User login
Primary Hyperparathyroidism: A Case-based Review
CE/CME No: CR-1705
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Differentiate primary hyperparathyroidism (PHPT) from other causes of hypercalcemia and types of hyerparathyroidism.
• Understand the calcium-parathyroid hormone feedback loop.
• Identify appropriate imaging studies and common laboratory findings in the patient with PHPT.
• Describe the common systemic manifestations of PHPT.
• Discuss medical versus surgical management of the patient with PHPT.
FACULTY
Barbara Austin is a Family Nurse Practitioner at Baptist Primary Care, Jacksonville, Florida, and is pursuing a Doctorate of Nursing Practice (DNP) at Jacksonville University.
The author has no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2017.
Article begins on next page >>
Primary hyperparathyroidism (PHPT) is most often detected as hypercalcemia in an asymptomatic patient during routine blood work. Knowing the appropriate work-up of hypercalcemia is essential, since untreated PHPT can have significant complications affecting multiple organ systems—most notably, renal and musculoskeletal. Parathyroidectomy is curative in up to 95% of cases, but prevention of long-term complications relies on prompt recognition and appropriate follow-up.
Primary hyperparathyroidism (PHPT) is a common endocrine disorder, with a prevalence of approximately 1 to 3 cases per 1,000 persons.1 PHPT results from inappropriate overproduction of parathyroid hormone (PTH), the primary regulator of calcium homeostasis, and is characterized by hypercalcemia in the setting of an elevated or high-normal PTH level. In most cases of PHPT, unregulated PTH production is caused by a single parathyroid adenoma.
PHPT is the most common cause of hypercalcemia in outpatients and is typically diagnosed following incidental discovery during routine blood work in an asymptomatic patient.1,2 It is two to three times more common in women than in men, and incidence increases with age; as such, postmenopausal women are most commonly affected.1,3 PHPT often has an insidious course, and recognition of its clinical manifestations followed by appropriate diagnostic work-up and management are necessary to prevent sequelae.3
PATIENT PRESENTATION
A 68-year-old Caucasian woman presented to her family practice office for a third visit with continued complaints of nontraumatic right lower leg pain. She had previously been diagnosed with tendonitis, which was treated conservatively. The pain failed to improve, and an x-ray was ordered. The x-ray revealed no acute findings but did show osteopenia, prompting an order for a bone mineral density (BMD) test. The BMD test demonstrated osteoporosis, which warranted further investigation. She was prescribed alendronate but refused it, against medical advice, due to concern over potential adverse effects.
Her medical history included hyperlipidemia and hypertension under fair control with lisinopril. She took a low-dose aspirin and flaxseed supplement daily. She also had a history of radiation to the neck, having undergone tonsillar irradiation as a child (a common practice from the 1930s through the 1950s).3 Surgical history included a total hysterectomy with bilateral salpingo-oophorectomy, appendectomy, and tonsillectomy. There was no personal or family history of cancer or endocrine disorders, hypercalcemia, or nephrolithiasis. She was up to date on vaccines and preventive health care measures. Allergies included penicillin and sulfa, both resulting in hives. She was a nonsmoker and did not drink alcohol or engage in illicit drug use.
Review of systems revealed right lower anterior leg pain for four months, characterized as aching, deep, sharp, and throbbing with radiation to the ankle. The pain was worse with activity and prolonged standing; ibuprofen and application of ice provided partial relief. She had experienced some mood changes, including irritability. Physical exam was normal except for dominant right-sided thyromegaly, marked bony point tenderness to the right midshin area, and an antalgic gait.
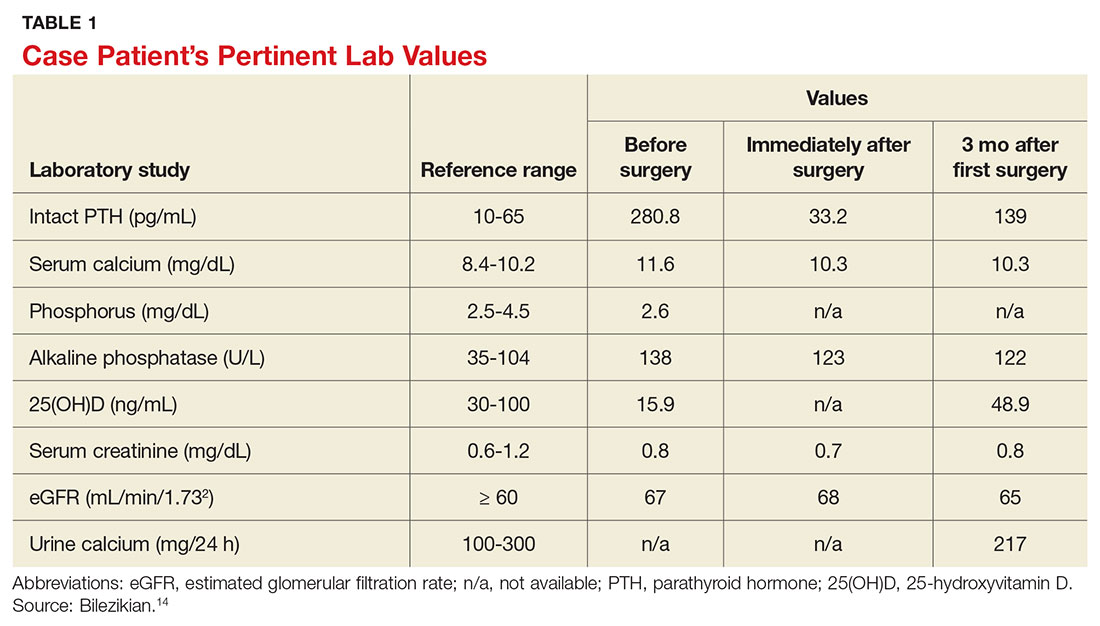
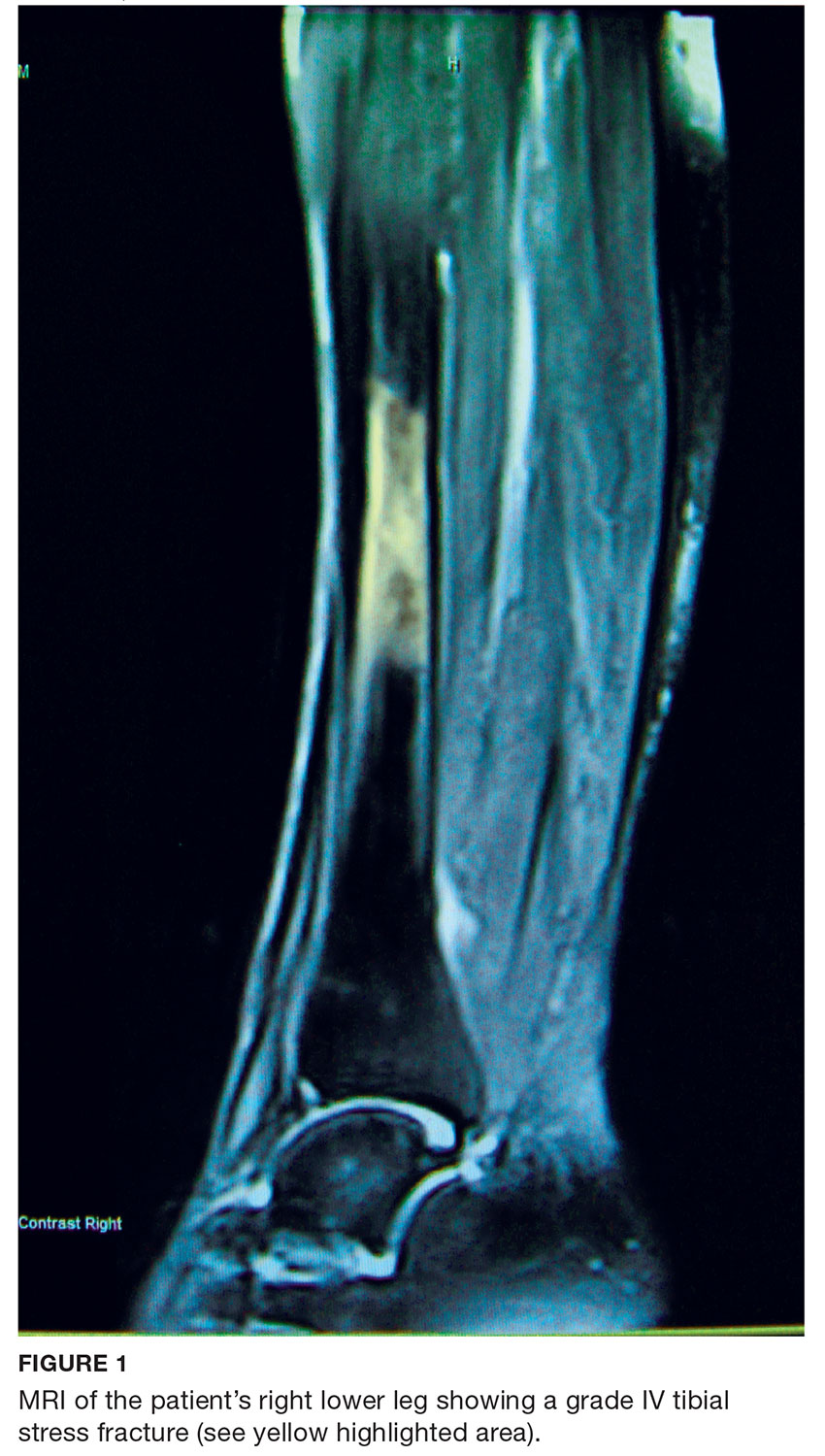
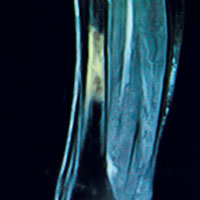
Laboratory work-up demonstrated elevated PTH, alkaline phosphatase, and calcium levels and a low 25-hydroxyvitamin D [25(OH)D] level (see Table 1). MRI of the right lower leg revealed a grade IV stress fracture (see Figure 1). The elevated serum calcium and PTH levels in addition to abnormal bone density findings led to the diagnosis of PHPT. She was referred to endocrinology and orthopedics for management of PHPT and the stress fracture, respectively, and was placed in an orthopedic walking boot for treatment of the midtibial stress fracture.
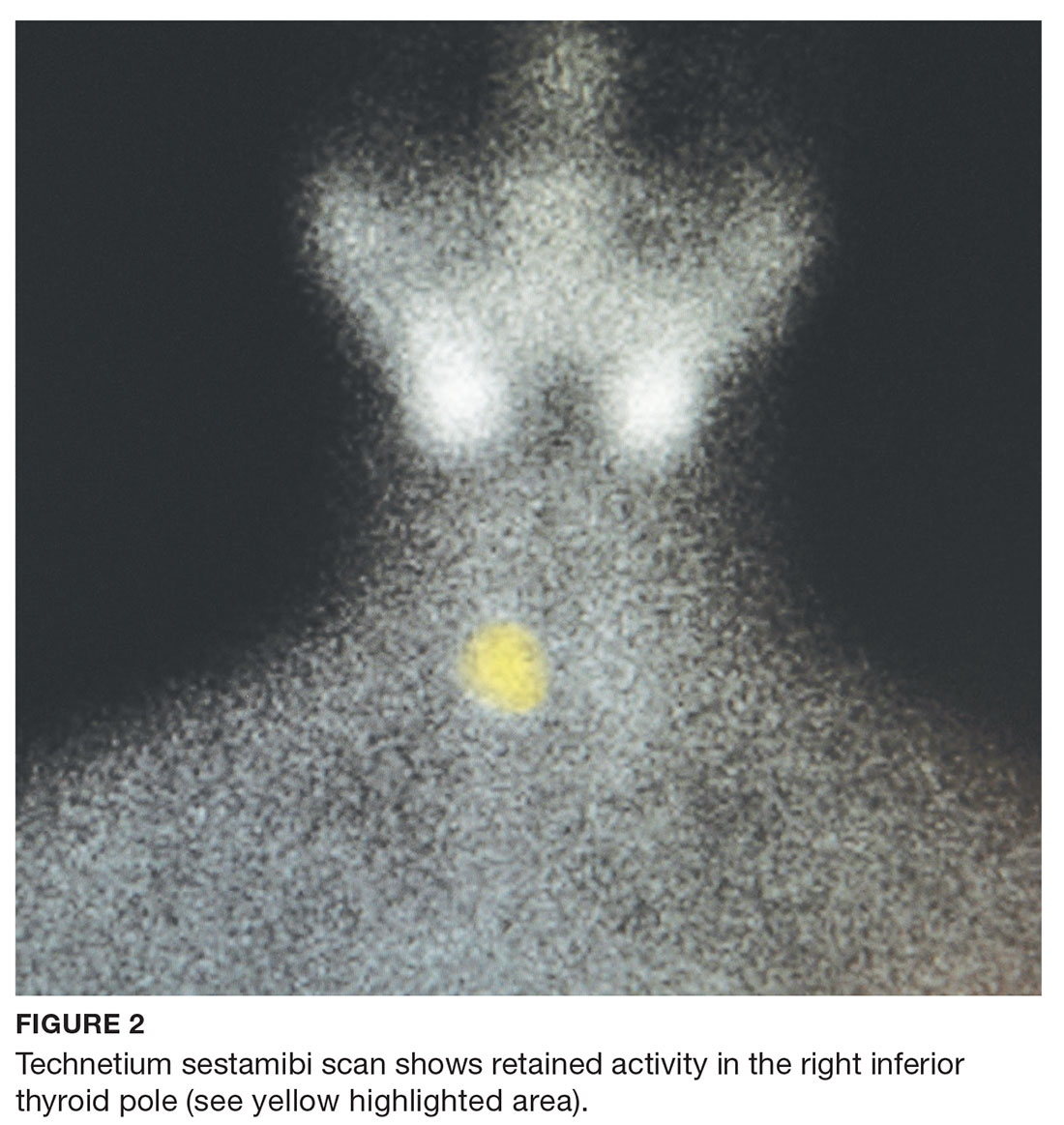
The endocrinologist referred her to an otolaryngologist trained in the surgical management of parathyroid adenomas, who ordered a thyroid ultrasound; this study was inconclusive. Additional imaging, including a Tc-99m sestamibi parathyroid scan and CT with contrast of the soft tissue of the neck, was obtained. The parathyroid scan of the neck and upper chest showed retained activity in the right inferior thyroid pole that was concerning for a parathyroid adenoma (see Figure 2). The CT identified a 1.5-cm parathyroid adenoma off the right inferior pole of the thyroid gland (concordant with the parathyroid scan). A single 300-mg parathyroid adenoma was removed from the right inferior pole of the thyroid. The surgery was deemed successful, with intraoperative normalization of the PTH level.
