User login
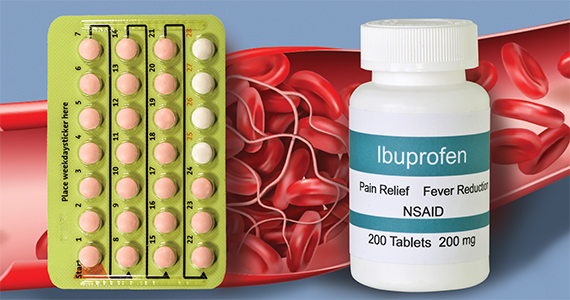
Does taking an NSAID while on hormonal contraception increase VTE risk?
Meaidi A, Mascolo A, Sessa M, et al. Venous thromboembolism with use of hormonal contraception and non-steroidal anti-inflammatory drugs: nationwide cohort study. BMJ. 2023;382:e074450. doi:10.1136/bmj-2022-074450
EXPERT COMMENTARY
Combination (estrogen plus progestin) hormonal contraceptives as well as non–aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk of VTE events, including lower extremity clots and pulmonary embolism. Taking contraceptives formulated with ethinyl estradiol increases hepatic production of clotting factors on a dose-related basis. Newer progestins, including desogestrel and drospirenone, also may contribute to an elevated VTE risk, although this association is controversial.1 NSAIDs promote platelet aggregation, thereby activating the clotting system and formation of clots. Although studies that assessed the association between NSAID use and thrombosis have focused on arterial clots, a substantial literature suggests that NSAIDs, including older NSAIDs (such as ibuprofen, diclofenac, and naproxen), also increase VTE risk.2
Although combination contraceptives (oral contraceptives, patches, vaginal rings) and NSAIDs are both commonly used by reproductive-age women, little data have assessed the impact of concomitant use of these medications on VTE risk. Accordingly, investigators in Denmark, using national databases, conducted a retrospective cohort study to assess the impact that independent as well as concomitant use of these medications have on VTE risk.
Details of the study
Meaidi and colleagues included in the cohort reproductive-age women living in Denmark between 1996 and 2017 with no history of thrombosis, thrombophilia, cancer, tubal sterilization, hysterectomy, bilateral oophorectomy, or infertility treatment. National prescription data were used to assess exposure to hormonal contraception.
The investigators classified hormonal contraception into 3 VTE risk categories:
- high risk—estrogen-progestin patches and vaginal rings; oral contraceptives containing 50 µg of ethinyl estradiol; or the progestins desogestrel, drospirenone, gestodene, or cyproterone (with the latter 2 progestins not available in the United States)
- medium risk—all other combination oral contraceptives, including those formulated with the progestins norethindrone, norethindrone acetate, norgestrel, and levonorgestrel, as well as depot medroxyprogesterone acetate
- low/no risk—progestin-only pills, implants, and progestin-containing intrauterine devices (IUDs).
Because in Denmark NSAIDs are prescribed as a single package containing no more than 30 tablets, time exposed to non–aspirin NSAIDs was assumed to last 1 week from the prescription date.
The authors considered first-time diagnoses of lower limb venous thrombosis or pulmonary embolism that were made in hospitals to represent VTE. They also constructed a subgroup of VTE patients in whom the diagnosis was either confirmed with imaging or followed by prescription of an anticoagulant.
To address potential confounding, the authors adjusted their analysis based on age, calendar year, educational attainment, occurrence of pregnancy, surgery, hypertension, diabetes, polycystic ovary syndrome, endometriosis, migraine, systemic connective tissue diseases, inflammatory polyarthropathies, and use of tranexamic acid (a medication that may increase VTE risk). They also censored (temporarily excluded women from analysis) episodes associated with a transiently elevated risk of VTE: pregnancy and 6 months following delivery, 12 weeks after other pregnancy terminations, 8 weeks following any surgery involving hospital admission, and 8 weeks following prescription of tranexamic acid.
Continue to: VTEs associated with risk category of hormonal contraception used...
VTEs associated with risk category of hormonal contraception used
Results. The overall cohort included more than 2 million women who were followed for a median of 10 years. During 21.0 million person-years, 8,710 VTE events were diagnosed; almost one-third of these were pulmonary embolisms, with the remainder diagnosed as lower extremity VTE. Of these 8,710 women diagnosed with VTE, 7,043 (81%) were confirmed with either diagnostic imaging or prescription of an anticoagulant. Unfortunately, 228 women (2.6%) died within 30 days of the diagnosis of VTE.
The investigators identified concomitant use of hormonal contraception and NSAIDs in more than 500,000 women. Among women with such concomitant use, 58% were using contraceptives that were high risk while 23% used medium-risk and 19% used low/no-risk contraceptives. Ibuprofen (60%) was the most commonly used NSAID, followed by diclofenac (20%) and naproxen (6%). Between 97% and 98% of high-risk and medium-risk contraceptives were combination pills; 89% of low/no-risk contraceptives were progestin IUDs.
Compared with nonuse of both hormonal contraceptives and NSAIDs, incidence rate ratios of VTE adjusted for age, calendar year, and education were 8.1 (95% confidence interval [CI], 6.9–9.6) for use of NSAIDs only, 4.2 (95% CI, 4.0–4.4) for use of high-risk contraceptives only, 3.0 (95% CI, 2.8–3.2) for medium-risk contraceptive use, and 1.1 (95% CI, 1.0–1.3) for use of low/no-risk hormonal contraception. Risk of VTE was approximately twice as high with the use of diclofenac only compared with the risks associated with ibuprofen or naproxen use only.
With respect to concomitant use of NSAIDs and hormonal contraception, incidence rate ratios of VTE were 50.6 (95% CI, 44.2–57.8), 26.1 (95% CI, 19.6–34.7), and 5.7 (95% CI, 3.3–10.1), respectively, with use of high-risk, medium-risk, and low/no-risk hormonal contraceptives. Adjusting for time updated information on occurrences of migraine, connective tissue disorder, inflammatory polyarthropathies, endometriosis, polycystic ovary syndrome, hypertension, and diabetes did not materially affect these associations.
