User login
Total Laparoscopic Hysterectomy
In 1989 Dr. Harry Reich performed the first laparoscopic hysterectomy using rather primitive instruments by today’s standards, and changed the gynecologic surgical landscape forever. Those of us who have performed laparoscopic hysterectomy during the 20-plus years since then have provided women with a faster recovery, less time in the hospital, and less pain than they would have experienced with a traditional abdominal approach.
The minimally invasive approach has become the mantra for most gynecologic procedures. Vaginal hysterectomy always has been considered its standard bearer, and as emphasized by the American College of Obstetricians and Gynecologists (ACOG) in its recent statement, it should remain the primary approach whenever it is feasible (Obstet. Gynecol. 2009;114:1156-8). In cases in which vaginal hysterectomy is not an option, however, laparoscopic hysterectomy is clearly the next-best technique.
In 2010, the AAGL published a position paper stating that most hysterectomies for benign disease should be performed either vaginally or laparoscopically, and that continued efforts should be taken to facilitate these approaches (J. Minimal. Invasive Gynecol. 2010 [doi: 10.1016/jmig.2010.10.001]). The recent consensus in the literature, moreover, has been that abdominal hysterectomy should be reserved for cases in which the patient cannot tolerate a laparoscopic approach. For example, a history of cardiopulmonary compromise or multiple gastrointestinal procedures may make a laparoscopic approach potentially too dangerous.
There have been some important refinements in laparoscopic hysterectomy over the past 20-plus years, including the ability to manage vascular pedicles with less thermal spread. This has decreased the potential for ureteral damage. Moreover, our ability to pinpoint the cervicovaginal margin, so as not to shorten the vagina, also has been an improvement. Overall, however, the technique has not changed significantly.
So what is new? The option in some hospitals of performing hysterectomies robotically is attracting surgeons who have not developed a skill set in traditional laparoscopy to perform the technique at bedside. This growing number of gynecologic surgeons is finding it easier and technically more enjoyable to use the da Vinci Surgical System.
Now, consequently, the focus of discussion and debate concerns the availability and cost of the robotic system, as well as the comparative complication rates and operating room times of robotic and traditional laparoscopic hysterectomy.
As gynecologic surgery faces this paradigm shift in the choice of surgical approaches, and as health care payment models continue to evolve, it is important to understand the value of each approach. One recent study found that robotic hysterectomies were associated with longer surgical times and cost an average of $2,600 more (J. Minim. Invasive Gynecol. 2010;17:730-8). Other studies have reported similar findings.
While some extra cost may be acceptable today, it will be less palatable if – or when – reimbursement models change and bundled payments become more common. Moreover, robotic technology is not universally available. Currently, the residents I teach do not have access to the da Vinci system. In the future, if access is available, their training in traditional laparoscopy will be valuable.
Thus, despite the increasing popularity of a robotic approach to hysterectomy, it is imperative that teaching the approach in laparoscopic hysterectomy not be pushed to the background.
While the learning curve of laparoscopic hysterectomy is not insignificant, it is more intimidating than it should be. Once the surgeon learns to suture laparoscopically, he or she has largely broken the barrier.
The Process and Technique
At the start of the procedure, before insufflation, I place a Rumi Uterine Manipulator and KOH colpotomizer (CooperSurgical, Trumbull, Conn.) to identify the anterior and posterior fornices in preparation for colpotomy. Other cupped manipulators such as the VCare uterine manipulator/elevator (CONMED EndoSurgery, Utica, N.Y.) and the McCarus-Volker system (LSI Solutions, Rochester, N.Y.) may be utilized, but I prefer the Rumi.
The application of the cup must be flush with the vaginal fornices, and the intrauterine stem must be the correct length to easily mobilize the uterus from side to side as well as anterior and posterior. If the manipulator is not used and placed properly, it will be more difficult to find the cervical cup when initiating the colpotomy and maintaining the pneumoperitoneum. Some surgeons suture the cup to the cervix to provide greater traction and easier retrieval of the specimen, but I don’t find this necessary.
I always employ a left upper quadrant approach for insufflation of the abdomen. I ask anesthesia to place an orogastric tube to empty the stomach of its contents and the air that has accumulated during intubation. The patient must not have had a prior splenectomy or surgery in the left upper quadrant.
In patients who have had any prior abdominal surgery (cesarean section, appendectomy, etc.), a 3-mm port is placed in the left upper quadrant to insufflate as well as visualize the anterior abdominal wall in order to identify any adhesions that may have formed after the prior surgery. If adhesions are present, then before the umbilical port is placed, two 5-mm left lateral ports are introduced and the anterior abdominal wall adhesions are lysed.
