User login
Updates on Cancer Survivorship Care Planning
With advances in treatment, supportive care, and early diagnosis, the prevalence of cancer is increasing. An individual is considered a cancer survivor from the time of diagnosis to the end of his or her life.1 Although many patients with cancer are cured, they experience various short-term and long-term effects of cancer treatment, a high risk of recurrence and second cancer, anxiety, chronic pain, fatigue, depression, sexual dysfunction, and infertility.1
As of January 1, 2014, there were about 14.5 million cancer survivors in the U.S. The most common cancers in this population include prostate (43%), colon and rectal (9%), and melanoma (8%) in males; breast (41%), uterine corpus (8%), and colon and rectal (8%) in females.2 This estimate does not include noninvasive cancers, but does include bladder, basal cell, and squamous cell skin cancers. By January 1, 2024, the population of cancer survivors is predicted to increase to almost 19 million: 9.3 million males and 9.6 million females.1 Most of the cancer survivors (64%) were diagnosed 5 or more years ago, and 15% were diagnosed 20 or more years ago. Nearly half (46%) of cancer survivors are aged ≥ 70 years, and only 5% are aged < 40 years.2
Moye and colleagues reported that 524,052 (11%) of veterans treated in 2007 were cancer survivors.3 The most common types of cancers among these veterans were prostate, skin (nonmelanoma), and colorectal cancers. Compared with the general population of cancer survivors in the SEER database, veteran survivors were older.3 Because of the increasing prevalence of cancer survivors, greater attention is focused on long-term complications of cancer treatment. Recent studies have demonstrated that cancer survivors are less likely to receive general preventive care and care associated with noncancer-related medical conditions than are individuals without cancer.4
Survivorship Care Components
In 2005, the Institute of Medicine (IOM) published From Cancer Patient to Cancer Survivor: Lost to Transition.5 The report addresses 4 essential components of survivorship care: (1) prevention of recurrence, new cancers, and other late effects; (2) surveillance for cancer spread, recurrence, second cancers, and medical and psychosocial adverse events (AEs); (3) interventions for consequences of cancer and its treatment (medical problems, symptoms, psychological distress experienced by cancer survivors and their caregivers, and concerns related to employment, insurance, and disability); and (4) coordination between the specialist and primary care providers (PCPs) to ensure that all the survivors’ health needs are met.5
Cancer Treatment Summary
To ensure better transition, the IOM recommended that survivorship plans be made with a summary of treatment provided by the primary oncologist who treated the patient, to improve communication among all health care providers and between the providers and the patient.5 The summary should include the date of diagnosis; diagnostic tests; stage of diagnosis; a medical, surgical, and radiation treatment summary; and a detailed follow-up care plan. The IOM also recommends preventive practices to maintain health and well-being; information on legal protection regarding employment and health insurance; the availability of psychosocial services in the community; and screening for psychosocial distress in cancer survivors.3,6 However, studies have shown that gaps in adherence with IOM recommendations exist even in dedicated survivorship centers.7,8
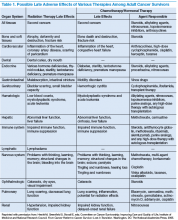
The American Society of Clinical Oncology (ASCO) and organizations such as Livestrong also have developed templates for survivorship care plans (SCPs). But there has been limited success in implementing SCPs.7,8 The American College of Surgeons Commission on Cancer (CoC) Standard 3.3 is scheduled to be implemented in 2015.9 The standard is expected to require that a cancer care committee develop and implement a process to disseminate a comprehensive care summary and follow-up plan for cancer survivors.9 To address this need, ASCO has formed a joint work group for improving cancer survivorship and a new version of an SCP template. This group recommended including contact information for the oncology providers who administered the treatment; basic diagnostic and staging information; and information on surgery, radiation therapy, systemic therapy (both chemotherapy and biologic therapy), and ongoing significant toxicities, including dates (Table 1).10
The ASCO also developed a follow-up care plan that includes a surveillance plan to detect recurrence and late AEs; interventions to manage ongoing problems resulting from cancer and its treatment; and age- and sex-appropriate health care, including cancer screening and general health promotion. It also recommended that the follow-up care plan should include a schedule of clinic visits in a table format, surveillance care testing to detect recurrence and second primary cancers, such as early breast cancer screening for women who underwent chest radiation for Hodgkin lymphoma. The person responsible for ordering these screening test should be included in the follow-up care plan.10 In addition, ASCO developed a cancer survivorship compendium, which included not only tools and resources, but also different models for cancer survivorship care.11 In addition, ASCO developed a Quality Oncology Practice Initiative (QOPI) to assess the quality of survivorship programs.9
The Central Arkansas Veterans Healthcare System (CAVHS) adopted National Comprehensive Cancer Network (NCCN), ASCO, and National Cancer Institute (NCI) guidelines and developed a template for a cancer treatment summary that includes the cancer type; grade; staging; and date of diagnosis and duration of treatment, including chemotherapy, surgery, and radiation with a disease-specific follow-up care plan for each type of cancer. The treatment summary is a useful tool to communicate a patient’s treatment and disease status to PCPs and patients.
