User login
An Electronic Chemotherapy Ordering Process and Template
In May 2008 the Kansas City VAMC in Missouri, the Chemotherapy Quality Improvement Team (CQIT) was formed to evaluate and improve the chemotherapy delivery process in response to a significant medication error. At the first meeting, the team quickly determined that the chemotherapy ordering process should be improved. Up until that point, chemotherapy was ordered on handwritten, self-duplicating forms. The finished order forms were often difficult to read. Further, the forms were not consistent with American Society of Health-System Pharmacists (ASHP) Guidelines for Preventing Medication Errors with Antineoplastic Agents.1 A solution using existing technology was needed and obtaining third-party software was not an option at the time.
Chemotherapy Ordering Options
The VA Computerized Patient Record System (CPRS)
electronic health record system could not support the
complexity of chemotherapy orders without some adjustments.
One consideration was to build “order sets,”
to allow sequential ordering. With this approach, once
an order set was engaged, all the chosen medication orders
would automatically fire in sequence.
Order Sets
The order set method of standardization in chemotherapy ordering would channel all chemotherapy orders to the pharmacy through the computerized physician order entry (CPOE). However, a major drawback to building this type of order set was its lack of flexibility. A veteran’s care plan rarely adheres to the “standard” and modifications are the norm. The ASHP recommendations regarding chemotherapy order contents could not be honored.
The final product presented to the pharmacy as a clump of orders with no sequencing, no explanation of deviations from standards, base doses, or name of the desired regimen. With this approach, no diagnosis or stage of cancer was offered to allow a pharmacist to check for the appropriateness of the regimen. Also, a complete treatment summary was not communicated, so details, including base doses and calculated parameters, such as body surface area (BSA), were not available for order checks. Probably the biggest drawback was the intense amount of support a program like that required. Order sets are difficult to write and maintain in this age of drug shortages and in the evershifting terrain of medical oncology practice.
Progress Notes
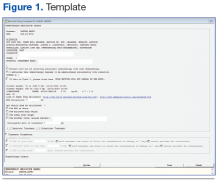
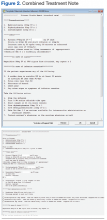
Another alternative identified was to work within the CPRS-based electronic progress note functionality. This option offered a number of positive features. First, the finished product appeared in a visually appealing sequential document. An option existed for the provider to detail calculated parameters and to discuss variances from standard. Course number and order sequence could be communicated to the nursing staff. Best of all, using a 2-template strategy, a simple system was developed that allowed much of the order to be prewritten. Order entry required minimal physician time while producing a high-quality chemotherapy order that was consistent with the ASHP recommendations for safe chemotherapy ordering (Figures 1 and 2; to download a sample of the template and treatment note, visit (http://www.fedprac.com/AVAHO).
Once the Kansas City VAMC decided chemotherapy ordering should be implemented through the electronic progress note functionality, Information Resources Management (IRM) staff and the oncology pharmacist worked together to develop a templated process that would make the most sense and require the least work for all parties. Strong consideration was given to the amount of labor needed to support the process, because that had been observed as a stumbling block in other sites’ attempts to develop similar processes.
Chemotherapy Ordering Process
When the provider engages the dedicated progress note, the more complex of the 2 templates engages. The template automatically pulls in identifiers and allergies, height, weight, creatinine, and age data and offers links to 2 dose calculators. It also prompts providers for information that is required for all orders, such as diagnosis, stage, protocol title, treatment date, inpatient or outpatient, BSA calculation, BSA qualifier (total, ideal, or adjusted body weight, capped doses, etc). Providers must indicate whether the patient is also to receive radiation, and there is an option to provide retreatment parameters for multiday orders. Once this dialogue is completed, the information provided populates into the note.
After the first template is completed and has populated into the note, the provider moves to the “shared templates” in CPRS and selects a treatment-specific template. Currently, > 150 treatment templates are in use at the facility for routine medical oncology practice. A parallel process also exists that has been modified for investigational protocols. Once a treatment template is selected, the provider populates the required fields with doses and completes the progress note. The 2 templates flow together to create 1 seamless treatment note. Providers can deviate from the template standard by making changes in the text of the note before signing.
Breaking down the process to 2 separate templates was a critical decision for the continued success of this system. Several other facilities have attempted to develop 1-click templates for each treatment mode. The Kansas City VAMC, however, decided that requiring IRM to install and maintain a full template for each of the > 150 chemotherapy orders was impractical. Because the complex portion of the ordering process was isolated in the first template, the individual treatment templates could be very simple, prewritten forms with a blank field for dose information. These forms were built on a combination of clinical guidelines and local practice, and an oncology provider approved each before installation. Once the note has been generated, providers are free to enter notes and make modifications to the order to reflect the patient’s needs.
Due to the simplicity of the treatment templates, a new one can be installed in the shared file in minutes. Development of the initial library of templates took about 2 months. The order sets were developed from previously written chemotherapy orders and adapted to the electronic format. The new system went live 3.5 months after the concept was proposed, and handwritten chemotherapy orders are no longer accepted.
Creating forms for > 100 orders was a daunting task, particularly considering the need to consider both local practice and guideline recommendations with regards to both dosing of chemotherapy and supportive medications. Extensive physician involvement was required. At first it seemed to be too much work for something that might be an interim measure; however, any thirdparty solution would require a similar process, so now the facility is prepared if third-party chemotherapy ordering software is purchased.
Why Is This A Best Practice?
The ASHP guidelines promote the importance of chemotherapy order standardization. However, done without careful attention, facilities can standardize errors into practice. To prevent errors and double check documents, an additional process for handling the templates was developed. The pharmacy department developed new order templates, incorporating both local practices and accepted guidelines. The template is first sent to the oncology physician for careful review. On physician approval, it goes to IRM for installation into CPRS as well as to the Pharmacy and Therapeutics Committee for final review. This process provides permanent and accessible documentation of pre-implementation review.
The pharmacy and nursing staff are automatically notified when an order is signed. The new order is printed and reviewed by a pharmacist, and the ordered items are entered in the same system for processing. Providers frequently enter the orders in advance, allowing careful review and medication profiling to occur well before the patient arrives. The orders can be processed during off-peak hours, simplifying workload and potentially reducing errors.
The order format also offers an effective communication tool. Since the template is in a checklist format, the nursing staff are instructed clearly from the order how to administer the treatment. In fact, the practice is to take the order to the room and log all treatment times and details on the order sheet, facilitating highquality documentation of administration. This option was not available with handwritten orders.
The orders are templated sequentially; nurses give the medications in the order they are presented, preserving sequencing preferences for certain regimens. Calls and pages to clarify doses are kept to a minimum by prompting the provider to indicate parameters for retreatment and dosing preferences used (ideal body weight, etc).
The treatment templates were locally developed and based on provider practices. Although guidelines are helpful, they cannot be uniformly applied to all facilities. VA practice, for example, requires less aggressive pretreatment for nausea in many cases due to the nature of the population. Since this process was developed locally, it mirrors the prior common practices.
Experience With The Program
Both medical and nursing staff quickly accepted the new ordering system. It is estimated that veterans’ turnaround time has decreased by as much as 45 minutes. There are several ways the process saves patient time. Transportation of written orders has been eliminated, a frequent stumbling block in the process. The orders are now delivered immediately on signing to both pharmacy and nursing staff.
There is no more time lost clarifying poorly written, smudged, or otherwise illegible orders. The finished product is clear, legible, standardized, and readily available in CPRS for all authorized personnel to review. Problems are often identified well in advance of the patient’s arrival. Nurses are seldom surprised with add-on directives, since the orders are entered when the plan is made even if that is a week or more before the start of treatment. Electronic notification of new orders allows nursing staff to predict workload and schedule staffing and treatments accordingly.
