User login
Post-stroke exercise rehabilitation: What we know about retraining the motor system and how it may apply to retraining the heart
Ideally, rehabilitation following a stroke that leads to functional deficit will result in a rapid return to normal function. In the real world, however, a rapid improvement in function is rarely achieved. Between 80% and 90% of stroke survivors have a motor deficit, with impairments in walking being the most common motor deficits.1 Most stroke survivors have a diminished fitness reserve that is stable and resistant to routine rehabilitative interventions. Recent research has begun to assess the value of exercise and other modalities of training during this period of stability to improve function long after cessation of other therapeutic interventions. This article will review this research and provide insight into those issues in post-stroke rehabilitation that remain to be addressed and may affect heart and brain physiology.
STROKE REDUCES AEROBIC CAPACITY
At all ages, the fitness level of stroke survivors, as measured by maximum oxygen consumption, is reduced by approximately 50% below that of an age-matched normal population. In a study comparing peak oxygen consumption during treadmill walking between stroke survivors and age-matched sedentary controls, we found that the stroke participants had an approximately 50% lower level of peak fitness relative to the control subjects.2 During treadmill walking at self-selected speeds, the stroke volunteers used 75% of their functional capacity, compared with 27% for the age-matched healthy controls. Furthermore, compared with the controls, the stroke subjects demonstrated a poorer economy of gait that required greater oxygen consumption to sustain their self-selected walking speeds.
CLINICAL TRIALS OF POST-STROKE EXERCISE REHABILITATION
In light of the efficacy of treadmill exercise in cardiac rehabilitation, we are evaluating whether treadmill exercise can similarly improve fitness, endurance, and walking velocity in stroke survivors. We have completed 6 months of treadmill training in two separate cohorts that show highly consistent results in terms of improved walking abilities in hemiparetic stroke subjects.3,4 A third cohort is in progress to confirm these findings and examine the effects of intensity on the functional benefits5 and mechanisms6 underlying the effects of treadmill training.
Treadmill exercise results in functional benefits and improved glucose metabolism
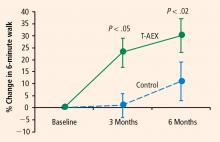
The first cohort was a before-and-after comparison of stable stroke survivors who underwent a three-times-weekly treadmill exercise program for 6 months.3 Peak exercise capacity testing (VO2peak) revealed functional benefits with minimal cardiac and injury risk compared with baseline, demonstrating the feasibility and safety of treadmill exercise therapy in stroke-impaired adults.
Potential mechanisms for the benefits
These findings raise the question of whether these beneficial effects of treadmill exercise are attributable to muscle training effects, cardiopulmonary circulatory training effects, or perhaps neural mechanisms involving economy of gait movements and neuroplasticity of the motor system.
This question is being examined in our third cohort, now under investigation. This cohort will evaluate the effects of treadmill exercise on 32 chronically disabled stroke survivors in a single-center study design that is randomizing 64 subjects to 6 months of three-times-weekly treadmill training or conventional physiotherapy.6 Similar to our prior studies, subjects are randomized at least 6 months after their index stroke; this lengthy interval is deliberate because subjects are considered to be in a “plateau” phase of recovery, as they have previously completed rehabilitative therapy.
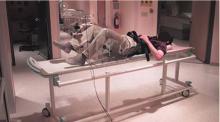
Activation will be measured in five prespecified “regions of interest”: the precentral gyrus, the postcentral gyrus, the supplementary motor area, the midbrain, and the cerebellum (anterior/posterior lobes). Difference activation maps of post-training minus pretraining fMRIs of paretic knee movement across all patients undergoing treadmill therapy will then be analyzed. The control group, which will receive dose-matched stretching activity from physical therapy, can be contrasted by comparing the patterns of pre/post differences in each region. This will allow for assessment of increased regional activation in the brain that should be specific to the treadmill training intervention. Furthermore, if a specifically localized regional activation difference is found, then individual fMRI and VO2 training responses (VO2peak, increase in walking speeds) can be correlated to further assess the relationship between regional activation and magnitude of functional response to the treadmill intervention.
