User login
The Pharmacist’s Role in Medication Optimization for Patients With Chronic Heart Failure (FULL)
In the U.S., about 5.1 million people have clinically manifested heart failure (HF).1 The absolute mortality rate for HF is about 50% within 5 years of diagnosis, and 1 in 9 death certificates in the U.S. list HF as a cause of death.2 Heart failure is the primary diagnosis in more than 1 million hospitalizations annually.1 Patients with HF who are at risk for all-cause rehospitalization have a 1-month readmission rate of 25%, and their median survival time decreases with each hospitalization.3,4 Heart failure is the top reason for discharge of veterans treated within the VA health care system.5
Some medications decrease morbidity and mortality in patients with systolic dysfunction.6 These medications include angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and β-blockers (BBs). Studies have demonstrated effectiveness of these medications in patients with reduced ejection fraction (EF) of less or equal to 40%. Other medications with proven success include aldosterone antagonists, hydralazine in combination with a nitrate, and digoxin. The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines provide medication recommendations based on the ACCF/AHA stages of HF and the New York Heart Association (NYHA) functional classifications, designated as guideline-directed medical therapy (GDMT).6,7 Therapeutic interventions are aimed at reducing morbidity and mortality for ACCF/AHA stage C HF. The ACCF/AHA guidelines also recommend establishing multidisciplinary HF disease management programs for patients at high risk for hospital readmission to facilitate implementation of GDMT, address different barriers to behavior change, and reduce the risk of subsequent rehospitalization for HF.6
In October 2010, the Jesse Brown VAMC (JBVAMC) in Chicago, Illinois, opened its Heart Failure Disease Management Program (HFDMP) to prevent readmissions by targeting patients discharged after HF exacerbations and arranging follow-up in the HFDMP clinic. Enrollment in the clinic is initiated when an inpatient physician places a consultation. A cardiology nurse practitioner (NP) receives the consultation and schedules an in-clinic appointment for the patient within 1 week of discharge. The patient goes to the clinic on average every 2 weeks until he or she is on a stable, optimal medication regimen and is competent in self-management. After 3 months, the patient transitions to the general cardiology clinic. This process allows the HFDMP to see new patients in need of intense care and education for HF. The multidisciplinary HFDMP began with a NP and a cardiologist and 6 months later in April 2011 added a pharmacist.
After enrolling in HFDMP, the patient can be referred to the pharmacist for independent optimization of medication therapy in the Pharmacy Medication Titration Clinic (PMTC). The PMTC at JBVAMC is different from other HF clinics in that the pharmacist has prescribing authority and can interact face-to-face with patients to titrate medications. Once a patient is on an optimal medication regimen, he or she is referred to the NP and cardiologist. The PMTC is open 4 hours twice per month and offers 30-minute time slots. The authors conducted a study of the effectiveness of face-to-face PMTC appointments within the HFDMP.
Methods
This study, approved by the institutional review board at the University of Illinois at Chicago and the research and development service at JBVAMC, was a retrospective electronic chart review of patients enrolled in the HFDMP. Study patients were aged ≥ 18 years and were enrolled in the HFDMP between April 15, 2011 and April 15, 2013. Exclusion criteria included EF higher than 40%, 1 or no appointment attended, and enrollment before April 15, 2011. There were 2 study groups: HFDMP patients enrolled in PMTC (PMTC group) and HFDMP patients not enrolled in PMTC (no-PMTC group). For 1:1 comparison, the number of patients who met the criteria for the PMTC group was used to determine the number of patients to include in the no-PMTC group. Baseline date was the date of enrollment into either HFDMP or PMTC.
Data collected at baseline included demographics, NYHA class of HF, blood pressure (BP), heart rate, ejection fraction (EF), date of HF diagnosis, number of hospitalizations for HF within previous 6 months, serum creatinine level, height, weight, comorbidities, and HF medications. Data collected at the end date included NYHA class of HF; BP; heart rate; EF; HF medications; reason for not achieving target dose of medication or GDMT; ACEI, ARB, or BB adherence, defined as 80% of medication refills 6 months after date of discharge from group; readmission for HF within 30 days and 90 days; length of stay (LOS), including bed type if readmitted; emergency department (ED) visits for HF within 6 months of date of discharge from group; and death within 6 months of date of discharge from group. Clinical GDMT was defined as reaching the maximum tolerable or target dose of each HF medication for each patient depending on clinical presentation, as recommended by the ACCF/AHA guidelines for HF.6 It incorporated NYHA class of HF, contraindications, hypotension, bradycardia, dizziness, and hyperkalemia as well as the prescribing of aldosterone antagonists, hydralazine and isosorbide dinitrate, and digoxin.
Study Outcomes
There were 2 primary endpoints: difference in percentage of patients who achieved target ACEI or ARB doses and difference in percentage of patients who achieved target BB doses. Secondary endpoints were difference between PMTC and no-PMTC groups in percentage of patient achievement in clinical GDMT; percentage medication adherence; percentage of patients with change in NYHA class of HF; percentage of patients with change in EF, including mean change; percentage of patients with 30-day and 90-day readmissions for HF; mean LOS if readmitted within 30 days and 90 days; percentage of patients with ED visits for HF within 6 months after baseline; and percentage mortality within 6 months after baseline.
Statistical Analysis
A 2-tailed Fisher exact test was used for nominal data, and a Student t test for continuous data. Statistical significance was set at P < .05.
Results
Of the 228 HFDMP enrollees, 29 were seen in the PMTC during the study period, and 199 were not seen in the PMTC. Of the 29 patients seen in the PMTC, 24 met the criteria for the PMTC study group. Charts of 106 of the 199 patients not seen in the PMTC were randomly reviewed until 24 patients who met the study criteria were selected for the no-PMTC study group. Thus, the PMTC group and the no-PMTC group each had 24 patients for 1:1 comparison. Eighty-seven patients were excluded from the study: 22 with EF > 40%, 50 with 1 or no appointment attended, and 15 who were enrolled in HFDMP before April 15, 2011.
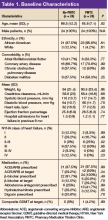
Mean age was 66 years. All patients were male, and most were African American. The baseline characteristics of the PMTC and no-PMTC groups were similar, except a higher percentage of patients in the PMTC group had NYHA class II or III of HF (Table 1).
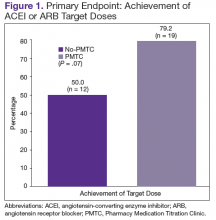
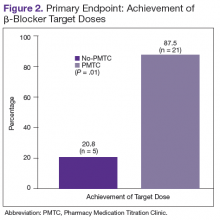
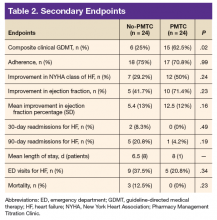
The ACEI or ARB target dose was achieved by a higher percentage of patients in the PMTC group, 79.2% (n = 19) vs 50% (n = 12), but the difference was not significant (P = .07) (Figure 1). However, BB target dose was achieved by a significantly higher (P = .01) percentage of patients in the PMTC group, 87.5% (n = 21) vs 20.8% (n = 5)(Figure 2). Furthermore, a significantly higher (P = .02) percentage of patients in the PMTC group, 62.5% (n = 15) vs 25% (n = 6), achieved composite clinical GDMT (Table 2). Last, there was not a statistically significant difference in 80% adherence with ACEI or ARB dosing or with BB dosing between the PMTC group (70.8%; n = 17) and the no-PMTC group (75%; n = 18).
For a higher percentage of patients in the PMTC group, 50% (n = 12) vs 29.2% (n = 7), NYHA class of HF improved, but the difference was not significant (P = .24). In addition, EF improved in a higher percentage of patients in the PMTC group, 71.4% (n = 10) vs 41.7% (n = 5), and mean (SD) improvement in EF was higher in the PMTC group, 12.5% (12%) vs 5.4% (13%), but neither difference was significant (P = .23 and P = .16, respectively).
A higher percentage of patients in the no-PMTC group were readmitted for HF within 30 days, 8.3% (n = 2) vs 0%, but the difference was not significant. Likewise, a higher percentage of patients in the no-PMTC group were readmitted for HF within 90 days, 20.8% (n = 5) vs 4.2% (n = 1; P = .19). Mean LOS for these readmissions was longer in the PMTC group, 8 days (n = 1) vs 6.5 days (n = 8). There was a higher percentage of ED visits for HF in the no-PMTC group, 37.5% (n = 9) vs 20.8% (n = 5), but did not reach statistical significance (P = .34). Last, the no-PMTC group had a higher percentage of deaths within 6 months after baseline, 37.5% (n = 9) vs 20.8% (n = 9), which also was not significant.
Discussion
This study demonstrated that, within HFDMPs, there is a role for a pharmacist who has prescribing authority and interacts face-to-face with patients in the clinic. Significantly more patients in the PMTC group achieved target BB doses by the end of the study. Target doses of BBs have been found to decrease morbidity and mortality.8-10
The present study also found a positive trend toward achieving target ACEI or ARB doses. Reasons for not achieving target doses included contraindication to the medication, medication discontinuation during hospital admission, hypotension, hyperkalemia, and titration not complete by end of study period. Two of the many reasons for titration not being complete were clinic enrollment timing and nonadherence. Although achievement of target ACEI or ARB doses did not reach statistical significance, statistically significantly more patients achieved composite clinical GDMT.
As defined in the study, clinical GDMT captured the prescribing of hydralazine and isosorbide dinitrate in patients intolerant to ACEIs and ARBs. This study, the first known to evaluate achievement in GDMT, demonstrated a pharmacist’s ability to titrate more than just ACEI, ARB, and BB doses. This finding is clinically important in that appropriate pharmacologic therapy can reduce the number of hospitalizations for HF and improve survival, even though the study found that its PMTC group showed only trends toward fewer 30-day and 90-day readmissions for HF, fewer ED visits for HF, and less mortality.6 This finding may be attributable to the small number of readmissions for HF and deaths among the study groups.
One endpoint that did not show an expected difference with pharmacist intervention was medication adherence. However, medication nonadherence likely was a reason for patient referral to the PMTC. Baseline medication adherence was not determined, so improvement in adherence could not be assessed. Findings might have been different, too, if medication adherence had been evaluated with patient interviews and refill history, not just refill history.
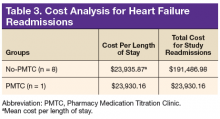
In the PMTC group, LOS for readmissions for HF did not improve. However, the group had only 1 readmission, which may have skewed the result. No studies have linked outpatient pharmacist intervention to decreased LOS for readmission for HF. The endpoint was evaluated to assess whether medication stability leads to reduced LOS and to complete a limited cost analysis. Analysis of mean cost based on number of readmissions, bed type, and LOS revealed a cost savings of $167,556.82 for the PMTC group (Table 3). Other potential cost savings that are difficult to quantify and that were not accounted for include extended time between ED visits or readmissions for HF and increased quality of life and daily functioning.
This is the first study known to evaluate a pharmacist who had prescribing authority and interacted face-to-face with patients. Other studies have evaluated the role of the pharmacist in the multidisciplinary management of patients with HF. In 1999, the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study reported the effect of direct HF-related patient care by a pharmacist who performed medication evaluations, provided patient education, and made medication recommendations to a physician.11
After a medication dosing change, the pharmacist provided telephone follow-up to assess for problems with the drug therapy and then, if any were identified, referred patients to the physician. Pharmacist intervention demonstrated a decrease in all-cause mortality and HF events and an increase in ACEI doses. Unlike the pharmacist in the present study, the pharmacist had to get recommendations approved and prescribed by a physician. The present PMTC allows for pharmacist intervention, including medication therapy changes and follow-up appointments without consultation with a physician. If needed, the HF cardiologist is available in the HFDMP clinic or by telephone.
Jain and colleagues evaluated a protocol-driven medication titration clinic staffed by nurse and pharmacist specialists.12 Although their study was limited by its descriptive nature, the authors concluded that the clinic increased the number of patients who achieved target ACEI/ARB or target BB doses. In the present study, the percentage of PMTC patients who achieved target doses increased during the study period, from 50% to 79.2% (ACEI/ARB) and from 20.8% to 87.5% (BB). Unlike other clinic pharmacists, however, the PMTC pharmacist titrates medications independently and does not follow a set clinic protocol.
Similar to the PHARM study, the Heart Failure Optimal Outcomes From Pharmacy Study (HOOPS) evaluated pharmacists who worked collaboratively with physicians to optimize HF therapy.13 As in the PMTC, patients and pharmacists had 30-minute appointments together. In HOOPS, however, physician agreement was needed before pharmacist recommendations were implemented. That study found that more patients with pharmacist intervention started an ACEI or ARB, had the medication titrated, and received recommended doses. More patients also either started a BB or increased its dose, but this did not increase the number of patients who received recommended BB doses. In addition, pharmacist intervention did not affect clinical outcomes. The authors acknowledged this finding might be attributable to the pharmacists’ lack of proper HF management training. Patients in the study were also more stable, whereas PMTC patients arrived after HF discharge and were followed until medication therapy was optimized. The HOOPS followed patients for only 3 or 4 visits, regardless of target dose achievement status.
In a study conducted within the VA health care system, Martinez and colleagues evaluated the use of pharmacists who had prescribing authority and were permitted to order laboratory tests under a scope of practice similar to that in the present study.14 However, their coordination agreement allowed them only to initiate and adjust doses of certain HF medications according to defined protocols. The pharmacist conducted monthly education classes and had medication titration appointments with individual patients by telephone over 2-week intervals. Face-to-face appointments were limited to medication reconciliations, whereas all appointments in the present study were face-to-face. In addition, Martinez and colleagues found that a higher percentage of patients who attended pharmacist appointments achieved target ACEI, ARB, and BB doses, whereas the present study found a higher percentage only of achieved target BB doses, not ACEI or ARB. However, the present study also found increased composite clinical GDMT achievement, which Martinez and colleagues did not evaluate. As mentioned, GDMT achievement may be a broader evaluation of optimal HF medical therapy, as it incorporates ACEI or ARB intolerance.