Limitations
Although proud of this project as a creative and effective solution, the oncology department staff recognize that there are some limitations with the process that preclude its use as a permanent tool. The main limitation of this approach to chemotherapy ordering stems from use of the CPRS progress note module to create an order. One important feature of any order is that it can be changed or discontinued. Due to the permanence of progress notes, an addendum must be made to the order to qualify it as discontinued, since a progress note cannot be discontinued. The users within the system are aware of this limitation and are vigilant for new addenda to these notes, but it could open a window for error.
This process is also not consistent with the ideal that all orders be entered by CPOE. In a perfect world, on signing the note at the end of this process, the orders would automatically generate pharmacy orders for the drug items. Unfortunately, that level of automation is not available at this time. Within the current infrastructure, that functionality would be devastating to the flexibility that is of greater importance for this process. It is exactly this problem that has led to the consideration of third-party software solutions.
Conclusion
The chemotherapy ordering process at the Kansas City VAMC is an effective communication tool. Ultimately, a physician’s order for treatment is a one-way communication to pharmacy and nursing staff. This process streamlines the communication and minimizes the need for callbacks and clarifications. It also permits anyone with access to the patient’s CPRS record to be able to review the plan. And it creates a standardized treatment checklist for more consistent care. The ASHP strongly recommends standardizing oncology ordering practices, and checklists are a recognized tool for improving the quality of care.2 The simplicity of the process and the no-cost maintenance of the technology are added benefits.
A novel solution was needed to improve safety and efficiency of chemotherapy ordering. The pharmacy department was the key in developing such a solution at the Kansas City VAMC. A transparent, standardized process was developed and implemented within a relatively short time frame. Built within existing software/hardware capabilities, the project had an immediate return on investment and avoided the overhead costs associated with implementing third-party ordering systems. In addition the process decreased turnaround time and increased throughput of the ordering process. An added benefit is that if a better tool (third party or otherwise) becomes available, the Kansas City VAMC is ready on a moment’s notice.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications,warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30(13):1553-1561.
2. Gawande A. The Checklist Manifesto: How to Get Things Right. Metropolitan Books: New York; 2009.
In May 2008 the Kansas City VAMC in Missouri, the Chemotherapy Quality Improvement Team (CQIT) was formed to evaluate and improve the chemotherapy delivery process in response to a significant medication error. At the first meeting, the team quickly determined that the chemotherapy ordering process should be improved. Up until that point, chemotherapy was ordered on handwritten, self-duplicating forms. The finished order forms were often difficult to read. Further, the forms were not consistent with American Society of Health-System Pharmacists (ASHP) Guidelines for Preventing Medication Errors with Antineoplastic Agents.1 A solution using existing technology was needed and obtaining third-party software was not an option at the time.
Chemotherapy Ordering Options
The VA Computerized Patient Record System (CPRS)
electronic health record system could not support the
complexity of chemotherapy orders without some adjustments.
One consideration was to build “order sets,”
to allow sequential ordering. With this approach, once
an order set was engaged, all the chosen medication orders
would automatically fire in sequence.
Order Sets
The order set method of standardization in chemotherapy ordering would channel all chemotherapy orders to the pharmacy through the computerized physician order entry (CPOE). However, a major drawback to building this type of order set was its lack of flexibility. A veteran’s care plan rarely adheres to the “standard” and modifications are the norm. The ASHP recommendations regarding chemotherapy order contents could not be honored.
The final product presented to the pharmacy as a clump of orders with no sequencing, no explanation of deviations from standards, base doses, or name of the desired regimen. With this approach, no diagnosis or stage of cancer was offered to allow a pharmacist to check for the appropriateness of the regimen. Also, a complete treatment summary was not communicated, so details, including base doses and calculated parameters, such as body surface area (BSA), were not available for order checks. Probably the biggest drawback was the intense amount of support a program like that required. Order sets are difficult to write and maintain in this age of drug shortages and in the evershifting terrain of medical oncology practice.
Progress Notes
Another alternative identified was to work within the CPRS-based electronic progress note functionality. This option offered a number of positive features. First, the finished product appeared in a visually appealing sequential document. An option existed for the provider to detail calculated parameters and to discuss variances from standard. Course number and order sequence could be communicated to the nursing staff. Best of all, using a 2-template strategy, a simple system was developed that allowed much of the order to be prewritten. Order entry required minimal physician time while producing a high-quality chemotherapy order that was consistent with the ASHP recommendations for safe chemotherapy ordering (Figures 1 and 2; to download a sample of the template and treatment note, visit (http://www.fedprac.com/AVAHO).
Once the Kansas City VAMC decided chemotherapy ordering should be implemented through the electronic progress note functionality, Information Resources Management (IRM) staff and the oncology pharmacist worked together to develop a templated process that would make the most sense and require the least work for all parties. Strong consideration was given to the amount of labor needed to support the process, because that had been observed as a stumbling block in other sites’ attempts to develop similar processes.
Chemotherapy Ordering Process
When the provider engages the dedicated progress note, the more complex of the 2 templates engages. The template automatically pulls in identifiers and allergies, height, weight, creatinine, and age data and offers links to 2 dose calculators. It also prompts providers for information that is required for all orders, such as diagnosis, stage, protocol title, treatment date, inpatient or outpatient, BSA calculation, BSA qualifier (total, ideal, or adjusted body weight, capped doses, etc). Providers must indicate whether the patient is also to receive radiation, and there is an option to provide retreatment parameters for multiday orders. Once this dialogue is completed, the information provided populates into the note.
After the first template is completed and has populated into the note, the provider moves to the “shared templates” in CPRS and selects a treatment-specific template. Currently, > 150 treatment templates are in use at the facility for routine medical oncology practice. A parallel process also exists that has been modified for investigational protocols. Once a treatment template is selected, the provider populates the required fields with doses and completes the progress note. The 2 templates flow together to create 1 seamless treatment note. Providers can deviate from the template standard by making changes in the text of the note before signing.
Breaking down the process to 2 separate templates was a critical decision for the continued success of this system. Several other facilities have attempted to develop 1-click templates for each treatment mode. The Kansas City VAMC, however, decided that requiring IRM to install and maintain a full template for each of the > 150 chemotherapy orders was impractical. Because the complex portion of the ordering process was isolated in the first template, the individual treatment templates could be very simple, prewritten forms with a blank field for dose information. These forms were built on a combination of clinical guidelines and local practice, and an oncology provider approved each before installation. Once the note has been generated, providers are free to enter notes and make modifications to the order to reflect the patient’s needs.
Due to the simplicity of the treatment templates, a new one can be installed in the shared file in minutes. Development of the initial library of templates took about 2 months. The order sets were developed from previously written chemotherapy orders and adapted to the electronic format. The new system went live 3.5 months after the concept was proposed, and handwritten chemotherapy orders are no longer accepted.
Creating forms for > 100 orders was a daunting task, particularly considering the need to consider both local practice and guideline recommendations with regards to both dosing of chemotherapy and supportive medications. Extensive physician involvement was required. At first it seemed to be too much work for something that might be an interim measure; however, any thirdparty solution would require a similar process, so now the facility is prepared if third-party chemotherapy ordering software is purchased.
Why Is This A Best Practice?
