User login
Diagnosis and 10-Year Follow-Up Of a Community-Based Hepatitis C Cohort
OBJECTIVE: To determine the health care follow-up and treatment associated with physician-diagnosed hepatitis C (HCV) in a community-based population.
STUDY DESIGN: We conducted a retrospective medical record review using records from all providers in Olmsted County, Minnesota.
POPULATION: The study incorporated all Olmsted County residents with physician-diagnosed hepatitis C from 1990 through 1999.
OUTCOMES MEASURED: We assessed demographic and health status information as well as health services use in subjects with physician-diagnosed HCV.
RESULTS: Physicians diagnosed hepatitis C in 355 subjects (219 men [62%], 136 women [38%]), mean age 43 years, in the 10-year period studied. About half of diagnoses (45%, n = 159) were confirmed with polymerase chain reaction or liver biopsies. Identified risk factors included IV drug use (50%), multiple sex partners (36%), and blood transfusion (30%). Follow-up assessment with aspartate aminotransferase/amino alanine transferase (AST/ALT) tests occurred in about half (49%) of subjects, while 202 subjects (60%) were referred for gastrointestinal (GI) specialist evaluation and 49 patients (14% of all, 25% of those referred to a GI specialist) had specific treatment for hepatitis C. Although well over half of patients (60%) had possible contraindications to HCV treatment, including heavy alcohol use, few were referred for chemical dependency therapy.
CONCLUSIONS: In this community, follow-up and treatment related to HCV were limited. Attention to prevention of disease-accelerating coinfections was only modest. Referral or documented recommendations for treatment of alcoholism or heavy chronic alcohol ingestion were minimal.
- Risk factors associated with acquiring hepatitis C in this small city are similar to those in urban areas: intravenous drug use, frequent sexual exposure, and blood transfusion before 1992.
- No follow-up of liver testing in people with known hepatitis C occurred in half of cases.
- Less than 15% of prevalent cases received hepatitis C–specific treatment; many people appeared to have contraindications for hepatitis C therapy.
- Preventive care measures appropriate for people with hepatitis C (eg, hepatitis B immunization, referral for treatment of known chemical dependency, and screening for HIV) were not universal in this population.
Hepatitis C is reportedly the most common chronic bloodborne infectious disease in the United States.1-3 Prevalence data based on modeling studies and extrapolation from studies such as the National Health and Nutrition Examination Survey (NHANES) and county surveillance projects3,4 report that 3.9 million Americans (1.8%) have been infected with the hepatitis C virus (HCV) and 2.7 million (1.2%) have chronic HCV infection.5 While the natural history of HCV infections is poorly understood,6-9 researchers and clinicians agree that most people with chronic HCV infection remain asymptomatic for many years while seeking medical care, often primary care, for problems other than their silent hepatitis C infections.8,9
Much of the hepatitis C literature is derived from studies of subspecialty clinic patient populations or other special populations such as those with blood transfusion or RhoGAM-acquired hepatitis C.9-12 The former data tend to concentrate on the more severe, symptomatic patients referred to specialty care, whereas the latter special-exposure groups provide little information about a large segment of the population with other risk factors and comorbidity. Neither of these sources provides data on the community population or community practice.1,9,11 Our study describes the people that have been diagnosed with HCV in a geographically defined community, including their physician-directed hepatitis C follow-up evaluations and HCV treatment. In addition, we report on testing and vaccination efforts for hepatitis A and B and on the recognition and treatment of alcoholism and chronic heavy alcohol ingestion (accelerating comorbidities). The ability to follow the patient across all types of care from ambulatory to inpatient and from primary to tertiary care provides a very broad overview of these population-based cases.
Methods
Study setting and instrument
This is a descriptive study of a geographically defined, population-based cohort of all persons living in Olmsted County, Minnesota, who received a physician or laboratory diagnosis of hepatitis C from January 1, 1990, through December 31, 1999. All physician diagnoses were captured using a communitywide diagnostic database, the Rochester Epidemiology Project.13,14 Olmsted County is a metropolitan statistical area that includes the city of Rochester and is served primarily by more than 200 primary care physicians employed by 2 medical facilities, the Olmsted Medical Center and the Mayo Clinic.
All patients with a physician diagnosis of hepatitis C were included, whether the diagnosis had been confirmed by liver biopsy or polymerase chain reaction (PCR) testing or by either positive recombinant immunoblast assay (RIBA) testing or indeterminate RIBA testing and the presence of 1 or more risk factors. Risk factors included blood transfusion before July 1,1992, a diagnosis of hemophilia before 1990, a history of intravenous drug use (IDU), selling sex or trading sex for drugs, having more than 10 sex partners, sexual exposure to a person infected with HCV, intranasal cocaine use, and work in a health care facility with exposure to blood products (eg, phlebotomists) or health care workers with a history of needlesticks. The immigration status of people from Africa or Southeast Asia, where hepatitis C is endemic, is reported when the medical record listed immigration as an HCV risk factor. Patients with acute hepatitis C, defined as those with acute symptoms at the time of diagnosis (N = 4), were included.
Only people who were residents of Olmsted County for at least 1 year before being diagnosed with hepatitis C are included. This step was necessary to ensure that all subjects were community members. Besides its large liver transplantation service, Olmsted County has several inpatient and outpatient chemical dependency treatment programs and halfway houses that may bring patients with hepatitis C to the community for short periods of time. Inclusion of these people would have skewed the community-based focus of the study. Prisoners incarcerated in local facilities were excluded as well.
Measures
For each subject in the cohort, we reviewed all medical records from the Mayo Clinic and hospitals, the Olmsted Medical Center and hospital, and all other care providers in the county. Data collected included information on the initial diagnostic process as well as on HCV-related follow-up; specifically, all aspartate aminotransferase/amino alanine transferase (AST/ALT) testing and all HCV treatment given. All diagnoses of cirrhosis, ascites, gastrointestinal (GI) bleeding, encephalopathy, jaundice, and hepatocellular carcinoma were recorded. Data on risk factors as well as on comorbid conditions believed to influence the progression of HCV-related liver disease (eg, alcoholism, chronic heavy alcohol ingestion, hepatitis B, and HIV disease) were noted.
Data analysis
We summarized demographic information and data on risk factors, comorbid conditions, the pattern of laboratory test follow-up, and HCV treatment and, when appropriate, stratified these data by date of diagnosis. We used logistic regression models to look for associations among personal, demographic, and clinical factors associated with continued AST/ALT follow-up 1 or more years after initial HCV diagnosis.
Results
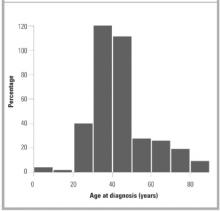
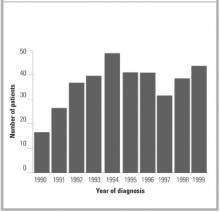
Of the 355 subjects with a diagnosis of hepatitis C between January 1, 1990, and December 31, 1999, 136 (38%) were women and 219 (62%) were men. The mean age at diagnosis was 42.6 years (Figure 1). The rate of new diagnoses of hepatitis C varied only slightly by year (Figure 2). After the period 1990–92, when HCV testing first became available, the difference in rates of new diagnoses is not statistically significant.
Complete follow-up data from the date of diagnosis until December 31, 1999, or the subject’s death were available in 78% of subjects with mean follow-up of 3.6 years, median 3.0 years, and range 0 to 9.8 years. Other subjects were lost to follow-up after they moved from the community; however, vital status (dead or alive) was obtained in 85% of all subjects as of January 1, 2000.
IDU was documented in 177 cases (50% of subjects) (Table 1) with the mean duration of 9.6 years (SD 7.9 years, range single use to 34 years). A single risk factor was recorded for 89 subjects (69 who had had a blood transfusion before 1992 and 20 health care workers with possible exposure to blood products or body fluids, including 5 with documented needlesticks). Sexual exposure and IDU were frequent coexisting risk factors.
All subjects had a positive anti-hepatitis C antibody test; 304 (86%) had a positive RIBA; 13 (4%) had an indeterminate RIBA with risk factors; 14 (3.9%) had PCR tests used in the diagnostic process; and the rest (n = 24) had only positive serology plus risk factors. Overall, 202 people (60%) were seen by a GI or hepatology specialist at least once after the diagnosis of HCV had been made. Confirmatory liver biopsies or PCR tests were used at some time in the follow-up of 157 subjects (44%), usually before the consideration of treatment or after referral to a hepatologist.
