User login
A 56-year-old with diarrhea and weakness
A 56-year-old man presents to the emergency department with nausea, weakness, and exertional dyspnea, which have been going on for 1 week. He is sent by his primary care physician after being noted to be hypotensive with a weak, thready pulse.
He has had diarrhea with intermittent abdominal pain over the past year, with 10 stools daily, including 3 or 4 at night. The stools are described as large, nonbloody, sticky, greasy, and occasionally watery. Stools are fewer when he curtails his food intake. The diarrhea is associated with occasional diffuse abdominal pain he describes as a burning sensation. He has no incontinence or tenesmus. He reports that he has unintentionally lost 137 lb (62 kg) over the past year. He has not taken over-the-counter antidiarrheal agents.
CHRONIC DIARRHEA
1. Chronic diarrhea is defined as lasting for at least how long?
- 1 week
- 2 weeks
- 3 weeks
- 4 weeks
Chronic diarrhea is defined as looser stools for more than 4 weeks,1 a period that allows most cases of acute, self-limited, infectious diarrhea to resolve.
Because individuals perceive diarrhea differently, reported prevalence rates of chronic diarrhea vary.2 Based on the definition of having excessive stool frequency, the prevalence in the United States is about 5%.1
In developing countries, the most common cause of chronic diarrhea is infection. In developed nations, irritable bowel syndrome, inflammatory bowel disease, malabsorption syndrome, and chronic infection predominate.1
Once chronicity is established, diarrhea should be characterized as inflammatory, fatty, or watery (Table 1).3
CASE CONTINUED: HISTORY OF HYPERTENSION, DIABETES
Our patient reports that he has never traveled outside the United States. He has a history of hypertension and type 2 diabetes mellitus that is controlled on oral agents. He has had surgery for a radial fracture and for reconstruction of his knees. He uses no tobacco, alcohol, or illicit drugs and works as a train engineer. He has no pets. He knows of no family history of inflammatory bowel disease or chronic diarrhea.
Comment. Patients with diabetes are at increased risk of gastrointestinal problems, with severity increasing with poorer control.4 Although our patient’s diabetes puts him at risk of diabetic autonomic neuropathy, his blood glucose control has been consistently good since his diagnosis, and his last measured hemoglobin A1c was 7.3% (reference range 4%–7%). His description of greasy stools in conjunction with his marked weight loss puts fatty diarrhea higher on the differential diagnosis.
DRUG-INDUCED DIARRHEA
His medications include glimepiride 1 mg twice daily, lisinopril 10 mg daily, metformin 500 mg twice daily, omeprazole 40 mg daily, and naproxen 220 mg daily. He has been taking metformin for at least 2 years. He is allergic to pentobarbital.
2. Which of his medications is least likely to be associated with his diarrhea?
- Lisinopril
- Metformin
- Glimepiride
- Naproxen
More than 700 drugs are known to cause diarrhea, often through the interplay of simultaneous mechanisms.5 The diagnosis of drug-induced diarrhea requires taking a careful medication history and establishing a temporal relationship between the drug and the diarrheal symptoms. Treatment consists of withdrawing the offending agent.
Nonsteroidal anti-inflammatory drugs (eg, naproxen) are associated with collagenous colitis that occurs mostly after long-term use (> 6 months). Metformin-induced diarrhea is related to fat malabsorption. Olmesartan, an angiotensin II receptor antagonist, has been associated with severe sprue-like enteropathy. On the other hand, the incidence of diarrhea with lisinopril is similar to that with placebo.7
CASE CONTINUED: EXAMINATION AND LABORATORY VALUES
The patient’s primary care physician had recently referred him to a gastroenterologist, and 4 days before presenting to the emergency department he had undergone abdominal and pelvic computed tomography (CT) with iodinated contrast, which had showed hepatic steatosis and pancreatic atrophy.
On examination now, the patient’s temperature is 97.5°F (36.4°C), heart rate 90 beats per minute, respirations 18 breaths per minute, oxygen saturation 99% on room air, and blood pressure 85/55 mm Hg. His body mass index is 32.5 kg/m2. His oral mucosa is dry. The rest of the examination is normal. No rash or ulcers are noted.
His laboratory values (Table 2) are notable for sodium 130 mmol/L, potassium 2.2 mmol/L, bicarbonate 9 mmol/L, blood urea nitrogen 32 mg/dL, creatinine 4.18 mg/dL, and international normalized ratio 5.4. Arterial blood gases drawn on admission reveal pH 7.32 and pCO2 19 mm Hg.
ACID-BASE DISTURBANCES
3. The patient’s acidosis is most likely related to which of the following?
- Sepsis
- Diarrhea
- Metformin
- Acute kidney injury
It is most likely related to diarrhea. The patient has a non-anion-gap metabolic acidosis. (The anion gap can be calculated by subtracting the sum of the serum bicarbonate and chloride values from the sodium—here, 130 – [112 + 9] = 9—and most textbooks list the reference range as 10–12 mmol/L.) Non-anion-gap metabolic acidosis results from excessive loss of bicarbonate or impaired ability of the kidney to excrete acid. Bicarbonate losses can occur in diarrhea or in ureteral diversion to the colon. Impairment in urinary acidification can occur in renal tubular acidosis.