DISCUSSION AND CONCLUSIONS
Central control of walking
Control of gait in animals is mediated by the cortex, brainstem/cerebellum,9,10 and spinal cord—the so-called cervical gait and lumbar gait pattern-generating areas of the spinal cord. In humans, cortical and spinal gait pattern areas are thought to be major regulatory centers of ambulation. Whether the cortical areas influence ambulatory recovery mediated by exercise training or whether the recruitment of spinal gait areas is needed to improve motor control after stroke is not known in humans. We will test the hypothesis that the recruitment of cortical and/or subcortical areas is relevant to some or all of the exercise-induced neuroplasticity response to treadmill rehabilitation. If a consistent pattern of brain regional activation is associated with an improvement in walking ability, this finding will suggest potential brain targets for neurally directed rehabilitation interventions. If brain targets for rehabilitation produce viable therapeutic improvement in walking and cardiocirculatory performance (such as VO2), this will be further evidence of heart-brain interactions.
Future research directions
Studies to date demonstrate that long-term treadmill exercise affects both the brain and cardiac physiology. This has holistic implications for the function of the whole person as well. Yet several pressing issues continue to confront researchers in post-stroke rehabilitation. One is the optimal therapeutic target and the intensity of the rehabilitative effort. Is this improvement solely a response of muscle and cardiac tissue to exercise, or is it possible that improved neuromotor control is a critical component to a major recovery of walking function? Furthermore, the most efficacious elements of rehabilitative therapy are not known. Should treadmill training be high- or low-intensity, and should it be accompanied by strength training, agility and flexibility activities, or other elements directed at reacquisition of finer degrees of gait-related motor training and neuropsychological input, as achieved by tai-chi or yoga? Another issue is the proper dose of rehabilitative therapy, which has barely been explored, although recent preliminary work suggests that the response is dose-dependent. Finally, predictors of response have not been established because the mechanisms of therapy and surrogate markers for early response are not well understood.
Our future research plans are to assess whether a better understanding of neural targets for rehabilitative treatment will be a fruitful avenue to improve recovery. Additionally, this plan will assess whether fMRI can serve as a surrogate marker of recovery by offering a noninvasive means to measure response to rehabilitation.
- Mayo NE, Wood-Dauphinee S, Ahmed S, et al. Disablement following stroke. Disabil Rehabil 1999; 21:258–268.
- Michael K, Macko RF. Ambulatory activity intensity profiles, fitness, and fatigue in chronic stroke. Top Stroke Rehabil 2007; 14:5–12.
- Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil 2001; 82:879–884.
- Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke. A randomized, controlled trial. Stroke 2005; 36:2206–2211.
- Ivey FM, Ryan AS, Hafer-Macko CE, Goldberg AP, Macko RF. Treadmill aerobic training improves glucose tolerance and indices of insulin sensitivity in disabled stroke survivors: a preliminary report. Stroke 2007; 38:2752–2758.
- Luft AR, Macko R, Forrester L, Villagra F, Hanley D. Subcortical reorganization induced by aerobic locomotor training in chronic stroke survivors [abstract]. Poster presented at: Annual Meeting of the Society for Neuroscience; November 15, 2005; Washington, DC.
- Luft AR, Smith GV, Forrester L, et al. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum Brain Mapping 2002; 17:131–140.
- Luft AR, Forrester L, Macko RF, et al. Brain activation of lower extremity movement in chronically impaired stroke survivors. Neuroimage 2005; 26:184–194.
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 1968; 91:1–14.
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain 1968; 91:15–36.
Ideally, rehabilitation following a stroke that leads to functional deficit will result in a rapid return to normal function. In the real world, however, a rapid improvement in function is rarely achieved. Between 80% and 90% of stroke survivors have a motor deficit, with impairments in walking being the most common motor deficits.1 Most stroke survivors have a diminished fitness reserve that is stable and resistant to routine rehabilitative interventions. Recent research has begun to assess the value of exercise and other modalities of training during this period of stability to improve function long after cessation of other therapeutic interventions. This article will review this research and provide insight into those issues in post-stroke rehabilitation that remain to be addressed and may affect heart and brain physiology.
STROKE REDUCES AEROBIC CAPACITY
At all ages, the fitness level of stroke survivors, as measured by maximum oxygen consumption, is reduced by approximately 50% below that of an age-matched normal population. In a study comparing peak oxygen consumption during treadmill walking between stroke survivors and age-matched sedentary controls, we found that the stroke participants had an approximately 50% lower level of peak fitness relative to the control subjects.2 During treadmill walking at self-selected speeds, the stroke volunteers used 75% of their functional capacity, compared with 27% for the age-matched healthy controls. Furthermore, compared with the controls, the stroke subjects demonstrated a poorer economy of gait that required greater oxygen consumption to sustain their self-selected walking speeds.