In all patients, an umbilical trocar is placed under direct vision for the 5-mm zero-degree laparoscope, and 5-mm ports (two on my operating side and one for my assistant) are placed lateral to the inferior epigastric vessels on the left and right. These lateral port sites are placed a fist-width apart along the lateral side wall, usually at a level just above the iliac crest. Exact placement is determined by uterine size. Depending on the uterine size, an additional port may be placed below the umbilicus.
When the ovaries are to be removed, both ureters are identified and the infundibulopelvic ligaments are isolated, then coagulated and transected. If there is any question about the path of the ureter and its proximity to the infundibulopelvic ligament or the uterine vasculature – as in the case of endometriosis or broad ligament fibroids – then ureterolysis will be performed.
The broad and round ligaments then are coagulated and transected, and the bladder flap is developed. The creation of the bladder flap is simple unless the patient has undergone multiple cesarean sections and has significant scarring. This dissection must be layer by layer, with the surgeon always working lateral to medial until the vaginal tissues are reached. When in doubt, it is helpful to backfill the bladder with normal saline to more easily identify the bladder margins. This is easily accomplished using a three-way Foley catheter placed at the beginning of the procedure in anticipation of a scarred lower uterine segment.
We then dissect the peritoneum posteriorly as well at the level of the uterosacral ligaments so that the uterine arteries may be isolated bilaterally. This also serves as another relaxing incision keeping the ureter away from our energy sources. At this point, we utilize a reusable bipolar forceps made by Storz (Karl Storz Endoscopy, El Segundo, Calif.) to coagulate the uterine vessels bilaterally and decrease back-bleeding, and I transect the vessels using the Harmonic Ace Shears (Ethicon Endo-Surgery, Somerville, N.J.). I prefer the reusable bipolar forceps over other instruments, and have learned not to overdesiccate the tissues.
It is imperative for the surgeon or the assistant to constantly push the uterus cephalad using the manipulator in order to protect the ureters from thermal damage as well as transection at the time of colpotomy. This should be the responsibility of the surgeon, unless tissue retraction is necessary. In this case, this task can be given to an assistant, but under constant scrutiny.
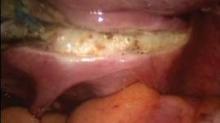
Once the uterine arteries are secured, the colpotomy is begun anteriorly using the active blade of the Harmonic Ace. Monopolar energy can be used as well, but I find that this results in more bleeding from the cuff, which in turn necessitates the use of bipolar energy to create hemostasis.
The cervix is circumscribed along the colpotomy cup and, once disconnected, is delivered vaginally. To make the colpotomy easier, it is best to thin out the tissue between the cut uterine vessels and the cervix, exposing the cup. This is accomplished by serially clamping and coagulating the soft tissue above the level of the cup, allowing the impression of the cup to be more easily seen, while at the same time always pushing the cup cephalad.
The delivery of the uterus through what appears to be a small colpotomy incision is accomplished by deflating the vaginal occlusion balloon and gently applying traction on the stem, bringing the cervix closer to the introitus. If the uterus is too large to be delivered through the incision, it can be easily morcellated as one would a large uterus during vaginal hysterectomy. To maintain pneumoperitoneum after delivery of the uterus, the uterus can be left in the vagina or, if the Rumi manipulator is used, the balloon can be removed and reinflated in the vagina.
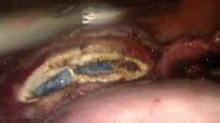
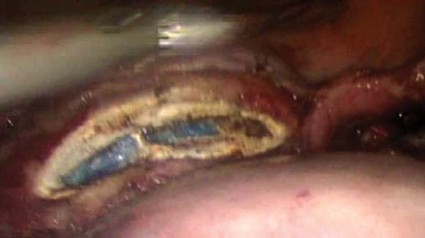
Once hemostasis is adequate, the cuff is closed with three figure-of-eight sutures of O-polydioxanone (PDS). ("Adequate" hemostasis, it must be noted, does not mean complete desiccation of the cuff; it just means no active bleeding. Leaving the cuff a little wet is not a bad thing.) Any dissolvable suture can be used, but I find that PDS slides nicely when used in extracorporeal knot tying. Even if the first knot loosens before the second knot is thrown, it will tighten as the second knot pushes the first toward the tissue. I suture the angles first and then the midline. To lower the risk of posthysterectomy prolapse, I include in my angle sutures the uterosacral ligament, vaginal mucosa, and anterior pericervical fascia. In my experience, inclusion of the vaginal mucosa eliminates the formation of granulation tissue.
A cystoscopy after injection of intravenous indigo carmine is performed to ensure that both ureters are patent, and that there is no injury to the bladder. If there is no flow of dye through one or both of the ureters, I would first cut one of the angle sutures to determine whether the ureter has been obstructed or kinked by one of these sutures. If doing so does not open the ureter, the course of the ureter must be traced to identify the obstruction.