Martinez and colleagues acknowledged several study limitations different from those of the present study.14 Most members of their study population were white men, unlike this study’s population. Combining these 2 studies’ results may support use of pharmacist intervention for both white and African American men. In addition, the authors noted that patients often forgot their medications or were confused about doses, and concluded that forgetfulness and confusion may stem from having only telephone interviews and lacking written instructions for the interval between clinic appointments. By contrast, all PMTC patients were seen face-to-face, and handouts detailing any changes helped minimized confusion. Even with face-to-face appointments, however, patient nonadherence persisted, making it difficult to optimize HF therapy. Patients did not always follow instructions to bring HF medications (or medication lists) and daily weight measurements to clinic visits, which complicated medication reconciliations, interventions, and educational efforts regarding dose changes. Furthermore, patients sometimes missed face-to-face appointments, often because of transportation difficulties. In these situations, telephone appointments may be beneficial. The obstacle of transportation is removed, and, during the at-home telephone call, the patient has easy access to medications and measurements.
Limitations
This study had several limitations. It was retrospective, and its sample size was too small for conclusions regarding morbidity and mortality. As the population was predominantly African American males, results may not be applicable to other races and females. Furthermore, not evaluating HF causes at baseline could have confounded results, as disease progression, response to medications, and prognosis can vary, depending on etiology. Moreover, as patients are referred from the HFDMP to the PMTC, there may have been a bias in patient selection for the PMTC group. Patients in the PMTC group may have been more clinically stable yet had a larger knowledge deficit, an adherence issue, or a need for difficult, frequent titrations. Patients also may have been less likely to be seen during the first 30 days after discharge. In addition, it could have been beneficial to match patients on NYHA class of HF at baseline to ensure HF severity was balanced between groups. Last, the adherence analysis may not be accurate, as it relied on refill history, which may not reflect how medications were taken at home.
It would be beneficial to expand this initial study with a larger sample. Presumed HF causes and medication adherence should be captured at baseline. Additional endpoints, including quality of life and patient cognition, could enhance results. Furthermore, comparing the HFDMP with the general cardiology clinic may reveal other benefits of a focused HFDMP and its PMTC. Last, evaluating patients who are recently discharged from HF admission yet not enrolled in the HFDMP may provide more information regarding the utility of both the HFDMP and the PMTC.
Conclusion
For the PMTC group in this study, achievement of target BB doses and achievement in composite clinical GDMT were significant, but achievement of target ACEI/ARB doses were not.
Click here to read the digital edition.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6-e245.
2. Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344-350.
3. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407-413.
4. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260-266.
5. U.S. Department of Veterans Affairs, VA Office of Research and Development, Executive Committee, Chronic Heart Failure Quality Enhancement Research Initiative (CHF-QUERI), Health Services Research and Development Service. Chronic heart failure [QUERI fact sheet]. https://www.queri.research.va.gov/about/factsheets/chf_factsheet.pdf. Published July 2014. Accessed October 9, 2017.
6. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
7. New York Heart Association Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Little Brown; 1994.
8. Parker M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349-1355.
9. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9-13.
10. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-2007.
11. Gattis WA, Hasselblad V, Whellan DJ, O’Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study. Arch Intern Med. 1999;159(16):1939-1945.
12. Jain A, Mills P, Nunn LM, et al. Success of a multidisciplinary heart failure clinic for initiation and up-titration of key therapeutic agents. Eur J Heart Fail. 2005;7(3):405-410.
13. Lowrie R, Mair FS, Greenlaw N, et al; Heart Failure Optimal Outcomes From Pharmacy Study (HOOPS) Investigators. Pharmacist intervention in primary care to improve outcomes in patients with left ventricular systolic dysfunction. Eur Heart J. 2012;33(3):314-324.
14. Martinez AS, Saef J, Paszczuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
In the U.S., about 5.1 million people have clinically manifested heart failure (HF).1 The absolute mortality rate for HF is about 50% within 5 years of diagnosis, and 1 in 9 death certificates in the U.S. list HF as a cause of death.2 Heart failure is the primary diagnosis in more than 1 million hospitalizations annually.1 Patients with HF who are at risk for all-cause rehospitalization have a 1-month readmission rate of 25%, and their median survival time decreases with each hospitalization.3,4 Heart failure is the top reason for discharge of veterans treated within the VA health care system.5
Some medications decrease morbidity and mortality in patients with systolic dysfunction.6 These medications include angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and β-blockers (BBs). Studies have demonstrated effectiveness of these medications in patients with reduced ejection fraction (EF) of less or equal to 40%. Other medications with proven success include aldosterone antagonists, hydralazine in combination with a nitrate, and digoxin. The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines provide medication recommendations based on the ACCF/AHA stages of HF and the New York Heart Association (NYHA) functional classifications, designated as guideline-directed medical therapy (GDMT).6,7 Therapeutic interventions are aimed at reducing morbidity and mortality for ACCF/AHA stage C HF. The ACCF/AHA guidelines also recommend establishing multidisciplinary HF disease management programs for patients at high risk for hospital readmission to facilitate implementation of GDMT, address different barriers to behavior change, and reduce the risk of subsequent rehospitalization for HF.6
In October 2010, the Jesse Brown VAMC (JBVAMC) in Chicago, Illinois, opened its Heart Failure Disease Management Program (HFDMP) to prevent readmissions by targeting patients discharged after HF exacerbations and arranging follow-up in the HFDMP clinic. Enrollment in the clinic is initiated when an inpatient physician places a consultation. A cardiology nurse practitioner (NP) receives the consultation and schedules an in-clinic appointment for the patient within 1 week of discharge. The patient goes to the clinic on average every 2 weeks until he or she is on a stable, optimal medication regimen and is competent in self-management. After 3 months, the patient transitions to the general cardiology clinic. This process allows the HFDMP to see new patients in need of intense care and education for HF. The multidisciplinary HFDMP began with a NP and a cardiologist and 6 months later in April 2011 added a pharmacist.
After enrolling in HFDMP, the patient can be referred to the pharmacist for independent optimization of medication therapy in the Pharmacy Medication Titration Clinic (PMTC). The PMTC at JBVAMC is different from other HF clinics in that the pharmacist has prescribing authority and can interact face-to-face with patients to titrate medications. Once a patient is on an optimal medication regimen, he or she is referred to the NP and cardiologist. The PMTC is open 4 hours twice per month and offers 30-minute time slots. The authors conducted a study of the effectiveness of face-to-face PMTC appointments within the HFDMP.
Methods
This study, approved by the institutional review board at the University of Illinois at Chicago and the research and development service at JBVAMC, was a retrospective electronic chart review of patients enrolled in the HFDMP. Study patients were aged ≥ 18 years and were enrolled in the HFDMP between April 15, 2011 and April 15, 2013. Exclusion criteria included EF higher than 40%, 1 or no appointment attended, and enrollment before April 15, 2011. There were 2 study groups: HFDMP patients enrolled in PMTC (PMTC group) and HFDMP patients not enrolled in PMTC (no-PMTC group). For 1:1 comparison, the number of patients who met the criteria for the PMTC group was used to determine the number of patients to include in the no-PMTC group. Baseline date was the date of enrollment into either HFDMP or PMTC.
Data collected at baseline included demographics, NYHA class of HF, blood pressure (BP), heart rate, ejection fraction (EF), date of HF diagnosis, number of hospitalizations for HF within previous 6 months, serum creatinine level, height, weight, comorbidities, and HF medications. Data collected at the end date included NYHA class of HF; BP; heart rate; EF; HF medications; reason for not achieving target dose of medication or GDMT; ACEI, ARB, or BB adherence, defined as 80% of medication refills 6 months after date of discharge from group; readmission for HF within 30 days and 90 days; length of stay (LOS), including bed type if readmitted; emergency department (ED) visits for HF within 6 months of date of discharge from group; and death within 6 months of date of discharge from group. Clinical GDMT was defined as reaching the maximum tolerable or target dose of each HF medication for each patient depending on clinical presentation, as recommended by the ACCF/AHA guidelines for HF.6 It incorporated NYHA class of HF, contraindications, hypotension, bradycardia, dizziness, and hyperkalemia as well as the prescribing of aldosterone antagonists, hydralazine and isosorbide dinitrate, and digoxin.
Study Outcomes
There were 2 primary endpoints: difference in percentage of patients who achieved target ACEI or ARB doses and difference in percentage of patients who achieved target BB doses. Secondary endpoints were difference between PMTC and no-PMTC groups in percentage of patient achievement in clinical GDMT; percentage medication adherence; percentage of patients with change in NYHA class of HF; percentage of patients with change in EF, including mean change; percentage of patients with 30-day and 90-day readmissions for HF; mean LOS if readmitted within 30 days and 90 days; percentage of patients with ED visits for HF within 6 months after baseline; and percentage mortality within 6 months after baseline.
Statistical Analysis
A 2-tailed Fisher exact test was used for nominal data, and a Student t test for continuous data. Statistical significance was set at P < .05.
Results
Of the 228 HFDMP enrollees, 29 were seen in the PMTC during the study period, and 199 were not seen in the PMTC. Of the 29 patients seen in the PMTC, 24 met the criteria for the PMTC study group. Charts of 106 of the 199 patients not seen in the PMTC were randomly reviewed until 24 patients who met the study criteria were selected for the no-PMTC study group. Thus, the PMTC group and the no-PMTC group each had 24 patients for 1:1 comparison. Eighty-seven patients were excluded from the study: 22 with EF > 40%, 50 with 1 or no appointment attended, and 15 who were enrolled in HFDMP before April 15, 2011.
Mean age was 66 years. All patients were male, and most were African American. The baseline characteristics of the PMTC and no-PMTC groups were similar, except a higher percentage of patients in the PMTC group had NYHA class II or III of HF (Table 1).
The ACEI or ARB target dose was achieved by a higher percentage of patients in the PMTC group, 79.2% (n = 19) vs 50% (n = 12), but the difference was not significant (P = .07) (Figure 1). However, BB target dose was achieved by a significantly higher (P = .01) percentage of patients in the PMTC group, 87.5% (n = 21) vs 20.8% (n = 5)(Figure 2). Furthermore, a significantly higher (P = .02) percentage of patients in the PMTC group, 62.5% (n = 15) vs 25% (n = 6), achieved composite clinical GDMT (Table 2). Last, there was not a statistically significant difference in 80% adherence with ACEI or ARB dosing or with BB dosing between the PMTC group (70.8%; n = 17) and the no-PMTC group (75%; n = 18).
For a higher percentage of patients in the PMTC group, 50% (n = 12) vs 29.2% (n = 7), NYHA class of HF improved, but the difference was not significant (P = .24). In addition, EF improved in a higher percentage of patients in the PMTC group, 71.4% (n = 10) vs 41.7% (n = 5), and mean (SD) improvement in EF was higher in the PMTC group, 12.5% (12%) vs 5.4% (13%), but neither difference was significant (P = .23 and P = .16, respectively).
A higher percentage of patients in the no-PMTC group were readmitted for HF within 30 days, 8.3% (n = 2) vs 0%, but the difference was not significant. Likewise, a higher percentage of patients in the no-PMTC group were readmitted for HF within 90 days, 20.8% (n = 5) vs 4.2% (n = 1; P = .19). Mean LOS for these readmissions was longer in the PMTC group, 8 days (n = 1) vs 6.5 days (n = 8). There was a higher percentage of ED visits for HF in the no-PMTC group, 37.5% (n = 9) vs 20.8% (n = 5), but did not reach statistical significance (P = .34). Last, the no-PMTC group had a higher percentage of deaths within 6 months after baseline, 37.5% (n = 9) vs 20.8% (n = 9), which also was not significant.
Discussion
This study demonstrated that, within HFDMPs, there is a role for a pharmacist who has prescribing authority and interacts face-to-face with patients in the clinic. Significantly more patients in the PMTC group achieved target BB doses by the end of the study. Target doses of BBs have been found to decrease morbidity and mortality.8-10
The present study also found a positive trend toward achieving target ACEI or ARB doses. Reasons for not achieving target doses included contraindication to the medication, medication discontinuation during hospital admission, hypotension, hyperkalemia, and titration not complete by end of study period. Two of the many reasons for titration not being complete were clinic enrollment timing and nonadherence. Although achievement of target ACEI or ARB doses did not reach statistical significance, statistically significantly more patients achieved composite clinical GDMT.
As defined in the study, clinical GDMT captured the prescribing of hydralazine and isosorbide dinitrate in patients intolerant to ACEIs and ARBs. This study, the first known to evaluate achievement in GDMT, demonstrated a pharmacist’s ability to titrate more than just ACEI, ARB, and BB doses. This finding is clinically important in that appropriate pharmacologic therapy can reduce the number of hospitalizations for HF and improve survival, even though the study found that its PMTC group showed only trends toward fewer 30-day and 90-day readmissions for HF, fewer ED visits for HF, and less mortality.6 This finding may be attributable to the small number of readmissions for HF and deaths among the study groups.
One endpoint that did not show an expected difference with pharmacist intervention was medication adherence. However, medication nonadherence likely was a reason for patient referral to the PMTC. Baseline medication adherence was not determined, so improvement in adherence could not be assessed. Findings might have been different, too, if medication adherence had been evaluated with patient interviews and refill history, not just refill history.