When analysis was limited to women without these occurring conditions, rate ratios were somewhat higher (5.7 and 4.1) for use of high-risk and medium-risk contraceptives only. Incidence rate ratios in this subcohort of healthier women were substantially higher for NSAID use only (15.0), and 111.7, 43.2, and 13.0, respectively, for concomitant use of NSAIDs with high-risk, medium-risk, and low/no-risk contraceptives. In this analysis of healthier women, diclofenac continued to be associated with substantially higher risks of VTE than ibuprofen or naproxen. When the stricter definition of VTE (confirmed cases) was used, adjusted rate ratios remained similar.
Absolute risks of VTE
Although some of the elevated rate ratios noted in this study might appear alarming, it is important to keep in mind that the baseline incidence of VTE in healthy reproductive-age women is low. Accordingly, as the authors pointed out, even among women who used NSAIDs concomitantly with high-risk combination hormonal contraceptives, the absolute risk of VTE was 2/10,000.
Study strengths and limitations
Strengths of this analysis by Meaidi and colleagues include the use of large, essentially all-inclusive national registries. In addition, nationwide Danish registry data that indicate a diagnosis of VTE have been found to have a high positive predictive value.3 Another strength is the large number of potentially confounding factors that the authors controlled for.
One potential limitation of their analysis is that the use of only prescribed NSAIDs was considered. Fortunately, however, the prevalence of over-the-counter ibuprofen use in Denmark is not high enough to materially affect the authors’ findings.4 Another potential limitation was that information on smoking and body mass index was not available for most of the women included in the study cohort. The authors countered this limitation by pointing out that, in Denmark, smoking and obesity are highly correlated with educational status, and that all analyses were adjusted for educational status. ●
It is important for clinicians and our patients to recognize that pregnancy—the condition prevented by hormonal contraception— is associated with far higher risks of VTE (10–14 VTE events per 10,000 deliveries) than the use of any modern hormonal contraceptive.5 Although concomitant use of combination contraceptives and NSAIDs increases VTE risk, the absolute risk is modest, particularly when the NSAID is ibuprofen or naproxen (these are the non–aspirin NSAIDs most commonly used in the United States6). Women who regularly take NSAIDs can minimize VTE risk by choosing hormonal contraceptives with little or no impact on the risk of VTE: the progestin implant, progestin IUDs, and progestinonly pills.
ANDREW M. KAUNITZ, MD, MSCP
- Reid RL. Oral hormonal contraception and venous thromboembolism (VTE). Contraception. 2014;89:235-236. doi:10.1016/j.contraception.2014.02.002
- Ungprasert P, Srivali N, Wijarnpreecha K, et al. Nonsteroidal anti-inflammatory drugs and risk of venous thromboembolism: a systematic review and meta-analysis. Rheumatology (Oxford). 2015;54:736-742. doi:10.1093 /rheumatology/keu408
- Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. doi:10.1136/bmjopen-2016-012832
- Gaster N, Hallas J, Pottegård A, et al. The validity of Danish prescription data to measure use of aspirin and other nonsteroidal anti-inflammatory drugs and quantification of bias due to non-prescription drug use. Clin Epidemiol. 2021;13:569-579. doi:10.2147/CLEP.S311450
- Maughan BC, Marin M, Han J, et al. Venous thromboembolism during pregnancy and the postpartum period: risk factors, diagnostic testing, and treatment. Obstet Gynecol Surv. 2022;77:433-444. doi:10.1097/OGX.0000000000001043
- Chu A. Ibuprofen, naproxen, and more: the 8 most common NSAIDs. GoodRx. July 20, 2023. Accessed October 4, 2023. https://www.goodrx.com/classes/nsaids/nsaid-list
Meaidi A, Mascolo A, Sessa M, et al. Venous thromboembolism with use of hormonal contraception and non-steroidal anti-inflammatory drugs: nationwide cohort study. BMJ. 2023;382:e074450. doi:10.1136/bmj-2022-074450
EXPERT COMMENTARY
Combination (estrogen plus progestin) hormonal contraceptives as well as non–aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk of VTE events, including lower extremity clots and pulmonary embolism. Taking contraceptives formulated with ethinyl estradiol increases hepatic production of clotting factors on a dose-related basis. Newer progestins, including desogestrel and drospirenone, also may contribute to an elevated VTE risk, although this association is controversial.1 NSAIDs promote platelet aggregation, thereby activating the clotting system and formation of clots. Although studies that assessed the association between NSAID use and thrombosis have focused on arterial clots, a substantial literature suggests that NSAIDs, including older NSAIDs (such as ibuprofen, diclofenac, and naproxen), also increase VTE risk.2
Although combination contraceptives (oral contraceptives, patches, vaginal rings) and NSAIDs are both commonly used by reproductive-age women, little data have assessed the impact of concomitant use of these medications on VTE risk. Accordingly, investigators in Denmark, using national databases, conducted a retrospective cohort study to assess the impact that independent as well as concomitant use of these medications have on VTE risk.
Details of the study
Meaidi and colleagues included in the cohort reproductive-age women living in Denmark between 1996 and 2017 with no history of thrombosis, thrombophilia, cancer, tubal sterilization, hysterectomy, bilateral oophorectomy, or infertility treatment. National prescription data were used to assess exposure to hormonal contraception.
The investigators classified hormonal contraception into 3 VTE risk categories:
- high risk—estrogen-progestin patches and vaginal rings; oral contraceptives containing 50 µg of ethinyl estradiol; or the progestins desogestrel, drospirenone, gestodene, or cyproterone (with the latter 2 progestins not available in the United States)
- medium risk—all other combination oral contraceptives, including those formulated with the progestins norethindrone, norethindrone acetate, norgestrel, and levonorgestrel, as well as depot medroxyprogesterone acetate
- low/no risk—progestin-only pills, implants, and progestin-containing intrauterine devices (IUDs).
Because in Denmark NSAIDs are prescribed as a single package containing no more than 30 tablets, time exposed to non–aspirin NSAIDs was assumed to last 1 week from the prescription date.
The authors considered first-time diagnoses of lower limb venous thrombosis or pulmonary embolism that were made in hospitals to represent VTE. They also constructed a subgroup of VTE patients in whom the diagnosis was either confirmed with imaging or followed by prescription of an anticoagulant.
