No. Studies show no evidence that reducing dietary fat decreases a woman’s risk of developing postmenopausal breast cancer within the subsequent 14 years (strength of recommendation [SOR]: B, based on large heterogeneous prospective cohort studies and appropriate meta-analyses of these studies). Overall, evidence is insufficient to recommend for or against reduction in dietary fat to reduce risk of breast cancer for women, although recommendations for prudent fat intake may be justified on other grounds.
Losing weight is still a good strategy
Kathryn Kolasa, PhD, RD, LDN
East Carolina University, Greenville, NC
Women at risk for breast cancer—and cancer survivors—want to know about lifestyle changes that can reduce their risks for cancer or recurrence. There is growing evidence that obesity plays a role in cancer development and promotion.
A low-fat diet has been demonstrated as a successful strategy for weight loss. However, for most women, making these changes can be difficult without extensive instruction, support, and motivation. Limiting sweetened beverages, increasing consumption of fruits and vegetables, and limiting fat intake are 3 strategies women can use to achieve a healthy weight. If this turns out to reduce their risk of breast cancer, so much the better!
Evidence summary
Our Medline search retrieved 1114 English-language studies published from 1960 through October 2006. We limited this set to randomized controlled trials and cohort studies, leaving 212 articles. We then excluded articles that had small sample sizes, did not follow subjects for at least 5 years, did not include original data, included men, did not give prevalence or incidence rate of breast cancer in the subjects, or did not discuss diet assessment tools. Of the remaining articles, we selected the 11 best studies to include in the review.
Early studies evaluating national average dietary fat intake and breast cancer incidence rates showed an almost linear relationship between increased dietary fat and increased breast cancer incidence.1 However, increased fat intake occurs primarily in industrialized nations, providing multiple possible confounders for increased rates of breast cancer, such as pollutants and increased consumption of preservatives, pesticides, and other chemicals.
Case-control studies have shown some minimally increased risk related to dietary fat consumption, but there is concern about recall bias in these studies.2 Since the late 1970s, 7 large, well-designed prospective cohort studies have examined the possible relationship between dietary fat and breast cancer.1 The findings have been somewhat contradictory, with some studies showing statistically significant associations toward increased risk with higher fat intake.3-5
Since the late 1990s, several meta-analyses, a systematic review of these cohort studies, and the Women’s Health Initiative Randomized Controlled Diet Initiative have largely concluded that there is no difference in breast cancer incidence between women with a low-fat diet (<20% of total calories from fat) and women with average or high-fat diets (>40% total calories from fat).1,3,6,7
The meta-analysis performed by Boyd et al did find a statistically significant difference, with relative risks ranging from 1.11 for overall to 1.19 for high-saturated-fat diets.8 The upper limit of all confidence intervals was no higher than 1.35, however, suggesting a lack of clinical significance. The best-designed studies also evaluated dietary composition with regard to key types of fat (saturated, mono- and poly-unsaturated; animal vs vegetable vs marine) and found no significant differences based on type of fat consumed.1
Preliminary evidence indicates that lowering dietary fat consumption may help with secondary prevention of breast cancer, but no large studies have been performed to date.9 Recently, a nested study within the Women’s Intervention Nutrition Study did show that women with breast cancer who decreased their fat intake to a median of 33 g/day had a hazard ratio of 0.76 for relapse over 60 months (compared with controls who ate a median of 51 g/day).10
Recommendations from others
There are no evidence-based or specific recommendations for the primary prevention of postmenopausal breast cancer for women through dietary fat reduction. In particular, neither the American Academy of Family Physicians, American College of Surgeons, National Institutes of Health, American College of Obstetricians and Gynecologists, American College of Physicians, US Preventive Services Task Force, or the Centers for Disease Control and Prevention provide any guidelines on dietary fat restriction for primary prevention of postmenopausal breast cancer.
The American Heart Association does have guidelines for coronary artery disease prevention for women, which include a low-fat diet.11 The USPSTF has no specific guidelines regarding dietary fat consumption for the general population.
1. Willett WC. Diet and breast cancer. J Intern Med 2001;249:395-411.
2. Bingham SA, Luben R, Welch A, Wareham N, Khaw KT, Day N. Are imprecise methods obscuring a relation between fat and breast cancer?. Lancet 2003;362:212-214.
3. Mattisson I, Wirfalt E, Wallstrom P, Gullberg B, Olsson H, Berglund G. High fat and alcohol intakes are risk factors of postmenopausal breast cancer: a prospective study from the Malmo diet and cancer cohort. Int J Cancer 2004;110:589-597.
4. Sieri S, Krogh V, Muti P, et al. Fat and Protein Intake and subsequent Breast Cancer risk in Postmenopausal Women. Nutr Cancer 2004;42:10-17.
5. Velie E, Kulldorff M, Schairer C, Block G, Albanes D, Schatzkin A. Dietary fat, fat subtypes, and breast cancer in postmenopausal women: a prospective cohort study. J Natl Cancer Inst 2000;92:833-839.
6. Holmes MD, Hunter DJ, Colditz GA, et al. Association of dietary intake of fat and fatty acids with risk of breast cancer. JAMA 1999;281:914-920.
7. Low-Fat Dietary Pattern and risk of Breast Cancer, Colorectal Cancer, and Cardiovascular Disease: The Women’s Health Initiative randomized Controlled Dietary Modification Trial. Available at: www.whi.org/findings/dm/dm.php. Accessed on June 14, 2007.
8. Boyd NF, Stone J, Vogt KN, Connelly BS, Martin LJ, Minkin S. Dietary fat and breast cancer risk revisited: a meta-analysis of the published literature. Br J Cancer 2003;89:1672-1685.
9. Rock CL. Diet and breast cancer: can dietary factors influence survival? J Mammary Gland Biol Neoplasia 2003;8:119-132.
10. Rowan T, Chlebowski GL, Blackburn CA, et al. Dietary Fat Reduction and Breast Cancer Outcome: Interim Efficacy Results From the Women’s Intervention Nutrition Study. J Natl Cancer Inst 2006;98:1767-1776.
11. Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation 2004;109:672-693.
1. Willett WC. Diet and breast cancer. J Intern Med 2001;249:395-411.
2. Bingham SA, Luben R, Welch A, Wareham N, Khaw KT, Day N. Are imprecise methods obscuring a relation between fat and breast cancer?. Lancet 2003;362:212-214.
3. Mattisson I, Wirfalt E, Wallstrom P, Gullberg B, Olsson H, Berglund G. High fat and alcohol intakes are risk factors of postmenopausal breast cancer: a prospective study from the Malmo diet and cancer cohort. Int J Cancer 2004;110:589-597.
4. Sieri S, Krogh V, Muti P, et al. Fat and Protein Intake and subsequent Breast Cancer risk in Postmenopausal Women. Nutr Cancer 2004;42:10-17.
5. Velie E, Kulldorff M, Schairer C, Block G, Albanes D, Schatzkin A. Dietary fat, fat subtypes, and breast cancer in postmenopausal women: a prospective cohort study. J Natl Cancer Inst 2000;92:833-839.
6. Holmes MD, Hunter DJ, Colditz GA, et al. Association of dietary intake of fat and fatty acids with risk of breast cancer. JAMA 1999;281:914-920.
7. Low-Fat Dietary Pattern and risk of Breast Cancer, Colorectal Cancer, and Cardiovascular Disease: The Women’s Health Initiative randomized Controlled Dietary Modification Trial. Available at: www.whi.org/findings/dm/dm.php. Accessed on June 14, 2007.
8. Boyd NF, Stone J, Vogt KN, Connelly BS, Martin LJ, Minkin S. Dietary fat and breast cancer risk revisited: a meta-analysis of the published literature. Br J Cancer 2003;89:1672-1685.
9. Rock CL. Diet and breast cancer: can dietary factors influence survival? J Mammary Gland Biol Neoplasia 2003;8:119-132.
10. Rowan T, Chlebowski GL, Blackburn CA, et al. Dietary Fat Reduction and Breast Cancer Outcome: Interim Efficacy Results From the Women’s Intervention Nutrition Study. J Natl Cancer Inst 2006;98:1767-1776.
11. Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation 2004;109:672-693.
Evidence-based answers from the Family Physicians Inquiries Network
What are safe sleeping arrangements for infants?
Non-supine sleep position and parental tobacco use are known risk factors for sudden infant death syndrome (SIDS). Recent studies show that co-sleeping (bed sharing) slightly increases the overall risk of SIDS (strength of recommendation [SOR]: B) and is greatest for infants less than 11 weeks old (SOR: B). The relationship between bed sharing and SIDS is strongest for infants whose parents use tobacco (SOR: B). Infants who sleep in a room separate from their caregivers or on a couch or an armchair are at increased risk for SIDS (SOR: B). Using bedding accessories such as duvets or pillows may increase an infant’s risk of SIDS (SOR: B).
Despite its weakness, counsel families based on what evidence is available
Perry Brown, MD, FAAP
Family Medicine Residency of Idaho, Boise; University of Washington School of Medicine, Seattle
This Clinical Inquiry reviews evidence about one of the most controversial and emotion-laden issues of infancy—where should baby sleep? Of course a parent wants to minimize the risk of SIDS, and this review has some evidence of how to accomplish this.