In the PMTC group, LOS for readmissions for HF did not improve. However, the group had only 1 readmission, which may have skewed the result. No studies have linked outpatient pharmacist intervention to decreased LOS for readmission for HF. The endpoint was evaluated to assess whether medication stability leads to reduced LOS and to complete a limited cost analysis. Analysis of mean cost based on number of readmissions, bed type, and LOS revealed a cost savings of $167,556.82 for the PMTC group (Table 3). Other potential cost savings that are difficult to quantify and that were not accounted for include extended time between ED visits or readmissions for HF and increased quality of life and daily functioning.
This is the first study known to evaluate a pharmacist who had prescribing authority and interacted face-to-face with patients. Other studies have evaluated the role of the pharmacist in the multidisciplinary management of patients with HF. In 1999, the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study reported the effect of direct HF-related patient care by a pharmacist who performed medication evaluations, provided patient education, and made medication recommendations to a physician.11
After a medication dosing change, the pharmacist provided telephone follow-up to assess for problems with the drug therapy and then, if any were identified, referred patients to the physician. Pharmacist intervention demonstrated a decrease in all-cause mortality and HF events and an increase in ACEI doses. Unlike the pharmacist in the present study, the pharmacist had to get recommendations approved and prescribed by a physician. The present PMTC allows for pharmacist intervention, including medication therapy changes and follow-up appointments without consultation with a physician. If needed, the HF cardiologist is available in the HFDMP clinic or by telephone.
Jain and colleagues evaluated a protocol-driven medication titration clinic staffed by nurse and pharmacist specialists.12 Although their study was limited by its descriptive nature, the authors concluded that the clinic increased the number of patients who achieved target ACEI/ARB or target BB doses. In the present study, the percentage of PMTC patients who achieved target doses increased during the study period, from 50% to 79.2% (ACEI/ARB) and from 20.8% to 87.5% (BB). Unlike other clinic pharmacists, however, the PMTC pharmacist titrates medications independently and does not follow a set clinic protocol.
Similar to the PHARM study, the Heart Failure Optimal Outcomes From Pharmacy Study (HOOPS) evaluated pharmacists who worked collaboratively with physicians to optimize HF therapy.13 As in the PMTC, patients and pharmacists had 30-minute appointments together. In HOOPS, however, physician agreement was needed before pharmacist recommendations were implemented. That study found that more patients with pharmacist intervention started an ACEI or ARB, had the medication titrated, and received recommended doses. More patients also either started a BB or increased its dose, but this did not increase the number of patients who received recommended BB doses. In addition, pharmacist intervention did not affect clinical outcomes. The authors acknowledged this finding might be attributable to the pharmacists’ lack of proper HF management training. Patients in the study were also more stable, whereas PMTC patients arrived after HF discharge and were followed until medication therapy was optimized. The HOOPS followed patients for only 3 or 4 visits, regardless of target dose achievement status.
In a study conducted within the VA health care system, Martinez and colleagues evaluated the use of pharmacists who had prescribing authority and were permitted to order laboratory tests under a scope of practice similar to that in the present study.14 However, their coordination agreement allowed them only to initiate and adjust doses of certain HF medications according to defined protocols. The pharmacist conducted monthly education classes and had medication titration appointments with individual patients by telephone over 2-week intervals. Face-to-face appointments were limited to medication reconciliations, whereas all appointments in the present study were face-to-face. In addition, Martinez and colleagues found that a higher percentage of patients who attended pharmacist appointments achieved target ACEI, ARB, and BB doses, whereas the present study found a higher percentage only of achieved target BB doses, not ACEI or ARB. However, the present study also found increased composite clinical GDMT achievement, which Martinez and colleagues did not evaluate. As mentioned, GDMT achievement may be a broader evaluation of optimal HF medical therapy, as it incorporates ACEI or ARB intolerance.
Martinez and colleagues acknowledged several study limitations different from those of the present study.14 Most members of their study population were white men, unlike this study’s population. Combining these 2 studies’ results may support use of pharmacist intervention for both white and African American men. In addition, the authors noted that patients often forgot their medications or were confused about doses, and concluded that forgetfulness and confusion may stem from having only telephone interviews and lacking written instructions for the interval between clinic appointments. By contrast, all PMTC patients were seen face-to-face, and handouts detailing any changes helped minimized confusion. Even with face-to-face appointments, however, patient nonadherence persisted, making it difficult to optimize HF therapy. Patients did not always follow instructions to bring HF medications (or medication lists) and daily weight measurements to clinic visits, which complicated medication reconciliations, interventions, and educational efforts regarding dose changes. Furthermore, patients sometimes missed face-to-face appointments, often because of transportation difficulties. In these situations, telephone appointments may be beneficial. The obstacle of transportation is removed, and, during the at-home telephone call, the patient has easy access to medications and measurements.
Limitations
This study had several limitations. It was retrospective, and its sample size was too small for conclusions regarding morbidity and mortality. As the population was predominantly African American males, results may not be applicable to other races and females. Furthermore, not evaluating HF causes at baseline could have confounded results, as disease progression, response to medications, and prognosis can vary, depending on etiology. Moreover, as patients are referred from the HFDMP to the PMTC, there may have been a bias in patient selection for the PMTC group. Patients in the PMTC group may have been more clinically stable yet had a larger knowledge deficit, an adherence issue, or a need for difficult, frequent titrations. Patients also may have been less likely to be seen during the first 30 days after discharge. In addition, it could have been beneficial to match patients on NYHA class of HF at baseline to ensure HF severity was balanced between groups. Last, the adherence analysis may not be accurate, as it relied on refill history, which may not reflect how medications were taken at home.
It would be beneficial to expand this initial study with a larger sample. Presumed HF causes and medication adherence should be captured at baseline. Additional endpoints, including quality of life and patient cognition, could enhance results. Furthermore, comparing the HFDMP with the general cardiology clinic may reveal other benefits of a focused HFDMP and its PMTC. Last, evaluating patients who are recently discharged from HF admission yet not enrolled in the HFDMP may provide more information regarding the utility of both the HFDMP and the PMTC.
Conclusion
For the PMTC group in this study, achievement of target BB doses and achievement in composite clinical GDMT were significant, but achievement of target ACEI/ARB doses were not.
Click here to read the digital edition.
In the U.S., about 5.1 million people have clinically manifested heart failure (HF).1 The absolute mortality rate for HF is about 50% within 5 years of diagnosis, and 1 in 9 death certificates in the U.S. list HF as a cause of death.2 Heart failure is the primary diagnosis in more than 1 million hospitalizations annually.1 Patients with HF who are at risk for all-cause rehospitalization have a 1-month readmission rate of 25%, and their median survival time decreases with each hospitalization.3,4 Heart failure is the top reason for discharge of veterans treated within the VA health care system.5
Some medications decrease morbidity and mortality in patients with systolic dysfunction.6 These medications include angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and β-blockers (BBs). Studies have demonstrated effectiveness of these medications in patients with reduced ejection fraction (EF) of less or equal to 40%. Other medications with proven success include aldosterone antagonists, hydralazine in combination with a nitrate, and digoxin. The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines provide medication recommendations based on the ACCF/AHA stages of HF and the New York Heart Association (NYHA) functional classifications, designated as guideline-directed medical therapy (GDMT).6,7 Therapeutic interventions are aimed at reducing morbidity and mortality for ACCF/AHA stage C HF. The ACCF/AHA guidelines also recommend establishing multidisciplinary HF disease management programs for patients at high risk for hospital readmission to facilitate implementation of GDMT, address different barriers to behavior change, and reduce the risk of subsequent rehospitalization for HF.6
In October 2010, the Jesse Brown VAMC (JBVAMC) in Chicago, Illinois, opened its Heart Failure Disease Management Program (HFDMP) to prevent readmissions by targeting patients discharged after HF exacerbations and arranging follow-up in the HFDMP clinic. Enrollment in the clinic is initiated when an inpatient physician places a consultation. A cardiology nurse practitioner (NP) receives the consultation and schedules an in-clinic appointment for the patient within 1 week of discharge. The patient goes to the clinic on average every 2 weeks until he or she is on a stable, optimal medication regimen and is competent in self-management. After 3 months, the patient transitions to the general cardiology clinic. This process allows the HFDMP to see new patients in need of intense care and education for HF. The multidisciplinary HFDMP began with a NP and a cardiologist and 6 months later in April 2011 added a pharmacist.
After enrolling in HFDMP, the patient can be referred to the pharmacist for independent optimization of medication therapy in the Pharmacy Medication Titration Clinic (PMTC). The PMTC at JBVAMC is different from other HF clinics in that the pharmacist has prescribing authority and can interact face-to-face with patients to titrate medications. Once a patient is on an optimal medication regimen, he or she is referred to the NP and cardiologist. The PMTC is open 4 hours twice per month and offers 30-minute time slots. The authors conducted a study of the effectiveness of face-to-face PMTC appointments within the HFDMP.
Methods
This study, approved by the institutional review board at the University of Illinois at Chicago and the research and development service at JBVAMC, was a retrospective electronic chart review of patients enrolled in the HFDMP. Study patients were aged ≥ 18 years and were enrolled in the HFDMP between April 15, 2011 and April 15, 2013. Exclusion criteria included EF higher than 40%, 1 or no appointment attended, and enrollment before April 15, 2011. There were 2 study groups: HFDMP patients enrolled in PMTC (PMTC group) and HFDMP patients not enrolled in PMTC (no-PMTC group). For 1:1 comparison, the number of patients who met the criteria for the PMTC group was used to determine the number of patients to include in the no-PMTC group. Baseline date was the date of enrollment into either HFDMP or PMTC.
Data collected at baseline included demographics, NYHA class of HF, blood pressure (BP), heart rate, ejection fraction (EF), date of HF diagnosis, number of hospitalizations for HF within previous 6 months, serum creatinine level, height, weight, comorbidities, and HF medications. Data collected at the end date included NYHA class of HF; BP; heart rate; EF; HF medications; reason for not achieving target dose of medication or GDMT; ACEI, ARB, or BB adherence, defined as 80% of medication refills 6 months after date of discharge from group; readmission for HF within 30 days and 90 days; length of stay (LOS), including bed type if readmitted; emergency department (ED) visits for HF within 6 months of date of discharge from group; and death within 6 months of date of discharge from group. Clinical GDMT was defined as reaching the maximum tolerable or target dose of each HF medication for each patient depending on clinical presentation, as recommended by the ACCF/AHA guidelines for HF.6 It incorporated NYHA class of HF, contraindications, hypotension, bradycardia, dizziness, and hyperkalemia as well as the prescribing of aldosterone antagonists, hydralazine and isosorbide dinitrate, and digoxin.
Study Outcomes
There were 2 primary endpoints: difference in percentage of patients who achieved target ACEI or ARB doses and difference in percentage of patients who achieved target BB doses. Secondary endpoints were difference between PMTC and no-PMTC groups in percentage of patient achievement in clinical GDMT; percentage medication adherence; percentage of patients with change in NYHA class of HF; percentage of patients with change in EF, including mean change; percentage of patients with 30-day and 90-day readmissions for HF; mean LOS if readmitted within 30 days and 90 days; percentage of patients with ED visits for HF within 6 months after baseline; and percentage mortality within 6 months after baseline.
Statistical Analysis
A 2-tailed Fisher exact test was used for nominal data, and a Student t test for continuous data. Statistical significance was set at P < .05.
Results
Of the 228 HFDMP enrollees, 29 were seen in the PMTC during the study period, and 199 were not seen in the PMTC. Of the 29 patients seen in the PMTC, 24 met the criteria for the PMTC study group. Charts of 106 of the 199 patients not seen in the PMTC were randomly reviewed until 24 patients who met the study criteria were selected for the no-PMTC study group. Thus, the PMTC group and the no-PMTC group each had 24 patients for 1:1 comparison. Eighty-seven patients were excluded from the study: 22 with EF > 40%, 50 with 1 or no appointment attended, and 15 who were enrolled in HFDMP before April 15, 2011.
Mean age was 66 years. All patients were male, and most were African American. The baseline characteristics of the PMTC and no-PMTC groups were similar, except a higher percentage of patients in the PMTC group had NYHA class II or III of HF (Table 1).
The ACEI or ARB target dose was achieved by a higher percentage of patients in the PMTC group, 79.2% (n = 19) vs 50% (n = 12), but the difference was not significant (P = .07) (Figure 1). However, BB target dose was achieved by a significantly higher (P = .01) percentage of patients in the PMTC group, 87.5% (n = 21) vs 20.8% (n = 5)(Figure 2). Furthermore, a significantly higher (P = .02) percentage of patients in the PMTC group, 62.5% (n = 15) vs 25% (n = 6), achieved composite clinical GDMT (Table 2). Last, there was not a statistically significant difference in 80% adherence with ACEI or ARB dosing or with BB dosing between the PMTC group (70.8%; n = 17) and the no-PMTC group (75%; n = 18).
For a higher percentage of patients in the PMTC group, 50% (n = 12) vs 29.2% (n = 7), NYHA class of HF improved, but the difference was not significant (P = .24). In addition, EF improved in a higher percentage of patients in the PMTC group, 71.4% (n = 10) vs 41.7% (n = 5), and mean (SD) improvement in EF was higher in the PMTC group, 12.5% (12%) vs 5.4% (13%), but neither difference was significant (P = .23 and P = .16, respectively).