To address potential confounding, the authors adjusted their analysis based on age, calendar year, educational attainment, occurrence of pregnancy, surgery, hypertension, diabetes, polycystic ovary syndrome, endometriosis, migraine, systemic connective tissue diseases, inflammatory polyarthropathies, and use of tranexamic acid (a medication that may increase VTE risk). They also censored (temporarily excluded women from analysis) episodes associated with a transiently elevated risk of VTE: pregnancy and 6 months following delivery, 12 weeks after other pregnancy terminations, 8 weeks following any surgery involving hospital admission, and 8 weeks following prescription of tranexamic acid.
Continue to: VTEs associated with risk category of hormonal contraception used...
VTEs associated with risk category of hormonal contraception used
Results. The overall cohort included more than 2 million women who were followed for a median of 10 years. During 21.0 million person-years, 8,710 VTE events were diagnosed; almost one-third of these were pulmonary embolisms, with the remainder diagnosed as lower extremity VTE. Of these 8,710 women diagnosed with VTE, 7,043 (81%) were confirmed with either diagnostic imaging or prescription of an anticoagulant. Unfortunately, 228 women (2.6%) died within 30 days of the diagnosis of VTE.
The investigators identified concomitant use of hormonal contraception and NSAIDs in more than 500,000 women. Among women with such concomitant use, 58% were using contraceptives that were high risk while 23% used medium-risk and 19% used low/no-risk contraceptives. Ibuprofen (60%) was the most commonly used NSAID, followed by diclofenac (20%) and naproxen (6%). Between 97% and 98% of high-risk and medium-risk contraceptives were combination pills; 89% of low/no-risk contraceptives were progestin IUDs.
Compared with nonuse of both hormonal contraceptives and NSAIDs, incidence rate ratios of VTE adjusted for age, calendar year, and education were 8.1 (95% confidence interval [CI], 6.9–9.6) for use of NSAIDs only, 4.2 (95% CI, 4.0–4.4) for use of high-risk contraceptives only, 3.0 (95% CI, 2.8–3.2) for medium-risk contraceptive use, and 1.1 (95% CI, 1.0–1.3) for use of low/no-risk hormonal contraception. Risk of VTE was approximately twice as high with the use of diclofenac only compared with the risks associated with ibuprofen or naproxen use only.
With respect to concomitant use of NSAIDs and hormonal contraception, incidence rate ratios of VTE were 50.6 (95% CI, 44.2–57.8), 26.1 (95% CI, 19.6–34.7), and 5.7 (95% CI, 3.3–10.1), respectively, with use of high-risk, medium-risk, and low/no-risk hormonal contraceptives. Adjusting for time updated information on occurrences of migraine, connective tissue disorder, inflammatory polyarthropathies, endometriosis, polycystic ovary syndrome, hypertension, and diabetes did not materially affect these associations.
When analysis was limited to women without these occurring conditions, rate ratios were somewhat higher (5.7 and 4.1) for use of high-risk and medium-risk contraceptives only. Incidence rate ratios in this subcohort of healthier women were substantially higher for NSAID use only (15.0), and 111.7, 43.2, and 13.0, respectively, for concomitant use of NSAIDs with high-risk, medium-risk, and low/no-risk contraceptives. In this analysis of healthier women, diclofenac continued to be associated with substantially higher risks of VTE than ibuprofen or naproxen. When the stricter definition of VTE (confirmed cases) was used, adjusted rate ratios remained similar.
Absolute risks of VTE
Although some of the elevated rate ratios noted in this study might appear alarming, it is important to keep in mind that the baseline incidence of VTE in healthy reproductive-age women is low. Accordingly, as the authors pointed out, even among women who used NSAIDs concomitantly with high-risk combination hormonal contraceptives, the absolute risk of VTE was 2/10,000.
Study strengths and limitations
Strengths of this analysis by Meaidi and colleagues include the use of large, essentially all-inclusive national registries. In addition, nationwide Danish registry data that indicate a diagnosis of VTE have been found to have a high positive predictive value.3 Another strength is the large number of potentially confounding factors that the authors controlled for.
One potential limitation of their analysis is that the use of only prescribed NSAIDs was considered. Fortunately, however, the prevalence of over-the-counter ibuprofen use in Denmark is not high enough to materially affect the authors’ findings.4 Another potential limitation was that information on smoking and body mass index was not available for most of the women included in the study cohort. The authors countered this limitation by pointing out that, in Denmark, smoking and obesity are highly correlated with educational status, and that all analyses were adjusted for educational status. ●
It is important for clinicians and our patients to recognize that pregnancy—the condition prevented by hormonal contraception— is associated with far higher risks of VTE (10–14 VTE events per 10,000 deliveries) than the use of any modern hormonal contraceptive.5 Although concomitant use of combination contraceptives and NSAIDs increases VTE risk, the absolute risk is modest, particularly when the NSAID is ibuprofen or naproxen (these are the non–aspirin NSAIDs most commonly used in the United States6). Women who regularly take NSAIDs can minimize VTE risk by choosing hormonal contraceptives with little or no impact on the risk of VTE: the progestin implant, progestin IUDs, and progestinonly pills.
ANDREW M. KAUNITZ, MD, MSCP
Meaidi A, Mascolo A, Sessa M, et al. Venous thromboembolism with use of hormonal contraception and non-steroidal anti-inflammatory drugs: nationwide cohort study. BMJ. 2023;382:e074450. doi:10.1136/bmj-2022-074450
EXPERT COMMENTARY
Combination (estrogen plus progestin) hormonal contraceptives as well as non–aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk of VTE events, including lower extremity clots and pulmonary embolism. Taking contraceptives formulated with ethinyl estradiol increases hepatic production of clotting factors on a dose-related basis. Newer progestins, including desogestrel and drospirenone, also may contribute to an elevated VTE risk, although this association is controversial.1 NSAIDs promote platelet aggregation, thereby activating the clotting system and formation of clots. Although studies that assessed the association between NSAID use and thrombosis have focused on arterial clots, a substantial literature suggests that NSAIDs, including older NSAIDs (such as ibuprofen, diclofenac, and naproxen), also increase VTE risk.2
Although combination contraceptives (oral contraceptives, patches, vaginal rings) and NSAIDs are both commonly used by reproductive-age women, little data have assessed the impact of concomitant use of these medications on VTE risk. Accordingly, investigators in Denmark, using national databases, conducted a retrospective cohort study to assess the impact that independent as well as concomitant use of these medications have on VTE risk.