To determine the cause of non-anion-gap acidosis, calculating the urine anion gap can be useful (Table 3), as it reflects the ability of the kidneys to excrete acid and is an indirect measure of ammonium excretion. Our patient’s urine anion gap is –45 mmol/L ([62 + 8] – 115), which supports diarrhea as the cause of his non-anion-gap acidosis. Sepsis, metformin use, or acute kidney injury would result in an anion-gap acidosis.
To manage acid-base disturbances, it is important to first determine whether there is a single primary disturbance with compensation or a mixed disorder. In the case of metabolic acidosis, for every 1-mmol/L decrease in bicarbonate, there should be a corresponding 1.3-mm Hg decrease in pCO2. Our patient’s laboratory data show that he had a pure non-anion-gap metabolic acidosis.8 His sensation of dyspnea was likely related to respiratory compensation as evidenced by an appropriately low pCO2.
CASE CONTINUED: HIS LABORATORY VALUES IMPROVE
The patient is admitted to the hospital for fluid resuscitation with normal saline and potassium and magnesium replacement.
Renal ultrasonography reveals normal-appearing kidneys without obstruction. The calculated fractional excretion of sodium is 3.4%. Urine microscopy reveals two to five hyaline casts per low-power field. His urine output remains adequate, and 3 days after hospitalization, his renal function starts to improve, as reflected in falling serum creatinine and blood urea nitrogen levels: his creatinine level has declined to 1.91 mg/dL and his blood urea nitrogen level has declined to 24 mg/dL. His acute kidney injury is attributed to intravenous contrast given for computed tomography, as well as to volume depletion and hypotension.
Stool studies for ova, parasites, and Clostridium difficile are negative. Fecal calprotectin and lactoferrin are useful noninvasive markers of intestinal inflammation but were not checked in this case.
Loperamide, taken as needed, is started for his diarrhea, along with empiric pancreatic enzyme replacement. After 3 days of treatment with oral vitamin K 10 mg, his international normalized ratio comes down to 1.4, from his admission value of 5.4. Given the clinical concern for fat malabsorption, vitamin D levels are also checked: his 25-hydroxyvitamin D level is less than 10 ng/mL (lower limit of normal 20). His fecal neutral fats are reported as normal, but split fats are increased.
STOOL FAT STUDIES
4. What does increased fecal split fats but normal fecal neutral fats imply?
- Pancreatic insufficiency
- Intestinal malabsorption
- Does not differentiate between the two
The finding does not differentiate between pancreatic insufficiency and intestinal malabsorption. The two-step Sudan stain has been used to differentiate maldigestion (eg, caused by pancreatic insufficiency) from malabsorption. Although patients with impaired digestion were once thought to excrete excessive amounts of intact triglyceride whereas those with malabsorption excrete more of the lipolytic or “split” product, the Sudan stain does not differentiate between the two.10 Stool fecal-elastase 1 testing correlates well with pancreatic exocrine function but was not performed in our patient.11
CASE CONTINUED: CELIAC DISEASE IS DIAGNOSED
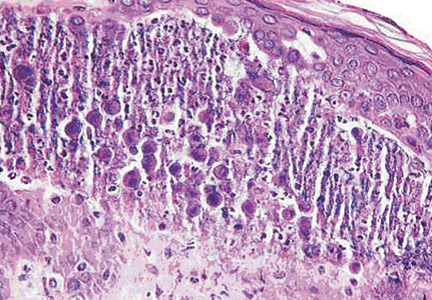
Given the description of his stools, unintentional weight loss, and improvement of stool frequency with fasting, serologic testing for celiac disease is performed (Table 4). The patient undergoes esophagogastroduodenoscopy, which shows mild duodenitis. Small-bowel biopsy reveals blunted villous architecture and increased mixed inflammatory cells of the epithelium and lamina propria, suggestive of celiac disease.
The patient is diagnosed with celiac disease and is counseled to follow a gluten-free diet. He is discharged home and scheduled to follow up with a gastroenterologist and nephrologist. His liver function test abnormalities are attributed to a combination of nonalcoholic steatohepatitis and celiac disease.
CELIAC DISEASE AND MALABSORPTION
Celiac disease is an immune-mediated disorder that causes mucosal injury to the small intestine, leading to malabsorption. It is triggered by gluten intake in genetically susceptible individuals. The HLA-DQ2 haplotype is expressed in nearly 90% of patients with the disease.
The worldwide prevalence of celiac disease is about 0.6% to 1%. Those with an affected first-degree relative, type 1 diabetes, Hashimoto thyroiditis, an autoimmune disease, Down syndrome, Turner syndrome, or IgA deficiency have an increased risk.
Celiac disease presents with chronic diarrhea, weight loss, and abdominal distention and pain. Sequelae of nutrient malabsorption such as iron-deficiency anemia, short stature, and osteopenia may be evident. Liver function may also be impaired. Dermatitis herpetiformis and gluten ataxia are rarer presentations of celiac disease.12
In the absence of immunoglobulin (Ig) A deficiency, measurement of serum IgA anti-tissue transglutaminase antibodies is recommended for initial testing. IgG antitissue transglutaminase antibodies can be measured in those with IgA deficiency.12
Duodenal biopsies to confirm the diagnosis are recommended in adults unless they have previously had biopsy-proven dermatitis herpetiformis.
