User login
Pharmacist-Assisted Varenicline Tobacco Cessation Treatment for Veterans
Tobacco smoking remains the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths annually.1 An estimated 50.6 million US adults (20.8%) identify as tobacco users, with even higher rates among veterans (29.2%).2,3 Tobacco use is estimated to cost the US more than $300 billion annually in direct and indirect medical costs.4 According to a 2015 report, more than two-thirds of adult smokers reported a desire to quit, while only 7.5% reported successfully quitting in the past year.5 According to that same report, only 57.2% of smokers who had seen a health professional in the past year reported receiving advice to quit.5 This statistic is unfortunate, as interventions that combine behavioral and pharmacologic support can drastically increase tobacco cessation rates compared with self-help materials or no treatment.6
Currently, 7 first-line medications (5 nicotine, 2 nonnicotine) have been shown to increase long-term smoking abstinence rates. Varenicline was approved by the US Food and Drug Administration (FDA) in 2006 for use in adults as an aid to smoking cessation treatment. As a partial agonist of the α4β2 nicotinic acetylcholine receptor, varenicline’s mechanism of action is believed to involve reduction of nicotine’s rewarding capacity.7 Varenicline not only aids in complete tobacco cessation but also has been found to be effective for reducing cigarette consumption among smokers not yet willing or able to make a quit attempt.8 Furthermore, varenicline has demonstrated efficacy among users of smokeless tobacco in achieving continuous abstinence.9
Widespread adoption of varenicline into clinical practice was perhaps slowed by early concerns of psychiatric complications, prompting the FDA to issue a boxed warning for risk of serious neuropsychiatric events. This boxed warning was removed in 2016 in response to publication of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES). In this randomized controlled trial of more than 8000 participants, among whom 50.5% had a psychiatric disorder determined to be stable, varenicline significantly increased rates of continuous tobacco cessation compared with bupropion or the nicotine patch without an increased risk of neuropsychiatric events.10 This study underscored not only the safety of varenicline, but also its superiority over other first-line cessation products. The most recently published clinical practice guidelines recommend varenicline as a first-line agent for helping patients achieve long-term smoking cessation.11,12
Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. Indeed, multiple studies have demonstrated the effectiveness of pharmacist-led interventions on tobacco cessation.13-15 As of 2019, only 12 states had statutes or regulations addressing pharmacist prescribing of tobacco cessation aids without a collaborative practice agreement or local standing order.16 Until recently, most of these states limited pharmacists’ prescriptive authority to
Within the US Department of Veterans Affairs (VA), the clinical pharmacy specialist (CPS) is credentialed as an advanced practitioner with authority to independently manage patient medication therapy for a variety of diseases specified under a scope of practice. Although CPSs have provided tobacco cessation services for years, expansion of their scope to include varenicline did not occur until June 26, 2019, at the Southern Arizona VA Health Care System (SAVAHCS). All VA prescribers must follow the same criteria for prescribing varenicline. Unless previously trialed on varenicline, patients must have failed an appropriate trial of first-line agents (NRT, bupropion, or combination therapy) or have a contraindication to use of these first-line therapies before varenicline can be considered. Exclusions to therapy would include history of serious hypersensitivity to varenicline; suicidal intent, plan, or attempt within the past 12 months; current substance use disorder other than nicotine (unless varenicline recommended or prescribed by mental health professional); or unstable mental health disorder.18
The purpose of this study was to evaluate the efficacy and safety of CPS management of varenicline compared with other clinicians. We hope that this study provides insight regarding how the expansion of CPS scope to include prescriptive authority for varenicline has affected patient outcomes.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
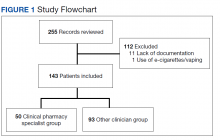
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
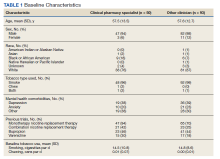
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
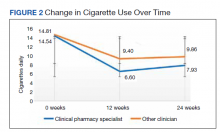
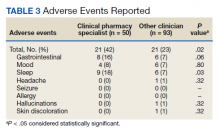
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.
Discussion
The results of this single center study suggest that management of varenicline by CPSs is associated with similar reductions in tobacco use and abstinence rates compared with management by other clinicians. These results provide evidence that CPS management of varenicline may be as safe and effective as management by other clinicians.
