User login
Do antibiotics shorten symptoms in patients with purulent nasal discharge?
NO. For most patients with purulent nasal discharge, antibiotics don’t decrease symptom duration; they do increase adverse events (strength of recommendation [SOR]: A, 3 meta-analyses and 2 randomized controlled trials [RCTs]).
Researchers in the field don’t recommend using antibiotics as routine treatment for purulent rhinorrhea associated with symptoms of upper respiratory infection ([SOR]: C, expert opinion).
Evidence summary
A Cochrane review of antibiotics for the common cold that included 5 RCTs with a total of 772 participants with purulent nasal discharge found no benefit from antibiotics.1 The relative risk (RR) for persistent acute purulent rhinitis with antibiotics compared with placebo was 0.63 (95% confidence interval [CI], 0.38-1.07; P=.087). The antibiotic groups showed an increase in adverse effects, with an RR of 1.46 (95% CI, 1.01-1.94; P=.047).
Benefits of antibiotics tempered by adverse effects
A meta-analysis of 6 RCTs with more than 1400 subjects showed persistent nasal discharge at 5 to 8 days, on average, in 23% of patients who received antibiotics compared with 46% of patients who received placebo (RR of benefits=1.18; 95% CI, 1.05-1.33; P=.05).2 Most subjects were between 12 and 50 years of age; 2 of the trials included children between 2 months and 16 years of age. All subjects had symptoms for fewer than 10 days.
The adverse effects of antibiotic treatment, primarily rash and diarrhea, were also addressed (RR of adverse effects=1.46; 95% CI, 1.10-1.94; P=.028). Given the overlap of the number needed to treat (7-15) and number needed to harm (12-78), the authors concluded that most patients get better without antibiotics, supporting “no antibiotic as first line” treatment advice.
Other studies show minimal benefit for antibiotics
A meta-analysis of 9 placebo-controlled RCTs (2640 adult subjects with rhinosinusitis-like complaints) found that antibiotics provided minimal benefit. For patients with visible purulent drainage in the pharynx, the NNT overlapped with the NNH; patients without visible purulent discharge showed even less benefit from antibiotics.3
Clinical improvement is insufficient to recommend antibiotic treatment
Three double-blinded RCTs studied patients older than 12 years who presented to a family practice clinic complaining of purulent rhinitis.4-6 All 3 studies compared amoxicillin treatment with placebo; outcomes were based primarily on patient diaries that recorded symptoms, including nasal discharge.
The first study randomized 135 patients to either amoxicillin (n=67) or placebo (n=68) for 10 days.4 At the end of 2 weeks, both groups had similar rates of symptom improvement—although in a subgroup of 57 patients who had complete symptom resolution at 2 weeks, the median number of days until resolution of purulent nasal discharge was 8 in the amoxicillin group compared with 12 days for the placebo group (P=.039). The authors could not identify clinical characteristics favoring antibiotic treatment.
In the second study, 207 patients received amoxicillin and 209 placebo.5 After 10 days of therapy, symptom resolution rates were not significantly different (35% for amoxicillin vs 29% for placebo). However, patients in the amoxicillin group had quicker resolution of purulent nasal discharge (9 vs 14 days for 75% of patients to be free of that symptom; P=.007).5
The third study (240 adults) didn’t find a significant decrease in duration of purulent nasal discharge in the antibiotic group compared with the placebo group.6
Despite the findings of decreased duration of purulent nasal discharge in the first 2 studies, the authors of all 3 studies concluded that the clinical difference in improvement between antibiotic and placebo groups was not enough to recommend treatment with antibiotics. Although the trials didn’t measure adverse outcomes, the authors advised clinicians to consider the potential for adverse reactions before recommending antibiotic treatment.
Recommendations
Both the American Academy of Otolaryngology and the American Academy of Allergy, Asthma, and Immunology recommend watchful waiting without antibiotics for acute sinusitis with mild pain or temperature lower than 101°F and consideration of antibiotics only if symptoms worsen or fail to improve by 7 days after diagnosis. Neither group offers specific recommendations regarding patients with purulent discharge.7,8
The Centers for Disease Control and Prevention recommend reserving antibiotic treatment of acute bacterial rhinosinusitis for patients with symptoms lasting longer than 7 days and patients who have unilateral symptoms with purulent nasal discharge.9
1. Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2005;(3):CD000247.-
2. Arroll B, Kenealy T. Are antibiotics effective for acute purulent rhinitis? Systematic review and meta-analysis of placebo controlled randomised trials. BMJ. 2006;333:279.-
3. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
4. Merenstein D, Whittaker C, Chadwell T, et al. Are antibiotics beneficial for patients with sinusitis complaints? A randomized double-blind clinical trial. J Fam Pract. 2005;54:144-151.
5. De Sutter AI, De Meyere MJ, Christiaens TC, et al. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double-blind controlled trial in family practice. J Fam Pract. 2002;51:317-323.
6. Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA. 2007;298:2487-2496.
7. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
8. Slavin RG, Spector SL, Bernstein IL, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116(6 suppl):S13-S47.
9. Hickner JM, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Emerg Med. 2001;37:703-710.
NO. For most patients with purulent nasal discharge, antibiotics don’t decrease symptom duration; they do increase adverse events (strength of recommendation [SOR]: A, 3 meta-analyses and 2 randomized controlled trials [RCTs]).
Researchers in the field don’t recommend using antibiotics as routine treatment for purulent rhinorrhea associated with symptoms of upper respiratory infection ([SOR]: C, expert opinion).
Evidence summary
A Cochrane review of antibiotics for the common cold that included 5 RCTs with a total of 772 participants with purulent nasal discharge found no benefit from antibiotics.1 The relative risk (RR) for persistent acute purulent rhinitis with antibiotics compared with placebo was 0.63 (95% confidence interval [CI], 0.38-1.07; P=.087). The antibiotic groups showed an increase in adverse effects, with an RR of 1.46 (95% CI, 1.01-1.94; P=.047).
Benefits of antibiotics tempered by adverse effects
A meta-analysis of 6 RCTs with more than 1400 subjects showed persistent nasal discharge at 5 to 8 days, on average, in 23% of patients who received antibiotics compared with 46% of patients who received placebo (RR of benefits=1.18; 95% CI, 1.05-1.33; P=.05).2 Most subjects were between 12 and 50 years of age; 2 of the trials included children between 2 months and 16 years of age. All subjects had symptoms for fewer than 10 days.
The adverse effects of antibiotic treatment, primarily rash and diarrhea, were also addressed (RR of adverse effects=1.46; 95% CI, 1.10-1.94; P=.028). Given the overlap of the number needed to treat (7-15) and number needed to harm (12-78), the authors concluded that most patients get better without antibiotics, supporting “no antibiotic as first line” treatment advice.
Other studies show minimal benefit for antibiotics
A meta-analysis of 9 placebo-controlled RCTs (2640 adult subjects with rhinosinusitis-like complaints) found that antibiotics provided minimal benefit. For patients with visible purulent drainage in the pharynx, the NNT overlapped with the NNH; patients without visible purulent discharge showed even less benefit from antibiotics.3
Clinical improvement is insufficient to recommend antibiotic treatment
Three double-blinded RCTs studied patients older than 12 years who presented to a family practice clinic complaining of purulent rhinitis.4-6 All 3 studies compared amoxicillin treatment with placebo; outcomes were based primarily on patient diaries that recorded symptoms, including nasal discharge.
The first study randomized 135 patients to either amoxicillin (n=67) or placebo (n=68) for 10 days.4 At the end of 2 weeks, both groups had similar rates of symptom improvement—although in a subgroup of 57 patients who had complete symptom resolution at 2 weeks, the median number of days until resolution of purulent nasal discharge was 8 in the amoxicillin group compared with 12 days for the placebo group (P=.039). The authors could not identify clinical characteristics favoring antibiotic treatment.
In the second study, 207 patients received amoxicillin and 209 placebo.5 After 10 days of therapy, symptom resolution rates were not significantly different (35% for amoxicillin vs 29% for placebo). However, patients in the amoxicillin group had quicker resolution of purulent nasal discharge (9 vs 14 days for 75% of patients to be free of that symptom; P=.007).5
The third study (240 adults) didn’t find a significant decrease in duration of purulent nasal discharge in the antibiotic group compared with the placebo group.6
Despite the findings of decreased duration of purulent nasal discharge in the first 2 studies, the authors of all 3 studies concluded that the clinical difference in improvement between antibiotic and placebo groups was not enough to recommend treatment with antibiotics. Although the trials didn’t measure adverse outcomes, the authors advised clinicians to consider the potential for adverse reactions before recommending antibiotic treatment.
Recommendations
Both the American Academy of Otolaryngology and the American Academy of Allergy, Asthma, and Immunology recommend watchful waiting without antibiotics for acute sinusitis with mild pain or temperature lower than 101°F and consideration of antibiotics only if symptoms worsen or fail to improve by 7 days after diagnosis. Neither group offers specific recommendations regarding patients with purulent discharge.7,8
The Centers for Disease Control and Prevention recommend reserving antibiotic treatment of acute bacterial rhinosinusitis for patients with symptoms lasting longer than 7 days and patients who have unilateral symptoms with purulent nasal discharge.9
NO. For most patients with purulent nasal discharge, antibiotics don’t decrease symptom duration; they do increase adverse events (strength of recommendation [SOR]: A, 3 meta-analyses and 2 randomized controlled trials [RCTs]).
Researchers in the field don’t recommend using antibiotics as routine treatment for purulent rhinorrhea associated with symptoms of upper respiratory infection ([SOR]: C, expert opinion).
Evidence summary
A Cochrane review of antibiotics for the common cold that included 5 RCTs with a total of 772 participants with purulent nasal discharge found no benefit from antibiotics.1 The relative risk (RR) for persistent acute purulent rhinitis with antibiotics compared with placebo was 0.63 (95% confidence interval [CI], 0.38-1.07; P=.087). The antibiotic groups showed an increase in adverse effects, with an RR of 1.46 (95% CI, 1.01-1.94; P=.047).
Benefits of antibiotics tempered by adverse effects
A meta-analysis of 6 RCTs with more than 1400 subjects showed persistent nasal discharge at 5 to 8 days, on average, in 23% of patients who received antibiotics compared with 46% of patients who received placebo (RR of benefits=1.18; 95% CI, 1.05-1.33; P=.05).2 Most subjects were between 12 and 50 years of age; 2 of the trials included children between 2 months and 16 years of age. All subjects had symptoms for fewer than 10 days.
The adverse effects of antibiotic treatment, primarily rash and diarrhea, were also addressed (RR of adverse effects=1.46; 95% CI, 1.10-1.94; P=.028). Given the overlap of the number needed to treat (7-15) and number needed to harm (12-78), the authors concluded that most patients get better without antibiotics, supporting “no antibiotic as first line” treatment advice.
Other studies show minimal benefit for antibiotics
A meta-analysis of 9 placebo-controlled RCTs (2640 adult subjects with rhinosinusitis-like complaints) found that antibiotics provided minimal benefit. For patients with visible purulent drainage in the pharynx, the NNT overlapped with the NNH; patients without visible purulent discharge showed even less benefit from antibiotics.3
Clinical improvement is insufficient to recommend antibiotic treatment
Three double-blinded RCTs studied patients older than 12 years who presented to a family practice clinic complaining of purulent rhinitis.4-6 All 3 studies compared amoxicillin treatment with placebo; outcomes were based primarily on patient diaries that recorded symptoms, including nasal discharge.
The first study randomized 135 patients to either amoxicillin (n=67) or placebo (n=68) for 10 days.4 At the end of 2 weeks, both groups had similar rates of symptom improvement—although in a subgroup of 57 patients who had complete symptom resolution at 2 weeks, the median number of days until resolution of purulent nasal discharge was 8 in the amoxicillin group compared with 12 days for the placebo group (P=.039). The authors could not identify clinical characteristics favoring antibiotic treatment.
In the second study, 207 patients received amoxicillin and 209 placebo.5 After 10 days of therapy, symptom resolution rates were not significantly different (35% for amoxicillin vs 29% for placebo). However, patients in the amoxicillin group had quicker resolution of purulent nasal discharge (9 vs 14 days for 75% of patients to be free of that symptom; P=.007).5
The third study (240 adults) didn’t find a significant decrease in duration of purulent nasal discharge in the antibiotic group compared with the placebo group.6
Despite the findings of decreased duration of purulent nasal discharge in the first 2 studies, the authors of all 3 studies concluded that the clinical difference in improvement between antibiotic and placebo groups was not enough to recommend treatment with antibiotics. Although the trials didn’t measure adverse outcomes, the authors advised clinicians to consider the potential for adverse reactions before recommending antibiotic treatment.
Recommendations
Both the American Academy of Otolaryngology and the American Academy of Allergy, Asthma, and Immunology recommend watchful waiting without antibiotics for acute sinusitis with mild pain or temperature lower than 101°F and consideration of antibiotics only if symptoms worsen or fail to improve by 7 days after diagnosis. Neither group offers specific recommendations regarding patients with purulent discharge.7,8
The Centers for Disease Control and Prevention recommend reserving antibiotic treatment of acute bacterial rhinosinusitis for patients with symptoms lasting longer than 7 days and patients who have unilateral symptoms with purulent nasal discharge.9
1. Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2005;(3):CD000247.-
2. Arroll B, Kenealy T. Are antibiotics effective for acute purulent rhinitis? Systematic review and meta-analysis of placebo controlled randomised trials. BMJ. 2006;333:279.-
3. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
4. Merenstein D, Whittaker C, Chadwell T, et al. Are antibiotics beneficial for patients with sinusitis complaints? A randomized double-blind clinical trial. J Fam Pract. 2005;54:144-151.
5. De Sutter AI, De Meyere MJ, Christiaens TC, et al. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double-blind controlled trial in family practice. J Fam Pract. 2002;51:317-323.
6. Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA. 2007;298:2487-2496.
7. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
8. Slavin RG, Spector SL, Bernstein IL, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116(6 suppl):S13-S47.
9. Hickner JM, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Emerg Med. 2001;37:703-710.
1. Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2005;(3):CD000247.-
2. Arroll B, Kenealy T. Are antibiotics effective for acute purulent rhinitis? Systematic review and meta-analysis of placebo controlled randomised trials. BMJ. 2006;333:279.-
3. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
4. Merenstein D, Whittaker C, Chadwell T, et al. Are antibiotics beneficial for patients with sinusitis complaints? A randomized double-blind clinical trial. J Fam Pract. 2005;54:144-151.
5. De Sutter AI, De Meyere MJ, Christiaens TC, et al. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double-blind controlled trial in family practice. J Fam Pract. 2002;51:317-323.
6. Williamson IG, Rumsby K, Benge S, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA. 2007;298:2487-2496.
7. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
8. Slavin RG, Spector SL, Bernstein IL, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116(6 suppl):S13-S47.
9. Hickner JM, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Emerg Med. 2001;37:703-710.
Evidence-based answers from the Family Physicians Inquiries Network
Is it safe to vaccinate children against varicella while they’re in close contact with a pregnant woman?
YES. All healthy children without evidence of immunity to varicella who are living in a household with a susceptible pregnant woman should be vaccinated (strength of recommendation [SOR]: C, expert opinion).
The risk of transmission of vaccine virus to household contacts is very low (SOR: B, observational studies). Transmission is higher, but still rare, among contacts of immunocompromised vaccinees (SOR: B, observational studies).
Varicella infection has not been reported in unborn babies of women who had contact with a recently vaccinated person.
Evidence summary
Pregnant women without immunity to varicella are at risk of developing chickenpox, which can cause congenital varicella syndrome. An estimated 44 cases of congenital varicella occurred each year in the prevaccine era.1
Varicella vaccine contains live attenuated virus. Approximately 2% to 3% of vaccinees develop either a localized rash around the injection site or a generalized rash.1 The vaccine virus can, theoretically, spread from vaccinees who develop a rash to other people. Nevertheless, the probability of contracting varicella after contact with a healthy vaccinee is very low.
Minimal transmission, no infection from contact with healthy vaccinees
A prospective vaccine efficacy study found that 3 of 446 (0.67%) contacts of healthy vaccinees seroconverted, but had no clinical evidence of varicella.2 In a smaller study, 30 immunocompromised siblings of 37 healthy children who received varicella vaccine showed no clinical or serological evidence of the virus.3
Five case reports document varicella infection in people who had contact with healthy vaccinees.1 One of these was a pregnant woman who chose to terminate the pregnancy, but subsequent tests showed no virus in the fetus.4 We couldn’t find any reports of congenital varicella attributable to infection of the mother from a recent vaccinee.
Transmission by immunocompromised vaccinees is slightly higher
The risk of contracting vaccine-associated varicella from contact with an immunocompromised vaccinee is slightly higher than for a healthy vaccinee. The National Institute of Allergy and Infectious Diseases Varicella Vaccine Collaborative Study evaluated transmission and infectivity of the varicella vaccine virus in the close contacts of 482 vaccinated children with leukemia.5 One hundred fifty-six vaccinees developed a rash approximately one month after vaccination. Among 88 healthy susceptible siblings in close contact with the 156 vaccinees, 15 (17%) showed evidence of virus transmission. Of the 15, 4 had subclinical infection and the other 11 had a mild rash.
Recommendations
The American Academy of Pediatrics, Advisory Committee on Immunization Practices, and Centers for Disease Control and Prevention say that no precautions are necessary after varicella vaccination of family members in households with pregnant women. If a vaccinee develops a rash, precautions such as separating the vaccinee and the pregnant woman until the rash resolves are advisable. Giving Varicella zoster immune globulin to pregnant women without immunity who are exposed to varicella should be considered. Varicella vaccines are contraindicated in people with malignancies, immunodeficiencies (congenital or acquired), and immunosuppression caused by medications.1,3,6,7
1. Centers for Disease Control and Prevention. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-4):1-40.
2. Weibel R, Neff B, Kuter B, et al. Live attenuated varicella virus vaccine: efficacy trial in healthy children. N Engl J Med. 1984;310:1409-1415.
3. Diaz PS, Au D, Smith S, et al. Lack of transmission of the live attenuated varicella vaccine virus to immunocompromised children after immunization of their siblings. Pediatrics. 1991;87:166-170.
4. Salzman MB, Sharrar RG, Steinberg S, et al. Transmission of varicella-vaccine virus from a healthy 12-month-old child to his pregnant mother. J Pediatr. 1997;131:151-154.
5. Tsolia M, Gershon AA, Steinberg SP, et al. Live attenuated varicella vaccine: evidence that the virus is attenuated and the importance of skin lesions in transmission of varicella-zoster virus. National Institute of Allergy and Infectious Diseases Varicella Vaccine Collaborative Study Group. J Pediatr. 1990;116:184-189.
6. American Academy of Pediatrics Committee on Infectious Diseases. Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine 2-dose varicella immunization schedule. Pediatrics. 2007;120:221-231.
7. Centers for Disease Control and Prevention. Varicella vaccine—Q&As about pregnancy. Available at: http://cdc.gov/vaccines/VPD-VAC/varicella/vac-faqs-clinic-preg.htm. Accessed October 11, 2010.
YES. All healthy children without evidence of immunity to varicella who are living in a household with a susceptible pregnant woman should be vaccinated (strength of recommendation [SOR]: C, expert opinion).
The risk of transmission of vaccine virus to household contacts is very low (SOR: B, observational studies). Transmission is higher, but still rare, among contacts of immunocompromised vaccinees (SOR: B, observational studies).
Varicella infection has not been reported in unborn babies of women who had contact with a recently vaccinated person.
Evidence summary
Pregnant women without immunity to varicella are at risk of developing chickenpox, which can cause congenital varicella syndrome. An estimated 44 cases of congenital varicella occurred each year in the prevaccine era.1
Varicella vaccine contains live attenuated virus. Approximately 2% to 3% of vaccinees develop either a localized rash around the injection site or a generalized rash.1 The vaccine virus can, theoretically, spread from vaccinees who develop a rash to other people. Nevertheless, the probability of contracting varicella after contact with a healthy vaccinee is very low.
Minimal transmission, no infection from contact with healthy vaccinees
A prospective vaccine efficacy study found that 3 of 446 (0.67%) contacts of healthy vaccinees seroconverted, but had no clinical evidence of varicella.2 In a smaller study, 30 immunocompromised siblings of 37 healthy children who received varicella vaccine showed no clinical or serological evidence of the virus.3
Five case reports document varicella infection in people who had contact with healthy vaccinees.1 One of these was a pregnant woman who chose to terminate the pregnancy, but subsequent tests showed no virus in the fetus.4 We couldn’t find any reports of congenital varicella attributable to infection of the mother from a recent vaccinee.
Transmission by immunocompromised vaccinees is slightly higher
The risk of contracting vaccine-associated varicella from contact with an immunocompromised vaccinee is slightly higher than for a healthy vaccinee. The National Institute of Allergy and Infectious Diseases Varicella Vaccine Collaborative Study evaluated transmission and infectivity of the varicella vaccine virus in the close contacts of 482 vaccinated children with leukemia.5 One hundred fifty-six vaccinees developed a rash approximately one month after vaccination. Among 88 healthy susceptible siblings in close contact with the 156 vaccinees, 15 (17%) showed evidence of virus transmission. Of the 15, 4 had subclinical infection and the other 11 had a mild rash.
Recommendations
The American Academy of Pediatrics, Advisory Committee on Immunization Practices, and Centers for Disease Control and Prevention say that no precautions are necessary after varicella vaccination of family members in households with pregnant women. If a vaccinee develops a rash, precautions such as separating the vaccinee and the pregnant woman until the rash resolves are advisable. Giving Varicella zoster immune globulin to pregnant women without immunity who are exposed to varicella should be considered. Varicella vaccines are contraindicated in people with malignancies, immunodeficiencies (congenital or acquired), and immunosuppression caused by medications.1,3,6,7
YES. All healthy children without evidence of immunity to varicella who are living in a household with a susceptible pregnant woman should be vaccinated (strength of recommendation [SOR]: C, expert opinion).
The risk of transmission of vaccine virus to household contacts is very low (SOR: B, observational studies). Transmission is higher, but still rare, among contacts of immunocompromised vaccinees (SOR: B, observational studies).
Varicella infection has not been reported in unborn babies of women who had contact with a recently vaccinated person.
Evidence summary
Pregnant women without immunity to varicella are at risk of developing chickenpox, which can cause congenital varicella syndrome. An estimated 44 cases of congenital varicella occurred each year in the prevaccine era.1
Varicella vaccine contains live attenuated virus. Approximately 2% to 3% of vaccinees develop either a localized rash around the injection site or a generalized rash.1 The vaccine virus can, theoretically, spread from vaccinees who develop a rash to other people. Nevertheless, the probability of contracting varicella after contact with a healthy vaccinee is very low.