The patient was managed postoperatively by the endocrinologist and was started on calcium and vitamin D supplements. She was prescribed a bisphosphonate, as she had refused to take alendronate following her abnormal BMD test.
DIFFERENTIAL DIAGNOSIS
PHPT and malignancy are the most common causes of hypercalcemia, accounting for 90% of cases.2,4 A less common cause is familial hypocalciuric hypercalcemia (FHH), a rare benign disorder that imitates PHPT.1 FHH is ruled out by measurement of 24-hour urine calcium excretion and is characterized by hypocalciuria, defined as a urine calcium level of less than 100 mg/24 h (reference range, 100-300 mg/24 h).2 Low calcium excretion can also be identified by a calcium-creatinine excretion ratio.2 FHH is a benign autosomal dominant condition caused by a heterozygous mutation of the parathyroid glands’ calcium-sensing receptors.2,5,6 Young adults with FHH are asymptomatic, and mild hypercalcemia and a normal or slightly elevated PTH are the only laboratory findings.4
Measuring PTH levels is key in determining the underlying mechanism of hypercalcemia.2,7 If the hypercalcemia is not PTH-mediated, malignancy and granulomatous diseases such as sarcoidosis must be considered.2,7 PTH is suppressed in malignancy except for rare cases of PTH-producing tumors.4 Bone metastases cause calcium resorption, and sarcoidosis causes an excess of vitamin D, both resulting in hypercalcemia. Lymphomas and sarcoid granulomas express 1α-hydroxylase, an enzyme that increases the conversion of 25(OH)D to 1,25-dihydroxyvitamin D [1,25(OH)2D].2 When malignancy is suspected, it is appropriate to check a 1,25(OH)2D level. Thiazide diuretics, such as hydrochlorothiazide, decrease urinary calcium excretion and may result in mild hypercalcemia.2 Other possibilities in the differential include hypervitaminosis A or D, dehydration, and excess calcium ingestion, but these are less common.6,7
CALCIUM REGULATION
The parathyroid glands stem from four poles on the back of the thyroid gland; there are typically four, but the number can vary from two to 11. Secreted PTH, the primary regulator of calcium homeostasis, maintains calcium levels within a narrow physiologic range.2,8 PTH increases bone resorption, stimulating release of calcium into the blood, and signals the kidneys to increase reabsorption of calcium and excrete phosphorus. It also converts 25(OH)D to 1,25(OH)2 D, the active form of vitamin D that increases gastrointestinal calcium absorption. In a negative feedback loop, PTH secretion is regulated by serum calcium levels, stimulated when levels are low and suppressed when levels are high (see Figure 3).3 Calcium-sensing receptors, located in the chief cells of parathyroid tissue, are essential to calcium homeostasis. These receptors will either increase or decrease PTH release in response to small changes in blood ionized calcium levels. The receptors also play an independent role in the renal tubules by promoting secretion of calcium in the setting of hypercalcemia.5,9 The precise regulation of intracellular and extracellular calcium is necessary for normal functioning of physiologic processes, including bone metabolism, hormone release and regulation, neuromuscular function, and cell signaling.5

PATHOPHYSIOLOGY
Hyperparathyroidism is defined as excess secretion of PTH and is categorized as primary, secondary, or tertiary based on pathophysiologic mechanisms.
Primary hyperparathyroidism
PHPT is defined as PTH levels that are elevated or inappropriately normal in patients with hypercalcemia and no known history of kidney disease.2,6 This occurs when the normal feedback mechanism fails to inhibit excess hormone secretion by one or more of the parathyroid glands.6 With uninhibited PTH secretion, hypercalcemia will result from increased gastrointestinal absorption and bone resorption.
The most common causes of PHPT are an abnormal proliferation of parathyroid cells (parathyroid adenomas) and parathyroid tissue overgrowth (hyperplasia). PHPT may result from a single parathyroid adenoma (80%-90%), multigland hyperplasia (10%-15%), multiple adenomas (2%-3%), or malignancy (< 1%).6,10 Adenomas can occur sporadically or less commonly as part of an inherited syndrome.1 It is estimated that more than 10% of patients with PHPT have a mutation in one of 11 genes associated with PHPT.11 Approximately 5% of PHPT cases are familial, resulting from adenomas or carcinomas associated with mutations in the tumor suppressor genes MEN1 and CDC73 and the RET proto-oncogene.5 Multiple endocrine neoplasia (MEN) syndrome type 1 or 2a is associated with the development of parathyroid adenomas and other endocrine tumors.1,5 Mutations in the CDC73 gene can lead to parathyroid cancer, familial isolated hyperparathyroidism, and familial hyperparathyroidism-jaw tumor syndrome.5 Parathyroid cancer is rare and is linked to a history of radiation to the head and neck.3 Ectopic parathyroid adenomas represent 3% to 4% of all parathyroid adenomas and are often found in the mediastinum.12PHPT is the third most common endocrine disorder, with a prevalence of 1 case per 1,000 men and 2 to 3 cases per 1,000 women.5 Most women with PHPT are postmenopausal and older than 50.1 The condition can occur in younger adults but is rare in childhood and adolescence, with an incidence of 2 to 5 cases per 100,000.13
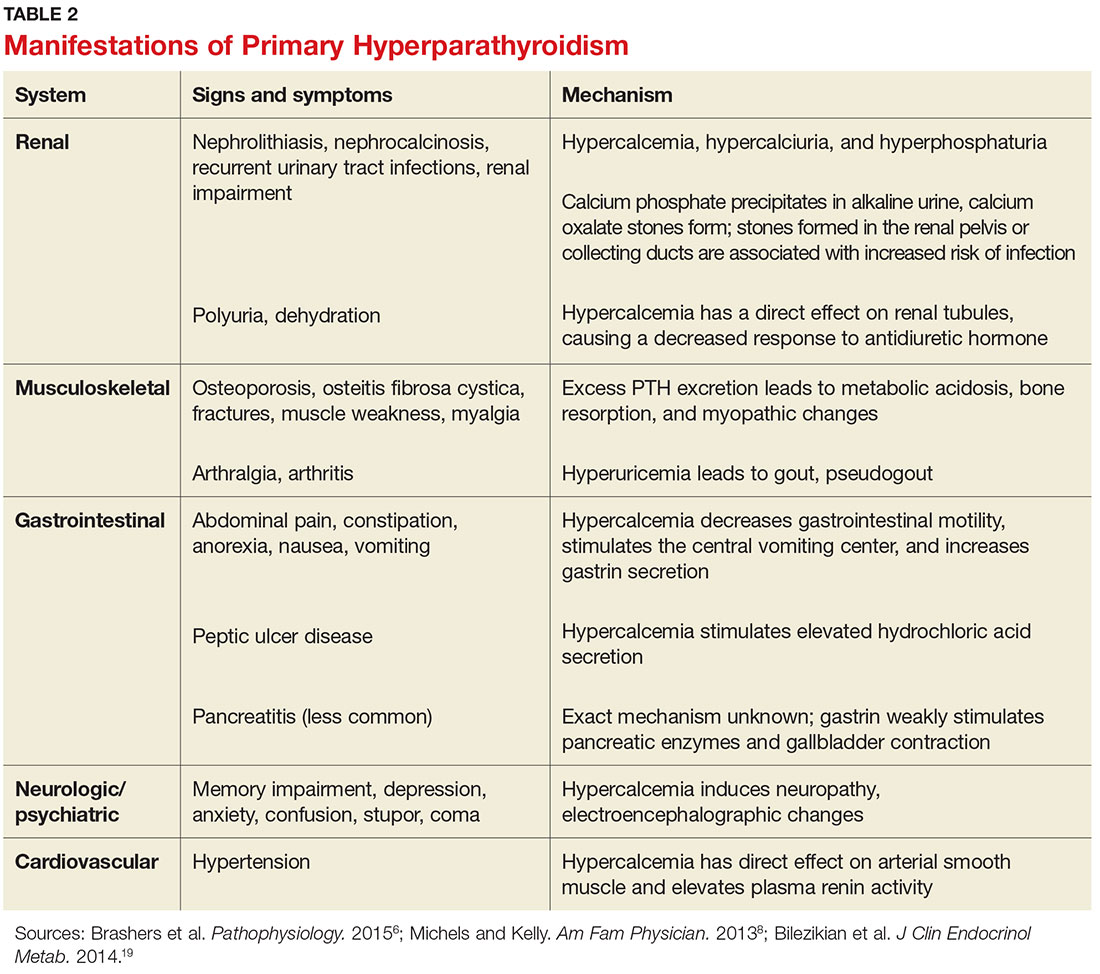
PHPT affects multiple organ systems, but the most commonly involved are the renal and musculoskeletal systems (see Table 2). The hypersecretory state causes excessive bone resorption and increased osteoclastic activity, resulting in osteoporosis and increased risk for pathologic fractures of the hip, wrist, and spine. The most common osteoporotic fractures are vertebral compression fractures.14 Fractures involving the thoracic spine contribute to the development of kyphosis.15
In the kidney, an increased filtered load of calcium leads to hypercalciuria, precipitation of calcium phosphate in the renal pelvis and collecting ducts, metabolic acidosis, alkaline urine, and hyperphosphaturia. The combination of alkaline urine, hyperphosphaturia, and hypercalciuria leads to the formation of kidney stones.6 Nephrolithiasis and alkaline urine predispose patients to recurrent urinary tract infections and subsequent renal impairment.6 In addition, hypercalcemia impairs the renal collecting system and decreases its response to antidiuretic hormone, resulting in polyuria.6
Secondary hyperparathyroidism
In secondary hyperparathyroidism, calcium levels are either normal or low. Normocalcemic hyperparathyroidism is characterized by normal ionized and total calcium levels and elevated PTH levels; it has no known cause.6 Secondary hyperparathyroidism occurs when excess PTH is excreted as a result of a chronic condition that leads to hypocalcemia. Examples of these disease states include vitamin D deficiency, chronic kidney disease (CKD), and intestinal malabsorption. The most common cause of secondary hyperparathyroidism is CKD; glomerular filtration insufficiency results in hyperphosphatemia, hypocalcemia, and low 1,25(OH)2D, stimulating the release of PTH. Other causes include deficient intake or decreased absorption of calcium or vitamin D; chronic use of medications such as lithium, phenobarbital, or phenytoin; bariatric surgery; celiac disease; and pancreatic disease.4,6,14 Lithium decreases urinary calcium excretion and reduces the sensitivity of the parathyroid gland to calcium.4
Tertiary hyperparathyroidism
Tertiary hyperparathyroidism, marked by hypercalcemia and excessive PTH secretion, can occur after prolonged secondary hyperparathyroidism. In this disorder, persistent parathyroid stimulation leads to gland hyperplasia, resulting in autonomous production of PTH despite correction of calcium levels.6 It most commonly occurs in patients with chronic secondary hyperparathyroidism with renal failure who receive a kidney transplant.2,6 In some cases, parathyroid hyperplasia may not regress after transplantation and parathyroidectomy may be necessary.
