User login
Medical Management of Ectopic Pregnancy: Early Diagnosis is Key
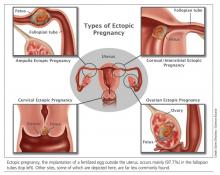
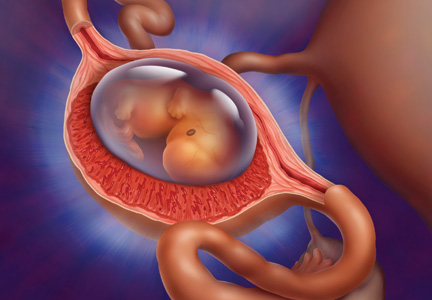
Ectopic pregnancy is a significant health risk to women during their childbearing years; approximately 6% of all pregnancy-related deaths are due to ectopic pregnancy.1-3 Some 1% to 2% of all pregnancies in the United States each year—approximately 100,000 cases—are ectopic, with an estimated annual cost of care approaching $1.1 billion.4 The incidence of ectopic pregnancy has increased in the past 20 years; in one analysis, ectopic pregnancy was diagnosed in 18% of women who presented to an emergency department (ED) with first trimester vaginal bleeding, abdominal pain, or both.5 This growing prevalence is attributed to a number of factors, including the sensitivity of current diagnostic methods in detecting early ectopic pregnancy, the greater incidence of salpingitis, and the growing use of assisted reproductive technologies.2,6
While the number of ectopic pregnancies is on the rise, the proportion of patients requiring hospitalization for surgical treatment of ectopic pregnancy has decreased significantly. Today, for appropriate patients, many clinicians manage ectopic pregnancy on an outpatient basis using the drug methotrexate.6
In this article, we will present an overview of the current status of medical management of ectopic pregnancy, along with a case study. The case study describes a patient diagnosed with an unruptured ectopic pregnancy who was managed medically with methotrexate. It illustrates how, with early diagnosis, clinicians can intervene to make medical management an effective treatment option in selected situations.
The patient reported a history of oral contraceptive use until approximately three months prior to this pregnancy. She was taking no medications and had no known drug allergies. Her previous pregnancies included two uncomplicated vaginal births at term and one miscarriage at six to seven weeks’ gestation two years ago. She also reported a dilation and curettage after the miscarriage. Her medical, surgical, and gynecologic histories were otherwise noncontributory. A review of systems was otherwise negative.
Sexual history revealed that the patient was married and monogamous with her husband of five years. She disclosed four previous sexual partners and inconsistent use of condoms with those partners; no current condom use was reported. Seven years ago, she tested positive for gonorrhea and chlamydia and was treated concurrently with her partner. Subsequent diagnostics were negative. She reported vaginal intercourse but no oral sex and denied any other sexual contact. All partners had been male.
On the next page: Diagnosis and case continuation >>
DIAGNOSIS
There is some variation in the presentation of women experiencing ectopic pregnancy; this may be due to differences in the pathologic mechanisms of ectopic pregnancy. Patients may be asymptomatic, hemodynamically compromised, or somewhere in between.3 Typical clinical signs include abdominal pain, amenorrhea, and vaginal bleeding. Approximately 40% to 50% of patients present with vaginal bleeding, 50% may have a palpable adnexal mass, and 75% may have abdominal tenderness.3 Only about 50% of women with ectopic pregnancies present with these typical symptoms.3
The patient may also experience common symptoms of early pregnancy, such as nausea, fatigue, and breast fullness. Worrisome signs and symptoms, including abdominal guarding, hypotension, tachycardia, shock, shoulder pain from peritoneal irritation, dizziness, fever, and vomiting, may also be present.3,7 Approximately 20% of patients with ectopic pregnancies are hemodynamically compromised at presentation, which is highly suggestive of rupture.3
Risk factors
Risk factors for ectopic pregnancy include previous ectopic pregnancy; previous tubal procedures; history of sexually transmitted disease or genital infections; infertility; use of assisted reproductive technology; previous abdominal or pelvic surgery; smoking; pelvic inflammatory disease; exposure in utero to diethylstilbestrol; and previous intrauterine device use.2,5,7,8 Knowledge of these risk factors can help identify a patient with an ectopic pregnancy.
The diagnosis of ectopic pregnancy is most certainly a clinical challenge. The differential diagnosis is based upon history and physical findings; the list can be lengthy if both vaginal bleeding and abdominal pain (nonspecific symptoms common in women who miscarry) are present.7 Prompt completion of diagnostic testing is critical in making a definitive diagnosis. Possible diagnoses are listed in Table 1.
CASE Upon examination, the patient appeared comfortable and relaxed, and there were no signs of distress. Blood pressure was 100/65 mm Hg, pulse rate was 72 beats/min, and temperature was 99.0°F. There was no tenderness upon abdominal examination. Pelvic examination revealed a small amount of brown vaginal discharge but no active bleeding or pooled blood, clots, or tissue. The cervical os was closed, and positive Chadwick sign was present. Bimanual examination revealed no cervical motion tenderness. The uterus was soft, mobile, and nontender, and consistent in size with a gestation at eight weeks. There were no palpable adnexa, ovaries, or masses. There was no pain with bimanual examination and no evidence of tenderness at the posterior fornix. The remainder of the physical examination was unremarkable.
It is important to note that examination results in the case patient are not unusual in a woman with a small, unruptured ectopic pregnancy. All findings were normal except for the scant brown vaginal discharge. Abdominal and adnexal tenderness are common, as is a palpable adnexal mass; but absence of a detectable mass does not exclude ectopic pregnancy.1 Pathologic findings may include severe abdominal tenderness and pain, significant vaginal bleeding, passage of clots, tachycardia, and orthostatic hypotension.
Diagnostic workup
Laboratory tests are critical to making an accurate diagnosis for women whose history and physical examination results are consistent with ectopic pregnancy. Assessment for ectopic pregnancy should include a urine pregnancy test, transvaginal ultrasound, measurement of serum ß-human chorionic gonadotropin (ß-hCG) level, and occasionally, diagnostic curettage.1 Once the diagnosis is confirmed, a complete blood count (CBC) is necessary to assess anemia and platelet functioning. Coagulation tests may be required for worrisome bleeding. Blood type, Rh status, and antibody screen are also necessary to determine whether a patient who is Rh D-negative will require Rh immune globulin. See Table 2 for the patient’s laboratory test results.