Details of the study
Meaidi and colleagues included in the cohort reproductive-age women living in Denmark between 1996 and 2017 with no history of thrombosis, thrombophilia, cancer, tubal sterilization, hysterectomy, bilateral oophorectomy, or infertility treatment. National prescription data were used to assess exposure to hormonal contraception.
The investigators classified hormonal contraception into 3 VTE risk categories:
- high risk—estrogen-progestin patches and vaginal rings; oral contraceptives containing 50 µg of ethinyl estradiol; or the progestins desogestrel, drospirenone, gestodene, or cyproterone (with the latter 2 progestins not available in the United States)
- medium risk—all other combination oral contraceptives, including those formulated with the progestins norethindrone, norethindrone acetate, norgestrel, and levonorgestrel, as well as depot medroxyprogesterone acetate
- low/no risk—progestin-only pills, implants, and progestin-containing intrauterine devices (IUDs).
Because in Denmark NSAIDs are prescribed as a single package containing no more than 30 tablets, time exposed to non–aspirin NSAIDs was assumed to last 1 week from the prescription date.
The authors considered first-time diagnoses of lower limb venous thrombosis or pulmonary embolism that were made in hospitals to represent VTE. They also constructed a subgroup of VTE patients in whom the diagnosis was either confirmed with imaging or followed by prescription of an anticoagulant.
To address potential confounding, the authors adjusted their analysis based on age, calendar year, educational attainment, occurrence of pregnancy, surgery, hypertension, diabetes, polycystic ovary syndrome, endometriosis, migraine, systemic connective tissue diseases, inflammatory polyarthropathies, and use of tranexamic acid (a medication that may increase VTE risk). They also censored (temporarily excluded women from analysis) episodes associated with a transiently elevated risk of VTE: pregnancy and 6 months following delivery, 12 weeks after other pregnancy terminations, 8 weeks following any surgery involving hospital admission, and 8 weeks following prescription of tranexamic acid.
Continue to: VTEs associated with risk category of hormonal contraception used...
VTEs associated with risk category of hormonal contraception used
Results. The overall cohort included more than 2 million women who were followed for a median of 10 years. During 21.0 million person-years, 8,710 VTE events were diagnosed; almost one-third of these were pulmonary embolisms, with the remainder diagnosed as lower extremity VTE. Of these 8,710 women diagnosed with VTE, 7,043 (81%) were confirmed with either diagnostic imaging or prescription of an anticoagulant. Unfortunately, 228 women (2.6%) died within 30 days of the diagnosis of VTE.
The investigators identified concomitant use of hormonal contraception and NSAIDs in more than 500,000 women. Among women with such concomitant use, 58% were using contraceptives that were high risk while 23% used medium-risk and 19% used low/no-risk contraceptives. Ibuprofen (60%) was the most commonly used NSAID, followed by diclofenac (20%) and naproxen (6%). Between 97% and 98% of high-risk and medium-risk contraceptives were combination pills; 89% of low/no-risk contraceptives were progestin IUDs.
Compared with nonuse of both hormonal contraceptives and NSAIDs, incidence rate ratios of VTE adjusted for age, calendar year, and education were 8.1 (95% confidence interval [CI], 6.9–9.6) for use of NSAIDs only, 4.2 (95% CI, 4.0–4.4) for use of high-risk contraceptives only, 3.0 (95% CI, 2.8–3.2) for medium-risk contraceptive use, and 1.1 (95% CI, 1.0–1.3) for use of low/no-risk hormonal contraception. Risk of VTE was approximately twice as high with the use of diclofenac only compared with the risks associated with ibuprofen or naproxen use only.
With respect to concomitant use of NSAIDs and hormonal contraception, incidence rate ratios of VTE were 50.6 (95% CI, 44.2–57.8), 26.1 (95% CI, 19.6–34.7), and 5.7 (95% CI, 3.3–10.1), respectively, with use of high-risk, medium-risk, and low/no-risk hormonal contraceptives. Adjusting for time updated information on occurrences of migraine, connective tissue disorder, inflammatory polyarthropathies, endometriosis, polycystic ovary syndrome, hypertension, and diabetes did not materially affect these associations.
When analysis was limited to women without these occurring conditions, rate ratios were somewhat higher (5.7 and 4.1) for use of high-risk and medium-risk contraceptives only. Incidence rate ratios in this subcohort of healthier women were substantially higher for NSAID use only (15.0), and 111.7, 43.2, and 13.0, respectively, for concomitant use of NSAIDs with high-risk, medium-risk, and low/no-risk contraceptives. In this analysis of healthier women, diclofenac continued to be associated with substantially higher risks of VTE than ibuprofen or naproxen. When the stricter definition of VTE (confirmed cases) was used, adjusted rate ratios remained similar.
Absolute risks of VTE
Although some of the elevated rate ratios noted in this study might appear alarming, it is important to keep in mind that the baseline incidence of VTE in healthy reproductive-age women is low. Accordingly, as the authors pointed out, even among women who used NSAIDs concomitantly with high-risk combination hormonal contraceptives, the absolute risk of VTE was 2/10,000.
Study strengths and limitations
Strengths of this analysis by Meaidi and colleagues include the use of large, essentially all-inclusive national registries. In addition, nationwide Danish registry data that indicate a diagnosis of VTE have been found to have a high positive predictive value.3 Another strength is the large number of potentially confounding factors that the authors controlled for.