EVALUATION AND DIAGNOSTIC WORK-UP
Laboratory tests
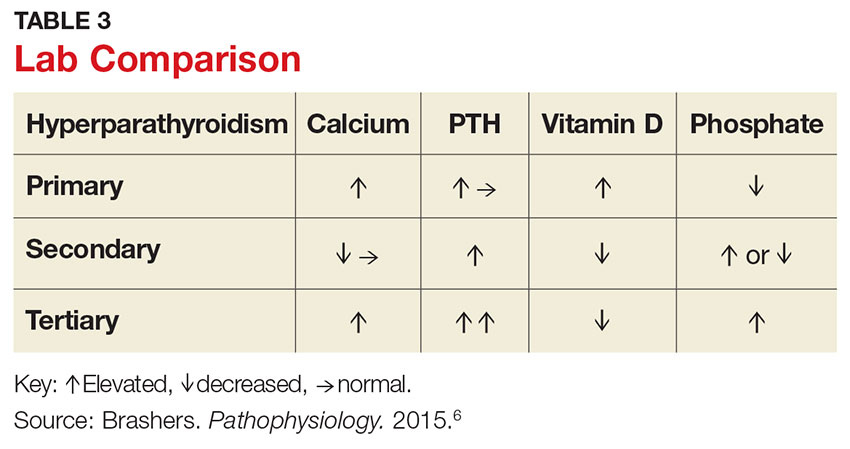
Hypercalcemia is the most common initial finding that leads to the diagnosis of PHPT. Elevated serum calcium and PTH is characteristic of the condition. When evaluating a patient with hypercalcemia, the diagnostic work-up includes tests to differentiate between PTH- and non–PTH-mediated causes of elevated calcium (see Table 3).7 Evaluation should begin with measurement of PTH by second- or third-generation immunoassay along with phosphorus, alkaline phosphatase, 25(OH)D, creatinine, estimated glomerular filtration rate (eGFR), and albumin. Additionally, a 24-hour urine collection for calcium, creatinine, and creatinine clearance should be considered in patients with overt nephrolithiasis or nephrocalcinosis. If the urine calcium is > 400 mg/24 h, a renal stone risk profile is indicated because nephrolithiasis is one of the most common complications of PHPT.14 There is a high prevalence of nephrolithiasis in patients with normocalcemic PHPT, even after parathyroidectomy.16 If the 24-hour urine calcium level is low, the diagnosis of FHH is considered. If the urine calcium is high and the intact PTH is elevated or inappropriately normal, the diagnosis of PHPT is considered; urine calcium will be normal in 60% of PHPT cases.4,11
Imaging studies
Imaging is useful for localization of adenomas and abnormal parathyroid tissue to guide surgical planning but is not necessary for diagnosis or medical management. Understanding the strengths and weaknesses of imaging modalities enables the clinician to order the most appropriate option. There are three primary imaging modalities used to locate parathyroid adenoma(s) or aberrant parathyroid tissue: ultrasound, nuclear medicine sestamibi parathyroid scans, and CT. Some clinicians start with an ultrasound, but its operator-dependent results can vary widely; in addition, ultrasound often provides poor anatomic definition and has limited value in locating ectopic parathyroid tissue.17
Nuclear medicine parathyroid scan with technetium-99m sestamibi is a sensitive method for localizing hyperfunctioning, enlarged parathyroid glands or tissue in normal anatomic positions or ectopic locations. Uptake is enhanced and prolonged in parathyroid adenomas as well as in aberrant tissue found in the mediastinum or subclavicular areas. Sestamibi parathyroid scan detects up to 89% of single adenomas, but studies of this imaging modality have demonstrated a wide range of sensitivities (44%-95%).5,17 A drawback of nuclear medicine studies is that they provide little anatomic detail.17 Nonetheless, the ability of the parathyroid scan to locate parathyroid glands has contributed to the success of the minimally invasive parathyroidectomy, and it is considered the most successful imaging modality available.5,10 Identifying the precise location of the parathyroid adenoma is essential for a successful surgical outcome; this is best achieved by combining the sestamibi parathyroid scan with CT.12
Emerging imaging modalities are the multidetector CT (MDCT) and 4D-CT techniques. In an evaluation of the diagnostic accuracy of contrast-enhanced MDCT in the detection of parathyroid adenomas and aberrant parathyroid tissue, MDCT demonstrated the ability to differentiate between adenomas and hyperplasia and display important anatomic structures such as nerves and blood vessels.17 The specificity of MDCT for ruling out abnormal parathyroid tissue was 75%, and the sensitivity for detecting a single adenoma was 80%. Overall, MDCT demonstrated an 88% positive predictive value (PPV) in localizing hyperfunctioning parathyroid glands but showed poor sensitivity in detecting multigland disease.17 The PPV is a key value in determining the ability of an imaging study to precisely locate aberrant parathyroid tissue. MDCT provides detailed definition of anatomy, locating ectopic parathyroid glands in the deeper paraesophageal areas and mediastinum while defining relationships between the tissue and its surrounding vasculature, lymph nodes, and thyroid tissue.17 The 4D-CT technique employs three-dimensional technology and accounts for the movement of the patient’s body over time (the “fourth dimension”). It is an accurate method for identifying parathyroid adenomas but exposes the patient to higher radiation doses.18 The sensitivity of 4D-CT in localizing abnormal parathyroid tissue is comparable to that of MDCT.16,18,19
Additional studies used during the management of the patient with PHPT are BMD testing and renal imaging. Secondary causes of bone loss are responsible for up to 30% of osteoporosis cases in postmenopausal women; one of these causes is PHPT.20 Elevated PTH causes increased bone turnover and results in decreased bone mass with subsequent increased fracture risk.9 Bone density should be measured by dual-energy x-ray absorptiometry (DEXA), and the skeletal survey should include the distal one-third of the radius, hip, and lumbar spine. The distal radius is rich in cortical bone and BMD is often lowest at this site in patients with PHPT, making it the most sensitive DEXA marker for early detection of bone loss.19,21 The hip contains an equal mix of cortical and trabecular bone and is the second most sensitive site for detecting bone loss in PHPT. The spine contains a high proportion of trabecular bone and is the least sensitive site.19,21 Renal imaging studies, including x-ray, ultrasound, and, less frequently, CT of the abdomen and pelvis, are used to assess for nephrolithiasis and nephrocalcinosis.19
TREATMENT/MANAGEMENT
Conservative medical management
PHPT is a complex disease process, and careful evaluation is required when determining whether medical versus surgical management is appropriate. Clinical presentation ranges from no symptoms to multisystem disease. Conservative medical management, which includes regular monitoring, is an acceptable strategy in an asymptomatic patient with a low fracture risk and no nephrolithiasis.1 Conservative care includes maintaining normal dietary calcium intake and adequate hydration, regular exercise, vitamin D supplementation, annual laboratory studies, BMD testing, and the avoidance of thiazide diuretics and lithium.1 Guidelines, from the Fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism, for monitoring asymptomatic PHPT patients recommend
- Annual measurement of serum calcium
- BMD measurement by DEXA every 1 to 2 years
- Annual assessment of eGFR and serum creatinine
- Renal imaging or a 24-h urine stone profile if nephrolithiasis is suspected.19
Long-term medical management of PHPT is difficult because no agents are available to suppress hypercalcemia or completely block PTH release.12
Maintaining serum 25(OH)D at a level > 20 ng/mL significantly reduces PTH secretion, in comparison to levels < 20 ng/mL, and does not aggravate hypercalcemia.22 The Endocrine Society recommends a minimum serum 25(OH)D level of 20 ng/mL and notes that targeting a higher threshold value of 30 ng/mL is reasonable.19 The daily requirement for vitamin D3, 800 IU to 1,000 IU, is a good starting point for supplementation.4 Measurement of 1,25(OH)2D levels lacks value and is not recommended for patients with PHPT. Calcium intake should follow established guidelines and is not limited in PHPT.19
Surgical management
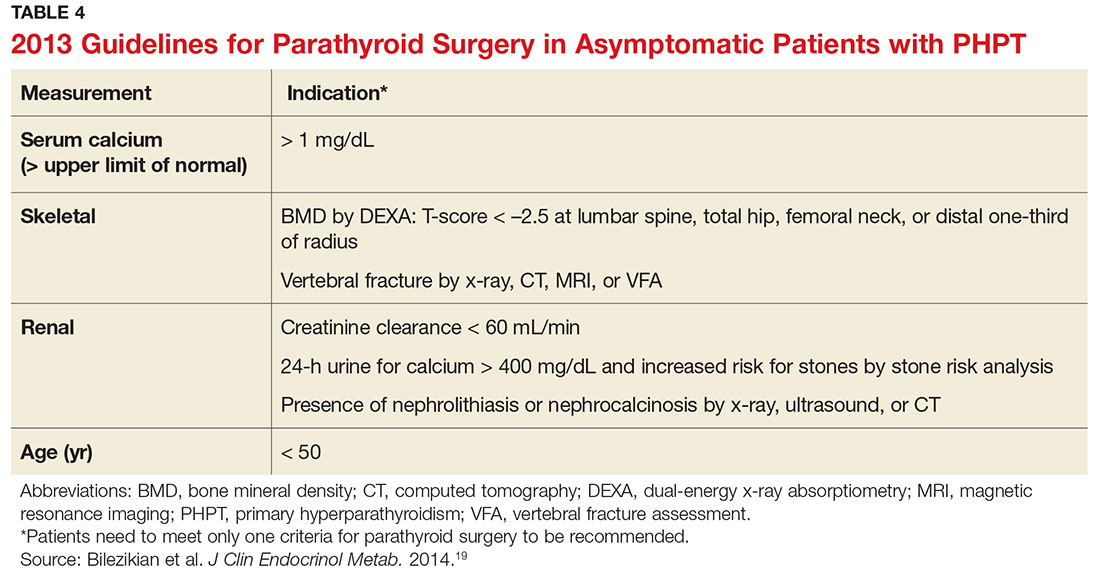
Surgical management is indicated for symptomatic patients.23 Indications include nephrolithiasis, nephrocalcinosis, osteitis fibrosa cystica, or osteoporosis. Surgery is considered appropriate for individuals who do not meet these criteria if there are no medical contraindications.14 The Fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism revised the indications for surgery in 2014 to include asymptomatic patients, since surgery is the only definitive treatment for PHPT. Current guidelines for when to recommend surgery in the asymptomatic patient with PHPT are listed in Table 4.19
Preoperative localization and referral to an experienced surgeon is of utmost importance for a good outcome. An expert surgeon will usually perform a minimally invasive parathyroidectomy (MIP) and obtain intraoperative PTH levels; in some cases, a full neck exploration is necessary. PTH has a half-life of less than five minutes and is an accurate tool for determining whether the culprit gland has been successfully removed.5
Modern imaging studies, less invasive surgical techniques, and intraoperative measurements of PTH have decreased the need to conduct full neck exploration. MIP offers a smaller incision, less tissue dissection, and lower morbidity, and can be offered without the risks associated with general anesthesia.10 The goal of surgery is to restore normocalcemia and, in turn, prevent bone mineral loss and systemic effects of hypercalcemia over the long term.10 Surgical management for an ectopic parathyroid adenoma is controversial because these are often found in the mediastinum, requiring invasive surgery.12
Surgery is curative in up to 95% of cases and has a low rate of complications.2,24 A joint decision regarding treatment options is made among the patient, primary care clinician, and surgeon. Complications include vocal cord paralysis resulting from injury to the recurrent laryngeal nerve, bleeding or hematoma, laryngospasm, symptomatic hypocalcemia, and persistent hyperparathyroidism; seizures are very rare but can occur from transient hypocalcemia and hypomagnesemia.5 PTH levels drop by more than 50% intraoperatively if the procedure is successful; otherwise, exploration for another adenoma is indicated.10 Postoperative calcium and vitamin D supplementation are warranted once lab values are stable.
When surgery is contraindicated/refused
If surgery is indicated but the patient is a poor candidate or refuses surgery, management of hypercalcemia and bone loss with pharmacologic agents is warranted. The calcimimetic cinacalcet is a reasonable medical alternative that has been shown to adequately control hypercalcemia and hypophosphatemia and has proven effective in various patient subgroups.25 This agent is useful in the treatment of patients who are asymptomatic and refuse surgery, patients with refractory PHPT after parathyroidectomy, and patients with contraindications to surgery.24,25 The medication reduces calcium and modestly reduces PTH levels by binding parathyroid calcium-sensing receptors but does not improve bone density.2,12 Cinacalcet is approved by the FDA for use in patients with moderate to severe disease when surgery is contraindicated.24
Treatment options for osteoporosis, vertebral fractures, and progressive bone loss in the patient with PHPT include bisphosphonates. Raloxifene and estrogen replacements may be used in postmenopausal women. Oral bisphosphonates (alendronate or risedronate) are firstline therapies and have been shown to inhibit progression to osteoporosis in PHPT.9,26 They prevent osteoclastic activity, reducing bone resorption and turnover. Contraindications to oral bisphosphonates include esophageal disorders, gastrointestinal intolerance, or inability to follow the dosing requirements. Intravenous zoledronic acid provides an alternative route of administration.
