User login
Fetal thrombophilia, perinatal stroke, and novel ideas about CP
The authors report no financial relationships relevant to this article.
Thrombosis is hypothesized to be the more common mechanism underlying cerebral palsy in many cases of maternal or fetal thrombophilia; for that reason, understanding the impact of maternal and fetal thrombophilia on pregnancy outcome is of paramount importance when counseling patients.
Is a maternal and fetal thrombophilia work-up needed in women who give birth to a term infant with cerebral palsy? Prospective studies are needed to evaluate whether that is the case. In this article, we review the literature on fetal thrombophilia and its role in explaining some cases of perinatal stroke that lead, ultimately, to cerebral palsy.
The several causes of cerebral palsy
Cerebral palsy is the most common chronic motor disability of childhood. Approximately 2 to 2.5 of every 1,000 children are given a diagnosis of this disorder every year.1,2 The condition appears early in life; it is not the result of recognized progressive disease.1 Risk factors for cerebral palsy are multiple and heterogenous1,3,4-6:
- Prematurity. The risk of developing cerebral palsy correlates inversely with gestational age.7,8 A premature infant who weighs less than 1,500 g at birth has a risk of cerebral palsy that is 20 to 30 times greater than that of a full-term, normal-weight newborn.3,4
- Hypoxia and ischemia. These are the conditions most often implicated as the cause of cerebral palsy. Fetal heart-rate monitoring was introduced in the 1960s in the hope that interventions to prevent hypoxia and ischemia would reduce the incidence of cerebral palsy. But monitoring has not had that effect—most likely, because some cases of cerebral palsy are caused by perinatal stroke.9 In fact, a large, population-based study has demonstrated that potentially asphyxiating obstetrical conditions account for only about 6% of cases of cerebral palsy.6
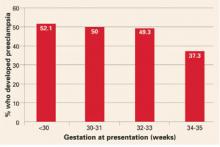
- Thrombophilia. Several recent studies report an association between fetal thrombophilia and both neonatal stroke and cerebral palsy.10-14 That association provides a possible explanation for adverse pregnancy outcomes that have otherwise been ascribed to events during delivery.15-23 Although thrombophilia is a recognized risk factor for cerebral palsy, the strength of the association has still not been fully investigated. TABLE 1 and TABLE 2 summarize studies that have examined this association. Given the rarity of both inherited thrombophilias and cerebral palsy, however, an enormous number of cases would be required to fully establish a causal relationship.
TABLE 1
Case reports reveal an association
between fetal thrombophilias and cerebral palsy
| Thrombophilias present | |||
|---|---|---|---|
| Study (type) | Cases of CP | Number | Type |
| Harum et al36 (case report) | 1 | 1 | Factor V Leiden |
| Thorarensen et al37 (case report) | 3 | 3 | Factor V Leiden |
| Lynch et al2 (case series) | 8 | 8 | Factor V Leiden |
| Halliday et al38 (case series) | 55 | 5 | Factor V Leiden; prothrombin mutation |
| Smith et al39 (case series) | 38 | 7 | Factor VIIIc |
| Nelson et al40 (case series) | 31 | 20 | Factor V Leiden; protein C deficiency |
TABLE 2
How often is a fetal thrombophilia
the likely underlying cause of cerebral palsy?
| Thrombophilia* | Prevalence of CP† | Odds ratio |
|---|---|---|
| Factor V Leiden | 6.3% | 0.62 (0.37–1.05) |
| Prothrombin gene | 5.2% | 1.11 (0.59–2.06) |
| MTHFR 677 | 54.1% | 1.27 (0.97–1.66) |
| MTHFR 1298 | 39.4% | 1.08 (0.69–1.19) |
| MTHFR 677/1298 | 15.1% | 1.18 (0.82–1.69) |
| * Heterozygous or homozygous | ||
| † Among 354 subjects with thrombophilia studied41 | ||
| Key: MTHFR, methyltetrahydrofolate reductase | ||
“Thrombophilia” describes a spectrum of congenital or acquired coagulation disorders associated with venous and arterial thrombosis.24 These disorders can occur in the mother or in the fetus, or in both concomitantly.
Fetal thrombophilia has a reported incidence of 2.4 to 5.1 cases for every 100,000 births.25 Whereas maternal thrombophilia has a substantially higher incidence, both maternal and fetal thrombophilia can lead to adverse maternal and fetal events.
The incidence of specific inherited fetal thrombophilias is summarized in TABLE 3. Maternal thrombophilia is generally associated with various adverse pregnancy outcomes, particularly cerebral palsy and perinatal stroke.9,26
TABLE 3
Inherited thrombophilias among the general population
| Study | Number | Factor V Leiden | Protein gene mutation | MTHFR |
|---|---|---|---|---|
| Gibson et al41 (2003) | 708 | 9.8% | 4.7% | 15.1%* |
| Dizon-Townson et al42 (2005) | 4,033 | 3.0% | Not reported | Not reported |
| Infante-Rivard et al43 (2002) | 472 | 3.3% | 1.3% | 43% to 49% |
| Stanley-Christian et al44 (2005) | 14 | 0 | 0 | 0 |
| Currie et al45 (2002) | 46 | 13.0% | Not reported | Not reported |
| Livingston et al46 (2001) | 92 | 0 | 2% | 4% |
| Schlembach et al47 (2003) | 28 | 4.0% | 2% | Not reported |
| Dizon-Townson et al48 (1997) | 130 | 8.6% | Not reported | Not reported |
| * Heterozygous and homozygous carriers of MTHFR C677T and A1298C | ||||
| Key: MTHFR, methyltetrahydrofolate reductase | ||||
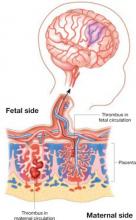
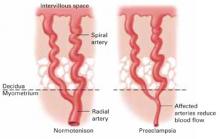
Thrombophilia leads to thrombosis at the maternal or fetal interface (FIGURE):
- When thrombosis occurs on the maternal side, the consequence may be severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss.27-29
- Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain.30 As a result, the newborn can sustain a catastrophic event such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.25
Thrombophilia can lead to thrombosis at the maternal or the fetal interface
Thrombosis on the maternal side may lead to severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss. Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain and cause a catastrophic event, such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.
Perinatal and neonatal stroke
Perinatal stroke is defined as a cerebrovascular event that occurs between 28 weeks of gestation and 28 days of postnatal age.30 Incidence is approximately 17 to 93 cases for every 100,000 live births.9
Neonatal stroke occurs in approximately 1 of every 4,000 live births.30 In addition, 1 in every 2,300 to 4,000 newborns is given a diagnosis of ischemic stroke in the nursery.9
Stroke and cerebral palsy
Arterial ischemic stroke in the newborn accounts for 50% to 70% of cases of congenital hemiplegic cerebral palsy.11 Factor V Leiden mutation, prothrombin gene mutation, and a deficiency of protein C, protein S, and antithrombin III have, taken together in two studies, been identified in more than 50% of cerebral ischemic strokes.31,32 In addition to these thrombophilias, important risk factors for perinatal and neonatal stroke include:
- thrombosis in placental villi or vessels
- infection
- use of an intravascular catheter.33
The mechanism that underlies perinatal stroke is a thromboembolic event that originates from either an intracranial or extracranial vessel, the heart, or the placenta.10 A recent meta-analysis by Haywood and colleagues found a statistically significant correlation between protein C deficiency, MTHFR C677T (methyltetrahydrofolate reductase), and the first occurrence of arterial ischemic stroke in a pediatric population.34 Associations between specific thrombophilias and perinatal stroke, as well as pediatric stroke, have been demonstrated (TABLE 4), but we want to emphasize that the absolute risks in these populations are very small.34,35 In addition, the infrequency of these thrombophilias in the general population (TABLE 3) means that their positive predictive value is extremely low.
TABLE 4
Fetal thrombophilia is detected in as many as two thirds of study cases of perinatal and neonatal stroke
| Type of thrombophilia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Infants | Thrombophilia | FVL | APCR | ACA | AT | PC | PS |
| Golomb et al31 | 22 | 14 (63%) * | 1 * | 3 * | 12 * | 0 | 0 | 0 |
| Bonduel et al32 | 30 | 9 (30%) † | n/a | n/a | n/a | 2 | 1 | 2 |
| deVeber et al49 | 92 | 35 (38%) ‡ | 0 | 6 | 23‡ | 10‡ | 6‡ | 3‡ |
| Mercuri et al50 | 24 | 10 (42%) | 5 | n/a | n/a | 0 | 0 | 0 |
| Günther et al35 | 91 | 62 (68%) | 17 | n/a | 3 | 0 | 6 | 0 |
| Govaert et al51 | 40 | 3 (8%) | 3 | n/a | n/a | n/a | n/a | n/a |
| * FVL, APCR, and ACA diagnoses overlapped. | ||||||||
| † Three patients had anticardiolipin antibody and plasminogen deficiency. | ||||||||
| ‡ Of 35 children, 21 had multiple abnormalities (combined coagulation deficiencies). | ||||||||
| Key: ACA, anticardiolipin antibody; APCR, activated protein C resistance; AT, antithrombin deficiency; FVL, factor V Leiden; PC, protein C deficiency; PS, protein S deficiency; n/a, not available or not studied. | ||||||||
Brain injury
The brain is the largest and most vulnerable fetal organ susceptible to thrombi that are formed either in the placenta or elsewhere.16 A review of cases of cerebral palsy has revealed a pathologic finding, fetal thrombotic vasculopathy (FTV), that has been associated with brain injury.16 Arias and colleagues17 and Kraus18 have observed a correlation among cerebral palsy, a thrombophilic state, and FTV.
Furthermore, Redline found that the presence of severe fetal vascular lesions correlated highly with neurologic impairment and cerebral palsy.19
What is the take-home message?
Regrettably for patients and their offspring, evidence about the relationship between thrombophilia and an adverse neurologic outcome is insufficiently strong to offer much in the way of definitive recommendations for the obstetrician.
We can, however, make some tentative recommendations on management:
Consider screening. When cerebral palsy occurs in association with perinatal stroke, fetal and maternal screening for thrombophilia can be performed.34 The recommended thrombophilia panel comprises tests for:
- factor V Leiden
- prothrombin G20210
- anticardiolipin antibody
- MTHFR mutation.10
Family screening has also been suggested in cases of 1) multiple prothrombotic risk factors in an affected newborn and 2) a positive family history.9
The cost-effectiveness of screening for thrombophilia has not been evaluated in prospective studies, because the positive predictive value of such screening is extremely low.
Consider offering prophylaxis, with cautions. A mother whose baby has been given a diagnosis of thrombophilia and fetal or neonatal stroke can be offered thromboprophylaxis (heparin and aspirin) during any subsequent pregnancy. The usefulness of this intervention has not been well studied and is based solely on expert opinion, however, so it is imperative to counsel patients on the risks and benefits of prophylactic therapy beforehand.
1. American College of Obstetricians and Gynecologists and American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington DC: The American College of Obstetricians and Gynecologists; September 2003.
2. Lynch JK, Nelson KB, Curry CJ, Grether JK. Cerebrovascular disorders in children with the factor V Leiden mutation. J Child Neurol. 2001;16:735-744.
3. Gibson CS, MacLennan AH, Goldwater PN, Dekker GA. Antenatal causes of cerebral palsy: associations between inherited thrombophilias, viral and bacterial infection, and inherited susceptibility to infection. Obstet Gynecol Surv. 2003;58:209-220.
4. Ramin SM, Gilstrap LC. Other factors/conditions associated with cerebral palsy. Semin Perinatol. 2000;24:196-199.
5. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613-618.
6. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507-513.
7. Himmelman K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287-294.
8. Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics. 2002;110:1220-1225.
9. Nelson KB. Thrombophilias, Thrombosis and Outcome in Pregnancy, Mother, and Child Symposium. Society of Maternal– Fetal Medicine 26th Annual Meeting. Miami Beach, Fla; 2006.
10. Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150-158.
11. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723-729.
12. Sarig G, Brenner B. Coagulation, inflammation and pregnancy complications. Lancet. 2004;363:96-97.
13. Fattal-Valevski A, Kenet G, Kupferminc MJ, et al. Role of thrombophilic risk factors in children with non-stroke cerebral palsy. Thromb Res. 2005;116:133-137.
14. Steiner M, Hodes MZ, Shreve M, Sundberg S, Edson JR. Postoperative stroke in a child with cerebral palsy heterozygous for factor V Leiden. J Pediatr Hematol Oncol. 2000;22:262-264.
15. Kraus FT. Perinatal pathology, the placenta and litigation. Human Pathol. 2003;34:517-521.
16. Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies and cerebral palsy. Hum Pathol. 1999;30:759-769.
17. Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med. 1998;7:277-286.
18. Kraus FT. Cerebral palsy and thrombi in placental vessels in the fetus: insights from litigation. Hum Pathol. 1997;28:246-248.
19. Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452-457.
20. Kraus FT. Placental thrombi and related problems. Semin Diagn Pathol. 1993;10:275-283.
21. Rayne SC, Kraus FT. Placental thrombi and other vascular lesions: classification, morphology and clinical correlations. Pathol Res Pract. 1993;189:2-17.
22. Grafe MR. The correlation of prenatal brain damage and placental pathology. J Neuropathol Exp Neurol. 1994;53:407-415.
23. Redline RW, O’Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med. 2000;124:1785-1791.
24. Paidas MJ, Ku DH, Arkel YS. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 2004;31:783-805.
25. Kenet G, Nowak-Göttl U. Fetal and neonatal thrombophilia. Obstet Gynecol Clin North Am. 2006;33:457-466.
26. Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412-414.
27. Stella CL, How HY, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome: controversies in screening and management. Am J Perinatol. 2006;23:499-506.
28. Stella CL, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome. Clin Obstet Gynecol. 2006;49:850-860.
29. Sibai BM. Thrombophilia and severe preeclampsia: time to screen and treat in future pregnancies? Hypertension. 2005;46:1252-1253.
30. Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurologic Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116-123.
31. Golomb MR, MacGregor DL, Domi T, et al. Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol. 2001;50:163-168.
32. Bonduel M, Sciuccati G, Hepner M, Torres AF, Pieroni G, Frontroth JP. Prethrombotic disorders in children with arterial ischemic stroke and sinovenous thrombosis. Arch Neurol. 1999;56:967-971.
33. Andrew ME, Monagle P, deVeber G, Chan AK. Thromboembolic disease and antithrombotic therapy in newborns. Hematology Am Soc Hematol Educ Program. 2001;358:374.-
34. Haywood S, Leisner R, Pindora S, Ganesan V. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child. 2005;90:402-405.
35. Günther G, Junker R, Sträter R, et al. Childhood Stroke Study Group. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic prothrombotic risk factors. Stroke. 2000;31:2437-2441.
36. Harum KH, Hoon AH, Jr, Kato GJ, Casella JF, Breiter SN, Johnston MV. Homozygous factor-V mutation as a genetic cause of perinatal thrombosis and cerebral palsy. Dev Med Child Neurol. 1999;41:777-780.
37. Thorarensen O, Ryan S, Hunter J, Younkin DP. Factor V Leiden mutation: an unrecognized cause of hemiplegic cerebral palsy, neonatal stroke, and placental thrombosis. Ann Neurol. 1997;42:372-375.
38. Halliday JL, Reddihough D, Byron K, Ekert H, Ditchfield M. Hemiplegic cerebral palsy and factor V Leiden mutation. J Med Genet. 2000;37:787-789.
39. Smith RA, Skelton M, Howard M, Levene M. Is thrombophilia a factor in the development of hemiplegic cerebral palsy? Dev Med Child Neurol. 2001;43:724-730.
40. Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665-675.
41. Gibson CS, MacLennan A, Hague B, et al. Fetal thrombophilic polymorphisms are not a risk factor for cerebral palsy. Am J Obstet Gynecol. 2003;189 Suppl 1:S75.-
42. Dizon-Townson D, Miller C, Sibai BM, et al. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517-524.
43. Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19-25.
44. Stanley-Christian H, Ghidini A, Sacher R, Shemirani M. Fetal genotype for specific inherited thrombophilia is not associated with severe preeclampsia. J Soc Gynecol Investig. 2005;12:198-201.
45. Currie L, Peek M, McNiven M, Prosser I, Mansour J, Ridgway J. Is there an increased maternal–infant prevalence of Factor V Leiden in association with severe pre-eclampsia? BJOG. 2002;109:191-196.
46. Livingston JC, Barton JR, Park V, Haddad B, Phillips O, Sibai BM. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:153-157.
47. Schlembach D, Beinder E, Zingsem J, Wunsiedler U, Beckmann MW, Fischer T. Association of maternal and/or fetal factor V Leiden and G20210A prothrombin mutation with HELLP syndrome and intrauterine growth restriction. Clin Sci (Lond). 2003;105:279-285.
48. Dizon-Townson DS, Meline L, Nelson LM, Varner M, Ward K. Fetal carriers of the factor V Leiden mutation are prone to miscarriage and placental infarction. Am J Obstet Gynecol. 1997;177:402-405.
49. deVeber G, Monagle P, Chan A, et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998;55:1539-1543.
50. Mercuri E, Cowan F, Gupte G, et al. Prothrombotic disorders and abnormal neurodevelopmental outcome in infants with neonatal cerebral infarction. Pediatrics. 2001;107:1400-1404.
51. Govaert P, Matthys E, Zecic A, Roelens F, Oostra A, Vanzieleghem B. Perinatal cortical infarction within middle cerebral artery trunks. Arch Dis Child Fetal Neonatal Ed. 2000;82:F59-F63.
The authors report no financial relationships relevant to this article.
Thrombosis is hypothesized to be the more common mechanism underlying cerebral palsy in many cases of maternal or fetal thrombophilia; for that reason, understanding the impact of maternal and fetal thrombophilia on pregnancy outcome is of paramount importance when counseling patients.
Is a maternal and fetal thrombophilia work-up needed in women who give birth to a term infant with cerebral palsy? Prospective studies are needed to evaluate whether that is the case. In this article, we review the literature on fetal thrombophilia and its role in explaining some cases of perinatal stroke that lead, ultimately, to cerebral palsy.
The several causes of cerebral palsy
Cerebral palsy is the most common chronic motor disability of childhood. Approximately 2 to 2.5 of every 1,000 children are given a diagnosis of this disorder every year.1,2 The condition appears early in life; it is not the result of recognized progressive disease.1 Risk factors for cerebral palsy are multiple and heterogenous1,3,4-6:
- Prematurity. The risk of developing cerebral palsy correlates inversely with gestational age.7,8 A premature infant who weighs less than 1,500 g at birth has a risk of cerebral palsy that is 20 to 30 times greater than that of a full-term, normal-weight newborn.3,4
- Hypoxia and ischemia. These are the conditions most often implicated as the cause of cerebral palsy. Fetal heart-rate monitoring was introduced in the 1960s in the hope that interventions to prevent hypoxia and ischemia would reduce the incidence of cerebral palsy. But monitoring has not had that effect—most likely, because some cases of cerebral palsy are caused by perinatal stroke.9 In fact, a large, population-based study has demonstrated that potentially asphyxiating obstetrical conditions account for only about 6% of cases of cerebral palsy.6
- Thrombophilia. Several recent studies report an association between fetal thrombophilia and both neonatal stroke and cerebral palsy.10-14 That association provides a possible explanation for adverse pregnancy outcomes that have otherwise been ascribed to events during delivery.15-23 Although thrombophilia is a recognized risk factor for cerebral palsy, the strength of the association has still not been fully investigated. TABLE 1 and TABLE 2 summarize studies that have examined this association. Given the rarity of both inherited thrombophilias and cerebral palsy, however, an enormous number of cases would be required to fully establish a causal relationship.
TABLE 1
Case reports reveal an association
between fetal thrombophilias and cerebral palsy
| Thrombophilias present | |||
|---|---|---|---|
| Study (type) | Cases of CP | Number | Type |
| Harum et al36 (case report) | 1 | 1 | Factor V Leiden |
| Thorarensen et al37 (case report) | 3 | 3 | Factor V Leiden |
| Lynch et al2 (case series) | 8 | 8 | Factor V Leiden |
| Halliday et al38 (case series) | 55 | 5 | Factor V Leiden; prothrombin mutation |
| Smith et al39 (case series) | 38 | 7 | Factor VIIIc |
| Nelson et al40 (case series) | 31 | 20 | Factor V Leiden; protein C deficiency |
TABLE 2
How often is a fetal thrombophilia
the likely underlying cause of cerebral palsy?
| Thrombophilia* | Prevalence of CP† | Odds ratio |
|---|---|---|
| Factor V Leiden | 6.3% | 0.62 (0.37–1.05) |
| Prothrombin gene | 5.2% | 1.11 (0.59–2.06) |
| MTHFR 677 | 54.1% | 1.27 (0.97–1.66) |
| MTHFR 1298 | 39.4% | 1.08 (0.69–1.19) |
| MTHFR 677/1298 | 15.1% | 1.18 (0.82–1.69) |
| * Heterozygous or homozygous | ||
| † Among 354 subjects with thrombophilia studied41 | ||
| Key: MTHFR, methyltetrahydrofolate reductase | ||
“Thrombophilia” describes a spectrum of congenital or acquired coagulation disorders associated with venous and arterial thrombosis.24 These disorders can occur in the mother or in the fetus, or in both concomitantly.
Fetal thrombophilia has a reported incidence of 2.4 to 5.1 cases for every 100,000 births.25 Whereas maternal thrombophilia has a substantially higher incidence, both maternal and fetal thrombophilia can lead to adverse maternal and fetal events.
The incidence of specific inherited fetal thrombophilias is summarized in TABLE 3. Maternal thrombophilia is generally associated with various adverse pregnancy outcomes, particularly cerebral palsy and perinatal stroke.9,26
TABLE 3
Inherited thrombophilias among the general population
| Study | Number | Factor V Leiden | Protein gene mutation | MTHFR |
|---|---|---|---|---|
| Gibson et al41 (2003) | 708 | 9.8% | 4.7% | 15.1%* |
| Dizon-Townson et al42 (2005) | 4,033 | 3.0% | Not reported | Not reported |
| Infante-Rivard et al43 (2002) | 472 | 3.3% | 1.3% | 43% to 49% |
| Stanley-Christian et al44 (2005) | 14 | 0 | 0 | 0 |
| Currie et al45 (2002) | 46 | 13.0% | Not reported | Not reported |
| Livingston et al46 (2001) | 92 | 0 | 2% | 4% |
| Schlembach et al47 (2003) | 28 | 4.0% | 2% | Not reported |
| Dizon-Townson et al48 (1997) | 130 | 8.6% | Not reported | Not reported |
| * Heterozygous and homozygous carriers of MTHFR C677T and A1298C | ||||
| Key: MTHFR, methyltetrahydrofolate reductase | ||||
Thrombophilia leads to thrombosis at the maternal or fetal interface (FIGURE):
- When thrombosis occurs on the maternal side, the consequence may be severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss.27-29
- Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain.30 As a result, the newborn can sustain a catastrophic event such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.25
Thrombophilia can lead to thrombosis at the maternal or the fetal interface
Thrombosis on the maternal side may lead to severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss. Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain and cause a catastrophic event, such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.
Perinatal and neonatal stroke
Perinatal stroke is defined as a cerebrovascular event that occurs between 28 weeks of gestation and 28 days of postnatal age.30 Incidence is approximately 17 to 93 cases for every 100,000 live births.9
Neonatal stroke occurs in approximately 1 of every 4,000 live births.30 In addition, 1 in every 2,300 to 4,000 newborns is given a diagnosis of ischemic stroke in the nursery.9
Stroke and cerebral palsy
Arterial ischemic stroke in the newborn accounts for 50% to 70% of cases of congenital hemiplegic cerebral palsy.11 Factor V Leiden mutation, prothrombin gene mutation, and a deficiency of protein C, protein S, and antithrombin III have, taken together in two studies, been identified in more than 50% of cerebral ischemic strokes.31,32 In addition to these thrombophilias, important risk factors for perinatal and neonatal stroke include:
- thrombosis in placental villi or vessels
- infection
- use of an intravascular catheter.33
The mechanism that underlies perinatal stroke is a thromboembolic event that originates from either an intracranial or extracranial vessel, the heart, or the placenta.10 A recent meta-analysis by Haywood and colleagues found a statistically significant correlation between protein C deficiency, MTHFR C677T (methyltetrahydrofolate reductase), and the first occurrence of arterial ischemic stroke in a pediatric population.34 Associations between specific thrombophilias and perinatal stroke, as well as pediatric stroke, have been demonstrated (TABLE 4), but we want to emphasize that the absolute risks in these populations are very small.34,35 In addition, the infrequency of these thrombophilias in the general population (TABLE 3) means that their positive predictive value is extremely low.
TABLE 4
Fetal thrombophilia is detected in as many as two thirds of study cases of perinatal and neonatal stroke
| Type of thrombophilia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Infants | Thrombophilia | FVL | APCR | ACA | AT | PC | PS |
| Golomb et al31 | 22 | 14 (63%) * | 1 * | 3 * | 12 * | 0 | 0 | 0 |
| Bonduel et al32 | 30 | 9 (30%) † | n/a | n/a | n/a | 2 | 1 | 2 |
| deVeber et al49 | 92 | 35 (38%) ‡ | 0 | 6 | 23‡ | 10‡ | 6‡ | 3‡ |
| Mercuri et al50 | 24 | 10 (42%) | 5 | n/a | n/a | 0 | 0 | 0 |
| Günther et al35 | 91 | 62 (68%) | 17 | n/a | 3 | 0 | 6 | 0 |
| Govaert et al51 | 40 | 3 (8%) | 3 | n/a | n/a | n/a | n/a | n/a |
| * FVL, APCR, and ACA diagnoses overlapped. | ||||||||
| † Three patients had anticardiolipin antibody and plasminogen deficiency. | ||||||||
| ‡ Of 35 children, 21 had multiple abnormalities (combined coagulation deficiencies). | ||||||||
| Key: ACA, anticardiolipin antibody; APCR, activated protein C resistance; AT, antithrombin deficiency; FVL, factor V Leiden; PC, protein C deficiency; PS, protein S deficiency; n/a, not available or not studied. | ||||||||
Brain injury
The brain is the largest and most vulnerable fetal organ susceptible to thrombi that are formed either in the placenta or elsewhere.16 A review of cases of cerebral palsy has revealed a pathologic finding, fetal thrombotic vasculopathy (FTV), that has been associated with brain injury.16 Arias and colleagues17 and Kraus18 have observed a correlation among cerebral palsy, a thrombophilic state, and FTV.
Furthermore, Redline found that the presence of severe fetal vascular lesions correlated highly with neurologic impairment and cerebral palsy.19
What is the take-home message?
Regrettably for patients and their offspring, evidence about the relationship between thrombophilia and an adverse neurologic outcome is insufficiently strong to offer much in the way of definitive recommendations for the obstetrician.
We can, however, make some tentative recommendations on management:
Consider screening. When cerebral palsy occurs in association with perinatal stroke, fetal and maternal screening for thrombophilia can be performed.34 The recommended thrombophilia panel comprises tests for:
- factor V Leiden
- prothrombin G20210
- anticardiolipin antibody
- MTHFR mutation.10
Family screening has also been suggested in cases of 1) multiple prothrombotic risk factors in an affected newborn and 2) a positive family history.9
The cost-effectiveness of screening for thrombophilia has not been evaluated in prospective studies, because the positive predictive value of such screening is extremely low.
Consider offering prophylaxis, with cautions. A mother whose baby has been given a diagnosis of thrombophilia and fetal or neonatal stroke can be offered thromboprophylaxis (heparin and aspirin) during any subsequent pregnancy. The usefulness of this intervention has not been well studied and is based solely on expert opinion, however, so it is imperative to counsel patients on the risks and benefits of prophylactic therapy beforehand.
The authors report no financial relationships relevant to this article.
Thrombosis is hypothesized to be the more common mechanism underlying cerebral palsy in many cases of maternal or fetal thrombophilia; for that reason, understanding the impact of maternal and fetal thrombophilia on pregnancy outcome is of paramount importance when counseling patients.
Is a maternal and fetal thrombophilia work-up needed in women who give birth to a term infant with cerebral palsy? Prospective studies are needed to evaluate whether that is the case. In this article, we review the literature on fetal thrombophilia and its role in explaining some cases of perinatal stroke that lead, ultimately, to cerebral palsy.
The several causes of cerebral palsy
Cerebral palsy is the most common chronic motor disability of childhood. Approximately 2 to 2.5 of every 1,000 children are given a diagnosis of this disorder every year.1,2 The condition appears early in life; it is not the result of recognized progressive disease.1 Risk factors for cerebral palsy are multiple and heterogenous1,3,4-6:
- Prematurity. The risk of developing cerebral palsy correlates inversely with gestational age.7,8 A premature infant who weighs less than 1,500 g at birth has a risk of cerebral palsy that is 20 to 30 times greater than that of a full-term, normal-weight newborn.3,4
- Hypoxia and ischemia. These are the conditions most often implicated as the cause of cerebral palsy. Fetal heart-rate monitoring was introduced in the 1960s in the hope that interventions to prevent hypoxia and ischemia would reduce the incidence of cerebral palsy. But monitoring has not had that effect—most likely, because some cases of cerebral palsy are caused by perinatal stroke.9 In fact, a large, population-based study has demonstrated that potentially asphyxiating obstetrical conditions account for only about 6% of cases of cerebral palsy.6
- Thrombophilia. Several recent studies report an association between fetal thrombophilia and both neonatal stroke and cerebral palsy.10-14 That association provides a possible explanation for adverse pregnancy outcomes that have otherwise been ascribed to events during delivery.15-23 Although thrombophilia is a recognized risk factor for cerebral palsy, the strength of the association has still not been fully investigated. TABLE 1 and TABLE 2 summarize studies that have examined this association. Given the rarity of both inherited thrombophilias and cerebral palsy, however, an enormous number of cases would be required to fully establish a causal relationship.
TABLE 1
Case reports reveal an association
between fetal thrombophilias and cerebral palsy
| Thrombophilias present | |||
|---|---|---|---|
| Study (type) | Cases of CP | Number | Type |
| Harum et al36 (case report) | 1 | 1 | Factor V Leiden |
| Thorarensen et al37 (case report) | 3 | 3 | Factor V Leiden |
| Lynch et al2 (case series) | 8 | 8 | Factor V Leiden |
| Halliday et al38 (case series) | 55 | 5 | Factor V Leiden; prothrombin mutation |
| Smith et al39 (case series) | 38 | 7 | Factor VIIIc |
| Nelson et al40 (case series) | 31 | 20 | Factor V Leiden; protein C deficiency |
TABLE 2
How often is a fetal thrombophilia
the likely underlying cause of cerebral palsy?
| Thrombophilia* | Prevalence of CP† | Odds ratio |
|---|---|---|
| Factor V Leiden | 6.3% | 0.62 (0.37–1.05) |
| Prothrombin gene | 5.2% | 1.11 (0.59–2.06) |
| MTHFR 677 | 54.1% | 1.27 (0.97–1.66) |
| MTHFR 1298 | 39.4% | 1.08 (0.69–1.19) |
| MTHFR 677/1298 | 15.1% | 1.18 (0.82–1.69) |
| * Heterozygous or homozygous | ||
| † Among 354 subjects with thrombophilia studied41 | ||
| Key: MTHFR, methyltetrahydrofolate reductase | ||
“Thrombophilia” describes a spectrum of congenital or acquired coagulation disorders associated with venous and arterial thrombosis.24 These disorders can occur in the mother or in the fetus, or in both concomitantly.
Fetal thrombophilia has a reported incidence of 2.4 to 5.1 cases for every 100,000 births.25 Whereas maternal thrombophilia has a substantially higher incidence, both maternal and fetal thrombophilia can lead to adverse maternal and fetal events.
The incidence of specific inherited fetal thrombophilias is summarized in TABLE 3. Maternal thrombophilia is generally associated with various adverse pregnancy outcomes, particularly cerebral palsy and perinatal stroke.9,26
TABLE 3
Inherited thrombophilias among the general population
| Study | Number | Factor V Leiden | Protein gene mutation | MTHFR |
|---|---|---|---|---|
| Gibson et al41 (2003) | 708 | 9.8% | 4.7% | 15.1%* |
| Dizon-Townson et al42 (2005) | 4,033 | 3.0% | Not reported | Not reported |
| Infante-Rivard et al43 (2002) | 472 | 3.3% | 1.3% | 43% to 49% |
| Stanley-Christian et al44 (2005) | 14 | 0 | 0 | 0 |
| Currie et al45 (2002) | 46 | 13.0% | Not reported | Not reported |
| Livingston et al46 (2001) | 92 | 0 | 2% | 4% |
| Schlembach et al47 (2003) | 28 | 4.0% | 2% | Not reported |
| Dizon-Townson et al48 (1997) | 130 | 8.6% | Not reported | Not reported |
| * Heterozygous and homozygous carriers of MTHFR C677T and A1298C | ||||
| Key: MTHFR, methyltetrahydrofolate reductase | ||||
Thrombophilia leads to thrombosis at the maternal or fetal interface (FIGURE):
- When thrombosis occurs on the maternal side, the consequence may be severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss.27-29
- Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain.30 As a result, the newborn can sustain a catastrophic event such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.25
Thrombophilia can lead to thrombosis at the maternal or the fetal interface
Thrombosis on the maternal side may lead to severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss. Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain and cause a catastrophic event, such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.
Perinatal and neonatal stroke
Perinatal stroke is defined as a cerebrovascular event that occurs between 28 weeks of gestation and 28 days of postnatal age.30 Incidence is approximately 17 to 93 cases for every 100,000 live births.9
Neonatal stroke occurs in approximately 1 of every 4,000 live births.30 In addition, 1 in every 2,300 to 4,000 newborns is given a diagnosis of ischemic stroke in the nursery.9
Stroke and cerebral palsy
Arterial ischemic stroke in the newborn accounts for 50% to 70% of cases of congenital hemiplegic cerebral palsy.11 Factor V Leiden mutation, prothrombin gene mutation, and a deficiency of protein C, protein S, and antithrombin III have, taken together in two studies, been identified in more than 50% of cerebral ischemic strokes.31,32 In addition to these thrombophilias, important risk factors for perinatal and neonatal stroke include:
- thrombosis in placental villi or vessels
- infection
- use of an intravascular catheter.33
The mechanism that underlies perinatal stroke is a thromboembolic event that originates from either an intracranial or extracranial vessel, the heart, or the placenta.10 A recent meta-analysis by Haywood and colleagues found a statistically significant correlation between protein C deficiency, MTHFR C677T (methyltetrahydrofolate reductase), and the first occurrence of arterial ischemic stroke in a pediatric population.34 Associations between specific thrombophilias and perinatal stroke, as well as pediatric stroke, have been demonstrated (TABLE 4), but we want to emphasize that the absolute risks in these populations are very small.34,35 In addition, the infrequency of these thrombophilias in the general population (TABLE 3) means that their positive predictive value is extremely low.
TABLE 4
Fetal thrombophilia is detected in as many as two thirds of study cases of perinatal and neonatal stroke
| Type of thrombophilia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Infants | Thrombophilia | FVL | APCR | ACA | AT | PC | PS |
| Golomb et al31 | 22 | 14 (63%) * | 1 * | 3 * | 12 * | 0 | 0 | 0 |
| Bonduel et al32 | 30 | 9 (30%) † | n/a | n/a | n/a | 2 | 1 | 2 |
| deVeber et al49 | 92 | 35 (38%) ‡ | 0 | 6 | 23‡ | 10‡ | 6‡ | 3‡ |
| Mercuri et al50 | 24 | 10 (42%) | 5 | n/a | n/a | 0 | 0 | 0 |
| Günther et al35 | 91 | 62 (68%) | 17 | n/a | 3 | 0 | 6 | 0 |
| Govaert et al51 | 40 | 3 (8%) | 3 | n/a | n/a | n/a | n/a | n/a |
| * FVL, APCR, and ACA diagnoses overlapped. | ||||||||
| † Three patients had anticardiolipin antibody and plasminogen deficiency. | ||||||||
| ‡ Of 35 children, 21 had multiple abnormalities (combined coagulation deficiencies). | ||||||||
| Key: ACA, anticardiolipin antibody; APCR, activated protein C resistance; AT, antithrombin deficiency; FVL, factor V Leiden; PC, protein C deficiency; PS, protein S deficiency; n/a, not available or not studied. | ||||||||
Brain injury
The brain is the largest and most vulnerable fetal organ susceptible to thrombi that are formed either in the placenta or elsewhere.16 A review of cases of cerebral palsy has revealed a pathologic finding, fetal thrombotic vasculopathy (FTV), that has been associated with brain injury.16 Arias and colleagues17 and Kraus18 have observed a correlation among cerebral palsy, a thrombophilic state, and FTV.
Furthermore, Redline found that the presence of severe fetal vascular lesions correlated highly with neurologic impairment and cerebral palsy.19
What is the take-home message?
Regrettably for patients and their offspring, evidence about the relationship between thrombophilia and an adverse neurologic outcome is insufficiently strong to offer much in the way of definitive recommendations for the obstetrician.
We can, however, make some tentative recommendations on management:
Consider screening. When cerebral palsy occurs in association with perinatal stroke, fetal and maternal screening for thrombophilia can be performed.34 The recommended thrombophilia panel comprises tests for:
- factor V Leiden
- prothrombin G20210
- anticardiolipin antibody
- MTHFR mutation.10
Family screening has also been suggested in cases of 1) multiple prothrombotic risk factors in an affected newborn and 2) a positive family history.9
The cost-effectiveness of screening for thrombophilia has not been evaluated in prospective studies, because the positive predictive value of such screening is extremely low.
Consider offering prophylaxis, with cautions. A mother whose baby has been given a diagnosis of thrombophilia and fetal or neonatal stroke can be offered thromboprophylaxis (heparin and aspirin) during any subsequent pregnancy. The usefulness of this intervention has not been well studied and is based solely on expert opinion, however, so it is imperative to counsel patients on the risks and benefits of prophylactic therapy beforehand.
1. American College of Obstetricians and Gynecologists and American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington DC: The American College of Obstetricians and Gynecologists; September 2003.
2. Lynch JK, Nelson KB, Curry CJ, Grether JK. Cerebrovascular disorders in children with the factor V Leiden mutation. J Child Neurol. 2001;16:735-744.
3. Gibson CS, MacLennan AH, Goldwater PN, Dekker GA. Antenatal causes of cerebral palsy: associations between inherited thrombophilias, viral and bacterial infection, and inherited susceptibility to infection. Obstet Gynecol Surv. 2003;58:209-220.
4. Ramin SM, Gilstrap LC. Other factors/conditions associated with cerebral palsy. Semin Perinatol. 2000;24:196-199.
5. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613-618.
6. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507-513.
7. Himmelman K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287-294.
8. Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics. 2002;110:1220-1225.
9. Nelson KB. Thrombophilias, Thrombosis and Outcome in Pregnancy, Mother, and Child Symposium. Society of Maternal– Fetal Medicine 26th Annual Meeting. Miami Beach, Fla; 2006.
10. Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150-158.
11. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723-729.
12. Sarig G, Brenner B. Coagulation, inflammation and pregnancy complications. Lancet. 2004;363:96-97.
13. Fattal-Valevski A, Kenet G, Kupferminc MJ, et al. Role of thrombophilic risk factors in children with non-stroke cerebral palsy. Thromb Res. 2005;116:133-137.
14. Steiner M, Hodes MZ, Shreve M, Sundberg S, Edson JR. Postoperative stroke in a child with cerebral palsy heterozygous for factor V Leiden. J Pediatr Hematol Oncol. 2000;22:262-264.
15. Kraus FT. Perinatal pathology, the placenta and litigation. Human Pathol. 2003;34:517-521.
16. Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies and cerebral palsy. Hum Pathol. 1999;30:759-769.
17. Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med. 1998;7:277-286.
18. Kraus FT. Cerebral palsy and thrombi in placental vessels in the fetus: insights from litigation. Hum Pathol. 1997;28:246-248.
19. Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452-457.
20. Kraus FT. Placental thrombi and related problems. Semin Diagn Pathol. 1993;10:275-283.
21. Rayne SC, Kraus FT. Placental thrombi and other vascular lesions: classification, morphology and clinical correlations. Pathol Res Pract. 1993;189:2-17.
22. Grafe MR. The correlation of prenatal brain damage and placental pathology. J Neuropathol Exp Neurol. 1994;53:407-415.
23. Redline RW, O’Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med. 2000;124:1785-1791.
24. Paidas MJ, Ku DH, Arkel YS. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 2004;31:783-805.
25. Kenet G, Nowak-Göttl U. Fetal and neonatal thrombophilia. Obstet Gynecol Clin North Am. 2006;33:457-466.
26. Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412-414.
27. Stella CL, How HY, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome: controversies in screening and management. Am J Perinatol. 2006;23:499-506.
28. Stella CL, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome. Clin Obstet Gynecol. 2006;49:850-860.
29. Sibai BM. Thrombophilia and severe preeclampsia: time to screen and treat in future pregnancies? Hypertension. 2005;46:1252-1253.
30. Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurologic Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116-123.
31. Golomb MR, MacGregor DL, Domi T, et al. Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol. 2001;50:163-168.
32. Bonduel M, Sciuccati G, Hepner M, Torres AF, Pieroni G, Frontroth JP. Prethrombotic disorders in children with arterial ischemic stroke and sinovenous thrombosis. Arch Neurol. 1999;56:967-971.
33. Andrew ME, Monagle P, deVeber G, Chan AK. Thromboembolic disease and antithrombotic therapy in newborns. Hematology Am Soc Hematol Educ Program. 2001;358:374.-
34. Haywood S, Leisner R, Pindora S, Ganesan V. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child. 2005;90:402-405.
35. Günther G, Junker R, Sträter R, et al. Childhood Stroke Study Group. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic prothrombotic risk factors. Stroke. 2000;31:2437-2441.
36. Harum KH, Hoon AH, Jr, Kato GJ, Casella JF, Breiter SN, Johnston MV. Homozygous factor-V mutation as a genetic cause of perinatal thrombosis and cerebral palsy. Dev Med Child Neurol. 1999;41:777-780.
37. Thorarensen O, Ryan S, Hunter J, Younkin DP. Factor V Leiden mutation: an unrecognized cause of hemiplegic cerebral palsy, neonatal stroke, and placental thrombosis. Ann Neurol. 1997;42:372-375.
38. Halliday JL, Reddihough D, Byron K, Ekert H, Ditchfield M. Hemiplegic cerebral palsy and factor V Leiden mutation. J Med Genet. 2000;37:787-789.
39. Smith RA, Skelton M, Howard M, Levene M. Is thrombophilia a factor in the development of hemiplegic cerebral palsy? Dev Med Child Neurol. 2001;43:724-730.
40. Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665-675.
41. Gibson CS, MacLennan A, Hague B, et al. Fetal thrombophilic polymorphisms are not a risk factor for cerebral palsy. Am J Obstet Gynecol. 2003;189 Suppl 1:S75.-
42. Dizon-Townson D, Miller C, Sibai BM, et al. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517-524.
43. Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19-25.
44. Stanley-Christian H, Ghidini A, Sacher R, Shemirani M. Fetal genotype for specific inherited thrombophilia is not associated with severe preeclampsia. J Soc Gynecol Investig. 2005;12:198-201.
45. Currie L, Peek M, McNiven M, Prosser I, Mansour J, Ridgway J. Is there an increased maternal–infant prevalence of Factor V Leiden in association with severe pre-eclampsia? BJOG. 2002;109:191-196.
46. Livingston JC, Barton JR, Park V, Haddad B, Phillips O, Sibai BM. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:153-157.
47. Schlembach D, Beinder E, Zingsem J, Wunsiedler U, Beckmann MW, Fischer T. Association of maternal and/or fetal factor V Leiden and G20210A prothrombin mutation with HELLP syndrome and intrauterine growth restriction. Clin Sci (Lond). 2003;105:279-285.
48. Dizon-Townson DS, Meline L, Nelson LM, Varner M, Ward K. Fetal carriers of the factor V Leiden mutation are prone to miscarriage and placental infarction. Am J Obstet Gynecol. 1997;177:402-405.
49. deVeber G, Monagle P, Chan A, et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998;55:1539-1543.
50. Mercuri E, Cowan F, Gupte G, et al. Prothrombotic disorders and abnormal neurodevelopmental outcome in infants with neonatal cerebral infarction. Pediatrics. 2001;107:1400-1404.
51. Govaert P, Matthys E, Zecic A, Roelens F, Oostra A, Vanzieleghem B. Perinatal cortical infarction within middle cerebral artery trunks. Arch Dis Child Fetal Neonatal Ed. 2000;82:F59-F63.
1. American College of Obstetricians and Gynecologists and American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington DC: The American College of Obstetricians and Gynecologists; September 2003.
2. Lynch JK, Nelson KB, Curry CJ, Grether JK. Cerebrovascular disorders in children with the factor V Leiden mutation. J Child Neurol. 2001;16:735-744.
3. Gibson CS, MacLennan AH, Goldwater PN, Dekker GA. Antenatal causes of cerebral palsy: associations between inherited thrombophilias, viral and bacterial infection, and inherited susceptibility to infection. Obstet Gynecol Surv. 2003;58:209-220.
4. Ramin SM, Gilstrap LC. Other factors/conditions associated with cerebral palsy. Semin Perinatol. 2000;24:196-199.
5. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613-618.
6. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507-513.
7. Himmelman K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287-294.
8. Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics. 2002;110:1220-1225.
9. Nelson KB. Thrombophilias, Thrombosis and Outcome in Pregnancy, Mother, and Child Symposium. Society of Maternal– Fetal Medicine 26th Annual Meeting. Miami Beach, Fla; 2006.
10. Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150-158.
11. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723-729.
12. Sarig G, Brenner B. Coagulation, inflammation and pregnancy complications. Lancet. 2004;363:96-97.
13. Fattal-Valevski A, Kenet G, Kupferminc MJ, et al. Role of thrombophilic risk factors in children with non-stroke cerebral palsy. Thromb Res. 2005;116:133-137.
14. Steiner M, Hodes MZ, Shreve M, Sundberg S, Edson JR. Postoperative stroke in a child with cerebral palsy heterozygous for factor V Leiden. J Pediatr Hematol Oncol. 2000;22:262-264.
15. Kraus FT. Perinatal pathology, the placenta and litigation. Human Pathol. 2003;34:517-521.
16. Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies and cerebral palsy. Hum Pathol. 1999;30:759-769.
17. Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med. 1998;7:277-286.
18. Kraus FT. Cerebral palsy and thrombi in placental vessels in the fetus: insights from litigation. Hum Pathol. 1997;28:246-248.
19. Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452-457.
20. Kraus FT. Placental thrombi and related problems. Semin Diagn Pathol. 1993;10:275-283.
21. Rayne SC, Kraus FT. Placental thrombi and other vascular lesions: classification, morphology and clinical correlations. Pathol Res Pract. 1993;189:2-17.
22. Grafe MR. The correlation of prenatal brain damage and placental pathology. J Neuropathol Exp Neurol. 1994;53:407-415.
23. Redline RW, O’Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med. 2000;124:1785-1791.
24. Paidas MJ, Ku DH, Arkel YS. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 2004;31:783-805.
25. Kenet G, Nowak-Göttl U. Fetal and neonatal thrombophilia. Obstet Gynecol Clin North Am. 2006;33:457-466.
26. Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412-414.
27. Stella CL, How HY, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome: controversies in screening and management. Am J Perinatol. 2006;23:499-506.
28. Stella CL, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome. Clin Obstet Gynecol. 2006;49:850-860.
29. Sibai BM. Thrombophilia and severe preeclampsia: time to screen and treat in future pregnancies? Hypertension. 2005;46:1252-1253.
30. Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurologic Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116-123.
31. Golomb MR, MacGregor DL, Domi T, et al. Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol. 2001;50:163-168.
32. Bonduel M, Sciuccati G, Hepner M, Torres AF, Pieroni G, Frontroth JP. Prethrombotic disorders in children with arterial ischemic stroke and sinovenous thrombosis. Arch Neurol. 1999;56:967-971.
33. Andrew ME, Monagle P, deVeber G, Chan AK. Thromboembolic disease and antithrombotic therapy in newborns. Hematology Am Soc Hematol Educ Program. 2001;358:374.-
34. Haywood S, Leisner R, Pindora S, Ganesan V. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child. 2005;90:402-405.
35. Günther G, Junker R, Sträter R, et al. Childhood Stroke Study Group. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic prothrombotic risk factors. Stroke. 2000;31:2437-2441.
36. Harum KH, Hoon AH, Jr, Kato GJ, Casella JF, Breiter SN, Johnston MV. Homozygous factor-V mutation as a genetic cause of perinatal thrombosis and cerebral palsy. Dev Med Child Neurol. 1999;41:777-780.
37. Thorarensen O, Ryan S, Hunter J, Younkin DP. Factor V Leiden mutation: an unrecognized cause of hemiplegic cerebral palsy, neonatal stroke, and placental thrombosis. Ann Neurol. 1997;42:372-375.
38. Halliday JL, Reddihough D, Byron K, Ekert H, Ditchfield M. Hemiplegic cerebral palsy and factor V Leiden mutation. J Med Genet. 2000;37:787-789.
39. Smith RA, Skelton M, Howard M, Levene M. Is thrombophilia a factor in the development of hemiplegic cerebral palsy? Dev Med Child Neurol. 2001;43:724-730.
40. Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665-675.
41. Gibson CS, MacLennan A, Hague B, et al. Fetal thrombophilic polymorphisms are not a risk factor for cerebral palsy. Am J Obstet Gynecol. 2003;189 Suppl 1:S75.-
42. Dizon-Townson D, Miller C, Sibai BM, et al. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517-524.
43. Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19-25.
44. Stanley-Christian H, Ghidini A, Sacher R, Shemirani M. Fetal genotype for specific inherited thrombophilia is not associated with severe preeclampsia. J Soc Gynecol Investig. 2005;12:198-201.
45. Currie L, Peek M, McNiven M, Prosser I, Mansour J, Ridgway J. Is there an increased maternal–infant prevalence of Factor V Leiden in association with severe pre-eclampsia? BJOG. 2002;109:191-196.
46. Livingston JC, Barton JR, Park V, Haddad B, Phillips O, Sibai BM. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:153-157.
47. Schlembach D, Beinder E, Zingsem J, Wunsiedler U, Beckmann MW, Fischer T. Association of maternal and/or fetal factor V Leiden and G20210A prothrombin mutation with HELLP syndrome and intrauterine growth restriction. Clin Sci (Lond). 2003;105:279-285.
48. Dizon-Townson DS, Meline L, Nelson LM, Varner M, Ward K. Fetal carriers of the factor V Leiden mutation are prone to miscarriage and placental infarction. Am J Obstet Gynecol. 1997;177:402-405.
49. deVeber G, Monagle P, Chan A, et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998;55:1539-1543.
50. Mercuri E, Cowan F, Gupte G, et al. Prothrombotic disorders and abnormal neurodevelopmental outcome in infants with neonatal cerebral infarction. Pediatrics. 2001;107:1400-1404.
51. Govaert P, Matthys E, Zecic A, Roelens F, Oostra A, Vanzieleghem B. Perinatal cortical infarction within middle cerebral artery trunks. Arch Dis Child Fetal Neonatal Ed. 2000;82:F59-F63.
How to manage hyperthyroid disease in pregnancy
The authors report no financial relationships relevant to this article.
CASE Life on the line
A 32-year-old woman in the 24th week of her fourth pregnancy arrives at the emergency department complaining of cough and congestion, shortness of breath, and swelling in her face, hands, and feet. The swelling has become worse over the past 2 weeks, and she had several episodes of bloody vomiting the day before her visit. The patient says she has not experienced any leakage of fluid, vaginal bleeding, or contractions. She reports good fetal movement.
The patient’s medical history is unremarkable, but a review of systems reveals a 15-lb weight loss over the past 2 weeks, racing heart, worsening edema and shortness of breath, and diarrhea.
Physical findings include exophthalmia and an enlarged thyroid with a nodule on the right side, as well as bilateral rales, tachycardia, tremor, and increased deep tendon reflexes. There is no evidence of fetal cardiac failure or goiter.
A computed tomography (CT) scan of the mother shows bilateral pleural effusions indicative of high-output cardiac failure. Thyroid ultrasonography (US) reveals a diffusely enlarged thyroid gland with a right-sided mass.
The thyroid-stimulating hormone (TSH) level is undetectable. Fetal heart rate is in the 160s, with normal variability and occasional variable deceleration. Fetal US is consistent with the estimated gestational age and shows adequate amniotic fluid and no gross fetal anomalies.
What is the likely diagnosis?
This is a classic example of undiagnosed hyperthyroidism in pregnancy manifesting as thyroid storm.
As the case illustrates, uncontrolled hyperthyroidism in pregnancy poses a significant challenge for the obstetrician. The condition can cause miscarriage, preterm delivery, intrauterine growth restriction, preeclampsia, and—at its most dangerous—thyroid storm.1 Thyroid storm is a life-threatening emergency, and treatment must be initiated even before hyperthyroidism is confirmed by thyroid function testing.2 The good news is that these complications can be successfully avoided with adequate control of thyroid function.
Overt hyperthyroidism, seen in 0.2% of pregnancies, requires active intervention to avert adverse pregnancy outcome and neurologic damage to the fetus. Subclinical disease, seen in 1.7% of pregnancies, can also create serious obstetrical problems.1
The effects of hyperthyroidism in pregnancy vary in severity, ranging from the fairly innocuous, transient, and self-limited state called gestational transient thyrotoxicosis to the life-threatening emergency of thyroid storm. This review will update you on how to manage this disorder for optimal pregnancy outcome.
To screen or not to screen
Routine screening for thyroid dysfunction has been recommended for women who have infertility, menstrual disorders, or type 1 diabetes mellitus, and for pregnant women who have signs and symptoms of the disorder. Some authors recommend screening all pregnant women, but routine screening is not endorsed by the American College of Obstetricians and Gynecologists.2,3
Thyroid testing in pregnancy is recommended in women who:
- have a family history of autoimmune thyroid disease
- are on thyroid therapy
- have a goiter or
- have insulin-dependent diabetes mellitus.
Pregnant women who have a history of high-dose neck radiation, thyroid therapy, postpartum thyroiditis, or an infant born with thyroid disease should also be tested at the first prenatal visit.4
Telltale signs and laboratory tests
The signs and symptoms of hyperthyroidism can include nervousness, heat intolerance, tachycardia, palpitations, goiter, weight loss, thyromegaly, exophthalmia, increased appetite, nausea and vomiting, sweating, and tremor.1 The difficulty here? Many of these symptoms are also seen in pregnant women who have normal thyroid function, so that symptoms alone are not a reliable guide.
Instead, the diagnosis of overt hyperthyroidism is made on the basis of laboratory tests indicating suppressed TSH and elevated levels of free thyroxine (FT4) and free triiodothyronine (FT3). Subclinical hyperthyroidism is defined as a suppressed TSH level with normal FT4 and FT3 levels.2
The effects of hyperthyroidism on laboratory values are shown in TABLE 1. A form of hyperthyroidism called the T3– toxicosis syndrome is diagnosed by suppressed TSH, normal FT4, and elevated FT3 levels.4
TABLE 1
Is your pregnant patient hyperthyroid? Five-test lab panel offers a guide
| TEST AND RESULT | |||||
|---|---|---|---|---|---|
| THYROID-STIMULATING HORMONE | FREE TRI-IODOTHYRONINE | FREE THYROXINE | TOTAL TRI-IODOTHYRONINE | TOTAL THYROXINE | THEN THE MOTHER’S CONDITION IS … |
| No change | No change | ↑ | ↑ | ↑ | Pregnancy |
| ↓ | ↑ | ↑ | ↑ | ↑ | Hyperthyroidism |
| ↓ | No change | No change | No change | No change | Subclinical hyperthyroidism |
What are the causes?
The most common cause of hyperthyroidism in pregnancy—accounting for some 95% of cases—is Graves’ disease.2 This autoimmune disorder is characterized by autoantibodies that activate the TSH receptor. These autoantibodies cross the placenta and can cause fetal and neonatal thyroid dysfunction even when the mother herself is in a euthyroid condition.4
Far less often, hyperthyroidism in pregnancy has a cause other than Graves’ disease; TABLE 2 summarizes the possibilities.1 Other causes of hyperthyroidism in early pregnancy include choriocarcinoma and gestational trophoblastic disease (partial and complete moles) (TABLE 3).
TABLE 2
Causes of hyperthyroidism in pregnancy
| Graves’ disease |
| Adenoma |
| Toxic nodular goiter |
| Thyroiditis |
| Excessive thyroid hormone intake |
| Choriocarcinoma |
| Molar pregnancy |
TABLE 3
What causes severe hyperthyroidism before 20 weeks’ gestation?
| Gestational transient thyrotoxicosis |
| Choriocarcinoma |
Gestational trophoblastic disease
|
Signs and symptoms of Graves’ disease
Women who have Graves’ disease usually have thyroid nodules and may have exophthalmia, pretibial myxedema, and tachycardia. They also display other classic signs and symptoms of hyperthyroidism, such as muscle weakness, tremor, and warm and moist skin.
During pregnancy, Graves’ disease usually becomes worse during the first trimester and postpartum period; symptoms resolve during the second and third trimesters.1
Thyrotoxin receptor and antithyroid antibodies
Antithyroid antibodies are common in patients with autoimmune thyroid disease, as a response to thyroid antigens. The two most common antithyroid antibodies are thyroglobulin and thyroid peroxidase (anti-TPO). Anti-TPO antibodies are associated with postpartum thyroiditis and fetal and neonatal hyperthyroidism. TSH-receptor antibodies include thyroid-stimulating immunoglobulin (TSI) and TSH-receptor antibody. TSI is associated with Graves’ disease. TSH-receptor antibody is associated with fetal goiter, congenital hypothyroidism, and chronic thyroiditis without goiter.4
Who do you test for antibodies? Test for maternal thyroid antibodies in patients who:
- had Graves’ disease with fetal or neonatal hyperthyroidism in a previous pregnancy
- have active Graves’ disease being treated with antithyroid drugs
- are euthyroid or have undergone ablative therapy and have fetal tachycardia or intrauterine growth restriction
- have chronic thyroiditis without goiter
- have fetal goiter on ultrasound.
Newborns who have congenital hypothyroidism should also be screened for thyroid antibodies.4
What are the consequences?
Hyperthyroidism can have multiple effects on the pregnant patient and her fetus, ranging in severity from the minimal to the catastrophic.
Gestational transient thyrotoxicosis
This condition is presumably related to high levels of human chorionic gonadotropin, a substance known to stimulate TSH receptors. Unhappily for your patient, the condition is usually heralded by severe bouts of nausea and vomiting starting at 4 to 8 weeks’ gestation. Laboratory tests show significantly elevated levels of FT4 and FT3 and suppressed TSH. Despite this significant derangement, patients generally have no evidence of a hypermetabolic state.
This condition resolves by 14 to 20 weeks of gestation, is not associated with poor pregnancy outcomes, and does not require treatment with antithyroid medication.1
Adverse pregnancy outcomes
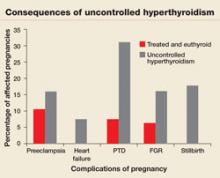
Pregnant women who have uncontrolled hyperthyroidism are at increased risk of spontaneous miscarriage, congestive heart failure, preterm delivery, intrauterine growth restriction, and preeclampsia.1 Studies that evaluated pregnancy outcomes in 239 women with overt hyperthyroidism showed increased risk of adverse pregnancy outcomes, compared with treated, euthyroid women (FIGURE 1).5-7
FIGURE 1 Consequences of uncontrolled hyperthyroidism
Several studies have found a much higher risk of pregnancy complications in women who have uncontrolled hyperthyroidism, compared with their treated and euthyroid peers.5-7
PTD=preterm delivery; FGR=fetal growth restrictions.
Fetal and neonatal hyperthyroidism
Hyperthyroidism in the fetus or newborn is caused by placental transfer of maternal immunoglobulin antibodies (TSI) to the fetus and is associated with maternal Graves’ disease. The incidence of neonatal hyperthyroidism is less than 1%. It can be predicted by rising levels of maternal TSI antibodies, to the point where levels in the third trimester are three to five times higher than they were at the beginning of pregnancy.4
Fetal hyperthyroidism develops at about 22 to 24 weeks’ gestation in mothers with a history of Graves’ disease who have been treated surgically or with ablative therapy prior to pregnancy. Even when these therapies achieve a euthyroid state in the mother, TSI levels may remain elevated and lead to fetal hyperthyroidism.
Characteristics of hyperthyroidism in the fetus include tachycardia, intrauterine growth restriction, congestive heart failure, oligohydramnios, and goiter. Treating the mother with antithyroid medications will ameliorate symptoms in the fetus.4
Thyroid storm
This is the worst-case scenario—a rare but potentially lethal complication of uncontrolled hyperthyroidism. Thyroid storm is a hypermetabolic state characterized by fever, nausea, vomiting, diarrhea, tachycardia, altered mental status, restlessness, nervousness, seizures, coma, and cardiac arrhythmias. It occurs in 1% to 2% of patients receiving thioamide therapy.8
In most instances, thyroid storm is a complication of uncontrolled hyperthyroidism, but it can also be precipitated by infection, surgery, thromboembolism, preeclampsia, labor, and delivery.
Thyroid storm is a medical emergency
This manifestation of uncontrolled hyperthyroidism is so urgent that treatment should be initiated before the results of TSH, FT4, and FT3 tests are available.2,8 Delivery should be avoided, if possible, until the mother’s condition can be stabilized but, if the status of the fetus is compromised, delivery is indicated.
Treatment of thyroid storm begins with stabilization of the patient, followed by initiation of a stepwise management approach (FIGURE 2).
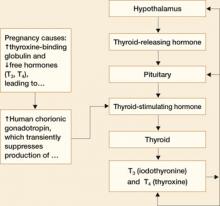
FIGURE 2 Management of thyroid storm
Aggressive management of thyroid storm is indicated, following a stepwise approach. Each medication used to treat thyroid storm plays a specific role in suppressing thyroid function. Propylthiouracil (PTU) blocks additional synthesis of thyroid hormone and inhibits the conversion of thyroxine (T4) to triiodothyronine (T3). Methimazole blocks additional synthesis of thyroid hormones. Saturated solution of potassium iodide (SSKI), Lugol’s solution, and sodium iodide block the release of thyroid hormone from the gland. Dexamethasone is used to decrease thyroid hormone release and peripheral conversion of T4 to T3. Propranolol is used to treat maternal tachycardia by inhibiting the adrenergic effects of excessive thyroid hormones. Finally, phenobarbital is used to treat maternal agitation and restlessness caused by the increased catabolism of thyroid hormones.
SOURCE: Adapted from ACOG.2
Treatment of hyperthyroidism in pregnancy
Two medications are available to treat hyperthyroidism in pregnancy: propylthiouracil (PTU) and methimazole. These medications are known as thioamides.1,2
PTU blocks the oxidation of iodine in the thyroid gland, thereby preventing the synthesis of T4 and T3. The initial dosage for hyperthyroid women who are not pregnant is usually 300 to 450 mg/day in three divided doses every 8 hours, and this dosing strategy can also be applied to the pregnant patient. Maintenance therapy is usually achieved with 100 to 150 mg/day in divided doses every 8 to 12 hours.9
Methimazole works by blocking the organification of iodide, which decreases thyroid hormone production. The usual dosing, given in three divided doses every 8 hours, is 15 mg/day for mild hyperthyroidism, 30 to 40 mg/day for moderately severe hyperthyroidism, and 60 mg/day for severe hyperthyroidism. Maintenance therapy with methimazole is usually given at a dosage of 5 to 15 mg/day.9
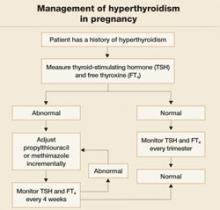
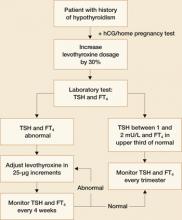
In the past, PTU was considered the drug of choice for treatment of hyperthyroidism in pregnancy because clinicians believed it crossed the placenta to a lesser degree than did methimazole, and because methimazole was associated with fetal esophageal and choanal atresia and fetal cutis aplasia (congenital skin defect of the scalp).1,2 Available evidence does not, however, support these conclusions.8,10 Whatever medication regimen you choose, thyroid function should be monitored 1) every 4 weeks until TSH and FT4 levels are within normal limits and 2) every trimester thereafter. FIGURE 3 presents an algorithm for managing hyperthyroidism in pregnancy.
FIGURE 3 Management of hyperthyroidism in pregnancy
CASE Resolved
The patient in thyroid storm described at the beginning of this article requires aggressive management, as outlined in the algorithm in FIGURE 2. As her symptoms diminish, fetal tachycardia resolves. The patient’s FT4 level begins to decline, consistent with appropriate treatment, and she is discharged home and instructed to continue PTU and labetalol and to follow up at the endocrinology and high-risk obstetrics clinics as soon as possible.
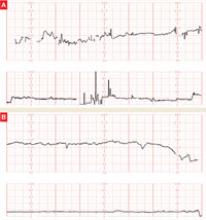
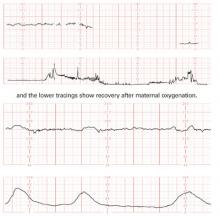
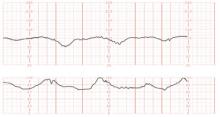
The patient does not follow this advice. Consequently, she presents at 33 5/7 weeks in a hypertensive crisis, with symptoms similar to those she first exhibited plus acute pulmonary edema. Fetal heart rate is initially in the 130s, with good variability and occasional decelerations (FIGURE 4A), but decelerations then become worse (FIGURE 4B) and emergency cesarean section is performed.
A male infant is delivered, weighing 2,390 g. Apgar scores are 0 at 1 minute and 9 at 5 minutes. A 25% placental abruption is noted at the time of delivery.
Mother and fetus are stabilized and discharged.
FIGURE 4 Weakening fetal status in a mother who is in thyroid storm
Fetal heart rate is initially in the 130s with good variability and occasional decelerations (A), but then deteriorates, with increasing decelerations (B), an indication for immediate delivery.
1. Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol. 2006;108:1283-1292.
2. American College of Obstetrics and Gynecology. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 37, August 2002. (Replaces Practice Bulletin Number 32, November 2001). Thyroid disease in pregnancy. Obstet Gynecol. 2002;100:387-396.
3. Mitchell ML, Klein RZ. The sequelae of untreated maternal hypothyroidism. Eur J Endocrinol. 2004;151 Suppl 3:U45-48.
4. Mestman JH. Endocrine diseases in pregnancy. In: Gabbe S, Niebyl JR, eds. Obstetrics: Normal and Problem Pregnancies. 4th ed. Philadelphia: Churchill Livingstone; 2002:1117-1168.
5. Davis LE, Leveno KJ, Cunningham FG. Hypothyroidism complicating pregnancy. Obstet Gynecol. 1988;72:108-112.
6. Davis LE, Lucas MJ, Hankins GD, Roark ML, Cunningham FG. Thyrotoxicosis complicating pregnancy. Am J Obstet Gynecol. 1989;160:63-70.
7. Kriplani A, Buckshee K, Bhargava VL, Takkar D, Ammini AC. Maternal and perinatal outcome in thyrotoxicosis complicating pregnancy. Eur J Obstet Gynecol Reprod Biol. 1994;54:159-163.
8. Belford MA. Navigating a thyroid storm. Contemporary OB/GYN. 2006; October:38–46.
9. Lazarus JH, Othman S. Thyroid disease in relation to pregnancy. Clin Endocrinol (Oxf). 1991;34:91-98.
10. Kent GN, Stuckey BG, Allen JR, Lambert T, Gee V. Postpartum thyroid dysfunction: clinical assessment and relationship to psychiatric affective morbidity. Clin Endocrinol (Oxf). 1999;51:429-438.
The authors report no financial relationships relevant to this article.
CASE Life on the line
A 32-year-old woman in the 24th week of her fourth pregnancy arrives at the emergency department complaining of cough and congestion, shortness of breath, and swelling in her face, hands, and feet. The swelling has become worse over the past 2 weeks, and she had several episodes of bloody vomiting the day before her visit. The patient says she has not experienced any leakage of fluid, vaginal bleeding, or contractions. She reports good fetal movement.
The patient’s medical history is unremarkable, but a review of systems reveals a 15-lb weight loss over the past 2 weeks, racing heart, worsening edema and shortness of breath, and diarrhea.
Physical findings include exophthalmia and an enlarged thyroid with a nodule on the right side, as well as bilateral rales, tachycardia, tremor, and increased deep tendon reflexes. There is no evidence of fetal cardiac failure or goiter.
A computed tomography (CT) scan of the mother shows bilateral pleural effusions indicative of high-output cardiac failure. Thyroid ultrasonography (US) reveals a diffusely enlarged thyroid gland with a right-sided mass.
The thyroid-stimulating hormone (TSH) level is undetectable. Fetal heart rate is in the 160s, with normal variability and occasional variable deceleration. Fetal US is consistent with the estimated gestational age and shows adequate amniotic fluid and no gross fetal anomalies.
What is the likely diagnosis?
This is a classic example of undiagnosed hyperthyroidism in pregnancy manifesting as thyroid storm.
As the case illustrates, uncontrolled hyperthyroidism in pregnancy poses a significant challenge for the obstetrician. The condition can cause miscarriage, preterm delivery, intrauterine growth restriction, preeclampsia, and—at its most dangerous—thyroid storm.1 Thyroid storm is a life-threatening emergency, and treatment must be initiated even before hyperthyroidism is confirmed by thyroid function testing.2 The good news is that these complications can be successfully avoided with adequate control of thyroid function.
Overt hyperthyroidism, seen in 0.2% of pregnancies, requires active intervention to avert adverse pregnancy outcome and neurologic damage to the fetus. Subclinical disease, seen in 1.7% of pregnancies, can also create serious obstetrical problems.1
The effects of hyperthyroidism in pregnancy vary in severity, ranging from the fairly innocuous, transient, and self-limited state called gestational transient thyrotoxicosis to the life-threatening emergency of thyroid storm. This review will update you on how to manage this disorder for optimal pregnancy outcome.
To screen or not to screen
Routine screening for thyroid dysfunction has been recommended for women who have infertility, menstrual disorders, or type 1 diabetes mellitus, and for pregnant women who have signs and symptoms of the disorder. Some authors recommend screening all pregnant women, but routine screening is not endorsed by the American College of Obstetricians and Gynecologists.2,3
Thyroid testing in pregnancy is recommended in women who:
- have a family history of autoimmune thyroid disease
- are on thyroid therapy
- have a goiter or
- have insulin-dependent diabetes mellitus.
Pregnant women who have a history of high-dose neck radiation, thyroid therapy, postpartum thyroiditis, or an infant born with thyroid disease should also be tested at the first prenatal visit.4
Telltale signs and laboratory tests
The signs and symptoms of hyperthyroidism can include nervousness, heat intolerance, tachycardia, palpitations, goiter, weight loss, thyromegaly, exophthalmia, increased appetite, nausea and vomiting, sweating, and tremor.1 The difficulty here? Many of these symptoms are also seen in pregnant women who have normal thyroid function, so that symptoms alone are not a reliable guide.
Instead, the diagnosis of overt hyperthyroidism is made on the basis of laboratory tests indicating suppressed TSH and elevated levels of free thyroxine (FT4) and free triiodothyronine (FT3). Subclinical hyperthyroidism is defined as a suppressed TSH level with normal FT4 and FT3 levels.2
The effects of hyperthyroidism on laboratory values are shown in TABLE 1. A form of hyperthyroidism called the T3– toxicosis syndrome is diagnosed by suppressed TSH, normal FT4, and elevated FT3 levels.4
TABLE 1
Is your pregnant patient hyperthyroid? Five-test lab panel offers a guide
| TEST AND RESULT | |||||
|---|---|---|---|---|---|
| THYROID-STIMULATING HORMONE | FREE TRI-IODOTHYRONINE | FREE THYROXINE | TOTAL TRI-IODOTHYRONINE | TOTAL THYROXINE | THEN THE MOTHER’S CONDITION IS … |
| No change | No change | ↑ | ↑ | ↑ | Pregnancy |
| ↓ | ↑ | ↑ | ↑ | ↑ | Hyperthyroidism |
| ↓ | No change | No change | No change | No change | Subclinical hyperthyroidism |
What are the causes?
The most common cause of hyperthyroidism in pregnancy—accounting for some 95% of cases—is Graves’ disease.2 This autoimmune disorder is characterized by autoantibodies that activate the TSH receptor. These autoantibodies cross the placenta and can cause fetal and neonatal thyroid dysfunction even when the mother herself is in a euthyroid condition.4
Far less often, hyperthyroidism in pregnancy has a cause other than Graves’ disease; TABLE 2 summarizes the possibilities.1 Other causes of hyperthyroidism in early pregnancy include choriocarcinoma and gestational trophoblastic disease (partial and complete moles) (TABLE 3).
TABLE 2
Causes of hyperthyroidism in pregnancy
| Graves’ disease |
| Adenoma |
| Toxic nodular goiter |
| Thyroiditis |
| Excessive thyroid hormone intake |
| Choriocarcinoma |
| Molar pregnancy |
TABLE 3
What causes severe hyperthyroidism before 20 weeks’ gestation?
| Gestational transient thyrotoxicosis |
| Choriocarcinoma |
Gestational trophoblastic disease
|
Signs and symptoms of Graves’ disease
Women who have Graves’ disease usually have thyroid nodules and may have exophthalmia, pretibial myxedema, and tachycardia. They also display other classic signs and symptoms of hyperthyroidism, such as muscle weakness, tremor, and warm and moist skin.