However, often there are pragmatic obstacles to an ideal sleeping arrangement for an infant. One obstacle is exhaustion. Parents are awake multiple times per night with a young infant, and having the infant bed-share is often easier and more efficient for breastfeeding mothers. Poverty is another obstacle—the family may be unable to afford a crib or bassinet. There can also be cultural obstacles, in that certain cultures traditionally bed-share with infants and children. Physicians are deterred from addressing bed-sharing with families, because the discussion is often lengthy and the family is sometimes defensive.
Despite generally weak evidence on this topic, we must counsel families based on what evidence is available, and not shy away from this discussion. Few things are worse than retrospectively wondering if a case of SIDS could have been prevented.
Evidence summary
SIDS is defined as the sudden death of an infant aged <1 year of age that remains unexplained after a thorough investigation. The SIDS mortality rate is 0.57 per 1000 infants, with peak incidence among 1- to 5-month-olds.1 Non-supine sleep position and parental tobacco use are established risk factors for SIDS and therefore are not explicitly addressed in this review. Using the 9 best-designed case-control studies published to date, each of which used multivariate analysis to control for infant sleep position and parental tobacco use (among other confounders), we evaluated co-sleeping, room sharing, sleep surfaces, and bedding accessories as risk factors for SIDS (TABLE).
TABLE
Sleeping arrangements and their relationship to SIDS
| SLEEP ARRANGEMENT | RISK ESTIMATE* |
|---|---|
| Co-sleeping2-10 | Overall OR: 2.0 (1.2–3.3)4 to 16.47 (3.72–72.75)9 |
| OR if parent is smoker: 4.55 (2.63–7.88)10 to 17.7 (10.3–20.0)8 | |
| OR if parent is nonsmoker: 0.98 (0.44–2.18)10 to 2.20 (0.99–4.91)7 | |
| Sleeping in separate rooms5,6,8,11 | OR: 3.13 (1.82–5.26)8 to 10.49 (4.26–25.89)5 |
| Sleeping on couch or chair4-6,9 | 55 non-bed sleepers among 772 total SIDS cases (7.1%) vs 8 non-bed sleepers among 1854 total controls (0.4%)† |
| Soft bedding accessories4,7-9 | OR for use of pillow: 1.03 (0.66–1.59)7 to 2.8 (1.3–6.2)4 |
| OR for use of duvet: 1.32 (0.41–4.15)9 vs 1.82 (1.30–2.58)8 | |
| *All studies used multivariate analyses and controlled for tobacco use and infant sleep position. Risk estimates are lowest to highest OR with 95% CI (unless otherwise specified). | |
| †Aggregated data from 4 studies given small numbers. | |
| SIDS, sudden infant death syndrome; OR, odds ratio; CI, confidence interval. | |
A number of factors complicated this review. First, although all studies evaluated infants through 1 year of age, some excluded infants <7 days or <28 days old. Second, studies examined different sleep periods; 2 focused on usual sleeping arrangements,2,3 5 on sleeping arrangement immediately prior to death,4-8 and 2 evaluated both usual and last sleep arrangements.9,10 Third, variations in definitions of each risk factor and differences in the confounders controlled for made comparing studies challenging. Fourth, given the difficulty in studying infant deaths, the best evidence available comes from case-control studies.
Co-sleeping. Overall, 5 of 6 studies demonstrated co-sleeping to be an independent risk factor for SIDS (odds ratio [OR]=2.0–16.5),2,4-7,9 especially for infants younger than 11 weeks old.6,8 Four stratified analyses indicate that the risk of co-sleeping is greatest among infants of smokers (OR=4.6–17.7) as compared with infants of nonsmokers (OR=1.0–2.2).3,7,8,10 Some descriptive studies suggest potential benefits of co-sleeping, such as improved breastfeeding and maternal-infant bonding, but these benefits have not been quantified.1
Room sharing. Three of 4 studies found that infants sleeping in separate rooms from their caregivers had a 3-fold increased risk of SIDS,5,6,11 while the fourth study found a 10-fold increased risk.8 One study found the risk was present in infants less than 20 weeks, but was inconclusive for those greater than 20 weeks.11
Sleep surface. All 4 studies evaluating sleep surface found a significantly increased risk of SIDS for infants sleeping on sofas or armchairs compared with infants sleeping in beds or cribs. Fifty-five of 772 total cases (7.1%) from the 4 studies slept on a non-bed surface compared with 8 of 1854 controls (0.4%).4-6,9
Bedding accessories. Two of 3 studies found pillow use unrelated to SIDS.4,7,9 The larger of 2 studies on duvet use found it to be a risk factor for SIDS (OR=1.82).8
Recommendations from others
The American Academy of Pediatrics recommends that infants should sleep supine in the same room, but not the same bed, as their caregivers, while on a firm surface without bedding accessories. They should never sleep on a couch or armchair. Infants may be brought into bed briefly for feeding or comforting. Parents should be encouraged to quit smoking.10
1. American Academy of Pediatrics. Task Force on Infant Sleep Position and SIDS. Changing concepts of SIDS: Implications for infant sleeping environment and sleep position. Pediatrics 2000;105:650-656.
2. Brooke H, Gibson A, Tappin D, Brown H. Case-control study of SIDS in Scotland, 1992–5. BMJ 1997;314:1516-1520.
3. Mitchell EA, Tuohy PG, Brunt JM, et al. Risk factors for SIDS following the prevention campaign in New Zealand: A prospective study. Pediatrics 1997;100:835-840.
4. Hauck FR, Herman SM, Donovan M, et al. Sleep environment and the risk of SIDS in an urban population: The Chicago infant mortality study. Pediatrics 2003;111:1207-1214.
5. Blair PS, Fleming PJ, Smith IJ, et al. Babies sleeping with parents: Case-control study of factors influencing the risk of SIDS. BMJ 1999;319:1457-1461.
6. Tappin D, Ecob R, Brooke H. Bedsharing, roomsharing, and SIDS in Scotland: A case-control study. J Pediatr 2005;147:32-37.
7. Vennemann MM, Findeisen M, Butterfass-Bahloul T, et al. Modifiable risk factors for SIDS in Germany: Results of GeSID. Acta Paediatrica 2005;94:655-660.
8. Carpenter RG, Irgens LM, Blair PS, et al. Sudden unexplained infant death in 20 regions in Europe: Case control study. Lancet 2004;363:185-191.
9. McGarvey C, McDonnell M, Chong A, et al. Factors relating to the infant’s last sleep environment in SIDS in the Republic of Ireland. Arch Dis Child 2003;88:1058-1064.
10. Scragg R, Mitchell EA, Taylor BJ, et al. Bed sharing, smoking, and alcohol in SIDS. BMJ 1993;307:1312-1318.
11. Scragg RK, Mitchell EA, Stewart AW, et al. Infant room-sharing and prone sleep position in SIDS. Lancet 1996;347:7-12.
Non-supine sleep position and parental tobacco use are known risk factors for sudden infant death syndrome (SIDS). Recent studies show that co-sleeping (bed sharing) slightly increases the overall risk of SIDS (strength of recommendation [SOR]: B) and is greatest for infants less than 11 weeks old (SOR: B). The relationship between bed sharing and SIDS is strongest for infants whose parents use tobacco (SOR: B). Infants who sleep in a room separate from their caregivers or on a couch or an armchair are at increased risk for SIDS (SOR: B). Using bedding accessories such as duvets or pillows may increase an infant’s risk of SIDS (SOR: B).
Despite its weakness, counsel families based on what evidence is available
Perry Brown, MD, FAAP
Family Medicine Residency of Idaho, Boise; University of Washington School of Medicine, Seattle
This Clinical Inquiry reviews evidence about one of the most controversial and emotion-laden issues of infancy—where should baby sleep? Of course a parent wants to minimize the risk of SIDS, and this review has some evidence of how to accomplish this.
However, often there are pragmatic obstacles to an ideal sleeping arrangement for an infant. One obstacle is exhaustion. Parents are awake multiple times per night with a young infant, and having the infant bed-share is often easier and more efficient for breastfeeding mothers. Poverty is another obstacle—the family may be unable to afford a crib or bassinet. There can also be cultural obstacles, in that certain cultures traditionally bed-share with infants and children. Physicians are deterred from addressing bed-sharing with families, because the discussion is often lengthy and the family is sometimes defensive.
Despite generally weak evidence on this topic, we must counsel families based on what evidence is available, and not shy away from this discussion. Few things are worse than retrospectively wondering if a case of SIDS could have been prevented.
Evidence summary
SIDS is defined as the sudden death of an infant aged <1 year of age that remains unexplained after a thorough investigation. The SIDS mortality rate is 0.57 per 1000 infants, with peak incidence among 1- to 5-month-olds.1 Non-supine sleep position and parental tobacco use are established risk factors for SIDS and therefore are not explicitly addressed in this review. Using the 9 best-designed case-control studies published to date, each of which used multivariate analysis to control for infant sleep position and parental tobacco use (among other confounders), we evaluated co-sleeping, room sharing, sleep surfaces, and bedding accessories as risk factors for SIDS (TABLE).