One potential limitation of their analysis is that the use of only prescribed NSAIDs was considered. Fortunately, however, the prevalence of over-the-counter ibuprofen use in Denmark is not high enough to materially affect the authors’ findings.4 Another potential limitation was that information on smoking and body mass index was not available for most of the women included in the study cohort. The authors countered this limitation by pointing out that, in Denmark, smoking and obesity are highly correlated with educational status, and that all analyses were adjusted for educational status. ●
It is important for clinicians and our patients to recognize that pregnancy—the condition prevented by hormonal contraception— is associated with far higher risks of VTE (10–14 VTE events per 10,000 deliveries) than the use of any modern hormonal contraceptive.5 Although concomitant use of combination contraceptives and NSAIDs increases VTE risk, the absolute risk is modest, particularly when the NSAID is ibuprofen or naproxen (these are the non–aspirin NSAIDs most commonly used in the United States6). Women who regularly take NSAIDs can minimize VTE risk by choosing hormonal contraceptives with little or no impact on the risk of VTE: the progestin implant, progestin IUDs, and progestinonly pills.
ANDREW M. KAUNITZ, MD, MSCP
- Reid RL. Oral hormonal contraception and venous thromboembolism (VTE). Contraception. 2014;89:235-236. doi:10.1016/j.contraception.2014.02.002
- Ungprasert P, Srivali N, Wijarnpreecha K, et al. Nonsteroidal anti-inflammatory drugs and risk of venous thromboembolism: a systematic review and meta-analysis. Rheumatology (Oxford). 2015;54:736-742. doi:10.1093 /rheumatology/keu408
- Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. doi:10.1136/bmjopen-2016-012832
- Gaster N, Hallas J, Pottegård A, et al. The validity of Danish prescription data to measure use of aspirin and other nonsteroidal anti-inflammatory drugs and quantification of bias due to non-prescription drug use. Clin Epidemiol. 2021;13:569-579. doi:10.2147/CLEP.S311450
- Maughan BC, Marin M, Han J, et al. Venous thromboembolism during pregnancy and the postpartum period: risk factors, diagnostic testing, and treatment. Obstet Gynecol Surv. 2022;77:433-444. doi:10.1097/OGX.0000000000001043
- Chu A. Ibuprofen, naproxen, and more: the 8 most common NSAIDs. GoodRx. July 20, 2023. Accessed October 4, 2023. https://www.goodrx.com/classes/nsaids/nsaid-list
- Reid RL. Oral hormonal contraception and venous thromboembolism (VTE). Contraception. 2014;89:235-236. doi:10.1016/j.contraception.2014.02.002
- Ungprasert P, Srivali N, Wijarnpreecha K, et al. Nonsteroidal anti-inflammatory drugs and risk of venous thromboembolism: a systematic review and meta-analysis. Rheumatology (Oxford). 2015;54:736-742. doi:10.1093 /rheumatology/keu408
- Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. doi:10.1136/bmjopen-2016-012832
- Gaster N, Hallas J, Pottegård A, et al. The validity of Danish prescription data to measure use of aspirin and other nonsteroidal anti-inflammatory drugs and quantification of bias due to non-prescription drug use. Clin Epidemiol. 2021;13:569-579. doi:10.2147/CLEP.S311450
- Maughan BC, Marin M, Han J, et al. Venous thromboembolism during pregnancy and the postpartum period: risk factors, diagnostic testing, and treatment. Obstet Gynecol Surv. 2022;77:433-444. doi:10.1097/OGX.0000000000001043
- Chu A. Ibuprofen, naproxen, and more: the 8 most common NSAIDs. GoodRx. July 20, 2023. Accessed October 4, 2023. https://www.goodrx.com/classes/nsaids/nsaid-list
Do screening mammograms in women aged 70 and older improve stage at diagnosis or breast cancer–specific mortality?
Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
EXPERT COMMENTARY
A screening test is performed to detect potential health disorders or diseases in people who do not have any symptoms of disease. The goal of screening is to detect the condition early enough to treat it most effectively, and ultimately to decrease morbidity and mortality related to the disease. Overdiagnosis refers to the finding of a cancer that would not have caused clinical problems during a person’s lifetime.
Current guidelines for the early detection of breast cancer vary considerably, including recommendations for what age to initiate screening, the cadence of screening (annual or biannual), the use of ancillary screening for people with dense breasts, and importantly the upper age limit for which screening is advised. The US Preventive Services Task Force recommends continuing screening to age 74. The American Cancer Society suggests ongoing screening if life expectancy is estimated at more than 10 years, and the American College of Physicians recommends stopping screening at age 75, or younger if life expectancy is less than 10 years. The American College of Obstetricians and Gynecologists states that women at average risk of breast cancer should continue screening mammography until at least age 75.
Overdiagnosis is a difficult concept for clinicians to understand let alone explain to our patients. Recently, Richman and colleagues published the results of their study aimed at estimating overdiagnosis associated with breast cancer screening among older women.1 As Dr. Otis Brawley, former Chief Medical and Scientific Officer of the American Cancer Society and current Distinguished Professor of Oncology and Epidemiology at Johns Hopkins University, states in the editorial that accompanies the study by Richman and colleagues, “Some tumors are not destined to grow, spread, and kill due to their genomics or their microenvironment. A second type of overdiagnosis involves small tumors that do have the potential to grow but will not grow fast enough to bother the patient within their natural lifetime.”2
Although screening mammography in older women results in frequent false positives that require additional imaging as well as biopsies, we have become more aware of the potential of overdiagnosis as an important downside of screening mammography in an elderly population.
Continue to: Details of the study...
Details of the study
Using the SEER registry to identify breast cancers linked to a 5% sample of Medicare beneficiaries, Richman and colleagues (funded by the National Cancer Institute and based at Yale University) conducted a retrospectivecohort study to estimate the likelihood of overdiagnosis associated with screening mammography among older women over 15 years of follow-up. Specifically, they assessed the difference in cumulative incidence of in situ and invasive breast cancer among women aged 70 years and older without a history of breast cancer when screened in 2002. During the subsequent 3 years, participants either continued screening (screened group) or did not (unscreened group). Women were followed through 2017.
Among almost 55,000 women followed, 88% were White, 6% were Black, and 3% were Hispanic. Mean follow-up was 13.7 years among women aged 70 to 74 years at baseline. For those aged 75 to 84 at baseline, mean follow-up was 10 years, and for those aged 85 years and older, mean follow-up was 5.7 years.
