User login
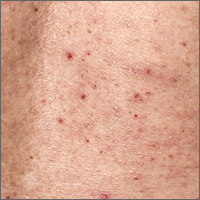
What is the most effective treatment for scabies?
EVIDENCE SUMMARY
A 2007 Cochrane review on scabies treatment identified 11 trials that evaluated permethrin for treating scabies.1 In 2 trials, 140 patients were randomized to receive either 200 mcg/kg of oral ivermectin or overnight application of 5% topical permethrin. Topical permethrin was superior to oral ivermectin with failure rates at 2 weeks of 8% and 39%, respectively (number needed to treat [NNT]=4; risk ratio [RR]=4.61; 95% confidence interval [CI], 2.07-10.26).
Two trials compared 5% topical permethrin with 10% topical crotamiton in 194 patients with follow-up at 28 days. Permethrin was superior to crotamiton with failure rates of 6% and 26%, respectively (NNT=6; RR=0.24; 95% CI, 0.10-0.55).
Five trials with 753 patients compared topical permethrin, 2.5% to 3.5%, with topical 1% lindane, but heterogeneity precluded pooling all the studies. In the 3 studies (554 patients) that were comparable, topical 3.5% permethrin was superior to lindane after a single application of each with failure rates of 9% and 15%, respectively (NNT=17; RR=0.59; 95% CI, 0.37-0.95).
Two trials that compared permethrin with topical benzyl benzoate (53 patients) and natural synergized pyrethrins (40 patients) showed no difference in treatment failures, but the trials were small and lacked sufficient statistical power.
Four additional studies included in the review compared crotamiton with lindane (100 patients), lindane with sulfur (68 patients), benzyl benzoate with sulfur (158 patients), and benzyl benzoate with natural synergized pyrethrins (240 patients). None demonstrated superiority, but all were small studies.1 A single small trial of 55 patients that compared oral ivermectin 200 mcg/kg with placebo showed failure rates at one week of 21% and 85%, respectively (NNT=2; RR=0.24; 95% CI, 0.12-0.51).1
Topical permethrin vs oral ivermectin
A 2014 systematic review of 5 studies included 2 new studies done after the 2007 Cochrane review.2 The new RCTs compared a single application of 5% topical permethrin with a single dose or 2 doses of oral ivermectin given 2 weeks apart. No statistically significant differences were found in these studies.2 Both underpowered studies favored topical permethrin, however.
The P value was .42 in one study of 242 adults and children, and this trial showed a clinical cure rate at 2 weeks of 93% using topical permethrin vs 86% using oral ivermectin.2
The other study of 120 adults and children didn’t report a P value or identify statistically significant differences between topical permethrin and oral ivermectin.2 This study reported a clinical cure rate of 87% with topical permethrin, 78% with a single dose of oral ivermectin, and 67% with 2 doses of oral ivermectin 2 weeks apart.2
Ivermectin may control endemic scabies better than permethrin
A 2015 randomized controlled trial with 2051 patients compared mass treatments in a scabies-endemic population in Fiji.3 The trial had 3 arms: a standard-care group treated with 5% topical permethrin if symptoms were present and retreated at 2 weeks if symptoms persisted; a permethrin group in which all participants, whether infected or not, received 5% permethrin followed by a second dose at 7 to 14 days if symptoms persisted; and an oral ivermectin group in which participants were treated with 200 mcg/kg, repeated in 7 to 14 days for those with baseline scabies.
At 12 months, the relative risk reductions were 94% (95% CI, 83%-100%) for the ivermectin group, 62% (95% CI, 49%-75%) for the permethrin group, and 49% (95% CI, 37%-60%) for the standard-care group.3 The study had multiple limitations, and all groups were permitted to receive standard care at any time during the 12-month follow-up period. Nevertheless, the findings suggest that endemic scabies control with ivermectin may be superior to topical permethrin.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)4 and the European Guideline for the Management of Scabies5 both recommend topical permethrin as first-line therapy for classical scabies and note that oral ivermectin may be safe and effective but isn’t licensed for scabies treatment in most countries. Ivermectin isn’t approved by the United States Food and Drug Administration for treating scabies.
The CDC recommendations note that the safety of ivermectin in children weighing less than 15 kg and pregnant women hasn’t been established.4
1. Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007;(3):CD000320.
2. Johnstone P, Strong M. Scabies. BMJ Clinical Evidence. 2014:1707.
3. Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
4. Centers for Disease Control and Prevention. Scabies. Treatment. Available at: www.cdc.gov/parasites/scabies/health_professionals/meds.html. Accessed February 26, 2016.
5. Scott G, Chosidow O. European guideline for the management of scabies, 2010. Int J STD AIDS. 2011;22:301-303.
EVIDENCE SUMMARY
A 2007 Cochrane review on scabies treatment identified 11 trials that evaluated permethrin for treating scabies.1 In 2 trials, 140 patients were randomized to receive either 200 mcg/kg of oral ivermectin or overnight application of 5% topical permethrin. Topical permethrin was superior to oral ivermectin with failure rates at 2 weeks of 8% and 39%, respectively (number needed to treat [NNT]=4; risk ratio [RR]=4.61; 95% confidence interval [CI], 2.07-10.26).
Two trials compared 5% topical permethrin with 10% topical crotamiton in 194 patients with follow-up at 28 days. Permethrin was superior to crotamiton with failure rates of 6% and 26%, respectively (NNT=6; RR=0.24; 95% CI, 0.10-0.55).
Five trials with 753 patients compared topical permethrin, 2.5% to 3.5%, with topical 1% lindane, but heterogeneity precluded pooling all the studies. In the 3 studies (554 patients) that were comparable, topical 3.5% permethrin was superior to lindane after a single application of each with failure rates of 9% and 15%, respectively (NNT=17; RR=0.59; 95% CI, 0.37-0.95).
Two trials that compared permethrin with topical benzyl benzoate (53 patients) and natural synergized pyrethrins (40 patients) showed no difference in treatment failures, but the trials were small and lacked sufficient statistical power.
Four additional studies included in the review compared crotamiton with lindane (100 patients), lindane with sulfur (68 patients), benzyl benzoate with sulfur (158 patients), and benzyl benzoate with natural synergized pyrethrins (240 patients). None demonstrated superiority, but all were small studies.1 A single small trial of 55 patients that compared oral ivermectin 200 mcg/kg with placebo showed failure rates at one week of 21% and 85%, respectively (NNT=2; RR=0.24; 95% CI, 0.12-0.51).1
Topical permethrin vs oral ivermectin
A 2014 systematic review of 5 studies included 2 new studies done after the 2007 Cochrane review.2 The new RCTs compared a single application of 5% topical permethrin with a single dose or 2 doses of oral ivermectin given 2 weeks apart. No statistically significant differences were found in these studies.2 Both underpowered studies favored topical permethrin, however.