TABLE
Sleeping arrangements and their relationship to SIDS
| SLEEP ARRANGEMENT | RISK ESTIMATE* |
|---|---|
| Co-sleeping2-10 | Overall OR: 2.0 (1.2–3.3)4 to 16.47 (3.72–72.75)9 |
| OR if parent is smoker: 4.55 (2.63–7.88)10 to 17.7 (10.3–20.0)8 | |
| OR if parent is nonsmoker: 0.98 (0.44–2.18)10 to 2.20 (0.99–4.91)7 | |
| Sleeping in separate rooms5,6,8,11 | OR: 3.13 (1.82–5.26)8 to 10.49 (4.26–25.89)5 |
| Sleeping on couch or chair4-6,9 | 55 non-bed sleepers among 772 total SIDS cases (7.1%) vs 8 non-bed sleepers among 1854 total controls (0.4%)† |
| Soft bedding accessories4,7-9 | OR for use of pillow: 1.03 (0.66–1.59)7 to 2.8 (1.3–6.2)4 |
| OR for use of duvet: 1.32 (0.41–4.15)9 vs 1.82 (1.30–2.58)8 | |
| *All studies used multivariate analyses and controlled for tobacco use and infant sleep position. Risk estimates are lowest to highest OR with 95% CI (unless otherwise specified). | |
| †Aggregated data from 4 studies given small numbers. | |
| SIDS, sudden infant death syndrome; OR, odds ratio; CI, confidence interval. | |
A number of factors complicated this review. First, although all studies evaluated infants through 1 year of age, some excluded infants <7 days or <28 days old. Second, studies examined different sleep periods; 2 focused on usual sleeping arrangements,2,3 5 on sleeping arrangement immediately prior to death,4-8 and 2 evaluated both usual and last sleep arrangements.9,10 Third, variations in definitions of each risk factor and differences in the confounders controlled for made comparing studies challenging. Fourth, given the difficulty in studying infant deaths, the best evidence available comes from case-control studies.
Co-sleeping. Overall, 5 of 6 studies demonstrated co-sleeping to be an independent risk factor for SIDS (odds ratio [OR]=2.0–16.5),2,4-7,9 especially for infants younger than 11 weeks old.6,8 Four stratified analyses indicate that the risk of co-sleeping is greatest among infants of smokers (OR=4.6–17.7) as compared with infants of nonsmokers (OR=1.0–2.2).3,7,8,10 Some descriptive studies suggest potential benefits of co-sleeping, such as improved breastfeeding and maternal-infant bonding, but these benefits have not been quantified.1
Room sharing. Three of 4 studies found that infants sleeping in separate rooms from their caregivers had a 3-fold increased risk of SIDS,5,6,11 while the fourth study found a 10-fold increased risk.8 One study found the risk was present in infants less than 20 weeks, but was inconclusive for those greater than 20 weeks.11
Sleep surface. All 4 studies evaluating sleep surface found a significantly increased risk of SIDS for infants sleeping on sofas or armchairs compared with infants sleeping in beds or cribs. Fifty-five of 772 total cases (7.1%) from the 4 studies slept on a non-bed surface compared with 8 of 1854 controls (0.4%).4-6,9
Bedding accessories. Two of 3 studies found pillow use unrelated to SIDS.4,7,9 The larger of 2 studies on duvet use found it to be a risk factor for SIDS (OR=1.82).8
Recommendations from others
The American Academy of Pediatrics recommends that infants should sleep supine in the same room, but not the same bed, as their caregivers, while on a firm surface without bedding accessories. They should never sleep on a couch or armchair. Infants may be brought into bed briefly for feeding or comforting. Parents should be encouraged to quit smoking.10
Non-supine sleep position and parental tobacco use are known risk factors for sudden infant death syndrome (SIDS). Recent studies show that co-sleeping (bed sharing) slightly increases the overall risk of SIDS (strength of recommendation [SOR]: B) and is greatest for infants less than 11 weeks old (SOR: B). The relationship between bed sharing and SIDS is strongest for infants whose parents use tobacco (SOR: B). Infants who sleep in a room separate from their caregivers or on a couch or an armchair are at increased risk for SIDS (SOR: B). Using bedding accessories such as duvets or pillows may increase an infant’s risk of SIDS (SOR: B).
Despite its weakness, counsel families based on what evidence is available
Perry Brown, MD, FAAP
Family Medicine Residency of Idaho, Boise; University of Washington School of Medicine, Seattle
This Clinical Inquiry reviews evidence about one of the most controversial and emotion-laden issues of infancy—where should baby sleep? Of course a parent wants to minimize the risk of SIDS, and this review has some evidence of how to accomplish this.
However, often there are pragmatic obstacles to an ideal sleeping arrangement for an infant. One obstacle is exhaustion. Parents are awake multiple times per night with a young infant, and having the infant bed-share is often easier and more efficient for breastfeeding mothers. Poverty is another obstacle—the family may be unable to afford a crib or bassinet. There can also be cultural obstacles, in that certain cultures traditionally bed-share with infants and children. Physicians are deterred from addressing bed-sharing with families, because the discussion is often lengthy and the family is sometimes defensive.
Despite generally weak evidence on this topic, we must counsel families based on what evidence is available, and not shy away from this discussion. Few things are worse than retrospectively wondering if a case of SIDS could have been prevented.
Evidence summary
SIDS is defined as the sudden death of an infant aged <1 year of age that remains unexplained after a thorough investigation. The SIDS mortality rate is 0.57 per 1000 infants, with peak incidence among 1- to 5-month-olds.1 Non-supine sleep position and parental tobacco use are established risk factors for SIDS and therefore are not explicitly addressed in this review. Using the 9 best-designed case-control studies published to date, each of which used multivariate analysis to control for infant sleep position and parental tobacco use (among other confounders), we evaluated co-sleeping, room sharing, sleep surfaces, and bedding accessories as risk factors for SIDS (TABLE).
TABLE
Sleeping arrangements and their relationship to SIDS
| SLEEP ARRANGEMENT | RISK ESTIMATE* |
|---|---|
| Co-sleeping2-10 | Overall OR: 2.0 (1.2–3.3)4 to 16.47 (3.72–72.75)9 |
| OR if parent is smoker: 4.55 (2.63–7.88)10 to 17.7 (10.3–20.0)8 | |
| OR if parent is nonsmoker: 0.98 (0.44–2.18)10 to 2.20 (0.99–4.91)7 | |
| Sleeping in separate rooms5,6,8,11 | OR: 3.13 (1.82–5.26)8 to 10.49 (4.26–25.89)5 |
| Sleeping on couch or chair4-6,9 | 55 non-bed sleepers among 772 total SIDS cases (7.1%) vs 8 non-bed sleepers among 1854 total controls (0.4%)† |
| Soft bedding accessories4,7-9 | OR for use of pillow: 1.03 (0.66–1.59)7 to 2.8 (1.3–6.2)4 |
| OR for use of duvet: 1.32 (0.41–4.15)9 vs 1.82 (1.30–2.58)8 | |
| *All studies used multivariate analyses and controlled for tobacco use and infant sleep position. Risk estimates are lowest to highest OR with 95% CI (unless otherwise specified). | |
| †Aggregated data from 4 studies given small numbers. | |
| SIDS, sudden infant death syndrome; OR, odds ratio; CI, confidence interval. | |
A number of factors complicated this review. First, although all studies evaluated infants through 1 year of age, some excluded infants <7 days or <28 days old. Second, studies examined different sleep periods; 2 focused on usual sleeping arrangements,2,3 5 on sleeping arrangement immediately prior to death,4-8 and 2 evaluated both usual and last sleep arrangements.9,10 Third, variations in definitions of each risk factor and differences in the confounders controlled for made comparing studies challenging. Fourth, given the difficulty in studying infant deaths, the best evidence available comes from case-control studies.
Co-sleeping. Overall, 5 of 6 studies demonstrated co-sleeping to be an independent risk factor for SIDS (odds ratio [OR]=2.0–16.5),2,4-7,9 especially for infants younger than 11 weeks old.6,8 Four stratified analyses indicate that the risk of co-sleeping is greatest among infants of smokers (OR=4.6–17.7) as compared with infants of nonsmokers (OR=1.0–2.2).3,7,8,10 Some descriptive studies suggest potential benefits of co-sleeping, such as improved breastfeeding and maternal-infant bonding, but these benefits have not been quantified.1
Room sharing. Three of 4 studies found that infants sleeping in separate rooms from their caregivers had a 3-fold increased risk of SIDS,5,6,11 while the fourth study found a 10-fold increased risk.8 One study found the risk was present in infants less than 20 weeks, but was inconclusive for those greater than 20 weeks.11
Sleep surface. All 4 studies evaluating sleep surface found a significantly increased risk of SIDS for infants sleeping on sofas or armchairs compared with infants sleeping in beds or cribs. Fifty-five of 772 total cases (7.1%) from the 4 studies slept on a non-bed surface compared with 8 of 1854 controls (0.4%).4-6,9
Bedding accessories. Two of 3 studies found pillow use unrelated to SIDS.4,7,9 The larger of 2 studies on duvet use found it to be a risk factor for SIDS (OR=1.82).8
Recommendations from others
The American Academy of Pediatrics recommends that infants should sleep supine in the same room, but not the same bed, as their caregivers, while on a firm surface without bedding accessories. They should never sleep on a couch or armchair. Infants may be brought into bed briefly for feeding or comforting. Parents should be encouraged to quit smoking.10
1. American Academy of Pediatrics. Task Force on Infant Sleep Position and SIDS. Changing concepts of SIDS: Implications for infant sleeping environment and sleep position. Pediatrics 2000;105:650-656.