Models of Care
Eight models for delivering survivorship care have been developed by ASCO11:
- Oncologist Specialist Care: Care occurs as a continuation in the oncology setting
- Multidisciplinary Survivor Clinic: Different specialists provide care
- General Survivorship Clinic: Care is provided by a physician or advanced care provider (APN) and implemented at a cancer center or private practice
- Consultative Survivorship Clinic: Initial follow-up is provided in an oncology setting with an eventual transition to a PCP; patients may be directed to the cancer center for needed services
- Integrated Survivorship Clinic: Care is provided by a physician or APN, and the care is coordinated with the PCP and other specialists as needed
- Community Generalist Model: The PCP, APN, or internist within the community provides care
- Shared-Care of Survivor: Care is coordinated and provided by any combination of specialists, PCPs, and nurses and is patient directed
Depending on the patients and the setting, practices can adopt various models to deliver survivorship care.
At CAVHS, cancer survivors are followed by an oncologist for their yearly examinations. This model is an illness model rather than a wellness model. A multidisciplinary clinic model can be initiated at CAVHS with the help of palliative care; complementary and alternative medicine for pain management; psychologists, chaplain services, and social workers for distress management; and coordination of survivorship care.
Implementation Barriers
There are many barriers to implementing SCPs, including time required by the providers to complete SCPs, inadequate reimbursement for the time and resources required to complete SCPs, challenges in coordinating care between survivors and providers, and lack of compatibility of the existing template with the electronic health record (EHR).7-10 A study regarding the barriers to implementation of SCPs, conducted at 14 NCI community cancer centers, demonstrated that the most common barrier is lack of personnel and time required to complete SCPs. The most widely used strategies was the use of a template with prespecified fields and delegating the completion of SCPs to one individual.12
Long-Term Complications for Survivors
Cancer survivors experience the physical and psychosocial effects of cancer treatment and have a very high risk of recurrence and second primary cancers. Common chronic AEs include fatigue, pain, neuropathy, infertility, sexual dysfunction, hypothyroidism, organ dysfunction, and urinary and bowel incontinence. In addition, patients also experience psychological AEs, including anxiety, depression, posttraumatic stress disorder, and sleep disturbances. Because of the AEs, cancer survivors have difficulty obtaining employment and insurance.1,5
Fatigue
Fatigue is the most common AE in cancer survivors.1 It may develop during treatment and persist for years. It is related to chemotherapy, radiation, surgical complications, depression, and insomnia.1 It is underrecognized and often untreated. It is important to assess and treat underlying comorbidities such as anemia, hypothyroidism, pain, depression and insomnia.13,14 Pharmacologic therapy with central nervous system stimulants and antidepressants have not shown any benefit.15 Studies on modafinil and armodafinil are ongoing.15 Exercise, treating underlying depression, sleep hygiene, behavioral and cognitive therapy, and yoga and mindfulness management of distress can help in treating fatigue.13 Meta-analyses showed that physical exercise helped reduce cancerrelated fatigue.15 A randomized controlled trial demonstrated that yoga led to significant improvement in fatigue in breast cancer survivors.16 Hence, it is important for providers to recognize and treat cancer-related fatigue and encourage patients to exercise.17
Psychological Adverse Effects
Cancer survivors also experience psychological AEs such as anxiety and depression because of the cancer diagnosis and the uncertainty of the outcome and the fear of relapse. Veterans may be at a higher risk of psychological AEs because of underlying mental illnesses. Counseling about the disease, psychotherapy interventions, and a mindfulness approach are recommended to treat anxiety and depression.14 The CAVHS cancer program has developed a mindfulness program as a multidisciplinary approach to manage psychological AEs.18
Sexual Dysfunction
Many patients may experience sexual dysfunction and infertility as a result of endocrine treatments, chemotherapy, radiation, and urologic and gynecologic surgeries.1 Over half of prostate cancer and breast cancer survivors report sexual dysfunction. Despite its high prevalence, sexual dysfunction often is not discussed with patients due to reluctance to discuss, lack of training, and lack of a standardized sexuality questionnaire.19 A brief sexual symptom checklist for women can be used as a primary screening tool. It is also recommended to screen for treatment-related infertility. Patients with sexual dysfunction should undergo screening for psychosocial problems such as anxiety, depression, and drug and alcohol use and treatment as these can contribute to sexual dysfunction.1