In a patient with a ß-hCG level greater than the discriminatory cutoff value of 1,500 to 1,800 mIU/mL, the level above which an intrauterine gestational sac is visible on transvaginal ultrasound in a normal pregnancy, an empty uterus is considered an ectopic pregnancy until proven otherwise.3 In a definite intrauterine pregnancy of about six weeks’ gestation, transvaginal ultrasound reveals a gestational sac that contains a yolk sac and a fetal pole.3
CASE The patient’s presenting symptoms, combined with a positive pregnancy test, ß-hCG level of 1,850 mIU/mL, and a complex adnexal mass in the right fallopian tube, were highly suggestive of an unruptured ectopic pregnancy (see Table 3 for the patient’s transvaginal ultrasound findings). There was also a secondary finding of a corpus luteum cyst. Other diagnoses were ruled out, and the patient was diagnosed with an unruptured ectopic pregnancy.
On the next page: Treatment >>
TREATMENT
A patient with an ectopic pregnancy who presents with pain and hemodynamic instability should be referred immediately for appropriate surgical care.7 Otherwise, once the diagnosis of ectopic pregnancy is confirmed, the patient should be referred to an obstetric specialist. Treatments for ectopic pregnancy include expectant management and surgery—which will be discussed briefly—and medical management, which is the focus of this review.5
Expectant management
Most ectopic pregnancies are diagnosed early as a result of accurate, minimally invasive and noninvasive diagnostic tools and greater awareness of risk factors. Since the natural course of early ectopic pregnancy is often self-limited, eventually resulting in tubal abortion or reabsorption, expectant management is a viable option.9
This treatment option may be considered if the patient is asymptomatic; ß-hCG is < 200 mIU/mL; the ectopic mass is < 3 cm; and no fetal heartbeat is present.1,2 With this approach, patients must be willing to accept the risk for tubal rupture and agree to close monitoring of ß-hCG levels. The ß-hCG level must be measured every 24 to 48 hours in order to determine if it is declining adequately, plateauing, or increasing.2,5
Surgery
For the hemodynamically unstable patient, the treatment decision is relatively straightforward. Optimal treatment for a ruptured ectopic pregnancy is immediate surgery, which may include salpingostomy or salpingectomy.10 Surgery may also be considered for hemodynamically stable patients with nonruptured ectopic pregnancies; in addition to her clinical presentation, overall management may be driven by a patient’s preferences.5 Salpingostomy and salpingectomy can be performed either laparoscopically or via laparotomy, depending on the specific situation.
Medical management
The use of methotrexate for the management of unruptured ectopic pregnancy was introduced in the early 1980s.11 Initially, protocols called for multiple doses administered during the course of an inpatient stay. Further research led to revised treatment recommendations and today, medical management most often consists of a single dose of methotrexate with outpatient follow-up.3
Methotrexate is a folic acid antagonist often used as an antimetabolite chemotherapeutic agent. In ectopic pregnancy, it inhibits growth of the rapidly dividing trophoblastic cells and ultimately ends the pregnancy.2 Outcomes of medical management are comparable to those of surgical treatment, including the potential for future normal pregnancies.2,5
An analysis of US trends in ectopic pregnancy management from 2002-2007 revealed that the use of methotrexate increased from 11.1% to 35.1% during that time, while the use of surgical approaches declined from 90% to 65%.10 Medical management of ectopic pregnancy eliminates the costs of surgery, anesthesia, and hospitalization and avoids potential complications of surgery and anesthesia.
Appropriate candidates
A hemodynamically stable patient with a confirmed or high clinical suspicion of ectopic pregnancy, an unruptured mass, no active bleeding, and low ß-hCG levels (< 5,000 mIU/mL) can be considered for methotrexate therapy.2,3,9 It is critical that medically managed patients be willing and able to adhere to all follow-up appointments.9 Before initiating treatment, normal serum creatinine and transaminase levels should be confirmed, and there should be no evidence of significant anemia, leukopenia, or thrombocytopenia.2 To detect any adverse effects of methotrexate on renal, hepatic, and hematologic functioning, these tests are repeated one week after administration.2
Contraindications
Contraindications to methotrexate treatment include breastfeeding, immunodeficiency, alcoholism, alcoholic liver disease or other chronic liver disease, preexisting blood dyscrasias (eg, bone marrow hypoplasia, leukopenia, thrombocytopenia, or significant anemia), known sensitivity to methotrexate, active pulmonary disease, peptic ulcer, and hepatic, renal, or hematologic dysfunction. Relative contraindications are a gestational sac larger than 3.5 cm and embryonic cardiac motion.2
On the next page: Patient education >>
PATIENT EDUCATION AND INFORMED CONSENT
A diagnosis of unruptured ectopic pregnancy requires patient education about the condition and its treatment options. The clinician should explain what an ectopic pregnancy is and distinguish between unruptured and ruptured. A discussion of the benefits and risks of each treatment option for which the patient is an appropriate candidate, as well as what to anticipate during treatment, is needed. Emotional support for impending pregnancy loss should also be provided.
For patients who choose medical management, education includes methotrexate-specific information and written instructions to follow after methotrexate administration. Patients must be instructed about the use of safety precautions after treatment (eg, the toilet should be double-flushed with the lid closed during the first 72 hours after treatment to prevent exposing others to methotrexate in urine and stool), the need for adherence to follow-up visits, and warning signs of a possible rupture.5 These warning signs are listed in Table 4.
The most common adverse effects of methotrexate are gastrointestinal (nausea, vomiting, stomatitis). Patients should be advised to avoid alcohol, NSAIDs, folic acid supplements, excessive sun exposure (due to photosensitivity), strenuous exercise, and sexual intercourse until ß-hCG has returned to nonpregnant levels. Other adverse effects may include a temporary elevation in liver enzymes and rarely, alopecia. Abdominal pain may occur a few days after methotrexate administration, likely from the cytotoxic effects of the drug on the trophoblastic tissue.
Informed consent is required prior to methotrexate administration. The patient must be advised of the potential risks of medical management with methotrexate, including rupture of the ectopic pregnancy during treatment, inadvertent administration of methotrexate in the presence of an early intrauterine embryo, allergic reaction to methotrexate, and methotrexate-induced pneumonitis.5
CASE After lengthy discussion of the treatment options, the patient chose medical management with methotrexate. She verbalized her understanding of the teaching provided and signed an informed consent document.