If the ureter can’t be easily seen through the peritoneum, a ureterolysis must be performed. I start at the pelvic brim as the ureter crosses over the anteromedial aspect of the psoas muscle and bifurcation of the common iliac artery. The peritoneum is tented and incised to follow the course of the ureter that lies just lateral to the peritoneal surface.
On the few occasions when I have cut the ureter, I knew it immediately and consulted a urologist to repair the damage. In cases in which there is no obvious ureteral damage, an intraoperative urologic consult is still appropriate. Of course, if a bladder perforation is identified, it must be closed and a catheter placed for 10-14 days. The choice of suture for cystotomy is normally a rapidly dissolving suture; closure should be performed in two layers, and cystoscopy is important to be sure that the trigone is not involved and the ureters are not compromised.
Vaginal Cuff Closure
Some surgeons are now using barbed suture to close the vaginal cuff in a double-layer closure, but I have continued using figure-of-eight sutures of O-PDS. I do not believe there are any disadvantages of a double-layer closure with barbed suture; rather, the question for me has been, is it better? Will there be any less incidence of dehiscence post procedure?
The authors of a new review on vaginal cuff dehiscence conclude that a two-layer cuff closure with bidirectional barbed suture – along with judicious use of electrocautery – may potentially decrease the risk of cuff dehiscence, although the extent of the effect is uncertain (Am. J. Obstet. Gynecol. 2012;206:284-8). Others have commented that laparoscopic cuff closure with or without robotic assistance fails to take large enough tissue bites, and therefore is inherently weaker than traditional abdominal or vaginal cuff closure.
I agree that both issues – overtreatment of the vaginal cuff with electrosurgery, and failure to take large enough bites when closing the cuff – are common denominators in vaginal cuff dehiscence. The magnified visualization that characterizes the laparoscopic and robotic approaches – and particularly the robotic approach – can inadvertently lead to smaller bites being sutured. When large enough bites of tissue are taken, the cuff will heal nicely and will not dehisce unless there is infection or tissue compromise.
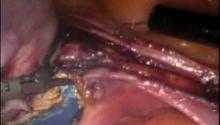
In general, suturing is the most challenging part of laparoscopic hysterectomy and a big part of the learning curve. The key lies in mastering a few maneuvers. I try to make the conversion from open suturing to laparoscopic suturing as seamless as possible. I suture from the left side of the patient with my ports a fist apart so that I don’t crowd myself. I introduce my needles into the abdomen by back-loading the needles and placing them through the 5-mm port.
I use a needle holder made by Olympus that is the same shape and has the same handle as the one I use for open surgery. The needle must always lie perpendicular to the needle holder and must be grasped at the tip of the needle holder. This way, the needle can be placed and, with a simple turn of the wrist, engaged into the tissue. The needle is then retrieved and the process repeated. There is no substitute for practice; with repetition, this maneuver becomes very natural.
Once the suture has been placed, the needle is removed from the abdomen by leaving an inch-long tail and by pulling the needle, suture first out, along with one of the 5-mm ports; this will guarantee that the needle exits without difficulty. A closed knot pusher allows the surgeon to apply enough tension to the suture so that the knots are nice and tight without the risk of the knot pusher slipping off the suture. Intracorporeal knot tying also has been performed very successfully, but it entails an additional level of skill.
Dr. Levine is the program director of minimally invasive gynecologic surgery and codirector of the Minimally Invasive Surgical Institute at Mercy Hospital, St. Louis. He reported that he has no disclosures relevant to this Master Class.
This column, "Master Class," appears regularly in Ob.Gyn. News.
In 1989 Dr. Harry Reich performed the first laparoscopic hysterectomy using rather primitive instruments by today’s standards, and changed the gynecologic surgical landscape forever. Those of us who have performed laparoscopic hysterectomy during the 20-plus years since then have provided women with a faster recovery, less time in the hospital, and less pain than they would have experienced with a traditional abdominal approach.
The minimally invasive approach has become the mantra for most gynecologic procedures. Vaginal hysterectomy always has been considered its standard bearer, and as emphasized by the American College of Obstetricians and Gynecologists (ACOG) in its recent statement, it should remain the primary approach whenever it is feasible (Obstet. Gynecol. 2009;114:1156-8). In cases in which vaginal hysterectomy is not an option, however, laparoscopic hysterectomy is clearly the next-best technique.
In 2010, the AAGL published a position paper stating that most hysterectomies for benign disease should be performed either vaginally or laparoscopically, and that continued efforts should be taken to facilitate these approaches (J. Minimal. Invasive Gynecol. 2010 [doi: 10.1016/jmig.2010.10.001]). The recent consensus in the literature, moreover, has been that abdominal hysterectomy should be reserved for cases in which the patient cannot tolerate a laparoscopic approach. For example, a history of cardiopulmonary compromise or multiple gastrointestinal procedures may make a laparoscopic approach potentially too dangerous.