During pregnancy, Graves’ disease usually becomes worse during the first trimester and postpartum period; symptoms resolve during the second and third trimesters.1
Thyrotoxin receptor and antithyroid antibodies
Antithyroid antibodies are common in patients with autoimmune thyroid disease, as a response to thyroid antigens. The two most common antithyroid antibodies are thyroglobulin and thyroid peroxidase (anti-TPO). Anti-TPO antibodies are associated with postpartum thyroiditis and fetal and neonatal hyperthyroidism. TSH-receptor antibodies include thyroid-stimulating immunoglobulin (TSI) and TSH-receptor antibody. TSI is associated with Graves’ disease. TSH-receptor antibody is associated with fetal goiter, congenital hypothyroidism, and chronic thyroiditis without goiter.4
Who do you test for antibodies? Test for maternal thyroid antibodies in patients who:
- had Graves’ disease with fetal or neonatal hyperthyroidism in a previous pregnancy
- have active Graves’ disease being treated with antithyroid drugs
- are euthyroid or have undergone ablative therapy and have fetal tachycardia or intrauterine growth restriction
- have chronic thyroiditis without goiter
- have fetal goiter on ultrasound.
Newborns who have congenital hypothyroidism should also be screened for thyroid antibodies.4
What are the consequences?
Hyperthyroidism can have multiple effects on the pregnant patient and her fetus, ranging in severity from the minimal to the catastrophic.
Gestational transient thyrotoxicosis
This condition is presumably related to high levels of human chorionic gonadotropin, a substance known to stimulate TSH receptors. Unhappily for your patient, the condition is usually heralded by severe bouts of nausea and vomiting starting at 4 to 8 weeks’ gestation. Laboratory tests show significantly elevated levels of FT4 and FT3 and suppressed TSH. Despite this significant derangement, patients generally have no evidence of a hypermetabolic state.
This condition resolves by 14 to 20 weeks of gestation, is not associated with poor pregnancy outcomes, and does not require treatment with antithyroid medication.1
Adverse pregnancy outcomes
Pregnant women who have uncontrolled hyperthyroidism are at increased risk of spontaneous miscarriage, congestive heart failure, preterm delivery, intrauterine growth restriction, and preeclampsia.1 Studies that evaluated pregnancy outcomes in 239 women with overt hyperthyroidism showed increased risk of adverse pregnancy outcomes, compared with treated, euthyroid women (FIGURE 1).5-7
FIGURE 1 Consequences of uncontrolled hyperthyroidism
Several studies have found a much higher risk of pregnancy complications in women who have uncontrolled hyperthyroidism, compared with their treated and euthyroid peers.5-7
PTD=preterm delivery; FGR=fetal growth restrictions.
Fetal and neonatal hyperthyroidism
Hyperthyroidism in the fetus or newborn is caused by placental transfer of maternal immunoglobulin antibodies (TSI) to the fetus and is associated with maternal Graves’ disease. The incidence of neonatal hyperthyroidism is less than 1%. It can be predicted by rising levels of maternal TSI antibodies, to the point where levels in the third trimester are three to five times higher than they were at the beginning of pregnancy.4
Fetal hyperthyroidism develops at about 22 to 24 weeks’ gestation in mothers with a history of Graves’ disease who have been treated surgically or with ablative therapy prior to pregnancy. Even when these therapies achieve a euthyroid state in the mother, TSI levels may remain elevated and lead to fetal hyperthyroidism.
Characteristics of hyperthyroidism in the fetus include tachycardia, intrauterine growth restriction, congestive heart failure, oligohydramnios, and goiter. Treating the mother with antithyroid medications will ameliorate symptoms in the fetus.4
Thyroid storm
This is the worst-case scenario—a rare but potentially lethal complication of uncontrolled hyperthyroidism. Thyroid storm is a hypermetabolic state characterized by fever, nausea, vomiting, diarrhea, tachycardia, altered mental status, restlessness, nervousness, seizures, coma, and cardiac arrhythmias. It occurs in 1% to 2% of patients receiving thioamide therapy.8
In most instances, thyroid storm is a complication of uncontrolled hyperthyroidism, but it can also be precipitated by infection, surgery, thromboembolism, preeclampsia, labor, and delivery.
Thyroid storm is a medical emergency
This manifestation of uncontrolled hyperthyroidism is so urgent that treatment should be initiated before the results of TSH, FT4, and FT3 tests are available.2,8 Delivery should be avoided, if possible, until the mother’s condition can be stabilized but, if the status of the fetus is compromised, delivery is indicated.
Treatment of thyroid storm begins with stabilization of the patient, followed by initiation of a stepwise management approach (FIGURE 2).
FIGURE 2 Management of thyroid storm
Aggressive management of thyroid storm is indicated, following a stepwise approach. Each medication used to treat thyroid storm plays a specific role in suppressing thyroid function. Propylthiouracil (PTU) blocks additional synthesis of thyroid hormone and inhibits the conversion of thyroxine (T4) to triiodothyronine (T3). Methimazole blocks additional synthesis of thyroid hormones. Saturated solution of potassium iodide (SSKI), Lugol’s solution, and sodium iodide block the release of thyroid hormone from the gland. Dexamethasone is used to decrease thyroid hormone release and peripheral conversion of T4 to T3. Propranolol is used to treat maternal tachycardia by inhibiting the adrenergic effects of excessive thyroid hormones. Finally, phenobarbital is used to treat maternal agitation and restlessness caused by the increased catabolism of thyroid hormones.
SOURCE: Adapted from ACOG.2
Treatment of hyperthyroidism in pregnancy
Two medications are available to treat hyperthyroidism in pregnancy: propylthiouracil (PTU) and methimazole. These medications are known as thioamides.1,2
PTU blocks the oxidation of iodine in the thyroid gland, thereby preventing the synthesis of T4 and T3. The initial dosage for hyperthyroid women who are not pregnant is usually 300 to 450 mg/day in three divided doses every 8 hours, and this dosing strategy can also be applied to the pregnant patient. Maintenance therapy is usually achieved with 100 to 150 mg/day in divided doses every 8 to 12 hours.9
Methimazole works by blocking the organification of iodide, which decreases thyroid hormone production. The usual dosing, given in three divided doses every 8 hours, is 15 mg/day for mild hyperthyroidism, 30 to 40 mg/day for moderately severe hyperthyroidism, and 60 mg/day for severe hyperthyroidism. Maintenance therapy with methimazole is usually given at a dosage of 5 to 15 mg/day.9
In the past, PTU was considered the drug of choice for treatment of hyperthyroidism in pregnancy because clinicians believed it crossed the placenta to a lesser degree than did methimazole, and because methimazole was associated with fetal esophageal and choanal atresia and fetal cutis aplasia (congenital skin defect of the scalp).1,2 Available evidence does not, however, support these conclusions.8,10 Whatever medication regimen you choose, thyroid function should be monitored 1) every 4 weeks until TSH and FT4 levels are within normal limits and 2) every trimester thereafter. FIGURE 3 presents an algorithm for managing hyperthyroidism in pregnancy.
FIGURE 3 Management of hyperthyroidism in pregnancy
CASE Resolved
The patient in thyroid storm described at the beginning of this article requires aggressive management, as outlined in the algorithm in FIGURE 2. As her symptoms diminish, fetal tachycardia resolves. The patient’s FT4 level begins to decline, consistent with appropriate treatment, and she is discharged home and instructed to continue PTU and labetalol and to follow up at the endocrinology and high-risk obstetrics clinics as soon as possible.
The patient does not follow this advice. Consequently, she presents at 33 5/7 weeks in a hypertensive crisis, with symptoms similar to those she first exhibited plus acute pulmonary edema. Fetal heart rate is initially in the 130s, with good variability and occasional decelerations (FIGURE 4A), but decelerations then become worse (FIGURE 4B) and emergency cesarean section is performed.
A male infant is delivered, weighing 2,390 g. Apgar scores are 0 at 1 minute and 9 at 5 minutes. A 25% placental abruption is noted at the time of delivery.
Mother and fetus are stabilized and discharged.
FIGURE 4 Weakening fetal status in a mother who is in thyroid storm
Fetal heart rate is initially in the 130s with good variability and occasional decelerations (A), but then deteriorates, with increasing decelerations (B), an indication for immediate delivery.
The authors report no financial relationships relevant to this article.
CASE Life on the line
A 32-year-old woman in the 24th week of her fourth pregnancy arrives at the emergency department complaining of cough and congestion, shortness of breath, and swelling in her face, hands, and feet. The swelling has become worse over the past 2 weeks, and she had several episodes of bloody vomiting the day before her visit. The patient says she has not experienced any leakage of fluid, vaginal bleeding, or contractions. She reports good fetal movement.
The patient’s medical history is unremarkable, but a review of systems reveals a 15-lb weight loss over the past 2 weeks, racing heart, worsening edema and shortness of breath, and diarrhea.
Physical findings include exophthalmia and an enlarged thyroid with a nodule on the right side, as well as bilateral rales, tachycardia, tremor, and increased deep tendon reflexes. There is no evidence of fetal cardiac failure or goiter.
A computed tomography (CT) scan of the mother shows bilateral pleural effusions indicative of high-output cardiac failure. Thyroid ultrasonography (US) reveals a diffusely enlarged thyroid gland with a right-sided mass.
The thyroid-stimulating hormone (TSH) level is undetectable. Fetal heart rate is in the 160s, with normal variability and occasional variable deceleration. Fetal US is consistent with the estimated gestational age and shows adequate amniotic fluid and no gross fetal anomalies.
What is the likely diagnosis?
This is a classic example of undiagnosed hyperthyroidism in pregnancy manifesting as thyroid storm.
As the case illustrates, uncontrolled hyperthyroidism in pregnancy poses a significant challenge for the obstetrician. The condition can cause miscarriage, preterm delivery, intrauterine growth restriction, preeclampsia, and—at its most dangerous—thyroid storm.1 Thyroid storm is a life-threatening emergency, and treatment must be initiated even before hyperthyroidism is confirmed by thyroid function testing.2 The good news is that these complications can be successfully avoided with adequate control of thyroid function.
Overt hyperthyroidism, seen in 0.2% of pregnancies, requires active intervention to avert adverse pregnancy outcome and neurologic damage to the fetus. Subclinical disease, seen in 1.7% of pregnancies, can also create serious obstetrical problems.1
The effects of hyperthyroidism in pregnancy vary in severity, ranging from the fairly innocuous, transient, and self-limited state called gestational transient thyrotoxicosis to the life-threatening emergency of thyroid storm. This review will update you on how to manage this disorder for optimal pregnancy outcome.
To screen or not to screen
Routine screening for thyroid dysfunction has been recommended for women who have infertility, menstrual disorders, or type 1 diabetes mellitus, and for pregnant women who have signs and symptoms of the disorder. Some authors recommend screening all pregnant women, but routine screening is not endorsed by the American College of Obstetricians and Gynecologists.2,3
Thyroid testing in pregnancy is recommended in women who:
- have a family history of autoimmune thyroid disease
- are on thyroid therapy
- have a goiter or
- have insulin-dependent diabetes mellitus.
Pregnant women who have a history of high-dose neck radiation, thyroid therapy, postpartum thyroiditis, or an infant born with thyroid disease should also be tested at the first prenatal visit.4
Telltale signs and laboratory tests
The signs and symptoms of hyperthyroidism can include nervousness, heat intolerance, tachycardia, palpitations, goiter, weight loss, thyromegaly, exophthalmia, increased appetite, nausea and vomiting, sweating, and tremor.1 The difficulty here? Many of these symptoms are also seen in pregnant women who have normal thyroid function, so that symptoms alone are not a reliable guide.
Instead, the diagnosis of overt hyperthyroidism is made on the basis of laboratory tests indicating suppressed TSH and elevated levels of free thyroxine (FT4) and free triiodothyronine (FT3). Subclinical hyperthyroidism is defined as a suppressed TSH level with normal FT4 and FT3 levels.2
The effects of hyperthyroidism on laboratory values are shown in TABLE 1. A form of hyperthyroidism called the T3– toxicosis syndrome is diagnosed by suppressed TSH, normal FT4, and elevated FT3 levels.4
TABLE 1
Is your pregnant patient hyperthyroid? Five-test lab panel offers a guide
| TEST AND RESULT | |||||
|---|---|---|---|---|---|
| THYROID-STIMULATING HORMONE | FREE TRI-IODOTHYRONINE | FREE THYROXINE | TOTAL TRI-IODOTHYRONINE | TOTAL THYROXINE | THEN THE MOTHER’S CONDITION IS … |
| No change | No change | ↑ | ↑ | ↑ | Pregnancy |
| ↓ | ↑ | ↑ | ↑ | ↑ | Hyperthyroidism |
| ↓ | No change | No change | No change | No change | Subclinical hyperthyroidism |
What are the causes?
The most common cause of hyperthyroidism in pregnancy—accounting for some 95% of cases—is Graves’ disease.2 This autoimmune disorder is characterized by autoantibodies that activate the TSH receptor. These autoantibodies cross the placenta and can cause fetal and neonatal thyroid dysfunction even when the mother herself is in a euthyroid condition.4
Far less often, hyperthyroidism in pregnancy has a cause other than Graves’ disease; TABLE 2 summarizes the possibilities.1 Other causes of hyperthyroidism in early pregnancy include choriocarcinoma and gestational trophoblastic disease (partial and complete moles) (TABLE 3).
TABLE 2
Causes of hyperthyroidism in pregnancy
| Graves’ disease |
| Adenoma |
| Toxic nodular goiter |
| Thyroiditis |
| Excessive thyroid hormone intake |
| Choriocarcinoma |
| Molar pregnancy |
TABLE 3
What causes severe hyperthyroidism before 20 weeks’ gestation?
| Gestational transient thyrotoxicosis |
| Choriocarcinoma |
Gestational trophoblastic disease
|
Signs and symptoms of Graves’ disease
Women who have Graves’ disease usually have thyroid nodules and may have exophthalmia, pretibial myxedema, and tachycardia. They also display other classic signs and symptoms of hyperthyroidism, such as muscle weakness, tremor, and warm and moist skin.
During pregnancy, Graves’ disease usually becomes worse during the first trimester and postpartum period; symptoms resolve during the second and third trimesters.1
Thyrotoxin receptor and antithyroid antibodies
Antithyroid antibodies are common in patients with autoimmune thyroid disease, as a response to thyroid antigens. The two most common antithyroid antibodies are thyroglobulin and thyroid peroxidase (anti-TPO). Anti-TPO antibodies are associated with postpartum thyroiditis and fetal and neonatal hyperthyroidism. TSH-receptor antibodies include thyroid-stimulating immunoglobulin (TSI) and TSH-receptor antibody. TSI is associated with Graves’ disease. TSH-receptor antibody is associated with fetal goiter, congenital hypothyroidism, and chronic thyroiditis without goiter.4
Who do you test for antibodies? Test for maternal thyroid antibodies in patients who:
- had Graves’ disease with fetal or neonatal hyperthyroidism in a previous pregnancy
- have active Graves’ disease being treated with antithyroid drugs
- are euthyroid or have undergone ablative therapy and have fetal tachycardia or intrauterine growth restriction
- have chronic thyroiditis without goiter
- have fetal goiter on ultrasound.
Newborns who have congenital hypothyroidism should also be screened for thyroid antibodies.4
What are the consequences?
Hyperthyroidism can have multiple effects on the pregnant patient and her fetus, ranging in severity from the minimal to the catastrophic.
Gestational transient thyrotoxicosis
This condition is presumably related to high levels of human chorionic gonadotropin, a substance known to stimulate TSH receptors. Unhappily for your patient, the condition is usually heralded by severe bouts of nausea and vomiting starting at 4 to 8 weeks’ gestation. Laboratory tests show significantly elevated levels of FT4 and FT3 and suppressed TSH. Despite this significant derangement, patients generally have no evidence of a hypermetabolic state.
This condition resolves by 14 to 20 weeks of gestation, is not associated with poor pregnancy outcomes, and does not require treatment with antithyroid medication.1
Adverse pregnancy outcomes
Pregnant women who have uncontrolled hyperthyroidism are at increased risk of spontaneous miscarriage, congestive heart failure, preterm delivery, intrauterine growth restriction, and preeclampsia.1 Studies that evaluated pregnancy outcomes in 239 women with overt hyperthyroidism showed increased risk of adverse pregnancy outcomes, compared with treated, euthyroid women (FIGURE 1).5-7
FIGURE 1 Consequences of uncontrolled hyperthyroidism
Several studies have found a much higher risk of pregnancy complications in women who have uncontrolled hyperthyroidism, compared with their treated and euthyroid peers.5-7
PTD=preterm delivery; FGR=fetal growth restrictions.
Fetal and neonatal hyperthyroidism
Hyperthyroidism in the fetus or newborn is caused by placental transfer of maternal immunoglobulin antibodies (TSI) to the fetus and is associated with maternal Graves’ disease. The incidence of neonatal hyperthyroidism is less than 1%. It can be predicted by rising levels of maternal TSI antibodies, to the point where levels in the third trimester are three to five times higher than they were at the beginning of pregnancy.4
Fetal hyperthyroidism develops at about 22 to 24 weeks’ gestation in mothers with a history of Graves’ disease who have been treated surgically or with ablative therapy prior to pregnancy. Even when these therapies achieve a euthyroid state in the mother, TSI levels may remain elevated and lead to fetal hyperthyroidism.
Characteristics of hyperthyroidism in the fetus include tachycardia, intrauterine growth restriction, congestive heart failure, oligohydramnios, and goiter. Treating the mother with antithyroid medications will ameliorate symptoms in the fetus.4
Thyroid storm
This is the worst-case scenario—a rare but potentially lethal complication of uncontrolled hyperthyroidism. Thyroid storm is a hypermetabolic state characterized by fever, nausea, vomiting, diarrhea, tachycardia, altered mental status, restlessness, nervousness, seizures, coma, and cardiac arrhythmias. It occurs in 1% to 2% of patients receiving thioamide therapy.8
In most instances, thyroid storm is a complication of uncontrolled hyperthyroidism, but it can also be precipitated by infection, surgery, thromboembolism, preeclampsia, labor, and delivery.
Thyroid storm is a medical emergency
This manifestation of uncontrolled hyperthyroidism is so urgent that treatment should be initiated before the results of TSH, FT4, and FT3 tests are available.2,8 Delivery should be avoided, if possible, until the mother’s condition can be stabilized but, if the status of the fetus is compromised, delivery is indicated.
Treatment of thyroid storm begins with stabilization of the patient, followed by initiation of a stepwise management approach (FIGURE 2).
FIGURE 2 Management of thyroid storm
Aggressive management of thyroid storm is indicated, following a stepwise approach. Each medication used to treat thyroid storm plays a specific role in suppressing thyroid function. Propylthiouracil (PTU) blocks additional synthesis of thyroid hormone and inhibits the conversion of thyroxine (T4) to triiodothyronine (T3). Methimazole blocks additional synthesis of thyroid hormones. Saturated solution of potassium iodide (SSKI), Lugol’s solution, and sodium iodide block the release of thyroid hormone from the gland. Dexamethasone is used to decrease thyroid hormone release and peripheral conversion of T4 to T3. Propranolol is used to treat maternal tachycardia by inhibiting the adrenergic effects of excessive thyroid hormones. Finally, phenobarbital is used to treat maternal agitation and restlessness caused by the increased catabolism of thyroid hormones.
SOURCE: Adapted from ACOG.2
Treatment of hyperthyroidism in pregnancy
Two medications are available to treat hyperthyroidism in pregnancy: propylthiouracil (PTU) and methimazole. These medications are known as thioamides.1,2
PTU blocks the oxidation of iodine in the thyroid gland, thereby preventing the synthesis of T4 and T3. The initial dosage for hyperthyroid women who are not pregnant is usually 300 to 450 mg/day in three divided doses every 8 hours, and this dosing strategy can also be applied to the pregnant patient. Maintenance therapy is usually achieved with 100 to 150 mg/day in divided doses every 8 to 12 hours.9
Methimazole works by blocking the organification of iodide, which decreases thyroid hormone production. The usual dosing, given in three divided doses every 8 hours, is 15 mg/day for mild hyperthyroidism, 30 to 40 mg/day for moderately severe hyperthyroidism, and 60 mg/day for severe hyperthyroidism. Maintenance therapy with methimazole is usually given at a dosage of 5 to 15 mg/day.9
In the past, PTU was considered the drug of choice for treatment of hyperthyroidism in pregnancy because clinicians believed it crossed the placenta to a lesser degree than did methimazole, and because methimazole was associated with fetal esophageal and choanal atresia and fetal cutis aplasia (congenital skin defect of the scalp).1,2 Available evidence does not, however, support these conclusions.8,10 Whatever medication regimen you choose, thyroid function should be monitored 1) every 4 weeks until TSH and FT4 levels are within normal limits and 2) every trimester thereafter. FIGURE 3 presents an algorithm for managing hyperthyroidism in pregnancy.
FIGURE 3 Management of hyperthyroidism in pregnancy
CASE Resolved
The patient in thyroid storm described at the beginning of this article requires aggressive management, as outlined in the algorithm in FIGURE 2. As her symptoms diminish, fetal tachycardia resolves. The patient’s FT4 level begins to decline, consistent with appropriate treatment, and she is discharged home and instructed to continue PTU and labetalol and to follow up at the endocrinology and high-risk obstetrics clinics as soon as possible.
The patient does not follow this advice. Consequently, she presents at 33 5/7 weeks in a hypertensive crisis, with symptoms similar to those she first exhibited plus acute pulmonary edema. Fetal heart rate is initially in the 130s, with good variability and occasional decelerations (FIGURE 4A), but decelerations then become worse (FIGURE 4B) and emergency cesarean section is performed.
A male infant is delivered, weighing 2,390 g. Apgar scores are 0 at 1 minute and 9 at 5 minutes. A 25% placental abruption is noted at the time of delivery.
Mother and fetus are stabilized and discharged.
FIGURE 4 Weakening fetal status in a mother who is in thyroid storm
Fetal heart rate is initially in the 130s with good variability and occasional decelerations (A), but then deteriorates, with increasing decelerations (B), an indication for immediate delivery.
1. Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol. 2006;108:1283-1292.
2. American College of Obstetrics and Gynecology. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 37, August 2002. (Replaces Practice Bulletin Number 32, November 2001). Thyroid disease in pregnancy. Obstet Gynecol. 2002;100:387-396.
3. Mitchell ML, Klein RZ. The sequelae of untreated maternal hypothyroidism. Eur J Endocrinol. 2004;151 Suppl 3:U45-48.
4. Mestman JH. Endocrine diseases in pregnancy. In: Gabbe S, Niebyl JR, eds. Obstetrics: Normal and Problem Pregnancies. 4th ed. Philadelphia: Churchill Livingstone; 2002:1117-1168.
5. Davis LE, Leveno KJ, Cunningham FG. Hypothyroidism complicating pregnancy. Obstet Gynecol. 1988;72:108-112.
6. Davis LE, Lucas MJ, Hankins GD, Roark ML, Cunningham FG. Thyrotoxicosis complicating pregnancy. Am J Obstet Gynecol. 1989;160:63-70.
7. Kriplani A, Buckshee K, Bhargava VL, Takkar D, Ammini AC. Maternal and perinatal outcome in thyrotoxicosis complicating pregnancy. Eur J Obstet Gynecol Reprod Biol. 1994;54:159-163.
8. Belford MA. Navigating a thyroid storm. Contemporary OB/GYN. 2006; October:38–46.
9. Lazarus JH, Othman S. Thyroid disease in relation to pregnancy. Clin Endocrinol (Oxf). 1991;34:91-98.
10. Kent GN, Stuckey BG, Allen JR, Lambert T, Gee V. Postpartum thyroid dysfunction: clinical assessment and relationship to psychiatric affective morbidity. Clin Endocrinol (Oxf). 1999;51:429-438.
1. Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol. 2006;108:1283-1292.
2. American College of Obstetrics and Gynecology. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 37, August 2002. (Replaces Practice Bulletin Number 32, November 2001). Thyroid disease in pregnancy. Obstet Gynecol. 2002;100:387-396.
3. Mitchell ML, Klein RZ. The sequelae of untreated maternal hypothyroidism. Eur J Endocrinol. 2004;151 Suppl 3:U45-48.
4. Mestman JH. Endocrine diseases in pregnancy. In: Gabbe S, Niebyl JR, eds. Obstetrics: Normal and Problem Pregnancies. 4th ed. Philadelphia: Churchill Livingstone; 2002:1117-1168.
5. Davis LE, Leveno KJ, Cunningham FG. Hypothyroidism complicating pregnancy. Obstet Gynecol. 1988;72:108-112.
6. Davis LE, Lucas MJ, Hankins GD, Roark ML, Cunningham FG. Thyrotoxicosis complicating pregnancy. Am J Obstet Gynecol. 1989;160:63-70.
7. Kriplani A, Buckshee K, Bhargava VL, Takkar D, Ammini AC. Maternal and perinatal outcome in thyrotoxicosis complicating pregnancy. Eur J Obstet Gynecol Reprod Biol. 1994;54:159-163.
8. Belford MA. Navigating a thyroid storm. Contemporary OB/GYN. 2006; October:38–46.
9. Lazarus JH, Othman S. Thyroid disease in relation to pregnancy. Clin Endocrinol (Oxf). 1991;34:91-98.
10. Kent GN, Stuckey BG, Allen JR, Lambert T, Gee V. Postpartum thyroid dysfunction: clinical assessment and relationship to psychiatric affective morbidity. Clin Endocrinol (Oxf). 1999;51:429-438.
How to manage hypothyroid disease in pregnancy
The authors report no financial relationships relevant to this article.
A pregnant woman whose thyroid gland isn’t doing its job presents a serious management problem for her obstetrician. If she has overt hypothyroidism, seen in between 0.3% and 2.5% of pregnancies, active intervention is required to prevent serious damage to the fetus.1,2 Even if she has subclinical disease, seen in 2% to 3% of pregnancies, current research indicates that intervention may be indicated.
Fetal thyroxine requirements increase as early as 5 weeks of gestation, when the fetus is still dependent on maternal thyroxine. A deficiency of maternal thyroxine can have severe adverse outcomes, affecting the course of the pregnancy and the neurologic development of the fetus. To prevent such sequelae, patients who were on thyroid medication before pregnancy should increase the dosage by 30% once pregnancy is confirmed, and hypothyroidism that develops in pregnancy should be managed aggressively and meticulously.
Here, we’ll examine the published research to advise you on evidence-based approaches for diagnosis and management of this complex condition.
Maternal thyroid function
An elaborate negative-feedback loop prevails before pregnancy
In a nonpregnant woman, thyroid function is controlled by a negative-feedback loop that works like this:
- The hypothalamus releases thyroid-releasing hormone (TRH)
- TRH acts on the pituitary gland to release thyroid-stimulating hormone (TSH)
- TSH, in turn, acts on the thyroid gland to release the thyroid hormones iodothyronine (T3) and thyroxine (T4) that regulate metabolism
- TRH and TSH concentrations are inversely related to T3 and T4 concentrations. That is, the more TRH and TSH circulating in the blood stream, the less T3 and T4 will be produced by the thyroid gland3
- Almost all (approximately 99%) circulating T3 and T4 is bound to a protein called thyroxine-binding globulin (TBG). Only 1% of these hormones circulate in the free form, and only the free forms are biologically active.3
This relationship is illustrated in FIGURE 1.
FIGURE 1 Thyroid physiology and the impact of pregnancy
Pregnancy reduces free forms of T3 and T4, and increases TSH slightly
Pregnancy alters thyroid function in significant ways:
- Increases in circulating estrogen lead to the production of more TBG
- When TBG increases, more T3 and T4 are bound and fewer free forms of these hormones are available
- Because the total T3 (TT3) and total free T4 (TT4) are decreased in pregnancy, they are not good measures of thyroid function. Maternal thyroid function in pregnancy should be monitored using free T4 (FT4) and TSH levels
- Increased TBG also leads to a slight increase in TSH between the first trimester and term
- Human chorionic gonadotropin (hCG) concentrations also increase in pregnancy. Because hCG has thyrotropin-like activity, these higher levels cause a transient decrease in TSH by suppression of TSH production between approximately 8 and 14 weeks of gestation.
Fetal thyroid function
During early gestation, the fetus receives thyroid hormone from the mother.1 Maternal T4 crosses the placenta actively—the only hormone that does so.4 The fetus’s need for thyroxine starts to increase as early as 5 weeks of gestation.5
Fetal thyroid development does not begin until 10 to 12 weeks of gestation, and then continues until term. The fetus relies on maternal T4 exclusively before 12 weeks and partially thereafter for normal fetal neurologic development. It follows that maternal hypothyroidism could be detrimental to fetal development if not detected and corrected very early in gestation.
How (and whom) to screen for maternal hypothyroidism
Routine screening has been recommended for women who have infertility, menstrual disorders, or type 1 diabetes mellitus, and for pregnant women who have signs and symptoms of deficient thyroid function.6 In recent years, some authors have recommended screening all pregnant women for thyroid dysfunction, but such recommendations remain controversial.3,7,8 Routine screening is not endorsed by the American College of Obstetricians and Gynecologists.6
Symptoms overlap typical conditions of pregnancy
The difficulty here is that the characteristic signs and symptoms of hypothyroidism are very similar to physiologic conditions seen in most pregnancies. They include fatigue, constipation, cold intolerance, muscle cramps, hair loss, dry skin, brittle nails, weight gain, intellectual slowness, bradycardia, depression, insomnia, periorbital edema, myxedema, and myxedema coma.6 A side-by-side comparison of pregnancy conditions and hypothyroidism symptoms is provided in TABLE 1.
TABLE 1
Distinguishing hypothyroidism from a normal gestation can be challenging
| SYMPTOM | HYPOTHYROIDISM | PREGNANCY |
|---|---|---|
| Fatigue | • | • |
| Constipation | • | • |
| Hair loss | • | |
| Dry skin | • | |
| Brittle nails | • | |
| Weight gain | • | • |
| Fluid retention | • | • |
| Bradycardia | • | • |
| Goiter | • | |
| Carpal tunnel syndrome | • | • |
Which laboratory tests are informative?
Because screening is controversial and symptomatology does not reliably distinguish hypothyroidism from normal pregnancy, laboratory tests are the standard for diagnosis. Overt hypothyroidism is diagnosed in a symptomatic patient by elevated TSH level and low levels of FT4 and free T3 (FT3). Subclinical hypothyroidism is defined as elevated TSH with normal FT4 and FT3 in an asymptomatic patient. Level changes characteristic of normal pregnancy, overt hypothyroidism, and subclinical hypothyroidism are given in TABLE 2.6
TABLE 2
Laboratory diagnosis of hypothyroidism
| MATERNAL CONDITION | TSH | FREE T3 | FREE T4 | TOTAL T3 | TOTAL T4 |
|---|---|---|---|---|---|
| Normal pregnancy | No change | No change | ↑ | ↑ | ↑ |
| Hypothyroidism | ↑ | ↓ | ↓ | ↓ | ↓ |
| Subclinical hypothyroidism | ↑ | No change | No change | ↓ | ↓ |
| Adapted from American College of Obstetricians and Gynecologists6 | |||||
What causes hypothyroidism?
The most common cause of hypothyroidism in most of the world is iodine deficiency. In developed countries, however, where lack of iodine in the diet is not a problem, Hashimoto’s thyroiditis, also known as chronic autoimmune thyroiditis, is the most common cause. Hashimoto’s thyroiditis is characterized by the presence of antithyroid antibodies, including both thyroid antimicrosomial and antithyroglobulin antibodies. Both iodine deficiency and Hashimoto’s thyroiditis are associated with goiter.5 Other causes of hypothyroidism include radioactive iodine therapy for Graves’ disease, a condition we will discuss in Part 2 of this series in February; thyroidectomy; viral thyroiditis; pituitary tumors; Sheehan’s syndrome; and a number of medications.
Causes of hypothyroidism are summarized in TABLE 3.3
TABLE 3
Causes of hypothyroidism
| Iodine deficiency |
| Hashimoto’s thyroiditis |
| Radioactive iodine therapy |
| Thyroidectomy |
| Viral thyroiditis |
| Sheehan’s syndrome |
Medications
|
Effects vary by medication
Medications alter thyroid function in different ways. Iodine and lithium inhibit thyroid function and, along with dopamine antagonists, increase TSH levels. Conversely, thioamides, glucocorticoids, dopamine agonists, and somatostatins decrease TSH levels. Finally, ferrous sulfate, sucrafate, cholestyramine, and aluminum hydroxide antacids all inhibit gastrointestinal absorption of thyroid hormone and therefore should not be taken within 4 hours of thyroid medication.6
Maternal hypothyroidism: Effects on fetus, newborn
The impact of maternal hypothyroidism on the fetus depends on the severity of the condition.
- Uncontrolled hypothyroidism. The consequences of this condition can be dire. The possibilities include intrauterine fetal demise and stillbirth, preterm delivery, low birth weight, preeclampsia, and developmental anomalies including reduced intelligence quotient (IQ).1,2,4,6 Blazer and colleagues correlated intrauterine growth with maternal TSH and fetal FT4 and concluded that impaired intrauterine growth is related to abnormal thyroid function and might reflect an insufficient level of hormone production by hypothyroid mothers during pregnancy.9 Maternal and congenital hypothyroidism resulting from severe iodine deficiency are associated with profound neurologic impairment and mental retardation.1,3,10 If the condition is left untreated, cretinism can occur. Congenital cretinism is associated with growth failure, mental retardation, and other neuropsychologic deficits including deaf-mutism.3,4 However, if cretinism is identified and treated in the first 3 months of life, near-normal growth and intelligence can be expected.6 For this reason, all 50 states and the District of Columbia require newborn screening for congenital hypothyroidism.6
- Asymptomatic overt hypothyroidism. Several studies have evaluated neonatal outcomes in pregnancy complicated by asymptomatic overt hypothyroidism—that is, women who had previously been diagnosed with hypothyroidism, who have abnormal TSH and FT4 levels, but who do not have symptoms. Pop and colleagues have shown impaired psychomotor development at 10 months in infants born to mothers who had low T4 during the first 12 weeks of gestation.7 Haddow and colleagues correlated elevated maternal TSH levels at less than 17 weeks’ gestation with low IQ scores in the offspring at 7 to 9 years of age.8 Klein and colleagues demonstrated an inverse correlation between a woman’s TSH level during pregnancy and the IQ of her offspring.11 Kooistra and colleagues confirmed that maternal hypothyroxinemia is a risk for neurodevelopmental abnormalities that can be identified as early as 3 weeks of age.12 Studies of this relationship are summarized in TABLE 4.
- Subclinical hypothyroidism. During the past decade, researchers have focused attention on neonatal neurologic function in infants born to mothers who had subclinical disease. Mitchell and Klein evaluated the prevalence of subclinical hypothyroidism at less than 17 weeks’ gestation and subsequently compared the IQs in these children with those of controls.4 They found the mean and standard-deviation IQs of the children in the control and treated groups to be significantly higher than those of the children whose mothers were not treated. Casey and colleagues evaluated pregnancy outcomes in women who had undiagnosed subclinical hypothyroidism.10 They found that such pregnancies were more likely to be complicated by placental abruption and preterm birth, and speculated that the reduced IQ demonstrated in the Mitchell and Klein study might have been related to the effects of prematurity.
TABLE 4
Fetal and neonatal effects of asymptomatic overt hypothyroidism
| STUDY | LABORATORY FINDINGS | OUTCOMES AND RECOMMENDATIONS |
|---|---|---|
| Kooistra et al12 | ↓ FT4 | Maternal hypothyroxinemia is a risk for neurodevelopmental abnormalities as early as 3 weeks of age |
| Casey et al10 | ↑ TSH | Pregnancies with undiagnosed subclinical hypothyroidism were more likely to be complicated by placental abruption and preterm birth. The reduced IQ seen in a prior study (Mitchell and Klein4) may be related to effects of prematurity |
| Mitchell and Klein4 | ↑ TSH | The mean and standard deviation of IQs of the children of treated mothers with hypothyroidism and the control group were significantly higher than those for children of untreated hypothyroid women |
| Blazer et al9 | ↑ maternal TSH, ↑ fetal FT4 | Impaired intrauterine growth may reflect insufficient levels of hormone replacement therapy in hypothyroid mothers during pregnancy |
| Pop et al7 | ↓ FT4 | Impaired psychomotor development at 10 months of age in offspring of mothers with low T4 at ≤12 weeks |
| Haddow et al8 | ↑ TSH, ↓ FT4 | Elevated TSH levels at <17 weeks’ gestation are associated with low IQ scores at 7 to 9 years of age. Routine screening for thyroid deficiency may be warranted |
| Klein et al11 | ↑ TSH, ↓ FT4, ↓ TT4 | Inverse correlation between TSH during pregnancy and IQ of offspring |
| FT4=free thyroxine, TSH=thyroid-stimulating hormone, TT4=total thyroxine | ||
Managing hypothyroidism in pregnancy
The treatment of choice for correction of hypothyroidism is synthetic T4, or levothyroxine (Levothyroid, Levoxyl, Synthroid, and Unithroid). Initial treatment in the nonpregnant patient is 1.7 μg/kg/day or 12.5 to 25 μg/day adjusted by 25 μg/day every 2 to 4 weeks until a euthyroid state is achieved.13
Patients who were on thyroxine therapy before pregnancy should increase the dose by 30% once pregnancy is confirmed.1,5 Serum thyrotropin levels should be monitored every 4 weeks to maintain a TSH level between 1 and 2 mU/L and FT4 in upper third of normal.1 Once a euthyroid state has been achieved, thyrotropin levels should be monitored every trimester until delivery. FIGURE 2 provides an algorithm for management of hypothyroidism in pregnancy.
FIGURE 2 During pregnancy, thyroid function merits regular monitoring, fine-tuning of treatment
Postpartum thyroiditis
About 5% of all obstetrical patients develop postpartum thyroiditis. Approximately 45% of these women present with hypothyroidism, with the rest evenly divided between thyrotoxicosis (hyperthyroidism) and thyrotoxicosis followed by hypothyroidism. Unfortunately, the signs and symptoms of hypo- and hyperthyroidism are similar to the postpartum state. Many of these patients are not diagnosed. A high index of suspicion warrants thyroid function testing. Women who have a history of type 1 diabetes mellitus have a 25% chance of developing postpartum thyroid dysfunction.
The diagnosis is made by documenting abnormal levels of TSH and FT4. Postpartum hyperthyroidism may be diagnosed by the presence of antimicrosomal or thyroperoxidase antithyroid peroxidase antibodies. Goiter may be present in up to 50% of patients.
Postpartum thyroiditis has two phases
The first phase, also known as the thyrotoxic phase, occurs 1 to 4 months after delivery when transient thyrotoxicosis develops from excessive release of thyroid hormones. The most common symptoms with early postpartum thyroiditis are fatigue and palpitations. Approximately 67% of these women will return to a euthyroid state, and thioamide therapy is generally considered ineffective. Hypothyroidism can develop within 1 month of the onset of thyroiditis.
The second phase occurs between 4 and 8 months postpartum, and these women present with hypothyroidism. Thyromegaly and associated symptoms are common. Unlike the first (thyrotoxic) phase, medical treatment is recommended. Thyroxine treatment should be initiated and maintained for 6 to 12 months. Postpartum thyroiditis carries a 30% risk of recurrence.14
Postpartum thyroiditis may be associated with depression or aggravate symptoms of depression, although the data on this association are conflicting. The largest study addressing this issue concluded that there was no difference in the clinical and psychiatric signs and symptoms between postpartum thyroiditis and controls.15 Nevertheless, it would seem prudent to evaluate thyroid function in postpartum depression if other signs of thyroid dysfunction are present.
1. Idris I, Srinivasan R, Simm A, Page RC. Effects of maternal hyperthyroidism during early gestation on neonatal and obstetric outcome. Clin Endocrinol. 2006;65:133-135.
2. Girling JC. Thyroid disorders in pregnancy. Curr Obstet Gynecol. 2006;16:47-53.
3. Creasy RK, Resnik R, Iams J. Maternal–Fetal Medicine. 5th ed. Philadelphia, Pa: Saunders Elsevier; 2004:1063-1082.
4. Mitchell ML, Klein RZ. The sequelae of untreated maternal hypothyroidism. Eur J Endocrinol. 2004;151 Suppl 3:U45-U48.
5. Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241-249.
6. American College of Obstetrics and Gynecology. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 37, August 2002. (Replaces Practice Bulletin Number 32, November 2001). Thyroid disease in pregnancy. Obstet Gynecol. 2002;100:387-396.
7. Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol. 1999;50:149-155.
8. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549-555.
9. Blazer S, Moreh-Waterman Y, Miller-Lotan R, Tamir A, Hochberg Z. Maternal hypothyroidism may affect fetal growth and neonatal thyroid function. Obstet Gynecol. 2003;102:232-241.
10. Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239-245.
11. Klein RZ, Haddow JE, Faix JD, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol. 1991;35:41-46.
12. Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161-167.
13. Levothyroxine: Drug information. Lexicomp. http://www.utdol.com/utd/content/topic.do?topicKey=drug_l_z/143814&type=A&selectedTitle=2~39. Accessed December 14, 2007.
14. Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol. 2006;108:1283-1292.
15. Kent GN, Stuckey BG, Allen JR, Lambert T, Gee V. Postpartum thyroid dysfunction: clinical assessment and relationship to psychiatric affective morbidity. Clin Endocrinol. 1999;51:429-438.
The authors report no financial relationships relevant to this article.
A pregnant woman whose thyroid gland isn’t doing its job presents a serious management problem for her obstetrician. If she has overt hypothyroidism, seen in between 0.3% and 2.5% of pregnancies, active intervention is required to prevent serious damage to the fetus.1,2 Even if she has subclinical disease, seen in 2% to 3% of pregnancies, current research indicates that intervention may be indicated.
Fetal thyroxine requirements increase as early as 5 weeks of gestation, when the fetus is still dependent on maternal thyroxine. A deficiency of maternal thyroxine can have severe adverse outcomes, affecting the course of the pregnancy and the neurologic development of the fetus. To prevent such sequelae, patients who were on thyroid medication before pregnancy should increase the dosage by 30% once pregnancy is confirmed, and hypothyroidism that develops in pregnancy should be managed aggressively and meticulously.
Here, we’ll examine the published research to advise you on evidence-based approaches for diagnosis and management of this complex condition.
Maternal thyroid function
An elaborate negative-feedback loop prevails before pregnancy
In a nonpregnant woman, thyroid function is controlled by a negative-feedback loop that works like this:
- The hypothalamus releases thyroid-releasing hormone (TRH)
- TRH acts on the pituitary gland to release thyroid-stimulating hormone (TSH)
- TSH, in turn, acts on the thyroid gland to release the thyroid hormones iodothyronine (T3) and thyroxine (T4) that regulate metabolism
- TRH and TSH concentrations are inversely related to T3 and T4 concentrations. That is, the more TRH and TSH circulating in the blood stream, the less T3 and T4 will be produced by the thyroid gland3
- Almost all (approximately 99%) circulating T3 and T4 is bound to a protein called thyroxine-binding globulin (TBG). Only 1% of these hormones circulate in the free form, and only the free forms are biologically active.3
This relationship is illustrated in FIGURE 1.
FIGURE 1 Thyroid physiology and the impact of pregnancy
Pregnancy reduces free forms of T3 and T4, and increases TSH slightly
Pregnancy alters thyroid function in significant ways:
- Increases in circulating estrogen lead to the production of more TBG
- When TBG increases, more T3 and T4 are bound and fewer free forms of these hormones are available
- Because the total T3 (TT3) and total free T4 (TT4) are decreased in pregnancy, they are not good measures of thyroid function. Maternal thyroid function in pregnancy should be monitored using free T4 (FT4) and TSH levels
- Increased TBG also leads to a slight increase in TSH between the first trimester and term
- Human chorionic gonadotropin (hCG) concentrations also increase in pregnancy. Because hCG has thyrotropin-like activity, these higher levels cause a transient decrease in TSH by suppression of TSH production between approximately 8 and 14 weeks of gestation.
Fetal thyroid function
During early gestation, the fetus receives thyroid hormone from the mother.1 Maternal T4 crosses the placenta actively—the only hormone that does so.4 The fetus’s need for thyroxine starts to increase as early as 5 weeks of gestation.5
Fetal thyroid development does not begin until 10 to 12 weeks of gestation, and then continues until term. The fetus relies on maternal T4 exclusively before 12 weeks and partially thereafter for normal fetal neurologic development. It follows that maternal hypothyroidism could be detrimental to fetal development if not detected and corrected very early in gestation.
How (and whom) to screen for maternal hypothyroidism
Routine screening has been recommended for women who have infertility, menstrual disorders, or type 1 diabetes mellitus, and for pregnant women who have signs and symptoms of deficient thyroid function.6 In recent years, some authors have recommended screening all pregnant women for thyroid dysfunction, but such recommendations remain controversial.3,7,8 Routine screening is not endorsed by the American College of Obstetricians and Gynecologists.6
Symptoms overlap typical conditions of pregnancy
The difficulty here is that the characteristic signs and symptoms of hypothyroidism are very similar to physiologic conditions seen in most pregnancies. They include fatigue, constipation, cold intolerance, muscle cramps, hair loss, dry skin, brittle nails, weight gain, intellectual slowness, bradycardia, depression, insomnia, periorbital edema, myxedema, and myxedema coma.6 A side-by-side comparison of pregnancy conditions and hypothyroidism symptoms is provided in TABLE 1.
TABLE 1
Distinguishing hypothyroidism from a normal gestation can be challenging
| SYMPTOM | HYPOTHYROIDISM | PREGNANCY |
|---|---|---|
| Fatigue | • | • |
| Constipation | • | • |
| Hair loss | • | |
| Dry skin | • | |
| Brittle nails | • | |
| Weight gain | • | • |
| Fluid retention | • | • |
| Bradycardia | • | • |
| Goiter | • | |
| Carpal tunnel syndrome | • | • |
Which laboratory tests are informative?
Because screening is controversial and symptomatology does not reliably distinguish hypothyroidism from normal pregnancy, laboratory tests are the standard for diagnosis. Overt hypothyroidism is diagnosed in a symptomatic patient by elevated TSH level and low levels of FT4 and free T3 (FT3). Subclinical hypothyroidism is defined as elevated TSH with normal FT4 and FT3 in an asymptomatic patient. Level changes characteristic of normal pregnancy, overt hypothyroidism, and subclinical hypothyroidism are given in TABLE 2.6
TABLE 2
Laboratory diagnosis of hypothyroidism
| MATERNAL CONDITION | TSH | FREE T3 | FREE T4 | TOTAL T3 | TOTAL T4 |
|---|---|---|---|---|---|
| Normal pregnancy | No change | No change | ↑ | ↑ | ↑ |
| Hypothyroidism | ↑ | ↓ | ↓ | ↓ | ↓ |
| Subclinical hypothyroidism | ↑ | No change | No change | ↓ | ↓ |
| Adapted from American College of Obstetricians and Gynecologists6 | |||||
What causes hypothyroidism?
The most common cause of hypothyroidism in most of the world is iodine deficiency. In developed countries, however, where lack of iodine in the diet is not a problem, Hashimoto’s thyroiditis, also known as chronic autoimmune thyroiditis, is the most common cause. Hashimoto’s thyroiditis is characterized by the presence of antithyroid antibodies, including both thyroid antimicrosomial and antithyroglobulin antibodies. Both iodine deficiency and Hashimoto’s thyroiditis are associated with goiter.5 Other causes of hypothyroidism include radioactive iodine therapy for Graves’ disease, a condition we will discuss in Part 2 of this series in February; thyroidectomy; viral thyroiditis; pituitary tumors; Sheehan’s syndrome; and a number of medications.
Causes of hypothyroidism are summarized in TABLE 3.3
TABLE 3
Causes of hypothyroidism
| Iodine deficiency |
| Hashimoto’s thyroiditis |
| Radioactive iodine therapy |
| Thyroidectomy |
| Viral thyroiditis |
| Sheehan’s syndrome |
Medications
|
Effects vary by medication
Medications alter thyroid function in different ways. Iodine and lithium inhibit thyroid function and, along with dopamine antagonists, increase TSH levels. Conversely, thioamides, glucocorticoids, dopamine agonists, and somatostatins decrease TSH levels. Finally, ferrous sulfate, sucrafate, cholestyramine, and aluminum hydroxide antacids all inhibit gastrointestinal absorption of thyroid hormone and therefore should not be taken within 4 hours of thyroid medication.6
Maternal hypothyroidism: Effects on fetus, newborn
The impact of maternal hypothyroidism on the fetus depends on the severity of the condition.
- Uncontrolled hypothyroidism. The consequences of this condition can be dire. The possibilities include intrauterine fetal demise and stillbirth, preterm delivery, low birth weight, preeclampsia, and developmental anomalies including reduced intelligence quotient (IQ).1,2,4,6 Blazer and colleagues correlated intrauterine growth with maternal TSH and fetal FT4 and concluded that impaired intrauterine growth is related to abnormal thyroid function and might reflect an insufficient level of hormone production by hypothyroid mothers during pregnancy.9 Maternal and congenital hypothyroidism resulting from severe iodine deficiency are associated with profound neurologic impairment and mental retardation.1,3,10 If the condition is left untreated, cretinism can occur. Congenital cretinism is associated with growth failure, mental retardation, and other neuropsychologic deficits including deaf-mutism.3,4 However, if cretinism is identified and treated in the first 3 months of life, near-normal growth and intelligence can be expected.6 For this reason, all 50 states and the District of Columbia require newborn screening for congenital hypothyroidism.6
- Asymptomatic overt hypothyroidism. Several studies have evaluated neonatal outcomes in pregnancy complicated by asymptomatic overt hypothyroidism—that is, women who had previously been diagnosed with hypothyroidism, who have abnormal TSH and FT4 levels, but who do not have symptoms. Pop and colleagues have shown impaired psychomotor development at 10 months in infants born to mothers who had low T4 during the first 12 weeks of gestation.7 Haddow and colleagues correlated elevated maternal TSH levels at less than 17 weeks’ gestation with low IQ scores in the offspring at 7 to 9 years of age.8 Klein and colleagues demonstrated an inverse correlation between a woman’s TSH level during pregnancy and the IQ of her offspring.11 Kooistra and colleagues confirmed that maternal hypothyroxinemia is a risk for neurodevelopmental abnormalities that can be identified as early as 3 weeks of age.12 Studies of this relationship are summarized in TABLE 4.
- Subclinical hypothyroidism. During the past decade, researchers have focused attention on neonatal neurologic function in infants born to mothers who had subclinical disease. Mitchell and Klein evaluated the prevalence of subclinical hypothyroidism at less than 17 weeks’ gestation and subsequently compared the IQs in these children with those of controls.4 They found the mean and standard-deviation IQs of the children in the control and treated groups to be significantly higher than those of the children whose mothers were not treated. Casey and colleagues evaluated pregnancy outcomes in women who had undiagnosed subclinical hypothyroidism.10 They found that such pregnancies were more likely to be complicated by placental abruption and preterm birth, and speculated that the reduced IQ demonstrated in the Mitchell and Klein study might have been related to the effects of prematurity.
TABLE 4
Fetal and neonatal effects of asymptomatic overt hypothyroidism
| STUDY | LABORATORY FINDINGS | OUTCOMES AND RECOMMENDATIONS |
|---|---|---|
| Kooistra et al12 | ↓ FT4 | Maternal hypothyroxinemia is a risk for neurodevelopmental abnormalities as early as 3 weeks of age |
| Casey et al10 | ↑ TSH | Pregnancies with undiagnosed subclinical hypothyroidism were more likely to be complicated by placental abruption and preterm birth. The reduced IQ seen in a prior study (Mitchell and Klein4) may be related to effects of prematurity |
| Mitchell and Klein4 | ↑ TSH | The mean and standard deviation of IQs of the children of treated mothers with hypothyroidism and the control group were significantly higher than those for children of untreated hypothyroid women |
| Blazer et al9 | ↑ maternal TSH, ↑ fetal FT4 | Impaired intrauterine growth may reflect insufficient levels of hormone replacement therapy in hypothyroid mothers during pregnancy |
| Pop et al7 | ↓ FT4 | Impaired psychomotor development at 10 months of age in offspring of mothers with low T4 at ≤12 weeks |
| Haddow et al8 | ↑ TSH, ↓ FT4 | Elevated TSH levels at <17 weeks’ gestation are associated with low IQ scores at 7 to 9 years of age. Routine screening for thyroid deficiency may be warranted |
| Klein et al11 | ↑ TSH, ↓ FT4, ↓ TT4 | Inverse correlation between TSH during pregnancy and IQ of offspring |
| FT4=free thyroxine, TSH=thyroid-stimulating hormone, TT4=total thyroxine | ||
Managing hypothyroidism in pregnancy
The treatment of choice for correction of hypothyroidism is synthetic T4, or levothyroxine (Levothyroid, Levoxyl, Synthroid, and Unithroid). Initial treatment in the nonpregnant patient is 1.7 μg/kg/day or 12.5 to 25 μg/day adjusted by 25 μg/day every 2 to 4 weeks until a euthyroid state is achieved.13
Patients who were on thyroxine therapy before pregnancy should increase the dose by 30% once pregnancy is confirmed.1,5 Serum thyrotropin levels should be monitored every 4 weeks to maintain a TSH level between 1 and 2 mU/L and FT4 in upper third of normal.1 Once a euthyroid state has been achieved, thyrotropin levels should be monitored every trimester until delivery. FIGURE 2 provides an algorithm for management of hypothyroidism in pregnancy.
FIGURE 2 During pregnancy, thyroid function merits regular monitoring, fine-tuning of treatment
Postpartum thyroiditis
About 5% of all obstetrical patients develop postpartum thyroiditis. Approximately 45% of these women present with hypothyroidism, with the rest evenly divided between thyrotoxicosis (hyperthyroidism) and thyrotoxicosis followed by hypothyroidism. Unfortunately, the signs and symptoms of hypo- and hyperthyroidism are similar to the postpartum state. Many of these patients are not diagnosed. A high index of suspicion warrants thyroid function testing. Women who have a history of type 1 diabetes mellitus have a 25% chance of developing postpartum thyroid dysfunction.
The diagnosis is made by documenting abnormal levels of TSH and FT4. Postpartum hyperthyroidism may be diagnosed by the presence of antimicrosomal or thyroperoxidase antithyroid peroxidase antibodies. Goiter may be present in up to 50% of patients.
Postpartum thyroiditis has two phases
The first phase, also known as the thyrotoxic phase, occurs 1 to 4 months after delivery when transient thyrotoxicosis develops from excessive release of thyroid hormones. The most common symptoms with early postpartum thyroiditis are fatigue and palpitations. Approximately 67% of these women will return to a euthyroid state, and thioamide therapy is generally considered ineffective. Hypothyroidism can develop within 1 month of the onset of thyroiditis.
The second phase occurs between 4 and 8 months postpartum, and these women present with hypothyroidism. Thyromegaly and associated symptoms are common. Unlike the first (thyrotoxic) phase, medical treatment is recommended. Thyroxine treatment should be initiated and maintained for 6 to 12 months. Postpartum thyroiditis carries a 30% risk of recurrence.14
Postpartum thyroiditis may be associated with depression or aggravate symptoms of depression, although the data on this association are conflicting. The largest study addressing this issue concluded that there was no difference in the clinical and psychiatric signs and symptoms between postpartum thyroiditis and controls.15 Nevertheless, it would seem prudent to evaluate thyroid function in postpartum depression if other signs of thyroid dysfunction are present.
The authors report no financial relationships relevant to this article.
A pregnant woman whose thyroid gland isn’t doing its job presents a serious management problem for her obstetrician. If she has overt hypothyroidism, seen in between 0.3% and 2.5% of pregnancies, active intervention is required to prevent serious damage to the fetus.1,2 Even if she has subclinical disease, seen in 2% to 3% of pregnancies, current research indicates that intervention may be indicated.
Fetal thyroxine requirements increase as early as 5 weeks of gestation, when the fetus is still dependent on maternal thyroxine. A deficiency of maternal thyroxine can have severe adverse outcomes, affecting the course of the pregnancy and the neurologic development of the fetus. To prevent such sequelae, patients who were on thyroid medication before pregnancy should increase the dosage by 30% once pregnancy is confirmed, and hypothyroidism that develops in pregnancy should be managed aggressively and meticulously.
Here, we’ll examine the published research to advise you on evidence-based approaches for diagnosis and management of this complex condition.
Maternal thyroid function
An elaborate negative-feedback loop prevails before pregnancy
In a nonpregnant woman, thyroid function is controlled by a negative-feedback loop that works like this:
- The hypothalamus releases thyroid-releasing hormone (TRH)
- TRH acts on the pituitary gland to release thyroid-stimulating hormone (TSH)
- TSH, in turn, acts on the thyroid gland to release the thyroid hormones iodothyronine (T3) and thyroxine (T4) that regulate metabolism
- TRH and TSH concentrations are inversely related to T3 and T4 concentrations. That is, the more TRH and TSH circulating in the blood stream, the less T3 and T4 will be produced by the thyroid gland3
- Almost all (approximately 99%) circulating T3 and T4 is bound to a protein called thyroxine-binding globulin (TBG). Only 1% of these hormones circulate in the free form, and only the free forms are biologically active.3
This relationship is illustrated in FIGURE 1.
FIGURE 1 Thyroid physiology and the impact of pregnancy
Pregnancy reduces free forms of T3 and T4, and increases TSH slightly
Pregnancy alters thyroid function in significant ways:
- Increases in circulating estrogen lead to the production of more TBG
- When TBG increases, more T3 and T4 are bound and fewer free forms of these hormones are available
- Because the total T3 (TT3) and total free T4 (TT4) are decreased in pregnancy, they are not good measures of thyroid function. Maternal thyroid function in pregnancy should be monitored using free T4 (FT4) and TSH levels
- Increased TBG also leads to a slight increase in TSH between the first trimester and term
- Human chorionic gonadotropin (hCG) concentrations also increase in pregnancy. Because hCG has thyrotropin-like activity, these higher levels cause a transient decrease in TSH by suppression of TSH production between approximately 8 and 14 weeks of gestation.
Fetal thyroid function
During early gestation, the fetus receives thyroid hormone from the mother.1 Maternal T4 crosses the placenta actively—the only hormone that does so.4 The fetus’s need for thyroxine starts to increase as early as 5 weeks of gestation.5
Fetal thyroid development does not begin until 10 to 12 weeks of gestation, and then continues until term. The fetus relies on maternal T4 exclusively before 12 weeks and partially thereafter for normal fetal neurologic development. It follows that maternal hypothyroidism could be detrimental to fetal development if not detected and corrected very early in gestation.
How (and whom) to screen for maternal hypothyroidism
Routine screening has been recommended for women who have infertility, menstrual disorders, or type 1 diabetes mellitus, and for pregnant women who have signs and symptoms of deficient thyroid function.6 In recent years, some authors have recommended screening all pregnant women for thyroid dysfunction, but such recommendations remain controversial.3,7,8 Routine screening is not endorsed by the American College of Obstetricians and Gynecologists.6
Symptoms overlap typical conditions of pregnancy
The difficulty here is that the characteristic signs and symptoms of hypothyroidism are very similar to physiologic conditions seen in most pregnancies. They include fatigue, constipation, cold intolerance, muscle cramps, hair loss, dry skin, brittle nails, weight gain, intellectual slowness, bradycardia, depression, insomnia, periorbital edema, myxedema, and myxedema coma.6 A side-by-side comparison of pregnancy conditions and hypothyroidism symptoms is provided in TABLE 1.
TABLE 1
Distinguishing hypothyroidism from a normal gestation can be challenging
| SYMPTOM | HYPOTHYROIDISM | PREGNANCY |
|---|---|---|
| Fatigue | • | • |
| Constipation | • | • |
| Hair loss | • | |
| Dry skin | • | |
| Brittle nails | • | |
| Weight gain | • | • |
| Fluid retention | • | • |
| Bradycardia | • | • |
| Goiter | • | |
| Carpal tunnel syndrome | • | • |
Which laboratory tests are informative?
Because screening is controversial and symptomatology does not reliably distinguish hypothyroidism from normal pregnancy, laboratory tests are the standard for diagnosis. Overt hypothyroidism is diagnosed in a symptomatic patient by elevated TSH level and low levels of FT4 and free T3 (FT3). Subclinical hypothyroidism is defined as elevated TSH with normal FT4 and FT3 in an asymptomatic patient. Level changes characteristic of normal pregnancy, overt hypothyroidism, and subclinical hypothyroidism are given in TABLE 2.6
TABLE 2
Laboratory diagnosis of hypothyroidism
| MATERNAL CONDITION | TSH | FREE T3 | FREE T4 | TOTAL T3 | TOTAL T4 |
|---|---|---|---|---|---|
| Normal pregnancy | No change | No change | ↑ | ↑ | ↑ |
| Hypothyroidism | ↑ | ↓ | ↓ | ↓ | ↓ |
| Subclinical hypothyroidism | ↑ | No change | No change | ↓ | ↓ |
| Adapted from American College of Obstetricians and Gynecologists6 | |||||
What causes hypothyroidism?
The most common cause of hypothyroidism in most of the world is iodine deficiency. In developed countries, however, where lack of iodine in the diet is not a problem, Hashimoto’s thyroiditis, also known as chronic autoimmune thyroiditis, is the most common cause. Hashimoto’s thyroiditis is characterized by the presence of antithyroid antibodies, including both thyroid antimicrosomial and antithyroglobulin antibodies. Both iodine deficiency and Hashimoto’s thyroiditis are associated with goiter.5 Other causes of hypothyroidism include radioactive iodine therapy for Graves’ disease, a condition we will discuss in Part 2 of this series in February; thyroidectomy; viral thyroiditis; pituitary tumors; Sheehan’s syndrome; and a number of medications.
Causes of hypothyroidism are summarized in TABLE 3.3
TABLE 3
Causes of hypothyroidism
| Iodine deficiency |
| Hashimoto’s thyroiditis |
| Radioactive iodine therapy |
| Thyroidectomy |
| Viral thyroiditis |
| Sheehan’s syndrome |
Medications
|
Effects vary by medication
Medications alter thyroid function in different ways. Iodine and lithium inhibit thyroid function and, along with dopamine antagonists, increase TSH levels. Conversely, thioamides, glucocorticoids, dopamine agonists, and somatostatins decrease TSH levels. Finally, ferrous sulfate, sucrafate, cholestyramine, and aluminum hydroxide antacids all inhibit gastrointestinal absorption of thyroid hormone and therefore should not be taken within 4 hours of thyroid medication.6
Maternal hypothyroidism: Effects on fetus, newborn
The impact of maternal hypothyroidism on the fetus depends on the severity of the condition.
- Uncontrolled hypothyroidism. The consequences of this condition can be dire. The possibilities include intrauterine fetal demise and stillbirth, preterm delivery, low birth weight, preeclampsia, and developmental anomalies including reduced intelligence quotient (IQ).1,2,4,6 Blazer and colleagues correlated intrauterine growth with maternal TSH and fetal FT4 and concluded that impaired intrauterine growth is related to abnormal thyroid function and might reflect an insufficient level of hormone production by hypothyroid mothers during pregnancy.9 Maternal and congenital hypothyroidism resulting from severe iodine deficiency are associated with profound neurologic impairment and mental retardation.1,3,10 If the condition is left untreated, cretinism can occur. Congenital cretinism is associated with growth failure, mental retardation, and other neuropsychologic deficits including deaf-mutism.3,4 However, if cretinism is identified and treated in the first 3 months of life, near-normal growth and intelligence can be expected.6 For this reason, all 50 states and the District of Columbia require newborn screening for congenital hypothyroidism.6
- Asymptomatic overt hypothyroidism. Several studies have evaluated neonatal outcomes in pregnancy complicated by asymptomatic overt hypothyroidism—that is, women who had previously been diagnosed with hypothyroidism, who have abnormal TSH and FT4 levels, but who do not have symptoms. Pop and colleagues have shown impaired psychomotor development at 10 months in infants born to mothers who had low T4 during the first 12 weeks of gestation.7 Haddow and colleagues correlated elevated maternal TSH levels at less than 17 weeks’ gestation with low IQ scores in the offspring at 7 to 9 years of age.8 Klein and colleagues demonstrated an inverse correlation between a woman’s TSH level during pregnancy and the IQ of her offspring.11 Kooistra and colleagues confirmed that maternal hypothyroxinemia is a risk for neurodevelopmental abnormalities that can be identified as early as 3 weeks of age.12 Studies of this relationship are summarized in TABLE 4.
- Subclinical hypothyroidism. During the past decade, researchers have focused attention on neonatal neurologic function in infants born to mothers who had subclinical disease. Mitchell and Klein evaluated the prevalence of subclinical hypothyroidism at less than 17 weeks’ gestation and subsequently compared the IQs in these children with those of controls.4 They found the mean and standard-deviation IQs of the children in the control and treated groups to be significantly higher than those of the children whose mothers were not treated. Casey and colleagues evaluated pregnancy outcomes in women who had undiagnosed subclinical hypothyroidism.10 They found that such pregnancies were more likely to be complicated by placental abruption and preterm birth, and speculated that the reduced IQ demonstrated in the Mitchell and Klein study might have been related to the effects of prematurity.
TABLE 4
Fetal and neonatal effects of asymptomatic overt hypothyroidism
| STUDY | LABORATORY FINDINGS | OUTCOMES AND RECOMMENDATIONS |
|---|---|---|
| Kooistra et al12 | ↓ FT4 | Maternal hypothyroxinemia is a risk for neurodevelopmental abnormalities as early as 3 weeks of age |
| Casey et al10 | ↑ TSH | Pregnancies with undiagnosed subclinical hypothyroidism were more likely to be complicated by placental abruption and preterm birth. The reduced IQ seen in a prior study (Mitchell and Klein4) may be related to effects of prematurity |
| Mitchell and Klein4 | ↑ TSH | The mean and standard deviation of IQs of the children of treated mothers with hypothyroidism and the control group were significantly higher than those for children of untreated hypothyroid women |
| Blazer et al9 | ↑ maternal TSH, ↑ fetal FT4 | Impaired intrauterine growth may reflect insufficient levels of hormone replacement therapy in hypothyroid mothers during pregnancy |
| Pop et al7 | ↓ FT4 | Impaired psychomotor development at 10 months of age in offspring of mothers with low T4 at ≤12 weeks |
| Haddow et al8 | ↑ TSH, ↓ FT4 | Elevated TSH levels at <17 weeks’ gestation are associated with low IQ scores at 7 to 9 years of age. Routine screening for thyroid deficiency may be warranted |
| Klein et al11 | ↑ TSH, ↓ FT4, ↓ TT4 | Inverse correlation between TSH during pregnancy and IQ of offspring |
| FT4=free thyroxine, TSH=thyroid-stimulating hormone, TT4=total thyroxine | ||
Managing hypothyroidism in pregnancy
The treatment of choice for correction of hypothyroidism is synthetic T4, or levothyroxine (Levothyroid, Levoxyl, Synthroid, and Unithroid). Initial treatment in the nonpregnant patient is 1.7 μg/kg/day or 12.5 to 25 μg/day adjusted by 25 μg/day every 2 to 4 weeks until a euthyroid state is achieved.13
Patients who were on thyroxine therapy before pregnancy should increase the dose by 30% once pregnancy is confirmed.1,5 Serum thyrotropin levels should be monitored every 4 weeks to maintain a TSH level between 1 and 2 mU/L and FT4 in upper third of normal.1 Once a euthyroid state has been achieved, thyrotropin levels should be monitored every trimester until delivery. FIGURE 2 provides an algorithm for management of hypothyroidism in pregnancy.
FIGURE 2 During pregnancy, thyroid function merits regular monitoring, fine-tuning of treatment
Postpartum thyroiditis
About 5% of all obstetrical patients develop postpartum thyroiditis. Approximately 45% of these women present with hypothyroidism, with the rest evenly divided between thyrotoxicosis (hyperthyroidism) and thyrotoxicosis followed by hypothyroidism. Unfortunately, the signs and symptoms of hypo- and hyperthyroidism are similar to the postpartum state. Many of these patients are not diagnosed. A high index of suspicion warrants thyroid function testing. Women who have a history of type 1 diabetes mellitus have a 25% chance of developing postpartum thyroid dysfunction.
The diagnosis is made by documenting abnormal levels of TSH and FT4. Postpartum hyperthyroidism may be diagnosed by the presence of antimicrosomal or thyroperoxidase antithyroid peroxidase antibodies. Goiter may be present in up to 50% of patients.
Postpartum thyroiditis has two phases
The first phase, also known as the thyrotoxic phase, occurs 1 to 4 months after delivery when transient thyrotoxicosis develops from excessive release of thyroid hormones. The most common symptoms with early postpartum thyroiditis are fatigue and palpitations. Approximately 67% of these women will return to a euthyroid state, and thioamide therapy is generally considered ineffective. Hypothyroidism can develop within 1 month of the onset of thyroiditis.
The second phase occurs between 4 and 8 months postpartum, and these women present with hypothyroidism. Thyromegaly and associated symptoms are common. Unlike the first (thyrotoxic) phase, medical treatment is recommended. Thyroxine treatment should be initiated and maintained for 6 to 12 months. Postpartum thyroiditis carries a 30% risk of recurrence.14
Postpartum thyroiditis may be associated with depression or aggravate symptoms of depression, although the data on this association are conflicting. The largest study addressing this issue concluded that there was no difference in the clinical and psychiatric signs and symptoms between postpartum thyroiditis and controls.15 Nevertheless, it would seem prudent to evaluate thyroid function in postpartum depression if other signs of thyroid dysfunction are present.
1. Idris I, Srinivasan R, Simm A, Page RC. Effects of maternal hyperthyroidism during early gestation on neonatal and obstetric outcome. Clin Endocrinol. 2006;65:133-135.
2. Girling JC. Thyroid disorders in pregnancy. Curr Obstet Gynecol. 2006;16:47-53.
3. Creasy RK, Resnik R, Iams J. Maternal–Fetal Medicine. 5th ed. Philadelphia, Pa: Saunders Elsevier; 2004:1063-1082.
4. Mitchell ML, Klein RZ. The sequelae of untreated maternal hypothyroidism. Eur J Endocrinol. 2004;151 Suppl 3:U45-U48.
5. Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241-249.
6. American College of Obstetrics and Gynecology. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 37, August 2002. (Replaces Practice Bulletin Number 32, November 2001). Thyroid disease in pregnancy. Obstet Gynecol. 2002;100:387-396.
7. Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol. 1999;50:149-155.
8. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549-555.
9. Blazer S, Moreh-Waterman Y, Miller-Lotan R, Tamir A, Hochberg Z. Maternal hypothyroidism may affect fetal growth and neonatal thyroid function. Obstet Gynecol. 2003;102:232-241.
10. Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239-245.
11. Klein RZ, Haddow JE, Faix JD, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol. 1991;35:41-46.
12. Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161-167.
13. Levothyroxine: Drug information. Lexicomp. http://www.utdol.com/utd/content/topic.do?topicKey=drug_l_z/143814&type=A&selectedTitle=2~39. Accessed December 14, 2007.
14. Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol. 2006;108:1283-1292.
15. Kent GN, Stuckey BG, Allen JR, Lambert T, Gee V. Postpartum thyroid dysfunction: clinical assessment and relationship to psychiatric affective morbidity. Clin Endocrinol. 1999;51:429-438.
1. Idris I, Srinivasan R, Simm A, Page RC. Effects of maternal hyperthyroidism during early gestation on neonatal and obstetric outcome. Clin Endocrinol. 2006;65:133-135.
2. Girling JC. Thyroid disorders in pregnancy. Curr Obstet Gynecol. 2006;16:47-53.
3. Creasy RK, Resnik R, Iams J. Maternal–Fetal Medicine. 5th ed. Philadelphia, Pa: Saunders Elsevier; 2004:1063-1082.
4. Mitchell ML, Klein RZ. The sequelae of untreated maternal hypothyroidism. Eur J Endocrinol. 2004;151 Suppl 3:U45-U48.
5. Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241-249.
6. American College of Obstetrics and Gynecology. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 37, August 2002. (Replaces Practice Bulletin Number 32, November 2001). Thyroid disease in pregnancy. Obstet Gynecol. 2002;100:387-396.
7. Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol. 1999;50:149-155.
8. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549-555.
9. Blazer S, Moreh-Waterman Y, Miller-Lotan R, Tamir A, Hochberg Z. Maternal hypothyroidism may affect fetal growth and neonatal thyroid function. Obstet Gynecol. 2003;102:232-241.
10. Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239-245.
11. Klein RZ, Haddow JE, Faix JD, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol. 1991;35:41-46.
12. Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161-167.
13. Levothyroxine: Drug information. Lexicomp. http://www.utdol.com/utd/content/topic.do?topicKey=drug_l_z/143814&type=A&selectedTitle=2~39. Accessed December 14, 2007.
14. Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol. 2006;108:1283-1292.
15. Kent GN, Stuckey BG, Allen JR, Lambert T, Gee V. Postpartum thyroid dysfunction: clinical assessment and relationship to psychiatric affective morbidity. Clin Endocrinol. 1999;51:429-438.
Thrombophilia in pregnancy: Whom to screen, when to treat
Why thrombophilia matters
During pregnancy, clotting factors I, VII, VIII, IX, and X rise; protein S and fibrinolytic activity diminish; and resistance to activated protein C develops.1,2 When compounded by thrombophilia—a broad spectrum of coagulation disorders that increase the risk for venous and arterial thrombosis—the hypercoagulable state of pregnancy may increase the risk of thromboembolism during pregnancy or postpartum.3
Pulmonary embolism is the leading cause of maternal death in the United States.1 Concern about this lethal sequela has led to numerous recommendations for screening and subsequent prophylaxis and therapy.
Two types
Thrombophilias are inherited or acquired (TABLE 1). The most common inherited disorders during pregnancy are mutations in factor V Leiden, prothrombin gene, and methylenetetrahydrofolate reductase (MTHFR) (TABLE 2). Caucasians have a higher rate of genetic thrombophilias than other racial groups.