METHOTREXATE REGIMENS
Protocols for single-dose, two-dose, and fixed multidose methotrexate regimens are described in the medical literature, according to a 2008 American Congress of Obstetricians and Gynecologists practice bulletin.2 A 2013 practice committee opinion of the American Society for Reproductive Medicine (ASRM) indicates that single-dose and multiple-dose regimens are used most often.12
With methotrexate treatment, complete resolution of ectopic pregnancy usually occurs in two to three weeks but may require up to six to eight weeks, depending on how high the ß-hCG level is when treatment begins.12
Single-dose
In the single-dose regimen, an intramuscular (IM) injection of methotrexate 50 mg/m2 is administered on day 1. The ß-hCG levels are measured on days 4 and 7 after administration; a decrease of at least 15% in the ß-hCG level should be observed. The ß-hCG level is then measured weekly until it reaches < 2 mIU/mL or is undetectable.2 If the level does not decline, a repeat dose of methotrexate can be given, with measurement of ß-hCG on days 4 and 7 after the repeat dose. If the ß-hCG level fails to decrease, additional methotrexate or surgical intervention should be considered.
The single-dose regimen is more frequently used and is most successful when ß-hCG levels are low (< 5,000 mIU/mL), the ectopic mass is small
(< 3.5 cm), and embryonic cardiac activity is not observed on ultrasound.2,3 Patients with ß-hCG levels > 5,000 mIU/mL may be appropriate candidates for additional doses of methotrexate.2 In fact, the single-dose protocol provides for repeat doses of methotrexate if the ß-hCG level is not decreasing adequately.12
Multiple-dose
With the multiple-dose regimen, methotrexate 1 mg/kg IM is administered on days 1, 3, 5, and 7; on days 2, 4, 6, and 8, the patient receives leucovorin (folinic acid) 0.1 mg/kg IM. The ß-hCG level is measured on days methotrexate is administered; once the minimum 15% decline is observed, ß-hCG is measured weekly until a nonpregnant level is reached.12
The patient received a single dose of methotrexate 50 mg/m2 IM on day 1 and returned to the clinic for follow-up on days 4 and 7 posttreatment. On day 4, her ß-hCG level was 1,060 mIU/mL; on day 7, it was 470 mIU/mL. Also on day 7, blood was drawn for a CBC and comprehensive metabolic panel; results were within normal limits. The patient continued weekly follow-up until her ß-hCG level decreased to < 2 mIU/mL.
On the next page: Follow-up and conclusion >>
FOLLOW-UP AND REFERRALS
Close monitoring of ß-hCG levels, as described previously, is essential after methotrexate treatment in order to confirm that the pregnancy has been terminated and reduce the risk for tubal rupture. Clinicians should also be sensitive to the sequelae of loss of a pregnancy and refer patients as needed to appropriate health care professionals for grief support.
CASE The patient was referred to an obstetrics clinic and reported for all scheduled follow-up appointments. She was discharged from care after a full reduction in her ß-hCG to nonpregnant levels. While at the clinic, the patient was referred to social services for psychosocial counseling.
CONCLUSION
Ectopic implantation is a serious complication that may occur during the first trimester of pregnancy. Worldwide, it is the leading cause of maternal death in the first trimester. For women who meet specific criteria, outpatient treatment of early ectopic pregnancy with methotrexate avoids surgery and decreases the overall cost of care. Medical management and conservative surgical management offer the patient comparable outcomes for tubal patency preservation and risk for ectopic pregnancy recurrence.11
REFERENCES
1. Lozeau AM, Potter B. Diagnosis and management of ectopic pregnancy. Am Fam Physician. 2005;72(9):1707-1714.
2. American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479-1485.
3. Sepilian VP, Wood E. Ectopic pregnancy. http://emedicine.medscape.com/article/2041923-overview. Medscape. Accessed June 19, 2014.
4. Stein JC, Wang R, Adler N, et al. Emergency physician ultrasonography for evaluating patients at risk for ectopic pregnancy: a meta-analysis. Ann Emerg Med. 2010;56(6):674-683.
5. Murtaza UI, Ortmann MJ, Mando-Vandrick J, Lee ASD. Management of first-trimester complications in the emergency department. Am J Health Syst Pharm. 2013;70(2):99-111.
6. Sewell CA, Cundiff GW. Trends for inpatient treatment of tubal pregnancy in Maryland. Am J Obstet Gynecol. 2002;186(3):404-408.
7. Nama V, Manyonda I. Tubal ectopic pregnancy: diagnosis and management. Arch Gynecol Obstet. 2009;279(4):443-453.
8. Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86(1):36-43.
9. Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;(1):CD000324.
10. Hoover KW, Tao G, Kent CK. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstet Gynecol. 2010;115(3): 495-502.
11. Autry A. Medical treatment of ectopic pregnancy: is there something new? Obstet Gynecol. 2013;122(4):733.
12. The Practice Committee of the American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100(3):638-644.
Ectopic pregnancy is a significant health risk to women during their childbearing years; approximately 6% of all pregnancy-related deaths are due to ectopic pregnancy.1-3 Some 1% to 2% of all pregnancies in the United States each year—approximately 100,000 cases—are ectopic, with an estimated annual cost of care approaching $1.1 billion.4 The incidence of ectopic pregnancy has increased in the past 20 years; in one analysis, ectopic pregnancy was diagnosed in 18% of women who presented to an emergency department (ED) with first trimester vaginal bleeding, abdominal pain, or both.5 This growing prevalence is attributed to a number of factors, including the sensitivity of current diagnostic methods in detecting early ectopic pregnancy, the greater incidence of salpingitis, and the growing use of assisted reproductive technologies.2,6
While the number of ectopic pregnancies is on the rise, the proportion of patients requiring hospitalization for surgical treatment of ectopic pregnancy has decreased significantly. Today, for appropriate patients, many clinicians manage ectopic pregnancy on an outpatient basis using the drug methotrexate.6
In this article, we will present an overview of the current status of medical management of ectopic pregnancy, along with a case study. The case study describes a patient diagnosed with an unruptured ectopic pregnancy who was managed medically with methotrexate. It illustrates how, with early diagnosis, clinicians can intervene to make medical management an effective treatment option in selected situations.
The patient reported a history of oral contraceptive use until approximately three months prior to this pregnancy. She was taking no medications and had no known drug allergies. Her previous pregnancies included two uncomplicated vaginal births at term and one miscarriage at six to seven weeks’ gestation two years ago. She also reported a dilation and curettage after the miscarriage. Her medical, surgical, and gynecologic histories were otherwise noncontributory. A review of systems was otherwise negative.