Gluten-free diet
The treatment for celiac disease is avoidance of gluten. Patients who consult with a nutritionist and participate in an advocacy group are more likely to adhere to a gluten-free diet, and the physician should strongly encourage and facilitate these activities.13
Untreated disease can lead to osteoporosis, impaired splenic function with increased risk of infection with encapsulated organisms, infertility or recurrent abortion, ulcerative jejunoileitis, and lymphoma.12 Patients should be monitored annually for adherence to the gluten-free diet and for the development of any associated condition. Despite adherence to a gluten-free diet, calcium absorption and bone mineral density are lower in patients with celiac disease than in controls.14 Careful monitoring of fracture risk and adequate calcium and vitamin D replacement are also important.
Our patient undergoes dual-emission x-ray absorptiometry after discharge, with results consistent with osteopenia. His T scores range from –0.2 at the right hip to –1.1 in the left femoral neck.
Recurrence or persistently abnormal levels of IgA anti-tissue transglutaminase antibodies usually indicates poor dietary compliance.12
5. In patients whose symptoms do not improve on gluten restriction, there should be concern for which of the following?
- Lymphoma
- Nonadherence to gluten restriction
- Microscopic colitis
- All of the above
The answer is all of the above. Up to 30% of patients have persistent symptoms on a gluten-free diet. Persistent exposure to gluten is the most common reason for lack of clinical improvement. In addition, bacterial overgrowth of the small bowel, lactose intolerance, pancreatic insufficiency, and microscopic colitis may coexist with celiac disease and may contribute to ongoing symptoms. In a small subset of patients with persistent villous atrophy and symptoms despite strict adherence to a gluten-free diet for 12 months, the disease is deemed “refractory.” Refractory celiac disease can be a precursor to enteropathy-associated T-cell lymphoma.13
CASE CONCLUDED
On telephone follow-up 3 weeks after discharge, the patient reports complete resolution of diarrhea and stabilization of his weight. He reports strict adherence to a gluten-free diet and feels he is coping well.
Diagnoses
- Presenting weakness secondary to dehydration and hypokalemia
- Dyspnea secondary to respiratory compensation for metabolic acidosis
- Non-anion-gap metabolic acidosis secondary to diarrhea
- Acute kidney injury secondary to iodinated contrast, volume depletion, hypotension
- Chronic diarrhea secondary to celiac disease
- Coagulopathy secondary to fat malabsorption secondary to celiac disease.
- Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology 1999; 116:1464–1486.
- Talley NJ, Weaver AL, Zinsmeister AR, Melton LJ 3rd. Self-reported diarrhea: what does it mean? Am J Gastroenterol 1994; 89:1160–1164.
- Sweetser S. Evaluating the patient with diarrhea: a case-based approach. Mayo Clin Proc 2012; 87:596–602.
- Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med 2001; 161:1989–1996.
- Chassany O, Michaux A, Bergmann JF. Drug-induced diarrhoea. Drug Saf 2000; 22:53–72.
- Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc 2012; 87:732–738.
- Zestril (lisinopril) tablets. www.accessdata.fda.gov/drugsatfda_docs/label/2012/019777s062lbl.pdf. Accessed September 8, 2015.
- Whittier WL, Rutecki GW. Primer on clinical acid-base problem solving. Dis Mon 2004; 50:122–162.
- Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 2008; 103:162–169.
- Khouri MR, Ng SN, Huang G, Shiau YF. Fecal triglyceride excretion is not excessive in pancreatic insufficiency. Gastroenterology 1989; 96:848–852.
- Dominici R, Franzini C. Fecal elastase-1 as a test for pancreatic function: a review. Clin Chem Lab Med 2002; 40:325–332.
- Fasano A, Catassi C. Celiac disease. New Engl J Med 2012; 367:2419–2426.
- Mooney PD, Hadjivassiliou M, Sanders DS. Coeliac disease. BMJ 2014; 348:g1561–g1561.
- Pazianas M, Butcher GP, Subhani JM, et al. Calcium absorption and bone mineral density in celiacs after long term treatment with gluten-free diet and adequate calcium intake. Osteoporos Int 2005; 16:56–63.
A 56-year-old man presents to the emergency department with nausea, weakness, and exertional dyspnea, which have been going on for 1 week. He is sent by his primary care physician after being noted to be hypotensive with a weak, thready pulse.
He has had diarrhea with intermittent abdominal pain over the past year, with 10 stools daily, including 3 or 4 at night. The stools are described as large, nonbloody, sticky, greasy, and occasionally watery. Stools are fewer when he curtails his food intake. The diarrhea is associated with occasional diffuse abdominal pain he describes as a burning sensation. He has no incontinence or tenesmus. He reports that he has unintentionally lost 137 lb (62 kg) over the past year. He has not taken over-the-counter antidiarrheal agents.
CHRONIC DIARRHEA
1. Chronic diarrhea is defined as lasting for at least how long?
- 1 week
- 2 weeks
- 3 weeks
- 4 weeks
Chronic diarrhea is defined as looser stools for more than 4 weeks,1 a period that allows most cases of acute, self-limited, infectious diarrhea to resolve.
Because individuals perceive diarrhea differently, reported prevalence rates of chronic diarrhea vary.2 Based on the definition of having excessive stool frequency, the prevalence in the United States is about 5%.1
In developing countries, the most common cause of chronic diarrhea is infection. In developed nations, irritable bowel syndrome, inflammatory bowel disease, malabsorption syndrome, and chronic infection predominate.1
Once chronicity is established, diarrhea should be characterized as inflammatory, fatty, or watery (Table 1).3
CASE CONTINUED: HISTORY OF HYPERTENSION, DIABETES
Our patient reports that he has never traveled outside the United States. He has a history of hypertension and type 2 diabetes mellitus that is controlled on oral agents. He has had surgery for a radial fracture and for reconstruction of his knees. He uses no tobacco, alcohol, or illicit drugs and works as a train engineer. He has no pets. He knows of no family history of inflammatory bowel disease or chronic diarrhea.