Among subjects, 21 (no gender differences) had hepatic decompensation, defined as cirrhosis with ascites, encephalopathy, or jaundice, or hepatocellular carcinoma identified either before or within 1 month of the hepatitis C diagnosis. These findings suggest that HCV evaluation was based on the presence of advanced liver disease. Thirty-seven (10%) of the patients, including 5 who died within days to weeks of the initial HCV diagnosis, died during the observation period.
At or around the time (±1 month) of diagnosis, serum albumin (n = 215, 61%), bilirubin (n = 265, 75%), and ALT or AST (n = 308, 87%) tests were commonly done. Albumin and bilirubin levels were normal in almost all cases (99% and 85%, respectively). The majority of the initial serum ALT/AST levels were elevated (262/308, 85%). Although the elevation was often modest, levels of 119 of the 246 initial tests (48%) were less than 2 times the upper limit of normal.
Follow-up of initial AST/ALT testing was not universal. Among subjects, 51% had one or more rechecks of liver function tests (LFTs) during the first year after diagnosis; 55%, 1 to 2 years after diagnosis; 56%, 2 to 3 years after diagnosis; and 45%, 3 to 4 years after diagnosis, based on the number of subjects not lost to follow-up for 1 to 4 years. Some subjects lost to hepatitis C follow-up had periods of active alcohol or drug abuse that appeared to disrupt hepatitis C care. Variations in rates of continued monitoring of AST/ALT, however, were not associated with risk factors such as IDU or transfusion nor with demographic factors such as age. Long-term follow-up (3 to 4 years after diagnosis) was associated with AST/ALT levels more than 2 times normal at diagnosis (P = .03) and a diagnosis of cirrhosis (P= .03). Women were more likely to have a repeat evaluation in the first year, but no gender differences were seen after that.
During the period of observation, which ended December 31, 1999, 49 subjects (14%) received interferon treatment specifically for their hepatitis C. Half of subjects (n = 25) received that treatment during clinical trials. Twenty people (12 in clinical trials) received ribavirin in addition to interferon; 1 received interferon and interleukin.
Many of the other 306 subjects had 1 or more documented contraindications to HCV therapy (Table 2). The 53 instances of chemical dependency may underestimate the effect of chronic alcohol ingestion on decisions not to treat, since among the 355 subjects, documentation of chronic heavy alcohol consumption (>6 drinks/day) was listed in the medical records of 182 (51%). The total number of subjects with one or more conditions that might be considered contraindications to therapy was 225 (63%). Although only a few of these contraindications were permanent conditions (eg, vegetative state) (Table 2), no subjects had a repeat reference to treatment after the documented condition (eg, depression) had resolved or improved.
Information on potentially accelerating comorbid conditions was available in many charts (ie, those of 11 subjects with known HIV infection). No HIV testing was documented, however, in 55 people who had HCV risk factors other than blood transfusion. Five people had documented chronic HBV infections (surface antigen positive). HBV screening was almost universal. Yet 159 subjects were not immune (including no documented HBV immunizations), and of these, 108 had HCV risk factors other than transfusion before 1992 and were therefore eligible for HBV immunization. Hepatitis A vaccination is now recommended for all nonimmune HCV patients,5 but immunization for hepatitis A was documented for only 25 subjects.
TABLE 1
RISK FACTORS NOTED AT DIAGNOSTIC VISIT
| Risk Factor | No. of Patients (%) (N=355) |
|---|---|
| History of intravenous drug use | 177 (50%) |
| Sexual exposure | 128 (36%) |
| Immigrant | 47 (13%) |
| Tattoos | 60 (17%) |
| History of blood transfusion | 107 (30%) |
| Occupational risks | 44 (12%) |
TABLE 2
REASONS DOCUMENTED FOR NOT RECEIVING TREATMENT
| Reason | No. of Patients (%) (N=306) |
|---|---|
| No reason stated | 81 (26%) |
| Chemical dependency | 53 (17%) |
| Comorbid condition | 28 (9%) |
| Refused/noncompliant | 44 (14%) |
| Age | 13 (4%) |
| Ineligible | 22 (7%)* |
| Depression | 25 (8%) |
| Psychiatric condition | 4 (1%) |
| Desired pregnancy | 5 (2%) |
| Cirrhosis | 6 (2%) |
| Hepatocellular carcinoma | 8 (3%) |
| HIV treatment | 7 (2%) |
| No insurance | 6 (1%) |
| No trial available | 4 (1%) |
| *Ineligible because of advanced liver disease or other terminal illness. | |
FIGURE 1
AGE AT DIAGNOSIS
FIGURE 2
NUMBER OF NEW DIAGNOSES PER YEAR
Discussion
Hepatitis C was an uncommon new diagnosis in Olmsted County and therefore not a frequent occurrence in the practice of any of the 200 primary care physicians in the community. Although the overall prevalence of hepatitis C was only about 25% of that reported for the Midwestern United States (1.3%),3,4 the ratio of men and women diagnosed with HCV and the distribution of recorded risk factors in our cohort are similar to those reported from the NHANES conducted in 1988 to 19944 and to those in other population-based studies.5,15-17
In this cohort, primary care physicians’ response to known HCV varied from occasional monitoring of AST/ALT tests to referral for specialty evaluation and HCV treatment. In a significant group of patients, no visits for HCV follow-up could be identified. The only other published data on primary care physicians’ follow-up care of patients with known HCV was self-report survey data.18 In response to a survey, primary care physicians reported they ordered yearly AST/ALT tests in all HCV patients and referred over 80% of people with known HCV to hepatologists. Self-reported care often overestimates the amount of care provided. This difference in reported and observed care emphasizes the importance of data on actual practice.
The community physicians’ lack of a uniform or aggressive approach to HCV infections may not be surprising in view of the wide disparity of available information on chronic HCV infection and its progression to symptomatic or progressive liver dis-ease.18 For example, published rates of progression to “chronic infection” after exposure vary from 85% of people receiving HCV-infected blood transfusions to 20% of women given HCV-contaminated RhoGAM.9 Even rates of progression in people with persistent viremia (+PCR) vary from 69 % to 88%.19 Unfortunately, progression to chronic HCV infection cannot be predicted from initial clinical or laboratory factors.20 In addition, the meaning of “chronic infection” is unclear.19 Reported rates of progression from chronic infection to cirrhosis are widely divergent and appear related to the type of group used to make predictions. Data from meta-analyses of clinical trial patients21 suggest progression rates of up to 69% in 30 years. Yet only 2.4% to 6% of community-based hepatitis C patients may develop cirrhosis after 17 to 40 years of follow-up.22,23
Wide variations in the progression of cirrhosis to decompensated cirrhosis have been reported.24 Studies of AST levels in people with known HCV show fluctuations over time, dispelling the idea that once elevated, AST or ALT will remain elevated or that AST levels are directly predictive of progression or resolution of liver injury or viral loads.25,26 Chronically elevated AST/ALT levels, however, have been shown to predict progression,27 making monitoring of AST/ALT important in all subjects.5,28
That HCV treatment was uncommon in this population may reflect the limited efficacy of single-agent therapy before 1998, as well as the large proportion of subjects with contraindications to therapy, primary care physician confusion regarding who should be treated, and current limited knowledge of the long-term outcomes of treatment.29 Cure rates are unknown and measures of cure are unclear, since clearing the virus from the bloodstream (negative PCR) may not confirm clearing of the virus from the liver.29 All these data together provide little experimental evidence for a standard set of recommendations for follow-up, nor do they support a clear rationale for the use of those follow-up data in determining HCV progression. The existing data have been used to develop consensus (expert opinion–based) guidelines published by NIH (1997)28 and CDC (1998,30 with an update in the summer of 2001). The substance of those consensus statements has changed over time5,28 as experts’ experience has increased and newer observations have become available. Recent changes in available treatments31-33 and FDA approval of a pegylated inferferon34,35 are likely to keep recommendations in flux for the near future.
The limited attention given to the identification, prevention, or treatment of comorbid accelerating conditions (HIV, HBV, HAV, and heavy alcohol intake)5,19,27,36-38 in this cohort is less understandable, since the literature is more consistent on these issues. Documented HIV testing was not universal even in those with a history of IDU or promiscuous sexual exposure. While testing for HBV was almost universal, HBV prevention in the form of vaccination was documented in only about one fourth (23%) of eligible subjects. Immunization for hepatitis A was documented in less than one tenth of subjects. Treatment or documented physician recommendations for treatment of ongoing alcohol abuse or heavy alccohol ingestion occurred in the minority of patients. Studies of combined chemical dependency and HCV therapy might be appropriate in this population.