Sexual history revealed that the patient was married and monogamous with her husband of five years. She disclosed four previous sexual partners and inconsistent use of condoms with those partners; no current condom use was reported. Seven years ago, she tested positive for gonorrhea and chlamydia and was treated concurrently with her partner. Subsequent diagnostics were negative. She reported vaginal intercourse but no oral sex and denied any other sexual contact. All partners had been male.
On the next page: Diagnosis and case continuation >>
DIAGNOSIS
There is some variation in the presentation of women experiencing ectopic pregnancy; this may be due to differences in the pathologic mechanisms of ectopic pregnancy. Patients may be asymptomatic, hemodynamically compromised, or somewhere in between.3 Typical clinical signs include abdominal pain, amenorrhea, and vaginal bleeding. Approximately 40% to 50% of patients present with vaginal bleeding, 50% may have a palpable adnexal mass, and 75% may have abdominal tenderness.3 Only about 50% of women with ectopic pregnancies present with these typical symptoms.3
The patient may also experience common symptoms of early pregnancy, such as nausea, fatigue, and breast fullness. Worrisome signs and symptoms, including abdominal guarding, hypotension, tachycardia, shock, shoulder pain from peritoneal irritation, dizziness, fever, and vomiting, may also be present.3,7 Approximately 20% of patients with ectopic pregnancies are hemodynamically compromised at presentation, which is highly suggestive of rupture.3
Risk factors
Risk factors for ectopic pregnancy include previous ectopic pregnancy; previous tubal procedures; history of sexually transmitted disease or genital infections; infertility; use of assisted reproductive technology; previous abdominal or pelvic surgery; smoking; pelvic inflammatory disease; exposure in utero to diethylstilbestrol; and previous intrauterine device use.2,5,7,8 Knowledge of these risk factors can help identify a patient with an ectopic pregnancy.
The diagnosis of ectopic pregnancy is most certainly a clinical challenge. The differential diagnosis is based upon history and physical findings; the list can be lengthy if both vaginal bleeding and abdominal pain (nonspecific symptoms common in women who miscarry) are present.7 Prompt completion of diagnostic testing is critical in making a definitive diagnosis. Possible diagnoses are listed in Table 1.
CASE Upon examination, the patient appeared comfortable and relaxed, and there were no signs of distress. Blood pressure was 100/65 mm Hg, pulse rate was 72 beats/min, and temperature was 99.0°F. There was no tenderness upon abdominal examination. Pelvic examination revealed a small amount of brown vaginal discharge but no active bleeding or pooled blood, clots, or tissue. The cervical os was closed, and positive Chadwick sign was present. Bimanual examination revealed no cervical motion tenderness. The uterus was soft, mobile, and nontender, and consistent in size with a gestation at eight weeks. There were no palpable adnexa, ovaries, or masses. There was no pain with bimanual examination and no evidence of tenderness at the posterior fornix. The remainder of the physical examination was unremarkable.
It is important to note that examination results in the case patient are not unusual in a woman with a small, unruptured ectopic pregnancy. All findings were normal except for the scant brown vaginal discharge. Abdominal and adnexal tenderness are common, as is a palpable adnexal mass; but absence of a detectable mass does not exclude ectopic pregnancy.1 Pathologic findings may include severe abdominal tenderness and pain, significant vaginal bleeding, passage of clots, tachycardia, and orthostatic hypotension.
Diagnostic workup
Laboratory tests are critical to making an accurate diagnosis for women whose history and physical examination results are consistent with ectopic pregnancy. Assessment for ectopic pregnancy should include a urine pregnancy test, transvaginal ultrasound, measurement of serum ß-human chorionic gonadotropin (ß-hCG) level, and occasionally, diagnostic curettage.1 Once the diagnosis is confirmed, a complete blood count (CBC) is necessary to assess anemia and platelet functioning. Coagulation tests may be required for worrisome bleeding. Blood type, Rh status, and antibody screen are also necessary to determine whether a patient who is Rh D-negative will require Rh immune globulin. See Table 2 for the patient’s laboratory test results.
In a patient with a ß-hCG level greater than the discriminatory cutoff value of 1,500 to 1,800 mIU/mL, the level above which an intrauterine gestational sac is visible on transvaginal ultrasound in a normal pregnancy, an empty uterus is considered an ectopic pregnancy until proven otherwise.3 In a definite intrauterine pregnancy of about six weeks’ gestation, transvaginal ultrasound reveals a gestational sac that contains a yolk sac and a fetal pole.3
CASE The patient’s presenting symptoms, combined with a positive pregnancy test, ß-hCG level of 1,850 mIU/mL, and a complex adnexal mass in the right fallopian tube, were highly suggestive of an unruptured ectopic pregnancy (see Table 3 for the patient’s transvaginal ultrasound findings). There was also a secondary finding of a corpus luteum cyst. Other diagnoses were ruled out, and the patient was diagnosed with an unruptured ectopic pregnancy.
On the next page: Treatment >>
TREATMENT
A patient with an ectopic pregnancy who presents with pain and hemodynamic instability should be referred immediately for appropriate surgical care.7 Otherwise, once the diagnosis of ectopic pregnancy is confirmed, the patient should be referred to an obstetric specialist. Treatments for ectopic pregnancy include expectant management and surgery—which will be discussed briefly—and medical management, which is the focus of this review.5
Expectant management
Most ectopic pregnancies are diagnosed early as a result of accurate, minimally invasive and noninvasive diagnostic tools and greater awareness of risk factors. Since the natural course of early ectopic pregnancy is often self-limited, eventually resulting in tubal abortion or reabsorption, expectant management is a viable option.9
This treatment option may be considered if the patient is asymptomatic; ß-hCG is < 200 mIU/mL; the ectopic mass is < 3 cm; and no fetal heartbeat is present.1,2 With this approach, patients must be willing to accept the risk for tubal rupture and agree to close monitoring of ß-hCG levels. The ß-hCG level must be measured every 24 to 48 hours in order to determine if it is declining adequately, plateauing, or increasing.2,5
Surgery
For the hemodynamically unstable patient, the treatment decision is relatively straightforward. Optimal treatment for a ruptured ectopic pregnancy is immediate surgery, which may include salpingostomy or salpingectomy.10 Surgery may also be considered for hemodynamically stable patients with nonruptured ectopic pregnancies; in addition to her clinical presentation, overall management may be driven by a patient’s preferences.5 Salpingostomy and salpingectomy can be performed either laparoscopically or via laparotomy, depending on the specific situation.