Alendronate has the best evidence for improving bone density and preventing progression to osteoporosis in patients with PHPT, but the medication does not affect calcium or PTH levels.1,19 There is limited data on the effects of combining bisphosphonates with calcimimetics. Raloxifene is a selective estrogen receptor modulator that decreases bone resorption; it is approved for treating osteoporosis and may be used when a patient is not a good candidate for a bisphosphonate.20 Denosumab, currently under study for the treatment of PHPT, is a human monoclonal antibody that improves bone density but does not affect serum calcium.20 Nonpharmacologic therapies include alcohol moderation, decreased caffeine intake, weight-bearing exercise, smoking cessation, adequate hydration, and dietary modifications.20
OUTCOME FOR THE CASE PATIENT
Although PHPT is often discovered incidentally in routine blood work with hypercalcemia, the case patient had developed osteoporosis and a grade IV tibial stress fracture before the diagnosis was made. Following parathyroidectomy, her hypertension worsened, requiring an additional antihypertensive medication. She developed recurrent disease and was referred to a tertiary care center for revision parathyroidectomy due to persistent elevated calcium levels. A 24-hour urine calcium test ruled out concurrent FHH. A full neck exploration was conducted and a 340-mg hypercellular parathyroid gland was removed from the left superior pole. She will be monitored for recurrent disease and will remain on a vitamin D3 supplement and treatment for osteoporosis.
CONCLUSION
Primary care clinicians should have a low threshold for initiating the work-up of mild hypercalcemia in an effort to prevent sequelae. Patient education is essential throughout the process. Understanding the condition and treatment options is necessary for a patient’s active participation in clinical decision making. Conservative management of an asymptomatic patient includes avoiding thiazide diuretics and lithium, staying well hydrated with water, maintaining moderate dietary calcium (1,000-1,200 mg/d) and vitamin D (400-600 IU/d) intake, regular exercise, and appropriate lab and bone density monitoring. Surgical treatment is recommended for symptomatic patients exhibiting decreased bone density, fractures, renal impairment, or nephrolithiasis. Treating bone loss with bisphosphonates and hypercalcemia with calcimimetics is useful. Postmenopausal women may benefit from estrogen therapy or selective estrogen receptor modulators. These agents improve bone density and lower calcium, but are often contraindicated or have adverse effects. Surgery is the only cure.3
1. Turner J. Hypercalcaemia and primary hyperparathyroidism. Medicine. 2009;37(9):461-464.
2. Crowley R, Gittoes N. How to approach hypercalcaemia. Clin Med. 2013;13(3):287-290.
3. Kapustin JF, Schofield DL. Hyperparathyroidism: an incidental finding. Nurse Pract. 2012;37(11):9-14.
4. Cordellat IM. Hyperparathyroidism: primary or secondary disease? Rheumatol Clin. 2012;8(5):287-291.
5. MacKenzie-Feder J, Sirrs S, Anderson D, Sharif J, Khan A. Primary hyperparathyroidism: an overview. Int J Endocrinol. 2011;2011:251410.
6. Brashers VL, Jones RE, Huether SE. Alterations of hormonal regulation. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 7th ed. St. Louis, MO: Mosby; 2015:731-733.
7. Osborne JL, Klocko DJ. Woman, 66, with persistent abdominal and back pain. Clinician Reviews. 2014;24(11):34-37, 40.
8. Michels TC, Kelly KM. Parathyroid disorders. Am Fam Physician. 2013;88(4):249-257.
9. Rolighed L, Rejnmark L, Christiansen P. Bone involvement in primary hyperparathyroidism and changes after parathyroidectomy. US Endocrinol. 2013;9(2):181-184.
10. Karahan Ö, Okus A, Sevinç B, et al. Minimally invasive parathyroidectomy under local anesthesia. J Postgrad Med. 2013;59(1):21-24.
11. Fuleihan GE, Silverberg SJ. Primary hyperparathyroidism: diagnosis, differential diagnosis, and evaluation. Up-to-Date. www.uptodate.com/contents/primary-hyperparathyroidism-diagnosis-differential-diagnosis-and-evaluation. Accessed April 20, 2017.
12. Panchani R, Varma T, Goyal A, et al. A challenging case of an ectopic parathyroid adenoma. Indian J Endocrinol Metab. 2012;16:S408-S410.
13. Otasowie J, Hambleton BA. Aggression and homicidal thoughts in a patient with primary hyperparathyroidism: a case report. Br J Medical Pract. 2013;6(4):a630.
14. Bilezikian JP. Primary hyperparathyroidism: new insights, concepts and guidelines. Presented at: American Association of Clinical Endocrinologists 24th Annual Scientific and Clinical Congress; May 13-17, 2015; Nashville, TN. am2015.aace.com/presentations/Friday/F31/PrimaryHyperparathyroidismNew InsightsConceptsandGuidelines.pdf. Accessed April 20, 2017.
15. Crowther-Radulewicz CL, McCance KL. Alterations of musculoskeletal function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 7th ed. St. Louis, MO: Mosby; 2015:1551-1555.
16. Amaral LM, Queiroz DC, Marques TF, et al. Clinical study: normocalcemic versus hypercalcemic primary hyperparathyroidism: more stone than bone? J Osteoporos. 2012;2012:128352.
17. Linda DD, Ng B, Rebello R, et al. The utility of multidetector computed tomography for detection of parathyroid disease in the setting of primary hyperparathyroidism. Can Assoc Radiol J. 2012;63(2):100-108.
18. Bann DV, Zacharia T, Goldenberg D, Goyal, N. Parathyroid localization using 4-D-computed tomography. Ear Nose Throat J. 2015;94(4-5):E55-E57.
19. Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3561-3569.
20. Jeremiah MP, Unwin BK, Greenawald MH, Casiano VE. Diagnosis and management of osteoporosis. Am Fam Physician. 2015;92(4):261-268.
21. Wood K, Dhital S, Chen H, Sippel RS. What is the utility of distal forearm DXA in primary hyperparathyroidism? Oncologist. 2012;17(3):322-325.
22. Jayasena CN, Modi M, Palazzo F, et al. Associations of serum 25-hydroxyvitamin D with circulating PTH, phosphate and calcium in patients with primary hyperparathyroidism. Clin Endocrinol (Oxf). 2013;78(6):838-843.
23. Grey A. Nonsurgical management of mild primary hyperparathyroidism—a reasonable option. Clin Endocrinol. 2012;77(5):639-644.
24. Saponaro F, Faggiano A, Grimaldi F, et al. Cinacalcet in the management of primary hyperparathyroidism: post marketing experience of an Italian multicenter group. Clin Endocrinol (Oxf). 2013;79(1):20-26.
25. Rothe HM, Liangos O, Biggar P, et al. Cinacalcet treatment of primary hyperparathyroidism. Int J Endocrinol. 2011;2011:415719.
26. Farag N, Delbanco T, Strewler GJ. Update: a 64-year-old woman with primary hyperparathyroidism. JAMA. 2008;300(17):2044-2045.
CE/CME No: CR-1705
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Differentiate primary hyperparathyroidism (PHPT) from other causes of hypercalcemia and types of hyerparathyroidism.
• Understand the calcium-parathyroid hormone feedback loop.
• Identify appropriate imaging studies and common laboratory findings in the patient with PHPT.
• Describe the common systemic manifestations of PHPT.
• Discuss medical versus surgical management of the patient with PHPT.
FACULTY
Barbara Austin is a Family Nurse Practitioner at Baptist Primary Care, Jacksonville, Florida, and is pursuing a Doctorate of Nursing Practice (DNP) at Jacksonville University.
The author has no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2017.
Article begins on next page >>
Primary hyperparathyroidism (PHPT) is most often detected as hypercalcemia in an asymptomatic patient during routine blood work. Knowing the appropriate work-up of hypercalcemia is essential, since untreated PHPT can have significant complications affecting multiple organ systems—most notably, renal and musculoskeletal. Parathyroidectomy is curative in up to 95% of cases, but prevention of long-term complications relies on prompt recognition and appropriate follow-up.
Primary hyperparathyroidism (PHPT) is a common endocrine disorder, with a prevalence of approximately 1 to 3 cases per 1,000 persons.1 PHPT results from inappropriate overproduction of parathyroid hormone (PTH), the primary regulator of calcium homeostasis, and is characterized by hypercalcemia in the setting of an elevated or high-normal PTH level. In most cases of PHPT, unregulated PTH production is caused by a single parathyroid adenoma.
PHPT is the most common cause of hypercalcemia in outpatients and is typically diagnosed following incidental discovery during routine blood work in an asymptomatic patient.1,2 It is two to three times more common in women than in men, and incidence increases with age; as such, postmenopausal women are most commonly affected.1,3 PHPT often has an insidious course, and recognition of its clinical manifestations followed by appropriate diagnostic work-up and management are necessary to prevent sequelae.3
PATIENT PRESENTATION
A 68-year-old Caucasian woman presented to her family practice office for a third visit with continued complaints of nontraumatic right lower leg pain. She had previously been diagnosed with tendonitis, which was treated conservatively. The pain failed to improve, and an x-ray was ordered. The x-ray revealed no acute findings but did show osteopenia, prompting an order for a bone mineral density (BMD) test. The BMD test demonstrated osteoporosis, which warranted further investigation. She was prescribed alendronate but refused it, against medical advice, due to concern over potential adverse effects.
Her medical history included hyperlipidemia and hypertension under fair control with lisinopril. She took a low-dose aspirin and flaxseed supplement daily. She also had a history of radiation to the neck, having undergone tonsillar irradiation as a child (a common practice from the 1930s through the 1950s).3 Surgical history included a total hysterectomy with bilateral salpingo-oophorectomy, appendectomy, and tonsillectomy. There was no personal or family history of cancer or endocrine disorders, hypercalcemia, or nephrolithiasis. She was up to date on vaccines and preventive health care measures. Allergies included penicillin and sulfa, both resulting in hives. She was a nonsmoker and did not drink alcohol or engage in illicit drug use.
Review of systems revealed right lower anterior leg pain for four months, characterized as aching, deep, sharp, and throbbing with radiation to the ankle. The pain was worse with activity and prolonged standing; ibuprofen and application of ice provided partial relief. She had experienced some mood changes, including irritability. Physical exam was normal except for dominant right-sided thyromegaly, marked bony point tenderness to the right midshin area, and an antalgic gait.
Laboratory work-up demonstrated elevated PTH, alkaline phosphatase, and calcium levels and a low 25-hydroxyvitamin D [25(OH)D] level (see Table 1). MRI of the right lower leg revealed a grade IV stress fracture (see Figure 1). The elevated serum calcium and PTH levels in addition to abnormal bone density findings led to the diagnosis of PHPT. She was referred to endocrinology and orthopedics for management of PHPT and the stress fracture, respectively, and was placed in an orthopedic walking boot for treatment of the midtibial stress fracture.
The endocrinologist referred her to an otolaryngologist trained in the surgical management of parathyroid adenomas, who ordered a thyroid ultrasound; this study was inconclusive. Additional imaging, including a Tc-99m sestamibi parathyroid scan and CT with contrast of the soft tissue of the neck, was obtained. The parathyroid scan of the neck and upper chest showed retained activity in the right inferior thyroid pole that was concerning for a parathyroid adenoma (see Figure 2). The CT identified a 1.5-cm parathyroid adenoma off the right inferior pole of the thyroid gland (concordant with the parathyroid scan). A single 300-mg parathyroid adenoma was removed from the right inferior pole of the thyroid. The surgery was deemed successful, with intraoperative normalization of the PTH level.
The patient was managed postoperatively by the endocrinologist and was started on calcium and vitamin D supplements. She was prescribed a bisphosphonate, as she had refused to take alendronate following her abnormal BMD test.