The P value was .42 in one study of 242 adults and children, and this trial showed a clinical cure rate at 2 weeks of 93% using topical permethrin vs 86% using oral ivermectin.2
The other study of 120 adults and children didn’t report a P value or identify statistically significant differences between topical permethrin and oral ivermectin.2 This study reported a clinical cure rate of 87% with topical permethrin, 78% with a single dose of oral ivermectin, and 67% with 2 doses of oral ivermectin 2 weeks apart.2
Ivermectin may control endemic scabies better than permethrin
A 2015 randomized controlled trial with 2051 patients compared mass treatments in a scabies-endemic population in Fiji.3 The trial had 3 arms: a standard-care group treated with 5% topical permethrin if symptoms were present and retreated at 2 weeks if symptoms persisted; a permethrin group in which all participants, whether infected or not, received 5% permethrin followed by a second dose at 7 to 14 days if symptoms persisted; and an oral ivermectin group in which participants were treated with 200 mcg/kg, repeated in 7 to 14 days for those with baseline scabies.
At 12 months, the relative risk reductions were 94% (95% CI, 83%-100%) for the ivermectin group, 62% (95% CI, 49%-75%) for the permethrin group, and 49% (95% CI, 37%-60%) for the standard-care group.3 The study had multiple limitations, and all groups were permitted to receive standard care at any time during the 12-month follow-up period. Nevertheless, the findings suggest that endemic scabies control with ivermectin may be superior to topical permethrin.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)4 and the European Guideline for the Management of Scabies5 both recommend topical permethrin as first-line therapy for classical scabies and note that oral ivermectin may be safe and effective but isn’t licensed for scabies treatment in most countries. Ivermectin isn’t approved by the United States Food and Drug Administration for treating scabies.
The CDC recommendations note that the safety of ivermectin in children weighing less than 15 kg and pregnant women hasn’t been established.4
EVIDENCE SUMMARY
A 2007 Cochrane review on scabies treatment identified 11 trials that evaluated permethrin for treating scabies.1 In 2 trials, 140 patients were randomized to receive either 200 mcg/kg of oral ivermectin or overnight application of 5% topical permethrin. Topical permethrin was superior to oral ivermectin with failure rates at 2 weeks of 8% and 39%, respectively (number needed to treat [NNT]=4; risk ratio [RR]=4.61; 95% confidence interval [CI], 2.07-10.26).
Two trials compared 5% topical permethrin with 10% topical crotamiton in 194 patients with follow-up at 28 days. Permethrin was superior to crotamiton with failure rates of 6% and 26%, respectively (NNT=6; RR=0.24; 95% CI, 0.10-0.55).
Five trials with 753 patients compared topical permethrin, 2.5% to 3.5%, with topical 1% lindane, but heterogeneity precluded pooling all the studies. In the 3 studies (554 patients) that were comparable, topical 3.5% permethrin was superior to lindane after a single application of each with failure rates of 9% and 15%, respectively (NNT=17; RR=0.59; 95% CI, 0.37-0.95).
Two trials that compared permethrin with topical benzyl benzoate (53 patients) and natural synergized pyrethrins (40 patients) showed no difference in treatment failures, but the trials were small and lacked sufficient statistical power.
Four additional studies included in the review compared crotamiton with lindane (100 patients), lindane with sulfur (68 patients), benzyl benzoate with sulfur (158 patients), and benzyl benzoate with natural synergized pyrethrins (240 patients). None demonstrated superiority, but all were small studies.1 A single small trial of 55 patients that compared oral ivermectin 200 mcg/kg with placebo showed failure rates at one week of 21% and 85%, respectively (NNT=2; RR=0.24; 95% CI, 0.12-0.51).1
Topical permethrin vs oral ivermectin
A 2014 systematic review of 5 studies included 2 new studies done after the 2007 Cochrane review.2 The new RCTs compared a single application of 5% topical permethrin with a single dose or 2 doses of oral ivermectin given 2 weeks apart. No statistically significant differences were found in these studies.2 Both underpowered studies favored topical permethrin, however.
The P value was .42 in one study of 242 adults and children, and this trial showed a clinical cure rate at 2 weeks of 93% using topical permethrin vs 86% using oral ivermectin.2
The other study of 120 adults and children didn’t report a P value or identify statistically significant differences between topical permethrin and oral ivermectin.2 This study reported a clinical cure rate of 87% with topical permethrin, 78% with a single dose of oral ivermectin, and 67% with 2 doses of oral ivermectin 2 weeks apart.2
Ivermectin may control endemic scabies better than permethrin
A 2015 randomized controlled trial with 2051 patients compared mass treatments in a scabies-endemic population in Fiji.3 The trial had 3 arms: a standard-care group treated with 5% topical permethrin if symptoms were present and retreated at 2 weeks if symptoms persisted; a permethrin group in which all participants, whether infected or not, received 5% permethrin followed by a second dose at 7 to 14 days if symptoms persisted; and an oral ivermectin group in which participants were treated with 200 mcg/kg, repeated in 7 to 14 days for those with baseline scabies.
At 12 months, the relative risk reductions were 94% (95% CI, 83%-100%) for the ivermectin group, 62% (95% CI, 49%-75%) for the permethrin group, and 49% (95% CI, 37%-60%) for the standard-care group.3 The study had multiple limitations, and all groups were permitted to receive standard care at any time during the 12-month follow-up period. Nevertheless, the findings suggest that endemic scabies control with ivermectin may be superior to topical permethrin.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)4 and the European Guideline for the Management of Scabies5 both recommend topical permethrin as first-line therapy for classical scabies and note that oral ivermectin may be safe and effective but isn’t licensed for scabies treatment in most countries. Ivermectin isn’t approved by the United States Food and Drug Administration for treating scabies.
The CDC recommendations note that the safety of ivermectin in children weighing less than 15 kg and pregnant women hasn’t been established.4
1. Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007;(3):CD000320.
2. Johnstone P, Strong M. Scabies. BMJ Clinical Evidence. 2014:1707.
3. Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
4. Centers for Disease Control and Prevention. Scabies. Treatment. Available at: www.cdc.gov/parasites/scabies/health_professionals/meds.html. Accessed February 26, 2016.