Antiphospholipid antibody (APA) syndrome is the most common acquired thrombophilia of pregnancy. It can be diagnosed when the immunoglobulin G or immunoglobulin M level is 20 g per liter or higher, when lupus anticoagulant is present, or both.4
TABLE 1
Thrombophilias are inherited or acquired
INHERITED
|
ACQUIRED
|
| MTHFR=methylenetetrahydrofolate reductase |
Prevalence of thrombophilias in women with normal pregnancy outcomes
| THROMBOPHILIA | PREVALENCE (%) |
|---|---|
| Factor V Leiden mutation | 2–10 |
| MTHFR mutation | 8–16 |
| Prothrombin gene mutation | 2–6 |
| Protein C and S deficiencies | 0.2–1.0* |
| Anticardiolipin antibodies | 1–7 |
| * Combined rate | |
| MTHFR=methylenetetrahydrofolate reductase | |
Link to adverse pregnancy outcomes
During the past 2 decades, several epidemiologic and case-control studies have explored the association between thrombophilias and adverse pregnancy outcomes,2-6 which include the following maternal effects:
- Venous thromboembolism, including deep vein thrombosis, pulmonary embolism, and cerebral vein thrombosis
- Arterial thrombosis (peripheral, cerebral)
- Severe preeclampsia
- Thrombosis and infarcts
- Abruptio placenta
- Recurrent miscarriage
- Fetal growth restriction
- Death
- Stroke
Preeclampsia and thrombophilia
The association between preeclampsia and thrombophilia remains somewhat unclear because of inconsistent data. Because of this, we do not recommend routine screening for thrombophilia in women with preeclampsia.
An association between inherited thrombophilias and preeclampsia was reported by Dekker et al in 1995.7 Since then, numerous retrospective and case-controlled studies have assessed the incidence of thrombophilia in women with severe preeclampsia.7-25 Their findings range from:
- Factor V Leiden: 3.7% to 26.5%
- Prothrombin gene mutation: 0 to 10.8%
- Protein S deficiency: 0.7% to 24.7%
- MTHFR variant: 6.7% to 24.0%
Other points of contention are the varying levels of severity of preeclampsia and of gestational age at delivery, as well as racial differences. For example, most studies found an association between thrombophilia and severe preeclampsia at less than 34 weeks’ gestation, but not between thrombophilia and mild preeclampsia at term. In addition, a recent prospective observational study at multiple centers involving 5,168 women found a factor V Leiden mutation rate of 6% among white women, 2.3% among Asians, 1.6% in Hispanics, and 0.8% in African Americans.8 This large study found no association between thrombophilia and preeclampsia in these women. Therefore, based on available data, we do not recommend routine screening for factor V Leiden in women with severe preeclampsia.
Preeclampsia and APA syndrome
In 1989, Branch et al26 first reported an association between APA syndrome and severe preeclampsia at less than 34 weeks’ gestation. They recommended that women with severe preeclampsia at this gestational age be screened for APA syndrome and treated when the screen is positive. Several later studies supported or refuted the association between APA syndrome and preeclampsia,26,27 and a recent report concluded that routine testing for APA syndrome in women with early-onset preeclampsia is unwarranted.26 Therefore, we do not recommend routine screening for APA in women with severe preeclampsia.
No need to screen women with abruptio placenta
The placental circulation is comparable to venous circulation, with low pressure and low flow velocity rendering it susceptible to thrombotic complications at the maternal–placental interface and consequent premature separation of the placenta.
It is difficult to confirm an association between thrombophilia and abruptio placenta because of confounding variables such as chronic hypertension, cigarette and cocaine use, and advanced maternal age.3 Studies reviewing this association are scarce, and screening for thrombophilia is discouraged in pregnancies marked by abruptio placenta.
Kupferminc et al28 found that 25%, 20%, and 15% of thrombophilia patients with placental abruption had mutations in factor V Leiden, prothrombin gene, and MTHFR, respectively. In contrast, Prochazka et al29 found 15.7% of their cohort of patients with abruptio placenta to have factor V Leiden mutation.
A large prospective, observational study of more than 5,000 asymptomatic pregnant women at multiple centers found no association between abruptio placenta and factor V Leiden mutation.8 Nor were there cases of abruptio placenta among 134 women who were heterozygous for factor V Leiden.
And no routine screening in cases of IUGR
Routine screening for thrombophilias in women with intrauterine growth restriction (IUGR) is not recommended. One reason: The prevalence of thrombophilias in these women ranges widely, depending on the study cited: from 2.8% to 35% for factor V Leiden and 2.8% to 15.4% for prothrombin gene mutation (TABLE 3). In addition, in contrast to earlier studies, a large case-control trial by Infante-Rivard et al30 found no increased risk of IUGR in women with thrombophilias, except for a subgroup of women with the MTHFR variant who did not take a prenatal multivitamin.
A recent meta-analysis of case-control studies by Howley et al31 found a significant association between factor V Leiden, the prothrombin gene variant, and IUGR, but the investigators cautioned that this strong association may be driven by small, poor-quality studies that yield extreme associations. A multicenter observational study by Dizon-Townson et al8 found no association between thrombophilia and IUGR in asymptomatic gravidas.
TABLE 3
Incidence of thrombophilias in women with intrauterine growth restriction
| STUDY | FACTOR V LEIDEN (%) | PROTHROMBIN GENE MUTATION (%) | ||
|---|---|---|---|---|
| IUGR | CONTROLS | IUGR | CONTROLS | |
| Kupferminc et al50 | 5/44 (11.4) | 7/110 (6.4) | 5/44 (11.4) | 3/110 (2.7) |
| Infante-Rivard et al30 | 22/488 (4.5) | 18/470 (3.8) | 12/488 (2.5) | 11/470 (2.3) |
| Verspyck et al51 | 4/97 (4.1) | 1/97 (1) | 3/97 (3.1) | 1/97 (1) |
| McCowan et al52 | 4/145 (2.8) | 11/290 (3.8) | 4/145 (2.8) | 9/290 (3.1) |
| Dizon-Townson et al*10 | 6/134 (4.5) | 233/4,753 (4.9) | NR | NR |
| Kupferminc**34 | 9/26 (35) | 2/52 (3.8) | 4/26 (15.4) | 2/52 (3.8) |
| * | ||||
| ** Mid-trimester severe intrauterine growth restriction | ||||
| IUGR=intrauterine growth restriction, NR=not recorded | ||||
| SOURCE: Adapted from Clin Obstet Gynecol. 2006;49:850–860 | ||||
Fetal loss is a complication of thrombophilia
One in 10 pregnancies ends in early death of the fetus (before 20 weeks), and 1 in 200 gestations ends in late fetal loss.32 When fetal loss occurs in the second and third trimesters, it is due to excessive thrombosis of the placental vessels, placental infarction, and secondary uteroplacental insufficiency.2,33 Women who are carriers of factor V or prothrombin gene mutations are at higher risk of late fetal loss than noncarriers are (TABLE 4).
Fetal loss is a well-established complication in women with thrombophilia, but not all thrombophilias are associated with fetal loss, according to a meta-analysis of 31 studies.33 In women with thrombophilia, first-trimester loss is generally associated with factor V Leiden, prothrombin gene mutation, and activated protein C resistance. Late, nonrecurrent fetal loss is associated with factor V Leiden, prothrombin gene mutation, and protein S deficiency.33
TABLE 4
Incidence of factor V Leiden mutation in women with recurrent pregnancy loss
| STUDY | PATIENT SELECTION | PATIENTS (%) | CONTROLS (%) | ODDS RATIO | 95% CONFIDENCE INTERVAL |
|---|---|---|---|---|---|
| Grandone et al53 | ≥2 unexplained fetal losses, other causes excluded | 7/43 (16.3) | 5/118 (4.2) | 4.4 | 1.3–14.7 |
| Ridker et al54 | Recurrent, spontaneous abortion, other causes not excluded | 9/113 (8) | 16/437 (3.7) | 2.3 | 1.0–5.2 |
| Sarig et al55 | ≥3 first- or second-trimester losses or ≥1 intrauterine fetal demise, other causes excluded* | 96/145 (66) | 41/145 (28) | 5.0 | 3.0–8.5 |
| * Excluded chromosomal abnormalities, infections, anatomic alterations, and endocrine dysfunction | |||||
History of adverse outcomes? Offer screening
It is well established that women with a history of fetal death, severe preeclampsia, IUGR, abruptio placenta, or recurrent miscarriage have an increased risk of recurrence in subsequent pregnancies.3,30,34-36 The rate of recurrence of any of these outcomes may be as high as 46% with a history of 2 or more adverse outcomes, even before any thrombophilia is taken into account.3 Although there are few studies describing the rate of recurrence of adverse pregnancy outcomes in women with thrombophilia and a previous adverse outcome (TABLE 5), it appears to range from 66% to 83% in untreated women.3,37
Based on these findings, some authors recommend screening for thrombophilia in women who have had adverse pregnancy outcomes3,9,38 and prophylactic therapy in subsequent pregnancies when the test is positive. Therapy includes low-dose aspirin with or without subcutaneous heparin, as well as folic acid and vitamin B6 supplements, according to the type of thrombophilia present as well as the nature of the previous adverse outcome.
TABLE 5
How women with a previous adverse outcome fare on anticoagulation therapy
| STUDY | PATIENTS | PREVIOUS ADVERSE PREGNANCY OUTCOME | ANTICOAGULANT | OUTCOME IN CURRENT PREGNANCY |
|---|---|---|---|---|
| Riyazi et al9 | 26 | Uteroplacental insufficiency | LMWH and low-dose aspirin | Decreased recurrence of preeclampsia (85% to 38%) and IUGR (54% to 15%) |
| Brenner37 | 50 | ≥3 first-trimester recurrent pregnancy losses with thrombophilia | LMWH | Higher live birth rate compared with historical controls (75% vs 20%) |
| Ogueh et al48 | 24 | Previous adverse pregnancy outcome plus history of thromboembolic disease, family history of thrombophilia | UFH | No significant mprovement |
| Kupferminc et al38 | 33 | Thrombophilia with history of preeclampsia or IUGR | LMWH and low-dose aspirin | With treatment, 3% recurrence of preeclampsia |
| Grandone et al53 | 25 | Repeated pregnancy loss, gestational hypertension, HELLP, or IUGR | UFH or LMWH | 90.3% treated with LMWH had good obstetric outcome |
| Paidas et al3 | 158 | Fetal loss, IUGR, placental abruption, or preeclampsia | UFH or LMWH | 80% reduction in risk of adverse pregnancy outcome, compared with historical controls (OR, 0.21; 95% CI, 0.11–0.39) |
| HELLP=hemolysis, elevated liver enzymes, and low platelets; IUGR=intrauterine growth restriction; LMWH=low-molecular-weight heparin; UFH=unfractionated heparin | ||||
| SOURCE: Adapted from Am J Perinatol. 2006;23:499–506 | ||||
No randomized trials on prophylaxis
We lack randomized trials evaluating thromboprophylaxis for prevention of recurrent adverse pregnancy outcomes in women with previous severe preeclampsia, IUGR, or abruptio placenta in association with genetic thrombophilia. Therefore, any recommendation to treat such women with low-molecular-weight heparin with or without low-dose aspirin in subsequent pregnancies should remain empiric and/or prescribed after appropriate counseling of the patients regarding risks and benefits.
TABLE 6 summarizes the risk of thromboembolism in women with thrombophilia—both for asymptomatic patients and for those with a history of thromboembolism. These percentages should be used when counseling women about their risk and determining management and therapy.
TABLE 6
Risk of thromboembolism during pregnancy and postpartum in women with thrombophilia
| THROMBOPHILIA | RISK (%) | |
|---|---|---|
| ASYMPTOMATIC WOMEN | HISTORY OF VENOUS THROMBOEMBOLISM | |
| Factor V Leiden | ||
| Heterozygous | 0.2 | 10 |
| Homozygous | 1–2 | 15–20 |
| Prothrombin gene mutation | ||
| Heterozygous | 0.5 | 10 |
| Homozygous | 2.3 | 20 |
| Factor V Leiden and prothrombin gene mutation | 5 | 20 |
| Antithrombin deficiency | 7 | 40 |
| Protein C deficiency | 0.5 | 5–15 |
| Protein S deficiency | 0.1 | Unknown |
Prophylaxis for APA syndrome and recurrent pregnancy loss
Several randomized trials have described the use of low-dose aspirin and heparin in women with APA syndrome and a history of recurrent pregnancy loss, although the results are inconsistent (TABLE 7).39-45 The inconsistency may be due to varying definitions of APA syndrome and gestational age at the time of randomization, as well as the population studied (previous thromboembolism, presence or absence of lupus anticoagulant, level of titer of anticardiolipin antibodies, presence or absence of previous stillbirth). Nevertheless, we recommend that women with true APA syndrome (presence of lupus anticoagulant, high titers of immunoglobulin G, history of thromboembolism or recurrent stillbirth) receive prophylaxis with low-dose aspirin, with subcutaneous heparin added once fetal cardiac activity is documented.46
TABLE 7
Live births in women with APA and a history of fetal loss
| STUDY | TREATMENT | CONTROL | NO. OF LIVE BIRTHS (%) | |
|---|---|---|---|---|
| TREATED WOMEN | CONTROL GROUP | |||
| Cowchock et al39 | Aspirin/heparin | Aspirin/prednisone | 9/12 (75) | 6/8 (75) |
| Laskin et al40 | Aspirin/prednisone | Placebo | 25/42 (60) | 24/46 (52) |
| Kutteh41 | Aspirin/heparin | Aspirin only | 20/25 (80) | 11/25 (44) |
| Rai et al42 | Aspirin/heparin | Aspirin only | 32/45 (71) | 19/45 (42) |
| Silver et al43 | Aspirin/prednisone | Aspirin only | 12/12 (100) | 22/22 (100) |
| Pattison et al44 | Aspirin | Placebo | 16/20 (80) | 17/20 (85) |
| Farquharson et al45 | Aspirin/LMWH | Aspirin only | 40/51 (78) | 34/47 (72) |
| LMWH=low-molecular-weight heparin | ||||
Genetic thrombophilias
Few published studies describe prophylactic use of low-molecular-weight heparin with or without low-dose aspirin in women with genetic thrombophilia and a history of adverse pregnancy outcomes. All but 1 of these studies are observational, comparing outcome in the treated pregnancy with that of previously untreated gestations in the same woman.3,9,38,44,45,47 These studies included a limited number of women and a heterogeneous group of patients with various thrombophilias; they also involved different therapies (TABLE 7).3,9,38,41,48,49
Gris et al47 performed a randomized trial in 160 women with at least 1 prior fetal loss after 10 weeks’ gestation who were heterozygous for factor V Leiden or prothrombin G20210A mutation, or had protein S deficiency. Beginning at 8 weeks’ gestation, these women were assigned to treatment with 40 mg of enoxaparin (n=80) or 100 mg of low-dose aspirin (n=80) daily. All women also received 5 mg of folic acid daily.
In the women treated with enoxaparin, 69 (86%) had a live birth, compared with 23 (29%) women treated with low-dose aspirin. The women treated with enoxaparin also had significantly higher median neonatal birth weights and a lower rate of IUGR (10% versus 30%). The authors concluded that women with factor V Leiden, prothrombin gene mutation, or protein S deficiency and a history of fetal loss should receive enoxaparin prophylaxis in subsequent pregnancies.
History of severe preeclampsia, IUGR, or abruptio placenta. No randomized trials have evaluated thromboprophylaxis in women with this history who have genetic thrombophilia. For this reason, any recommendation to treat these women with low-molecular-weight heparin with or without low-dose aspirin in subsequent pregnancies remains empiric. Prophylaxis can be prescribed after an appropriate discussion of risks and benefits with the patient.
Unresolved questions keep management experimental
What is the likelihood that a woman carrying a gene mutation that predisposes her to thrombophilia will have a serious complication during pregnancy? And how safe and effective is prophylaxis?
There is a prevailing need for a double-blind placebo-controlled trial to address these questions and evaluate the benefit of heparin in pregnant women with a history of adverse pregnancy outcomes and thrombophilia. Until then, screening and treatment for thrombophilia remain experimental in these women.
The authors report no financial relationships relevant to this article.
1. Thromboembolism in pregnancy. ACOG Practice Bulletin #19. Washington, DC: ACOG; 2000.
2. Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412-424.
3. Paidas MJ, De-Hui WK, Arkel YS. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 2004;31:783-805.
4. Lee RM, Brown MA, Branch DW, Ward K, Silver RM. Anticardiolipin and anti-B2 glycoprotein-I antibodies in preeclampsia. Obstet Gynecol. 2003;102:294-300.
5. Lin L, August P. Genetic thrombophilias and preeclampsia: a meta-analysis. Obstet Gynecol. 2005;105:182-192.
6. Mignini LE, Latthe PM, Villar J, et al. Mapping the theories of preeclampsia: the role of homocysteine. Obstet Gynecol. 2005;105:411-425.
7. Dekker GA, de Vries JI, Doelitzsch PM, et al. Underlying disorders associated with severe early-onset preeclampsia. Am J Obstet Gynecol. 1995;173:1042-1048.
8. Dizon-Townson D, Miller C, Sibai B, et al. The relationship of factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517-524.
9. Riyazi N, Leeda M, de Vries JIP, et al. Low molecular weight heparin combined with aspirin in pregnant women with thrombophilia and a history of preeclampsia or fetal growth restriction: a preliminary study. Eur J Obstet Gynecol Reprod Biol. 1998;80:49-54.
10. Dizon-Townson DS, Nelson LM, Easton K, Ward K. The factor V Leiden mutation may predispose women to severe preeclampsia. Am J Obstet Gynecol. 1996;175:902-905.
11. Nagy B. Detection of factor V Leiden mutation in severe preeclamptic Hungarian women. Clin Genet. 1998;53:478-481.
12. Krauss T. Activated protein C resistance and factor V Leiden in patients with hemolysis, elevated liver enzymes, low platelets syndrome. Obstet Gynecol. 1998;92:457-460.
13. Kupferminc MJ, Eldor A, Steinman N, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999;341:384.-
14. van Pampus EC. High prevalence of hemostatic abnormalities in women with a history of severe preeclampsia. Am J Obstet Gynecol. 1999;180:1146-1150.
15. DeGroot CJ, Bloemankamp KW, Duvekot EJ, et al. Preeclampsia and genetic factors for thrombosis: a case control study. Am J Obstet Gynecol. 1999;181:975-980.
16. Kupferminc MJ, Fait G, Many A, Girdon D, Eldor A, Lessing JB. Severe preeclampsia: high frequency of genetic thrombophilic mutations. Obstet Gynecol. 2000;96:45-49.
17. Rigo J, Nagy B, Fintor L, et al. Maternal and neonatal outcome of preeclamptic pregnancies: the potential roles of factor V Leiden mutations and 5,10 methylenetetrahydrofolate reductase. Hypertens Pregnancy. 2000;19(2):163-172.
18. von Tempelhoff GF. Incidence of factor V Leiden mutation, coagulation inhibitor deficiency, and elevated antiphospholipid-antibodies in patients with preeclampsia or HELLP syndrome (hemolysis, elevated liver enzymes, low platelets). Thromb Res. 2000;100:363-365.
19. Kupferminc MJ, Peri H, Zwang E, et al. High prevalence of the prothrombin gene mutation in women with intrauterine growth retardation, abruptio placentae and second trimester loss. Acta Obstet Gynecol Scand. 2000;79:963-967.
20. Kim YJ. Genetic susceptibility to preeclampsia: roles of cytosine-to-thymine substitution at nucleotide 677 of the gene for methylenetetrahydrofolate reductase, 68-base pair insertion at nucleotide 844 of the gene for cystathione [beta]-synthase, and factor V Leiden mutation. Am J Obstet Gynecol. 2001;184:1211-1217.
21. Livingston J, Barton JR, Park V, et al. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:153-157.
22. Currie L, Peek M, McNiven M, et al. Is there an increased maternal-infant prevalence of factor V Leiden in association with severe pre-eclampsia? BJOG. 2002;109:191-196.
23. Benedetto C, Marozio L, Salton L, et al. Factor V Leiden and factor II G20210A in preeclampsia and HELLP syndrome. Acta Obstet Gynecol. 2002;81:1095-1100.
24. Schlembach D, Beinder E, Zingsem J, et al. Association of maternal and/or fetal factor V Leiden and G20210A prothrombin mutation with HELLP syndrome and intrauterine growth restriction. Clin Sci. 2003;105:279-285.
25. Mello G, Parretti E, Marozio L, et al. Thrombophilia is significantly associated with severe preeclampsia: results of a large-scale, case-controlled study. Hypertension. 2005;46:1270-1274.
26. Branch DW, Andres R, Digre KB, Rote NS, Scott JR. The association of antiphospholipid antibodies with severe preeclampsia. Obstet Gynecol. 1989;73:541-545.
27. Dreyfus M, Hedelin G, Kutnahorsky R, et al. Antiphospholipid antibodies and preeclampsia: a case-control study. Obstet Gynecol. 2001;97:29-34.
28. Kupferminc MJ, Eldor A, Steinman N, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999;340:9-13.
29. Prochazka M, Happach C, Marsal K, Dahlback B, Lindqvist PG. Factor V Leiden in pregnancies complicated by placental abruption. BJOG. 2003;110:462-466.
30. Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19-25.
31. Howley HE, Walker M, Rodger MA. A systematic review of the association between factor V Leiden or prothrombin gene variant and intrauterine growth restriction. Am J Obstet Gynecol. 2005;192:694-708.
32. Martinelli I, Taioli E, Cetin I, et al. Mutations in coagulation factors in women with unexplained late fetal loss. N Engl J Med. 2000;343:1015-1018.
33. Rey E, Kahn SR, David M, et al. Thrombophilic disorders and fetal loss: a metaanalysis. Lancet. 2003;361:901-908.
34. Kupferminc MJ. Mid-trimester severe intrauterine growth restriction is associated with high prevalence of thrombophilia. BJOG. 2002;109:1373-1376.
35. Sibai BM, el-Nazer A, Gonzalez-Ruiz A. Severe preeclampsia-eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986;155:1011-1016.
36. Sibai BM, Mercer B, Sarinoglu C. Severe preeclampsia in the second trimester: recurrence risk and long-term prognosis. Am J Obstet Gynecol. 1991;165:1408-1412.
37. Brenner B. Thrombophilia and fetal loss. Semin Thromb Hemost. 2003;29:165-170.
38. Kupferminc MJ, Fait G, Many A, et al. Low molecular weight heparin for the prevention of obstetric complications in women with thrombophilias. Hypertens Pregnancy. 2001;20:35-44.
39. Cowchock FS, Reece EA, Balaban D, et al. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am J Obstet Gynecol. 1992;166:1318-1323.
40. Laskin CA, Bombardier C, Hannah ME, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. N Engl J Med. 1997;337:148-154.
41. Kutteh WH. Antiphospholipid antibody-associated recurrent pregnancy loss: treatment with heparin and low-dose aspirin is superior to low-dose aspirin alone. Am J Obstet Gynecol. 1996;174:1584-1589.
42. Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ. 1997;314:253-257.
43. Silver RK, MacGregor SN, Sholl JS, et al. Comparative trial of prednisone versus aspirin alone in the treatment of anticardiolipin antibody-positive obstetric patients. Am J Obstet Gynecol. 1993;169:1411-1417.
44. Pattison NS, Chamley LW, Birdsall M, et al. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. Am J Obstet Gynecol. 2000;183:1008-1012.
45. Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol. 2002;100:408-413.
46. Antiphospholipid syndrome. ACOG Practice Bulletin #68. Obstet Gynecol. 2005;106:1113-1121.
47. Gris JC, Mercier E, Quere I, et al. Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood. 2004;103:3695-3699.
48. Ogueh O, Chen MF, Spurll G, Benjamin A. Outcome of pregnancy in women with hereditary thrombophilia. Int J Gynecol Obstet. 2001;74:247-253.
49. Brenner B, Hoffman R, Blumenfeld Z, et al. Gestational outcome in thrombophilic women with recurrent pregnancy loss treated with enoxaparin. Thromb Haemost. 2000;83:693-697.
50. Kupferminc MJ, Fait G, Many A, et al. Low molecular weight heparin for the prevention of obstetric complications in women with thrombophilias. Hypertens Pregnancy. 2001;20:35-44.
51. Verspyck E, Borg JY, Le Cam-Duchez V, et al. Thrombophilia and fetal growth restriction. Eur J Obstet Gynecol Reprod Biol. 2004;113:36-40.
52. McCowan LME, Craigie S, Taylor RS, et al. Inherited thrombophilias are not increased in “idiopathic” small-for-gestationalage pregnancies. Am J Obstet Gynecol. 2003;188:981-992.
53. Grandone E, Brancaccio V, Colaizzo D, et al. Preventing adverse obstetric outcomes in women with genetic thrombophilia. Fertil Steril. 2002;78:371-375.
54. Ridker PM, Miletich JP, Buring JE, et al. Factor V Leiden mutation as a risk factor for recurrent pregnancy loss. Ann Intern Med. 1998;128:1000-1003.
55. Sarig G, Younis J, Hoffman R, et al. Thrombophilia is common in women with idiopathic pregnancy loss and is associated with late pregnancy wastage. Fertil Steril. 2002;77:342-347.
Why thrombophilia matters
During pregnancy, clotting factors I, VII, VIII, IX, and X rise; protein S and fibrinolytic activity diminish; and resistance to activated protein C develops.1,2 When compounded by thrombophilia—a broad spectrum of coagulation disorders that increase the risk for venous and arterial thrombosis—the hypercoagulable state of pregnancy may increase the risk of thromboembolism during pregnancy or postpartum.3
Pulmonary embolism is the leading cause of maternal death in the United States.1 Concern about this lethal sequela has led to numerous recommendations for screening and subsequent prophylaxis and therapy.
Two types
Thrombophilias are inherited or acquired (TABLE 1). The most common inherited disorders during pregnancy are mutations in factor V Leiden, prothrombin gene, and methylenetetrahydrofolate reductase (MTHFR) (TABLE 2). Caucasians have a higher rate of genetic thrombophilias than other racial groups.
Antiphospholipid antibody (APA) syndrome is the most common acquired thrombophilia of pregnancy. It can be diagnosed when the immunoglobulin G or immunoglobulin M level is 20 g per liter or higher, when lupus anticoagulant is present, or both.4
TABLE 1
Thrombophilias are inherited or acquired
INHERITED
|
ACQUIRED
|
| MTHFR=methylenetetrahydrofolate reductase |
Prevalence of thrombophilias in women with normal pregnancy outcomes
| THROMBOPHILIA | PREVALENCE (%) |
|---|---|
| Factor V Leiden mutation | 2–10 |
| MTHFR mutation | 8–16 |
| Prothrombin gene mutation | 2–6 |
| Protein C and S deficiencies | 0.2–1.0* |
| Anticardiolipin antibodies | 1–7 |
| * Combined rate | |
| MTHFR=methylenetetrahydrofolate reductase | |
Link to adverse pregnancy outcomes
During the past 2 decades, several epidemiologic and case-control studies have explored the association between thrombophilias and adverse pregnancy outcomes,2-6 which include the following maternal effects:
- Venous thromboembolism, including deep vein thrombosis, pulmonary embolism, and cerebral vein thrombosis
- Arterial thrombosis (peripheral, cerebral)
- Severe preeclampsia
- Thrombosis and infarcts
- Abruptio placenta
- Recurrent miscarriage
- Fetal growth restriction
- Death
- Stroke
Preeclampsia and thrombophilia
The association between preeclampsia and thrombophilia remains somewhat unclear because of inconsistent data. Because of this, we do not recommend routine screening for thrombophilia in women with preeclampsia.
An association between inherited thrombophilias and preeclampsia was reported by Dekker et al in 1995.7 Since then, numerous retrospective and case-controlled studies have assessed the incidence of thrombophilia in women with severe preeclampsia.7-25 Their findings range from:
- Factor V Leiden: 3.7% to 26.5%
- Prothrombin gene mutation: 0 to 10.8%
- Protein S deficiency: 0.7% to 24.7%
- MTHFR variant: 6.7% to 24.0%
Other points of contention are the varying levels of severity of preeclampsia and of gestational age at delivery, as well as racial differences. For example, most studies found an association between thrombophilia and severe preeclampsia at less than 34 weeks’ gestation, but not between thrombophilia and mild preeclampsia at term. In addition, a recent prospective observational study at multiple centers involving 5,168 women found a factor V Leiden mutation rate of 6% among white women, 2.3% among Asians, 1.6% in Hispanics, and 0.8% in African Americans.8 This large study found no association between thrombophilia and preeclampsia in these women. Therefore, based on available data, we do not recommend routine screening for factor V Leiden in women with severe preeclampsia.
Preeclampsia and APA syndrome
In 1989, Branch et al26 first reported an association between APA syndrome and severe preeclampsia at less than 34 weeks’ gestation. They recommended that women with severe preeclampsia at this gestational age be screened for APA syndrome and treated when the screen is positive. Several later studies supported or refuted the association between APA syndrome and preeclampsia,26,27 and a recent report concluded that routine testing for APA syndrome in women with early-onset preeclampsia is unwarranted.26 Therefore, we do not recommend routine screening for APA in women with severe preeclampsia.
No need to screen women with abruptio placenta
The placental circulation is comparable to venous circulation, with low pressure and low flow velocity rendering it susceptible to thrombotic complications at the maternal–placental interface and consequent premature separation of the placenta.
It is difficult to confirm an association between thrombophilia and abruptio placenta because of confounding variables such as chronic hypertension, cigarette and cocaine use, and advanced maternal age.3 Studies reviewing this association are scarce, and screening for thrombophilia is discouraged in pregnancies marked by abruptio placenta.
Kupferminc et al28 found that 25%, 20%, and 15% of thrombophilia patients with placental abruption had mutations in factor V Leiden, prothrombin gene, and MTHFR, respectively. In contrast, Prochazka et al29 found 15.7% of their cohort of patients with abruptio placenta to have factor V Leiden mutation.
A large prospective, observational study of more than 5,000 asymptomatic pregnant women at multiple centers found no association between abruptio placenta and factor V Leiden mutation.8 Nor were there cases of abruptio placenta among 134 women who were heterozygous for factor V Leiden.
And no routine screening in cases of IUGR
Routine screening for thrombophilias in women with intrauterine growth restriction (IUGR) is not recommended. One reason: The prevalence of thrombophilias in these women ranges widely, depending on the study cited: from 2.8% to 35% for factor V Leiden and 2.8% to 15.4% for prothrombin gene mutation (TABLE 3). In addition, in contrast to earlier studies, a large case-control trial by Infante-Rivard et al30 found no increased risk of IUGR in women with thrombophilias, except for a subgroup of women with the MTHFR variant who did not take a prenatal multivitamin.
A recent meta-analysis of case-control studies by Howley et al31 found a significant association between factor V Leiden, the prothrombin gene variant, and IUGR, but the investigators cautioned that this strong association may be driven by small, poor-quality studies that yield extreme associations. A multicenter observational study by Dizon-Townson et al8 found no association between thrombophilia and IUGR in asymptomatic gravidas.
TABLE 3
Incidence of thrombophilias in women with intrauterine growth restriction
| STUDY | FACTOR V LEIDEN (%) | PROTHROMBIN GENE MUTATION (%) | ||
|---|---|---|---|---|
| IUGR | CONTROLS | IUGR | CONTROLS | |
| Kupferminc et al50 | 5/44 (11.4) | 7/110 (6.4) | 5/44 (11.4) | 3/110 (2.7) |
| Infante-Rivard et al30 | 22/488 (4.5) | 18/470 (3.8) | 12/488 (2.5) | 11/470 (2.3) |
| Verspyck et al51 | 4/97 (4.1) | 1/97 (1) | 3/97 (3.1) | 1/97 (1) |
| McCowan et al52 | 4/145 (2.8) | 11/290 (3.8) | 4/145 (2.8) | 9/290 (3.1) |
| Dizon-Townson et al*10 | 6/134 (4.5) | 233/4,753 (4.9) | NR | NR |
| Kupferminc**34 | 9/26 (35) | 2/52 (3.8) | 4/26 (15.4) | 2/52 (3.8) |
| * | ||||
| ** Mid-trimester severe intrauterine growth restriction | ||||
| IUGR=intrauterine growth restriction, NR=not recorded | ||||
| SOURCE: Adapted from Clin Obstet Gynecol. 2006;49:850–860 | ||||
Fetal loss is a complication of thrombophilia
One in 10 pregnancies ends in early death of the fetus (before 20 weeks), and 1 in 200 gestations ends in late fetal loss.32 When fetal loss occurs in the second and third trimesters, it is due to excessive thrombosis of the placental vessels, placental infarction, and secondary uteroplacental insufficiency.2,33 Women who are carriers of factor V or prothrombin gene mutations are at higher risk of late fetal loss than noncarriers are (TABLE 4).
Fetal loss is a well-established complication in women with thrombophilia, but not all thrombophilias are associated with fetal loss, according to a meta-analysis of 31 studies.33 In women with thrombophilia, first-trimester loss is generally associated with factor V Leiden, prothrombin gene mutation, and activated protein C resistance. Late, nonrecurrent fetal loss is associated with factor V Leiden, prothrombin gene mutation, and protein S deficiency.33
TABLE 4
Incidence of factor V Leiden mutation in women with recurrent pregnancy loss
| STUDY | PATIENT SELECTION | PATIENTS (%) | CONTROLS (%) | ODDS RATIO | 95% CONFIDENCE INTERVAL |
|---|---|---|---|---|---|
| Grandone et al53 | ≥2 unexplained fetal losses, other causes excluded | 7/43 (16.3) | 5/118 (4.2) | 4.4 | 1.3–14.7 |
| Ridker et al54 | Recurrent, spontaneous abortion, other causes not excluded | 9/113 (8) | 16/437 (3.7) | 2.3 | 1.0–5.2 |
| Sarig et al55 | ≥3 first- or second-trimester losses or ≥1 intrauterine fetal demise, other causes excluded* | 96/145 (66) | 41/145 (28) | 5.0 | 3.0–8.5 |
| * Excluded chromosomal abnormalities, infections, anatomic alterations, and endocrine dysfunction | |||||
History of adverse outcomes? Offer screening
It is well established that women with a history of fetal death, severe preeclampsia, IUGR, abruptio placenta, or recurrent miscarriage have an increased risk of recurrence in subsequent pregnancies.3,30,34-36 The rate of recurrence of any of these outcomes may be as high as 46% with a history of 2 or more adverse outcomes, even before any thrombophilia is taken into account.3 Although there are few studies describing the rate of recurrence of adverse pregnancy outcomes in women with thrombophilia and a previous adverse outcome (TABLE 5), it appears to range from 66% to 83% in untreated women.3,37
Based on these findings, some authors recommend screening for thrombophilia in women who have had adverse pregnancy outcomes3,9,38 and prophylactic therapy in subsequent pregnancies when the test is positive. Therapy includes low-dose aspirin with or without subcutaneous heparin, as well as folic acid and vitamin B6 supplements, according to the type of thrombophilia present as well as the nature of the previous adverse outcome.
TABLE 5
How women with a previous adverse outcome fare on anticoagulation therapy
| STUDY | PATIENTS | PREVIOUS ADVERSE PREGNANCY OUTCOME | ANTICOAGULANT | OUTCOME IN CURRENT PREGNANCY |
|---|---|---|---|---|
| Riyazi et al9 | 26 | Uteroplacental insufficiency | LMWH and low-dose aspirin | Decreased recurrence of preeclampsia (85% to 38%) and IUGR (54% to 15%) |
| Brenner37 | 50 | ≥3 first-trimester recurrent pregnancy losses with thrombophilia | LMWH | Higher live birth rate compared with historical controls (75% vs 20%) |
| Ogueh et al48 | 24 | Previous adverse pregnancy outcome plus history of thromboembolic disease, family history of thrombophilia | UFH | No significant mprovement |
| Kupferminc et al38 | 33 | Thrombophilia with history of preeclampsia or IUGR | LMWH and low-dose aspirin | With treatment, 3% recurrence of preeclampsia |
| Grandone et al53 | 25 | Repeated pregnancy loss, gestational hypertension, HELLP, or IUGR | UFH or LMWH | 90.3% treated with LMWH had good obstetric outcome |
| Paidas et al3 | 158 | Fetal loss, IUGR, placental abruption, or preeclampsia | UFH or LMWH | 80% reduction in risk of adverse pregnancy outcome, compared with historical controls (OR, 0.21; 95% CI, 0.11–0.39) |
| HELLP=hemolysis, elevated liver enzymes, and low platelets; IUGR=intrauterine growth restriction; LMWH=low-molecular-weight heparin; UFH=unfractionated heparin | ||||
| SOURCE: Adapted from Am J Perinatol. 2006;23:499–506 | ||||
No randomized trials on prophylaxis
We lack randomized trials evaluating thromboprophylaxis for prevention of recurrent adverse pregnancy outcomes in women with previous severe preeclampsia, IUGR, or abruptio placenta in association with genetic thrombophilia. Therefore, any recommendation to treat such women with low-molecular-weight heparin with or without low-dose aspirin in subsequent pregnancies should remain empiric and/or prescribed after appropriate counseling of the patients regarding risks and benefits.
TABLE 6 summarizes the risk of thromboembolism in women with thrombophilia—both for asymptomatic patients and for those with a history of thromboembolism. These percentages should be used when counseling women about their risk and determining management and therapy.
TABLE 6
Risk of thromboembolism during pregnancy and postpartum in women with thrombophilia
| THROMBOPHILIA | RISK (%) | |
|---|---|---|
| ASYMPTOMATIC WOMEN | HISTORY OF VENOUS THROMBOEMBOLISM | |
| Factor V Leiden | ||
| Heterozygous | 0.2 | 10 |
| Homozygous | 1–2 | 15–20 |
| Prothrombin gene mutation | ||
| Heterozygous | 0.5 | 10 |
| Homozygous | 2.3 | 20 |
| Factor V Leiden and prothrombin gene mutation | 5 | 20 |
| Antithrombin deficiency | 7 | 40 |
| Protein C deficiency | 0.5 | 5–15 |
| Protein S deficiency | 0.1 | Unknown |
Prophylaxis for APA syndrome and recurrent pregnancy loss
Several randomized trials have described the use of low-dose aspirin and heparin in women with APA syndrome and a history of recurrent pregnancy loss, although the results are inconsistent (TABLE 7).39-45 The inconsistency may be due to varying definitions of APA syndrome and gestational age at the time of randomization, as well as the population studied (previous thromboembolism, presence or absence of lupus anticoagulant, level of titer of anticardiolipin antibodies, presence or absence of previous stillbirth). Nevertheless, we recommend that women with true APA syndrome (presence of lupus anticoagulant, high titers of immunoglobulin G, history of thromboembolism or recurrent stillbirth) receive prophylaxis with low-dose aspirin, with subcutaneous heparin added once fetal cardiac activity is documented.46
TABLE 7
Live births in women with APA and a history of fetal loss
| STUDY | TREATMENT | CONTROL | NO. OF LIVE BIRTHS (%) | |
|---|---|---|---|---|
| TREATED WOMEN | CONTROL GROUP | |||
| Cowchock et al39 | Aspirin/heparin | Aspirin/prednisone | 9/12 (75) | 6/8 (75) |
| Laskin et al40 | Aspirin/prednisone | Placebo | 25/42 (60) | 24/46 (52) |
| Kutteh41 | Aspirin/heparin | Aspirin only | 20/25 (80) | 11/25 (44) |
| Rai et al42 | Aspirin/heparin | Aspirin only | 32/45 (71) | 19/45 (42) |
| Silver et al43 | Aspirin/prednisone | Aspirin only | 12/12 (100) | 22/22 (100) |
| Pattison et al44 | Aspirin | Placebo | 16/20 (80) | 17/20 (85) |
| Farquharson et al45 | Aspirin/LMWH | Aspirin only | 40/51 (78) | 34/47 (72) |
| LMWH=low-molecular-weight heparin | ||||
Genetic thrombophilias
Few published studies describe prophylactic use of low-molecular-weight heparin with or without low-dose aspirin in women with genetic thrombophilia and a history of adverse pregnancy outcomes. All but 1 of these studies are observational, comparing outcome in the treated pregnancy with that of previously untreated gestations in the same woman.3,9,38,44,45,47 These studies included a limited number of women and a heterogeneous group of patients with various thrombophilias; they also involved different therapies (TABLE 7).3,9,38,41,48,49
Gris et al47 performed a randomized trial in 160 women with at least 1 prior fetal loss after 10 weeks’ gestation who were heterozygous for factor V Leiden or prothrombin G20210A mutation, or had protein S deficiency. Beginning at 8 weeks’ gestation, these women were assigned to treatment with 40 mg of enoxaparin (n=80) or 100 mg of low-dose aspirin (n=80) daily. All women also received 5 mg of folic acid daily.
In the women treated with enoxaparin, 69 (86%) had a live birth, compared with 23 (29%) women treated with low-dose aspirin. The women treated with enoxaparin also had significantly higher median neonatal birth weights and a lower rate of IUGR (10% versus 30%). The authors concluded that women with factor V Leiden, prothrombin gene mutation, or protein S deficiency and a history of fetal loss should receive enoxaparin prophylaxis in subsequent pregnancies.
History of severe preeclampsia, IUGR, or abruptio placenta. No randomized trials have evaluated thromboprophylaxis in women with this history who have genetic thrombophilia. For this reason, any recommendation to treat these women with low-molecular-weight heparin with or without low-dose aspirin in subsequent pregnancies remains empiric. Prophylaxis can be prescribed after an appropriate discussion of risks and benefits with the patient.
Unresolved questions keep management experimental
What is the likelihood that a woman carrying a gene mutation that predisposes her to thrombophilia will have a serious complication during pregnancy? And how safe and effective is prophylaxis?
There is a prevailing need for a double-blind placebo-controlled trial to address these questions and evaluate the benefit of heparin in pregnant women with a history of adverse pregnancy outcomes and thrombophilia. Until then, screening and treatment for thrombophilia remain experimental in these women.
The authors report no financial relationships relevant to this article.
Why thrombophilia matters
During pregnancy, clotting factors I, VII, VIII, IX, and X rise; protein S and fibrinolytic activity diminish; and resistance to activated protein C develops.1,2 When compounded by thrombophilia—a broad spectrum of coagulation disorders that increase the risk for venous and arterial thrombosis—the hypercoagulable state of pregnancy may increase the risk of thromboembolism during pregnancy or postpartum.3
Pulmonary embolism is the leading cause of maternal death in the United States.1 Concern about this lethal sequela has led to numerous recommendations for screening and subsequent prophylaxis and therapy.
Two types
Thrombophilias are inherited or acquired (TABLE 1). The most common inherited disorders during pregnancy are mutations in factor V Leiden, prothrombin gene, and methylenetetrahydrofolate reductase (MTHFR) (TABLE 2). Caucasians have a higher rate of genetic thrombophilias than other racial groups.
Antiphospholipid antibody (APA) syndrome is the most common acquired thrombophilia of pregnancy. It can be diagnosed when the immunoglobulin G or immunoglobulin M level is 20 g per liter or higher, when lupus anticoagulant is present, or both.4
TABLE 1
Thrombophilias are inherited or acquired
INHERITED
|
ACQUIRED
|
| MTHFR=methylenetetrahydrofolate reductase |
Prevalence of thrombophilias in women with normal pregnancy outcomes
| THROMBOPHILIA | PREVALENCE (%) |
|---|---|
| Factor V Leiden mutation | 2–10 |
| MTHFR mutation | 8–16 |
| Prothrombin gene mutation | 2–6 |
| Protein C and S deficiencies | 0.2–1.0* |
| Anticardiolipin antibodies | 1–7 |
| * Combined rate | |
| MTHFR=methylenetetrahydrofolate reductase | |
Link to adverse pregnancy outcomes
During the past 2 decades, several epidemiologic and case-control studies have explored the association between thrombophilias and adverse pregnancy outcomes,2-6 which include the following maternal effects:
- Venous thromboembolism, including deep vein thrombosis, pulmonary embolism, and cerebral vein thrombosis
- Arterial thrombosis (peripheral, cerebral)
- Severe preeclampsia
- Thrombosis and infarcts
- Abruptio placenta
- Recurrent miscarriage
- Fetal growth restriction
- Death
- Stroke
Preeclampsia and thrombophilia
The association between preeclampsia and thrombophilia remains somewhat unclear because of inconsistent data. Because of this, we do not recommend routine screening for thrombophilia in women with preeclampsia.
An association between inherited thrombophilias and preeclampsia was reported by Dekker et al in 1995.7 Since then, numerous retrospective and case-controlled studies have assessed the incidence of thrombophilia in women with severe preeclampsia.7-25 Their findings range from:
- Factor V Leiden: 3.7% to 26.5%
- Prothrombin gene mutation: 0 to 10.8%
- Protein S deficiency: 0.7% to 24.7%
- MTHFR variant: 6.7% to 24.0%
Other points of contention are the varying levels of severity of preeclampsia and of gestational age at delivery, as well as racial differences. For example, most studies found an association between thrombophilia and severe preeclampsia at less than 34 weeks’ gestation, but not between thrombophilia and mild preeclampsia at term. In addition, a recent prospective observational study at multiple centers involving 5,168 women found a factor V Leiden mutation rate of 6% among white women, 2.3% among Asians, 1.6% in Hispanics, and 0.8% in African Americans.8 This large study found no association between thrombophilia and preeclampsia in these women. Therefore, based on available data, we do not recommend routine screening for factor V Leiden in women with severe preeclampsia.
Preeclampsia and APA syndrome
In 1989, Branch et al26 first reported an association between APA syndrome and severe preeclampsia at less than 34 weeks’ gestation. They recommended that women with severe preeclampsia at this gestational age be screened for APA syndrome and treated when the screen is positive. Several later studies supported or refuted the association between APA syndrome and preeclampsia,26,27 and a recent report concluded that routine testing for APA syndrome in women with early-onset preeclampsia is unwarranted.26 Therefore, we do not recommend routine screening for APA in women with severe preeclampsia.
No need to screen women with abruptio placenta
The placental circulation is comparable to venous circulation, with low pressure and low flow velocity rendering it susceptible to thrombotic complications at the maternal–placental interface and consequent premature separation of the placenta.
It is difficult to confirm an association between thrombophilia and abruptio placenta because of confounding variables such as chronic hypertension, cigarette and cocaine use, and advanced maternal age.3 Studies reviewing this association are scarce, and screening for thrombophilia is discouraged in pregnancies marked by abruptio placenta.
Kupferminc et al28 found that 25%, 20%, and 15% of thrombophilia patients with placental abruption had mutations in factor V Leiden, prothrombin gene, and MTHFR, respectively. In contrast, Prochazka et al29 found 15.7% of their cohort of patients with abruptio placenta to have factor V Leiden mutation.
A large prospective, observational study of more than 5,000 asymptomatic pregnant women at multiple centers found no association between abruptio placenta and factor V Leiden mutation.8 Nor were there cases of abruptio placenta among 134 women who were heterozygous for factor V Leiden.
And no routine screening in cases of IUGR
Routine screening for thrombophilias in women with intrauterine growth restriction (IUGR) is not recommended. One reason: The prevalence of thrombophilias in these women ranges widely, depending on the study cited: from 2.8% to 35% for factor V Leiden and 2.8% to 15.4% for prothrombin gene mutation (TABLE 3). In addition, in contrast to earlier studies, a large case-control trial by Infante-Rivard et al30 found no increased risk of IUGR in women with thrombophilias, except for a subgroup of women with the MTHFR variant who did not take a prenatal multivitamin.
A recent meta-analysis of case-control studies by Howley et al31 found a significant association between factor V Leiden, the prothrombin gene variant, and IUGR, but the investigators cautioned that this strong association may be driven by small, poor-quality studies that yield extreme associations. A multicenter observational study by Dizon-Townson et al8 found no association between thrombophilia and IUGR in asymptomatic gravidas.
TABLE 3
Incidence of thrombophilias in women with intrauterine growth restriction
| STUDY | FACTOR V LEIDEN (%) | PROTHROMBIN GENE MUTATION (%) | ||
|---|---|---|---|---|
| IUGR | CONTROLS | IUGR | CONTROLS | |
| Kupferminc et al50 | 5/44 (11.4) | 7/110 (6.4) | 5/44 (11.4) | 3/110 (2.7) |
| Infante-Rivard et al30 | 22/488 (4.5) | 18/470 (3.8) | 12/488 (2.5) | 11/470 (2.3) |
| Verspyck et al51 | 4/97 (4.1) | 1/97 (1) | 3/97 (3.1) | 1/97 (1) |
| McCowan et al52 | 4/145 (2.8) | 11/290 (3.8) | 4/145 (2.8) | 9/290 (3.1) |
| Dizon-Townson et al*10 | 6/134 (4.5) | 233/4,753 (4.9) | NR | NR |
| Kupferminc**34 | 9/26 (35) | 2/52 (3.8) | 4/26 (15.4) | 2/52 (3.8) |
| * | ||||
| ** Mid-trimester severe intrauterine growth restriction | ||||
| IUGR=intrauterine growth restriction, NR=not recorded | ||||
| SOURCE: Adapted from Clin Obstet Gynecol. 2006;49:850–860 | ||||
Fetal loss is a complication of thrombophilia
One in 10 pregnancies ends in early death of the fetus (before 20 weeks), and 1 in 200 gestations ends in late fetal loss.32 When fetal loss occurs in the second and third trimesters, it is due to excessive thrombosis of the placental vessels, placental infarction, and secondary uteroplacental insufficiency.2,33 Women who are carriers of factor V or prothrombin gene mutations are at higher risk of late fetal loss than noncarriers are (TABLE 4).
Fetal loss is a well-established complication in women with thrombophilia, but not all thrombophilias are associated with fetal loss, according to a meta-analysis of 31 studies.33 In women with thrombophilia, first-trimester loss is generally associated with factor V Leiden, prothrombin gene mutation, and activated protein C resistance. Late, nonrecurrent fetal loss is associated with factor V Leiden, prothrombin gene mutation, and protein S deficiency.33
TABLE 4
Incidence of factor V Leiden mutation in women with recurrent pregnancy loss
| STUDY | PATIENT SELECTION | PATIENTS (%) | CONTROLS (%) | ODDS RATIO | 95% CONFIDENCE INTERVAL |
|---|---|---|---|---|---|
| Grandone et al53 | ≥2 unexplained fetal losses, other causes excluded | 7/43 (16.3) | 5/118 (4.2) | 4.4 | 1.3–14.7 |
| Ridker et al54 | Recurrent, spontaneous abortion, other causes not excluded | 9/113 (8) | 16/437 (3.7) | 2.3 | 1.0–5.2 |
| Sarig et al55 | ≥3 first- or second-trimester losses or ≥1 intrauterine fetal demise, other causes excluded* | 96/145 (66) | 41/145 (28) | 5.0 | 3.0–8.5 |
| * Excluded chromosomal abnormalities, infections, anatomic alterations, and endocrine dysfunction | |||||
History of adverse outcomes? Offer screening
It is well established that women with a history of fetal death, severe preeclampsia, IUGR, abruptio placenta, or recurrent miscarriage have an increased risk of recurrence in subsequent pregnancies.3,30,34-36 The rate of recurrence of any of these outcomes may be as high as 46% with a history of 2 or more adverse outcomes, even before any thrombophilia is taken into account.3 Although there are few studies describing the rate of recurrence of adverse pregnancy outcomes in women with thrombophilia and a previous adverse outcome (TABLE 5), it appears to range from 66% to 83% in untreated women.3,37
Based on these findings, some authors recommend screening for thrombophilia in women who have had adverse pregnancy outcomes3,9,38 and prophylactic therapy in subsequent pregnancies when the test is positive. Therapy includes low-dose aspirin with or without subcutaneous heparin, as well as folic acid and vitamin B6 supplements, according to the type of thrombophilia present as well as the nature of the previous adverse outcome.
TABLE 5
How women with a previous adverse outcome fare on anticoagulation therapy
| STUDY | PATIENTS | PREVIOUS ADVERSE PREGNANCY OUTCOME | ANTICOAGULANT | OUTCOME IN CURRENT PREGNANCY |
|---|---|---|---|---|
| Riyazi et al9 | 26 | Uteroplacental insufficiency | LMWH and low-dose aspirin | Decreased recurrence of preeclampsia (85% to 38%) and IUGR (54% to 15%) |
| Brenner37 | 50 | ≥3 first-trimester recurrent pregnancy losses with thrombophilia | LMWH | Higher live birth rate compared with historical controls (75% vs 20%) |
| Ogueh et al48 | 24 | Previous adverse pregnancy outcome plus history of thromboembolic disease, family history of thrombophilia | UFH | No significant mprovement |
| Kupferminc et al38 | 33 | Thrombophilia with history of preeclampsia or IUGR | LMWH and low-dose aspirin | With treatment, 3% recurrence of preeclampsia |
| Grandone et al53 | 25 | Repeated pregnancy loss, gestational hypertension, HELLP, or IUGR | UFH or LMWH | 90.3% treated with LMWH had good obstetric outcome |
| Paidas et al3 | 158 | Fetal loss, IUGR, placental abruption, or preeclampsia | UFH or LMWH | 80% reduction in risk of adverse pregnancy outcome, compared with historical controls (OR, 0.21; 95% CI, 0.11–0.39) |
| HELLP=hemolysis, elevated liver enzymes, and low platelets; IUGR=intrauterine growth restriction; LMWH=low-molecular-weight heparin; UFH=unfractionated heparin | ||||
| SOURCE: Adapted from Am J Perinatol. 2006;23:499–506 | ||||
No randomized trials on prophylaxis
We lack randomized trials evaluating thromboprophylaxis for prevention of recurrent adverse pregnancy outcomes in women with previous severe preeclampsia, IUGR, or abruptio placenta in association with genetic thrombophilia. Therefore, any recommendation to treat such women with low-molecular-weight heparin with or without low-dose aspirin in subsequent pregnancies should remain empiric and/or prescribed after appropriate counseling of the patients regarding risks and benefits.
TABLE 6 summarizes the risk of thromboembolism in women with thrombophilia—both for asymptomatic patients and for those with a history of thromboembolism. These percentages should be used when counseling women about their risk and determining management and therapy.
TABLE 6
Risk of thromboembolism during pregnancy and postpartum in women with thrombophilia
| THROMBOPHILIA | RISK (%) | |
|---|---|---|
| ASYMPTOMATIC WOMEN | HISTORY OF VENOUS THROMBOEMBOLISM | |
| Factor V Leiden | ||
| Heterozygous | 0.2 | 10 |
| Homozygous | 1–2 | 15–20 |
| Prothrombin gene mutation | ||
| Heterozygous | 0.5 | 10 |
| Homozygous | 2.3 | 20 |
| Factor V Leiden and prothrombin gene mutation | 5 | 20 |
| Antithrombin deficiency | 7 | 40 |
| Protein C deficiency | 0.5 | 5–15 |
| Protein S deficiency | 0.1 | Unknown |
Prophylaxis for APA syndrome and recurrent pregnancy loss
Several randomized trials have described the use of low-dose aspirin and heparin in women with APA syndrome and a history of recurrent pregnancy loss, although the results are inconsistent (TABLE 7).39-45 The inconsistency may be due to varying definitions of APA syndrome and gestational age at the time of randomization, as well as the population studied (previous thromboembolism, presence or absence of lupus anticoagulant, level of titer of anticardiolipin antibodies, presence or absence of previous stillbirth). Nevertheless, we recommend that women with true APA syndrome (presence of lupus anticoagulant, high titers of immunoglobulin G, history of thromboembolism or recurrent stillbirth) receive prophylaxis with low-dose aspirin, with subcutaneous heparin added once fetal cardiac activity is documented.46
TABLE 7
Live births in women with APA and a history of fetal loss
| STUDY | TREATMENT | CONTROL | NO. OF LIVE BIRTHS (%) | |
|---|---|---|---|---|
| TREATED WOMEN | CONTROL GROUP | |||
| Cowchock et al39 | Aspirin/heparin | Aspirin/prednisone | 9/12 (75) | 6/8 (75) |
| Laskin et al40 | Aspirin/prednisone | Placebo | 25/42 (60) | 24/46 (52) |
| Kutteh41 | Aspirin/heparin | Aspirin only | 20/25 (80) | 11/25 (44) |
| Rai et al42 | Aspirin/heparin | Aspirin only | 32/45 (71) | 19/45 (42) |
| Silver et al43 | Aspirin/prednisone | Aspirin only | 12/12 (100) | 22/22 (100) |
| Pattison et al44 | Aspirin | Placebo | 16/20 (80) | 17/20 (85) |
| Farquharson et al45 | Aspirin/LMWH | Aspirin only | 40/51 (78) | 34/47 (72) |
| LMWH=low-molecular-weight heparin | ||||
Genetic thrombophilias
Few published studies describe prophylactic use of low-molecular-weight heparin with or without low-dose aspirin in women with genetic thrombophilia and a history of adverse pregnancy outcomes. All but 1 of these studies are observational, comparing outcome in the treated pregnancy with that of previously untreated gestations in the same woman.3,9,38,44,45,47 These studies included a limited number of women and a heterogeneous group of patients with various thrombophilias; they also involved different therapies (TABLE 7).3,9,38,41,48,49
Gris et al47 performed a randomized trial in 160 women with at least 1 prior fetal loss after 10 weeks’ gestation who were heterozygous for factor V Leiden or prothrombin G20210A mutation, or had protein S deficiency. Beginning at 8 weeks’ gestation, these women were assigned to treatment with 40 mg of enoxaparin (n=80) or 100 mg of low-dose aspirin (n=80) daily. All women also received 5 mg of folic acid daily.
In the women treated with enoxaparin, 69 (86%) had a live birth, compared with 23 (29%) women treated with low-dose aspirin. The women treated with enoxaparin also had significantly higher median neonatal birth weights and a lower rate of IUGR (10% versus 30%). The authors concluded that women with factor V Leiden, prothrombin gene mutation, or protein S deficiency and a history of fetal loss should receive enoxaparin prophylaxis in subsequent pregnancies.
History of severe preeclampsia, IUGR, or abruptio placenta. No randomized trials have evaluated thromboprophylaxis in women with this history who have genetic thrombophilia. For this reason, any recommendation to treat these women with low-molecular-weight heparin with or without low-dose aspirin in subsequent pregnancies remains empiric. Prophylaxis can be prescribed after an appropriate discussion of risks and benefits with the patient.
Unresolved questions keep management experimental
What is the likelihood that a woman carrying a gene mutation that predisposes her to thrombophilia will have a serious complication during pregnancy? And how safe and effective is prophylaxis?
There is a prevailing need for a double-blind placebo-controlled trial to address these questions and evaluate the benefit of heparin in pregnant women with a history of adverse pregnancy outcomes and thrombophilia. Until then, screening and treatment for thrombophilia remain experimental in these women.
The authors report no financial relationships relevant to this article.
1. Thromboembolism in pregnancy. ACOG Practice Bulletin #19. Washington, DC: ACOG; 2000.
2. Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412-424.
3. Paidas MJ, De-Hui WK, Arkel YS. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 2004;31:783-805.
4. Lee RM, Brown MA, Branch DW, Ward K, Silver RM. Anticardiolipin and anti-B2 glycoprotein-I antibodies in preeclampsia. Obstet Gynecol. 2003;102:294-300.
5. Lin L, August P. Genetic thrombophilias and preeclampsia: a meta-analysis. Obstet Gynecol. 2005;105:182-192.
6. Mignini LE, Latthe PM, Villar J, et al. Mapping the theories of preeclampsia: the role of homocysteine. Obstet Gynecol. 2005;105:411-425.
7. Dekker GA, de Vries JI, Doelitzsch PM, et al. Underlying disorders associated with severe early-onset preeclampsia. Am J Obstet Gynecol. 1995;173:1042-1048.
8. Dizon-Townson D, Miller C, Sibai B, et al. The relationship of factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517-524.
9. Riyazi N, Leeda M, de Vries JIP, et al. Low molecular weight heparin combined with aspirin in pregnant women with thrombophilia and a history of preeclampsia or fetal growth restriction: a preliminary study. Eur J Obstet Gynecol Reprod Biol. 1998;80:49-54.
10. Dizon-Townson DS, Nelson LM, Easton K, Ward K. The factor V Leiden mutation may predispose women to severe preeclampsia. Am J Obstet Gynecol. 1996;175:902-905.
11. Nagy B. Detection of factor V Leiden mutation in severe preeclamptic Hungarian women. Clin Genet. 1998;53:478-481.
12. Krauss T. Activated protein C resistance and factor V Leiden in patients with hemolysis, elevated liver enzymes, low platelets syndrome. Obstet Gynecol. 1998;92:457-460.
13. Kupferminc MJ, Eldor A, Steinman N, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999;341:384.-
14. van Pampus EC. High prevalence of hemostatic abnormalities in women with a history of severe preeclampsia. Am J Obstet Gynecol. 1999;180:1146-1150.
15. DeGroot CJ, Bloemankamp KW, Duvekot EJ, et al. Preeclampsia and genetic factors for thrombosis: a case control study. Am J Obstet Gynecol. 1999;181:975-980.
16. Kupferminc MJ, Fait G, Many A, Girdon D, Eldor A, Lessing JB. Severe preeclampsia: high frequency of genetic thrombophilic mutations. Obstet Gynecol. 2000;96:45-49.
17. Rigo J, Nagy B, Fintor L, et al. Maternal and neonatal outcome of preeclamptic pregnancies: the potential roles of factor V Leiden mutations and 5,10 methylenetetrahydrofolate reductase. Hypertens Pregnancy. 2000;19(2):163-172.
18. von Tempelhoff GF. Incidence of factor V Leiden mutation, coagulation inhibitor deficiency, and elevated antiphospholipid-antibodies in patients with preeclampsia or HELLP syndrome (hemolysis, elevated liver enzymes, low platelets). Thromb Res. 2000;100:363-365.
19. Kupferminc MJ, Peri H, Zwang E, et al. High prevalence of the prothrombin gene mutation in women with intrauterine growth retardation, abruptio placentae and second trimester loss. Acta Obstet Gynecol Scand. 2000;79:963-967.
20. Kim YJ. Genetic susceptibility to preeclampsia: roles of cytosine-to-thymine substitution at nucleotide 677 of the gene for methylenetetrahydrofolate reductase, 68-base pair insertion at nucleotide 844 of the gene for cystathione [beta]-synthase, and factor V Leiden mutation. Am J Obstet Gynecol. 2001;184:1211-1217.
21. Livingston J, Barton JR, Park V, et al. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:153-157.
22. Currie L, Peek M, McNiven M, et al. Is there an increased maternal-infant prevalence of factor V Leiden in association with severe pre-eclampsia? BJOG. 2002;109:191-196.
23. Benedetto C, Marozio L, Salton L, et al. Factor V Leiden and factor II G20210A in preeclampsia and HELLP syndrome. Acta Obstet Gynecol. 2002;81:1095-1100.
24. Schlembach D, Beinder E, Zingsem J, et al. Association of maternal and/or fetal factor V Leiden and G20210A prothrombin mutation with HELLP syndrome and intrauterine growth restriction. Clin Sci. 2003;105:279-285.
25. Mello G, Parretti E, Marozio L, et al. Thrombophilia is significantly associated with severe preeclampsia: results of a large-scale, case-controlled study. Hypertension. 2005;46:1270-1274.
26. Branch DW, Andres R, Digre KB, Rote NS, Scott JR. The association of antiphospholipid antibodies with severe preeclampsia. Obstet Gynecol. 1989;73:541-545.
27. Dreyfus M, Hedelin G, Kutnahorsky R, et al. Antiphospholipid antibodies and preeclampsia: a case-control study. Obstet Gynecol. 2001;97:29-34.
28. Kupferminc MJ, Eldor A, Steinman N, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999;340:9-13.
29. Prochazka M, Happach C, Marsal K, Dahlback B, Lindqvist PG. Factor V Leiden in pregnancies complicated by placental abruption. BJOG. 2003;110:462-466.
30. Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19-25.
31. Howley HE, Walker M, Rodger MA. A systematic review of the association between factor V Leiden or prothrombin gene variant and intrauterine growth restriction. Am J Obstet Gynecol. 2005;192:694-708.
32. Martinelli I, Taioli E, Cetin I, et al. Mutations in coagulation factors in women with unexplained late fetal loss. N Engl J Med. 2000;343:1015-1018.
33. Rey E, Kahn SR, David M, et al. Thrombophilic disorders and fetal loss: a metaanalysis. Lancet. 2003;361:901-908.
34. Kupferminc MJ. Mid-trimester severe intrauterine growth restriction is associated with high prevalence of thrombophilia. BJOG. 2002;109:1373-1376.
35. Sibai BM, el-Nazer A, Gonzalez-Ruiz A. Severe preeclampsia-eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986;155:1011-1016.
36. Sibai BM, Mercer B, Sarinoglu C. Severe preeclampsia in the second trimester: recurrence risk and long-term prognosis. Am J Obstet Gynecol. 1991;165:1408-1412.
37. Brenner B. Thrombophilia and fetal loss. Semin Thromb Hemost. 2003;29:165-170.
38. Kupferminc MJ, Fait G, Many A, et al. Low molecular weight heparin for the prevention of obstetric complications in women with thrombophilias. Hypertens Pregnancy. 2001;20:35-44.
39. Cowchock FS, Reece EA, Balaban D, et al. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am J Obstet Gynecol. 1992;166:1318-1323.
40. Laskin CA, Bombardier C, Hannah ME, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. N Engl J Med. 1997;337:148-154.
41. Kutteh WH. Antiphospholipid antibody-associated recurrent pregnancy loss: treatment with heparin and low-dose aspirin is superior to low-dose aspirin alone. Am J Obstet Gynecol. 1996;174:1584-1589.
42. Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ. 1997;314:253-257.
43. Silver RK, MacGregor SN, Sholl JS, et al. Comparative trial of prednisone versus aspirin alone in the treatment of anticardiolipin antibody-positive obstetric patients. Am J Obstet Gynecol. 1993;169:1411-1417.
44. Pattison NS, Chamley LW, Birdsall M, et al. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. Am J Obstet Gynecol. 2000;183:1008-1012.
45. Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol. 2002;100:408-413.
46. Antiphospholipid syndrome. ACOG Practice Bulletin #68. Obstet Gynecol. 2005;106:1113-1121.
47. Gris JC, Mercier E, Quere I, et al. Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood. 2004;103:3695-3699.
48. Ogueh O, Chen MF, Spurll G, Benjamin A. Outcome of pregnancy in women with hereditary thrombophilia. Int J Gynecol Obstet. 2001;74:247-253.
49. Brenner B, Hoffman R, Blumenfeld Z, et al. Gestational outcome in thrombophilic women with recurrent pregnancy loss treated with enoxaparin. Thromb Haemost. 2000;83:693-697.
50. Kupferminc MJ, Fait G, Many A, et al. Low molecular weight heparin for the prevention of obstetric complications in women with thrombophilias. Hypertens Pregnancy. 2001;20:35-44.
51. Verspyck E, Borg JY, Le Cam-Duchez V, et al. Thrombophilia and fetal growth restriction. Eur J Obstet Gynecol Reprod Biol. 2004;113:36-40.
52. McCowan LME, Craigie S, Taylor RS, et al. Inherited thrombophilias are not increased in “idiopathic” small-for-gestationalage pregnancies. Am J Obstet Gynecol. 2003;188:981-992.
53. Grandone E, Brancaccio V, Colaizzo D, et al. Preventing adverse obstetric outcomes in women with genetic thrombophilia. Fertil Steril. 2002;78:371-375.
54. Ridker PM, Miletich JP, Buring JE, et al. Factor V Leiden mutation as a risk factor for recurrent pregnancy loss. Ann Intern Med. 1998;128:1000-1003.
55. Sarig G, Younis J, Hoffman R, et al. Thrombophilia is common in women with idiopathic pregnancy loss and is associated with late pregnancy wastage. Fertil Steril. 2002;77:342-347.
1. Thromboembolism in pregnancy. ACOG Practice Bulletin #19. Washington, DC: ACOG; 2000.
2. Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412-424.
3. Paidas MJ, De-Hui WK, Arkel YS. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 2004;31:783-805.
4. Lee RM, Brown MA, Branch DW, Ward K, Silver RM. Anticardiolipin and anti-B2 glycoprotein-I antibodies in preeclampsia. Obstet Gynecol. 2003;102:294-300.
5. Lin L, August P. Genetic thrombophilias and preeclampsia: a meta-analysis. Obstet Gynecol. 2005;105:182-192.
6. Mignini LE, Latthe PM, Villar J, et al. Mapping the theories of preeclampsia: the role of homocysteine. Obstet Gynecol. 2005;105:411-425.
7. Dekker GA, de Vries JI, Doelitzsch PM, et al. Underlying disorders associated with severe early-onset preeclampsia. Am J Obstet Gynecol. 1995;173:1042-1048.
8. Dizon-Townson D, Miller C, Sibai B, et al. The relationship of factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517-524.
9. Riyazi N, Leeda M, de Vries JIP, et al. Low molecular weight heparin combined with aspirin in pregnant women with thrombophilia and a history of preeclampsia or fetal growth restriction: a preliminary study. Eur J Obstet Gynecol Reprod Biol. 1998;80:49-54.
10. Dizon-Townson DS, Nelson LM, Easton K, Ward K. The factor V Leiden mutation may predispose women to severe preeclampsia. Am J Obstet Gynecol. 1996;175:902-905.
11. Nagy B. Detection of factor V Leiden mutation in severe preeclamptic Hungarian women. Clin Genet. 1998;53:478-481.
12. Krauss T. Activated protein C resistance and factor V Leiden in patients with hemolysis, elevated liver enzymes, low platelets syndrome. Obstet Gynecol. 1998;92:457-460.
13. Kupferminc MJ, Eldor A, Steinman N, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999;341:384.-
14. van Pampus EC. High prevalence of hemostatic abnormalities in women with a history of severe preeclampsia. Am J Obstet Gynecol. 1999;180:1146-1150.
15. DeGroot CJ, Bloemankamp KW, Duvekot EJ, et al. Preeclampsia and genetic factors for thrombosis: a case control study. Am J Obstet Gynecol. 1999;181:975-980.
16. Kupferminc MJ, Fait G, Many A, Girdon D, Eldor A, Lessing JB. Severe preeclampsia: high frequency of genetic thrombophilic mutations. Obstet Gynecol. 2000;96:45-49.
17. Rigo J, Nagy B, Fintor L, et al. Maternal and neonatal outcome of preeclamptic pregnancies: the potential roles of factor V Leiden mutations and 5,10 methylenetetrahydrofolate reductase. Hypertens Pregnancy. 2000;19(2):163-172.
18. von Tempelhoff GF. Incidence of factor V Leiden mutation, coagulation inhibitor deficiency, and elevated antiphospholipid-antibodies in patients with preeclampsia or HELLP syndrome (hemolysis, elevated liver enzymes, low platelets). Thromb Res. 2000;100:363-365.
19. Kupferminc MJ, Peri H, Zwang E, et al. High prevalence of the prothrombin gene mutation in women with intrauterine growth retardation, abruptio placentae and second trimester loss. Acta Obstet Gynecol Scand. 2000;79:963-967.
20. Kim YJ. Genetic susceptibility to preeclampsia: roles of cytosine-to-thymine substitution at nucleotide 677 of the gene for methylenetetrahydrofolate reductase, 68-base pair insertion at nucleotide 844 of the gene for cystathione [beta]-synthase, and factor V Leiden mutation. Am J Obstet Gynecol. 2001;184:1211-1217.
21. Livingston J, Barton JR, Park V, et al. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:153-157.
22. Currie L, Peek M, McNiven M, et al. Is there an increased maternal-infant prevalence of factor V Leiden in association with severe pre-eclampsia? BJOG. 2002;109:191-196.
23. Benedetto C, Marozio L, Salton L, et al. Factor V Leiden and factor II G20210A in preeclampsia and HELLP syndrome. Acta Obstet Gynecol. 2002;81:1095-1100.
24. Schlembach D, Beinder E, Zingsem J, et al. Association of maternal and/or fetal factor V Leiden and G20210A prothrombin mutation with HELLP syndrome and intrauterine growth restriction. Clin Sci. 2003;105:279-285.
25. Mello G, Parretti E, Marozio L, et al. Thrombophilia is significantly associated with severe preeclampsia: results of a large-scale, case-controlled study. Hypertension. 2005;46:1270-1274.
26. Branch DW, Andres R, Digre KB, Rote NS, Scott JR. The association of antiphospholipid antibodies with severe preeclampsia. Obstet Gynecol. 1989;73:541-545.
27. Dreyfus M, Hedelin G, Kutnahorsky R, et al. Antiphospholipid antibodies and preeclampsia: a case-control study. Obstet Gynecol. 2001;97:29-34.
28. Kupferminc MJ, Eldor A, Steinman N, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999;340:9-13.
29. Prochazka M, Happach C, Marsal K, Dahlback B, Lindqvist PG. Factor V Leiden in pregnancies complicated by placental abruption. BJOG. 2003;110:462-466.
30. Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19-25.
31. Howley HE, Walker M, Rodger MA. A systematic review of the association between factor V Leiden or prothrombin gene variant and intrauterine growth restriction. Am J Obstet Gynecol. 2005;192:694-708.
32. Martinelli I, Taioli E, Cetin I, et al. Mutations in coagulation factors in women with unexplained late fetal loss. N Engl J Med. 2000;343:1015-1018.
33. Rey E, Kahn SR, David M, et al. Thrombophilic disorders and fetal loss: a metaanalysis. Lancet. 2003;361:901-908.
34. Kupferminc MJ. Mid-trimester severe intrauterine growth restriction is associated with high prevalence of thrombophilia. BJOG. 2002;109:1373-1376.
35. Sibai BM, el-Nazer A, Gonzalez-Ruiz A. Severe preeclampsia-eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986;155:1011-1016.
36. Sibai BM, Mercer B, Sarinoglu C. Severe preeclampsia in the second trimester: recurrence risk and long-term prognosis. Am J Obstet Gynecol. 1991;165:1408-1412.
37. Brenner B. Thrombophilia and fetal loss. Semin Thromb Hemost. 2003;29:165-170.
38. Kupferminc MJ, Fait G, Many A, et al. Low molecular weight heparin for the prevention of obstetric complications in women with thrombophilias. Hypertens Pregnancy. 2001;20:35-44.
39. Cowchock FS, Reece EA, Balaban D, et al. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am J Obstet Gynecol. 1992;166:1318-1323.
40. Laskin CA, Bombardier C, Hannah ME, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. N Engl J Med. 1997;337:148-154.
41. Kutteh WH. Antiphospholipid antibody-associated recurrent pregnancy loss: treatment with heparin and low-dose aspirin is superior to low-dose aspirin alone. Am J Obstet Gynecol. 1996;174:1584-1589.
42. Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ. 1997;314:253-257.
43. Silver RK, MacGregor SN, Sholl JS, et al. Comparative trial of prednisone versus aspirin alone in the treatment of anticardiolipin antibody-positive obstetric patients. Am J Obstet Gynecol. 1993;169:1411-1417.