2. Brooke H, Gibson A, Tappin D, Brown H. Case-control study of SIDS in Scotland, 1992–5. BMJ 1997;314:1516-1520.
3. Mitchell EA, Tuohy PG, Brunt JM, et al. Risk factors for SIDS following the prevention campaign in New Zealand: A prospective study. Pediatrics 1997;100:835-840.
4. Hauck FR, Herman SM, Donovan M, et al. Sleep environment and the risk of SIDS in an urban population: The Chicago infant mortality study. Pediatrics 2003;111:1207-1214.
5. Blair PS, Fleming PJ, Smith IJ, et al. Babies sleeping with parents: Case-control study of factors influencing the risk of SIDS. BMJ 1999;319:1457-1461.
6. Tappin D, Ecob R, Brooke H. Bedsharing, roomsharing, and SIDS in Scotland: A case-control study. J Pediatr 2005;147:32-37.
7. Vennemann MM, Findeisen M, Butterfass-Bahloul T, et al. Modifiable risk factors for SIDS in Germany: Results of GeSID. Acta Paediatrica 2005;94:655-660.
8. Carpenter RG, Irgens LM, Blair PS, et al. Sudden unexplained infant death in 20 regions in Europe: Case control study. Lancet 2004;363:185-191.
9. McGarvey C, McDonnell M, Chong A, et al. Factors relating to the infant’s last sleep environment in SIDS in the Republic of Ireland. Arch Dis Child 2003;88:1058-1064.
10. Scragg R, Mitchell EA, Taylor BJ, et al. Bed sharing, smoking, and alcohol in SIDS. BMJ 1993;307:1312-1318.
11. Scragg RK, Mitchell EA, Stewart AW, et al. Infant room-sharing and prone sleep position in SIDS. Lancet 1996;347:7-12.
1. American Academy of Pediatrics. Task Force on Infant Sleep Position and SIDS. Changing concepts of SIDS: Implications for infant sleeping environment and sleep position. Pediatrics 2000;105:650-656.
2. Brooke H, Gibson A, Tappin D, Brown H. Case-control study of SIDS in Scotland, 1992–5. BMJ 1997;314:1516-1520.
3. Mitchell EA, Tuohy PG, Brunt JM, et al. Risk factors for SIDS following the prevention campaign in New Zealand: A prospective study. Pediatrics 1997;100:835-840.
4. Hauck FR, Herman SM, Donovan M, et al. Sleep environment and the risk of SIDS in an urban population: The Chicago infant mortality study. Pediatrics 2003;111:1207-1214.
5. Blair PS, Fleming PJ, Smith IJ, et al. Babies sleeping with parents: Case-control study of factors influencing the risk of SIDS. BMJ 1999;319:1457-1461.
6. Tappin D, Ecob R, Brooke H. Bedsharing, roomsharing, and SIDS in Scotland: A case-control study. J Pediatr 2005;147:32-37.
7. Vennemann MM, Findeisen M, Butterfass-Bahloul T, et al. Modifiable risk factors for SIDS in Germany: Results of GeSID. Acta Paediatrica 2005;94:655-660.
8. Carpenter RG, Irgens LM, Blair PS, et al. Sudden unexplained infant death in 20 regions in Europe: Case control study. Lancet 2004;363:185-191.
9. McGarvey C, McDonnell M, Chong A, et al. Factors relating to the infant’s last sleep environment in SIDS in the Republic of Ireland. Arch Dis Child 2003;88:1058-1064.
10. Scragg R, Mitchell EA, Taylor BJ, et al. Bed sharing, smoking, and alcohol in SIDS. BMJ 1993;307:1312-1318.
11. Scragg RK, Mitchell EA, Stewart AW, et al. Infant room-sharing and prone sleep position in SIDS. Lancet 1996;347:7-12.
Evidence-based answers from the Family Physicians Inquiries Network
What are appropriate screening tests for adolescents?
Screen all women of childbearing age, including adolescents, for rubella susceptibility (strength of recommendation [SOR]: B). Screen all sexually active adolescent females for chlamydia (SOR: A), gonorrhea (SOR: B), and cervical cancer (SOR: A). High-risk, sexually active adolescents should be screened for HIV and syphilis (SOR: A). Screen all adolescents at risk for tuberculosis (TB) infection (SOR: A).
Adolescent visits also provide opportunity to educate patients on nonmedical aspects of care
Andrea Darby-Stewart, MD
Department of Family Medicine, Mayo Clinic Arizona
Adolescent visits provide an opportunity to apply the biopsychosocial skills that enhance the care we provide as family physicians. In addition to screening for the diseases noted above, I take the opportunity to screen and educate these patients on “non-medical” aspects of care by using the HEADSSS assessment method. These open-ended questions regarding Home environment, Educational status and goals, extracurricular Activities, Drug use, Sexual activity and relationships, Suicide/depression risk, and Safety review allow me to get to know my patient better, and hopefully set the stage for open discussion of these topics in the future.
Evidence summary
The TABLE summarizes the recommendations of the US Preventive Services Task Force (USPSTF) with regard to adolescent screening.1 We identified no additional evidence-based recommendations for screening tests for adolescents.
As shown in the TABLE, rubella susceptibility screening is recommended for all adolescent females (SOR: B). Sexually active adolescent females should routinely be screened for chlamydia, gonorrhea, and cervical cancer. Adolescents at risk of contracting TB, HIV, or syphilis should be screened for those diseases.
Evidence is insufficient to recommend for or against performing the following tests for adolescents: hearing loss screening, anemia screening, clinical or self breast examination, blood pressure screening, screening for overweight, screening for alcohol misuse, screening for depression, and suicide risk screening. For males, evidence is insufficient to recommend for or against: rubella screening, routine rubella vaccination, and chlamydia or gonorrhea screening for sexually active males.
Do not perform the following tests on adolescents because evidence is good that the harms outweigh the benefits: testicular cancer screening using clinical or self-testicular examination, hepatitis B screening, screening for herpes, thyroid cancer screening, screening for scoliosis, and bacteriuria screening in asymptomatic non-pregnant adolescents. Screening for lipid disorders is recommended only for those over age 20 years who have significant risks for coronary artery disease.
TABLE
USPSTF evidence-supported screening tests for adolescents
| TEST (SOR) | POPULATION | USPSTF COMMENTS | AAFP | AAP AND AMA |
|---|---|---|---|---|
| Routine screening | ||||
| Rubella susceptibility (B) (with history of vaccination or serology) | All females of childbearing age | History of the disease is not adequate. For nonpregnant adolescents, an acceptable alternative is to offer vaccination against rubella without screening | Strongly recommends | Recommends |
| Chlamydia (A) | Sexually active females* | Insufficient evidence for or against screening males | Strongly recommends | Recommends |
| Gonorrhea (A) | Sexually active females* | Insufficient evidence for or against screening males | Recommends | Recommends |
| Cervical cancer (A) (with pap smear) | Sexually active females | Indirect evidence suggests screening should begin within 3 years of onset of sexual activity | Recommends | Recommends, and add HPV screening |
| High-risk screening | ||||
| HIV(A) | High risk† | Strongly recommends | Recommends | |
| Syphilis (A) | High risk‡ | Strongly recommends | Recommends | |
| Tuberculosis (A) (with PPD test) | High-risk** | Strongly recommends | Recommends | |
| Sources: USPSTF Guide to Clinical Preventive Services 1; AAFP Summary of Recommendations for Clinical Preventive Services2; AAP Recommendations for Preventive Pediatric Health Care3; AMA Guidelines for Adolescent Preventive Services (GAPS).4 | ||||
| SOR, strength of recommendation; USPSTF, US Preventive Services task Force; AAFP, American Academy of Family Physicians; AAP, American Academy of pediatricians; AM, American Medical Association | ||||
| * The interval for rescreening should take into account the frequency of changes in sexual partners. | ||||
| † Alist of HIV risks is available at: www.ahrq.gov/clinic/uspstf05/hiv/hivrs.htm#clinical. | ||||
| ‡ Alist of syphilis risks is available at: www.ahrq.gov/clinic/3rduspstf/syphilis/syphilrs.htm#clinical. | ||||
| ** Alist of tuberculosis risks is available at: www.ahrq.gov/clinic/2ndcps/tubercls.pdf (pp 282-283). | ||||
Recommendations from others
Several professional organizations provide recommendations for adolescent preventive services and screening tests. The American Academy of Family Physicians concurs with the USPSTF recommendations.2 The American Academy of Pediatrics (AAP)3 and the American Medical Association4 make several recommendations beyond those put forth by the USPSTF, including screening all adolescents for hypertension, risk for hyperlipidemia and adult coronary artery disease, eating disorders/obesity, and tobacco use. They also recommend extending chlamydia and gonorrhea screening to sexually active males.
The AAP also recommends conducting vision and hearing screening, developmental and behavioral assessment, hematocrit or hemoglobin for menstruating adolescents, urine leukocyte esterase for sexually active adolescents, and pelvic exams for sexually active females.
1. United States Preventive Services Task Force (USPSTF). Guide to Clinical Preventive Services. Available at www.ahrq.gov/clinic/cps3dix.htm. Accessed on September 6, 2006.
