User login
Man, 46, With Wrist Laceration
A right hand–dominant 46-year-old man presents to the emergency department (ED) with a 1-cm laceration of his volar right wrist that occurred after he slipped on a wet floor while carrying a ceramic dish. The patient fell with his hand outstretched and landed on the dish as it broke against the floor. The patient has no pain but complains of tingling in his fingers. Past medical history is negative for diabetes, hypertension, or any neurologic disorders. Social history includes smoking one-half pack of cigarettes per day and drinking 6 to 10 12-oz beers each weekend. He works as a machinist.
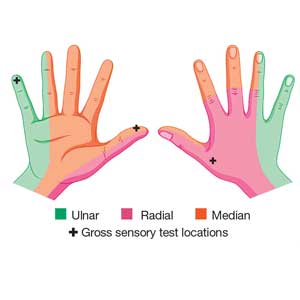
Physical examination shows no bony tenderness. There is a 1.0-cm transverse laceration at the base of the hand at the midline of the volar wrist crease. Flexion, extension, and strength of the fingers are intact, as are dull and sharp discrimination to the thumb and other fingers. A cotton-tip applicator is used for gross sensory testing. No other neuromuscular assessment of the hand is performed. An x-ray of the hand to rule out a fracture or ceramic foreign body is negative.
The wound is locally anesthetized with 1% xylocaine without epinephrine. The laceration is irrigated with normal saline solution and closed with 4-0 nylon sutures using conventional bedside-suturing technique. A sterile bandage is applied. After-care instructions include wound care and follow-up with the patient’s family physician in 1 week for suture removal.
The patient returns to the ED 4 days later, complaining of increased tingling and weakness of the thumb and index and middle fingers. Repeat neuromuscular examination shows decreased sensation and dull/sharp discrimination, and abnormal static 2-point discrimination of the thumb and index and middle fingers. Based on the location of the laceration, the follow-up provider suspects a median nerve injury. After a telephone consultation with a hand surgeon, the patient is told to come into the office in 2 days.
Subsequent follow-up by the hospital’s risk manager indicates that the hand surgeon found a transected median nerve, requiring surgery to repair it. The patient has resulting deficits in sensation and strength and requires extensive occupational therapy. The risk management team learns that the patient intends to file a malpractice suit.
DISCUSSION
Hand and finger injuries represent about 20% of ED visits and are among the most costly injuries for the employed population.1 Knife and glass lacerations of the fingers are most common.2 Failure to diagnose significant hand and finger injuries is also a major contributor to malpractice claims in the ED.3 It is imperative for the PA or NP working in a high-stress/high-volume environment to perform a thorough neuromuscular and vascular examination when encountering a traumatic hand injury or a laceration. This applies to all frontline practices, including urgent care, ED, and primary care and family practices.
Volar surface lacerations of the wrist and fingers are especially high risk.2 Small lacerations (< 2 cm for fingers and < 3 cm for wrist and forearm) may lead a provider to consider the injury minor; however, these have the greatest potential for missed significant deep injuries.2 Missed median nerve lacerations can result in major complications if not surgically repaired soon after the injury.4
Continue to: With our case patient...
With our case patient, a small glass cut at the volar wrist crease did not cause tendon lacerations or flexor deficits. The patient complained only of mild tingling to the fingers, and a detailed hand-and-finger examination was not performed to isolate further nerve injury.4
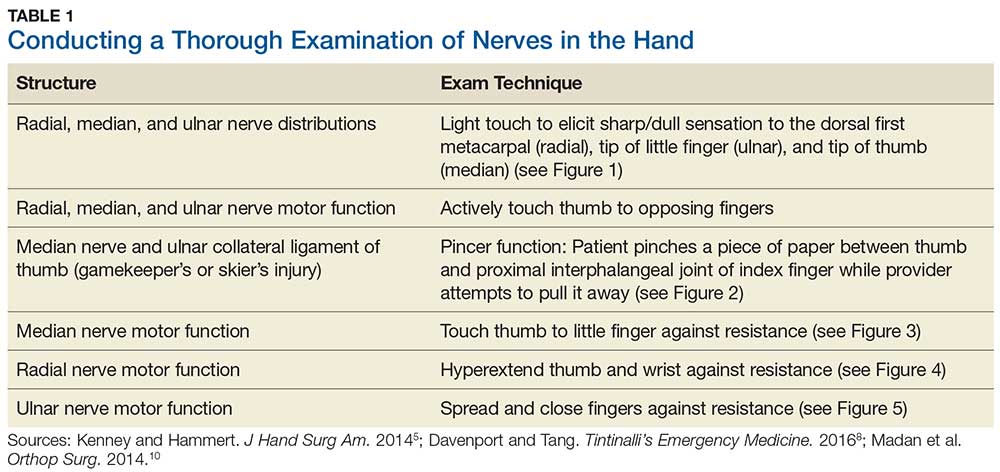
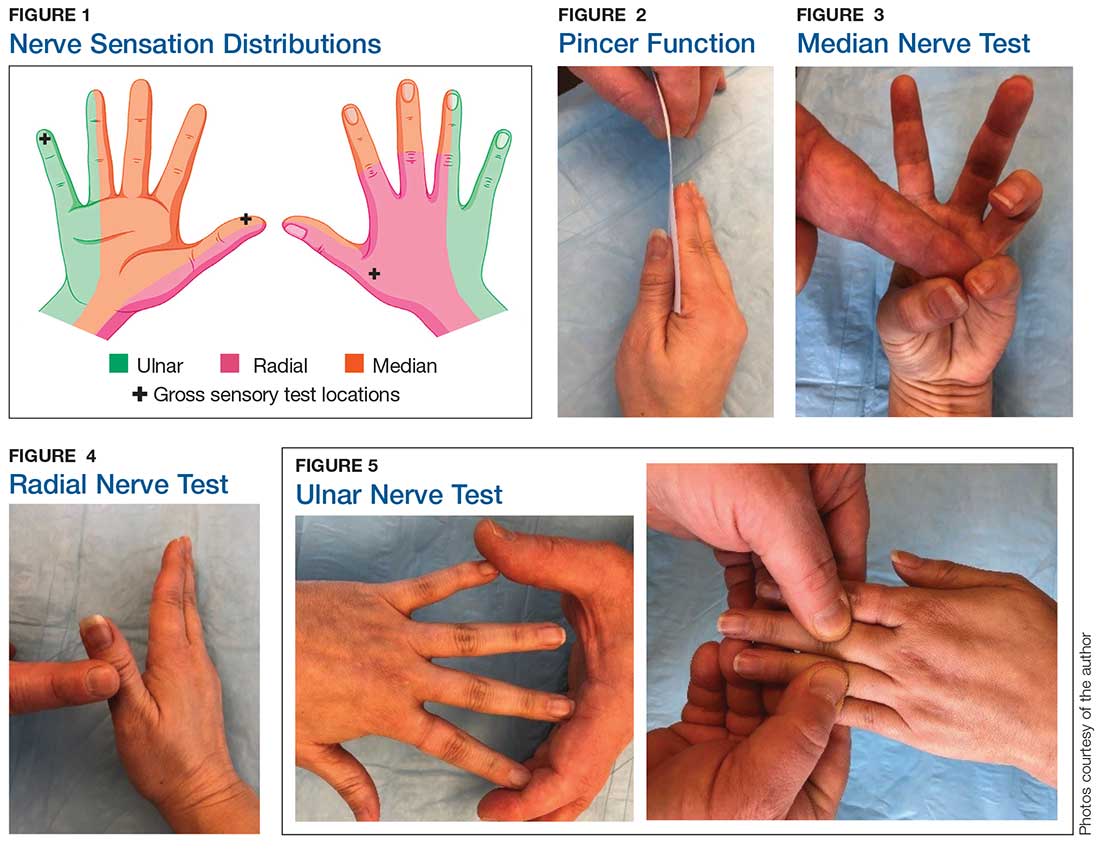
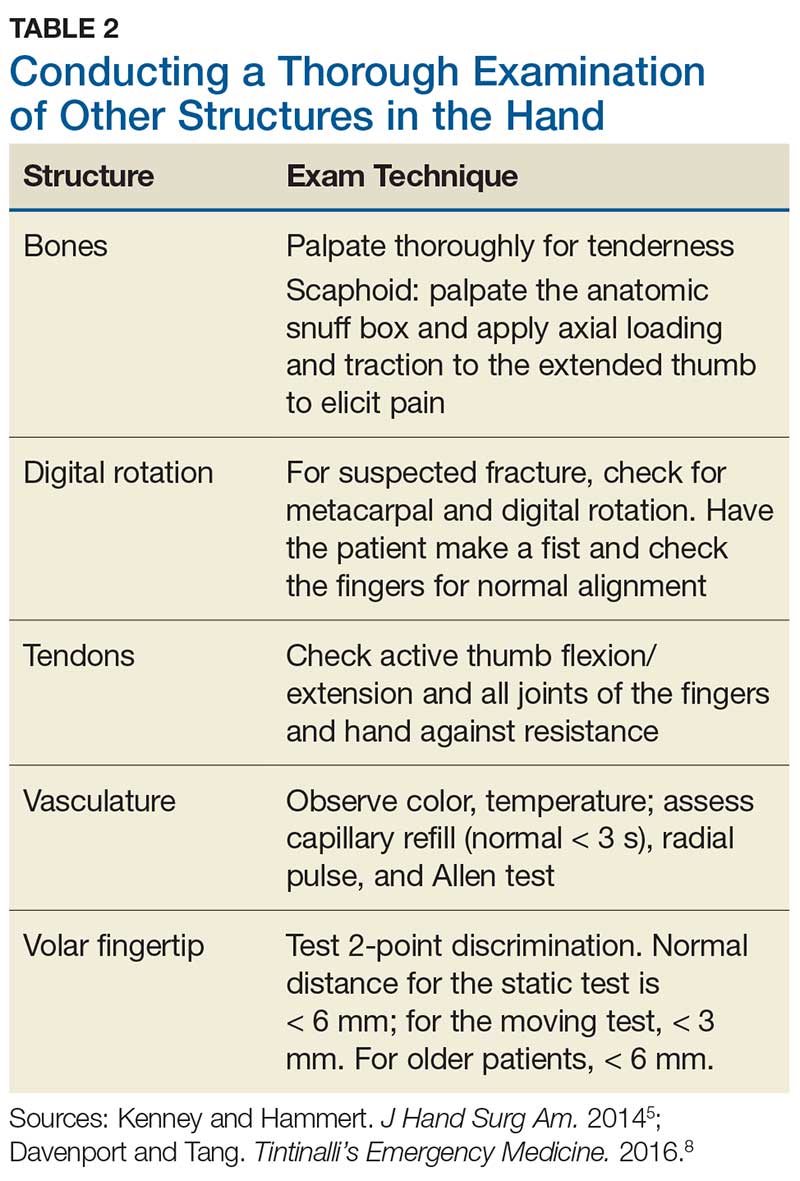
Although most nerve injuries result in a loss in sensory function, motor function must also be evaluated.5 With partial nerve lacerations, subtle loss of motor or sensory function can be missed by the examiner.4 It is imperative to conduct a thorough hand examination (outlined in Tables 1 and 2) to decrease the likelihood of missing a significant nerve or tendon injury.
Sensory testing basics
Nerve laceration vs nerve compression disorder. It is important to distinguish sensory testing for a nerve injury or laceration from testing for a nerve compression disorder, such as carpal tunnel syndrome. When examining compression neuropathies, light touch, tuning fork vibration, and monofilament testing are used. When a nerve injury or laceration is suspected, light touch and 2-point discrimination are used.5 Static 2-point discrimination (also known as the Weber static test) will be immediately abnormal if a nerve is lacerated. In a nerve compression disorder, 2-point discrimination is decreased progressively.5
Sensory testing evidence
Comparing light touch, monofilament, and 2-point discrimination. As seen with our case patient, testing dull-sharp discrimination using the cotton-tip applicator for “dull” and the broken end of the wooden applicator stick for “sharp” may not be the most complete way to assess sensation in the hand and fingers. The physical examination should include light touch and 2-point discrimination.5
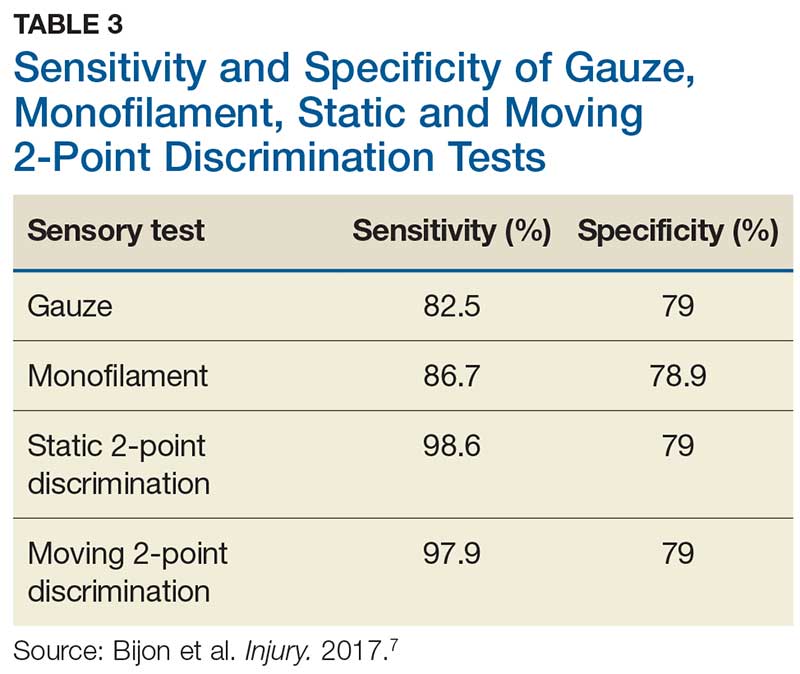
In one study, tests for sensation compared the gauze test (light touch), the static 2-point discrimination, the moving 2-point discrimination (m2PD; also known as the Weber dynamic test),6 and the monofilament test. The static and m2PD tests were statistically superior to the gauze and monofilament tests (see Table 3).7 Two-point discrimination abnormalities are detected immediately after a nerve is lacerated.5 This suggests performing 2-point discrimination, either moving or static, is superior to dull-sensation testing alone (gauze or cotton-tip applicator). This should be included in the motor and sensory examinations of the hand and fingers seen in Tables 1 and 2.
Continue to: Moving 2-point discrimination test
Moving 2-point discrimination test
The m2PD requires a 2-pointed instrument that can maintain a fixed 5 mm of width, such as a bent paperclip or EKG calipers. Commercially available devices specifically for 2-point discrimination can also be used.
When performing the m2PD test, the provider strokes 1 point in the proximal to distal direction in 5-mm increments on the finger and asks whether the patient feels “1 moving point.” The provider then holds 2 points and moves them in the proximal to distal direction in 5-mm increments and asks whether the patient feels “2 moving points.”
The m2PD test is then conducted comparing the ulnar and radial side of the injured finger with the ipsilateral noninjured finger. This should be done at least 4 times.8 The test is positive if there is a ≥ 2-mm difference between the affected and the unaffected side.7
Wound exploration
Data from a French insurance company indicate that 10% of ED malpractice claims in 2013 were related to inadequately examined hand lacerations. In an analysis of these claims, Mouton et al found that most injuries resulting in claims affected the thumb or the volar aspects of the fingers. Reasons for malpractice claims included residual stiffness, weakness, sensory deficit, retained foreign body, and wound infection. The researchers concluded that inadequate examination of hand wounds “carries a risk of lasting and sometimes severe residual impairment, and generates considerable societal costs.”3
In particular, small penetrating lacerations from broken glass or a knife should be considered high-risk injuries.2 In a study of small (< 2 cm) lacerations of the hand and fingers, 59% of the patients were found to have deep-structure injuries.2 Tuncali et al concluded that small lacerations increase the likelihood of missing deeper structural injuries because of failure to examine the wound.2 Furthermore, with glass lacerations, examiners tend to prioritize ruling out a foreign body and then fail to examine the wound. If a careful examination of the hand and fingers prompts suspicion of a tendon or nerve injury, referral to hand surgery for direct surgical exploration is indicated.