Minimal transmission, no infection from contact with healthy vaccinees
A prospective vaccine efficacy study found that 3 of 446 (0.67%) contacts of healthy vaccinees seroconverted, but had no clinical evidence of varicella.2 In a smaller study, 30 immunocompromised siblings of 37 healthy children who received varicella vaccine showed no clinical or serological evidence of the virus.3
Five case reports document varicella infection in people who had contact with healthy vaccinees.1 One of these was a pregnant woman who chose to terminate the pregnancy, but subsequent tests showed no virus in the fetus.4 We couldn’t find any reports of congenital varicella attributable to infection of the mother from a recent vaccinee.
Transmission by immunocompromised vaccinees is slightly higher
The risk of contracting vaccine-associated varicella from contact with an immunocompromised vaccinee is slightly higher than for a healthy vaccinee. The National Institute of Allergy and Infectious Diseases Varicella Vaccine Collaborative Study evaluated transmission and infectivity of the varicella vaccine virus in the close contacts of 482 vaccinated children with leukemia.5 One hundred fifty-six vaccinees developed a rash approximately one month after vaccination. Among 88 healthy susceptible siblings in close contact with the 156 vaccinees, 15 (17%) showed evidence of virus transmission. Of the 15, 4 had subclinical infection and the other 11 had a mild rash.
Recommendations
The American Academy of Pediatrics, Advisory Committee on Immunization Practices, and Centers for Disease Control and Prevention say that no precautions are necessary after varicella vaccination of family members in households with pregnant women. If a vaccinee develops a rash, precautions such as separating the vaccinee and the pregnant woman until the rash resolves are advisable. Giving Varicella zoster immune globulin to pregnant women without immunity who are exposed to varicella should be considered. Varicella vaccines are contraindicated in people with malignancies, immunodeficiencies (congenital or acquired), and immunosuppression caused by medications.1,3,6,7
1. Centers for Disease Control and Prevention. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-4):1-40.
2. Weibel R, Neff B, Kuter B, et al. Live attenuated varicella virus vaccine: efficacy trial in healthy children. N Engl J Med. 1984;310:1409-1415.
3. Diaz PS, Au D, Smith S, et al. Lack of transmission of the live attenuated varicella vaccine virus to immunocompromised children after immunization of their siblings. Pediatrics. 1991;87:166-170.
4. Salzman MB, Sharrar RG, Steinberg S, et al. Transmission of varicella-vaccine virus from a healthy 12-month-old child to his pregnant mother. J Pediatr. 1997;131:151-154.
5. Tsolia M, Gershon AA, Steinberg SP, et al. Live attenuated varicella vaccine: evidence that the virus is attenuated and the importance of skin lesions in transmission of varicella-zoster virus. National Institute of Allergy and Infectious Diseases Varicella Vaccine Collaborative Study Group. J Pediatr. 1990;116:184-189.
6. American Academy of Pediatrics Committee on Infectious Diseases. Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine 2-dose varicella immunization schedule. Pediatrics. 2007;120:221-231.
7. Centers for Disease Control and Prevention. Varicella vaccine—Q&As about pregnancy. Available at: http://cdc.gov/vaccines/VPD-VAC/varicella/vac-faqs-clinic-preg.htm. Accessed October 11, 2010.
1. Centers for Disease Control and Prevention. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-4):1-40.
2. Weibel R, Neff B, Kuter B, et al. Live attenuated varicella virus vaccine: efficacy trial in healthy children. N Engl J Med. 1984;310:1409-1415.
3. Diaz PS, Au D, Smith S, et al. Lack of transmission of the live attenuated varicella vaccine virus to immunocompromised children after immunization of their siblings. Pediatrics. 1991;87:166-170.
4. Salzman MB, Sharrar RG, Steinberg S, et al. Transmission of varicella-vaccine virus from a healthy 12-month-old child to his pregnant mother. J Pediatr. 1997;131:151-154.
5. Tsolia M, Gershon AA, Steinberg SP, et al. Live attenuated varicella vaccine: evidence that the virus is attenuated and the importance of skin lesions in transmission of varicella-zoster virus. National Institute of Allergy and Infectious Diseases Varicella Vaccine Collaborative Study Group. J Pediatr. 1990;116:184-189.
6. American Academy of Pediatrics Committee on Infectious Diseases. Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine 2-dose varicella immunization schedule. Pediatrics. 2007;120:221-231.
7. Centers for Disease Control and Prevention. Varicella vaccine—Q&As about pregnancy. Available at: http://cdc.gov/vaccines/VPD-VAC/varicella/vac-faqs-clinic-preg.htm. Accessed October 11, 2010.
Evidence-based answers from the Family Physicians Inquiries Network
Do nonmedicated topicals relieve childhood eczema?
Yes. Emollients are effective first-line treatment to decrease symptoms of eczema and reduce the need to use steroids in children (strength of recommendation [SOR]: A, consistent randomized, controlled trials [RCTs]).
Tar preparations work, but compliance may be limited (SOR: B, single small RCT). Gamma-linoleic acid preparations, borage oil, and evening primrose oil show efficacy in small studies (SOR: B, small RCTs). MAS063DP cream (Atopiclair) is effective (SOR: B, single RCT).
Chamomile (SOR: B, inconsistent RCTs) and bathing in acidic hot spring water (SOR: C, case-control study) may be effective, but these treatments have not been adequately evaluated. Wet wrap dressings may be effective but increase the risk of skin infections (SOR: B, single RCT).
Hamamelis distillate creams (SOR: B, limited RCT) and massage with essential oils/aromatherapy are ineffective (SOR: C, case-control study).
Evidence summary
Eczema is a chronic, inflammatory, pruritic skin disorder that affects infants, children, and adults. Therapeutic efficacy is defined as symptom relief and decreased inflammation. Topical corticosteroids and calcineurin inhibitors (such as tacrolimus and pimecrolimus) are the standard of care for prescription therapy in children, but their potentially harmful side effects argue for safer, nonmedicated treatments.
Topical treatments that work
Emollients have demonstrated efficacy in several RCTs compared with placebo and corticosteroids alone. No 1 preparation has proved superior to another; all reduce steroid use and improve skin hydration.1-3
Tar. Only 1 study has evaluated the use of tar: a comparison of 30 patients (mean age 11.8 years) who were treated with tar on one side of the body and 1% hydrocortisone on the other. Both treatments produced comparable results and were well tolerated. But compliance can be a problem with tar products because they smell unpleasant and stain clothing.4
Gamma-linoleic acid. Small studies have evaluated the efficacy of gamma-linoleic acid (GLA)—including borage oil (24% GLA) and evening primrose oil (7%-10% GLA). An RCT of 12 patients (ages 4-46 years, mean 18 years) that compared evening primrose oil with placebo found that patients treated with primrose oil showed a subjective improvement in skin scaling, dryness, redness, and itching.5
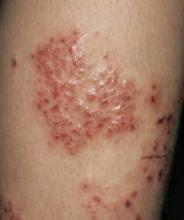
Eczema on the leg of a 9-year-old boy.A double-blind, placebo-controlled trial of 32 children that assessed the effects of undershirts coated with borage oil compared with noncoated undershirts found statistically significant improvements in both itching and erythema.6
MAS063DP is a nonsteroidal, hydrolipidic cream containing glycyrrhetinic acid (GrA), vitis vinifera (grapevine extract), and telmestine. A recent multicenter RCT of 142 children compared MAS063DP to vehicle cream alone. The primary outcome was treatment success defined as an Investigator’s Global Assessment score of ≤1 (range 0-5), measured on day 22. Therapy was successful in 77% of the treatment group vs 0% of the vehicle-only group (number needed to treat=1).7
Hot spring baths, chamomile may help
In a case control study of 70 patients (ages 12-80 years, mean 23 years,) bathing in acidic hot spring water (42° C) helped control edema, erythema, exudation, and excoriation in refractory cases of eczema.8
Several adult and mixed adult-child studies have found mild efficacy for chamomile extracts. One RCT demonstrated topical chamomile to be equivalent to 0.25% hydrocortisone cream for treating mild eczema.9
Wet wraps may help, but may raise skin infection risk
A critical review suggests that short-term use of wet wraps in combination with topical steroids and emollients is effective for severe eczema. However, a small RCT of 50 children found no additional benefit over standard care and an increased risk of skin infection (95% CI, 5%-42%; P=.05) with a number needed to harm of 5.10,11
Essential oils, hamamelis distillate don’t work
In 1 case control study, massage with essential oils didn’t improve eczema compared with massage without essential oils.12 Hamamelis (witch hazel) distillate cream was inferior to steroid creams.13
Recommendations
The American Academy of Dermatology guidelines state that emollients are the standard of care for childhood eczema and have a steroid-sparing effect (level of evidence [LOE]: A). Tar preparations have therapeutic benefits, but compliance is a major limitation (LOE: B). Not enough evidence exists to recommend acidic baths. The guidelines make no recommendations about other topical therapies.
A task force to formulate practice parameters has been created by the American College of Allergy, Asthma, and Immunology; the American Academy of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology. The task force’s latest recommendations suggest that emollients, tar preparations, and wet dressings are beneficial for treating eczema.2
1. Grimalt R, Mengeaud V, Cambazard F. Study Investigators’ Group. The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology. 2007;214:61-67.
2. Leung DY, Nicklas RA, Li JT, et al. Disease management of atopic dermatitis: an updated practice parameter. Joint Task Force on Practice Parameters. Ann Allergy Asthma Immunol. 2004;93(3 suppl 2):S1-S21.
3. Hanifin JM, Cooper KD, Ho VC, et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (ADA)/American Academy of Dermatology Association “Administrative Regulations for Evidence-Based Clinical Practice Guidelines.” J Am Acad Dermatol. 2004;50:391-404.
4. Munkvad M. A comparative trial of Clinitar versus hydrocortisone cream in the treatment of atopic eczema. Br J Dermatol. 1989;121:763-766.
5. Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatol Treat. 1990;1:199-201.
6. Kanehara S, Ohtani T, Uede K, et al. Clinical effects of undershirts coated with borage oil on children with atopic dermatitis: a double-blind, placebo-controlled trial. J Dermatol. 2007;34:811-815.
7. Boguniewicz M, Ziechner JA, Eichenfield LF, et al. MAS063DP is effective monotherapy for mild to moderate atopic dermatitis in infants and children: a multicenter, randomized, vehicle-controlled study. J Pediatr. 2008;152:854-859.
8. Kubota K, Machida I, Tamura K, et al. Treatment of refractory cases of atopic dermatitis with acidic hot-spring bathing. Acta Derm Venereol. 1997;77:452-454.
9. Ross SM. An integrative approach to eczema atopic dermatitis. Holist Nurs Pract. 2003;17:56-62.
10. Devillers AC, Oranje AP. Efficacy and safety of “wet-wrap” dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154:579-585.
11. Hindley D, Galloway G, Murray J, et al. A randomised study of “wet wraps” versus conventional treatment for atopic eczema. Arch Dis Child. 2006;91:164-168.
12. Anderson C, Lis-Balchin M, Kirk-Smith M. Evaluation of massage with essential oils on childhood eczema. Phytother Res. 2000;14:452-456.
13. Korting HC, Schäfer-Korting M, Klövekorn W, et al. Comparative efficacy of hamamelis distillate and hydrocortisone cream in atopic eczema. Eur J Clin Pharmacol. 1995;48:461-465.
Yes. Emollients are effective first-line treatment to decrease symptoms of eczema and reduce the need to use steroids in children (strength of recommendation [SOR]: A, consistent randomized, controlled trials [RCTs]).
Tar preparations work, but compliance may be limited (SOR: B, single small RCT). Gamma-linoleic acid preparations, borage oil, and evening primrose oil show efficacy in small studies (SOR: B, small RCTs). MAS063DP cream (Atopiclair) is effective (SOR: B, single RCT).
Chamomile (SOR: B, inconsistent RCTs) and bathing in acidic hot spring water (SOR: C, case-control study) may be effective, but these treatments have not been adequately evaluated. Wet wrap dressings may be effective but increase the risk of skin infections (SOR: B, single RCT).
Hamamelis distillate creams (SOR: B, limited RCT) and massage with essential oils/aromatherapy are ineffective (SOR: C, case-control study).
Evidence summary
Eczema is a chronic, inflammatory, pruritic skin disorder that affects infants, children, and adults. Therapeutic efficacy is defined as symptom relief and decreased inflammation. Topical corticosteroids and calcineurin inhibitors (such as tacrolimus and pimecrolimus) are the standard of care for prescription therapy in children, but their potentially harmful side effects argue for safer, nonmedicated treatments.
Topical treatments that work
Emollients have demonstrated efficacy in several RCTs compared with placebo and corticosteroids alone. No 1 preparation has proved superior to another; all reduce steroid use and improve skin hydration.1-3
Tar. Only 1 study has evaluated the use of tar: a comparison of 30 patients (mean age 11.8 years) who were treated with tar on one side of the body and 1% hydrocortisone on the other. Both treatments produced comparable results and were well tolerated. But compliance can be a problem with tar products because they smell unpleasant and stain clothing.4
Gamma-linoleic acid. Small studies have evaluated the efficacy of gamma-linoleic acid (GLA)—including borage oil (24% GLA) and evening primrose oil (7%-10% GLA). An RCT of 12 patients (ages 4-46 years, mean 18 years) that compared evening primrose oil with placebo found that patients treated with primrose oil showed a subjective improvement in skin scaling, dryness, redness, and itching.5
Eczema on the leg of a 9-year-old boy.A double-blind, placebo-controlled trial of 32 children that assessed the effects of undershirts coated with borage oil compared with noncoated undershirts found statistically significant improvements in both itching and erythema.6
MAS063DP is a nonsteroidal, hydrolipidic cream containing glycyrrhetinic acid (GrA), vitis vinifera (grapevine extract), and telmestine. A recent multicenter RCT of 142 children compared MAS063DP to vehicle cream alone. The primary outcome was treatment success defined as an Investigator’s Global Assessment score of ≤1 (range 0-5), measured on day 22. Therapy was successful in 77% of the treatment group vs 0% of the vehicle-only group (number needed to treat=1).7
Hot spring baths, chamomile may help
In a case control study of 70 patients (ages 12-80 years, mean 23 years,) bathing in acidic hot spring water (42° C) helped control edema, erythema, exudation, and excoriation in refractory cases of eczema.8
Several adult and mixed adult-child studies have found mild efficacy for chamomile extracts. One RCT demonstrated topical chamomile to be equivalent to 0.25% hydrocortisone cream for treating mild eczema.9
Wet wraps may help, but may raise skin infection risk
A critical review suggests that short-term use of wet wraps in combination with topical steroids and emollients is effective for severe eczema. However, a small RCT of 50 children found no additional benefit over standard care and an increased risk of skin infection (95% CI, 5%-42%; P=.05) with a number needed to harm of 5.10,11
Essential oils, hamamelis distillate don’t work
In 1 case control study, massage with essential oils didn’t improve eczema compared with massage without essential oils.12 Hamamelis (witch hazel) distillate cream was inferior to steroid creams.13
Recommendations
The American Academy of Dermatology guidelines state that emollients are the standard of care for childhood eczema and have a steroid-sparing effect (level of evidence [LOE]: A). Tar preparations have therapeutic benefits, but compliance is a major limitation (LOE: B). Not enough evidence exists to recommend acidic baths. The guidelines make no recommendations about other topical therapies.
A task force to formulate practice parameters has been created by the American College of Allergy, Asthma, and Immunology; the American Academy of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology. The task force’s latest recommendations suggest that emollients, tar preparations, and wet dressings are beneficial for treating eczema.2
Yes. Emollients are effective first-line treatment to decrease symptoms of eczema and reduce the need to use steroids in children (strength of recommendation [SOR]: A, consistent randomized, controlled trials [RCTs]).
Tar preparations work, but compliance may be limited (SOR: B, single small RCT). Gamma-linoleic acid preparations, borage oil, and evening primrose oil show efficacy in small studies (SOR: B, small RCTs). MAS063DP cream (Atopiclair) is effective (SOR: B, single RCT).
Chamomile (SOR: B, inconsistent RCTs) and bathing in acidic hot spring water (SOR: C, case-control study) may be effective, but these treatments have not been adequately evaluated. Wet wrap dressings may be effective but increase the risk of skin infections (SOR: B, single RCT).
Hamamelis distillate creams (SOR: B, limited RCT) and massage with essential oils/aromatherapy are ineffective (SOR: C, case-control study).
Evidence summary
Eczema is a chronic, inflammatory, pruritic skin disorder that affects infants, children, and adults. Therapeutic efficacy is defined as symptom relief and decreased inflammation. Topical corticosteroids and calcineurin inhibitors (such as tacrolimus and pimecrolimus) are the standard of care for prescription therapy in children, but their potentially harmful side effects argue for safer, nonmedicated treatments.
Topical treatments that work
Emollients have demonstrated efficacy in several RCTs compared with placebo and corticosteroids alone. No 1 preparation has proved superior to another; all reduce steroid use and improve skin hydration.1-3
Tar. Only 1 study has evaluated the use of tar: a comparison of 30 patients (mean age 11.8 years) who were treated with tar on one side of the body and 1% hydrocortisone on the other. Both treatments produced comparable results and were well tolerated. But compliance can be a problem with tar products because they smell unpleasant and stain clothing.4
Gamma-linoleic acid. Small studies have evaluated the efficacy of gamma-linoleic acid (GLA)—including borage oil (24% GLA) and evening primrose oil (7%-10% GLA). An RCT of 12 patients (ages 4-46 years, mean 18 years) that compared evening primrose oil with placebo found that patients treated with primrose oil showed a subjective improvement in skin scaling, dryness, redness, and itching.5
Eczema on the leg of a 9-year-old boy.A double-blind, placebo-controlled trial of 32 children that assessed the effects of undershirts coated with borage oil compared with noncoated undershirts found statistically significant improvements in both itching and erythema.6
MAS063DP is a nonsteroidal, hydrolipidic cream containing glycyrrhetinic acid (GrA), vitis vinifera (grapevine extract), and telmestine. A recent multicenter RCT of 142 children compared MAS063DP to vehicle cream alone. The primary outcome was treatment success defined as an Investigator’s Global Assessment score of ≤1 (range 0-5), measured on day 22. Therapy was successful in 77% of the treatment group vs 0% of the vehicle-only group (number needed to treat=1).7
Hot spring baths, chamomile may help
In a case control study of 70 patients (ages 12-80 years, mean 23 years,) bathing in acidic hot spring water (42° C) helped control edema, erythema, exudation, and excoriation in refractory cases of eczema.8
Several adult and mixed adult-child studies have found mild efficacy for chamomile extracts. One RCT demonstrated topical chamomile to be equivalent to 0.25% hydrocortisone cream for treating mild eczema.9
Wet wraps may help, but may raise skin infection risk
A critical review suggests that short-term use of wet wraps in combination with topical steroids and emollients is effective for severe eczema. However, a small RCT of 50 children found no additional benefit over standard care and an increased risk of skin infection (95% CI, 5%-42%; P=.05) with a number needed to harm of 5.10,11
Essential oils, hamamelis distillate don’t work
In 1 case control study, massage with essential oils didn’t improve eczema compared with massage without essential oils.12 Hamamelis (witch hazel) distillate cream was inferior to steroid creams.13
Recommendations
The American Academy of Dermatology guidelines state that emollients are the standard of care for childhood eczema and have a steroid-sparing effect (level of evidence [LOE]: A). Tar preparations have therapeutic benefits, but compliance is a major limitation (LOE: B). Not enough evidence exists to recommend acidic baths. The guidelines make no recommendations about other topical therapies.
A task force to formulate practice parameters has been created by the American College of Allergy, Asthma, and Immunology; the American Academy of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology. The task force’s latest recommendations suggest that emollients, tar preparations, and wet dressings are beneficial for treating eczema.2
1. Grimalt R, Mengeaud V, Cambazard F. Study Investigators’ Group. The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology. 2007;214:61-67.
2. Leung DY, Nicklas RA, Li JT, et al. Disease management of atopic dermatitis: an updated practice parameter. Joint Task Force on Practice Parameters. Ann Allergy Asthma Immunol. 2004;93(3 suppl 2):S1-S21.
3. Hanifin JM, Cooper KD, Ho VC, et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (ADA)/American Academy of Dermatology Association “Administrative Regulations for Evidence-Based Clinical Practice Guidelines.” J Am Acad Dermatol. 2004;50:391-404.
4. Munkvad M. A comparative trial of Clinitar versus hydrocortisone cream in the treatment of atopic eczema. Br J Dermatol. 1989;121:763-766.
5. Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatol Treat. 1990;1:199-201.
6. Kanehara S, Ohtani T, Uede K, et al. Clinical effects of undershirts coated with borage oil on children with atopic dermatitis: a double-blind, placebo-controlled trial. J Dermatol. 2007;34:811-815.
7. Boguniewicz M, Ziechner JA, Eichenfield LF, et al. MAS063DP is effective monotherapy for mild to moderate atopic dermatitis in infants and children: a multicenter, randomized, vehicle-controlled study. J Pediatr. 2008;152:854-859.
8. Kubota K, Machida I, Tamura K, et al. Treatment of refractory cases of atopic dermatitis with acidic hot-spring bathing. Acta Derm Venereol. 1997;77:452-454.
9. Ross SM. An integrative approach to eczema atopic dermatitis. Holist Nurs Pract. 2003;17:56-62.
10. Devillers AC, Oranje AP. Efficacy and safety of “wet-wrap” dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154:579-585.
11. Hindley D, Galloway G, Murray J, et al. A randomised study of “wet wraps” versus conventional treatment for atopic eczema. Arch Dis Child. 2006;91:164-168.
12. Anderson C, Lis-Balchin M, Kirk-Smith M. Evaluation of massage with essential oils on childhood eczema. Phytother Res. 2000;14:452-456.
13. Korting HC, Schäfer-Korting M, Klövekorn W, et al. Comparative efficacy of hamamelis distillate and hydrocortisone cream in atopic eczema. Eur J Clin Pharmacol. 1995;48:461-465.
1. Grimalt R, Mengeaud V, Cambazard F. Study Investigators’ Group. The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology. 2007;214:61-67.
2. Leung DY, Nicklas RA, Li JT, et al. Disease management of atopic dermatitis: an updated practice parameter. Joint Task Force on Practice Parameters. Ann Allergy Asthma Immunol. 2004;93(3 suppl 2):S1-S21.
3. Hanifin JM, Cooper KD, Ho VC, et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (ADA)/American Academy of Dermatology Association “Administrative Regulations for Evidence-Based Clinical Practice Guidelines.” J Am Acad Dermatol. 2004;50:391-404.
4. Munkvad M. A comparative trial of Clinitar versus hydrocortisone cream in the treatment of atopic eczema. Br J Dermatol. 1989;121:763-766.
5. Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatol Treat. 1990;1:199-201.
6. Kanehara S, Ohtani T, Uede K, et al. Clinical effects of undershirts coated with borage oil on children with atopic dermatitis: a double-blind, placebo-controlled trial. J Dermatol. 2007;34:811-815.
7. Boguniewicz M, Ziechner JA, Eichenfield LF, et al. MAS063DP is effective monotherapy for mild to moderate atopic dermatitis in infants and children: a multicenter, randomized, vehicle-controlled study. J Pediatr. 2008;152:854-859.
8. Kubota K, Machida I, Tamura K, et al. Treatment of refractory cases of atopic dermatitis with acidic hot-spring bathing. Acta Derm Venereol. 1997;77:452-454.
9. Ross SM. An integrative approach to eczema atopic dermatitis. Holist Nurs Pract. 2003;17:56-62.
10. Devillers AC, Oranje AP. Efficacy and safety of “wet-wrap” dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154:579-585.
11. Hindley D, Galloway G, Murray J, et al. A randomised study of “wet wraps” versus conventional treatment for atopic eczema. Arch Dis Child. 2006;91:164-168.
12. Anderson C, Lis-Balchin M, Kirk-Smith M. Evaluation of massage with essential oils on childhood eczema. Phytother Res. 2000;14:452-456.
13. Korting HC, Schäfer-Korting M, Klövekorn W, et al. Comparative efficacy of hamamelis distillate and hydrocortisone cream in atopic eczema. Eur J Clin Pharmacol. 1995;48:461-465.
Evidence-based answers from the Family Physicians Inquiries Network
Prophylactic oxytocin: Before or after placental delivery?
Either is fine.
Timing alone doesn’t influence the drug’s efficacy in preventing postpartum bleeding (strength of recommendation: B, randomized controlled trial [RCT] and prospective cohort studies).
Evidence summary
The prophylactic use of oxytocic drugs reduces the risk of postpartum hemorrhage (PPH) by about 40% and has been widely adopted as a routine policy in the active management of the third stage of labor.1 A number of studies have evaluated the timing of oxytocin after delivery (TABLE).
TABLE
What studies say about the timing of oxytocin and PPH risk
| STUDY TYPE (YEAR) | OXYTOCIN GIVEN AFTER | OUTCOMES (RISK OF PPH) | |
|---|---|---|---|
| DELIVERY OF ANTERIOR SHOULDER (N) | DELIVERY OF PLACENTA (N) | ||
| DBRCT (2001)2 | 745 | 741 | No difference (OR=0.92; 95% CI, 0.59-1.43) |
| DBRCT (2004)3 | 27 | 24 | Incidence lower when given after delivery of placenta (P=.049) |
| Cohort (2006)4 | 82 | 52 | Incidence lower when given after delivery of anterior shoulder (OR=0.33; 95% CI, 0.11-0.98) |
| RCT (1997)5 | 827 | 821 | Incidence lower when given after delivery of anterior shoulder (OR=0.50; 95% CI, 0.34-0.73) |
| Cohort (1996)6 | 524 (given after delivery of head) | 478 | Incidence lower when given after delivery of head (OR=0.60; 95% CI, 0.41-0.87) |
| CI, confidence interval; DBRCT, double-blinded randomized controlled trial; OR, odds ratio; PPH, postpartum hemorrhage; RCT, randomized controlled trial. | |||
Which timing is best? It depends on the study
A well-constructed double-blinded RCT found no significant difference in the incidence of PPH when oxytocin was given after delivery of the anterior shoulder or the placenta.2 The study included 1486 patients; 745 received 20 units of oxytocin on delivery of the anterior shoulder, and 741 received an identical dose of oxytocin on delivery of the placenta. The incidence of PPH was 5.4% for the anterior shoulder group and 5.8% for the placenta group (P=.72). Likewise, no significant difference between the groups was noted in the proportion of women with estimated blood loss (EBL) ≥500 mL (7.5% vs 9.7%; P=.15).
A much smaller double-blinded RCT found that PPH occurred significantly less often when oxytocin was delayed until after delivery of the placenta.3 The study comprised 51 patients; 27 received 10 units of oxytocin on delivery of the anterior shoulder and 24 received an identical dose after delivery of the placenta. The incidence of PPH ≥500 mL was 0% when oxytocin was given after delivery of the placenta vs 14.8% when it was given on delivery of the anterior shoulder (P=.049). However, the study was limited by its size and potential inaccuracies in estimating blood loss.
A prospective cohort study noted a significant reduction in the risk of PPH when oxytocin was given after delivery of the anterior shoulder, compared with the placenta.4 In this study, 82 patients received 5 units of oxytocin on delivery of the anterior shoulder, and 52 received an identical dose after delivery of the placenta. The incidence of PPH ≥500 mL was 7.3% in the anterior shoulder group and 19.2% in the placenta group. However, the study was not blinded and was limited by its small sample size.
Two earlier studies, an RCT and a prospective cohort study, concluded that oxytocin is more effective in reducing PPH when given before placental delivery (after delivery of the anterior shoulder and head, respectively).5,6 Neither of these studies was blinded nor controlled for nonpharmacologic interventions, however.
Recommendations
The American College of Obstetricians and Gynecologists (ACOG) states that ongoing blood loss accompanied by decreased uterine tone requires uterotonic agents as first-line treatment for PPH.7 ACOG doesn’t make specific recommendations regarding the timing of oxytocin administration.
The American Academy of Family Physicians (AAFP) recommends oxytocin as the uterotonic agent of choice for preventing PPH.8 The AAFP further advocates active management of the third stage of labor to decrease PPH by administering oxytocin as soon as possible after delivery of the anterior shoulder and before delivery of the placenta.
The World Health Organization (WHO) also recommends oxytocin as the uterotonic of choice.9 WHO advocates administration within 1 minute of delivery of the baby.
1. Prendiville W, Elbourne D, Chalmers I. The effects of routine oxytocic administration in the management of the third stage of labour: an overview of the evidence from controlled trials. Br J Obstet Gynaecol. 1988;95:3-16.
2. Jackson KW, Jr, Allbert JR, Schemmer GK, et al. A randomized, controlled trial comparing oxytocin administration before and after placental delivery in the prevention of postpartum hemorrhage. Am J Obstet Gynecol. 2001;185:873-877.
3. Huh WK, Chelmow D, Malone F. A double-blinded, randomized controlled trial of oxytocin at the beginning versus the end of the third stage of labor for prevention of postpartum hemorrhage. Gynecol Obstet Invest. 2004;58:72-76.
4. Fujimoto M, Takeuchi K, Sugimoto M, et al. Prevention of postpartum hemorrhage by uterotonic agents: comparison of oxytocin and methylergometrine in the management of the third stage of labor. Acta Obstet Gynecol Scand. 2006;85:1310-1314.
5. Khan GQ, John IS, Wani S, et al. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomized controlled trial. Am J Obstet Gynecol. 1997;177:770-774.
6. Soriano D, Dulitzki M, Schiff E, et al. A prospective cohort study of oxytocin plus ergometrine compared with oxytocin alone for prevention of postpartum haemorrhage. Br J Obstet Gynaecol. 1996;103:1068-1073.
7. American College of Obstetricians and Gynecologists. Practice Bulletin Number 76, June 2006. Postpartum hemorrhage. Obstet Gynecol. 2006;76:1-9.
8. Quinlan J, Bailey E, Dresang L, et al. for the Advanced Life Support in Obstetrics Advisory Board. 2007-2008 Advanced Life Support in Obstetrics Course Syllabus. Leawood, Kan: American Academy of Family Physicians; 2006.
9. Managing Complications in Pregnancy and Child-birth: A Guide for Midwives and Doctors. Geneva, Switzerland: World Health Organization, 2003. Available at: www.who.int/reproductivehealth/impac/clinical_principles/normal_lobour_C57_C76.html. Accessed May 12, 2008.
Either is fine.
Timing alone doesn’t influence the drug’s efficacy in preventing postpartum bleeding (strength of recommendation: B, randomized controlled trial [RCT] and prospective cohort studies).
Evidence summary
The prophylactic use of oxytocic drugs reduces the risk of postpartum hemorrhage (PPH) by about 40% and has been widely adopted as a routine policy in the active management of the third stage of labor.1 A number of studies have evaluated the timing of oxytocin after delivery (TABLE).
TABLE
What studies say about the timing of oxytocin and PPH risk
| STUDY TYPE (YEAR) | OXYTOCIN GIVEN AFTER | OUTCOMES (RISK OF PPH) | |
|---|---|---|---|
| DELIVERY OF ANTERIOR SHOULDER (N) | DELIVERY OF PLACENTA (N) | ||
| DBRCT (2001)2 | 745 | 741 | No difference (OR=0.92; 95% CI, 0.59-1.43) |
| DBRCT (2004)3 | 27 | 24 | Incidence lower when given after delivery of placenta (P=.049) |
| Cohort (2006)4 | 82 | 52 | Incidence lower when given after delivery of anterior shoulder (OR=0.33; 95% CI, 0.11-0.98) |
| RCT (1997)5 | 827 | 821 | Incidence lower when given after delivery of anterior shoulder (OR=0.50; 95% CI, 0.34-0.73) |
| Cohort (1996)6 | 524 (given after delivery of head) | 478 | Incidence lower when given after delivery of head (OR=0.60; 95% CI, 0.41-0.87) |
| CI, confidence interval; DBRCT, double-blinded randomized controlled trial; OR, odds ratio; PPH, postpartum hemorrhage; RCT, randomized controlled trial. | |||
Which timing is best? It depends on the study
A well-constructed double-blinded RCT found no significant difference in the incidence of PPH when oxytocin was given after delivery of the anterior shoulder or the placenta.2 The study included 1486 patients; 745 received 20 units of oxytocin on delivery of the anterior shoulder, and 741 received an identical dose of oxytocin on delivery of the placenta. The incidence of PPH was 5.4% for the anterior shoulder group and 5.8% for the placenta group (P=.72). Likewise, no significant difference between the groups was noted in the proportion of women with estimated blood loss (EBL) ≥500 mL (7.5% vs 9.7%; P=.15).
A much smaller double-blinded RCT found that PPH occurred significantly less often when oxytocin was delayed until after delivery of the placenta.3 The study comprised 51 patients; 27 received 10 units of oxytocin on delivery of the anterior shoulder and 24 received an identical dose after delivery of the placenta. The incidence of PPH ≥500 mL was 0% when oxytocin was given after delivery of the placenta vs 14.8% when it was given on delivery of the anterior shoulder (P=.049). However, the study was limited by its size and potential inaccuracies in estimating blood loss.
A prospective cohort study noted a significant reduction in the risk of PPH when oxytocin was given after delivery of the anterior shoulder, compared with the placenta.4 In this study, 82 patients received 5 units of oxytocin on delivery of the anterior shoulder, and 52 received an identical dose after delivery of the placenta. The incidence of PPH ≥500 mL was 7.3% in the anterior shoulder group and 19.2% in the placenta group. However, the study was not blinded and was limited by its small sample size.
Two earlier studies, an RCT and a prospective cohort study, concluded that oxytocin is more effective in reducing PPH when given before placental delivery (after delivery of the anterior shoulder and head, respectively).5,6 Neither of these studies was blinded nor controlled for nonpharmacologic interventions, however.
Recommendations
The American College of Obstetricians and Gynecologists (ACOG) states that ongoing blood loss accompanied by decreased uterine tone requires uterotonic agents as first-line treatment for PPH.7 ACOG doesn’t make specific recommendations regarding the timing of oxytocin administration.
The American Academy of Family Physicians (AAFP) recommends oxytocin as the uterotonic agent of choice for preventing PPH.8 The AAFP further advocates active management of the third stage of labor to decrease PPH by administering oxytocin as soon as possible after delivery of the anterior shoulder and before delivery of the placenta.
The World Health Organization (WHO) also recommends oxytocin as the uterotonic of choice.9 WHO advocates administration within 1 minute of delivery of the baby.
Either is fine.
Timing alone doesn’t influence the drug’s efficacy in preventing postpartum bleeding (strength of recommendation: B, randomized controlled trial [RCT] and prospective cohort studies).
Evidence summary
The prophylactic use of oxytocic drugs reduces the risk of postpartum hemorrhage (PPH) by about 40% and has been widely adopted as a routine policy in the active management of the third stage of labor.1 A number of studies have evaluated the timing of oxytocin after delivery (TABLE).
TABLE
What studies say about the timing of oxytocin and PPH risk
| STUDY TYPE (YEAR) | OXYTOCIN GIVEN AFTER | OUTCOMES (RISK OF PPH) | |
|---|---|---|---|
| DELIVERY OF ANTERIOR SHOULDER (N) | DELIVERY OF PLACENTA (N) | ||
| DBRCT (2001)2 | 745 | 741 | No difference (OR=0.92; 95% CI, 0.59-1.43) |
| DBRCT (2004)3 | 27 | 24 | Incidence lower when given after delivery of placenta (P=.049) |
| Cohort (2006)4 | 82 | 52 | Incidence lower when given after delivery of anterior shoulder (OR=0.33; 95% CI, 0.11-0.98) |
| RCT (1997)5 | 827 | 821 | Incidence lower when given after delivery of anterior shoulder (OR=0.50; 95% CI, 0.34-0.73) |
| Cohort (1996)6 | 524 (given after delivery of head) | 478 | Incidence lower when given after delivery of head (OR=0.60; 95% CI, 0.41-0.87) |
| CI, confidence interval; DBRCT, double-blinded randomized controlled trial; OR, odds ratio; PPH, postpartum hemorrhage; RCT, randomized controlled trial. | |||
Which timing is best? It depends on the study
A well-constructed double-blinded RCT found no significant difference in the incidence of PPH when oxytocin was given after delivery of the anterior shoulder or the placenta.2 The study included 1486 patients; 745 received 20 units of oxytocin on delivery of the anterior shoulder, and 741 received an identical dose of oxytocin on delivery of the placenta. The incidence of PPH was 5.4% for the anterior shoulder group and 5.8% for the placenta group (P=.72). Likewise, no significant difference between the groups was noted in the proportion of women with estimated blood loss (EBL) ≥500 mL (7.5% vs 9.7%; P=.15).
A much smaller double-blinded RCT found that PPH occurred significantly less often when oxytocin was delayed until after delivery of the placenta.3 The study comprised 51 patients; 27 received 10 units of oxytocin on delivery of the anterior shoulder and 24 received an identical dose after delivery of the placenta. The incidence of PPH ≥500 mL was 0% when oxytocin was given after delivery of the placenta vs 14.8% when it was given on delivery of the anterior shoulder (P=.049). However, the study was limited by its size and potential inaccuracies in estimating blood loss.
A prospective cohort study noted a significant reduction in the risk of PPH when oxytocin was given after delivery of the anterior shoulder, compared with the placenta.4 In this study, 82 patients received 5 units of oxytocin on delivery of the anterior shoulder, and 52 received an identical dose after delivery of the placenta. The incidence of PPH ≥500 mL was 7.3% in the anterior shoulder group and 19.2% in the placenta group. However, the study was not blinded and was limited by its small sample size.
Two earlier studies, an RCT and a prospective cohort study, concluded that oxytocin is more effective in reducing PPH when given before placental delivery (after delivery of the anterior shoulder and head, respectively).5,6 Neither of these studies was blinded nor controlled for nonpharmacologic interventions, however.
Recommendations
The American College of Obstetricians and Gynecologists (ACOG) states that ongoing blood loss accompanied by decreased uterine tone requires uterotonic agents as first-line treatment for PPH.7 ACOG doesn’t make specific recommendations regarding the timing of oxytocin administration.
The American Academy of Family Physicians (AAFP) recommends oxytocin as the uterotonic agent of choice for preventing PPH.8 The AAFP further advocates active management of the third stage of labor to decrease PPH by administering oxytocin as soon as possible after delivery of the anterior shoulder and before delivery of the placenta.
The World Health Organization (WHO) also recommends oxytocin as the uterotonic of choice.9 WHO advocates administration within 1 minute of delivery of the baby.
1. Prendiville W, Elbourne D, Chalmers I. The effects of routine oxytocic administration in the management of the third stage of labour: an overview of the evidence from controlled trials. Br J Obstet Gynaecol. 1988;95:3-16.
2. Jackson KW, Jr, Allbert JR, Schemmer GK, et al. A randomized, controlled trial comparing oxytocin administration before and after placental delivery in the prevention of postpartum hemorrhage. Am J Obstet Gynecol. 2001;185:873-877.
3. Huh WK, Chelmow D, Malone F. A double-blinded, randomized controlled trial of oxytocin at the beginning versus the end of the third stage of labor for prevention of postpartum hemorrhage. Gynecol Obstet Invest. 2004;58:72-76.
4. Fujimoto M, Takeuchi K, Sugimoto M, et al. Prevention of postpartum hemorrhage by uterotonic agents: comparison of oxytocin and methylergometrine in the management of the third stage of labor. Acta Obstet Gynecol Scand. 2006;85:1310-1314.
5. Khan GQ, John IS, Wani S, et al. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomized controlled trial. Am J Obstet Gynecol. 1997;177:770-774.
6. Soriano D, Dulitzki M, Schiff E, et al. A prospective cohort study of oxytocin plus ergometrine compared with oxytocin alone for prevention of postpartum haemorrhage. Br J Obstet Gynaecol. 1996;103:1068-1073.
7. American College of Obstetricians and Gynecologists. Practice Bulletin Number 76, June 2006. Postpartum hemorrhage. Obstet Gynecol. 2006;76:1-9.
8. Quinlan J, Bailey E, Dresang L, et al. for the Advanced Life Support in Obstetrics Advisory Board. 2007-2008 Advanced Life Support in Obstetrics Course Syllabus. Leawood, Kan: American Academy of Family Physicians; 2006.
9. Managing Complications in Pregnancy and Child-birth: A Guide for Midwives and Doctors. Geneva, Switzerland: World Health Organization, 2003. Available at: www.who.int/reproductivehealth/impac/clinical_principles/normal_lobour_C57_C76.html. Accessed May 12, 2008.
1. Prendiville W, Elbourne D, Chalmers I. The effects of routine oxytocic administration in the management of the third stage of labour: an overview of the evidence from controlled trials. Br J Obstet Gynaecol. 1988;95:3-16.
2. Jackson KW, Jr, Allbert JR, Schemmer GK, et al. A randomized, controlled trial comparing oxytocin administration before and after placental delivery in the prevention of postpartum hemorrhage. Am J Obstet Gynecol. 2001;185:873-877.
3. Huh WK, Chelmow D, Malone F. A double-blinded, randomized controlled trial of oxytocin at the beginning versus the end of the third stage of labor for prevention of postpartum hemorrhage. Gynecol Obstet Invest. 2004;58:72-76.
4. Fujimoto M, Takeuchi K, Sugimoto M, et al. Prevention of postpartum hemorrhage by uterotonic agents: comparison of oxytocin and methylergometrine in the management of the third stage of labor. Acta Obstet Gynecol Scand. 2006;85:1310-1314.
5. Khan GQ, John IS, Wani S, et al. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomized controlled trial. Am J Obstet Gynecol. 1997;177:770-774.
6. Soriano D, Dulitzki M, Schiff E, et al. A prospective cohort study of oxytocin plus ergometrine compared with oxytocin alone for prevention of postpartum haemorrhage. Br J Obstet Gynaecol. 1996;103:1068-1073.
7. American College of Obstetricians and Gynecologists. Practice Bulletin Number 76, June 2006. Postpartum hemorrhage. Obstet Gynecol. 2006;76:1-9.
8. Quinlan J, Bailey E, Dresang L, et al. for the Advanced Life Support in Obstetrics Advisory Board. 2007-2008 Advanced Life Support in Obstetrics Course Syllabus. Leawood, Kan: American Academy of Family Physicians; 2006.
9. Managing Complications in Pregnancy and Child-birth: A Guide for Midwives and Doctors. Geneva, Switzerland: World Health Organization, 2003. Available at: www.who.int/reproductivehealth/impac/clinical_principles/normal_lobour_C57_C76.html. Accessed May 12, 2008.
Evidence-based answers from the Family Physicians Inquiries Network
How useful are autoantibodies in diagnosing thyroid disorders?
They’re useful in diagnosing Graves’ disease and, to a lesser extent, autoimmune thyroid disease; they can also help predict hypothyroidism. thyrotropin receptor antibodies (TRAb) may be mildly elevated in a variety of thyroid disorders, but a TRAb level >10 U/L increases the probability of Graves’ disease by a moderate to large degree (strength of recommendation [SOR]: B, cross-sectional study). A positive or negative thyroid peroxidase antibody (TPOAb) test increases or decreases the probability of autoimmune thyroid disease by only a small to moderate degree (SOR: B, 3 cross-sectional studies).
Thyroid-stimulating hormone (TSH) levels >2 mU/L, although still in the normal range, can be followed up with TPOAb testing to determine whether the patient has an increased probability of developing hypothyroidism (SOR: B, cohort study with a vague hypothyroidism reference standard).
Evidence summary
Although TSH followed by free T4 remain the initial screening tests for thyroid disorders, adding thyroid autoantibodies may refine the diagnosis. Three principal thyroid antibodies—TPOAb, thyroglobulin, and TRAb—can be positive in a variety of autoimmune thyroid disorders. TPOAb represents a specific antigen of antimicrosomal antibody (AMA). It has largely replaced AMA testing in most laboratories and clinical settings.
Antibodies point to Graves’, autoimmune disorders
A cross-sectional study of 267 Singaporean patients with previously diagnosed thyroid disorders measured TRAb, AMA, and thyroglobulin (TABLE). TRAb levels >10 U/L were found to have a positive likelihood ratio (LR+) of 13 and a negative likelihood ratio (LR–) of 0.2 for Graves’ disease.1
Two cross-sectional studies compared AMA to TPOAb in healthy patients and those with autoimmune thyroid and nonthyroid disorders. One study of 235 people in a university endocrinology department found that a TPOAb level >190 U/mL yielded an LR+ of 10.75 and an LR– of 0.15 for chronic autoimmune (Hashimoto’s) thyroiditis [CAHT]; the AMA-positive sera yielded an LR+ of 13.67 and an LR– of 0.19. Both TPOAb and AMA test characteristics were highly associated with CAHT (P<.001).