2. The American Academy of Family Practice (AAFP). Summary of Recommendations for Clinical Preventive Services, August 2005. Leawood, Kansas: AAFP; 2005. Available at www.aafp.org/PreBuilt/RCPS_August2005.pdf. Accessed on September 6, 2006.
3. American Academy of Pediatrics, Committee on Practice and Ambulatory Medicine. Recommendations for Preventive Pediatric Health Care, March 2000. Pediatrics 2000;105:645-646.Available at: aappolicy.aappublications.org/cgi/content/full/pediatrics;105/3/645. Accessed on September 6, 2006.
4. American Medical Association, Department of Adolescent Health. Guidelines for Adolescent Preventive Services (GAPS). Recommendations Monograph 1997. Chicago, Ill. Available at: www.ama-assn.org/ama/upload/mm/39/gapsmono.pdf. Accessed on September 6, 2006.
Screen all women of childbearing age, including adolescents, for rubella susceptibility (strength of recommendation [SOR]: B). Screen all sexually active adolescent females for chlamydia (SOR: A), gonorrhea (SOR: B), and cervical cancer (SOR: A). High-risk, sexually active adolescents should be screened for HIV and syphilis (SOR: A). Screen all adolescents at risk for tuberculosis (TB) infection (SOR: A).
Adolescent visits also provide opportunity to educate patients on nonmedical aspects of care
Andrea Darby-Stewart, MD
Department of Family Medicine, Mayo Clinic Arizona
Adolescent visits provide an opportunity to apply the biopsychosocial skills that enhance the care we provide as family physicians. In addition to screening for the diseases noted above, I take the opportunity to screen and educate these patients on “non-medical” aspects of care by using the HEADSSS assessment method. These open-ended questions regarding Home environment, Educational status and goals, extracurricular Activities, Drug use, Sexual activity and relationships, Suicide/depression risk, and Safety review allow me to get to know my patient better, and hopefully set the stage for open discussion of these topics in the future.
Evidence summary
The TABLE summarizes the recommendations of the US Preventive Services Task Force (USPSTF) with regard to adolescent screening.1 We identified no additional evidence-based recommendations for screening tests for adolescents.
As shown in the TABLE, rubella susceptibility screening is recommended for all adolescent females (SOR: B). Sexually active adolescent females should routinely be screened for chlamydia, gonorrhea, and cervical cancer. Adolescents at risk of contracting TB, HIV, or syphilis should be screened for those diseases.
Evidence is insufficient to recommend for or against performing the following tests for adolescents: hearing loss screening, anemia screening, clinical or self breast examination, blood pressure screening, screening for overweight, screening for alcohol misuse, screening for depression, and suicide risk screening. For males, evidence is insufficient to recommend for or against: rubella screening, routine rubella vaccination, and chlamydia or gonorrhea screening for sexually active males.
Do not perform the following tests on adolescents because evidence is good that the harms outweigh the benefits: testicular cancer screening using clinical or self-testicular examination, hepatitis B screening, screening for herpes, thyroid cancer screening, screening for scoliosis, and bacteriuria screening in asymptomatic non-pregnant adolescents. Screening for lipid disorders is recommended only for those over age 20 years who have significant risks for coronary artery disease.
TABLE
USPSTF evidence-supported screening tests for adolescents
| TEST (SOR) | POPULATION | USPSTF COMMENTS | AAFP | AAP AND AMA |
|---|---|---|---|---|
| Routine screening | ||||
| Rubella susceptibility (B) (with history of vaccination or serology) | All females of childbearing age | History of the disease is not adequate. For nonpregnant adolescents, an acceptable alternative is to offer vaccination against rubella without screening | Strongly recommends | Recommends |
| Chlamydia (A) | Sexually active females* | Insufficient evidence for or against screening males | Strongly recommends | Recommends |
| Gonorrhea (A) | Sexually active females* | Insufficient evidence for or against screening males | Recommends | Recommends |
| Cervical cancer (A) (with pap smear) | Sexually active females | Indirect evidence suggests screening should begin within 3 years of onset of sexual activity | Recommends | Recommends, and add HPV screening |
| High-risk screening | ||||
| HIV(A) | High risk† | Strongly recommends | Recommends | |
| Syphilis (A) | High risk‡ | Strongly recommends | Recommends | |
| Tuberculosis (A) (with PPD test) | High-risk** | Strongly recommends | Recommends | |
| Sources: USPSTF Guide to Clinical Preventive Services 1; AAFP Summary of Recommendations for Clinical Preventive Services2; AAP Recommendations for Preventive Pediatric Health Care3; AMA Guidelines for Adolescent Preventive Services (GAPS).4 | ||||
| SOR, strength of recommendation; USPSTF, US Preventive Services task Force; AAFP, American Academy of Family Physicians; AAP, American Academy of pediatricians; AM, American Medical Association | ||||
| * The interval for rescreening should take into account the frequency of changes in sexual partners. | ||||
| † Alist of HIV risks is available at: www.ahrq.gov/clinic/uspstf05/hiv/hivrs.htm#clinical. | ||||
| ‡ Alist of syphilis risks is available at: www.ahrq.gov/clinic/3rduspstf/syphilis/syphilrs.htm#clinical. | ||||
| ** Alist of tuberculosis risks is available at: www.ahrq.gov/clinic/2ndcps/tubercls.pdf (pp 282-283). | ||||
Recommendations from others
Several professional organizations provide recommendations for adolescent preventive services and screening tests. The American Academy of Family Physicians concurs with the USPSTF recommendations.2 The American Academy of Pediatrics (AAP)3 and the American Medical Association4 make several recommendations beyond those put forth by the USPSTF, including screening all adolescents for hypertension, risk for hyperlipidemia and adult coronary artery disease, eating disorders/obesity, and tobacco use. They also recommend extending chlamydia and gonorrhea screening to sexually active males.
The AAP also recommends conducting vision and hearing screening, developmental and behavioral assessment, hematocrit or hemoglobin for menstruating adolescents, urine leukocyte esterase for sexually active adolescents, and pelvic exams for sexually active females.
Screen all women of childbearing age, including adolescents, for rubella susceptibility (strength of recommendation [SOR]: B). Screen all sexually active adolescent females for chlamydia (SOR: A), gonorrhea (SOR: B), and cervical cancer (SOR: A). High-risk, sexually active adolescents should be screened for HIV and syphilis (SOR: A). Screen all adolescents at risk for tuberculosis (TB) infection (SOR: A).
Adolescent visits also provide opportunity to educate patients on nonmedical aspects of care
Andrea Darby-Stewart, MD
Department of Family Medicine, Mayo Clinic Arizona
Adolescent visits provide an opportunity to apply the biopsychosocial skills that enhance the care we provide as family physicians. In addition to screening for the diseases noted above, I take the opportunity to screen and educate these patients on “non-medical” aspects of care by using the HEADSSS assessment method. These open-ended questions regarding Home environment, Educational status and goals, extracurricular Activities, Drug use, Sexual activity and relationships, Suicide/depression risk, and Safety review allow me to get to know my patient better, and hopefully set the stage for open discussion of these topics in the future.
Evidence summary
The TABLE summarizes the recommendations of the US Preventive Services Task Force (USPSTF) with regard to adolescent screening.1 We identified no additional evidence-based recommendations for screening tests for adolescents.
As shown in the TABLE, rubella susceptibility screening is recommended for all adolescent females (SOR: B). Sexually active adolescent females should routinely be screened for chlamydia, gonorrhea, and cervical cancer. Adolescents at risk of contracting TB, HIV, or syphilis should be screened for those diseases.
Evidence is insufficient to recommend for or against performing the following tests for adolescents: hearing loss screening, anemia screening, clinical or self breast examination, blood pressure screening, screening for overweight, screening for alcohol misuse, screening for depression, and suicide risk screening. For males, evidence is insufficient to recommend for or against: rubella screening, routine rubella vaccination, and chlamydia or gonorrhea screening for sexually active males.
Do not perform the following tests on adolescents because evidence is good that the harms outweigh the benefits: testicular cancer screening using clinical or self-testicular examination, hepatitis B screening, screening for herpes, thyroid cancer screening, screening for scoliosis, and bacteriuria screening in asymptomatic non-pregnant adolescents. Screening for lipid disorders is recommended only for those over age 20 years who have significant risks for coronary artery disease.