The Olmsted County population from which the subjects were identified is more than 90% white. Therefore, community-based prevalence rates of physician diagnoses may not be representative of other racial or ethnic groups. The high frequency of drug use and sexual exposure suggests that our community’s problems associated with HCV are similar to those identified in other communities with greater economic, ethnic, and racial diversity. We did not perform PCR, liver biopsy, or yearly liver function tests on all subjects. One aim of our study, however, was to understand community practice and the resulting variations in information and testing completed for each subject. The existence of a large medical education program and the local availability of hepatologists may affect the care provided. Yet even in this setting, additional attention to follow-up of liver disease and comorbid conditions appeared indicated.
Conclusions
Primary care physicians make most diagnoses and perform most initial management of hepatitis C. However, primary care directed long term follow-up care is inconsistent and management of accelerating comorbidities is incomplete. Family physicians can offer important additional services to their patients who have hepatitis C.
1. Centers for Disease Control. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep 1998;47(No RR-19):1-39.
2. McQuillan G, Alter M, Moyer L, Lambert S, Margolis H. A population-based serologic study of hepatitis C virus infection in the United States. Am J Epidemiol 1996;143(suppl):S32.-
3. Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 2000;31:777-82.
4. Alter M, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999;341:556-62.
5. Management of hepatitis C. NIH Consens Statement Online 1997 Mar 24-26 [cited 2001, July 23]; 15(3):1-41.
6. Takahashi M, Yamada G, Miyamoto R, Doi T, Endo H, Tsuji T. Natural course of chronic hepatitis C. Am J Gastroenterol 1993;88:240-3.
7. Kiyosawa K, Tanaka E, Sodeyama T, Furuta S. Natural history of hepatitis C. Intervirology 1994;37:101-7.
8. Muller R. The natural history of hepatitis C: clinical experiences. J Hepatology 1996;24(suppl 2):52-4.
9. Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. N Engl J Med 1999;340:1228-33.
10. Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med 1995;332:1463-6.
11. Kaur S, Rybicki L, Bacon BR, Gollan JL, Rustgi VK, Carey WD, and the National Hepatitis Surveillance Group. Performance characteristics and results of a large-scale screening program for viral hepatitis and risk factors associated with exposure to viral hepatitis B and C: results of the National Hepatitis Screening Survey. Hepatology 1996;24:979-86.
12. Makris M, Preston FE, Rosendaal FR, Underwood JCE, Rice KM, Triger DR. The natural history of chronic hepatitis C in haemophiliacs. Br J Haematol 1996;94:746-52.
13. Melton LJ, III. History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71:266-74.
14. Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am 1981;245:54-63.
15. Alter MJ. Epidemiology of hepatitis C in the West. Semin Liver Dis 1995;15:5-14.
16. Tillman HL, Manns MP. Mode of hepatitis C virus infection, epidemiology, and chronicity rate in the general population and risk groups. Dig Dis Sci 1996;41(12 suppl):27S-40S.
17. Murphy EL, Bryzman SM, Glynn SA, et al. Risk factors for hepatitis C virus infection in United States blood donors. Hepatology 2000;31:756-62.
18. Shehab TM, Sonnad SS, Jeffries M, Gunaratnum N, Lok ASF. Current practice patterns of primary care physicians in the management of patients with hepatitis C. Hepatology 1999;30:794-800.
19. Muir AJ. The natural history of hepatitis C viral infection. Semin Gastrointest Dis 2000;11:54-61.
20. Villano SA, Vlahov D, Nelson K, et al. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology 1999;29:908-14.
21. Poynard T, Bedossa P, Opolon P, et al. Natural history of liver fibro-sis progression in patients with chronic hepatitis C. Lancet 1997;346:825-32.
22. Crowe J, Doyle C, Fielding JF, et al. Presentation of hepatitis C in a unique uniform cohort 17 years from inoculation. Gastroenterology 1995;108:A1054. Abstract.-
23. Seeff LB, Miller RN, Rabkin CS, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med 2000;132:105-11.
24. Lionis C, Vlachonikolis IG, Skliros S, Symeonidis A, Merjiyrus BP, Kouroumalis E. Do undefined sources of hepatitis C transmission exist? The Greek study in general practice. J Viral Hepatitis 2000;7:218-24.
25. Bellentani S, Pozzato G, Saccoccio G, et al. Clinical course and risk factors of HCV related liver disease in the general population: report from the Dionysos study. Gut 1999;44:874-80.
26. Inglesby TV, Rai R, Astemborski J, et al. A prospective, community-based evaluation of liver enzymes in individuals with hepatitis C after drug use. Hepatology 1999;29:590-6.
27. Liang TJ (moderator), Rehermann B, Seef LB, Hofnagle JH (disscussants). Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med 2000;132:296-305.
28. Management of Hepatitis C. NIH Consens Statement Online 1997 Mar 24-26 [cited 2001, July 23]; 15(3):-41.
29. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep 1998;47(RR-19):1-39 [158 ].
30. Haydon GH, Jarvis LM, Blair CS, et al. Clinical significance of intra-hepatic hepatitis C virus levels in patients with chronic HCV infection. Gut 1998;42:570-5.
31. Wong JB, Bennett WG, Koff RS, Pauker SG. Pretreatment evaluation of chronic hepatitis C. JAMA 1998;280:2088-2093.
32. McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med 1998;339:1485-92.
33. Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha-2b plus ribavirin for 48 weeks or for 24 weeks versus inter-feron alpha-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 1998;352:1426-32.
34. Zeuzem S, Feinman SV, Rasenack J, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med 2000;343:1666-72.
35. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2a plus ribavirin for initial treatment of chronic hepatitis C: Results of a randomised trial. Lancet 2001;358:958-65.
36. Xiaowei F. Hepatitis C infection: a review. Lippincott Prim Care Pract 1999;3:345-53.
37. Serfaty L, Chazouilleres O, Poujol-Robert A, et al. Risk factors for cirrhosis in patients with chronic HCV infection: results of a case-control study. Hepatology 1997;26:776-9.
38. Jenkins PJ, Cromie SL, Roberts SK, et al. Chronic hepatitis C, alcohol and hepatic fibrosis. Hepatology 1996;24:153A. Abstract.
OBJECTIVE: To determine the health care follow-up and treatment associated with physician-diagnosed hepatitis C (HCV) in a community-based population.
STUDY DESIGN: We conducted a retrospective medical record review using records from all providers in Olmsted County, Minnesota.
POPULATION: The study incorporated all Olmsted County residents with physician-diagnosed hepatitis C from 1990 through 1999.
OUTCOMES MEASURED: We assessed demographic and health status information as well as health services use in subjects with physician-diagnosed HCV.
RESULTS: Physicians diagnosed hepatitis C in 355 subjects (219 men [62%], 136 women [38%]), mean age 43 years, in the 10-year period studied. About half of diagnoses (45%, n = 159) were confirmed with polymerase chain reaction or liver biopsies. Identified risk factors included IV drug use (50%), multiple sex partners (36%), and blood transfusion (30%). Follow-up assessment with aspartate aminotransferase/amino alanine transferase (AST/ALT) tests occurred in about half (49%) of subjects, while 202 subjects (60%) were referred for gastrointestinal (GI) specialist evaluation and 49 patients (14% of all, 25% of those referred to a GI specialist) had specific treatment for hepatitis C. Although well over half of patients (60%) had possible contraindications to HCV treatment, including heavy alcohol use, few were referred for chemical dependency therapy.
CONCLUSIONS: In this community, follow-up and treatment related to HCV were limited. Attention to prevention of disease-accelerating coinfections was only modest. Referral or documented recommendations for treatment of alcoholism or heavy chronic alcohol ingestion were minimal.
- Risk factors associated with acquiring hepatitis C in this small city are similar to those in urban areas: intravenous drug use, frequent sexual exposure, and blood transfusion before 1992.
- No follow-up of liver testing in people with known hepatitis C occurred in half of cases.
- Less than 15% of prevalent cases received hepatitis C–specific treatment; many people appeared to have contraindications for hepatitis C therapy.
- Preventive care measures appropriate for people with hepatitis C (eg, hepatitis B immunization, referral for treatment of known chemical dependency, and screening for HIV) were not universal in this population.