44. Pattison NS, Chamley LW, Birdsall M, et al. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. Am J Obstet Gynecol. 2000;183:1008-1012.
45. Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol. 2002;100:408-413.
46. Antiphospholipid syndrome. ACOG Practice Bulletin #68. Obstet Gynecol. 2005;106:1113-1121.
47. Gris JC, Mercier E, Quere I, et al. Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood. 2004;103:3695-3699.
48. Ogueh O, Chen MF, Spurll G, Benjamin A. Outcome of pregnancy in women with hereditary thrombophilia. Int J Gynecol Obstet. 2001;74:247-253.
49. Brenner B, Hoffman R, Blumenfeld Z, et al. Gestational outcome in thrombophilic women with recurrent pregnancy loss treated with enoxaparin. Thromb Haemost. 2000;83:693-697.
50. Kupferminc MJ, Fait G, Many A, et al. Low molecular weight heparin for the prevention of obstetric complications in women with thrombophilias. Hypertens Pregnancy. 2001;20:35-44.
51. Verspyck E, Borg JY, Le Cam-Duchez V, et al. Thrombophilia and fetal growth restriction. Eur J Obstet Gynecol Reprod Biol. 2004;113:36-40.
52. McCowan LME, Craigie S, Taylor RS, et al. Inherited thrombophilias are not increased in “idiopathic” small-for-gestationalage pregnancies. Am J Obstet Gynecol. 2003;188:981-992.
53. Grandone E, Brancaccio V, Colaizzo D, et al. Preventing adverse obstetric outcomes in women with genetic thrombophilia. Fertil Steril. 2002;78:371-375.
54. Ridker PM, Miletich JP, Buring JE, et al. Factor V Leiden mutation as a risk factor for recurrent pregnancy loss. Ann Intern Med. 1998;128:1000-1003.
55. Sarig G, Younis J, Hoffman R, et al. Thrombophilia is common in women with idiopathic pregnancy loss and is associated with late pregnancy wastage. Fertil Steril. 2002;77:342-347.
Controlling chronic hypertension in pregnancy
One unhappy effect of the obesity epidemic and the increasing age of women at childbirth is the rising prevalence of chronic hypertension, which climbed from 4.6% to 22.3% in women aged 30 to 39 years, and from 0.6% to 2.0% in women aged 18 to 29 years, according to the National Health and Nutrition Examination Survey for 1988–1991. These trends are expected to continue, and so are the rates of chronic hypertension in pregnancy, with its increased possibility of super-imposed preeclampsia.
This article outlines diagnosis and management, including:
- how to tell when drug therapy is needed
- how to detect superimposed preeclampsia
- when to discontinue drug regimens
- which drugs and doses should be used during pregnancy and after delivery.
When is hypertension “chronic”?
Hypertension is chronic if the elevated blood pressure was documented before pregnancy. If prepregnancy blood pressure is unknown, the patient is thought to have chronic hypertension if it is consistently elevated before 20 weeks of gestation.
Blood pressure is elevated if systolic pressure is at least 140 mm Hg or diastolic pressure is at least 90 mm Hg. These blood pressure ranges should be documented on at least 2 occasions at least 4 hours apart.1
Diagnosis may be difficult in women with previously undiagnosed chronic hypertension who begin prenatal care after 16 weeks’ gestation, because a physiologic decrease in blood pressure usually begins at that time. These women are more likely to be erroneously diagnosed as having gestational hypertension.2
Chronic hypertension is primary (essential) in approximately 80% to 90% of cases, and, in 10% to 20% of cases, secondary to collagen vascular disease, or renal, endocrine, or vascular disorders.
Outside of pregnancy, hypertension is categorized into 3 stages: prehypertension, stage 1 hypertension, and stage 2 hypertension.3
Mild vs severe, low-risk vs high-risk
During pregnancy, chronic hypertension is classified according to its severity, depending on the systolic and diastolic blood pressures. Systolic pressures of at least 160 mm Hg and/or diastolic pressures of at least 110 mm Hg constitute severe hypertension (Korotkoff phase V). The diagnosis requires documented evidence of hypertension before pregnancy and/or before 20 weeks’ gestation.
Korotkoff phase V readings are more precise. This phase occurs when the sound disappears, as opposed to phase IV, in which the sound is muffled. Phase V is more accurate because it correlates with actual intra-arterial pressure. Moreover, phase IV cannot be recorded in at least 10% of gravidas because of hemodynamic changes of pregnancy.
Low vs high risk. For management and counseling purposes, chronic hypertension in pregnancy also is classified as low- or high-risk (TABLE 1). A gravida has a low risk when she has mild essential hypertension without any organ involvement.
Blood pressure criteria are based on measurements at the initial visit regardless of whether the patient is taking antihypertensive drugs. For example, if the patient has blood pressure of 140/80 mm Hg and is taking antihypertensive agents, she is nevertheless classified as low-risk. Her medications are discontinued, and blood pressure is monitored very closely. If readings reach severe levels, she is then classified as high-risk and managed as such.
Risk classification may change. A woman initially classified as low-risk early in pregnancy may become high-risk if preeclampsia or severe hypertension develops.
TABLE 1
Low- and high-risk criteria
| LOW RISK | HIGH RISK |
|---|---|
| Uncomplicated essential hypertension | Secondary hypertension |
| Target organ damage* | |
| No previous perinatal loss | Previous perinatal loss |
| Systolic pressure <160 mm Hg and diastolic pressure <110 mm Hg | Maternal age >40 years |
| Systolic pressure ≥160 mm Hg or diastolic pressure ≥110 mm Hg | |
| *Left ventricular dysfunction, retinopathy, dyslipidemia, microvascular disease, or stroke. | |
Risk factors for preeclampsia
Pregnancies complicated by chronic hypertension carry a heightened risk of superimposed preeclampsia, which is associated with high rates of adverse maternal and perinatal outcomes.4 Sibai and colleagues4 documented the rate of superimposed preeclampsia among 763 women with chronic hypertension who were followed prospectively at several medical centers in the United States. The overall rate of superimposed preeclampsia was 25%.
Specific characteristics affected the risk of preeclampsia: age, previous preeclampsia, duration of hypertension, diastolic blood pressure, thrombophilia, diabetes, proteinuria, multifetal gestation, and use of assisted reproductive technology (TABLE 2).
Diagnostic criteria
In women with hypertension only, superimposed preeclampsia is diagnosed when there is proteinuria of at least 500 mg in 24 hours or thrombocytopenia or abnormal liver enzymes (TABLE 3).
In women with hypertension and proteinuria (renal disease or class F diabetes), new onset of persistent symptoms (severe headache, visual changes) and/or thrombocytopenia, and/or elevated liver enzymes makes the diagnosis of preeclampsia.
Risk of abruption and other complications
Gravidas with chronic hypertension also have an increased risk for abruptio placentae.
In addition, women with high-risk chronic hypertension are at increased risk for life-threatening maternal complications such as pulmonary edema, hypertensive encephalopathy, retinopathy, cerebral hemorrhage, and acute renal failure.5 These risks are particularly acute in women with uncontrolled severe hypertension, renal dysfunction early in pregnancy, or left ventricular dysfunction prior to conception. The risk of these and other complications increases further when superimposed preeclampsia develops (TABLE 4).
Fetal and neonatal complications in women with chronic hypertension are 3 to 4 times more likely than in the general obstetric population.1 These complications include premature delivery and small-for-gestational-age infants (TABLE 5).
Benefits vs risks of drug treatment
Although long-term blood pressure control greatly reduces stroke, cardiovascular morbidity, and mortality in nonpregnant persons,3 hypertension in pregnancy is different because the duration of therapy is shorter. In people with mild to moderate hypertension, the benefit is achieved after at least 5 years of treatment.2 In pregnancy, the benefits to the mother may not be obvious during the short time of treatment, and exposure to drugs includes both mother and fetus.6 Thus, in pregnancy, one must balance the potential short-term maternal benefits against possible short- and long-term benefits and risks to the fetus and infant.1,5,6
Low-risk: No benefit
We lack compelling evidence that shortterm antihypertensive therapy is beneficial in these women except for a reduction in the exacerbation of hypertension.5,7
High-risk: Drug therapy is needed
We lack placebo-controlled trials of antihypertensive therapy in gravidas with severe hypertension, and none are likely to be performed because of the potential risks of untreated severe hypertension.
In these women, drug therapy reduces the acute risk of stroke, congestive heart failure, and renal failure.2 Control of severe hypertension may also prolong the pregnancy and thereby improve perinatal outcome. However, there is no evidence that control of severe hypertension reduces the rates of superimposed preeclampsia or abruptio placentae.2,4,5
Adverse effects
The potential adverse effects of the most commonly prescribed antihypertensive agents are poorly established or unclearly quantified.1 In general, we have limited and selective information about teratogenicity except in laboratory animals, and minimal data on the benefits and risks of most antihypertensive drugs when used during pregnancy. Nevertheless, the limited data available suggest that some drugs carry the potential for adverse fetal effects and should be avoided (TABLE 6).
Chronic hypertension heightens risk of placental abruption
Gravidas with chronic hypertension are at increased risk for abruptio placentae, which ranges from 0.7% to 1.5% in women with mild chronic hypertension, and from 5% to 10% in women with severe or high-risk hypertension. The rate increases to 30% with superimposed preeclampsia.
Drug treatment of comorbidities
According to data from clinical trials in nonpregnant subjects, selected comorbidities can be treated as follows:
- Ischemic heart disease. Beta-blockers are the first line of treatment, particularly labetalol. Alternatively, calciumchannel blockers can be used.
- Heart failure. In asymptomatic gravidas, beta-blockers should be used. In symptomatic gravidas, both beta-blockers and diuretics are recommended.
- Diabetes. Two or more drugs are usually needed to control blood pressure in this population. Although angiotensin-converting enzyme (ACE) inhibitors have a beneficial effect outside of pregnancy, calcium-channel blockers and diuretics are safer for gravidas.
- Chronic kidney disease warrants aggressive management, typically with 3 or more medications. Again, while ACE inhibitors have a favorable effect outside of pregnancy, calcium-channel blockers, beta-blockers, and diuretics are better choices.
ACE inhibitors are contraindicated in pregnancy due to the risk of oligohydramnios, renal dysplasia, pulmonary hypoplasia, and intrauterine growth restriction.8
Management goals
The primary objectives in managing chronic hypertension in pregnancy are to reduce maternal risks and achieve optimal perinatal survival. These objectives call for a rational approach that includes:
- preconception education and counseling,
- early antenatal care,
- frequent antepartum visits to monitor both mother and fetus,
- timely delivery with intensive intrapartum monitoring, and
- proper postpartum care.
Ideally, management should begin prior to pregnancy, with extensive evaluation and a complete workup to assess the cause and severity of the hypertension, determine whether other medical illnesses are present, and rule out target organ damage associated with longstanding hypertension (TABLE 7).
TABLE 2
Characteristics that affect risk of preeclampsia
| CHARACTERISTIC | PREECLAMPSIA (%) |
|---|---|
| Age (yr) | |
| ≤35 | 26 |
| >35 | 25 |
| Previous preeclampsia | |
| Yes | 32 |
| No | 23 |
| Duration of hypertension | |
| <4 years | 23 |
| ≥4 years | 32 |
| Diastolic blood pressure (mm Hg) | |
| <100 | 24 |
| 100-109 | 25 |
| ≥110 | 40-50 |
| Thrombophilia | 40-50 |
| Diabetes mellitus | 30-40 |
| Proteinuria | |
| No | 25 |
| Yes | 27 |
| Note: Risk is also increased in women with multifetal gestation and in those who have conceived using assisted reproductive technology. | |
| Source: Sibai BM, et al4 | |
Low-risk hypertension
Stop drugs at first visit
Women with low-risk chronic hypertension without superimposed preeclampsia usually have pregnancy outcomes similar to those in the general obstetric population.2,5,9
Discontinuation of antihypertensive therapy early in pregnancy does not increase the rates of preeclampsia, abruptio placentae, and preterm delivery in these women.2,9
Our policy is to discontinue antihypertensive treatment in low-risk women at the first prenatal visit, because most of these women have good outcomes without such therapy.
Follow-up strategy
During subsequent visits, we educate the patient about nutritional requirements, weight gain, and sodium intake (maximum of 2.4 g sodium per day). We also remind them that alcohol use and smoking during pregnancy can aggravate maternal hypertension and cause adverse effects in the fetus such as fetal growth restriction and abruptio placentae.
During the remainder of the pregnancy, we observe the gravida very closely for appropriate fetal growth and early signs of preeclampsia.
Fetal evaluation should include an ultrasound examination at 16 to 20 weeks’ gestation, to be repeated at 32 to 34 weeks and monthly thereafter until term. In addition, all women with low-risk hypertension should undergo growth scans starting at 32 to 34 weeks, especially obese women in whom fundal height measurements are unreliable, because of the increased risk of intrauterine growth restriction.
If severe hypertension develops before term, start either nifedipine or labetalol (TABLE 6).
Immediate fetal testing with the nonstress test or biophysical profile is necessary if severe hypertension, preeclampsia, abnormal fetal growth, or evidence of oligohydramnios develops.
Hospitalization and delivery are necessary if severe hypertension, fetal growth restriction documented by ultrasound, or superimposed preeclampsia develops at or beyond 37 weeks.
If none of these complications is present, pregnancy can continue until 40 weeks’ gestation.5
TABLE 3
Diagnosis of preeclampsia in women with preexisting conditions
| PREEXISTING CONDITION | PREECLAMPSIA IS PRESENT IF SHE HAS… |
|---|---|
| Hypertension | Proteinuria ≥500 mg/24 hours or thrombocytopenia or abnormal liver enzymes |
| Proteinuria | New onset hypertension plus symptoms and/or thrombocytopenia or elevated liver enzymes |
| Hypertension plus proteinuria (renal disease or class F diabetes) | New onset of persistent symptoms (severe headache, visual changes) or thrombocytopenia or elevated liver enzymes |
TABLE 4
Complication rates in women with superimposed preeclampsia vs women without hypertension*
| COMPLICATION | WITHOUT HYPERTENSION (PER 1,000 CASES) | PREECLAMPSIA SUPERIMPOSED ON CHRONIC HYPERTENSION (PER 1,000 CASES) |
|---|---|---|
| Abruptio placentae | 9.6 | 30.6 |
| Thrombocytopenia | 1.6 | 11.5 |
| Disseminated intravascular coagulation | 2.9 | 17.4 |
| Pulmonary edema | 0.2 | 6.4 |
| Blood transfusion | 1.5 | 16.3 |
| Mechanical ventilation | 0.2 | 17.0 |
| *US women, 1988–1997 | ||
| Source: Zhang J, et al15 | ||
High-risk hypertension
The frequency and nature of maternalfetal adverse effects depends on the cause of the hypertension and the extent of target organ damage.
Realistic preconception counseling
Women with substantial renal insufficiency (ie, serum creatinine >1.4 mg/dL), diabetes with vascular involvement (class R/F), severe collagen vascular disease, cardiomyopathy, or coarctation of the aorta should be advised that the pregnancy might exacerbate their condition. These patients should be made aware of the potential for congestive heart failure, acute renal failure requiring dialysis, and even death. In addition, perinatal loss and neonatal complications are markedly increased in these women.
Refer or consult a specialist
All women with severe hypertension should be managed in consultation with a subspecialist in maternal-fetal medicine, as well as any other specialists who may be indicated.
They also should be observed and delivered at a tertiary care center with adequate maternal-neonatal care facilities.5
TABLE 5
Adverse pregnancy outcomes in women with mild chronic hypertension
| OBSERVATIONAL STUDY | PREECLAMPSIA (%) | ABRUPTIO PLACENTAE (%) | DELIVERY AT <37 WEEKS (%) | SMALL FOR GESTATIONAL AGE (%) |
|---|---|---|---|---|
| Sibai et al2 (n=211) | 10.0 | 1.4 | 12.0 | 8.0 |
| Rey and Couturier16 (n=337) | 21.0 | 0.7 | 34.4 | 15.5 |
| McCowan et al17 (n=142) | 14.0 | Not reported | 16.0 | 11.0 |
| Sibai et al4 (n=763) | 25.0 | 1.5 | 33.3 | 11.1 |
| August et al18 (n=110) | 34.0 | Not reported | Not reported | 8.0 |
Management strategy
Our policy is to hospitalize women with high-risk hypertension at the time of the first prenatal visit to evaluate their cardiovascular and renal status and regulate antihypertensive medications, as well as other prescribed drugs (eg, insulin, cardiac drugs, thyroid drugs). Women receiving atenolol, ACE inhibitors, or angiotensin II receptor antagonists should have these medications discontinued under close observation.
In women without target organ damage, the aim of antihypertensive therapy is to keep systolic pressure between 140 and 150 mm Hg and diastolic pressure between 90 and 100 mm Hg.
In women with target organ damage and mild hypertension, antihypertensive therapy is also indicated, because there are short-term maternal benefits to lowering blood pressure. We recommend keeping systolic pressure below 140 mm Hg and diastolic pressure below 90 mm Hg.
Early, frequent visits.Women with high-risk chronic hypertension need close observation throughout pregnancy and may require serial evaluation of 24-hour urine protein excretion and a complete blood count with a metabolic profile at least once every trimester. Further laboratory testing depends on the clinical progress of the pregnancy. At each visit, remind the woman about the adverse effects of smoking and alcohol use, and counsel her about the importance of diet and minimal salt intake.5
Fetal surveillance includes ultrasound, growth scans, and nonstress testing (TABLE 8).
Hospitalization is warranted if uncontrolled severe hypertension, preeclampsia, or evidence of fetal growth restriction develops, so that more frequent evaluation of maternal and fetal well-being can be performed.
Delivery is indicated if any of these complications develop at or beyond 34 weeks’ gestation. If there are none of these complications, consider delivery at 36 to 37 weeks after documenting fetal lung maturity.5
Postpartum care
Women with high-risk chronic hypertension are at risk for postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure.10,11 These risks are heightened in women with target organ involvement, superimposed preeclampsia, or abruptio placentae.10
Blood pressure must be closely controlled for at least 48 hours after delivery. Intravenous labetalol or hydralazine can be used as needed, and diuretics may be appropriate in women with circulatory congestion and pulmonary edema.12 Oral therapy may be needed to control blood pressure after delivery. In some women, it may be necessary to switch to a new agent such as an ACE inhibitor, particularly in women who had pregestational diabetes or cardiomyopathy.
All antihypertensive drugs are found in breast milk, although differences in the milk-to-plasma ratio do occur. The longterm effects of maternal antihypertensive drugs on breastfeeding infants has not been studied. However, methyldopa appears to be a reasonable first-line oral therapy (if it is contraindicated, use labetalol). Milk concentrations of methyldopa appear to be low and are considered safe. Beta-blockers (atenolol and metoprolol) are concentrated in breast milk, whereas labetalol or propanolol have low concentrations.13,14 Concentrations of diuretics in breast milk are low, but may diminish milk production.13 Little is known about the transfer of calcium-channel blockers to breast milk, but there are no apparent side effects. ACE inhibitors and angiotensin II receptor antagonists should be avoided because of their effects on neonatal renal function, even though their concentrations in breast milk appear to be low.
The authors report no financial relationships relevant to this article.
TABLE 6
Acute and long-term drug treatment
| DRUG | STARTING DOSE | MAXIMUM DOSE | COMMENTS |
|---|---|---|---|
| ACUTE TREATMENT OF SEVERE HYPERTENSION | |||
| Hydralazine | 5-10 mg IV every 20 min | 30 mg* | |
| Labetalol | 20-40 mg IV every 10-15 min | 220 mg* | Avoid in women with asthma or congestive heart failure |
| Nifedipine | 10-20 mg orally every 30 min | 50 mg* | |
| LONG-TERM TREATMENT OF HYPERTENSION | |||
| Methyldopa | 250 mg BID | 4 g/day | Rarely indicated |
| Labetalol | 100 mg BID | 2,400 mg/day | First choice |
| Atenolol | 50 mg/day | 100 mg/day | Associated with intrauterine growth restriction |
| Propanolol | 40 mg BID | 640 mg/day | Use with associated thyroid disease |
| Hydralazine | 10 mg TID | 100 mg/day | Use in cases of left ventricular hypertrophy |
| Nifedipine | 10 mg BID | 120 mg/day | Use in women with diabetes |
| Diltiazem | 120-180 mg/day | 540 mg/day | |
| Thiazide diuretic | 12.5 mg BID | 50 mg/day | Use in salt-sensitive hypertension and/or congestive heart failure |
| May be added as second agent | |||
| Avoid if preeclampsia develops or intrauterine growth restriction is present | |||
| Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers | — | — | Do not use after 16-18 weeks |
| *If desired blood pressure levels are not achieved, switch to another drug. | |||
TABLE 7
How to evaluate gravidas with chronic hypertension
| POPULATION | RECOMMENDED TESTS |
|---|---|
| All |
|
| Gravidas with longstanding hypertension, poor compliance, or poor control |
|
TABLE 8
Recommended antenatal testing
| LEVEL OF RISK | TEST |
|---|---|
| Low (uncomplicated) |
|
| High (complicated) |
|
1. Ferrer RL, Sibai BM, Murlow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy:a review. Obstet Gynecol. 2000;96:849-860.
2. Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571-576.
3. Chobanian AB, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003;289:2560-2572.
4. Sibai BM, Lindheimer M, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. N Engl J Med. 1998;339:667-671.
5. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
6. Umans JG, Lindheimer MD. Antihypertensive treatment. In:Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Norwalk, Conn:Appleton and Lange; 1998:581-604.
7. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. In: The Cochrane Library, Issue 12, 2003. Oxford:Update Software.
8. How HY, Sibai BM. Use of angiotensin-converting enzyme inhibitors in patients with diabetic nephropathy. J Matern Fetal Neonatal Med. 2002;12:402-407.
9. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-966.
10. Sibai BM, Villar MA, Mabie BC. Acute renal failure in hypertensive disorders of pregnancy:pregnancy outcome and remote prognosis in thirty-one consecutive cases. Am J Obstet Gynecol. 1990;62:777.-
11. Mabie WC, Ratts TE, Ramanathan KB, Sibai BM. Circulatory congestion in obese hypertensive women:a subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553-558.
12. Lie RT, Rasmussen S, Brunborg H, et al. Fetal and maternal contributions to risk of pre-eclampsia:a population based study. Br Med J. 1998;316:1343-1347.
13. Briggs GG, Freeman RK, Yaffee SJ. Drugs in Pregnancy and Lactation:A Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore:Williams & Wilkins;1998.
14. White WB. Management of hypertension during lactation. Hypertension. 1984;6:297-300.
15. Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203-212.
16. Rey E, Couturier A. The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol. 1994;171:410-416.
17. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123-129.
18. August P, Helseth G, Cook EF, Silson C. A prediction model for superimposed preeclampsia in women with chronic hypertension during pregnancy. Am J Obstet Gynecol. 2004;191:1666-1672.
One unhappy effect of the obesity epidemic and the increasing age of women at childbirth is the rising prevalence of chronic hypertension, which climbed from 4.6% to 22.3% in women aged 30 to 39 years, and from 0.6% to 2.0% in women aged 18 to 29 years, according to the National Health and Nutrition Examination Survey for 1988–1991. These trends are expected to continue, and so are the rates of chronic hypertension in pregnancy, with its increased possibility of super-imposed preeclampsia.
This article outlines diagnosis and management, including:
- how to tell when drug therapy is needed
- how to detect superimposed preeclampsia
- when to discontinue drug regimens
- which drugs and doses should be used during pregnancy and after delivery.
When is hypertension “chronic”?
Hypertension is chronic if the elevated blood pressure was documented before pregnancy. If prepregnancy blood pressure is unknown, the patient is thought to have chronic hypertension if it is consistently elevated before 20 weeks of gestation.
Blood pressure is elevated if systolic pressure is at least 140 mm Hg or diastolic pressure is at least 90 mm Hg. These blood pressure ranges should be documented on at least 2 occasions at least 4 hours apart.1
Diagnosis may be difficult in women with previously undiagnosed chronic hypertension who begin prenatal care after 16 weeks’ gestation, because a physiologic decrease in blood pressure usually begins at that time. These women are more likely to be erroneously diagnosed as having gestational hypertension.2
Chronic hypertension is primary (essential) in approximately 80% to 90% of cases, and, in 10% to 20% of cases, secondary to collagen vascular disease, or renal, endocrine, or vascular disorders.
Outside of pregnancy, hypertension is categorized into 3 stages: prehypertension, stage 1 hypertension, and stage 2 hypertension.3
Mild vs severe, low-risk vs high-risk
During pregnancy, chronic hypertension is classified according to its severity, depending on the systolic and diastolic blood pressures. Systolic pressures of at least 160 mm Hg and/or diastolic pressures of at least 110 mm Hg constitute severe hypertension (Korotkoff phase V). The diagnosis requires documented evidence of hypertension before pregnancy and/or before 20 weeks’ gestation.
Korotkoff phase V readings are more precise. This phase occurs when the sound disappears, as opposed to phase IV, in which the sound is muffled. Phase V is more accurate because it correlates with actual intra-arterial pressure. Moreover, phase IV cannot be recorded in at least 10% of gravidas because of hemodynamic changes of pregnancy.
Low vs high risk. For management and counseling purposes, chronic hypertension in pregnancy also is classified as low- or high-risk (TABLE 1). A gravida has a low risk when she has mild essential hypertension without any organ involvement.
Blood pressure criteria are based on measurements at the initial visit regardless of whether the patient is taking antihypertensive drugs. For example, if the patient has blood pressure of 140/80 mm Hg and is taking antihypertensive agents, she is nevertheless classified as low-risk. Her medications are discontinued, and blood pressure is monitored very closely. If readings reach severe levels, she is then classified as high-risk and managed as such.
Risk classification may change. A woman initially classified as low-risk early in pregnancy may become high-risk if preeclampsia or severe hypertension develops.
TABLE 1
Low- and high-risk criteria
| LOW RISK | HIGH RISK |
|---|---|
| Uncomplicated essential hypertension | Secondary hypertension |
| Target organ damage* | |
| No previous perinatal loss | Previous perinatal loss |
| Systolic pressure <160 mm Hg and diastolic pressure <110 mm Hg | Maternal age >40 years |
| Systolic pressure ≥160 mm Hg or diastolic pressure ≥110 mm Hg | |
| *Left ventricular dysfunction, retinopathy, dyslipidemia, microvascular disease, or stroke. | |
Risk factors for preeclampsia
Pregnancies complicated by chronic hypertension carry a heightened risk of superimposed preeclampsia, which is associated with high rates of adverse maternal and perinatal outcomes.4 Sibai and colleagues4 documented the rate of superimposed preeclampsia among 763 women with chronic hypertension who were followed prospectively at several medical centers in the United States. The overall rate of superimposed preeclampsia was 25%.
Specific characteristics affected the risk of preeclampsia: age, previous preeclampsia, duration of hypertension, diastolic blood pressure, thrombophilia, diabetes, proteinuria, multifetal gestation, and use of assisted reproductive technology (TABLE 2).
Diagnostic criteria
In women with hypertension only, superimposed preeclampsia is diagnosed when there is proteinuria of at least 500 mg in 24 hours or thrombocytopenia or abnormal liver enzymes (TABLE 3).
In women with hypertension and proteinuria (renal disease or class F diabetes), new onset of persistent symptoms (severe headache, visual changes) and/or thrombocytopenia, and/or elevated liver enzymes makes the diagnosis of preeclampsia.
Risk of abruption and other complications
Gravidas with chronic hypertension also have an increased risk for abruptio placentae.
In addition, women with high-risk chronic hypertension are at increased risk for life-threatening maternal complications such as pulmonary edema, hypertensive encephalopathy, retinopathy, cerebral hemorrhage, and acute renal failure.5 These risks are particularly acute in women with uncontrolled severe hypertension, renal dysfunction early in pregnancy, or left ventricular dysfunction prior to conception. The risk of these and other complications increases further when superimposed preeclampsia develops (TABLE 4).
Fetal and neonatal complications in women with chronic hypertension are 3 to 4 times more likely than in the general obstetric population.1 These complications include premature delivery and small-for-gestational-age infants (TABLE 5).
Benefits vs risks of drug treatment
Although long-term blood pressure control greatly reduces stroke, cardiovascular morbidity, and mortality in nonpregnant persons,3 hypertension in pregnancy is different because the duration of therapy is shorter. In people with mild to moderate hypertension, the benefit is achieved after at least 5 years of treatment.2 In pregnancy, the benefits to the mother may not be obvious during the short time of treatment, and exposure to drugs includes both mother and fetus.6 Thus, in pregnancy, one must balance the potential short-term maternal benefits against possible short- and long-term benefits and risks to the fetus and infant.1,5,6
Low-risk: No benefit
We lack compelling evidence that shortterm antihypertensive therapy is beneficial in these women except for a reduction in the exacerbation of hypertension.5,7
High-risk: Drug therapy is needed
We lack placebo-controlled trials of antihypertensive therapy in gravidas with severe hypertension, and none are likely to be performed because of the potential risks of untreated severe hypertension.
In these women, drug therapy reduces the acute risk of stroke, congestive heart failure, and renal failure.2 Control of severe hypertension may also prolong the pregnancy and thereby improve perinatal outcome. However, there is no evidence that control of severe hypertension reduces the rates of superimposed preeclampsia or abruptio placentae.2,4,5
Adverse effects
The potential adverse effects of the most commonly prescribed antihypertensive agents are poorly established or unclearly quantified.1 In general, we have limited and selective information about teratogenicity except in laboratory animals, and minimal data on the benefits and risks of most antihypertensive drugs when used during pregnancy. Nevertheless, the limited data available suggest that some drugs carry the potential for adverse fetal effects and should be avoided (TABLE 6).
Chronic hypertension heightens risk of placental abruption
Gravidas with chronic hypertension are at increased risk for abruptio placentae, which ranges from 0.7% to 1.5% in women with mild chronic hypertension, and from 5% to 10% in women with severe or high-risk hypertension. The rate increases to 30% with superimposed preeclampsia.
Drug treatment of comorbidities
According to data from clinical trials in nonpregnant subjects, selected comorbidities can be treated as follows:
- Ischemic heart disease. Beta-blockers are the first line of treatment, particularly labetalol. Alternatively, calciumchannel blockers can be used.
- Heart failure. In asymptomatic gravidas, beta-blockers should be used. In symptomatic gravidas, both beta-blockers and diuretics are recommended.
- Diabetes. Two or more drugs are usually needed to control blood pressure in this population. Although angiotensin-converting enzyme (ACE) inhibitors have a beneficial effect outside of pregnancy, calcium-channel blockers and diuretics are safer for gravidas.
- Chronic kidney disease warrants aggressive management, typically with 3 or more medications. Again, while ACE inhibitors have a favorable effect outside of pregnancy, calcium-channel blockers, beta-blockers, and diuretics are better choices.
ACE inhibitors are contraindicated in pregnancy due to the risk of oligohydramnios, renal dysplasia, pulmonary hypoplasia, and intrauterine growth restriction.8
Management goals
The primary objectives in managing chronic hypertension in pregnancy are to reduce maternal risks and achieve optimal perinatal survival. These objectives call for a rational approach that includes:
- preconception education and counseling,
- early antenatal care,
- frequent antepartum visits to monitor both mother and fetus,
- timely delivery with intensive intrapartum monitoring, and
- proper postpartum care.
Ideally, management should begin prior to pregnancy, with extensive evaluation and a complete workup to assess the cause and severity of the hypertension, determine whether other medical illnesses are present, and rule out target organ damage associated with longstanding hypertension (TABLE 7).
TABLE 2
Characteristics that affect risk of preeclampsia
| CHARACTERISTIC | PREECLAMPSIA (%) |
|---|---|
| Age (yr) | |
| ≤35 | 26 |
| >35 | 25 |
| Previous preeclampsia | |
| Yes | 32 |
| No | 23 |
| Duration of hypertension | |
| <4 years | 23 |
| ≥4 years | 32 |
| Diastolic blood pressure (mm Hg) | |
| <100 | 24 |
| 100-109 | 25 |
| ≥110 | 40-50 |
| Thrombophilia | 40-50 |
| Diabetes mellitus | 30-40 |
| Proteinuria | |
| No | 25 |
| Yes | 27 |
| Note: Risk is also increased in women with multifetal gestation and in those who have conceived using assisted reproductive technology. | |
| Source: Sibai BM, et al4 | |
Low-risk hypertension
Stop drugs at first visit
Women with low-risk chronic hypertension without superimposed preeclampsia usually have pregnancy outcomes similar to those in the general obstetric population.2,5,9
Discontinuation of antihypertensive therapy early in pregnancy does not increase the rates of preeclampsia, abruptio placentae, and preterm delivery in these women.2,9
Our policy is to discontinue antihypertensive treatment in low-risk women at the first prenatal visit, because most of these women have good outcomes without such therapy.
Follow-up strategy
During subsequent visits, we educate the patient about nutritional requirements, weight gain, and sodium intake (maximum of 2.4 g sodium per day). We also remind them that alcohol use and smoking during pregnancy can aggravate maternal hypertension and cause adverse effects in the fetus such as fetal growth restriction and abruptio placentae.
During the remainder of the pregnancy, we observe the gravida very closely for appropriate fetal growth and early signs of preeclampsia.
Fetal evaluation should include an ultrasound examination at 16 to 20 weeks’ gestation, to be repeated at 32 to 34 weeks and monthly thereafter until term. In addition, all women with low-risk hypertension should undergo growth scans starting at 32 to 34 weeks, especially obese women in whom fundal height measurements are unreliable, because of the increased risk of intrauterine growth restriction.
If severe hypertension develops before term, start either nifedipine or labetalol (TABLE 6).
Immediate fetal testing with the nonstress test or biophysical profile is necessary if severe hypertension, preeclampsia, abnormal fetal growth, or evidence of oligohydramnios develops.
Hospitalization and delivery are necessary if severe hypertension, fetal growth restriction documented by ultrasound, or superimposed preeclampsia develops at or beyond 37 weeks.
If none of these complications is present, pregnancy can continue until 40 weeks’ gestation.5
TABLE 3
Diagnosis of preeclampsia in women with preexisting conditions
| PREEXISTING CONDITION | PREECLAMPSIA IS PRESENT IF SHE HAS… |
|---|---|
| Hypertension | Proteinuria ≥500 mg/24 hours or thrombocytopenia or abnormal liver enzymes |
| Proteinuria | New onset hypertension plus symptoms and/or thrombocytopenia or elevated liver enzymes |
| Hypertension plus proteinuria (renal disease or class F diabetes) | New onset of persistent symptoms (severe headache, visual changes) or thrombocytopenia or elevated liver enzymes |
TABLE 4
Complication rates in women with superimposed preeclampsia vs women without hypertension*
| COMPLICATION | WITHOUT HYPERTENSION (PER 1,000 CASES) | PREECLAMPSIA SUPERIMPOSED ON CHRONIC HYPERTENSION (PER 1,000 CASES) |
|---|---|---|
| Abruptio placentae | 9.6 | 30.6 |
| Thrombocytopenia | 1.6 | 11.5 |
| Disseminated intravascular coagulation | 2.9 | 17.4 |
| Pulmonary edema | 0.2 | 6.4 |
| Blood transfusion | 1.5 | 16.3 |
| Mechanical ventilation | 0.2 | 17.0 |
| *US women, 1988–1997 | ||
| Source: Zhang J, et al15 | ||
High-risk hypertension
The frequency and nature of maternalfetal adverse effects depends on the cause of the hypertension and the extent of target organ damage.
Realistic preconception counseling
Women with substantial renal insufficiency (ie, serum creatinine >1.4 mg/dL), diabetes with vascular involvement (class R/F), severe collagen vascular disease, cardiomyopathy, or coarctation of the aorta should be advised that the pregnancy might exacerbate their condition. These patients should be made aware of the potential for congestive heart failure, acute renal failure requiring dialysis, and even death. In addition, perinatal loss and neonatal complications are markedly increased in these women.
Refer or consult a specialist
All women with severe hypertension should be managed in consultation with a subspecialist in maternal-fetal medicine, as well as any other specialists who may be indicated.
They also should be observed and delivered at a tertiary care center with adequate maternal-neonatal care facilities.5
TABLE 5
Adverse pregnancy outcomes in women with mild chronic hypertension
| OBSERVATIONAL STUDY | PREECLAMPSIA (%) | ABRUPTIO PLACENTAE (%) | DELIVERY AT <37 WEEKS (%) | SMALL FOR GESTATIONAL AGE (%) |
|---|---|---|---|---|
| Sibai et al2 (n=211) | 10.0 | 1.4 | 12.0 | 8.0 |
| Rey and Couturier16 (n=337) | 21.0 | 0.7 | 34.4 | 15.5 |
| McCowan et al17 (n=142) | 14.0 | Not reported | 16.0 | 11.0 |
| Sibai et al4 (n=763) | 25.0 | 1.5 | 33.3 | 11.1 |
| August et al18 (n=110) | 34.0 | Not reported | Not reported | 8.0 |
Management strategy
Our policy is to hospitalize women with high-risk hypertension at the time of the first prenatal visit to evaluate their cardiovascular and renal status and regulate antihypertensive medications, as well as other prescribed drugs (eg, insulin, cardiac drugs, thyroid drugs). Women receiving atenolol, ACE inhibitors, or angiotensin II receptor antagonists should have these medications discontinued under close observation.
In women without target organ damage, the aim of antihypertensive therapy is to keep systolic pressure between 140 and 150 mm Hg and diastolic pressure between 90 and 100 mm Hg.
In women with target organ damage and mild hypertension, antihypertensive therapy is also indicated, because there are short-term maternal benefits to lowering blood pressure. We recommend keeping systolic pressure below 140 mm Hg and diastolic pressure below 90 mm Hg.
Early, frequent visits.Women with high-risk chronic hypertension need close observation throughout pregnancy and may require serial evaluation of 24-hour urine protein excretion and a complete blood count with a metabolic profile at least once every trimester. Further laboratory testing depends on the clinical progress of the pregnancy. At each visit, remind the woman about the adverse effects of smoking and alcohol use, and counsel her about the importance of diet and minimal salt intake.5
Fetal surveillance includes ultrasound, growth scans, and nonstress testing (TABLE 8).
Hospitalization is warranted if uncontrolled severe hypertension, preeclampsia, or evidence of fetal growth restriction develops, so that more frequent evaluation of maternal and fetal well-being can be performed.
Delivery is indicated if any of these complications develop at or beyond 34 weeks’ gestation. If there are none of these complications, consider delivery at 36 to 37 weeks after documenting fetal lung maturity.5
Postpartum care
Women with high-risk chronic hypertension are at risk for postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure.10,11 These risks are heightened in women with target organ involvement, superimposed preeclampsia, or abruptio placentae.10
Blood pressure must be closely controlled for at least 48 hours after delivery. Intravenous labetalol or hydralazine can be used as needed, and diuretics may be appropriate in women with circulatory congestion and pulmonary edema.12 Oral therapy may be needed to control blood pressure after delivery. In some women, it may be necessary to switch to a new agent such as an ACE inhibitor, particularly in women who had pregestational diabetes or cardiomyopathy.
All antihypertensive drugs are found in breast milk, although differences in the milk-to-plasma ratio do occur. The longterm effects of maternal antihypertensive drugs on breastfeeding infants has not been studied. However, methyldopa appears to be a reasonable first-line oral therapy (if it is contraindicated, use labetalol). Milk concentrations of methyldopa appear to be low and are considered safe. Beta-blockers (atenolol and metoprolol) are concentrated in breast milk, whereas labetalol or propanolol have low concentrations.13,14 Concentrations of diuretics in breast milk are low, but may diminish milk production.13 Little is known about the transfer of calcium-channel blockers to breast milk, but there are no apparent side effects. ACE inhibitors and angiotensin II receptor antagonists should be avoided because of their effects on neonatal renal function, even though their concentrations in breast milk appear to be low.
The authors report no financial relationships relevant to this article.
TABLE 6
Acute and long-term drug treatment
| DRUG | STARTING DOSE | MAXIMUM DOSE | COMMENTS |
|---|---|---|---|
| ACUTE TREATMENT OF SEVERE HYPERTENSION | |||
| Hydralazine | 5-10 mg IV every 20 min | 30 mg* | |
| Labetalol | 20-40 mg IV every 10-15 min | 220 mg* | Avoid in women with asthma or congestive heart failure |
| Nifedipine | 10-20 mg orally every 30 min | 50 mg* | |
| LONG-TERM TREATMENT OF HYPERTENSION | |||
| Methyldopa | 250 mg BID | 4 g/day | Rarely indicated |
| Labetalol | 100 mg BID | 2,400 mg/day | First choice |
| Atenolol | 50 mg/day | 100 mg/day | Associated with intrauterine growth restriction |
| Propanolol | 40 mg BID | 640 mg/day | Use with associated thyroid disease |
| Hydralazine | 10 mg TID | 100 mg/day | Use in cases of left ventricular hypertrophy |
| Nifedipine | 10 mg BID | 120 mg/day | Use in women with diabetes |
| Diltiazem | 120-180 mg/day | 540 mg/day | |
| Thiazide diuretic | 12.5 mg BID | 50 mg/day | Use in salt-sensitive hypertension and/or congestive heart failure |
| May be added as second agent | |||
| Avoid if preeclampsia develops or intrauterine growth restriction is present | |||
| Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers | — | — | Do not use after 16-18 weeks |
| *If desired blood pressure levels are not achieved, switch to another drug. | |||
TABLE 7
How to evaluate gravidas with chronic hypertension
| POPULATION | RECOMMENDED TESTS |
|---|---|
| All |
|
| Gravidas with longstanding hypertension, poor compliance, or poor control |
|
TABLE 8
Recommended antenatal testing
| LEVEL OF RISK | TEST |
|---|---|
| Low (uncomplicated) |
|
| High (complicated) |
|
One unhappy effect of the obesity epidemic and the increasing age of women at childbirth is the rising prevalence of chronic hypertension, which climbed from 4.6% to 22.3% in women aged 30 to 39 years, and from 0.6% to 2.0% in women aged 18 to 29 years, according to the National Health and Nutrition Examination Survey for 1988–1991. These trends are expected to continue, and so are the rates of chronic hypertension in pregnancy, with its increased possibility of super-imposed preeclampsia.
This article outlines diagnosis and management, including:
- how to tell when drug therapy is needed
- how to detect superimposed preeclampsia
- when to discontinue drug regimens
- which drugs and doses should be used during pregnancy and after delivery.
When is hypertension “chronic”?
Hypertension is chronic if the elevated blood pressure was documented before pregnancy. If prepregnancy blood pressure is unknown, the patient is thought to have chronic hypertension if it is consistently elevated before 20 weeks of gestation.
Blood pressure is elevated if systolic pressure is at least 140 mm Hg or diastolic pressure is at least 90 mm Hg. These blood pressure ranges should be documented on at least 2 occasions at least 4 hours apart.1
Diagnosis may be difficult in women with previously undiagnosed chronic hypertension who begin prenatal care after 16 weeks’ gestation, because a physiologic decrease in blood pressure usually begins at that time. These women are more likely to be erroneously diagnosed as having gestational hypertension.2
Chronic hypertension is primary (essential) in approximately 80% to 90% of cases, and, in 10% to 20% of cases, secondary to collagen vascular disease, or renal, endocrine, or vascular disorders.
Outside of pregnancy, hypertension is categorized into 3 stages: prehypertension, stage 1 hypertension, and stage 2 hypertension.3
Mild vs severe, low-risk vs high-risk
During pregnancy, chronic hypertension is classified according to its severity, depending on the systolic and diastolic blood pressures. Systolic pressures of at least 160 mm Hg and/or diastolic pressures of at least 110 mm Hg constitute severe hypertension (Korotkoff phase V). The diagnosis requires documented evidence of hypertension before pregnancy and/or before 20 weeks’ gestation.
Korotkoff phase V readings are more precise. This phase occurs when the sound disappears, as opposed to phase IV, in which the sound is muffled. Phase V is more accurate because it correlates with actual intra-arterial pressure. Moreover, phase IV cannot be recorded in at least 10% of gravidas because of hemodynamic changes of pregnancy.
Low vs high risk. For management and counseling purposes, chronic hypertension in pregnancy also is classified as low- or high-risk (TABLE 1). A gravida has a low risk when she has mild essential hypertension without any organ involvement.
Blood pressure criteria are based on measurements at the initial visit regardless of whether the patient is taking antihypertensive drugs. For example, if the patient has blood pressure of 140/80 mm Hg and is taking antihypertensive agents, she is nevertheless classified as low-risk. Her medications are discontinued, and blood pressure is monitored very closely. If readings reach severe levels, she is then classified as high-risk and managed as such.
Risk classification may change. A woman initially classified as low-risk early in pregnancy may become high-risk if preeclampsia or severe hypertension develops.
TABLE 1
Low- and high-risk criteria
| LOW RISK | HIGH RISK |
|---|---|
| Uncomplicated essential hypertension | Secondary hypertension |
| Target organ damage* | |
| No previous perinatal loss | Previous perinatal loss |
| Systolic pressure <160 mm Hg and diastolic pressure <110 mm Hg | Maternal age >40 years |
| Systolic pressure ≥160 mm Hg or diastolic pressure ≥110 mm Hg | |
| *Left ventricular dysfunction, retinopathy, dyslipidemia, microvascular disease, or stroke. | |
Risk factors for preeclampsia
Pregnancies complicated by chronic hypertension carry a heightened risk of superimposed preeclampsia, which is associated with high rates of adverse maternal and perinatal outcomes.4 Sibai and colleagues4 documented the rate of superimposed preeclampsia among 763 women with chronic hypertension who were followed prospectively at several medical centers in the United States. The overall rate of superimposed preeclampsia was 25%.
Specific characteristics affected the risk of preeclampsia: age, previous preeclampsia, duration of hypertension, diastolic blood pressure, thrombophilia, diabetes, proteinuria, multifetal gestation, and use of assisted reproductive technology (TABLE 2).
Diagnostic criteria
In women with hypertension only, superimposed preeclampsia is diagnosed when there is proteinuria of at least 500 mg in 24 hours or thrombocytopenia or abnormal liver enzymes (TABLE 3).
In women with hypertension and proteinuria (renal disease or class F diabetes), new onset of persistent symptoms (severe headache, visual changes) and/or thrombocytopenia, and/or elevated liver enzymes makes the diagnosis of preeclampsia.
Risk of abruption and other complications
Gravidas with chronic hypertension also have an increased risk for abruptio placentae.
In addition, women with high-risk chronic hypertension are at increased risk for life-threatening maternal complications such as pulmonary edema, hypertensive encephalopathy, retinopathy, cerebral hemorrhage, and acute renal failure.5 These risks are particularly acute in women with uncontrolled severe hypertension, renal dysfunction early in pregnancy, or left ventricular dysfunction prior to conception. The risk of these and other complications increases further when superimposed preeclampsia develops (TABLE 4).
Fetal and neonatal complications in women with chronic hypertension are 3 to 4 times more likely than in the general obstetric population.1 These complications include premature delivery and small-for-gestational-age infants (TABLE 5).
Benefits vs risks of drug treatment
Although long-term blood pressure control greatly reduces stroke, cardiovascular morbidity, and mortality in nonpregnant persons,3 hypertension in pregnancy is different because the duration of therapy is shorter. In people with mild to moderate hypertension, the benefit is achieved after at least 5 years of treatment.2 In pregnancy, the benefits to the mother may not be obvious during the short time of treatment, and exposure to drugs includes both mother and fetus.6 Thus, in pregnancy, one must balance the potential short-term maternal benefits against possible short- and long-term benefits and risks to the fetus and infant.1,5,6
Low-risk: No benefit
We lack compelling evidence that shortterm antihypertensive therapy is beneficial in these women except for a reduction in the exacerbation of hypertension.5,7
High-risk: Drug therapy is needed
We lack placebo-controlled trials of antihypertensive therapy in gravidas with severe hypertension, and none are likely to be performed because of the potential risks of untreated severe hypertension.
In these women, drug therapy reduces the acute risk of stroke, congestive heart failure, and renal failure.2 Control of severe hypertension may also prolong the pregnancy and thereby improve perinatal outcome. However, there is no evidence that control of severe hypertension reduces the rates of superimposed preeclampsia or abruptio placentae.2,4,5
Adverse effects
The potential adverse effects of the most commonly prescribed antihypertensive agents are poorly established or unclearly quantified.1 In general, we have limited and selective information about teratogenicity except in laboratory animals, and minimal data on the benefits and risks of most antihypertensive drugs when used during pregnancy. Nevertheless, the limited data available suggest that some drugs carry the potential for adverse fetal effects and should be avoided (TABLE 6).
Chronic hypertension heightens risk of placental abruption
Gravidas with chronic hypertension are at increased risk for abruptio placentae, which ranges from 0.7% to 1.5% in women with mild chronic hypertension, and from 5% to 10% in women with severe or high-risk hypertension. The rate increases to 30% with superimposed preeclampsia.
Drug treatment of comorbidities
According to data from clinical trials in nonpregnant subjects, selected comorbidities can be treated as follows:
- Ischemic heart disease. Beta-blockers are the first line of treatment, particularly labetalol. Alternatively, calciumchannel blockers can be used.
- Heart failure. In asymptomatic gravidas, beta-blockers should be used. In symptomatic gravidas, both beta-blockers and diuretics are recommended.
- Diabetes. Two or more drugs are usually needed to control blood pressure in this population. Although angiotensin-converting enzyme (ACE) inhibitors have a beneficial effect outside of pregnancy, calcium-channel blockers and diuretics are safer for gravidas.
- Chronic kidney disease warrants aggressive management, typically with 3 or more medications. Again, while ACE inhibitors have a favorable effect outside of pregnancy, calcium-channel blockers, beta-blockers, and diuretics are better choices.
ACE inhibitors are contraindicated in pregnancy due to the risk of oligohydramnios, renal dysplasia, pulmonary hypoplasia, and intrauterine growth restriction.8
Management goals
The primary objectives in managing chronic hypertension in pregnancy are to reduce maternal risks and achieve optimal perinatal survival. These objectives call for a rational approach that includes:
- preconception education and counseling,
- early antenatal care,
- frequent antepartum visits to monitor both mother and fetus,
- timely delivery with intensive intrapartum monitoring, and
- proper postpartum care.
Ideally, management should begin prior to pregnancy, with extensive evaluation and a complete workup to assess the cause and severity of the hypertension, determine whether other medical illnesses are present, and rule out target organ damage associated with longstanding hypertension (TABLE 7).
TABLE 2
Characteristics that affect risk of preeclampsia
| CHARACTERISTIC | PREECLAMPSIA (%) |
|---|---|
| Age (yr) | |
| ≤35 | 26 |
| >35 | 25 |
| Previous preeclampsia | |
| Yes | 32 |
| No | 23 |
| Duration of hypertension | |
| <4 years | 23 |
| ≥4 years | 32 |
| Diastolic blood pressure (mm Hg) | |
| <100 | 24 |
| 100-109 | 25 |
| ≥110 | 40-50 |
| Thrombophilia | 40-50 |
| Diabetes mellitus | 30-40 |
| Proteinuria | |
| No | 25 |
| Yes | 27 |
| Note: Risk is also increased in women with multifetal gestation and in those who have conceived using assisted reproductive technology. | |
| Source: Sibai BM, et al4 | |
Low-risk hypertension
Stop drugs at first visit
Women with low-risk chronic hypertension without superimposed preeclampsia usually have pregnancy outcomes similar to those in the general obstetric population.2,5,9
Discontinuation of antihypertensive therapy early in pregnancy does not increase the rates of preeclampsia, abruptio placentae, and preterm delivery in these women.2,9
Our policy is to discontinue antihypertensive treatment in low-risk women at the first prenatal visit, because most of these women have good outcomes without such therapy.
Follow-up strategy
During subsequent visits, we educate the patient about nutritional requirements, weight gain, and sodium intake (maximum of 2.4 g sodium per day). We also remind them that alcohol use and smoking during pregnancy can aggravate maternal hypertension and cause adverse effects in the fetus such as fetal growth restriction and abruptio placentae.
During the remainder of the pregnancy, we observe the gravida very closely for appropriate fetal growth and early signs of preeclampsia.
Fetal evaluation should include an ultrasound examination at 16 to 20 weeks’ gestation, to be repeated at 32 to 34 weeks and monthly thereafter until term. In addition, all women with low-risk hypertension should undergo growth scans starting at 32 to 34 weeks, especially obese women in whom fundal height measurements are unreliable, because of the increased risk of intrauterine growth restriction.
If severe hypertension develops before term, start either nifedipine or labetalol (TABLE 6).
Immediate fetal testing with the nonstress test or biophysical profile is necessary if severe hypertension, preeclampsia, abnormal fetal growth, or evidence of oligohydramnios develops.
Hospitalization and delivery are necessary if severe hypertension, fetal growth restriction documented by ultrasound, or superimposed preeclampsia develops at or beyond 37 weeks.
If none of these complications is present, pregnancy can continue until 40 weeks’ gestation.5
TABLE 3
Diagnosis of preeclampsia in women with preexisting conditions
| PREEXISTING CONDITION | PREECLAMPSIA IS PRESENT IF SHE HAS… |
|---|---|
| Hypertension | Proteinuria ≥500 mg/24 hours or thrombocytopenia or abnormal liver enzymes |
| Proteinuria | New onset hypertension plus symptoms and/or thrombocytopenia or elevated liver enzymes |
| Hypertension plus proteinuria (renal disease or class F diabetes) | New onset of persistent symptoms (severe headache, visual changes) or thrombocytopenia or elevated liver enzymes |
TABLE 4
Complication rates in women with superimposed preeclampsia vs women without hypertension*
| COMPLICATION | WITHOUT HYPERTENSION (PER 1,000 CASES) | PREECLAMPSIA SUPERIMPOSED ON CHRONIC HYPERTENSION (PER 1,000 CASES) |
|---|---|---|
| Abruptio placentae | 9.6 | 30.6 |
| Thrombocytopenia | 1.6 | 11.5 |
| Disseminated intravascular coagulation | 2.9 | 17.4 |
| Pulmonary edema | 0.2 | 6.4 |
| Blood transfusion | 1.5 | 16.3 |
| Mechanical ventilation | 0.2 | 17.0 |
| *US women, 1988–1997 | ||
| Source: Zhang J, et al15 | ||
High-risk hypertension
The frequency and nature of maternalfetal adverse effects depends on the cause of the hypertension and the extent of target organ damage.
Realistic preconception counseling
Women with substantial renal insufficiency (ie, serum creatinine >1.4 mg/dL), diabetes with vascular involvement (class R/F), severe collagen vascular disease, cardiomyopathy, or coarctation of the aorta should be advised that the pregnancy might exacerbate their condition. These patients should be made aware of the potential for congestive heart failure, acute renal failure requiring dialysis, and even death. In addition, perinatal loss and neonatal complications are markedly increased in these women.
Refer or consult a specialist
All women with severe hypertension should be managed in consultation with a subspecialist in maternal-fetal medicine, as well as any other specialists who may be indicated.
They also should be observed and delivered at a tertiary care center with adequate maternal-neonatal care facilities.5
TABLE 5
Adverse pregnancy outcomes in women with mild chronic hypertension
| OBSERVATIONAL STUDY | PREECLAMPSIA (%) | ABRUPTIO PLACENTAE (%) | DELIVERY AT <37 WEEKS (%) | SMALL FOR GESTATIONAL AGE (%) |
|---|---|---|---|---|
| Sibai et al2 (n=211) | 10.0 | 1.4 | 12.0 | 8.0 |
| Rey and Couturier16 (n=337) | 21.0 | 0.7 | 34.4 | 15.5 |
| McCowan et al17 (n=142) | 14.0 | Not reported | 16.0 | 11.0 |
| Sibai et al4 (n=763) | 25.0 | 1.5 | 33.3 | 11.1 |
| August et al18 (n=110) | 34.0 | Not reported | Not reported | 8.0 |
Management strategy
Our policy is to hospitalize women with high-risk hypertension at the time of the first prenatal visit to evaluate their cardiovascular and renal status and regulate antihypertensive medications, as well as other prescribed drugs (eg, insulin, cardiac drugs, thyroid drugs). Women receiving atenolol, ACE inhibitors, or angiotensin II receptor antagonists should have these medications discontinued under close observation.
In women without target organ damage, the aim of antihypertensive therapy is to keep systolic pressure between 140 and 150 mm Hg and diastolic pressure between 90 and 100 mm Hg.
In women with target organ damage and mild hypertension, antihypertensive therapy is also indicated, because there are short-term maternal benefits to lowering blood pressure. We recommend keeping systolic pressure below 140 mm Hg and diastolic pressure below 90 mm Hg.
Early, frequent visits.Women with high-risk chronic hypertension need close observation throughout pregnancy and may require serial evaluation of 24-hour urine protein excretion and a complete blood count with a metabolic profile at least once every trimester. Further laboratory testing depends on the clinical progress of the pregnancy. At each visit, remind the woman about the adverse effects of smoking and alcohol use, and counsel her about the importance of diet and minimal salt intake.5
Fetal surveillance includes ultrasound, growth scans, and nonstress testing (TABLE 8).
Hospitalization is warranted if uncontrolled severe hypertension, preeclampsia, or evidence of fetal growth restriction develops, so that more frequent evaluation of maternal and fetal well-being can be performed.
Delivery is indicated if any of these complications develop at or beyond 34 weeks’ gestation. If there are none of these complications, consider delivery at 36 to 37 weeks after documenting fetal lung maturity.5
Postpartum care
Women with high-risk chronic hypertension are at risk for postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure.10,11 These risks are heightened in women with target organ involvement, superimposed preeclampsia, or abruptio placentae.10
Blood pressure must be closely controlled for at least 48 hours after delivery. Intravenous labetalol or hydralazine can be used as needed, and diuretics may be appropriate in women with circulatory congestion and pulmonary edema.12 Oral therapy may be needed to control blood pressure after delivery. In some women, it may be necessary to switch to a new agent such as an ACE inhibitor, particularly in women who had pregestational diabetes or cardiomyopathy.
All antihypertensive drugs are found in breast milk, although differences in the milk-to-plasma ratio do occur. The longterm effects of maternal antihypertensive drugs on breastfeeding infants has not been studied. However, methyldopa appears to be a reasonable first-line oral therapy (if it is contraindicated, use labetalol). Milk concentrations of methyldopa appear to be low and are considered safe. Beta-blockers (atenolol and metoprolol) are concentrated in breast milk, whereas labetalol or propanolol have low concentrations.13,14 Concentrations of diuretics in breast milk are low, but may diminish milk production.13 Little is known about the transfer of calcium-channel blockers to breast milk, but there are no apparent side effects. ACE inhibitors and angiotensin II receptor antagonists should be avoided because of their effects on neonatal renal function, even though their concentrations in breast milk appear to be low.
The authors report no financial relationships relevant to this article.
TABLE 6
Acute and long-term drug treatment
| DRUG | STARTING DOSE | MAXIMUM DOSE | COMMENTS |
|---|---|---|---|
| ACUTE TREATMENT OF SEVERE HYPERTENSION | |||
| Hydralazine | 5-10 mg IV every 20 min | 30 mg* | |
| Labetalol | 20-40 mg IV every 10-15 min | 220 mg* | Avoid in women with asthma or congestive heart failure |
| Nifedipine | 10-20 mg orally every 30 min | 50 mg* | |
| LONG-TERM TREATMENT OF HYPERTENSION | |||
| Methyldopa | 250 mg BID | 4 g/day | Rarely indicated |
| Labetalol | 100 mg BID | 2,400 mg/day | First choice |
| Atenolol | 50 mg/day | 100 mg/day | Associated with intrauterine growth restriction |
| Propanolol | 40 mg BID | 640 mg/day | Use with associated thyroid disease |
| Hydralazine | 10 mg TID | 100 mg/day | Use in cases of left ventricular hypertrophy |
| Nifedipine | 10 mg BID | 120 mg/day | Use in women with diabetes |
| Diltiazem | 120-180 mg/day | 540 mg/day | |
| Thiazide diuretic | 12.5 mg BID | 50 mg/day | Use in salt-sensitive hypertension and/or congestive heart failure |
| May be added as second agent | |||
| Avoid if preeclampsia develops or intrauterine growth restriction is present | |||
| Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers | — | — | Do not use after 16-18 weeks |
| *If desired blood pressure levels are not achieved, switch to another drug. | |||
TABLE 7
How to evaluate gravidas with chronic hypertension
| POPULATION | RECOMMENDED TESTS |
|---|---|
| All |
|
| Gravidas with longstanding hypertension, poor compliance, or poor control |
|
TABLE 8
Recommended antenatal testing
| LEVEL OF RISK | TEST |
|---|---|
| Low (uncomplicated) |
|
| High (complicated) |
|
1. Ferrer RL, Sibai BM, Murlow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy:a review. Obstet Gynecol. 2000;96:849-860.
2. Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571-576.
3. Chobanian AB, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003;289:2560-2572.
4. Sibai BM, Lindheimer M, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. N Engl J Med. 1998;339:667-671.
5. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
6. Umans JG, Lindheimer MD. Antihypertensive treatment. In:Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Norwalk, Conn:Appleton and Lange; 1998:581-604.
7. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. In: The Cochrane Library, Issue 12, 2003. Oxford:Update Software.
8. How HY, Sibai BM. Use of angiotensin-converting enzyme inhibitors in patients with diabetic nephropathy. J Matern Fetal Neonatal Med. 2002;12:402-407.
9. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-966.
10. Sibai BM, Villar MA, Mabie BC. Acute renal failure in hypertensive disorders of pregnancy:pregnancy outcome and remote prognosis in thirty-one consecutive cases. Am J Obstet Gynecol. 1990;62:777.-
11. Mabie WC, Ratts TE, Ramanathan KB, Sibai BM. Circulatory congestion in obese hypertensive women:a subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553-558.
12. Lie RT, Rasmussen S, Brunborg H, et al. Fetal and maternal contributions to risk of pre-eclampsia:a population based study. Br Med J. 1998;316:1343-1347.
13. Briggs GG, Freeman RK, Yaffee SJ. Drugs in Pregnancy and Lactation:A Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore:Williams & Wilkins;1998.
14. White WB. Management of hypertension during lactation. Hypertension. 1984;6:297-300.
15. Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203-212.
16. Rey E, Couturier A. The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol. 1994;171:410-416.
17. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123-129.
18. August P, Helseth G, Cook EF, Silson C. A prediction model for superimposed preeclampsia in women with chronic hypertension during pregnancy. Am J Obstet Gynecol. 2004;191:1666-1672.
1. Ferrer RL, Sibai BM, Murlow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy:a review. Obstet Gynecol. 2000;96:849-860.
2. Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571-576.
3. Chobanian AB, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003;289:2560-2572.
4. Sibai BM, Lindheimer M, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. N Engl J Med. 1998;339:667-671.
5. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
6. Umans JG, Lindheimer MD. Antihypertensive treatment. In:Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Norwalk, Conn:Appleton and Lange; 1998:581-604.
7. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. In: The Cochrane Library, Issue 12, 2003. Oxford:Update Software.
8. How HY, Sibai BM. Use of angiotensin-converting enzyme inhibitors in patients with diabetic nephropathy. J Matern Fetal Neonatal Med. 2002;12:402-407.
9. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-966.
10. Sibai BM, Villar MA, Mabie BC. Acute renal failure in hypertensive disorders of pregnancy:pregnancy outcome and remote prognosis in thirty-one consecutive cases. Am J Obstet Gynecol. 1990;62:777.-
11. Mabie WC, Ratts TE, Ramanathan KB, Sibai BM. Circulatory congestion in obese hypertensive women:a subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553-558.
12. Lie RT, Rasmussen S, Brunborg H, et al. Fetal and maternal contributions to risk of pre-eclampsia:a population based study. Br Med J. 1998;316:1343-1347.
13. Briggs GG, Freeman RK, Yaffee SJ. Drugs in Pregnancy and Lactation:A Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore:Williams & Wilkins;1998.
14. White WB. Management of hypertension during lactation. Hypertension. 1984;6:297-300.
15. Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203-212.
16. Rey E, Couturier A. The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol. 1994;171:410-416.
17. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123-129.
18. August P, Helseth G, Cook EF, Silson C. A prediction model for superimposed preeclampsia in women with chronic hypertension during pregnancy. Am J Obstet Gynecol. 2004;191:1666-1672.
Managing an eclamptic patient
An eclamptic convulsion is frightening to behold. First, the woman’s face becomes distorted, and her eyes protrude. Then her face acquires a congested expression, and foam may exude from her mouth. Breathing stops.
Because eclampsia is so frightening, the natural tendency is to try to stop a convulsion, but this is not the wisest strategy.
Rather, the foremost priorities are to avoid maternal injury and support cardiovascular functions. How to do this, and how to prevent further convulsions, monitor and deliver the fetus, and avert complications are the focus of this article.
Since eclampsia may be fatal for both mother and fetus, all labor and delivery units and all obstetricians should be prepared to diagnose and manage this grave threat. However, few obstetric units encounter more than 1 or 2 cases a year; most obstetricians have little or no experience managing acute eclampsia. In the Western world, it affects only 1 in 2,000 to 1 in 3,448 pregnancies.1-4
How a convulsion happens
Most convulsions occur in 2 phases and last for 60 to 75 seconds. The first phase, lasting 15 to 20 seconds, begins with facial twitching, soon followed by a rigid body with generalized muscular contractions.
In the second phase, which lasts about a minute, the muscles of the body alternately contract and relax in rapid succession. This phase begins with the muscles of the jaw and rapidly encompasses eyelids, other facial muscles, and body. If the tongue is unprotected, the woman often bites it.
Coma sometimes follows the convulsion, and deep, rapid breathing usually begins as soon as the convulsion stops. In fact, maintaining oxygenation typically is not a problem after a single convulsion, and the risk of aspiration is low in a well managed patient.
Upon reviving, the woman typically remembers nothing about the seizure.
If convulsions recur, some degree of consciousness returns after each one, although the woman may become combative, agitated, and difficult to control.
Harbingers of complications
In the developed world, eclampsia increases the risk of maternal death (range: 0 to 1.8%).1-5 A recent review of all reported pregnancy-related deaths in the United States from 1979 to 1992 found 4,024 cases.6 Of these, 790 (19.6%) were due to preeclampsia-eclampsia, 49% of which were caused by eclampsia. The risk of death from preeclampsia or eclampsia was higher for the following groups:
- women over 30,
- no prenatal care,
- African Americans, and
- onset of preeclampsia or eclampsia before 28 weeks.6
Maternal morbidity
Pregnancies complicated by eclampsia also have higher rates of maternal morbidity such as pulmonary edema and HELLP syndrome (TABLE 1). Complications are substantially higher among women who develop antepartum eclampsia, especially when it is remote from term.1-3
TABLE 1
Maternal complications
Antepartum eclampsia, especially when it is remote from term, is much more likely to lead to complications
| COMPLICATION | RATE (%) | REMARKS |
|---|---|---|
| Death | 0.5-–2 | Risk of death is higher:
|
| Intracerebral hemorrhage | <1 | Usually related to several risk factors |
| Aspiration pneumonia | 2–3 | Heightened risk of maternal hypoxemia and acidosis |
| Disseminated coagulopathy | 3–5 | Regional anesthesia is contraindicated in these patients, and there is a heightened risk of hemorrhagic shock |
| Pulmonary edema | 3–5 | Heightened risk of maternal hypoxemia and acidosis |
| Acute renal failure | 5–9 | Usually seen in association with abruptio placentae, maternal hemorrhage, and prolonged maternal hypotension |
| Abruptio placentae | 7–10 | Can occur after a convulsion; suspect it if fetal bradycardia or late decelerations persist |
| HELLP syndrome | 10–15 |
Adverse perinatal outcomes
Perinatal mortality and morbidity are high in eclampsia, with a perinatal death rate in recent series of 5.6% to 11.8%.1,7 This high rate is related to prematurity, abruptio placentae, and severe growth restriction.1
Preterm deliveries occur in approximately 50% of cases, and about 25% occur before 32 weeks’ gestation.1
Diagnosis can be tricky
When the patient has generalized edema, hypertension, proteinuria, and convulsions, diagnosis of eclampsia is straightforward. Unfortunately, women with eclampsia exhibit a broad spectrum of signs, ranging from severe hypertension, severe proteinuria, and generalized edema, to absent or minimal hypertension, nonexistent proteinuria, and no edema (TABLE 2).1
Hypertension is the hallmark of diagnosis. Hypertension is severe (at least 160 mm Hg systolic and/or at least 110 mm Hg diastolic) in 20% to 54% of cases, and it is mild (systolic pressure between 140 and 160 mm Hg or diastolic pressure between 90 and 110 mm Hg) in 30% to 60% of cases.2,3 In 16% of cases, there may be no hypertension at all.2
Proteinuria. Eclampsia usually is associated with proteinuria (at least 1+ on dipstick).1 However, when I studied a series of 399 women with eclampsia, I found substantial proteinuria (3+ or above on dipstick) in only 48% of cases; proteinuria was absent in 14%.2
Edema. A weight gain of more than 2 lb per week (with or without clinical edema) during the third trimester may be the first sign of eclampsia. However, in my series of 399 women, edema was absent in 26% of cases.2
TABLE 2
Signs and symptoms of eclampsia*
Hypertension and proteinuria in eclampsia may be severe, mild, or even absent
| CONDITION | FREQUENCY (%) IN WOMEN WITH ECLAMPSIA | REMARKS |
|---|---|---|
| SIGNS | ||
| Hypertension | 85 | Should be documented on at least 2 occasions more than 6 hours apart |
| Severe: 160/110 mm Hg or more | 20–54 | |
| Mild: 140–160/90–110 mm Hg | 30–60 | |
| No hypertension2 | 16 | |
| Proteinuria | 85 | |
| At least 1+ on dipstick2 | 48 | |
| At least 3+ on dipstick | 14 | |
| No proteinuria | 15 | |
| SYMPTOMS | ||
| At least 1 of the following: | 33–75 | Clinical symptoms may occur before or after a convulsion |
| Headache | 30–70 | Persistent, occipital, or frontal |
| Right upper quadrant or epigastric pain | 12–20 | |
| Visual changes | 19–32 | Blurred vision, photophobia |
| Altered mental changes | 4–5 | |
| * Summary of 5 series | ||
Symptoms of eclampsia
Several clinical symptoms can occur before or after a convulsion1:
- persistent occipital or frontal headaches,
- blurred vision,
- photophobia,
- epigastric and/or right upper quadrant pain, and
- altered mental status.
Usual times of onset
Eclamptic convulsions can occur during pregnancy or delivery, or after delivery ( TABLE 3).1-5
Approximately 91% of antepartum cases develop at 28 weeks or beyond. The remaining cases tend to occur between 21 and 27 weeks’ gestation (7.5%), or at or before 20 weeks (1.5%).2
When eclampsia occurs before 20 weeks, it usually involves molar or hydropic degeneration of the placenta, with or without a fetus.1 However, eclampsia can occur in the first half of pregnancy without molar degeneration of the placenta, although this is rare.1,2 These women are sometimes misdiagnosed as having hypertensive encephalopathy, seizure disorder, or thrombotic thrombocytopenia purpura. Thus, women who develop convulsions in association with hypertension and proteinuria in the first half of pregnancy should be assumed to have eclampsia until proven otherwise,1 and require ultrasound examination of the uterus to rule out molar pregnancy and/or hydropic or cystic degeneration of the placenta.
Postpartum cases tend to occur within the first 48 hours, although some develop beyond this limit and have been reported as late as 23 days postpartum.8
Late postpartum eclampsia occurs more than 48 hours but less than 4 weeks after delivery.8 These women have signs and symptoms consistent with preeclampsia along with their convulsions.8,9 Thus, women who develop convulsions with hypertension and/or proteinuria, or with headaches or blurred vision, after 48 hours postpartum should be assumed to have eclampsia and treated accordingly.8,9
When eclampsia occurs especially late, perform an extensive neurologic examination to rule out other cerebral pathology.1,10
Eclampsia is atypical if convulsions occur before 20 weeks’ gestation or beyond 48 hours postpartum. It also is atypical if convulsions develop or persist despite adequate magnesium sulfate, or if the patient develops focal neurologic deficits, disorientation, blindness, or coma. In these cases, conduct a neurologic exam and cerebral imaging to exclude neurologic pathology.8-10
TABLE 3
Usual times of onset*
91% of antepartum eclampsia cases occur at 28 weeks or later, although eclamptic convulsions can occur at any time during pregnancy or delivery, or postpartum
| ONSET | FREQUENCY (%) | REMARKS |
|---|---|---|
| Antepartum | 38–53 | Maternal and perinatal mortality, and the incidence of complications and underlying disease, are higher in antepartum eclampsia, especially in early cases |
| ≤20 weeks | 1.5 | |
| 21 to 27 weeks | 7.5 | |
| ≥28 weeks | 91 | |
| Intrapartum | 18–36 | Intrapartum eclampsia more closely resembles postpartum disease than antepartum cases |
| Postpartum | 11–44 | Late postpartum eclampsia occurs more than 48 hours but less than 4 weeks after delivery |
| ≤48 hours | 7–39 | |
| >48 hours | 5–26 | |
| * Summary of 5 series | ||
Differential diagnosis
As with other aspects of preeclampsia, the presenting symptoms, clinical findings, and many laboratory results in eclampsia overlap several other medical and surgical conditions.1,10 Of course, eclampsia is the most common cause of convulsions in a woman with hypertension and/or proteinuria during pregnancy or immediately postpartum. On rare occasions, other causes of convulsions in pregnancy or postpartum may mimic eclampsia.1 These potential diagnoses are particularly important when the woman has focal neurologic deficits, prolonged coma, or atypical eclampsia.
The differential diagnosis encompasses a variety of cerebrovascular and metabolic disorders:
- hemorrhage,
- ruptured aneurysm or malformation,
- arterial embolism, thrombosis,
- venous thrombosis,
- hypoxic ischemic encephalopathy,
- angiomas,
- hypertensive encephalopathy,
- seizure disorder,
- hypoglycemia, hyponatremia,
- posterior leukoencephalopathy syndrome,
- thrombotic thrombocytopenic purpura,
- postdural puncture syndrome, and
- cerebral vasculitis/angiopathy.