TABLE
USPSTF evidence-supported screening tests for adolescents
| TEST (SOR) | POPULATION | USPSTF COMMENTS | AAFP | AAP AND AMA |
|---|---|---|---|---|
| Routine screening | ||||
| Rubella susceptibility (B) (with history of vaccination or serology) | All females of childbearing age | History of the disease is not adequate. For nonpregnant adolescents, an acceptable alternative is to offer vaccination against rubella without screening | Strongly recommends | Recommends |
| Chlamydia (A) | Sexually active females* | Insufficient evidence for or against screening males | Strongly recommends | Recommends |
| Gonorrhea (A) | Sexually active females* | Insufficient evidence for or against screening males | Recommends | Recommends |
| Cervical cancer (A) (with pap smear) | Sexually active females | Indirect evidence suggests screening should begin within 3 years of onset of sexual activity | Recommends | Recommends, and add HPV screening |
| High-risk screening | ||||
| HIV(A) | High risk† | Strongly recommends | Recommends | |
| Syphilis (A) | High risk‡ | Strongly recommends | Recommends | |
| Tuberculosis (A) (with PPD test) | High-risk** | Strongly recommends | Recommends | |
| Sources: USPSTF Guide to Clinical Preventive Services 1; AAFP Summary of Recommendations for Clinical Preventive Services2; AAP Recommendations for Preventive Pediatric Health Care3; AMA Guidelines for Adolescent Preventive Services (GAPS).4 | ||||
| SOR, strength of recommendation; USPSTF, US Preventive Services task Force; AAFP, American Academy of Family Physicians; AAP, American Academy of pediatricians; AM, American Medical Association | ||||
| * The interval for rescreening should take into account the frequency of changes in sexual partners. | ||||
| † Alist of HIV risks is available at: www.ahrq.gov/clinic/uspstf05/hiv/hivrs.htm#clinical. | ||||
| ‡ Alist of syphilis risks is available at: www.ahrq.gov/clinic/3rduspstf/syphilis/syphilrs.htm#clinical. | ||||
| ** Alist of tuberculosis risks is available at: www.ahrq.gov/clinic/2ndcps/tubercls.pdf (pp 282-283). | ||||
Recommendations from others
Several professional organizations provide recommendations for adolescent preventive services and screening tests. The American Academy of Family Physicians concurs with the USPSTF recommendations.2 The American Academy of Pediatrics (AAP)3 and the American Medical Association4 make several recommendations beyond those put forth by the USPSTF, including screening all adolescents for hypertension, risk for hyperlipidemia and adult coronary artery disease, eating disorders/obesity, and tobacco use. They also recommend extending chlamydia and gonorrhea screening to sexually active males.
The AAP also recommends conducting vision and hearing screening, developmental and behavioral assessment, hematocrit or hemoglobin for menstruating adolescents, urine leukocyte esterase for sexually active adolescents, and pelvic exams for sexually active females.
1. United States Preventive Services Task Force (USPSTF). Guide to Clinical Preventive Services. Available at www.ahrq.gov/clinic/cps3dix.htm. Accessed on September 6, 2006.
2. The American Academy of Family Practice (AAFP). Summary of Recommendations for Clinical Preventive Services, August 2005. Leawood, Kansas: AAFP; 2005. Available at www.aafp.org/PreBuilt/RCPS_August2005.pdf. Accessed on September 6, 2006.
3. American Academy of Pediatrics, Committee on Practice and Ambulatory Medicine. Recommendations for Preventive Pediatric Health Care, March 2000. Pediatrics 2000;105:645-646.Available at: aappolicy.aappublications.org/cgi/content/full/pediatrics;105/3/645. Accessed on September 6, 2006.
4. American Medical Association, Department of Adolescent Health. Guidelines for Adolescent Preventive Services (GAPS). Recommendations Monograph 1997. Chicago, Ill. Available at: www.ama-assn.org/ama/upload/mm/39/gapsmono.pdf. Accessed on September 6, 2006.
1. United States Preventive Services Task Force (USPSTF). Guide to Clinical Preventive Services. Available at www.ahrq.gov/clinic/cps3dix.htm. Accessed on September 6, 2006.
2. The American Academy of Family Practice (AAFP). Summary of Recommendations for Clinical Preventive Services, August 2005. Leawood, Kansas: AAFP; 2005. Available at www.aafp.org/PreBuilt/RCPS_August2005.pdf. Accessed on September 6, 2006.
3. American Academy of Pediatrics, Committee on Practice and Ambulatory Medicine. Recommendations for Preventive Pediatric Health Care, March 2000. Pediatrics 2000;105:645-646.Available at: aappolicy.aappublications.org/cgi/content/full/pediatrics;105/3/645. Accessed on September 6, 2006.
4. American Medical Association, Department of Adolescent Health. Guidelines for Adolescent Preventive Services (GAPS). Recommendations Monograph 1997. Chicago, Ill. Available at: www.ama-assn.org/ama/upload/mm/39/gapsmono.pdf. Accessed on September 6, 2006.
Evidence-based answers from the Family Physicians Inquiries Network
What are appropriate screening tests for infants and children?
There is adequate evidence for screening neonates for hemoglobinopathies, congenital hypothyroidism, phenylketonuria (strength of recommendation [SOR]: A), and cystic fibrosis (SOR: B). Vision screening should be done for those younger than age 5 years (SOR: B). High-risk children should be tested for tuberculosis (TB) (SOR: B) and lead toxicity (SOR: B). Few data exist to guide frequency and timing of these screening tests, so the following timing recommendations are based on consensus opinion (SOR: C): test for visual acuity yearly starting at age 3 years; test for TB and lead once between the ages of 9 and 12 months, and repeat for high risk or exposure.
Obtain family history; order additional screening tests if history suggests them
Vince WinklerPrins, MD, FAAFP
Michigan State University, East Lansing
Why do states differ so much in the neonatal screening tests that they routinely perform? Some states screen for only a few genetic diseases, others for more than 40. Most states do neonatal hearing screening despite limited evidence of utility, while only one quarter of all states have neonatal cystic fibrosis screening programs, a condition for which there is probably better evidence for screening. While we might like to think that good science alone would dictate screening policy, the economic circumstances of each state, variable interpretation/quality of the research reviewed, and legislative priorities (among many reasons) probably play at least as much a roll. For any test, its accuracy is only as good as the pretest probability of the disease for which it is being used. Our yield for cystic fibrosis screening will be higher in families with a history of cystic fibrosis. This is the key point—you still need to obtain a family history and order additional screening tests if the history suggests them.
Evidence summary
There are many opinions and recommendations about what constitutes quality health surveillance for children. However, many screening tests for children lack evidence of effectiveness and information on harms.1 The scope of this question required use of evidence published in high-quality systematic reviews. The US Preventive Services Task Force (USPSTF) provides the most rigorous evidence on which to base recommendations.2 Medline was searched for any additional individual studies of interest. The USPSTF has conducted reviews for selected screening tests for children; the TABLE summarizes those with sufficient evidence to recommend them. We identified 1 additional evidence-based recommendation from the Centers for Disease Control and Prevention. This report, based on a systematic review, recommends cystic fibrosis screening in neonates based on moderate benefits and low risks of harm.3
The TABLE summarizes the evidence supporting universal childhood screening for hemoglobinopathies, congenital hypothyroidism, phenylketonuria, and visual defects; and high-risk childhood screening for tuberculosis and lead toxicity. The TABLE also lists the recommendations from the American Academy of Pediatrics (AAP) on frequency and timing of screening as guided by consensus opinion.
The USPSTF recommendations supporting screening for hemoglobinopathies, congenital hypothyroidism, and phenylketonuria are considered standard of care. The USPSTF believes that updating these 1996 recommendations would have little impact on clinical practice.
The USPSTF recommendations supporting vision screening found no direct evidence supporting screening for visual acuity. One fair-quality controlled study (N=3490) showed a decreased prevalence of amblyopia in the screened group and evidence that treatment of amblyotic risk factors prevents amblyopia. A Cochrane review of this topic showed insufficient evidence for visual screening of older (school-aged) children; for amblyopia, no data sufficient for analysis was found.4,5
The USPSTF recommendation to screen asymptomatic high-risk children for TB is based on the effectiveness of early intervention (14 controlled trials) and the accuracy of the Mantoux test.
TABLE
US Preventive Services Task Force evidence-supported testing for children
| TEST(SOR) | POPULATION | USPSTF COMMENTS | AAFP | AAP |
| UNIVERSALSCREENING | ||||
| Neonatal hemoglobinopathy (A) | Newborns | Strongly recommends | Recommends once at 2 to 4 days of life, but before age 1 month | |
| Neonatal phenylketonuria (A) | Newborns—repeat at 2 weeks if <24 hrs old at discharge | Strongly recommends | ||
| Congenital hypothyroidism (A) | Newborns | Strongly recommends | ||
| Vision screening for strabismus, amblyopia, and refractive error (B) | Before age 5 | Type of screening tests vary with age; evidence inadequate to recommend specific test | Recommends | Start objective testing yearly at age 3 |
| HIGH-RISK SCREENING | ||||
| PPD test for tuberculosis (A) | Children at high-risk for TB | Risks for TB: HIV,close contacts of persons with TB, immigrants from countries with high TB prevalence, low income populations, and residents of long-term care facilities | Strongly recommends screening high-risk Children | Screen high-risk children at 12 months and or upon recognition of high-risk factors |
| Lead toxicity (B)* | Infants at risk at 12 months of age at risk for lead exposure | Risks for lead† | Screen infants at high risk | Screen high-risk infants at 9 to 12 months. Repeat at age 24 months for those at high-risk |
| * This document is currently being updated; the recommendation may or may not change. | ||||
| † Risk are living in a house older than 1950 with peeling paint or remodeling, living near heavy traffic or lead industry, living with someone who has elevated lead levels or whose job/hobby involves lead exposure, using lead-based pottery, or taking remedies that contain lead. | ||||
| AAFP: American Academy of Family Practice recommendations from: www.aafp.org/PreBuilt/RCPS_August2005.pdf. | ||||
| AAP: American Academy of Pediatrics recommendations from: aappolicy.aappublications.org/cgi/content/full/pediatrics;105/3/645. | ||||
The USPSTF document on screening for lead levels is currently being revised and the recommendation may change. Although no controlled studies directly show that screening high-risk children for lead exposure improves clinical outcomes, several lesser-quality studies create a logical path to this conclusion.