DIFFERENTIAL DIAGNOSIS
PHPT and malignancy are the most common causes of hypercalcemia, accounting for 90% of cases.2,4 A less common cause is familial hypocalciuric hypercalcemia (FHH), a rare benign disorder that imitates PHPT.1 FHH is ruled out by measurement of 24-hour urine calcium excretion and is characterized by hypocalciuria, defined as a urine calcium level of less than 100 mg/24 h (reference range, 100-300 mg/24 h).2 Low calcium excretion can also be identified by a calcium-creatinine excretion ratio.2 FHH is a benign autosomal dominant condition caused by a heterozygous mutation of the parathyroid glands’ calcium-sensing receptors.2,5,6 Young adults with FHH are asymptomatic, and mild hypercalcemia and a normal or slightly elevated PTH are the only laboratory findings.4
Measuring PTH levels is key in determining the underlying mechanism of hypercalcemia.2,7 If the hypercalcemia is not PTH-mediated, malignancy and granulomatous diseases such as sarcoidosis must be considered.2,7 PTH is suppressed in malignancy except for rare cases of PTH-producing tumors.4 Bone metastases cause calcium resorption, and sarcoidosis causes an excess of vitamin D, both resulting in hypercalcemia. Lymphomas and sarcoid granulomas express 1α-hydroxylase, an enzyme that increases the conversion of 25(OH)D to 1,25-dihydroxyvitamin D [1,25(OH)2D].2 When malignancy is suspected, it is appropriate to check a 1,25(OH)2D level. Thiazide diuretics, such as hydrochlorothiazide, decrease urinary calcium excretion and may result in mild hypercalcemia.2 Other possibilities in the differential include hypervitaminosis A or D, dehydration, and excess calcium ingestion, but these are less common.6,7
CALCIUM REGULATION
The parathyroid glands stem from four poles on the back of the thyroid gland; there are typically four, but the number can vary from two to 11. Secreted PTH, the primary regulator of calcium homeostasis, maintains calcium levels within a narrow physiologic range.2,8 PTH increases bone resorption, stimulating release of calcium into the blood, and signals the kidneys to increase reabsorption of calcium and excrete phosphorus. It also converts 25(OH)D to 1,25(OH)2 D, the active form of vitamin D that increases gastrointestinal calcium absorption. In a negative feedback loop, PTH secretion is regulated by serum calcium levels, stimulated when levels are low and suppressed when levels are high (see Figure 3).3 Calcium-sensing receptors, located in the chief cells of parathyroid tissue, are essential to calcium homeostasis. These receptors will either increase or decrease PTH release in response to small changes in blood ionized calcium levels. The receptors also play an independent role in the renal tubules by promoting secretion of calcium in the setting of hypercalcemia.5,9 The precise regulation of intracellular and extracellular calcium is necessary for normal functioning of physiologic processes, including bone metabolism, hormone release and regulation, neuromuscular function, and cell signaling.5

PATHOPHYSIOLOGY
Hyperparathyroidism is defined as excess secretion of PTH and is categorized as primary, secondary, or tertiary based on pathophysiologic mechanisms.
Primary hyperparathyroidism
PHPT is defined as PTH levels that are elevated or inappropriately normal in patients with hypercalcemia and no known history of kidney disease.2,6 This occurs when the normal feedback mechanism fails to inhibit excess hormone secretion by one or more of the parathyroid glands.6 With uninhibited PTH secretion, hypercalcemia will result from increased gastrointestinal absorption and bone resorption.
The most common causes of PHPT are an abnormal proliferation of parathyroid cells (parathyroid adenomas) and parathyroid tissue overgrowth (hyperplasia). PHPT may result from a single parathyroid adenoma (80%-90%), multigland hyperplasia (10%-15%), multiple adenomas (2%-3%), or malignancy (< 1%).6,10 Adenomas can occur sporadically or less commonly as part of an inherited syndrome.1 It is estimated that more than 10% of patients with PHPT have a mutation in one of 11 genes associated with PHPT.11 Approximately 5% of PHPT cases are familial, resulting from adenomas or carcinomas associated with mutations in the tumor suppressor genes MEN1 and CDC73 and the RET proto-oncogene.5 Multiple endocrine neoplasia (MEN) syndrome type 1 or 2a is associated with the development of parathyroid adenomas and other endocrine tumors.1,5 Mutations in the CDC73 gene can lead to parathyroid cancer, familial isolated hyperparathyroidism, and familial hyperparathyroidism-jaw tumor syndrome.5 Parathyroid cancer is rare and is linked to a history of radiation to the head and neck.3 Ectopic parathyroid adenomas represent 3% to 4% of all parathyroid adenomas and are often found in the mediastinum.12PHPT is the third most common endocrine disorder, with a prevalence of 1 case per 1,000 men and 2 to 3 cases per 1,000 women.5 Most women with PHPT are postmenopausal and older than 50.1 The condition can occur in younger adults but is rare in childhood and adolescence, with an incidence of 2 to 5 cases per 100,000.13
PHPT affects multiple organ systems, but the most commonly involved are the renal and musculoskeletal systems (see Table 2). The hypersecretory state causes excessive bone resorption and increased osteoclastic activity, resulting in osteoporosis and increased risk for pathologic fractures of the hip, wrist, and spine. The most common osteoporotic fractures are vertebral compression fractures.14 Fractures involving the thoracic spine contribute to the development of kyphosis.15
In the kidney, an increased filtered load of calcium leads to hypercalciuria, precipitation of calcium phosphate in the renal pelvis and collecting ducts, metabolic acidosis, alkaline urine, and hyperphosphaturia. The combination of alkaline urine, hyperphosphaturia, and hypercalciuria leads to the formation of kidney stones.6 Nephrolithiasis and alkaline urine predispose patients to recurrent urinary tract infections and subsequent renal impairment.6 In addition, hypercalcemia impairs the renal collecting system and decreases its response to antidiuretic hormone, resulting in polyuria.6
Secondary hyperparathyroidism
In secondary hyperparathyroidism, calcium levels are either normal or low. Normocalcemic hyperparathyroidism is characterized by normal ionized and total calcium levels and elevated PTH levels; it has no known cause.6 Secondary hyperparathyroidism occurs when excess PTH is excreted as a result of a chronic condition that leads to hypocalcemia. Examples of these disease states include vitamin D deficiency, chronic kidney disease (CKD), and intestinal malabsorption. The most common cause of secondary hyperparathyroidism is CKD; glomerular filtration insufficiency results in hyperphosphatemia, hypocalcemia, and low 1,25(OH)2D, stimulating the release of PTH. Other causes include deficient intake or decreased absorption of calcium or vitamin D; chronic use of medications such as lithium, phenobarbital, or phenytoin; bariatric surgery; celiac disease; and pancreatic disease.4,6,14 Lithium decreases urinary calcium excretion and reduces the sensitivity of the parathyroid gland to calcium.4
Tertiary hyperparathyroidism
Tertiary hyperparathyroidism, marked by hypercalcemia and excessive PTH secretion, can occur after prolonged secondary hyperparathyroidism. In this disorder, persistent parathyroid stimulation leads to gland hyperplasia, resulting in autonomous production of PTH despite correction of calcium levels.6 It most commonly occurs in patients with chronic secondary hyperparathyroidism with renal failure who receive a kidney transplant.2,6 In some cases, parathyroid hyperplasia may not regress after transplantation and parathyroidectomy may be necessary.
EVALUATION AND DIAGNOSTIC WORK-UP
Laboratory tests
Hypercalcemia is the most common initial finding that leads to the diagnosis of PHPT. Elevated serum calcium and PTH is characteristic of the condition. When evaluating a patient with hypercalcemia, the diagnostic work-up includes tests to differentiate between PTH- and non–PTH-mediated causes of elevated calcium (see Table 3).7 Evaluation should begin with measurement of PTH by second- or third-generation immunoassay along with phosphorus, alkaline phosphatase, 25(OH)D, creatinine, estimated glomerular filtration rate (eGFR), and albumin. Additionally, a 24-hour urine collection for calcium, creatinine, and creatinine clearance should be considered in patients with overt nephrolithiasis or nephrocalcinosis. If the urine calcium is > 400 mg/24 h, a renal stone risk profile is indicated because nephrolithiasis is one of the most common complications of PHPT.14 There is a high prevalence of nephrolithiasis in patients with normocalcemic PHPT, even after parathyroidectomy.16 If the 24-hour urine calcium level is low, the diagnosis of FHH is considered. If the urine calcium is high and the intact PTH is elevated or inappropriately normal, the diagnosis of PHPT is considered; urine calcium will be normal in 60% of PHPT cases.4,11

Imaging studies
Imaging is useful for localization of adenomas and abnormal parathyroid tissue to guide surgical planning but is not necessary for diagnosis or medical management. Understanding the strengths and weaknesses of imaging modalities enables the clinician to order the most appropriate option. There are three primary imaging modalities used to locate parathyroid adenoma(s) or aberrant parathyroid tissue: ultrasound, nuclear medicine sestamibi parathyroid scans, and CT. Some clinicians start with an ultrasound, but its operator-dependent results can vary widely; in addition, ultrasound often provides poor anatomic definition and has limited value in locating ectopic parathyroid tissue.17
Nuclear medicine parathyroid scan with technetium-99m sestamibi is a sensitive method for localizing hyperfunctioning, enlarged parathyroid glands or tissue in normal anatomic positions or ectopic locations. Uptake is enhanced and prolonged in parathyroid adenomas as well as in aberrant tissue found in the mediastinum or subclavicular areas. Sestamibi parathyroid scan detects up to 89% of single adenomas, but studies of this imaging modality have demonstrated a wide range of sensitivities (44%-95%).5,17 A drawback of nuclear medicine studies is that they provide little anatomic detail.17 Nonetheless, the ability of the parathyroid scan to locate parathyroid glands has contributed to the success of the minimally invasive parathyroidectomy, and it is considered the most successful imaging modality available.5,10 Identifying the precise location of the parathyroid adenoma is essential for a successful surgical outcome; this is best achieved by combining the sestamibi parathyroid scan with CT.12
Emerging imaging modalities are the multidetector CT (MDCT) and 4D-CT techniques. In an evaluation of the diagnostic accuracy of contrast-enhanced MDCT in the detection of parathyroid adenomas and aberrant parathyroid tissue, MDCT demonstrated the ability to differentiate between adenomas and hyperplasia and display important anatomic structures such as nerves and blood vessels.17 The specificity of MDCT for ruling out abnormal parathyroid tissue was 75%, and the sensitivity for detecting a single adenoma was 80%. Overall, MDCT demonstrated an 88% positive predictive value (PPV) in localizing hyperfunctioning parathyroid glands but showed poor sensitivity in detecting multigland disease.17 The PPV is a key value in determining the ability of an imaging study to precisely locate aberrant parathyroid tissue. MDCT provides detailed definition of anatomy, locating ectopic parathyroid glands in the deeper paraesophageal areas and mediastinum while defining relationships between the tissue and its surrounding vasculature, lymph nodes, and thyroid tissue.17 The 4D-CT technique employs three-dimensional technology and accounts for the movement of the patient’s body over time (the “fourth dimension”). It is an accurate method for identifying parathyroid adenomas but exposes the patient to higher radiation doses.18 The sensitivity of 4D-CT in localizing abnormal parathyroid tissue is comparable to that of MDCT.16,18,19
Additional studies used during the management of the patient with PHPT are BMD testing and renal imaging. Secondary causes of bone loss are responsible for up to 30% of osteoporosis cases in postmenopausal women; one of these causes is PHPT.20 Elevated PTH causes increased bone turnover and results in decreased bone mass with subsequent increased fracture risk.9 Bone density should be measured by dual-energy x-ray absorptiometry (DEXA), and the skeletal survey should include the distal one-third of the radius, hip, and lumbar spine. The distal radius is rich in cortical bone and BMD is often lowest at this site in patients with PHPT, making it the most sensitive DEXA marker for early detection of bone loss.19,21 The hip contains an equal mix of cortical and trabecular bone and is the second most sensitive site for detecting bone loss in PHPT. The spine contains a high proportion of trabecular bone and is the least sensitive site.19,21 Renal imaging studies, including x-ray, ultrasound, and, less frequently, CT of the abdomen and pelvis, are used to assess for nephrolithiasis and nephrocalcinosis.19
TREATMENT/MANAGEMENT
Conservative medical management
PHPT is a complex disease process, and careful evaluation is required when determining whether medical versus surgical management is appropriate. Clinical presentation ranges from no symptoms to multisystem disease. Conservative medical management, which includes regular monitoring, is an acceptable strategy in an asymptomatic patient with a low fracture risk and no nephrolithiasis.1 Conservative care includes maintaining normal dietary calcium intake and adequate hydration, regular exercise, vitamin D supplementation, annual laboratory studies, BMD testing, and the avoidance of thiazide diuretics and lithium.1 Guidelines, from the Fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism, for monitoring asymptomatic PHPT patients recommend
- Annual measurement of serum calcium
- BMD measurement by DEXA every 1 to 2 years
- Annual assessment of eGFR and serum creatinine
- Renal imaging or a 24-h urine stone profile if nephrolithiasis is suspected.19
Long-term medical management of PHPT is difficult because no agents are available to suppress hypercalcemia or completely block PTH release.12
Maintaining serum 25(OH)D at a level > 20 ng/mL significantly reduces PTH secretion, in comparison to levels < 20 ng/mL, and does not aggravate hypercalcemia.22 The Endocrine Society recommends a minimum serum 25(OH)D level of 20 ng/mL and notes that targeting a higher threshold value of 30 ng/mL is reasonable.19 The daily requirement for vitamin D3, 800 IU to 1,000 IU, is a good starting point for supplementation.4 Measurement of 1,25(OH)2D levels lacks value and is not recommended for patients with PHPT. Calcium intake should follow established guidelines and is not limited in PHPT.19
Surgical management
Surgical management is indicated for symptomatic patients.23 Indications include nephrolithiasis, nephrocalcinosis, osteitis fibrosa cystica, or osteoporosis. Surgery is considered appropriate for individuals who do not meet these criteria if there are no medical contraindications.14 The Fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism revised the indications for surgery in 2014 to include asymptomatic patients, since surgery is the only definitive treatment for PHPT. Current guidelines for when to recommend surgery in the asymptomatic patient with PHPT are listed in Table 4.19
Preoperative localization and referral to an experienced surgeon is of utmost importance for a good outcome. An expert surgeon will usually perform a minimally invasive parathyroidectomy (MIP) and obtain intraoperative PTH levels; in some cases, a full neck exploration is necessary. PTH has a half-life of less than five minutes and is an accurate tool for determining whether the culprit gland has been successfully removed.5
Modern imaging studies, less invasive surgical techniques, and intraoperative measurements of PTH have decreased the need to conduct full neck exploration. MIP offers a smaller incision, less tissue dissection, and lower morbidity, and can be offered without the risks associated with general anesthesia.10 The goal of surgery is to restore normocalcemia and, in turn, prevent bone mineral loss and systemic effects of hypercalcemia over the long term.10 Surgical management for an ectopic parathyroid adenoma is controversial because these are often found in the mediastinum, requiring invasive surgery.12
Surgery is curative in up to 95% of cases and has a low rate of complications.2,24 A joint decision regarding treatment options is made among the patient, primary care clinician, and surgeon. Complications include vocal cord paralysis resulting from injury to the recurrent laryngeal nerve, bleeding or hematoma, laryngospasm, symptomatic hypocalcemia, and persistent hyperparathyroidism; seizures are very rare but can occur from transient hypocalcemia and hypomagnesemia.5 PTH levels drop by more than 50% intraoperatively if the procedure is successful; otherwise, exploration for another adenoma is indicated.10 Postoperative calcium and vitamin D supplementation are warranted once lab values are stable.