Continue to: CONCLUSION
CONCLUSION
Busy health care providers must be aware that approximately 10% to 15% of the negative outcomes in patient care result from diagnostic errors and are most common in the internal medicine, family medicine, and emergency medicine clinical environments.9 With hand and finger lacerations, small size can give a provider a false sense that the laceration is minor, resulting in a failure to diagnose a deeper injury (eg, tendon or nerve).1
When evaluating a traumatic injury or laceration to the hand or fingers, it is important to conduct a thorough sensory and motor examination. Experts recommend light touch and 2-point discrimination be included in the sensory exam to avoid missing nerve injuries. If a deeper structural injury is suspected, the patient should be referred to hand surgery and the wound surgically explored.2
1. Robinson LS, Sarkies M, Brown T, et al. Direct, indirect and intangible costs of acute hand and wrist injuries: a systematic review. Injury. 2016;47:2614-2626.
2. Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55:146-148.
3. Mouton J, Houdre H, Beccari R, et al. Surgical exploration of hand wounds in the emergency room: preliminary study of 80 personal injury claims. Orthop Traumatol Surg Res. 2016;102:1009-1012.
4. Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39(6): 1216-1222.
5. Kenney RJ, Hammert WC. Physical examination of the hand. J Hand Surg Am. 2014;39(11):2324-2334.
6. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474-481.
7. Bijon C, Hidalgo-Diaz JJ, Chiara P, et al. Nerve injuries to the volar aspect of the hand: a comparison of the reliability of the Weber static test versus the gauze test. Injury. 2017;48:2582-2585.
8. Davenport M, Tang P. Injuries to the hand and digits. In: Tintinalli JE, Stapczynski J, Ma OJ, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016:1667.
9. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41:155-162.
10. Madan SS, Pai DR, Kaur A, Dixit R. Injury to the ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7.
A right hand–dominant 46-year-old man presents to the emergency department (ED) with a 1-cm laceration of his volar right wrist that occurred after he slipped on a wet floor while carrying a ceramic dish. The patient fell with his hand outstretched and landed on the dish as it broke against the floor. The patient has no pain but complains of tingling in his fingers. Past medical history is negative for diabetes, hypertension, or any neurologic disorders. Social history includes smoking one-half pack of cigarettes per day and drinking 6 to 10 12-oz beers each weekend. He works as a machinist.
Physical examination shows no bony tenderness. There is a 1.0-cm transverse laceration at the base of the hand at the midline of the volar wrist crease. Flexion, extension, and strength of the fingers are intact, as are dull and sharp discrimination to the thumb and other fingers. A cotton-tip applicator is used for gross sensory testing. No other neuromuscular assessment of the hand is performed. An x-ray of the hand to rule out a fracture or ceramic foreign body is negative.
The wound is locally anesthetized with 1% xylocaine without epinephrine. The laceration is irrigated with normal saline solution and closed with 4-0 nylon sutures using conventional bedside-suturing technique. A sterile bandage is applied. After-care instructions include wound care and follow-up with the patient’s family physician in 1 week for suture removal.
The patient returns to the ED 4 days later, complaining of increased tingling and weakness of the thumb and index and middle fingers. Repeat neuromuscular examination shows decreased sensation and dull/sharp discrimination, and abnormal static 2-point discrimination of the thumb and index and middle fingers. Based on the location of the laceration, the follow-up provider suspects a median nerve injury. After a telephone consultation with a hand surgeon, the patient is told to come into the office in 2 days.
Subsequent follow-up by the hospital’s risk manager indicates that the hand surgeon found a transected median nerve, requiring surgery to repair it. The patient has resulting deficits in sensation and strength and requires extensive occupational therapy. The risk management team learns that the patient intends to file a malpractice suit.
DISCUSSION
Hand and finger injuries represent about 20% of ED visits and are among the most costly injuries for the employed population.1 Knife and glass lacerations of the fingers are most common.2 Failure to diagnose significant hand and finger injuries is also a major contributor to malpractice claims in the ED.3 It is imperative for the PA or NP working in a high-stress/high-volume environment to perform a thorough neuromuscular and vascular examination when encountering a traumatic hand injury or a laceration. This applies to all frontline practices, including urgent care, ED, and primary care and family practices.
Volar surface lacerations of the wrist and fingers are especially high risk.2 Small lacerations (< 2 cm for fingers and < 3 cm for wrist and forearm) may lead a provider to consider the injury minor; however, these have the greatest potential for missed significant deep injuries.2 Missed median nerve lacerations can result in major complications if not surgically repaired soon after the injury.4
Continue to: With our case patient...
With our case patient, a small glass cut at the volar wrist crease did not cause tendon lacerations or flexor deficits. The patient complained only of mild tingling to the fingers, and a detailed hand-and-finger examination was not performed to isolate further nerve injury.4
Although most nerve injuries result in a loss in sensory function, motor function must also be evaluated.5 With partial nerve lacerations, subtle loss of motor or sensory function can be missed by the examiner.4 It is imperative to conduct a thorough hand examination (outlined in Tables 1 and 2) to decrease the likelihood of missing a significant nerve or tendon injury.
Sensory testing basics
Nerve laceration vs nerve compression disorder. It is important to distinguish sensory testing for a nerve injury or laceration from testing for a nerve compression disorder, such as carpal tunnel syndrome. When examining compression neuropathies, light touch, tuning fork vibration, and monofilament testing are used. When a nerve injury or laceration is suspected, light touch and 2-point discrimination are used.5 Static 2-point discrimination (also known as the Weber static test) will be immediately abnormal if a nerve is lacerated. In a nerve compression disorder, 2-point discrimination is decreased progressively.5
Sensory testing evidence
Comparing light touch, monofilament, and 2-point discrimination. As seen with our case patient, testing dull-sharp discrimination using the cotton-tip applicator for “dull” and the broken end of the wooden applicator stick for “sharp” may not be the most complete way to assess sensation in the hand and fingers. The physical examination should include light touch and 2-point discrimination.5
In one study, tests for sensation compared the gauze test (light touch), the static 2-point discrimination, the moving 2-point discrimination (m2PD; also known as the Weber dynamic test),6 and the monofilament test. The static and m2PD tests were statistically superior to the gauze and monofilament tests (see Table 3).7 Two-point discrimination abnormalities are detected immediately after a nerve is lacerated.5 This suggests performing 2-point discrimination, either moving or static, is superior to dull-sensation testing alone (gauze or cotton-tip applicator). This should be included in the motor and sensory examinations of the hand and fingers seen in Tables 1 and 2.
Continue to: Moving 2-point discrimination test
Moving 2-point discrimination test
The m2PD requires a 2-pointed instrument that can maintain a fixed 5 mm of width, such as a bent paperclip or EKG calipers. Commercially available devices specifically for 2-point discrimination can also be used.
When performing the m2PD test, the provider strokes 1 point in the proximal to distal direction in 5-mm increments on the finger and asks whether the patient feels “1 moving point.” The provider then holds 2 points and moves them in the proximal to distal direction in 5-mm increments and asks whether the patient feels “2 moving points.”
The m2PD test is then conducted comparing the ulnar and radial side of the injured finger with the ipsilateral noninjured finger. This should be done at least 4 times.8 The test is positive if there is a ≥ 2-mm difference between the affected and the unaffected side.7
Wound exploration
Data from a French insurance company indicate that 10% of ED malpractice claims in 2013 were related to inadequately examined hand lacerations. In an analysis of these claims, Mouton et al found that most injuries resulting in claims affected the thumb or the volar aspects of the fingers. Reasons for malpractice claims included residual stiffness, weakness, sensory deficit, retained foreign body, and wound infection. The researchers concluded that inadequate examination of hand wounds “carries a risk of lasting and sometimes severe residual impairment, and generates considerable societal costs.”3
In particular, small penetrating lacerations from broken glass or a knife should be considered high-risk injuries.2 In a study of small (< 2 cm) lacerations of the hand and fingers, 59% of the patients were found to have deep-structure injuries.2 Tuncali et al concluded that small lacerations increase the likelihood of missing deeper structural injuries because of failure to examine the wound.2 Furthermore, with glass lacerations, examiners tend to prioritize ruling out a foreign body and then fail to examine the wound. If a careful examination of the hand and fingers prompts suspicion of a tendon or nerve injury, referral to hand surgery for direct surgical exploration is indicated.
Continue to: CONCLUSION
CONCLUSION
Busy health care providers must be aware that approximately 10% to 15% of the negative outcomes in patient care result from diagnostic errors and are most common in the internal medicine, family medicine, and emergency medicine clinical environments.9 With hand and finger lacerations, small size can give a provider a false sense that the laceration is minor, resulting in a failure to diagnose a deeper injury (eg, tendon or nerve).1
When evaluating a traumatic injury or laceration to the hand or fingers, it is important to conduct a thorough sensory and motor examination. Experts recommend light touch and 2-point discrimination be included in the sensory exam to avoid missing nerve injuries. If a deeper structural injury is suspected, the patient should be referred to hand surgery and the wound surgically explored.2
A right hand–dominant 46-year-old man presents to the emergency department (ED) with a 1-cm laceration of his volar right wrist that occurred after he slipped on a wet floor while carrying a ceramic dish. The patient fell with his hand outstretched and landed on the dish as it broke against the floor. The patient has no pain but complains of tingling in his fingers. Past medical history is negative for diabetes, hypertension, or any neurologic disorders. Social history includes smoking one-half pack of cigarettes per day and drinking 6 to 10 12-oz beers each weekend. He works as a machinist.
Physical examination shows no bony tenderness. There is a 1.0-cm transverse laceration at the base of the hand at the midline of the volar wrist crease. Flexion, extension, and strength of the fingers are intact, as are dull and sharp discrimination to the thumb and other fingers. A cotton-tip applicator is used for gross sensory testing. No other neuromuscular assessment of the hand is performed. An x-ray of the hand to rule out a fracture or ceramic foreign body is negative.
The wound is locally anesthetized with 1% xylocaine without epinephrine. The laceration is irrigated with normal saline solution and closed with 4-0 nylon sutures using conventional bedside-suturing technique. A sterile bandage is applied. After-care instructions include wound care and follow-up with the patient’s family physician in 1 week for suture removal.
The patient returns to the ED 4 days later, complaining of increased tingling and weakness of the thumb and index and middle fingers. Repeat neuromuscular examination shows decreased sensation and dull/sharp discrimination, and abnormal static 2-point discrimination of the thumb and index and middle fingers. Based on the location of the laceration, the follow-up provider suspects a median nerve injury. After a telephone consultation with a hand surgeon, the patient is told to come into the office in 2 days.
Subsequent follow-up by the hospital’s risk manager indicates that the hand surgeon found a transected median nerve, requiring surgery to repair it. The patient has resulting deficits in sensation and strength and requires extensive occupational therapy. The risk management team learns that the patient intends to file a malpractice suit.
DISCUSSION
Hand and finger injuries represent about 20% of ED visits and are among the most costly injuries for the employed population.1 Knife and glass lacerations of the fingers are most common.2 Failure to diagnose significant hand and finger injuries is also a major contributor to malpractice claims in the ED.3 It is imperative for the PA or NP working in a high-stress/high-volume environment to perform a thorough neuromuscular and vascular examination when encountering a traumatic hand injury or a laceration. This applies to all frontline practices, including urgent care, ED, and primary care and family practices.
Volar surface lacerations of the wrist and fingers are especially high risk.2 Small lacerations (< 2 cm for fingers and < 3 cm for wrist and forearm) may lead a provider to consider the injury minor; however, these have the greatest potential for missed significant deep injuries.2 Missed median nerve lacerations can result in major complications if not surgically repaired soon after the injury.4
Continue to: With our case patient...
With our case patient, a small glass cut at the volar wrist crease did not cause tendon lacerations or flexor deficits. The patient complained only of mild tingling to the fingers, and a detailed hand-and-finger examination was not performed to isolate further nerve injury.4
Although most nerve injuries result in a loss in sensory function, motor function must also be evaluated.5 With partial nerve lacerations, subtle loss of motor or sensory function can be missed by the examiner.4 It is imperative to conduct a thorough hand examination (outlined in Tables 1 and 2) to decrease the likelihood of missing a significant nerve or tendon injury.
Sensory testing basics
Nerve laceration vs nerve compression disorder. It is important to distinguish sensory testing for a nerve injury or laceration from testing for a nerve compression disorder, such as carpal tunnel syndrome. When examining compression neuropathies, light touch, tuning fork vibration, and monofilament testing are used. When a nerve injury or laceration is suspected, light touch and 2-point discrimination are used.5 Static 2-point discrimination (also known as the Weber static test) will be immediately abnormal if a nerve is lacerated. In a nerve compression disorder, 2-point discrimination is decreased progressively.5
Sensory testing evidence
Comparing light touch, monofilament, and 2-point discrimination. As seen with our case patient, testing dull-sharp discrimination using the cotton-tip applicator for “dull” and the broken end of the wooden applicator stick for “sharp” may not be the most complete way to assess sensation in the hand and fingers. The physical examination should include light touch and 2-point discrimination.5
In one study, tests for sensation compared the gauze test (light touch), the static 2-point discrimination, the moving 2-point discrimination (m2PD; also known as the Weber dynamic test),6 and the monofilament test. The static and m2PD tests were statistically superior to the gauze and monofilament tests (see Table 3).7 Two-point discrimination abnormalities are detected immediately after a nerve is lacerated.5 This suggests performing 2-point discrimination, either moving or static, is superior to dull-sensation testing alone (gauze or cotton-tip applicator). This should be included in the motor and sensory examinations of the hand and fingers seen in Tables 1 and 2.
Continue to: Moving 2-point discrimination test
Moving 2-point discrimination test
The m2PD requires a 2-pointed instrument that can maintain a fixed 5 mm of width, such as a bent paperclip or EKG calipers. Commercially available devices specifically for 2-point discrimination can also be used.
When performing the m2PD test, the provider strokes 1 point in the proximal to distal direction in 5-mm increments on the finger and asks whether the patient feels “1 moving point.” The provider then holds 2 points and moves them in the proximal to distal direction in 5-mm increments and asks whether the patient feels “2 moving points.”
The m2PD test is then conducted comparing the ulnar and radial side of the injured finger with the ipsilateral noninjured finger. This should be done at least 4 times.8 The test is positive if there is a ≥ 2-mm difference between the affected and the unaffected side.7
Wound exploration
Data from a French insurance company indicate that 10% of ED malpractice claims in 2013 were related to inadequately examined hand lacerations. In an analysis of these claims, Mouton et al found that most injuries resulting in claims affected the thumb or the volar aspects of the fingers. Reasons for malpractice claims included residual stiffness, weakness, sensory deficit, retained foreign body, and wound infection. The researchers concluded that inadequate examination of hand wounds “carries a risk of lasting and sometimes severe residual impairment, and generates considerable societal costs.”3
In particular, small penetrating lacerations from broken glass or a knife should be considered high-risk injuries.2 In a study of small (< 2 cm) lacerations of the hand and fingers, 59% of the patients were found to have deep-structure injuries.2 Tuncali et al concluded that small lacerations increase the likelihood of missing deeper structural injuries because of failure to examine the wound.2 Furthermore, with glass lacerations, examiners tend to prioritize ruling out a foreign body and then fail to examine the wound. If a careful examination of the hand and fingers prompts suspicion of a tendon or nerve injury, referral to hand surgery for direct surgical exploration is indicated.
Continue to: CONCLUSION
CONCLUSION
Busy health care providers must be aware that approximately 10% to 15% of the negative outcomes in patient care result from diagnostic errors and are most common in the internal medicine, family medicine, and emergency medicine clinical environments.9 With hand and finger lacerations, small size can give a provider a false sense that the laceration is minor, resulting in a failure to diagnose a deeper injury (eg, tendon or nerve).1
When evaluating a traumatic injury or laceration to the hand or fingers, it is important to conduct a thorough sensory and motor examination. Experts recommend light touch and 2-point discrimination be included in the sensory exam to avoid missing nerve injuries. If a deeper structural injury is suspected, the patient should be referred to hand surgery and the wound surgically explored.2
1. Robinson LS, Sarkies M, Brown T, et al. Direct, indirect and intangible costs of acute hand and wrist injuries: a systematic review. Injury. 2016;47:2614-2626.
2. Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55:146-148.
3. Mouton J, Houdre H, Beccari R, et al. Surgical exploration of hand wounds in the emergency room: preliminary study of 80 personal injury claims. Orthop Traumatol Surg Res. 2016;102:1009-1012.
4. Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39(6): 1216-1222.
5. Kenney RJ, Hammert WC. Physical examination of the hand. J Hand Surg Am. 2014;39(11):2324-2334.
6. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474-481.
7. Bijon C, Hidalgo-Diaz JJ, Chiara P, et al. Nerve injuries to the volar aspect of the hand: a comparison of the reliability of the Weber static test versus the gauze test. Injury. 2017;48:2582-2585.
8. Davenport M, Tang P. Injuries to the hand and digits. In: Tintinalli JE, Stapczynski J, Ma OJ, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016:1667.
9. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41:155-162.
10. Madan SS, Pai DR, Kaur A, Dixit R. Injury to the ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7.
1. Robinson LS, Sarkies M, Brown T, et al. Direct, indirect and intangible costs of acute hand and wrist injuries: a systematic review. Injury. 2016;47:2614-2626.
2. Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55:146-148.
3. Mouton J, Houdre H, Beccari R, et al. Surgical exploration of hand wounds in the emergency room: preliminary study of 80 personal injury claims. Orthop Traumatol Surg Res. 2016;102:1009-1012.
4. Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39(6): 1216-1222.
5. Kenney RJ, Hammert WC. Physical examination of the hand. J Hand Surg Am. 2014;39(11):2324-2334.
6. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474-481.
7. Bijon C, Hidalgo-Diaz JJ, Chiara P, et al. Nerve injuries to the volar aspect of the hand: a comparison of the reliability of the Weber static test versus the gauze test. Injury. 2017;48:2582-2585.
8. Davenport M, Tang P. Injuries to the hand and digits. In: Tintinalli JE, Stapczynski J, Ma OJ, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016:1667.
9. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41:155-162.
10. Madan SS, Pai DR, Kaur A, Dixit R. Injury to the ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7.
Are Cognitive Biases Influencing Your Clinical Decisions?
CE/CME No: CR-1603
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• List the characteristics of System 1 and System 2 thinking.
• Explain how System 1 and System 2 thinking affects clinical decisions.
• Define the characteristics of no-fault, system, and cognitive errors and how they affect health care delivery.
• Describe how biases and cognitive dispositions to respond cause health care providers to make clinical decision errors.
• List some effective debiasing techniques to improve clinical decisions and patient safety.
FACULTY
David J. Klocko is an Associate Professor and Academic Coordinator in the Department of Physician Assistant Studies at the University of Texas Southwestern Medical Center, School of Health Professions, Dallas.
The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2016.
Article begins on next page >>
Diagnostic errors occur for many reasons, some of which are based in cognitive biases. Also called cognitive dispositions to respond (CDR), these can result from failures in perception, faulty mental shortcuts, or unconscious biases, and clinicians are usually unaware they exist. This article discusses the influence CDRs have on clinical decisions and walks you through methods for purposeful debiasing.
Diagnosis is the foundation of medicine ... [and] diagnostic reasoning is a critical aspect of clinical performance.1
— Pat Croskerry, MD, PhD
Diagnostic errors compromise patient safety and the quality of health care and account for the majority of paid malpractice claims. They are especially common in family medicine, internal medicine, emergency medicine, and urgent care, wherethe error rate can be as high as 15%.2 However, all health care providers are subject to errors in clinical judgment, regardless of the setting or specialty in which they practice.3
Clinical disciplines such as internal medicine and emergency medicine have higher error rates than the perceptual disciplines, radiology and pathology. Higher diagnostic error rates in the clinical disciplines are due to the elevated case complexity and the need for rapid interpretation of diagnostic studies. In the perceptual disciplines such as pathology and radiology, fewer time pressures and the ability to obtain a second opinion before making a diagnosis decrease error rates.3 In a National Practitioner Data Bank analysis, more diagnostic error claims occurred in the outpatient setting than in the inpatient setting.4
Quality assurance and performance improvement have become paramount for all health care providers. The modern patient safety movement began in 1999 with the Institute of Medicine (IOM) report To Err Is Human, which highlighted how a faulty health care system causes people to make mistakes and negatively impacts patient safety.5 Some examples of errors arising from imperfections in the health system include medication errors, patient falls, wrong-site surgeries, and improper patient identification. Despite an increased emphasis on patient safety and quality improvement, diagnostic error had not been a focus of attention for policy makers and institutions. Only since the IOM report was released have the medical profession and health policy makers begun to pay attention to diagnostic errors as a serious patient safety issue.5
Cognitive biases, or cognitive dispositions to respond (CDR), can influence clinical decision-making and lead to diagnostic errors. By understanding the thinking processes involved in diagnostic reasoning and the interaction between these processes and cognitive biases, clinicians can take steps to counteract the influence of cognitive biases on their clinical decisions. Here, a brief introduction to dual processing theory is provided, along with information to help clinicians identify potential cognitive biases. Workplace and educational debiasing techniques to counter biases that lead to cognitive decision errors are presented as well.
DIAGNOSTIC ERRORS
All advanced practice providers are at risk for making a clinical decision error. The diagnostic errors that are made in clinical practice can be classified into three broad etiologic categories6:
No-fault errors occur when a rare disease is misdiagnosed as something more common or a disease is silent or presents in an atypical manner. An example of an error that falls into this category is a delayed diagnosis of ischemic bowel in a diabetic patient with no abdominal pain. Another example is a patient with a language barrier who is not able to describe his or her symptoms clearly, leading the clinician to misinterpret the history. Patient nonadherence to recommended care can also be viewed as no-fault, as in the case of a patient diagnosed with colon cancer who did not obtain a recommended screening colonoscopy.6 In one study, no-fault errors accounted for 7% of diagnostic errors.7
System errors occur as a result of “latent” faults in the process of delivering care and can be technical or organizational in nature.6 Examples of diagnostic errors related to technical issues are misdiagnosis or delayed diagnosis resulting from lack of appropriate testing or equipment or from incorrect laboratory results caused by technical problems with equipment. Organizational shortcomings that contribute to diagnostic errors include imperfections in department policies, error tolerance culture, poor patient care coordination, communication problems, inadequate staff training, poor working conditions, unavailability of acute specialty care, and failing to follow up with patients having abnormal diagnostic study results.6 Excessive workload and heavy administrative responsibilities also can contribute to clinician decision errors.
An example of a specific clinical organizational system error would be a missed or delayed diagnosis of a cancer on a chest x-ray due to lack of an “over-read” by a radiologist. Due to cost, many private practices do not send all radiographs for a radiologist’s interpretation. Another example is a patient with a severe eye injury who develops complications after being transferred to another hospital because there is not an on-call ophthalmologist at the presenting hospital.6 Delays in reviewing patient laboratory results are a significant system-based source of medical errors. In one study, 83% of the physician respondents reported at least one delay in reviewing test results in the past two months, with 18% reporting five or more delays in reviewing test results over the same time period.8
Cognitive errors are caused by gaps in knowledge or experience, inadequate interpretation of diagnostic studies, or succumbing to faulty heuristics and biases.6 With cognitive errors, incorrect perception or interpretation of a clinical situation results in faulty differential diagnosis development. Confirmation bias is one type of cognitive error—once supporting information is found for a diagnosis, the search for information to rule out the diagnosis stops.6
An example of this would be a patient with an ankle fracture who is discharged with a missed proximal fibula fracture after the clinician performs a physical exam only on the ankle and orders an ankle x-ray. A cognitive error like this would occur due to inadvertent omission of an important physical exam component or the clinician not knowing the importance of examining the knee when evaluating an ankle fracture.
It is important to note that clinical decision errors are usually multifactorial. In a study involving 100 cases of diagnostic error in internal medicine, Graber and colleagues determined that in 46% of the cases errors were caused by a combination of system-related and cognitive factors.7
Continue for decision making >>
Decision Making: Dual Process Theory
Over the past two decades, dual process theory (DPT) has been recognized as a reliable model of the decision-making process in the psychology literature.9 DPT proposes two unique processes of thinking during decision making, referred to as System 1 and System 2, or Type 1 and Type 2, processes. A brief introduction to DPT is given here for practicing clinicians, but a detailed discussion of the literature pertaining to this concept is beyond the scope of this review.
System 1 processes are “intuitive,” utilize pattern recognition and heuristics, and rely heavily on the context or conditions in which the decision is made. The intuitive System 1 mode of thinking uses a pattern recognition or “gut reaction” approach.10 It is fast and reflexive but can be subject to deficits in predictive power and reliability.10 Experienced clinicians use pattern recognition in conditions presenting with classic signs and symptoms.10 For example, the clinician who evaluates a 12-year-old child with an annular, erythemic patch with central clearing on the forearm and immediately diagnoses ringworm is thinking in the intuitive mode. Generally, human beings are most comfortable in this decision mode because it involves intuition and requires less mental effort and concentration. For clinicians, System 1 thinking is the default defense mechanism against “decision fatigue” and “cognitive overload” during a busy shift, and it is the thinking mode used when clinicians are stressed, hurried, tired, and working with a lack of resources.9,10 Croskerry maintains, however, that such clinical situations, and the reliance on System 1 thinking that such situations entail, can make clinicians more vulnerable to certain biases.9
System 2 thinking is analytic, deductive, slow, and deliberate. This mode of thinking has high predictive power with high reliability, and it is less influenced by the context or conditions in which the decision is being made.10 Clinicians use this mode of thinking when patients present with vague signs and symptoms and a diagnosis is not instantly recognized.10 System 2 decision making would be required, for example, when evaluating a 55-year-old woman with chest pain. The clinical condition requires the clinician to acquire more data and make a conscious effort to analyze results, and arriving at a clinical decision in this situation takes more time. Shortcuts due to time pressures can have devastating outcomes in this setting. It should be mentioned, however, that psychology research has shown that the System 2 analytic approach is mentally taxing and may also result in poor decisions (“thinking too much”).11
Intuitive and analytic thinking are not independent of each other. During a clinical encounter, there is unconscious switching back and forth between the two modes as the clinician evaluates the information at hand in order to produce a decision.12 A patient presenting with a chief complaint may trigger a System 1 decision, but due to uncertainty there may be a “System 2 override”where the clinician consciously forces herself to reassess and perform further analysis.10 System 1 intuitive decision processes become more dominant with experience. Many encounters requiring System 2 thinking early in a clinician’s career may become System 1 decisions as the clinician gains expertise.10 This results as the clinician develops a “mental library” of previous encounters with commonly seen medical conditions.13 It is important to note that clinical decision errors often result from a combination of knowledge gaps and processing malfunctions and not from one process alone.14
Similarly, diagnostic errors are not purely a result of cognitive biases or reliance on System 1 or System 2 thinking, but rather are a result of multiple factors.In a study that looked at provider time to diagnosis and accuracy of diagnosis, results indicated that System 1 reasoning was not more error prone than System 2 thinking.15 Experienced clinicians emphasize that errors can occur at any time or in any context in both System 1 and 2 modes of thinking.16
The vast majority of human decisions—95%—are made in System 1 mode, while only 5% of our “thinking” is conscious analytic thought.17 Croskerry suggests that clinical reasoning defaults to the faster, more mentally economic System 1 thinking, which can make clinicians prone to error by allowing intuition, heuristics, and processes that are most vulnerable to mistakes—stereotyping, prejudices, and biases—to influence a decision.9,18 Both novice and expert clinicians should be encouraged to develop insight into their intuitive and analytic decision-making processes and become aware of which thinking mode they are using in a specific clinical situation.
Continue for cognitive dispositions to respond >>
Cognitive Dispositions to Respond
Diagnostic errors are often associated with cognitive errors such as failures in perception, failed heuristics, and biases; as a group, these cognitive errors have been labeled cognitive dispositions to respond.1 In the medical and psychology literature, more than 100 CDRs have been identified.19 Common CDR/bias definitions are provided in the graphic.
In everyday practice, clinicians encounter clinical scenarios or situations where CDRs can affect decision making. The following brief clinical examples further illustrate the defining characteristics of the CDRs. Cognitive errors related to these CDRs can occur if a clinician does not remain completely objective.
Availability is a bias that applies the saying “more common diseases are common.” An example of this bias in practice would be a provider who has seen three patients with abdominal pain and diagnosed gastritis for each. A fourth patient presents with abdominal pain, is diagnosed with gastritis, but actually has appendicitis.
Search satisficing, or premature closure, occurs when one has found enough information to make a diagnosis and then stops looking for further causes or additional problems. For example, a PA rounds on a patient who is post-op day 1 from coronary bypass surgery and develops decreasing oxygen saturation. A chest x-ray reveals right lower lobe opacity consistent with either pneumonia or pleural effusion; antibiotics are started and oxygen concentration is increased on the ventilator. The radiologist later informs the PA that the patient also has a left-sided pneumothorax. The PA did not treat that because he stopped looking for other causes of the oxygen desaturation once the right lower lobe pneumonia was found.
Continue for confirmation >>
Confirmation bias occurs when clinicians seek to confirm a diagnosis rather than rule it out. For example, a patient presents with first-time, new-onset “classic” migraine symptoms, characterized as “the worst headache of her life.” The provider asks patient history questions to confirm the initial impression of a migraine headache and does not order a CT scan.
Posterior probability is a bias whereby the clinician gives excessive weight to a patient’s previous medical history. It occurs, for example, when a patient with chronic back pain is diagnosed with musculoskeletal back pain without considering other causes, such as urinary tract infection or pyelonephritis.
Diagnosis momentum bias occurs when a clinician relies on information handed down from numerous parties involved with the patient. An example is a patient who has a syncopal episode in church and several tonic-clonic movements while briefly unconscious. Nearby witnesses describe the event as a “seizure,” and paramedics relaying information to the emergency department indicate that the patient had a “seizure.” Ultimately, the triage information records “seizure” as the diagnosis. A cognitive error can occur if the treating clinician does not take a thorough history to consider an alternative diagnosis.
Fundamental attribution error bias occurs when a provider is judgmental and blames the patient for their disease. A provider who quips, “No wonder that patient has diabetes and hypertension; she weighs 325 lb,” is exhibiting fundamental attribution error bias.
Ascertainment bias allows preconceived notions, including stereotypes, to influence a clinician’s thinking. A provider who determines that all female patients with multiple somatic complaints have anxiety and depression is subject to this bias.
Triage cueing occurs when some aspect of the triage process influences the clinician’s thinking, such as when the clinician assumes that patients who are placed in the fast track are low acuity and therefore gives no consideration to higher acuity diagnoses.
Playing the odds assumes that a patient with a vague presentation has a benign condition rather than a serious one because the odds favor that. An example of this bias occurs when a 65-year-old woman with vomiting during flu season is quickly diagnosed with gastroenteritis. Fortunately, the patient is on a telemetry monitor while getting IV fluids and antinausea medication. The monitor results indicate that her vomiting episodes are occurring during long periods of sinus arrest.
Psych-out bias applies when signs or symptoms in a patient with a psychiatric diagnosis are ascribed to the underlying psychiatric condition and other serious possibilities are quickly dismissed. For example, a provider who assumes that an unstable psychiatric patient is nonadherent with her prescribed medication or is abusing substances rather than considering an underlying medical illness is demonstrating psych-out bias.
Illusory correlation bias occurs, for example, when the provider makes the assumption that the emergency department will be busy because there is a full moon.
Continue to find out if you are at risk for being wrong >>
AM I AT RISK FOR BEING WRONG?
Autonomous advanced practice clinicians in high-risk practice settings have an immense responsibility to ensure that their patients are getting the best possible care. It is documented that as expertise develops, knowledge and decision processes change. Ordinarily, highly experienced clinicians use the more time-efficient System 1 process when faced with common disorders; for more complex disorders, they change to System 2 thinking to facilitate a more comprehensive evaluation.13 In many instances, however, a provider may inadvertently take shortcuts to conclude the clinical encounter, including relying on intuitive thinking—which can be prone to bias—when analytic thinking is necessary.