A higher percentage of patients in the no-PMTC group were readmitted for HF within 30 days, 8.3% (n = 2) vs 0%, but the difference was not significant. Likewise, a higher percentage of patients in the no-PMTC group were readmitted for HF within 90 days, 20.8% (n = 5) vs 4.2% (n = 1; P = .19). Mean LOS for these readmissions was longer in the PMTC group, 8 days (n = 1) vs 6.5 days (n = 8). There was a higher percentage of ED visits for HF in the no-PMTC group, 37.5% (n = 9) vs 20.8% (n = 5), but did not reach statistical significance (P = .34). Last, the no-PMTC group had a higher percentage of deaths within 6 months after baseline, 37.5% (n = 9) vs 20.8% (n = 9), which also was not significant.
Discussion
This study demonstrated that, within HFDMPs, there is a role for a pharmacist who has prescribing authority and interacts face-to-face with patients in the clinic. Significantly more patients in the PMTC group achieved target BB doses by the end of the study. Target doses of BBs have been found to decrease morbidity and mortality.8-10
The present study also found a positive trend toward achieving target ACEI or ARB doses. Reasons for not achieving target doses included contraindication to the medication, medication discontinuation during hospital admission, hypotension, hyperkalemia, and titration not complete by end of study period. Two of the many reasons for titration not being complete were clinic enrollment timing and nonadherence. Although achievement of target ACEI or ARB doses did not reach statistical significance, statistically significantly more patients achieved composite clinical GDMT.
As defined in the study, clinical GDMT captured the prescribing of hydralazine and isosorbide dinitrate in patients intolerant to ACEIs and ARBs. This study, the first known to evaluate achievement in GDMT, demonstrated a pharmacist’s ability to titrate more than just ACEI, ARB, and BB doses. This finding is clinically important in that appropriate pharmacologic therapy can reduce the number of hospitalizations for HF and improve survival, even though the study found that its PMTC group showed only trends toward fewer 30-day and 90-day readmissions for HF, fewer ED visits for HF, and less mortality.6 This finding may be attributable to the small number of readmissions for HF and deaths among the study groups.
One endpoint that did not show an expected difference with pharmacist intervention was medication adherence. However, medication nonadherence likely was a reason for patient referral to the PMTC. Baseline medication adherence was not determined, so improvement in adherence could not be assessed. Findings might have been different, too, if medication adherence had been evaluated with patient interviews and refill history, not just refill history.
In the PMTC group, LOS for readmissions for HF did not improve. However, the group had only 1 readmission, which may have skewed the result. No studies have linked outpatient pharmacist intervention to decreased LOS for readmission for HF. The endpoint was evaluated to assess whether medication stability leads to reduced LOS and to complete a limited cost analysis. Analysis of mean cost based on number of readmissions, bed type, and LOS revealed a cost savings of $167,556.82 for the PMTC group (Table 3). Other potential cost savings that are difficult to quantify and that were not accounted for include extended time between ED visits or readmissions for HF and increased quality of life and daily functioning.
This is the first study known to evaluate a pharmacist who had prescribing authority and interacted face-to-face with patients. Other studies have evaluated the role of the pharmacist in the multidisciplinary management of patients with HF. In 1999, the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study reported the effect of direct HF-related patient care by a pharmacist who performed medication evaluations, provided patient education, and made medication recommendations to a physician.11
After a medication dosing change, the pharmacist provided telephone follow-up to assess for problems with the drug therapy and then, if any were identified, referred patients to the physician. Pharmacist intervention demonstrated a decrease in all-cause mortality and HF events and an increase in ACEI doses. Unlike the pharmacist in the present study, the pharmacist had to get recommendations approved and prescribed by a physician. The present PMTC allows for pharmacist intervention, including medication therapy changes and follow-up appointments without consultation with a physician. If needed, the HF cardiologist is available in the HFDMP clinic or by telephone.
Jain and colleagues evaluated a protocol-driven medication titration clinic staffed by nurse and pharmacist specialists.12 Although their study was limited by its descriptive nature, the authors concluded that the clinic increased the number of patients who achieved target ACEI/ARB or target BB doses. In the present study, the percentage of PMTC patients who achieved target doses increased during the study period, from 50% to 79.2% (ACEI/ARB) and from 20.8% to 87.5% (BB). Unlike other clinic pharmacists, however, the PMTC pharmacist titrates medications independently and does not follow a set clinic protocol.
Similar to the PHARM study, the Heart Failure Optimal Outcomes From Pharmacy Study (HOOPS) evaluated pharmacists who worked collaboratively with physicians to optimize HF therapy.13 As in the PMTC, patients and pharmacists had 30-minute appointments together. In HOOPS, however, physician agreement was needed before pharmacist recommendations were implemented. That study found that more patients with pharmacist intervention started an ACEI or ARB, had the medication titrated, and received recommended doses. More patients also either started a BB or increased its dose, but this did not increase the number of patients who received recommended BB doses. In addition, pharmacist intervention did not affect clinical outcomes. The authors acknowledged this finding might be attributable to the pharmacists’ lack of proper HF management training. Patients in the study were also more stable, whereas PMTC patients arrived after HF discharge and were followed until medication therapy was optimized. The HOOPS followed patients for only 3 or 4 visits, regardless of target dose achievement status.
In a study conducted within the VA health care system, Martinez and colleagues evaluated the use of pharmacists who had prescribing authority and were permitted to order laboratory tests under a scope of practice similar to that in the present study.14 However, their coordination agreement allowed them only to initiate and adjust doses of certain HF medications according to defined protocols. The pharmacist conducted monthly education classes and had medication titration appointments with individual patients by telephone over 2-week intervals. Face-to-face appointments were limited to medication reconciliations, whereas all appointments in the present study were face-to-face. In addition, Martinez and colleagues found that a higher percentage of patients who attended pharmacist appointments achieved target ACEI, ARB, and BB doses, whereas the present study found a higher percentage only of achieved target BB doses, not ACEI or ARB. However, the present study also found increased composite clinical GDMT achievement, which Martinez and colleagues did not evaluate. As mentioned, GDMT achievement may be a broader evaluation of optimal HF medical therapy, as it incorporates ACEI or ARB intolerance.
Martinez and colleagues acknowledged several study limitations different from those of the present study.14 Most members of their study population were white men, unlike this study’s population. Combining these 2 studies’ results may support use of pharmacist intervention for both white and African American men. In addition, the authors noted that patients often forgot their medications or were confused about doses, and concluded that forgetfulness and confusion may stem from having only telephone interviews and lacking written instructions for the interval between clinic appointments. By contrast, all PMTC patients were seen face-to-face, and handouts detailing any changes helped minimized confusion. Even with face-to-face appointments, however, patient nonadherence persisted, making it difficult to optimize HF therapy. Patients did not always follow instructions to bring HF medications (or medication lists) and daily weight measurements to clinic visits, which complicated medication reconciliations, interventions, and educational efforts regarding dose changes. Furthermore, patients sometimes missed face-to-face appointments, often because of transportation difficulties. In these situations, telephone appointments may be beneficial. The obstacle of transportation is removed, and, during the at-home telephone call, the patient has easy access to medications and measurements.
Limitations
This study had several limitations. It was retrospective, and its sample size was too small for conclusions regarding morbidity and mortality. As the population was predominantly African American males, results may not be applicable to other races and females. Furthermore, not evaluating HF causes at baseline could have confounded results, as disease progression, response to medications, and prognosis can vary, depending on etiology. Moreover, as patients are referred from the HFDMP to the PMTC, there may have been a bias in patient selection for the PMTC group. Patients in the PMTC group may have been more clinically stable yet had a larger knowledge deficit, an adherence issue, or a need for difficult, frequent titrations. Patients also may have been less likely to be seen during the first 30 days after discharge. In addition, it could have been beneficial to match patients on NYHA class of HF at baseline to ensure HF severity was balanced between groups. Last, the adherence analysis may not be accurate, as it relied on refill history, which may not reflect how medications were taken at home.
It would be beneficial to expand this initial study with a larger sample. Presumed HF causes and medication adherence should be captured at baseline. Additional endpoints, including quality of life and patient cognition, could enhance results. Furthermore, comparing the HFDMP with the general cardiology clinic may reveal other benefits of a focused HFDMP and its PMTC. Last, evaluating patients who are recently discharged from HF admission yet not enrolled in the HFDMP may provide more information regarding the utility of both the HFDMP and the PMTC.
Conclusion
For the PMTC group in this study, achievement of target BB doses and achievement in composite clinical GDMT were significant, but achievement of target ACEI/ARB doses were not.
Click here to read the digital edition.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6-e245.
2. Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344-350.
3. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407-413.
4. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260-266.
5. U.S. Department of Veterans Affairs, VA Office of Research and Development, Executive Committee, Chronic Heart Failure Quality Enhancement Research Initiative (CHF-QUERI), Health Services Research and Development Service. Chronic heart failure [QUERI fact sheet]. https://www.queri.research.va.gov/about/factsheets/chf_factsheet.pdf. Published July 2014. Accessed October 9, 2017.
6. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
7. New York Heart Association Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Little Brown; 1994.
8. Parker M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349-1355.
9. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9-13.
10. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-2007.
11. Gattis WA, Hasselblad V, Whellan DJ, O’Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study. Arch Intern Med. 1999;159(16):1939-1945.
12. Jain A, Mills P, Nunn LM, et al. Success of a multidisciplinary heart failure clinic for initiation and up-titration of key therapeutic agents. Eur J Heart Fail. 2005;7(3):405-410.
13. Lowrie R, Mair FS, Greenlaw N, et al; Heart Failure Optimal Outcomes From Pharmacy Study (HOOPS) Investigators. Pharmacist intervention in primary care to improve outcomes in patients with left ventricular systolic dysfunction. Eur Heart J. 2012;33(3):314-324.
14. Martinez AS, Saef J, Paszczuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6-e245.
2. Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344-350.
3. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407-413.
4. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260-266.
5. U.S. Department of Veterans Affairs, VA Office of Research and Development, Executive Committee, Chronic Heart Failure Quality Enhancement Research Initiative (CHF-QUERI), Health Services Research and Development Service. Chronic heart failure [QUERI fact sheet]. https://www.queri.research.va.gov/about/factsheets/chf_factsheet.pdf. Published July 2014. Accessed October 9, 2017.
6. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
7. New York Heart Association Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Little Brown; 1994.
8. Parker M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349-1355.
9. CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9-13.
10. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-2007.
11. Gattis WA, Hasselblad V, Whellan DJ, O’Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study. Arch Intern Med. 1999;159(16):1939-1945.
12. Jain A, Mills P, Nunn LM, et al. Success of a multidisciplinary heart failure clinic for initiation and up-titration of key therapeutic agents. Eur J Heart Fail. 2005;7(3):405-410.
13. Lowrie R, Mair FS, Greenlaw N, et al; Heart Failure Optimal Outcomes From Pharmacy Study (HOOPS) Investigators. Pharmacist intervention in primary care to improve outcomes in patients with left ventricular systolic dysfunction. Eur Heart J. 2012;33(3):314-324.
14. Martinez AS, Saef J, Paszczuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
Factors Affecting Heart Failure Readmission Rates in VA Patients
Heart failure (HF) continues to grow as a significant health problem in the U.S., accounting for 1.1 million hospitalizations annually.1 About 5.8 million Americans have HF, and 670,000 new cases are diagnosed each year.1 The prevalence of HF increases with age. Persons aged > 65 years comprise the largest group of patients hospitalized for the condition. Heart failure-related hospitalizations place a major financial burden on patients, caregivers, and the national health care system. In 2010, the estimated cost of health care for HF was $35 billion with hospitalizations accounting for 1% to 2% of the total annual health care costs.1-3 Furthermore, > 50% of patients with HF are rehospitalized before their first outpatient follow-up.4
To ensure patients are ready for discharge, HF guidelines recommend specific interventions for all hospitalized patients. These recommendations include successful transition from IV to oral diuretic therapy as well as the initiation of a ß-blocker and an angiotensin-converting enzyme inhibitor (ACE-I) or an angiotensin receptor blocker (ARB) in stable patients with a left ventricular ejection fraction (LVEF) < 40% and without contraindications. Additionally, patients and their caregivers should receive comprehensive discharge instructions regarding medications, the importance of adherence and regular follow-up, sodium and fluid restriction, weight monitoring, physical activity, and a plan for worsening symptoms. When available, assistance with the hospital-to-home transition should also be provided.5,6
Heart Failure Measures
Recognizing common factors essential to HF care, the Joint Commission has implemented HF core measures that all U.S. hospitals are required to meet to maintain accreditation status. These guideline-supported measures include receipt of diet, weight, and medication instructions; measured or scheduled assessment of LVEF; ACE-I or an ARB prescribed in patients with LVEF < 40%; and smoking cessation counseling before discharge for all patients with HF.
In addition to HF core measures, 30-day HF readmission rates have also become available to the general public as another hospital quality indicator. In 2009, the Centers for Medicare & Medicaid Services began publicly reporting 30-day HF readmission rates for Medicare patients. A CMS report indicated a 24.8% national 30-day HF readmission rate from July 1, 2007, through June 30, 2010.7 Unfortunately, even with the increased quality improvement effort, national HF rehospitalization rates have remained relatively steady in recent years.3
The VA health care system has a growing number of veterans with HF, and it is the leading discharge diagnosis in patients treated at VA hospitals. The number of HF-related hospitalizations at the VA health care system increased from just over 74,000 in fiscal year 2002 to 96,000 in 2009.8
To advance the care of veterans with HF and implement best practices, the VA launched the Chronic HF-Quality Enhancement Research Initiative (CHF-QUERI). The major goals of this initiative are to reduce hospitalization rates, increase use of life-prolonging care, empower patients and their caregivers in self-management, and improve appropriateness of HF therapies and tests. As part of its efforts, CHF-QUERI launched the HF Provider Network (HF Network), involving more than 712 VA health care providers (as of July 2014 there were more than 900 providers) committed to improving HF management throughout the entire VA health care system. The HF Network has already put into practice several quality improvement initiatives.