Hepatitis C is reportedly the most common chronic bloodborne infectious disease in the United States.1-3 Prevalence data based on modeling studies and extrapolation from studies such as the National Health and Nutrition Examination Survey (NHANES) and county surveillance projects3,4 report that 3.9 million Americans (1.8%) have been infected with the hepatitis C virus (HCV) and 2.7 million (1.2%) have chronic HCV infection.5 While the natural history of HCV infections is poorly understood,6-9 researchers and clinicians agree that most people with chronic HCV infection remain asymptomatic for many years while seeking medical care, often primary care, for problems other than their silent hepatitis C infections.8,9
Much of the hepatitis C literature is derived from studies of subspecialty clinic patient populations or other special populations such as those with blood transfusion or RhoGAM-acquired hepatitis C.9-12 The former data tend to concentrate on the more severe, symptomatic patients referred to specialty care, whereas the latter special-exposure groups provide little information about a large segment of the population with other risk factors and comorbidity. Neither of these sources provides data on the community population or community practice.1,9,11 Our study describes the people that have been diagnosed with HCV in a geographically defined community, including their physician-directed hepatitis C follow-up evaluations and HCV treatment. In addition, we report on testing and vaccination efforts for hepatitis A and B and on the recognition and treatment of alcoholism and chronic heavy alcohol ingestion (accelerating comorbidities). The ability to follow the patient across all types of care from ambulatory to inpatient and from primary to tertiary care provides a very broad overview of these population-based cases.
Methods
Study setting and instrument
This is a descriptive study of a geographically defined, population-based cohort of all persons living in Olmsted County, Minnesota, who received a physician or laboratory diagnosis of hepatitis C from January 1, 1990, through December 31, 1999. All physician diagnoses were captured using a communitywide diagnostic database, the Rochester Epidemiology Project.13,14 Olmsted County is a metropolitan statistical area that includes the city of Rochester and is served primarily by more than 200 primary care physicians employed by 2 medical facilities, the Olmsted Medical Center and the Mayo Clinic.
All patients with a physician diagnosis of hepatitis C were included, whether the diagnosis had been confirmed by liver biopsy or polymerase chain reaction (PCR) testing or by either positive recombinant immunoblast assay (RIBA) testing or indeterminate RIBA testing and the presence of 1 or more risk factors. Risk factors included blood transfusion before July 1,1992, a diagnosis of hemophilia before 1990, a history of intravenous drug use (IDU), selling sex or trading sex for drugs, having more than 10 sex partners, sexual exposure to a person infected with HCV, intranasal cocaine use, and work in a health care facility with exposure to blood products (eg, phlebotomists) or health care workers with a history of needlesticks. The immigration status of people from Africa or Southeast Asia, where hepatitis C is endemic, is reported when the medical record listed immigration as an HCV risk factor. Patients with acute hepatitis C, defined as those with acute symptoms at the time of diagnosis (N = 4), were included.
Only people who were residents of Olmsted County for at least 1 year before being diagnosed with hepatitis C are included. This step was necessary to ensure that all subjects were community members. Besides its large liver transplantation service, Olmsted County has several inpatient and outpatient chemical dependency treatment programs and halfway houses that may bring patients with hepatitis C to the community for short periods of time. Inclusion of these people would have skewed the community-based focus of the study. Prisoners incarcerated in local facilities were excluded as well.
Measures
For each subject in the cohort, we reviewed all medical records from the Mayo Clinic and hospitals, the Olmsted Medical Center and hospital, and all other care providers in the county. Data collected included information on the initial diagnostic process as well as on HCV-related follow-up; specifically, all aspartate aminotransferase/amino alanine transferase (AST/ALT) testing and all HCV treatment given. All diagnoses of cirrhosis, ascites, gastrointestinal (GI) bleeding, encephalopathy, jaundice, and hepatocellular carcinoma were recorded. Data on risk factors as well as on comorbid conditions believed to influence the progression of HCV-related liver disease (eg, alcoholism, chronic heavy alcohol ingestion, hepatitis B, and HIV disease) were noted.
Data analysis
We summarized demographic information and data on risk factors, comorbid conditions, the pattern of laboratory test follow-up, and HCV treatment and, when appropriate, stratified these data by date of diagnosis. We used logistic regression models to look for associations among personal, demographic, and clinical factors associated with continued AST/ALT follow-up 1 or more years after initial HCV diagnosis.
Results
Of the 355 subjects with a diagnosis of hepatitis C between January 1, 1990, and December 31, 1999, 136 (38%) were women and 219 (62%) were men. The mean age at diagnosis was 42.6 years (Figure 1). The rate of new diagnoses of hepatitis C varied only slightly by year (Figure 2). After the period 1990–92, when HCV testing first became available, the difference in rates of new diagnoses is not statistically significant.
Complete follow-up data from the date of diagnosis until December 31, 1999, or the subject’s death were available in 78% of subjects with mean follow-up of 3.6 years, median 3.0 years, and range 0 to 9.8 years. Other subjects were lost to follow-up after they moved from the community; however, vital status (dead or alive) was obtained in 85% of all subjects as of January 1, 2000.
IDU was documented in 177 cases (50% of subjects) (Table 1) with the mean duration of 9.6 years (SD 7.9 years, range single use to 34 years). A single risk factor was recorded for 89 subjects (69 who had had a blood transfusion before 1992 and 20 health care workers with possible exposure to blood products or body fluids, including 5 with documented needlesticks). Sexual exposure and IDU were frequent coexisting risk factors.
All subjects had a positive anti-hepatitis C antibody test; 304 (86%) had a positive RIBA; 13 (4%) had an indeterminate RIBA with risk factors; 14 (3.9%) had PCR tests used in the diagnostic process; and the rest (n = 24) had only positive serology plus risk factors. Overall, 202 people (60%) were seen by a GI or hepatology specialist at least once after the diagnosis of HCV had been made. Confirmatory liver biopsies or PCR tests were used at some time in the follow-up of 157 subjects (44%), usually before the consideration of treatment or after referral to a hepatologist.
Among subjects, 21 (no gender differences) had hepatic decompensation, defined as cirrhosis with ascites, encephalopathy, or jaundice, or hepatocellular carcinoma identified either before or within 1 month of the hepatitis C diagnosis. These findings suggest that HCV evaluation was based on the presence of advanced liver disease. Thirty-seven (10%) of the patients, including 5 who died within days to weeks of the initial HCV diagnosis, died during the observation period.
At or around the time (±1 month) of diagnosis, serum albumin (n = 215, 61%), bilirubin (n = 265, 75%), and ALT or AST (n = 308, 87%) tests were commonly done. Albumin and bilirubin levels were normal in almost all cases (99% and 85%, respectively). The majority of the initial serum ALT/AST levels were elevated (262/308, 85%). Although the elevation was often modest, levels of 119 of the 246 initial tests (48%) were less than 2 times the upper limit of normal.
Follow-up of initial AST/ALT testing was not universal. Among subjects, 51% had one or more rechecks of liver function tests (LFTs) during the first year after diagnosis; 55%, 1 to 2 years after diagnosis; 56%, 2 to 3 years after diagnosis; and 45%, 3 to 4 years after diagnosis, based on the number of subjects not lost to follow-up for 1 to 4 years. Some subjects lost to hepatitis C follow-up had periods of active alcohol or drug abuse that appeared to disrupt hepatitis C care. Variations in rates of continued monitoring of AST/ALT, however, were not associated with risk factors such as IDU or transfusion nor with demographic factors such as age. Long-term follow-up (3 to 4 years after diagnosis) was associated with AST/ALT levels more than 2 times normal at diagnosis (P = .03) and a diagnosis of cirrhosis (P= .03). Women were more likely to have a repeat evaluation in the first year, but no gender differences were seen after that.
During the period of observation, which ended December 31, 1999, 49 subjects (14%) received interferon treatment specifically for their hepatitis C. Half of subjects (n = 25) received that treatment during clinical trials. Twenty people (12 in clinical trials) received ribavirin in addition to interferon; 1 received interferon and interleukin.
Many of the other 306 subjects had 1 or more documented contraindications to HCV therapy (Table 2). The 53 instances of chemical dependency may underestimate the effect of chronic alcohol ingestion on decisions not to treat, since among the 355 subjects, documentation of chronic heavy alcohol consumption (>6 drinks/day) was listed in the medical records of 182 (51%). The total number of subjects with one or more conditions that might be considered contraindications to therapy was 225 (63%). Although only a few of these contraindications were permanent conditions (eg, vegetative state) (Table 2), no subjects had a repeat reference to treatment after the documented condition (eg, depression) had resolved or improved.
Information on potentially accelerating comorbid conditions was available in many charts (ie, those of 11 subjects with known HIV infection). No HIV testing was documented, however, in 55 people who had HCV risk factors other than blood transfusion. Five people had documented chronic HBV infections (surface antigen positive). HBV screening was almost universal. Yet 159 subjects were not immune (including no documented HBV immunizations), and of these, 108 had HCV risk factors other than transfusion before 1992 and were therefore eligible for HBV immunization. Hepatitis A vaccination is now recommended for all nonimmune HCV patients,5 but immunization for hepatitis A was documented for only 25 subjects.