Comment. Patients with diabetes are at increased risk of gastrointestinal problems, with severity increasing with poorer control.4 Although our patient’s diabetes puts him at risk of diabetic autonomic neuropathy, his blood glucose control has been consistently good since his diagnosis, and his last measured hemoglobin A1c was 7.3% (reference range 4%–7%). His description of greasy stools in conjunction with his marked weight loss puts fatty diarrhea higher on the differential diagnosis.
DRUG-INDUCED DIARRHEA
His medications include glimepiride 1 mg twice daily, lisinopril 10 mg daily, metformin 500 mg twice daily, omeprazole 40 mg daily, and naproxen 220 mg daily. He has been taking metformin for at least 2 years. He is allergic to pentobarbital.
2. Which of his medications is least likely to be associated with his diarrhea?
- Lisinopril
- Metformin
- Glimepiride
- Naproxen
More than 700 drugs are known to cause diarrhea, often through the interplay of simultaneous mechanisms.5 The diagnosis of drug-induced diarrhea requires taking a careful medication history and establishing a temporal relationship between the drug and the diarrheal symptoms. Treatment consists of withdrawing the offending agent.
Nonsteroidal anti-inflammatory drugs (eg, naproxen) are associated with collagenous colitis that occurs mostly after long-term use (> 6 months). Metformin-induced diarrhea is related to fat malabsorption. Olmesartan, an angiotensin II receptor antagonist, has been associated with severe sprue-like enteropathy. On the other hand, the incidence of diarrhea with lisinopril is similar to that with placebo.7
CASE CONTINUED: EXAMINATION AND LABORATORY VALUES
The patient’s primary care physician had recently referred him to a gastroenterologist, and 4 days before presenting to the emergency department he had undergone abdominal and pelvic computed tomography (CT) with iodinated contrast, which had showed hepatic steatosis and pancreatic atrophy.
On examination now, the patient’s temperature is 97.5°F (36.4°C), heart rate 90 beats per minute, respirations 18 breaths per minute, oxygen saturation 99% on room air, and blood pressure 85/55 mm Hg. His body mass index is 32.5 kg/m2. His oral mucosa is dry. The rest of the examination is normal. No rash or ulcers are noted.
His laboratory values (Table 2) are notable for sodium 130 mmol/L, potassium 2.2 mmol/L, bicarbonate 9 mmol/L, blood urea nitrogen 32 mg/dL, creatinine 4.18 mg/dL, and international normalized ratio 5.4. Arterial blood gases drawn on admission reveal pH 7.32 and pCO2 19 mm Hg.
ACID-BASE DISTURBANCES
3. The patient’s acidosis is most likely related to which of the following?
- Sepsis
- Diarrhea
- Metformin
- Acute kidney injury
It is most likely related to diarrhea. The patient has a non-anion-gap metabolic acidosis. (The anion gap can be calculated by subtracting the sum of the serum bicarbonate and chloride values from the sodium—here, 130 – [112 + 9] = 9—and most textbooks list the reference range as 10–12 mmol/L.) Non-anion-gap metabolic acidosis results from excessive loss of bicarbonate or impaired ability of the kidney to excrete acid. Bicarbonate losses can occur in diarrhea or in ureteral diversion to the colon. Impairment in urinary acidification can occur in renal tubular acidosis.
To determine the cause of non-anion-gap acidosis, calculating the urine anion gap can be useful (Table 3), as it reflects the ability of the kidneys to excrete acid and is an indirect measure of ammonium excretion. Our patient’s urine anion gap is –45 mmol/L ([62 + 8] – 115), which supports diarrhea as the cause of his non-anion-gap acidosis. Sepsis, metformin use, or acute kidney injury would result in an anion-gap acidosis.
To manage acid-base disturbances, it is important to first determine whether there is a single primary disturbance with compensation or a mixed disorder. In the case of metabolic acidosis, for every 1-mmol/L decrease in bicarbonate, there should be a corresponding 1.3-mm Hg decrease in pCO2. Our patient’s laboratory data show that he had a pure non-anion-gap metabolic acidosis.8 His sensation of dyspnea was likely related to respiratory compensation as evidenced by an appropriately low pCO2.
CASE CONTINUED: HIS LABORATORY VALUES IMPROVE
The patient is admitted to the hospital for fluid resuscitation with normal saline and potassium and magnesium replacement.
Renal ultrasonography reveals normal-appearing kidneys without obstruction. The calculated fractional excretion of sodium is 3.4%. Urine microscopy reveals two to five hyaline casts per low-power field. His urine output remains adequate, and 3 days after hospitalization, his renal function starts to improve, as reflected in falling serum creatinine and blood urea nitrogen levels: his creatinine level has declined to 1.91 mg/dL and his blood urea nitrogen level has declined to 24 mg/dL. His acute kidney injury is attributed to intravenous contrast given for computed tomography, as well as to volume depletion and hypotension.