The USPSTF finds there is insufficient evidence to recommend for or against performing the following screening tests in children: blood pressure screening; screening for overweight in children and adolescents; and iron deficiency screening in asymptomatic infants. Both Cochrane Systematic Reviews and USPSTF found insufficient evidence to support universal hearing screening, including neonatal hearing screening.6 The USPSTF makes no recommendation regarding screening high-risk children for hyperlipidemia.
The USPSTF recommends that the following tests should not be performed in children because there is good evidence that the harms outweigh the benefits: thyroid cancer screening in children and bacteriuria screening in asymptomatic nonpregnant children.
Recommendations from others
There are numerous guidelines recommending various sets of preventive services for children, but there are few evidence-based recommendations. The AAP recommendations can be found in Guidelines for Health Supervision III.7 The AAP also publishes policy statements and guidelines in the journal Pediatrics. The American Academy of Family Practice’s (AAFP) recommendations on health supervision can be found at: www.aafp.org/PreBuilt/RCPS_August2005.pdf.
A summary of the AAFP and the AAP recommendations on each of the USPSTF supported tests is in the TABLE. While AAFP and USPSTF recommendations concur, AAP recommendations differ in recommending hearing screening for all newborns, iron deficiency screening at 9 months of age, screening for lipid disorders in children at risk starting at 24 months, and screening urinalysis at age 5 years.
1. Moyer VA, Butler M. Gaps in the evidence for well-child care: A challenge to our profession. Pediatrics 2004;114:1511-1521.
2. US Preventive Services Task Force. Guide to Clinical Preventive Services [website]. Available at: www.ahrq.gov/clinic/cps3dix.htm. Accessed on June 28, 2006.
3. Grosse SD, Boyle CA, Botkin JR, et al. Newborn screening for cystic fibrosis: evaluation of benefits and risks and recommendations for state newborn screening programs. MMWR Recomm Rep 2004;53(RR-13):1-36.
4. Powell C, Porooshani H, Bohorquez MC, Richardson S. Screening for amblyopia in childhood. Cochrane Database Syst Rev 2005;(3)::CD005020.-
5. Powell C, Wedner S, Richardson S. Screening for correctable visual acuity deficits in school-age children and adolescents. Cochrane Database Syst Rev 2005;(1):CD005023.-
6. Puig T, Municio A, Meda C. Universal neonatal hearing screening versus selective screening as part of the management of childhood deafness. Cochrane Database Syst Rev 2005;(2):CD003731.-
7. Guidelines for Health Supervision III. Elk Grove Village, Ill: American Academy of Pediatrics; 1997. (Updated 2002).
There is adequate evidence for screening neonates for hemoglobinopathies, congenital hypothyroidism, phenylketonuria (strength of recommendation [SOR]: A), and cystic fibrosis (SOR: B). Vision screening should be done for those younger than age 5 years (SOR: B). High-risk children should be tested for tuberculosis (TB) (SOR: B) and lead toxicity (SOR: B). Few data exist to guide frequency and timing of these screening tests, so the following timing recommendations are based on consensus opinion (SOR: C): test for visual acuity yearly starting at age 3 years; test for TB and lead once between the ages of 9 and 12 months, and repeat for high risk or exposure.
Obtain family history; order additional screening tests if history suggests them
Vince WinklerPrins, MD, FAAFP
Michigan State University, East Lansing
Why do states differ so much in the neonatal screening tests that they routinely perform? Some states screen for only a few genetic diseases, others for more than 40. Most states do neonatal hearing screening despite limited evidence of utility, while only one quarter of all states have neonatal cystic fibrosis screening programs, a condition for which there is probably better evidence for screening. While we might like to think that good science alone would dictate screening policy, the economic circumstances of each state, variable interpretation/quality of the research reviewed, and legislative priorities (among many reasons) probably play at least as much a roll. For any test, its accuracy is only as good as the pretest probability of the disease for which it is being used. Our yield for cystic fibrosis screening will be higher in families with a history of cystic fibrosis. This is the key point—you still need to obtain a family history and order additional screening tests if the history suggests them.
Evidence summary
There are many opinions and recommendations about what constitutes quality health surveillance for children. However, many screening tests for children lack evidence of effectiveness and information on harms.1 The scope of this question required use of evidence published in high-quality systematic reviews. The US Preventive Services Task Force (USPSTF) provides the most rigorous evidence on which to base recommendations.2 Medline was searched for any additional individual studies of interest. The USPSTF has conducted reviews for selected screening tests for children; the TABLE summarizes those with sufficient evidence to recommend them. We identified 1 additional evidence-based recommendation from the Centers for Disease Control and Prevention. This report, based on a systematic review, recommends cystic fibrosis screening in neonates based on moderate benefits and low risks of harm.3
The TABLE summarizes the evidence supporting universal childhood screening for hemoglobinopathies, congenital hypothyroidism, phenylketonuria, and visual defects; and high-risk childhood screening for tuberculosis and lead toxicity. The TABLE also lists the recommendations from the American Academy of Pediatrics (AAP) on frequency and timing of screening as guided by consensus opinion.
The USPSTF recommendations supporting screening for hemoglobinopathies, congenital hypothyroidism, and phenylketonuria are considered standard of care. The USPSTF believes that updating these 1996 recommendations would have little impact on clinical practice.
The USPSTF recommendations supporting vision screening found no direct evidence supporting screening for visual acuity. One fair-quality controlled study (N=3490) showed a decreased prevalence of amblyopia in the screened group and evidence that treatment of amblyotic risk factors prevents amblyopia. A Cochrane review of this topic showed insufficient evidence for visual screening of older (school-aged) children; for amblyopia, no data sufficient for analysis was found.4,5
The USPSTF recommendation to screen asymptomatic high-risk children for TB is based on the effectiveness of early intervention (14 controlled trials) and the accuracy of the Mantoux test.
TABLE
US Preventive Services Task Force evidence-supported testing for children
| TEST(SOR) | POPULATION | USPSTF COMMENTS | AAFP | AAP |
| UNIVERSALSCREENING | ||||
| Neonatal hemoglobinopathy (A) | Newborns | Strongly recommends | Recommends once at 2 to 4 days of life, but before age 1 month | |
| Neonatal phenylketonuria (A) | Newborns—repeat at 2 weeks if <24 hrs old at discharge | Strongly recommends | ||
| Congenital hypothyroidism (A) | Newborns | Strongly recommends | ||
| Vision screening for strabismus, amblyopia, and refractive error (B) | Before age 5 | Type of screening tests vary with age; evidence inadequate to recommend specific test | Recommends | Start objective testing yearly at age 3 |
| HIGH-RISK SCREENING | ||||
| PPD test for tuberculosis (A) | Children at high-risk for TB | Risks for TB: HIV,close contacts of persons with TB, immigrants from countries with high TB prevalence, low income populations, and residents of long-term care facilities | Strongly recommends screening high-risk Children | Screen high-risk children at 12 months and or upon recognition of high-risk factors |
| Lead toxicity (B)* | Infants at risk at 12 months of age at risk for lead exposure | Risks for lead† | Screen infants at high risk | Screen high-risk infants at 9 to 12 months. Repeat at age 24 months for those at high-risk |
| * This document is currently being updated; the recommendation may or may not change. | ||||
| † Risk are living in a house older than 1950 with peeling paint or remodeling, living near heavy traffic or lead industry, living with someone who has elevated lead levels or whose job/hobby involves lead exposure, using lead-based pottery, or taking remedies that contain lead. | ||||
| AAFP: American Academy of Family Practice recommendations from: www.aafp.org/PreBuilt/RCPS_August2005.pdf. | ||||
| AAP: American Academy of Pediatrics recommendations from: aappolicy.aappublications.org/cgi/content/full/pediatrics;105/3/645. | ||||
The USPSTF document on screening for lead levels is currently being revised and the recommendation may change. Although no controlled studies directly show that screening high-risk children for lead exposure improves clinical outcomes, several lesser-quality studies create a logical path to this conclusion.
The USPSTF finds there is insufficient evidence to recommend for or against performing the following screening tests in children: blood pressure screening; screening for overweight in children and adolescents; and iron deficiency screening in asymptomatic infants. Both Cochrane Systematic Reviews and USPSTF found insufficient evidence to support universal hearing screening, including neonatal hearing screening.6 The USPSTF makes no recommendation regarding screening high-risk children for hyperlipidemia.
The USPSTF recommends that the following tests should not be performed in children because there is good evidence that the harms outweigh the benefits: thyroid cancer screening in children and bacteriuria screening in asymptomatic nonpregnant children.
Recommendations from others
There are numerous guidelines recommending various sets of preventive services for children, but there are few evidence-based recommendations. The AAP recommendations can be found in Guidelines for Health Supervision III.7 The AAP also publishes policy statements and guidelines in the journal Pediatrics. The American Academy of Family Practice’s (AAFP) recommendations on health supervision can be found at: www.aafp.org/PreBuilt/RCPS_August2005.pdf.
A summary of the AAFP and the AAP recommendations on each of the USPSTF supported tests is in the TABLE. While AAFP and USPSTF recommendations concur, AAP recommendations differ in recommending hearing screening for all newborns, iron deficiency screening at 9 months of age, screening for lipid disorders in children at risk starting at 24 months, and screening urinalysis at age 5 years.