Clinicians are usually unaware of the influence that biases may have on their decision making and should reflect on their behavior to determine if any biases exist. To improve patient safety and facilitate better care, all providers should perform a personal inventory to identify CDRs they may have developed. Questions that will help to reveal CDRs include
- Am I rushing to get off my shift on time?
- Was the patient “turned over” to me at the shift change?
- Have I allowed a previously negative experience with this patient to influence my objectivity and clinical decision-making?
- Am I tired?
- Has the diagnosis been suggested by the nurse, paramedic, or the patient’s family?9
- Has the diagnosis been suggested by the nurse,
If one or more biases are found, a purposeful effort to mentally “uncouple” from a bias should be done. This process is referred to as metacognition, or thinking about one’s own thought processes.9 Paramount among the thinking processes that may be at play is an awareness of how System 1 and System 2 thinking interact and affect clinical decision making, as this enables the clinician to recognize which mode of thinking they use to arrive at a decision and when they need to shift from intuitive to analytic thinking.
Another factor to consider is overconfidence: Berner and Graber note that a provider’s overconfidence3 in his or her own knowledge and experience and lack of awareness of when an “override” is needed can be a cause of diagnostic errors.18 The tendency to shore up existing beliefs rather than force a new cognitive strategy is a sign of a rigid thinking process that may ultimately result in a poor clinical decision.9 Finally, providers should be aware of their surroundings and practice environments. As noted earlier, emergency medicine, family medicine, internal medicine, and urgent care have high diagnostic error rates due, in part, to high patient volumes.1
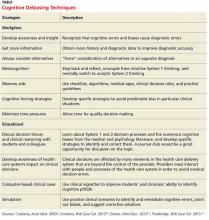
Once a tendency for a certain cognitive bias is recognized, the next step is to develop a sustainable method to counteract it, a process referred to as debiasing, to prevent cognitive errors. The table lists some workplace and educational debiasing techniques that have been described in the literature.20,21 Critics of cognitive debiasing argue that CDRs are preconscious, that awareness of CDRs is not enough to counteract their effects, and that there is no ability for one to develop “generic” conscious efforts to counter them.14 Their concern here is that a clinician may be able to counter a bias in one clinical context but not in another.14 It is clear that clinical reasoning is complex and involves many interrelated elements, such as clinical knowledge and critical thinking, with System 1 and 2 thinking working in tandem and metacognition overarching the whole process.21 Errors in diagnosis can have multiple causes and no single cognitive approach can be effective in addressing all of these causes. Knowing about cognitive bias helps clinicians address one possible element underlying diagnostic errors. Efforts to eliminate bias in clinical reasoning should begin early in clinical education; this can be done by incorporating instruction on clinical reasoning, including the relationship between intuitive and analytic decisions, metacognition, and awareness of the strengths and weaknesses of heuristics.22
In summary, in clinical situations where bias or uncertainty might exist, a clinician can make an effort to avoid a bad decision by
- Stepping back and reflecting to consider if a bias exists.
- Developing rules and mental procedures to reject a reflexive automatic response and force a “System 2 override.”9
- Developing “mental-ware” (mental techniques) to uncouple from a recognized or recurring cognitive bias.9
Continue to conclusion >>
Conclusion
This article reminds health care providers that cognitive biases can influence clinical decision-making. Clinicians should be aware of how System 1 and System 2 thinking couple with unconscious cognitive biases to affect clinical decisions and patient safety. Once a provider identifies a bias, he or she should attempt to employ one or more debiasing techniques. Medical decision errors usually occur due to multiple factors, and one thinking mode is not more error prone than the other (analytic versus intuitive). Cognitive errors are also caused by knowledge gaps and faulty patient data processing. Future research is needed to assess outcomes of quality improvement projects that include these components.
1. Croskerry P. Diagnostic failure: a cognitive and affective approach. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville, MD: Agency for Healthcare Research and Quality; 2005:241-254.
2. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
3. Berner ES, Graber ML. Overconfidence as a cause of diagnostic error in medicine. Am J Med. 2008;121(5A):S2-S23.
4. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680.
5. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy of Sciences; 1999.
6. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77:981-992.
7. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499.
8. Poon EG, Gandhi TK, Sequist TD, et al. “I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223-2228.
9. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58-ii64.
10. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41(2):155-162.
11. Wilson TD, Schooler JW. Thinking too much: introspection can reduce the quality of p and decisions. J Pers Soc Psychol. 1991;60(2): 181-192.
12. Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009; 84(8):1022-1028.
13. Norman G, Young M, Brooks L. Non-analytical models of clinical reasoning: the role of experience. Med Educ. 2007;41(12):1140-1145.
14. Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
15. Sherbino J, Dore KL, Wood TJ, et al. The relationship between response time and diagnostic accuracy. Acad Med. 2012;87(6):785-791.
16. Petrie D, Campbell S. Clinical decision making, fast and slow. Acad Med. 2013;88(5):557.
17. Lakoff G, Johnson M. Philosophy in the Flesh: The Embodied Mind and Its Challenge to Western Thought. New York, NY: Basic Books; 1999.
18. Sinclair D, Croskerry P. Patient safety and diagnostic error: tips for your next shift. Can Fam Physician. 2010;56(1):28-30.
19. Croskerry P. From mindless to mindful practice—cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448.
20. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-ii72.
21. Groves M. Understanding clinical reasoning: the next step in working out how it really works. Med Educ. 2012;46(5):444-446.
22. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(suppl 2):ii28-ii32.
CE/CME No: CR-1603
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• List the characteristics of System 1 and System 2 thinking.
• Explain how System 1 and System 2 thinking affects clinical decisions.
• Define the characteristics of no-fault, system, and cognitive errors and how they affect health care delivery.
• Describe how biases and cognitive dispositions to respond cause health care providers to make clinical decision errors.
• List some effective debiasing techniques to improve clinical decisions and patient safety.
FACULTY
David J. Klocko is an Associate Professor and Academic Coordinator in the Department of Physician Assistant Studies at the University of Texas Southwestern Medical Center, School of Health Professions, Dallas.
The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2016.
Article begins on next page >>
Diagnostic errors occur for many reasons, some of which are based in cognitive biases. Also called cognitive dispositions to respond (CDR), these can result from failures in perception, faulty mental shortcuts, or unconscious biases, and clinicians are usually unaware they exist. This article discusses the influence CDRs have on clinical decisions and walks you through methods for purposeful debiasing.
Diagnosis is the foundation of medicine ... [and] diagnostic reasoning is a critical aspect of clinical performance.1
— Pat Croskerry, MD, PhD
Diagnostic errors compromise patient safety and the quality of health care and account for the majority of paid malpractice claims. They are especially common in family medicine, internal medicine, emergency medicine, and urgent care, wherethe error rate can be as high as 15%.2 However, all health care providers are subject to errors in clinical judgment, regardless of the setting or specialty in which they practice.3
Clinical disciplines such as internal medicine and emergency medicine have higher error rates than the perceptual disciplines, radiology and pathology. Higher diagnostic error rates in the clinical disciplines are due to the elevated case complexity and the need for rapid interpretation of diagnostic studies. In the perceptual disciplines such as pathology and radiology, fewer time pressures and the ability to obtain a second opinion before making a diagnosis decrease error rates.3 In a National Practitioner Data Bank analysis, more diagnostic error claims occurred in the outpatient setting than in the inpatient setting.4
Quality assurance and performance improvement have become paramount for all health care providers. The modern patient safety movement began in 1999 with the Institute of Medicine (IOM) report To Err Is Human, which highlighted how a faulty health care system causes people to make mistakes and negatively impacts patient safety.5 Some examples of errors arising from imperfections in the health system include medication errors, patient falls, wrong-site surgeries, and improper patient identification. Despite an increased emphasis on patient safety and quality improvement, diagnostic error had not been a focus of attention for policy makers and institutions. Only since the IOM report was released have the medical profession and health policy makers begun to pay attention to diagnostic errors as a serious patient safety issue.5
Cognitive biases, or cognitive dispositions to respond (CDR), can influence clinical decision-making and lead to diagnostic errors. By understanding the thinking processes involved in diagnostic reasoning and the interaction between these processes and cognitive biases, clinicians can take steps to counteract the influence of cognitive biases on their clinical decisions. Here, a brief introduction to dual processing theory is provided, along with information to help clinicians identify potential cognitive biases. Workplace and educational debiasing techniques to counter biases that lead to cognitive decision errors are presented as well.
DIAGNOSTIC ERRORS
All advanced practice providers are at risk for making a clinical decision error. The diagnostic errors that are made in clinical practice can be classified into three broad etiologic categories6:
No-fault errors occur when a rare disease is misdiagnosed as something more common or a disease is silent or presents in an atypical manner. An example of an error that falls into this category is a delayed diagnosis of ischemic bowel in a diabetic patient with no abdominal pain. Another example is a patient with a language barrier who is not able to describe his or her symptoms clearly, leading the clinician to misinterpret the history. Patient nonadherence to recommended care can also be viewed as no-fault, as in the case of a patient diagnosed with colon cancer who did not obtain a recommended screening colonoscopy.6 In one study, no-fault errors accounted for 7% of diagnostic errors.7
System errors occur as a result of “latent” faults in the process of delivering care and can be technical or organizational in nature.6 Examples of diagnostic errors related to technical issues are misdiagnosis or delayed diagnosis resulting from lack of appropriate testing or equipment or from incorrect laboratory results caused by technical problems with equipment. Organizational shortcomings that contribute to diagnostic errors include imperfections in department policies, error tolerance culture, poor patient care coordination, communication problems, inadequate staff training, poor working conditions, unavailability of acute specialty care, and failing to follow up with patients having abnormal diagnostic study results.6 Excessive workload and heavy administrative responsibilities also can contribute to clinician decision errors.
An example of a specific clinical organizational system error would be a missed or delayed diagnosis of a cancer on a chest x-ray due to lack of an “over-read” by a radiologist. Due to cost, many private practices do not send all radiographs for a radiologist’s interpretation. Another example is a patient with a severe eye injury who develops complications after being transferred to another hospital because there is not an on-call ophthalmologist at the presenting hospital.6 Delays in reviewing patient laboratory results are a significant system-based source of medical errors. In one study, 83% of the physician respondents reported at least one delay in reviewing test results in the past two months, with 18% reporting five or more delays in reviewing test results over the same time period.8
Cognitive errors are caused by gaps in knowledge or experience, inadequate interpretation of diagnostic studies, or succumbing to faulty heuristics and biases.6 With cognitive errors, incorrect perception or interpretation of a clinical situation results in faulty differential diagnosis development. Confirmation bias is one type of cognitive error—once supporting information is found for a diagnosis, the search for information to rule out the diagnosis stops.6
An example of this would be a patient with an ankle fracture who is discharged with a missed proximal fibula fracture after the clinician performs a physical exam only on the ankle and orders an ankle x-ray. A cognitive error like this would occur due to inadvertent omission of an important physical exam component or the clinician not knowing the importance of examining the knee when evaluating an ankle fracture.
It is important to note that clinical decision errors are usually multifactorial. In a study involving 100 cases of diagnostic error in internal medicine, Graber and colleagues determined that in 46% of the cases errors were caused by a combination of system-related and cognitive factors.7
Continue for decision making >>
Decision Making: Dual Process Theory
Over the past two decades, dual process theory (DPT) has been recognized as a reliable model of the decision-making process in the psychology literature.9 DPT proposes two unique processes of thinking during decision making, referred to as System 1 and System 2, or Type 1 and Type 2, processes. A brief introduction to DPT is given here for practicing clinicians, but a detailed discussion of the literature pertaining to this concept is beyond the scope of this review.
System 1 processes are “intuitive,” utilize pattern recognition and heuristics, and rely heavily on the context or conditions in which the decision is made. The intuitive System 1 mode of thinking uses a pattern recognition or “gut reaction” approach.10 It is fast and reflexive but can be subject to deficits in predictive power and reliability.10 Experienced clinicians use pattern recognition in conditions presenting with classic signs and symptoms.10 For example, the clinician who evaluates a 12-year-old child with an annular, erythemic patch with central clearing on the forearm and immediately diagnoses ringworm is thinking in the intuitive mode. Generally, human beings are most comfortable in this decision mode because it involves intuition and requires less mental effort and concentration. For clinicians, System 1 thinking is the default defense mechanism against “decision fatigue” and “cognitive overload” during a busy shift, and it is the thinking mode used when clinicians are stressed, hurried, tired, and working with a lack of resources.9,10 Croskerry maintains, however, that such clinical situations, and the reliance on System 1 thinking that such situations entail, can make clinicians more vulnerable to certain biases.9
System 2 thinking is analytic, deductive, slow, and deliberate. This mode of thinking has high predictive power with high reliability, and it is less influenced by the context or conditions in which the decision is being made.10 Clinicians use this mode of thinking when patients present with vague signs and symptoms and a diagnosis is not instantly recognized.10 System 2 decision making would be required, for example, when evaluating a 55-year-old woman with chest pain. The clinical condition requires the clinician to acquire more data and make a conscious effort to analyze results, and arriving at a clinical decision in this situation takes more time. Shortcuts due to time pressures can have devastating outcomes in this setting. It should be mentioned, however, that psychology research has shown that the System 2 analytic approach is mentally taxing and may also result in poor decisions (“thinking too much”).11
Intuitive and analytic thinking are not independent of each other. During a clinical encounter, there is unconscious switching back and forth between the two modes as the clinician evaluates the information at hand in order to produce a decision.12 A patient presenting with a chief complaint may trigger a System 1 decision, but due to uncertainty there may be a “System 2 override”where the clinician consciously forces herself to reassess and perform further analysis.10 System 1 intuitive decision processes become more dominant with experience. Many encounters requiring System 2 thinking early in a clinician’s career may become System 1 decisions as the clinician gains expertise.10 This results as the clinician develops a “mental library” of previous encounters with commonly seen medical conditions.13 It is important to note that clinical decision errors often result from a combination of knowledge gaps and processing malfunctions and not from one process alone.14
Similarly, diagnostic errors are not purely a result of cognitive biases or reliance on System 1 or System 2 thinking, but rather are a result of multiple factors.In a study that looked at provider time to diagnosis and accuracy of diagnosis, results indicated that System 1 reasoning was not more error prone than System 2 thinking.15 Experienced clinicians emphasize that errors can occur at any time or in any context in both System 1 and 2 modes of thinking.16
The vast majority of human decisions—95%—are made in System 1 mode, while only 5% of our “thinking” is conscious analytic thought.17 Croskerry suggests that clinical reasoning defaults to the faster, more mentally economic System 1 thinking, which can make clinicians prone to error by allowing intuition, heuristics, and processes that are most vulnerable to mistakes—stereotyping, prejudices, and biases—to influence a decision.9,18 Both novice and expert clinicians should be encouraged to develop insight into their intuitive and analytic decision-making processes and become aware of which thinking mode they are using in a specific clinical situation.
Continue for cognitive dispositions to respond >>
Cognitive Dispositions to Respond
Diagnostic errors are often associated with cognitive errors such as failures in perception, failed heuristics, and biases; as a group, these cognitive errors have been labeled cognitive dispositions to respond.1 In the medical and psychology literature, more than 100 CDRs have been identified.19 Common CDR/bias definitions are provided in the graphic.
In everyday practice, clinicians encounter clinical scenarios or situations where CDRs can affect decision making. The following brief clinical examples further illustrate the defining characteristics of the CDRs. Cognitive errors related to these CDRs can occur if a clinician does not remain completely objective.
Availability is a bias that applies the saying “more common diseases are common.” An example of this bias in practice would be a provider who has seen three patients with abdominal pain and diagnosed gastritis for each. A fourth patient presents with abdominal pain, is diagnosed with gastritis, but actually has appendicitis.