TABLE
Autoimmune markers in thyroid disorders
| % TRA b >3.4 U/L | % TRA b >10 U/L | % AMA positive | % thyroglobulin positive | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Thyroid disorders | % of study patie nts | LR + | LR – | LR + | LR – | LR + | LR – | LR + | LR – |
| Graves’ disease | 68 | 4.6 | 0.1 | 13 | 0.2 | 1.3 | 0.6 | 1.1 | 0.9 |
| CAHT | 20 | 0.2 | 4.7 | 0.1 | 2.8 | 1.4 | 0.2 | 1.4 | 0.6 |
| Subacute thyroiditis | 4 | 0.2 | 3.0 | 0 | 2.4 | 0.1 | 3.6 | 0.5 | 1.5 |
| Thyroid nodules | 6 | 0.2 | 3.4 | 0 | 2.4 | 0.1 | 4.1 | 0.1 | 2.0 |
| Others | 2 | 0.8 | 1.4 | 0 | 2.3 | 0 | 2.8 | 0 | 2.0 |
| AMA, antimicrosomal antibodies; CAHT, chronic autoimmune (Hashimoto’s) thyroiditis; LR +, positive likelihood ratio; LR –, negative likelihood ratio; TRAb, thyrotropin receptor antibodies | |||||||||
| Source: Khoo DHC, et al.1 | |||||||||
TPOAb is more sensitive than AMA and thyroglobulin
In the second study comparing AMA to TPOAb, the thyroid antibody test results of 32 healthy patients were compared with those of 262 clinic patients. In those with known thyroid dysfunction, TPOAb was found to be a more sensitive assay than AMA for autoimmune thyroid disorders. The sensitivity of TPOAb levels >3.1 U/mL was 88.1%; AMA sensitivity was 70.2% (P<.001).2,3
A cross-sectional study (National Health and Nutrition Examination Survey [NHANES III]) evaluated the presence of thyroid antibodies in 17,353 people representing the geographic and ethnic distribution of the United States, 95% of whom were categorized as free of thyroid disease.4 The study found that TPOAb was more sensitive than thyroglobulin for diagnosing nonspecific thyroid disease. The diagnosis of thyroid disease was based on abnormal TSH and free T4 levels. Abnormally high levels of TPOAb had an LR+ of 4.3 and LR– of 0.6 (P<.0001) for thyroid disease, compared with an LR+ of 3.4 and LR– of 0.7 (P<.01) for abnormally elevated thyroglobulin.
TSH + TPOAb more accurate than TSH in women
In the early 1970s, a cohort study of 2779 adults from Great Britain attempted to establish the incidence of thyroid disease in the general population by measuring TSH and TPOAb. Twenty years later, investigators restudied 1708 people from the original sample to determine the incidence of hypothyroidism and the prognostic value of these 2 biochemical markers for its development. At follow-up, the definition of a new case of hypothyroidism was based on an “intention to treat by the general practitioner by meeting clear biochemical criteria and/or symptoms.”
The initial presence of abnormally high serum TPOAb levels and TSH >2.0 mU/L predicted a 4.3% annual risk of developing hypothyroidism compared with a 2.6% annual risk with serum TSH >6.0 mU/L alone in women. This risk was not estimated for men because of the small number of cases.5
Recommendations
The American Association of Clinical Endocrinologists (AACE) makes no specific recommendations about laboratory testing of thyroid antibodies. Based on clinical judgment, the AACE states that antibodies may be considered in the workup of hyperthyroidism and hypothyroidism and to determine potential risk to the fetus in pregnant women diagnosed with Graves’ disease.6
The National Academy of Clinical Biochemistry (NACB) recommends TPOAb measurements in patients who have Down syndrome, are pregnant, or have miscarried or failed in vitro fertilization. The NACB also advocates measuring TPOAb before treatment with amiodarone, lithium, interferon-α, or interleukin-2.7
1. Khoo DHC, Fok ACK, Tan CE, et al. Thyroid stimulating hormone receptor antibody levels in Singaporean patients with autoimmune thyroid disease. Ann Acad Med Singapore. 1997;26:435-438.
2. Feldt-Rasmussen U, Hoier-Madsen M, Bech K, et al. Antithyroid peroxidase antibodies in thyroid disorders and nonthyroid autoimmune diseases. Autoimmunity. 1991;9:245-254.
3. Doullay F, Ruf J, Codaccioni JL, Carayon P. Prevalence of autoantibodies to thyroperoxidase in patients with various thyroid and autoimmune diseases. Autoimmunity. 1991;9:237-244.
4. Hollowell JG, Staehling NW, Flanders WD, et al. Srum TSH, T4, and thyroid antibodies in the united states population (1998 to 1994): National Health and Nutrition Examination Survey (NHANES III.) J Clin Endocrinol Metab. 2002;87:489-499.
5. Vanderpump MPJ, Tunbridge WMG, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow up of the Whickham survey. Clin Endocrinol (Oxf). 1995;43:55-68.
6. American Association of Clinical Endocrinologists Thyroid Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Available at: www.aace.com/pub/pdf/guidelines/hypo_hyper.pdf. Accessed June 8, 2007.
7. Demers LM, Spencer CA. Laboratory Medicine Practice Guidelines: Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease. Washington, DC: AACC press; 2003.
They’re useful in diagnosing Graves’ disease and, to a lesser extent, autoimmune thyroid disease; they can also help predict hypothyroidism. thyrotropin receptor antibodies (TRAb) may be mildly elevated in a variety of thyroid disorders, but a TRAb level >10 U/L increases the probability of Graves’ disease by a moderate to large degree (strength of recommendation [SOR]: B, cross-sectional study). A positive or negative thyroid peroxidase antibody (TPOAb) test increases or decreases the probability of autoimmune thyroid disease by only a small to moderate degree (SOR: B, 3 cross-sectional studies).
Thyroid-stimulating hormone (TSH) levels >2 mU/L, although still in the normal range, can be followed up with TPOAb testing to determine whether the patient has an increased probability of developing hypothyroidism (SOR: B, cohort study with a vague hypothyroidism reference standard).
Evidence summary
Although TSH followed by free T4 remain the initial screening tests for thyroid disorders, adding thyroid autoantibodies may refine the diagnosis. Three principal thyroid antibodies—TPOAb, thyroglobulin, and TRAb—can be positive in a variety of autoimmune thyroid disorders. TPOAb represents a specific antigen of antimicrosomal antibody (AMA). It has largely replaced AMA testing in most laboratories and clinical settings.
Antibodies point to Graves’, autoimmune disorders
A cross-sectional study of 267 Singaporean patients with previously diagnosed thyroid disorders measured TRAb, AMA, and thyroglobulin (TABLE). TRAb levels >10 U/L were found to have a positive likelihood ratio (LR+) of 13 and a negative likelihood ratio (LR–) of 0.2 for Graves’ disease.1
Two cross-sectional studies compared AMA to TPOAb in healthy patients and those with autoimmune thyroid and nonthyroid disorders. One study of 235 people in a university endocrinology department found that a TPOAb level >190 U/mL yielded an LR+ of 10.75 and an LR– of 0.15 for chronic autoimmune (Hashimoto’s) thyroiditis [CAHT]; the AMA-positive sera yielded an LR+ of 13.67 and an LR– of 0.19. Both TPOAb and AMA test characteristics were highly associated with CAHT (P<.001).
TABLE
Autoimmune markers in thyroid disorders
| % TRA b >3.4 U/L | % TRA b >10 U/L | % AMA positive | % thyroglobulin positive | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Thyroid disorders | % of study patie nts | LR + | LR – | LR + | LR – | LR + | LR – | LR + | LR – |
| Graves’ disease | 68 | 4.6 | 0.1 | 13 | 0.2 | 1.3 | 0.6 | 1.1 | 0.9 |
| CAHT | 20 | 0.2 | 4.7 | 0.1 | 2.8 | 1.4 | 0.2 | 1.4 | 0.6 |
| Subacute thyroiditis | 4 | 0.2 | 3.0 | 0 | 2.4 | 0.1 | 3.6 | 0.5 | 1.5 |
| Thyroid nodules | 6 | 0.2 | 3.4 | 0 | 2.4 | 0.1 | 4.1 | 0.1 | 2.0 |
| Others | 2 | 0.8 | 1.4 | 0 | 2.3 | 0 | 2.8 | 0 | 2.0 |
| AMA, antimicrosomal antibodies; CAHT, chronic autoimmune (Hashimoto’s) thyroiditis; LR +, positive likelihood ratio; LR –, negative likelihood ratio; TRAb, thyrotropin receptor antibodies | |||||||||
| Source: Khoo DHC, et al.1 | |||||||||
TPOAb is more sensitive than AMA and thyroglobulin
In the second study comparing AMA to TPOAb, the thyroid antibody test results of 32 healthy patients were compared with those of 262 clinic patients. In those with known thyroid dysfunction, TPOAb was found to be a more sensitive assay than AMA for autoimmune thyroid disorders. The sensitivity of TPOAb levels >3.1 U/mL was 88.1%; AMA sensitivity was 70.2% (P<.001).2,3
A cross-sectional study (National Health and Nutrition Examination Survey [NHANES III]) evaluated the presence of thyroid antibodies in 17,353 people representing the geographic and ethnic distribution of the United States, 95% of whom were categorized as free of thyroid disease.4 The study found that TPOAb was more sensitive than thyroglobulin for diagnosing nonspecific thyroid disease. The diagnosis of thyroid disease was based on abnormal TSH and free T4 levels. Abnormally high levels of TPOAb had an LR+ of 4.3 and LR– of 0.6 (P<.0001) for thyroid disease, compared with an LR+ of 3.4 and LR– of 0.7 (P<.01) for abnormally elevated thyroglobulin.
TSH + TPOAb more accurate than TSH in women
In the early 1970s, a cohort study of 2779 adults from Great Britain attempted to establish the incidence of thyroid disease in the general population by measuring TSH and TPOAb. Twenty years later, investigators restudied 1708 people from the original sample to determine the incidence of hypothyroidism and the prognostic value of these 2 biochemical markers for its development. At follow-up, the definition of a new case of hypothyroidism was based on an “intention to treat by the general practitioner by meeting clear biochemical criteria and/or symptoms.”
The initial presence of abnormally high serum TPOAb levels and TSH >2.0 mU/L predicted a 4.3% annual risk of developing hypothyroidism compared with a 2.6% annual risk with serum TSH >6.0 mU/L alone in women. This risk was not estimated for men because of the small number of cases.5
Recommendations
The American Association of Clinical Endocrinologists (AACE) makes no specific recommendations about laboratory testing of thyroid antibodies. Based on clinical judgment, the AACE states that antibodies may be considered in the workup of hyperthyroidism and hypothyroidism and to determine potential risk to the fetus in pregnant women diagnosed with Graves’ disease.6
The National Academy of Clinical Biochemistry (NACB) recommends TPOAb measurements in patients who have Down syndrome, are pregnant, or have miscarried or failed in vitro fertilization. The NACB also advocates measuring TPOAb before treatment with amiodarone, lithium, interferon-α, or interleukin-2.7
They’re useful in diagnosing Graves’ disease and, to a lesser extent, autoimmune thyroid disease; they can also help predict hypothyroidism. thyrotropin receptor antibodies (TRAb) may be mildly elevated in a variety of thyroid disorders, but a TRAb level >10 U/L increases the probability of Graves’ disease by a moderate to large degree (strength of recommendation [SOR]: B, cross-sectional study). A positive or negative thyroid peroxidase antibody (TPOAb) test increases or decreases the probability of autoimmune thyroid disease by only a small to moderate degree (SOR: B, 3 cross-sectional studies).
Thyroid-stimulating hormone (TSH) levels >2 mU/L, although still in the normal range, can be followed up with TPOAb testing to determine whether the patient has an increased probability of developing hypothyroidism (SOR: B, cohort study with a vague hypothyroidism reference standard).
Evidence summary
Although TSH followed by free T4 remain the initial screening tests for thyroid disorders, adding thyroid autoantibodies may refine the diagnosis. Three principal thyroid antibodies—TPOAb, thyroglobulin, and TRAb—can be positive in a variety of autoimmune thyroid disorders. TPOAb represents a specific antigen of antimicrosomal antibody (AMA). It has largely replaced AMA testing in most laboratories and clinical settings.
Antibodies point to Graves’, autoimmune disorders
A cross-sectional study of 267 Singaporean patients with previously diagnosed thyroid disorders measured TRAb, AMA, and thyroglobulin (TABLE). TRAb levels >10 U/L were found to have a positive likelihood ratio (LR+) of 13 and a negative likelihood ratio (LR–) of 0.2 for Graves’ disease.1
Two cross-sectional studies compared AMA to TPOAb in healthy patients and those with autoimmune thyroid and nonthyroid disorders. One study of 235 people in a university endocrinology department found that a TPOAb level >190 U/mL yielded an LR+ of 10.75 and an LR– of 0.15 for chronic autoimmune (Hashimoto’s) thyroiditis [CAHT]; the AMA-positive sera yielded an LR+ of 13.67 and an LR– of 0.19. Both TPOAb and AMA test characteristics were highly associated with CAHT (P<.001).
TABLE
Autoimmune markers in thyroid disorders
| % TRA b >3.4 U/L | % TRA b >10 U/L | % AMA positive | % thyroglobulin positive | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Thyroid disorders | % of study patie nts | LR + | LR – | LR + | LR – | LR + | LR – | LR + | LR – |
| Graves’ disease | 68 | 4.6 | 0.1 | 13 | 0.2 | 1.3 | 0.6 | 1.1 | 0.9 |
| CAHT | 20 | 0.2 | 4.7 | 0.1 | 2.8 | 1.4 | 0.2 | 1.4 | 0.6 |
| Subacute thyroiditis | 4 | 0.2 | 3.0 | 0 | 2.4 | 0.1 | 3.6 | 0.5 | 1.5 |
| Thyroid nodules | 6 | 0.2 | 3.4 | 0 | 2.4 | 0.1 | 4.1 | 0.1 | 2.0 |
| Others | 2 | 0.8 | 1.4 | 0 | 2.3 | 0 | 2.8 | 0 | 2.0 |
| AMA, antimicrosomal antibodies; CAHT, chronic autoimmune (Hashimoto’s) thyroiditis; LR +, positive likelihood ratio; LR –, negative likelihood ratio; TRAb, thyrotropin receptor antibodies | |||||||||
| Source: Khoo DHC, et al.1 | |||||||||
TPOAb is more sensitive than AMA and thyroglobulin
In the second study comparing AMA to TPOAb, the thyroid antibody test results of 32 healthy patients were compared with those of 262 clinic patients. In those with known thyroid dysfunction, TPOAb was found to be a more sensitive assay than AMA for autoimmune thyroid disorders. The sensitivity of TPOAb levels >3.1 U/mL was 88.1%; AMA sensitivity was 70.2% (P<.001).2,3
A cross-sectional study (National Health and Nutrition Examination Survey [NHANES III]) evaluated the presence of thyroid antibodies in 17,353 people representing the geographic and ethnic distribution of the United States, 95% of whom were categorized as free of thyroid disease.4 The study found that TPOAb was more sensitive than thyroglobulin for diagnosing nonspecific thyroid disease. The diagnosis of thyroid disease was based on abnormal TSH and free T4 levels. Abnormally high levels of TPOAb had an LR+ of 4.3 and LR– of 0.6 (P<.0001) for thyroid disease, compared with an LR+ of 3.4 and LR– of 0.7 (P<.01) for abnormally elevated thyroglobulin.
TSH + TPOAb more accurate than TSH in women
In the early 1970s, a cohort study of 2779 adults from Great Britain attempted to establish the incidence of thyroid disease in the general population by measuring TSH and TPOAb. Twenty years later, investigators restudied 1708 people from the original sample to determine the incidence of hypothyroidism and the prognostic value of these 2 biochemical markers for its development. At follow-up, the definition of a new case of hypothyroidism was based on an “intention to treat by the general practitioner by meeting clear biochemical criteria and/or symptoms.”
The initial presence of abnormally high serum TPOAb levels and TSH >2.0 mU/L predicted a 4.3% annual risk of developing hypothyroidism compared with a 2.6% annual risk with serum TSH >6.0 mU/L alone in women. This risk was not estimated for men because of the small number of cases.5
Recommendations
The American Association of Clinical Endocrinologists (AACE) makes no specific recommendations about laboratory testing of thyroid antibodies. Based on clinical judgment, the AACE states that antibodies may be considered in the workup of hyperthyroidism and hypothyroidism and to determine potential risk to the fetus in pregnant women diagnosed with Graves’ disease.6
The National Academy of Clinical Biochemistry (NACB) recommends TPOAb measurements in patients who have Down syndrome, are pregnant, or have miscarried or failed in vitro fertilization. The NACB also advocates measuring TPOAb before treatment with amiodarone, lithium, interferon-α, or interleukin-2.7
1. Khoo DHC, Fok ACK, Tan CE, et al. Thyroid stimulating hormone receptor antibody levels in Singaporean patients with autoimmune thyroid disease. Ann Acad Med Singapore. 1997;26:435-438.
2. Feldt-Rasmussen U, Hoier-Madsen M, Bech K, et al. Antithyroid peroxidase antibodies in thyroid disorders and nonthyroid autoimmune diseases. Autoimmunity. 1991;9:245-254.
3. Doullay F, Ruf J, Codaccioni JL, Carayon P. Prevalence of autoantibodies to thyroperoxidase in patients with various thyroid and autoimmune diseases. Autoimmunity. 1991;9:237-244.
4. Hollowell JG, Staehling NW, Flanders WD, et al. Srum TSH, T4, and thyroid antibodies in the united states population (1998 to 1994): National Health and Nutrition Examination Survey (NHANES III.) J Clin Endocrinol Metab. 2002;87:489-499.
5. Vanderpump MPJ, Tunbridge WMG, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow up of the Whickham survey. Clin Endocrinol (Oxf). 1995;43:55-68.
6. American Association of Clinical Endocrinologists Thyroid Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Available at: www.aace.com/pub/pdf/guidelines/hypo_hyper.pdf. Accessed June 8, 2007.
7. Demers LM, Spencer CA. Laboratory Medicine Practice Guidelines: Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease. Washington, DC: AACC press; 2003.
1. Khoo DHC, Fok ACK, Tan CE, et al. Thyroid stimulating hormone receptor antibody levels in Singaporean patients with autoimmune thyroid disease. Ann Acad Med Singapore. 1997;26:435-438.
2. Feldt-Rasmussen U, Hoier-Madsen M, Bech K, et al. Antithyroid peroxidase antibodies in thyroid disorders and nonthyroid autoimmune diseases. Autoimmunity. 1991;9:245-254.
3. Doullay F, Ruf J, Codaccioni JL, Carayon P. Prevalence of autoantibodies to thyroperoxidase in patients with various thyroid and autoimmune diseases. Autoimmunity. 1991;9:237-244.
4. Hollowell JG, Staehling NW, Flanders WD, et al. Srum TSH, T4, and thyroid antibodies in the united states population (1998 to 1994): National Health and Nutrition Examination Survey (NHANES III.) J Clin Endocrinol Metab. 2002;87:489-499.
5. Vanderpump MPJ, Tunbridge WMG, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow up of the Whickham survey. Clin Endocrinol (Oxf). 1995;43:55-68.
6. American Association of Clinical Endocrinologists Thyroid Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Available at: www.aace.com/pub/pdf/guidelines/hypo_hyper.pdf. Accessed June 8, 2007.
7. Demers LM, Spencer CA. Laboratory Medicine Practice Guidelines: Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease. Washington, DC: AACC press; 2003.
Evidence-based answers from the Family Physicians Inquiries Network
Is there much risk in using fluoroquinolones in children?
No, the risks seem to be minimal. Arthralgias and myalgias have been observed clinically in children and adolescents exposed to fluoroquinolones, but they’re transient, disappear when the drug is discontinued, and appear to be no more prevalent than with other antibiotics (strength of recommendation [SOR]: B, 1 structured review and 2 prospective cohort studies). No apparent long-term risk of developmental skeletal growth delay is associated with fluoroquinolone exposure (SOR: B, 1 prospective controlled study). Fluoroquinolone use in children isn’t associated with tendonopathy (SOR: B, 1 prospective controlled study), but it probably carries a very low risk of tendon rupture (SOR: C, extrapolation from a national passive postmarketing monitoring system study predominantly in adults).
Be Judicious
Stefan M. Groetsch, MD
Naval Branch Health Clinic, Atsugi, Japan
Just because you can do something doesn’t mean you should. It’s reassuring that quinolones can be given to pediatric patients if necessary inasmuch as the drugs don’t appear to cause long-term skeletal side effects, and the infrequent arthralgias and myalgias they produce seem to be transient and benign. However, in an era of increasing microbial drug resistance and escalating pharmaceutical costs, we should strive for rational prescribing and reserve quinolones for patients who truly need them.
Evidence summary
Few short-term joint complaints, no long-term skeletal harm
A 1997 database review compiled reports of skeletally immature patients ranging in age from 4 days to 26 years who were exposed to quinolones.1 Thirty-one reports met search term criteria, for a total of 7045 patients. No incidences of quinolone-associated arthralgia were documented in 30 reports (>5000 patients). The review didn’t report the incidence of tendonopathy. One report of 1795 pediatric patients documented a small incidence of arthralgias (~1.5%), which was considered to be reversible and no more than expected for a comparable quinolone-naïve population.
Follow-up data on safety and adverse findings, from as long as 12 years after treatment, were reported for 530 (28%) of the 7045 patients. Changes in skeletal growth were evaluated using various diagnostic techniques. Clinical observation was the most common method of assessment (N=357), however. The follow-up data revealed no arthropathy or abnormal skeletal growth (rate=0%; estimated 95% confidence interval [CI]=0%-0.04%).
A prospective study published in 2006 monitored joint toxicities (swelling, tenderness, or restricted movement) during acute treatment with ciprofloxacin as well as skeletal growth at follow-up based on physical examination.2 Preterm neonates with septicemia were treated with either ciprofloxacin (n=48) or other antibiotics (n=66). Forty infants in the ciprofloxacin group completed an average of 28 months of follow-up. No complaints or physical findings of osteoarticular joint abnormalities or skeletal growth delay were noted in either group during acute treatment or at follow-up. The incidence of tendonopathy was not reported.
Arthralgias, myalgias are transient
A large multicenter, prospective, non-blinded cohort study evaluated adverse effects in children receiving fluoroquinolones versus other antibiotics.3 Duration of fluoroquinolone use was 1 to 23 days. Arthralgias or myalgias, which were only evaluated clinically, occurred more often in children receiving fluoroquinolones—10 of 276 children (3.6%) vs 1 of 249 (0.3%), respectively (odds ratio [OR]=9.3; 95% CI, 1.2-195; P=.02). All events occurred within the first 2 weeks of fluoroquinolone treatment and resolved within 20 days. No tendonopathies were reported.
Tendon rupture is rare, especially in children
A 1996 study reported the incidence of tendon disorders related to fluoroquinolones using drug surveillance data from the general population. The average age of the patients was 55 years.4
The author estimated the risk of tendon rupture associated with norfloxacin or ofloxacin to be 1 case per 23,130 days of treatment and only 1 case per 779,600 days of ciprofloxacin treatment. The estimated risk would likely be even lower in children, the author noted, because the risk of tendon rupture increases with age.