5. Scott G, Chosidow O. European guideline for the management of scabies, 2010. Int J STD AIDS. 2011;22:301-303.
1. Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007;(3):CD000320.
2. Johnstone P, Strong M. Scabies. BMJ Clinical Evidence. 2014:1707.
3. Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
4. Centers for Disease Control and Prevention. Scabies. Treatment. Available at: www.cdc.gov/parasites/scabies/health_professionals/meds.html. Accessed February 26, 2016.
5. Scott G, Chosidow O. European guideline for the management of scabies, 2010. Int J STD AIDS. 2011;22:301-303.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Topical permethrin is the most effective treatment for classic scabies (strength of recommendation [SOR]: A, meta-analyses with consistent results).
Topical lindane and crotamiton are inferior to permethrin but appear equivalent to each other and benzyl benzoate, sulfur, and natural synergized pyrethrins (SOR: B, limited randomized trials).
Although not as effective as topical permethrin, oral ivermectin is an effective treatment compared with placebo (SOR: B, a single small randomized trial).
Oral ivermectin may reduce the prevalence of scabies at one year in populations with endemic disease more than topical permethrin (SOR: B, a single randomized trial).
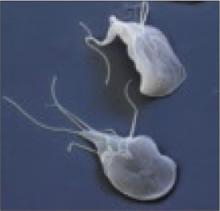
What’s the most effective treatment for giardiasis?
A single 2-g dose of tinidazole is the best treatment (strength of recommendation [SOR]: A, based on meta-analysis). Other drugs, such as nitazoxanide, metronidazole, mebendazole, and albendazole, can also be used (SOR: A, based on randomized controlled trial [RCT] of patient-oriented outcomes), but tinidazole has a higher clinical cure rate than these drugs. It also has a comparable side-effect profile and requires only 1 dose.
The real challenge is diagnosis
Cynthia Brown, MD
University of Nevada, Reno
As this review points out, all the available treatments for giardiasis are effective. Additional prescribing considerations include cost (500 mg metronidazole costs about 30 cents, for example, while 2 mg tinidazole costs $18) and insurance coverage. Tinidazole and metronidazole, unlike the other medications, require that the patient abstain from alcohol for 72 hours after dosing.
In my experience, the biggest challenge in treating giardiasis is deciding when to consider it in the differential and when to test for it. Presentations vary from vague symptoms such as bloating to severe diarrhea. Often the patient has not been exposed to well or stream water. You can test stool samples for ova and parasites, or serum for fluorescent antibody or enzyme-linked immunosorbent assay (ELISA).
Evidence summary
Giardia lamblia is a protozoan parasite found worldwide. Infection typically results from ingesting cysts in contaminated food or water. Patients with giardiasis may be asymptomatic or have mild to severe gastrointestinal symptoms, including explosive diarrhea, abdominal pain, steatorrhea, flatulence, bloating, nausea, and vomiting. Treatment varies widely based on geographic location, physician preference, and availability and cost of medication (TABLE).1
TABLE 1
Drugs commonly used to treat giardiasis
| DRUG | ADULT DOSE | SCHEDULE | COMMENT |
|---|---|---|---|
| Tinidazole | 2 g | 1 time | Can be given to children 3 years of age and older Pregnancy drug class C |
| Metronidazole | 250, 500,or 750 mg | 1 time or 3 times daily for 5 days. (Usually 250 mg, 3 times a day, for 5 days) | Contraindicated in first trimester of pregnancy |
| Mebendazole | 100 mg | Twice daily for 5 days | Contraindicated in first trimester of pregnancy Pregnancy drug class B |
| Nitazoxanide | 500 mg | Twice daily for 3 days | Can be given to children 1 year of age and older Available in liquid form Pregnancy drug class B |
| Albendazole | 200-400 mg | Twice daily for 5 days | Pregnancy drug class C |
| Sources: Beach M,1 and Gilbert DM et al.8 | |||
Tinidazole is the treatment of choice
A 2006 Cochrane Review compared 34 trials of many drug therapies for giardiasis.2 The review, which is being updated to include additional publications, evaluated both head-to-head and placebo-controlled studies, looking at dosage as well as length of drug therapy.
The review found that a single dose of tinidazole had a higher clinical cure rate than other therapies such as metronidazole (odds ratio [OR]=5.33; 95% confidence interval [CI], 2.66-10.67)2 along with a comparable side-effect profile. These findings favor tinidazole as the treatment of choice for symptomatic giardiasis.
How effective are other drugs?
The 2006 Cochrane Review found no difference in clinical cure rate between short-term treatment (3 days) with metronidazole and longer therapy with metronidazole or other drugs. Subsequently, a single dose of metronidazole was found to be as effective as treatment for 5 days or longer (OR=0.33, 95% CI 0.08-1.34).
Since publication of the Cochrane review, several studies have further evaluated mebendazole.
- An RCT in Cuban children 5 to 15 years of age found no difference in clinical cure rate between a 5-day course of mebendazole and more traditional therapy with quinacrine.3
- Another RCT comparing 5 days of mebendazole with 7 days of metronidazole in 7- to 12-year-old Iranian children showed no statistical difference in microbiologic cure between the 2 regimens.4
- Single-dose tinidazole was superior to 3 doses of mebendazole in a single day in an RCT of 122 Cuban children that measured microbiologic cure (NNT=5.5 patients with tinidazole vs mebendazole).5
Two RCTs found nitazoxanide to be effective (number needed to treat [NNT]=1.82) compared to placebo in adolescents and adults.6 A 3-day course of nitazoxanide was as effective as 5 days of metronidazole (80% vs 85%, P=0.61) in resolving clinical giardiasis.7
An RCT of albendazole, 400 mg for 5 days, in 28 adults found it to be as effective as 500 mg metronidazole given 3 times a day for 5 days (80% vs 83%) but less likely than metronidazole (2% vs 18%) to cause anorexia (number needed to harm [NNH]=6.25).
Recommendations
The Centers for Disease Control and Prevention recommends tinidazole, metronidazole, quinacrine, albendazole, or nitazoxanide to treat giardiasis; however, it doesn’t indicate a preference for 1 medicine over another.1 The Infectious Diseases Society of America has no guideline. The Sanford Guide to Antimicrobial Therapy recommends either a single 2-g dose of tinidazole or 500 mg of nitazoxanide PO bid for 3 days as primary treatment.8
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Air Force Medical Service, nor the US Air Force.