Search satisficing, or premature closure, occurs when one has found enough information to make a diagnosis and then stops looking for further causes or additional problems. For example, a PA rounds on a patient who is post-op day 1 from coronary bypass surgery and develops decreasing oxygen saturation. A chest x-ray reveals right lower lobe opacity consistent with either pneumonia or pleural effusion; antibiotics are started and oxygen concentration is increased on the ventilator. The radiologist later informs the PA that the patient also has a left-sided pneumothorax. The PA did not treat that because he stopped looking for other causes of the oxygen desaturation once the right lower lobe pneumonia was found.
Continue for confirmation >>
Confirmation bias occurs when clinicians seek to confirm a diagnosis rather than rule it out. For example, a patient presents with first-time, new-onset “classic” migraine symptoms, characterized as “the worst headache of her life.” The provider asks patient history questions to confirm the initial impression of a migraine headache and does not order a CT scan.
Posterior probability is a bias whereby the clinician gives excessive weight to a patient’s previous medical history. It occurs, for example, when a patient with chronic back pain is diagnosed with musculoskeletal back pain without considering other causes, such as urinary tract infection or pyelonephritis.
Diagnosis momentum bias occurs when a clinician relies on information handed down from numerous parties involved with the patient. An example is a patient who has a syncopal episode in church and several tonic-clonic movements while briefly unconscious. Nearby witnesses describe the event as a “seizure,” and paramedics relaying information to the emergency department indicate that the patient had a “seizure.” Ultimately, the triage information records “seizure” as the diagnosis. A cognitive error can occur if the treating clinician does not take a thorough history to consider an alternative diagnosis.
Fundamental attribution error bias occurs when a provider is judgmental and blames the patient for their disease. A provider who quips, “No wonder that patient has diabetes and hypertension; she weighs 325 lb,” is exhibiting fundamental attribution error bias.
Ascertainment bias allows preconceived notions, including stereotypes, to influence a clinician’s thinking. A provider who determines that all female patients with multiple somatic complaints have anxiety and depression is subject to this bias.
Triage cueing occurs when some aspect of the triage process influences the clinician’s thinking, such as when the clinician assumes that patients who are placed in the fast track are low acuity and therefore gives no consideration to higher acuity diagnoses.
Playing the odds assumes that a patient with a vague presentation has a benign condition rather than a serious one because the odds favor that. An example of this bias occurs when a 65-year-old woman with vomiting during flu season is quickly diagnosed with gastroenteritis. Fortunately, the patient is on a telemetry monitor while getting IV fluids and antinausea medication. The monitor results indicate that her vomiting episodes are occurring during long periods of sinus arrest.
Psych-out bias applies when signs or symptoms in a patient with a psychiatric diagnosis are ascribed to the underlying psychiatric condition and other serious possibilities are quickly dismissed. For example, a provider who assumes that an unstable psychiatric patient is nonadherent with her prescribed medication or is abusing substances rather than considering an underlying medical illness is demonstrating psych-out bias.
Illusory correlation bias occurs, for example, when the provider makes the assumption that the emergency department will be busy because there is a full moon.
Continue to find out if you are at risk for being wrong >>
AM I AT RISK FOR BEING WRONG?
Autonomous advanced practice clinicians in high-risk practice settings have an immense responsibility to ensure that their patients are getting the best possible care. It is documented that as expertise develops, knowledge and decision processes change. Ordinarily, highly experienced clinicians use the more time-efficient System 1 process when faced with common disorders; for more complex disorders, they change to System 2 thinking to facilitate a more comprehensive evaluation.13 In many instances, however, a provider may inadvertently take shortcuts to conclude the clinical encounter, including relying on intuitive thinking—which can be prone to bias—when analytic thinking is necessary.
Clinicians are usually unaware of the influence that biases may have on their decision making and should reflect on their behavior to determine if any biases exist. To improve patient safety and facilitate better care, all providers should perform a personal inventory to identify CDRs they may have developed. Questions that will help to reveal CDRs include
- Am I rushing to get off my shift on time?
- Was the patient “turned over” to me at the shift change?
- Have I allowed a previously negative experience with this patient to influence my objectivity and clinical decision-making?
- Am I tired?
- Has the diagnosis been suggested by the nurse, paramedic, or the patient’s family?9
- Has the diagnosis been suggested by the nurse,
If one or more biases are found, a purposeful effort to mentally “uncouple” from a bias should be done. This process is referred to as metacognition, or thinking about one’s own thought processes.9 Paramount among the thinking processes that may be at play is an awareness of how System 1 and System 2 thinking interact and affect clinical decision making, as this enables the clinician to recognize which mode of thinking they use to arrive at a decision and when they need to shift from intuitive to analytic thinking.
Another factor to consider is overconfidence: Berner and Graber note that a provider’s overconfidence3 in his or her own knowledge and experience and lack of awareness of when an “override” is needed can be a cause of diagnostic errors.18 The tendency to shore up existing beliefs rather than force a new cognitive strategy is a sign of a rigid thinking process that may ultimately result in a poor clinical decision.9 Finally, providers should be aware of their surroundings and practice environments. As noted earlier, emergency medicine, family medicine, internal medicine, and urgent care have high diagnostic error rates due, in part, to high patient volumes.1
Once a tendency for a certain cognitive bias is recognized, the next step is to develop a sustainable method to counteract it, a process referred to as debiasing, to prevent cognitive errors. The table lists some workplace and educational debiasing techniques that have been described in the literature.20,21 Critics of cognitive debiasing argue that CDRs are preconscious, that awareness of CDRs is not enough to counteract their effects, and that there is no ability for one to develop “generic” conscious efforts to counter them.14 Their concern here is that a clinician may be able to counter a bias in one clinical context but not in another.14 It is clear that clinical reasoning is complex and involves many interrelated elements, such as clinical knowledge and critical thinking, with System 1 and 2 thinking working in tandem and metacognition overarching the whole process.21 Errors in diagnosis can have multiple causes and no single cognitive approach can be effective in addressing all of these causes. Knowing about cognitive bias helps clinicians address one possible element underlying diagnostic errors. Efforts to eliminate bias in clinical reasoning should begin early in clinical education; this can be done by incorporating instruction on clinical reasoning, including the relationship between intuitive and analytic decisions, metacognition, and awareness of the strengths and weaknesses of heuristics.22
In summary, in clinical situations where bias or uncertainty might exist, a clinician can make an effort to avoid a bad decision by
- Stepping back and reflecting to consider if a bias exists.
- Developing rules and mental procedures to reject a reflexive automatic response and force a “System 2 override.”9
- Developing “mental-ware” (mental techniques) to uncouple from a recognized or recurring cognitive bias.9
Continue to conclusion >>
Conclusion
This article reminds health care providers that cognitive biases can influence clinical decision-making. Clinicians should be aware of how System 1 and System 2 thinking couple with unconscious cognitive biases to affect clinical decisions and patient safety. Once a provider identifies a bias, he or she should attempt to employ one or more debiasing techniques. Medical decision errors usually occur due to multiple factors, and one thinking mode is not more error prone than the other (analytic versus intuitive). Cognitive errors are also caused by knowledge gaps and faulty patient data processing. Future research is needed to assess outcomes of quality improvement projects that include these components.
CE/CME No: CR-1603
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• List the characteristics of System 1 and System 2 thinking.
• Explain how System 1 and System 2 thinking affects clinical decisions.
• Define the characteristics of no-fault, system, and cognitive errors and how they affect health care delivery.
• Describe how biases and cognitive dispositions to respond cause health care providers to make clinical decision errors.
• List some effective debiasing techniques to improve clinical decisions and patient safety.
FACULTY
David J. Klocko is an Associate Professor and Academic Coordinator in the Department of Physician Assistant Studies at the University of Texas Southwestern Medical Center, School of Health Professions, Dallas.
The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2016.
Article begins on next page >>
Diagnostic errors occur for many reasons, some of which are based in cognitive biases. Also called cognitive dispositions to respond (CDR), these can result from failures in perception, faulty mental shortcuts, or unconscious biases, and clinicians are usually unaware they exist. This article discusses the influence CDRs have on clinical decisions and walks you through methods for purposeful debiasing.
Diagnosis is the foundation of medicine ... [and] diagnostic reasoning is a critical aspect of clinical performance.1
— Pat Croskerry, MD, PhD
Diagnostic errors compromise patient safety and the quality of health care and account for the majority of paid malpractice claims. They are especially common in family medicine, internal medicine, emergency medicine, and urgent care, wherethe error rate can be as high as 15%.2 However, all health care providers are subject to errors in clinical judgment, regardless of the setting or specialty in which they practice.3
Clinical disciplines such as internal medicine and emergency medicine have higher error rates than the perceptual disciplines, radiology and pathology. Higher diagnostic error rates in the clinical disciplines are due to the elevated case complexity and the need for rapid interpretation of diagnostic studies. In the perceptual disciplines such as pathology and radiology, fewer time pressures and the ability to obtain a second opinion before making a diagnosis decrease error rates.3 In a National Practitioner Data Bank analysis, more diagnostic error claims occurred in the outpatient setting than in the inpatient setting.4
Quality assurance and performance improvement have become paramount for all health care providers. The modern patient safety movement began in 1999 with the Institute of Medicine (IOM) report To Err Is Human, which highlighted how a faulty health care system causes people to make mistakes and negatively impacts patient safety.5 Some examples of errors arising from imperfections in the health system include medication errors, patient falls, wrong-site surgeries, and improper patient identification. Despite an increased emphasis on patient safety and quality improvement, diagnostic error had not been a focus of attention for policy makers and institutions. Only since the IOM report was released have the medical profession and health policy makers begun to pay attention to diagnostic errors as a serious patient safety issue.5
Cognitive biases, or cognitive dispositions to respond (CDR), can influence clinical decision-making and lead to diagnostic errors. By understanding the thinking processes involved in diagnostic reasoning and the interaction between these processes and cognitive biases, clinicians can take steps to counteract the influence of cognitive biases on their clinical decisions. Here, a brief introduction to dual processing theory is provided, along with information to help clinicians identify potential cognitive biases. Workplace and educational debiasing techniques to counter biases that lead to cognitive decision errors are presented as well.
DIAGNOSTIC ERRORS
All advanced practice providers are at risk for making a clinical decision error. The diagnostic errors that are made in clinical practice can be classified into three broad etiologic categories6:
No-fault errors occur when a rare disease is misdiagnosed as something more common or a disease is silent or presents in an atypical manner. An example of an error that falls into this category is a delayed diagnosis of ischemic bowel in a diabetic patient with no abdominal pain. Another example is a patient with a language barrier who is not able to describe his or her symptoms clearly, leading the clinician to misinterpret the history. Patient nonadherence to recommended care can also be viewed as no-fault, as in the case of a patient diagnosed with colon cancer who did not obtain a recommended screening colonoscopy.6 In one study, no-fault errors accounted for 7% of diagnostic errors.7
System errors occur as a result of “latent” faults in the process of delivering care and can be technical or organizational in nature.6 Examples of diagnostic errors related to technical issues are misdiagnosis or delayed diagnosis resulting from lack of appropriate testing or equipment or from incorrect laboratory results caused by technical problems with equipment. Organizational shortcomings that contribute to diagnostic errors include imperfections in department policies, error tolerance culture, poor patient care coordination, communication problems, inadequate staff training, poor working conditions, unavailability of acute specialty care, and failing to follow up with patients having abnormal diagnostic study results.6 Excessive workload and heavy administrative responsibilities also can contribute to clinician decision errors.
An example of a specific clinical organizational system error would be a missed or delayed diagnosis of a cancer on a chest x-ray due to lack of an “over-read” by a radiologist. Due to cost, many private practices do not send all radiographs for a radiologist’s interpretation. Another example is a patient with a severe eye injury who develops complications after being transferred to another hospital because there is not an on-call ophthalmologist at the presenting hospital.6 Delays in reviewing patient laboratory results are a significant system-based source of medical errors. In one study, 83% of the physician respondents reported at least one delay in reviewing test results in the past two months, with 18% reporting five or more delays in reviewing test results over the same time period.8
Cognitive errors are caused by gaps in knowledge or experience, inadequate interpretation of diagnostic studies, or succumbing to faulty heuristics and biases.6 With cognitive errors, incorrect perception or interpretation of a clinical situation results in faulty differential diagnosis development. Confirmation bias is one type of cognitive error—once supporting information is found for a diagnosis, the search for information to rule out the diagnosis stops.6
An example of this would be a patient with an ankle fracture who is discharged with a missed proximal fibula fracture after the clinician performs a physical exam only on the ankle and orders an ankle x-ray. A cognitive error like this would occur due to inadvertent omission of an important physical exam component or the clinician not knowing the importance of examining the knee when evaluating an ankle fracture.
It is important to note that clinical decision errors are usually multifactorial. In a study involving 100 cases of diagnostic error in internal medicine, Graber and colleagues determined that in 46% of the cases errors were caused by a combination of system-related and cognitive factors.7
Continue for decision making >>
Decision Making: Dual Process Theory
Over the past two decades, dual process theory (DPT) has been recognized as a reliable model of the decision-making process in the psychology literature.9 DPT proposes two unique processes of thinking during decision making, referred to as System 1 and System 2, or Type 1 and Type 2, processes. A brief introduction to DPT is given here for practicing clinicians, but a detailed discussion of the literature pertaining to this concept is beyond the scope of this review.
System 1 processes are “intuitive,” utilize pattern recognition and heuristics, and rely heavily on the context or conditions in which the decision is made. The intuitive System 1 mode of thinking uses a pattern recognition or “gut reaction” approach.10 It is fast and reflexive but can be subject to deficits in predictive power and reliability.10 Experienced clinicians use pattern recognition in conditions presenting with classic signs and symptoms.10 For example, the clinician who evaluates a 12-year-old child with an annular, erythemic patch with central clearing on the forearm and immediately diagnoses ringworm is thinking in the intuitive mode. Generally, human beings are most comfortable in this decision mode because it involves intuition and requires less mental effort and concentration. For clinicians, System 1 thinking is the default defense mechanism against “decision fatigue” and “cognitive overload” during a busy shift, and it is the thinking mode used when clinicians are stressed, hurried, tired, and working with a lack of resources.9,10 Croskerry maintains, however, that such clinical situations, and the reliance on System 1 thinking that such situations entail, can make clinicians more vulnerable to certain biases.9
System 2 thinking is analytic, deductive, slow, and deliberate. This mode of thinking has high predictive power with high reliability, and it is less influenced by the context or conditions in which the decision is being made.10 Clinicians use this mode of thinking when patients present with vague signs and symptoms and a diagnosis is not instantly recognized.10 System 2 decision making would be required, for example, when evaluating a 55-year-old woman with chest pain. The clinical condition requires the clinician to acquire more data and make a conscious effort to analyze results, and arriving at a clinical decision in this situation takes more time. Shortcuts due to time pressures can have devastating outcomes in this setting. It should be mentioned, however, that psychology research has shown that the System 2 analytic approach is mentally taxing and may also result in poor decisions (“thinking too much”).11
Intuitive and analytic thinking are not independent of each other. During a clinical encounter, there is unconscious switching back and forth between the two modes as the clinician evaluates the information at hand in order to produce a decision.12 A patient presenting with a chief complaint may trigger a System 1 decision, but due to uncertainty there may be a “System 2 override”where the clinician consciously forces herself to reassess and perform further analysis.10 System 1 intuitive decision processes become more dominant with experience. Many encounters requiring System 2 thinking early in a clinician’s career may become System 1 decisions as the clinician gains expertise.10 This results as the clinician develops a “mental library” of previous encounters with commonly seen medical conditions.13 It is important to note that clinical decision errors often result from a combination of knowledge gaps and processing malfunctions and not from one process alone.14
Similarly, diagnostic errors are not purely a result of cognitive biases or reliance on System 1 or System 2 thinking, but rather are a result of multiple factors.In a study that looked at provider time to diagnosis and accuracy of diagnosis, results indicated that System 1 reasoning was not more error prone than System 2 thinking.15 Experienced clinicians emphasize that errors can occur at any time or in any context in both System 1 and 2 modes of thinking.16
The vast majority of human decisions—95%—are made in System 1 mode, while only 5% of our “thinking” is conscious analytic thought.17 Croskerry suggests that clinical reasoning defaults to the faster, more mentally economic System 1 thinking, which can make clinicians prone to error by allowing intuition, heuristics, and processes that are most vulnerable to mistakes—stereotyping, prejudices, and biases—to influence a decision.9,18 Both novice and expert clinicians should be encouraged to develop insight into their intuitive and analytic decision-making processes and become aware of which thinking mode they are using in a specific clinical situation.