Recommendations
Ciprofloxacin is the only fluoroquinolone approved by the US Food and Drug Administration for pediatric indications. The FDA recently ordered the addition of a Boxed Warning to fluoroquinolones regarding the increased risk of tendonitis and tendon rupture. The FDA made no comments specifically about children or adolescents, and stated that the risks are increased in people older than 60.
The American Academy of Pediatrics recommends limiting fluoroquinolone use to children with infections caused by multidrug-resistant pathogens or children for whom parenteral therapy is not feasible and no other effective oral medication is available.5
The Agency for Healthcare Research and Quality (AHRQ) recommends fluoroquinolones as first-line treatment for children with uncomplicated gonorrhea who weigh more than 45 kg,6 and second-line therapy for children with bacterial meningitis,7 nongonococcal urethritis, chlamydia,6 or pelvic inflammatory disease.8
Acknowledgments
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government.
1. Burkhardt JE, Walterspiel JN, Schaad RB. Quinolone arthropathy in animals versus children. Clin Infect Dis. 1997;25:1196-1204.
2. Ahmed AS, Khan NZ, Saha SK, et al. Ciprofloxacin treatment in preterm neonates in Bangladesh. Pediatr Infect Dis J. 2006;25:1137-1141.
3. Chalumeau M, Tonnelier S, D’Athis P, et al. Fluoroquinolone safety in pediatric patients: a prospective, multicenter, comparative cohort study in France. Pediatrics. 2003;111:e714-e719.
4. Royer RJ. Adverse drug reactions with fluoroquinolones. Therapie. 1998;51:414-416.
5. Committee on Infectious Diseases. The use of systematic fluoroquinolones. Pediatrics. 2006;118:1287-1292.
6. Workowski KA, Berman SM. and the Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. Diseases characterized by urethritis and cervicitis. MMWR Morb Mortal Wkly Rep. 2006;55(RR-11):35-49.
7. Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267-1284.
8. Workowski KA, Berman SM. and the Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. Pelvic inflammatory disease. MMWR Morb Mortal Wkly Rep. 2006;55(RR-11):56-61.
No, the risks seem to be minimal. Arthralgias and myalgias have been observed clinically in children and adolescents exposed to fluoroquinolones, but they’re transient, disappear when the drug is discontinued, and appear to be no more prevalent than with other antibiotics (strength of recommendation [SOR]: B, 1 structured review and 2 prospective cohort studies). No apparent long-term risk of developmental skeletal growth delay is associated with fluoroquinolone exposure (SOR: B, 1 prospective controlled study). Fluoroquinolone use in children isn’t associated with tendonopathy (SOR: B, 1 prospective controlled study), but it probably carries a very low risk of tendon rupture (SOR: C, extrapolation from a national passive postmarketing monitoring system study predominantly in adults).
Be Judicious
Stefan M. Groetsch, MD
Naval Branch Health Clinic, Atsugi, Japan
Just because you can do something doesn’t mean you should. It’s reassuring that quinolones can be given to pediatric patients if necessary inasmuch as the drugs don’t appear to cause long-term skeletal side effects, and the infrequent arthralgias and myalgias they produce seem to be transient and benign. However, in an era of increasing microbial drug resistance and escalating pharmaceutical costs, we should strive for rational prescribing and reserve quinolones for patients who truly need them.
Evidence summary
Few short-term joint complaints, no long-term skeletal harm
A 1997 database review compiled reports of skeletally immature patients ranging in age from 4 days to 26 years who were exposed to quinolones.1 Thirty-one reports met search term criteria, for a total of 7045 patients. No incidences of quinolone-associated arthralgia were documented in 30 reports (>5000 patients). The review didn’t report the incidence of tendonopathy. One report of 1795 pediatric patients documented a small incidence of arthralgias (~1.5%), which was considered to be reversible and no more than expected for a comparable quinolone-naïve population.
Follow-up data on safety and adverse findings, from as long as 12 years after treatment, were reported for 530 (28%) of the 7045 patients. Changes in skeletal growth were evaluated using various diagnostic techniques. Clinical observation was the most common method of assessment (N=357), however. The follow-up data revealed no arthropathy or abnormal skeletal growth (rate=0%; estimated 95% confidence interval [CI]=0%-0.04%).
A prospective study published in 2006 monitored joint toxicities (swelling, tenderness, or restricted movement) during acute treatment with ciprofloxacin as well as skeletal growth at follow-up based on physical examination.2 Preterm neonates with septicemia were treated with either ciprofloxacin (n=48) or other antibiotics (n=66). Forty infants in the ciprofloxacin group completed an average of 28 months of follow-up. No complaints or physical findings of osteoarticular joint abnormalities or skeletal growth delay were noted in either group during acute treatment or at follow-up. The incidence of tendonopathy was not reported.
Arthralgias, myalgias are transient
A large multicenter, prospective, non-blinded cohort study evaluated adverse effects in children receiving fluoroquinolones versus other antibiotics.3 Duration of fluoroquinolone use was 1 to 23 days. Arthralgias or myalgias, which were only evaluated clinically, occurred more often in children receiving fluoroquinolones—10 of 276 children (3.6%) vs 1 of 249 (0.3%), respectively (odds ratio [OR]=9.3; 95% CI, 1.2-195; P=.02). All events occurred within the first 2 weeks of fluoroquinolone treatment and resolved within 20 days. No tendonopathies were reported.
Tendon rupture is rare, especially in children
A 1996 study reported the incidence of tendon disorders related to fluoroquinolones using drug surveillance data from the general population. The average age of the patients was 55 years.4
The author estimated the risk of tendon rupture associated with norfloxacin or ofloxacin to be 1 case per 23,130 days of treatment and only 1 case per 779,600 days of ciprofloxacin treatment. The estimated risk would likely be even lower in children, the author noted, because the risk of tendon rupture increases with age.
Recommendations
Ciprofloxacin is the only fluoroquinolone approved by the US Food and Drug Administration for pediatric indications. The FDA recently ordered the addition of a Boxed Warning to fluoroquinolones regarding the increased risk of tendonitis and tendon rupture. The FDA made no comments specifically about children or adolescents, and stated that the risks are increased in people older than 60.
The American Academy of Pediatrics recommends limiting fluoroquinolone use to children with infections caused by multidrug-resistant pathogens or children for whom parenteral therapy is not feasible and no other effective oral medication is available.5
The Agency for Healthcare Research and Quality (AHRQ) recommends fluoroquinolones as first-line treatment for children with uncomplicated gonorrhea who weigh more than 45 kg,6 and second-line therapy for children with bacterial meningitis,7 nongonococcal urethritis, chlamydia,6 or pelvic inflammatory disease.8
Acknowledgments
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government.
No, the risks seem to be minimal. Arthralgias and myalgias have been observed clinically in children and adolescents exposed to fluoroquinolones, but they’re transient, disappear when the drug is discontinued, and appear to be no more prevalent than with other antibiotics (strength of recommendation [SOR]: B, 1 structured review and 2 prospective cohort studies). No apparent long-term risk of developmental skeletal growth delay is associated with fluoroquinolone exposure (SOR: B, 1 prospective controlled study). Fluoroquinolone use in children isn’t associated with tendonopathy (SOR: B, 1 prospective controlled study), but it probably carries a very low risk of tendon rupture (SOR: C, extrapolation from a national passive postmarketing monitoring system study predominantly in adults).
Be Judicious
Stefan M. Groetsch, MD
Naval Branch Health Clinic, Atsugi, Japan
Just because you can do something doesn’t mean you should. It’s reassuring that quinolones can be given to pediatric patients if necessary inasmuch as the drugs don’t appear to cause long-term skeletal side effects, and the infrequent arthralgias and myalgias they produce seem to be transient and benign. However, in an era of increasing microbial drug resistance and escalating pharmaceutical costs, we should strive for rational prescribing and reserve quinolones for patients who truly need them.
Evidence summary
Few short-term joint complaints, no long-term skeletal harm
A 1997 database review compiled reports of skeletally immature patients ranging in age from 4 days to 26 years who were exposed to quinolones.1 Thirty-one reports met search term criteria, for a total of 7045 patients. No incidences of quinolone-associated arthralgia were documented in 30 reports (>5000 patients). The review didn’t report the incidence of tendonopathy. One report of 1795 pediatric patients documented a small incidence of arthralgias (~1.5%), which was considered to be reversible and no more than expected for a comparable quinolone-naïve population.
Follow-up data on safety and adverse findings, from as long as 12 years after treatment, were reported for 530 (28%) of the 7045 patients. Changes in skeletal growth were evaluated using various diagnostic techniques. Clinical observation was the most common method of assessment (N=357), however. The follow-up data revealed no arthropathy or abnormal skeletal growth (rate=0%; estimated 95% confidence interval [CI]=0%-0.04%).
A prospective study published in 2006 monitored joint toxicities (swelling, tenderness, or restricted movement) during acute treatment with ciprofloxacin as well as skeletal growth at follow-up based on physical examination.2 Preterm neonates with septicemia were treated with either ciprofloxacin (n=48) or other antibiotics (n=66). Forty infants in the ciprofloxacin group completed an average of 28 months of follow-up. No complaints or physical findings of osteoarticular joint abnormalities or skeletal growth delay were noted in either group during acute treatment or at follow-up. The incidence of tendonopathy was not reported.
Arthralgias, myalgias are transient
A large multicenter, prospective, non-blinded cohort study evaluated adverse effects in children receiving fluoroquinolones versus other antibiotics.3 Duration of fluoroquinolone use was 1 to 23 days. Arthralgias or myalgias, which were only evaluated clinically, occurred more often in children receiving fluoroquinolones—10 of 276 children (3.6%) vs 1 of 249 (0.3%), respectively (odds ratio [OR]=9.3; 95% CI, 1.2-195; P=.02). All events occurred within the first 2 weeks of fluoroquinolone treatment and resolved within 20 days. No tendonopathies were reported.
Tendon rupture is rare, especially in children
A 1996 study reported the incidence of tendon disorders related to fluoroquinolones using drug surveillance data from the general population. The average age of the patients was 55 years.4
The author estimated the risk of tendon rupture associated with norfloxacin or ofloxacin to be 1 case per 23,130 days of treatment and only 1 case per 779,600 days of ciprofloxacin treatment. The estimated risk would likely be even lower in children, the author noted, because the risk of tendon rupture increases with age.
Recommendations
Ciprofloxacin is the only fluoroquinolone approved by the US Food and Drug Administration for pediatric indications. The FDA recently ordered the addition of a Boxed Warning to fluoroquinolones regarding the increased risk of tendonitis and tendon rupture. The FDA made no comments specifically about children or adolescents, and stated that the risks are increased in people older than 60.
The American Academy of Pediatrics recommends limiting fluoroquinolone use to children with infections caused by multidrug-resistant pathogens or children for whom parenteral therapy is not feasible and no other effective oral medication is available.5
The Agency for Healthcare Research and Quality (AHRQ) recommends fluoroquinolones as first-line treatment for children with uncomplicated gonorrhea who weigh more than 45 kg,6 and second-line therapy for children with bacterial meningitis,7 nongonococcal urethritis, chlamydia,6 or pelvic inflammatory disease.8
Acknowledgments
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government.
1. Burkhardt JE, Walterspiel JN, Schaad RB. Quinolone arthropathy in animals versus children. Clin Infect Dis. 1997;25:1196-1204.
2. Ahmed AS, Khan NZ, Saha SK, et al. Ciprofloxacin treatment in preterm neonates in Bangladesh. Pediatr Infect Dis J. 2006;25:1137-1141.
3. Chalumeau M, Tonnelier S, D’Athis P, et al. Fluoroquinolone safety in pediatric patients: a prospective, multicenter, comparative cohort study in France. Pediatrics. 2003;111:e714-e719.
4. Royer RJ. Adverse drug reactions with fluoroquinolones. Therapie. 1998;51:414-416.
5. Committee on Infectious Diseases. The use of systematic fluoroquinolones. Pediatrics. 2006;118:1287-1292.
6. Workowski KA, Berman SM. and the Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. Diseases characterized by urethritis and cervicitis. MMWR Morb Mortal Wkly Rep. 2006;55(RR-11):35-49.
7. Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267-1284.
8. Workowski KA, Berman SM. and the Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. Pelvic inflammatory disease. MMWR Morb Mortal Wkly Rep. 2006;55(RR-11):56-61.
1. Burkhardt JE, Walterspiel JN, Schaad RB. Quinolone arthropathy in animals versus children. Clin Infect Dis. 1997;25:1196-1204.
2. Ahmed AS, Khan NZ, Saha SK, et al. Ciprofloxacin treatment in preterm neonates in Bangladesh. Pediatr Infect Dis J. 2006;25:1137-1141.
3. Chalumeau M, Tonnelier S, D’Athis P, et al. Fluoroquinolone safety in pediatric patients: a prospective, multicenter, comparative cohort study in France. Pediatrics. 2003;111:e714-e719.
4. Royer RJ. Adverse drug reactions with fluoroquinolones. Therapie. 1998;51:414-416.
5. Committee on Infectious Diseases. The use of systematic fluoroquinolones. Pediatrics. 2006;118:1287-1292.
6. Workowski KA, Berman SM. and the Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. Diseases characterized by urethritis and cervicitis. MMWR Morb Mortal Wkly Rep. 2006;55(RR-11):35-49.
7. Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267-1284.
8. Workowski KA, Berman SM. and the Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. Pelvic inflammatory disease. MMWR Morb Mortal Wkly Rep. 2006;55(RR-11):56-61.
Evidence-based answers from the Family Physicians Inquiries Network
Alcoholic liver disease: Is acetaminophen safe?
Yes—acetaminophen is a safe and effective analgesic that can be appropriately used for adult patients with stable chronic alcoholic liver disease for at least a short period of time (studies have been limited to a maximum of 48–72 hours), up to the maximum recommended dosage of 4 g daily (strength of recommendation: A, based on 2 RCTs and other studies). There are little data to guide longer-term use of acetaminophen in this situation.
“Lesser of all evils”
Joseph J. Saseen, PharmD, FCCP, BCPS
University of Colorado at Denver and Health Sciences Center, Denver
Selecting an appropriate analgesic for patients with chronic alcoholic liver disease is complicated. Narcotics are potentially addictive, and nonsteroidal anti-inflammatory drugs (NSAIDs) can cause gastrointestinal bleeding and other adverse events. Alcoholic liver disease predisposes patients to these potential drug-related complications, so these options are not ideal.
Acetaminophen is the “lesser of all evils” in this population, based on some data suggesting it is safe when used within approved dosing parameters. However, these parameters vary significantly.
Although a maximum daily dose of 4 g is widely accepted as normal, the American Geriatric Society recommends no more than 2 to 3 g daily for older patients with hepatic insufficiency or a history of alcohol abuse.
Moreover, the American Liver Foundation issued a warning to not exceed 3 g daily for any prolonged period of time in response to a 2006 clinical trial that demonstrated aminotransferase increases in healthy volunteers treated with 4 g of acetaminophen daily for 14 days.1
Regardless of the exact maximum dose, none are greater than 4 g daily.
Always judiciously monitor dosing of acetaminophen because patients continue to experience unintentional overdose and hepatic failure caused by inadvertent use of multiple acetaminophen-containing products.2
Evidence summary
Acetaminophen, while widely used, is hepatotoxic in supra-therapeutic doses.3 Many studies purporting to show evidence of hepatic damage from therapeutic doses of acetaminophen have also been reported. Particularly in the 1970s and 1980s, there were a number of case reports and small literature reviews indicating that hepatic injury among regular users of alcohol (particularly chronic alcoholics) who take acetaminophen with therapeutic intent could be a “therapeutic misadventure.”4
Recent studies suggest short-term safety
- A systematic review (published in 2000) identified reports of acetaminophen toxicity, poisoning, or adverse events for alcohol patients.5
- In a randomized, double-blinded, placebo-controlled study, 102 alcoholic patients were given 4 g of acetaminophen daily for 2 days.6
Recommendations from others
American College of Gastroenterology. The American College of Gastroenterology states that it’s generally safe to take acetaminophen in the amount specified in the package labeling. Furthermore, they recommend that patients diagnosed with liver conditions consult their physician for advice on dosing for acetaminophen or any other pain reliever.7
In Liver and Biliary Disease, the author concludes that chronic alcoholics are at increased risk for hepatotoxicity secondary to acetaminophen even at therapeutic doses; therefore, advise them to take no more than 2 g daily.8
1. Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily. A randomized controlled trial. JAMA 2006;296:87-93.
2. Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005;42:1364-1372.
3. Barker JD, Jr, de Carle DJ, Anuras S. Chronic excessive acetaminophen use and liver damage. Ann Intern Med 1977;87:299-301.
4. Zimmerman HJ, Maddery WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 1995;22:767-773.
5. Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. Am J Therapeutics 2000;7:123-134.
6. Kuffner EK, Dart RC, Bogdan GM, Hill RE, Casper E, Darton L. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Inter Med 2001;161:2247-2252.
7. Herrera JL, O’Brien BL. Important information for patients with chronic liver disease and/or cirrhosis. In McNally PR, DeVault KR, Surawicz CM, eds. Common GI Problems. vol 3. Available at: www.acg.gi.org/patients/cgp/cgpvol3.asp#liver. Accessed on June 26, 2007.
8. Kaplowitz N. Liver and Biliary Diseases. Baltimore, Md: Williams and Wilkins; 1996.
Yes—acetaminophen is a safe and effective analgesic that can be appropriately used for adult patients with stable chronic alcoholic liver disease for at least a short period of time (studies have been limited to a maximum of 48–72 hours), up to the maximum recommended dosage of 4 g daily (strength of recommendation: A, based on 2 RCTs and other studies). There are little data to guide longer-term use of acetaminophen in this situation.
“Lesser of all evils”
Joseph J. Saseen, PharmD, FCCP, BCPS
University of Colorado at Denver and Health Sciences Center, Denver
Selecting an appropriate analgesic for patients with chronic alcoholic liver disease is complicated. Narcotics are potentially addictive, and nonsteroidal anti-inflammatory drugs (NSAIDs) can cause gastrointestinal bleeding and other adverse events. Alcoholic liver disease predisposes patients to these potential drug-related complications, so these options are not ideal.
Acetaminophen is the “lesser of all evils” in this population, based on some data suggesting it is safe when used within approved dosing parameters. However, these parameters vary significantly.
Although a maximum daily dose of 4 g is widely accepted as normal, the American Geriatric Society recommends no more than 2 to 3 g daily for older patients with hepatic insufficiency or a history of alcohol abuse.
Moreover, the American Liver Foundation issued a warning to not exceed 3 g daily for any prolonged period of time in response to a 2006 clinical trial that demonstrated aminotransferase increases in healthy volunteers treated with 4 g of acetaminophen daily for 14 days.1
Regardless of the exact maximum dose, none are greater than 4 g daily.
Always judiciously monitor dosing of acetaminophen because patients continue to experience unintentional overdose and hepatic failure caused by inadvertent use of multiple acetaminophen-containing products.2
Evidence summary
Acetaminophen, while widely used, is hepatotoxic in supra-therapeutic doses.3 Many studies purporting to show evidence of hepatic damage from therapeutic doses of acetaminophen have also been reported. Particularly in the 1970s and 1980s, there were a number of case reports and small literature reviews indicating that hepatic injury among regular users of alcohol (particularly chronic alcoholics) who take acetaminophen with therapeutic intent could be a “therapeutic misadventure.”4
Recent studies suggest short-term safety
- A systematic review (published in 2000) identified reports of acetaminophen toxicity, poisoning, or adverse events for alcohol patients.5
- In a randomized, double-blinded, placebo-controlled study, 102 alcoholic patients were given 4 g of acetaminophen daily for 2 days.6
Recommendations from others
American College of Gastroenterology. The American College of Gastroenterology states that it’s generally safe to take acetaminophen in the amount specified in the package labeling. Furthermore, they recommend that patients diagnosed with liver conditions consult their physician for advice on dosing for acetaminophen or any other pain reliever.7
In Liver and Biliary Disease, the author concludes that chronic alcoholics are at increased risk for hepatotoxicity secondary to acetaminophen even at therapeutic doses; therefore, advise them to take no more than 2 g daily.8
Yes—acetaminophen is a safe and effective analgesic that can be appropriately used for adult patients with stable chronic alcoholic liver disease for at least a short period of time (studies have been limited to a maximum of 48–72 hours), up to the maximum recommended dosage of 4 g daily (strength of recommendation: A, based on 2 RCTs and other studies). There are little data to guide longer-term use of acetaminophen in this situation.
“Lesser of all evils”
Joseph J. Saseen, PharmD, FCCP, BCPS
University of Colorado at Denver and Health Sciences Center, Denver
Selecting an appropriate analgesic for patients with chronic alcoholic liver disease is complicated. Narcotics are potentially addictive, and nonsteroidal anti-inflammatory drugs (NSAIDs) can cause gastrointestinal bleeding and other adverse events. Alcoholic liver disease predisposes patients to these potential drug-related complications, so these options are not ideal.
Acetaminophen is the “lesser of all evils” in this population, based on some data suggesting it is safe when used within approved dosing parameters. However, these parameters vary significantly.
Although a maximum daily dose of 4 g is widely accepted as normal, the American Geriatric Society recommends no more than 2 to 3 g daily for older patients with hepatic insufficiency or a history of alcohol abuse.
Moreover, the American Liver Foundation issued a warning to not exceed 3 g daily for any prolonged period of time in response to a 2006 clinical trial that demonstrated aminotransferase increases in healthy volunteers treated with 4 g of acetaminophen daily for 14 days.1
Regardless of the exact maximum dose, none are greater than 4 g daily.
Always judiciously monitor dosing of acetaminophen because patients continue to experience unintentional overdose and hepatic failure caused by inadvertent use of multiple acetaminophen-containing products.2
Evidence summary
Acetaminophen, while widely used, is hepatotoxic in supra-therapeutic doses.3 Many studies purporting to show evidence of hepatic damage from therapeutic doses of acetaminophen have also been reported. Particularly in the 1970s and 1980s, there were a number of case reports and small literature reviews indicating that hepatic injury among regular users of alcohol (particularly chronic alcoholics) who take acetaminophen with therapeutic intent could be a “therapeutic misadventure.”4
Recent studies suggest short-term safety
- A systematic review (published in 2000) identified reports of acetaminophen toxicity, poisoning, or adverse events for alcohol patients.5
- In a randomized, double-blinded, placebo-controlled study, 102 alcoholic patients were given 4 g of acetaminophen daily for 2 days.6
Recommendations from others
American College of Gastroenterology. The American College of Gastroenterology states that it’s generally safe to take acetaminophen in the amount specified in the package labeling. Furthermore, they recommend that patients diagnosed with liver conditions consult their physician for advice on dosing for acetaminophen or any other pain reliever.7
In Liver and Biliary Disease, the author concludes that chronic alcoholics are at increased risk for hepatotoxicity secondary to acetaminophen even at therapeutic doses; therefore, advise them to take no more than 2 g daily.8
1. Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily. A randomized controlled trial. JAMA 2006;296:87-93.
2. Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005;42:1364-1372.
3. Barker JD, Jr, de Carle DJ, Anuras S. Chronic excessive acetaminophen use and liver damage. Ann Intern Med 1977;87:299-301.
4. Zimmerman HJ, Maddery WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 1995;22:767-773.
5. Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. Am J Therapeutics 2000;7:123-134.
6. Kuffner EK, Dart RC, Bogdan GM, Hill RE, Casper E, Darton L. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Inter Med 2001;161:2247-2252.
7. Herrera JL, O’Brien BL. Important information for patients with chronic liver disease and/or cirrhosis. In McNally PR, DeVault KR, Surawicz CM, eds. Common GI Problems. vol 3. Available at: www.acg.gi.org/patients/cgp/cgpvol3.asp#liver. Accessed on June 26, 2007.
8. Kaplowitz N. Liver and Biliary Diseases. Baltimore, Md: Williams and Wilkins; 1996.
1. Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily. A randomized controlled trial. JAMA 2006;296:87-93.
2. Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005;42:1364-1372.
3. Barker JD, Jr, de Carle DJ, Anuras S. Chronic excessive acetaminophen use and liver damage. Ann Intern Med 1977;87:299-301.
4. Zimmerman HJ, Maddery WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 1995;22:767-773.
5. Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. Am J Therapeutics 2000;7:123-134.
6. Kuffner EK, Dart RC, Bogdan GM, Hill RE, Casper E, Darton L. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Inter Med 2001;161:2247-2252.
7. Herrera JL, O’Brien BL. Important information for patients with chronic liver disease and/or cirrhosis. In McNally PR, DeVault KR, Surawicz CM, eds. Common GI Problems. vol 3. Available at: www.acg.gi.org/patients/cgp/cgpvol3.asp#liver. Accessed on June 26, 2007.
8. Kaplowitz N. Liver and Biliary Diseases. Baltimore, Md: Williams and Wilkins; 1996.
Evidence-based answers from the Family Physicians Inquiries Network
What are the indications for meningococcal vaccination?
Routine vaccination with the meningococcal conjugate vaccine MCV4 (Menactra) is indicated for all US adolescents entering high school and for college freshmen living in dormitories (strength of recommendation [SOR]: B, based on observational studies). For convenience, MCV4 can be given at the 11- to 12-year-old visit.
High-risk individuals (ages 2 and older) who should receive meningococcal vaccine (MCV4 or the unconjugated polysaccharide vaccine [MPSV4]) include those with terminal complement deficiencies, asplenia, or HIV, as well as military recruits, laboratory personnel exposed to aerosolized meningococci, and travelers to areas hyperendemic or epidemic for Neisseria meningitides (SOR: C, based on consensus guidelines). Routine vaccination of infants and toddlers with conjugate vaccine may be more cost-effective than targeting adolescents, but conjugate meningococcal vaccine for this age group is not yet available in the US (SOR: B, based on cohort studies).
The vaccine is available and efficacious—use it well
Mark Stephens, MD
Uniformed Services University, Bethesda, Md
As a junior military medical officer, my first assignment was in San Diego, California near the former Naval Training Center (NTC). The NTC was the site of one of the last major outbreaks of meningococcal disease in a military barracks setting. I recall with alacrity the rapidity with which this disease overcomes its host, and the overwhelming morbidity (and mortality) the disease leaves behind if treatment is delayed. This is truly a “not-to-be-missed” diagnosis.
The historical parallels between smallpox and meningococcal disease are striking. Each is spread primarily by respiratory means, particularly in close quarters. While meningococcal disease is amenable to antibiotic treatment when recognized early (contrary to smallpox), the principles of high-risk “herd” immunization hold true for both conditions. By focusing on high-risk groups and adhering to ACIP recommendations, control of meningococcal disease is within the grasp of modern medical science. The vaccine is available. The vaccine is efficacious. Use it well.
Evidence summary
Two meningococcal vaccines are currently available in the US: tetravalent polysaccharide vaccine (MPSV4) and tetravalent polysaccharide-protein conjugate vaccine (MCV4). Both protect against serogroups A, C, Y, and W-135, but not against serogroup B, which is the most prevalent. A vaccine for serogroup B is under development.
MPSV4 is licensed for ages 2 years and up, but its poor immunogenicity in infants, lack of memory and booster response, and relatively short duration of protection have restricted its use. MCV4 is licensed for 11- to 55-year-olds and is the preferred vaccine in this age group, since it provides longer duration of immunity and reduces nasopharyngeal carriage.1
Infants and freshman are especially vulnerable
Using active community surveillance from 1991 to 2002, Centers for Disease Control and Prevention (CDC) data2 found annual rates of meningococcal disease in the US of 0.5 to 1.1 per 100,000. The highest rates were found in children under age 2. Infants younger than 12 months of age were especially vulnerable (rate 9/100,000), with more than 50% of cases caused by serogroup B.
A 1998–1999 prospective surveillance study3 including 50 state health departments and 231 college health centers identified 96 cases of meningococcal disease in college students (incidence 0.7/100,000). Freshmen living in dormitories had an elevated risk of meningococcal disease compared with other undergraduates or nonstudents of the same age (incidence 5.1/100,000; adjusted relative risk=3.6 [95% confidence interval [CI], 1.6–8.5). Sixty-eight percent had illness due to a vaccine-preventable serogroup.
Using CDC incidence data, a cost-effectiveness model4 compared hypothetical vaccination strategies targeting US infants (3 doses), toddlers (1 dose), or 11-year-olds (1 dose). Routine MCV4 vaccination of all 11-year-olds would prevent 270 cases and 36 deaths in this cohort over their next 22 years. For a toddler cohort, vaccination would prevent 385 cases and 33 deaths; for infants, 447 cases and 36 deaths. Conjugate meningococcal vaccines for serogroups A and C have been tested and used in children in other countries, and appear safe and effective, but are not yet available in the US. An application has been submitted for FDA approval of MCV4 for 2- to 10-year-olds.
Herd immunity may expand benefit of vaccination
A British study compared attack rates for meningococcal C disease in children from infancy to age 18 before and 1 to 2 years after the institution of a nationwide meningococcal serogroup C conjugate vaccination. Vaccine coverage ranged from 66% (adolescents) to 87% (schoolchildren), and vaccine efficacy was 94% to 96%. Incidence of meningococcal C disease in the unvaccinated children also decreased by 52% to 67% (from 4.08/100,000 to 1.36/100,000).5
Vaccinating adolescents may be particularly helpful for building herd immunity. A Norwegian study of nasopharyngeal meningococcal carriage among 943 unimmunized individuals ages 2 months to 95 years found a carriage rate of 28% among 15- to 24-year-olds, compared with 9.6% overall.6
High hospitalization rates in US military recruits during 1964 to 1970 (25.2/100,000) led to the development of the meningococcal polysaccharide vaccine. Since 1971, all new military recruits have received polysaccharide meningococcal vaccine, and for the period 1990 to 1998 the hospitalization rate for meningococcal disease among active duty service members had decreased by 98% (to 0.51/100,000).7
Recommendations from others
The Advisory Committee on Immunization Practices,2 American Academy of Pediatrics,8 American Academy of Family Physicians,9 and American College Association10 recommendations are summarized in the TABLE. Recommendations for vaccination during meningococcal disease outbreaks can be found at www.cdc.gov.2
TABLE
Who should get vaccinated—and when
| TARGET POPULATION | VACCINE TYPE |
|---|---|
| Children 2–10 years at increased risk* | MPSV4† |
| Adolescents 11–12 years | MCV4 |
| Adolescents at high school entry or 15 years of age without prior vaccination | MCV4 |
| College freshmen planning to reside in dormitories | MCV4‡ |
| Patients ages 11–55 at increased risk* | MCV4‡ |
| Patients older than 55 years at increased risk* | MPSV4 |
| Microbiologist, lab personnel exposed to N meningitides | MCV4‡ |
| Military recruits | MCV4‡ |
| *“Increased risk” is defined by terminal complement deficiency, anatomic or functional asplenia, travel to endemic areas, HIV infection (optional). | |
| †May be repeated every 3 to 5 years if increased risk continues. | |
| ‡MPSV4 is an acceptable alternative. | |
| MPSV4, meningococcal polysaccharide vaccine; MCV4, meningococcal polysaccharide diphtheria toxoid conjugate vaccine. | |
| Adapted from Harrison, Clinical Microbiology Reviews 2006;1 Kimmel, Am Fam Physician 2005.9 | |
1. Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev 2006;19:142-164.
2. Bilukha OO, Rosenstein N. National Center for Infectious Diseases; Center for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54:1-21. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5407a1.htm. Accessed on April 19, 2007.
3. Bruce MG, Rosenstein NE, Capparella JM, et al. Risk factors for meningococcal disease in college students. JAMA 2001;286:688-693.
4. Shepard CW, Ortega-Sanchez IR, Scott II RD, Rosenstein NE, ABCs Team. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 2005;115:1220-1232.
5. Ramsay ME, Andrews NJ, Trotter CL, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: data analysis. Br Med J 2003;326:365-366.
6. Caugant DA, Hoiby EA, Magnus P, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiology 1994;32:323-330.
7. US Department of Health and Human Services. Meningococcal disease and college students: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2000;49(RR-07):11-20.
8. American Academy of Pediatrics Committee on Infectious Diseases. Prevention and control of meningococcal disease: recommendations for use of meningococcal vaccines in pediatric patients. Pediatrics 2005;116:496-505.
9. Kimmel SR. Prevention of meningococcal disease. Am Fam Physician 2005;72:2049-2056.
10. American College Health Association. ACHA guidelines: recommendations for institutional prematriculation immunizations, 2006. Available at www.acha.org/info_resources/RIPIstatement.pdf. Accessed on April 3, 2007.
Routine vaccination with the meningococcal conjugate vaccine MCV4 (Menactra) is indicated for all US adolescents entering high school and for college freshmen living in dormitories (strength of recommendation [SOR]: B, based on observational studies). For convenience, MCV4 can be given at the 11- to 12-year-old visit.
High-risk individuals (ages 2 and older) who should receive meningococcal vaccine (MCV4 or the unconjugated polysaccharide vaccine [MPSV4]) include those with terminal complement deficiencies, asplenia, or HIV, as well as military recruits, laboratory personnel exposed to aerosolized meningococci, and travelers to areas hyperendemic or epidemic for Neisseria meningitides (SOR: C, based on consensus guidelines). Routine vaccination of infants and toddlers with conjugate vaccine may be more cost-effective than targeting adolescents, but conjugate meningococcal vaccine for this age group is not yet available in the US (SOR: B, based on cohort studies).
The vaccine is available and efficacious—use it well
Mark Stephens, MD
Uniformed Services University, Bethesda, Md
As a junior military medical officer, my first assignment was in San Diego, California near the former Naval Training Center (NTC). The NTC was the site of one of the last major outbreaks of meningococcal disease in a military barracks setting. I recall with alacrity the rapidity with which this disease overcomes its host, and the overwhelming morbidity (and mortality) the disease leaves behind if treatment is delayed. This is truly a “not-to-be-missed” diagnosis.
The historical parallels between smallpox and meningococcal disease are striking. Each is spread primarily by respiratory means, particularly in close quarters. While meningococcal disease is amenable to antibiotic treatment when recognized early (contrary to smallpox), the principles of high-risk “herd” immunization hold true for both conditions. By focusing on high-risk groups and adhering to ACIP recommendations, control of meningococcal disease is within the grasp of modern medical science. The vaccine is available. The vaccine is efficacious. Use it well.
Evidence summary
Two meningococcal vaccines are currently available in the US: tetravalent polysaccharide vaccine (MPSV4) and tetravalent polysaccharide-protein conjugate vaccine (MCV4). Both protect against serogroups A, C, Y, and W-135, but not against serogroup B, which is the most prevalent. A vaccine for serogroup B is under development.
MPSV4 is licensed for ages 2 years and up, but its poor immunogenicity in infants, lack of memory and booster response, and relatively short duration of protection have restricted its use. MCV4 is licensed for 11- to 55-year-olds and is the preferred vaccine in this age group, since it provides longer duration of immunity and reduces nasopharyngeal carriage.1
Infants and freshman are especially vulnerable
Using active community surveillance from 1991 to 2002, Centers for Disease Control and Prevention (CDC) data2 found annual rates of meningococcal disease in the US of 0.5 to 1.1 per 100,000. The highest rates were found in children under age 2. Infants younger than 12 months of age were especially vulnerable (rate 9/100,000), with more than 50% of cases caused by serogroup B.
A 1998–1999 prospective surveillance study3 including 50 state health departments and 231 college health centers identified 96 cases of meningococcal disease in college students (incidence 0.7/100,000). Freshmen living in dormitories had an elevated risk of meningococcal disease compared with other undergraduates or nonstudents of the same age (incidence 5.1/100,000; adjusted relative risk=3.6 [95% confidence interval [CI], 1.6–8.5). Sixty-eight percent had illness due to a vaccine-preventable serogroup.
Using CDC incidence data, a cost-effectiveness model4 compared hypothetical vaccination strategies targeting US infants (3 doses), toddlers (1 dose), or 11-year-olds (1 dose). Routine MCV4 vaccination of all 11-year-olds would prevent 270 cases and 36 deaths in this cohort over their next 22 years. For a toddler cohort, vaccination would prevent 385 cases and 33 deaths; for infants, 447 cases and 36 deaths. Conjugate meningococcal vaccines for serogroups A and C have been tested and used in children in other countries, and appear safe and effective, but are not yet available in the US. An application has been submitted for FDA approval of MCV4 for 2- to 10-year-olds.
Herd immunity may expand benefit of vaccination
A British study compared attack rates for meningococcal C disease in children from infancy to age 18 before and 1 to 2 years after the institution of a nationwide meningococcal serogroup C conjugate vaccination. Vaccine coverage ranged from 66% (adolescents) to 87% (schoolchildren), and vaccine efficacy was 94% to 96%. Incidence of meningococcal C disease in the unvaccinated children also decreased by 52% to 67% (from 4.08/100,000 to 1.36/100,000).5
Vaccinating adolescents may be particularly helpful for building herd immunity. A Norwegian study of nasopharyngeal meningococcal carriage among 943 unimmunized individuals ages 2 months to 95 years found a carriage rate of 28% among 15- to 24-year-olds, compared with 9.6% overall.6
High hospitalization rates in US military recruits during 1964 to 1970 (25.2/100,000) led to the development of the meningococcal polysaccharide vaccine. Since 1971, all new military recruits have received polysaccharide meningococcal vaccine, and for the period 1990 to 1998 the hospitalization rate for meningococcal disease among active duty service members had decreased by 98% (to 0.51/100,000).7
Recommendations from others
The Advisory Committee on Immunization Practices,2 American Academy of Pediatrics,8 American Academy of Family Physicians,9 and American College Association10 recommendations are summarized in the TABLE. Recommendations for vaccination during meningococcal disease outbreaks can be found at www.cdc.gov.2
TABLE
Who should get vaccinated—and when
| TARGET POPULATION | VACCINE TYPE |
|---|---|
| Children 2–10 years at increased risk* | MPSV4† |
| Adolescents 11–12 years | MCV4 |
| Adolescents at high school entry or 15 years of age without prior vaccination | MCV4 |
| College freshmen planning to reside in dormitories | MCV4‡ |
| Patients ages 11–55 at increased risk* | MCV4‡ |
| Patients older than 55 years at increased risk* | MPSV4 |
| Microbiologist, lab personnel exposed to N meningitides | MCV4‡ |
| Military recruits | MCV4‡ |
| *“Increased risk” is defined by terminal complement deficiency, anatomic or functional asplenia, travel to endemic areas, HIV infection (optional). | |
| †May be repeated every 3 to 5 years if increased risk continues. | |
| ‡MPSV4 is an acceptable alternative. | |
| MPSV4, meningococcal polysaccharide vaccine; MCV4, meningococcal polysaccharide diphtheria toxoid conjugate vaccine. | |
| Adapted from Harrison, Clinical Microbiology Reviews 2006;1 Kimmel, Am Fam Physician 2005.9 | |
Routine vaccination with the meningococcal conjugate vaccine MCV4 (Menactra) is indicated for all US adolescents entering high school and for college freshmen living in dormitories (strength of recommendation [SOR]: B, based on observational studies). For convenience, MCV4 can be given at the 11- to 12-year-old visit.
High-risk individuals (ages 2 and older) who should receive meningococcal vaccine (MCV4 or the unconjugated polysaccharide vaccine [MPSV4]) include those with terminal complement deficiencies, asplenia, or HIV, as well as military recruits, laboratory personnel exposed to aerosolized meningococci, and travelers to areas hyperendemic or epidemic for Neisseria meningitides (SOR: C, based on consensus guidelines). Routine vaccination of infants and toddlers with conjugate vaccine may be more cost-effective than targeting adolescents, but conjugate meningococcal vaccine for this age group is not yet available in the US (SOR: B, based on cohort studies).
The vaccine is available and efficacious—use it well
Mark Stephens, MD
Uniformed Services University, Bethesda, Md
As a junior military medical officer, my first assignment was in San Diego, California near the former Naval Training Center (NTC). The NTC was the site of one of the last major outbreaks of meningococcal disease in a military barracks setting. I recall with alacrity the rapidity with which this disease overcomes its host, and the overwhelming morbidity (and mortality) the disease leaves behind if treatment is delayed. This is truly a “not-to-be-missed” diagnosis.
The historical parallels between smallpox and meningococcal disease are striking. Each is spread primarily by respiratory means, particularly in close quarters. While meningococcal disease is amenable to antibiotic treatment when recognized early (contrary to smallpox), the principles of high-risk “herd” immunization hold true for both conditions. By focusing on high-risk groups and adhering to ACIP recommendations, control of meningococcal disease is within the grasp of modern medical science. The vaccine is available. The vaccine is efficacious. Use it well.
Evidence summary
Two meningococcal vaccines are currently available in the US: tetravalent polysaccharide vaccine (MPSV4) and tetravalent polysaccharide-protein conjugate vaccine (MCV4). Both protect against serogroups A, C, Y, and W-135, but not against serogroup B, which is the most prevalent. A vaccine for serogroup B is under development.
MPSV4 is licensed for ages 2 years and up, but its poor immunogenicity in infants, lack of memory and booster response, and relatively short duration of protection have restricted its use. MCV4 is licensed for 11- to 55-year-olds and is the preferred vaccine in this age group, since it provides longer duration of immunity and reduces nasopharyngeal carriage.1
Infants and freshman are especially vulnerable
Using active community surveillance from 1991 to 2002, Centers for Disease Control and Prevention (CDC) data2 found annual rates of meningococcal disease in the US of 0.5 to 1.1 per 100,000. The highest rates were found in children under age 2. Infants younger than 12 months of age were especially vulnerable (rate 9/100,000), with more than 50% of cases caused by serogroup B.
A 1998–1999 prospective surveillance study3 including 50 state health departments and 231 college health centers identified 96 cases of meningococcal disease in college students (incidence 0.7/100,000). Freshmen living in dormitories had an elevated risk of meningococcal disease compared with other undergraduates or nonstudents of the same age (incidence 5.1/100,000; adjusted relative risk=3.6 [95% confidence interval [CI], 1.6–8.5). Sixty-eight percent had illness due to a vaccine-preventable serogroup.
Using CDC incidence data, a cost-effectiveness model4 compared hypothetical vaccination strategies targeting US infants (3 doses), toddlers (1 dose), or 11-year-olds (1 dose). Routine MCV4 vaccination of all 11-year-olds would prevent 270 cases and 36 deaths in this cohort over their next 22 years. For a toddler cohort, vaccination would prevent 385 cases and 33 deaths; for infants, 447 cases and 36 deaths. Conjugate meningococcal vaccines for serogroups A and C have been tested and used in children in other countries, and appear safe and effective, but are not yet available in the US. An application has been submitted for FDA approval of MCV4 for 2- to 10-year-olds.
Herd immunity may expand benefit of vaccination
A British study compared attack rates for meningococcal C disease in children from infancy to age 18 before and 1 to 2 years after the institution of a nationwide meningococcal serogroup C conjugate vaccination. Vaccine coverage ranged from 66% (adolescents) to 87% (schoolchildren), and vaccine efficacy was 94% to 96%. Incidence of meningococcal C disease in the unvaccinated children also decreased by 52% to 67% (from 4.08/100,000 to 1.36/100,000).5
Vaccinating adolescents may be particularly helpful for building herd immunity. A Norwegian study of nasopharyngeal meningococcal carriage among 943 unimmunized individuals ages 2 months to 95 years found a carriage rate of 28% among 15- to 24-year-olds, compared with 9.6% overall.6
High hospitalization rates in US military recruits during 1964 to 1970 (25.2/100,000) led to the development of the meningococcal polysaccharide vaccine. Since 1971, all new military recruits have received polysaccharide meningococcal vaccine, and for the period 1990 to 1998 the hospitalization rate for meningococcal disease among active duty service members had decreased by 98% (to 0.51/100,000).7
Recommendations from others
The Advisory Committee on Immunization Practices,2 American Academy of Pediatrics,8 American Academy of Family Physicians,9 and American College Association10 recommendations are summarized in the TABLE. Recommendations for vaccination during meningococcal disease outbreaks can be found at www.cdc.gov.2
TABLE
Who should get vaccinated—and when
| TARGET POPULATION | VACCINE TYPE |
|---|---|
| Children 2–10 years at increased risk* | MPSV4† |
| Adolescents 11–12 years | MCV4 |
| Adolescents at high school entry or 15 years of age without prior vaccination | MCV4 |
| College freshmen planning to reside in dormitories | MCV4‡ |
| Patients ages 11–55 at increased risk* | MCV4‡ |
| Patients older than 55 years at increased risk* | MPSV4 |
| Microbiologist, lab personnel exposed to N meningitides | MCV4‡ |
| Military recruits | MCV4‡ |
| *“Increased risk” is defined by terminal complement deficiency, anatomic or functional asplenia, travel to endemic areas, HIV infection (optional). | |
| †May be repeated every 3 to 5 years if increased risk continues. | |
| ‡MPSV4 is an acceptable alternative. | |
| MPSV4, meningococcal polysaccharide vaccine; MCV4, meningococcal polysaccharide diphtheria toxoid conjugate vaccine. | |
| Adapted from Harrison, Clinical Microbiology Reviews 2006;1 Kimmel, Am Fam Physician 2005.9 | |
1. Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev 2006;19:142-164.
2. Bilukha OO, Rosenstein N. National Center for Infectious Diseases; Center for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54:1-21. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5407a1.htm. Accessed on April 19, 2007.