CLINICAL TRIALS OF POST-STROKE EXERCISE REHABILITATION
In light of the efficacy of treadmill exercise in cardiac rehabilitation, we are evaluating whether treadmill exercise can similarly improve fitness, endurance, and walking velocity in stroke survivors. We have completed 6 months of treadmill training in two separate cohorts that show highly consistent results in terms of improved walking abilities in hemiparetic stroke subjects.3,4 A third cohort is in progress to confirm these findings and examine the effects of intensity on the functional benefits5 and mechanisms6 underlying the effects of treadmill training.
Treadmill exercise results in functional benefits and improved glucose metabolism
The first cohort was a before-and-after comparison of stable stroke survivors who underwent a three-times-weekly treadmill exercise program for 6 months.3 Peak exercise capacity testing (VO2peak) revealed functional benefits with minimal cardiac and injury risk compared with baseline, demonstrating the feasibility and safety of treadmill exercise therapy in stroke-impaired adults.
Potential mechanisms for the benefits
These findings raise the question of whether these beneficial effects of treadmill exercise are attributable to muscle training effects, cardiopulmonary circulatory training effects, or perhaps neural mechanisms involving economy of gait movements and neuroplasticity of the motor system.
This question is being examined in our third cohort, now under investigation. This cohort will evaluate the effects of treadmill exercise on 32 chronically disabled stroke survivors in a single-center study design that is randomizing 64 subjects to 6 months of three-times-weekly treadmill training or conventional physiotherapy.6 Similar to our prior studies, subjects are randomized at least 6 months after their index stroke; this lengthy interval is deliberate because subjects are considered to be in a “plateau” phase of recovery, as they have previously completed rehabilitative therapy.
Activation will be measured in five prespecified “regions of interest”: the precentral gyrus, the postcentral gyrus, the supplementary motor area, the midbrain, and the cerebellum (anterior/posterior lobes). Difference activation maps of post-training minus pretraining fMRIs of paretic knee movement across all patients undergoing treadmill therapy will then be analyzed. The control group, which will receive dose-matched stretching activity from physical therapy, can be contrasted by comparing the patterns of pre/post differences in each region. This will allow for assessment of increased regional activation in the brain that should be specific to the treadmill training intervention. Furthermore, if a specifically localized regional activation difference is found, then individual fMRI and VO2 training responses (VO2peak, increase in walking speeds) can be correlated to further assess the relationship between regional activation and magnitude of functional response to the treadmill intervention.
DISCUSSION AND CONCLUSIONS
Central control of walking
Control of gait in animals is mediated by the cortex, brainstem/cerebellum,9,10 and spinal cord—the so-called cervical gait and lumbar gait pattern-generating areas of the spinal cord. In humans, cortical and spinal gait pattern areas are thought to be major regulatory centers of ambulation. Whether the cortical areas influence ambulatory recovery mediated by exercise training or whether the recruitment of spinal gait areas is needed to improve motor control after stroke is not known in humans. We will test the hypothesis that the recruitment of cortical and/or subcortical areas is relevant to some or all of the exercise-induced neuroplasticity response to treadmill rehabilitation. If a consistent pattern of brain regional activation is associated with an improvement in walking ability, this finding will suggest potential brain targets for neurally directed rehabilitation interventions. If brain targets for rehabilitation produce viable therapeutic improvement in walking and cardiocirculatory performance (such as VO2), this will be further evidence of heart-brain interactions.
Future research directions
Studies to date demonstrate that long-term treadmill exercise affects both the brain and cardiac physiology. This has holistic implications for the function of the whole person as well. Yet several pressing issues continue to confront researchers in post-stroke rehabilitation. One is the optimal therapeutic target and the intensity of the rehabilitative effort. Is this improvement solely a response of muscle and cardiac tissue to exercise, or is it possible that improved neuromotor control is a critical component to a major recovery of walking function? Furthermore, the most efficacious elements of rehabilitative therapy are not known. Should treadmill training be high- or low-intensity, and should it be accompanied by strength training, agility and flexibility activities, or other elements directed at reacquisition of finer degrees of gait-related motor training and neuropsychological input, as achieved by tai-chi or yoga? Another issue is the proper dose of rehabilitative therapy, which has barely been explored, although recent preliminary work suggests that the response is dose-dependent. Finally, predictors of response have not been established because the mechanisms of therapy and surrogate markers for early response are not well understood.