Estimated rates of overdiagnosis. Overall, among women aged 70 to 74 at baseline who were eventually diagnosed with breast cancer, the investigators estimated that 31% of these cancers were overdiagnosed. The corresponding percentage of breast cancers estimated to represent overdiagnosis climbed to 47% for those aged 75 to 84 years at baseline and to 54% for those aged 85 years and older at baseline.
The investigators assessed the impact of greater screening among women with a first-degree relative with a diagnosis of breast cancer and determined that this did not explain their results. With respect to cancer stage, the investigators noted that overdiagnosis was more prevalent among in situ and localized invasive cancers compared with those with regional or distant spread. Of note, the incidence of cancer with regional or distant spread was neither higher nor lower among those who were screened. Finally, the investigators did not observe significant differences in breast cancer–specific mortality by screening status.
The proportion of cancers that were overdiagnosed was particularly high among women with in situ as well as those with localized invasive disease. The investigators pointed out that as many as 90% of women aged 80 and older diagnosed with localized cancer undergo surgery, and almost two-thirds of those older than 70 years have radiation therapy for early-stage disease. In addition to the burdens associated with these treatments for overdiagnosed cancers in older women, simply being diagnosed with breast cancer profoundly affects the health and well-being of women, resulting in anxiety and substantial reductions in quality of life.
The authors also noted that some studies suggest that, among breast cancers diagnosed with screening, chemotherapy is less likely to be employed among older women, a screening benefit that must be weighed against the high likelihood of overdiagnosis. However, this benefit is unlikely to be meaningful for the majority of patients in this study who presented with in situ or early invasive lesions since chemotherapy often is not recommended for such women.
Study strengths and limitations
If screening mammography is effective, the incidence of advanced-stage tumors and breast cancer–specific mortality should be reduced in screened populations. Accordingly, in this large, long-term study using reliable sources of data, the findings that the incidence of advanced-stage disease as well as breast cancer–specific mortality were similar in the screened and unscreened cohorts provides powerful evidence that screening mammography is not effective in older women.3
As the authors pointed out, their findings regarding a high prevalence of overdiagnosis associated with screening mammography in older women are consistent with findings of other studies, some of which used different methodology.
The authors acknowledged that some women in their Medicare cohort who initially continued screening likely stopped screening subsequently, while some who initially did not continue screening might have been screened subsequently. They went on to indicate that if patients were completely adherent with subsequent screening (or not getting screened) the likelihood that cancers among screened women were overdiagnosed would be even higher.
Lead-time bias occurs when screening finds a cancer earlier than that cancer would have been diagnosed because of symptoms. This study followed the cohorts over a long timeframe to reduce the possibility that lead time was inappropriately identified as overdiagnosis. They also observed that, among women aged 85 and older, most cohort members had died by the end of study follow-up; accordingly, lead time is not likely to have explained their findings.
Limitations. The authors acknowledged that miscoding the mammogram type (screening vs diagnostic) could result in higher estimates of overdiagnosis. In their most conservative sensitivity analysis, the overdiagnosis rates could be as low as 15% for women aged 70 to 74, 36% for those aged 75 to 84, and 44% for people aged 85 and older.
Because this was an observational cohort study, unmeasured differences in breast cancer risk and underlying health factors may have been confounders. Specifically, people with severe life-threatening conditions that limited their expected life span may have chosen not to undergo regular screening. Although the authors did attempt to adjust for these factors, there may have been unrecognized confounders. This study was designed to estimate overdiagnosis, and therefore the specific benefits and harms of screening could not be addressed based on the data collected. ●
The high prevalence of overdiagnosis and lack of a breast cancer–specific mortality benefit among older women who undergo screening mammography is sobering. Clinician recommendations and shared decision making with our patients regarding screening mammography should take into consideration overdiagnosis and the considerable harms associated with overtreatment. Although we may recognize that overdiagnosed cancers are often indolent tumors with a long presymptomatic phase, in older women, even finding a biologically aggressive cancer may represent overdiagnosis if life expectancy is limited.
BARBARA LEVY, MD, MSCP; ANDREW M. KAUNITZ, MD, MSCP.
- Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
- Brawley OW, Ramalingam R. Understanding the varying biological behaviors of breast and other types of cancer to avoid overdiagnosis. Ann Intern Med. 2023;176:1273-1274. doi:10.7326/M23-18953
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer—lessons in cancer dynamics. N Engl J Med. 2015;373:1685-1687. doi:10.1056/NEJM p1510443

Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
EXPERT COMMENTARY
A screening test is performed to detect potential health disorders or diseases in people who do not have any symptoms of disease. The goal of screening is to detect the condition early enough to treat it most effectively, and ultimately to decrease morbidity and mortality related to the disease. Overdiagnosis refers to the finding of a cancer that would not have caused clinical problems during a person’s lifetime.
Current guidelines for the early detection of breast cancer vary considerably, including recommendations for what age to initiate screening, the cadence of screening (annual or biannual), the use of ancillary screening for people with dense breasts, and importantly the upper age limit for which screening is advised. The US Preventive Services Task Force recommends continuing screening to age 74. The American Cancer Society suggests ongoing screening if life expectancy is estimated at more than 10 years, and the American College of Physicians recommends stopping screening at age 75, or younger if life expectancy is less than 10 years. The American College of Obstetricians and Gynecologists states that women at average risk of breast cancer should continue screening mammography until at least age 75.
Overdiagnosis is a difficult concept for clinicians to understand let alone explain to our patients. Recently, Richman and colleagues published the results of their study aimed at estimating overdiagnosis associated with breast cancer screening among older women.1 As Dr. Otis Brawley, former Chief Medical and Scientific Officer of the American Cancer Society and current Distinguished Professor of Oncology and Epidemiology at Johns Hopkins University, states in the editorial that accompanies the study by Richman and colleagues, “Some tumors are not destined to grow, spread, and kill due to their genomics or their microenvironment. A second type of overdiagnosis involves small tumors that do have the potential to grow but will not grow fast enough to bother the patient within their natural lifetime.”2
Although screening mammography in older women results in frequent false positives that require additional imaging as well as biopsies, we have become more aware of the potential of overdiagnosis as an important downside of screening mammography in an elderly population.
Continue to: Details of the study...