The ASHP guidelines promote the importance of chemotherapy order standardization. However, done without careful attention, facilities can standardize errors into practice. To prevent errors and double check documents, an additional process for handling the templates was developed. The pharmacy department developed new order templates, incorporating both local practices and accepted guidelines. The template is first sent to the oncology physician for careful review. On physician approval, it goes to IRM for installation into CPRS as well as to the Pharmacy and Therapeutics Committee for final review. This process provides permanent and accessible documentation of pre-implementation review.
The pharmacy and nursing staff are automatically notified when an order is signed. The new order is printed and reviewed by a pharmacist, and the ordered items are entered in the same system for processing. Providers frequently enter the orders in advance, allowing careful review and medication profiling to occur well before the patient arrives. The orders can be processed during off-peak hours, simplifying workload and potentially reducing errors.
The order format also offers an effective communication tool. Since the template is in a checklist format, the nursing staff are instructed clearly from the order how to administer the treatment. In fact, the practice is to take the order to the room and log all treatment times and details on the order sheet, facilitating highquality documentation of administration. This option was not available with handwritten orders.
The orders are templated sequentially; nurses give the medications in the order they are presented, preserving sequencing preferences for certain regimens. Calls and pages to clarify doses are kept to a minimum by prompting the provider to indicate parameters for retreatment and dosing preferences used (ideal body weight, etc).
The treatment templates were locally developed and based on provider practices. Although guidelines are helpful, they cannot be uniformly applied to all facilities. VA practice, for example, requires less aggressive pretreatment for nausea in many cases due to the nature of the population. Since this process was developed locally, it mirrors the prior common practices.
Experience With The Program
Both medical and nursing staff quickly accepted the new ordering system. It is estimated that veterans’ turnaround time has decreased by as much as 45 minutes. There are several ways the process saves patient time. Transportation of written orders has been eliminated, a frequent stumbling block in the process. The orders are now delivered immediately on signing to both pharmacy and nursing staff.
There is no more time lost clarifying poorly written, smudged, or otherwise illegible orders. The finished product is clear, legible, standardized, and readily available in CPRS for all authorized personnel to review. Problems are often identified well in advance of the patient’s arrival. Nurses are seldom surprised with add-on directives, since the orders are entered when the plan is made even if that is a week or more before the start of treatment. Electronic notification of new orders allows nursing staff to predict workload and schedule staffing and treatments accordingly.
Limitations
Although proud of this project as a creative and effective solution, the oncology department staff recognize that there are some limitations with the process that preclude its use as a permanent tool. The main limitation of this approach to chemotherapy ordering stems from use of the CPRS progress note module to create an order. One important feature of any order is that it can be changed or discontinued. Due to the permanence of progress notes, an addendum must be made to the order to qualify it as discontinued, since a progress note cannot be discontinued. The users within the system are aware of this limitation and are vigilant for new addenda to these notes, but it could open a window for error.
This process is also not consistent with the ideal that all orders be entered by CPOE. In a perfect world, on signing the note at the end of this process, the orders would automatically generate pharmacy orders for the drug items. Unfortunately, that level of automation is not available at this time. Within the current infrastructure, that functionality would be devastating to the flexibility that is of greater importance for this process. It is exactly this problem that has led to the consideration of third-party software solutions.
Conclusion
The chemotherapy ordering process at the Kansas City VAMC is an effective communication tool. Ultimately, a physician’s order for treatment is a one-way communication to pharmacy and nursing staff. This process streamlines the communication and minimizes the need for callbacks and clarifications. It also permits anyone with access to the patient’s CPRS record to be able to review the plan. And it creates a standardized treatment checklist for more consistent care. The ASHP strongly recommends standardizing oncology ordering practices, and checklists are a recognized tool for improving the quality of care.2 The simplicity of the process and the no-cost maintenance of the technology are added benefits.
A novel solution was needed to improve safety and efficiency of chemotherapy ordering. The pharmacy department was the key in developing such a solution at the Kansas City VAMC. A transparent, standardized process was developed and implemented within a relatively short time frame. Built within existing software/hardware capabilities, the project had an immediate return on investment and avoided the overhead costs associated with implementing third-party ordering systems. In addition the process decreased turnaround time and increased throughput of the ordering process. An added benefit is that if a better tool (third party or otherwise) becomes available, the Kansas City VAMC is ready on a moment’s notice.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications,warnings, and adverse effects—before administering pharmacologic therapy to patients.
In May 2008 the Kansas City VAMC in Missouri, the Chemotherapy Quality Improvement Team (CQIT) was formed to evaluate and improve the chemotherapy delivery process in response to a significant medication error. At the first meeting, the team quickly determined that the chemotherapy ordering process should be improved. Up until that point, chemotherapy was ordered on handwritten, self-duplicating forms. The finished order forms were often difficult to read. Further, the forms were not consistent with American Society of Health-System Pharmacists (ASHP) Guidelines for Preventing Medication Errors with Antineoplastic Agents.1 A solution using existing technology was needed and obtaining third-party software was not an option at the time.
Chemotherapy Ordering Options
The VA Computerized Patient Record System (CPRS)
electronic health record system could not support the
complexity of chemotherapy orders without some adjustments.
One consideration was to build “order sets,”
to allow sequential ordering. With this approach, once
an order set was engaged, all the chosen medication orders
would automatically fire in sequence.
Order Sets
The order set method of standardization in chemotherapy ordering would channel all chemotherapy orders to the pharmacy through the computerized physician order entry (CPOE). However, a major drawback to building this type of order set was its lack of flexibility. A veteran’s care plan rarely adheres to the “standard” and modifications are the norm. The ASHP recommendations regarding chemotherapy order contents could not be honored.
The final product presented to the pharmacy as a clump of orders with no sequencing, no explanation of deviations from standards, base doses, or name of the desired regimen. With this approach, no diagnosis or stage of cancer was offered to allow a pharmacist to check for the appropriateness of the regimen. Also, a complete treatment summary was not communicated, so details, including base doses and calculated parameters, such as body surface area (BSA), were not available for order checks. Probably the biggest drawback was the intense amount of support a program like that required. Order sets are difficult to write and maintain in this age of drug shortages and in the evershifting terrain of medical oncology practice.
Progress Notes
Another alternative identified was to work within the CPRS-based electronic progress note functionality. This option offered a number of positive features. First, the finished product appeared in a visually appealing sequential document. An option existed for the provider to detail calculated parameters and to discuss variances from standard. Course number and order sequence could be communicated to the nursing staff. Best of all, using a 2-template strategy, a simple system was developed that allowed much of the order to be prewritten. Order entry required minimal physician time while producing a high-quality chemotherapy order that was consistent with the ASHP recommendations for safe chemotherapy ordering (Figures 1 and 2; to download a sample of the template and treatment note, visit (http://www.fedprac.com/AVAHO).
Once the Kansas City VAMC decided chemotherapy ordering should be implemented through the electronic progress note functionality, Information Resources Management (IRM) staff and the oncology pharmacist worked together to develop a templated process that would make the most sense and require the least work for all parties. Strong consideration was given to the amount of labor needed to support the process, because that had been observed as a stumbling block in other sites’ attempts to develop similar processes.
Chemotherapy Ordering Process
When the provider engages the dedicated progress note, the more complex of the 2 templates engages. The template automatically pulls in identifiers and allergies, height, weight, creatinine, and age data and offers links to 2 dose calculators. It also prompts providers for information that is required for all orders, such as diagnosis, stage, protocol title, treatment date, inpatient or outpatient, BSA calculation, BSA qualifier (total, ideal, or adjusted body weight, capped doses, etc). Providers must indicate whether the patient is also to receive radiation, and there is an option to provide retreatment parameters for multiday orders. Once this dialogue is completed, the information provided populates into the note.