TABLE 1
RISK FACTORS NOTED AT DIAGNOSTIC VISIT
| Risk Factor | No. of Patients (%) (N=355) |
|---|---|
| History of intravenous drug use | 177 (50%) |
| Sexual exposure | 128 (36%) |
| Immigrant | 47 (13%) |
| Tattoos | 60 (17%) |
| History of blood transfusion | 107 (30%) |
| Occupational risks | 44 (12%) |
TABLE 2
REASONS DOCUMENTED FOR NOT RECEIVING TREATMENT
| Reason | No. of Patients (%) (N=306) |
|---|---|
| No reason stated | 81 (26%) |
| Chemical dependency | 53 (17%) |
| Comorbid condition | 28 (9%) |
| Refused/noncompliant | 44 (14%) |
| Age | 13 (4%) |
| Ineligible | 22 (7%)* |
| Depression | 25 (8%) |
| Psychiatric condition | 4 (1%) |
| Desired pregnancy | 5 (2%) |
| Cirrhosis | 6 (2%) |
| Hepatocellular carcinoma | 8 (3%) |
| HIV treatment | 7 (2%) |
| No insurance | 6 (1%) |
| No trial available | 4 (1%) |
| *Ineligible because of advanced liver disease or other terminal illness. | |
FIGURE 1
AGE AT DIAGNOSIS
FIGURE 2
NUMBER OF NEW DIAGNOSES PER YEAR
Discussion
Hepatitis C was an uncommon new diagnosis in Olmsted County and therefore not a frequent occurrence in the practice of any of the 200 primary care physicians in the community. Although the overall prevalence of hepatitis C was only about 25% of that reported for the Midwestern United States (1.3%),3,4 the ratio of men and women diagnosed with HCV and the distribution of recorded risk factors in our cohort are similar to those reported from the NHANES conducted in 1988 to 19944 and to those in other population-based studies.5,15-17
In this cohort, primary care physicians’ response to known HCV varied from occasional monitoring of AST/ALT tests to referral for specialty evaluation and HCV treatment. In a significant group of patients, no visits for HCV follow-up could be identified. The only other published data on primary care physicians’ follow-up care of patients with known HCV was self-report survey data.18 In response to a survey, primary care physicians reported they ordered yearly AST/ALT tests in all HCV patients and referred over 80% of people with known HCV to hepatologists. Self-reported care often overestimates the amount of care provided. This difference in reported and observed care emphasizes the importance of data on actual practice.
The community physicians’ lack of a uniform or aggressive approach to HCV infections may not be surprising in view of the wide disparity of available information on chronic HCV infection and its progression to symptomatic or progressive liver dis-ease.18 For example, published rates of progression to “chronic infection” after exposure vary from 85% of people receiving HCV-infected blood transfusions to 20% of women given HCV-contaminated RhoGAM.9 Even rates of progression in people with persistent viremia (+PCR) vary from 69 % to 88%.19 Unfortunately, progression to chronic HCV infection cannot be predicted from initial clinical or laboratory factors.20 In addition, the meaning of “chronic infection” is unclear.19 Reported rates of progression from chronic infection to cirrhosis are widely divergent and appear related to the type of group used to make predictions. Data from meta-analyses of clinical trial patients21 suggest progression rates of up to 69% in 30 years. Yet only 2.4% to 6% of community-based hepatitis C patients may develop cirrhosis after 17 to 40 years of follow-up.22,23
Wide variations in the progression of cirrhosis to decompensated cirrhosis have been reported.24 Studies of AST levels in people with known HCV show fluctuations over time, dispelling the idea that once elevated, AST or ALT will remain elevated or that AST levels are directly predictive of progression or resolution of liver injury or viral loads.25,26 Chronically elevated AST/ALT levels, however, have been shown to predict progression,27 making monitoring of AST/ALT important in all subjects.5,28
That HCV treatment was uncommon in this population may reflect the limited efficacy of single-agent therapy before 1998, as well as the large proportion of subjects with contraindications to therapy, primary care physician confusion regarding who should be treated, and current limited knowledge of the long-term outcomes of treatment.29 Cure rates are unknown and measures of cure are unclear, since clearing the virus from the bloodstream (negative PCR) may not confirm clearing of the virus from the liver.29 All these data together provide little experimental evidence for a standard set of recommendations for follow-up, nor do they support a clear rationale for the use of those follow-up data in determining HCV progression. The existing data have been used to develop consensus (expert opinion–based) guidelines published by NIH (1997)28 and CDC (1998,30 with an update in the summer of 2001). The substance of those consensus statements has changed over time5,28 as experts’ experience has increased and newer observations have become available. Recent changes in available treatments31-33 and FDA approval of a pegylated inferferon34,35 are likely to keep recommendations in flux for the near future.
The limited attention given to the identification, prevention, or treatment of comorbid accelerating conditions (HIV, HBV, HAV, and heavy alcohol intake)5,19,27,36-38 in this cohort is less understandable, since the literature is more consistent on these issues. Documented HIV testing was not universal even in those with a history of IDU or promiscuous sexual exposure. While testing for HBV was almost universal, HBV prevention in the form of vaccination was documented in only about one fourth (23%) of eligible subjects. Immunization for hepatitis A was documented in less than one tenth of subjects. Treatment or documented physician recommendations for treatment of ongoing alcohol abuse or heavy alccohol ingestion occurred in the minority of patients. Studies of combined chemical dependency and HCV therapy might be appropriate in this population.
The Olmsted County population from which the subjects were identified is more than 90% white. Therefore, community-based prevalence rates of physician diagnoses may not be representative of other racial or ethnic groups. The high frequency of drug use and sexual exposure suggests that our community’s problems associated with HCV are similar to those identified in other communities with greater economic, ethnic, and racial diversity. We did not perform PCR, liver biopsy, or yearly liver function tests on all subjects. One aim of our study, however, was to understand community practice and the resulting variations in information and testing completed for each subject. The existence of a large medical education program and the local availability of hepatologists may affect the care provided. Yet even in this setting, additional attention to follow-up of liver disease and comorbid conditions appeared indicated.
Conclusions
Primary care physicians make most diagnoses and perform most initial management of hepatitis C. However, primary care directed long term follow-up care is inconsistent and management of accelerating comorbidities is incomplete. Family physicians can offer important additional services to their patients who have hepatitis C.
OBJECTIVE: To determine the health care follow-up and treatment associated with physician-diagnosed hepatitis C (HCV) in a community-based population.
STUDY DESIGN: We conducted a retrospective medical record review using records from all providers in Olmsted County, Minnesota.
POPULATION: The study incorporated all Olmsted County residents with physician-diagnosed hepatitis C from 1990 through 1999.
OUTCOMES MEASURED: We assessed demographic and health status information as well as health services use in subjects with physician-diagnosed HCV.
RESULTS: Physicians diagnosed hepatitis C in 355 subjects (219 men [62%], 136 women [38%]), mean age 43 years, in the 10-year period studied. About half of diagnoses (45%, n = 159) were confirmed with polymerase chain reaction or liver biopsies. Identified risk factors included IV drug use (50%), multiple sex partners (36%), and blood transfusion (30%). Follow-up assessment with aspartate aminotransferase/amino alanine transferase (AST/ALT) tests occurred in about half (49%) of subjects, while 202 subjects (60%) were referred for gastrointestinal (GI) specialist evaluation and 49 patients (14% of all, 25% of those referred to a GI specialist) had specific treatment for hepatitis C. Although well over half of patients (60%) had possible contraindications to HCV treatment, including heavy alcohol use, few were referred for chemical dependency therapy.
CONCLUSIONS: In this community, follow-up and treatment related to HCV were limited. Attention to prevention of disease-accelerating coinfections was only modest. Referral or documented recommendations for treatment of alcoholism or heavy chronic alcohol ingestion were minimal.
- Risk factors associated with acquiring hepatitis C in this small city are similar to those in urban areas: intravenous drug use, frequent sexual exposure, and blood transfusion before 1992.
- No follow-up of liver testing in people with known hepatitis C occurred in half of cases.
- Less than 15% of prevalent cases received hepatitis C–specific treatment; many people appeared to have contraindications for hepatitis C therapy.
- Preventive care measures appropriate for people with hepatitis C (eg, hepatitis B immunization, referral for treatment of known chemical dependency, and screening for HIV) were not universal in this population.