Stool studies for ova, parasites, and Clostridium difficile are negative. Fecal calprotectin and lactoferrin are useful noninvasive markers of intestinal inflammation but were not checked in this case.
Loperamide, taken as needed, is started for his diarrhea, along with empiric pancreatic enzyme replacement. After 3 days of treatment with oral vitamin K 10 mg, his international normalized ratio comes down to 1.4, from his admission value of 5.4. Given the clinical concern for fat malabsorption, vitamin D levels are also checked: his 25-hydroxyvitamin D level is less than 10 ng/mL (lower limit of normal 20). His fecal neutral fats are reported as normal, but split fats are increased.
STOOL FAT STUDIES
4. What does increased fecal split fats but normal fecal neutral fats imply?
- Pancreatic insufficiency
- Intestinal malabsorption
- Does not differentiate between the two
The finding does not differentiate between pancreatic insufficiency and intestinal malabsorption. The two-step Sudan stain has been used to differentiate maldigestion (eg, caused by pancreatic insufficiency) from malabsorption. Although patients with impaired digestion were once thought to excrete excessive amounts of intact triglyceride whereas those with malabsorption excrete more of the lipolytic or “split” product, the Sudan stain does not differentiate between the two.10 Stool fecal-elastase 1 testing correlates well with pancreatic exocrine function but was not performed in our patient.11
CASE CONTINUED: CELIAC DISEASE IS DIAGNOSED
Given the description of his stools, unintentional weight loss, and improvement of stool frequency with fasting, serologic testing for celiac disease is performed (Table 4). The patient undergoes esophagogastroduodenoscopy, which shows mild duodenitis. Small-bowel biopsy reveals blunted villous architecture and increased mixed inflammatory cells of the epithelium and lamina propria, suggestive of celiac disease.
The patient is diagnosed with celiac disease and is counseled to follow a gluten-free diet. He is discharged home and scheduled to follow up with a gastroenterologist and nephrologist. His liver function test abnormalities are attributed to a combination of nonalcoholic steatohepatitis and celiac disease.
CELIAC DISEASE AND MALABSORPTION
Celiac disease is an immune-mediated disorder that causes mucosal injury to the small intestine, leading to malabsorption. It is triggered by gluten intake in genetically susceptible individuals. The HLA-DQ2 haplotype is expressed in nearly 90% of patients with the disease.
The worldwide prevalence of celiac disease is about 0.6% to 1%. Those with an affected first-degree relative, type 1 diabetes, Hashimoto thyroiditis, an autoimmune disease, Down syndrome, Turner syndrome, or IgA deficiency have an increased risk.
Celiac disease presents with chronic diarrhea, weight loss, and abdominal distention and pain. Sequelae of nutrient malabsorption such as iron-deficiency anemia, short stature, and osteopenia may be evident. Liver function may also be impaired. Dermatitis herpetiformis and gluten ataxia are rarer presentations of celiac disease.12
In the absence of immunoglobulin (Ig) A deficiency, measurement of serum IgA anti-tissue transglutaminase antibodies is recommended for initial testing. IgG antitissue transglutaminase antibodies can be measured in those with IgA deficiency.12
Duodenal biopsies to confirm the diagnosis are recommended in adults unless they have previously had biopsy-proven dermatitis herpetiformis.
Gluten-free diet
The treatment for celiac disease is avoidance of gluten. Patients who consult with a nutritionist and participate in an advocacy group are more likely to adhere to a gluten-free diet, and the physician should strongly encourage and facilitate these activities.13
Untreated disease can lead to osteoporosis, impaired splenic function with increased risk of infection with encapsulated organisms, infertility or recurrent abortion, ulcerative jejunoileitis, and lymphoma.12 Patients should be monitored annually for adherence to the gluten-free diet and for the development of any associated condition. Despite adherence to a gluten-free diet, calcium absorption and bone mineral density are lower in patients with celiac disease than in controls.14 Careful monitoring of fracture risk and adequate calcium and vitamin D replacement are also important.
Our patient undergoes dual-emission x-ray absorptiometry after discharge, with results consistent with osteopenia. His T scores range from –0.2 at the right hip to –1.1 in the left femoral neck.
Recurrence or persistently abnormal levels of IgA anti-tissue transglutaminase antibodies usually indicates poor dietary compliance.12
5. In patients whose symptoms do not improve on gluten restriction, there should be concern for which of the following?
- Lymphoma
- Nonadherence to gluten restriction
- Microscopic colitis
- All of the above
The answer is all of the above. Up to 30% of patients have persistent symptoms on a gluten-free diet. Persistent exposure to gluten is the most common reason for lack of clinical improvement. In addition, bacterial overgrowth of the small bowel, lactose intolerance, pancreatic insufficiency, and microscopic colitis may coexist with celiac disease and may contribute to ongoing symptoms. In a small subset of patients with persistent villous atrophy and symptoms despite strict adherence to a gluten-free diet for 12 months, the disease is deemed “refractory.” Refractory celiac disease can be a precursor to enteropathy-associated T-cell lymphoma.13
CASE CONCLUDED
On telephone follow-up 3 weeks after discharge, the patient reports complete resolution of diarrhea and stabilization of his weight. He reports strict adherence to a gluten-free diet and feels he is coping well.