After the first template is completed and has populated into the note, the provider moves to the “shared templates” in CPRS and selects a treatment-specific template. Currently, > 150 treatment templates are in use at the facility for routine medical oncology practice. A parallel process also exists that has been modified for investigational protocols. Once a treatment template is selected, the provider populates the required fields with doses and completes the progress note. The 2 templates flow together to create 1 seamless treatment note. Providers can deviate from the template standard by making changes in the text of the note before signing.
Breaking down the process to 2 separate templates was a critical decision for the continued success of this system. Several other facilities have attempted to develop 1-click templates for each treatment mode. The Kansas City VAMC, however, decided that requiring IRM to install and maintain a full template for each of the > 150 chemotherapy orders was impractical. Because the complex portion of the ordering process was isolated in the first template, the individual treatment templates could be very simple, prewritten forms with a blank field for dose information. These forms were built on a combination of clinical guidelines and local practice, and an oncology provider approved each before installation. Once the note has been generated, providers are free to enter notes and make modifications to the order to reflect the patient’s needs.
Due to the simplicity of the treatment templates, a new one can be installed in the shared file in minutes. Development of the initial library of templates took about 2 months. The order sets were developed from previously written chemotherapy orders and adapted to the electronic format. The new system went live 3.5 months after the concept was proposed, and handwritten chemotherapy orders are no longer accepted.
Creating forms for > 100 orders was a daunting task, particularly considering the need to consider both local practice and guideline recommendations with regards to both dosing of chemotherapy and supportive medications. Extensive physician involvement was required. At first it seemed to be too much work for something that might be an interim measure; however, any thirdparty solution would require a similar process, so now the facility is prepared if third-party chemotherapy ordering software is purchased.
Why Is This A Best Practice?
The ASHP guidelines promote the importance of chemotherapy order standardization. However, done without careful attention, facilities can standardize errors into practice. To prevent errors and double check documents, an additional process for handling the templates was developed. The pharmacy department developed new order templates, incorporating both local practices and accepted guidelines. The template is first sent to the oncology physician for careful review. On physician approval, it goes to IRM for installation into CPRS as well as to the Pharmacy and Therapeutics Committee for final review. This process provides permanent and accessible documentation of pre-implementation review.
The pharmacy and nursing staff are automatically notified when an order is signed. The new order is printed and reviewed by a pharmacist, and the ordered items are entered in the same system for processing. Providers frequently enter the orders in advance, allowing careful review and medication profiling to occur well before the patient arrives. The orders can be processed during off-peak hours, simplifying workload and potentially reducing errors.
The order format also offers an effective communication tool. Since the template is in a checklist format, the nursing staff are instructed clearly from the order how to administer the treatment. In fact, the practice is to take the order to the room and log all treatment times and details on the order sheet, facilitating highquality documentation of administration. This option was not available with handwritten orders.
The orders are templated sequentially; nurses give the medications in the order they are presented, preserving sequencing preferences for certain regimens. Calls and pages to clarify doses are kept to a minimum by prompting the provider to indicate parameters for retreatment and dosing preferences used (ideal body weight, etc).
The treatment templates were locally developed and based on provider practices. Although guidelines are helpful, they cannot be uniformly applied to all facilities. VA practice, for example, requires less aggressive pretreatment for nausea in many cases due to the nature of the population. Since this process was developed locally, it mirrors the prior common practices.
Experience With The Program
Both medical and nursing staff quickly accepted the new ordering system. It is estimated that veterans’ turnaround time has decreased by as much as 45 minutes. There are several ways the process saves patient time. Transportation of written orders has been eliminated, a frequent stumbling block in the process. The orders are now delivered immediately on signing to both pharmacy and nursing staff.
There is no more time lost clarifying poorly written, smudged, or otherwise illegible orders. The finished product is clear, legible, standardized, and readily available in CPRS for all authorized personnel to review. Problems are often identified well in advance of the patient’s arrival. Nurses are seldom surprised with add-on directives, since the orders are entered when the plan is made even if that is a week or more before the start of treatment. Electronic notification of new orders allows nursing staff to predict workload and schedule staffing and treatments accordingly.
Limitations
Although proud of this project as a creative and effective solution, the oncology department staff recognize that there are some limitations with the process that preclude its use as a permanent tool. The main limitation of this approach to chemotherapy ordering stems from use of the CPRS progress note module to create an order. One important feature of any order is that it can be changed or discontinued. Due to the permanence of progress notes, an addendum must be made to the order to qualify it as discontinued, since a progress note cannot be discontinued. The users within the system are aware of this limitation and are vigilant for new addenda to these notes, but it could open a window for error.
This process is also not consistent with the ideal that all orders be entered by CPOE. In a perfect world, on signing the note at the end of this process, the orders would automatically generate pharmacy orders for the drug items. Unfortunately, that level of automation is not available at this time. Within the current infrastructure, that functionality would be devastating to the flexibility that is of greater importance for this process. It is exactly this problem that has led to the consideration of third-party software solutions.
Conclusion
The chemotherapy ordering process at the Kansas City VAMC is an effective communication tool. Ultimately, a physician’s order for treatment is a one-way communication to pharmacy and nursing staff. This process streamlines the communication and minimizes the need for callbacks and clarifications. It also permits anyone with access to the patient’s CPRS record to be able to review the plan. And it creates a standardized treatment checklist for more consistent care. The ASHP strongly recommends standardizing oncology ordering practices, and checklists are a recognized tool for improving the quality of care.2 The simplicity of the process and the no-cost maintenance of the technology are added benefits.
A novel solution was needed to improve safety and efficiency of chemotherapy ordering. The pharmacy department was the key in developing such a solution at the Kansas City VAMC. A transparent, standardized process was developed and implemented within a relatively short time frame. Built within existing software/hardware capabilities, the project had an immediate return on investment and avoided the overhead costs associated with implementing third-party ordering systems. In addition the process decreased turnaround time and increased throughput of the ordering process. An added benefit is that if a better tool (third party or otherwise) becomes available, the Kansas City VAMC is ready on a moment’s notice.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications,warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30(13):1553-1561.
2. Gawande A. The Checklist Manifesto: How to Get Things Right. Metropolitan Books: New York; 2009.
1. Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30(13):1553-1561.
2. Gawande A. The Checklist Manifesto: How to Get Things Right. Metropolitan Books: New York; 2009.
Blast Phase Chronic Myelogenous Leukemia
Chronic myelogenous leukemia (CML) is caused by the constitutively active BCR-ABL fusion protein that results from t(9;22), the Philadelphia (Ph+) chromosome. Chronic myelogenous leukemia typically evolves through 3 clinical phases: an indolent chronic phase, an accelerated phase, and a terminal blast phase analogous to acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL). Fortunately, today more than 80% of patients are diagnosed in the chronic phase of the disease.1
Before the development of the tyrosine kinase inhibitor (TKI) imatinib, > 20% of the patients with chronic phase CML progressed to the blast phase every year.2 Based on data from 8 years of follow-up with imatinib therapy, the rate of progression to the advanced phases of CML is about 1% per year, with freedom from progression at 92%.3 For the majority of patients with chronic phase CML, due to advances in treatment, the disease does not affect mortality.
For those who progress to the terminal blast phase of CML, survival is typically measured in months unless allogeneic stem cell transplant (allo-SCT) is an option. This article will review one of the major remaining problems in CML: how to manage blast phase CML.