1. Beach M. Prevention of specific infectious diseases—giardiasis. In: Arguin PM, Kozarsky PE, Navin AW eds. Centers for Disease Control and Prevention. Health Information for International Travel 2005-2006. Atlanta: US Department of Health and Human Services, Public Health Service; 2005. Available at: www2.ncid.cdc.gov/travel/yb/utils/ybGet.asp?section=dis&obj=giardiasis.htm. Accessed March 7, 2008.
2. Zaat JO, Mank T, Assendelft WJ. Drugs for treating giardiasis. Cochrane Database Syst Rev. 2005:CD000217.-
3. Canete R, Escobedo A, Gonzalez M, et al. Randomized clinical study of five days’ therapy with mebendazole compared to quinacrine in the treatment of symptomatic giardiasis in children. World J Gastroenterol. 2006;12:6366-6370.
4. Sadjjadi SM, Alborzi AW, Mostovfi H. Comparative clinical trial of mebendazole and metronidazole in giardiasis of children. J Trop Pediatr. 2001;47:176-178.
5. Canete R, Escobedo A, Gonzalez M, et al. A randomized, controlled, open-label trial of a single day of mebendazole versus a single dose of tinidazole in the treatment of giardiasis in children. Curr Med Res Opin. 2006;22:2131-2136.
6. Rossignol JF, Ayoub A, Ayers MS, et al. Treatment of diarrhea caused by Giardia intestinalis and Entameba histolytica or E dispar: A Randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis. 2001;184:381-384.
7. Ortiz JJ, Ayoub A, Gargala G, et al. Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from northern Peru. Aliment Pharmacol Ther. 2001;15:1409-1415.
8. Gilbert DM, Eliopoulos GM, Moellering RC, et al. The Sanford Guide to Antimicrobial Therapy 2006. 36th ed. Sperryville, Va: Antimicrobial Therapy; 2006:95.
A single 2-g dose of tinidazole is the best treatment (strength of recommendation [SOR]: A, based on meta-analysis). Other drugs, such as nitazoxanide, metronidazole, mebendazole, and albendazole, can also be used (SOR: A, based on randomized controlled trial [RCT] of patient-oriented outcomes), but tinidazole has a higher clinical cure rate than these drugs. It also has a comparable side-effect profile and requires only 1 dose.
The real challenge is diagnosis
Cynthia Brown, MD
University of Nevada, Reno
As this review points out, all the available treatments for giardiasis are effective. Additional prescribing considerations include cost (500 mg metronidazole costs about 30 cents, for example, while 2 mg tinidazole costs $18) and insurance coverage. Tinidazole and metronidazole, unlike the other medications, require that the patient abstain from alcohol for 72 hours after dosing.
In my experience, the biggest challenge in treating giardiasis is deciding when to consider it in the differential and when to test for it. Presentations vary from vague symptoms such as bloating to severe diarrhea. Often the patient has not been exposed to well or stream water. You can test stool samples for ova and parasites, or serum for fluorescent antibody or enzyme-linked immunosorbent assay (ELISA).
Evidence summary
Giardia lamblia is a protozoan parasite found worldwide. Infection typically results from ingesting cysts in contaminated food or water. Patients with giardiasis may be asymptomatic or have mild to severe gastrointestinal symptoms, including explosive diarrhea, abdominal pain, steatorrhea, flatulence, bloating, nausea, and vomiting. Treatment varies widely based on geographic location, physician preference, and availability and cost of medication (TABLE).1
TABLE 1
Drugs commonly used to treat giardiasis
| DRUG | ADULT DOSE | SCHEDULE | COMMENT |
|---|---|---|---|
| Tinidazole | 2 g | 1 time | Can be given to children 3 years of age and older Pregnancy drug class C |
| Metronidazole | 250, 500,or 750 mg | 1 time or 3 times daily for 5 days. (Usually 250 mg, 3 times a day, for 5 days) | Contraindicated in first trimester of pregnancy |
| Mebendazole | 100 mg | Twice daily for 5 days | Contraindicated in first trimester of pregnancy Pregnancy drug class B |
| Nitazoxanide | 500 mg | Twice daily for 3 days | Can be given to children 1 year of age and older Available in liquid form Pregnancy drug class B |
| Albendazole | 200-400 mg | Twice daily for 5 days | Pregnancy drug class C |
| Sources: Beach M,1 and Gilbert DM et al.8 | |||
Tinidazole is the treatment of choice
A 2006 Cochrane Review compared 34 trials of many drug therapies for giardiasis.2 The review, which is being updated to include additional publications, evaluated both head-to-head and placebo-controlled studies, looking at dosage as well as length of drug therapy.
The review found that a single dose of tinidazole had a higher clinical cure rate than other therapies such as metronidazole (odds ratio [OR]=5.33; 95% confidence interval [CI], 2.66-10.67)2 along with a comparable side-effect profile. These findings favor tinidazole as the treatment of choice for symptomatic giardiasis.
How effective are other drugs?
The 2006 Cochrane Review found no difference in clinical cure rate between short-term treatment (3 days) with metronidazole and longer therapy with metronidazole or other drugs. Subsequently, a single dose of metronidazole was found to be as effective as treatment for 5 days or longer (OR=0.33, 95% CI 0.08-1.34).
Since publication of the Cochrane review, several studies have further evaluated mebendazole.
- An RCT in Cuban children 5 to 15 years of age found no difference in clinical cure rate between a 5-day course of mebendazole and more traditional therapy with quinacrine.3
- Another RCT comparing 5 days of mebendazole with 7 days of metronidazole in 7- to 12-year-old Iranian children showed no statistical difference in microbiologic cure between the 2 regimens.4
- Single-dose tinidazole was superior to 3 doses of mebendazole in a single day in an RCT of 122 Cuban children that measured microbiologic cure (NNT=5.5 patients with tinidazole vs mebendazole).5
Two RCTs found nitazoxanide to be effective (number needed to treat [NNT]=1.82) compared to placebo in adolescents and adults.6 A 3-day course of nitazoxanide was as effective as 5 days of metronidazole (80% vs 85%, P=0.61) in resolving clinical giardiasis.7
An RCT of albendazole, 400 mg for 5 days, in 28 adults found it to be as effective as 500 mg metronidazole given 3 times a day for 5 days (80% vs 83%) but less likely than metronidazole (2% vs 18%) to cause anorexia (number needed to harm [NNH]=6.25).