Vaginal lubricants are recommended to treat vaginal dryness. Vaginal estrogen creams are effective in patients with nonhormone-dependent gynecologic cancer without risk of breast cancer. The FDA approved the selective estrogen receptor modulator ospemifene for treating dyspareunia in postmenopausal women without risk of breast cancer.1 Oral phosphodiesterase-5 inhibitors, such as sildenafil, vacuum erection devices, penile prosthesis, and intracavernous injections, have shown to be effective in treating male erectile dysfunction (ED).19
Cardiovascular risk should be estimated in all patients with ED, because most of these patients will have common risk factors and need to be referred to a cardiologist before treating ED.1
Chronic Pain
Patients may also experience chronic pain as a late complication of chemotherapy, radiation, and surgery.1 More than one-third of cancer survivors experience pain, and it is often ineffectively managed due to lack of training, fear of AEs, and addiction.20 The goals of pain management are to increase comfort and improve quality of life. Short-acting and long-acting opioids remain an important treatment for cancer pain. Drug selection should be guided by previous exposure and comorbidities.15 Opioid therapy AEs, such as constipation, sleep apnea, and hypogonadism, should be recognized and treated. Antidepressants and anticonvulsants, such as gabapentin and pregabalin, may be used to manage neuropathic pain. A multidisciplinary approach using pharmacologic therapy, psychosocial therapy, behavioral interventions, exercise, and physical therapy is recommended.15 Patients with refractory pain are treated with interventional approaches, such as a neuronal blockade.
Survivorship Resources
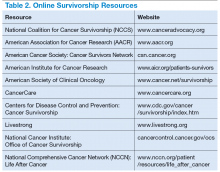
Survivorship resources were developed by various cancer societies, such as NCCN, ASCO, Livestrong, and the Children’s Oncology Group. These resources help providers develop and deliver a quality survivorship care program (Table 2).5 The NCCN provides a template to help create cancer treatment summaries; guidelines for follow-up care for different cancers; and information for patients on legal and employment issues, smoking cessation, nutrition, and weight loss. In addition, the NCCN provides tools to assess anthracyclineinduced cardiac toxicity, anxiety, depression, cognitive dysfunction, pain, sexual dysfunction, and fatigue.
The CAVHS has several resources to develop a survivor ship progr am: It offers complementary and alternative therapy (a multidisciplinary approach) to help veterans with chronic pain and psychological distress. This resource incorporates acupuncture, yoga, hypnotherapy, biofeedback, mind-body approaches, stress management, nutritional counseling, physical therapy, and other mental and behavioral health support.
In addition, VA is implementing a patient-centered care and cultural transformation program to help veterans establish a relationship with their health care providers.21 The patient-centered approach along with the complementary and alternative therapy program, mindfulness program, and telehealth exercise motivation program can be incorporated to develop a multidisciplinary survivorship program.
Survivorship Research
Survivorship is an emerging field, and there is need for research to improve the implementation of quality survivorship care. Cancer survivorship research encompasses the physical, psychosocial, and economic sequelae of cancer diagnosis and its treatment among both pediatric and adult survivors of cancer. It also includes issues related to health care delivery, access, and follow-up care.
Mayer and colleagues reported that there were 42 published studies of SCPs in adult cancer survivors.22 Eleven studies reported that SCP use was limited and that < 25% of oncology providers never used an SCP. Research also showed that oncologists who have had training in long-term AEs of cancer and those who have used the EHRs were more likely to use SCPs.23 An integrated review of studies of SCPs recommended areas of research for survivorship. Thus the quality and quantity of SCP research are limited, and there is more need for quality research in survivorship to endorse the effective use of SCPs.
Conclusion
Cancer patients experience various AEs of cancer treatment, psychosocial distress, and can be lost to follow-up. It is important for health care providers to recognize and screen for the complications and provide SCPs, which includes a treatment summary and follow-up care to communicate and coordinate quality cancer survivorship care. Providers can use tools and resources developed by various organizations to develop and implement SCPs. The CoC surveys and accredits the cancer programs for quality measures, and has developed a new standard to provide SCPs for all patients effective 2015.