There is adequate evidence for screening neonates for hemoglobinopathies, congenital hypothyroidism, phenylketonuria (strength of recommendation [SOR]: A), and cystic fibrosis (SOR: B). Vision screening should be done for those younger than age 5 years (SOR: B). High-risk children should be tested for tuberculosis (TB) (SOR: B) and lead toxicity (SOR: B). Few data exist to guide frequency and timing of these screening tests, so the following timing recommendations are based on consensus opinion (SOR: C): test for visual acuity yearly starting at age 3 years; test for TB and lead once between the ages of 9 and 12 months, and repeat for high risk or exposure.
Obtain family history; order additional screening tests if history suggests them
Vince WinklerPrins, MD, FAAFP
Michigan State University, East Lansing
Why do states differ so much in the neonatal screening tests that they routinely perform? Some states screen for only a few genetic diseases, others for more than 40. Most states do neonatal hearing screening despite limited evidence of utility, while only one quarter of all states have neonatal cystic fibrosis screening programs, a condition for which there is probably better evidence for screening. While we might like to think that good science alone would dictate screening policy, the economic circumstances of each state, variable interpretation/quality of the research reviewed, and legislative priorities (among many reasons) probably play at least as much a roll. For any test, its accuracy is only as good as the pretest probability of the disease for which it is being used. Our yield for cystic fibrosis screening will be higher in families with a history of cystic fibrosis. This is the key point—you still need to obtain a family history and order additional screening tests if the history suggests them.
Evidence summary
There are many opinions and recommendations about what constitutes quality health surveillance for children. However, many screening tests for children lack evidence of effectiveness and information on harms.1 The scope of this question required use of evidence published in high-quality systematic reviews. The US Preventive Services Task Force (USPSTF) provides the most rigorous evidence on which to base recommendations.2 Medline was searched for any additional individual studies of interest. The USPSTF has conducted reviews for selected screening tests for children; the TABLE summarizes those with sufficient evidence to recommend them. We identified 1 additional evidence-based recommendation from the Centers for Disease Control and Prevention. This report, based on a systematic review, recommends cystic fibrosis screening in neonates based on moderate benefits and low risks of harm.3
The TABLE summarizes the evidence supporting universal childhood screening for hemoglobinopathies, congenital hypothyroidism, phenylketonuria, and visual defects; and high-risk childhood screening for tuberculosis and lead toxicity. The TABLE also lists the recommendations from the American Academy of Pediatrics (AAP) on frequency and timing of screening as guided by consensus opinion.
The USPSTF recommendations supporting screening for hemoglobinopathies, congenital hypothyroidism, and phenylketonuria are considered standard of care. The USPSTF believes that updating these 1996 recommendations would have little impact on clinical practice.
The USPSTF recommendations supporting vision screening found no direct evidence supporting screening for visual acuity. One fair-quality controlled study (N=3490) showed a decreased prevalence of amblyopia in the screened group and evidence that treatment of amblyotic risk factors prevents amblyopia. A Cochrane review of this topic showed insufficient evidence for visual screening of older (school-aged) children; for amblyopia, no data sufficient for analysis was found.4,5
The USPSTF recommendation to screen asymptomatic high-risk children for TB is based on the effectiveness of early intervention (14 controlled trials) and the accuracy of the Mantoux test.
TABLE
US Preventive Services Task Force evidence-supported testing for children
| TEST(SOR) | POPULATION | USPSTF COMMENTS | AAFP | AAP |
| UNIVERSALSCREENING | ||||
| Neonatal hemoglobinopathy (A) | Newborns | Strongly recommends | Recommends once at 2 to 4 days of life, but before age 1 month | |
| Neonatal phenylketonuria (A) | Newborns—repeat at 2 weeks if <24 hrs old at discharge | Strongly recommends | ||
| Congenital hypothyroidism (A) | Newborns | Strongly recommends | ||
| Vision screening for strabismus, amblyopia, and refractive error (B) | Before age 5 | Type of screening tests vary with age; evidence inadequate to recommend specific test | Recommends | Start objective testing yearly at age 3 |
| HIGH-RISK SCREENING | ||||
| PPD test for tuberculosis (A) | Children at high-risk for TB | Risks for TB: HIV,close contacts of persons with TB, immigrants from countries with high TB prevalence, low income populations, and residents of long-term care facilities | Strongly recommends screening high-risk Children | Screen high-risk children at 12 months and or upon recognition of high-risk factors |
| Lead toxicity (B)* | Infants at risk at 12 months of age at risk for lead exposure | Risks for lead† | Screen infants at high risk | Screen high-risk infants at 9 to 12 months. Repeat at age 24 months for those at high-risk |
| * This document is currently being updated; the recommendation may or may not change. | ||||
| † Risk are living in a house older than 1950 with peeling paint or remodeling, living near heavy traffic or lead industry, living with someone who has elevated lead levels or whose job/hobby involves lead exposure, using lead-based pottery, or taking remedies that contain lead. | ||||
| AAFP: American Academy of Family Practice recommendations from: www.aafp.org/PreBuilt/RCPS_August2005.pdf. | ||||
| AAP: American Academy of Pediatrics recommendations from: aappolicy.aappublications.org/cgi/content/full/pediatrics;105/3/645. | ||||
The USPSTF document on screening for lead levels is currently being revised and the recommendation may change. Although no controlled studies directly show that screening high-risk children for lead exposure improves clinical outcomes, several lesser-quality studies create a logical path to this conclusion.
The USPSTF finds there is insufficient evidence to recommend for or against performing the following screening tests in children: blood pressure screening; screening for overweight in children and adolescents; and iron deficiency screening in asymptomatic infants. Both Cochrane Systematic Reviews and USPSTF found insufficient evidence to support universal hearing screening, including neonatal hearing screening.6 The USPSTF makes no recommendation regarding screening high-risk children for hyperlipidemia.
The USPSTF recommends that the following tests should not be performed in children because there is good evidence that the harms outweigh the benefits: thyroid cancer screening in children and bacteriuria screening in asymptomatic nonpregnant children.
Recommendations from others
There are numerous guidelines recommending various sets of preventive services for children, but there are few evidence-based recommendations. The AAP recommendations can be found in Guidelines for Health Supervision III.7 The AAP also publishes policy statements and guidelines in the journal Pediatrics. The American Academy of Family Practice’s (AAFP) recommendations on health supervision can be found at: www.aafp.org/PreBuilt/RCPS_August2005.pdf.
A summary of the AAFP and the AAP recommendations on each of the USPSTF supported tests is in the TABLE. While AAFP and USPSTF recommendations concur, AAP recommendations differ in recommending hearing screening for all newborns, iron deficiency screening at 9 months of age, screening for lipid disorders in children at risk starting at 24 months, and screening urinalysis at age 5 years.
1. Moyer VA, Butler M. Gaps in the evidence for well-child care: A challenge to our profession. Pediatrics 2004;114:1511-1521.
2. US Preventive Services Task Force. Guide to Clinical Preventive Services [website]. Available at: www.ahrq.gov/clinic/cps3dix.htm. Accessed on June 28, 2006.
3. Grosse SD, Boyle CA, Botkin JR, et al. Newborn screening for cystic fibrosis: evaluation of benefits and risks and recommendations for state newborn screening programs. MMWR Recomm Rep 2004;53(RR-13):1-36.
4. Powell C, Porooshani H, Bohorquez MC, Richardson S. Screening for amblyopia in childhood. Cochrane Database Syst Rev 2005;(3)::CD005020.-
5. Powell C, Wedner S, Richardson S. Screening for correctable visual acuity deficits in school-age children and adolescents. Cochrane Database Syst Rev 2005;(1):CD005023.-
6. Puig T, Municio A, Meda C. Universal neonatal hearing screening versus selective screening as part of the management of childhood deafness. Cochrane Database Syst Rev 2005;(2):CD003731.-
7. Guidelines for Health Supervision III. Elk Grove Village, Ill: American Academy of Pediatrics; 1997. (Updated 2002).
1. Moyer VA, Butler M. Gaps in the evidence for well-child care: A challenge to our profession. Pediatrics 2004;114:1511-1521.
2. US Preventive Services Task Force. Guide to Clinical Preventive Services [website]. Available at: www.ahrq.gov/clinic/cps3dix.htm. Accessed on June 28, 2006.
3. Grosse SD, Boyle CA, Botkin JR, et al. Newborn screening for cystic fibrosis: evaluation of benefits and risks and recommendations for state newborn screening programs. MMWR Recomm Rep 2004;53(RR-13):1-36.
4. Powell C, Porooshani H, Bohorquez MC, Richardson S. Screening for amblyopia in childhood. Cochrane Database Syst Rev 2005;(3)::CD005020.-
5. Powell C, Wedner S, Richardson S. Screening for correctable visual acuity deficits in school-age children and adolescents. Cochrane Database Syst Rev 2005;(1):CD005023.-
6. Puig T, Municio A, Meda C. Universal neonatal hearing screening versus selective screening as part of the management of childhood deafness. Cochrane Database Syst Rev 2005;(2):CD003731.-
7. Guidelines for Health Supervision III. Elk Grove Village, Ill: American Academy of Pediatrics; 1997. (Updated 2002).
The Journal of Family Practice ©2006 Dowden Health Media