Other diseases may factor in
Some women develop gestational hypertension or preeclampsia in association with connective tissue disease, thrombophilias, seizure disorder, or hypertensive encephalopathy, further confounding the diagnosis.
Thus, make every effort to ensure a correct diagnosis, since management may differ among these conditions.
Managing convulsions
Do not try to stop the first convulsion
The natural tendency is to try and interrupt the convulsion, but this is not recommended. Nor should you give a drug such as diazepam to shorten or stop the convulsion, especially if the patient lacks an intravenous line and no one skilled in intubation is immediately available. If diazepam is given, do not exceed 5 mg over 60 seconds. Rapid administration of this drug may lead to apnea or cardiac arrest, or both.
Steps to prevent maternal injury
During or immediately after the acute convulsive episode, take steps to prevent serious maternal injury and aspiration, assess and establish airway patency, and ensure maternal oxygenation (TABLE 4).
Elevate and pad the bed’s side rails and insert a padded tongue blade between the patient’s teeth, taking care not to trigger the gag reflex.
Physical restraints also may be needed.
Prevent aspiration. To minimize the risk of aspiration, place the patient in the lateral decubitus position, and suction vomitus and oral secretions as needed. Be aware that aspiration can occur when the padded tongue blade is forced to the back of the throat, stimulating the gag reflex and resultant vomiting.
TABLE 4
During a convulsion, 3 spheres of concern
| AVOID MATERNAL INJURY |
| Insert padded tongue blade |
| Avoid inducing gag reflex |
| Elevate padded bedside rails |
| Use physical restraints as needed |
| MAINTAIN OXYGENATION TO MOTHER AND FETUS |
| Apply face mask with or without oxygen reservoir at 8–10 L/minute |
| Monitor oxygenation and metabolic status via |
| Transcutaneous pulse oximetry |
| Arterial blood gases (sodium bicarbonate administered accordingly) |
| Correct oxygenation and metabolic status before administering anesthetics that may depress myocardial function |
| MINIMIZE ASPIRATION |
| Place patient in lateral decubitus position (which also maximizes uterine blood flow and venous return) |
| Suction vomitus and oral secretions |
| Obtain chest x-ray after the convulsion is controlled to rule out aspiration |
Tips on supplemental oxygenation
Although the initial seizure lasts only a minute or 2, it is important to maintain oxygenation by giving supplemental oxygen via a face mask, with or without an oxygen reservoir, at 8 to 10 L per minute. This is important because hypoventilation and respiratory acidosis often occur.
Once the convulsion ends and the patient resumes breathing, oxygenation is rarely a problem. However, maternal hypoxemia and acidosis can develop in women with repetitive convulsions, aspiration pneumonia, pulmonary edema, or a combination of these factors. Thus, transcutaneous pulse oximetry is advisable to monitor oxygenation in eclamptic patients.
Arterial blood gas analysis is necessary if pulse oximetry shows abnormal oxygen saturation (ie, at or below 92%).
Strategy to prevent recurrence
Magnesium sulfate is the drug of choice to treat and prevent subsequent convulsions in women with eclampsia.1,2
Dosage. I give a loading dose of 6 g over 15 to 20 minutes, followed by a maintenance dose of 2 g per hour as a continuous intravenous solution.
Approximately 10% of eclamptic women have a second convulsion after receiving magnesium sulfate.1,2 When this occurs, I give another 2-g bolus intravenously over 3 to 5 minutes.
More rarely, a woman will continue to have convulsions while receiving adequate and therapeutic doses of magnesium sulfate.
I treat such patients with sodium amobarbital, 250 mg, intravenously over 3 to 5 minutes.
Monitor maternal magnesium levels
Plasma levels should be in the range of 4 to 8 mg/dL during treatment for eclampsia, and are determined by the volume of distribution and by renal excretion. Thus, it is important to monitor the patient for magnesium toxicity, particularly if she has renal dysfunction (serum creatinine of 1.2 mg/dL or above) or urine output below 100 mL in 4 hours. In these women, adjust the maintenance dose according to plasma levels.
Side effects of magnesium including flushing, a feeling of warmth, nausea and vomiting, double vision, and slurred speech (TABLE 5).
If magnesium toxicity is suspected, immediately discontinue the infusion and administer supplemental oxygen along with 10 mL of 10% calcium gluconate (1 g total) as an intravenous push slowly over 5 minutes.
If respiratory arrest occurs, prompt resuscitation—including intubation and assisted ventilation—is vital.
TABLE 5
Clinical manifestations of magnesium toxicity
Maternal plasma levels of 4 to 8 mg/dL are appropriate during treatment for eclampsia; higher levels signal toxicity
| IF THE MAGNESIUM LEVEL IS… | THE CLINICAL MANIFESTATION IS… |
|---|---|
| 8–12 mg/dL | Loss of patellar reflex |
| Double or blurred vision | |
| Headache and nausea | |
| 9–12 mg/dL | Feeling of warmth, flushing |
| 10–12 mg/dL | Somnolence |
| Slurred speech | |
| 15–17 mg/dL | Muscular paralysis |
| Respiratory arrest | |
| 30–35 mg/dL | Cardiac arrest |
Controlling severe hypertension
The next step is to reduce blood pressure to a safe range. The objective: to preserve cerebral autoregulation and prevent congestive heart failure without compromising cerebral perfusion or jeopardizing uteroplacental blood flow, which is already reduced in many women with eclampsia.1
To these ends, try to keep systolic blood pressure between 140 and 160 mm Hg and diastolic pressure between 90 and 110 mm Hg. This can be achieved with:
- bolus 5- to 10-mg doses of hydralazine,
- 20 to 40 mg labetalol intravenously every 15 minutes, as needed, or
- 10 to 20 mg oral nifedipine every 30 minutes.
Other potent antihypertensive drugs such as sodium nitroprusside or nitroglycerine are rarely needed in eclampsia, and diuretics are indicated only in the presence of pulmonary edema.
Intrapartum management
Maternal hypoxemia and hypercarbia cause fetal heart rate and uterine activity changes during and immediately following a convulsion.
Fetal heart rate changes
These can include bradycardia, transient late decelerations, decreased beat-to-beat variability, and compensatory tachycardia.
The interval from onset of the seizure to the fall in fetal heart rate is approximately 5 minutes (FIGURE 1). Transitory fetal tachycardia frequently occurs after the prolonged bradycardia. The loss of beat-to-beat variability, with transitory late decelerations, occurs during the recovery phase.
The mechanism for the transient fetal bradycardia may be intense vasospasm and uterine hyperactivity, which may decrease uterine blood flow. The absence of maternal respiration during the convulsion may also contribute to fetal heart rate changes.
Since the fetal heart rate usually returns to normal after a convulsion, other conditions should be considered if an abnormal pattern persists.
In some cases, it may take longer for the heart rate pattern to return to baseline if the fetus is preterm with growth restriction.
Placental abruption may occur after the convulsion and should be suspected if fetal bradycardia or repetitive late decelerations persist (FIGURE 2).
FIGURE 1 Fetal response to a convulsion The top 2 tracings show fetal bradycardia during an eclamptic convulsion
FIGURE 2 Abruptio placentae Repetitive late decelerations secondary to abruptio placentae, necessitating cesarean section
Uterine activity
During a convulsion, contractions can increase in frequency and tone. The duration of increased uterine activity varies from 2 to 14 minutes.
These changes usually resolve spontaneously within 3 to 10 minutes following the termination of convulsions and correction of maternal hypoxemia (FIGURE 1).
If uterine hyperactivity persists, suspect placental abruption (FIGURE 2).
Do not rush to cesarean
It benefits the fetus to allow in utero recovery from the maternal convulsion, hypoxia, and hypercarbia before delivery. However, if the bradycardia and/or recurrent late decelerations persist beyond 10 to 15 minutes despite all efforts, suspect abruptio placentae or nonreassuring fetal status.
Once the patient regains consciousness and is oriented to name, place, and time, and her convulsions are controlled and condition stabilized, proceed with delivery.
Choosing a delivery route
Eclampsia is not an indication for cesarean. The decision to perform a cesarean should be based on fetal gestational age, fetal condition, presence of labor, and cervical Bishop score. I recommend:
- Cesarean section for women with eclampsia before 30 weeks’ gestation who are not in labor and whose Bishop score is below 5.
- Vaginal delivery for women in labor or with rupture of membranes, provided there are no obstetric complications.
- Labor induction with oxytocin infusion or prostaglandins in all women at or after 30 weeks, regardless of the Bishop score, and in women before 30 weeks when the Bishop score is 5 or above.
Maternal pain relief
During labor and delivery, systemic opioids or epidural anesthesia can provide pain relief—the same recommendations as for women with severe preeclampsia.
For cesarean delivery, an epidural, spinal, or combined techniques of regional anesthesia are suitable.
Do not use regional anesthesia if there is coagulopathy or severe thrombocytopenia (platelet count less than 50,000/mm3). In women with eclampsia, general anesthesia increases the risk of aspiration and failed intubation due to airway edema, and is associated with marked increases in systemic and cerebral pressures during intubation and extubation.
Women with airway or laryngeal edema may require awake intubation under fiber optic observation, with tracheostomy immediately available.
Changes in systemic or cerebral pressures may be attenuated by pretreatment with labetalol or nitroglycerine injections.
Postpartum management
After delivery, women with eclampsia require close monitoring of vital signs, fluid intake and output, and symptoms for at least 48 hours.
Risk of pulmonary edema
These women usually receive large amounts of intravenous fluids during labor, delivery, and postpartum. In addition, during the postpartum period, extracellular fluid mobilizes, leading to increased intravascular volume.
As a result, women who develop eclampsia—particularly those with abnormal renal function, abruptio placentae, and/or preexisting chronic hypertension— face an increased risk for pulmonary edema and exacerbation of severe hypertension.2
Frequent evaluation of the amount of intravenous fluids is necessary, as well as oral intake, blood products, and urine output. They also need pulse oximetry and pulmonary auscultation.
Continue magnesium sulfate
Parenteral magnesium sulfate should be given for at least 24 hours after delivery and/or for at least 24 hours after the last convulsion.
If the patient has oliguria (less than 100 mL over 4 hours), both fluid administration and the dose of magnesium sulfate should be reduced.
Oral antihypertensives
Other oral antihypertensive agents such as labetalol or nifedipine can be given to keep systolic blood pressure below 155 mm Hg and diastolic blood pressure below 105 mm Hg. Nifedipine offers the benefit of improved diuresis in the postpartum period.
The author reports no financial relationships relevant to this article.
1. Sibai BM. Diagnosis, differential diagnosis, and management of eclampsia. Obstet Gynecol. 2005;105:402-410.
2. Mattar F, Sibai BM. Eclampsia VIII. Risk factors for maternal morbidity. Am J Obstet Gynecol. 2000;182:307-312.
3. Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ. 1994;309:1395-1400.
4. Rugard O, Carling MS, Berg G. Eclampsia at a tertiary hospital, 1973–99. Acta Obstet Gynecol Scand. 2004;83:240-245.
5. Katz VL, Farmer R, Kuller J. Preeclampsia into eclampsia: toward a new paradigm. Am J Obstet Gynecol. 2000;182:1389-1396.
6. MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97:533-538.
7. Leitch CR, Cameron AD, Walker JJ. The changing pattern of eclampsia over a 60-year period. Br J Obstet Gynaecol. 1997;104:917-922.
8. Lubarsky SL, Barton JR, Friedman SA, Nasreddine S, Ramaddan MK, Sibai BM. Late postpartum eclampsia revisited. Obstet Gynaecol. 1994;83:502-505.
9. Chames MC, Livingston JC, Ivester TS, Barton JR, Sibai BM. Late postpartum eclampsia: a preventable disease? Am J Obstet Gynecol. 2002;186:1174-1177.
10. Witlin AG, Friedman SA, Egerman RS, Frangieh AY, Sibai BM. Cerebrovascular disorders complicating pregnancy. Beyond eclampsia. Am J Obstet Gynecol. 1997;176:139-148.
An eclamptic convulsion is frightening to behold. First, the woman’s face becomes distorted, and her eyes protrude. Then her face acquires a congested expression, and foam may exude from her mouth. Breathing stops.
Because eclampsia is so frightening, the natural tendency is to try to stop a convulsion, but this is not the wisest strategy.
Rather, the foremost priorities are to avoid maternal injury and support cardiovascular functions. How to do this, and how to prevent further convulsions, monitor and deliver the fetus, and avert complications are the focus of this article.
Since eclampsia may be fatal for both mother and fetus, all labor and delivery units and all obstetricians should be prepared to diagnose and manage this grave threat. However, few obstetric units encounter more than 1 or 2 cases a year; most obstetricians have little or no experience managing acute eclampsia. In the Western world, it affects only 1 in 2,000 to 1 in 3,448 pregnancies.1-4
How a convulsion happens
Most convulsions occur in 2 phases and last for 60 to 75 seconds. The first phase, lasting 15 to 20 seconds, begins with facial twitching, soon followed by a rigid body with generalized muscular contractions.
In the second phase, which lasts about a minute, the muscles of the body alternately contract and relax in rapid succession. This phase begins with the muscles of the jaw and rapidly encompasses eyelids, other facial muscles, and body. If the tongue is unprotected, the woman often bites it.
Coma sometimes follows the convulsion, and deep, rapid breathing usually begins as soon as the convulsion stops. In fact, maintaining oxygenation typically is not a problem after a single convulsion, and the risk of aspiration is low in a well managed patient.
Upon reviving, the woman typically remembers nothing about the seizure.
If convulsions recur, some degree of consciousness returns after each one, although the woman may become combative, agitated, and difficult to control.
Harbingers of complications
In the developed world, eclampsia increases the risk of maternal death (range: 0 to 1.8%).1-5 A recent review of all reported pregnancy-related deaths in the United States from 1979 to 1992 found 4,024 cases.6 Of these, 790 (19.6%) were due to preeclampsia-eclampsia, 49% of which were caused by eclampsia. The risk of death from preeclampsia or eclampsia was higher for the following groups:
- women over 30,
- no prenatal care,
- African Americans, and
- onset of preeclampsia or eclampsia before 28 weeks.6
Maternal morbidity
Pregnancies complicated by eclampsia also have higher rates of maternal morbidity such as pulmonary edema and HELLP syndrome (TABLE 1). Complications are substantially higher among women who develop antepartum eclampsia, especially when it is remote from term.1-3
TABLE 1
Maternal complications
Antepartum eclampsia, especially when it is remote from term, is much more likely to lead to complications
| COMPLICATION | RATE (%) | REMARKS |
|---|---|---|
| Death | 0.5-–2 | Risk of death is higher:
|
| Intracerebral hemorrhage | <1 | Usually related to several risk factors |
| Aspiration pneumonia | 2–3 | Heightened risk of maternal hypoxemia and acidosis |
| Disseminated coagulopathy | 3–5 | Regional anesthesia is contraindicated in these patients, and there is a heightened risk of hemorrhagic shock |
| Pulmonary edema | 3–5 | Heightened risk of maternal hypoxemia and acidosis |
| Acute renal failure | 5–9 | Usually seen in association with abruptio placentae, maternal hemorrhage, and prolonged maternal hypotension |
| Abruptio placentae | 7–10 | Can occur after a convulsion; suspect it if fetal bradycardia or late decelerations persist |
| HELLP syndrome | 10–15 |
Adverse perinatal outcomes
Perinatal mortality and morbidity are high in eclampsia, with a perinatal death rate in recent series of 5.6% to 11.8%.1,7 This high rate is related to prematurity, abruptio placentae, and severe growth restriction.1
Preterm deliveries occur in approximately 50% of cases, and about 25% occur before 32 weeks’ gestation.1
Diagnosis can be tricky
When the patient has generalized edema, hypertension, proteinuria, and convulsions, diagnosis of eclampsia is straightforward. Unfortunately, women with eclampsia exhibit a broad spectrum of signs, ranging from severe hypertension, severe proteinuria, and generalized edema, to absent or minimal hypertension, nonexistent proteinuria, and no edema (TABLE 2).1
Hypertension is the hallmark of diagnosis. Hypertension is severe (at least 160 mm Hg systolic and/or at least 110 mm Hg diastolic) in 20% to 54% of cases, and it is mild (systolic pressure between 140 and 160 mm Hg or diastolic pressure between 90 and 110 mm Hg) in 30% to 60% of cases.2,3 In 16% of cases, there may be no hypertension at all.2
Proteinuria. Eclampsia usually is associated with proteinuria (at least 1+ on dipstick).1 However, when I studied a series of 399 women with eclampsia, I found substantial proteinuria (3+ or above on dipstick) in only 48% of cases; proteinuria was absent in 14%.2
Edema. A weight gain of more than 2 lb per week (with or without clinical edema) during the third trimester may be the first sign of eclampsia. However, in my series of 399 women, edema was absent in 26% of cases.2
TABLE 2
Signs and symptoms of eclampsia*
Hypertension and proteinuria in eclampsia may be severe, mild, or even absent
| CONDITION | FREQUENCY (%) IN WOMEN WITH ECLAMPSIA | REMARKS |
|---|---|---|
| SIGNS | ||
| Hypertension | 85 | Should be documented on at least 2 occasions more than 6 hours apart |
| Severe: 160/110 mm Hg or more | 20–54 | |
| Mild: 140–160/90–110 mm Hg | 30–60 | |
| No hypertension2 | 16 | |
| Proteinuria | 85 | |
| At least 1+ on dipstick2 | 48 | |
| At least 3+ on dipstick | 14 | |
| No proteinuria | 15 | |
| SYMPTOMS | ||
| At least 1 of the following: | 33–75 | Clinical symptoms may occur before or after a convulsion |
| Headache | 30–70 | Persistent, occipital, or frontal |
| Right upper quadrant or epigastric pain | 12–20 | |
| Visual changes | 19–32 | Blurred vision, photophobia |
| Altered mental changes | 4–5 | |
| * Summary of 5 series | ||
Symptoms of eclampsia
Several clinical symptoms can occur before or after a convulsion1:
- persistent occipital or frontal headaches,
- blurred vision,
- photophobia,
- epigastric and/or right upper quadrant pain, and
- altered mental status.
Usual times of onset
Eclamptic convulsions can occur during pregnancy or delivery, or after delivery ( TABLE 3).1-5
Approximately 91% of antepartum cases develop at 28 weeks or beyond. The remaining cases tend to occur between 21 and 27 weeks’ gestation (7.5%), or at or before 20 weeks (1.5%).2
When eclampsia occurs before 20 weeks, it usually involves molar or hydropic degeneration of the placenta, with or without a fetus.1 However, eclampsia can occur in the first half of pregnancy without molar degeneration of the placenta, although this is rare.1,2 These women are sometimes misdiagnosed as having hypertensive encephalopathy, seizure disorder, or thrombotic thrombocytopenia purpura. Thus, women who develop convulsions in association with hypertension and proteinuria in the first half of pregnancy should be assumed to have eclampsia until proven otherwise,1 and require ultrasound examination of the uterus to rule out molar pregnancy and/or hydropic or cystic degeneration of the placenta.
Postpartum cases tend to occur within the first 48 hours, although some develop beyond this limit and have been reported as late as 23 days postpartum.8
Late postpartum eclampsia occurs more than 48 hours but less than 4 weeks after delivery.8 These women have signs and symptoms consistent with preeclampsia along with their convulsions.8,9 Thus, women who develop convulsions with hypertension and/or proteinuria, or with headaches or blurred vision, after 48 hours postpartum should be assumed to have eclampsia and treated accordingly.8,9
When eclampsia occurs especially late, perform an extensive neurologic examination to rule out other cerebral pathology.1,10
Eclampsia is atypical if convulsions occur before 20 weeks’ gestation or beyond 48 hours postpartum. It also is atypical if convulsions develop or persist despite adequate magnesium sulfate, or if the patient develops focal neurologic deficits, disorientation, blindness, or coma. In these cases, conduct a neurologic exam and cerebral imaging to exclude neurologic pathology.8-10
TABLE 3
Usual times of onset*
91% of antepartum eclampsia cases occur at 28 weeks or later, although eclamptic convulsions can occur at any time during pregnancy or delivery, or postpartum
| ONSET | FREQUENCY (%) | REMARKS |
|---|---|---|
| Antepartum | 38–53 | Maternal and perinatal mortality, and the incidence of complications and underlying disease, are higher in antepartum eclampsia, especially in early cases |
| ≤20 weeks | 1.5 | |
| 21 to 27 weeks | 7.5 | |
| ≥28 weeks | 91 | |
| Intrapartum | 18–36 | Intrapartum eclampsia more closely resembles postpartum disease than antepartum cases |
| Postpartum | 11–44 | Late postpartum eclampsia occurs more than 48 hours but less than 4 weeks after delivery |
| ≤48 hours | 7–39 | |
| >48 hours | 5–26 | |
| * Summary of 5 series | ||
Differential diagnosis
As with other aspects of preeclampsia, the presenting symptoms, clinical findings, and many laboratory results in eclampsia overlap several other medical and surgical conditions.1,10 Of course, eclampsia is the most common cause of convulsions in a woman with hypertension and/or proteinuria during pregnancy or immediately postpartum. On rare occasions, other causes of convulsions in pregnancy or postpartum may mimic eclampsia.1 These potential diagnoses are particularly important when the woman has focal neurologic deficits, prolonged coma, or atypical eclampsia.
The differential diagnosis encompasses a variety of cerebrovascular and metabolic disorders:
- hemorrhage,
- ruptured aneurysm or malformation,
- arterial embolism, thrombosis,
- venous thrombosis,
- hypoxic ischemic encephalopathy,
- angiomas,
- hypertensive encephalopathy,
- seizure disorder,
- hypoglycemia, hyponatremia,
- posterior leukoencephalopathy syndrome,
- thrombotic thrombocytopenic purpura,
- postdural puncture syndrome, and
- cerebral vasculitis/angiopathy.
Other diseases may factor in
Some women develop gestational hypertension or preeclampsia in association with connective tissue disease, thrombophilias, seizure disorder, or hypertensive encephalopathy, further confounding the diagnosis.
Thus, make every effort to ensure a correct diagnosis, since management may differ among these conditions.
Managing convulsions
Do not try to stop the first convulsion
The natural tendency is to try and interrupt the convulsion, but this is not recommended. Nor should you give a drug such as diazepam to shorten or stop the convulsion, especially if the patient lacks an intravenous line and no one skilled in intubation is immediately available. If diazepam is given, do not exceed 5 mg over 60 seconds. Rapid administration of this drug may lead to apnea or cardiac arrest, or both.
Steps to prevent maternal injury
During or immediately after the acute convulsive episode, take steps to prevent serious maternal injury and aspiration, assess and establish airway patency, and ensure maternal oxygenation (TABLE 4).
Elevate and pad the bed’s side rails and insert a padded tongue blade between the patient’s teeth, taking care not to trigger the gag reflex.
Physical restraints also may be needed.
Prevent aspiration. To minimize the risk of aspiration, place the patient in the lateral decubitus position, and suction vomitus and oral secretions as needed. Be aware that aspiration can occur when the padded tongue blade is forced to the back of the throat, stimulating the gag reflex and resultant vomiting.
TABLE 4
During a convulsion, 3 spheres of concern
| AVOID MATERNAL INJURY |
| Insert padded tongue blade |
| Avoid inducing gag reflex |
| Elevate padded bedside rails |
| Use physical restraints as needed |
| MAINTAIN OXYGENATION TO MOTHER AND FETUS |
| Apply face mask with or without oxygen reservoir at 8–10 L/minute |
| Monitor oxygenation and metabolic status via |
| Transcutaneous pulse oximetry |
| Arterial blood gases (sodium bicarbonate administered accordingly) |
| Correct oxygenation and metabolic status before administering anesthetics that may depress myocardial function |
| MINIMIZE ASPIRATION |
| Place patient in lateral decubitus position (which also maximizes uterine blood flow and venous return) |
| Suction vomitus and oral secretions |
| Obtain chest x-ray after the convulsion is controlled to rule out aspiration |
Tips on supplemental oxygenation
Although the initial seizure lasts only a minute or 2, it is important to maintain oxygenation by giving supplemental oxygen via a face mask, with or without an oxygen reservoir, at 8 to 10 L per minute. This is important because hypoventilation and respiratory acidosis often occur.
Once the convulsion ends and the patient resumes breathing, oxygenation is rarely a problem. However, maternal hypoxemia and acidosis can develop in women with repetitive convulsions, aspiration pneumonia, pulmonary edema, or a combination of these factors. Thus, transcutaneous pulse oximetry is advisable to monitor oxygenation in eclamptic patients.
Arterial blood gas analysis is necessary if pulse oximetry shows abnormal oxygen saturation (ie, at or below 92%).
Strategy to prevent recurrence
Magnesium sulfate is the drug of choice to treat and prevent subsequent convulsions in women with eclampsia.1,2
Dosage. I give a loading dose of 6 g over 15 to 20 minutes, followed by a maintenance dose of 2 g per hour as a continuous intravenous solution.
Approximately 10% of eclamptic women have a second convulsion after receiving magnesium sulfate.1,2 When this occurs, I give another 2-g bolus intravenously over 3 to 5 minutes.
More rarely, a woman will continue to have convulsions while receiving adequate and therapeutic doses of magnesium sulfate.
I treat such patients with sodium amobarbital, 250 mg, intravenously over 3 to 5 minutes.
Monitor maternal magnesium levels
Plasma levels should be in the range of 4 to 8 mg/dL during treatment for eclampsia, and are determined by the volume of distribution and by renal excretion. Thus, it is important to monitor the patient for magnesium toxicity, particularly if she has renal dysfunction (serum creatinine of 1.2 mg/dL or above) or urine output below 100 mL in 4 hours. In these women, adjust the maintenance dose according to plasma levels.
Side effects of magnesium including flushing, a feeling of warmth, nausea and vomiting, double vision, and slurred speech (TABLE 5).
If magnesium toxicity is suspected, immediately discontinue the infusion and administer supplemental oxygen along with 10 mL of 10% calcium gluconate (1 g total) as an intravenous push slowly over 5 minutes.
If respiratory arrest occurs, prompt resuscitation—including intubation and assisted ventilation—is vital.
TABLE 5
Clinical manifestations of magnesium toxicity
Maternal plasma levels of 4 to 8 mg/dL are appropriate during treatment for eclampsia; higher levels signal toxicity
| IF THE MAGNESIUM LEVEL IS… | THE CLINICAL MANIFESTATION IS… |
|---|---|
| 8–12 mg/dL | Loss of patellar reflex |
| Double or blurred vision | |
| Headache and nausea | |
| 9–12 mg/dL | Feeling of warmth, flushing |
| 10–12 mg/dL | Somnolence |
| Slurred speech | |
| 15–17 mg/dL | Muscular paralysis |
| Respiratory arrest | |
| 30–35 mg/dL | Cardiac arrest |
Controlling severe hypertension
The next step is to reduce blood pressure to a safe range. The objective: to preserve cerebral autoregulation and prevent congestive heart failure without compromising cerebral perfusion or jeopardizing uteroplacental blood flow, which is already reduced in many women with eclampsia.1
To these ends, try to keep systolic blood pressure between 140 and 160 mm Hg and diastolic pressure between 90 and 110 mm Hg. This can be achieved with:
- bolus 5- to 10-mg doses of hydralazine,
- 20 to 40 mg labetalol intravenously every 15 minutes, as needed, or
- 10 to 20 mg oral nifedipine every 30 minutes.
Other potent antihypertensive drugs such as sodium nitroprusside or nitroglycerine are rarely needed in eclampsia, and diuretics are indicated only in the presence of pulmonary edema.
Intrapartum management
Maternal hypoxemia and hypercarbia cause fetal heart rate and uterine activity changes during and immediately following a convulsion.
Fetal heart rate changes
These can include bradycardia, transient late decelerations, decreased beat-to-beat variability, and compensatory tachycardia.
The interval from onset of the seizure to the fall in fetal heart rate is approximately 5 minutes (FIGURE 1). Transitory fetal tachycardia frequently occurs after the prolonged bradycardia. The loss of beat-to-beat variability, with transitory late decelerations, occurs during the recovery phase.
The mechanism for the transient fetal bradycardia may be intense vasospasm and uterine hyperactivity, which may decrease uterine blood flow. The absence of maternal respiration during the convulsion may also contribute to fetal heart rate changes.
Since the fetal heart rate usually returns to normal after a convulsion, other conditions should be considered if an abnormal pattern persists.
In some cases, it may take longer for the heart rate pattern to return to baseline if the fetus is preterm with growth restriction.
Placental abruption may occur after the convulsion and should be suspected if fetal bradycardia or repetitive late decelerations persist (FIGURE 2).
FIGURE 1 Fetal response to a convulsion The top 2 tracings show fetal bradycardia during an eclamptic convulsion
FIGURE 2 Abruptio placentae Repetitive late decelerations secondary to abruptio placentae, necessitating cesarean section
Uterine activity
During a convulsion, contractions can increase in frequency and tone. The duration of increased uterine activity varies from 2 to 14 minutes.
These changes usually resolve spontaneously within 3 to 10 minutes following the termination of convulsions and correction of maternal hypoxemia (FIGURE 1).
If uterine hyperactivity persists, suspect placental abruption (FIGURE 2).
Do not rush to cesarean
It benefits the fetus to allow in utero recovery from the maternal convulsion, hypoxia, and hypercarbia before delivery. However, if the bradycardia and/or recurrent late decelerations persist beyond 10 to 15 minutes despite all efforts, suspect abruptio placentae or nonreassuring fetal status.
Once the patient regains consciousness and is oriented to name, place, and time, and her convulsions are controlled and condition stabilized, proceed with delivery.
Choosing a delivery route
Eclampsia is not an indication for cesarean. The decision to perform a cesarean should be based on fetal gestational age, fetal condition, presence of labor, and cervical Bishop score. I recommend:
- Cesarean section for women with eclampsia before 30 weeks’ gestation who are not in labor and whose Bishop score is below 5.
- Vaginal delivery for women in labor or with rupture of membranes, provided there are no obstetric complications.
- Labor induction with oxytocin infusion or prostaglandins in all women at or after 30 weeks, regardless of the Bishop score, and in women before 30 weeks when the Bishop score is 5 or above.
Maternal pain relief
During labor and delivery, systemic opioids or epidural anesthesia can provide pain relief—the same recommendations as for women with severe preeclampsia.
For cesarean delivery, an epidural, spinal, or combined techniques of regional anesthesia are suitable.
Do not use regional anesthesia if there is coagulopathy or severe thrombocytopenia (platelet count less than 50,000/mm3). In women with eclampsia, general anesthesia increases the risk of aspiration and failed intubation due to airway edema, and is associated with marked increases in systemic and cerebral pressures during intubation and extubation.
Women with airway or laryngeal edema may require awake intubation under fiber optic observation, with tracheostomy immediately available.
Changes in systemic or cerebral pressures may be attenuated by pretreatment with labetalol or nitroglycerine injections.
Postpartum management
After delivery, women with eclampsia require close monitoring of vital signs, fluid intake and output, and symptoms for at least 48 hours.
Risk of pulmonary edema
These women usually receive large amounts of intravenous fluids during labor, delivery, and postpartum. In addition, during the postpartum period, extracellular fluid mobilizes, leading to increased intravascular volume.
As a result, women who develop eclampsia—particularly those with abnormal renal function, abruptio placentae, and/or preexisting chronic hypertension— face an increased risk for pulmonary edema and exacerbation of severe hypertension.2
Frequent evaluation of the amount of intravenous fluids is necessary, as well as oral intake, blood products, and urine output. They also need pulse oximetry and pulmonary auscultation.
Continue magnesium sulfate
Parenteral magnesium sulfate should be given for at least 24 hours after delivery and/or for at least 24 hours after the last convulsion.
If the patient has oliguria (less than 100 mL over 4 hours), both fluid administration and the dose of magnesium sulfate should be reduced.
Oral antihypertensives
Other oral antihypertensive agents such as labetalol or nifedipine can be given to keep systolic blood pressure below 155 mm Hg and diastolic blood pressure below 105 mm Hg. Nifedipine offers the benefit of improved diuresis in the postpartum period.
The author reports no financial relationships relevant to this article.
An eclamptic convulsion is frightening to behold. First, the woman’s face becomes distorted, and her eyes protrude. Then her face acquires a congested expression, and foam may exude from her mouth. Breathing stops.
Because eclampsia is so frightening, the natural tendency is to try to stop a convulsion, but this is not the wisest strategy.
Rather, the foremost priorities are to avoid maternal injury and support cardiovascular functions. How to do this, and how to prevent further convulsions, monitor and deliver the fetus, and avert complications are the focus of this article.
Since eclampsia may be fatal for both mother and fetus, all labor and delivery units and all obstetricians should be prepared to diagnose and manage this grave threat. However, few obstetric units encounter more than 1 or 2 cases a year; most obstetricians have little or no experience managing acute eclampsia. In the Western world, it affects only 1 in 2,000 to 1 in 3,448 pregnancies.1-4
How a convulsion happens
Most convulsions occur in 2 phases and last for 60 to 75 seconds. The first phase, lasting 15 to 20 seconds, begins with facial twitching, soon followed by a rigid body with generalized muscular contractions.
In the second phase, which lasts about a minute, the muscles of the body alternately contract and relax in rapid succession. This phase begins with the muscles of the jaw and rapidly encompasses eyelids, other facial muscles, and body. If the tongue is unprotected, the woman often bites it.
Coma sometimes follows the convulsion, and deep, rapid breathing usually begins as soon as the convulsion stops. In fact, maintaining oxygenation typically is not a problem after a single convulsion, and the risk of aspiration is low in a well managed patient.
Upon reviving, the woman typically remembers nothing about the seizure.
If convulsions recur, some degree of consciousness returns after each one, although the woman may become combative, agitated, and difficult to control.
Harbingers of complications
In the developed world, eclampsia increases the risk of maternal death (range: 0 to 1.8%).1-5 A recent review of all reported pregnancy-related deaths in the United States from 1979 to 1992 found 4,024 cases.6 Of these, 790 (19.6%) were due to preeclampsia-eclampsia, 49% of which were caused by eclampsia. The risk of death from preeclampsia or eclampsia was higher for the following groups:
- women over 30,
- no prenatal care,
- African Americans, and
- onset of preeclampsia or eclampsia before 28 weeks.6
Maternal morbidity
Pregnancies complicated by eclampsia also have higher rates of maternal morbidity such as pulmonary edema and HELLP syndrome (TABLE 1). Complications are substantially higher among women who develop antepartum eclampsia, especially when it is remote from term.1-3
TABLE 1
Maternal complications
Antepartum eclampsia, especially when it is remote from term, is much more likely to lead to complications
| COMPLICATION | RATE (%) | REMARKS |
|---|---|---|
| Death | 0.5-–2 | Risk of death is higher:
|
| Intracerebral hemorrhage | <1 | Usually related to several risk factors |
| Aspiration pneumonia | 2–3 | Heightened risk of maternal hypoxemia and acidosis |
| Disseminated coagulopathy | 3–5 | Regional anesthesia is contraindicated in these patients, and there is a heightened risk of hemorrhagic shock |
| Pulmonary edema | 3–5 | Heightened risk of maternal hypoxemia and acidosis |
| Acute renal failure | 5–9 | Usually seen in association with abruptio placentae, maternal hemorrhage, and prolonged maternal hypotension |
| Abruptio placentae | 7–10 | Can occur after a convulsion; suspect it if fetal bradycardia or late decelerations persist |
| HELLP syndrome | 10–15 |
Adverse perinatal outcomes
Perinatal mortality and morbidity are high in eclampsia, with a perinatal death rate in recent series of 5.6% to 11.8%.1,7 This high rate is related to prematurity, abruptio placentae, and severe growth restriction.1
Preterm deliveries occur in approximately 50% of cases, and about 25% occur before 32 weeks’ gestation.1
Diagnosis can be tricky
When the patient has generalized edema, hypertension, proteinuria, and convulsions, diagnosis of eclampsia is straightforward. Unfortunately, women with eclampsia exhibit a broad spectrum of signs, ranging from severe hypertension, severe proteinuria, and generalized edema, to absent or minimal hypertension, nonexistent proteinuria, and no edema (TABLE 2).1
Hypertension is the hallmark of diagnosis. Hypertension is severe (at least 160 mm Hg systolic and/or at least 110 mm Hg diastolic) in 20% to 54% of cases, and it is mild (systolic pressure between 140 and 160 mm Hg or diastolic pressure between 90 and 110 mm Hg) in 30% to 60% of cases.2,3 In 16% of cases, there may be no hypertension at all.2
Proteinuria. Eclampsia usually is associated with proteinuria (at least 1+ on dipstick).1 However, when I studied a series of 399 women with eclampsia, I found substantial proteinuria (3+ or above on dipstick) in only 48% of cases; proteinuria was absent in 14%.2
Edema. A weight gain of more than 2 lb per week (with or without clinical edema) during the third trimester may be the first sign of eclampsia. However, in my series of 399 women, edema was absent in 26% of cases.2
TABLE 2
Signs and symptoms of eclampsia*
Hypertension and proteinuria in eclampsia may be severe, mild, or even absent
| CONDITION | FREQUENCY (%) IN WOMEN WITH ECLAMPSIA | REMARKS |
|---|---|---|
| SIGNS | ||
| Hypertension | 85 | Should be documented on at least 2 occasions more than 6 hours apart |
| Severe: 160/110 mm Hg or more | 20–54 | |
| Mild: 140–160/90–110 mm Hg | 30–60 | |
| No hypertension2 | 16 | |
| Proteinuria | 85 | |
| At least 1+ on dipstick2 | 48 | |
| At least 3+ on dipstick | 14 | |
| No proteinuria | 15 | |
| SYMPTOMS | ||
| At least 1 of the following: | 33–75 | Clinical symptoms may occur before or after a convulsion |
| Headache | 30–70 | Persistent, occipital, or frontal |
| Right upper quadrant or epigastric pain | 12–20 | |
| Visual changes | 19–32 | Blurred vision, photophobia |
| Altered mental changes | 4–5 | |
| * Summary of 5 series | ||
Symptoms of eclampsia
Several clinical symptoms can occur before or after a convulsion1:
- persistent occipital or frontal headaches,
- blurred vision,
- photophobia,
- epigastric and/or right upper quadrant pain, and
- altered mental status.
Usual times of onset
Eclamptic convulsions can occur during pregnancy or delivery, or after delivery ( TABLE 3).1-5
Approximately 91% of antepartum cases develop at 28 weeks or beyond. The remaining cases tend to occur between 21 and 27 weeks’ gestation (7.5%), or at or before 20 weeks (1.5%).2
When eclampsia occurs before 20 weeks, it usually involves molar or hydropic degeneration of the placenta, with or without a fetus.1 However, eclampsia can occur in the first half of pregnancy without molar degeneration of the placenta, although this is rare.1,2 These women are sometimes misdiagnosed as having hypertensive encephalopathy, seizure disorder, or thrombotic thrombocytopenia purpura. Thus, women who develop convulsions in association with hypertension and proteinuria in the first half of pregnancy should be assumed to have eclampsia until proven otherwise,1 and require ultrasound examination of the uterus to rule out molar pregnancy and/or hydropic or cystic degeneration of the placenta.
Postpartum cases tend to occur within the first 48 hours, although some develop beyond this limit and have been reported as late as 23 days postpartum.8
Late postpartum eclampsia occurs more than 48 hours but less than 4 weeks after delivery.8 These women have signs and symptoms consistent with preeclampsia along with their convulsions.8,9 Thus, women who develop convulsions with hypertension and/or proteinuria, or with headaches or blurred vision, after 48 hours postpartum should be assumed to have eclampsia and treated accordingly.8,9
When eclampsia occurs especially late, perform an extensive neurologic examination to rule out other cerebral pathology.1,10
Eclampsia is atypical if convulsions occur before 20 weeks’ gestation or beyond 48 hours postpartum. It also is atypical if convulsions develop or persist despite adequate magnesium sulfate, or if the patient develops focal neurologic deficits, disorientation, blindness, or coma. In these cases, conduct a neurologic exam and cerebral imaging to exclude neurologic pathology.8-10
TABLE 3
Usual times of onset*
91% of antepartum eclampsia cases occur at 28 weeks or later, although eclamptic convulsions can occur at any time during pregnancy or delivery, or postpartum
| ONSET | FREQUENCY (%) | REMARKS |
|---|---|---|
| Antepartum | 38–53 | Maternal and perinatal mortality, and the incidence of complications and underlying disease, are higher in antepartum eclampsia, especially in early cases |
| ≤20 weeks | 1.5 | |
| 21 to 27 weeks | 7.5 | |
| ≥28 weeks | 91 | |
| Intrapartum | 18–36 | Intrapartum eclampsia more closely resembles postpartum disease than antepartum cases |
| Postpartum | 11–44 | Late postpartum eclampsia occurs more than 48 hours but less than 4 weeks after delivery |
| ≤48 hours | 7–39 | |
| >48 hours | 5–26 | |
| * Summary of 5 series | ||
Differential diagnosis
As with other aspects of preeclampsia, the presenting symptoms, clinical findings, and many laboratory results in eclampsia overlap several other medical and surgical conditions.1,10 Of course, eclampsia is the most common cause of convulsions in a woman with hypertension and/or proteinuria during pregnancy or immediately postpartum. On rare occasions, other causes of convulsions in pregnancy or postpartum may mimic eclampsia.1 These potential diagnoses are particularly important when the woman has focal neurologic deficits, prolonged coma, or atypical eclampsia.
The differential diagnosis encompasses a variety of cerebrovascular and metabolic disorders:
- hemorrhage,
- ruptured aneurysm or malformation,
- arterial embolism, thrombosis,
- venous thrombosis,
- hypoxic ischemic encephalopathy,
- angiomas,
- hypertensive encephalopathy,
- seizure disorder,
- hypoglycemia, hyponatremia,
- posterior leukoencephalopathy syndrome,
- thrombotic thrombocytopenic purpura,
- postdural puncture syndrome, and
- cerebral vasculitis/angiopathy.
Other diseases may factor in
Some women develop gestational hypertension or preeclampsia in association with connective tissue disease, thrombophilias, seizure disorder, or hypertensive encephalopathy, further confounding the diagnosis.
Thus, make every effort to ensure a correct diagnosis, since management may differ among these conditions.
Managing convulsions
Do not try to stop the first convulsion
The natural tendency is to try and interrupt the convulsion, but this is not recommended. Nor should you give a drug such as diazepam to shorten or stop the convulsion, especially if the patient lacks an intravenous line and no one skilled in intubation is immediately available. If diazepam is given, do not exceed 5 mg over 60 seconds. Rapid administration of this drug may lead to apnea or cardiac arrest, or both.
Steps to prevent maternal injury
During or immediately after the acute convulsive episode, take steps to prevent serious maternal injury and aspiration, assess and establish airway patency, and ensure maternal oxygenation (TABLE 4).
Elevate and pad the bed’s side rails and insert a padded tongue blade between the patient’s teeth, taking care not to trigger the gag reflex.
Physical restraints also may be needed.
Prevent aspiration. To minimize the risk of aspiration, place the patient in the lateral decubitus position, and suction vomitus and oral secretions as needed. Be aware that aspiration can occur when the padded tongue blade is forced to the back of the throat, stimulating the gag reflex and resultant vomiting.
TABLE 4
During a convulsion, 3 spheres of concern
| AVOID MATERNAL INJURY |
| Insert padded tongue blade |
| Avoid inducing gag reflex |
| Elevate padded bedside rails |
| Use physical restraints as needed |
| MAINTAIN OXYGENATION TO MOTHER AND FETUS |
| Apply face mask with or without oxygen reservoir at 8–10 L/minute |
| Monitor oxygenation and metabolic status via |
| Transcutaneous pulse oximetry |
| Arterial blood gases (sodium bicarbonate administered accordingly) |
| Correct oxygenation and metabolic status before administering anesthetics that may depress myocardial function |
| MINIMIZE ASPIRATION |
| Place patient in lateral decubitus position (which also maximizes uterine blood flow and venous return) |
| Suction vomitus and oral secretions |
| Obtain chest x-ray after the convulsion is controlled to rule out aspiration |
Tips on supplemental oxygenation
Although the initial seizure lasts only a minute or 2, it is important to maintain oxygenation by giving supplemental oxygen via a face mask, with or without an oxygen reservoir, at 8 to 10 L per minute. This is important because hypoventilation and respiratory acidosis often occur.
Once the convulsion ends and the patient resumes breathing, oxygenation is rarely a problem. However, maternal hypoxemia and acidosis can develop in women with repetitive convulsions, aspiration pneumonia, pulmonary edema, or a combination of these factors. Thus, transcutaneous pulse oximetry is advisable to monitor oxygenation in eclamptic patients.
Arterial blood gas analysis is necessary if pulse oximetry shows abnormal oxygen saturation (ie, at or below 92%).
Strategy to prevent recurrence
Magnesium sulfate is the drug of choice to treat and prevent subsequent convulsions in women with eclampsia.1,2
Dosage. I give a loading dose of 6 g over 15 to 20 minutes, followed by a maintenance dose of 2 g per hour as a continuous intravenous solution.
Approximately 10% of eclamptic women have a second convulsion after receiving magnesium sulfate.1,2 When this occurs, I give another 2-g bolus intravenously over 3 to 5 minutes.
More rarely, a woman will continue to have convulsions while receiving adequate and therapeutic doses of magnesium sulfate.
I treat such patients with sodium amobarbital, 250 mg, intravenously over 3 to 5 minutes.
Monitor maternal magnesium levels
Plasma levels should be in the range of 4 to 8 mg/dL during treatment for eclampsia, and are determined by the volume of distribution and by renal excretion. Thus, it is important to monitor the patient for magnesium toxicity, particularly if she has renal dysfunction (serum creatinine of 1.2 mg/dL or above) or urine output below 100 mL in 4 hours. In these women, adjust the maintenance dose according to plasma levels.
Side effects of magnesium including flushing, a feeling of warmth, nausea and vomiting, double vision, and slurred speech (TABLE 5).
If magnesium toxicity is suspected, immediately discontinue the infusion and administer supplemental oxygen along with 10 mL of 10% calcium gluconate (1 g total) as an intravenous push slowly over 5 minutes.
If respiratory arrest occurs, prompt resuscitation—including intubation and assisted ventilation—is vital.
TABLE 5
Clinical manifestations of magnesium toxicity
Maternal plasma levels of 4 to 8 mg/dL are appropriate during treatment for eclampsia; higher levels signal toxicity
| IF THE MAGNESIUM LEVEL IS… | THE CLINICAL MANIFESTATION IS… |
|---|---|
| 8–12 mg/dL | Loss of patellar reflex |
| Double or blurred vision | |
| Headache and nausea | |
| 9–12 mg/dL | Feeling of warmth, flushing |
| 10–12 mg/dL | Somnolence |
| Slurred speech | |
| 15–17 mg/dL | Muscular paralysis |
| Respiratory arrest | |
| 30–35 mg/dL | Cardiac arrest |
Controlling severe hypertension
The next step is to reduce blood pressure to a safe range. The objective: to preserve cerebral autoregulation and prevent congestive heart failure without compromising cerebral perfusion or jeopardizing uteroplacental blood flow, which is already reduced in many women with eclampsia.1
To these ends, try to keep systolic blood pressure between 140 and 160 mm Hg and diastolic pressure between 90 and 110 mm Hg. This can be achieved with:
- bolus 5- to 10-mg doses of hydralazine,
- 20 to 40 mg labetalol intravenously every 15 minutes, as needed, or
- 10 to 20 mg oral nifedipine every 30 minutes.
Other potent antihypertensive drugs such as sodium nitroprusside or nitroglycerine are rarely needed in eclampsia, and diuretics are indicated only in the presence of pulmonary edema.
Intrapartum management
Maternal hypoxemia and hypercarbia cause fetal heart rate and uterine activity changes during and immediately following a convulsion.
Fetal heart rate changes
These can include bradycardia, transient late decelerations, decreased beat-to-beat variability, and compensatory tachycardia.
The interval from onset of the seizure to the fall in fetal heart rate is approximately 5 minutes (FIGURE 1). Transitory fetal tachycardia frequently occurs after the prolonged bradycardia. The loss of beat-to-beat variability, with transitory late decelerations, occurs during the recovery phase.
The mechanism for the transient fetal bradycardia may be intense vasospasm and uterine hyperactivity, which may decrease uterine blood flow. The absence of maternal respiration during the convulsion may also contribute to fetal heart rate changes.
Since the fetal heart rate usually returns to normal after a convulsion, other conditions should be considered if an abnormal pattern persists.
In some cases, it may take longer for the heart rate pattern to return to baseline if the fetus is preterm with growth restriction.
Placental abruption may occur after the convulsion and should be suspected if fetal bradycardia or repetitive late decelerations persist (FIGURE 2).
FIGURE 1 Fetal response to a convulsion The top 2 tracings show fetal bradycardia during an eclamptic convulsion
FIGURE 2 Abruptio placentae Repetitive late decelerations secondary to abruptio placentae, necessitating cesarean section
Uterine activity
During a convulsion, contractions can increase in frequency and tone. The duration of increased uterine activity varies from 2 to 14 minutes.
These changes usually resolve spontaneously within 3 to 10 minutes following the termination of convulsions and correction of maternal hypoxemia (FIGURE 1).
If uterine hyperactivity persists, suspect placental abruption (FIGURE 2).
Do not rush to cesarean
It benefits the fetus to allow in utero recovery from the maternal convulsion, hypoxia, and hypercarbia before delivery. However, if the bradycardia and/or recurrent late decelerations persist beyond 10 to 15 minutes despite all efforts, suspect abruptio placentae or nonreassuring fetal status.
Once the patient regains consciousness and is oriented to name, place, and time, and her convulsions are controlled and condition stabilized, proceed with delivery.
Choosing a delivery route
Eclampsia is not an indication for cesarean. The decision to perform a cesarean should be based on fetal gestational age, fetal condition, presence of labor, and cervical Bishop score. I recommend:
- Cesarean section for women with eclampsia before 30 weeks’ gestation who are not in labor and whose Bishop score is below 5.
- Vaginal delivery for women in labor or with rupture of membranes, provided there are no obstetric complications.
- Labor induction with oxytocin infusion or prostaglandins in all women at or after 30 weeks, regardless of the Bishop score, and in women before 30 weeks when the Bishop score is 5 or above.
Maternal pain relief
During labor and delivery, systemic opioids or epidural anesthesia can provide pain relief—the same recommendations as for women with severe preeclampsia.
For cesarean delivery, an epidural, spinal, or combined techniques of regional anesthesia are suitable.
Do not use regional anesthesia if there is coagulopathy or severe thrombocytopenia (platelet count less than 50,000/mm3). In women with eclampsia, general anesthesia increases the risk of aspiration and failed intubation due to airway edema, and is associated with marked increases in systemic and cerebral pressures during intubation and extubation.
Women with airway or laryngeal edema may require awake intubation under fiber optic observation, with tracheostomy immediately available.
Changes in systemic or cerebral pressures may be attenuated by pretreatment with labetalol or nitroglycerine injections.
Postpartum management
After delivery, women with eclampsia require close monitoring of vital signs, fluid intake and output, and symptoms for at least 48 hours.
Risk of pulmonary edema
These women usually receive large amounts of intravenous fluids during labor, delivery, and postpartum. In addition, during the postpartum period, extracellular fluid mobilizes, leading to increased intravascular volume.
As a result, women who develop eclampsia—particularly those with abnormal renal function, abruptio placentae, and/or preexisting chronic hypertension— face an increased risk for pulmonary edema and exacerbation of severe hypertension.2
Frequent evaluation of the amount of intravenous fluids is necessary, as well as oral intake, blood products, and urine output. They also need pulse oximetry and pulmonary auscultation.
Continue magnesium sulfate
Parenteral magnesium sulfate should be given for at least 24 hours after delivery and/or for at least 24 hours after the last convulsion.
If the patient has oliguria (less than 100 mL over 4 hours), both fluid administration and the dose of magnesium sulfate should be reduced.
Oral antihypertensives
Other oral antihypertensive agents such as labetalol or nifedipine can be given to keep systolic blood pressure below 155 mm Hg and diastolic blood pressure below 105 mm Hg. Nifedipine offers the benefit of improved diuresis in the postpartum period.
The author reports no financial relationships relevant to this article.
1. Sibai BM. Diagnosis, differential diagnosis, and management of eclampsia. Obstet Gynecol. 2005;105:402-410.
2. Mattar F, Sibai BM. Eclampsia VIII. Risk factors for maternal morbidity. Am J Obstet Gynecol. 2000;182:307-312.
3. Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ. 1994;309:1395-1400.
4. Rugard O, Carling MS, Berg G. Eclampsia at a tertiary hospital, 1973–99. Acta Obstet Gynecol Scand. 2004;83:240-245.
5. Katz VL, Farmer R, Kuller J. Preeclampsia into eclampsia: toward a new paradigm. Am J Obstet Gynecol. 2000;182:1389-1396.
6. MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97:533-538.
7. Leitch CR, Cameron AD, Walker JJ. The changing pattern of eclampsia over a 60-year period. Br J Obstet Gynaecol. 1997;104:917-922.
8. Lubarsky SL, Barton JR, Friedman SA, Nasreddine S, Ramaddan MK, Sibai BM. Late postpartum eclampsia revisited. Obstet Gynaecol. 1994;83:502-505.
9. Chames MC, Livingston JC, Ivester TS, Barton JR, Sibai BM. Late postpartum eclampsia: a preventable disease? Am J Obstet Gynecol. 2002;186:1174-1177.
10. Witlin AG, Friedman SA, Egerman RS, Frangieh AY, Sibai BM. Cerebrovascular disorders complicating pregnancy. Beyond eclampsia. Am J Obstet Gynecol. 1997;176:139-148.
1. Sibai BM. Diagnosis, differential diagnosis, and management of eclampsia. Obstet Gynecol. 2005;105:402-410.
2. Mattar F, Sibai BM. Eclampsia VIII. Risk factors for maternal morbidity. Am J Obstet Gynecol. 2000;182:307-312.
3. Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ. 1994;309:1395-1400.
4. Rugard O, Carling MS, Berg G. Eclampsia at a tertiary hospital, 1973–99. Acta Obstet Gynecol Scand. 2004;83:240-245.
5. Katz VL, Farmer R, Kuller J. Preeclampsia into eclampsia: toward a new paradigm. Am J Obstet Gynecol. 2000;182:1389-1396.
6. MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97:533-538.
7. Leitch CR, Cameron AD, Walker JJ. The changing pattern of eclampsia over a 60-year period. Br J Obstet Gynaecol. 1997;104:917-922.
8. Lubarsky SL, Barton JR, Friedman SA, Nasreddine S, Ramaddan MK, Sibai BM. Late postpartum eclampsia revisited. Obstet Gynaecol. 1994;83:502-505.
9. Chames MC, Livingston JC, Ivester TS, Barton JR, Sibai BM. Late postpartum eclampsia: a preventable disease? Am J Obstet Gynecol. 2002;186:1174-1177.
10. Witlin AG, Friedman SA, Egerman RS, Frangieh AY, Sibai BM. Cerebrovascular disorders complicating pregnancy. Beyond eclampsia. Am J Obstet Gynecol. 1997;176:139-148.
A practical plan to detect and manage HELLP syndrome
Here’s a disturbing fact: If it looks like HELLP syndrome, and impairs the patient like HELLP syndrome, it isn’t necessarily HELLP syndrome. A plethora of diagnostic criteria from different investigators over the years has confused the issue of what constitutes this syndrome—not to mention how to manage it.
A management issue has also attracted recent attention: use of corticosteroids either antepartum to enhance maternal status so that epidural anesthesia can be administered, or postpartum to improve platelets. Such improvements are only transient, however, and we lack definitive data on the benefits.
One thing is certain, however. The combination of hemolysis, liver dysfunction or injury, and platelet consumption in women with preeclampsia makes adverse maternal and perinatal outcomes more likely and leaves no room for expectant management.
HELLP syndrome also has become a major issue in litigation against obstetricians and medical and surgical consultants. Lawsuits usually allege misdiagnosed preeclampsia, delayed delivery, or improper recognition and management of complications.
Pinning HELLP Down
One of the best tools to identify HELLP syndrome is a healthy dose of suspicion, since it can affect any pregnant woman at any time: antepartum, intrapartum, or within 1 week postpartum. Approximately 72% of cases are diagnosed before delivery, and the rest are diagnosed during the first week postpartum.
Weinstein noted that the signs and symptoms of HELLP syndrome can occur without clinical evidence of severe preeclampsia (severe hypertension and/or severe proteinuria). Indeed, he reported that hypertension can be mild or absent in most patients with HELLP, and proteinuria can be mild.
Weinstein coined the term HELLP syndrome in 1982 to describe these abnormalities in women with preeclampsia:
- H = hemolysis
- EL = elevated liver enzymes
- LP = low platelets
Another obstacle to early detection: Patients may have nonspecific signs and symptoms, none of which are diagnostic of classical preeclampsia.
However, HELLP syndrome is most common in women who have already been diagnosed with gestational hypertension and/or preeclampsia.
HELLP is more likely with severe hypertension
Overall, the incidence of HELLP syndrome in women with gestational hypertension/preeclampsia increases with the severity of the condition. HELLP syndrome also is more likely in women with early-onset hypertension/preeclampsia (before 34 weeks’ gestation).
Making The Diagnosis
HELLP syndrome is diagnosed when all 3 of the following are present:
- Hemolysis, defined as the presence of microangiopathic hemolytic anemia. This is the hallmark of the triad.
- Elevated liver enzymes (either aspartate aminotransferase [AST] or alanine aminotransferase [ALT]). This component signifies liver cell ischemia and/or necrosis.
- Low platelet count (<100,000/mm3). TABLE 1 summarizes the laboratory criteria for the diagnosis.
When to begin testing
In women with new-onset hypertension, order a complete blood count with platelets and liver enzyme analysis at the time of diagnosis and serially thereafter. The frequency of these tests depends on the initial test results, severity of disease, and onset of symptoms.
In women without hypertension, I recommend obtaining the same blood tests at the onset of any of the signs and symptoms listed in TABLE 2.
TABLE 2
Conditions that heighten the risk of HELLP
|
Assessing test results
Clinicians should be familiar with the upper limit for liverenzyme tests in their laboratory. I suggest a cutoff more than twice the upper limit for a particular test.
Also keep in mind that these parameters are dynamic; some women will meet only some of the criteria early in the disease process. Moreover, maternal complications are substantially higher when all 3 components are present than when only 1 or 2 are present.
Look for these clinical findings
Hypertension. Most women with HELLP syndrome have hypertension. In 15% to 50% of cases, the hypertension is mild, but it may be absent in 15%.
Proteinuria. Most patients also have proteinuria by dipstick (≥1+). Proteinuria may be absent in approximately 13% of women with HELLP syndrome, although they will likely have many of the symptoms reported by women with severe preeclampsia.
TABLE 3 lists the signs and symptoms to be expected in these patients, along with their frequency.
TABLE 3
Signs and symptoms
| CONDITION | FREQUENCY (%) |
|---|---|
| Hypertension | 85 |
| Proteinuria | 87 |
| Right upper quadrant or epigastric pain | 40–90 |
| Nausea or vomiting | 29–84 |
| Headaches | 33–60 |
| Visual changes | 10–20 |
| Mucosal bleeding | 10 |
| Jaundice | 5 |
The usual times of onset
Antepartum cases. As was previously noted, HELLP syndrome usually develops before delivery, with the most frequent onset being before 37 weeks’ gestation ( TABLE 4).
In the postpartum period, most cases develop within 48 hours after delivery. Of these, approximately 90% occur in women who had antepartum preeclampsia that progressed to HELLP syndrome in the postpartum period. However, approximately 20% of postpartum cases develop more than 48 hours after delivery.
Another important point: HELLP syndrome can develop for the first time postpartum in women who had no evidence of preeclampsia before or during labor. Thus, it is important to educate all postpartum women to report new symptoms (listed in TABLE 3) as soon as possible. When these symptoms develop, evaluate the patient for both preeclampsia and HELLP syndrome.
TABLE 4
Usual times of onset*
| RELATION TO DELIVERY | PERCENTAGE |
| Antepartum | 72 |
| Postpartum | 28 |
| ≤48 hours | 80 |
| >48 hours | 20 |
| GESTATIONAL AGE (WEEKS) | PERCENTAGE |
| 17–20 | 2 |
| 21–27 | 10 |
| 28–36 | 68 |
| >37 | 20 |
| * Based on 700 cases | |
Risk for life-threatening maternal complications
When all components of HELLP syndrome are present in a woman with preeclampsia, the risk of maternal death and serious maternal morbidities increases substantially (TABLE 5). The rate of these complications depends on gestational age at onset, presence of associated obstetric complications (eclampsia, abruptio placentae, peripartum hemorrhage, or fetal demise) or preexisting conditions (lupus, renal disease, chronic hypertension, or type 1 diabetes).
Abruptio placentae increases the risk of disseminated intravascular coagulopathy (DIC), as well as the need for blood transfusions.
Marked ascites (>1 L) leads to higher rates of cardiopulmonary complications.
TABLE 5
Maternal complications
| COMPLICATION | FREQUENCY (%) |
|---|---|
| Death | 1 |
| Adult respiratory distress syndrome | 1 |
| Laryngeal edema | 1–2 |
| Liver failure or hemorrhage | 1–2 |
| Acute renal failure | 5–8 |
| Pulmonary edema | 6–8 |
| Pleural effusions | 6–10 |
| Abruptio placentae | 10–15 |
| Disseminated intravascular coagulopathy | 10–15 |
| Marked ascites | 10–15 |
Differential diagnosis
When diagnosing HELLP syndrome, confirm or exclude the conditions listed in TABLE 6, since the presenting symptoms and clinical and laboratory findings in women with HELLP syndrome overlap those of several microangiopathic disorders that can develop during pregnancy and/or postpartum. In some women, preeclampsia may be superimposed on one of these disorders, further confounding an already difficult differential diagnosis.
Because of the remarkably similar clinical and laboratory findings of these diseases, make every effort to achieve an accurate diagnosis, since management and outcomes may differ among these conditions.
TABLE 6
Differential diagnosis
|
Initial Management
Hospitalize the patient
Because HELLP syndrome usually is characterized by progressive and sometimes sudden deterioration in maternal and fetal conditions, patients should be hospitalized and observed in a labor and delivery unit.
Initially, assume the patient has severe preeclampsia and treat her with intravenous magnesium sulfate to prevent convulsions and antihypertensive medications as needed to keep systolic blood pressure below 160 mm Hg and diastolic blood pressure below 105 mm Hg.
Blood tests should include:
- complete blood count with platelet count,
- peripheral smear evaluation,
- serum AST,
- lactate dehydrogenase,
- creatinine,
- bilirubin, and
- coagulation studies.
These tests help confirm the diagnosis and check for the presence of DIC, massive hemolysis, severe anemia, or renal failure.
The first priority is to assess the patient for the presence of cardiovascular complications, signs of liver hematoma or hemorrhage, and abruptio placentae. If any is present—particularly hypotension, hypovolemia, DIC, or pulmonary edema—make every effort to stabilize the maternal condition.
Can delivery wait 48 hours for corticosteroids?
Evaluate fetal status by heart rate monitoring or biophysical profile, and confirm gestational age. Then decide whether delivery is indicated or can be delayed for 48 hours so that corticosteroids can be given.
No room for expectant management. Do not consider expectant management in women with true HELLP syndrome. Delivery can only be delayed for a maximum of 48 hours—and only when both mother and fetus are stable, at 24 to 34 weeks’ gestation, and awaiting the benefit of corticosteroids.
Corticosteroid dosing. My practice is to give 2 doses of either betamethasone 12 mg intramuscularly every 12 hours or dexamethasone 12 mg intravenously every 12 hours. This is to improve maternal status, at least temporarily.
Initiate delivery within 24 hours after the last steroid dose, with continuous monitoring in the labor and delivery unit.
Although some women may demonstrate transient improvement in their blood tests (eg, increased platelet count or decreased AST levels), delivery is still indicated. Conversely, in some cases, maternal and fetal conditions may deteriorate, mandating delivery before the 2 doses of steroids are completed.
Delivery Considerations
HELLP syndrome does not justify immediate cesarean
Patients with HELLP syndrome in labor or with rupture of membranes can deliver vaginally in the absence of obstetric complications. In addition, induction or augmentation of labor is acceptable with either oxytocin infusion or prostaglandins if the fetal gestational age is 32 weeks or more and the cervical Bishop score exceeds 5.
TABLE 7 lists the indications for elective cesarean delivery and summarizes management during surgery. It is important to stabilize the maternal condition, correct coagulopathy, and have blood or blood products available before initiating surgery.
TABLE 7
Cesarean delivery: Indications and management
| Indications for cesarean |
|
| Management during cesarean |
|
Watch for oozing from surgical sites
In a cesarean section, generalized oozing from the surgical site can occur during the operation or immediately postpartum because of the continued drop in platelet count in some of these patients. Thus, it is advisable to insert a subfascial drain and to leave the skin incision open for at least 48 hours to avoid hematoma formation in these areas (FIGURE 1).
FIGURE 1 Insert subfascial drain at cesarean section
Because generalized oozing from the surgical site can occur intraoperatively or immediately postpartum, insert a subfascial drain and leave the skin incision open for at least 48 hours to avoid hematoma formation.
Small doses of systemic opioids are best
For maternal analgesia during labor, give small, intermittent doses of systemic opioids. For repair of episiotomy or vulvar or vaginal lacerations, use local infiltration anesthesia.
Avoid pudendal block because of the potential for bleeding and hematoma formation in this area. Epidural anesthesia may be used after consultation with the anesthesiologist if the platelet count exceeds 75,000/mm3.
Some authors report rising platelet counts after intravenous dexamethasone and, with the improved platelets, greater use of epidural anesthesia, especially in women who achieved a 24-hour latency period before delivery. However, since the platelet count may drop again, insert the epidural catheter once the desired platelet level (with anesthesiologist approval) is reached.
Suspected Liver Hematoma
A rare and potentially life-threatening complication of HELLP syndrome is subcapsular liver hematoma (FIGURE 2). Unfortunately, the rarity of this complication sometimes causes it to be overlooked.
FIGURE 2 Rare but life-threatening: Subcapsular liver hematoma
Liver hematomas can develop antepartum, intrapartum, or postpartum. Presenting symptoms may include severe epigastric or retrosternal pain in association with respiratory difficulty (pain on inspiration), with or without shoulder or neck pain.
Early signs and symptoms
Liver hematomas can develop antepartum, during labor, or in the postpartum period. Presenting symptoms may include severe epigastric or retrosternal pain in association with breathing difficulty (pain on inspiration), with or without shoulder or neck pain.
When profound hypovolemic shock occurs in a previously hypertensive patient, suspect rupture of a liver hematoma. Diagnosis can be made by ultrasound or computed tomography (CT) imaging of the liver, both of which can also confirm intraperitoneal bleeding.
In most cases, rupture involves the right lobe of the liver and is preceded by a parenchymal liver hematoma.
Mortality can exceed 50%
Maternal and fetal mortality increase substantially when a subcapsular liver hematoma is present. In fact, mortality may exceed 50% when frank rupture of the capsule involves liver tissue.
Choose conservative management whenever possible
Management of subcapsular liver hematoma depends on maternal hemodynamic status, integrity of the capsule (ruptured or intact), and the fetal condition.
Conservative management is preferable in hemodynamically stable women with an unruptured hematoma. It consists of close monitoring of the patient’s hemodynamic and coagulation status and serial assessment of the hematoma with ultrasound or CT scan.
Avoid exogenous trauma to the liver, such as frequent abdominal palpation, emesis, or convulsions. Any sudden increase in intraabdominal pressure can led to rupture of the hematoma.
When rupture occurs
This surgical emergency requires an acute multidisciplinary team, including an Ob/Gyn, anesthesiologist, highly qualified surgeon, and a representative of the hospital’s blood bank.
Maternal resuscitation should include:
- transfusion of packed red blood cells to maintain blood pressure and tissue perfusion,
- correction of coagulopathy with fresh frozen plasma and platelets, and
- laparotomy, preferably using a cell saver.
Options at laparotomy include:
- packing and drainage (preferred),
- ligation of the hepatic lacerations,
- embolization of the hepatic artery to the affected liver segment, and
- loosely suturing omentum or surgical mesh to the liver surface.
Postpartum Care
In women who develop HELLP prior to delivery, closely monitor postpartum vital signs, intake and output, and symptoms in intensive care or a similar facility for at least 48 hours.
During this time, my practice is to give the patient intravenous magnesium sulfate and antihypertensive medications as needed to keep systolic blood pressure below 155 mm Hg (the standard is 160 mm Hg) and diastolic blood pressure below 105 mm Hg.
The rationale for this treatment is to prevent bleeding in the brain if the woman has thrombocytopenia.
When HELLP appears in the postpartum period
Several maternal complications from HELLP syndrome may not appear until immediately postpartum. Thus, all women with preeclampsia require close monitoring of vital signs, fluid intake and output, laboratory values, and pulse oximetry for at least 48 hours.
Also continue magnesium sulfate in the postpartum period and keep maternal blood pressure below 155 mm Hg systolic and 105 mm Hg diastolic.
Time to recovery
Most patients begin to improve or completely recover within 72 hours, while others deteriorate further or fail to recover for as long as 1 week after delivery. Thus, some women may require intensive monitoring for several days because of the risk of pulmonary edema, renal failure, or adult respiratory distress syndrome.
Keep in mind that, in some of these women, the cause of the postpartum deterioration may be something other than HELLP syndrome(TABLE 6).
Watch for sudden hypotension
A sudden drop in blood pressure to hypotensive levels can be an early sign of severe hemolysis or unrecognized intraperitoneal blood loss (from surgical sites or ruptured liver hematoma), as well as sepsis.
In a woman with severe hemoconcentration (ie, severe vasoconstriction), sudden hypotension also may indicate excessive vasodilation from antihypertensive drugs such as hydralazine or nifedipine, resulting in relative hypovolemia.
Such a case requires volume resuscitation, blood transfusion (if indicated), and evaluation for unrecognized bleeding.
Use of steroids
Some authors recommend giving intravenous dexamethasone (5 to 10 mg every 12 hours) for approximately 48 hours after delivery in women who develop antepartum or postpartum HELLP. They claim this treatment improves maternal blood tests, shortens recovery, and reduces maternal morbidity.
However, at present, no data indicate this approach has clinical benefit—and the risks are unknown. For these reasons, treatment with intravenous dexamethasone after delivery remains empiric.
The author reports no financial relationships relevant to this article.
BIBLIOGRAPHY
1. Abramovici D, Friedman SA, Mercer BM, Audibert F, Kao L, Sibai BM. Neonatal outcome in severe preeclampsia at 24 to 36 weeks’ gestation. Does HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome matter? Am J Obstet Gynecol. 1999;180:221-225.
2. Audibert F, Friedman SA, Frangieh AY, Sibai BM. Clinical utility of strict diagnostic criteria for the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol. 1996;175:460-464.
3. Egerman RS, Sibai BM. Recognizing and managing HELLP syndrome and its imitators. Contemporary Ob/Gyn. 1997;(October):129-149.
4. Magann EF, Perry KG, Jr, Meydrech EF, Harris RL, Chauchan SP, Martin JN, Jr. Postpartum corticosteroids: accelerated recovery from the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP). Am J Obstet Gynecol. 1994;171:1154-1158.
5. Martin JN, Jr, Thigsen BD, Rose CH, et al. Maternal benefit of high-dose intravenous corticosteroid therapy for HELLP. Am J Obstet Gynecol. 2003;189:830-834.
6. O’Brien JM, Milligan DA, Barton JR. Impact of high-dose corticosteroid therapy for patients with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Am J Obstet Gynecol. 2000;183:921-924.
7. O’Brien JM, Shumate SA, Satchwell SL, Milligan DA, Barton JR. Maternal benefit to corticosteroid therapy in patients with HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome: impact on the rate of regional anesthesia. Am J Obstet Gynecol. 2002;186:475-479.
8. Rinehart BK, Terrone DA, Magann EF, Martin RW, May WL, Martin JN, Jr. Preeclampsia-associated hepatic hemorrhage and rupture: mode of management related to maternal and perinatal outcome. Obstet Gynecol Surv. 1999;196-202.
9. Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169:1000-1006.
10. Sibai BM. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): much ado about nothing? Am J Obstet Gynecol. 1990;162:311-316.
11. Sibai BM. Diagnosis, controversies, and management of HELLP syndrome. Obstet Gynecol. 2004;103:981-991.
12. Tompkins MJ, Thiagarajah S. HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome: the benefit of corticosteroids. Am J Obstet Gynecol. 1999;181:304-309.
13. VanPampus MG, Wolf H, et al. Maternal and perinatal outcome after expectant management of the HELLP syndrome compared with preeclampsia without HELLP syndrome. Eur J Obstet Gynecol Reprod Biol. 1998;76:31.-
14. Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: A severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142:159-167.
15. Weinstein L. Preeclampsia/eclampsia with hemolysis, elevated liver enzymes and thrombocytopenia. Obstet Gynecol. 1985;66:657-660.
Here’s a disturbing fact: If it looks like HELLP syndrome, and impairs the patient like HELLP syndrome, it isn’t necessarily HELLP syndrome. A plethora of diagnostic criteria from different investigators over the years has confused the issue of what constitutes this syndrome—not to mention how to manage it.
A management issue has also attracted recent attention: use of corticosteroids either antepartum to enhance maternal status so that epidural anesthesia can be administered, or postpartum to improve platelets. Such improvements are only transient, however, and we lack definitive data on the benefits.
One thing is certain, however. The combination of hemolysis, liver dysfunction or injury, and platelet consumption in women with preeclampsia makes adverse maternal and perinatal outcomes more likely and leaves no room for expectant management.
HELLP syndrome also has become a major issue in litigation against obstetricians and medical and surgical consultants. Lawsuits usually allege misdiagnosed preeclampsia, delayed delivery, or improper recognition and management of complications.
Pinning HELLP Down
One of the best tools to identify HELLP syndrome is a healthy dose of suspicion, since it can affect any pregnant woman at any time: antepartum, intrapartum, or within 1 week postpartum. Approximately 72% of cases are diagnosed before delivery, and the rest are diagnosed during the first week postpartum.