Definition and Diagnosis
Defining blast phase CML can be confusing, because different criteria have been proposed, none of which are biologically based. The most widely used definition is set forth by the European LeukemiaNet, recommending 30% blasts in the blood or bone marrow or the presence of extramedullary disease.1 Clinically, blast phase CML may present with constitutional symptoms, bone pain, or symptoms related to cytopenias (fatigue, dyspnea, bleeding, infections).
Diagnostic workup should include a complete blood cell count (CBC) with differential, bone marrow analysis with conventional cytogenetics, flow cytometry to determine whether the blast phase is of myeloid or lymphoid origin, and molecular mutational analysis of the BCR-ABL tyrosine kinase domain to help guide the choice of TKI. If age and performance status are favorable, a donor search for allo-SCT should be started promptly.
Chronic myelogenous leukemia cells that contain the BCR-ABL kinase protein are genetically unstable.4,5 Additional cytogenetic aberrations (ACAs) are seen in up to 80% of those with blast phase CML and are the most consistent predictor of blast transformation in those with chronic phase CML.6 Chromosomal changes are broken down into the nonrandom, “major route” ACAs (trisomy 8, additional Ph+ chromosome, isochromosome 17q, trisomy 19), considered likely to be involved in the evolution of CML, and the more random “minor route” ACAs, which may denote nothing more than the instability of the genome.5,7 Mutations of the BCR-ABL tyrosine kinase domain are also seen in the majority of those in blast phase CML and, depending on the specific mutation, can negatively predict the response to certain TKI therapies.4
Prognosis
The single most important prognostic indicator for patients with CML remains the length of response to initial BCR-ABL–specific TKI therapy. Only a very small minority of patients are refractory to TKIs from the beginning; these are the patients with the worst prognosis.8 If the response to treatment seems inadequate, then the health care professional should first verify with the patient that he or she is taking the medicine as prescribed.1 Lack of adherence continues to be the most common reason for less-than-ideal outcomes or fluctuations in response and plays a critical role in treatment with TKI therapy, with worse outcomes when < 90% of doses are taken.9
Other features associated with a poor prognosis include cytogenetic clonal evolution, > 50% blasts, and/or extramedullary disease.7,10,11 At the time of imatinib failure, detection of mutations of the BCR-ABL tyrosine kinase domain correlates to worse 4-year event-free survival.12 Showing the instability of the genome in CML, patients who harbor mutations of the BCR-ABL domain have a higher likelihood of relapse associated with further mutations and, therefore, potentially further TKI resistance.13 Once CML has progressed to the blast phase, life expectancy is, on average, less than a year.11
Treatment Strategy
Currently, the most effective treatment strategy in blast phase CML is to prevent the transformation from chronic phase from ever occurring. Management of blast phase CML depends on 2 factors: (1) previous therapies; and (2) type of blast phase—myeloid or lymphoid. The goal of treatment is to knock the disease back to a clinical remission and/or a chronic phase for a long enough period to get the patient to allo-SCT if age, performance status, and suitable donor allow for it.
Using single-agent imatinib for blast phase CML has been tried in patients who have never been on TKI therapy before. Hematologic responses were seen in the majority of patients, but any form of cytogenetic response was seen in fewer than 20% of patients. Median overall survival, although better than with previous conventional chemotherapies, was still measured in months.6 A patient with blast phase CML who has never been on BCR-ABL–specific TKIs is very rare now; at a minimum, the patient has usually tried at least 1 TKI previously.
If blast phase CML develops while a patient is taking imatinib, treatment with a second-generation TKIs—nilotinib or dasatinib— should be attempted if the BCR-ABL tyrosine kinase domain analysis shows no resistant mutations.14 Both nilotinib and dasatinib have been tried as single agents in patients with imatinib-refractory CML or who are unable to tolerate imatinib.15,16 Cytogenetic response rates were 2 to 4 times higher for these agents than for imatinib when used in blast phase CML.
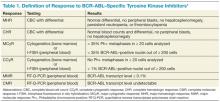
Table 1 reviews the common definitions of response, including cytogenetic response, to TKIs in CML. The pattern of response is usually very predictable: First, a hematologic response is seen, then a cytogenetic response, and finally, a hoped-for molecular response. Interestingly, in these studies, not all patients with blast phase CML who experienced a cytogenetic response had a hematologic response. This makes CBCs less reliable for assessing response and other peripheral blood tests, such as the interphase fluorescence in situ hybridization (I-FISH) test or the quantitative reverse transcriptase polymerase chain reaction (RT-Q-PCR) test, more important. Unfortunately, this improved cytogenetic response in blast phase CML did not translate to long-term survival advantage; median survival with these second- generation TKIs was still less than a year without transplant. If the T315I mutation is present, then clinical trials involving ponatinib or one of the newest non–FDA-approved TKIs should be considered.
Recent data involving ponatinib suggest similar response and survival rates to nilotinib and dasatinib, but this was in more heavily-pretreated CML patients who had resistance to, or unacceptable adverse effects from the second-generation TKIs or who had the BCR-ABL T315I mutation.17
In late 2013, ponatinib was voluntarily suspended from marketing and sales by its manufacturer due to a worrisome rate of serious arterial thromboembolic events reported in clinical trials and in postmarketing experience. However, the FDA reintroduced ponatinib in 2014 once additional safety measures were put in place, such as changes to the black box warning and review of the risk of arterial and venous thrombosis and occlusions.18
Table 2 compares the results between these newer TKIs in blast phase CML. If the patient can tolerate it, a combination of TKI with AML or ALL-type induction chemotherapy, preferably in a clinical trial setting, provides the best opportunity to return the patient to the chronic phase. If this is achieved, then allo-SCT represents the best chance for sustained remission or cure; allo-SCT was standard first-line therapy prior to the advent of BCR-ABL–specific TKIs. Tyrosine kinase inhibitor exposure prior to allo-SCT does not seem to affect transplantation outcomes.19 Allo-SCT while still in blast phase is discouraged because of its high risks with minimal benefit; disease-free survival rates are <10%.19 Although no scientific data support this, maintenance TKI posttransplantation seems logical, with monitoring of BCR-ABL transcript levels every 3 months.
Conclusion
With the advent of TKI therapy, the overall prognosis of CML has changed drastically. Unfortunately, the success seen with these novel agents in the chronic phase of CML has not translated into success in the blast phase of CML. Therefore, the best way to manage blast phase CML is to prevent this transformation from ever happening. The deeper and more rapid the cytogenetic and molecular response after TKI initiation, the better the long-term outcome for the patient.
If the patient progresses though TKI therapy, then combining a different TKI with a conventional induction chemotherapy regimen for acute leukemia should be tried; the goal is to achieve a remission that lasts long enough for the patient to be able to undergo allo-SCT. If the patient is not a candidate for allo-SCT, then the prognosis is extremely poor, and clinical trials with best supportive care should be considered.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Baccarani M, Pileri S, Steegmann JL, Muller M, Soverini S, Dreyling M; ESMO Guidelines Working Group. Chronic myeloid leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(7):vii72-vii77.
2. Sokal JE. Evaluation of survival data for chronic myelocytic leukemia. Am J Hematol. 1976;1(4):493-500.
3. Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood (ASH Annual Meeting Abstracts). 2009;114(22):abstract 1126.
4. Fabarius A, Leitner A, Hochhaus A, et al, Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung (SAKK) and the German CML Study Group. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118(26):6760-6768.
5. Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 2002;107(2):76-94.
6. Hehlmann R. How I treat CML blast crisis. Blood. 2012;120(4):737-747.
7. Jabbour EJ, Hughes TP, Cortes JE, Kantarjian HM, Hochhaus A. Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia [published online ahead of print November 12, 2013]. Leuk Lymphoma. doi:10.3109/10428194.2013.845883.
8. Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118(17):4541-4546.
9. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388.
10. Cervantes F, Rozman M, Rosell J, Urbano-Ispizua A, Montserrat E, Rozman C. A study of prognostic factors in blast crisis of Philadelphia chromosome-positive chronic myelogenous leukemia. Br J Haematol. 1990;76(1):27-32.
11. Wadhwa J, Szydlo RM, Apperley JF, et al. Factors affecting duration of survival after onset of blastic transformation of chronic myeloid leukemia. Blood. 2002;99(7):2304-2309.
12. Quintas-Cardama A, Kantarjian H, O’Brien S, et al. Outcome of patients with chronic myeloid leukemia with multiple ABL1 kinase domain mutations receiving tyrosine kinase inhibitor therapy. Haematologica. 2011;96(6):918-921.
13. Soverini S, Gnani A, Colarossi S, et al. Philadelphia-positive patients who already harbor imatinib-resistant BCR-ABL kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. 2009;114(10):2168-2171.
14. Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118(5):1208-1215.
15. Giles FJ, Kantarjian HM, le Coutre PD, et al. Nilotinib is effective in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blastic phase. Leukemia. 2012;26(5):959-962.
16. Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116(16):3852-3861.
17. Cortes JE, Kim D-W, Pinilla-Ibarz J, et al; PACE Investigators. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783-1796.
18. Food and Drug Administration. FDA Drug Safety Communication: FDA requires multiple new safety measures for leukemia drug Iclusig; company expected to resume marketing. U.S. Food and Drug Administration Website. http://www.fda.gov/drugs/drugsafety/ucm379554.htm. Updated December 20, 2013. Accessed June 13, 2014.
19. Khoury HJ, Kukreja M, Goldman JM, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012;47(6):810-816.
Chronic myelogenous leukemia (CML) is caused by the constitutively active BCR-ABL fusion protein that results from t(9;22), the Philadelphia (Ph+) chromosome. Chronic myelogenous leukemia typically evolves through 3 clinical phases: an indolent chronic phase, an accelerated phase, and a terminal blast phase analogous to acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL). Fortunately, today more than 80% of patients are diagnosed in the chronic phase of the disease.1
Before the development of the tyrosine kinase inhibitor (TKI) imatinib, > 20% of the patients with chronic phase CML progressed to the blast phase every year.2 Based on data from 8 years of follow-up with imatinib therapy, the rate of progression to the advanced phases of CML is about 1% per year, with freedom from progression at 92%.3 For the majority of patients with chronic phase CML, due to advances in treatment, the disease does not affect mortality.
For those who progress to the terminal blast phase of CML, survival is typically measured in months unless allogeneic stem cell transplant (allo-SCT) is an option. This article will review one of the major remaining problems in CML: how to manage blast phase CML.
Definition and Diagnosis
Defining blast phase CML can be confusing, because different criteria have been proposed, none of which are biologically based. The most widely used definition is set forth by the European LeukemiaNet, recommending 30% blasts in the blood or bone marrow or the presence of extramedullary disease.1 Clinically, blast phase CML may present with constitutional symptoms, bone pain, or symptoms related to cytopenias (fatigue, dyspnea, bleeding, infections).
Diagnostic workup should include a complete blood cell count (CBC) with differential, bone marrow analysis with conventional cytogenetics, flow cytometry to determine whether the blast phase is of myeloid or lymphoid origin, and molecular mutational analysis of the BCR-ABL tyrosine kinase domain to help guide the choice of TKI. If age and performance status are favorable, a donor search for allo-SCT should be started promptly.
Chronic myelogenous leukemia cells that contain the BCR-ABL kinase protein are genetically unstable.4,5 Additional cytogenetic aberrations (ACAs) are seen in up to 80% of those with blast phase CML and are the most consistent predictor of blast transformation in those with chronic phase CML.6 Chromosomal changes are broken down into the nonrandom, “major route” ACAs (trisomy 8, additional Ph+ chromosome, isochromosome 17q, trisomy 19), considered likely to be involved in the evolution of CML, and the more random “minor route” ACAs, which may denote nothing more than the instability of the genome.5,7 Mutations of the BCR-ABL tyrosine kinase domain are also seen in the majority of those in blast phase CML and, depending on the specific mutation, can negatively predict the response to certain TKI therapies.4
Prognosis
The single most important prognostic indicator for patients with CML remains the length of response to initial BCR-ABL–specific TKI therapy. Only a very small minority of patients are refractory to TKIs from the beginning; these are the patients with the worst prognosis.8 If the response to treatment seems inadequate, then the health care professional should first verify with the patient that he or she is taking the medicine as prescribed.1 Lack of adherence continues to be the most common reason for less-than-ideal outcomes or fluctuations in response and plays a critical role in treatment with TKI therapy, with worse outcomes when < 90% of doses are taken.9
Other features associated with a poor prognosis include cytogenetic clonal evolution, > 50% blasts, and/or extramedullary disease.7,10,11 At the time of imatinib failure, detection of mutations of the BCR-ABL tyrosine kinase domain correlates to worse 4-year event-free survival.12 Showing the instability of the genome in CML, patients who harbor mutations of the BCR-ABL domain have a higher likelihood of relapse associated with further mutations and, therefore, potentially further TKI resistance.13 Once CML has progressed to the blast phase, life expectancy is, on average, less than a year.11
Treatment Strategy
Currently, the most effective treatment strategy in blast phase CML is to prevent the transformation from chronic phase from ever occurring. Management of blast phase CML depends on 2 factors: (1) previous therapies; and (2) type of blast phase—myeloid or lymphoid. The goal of treatment is to knock the disease back to a clinical remission and/or a chronic phase for a long enough period to get the patient to allo-SCT if age, performance status, and suitable donor allow for it.
Using single-agent imatinib for blast phase CML has been tried in patients who have never been on TKI therapy before. Hematologic responses were seen in the majority of patients, but any form of cytogenetic response was seen in fewer than 20% of patients. Median overall survival, although better than with previous conventional chemotherapies, was still measured in months.6 A patient with blast phase CML who has never been on BCR-ABL–specific TKIs is very rare now; at a minimum, the patient has usually tried at least 1 TKI previously.
If blast phase CML develops while a patient is taking imatinib, treatment with a second-generation TKIs—nilotinib or dasatinib— should be attempted if the BCR-ABL tyrosine kinase domain analysis shows no resistant mutations.14 Both nilotinib and dasatinib have been tried as single agents in patients with imatinib-refractory CML or who are unable to tolerate imatinib.15,16 Cytogenetic response rates were 2 to 4 times higher for these agents than for imatinib when used in blast phase CML.
Table 1 reviews the common definitions of response, including cytogenetic response, to TKIs in CML. The pattern of response is usually very predictable: First, a hematologic response is seen, then a cytogenetic response, and finally, a hoped-for molecular response. Interestingly, in these studies, not all patients with blast phase CML who experienced a cytogenetic response had a hematologic response. This makes CBCs less reliable for assessing response and other peripheral blood tests, such as the interphase fluorescence in situ hybridization (I-FISH) test or the quantitative reverse transcriptase polymerase chain reaction (RT-Q-PCR) test, more important. Unfortunately, this improved cytogenetic response in blast phase CML did not translate to long-term survival advantage; median survival with these second- generation TKIs was still less than a year without transplant. If the T315I mutation is present, then clinical trials involving ponatinib or one of the newest non–FDA-approved TKIs should be considered.