The National Hospital to Home initiative led by the American College of Cardiology and the Institute for Healthcare Improvement was launched throughout the VA system in January 2010.9 The main goal of this initiative is to reduce all-cause hospital readmission rates in patients with a discharge diagnosis of HF by improving medication management, early follow-up after discharge, and symptom management.
The Jesse Brown VAMC (JBVAMC) is an active participant of the Hospital to Home initiative, embracing the goals of reducing HF readmission rates and improving the transition of veterans from inpatient to outpatient care. The JBVAMC also has been successfully meeting or exceeding HF core measures except for providing discharge instructions. In May 2011, 91% of patients received discharge instructions, falling just slightly below the 93% target goal. Despite the implementation of HF care improvement initiatives and successful core measure performance, from July 1, 2007, to June 30, 2010, the average HF 30-day readmission rate at JBVAMC was reported to be 28.4%, compared with the national average of 24.8%. Additionally, the average readmission rate for fiscal year 2011 was 31% at JBVAMC, showing a further increase in readmission rates.
The cost of a hospital bed at JBVAMC ranges from about $2,000 to $5,000 per day. According to the American Heart Association’s Get With the Guidelines-HF registry, the mean hospital length of stay for HF in 2009 was 5.5 days.1 Consequently, HF hospitalizations could potentially cost JBVAMC nearly $7 million annually. Therefore, HF readmissions not only affect patients and caregivers, but also represent a financial burden for JBVAMC.
METHODS
The purpose of this study was to identify factors contributing to the high HF readmission rates in veterans enrolled at JBVAMC. This study was an Institutional Review Board and VA Research and Development Committee-approved retrospective, electronic chart review of patients with an ICD-9 principal discharge diagnosis code for HF and hospitalization for HF exacerbation anytime between October 1, 2010, and March 1, 2011. A patient chart was reviewed for 6 months after inclusion. A report was generated to identify patients discharged from JBVAMC with a principal discharge diagnosis of HF between October 1, 2010, and March 1, 2011, using the following ICD-9 HF codes: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, and 428.9.
Patients were included if aged ≥ 18 years with one of the ICD-9 HF codes as the principal discharge diagnosis within the study period. Patients were excluded from the study if transferred to or from an outside hospital, discharged without an ICD-9 principal diagnosis code for HF, electively admitted for HF, not treated for HF during hospitalization, left the hospital against medical advice, had chart documentation with comfort measures only, were discharged/transferred to hospice, had active HF medications listed under non-VA medications in the electronic medication profile, or did not receive follow-up at JBVAMC. Study participants were included in the study once, which was classified as their index HF hospitalization, and were followed for 6 months thereafter.
The primary endpoint was the difference in patient characteristics between 2 groups of patients: those readmitted for HF within 30 days of the index hospitalization and those readmitted after 30 days or not at all.
The study had multiple secondary endpoints. One was the difference in patient characteristics between 2 groups of patients: those readmitted for HF within 90 days of the index hospitalization and those readmitted after 90 days or not at all. Another secondary endpoint was the difference in patient characteristics between 2 groups of patients: those with ≥ 2 readmissions for HF within 6 months and those with < 2 HF readmissions within 6 months. Additional secondary endpoints included percentage of patients readmitted for HF within 30 days of the index HF hospitalization, time to readmission if applicable, time to death if applicable, and average number of readmissions per patient within 6 months.
Index data collected included age, gender, ethnicity, prior diagnosis of HF, date of diagnosis, hospitalization for HF within 30 days of the index HF admission, in-hospital cardiac arrest, comorbid conditions, systolic blood pressure (BP), heart rate, respiratory rate, weight, serum sodium, blood urea nitrogen, serum creatinine, hematocrit, and glucose. For this study, comorbid conditions gathered were diabetes mellitus, coronary artery disease, prior percutaneous coronary intervention, aortic stenosis, stroke, chronic obstructive pulmonary disease, and dementia.
Medication profiles were reviewed at the time of admission to determine whether the patient was prescribed an ACE-I/ARB, ß-blocker, diuretic, hydralazine and isosorbide dinitrate, aldosterone antagonist, digoxin, NSAIDs, nonvasoselective calcium channel blocker, and an antiarrhytmic other than amiodarone and dofetilide. Hospitalization data included the most recent LVEF, the number of days on oral diuretic therapy after stopping IV diuretics, the number of days admitted, and documentation of an in-person inpatient dietitian consultation.
Data collected at discharge included diet/weight/medication instructions, weight, BP, American College of Cardiology/American Heart Association HF stage and New York Heart Association (NYHA) HF functional class, if documented. Discharge medication profiles were assessed for the number of medications (< 9 or ≥ 9), documentation of active prescriptions for an ACE-I/ARB and a ß-blocker (or contraindication documented), diuretic, hydralazine and isosorbide dinitrate, aldosterone antagonist, and digoxin. Other data collected were documentation of a scheduled follow-up appointment with primary care physician, urgent care, chronic HF (CHF) clinic, or cardiologist, and whether the patient was discharged to home, skilled nursing facility, shelter, or homeless. Additionally, if the patient was discharged on a diuretic, the dose was compared with the baseline diuretic. If the diuretic at discharge was different from the home diuretic, equivalent doses were used for comparison with that of the baseline diuretic.
Postdischarge data collection included telephone follow-up within 48 hours of discharge, medication compliance since the initial hospitalization, date of first outpatient follow-up after initial hospital discharge, enrollment in CHF clinic/CHF-PharmD/Care Coordination Home Telehealth (CCHT) program, outpatient dietitian consultations, and date of death if applicable. Medication adherence was defined as ≥ 80% of lowest percentage filled medication of all HF medications, determined by the refill history in the computerized patient record system (CPRS). First outpatient follow-up was defined as a visit in which HF was addressed in the assessment and plan.
If readmitted within the study period, data collection included the date of first nonelective hospital readmission for HF, BP, heart rate, weight, serum digoxin level, serum creatinine, serum potassium, and whether the patient was on a target dose of HF recommended medications (if LVEF < 40% and no contraindication). Heart failure recommended medications for which target doses are established include ACE-I/ARB and ß-blockers. For this study, target doses of ACE-Is were captopril 50 mg 3 times daily, enalapril 10 mg twice daily, fosinopril 40 mg daily, lisinopril 20 mg daily, ramipril 10 mg daily, and trandolapril 4 mg daily. Target doses for ARBs were candesartan 32 mg daily, losartan 50 mg daily, and valsartan 160 mg twice daily. ß-blocker target doses were bisoprolol 10 mg daily, carvedilol 25 mg twice daily (50 mg twice daily if patients’ weight was > 85 kg), and metoprolol succinate 200 mg daily.5,6 A statistical analysis was not performed on the data.
RESULTS
A total of 137 patient charts were reviewed, and 109 patients were included in the study. Patients were excluded if they transferred to or from an outside hospital (n = 8), had no follow-up at JBVAMC (n = 8), left the hospital against medical advice (n = 4), were electively admitted (n = 4), were not treated for HF (n = 3), or only had comfort measures documented in the chart (n = 1). The patients included were predominantly male (99%) and African American (78%) and had a mean age of 70 years. The majority of the patients had a prior diagnosis of HF (87%) and a history of systolic HF (58%). Most patients were previously prescribed an ACE-I/ARB (83%) and a ß-blocker (76%) at the time of admission (Table 1).
Six patients were readmitted within 30 days of the index hospitalization, whereas 103 patients were readmitted after 30 days or not at all. With respect to secondary endpoints, there were 21 patients readmitted within 90 days of the index hospitalization, whereas 88 patients were readmitted after 90 days or not at all. Additionally, 6 patients were readmitted ≥ 2 times within 6 months of the index hospitalization, whereas 103 patients were readmitted < 2 times within 6 months.
Baseline characteristics seemed similar across the study groups, except a greater percentage of patients readmitted within 30 days of the index HF hospitalization had a prior history of systolic HF and were hospitalized for HF 30 days prior to the index hospitalization (Table 2). In addition, patients readmitted within 30 days tended to receive a shorter duration of oral diuretic therapy after discontinuation of IV diuretics (mean 0.2 days vs 1.1 days). Patients in this group with an LVEF < 40% were less likely to be discharged on an ACE-I/ARB (75% vs 95%) and a ß-blocker (50% vs 85%) than were the patients who were readmitted after 30 days or not at all. These trends continued for patients readmitted within 90 days of the index hospitalization and for those readmitted after 90 days or not at all. The mean length of stay for the index HF hospitalization was about 5 days and was comparable among all study groups.
From the evaluation of postdischarge characteristics, no patients readmitted within 30 days had a follow-up appointment scheduled with the CHF clinic. In comparison with patients readmitted after 30 days or not at all, more patients had follow-up at an urgent care clinic (33% vs 6%) or no follow-up appointment scheduled at the time of discharge (17% vs 2%). Half of all the patients with a scheduled follow-up missed their appointment. Additionally, medication adherence was lower (33% vs 80%), and none of the patients were enrolled in the CHF-PharmD clinic (0% vs 5%). A similar trend continued for the secondary endpoint groups (Table 3). Last, none of the study patients had an outpatient dietitian consultation.
On readmission, the majority of patients readmitted within 30 days were not on a target dose of an ACE-I/ARB (75%), and none were on a target dose of a ß-blocker. The same trend continued for the secondary endpoint groups. None of the study patients had a serum digoxin level > 0.9 ng/mL. However, serum digoxin level was not measured in all readmitted patients prescribed digoxin (Table 4).
In regard to other secondary endpoints, 6 patients (5.5%) were readmitted for HF within 30 days of the index HF hospitalization. The average number of readmissions per patient in 6 months was < 1, mean time to readmission was 85 days (n = 33), and mean time to death was 88 days (n = 5) when applicable.
DISCUSSION
Based on the trends observed in this study, multiple recommendations can be made to further improve the quality of care and reduce HF readmissions at JBVAMC. The medical center physicians currently use a discharge note template, which already includes sections such as HF discharge instructions and follow-up appointments. The template also prompts providers to prescribe an ACE-I in appropriate patients.
When JBVAMC providers are ready to enter discharge notes into the CPRS, they first select the discharge note template from available note template options. The electronic template contains spaces for the provider to enter a patient’s primary reason for hospitalization, date of admission, discharge medication list, specific or suggested dates for follow-up with outpatient provider(s), general diet/weight/medication instructions, a space to answer whether the patient has HF, a space to record NYHA HF class if applicable, and a space to record whether the patient is prescribed or will be prescribed an ACE-I if appropriate, or whether ACE-I is contraindicated. The providers are able to modify and add information to the discharge note template as they see appropriate.
The findings of this study suggest that modifying the existing discharge template to include additional provider prompts in a form of designated spaces asking for specific information may help improve HF care outcomes. If providers are prompted to answer whether an oral diuretic was continued for at least 24 hours after stopping IV diuretics for HF, adherence to the HF guideline-recommended duration of oral diuretic therapy may improve. Additionally, ß-blocker prescribing in appropriate systolic HF patients may increase if providers are prompted. To enhance continuity of care, the discharge note template may be modified to include a section in which the providers can document patients followed by outside providers. This can be done by incorporating a space in the discharge template to enter the patient’s non-VA provider information if applicable and may help further coordinate the care of such patients to ensure that they are not lost.
Furthermore, the discharge template may be modified to include a prompt to place a CHF clinic consult to increase provider awareness about the availability of CHF and CHF-PharmD clinics at JBVAMC. CHF and CHF-PharmD clinics collaborate to provide comprehensive care to HF patients. After an initial evaluation at the CHF clinic, patients are referred to the clinical pharmacist for further medication therapy management when necessary. Currently, the physicians are encouraged to refer HF patients to the CHF clinic after discharge, but not all providers know that such a service is available. The prompt within the discharge note template would provide CHF/CHF-PharmD clinic provider contact information, clinic times, and a link that would take the provider to an appropriate screen for placing the consult.
Limitations
There are several limitations to this study, including its retrospective design and small sample size. Another source of potential study limitation was the initial process for creating a study patient list. The study list was designed to use ICD-9 codes to capture readmissions only for HF and only at JBVAMC. This was achieved by specifying any of the HF ICD-9 codes as the principal discharge diagnosis. However, the providers may not have always used a HF specific ICD-9 code for the principal discharge diagnosis, even if a patient was admitted primarily for HF. The provider may have chosen another principal discharge diagnosis for which the patient received treatment during the hospitalization.
There are multiple ways to obtain HF patient lists, one includes using the diagnosis-related group codes instead of ICD-9 codes. Due to the way the patient list was obtained and an inherent possibility that some patients admitted for HF had a non-HF ICD-9 code recorded as their principal discharge diagnosis, some eligible patients may not have appeared on the generated list. Additionally, this study captured readmission rates for only HF whereas the national HF 30-day readmission rate represents all-cause readmissions for HF patients. This difference may be reflected in the low 30-day readmission rate observed.
Another possible limitation was the timing of the launch of the CHF-PharmD clinic and the initiative for telephone follow-up 48 hours postdischarge. The CHF-PharmD clinic was launched in April 2011, and the initiative for telephone follow-up 48 hours postdischarge began in January 2011. As the start dates fell within the study period, these services may not have been available to all patients. Therefore, the data describing patient enrollment in CHF-PharmD clinic and those who received postdischarge telephone follow-up may not accurately reflect current practice. Last, statistical tests were not used in the study data analysis leaving any differences found open to interpretation. To minimize these limitations, larger prospective studies with statistical analysis capturing all-cause readmissions are necessary to further evaluate patient characteristics that may be contributing to HF readmissions at JBVAMC.