Weinstein noted that the signs and symptoms of HELLP syndrome can occur without clinical evidence of severe preeclampsia (severe hypertension and/or severe proteinuria). Indeed, he reported that hypertension can be mild or absent in most patients with HELLP, and proteinuria can be mild.
Weinstein coined the term HELLP syndrome in 1982 to describe these abnormalities in women with preeclampsia:
- H = hemolysis
- EL = elevated liver enzymes
- LP = low platelets
Another obstacle to early detection: Patients may have nonspecific signs and symptoms, none of which are diagnostic of classical preeclampsia.
However, HELLP syndrome is most common in women who have already been diagnosed with gestational hypertension and/or preeclampsia.
HELLP is more likely with severe hypertension
Overall, the incidence of HELLP syndrome in women with gestational hypertension/preeclampsia increases with the severity of the condition. HELLP syndrome also is more likely in women with early-onset hypertension/preeclampsia (before 34 weeks’ gestation).
Making The Diagnosis
HELLP syndrome is diagnosed when all 3 of the following are present:
- Hemolysis, defined as the presence of microangiopathic hemolytic anemia. This is the hallmark of the triad.
- Elevated liver enzymes (either aspartate aminotransferase [AST] or alanine aminotransferase [ALT]). This component signifies liver cell ischemia and/or necrosis.
- Low platelet count (<100,000/mm3). TABLE 1 summarizes the laboratory criteria for the diagnosis.
When to begin testing
In women with new-onset hypertension, order a complete blood count with platelets and liver enzyme analysis at the time of diagnosis and serially thereafter. The frequency of these tests depends on the initial test results, severity of disease, and onset of symptoms.
In women without hypertension, I recommend obtaining the same blood tests at the onset of any of the signs and symptoms listed in TABLE 2.
TABLE 2
Conditions that heighten the risk of HELLP
|
Assessing test results
Clinicians should be familiar with the upper limit for liverenzyme tests in their laboratory. I suggest a cutoff more than twice the upper limit for a particular test.
Also keep in mind that these parameters are dynamic; some women will meet only some of the criteria early in the disease process. Moreover, maternal complications are substantially higher when all 3 components are present than when only 1 or 2 are present.
Look for these clinical findings
Hypertension. Most women with HELLP syndrome have hypertension. In 15% to 50% of cases, the hypertension is mild, but it may be absent in 15%.
Proteinuria. Most patients also have proteinuria by dipstick (≥1+). Proteinuria may be absent in approximately 13% of women with HELLP syndrome, although they will likely have many of the symptoms reported by women with severe preeclampsia.
TABLE 3 lists the signs and symptoms to be expected in these patients, along with their frequency.
TABLE 3
Signs and symptoms
| CONDITION | FREQUENCY (%) |
|---|---|
| Hypertension | 85 |
| Proteinuria | 87 |
| Right upper quadrant or epigastric pain | 40–90 |
| Nausea or vomiting | 29–84 |
| Headaches | 33–60 |
| Visual changes | 10–20 |
| Mucosal bleeding | 10 |
| Jaundice | 5 |
The usual times of onset
Antepartum cases. As was previously noted, HELLP syndrome usually develops before delivery, with the most frequent onset being before 37 weeks’ gestation ( TABLE 4).
In the postpartum period, most cases develop within 48 hours after delivery. Of these, approximately 90% occur in women who had antepartum preeclampsia that progressed to HELLP syndrome in the postpartum period. However, approximately 20% of postpartum cases develop more than 48 hours after delivery.
Another important point: HELLP syndrome can develop for the first time postpartum in women who had no evidence of preeclampsia before or during labor. Thus, it is important to educate all postpartum women to report new symptoms (listed in TABLE 3) as soon as possible. When these symptoms develop, evaluate the patient for both preeclampsia and HELLP syndrome.
TABLE 4
Usual times of onset*
| RELATION TO DELIVERY | PERCENTAGE |
| Antepartum | 72 |
| Postpartum | 28 |
| ≤48 hours | 80 |
| >48 hours | 20 |
| GESTATIONAL AGE (WEEKS) | PERCENTAGE |
| 17–20 | 2 |
| 21–27 | 10 |
| 28–36 | 68 |
| >37 | 20 |
| * Based on 700 cases | |
Risk for life-threatening maternal complications
When all components of HELLP syndrome are present in a woman with preeclampsia, the risk of maternal death and serious maternal morbidities increases substantially (TABLE 5). The rate of these complications depends on gestational age at onset, presence of associated obstetric complications (eclampsia, abruptio placentae, peripartum hemorrhage, or fetal demise) or preexisting conditions (lupus, renal disease, chronic hypertension, or type 1 diabetes).
Abruptio placentae increases the risk of disseminated intravascular coagulopathy (DIC), as well as the need for blood transfusions.
Marked ascites (>1 L) leads to higher rates of cardiopulmonary complications.
TABLE 5
Maternal complications
| COMPLICATION | FREQUENCY (%) |
|---|---|
| Death | 1 |
| Adult respiratory distress syndrome | 1 |
| Laryngeal edema | 1–2 |
| Liver failure or hemorrhage | 1–2 |
| Acute renal failure | 5–8 |
| Pulmonary edema | 6–8 |
| Pleural effusions | 6–10 |
| Abruptio placentae | 10–15 |
| Disseminated intravascular coagulopathy | 10–15 |
| Marked ascites | 10–15 |
Differential diagnosis
When diagnosing HELLP syndrome, confirm or exclude the conditions listed in TABLE 6, since the presenting symptoms and clinical and laboratory findings in women with HELLP syndrome overlap those of several microangiopathic disorders that can develop during pregnancy and/or postpartum. In some women, preeclampsia may be superimposed on one of these disorders, further confounding an already difficult differential diagnosis.
Because of the remarkably similar clinical and laboratory findings of these diseases, make every effort to achieve an accurate diagnosis, since management and outcomes may differ among these conditions.
TABLE 6
Differential diagnosis
|
Initial Management
Hospitalize the patient
Because HELLP syndrome usually is characterized by progressive and sometimes sudden deterioration in maternal and fetal conditions, patients should be hospitalized and observed in a labor and delivery unit.
Initially, assume the patient has severe preeclampsia and treat her with intravenous magnesium sulfate to prevent convulsions and antihypertensive medications as needed to keep systolic blood pressure below 160 mm Hg and diastolic blood pressure below 105 mm Hg.
Blood tests should include:
- complete blood count with platelet count,
- peripheral smear evaluation,
- serum AST,
- lactate dehydrogenase,
- creatinine,
- bilirubin, and
- coagulation studies.
These tests help confirm the diagnosis and check for the presence of DIC, massive hemolysis, severe anemia, or renal failure.
The first priority is to assess the patient for the presence of cardiovascular complications, signs of liver hematoma or hemorrhage, and abruptio placentae. If any is present—particularly hypotension, hypovolemia, DIC, or pulmonary edema—make every effort to stabilize the maternal condition.
Can delivery wait 48 hours for corticosteroids?
Evaluate fetal status by heart rate monitoring or biophysical profile, and confirm gestational age. Then decide whether delivery is indicated or can be delayed for 48 hours so that corticosteroids can be given.
No room for expectant management. Do not consider expectant management in women with true HELLP syndrome. Delivery can only be delayed for a maximum of 48 hours—and only when both mother and fetus are stable, at 24 to 34 weeks’ gestation, and awaiting the benefit of corticosteroids.
Corticosteroid dosing. My practice is to give 2 doses of either betamethasone 12 mg intramuscularly every 12 hours or dexamethasone 12 mg intravenously every 12 hours. This is to improve maternal status, at least temporarily.
Initiate delivery within 24 hours after the last steroid dose, with continuous monitoring in the labor and delivery unit.
Although some women may demonstrate transient improvement in their blood tests (eg, increased platelet count or decreased AST levels), delivery is still indicated. Conversely, in some cases, maternal and fetal conditions may deteriorate, mandating delivery before the 2 doses of steroids are completed.
Delivery Considerations
HELLP syndrome does not justify immediate cesarean
Patients with HELLP syndrome in labor or with rupture of membranes can deliver vaginally in the absence of obstetric complications. In addition, induction or augmentation of labor is acceptable with either oxytocin infusion or prostaglandins if the fetal gestational age is 32 weeks or more and the cervical Bishop score exceeds 5.
TABLE 7 lists the indications for elective cesarean delivery and summarizes management during surgery. It is important to stabilize the maternal condition, correct coagulopathy, and have blood or blood products available before initiating surgery.
TABLE 7
Cesarean delivery: Indications and management
| Indications for cesarean |
|
| Management during cesarean |
|
Watch for oozing from surgical sites
In a cesarean section, generalized oozing from the surgical site can occur during the operation or immediately postpartum because of the continued drop in platelet count in some of these patients. Thus, it is advisable to insert a subfascial drain and to leave the skin incision open for at least 48 hours to avoid hematoma formation in these areas (FIGURE 1).
FIGURE 1 Insert subfascial drain at cesarean section
Because generalized oozing from the surgical site can occur intraoperatively or immediately postpartum, insert a subfascial drain and leave the skin incision open for at least 48 hours to avoid hematoma formation.
Small doses of systemic opioids are best
For maternal analgesia during labor, give small, intermittent doses of systemic opioids. For repair of episiotomy or vulvar or vaginal lacerations, use local infiltration anesthesia.
Avoid pudendal block because of the potential for bleeding and hematoma formation in this area. Epidural anesthesia may be used after consultation with the anesthesiologist if the platelet count exceeds 75,000/mm3.
Some authors report rising platelet counts after intravenous dexamethasone and, with the improved platelets, greater use of epidural anesthesia, especially in women who achieved a 24-hour latency period before delivery. However, since the platelet count may drop again, insert the epidural catheter once the desired platelet level (with anesthesiologist approval) is reached.
Suspected Liver Hematoma
A rare and potentially life-threatening complication of HELLP syndrome is subcapsular liver hematoma (FIGURE 2). Unfortunately, the rarity of this complication sometimes causes it to be overlooked.
FIGURE 2 Rare but life-threatening: Subcapsular liver hematoma
Liver hematomas can develop antepartum, intrapartum, or postpartum. Presenting symptoms may include severe epigastric or retrosternal pain in association with respiratory difficulty (pain on inspiration), with or without shoulder or neck pain.
Early signs and symptoms
Liver hematomas can develop antepartum, during labor, or in the postpartum period. Presenting symptoms may include severe epigastric or retrosternal pain in association with breathing difficulty (pain on inspiration), with or without shoulder or neck pain.
When profound hypovolemic shock occurs in a previously hypertensive patient, suspect rupture of a liver hematoma. Diagnosis can be made by ultrasound or computed tomography (CT) imaging of the liver, both of which can also confirm intraperitoneal bleeding.
In most cases, rupture involves the right lobe of the liver and is preceded by a parenchymal liver hematoma.
Mortality can exceed 50%
Maternal and fetal mortality increase substantially when a subcapsular liver hematoma is present. In fact, mortality may exceed 50% when frank rupture of the capsule involves liver tissue.
Choose conservative management whenever possible
Management of subcapsular liver hematoma depends on maternal hemodynamic status, integrity of the capsule (ruptured or intact), and the fetal condition.
Conservative management is preferable in hemodynamically stable women with an unruptured hematoma. It consists of close monitoring of the patient’s hemodynamic and coagulation status and serial assessment of the hematoma with ultrasound or CT scan.
Avoid exogenous trauma to the liver, such as frequent abdominal palpation, emesis, or convulsions. Any sudden increase in intraabdominal pressure can led to rupture of the hematoma.
When rupture occurs
This surgical emergency requires an acute multidisciplinary team, including an Ob/Gyn, anesthesiologist, highly qualified surgeon, and a representative of the hospital’s blood bank.
Maternal resuscitation should include:
- transfusion of packed red blood cells to maintain blood pressure and tissue perfusion,
- correction of coagulopathy with fresh frozen plasma and platelets, and
- laparotomy, preferably using a cell saver.
Options at laparotomy include:
- packing and drainage (preferred),
- ligation of the hepatic lacerations,
- embolization of the hepatic artery to the affected liver segment, and
- loosely suturing omentum or surgical mesh to the liver surface.
Postpartum Care
In women who develop HELLP prior to delivery, closely monitor postpartum vital signs, intake and output, and symptoms in intensive care or a similar facility for at least 48 hours.
During this time, my practice is to give the patient intravenous magnesium sulfate and antihypertensive medications as needed to keep systolic blood pressure below 155 mm Hg (the standard is 160 mm Hg) and diastolic blood pressure below 105 mm Hg.
The rationale for this treatment is to prevent bleeding in the brain if the woman has thrombocytopenia.
When HELLP appears in the postpartum period
Several maternal complications from HELLP syndrome may not appear until immediately postpartum. Thus, all women with preeclampsia require close monitoring of vital signs, fluid intake and output, laboratory values, and pulse oximetry for at least 48 hours.
Also continue magnesium sulfate in the postpartum period and keep maternal blood pressure below 155 mm Hg systolic and 105 mm Hg diastolic.
Time to recovery
Most patients begin to improve or completely recover within 72 hours, while others deteriorate further or fail to recover for as long as 1 week after delivery. Thus, some women may require intensive monitoring for several days because of the risk of pulmonary edema, renal failure, or adult respiratory distress syndrome.
Keep in mind that, in some of these women, the cause of the postpartum deterioration may be something other than HELLP syndrome(TABLE 6).
Watch for sudden hypotension
A sudden drop in blood pressure to hypotensive levels can be an early sign of severe hemolysis or unrecognized intraperitoneal blood loss (from surgical sites or ruptured liver hematoma), as well as sepsis.
In a woman with severe hemoconcentration (ie, severe vasoconstriction), sudden hypotension also may indicate excessive vasodilation from antihypertensive drugs such as hydralazine or nifedipine, resulting in relative hypovolemia.
Such a case requires volume resuscitation, blood transfusion (if indicated), and evaluation for unrecognized bleeding.
Use of steroids
Some authors recommend giving intravenous dexamethasone (5 to 10 mg every 12 hours) for approximately 48 hours after delivery in women who develop antepartum or postpartum HELLP. They claim this treatment improves maternal blood tests, shortens recovery, and reduces maternal morbidity.
However, at present, no data indicate this approach has clinical benefit—and the risks are unknown. For these reasons, treatment with intravenous dexamethasone after delivery remains empiric.
The author reports no financial relationships relevant to this article.
Here’s a disturbing fact: If it looks like HELLP syndrome, and impairs the patient like HELLP syndrome, it isn’t necessarily HELLP syndrome. A plethora of diagnostic criteria from different investigators over the years has confused the issue of what constitutes this syndrome—not to mention how to manage it.
A management issue has also attracted recent attention: use of corticosteroids either antepartum to enhance maternal status so that epidural anesthesia can be administered, or postpartum to improve platelets. Such improvements are only transient, however, and we lack definitive data on the benefits.
One thing is certain, however. The combination of hemolysis, liver dysfunction or injury, and platelet consumption in women with preeclampsia makes adverse maternal and perinatal outcomes more likely and leaves no room for expectant management.
HELLP syndrome also has become a major issue in litigation against obstetricians and medical and surgical consultants. Lawsuits usually allege misdiagnosed preeclampsia, delayed delivery, or improper recognition and management of complications.
Pinning HELLP Down
One of the best tools to identify HELLP syndrome is a healthy dose of suspicion, since it can affect any pregnant woman at any time: antepartum, intrapartum, or within 1 week postpartum. Approximately 72% of cases are diagnosed before delivery, and the rest are diagnosed during the first week postpartum.
Weinstein noted that the signs and symptoms of HELLP syndrome can occur without clinical evidence of severe preeclampsia (severe hypertension and/or severe proteinuria). Indeed, he reported that hypertension can be mild or absent in most patients with HELLP, and proteinuria can be mild.
Weinstein coined the term HELLP syndrome in 1982 to describe these abnormalities in women with preeclampsia:
- H = hemolysis
- EL = elevated liver enzymes
- LP = low platelets
Another obstacle to early detection: Patients may have nonspecific signs and symptoms, none of which are diagnostic of classical preeclampsia.
However, HELLP syndrome is most common in women who have already been diagnosed with gestational hypertension and/or preeclampsia.
HELLP is more likely with severe hypertension
Overall, the incidence of HELLP syndrome in women with gestational hypertension/preeclampsia increases with the severity of the condition. HELLP syndrome also is more likely in women with early-onset hypertension/preeclampsia (before 34 weeks’ gestation).
Making The Diagnosis
HELLP syndrome is diagnosed when all 3 of the following are present:
- Hemolysis, defined as the presence of microangiopathic hemolytic anemia. This is the hallmark of the triad.
- Elevated liver enzymes (either aspartate aminotransferase [AST] or alanine aminotransferase [ALT]). This component signifies liver cell ischemia and/or necrosis.
- Low platelet count (<100,000/mm3). TABLE 1 summarizes the laboratory criteria for the diagnosis.
When to begin testing
In women with new-onset hypertension, order a complete blood count with platelets and liver enzyme analysis at the time of diagnosis and serially thereafter. The frequency of these tests depends on the initial test results, severity of disease, and onset of symptoms.
In women without hypertension, I recommend obtaining the same blood tests at the onset of any of the signs and symptoms listed in TABLE 2.
TABLE 2
Conditions that heighten the risk of HELLP
|
Assessing test results
Clinicians should be familiar with the upper limit for liverenzyme tests in their laboratory. I suggest a cutoff more than twice the upper limit for a particular test.
Also keep in mind that these parameters are dynamic; some women will meet only some of the criteria early in the disease process. Moreover, maternal complications are substantially higher when all 3 components are present than when only 1 or 2 are present.
Look for these clinical findings
Hypertension. Most women with HELLP syndrome have hypertension. In 15% to 50% of cases, the hypertension is mild, but it may be absent in 15%.
Proteinuria. Most patients also have proteinuria by dipstick (≥1+). Proteinuria may be absent in approximately 13% of women with HELLP syndrome, although they will likely have many of the symptoms reported by women with severe preeclampsia.
TABLE 3 lists the signs and symptoms to be expected in these patients, along with their frequency.
TABLE 3
Signs and symptoms
| CONDITION | FREQUENCY (%) |
|---|---|
| Hypertension | 85 |
| Proteinuria | 87 |
| Right upper quadrant or epigastric pain | 40–90 |
| Nausea or vomiting | 29–84 |
| Headaches | 33–60 |
| Visual changes | 10–20 |
| Mucosal bleeding | 10 |
| Jaundice | 5 |
The usual times of onset
Antepartum cases. As was previously noted, HELLP syndrome usually develops before delivery, with the most frequent onset being before 37 weeks’ gestation ( TABLE 4).
In the postpartum period, most cases develop within 48 hours after delivery. Of these, approximately 90% occur in women who had antepartum preeclampsia that progressed to HELLP syndrome in the postpartum period. However, approximately 20% of postpartum cases develop more than 48 hours after delivery.
Another important point: HELLP syndrome can develop for the first time postpartum in women who had no evidence of preeclampsia before or during labor. Thus, it is important to educate all postpartum women to report new symptoms (listed in TABLE 3) as soon as possible. When these symptoms develop, evaluate the patient for both preeclampsia and HELLP syndrome.
TABLE 4
Usual times of onset*
| RELATION TO DELIVERY | PERCENTAGE |
| Antepartum | 72 |
| Postpartum | 28 |
| ≤48 hours | 80 |
| >48 hours | 20 |
| GESTATIONAL AGE (WEEKS) | PERCENTAGE |
| 17–20 | 2 |
| 21–27 | 10 |
| 28–36 | 68 |
| >37 | 20 |
| * Based on 700 cases | |
Risk for life-threatening maternal complications
When all components of HELLP syndrome are present in a woman with preeclampsia, the risk of maternal death and serious maternal morbidities increases substantially (TABLE 5). The rate of these complications depends on gestational age at onset, presence of associated obstetric complications (eclampsia, abruptio placentae, peripartum hemorrhage, or fetal demise) or preexisting conditions (lupus, renal disease, chronic hypertension, or type 1 diabetes).
Abruptio placentae increases the risk of disseminated intravascular coagulopathy (DIC), as well as the need for blood transfusions.
Marked ascites (>1 L) leads to higher rates of cardiopulmonary complications.
TABLE 5
Maternal complications
| COMPLICATION | FREQUENCY (%) |
|---|---|
| Death | 1 |
| Adult respiratory distress syndrome | 1 |
| Laryngeal edema | 1–2 |
| Liver failure or hemorrhage | 1–2 |
| Acute renal failure | 5–8 |
| Pulmonary edema | 6–8 |
| Pleural effusions | 6–10 |
| Abruptio placentae | 10–15 |
| Disseminated intravascular coagulopathy | 10–15 |
| Marked ascites | 10–15 |
Differential diagnosis
When diagnosing HELLP syndrome, confirm or exclude the conditions listed in TABLE 6, since the presenting symptoms and clinical and laboratory findings in women with HELLP syndrome overlap those of several microangiopathic disorders that can develop during pregnancy and/or postpartum. In some women, preeclampsia may be superimposed on one of these disorders, further confounding an already difficult differential diagnosis.
Because of the remarkably similar clinical and laboratory findings of these diseases, make every effort to achieve an accurate diagnosis, since management and outcomes may differ among these conditions.
TABLE 6
Differential diagnosis
|
Initial Management
Hospitalize the patient
Because HELLP syndrome usually is characterized by progressive and sometimes sudden deterioration in maternal and fetal conditions, patients should be hospitalized and observed in a labor and delivery unit.
Initially, assume the patient has severe preeclampsia and treat her with intravenous magnesium sulfate to prevent convulsions and antihypertensive medications as needed to keep systolic blood pressure below 160 mm Hg and diastolic blood pressure below 105 mm Hg.
Blood tests should include:
- complete blood count with platelet count,
- peripheral smear evaluation,
- serum AST,
- lactate dehydrogenase,
- creatinine,
- bilirubin, and
- coagulation studies.
These tests help confirm the diagnosis and check for the presence of DIC, massive hemolysis, severe anemia, or renal failure.
The first priority is to assess the patient for the presence of cardiovascular complications, signs of liver hematoma or hemorrhage, and abruptio placentae. If any is present—particularly hypotension, hypovolemia, DIC, or pulmonary edema—make every effort to stabilize the maternal condition.
Can delivery wait 48 hours for corticosteroids?
Evaluate fetal status by heart rate monitoring or biophysical profile, and confirm gestational age. Then decide whether delivery is indicated or can be delayed for 48 hours so that corticosteroids can be given.
No room for expectant management. Do not consider expectant management in women with true HELLP syndrome. Delivery can only be delayed for a maximum of 48 hours—and only when both mother and fetus are stable, at 24 to 34 weeks’ gestation, and awaiting the benefit of corticosteroids.
Corticosteroid dosing. My practice is to give 2 doses of either betamethasone 12 mg intramuscularly every 12 hours or dexamethasone 12 mg intravenously every 12 hours. This is to improve maternal status, at least temporarily.
Initiate delivery within 24 hours after the last steroid dose, with continuous monitoring in the labor and delivery unit.
Although some women may demonstrate transient improvement in their blood tests (eg, increased platelet count or decreased AST levels), delivery is still indicated. Conversely, in some cases, maternal and fetal conditions may deteriorate, mandating delivery before the 2 doses of steroids are completed.
Delivery Considerations
HELLP syndrome does not justify immediate cesarean
Patients with HELLP syndrome in labor or with rupture of membranes can deliver vaginally in the absence of obstetric complications. In addition, induction or augmentation of labor is acceptable with either oxytocin infusion or prostaglandins if the fetal gestational age is 32 weeks or more and the cervical Bishop score exceeds 5.
TABLE 7 lists the indications for elective cesarean delivery and summarizes management during surgery. It is important to stabilize the maternal condition, correct coagulopathy, and have blood or blood products available before initiating surgery.
TABLE 7
Cesarean delivery: Indications and management
| Indications for cesarean |
|
| Management during cesarean |
|
Watch for oozing from surgical sites
In a cesarean section, generalized oozing from the surgical site can occur during the operation or immediately postpartum because of the continued drop in platelet count in some of these patients. Thus, it is advisable to insert a subfascial drain and to leave the skin incision open for at least 48 hours to avoid hematoma formation in these areas (FIGURE 1).
FIGURE 1 Insert subfascial drain at cesarean section
Because generalized oozing from the surgical site can occur intraoperatively or immediately postpartum, insert a subfascial drain and leave the skin incision open for at least 48 hours to avoid hematoma formation.
Small doses of systemic opioids are best
For maternal analgesia during labor, give small, intermittent doses of systemic opioids. For repair of episiotomy or vulvar or vaginal lacerations, use local infiltration anesthesia.
Avoid pudendal block because of the potential for bleeding and hematoma formation in this area. Epidural anesthesia may be used after consultation with the anesthesiologist if the platelet count exceeds 75,000/mm3.
Some authors report rising platelet counts after intravenous dexamethasone and, with the improved platelets, greater use of epidural anesthesia, especially in women who achieved a 24-hour latency period before delivery. However, since the platelet count may drop again, insert the epidural catheter once the desired platelet level (with anesthesiologist approval) is reached.
Suspected Liver Hematoma
A rare and potentially life-threatening complication of HELLP syndrome is subcapsular liver hematoma (FIGURE 2). Unfortunately, the rarity of this complication sometimes causes it to be overlooked.
FIGURE 2 Rare but life-threatening: Subcapsular liver hematoma
Liver hematomas can develop antepartum, intrapartum, or postpartum. Presenting symptoms may include severe epigastric or retrosternal pain in association with respiratory difficulty (pain on inspiration), with or without shoulder or neck pain.
Early signs and symptoms
Liver hematomas can develop antepartum, during labor, or in the postpartum period. Presenting symptoms may include severe epigastric or retrosternal pain in association with breathing difficulty (pain on inspiration), with or without shoulder or neck pain.
When profound hypovolemic shock occurs in a previously hypertensive patient, suspect rupture of a liver hematoma. Diagnosis can be made by ultrasound or computed tomography (CT) imaging of the liver, both of which can also confirm intraperitoneal bleeding.
In most cases, rupture involves the right lobe of the liver and is preceded by a parenchymal liver hematoma.
Mortality can exceed 50%
Maternal and fetal mortality increase substantially when a subcapsular liver hematoma is present. In fact, mortality may exceed 50% when frank rupture of the capsule involves liver tissue.
Choose conservative management whenever possible
Management of subcapsular liver hematoma depends on maternal hemodynamic status, integrity of the capsule (ruptured or intact), and the fetal condition.
Conservative management is preferable in hemodynamically stable women with an unruptured hematoma. It consists of close monitoring of the patient’s hemodynamic and coagulation status and serial assessment of the hematoma with ultrasound or CT scan.
Avoid exogenous trauma to the liver, such as frequent abdominal palpation, emesis, or convulsions. Any sudden increase in intraabdominal pressure can led to rupture of the hematoma.
When rupture occurs
This surgical emergency requires an acute multidisciplinary team, including an Ob/Gyn, anesthesiologist, highly qualified surgeon, and a representative of the hospital’s blood bank.
Maternal resuscitation should include:
- transfusion of packed red blood cells to maintain blood pressure and tissue perfusion,
- correction of coagulopathy with fresh frozen plasma and platelets, and
- laparotomy, preferably using a cell saver.
Options at laparotomy include:
- packing and drainage (preferred),
- ligation of the hepatic lacerations,
- embolization of the hepatic artery to the affected liver segment, and
- loosely suturing omentum or surgical mesh to the liver surface.
Postpartum Care
In women who develop HELLP prior to delivery, closely monitor postpartum vital signs, intake and output, and symptoms in intensive care or a similar facility for at least 48 hours.
During this time, my practice is to give the patient intravenous magnesium sulfate and antihypertensive medications as needed to keep systolic blood pressure below 155 mm Hg (the standard is 160 mm Hg) and diastolic blood pressure below 105 mm Hg.
The rationale for this treatment is to prevent bleeding in the brain if the woman has thrombocytopenia.
When HELLP appears in the postpartum period
Several maternal complications from HELLP syndrome may not appear until immediately postpartum. Thus, all women with preeclampsia require close monitoring of vital signs, fluid intake and output, laboratory values, and pulse oximetry for at least 48 hours.
Also continue magnesium sulfate in the postpartum period and keep maternal blood pressure below 155 mm Hg systolic and 105 mm Hg diastolic.
Time to recovery
Most patients begin to improve or completely recover within 72 hours, while others deteriorate further or fail to recover for as long as 1 week after delivery. Thus, some women may require intensive monitoring for several days because of the risk of pulmonary edema, renal failure, or adult respiratory distress syndrome.
Keep in mind that, in some of these women, the cause of the postpartum deterioration may be something other than HELLP syndrome(TABLE 6).
Watch for sudden hypotension
A sudden drop in blood pressure to hypotensive levels can be an early sign of severe hemolysis or unrecognized intraperitoneal blood loss (from surgical sites or ruptured liver hematoma), as well as sepsis.
In a woman with severe hemoconcentration (ie, severe vasoconstriction), sudden hypotension also may indicate excessive vasodilation from antihypertensive drugs such as hydralazine or nifedipine, resulting in relative hypovolemia.
Such a case requires volume resuscitation, blood transfusion (if indicated), and evaluation for unrecognized bleeding.
Use of steroids
Some authors recommend giving intravenous dexamethasone (5 to 10 mg every 12 hours) for approximately 48 hours after delivery in women who develop antepartum or postpartum HELLP. They claim this treatment improves maternal blood tests, shortens recovery, and reduces maternal morbidity.
However, at present, no data indicate this approach has clinical benefit—and the risks are unknown. For these reasons, treatment with intravenous dexamethasone after delivery remains empiric.
The author reports no financial relationships relevant to this article.
BIBLIOGRAPHY
1. Abramovici D, Friedman SA, Mercer BM, Audibert F, Kao L, Sibai BM. Neonatal outcome in severe preeclampsia at 24 to 36 weeks’ gestation. Does HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome matter? Am J Obstet Gynecol. 1999;180:221-225.
2. Audibert F, Friedman SA, Frangieh AY, Sibai BM. Clinical utility of strict diagnostic criteria for the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol. 1996;175:460-464.
3. Egerman RS, Sibai BM. Recognizing and managing HELLP syndrome and its imitators. Contemporary Ob/Gyn. 1997;(October):129-149.
4. Magann EF, Perry KG, Jr, Meydrech EF, Harris RL, Chauchan SP, Martin JN, Jr. Postpartum corticosteroids: accelerated recovery from the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP). Am J Obstet Gynecol. 1994;171:1154-1158.
5. Martin JN, Jr, Thigsen BD, Rose CH, et al. Maternal benefit of high-dose intravenous corticosteroid therapy for HELLP. Am J Obstet Gynecol. 2003;189:830-834.
6. O’Brien JM, Milligan DA, Barton JR. Impact of high-dose corticosteroid therapy for patients with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Am J Obstet Gynecol. 2000;183:921-924.
7. O’Brien JM, Shumate SA, Satchwell SL, Milligan DA, Barton JR. Maternal benefit to corticosteroid therapy in patients with HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome: impact on the rate of regional anesthesia. Am J Obstet Gynecol. 2002;186:475-479.
8. Rinehart BK, Terrone DA, Magann EF, Martin RW, May WL, Martin JN, Jr. Preeclampsia-associated hepatic hemorrhage and rupture: mode of management related to maternal and perinatal outcome. Obstet Gynecol Surv. 1999;196-202.
9. Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169:1000-1006.
10. Sibai BM. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): much ado about nothing? Am J Obstet Gynecol. 1990;162:311-316.
11. Sibai BM. Diagnosis, controversies, and management of HELLP syndrome. Obstet Gynecol. 2004;103:981-991.
12. Tompkins MJ, Thiagarajah S. HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome: the benefit of corticosteroids. Am J Obstet Gynecol. 1999;181:304-309.
13. VanPampus MG, Wolf H, et al. Maternal and perinatal outcome after expectant management of the HELLP syndrome compared with preeclampsia without HELLP syndrome. Eur J Obstet Gynecol Reprod Biol. 1998;76:31.-
14. Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: A severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142:159-167.
15. Weinstein L. Preeclampsia/eclampsia with hemolysis, elevated liver enzymes and thrombocytopenia. Obstet Gynecol. 1985;66:657-660.
BIBLIOGRAPHY
1. Abramovici D, Friedman SA, Mercer BM, Audibert F, Kao L, Sibai BM. Neonatal outcome in severe preeclampsia at 24 to 36 weeks’ gestation. Does HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome matter? Am J Obstet Gynecol. 1999;180:221-225.
2. Audibert F, Friedman SA, Frangieh AY, Sibai BM. Clinical utility of strict diagnostic criteria for the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol. 1996;175:460-464.
3. Egerman RS, Sibai BM. Recognizing and managing HELLP syndrome and its imitators. Contemporary Ob/Gyn. 1997;(October):129-149.
4. Magann EF, Perry KG, Jr, Meydrech EF, Harris RL, Chauchan SP, Martin JN, Jr. Postpartum corticosteroids: accelerated recovery from the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP). Am J Obstet Gynecol. 1994;171:1154-1158.
5. Martin JN, Jr, Thigsen BD, Rose CH, et al. Maternal benefit of high-dose intravenous corticosteroid therapy for HELLP. Am J Obstet Gynecol. 2003;189:830-834.
6. O’Brien JM, Milligan DA, Barton JR. Impact of high-dose corticosteroid therapy for patients with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Am J Obstet Gynecol. 2000;183:921-924.
7. O’Brien JM, Shumate SA, Satchwell SL, Milligan DA, Barton JR. Maternal benefit to corticosteroid therapy in patients with HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome: impact on the rate of regional anesthesia. Am J Obstet Gynecol. 2002;186:475-479.
8. Rinehart BK, Terrone DA, Magann EF, Martin RW, May WL, Martin JN, Jr. Preeclampsia-associated hepatic hemorrhage and rupture: mode of management related to maternal and perinatal outcome. Obstet Gynecol Surv. 1999;196-202.
9. Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169:1000-1006.
10. Sibai BM. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): much ado about nothing? Am J Obstet Gynecol. 1990;162:311-316.
11. Sibai BM. Diagnosis, controversies, and management of HELLP syndrome. Obstet Gynecol. 2004;103:981-991.
12. Tompkins MJ, Thiagarajah S. HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome: the benefit of corticosteroids. Am J Obstet Gynecol. 1999;181:304-309.
13. VanPampus MG, Wolf H, et al. Maternal and perinatal outcome after expectant management of the HELLP syndrome compared with preeclampsia without HELLP syndrome. Eur J Obstet Gynecol Reprod Biol. 1998;76:31.-
14. Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: A severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142:159-167.
15. Weinstein L. Preeclampsia/eclampsia with hemolysis, elevated liver enzymes and thrombocytopenia. Obstet Gynecol. 1985;66:657-660.
Expectant management of preeclampsia
Once you decide to expectantly manage a patient with preeclampsia, the balancing act begins. That means weighing fetal benefits against maternal risks, since the only justification for expectant management is to prolong pregnancy for fetal gain—there is no advantage to the mother.
The best approach is to classify the woman’s preeclampsia by the degree of severity and gestational age at the time of diagnosis, then follow recommendations tailored to that particular category.
This article offers guidelines for expectant management of mild and severe preeclampsia, preeclampsia superimposed on a preexisting medical condition, and intrapartum and postpartum care.
Mild preeclampsia
The earlier preeclampsia develops, the greater the risk it will become severe. The need for hospitalization depends on gestational age, blood pressure, proteinuria levels, maternal symptoms, and reliability of the patient.
Preeclampsia is mild when systolic blood pressure reaches 140 to 159 mm Hg or diastolic pressure measures 90 to 109 mm Hg on at least 2 occasions more than 6 hours apart after 20 weeks’ gestation in a woman who previously had normal blood pressure. In preeclampsia, this hypertension is accompanied by proteinuria of 0.3 to 4.9 g in a 24-hour urine sample (1+ or 2+ by dipstick on 2 occasions).
At or beyond 37 weeks’ gestation
In general, women diagnosed with preeclampsia at this gestational age have pregnancy outcomes similar to those of normotensive gravidas. Thus, they benefit from induction of labor and delivery.
32 to 36 weeks’ gestation
Close maternal and fetal evaluation is essential. (It is assumed these women have no labor or membrane rupture and normal fetal testing; otherwise, delivery is indicated at 34 weeks or beyond.)
In general, hospitalization is indicated when any of the following circumstances are present (FIGURE 1):
- the patient is unreliable,
- 2 or more systolic blood pressure readings exceed 150 mm Hg,
- 2 or more diastolic blood pressure readings exceed 100 mm Hg,
- proteinuria occurs at a rate exceeding 1 g/24 hours, or
- persistent maternal symptoms are present.
FIGURE 1 Treatment of mild preeclampsia in healthy women
Before 32 weeks’ gestation
These women are at high risk of progressing to severe disease. They also are more likely to have adverse perinatal outcomes such as intrauterine growth restriction (IUGR) (15% to 20%), preterm delivery (50%), and abruptio placentae (1% to 2%), compared with women diagnosed with preeclampsia at 32 to 36 weeks. In addition, they require more antenatal surveillance than women who develop preeclampsia later in pregnancy.
I recommend hospitalization at the time of diagnosis when women develop mild preeclampsia before 32 weeks.
What and when to monitor
Maternal evaluation should include:
- monitoring of blood pressure at least daily (at home or in the hospital),
- daily urine dipstick evaluation to monitor changes in proteinuria,
- twice-weekly platelet count and liver enzymes, and
- documentation of symptoms. (Instruct all women to report the onset of severe headaches, visual changes, altered mental status, epigastric or right upper quadrant pain, and any nausea or vomiting.)
Fetal evaluation should include:
- serial ultrasound every 3 weeks to estimate fetal weight and amniotic fluid status,
- nonstress testing every week, and
- daily fetal movement counts.
If a nonstress test is nonreactive, it should be confirmed by biophysical profile.
All testing should be promptly repeated if the maternal clinical condition deteriorates.
No need for bed rest, diuretics, or antihypertensive medications
Although expectantly managed patients with mild preeclampsia should be advised to restrict daily activity, there is no need for complete bed rest. Nor have diuretics or other antihypertensive drugs been shown to prolong gestation. On the contrary, these medications may mask severe preeclampsia.
Antihypertensive medications reduce the rate of severe hypertension but do not improve perinatal outcome. If these drugs are used to treat mild disease remote from term, hospitalize the patient and manage her as though she has severe preeclampsia.
Hospitalization versus outpatient management
Although she may be hospitalized at the time of diagnosis, a woman with preeclampsia may switch to outpatient management if systolic or diastolic blood pressure declines, proteinuria diminishes to 1 g/24 hours or less, and there are no maternal symptoms or evidence of severe IUGR. Otherwise, these women should remain hospitalized until delivery.
In cases that begin with outpatient management, prompt hospitalization is indicated if there is clinical evidence that the disease is progressing (ie, new symptoms, labor or rupture of membranes, vaginal bleeding, or increased blood pressures or proteinuria) or IUGR and/or oligohydramnios.
Instruct all women to report symptoms and changes in fetal movement.
When to deliver
Whether the gravida is hospitalized or an outpatient, delivery is indicated at 37 weeks. Earlier delivery may be warranted if nonreassuring maternal or fetal conditions develop. (FIGURE 1 summarizes management of mild preeclampsia.)
Severe preeclampsia
Expectant management is safe in properly selected women with severe disease, although maternal and fetal conditions can deteriorate. Hospitalization and daily monitoring are required.
Preeclampsia is severe when any of the following are present:
- systolic blood pressure of 160 mm Hg or higher or diastolic pressure of 110 mm Hg or above on 2 occasions at least 6 hours apart while the patient is on bed rest
- proteinuria of 5 g or more in a 24-hour urine specimen,
- oliguria of less than 500 mL in 24 hours,
- cerebral or visual disturbances,
- pulmonary edema or cyanosis,
- severe epigastric or right upper-quadrant pain, or
- thrombocytopenia.
When gestational hypertension or preeclampsia is severe, hospitalization in the labor and delivery suite is warranted. These women should receive intravenous (IV) magnesium sulfate to reduce the risk of convulsions and antihypertensive drugs to treat severe levels of hypertension, if present. The aim of antihypertensive treatment is to keep diastolic blood pressure between 90 and 105 mm Hg and systolic blood pressure below 160 mm Hg.
During observation, assess maternal and fetal conditions and decide whether delivery is indicated (FIGURE 2).
Expectant management is warranted only for gestations between 23 and 32 weeks’ gestation, provided maternal and fetal conditions are stable (FIGURE 2).
Keep in mind that both maternal and fetal conditions may progressively deteriorate. Thus, these pregnancies involve higher rates of maternal morbidity and significant risk of neonatal morbidity. For this reason, expectant management should proceed only in a tertiary-care center with adequate maternal and neonatal facilities.
Recommended counseling
Advise these patients of the potential risks and benefits of expectant management, which requires daily monitoring of maternal and fetal conditions. Also explain that the decision to continue expectant management will be revisited on a daily basis and that the median number of days pregnancy is prolonged in these cases is 7 (range 2 to 35).
Another important fact to relay: Only 2 randomized trials involving 133 women have compared expectant management to aggressive management in early-onset preeclampsia. However, retrospective and observational studies involving more than 700 women suggest expectant management reduces short-term neonatal morbidity with minimal risk to the mother.
Superimposed preeclampsia
Women who develop preeclampsia on top of chronic hypertension, renal disease, or type 1 diabetes have a markedly higher risk of morbidity, including perinatal morbidity, than women without preexisting conditions.
Women with superimposed preeclampsia may be managed in the hospital, since these pregnancies are associated with higher rates of abruptio placentae (2% to 5%), preterm delivery (56%), IUGR (13% to 15%), and perinatal death (8%). Thus, these women benefit from very close maternal and fetal monitoring.
Superimposed preeclampsia is not classified according to severity.
In general, maternal and perinatal morbidities are substantially higher in women who have preexisting conditions than in healthy women who develop preeclampsia.
Chronic hypertension
Indications for delivery are similar to those described for healthy women with preeclampsia, as is antihypertensive therapy.
If the woman develops preeclampsia while using antihypertensive drugs, delivery should be considered beyond 34 weeks’ gestation.
How preeclampsia affects renal function
Women with renal disease or dysfunction (serum creatinine ≥1.2 mg/dL) prior to or early in pregnancy face an increased risk of adverse neonatal outcomes, regardless of whether preeclampsia also develops. These women also face an increased risk of deteriorating renal function during pregnancy (particularly if preeclampsia or severe hypertension develops) and beyond (more than 6 months postpartum).
Start antihypertensive medications as soon as possible, with the goal of keeping systolic blood pressure below 140 mm Hg and diastolic blood pressure below 90 mm Hg throughout gestation.
Delivery is indicated with the onset of preeclampsia or significant deterioration in renal function.
Diabetes warrants aggressive therapy
Women with type 1 diabetes have a higher risk of preeclampsia, maternal and fetal morbidity, and perinatal mortality. These risks multiply in women who have hypertension and/or diabetic nephropathy. Worsening of retinopathy and nephropathy also is more likely in women who have hypertension. Thus, aggressive management of blood sugars with insulin should be accompanied by aggressive control of blood pressure, with the goal of keeping systolic pressure below 130 mm Hg and diastolic pressure below 85 mm Hg.
Choosing antihypertensive drugs. Calcium-channel blockers are preferred to control blood pressure during pregnancy in women with diabetes. Outside of pregnancy, angiotensin-converting enzyme (ACE) inhibitors are best to avert long-term complications, but avoid these drugs in pregnancy (along with angiotensin-receptor blockers), particularly beyond 16 weeks.
Delivery is indicated in all women with vascular diabetes mellitus beyond 34 weeks when preeclampsia is present.
Intrapartum management
Close fetal heart rate and maternal blood pressure monitoring are mainstays, along with magnesium sulfate and antihypertensive therapy.
All women with preeclampsia should receive continuous monitoring of fetal heart rate and uterine activity, with special vigilance for hyperstimulation and onset of vaginal bleeding during labor. (For a description of potential maternal complications, see TABLE 1; fetal complications are described in FIGURE 3.)
Uterine irritability, recurrent variable or late decelerations, and the development of vaginal bleeding may be the first signs of abruptio placentae.
I recommend recording maternal blood pressure at least hourly to detect progression from mild to severe hypertension and to determine the need for antihypertensive therapy.
TABLE 1
Likelihood of maternal complications
| Disease progresses during labor (from mild to severe) | 10% |
| Eclampsia | |
| • Mild disease | <0.5% |
| • Severe preeclampsia | 1–2% |
| Stroke (encephalopathy or hemorrhage) | <1% |
| Mainly with severe or early onset disease | |
| Pulmonary edema | 1–2% |
| Usually associated with fluid overload or long-standing chronic hypertension | |
Prevent progression to eclampsia
Magnesium sulfate is the drug of choice in women with preeclampsia. Recent reviews indicate that it reduces the rate of convulsions from 2% to 0.6% in women with severe preeclampsia. In women with mild preeclampsia, the benefit of magnesium sulfate remains unclear.
I recommend IV magnesium sulfate during labor and postpartum when a woman has the indications listed in TABLE 2.
The dose of magnesium sulfate is 6 g IV loading over 20 minutes, followed by a maintenance dose of 2 g/hour.
Magnesium sulfate should be started before surgery (elective cesarean delivery) and continued for at least 12 hours postpartum (I prefer 24 hours).
TABLE 2
When to give prophylactic magnesium sulfate
| Use intrapartum and for at least 12 hours postpartum |
|---|
When the patient has:
|
When treating hypertension in labor, avoid “hypotensive overshoot”
The goal of intrapartum treatment is to lower maternal blood pressure without causing precipitous hypotensive overshoot that may lead to reduced maternal organ perfusion, particularly uteroplacental blood flow. Such acute lowering of maternal blood pressure is a common cause of nonreassuring fetal heart rate patterns during labor.
What blood pressure necessitates treatment? There is no doubt that severe levels of hypertension should be treated to avoid potential cerebrovascular and cardiovascular complications in healthy women. However, there is disagreement about what constitutes severe hypertension.
In previously healthy women, I recommend antihypertensive therapy for systolic pressures of 170 mm Hg or above and/or for diastolic pressures of 110 mm Hg or above.
For women with thrombocytopenia, disseminated intravascular coagulation, or pulmonary edema, I recommend treatment for systolic pressures of 160 mm Hg or above and diastolic pressures of 105 mm Hg or above. This latter group should also be given IV furosemide (20 to 40 mg) to promote diuresis. I also recommend treatment at these levels in the postpartum period.
For women with diabetes, renal disease, or left ventricular cardiac disease, antihypertensive medications should be used to keep systolic pressure below 140 mm Hg and diastolic pressure below 90 mm Hg during labor and postpartum. Further, patients in congestive heart failure or with left ventricular diastolic dysfunction should receive furosemide in addition to antihypertensive drugs.
Choosing a drug. My drugs of choice are IV labetalol and oral nifedipine. These 2 drugs, along with IV hydralazine, are the most commonly recommended medications for severe hypertension in pregnancy (TABLE 3).
Although many authorities prefer hydralazine, recent data indicate that, compared with IV labetalol and oral nifedipine, IV hydralazine is associated with more maternal side effects and worse perinatal outcomes (more fetal distress in labor).
TABLE 3
Drug profiles: Dosing and side effects of antihypertensives used in pregnancy
| MEDICATION | ONSET OF ACTION | DOSE | SIDE EFFECTS |
|---|---|---|---|
| Hydralazine | 10-20 minutes | 5-10 mg intravenously every 20 minutes up to maximum dose of 30 mg | More maternal side effects and worse perinatal outcomes than labetalol or nifedipine. |
| Skin blisters; chest pain; general feeling of discomfort, illness, or weakness; joint or muscle pain; sore throat and fever; swollen lymph glands | |||
| Labetalol* | 10-15 minutes | 10-20 mg intravenously, then 40-80 mg every 10 minutes up to maximum dose of 220 mg/hour or continuous infusion of 1-2 mg/minute | Breathing difficulty and/or wheezing, cold hands and feet, mental depression, shortness of breath, slow heartbeat, swelling of lower extremities, back or joint pain, chest pain, confusion, fever and sore throat, hallucinations, irregular heartbeat, unusual bleeding and bruising, yellow eyes or skin |
| Nifedipine | 5-10 minutes | 10-20 mg orally, repeated in 30 minutes, up to maximum dose of 50 mg/hour | Breathing difficulty, coughing, or wheezing; irregular or fast, poundingheartbeat; skin rash; swelling of lower extremities; chest pain; fainting; painful, swollen joints; vision impairment |
| Sodium nitroprusside† | 0.5-5 minutes | 0.25-5 μg/kg/minute by intravenous infusion | Risk of fetal cyanide poisoning with prolonged treatment. |
| Maternal effects include symptoms of hypothyroidism, headache, abdominal pain, drowsiness, nausea, involuntary muscle movements, perspiration, restlessness, paraesthesia, palpitations, dizziness, retching, tachycardia | |||
| *In women with asthma and congestive heart failure | |||
| †Rarely needed except in hypertensive encephalopathy or cerebral hemorrhage | |||
Postpartum management
Because preeclampsia can worsen, or first appear, in the postpartum period, extra vigilance is important, and pharmacotherapy may be appropriate.
Management of preeclampsia does not end with delivery of the fetus and the placenta. These events do signal the beginning of the curative process, but complications can occur in the postpartum period. Indeed, in some women, the disease process worsens immediately postpartum. Therefore, women with diagnosed preeclampsia or severe gestational hypertension require close monitoring of blood pressure and maternal symptoms and accurate measurement of fluid intake and urine output. Some of these women are at increased risk for pulmonary edema; exacerbation of severe hypertension; eclampsia; and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome.
Treating postpartum hypertension
Women who continue to have severe hypertension (systolic pressure at or above 155 mm Hg or diastolic pressure of 105 mm Hg or higher) will benefit from oral nifedipine (10 mg every 6 hours) or long-acting nifedipine (10 to 20 mg twice daily), the drugs of choice because of their favorable effects on renal function.
Women with severe hypertension also may require diuretics for better control of blood pressure, as may women with a history of congestive heart failure or left ventricular dysfunction.
Start women with vascular diabetes mellitus or diabetic nephropathy on ACE inhibitors immediately postpartum.
Patients can be discharged home once blood pressure is stable, provided there are no maternal symptoms of preeclampsia.
Postpartum preeclampsia can develop even in healthy women
Because severe hypertension or preeclampsia may develop for the first time in the postpartum period, it is important to educate all gravidas about the signs and symptoms. All health-care providers should be on the lookout for these symptoms as well.
The author reports no financial relationships relevant to this article.
BIBLIOGRAPHY
Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev (England). 2001;(2)pCD002252.-
Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur J Obstet Gynecol Reprod Biol. 2002;101:6-14.
Amorim MMR, Santas LC, Faundes A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. Am J Obstet Gynecol. 1999;180:1283-1288.
Duley L, Galmezoglu AM, Henderson-Smart DJ. Magnesium sulfate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev (England). 2003;(2)pCD000025.-
Friedman SA, Lubarsky S, Schiff E. Expectant management of severe preeclampsia remote from term. Clin Obstet Gynecol. 1999;42:470-478.
Haddad B, Deis S, Goffinet F, et al. Maternal and perinatal outcomes during expectant management of 239 severe preeclamptic women between 24 and 33 weeks’ gestation. Am J Obstet Gynecol. 2004;190:1590-1597.
Hall DR, Odendaal HJ, Kirten GF, Smith J. Expectant management of early onset, severe preeclampsia, perinatal outcome. BJOG. 2000;107:1258-1264.
Kupferminc MJ, Fait G, Many A, et al. Low molecular weight heparin for the prevention of obstetric complications in women with thrombophilia. Hypertension in Pregnancy. 2001;20:35-44.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111-166.
Magee LA, Cham C, Waterman EJ, Ohlsson A, Von Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ. 2003;327:1-10.
Magee LA, Ornstein MP, Von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999;318:1332-1336.
Magpie Trial Group. Do women with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised, placebo-controlled trial. Lancet. 2002;359:1877-1890.
Report of the National High Blood Pressure Education Program. Working group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1-22.
Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
Sibai BM, Lindheimer MD, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;229:667-671.
Sibai BM. Magnesium sulfate prophylaxis in preeclampsia. Lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520-1526.
Vigil-DeGracia P, Montufar-Rueda C, Ruiz J. Expectant management of severe preeclampsia and preeclampsia superimposed on chronic hypertension between 24 and 34 weeks’ gestation. Eur J Obstet Gynecol Reprod Biol. 2003;107:24-27.
Once you decide to expectantly manage a patient with preeclampsia, the balancing act begins. That means weighing fetal benefits against maternal risks, since the only justification for expectant management is to prolong pregnancy for fetal gain—there is no advantage to the mother.
The best approach is to classify the woman’s preeclampsia by the degree of severity and gestational age at the time of diagnosis, then follow recommendations tailored to that particular category.
This article offers guidelines for expectant management of mild and severe preeclampsia, preeclampsia superimposed on a preexisting medical condition, and intrapartum and postpartum care.
Mild preeclampsia
The earlier preeclampsia develops, the greater the risk it will become severe. The need for hospitalization depends on gestational age, blood pressure, proteinuria levels, maternal symptoms, and reliability of the patient.
Preeclampsia is mild when systolic blood pressure reaches 140 to 159 mm Hg or diastolic pressure measures 90 to 109 mm Hg on at least 2 occasions more than 6 hours apart after 20 weeks’ gestation in a woman who previously had normal blood pressure. In preeclampsia, this hypertension is accompanied by proteinuria of 0.3 to 4.9 g in a 24-hour urine sample (1+ or 2+ by dipstick on 2 occasions).
At or beyond 37 weeks’ gestation
In general, women diagnosed with preeclampsia at this gestational age have pregnancy outcomes similar to those of normotensive gravidas. Thus, they benefit from induction of labor and delivery.
32 to 36 weeks’ gestation
Close maternal and fetal evaluation is essential. (It is assumed these women have no labor or membrane rupture and normal fetal testing; otherwise, delivery is indicated at 34 weeks or beyond.)
In general, hospitalization is indicated when any of the following circumstances are present (FIGURE 1):
- the patient is unreliable,
- 2 or more systolic blood pressure readings exceed 150 mm Hg,
- 2 or more diastolic blood pressure readings exceed 100 mm Hg,
- proteinuria occurs at a rate exceeding 1 g/24 hours, or
- persistent maternal symptoms are present.
FIGURE 1 Treatment of mild preeclampsia in healthy women
Before 32 weeks’ gestation
These women are at high risk of progressing to severe disease. They also are more likely to have adverse perinatal outcomes such as intrauterine growth restriction (IUGR) (15% to 20%), preterm delivery (50%), and abruptio placentae (1% to 2%), compared with women diagnosed with preeclampsia at 32 to 36 weeks. In addition, they require more antenatal surveillance than women who develop preeclampsia later in pregnancy.
I recommend hospitalization at the time of diagnosis when women develop mild preeclampsia before 32 weeks.
What and when to monitor
Maternal evaluation should include:
- monitoring of blood pressure at least daily (at home or in the hospital),
- daily urine dipstick evaluation to monitor changes in proteinuria,
- twice-weekly platelet count and liver enzymes, and
- documentation of symptoms. (Instruct all women to report the onset of severe headaches, visual changes, altered mental status, epigastric or right upper quadrant pain, and any nausea or vomiting.)
Fetal evaluation should include:
- serial ultrasound every 3 weeks to estimate fetal weight and amniotic fluid status,
- nonstress testing every week, and
- daily fetal movement counts.
If a nonstress test is nonreactive, it should be confirmed by biophysical profile.
All testing should be promptly repeated if the maternal clinical condition deteriorates.
No need for bed rest, diuretics, or antihypertensive medications
Although expectantly managed patients with mild preeclampsia should be advised to restrict daily activity, there is no need for complete bed rest. Nor have diuretics or other antihypertensive drugs been shown to prolong gestation. On the contrary, these medications may mask severe preeclampsia.
Antihypertensive medications reduce the rate of severe hypertension but do not improve perinatal outcome. If these drugs are used to treat mild disease remote from term, hospitalize the patient and manage her as though she has severe preeclampsia.
Hospitalization versus outpatient management
Although she may be hospitalized at the time of diagnosis, a woman with preeclampsia may switch to outpatient management if systolic or diastolic blood pressure declines, proteinuria diminishes to 1 g/24 hours or less, and there are no maternal symptoms or evidence of severe IUGR. Otherwise, these women should remain hospitalized until delivery.
In cases that begin with outpatient management, prompt hospitalization is indicated if there is clinical evidence that the disease is progressing (ie, new symptoms, labor or rupture of membranes, vaginal bleeding, or increased blood pressures or proteinuria) or IUGR and/or oligohydramnios.
Instruct all women to report symptoms and changes in fetal movement.
When to deliver
Whether the gravida is hospitalized or an outpatient, delivery is indicated at 37 weeks. Earlier delivery may be warranted if nonreassuring maternal or fetal conditions develop. (FIGURE 1 summarizes management of mild preeclampsia.)
Severe preeclampsia
Expectant management is safe in properly selected women with severe disease, although maternal and fetal conditions can deteriorate. Hospitalization and daily monitoring are required.
Preeclampsia is severe when any of the following are present:
- systolic blood pressure of 160 mm Hg or higher or diastolic pressure of 110 mm Hg or above on 2 occasions at least 6 hours apart while the patient is on bed rest
- proteinuria of 5 g or more in a 24-hour urine specimen,
- oliguria of less than 500 mL in 24 hours,
- cerebral or visual disturbances,
- pulmonary edema or cyanosis,
- severe epigastric or right upper-quadrant pain, or
- thrombocytopenia.
When gestational hypertension or preeclampsia is severe, hospitalization in the labor and delivery suite is warranted. These women should receive intravenous (IV) magnesium sulfate to reduce the risk of convulsions and antihypertensive drugs to treat severe levels of hypertension, if present. The aim of antihypertensive treatment is to keep diastolic blood pressure between 90 and 105 mm Hg and systolic blood pressure below 160 mm Hg.
During observation, assess maternal and fetal conditions and decide whether delivery is indicated (FIGURE 2).
Expectant management is warranted only for gestations between 23 and 32 weeks’ gestation, provided maternal and fetal conditions are stable (FIGURE 2).
Keep in mind that both maternal and fetal conditions may progressively deteriorate. Thus, these pregnancies involve higher rates of maternal morbidity and significant risk of neonatal morbidity. For this reason, expectant management should proceed only in a tertiary-care center with adequate maternal and neonatal facilities.
Recommended counseling
Advise these patients of the potential risks and benefits of expectant management, which requires daily monitoring of maternal and fetal conditions. Also explain that the decision to continue expectant management will be revisited on a daily basis and that the median number of days pregnancy is prolonged in these cases is 7 (range 2 to 35).
Another important fact to relay: Only 2 randomized trials involving 133 women have compared expectant management to aggressive management in early-onset preeclampsia. However, retrospective and observational studies involving more than 700 women suggest expectant management reduces short-term neonatal morbidity with minimal risk to the mother.
Superimposed preeclampsia
Women who develop preeclampsia on top of chronic hypertension, renal disease, or type 1 diabetes have a markedly higher risk of morbidity, including perinatal morbidity, than women without preexisting conditions.
Women with superimposed preeclampsia may be managed in the hospital, since these pregnancies are associated with higher rates of abruptio placentae (2% to 5%), preterm delivery (56%), IUGR (13% to 15%), and perinatal death (8%). Thus, these women benefit from very close maternal and fetal monitoring.
Superimposed preeclampsia is not classified according to severity.
In general, maternal and perinatal morbidities are substantially higher in women who have preexisting conditions than in healthy women who develop preeclampsia.
Chronic hypertension
Indications for delivery are similar to those described for healthy women with preeclampsia, as is antihypertensive therapy.
If the woman develops preeclampsia while using antihypertensive drugs, delivery should be considered beyond 34 weeks’ gestation.
How preeclampsia affects renal function
Women with renal disease or dysfunction (serum creatinine ≥1.2 mg/dL) prior to or early in pregnancy face an increased risk of adverse neonatal outcomes, regardless of whether preeclampsia also develops. These women also face an increased risk of deteriorating renal function during pregnancy (particularly if preeclampsia or severe hypertension develops) and beyond (more than 6 months postpartum).
Start antihypertensive medications as soon as possible, with the goal of keeping systolic blood pressure below 140 mm Hg and diastolic blood pressure below 90 mm Hg throughout gestation.
Delivery is indicated with the onset of preeclampsia or significant deterioration in renal function.
Diabetes warrants aggressive therapy
Women with type 1 diabetes have a higher risk of preeclampsia, maternal and fetal morbidity, and perinatal mortality. These risks multiply in women who have hypertension and/or diabetic nephropathy. Worsening of retinopathy and nephropathy also is more likely in women who have hypertension. Thus, aggressive management of blood sugars with insulin should be accompanied by aggressive control of blood pressure, with the goal of keeping systolic pressure below 130 mm Hg and diastolic pressure below 85 mm Hg.
Choosing antihypertensive drugs. Calcium-channel blockers are preferred to control blood pressure during pregnancy in women with diabetes. Outside of pregnancy, angiotensin-converting enzyme (ACE) inhibitors are best to avert long-term complications, but avoid these drugs in pregnancy (along with angiotensin-receptor blockers), particularly beyond 16 weeks.
Delivery is indicated in all women with vascular diabetes mellitus beyond 34 weeks when preeclampsia is present.
Intrapartum management
Close fetal heart rate and maternal blood pressure monitoring are mainstays, along with magnesium sulfate and antihypertensive therapy.
All women with preeclampsia should receive continuous monitoring of fetal heart rate and uterine activity, with special vigilance for hyperstimulation and onset of vaginal bleeding during labor. (For a description of potential maternal complications, see TABLE 1; fetal complications are described in FIGURE 3.)
Uterine irritability, recurrent variable or late decelerations, and the development of vaginal bleeding may be the first signs of abruptio placentae.
I recommend recording maternal blood pressure at least hourly to detect progression from mild to severe hypertension and to determine the need for antihypertensive therapy.
TABLE 1
Likelihood of maternal complications
| Disease progresses during labor (from mild to severe) | 10% |
| Eclampsia | |
| • Mild disease | <0.5% |
| • Severe preeclampsia | 1–2% |
| Stroke (encephalopathy or hemorrhage) | <1% |
| Mainly with severe or early onset disease | |
| Pulmonary edema | 1–2% |
| Usually associated with fluid overload or long-standing chronic hypertension | |
Prevent progression to eclampsia
Magnesium sulfate is the drug of choice in women with preeclampsia. Recent reviews indicate that it reduces the rate of convulsions from 2% to 0.6% in women with severe preeclampsia. In women with mild preeclampsia, the benefit of magnesium sulfate remains unclear.
I recommend IV magnesium sulfate during labor and postpartum when a woman has the indications listed in TABLE 2.
The dose of magnesium sulfate is 6 g IV loading over 20 minutes, followed by a maintenance dose of 2 g/hour.
Magnesium sulfate should be started before surgery (elective cesarean delivery) and continued for at least 12 hours postpartum (I prefer 24 hours).
TABLE 2
When to give prophylactic magnesium sulfate
| Use intrapartum and for at least 12 hours postpartum |
|---|
When the patient has:
|
When treating hypertension in labor, avoid “hypotensive overshoot”
The goal of intrapartum treatment is to lower maternal blood pressure without causing precipitous hypotensive overshoot that may lead to reduced maternal organ perfusion, particularly uteroplacental blood flow. Such acute lowering of maternal blood pressure is a common cause of nonreassuring fetal heart rate patterns during labor.
What blood pressure necessitates treatment? There is no doubt that severe levels of hypertension should be treated to avoid potential cerebrovascular and cardiovascular complications in healthy women. However, there is disagreement about what constitutes severe hypertension.
In previously healthy women, I recommend antihypertensive therapy for systolic pressures of 170 mm Hg or above and/or for diastolic pressures of 110 mm Hg or above.
For women with thrombocytopenia, disseminated intravascular coagulation, or pulmonary edema, I recommend treatment for systolic pressures of 160 mm Hg or above and diastolic pressures of 105 mm Hg or above. This latter group should also be given IV furosemide (20 to 40 mg) to promote diuresis. I also recommend treatment at these levels in the postpartum period.
For women with diabetes, renal disease, or left ventricular cardiac disease, antihypertensive medications should be used to keep systolic pressure below 140 mm Hg and diastolic pressure below 90 mm Hg during labor and postpartum. Further, patients in congestive heart failure or with left ventricular diastolic dysfunction should receive furosemide in addition to antihypertensive drugs.
Choosing a drug. My drugs of choice are IV labetalol and oral nifedipine. These 2 drugs, along with IV hydralazine, are the most commonly recommended medications for severe hypertension in pregnancy (TABLE 3).
Although many authorities prefer hydralazine, recent data indicate that, compared with IV labetalol and oral nifedipine, IV hydralazine is associated with more maternal side effects and worse perinatal outcomes (more fetal distress in labor).
TABLE 3
Drug profiles: Dosing and side effects of antihypertensives used in pregnancy
| MEDICATION | ONSET OF ACTION | DOSE | SIDE EFFECTS |
|---|---|---|---|
| Hydralazine | 10-20 minutes | 5-10 mg intravenously every 20 minutes up to maximum dose of 30 mg | More maternal side effects and worse perinatal outcomes than labetalol or nifedipine. |
| Skin blisters; chest pain; general feeling of discomfort, illness, or weakness; joint or muscle pain; sore throat and fever; swollen lymph glands | |||
| Labetalol* | 10-15 minutes | 10-20 mg intravenously, then 40-80 mg every 10 minutes up to maximum dose of 220 mg/hour or continuous infusion of 1-2 mg/minute | Breathing difficulty and/or wheezing, cold hands and feet, mental depression, shortness of breath, slow heartbeat, swelling of lower extremities, back or joint pain, chest pain, confusion, fever and sore throat, hallucinations, irregular heartbeat, unusual bleeding and bruising, yellow eyes or skin |
| Nifedipine | 5-10 minutes | 10-20 mg orally, repeated in 30 minutes, up to maximum dose of 50 mg/hour | Breathing difficulty, coughing, or wheezing; irregular or fast, poundingheartbeat; skin rash; swelling of lower extremities; chest pain; fainting; painful, swollen joints; vision impairment |
| Sodium nitroprusside† | 0.5-5 minutes | 0.25-5 μg/kg/minute by intravenous infusion | Risk of fetal cyanide poisoning with prolonged treatment. |
| Maternal effects include symptoms of hypothyroidism, headache, abdominal pain, drowsiness, nausea, involuntary muscle movements, perspiration, restlessness, paraesthesia, palpitations, dizziness, retching, tachycardia | |||
| *In women with asthma and congestive heart failure | |||
| †Rarely needed except in hypertensive encephalopathy or cerebral hemorrhage | |||
Postpartum management
Because preeclampsia can worsen, or first appear, in the postpartum period, extra vigilance is important, and pharmacotherapy may be appropriate.
Management of preeclampsia does not end with delivery of the fetus and the placenta. These events do signal the beginning of the curative process, but complications can occur in the postpartum period. Indeed, in some women, the disease process worsens immediately postpartum. Therefore, women with diagnosed preeclampsia or severe gestational hypertension require close monitoring of blood pressure and maternal symptoms and accurate measurement of fluid intake and urine output. Some of these women are at increased risk for pulmonary edema; exacerbation of severe hypertension; eclampsia; and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome.
Treating postpartum hypertension
Women who continue to have severe hypertension (systolic pressure at or above 155 mm Hg or diastolic pressure of 105 mm Hg or higher) will benefit from oral nifedipine (10 mg every 6 hours) or long-acting nifedipine (10 to 20 mg twice daily), the drugs of choice because of their favorable effects on renal function.
Women with severe hypertension also may require diuretics for better control of blood pressure, as may women with a history of congestive heart failure or left ventricular dysfunction.
Start women with vascular diabetes mellitus or diabetic nephropathy on ACE inhibitors immediately postpartum.
Patients can be discharged home once blood pressure is stable, provided there are no maternal symptoms of preeclampsia.
Postpartum preeclampsia can develop even in healthy women
Because severe hypertension or preeclampsia may develop for the first time in the postpartum period, it is important to educate all gravidas about the signs and symptoms. All health-care providers should be on the lookout for these symptoms as well.
The author reports no financial relationships relevant to this article.
Once you decide to expectantly manage a patient with preeclampsia, the balancing act begins. That means weighing fetal benefits against maternal risks, since the only justification for expectant management is to prolong pregnancy for fetal gain—there is no advantage to the mother.
The best approach is to classify the woman’s preeclampsia by the degree of severity and gestational age at the time of diagnosis, then follow recommendations tailored to that particular category.
This article offers guidelines for expectant management of mild and severe preeclampsia, preeclampsia superimposed on a preexisting medical condition, and intrapartum and postpartum care.
Mild preeclampsia
The earlier preeclampsia develops, the greater the risk it will become severe. The need for hospitalization depends on gestational age, blood pressure, proteinuria levels, maternal symptoms, and reliability of the patient.
Preeclampsia is mild when systolic blood pressure reaches 140 to 159 mm Hg or diastolic pressure measures 90 to 109 mm Hg on at least 2 occasions more than 6 hours apart after 20 weeks’ gestation in a woman who previously had normal blood pressure. In preeclampsia, this hypertension is accompanied by proteinuria of 0.3 to 4.9 g in a 24-hour urine sample (1+ or 2+ by dipstick on 2 occasions).
At or beyond 37 weeks’ gestation
In general, women diagnosed with preeclampsia at this gestational age have pregnancy outcomes similar to those of normotensive gravidas. Thus, they benefit from induction of labor and delivery.
32 to 36 weeks’ gestation
Close maternal and fetal evaluation is essential. (It is assumed these women have no labor or membrane rupture and normal fetal testing; otherwise, delivery is indicated at 34 weeks or beyond.)
In general, hospitalization is indicated when any of the following circumstances are present (FIGURE 1):
- the patient is unreliable,
- 2 or more systolic blood pressure readings exceed 150 mm Hg,
- 2 or more diastolic blood pressure readings exceed 100 mm Hg,
- proteinuria occurs at a rate exceeding 1 g/24 hours, or
- persistent maternal symptoms are present.
FIGURE 1 Treatment of mild preeclampsia in healthy women
Before 32 weeks’ gestation
These women are at high risk of progressing to severe disease. They also are more likely to have adverse perinatal outcomes such as intrauterine growth restriction (IUGR) (15% to 20%), preterm delivery (50%), and abruptio placentae (1% to 2%), compared with women diagnosed with preeclampsia at 32 to 36 weeks. In addition, they require more antenatal surveillance than women who develop preeclampsia later in pregnancy.
I recommend hospitalization at the time of diagnosis when women develop mild preeclampsia before 32 weeks.
What and when to monitor
Maternal evaluation should include:
- monitoring of blood pressure at least daily (at home or in the hospital),
- daily urine dipstick evaluation to monitor changes in proteinuria,
- twice-weekly platelet count and liver enzymes, and
- documentation of symptoms. (Instruct all women to report the onset of severe headaches, visual changes, altered mental status, epigastric or right upper quadrant pain, and any nausea or vomiting.)
Fetal evaluation should include:
- serial ultrasound every 3 weeks to estimate fetal weight and amniotic fluid status,
- nonstress testing every week, and
- daily fetal movement counts.
If a nonstress test is nonreactive, it should be confirmed by biophysical profile.
All testing should be promptly repeated if the maternal clinical condition deteriorates.
No need for bed rest, diuretics, or antihypertensive medications
Although expectantly managed patients with mild preeclampsia should be advised to restrict daily activity, there is no need for complete bed rest. Nor have diuretics or other antihypertensive drugs been shown to prolong gestation. On the contrary, these medications may mask severe preeclampsia.
Antihypertensive medications reduce the rate of severe hypertension but do not improve perinatal outcome. If these drugs are used to treat mild disease remote from term, hospitalize the patient and manage her as though she has severe preeclampsia.
Hospitalization versus outpatient management
Although she may be hospitalized at the time of diagnosis, a woman with preeclampsia may switch to outpatient management if systolic or diastolic blood pressure declines, proteinuria diminishes to 1 g/24 hours or less, and there are no maternal symptoms or evidence of severe IUGR. Otherwise, these women should remain hospitalized until delivery.
In cases that begin with outpatient management, prompt hospitalization is indicated if there is clinical evidence that the disease is progressing (ie, new symptoms, labor or rupture of membranes, vaginal bleeding, or increased blood pressures or proteinuria) or IUGR and/or oligohydramnios.
Instruct all women to report symptoms and changes in fetal movement.
When to deliver
Whether the gravida is hospitalized or an outpatient, delivery is indicated at 37 weeks. Earlier delivery may be warranted if nonreassuring maternal or fetal conditions develop. (FIGURE 1 summarizes management of mild preeclampsia.)
Severe preeclampsia
Expectant management is safe in properly selected women with severe disease, although maternal and fetal conditions can deteriorate. Hospitalization and daily monitoring are required.
Preeclampsia is severe when any of the following are present:
- systolic blood pressure of 160 mm Hg or higher or diastolic pressure of 110 mm Hg or above on 2 occasions at least 6 hours apart while the patient is on bed rest
- proteinuria of 5 g or more in a 24-hour urine specimen,
- oliguria of less than 500 mL in 24 hours,
- cerebral or visual disturbances,
- pulmonary edema or cyanosis,
- severe epigastric or right upper-quadrant pain, or
- thrombocytopenia.
When gestational hypertension or preeclampsia is severe, hospitalization in the labor and delivery suite is warranted. These women should receive intravenous (IV) magnesium sulfate to reduce the risk of convulsions and antihypertensive drugs to treat severe levels of hypertension, if present. The aim of antihypertensive treatment is to keep diastolic blood pressure between 90 and 105 mm Hg and systolic blood pressure below 160 mm Hg.
During observation, assess maternal and fetal conditions and decide whether delivery is indicated (FIGURE 2).
Expectant management is warranted only for gestations between 23 and 32 weeks’ gestation, provided maternal and fetal conditions are stable (FIGURE 2).
Keep in mind that both maternal and fetal conditions may progressively deteriorate. Thus, these pregnancies involve higher rates of maternal morbidity and significant risk of neonatal morbidity. For this reason, expectant management should proceed only in a tertiary-care center with adequate maternal and neonatal facilities.