There have been some important refinements in laparoscopic hysterectomy over the past 20-plus years, including the ability to manage vascular pedicles with less thermal spread. This has decreased the potential for ureteral damage. Moreover, our ability to pinpoint the cervicovaginal margin, so as not to shorten the vagina, also has been an improvement. Overall, however, the technique has not changed significantly.
So what is new? The option in some hospitals of performing hysterectomies robotically is attracting surgeons who have not developed a skill set in traditional laparoscopy to perform the technique at bedside. This growing number of gynecologic surgeons is finding it easier and technically more enjoyable to use the da Vinci Surgical System.
Now, consequently, the focus of discussion and debate concerns the availability and cost of the robotic system, as well as the comparative complication rates and operating room times of robotic and traditional laparoscopic hysterectomy.
As gynecologic surgery faces this paradigm shift in the choice of surgical approaches, and as health care payment models continue to evolve, it is important to understand the value of each approach. One recent study found that robotic hysterectomies were associated with longer surgical times and cost an average of $2,600 more (J. Minim. Invasive Gynecol. 2010;17:730-8). Other studies have reported similar findings.
While some extra cost may be acceptable today, it will be less palatable if – or when – reimbursement models change and bundled payments become more common. Moreover, robotic technology is not universally available. Currently, the residents I teach do not have access to the da Vinci system. In the future, if access is available, their training in traditional laparoscopy will be valuable.
Thus, despite the increasing popularity of a robotic approach to hysterectomy, it is imperative that teaching the approach in laparoscopic hysterectomy not be pushed to the background.
While the learning curve of laparoscopic hysterectomy is not insignificant, it is more intimidating than it should be. Once the surgeon learns to suture laparoscopically, he or she has largely broken the barrier.
The Process and Technique
At the start of the procedure, before insufflation, I place a Rumi Uterine Manipulator and KOH colpotomizer (CooperSurgical, Trumbull, Conn.) to identify the anterior and posterior fornices in preparation for colpotomy. Other cupped manipulators such as the VCare uterine manipulator/elevator (CONMED EndoSurgery, Utica, N.Y.) and the McCarus-Volker system (LSI Solutions, Rochester, N.Y.) may be utilized, but I prefer the Rumi.
The application of the cup must be flush with the vaginal fornices, and the intrauterine stem must be the correct length to easily mobilize the uterus from side to side as well as anterior and posterior. If the manipulator is not used and placed properly, it will be more difficult to find the cervical cup when initiating the colpotomy and maintaining the pneumoperitoneum. Some surgeons suture the cup to the cervix to provide greater traction and easier retrieval of the specimen, but I don’t find this necessary.
I always employ a left upper quadrant approach for insufflation of the abdomen. I ask anesthesia to place an orogastric tube to empty the stomach of its contents and the air that has accumulated during intubation. The patient must not have had a prior splenectomy or surgery in the left upper quadrant.
In patients who have had any prior abdominal surgery (cesarean section, appendectomy, etc.), a 3-mm port is placed in the left upper quadrant to insufflate as well as visualize the anterior abdominal wall in order to identify any adhesions that may have formed after the prior surgery. If adhesions are present, then before the umbilical port is placed, two 5-mm left lateral ports are introduced and the anterior abdominal wall adhesions are lysed.
In all patients, an umbilical trocar is placed under direct vision for the 5-mm zero-degree laparoscope, and 5-mm ports (two on my operating side and one for my assistant) are placed lateral to the inferior epigastric vessels on the left and right. These lateral port sites are placed a fist-width apart along the lateral side wall, usually at a level just above the iliac crest. Exact placement is determined by uterine size. Depending on the uterine size, an additional port may be placed below the umbilicus.
When the ovaries are to be removed, both ureters are identified and the infundibulopelvic ligaments are isolated, then coagulated and transected. If there is any question about the path of the ureter and its proximity to the infundibulopelvic ligament or the uterine vasculature – as in the case of endometriosis or broad ligament fibroids – then ureterolysis will be performed.
The broad and round ligaments then are coagulated and transected, and the bladder flap is developed. The creation of the bladder flap is simple unless the patient has undergone multiple cesarean sections and has significant scarring. This dissection must be layer by layer, with the surgeon always working lateral to medial until the vaginal tissues are reached. When in doubt, it is helpful to backfill the bladder with normal saline to more easily identify the bladder margins. This is easily accomplished using a three-way Foley catheter placed at the beginning of the procedure in anticipation of a scarred lower uterine segment.