Continue for cognitive dispositions to respond >>
Cognitive Dispositions to Respond
Diagnostic errors are often associated with cognitive errors such as failures in perception, failed heuristics, and biases; as a group, these cognitive errors have been labeled cognitive dispositions to respond.1 In the medical and psychology literature, more than 100 CDRs have been identified.19 Common CDR/bias definitions are provided in the graphic.
In everyday practice, clinicians encounter clinical scenarios or situations where CDRs can affect decision making. The following brief clinical examples further illustrate the defining characteristics of the CDRs. Cognitive errors related to these CDRs can occur if a clinician does not remain completely objective.
Availability is a bias that applies the saying “more common diseases are common.” An example of this bias in practice would be a provider who has seen three patients with abdominal pain and diagnosed gastritis for each. A fourth patient presents with abdominal pain, is diagnosed with gastritis, but actually has appendicitis.
Search satisficing, or premature closure, occurs when one has found enough information to make a diagnosis and then stops looking for further causes or additional problems. For example, a PA rounds on a patient who is post-op day 1 from coronary bypass surgery and develops decreasing oxygen saturation. A chest x-ray reveals right lower lobe opacity consistent with either pneumonia or pleural effusion; antibiotics are started and oxygen concentration is increased on the ventilator. The radiologist later informs the PA that the patient also has a left-sided pneumothorax. The PA did not treat that because he stopped looking for other causes of the oxygen desaturation once the right lower lobe pneumonia was found.
Continue for confirmation >>
Confirmation bias occurs when clinicians seek to confirm a diagnosis rather than rule it out. For example, a patient presents with first-time, new-onset “classic” migraine symptoms, characterized as “the worst headache of her life.” The provider asks patient history questions to confirm the initial impression of a migraine headache and does not order a CT scan.
Posterior probability is a bias whereby the clinician gives excessive weight to a patient’s previous medical history. It occurs, for example, when a patient with chronic back pain is diagnosed with musculoskeletal back pain without considering other causes, such as urinary tract infection or pyelonephritis.
Diagnosis momentum bias occurs when a clinician relies on information handed down from numerous parties involved with the patient. An example is a patient who has a syncopal episode in church and several tonic-clonic movements while briefly unconscious. Nearby witnesses describe the event as a “seizure,” and paramedics relaying information to the emergency department indicate that the patient had a “seizure.” Ultimately, the triage information records “seizure” as the diagnosis. A cognitive error can occur if the treating clinician does not take a thorough history to consider an alternative diagnosis.
Fundamental attribution error bias occurs when a provider is judgmental and blames the patient for their disease. A provider who quips, “No wonder that patient has diabetes and hypertension; she weighs 325 lb,” is exhibiting fundamental attribution error bias.
Ascertainment bias allows preconceived notions, including stereotypes, to influence a clinician’s thinking. A provider who determines that all female patients with multiple somatic complaints have anxiety and depression is subject to this bias.
Triage cueing occurs when some aspect of the triage process influences the clinician’s thinking, such as when the clinician assumes that patients who are placed in the fast track are low acuity and therefore gives no consideration to higher acuity diagnoses.
Playing the odds assumes that a patient with a vague presentation has a benign condition rather than a serious one because the odds favor that. An example of this bias occurs when a 65-year-old woman with vomiting during flu season is quickly diagnosed with gastroenteritis. Fortunately, the patient is on a telemetry monitor while getting IV fluids and antinausea medication. The monitor results indicate that her vomiting episodes are occurring during long periods of sinus arrest.
Psych-out bias applies when signs or symptoms in a patient with a psychiatric diagnosis are ascribed to the underlying psychiatric condition and other serious possibilities are quickly dismissed. For example, a provider who assumes that an unstable psychiatric patient is nonadherent with her prescribed medication or is abusing substances rather than considering an underlying medical illness is demonstrating psych-out bias.
Illusory correlation bias occurs, for example, when the provider makes the assumption that the emergency department will be busy because there is a full moon.
Continue to find out if you are at risk for being wrong >>
AM I AT RISK FOR BEING WRONG?
Autonomous advanced practice clinicians in high-risk practice settings have an immense responsibility to ensure that their patients are getting the best possible care. It is documented that as expertise develops, knowledge and decision processes change. Ordinarily, highly experienced clinicians use the more time-efficient System 1 process when faced with common disorders; for more complex disorders, they change to System 2 thinking to facilitate a more comprehensive evaluation.13 In many instances, however, a provider may inadvertently take shortcuts to conclude the clinical encounter, including relying on intuitive thinking—which can be prone to bias—when analytic thinking is necessary.
Clinicians are usually unaware of the influence that biases may have on their decision making and should reflect on their behavior to determine if any biases exist. To improve patient safety and facilitate better care, all providers should perform a personal inventory to identify CDRs they may have developed. Questions that will help to reveal CDRs include
- Am I rushing to get off my shift on time?
- Was the patient “turned over” to me at the shift change?
- Have I allowed a previously negative experience with this patient to influence my objectivity and clinical decision-making?
- Am I tired?
- Has the diagnosis been suggested by the nurse, paramedic, or the patient’s family?9
- Has the diagnosis been suggested by the nurse,
If one or more biases are found, a purposeful effort to mentally “uncouple” from a bias should be done. This process is referred to as metacognition, or thinking about one’s own thought processes.9 Paramount among the thinking processes that may be at play is an awareness of how System 1 and System 2 thinking interact and affect clinical decision making, as this enables the clinician to recognize which mode of thinking they use to arrive at a decision and when they need to shift from intuitive to analytic thinking.
Another factor to consider is overconfidence: Berner and Graber note that a provider’s overconfidence3 in his or her own knowledge and experience and lack of awareness of when an “override” is needed can be a cause of diagnostic errors.18 The tendency to shore up existing beliefs rather than force a new cognitive strategy is a sign of a rigid thinking process that may ultimately result in a poor clinical decision.9 Finally, providers should be aware of their surroundings and practice environments. As noted earlier, emergency medicine, family medicine, internal medicine, and urgent care have high diagnostic error rates due, in part, to high patient volumes.1
Once a tendency for a certain cognitive bias is recognized, the next step is to develop a sustainable method to counteract it, a process referred to as debiasing, to prevent cognitive errors. The table lists some workplace and educational debiasing techniques that have been described in the literature.20,21 Critics of cognitive debiasing argue that CDRs are preconscious, that awareness of CDRs is not enough to counteract their effects, and that there is no ability for one to develop “generic” conscious efforts to counter them.14 Their concern here is that a clinician may be able to counter a bias in one clinical context but not in another.14 It is clear that clinical reasoning is complex and involves many interrelated elements, such as clinical knowledge and critical thinking, with System 1 and 2 thinking working in tandem and metacognition overarching the whole process.21 Errors in diagnosis can have multiple causes and no single cognitive approach can be effective in addressing all of these causes. Knowing about cognitive bias helps clinicians address one possible element underlying diagnostic errors. Efforts to eliminate bias in clinical reasoning should begin early in clinical education; this can be done by incorporating instruction on clinical reasoning, including the relationship between intuitive and analytic decisions, metacognition, and awareness of the strengths and weaknesses of heuristics.22
In summary, in clinical situations where bias or uncertainty might exist, a clinician can make an effort to avoid a bad decision by
- Stepping back and reflecting to consider if a bias exists.
- Developing rules and mental procedures to reject a reflexive automatic response and force a “System 2 override.”9
- Developing “mental-ware” (mental techniques) to uncouple from a recognized or recurring cognitive bias.9
Continue to conclusion >>
Conclusion
This article reminds health care providers that cognitive biases can influence clinical decision-making. Clinicians should be aware of how System 1 and System 2 thinking couple with unconscious cognitive biases to affect clinical decisions and patient safety. Once a provider identifies a bias, he or she should attempt to employ one or more debiasing techniques. Medical decision errors usually occur due to multiple factors, and one thinking mode is not more error prone than the other (analytic versus intuitive). Cognitive errors are also caused by knowledge gaps and faulty patient data processing. Future research is needed to assess outcomes of quality improvement projects that include these components.
1. Croskerry P. Diagnostic failure: a cognitive and affective approach. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville, MD: Agency for Healthcare Research and Quality; 2005:241-254.
2. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
3. Berner ES, Graber ML. Overconfidence as a cause of diagnostic error in medicine. Am J Med. 2008;121(5A):S2-S23.
4. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680.
5. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy of Sciences; 1999.
6. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77:981-992.
7. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499.
8. Poon EG, Gandhi TK, Sequist TD, et al. “I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223-2228.
9. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58-ii64.
10. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41(2):155-162.
11. Wilson TD, Schooler JW. Thinking too much: introspection can reduce the quality of p and decisions. J Pers Soc Psychol. 1991;60(2): 181-192.
12. Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009; 84(8):1022-1028.
13. Norman G, Young M, Brooks L. Non-analytical models of clinical reasoning: the role of experience. Med Educ. 2007;41(12):1140-1145.
14. Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
15. Sherbino J, Dore KL, Wood TJ, et al. The relationship between response time and diagnostic accuracy. Acad Med. 2012;87(6):785-791.
16. Petrie D, Campbell S. Clinical decision making, fast and slow. Acad Med. 2013;88(5):557.
17. Lakoff G, Johnson M. Philosophy in the Flesh: The Embodied Mind and Its Challenge to Western Thought. New York, NY: Basic Books; 1999.
18. Sinclair D, Croskerry P. Patient safety and diagnostic error: tips for your next shift. Can Fam Physician. 2010;56(1):28-30.
19. Croskerry P. From mindless to mindful practice—cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448.
20. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-ii72.
21. Groves M. Understanding clinical reasoning: the next step in working out how it really works. Med Educ. 2012;46(5):444-446.
22. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(suppl 2):ii28-ii32.
1. Croskerry P. Diagnostic failure: a cognitive and affective approach. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville, MD: Agency for Healthcare Research and Quality; 2005:241-254.
2. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
3. Berner ES, Graber ML. Overconfidence as a cause of diagnostic error in medicine. Am J Med. 2008;121(5A):S2-S23.
4. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680.
5. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy of Sciences; 1999.
6. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77:981-992.
7. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499.
8. Poon EG, Gandhi TK, Sequist TD, et al. “I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223-2228.
9. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58-ii64.
10. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41(2):155-162.
11. Wilson TD, Schooler JW. Thinking too much: introspection can reduce the quality of p and decisions. J Pers Soc Psychol. 1991;60(2): 181-192.
12. Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009; 84(8):1022-1028.
13. Norman G, Young M, Brooks L. Non-analytical models of clinical reasoning: the role of experience. Med Educ. 2007;41(12):1140-1145.
14. Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
15. Sherbino J, Dore KL, Wood TJ, et al. The relationship between response time and diagnostic accuracy. Acad Med. 2012;87(6):785-791.
16. Petrie D, Campbell S. Clinical decision making, fast and slow. Acad Med. 2013;88(5):557.
17. Lakoff G, Johnson M. Philosophy in the Flesh: The Embodied Mind and Its Challenge to Western Thought. New York, NY: Basic Books; 1999.
18. Sinclair D, Croskerry P. Patient safety and diagnostic error: tips for your next shift. Can Fam Physician. 2010;56(1):28-30.
19. Croskerry P. From mindless to mindful practice—cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448.
20. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-ii72.
21. Groves M. Understanding clinical reasoning: the next step in working out how it really works. Med Educ. 2012;46(5):444-446.
22. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(suppl 2):ii28-ii32.
Woman, 66, With Persistent Abdominal and Back Pain
A 66-year-old Latin American woman presented to the emergency department (ED) with persistent abdominal and back pain of about one month’s duration. She had visited another ED eight days earlier for similar symptoms and was discharged home with a mild opioid pain medication and a proton pump inhibitor. However, she said that she had received neither a diagnosis nor an explanation for her symptoms.
Medical history, obtained with the assistance of an interpreter because the patient was not fluent in English, included hypertension, coronary artery disease, and hyperlipidemia; these had gone untreated for at least two years. She denied any personal or family history of cancer or endocrine disorders. Surgical history included a cholecystectomy and a percutaneous coronary intervention for an unknown coronary artery lesion.
She had a 14-pack-year history of cigarette smoking. Her medications included only ibuprofen and hydrocodone, and she had no known drug allergies. The patient denied use of herbal preparations or vitamin supplements and unusual dietary practices.
Review of systems revealed occasional dizziness, constipation, decreased appetite, and some mild confusion noted by family members, but no fever, chills, palpitations, chest pain, shortness of breath, muscle spasm, or weakness. Vital signs were normal. Physical examination was remarkable for tenderness of the upper quadrants of the abdomen with deep palpation, without guarding or rebound. Bony tenderness at the right anterior costal margin of the rib cage was also noted.
Laboratory work-up revealed marked hypercalcemia (15.4 mg/dL), electrolyte abnormalities, anemia, impaired renal function, and elevated alkaline phosphatase and globulin levels (see Table 1). In addition, a plain abdominal x-ray series was negative for acute findings, but x-rays of the right ribs revealed a fracture of the sixth rib and osteopenia.
Continued >>
The patient was admitted to the hospital for treatment of hypercalcemia and hypokalemia and for work-up of elevated alkaline phosphatase and abdominal pain. Upon admission, serum ionized calcium measurement confirmed true hypercalcemia. Additional diagnostic tests were then ordered to help differentiate between parathyroid hormone (PTH)–mediated and non-PTH–mediated causes for the hypercalcemia (see Table 2).
The patient’s PTH level was normal and the urine fractional excretion of calcium level was high, ruling out familial hypocalciuric hypercalcemia (FHH), in which urine calcium level is low. A measurement of PTH-related protein (PTHrP), secreted by some cancers, was normal, suggesting exclusion of solid tumor malignancy. Vitamin D toxicity was ruled out because the patient’s 1,25-dihydroxyvitamin D level was low.
The patient continued to experience vague abdominal, back, and rib pain that seemed to migrate daily and worsened with movement. A skeletal x-ray was performed and revealed numerous lytic lesions of the skull (Figure 1), midright humerus (Figure 2), and distal left radius.
Continue for discussion >>
DISCUSSION
Hypercalcemia is a relatively common presentation in primary care. The most frequent causes are primary hyperparathyroidism and malignancy.1 One in 500 patients will be diagnosed incidentally with asymptomatic hypercalcemia caused by underlying hyperparathyroidism.1
Clinical manifestations of hypercalcemia can range from no symptoms to multisystem disease. Fatigue, nausea, vomiting, constipation, bone pain, osteoporosis, nephrolithiasis, mental status changes, hypertension, anemia, elevated creatinine, and cardiac arrhythmias are among the more common clinical conditions associated with hypercalcemia (hence the mnemonic “stones, bones, abdominal moans, and psychic groans” for its signs and symptoms).1
Diagnostic overview
Causes of hypercalcemia are numerous and can be broken down into two categories: PTH-mediated and non-PTH–mediated. PTH-mediated causes include primary and secondary hyperparathyroidism and FHH. Non-PTH–mediated causes include vitamin D toxicity, solid tumor malignancy with or without metastasis, multiple myeloma (MM) and other plasma cell dyscrasias, granulomatous disease such as sarcoid, and some medications.1
The differential diagnosis for hypercalcemia begins with measurement of the patient’s intact PTH level. An elevated or high-normal result indicates a PTH-mediated cause, so 24-hour measurement of excretion of urinary calcium is the next step. A low or low-normal PTH level (< 20 pg/mL), however, suggests the cause is non-PTH-mediated.2 The diagnostic approach in this situation is more challenging because testing to exclude or confirm various potential causes can be expensive and time-consuming. The degree of hypercalcemia, however, can aid in the diagnosis: Primary hyperparathyroidism is often associated with borderline or mild hypercalcemia, while calcium values > 13 mg/dL are more common in patients with malignancies.2
PTH elevates calcium levels in the blood when ionized (free) calcium levels are low by increasing gastrointestinal absorption, decreasing urinary excretion, and increasing bone resorption.3 With malignant tumors such as lung, breast, and renal cell, osteolytic metastases can destroy the bone, resulting in release of calcium. In other cases, solid-tumor cancers produce PTHrP, which increases serum calcium. This latter situation is referred to as humoral hypercalcemia of malignancy.3 Lymphoma and granulomatous disease, such as sarcoid, can be associated with excess production of 1,25-dihydroxyvitamin D.2 If vitamin D is elevated but PTH and PTHrP are normal, a chest x-ray should be obtained to evaluate the patient for sarcoid or lymphoma.