Diagnoses
- Presenting weakness secondary to dehydration and hypokalemia
- Dyspnea secondary to respiratory compensation for metabolic acidosis
- Non-anion-gap metabolic acidosis secondary to diarrhea
- Acute kidney injury secondary to iodinated contrast, volume depletion, hypotension
- Chronic diarrhea secondary to celiac disease
- Coagulopathy secondary to fat malabsorption secondary to celiac disease.
A 56-year-old man presents to the emergency department with nausea, weakness, and exertional dyspnea, which have been going on for 1 week. He is sent by his primary care physician after being noted to be hypotensive with a weak, thready pulse.
He has had diarrhea with intermittent abdominal pain over the past year, with 10 stools daily, including 3 or 4 at night. The stools are described as large, nonbloody, sticky, greasy, and occasionally watery. Stools are fewer when he curtails his food intake. The diarrhea is associated with occasional diffuse abdominal pain he describes as a burning sensation. He has no incontinence or tenesmus. He reports that he has unintentionally lost 137 lb (62 kg) over the past year. He has not taken over-the-counter antidiarrheal agents.
CHRONIC DIARRHEA
1. Chronic diarrhea is defined as lasting for at least how long?
- 1 week
- 2 weeks
- 3 weeks
- 4 weeks
Chronic diarrhea is defined as looser stools for more than 4 weeks,1 a period that allows most cases of acute, self-limited, infectious diarrhea to resolve.
Because individuals perceive diarrhea differently, reported prevalence rates of chronic diarrhea vary.2 Based on the definition of having excessive stool frequency, the prevalence in the United States is about 5%.1
In developing countries, the most common cause of chronic diarrhea is infection. In developed nations, irritable bowel syndrome, inflammatory bowel disease, malabsorption syndrome, and chronic infection predominate.1
Once chronicity is established, diarrhea should be characterized as inflammatory, fatty, or watery (Table 1).3
CASE CONTINUED: HISTORY OF HYPERTENSION, DIABETES
Our patient reports that he has never traveled outside the United States. He has a history of hypertension and type 2 diabetes mellitus that is controlled on oral agents. He has had surgery for a radial fracture and for reconstruction of his knees. He uses no tobacco, alcohol, or illicit drugs and works as a train engineer. He has no pets. He knows of no family history of inflammatory bowel disease or chronic diarrhea.
Comment. Patients with diabetes are at increased risk of gastrointestinal problems, with severity increasing with poorer control.4 Although our patient’s diabetes puts him at risk of diabetic autonomic neuropathy, his blood glucose control has been consistently good since his diagnosis, and his last measured hemoglobin A1c was 7.3% (reference range 4%–7%). His description of greasy stools in conjunction with his marked weight loss puts fatty diarrhea higher on the differential diagnosis.
DRUG-INDUCED DIARRHEA
His medications include glimepiride 1 mg twice daily, lisinopril 10 mg daily, metformin 500 mg twice daily, omeprazole 40 mg daily, and naproxen 220 mg daily. He has been taking metformin for at least 2 years. He is allergic to pentobarbital.
2. Which of his medications is least likely to be associated with his diarrhea?
- Lisinopril
- Metformin
- Glimepiride
- Naproxen
More than 700 drugs are known to cause diarrhea, often through the interplay of simultaneous mechanisms.5 The diagnosis of drug-induced diarrhea requires taking a careful medication history and establishing a temporal relationship between the drug and the diarrheal symptoms. Treatment consists of withdrawing the offending agent.
Nonsteroidal anti-inflammatory drugs (eg, naproxen) are associated with collagenous colitis that occurs mostly after long-term use (> 6 months). Metformin-induced diarrhea is related to fat malabsorption. Olmesartan, an angiotensin II receptor antagonist, has been associated with severe sprue-like enteropathy. On the other hand, the incidence of diarrhea with lisinopril is similar to that with placebo.7
CASE CONTINUED: EXAMINATION AND LABORATORY VALUES
The patient’s primary care physician had recently referred him to a gastroenterologist, and 4 days before presenting to the emergency department he had undergone abdominal and pelvic computed tomography (CT) with iodinated contrast, which had showed hepatic steatosis and pancreatic atrophy.
On examination now, the patient’s temperature is 97.5°F (36.4°C), heart rate 90 beats per minute, respirations 18 breaths per minute, oxygen saturation 99% on room air, and blood pressure 85/55 mm Hg. His body mass index is 32.5 kg/m2. His oral mucosa is dry. The rest of the examination is normal. No rash or ulcers are noted.
His laboratory values (Table 2) are notable for sodium 130 mmol/L, potassium 2.2 mmol/L, bicarbonate 9 mmol/L, blood urea nitrogen 32 mg/dL, creatinine 4.18 mg/dL, and international normalized ratio 5.4. Arterial blood gases drawn on admission reveal pH 7.32 and pCO2 19 mm Hg.
ACID-BASE DISTURBANCES
3. The patient’s acidosis is most likely related to which of the following?
- Sepsis
- Diarrhea
- Metformin
- Acute kidney injury
It is most likely related to diarrhea. The patient has a non-anion-gap metabolic acidosis. (The anion gap can be calculated by subtracting the sum of the serum bicarbonate and chloride values from the sodium—here, 130 – [112 + 9] = 9—and most textbooks list the reference range as 10–12 mmol/L.) Non-anion-gap metabolic acidosis results from excessive loss of bicarbonate or impaired ability of the kidney to excrete acid. Bicarbonate losses can occur in diarrhea or in ureteral diversion to the colon. Impairment in urinary acidification can occur in renal tubular acidosis.
