User login
Suicidal while receiving treatment for breast cancer
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6
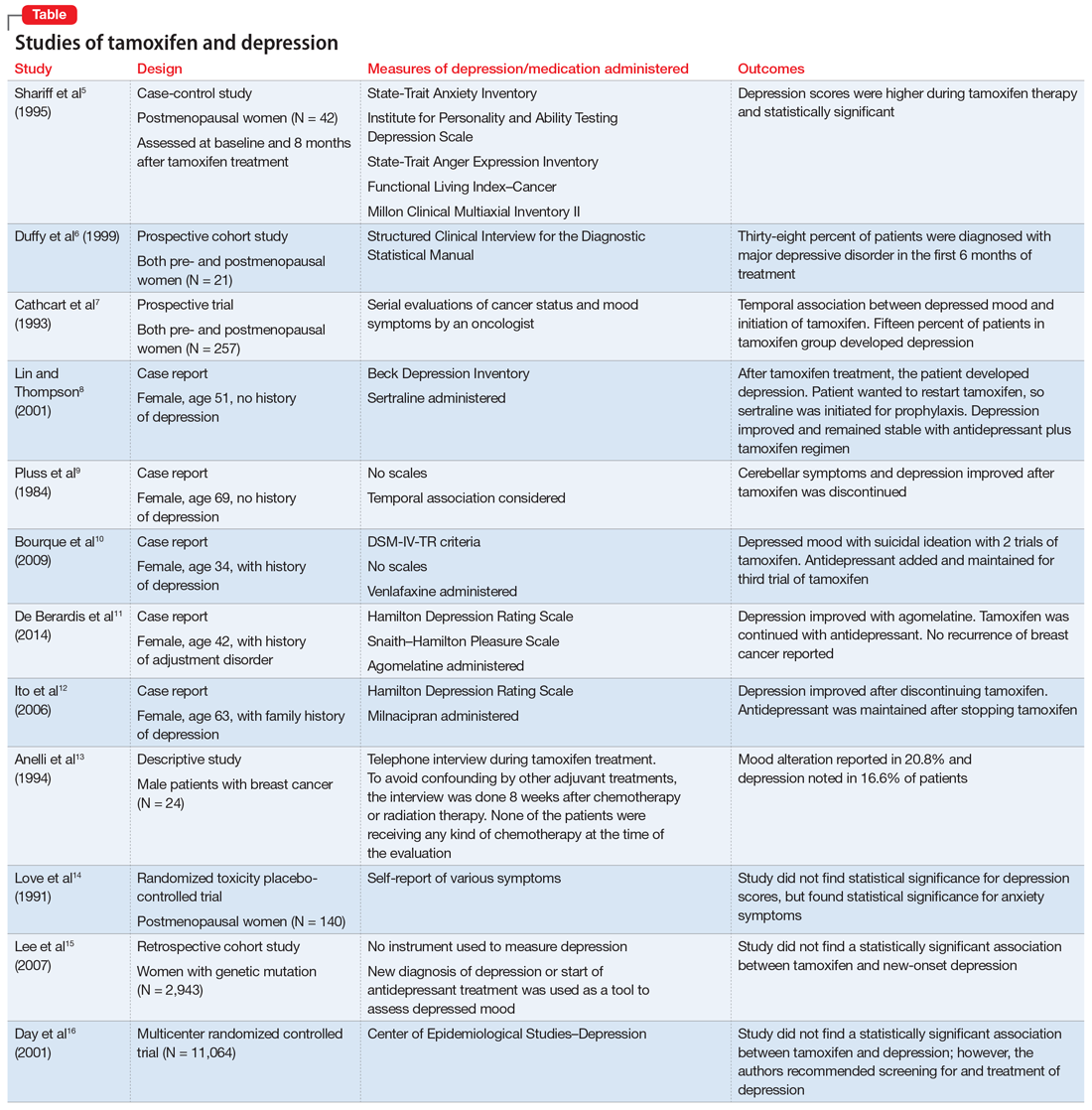
In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6

In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6

In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