The CAVHS has already adopted this standard and has developed a template to complete a treatment summary and follow-up care planning in the EHR. The cancer treatment summary is given to the patient and is available to the PCP for review. There is also a need for more quality research in survivorship.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: survivorship. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. Updated February 27, 2015. Accessed July 20, 2015.
2. American Cancer Society. Cancer treatment and survivorship facts and figures 2014-2015. American Cancer Society Website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf. Accessed July 8, 2015.
3. Moye J, Schuster J, Latini D, Naik A. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
4. Snyder CF, Frick KD, Kantisiper ME, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27(7):1054-1061.
5. Hewitt M, Greenfield S, Stovall E, eds; Committee on Cancer Survivorship: Improving Care and Quality of Life, Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2005.
6. Adler NE, Page AEK, eds; Institute of Medicine (US) Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press; 2008. http://www.ncbi.nlm.nih.gov/books/NBK4015/. Accessed July 10, 2015.
7. Stricker CT, Jacobs LA, Risendal B, et al. Survivorship care planning after the institute of medicine recommendations: how are we faring? J Cancer Surviv. 2011;5(4):358-370.
8. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
9. American College of Surgeons Commission on Cancer. Cancer Program Standards 2012: Ensuring Patient-Centered Care.V1.2.1. Chicago, IL: American College of Surgeons;2012. https://www.facs.org/~/media/files/quality%20programs/cancer
/coc/programstandards2012.ashx. Accessed July 8, 2015.
10. Mayer DK, Nekhlyudov L, Snyder CF, Merill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
11. American Society of Clinical Oncology. ASCO cancer survivorship compendium. American Society of Clinical Oncology Website. http://www.asco.org/practice-research/asco-cancer-survivorship-compendium. Accessed July 10, 2015.
12. Forsythe LP, Alfano CM, Leach CR, Ganz PA, Stefanek ME, Rowland JH. Who provides psychosocial follow-up care for post-treatment cancer survivors? A survey of medical oncologists and primary care physicians. J Clin Oncol. 2012;30(23):2897-2905.
13. Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following
treatment. Clin J Oncol Nurs. 2014;18(suppl):38-58.
14. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for
adult cancer survivors: highlighing ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015;35:188-194.
15. Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30(30):3687-3696.
16. Cramp F, Daniel J. Exercise for the management of cancer–related fatigue in adults. Cochrane Database Syst Rev. 2008(2):CD006145.
17. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2011;118(15):3766-3775.
18. Mesidor M, Kunthur A, Mehta P, et al. Mindfulness and cancer. Paper presented at: Annual meeting of Association of VA Hematologists/Oncologists; October 2015; Washington, DC.
19. Goncalves P, Groninger H. Sexual dysfunction in cancer patients and survivors #293. J Palliat Med. 2015 [Epub ahead of print].
20. Pargeon KL, Hailey BJ. Barriers to effective pain management: a review of the literature. J Pain Symptom Manage. 1999;18(5):358-368.
21. Heneghan C. The future of veteran care? Health Care Journal of Little Rock. May/June. http://www.healthcarejournallr.com/the-journal/contents-index/features/567-integrative-medicine.html. Accessed July 10, 2015.
22. Mayer DK, Birken SA, Check DK, Chen RC. Summing it up: an integrated
review of studies of cancer survivorship care plans (2006-2013). Cancer. 2015;121(7):978-996.
23. Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol. 2014;32(15):1578-1585.