We then dissect the peritoneum posteriorly as well at the level of the uterosacral ligaments so that the uterine arteries may be isolated bilaterally. This also serves as another relaxing incision keeping the ureter away from our energy sources. At this point, we utilize a reusable bipolar forceps made by Storz (Karl Storz Endoscopy, El Segundo, Calif.) to coagulate the uterine vessels bilaterally and decrease back-bleeding, and I transect the vessels using the Harmonic Ace Shears (Ethicon Endo-Surgery, Somerville, N.J.). I prefer the reusable bipolar forceps over other instruments, and have learned not to overdesiccate the tissues.
It is imperative for the surgeon or the assistant to constantly push the uterus cephalad using the manipulator in order to protect the ureters from thermal damage as well as transection at the time of colpotomy. This should be the responsibility of the surgeon, unless tissue retraction is necessary. In this case, this task can be given to an assistant, but under constant scrutiny.
Once the uterine arteries are secured, the colpotomy is begun anteriorly using the active blade of the Harmonic Ace. Monopolar energy can be used as well, but I find that this results in more bleeding from the cuff, which in turn necessitates the use of bipolar energy to create hemostasis.
The cervix is circumscribed along the colpotomy cup and, once disconnected, is delivered vaginally. To make the colpotomy easier, it is best to thin out the tissue between the cut uterine vessels and the cervix, exposing the cup. This is accomplished by serially clamping and coagulating the soft tissue above the level of the cup, allowing the impression of the cup to be more easily seen, while at the same time always pushing the cup cephalad.
The delivery of the uterus through what appears to be a small colpotomy incision is accomplished by deflating the vaginal occlusion balloon and gently applying traction on the stem, bringing the cervix closer to the introitus. If the uterus is too large to be delivered through the incision, it can be easily morcellated as one would a large uterus during vaginal hysterectomy. To maintain pneumoperitoneum after delivery of the uterus, the uterus can be left in the vagina or, if the Rumi manipulator is used, the balloon can be removed and reinflated in the vagina.
Once hemostasis is adequate, the cuff is closed with three figure-of-eight sutures of O-polydioxanone (PDS). ("Adequate" hemostasis, it must be noted, does not mean complete desiccation of the cuff; it just means no active bleeding. Leaving the cuff a little wet is not a bad thing.) Any dissolvable suture can be used, but I find that PDS slides nicely when used in extracorporeal knot tying. Even if the first knot loosens before the second knot is thrown, it will tighten as the second knot pushes the first toward the tissue. I suture the angles first and then the midline. To lower the risk of posthysterectomy prolapse, I include in my angle sutures the uterosacral ligament, vaginal mucosa, and anterior pericervical fascia. In my experience, inclusion of the vaginal mucosa eliminates the formation of granulation tissue.
A cystoscopy after injection of intravenous indigo carmine is performed to ensure that both ureters are patent, and that there is no injury to the bladder. If there is no flow of dye through one or both of the ureters, I would first cut one of the angle sutures to determine whether the ureter has been obstructed or kinked by one of these sutures. If doing so does not open the ureter, the course of the ureter must be traced to identify the obstruction.
If the ureter can’t be easily seen through the peritoneum, a ureterolysis must be performed. I start at the pelvic brim as the ureter crosses over the anteromedial aspect of the psoas muscle and bifurcation of the common iliac artery. The peritoneum is tented and incised to follow the course of the ureter that lies just lateral to the peritoneal surface.
On the few occasions when I have cut the ureter, I knew it immediately and consulted a urologist to repair the damage. In cases in which there is no obvious ureteral damage, an intraoperative urologic consult is still appropriate. Of course, if a bladder perforation is identified, it must be closed and a catheter placed for 10-14 days. The choice of suture for cystotomy is normally a rapidly dissolving suture; closure should be performed in two layers, and cystoscopy is important to be sure that the trigone is not involved and the ureters are not compromised.
Vaginal Cuff Closure
Some surgeons are now using barbed suture to close the vaginal cuff in a double-layer closure, but I have continued using figure-of-eight sutures of O-PDS. I do not believe there are any disadvantages of a double-layer closure with barbed suture; rather, the question for me has been, is it better? Will there be any less incidence of dehiscence post procedure?
The authors of a new review on vaginal cuff dehiscence conclude that a two-layer cuff closure with bidirectional barbed suture – along with judicious use of electrocautery – may potentially decrease the risk of cuff dehiscence, although the extent of the effect is uncertain (Am. J. Obstet. Gynecol. 2012;206:284-8). Others have commented that laparoscopic cuff closure with or without robotic assistance fails to take large enough tissue bites, and therefore is inherently weaker than traditional abdominal or vaginal cuff closure.