3. Bruce MG, Rosenstein NE, Capparella JM, et al. Risk factors for meningococcal disease in college students. JAMA 2001;286:688-693.
4. Shepard CW, Ortega-Sanchez IR, Scott II RD, Rosenstein NE, ABCs Team. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 2005;115:1220-1232.
5. Ramsay ME, Andrews NJ, Trotter CL, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: data analysis. Br Med J 2003;326:365-366.
6. Caugant DA, Hoiby EA, Magnus P, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiology 1994;32:323-330.
7. US Department of Health and Human Services. Meningococcal disease and college students: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2000;49(RR-07):11-20.
8. American Academy of Pediatrics Committee on Infectious Diseases. Prevention and control of meningococcal disease: recommendations for use of meningococcal vaccines in pediatric patients. Pediatrics 2005;116:496-505.
9. Kimmel SR. Prevention of meningococcal disease. Am Fam Physician 2005;72:2049-2056.
10. American College Health Association. ACHA guidelines: recommendations for institutional prematriculation immunizations, 2006. Available at www.acha.org/info_resources/RIPIstatement.pdf. Accessed on April 3, 2007.
1. Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev 2006;19:142-164.
2. Bilukha OO, Rosenstein N. National Center for Infectious Diseases; Center for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54:1-21. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5407a1.htm. Accessed on April 19, 2007.
3. Bruce MG, Rosenstein NE, Capparella JM, et al. Risk factors for meningococcal disease in college students. JAMA 2001;286:688-693.
4. Shepard CW, Ortega-Sanchez IR, Scott II RD, Rosenstein NE, ABCs Team. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 2005;115:1220-1232.
5. Ramsay ME, Andrews NJ, Trotter CL, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: data analysis. Br Med J 2003;326:365-366.
6. Caugant DA, Hoiby EA, Magnus P, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiology 1994;32:323-330.
7. US Department of Health and Human Services. Meningococcal disease and college students: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2000;49(RR-07):11-20.
8. American Academy of Pediatrics Committee on Infectious Diseases. Prevention and control of meningococcal disease: recommendations for use of meningococcal vaccines in pediatric patients. Pediatrics 2005;116:496-505.
9. Kimmel SR. Prevention of meningococcal disease. Am Fam Physician 2005;72:2049-2056.
10. American College Health Association. ACHA guidelines: recommendations for institutional prematriculation immunizations, 2006. Available at www.acha.org/info_resources/RIPIstatement.pdf. Accessed on April 3, 2007.
Evidence-based answers from the Family Physicians Inquiries Network
What are the risks and benefits of elective induction for uncomplicated term pregnancies?
Elective induction of labor for term, singleton, uncomplicated pregnancies appears safe for both the mother and infant (strength of recommendation [SOR]: B). The benefit of elective induction for nonmedical reasons is unclear (SOR: B).
Elective inductions can add costs and legal risks
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
Family physicians cherish having long, collaborative relationships with patients. But when they practice obstetrics, this desire can result in feeling pressured to grant requests by pregnant patients for elective inductions. As indicated in this Clinical Inquiry, elective inductions may be relatively safe in some situations, but they always incur added costs. The cost of cervical ripening, extra monitoring, and medications to promote uterine contractions fall to the medical system. There also may be added legal risk to the provider. Eventually, some elective induction will have a bad outcome and there will be no way to defend the decision to induce as medically necessary.
Evidence summary
Induction of labor is a viable therapeutic option when the benefits of timely delivery outweigh the risks of unnecessary cesarean section or prematurity. Two large retrospective studies support the concept that cesarean section rates and admissions to neonatal intensive care units are higher with elective induction as opposed to expectant management (TABLE).1,2 A large population-based study suggests that the higher cesarean section rates in elective induction is present only among nulliparous women; in multiparous women, the rate is the same as expectant management.3 Contrasting these results are those of a large systematic review, which found lower cesarean section rates in electively induced women. Two more recent studies, a retrospective cohort study4 and a randomized controlled trial,5 found a much lower incidence of cesarean section and operative vaginal deliveries among induced vs expectantly managed women at term.
TABLE
Summary of evidence regarding induction of labor
| STUDY | METHODS | CESAREAN DELIVERY RATE | OPERATIVE VAGINAL DELIVERY | PERINATAL COMPLICATIONS |
|---|---|---|---|---|
| Cammu, 20021 | Matched cohort study. 7683 women in IND group, 7683 women in EM group. 38–410/7 weeks gestation. | 9.9% vs 6.5% (P=.001); NNH=30. | 31.67% vs 29.1% (P=.001); NNH=39. | NICU admission 10.7% vs 9.4% (RR=1.03<1.14<1.25; P=.001). |
| Boulvain, 20012 | Retrospective cohort study.7430 women between 38 and 40 6/7weeks.531women in induced group vs 3353 women in spontaneous labor group. | Induction of labor was found to be associated with higher risk of cesarean delivery (7.7% to 3.6%) (RR=2.4; 95% CI, 1.1–3.4). | IND vs spontaneous labor 28.1% vs 30.1% (RR=1.0; 95% CI, 0.9–1.2); not statistically significant. | NICU admission 4.1% vs 2.8% (RR=1.6; 95% CI, 1.0–2.4). |
| Dublin, 20003 | Population-based cohort study.2886 induced vs 9648 spontaneous labor. 37–41weeks gestation. | In nulliparous women 19% of IND group had cesarean delivery vs 10% nulliparous of women in spontaneous labor group (NNH=11). No association was seen in multiparous women. | 18.6% vs 15.5% (RR=1.2; 95% CI, 1.02–1.32). | Shoulder dystocia 3.0% vs 1.7% (RR=1.32; 95% CI, 1.02–1.69); NNH=77. |
| Nicholson, 20044 | Retrospective cohort study.100 women in active management (AM) group, 300 selected subjects in standard management (SM) group. 38 to to 410/7 weeks gestation. | AM group vs SM group had higher rates of induction (63% vs 23.7%; risk ratio=2.66 [95% CI, 2.07–3.43]). AM group vs SM group had a lower cesarean delivery rate (4% vs 16.7%; risk ratio=0.24; 95% CI, 0.09–0.65; NNT=7). | AM group vs SM group 16% vs 15.3%. Not statically significant. | No significant differences. |
| Nielson, 20055 | 116 women (45 nulliparous) randomized at ≥39 wks to expectant management or induction with oxytocin and/or amniotomy. | 6.9% (8/116) IND group. vs 7.3% (8/110) in EM group. Not statistically significant. | 6.9% (8/116) IND group vs 8.2% (9/116) EM group. Not statistically significant. | No mention. |
| Sanchez-Ramos, 20036 | Systematic review of 16 randomized controlled trials (6588 women). Included women at 41 weeks gestation. | 20.1% in IND group vs 22.0% in EM group. NNT=52; odds reduction of 12% (95% CI, 0.78–0.99). Statistically significant. | No mention. | Perinatal mortality rate: 0.09% IND group vs 0.33% EM group. Not statistically significant. |
| IND, induction; EM, expectant management; AM, active management; SM, standard management; NICU, neonatal intensive care unit; RR, relative risk; CI, confidence interval; NNT, number needed to treat; NNH, number needed to harm. | ||||
Recommendations from others
A 1999 American College of Obstetricians and Gynecologists (ACOG) practice bulletin states that labor may be induced for logistic reasons such as psychosocial factors and distance from hospital, as long as 1 of these 4 criteria is met: (1) fetal heart tones have been documented for 20 weeks by nonelectronic fetoscope or for 30 weeks by Doppler; (2) it has been 36 weeks since a positive serum or urine human chorionic gonadotropin pregnancy test was performed; (3) ultrasound measurement of crown-rump length, obtained at 6 to 12 weeks, supports a gestational age of at least 39 weeks; (4) ultrasound obtained at 13 to 20 weeks confirms the gestational age of at least 39 weeks determined by clinical history and physical examination. The ACOG recommendation (which dates back to 1989) is for induction of low-risk pregnancy at the 43rd week of gestation.7
The Royal College of Obstetricians and Gynaecologists recommends that women with uncomplicated pregnancies be offered induction of labor beyond 41 weeks.8 The Department of Obstetrics and Gynecology and Reproductive Biology at Harvard Medical School recommends routine induction of labor be recommended at 41 weeks’ gestation.9
1. Cammu H, Martens G, Ruyssinck G, Amy JJ. Outcome after elective labor induction in nulliparous women: A matched cohort study. Am J Obstet Gynecol 2002;186:240-244.
2. Boulvain M, Marcoux S, Bureau M, Fortier M, Fraser W. Risks of induction of labour in uncomplicated term pregnancies. Paediatric Perinatal Epidemiology 2001;15:131-139.
3. Dublin S, Lydon-Rochelle M, Kaplan RC, Watts DH, Critchlow CW. Maternal and neonatal outcomes after induction of labor without an identified indication. Am J Obstet Gynecol 2000;183:986-994.
4. Nicholson JM, Kellar LC, Cronholm PF, Macones GA. Active management of risk in pregnancy at term in an urban population: An association between a higher induction of labor rate and a lower cesarean delivery rate. Am J Obstet Gynecol 2004;191:1516-1528.
5. Nielsen PE, Howard BC, Hill CC, Larson PL, Holland RHB, Smith PN. Comparison of elective induction of labor with favorable Bishop scores versus expectant management: A randomized clinical trial. J Maternal Fetal Neonatal Med 2005;18:59-64.
6. Sanchez-Ramos L, Olivier F, Delke I, Kaunitz A. Labor induction versus expectant management for postterm pregnancies: A systematic review with meta-analysis. Obstet Gynecol 2003;101:1312-1318.
7. ACOG Practice Bulletin. Induction of labor. Int J Gynecol Obstet 2000;69:283-292.
8. Royal College of Obstetricians and Gynaecologists. Induction of Labour London: RCOG Press; 2001.
9. Rand L, Robinson JN, Economy KE, Norwitz ER. Post-term induction of labor revisited. Obstet Gynecol 2000;96:779-783.
Elective induction of labor for term, singleton, uncomplicated pregnancies appears safe for both the mother and infant (strength of recommendation [SOR]: B). The benefit of elective induction for nonmedical reasons is unclear (SOR: B).
Elective inductions can add costs and legal risks
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
Family physicians cherish having long, collaborative relationships with patients. But when they practice obstetrics, this desire can result in feeling pressured to grant requests by pregnant patients for elective inductions. As indicated in this Clinical Inquiry, elective inductions may be relatively safe in some situations, but they always incur added costs. The cost of cervical ripening, extra monitoring, and medications to promote uterine contractions fall to the medical system. There also may be added legal risk to the provider. Eventually, some elective induction will have a bad outcome and there will be no way to defend the decision to induce as medically necessary.
Evidence summary
Induction of labor is a viable therapeutic option when the benefits of timely delivery outweigh the risks of unnecessary cesarean section or prematurity. Two large retrospective studies support the concept that cesarean section rates and admissions to neonatal intensive care units are higher with elective induction as opposed to expectant management (TABLE).1,2 A large population-based study suggests that the higher cesarean section rates in elective induction is present only among nulliparous women; in multiparous women, the rate is the same as expectant management.3 Contrasting these results are those of a large systematic review, which found lower cesarean section rates in electively induced women. Two more recent studies, a retrospective cohort study4 and a randomized controlled trial,5 found a much lower incidence of cesarean section and operative vaginal deliveries among induced vs expectantly managed women at term.
TABLE
Summary of evidence regarding induction of labor
| STUDY | METHODS | CESAREAN DELIVERY RATE | OPERATIVE VAGINAL DELIVERY | PERINATAL COMPLICATIONS |
|---|---|---|---|---|
| Cammu, 20021 | Matched cohort study. 7683 women in IND group, 7683 women in EM group. 38–410/7 weeks gestation. | 9.9% vs 6.5% (P=.001); NNH=30. | 31.67% vs 29.1% (P=.001); NNH=39. | NICU admission 10.7% vs 9.4% (RR=1.03<1.14<1.25; P=.001). |
| Boulvain, 20012 | Retrospective cohort study.7430 women between 38 and 40 6/7weeks.531women in induced group vs 3353 women in spontaneous labor group. | Induction of labor was found to be associated with higher risk of cesarean delivery (7.7% to 3.6%) (RR=2.4; 95% CI, 1.1–3.4). | IND vs spontaneous labor 28.1% vs 30.1% (RR=1.0; 95% CI, 0.9–1.2); not statistically significant. | NICU admission 4.1% vs 2.8% (RR=1.6; 95% CI, 1.0–2.4). |
| Dublin, 20003 | Population-based cohort study.2886 induced vs 9648 spontaneous labor. 37–41weeks gestation. | In nulliparous women 19% of IND group had cesarean delivery vs 10% nulliparous of women in spontaneous labor group (NNH=11). No association was seen in multiparous women. | 18.6% vs 15.5% (RR=1.2; 95% CI, 1.02–1.32). | Shoulder dystocia 3.0% vs 1.7% (RR=1.32; 95% CI, 1.02–1.69); NNH=77. |
| Nicholson, 20044 | Retrospective cohort study.100 women in active management (AM) group, 300 selected subjects in standard management (SM) group. 38 to to 410/7 weeks gestation. | AM group vs SM group had higher rates of induction (63% vs 23.7%; risk ratio=2.66 [95% CI, 2.07–3.43]). AM group vs SM group had a lower cesarean delivery rate (4% vs 16.7%; risk ratio=0.24; 95% CI, 0.09–0.65; NNT=7). | AM group vs SM group 16% vs 15.3%. Not statically significant. | No significant differences. |
| Nielson, 20055 | 116 women (45 nulliparous) randomized at ≥39 wks to expectant management or induction with oxytocin and/or amniotomy. | 6.9% (8/116) IND group. vs 7.3% (8/110) in EM group. Not statistically significant. | 6.9% (8/116) IND group vs 8.2% (9/116) EM group. Not statistically significant. | No mention. |
| Sanchez-Ramos, 20036 | Systematic review of 16 randomized controlled trials (6588 women). Included women at 41 weeks gestation. | 20.1% in IND group vs 22.0% in EM group. NNT=52; odds reduction of 12% (95% CI, 0.78–0.99). Statistically significant. | No mention. | Perinatal mortality rate: 0.09% IND group vs 0.33% EM group. Not statistically significant. |
| IND, induction; EM, expectant management; AM, active management; SM, standard management; NICU, neonatal intensive care unit; RR, relative risk; CI, confidence interval; NNT, number needed to treat; NNH, number needed to harm. | ||||
Recommendations from others
A 1999 American College of Obstetricians and Gynecologists (ACOG) practice bulletin states that labor may be induced for logistic reasons such as psychosocial factors and distance from hospital, as long as 1 of these 4 criteria is met: (1) fetal heart tones have been documented for 20 weeks by nonelectronic fetoscope or for 30 weeks by Doppler; (2) it has been 36 weeks since a positive serum or urine human chorionic gonadotropin pregnancy test was performed; (3) ultrasound measurement of crown-rump length, obtained at 6 to 12 weeks, supports a gestational age of at least 39 weeks; (4) ultrasound obtained at 13 to 20 weeks confirms the gestational age of at least 39 weeks determined by clinical history and physical examination. The ACOG recommendation (which dates back to 1989) is for induction of low-risk pregnancy at the 43rd week of gestation.7
The Royal College of Obstetricians and Gynaecologists recommends that women with uncomplicated pregnancies be offered induction of labor beyond 41 weeks.8 The Department of Obstetrics and Gynecology and Reproductive Biology at Harvard Medical School recommends routine induction of labor be recommended at 41 weeks’ gestation.9
Elective induction of labor for term, singleton, uncomplicated pregnancies appears safe for both the mother and infant (strength of recommendation [SOR]: B). The benefit of elective induction for nonmedical reasons is unclear (SOR: B).
Elective inductions can add costs and legal risks
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
Family physicians cherish having long, collaborative relationships with patients. But when they practice obstetrics, this desire can result in feeling pressured to grant requests by pregnant patients for elective inductions. As indicated in this Clinical Inquiry, elective inductions may be relatively safe in some situations, but they always incur added costs. The cost of cervical ripening, extra monitoring, and medications to promote uterine contractions fall to the medical system. There also may be added legal risk to the provider. Eventually, some elective induction will have a bad outcome and there will be no way to defend the decision to induce as medically necessary.
Evidence summary
Induction of labor is a viable therapeutic option when the benefits of timely delivery outweigh the risks of unnecessary cesarean section or prematurity. Two large retrospective studies support the concept that cesarean section rates and admissions to neonatal intensive care units are higher with elective induction as opposed to expectant management (TABLE).1,2 A large population-based study suggests that the higher cesarean section rates in elective induction is present only among nulliparous women; in multiparous women, the rate is the same as expectant management.3 Contrasting these results are those of a large systematic review, which found lower cesarean section rates in electively induced women. Two more recent studies, a retrospective cohort study4 and a randomized controlled trial,5 found a much lower incidence of cesarean section and operative vaginal deliveries among induced vs expectantly managed women at term.
TABLE
Summary of evidence regarding induction of labor
| STUDY | METHODS | CESAREAN DELIVERY RATE | OPERATIVE VAGINAL DELIVERY | PERINATAL COMPLICATIONS |
|---|---|---|---|---|
| Cammu, 20021 | Matched cohort study. 7683 women in IND group, 7683 women in EM group. 38–410/7 weeks gestation. | 9.9% vs 6.5% (P=.001); NNH=30. | 31.67% vs 29.1% (P=.001); NNH=39. | NICU admission 10.7% vs 9.4% (RR=1.03<1.14<1.25; P=.001). |
| Boulvain, 20012 | Retrospective cohort study.7430 women between 38 and 40 6/7weeks.531women in induced group vs 3353 women in spontaneous labor group. | Induction of labor was found to be associated with higher risk of cesarean delivery (7.7% to 3.6%) (RR=2.4; 95% CI, 1.1–3.4). | IND vs spontaneous labor 28.1% vs 30.1% (RR=1.0; 95% CI, 0.9–1.2); not statistically significant. | NICU admission 4.1% vs 2.8% (RR=1.6; 95% CI, 1.0–2.4). |
| Dublin, 20003 | Population-based cohort study.2886 induced vs 9648 spontaneous labor. 37–41weeks gestation. | In nulliparous women 19% of IND group had cesarean delivery vs 10% nulliparous of women in spontaneous labor group (NNH=11). No association was seen in multiparous women. | 18.6% vs 15.5% (RR=1.2; 95% CI, 1.02–1.32). | Shoulder dystocia 3.0% vs 1.7% (RR=1.32; 95% CI, 1.02–1.69); NNH=77. |
| Nicholson, 20044 | Retrospective cohort study.100 women in active management (AM) group, 300 selected subjects in standard management (SM) group. 38 to to 410/7 weeks gestation. | AM group vs SM group had higher rates of induction (63% vs 23.7%; risk ratio=2.66 [95% CI, 2.07–3.43]). AM group vs SM group had a lower cesarean delivery rate (4% vs 16.7%; risk ratio=0.24; 95% CI, 0.09–0.65; NNT=7). | AM group vs SM group 16% vs 15.3%. Not statically significant. | No significant differences. |
| Nielson, 20055 | 116 women (45 nulliparous) randomized at ≥39 wks to expectant management or induction with oxytocin and/or amniotomy. | 6.9% (8/116) IND group. vs 7.3% (8/110) in EM group. Not statistically significant. | 6.9% (8/116) IND group vs 8.2% (9/116) EM group. Not statistically significant. | No mention. |
| Sanchez-Ramos, 20036 | Systematic review of 16 randomized controlled trials (6588 women). Included women at 41 weeks gestation. | 20.1% in IND group vs 22.0% in EM group. NNT=52; odds reduction of 12% (95% CI, 0.78–0.99). Statistically significant. | No mention. | Perinatal mortality rate: 0.09% IND group vs 0.33% EM group. Not statistically significant. |
| IND, induction; EM, expectant management; AM, active management; SM, standard management; NICU, neonatal intensive care unit; RR, relative risk; CI, confidence interval; NNT, number needed to treat; NNH, number needed to harm. | ||||
Recommendations from others
A 1999 American College of Obstetricians and Gynecologists (ACOG) practice bulletin states that labor may be induced for logistic reasons such as psychosocial factors and distance from hospital, as long as 1 of these 4 criteria is met: (1) fetal heart tones have been documented for 20 weeks by nonelectronic fetoscope or for 30 weeks by Doppler; (2) it has been 36 weeks since a positive serum or urine human chorionic gonadotropin pregnancy test was performed; (3) ultrasound measurement of crown-rump length, obtained at 6 to 12 weeks, supports a gestational age of at least 39 weeks; (4) ultrasound obtained at 13 to 20 weeks confirms the gestational age of at least 39 weeks determined by clinical history and physical examination. The ACOG recommendation (which dates back to 1989) is for induction of low-risk pregnancy at the 43rd week of gestation.7
The Royal College of Obstetricians and Gynaecologists recommends that women with uncomplicated pregnancies be offered induction of labor beyond 41 weeks.8 The Department of Obstetrics and Gynecology and Reproductive Biology at Harvard Medical School recommends routine induction of labor be recommended at 41 weeks’ gestation.9
1. Cammu H, Martens G, Ruyssinck G, Amy JJ. Outcome after elective labor induction in nulliparous women: A matched cohort study. Am J Obstet Gynecol 2002;186:240-244.
2. Boulvain M, Marcoux S, Bureau M, Fortier M, Fraser W. Risks of induction of labour in uncomplicated term pregnancies. Paediatric Perinatal Epidemiology 2001;15:131-139.
3. Dublin S, Lydon-Rochelle M, Kaplan RC, Watts DH, Critchlow CW. Maternal and neonatal outcomes after induction of labor without an identified indication. Am J Obstet Gynecol 2000;183:986-994.
4. Nicholson JM, Kellar LC, Cronholm PF, Macones GA. Active management of risk in pregnancy at term in an urban population: An association between a higher induction of labor rate and a lower cesarean delivery rate. Am J Obstet Gynecol 2004;191:1516-1528.
5. Nielsen PE, Howard BC, Hill CC, Larson PL, Holland RHB, Smith PN. Comparison of elective induction of labor with favorable Bishop scores versus expectant management: A randomized clinical trial. J Maternal Fetal Neonatal Med 2005;18:59-64.
6. Sanchez-Ramos L, Olivier F, Delke I, Kaunitz A. Labor induction versus expectant management for postterm pregnancies: A systematic review with meta-analysis. Obstet Gynecol 2003;101:1312-1318.