With advances in treatment, supportive care, and early diagnosis, the prevalence of cancer is increasing. An individual is considered a cancer survivor from the time of diagnosis to the end of his or her life.1 Although many patients with cancer are cured, they experience various short-term and long-term effects of cancer treatment, a high risk of recurrence and second cancer, anxiety, chronic pain, fatigue, depression, sexual dysfunction, and infertility.1
As of January 1, 2014, there were about 14.5 million cancer survivors in the U.S. The most common cancers in this population include prostate (43%), colon and rectal (9%), and melanoma (8%) in males; breast (41%), uterine corpus (8%), and colon and rectal (8%) in females.2 This estimate does not include noninvasive cancers, but does include bladder, basal cell, and squamous cell skin cancers. By January 1, 2024, the population of cancer survivors is predicted to increase to almost 19 million: 9.3 million males and 9.6 million females.1 Most of the cancer survivors (64%) were diagnosed 5 or more years ago, and 15% were diagnosed 20 or more years ago. Nearly half (46%) of cancer survivors are aged ≥ 70 years, and only 5% are aged < 40 years.2
Moye and colleagues reported that 524,052 (11%) of veterans treated in 2007 were cancer survivors.3 The most common types of cancers among these veterans were prostate, skin (nonmelanoma), and colorectal cancers. Compared with the general population of cancer survivors in the SEER database, veteran survivors were older.3 Because of the increasing prevalence of cancer survivors, greater attention is focused on long-term complications of cancer treatment. Recent studies have demonstrated that cancer survivors are less likely to receive general preventive care and care associated with noncancer-related medical conditions than are individuals without cancer.4
Survivorship Care Components
In 2005, the Institute of Medicine (IOM) published From Cancer Patient to Cancer Survivor: Lost to Transition.5 The report addresses 4 essential components of survivorship care: (1) prevention of recurrence, new cancers, and other late effects; (2) surveillance for cancer spread, recurrence, second cancers, and medical and psychosocial adverse events (AEs); (3) interventions for consequences of cancer and its treatment (medical problems, symptoms, psychological distress experienced by cancer survivors and their caregivers, and concerns related to employment, insurance, and disability); and (4) coordination between the specialist and primary care providers (PCPs) to ensure that all the survivors’ health needs are met.5
Cancer Treatment Summary
To ensure better transition, the IOM recommended that survivorship plans be made with a summary of treatment provided by the primary oncologist who treated the patient, to improve communication among all health care providers and between the providers and the patient.5 The summary should include the date of diagnosis; diagnostic tests; stage of diagnosis; a medical, surgical, and radiation treatment summary; and a detailed follow-up care plan. The IOM also recommends preventive practices to maintain health and well-being; information on legal protection regarding employment and health insurance; the availability of psychosocial services in the community; and screening for psychosocial distress in cancer survivors.3,6 However, studies have shown that gaps in adherence with IOM recommendations exist even in dedicated survivorship centers.7,8
The American Society of Clinical Oncology (ASCO) and organizations such as Livestrong also have developed templates for survivorship care plans (SCPs). But there has been limited success in implementing SCPs.7,8 The American College of Surgeons Commission on Cancer (CoC) Standard 3.3 is scheduled to be implemented in 2015.9 The standard is expected to require that a cancer care committee develop and implement a process to disseminate a comprehensive care summary and follow-up plan for cancer survivors.9 To address this need, ASCO has formed a joint work group for improving cancer survivorship and a new version of an SCP template. This group recommended including contact information for the oncology providers who administered the treatment; basic diagnostic and staging information; and information on surgery, radiation therapy, systemic therapy (both chemotherapy and biologic therapy), and ongoing significant toxicities, including dates (Table 1).10
The ASCO also developed a follow-up care plan that includes a surveillance plan to detect recurrence and late AEs; interventions to manage ongoing problems resulting from cancer and its treatment; and age- and sex-appropriate health care, including cancer screening and general health promotion. It also recommended that the follow-up care plan should include a schedule of clinic visits in a table format, surveillance care testing to detect recurrence and second primary cancers, such as early breast cancer screening for women who underwent chest radiation for Hodgkin lymphoma. The person responsible for ordering these screening test should be included in the follow-up care plan.10 In addition, ASCO developed a cancer survivorship compendium, which included not only tools and resources, but also different models for cancer survivorship care.11 In addition, ASCO developed a Quality Oncology Practice Initiative (QOPI) to assess the quality of survivorship programs.9
The Central Arkansas Veterans Healthcare System (CAVHS) adopted National Comprehensive Cancer Network (NCCN), ASCO, and National Cancer Institute (NCI) guidelines and developed a template for a cancer treatment summary that includes the cancer type; grade; staging; and date of diagnosis and duration of treatment, including chemotherapy, surgery, and radiation with a disease-specific follow-up care plan for each type of cancer. The treatment summary is a useful tool to communicate a patient’s treatment and disease status to PCPs and patients.
Models of Care
Eight models for delivering survivorship care have been developed by ASCO11:
- Oncologist Specialist Care: Care occurs as a continuation in the oncology setting
- Multidisciplinary Survivor Clinic: Different specialists provide care
- General Survivorship Clinic: Care is provided by a physician or advanced care provider (APN) and implemented at a cancer center or private practice
- Consultative Survivorship Clinic: Initial follow-up is provided in an oncology setting with an eventual transition to a PCP; patients may be directed to the cancer center for needed services
- Integrated Survivorship Clinic: Care is provided by a physician or APN, and the care is coordinated with the PCP and other specialists as needed
- Community Generalist Model: The PCP, APN, or internist within the community provides care
- Shared-Care of Survivor: Care is coordinated and provided by any combination of specialists, PCPs, and nurses and is patient directed
Depending on the patients and the setting, practices can adopt various models to deliver survivorship care.