Medical management
The use of methotrexate for the management of unruptured ectopic pregnancy was introduced in the early 1980s.11 Initially, protocols called for multiple doses administered during the course of an inpatient stay. Further research led to revised treatment recommendations and today, medical management most often consists of a single dose of methotrexate with outpatient follow-up.3
Methotrexate is a folic acid antagonist often used as an antimetabolite chemotherapeutic agent. In ectopic pregnancy, it inhibits growth of the rapidly dividing trophoblastic cells and ultimately ends the pregnancy.2 Outcomes of medical management are comparable to those of surgical treatment, including the potential for future normal pregnancies.2,5
An analysis of US trends in ectopic pregnancy management from 2002-2007 revealed that the use of methotrexate increased from 11.1% to 35.1% during that time, while the use of surgical approaches declined from 90% to 65%.10 Medical management of ectopic pregnancy eliminates the costs of surgery, anesthesia, and hospitalization and avoids potential complications of surgery and anesthesia.
Appropriate candidates
A hemodynamically stable patient with a confirmed or high clinical suspicion of ectopic pregnancy, an unruptured mass, no active bleeding, and low ß-hCG levels (< 5,000 mIU/mL) can be considered for methotrexate therapy.2,3,9 It is critical that medically managed patients be willing and able to adhere to all follow-up appointments.9 Before initiating treatment, normal serum creatinine and transaminase levels should be confirmed, and there should be no evidence of significant anemia, leukopenia, or thrombocytopenia.2 To detect any adverse effects of methotrexate on renal, hepatic, and hematologic functioning, these tests are repeated one week after administration.2
Contraindications
Contraindications to methotrexate treatment include breastfeeding, immunodeficiency, alcoholism, alcoholic liver disease or other chronic liver disease, preexisting blood dyscrasias (eg, bone marrow hypoplasia, leukopenia, thrombocytopenia, or significant anemia), known sensitivity to methotrexate, active pulmonary disease, peptic ulcer, and hepatic, renal, or hematologic dysfunction. Relative contraindications are a gestational sac larger than 3.5 cm and embryonic cardiac motion.2
On the next page: Patient education >>
PATIENT EDUCATION AND INFORMED CONSENT
A diagnosis of unruptured ectopic pregnancy requires patient education about the condition and its treatment options. The clinician should explain what an ectopic pregnancy is and distinguish between unruptured and ruptured. A discussion of the benefits and risks of each treatment option for which the patient is an appropriate candidate, as well as what to anticipate during treatment, is needed. Emotional support for impending pregnancy loss should also be provided.
For patients who choose medical management, education includes methotrexate-specific information and written instructions to follow after methotrexate administration. Patients must be instructed about the use of safety precautions after treatment (eg, the toilet should be double-flushed with the lid closed during the first 72 hours after treatment to prevent exposing others to methotrexate in urine and stool), the need for adherence to follow-up visits, and warning signs of a possible rupture.5 These warning signs are listed in Table 4.
The most common adverse effects of methotrexate are gastrointestinal (nausea, vomiting, stomatitis). Patients should be advised to avoid alcohol, NSAIDs, folic acid supplements, excessive sun exposure (due to photosensitivity), strenuous exercise, and sexual intercourse until ß-hCG has returned to nonpregnant levels. Other adverse effects may include a temporary elevation in liver enzymes and rarely, alopecia. Abdominal pain may occur a few days after methotrexate administration, likely from the cytotoxic effects of the drug on the trophoblastic tissue.
Informed consent is required prior to methotrexate administration. The patient must be advised of the potential risks of medical management with methotrexate, including rupture of the ectopic pregnancy during treatment, inadvertent administration of methotrexate in the presence of an early intrauterine embryo, allergic reaction to methotrexate, and methotrexate-induced pneumonitis.5
CASE After lengthy discussion of the treatment options, the patient chose medical management with methotrexate. She verbalized her understanding of the teaching provided and signed an informed consent document.
METHOTREXATE REGIMENS
Protocols for single-dose, two-dose, and fixed multidose methotrexate regimens are described in the medical literature, according to a 2008 American Congress of Obstetricians and Gynecologists practice bulletin.2 A 2013 practice committee opinion of the American Society for Reproductive Medicine (ASRM) indicates that single-dose and multiple-dose regimens are used most often.12
With methotrexate treatment, complete resolution of ectopic pregnancy usually occurs in two to three weeks but may require up to six to eight weeks, depending on how high the ß-hCG level is when treatment begins.12
Single-dose
In the single-dose regimen, an intramuscular (IM) injection of methotrexate 50 mg/m2 is administered on day 1. The ß-hCG levels are measured on days 4 and 7 after administration; a decrease of at least 15% in the ß-hCG level should be observed. The ß-hCG level is then measured weekly until it reaches < 2 mIU/mL or is undetectable.2 If the level does not decline, a repeat dose of methotrexate can be given, with measurement of ß-hCG on days 4 and 7 after the repeat dose. If the ß-hCG level fails to decrease, additional methotrexate or surgical intervention should be considered.
The single-dose regimen is more frequently used and is most successful when ß-hCG levels are low (< 5,000 mIU/mL), the ectopic mass is small
(< 3.5 cm), and embryonic cardiac activity is not observed on ultrasound.2,3 Patients with ß-hCG levels > 5,000 mIU/mL may be appropriate candidates for additional doses of methotrexate.2 In fact, the single-dose protocol provides for repeat doses of methotrexate if the ß-hCG level is not decreasing adequately.12
Multiple-dose
With the multiple-dose regimen, methotrexate 1 mg/kg IM is administered on days 1, 3, 5, and 7; on days 2, 4, 6, and 8, the patient receives leucovorin (folinic acid) 0.1 mg/kg IM. The ß-hCG level is measured on days methotrexate is administered; once the minimum 15% decline is observed, ß-hCG is measured weekly until a nonpregnant level is reached.12
The patient received a single dose of methotrexate 50 mg/m2 IM on day 1 and returned to the clinic for follow-up on days 4 and 7 posttreatment. On day 4, her ß-hCG level was 1,060 mIU/mL; on day 7, it was 470 mIU/mL. Also on day 7, blood was drawn for a CBC and comprehensive metabolic panel; results were within normal limits. The patient continued weekly follow-up until her ß-hCG level decreased to < 2 mIU/mL.