Our future research plans are to assess whether a better understanding of neural targets for rehabilitative treatment will be a fruitful avenue to improve recovery. Additionally, this plan will assess whether fMRI can serve as a surrogate marker of recovery by offering a noninvasive means to measure response to rehabilitation.
Ideally, rehabilitation following a stroke that leads to functional deficit will result in a rapid return to normal function. In the real world, however, a rapid improvement in function is rarely achieved. Between 80% and 90% of stroke survivors have a motor deficit, with impairments in walking being the most common motor deficits.1 Most stroke survivors have a diminished fitness reserve that is stable and resistant to routine rehabilitative interventions. Recent research has begun to assess the value of exercise and other modalities of training during this period of stability to improve function long after cessation of other therapeutic interventions. This article will review this research and provide insight into those issues in post-stroke rehabilitation that remain to be addressed and may affect heart and brain physiology.
STROKE REDUCES AEROBIC CAPACITY
At all ages, the fitness level of stroke survivors, as measured by maximum oxygen consumption, is reduced by approximately 50% below that of an age-matched normal population. In a study comparing peak oxygen consumption during treadmill walking between stroke survivors and age-matched sedentary controls, we found that the stroke participants had an approximately 50% lower level of peak fitness relative to the control subjects.2 During treadmill walking at self-selected speeds, the stroke volunteers used 75% of their functional capacity, compared with 27% for the age-matched healthy controls. Furthermore, compared with the controls, the stroke subjects demonstrated a poorer economy of gait that required greater oxygen consumption to sustain their self-selected walking speeds.
CLINICAL TRIALS OF POST-STROKE EXERCISE REHABILITATION
In light of the efficacy of treadmill exercise in cardiac rehabilitation, we are evaluating whether treadmill exercise can similarly improve fitness, endurance, and walking velocity in stroke survivors. We have completed 6 months of treadmill training in two separate cohorts that show highly consistent results in terms of improved walking abilities in hemiparetic stroke subjects.3,4 A third cohort is in progress to confirm these findings and examine the effects of intensity on the functional benefits5 and mechanisms6 underlying the effects of treadmill training.
Treadmill exercise results in functional benefits and improved glucose metabolism
The first cohort was a before-and-after comparison of stable stroke survivors who underwent a three-times-weekly treadmill exercise program for 6 months.3 Peak exercise capacity testing (VO2peak) revealed functional benefits with minimal cardiac and injury risk compared with baseline, demonstrating the feasibility and safety of treadmill exercise therapy in stroke-impaired adults.
Potential mechanisms for the benefits
These findings raise the question of whether these beneficial effects of treadmill exercise are attributable to muscle training effects, cardiopulmonary circulatory training effects, or perhaps neural mechanisms involving economy of gait movements and neuroplasticity of the motor system.
This question is being examined in our third cohort, now under investigation. This cohort will evaluate the effects of treadmill exercise on 32 chronically disabled stroke survivors in a single-center study design that is randomizing 64 subjects to 6 months of three-times-weekly treadmill training or conventional physiotherapy.6 Similar to our prior studies, subjects are randomized at least 6 months after their index stroke; this lengthy interval is deliberate because subjects are considered to be in a “plateau” phase of recovery, as they have previously completed rehabilitative therapy.
Activation will be measured in five prespecified “regions of interest”: the precentral gyrus, the postcentral gyrus, the supplementary motor area, the midbrain, and the cerebellum (anterior/posterior lobes). Difference activation maps of post-training minus pretraining fMRIs of paretic knee movement across all patients undergoing treadmill therapy will then be analyzed. The control group, which will receive dose-matched stretching activity from physical therapy, can be contrasted by comparing the patterns of pre/post differences in each region. This will allow for assessment of increased regional activation in the brain that should be specific to the treadmill training intervention. Furthermore, if a specifically localized regional activation difference is found, then individual fMRI and VO2 training responses (VO2peak, increase in walking speeds) can be correlated to further assess the relationship between regional activation and magnitude of functional response to the treadmill intervention.