Recommendations
The Centers for Disease Control and Prevention recommends tinidazole, metronidazole, quinacrine, albendazole, or nitazoxanide to treat giardiasis; however, it doesn’t indicate a preference for 1 medicine over another.1 The Infectious Diseases Society of America has no guideline. The Sanford Guide to Antimicrobial Therapy recommends either a single 2-g dose of tinidazole or 500 mg of nitazoxanide PO bid for 3 days as primary treatment.8
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Air Force Medical Service, nor the US Air Force.
A single 2-g dose of tinidazole is the best treatment (strength of recommendation [SOR]: A, based on meta-analysis). Other drugs, such as nitazoxanide, metronidazole, mebendazole, and albendazole, can also be used (SOR: A, based on randomized controlled trial [RCT] of patient-oriented outcomes), but tinidazole has a higher clinical cure rate than these drugs. It also has a comparable side-effect profile and requires only 1 dose.
The real challenge is diagnosis
Cynthia Brown, MD
University of Nevada, Reno
As this review points out, all the available treatments for giardiasis are effective. Additional prescribing considerations include cost (500 mg metronidazole costs about 30 cents, for example, while 2 mg tinidazole costs $18) and insurance coverage. Tinidazole and metronidazole, unlike the other medications, require that the patient abstain from alcohol for 72 hours after dosing.
In my experience, the biggest challenge in treating giardiasis is deciding when to consider it in the differential and when to test for it. Presentations vary from vague symptoms such as bloating to severe diarrhea. Often the patient has not been exposed to well or stream water. You can test stool samples for ova and parasites, or serum for fluorescent antibody or enzyme-linked immunosorbent assay (ELISA).
Evidence summary
Giardia lamblia is a protozoan parasite found worldwide. Infection typically results from ingesting cysts in contaminated food or water. Patients with giardiasis may be asymptomatic or have mild to severe gastrointestinal symptoms, including explosive diarrhea, abdominal pain, steatorrhea, flatulence, bloating, nausea, and vomiting. Treatment varies widely based on geographic location, physician preference, and availability and cost of medication (TABLE).1
TABLE 1
Drugs commonly used to treat giardiasis
| DRUG | ADULT DOSE | SCHEDULE | COMMENT |
|---|---|---|---|
| Tinidazole | 2 g | 1 time | Can be given to children 3 years of age and older Pregnancy drug class C |
| Metronidazole | 250, 500,or 750 mg | 1 time or 3 times daily for 5 days. (Usually 250 mg, 3 times a day, for 5 days) | Contraindicated in first trimester of pregnancy |
| Mebendazole | 100 mg | Twice daily for 5 days | Contraindicated in first trimester of pregnancy Pregnancy drug class B |
| Nitazoxanide | 500 mg | Twice daily for 3 days | Can be given to children 1 year of age and older Available in liquid form Pregnancy drug class B |
| Albendazole | 200-400 mg | Twice daily for 5 days | Pregnancy drug class C |
| Sources: Beach M,1 and Gilbert DM et al.8 | |||
Tinidazole is the treatment of choice
A 2006 Cochrane Review compared 34 trials of many drug therapies for giardiasis.2 The review, which is being updated to include additional publications, evaluated both head-to-head and placebo-controlled studies, looking at dosage as well as length of drug therapy.
The review found that a single dose of tinidazole had a higher clinical cure rate than other therapies such as metronidazole (odds ratio [OR]=5.33; 95% confidence interval [CI], 2.66-10.67)2 along with a comparable side-effect profile. These findings favor tinidazole as the treatment of choice for symptomatic giardiasis.
How effective are other drugs?
The 2006 Cochrane Review found no difference in clinical cure rate between short-term treatment (3 days) with metronidazole and longer therapy with metronidazole or other drugs. Subsequently, a single dose of metronidazole was found to be as effective as treatment for 5 days or longer (OR=0.33, 95% CI 0.08-1.34).
Since publication of the Cochrane review, several studies have further evaluated mebendazole.
- An RCT in Cuban children 5 to 15 years of age found no difference in clinical cure rate between a 5-day course of mebendazole and more traditional therapy with quinacrine.3
- Another RCT comparing 5 days of mebendazole with 7 days of metronidazole in 7- to 12-year-old Iranian children showed no statistical difference in microbiologic cure between the 2 regimens.4
- Single-dose tinidazole was superior to 3 doses of mebendazole in a single day in an RCT of 122 Cuban children that measured microbiologic cure (NNT=5.5 patients with tinidazole vs mebendazole).5
Two RCTs found nitazoxanide to be effective (number needed to treat [NNT]=1.82) compared to placebo in adolescents and adults.6 A 3-day course of nitazoxanide was as effective as 5 days of metronidazole (80% vs 85%, P=0.61) in resolving clinical giardiasis.7
An RCT of albendazole, 400 mg for 5 days, in 28 adults found it to be as effective as 500 mg metronidazole given 3 times a day for 5 days (80% vs 83%) but less likely than metronidazole (2% vs 18%) to cause anorexia (number needed to harm [NNH]=6.25).
Recommendations
The Centers for Disease Control and Prevention recommends tinidazole, metronidazole, quinacrine, albendazole, or nitazoxanide to treat giardiasis; however, it doesn’t indicate a preference for 1 medicine over another.1 The Infectious Diseases Society of America has no guideline. The Sanford Guide to Antimicrobial Therapy recommends either a single 2-g dose of tinidazole or 500 mg of nitazoxanide PO bid for 3 days as primary treatment.8
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Air Force Medical Service, nor the US Air Force.
1. Beach M. Prevention of specific infectious diseases—giardiasis. In: Arguin PM, Kozarsky PE, Navin AW eds. Centers for Disease Control and Prevention. Health Information for International Travel 2005-2006. Atlanta: US Department of Health and Human Services, Public Health Service; 2005. Available at: www2.ncid.cdc.gov/travel/yb/utils/ybGet.asp?section=dis&obj=giardiasis.htm. Accessed March 7, 2008.
2. Zaat JO, Mank T, Assendelft WJ. Drugs for treating giardiasis. Cochrane Database Syst Rev. 2005:CD000217.-
3. Canete R, Escobedo A, Gonzalez M, et al. Randomized clinical study of five days’ therapy with mebendazole compared to quinacrine in the treatment of symptomatic giardiasis in children. World J Gastroenterol. 2006;12:6366-6370.