Hepatitis C is reportedly the most common chronic bloodborne infectious disease in the United States.1-3 Prevalence data based on modeling studies and extrapolation from studies such as the National Health and Nutrition Examination Survey (NHANES) and county surveillance projects3,4 report that 3.9 million Americans (1.8%) have been infected with the hepatitis C virus (HCV) and 2.7 million (1.2%) have chronic HCV infection.5 While the natural history of HCV infections is poorly understood,6-9 researchers and clinicians agree that most people with chronic HCV infection remain asymptomatic for many years while seeking medical care, often primary care, for problems other than their silent hepatitis C infections.8,9
Much of the hepatitis C literature is derived from studies of subspecialty clinic patient populations or other special populations such as those with blood transfusion or RhoGAM-acquired hepatitis C.9-12 The former data tend to concentrate on the more severe, symptomatic patients referred to specialty care, whereas the latter special-exposure groups provide little information about a large segment of the population with other risk factors and comorbidity. Neither of these sources provides data on the community population or community practice.1,9,11 Our study describes the people that have been diagnosed with HCV in a geographically defined community, including their physician-directed hepatitis C follow-up evaluations and HCV treatment. In addition, we report on testing and vaccination efforts for hepatitis A and B and on the recognition and treatment of alcoholism and chronic heavy alcohol ingestion (accelerating comorbidities). The ability to follow the patient across all types of care from ambulatory to inpatient and from primary to tertiary care provides a very broad overview of these population-based cases.
Methods
Study setting and instrument
This is a descriptive study of a geographically defined, population-based cohort of all persons living in Olmsted County, Minnesota, who received a physician or laboratory diagnosis of hepatitis C from January 1, 1990, through December 31, 1999. All physician diagnoses were captured using a communitywide diagnostic database, the Rochester Epidemiology Project.13,14 Olmsted County is a metropolitan statistical area that includes the city of Rochester and is served primarily by more than 200 primary care physicians employed by 2 medical facilities, the Olmsted Medical Center and the Mayo Clinic.
All patients with a physician diagnosis of hepatitis C were included, whether the diagnosis had been confirmed by liver biopsy or polymerase chain reaction (PCR) testing or by either positive recombinant immunoblast assay (RIBA) testing or indeterminate RIBA testing and the presence of 1 or more risk factors. Risk factors included blood transfusion before July 1,1992, a diagnosis of hemophilia before 1990, a history of intravenous drug use (IDU), selling sex or trading sex for drugs, having more than 10 sex partners, sexual exposure to a person infected with HCV, intranasal cocaine use, and work in a health care facility with exposure to blood products (eg, phlebotomists) or health care workers with a history of needlesticks. The immigration status of people from Africa or Southeast Asia, where hepatitis C is endemic, is reported when the medical record listed immigration as an HCV risk factor. Patients with acute hepatitis C, defined as those with acute symptoms at the time of diagnosis (N = 4), were included.
Only people who were residents of Olmsted County for at least 1 year before being diagnosed with hepatitis C are included. This step was necessary to ensure that all subjects were community members. Besides its large liver transplantation service, Olmsted County has several inpatient and outpatient chemical dependency treatment programs and halfway houses that may bring patients with hepatitis C to the community for short periods of time. Inclusion of these people would have skewed the community-based focus of the study. Prisoners incarcerated in local facilities were excluded as well.
Measures
For each subject in the cohort, we reviewed all medical records from the Mayo Clinic and hospitals, the Olmsted Medical Center and hospital, and all other care providers in the county. Data collected included information on the initial diagnostic process as well as on HCV-related follow-up; specifically, all aspartate aminotransferase/amino alanine transferase (AST/ALT) testing and all HCV treatment given. All diagnoses of cirrhosis, ascites, gastrointestinal (GI) bleeding, encephalopathy, jaundice, and hepatocellular carcinoma were recorded. Data on risk factors as well as on comorbid conditions believed to influence the progression of HCV-related liver disease (eg, alcoholism, chronic heavy alcohol ingestion, hepatitis B, and HIV disease) were noted.
Data analysis
We summarized demographic information and data on risk factors, comorbid conditions, the pattern of laboratory test follow-up, and HCV treatment and, when appropriate, stratified these data by date of diagnosis. We used logistic regression models to look for associations among personal, demographic, and clinical factors associated with continued AST/ALT follow-up 1 or more years after initial HCV diagnosis.
Results
Of the 355 subjects with a diagnosis of hepatitis C between January 1, 1990, and December 31, 1999, 136 (38%) were women and 219 (62%) were men. The mean age at diagnosis was 42.6 years (Figure 1). The rate of new diagnoses of hepatitis C varied only slightly by year (Figure 2). After the period 1990–92, when HCV testing first became available, the difference in rates of new diagnoses is not statistically significant.
Complete follow-up data from the date of diagnosis until December 31, 1999, or the subject’s death were available in 78% of subjects with mean follow-up of 3.6 years, median 3.0 years, and range 0 to 9.8 years. Other subjects were lost to follow-up after they moved from the community; however, vital status (dead or alive) was obtained in 85% of all subjects as of January 1, 2000.
IDU was documented in 177 cases (50% of subjects) (Table 1) with the mean duration of 9.6 years (SD 7.9 years, range single use to 34 years). A single risk factor was recorded for 89 subjects (69 who had had a blood transfusion before 1992 and 20 health care workers with possible exposure to blood products or body fluids, including 5 with documented needlesticks). Sexual exposure and IDU were frequent coexisting risk factors.
All subjects had a positive anti-hepatitis C antibody test; 304 (86%) had a positive RIBA; 13 (4%) had an indeterminate RIBA with risk factors; 14 (3.9%) had PCR tests used in the diagnostic process; and the rest (n = 24) had only positive serology plus risk factors. Overall, 202 people (60%) were seen by a GI or hepatology specialist at least once after the diagnosis of HCV had been made. Confirmatory liver biopsies or PCR tests were used at some time in the follow-up of 157 subjects (44%), usually before the consideration of treatment or after referral to a hepatologist.
Among subjects, 21 (no gender differences) had hepatic decompensation, defined as cirrhosis with ascites, encephalopathy, or jaundice, or hepatocellular carcinoma identified either before or within 1 month of the hepatitis C diagnosis. These findings suggest that HCV evaluation was based on the presence of advanced liver disease. Thirty-seven (10%) of the patients, including 5 who died within days to weeks of the initial HCV diagnosis, died during the observation period.
At or around the time (±1 month) of diagnosis, serum albumin (n = 215, 61%), bilirubin (n = 265, 75%), and ALT or AST (n = 308, 87%) tests were commonly done. Albumin and bilirubin levels were normal in almost all cases (99% and 85%, respectively). The majority of the initial serum ALT/AST levels were elevated (262/308, 85%). Although the elevation was often modest, levels of 119 of the 246 initial tests (48%) were less than 2 times the upper limit of normal.
Follow-up of initial AST/ALT testing was not universal. Among subjects, 51% had one or more rechecks of liver function tests (LFTs) during the first year after diagnosis; 55%, 1 to 2 years after diagnosis; 56%, 2 to 3 years after diagnosis; and 45%, 3 to 4 years after diagnosis, based on the number of subjects not lost to follow-up for 1 to 4 years. Some subjects lost to hepatitis C follow-up had periods of active alcohol or drug abuse that appeared to disrupt hepatitis C care. Variations in rates of continued monitoring of AST/ALT, however, were not associated with risk factors such as IDU or transfusion nor with demographic factors such as age. Long-term follow-up (3 to 4 years after diagnosis) was associated with AST/ALT levels more than 2 times normal at diagnosis (P = .03) and a diagnosis of cirrhosis (P= .03). Women were more likely to have a repeat evaluation in the first year, but no gender differences were seen after that.
During the period of observation, which ended December 31, 1999, 49 subjects (14%) received interferon treatment specifically for their hepatitis C. Half of subjects (n = 25) received that treatment during clinical trials. Twenty people (12 in clinical trials) received ribavirin in addition to interferon; 1 received interferon and interleukin.
Many of the other 306 subjects had 1 or more documented contraindications to HCV therapy (Table 2). The 53 instances of chemical dependency may underestimate the effect of chronic alcohol ingestion on decisions not to treat, since among the 355 subjects, documentation of chronic heavy alcohol consumption (>6 drinks/day) was listed in the medical records of 182 (51%). The total number of subjects with one or more conditions that might be considered contraindications to therapy was 225 (63%). Although only a few of these contraindications were permanent conditions (eg, vegetative state) (Table 2), no subjects had a repeat reference to treatment after the documented condition (eg, depression) had resolved or improved.