Hypercalcemia work-up
The work-up for suspected hypercalcemia begins with measurement of the patient’s calcium level. Because calcium is bound to albumin in the blood, the standard serum calcium test may not reflect the true calcium level. (If albumin is high, the calcium level will be high, and vice versa.)1 The true (serum ionized) calcium level (also known as corrected calcium level) should always be calculated to confirm true hypercalcemia. A formula commonly used to calculate the corrected calcium level is
Corrected calcium (mg/dL) = (measured calcium [mg/dL]) + 0.8 (4.0 – serum albumin [mg/dL])
Direct measurement of the serum ionized calcium level is not affected by the albumin level and can also confirm true hypercalcemia.4
Once hypercalcemia is confirmed, the next step is to measure the patient’s intact PTH level.
If intact PTH is elevated or high normal, consider primary hyperparathyroidism or FHH and confirm by obtaining the urine calcium level.
• If urine calcium level is high (> 200 mg/24 h), the diagnosis is primary hyperparathyroidism.
• If urine calcium level is low (< 100 mg/24 h), the diagnosis is FHH.
If intact PTH is low, consider non–PTH-mediated causes and confirm by obtaining PTHrP and vitamin D levels.
• If PTHrP level is elevated, scan for malignancy.
• If 1,25-dihydroxyvitamin D level is elevated, order a chest x-ray to rule out sarcoid or lymphoma.
• If both PTHrP and vitamin D levels are normal, order both serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP) with immunofixation to rule out MM.
• If vitamin D level is elevated, check vitamin and herbal supplement use for excessive vitamin D intake.
Treatment of symptomatic hypercalcemia
The goals of treatment of symptomatic hypercalcemia are to reduce the serum calcium level to the normal range and to treat the underlying cause.1 Mild hypercalcemia (calcium level, 10-12 mg/dL) is typically asymptomatic and does not need to be treated. Moderate hypercalcemia (calcium level, 12-14 mg/dL) may not require treatment unless the patient is symptomatic and/or has had an acute rise in calcium level.1 In mild to moderate hypercalcemia, the serum calcium level should be monitored to establish a trend.
Treatment for symptomatic moderate and severe hypercalcemia (calcium level, > 14 mg/dL) typically involves a similar regimen:
• Volume expansion with isotonic saline at an initial rate of 2 to 4 L/d, which is then adjusted to achieve 200 mL/h of continuous urine output. IV furosemide can be used with caution (10-20 mg IV as needed) to promote diuresis if volume overload is a concern (furosemide promotes renal excretion of calcium).
• Administration of subcutaneous calcitonin (4-8 IU/kg, repeated every 6 h for 24 h). Calcitonin works rapidly to lower calcium levels in 4 to 6 h.
• Concurrent administration of IV bisphosphonate (zoledronic acid [4 mg over 15 min] or pamidronate [60-90 mg over 4 h]). Pamidronate is superior for reversal of malignancy-related hypercalcemia.1
Hypercalcemia and multiple myeloma
MM is a malignant neoplasm of plasma cells that accounts for approximately 1% of all cancers and about 10% of hematologic malignancies in the United States, with a median patient age of 70 at diagnosis.5-7 In MM, myeloma cells induce the secretion of cytokines and growth factors that alter plasma cells, activate osteoclasts, suppress osteoblasts, cause abnormal interactions between plasma cells and bone marrow, and stimulate aberrant angiogenesis.8 Osteoclastic bone resorption produces hypercalcemia as well as the lytic lesions seen on x-ray.7
Approximately 74% of patients present with typical MM symptoms of calcium elevation in the blood, renal insufficiency, anemia, and bone lesions, known as CRAB symptoms, but other myeloma-related manifestations may be present.9
Diagnostic criteria for MM include the following (all three must be present):10
• Monoclonal bone marrow plasma cells ≥ 10% and/or a biopsy-proven plasmacytoma
• Monoclonal protein in the serum and/or urine (if none is detected, disease is nonsecretory and diagnosis requires ≥ 30% bone marrow plasma cells and/or biopsy-proven plasmacytoma)
• Myeloma-related organ dysfunction, indicated by at least one of the CRAB symptoms.
In the absence of CRAB symptoms, an asymptomatic patient may have an MM precursor syndrome: monoclonal gammopathy of undetermined significance or smoldering (or indolent) MM.10
Treatment of multiple myeloma
In recent years, the use of induction therapy followed by autologous stem cell transplantation and the development of novel therapeutic agents have extended overall survival for patients with MM. These agents include proteasome inhibitors (bortezomib and the second-generation carfilzomib)11 and immunomodulators (thalidomide and the second-generation lenalidomide). Early diagnosis and treatment can improve progression-free survival as well as overall survival, including recovery of renal function for patients with renal failure.12 With survival ranging from one year or less—with aggressive disease—to 10 years or more for patients with responsive disease,7 there remains no cure for MM.
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
Further work-up included SPEP and UPEP with immunofixation, which revealed marked IgG free λ light chains with an M (monoclonal) component, making MM a very likely diagnosis. Confirmation by means of bone marrow biopsy was indicated, but the patient refused the procedure.
The patient’s hypercalcemia was treated by IV administration of calcitonin with isotonic saline. This reduced the serum calcium level from 15.4 mg/dL to 10.6 mg/dL within 48 hours. One dose of ergocalciferol (vitamin D2) was then administered to promote intestinal absorption of calcium and support bone mineralization, further lowering the patient’s serum calcium level to a normal 8.9 mg/dL. Hypokalemia was treated with oral potassium supplementation.
The patient, now stable, was referred to the hematology/oncology and bone mineral metabolism clinics and was discharged from the hospital. She did not keep those appointments and was lost to follow-up.
CONCLUSION
The most common causes of hypercalcemia are hyperparathyroidism and malignancy. Most cases do not require treatment unless the calcium level is >14 mg/dL and/or the patient is symptomatic. Red flag symptoms include weakness, abdominal pain, mental status changes, and coma.4 Primary care clinicians should suspect MM in older patients with laboratory findings of hypercalcemia, anemia, and renal dysfunction, with lytic lesions on x-ray.
REFERENCES
1. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959-1966.
2. Endres DB. Investigation of hypercalcemia. Clin Biochem. 2012;45(12):
954-963.
3. Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care. 2008;35(2):215-237.
4. Sharma B, Misicko NE. How should you evaluate elevated calcium in an asymptomatic patient? J Fam Pract. 2008;57(4):267-269.
5. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
6. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
7. Shaheen SP, Talwalkar SS, Medeiros LJ. Multiple myeloma and immunosecretory disorders: an update. Adv Anat Pathol. 2008;15(4):196-210.
8. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11): 1046-1060.
9. Talamo G, Farooq U, Zangari M, et al. Beyond the CRAB symptoms: a study of presenting clinical manifestations of multiple myeloma. Clin Lymphoma Myeloma Leuk. 2010;10(6):464-468.
10. Palumbo A, Sezer O, Kyle R, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transportation. Leukemia. 2009;23(10):1716-1730.
11. Kyprolis [package insert]. South San Francisco, CA: Onyx Pharmaceuticals, Inc; 2012.
12. Suyani E, Sucak GT, Erten Y, et al. Evaluation of multiple myeloma patients presenting with renal failure in a university hospital in the year 2010. Ren Fail. 2012;34(2):257-262.
A 66-year-old Latin American woman presented to the emergency department (ED) with persistent abdominal and back pain of about one month’s duration. She had visited another ED eight days earlier for similar symptoms and was discharged home with a mild opioid pain medication and a proton pump inhibitor. However, she said that she had received neither a diagnosis nor an explanation for her symptoms.
Medical history, obtained with the assistance of an interpreter because the patient was not fluent in English, included hypertension, coronary artery disease, and hyperlipidemia; these had gone untreated for at least two years. She denied any personal or family history of cancer or endocrine disorders. Surgical history included a cholecystectomy and a percutaneous coronary intervention for an unknown coronary artery lesion.
She had a 14-pack-year history of cigarette smoking. Her medications included only ibuprofen and hydrocodone, and she had no known drug allergies. The patient denied use of herbal preparations or vitamin supplements and unusual dietary practices.
Review of systems revealed occasional dizziness, constipation, decreased appetite, and some mild confusion noted by family members, but no fever, chills, palpitations, chest pain, shortness of breath, muscle spasm, or weakness. Vital signs were normal. Physical examination was remarkable for tenderness of the upper quadrants of the abdomen with deep palpation, without guarding or rebound. Bony tenderness at the right anterior costal margin of the rib cage was also noted.
Laboratory work-up revealed marked hypercalcemia (15.4 mg/dL), electrolyte abnormalities, anemia, impaired renal function, and elevated alkaline phosphatase and globulin levels (see Table 1). In addition, a plain abdominal x-ray series was negative for acute findings, but x-rays of the right ribs revealed a fracture of the sixth rib and osteopenia.
Continued >>
The patient was admitted to the hospital for treatment of hypercalcemia and hypokalemia and for work-up of elevated alkaline phosphatase and abdominal pain. Upon admission, serum ionized calcium measurement confirmed true hypercalcemia. Additional diagnostic tests were then ordered to help differentiate between parathyroid hormone (PTH)–mediated and non-PTH–mediated causes for the hypercalcemia (see Table 2).
The patient’s PTH level was normal and the urine fractional excretion of calcium level was high, ruling out familial hypocalciuric hypercalcemia (FHH), in which urine calcium level is low. A measurement of PTH-related protein (PTHrP), secreted by some cancers, was normal, suggesting exclusion of solid tumor malignancy. Vitamin D toxicity was ruled out because the patient’s 1,25-dihydroxyvitamin D level was low.
The patient continued to experience vague abdominal, back, and rib pain that seemed to migrate daily and worsened with movement. A skeletal x-ray was performed and revealed numerous lytic lesions of the skull (Figure 1), midright humerus (Figure 2), and distal left radius.
Continue for discussion >>
DISCUSSION
Hypercalcemia is a relatively common presentation in primary care. The most frequent causes are primary hyperparathyroidism and malignancy.1 One in 500 patients will be diagnosed incidentally with asymptomatic hypercalcemia caused by underlying hyperparathyroidism.1
Clinical manifestations of hypercalcemia can range from no symptoms to multisystem disease. Fatigue, nausea, vomiting, constipation, bone pain, osteoporosis, nephrolithiasis, mental status changes, hypertension, anemia, elevated creatinine, and cardiac arrhythmias are among the more common clinical conditions associated with hypercalcemia (hence the mnemonic “stones, bones, abdominal moans, and psychic groans” for its signs and symptoms).1
Diagnostic overview
Causes of hypercalcemia are numerous and can be broken down into two categories: PTH-mediated and non-PTH–mediated. PTH-mediated causes include primary and secondary hyperparathyroidism and FHH. Non-PTH–mediated causes include vitamin D toxicity, solid tumor malignancy with or without metastasis, multiple myeloma (MM) and other plasma cell dyscrasias, granulomatous disease such as sarcoid, and some medications.1
The differential diagnosis for hypercalcemia begins with measurement of the patient’s intact PTH level. An elevated or high-normal result indicates a PTH-mediated cause, so 24-hour measurement of excretion of urinary calcium is the next step. A low or low-normal PTH level (< 20 pg/mL), however, suggests the cause is non-PTH-mediated.2 The diagnostic approach in this situation is more challenging because testing to exclude or confirm various potential causes can be expensive and time-consuming. The degree of hypercalcemia, however, can aid in the diagnosis: Primary hyperparathyroidism is often associated with borderline or mild hypercalcemia, while calcium values > 13 mg/dL are more common in patients with malignancies.2
PTH elevates calcium levels in the blood when ionized (free) calcium levels are low by increasing gastrointestinal absorption, decreasing urinary excretion, and increasing bone resorption.3 With malignant tumors such as lung, breast, and renal cell, osteolytic metastases can destroy the bone, resulting in release of calcium. In other cases, solid-tumor cancers produce PTHrP, which increases serum calcium. This latter situation is referred to as humoral hypercalcemia of malignancy.3 Lymphoma and granulomatous disease, such as sarcoid, can be associated with excess production of 1,25-dihydroxyvitamin D.2 If vitamin D is elevated but PTH and PTHrP are normal, a chest x-ray should be obtained to evaluate the patient for sarcoid or lymphoma.
Hypercalcemia work-up
The work-up for suspected hypercalcemia begins with measurement of the patient’s calcium level. Because calcium is bound to albumin in the blood, the standard serum calcium test may not reflect the true calcium level. (If albumin is high, the calcium level will be high, and vice versa.)1 The true (serum ionized) calcium level (also known as corrected calcium level) should always be calculated to confirm true hypercalcemia. A formula commonly used to calculate the corrected calcium level is
Corrected calcium (mg/dL) = (measured calcium [mg/dL]) + 0.8 (4.0 – serum albumin [mg/dL])
Direct measurement of the serum ionized calcium level is not affected by the albumin level and can also confirm true hypercalcemia.4
Once hypercalcemia is confirmed, the next step is to measure the patient’s intact PTH level.
If intact PTH is elevated or high normal, consider primary hyperparathyroidism or FHH and confirm by obtaining the urine calcium level.
• If urine calcium level is high (> 200 mg/24 h), the diagnosis is primary hyperparathyroidism.
• If urine calcium level is low (< 100 mg/24 h), the diagnosis is FHH.
If intact PTH is low, consider non–PTH-mediated causes and confirm by obtaining PTHrP and vitamin D levels.
• If PTHrP level is elevated, scan for malignancy.
• If 1,25-dihydroxyvitamin D level is elevated, order a chest x-ray to rule out sarcoid or lymphoma.
• If both PTHrP and vitamin D levels are normal, order both serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP) with immunofixation to rule out MM.
• If vitamin D level is elevated, check vitamin and herbal supplement use for excessive vitamin D intake.
Treatment of symptomatic hypercalcemia
The goals of treatment of symptomatic hypercalcemia are to reduce the serum calcium level to the normal range and to treat the underlying cause.1 Mild hypercalcemia (calcium level, 10-12 mg/dL) is typically asymptomatic and does not need to be treated. Moderate hypercalcemia (calcium level, 12-14 mg/dL) may not require treatment unless the patient is symptomatic and/or has had an acute rise in calcium level.1 In mild to moderate hypercalcemia, the serum calcium level should be monitored to establish a trend.
Treatment for symptomatic moderate and severe hypercalcemia (calcium level, > 14 mg/dL) typically involves a similar regimen:
• Volume expansion with isotonic saline at an initial rate of 2 to 4 L/d, which is then adjusted to achieve 200 mL/h of continuous urine output. IV furosemide can be used with caution (10-20 mg IV as needed) to promote diuresis if volume overload is a concern (furosemide promotes renal excretion of calcium).
• Administration of subcutaneous calcitonin (4-8 IU/kg, repeated every 6 h for 24 h). Calcitonin works rapidly to lower calcium levels in 4 to 6 h.