Recommended counseling
Advise these patients of the potential risks and benefits of expectant management, which requires daily monitoring of maternal and fetal conditions. Also explain that the decision to continue expectant management will be revisited on a daily basis and that the median number of days pregnancy is prolonged in these cases is 7 (range 2 to 35).
Another important fact to relay: Only 2 randomized trials involving 133 women have compared expectant management to aggressive management in early-onset preeclampsia. However, retrospective and observational studies involving more than 700 women suggest expectant management reduces short-term neonatal morbidity with minimal risk to the mother.
Superimposed preeclampsia
Women who develop preeclampsia on top of chronic hypertension, renal disease, or type 1 diabetes have a markedly higher risk of morbidity, including perinatal morbidity, than women without preexisting conditions.
Women with superimposed preeclampsia may be managed in the hospital, since these pregnancies are associated with higher rates of abruptio placentae (2% to 5%), preterm delivery (56%), IUGR (13% to 15%), and perinatal death (8%). Thus, these women benefit from very close maternal and fetal monitoring.
Superimposed preeclampsia is not classified according to severity.
In general, maternal and perinatal morbidities are substantially higher in women who have preexisting conditions than in healthy women who develop preeclampsia.
Chronic hypertension
Indications for delivery are similar to those described for healthy women with preeclampsia, as is antihypertensive therapy.
If the woman develops preeclampsia while using antihypertensive drugs, delivery should be considered beyond 34 weeks’ gestation.
How preeclampsia affects renal function
Women with renal disease or dysfunction (serum creatinine ≥1.2 mg/dL) prior to or early in pregnancy face an increased risk of adverse neonatal outcomes, regardless of whether preeclampsia also develops. These women also face an increased risk of deteriorating renal function during pregnancy (particularly if preeclampsia or severe hypertension develops) and beyond (more than 6 months postpartum).
Start antihypertensive medications as soon as possible, with the goal of keeping systolic blood pressure below 140 mm Hg and diastolic blood pressure below 90 mm Hg throughout gestation.
Delivery is indicated with the onset of preeclampsia or significant deterioration in renal function.
Diabetes warrants aggressive therapy
Women with type 1 diabetes have a higher risk of preeclampsia, maternal and fetal morbidity, and perinatal mortality. These risks multiply in women who have hypertension and/or diabetic nephropathy. Worsening of retinopathy and nephropathy also is more likely in women who have hypertension. Thus, aggressive management of blood sugars with insulin should be accompanied by aggressive control of blood pressure, with the goal of keeping systolic pressure below 130 mm Hg and diastolic pressure below 85 mm Hg.
Choosing antihypertensive drugs. Calcium-channel blockers are preferred to control blood pressure during pregnancy in women with diabetes. Outside of pregnancy, angiotensin-converting enzyme (ACE) inhibitors are best to avert long-term complications, but avoid these drugs in pregnancy (along with angiotensin-receptor blockers), particularly beyond 16 weeks.
Delivery is indicated in all women with vascular diabetes mellitus beyond 34 weeks when preeclampsia is present.
Intrapartum management
Close fetal heart rate and maternal blood pressure monitoring are mainstays, along with magnesium sulfate and antihypertensive therapy.
All women with preeclampsia should receive continuous monitoring of fetal heart rate and uterine activity, with special vigilance for hyperstimulation and onset of vaginal bleeding during labor. (For a description of potential maternal complications, see TABLE 1; fetal complications are described in FIGURE 3.)
Uterine irritability, recurrent variable or late decelerations, and the development of vaginal bleeding may be the first signs of abruptio placentae.
I recommend recording maternal blood pressure at least hourly to detect progression from mild to severe hypertension and to determine the need for antihypertensive therapy.
TABLE 1
Likelihood of maternal complications
| Disease progresses during labor (from mild to severe) | 10% |
| Eclampsia | |
| • Mild disease | <0.5% |
| • Severe preeclampsia | 1–2% |
| Stroke (encephalopathy or hemorrhage) | <1% |
| Mainly with severe or early onset disease | |
| Pulmonary edema | 1–2% |
| Usually associated with fluid overload or long-standing chronic hypertension | |
Prevent progression to eclampsia
Magnesium sulfate is the drug of choice in women with preeclampsia. Recent reviews indicate that it reduces the rate of convulsions from 2% to 0.6% in women with severe preeclampsia. In women with mild preeclampsia, the benefit of magnesium sulfate remains unclear.
I recommend IV magnesium sulfate during labor and postpartum when a woman has the indications listed in TABLE 2.
The dose of magnesium sulfate is 6 g IV loading over 20 minutes, followed by a maintenance dose of 2 g/hour.
Magnesium sulfate should be started before surgery (elective cesarean delivery) and continued for at least 12 hours postpartum (I prefer 24 hours).
TABLE 2
When to give prophylactic magnesium sulfate
| Use intrapartum and for at least 12 hours postpartum |
|---|
When the patient has:
|
When treating hypertension in labor, avoid “hypotensive overshoot”
The goal of intrapartum treatment is to lower maternal blood pressure without causing precipitous hypotensive overshoot that may lead to reduced maternal organ perfusion, particularly uteroplacental blood flow. Such acute lowering of maternal blood pressure is a common cause of nonreassuring fetal heart rate patterns during labor.
What blood pressure necessitates treatment? There is no doubt that severe levels of hypertension should be treated to avoid potential cerebrovascular and cardiovascular complications in healthy women. However, there is disagreement about what constitutes severe hypertension.
In previously healthy women, I recommend antihypertensive therapy for systolic pressures of 170 mm Hg or above and/or for diastolic pressures of 110 mm Hg or above.
For women with thrombocytopenia, disseminated intravascular coagulation, or pulmonary edema, I recommend treatment for systolic pressures of 160 mm Hg or above and diastolic pressures of 105 mm Hg or above. This latter group should also be given IV furosemide (20 to 40 mg) to promote diuresis. I also recommend treatment at these levels in the postpartum period.
For women with diabetes, renal disease, or left ventricular cardiac disease, antihypertensive medications should be used to keep systolic pressure below 140 mm Hg and diastolic pressure below 90 mm Hg during labor and postpartum. Further, patients in congestive heart failure or with left ventricular diastolic dysfunction should receive furosemide in addition to antihypertensive drugs.
Choosing a drug. My drugs of choice are IV labetalol and oral nifedipine. These 2 drugs, along with IV hydralazine, are the most commonly recommended medications for severe hypertension in pregnancy (TABLE 3).
Although many authorities prefer hydralazine, recent data indicate that, compared with IV labetalol and oral nifedipine, IV hydralazine is associated with more maternal side effects and worse perinatal outcomes (more fetal distress in labor).
TABLE 3
Drug profiles: Dosing and side effects of antihypertensives used in pregnancy
| MEDICATION | ONSET OF ACTION | DOSE | SIDE EFFECTS |
|---|---|---|---|
| Hydralazine | 10-20 minutes | 5-10 mg intravenously every 20 minutes up to maximum dose of 30 mg | More maternal side effects and worse perinatal outcomes than labetalol or nifedipine. |
| Skin blisters; chest pain; general feeling of discomfort, illness, or weakness; joint or muscle pain; sore throat and fever; swollen lymph glands | |||
| Labetalol* | 10-15 minutes | 10-20 mg intravenously, then 40-80 mg every 10 minutes up to maximum dose of 220 mg/hour or continuous infusion of 1-2 mg/minute | Breathing difficulty and/or wheezing, cold hands and feet, mental depression, shortness of breath, slow heartbeat, swelling of lower extremities, back or joint pain, chest pain, confusion, fever and sore throat, hallucinations, irregular heartbeat, unusual bleeding and bruising, yellow eyes or skin |
| Nifedipine | 5-10 minutes | 10-20 mg orally, repeated in 30 minutes, up to maximum dose of 50 mg/hour | Breathing difficulty, coughing, or wheezing; irregular or fast, poundingheartbeat; skin rash; swelling of lower extremities; chest pain; fainting; painful, swollen joints; vision impairment |
| Sodium nitroprusside† | 0.5-5 minutes | 0.25-5 μg/kg/minute by intravenous infusion | Risk of fetal cyanide poisoning with prolonged treatment. |
| Maternal effects include symptoms of hypothyroidism, headache, abdominal pain, drowsiness, nausea, involuntary muscle movements, perspiration, restlessness, paraesthesia, palpitations, dizziness, retching, tachycardia | |||
| *In women with asthma and congestive heart failure | |||
| †Rarely needed except in hypertensive encephalopathy or cerebral hemorrhage | |||
Postpartum management
Because preeclampsia can worsen, or first appear, in the postpartum period, extra vigilance is important, and pharmacotherapy may be appropriate.
Management of preeclampsia does not end with delivery of the fetus and the placenta. These events do signal the beginning of the curative process, but complications can occur in the postpartum period. Indeed, in some women, the disease process worsens immediately postpartum. Therefore, women with diagnosed preeclampsia or severe gestational hypertension require close monitoring of blood pressure and maternal symptoms and accurate measurement of fluid intake and urine output. Some of these women are at increased risk for pulmonary edema; exacerbation of severe hypertension; eclampsia; and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome.
Treating postpartum hypertension
Women who continue to have severe hypertension (systolic pressure at or above 155 mm Hg or diastolic pressure of 105 mm Hg or higher) will benefit from oral nifedipine (10 mg every 6 hours) or long-acting nifedipine (10 to 20 mg twice daily), the drugs of choice because of their favorable effects on renal function.
Women with severe hypertension also may require diuretics for better control of blood pressure, as may women with a history of congestive heart failure or left ventricular dysfunction.
Start women with vascular diabetes mellitus or diabetic nephropathy on ACE inhibitors immediately postpartum.
Patients can be discharged home once blood pressure is stable, provided there are no maternal symptoms of preeclampsia.
Postpartum preeclampsia can develop even in healthy women
Because severe hypertension or preeclampsia may develop for the first time in the postpartum period, it is important to educate all gravidas about the signs and symptoms. All health-care providers should be on the lookout for these symptoms as well.
The author reports no financial relationships relevant to this article.
BIBLIOGRAPHY
Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev (England). 2001;(2)pCD002252.-
Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur J Obstet Gynecol Reprod Biol. 2002;101:6-14.
Amorim MMR, Santas LC, Faundes A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. Am J Obstet Gynecol. 1999;180:1283-1288.
Duley L, Galmezoglu AM, Henderson-Smart DJ. Magnesium sulfate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev (England). 2003;(2)pCD000025.-
Friedman SA, Lubarsky S, Schiff E. Expectant management of severe preeclampsia remote from term. Clin Obstet Gynecol. 1999;42:470-478.
Haddad B, Deis S, Goffinet F, et al. Maternal and perinatal outcomes during expectant management of 239 severe preeclamptic women between 24 and 33 weeks’ gestation. Am J Obstet Gynecol. 2004;190:1590-1597.
Hall DR, Odendaal HJ, Kirten GF, Smith J. Expectant management of early onset, severe preeclampsia, perinatal outcome. BJOG. 2000;107:1258-1264.
Kupferminc MJ, Fait G, Many A, et al. Low molecular weight heparin for the prevention of obstetric complications in women with thrombophilia. Hypertension in Pregnancy. 2001;20:35-44.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111-166.
Magee LA, Cham C, Waterman EJ, Ohlsson A, Von Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ. 2003;327:1-10.
Magee LA, Ornstein MP, Von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999;318:1332-1336.
Magpie Trial Group. Do women with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised, placebo-controlled trial. Lancet. 2002;359:1877-1890.
Report of the National High Blood Pressure Education Program. Working group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1-22.
Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
Sibai BM, Lindheimer MD, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;229:667-671.
Sibai BM. Magnesium sulfate prophylaxis in preeclampsia. Lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520-1526.
Vigil-DeGracia P, Montufar-Rueda C, Ruiz J. Expectant management of severe preeclampsia and preeclampsia superimposed on chronic hypertension between 24 and 34 weeks’ gestation. Eur J Obstet Gynecol Reprod Biol. 2003;107:24-27.
BIBLIOGRAPHY
Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev (England). 2001;(2)pCD002252.-
Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur J Obstet Gynecol Reprod Biol. 2002;101:6-14.
Amorim MMR, Santas LC, Faundes A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. Am J Obstet Gynecol. 1999;180:1283-1288.
Duley L, Galmezoglu AM, Henderson-Smart DJ. Magnesium sulfate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev (England). 2003;(2)pCD000025.-
Friedman SA, Lubarsky S, Schiff E. Expectant management of severe preeclampsia remote from term. Clin Obstet Gynecol. 1999;42:470-478.
Haddad B, Deis S, Goffinet F, et al. Maternal and perinatal outcomes during expectant management of 239 severe preeclamptic women between 24 and 33 weeks’ gestation. Am J Obstet Gynecol. 2004;190:1590-1597.
Hall DR, Odendaal HJ, Kirten GF, Smith J. Expectant management of early onset, severe preeclampsia, perinatal outcome. BJOG. 2000;107:1258-1264.
Kupferminc MJ, Fait G, Many A, et al. Low molecular weight heparin for the prevention of obstetric complications in women with thrombophilia. Hypertension in Pregnancy. 2001;20:35-44.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111-166.
Magee LA, Cham C, Waterman EJ, Ohlsson A, Von Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ. 2003;327:1-10.
Magee LA, Ornstein MP, Von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999;318:1332-1336.
Magpie Trial Group. Do women with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised, placebo-controlled trial. Lancet. 2002;359:1877-1890.
Report of the National High Blood Pressure Education Program. Working group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1-22.
Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
Sibai BM, Lindheimer MD, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;229:667-671.
Sibai BM. Magnesium sulfate prophylaxis in preeclampsia. Lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520-1526.
Vigil-DeGracia P, Montufar-Rueda C, Ruiz J. Expectant management of severe preeclampsia and preeclampsia superimposed on chronic hypertension between 24 and 34 weeks’ gestation. Eur J Obstet Gynecol Reprod Biol. 2003;107:24-27.
Preeclampsia: 3 preemptive tactics
We routinely use every means possible to overcome the complications of hypertensive disorders and related preterm births. Yet our best opportunity to reduce morbidity and mortality could be before preeclampsia develops.
Preemptive tactics can be effective in preventing or reducing severity of preeclampsia. The patient’s active cooperation is a must, but the effort to recruit her cooperation can mean a better outcome.
If a diabetic or hypertensive woman doesn’t take her medications properly or if an obese woman postpones weight loss until after preeclampsia develops, it is too late to reduce the level of risk.
At-risk patients can benefit from being informed of any other ways to reduce risk as well; for example, by controlling the number of fetuses transferred via assisted reproductive techniques.
Trends that are driving up the prevalence of risk factors will only increase the number of preconception and obstetric cases with high-risk potential:
- The increased proportion of births among nulliparous women and women older than 35 years.
- The increased proportion of multifetal gestation as a result of assisted reproductive therapy.
- The increased prevalence of obesity in women, which is likely to lead to greater frequency of gestational diabetes, insulin resistance, and chronic hypertension.
Step 1Start risk-reducing tactics as early as possible
Retrospective studies have identified factors that multiply the risk of preeclampsia. Some are identifiable—and modifiable—before conception or beginning at the first prenatal visit (TABLE 1).
- Identify risk factors and recruit the patient’s efforts to reduce risks—before conception whenever possible.
- Set up prenatal care to watch closely for signal findings and make a prompt diagnosis.
- Develop a delivery plan that balances maternal and fetal needs. Identify indications for delivery.
Preconception risk factors
Obesity carries a 10 to 15% risk for preeclampsia. Prevention or effective treatment can greatly reduce risk.
Hypertension.Women with uncontrolled hypertension should have their blood pressure controlled prior to conception and as early as possible in the first trimester. In these women, the risk of preeclampsia may be reduced to below the 10 to 40% rate, depending on severity.
Renal disease. Risk for an adverse pregnancy outcome depends on maternal renal function at time of conception. Women should be encouraged to conceive while serum creatinine is less than 1.2 mg/dl.
Pregestational diabetes mellitus. Risk for preeclampsia and adverse outcomes depends on duration of diabetes, as well as vascular complications and blood sugar control prior to conception and early in pregnancy. Encourage these women to complete childbearing as early as possible and before vascular complications develop, and to aggressively control their diabetes and hypertension (if present) at least a few months prior to conception and throughout pregnancy.
Maternal age older than 35 years increases risk depending on associated medical conditions, nulliparity, and need for assisted reproductive therapy. These women are more likely to be nulliparous, overweight, chronically hypertensive, and to require assisted reproductive therapy. ART may involve multifetal gestation and donor insemination or oocyte donation—both of which increase risk and severity of preeclampsia. Therefore, these patients need to be made aware of their risks and helped to take steps to minimize risks.
TABLE 1
Preconception risk factors for preeclampsia
| 20 to 30% | Previous preeclampsia |
| 50% | Previous preeclampsia at 28 weeks |
| 15 to 25% | Chronic hypertension |
| 40% | Severe hypertension |
| 25% | Renal disease |
| 20% | Pregestational diabetes mellitus |
| 10 to 15% | Class B/C diabetes |
| 35% | Class F/R diabetes |
| 10 to 40% | Thrombophilia |
| 10 to 15% | Obesity/insulin resistance |
| 10 to 20% | Age >35 years |
| 10 to 15% | Family history of preeclampsia |
| 6 to 7% | Nulliparity/primipaternity |
Pregnancy-related risk factors
Many risk factors may be identified for the first time during pregnancy (TABLE 2). It is important to realize that the magnitude of risk depends on number of risk factors.
Nulliparity and primipaternity. Over the past decade, several epidemiologic studies suggested that immune maladaptation plays an important pathogenetic role in development of preeclampsia.
Generally, preeclampsia is considered a disease of first pregnancy. Indeed, a previous miscarriage of a previous normotensive pregnancy with the same partner is has a lowered frequency of preeclampsia. This protective effect is lost, however, with change of partner, suggesting that primipaternity increases the rate of preeclampsia.
A large prospective study on the relation between duration of sperm exposure with a partner and the rate of preeclampsia showed that women who conceive after a cohabitation period of 0 to 4 months have a 10-fold rate of preeclampsia, compared to those who conceive after a cohabitation period of at least 12 months. A similar study confirmed these findings.
The protective effects of long-term sperm exposure could explain the high frequency of preeclampsia in teenage pregnancy. (These women tend to have limited sperm exposure with a partner, or multiple partners). Thus, it is important to teach these women about their risks and the need for regular prenatal care.
Multifetal gestation increases the rate as well as the severity of preeclampsia, and the rate increases with the number of fetuses. Lowering the number of embryos transferred will substantially reduce the risk of preeclampsia and adverse outcomes.
There is no therapy to prevent preeclampsia in these women; however, we should acknowledge the increased risk and develop antenatal-care programs that allow close observation and early detection of preeclampsia in these women.
Hydropic degeneration of placenta. It is well-established that pregnancies complicated by fetal hydrops or hydropic degeneration of the placenta (with or without a coexisting fetus) are at very high risk for preeclampsia. In these cases, preeclampsia usually develops in the second trimester and is usually severe, and therefore causes substantial maternal and perinatal morbidities. Development of preeclampsia in such pregnancies requires immediate hospitalization and consideration for prompt delivery.
Unexplained elevated serum markers in the second trimester. Maternal serum screening with alpha fetoprotein (AFP), human chorionic gonadotropin (HCG) and inhibin A is commonly used to identify those at risk for aneuploidy or neural tube defects.
Unexplained elevations in AFP, HCG or inhibin A have been associated with increased adverse pregnancy outcome such as fetal death, intrauterine growth restriction (IUGR), preterm delivery, and preeclampsia. However, the data on the association between abnormalities in these biomarkers and preeclampsia have been inconsistent. Nevertheless, retrospective studies suggest that elevation in these serum markers during the second trimester increases the risk of preeclampsia by at least twofold. The risk is probably higher in those who have abnormalities in more than 1 of these markers. Since unexplained abnormalities of these serum markers may reflect early placental pathology, it is suggested that these pregnancies may benefit from close obstetric surveillance.
Serum and urinary markers of abnormal angiogenesis and subsequent preeclampsia were strongly associate, in newly published studies reported by Levine and colleagues. For example, circulating soluble fms-like tyrosine kinase (sFLt1) is elevated in pregnant women prior to onset of preeclampsia, whereas urinary placental growth factor is reduced several weeks prior to clinical onset of preeclampsia. Both of these markers appear to hold some promise.
Unexplained proteinuria or hematuria. Generally, proteinuria is considered a late manifestation of preeclampsia. However, recent retrospective studies suggest that some women with preeclampsia, particularly those with HELLP syndrome, might not have hypertension (>140 mm Hg systolic or >90 mm Hg diastolic). In some women, persistent proteinuria (3+ on dipstick) or >300 mg/24 hour may be the first sign of preeclampsia or could be a marker of silent renal disease.
No prospective studies have evaluated the risk of preeclampsia in asymptomatic women with persistent proteinuria. I suggest, however, that women with this finding will benefit from intensified obstetric surveillance (more frequent prenatal visits) and/or biochemical evaluation (platelet count, liver enzymes), particularly if they have headaches, visual changes, epigastric or right upper quadrant pain, nausea or vomiting, or respiratory symptoms (chest pain or shortness of breath)—likewise, for pregnant women with persistent hematuria of unknown origin.
Unexplained fetal growth restriction. Impaired trophoblast invasion is a key features of pregnancies complicated by preeclampsia or unexplained IUGR. Preeclampsia can manifest either as a maternal syndrome (hypertension and proteinuria with or without symptoms) or a fetal abnormal growth syndrome.
In clinical practice, most cases of unexplained IUGR are probably delivered before the maternal syndrome develops. In some cases, unexplained IUGR may be the first manifestation of preeclampsia, particularly those with IUGR before 34 weeks’ gestation. The absolute risk of clinical preeclampsia in such women is unknown because of lack of prospective data. Nevertheless, a woman with idiopathic IUGR prior to 34 weeks’ gestation whose pregnancy is managed expectantly is at increased risk for future preeclampsia. These women should receive intensive maternal surveillance for preeclampsia, and a diagnosis of preeclampsia should be considered in those who develop maternal symptoms or abnormal blood tests.
Abnormal uterine artery Doppler velocimetry at 18 to 24 weeks’ gestation. Several observational studies reported an association between elevated uterine artery resistance as measured by Doppler (with or without presence of a notch) in the second trimester and subsequent preeclampsia and/or IUGR. The reported rates of preeclampsia among women with abnormal Doppler results range from 6% to 40%. The risk varies depending on the site measured, gestational age at time of measurement, normal indices used, abnormality on repeat measurement, and population studied.
A systemic review of 27 studies, which included approximately 13,000 women, revealed that an abnormal uterine artery Doppler waveform increases the risk of preeclampsia by 4- to 6-fold, compared to normal Doppler results. The review concluded that uterine artery Doppler evaluation has a limited value as a screening test to predict preeclampsia.
What should the physician do when faced with an ultrasound report indicating an abnormal uterine artery Doppler finding?
Is low-dose aspirin helpful? Several randomized trials evaluated the potential role of low-dose aspirin in reducing the risk of preeclampsia in women with abnormal uterine artery Doppler indices. A meta-analysis suggested that low-dose aspirin significantly reduced the rate of preeclampsia (16% in placebo versus 10% with aspirin, odds ratio of 0.55). This analysis included a total of 498 subjects.
In contrast, a recent randomized trial in 560 women with abnormal uterine artery Doppler at 23 weeks’, who were assigned to aspirin 150 mg or placebo, found no differences in rates of preeclampsia (18% versus 19%) or in preeclampsia requiring delivery before 34 weeks’ (6% versus 8%). A similar randomized trial using 100 mg aspirin daily in 237 women with abnormal uterine artery Doppler at 22 to 24 weeks revealed no reduction in rate of preeclampsia compared to placebo.
Consequently, low-dose aspirin is not recommended for prevention of preeclamp-sia in these women.
Close surveillance is warranted. Although there is no available proven therapy to reduce the risk of preeclampsia in these women, they should be closely observed because of the increased rate of adverse outcomes, including preeclampsia.
TABLE 2
Pregnancy-related risk factors for preeclampsia
| Magnitude of risk depends on the number of factors | |
|---|---|
| 2-fold normal | Unexplained midtrimester elevations of serum AFP, HCG, inhibin-A |
| 10 to 30% | Abnormal uterine artery Doppler velocimetry |
| 0 to 30% | Hydrops/hydropic degeneration of placenta |
| 10 to 20% | Multifetal gestation (depends on number of fetuses and maternal age) |
| 10% | Partner who fathered preeclampsia in another woman |
| 8 to 10% | Gestational diabetes mellitus |
| 8 to 10% | Limited sperm exposure (teenage pregnancy) |
| 6 to 7% | Nulliparity/primipaternity |
| Limited data | Donor insemination, oocyte donation |
| Limited data | Unexplained persistent proteinuria or hematuria |
| Unknown | Unexplained fetal growth restriction |
Step 2Watch for signal findings, diagnose preeclampsia early
Signs and symptoms may call for close surveillance at any time. Early detection of preeclampsia is the best way to reduce adverse outcomes.
Prenatal care does not prevent preeclampsia, of course. All pregnant women are at risk, some more than others. Still, adequate and proper prenatal care is the best strategy to detect preeclampsia early.
We may need to modify the frequency and type of maternal and fetal surveillance at any time. Thus, patients with multiple risk factors or risk exceeding 10% should have more frequent visits, especially beyond 24 weeks. Maternal blood pressure (both systolic and diastolic), urine protein values, abrupt and excessive weight gain, maternal symptoms, and fetal growth warrant particular attention.
Diagnostic criteria vary with risk
The diagnosis of preeclampsia is different in patients with different risk factors. In healthy nulliparous women, the diagnosis requires persistent hypertension and proteinuria (new onset after 20 weeks’ gestation). However, in some patients the diagnosis should be made based on new onset hypertension and maternal symptoms or abnormal blood tests (low platelets or elevated liver enzymes).
Urine dipstick is a reliable screening test in women who remain normotensive.
24-hour urine measurement is the best test to confirm proteinuria if hypertension develops. Several studies found that urine dipstick values less than (1+) and random urine protein to creatinine ratio measurements are not accurate to predict proteinuria in women with gestational hypertension.
When is it gestational hypertension? The term applies only women with all of these findings:
- mild hypertension <160/<110 mm Hg
- proteinuria <300 mg/24-hour urine
- normal platelet count
- normal liver enzymes
- normal fetal growth
- no maternal symptoms
Once gestational hypertension is diagnosed, obtain blood tests and ultrasound evaluation to document fetal growth and amniotic fluid status.
Women with severe gestational hypertension and those with abnormal tests should be diagnosed as having preeclampsia and managed as such.
Women with gestational hypertension are at high risk for preeclampsia, and risk of progression depends on gestational age at time of diagnosis. Women who develop gestational hypertension at 24 to 35 weeks have a 46% chance of developing preeclampsia with a high rate of preterm delivery (32% <36 weeks and 12.5% <34 weeks) (FIGURE). These women require very close surveillance. In contrast, maternal and perinatal outcome is usually favorable when only mild gestational hypertension develops at or beyond 36 weeks.
When hypertension, proteinuria occur before 20 weeks
The traditional diagnostic criteria for preeclampsia in healthy women are not reliable in women who have either hypertension or proteinuria prior to 20 weeks’ gestation, particularly in those taking antihypertensive medications and in those who have class F diabetes mellitus. Because of the physiologic changes during pregnancy, women with diabetes and renal disease will have serial increases in blood pressure as well as protein excretion with advanced gestational age, particularly in the third trimester. Diagnostic criteria (TABLE 3) should be individualized based on medical conditions and current therapy. Antihypertensive drugs and preexisting proteinuria make it more difficult to classify preeclampsia as mild or severe.
TABLE 3
Diagnostic criteria
| GESTATIONAL HYPERTENSION IN HEALTHY WOMEN | |
| Blood pressure <160 mm Hg diastolic and <110 mm Hg systolic | |
| Proteinuria <300 mg/24-hour collection | |
| Platelet count >100,000/mm3 | |
| Normal liver enzymes | |
| No maternal symptoms | |
| No intrauterine growth restriction or oligohydraminos by ultrasound | |
| PREECLAMPSIA IN WOMEN WITH PREEXISTING MEDICAL CONDITIONS | |
| Condition | Criteria |
| Hypertension only | Proteinuria >500 mg/24-hours or thrombocytopenia |
| Proteinuria only | New onset hypertension plus symptoms or thrombocytopenia or elevated liver enzymes |
| Hypertension plus proteinuria (renal disease or class F diabetes) | Worsening severe hypertension and/or new onset of symptoms, thrombocytopenia, elevated liver enzymes |
FIGURE Whether preeclampsia will develop depends on when gestational hypertension begins
Adapted from Barton JR, et al. Am J Obstet Gynecol. 2001;184:979-983.
Step 3Consider how to balance risk to mother and fetus
Once a diagnosis is made, promptly evaluate mother and fetus, continue close surveillance, select those who will benefit from hospitalization, and identify indications for delivery (TABLE 4).
Delivery will always reduce the risks for the mother, but in certain situations, it might not be the best option for an extremely premature fetus. Sometimes delivery is best for both mother and fetus.
The best strategy takes into consideration:
- maternal and fetal status at initial evaluation,
- preexisting medical conditions that could affect pregnancy outcome,
- fetal gestational age at time of diagnosis,
- labor or rupture of fetal membranes (both could affect management), and
- maternal choice of available options.
Women who remain undelivered require close maternal and fetal evaluation. In otherwise healthy women, management depends on whether the preeclampsia is mild or severe, and, if there are other medical conditions, on the status of those conditions, as well.
TABLE 4
Indications for delivery
| Consider delivery in gravidas with 1 or more indications |
|---|
| Gestational age ≥38 weeks for mild disease |
| Gestational age ≥34 weeks for severe disease |
| 33-34 weeks with severe disease after steroids |
| Onset of labor and/or membrane rupture ≥34 weeks |
| Eclampsia or pulmonary edema (any gestational age) |
| HELLP syndrome (any gestational age) |
| Severe cerebral symptoms or epigastric pain |
| Acute renal insufficiency (serum creatinine >1.2 mg/dl) |
| Persistent thrombocytopenia (platelet count <100,000) |
| Maternal desire for delivery |
| Severe oligohydraminos or IUGR < 5th percentile |
| Nonreassuring fetal testing |
Chronic hypertension
- Underlies 30% of cases of hypertension during pregnancy.
- Begins before pregnancy or before 20 weeks’ gestation.
Gestational hypertension
- The most common form of hypertension during pregnancy.
- Acute onset beyond 20 weeks’ gestation in a woman known to be normotensive before pregnancy or prior to 20 weeks’ gestation.
Preeclampsia
- Can superimpose upon chronic hypertension, renal disease, or connective tissue disease, or develop in women with gestational hypertension.
- Preeclampsia in healthy nulliparous women: hypertension and proteinuria after 20 weeks’ gestation.
- Preeclampsia in women with preexisting chronic hypertension and absent proteinuria: an exacerbation of hypertension and new onset proteinuria.
Eclampsia
- Development of convulsions in women with hypertensive disorders of pregnancy.
“HELLP syndrome”
- Hemolysis,
- Elevated liver enzymes, and
- Low platelet count
Suspected or confirmed preeclampsia in a woman who has documented evidence of hemolysis (abnormal peripheral smear, or elevated bilirubin, or anemia, or low heptoglobin levels), plus elevated liver enzymes (AST or ALT), and thrombocytopenia (platelet count below 100,000).
The author reports no financial relationships relevant to this article.
BIBLIOGRAPHY
Barton JR, O’Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: Progression and outcome. Am J Obstet Gynecol. 2001;184:979-983.
Boggess KA, Lief S, Martha AP, Moos K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003;101:227-231.
Buchbinder A, Sibai BM, Caritis S, MacPherson C, Hauth J, Lindheimer MD. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66-71.
Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E. Low-dose aspirin to prevent preeclampsia in women at high risk. N Engl J Med. 1998;338:701-705.
Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219-224.
Chien PE, Arnott N, Gordon A, Owen P, Khan KS. How useful is uterine artery Doppler flow velocimetry in the prediction of pre-eclampsia, intrauterine growth retardation and perinatal death? An Overview. Br J Obstet Gynaecol. 2000;107:196-208.
Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for the prevention of preeclampsia in women with abnormal uterine artery Doppler: A meta-analysis. Obstet Gynecol. 2001;98:861-866.
Curet LB. Pregnancy outcomes in healthy nulliparous women who subsequently developed hypertension. Obstet Gynecol. 2000;95:24-28.
Dekker G, Robillard PY. The birth interval hypothesis - Does it really indicate the end of the primipaternity hypothesis? J Reprod Immunol. 2003;59:245-251.
Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet. 2001;357:209-215.
Durnwald C, Mercer B. A prospective comparison of total protein/creatinine ratio versus 24-hour urine protein in women with suspected preeclampsia. Am J Obstet Gynecol. 2003;189:848-52.
Einarsson JI, Sangi-Haghpeykar H, Gardner NO. Sperm exposure and development of preeclampsia. Am J Obstet Gynecol. 2003;188:1241-1243.
Hauth JC, Ewell MG, Levine RL, Esterlitz JR, Sibai BM, Curet LB. Pregnancy outcomes in healthy nulliparous women who subsequently developed hypertension. Obstet Gynecol. 2000;95:24-28.
Hnat MD, Sibai BM, Caritis S, Hiouth J, Lindheimer MD, MacPherson C. Perinatal outcome in women with recurrent preeclampsia compared with women who develop preeclampsia as nulliparous. Am J Obstet Gynecol. 2002;186:422-426.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111-166.
Levine RG, Thadhani R, Qian C, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77-85.
Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
Nilsson E, Salonen Ros H, Cnattingius S, Lichtenstein P. The importance of genetic and environmental effects for preeclampsia and gestational hypertension: a family study. Br J Obstet Gynaecol. 2004;111:200-206.
O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368-374.
Ragip A Al, Baykal C, Karacay O, Geyik PO, Altun S, Dolen I. Random urine protein-creatinine ratio to predict proteinuria in new-onset mild hypertension in late pregnancy. Obstet Gynecol. 2004;104:367-371.
Saftlas AF, Levine RJ, Klebanoff MA, Martz KL, Ewell MG, Morris CD, Sibai BM. Abortion, changed paternity, and risk of preeclampsia in nulliparous women. Am J Epidemiol. 2003;157:1108-1114.
Sibai BM, Caritis S, Hauth J, Lilndheimer MD, MacPherson C, Klebanoff M, et al. Hypertensive disorders in twin versus singleton gestations. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 2000;182:938-942.
Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-33377.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
Subtil D, Goeusse P, Houfflin-Debarge V, Puech F, Lequien P, Breart G, Uzan S, Quandalle F, Delcourt YM, Malek YM. Essai Regional Aspirine Mere-Enfant (ERASME) Collaborative Group. Randomised comparison of uterine artery Doppler and aspirin (100 mg) with placebo in nulliparous women: the Essai Regional Aspirine Mere-Enfant study (Part 2). Br J Obstet Gynaecol. 2003;110:485-491.
Wen SW, Demissie K, Yang Q, Walker MC. Maternal morbidity and obstetric complications in triplet pregnancies and quadruplet and higher-order multiple pregnancies. Am J Obstet Gynecol. 2004;191:254-258.
Yu CKH, Papageorghiou AT, Parra M, Dias RP, Nicolaides KH. Randomized controlled trial using low-dose aspirin in the prevention of pre-eclampsia in women with abnormal uterine artery Doppler at 23 weeks’ gestation. Ultrasound Obstet Gynecol. 2003;22:233-239.
We routinely use every means possible to overcome the complications of hypertensive disorders and related preterm births. Yet our best opportunity to reduce morbidity and mortality could be before preeclampsia develops.
Preemptive tactics can be effective in preventing or reducing severity of preeclampsia. The patient’s active cooperation is a must, but the effort to recruit her cooperation can mean a better outcome.
If a diabetic or hypertensive woman doesn’t take her medications properly or if an obese woman postpones weight loss until after preeclampsia develops, it is too late to reduce the level of risk.
At-risk patients can benefit from being informed of any other ways to reduce risk as well; for example, by controlling the number of fetuses transferred via assisted reproductive techniques.
Trends that are driving up the prevalence of risk factors will only increase the number of preconception and obstetric cases with high-risk potential:
- The increased proportion of births among nulliparous women and women older than 35 years.
- The increased proportion of multifetal gestation as a result of assisted reproductive therapy.
- The increased prevalence of obesity in women, which is likely to lead to greater frequency of gestational diabetes, insulin resistance, and chronic hypertension.
Step 1Start risk-reducing tactics as early as possible
Retrospective studies have identified factors that multiply the risk of preeclampsia. Some are identifiable—and modifiable—before conception or beginning at the first prenatal visit (TABLE 1).
- Identify risk factors and recruit the patient’s efforts to reduce risks—before conception whenever possible.
- Set up prenatal care to watch closely for signal findings and make a prompt diagnosis.
- Develop a delivery plan that balances maternal and fetal needs. Identify indications for delivery.
Preconception risk factors
Obesity carries a 10 to 15% risk for preeclampsia. Prevention or effective treatment can greatly reduce risk.
Hypertension.Women with uncontrolled hypertension should have their blood pressure controlled prior to conception and as early as possible in the first trimester. In these women, the risk of preeclampsia may be reduced to below the 10 to 40% rate, depending on severity.
Renal disease. Risk for an adverse pregnancy outcome depends on maternal renal function at time of conception. Women should be encouraged to conceive while serum creatinine is less than 1.2 mg/dl.
Pregestational diabetes mellitus. Risk for preeclampsia and adverse outcomes depends on duration of diabetes, as well as vascular complications and blood sugar control prior to conception and early in pregnancy. Encourage these women to complete childbearing as early as possible and before vascular complications develop, and to aggressively control their diabetes and hypertension (if present) at least a few months prior to conception and throughout pregnancy.
Maternal age older than 35 years increases risk depending on associated medical conditions, nulliparity, and need for assisted reproductive therapy. These women are more likely to be nulliparous, overweight, chronically hypertensive, and to require assisted reproductive therapy. ART may involve multifetal gestation and donor insemination or oocyte donation—both of which increase risk and severity of preeclampsia. Therefore, these patients need to be made aware of their risks and helped to take steps to minimize risks.
TABLE 1
Preconception risk factors for preeclampsia
| 20 to 30% | Previous preeclampsia |
| 50% | Previous preeclampsia at 28 weeks |
| 15 to 25% | Chronic hypertension |
| 40% | Severe hypertension |
| 25% | Renal disease |
| 20% | Pregestational diabetes mellitus |
| 10 to 15% | Class B/C diabetes |
| 35% | Class F/R diabetes |
| 10 to 40% | Thrombophilia |
| 10 to 15% | Obesity/insulin resistance |
| 10 to 20% | Age >35 years |
| 10 to 15% | Family history of preeclampsia |
| 6 to 7% | Nulliparity/primipaternity |
Pregnancy-related risk factors
Many risk factors may be identified for the first time during pregnancy (TABLE 2). It is important to realize that the magnitude of risk depends on number of risk factors.
Nulliparity and primipaternity. Over the past decade, several epidemiologic studies suggested that immune maladaptation plays an important pathogenetic role in development of preeclampsia.
Generally, preeclampsia is considered a disease of first pregnancy. Indeed, a previous miscarriage of a previous normotensive pregnancy with the same partner is has a lowered frequency of preeclampsia. This protective effect is lost, however, with change of partner, suggesting that primipaternity increases the rate of preeclampsia.
A large prospective study on the relation between duration of sperm exposure with a partner and the rate of preeclampsia showed that women who conceive after a cohabitation period of 0 to 4 months have a 10-fold rate of preeclampsia, compared to those who conceive after a cohabitation period of at least 12 months. A similar study confirmed these findings.
The protective effects of long-term sperm exposure could explain the high frequency of preeclampsia in teenage pregnancy. (These women tend to have limited sperm exposure with a partner, or multiple partners). Thus, it is important to teach these women about their risks and the need for regular prenatal care.
Multifetal gestation increases the rate as well as the severity of preeclampsia, and the rate increases with the number of fetuses. Lowering the number of embryos transferred will substantially reduce the risk of preeclampsia and adverse outcomes.
There is no therapy to prevent preeclampsia in these women; however, we should acknowledge the increased risk and develop antenatal-care programs that allow close observation and early detection of preeclampsia in these women.
Hydropic degeneration of placenta. It is well-established that pregnancies complicated by fetal hydrops or hydropic degeneration of the placenta (with or without a coexisting fetus) are at very high risk for preeclampsia. In these cases, preeclampsia usually develops in the second trimester and is usually severe, and therefore causes substantial maternal and perinatal morbidities. Development of preeclampsia in such pregnancies requires immediate hospitalization and consideration for prompt delivery.
Unexplained elevated serum markers in the second trimester. Maternal serum screening with alpha fetoprotein (AFP), human chorionic gonadotropin (HCG) and inhibin A is commonly used to identify those at risk for aneuploidy or neural tube defects.
Unexplained elevations in AFP, HCG or inhibin A have been associated with increased adverse pregnancy outcome such as fetal death, intrauterine growth restriction (IUGR), preterm delivery, and preeclampsia. However, the data on the association between abnormalities in these biomarkers and preeclampsia have been inconsistent. Nevertheless, retrospective studies suggest that elevation in these serum markers during the second trimester increases the risk of preeclampsia by at least twofold. The risk is probably higher in those who have abnormalities in more than 1 of these markers. Since unexplained abnormalities of these serum markers may reflect early placental pathology, it is suggested that these pregnancies may benefit from close obstetric surveillance.
Serum and urinary markers of abnormal angiogenesis and subsequent preeclampsia were strongly associate, in newly published studies reported by Levine and colleagues. For example, circulating soluble fms-like tyrosine kinase (sFLt1) is elevated in pregnant women prior to onset of preeclampsia, whereas urinary placental growth factor is reduced several weeks prior to clinical onset of preeclampsia. Both of these markers appear to hold some promise.
Unexplained proteinuria or hematuria. Generally, proteinuria is considered a late manifestation of preeclampsia. However, recent retrospective studies suggest that some women with preeclampsia, particularly those with HELLP syndrome, might not have hypertension (>140 mm Hg systolic or >90 mm Hg diastolic). In some women, persistent proteinuria (3+ on dipstick) or >300 mg/24 hour may be the first sign of preeclampsia or could be a marker of silent renal disease.
No prospective studies have evaluated the risk of preeclampsia in asymptomatic women with persistent proteinuria. I suggest, however, that women with this finding will benefit from intensified obstetric surveillance (more frequent prenatal visits) and/or biochemical evaluation (platelet count, liver enzymes), particularly if they have headaches, visual changes, epigastric or right upper quadrant pain, nausea or vomiting, or respiratory symptoms (chest pain or shortness of breath)—likewise, for pregnant women with persistent hematuria of unknown origin.
Unexplained fetal growth restriction. Impaired trophoblast invasion is a key features of pregnancies complicated by preeclampsia or unexplained IUGR. Preeclampsia can manifest either as a maternal syndrome (hypertension and proteinuria with or without symptoms) or a fetal abnormal growth syndrome.
In clinical practice, most cases of unexplained IUGR are probably delivered before the maternal syndrome develops. In some cases, unexplained IUGR may be the first manifestation of preeclampsia, particularly those with IUGR before 34 weeks’ gestation. The absolute risk of clinical preeclampsia in such women is unknown because of lack of prospective data. Nevertheless, a woman with idiopathic IUGR prior to 34 weeks’ gestation whose pregnancy is managed expectantly is at increased risk for future preeclampsia. These women should receive intensive maternal surveillance for preeclampsia, and a diagnosis of preeclampsia should be considered in those who develop maternal symptoms or abnormal blood tests.
Abnormal uterine artery Doppler velocimetry at 18 to 24 weeks’ gestation. Several observational studies reported an association between elevated uterine artery resistance as measured by Doppler (with or without presence of a notch) in the second trimester and subsequent preeclampsia and/or IUGR. The reported rates of preeclampsia among women with abnormal Doppler results range from 6% to 40%. The risk varies depending on the site measured, gestational age at time of measurement, normal indices used, abnormality on repeat measurement, and population studied.
A systemic review of 27 studies, which included approximately 13,000 women, revealed that an abnormal uterine artery Doppler waveform increases the risk of preeclampsia by 4- to 6-fold, compared to normal Doppler results. The review concluded that uterine artery Doppler evaluation has a limited value as a screening test to predict preeclampsia.
What should the physician do when faced with an ultrasound report indicating an abnormal uterine artery Doppler finding?
Is low-dose aspirin helpful? Several randomized trials evaluated the potential role of low-dose aspirin in reducing the risk of preeclampsia in women with abnormal uterine artery Doppler indices. A meta-analysis suggested that low-dose aspirin significantly reduced the rate of preeclampsia (16% in placebo versus 10% with aspirin, odds ratio of 0.55). This analysis included a total of 498 subjects.
In contrast, a recent randomized trial in 560 women with abnormal uterine artery Doppler at 23 weeks’, who were assigned to aspirin 150 mg or placebo, found no differences in rates of preeclampsia (18% versus 19%) or in preeclampsia requiring delivery before 34 weeks’ (6% versus 8%). A similar randomized trial using 100 mg aspirin daily in 237 women with abnormal uterine artery Doppler at 22 to 24 weeks revealed no reduction in rate of preeclampsia compared to placebo.
Consequently, low-dose aspirin is not recommended for prevention of preeclamp-sia in these women.
Close surveillance is warranted. Although there is no available proven therapy to reduce the risk of preeclampsia in these women, they should be closely observed because of the increased rate of adverse outcomes, including preeclampsia.
TABLE 2
Pregnancy-related risk factors for preeclampsia
| Magnitude of risk depends on the number of factors | |
|---|---|
| 2-fold normal | Unexplained midtrimester elevations of serum AFP, HCG, inhibin-A |
| 10 to 30% | Abnormal uterine artery Doppler velocimetry |
| 0 to 30% | Hydrops/hydropic degeneration of placenta |
| 10 to 20% | Multifetal gestation (depends on number of fetuses and maternal age) |
| 10% | Partner who fathered preeclampsia in another woman |
| 8 to 10% | Gestational diabetes mellitus |
| 8 to 10% | Limited sperm exposure (teenage pregnancy) |
| 6 to 7% | Nulliparity/primipaternity |
| Limited data | Donor insemination, oocyte donation |
| Limited data | Unexplained persistent proteinuria or hematuria |
| Unknown | Unexplained fetal growth restriction |
Step 2Watch for signal findings, diagnose preeclampsia early
Signs and symptoms may call for close surveillance at any time. Early detection of preeclampsia is the best way to reduce adverse outcomes.
Prenatal care does not prevent preeclampsia, of course. All pregnant women are at risk, some more than others. Still, adequate and proper prenatal care is the best strategy to detect preeclampsia early.
We may need to modify the frequency and type of maternal and fetal surveillance at any time. Thus, patients with multiple risk factors or risk exceeding 10% should have more frequent visits, especially beyond 24 weeks. Maternal blood pressure (both systolic and diastolic), urine protein values, abrupt and excessive weight gain, maternal symptoms, and fetal growth warrant particular attention.
Diagnostic criteria vary with risk
The diagnosis of preeclampsia is different in patients with different risk factors. In healthy nulliparous women, the diagnosis requires persistent hypertension and proteinuria (new onset after 20 weeks’ gestation). However, in some patients the diagnosis should be made based on new onset hypertension and maternal symptoms or abnormal blood tests (low platelets or elevated liver enzymes).
Urine dipstick is a reliable screening test in women who remain normotensive.
24-hour urine measurement is the best test to confirm proteinuria if hypertension develops. Several studies found that urine dipstick values less than (1+) and random urine protein to creatinine ratio measurements are not accurate to predict proteinuria in women with gestational hypertension.
When is it gestational hypertension? The term applies only women with all of these findings:
- mild hypertension <160/<110 mm Hg
- proteinuria <300 mg/24-hour urine
- normal platelet count
- normal liver enzymes
- normal fetal growth
- no maternal symptoms
Once gestational hypertension is diagnosed, obtain blood tests and ultrasound evaluation to document fetal growth and amniotic fluid status.
Women with severe gestational hypertension and those with abnormal tests should be diagnosed as having preeclampsia and managed as such.
Women with gestational hypertension are at high risk for preeclampsia, and risk of progression depends on gestational age at time of diagnosis. Women who develop gestational hypertension at 24 to 35 weeks have a 46% chance of developing preeclampsia with a high rate of preterm delivery (32% <36 weeks and 12.5% <34 weeks) (FIGURE). These women require very close surveillance. In contrast, maternal and perinatal outcome is usually favorable when only mild gestational hypertension develops at or beyond 36 weeks.
When hypertension, proteinuria occur before 20 weeks
The traditional diagnostic criteria for preeclampsia in healthy women are not reliable in women who have either hypertension or proteinuria prior to 20 weeks’ gestation, particularly in those taking antihypertensive medications and in those who have class F diabetes mellitus. Because of the physiologic changes during pregnancy, women with diabetes and renal disease will have serial increases in blood pressure as well as protein excretion with advanced gestational age, particularly in the third trimester. Diagnostic criteria (TABLE 3) should be individualized based on medical conditions and current therapy. Antihypertensive drugs and preexisting proteinuria make it more difficult to classify preeclampsia as mild or severe.
TABLE 3
Diagnostic criteria
| GESTATIONAL HYPERTENSION IN HEALTHY WOMEN | |
| Blood pressure <160 mm Hg diastolic and <110 mm Hg systolic | |
| Proteinuria <300 mg/24-hour collection | |
| Platelet count >100,000/mm3 | |
| Normal liver enzymes | |
| No maternal symptoms | |
| No intrauterine growth restriction or oligohydraminos by ultrasound | |
| PREECLAMPSIA IN WOMEN WITH PREEXISTING MEDICAL CONDITIONS | |
| Condition | Criteria |
| Hypertension only | Proteinuria >500 mg/24-hours or thrombocytopenia |
| Proteinuria only | New onset hypertension plus symptoms or thrombocytopenia or elevated liver enzymes |
| Hypertension plus proteinuria (renal disease or class F diabetes) | Worsening severe hypertension and/or new onset of symptoms, thrombocytopenia, elevated liver enzymes |
FIGURE Whether preeclampsia will develop depends on when gestational hypertension begins
Adapted from Barton JR, et al. Am J Obstet Gynecol. 2001;184:979-983.
Step 3Consider how to balance risk to mother and fetus
Once a diagnosis is made, promptly evaluate mother and fetus, continue close surveillance, select those who will benefit from hospitalization, and identify indications for delivery (TABLE 4).
Delivery will always reduce the risks for the mother, but in certain situations, it might not be the best option for an extremely premature fetus. Sometimes delivery is best for both mother and fetus.
The best strategy takes into consideration:
- maternal and fetal status at initial evaluation,
- preexisting medical conditions that could affect pregnancy outcome,
- fetal gestational age at time of diagnosis,
- labor or rupture of fetal membranes (both could affect management), and
- maternal choice of available options.
Women who remain undelivered require close maternal and fetal evaluation. In otherwise healthy women, management depends on whether the preeclampsia is mild or severe, and, if there are other medical conditions, on the status of those conditions, as well.
TABLE 4
Indications for delivery
| Consider delivery in gravidas with 1 or more indications |
|---|
| Gestational age ≥38 weeks for mild disease |
| Gestational age ≥34 weeks for severe disease |
| 33-34 weeks with severe disease after steroids |
| Onset of labor and/or membrane rupture ≥34 weeks |
| Eclampsia or pulmonary edema (any gestational age) |
| HELLP syndrome (any gestational age) |
| Severe cerebral symptoms or epigastric pain |
| Acute renal insufficiency (serum creatinine >1.2 mg/dl) |
| Persistent thrombocytopenia (platelet count <100,000) |
| Maternal desire for delivery |
| Severe oligohydraminos or IUGR < 5th percentile |
| Nonreassuring fetal testing |
Chronic hypertension
- Underlies 30% of cases of hypertension during pregnancy.
- Begins before pregnancy or before 20 weeks’ gestation.
Gestational hypertension
- The most common form of hypertension during pregnancy.
- Acute onset beyond 20 weeks’ gestation in a woman known to be normotensive before pregnancy or prior to 20 weeks’ gestation.
Preeclampsia
- Can superimpose upon chronic hypertension, renal disease, or connective tissue disease, or develop in women with gestational hypertension.
- Preeclampsia in healthy nulliparous women: hypertension and proteinuria after 20 weeks’ gestation.
- Preeclampsia in women with preexisting chronic hypertension and absent proteinuria: an exacerbation of hypertension and new onset proteinuria.
Eclampsia
- Development of convulsions in women with hypertensive disorders of pregnancy.
“HELLP syndrome”
- Hemolysis,
- Elevated liver enzymes, and
- Low platelet count
Suspected or confirmed preeclampsia in a woman who has documented evidence of hemolysis (abnormal peripheral smear, or elevated bilirubin, or anemia, or low heptoglobin levels), plus elevated liver enzymes (AST or ALT), and thrombocytopenia (platelet count below 100,000).
The author reports no financial relationships relevant to this article.
We routinely use every means possible to overcome the complications of hypertensive disorders and related preterm births. Yet our best opportunity to reduce morbidity and mortality could be before preeclampsia develops.
Preemptive tactics can be effective in preventing or reducing severity of preeclampsia. The patient’s active cooperation is a must, but the effort to recruit her cooperation can mean a better outcome.
If a diabetic or hypertensive woman doesn’t take her medications properly or if an obese woman postpones weight loss until after preeclampsia develops, it is too late to reduce the level of risk.
At-risk patients can benefit from being informed of any other ways to reduce risk as well; for example, by controlling the number of fetuses transferred via assisted reproductive techniques.
Trends that are driving up the prevalence of risk factors will only increase the number of preconception and obstetric cases with high-risk potential:
- The increased proportion of births among nulliparous women and women older than 35 years.
- The increased proportion of multifetal gestation as a result of assisted reproductive therapy.
- The increased prevalence of obesity in women, which is likely to lead to greater frequency of gestational diabetes, insulin resistance, and chronic hypertension.
Step 1Start risk-reducing tactics as early as possible
Retrospective studies have identified factors that multiply the risk of preeclampsia. Some are identifiable—and modifiable—before conception or beginning at the first prenatal visit (TABLE 1).
- Identify risk factors and recruit the patient’s efforts to reduce risks—before conception whenever possible.
- Set up prenatal care to watch closely for signal findings and make a prompt diagnosis.
- Develop a delivery plan that balances maternal and fetal needs. Identify indications for delivery.
Preconception risk factors
Obesity carries a 10 to 15% risk for preeclampsia. Prevention or effective treatment can greatly reduce risk.
Hypertension.Women with uncontrolled hypertension should have their blood pressure controlled prior to conception and as early as possible in the first trimester. In these women, the risk of preeclampsia may be reduced to below the 10 to 40% rate, depending on severity.
Renal disease. Risk for an adverse pregnancy outcome depends on maternal renal function at time of conception. Women should be encouraged to conceive while serum creatinine is less than 1.2 mg/dl.
Pregestational diabetes mellitus. Risk for preeclampsia and adverse outcomes depends on duration of diabetes, as well as vascular complications and blood sugar control prior to conception and early in pregnancy. Encourage these women to complete childbearing as early as possible and before vascular complications develop, and to aggressively control their diabetes and hypertension (if present) at least a few months prior to conception and throughout pregnancy.
Maternal age older than 35 years increases risk depending on associated medical conditions, nulliparity, and need for assisted reproductive therapy. These women are more likely to be nulliparous, overweight, chronically hypertensive, and to require assisted reproductive therapy. ART may involve multifetal gestation and donor insemination or oocyte donation—both of which increase risk and severity of preeclampsia. Therefore, these patients need to be made aware of their risks and helped to take steps to minimize risks.
TABLE 1
Preconception risk factors for preeclampsia
| 20 to 30% | Previous preeclampsia |
| 50% | Previous preeclampsia at 28 weeks |
| 15 to 25% | Chronic hypertension |
| 40% | Severe hypertension |
| 25% | Renal disease |
| 20% | Pregestational diabetes mellitus |
| 10 to 15% | Class B/C diabetes |
| 35% | Class F/R diabetes |
| 10 to 40% | Thrombophilia |
| 10 to 15% | Obesity/insulin resistance |
| 10 to 20% | Age >35 years |
| 10 to 15% | Family history of preeclampsia |
| 6 to 7% | Nulliparity/primipaternity |
Pregnancy-related risk factors
Many risk factors may be identified for the first time during pregnancy (TABLE 2). It is important to realize that the magnitude of risk depends on number of risk factors.
Nulliparity and primipaternity. Over the past decade, several epidemiologic studies suggested that immune maladaptation plays an important pathogenetic role in development of preeclampsia.
Generally, preeclampsia is considered a disease of first pregnancy. Indeed, a previous miscarriage of a previous normotensive pregnancy with the same partner is has a lowered frequency of preeclampsia. This protective effect is lost, however, with change of partner, suggesting that primipaternity increases the rate of preeclampsia.
A large prospective study on the relation between duration of sperm exposure with a partner and the rate of preeclampsia showed that women who conceive after a cohabitation period of 0 to 4 months have a 10-fold rate of preeclampsia, compared to those who conceive after a cohabitation period of at least 12 months. A similar study confirmed these findings.
The protective effects of long-term sperm exposure could explain the high frequency of preeclampsia in teenage pregnancy. (These women tend to have limited sperm exposure with a partner, or multiple partners). Thus, it is important to teach these women about their risks and the need for regular prenatal care.
Multifetal gestation increases the rate as well as the severity of preeclampsia, and the rate increases with the number of fetuses. Lowering the number of embryos transferred will substantially reduce the risk of preeclampsia and adverse outcomes.
There is no therapy to prevent preeclampsia in these women; however, we should acknowledge the increased risk and develop antenatal-care programs that allow close observation and early detection of preeclampsia in these women.
Hydropic degeneration of placenta. It is well-established that pregnancies complicated by fetal hydrops or hydropic degeneration of the placenta (with or without a coexisting fetus) are at very high risk for preeclampsia. In these cases, preeclampsia usually develops in the second trimester and is usually severe, and therefore causes substantial maternal and perinatal morbidities. Development of preeclampsia in such pregnancies requires immediate hospitalization and consideration for prompt delivery.
Unexplained elevated serum markers in the second trimester. Maternal serum screening with alpha fetoprotein (AFP), human chorionic gonadotropin (HCG) and inhibin A is commonly used to identify those at risk for aneuploidy or neural tube defects.
Unexplained elevations in AFP, HCG or inhibin A have been associated with increased adverse pregnancy outcome such as fetal death, intrauterine growth restriction (IUGR), preterm delivery, and preeclampsia. However, the data on the association between abnormalities in these biomarkers and preeclampsia have been inconsistent. Nevertheless, retrospective studies suggest that elevation in these serum markers during the second trimester increases the risk of preeclampsia by at least twofold. The risk is probably higher in those who have abnormalities in more than 1 of these markers. Since unexplained abnormalities of these serum markers may reflect early placental pathology, it is suggested that these pregnancies may benefit from close obstetric surveillance.
Serum and urinary markers of abnormal angiogenesis and subsequent preeclampsia were strongly associate, in newly published studies reported by Levine and colleagues. For example, circulating soluble fms-like tyrosine kinase (sFLt1) is elevated in pregnant women prior to onset of preeclampsia, whereas urinary placental growth factor is reduced several weeks prior to clinical onset of preeclampsia. Both of these markers appear to hold some promise.
Unexplained proteinuria or hematuria. Generally, proteinuria is considered a late manifestation of preeclampsia. However, recent retrospective studies suggest that some women with preeclampsia, particularly those with HELLP syndrome, might not have hypertension (>140 mm Hg systolic or >90 mm Hg diastolic). In some women, persistent proteinuria (3+ on dipstick) or >300 mg/24 hour may be the first sign of preeclampsia or could be a marker of silent renal disease.
No prospective studies have evaluated the risk of preeclampsia in asymptomatic women with persistent proteinuria. I suggest, however, that women with this finding will benefit from intensified obstetric surveillance (more frequent prenatal visits) and/or biochemical evaluation (platelet count, liver enzymes), particularly if they have headaches, visual changes, epigastric or right upper quadrant pain, nausea or vomiting, or respiratory symptoms (chest pain or shortness of breath)—likewise, for pregnant women with persistent hematuria of unknown origin.
Unexplained fetal growth restriction. Impaired trophoblast invasion is a key features of pregnancies complicated by preeclampsia or unexplained IUGR. Preeclampsia can manifest either as a maternal syndrome (hypertension and proteinuria with or without symptoms) or a fetal abnormal growth syndrome.
In clinical practice, most cases of unexplained IUGR are probably delivered before the maternal syndrome develops. In some cases, unexplained IUGR may be the first manifestation of preeclampsia, particularly those with IUGR before 34 weeks’ gestation. The absolute risk of clinical preeclampsia in such women is unknown because of lack of prospective data. Nevertheless, a woman with idiopathic IUGR prior to 34 weeks’ gestation whose pregnancy is managed expectantly is at increased risk for future preeclampsia. These women should receive intensive maternal surveillance for preeclampsia, and a diagnosis of preeclampsia should be considered in those who develop maternal symptoms or abnormal blood tests.
Abnormal uterine artery Doppler velocimetry at 18 to 24 weeks’ gestation. Several observational studies reported an association between elevated uterine artery resistance as measured by Doppler (with or without presence of a notch) in the second trimester and subsequent preeclampsia and/or IUGR. The reported rates of preeclampsia among women with abnormal Doppler results range from 6% to 40%. The risk varies depending on the site measured, gestational age at time of measurement, normal indices used, abnormality on repeat measurement, and population studied.
A systemic review of 27 studies, which included approximately 13,000 women, revealed that an abnormal uterine artery Doppler waveform increases the risk of preeclampsia by 4- to 6-fold, compared to normal Doppler results. The review concluded that uterine artery Doppler evaluation has a limited value as a screening test to predict preeclampsia.
What should the physician do when faced with an ultrasound report indicating an abnormal uterine artery Doppler finding?
Is low-dose aspirin helpful? Several randomized trials evaluated the potential role of low-dose aspirin in reducing the risk of preeclampsia in women with abnormal uterine artery Doppler indices. A meta-analysis suggested that low-dose aspirin significantly reduced the rate of preeclampsia (16% in placebo versus 10% with aspirin, odds ratio of 0.55). This analysis included a total of 498 subjects.
In contrast, a recent randomized trial in 560 women with abnormal uterine artery Doppler at 23 weeks’, who were assigned to aspirin 150 mg or placebo, found no differences in rates of preeclampsia (18% versus 19%) or in preeclampsia requiring delivery before 34 weeks’ (6% versus 8%). A similar randomized trial using 100 mg aspirin daily in 237 women with abnormal uterine artery Doppler at 22 to 24 weeks revealed no reduction in rate of preeclampsia compared to placebo.
Consequently, low-dose aspirin is not recommended for prevention of preeclamp-sia in these women.
Close surveillance is warranted. Although there is no available proven therapy to reduce the risk of preeclampsia in these women, they should be closely observed because of the increased rate of adverse outcomes, including preeclampsia.
TABLE 2
Pregnancy-related risk factors for preeclampsia
| Magnitude of risk depends on the number of factors | |
|---|---|
| 2-fold normal | Unexplained midtrimester elevations of serum AFP, HCG, inhibin-A |
| 10 to 30% | Abnormal uterine artery Doppler velocimetry |
| 0 to 30% | Hydrops/hydropic degeneration of placenta |
| 10 to 20% | Multifetal gestation (depends on number of fetuses and maternal age) |
| 10% | Partner who fathered preeclampsia in another woman |
| 8 to 10% | Gestational diabetes mellitus |
| 8 to 10% | Limited sperm exposure (teenage pregnancy) |
| 6 to 7% | Nulliparity/primipaternity |
| Limited data | Donor insemination, oocyte donation |
| Limited data | Unexplained persistent proteinuria or hematuria |
| Unknown | Unexplained fetal growth restriction |
Step 2Watch for signal findings, diagnose preeclampsia early
Signs and symptoms may call for close surveillance at any time. Early detection of preeclampsia is the best way to reduce adverse outcomes.
Prenatal care does not prevent preeclampsia, of course. All pregnant women are at risk, some more than others. Still, adequate and proper prenatal care is the best strategy to detect preeclampsia early.
We may need to modify the frequency and type of maternal and fetal surveillance at any time. Thus, patients with multiple risk factors or risk exceeding 10% should have more frequent visits, especially beyond 24 weeks. Maternal blood pressure (both systolic and diastolic), urine protein values, abrupt and excessive weight gain, maternal symptoms, and fetal growth warrant particular attention.
Diagnostic criteria vary with risk
The diagnosis of preeclampsia is different in patients with different risk factors. In healthy nulliparous women, the diagnosis requires persistent hypertension and proteinuria (new onset after 20 weeks’ gestation). However, in some patients the diagnosis should be made based on new onset hypertension and maternal symptoms or abnormal blood tests (low platelets or elevated liver enzymes).
Urine dipstick is a reliable screening test in women who remain normotensive.
24-hour urine measurement is the best test to confirm proteinuria if hypertension develops. Several studies found that urine dipstick values less than (1+) and random urine protein to creatinine ratio measurements are not accurate to predict proteinuria in women with gestational hypertension.
When is it gestational hypertension? The term applies only women with all of these findings:
- mild hypertension <160/<110 mm Hg
- proteinuria <300 mg/24-hour urine
- normal platelet count
- normal liver enzymes
- normal fetal growth
- no maternal symptoms
Once gestational hypertension is diagnosed, obtain blood tests and ultrasound evaluation to document fetal growth and amniotic fluid status.
Women with severe gestational hypertension and those with abnormal tests should be diagnosed as having preeclampsia and managed as such.
Women with gestational hypertension are at high risk for preeclampsia, and risk of progression depends on gestational age at time of diagnosis. Women who develop gestational hypertension at 24 to 35 weeks have a 46% chance of developing preeclampsia with a high rate of preterm delivery (32% <36 weeks and 12.5% <34 weeks) (FIGURE). These women require very close surveillance. In contrast, maternal and perinatal outcome is usually favorable when only mild gestational hypertension develops at or beyond 36 weeks.
When hypertension, proteinuria occur before 20 weeks
The traditional diagnostic criteria for preeclampsia in healthy women are not reliable in women who have either hypertension or proteinuria prior to 20 weeks’ gestation, particularly in those taking antihypertensive medications and in those who have class F diabetes mellitus. Because of the physiologic changes during pregnancy, women with diabetes and renal disease will have serial increases in blood pressure as well as protein excretion with advanced gestational age, particularly in the third trimester. Diagnostic criteria (TABLE 3) should be individualized based on medical conditions and current therapy. Antihypertensive drugs and preexisting proteinuria make it more difficult to classify preeclampsia as mild or severe.
TABLE 3
Diagnostic criteria
| GESTATIONAL HYPERTENSION IN HEALTHY WOMEN | |
| Blood pressure <160 mm Hg diastolic and <110 mm Hg systolic | |
| Proteinuria <300 mg/24-hour collection | |
| Platelet count >100,000/mm3 | |
| Normal liver enzymes | |
| No maternal symptoms | |
| No intrauterine growth restriction or oligohydraminos by ultrasound | |
| PREECLAMPSIA IN WOMEN WITH PREEXISTING MEDICAL CONDITIONS | |
| Condition | Criteria |
| Hypertension only | Proteinuria >500 mg/24-hours or thrombocytopenia |
| Proteinuria only | New onset hypertension plus symptoms or thrombocytopenia or elevated liver enzymes |
| Hypertension plus proteinuria (renal disease or class F diabetes) | Worsening severe hypertension and/or new onset of symptoms, thrombocytopenia, elevated liver enzymes |
FIGURE Whether preeclampsia will develop depends on when gestational hypertension begins
Adapted from Barton JR, et al. Am J Obstet Gynecol. 2001;184:979-983.
Step 3Consider how to balance risk to mother and fetus
Once a diagnosis is made, promptly evaluate mother and fetus, continue close surveillance, select those who will benefit from hospitalization, and identify indications for delivery (TABLE 4).
Delivery will always reduce the risks for the mother, but in certain situations, it might not be the best option for an extremely premature fetus. Sometimes delivery is best for both mother and fetus.
The best strategy takes into consideration:
- maternal and fetal status at initial evaluation,
- preexisting medical conditions that could affect pregnancy outcome,
- fetal gestational age at time of diagnosis,
- labor or rupture of fetal membranes (both could affect management), and
- maternal choice of available options.
Women who remain undelivered require close maternal and fetal evaluation. In otherwise healthy women, management depends on whether the preeclampsia is mild or severe, and, if there are other medical conditions, on the status of those conditions, as well.
TABLE 4
Indications for delivery
| Consider delivery in gravidas with 1 or more indications |
|---|
| Gestational age ≥38 weeks for mild disease |
| Gestational age ≥34 weeks for severe disease |
| 33-34 weeks with severe disease after steroids |
| Onset of labor and/or membrane rupture ≥34 weeks |
| Eclampsia or pulmonary edema (any gestational age) |
| HELLP syndrome (any gestational age) |
| Severe cerebral symptoms or epigastric pain |
| Acute renal insufficiency (serum creatinine >1.2 mg/dl) |
| Persistent thrombocytopenia (platelet count <100,000) |
| Maternal desire for delivery |
| Severe oligohydraminos or IUGR < 5th percentile |
| Nonreassuring fetal testing |
Chronic hypertension
- Underlies 30% of cases of hypertension during pregnancy.
- Begins before pregnancy or before 20 weeks’ gestation.
Gestational hypertension
- The most common form of hypertension during pregnancy.
- Acute onset beyond 20 weeks’ gestation in a woman known to be normotensive before pregnancy or prior to 20 weeks’ gestation.
Preeclampsia
- Can superimpose upon chronic hypertension, renal disease, or connective tissue disease, or develop in women with gestational hypertension.
- Preeclampsia in healthy nulliparous women: hypertension and proteinuria after 20 weeks’ gestation.
- Preeclampsia in women with preexisting chronic hypertension and absent proteinuria: an exacerbation of hypertension and new onset proteinuria.
Eclampsia
- Development of convulsions in women with hypertensive disorders of pregnancy.
“HELLP syndrome”
- Hemolysis,
- Elevated liver enzymes, and
- Low platelet count
Suspected or confirmed preeclampsia in a woman who has documented evidence of hemolysis (abnormal peripheral smear, or elevated bilirubin, or anemia, or low heptoglobin levels), plus elevated liver enzymes (AST or ALT), and thrombocytopenia (platelet count below 100,000).
The author reports no financial relationships relevant to this article.
BIBLIOGRAPHY
Barton JR, O’Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: Progression and outcome. Am J Obstet Gynecol. 2001;184:979-983.
Boggess KA, Lief S, Martha AP, Moos K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003;101:227-231.
Buchbinder A, Sibai BM, Caritis S, MacPherson C, Hauth J, Lindheimer MD. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66-71.
Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E. Low-dose aspirin to prevent preeclampsia in women at high risk. N Engl J Med. 1998;338:701-705.
Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219-224.
Chien PE, Arnott N, Gordon A, Owen P, Khan KS. How useful is uterine artery Doppler flow velocimetry in the prediction of pre-eclampsia, intrauterine growth retardation and perinatal death? An Overview. Br J Obstet Gynaecol. 2000;107:196-208.
Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for the prevention of preeclampsia in women with abnormal uterine artery Doppler: A meta-analysis. Obstet Gynecol. 2001;98:861-866.
Curet LB. Pregnancy outcomes in healthy nulliparous women who subsequently developed hypertension. Obstet Gynecol. 2000;95:24-28.
Dekker G, Robillard PY. The birth interval hypothesis - Does it really indicate the end of the primipaternity hypothesis? J Reprod Immunol. 2003;59:245-251.
Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet. 2001;357:209-215.
Durnwald C, Mercer B. A prospective comparison of total protein/creatinine ratio versus 24-hour urine protein in women with suspected preeclampsia. Am J Obstet Gynecol. 2003;189:848-52.
Einarsson JI, Sangi-Haghpeykar H, Gardner NO. Sperm exposure and development of preeclampsia. Am J Obstet Gynecol. 2003;188:1241-1243.
Hauth JC, Ewell MG, Levine RL, Esterlitz JR, Sibai BM, Curet LB. Pregnancy outcomes in healthy nulliparous women who subsequently developed hypertension. Obstet Gynecol. 2000;95:24-28.
Hnat MD, Sibai BM, Caritis S, Hiouth J, Lindheimer MD, MacPherson C. Perinatal outcome in women with recurrent preeclampsia compared with women who develop preeclampsia as nulliparous. Am J Obstet Gynecol. 2002;186:422-426.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111-166.
Levine RG, Thadhani R, Qian C, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77-85.
Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
Nilsson E, Salonen Ros H, Cnattingius S, Lichtenstein P. The importance of genetic and environmental effects for preeclampsia and gestational hypertension: a family study. Br J Obstet Gynaecol. 2004;111:200-206.
O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368-374.
Ragip A Al, Baykal C, Karacay O, Geyik PO, Altun S, Dolen I. Random urine protein-creatinine ratio to predict proteinuria in new-onset mild hypertension in late pregnancy. Obstet Gynecol. 2004;104:367-371.
Saftlas AF, Levine RJ, Klebanoff MA, Martz KL, Ewell MG, Morris CD, Sibai BM. Abortion, changed paternity, and risk of preeclampsia in nulliparous women. Am J Epidemiol. 2003;157:1108-1114.
Sibai BM, Caritis S, Hauth J, Lilndheimer MD, MacPherson C, Klebanoff M, et al. Hypertensive disorders in twin versus singleton gestations. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 2000;182:938-942.
Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-33377.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
Subtil D, Goeusse P, Houfflin-Debarge V, Puech F, Lequien P, Breart G, Uzan S, Quandalle F, Delcourt YM, Malek YM. Essai Regional Aspirine Mere-Enfant (ERASME) Collaborative Group. Randomised comparison of uterine artery Doppler and aspirin (100 mg) with placebo in nulliparous women: the Essai Regional Aspirine Mere-Enfant study (Part 2). Br J Obstet Gynaecol. 2003;110:485-491.