DISCUSSION AND CONCLUSIONS
Central control of walking
Control of gait in animals is mediated by the cortex, brainstem/cerebellum,9,10 and spinal cord—the so-called cervical gait and lumbar gait pattern-generating areas of the spinal cord. In humans, cortical and spinal gait pattern areas are thought to be major regulatory centers of ambulation. Whether the cortical areas influence ambulatory recovery mediated by exercise training or whether the recruitment of spinal gait areas is needed to improve motor control after stroke is not known in humans. We will test the hypothesis that the recruitment of cortical and/or subcortical areas is relevant to some or all of the exercise-induced neuroplasticity response to treadmill rehabilitation. If a consistent pattern of brain regional activation is associated with an improvement in walking ability, this finding will suggest potential brain targets for neurally directed rehabilitation interventions. If brain targets for rehabilitation produce viable therapeutic improvement in walking and cardiocirculatory performance (such as VO2), this will be further evidence of heart-brain interactions.
Future research directions
Studies to date demonstrate that long-term treadmill exercise affects both the brain and cardiac physiology. This has holistic implications for the function of the whole person as well. Yet several pressing issues continue to confront researchers in post-stroke rehabilitation. One is the optimal therapeutic target and the intensity of the rehabilitative effort. Is this improvement solely a response of muscle and cardiac tissue to exercise, or is it possible that improved neuromotor control is a critical component to a major recovery of walking function? Furthermore, the most efficacious elements of rehabilitative therapy are not known. Should treadmill training be high- or low-intensity, and should it be accompanied by strength training, agility and flexibility activities, or other elements directed at reacquisition of finer degrees of gait-related motor training and neuropsychological input, as achieved by tai-chi or yoga? Another issue is the proper dose of rehabilitative therapy, which has barely been explored, although recent preliminary work suggests that the response is dose-dependent. Finally, predictors of response have not been established because the mechanisms of therapy and surrogate markers for early response are not well understood.
Our future research plans are to assess whether a better understanding of neural targets for rehabilitative treatment will be a fruitful avenue to improve recovery. Additionally, this plan will assess whether fMRI can serve as a surrogate marker of recovery by offering a noninvasive means to measure response to rehabilitation.
- Mayo NE, Wood-Dauphinee S, Ahmed S, et al. Disablement following stroke. Disabil Rehabil 1999; 21:258–268.
- Michael K, Macko RF. Ambulatory activity intensity profiles, fitness, and fatigue in chronic stroke. Top Stroke Rehabil 2007; 14:5–12.
- Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil 2001; 82:879–884.
- Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke. A randomized, controlled trial. Stroke 2005; 36:2206–2211.
- Ivey FM, Ryan AS, Hafer-Macko CE, Goldberg AP, Macko RF. Treadmill aerobic training improves glucose tolerance and indices of insulin sensitivity in disabled stroke survivors: a preliminary report. Stroke 2007; 38:2752–2758.
- Luft AR, Macko R, Forrester L, Villagra F, Hanley D. Subcortical reorganization induced by aerobic locomotor training in chronic stroke survivors [abstract]. Poster presented at: Annual Meeting of the Society for Neuroscience; November 15, 2005; Washington, DC.
- Luft AR, Smith GV, Forrester L, et al. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum Brain Mapping 2002; 17:131–140.
- Luft AR, Forrester L, Macko RF, et al. Brain activation of lower extremity movement in chronically impaired stroke survivors. Neuroimage 2005; 26:184–194.
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 1968; 91:1–14.
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain 1968; 91:15–36.
- Mayo NE, Wood-Dauphinee S, Ahmed S, et al. Disablement following stroke. Disabil Rehabil 1999; 21:258–268.
- Michael K, Macko RF. Ambulatory activity intensity profiles, fitness, and fatigue in chronic stroke. Top Stroke Rehabil 2007; 14:5–12.
- Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil 2001; 82:879–884.
- Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke. A randomized, controlled trial. Stroke 2005; 36:2206–2211.
- Ivey FM, Ryan AS, Hafer-Macko CE, Goldberg AP, Macko RF. Treadmill aerobic training improves glucose tolerance and indices of insulin sensitivity in disabled stroke survivors: a preliminary report. Stroke 2007; 38:2752–2758.
- Luft AR, Macko R, Forrester L, Villagra F, Hanley D. Subcortical reorganization induced by aerobic locomotor training in chronic stroke survivors [abstract]. Poster presented at: Annual Meeting of the Society for Neuroscience; November 15, 2005; Washington, DC.
- Luft AR, Smith GV, Forrester L, et al. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum Brain Mapping 2002; 17:131–140.
- Luft AR, Forrester L, Macko RF, et al. Brain activation of lower extremity movement in chronically impaired stroke survivors. Neuroimage 2005; 26:184–194.
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 1968; 91:1–14.
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain 1968; 91:15–36.