Details of the study
Using the SEER registry to identify breast cancers linked to a 5% sample of Medicare beneficiaries, Richman and colleagues (funded by the National Cancer Institute and based at Yale University) conducted a retrospectivecohort study to estimate the likelihood of overdiagnosis associated with screening mammography among older women over 15 years of follow-up. Specifically, they assessed the difference in cumulative incidence of in situ and invasive breast cancer among women aged 70 years and older without a history of breast cancer when screened in 2002. During the subsequent 3 years, participants either continued screening (screened group) or did not (unscreened group). Women were followed through 2017.
Among almost 55,000 women followed, 88% were White, 6% were Black, and 3% were Hispanic. Mean follow-up was 13.7 years among women aged 70 to 74 years at baseline. For those aged 75 to 84 at baseline, mean follow-up was 10 years, and for those aged 85 years and older, mean follow-up was 5.7 years.
Estimated rates of overdiagnosis. Overall, among women aged 70 to 74 at baseline who were eventually diagnosed with breast cancer, the investigators estimated that 31% of these cancers were overdiagnosed. The corresponding percentage of breast cancers estimated to represent overdiagnosis climbed to 47% for those aged 75 to 84 years at baseline and to 54% for those aged 85 years and older at baseline.
The investigators assessed the impact of greater screening among women with a first-degree relative with a diagnosis of breast cancer and determined that this did not explain their results. With respect to cancer stage, the investigators noted that overdiagnosis was more prevalent among in situ and localized invasive cancers compared with those with regional or distant spread. Of note, the incidence of cancer with regional or distant spread was neither higher nor lower among those who were screened. Finally, the investigators did not observe significant differences in breast cancer–specific mortality by screening status.
The proportion of cancers that were overdiagnosed was particularly high among women with in situ as well as those with localized invasive disease. The investigators pointed out that as many as 90% of women aged 80 and older diagnosed with localized cancer undergo surgery, and almost two-thirds of those older than 70 years have radiation therapy for early-stage disease. In addition to the burdens associated with these treatments for overdiagnosed cancers in older women, simply being diagnosed with breast cancer profoundly affects the health and well-being of women, resulting in anxiety and substantial reductions in quality of life.
The authors also noted that some studies suggest that, among breast cancers diagnosed with screening, chemotherapy is less likely to be employed among older women, a screening benefit that must be weighed against the high likelihood of overdiagnosis. However, this benefit is unlikely to be meaningful for the majority of patients in this study who presented with in situ or early invasive lesions since chemotherapy often is not recommended for such women.
Study strengths and limitations
If screening mammography is effective, the incidence of advanced-stage tumors and breast cancer–specific mortality should be reduced in screened populations. Accordingly, in this large, long-term study using reliable sources of data, the findings that the incidence of advanced-stage disease as well as breast cancer–specific mortality were similar in the screened and unscreened cohorts provides powerful evidence that screening mammography is not effective in older women.3
As the authors pointed out, their findings regarding a high prevalence of overdiagnosis associated with screening mammography in older women are consistent with findings of other studies, some of which used different methodology.
The authors acknowledged that some women in their Medicare cohort who initially continued screening likely stopped screening subsequently, while some who initially did not continue screening might have been screened subsequently. They went on to indicate that if patients were completely adherent with subsequent screening (or not getting screened) the likelihood that cancers among screened women were overdiagnosed would be even higher.
Lead-time bias occurs when screening finds a cancer earlier than that cancer would have been diagnosed because of symptoms. This study followed the cohorts over a long timeframe to reduce the possibility that lead time was inappropriately identified as overdiagnosis. They also observed that, among women aged 85 and older, most cohort members had died by the end of study follow-up; accordingly, lead time is not likely to have explained their findings.
Limitations. The authors acknowledged that miscoding the mammogram type (screening vs diagnostic) could result in higher estimates of overdiagnosis. In their most conservative sensitivity analysis, the overdiagnosis rates could be as low as 15% for women aged 70 to 74, 36% for those aged 75 to 84, and 44% for people aged 85 and older.
Because this was an observational cohort study, unmeasured differences in breast cancer risk and underlying health factors may have been confounders. Specifically, people with severe life-threatening conditions that limited their expected life span may have chosen not to undergo regular screening. Although the authors did attempt to adjust for these factors, there may have been unrecognized confounders. This study was designed to estimate overdiagnosis, and therefore the specific benefits and harms of screening could not be addressed based on the data collected. ●
The high prevalence of overdiagnosis and lack of a breast cancer–specific mortality benefit among older women who undergo screening mammography is sobering. Clinician recommendations and shared decision making with our patients regarding screening mammography should take into consideration overdiagnosis and the considerable harms associated with overtreatment. Although we may recognize that overdiagnosed cancers are often indolent tumors with a long presymptomatic phase, in older women, even finding a biologically aggressive cancer may represent overdiagnosis if life expectancy is limited.
BARBARA LEVY, MD, MSCP; ANDREW M. KAUNITZ, MD, MSCP.

Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
EXPERT COMMENTARY
A screening test is performed to detect potential health disorders or diseases in people who do not have any symptoms of disease. The goal of screening is to detect the condition early enough to treat it most effectively, and ultimately to decrease morbidity and mortality related to the disease. Overdiagnosis refers to the finding of a cancer that would not have caused clinical problems during a person’s lifetime.
Current guidelines for the early detection of breast cancer vary considerably, including recommendations for what age to initiate screening, the cadence of screening (annual or biannual), the use of ancillary screening for people with dense breasts, and importantly the upper age limit for which screening is advised. The US Preventive Services Task Force recommends continuing screening to age 74. The American Cancer Society suggests ongoing screening if life expectancy is estimated at more than 10 years, and the American College of Physicians recommends stopping screening at age 75, or younger if life expectancy is less than 10 years. The American College of Obstetricians and Gynecologists states that women at average risk of breast cancer should continue screening mammography until at least age 75.