Adherence rates (reported as proportion of days covered when assessing varenicline refill data) were higher on average among patients managed by a CPS compared with patients managed by other clinicians. However, this outcome may not be as reflective of adherence as initially intended, given delays in follow-up (see limitations section). Time to first follow-up was drastically different between the groups, with much sooner follow-up by CPSs compared with other clinicians. Despite similar tobacco cessation rates between groups, more frequent follow-up by CPSs helps to assess patient barriers to cessation, adherence to therapy, and AEs with varenicline. A higher percentage of AEs were documented within the CPS group that could be attributed to disparities in documentation rather than true rates of AEs. While rates of AEs were initially intended to serve as the primary safety outcome, they may instead reflect pharmacists’ diligence in monitoring and documenting tolerability of medication therapy.
Limitations
Several limitations to this study should be noted. First, the data collected were only as detailed as the extent to which prescribers documented tobacco use, previous cessation trials, and AEs; thus, various data points are likely missing within this study that could impact the results presented. In line with lack of documentation, delays in follow-up (ie, annual primary care visits) sorely undermined proportion of days covered, making these data less indicative of true medication adherence. Furthermore, this study did not account for concurrent therapies, such as combination varenicline and nicotine gum/lozenges, or behavioral treatment strategies like cessation classes.
Another limitation was that some primary care practitioners prescribed varenicline but then referred these patients to a CPS for tobacco cessation follow-up. Per the study’s protocol, these patients were included within the other clinician group, which could have brought results closer to the null. Finally, the timing of this chart review (July 1, 2019, to July 31, 2020) intersects with the start of the COVID-19 pandemic, presenting a possible confounding factor if patients’ quit attempts were hindered by the stress and isolation of the pandemic.19 All pharmacist visits during the pandemic were conducted by telephone, which may have affected results.
Conclusions
In this study of veterans receiving varenicline, management by CPSs resulted in similar reductions of tobacco use and rates of complete abstinence compared with management by other clinicians. Pharmacist management was associated with greater adherence and shorter time to first follow-up compared with other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of clinical pharmacist scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
It would be interesting to see more studies outside of the VA system to determine the impact of pharmacist management of varenicline for a more heterogenous patient population. At some point, a prospective controlled trial should be conducted to overcome the various confounding factors that limit the results of retrospective chart reviews
Acknowledgments
This article was prepared, and research was conducted with resources and the use of facilities at the Southern Arizona Veterans Affairs Health Care System in Tucson.
1. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Updated March 17, 2022. Accessed May 31, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736-1742. doi:10.15585/mmwr.mm6946a4
3. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
4. Hall W, Doran C. How much can the USA reduce health care costs by reducing smoking? PLoS Med. 2016;13(5):e1002021. doi:10.1371/journal.pmed.1002021.
5. Centers for Disease Control and Prevention. Smoking cessation: fast facts. Updated March 21, 2022. Accessed June 1, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
6. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Chapter 6, Interventions for smoking cessation and treatments for nicotine dependence. In: Smoking Cessation: A Report of the Surgeon General [Internet]. Washington, DC: US Department of Health and Human Services; 2020. Accessed June 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555596
7. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. doi:10.1016/j.neuropharm.2006.10.016
8. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi:10.1001/jama.2015.280
9. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi:10.1136/bmj.c6549
10. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0
11. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
12. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
13. Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247. doi:10.1111/jcpt.12131
14. Augustine JM, Taylor AM, Pelger M, Schiefer D, Warholak TL. Smoking quit rates among patients receiving pharmacist-provided pharmacotherapy and telephonic smoking cessation counseling. J Am Pharm Assoc. 2016;56(2):129-136. doi:10.1016/j.japh.2016.02.001
15. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040-1051. doi:10.1592/phco.27.7.1040
16. National Alliance of State Pharmacy Associations. Pharmacist prescribing: tobacco cessation aids. February 10, 2021. Accessed June 1, 2022. https://naspa.us/resource/tobacco-cessation