When surgery is contraindicated/refused
If surgery is indicated but the patient is a poor candidate or refuses surgery, management of hypercalcemia and bone loss with pharmacologic agents is warranted. The calcimimetic cinacalcet is a reasonable medical alternative that has been shown to adequately control hypercalcemia and hypophosphatemia and has proven effective in various patient subgroups.25 This agent is useful in the treatment of patients who are asymptomatic and refuse surgery, patients with refractory PHPT after parathyroidectomy, and patients with contraindications to surgery.24,25 The medication reduces calcium and modestly reduces PTH levels by binding parathyroid calcium-sensing receptors but does not improve bone density.2,12 Cinacalcet is approved by the FDA for use in patients with moderate to severe disease when surgery is contraindicated.24
Treatment options for osteoporosis, vertebral fractures, and progressive bone loss in the patient with PHPT include bisphosphonates. Raloxifene and estrogen replacements may be used in postmenopausal women. Oral bisphosphonates (alendronate or risedronate) are firstline therapies and have been shown to inhibit progression to osteoporosis in PHPT.9,26 They prevent osteoclastic activity, reducing bone resorption and turnover. Contraindications to oral bisphosphonates include esophageal disorders, gastrointestinal intolerance, or inability to follow the dosing requirements. Intravenous zoledronic acid provides an alternative route of administration.
Alendronate has the best evidence for improving bone density and preventing progression to osteoporosis in patients with PHPT, but the medication does not affect calcium or PTH levels.1,19 There is limited data on the effects of combining bisphosphonates with calcimimetics. Raloxifene is a selective estrogen receptor modulator that decreases bone resorption; it is approved for treating osteoporosis and may be used when a patient is not a good candidate for a bisphosphonate.20 Denosumab, currently under study for the treatment of PHPT, is a human monoclonal antibody that improves bone density but does not affect serum calcium.20 Nonpharmacologic therapies include alcohol moderation, decreased caffeine intake, weight-bearing exercise, smoking cessation, adequate hydration, and dietary modifications.20
OUTCOME FOR THE CASE PATIENT
Although PHPT is often discovered incidentally in routine blood work with hypercalcemia, the case patient had developed osteoporosis and a grade IV tibial stress fracture before the diagnosis was made. Following parathyroidectomy, her hypertension worsened, requiring an additional antihypertensive medication. She developed recurrent disease and was referred to a tertiary care center for revision parathyroidectomy due to persistent elevated calcium levels. A 24-hour urine calcium test ruled out concurrent FHH. A full neck exploration was conducted and a 340-mg hypercellular parathyroid gland was removed from the left superior pole. She will be monitored for recurrent disease and will remain on a vitamin D3 supplement and treatment for osteoporosis.
CONCLUSION
Primary care clinicians should have a low threshold for initiating the work-up of mild hypercalcemia in an effort to prevent sequelae. Patient education is essential throughout the process. Understanding the condition and treatment options is necessary for a patient’s active participation in clinical decision making. Conservative management of an asymptomatic patient includes avoiding thiazide diuretics and lithium, staying well hydrated with water, maintaining moderate dietary calcium (1,000-1,200 mg/d) and vitamin D (400-600 IU/d) intake, regular exercise, and appropriate lab and bone density monitoring. Surgical treatment is recommended for symptomatic patients exhibiting decreased bone density, fractures, renal impairment, or nephrolithiasis. Treating bone loss with bisphosphonates and hypercalcemia with calcimimetics is useful. Postmenopausal women may benefit from estrogen therapy or selective estrogen receptor modulators. These agents improve bone density and lower calcium, but are often contraindicated or have adverse effects. Surgery is the only cure.3
CE/CME No: CR-1705
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Differentiate primary hyperparathyroidism (PHPT) from other causes of hypercalcemia and types of hyerparathyroidism.
• Understand the calcium-parathyroid hormone feedback loop.
• Identify appropriate imaging studies and common laboratory findings in the patient with PHPT.
• Describe the common systemic manifestations of PHPT.
• Discuss medical versus surgical management of the patient with PHPT.
FACULTY
Barbara Austin is a Family Nurse Practitioner at Baptist Primary Care, Jacksonville, Florida, and is pursuing a Doctorate of Nursing Practice (DNP) at Jacksonville University.
The author has no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2017.
Article begins on next page >>
Primary hyperparathyroidism (PHPT) is most often detected as hypercalcemia in an asymptomatic patient during routine blood work. Knowing the appropriate work-up of hypercalcemia is essential, since untreated PHPT can have significant complications affecting multiple organ systems—most notably, renal and musculoskeletal. Parathyroidectomy is curative in up to 95% of cases, but prevention of long-term complications relies on prompt recognition and appropriate follow-up.
Primary hyperparathyroidism (PHPT) is a common endocrine disorder, with a prevalence of approximately 1 to 3 cases per 1,000 persons.1 PHPT results from inappropriate overproduction of parathyroid hormone (PTH), the primary regulator of calcium homeostasis, and is characterized by hypercalcemia in the setting of an elevated or high-normal PTH level. In most cases of PHPT, unregulated PTH production is caused by a single parathyroid adenoma.
PHPT is the most common cause of hypercalcemia in outpatients and is typically diagnosed following incidental discovery during routine blood work in an asymptomatic patient.1,2 It is two to three times more common in women than in men, and incidence increases with age; as such, postmenopausal women are most commonly affected.1,3 PHPT often has an insidious course, and recognition of its clinical manifestations followed by appropriate diagnostic work-up and management are necessary to prevent sequelae.3
PATIENT PRESENTATION
A 68-year-old Caucasian woman presented to her family practice office for a third visit with continued complaints of nontraumatic right lower leg pain. She had previously been diagnosed with tendonitis, which was treated conservatively. The pain failed to improve, and an x-ray was ordered. The x-ray revealed no acute findings but did show osteopenia, prompting an order for a bone mineral density (BMD) test. The BMD test demonstrated osteoporosis, which warranted further investigation. She was prescribed alendronate but refused it, against medical advice, due to concern over potential adverse effects.
Her medical history included hyperlipidemia and hypertension under fair control with lisinopril. She took a low-dose aspirin and flaxseed supplement daily. She also had a history of radiation to the neck, having undergone tonsillar irradiation as a child (a common practice from the 1930s through the 1950s).3 Surgical history included a total hysterectomy with bilateral salpingo-oophorectomy, appendectomy, and tonsillectomy. There was no personal or family history of cancer or endocrine disorders, hypercalcemia, or nephrolithiasis. She was up to date on vaccines and preventive health care measures. Allergies included penicillin and sulfa, both resulting in hives. She was a nonsmoker and did not drink alcohol or engage in illicit drug use.
Review of systems revealed right lower anterior leg pain for four months, characterized as aching, deep, sharp, and throbbing with radiation to the ankle. The pain was worse with activity and prolonged standing; ibuprofen and application of ice provided partial relief. She had experienced some mood changes, including irritability. Physical exam was normal except for dominant right-sided thyromegaly, marked bony point tenderness to the right midshin area, and an antalgic gait.
Laboratory work-up demonstrated elevated PTH, alkaline phosphatase, and calcium levels and a low 25-hydroxyvitamin D [25(OH)D] level (see Table 1). MRI of the right lower leg revealed a grade IV stress fracture (see Figure 1). The elevated serum calcium and PTH levels in addition to abnormal bone density findings led to the diagnosis of PHPT. She was referred to endocrinology and orthopedics for management of PHPT and the stress fracture, respectively, and was placed in an orthopedic walking boot for treatment of the midtibial stress fracture.
The endocrinologist referred her to an otolaryngologist trained in the surgical management of parathyroid adenomas, who ordered a thyroid ultrasound; this study was inconclusive. Additional imaging, including a Tc-99m sestamibi parathyroid scan and CT with contrast of the soft tissue of the neck, was obtained. The parathyroid scan of the neck and upper chest showed retained activity in the right inferior thyroid pole that was concerning for a parathyroid adenoma (see Figure 2). The CT identified a 1.5-cm parathyroid adenoma off the right inferior pole of the thyroid gland (concordant with the parathyroid scan). A single 300-mg parathyroid adenoma was removed from the right inferior pole of the thyroid. The surgery was deemed successful, with intraoperative normalization of the PTH level.
The patient was managed postoperatively by the endocrinologist and was started on calcium and vitamin D supplements. She was prescribed a bisphosphonate, as she had refused to take alendronate following her abnormal BMD test.
DIFFERENTIAL DIAGNOSIS
PHPT and malignancy are the most common causes of hypercalcemia, accounting for 90% of cases.2,4 A less common cause is familial hypocalciuric hypercalcemia (FHH), a rare benign disorder that imitates PHPT.1 FHH is ruled out by measurement of 24-hour urine calcium excretion and is characterized by hypocalciuria, defined as a urine calcium level of less than 100 mg/24 h (reference range, 100-300 mg/24 h).2 Low calcium excretion can also be identified by a calcium-creatinine excretion ratio.2 FHH is a benign autosomal dominant condition caused by a heterozygous mutation of the parathyroid glands’ calcium-sensing receptors.2,5,6 Young adults with FHH are asymptomatic, and mild hypercalcemia and a normal or slightly elevated PTH are the only laboratory findings.4
Measuring PTH levels is key in determining the underlying mechanism of hypercalcemia.2,7 If the hypercalcemia is not PTH-mediated, malignancy and granulomatous diseases such as sarcoidosis must be considered.2,7 PTH is suppressed in malignancy except for rare cases of PTH-producing tumors.4 Bone metastases cause calcium resorption, and sarcoidosis causes an excess of vitamin D, both resulting in hypercalcemia. Lymphomas and sarcoid granulomas express 1α-hydroxylase, an enzyme that increases the conversion of 25(OH)D to 1,25-dihydroxyvitamin D [1,25(OH)2D].2 When malignancy is suspected, it is appropriate to check a 1,25(OH)2D level. Thiazide diuretics, such as hydrochlorothiazide, decrease urinary calcium excretion and may result in mild hypercalcemia.2 Other possibilities in the differential include hypervitaminosis A or D, dehydration, and excess calcium ingestion, but these are less common.6,7
CALCIUM REGULATION
The parathyroid glands stem from four poles on the back of the thyroid gland; there are typically four, but the number can vary from two to 11. Secreted PTH, the primary regulator of calcium homeostasis, maintains calcium levels within a narrow physiologic range.2,8 PTH increases bone resorption, stimulating release of calcium into the blood, and signals the kidneys to increase reabsorption of calcium and excrete phosphorus. It also converts 25(OH)D to 1,25(OH)2 D, the active form of vitamin D that increases gastrointestinal calcium absorption. In a negative feedback loop, PTH secretion is regulated by serum calcium levels, stimulated when levels are low and suppressed when levels are high (see Figure 3).3 Calcium-sensing receptors, located in the chief cells of parathyroid tissue, are essential to calcium homeostasis. These receptors will either increase or decrease PTH release in response to small changes in blood ionized calcium levels. The receptors also play an independent role in the renal tubules by promoting secretion of calcium in the setting of hypercalcemia.5,9 The precise regulation of intracellular and extracellular calcium is necessary for normal functioning of physiologic processes, including bone metabolism, hormone release and regulation, neuromuscular function, and cell signaling.5

PATHOPHYSIOLOGY
Hyperparathyroidism is defined as excess secretion of PTH and is categorized as primary, secondary, or tertiary based on pathophysiologic mechanisms.