On the next page: Follow-up and conclusion >>
FOLLOW-UP AND REFERRALS
Close monitoring of ß-hCG levels, as described previously, is essential after methotrexate treatment in order to confirm that the pregnancy has been terminated and reduce the risk for tubal rupture. Clinicians should also be sensitive to the sequelae of loss of a pregnancy and refer patients as needed to appropriate health care professionals for grief support.
CASE The patient was referred to an obstetrics clinic and reported for all scheduled follow-up appointments. She was discharged from care after a full reduction in her ß-hCG to nonpregnant levels. While at the clinic, the patient was referred to social services for psychosocial counseling.
CONCLUSION
Ectopic implantation is a serious complication that may occur during the first trimester of pregnancy. Worldwide, it is the leading cause of maternal death in the first trimester. For women who meet specific criteria, outpatient treatment of early ectopic pregnancy with methotrexate avoids surgery and decreases the overall cost of care. Medical management and conservative surgical management offer the patient comparable outcomes for tubal patency preservation and risk for ectopic pregnancy recurrence.11
REFERENCES
1. Lozeau AM, Potter B. Diagnosis and management of ectopic pregnancy. Am Fam Physician. 2005;72(9):1707-1714.
2. American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479-1485.
3. Sepilian VP, Wood E. Ectopic pregnancy. http://emedicine.medscape.com/article/2041923-overview. Medscape. Accessed June 19, 2014.
4. Stein JC, Wang R, Adler N, et al. Emergency physician ultrasonography for evaluating patients at risk for ectopic pregnancy: a meta-analysis. Ann Emerg Med. 2010;56(6):674-683.
5. Murtaza UI, Ortmann MJ, Mando-Vandrick J, Lee ASD. Management of first-trimester complications in the emergency department. Am J Health Syst Pharm. 2013;70(2):99-111.
6. Sewell CA, Cundiff GW. Trends for inpatient treatment of tubal pregnancy in Maryland. Am J Obstet Gynecol. 2002;186(3):404-408.
7. Nama V, Manyonda I. Tubal ectopic pregnancy: diagnosis and management. Arch Gynecol Obstet. 2009;279(4):443-453.
8. Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86(1):36-43.
9. Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;(1):CD000324.
10. Hoover KW, Tao G, Kent CK. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstet Gynecol. 2010;115(3): 495-502.
11. Autry A. Medical treatment of ectopic pregnancy: is there something new? Obstet Gynecol. 2013;122(4):733.
12. The Practice Committee of the American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100(3):638-644.
Ectopic pregnancy is a significant health risk to women during their childbearing years; approximately 6% of all pregnancy-related deaths are due to ectopic pregnancy.1-3 Some 1% to 2% of all pregnancies in the United States each year—approximately 100,000 cases—are ectopic, with an estimated annual cost of care approaching $1.1 billion.4 The incidence of ectopic pregnancy has increased in the past 20 years; in one analysis, ectopic pregnancy was diagnosed in 18% of women who presented to an emergency department (ED) with first trimester vaginal bleeding, abdominal pain, or both.5 This growing prevalence is attributed to a number of factors, including the sensitivity of current diagnostic methods in detecting early ectopic pregnancy, the greater incidence of salpingitis, and the growing use of assisted reproductive technologies.2,6
While the number of ectopic pregnancies is on the rise, the proportion of patients requiring hospitalization for surgical treatment of ectopic pregnancy has decreased significantly. Today, for appropriate patients, many clinicians manage ectopic pregnancy on an outpatient basis using the drug methotrexate.6
In this article, we will present an overview of the current status of medical management of ectopic pregnancy, along with a case study. The case study describes a patient diagnosed with an unruptured ectopic pregnancy who was managed medically with methotrexate. It illustrates how, with early diagnosis, clinicians can intervene to make medical management an effective treatment option in selected situations.
The patient reported a history of oral contraceptive use until approximately three months prior to this pregnancy. She was taking no medications and had no known drug allergies. Her previous pregnancies included two uncomplicated vaginal births at term and one miscarriage at six to seven weeks’ gestation two years ago. She also reported a dilation and curettage after the miscarriage. Her medical, surgical, and gynecologic histories were otherwise noncontributory. A review of systems was otherwise negative.
Sexual history revealed that the patient was married and monogamous with her husband of five years. She disclosed four previous sexual partners and inconsistent use of condoms with those partners; no current condom use was reported. Seven years ago, she tested positive for gonorrhea and chlamydia and was treated concurrently with her partner. Subsequent diagnostics were negative. She reported vaginal intercourse but no oral sex and denied any other sexual contact. All partners had been male.
On the next page: Diagnosis and case continuation >>
DIAGNOSIS
There is some variation in the presentation of women experiencing ectopic pregnancy; this may be due to differences in the pathologic mechanisms of ectopic pregnancy. Patients may be asymptomatic, hemodynamically compromised, or somewhere in between.3 Typical clinical signs include abdominal pain, amenorrhea, and vaginal bleeding. Approximately 40% to 50% of patients present with vaginal bleeding, 50% may have a palpable adnexal mass, and 75% may have abdominal tenderness.3 Only about 50% of women with ectopic pregnancies present with these typical symptoms.3
The patient may also experience common symptoms of early pregnancy, such as nausea, fatigue, and breast fullness. Worrisome signs and symptoms, including abdominal guarding, hypotension, tachycardia, shock, shoulder pain from peritoneal irritation, dizziness, fever, and vomiting, may also be present.3,7 Approximately 20% of patients with ectopic pregnancies are hemodynamically compromised at presentation, which is highly suggestive of rupture.3
Risk factors
Risk factors for ectopic pregnancy include previous ectopic pregnancy; previous tubal procedures; history of sexually transmitted disease or genital infections; infertility; use of assisted reproductive technology; previous abdominal or pelvic surgery; smoking; pelvic inflammatory disease; exposure in utero to diethylstilbestrol; and previous intrauterine device use.2,5,7,8 Knowledge of these risk factors can help identify a patient with an ectopic pregnancy.
The diagnosis of ectopic pregnancy is most certainly a clinical challenge. The differential diagnosis is based upon history and physical findings; the list can be lengthy if both vaginal bleeding and abdominal pain (nonspecific symptoms common in women who miscarry) are present.7 Prompt completion of diagnostic testing is critical in making a definitive diagnosis. Possible diagnoses are listed in Table 1.