Overdiagnosis is a difficult concept for clinicians to understand let alone explain to our patients. Recently, Richman and colleagues published the results of their study aimed at estimating overdiagnosis associated with breast cancer screening among older women.1 As Dr. Otis Brawley, former Chief Medical and Scientific Officer of the American Cancer Society and current Distinguished Professor of Oncology and Epidemiology at Johns Hopkins University, states in the editorial that accompanies the study by Richman and colleagues, “Some tumors are not destined to grow, spread, and kill due to their genomics or their microenvironment. A second type of overdiagnosis involves small tumors that do have the potential to grow but will not grow fast enough to bother the patient within their natural lifetime.”2
Although screening mammography in older women results in frequent false positives that require additional imaging as well as biopsies, we have become more aware of the potential of overdiagnosis as an important downside of screening mammography in an elderly population.
Continue to: Details of the study...
Details of the study
Using the SEER registry to identify breast cancers linked to a 5% sample of Medicare beneficiaries, Richman and colleagues (funded by the National Cancer Institute and based at Yale University) conducted a retrospectivecohort study to estimate the likelihood of overdiagnosis associated with screening mammography among older women over 15 years of follow-up. Specifically, they assessed the difference in cumulative incidence of in situ and invasive breast cancer among women aged 70 years and older without a history of breast cancer when screened in 2002. During the subsequent 3 years, participants either continued screening (screened group) or did not (unscreened group). Women were followed through 2017.
Among almost 55,000 women followed, 88% were White, 6% were Black, and 3% were Hispanic. Mean follow-up was 13.7 years among women aged 70 to 74 years at baseline. For those aged 75 to 84 at baseline, mean follow-up was 10 years, and for those aged 85 years and older, mean follow-up was 5.7 years.
Estimated rates of overdiagnosis. Overall, among women aged 70 to 74 at baseline who were eventually diagnosed with breast cancer, the investigators estimated that 31% of these cancers were overdiagnosed. The corresponding percentage of breast cancers estimated to represent overdiagnosis climbed to 47% for those aged 75 to 84 years at baseline and to 54% for those aged 85 years and older at baseline.
The investigators assessed the impact of greater screening among women with a first-degree relative with a diagnosis of breast cancer and determined that this did not explain their results. With respect to cancer stage, the investigators noted that overdiagnosis was more prevalent among in situ and localized invasive cancers compared with those with regional or distant spread. Of note, the incidence of cancer with regional or distant spread was neither higher nor lower among those who were screened. Finally, the investigators did not observe significant differences in breast cancer–specific mortality by screening status.
The proportion of cancers that were overdiagnosed was particularly high among women with in situ as well as those with localized invasive disease. The investigators pointed out that as many as 90% of women aged 80 and older diagnosed with localized cancer undergo surgery, and almost two-thirds of those older than 70 years have radiation therapy for early-stage disease. In addition to the burdens associated with these treatments for overdiagnosed cancers in older women, simply being diagnosed with breast cancer profoundly affects the health and well-being of women, resulting in anxiety and substantial reductions in quality of life.
The authors also noted that some studies suggest that, among breast cancers diagnosed with screening, chemotherapy is less likely to be employed among older women, a screening benefit that must be weighed against the high likelihood of overdiagnosis. However, this benefit is unlikely to be meaningful for the majority of patients in this study who presented with in situ or early invasive lesions since chemotherapy often is not recommended for such women.
Study strengths and limitations
If screening mammography is effective, the incidence of advanced-stage tumors and breast cancer–specific mortality should be reduced in screened populations. Accordingly, in this large, long-term study using reliable sources of data, the findings that the incidence of advanced-stage disease as well as breast cancer–specific mortality were similar in the screened and unscreened cohorts provides powerful evidence that screening mammography is not effective in older women.3
As the authors pointed out, their findings regarding a high prevalence of overdiagnosis associated with screening mammography in older women are consistent with findings of other studies, some of which used different methodology.
The authors acknowledged that some women in their Medicare cohort who initially continued screening likely stopped screening subsequently, while some who initially did not continue screening might have been screened subsequently. They went on to indicate that if patients were completely adherent with subsequent screening (or not getting screened) the likelihood that cancers among screened women were overdiagnosed would be even higher.
Lead-time bias occurs when screening finds a cancer earlier than that cancer would have been diagnosed because of symptoms. This study followed the cohorts over a long timeframe to reduce the possibility that lead time was inappropriately identified as overdiagnosis. They also observed that, among women aged 85 and older, most cohort members had died by the end of study follow-up; accordingly, lead time is not likely to have explained their findings.
Limitations. The authors acknowledged that miscoding the mammogram type (screening vs diagnostic) could result in higher estimates of overdiagnosis. In their most conservative sensitivity analysis, the overdiagnosis rates could be as low as 15% for women aged 70 to 74, 36% for those aged 75 to 84, and 44% for people aged 85 and older.
Because this was an observational cohort study, unmeasured differences in breast cancer risk and underlying health factors may have been confounders. Specifically, people with severe life-threatening conditions that limited their expected life span may have chosen not to undergo regular screening. Although the authors did attempt to adjust for these factors, there may have been unrecognized confounders. This study was designed to estimate overdiagnosis, and therefore the specific benefits and harms of screening could not be addressed based on the data collected. ●
The high prevalence of overdiagnosis and lack of a breast cancer–specific mortality benefit among older women who undergo screening mammography is sobering. Clinician recommendations and shared decision making with our patients regarding screening mammography should take into consideration overdiagnosis and the considerable harms associated with overtreatment. Although we may recognize that overdiagnosed cancers are often indolent tumors with a long presymptomatic phase, in older women, even finding a biologically aggressive cancer may represent overdiagnosis if life expectancy is limited.
BARBARA LEVY, MD, MSCP; ANDREW M. KAUNITZ, MD, MSCP.
- Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
- Brawley OW, Ramalingam R. Understanding the varying biological behaviors of breast and other types of cancer to avoid overdiagnosis. Ann Intern Med. 2023;176:1273-1274. doi:10.7326/M23-18953
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer—lessons in cancer dynamics. N Engl J Med. 2015;373:1685-1687. doi:10.1056/NEJM p1510443
- Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
- Brawley OW, Ramalingam R. Understanding the varying biological behaviors of breast and other types of cancer to avoid overdiagnosis. Ann Intern Med. 2023;176:1273-1274. doi:10.7326/M23-18953
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer—lessons in cancer dynamics. N Engl J Med. 2015;373:1685-1687. doi:10.1056/NEJM p1510443