17. Shen X, Bachyrycz A, Anderson JR, Tinker D, Raisch DW. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20(6):579-587. doi:10.18553/jmcp.2014.20.6.579
18. VA Center for Medication Safety, Tobacco Use Cessation Technical Advisory Group, Public Health Strategic Healthcare Group, VA Pharmacy Benefits Management Services, VISN Pharmacist Executives, and Medical Advisory Panel. Varenicline criteria for prescribing. 2008. Updated July 2011. Accessed June 9, 2022. https://www.healthquality.va.gov/tuc/VareniclineCriteriaforPrescribing.pdf
19. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929-1930. doi:10.1001/jama.2021.5601
Tobacco smoking remains the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths annually.1 An estimated 50.6 million US adults (20.8%) identify as tobacco users, with even higher rates among veterans (29.2%).2,3 Tobacco use is estimated to cost the US more than $300 billion annually in direct and indirect medical costs.4 According to a 2015 report, more than two-thirds of adult smokers reported a desire to quit, while only 7.5% reported successfully quitting in the past year.5 According to that same report, only 57.2% of smokers who had seen a health professional in the past year reported receiving advice to quit.5 This statistic is unfortunate, as interventions that combine behavioral and pharmacologic support can drastically increase tobacco cessation rates compared with self-help materials or no treatment.6
Currently, 7 first-line medications (5 nicotine, 2 nonnicotine) have been shown to increase long-term smoking abstinence rates. Varenicline was approved by the US Food and Drug Administration (FDA) in 2006 for use in adults as an aid to smoking cessation treatment. As a partial agonist of the α4β2 nicotinic acetylcholine receptor, varenicline’s mechanism of action is believed to involve reduction of nicotine’s rewarding capacity.7 Varenicline not only aids in complete tobacco cessation but also has been found to be effective for reducing cigarette consumption among smokers not yet willing or able to make a quit attempt.8 Furthermore, varenicline has demonstrated efficacy among users of smokeless tobacco in achieving continuous abstinence.9
Widespread adoption of varenicline into clinical practice was perhaps slowed by early concerns of psychiatric complications, prompting the FDA to issue a boxed warning for risk of serious neuropsychiatric events. This boxed warning was removed in 2016 in response to publication of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES). In this randomized controlled trial of more than 8000 participants, among whom 50.5% had a psychiatric disorder determined to be stable, varenicline significantly increased rates of continuous tobacco cessation compared with bupropion or the nicotine patch without an increased risk of neuropsychiatric events.10 This study underscored not only the safety of varenicline, but also its superiority over other first-line cessation products. The most recently published clinical practice guidelines recommend varenicline as a first-line agent for helping patients achieve long-term smoking cessation.11,12
Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. Indeed, multiple studies have demonstrated the effectiveness of pharmacist-led interventions on tobacco cessation.13-15 As of 2019, only 12 states had statutes or regulations addressing pharmacist prescribing of tobacco cessation aids without a collaborative practice agreement or local standing order.16 Until recently, most of these states limited pharmacists’ prescriptive authority to
Within the US Department of Veterans Affairs (VA), the clinical pharmacy specialist (CPS) is credentialed as an advanced practitioner with authority to independently manage patient medication therapy for a variety of diseases specified under a scope of practice. Although CPSs have provided tobacco cessation services for years, expansion of their scope to include varenicline did not occur until June 26, 2019, at the Southern Arizona VA Health Care System (SAVAHCS). All VA prescribers must follow the same criteria for prescribing varenicline. Unless previously trialed on varenicline, patients must have failed an appropriate trial of first-line agents (NRT, bupropion, or combination therapy) or have a contraindication to use of these first-line therapies before varenicline can be considered. Exclusions to therapy would include history of serious hypersensitivity to varenicline; suicidal intent, plan, or attempt within the past 12 months; current substance use disorder other than nicotine (unless varenicline recommended or prescribed by mental health professional); or unstable mental health disorder.18
The purpose of this study was to evaluate the efficacy and safety of CPS management of varenicline compared with other clinicians. We hope that this study provides insight regarding how the expansion of CPS scope to include prescriptive authority for varenicline has affected patient outcomes.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.
Discussion
The results of this single center study suggest that management of varenicline by CPSs is associated with similar reductions in tobacco use and abstinence rates compared with management by other clinicians. These results provide evidence that CPS management of varenicline may be as safe and effective as management by other clinicians.