4. Sadjjadi SM, Alborzi AW, Mostovfi H. Comparative clinical trial of mebendazole and metronidazole in giardiasis of children. J Trop Pediatr. 2001;47:176-178.
5. Canete R, Escobedo A, Gonzalez M, et al. A randomized, controlled, open-label trial of a single day of mebendazole versus a single dose of tinidazole in the treatment of giardiasis in children. Curr Med Res Opin. 2006;22:2131-2136.
6. Rossignol JF, Ayoub A, Ayers MS, et al. Treatment of diarrhea caused by Giardia intestinalis and Entameba histolytica or E dispar: A Randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis. 2001;184:381-384.
7. Ortiz JJ, Ayoub A, Gargala G, et al. Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from northern Peru. Aliment Pharmacol Ther. 2001;15:1409-1415.
8. Gilbert DM, Eliopoulos GM, Moellering RC, et al. The Sanford Guide to Antimicrobial Therapy 2006. 36th ed. Sperryville, Va: Antimicrobial Therapy; 2006:95.
1. Beach M. Prevention of specific infectious diseases—giardiasis. In: Arguin PM, Kozarsky PE, Navin AW eds. Centers for Disease Control and Prevention. Health Information for International Travel 2005-2006. Atlanta: US Department of Health and Human Services, Public Health Service; 2005. Available at: www2.ncid.cdc.gov/travel/yb/utils/ybGet.asp?section=dis&obj=giardiasis.htm. Accessed March 7, 2008.
2. Zaat JO, Mank T, Assendelft WJ. Drugs for treating giardiasis. Cochrane Database Syst Rev. 2005:CD000217.-
3. Canete R, Escobedo A, Gonzalez M, et al. Randomized clinical study of five days’ therapy with mebendazole compared to quinacrine in the treatment of symptomatic giardiasis in children. World J Gastroenterol. 2006;12:6366-6370.
4. Sadjjadi SM, Alborzi AW, Mostovfi H. Comparative clinical trial of mebendazole and metronidazole in giardiasis of children. J Trop Pediatr. 2001;47:176-178.
5. Canete R, Escobedo A, Gonzalez M, et al. A randomized, controlled, open-label trial of a single day of mebendazole versus a single dose of tinidazole in the treatment of giardiasis in children. Curr Med Res Opin. 2006;22:2131-2136.
6. Rossignol JF, Ayoub A, Ayers MS, et al. Treatment of diarrhea caused by Giardia intestinalis and Entameba histolytica or E dispar: A Randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis. 2001;184:381-384.
7. Ortiz JJ, Ayoub A, Gargala G, et al. Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from northern Peru. Aliment Pharmacol Ther. 2001;15:1409-1415.
8. Gilbert DM, Eliopoulos GM, Moellering RC, et al. The Sanford Guide to Antimicrobial Therapy 2006. 36th ed. Sperryville, Va: Antimicrobial Therapy; 2006:95.
Evidence-based answers from the Family Physicians Inquiries Network
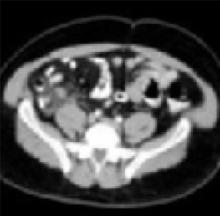
History, exam, and labs: Is one enough to diagnose acute adult appendicitis?
No, none of the 3—history, exam, or labs— is sufficiently accurate to diagnose acute appendicitis (strength of recommendation [SOR]: A, based on meta-analysis of high-quality studies). When combined, the following tests are helpful: an elevated C-reactive protein (CRP), elevated total white blood cell (WBC) count, elevated percentage of polymorphonuclear leukocyte (PMN) cells (left shift), and the presence of guarding or rebound on physical examination. The combination of any 2 of these tests yields a very high positive likelihood ratio (LR+), but the absence of these does not exclude appendicitis (SOR: A, based on meta-analysis of high-quality studies).
2 inexpensive tests can lower costs in low-probability presentations
Fereshteh Gerayli, MD
East Tennessee State University, Johnson City
Unlike physicians in other parts of the world, us physicians rely heavily on imaging studies to diagnose acute appendicitis. This has decreased the rate of negative appendectomies by 15% to 20%. However, the liberal and indiscriminate use of imaging studies increases medical costs while diminishing physicians’ clinical diagnostic skills.
The systematic review our authors cited demonstrated a high likelihood ratio for the presence of appendicitis by combining 2 inexpensive tests. Adding a thorough history and physical exam and a clinical scoring system can further enhance our clinical diagnosis. Considering the cost and the wide range of diagnostic accuracy of imaging studies (which depend on the experience of the reader), it is reasonable to skip CT scan in low probability presentations.
Evidence summary
Radiographic imaging to rule out appendicitis has become more commonplace, but it comes with an increased financial cost and additional delay in surgical intervention. Knowing the accuracy of common diagnostic tests may reduce the need for confirmatory imaging studies that increase both cost and time to surgery.
High levels of 2 or more inflammatory values are helpful
A meta-analysis of patients hospitalized for suspected acute appendicitis analyzed 28 different diagnostic variables in 24 studies.1 Variables included WBC, granulocyte count, PMN proportion, CRP level, and body temperature; histopathology was the gold standard. In no circumstance did an isolated elevation of any 1 factor result in a significant LR+. In addition, the absence of any 1 variable failed to yield a LR– <0.01 (low enough to exclude appendicitis).
Clinicians inherently combine multiple variables when evaluating patients, and when evaluating patients with abdominal pain, this technique can result in identification of adequate likelihood ratios (TABLE).1 In general, when 2 or more of the aforementioned inflammatory variables are present, the diagnosis of acute appendicitis is likely. When all markers of inflammation are normal, though acute appendicitis is less likely, the power is insufficient to exclude it as a possible diagnosis.
The value of CRP in the evaluation of suspected appendicitis was confirmed in a retrospective evaluation of 566 patients who underwent appendectomies.2 The sensitivity and specificity of the test improved depending on the duration of symptoms for both appendicitis and ruptured appendicitis. For appendicitis, CRP levels >1.4, 4.0, and 10.5 on Days 1, 2, and 3 had sensitivities/specificities of 0.38/0.81, 0.63/0.78, and 0.72/0.83, respectively. For ruptured appendicitis, levels of 3.3, 8.5, and 12.0 on Days 1, 2, and 3 had sensitivities/specificities of 0.77/0.89, 0.70/0.95, and 0.90/0.96, respectively.