CASE Upon examination, the patient appeared comfortable and relaxed, and there were no signs of distress. Blood pressure was 100/65 mm Hg, pulse rate was 72 beats/min, and temperature was 99.0°F. There was no tenderness upon abdominal examination. Pelvic examination revealed a small amount of brown vaginal discharge but no active bleeding or pooled blood, clots, or tissue. The cervical os was closed, and positive Chadwick sign was present. Bimanual examination revealed no cervical motion tenderness. The uterus was soft, mobile, and nontender, and consistent in size with a gestation at eight weeks. There were no palpable adnexa, ovaries, or masses. There was no pain with bimanual examination and no evidence of tenderness at the posterior fornix. The remainder of the physical examination was unremarkable.
It is important to note that examination results in the case patient are not unusual in a woman with a small, unruptured ectopic pregnancy. All findings were normal except for the scant brown vaginal discharge. Abdominal and adnexal tenderness are common, as is a palpable adnexal mass; but absence of a detectable mass does not exclude ectopic pregnancy.1 Pathologic findings may include severe abdominal tenderness and pain, significant vaginal bleeding, passage of clots, tachycardia, and orthostatic hypotension.
Diagnostic workup
Laboratory tests are critical to making an accurate diagnosis for women whose history and physical examination results are consistent with ectopic pregnancy. Assessment for ectopic pregnancy should include a urine pregnancy test, transvaginal ultrasound, measurement of serum ß-human chorionic gonadotropin (ß-hCG) level, and occasionally, diagnostic curettage.1 Once the diagnosis is confirmed, a complete blood count (CBC) is necessary to assess anemia and platelet functioning. Coagulation tests may be required for worrisome bleeding. Blood type, Rh status, and antibody screen are also necessary to determine whether a patient who is Rh D-negative will require Rh immune globulin. See Table 2 for the patient’s laboratory test results.
In a patient with a ß-hCG level greater than the discriminatory cutoff value of 1,500 to 1,800 mIU/mL, the level above which an intrauterine gestational sac is visible on transvaginal ultrasound in a normal pregnancy, an empty uterus is considered an ectopic pregnancy until proven otherwise.3 In a definite intrauterine pregnancy of about six weeks’ gestation, transvaginal ultrasound reveals a gestational sac that contains a yolk sac and a fetal pole.3
CASE The patient’s presenting symptoms, combined with a positive pregnancy test, ß-hCG level of 1,850 mIU/mL, and a complex adnexal mass in the right fallopian tube, were highly suggestive of an unruptured ectopic pregnancy (see Table 3 for the patient’s transvaginal ultrasound findings). There was also a secondary finding of a corpus luteum cyst. Other diagnoses were ruled out, and the patient was diagnosed with an unruptured ectopic pregnancy.
On the next page: Treatment >>
TREATMENT
A patient with an ectopic pregnancy who presents with pain and hemodynamic instability should be referred immediately for appropriate surgical care.7 Otherwise, once the diagnosis of ectopic pregnancy is confirmed, the patient should be referred to an obstetric specialist. Treatments for ectopic pregnancy include expectant management and surgery—which will be discussed briefly—and medical management, which is the focus of this review.5
Expectant management
Most ectopic pregnancies are diagnosed early as a result of accurate, minimally invasive and noninvasive diagnostic tools and greater awareness of risk factors. Since the natural course of early ectopic pregnancy is often self-limited, eventually resulting in tubal abortion or reabsorption, expectant management is a viable option.9
This treatment option may be considered if the patient is asymptomatic; ß-hCG is < 200 mIU/mL; the ectopic mass is < 3 cm; and no fetal heartbeat is present.1,2 With this approach, patients must be willing to accept the risk for tubal rupture and agree to close monitoring of ß-hCG levels. The ß-hCG level must be measured every 24 to 48 hours in order to determine if it is declining adequately, plateauing, or increasing.2,5
Surgery
For the hemodynamically unstable patient, the treatment decision is relatively straightforward. Optimal treatment for a ruptured ectopic pregnancy is immediate surgery, which may include salpingostomy or salpingectomy.10 Surgery may also be considered for hemodynamically stable patients with nonruptured ectopic pregnancies; in addition to her clinical presentation, overall management may be driven by a patient’s preferences.5 Salpingostomy and salpingectomy can be performed either laparoscopically or via laparotomy, depending on the specific situation.
Medical management
The use of methotrexate for the management of unruptured ectopic pregnancy was introduced in the early 1980s.11 Initially, protocols called for multiple doses administered during the course of an inpatient stay. Further research led to revised treatment recommendations and today, medical management most often consists of a single dose of methotrexate with outpatient follow-up.3
Methotrexate is a folic acid antagonist often used as an antimetabolite chemotherapeutic agent. In ectopic pregnancy, it inhibits growth of the rapidly dividing trophoblastic cells and ultimately ends the pregnancy.2 Outcomes of medical management are comparable to those of surgical treatment, including the potential for future normal pregnancies.2,5
An analysis of US trends in ectopic pregnancy management from 2002-2007 revealed that the use of methotrexate increased from 11.1% to 35.1% during that time, while the use of surgical approaches declined from 90% to 65%.10 Medical management of ectopic pregnancy eliminates the costs of surgery, anesthesia, and hospitalization and avoids potential complications of surgery and anesthesia.
Appropriate candidates
A hemodynamically stable patient with a confirmed or high clinical suspicion of ectopic pregnancy, an unruptured mass, no active bleeding, and low ß-hCG levels (< 5,000 mIU/mL) can be considered for methotrexate therapy.2,3,9 It is critical that medically managed patients be willing and able to adhere to all follow-up appointments.9 Before initiating treatment, normal serum creatinine and transaminase levels should be confirmed, and there should be no evidence of significant anemia, leukopenia, or thrombocytopenia.2 To detect any adverse effects of methotrexate on renal, hepatic, and hematologic functioning, these tests are repeated one week after administration.2
Contraindications
Contraindications to methotrexate treatment include breastfeeding, immunodeficiency, alcoholism, alcoholic liver disease or other chronic liver disease, preexisting blood dyscrasias (eg, bone marrow hypoplasia, leukopenia, thrombocytopenia, or significant anemia), known sensitivity to methotrexate, active pulmonary disease, peptic ulcer, and hepatic, renal, or hematologic dysfunction. Relative contraindications are a gestational sac larger than 3.5 cm and embryonic cardiac motion.2
On the next page: Patient education >>
PATIENT EDUCATION AND INFORMED CONSENT
A diagnosis of unruptured ectopic pregnancy requires patient education about the condition and its treatment options. The clinician should explain what an ectopic pregnancy is and distinguish between unruptured and ruptured. A discussion of the benefits and risks of each treatment option for which the patient is an appropriate candidate, as well as what to anticipate during treatment, is needed. Emotional support for impending pregnancy loss should also be provided.