Primary hyperparathyroidism
PHPT is defined as PTH levels that are elevated or inappropriately normal in patients with hypercalcemia and no known history of kidney disease.2,6 This occurs when the normal feedback mechanism fails to inhibit excess hormone secretion by one or more of the parathyroid glands.6 With uninhibited PTH secretion, hypercalcemia will result from increased gastrointestinal absorption and bone resorption.
The most common causes of PHPT are an abnormal proliferation of parathyroid cells (parathyroid adenomas) and parathyroid tissue overgrowth (hyperplasia). PHPT may result from a single parathyroid adenoma (80%-90%), multigland hyperplasia (10%-15%), multiple adenomas (2%-3%), or malignancy (< 1%).6,10 Adenomas can occur sporadically or less commonly as part of an inherited syndrome.1 It is estimated that more than 10% of patients with PHPT have a mutation in one of 11 genes associated with PHPT.11 Approximately 5% of PHPT cases are familial, resulting from adenomas or carcinomas associated with mutations in the tumor suppressor genes MEN1 and CDC73 and the RET proto-oncogene.5 Multiple endocrine neoplasia (MEN) syndrome type 1 or 2a is associated with the development of parathyroid adenomas and other endocrine tumors.1,5 Mutations in the CDC73 gene can lead to parathyroid cancer, familial isolated hyperparathyroidism, and familial hyperparathyroidism-jaw tumor syndrome.5 Parathyroid cancer is rare and is linked to a history of radiation to the head and neck.3 Ectopic parathyroid adenomas represent 3% to 4% of all parathyroid adenomas and are often found in the mediastinum.12PHPT is the third most common endocrine disorder, with a prevalence of 1 case per 1,000 men and 2 to 3 cases per 1,000 women.5 Most women with PHPT are postmenopausal and older than 50.1 The condition can occur in younger adults but is rare in childhood and adolescence, with an incidence of 2 to 5 cases per 100,000.13
PHPT affects multiple organ systems, but the most commonly involved are the renal and musculoskeletal systems (see Table 2). The hypersecretory state causes excessive bone resorption and increased osteoclastic activity, resulting in osteoporosis and increased risk for pathologic fractures of the hip, wrist, and spine. The most common osteoporotic fractures are vertebral compression fractures.14 Fractures involving the thoracic spine contribute to the development of kyphosis.15
In the kidney, an increased filtered load of calcium leads to hypercalciuria, precipitation of calcium phosphate in the renal pelvis and collecting ducts, metabolic acidosis, alkaline urine, and hyperphosphaturia. The combination of alkaline urine, hyperphosphaturia, and hypercalciuria leads to the formation of kidney stones.6 Nephrolithiasis and alkaline urine predispose patients to recurrent urinary tract infections and subsequent renal impairment.6 In addition, hypercalcemia impairs the renal collecting system and decreases its response to antidiuretic hormone, resulting in polyuria.6
Secondary hyperparathyroidism
In secondary hyperparathyroidism, calcium levels are either normal or low. Normocalcemic hyperparathyroidism is characterized by normal ionized and total calcium levels and elevated PTH levels; it has no known cause.6 Secondary hyperparathyroidism occurs when excess PTH is excreted as a result of a chronic condition that leads to hypocalcemia. Examples of these disease states include vitamin D deficiency, chronic kidney disease (CKD), and intestinal malabsorption. The most common cause of secondary hyperparathyroidism is CKD; glomerular filtration insufficiency results in hyperphosphatemia, hypocalcemia, and low 1,25(OH)2D, stimulating the release of PTH. Other causes include deficient intake or decreased absorption of calcium or vitamin D; chronic use of medications such as lithium, phenobarbital, or phenytoin; bariatric surgery; celiac disease; and pancreatic disease.4,6,14 Lithium decreases urinary calcium excretion and reduces the sensitivity of the parathyroid gland to calcium.4
Tertiary hyperparathyroidism
Tertiary hyperparathyroidism, marked by hypercalcemia and excessive PTH secretion, can occur after prolonged secondary hyperparathyroidism. In this disorder, persistent parathyroid stimulation leads to gland hyperplasia, resulting in autonomous production of PTH despite correction of calcium levels.6 It most commonly occurs in patients with chronic secondary hyperparathyroidism with renal failure who receive a kidney transplant.2,6 In some cases, parathyroid hyperplasia may not regress after transplantation and parathyroidectomy may be necessary.
EVALUATION AND DIAGNOSTIC WORK-UP
Laboratory tests
Hypercalcemia is the most common initial finding that leads to the diagnosis of PHPT. Elevated serum calcium and PTH is characteristic of the condition. When evaluating a patient with hypercalcemia, the diagnostic work-up includes tests to differentiate between PTH- and non–PTH-mediated causes of elevated calcium (see Table 3).7 Evaluation should begin with measurement of PTH by second- or third-generation immunoassay along with phosphorus, alkaline phosphatase, 25(OH)D, creatinine, estimated glomerular filtration rate (eGFR), and albumin. Additionally, a 24-hour urine collection for calcium, creatinine, and creatinine clearance should be considered in patients with overt nephrolithiasis or nephrocalcinosis. If the urine calcium is > 400 mg/24 h, a renal stone risk profile is indicated because nephrolithiasis is one of the most common complications of PHPT.14 There is a high prevalence of nephrolithiasis in patients with normocalcemic PHPT, even after parathyroidectomy.16 If the 24-hour urine calcium level is low, the diagnosis of FHH is considered. If the urine calcium is high and the intact PTH is elevated or inappropriately normal, the diagnosis of PHPT is considered; urine calcium will be normal in 60% of PHPT cases.4,11

Imaging studies
Imaging is useful for localization of adenomas and abnormal parathyroid tissue to guide surgical planning but is not necessary for diagnosis or medical management. Understanding the strengths and weaknesses of imaging modalities enables the clinician to order the most appropriate option. There are three primary imaging modalities used to locate parathyroid adenoma(s) or aberrant parathyroid tissue: ultrasound, nuclear medicine sestamibi parathyroid scans, and CT. Some clinicians start with an ultrasound, but its operator-dependent results can vary widely; in addition, ultrasound often provides poor anatomic definition and has limited value in locating ectopic parathyroid tissue.17
Nuclear medicine parathyroid scan with technetium-99m sestamibi is a sensitive method for localizing hyperfunctioning, enlarged parathyroid glands or tissue in normal anatomic positions or ectopic locations. Uptake is enhanced and prolonged in parathyroid adenomas as well as in aberrant tissue found in the mediastinum or subclavicular areas. Sestamibi parathyroid scan detects up to 89% of single adenomas, but studies of this imaging modality have demonstrated a wide range of sensitivities (44%-95%).5,17 A drawback of nuclear medicine studies is that they provide little anatomic detail.17 Nonetheless, the ability of the parathyroid scan to locate parathyroid glands has contributed to the success of the minimally invasive parathyroidectomy, and it is considered the most successful imaging modality available.5,10 Identifying the precise location of the parathyroid adenoma is essential for a successful surgical outcome; this is best achieved by combining the sestamibi parathyroid scan with CT.12
Emerging imaging modalities are the multidetector CT (MDCT) and 4D-CT techniques. In an evaluation of the diagnostic accuracy of contrast-enhanced MDCT in the detection of parathyroid adenomas and aberrant parathyroid tissue, MDCT demonstrated the ability to differentiate between adenomas and hyperplasia and display important anatomic structures such as nerves and blood vessels.17 The specificity of MDCT for ruling out abnormal parathyroid tissue was 75%, and the sensitivity for detecting a single adenoma was 80%. Overall, MDCT demonstrated an 88% positive predictive value (PPV) in localizing hyperfunctioning parathyroid glands but showed poor sensitivity in detecting multigland disease.17 The PPV is a key value in determining the ability of an imaging study to precisely locate aberrant parathyroid tissue. MDCT provides detailed definition of anatomy, locating ectopic parathyroid glands in the deeper paraesophageal areas and mediastinum while defining relationships between the tissue and its surrounding vasculature, lymph nodes, and thyroid tissue.17 The 4D-CT technique employs three-dimensional technology and accounts for the movement of the patient’s body over time (the “fourth dimension”). It is an accurate method for identifying parathyroid adenomas but exposes the patient to higher radiation doses.18 The sensitivity of 4D-CT in localizing abnormal parathyroid tissue is comparable to that of MDCT.16,18,19
Additional studies used during the management of the patient with PHPT are BMD testing and renal imaging. Secondary causes of bone loss are responsible for up to 30% of osteoporosis cases in postmenopausal women; one of these causes is PHPT.20 Elevated PTH causes increased bone turnover and results in decreased bone mass with subsequent increased fracture risk.9 Bone density should be measured by dual-energy x-ray absorptiometry (DEXA), and the skeletal survey should include the distal one-third of the radius, hip, and lumbar spine. The distal radius is rich in cortical bone and BMD is often lowest at this site in patients with PHPT, making it the most sensitive DEXA marker for early detection of bone loss.19,21 The hip contains an equal mix of cortical and trabecular bone and is the second most sensitive site for detecting bone loss in PHPT. The spine contains a high proportion of trabecular bone and is the least sensitive site.19,21 Renal imaging studies, including x-ray, ultrasound, and, less frequently, CT of the abdomen and pelvis, are used to assess for nephrolithiasis and nephrocalcinosis.19
TREATMENT/MANAGEMENT
Conservative medical management
PHPT is a complex disease process, and careful evaluation is required when determining whether medical versus surgical management is appropriate. Clinical presentation ranges from no symptoms to multisystem disease. Conservative medical management, which includes regular monitoring, is an acceptable strategy in an asymptomatic patient with a low fracture risk and no nephrolithiasis.1 Conservative care includes maintaining normal dietary calcium intake and adequate hydration, regular exercise, vitamin D supplementation, annual laboratory studies, BMD testing, and the avoidance of thiazide diuretics and lithium.1 Guidelines, from the Fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism, for monitoring asymptomatic PHPT patients recommend
- Annual measurement of serum calcium
- BMD measurement by DEXA every 1 to 2 years
- Annual assessment of eGFR and serum creatinine
- Renal imaging or a 24-h urine stone profile if nephrolithiasis is suspected.19
Long-term medical management of PHPT is difficult because no agents are available to suppress hypercalcemia or completely block PTH release.12
Maintaining serum 25(OH)D at a level > 20 ng/mL significantly reduces PTH secretion, in comparison to levels < 20 ng/mL, and does not aggravate hypercalcemia.22 The Endocrine Society recommends a minimum serum 25(OH)D level of 20 ng/mL and notes that targeting a higher threshold value of 30 ng/mL is reasonable.19 The daily requirement for vitamin D3, 800 IU to 1,000 IU, is a good starting point for supplementation.4 Measurement of 1,25(OH)2D levels lacks value and is not recommended for patients with PHPT. Calcium intake should follow established guidelines and is not limited in PHPT.19
Surgical management
Surgical management is indicated for symptomatic patients.23 Indications include nephrolithiasis, nephrocalcinosis, osteitis fibrosa cystica, or osteoporosis. Surgery is considered appropriate for individuals who do not meet these criteria if there are no medical contraindications.14 The Fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism revised the indications for surgery in 2014 to include asymptomatic patients, since surgery is the only definitive treatment for PHPT. Current guidelines for when to recommend surgery in the asymptomatic patient with PHPT are listed in Table 4.19
Preoperative localization and referral to an experienced surgeon is of utmost importance for a good outcome. An expert surgeon will usually perform a minimally invasive parathyroidectomy (MIP) and obtain intraoperative PTH levels; in some cases, a full neck exploration is necessary. PTH has a half-life of less than five minutes and is an accurate tool for determining whether the culprit gland has been successfully removed.5
Modern imaging studies, less invasive surgical techniques, and intraoperative measurements of PTH have decreased the need to conduct full neck exploration. MIP offers a smaller incision, less tissue dissection, and lower morbidity, and can be offered without the risks associated with general anesthesia.10 The goal of surgery is to restore normocalcemia and, in turn, prevent bone mineral loss and systemic effects of hypercalcemia over the long term.10 Surgical management for an ectopic parathyroid adenoma is controversial because these are often found in the mediastinum, requiring invasive surgery.12
Surgery is curative in up to 95% of cases and has a low rate of complications.2,24 A joint decision regarding treatment options is made among the patient, primary care clinician, and surgeon. Complications include vocal cord paralysis resulting from injury to the recurrent laryngeal nerve, bleeding or hematoma, laryngospasm, symptomatic hypocalcemia, and persistent hyperparathyroidism; seizures are very rare but can occur from transient hypocalcemia and hypomagnesemia.5 PTH levels drop by more than 50% intraoperatively if the procedure is successful; otherwise, exploration for another adenoma is indicated.10 Postoperative calcium and vitamin D supplementation are warranted once lab values are stable.