Recent data involving ponatinib suggest similar response and survival rates to nilotinib and dasatinib, but this was in more heavily-pretreated CML patients who had resistance to, or unacceptable adverse effects from the second-generation TKIs or who had the BCR-ABL T315I mutation.17
In late 2013, ponatinib was voluntarily suspended from marketing and sales by its manufacturer due to a worrisome rate of serious arterial thromboembolic events reported in clinical trials and in postmarketing experience. However, the FDA reintroduced ponatinib in 2014 once additional safety measures were put in place, such as changes to the black box warning and review of the risk of arterial and venous thrombosis and occlusions.18
Table 2 compares the results between these newer TKIs in blast phase CML. If the patient can tolerate it, a combination of TKI with AML or ALL-type induction chemotherapy, preferably in a clinical trial setting, provides the best opportunity to return the patient to the chronic phase. If this is achieved, then allo-SCT represents the best chance for sustained remission or cure; allo-SCT was standard first-line therapy prior to the advent of BCR-ABL–specific TKIs. Tyrosine kinase inhibitor exposure prior to allo-SCT does not seem to affect transplantation outcomes.19 Allo-SCT while still in blast phase is discouraged because of its high risks with minimal benefit; disease-free survival rates are <10%.19 Although no scientific data support this, maintenance TKI posttransplantation seems logical, with monitoring of BCR-ABL transcript levels every 3 months.
Conclusion
With the advent of TKI therapy, the overall prognosis of CML has changed drastically. Unfortunately, the success seen with these novel agents in the chronic phase of CML has not translated into success in the blast phase of CML. Therefore, the best way to manage blast phase CML is to prevent this transformation from ever happening. The deeper and more rapid the cytogenetic and molecular response after TKI initiation, the better the long-term outcome for the patient.
If the patient progresses though TKI therapy, then combining a different TKI with a conventional induction chemotherapy regimen for acute leukemia should be tried; the goal is to achieve a remission that lasts long enough for the patient to be able to undergo allo-SCT. If the patient is not a candidate for allo-SCT, then the prognosis is extremely poor, and clinical trials with best supportive care should be considered.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Chronic myelogenous leukemia (CML) is caused by the constitutively active BCR-ABL fusion protein that results from t(9;22), the Philadelphia (Ph+) chromosome. Chronic myelogenous leukemia typically evolves through 3 clinical phases: an indolent chronic phase, an accelerated phase, and a terminal blast phase analogous to acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL). Fortunately, today more than 80% of patients are diagnosed in the chronic phase of the disease.1
Before the development of the tyrosine kinase inhibitor (TKI) imatinib, > 20% of the patients with chronic phase CML progressed to the blast phase every year.2 Based on data from 8 years of follow-up with imatinib therapy, the rate of progression to the advanced phases of CML is about 1% per year, with freedom from progression at 92%.3 For the majority of patients with chronic phase CML, due to advances in treatment, the disease does not affect mortality.
For those who progress to the terminal blast phase of CML, survival is typically measured in months unless allogeneic stem cell transplant (allo-SCT) is an option. This article will review one of the major remaining problems in CML: how to manage blast phase CML.
Definition and Diagnosis
Defining blast phase CML can be confusing, because different criteria have been proposed, none of which are biologically based. The most widely used definition is set forth by the European LeukemiaNet, recommending 30% blasts in the blood or bone marrow or the presence of extramedullary disease.1 Clinically, blast phase CML may present with constitutional symptoms, bone pain, or symptoms related to cytopenias (fatigue, dyspnea, bleeding, infections).
Diagnostic workup should include a complete blood cell count (CBC) with differential, bone marrow analysis with conventional cytogenetics, flow cytometry to determine whether the blast phase is of myeloid or lymphoid origin, and molecular mutational analysis of the BCR-ABL tyrosine kinase domain to help guide the choice of TKI. If age and performance status are favorable, a donor search for allo-SCT should be started promptly.
Chronic myelogenous leukemia cells that contain the BCR-ABL kinase protein are genetically unstable.4,5 Additional cytogenetic aberrations (ACAs) are seen in up to 80% of those with blast phase CML and are the most consistent predictor of blast transformation in those with chronic phase CML.6 Chromosomal changes are broken down into the nonrandom, “major route” ACAs (trisomy 8, additional Ph+ chromosome, isochromosome 17q, trisomy 19), considered likely to be involved in the evolution of CML, and the more random “minor route” ACAs, which may denote nothing more than the instability of the genome.5,7 Mutations of the BCR-ABL tyrosine kinase domain are also seen in the majority of those in blast phase CML and, depending on the specific mutation, can negatively predict the response to certain TKI therapies.4
Prognosis
The single most important prognostic indicator for patients with CML remains the length of response to initial BCR-ABL–specific TKI therapy. Only a very small minority of patients are refractory to TKIs from the beginning; these are the patients with the worst prognosis.8 If the response to treatment seems inadequate, then the health care professional should first verify with the patient that he or she is taking the medicine as prescribed.1 Lack of adherence continues to be the most common reason for less-than-ideal outcomes or fluctuations in response and plays a critical role in treatment with TKI therapy, with worse outcomes when < 90% of doses are taken.9
Other features associated with a poor prognosis include cytogenetic clonal evolution, > 50% blasts, and/or extramedullary disease.7,10,11 At the time of imatinib failure, detection of mutations of the BCR-ABL tyrosine kinase domain correlates to worse 4-year event-free survival.12 Showing the instability of the genome in CML, patients who harbor mutations of the BCR-ABL domain have a higher likelihood of relapse associated with further mutations and, therefore, potentially further TKI resistance.13 Once CML has progressed to the blast phase, life expectancy is, on average, less than a year.11
Treatment Strategy
Currently, the most effective treatment strategy in blast phase CML is to prevent the transformation from chronic phase from ever occurring. Management of blast phase CML depends on 2 factors: (1) previous therapies; and (2) type of blast phase—myeloid or lymphoid. The goal of treatment is to knock the disease back to a clinical remission and/or a chronic phase for a long enough period to get the patient to allo-SCT if age, performance status, and suitable donor allow for it.
Using single-agent imatinib for blast phase CML has been tried in patients who have never been on TKI therapy before. Hematologic responses were seen in the majority of patients, but any form of cytogenetic response was seen in fewer than 20% of patients. Median overall survival, although better than with previous conventional chemotherapies, was still measured in months.6 A patient with blast phase CML who has never been on BCR-ABL–specific TKIs is very rare now; at a minimum, the patient has usually tried at least 1 TKI previously.
If blast phase CML develops while a patient is taking imatinib, treatment with a second-generation TKIs—nilotinib or dasatinib— should be attempted if the BCR-ABL tyrosine kinase domain analysis shows no resistant mutations.14 Both nilotinib and dasatinib have been tried as single agents in patients with imatinib-refractory CML or who are unable to tolerate imatinib.15,16 Cytogenetic response rates were 2 to 4 times higher for these agents than for imatinib when used in blast phase CML.
Table 1 reviews the common definitions of response, including cytogenetic response, to TKIs in CML. The pattern of response is usually very predictable: First, a hematologic response is seen, then a cytogenetic response, and finally, a hoped-for molecular response. Interestingly, in these studies, not all patients with blast phase CML who experienced a cytogenetic response had a hematologic response. This makes CBCs less reliable for assessing response and other peripheral blood tests, such as the interphase fluorescence in situ hybridization (I-FISH) test or the quantitative reverse transcriptase polymerase chain reaction (RT-Q-PCR) test, more important. Unfortunately, this improved cytogenetic response in blast phase CML did not translate to long-term survival advantage; median survival with these second- generation TKIs was still less than a year without transplant. If the T315I mutation is present, then clinical trials involving ponatinib or one of the newest non–FDA-approved TKIs should be considered.