For patients who choose medical management, education includes methotrexate-specific information and written instructions to follow after methotrexate administration. Patients must be instructed about the use of safety precautions after treatment (eg, the toilet should be double-flushed with the lid closed during the first 72 hours after treatment to prevent exposing others to methotrexate in urine and stool), the need for adherence to follow-up visits, and warning signs of a possible rupture.5 These warning signs are listed in Table 4.
The most common adverse effects of methotrexate are gastrointestinal (nausea, vomiting, stomatitis). Patients should be advised to avoid alcohol, NSAIDs, folic acid supplements, excessive sun exposure (due to photosensitivity), strenuous exercise, and sexual intercourse until ß-hCG has returned to nonpregnant levels. Other adverse effects may include a temporary elevation in liver enzymes and rarely, alopecia. Abdominal pain may occur a few days after methotrexate administration, likely from the cytotoxic effects of the drug on the trophoblastic tissue.
Informed consent is required prior to methotrexate administration. The patient must be advised of the potential risks of medical management with methotrexate, including rupture of the ectopic pregnancy during treatment, inadvertent administration of methotrexate in the presence of an early intrauterine embryo, allergic reaction to methotrexate, and methotrexate-induced pneumonitis.5
CASE After lengthy discussion of the treatment options, the patient chose medical management with methotrexate. She verbalized her understanding of the teaching provided and signed an informed consent document.
METHOTREXATE REGIMENS
Protocols for single-dose, two-dose, and fixed multidose methotrexate regimens are described in the medical literature, according to a 2008 American Congress of Obstetricians and Gynecologists practice bulletin.2 A 2013 practice committee opinion of the American Society for Reproductive Medicine (ASRM) indicates that single-dose and multiple-dose regimens are used most often.12
With methotrexate treatment, complete resolution of ectopic pregnancy usually occurs in two to three weeks but may require up to six to eight weeks, depending on how high the ß-hCG level is when treatment begins.12
Single-dose
In the single-dose regimen, an intramuscular (IM) injection of methotrexate 50 mg/m2 is administered on day 1. The ß-hCG levels are measured on days 4 and 7 after administration; a decrease of at least 15% in the ß-hCG level should be observed. The ß-hCG level is then measured weekly until it reaches < 2 mIU/mL or is undetectable.2 If the level does not decline, a repeat dose of methotrexate can be given, with measurement of ß-hCG on days 4 and 7 after the repeat dose. If the ß-hCG level fails to decrease, additional methotrexate or surgical intervention should be considered.
The single-dose regimen is more frequently used and is most successful when ß-hCG levels are low (< 5,000 mIU/mL), the ectopic mass is small
(< 3.5 cm), and embryonic cardiac activity is not observed on ultrasound.2,3 Patients with ß-hCG levels > 5,000 mIU/mL may be appropriate candidates for additional doses of methotrexate.2 In fact, the single-dose protocol provides for repeat doses of methotrexate if the ß-hCG level is not decreasing adequately.12
Multiple-dose
With the multiple-dose regimen, methotrexate 1 mg/kg IM is administered on days 1, 3, 5, and 7; on days 2, 4, 6, and 8, the patient receives leucovorin (folinic acid) 0.1 mg/kg IM. The ß-hCG level is measured on days methotrexate is administered; once the minimum 15% decline is observed, ß-hCG is measured weekly until a nonpregnant level is reached.12
The patient received a single dose of methotrexate 50 mg/m2 IM on day 1 and returned to the clinic for follow-up on days 4 and 7 posttreatment. On day 4, her ß-hCG level was 1,060 mIU/mL; on day 7, it was 470 mIU/mL. Also on day 7, blood was drawn for a CBC and comprehensive metabolic panel; results were within normal limits. The patient continued weekly follow-up until her ß-hCG level decreased to < 2 mIU/mL.
On the next page: Follow-up and conclusion >>
FOLLOW-UP AND REFERRALS
Close monitoring of ß-hCG levels, as described previously, is essential after methotrexate treatment in order to confirm that the pregnancy has been terminated and reduce the risk for tubal rupture. Clinicians should also be sensitive to the sequelae of loss of a pregnancy and refer patients as needed to appropriate health care professionals for grief support.
CASE The patient was referred to an obstetrics clinic and reported for all scheduled follow-up appointments. She was discharged from care after a full reduction in her ß-hCG to nonpregnant levels. While at the clinic, the patient was referred to social services for psychosocial counseling.
CONCLUSION
Ectopic implantation is a serious complication that may occur during the first trimester of pregnancy. Worldwide, it is the leading cause of maternal death in the first trimester. For women who meet specific criteria, outpatient treatment of early ectopic pregnancy with methotrexate avoids surgery and decreases the overall cost of care. Medical management and conservative surgical management offer the patient comparable outcomes for tubal patency preservation and risk for ectopic pregnancy recurrence.11
REFERENCES
1. Lozeau AM, Potter B. Diagnosis and management of ectopic pregnancy. Am Fam Physician. 2005;72(9):1707-1714.
2. American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479-1485.
3. Sepilian VP, Wood E. Ectopic pregnancy. http://emedicine.medscape.com/article/2041923-overview. Medscape. Accessed June 19, 2014.
4. Stein JC, Wang R, Adler N, et al. Emergency physician ultrasonography for evaluating patients at risk for ectopic pregnancy: a meta-analysis. Ann Emerg Med. 2010;56(6):674-683.
5. Murtaza UI, Ortmann MJ, Mando-Vandrick J, Lee ASD. Management of first-trimester complications in the emergency department. Am J Health Syst Pharm. 2013;70(2):99-111.
6. Sewell CA, Cundiff GW. Trends for inpatient treatment of tubal pregnancy in Maryland. Am J Obstet Gynecol. 2002;186(3):404-408.
7. Nama V, Manyonda I. Tubal ectopic pregnancy: diagnosis and management. Arch Gynecol Obstet. 2009;279(4):443-453.
8. Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86(1):36-43.
9. Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;(1):CD000324.
10. Hoover KW, Tao G, Kent CK. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstet Gynecol. 2010;115(3): 495-502.
11. Autry A. Medical treatment of ectopic pregnancy: is there something new? Obstet Gynecol. 2013;122(4):733.
12. The Practice Committee of the American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100(3):638-644.