Conclusions
In general, earlier and more frequent readmissions were more common in patients who were converted to oral diuretic therapy for < 24 hours before discharge and were not discharged on an ACE-I/ARB and a b-blocker when appropriate. Additionally, most of the readmitted patients had no follow-up scheduled at discharge, were nonadherent with medications and follow-up appointments, and were not enrolled in the CHF and/or CHF-PharmD clinic. The majority of patients with systolic HF were not at target doses of either the ACE-I/ARB or the ß-blocker when readmitted. Overall, JBVAMC had a low percentage of patients readmitted for HF within 30 days, but there is still room for improvement in reducing HF readmissions.
At the time of discharge, all JBVAMC patients receive printed instructions and recommendations for their care after hospitalization. The patient handout includes the most current medications, diet/weight/medication instructions, and actual or suggested dates for follow-up appointments and/or tests. It may enhance awareness regarding dietician services to patients if the current discharge instruction template can be modified to provide information regarding the outpatient dietitian class. This could include date, time, and location of classes as well as dietician contact information. (See Appendixes 1 and 2.)
When these recommendations have been implemented, further studies will be warranted to assess the impact of the interventions.
Acknowledgments
The authors thank Ms. Yvette Bloodson for her assistance in generating the initial patient list.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2011 Update. A Report from the American Heart Association. Circulation. 2011;123(4):e18-e209.
2. National Heart, Lung, and Blood Institute. Incidence and Prevalence: 2009 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Institutes of Health; 2009.
3. Ross JS, Chen J, Lin ZQ, et al. Recent national trends in readmission rates after HF hospitalization. Circ Heart Fail. 2010;3(1):97-103.
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
5. Hunt SA, Abraham WT, Chin MH., et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391-e479.
6. Lindenfeld J, Albert NM, Boehmer JP, et al. Executive Summary: HFSA 2010 Comprehensive HF Practice Guideline. J Card Fail. 2010;16(6):475-539.
7. U.S. Department of Health and Human Services. Hospital Compare. https://data.medicare.gov/data/archives/hospital-compare. Updated August 22, 2011. Accessed October 15, 2014.
8. Heidenreich PA. Chronic HF QUERI Center Application: Strategic Plan 2009. U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI) Website. http://www.queri.research.va.gov/about/strategic_plans/chf.pdf. Updated August 22, 2011. Accessed September 2, 2014.
9. U.S. Department of Veterans Affairs. Chronic HF Quality Enhancement Research Initiative: VA Hospital to Home (H2H) Initiative. U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI) Website. http://www.queri.research.va.gov/chf/products/h2h. Updated August 19, 2011. Accessed September 2, 2014.
Heart failure (HF) continues to grow as a significant health problem in the U.S., accounting for 1.1 million hospitalizations annually.1 About 5.8 million Americans have HF, and 670,000 new cases are diagnosed each year.1 The prevalence of HF increases with age. Persons aged > 65 years comprise the largest group of patients hospitalized for the condition. Heart failure-related hospitalizations place a major financial burden on patients, caregivers, and the national health care system. In 2010, the estimated cost of health care for HF was $35 billion with hospitalizations accounting for 1% to 2% of the total annual health care costs.1-3 Furthermore, > 50% of patients with HF are rehospitalized before their first outpatient follow-up.4
To ensure patients are ready for discharge, HF guidelines recommend specific interventions for all hospitalized patients. These recommendations include successful transition from IV to oral diuretic therapy as well as the initiation of a ß-blocker and an angiotensin-converting enzyme inhibitor (ACE-I) or an angiotensin receptor blocker (ARB) in stable patients with a left ventricular ejection fraction (LVEF) < 40% and without contraindications. Additionally, patients and their caregivers should receive comprehensive discharge instructions regarding medications, the importance of adherence and regular follow-up, sodium and fluid restriction, weight monitoring, physical activity, and a plan for worsening symptoms. When available, assistance with the hospital-to-home transition should also be provided.5,6
Heart Failure Measures
Recognizing common factors essential to HF care, the Joint Commission has implemented HF core measures that all U.S. hospitals are required to meet to maintain accreditation status. These guideline-supported measures include receipt of diet, weight, and medication instructions; measured or scheduled assessment of LVEF; ACE-I or an ARB prescribed in patients with LVEF < 40%; and smoking cessation counseling before discharge for all patients with HF.
In addition to HF core measures, 30-day HF readmission rates have also become available to the general public as another hospital quality indicator. In 2009, the Centers for Medicare & Medicaid Services began publicly reporting 30-day HF readmission rates for Medicare patients. A CMS report indicated a 24.8% national 30-day HF readmission rate from July 1, 2007, through June 30, 2010.7 Unfortunately, even with the increased quality improvement effort, national HF rehospitalization rates have remained relatively steady in recent years.3
The VA health care system has a growing number of veterans with HF, and it is the leading discharge diagnosis in patients treated at VA hospitals. The number of HF-related hospitalizations at the VA health care system increased from just over 74,000 in fiscal year 2002 to 96,000 in 2009.8
To advance the care of veterans with HF and implement best practices, the VA launched the Chronic HF-Quality Enhancement Research Initiative (CHF-QUERI). The major goals of this initiative are to reduce hospitalization rates, increase use of life-prolonging care, empower patients and their caregivers in self-management, and improve appropriateness of HF therapies and tests. As part of its efforts, CHF-QUERI launched the HF Provider Network (HF Network), involving more than 712 VA health care providers (as of July 2014 there were more than 900 providers) committed to improving HF management throughout the entire VA health care system. The HF Network has already put into practice several quality improvement initiatives.
The National Hospital to Home initiative led by the American College of Cardiology and the Institute for Healthcare Improvement was launched throughout the VA system in January 2010.9 The main goal of this initiative is to reduce all-cause hospital readmission rates in patients with a discharge diagnosis of HF by improving medication management, early follow-up after discharge, and symptom management.
The Jesse Brown VAMC (JBVAMC) is an active participant of the Hospital to Home initiative, embracing the goals of reducing HF readmission rates and improving the transition of veterans from inpatient to outpatient care. The JBVAMC also has been successfully meeting or exceeding HF core measures except for providing discharge instructions. In May 2011, 91% of patients received discharge instructions, falling just slightly below the 93% target goal. Despite the implementation of HF care improvement initiatives and successful core measure performance, from July 1, 2007, to June 30, 2010, the average HF 30-day readmission rate at JBVAMC was reported to be 28.4%, compared with the national average of 24.8%. Additionally, the average readmission rate for fiscal year 2011 was 31% at JBVAMC, showing a further increase in readmission rates.
The cost of a hospital bed at JBVAMC ranges from about $2,000 to $5,000 per day. According to the American Heart Association’s Get With the Guidelines-HF registry, the mean hospital length of stay for HF in 2009 was 5.5 days.1 Consequently, HF hospitalizations could potentially cost JBVAMC nearly $7 million annually. Therefore, HF readmissions not only affect patients and caregivers, but also represent a financial burden for JBVAMC.
METHODS
The purpose of this study was to identify factors contributing to the high HF readmission rates in veterans enrolled at JBVAMC. This study was an Institutional Review Board and VA Research and Development Committee-approved retrospective, electronic chart review of patients with an ICD-9 principal discharge diagnosis code for HF and hospitalization for HF exacerbation anytime between October 1, 2010, and March 1, 2011. A patient chart was reviewed for 6 months after inclusion. A report was generated to identify patients discharged from JBVAMC with a principal discharge diagnosis of HF between October 1, 2010, and March 1, 2011, using the following ICD-9 HF codes: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, and 428.9.
Patients were included if aged ≥ 18 years with one of the ICD-9 HF codes as the principal discharge diagnosis within the study period. Patients were excluded from the study if transferred to or from an outside hospital, discharged without an ICD-9 principal diagnosis code for HF, electively admitted for HF, not treated for HF during hospitalization, left the hospital against medical advice, had chart documentation with comfort measures only, were discharged/transferred to hospice, had active HF medications listed under non-VA medications in the electronic medication profile, or did not receive follow-up at JBVAMC. Study participants were included in the study once, which was classified as their index HF hospitalization, and were followed for 6 months thereafter.
The primary endpoint was the difference in patient characteristics between 2 groups of patients: those readmitted for HF within 30 days of the index hospitalization and those readmitted after 30 days or not at all.
The study had multiple secondary endpoints. One was the difference in patient characteristics between 2 groups of patients: those readmitted for HF within 90 days of the index hospitalization and those readmitted after 90 days or not at all. Another secondary endpoint was the difference in patient characteristics between 2 groups of patients: those with ≥ 2 readmissions for HF within 6 months and those with < 2 HF readmissions within 6 months. Additional secondary endpoints included percentage of patients readmitted for HF within 30 days of the index HF hospitalization, time to readmission if applicable, time to death if applicable, and average number of readmissions per patient within 6 months.
Index data collected included age, gender, ethnicity, prior diagnosis of HF, date of diagnosis, hospitalization for HF within 30 days of the index HF admission, in-hospital cardiac arrest, comorbid conditions, systolic blood pressure (BP), heart rate, respiratory rate, weight, serum sodium, blood urea nitrogen, serum creatinine, hematocrit, and glucose. For this study, comorbid conditions gathered were diabetes mellitus, coronary artery disease, prior percutaneous coronary intervention, aortic stenosis, stroke, chronic obstructive pulmonary disease, and dementia.
Medication profiles were reviewed at the time of admission to determine whether the patient was prescribed an ACE-I/ARB, ß-blocker, diuretic, hydralazine and isosorbide dinitrate, aldosterone antagonist, digoxin, NSAIDs, nonvasoselective calcium channel blocker, and an antiarrhytmic other than amiodarone and dofetilide. Hospitalization data included the most recent LVEF, the number of days on oral diuretic therapy after stopping IV diuretics, the number of days admitted, and documentation of an in-person inpatient dietitian consultation.
Data collected at discharge included diet/weight/medication instructions, weight, BP, American College of Cardiology/American Heart Association HF stage and New York Heart Association (NYHA) HF functional class, if documented. Discharge medication profiles were assessed for the number of medications (< 9 or ≥ 9), documentation of active prescriptions for an ACE-I/ARB and a ß-blocker (or contraindication documented), diuretic, hydralazine and isosorbide dinitrate, aldosterone antagonist, and digoxin. Other data collected were documentation of a scheduled follow-up appointment with primary care physician, urgent care, chronic HF (CHF) clinic, or cardiologist, and whether the patient was discharged to home, skilled nursing facility, shelter, or homeless. Additionally, if the patient was discharged on a diuretic, the dose was compared with the baseline diuretic. If the diuretic at discharge was different from the home diuretic, equivalent doses were used for comparison with that of the baseline diuretic.
Postdischarge data collection included telephone follow-up within 48 hours of discharge, medication compliance since the initial hospitalization, date of first outpatient follow-up after initial hospital discharge, enrollment in CHF clinic/CHF-PharmD/Care Coordination Home Telehealth (CCHT) program, outpatient dietitian consultations, and date of death if applicable. Medication adherence was defined as ≥ 80% of lowest percentage filled medication of all HF medications, determined by the refill history in the computerized patient record system (CPRS). First outpatient follow-up was defined as a visit in which HF was addressed in the assessment and plan.
If readmitted within the study period, data collection included the date of first nonelective hospital readmission for HF, BP, heart rate, weight, serum digoxin level, serum creatinine, serum potassium, and whether the patient was on a target dose of HF recommended medications (if LVEF < 40% and no contraindication). Heart failure recommended medications for which target doses are established include ACE-I/ARB and ß-blockers. For this study, target doses of ACE-Is were captopril 50 mg 3 times daily, enalapril 10 mg twice daily, fosinopril 40 mg daily, lisinopril 20 mg daily, ramipril 10 mg daily, and trandolapril 4 mg daily. Target doses for ARBs were candesartan 32 mg daily, losartan 50 mg daily, and valsartan 160 mg twice daily. ß-blocker target doses were bisoprolol 10 mg daily, carvedilol 25 mg twice daily (50 mg twice daily if patients’ weight was > 85 kg), and metoprolol succinate 200 mg daily.5,6 A statistical analysis was not performed on the data.
RESULTS
A total of 137 patient charts were reviewed, and 109 patients were included in the study. Patients were excluded if they transferred to or from an outside hospital (n = 8), had no follow-up at JBVAMC (n = 8), left the hospital against medical advice (n = 4), were electively admitted (n = 4), were not treated for HF (n = 3), or only had comfort measures documented in the chart (n = 1). The patients included were predominantly male (99%) and African American (78%) and had a mean age of 70 years. The majority of the patients had a prior diagnosis of HF (87%) and a history of systolic HF (58%). Most patients were previously prescribed an ACE-I/ARB (83%) and a ß-blocker (76%) at the time of admission (Table 1).
Six patients were readmitted within 30 days of the index hospitalization, whereas 103 patients were readmitted after 30 days or not at all. With respect to secondary endpoints, there were 21 patients readmitted within 90 days of the index hospitalization, whereas 88 patients were readmitted after 90 days or not at all. Additionally, 6 patients were readmitted ≥ 2 times within 6 months of the index hospitalization, whereas 103 patients were readmitted < 2 times within 6 months.