Wen SW, Demissie K, Yang Q, Walker MC. Maternal morbidity and obstetric complications in triplet pregnancies and quadruplet and higher-order multiple pregnancies. Am J Obstet Gynecol. 2004;191:254-258.
Yu CKH, Papageorghiou AT, Parra M, Dias RP, Nicolaides KH. Randomized controlled trial using low-dose aspirin in the prevention of pre-eclampsia in women with abnormal uterine artery Doppler at 23 weeks’ gestation. Ultrasound Obstet Gynecol. 2003;22:233-239.
BIBLIOGRAPHY
Barton JR, O’Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: Progression and outcome. Am J Obstet Gynecol. 2001;184:979-983.
Boggess KA, Lief S, Martha AP, Moos K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003;101:227-231.
Buchbinder A, Sibai BM, Caritis S, MacPherson C, Hauth J, Lindheimer MD. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66-71.
Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E. Low-dose aspirin to prevent preeclampsia in women at high risk. N Engl J Med. 1998;338:701-705.
Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219-224.
Chien PE, Arnott N, Gordon A, Owen P, Khan KS. How useful is uterine artery Doppler flow velocimetry in the prediction of pre-eclampsia, intrauterine growth retardation and perinatal death? An Overview. Br J Obstet Gynaecol. 2000;107:196-208.
Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for the prevention of preeclampsia in women with abnormal uterine artery Doppler: A meta-analysis. Obstet Gynecol. 2001;98:861-866.
Curet LB. Pregnancy outcomes in healthy nulliparous women who subsequently developed hypertension. Obstet Gynecol. 2000;95:24-28.
Dekker G, Robillard PY. The birth interval hypothesis - Does it really indicate the end of the primipaternity hypothesis? J Reprod Immunol. 2003;59:245-251.
Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet. 2001;357:209-215.
Durnwald C, Mercer B. A prospective comparison of total protein/creatinine ratio versus 24-hour urine protein in women with suspected preeclampsia. Am J Obstet Gynecol. 2003;189:848-52.
Einarsson JI, Sangi-Haghpeykar H, Gardner NO. Sperm exposure and development of preeclampsia. Am J Obstet Gynecol. 2003;188:1241-1243.
Hauth JC, Ewell MG, Levine RL, Esterlitz JR, Sibai BM, Curet LB. Pregnancy outcomes in healthy nulliparous women who subsequently developed hypertension. Obstet Gynecol. 2000;95:24-28.
Hnat MD, Sibai BM, Caritis S, Hiouth J, Lindheimer MD, MacPherson C. Perinatal outcome in women with recurrent preeclampsia compared with women who develop preeclampsia as nulliparous. Am J Obstet Gynecol. 2002;186:422-426.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111-166.
Levine RG, Thadhani R, Qian C, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77-85.
Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
Nilsson E, Salonen Ros H, Cnattingius S, Lichtenstein P. The importance of genetic and environmental effects for preeclampsia and gestational hypertension: a family study. Br J Obstet Gynaecol. 2004;111:200-206.
O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368-374.
Ragip A Al, Baykal C, Karacay O, Geyik PO, Altun S, Dolen I. Random urine protein-creatinine ratio to predict proteinuria in new-onset mild hypertension in late pregnancy. Obstet Gynecol. 2004;104:367-371.
Saftlas AF, Levine RJ, Klebanoff MA, Martz KL, Ewell MG, Morris CD, Sibai BM. Abortion, changed paternity, and risk of preeclampsia in nulliparous women. Am J Epidemiol. 2003;157:1108-1114.
Sibai BM, Caritis S, Hauth J, Lilndheimer MD, MacPherson C, Klebanoff M, et al. Hypertensive disorders in twin versus singleton gestations. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 2000;182:938-942.
Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-33377.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
Subtil D, Goeusse P, Houfflin-Debarge V, Puech F, Lequien P, Breart G, Uzan S, Quandalle F, Delcourt YM, Malek YM. Essai Regional Aspirine Mere-Enfant (ERASME) Collaborative Group. Randomised comparison of uterine artery Doppler and aspirin (100 mg) with placebo in nulliparous women: the Essai Regional Aspirine Mere-Enfant study (Part 2). Br J Obstet Gynaecol. 2003;110:485-491.
Wen SW, Demissie K, Yang Q, Walker MC. Maternal morbidity and obstetric complications in triplet pregnancies and quadruplet and higher-order multiple pregnancies. Am J Obstet Gynecol. 2004;191:254-258.
Yu CKH, Papageorghiou AT, Parra M, Dias RP, Nicolaides KH. Randomized controlled trial using low-dose aspirin in the prevention of pre-eclampsia in women with abnormal uterine artery Doppler at 23 weeks’ gestation. Ultrasound Obstet Gynecol. 2003;22:233-239.
Hypertension in pregnancy: Tailoring treatment to risk
- The treatment goal is to reduce blood pressure to a safe level to prevent maternal cerebral complications.This goal must be weighed against the risks of fetal exposure to antihypertensive drugs and the effects on uteroplacental blood flow.
- Gravidas with uncomplicated mild hypertension are at low risk; however, those with severe hypertension or associated complicating factors are at high risk of complications and adverse outcomes.
- Antihypertensive medications should not be used routinely in low-risk patients.
- Women with high-risk chronic hypertension are at risk for postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure.
The decision to use antihypertensive drug therapy in pregnant women is a tricky one—especially considering the ever-evolving nature of treatment. For instance, we now know that in some hypertensive gravidas, medical interventions may actually be deleterious.
With the aging of the obstetric population in the United States, hypertension in pregnancy—which currently affects 7% of gestations—will remain a major issue in preconception and prenatal care. Its reported risks, which include stroke, pulmonary edema, and death, underscore the importance of careful management (TABLE 1).
This article describes the indications for antihypertensive therapy in pregnancy, focusing on 2 basic categories—high-risk and lowrisk patients—and offers guidance in choosing the optimal agent for each patient.
TABLE 1
Maternal risks of severe hypertension in pregnancy
| Stroke |
| Cerebral hemorrhage |
| Hypertensive encephalopathy |
| Congestive heart failure/pulmonary edema |
| Acute renal dysfunction/acute renal failure |
| Abruptio placentae |
| Disseminated intravascular coagulopathy |
| Death |
Correct classification helps direct management
First, identify chronic hypertension. Chronic hypertension is defined as an elevation in blood pressure (BP) that exists prior to pregnancy. Unfortunately, because the pregestational BP is not always known, the diagnosis in many cases must be made on the basis of specific levels: systolic BP of at least 140 mm Hg or diastolic BP of at least 90 mm Hg on at least 2 occasions at least 4 hours apart prior to 20 weeks’ gestation.1
Even with these guidelines, however, diagnosis may be difficult, since early manifestations of preeclampsia can include hypertension prior to 20 weeks’ gestation.2,3 In addition, the physiologic decrease in BP during the first and second trimesters—seen in many patients with chronic hypertension—may obscure the condition early in gestation and lead to the erroneous diagnosis of gestational hypertension or preeclampsia later in pregnancy.4-6
Once a diagnosis of chronic hypertension is made, an accurate classification of the disease will help guide management and initiation of antihypertensive medication.
Mild versus severe hypertension. In pregnancy, chronic hypertension is classified as mild or severe. Mild hypertension has traditionally been defined as systolic BP less than 160 mm Hg and diastolic blood pressure less than 110 mm Hg.1,7 However, the American College of Obstetricians and Gynecologists recently changed its definition of mild hypertension to systolic BP less than 180 mm Hg.8,9 Most women with chronic hypertension in pregnancy have the mild form of the disease.
Low-risk hypertension. Patients with uncomplicated chronic mild hypertension are at low risk.
High-risk hypertension. Patients at high risk have either chronic severe hypertension or chronic mild hypertension in association with any of the complicating factors listed in TABLE 2.
History and laboratory studies. To properly classify the disease when first evaluating a patient with chronic hypertension, a thorough history is essential. Ask about related medical illnesses as well as target organ damage. Pay special attention to cardiac, renal, thyroid, and cerebrovascular disease, as well as diabetes. The outcomes of prior pregnancies also are important, especially complications such as abruptio placentae, preeclampsia, preterm delivery, growth restriction, fetal death, and neonatal complications.
Overall, regardless of the treatment, perinatal mortality is not improved with antihypertensive medications for mild hypertension.
Finally, laboratory evaluation should include urine analysis, urine culture, 24-hour urine protein, electrolytes, complete blood count, and glucose tolerance testing.
Other key examinations. In women with long-standing disease, ophthalmologic evaluation, electrocardiography, echocardiography, and assessment of creatinine clearance may be indicated.
TABLE 2
Criteria for low- versus high-risk chronic hypertension
| LOW-RISK CHRONIC HYPERTENSION |
| Chronic mild hypertension (systolic blood pressure 140–160 mm Hg and diastolic blood pressure 90–110 mm Hg) in the absence of complicating factors |
| HIGH-RISK CHRONIC HYPERTENSION |
| Either: |
| Chronic severe hypertension (systolic blood pressure ≥180 mm Hg and diastolic blood pressure ≥110 mm Hg) |
| or |
Chronic mild hypertension (systolic blood pressure 140–160 mm Hg and diastolic blood pressure 90–110 mm Hg) in association with anyof the following:
|
Objective of treatment: Prevent complications
In the nonpregnant state, the aim of hypertensive management is to prevent longterm vascular complications such as stroke and cardiovascular disease.10 A reasonable treatment goal for patients with mild to moderate hypertension may be benefits that are apparent after 5 years of therapy10—an acceptable time frame due to the long-term nature of the disease.
In pregnant women, however, the duration of the condition (pregnant and hypertensive) is finite and relatively short; as a result, maternal health benefits may not be clear. For that reason, the objective is to reduce BP to a safe level to prevent maternal cerebral complications (systolic BP below 160 mm Hg and diastolic BP below 110 mm Hg).Of course, these short-term maternal benefits must be weighed against the potential risks of fetal exposure (TABLE 3).11,12
TABLE 3
Rates of adverse pregnancy outcomes among patients with mild and severe chronic hypertension
| OUTCOME | MILD HYPERTENSION (%) | SEVERE HYPERTENSION (%) |
|---|---|---|
| Preeclampsia | 10-25 | 25-50 |
| Abruptio placentae | 0.7-1.5 | 2-5 |
| Fetal growth restriction | 8.0-15.5 | 10-20 |
| Preterm birth | 12-34.4 | 25-30 |
Low-risk disease: Avoid routine antihypertensive therapy
A limited number of randomized trials have studied the effectiveness of antihypertensive treatment in preventing adverse maternal outcomes such as superimposed preeclampsia and abruptio placentae. Here are 2 key findings:
- No demonstrable maternal benefit. Overall, there appears to be no clear benefit of antihypertensive treatment in women with mild hypertension. Indeed, the 2 largest studies had contradictory findings regarding preeclampsia,6,13 and there was no demonstrable benefit in regard to abruptio placentae.
- Antihypertensive drugs may adversely affect fetal growth. A recent meta-analysis examining antihypertensive medications in patients with mild to moderate hypertension investigated the relationship between a fall in mean arterial blood pressure and the delivery of small-for-gestational-age (SGA) infants.14 The authors concluded that antihypertensive medications induce BP drops that may adversely affect fetal growth (see FIGURE). Prior to this observation, prospective studies had shown no association between antihypertensive medications and SGA infants. (The only exception was atenolol; 3 separate studies found a relationship between treatment with atenolol and low birth weight.15-17)
Overall, maternal and perinatal data indicate that, regardless of the treatment, perinatal mortality is not improved with antihypertensive medications for mild hypertension. In fact, the indiscriminate use of such medications may have deleterious effects. Consequently, antihypertensive medications should not be used routinely in low-risk patients.18,19
Clinical care. When a woman with low-risk hypertension presents for prenatal care, it is our policy to discontinue antihypertensive medications at the first prenatal visit. Although many women will not require antihypertensive treatment during the pregnancy, careful management remains essential, as such patients can become high-risk at any time. Therapy should be initiated if her condition changes to severe hypertension (systolic BP of 180 mm Hg or more, or a diastolic blood pressure of 110 mm Hg or more).8
Low-risk women should be monitored closely for evidence of preeclampsia and fetal growth restriction. Thus, they should have a baseline ultrasound at 16 to 20 weeks’ gestation, with serial monthly ultrasounds beginning at 30 to 32 weeks to follow fetal growth. Nonstress testing or biophysical profiles are indicated in the presence of severe hypertension, preeclampsia, or abnormal fetal growth.
Patients with uncomplicated lowrisk hypertension may continue pregnancy until 40 weeks’ gestation. However, beyond 37 weeks, the presence of complications such as severe hypertension, documented growth restriction, and superimposed preeclampsia are indications for hospitalization and delivery.
FIGURE Placental effects of hypertension
In hypertensive gravidas, placental blood flow is reduced—particularly in cases of preeclampsia. Antihypertensive therapy in low-risk women may induce blood pressure drops that further compromise fetal growth.
High-risk disease: Initiate medical therapy
Randomized, controlled trials do not exist for gravidas with high-risk hypertension—that is, women with severe hypertension or complicating factors—due to concerns about the potential adverse consequences of uncontrolled disease, such as cerebrovascular accident, congestive heart failure, and renal failure.20 It is interesting to note, however, that although controlling hypertension in such patients may help prolong pregnancy, there is no evidence that it reduces the rates of preeclampsia or abruptio placentae.20,21
Labetalol provides the added benefit of alpha-adrenergic blockade, which offers the theoretical advantage of vasodilation.
When to start treatment. For women with high-risk hypertension, hospitalization at the time of the first prenatal visit facilitates complete cardiovascular and renal evaluation, and is therefore often beneficial. If a woman has target organ damage, treatment should be initiated at a systolic BP of 140 mm Hg or a diastolicBP of 90 mm Hg. Indeed, many such women are already receiving treatment for their hypertension, in which case antihypertensive medications should be continued, though physicians should consider altering the regimen to optimize fetal safety.
Choosing the best agent. Before choosing an antihypertensive drug, review the patient’s history. If her disease was well controlled on a particular medication, that agent is probably a reasonable first choice, provided there is adequate published literature establishing the safety of her medication during pregnancy. Obviously, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II antagonists, and atenolol should be avoided because of the potential adverse effects on the fetus, including renal failure.The most commonly used medications for control of hypertension during pregnancy are listed in TABLE 4.
- Labetalol. We consider labetalol a first-line agent for controlling hypertension in pregnancy. This beta-blocking drug provides the added benefit of alpha-adrenergic blockade, which offers the theoretical advantage of vasodilation—not seen with traditional beta-blockers. Overall, labetalol has an excellent record of safety in pregnancy.
In a randomized, controlled trial involving 86 mildly hypertensive patients who initiated labetalol therapy between 6 and 13 weeks’ gestation, no major congenital malformations were identified.6 Although there have been reports of an increased risk for SGA infants in patients treated with labetalol for mild pregnancy-induced hypertension during the second and third trimesters, this association has not been documented in women with chronic hypertension.6
- Thiazide diuretics. If labetalol fails to control blood pressure, we typically add either the calcium-channel blocker nifedipine or a thiazide diuretic. Use of the latter has been well documented in pregnancy. Indeed, thiazide diuretics can be given in the first trimester and throughout gestation without associated risks of major fetal malformations or adverse fetal-neonatal complications.
- Calcium-channel blockers. Calcium-channel–blocking agents also have an excellent safety profile in pregnancy. They have been studied both as antihypertensive medications (primarily in the second and third trimesters) and as tocolytic agents. In a prospective, multicenter, cohort study in which 78 women were exposed to calcium-channel blockers (mainly nifedipine and verapamil) during the first trimester, there was no increase in the rate of birth defects.22
A separate prospective, randomized trial evaluated the benefit of nifedipine in pregnancy. A total of 283 women—47% of whom had chronic hypertension—were enrolled between 12 and 34 weeks’ gestation (mean: 24 weeks). Researchers found patients on nifedipine therapy experienced no improvement in maternal or neonatal outcomes compared to subjects assigned to no treatment.23 Follow-up at 18 months of 94 of the infants exposed to nifedipine in utero showed no adverse effects on development.24
- Methyldopa. For many obstetricians, methyldopa remains a first-line agent for the treatment of chronic hypertension in pregnancy.1 It has a well-documented safety record in both short-term25 and long-term follow-up of children exposed in utero.26 Indeed, many studies have evaluated use of this medication to manage mild to moderate hypertension, with no evidence of adverse maternal or fetal outcome. However, it is now rarely used in the nonpregnant population, and the safety of other medications, such as labetalol and nifedipine, has prompted us to stop giving it.
- Other considerations. Finally, when choosing an antihypertensive drug, the physician must consider the benefits and response of specific agents in particular risk groups (TABLE 5).
In women with diabetes, calcium-channel blockers have a reno-protective effect and are our first-line agent in pregnancy, since ACE inhibitors, which also offer this benefit, must be avoided beyond 16 weeks’ gestation because of the potential adverse fetal effects.
In women with diabetes, calcium-channel blockers have a reno-protective effect and are our first-line agent in pregnancy.
Young African-American women frequently have low-renin, salt-sensitive hypertension, and therefore thiazide diuretics or nifedipine may be better first-line agents in this population.
TABLE 4
Agents for treating chronic hypertension in pregnancy
| DRUG | STARTING DOSE | MAXIMUM DAILY DOSE | COMMON SIDE EFFECTS |
|---|---|---|---|
| Labetalol | 100 mg every 8 h | 1,200–2,400 mg | Headache Tremulousness |
| Thiazide diuretic | 12.5 mg twice daily | 50 mg | Hypokalemia |
| Nifedipine | Hypotension | ||
| Short acting | 10 mg every 8 h | 120 mg | Headache |
| Long acting | 30 mg/d | 240 mg | Tachycardia |
| Alpha methyldopa | 250 mg twice daily | 4 g | Thirst Drowsiness Elevation of liver enzymes |
TABLE 5
Medical factors guiding selection of antihypertensive medication
| If the patient has… | It’s generally best to start with… |
|---|---|
| Diabetes | Calcium-channel blocker |
| Vascular disease | |
| Salt-wasting hypertension* | Thiazide diuretic |
| Left ventricular systolic dysfunction | |
| Mitral stenosis | |
| *Mostly African-American women | |
Severe gestational hypertension and preeclampsia
Women who develop severe gestational hypertension (systolic BP of 160 mm Hg or more or diastolic BP of 110 mm Hg or more) and/or preeclampsia require antihypertensive treatment during management remote from term. In this case, the aim of antihypertensive drug treatment is to keep systolic BP between 150 and 159 mm Hg and diastolic BP between 100 and 109 mm Hg in order to not compromise uteroplacental blood flow.
Because nitroprusside is both a vasodilator and a venodilator, it is an ideal agent for gravidas with hypertensive encephalopathy.
The drugs to use are oral labetalol and/or oral nifedipine. If maternal BP is not adequately controlled with maximum doses of labetalol plus nifedipine, the patient should be delivered.
Severe hypertension and encephalopathy
Hypertensive encephalopathy is a medical emergency. This rare complication of hypertension in pregnancy27 is marked by severely elevated BP, with the diastolic level frequently exceeding 130 mm Hg. Associated findings include headache, visual disturbances, nausea, vomiting, seizures, confusion, stupor, and coma. Also possible are retinal hemorrhage, exudates, papilledema, and evidence of renal or cardiac disease. Transient focal neurologic findings may be present as well, but more often suggest vascular disease, hemorrhage, embolism, or thrombosis.
Pathophysiology. In hypertensive encephalopathy, loss of autoregulation leads to generalized cerebral vasodilation. Under normal conditions, when the mean arterial pressure is between 60 and 130 mm Hg, patients maintain constant cerebral blood flow. In hypertensive patients, however, autoregulation occurs between mean arterial pressures of 110 and 180 mm Hg as a result of arteriolar thickening. When BP exceeds the ability of the vessels to autoregulate, blood flow hyperperfuses the brain, causing fluid to leak into the perivascular tissue and resulting in vasogenic cerebral edema. Altered vascular reactivity to normally circulating pressor agents, deficient levels of vasodilating prostaglandins, endothelial dysfunction, and activation of the coagulation cascade may further exacerbate this condition.28
Treatment options. Clinically, it may be impossible to differentiate hypertensive encephalopathy from eclampsia, and magnesium sulfate should be considered for seizure prophylaxis. The most frequently used antihypertensive medications for this syndrome are shown in TABLE 6. Nitroprusside lowers BP most predictably, but because of the associated risks of fetal cyanide toxicity, other medications may be more desirable first-line agents in the pregnant woman.
Importantly, because sudden drops in BP may impair cerebral perfusion, we recommend that the mean arterial pressure be lowered no more than 25% from baseline (TABLE 7). If pulmonary edema develops, oxygen and furosemide should be administered, and consultation with subspecialists considered. (We suggest such consultation for cases of renal dysfunction and cerebral complications, as well.) Because nitroprusside is both a vasodilator and a venodilator, it is an ideal agent in this situation.
TABLE 6
Medications for treating acute severe hypertension
| DRUG | DOSE | ONSET OF ACTION | DURATION OF ACTION | SIDE EFFECTS |
|---|---|---|---|---|
| Hydralazine | 5-10 mg IV every 20 min | 10-20 min | 3-6 h | Tachycardia Headache Flushing Angina |
| Labetalol | 20-80 mg IV every 10 min | 5-10 min | 3-6 h | Scalp tingling Vomiting Heart block |
| Sodium nitroprusside* | 0.25-5 mcg/kg/min | Immediate | 1-2 min | Nausea Vomiting Muscle twitching Thiocyanate and cyanide intoxication |
| Nicardipine* | 5-15 mg/h IV | 5-10 min | 1-4 h | Tachycardia Headache Phlebitis |
| *Drugs to use in the presence of hypertensive encephalopathy. | ||||
TABLE 7
Principles of management for severe hypertension and encephalopathy
Acute severe hypertension
|
Hypertensive encephalopathy
|
| *Lower mean arterial pressure no more than 25% from baseline. |
Postpartum management
Monitor BP for at least 48 hours. Women with high-risk chronic hypertension are more likely to suffer postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure than normotensive patients.8 This risk is even higher when these women also have target organ involvement, superimposed preeclampsia, abruptio placentae, morbid obesity, or longstanding hypertension.
In these patients, BP must be closely monitored and controlled for at least 48 hours after delivery. Intravenous labetalol or hydralazine can be administered for acute elevations of BP29; diuretics should also be used for women with circulatory congestion and pulmonary edema.30
Methyldopa remains the first-line agent for breastfeeding patients without compelling indications for another drug.
Oral antihypertensive therapy may be needed to maintain BP control. In choosing the appropriate agent, it is important to consider whether factors compel the choice of one medication over another. For example, for patients with a history of myocardial infarction, beta blockers and ACE inhibitors are excellent choices to decrease mortality.31 In patients with diabetes mellitus, as mentioned earlier, ACE inhibitors offer a renoprotective effect.10
Consider drug concentrations in breast milk. Another significant consideration in the postpartum period is whether the mother wishes to breastfeed her infant. All antihypertensive medications are found in breast milk to varying degrees,32 and the long-term effects of these medications on breastfeeding infants has not been specifically studied.
Because concentrations of methyldopa in milk are low and considered safe, it remains the first-line agent for patients without compelling indications for another antihypertensive drug. Concentrations of labetalol and propranolol also are low in breast milk; therefore, these may be better choices than atenolol and metoprolol, which are more highly concentrated in breast milk.12
Although diuretic agents have low concentrations in breast milk, they may decrease milk production.32
Little information exists regarding the excretion of calcium-channel blockers in breast milk, but no untoward effects are apparent.33 ACE inhibitors and angiotensin II receptor antagonists should be avoided because of potential deleterious effects on neonatal renal function, even though their concentrations in breast milk appear to be low. If ACE inhibitors are indicated for the breastfeeding mother, current data suggest that captopril and enalapril are safe.34
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. National High Blood Pressure Education Program Working Group. National High Blood Pressure Education Program Working Group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
2. Sibai BM, Akl S, Fairlie F, Moretti M. A protocol for managing severe preeclampsia in the second trimester. Am J Obstet Gynecol. 1990;163:733-738.
3. Hermida RC, Ayala DA, Mojon A, et al. Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension. 2000;36:149-158.
4. Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571-576.
5. Benedetto C, Zonca M, Marozio L, Dolci C, Carandente F, Massobrio M. Blood pressure patterns in normal pregnancy and in pregnancy-induced hypertension, preeclampsia, and chronic hypertension. Obstet Gynecol. 1996;88:503-510.
6. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-966.
7. Sibai BM. Diagnosis and management of chronic hypertension in pregnancy. Obstet Gynecol. 1991;78:451-461.
8. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #29: Chronic hypertension in pregnancy. Washington, DC: ACOG; 2001.
9. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
10. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413-2446.
11. Ferrer RL, Sibai BM, Murlow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol. 2000;96:849-860.
12. Umans JG, Lindheimer MD. Antihypertensive treatment. In: Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Norwalk, Conn: Appleton and Lange; 1998;581-604.
13. Steyn DW, Odendaal HJ. Randomized controlled trial of ketanserin and aspirin in prevention of pre-eclampsia. Lancet. 1997;350:1267-1271.
14. von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet. 2000;355:87-92.
15. Lip GY, Beevers M, Churchill D, Shaffer LM, Beevers DG. Effect of atenolol on birth weight. Am J Cardiol. 1997;79:1436-1438.
16. Easterling TR, Brateng D, Schmucker B, Brown Z, Millard SP. Prevention of preeclampsia: a randomized trial of atenolol in hyperdynamic patients before onset of hypertension. Obstet Gynecol. 1999;93:725-733.
17. Lydakis C, Lip GY, Beevers M, Beevers G. Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens. 1999;12:541-547.
18. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335:257-265.
19. Magee LA, Ornstein MP, von Dadelszen P. Management of hypertension in pregnancy. BMJ. 1999;318:1332-1336.
20. Sibai BM, Anderson GD. Pregnancy outcome of intensive therapy in severe hypertension in first trimester. Obstet Gynecol. 1986;67:517-522.
21. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123-129.
22. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174:823-828.
23. Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. Br J Obstet Gynaecol. 1998;105:718-722.
24. Bortolus R, Ricci E, Chatenoud L, Parazzini F. Nifedipine administered in pregnancy: effect on the development of children at 18 months. Br J Obstet Gynaecol. 2000;107:792-794.
25. Montan S, Anandakumar C, Arulkumaran S, Ingemarsson I, Ratnam SS. Effects of methyldopa on uteroplacental and fetal hemodynamics in pregnancy-induced hypertension. Am J Obstet Gynecol. 1993;168:152-156.
26. Cockburn J, Moar VA, Ounsted M, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet. 1982;1:647-649.
27. Witlin AG, Friedman SA, Egerman RS, et al. Cerebrovascular disorders complicating pregnancy—beyond eclampsia. Am J Obstet Gynecol. 1997;176:1139-1148.
28. Cotton DB, Janusz CA, Berman RF. Anticonvulsant effects of magnesium sulfate on hippocampal seizures: therapeutic implication in preeclampsia-eclampsia. Am J Obstet Gynecol. 1992;166:1127-1136.
29. Mabie WC, Gonzalez AR, Sibai BM, Amon E. A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Obstet Gynecol. 1987;70:328-333.
30. Mabie WC, Ratts TE, Ramanathan KB, Sibai BM. Circulatory congestion in obese hypertensive women: a subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553-558.
31. Pfeffer MA, Braunwald E, Moye LA. for the SAVE investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement Trials. N Engl J Med. 1992;327:669-677.
32. White WB. Management of hypertension during lactation. Hypertension. 1984;6:297-300.
33. Briggs GG, Freeman RK, Yaffee SJ. Drugs in Pregnancy and Lactation: a Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore, Md: Williams and Wilkins; 1998.
34. Committee on Drugs, American Academy of Pediatrics. The transfer of drugs and other chemicals into human milk. Pediatrics. 1994;93:137-150.
- The treatment goal is to reduce blood pressure to a safe level to prevent maternal cerebral complications.This goal must be weighed against the risks of fetal exposure to antihypertensive drugs and the effects on uteroplacental blood flow.
- Gravidas with uncomplicated mild hypertension are at low risk; however, those with severe hypertension or associated complicating factors are at high risk of complications and adverse outcomes.
- Antihypertensive medications should not be used routinely in low-risk patients.
- Women with high-risk chronic hypertension are at risk for postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure.
The decision to use antihypertensive drug therapy in pregnant women is a tricky one—especially considering the ever-evolving nature of treatment. For instance, we now know that in some hypertensive gravidas, medical interventions may actually be deleterious.
With the aging of the obstetric population in the United States, hypertension in pregnancy—which currently affects 7% of gestations—will remain a major issue in preconception and prenatal care. Its reported risks, which include stroke, pulmonary edema, and death, underscore the importance of careful management (TABLE 1).
This article describes the indications for antihypertensive therapy in pregnancy, focusing on 2 basic categories—high-risk and lowrisk patients—and offers guidance in choosing the optimal agent for each patient.
TABLE 1
Maternal risks of severe hypertension in pregnancy
| Stroke |
| Cerebral hemorrhage |
| Hypertensive encephalopathy |
| Congestive heart failure/pulmonary edema |
| Acute renal dysfunction/acute renal failure |
| Abruptio placentae |
| Disseminated intravascular coagulopathy |
| Death |
Correct classification helps direct management
First, identify chronic hypertension. Chronic hypertension is defined as an elevation in blood pressure (BP) that exists prior to pregnancy. Unfortunately, because the pregestational BP is not always known, the diagnosis in many cases must be made on the basis of specific levels: systolic BP of at least 140 mm Hg or diastolic BP of at least 90 mm Hg on at least 2 occasions at least 4 hours apart prior to 20 weeks’ gestation.1
Even with these guidelines, however, diagnosis may be difficult, since early manifestations of preeclampsia can include hypertension prior to 20 weeks’ gestation.2,3 In addition, the physiologic decrease in BP during the first and second trimesters—seen in many patients with chronic hypertension—may obscure the condition early in gestation and lead to the erroneous diagnosis of gestational hypertension or preeclampsia later in pregnancy.4-6
Once a diagnosis of chronic hypertension is made, an accurate classification of the disease will help guide management and initiation of antihypertensive medication.
Mild versus severe hypertension. In pregnancy, chronic hypertension is classified as mild or severe. Mild hypertension has traditionally been defined as systolic BP less than 160 mm Hg and diastolic blood pressure less than 110 mm Hg.1,7 However, the American College of Obstetricians and Gynecologists recently changed its definition of mild hypertension to systolic BP less than 180 mm Hg.8,9 Most women with chronic hypertension in pregnancy have the mild form of the disease.
Low-risk hypertension. Patients with uncomplicated chronic mild hypertension are at low risk.
High-risk hypertension. Patients at high risk have either chronic severe hypertension or chronic mild hypertension in association with any of the complicating factors listed in TABLE 2.
History and laboratory studies. To properly classify the disease when first evaluating a patient with chronic hypertension, a thorough history is essential. Ask about related medical illnesses as well as target organ damage. Pay special attention to cardiac, renal, thyroid, and cerebrovascular disease, as well as diabetes. The outcomes of prior pregnancies also are important, especially complications such as abruptio placentae, preeclampsia, preterm delivery, growth restriction, fetal death, and neonatal complications.
Overall, regardless of the treatment, perinatal mortality is not improved with antihypertensive medications for mild hypertension.
Finally, laboratory evaluation should include urine analysis, urine culture, 24-hour urine protein, electrolytes, complete blood count, and glucose tolerance testing.
Other key examinations. In women with long-standing disease, ophthalmologic evaluation, electrocardiography, echocardiography, and assessment of creatinine clearance may be indicated.
TABLE 2
Criteria for low- versus high-risk chronic hypertension
| LOW-RISK CHRONIC HYPERTENSION |
| Chronic mild hypertension (systolic blood pressure 140–160 mm Hg and diastolic blood pressure 90–110 mm Hg) in the absence of complicating factors |
| HIGH-RISK CHRONIC HYPERTENSION |
| Either: |
| Chronic severe hypertension (systolic blood pressure ≥180 mm Hg and diastolic blood pressure ≥110 mm Hg) |
| or |
Chronic mild hypertension (systolic blood pressure 140–160 mm Hg and diastolic blood pressure 90–110 mm Hg) in association with anyof the following:
|
Objective of treatment: Prevent complications
In the nonpregnant state, the aim of hypertensive management is to prevent longterm vascular complications such as stroke and cardiovascular disease.10 A reasonable treatment goal for patients with mild to moderate hypertension may be benefits that are apparent after 5 years of therapy10—an acceptable time frame due to the long-term nature of the disease.
In pregnant women, however, the duration of the condition (pregnant and hypertensive) is finite and relatively short; as a result, maternal health benefits may not be clear. For that reason, the objective is to reduce BP to a safe level to prevent maternal cerebral complications (systolic BP below 160 mm Hg and diastolic BP below 110 mm Hg).Of course, these short-term maternal benefits must be weighed against the potential risks of fetal exposure (TABLE 3).11,12
TABLE 3
Rates of adverse pregnancy outcomes among patients with mild and severe chronic hypertension
| OUTCOME | MILD HYPERTENSION (%) | SEVERE HYPERTENSION (%) |
|---|---|---|
| Preeclampsia | 10-25 | 25-50 |
| Abruptio placentae | 0.7-1.5 | 2-5 |
| Fetal growth restriction | 8.0-15.5 | 10-20 |
| Preterm birth | 12-34.4 | 25-30 |
Low-risk disease: Avoid routine antihypertensive therapy
A limited number of randomized trials have studied the effectiveness of antihypertensive treatment in preventing adverse maternal outcomes such as superimposed preeclampsia and abruptio placentae. Here are 2 key findings:
- No demonstrable maternal benefit. Overall, there appears to be no clear benefit of antihypertensive treatment in women with mild hypertension. Indeed, the 2 largest studies had contradictory findings regarding preeclampsia,6,13 and there was no demonstrable benefit in regard to abruptio placentae.
- Antihypertensive drugs may adversely affect fetal growth. A recent meta-analysis examining antihypertensive medications in patients with mild to moderate hypertension investigated the relationship between a fall in mean arterial blood pressure and the delivery of small-for-gestational-age (SGA) infants.14 The authors concluded that antihypertensive medications induce BP drops that may adversely affect fetal growth (see FIGURE). Prior to this observation, prospective studies had shown no association between antihypertensive medications and SGA infants. (The only exception was atenolol; 3 separate studies found a relationship between treatment with atenolol and low birth weight.15-17)
Overall, maternal and perinatal data indicate that, regardless of the treatment, perinatal mortality is not improved with antihypertensive medications for mild hypertension. In fact, the indiscriminate use of such medications may have deleterious effects. Consequently, antihypertensive medications should not be used routinely in low-risk patients.18,19
Clinical care. When a woman with low-risk hypertension presents for prenatal care, it is our policy to discontinue antihypertensive medications at the first prenatal visit. Although many women will not require antihypertensive treatment during the pregnancy, careful management remains essential, as such patients can become high-risk at any time. Therapy should be initiated if her condition changes to severe hypertension (systolic BP of 180 mm Hg or more, or a diastolic blood pressure of 110 mm Hg or more).8
Low-risk women should be monitored closely for evidence of preeclampsia and fetal growth restriction. Thus, they should have a baseline ultrasound at 16 to 20 weeks’ gestation, with serial monthly ultrasounds beginning at 30 to 32 weeks to follow fetal growth. Nonstress testing or biophysical profiles are indicated in the presence of severe hypertension, preeclampsia, or abnormal fetal growth.
Patients with uncomplicated lowrisk hypertension may continue pregnancy until 40 weeks’ gestation. However, beyond 37 weeks, the presence of complications such as severe hypertension, documented growth restriction, and superimposed preeclampsia are indications for hospitalization and delivery.
FIGURE Placental effects of hypertension
In hypertensive gravidas, placental blood flow is reduced—particularly in cases of preeclampsia. Antihypertensive therapy in low-risk women may induce blood pressure drops that further compromise fetal growth.
High-risk disease: Initiate medical therapy
Randomized, controlled trials do not exist for gravidas with high-risk hypertension—that is, women with severe hypertension or complicating factors—due to concerns about the potential adverse consequences of uncontrolled disease, such as cerebrovascular accident, congestive heart failure, and renal failure.20 It is interesting to note, however, that although controlling hypertension in such patients may help prolong pregnancy, there is no evidence that it reduces the rates of preeclampsia or abruptio placentae.20,21
Labetalol provides the added benefit of alpha-adrenergic blockade, which offers the theoretical advantage of vasodilation.
When to start treatment. For women with high-risk hypertension, hospitalization at the time of the first prenatal visit facilitates complete cardiovascular and renal evaluation, and is therefore often beneficial. If a woman has target organ damage, treatment should be initiated at a systolic BP of 140 mm Hg or a diastolicBP of 90 mm Hg. Indeed, many such women are already receiving treatment for their hypertension, in which case antihypertensive medications should be continued, though physicians should consider altering the regimen to optimize fetal safety.
Choosing the best agent. Before choosing an antihypertensive drug, review the patient’s history. If her disease was well controlled on a particular medication, that agent is probably a reasonable first choice, provided there is adequate published literature establishing the safety of her medication during pregnancy. Obviously, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II antagonists, and atenolol should be avoided because of the potential adverse effects on the fetus, including renal failure.The most commonly used medications for control of hypertension during pregnancy are listed in TABLE 4.
- Labetalol. We consider labetalol a first-line agent for controlling hypertension in pregnancy. This beta-blocking drug provides the added benefit of alpha-adrenergic blockade, which offers the theoretical advantage of vasodilation—not seen with traditional beta-blockers. Overall, labetalol has an excellent record of safety in pregnancy.
In a randomized, controlled trial involving 86 mildly hypertensive patients who initiated labetalol therapy between 6 and 13 weeks’ gestation, no major congenital malformations were identified.6 Although there have been reports of an increased risk for SGA infants in patients treated with labetalol for mild pregnancy-induced hypertension during the second and third trimesters, this association has not been documented in women with chronic hypertension.6
- Thiazide diuretics. If labetalol fails to control blood pressure, we typically add either the calcium-channel blocker nifedipine or a thiazide diuretic. Use of the latter has been well documented in pregnancy. Indeed, thiazide diuretics can be given in the first trimester and throughout gestation without associated risks of major fetal malformations or adverse fetal-neonatal complications.
- Calcium-channel blockers. Calcium-channel–blocking agents also have an excellent safety profile in pregnancy. They have been studied both as antihypertensive medications (primarily in the second and third trimesters) and as tocolytic agents. In a prospective, multicenter, cohort study in which 78 women were exposed to calcium-channel blockers (mainly nifedipine and verapamil) during the first trimester, there was no increase in the rate of birth defects.22
A separate prospective, randomized trial evaluated the benefit of nifedipine in pregnancy. A total of 283 women—47% of whom had chronic hypertension—were enrolled between 12 and 34 weeks’ gestation (mean: 24 weeks). Researchers found patients on nifedipine therapy experienced no improvement in maternal or neonatal outcomes compared to subjects assigned to no treatment.23 Follow-up at 18 months of 94 of the infants exposed to nifedipine in utero showed no adverse effects on development.24
- Methyldopa. For many obstetricians, methyldopa remains a first-line agent for the treatment of chronic hypertension in pregnancy.1 It has a well-documented safety record in both short-term25 and long-term follow-up of children exposed in utero.26 Indeed, many studies have evaluated use of this medication to manage mild to moderate hypertension, with no evidence of adverse maternal or fetal outcome. However, it is now rarely used in the nonpregnant population, and the safety of other medications, such as labetalol and nifedipine, has prompted us to stop giving it.
- Other considerations. Finally, when choosing an antihypertensive drug, the physician must consider the benefits and response of specific agents in particular risk groups (TABLE 5).
In women with diabetes, calcium-channel blockers have a reno-protective effect and are our first-line agent in pregnancy, since ACE inhibitors, which also offer this benefit, must be avoided beyond 16 weeks’ gestation because of the potential adverse fetal effects.
In women with diabetes, calcium-channel blockers have a reno-protective effect and are our first-line agent in pregnancy.
Young African-American women frequently have low-renin, salt-sensitive hypertension, and therefore thiazide diuretics or nifedipine may be better first-line agents in this population.
TABLE 4
Agents for treating chronic hypertension in pregnancy
| DRUG | STARTING DOSE | MAXIMUM DAILY DOSE | COMMON SIDE EFFECTS |
|---|---|---|---|
| Labetalol | 100 mg every 8 h | 1,200–2,400 mg | Headache Tremulousness |
| Thiazide diuretic | 12.5 mg twice daily | 50 mg | Hypokalemia |
| Nifedipine | Hypotension | ||
| Short acting | 10 mg every 8 h | 120 mg | Headache |
| Long acting | 30 mg/d | 240 mg | Tachycardia |
| Alpha methyldopa | 250 mg twice daily | 4 g | Thirst Drowsiness Elevation of liver enzymes |
TABLE 5
Medical factors guiding selection of antihypertensive medication
| If the patient has… | It’s generally best to start with… |
|---|---|
| Diabetes | Calcium-channel blocker |
| Vascular disease | |
| Salt-wasting hypertension* | Thiazide diuretic |
| Left ventricular systolic dysfunction | |
| Mitral stenosis | |
| *Mostly African-American women | |
Severe gestational hypertension and preeclampsia
Women who develop severe gestational hypertension (systolic BP of 160 mm Hg or more or diastolic BP of 110 mm Hg or more) and/or preeclampsia require antihypertensive treatment during management remote from term. In this case, the aim of antihypertensive drug treatment is to keep systolic BP between 150 and 159 mm Hg and diastolic BP between 100 and 109 mm Hg in order to not compromise uteroplacental blood flow.
Because nitroprusside is both a vasodilator and a venodilator, it is an ideal agent for gravidas with hypertensive encephalopathy.
The drugs to use are oral labetalol and/or oral nifedipine. If maternal BP is not adequately controlled with maximum doses of labetalol plus nifedipine, the patient should be delivered.
Severe hypertension and encephalopathy
Hypertensive encephalopathy is a medical emergency. This rare complication of hypertension in pregnancy27 is marked by severely elevated BP, with the diastolic level frequently exceeding 130 mm Hg. Associated findings include headache, visual disturbances, nausea, vomiting, seizures, confusion, stupor, and coma. Also possible are retinal hemorrhage, exudates, papilledema, and evidence of renal or cardiac disease. Transient focal neurologic findings may be present as well, but more often suggest vascular disease, hemorrhage, embolism, or thrombosis.
Pathophysiology. In hypertensive encephalopathy, loss of autoregulation leads to generalized cerebral vasodilation. Under normal conditions, when the mean arterial pressure is between 60 and 130 mm Hg, patients maintain constant cerebral blood flow. In hypertensive patients, however, autoregulation occurs between mean arterial pressures of 110 and 180 mm Hg as a result of arteriolar thickening. When BP exceeds the ability of the vessels to autoregulate, blood flow hyperperfuses the brain, causing fluid to leak into the perivascular tissue and resulting in vasogenic cerebral edema. Altered vascular reactivity to normally circulating pressor agents, deficient levels of vasodilating prostaglandins, endothelial dysfunction, and activation of the coagulation cascade may further exacerbate this condition.28
Treatment options. Clinically, it may be impossible to differentiate hypertensive encephalopathy from eclampsia, and magnesium sulfate should be considered for seizure prophylaxis. The most frequently used antihypertensive medications for this syndrome are shown in TABLE 6. Nitroprusside lowers BP most predictably, but because of the associated risks of fetal cyanide toxicity, other medications may be more desirable first-line agents in the pregnant woman.
Importantly, because sudden drops in BP may impair cerebral perfusion, we recommend that the mean arterial pressure be lowered no more than 25% from baseline (TABLE 7). If pulmonary edema develops, oxygen and furosemide should be administered, and consultation with subspecialists considered. (We suggest such consultation for cases of renal dysfunction and cerebral complications, as well.) Because nitroprusside is both a vasodilator and a venodilator, it is an ideal agent in this situation.
TABLE 6
Medications for treating acute severe hypertension
| DRUG | DOSE | ONSET OF ACTION | DURATION OF ACTION | SIDE EFFECTS |
|---|---|---|---|---|
| Hydralazine | 5-10 mg IV every 20 min | 10-20 min | 3-6 h | Tachycardia Headache Flushing Angina |
| Labetalol | 20-80 mg IV every 10 min | 5-10 min | 3-6 h | Scalp tingling Vomiting Heart block |
| Sodium nitroprusside* | 0.25-5 mcg/kg/min | Immediate | 1-2 min | Nausea Vomiting Muscle twitching Thiocyanate and cyanide intoxication |
| Nicardipine* | 5-15 mg/h IV | 5-10 min | 1-4 h | Tachycardia Headache Phlebitis |
| *Drugs to use in the presence of hypertensive encephalopathy. | ||||
TABLE 7
Principles of management for severe hypertension and encephalopathy
Acute severe hypertension
|
Hypertensive encephalopathy
|
| *Lower mean arterial pressure no more than 25% from baseline. |
Postpartum management
Monitor BP for at least 48 hours. Women with high-risk chronic hypertension are more likely to suffer postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure than normotensive patients.8 This risk is even higher when these women also have target organ involvement, superimposed preeclampsia, abruptio placentae, morbid obesity, or longstanding hypertension.
In these patients, BP must be closely monitored and controlled for at least 48 hours after delivery. Intravenous labetalol or hydralazine can be administered for acute elevations of BP29; diuretics should also be used for women with circulatory congestion and pulmonary edema.30
Methyldopa remains the first-line agent for breastfeeding patients without compelling indications for another drug.
Oral antihypertensive therapy may be needed to maintain BP control. In choosing the appropriate agent, it is important to consider whether factors compel the choice of one medication over another. For example, for patients with a history of myocardial infarction, beta blockers and ACE inhibitors are excellent choices to decrease mortality.31 In patients with diabetes mellitus, as mentioned earlier, ACE inhibitors offer a renoprotective effect.10
Consider drug concentrations in breast milk. Another significant consideration in the postpartum period is whether the mother wishes to breastfeed her infant. All antihypertensive medications are found in breast milk to varying degrees,32 and the long-term effects of these medications on breastfeeding infants has not been specifically studied.
Because concentrations of methyldopa in milk are low and considered safe, it remains the first-line agent for patients without compelling indications for another antihypertensive drug. Concentrations of labetalol and propranolol also are low in breast milk; therefore, these may be better choices than atenolol and metoprolol, which are more highly concentrated in breast milk.12
Although diuretic agents have low concentrations in breast milk, they may decrease milk production.32
Little information exists regarding the excretion of calcium-channel blockers in breast milk, but no untoward effects are apparent.33 ACE inhibitors and angiotensin II receptor antagonists should be avoided because of potential deleterious effects on neonatal renal function, even though their concentrations in breast milk appear to be low. If ACE inhibitors are indicated for the breastfeeding mother, current data suggest that captopril and enalapril are safe.34
The authors report no financial relationship with any companies whose products are mentioned in this article.
- The treatment goal is to reduce blood pressure to a safe level to prevent maternal cerebral complications.This goal must be weighed against the risks of fetal exposure to antihypertensive drugs and the effects on uteroplacental blood flow.
- Gravidas with uncomplicated mild hypertension are at low risk; however, those with severe hypertension or associated complicating factors are at high risk of complications and adverse outcomes.
- Antihypertensive medications should not be used routinely in low-risk patients.
- Women with high-risk chronic hypertension are at risk for postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure.
The decision to use antihypertensive drug therapy in pregnant women is a tricky one—especially considering the ever-evolving nature of treatment. For instance, we now know that in some hypertensive gravidas, medical interventions may actually be deleterious.
With the aging of the obstetric population in the United States, hypertension in pregnancy—which currently affects 7% of gestations—will remain a major issue in preconception and prenatal care. Its reported risks, which include stroke, pulmonary edema, and death, underscore the importance of careful management (TABLE 1).
This article describes the indications for antihypertensive therapy in pregnancy, focusing on 2 basic categories—high-risk and lowrisk patients—and offers guidance in choosing the optimal agent for each patient.
TABLE 1
Maternal risks of severe hypertension in pregnancy
| Stroke |
| Cerebral hemorrhage |
| Hypertensive encephalopathy |
| Congestive heart failure/pulmonary edema |
| Acute renal dysfunction/acute renal failure |
| Abruptio placentae |
| Disseminated intravascular coagulopathy |
| Death |
Correct classification helps direct management
First, identify chronic hypertension. Chronic hypertension is defined as an elevation in blood pressure (BP) that exists prior to pregnancy. Unfortunately, because the pregestational BP is not always known, the diagnosis in many cases must be made on the basis of specific levels: systolic BP of at least 140 mm Hg or diastolic BP of at least 90 mm Hg on at least 2 occasions at least 4 hours apart prior to 20 weeks’ gestation.1
Even with these guidelines, however, diagnosis may be difficult, since early manifestations of preeclampsia can include hypertension prior to 20 weeks’ gestation.2,3 In addition, the physiologic decrease in BP during the first and second trimesters—seen in many patients with chronic hypertension—may obscure the condition early in gestation and lead to the erroneous diagnosis of gestational hypertension or preeclampsia later in pregnancy.4-6
Once a diagnosis of chronic hypertension is made, an accurate classification of the disease will help guide management and initiation of antihypertensive medication.
Mild versus severe hypertension. In pregnancy, chronic hypertension is classified as mild or severe. Mild hypertension has traditionally been defined as systolic BP less than 160 mm Hg and diastolic blood pressure less than 110 mm Hg.1,7 However, the American College of Obstetricians and Gynecologists recently changed its definition of mild hypertension to systolic BP less than 180 mm Hg.8,9 Most women with chronic hypertension in pregnancy have the mild form of the disease.
Low-risk hypertension. Patients with uncomplicated chronic mild hypertension are at low risk.
High-risk hypertension. Patients at high risk have either chronic severe hypertension or chronic mild hypertension in association with any of the complicating factors listed in TABLE 2.
History and laboratory studies. To properly classify the disease when first evaluating a patient with chronic hypertension, a thorough history is essential. Ask about related medical illnesses as well as target organ damage. Pay special attention to cardiac, renal, thyroid, and cerebrovascular disease, as well as diabetes. The outcomes of prior pregnancies also are important, especially complications such as abruptio placentae, preeclampsia, preterm delivery, growth restriction, fetal death, and neonatal complications.
Overall, regardless of the treatment, perinatal mortality is not improved with antihypertensive medications for mild hypertension.
Finally, laboratory evaluation should include urine analysis, urine culture, 24-hour urine protein, electrolytes, complete blood count, and glucose tolerance testing.
Other key examinations. In women with long-standing disease, ophthalmologic evaluation, electrocardiography, echocardiography, and assessment of creatinine clearance may be indicated.
TABLE 2
Criteria for low- versus high-risk chronic hypertension
| LOW-RISK CHRONIC HYPERTENSION |
| Chronic mild hypertension (systolic blood pressure 140–160 mm Hg and diastolic blood pressure 90–110 mm Hg) in the absence of complicating factors |
| HIGH-RISK CHRONIC HYPERTENSION |
| Either: |
| Chronic severe hypertension (systolic blood pressure ≥180 mm Hg and diastolic blood pressure ≥110 mm Hg) |
| or |
Chronic mild hypertension (systolic blood pressure 140–160 mm Hg and diastolic blood pressure 90–110 mm Hg) in association with anyof the following:
|
Objective of treatment: Prevent complications
In the nonpregnant state, the aim of hypertensive management is to prevent longterm vascular complications such as stroke and cardiovascular disease.10 A reasonable treatment goal for patients with mild to moderate hypertension may be benefits that are apparent after 5 years of therapy10—an acceptable time frame due to the long-term nature of the disease.
In pregnant women, however, the duration of the condition (pregnant and hypertensive) is finite and relatively short; as a result, maternal health benefits may not be clear. For that reason, the objective is to reduce BP to a safe level to prevent maternal cerebral complications (systolic BP below 160 mm Hg and diastolic BP below 110 mm Hg).Of course, these short-term maternal benefits must be weighed against the potential risks of fetal exposure (TABLE 3).11,12
TABLE 3
Rates of adverse pregnancy outcomes among patients with mild and severe chronic hypertension
| OUTCOME | MILD HYPERTENSION (%) | SEVERE HYPERTENSION (%) |
|---|---|---|
| Preeclampsia | 10-25 | 25-50 |
| Abruptio placentae | 0.7-1.5 | 2-5 |
| Fetal growth restriction | 8.0-15.5 | 10-20 |
| Preterm birth | 12-34.4 | 25-30 |
Low-risk disease: Avoid routine antihypertensive therapy
A limited number of randomized trials have studied the effectiveness of antihypertensive treatment in preventing adverse maternal outcomes such as superimposed preeclampsia and abruptio placentae. Here are 2 key findings:
- No demonstrable maternal benefit. Overall, there appears to be no clear benefit of antihypertensive treatment in women with mild hypertension. Indeed, the 2 largest studies had contradictory findings regarding preeclampsia,6,13 and there was no demonstrable benefit in regard to abruptio placentae.
- Antihypertensive drugs may adversely affect fetal growth. A recent meta-analysis examining antihypertensive medications in patients with mild to moderate hypertension investigated the relationship between a fall in mean arterial blood pressure and the delivery of small-for-gestational-age (SGA) infants.14 The authors concluded that antihypertensive medications induce BP drops that may adversely affect fetal growth (see FIGURE). Prior to this observation, prospective studies had shown no association between antihypertensive medications and SGA infants. (The only exception was atenolol; 3 separate studies found a relationship between treatment with atenolol and low birth weight.15-17)
Overall, maternal and perinatal data indicate that, regardless of the treatment, perinatal mortality is not improved with antihypertensive medications for mild hypertension. In fact, the indiscriminate use of such medications may have deleterious effects. Consequently, antihypertensive medications should not be used routinely in low-risk patients.18,19
Clinical care. When a woman with low-risk hypertension presents for prenatal care, it is our policy to discontinue antihypertensive medications at the first prenatal visit. Although many women will not require antihypertensive treatment during the pregnancy, careful management remains essential, as such patients can become high-risk at any time. Therapy should be initiated if her condition changes to severe hypertension (systolic BP of 180 mm Hg or more, or a diastolic blood pressure of 110 mm Hg or more).8
Low-risk women should be monitored closely for evidence of preeclampsia and fetal growth restriction. Thus, they should have a baseline ultrasound at 16 to 20 weeks’ gestation, with serial monthly ultrasounds beginning at 30 to 32 weeks to follow fetal growth. Nonstress testing or biophysical profiles are indicated in the presence of severe hypertension, preeclampsia, or abnormal fetal growth.
Patients with uncomplicated lowrisk hypertension may continue pregnancy until 40 weeks’ gestation. However, beyond 37 weeks, the presence of complications such as severe hypertension, documented growth restriction, and superimposed preeclampsia are indications for hospitalization and delivery.
FIGURE Placental effects of hypertension
In hypertensive gravidas, placental blood flow is reduced—particularly in cases of preeclampsia. Antihypertensive therapy in low-risk women may induce blood pressure drops that further compromise fetal growth.
High-risk disease: Initiate medical therapy
Randomized, controlled trials do not exist for gravidas with high-risk hypertension—that is, women with severe hypertension or complicating factors—due to concerns about the potential adverse consequences of uncontrolled disease, such as cerebrovascular accident, congestive heart failure, and renal failure.20 It is interesting to note, however, that although controlling hypertension in such patients may help prolong pregnancy, there is no evidence that it reduces the rates of preeclampsia or abruptio placentae.20,21
Labetalol provides the added benefit of alpha-adrenergic blockade, which offers the theoretical advantage of vasodilation.
When to start treatment. For women with high-risk hypertension, hospitalization at the time of the first prenatal visit facilitates complete cardiovascular and renal evaluation, and is therefore often beneficial. If a woman has target organ damage, treatment should be initiated at a systolic BP of 140 mm Hg or a diastolicBP of 90 mm Hg. Indeed, many such women are already receiving treatment for their hypertension, in which case antihypertensive medications should be continued, though physicians should consider altering the regimen to optimize fetal safety.
Choosing the best agent. Before choosing an antihypertensive drug, review the patient’s history. If her disease was well controlled on a particular medication, that agent is probably a reasonable first choice, provided there is adequate published literature establishing the safety of her medication during pregnancy. Obviously, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II antagonists, and atenolol should be avoided because of the potential adverse effects on the fetus, including renal failure.The most commonly used medications for control of hypertension during pregnancy are listed in TABLE 4.
- Labetalol. We consider labetalol a first-line agent for controlling hypertension in pregnancy. This beta-blocking drug provides the added benefit of alpha-adrenergic blockade, which offers the theoretical advantage of vasodilation—not seen with traditional beta-blockers. Overall, labetalol has an excellent record of safety in pregnancy.
In a randomized, controlled trial involving 86 mildly hypertensive patients who initiated labetalol therapy between 6 and 13 weeks’ gestation, no major congenital malformations were identified.6 Although there have been reports of an increased risk for SGA infants in patients treated with labetalol for mild pregnancy-induced hypertension during the second and third trimesters, this association has not been documented in women with chronic hypertension.6
- Thiazide diuretics. If labetalol fails to control blood pressure, we typically add either the calcium-channel blocker nifedipine or a thiazide diuretic. Use of the latter has been well documented in pregnancy. Indeed, thiazide diuretics can be given in the first trimester and throughout gestation without associated risks of major fetal malformations or adverse fetal-neonatal complications.
- Calcium-channel blockers. Calcium-channel–blocking agents also have an excellent safety profile in pregnancy. They have been studied both as antihypertensive medications (primarily in the second and third trimesters) and as tocolytic agents. In a prospective, multicenter, cohort study in which 78 women were exposed to calcium-channel blockers (mainly nifedipine and verapamil) during the first trimester, there was no increase in the rate of birth defects.22
A separate prospective, randomized trial evaluated the benefit of nifedipine in pregnancy. A total of 283 women—47% of whom had chronic hypertension—were enrolled between 12 and 34 weeks’ gestation (mean: 24 weeks). Researchers found patients on nifedipine therapy experienced no improvement in maternal or neonatal outcomes compared to subjects assigned to no treatment.23 Follow-up at 18 months of 94 of the infants exposed to nifedipine in utero showed no adverse effects on development.24
- Methyldopa. For many obstetricians, methyldopa remains a first-line agent for the treatment of chronic hypertension in pregnancy.1 It has a well-documented safety record in both short-term25 and long-term follow-up of children exposed in utero.26 Indeed, many studies have evaluated use of this medication to manage mild to moderate hypertension, with no evidence of adverse maternal or fetal outcome. However, it is now rarely used in the nonpregnant population, and the safety of other medications, such as labetalol and nifedipine, has prompted us to stop giving it.
- Other considerations. Finally, when choosing an antihypertensive drug, the physician must consider the benefits and response of specific agents in particular risk groups (TABLE 5).
In women with diabetes, calcium-channel blockers have a reno-protective effect and are our first-line agent in pregnancy, since ACE inhibitors, which also offer this benefit, must be avoided beyond 16 weeks’ gestation because of the potential adverse fetal effects.
In women with diabetes, calcium-channel blockers have a reno-protective effect and are our first-line agent in pregnancy.
Young African-American women frequently have low-renin, salt-sensitive hypertension, and therefore thiazide diuretics or nifedipine may be better first-line agents in this population.
TABLE 4
Agents for treating chronic hypertension in pregnancy
| DRUG | STARTING DOSE | MAXIMUM DAILY DOSE | COMMON SIDE EFFECTS |
|---|---|---|---|
| Labetalol | 100 mg every 8 h | 1,200–2,400 mg | Headache Tremulousness |
| Thiazide diuretic | 12.5 mg twice daily | 50 mg | Hypokalemia |
| Nifedipine | Hypotension | ||
| Short acting | 10 mg every 8 h | 120 mg | Headache |
| Long acting | 30 mg/d | 240 mg | Tachycardia |
| Alpha methyldopa | 250 mg twice daily | 4 g | Thirst Drowsiness Elevation of liver enzymes |
TABLE 5
Medical factors guiding selection of antihypertensive medication
| If the patient has… | It’s generally best to start with… |
|---|---|
| Diabetes | Calcium-channel blocker |
| Vascular disease | |
| Salt-wasting hypertension* | Thiazide diuretic |
| Left ventricular systolic dysfunction | |
| Mitral stenosis | |
| *Mostly African-American women | |
Severe gestational hypertension and preeclampsia
Women who develop severe gestational hypertension (systolic BP of 160 mm Hg or more or diastolic BP of 110 mm Hg or more) and/or preeclampsia require antihypertensive treatment during management remote from term. In this case, the aim of antihypertensive drug treatment is to keep systolic BP between 150 and 159 mm Hg and diastolic BP between 100 and 109 mm Hg in order to not compromise uteroplacental blood flow.
Because nitroprusside is both a vasodilator and a venodilator, it is an ideal agent for gravidas with hypertensive encephalopathy.
The drugs to use are oral labetalol and/or oral nifedipine. If maternal BP is not adequately controlled with maximum doses of labetalol plus nifedipine, the patient should be delivered.
Severe hypertension and encephalopathy
Hypertensive encephalopathy is a medical emergency. This rare complication of hypertension in pregnancy27 is marked by severely elevated BP, with the diastolic level frequently exceeding 130 mm Hg. Associated findings include headache, visual disturbances, nausea, vomiting, seizures, confusion, stupor, and coma. Also possible are retinal hemorrhage, exudates, papilledema, and evidence of renal or cardiac disease. Transient focal neurologic findings may be present as well, but more often suggest vascular disease, hemorrhage, embolism, or thrombosis.
Pathophysiology. In hypertensive encephalopathy, loss of autoregulation leads to generalized cerebral vasodilation. Under normal conditions, when the mean arterial pressure is between 60 and 130 mm Hg, patients maintain constant cerebral blood flow. In hypertensive patients, however, autoregulation occurs between mean arterial pressures of 110 and 180 mm Hg as a result of arteriolar thickening. When BP exceeds the ability of the vessels to autoregulate, blood flow hyperperfuses the brain, causing fluid to leak into the perivascular tissue and resulting in vasogenic cerebral edema. Altered vascular reactivity to normally circulating pressor agents, deficient levels of vasodilating prostaglandins, endothelial dysfunction, and activation of the coagulation cascade may further exacerbate this condition.28
Treatment options. Clinically, it may be impossible to differentiate hypertensive encephalopathy from eclampsia, and magnesium sulfate should be considered for seizure prophylaxis. The most frequently used antihypertensive medications for this syndrome are shown in TABLE 6. Nitroprusside lowers BP most predictably, but because of the associated risks of fetal cyanide toxicity, other medications may be more desirable first-line agents in the pregnant woman.
Importantly, because sudden drops in BP may impair cerebral perfusion, we recommend that the mean arterial pressure be lowered no more than 25% from baseline (TABLE 7). If pulmonary edema develops, oxygen and furosemide should be administered, and consultation with subspecialists considered. (We suggest such consultation for cases of renal dysfunction and cerebral complications, as well.) Because nitroprusside is both a vasodilator and a venodilator, it is an ideal agent in this situation.
TABLE 6
Medications for treating acute severe hypertension
| DRUG | DOSE | ONSET OF ACTION | DURATION OF ACTION | SIDE EFFECTS |
|---|---|---|---|---|
| Hydralazine | 5-10 mg IV every 20 min | 10-20 min | 3-6 h | Tachycardia Headache Flushing Angina |
| Labetalol | 20-80 mg IV every 10 min | 5-10 min | 3-6 h | Scalp tingling Vomiting Heart block |
| Sodium nitroprusside* | 0.25-5 mcg/kg/min | Immediate | 1-2 min | Nausea Vomiting Muscle twitching Thiocyanate and cyanide intoxication |
| Nicardipine* | 5-15 mg/h IV | 5-10 min | 1-4 h | Tachycardia Headache Phlebitis |
| *Drugs to use in the presence of hypertensive encephalopathy. | ||||
TABLE 7
Principles of management for severe hypertension and encephalopathy
Acute severe hypertension
|
Hypertensive encephalopathy
|
| *Lower mean arterial pressure no more than 25% from baseline. |
Postpartum management
Monitor BP for at least 48 hours. Women with high-risk chronic hypertension are more likely to suffer postpartum complications such as pulmonary edema, hypertensive encephalopathy, and renal failure than normotensive patients.8 This risk is even higher when these women also have target organ involvement, superimposed preeclampsia, abruptio placentae, morbid obesity, or longstanding hypertension.
In these patients, BP must be closely monitored and controlled for at least 48 hours after delivery. Intravenous labetalol or hydralazine can be administered for acute elevations of BP29; diuretics should also be used for women with circulatory congestion and pulmonary edema.30
Methyldopa remains the first-line agent for breastfeeding patients without compelling indications for another drug.
Oral antihypertensive therapy may be needed to maintain BP control. In choosing the appropriate agent, it is important to consider whether factors compel the choice of one medication over another. For example, for patients with a history of myocardial infarction, beta blockers and ACE inhibitors are excellent choices to decrease mortality.31 In patients with diabetes mellitus, as mentioned earlier, ACE inhibitors offer a renoprotective effect.10
Consider drug concentrations in breast milk. Another significant consideration in the postpartum period is whether the mother wishes to breastfeed her infant. All antihypertensive medications are found in breast milk to varying degrees,32 and the long-term effects of these medications on breastfeeding infants has not been specifically studied.
Because concentrations of methyldopa in milk are low and considered safe, it remains the first-line agent for patients without compelling indications for another antihypertensive drug. Concentrations of labetalol and propranolol also are low in breast milk; therefore, these may be better choices than atenolol and metoprolol, which are more highly concentrated in breast milk.12
Although diuretic agents have low concentrations in breast milk, they may decrease milk production.32
Little information exists regarding the excretion of calcium-channel blockers in breast milk, but no untoward effects are apparent.33 ACE inhibitors and angiotensin II receptor antagonists should be avoided because of potential deleterious effects on neonatal renal function, even though their concentrations in breast milk appear to be low. If ACE inhibitors are indicated for the breastfeeding mother, current data suggest that captopril and enalapril are safe.34
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. National High Blood Pressure Education Program Working Group. National High Blood Pressure Education Program Working Group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
2. Sibai BM, Akl S, Fairlie F, Moretti M. A protocol for managing severe preeclampsia in the second trimester. Am J Obstet Gynecol. 1990;163:733-738.
3. Hermida RC, Ayala DA, Mojon A, et al. Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension. 2000;36:149-158.
4. Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571-576.
5. Benedetto C, Zonca M, Marozio L, Dolci C, Carandente F, Massobrio M. Blood pressure patterns in normal pregnancy and in pregnancy-induced hypertension, preeclampsia, and chronic hypertension. Obstet Gynecol. 1996;88:503-510.
6. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-966.
7. Sibai BM. Diagnosis and management of chronic hypertension in pregnancy. Obstet Gynecol. 1991;78:451-461.
8. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #29: Chronic hypertension in pregnancy. Washington, DC: ACOG; 2001.
9. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
10. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413-2446.
11. Ferrer RL, Sibai BM, Murlow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol. 2000;96:849-860.
12. Umans JG, Lindheimer MD. Antihypertensive treatment. In: Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Norwalk, Conn: Appleton and Lange; 1998;581-604.
13. Steyn DW, Odendaal HJ. Randomized controlled trial of ketanserin and aspirin in prevention of pre-eclampsia. Lancet. 1997;350:1267-1271.
14. von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet. 2000;355:87-92.
15. Lip GY, Beevers M, Churchill D, Shaffer LM, Beevers DG. Effect of atenolol on birth weight. Am J Cardiol. 1997;79:1436-1438.
16. Easterling TR, Brateng D, Schmucker B, Brown Z, Millard SP. Prevention of preeclampsia: a randomized trial of atenolol in hyperdynamic patients before onset of hypertension. Obstet Gynecol. 1999;93:725-733.
17. Lydakis C, Lip GY, Beevers M, Beevers G. Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens. 1999;12:541-547.
18. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335:257-265.
19. Magee LA, Ornstein MP, von Dadelszen P. Management of hypertension in pregnancy. BMJ. 1999;318:1332-1336.
20. Sibai BM, Anderson GD. Pregnancy outcome of intensive therapy in severe hypertension in first trimester. Obstet Gynecol. 1986;67:517-522.
21. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123-129.
22. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174:823-828.
23. Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. Br J Obstet Gynaecol. 1998;105:718-722.
24. Bortolus R, Ricci E, Chatenoud L, Parazzini F. Nifedipine administered in pregnancy: effect on the development of children at 18 months. Br J Obstet Gynaecol. 2000;107:792-794.
25. Montan S, Anandakumar C, Arulkumaran S, Ingemarsson I, Ratnam SS. Effects of methyldopa on uteroplacental and fetal hemodynamics in pregnancy-induced hypertension. Am J Obstet Gynecol. 1993;168:152-156.
26. Cockburn J, Moar VA, Ounsted M, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet. 1982;1:647-649.
27. Witlin AG, Friedman SA, Egerman RS, et al. Cerebrovascular disorders complicating pregnancy—beyond eclampsia. Am J Obstet Gynecol. 1997;176:1139-1148.
28. Cotton DB, Janusz CA, Berman RF. Anticonvulsant effects of magnesium sulfate on hippocampal seizures: therapeutic implication in preeclampsia-eclampsia. Am J Obstet Gynecol. 1992;166:1127-1136.
29. Mabie WC, Gonzalez AR, Sibai BM, Amon E. A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Obstet Gynecol. 1987;70:328-333.
30. Mabie WC, Ratts TE, Ramanathan KB, Sibai BM. Circulatory congestion in obese hypertensive women: a subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553-558.
31. Pfeffer MA, Braunwald E, Moye LA. for the SAVE investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement Trials. N Engl J Med. 1992;327:669-677.
32. White WB. Management of hypertension during lactation. Hypertension. 1984;6:297-300.
33. Briggs GG, Freeman RK, Yaffee SJ. Drugs in Pregnancy and Lactation: a Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore, Md: Williams and Wilkins; 1998.
34. Committee on Drugs, American Academy of Pediatrics. The transfer of drugs and other chemicals into human milk. Pediatrics. 1994;93:137-150.
1. National High Blood Pressure Education Program Working Group. National High Blood Pressure Education Program Working Group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
2. Sibai BM, Akl S, Fairlie F, Moretti M. A protocol for managing severe preeclampsia in the second trimester. Am J Obstet Gynecol. 1990;163:733-738.
3. Hermida RC, Ayala DA, Mojon A, et al. Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension. 2000;36:149-158.
4. Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571-576.
5. Benedetto C, Zonca M, Marozio L, Dolci C, Carandente F, Massobrio M. Blood pressure patterns in normal pregnancy and in pregnancy-induced hypertension, preeclampsia, and chronic hypertension. Obstet Gynecol. 1996;88:503-510.
6. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-966.
7. Sibai BM. Diagnosis and management of chronic hypertension in pregnancy. Obstet Gynecol. 1991;78:451-461.
8. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #29: Chronic hypertension in pregnancy. Washington, DC: ACOG; 2001.
9. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
10. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413-2446.
11. Ferrer RL, Sibai BM, Murlow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol. 2000;96:849-860.
12. Umans JG, Lindheimer MD. Antihypertensive treatment. In: Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesley’s Hypertensive Disorders in Pregnancy. 2nd ed. Norwalk, Conn: Appleton and Lange; 1998;581-604.
13. Steyn DW, Odendaal HJ. Randomized controlled trial of ketanserin and aspirin in prevention of pre-eclampsia. Lancet. 1997;350:1267-1271.
14. von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet. 2000;355:87-92.
15. Lip GY, Beevers M, Churchill D, Shaffer LM, Beevers DG. Effect of atenolol on birth weight. Am J Cardiol. 1997;79:1436-1438.
16. Easterling TR, Brateng D, Schmucker B, Brown Z, Millard SP. Prevention of preeclampsia: a randomized trial of atenolol in hyperdynamic patients before onset of hypertension. Obstet Gynecol. 1999;93:725-733.
17. Lydakis C, Lip GY, Beevers M, Beevers G. Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens. 1999;12:541-547.
18. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335:257-265.
19. Magee LA, Ornstein MP, von Dadelszen P. Management of hypertension in pregnancy. BMJ. 1999;318:1332-1336.
20. Sibai BM, Anderson GD. Pregnancy outcome of intensive therapy in severe hypertension in first trimester. Obstet Gynecol. 1986;67:517-522.
21. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123-129.
22. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174:823-828.
23. Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. Br J Obstet Gynaecol. 1998;105:718-722.
24. Bortolus R, Ricci E, Chatenoud L, Parazzini F. Nifedipine administered in pregnancy: effect on the development of children at 18 months. Br J Obstet Gynaecol. 2000;107:792-794.
25. Montan S, Anandakumar C, Arulkumaran S, Ingemarsson I, Ratnam SS. Effects of methyldopa on uteroplacental and fetal hemodynamics in pregnancy-induced hypertension. Am J Obstet Gynecol. 1993;168:152-156.
26. Cockburn J, Moar VA, Ounsted M, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet. 1982;1:647-649.
27. Witlin AG, Friedman SA, Egerman RS, et al. Cerebrovascular disorders complicating pregnancy—beyond eclampsia. Am J Obstet Gynecol. 1997;176:1139-1148.
28. Cotton DB, Janusz CA, Berman RF. Anticonvulsant effects of magnesium sulfate on hippocampal seizures: therapeutic implication in preeclampsia-eclampsia. Am J Obstet Gynecol. 1992;166:1127-1136.
29. Mabie WC, Gonzalez AR, Sibai BM, Amon E. A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Obstet Gynecol. 1987;70:328-333.
30. Mabie WC, Ratts TE, Ramanathan KB, Sibai BM. Circulatory congestion in obese hypertensive women: a subset of pulmonary edema in pregnancy. Obstet Gynecol. 1988;72:553-558.
31. Pfeffer MA, Braunwald E, Moye LA. for the SAVE investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement Trials. N Engl J Med. 1992;327:669-677.
32. White WB. Management of hypertension during lactation. Hypertension. 1984;6:297-300.
33. Briggs GG, Freeman RK, Yaffee SJ. Drugs in Pregnancy and Lactation: a Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore, Md: Williams and Wilkins; 1998.
34. Committee on Drugs, American Academy of Pediatrics. The transfer of drugs and other chemicals into human milk. Pediatrics. 1994;93:137-150.