Enlarged appendix with inflammatory changes to mesenteric fatIn a series of 439 patients with symptoms suggestive of acute appendicitis, those with confirmed appendicitis (n=101) had a mean WBC count of 14.8 K/μL (95% CI, 13.9–15.8) and a mean neutrophil percentage of 82 (95% CI, 80–84).1 In contrast, those without appendicitis (n=338) had a mean WBC count of 9.2 K/μL (95% CI, 9.0–9.4) and a mean neutrophil percentage of 68 (95% CI, 66–70).
TABLE
How much do the inflammatory markers tell us? A look at likelihood ratios for appendicitis
| COMBINATION OF TESTS | LIKELIHOOD RATIOS | |
|---|---|---|
| POSITIVE (>10=STRONG EVIDENCE FOR DIAGNOSIS) | NEGATIVE (<0.1=EVIDENCE AGAINST DIAGNOSIS) | |
| WBC >10.0 × 109/L CRP >8 mg/L | 23.32 (95% CI, 6.87–84.79) | 0.03 (95% CI, 0.00–0.14) |
| WBC >10.0 × 109/L PMN cells >70% CRP >12 mg/L | 20.85 (95% CI, 5.47–80.27) | 0.03 (95% CI, 0.01–0.16) |
| Guarding/rebound tenderness WBC >10.0×109 | 11.34 (95% CI, 6.65–19.56) | 0.14 (95% CI, 0.08–0.24) |
| WBC, white blood cell count; CRP, C-reactive protein; PMN, polymorphonuclear leukocyte; CI, confidence interval. | ||
| Source: Andersson, Br J Surg 2004.1 | ||
Recommendations from others
A review of medical and professional associations revealed no official guidelines regarding the evaluation of suspected acute appendicitis. Surgical textbooks confirm that the diagnosis of acute appendicitis is made primarily by history and examination, with help from laboratory and radiographic studies.3
1. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg 2004;91:28-37.
2. Birkhahn R, Briggs M, Datillo PA, Van Deusen SK, Gaeta TJ. Classifying patient suspected of appendicitis with regard to likelihood. Am J Surgery 2006;191:497-502.
3. Townsend CM, Sabiston DC. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders, 2004:1381–1395.
No, none of the 3—history, exam, or labs— is sufficiently accurate to diagnose acute appendicitis (strength of recommendation [SOR]: A, based on meta-analysis of high-quality studies). When combined, the following tests are helpful: an elevated C-reactive protein (CRP), elevated total white blood cell (WBC) count, elevated percentage of polymorphonuclear leukocyte (PMN) cells (left shift), and the presence of guarding or rebound on physical examination. The combination of any 2 of these tests yields a very high positive likelihood ratio (LR+), but the absence of these does not exclude appendicitis (SOR: A, based on meta-analysis of high-quality studies).
2 inexpensive tests can lower costs in low-probability presentations
Fereshteh Gerayli, MD
East Tennessee State University, Johnson City
Unlike physicians in other parts of the world, us physicians rely heavily on imaging studies to diagnose acute appendicitis. This has decreased the rate of negative appendectomies by 15% to 20%. However, the liberal and indiscriminate use of imaging studies increases medical costs while diminishing physicians’ clinical diagnostic skills.
The systematic review our authors cited demonstrated a high likelihood ratio for the presence of appendicitis by combining 2 inexpensive tests. Adding a thorough history and physical exam and a clinical scoring system can further enhance our clinical diagnosis. Considering the cost and the wide range of diagnostic accuracy of imaging studies (which depend on the experience of the reader), it is reasonable to skip CT scan in low probability presentations.
Evidence summary
Radiographic imaging to rule out appendicitis has become more commonplace, but it comes with an increased financial cost and additional delay in surgical intervention. Knowing the accuracy of common diagnostic tests may reduce the need for confirmatory imaging studies that increase both cost and time to surgery.
High levels of 2 or more inflammatory values are helpful
A meta-analysis of patients hospitalized for suspected acute appendicitis analyzed 28 different diagnostic variables in 24 studies.1 Variables included WBC, granulocyte count, PMN proportion, CRP level, and body temperature; histopathology was the gold standard. In no circumstance did an isolated elevation of any 1 factor result in a significant LR+. In addition, the absence of any 1 variable failed to yield a LR– <0.01 (low enough to exclude appendicitis).
Clinicians inherently combine multiple variables when evaluating patients, and when evaluating patients with abdominal pain, this technique can result in identification of adequate likelihood ratios (TABLE).1 In general, when 2 or more of the aforementioned inflammatory variables are present, the diagnosis of acute appendicitis is likely. When all markers of inflammation are normal, though acute appendicitis is less likely, the power is insufficient to exclude it as a possible diagnosis.
The value of CRP in the evaluation of suspected appendicitis was confirmed in a retrospective evaluation of 566 patients who underwent appendectomies.2 The sensitivity and specificity of the test improved depending on the duration of symptoms for both appendicitis and ruptured appendicitis. For appendicitis, CRP levels >1.4, 4.0, and 10.5 on Days 1, 2, and 3 had sensitivities/specificities of 0.38/0.81, 0.63/0.78, and 0.72/0.83, respectively. For ruptured appendicitis, levels of 3.3, 8.5, and 12.0 on Days 1, 2, and 3 had sensitivities/specificities of 0.77/0.89, 0.70/0.95, and 0.90/0.96, respectively.
Enlarged appendix with inflammatory changes to mesenteric fatIn a series of 439 patients with symptoms suggestive of acute appendicitis, those with confirmed appendicitis (n=101) had a mean WBC count of 14.8 K/μL (95% CI, 13.9–15.8) and a mean neutrophil percentage of 82 (95% CI, 80–84).1 In contrast, those without appendicitis (n=338) had a mean WBC count of 9.2 K/μL (95% CI, 9.0–9.4) and a mean neutrophil percentage of 68 (95% CI, 66–70).