Information on potentially accelerating comorbid conditions was available in many charts (ie, those of 11 subjects with known HIV infection). No HIV testing was documented, however, in 55 people who had HCV risk factors other than blood transfusion. Five people had documented chronic HBV infections (surface antigen positive). HBV screening was almost universal. Yet 159 subjects were not immune (including no documented HBV immunizations), and of these, 108 had HCV risk factors other than transfusion before 1992 and were therefore eligible for HBV immunization. Hepatitis A vaccination is now recommended for all nonimmune HCV patients,5 but immunization for hepatitis A was documented for only 25 subjects.
TABLE 1
RISK FACTORS NOTED AT DIAGNOSTIC VISIT
| Risk Factor | No. of Patients (%) (N=355) |
|---|---|
| History of intravenous drug use | 177 (50%) |
| Sexual exposure | 128 (36%) |
| Immigrant | 47 (13%) |
| Tattoos | 60 (17%) |
| History of blood transfusion | 107 (30%) |
| Occupational risks | 44 (12%) |
TABLE 2
REASONS DOCUMENTED FOR NOT RECEIVING TREATMENT
| Reason | No. of Patients (%) (N=306) |
|---|---|
| No reason stated | 81 (26%) |
| Chemical dependency | 53 (17%) |
| Comorbid condition | 28 (9%) |
| Refused/noncompliant | 44 (14%) |
| Age | 13 (4%) |
| Ineligible | 22 (7%)* |
| Depression | 25 (8%) |
| Psychiatric condition | 4 (1%) |
| Desired pregnancy | 5 (2%) |
| Cirrhosis | 6 (2%) |
| Hepatocellular carcinoma | 8 (3%) |
| HIV treatment | 7 (2%) |
| No insurance | 6 (1%) |
| No trial available | 4 (1%) |
| *Ineligible because of advanced liver disease or other terminal illness. | |
FIGURE 1
AGE AT DIAGNOSIS
FIGURE 2
NUMBER OF NEW DIAGNOSES PER YEAR
Discussion
Hepatitis C was an uncommon new diagnosis in Olmsted County and therefore not a frequent occurrence in the practice of any of the 200 primary care physicians in the community. Although the overall prevalence of hepatitis C was only about 25% of that reported for the Midwestern United States (1.3%),3,4 the ratio of men and women diagnosed with HCV and the distribution of recorded risk factors in our cohort are similar to those reported from the NHANES conducted in 1988 to 19944 and to those in other population-based studies.5,15-17
In this cohort, primary care physicians’ response to known HCV varied from occasional monitoring of AST/ALT tests to referral for specialty evaluation and HCV treatment. In a significant group of patients, no visits for HCV follow-up could be identified. The only other published data on primary care physicians’ follow-up care of patients with known HCV was self-report survey data.18 In response to a survey, primary care physicians reported they ordered yearly AST/ALT tests in all HCV patients and referred over 80% of people with known HCV to hepatologists. Self-reported care often overestimates the amount of care provided. This difference in reported and observed care emphasizes the importance of data on actual practice.
The community physicians’ lack of a uniform or aggressive approach to HCV infections may not be surprising in view of the wide disparity of available information on chronic HCV infection and its progression to symptomatic or progressive liver dis-ease.18 For example, published rates of progression to “chronic infection” after exposure vary from 85% of people receiving HCV-infected blood transfusions to 20% of women given HCV-contaminated RhoGAM.9 Even rates of progression in people with persistent viremia (+PCR) vary from 69 % to 88%.19 Unfortunately, progression to chronic HCV infection cannot be predicted from initial clinical or laboratory factors.20 In addition, the meaning of “chronic infection” is unclear.19 Reported rates of progression from chronic infection to cirrhosis are widely divergent and appear related to the type of group used to make predictions. Data from meta-analyses of clinical trial patients21 suggest progression rates of up to 69% in 30 years. Yet only 2.4% to 6% of community-based hepatitis C patients may develop cirrhosis after 17 to 40 years of follow-up.22,23
Wide variations in the progression of cirrhosis to decompensated cirrhosis have been reported.24 Studies of AST levels in people with known HCV show fluctuations over time, dispelling the idea that once elevated, AST or ALT will remain elevated or that AST levels are directly predictive of progression or resolution of liver injury or viral loads.25,26 Chronically elevated AST/ALT levels, however, have been shown to predict progression,27 making monitoring of AST/ALT important in all subjects.5,28
That HCV treatment was uncommon in this population may reflect the limited efficacy of single-agent therapy before 1998, as well as the large proportion of subjects with contraindications to therapy, primary care physician confusion regarding who should be treated, and current limited knowledge of the long-term outcomes of treatment.29 Cure rates are unknown and measures of cure are unclear, since clearing the virus from the bloodstream (negative PCR) may not confirm clearing of the virus from the liver.29 All these data together provide little experimental evidence for a standard set of recommendations for follow-up, nor do they support a clear rationale for the use of those follow-up data in determining HCV progression. The existing data have been used to develop consensus (expert opinion–based) guidelines published by NIH (1997)28 and CDC (1998,30 with an update in the summer of 2001). The substance of those consensus statements has changed over time5,28 as experts’ experience has increased and newer observations have become available. Recent changes in available treatments31-33 and FDA approval of a pegylated inferferon34,35 are likely to keep recommendations in flux for the near future.
The limited attention given to the identification, prevention, or treatment of comorbid accelerating conditions (HIV, HBV, HAV, and heavy alcohol intake)5,19,27,36-38 in this cohort is less understandable, since the literature is more consistent on these issues. Documented HIV testing was not universal even in those with a history of IDU or promiscuous sexual exposure. While testing for HBV was almost universal, HBV prevention in the form of vaccination was documented in only about one fourth (23%) of eligible subjects. Immunization for hepatitis A was documented in less than one tenth of subjects. Treatment or documented physician recommendations for treatment of ongoing alcohol abuse or heavy alccohol ingestion occurred in the minority of patients. Studies of combined chemical dependency and HCV therapy might be appropriate in this population.
The Olmsted County population from which the subjects were identified is more than 90% white. Therefore, community-based prevalence rates of physician diagnoses may not be representative of other racial or ethnic groups. The high frequency of drug use and sexual exposure suggests that our community’s problems associated with HCV are similar to those identified in other communities with greater economic, ethnic, and racial diversity. We did not perform PCR, liver biopsy, or yearly liver function tests on all subjects. One aim of our study, however, was to understand community practice and the resulting variations in information and testing completed for each subject. The existence of a large medical education program and the local availability of hepatologists may affect the care provided. Yet even in this setting, additional attention to follow-up of liver disease and comorbid conditions appeared indicated.
Conclusions
Primary care physicians make most diagnoses and perform most initial management of hepatitis C. However, primary care directed long term follow-up care is inconsistent and management of accelerating comorbidities is incomplete. Family physicians can offer important additional services to their patients who have hepatitis C.
1. Centers for Disease Control. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep 1998;47(No RR-19):1-39.
2. McQuillan G, Alter M, Moyer L, Lambert S, Margolis H. A population-based serologic study of hepatitis C virus infection in the United States. Am J Epidemiol 1996;143(suppl):S32.-
3. Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 2000;31:777-82.
4. Alter M, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999;341:556-62.
5. Management of hepatitis C. NIH Consens Statement Online 1997 Mar 24-26 [cited 2001, July 23]; 15(3):1-41.
6. Takahashi M, Yamada G, Miyamoto R, Doi T, Endo H, Tsuji T. Natural course of chronic hepatitis C. Am J Gastroenterol 1993;88:240-3.
7. Kiyosawa K, Tanaka E, Sodeyama T, Furuta S. Natural history of hepatitis C. Intervirology 1994;37:101-7.
8. Muller R. The natural history of hepatitis C: clinical experiences. J Hepatology 1996;24(suppl 2):52-4.
9. Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. N Engl J Med 1999;340:1228-33.
10. Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med 1995;332:1463-6.
11. Kaur S, Rybicki L, Bacon BR, Gollan JL, Rustgi VK, Carey WD, and the National Hepatitis Surveillance Group. Performance characteristics and results of a large-scale screening program for viral hepatitis and risk factors associated with exposure to viral hepatitis B and C: results of the National Hepatitis Screening Survey. Hepatology 1996;24:979-86.
12. Makris M, Preston FE, Rosendaal FR, Underwood JCE, Rice KM, Triger DR. The natural history of chronic hepatitis C in haemophiliacs. Br J Haematol 1996;94:746-52.
13. Melton LJ, III. History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71:266-74.
14. Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am 1981;245:54-63.
15. Alter MJ. Epidemiology of hepatitis C in the West. Semin Liver Dis 1995;15:5-14.
16. Tillman HL, Manns MP. Mode of hepatitis C virus infection, epidemiology, and chronicity rate in the general population and risk groups. Dig Dis Sci 1996;41(12 suppl):27S-40S.