Baseline characteristics seemed similar across the study groups, except a greater percentage of patients readmitted within 30 days of the index HF hospitalization had a prior history of systolic HF and were hospitalized for HF 30 days prior to the index hospitalization (Table 2). In addition, patients readmitted within 30 days tended to receive a shorter duration of oral diuretic therapy after discontinuation of IV diuretics (mean 0.2 days vs 1.1 days). Patients in this group with an LVEF < 40% were less likely to be discharged on an ACE-I/ARB (75% vs 95%) and a ß-blocker (50% vs 85%) than were the patients who were readmitted after 30 days or not at all. These trends continued for patients readmitted within 90 days of the index hospitalization and for those readmitted after 90 days or not at all. The mean length of stay for the index HF hospitalization was about 5 days and was comparable among all study groups.
From the evaluation of postdischarge characteristics, no patients readmitted within 30 days had a follow-up appointment scheduled with the CHF clinic. In comparison with patients readmitted after 30 days or not at all, more patients had follow-up at an urgent care clinic (33% vs 6%) or no follow-up appointment scheduled at the time of discharge (17% vs 2%). Half of all the patients with a scheduled follow-up missed their appointment. Additionally, medication adherence was lower (33% vs 80%), and none of the patients were enrolled in the CHF-PharmD clinic (0% vs 5%). A similar trend continued for the secondary endpoint groups (Table 3). Last, none of the study patients had an outpatient dietitian consultation.
On readmission, the majority of patients readmitted within 30 days were not on a target dose of an ACE-I/ARB (75%), and none were on a target dose of a ß-blocker. The same trend continued for the secondary endpoint groups. None of the study patients had a serum digoxin level > 0.9 ng/mL. However, serum digoxin level was not measured in all readmitted patients prescribed digoxin (Table 4).
In regard to other secondary endpoints, 6 patients (5.5%) were readmitted for HF within 30 days of the index HF hospitalization. The average number of readmissions per patient in 6 months was < 1, mean time to readmission was 85 days (n = 33), and mean time to death was 88 days (n = 5) when applicable.
DISCUSSION
Based on the trends observed in this study, multiple recommendations can be made to further improve the quality of care and reduce HF readmissions at JBVAMC. The medical center physicians currently use a discharge note template, which already includes sections such as HF discharge instructions and follow-up appointments. The template also prompts providers to prescribe an ACE-I in appropriate patients.
When JBVAMC providers are ready to enter discharge notes into the CPRS, they first select the discharge note template from available note template options. The electronic template contains spaces for the provider to enter a patient’s primary reason for hospitalization, date of admission, discharge medication list, specific or suggested dates for follow-up with outpatient provider(s), general diet/weight/medication instructions, a space to answer whether the patient has HF, a space to record NYHA HF class if applicable, and a space to record whether the patient is prescribed or will be prescribed an ACE-I if appropriate, or whether ACE-I is contraindicated. The providers are able to modify and add information to the discharge note template as they see appropriate.
The findings of this study suggest that modifying the existing discharge template to include additional provider prompts in a form of designated spaces asking for specific information may help improve HF care outcomes. If providers are prompted to answer whether an oral diuretic was continued for at least 24 hours after stopping IV diuretics for HF, adherence to the HF guideline-recommended duration of oral diuretic therapy may improve. Additionally, ß-blocker prescribing in appropriate systolic HF patients may increase if providers are prompted. To enhance continuity of care, the discharge note template may be modified to include a section in which the providers can document patients followed by outside providers. This can be done by incorporating a space in the discharge template to enter the patient’s non-VA provider information if applicable and may help further coordinate the care of such patients to ensure that they are not lost.
Furthermore, the discharge template may be modified to include a prompt to place a CHF clinic consult to increase provider awareness about the availability of CHF and CHF-PharmD clinics at JBVAMC. CHF and CHF-PharmD clinics collaborate to provide comprehensive care to HF patients. After an initial evaluation at the CHF clinic, patients are referred to the clinical pharmacist for further medication therapy management when necessary. Currently, the physicians are encouraged to refer HF patients to the CHF clinic after discharge, but not all providers know that such a service is available. The prompt within the discharge note template would provide CHF/CHF-PharmD clinic provider contact information, clinic times, and a link that would take the provider to an appropriate screen for placing the consult.
Limitations
There are several limitations to this study, including its retrospective design and small sample size. Another source of potential study limitation was the initial process for creating a study patient list. The study list was designed to use ICD-9 codes to capture readmissions only for HF and only at JBVAMC. This was achieved by specifying any of the HF ICD-9 codes as the principal discharge diagnosis. However, the providers may not have always used a HF specific ICD-9 code for the principal discharge diagnosis, even if a patient was admitted primarily for HF. The provider may have chosen another principal discharge diagnosis for which the patient received treatment during the hospitalization.
There are multiple ways to obtain HF patient lists, one includes using the diagnosis-related group codes instead of ICD-9 codes. Due to the way the patient list was obtained and an inherent possibility that some patients admitted for HF had a non-HF ICD-9 code recorded as their principal discharge diagnosis, some eligible patients may not have appeared on the generated list. Additionally, this study captured readmission rates for only HF whereas the national HF 30-day readmission rate represents all-cause readmissions for HF patients. This difference may be reflected in the low 30-day readmission rate observed.
Another possible limitation was the timing of the launch of the CHF-PharmD clinic and the initiative for telephone follow-up 48 hours postdischarge. The CHF-PharmD clinic was launched in April 2011, and the initiative for telephone follow-up 48 hours postdischarge began in January 2011. As the start dates fell within the study period, these services may not have been available to all patients. Therefore, the data describing patient enrollment in CHF-PharmD clinic and those who received postdischarge telephone follow-up may not accurately reflect current practice. Last, statistical tests were not used in the study data analysis leaving any differences found open to interpretation. To minimize these limitations, larger prospective studies with statistical analysis capturing all-cause readmissions are necessary to further evaluate patient characteristics that may be contributing to HF readmissions at JBVAMC.
Conclusions
In general, earlier and more frequent readmissions were more common in patients who were converted to oral diuretic therapy for < 24 hours before discharge and were not discharged on an ACE-I/ARB and a b-blocker when appropriate. Additionally, most of the readmitted patients had no follow-up scheduled at discharge, were nonadherent with medications and follow-up appointments, and were not enrolled in the CHF and/or CHF-PharmD clinic. The majority of patients with systolic HF were not at target doses of either the ACE-I/ARB or the ß-blocker when readmitted. Overall, JBVAMC had a low percentage of patients readmitted for HF within 30 days, but there is still room for improvement in reducing HF readmissions.
At the time of discharge, all JBVAMC patients receive printed instructions and recommendations for their care after hospitalization. The patient handout includes the most current medications, diet/weight/medication instructions, and actual or suggested dates for follow-up appointments and/or tests. It may enhance awareness regarding dietician services to patients if the current discharge instruction template can be modified to provide information regarding the outpatient dietitian class. This could include date, time, and location of classes as well as dietician contact information. (See Appendixes 1 and 2.)
When these recommendations have been implemented, further studies will be warranted to assess the impact of the interventions.
Acknowledgments
The authors thank Ms. Yvette Bloodson for her assistance in generating the initial patient list.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Heart failure (HF) continues to grow as a significant health problem in the U.S., accounting for 1.1 million hospitalizations annually.1 About 5.8 million Americans have HF, and 670,000 new cases are diagnosed each year.1 The prevalence of HF increases with age. Persons aged > 65 years comprise the largest group of patients hospitalized for the condition. Heart failure-related hospitalizations place a major financial burden on patients, caregivers, and the national health care system. In 2010, the estimated cost of health care for HF was $35 billion with hospitalizations accounting for 1% to 2% of the total annual health care costs.1-3 Furthermore, > 50% of patients with HF are rehospitalized before their first outpatient follow-up.4
To ensure patients are ready for discharge, HF guidelines recommend specific interventions for all hospitalized patients. These recommendations include successful transition from IV to oral diuretic therapy as well as the initiation of a ß-blocker and an angiotensin-converting enzyme inhibitor (ACE-I) or an angiotensin receptor blocker (ARB) in stable patients with a left ventricular ejection fraction (LVEF) < 40% and without contraindications. Additionally, patients and their caregivers should receive comprehensive discharge instructions regarding medications, the importance of adherence and regular follow-up, sodium and fluid restriction, weight monitoring, physical activity, and a plan for worsening symptoms. When available, assistance with the hospital-to-home transition should also be provided.5,6
Heart Failure Measures
Recognizing common factors essential to HF care, the Joint Commission has implemented HF core measures that all U.S. hospitals are required to meet to maintain accreditation status. These guideline-supported measures include receipt of diet, weight, and medication instructions; measured or scheduled assessment of LVEF; ACE-I or an ARB prescribed in patients with LVEF < 40%; and smoking cessation counseling before discharge for all patients with HF.
In addition to HF core measures, 30-day HF readmission rates have also become available to the general public as another hospital quality indicator. In 2009, the Centers for Medicare & Medicaid Services began publicly reporting 30-day HF readmission rates for Medicare patients. A CMS report indicated a 24.8% national 30-day HF readmission rate from July 1, 2007, through June 30, 2010.7 Unfortunately, even with the increased quality improvement effort, national HF rehospitalization rates have remained relatively steady in recent years.3
The VA health care system has a growing number of veterans with HF, and it is the leading discharge diagnosis in patients treated at VA hospitals. The number of HF-related hospitalizations at the VA health care system increased from just over 74,000 in fiscal year 2002 to 96,000 in 2009.8
To advance the care of veterans with HF and implement best practices, the VA launched the Chronic HF-Quality Enhancement Research Initiative (CHF-QUERI). The major goals of this initiative are to reduce hospitalization rates, increase use of life-prolonging care, empower patients and their caregivers in self-management, and improve appropriateness of HF therapies and tests. As part of its efforts, CHF-QUERI launched the HF Provider Network (HF Network), involving more than 712 VA health care providers (as of July 2014 there were more than 900 providers) committed to improving HF management throughout the entire VA health care system. The HF Network has already put into practice several quality improvement initiatives.
The National Hospital to Home initiative led by the American College of Cardiology and the Institute for Healthcare Improvement was launched throughout the VA system in January 2010.9 The main goal of this initiative is to reduce all-cause hospital readmission rates in patients with a discharge diagnosis of HF by improving medication management, early follow-up after discharge, and symptom management.
The Jesse Brown VAMC (JBVAMC) is an active participant of the Hospital to Home initiative, embracing the goals of reducing HF readmission rates and improving the transition of veterans from inpatient to outpatient care. The JBVAMC also has been successfully meeting or exceeding HF core measures except for providing discharge instructions. In May 2011, 91% of patients received discharge instructions, falling just slightly below the 93% target goal. Despite the implementation of HF care improvement initiatives and successful core measure performance, from July 1, 2007, to June 30, 2010, the average HF 30-day readmission rate at JBVAMC was reported to be 28.4%, compared with the national average of 24.8%. Additionally, the average readmission rate for fiscal year 2011 was 31% at JBVAMC, showing a further increase in readmission rates.
The cost of a hospital bed at JBVAMC ranges from about $2,000 to $5,000 per day. According to the American Heart Association’s Get With the Guidelines-HF registry, the mean hospital length of stay for HF in 2009 was 5.5 days.1 Consequently, HF hospitalizations could potentially cost JBVAMC nearly $7 million annually. Therefore, HF readmissions not only affect patients and caregivers, but also represent a financial burden for JBVAMC.
METHODS
The purpose of this study was to identify factors contributing to the high HF readmission rates in veterans enrolled at JBVAMC. This study was an Institutional Review Board and VA Research and Development Committee-approved retrospective, electronic chart review of patients with an ICD-9 principal discharge diagnosis code for HF and hospitalization for HF exacerbation anytime between October 1, 2010, and March 1, 2011. A patient chart was reviewed for 6 months after inclusion. A report was generated to identify patients discharged from JBVAMC with a principal discharge diagnosis of HF between October 1, 2010, and March 1, 2011, using the following ICD-9 HF codes: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, and 428.9.
Patients were included if aged ≥ 18 years with one of the ICD-9 HF codes as the principal discharge diagnosis within the study period. Patients were excluded from the study if transferred to or from an outside hospital, discharged without an ICD-9 principal diagnosis code for HF, electively admitted for HF, not treated for HF during hospitalization, left the hospital against medical advice, had chart documentation with comfort measures only, were discharged/transferred to hospice, had active HF medications listed under non-VA medications in the electronic medication profile, or did not receive follow-up at JBVAMC. Study participants were included in the study once, which was classified as their index HF hospitalization, and were followed for 6 months thereafter.
The primary endpoint was the difference in patient characteristics between 2 groups of patients: those readmitted for HF within 30 days of the index hospitalization and those readmitted after 30 days or not at all.
The study had multiple secondary endpoints. One was the difference in patient characteristics between 2 groups of patients: those readmitted for HF within 90 days of the index hospitalization and those readmitted after 90 days or not at all. Another secondary endpoint was the difference in patient characteristics between 2 groups of patients: those with ≥ 2 readmissions for HF within 6 months and those with < 2 HF readmissions within 6 months. Additional secondary endpoints included percentage of patients readmitted for HF within 30 days of the index HF hospitalization, time to readmission if applicable, time to death if applicable, and average number of readmissions per patient within 6 months.
Index data collected included age, gender, ethnicity, prior diagnosis of HF, date of diagnosis, hospitalization for HF within 30 days of the index HF admission, in-hospital cardiac arrest, comorbid conditions, systolic blood pressure (BP), heart rate, respiratory rate, weight, serum sodium, blood urea nitrogen, serum creatinine, hematocrit, and glucose. For this study, comorbid conditions gathered were diabetes mellitus, coronary artery disease, prior percutaneous coronary intervention, aortic stenosis, stroke, chronic obstructive pulmonary disease, and dementia.