To determine the cause of non-anion-gap acidosis, calculating the urine anion gap can be useful (Table 3), as it reflects the ability of the kidneys to excrete acid and is an indirect measure of ammonium excretion. Our patient’s urine anion gap is –45 mmol/L ([62 + 8] – 115), which supports diarrhea as the cause of his non-anion-gap acidosis. Sepsis, metformin use, or acute kidney injury would result in an anion-gap acidosis.
To manage acid-base disturbances, it is important to first determine whether there is a single primary disturbance with compensation or a mixed disorder. In the case of metabolic acidosis, for every 1-mmol/L decrease in bicarbonate, there should be a corresponding 1.3-mm Hg decrease in pCO2. Our patient’s laboratory data show that he had a pure non-anion-gap metabolic acidosis.8 His sensation of dyspnea was likely related to respiratory compensation as evidenced by an appropriately low pCO2.
CASE CONTINUED: HIS LABORATORY VALUES IMPROVE
The patient is admitted to the hospital for fluid resuscitation with normal saline and potassium and magnesium replacement.
Renal ultrasonography reveals normal-appearing kidneys without obstruction. The calculated fractional excretion of sodium is 3.4%. Urine microscopy reveals two to five hyaline casts per low-power field. His urine output remains adequate, and 3 days after hospitalization, his renal function starts to improve, as reflected in falling serum creatinine and blood urea nitrogen levels: his creatinine level has declined to 1.91 mg/dL and his blood urea nitrogen level has declined to 24 mg/dL. His acute kidney injury is attributed to intravenous contrast given for computed tomography, as well as to volume depletion and hypotension.
Stool studies for ova, parasites, and Clostridium difficile are negative. Fecal calprotectin and lactoferrin are useful noninvasive markers of intestinal inflammation but were not checked in this case.
Loperamide, taken as needed, is started for his diarrhea, along with empiric pancreatic enzyme replacement. After 3 days of treatment with oral vitamin K 10 mg, his international normalized ratio comes down to 1.4, from his admission value of 5.4. Given the clinical concern for fat malabsorption, vitamin D levels are also checked: his 25-hydroxyvitamin D level is less than 10 ng/mL (lower limit of normal 20). His fecal neutral fats are reported as normal, but split fats are increased.
STOOL FAT STUDIES
4. What does increased fecal split fats but normal fecal neutral fats imply?
- Pancreatic insufficiency
- Intestinal malabsorption
- Does not differentiate between the two
The finding does not differentiate between pancreatic insufficiency and intestinal malabsorption. The two-step Sudan stain has been used to differentiate maldigestion (eg, caused by pancreatic insufficiency) from malabsorption. Although patients with impaired digestion were once thought to excrete excessive amounts of intact triglyceride whereas those with malabsorption excrete more of the lipolytic or “split” product, the Sudan stain does not differentiate between the two.10 Stool fecal-elastase 1 testing correlates well with pancreatic exocrine function but was not performed in our patient.11
CASE CONTINUED: CELIAC DISEASE IS DIAGNOSED
Given the description of his stools, unintentional weight loss, and improvement of stool frequency with fasting, serologic testing for celiac disease is performed (Table 4). The patient undergoes esophagogastroduodenoscopy, which shows mild duodenitis. Small-bowel biopsy reveals blunted villous architecture and increased mixed inflammatory cells of the epithelium and lamina propria, suggestive of celiac disease.
The patient is diagnosed with celiac disease and is counseled to follow a gluten-free diet. He is discharged home and scheduled to follow up with a gastroenterologist and nephrologist. His liver function test abnormalities are attributed to a combination of nonalcoholic steatohepatitis and celiac disease.
CELIAC DISEASE AND MALABSORPTION
Celiac disease is an immune-mediated disorder that causes mucosal injury to the small intestine, leading to malabsorption. It is triggered by gluten intake in genetically susceptible individuals. The HLA-DQ2 haplotype is expressed in nearly 90% of patients with the disease.
The worldwide prevalence of celiac disease is about 0.6% to 1%. Those with an affected first-degree relative, type 1 diabetes, Hashimoto thyroiditis, an autoimmune disease, Down syndrome, Turner syndrome, or IgA deficiency have an increased risk.
Celiac disease presents with chronic diarrhea, weight loss, and abdominal distention and pain. Sequelae of nutrient malabsorption such as iron-deficiency anemia, short stature, and osteopenia may be evident. Liver function may also be impaired. Dermatitis herpetiformis and gluten ataxia are rarer presentations of celiac disease.12
In the absence of immunoglobulin (Ig) A deficiency, measurement of serum IgA anti-tissue transglutaminase antibodies is recommended for initial testing. IgG antitissue transglutaminase antibodies can be measured in those with IgA deficiency.12
Duodenal biopsies to confirm the diagnosis are recommended in adults unless they have previously had biopsy-proven dermatitis herpetiformis.
Gluten-free diet
The treatment for celiac disease is avoidance of gluten. Patients who consult with a nutritionist and participate in an advocacy group are more likely to adhere to a gluten-free diet, and the physician should strongly encourage and facilitate these activities.13
Untreated disease can lead to osteoporosis, impaired splenic function with increased risk of infection with encapsulated organisms, infertility or recurrent abortion, ulcerative jejunoileitis, and lymphoma.12 Patients should be monitored annually for adherence to the gluten-free diet and for the development of any associated condition. Despite adherence to a gluten-free diet, calcium absorption and bone mineral density are lower in patients with celiac disease than in controls.14 Careful monitoring of fracture risk and adequate calcium and vitamin D replacement are also important.