17. Murphy EL, Bryzman SM, Glynn SA, et al. Risk factors for hepatitis C virus infection in United States blood donors. Hepatology 2000;31:756-62.
18. Shehab TM, Sonnad SS, Jeffries M, Gunaratnum N, Lok ASF. Current practice patterns of primary care physicians in the management of patients with hepatitis C. Hepatology 1999;30:794-800.
19. Muir AJ. The natural history of hepatitis C viral infection. Semin Gastrointest Dis 2000;11:54-61.
20. Villano SA, Vlahov D, Nelson K, et al. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology 1999;29:908-14.
21. Poynard T, Bedossa P, Opolon P, et al. Natural history of liver fibro-sis progression in patients with chronic hepatitis C. Lancet 1997;346:825-32.
22. Crowe J, Doyle C, Fielding JF, et al. Presentation of hepatitis C in a unique uniform cohort 17 years from inoculation. Gastroenterology 1995;108:A1054. Abstract.-
23. Seeff LB, Miller RN, Rabkin CS, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med 2000;132:105-11.
24. Lionis C, Vlachonikolis IG, Skliros S, Symeonidis A, Merjiyrus BP, Kouroumalis E. Do undefined sources of hepatitis C transmission exist? The Greek study in general practice. J Viral Hepatitis 2000;7:218-24.
25. Bellentani S, Pozzato G, Saccoccio G, et al. Clinical course and risk factors of HCV related liver disease in the general population: report from the Dionysos study. Gut 1999;44:874-80.
26. Inglesby TV, Rai R, Astemborski J, et al. A prospective, community-based evaluation of liver enzymes in individuals with hepatitis C after drug use. Hepatology 1999;29:590-6.
27. Liang TJ (moderator), Rehermann B, Seef LB, Hofnagle JH (disscussants). Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med 2000;132:296-305.
28. Management of Hepatitis C. NIH Consens Statement Online 1997 Mar 24-26 [cited 2001, July 23]; 15(3):-41.
29. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep 1998;47(RR-19):1-39 [158 ].
30. Haydon GH, Jarvis LM, Blair CS, et al. Clinical significance of intra-hepatic hepatitis C virus levels in patients with chronic HCV infection. Gut 1998;42:570-5.
31. Wong JB, Bennett WG, Koff RS, Pauker SG. Pretreatment evaluation of chronic hepatitis C. JAMA 1998;280:2088-2093.
32. McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med 1998;339:1485-92.
33. Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha-2b plus ribavirin for 48 weeks or for 24 weeks versus inter-feron alpha-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 1998;352:1426-32.
34. Zeuzem S, Feinman SV, Rasenack J, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med 2000;343:1666-72.
35. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2a plus ribavirin for initial treatment of chronic hepatitis C: Results of a randomised trial. Lancet 2001;358:958-65.
36. Xiaowei F. Hepatitis C infection: a review. Lippincott Prim Care Pract 1999;3:345-53.
37. Serfaty L, Chazouilleres O, Poujol-Robert A, et al. Risk factors for cirrhosis in patients with chronic HCV infection: results of a case-control study. Hepatology 1997;26:776-9.
38. Jenkins PJ, Cromie SL, Roberts SK, et al. Chronic hepatitis C, alcohol and hepatic fibrosis. Hepatology 1996;24:153A. Abstract.
1. Centers for Disease Control. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep 1998;47(No RR-19):1-39.
2. McQuillan G, Alter M, Moyer L, Lambert S, Margolis H. A population-based serologic study of hepatitis C virus infection in the United States. Am J Epidemiol 1996;143(suppl):S32.-
3. Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 2000;31:777-82.
4. Alter M, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999;341:556-62.
5. Management of hepatitis C. NIH Consens Statement Online 1997 Mar 24-26 [cited 2001, July 23]; 15(3):1-41.
6. Takahashi M, Yamada G, Miyamoto R, Doi T, Endo H, Tsuji T. Natural course of chronic hepatitis C. Am J Gastroenterol 1993;88:240-3.
7. Kiyosawa K, Tanaka E, Sodeyama T, Furuta S. Natural history of hepatitis C. Intervirology 1994;37:101-7.
8. Muller R. The natural history of hepatitis C: clinical experiences. J Hepatology 1996;24(suppl 2):52-4.
9. Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. N Engl J Med 1999;340:1228-33.
10. Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med 1995;332:1463-6.
11. Kaur S, Rybicki L, Bacon BR, Gollan JL, Rustgi VK, Carey WD, and the National Hepatitis Surveillance Group. Performance characteristics and results of a large-scale screening program for viral hepatitis and risk factors associated with exposure to viral hepatitis B and C: results of the National Hepatitis Screening Survey. Hepatology 1996;24:979-86.
12. Makris M, Preston FE, Rosendaal FR, Underwood JCE, Rice KM, Triger DR. The natural history of chronic hepatitis C in haemophiliacs. Br J Haematol 1996;94:746-52.
13. Melton LJ, III. History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71:266-74.
14. Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am 1981;245:54-63.
15. Alter MJ. Epidemiology of hepatitis C in the West. Semin Liver Dis 1995;15:5-14.
16. Tillman HL, Manns MP. Mode of hepatitis C virus infection, epidemiology, and chronicity rate in the general population and risk groups. Dig Dis Sci 1996;41(12 suppl):27S-40S.
17. Murphy EL, Bryzman SM, Glynn SA, et al. Risk factors for hepatitis C virus infection in United States blood donors. Hepatology 2000;31:756-62.
18. Shehab TM, Sonnad SS, Jeffries M, Gunaratnum N, Lok ASF. Current practice patterns of primary care physicians in the management of patients with hepatitis C. Hepatology 1999;30:794-800.
19. Muir AJ. The natural history of hepatitis C viral infection. Semin Gastrointest Dis 2000;11:54-61.
20. Villano SA, Vlahov D, Nelson K, et al. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology 1999;29:908-14.
21. Poynard T, Bedossa P, Opolon P, et al. Natural history of liver fibro-sis progression in patients with chronic hepatitis C. Lancet 1997;346:825-32.
22. Crowe J, Doyle C, Fielding JF, et al. Presentation of hepatitis C in a unique uniform cohort 17 years from inoculation. Gastroenterology 1995;108:A1054. Abstract.-
23. Seeff LB, Miller RN, Rabkin CS, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med 2000;132:105-11.
24. Lionis C, Vlachonikolis IG, Skliros S, Symeonidis A, Merjiyrus BP, Kouroumalis E. Do undefined sources of hepatitis C transmission exist? The Greek study in general practice. J Viral Hepatitis 2000;7:218-24.
25. Bellentani S, Pozzato G, Saccoccio G, et al. Clinical course and risk factors of HCV related liver disease in the general population: report from the Dionysos study. Gut 1999;44:874-80.
26. Inglesby TV, Rai R, Astemborski J, et al. A prospective, community-based evaluation of liver enzymes in individuals with hepatitis C after drug use. Hepatology 1999;29:590-6.
27. Liang TJ (moderator), Rehermann B, Seef LB, Hofnagle JH (disscussants). Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med 2000;132:296-305.
28. Management of Hepatitis C. NIH Consens Statement Online 1997 Mar 24-26 [cited 2001, July 23]; 15(3):-41.
29. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep 1998;47(RR-19):1-39 [158 ].
30. Haydon GH, Jarvis LM, Blair CS, et al. Clinical significance of intra-hepatic hepatitis C virus levels in patients with chronic HCV infection. Gut 1998;42:570-5.
31. Wong JB, Bennett WG, Koff RS, Pauker SG. Pretreatment evaluation of chronic hepatitis C. JAMA 1998;280:2088-2093.
32. McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med 1998;339:1485-92.
33. Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha-2b plus ribavirin for 48 weeks or for 24 weeks versus inter-feron alpha-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 1998;352:1426-32.
34. Zeuzem S, Feinman SV, Rasenack J, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med 2000;343:1666-72.
35. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2a plus ribavirin for initial treatment of chronic hepatitis C: Results of a randomised trial. Lancet 2001;358:958-65.
36. Xiaowei F. Hepatitis C infection: a review. Lippincott Prim Care Pract 1999;3:345-53.
37. Serfaty L, Chazouilleres O, Poujol-Robert A, et al. Risk factors for cirrhosis in patients with chronic HCV infection: results of a case-control study. Hepatology 1997;26:776-9.
38. Jenkins PJ, Cromie SL, Roberts SK, et al. Chronic hepatitis C, alcohol and hepatic fibrosis. Hepatology 1996;24:153A. Abstract.