7. ACOG Practice Bulletin. Induction of labor. Int J Gynecol Obstet 2000;69:283-292.
8. Royal College of Obstetricians and Gynaecologists. Induction of Labour London: RCOG Press; 2001.
9. Rand L, Robinson JN, Economy KE, Norwitz ER. Post-term induction of labor revisited. Obstet Gynecol 2000;96:779-783.
1. Cammu H, Martens G, Ruyssinck G, Amy JJ. Outcome after elective labor induction in nulliparous women: A matched cohort study. Am J Obstet Gynecol 2002;186:240-244.
2. Boulvain M, Marcoux S, Bureau M, Fortier M, Fraser W. Risks of induction of labour in uncomplicated term pregnancies. Paediatric Perinatal Epidemiology 2001;15:131-139.
3. Dublin S, Lydon-Rochelle M, Kaplan RC, Watts DH, Critchlow CW. Maternal and neonatal outcomes after induction of labor without an identified indication. Am J Obstet Gynecol 2000;183:986-994.
4. Nicholson JM, Kellar LC, Cronholm PF, Macones GA. Active management of risk in pregnancy at term in an urban population: An association between a higher induction of labor rate and a lower cesarean delivery rate. Am J Obstet Gynecol 2004;191:1516-1528.
5. Nielsen PE, Howard BC, Hill CC, Larson PL, Holland RHB, Smith PN. Comparison of elective induction of labor with favorable Bishop scores versus expectant management: A randomized clinical trial. J Maternal Fetal Neonatal Med 2005;18:59-64.
6. Sanchez-Ramos L, Olivier F, Delke I, Kaunitz A. Labor induction versus expectant management for postterm pregnancies: A systematic review with meta-analysis. Obstet Gynecol 2003;101:1312-1318.
7. ACOG Practice Bulletin. Induction of labor. Int J Gynecol Obstet 2000;69:283-292.
8. Royal College of Obstetricians and Gynaecologists. Induction of Labour London: RCOG Press; 2001.
9. Rand L, Robinson JN, Economy KE, Norwitz ER. Post-term induction of labor revisited. Obstet Gynecol 2000;96:779-783.
Evidence-based answers from the Family Physicians Inquiries Network
What precautions should we use with statins for women of childbearing age?
Statins are contraindicated for women who are pregnant or breastfeeding. Data evaluating statin use for women of childbearing age is limited; however, they may be used cautiously with adequate contraception. Pravastatin may be preferred based on its low tissue-penetration properties. Cholesterol-lowering with simvastatin 40 mg/d did not disrupt menstrual cycles or effect luteal phase duration (strength of recommendation: C).
Use statins only as a last resort for women of childbearing age
Ariel Smits, MD
Department of Family Medicine, Oregon Health & Science University, Portland
I try to follow the USPSTF recommendations and not screen women aged <45 years without coronary artery disease risk factors for hyperlipidemia. When a woman of any age needs treatment, my first-line therapy is lifestyle modification. Given the risks of statin drugs to the developing fetus, women with childbearing potential should give fully informed consent and be offered reliable contraception before stating statin therapy.
Before reading this review, I had not been aware of the serious effects of statin medications on the developing fetus. In conversations with my colleagues, I found that the adverse effects of statins during pregnancy are not readily known. Such information needs to be more widely disseminated.
Evidence summary
Hydroxymethyl glutaryl coenzyme A (HMG CoA) reductase inhibitors, commonly called statins, have been on the market since the late 1980s. Statins are primarily used to treat hypercholesterolemia, and in recent years have been shown to reduce the risks of coronary events, stroke, and cardiovascular mortality.1
Use of statins is contraindicated during pregnancy based on pre-marketing animal studies showing developmental toxicities in animal fetuses; consequently they are pregnancy category X.2 To date, no controlled studies demonstrate teratogenic effects in humans; however, numerous case reports have documented congenital anomalies, including vertebral, anal, cardiac, tracheal, esophageal, renal, and limb deficiency (VACTERL association), intrauterine growth retardation (IUGR), and demise in fetuses exposed during pregnancy, especially in the first trimester. It is thought that adverse events are under-reported and likely biased toward severe outcomes, thereby limiting actual reported exposures. Despite this limitation, the likelihood of observing specific anomalies has been predicted based upon prescription data and birth rates. The overall birth prevalence of any isolated lower-limb defect or VACTERL anomaly is estimated as 1:100,000 and ranges from 1:50,000 for simvastatin (Zocor) to 1:500,000 for lovastatin (Mevacor).3 These congenital anomaly frequencies do not exceed general population rates.
One study suggests that short-term use of simvastatin does not affect menstruation or ovulation of premenopausal women. This double-blind, randomized, placebo-controlled trial enrolled 86 normally cycling women. Mean age of women completing the study was 35. Simvastatin 40 mg/d was studied for cholesterol effects and female reproductive effects. Urinary luteinizing hormone (LH) and pregnanediol glucuronide (PDG), a progesterone metabolite, were assessed to determine if treatment with simvastatin adversely affects luteal function. Simvastatin lowered low-density lipoprotein (LDL) cholesterol by 34.3% (P<.001). Normal luteal phase duration and peak were confirmed by urinary PDG and LH levels. This study demonstrated that treatment with simvastatin for 4 months had no significant clinical changes on reproductive gonadal function compared with placebo.4
Although ovulation may not be affected by simvastatin, do statins provide a reward worth the risk of other adverse effects? A recent meta-analysis evaluated the benefits of lipid-lowering medication in trials of at least 1 year duration that included women. Total and coronary heart disease (CHD) mortality, nonfatal myocardial infarction, revascularization, and total CHD events were assessed among women with and without cardiovascular disease (CVD). Ten trials included statins. Of the 5 studies that reported age, the average was 61 years. For women without CVD, lipid-lowering treatment was not shown to affect total or CHD mortality. For women with known CVD, hyperlipidemia treatment did not affect total mortality, but was shown effective in reducing CHD events, CHD mortality, nonfatal myocardial infarction, and revascularization; the relative risk of CHD events for statin users was 0.80 (95% confidence interval [CI], 0.71–0.91). The number of women needed to treat (NNT) to prevent an initial CHD event was 140. For secondary prevention, the NNT to prevent 1 CHD event was 26. Since women of child-bearing potential have lower probability of CHD events compared to the older women studied in this meta-analysis, the expected benefit for younger women is likely to be substantially lower.5
Consider initial pregnancy tests and inform all women of childbearing age of the possibility of fetal injury before starting statin therapy.2 Highly lipophilic statins—such as simvastatin, atorvastatin (Lipitor), and lovastatin—achieve embryoplacental concentrations similar to those of maternal plasma. For this reason, if statin therapy is needed, these agents should be avoided. Pravastatin (Pravachol) is the most hydrophilic statin and has no reports of abnormal pregnancy outcomes, even in animal research.3
Recommendations from others
The National Cholesterol Education Program Expert Panel and the American Heart Association make no specific recommendations regarding precautions with statin use for women of childbearing age who require treatment for hypercholesterolemia or coronary heart disease.6,7 The American College of Obstetrics and Gynecologists makes no distinction regarding recommendations for pharmacological treatment of hyperlipidemia for women aged 20 to 45 years.8
The US Preventive Services Task Force makes no recommendations on treatment with statins; they only address screening for hypercholesterolemia.9 The Food and Drug Administration has given statin agents a pregnancy category of X (risks involved in use of the drug by pregnant women clearly outweigh potential benefits).
1. Moore TH, Bartlett C, Burke MA, Davey Smith G, Ebrahim SB. Statins for preventing cardiovascular disease. Cochrane Database Syst Rev 2004;(2):CD004816.
2. Draft summary of reproductive toxicology studies on Mevacor NDA 21-213: Joint Meeting of the Nonprescription Drugs Advisory Committee and Endocrinologic and Metabolic Drugs Advisory Committee of the Federal Drug Administration, Merck & Co (July 13, 2000). Available at: www.fda.gov/ohrms/dockets/ac/00/backgrd/3622b1b_summary.pdf. Accessed on December 7, 2005.
3. Edison RJ, Muenke M. Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am J Med Genet A 2004;131:287-298.
4. Plotkin D, Miller S, Nakajima S, et al. Lowering low density lipoprotein cholesterol with simvastatin, a hydroxyl-3-methylglutaryl-coenzyme a reductase inhibitor, does not affect luteal function in premenopausal women. J Clin Endocrinol Metabol 2002;87:3155-3161.
5. Walsh JME, Pignone M. Drug treatment of hyperlipidemia in women. JAMA 2004;291:2243-2252.
6. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-2497.
7. Mosca L, Appel JA, Benjamin EJ, et al. AHA guidelines: Evidence-based Guidelines for Cardiovascular Disease Prevention in Women. Circulation 2004;109:672-693.
8. Herbert WNP, Braly PS, Barss VA, et al. ACOG: Guidelines for Women’s Health Care. 2nd ed. Washington, DC: ACOG; 2002;209-211.
9. US Preventive Services Task Force. Screening for lipid disorder in adults: recommendations and rationale. Internet J Intern Med 2002;3(2).-Available at: www.ispub.com/ostia/index.php?xmlFilePath=journals/ijim/vol3n2/lipid.xml. Accessed on December 8, 2005.
Statins are contraindicated for women who are pregnant or breastfeeding. Data evaluating statin use for women of childbearing age is limited; however, they may be used cautiously with adequate contraception. Pravastatin may be preferred based on its low tissue-penetration properties. Cholesterol-lowering with simvastatin 40 mg/d did not disrupt menstrual cycles or effect luteal phase duration (strength of recommendation: C).
Use statins only as a last resort for women of childbearing age
Ariel Smits, MD
Department of Family Medicine, Oregon Health & Science University, Portland
I try to follow the USPSTF recommendations and not screen women aged <45 years without coronary artery disease risk factors for hyperlipidemia. When a woman of any age needs treatment, my first-line therapy is lifestyle modification. Given the risks of statin drugs to the developing fetus, women with childbearing potential should give fully informed consent and be offered reliable contraception before stating statin therapy.
Before reading this review, I had not been aware of the serious effects of statin medications on the developing fetus. In conversations with my colleagues, I found that the adverse effects of statins during pregnancy are not readily known. Such information needs to be more widely disseminated.
Evidence summary
Hydroxymethyl glutaryl coenzyme A (HMG CoA) reductase inhibitors, commonly called statins, have been on the market since the late 1980s. Statins are primarily used to treat hypercholesterolemia, and in recent years have been shown to reduce the risks of coronary events, stroke, and cardiovascular mortality.1
Use of statins is contraindicated during pregnancy based on pre-marketing animal studies showing developmental toxicities in animal fetuses; consequently they are pregnancy category X.2 To date, no controlled studies demonstrate teratogenic effects in humans; however, numerous case reports have documented congenital anomalies, including vertebral, anal, cardiac, tracheal, esophageal, renal, and limb deficiency (VACTERL association), intrauterine growth retardation (IUGR), and demise in fetuses exposed during pregnancy, especially in the first trimester. It is thought that adverse events are under-reported and likely biased toward severe outcomes, thereby limiting actual reported exposures. Despite this limitation, the likelihood of observing specific anomalies has been predicted based upon prescription data and birth rates. The overall birth prevalence of any isolated lower-limb defect or VACTERL anomaly is estimated as 1:100,000 and ranges from 1:50,000 for simvastatin (Zocor) to 1:500,000 for lovastatin (Mevacor).3 These congenital anomaly frequencies do not exceed general population rates.
One study suggests that short-term use of simvastatin does not affect menstruation or ovulation of premenopausal women. This double-blind, randomized, placebo-controlled trial enrolled 86 normally cycling women. Mean age of women completing the study was 35. Simvastatin 40 mg/d was studied for cholesterol effects and female reproductive effects. Urinary luteinizing hormone (LH) and pregnanediol glucuronide (PDG), a progesterone metabolite, were assessed to determine if treatment with simvastatin adversely affects luteal function. Simvastatin lowered low-density lipoprotein (LDL) cholesterol by 34.3% (P<.001). Normal luteal phase duration and peak were confirmed by urinary PDG and LH levels. This study demonstrated that treatment with simvastatin for 4 months had no significant clinical changes on reproductive gonadal function compared with placebo.4
Although ovulation may not be affected by simvastatin, do statins provide a reward worth the risk of other adverse effects? A recent meta-analysis evaluated the benefits of lipid-lowering medication in trials of at least 1 year duration that included women. Total and coronary heart disease (CHD) mortality, nonfatal myocardial infarction, revascularization, and total CHD events were assessed among women with and without cardiovascular disease (CVD). Ten trials included statins. Of the 5 studies that reported age, the average was 61 years. For women without CVD, lipid-lowering treatment was not shown to affect total or CHD mortality. For women with known CVD, hyperlipidemia treatment did not affect total mortality, but was shown effective in reducing CHD events, CHD mortality, nonfatal myocardial infarction, and revascularization; the relative risk of CHD events for statin users was 0.80 (95% confidence interval [CI], 0.71–0.91). The number of women needed to treat (NNT) to prevent an initial CHD event was 140. For secondary prevention, the NNT to prevent 1 CHD event was 26. Since women of child-bearing potential have lower probability of CHD events compared to the older women studied in this meta-analysis, the expected benefit for younger women is likely to be substantially lower.5
Consider initial pregnancy tests and inform all women of childbearing age of the possibility of fetal injury before starting statin therapy.2 Highly lipophilic statins—such as simvastatin, atorvastatin (Lipitor), and lovastatin—achieve embryoplacental concentrations similar to those of maternal plasma. For this reason, if statin therapy is needed, these agents should be avoided. Pravastatin (Pravachol) is the most hydrophilic statin and has no reports of abnormal pregnancy outcomes, even in animal research.3
Recommendations from others
The National Cholesterol Education Program Expert Panel and the American Heart Association make no specific recommendations regarding precautions with statin use for women of childbearing age who require treatment for hypercholesterolemia or coronary heart disease.6,7 The American College of Obstetrics and Gynecologists makes no distinction regarding recommendations for pharmacological treatment of hyperlipidemia for women aged 20 to 45 years.8
The US Preventive Services Task Force makes no recommendations on treatment with statins; they only address screening for hypercholesterolemia.9 The Food and Drug Administration has given statin agents a pregnancy category of X (risks involved in use of the drug by pregnant women clearly outweigh potential benefits).
Statins are contraindicated for women who are pregnant or breastfeeding. Data evaluating statin use for women of childbearing age is limited; however, they may be used cautiously with adequate contraception. Pravastatin may be preferred based on its low tissue-penetration properties. Cholesterol-lowering with simvastatin 40 mg/d did not disrupt menstrual cycles or effect luteal phase duration (strength of recommendation: C).
Use statins only as a last resort for women of childbearing age
Ariel Smits, MD
Department of Family Medicine, Oregon Health & Science University, Portland
I try to follow the USPSTF recommendations and not screen women aged <45 years without coronary artery disease risk factors for hyperlipidemia. When a woman of any age needs treatment, my first-line therapy is lifestyle modification. Given the risks of statin drugs to the developing fetus, women with childbearing potential should give fully informed consent and be offered reliable contraception before stating statin therapy.
Before reading this review, I had not been aware of the serious effects of statin medications on the developing fetus. In conversations with my colleagues, I found that the adverse effects of statins during pregnancy are not readily known. Such information needs to be more widely disseminated.
Evidence summary
Hydroxymethyl glutaryl coenzyme A (HMG CoA) reductase inhibitors, commonly called statins, have been on the market since the late 1980s. Statins are primarily used to treat hypercholesterolemia, and in recent years have been shown to reduce the risks of coronary events, stroke, and cardiovascular mortality.1
Use of statins is contraindicated during pregnancy based on pre-marketing animal studies showing developmental toxicities in animal fetuses; consequently they are pregnancy category X.2 To date, no controlled studies demonstrate teratogenic effects in humans; however, numerous case reports have documented congenital anomalies, including vertebral, anal, cardiac, tracheal, esophageal, renal, and limb deficiency (VACTERL association), intrauterine growth retardation (IUGR), and demise in fetuses exposed during pregnancy, especially in the first trimester. It is thought that adverse events are under-reported and likely biased toward severe outcomes, thereby limiting actual reported exposures. Despite this limitation, the likelihood of observing specific anomalies has been predicted based upon prescription data and birth rates. The overall birth prevalence of any isolated lower-limb defect or VACTERL anomaly is estimated as 1:100,000 and ranges from 1:50,000 for simvastatin (Zocor) to 1:500,000 for lovastatin (Mevacor).3 These congenital anomaly frequencies do not exceed general population rates.
One study suggests that short-term use of simvastatin does not affect menstruation or ovulation of premenopausal women. This double-blind, randomized, placebo-controlled trial enrolled 86 normally cycling women. Mean age of women completing the study was 35. Simvastatin 40 mg/d was studied for cholesterol effects and female reproductive effects. Urinary luteinizing hormone (LH) and pregnanediol glucuronide (PDG), a progesterone metabolite, were assessed to determine if treatment with simvastatin adversely affects luteal function. Simvastatin lowered low-density lipoprotein (LDL) cholesterol by 34.3% (P<.001). Normal luteal phase duration and peak were confirmed by urinary PDG and LH levels. This study demonstrated that treatment with simvastatin for 4 months had no significant clinical changes on reproductive gonadal function compared with placebo.4
Although ovulation may not be affected by simvastatin, do statins provide a reward worth the risk of other adverse effects? A recent meta-analysis evaluated the benefits of lipid-lowering medication in trials of at least 1 year duration that included women. Total and coronary heart disease (CHD) mortality, nonfatal myocardial infarction, revascularization, and total CHD events were assessed among women with and without cardiovascular disease (CVD). Ten trials included statins. Of the 5 studies that reported age, the average was 61 years. For women without CVD, lipid-lowering treatment was not shown to affect total or CHD mortality. For women with known CVD, hyperlipidemia treatment did not affect total mortality, but was shown effective in reducing CHD events, CHD mortality, nonfatal myocardial infarction, and revascularization; the relative risk of CHD events for statin users was 0.80 (95% confidence interval [CI], 0.71–0.91). The number of women needed to treat (NNT) to prevent an initial CHD event was 140. For secondary prevention, the NNT to prevent 1 CHD event was 26. Since women of child-bearing potential have lower probability of CHD events compared to the older women studied in this meta-analysis, the expected benefit for younger women is likely to be substantially lower.5
Consider initial pregnancy tests and inform all women of childbearing age of the possibility of fetal injury before starting statin therapy.2 Highly lipophilic statins—such as simvastatin, atorvastatin (Lipitor), and lovastatin—achieve embryoplacental concentrations similar to those of maternal plasma. For this reason, if statin therapy is needed, these agents should be avoided. Pravastatin (Pravachol) is the most hydrophilic statin and has no reports of abnormal pregnancy outcomes, even in animal research.3
Recommendations from others
The National Cholesterol Education Program Expert Panel and the American Heart Association make no specific recommendations regarding precautions with statin use for women of childbearing age who require treatment for hypercholesterolemia or coronary heart disease.6,7 The American College of Obstetrics and Gynecologists makes no distinction regarding recommendations for pharmacological treatment of hyperlipidemia for women aged 20 to 45 years.8
The US Preventive Services Task Force makes no recommendations on treatment with statins; they only address screening for hypercholesterolemia.9 The Food and Drug Administration has given statin agents a pregnancy category of X (risks involved in use of the drug by pregnant women clearly outweigh potential benefits).
1. Moore TH, Bartlett C, Burke MA, Davey Smith G, Ebrahim SB. Statins for preventing cardiovascular disease. Cochrane Database Syst Rev 2004;(2):CD004816.
2. Draft summary of reproductive toxicology studies on Mevacor NDA 21-213: Joint Meeting of the Nonprescription Drugs Advisory Committee and Endocrinologic and Metabolic Drugs Advisory Committee of the Federal Drug Administration, Merck & Co (July 13, 2000). Available at: www.fda.gov/ohrms/dockets/ac/00/backgrd/3622b1b_summary.pdf. Accessed on December 7, 2005.
3. Edison RJ, Muenke M. Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am J Med Genet A 2004;131:287-298.
4. Plotkin D, Miller S, Nakajima S, et al. Lowering low density lipoprotein cholesterol with simvastatin, a hydroxyl-3-methylglutaryl-coenzyme a reductase inhibitor, does not affect luteal function in premenopausal women. J Clin Endocrinol Metabol 2002;87:3155-3161.
5. Walsh JME, Pignone M. Drug treatment of hyperlipidemia in women. JAMA 2004;291:2243-2252.
6. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-2497.
7. Mosca L, Appel JA, Benjamin EJ, et al. AHA guidelines: Evidence-based Guidelines for Cardiovascular Disease Prevention in Women. Circulation 2004;109:672-693.
8. Herbert WNP, Braly PS, Barss VA, et al. ACOG: Guidelines for Women’s Health Care. 2nd ed. Washington, DC: ACOG; 2002;209-211.
9. US Preventive Services Task Force. Screening for lipid disorder in adults: recommendations and rationale. Internet J Intern Med 2002;3(2).-Available at: www.ispub.com/ostia/index.php?xmlFilePath=journals/ijim/vol3n2/lipid.xml. Accessed on December 8, 2005.
1. Moore TH, Bartlett C, Burke MA, Davey Smith G, Ebrahim SB. Statins for preventing cardiovascular disease. Cochrane Database Syst Rev 2004;(2):CD004816.
2. Draft summary of reproductive toxicology studies on Mevacor NDA 21-213: Joint Meeting of the Nonprescription Drugs Advisory Committee and Endocrinologic and Metabolic Drugs Advisory Committee of the Federal Drug Administration, Merck & Co (July 13, 2000). Available at: www.fda.gov/ohrms/dockets/ac/00/backgrd/3622b1b_summary.pdf. Accessed on December 7, 2005.
3. Edison RJ, Muenke M. Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am J Med Genet A 2004;131:287-298.
4. Plotkin D, Miller S, Nakajima S, et al. Lowering low density lipoprotein cholesterol with simvastatin, a hydroxyl-3-methylglutaryl-coenzyme a reductase inhibitor, does not affect luteal function in premenopausal women. J Clin Endocrinol Metabol 2002;87:3155-3161.
5. Walsh JME, Pignone M. Drug treatment of hyperlipidemia in women. JAMA 2004;291:2243-2252.
6. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-2497.
7. Mosca L, Appel JA, Benjamin EJ, et al. AHA guidelines: Evidence-based Guidelines for Cardiovascular Disease Prevention in Women. Circulation 2004;109:672-693.
8. Herbert WNP, Braly PS, Barss VA, et al. ACOG: Guidelines for Women’s Health Care. 2nd ed. Washington, DC: ACOG; 2002;209-211.
9. US Preventive Services Task Force. Screening for lipid disorder in adults: recommendations and rationale. Internet J Intern Med 2002;3(2).-Available at: www.ispub.com/ostia/index.php?xmlFilePath=journals/ijim/vol3n2/lipid.xml. Accessed on December 8, 2005.
Evidence-based answers from the Family Physicians Inquiries Network