TABLE
How much do the inflammatory markers tell us? A look at likelihood ratios for appendicitis
| COMBINATION OF TESTS | LIKELIHOOD RATIOS | |
|---|---|---|
| POSITIVE (>10=STRONG EVIDENCE FOR DIAGNOSIS) | NEGATIVE (<0.1=EVIDENCE AGAINST DIAGNOSIS) | |
| WBC >10.0 × 109/L CRP >8 mg/L | 23.32 (95% CI, 6.87–84.79) | 0.03 (95% CI, 0.00–0.14) |
| WBC >10.0 × 109/L PMN cells >70% CRP >12 mg/L | 20.85 (95% CI, 5.47–80.27) | 0.03 (95% CI, 0.01–0.16) |
| Guarding/rebound tenderness WBC >10.0×109 | 11.34 (95% CI, 6.65–19.56) | 0.14 (95% CI, 0.08–0.24) |
| WBC, white blood cell count; CRP, C-reactive protein; PMN, polymorphonuclear leukocyte; CI, confidence interval. | ||
| Source: Andersson, Br J Surg 2004.1 | ||
Recommendations from others
A review of medical and professional associations revealed no official guidelines regarding the evaluation of suspected acute appendicitis. Surgical textbooks confirm that the diagnosis of acute appendicitis is made primarily by history and examination, with help from laboratory and radiographic studies.3
No, none of the 3—history, exam, or labs— is sufficiently accurate to diagnose acute appendicitis (strength of recommendation [SOR]: A, based on meta-analysis of high-quality studies). When combined, the following tests are helpful: an elevated C-reactive protein (CRP), elevated total white blood cell (WBC) count, elevated percentage of polymorphonuclear leukocyte (PMN) cells (left shift), and the presence of guarding or rebound on physical examination. The combination of any 2 of these tests yields a very high positive likelihood ratio (LR+), but the absence of these does not exclude appendicitis (SOR: A, based on meta-analysis of high-quality studies).
2 inexpensive tests can lower costs in low-probability presentations
Fereshteh Gerayli, MD
East Tennessee State University, Johnson City
Unlike physicians in other parts of the world, us physicians rely heavily on imaging studies to diagnose acute appendicitis. This has decreased the rate of negative appendectomies by 15% to 20%. However, the liberal and indiscriminate use of imaging studies increases medical costs while diminishing physicians’ clinical diagnostic skills.
The systematic review our authors cited demonstrated a high likelihood ratio for the presence of appendicitis by combining 2 inexpensive tests. Adding a thorough history and physical exam and a clinical scoring system can further enhance our clinical diagnosis. Considering the cost and the wide range of diagnostic accuracy of imaging studies (which depend on the experience of the reader), it is reasonable to skip CT scan in low probability presentations.
Evidence summary
Radiographic imaging to rule out appendicitis has become more commonplace, but it comes with an increased financial cost and additional delay in surgical intervention. Knowing the accuracy of common diagnostic tests may reduce the need for confirmatory imaging studies that increase both cost and time to surgery.
High levels of 2 or more inflammatory values are helpful
A meta-analysis of patients hospitalized for suspected acute appendicitis analyzed 28 different diagnostic variables in 24 studies.1 Variables included WBC, granulocyte count, PMN proportion, CRP level, and body temperature; histopathology was the gold standard. In no circumstance did an isolated elevation of any 1 factor result in a significant LR+. In addition, the absence of any 1 variable failed to yield a LR– <0.01 (low enough to exclude appendicitis).
Clinicians inherently combine multiple variables when evaluating patients, and when evaluating patients with abdominal pain, this technique can result in identification of adequate likelihood ratios (TABLE).1 In general, when 2 or more of the aforementioned inflammatory variables are present, the diagnosis of acute appendicitis is likely. When all markers of inflammation are normal, though acute appendicitis is less likely, the power is insufficient to exclude it as a possible diagnosis.
The value of CRP in the evaluation of suspected appendicitis was confirmed in a retrospective evaluation of 566 patients who underwent appendectomies.2 The sensitivity and specificity of the test improved depending on the duration of symptoms for both appendicitis and ruptured appendicitis. For appendicitis, CRP levels >1.4, 4.0, and 10.5 on Days 1, 2, and 3 had sensitivities/specificities of 0.38/0.81, 0.63/0.78, and 0.72/0.83, respectively. For ruptured appendicitis, levels of 3.3, 8.5, and 12.0 on Days 1, 2, and 3 had sensitivities/specificities of 0.77/0.89, 0.70/0.95, and 0.90/0.96, respectively.
Enlarged appendix with inflammatory changes to mesenteric fatIn a series of 439 patients with symptoms suggestive of acute appendicitis, those with confirmed appendicitis (n=101) had a mean WBC count of 14.8 K/μL (95% CI, 13.9–15.8) and a mean neutrophil percentage of 82 (95% CI, 80–84).1 In contrast, those without appendicitis (n=338) had a mean WBC count of 9.2 K/μL (95% CI, 9.0–9.4) and a mean neutrophil percentage of 68 (95% CI, 66–70).
TABLE
How much do the inflammatory markers tell us? A look at likelihood ratios for appendicitis
| COMBINATION OF TESTS | LIKELIHOOD RATIOS | |
|---|---|---|
| POSITIVE (>10=STRONG EVIDENCE FOR DIAGNOSIS) | NEGATIVE (<0.1=EVIDENCE AGAINST DIAGNOSIS) | |
| WBC >10.0 × 109/L CRP >8 mg/L | 23.32 (95% CI, 6.87–84.79) | 0.03 (95% CI, 0.00–0.14) |
| WBC >10.0 × 109/L PMN cells >70% CRP >12 mg/L | 20.85 (95% CI, 5.47–80.27) | 0.03 (95% CI, 0.01–0.16) |
| Guarding/rebound tenderness WBC >10.0×109 | 11.34 (95% CI, 6.65–19.56) | 0.14 (95% CI, 0.08–0.24) |
| WBC, white blood cell count; CRP, C-reactive protein; PMN, polymorphonuclear leukocyte; CI, confidence interval. | ||
| Source: Andersson, Br J Surg 2004.1 | ||
Recommendations from others
A review of medical and professional associations revealed no official guidelines regarding the evaluation of suspected acute appendicitis. Surgical textbooks confirm that the diagnosis of acute appendicitis is made primarily by history and examination, with help from laboratory and radiographic studies.3
1. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg 2004;91:28-37.
2. Birkhahn R, Briggs M, Datillo PA, Van Deusen SK, Gaeta TJ. Classifying patient suspected of appendicitis with regard to likelihood. Am J Surgery 2006;191:497-502.
3. Townsend CM, Sabiston DC. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders, 2004:1381–1395.
1. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg 2004;91:28-37.
2. Birkhahn R, Briggs M, Datillo PA, Van Deusen SK, Gaeta TJ. Classifying patient suspected of appendicitis with regard to likelihood. Am J Surgery 2006;191:497-502.
3. Townsend CM, Sabiston DC. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders, 2004:1381–1395.
Evidence-based answers from the Family Physicians Inquiries Network