I agree that both issues – overtreatment of the vaginal cuff with electrosurgery, and failure to take large enough bites when closing the cuff – are common denominators in vaginal cuff dehiscence. The magnified visualization that characterizes the laparoscopic and robotic approaches – and particularly the robotic approach – can inadvertently lead to smaller bites being sutured. When large enough bites of tissue are taken, the cuff will heal nicely and will not dehisce unless there is infection or tissue compromise.
In general, suturing is the most challenging part of laparoscopic hysterectomy and a big part of the learning curve. The key lies in mastering a few maneuvers. I try to make the conversion from open suturing to laparoscopic suturing as seamless as possible. I suture from the left side of the patient with my ports a fist apart so that I don’t crowd myself. I introduce my needles into the abdomen by back-loading the needles and placing them through the 5-mm port.
I use a needle holder made by Olympus that is the same shape and has the same handle as the one I use for open surgery. The needle must always lie perpendicular to the needle holder and must be grasped at the tip of the needle holder. This way, the needle can be placed and, with a simple turn of the wrist, engaged into the tissue. The needle is then retrieved and the process repeated. There is no substitute for practice; with repetition, this maneuver becomes very natural.
Once the suture has been placed, the needle is removed from the abdomen by leaving an inch-long tail and by pulling the needle, suture first out, along with one of the 5-mm ports; this will guarantee that the needle exits without difficulty. A closed knot pusher allows the surgeon to apply enough tension to the suture so that the knots are nice and tight without the risk of the knot pusher slipping off the suture. Intracorporeal knot tying also has been performed very successfully, but it entails an additional level of skill.
Dr. Levine is the program director of minimally invasive gynecologic surgery and codirector of the Minimally Invasive Surgical Institute at Mercy Hospital, St. Louis. He reported that he has no disclosures relevant to this Master Class.
This column, "Master Class," appears regularly in Ob.Gyn. News.
In 1989 Dr. Harry Reich performed the first laparoscopic hysterectomy using rather primitive instruments by today’s standards, and changed the gynecologic surgical landscape forever. Those of us who have performed laparoscopic hysterectomy during the 20-plus years since then have provided women with a faster recovery, less time in the hospital, and less pain than they would have experienced with a traditional abdominal approach.
The minimally invasive approach has become the mantra for most gynecologic procedures. Vaginal hysterectomy always has been considered its standard bearer, and as emphasized by the American College of Obstetricians and Gynecologists (ACOG) in its recent statement, it should remain the primary approach whenever it is feasible (Obstet. Gynecol. 2009;114:1156-8). In cases in which vaginal hysterectomy is not an option, however, laparoscopic hysterectomy is clearly the next-best technique.
In 2010, the AAGL published a position paper stating that most hysterectomies for benign disease should be performed either vaginally or laparoscopically, and that continued efforts should be taken to facilitate these approaches (J. Minimal. Invasive Gynecol. 2010 [doi: 10.1016/jmig.2010.10.001]). The recent consensus in the literature, moreover, has been that abdominal hysterectomy should be reserved for cases in which the patient cannot tolerate a laparoscopic approach. For example, a history of cardiopulmonary compromise or multiple gastrointestinal procedures may make a laparoscopic approach potentially too dangerous.
There have been some important refinements in laparoscopic hysterectomy over the past 20-plus years, including the ability to manage vascular pedicles with less thermal spread. This has decreased the potential for ureteral damage. Moreover, our ability to pinpoint the cervicovaginal margin, so as not to shorten the vagina, also has been an improvement. Overall, however, the technique has not changed significantly.
So what is new? The option in some hospitals of performing hysterectomies robotically is attracting surgeons who have not developed a skill set in traditional laparoscopy to perform the technique at bedside. This growing number of gynecologic surgeons is finding it easier and technically more enjoyable to use the da Vinci Surgical System.
Now, consequently, the focus of discussion and debate concerns the availability and cost of the robotic system, as well as the comparative complication rates and operating room times of robotic and traditional laparoscopic hysterectomy.
As gynecologic surgery faces this paradigm shift in the choice of surgical approaches, and as health care payment models continue to evolve, it is important to understand the value of each approach. One recent study found that robotic hysterectomies were associated with longer surgical times and cost an average of $2,600 more (J. Minim. Invasive Gynecol. 2010;17:730-8). Other studies have reported similar findings.
While some extra cost may be acceptable today, it will be less palatable if – or when – reimbursement models change and bundled payments become more common. Moreover, robotic technology is not universally available. Currently, the residents I teach do not have access to the da Vinci system. In the future, if access is available, their training in traditional laparoscopy will be valuable.
Thus, despite the increasing popularity of a robotic approach to hysterectomy, it is imperative that teaching the approach in laparoscopic hysterectomy not be pushed to the background.