Adherence rates (reported as proportion of days covered when assessing varenicline refill data) were higher on average among patients managed by a CPS compared with patients managed by other clinicians. However, this outcome may not be as reflective of adherence as initially intended, given delays in follow-up (see limitations section). Time to first follow-up was drastically different between the groups, with much sooner follow-up by CPSs compared with other clinicians. Despite similar tobacco cessation rates between groups, more frequent follow-up by CPSs helps to assess patient barriers to cessation, adherence to therapy, and AEs with varenicline. A higher percentage of AEs were documented within the CPS group that could be attributed to disparities in documentation rather than true rates of AEs. While rates of AEs were initially intended to serve as the primary safety outcome, they may instead reflect pharmacists’ diligence in monitoring and documenting tolerability of medication therapy.
Limitations
Several limitations to this study should be noted. First, the data collected were only as detailed as the extent to which prescribers documented tobacco use, previous cessation trials, and AEs; thus, various data points are likely missing within this study that could impact the results presented. In line with lack of documentation, delays in follow-up (ie, annual primary care visits) sorely undermined proportion of days covered, making these data less indicative of true medication adherence. Furthermore, this study did not account for concurrent therapies, such as combination varenicline and nicotine gum/lozenges, or behavioral treatment strategies like cessation classes.
Another limitation was that some primary care practitioners prescribed varenicline but then referred these patients to a CPS for tobacco cessation follow-up. Per the study’s protocol, these patients were included within the other clinician group, which could have brought results closer to the null. Finally, the timing of this chart review (July 1, 2019, to July 31, 2020) intersects with the start of the COVID-19 pandemic, presenting a possible confounding factor if patients’ quit attempts were hindered by the stress and isolation of the pandemic.19 All pharmacist visits during the pandemic were conducted by telephone, which may have affected results.
Conclusions
In this study of veterans receiving varenicline, management by CPSs resulted in similar reductions of tobacco use and rates of complete abstinence compared with management by other clinicians. Pharmacist management was associated with greater adherence and shorter time to first follow-up compared with other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of clinical pharmacist scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
It would be interesting to see more studies outside of the VA system to determine the impact of pharmacist management of varenicline for a more heterogenous patient population. At some point, a prospective controlled trial should be conducted to overcome the various confounding factors that limit the results of retrospective chart reviews
Acknowledgments
This article was prepared, and research was conducted with resources and the use of facilities at the Southern Arizona Veterans Affairs Health Care System in Tucson.
Tobacco smoking remains the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths annually.1 An estimated 50.6 million US adults (20.8%) identify as tobacco users, with even higher rates among veterans (29.2%).2,3 Tobacco use is estimated to cost the US more than $300 billion annually in direct and indirect medical costs.4 According to a 2015 report, more than two-thirds of adult smokers reported a desire to quit, while only 7.5% reported successfully quitting in the past year.5 According to that same report, only 57.2% of smokers who had seen a health professional in the past year reported receiving advice to quit.5 This statistic is unfortunate, as interventions that combine behavioral and pharmacologic support can drastically increase tobacco cessation rates compared with self-help materials or no treatment.6
Currently, 7 first-line medications (5 nicotine, 2 nonnicotine) have been shown to increase long-term smoking abstinence rates. Varenicline was approved by the US Food and Drug Administration (FDA) in 2006 for use in adults as an aid to smoking cessation treatment. As a partial agonist of the α4β2 nicotinic acetylcholine receptor, varenicline’s mechanism of action is believed to involve reduction of nicotine’s rewarding capacity.7 Varenicline not only aids in complete tobacco cessation but also has been found to be effective for reducing cigarette consumption among smokers not yet willing or able to make a quit attempt.8 Furthermore, varenicline has demonstrated efficacy among users of smokeless tobacco in achieving continuous abstinence.9
Widespread adoption of varenicline into clinical practice was perhaps slowed by early concerns of psychiatric complications, prompting the FDA to issue a boxed warning for risk of serious neuropsychiatric events. This boxed warning was removed in 2016 in response to publication of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES). In this randomized controlled trial of more than 8000 participants, among whom 50.5% had a psychiatric disorder determined to be stable, varenicline significantly increased rates of continuous tobacco cessation compared with bupropion or the nicotine patch without an increased risk of neuropsychiatric events.10 This study underscored not only the safety of varenicline, but also its superiority over other first-line cessation products. The most recently published clinical practice guidelines recommend varenicline as a first-line agent for helping patients achieve long-term smoking cessation.11,12
Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. Indeed, multiple studies have demonstrated the effectiveness of pharmacist-led interventions on tobacco cessation.13-15 As of 2019, only 12 states had statutes or regulations addressing pharmacist prescribing of tobacco cessation aids without a collaborative practice agreement or local standing order.16 Until recently, most of these states limited pharmacists’ prescriptive authority to
Within the US Department of Veterans Affairs (VA), the clinical pharmacy specialist (CPS) is credentialed as an advanced practitioner with authority to independently manage patient medication therapy for a variety of diseases specified under a scope of practice. Although CPSs have provided tobacco cessation services for years, expansion of their scope to include varenicline did not occur until June 26, 2019, at the Southern Arizona VA Health Care System (SAVAHCS). All VA prescribers must follow the same criteria for prescribing varenicline. Unless previously trialed on varenicline, patients must have failed an appropriate trial of first-line agents (NRT, bupropion, or combination therapy) or have a contraindication to use of these first-line therapies before varenicline can be considered. Exclusions to therapy would include history of serious hypersensitivity to varenicline; suicidal intent, plan, or attempt within the past 12 months; current substance use disorder other than nicotine (unless varenicline recommended or prescribed by mental health professional); or unstable mental health disorder.18
The purpose of this study was to evaluate the efficacy and safety of CPS management of varenicline compared with other clinicians. We hope that this study provides insight regarding how the expansion of CPS scope to include prescriptive authority for varenicline has affected patient outcomes.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.
Discussion
The results of this single center study suggest that management of varenicline by CPSs is associated with similar reductions in tobacco use and abstinence rates compared with management by other clinicians. These results provide evidence that CPS management of varenicline may be as safe and effective as management by other clinicians.
Adherence rates (reported as proportion of days covered when assessing varenicline refill data) were higher on average among patients managed by a CPS compared with patients managed by other clinicians. However, this outcome may not be as reflective of adherence as initially intended, given delays in follow-up (see limitations section). Time to first follow-up was drastically different between the groups, with much sooner follow-up by CPSs compared with other clinicians. Despite similar tobacco cessation rates between groups, more frequent follow-up by CPSs helps to assess patient barriers to cessation, adherence to therapy, and AEs with varenicline. A higher percentage of AEs were documented within the CPS group that could be attributed to disparities in documentation rather than true rates of AEs. While rates of AEs were initially intended to serve as the primary safety outcome, they may instead reflect pharmacists’ diligence in monitoring and documenting tolerability of medication therapy.
Limitations
Several limitations to this study should be noted. First, the data collected were only as detailed as the extent to which prescribers documented tobacco use, previous cessation trials, and AEs; thus, various data points are likely missing within this study that could impact the results presented. In line with lack of documentation, delays in follow-up (ie, annual primary care visits) sorely undermined proportion of days covered, making these data less indicative of true medication adherence. Furthermore, this study did not account for concurrent therapies, such as combination varenicline and nicotine gum/lozenges, or behavioral treatment strategies like cessation classes.
Another limitation was that some primary care practitioners prescribed varenicline but then referred these patients to a CPS for tobacco cessation follow-up. Per the study’s protocol, these patients were included within the other clinician group, which could have brought results closer to the null. Finally, the timing of this chart review (July 1, 2019, to July 31, 2020) intersects with the start of the COVID-19 pandemic, presenting a possible confounding factor if patients’ quit attempts were hindered by the stress and isolation of the pandemic.19 All pharmacist visits during the pandemic were conducted by telephone, which may have affected results.
Conclusions
In this study of veterans receiving varenicline, management by CPSs resulted in similar reductions of tobacco use and rates of complete abstinence compared with management by other clinicians. Pharmacist management was associated with greater adherence and shorter time to first follow-up compared with other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of clinical pharmacist scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
It would be interesting to see more studies outside of the VA system to determine the impact of pharmacist management of varenicline for a more heterogenous patient population. At some point, a prospective controlled trial should be conducted to overcome the various confounding factors that limit the results of retrospective chart reviews
Acknowledgments
This article was prepared, and research was conducted with resources and the use of facilities at the Southern Arizona Veterans Affairs Health Care System in Tucson.
1. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Updated March 17, 2022. Accessed May 31, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736-1742. doi:10.15585/mmwr.mm6946a4
3. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
4. Hall W, Doran C. How much can the USA reduce health care costs by reducing smoking? PLoS Med. 2016;13(5):e1002021. doi:10.1371/journal.pmed.1002021.
5. Centers for Disease Control and Prevention. Smoking cessation: fast facts. Updated March 21, 2022. Accessed June 1, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
6. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Chapter 6, Interventions for smoking cessation and treatments for nicotine dependence. In: Smoking Cessation: A Report of the Surgeon General [Internet]. Washington, DC: US Department of Health and Human Services; 2020. Accessed June 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555596
7. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. doi:10.1016/j.neuropharm.2006.10.016
8. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi:10.1001/jama.2015.280
9. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi:10.1136/bmj.c6549
10. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0
11. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
12. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
13. Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247. doi:10.1111/jcpt.12131
14. Augustine JM, Taylor AM, Pelger M, Schiefer D, Warholak TL. Smoking quit rates among patients receiving pharmacist-provided pharmacotherapy and telephonic smoking cessation counseling. J Am Pharm Assoc. 2016;56(2):129-136. doi:10.1016/j.japh.2016.02.001
15. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040-1051. doi:10.1592/phco.27.7.1040
16. National Alliance of State Pharmacy Associations. Pharmacist prescribing: tobacco cessation aids. February 10, 2021. Accessed June 1, 2022. https://naspa.us/resource/tobacco-cessation
17. Shen X, Bachyrycz A, Anderson JR, Tinker D, Raisch DW. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20(6):579-587. doi:10.18553/jmcp.2014.20.6.579
18. VA Center for Medication Safety, Tobacco Use Cessation Technical Advisory Group, Public Health Strategic Healthcare Group, VA Pharmacy Benefits Management Services, VISN Pharmacist Executives, and Medical Advisory Panel. Varenicline criteria for prescribing. 2008. Updated July 2011. Accessed June 9, 2022. https://www.healthquality.va.gov/tuc/VareniclineCriteriaforPrescribing.pdf
19. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929-1930. doi:10.1001/jama.2021.5601
1. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Updated March 17, 2022. Accessed May 31, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736-1742. doi:10.15585/mmwr.mm6946a4
3. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
4. Hall W, Doran C. How much can the USA reduce health care costs by reducing smoking? PLoS Med. 2016;13(5):e1002021. doi:10.1371/journal.pmed.1002021.
5. Centers for Disease Control and Prevention. Smoking cessation: fast facts. Updated March 21, 2022. Accessed June 1, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
6. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Chapter 6, Interventions for smoking cessation and treatments for nicotine dependence. In: Smoking Cessation: A Report of the Surgeon General [Internet]. Washington, DC: US Department of Health and Human Services; 2020. Accessed June 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555596
7. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. doi:10.1016/j.neuropharm.2006.10.016
8. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi:10.1001/jama.2015.280
9. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi:10.1136/bmj.c6549
10. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0
11. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
12. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
13. Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247. doi:10.1111/jcpt.12131
14. Augustine JM, Taylor AM, Pelger M, Schiefer D, Warholak TL. Smoking quit rates among patients receiving pharmacist-provided pharmacotherapy and telephonic smoking cessation counseling. J Am Pharm Assoc. 2016;56(2):129-136. doi:10.1016/j.japh.2016.02.001
15. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040-1051. doi:10.1592/phco.27.7.1040
16. National Alliance of State Pharmacy Associations. Pharmacist prescribing: tobacco cessation aids. February 10, 2021. Accessed June 1, 2022. https://naspa.us/resource/tobacco-cessation
17. Shen X, Bachyrycz A, Anderson JR, Tinker D, Raisch DW. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20(6):579-587. doi:10.18553/jmcp.2014.20.6.579
18. VA Center for Medication Safety, Tobacco Use Cessation Technical Advisory Group, Public Health Strategic Healthcare Group, VA Pharmacy Benefits Management Services, VISN Pharmacist Executives, and Medical Advisory Panel. Varenicline criteria for prescribing. 2008. Updated July 2011. Accessed June 9, 2022. https://www.healthquality.va.gov/tuc/VareniclineCriteriaforPrescribing.pdf
19. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929-1930. doi:10.1001/jama.2021.5601