Recent data involving ponatinib suggest similar response and survival rates to nilotinib and dasatinib, but this was in more heavily-pretreated CML patients who had resistance to, or unacceptable adverse effects from the second-generation TKIs or who had the BCR-ABL T315I mutation.17
In late 2013, ponatinib was voluntarily suspended from marketing and sales by its manufacturer due to a worrisome rate of serious arterial thromboembolic events reported in clinical trials and in postmarketing experience. However, the FDA reintroduced ponatinib in 2014 once additional safety measures were put in place, such as changes to the black box warning and review of the risk of arterial and venous thrombosis and occlusions.18
Table 2 compares the results between these newer TKIs in blast phase CML. If the patient can tolerate it, a combination of TKI with AML or ALL-type induction chemotherapy, preferably in a clinical trial setting, provides the best opportunity to return the patient to the chronic phase. If this is achieved, then allo-SCT represents the best chance for sustained remission or cure; allo-SCT was standard first-line therapy prior to the advent of BCR-ABL–specific TKIs. Tyrosine kinase inhibitor exposure prior to allo-SCT does not seem to affect transplantation outcomes.19 Allo-SCT while still in blast phase is discouraged because of its high risks with minimal benefit; disease-free survival rates are <10%.19 Although no scientific data support this, maintenance TKI posttransplantation seems logical, with monitoring of BCR-ABL transcript levels every 3 months.
Conclusion
With the advent of TKI therapy, the overall prognosis of CML has changed drastically. Unfortunately, the success seen with these novel agents in the chronic phase of CML has not translated into success in the blast phase of CML. Therefore, the best way to manage blast phase CML is to prevent this transformation from ever happening. The deeper and more rapid the cytogenetic and molecular response after TKI initiation, the better the long-term outcome for the patient.
If the patient progresses though TKI therapy, then combining a different TKI with a conventional induction chemotherapy regimen for acute leukemia should be tried; the goal is to achieve a remission that lasts long enough for the patient to be able to undergo allo-SCT. If the patient is not a candidate for allo-SCT, then the prognosis is extremely poor, and clinical trials with best supportive care should be considered.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Baccarani M, Pileri S, Steegmann JL, Muller M, Soverini S, Dreyling M; ESMO Guidelines Working Group. Chronic myeloid leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(7):vii72-vii77.
2. Sokal JE. Evaluation of survival data for chronic myelocytic leukemia. Am J Hematol. 1976;1(4):493-500.
3. Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood (ASH Annual Meeting Abstracts). 2009;114(22):abstract 1126.
4. Fabarius A, Leitner A, Hochhaus A, et al, Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung (SAKK) and the German CML Study Group. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118(26):6760-6768.
5. Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 2002;107(2):76-94.
6. Hehlmann R. How I treat CML blast crisis. Blood. 2012;120(4):737-747.
7. Jabbour EJ, Hughes TP, Cortes JE, Kantarjian HM, Hochhaus A. Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia [published online ahead of print November 12, 2013]. Leuk Lymphoma. doi:10.3109/10428194.2013.845883.
8. Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118(17):4541-4546.
9. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388.
10. Cervantes F, Rozman M, Rosell J, Urbano-Ispizua A, Montserrat E, Rozman C. A study of prognostic factors in blast crisis of Philadelphia chromosome-positive chronic myelogenous leukemia. Br J Haematol. 1990;76(1):27-32.
11. Wadhwa J, Szydlo RM, Apperley JF, et al. Factors affecting duration of survival after onset of blastic transformation of chronic myeloid leukemia. Blood. 2002;99(7):2304-2309.
12. Quintas-Cardama A, Kantarjian H, O’Brien S, et al. Outcome of patients with chronic myeloid leukemia with multiple ABL1 kinase domain mutations receiving tyrosine kinase inhibitor therapy. Haematologica. 2011;96(6):918-921.
13. Soverini S, Gnani A, Colarossi S, et al. Philadelphia-positive patients who already harbor imatinib-resistant BCR-ABL kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. 2009;114(10):2168-2171.
14. Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118(5):1208-1215.
15. Giles FJ, Kantarjian HM, le Coutre PD, et al. Nilotinib is effective in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blastic phase. Leukemia. 2012;26(5):959-962.
16. Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116(16):3852-3861.
17. Cortes JE, Kim D-W, Pinilla-Ibarz J, et al; PACE Investigators. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783-1796.
18. Food and Drug Administration. FDA Drug Safety Communication: FDA requires multiple new safety measures for leukemia drug Iclusig; company expected to resume marketing. U.S. Food and Drug Administration Website. http://www.fda.gov/drugs/drugsafety/ucm379554.htm. Updated December 20, 2013. Accessed June 13, 2014.
19. Khoury HJ, Kukreja M, Goldman JM, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012;47(6):810-816.
1. Baccarani M, Pileri S, Steegmann JL, Muller M, Soverini S, Dreyling M; ESMO Guidelines Working Group. Chronic myeloid leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(7):vii72-vii77.
2. Sokal JE. Evaluation of survival data for chronic myelocytic leukemia. Am J Hematol. 1976;1(4):493-500.
3. Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood (ASH Annual Meeting Abstracts). 2009;114(22):abstract 1126.
4. Fabarius A, Leitner A, Hochhaus A, et al, Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung (SAKK) and the German CML Study Group. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118(26):6760-6768.
5. Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 2002;107(2):76-94.
6. Hehlmann R. How I treat CML blast crisis. Blood. 2012;120(4):737-747.
7. Jabbour EJ, Hughes TP, Cortes JE, Kantarjian HM, Hochhaus A. Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia [published online ahead of print November 12, 2013]. Leuk Lymphoma. doi:10.3109/10428194.2013.845883.
8. Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118(17):4541-4546.
9. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388.
10. Cervantes F, Rozman M, Rosell J, Urbano-Ispizua A, Montserrat E, Rozman C. A study of prognostic factors in blast crisis of Philadelphia chromosome-positive chronic myelogenous leukemia. Br J Haematol. 1990;76(1):27-32.
11. Wadhwa J, Szydlo RM, Apperley JF, et al. Factors affecting duration of survival after onset of blastic transformation of chronic myeloid leukemia. Blood. 2002;99(7):2304-2309.
12. Quintas-Cardama A, Kantarjian H, O’Brien S, et al. Outcome of patients with chronic myeloid leukemia with multiple ABL1 kinase domain mutations receiving tyrosine kinase inhibitor therapy. Haematologica. 2011;96(6):918-921.
13. Soverini S, Gnani A, Colarossi S, et al. Philadelphia-positive patients who already harbor imatinib-resistant BCR-ABL kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. 2009;114(10):2168-2171.
14. Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118(5):1208-1215.
15. Giles FJ, Kantarjian HM, le Coutre PD, et al. Nilotinib is effective in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blastic phase. Leukemia. 2012;26(5):959-962.
16. Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116(16):3852-3861.
17. Cortes JE, Kim D-W, Pinilla-Ibarz J, et al; PACE Investigators. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783-1796.
18. Food and Drug Administration. FDA Drug Safety Communication: FDA requires multiple new safety measures for leukemia drug Iclusig; company expected to resume marketing. U.S. Food and Drug Administration Website. http://www.fda.gov/drugs/drugsafety/ucm379554.htm. Updated December 20, 2013. Accessed June 13, 2014.
19. Khoury HJ, Kukreja M, Goldman JM, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012;47(6):810-816.