While the learning curve of laparoscopic hysterectomy is not insignificant, it is more intimidating than it should be. Once the surgeon learns to suture laparoscopically, he or she has largely broken the barrier.
The Process and Technique
At the start of the procedure, before insufflation, I place a Rumi Uterine Manipulator and KOH colpotomizer (CooperSurgical, Trumbull, Conn.) to identify the anterior and posterior fornices in preparation for colpotomy. Other cupped manipulators such as the VCare uterine manipulator/elevator (CONMED EndoSurgery, Utica, N.Y.) and the McCarus-Volker system (LSI Solutions, Rochester, N.Y.) may be utilized, but I prefer the Rumi.
The application of the cup must be flush with the vaginal fornices, and the intrauterine stem must be the correct length to easily mobilize the uterus from side to side as well as anterior and posterior. If the manipulator is not used and placed properly, it will be more difficult to find the cervical cup when initiating the colpotomy and maintaining the pneumoperitoneum. Some surgeons suture the cup to the cervix to provide greater traction and easier retrieval of the specimen, but I don’t find this necessary.
I always employ a left upper quadrant approach for insufflation of the abdomen. I ask anesthesia to place an orogastric tube to empty the stomach of its contents and the air that has accumulated during intubation. The patient must not have had a prior splenectomy or surgery in the left upper quadrant.
In patients who have had any prior abdominal surgery (cesarean section, appendectomy, etc.), a 3-mm port is placed in the left upper quadrant to insufflate as well as visualize the anterior abdominal wall in order to identify any adhesions that may have formed after the prior surgery. If adhesions are present, then before the umbilical port is placed, two 5-mm left lateral ports are introduced and the anterior abdominal wall adhesions are lysed.
In all patients, an umbilical trocar is placed under direct vision for the 5-mm zero-degree laparoscope, and 5-mm ports (two on my operating side and one for my assistant) are placed lateral to the inferior epigastric vessels on the left and right. These lateral port sites are placed a fist-width apart along the lateral side wall, usually at a level just above the iliac crest. Exact placement is determined by uterine size. Depending on the uterine size, an additional port may be placed below the umbilicus.
When the ovaries are to be removed, both ureters are identified and the infundibulopelvic ligaments are isolated, then coagulated and transected. If there is any question about the path of the ureter and its proximity to the infundibulopelvic ligament or the uterine vasculature – as in the case of endometriosis or broad ligament fibroids – then ureterolysis will be performed.
The broad and round ligaments then are coagulated and transected, and the bladder flap is developed. The creation of the bladder flap is simple unless the patient has undergone multiple cesarean sections and has significant scarring. This dissection must be layer by layer, with the surgeon always working lateral to medial until the vaginal tissues are reached. When in doubt, it is helpful to backfill the bladder with normal saline to more easily identify the bladder margins. This is easily accomplished using a three-way Foley catheter placed at the beginning of the procedure in anticipation of a scarred lower uterine segment.
We then dissect the peritoneum posteriorly as well at the level of the uterosacral ligaments so that the uterine arteries may be isolated bilaterally. This also serves as another relaxing incision keeping the ureter away from our energy sources. At this point, we utilize a reusable bipolar forceps made by Storz (Karl Storz Endoscopy, El Segundo, Calif.) to coagulate the uterine vessels bilaterally and decrease back-bleeding, and I transect the vessels using the Harmonic Ace Shears (Ethicon Endo-Surgery, Somerville, N.J.). I prefer the reusable bipolar forceps over other instruments, and have learned not to overdesiccate the tissues.
It is imperative for the surgeon or the assistant to constantly push the uterus cephalad using the manipulator in order to protect the ureters from thermal damage as well as transection at the time of colpotomy. This should be the responsibility of the surgeon, unless tissue retraction is necessary. In this case, this task can be given to an assistant, but under constant scrutiny.
Once the uterine arteries are secured, the colpotomy is begun anteriorly using the active blade of the Harmonic Ace. Monopolar energy can be used as well, but I find that this results in more bleeding from the cuff, which in turn necessitates the use of bipolar energy to create hemostasis.
The cervix is circumscribed along the colpotomy cup and, once disconnected, is delivered vaginally. To make the colpotomy easier, it is best to thin out the tissue between the cut uterine vessels and the cervix, exposing the cup. This is accomplished by serially clamping and coagulating the soft tissue above the level of the cup, allowing the impression of the cup to be more easily seen, while at the same time always pushing the cup cephalad.
The delivery of the uterus through what appears to be a small colpotomy incision is accomplished by deflating the vaginal occlusion balloon and gently applying traction on the stem, bringing the cervix closer to the introitus. If the uterus is too large to be delivered through the incision, it can be easily morcellated as one would a large uterus during vaginal hysterectomy. To maintain pneumoperitoneum after delivery of the uterus, the uterus can be left in the vagina or, if the Rumi manipulator is used, the balloon can be removed and reinflated in the vagina.