When surgery is contraindicated/refused
If surgery is indicated but the patient is a poor candidate or refuses surgery, management of hypercalcemia and bone loss with pharmacologic agents is warranted. The calcimimetic cinacalcet is a reasonable medical alternative that has been shown to adequately control hypercalcemia and hypophosphatemia and has proven effective in various patient subgroups.25 This agent is useful in the treatment of patients who are asymptomatic and refuse surgery, patients with refractory PHPT after parathyroidectomy, and patients with contraindications to surgery.24,25 The medication reduces calcium and modestly reduces PTH levels by binding parathyroid calcium-sensing receptors but does not improve bone density.2,12 Cinacalcet is approved by the FDA for use in patients with moderate to severe disease when surgery is contraindicated.24
Treatment options for osteoporosis, vertebral fractures, and progressive bone loss in the patient with PHPT include bisphosphonates. Raloxifene and estrogen replacements may be used in postmenopausal women. Oral bisphosphonates (alendronate or risedronate) are firstline therapies and have been shown to inhibit progression to osteoporosis in PHPT.9,26 They prevent osteoclastic activity, reducing bone resorption and turnover. Contraindications to oral bisphosphonates include esophageal disorders, gastrointestinal intolerance, or inability to follow the dosing requirements. Intravenous zoledronic acid provides an alternative route of administration.
Alendronate has the best evidence for improving bone density and preventing progression to osteoporosis in patients with PHPT, but the medication does not affect calcium or PTH levels.1,19 There is limited data on the effects of combining bisphosphonates with calcimimetics. Raloxifene is a selective estrogen receptor modulator that decreases bone resorption; it is approved for treating osteoporosis and may be used when a patient is not a good candidate for a bisphosphonate.20 Denosumab, currently under study for the treatment of PHPT, is a human monoclonal antibody that improves bone density but does not affect serum calcium.20 Nonpharmacologic therapies include alcohol moderation, decreased caffeine intake, weight-bearing exercise, smoking cessation, adequate hydration, and dietary modifications.20
OUTCOME FOR THE CASE PATIENT
Although PHPT is often discovered incidentally in routine blood work with hypercalcemia, the case patient had developed osteoporosis and a grade IV tibial stress fracture before the diagnosis was made. Following parathyroidectomy, her hypertension worsened, requiring an additional antihypertensive medication. She developed recurrent disease and was referred to a tertiary care center for revision parathyroidectomy due to persistent elevated calcium levels. A 24-hour urine calcium test ruled out concurrent FHH. A full neck exploration was conducted and a 340-mg hypercellular parathyroid gland was removed from the left superior pole. She will be monitored for recurrent disease and will remain on a vitamin D3 supplement and treatment for osteoporosis.
CONCLUSION
Primary care clinicians should have a low threshold for initiating the work-up of mild hypercalcemia in an effort to prevent sequelae. Patient education is essential throughout the process. Understanding the condition and treatment options is necessary for a patient’s active participation in clinical decision making. Conservative management of an asymptomatic patient includes avoiding thiazide diuretics and lithium, staying well hydrated with water, maintaining moderate dietary calcium (1,000-1,200 mg/d) and vitamin D (400-600 IU/d) intake, regular exercise, and appropriate lab and bone density monitoring. Surgical treatment is recommended for symptomatic patients exhibiting decreased bone density, fractures, renal impairment, or nephrolithiasis. Treating bone loss with bisphosphonates and hypercalcemia with calcimimetics is useful. Postmenopausal women may benefit from estrogen therapy or selective estrogen receptor modulators. These agents improve bone density and lower calcium, but are often contraindicated or have adverse effects. Surgery is the only cure.3
1. Turner J. Hypercalcaemia and primary hyperparathyroidism. Medicine. 2009;37(9):461-464.
2. Crowley R, Gittoes N. How to approach hypercalcaemia. Clin Med. 2013;13(3):287-290.
3. Kapustin JF, Schofield DL. Hyperparathyroidism: an incidental finding. Nurse Pract. 2012;37(11):9-14.
4. Cordellat IM. Hyperparathyroidism: primary or secondary disease? Rheumatol Clin. 2012;8(5):287-291.
5. MacKenzie-Feder J, Sirrs S, Anderson D, Sharif J, Khan A. Primary hyperparathyroidism: an overview. Int J Endocrinol. 2011;2011:251410.
6. Brashers VL, Jones RE, Huether SE. Alterations of hormonal regulation. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 7th ed. St. Louis, MO: Mosby; 2015:731-733.
7. Osborne JL, Klocko DJ. Woman, 66, with persistent abdominal and back pain. Clinician Reviews. 2014;24(11):34-37, 40.
8. Michels TC, Kelly KM. Parathyroid disorders. Am Fam Physician. 2013;88(4):249-257.
9. Rolighed L, Rejnmark L, Christiansen P. Bone involvement in primary hyperparathyroidism and changes after parathyroidectomy. US Endocrinol. 2013;9(2):181-184.
10. Karahan Ö, Okus A, Sevinç B, et al. Minimally invasive parathyroidectomy under local anesthesia. J Postgrad Med. 2013;59(1):21-24.
11. Fuleihan GE, Silverberg SJ. Primary hyperparathyroidism: diagnosis, differential diagnosis, and evaluation. Up-to-Date. www.uptodate.com/contents/primary-hyperparathyroidism-diagnosis-differential-diagnosis-and-evaluation. Accessed April 20, 2017.
12. Panchani R, Varma T, Goyal A, et al. A challenging case of an ectopic parathyroid adenoma. Indian J Endocrinol Metab. 2012;16:S408-S410.
13. Otasowie J, Hambleton BA. Aggression and homicidal thoughts in a patient with primary hyperparathyroidism: a case report. Br J Medical Pract. 2013;6(4):a630.
14. Bilezikian JP. Primary hyperparathyroidism: new insights, concepts and guidelines. Presented at: American Association of Clinical Endocrinologists 24th Annual Scientific and Clinical Congress; May 13-17, 2015; Nashville, TN. am2015.aace.com/presentations/Friday/F31/PrimaryHyperparathyroidismNew InsightsConceptsandGuidelines.pdf. Accessed April 20, 2017.
15. Crowther-Radulewicz CL, McCance KL. Alterations of musculoskeletal function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 7th ed. St. Louis, MO: Mosby; 2015:1551-1555.
16. Amaral LM, Queiroz DC, Marques TF, et al. Clinical study: normocalcemic versus hypercalcemic primary hyperparathyroidism: more stone than bone? J Osteoporos. 2012;2012:128352.
17. Linda DD, Ng B, Rebello R, et al. The utility of multidetector computed tomography for detection of parathyroid disease in the setting of primary hyperparathyroidism. Can Assoc Radiol J. 2012;63(2):100-108.
18. Bann DV, Zacharia T, Goldenberg D, Goyal, N. Parathyroid localization using 4-D-computed tomography. Ear Nose Throat J. 2015;94(4-5):E55-E57.
19. Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3561-3569.
20. Jeremiah MP, Unwin BK, Greenawald MH, Casiano VE. Diagnosis and management of osteoporosis. Am Fam Physician. 2015;92(4):261-268.
21. Wood K, Dhital S, Chen H, Sippel RS. What is the utility of distal forearm DXA in primary hyperparathyroidism? Oncologist. 2012;17(3):322-325.
22. Jayasena CN, Modi M, Palazzo F, et al. Associations of serum 25-hydroxyvitamin D with circulating PTH, phosphate and calcium in patients with primary hyperparathyroidism. Clin Endocrinol (Oxf). 2013;78(6):838-843.
23. Grey A. Nonsurgical management of mild primary hyperparathyroidism—a reasonable option. Clin Endocrinol. 2012;77(5):639-644.
24. Saponaro F, Faggiano A, Grimaldi F, et al. Cinacalcet in the management of primary hyperparathyroidism: post marketing experience of an Italian multicenter group. Clin Endocrinol (Oxf). 2013;79(1):20-26.
25. Rothe HM, Liangos O, Biggar P, et al. Cinacalcet treatment of primary hyperparathyroidism. Int J Endocrinol. 2011;2011:415719.
26. Farag N, Delbanco T, Strewler GJ. Update: a 64-year-old woman with primary hyperparathyroidism. JAMA. 2008;300(17):2044-2045.
1. Turner J. Hypercalcaemia and primary hyperparathyroidism. Medicine. 2009;37(9):461-464.
2. Crowley R, Gittoes N. How to approach hypercalcaemia. Clin Med. 2013;13(3):287-290.
3. Kapustin JF, Schofield DL. Hyperparathyroidism: an incidental finding. Nurse Pract. 2012;37(11):9-14.
4. Cordellat IM. Hyperparathyroidism: primary or secondary disease? Rheumatol Clin. 2012;8(5):287-291.
5. MacKenzie-Feder J, Sirrs S, Anderson D, Sharif J, Khan A. Primary hyperparathyroidism: an overview. Int J Endocrinol. 2011;2011:251410.
6. Brashers VL, Jones RE, Huether SE. Alterations of hormonal regulation. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 7th ed. St. Louis, MO: Mosby; 2015:731-733.
7. Osborne JL, Klocko DJ. Woman, 66, with persistent abdominal and back pain. Clinician Reviews. 2014;24(11):34-37, 40.
8. Michels TC, Kelly KM. Parathyroid disorders. Am Fam Physician. 2013;88(4):249-257.
9. Rolighed L, Rejnmark L, Christiansen P. Bone involvement in primary hyperparathyroidism and changes after parathyroidectomy. US Endocrinol. 2013;9(2):181-184.
10. Karahan Ö, Okus A, Sevinç B, et al. Minimally invasive parathyroidectomy under local anesthesia. J Postgrad Med. 2013;59(1):21-24.
11. Fuleihan GE, Silverberg SJ. Primary hyperparathyroidism: diagnosis, differential diagnosis, and evaluation. Up-to-Date. www.uptodate.com/contents/primary-hyperparathyroidism-diagnosis-differential-diagnosis-and-evaluation. Accessed April 20, 2017.
12. Panchani R, Varma T, Goyal A, et al. A challenging case of an ectopic parathyroid adenoma. Indian J Endocrinol Metab. 2012;16:S408-S410.
13. Otasowie J, Hambleton BA. Aggression and homicidal thoughts in a patient with primary hyperparathyroidism: a case report. Br J Medical Pract. 2013;6(4):a630.
14. Bilezikian JP. Primary hyperparathyroidism: new insights, concepts and guidelines. Presented at: American Association of Clinical Endocrinologists 24th Annual Scientific and Clinical Congress; May 13-17, 2015; Nashville, TN. am2015.aace.com/presentations/Friday/F31/PrimaryHyperparathyroidismNew InsightsConceptsandGuidelines.pdf. Accessed April 20, 2017.
15. Crowther-Radulewicz CL, McCance KL. Alterations of musculoskeletal function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 7th ed. St. Louis, MO: Mosby; 2015:1551-1555.
16. Amaral LM, Queiroz DC, Marques TF, et al. Clinical study: normocalcemic versus hypercalcemic primary hyperparathyroidism: more stone than bone? J Osteoporos. 2012;2012:128352.
17. Linda DD, Ng B, Rebello R, et al. The utility of multidetector computed tomography for detection of parathyroid disease in the setting of primary hyperparathyroidism. Can Assoc Radiol J. 2012;63(2):100-108.
18. Bann DV, Zacharia T, Goldenberg D, Goyal, N. Parathyroid localization using 4-D-computed tomography. Ear Nose Throat J. 2015;94(4-5):E55-E57.
19. Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3561-3569.
20. Jeremiah MP, Unwin BK, Greenawald MH, Casiano VE. Diagnosis and management of osteoporosis. Am Fam Physician. 2015;92(4):261-268.
21. Wood K, Dhital S, Chen H, Sippel RS. What is the utility of distal forearm DXA in primary hyperparathyroidism? Oncologist. 2012;17(3):322-325.
22. Jayasena CN, Modi M, Palazzo F, et al. Associations of serum 25-hydroxyvitamin D with circulating PTH, phosphate and calcium in patients with primary hyperparathyroidism. Clin Endocrinol (Oxf). 2013;78(6):838-843.
23. Grey A. Nonsurgical management of mild primary hyperparathyroidism—a reasonable option. Clin Endocrinol. 2012;77(5):639-644.
24. Saponaro F, Faggiano A, Grimaldi F, et al. Cinacalcet in the management of primary hyperparathyroidism: post marketing experience of an Italian multicenter group. Clin Endocrinol (Oxf). 2013;79(1):20-26.
25. Rothe HM, Liangos O, Biggar P, et al. Cinacalcet treatment of primary hyperparathyroidism. Int J Endocrinol. 2011;2011:415719.
26. Farag N, Delbanco T, Strewler GJ. Update: a 64-year-old woman with primary hyperparathyroidism. JAMA. 2008;300(17):2044-2045.