Our patient undergoes dual-emission x-ray absorptiometry after discharge, with results consistent with osteopenia. His T scores range from –0.2 at the right hip to –1.1 in the left femoral neck.
Recurrence or persistently abnormal levels of IgA anti-tissue transglutaminase antibodies usually indicates poor dietary compliance.12
5. In patients whose symptoms do not improve on gluten restriction, there should be concern for which of the following?
- Lymphoma
- Nonadherence to gluten restriction
- Microscopic colitis
- All of the above
The answer is all of the above. Up to 30% of patients have persistent symptoms on a gluten-free diet. Persistent exposure to gluten is the most common reason for lack of clinical improvement. In addition, bacterial overgrowth of the small bowel, lactose intolerance, pancreatic insufficiency, and microscopic colitis may coexist with celiac disease and may contribute to ongoing symptoms. In a small subset of patients with persistent villous atrophy and symptoms despite strict adherence to a gluten-free diet for 12 months, the disease is deemed “refractory.” Refractory celiac disease can be a precursor to enteropathy-associated T-cell lymphoma.13
CASE CONCLUDED
On telephone follow-up 3 weeks after discharge, the patient reports complete resolution of diarrhea and stabilization of his weight. He reports strict adherence to a gluten-free diet and feels he is coping well.
Diagnoses
- Presenting weakness secondary to dehydration and hypokalemia
- Dyspnea secondary to respiratory compensation for metabolic acidosis
- Non-anion-gap metabolic acidosis secondary to diarrhea
- Acute kidney injury secondary to iodinated contrast, volume depletion, hypotension
- Chronic diarrhea secondary to celiac disease
- Coagulopathy secondary to fat malabsorption secondary to celiac disease.
- Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology 1999; 116:1464–1486.
- Talley NJ, Weaver AL, Zinsmeister AR, Melton LJ 3rd. Self-reported diarrhea: what does it mean? Am J Gastroenterol 1994; 89:1160–1164.
- Sweetser S. Evaluating the patient with diarrhea: a case-based approach. Mayo Clin Proc 2012; 87:596–602.
- Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med 2001; 161:1989–1996.
- Chassany O, Michaux A, Bergmann JF. Drug-induced diarrhoea. Drug Saf 2000; 22:53–72.
- Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc 2012; 87:732–738.
- Zestril (lisinopril) tablets. www.accessdata.fda.gov/drugsatfda_docs/label/2012/019777s062lbl.pdf. Accessed September 8, 2015.
- Whittier WL, Rutecki GW. Primer on clinical acid-base problem solving. Dis Mon 2004; 50:122–162.
- Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 2008; 103:162–169.
- Khouri MR, Ng SN, Huang G, Shiau YF. Fecal triglyceride excretion is not excessive in pancreatic insufficiency. Gastroenterology 1989; 96:848–852.
- Dominici R, Franzini C. Fecal elastase-1 as a test for pancreatic function: a review. Clin Chem Lab Med 2002; 40:325–332.
- Fasano A, Catassi C. Celiac disease. New Engl J Med 2012; 367:2419–2426.
- Mooney PD, Hadjivassiliou M, Sanders DS. Coeliac disease. BMJ 2014; 348:g1561–g1561.
- Pazianas M, Butcher GP, Subhani JM, et al. Calcium absorption and bone mineral density in celiacs after long term treatment with gluten-free diet and adequate calcium intake. Osteoporos Int 2005; 16:56–63.
- Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology 1999; 116:1464–1486.
- Talley NJ, Weaver AL, Zinsmeister AR, Melton LJ 3rd. Self-reported diarrhea: what does it mean? Am J Gastroenterol 1994; 89:1160–1164.
- Sweetser S. Evaluating the patient with diarrhea: a case-based approach. Mayo Clin Proc 2012; 87:596–602.
- Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med 2001; 161:1989–1996.
- Chassany O, Michaux A, Bergmann JF. Drug-induced diarrhoea. Drug Saf 2000; 22:53–72.
- Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc 2012; 87:732–738.
- Zestril (lisinopril) tablets. www.accessdata.fda.gov/drugsatfda_docs/label/2012/019777s062lbl.pdf. Accessed September 8, 2015.
- Whittier WL, Rutecki GW. Primer on clinical acid-base problem solving. Dis Mon 2004; 50:122–162.
- Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 2008; 103:162–169.
- Khouri MR, Ng SN, Huang G, Shiau YF. Fecal triglyceride excretion is not excessive in pancreatic insufficiency. Gastroenterology 1989; 96:848–852.
- Dominici R, Franzini C. Fecal elastase-1 as a test for pancreatic function: a review. Clin Chem Lab Med 2002; 40:325–332.
- Fasano A, Catassi C. Celiac disease. New Engl J Med 2012; 367:2419–2426.
- Mooney PD, Hadjivassiliou M, Sanders DS. Coeliac disease. BMJ 2014; 348:g1561–g1561.
- Pazianas M, Butcher GP, Subhani JM, et al. Calcium absorption and bone mineral density in celiacs after long term treatment with gluten-free diet and adequate calcium intake. Osteoporos Int 2005; 16:56–63.