Once hemostasis is adequate, the cuff is closed with three figure-of-eight sutures of O-polydioxanone (PDS). ("Adequate" hemostasis, it must be noted, does not mean complete desiccation of the cuff; it just means no active bleeding. Leaving the cuff a little wet is not a bad thing.) Any dissolvable suture can be used, but I find that PDS slides nicely when used in extracorporeal knot tying. Even if the first knot loosens before the second knot is thrown, it will tighten as the second knot pushes the first toward the tissue. I suture the angles first and then the midline. To lower the risk of posthysterectomy prolapse, I include in my angle sutures the uterosacral ligament, vaginal mucosa, and anterior pericervical fascia. In my experience, inclusion of the vaginal mucosa eliminates the formation of granulation tissue.
A cystoscopy after injection of intravenous indigo carmine is performed to ensure that both ureters are patent, and that there is no injury to the bladder. If there is no flow of dye through one or both of the ureters, I would first cut one of the angle sutures to determine whether the ureter has been obstructed or kinked by one of these sutures. If doing so does not open the ureter, the course of the ureter must be traced to identify the obstruction.
If the ureter can’t be easily seen through the peritoneum, a ureterolysis must be performed. I start at the pelvic brim as the ureter crosses over the anteromedial aspect of the psoas muscle and bifurcation of the common iliac artery. The peritoneum is tented and incised to follow the course of the ureter that lies just lateral to the peritoneal surface.
On the few occasions when I have cut the ureter, I knew it immediately and consulted a urologist to repair the damage. In cases in which there is no obvious ureteral damage, an intraoperative urologic consult is still appropriate. Of course, if a bladder perforation is identified, it must be closed and a catheter placed for 10-14 days. The choice of suture for cystotomy is normally a rapidly dissolving suture; closure should be performed in two layers, and cystoscopy is important to be sure that the trigone is not involved and the ureters are not compromised.
Vaginal Cuff Closure
Some surgeons are now using barbed suture to close the vaginal cuff in a double-layer closure, but I have continued using figure-of-eight sutures of O-PDS. I do not believe there are any disadvantages of a double-layer closure with barbed suture; rather, the question for me has been, is it better? Will there be any less incidence of dehiscence post procedure?
The authors of a new review on vaginal cuff dehiscence conclude that a two-layer cuff closure with bidirectional barbed suture – along with judicious use of electrocautery – may potentially decrease the risk of cuff dehiscence, although the extent of the effect is uncertain (Am. J. Obstet. Gynecol. 2012;206:284-8). Others have commented that laparoscopic cuff closure with or without robotic assistance fails to take large enough tissue bites, and therefore is inherently weaker than traditional abdominal or vaginal cuff closure.
I agree that both issues – overtreatment of the vaginal cuff with electrosurgery, and failure to take large enough bites when closing the cuff – are common denominators in vaginal cuff dehiscence. The magnified visualization that characterizes the laparoscopic and robotic approaches – and particularly the robotic approach – can inadvertently lead to smaller bites being sutured. When large enough bites of tissue are taken, the cuff will heal nicely and will not dehisce unless there is infection or tissue compromise.
In general, suturing is the most challenging part of laparoscopic hysterectomy and a big part of the learning curve. The key lies in mastering a few maneuvers. I try to make the conversion from open suturing to laparoscopic suturing as seamless as possible. I suture from the left side of the patient with my ports a fist apart so that I don’t crowd myself. I introduce my needles into the abdomen by back-loading the needles and placing them through the 5-mm port.
I use a needle holder made by Olympus that is the same shape and has the same handle as the one I use for open surgery. The needle must always lie perpendicular to the needle holder and must be grasped at the tip of the needle holder. This way, the needle can be placed and, with a simple turn of the wrist, engaged into the tissue. The needle is then retrieved and the process repeated. There is no substitute for practice; with repetition, this maneuver becomes very natural.
Once the suture has been placed, the needle is removed from the abdomen by leaving an inch-long tail and by pulling the needle, suture first out, along with one of the 5-mm ports; this will guarantee that the needle exits without difficulty. A closed knot pusher allows the surgeon to apply enough tension to the suture so that the knots are nice and tight without the risk of the knot pusher slipping off the suture. Intracorporeal knot tying also has been performed very successfully, but it entails an additional level of skill.
Dr. Levine is the program director of minimally invasive gynecologic surgery and codirector of the Minimally Invasive Surgical Institute at Mercy Hospital, St. Louis. He reported that he has no disclosures relevant to this Master Class.
This column, "Master Class," appears regularly in Ob.Gyn. News.