Medication profiles were reviewed at the time of admission to determine whether the patient was prescribed an ACE-I/ARB, ß-blocker, diuretic, hydralazine and isosorbide dinitrate, aldosterone antagonist, digoxin, NSAIDs, nonvasoselective calcium channel blocker, and an antiarrhytmic other than amiodarone and dofetilide. Hospitalization data included the most recent LVEF, the number of days on oral diuretic therapy after stopping IV diuretics, the number of days admitted, and documentation of an in-person inpatient dietitian consultation.
Data collected at discharge included diet/weight/medication instructions, weight, BP, American College of Cardiology/American Heart Association HF stage and New York Heart Association (NYHA) HF functional class, if documented. Discharge medication profiles were assessed for the number of medications (< 9 or ≥ 9), documentation of active prescriptions for an ACE-I/ARB and a ß-blocker (or contraindication documented), diuretic, hydralazine and isosorbide dinitrate, aldosterone antagonist, and digoxin. Other data collected were documentation of a scheduled follow-up appointment with primary care physician, urgent care, chronic HF (CHF) clinic, or cardiologist, and whether the patient was discharged to home, skilled nursing facility, shelter, or homeless. Additionally, if the patient was discharged on a diuretic, the dose was compared with the baseline diuretic. If the diuretic at discharge was different from the home diuretic, equivalent doses were used for comparison with that of the baseline diuretic.
Postdischarge data collection included telephone follow-up within 48 hours of discharge, medication compliance since the initial hospitalization, date of first outpatient follow-up after initial hospital discharge, enrollment in CHF clinic/CHF-PharmD/Care Coordination Home Telehealth (CCHT) program, outpatient dietitian consultations, and date of death if applicable. Medication adherence was defined as ≥ 80% of lowest percentage filled medication of all HF medications, determined by the refill history in the computerized patient record system (CPRS). First outpatient follow-up was defined as a visit in which HF was addressed in the assessment and plan.
If readmitted within the study period, data collection included the date of first nonelective hospital readmission for HF, BP, heart rate, weight, serum digoxin level, serum creatinine, serum potassium, and whether the patient was on a target dose of HF recommended medications (if LVEF < 40% and no contraindication). Heart failure recommended medications for which target doses are established include ACE-I/ARB and ß-blockers. For this study, target doses of ACE-Is were captopril 50 mg 3 times daily, enalapril 10 mg twice daily, fosinopril 40 mg daily, lisinopril 20 mg daily, ramipril 10 mg daily, and trandolapril 4 mg daily. Target doses for ARBs were candesartan 32 mg daily, losartan 50 mg daily, and valsartan 160 mg twice daily. ß-blocker target doses were bisoprolol 10 mg daily, carvedilol 25 mg twice daily (50 mg twice daily if patients’ weight was > 85 kg), and metoprolol succinate 200 mg daily.5,6 A statistical analysis was not performed on the data.
RESULTS
A total of 137 patient charts were reviewed, and 109 patients were included in the study. Patients were excluded if they transferred to or from an outside hospital (n = 8), had no follow-up at JBVAMC (n = 8), left the hospital against medical advice (n = 4), were electively admitted (n = 4), were not treated for HF (n = 3), or only had comfort measures documented in the chart (n = 1). The patients included were predominantly male (99%) and African American (78%) and had a mean age of 70 years. The majority of the patients had a prior diagnosis of HF (87%) and a history of systolic HF (58%). Most patients were previously prescribed an ACE-I/ARB (83%) and a ß-blocker (76%) at the time of admission (Table 1).
Six patients were readmitted within 30 days of the index hospitalization, whereas 103 patients were readmitted after 30 days or not at all. With respect to secondary endpoints, there were 21 patients readmitted within 90 days of the index hospitalization, whereas 88 patients were readmitted after 90 days or not at all. Additionally, 6 patients were readmitted ≥ 2 times within 6 months of the index hospitalization, whereas 103 patients were readmitted < 2 times within 6 months.
Baseline characteristics seemed similar across the study groups, except a greater percentage of patients readmitted within 30 days of the index HF hospitalization had a prior history of systolic HF and were hospitalized for HF 30 days prior to the index hospitalization (Table 2). In addition, patients readmitted within 30 days tended to receive a shorter duration of oral diuretic therapy after discontinuation of IV diuretics (mean 0.2 days vs 1.1 days). Patients in this group with an LVEF < 40% were less likely to be discharged on an ACE-I/ARB (75% vs 95%) and a ß-blocker (50% vs 85%) than were the patients who were readmitted after 30 days or not at all. These trends continued for patients readmitted within 90 days of the index hospitalization and for those readmitted after 90 days or not at all. The mean length of stay for the index HF hospitalization was about 5 days and was comparable among all study groups.
From the evaluation of postdischarge characteristics, no patients readmitted within 30 days had a follow-up appointment scheduled with the CHF clinic. In comparison with patients readmitted after 30 days or not at all, more patients had follow-up at an urgent care clinic (33% vs 6%) or no follow-up appointment scheduled at the time of discharge (17% vs 2%). Half of all the patients with a scheduled follow-up missed their appointment. Additionally, medication adherence was lower (33% vs 80%), and none of the patients were enrolled in the CHF-PharmD clinic (0% vs 5%). A similar trend continued for the secondary endpoint groups (Table 3). Last, none of the study patients had an outpatient dietitian consultation.
On readmission, the majority of patients readmitted within 30 days were not on a target dose of an ACE-I/ARB (75%), and none were on a target dose of a ß-blocker. The same trend continued for the secondary endpoint groups. None of the study patients had a serum digoxin level > 0.9 ng/mL. However, serum digoxin level was not measured in all readmitted patients prescribed digoxin (Table 4).
In regard to other secondary endpoints, 6 patients (5.5%) were readmitted for HF within 30 days of the index HF hospitalization. The average number of readmissions per patient in 6 months was < 1, mean time to readmission was 85 days (n = 33), and mean time to death was 88 days (n = 5) when applicable.
DISCUSSION
Based on the trends observed in this study, multiple recommendations can be made to further improve the quality of care and reduce HF readmissions at JBVAMC. The medical center physicians currently use a discharge note template, which already includes sections such as HF discharge instructions and follow-up appointments. The template also prompts providers to prescribe an ACE-I in appropriate patients.
When JBVAMC providers are ready to enter discharge notes into the CPRS, they first select the discharge note template from available note template options. The electronic template contains spaces for the provider to enter a patient’s primary reason for hospitalization, date of admission, discharge medication list, specific or suggested dates for follow-up with outpatient provider(s), general diet/weight/medication instructions, a space to answer whether the patient has HF, a space to record NYHA HF class if applicable, and a space to record whether the patient is prescribed or will be prescribed an ACE-I if appropriate, or whether ACE-I is contraindicated. The providers are able to modify and add information to the discharge note template as they see appropriate.
The findings of this study suggest that modifying the existing discharge template to include additional provider prompts in a form of designated spaces asking for specific information may help improve HF care outcomes. If providers are prompted to answer whether an oral diuretic was continued for at least 24 hours after stopping IV diuretics for HF, adherence to the HF guideline-recommended duration of oral diuretic therapy may improve. Additionally, ß-blocker prescribing in appropriate systolic HF patients may increase if providers are prompted. To enhance continuity of care, the discharge note template may be modified to include a section in which the providers can document patients followed by outside providers. This can be done by incorporating a space in the discharge template to enter the patient’s non-VA provider information if applicable and may help further coordinate the care of such patients to ensure that they are not lost.
Furthermore, the discharge template may be modified to include a prompt to place a CHF clinic consult to increase provider awareness about the availability of CHF and CHF-PharmD clinics at JBVAMC. CHF and CHF-PharmD clinics collaborate to provide comprehensive care to HF patients. After an initial evaluation at the CHF clinic, patients are referred to the clinical pharmacist for further medication therapy management when necessary. Currently, the physicians are encouraged to refer HF patients to the CHF clinic after discharge, but not all providers know that such a service is available. The prompt within the discharge note template would provide CHF/CHF-PharmD clinic provider contact information, clinic times, and a link that would take the provider to an appropriate screen for placing the consult.
Limitations
There are several limitations to this study, including its retrospective design and small sample size. Another source of potential study limitation was the initial process for creating a study patient list. The study list was designed to use ICD-9 codes to capture readmissions only for HF and only at JBVAMC. This was achieved by specifying any of the HF ICD-9 codes as the principal discharge diagnosis. However, the providers may not have always used a HF specific ICD-9 code for the principal discharge diagnosis, even if a patient was admitted primarily for HF. The provider may have chosen another principal discharge diagnosis for which the patient received treatment during the hospitalization.
There are multiple ways to obtain HF patient lists, one includes using the diagnosis-related group codes instead of ICD-9 codes. Due to the way the patient list was obtained and an inherent possibility that some patients admitted for HF had a non-HF ICD-9 code recorded as their principal discharge diagnosis, some eligible patients may not have appeared on the generated list. Additionally, this study captured readmission rates for only HF whereas the national HF 30-day readmission rate represents all-cause readmissions for HF patients. This difference may be reflected in the low 30-day readmission rate observed.
Another possible limitation was the timing of the launch of the CHF-PharmD clinic and the initiative for telephone follow-up 48 hours postdischarge. The CHF-PharmD clinic was launched in April 2011, and the initiative for telephone follow-up 48 hours postdischarge began in January 2011. As the start dates fell within the study period, these services may not have been available to all patients. Therefore, the data describing patient enrollment in CHF-PharmD clinic and those who received postdischarge telephone follow-up may not accurately reflect current practice. Last, statistical tests were not used in the study data analysis leaving any differences found open to interpretation. To minimize these limitations, larger prospective studies with statistical analysis capturing all-cause readmissions are necessary to further evaluate patient characteristics that may be contributing to HF readmissions at JBVAMC.
Conclusions
In general, earlier and more frequent readmissions were more common in patients who were converted to oral diuretic therapy for < 24 hours before discharge and were not discharged on an ACE-I/ARB and a b-blocker when appropriate. Additionally, most of the readmitted patients had no follow-up scheduled at discharge, were nonadherent with medications and follow-up appointments, and were not enrolled in the CHF and/or CHF-PharmD clinic. The majority of patients with systolic HF were not at target doses of either the ACE-I/ARB or the ß-blocker when readmitted. Overall, JBVAMC had a low percentage of patients readmitted for HF within 30 days, but there is still room for improvement in reducing HF readmissions.
At the time of discharge, all JBVAMC patients receive printed instructions and recommendations for their care after hospitalization. The patient handout includes the most current medications, diet/weight/medication instructions, and actual or suggested dates for follow-up appointments and/or tests. It may enhance awareness regarding dietician services to patients if the current discharge instruction template can be modified to provide information regarding the outpatient dietitian class. This could include date, time, and location of classes as well as dietician contact information. (See Appendixes 1 and 2.)
When these recommendations have been implemented, further studies will be warranted to assess the impact of the interventions.
Acknowledgments
The authors thank Ms. Yvette Bloodson for her assistance in generating the initial patient list.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2011 Update. A Report from the American Heart Association. Circulation. 2011;123(4):e18-e209.
2. National Heart, Lung, and Blood Institute. Incidence and Prevalence: 2009 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Institutes of Health; 2009.
3. Ross JS, Chen J, Lin ZQ, et al. Recent national trends in readmission rates after HF hospitalization. Circ Heart Fail. 2010;3(1):97-103.
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
5. Hunt SA, Abraham WT, Chin MH., et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391-e479.
6. Lindenfeld J, Albert NM, Boehmer JP, et al. Executive Summary: HFSA 2010 Comprehensive HF Practice Guideline. J Card Fail. 2010;16(6):475-539.
7. U.S. Department of Health and Human Services. Hospital Compare. https://data.medicare.gov/data/archives/hospital-compare. Updated August 22, 2011. Accessed October 15, 2014.
8. Heidenreich PA. Chronic HF QUERI Center Application: Strategic Plan 2009. U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI) Website. http://www.queri.research.va.gov/about/strategic_plans/chf.pdf. Updated August 22, 2011. Accessed September 2, 2014.
9. U.S. Department of Veterans Affairs. Chronic HF Quality Enhancement Research Initiative: VA Hospital to Home (H2H) Initiative. U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI) Website. http://www.queri.research.va.gov/chf/products/h2h. Updated August 19, 2011. Accessed September 2, 2014.
1. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2011 Update. A Report from the American Heart Association. Circulation. 2011;123(4):e18-e209.
2. National Heart, Lung, and Blood Institute. Incidence and Prevalence: 2009 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Institutes of Health; 2009.
3. Ross JS, Chen J, Lin ZQ, et al. Recent national trends in readmission rates after HF hospitalization. Circ Heart Fail. 2010;3(1):97-103.
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
5. Hunt SA, Abraham WT, Chin MH., et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391-e479.
6. Lindenfeld J, Albert NM, Boehmer JP, et al. Executive Summary: HFSA 2010 Comprehensive HF Practice Guideline. J Card Fail. 2010;16(6):475-539.
7. U.S. Department of Health and Human Services. Hospital Compare. https://data.medicare.gov/data/archives/hospital-compare. Updated August 22, 2011. Accessed October 15, 2014.
8. Heidenreich PA. Chronic HF QUERI Center Application: Strategic Plan 2009. U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI) Website. http://www.queri.research.va.gov/about/strategic_plans/chf.pdf. Updated August 22, 2011. Accessed September 2, 2014.
9. U.S. Department of Veterans Affairs. Chronic HF Quality Enhancement Research Initiative: VA Hospital to Home (H2H) Initiative. U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI) Website. http://www.queri.research.va.gov/chf/products/h2h. Updated August 19, 2011. Accessed September 2, 2014.