• Concurrent administration of IV bisphosphonate (zoledronic acid [4 mg over 15 min] or pamidronate [60-90 mg over 4 h]). Pamidronate is superior for reversal of malignancy-related hypercalcemia.1
Hypercalcemia and multiple myeloma
MM is a malignant neoplasm of plasma cells that accounts for approximately 1% of all cancers and about 10% of hematologic malignancies in the United States, with a median patient age of 70 at diagnosis.5-7 In MM, myeloma cells induce the secretion of cytokines and growth factors that alter plasma cells, activate osteoclasts, suppress osteoblasts, cause abnormal interactions between plasma cells and bone marrow, and stimulate aberrant angiogenesis.8 Osteoclastic bone resorption produces hypercalcemia as well as the lytic lesions seen on x-ray.7
Approximately 74% of patients present with typical MM symptoms of calcium elevation in the blood, renal insufficiency, anemia, and bone lesions, known as CRAB symptoms, but other myeloma-related manifestations may be present.9
Diagnostic criteria for MM include the following (all three must be present):10
• Monoclonal bone marrow plasma cells ≥ 10% and/or a biopsy-proven plasmacytoma
• Monoclonal protein in the serum and/or urine (if none is detected, disease is nonsecretory and diagnosis requires ≥ 30% bone marrow plasma cells and/or biopsy-proven plasmacytoma)
• Myeloma-related organ dysfunction, indicated by at least one of the CRAB symptoms.
In the absence of CRAB symptoms, an asymptomatic patient may have an MM precursor syndrome: monoclonal gammopathy of undetermined significance or smoldering (or indolent) MM.10
Treatment of multiple myeloma
In recent years, the use of induction therapy followed by autologous stem cell transplantation and the development of novel therapeutic agents have extended overall survival for patients with MM. These agents include proteasome inhibitors (bortezomib and the second-generation carfilzomib)11 and immunomodulators (thalidomide and the second-generation lenalidomide). Early diagnosis and treatment can improve progression-free survival as well as overall survival, including recovery of renal function for patients with renal failure.12 With survival ranging from one year or less—with aggressive disease—to 10 years or more for patients with responsive disease,7 there remains no cure for MM.
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
Further work-up included SPEP and UPEP with immunofixation, which revealed marked IgG free λ light chains with an M (monoclonal) component, making MM a very likely diagnosis. Confirmation by means of bone marrow biopsy was indicated, but the patient refused the procedure.
The patient’s hypercalcemia was treated by IV administration of calcitonin with isotonic saline. This reduced the serum calcium level from 15.4 mg/dL to 10.6 mg/dL within 48 hours. One dose of ergocalciferol (vitamin D2) was then administered to promote intestinal absorption of calcium and support bone mineralization, further lowering the patient’s serum calcium level to a normal 8.9 mg/dL. Hypokalemia was treated with oral potassium supplementation.
The patient, now stable, was referred to the hematology/oncology and bone mineral metabolism clinics and was discharged from the hospital. She did not keep those appointments and was lost to follow-up.
CONCLUSION
The most common causes of hypercalcemia are hyperparathyroidism and malignancy. Most cases do not require treatment unless the calcium level is >14 mg/dL and/or the patient is symptomatic. Red flag symptoms include weakness, abdominal pain, mental status changes, and coma.4 Primary care clinicians should suspect MM in older patients with laboratory findings of hypercalcemia, anemia, and renal dysfunction, with lytic lesions on x-ray.
REFERENCES
1. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959-1966.
2. Endres DB. Investigation of hypercalcemia. Clin Biochem. 2012;45(12):
954-963.
3. Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care. 2008;35(2):215-237.
4. Sharma B, Misicko NE. How should you evaluate elevated calcium in an asymptomatic patient? J Fam Pract. 2008;57(4):267-269.
5. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
6. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
7. Shaheen SP, Talwalkar SS, Medeiros LJ. Multiple myeloma and immunosecretory disorders: an update. Adv Anat Pathol. 2008;15(4):196-210.
8. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11): 1046-1060.
9. Talamo G, Farooq U, Zangari M, et al. Beyond the CRAB symptoms: a study of presenting clinical manifestations of multiple myeloma. Clin Lymphoma Myeloma Leuk. 2010;10(6):464-468.
10. Palumbo A, Sezer O, Kyle R, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transportation. Leukemia. 2009;23(10):1716-1730.
11. Kyprolis [package insert]. South San Francisco, CA: Onyx Pharmaceuticals, Inc; 2012.
12. Suyani E, Sucak GT, Erten Y, et al. Evaluation of multiple myeloma patients presenting with renal failure in a university hospital in the year 2010. Ren Fail. 2012;34(2):257-262.
A 66-year-old Latin American woman presented to the emergency department (ED) with persistent abdominal and back pain of about one month’s duration. She had visited another ED eight days earlier for similar symptoms and was discharged home with a mild opioid pain medication and a proton pump inhibitor. However, she said that she had received neither a diagnosis nor an explanation for her symptoms.
Medical history, obtained with the assistance of an interpreter because the patient was not fluent in English, included hypertension, coronary artery disease, and hyperlipidemia; these had gone untreated for at least two years. She denied any personal or family history of cancer or endocrine disorders. Surgical history included a cholecystectomy and a percutaneous coronary intervention for an unknown coronary artery lesion.
She had a 14-pack-year history of cigarette smoking. Her medications included only ibuprofen and hydrocodone, and she had no known drug allergies. The patient denied use of herbal preparations or vitamin supplements and unusual dietary practices.
Review of systems revealed occasional dizziness, constipation, decreased appetite, and some mild confusion noted by family members, but no fever, chills, palpitations, chest pain, shortness of breath, muscle spasm, or weakness. Vital signs were normal. Physical examination was remarkable for tenderness of the upper quadrants of the abdomen with deep palpation, without guarding or rebound. Bony tenderness at the right anterior costal margin of the rib cage was also noted.
Laboratory work-up revealed marked hypercalcemia (15.4 mg/dL), electrolyte abnormalities, anemia, impaired renal function, and elevated alkaline phosphatase and globulin levels (see Table 1). In addition, a plain abdominal x-ray series was negative for acute findings, but x-rays of the right ribs revealed a fracture of the sixth rib and osteopenia.
Continued >>
The patient was admitted to the hospital for treatment of hypercalcemia and hypokalemia and for work-up of elevated alkaline phosphatase and abdominal pain. Upon admission, serum ionized calcium measurement confirmed true hypercalcemia. Additional diagnostic tests were then ordered to help differentiate between parathyroid hormone (PTH)–mediated and non-PTH–mediated causes for the hypercalcemia (see Table 2).
The patient’s PTH level was normal and the urine fractional excretion of calcium level was high, ruling out familial hypocalciuric hypercalcemia (FHH), in which urine calcium level is low. A measurement of PTH-related protein (PTHrP), secreted by some cancers, was normal, suggesting exclusion of solid tumor malignancy. Vitamin D toxicity was ruled out because the patient’s 1,25-dihydroxyvitamin D level was low.
The patient continued to experience vague abdominal, back, and rib pain that seemed to migrate daily and worsened with movement. A skeletal x-ray was performed and revealed numerous lytic lesions of the skull (Figure 1), midright humerus (Figure 2), and distal left radius.
Continue for discussion >>
DISCUSSION
Hypercalcemia is a relatively common presentation in primary care. The most frequent causes are primary hyperparathyroidism and malignancy.1 One in 500 patients will be diagnosed incidentally with asymptomatic hypercalcemia caused by underlying hyperparathyroidism.1
Clinical manifestations of hypercalcemia can range from no symptoms to multisystem disease. Fatigue, nausea, vomiting, constipation, bone pain, osteoporosis, nephrolithiasis, mental status changes, hypertension, anemia, elevated creatinine, and cardiac arrhythmias are among the more common clinical conditions associated with hypercalcemia (hence the mnemonic “stones, bones, abdominal moans, and psychic groans” for its signs and symptoms).1
Diagnostic overview
Causes of hypercalcemia are numerous and can be broken down into two categories: PTH-mediated and non-PTH–mediated. PTH-mediated causes include primary and secondary hyperparathyroidism and FHH. Non-PTH–mediated causes include vitamin D toxicity, solid tumor malignancy with or without metastasis, multiple myeloma (MM) and other plasma cell dyscrasias, granulomatous disease such as sarcoid, and some medications.1
The differential diagnosis for hypercalcemia begins with measurement of the patient’s intact PTH level. An elevated or high-normal result indicates a PTH-mediated cause, so 24-hour measurement of excretion of urinary calcium is the next step. A low or low-normal PTH level (< 20 pg/mL), however, suggests the cause is non-PTH-mediated.2 The diagnostic approach in this situation is more challenging because testing to exclude or confirm various potential causes can be expensive and time-consuming. The degree of hypercalcemia, however, can aid in the diagnosis: Primary hyperparathyroidism is often associated with borderline or mild hypercalcemia, while calcium values > 13 mg/dL are more common in patients with malignancies.2
PTH elevates calcium levels in the blood when ionized (free) calcium levels are low by increasing gastrointestinal absorption, decreasing urinary excretion, and increasing bone resorption.3 With malignant tumors such as lung, breast, and renal cell, osteolytic metastases can destroy the bone, resulting in release of calcium. In other cases, solid-tumor cancers produce PTHrP, which increases serum calcium. This latter situation is referred to as humoral hypercalcemia of malignancy.3 Lymphoma and granulomatous disease, such as sarcoid, can be associated with excess production of 1,25-dihydroxyvitamin D.2 If vitamin D is elevated but PTH and PTHrP are normal, a chest x-ray should be obtained to evaluate the patient for sarcoid or lymphoma.
Hypercalcemia work-up
The work-up for suspected hypercalcemia begins with measurement of the patient’s calcium level. Because calcium is bound to albumin in the blood, the standard serum calcium test may not reflect the true calcium level. (If albumin is high, the calcium level will be high, and vice versa.)1 The true (serum ionized) calcium level (also known as corrected calcium level) should always be calculated to confirm true hypercalcemia. A formula commonly used to calculate the corrected calcium level is
Corrected calcium (mg/dL) = (measured calcium [mg/dL]) + 0.8 (4.0 – serum albumin [mg/dL])
Direct measurement of the serum ionized calcium level is not affected by the albumin level and can also confirm true hypercalcemia.4
Once hypercalcemia is confirmed, the next step is to measure the patient’s intact PTH level.
If intact PTH is elevated or high normal, consider primary hyperparathyroidism or FHH and confirm by obtaining the urine calcium level.
• If urine calcium level is high (> 200 mg/24 h), the diagnosis is primary hyperparathyroidism.
• If urine calcium level is low (< 100 mg/24 h), the diagnosis is FHH.
If intact PTH is low, consider non–PTH-mediated causes and confirm by obtaining PTHrP and vitamin D levels.
• If PTHrP level is elevated, scan for malignancy.
• If 1,25-dihydroxyvitamin D level is elevated, order a chest x-ray to rule out sarcoid or lymphoma.
• If both PTHrP and vitamin D levels are normal, order both serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP) with immunofixation to rule out MM.
• If vitamin D level is elevated, check vitamin and herbal supplement use for excessive vitamin D intake.
Treatment of symptomatic hypercalcemia
The goals of treatment of symptomatic hypercalcemia are to reduce the serum calcium level to the normal range and to treat the underlying cause.1 Mild hypercalcemia (calcium level, 10-12 mg/dL) is typically asymptomatic and does not need to be treated. Moderate hypercalcemia (calcium level, 12-14 mg/dL) may not require treatment unless the patient is symptomatic and/or has had an acute rise in calcium level.1 In mild to moderate hypercalcemia, the serum calcium level should be monitored to establish a trend.
Treatment for symptomatic moderate and severe hypercalcemia (calcium level, > 14 mg/dL) typically involves a similar regimen:
• Volume expansion with isotonic saline at an initial rate of 2 to 4 L/d, which is then adjusted to achieve 200 mL/h of continuous urine output. IV furosemide can be used with caution (10-20 mg IV as needed) to promote diuresis if volume overload is a concern (furosemide promotes renal excretion of calcium).
• Administration of subcutaneous calcitonin (4-8 IU/kg, repeated every 6 h for 24 h). Calcitonin works rapidly to lower calcium levels in 4 to 6 h.
• Concurrent administration of IV bisphosphonate (zoledronic acid [4 mg over 15 min] or pamidronate [60-90 mg over 4 h]). Pamidronate is superior for reversal of malignancy-related hypercalcemia.1
Hypercalcemia and multiple myeloma
MM is a malignant neoplasm of plasma cells that accounts for approximately 1% of all cancers and about 10% of hematologic malignancies in the United States, with a median patient age of 70 at diagnosis.5-7 In MM, myeloma cells induce the secretion of cytokines and growth factors that alter plasma cells, activate osteoclasts, suppress osteoblasts, cause abnormal interactions between plasma cells and bone marrow, and stimulate aberrant angiogenesis.8 Osteoclastic bone resorption produces hypercalcemia as well as the lytic lesions seen on x-ray.7
Approximately 74% of patients present with typical MM symptoms of calcium elevation in the blood, renal insufficiency, anemia, and bone lesions, known as CRAB symptoms, but other myeloma-related manifestations may be present.9
Diagnostic criteria for MM include the following (all three must be present):10
• Monoclonal bone marrow plasma cells ≥ 10% and/or a biopsy-proven plasmacytoma
• Monoclonal protein in the serum and/or urine (if none is detected, disease is nonsecretory and diagnosis requires ≥ 30% bone marrow plasma cells and/or biopsy-proven plasmacytoma)
• Myeloma-related organ dysfunction, indicated by at least one of the CRAB symptoms.
In the absence of CRAB symptoms, an asymptomatic patient may have an MM precursor syndrome: monoclonal gammopathy of undetermined significance or smoldering (or indolent) MM.10
Treatment of multiple myeloma
In recent years, the use of induction therapy followed by autologous stem cell transplantation and the development of novel therapeutic agents have extended overall survival for patients with MM. These agents include proteasome inhibitors (bortezomib and the second-generation carfilzomib)11 and immunomodulators (thalidomide and the second-generation lenalidomide). Early diagnosis and treatment can improve progression-free survival as well as overall survival, including recovery of renal function for patients with renal failure.12 With survival ranging from one year or less—with aggressive disease—to 10 years or more for patients with responsive disease,7 there remains no cure for MM.
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
Further work-up included SPEP and UPEP with immunofixation, which revealed marked IgG free λ light chains with an M (monoclonal) component, making MM a very likely diagnosis. Confirmation by means of bone marrow biopsy was indicated, but the patient refused the procedure.
The patient’s hypercalcemia was treated by IV administration of calcitonin with isotonic saline. This reduced the serum calcium level from 15.4 mg/dL to 10.6 mg/dL within 48 hours. One dose of ergocalciferol (vitamin D2) was then administered to promote intestinal absorption of calcium and support bone mineralization, further lowering the patient’s serum calcium level to a normal 8.9 mg/dL. Hypokalemia was treated with oral potassium supplementation.
The patient, now stable, was referred to the hematology/oncology and bone mineral metabolism clinics and was discharged from the hospital. She did not keep those appointments and was lost to follow-up.
CONCLUSION
The most common causes of hypercalcemia are hyperparathyroidism and malignancy. Most cases do not require treatment unless the calcium level is >14 mg/dL and/or the patient is symptomatic. Red flag symptoms include weakness, abdominal pain, mental status changes, and coma.4 Primary care clinicians should suspect MM in older patients with laboratory findings of hypercalcemia, anemia, and renal dysfunction, with lytic lesions on x-ray.
REFERENCES
1. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959-1966.
2. Endres DB. Investigation of hypercalcemia. Clin Biochem. 2012;45(12):
954-963.
3. Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care. 2008;35(2):215-237.
4. Sharma B, Misicko NE. How should you evaluate elevated calcium in an asymptomatic patient? J Fam Pract. 2008;57(4):267-269.
5. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
6. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
7. Shaheen SP, Talwalkar SS, Medeiros LJ. Multiple myeloma and immunosecretory disorders: an update. Adv Anat Pathol. 2008;15(4):196-210.
8. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11): 1046-1060.
9. Talamo G, Farooq U, Zangari M, et al. Beyond the CRAB symptoms: a study of presenting clinical manifestations of multiple myeloma. Clin Lymphoma Myeloma Leuk. 2010;10(6):464-468.
10. Palumbo A, Sezer O, Kyle R, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transportation. Leukemia. 2009;23(10):1716-1730.
11. Kyprolis [package insert]. South San Francisco, CA: Onyx Pharmaceuticals, Inc; 2012.
12. Suyani E, Sucak GT, Erten Y, et al. Evaluation of multiple myeloma patients presenting with renal failure in a university hospital in the year 2010. Ren Fail. 2012;34(2):257-262.