At CAVHS, cancer survivors are followed by an oncologist for their yearly examinations. This model is an illness model rather than a wellness model. A multidisciplinary clinic model can be initiated at CAVHS with the help of palliative care; complementary and alternative medicine for pain management; psychologists, chaplain services, and social workers for distress management; and coordination of survivorship care.
Implementation Barriers
There are many barriers to implementing SCPs, including time required by the providers to complete SCPs, inadequate reimbursement for the time and resources required to complete SCPs, challenges in coordinating care between survivors and providers, and lack of compatibility of the existing template with the electronic health record (EHR).7-10 A study regarding the barriers to implementation of SCPs, conducted at 14 NCI community cancer centers, demonstrated that the most common barrier is lack of personnel and time required to complete SCPs. The most widely used strategies was the use of a template with prespecified fields and delegating the completion of SCPs to one individual.12
Long-Term Complications for Survivors
Cancer survivors experience the physical and psychosocial effects of cancer treatment and have a very high risk of recurrence and second primary cancers. Common chronic AEs include fatigue, pain, neuropathy, infertility, sexual dysfunction, hypothyroidism, organ dysfunction, and urinary and bowel incontinence. In addition, patients also experience psychological AEs, including anxiety, depression, posttraumatic stress disorder, and sleep disturbances. Because of the AEs, cancer survivors have difficulty obtaining employment and insurance.1,5
Fatigue
Fatigue is the most common AE in cancer survivors.1 It may develop during treatment and persist for years. It is related to chemotherapy, radiation, surgical complications, depression, and insomnia.1 It is underrecognized and often untreated. It is important to assess and treat underlying comorbidities such as anemia, hypothyroidism, pain, depression and insomnia.13,14 Pharmacologic therapy with central nervous system stimulants and antidepressants have not shown any benefit.15 Studies on modafinil and armodafinil are ongoing.15 Exercise, treating underlying depression, sleep hygiene, behavioral and cognitive therapy, and yoga and mindfulness management of distress can help in treating fatigue.13 Meta-analyses showed that physical exercise helped reduce cancerrelated fatigue.15 A randomized controlled trial demonstrated that yoga led to significant improvement in fatigue in breast cancer survivors.16 Hence, it is important for providers to recognize and treat cancer-related fatigue and encourage patients to exercise.17
Psychological Adverse Effects
Cancer survivors also experience psychological AEs such as anxiety and depression because of the cancer diagnosis and the uncertainty of the outcome and the fear of relapse. Veterans may be at a higher risk of psychological AEs because of underlying mental illnesses. Counseling about the disease, psychotherapy interventions, and a mindfulness approach are recommended to treat anxiety and depression.14 The CAVHS cancer program has developed a mindfulness program as a multidisciplinary approach to manage psychological AEs.18
Sexual Dysfunction
Many patients may experience sexual dysfunction and infertility as a result of endocrine treatments, chemotherapy, radiation, and urologic and gynecologic surgeries.1 Over half of prostate cancer and breast cancer survivors report sexual dysfunction. Despite its high prevalence, sexual dysfunction often is not discussed with patients due to reluctance to discuss, lack of training, and lack of a standardized sexuality questionnaire.19 A brief sexual symptom checklist for women can be used as a primary screening tool. It is also recommended to screen for treatment-related infertility. Patients with sexual dysfunction should undergo screening for psychosocial problems such as anxiety, depression, and drug and alcohol use and treatment as these can contribute to sexual dysfunction.1
Vaginal lubricants are recommended to treat vaginal dryness. Vaginal estrogen creams are effective in patients with nonhormone-dependent gynecologic cancer without risk of breast cancer. The FDA approved the selective estrogen receptor modulator ospemifene for treating dyspareunia in postmenopausal women without risk of breast cancer.1 Oral phosphodiesterase-5 inhibitors, such as sildenafil, vacuum erection devices, penile prosthesis, and intracavernous injections, have shown to be effective in treating male erectile dysfunction (ED).19
Cardiovascular risk should be estimated in all patients with ED, because most of these patients will have common risk factors and need to be referred to a cardiologist before treating ED.1
Chronic Pain
Patients may also experience chronic pain as a late complication of chemotherapy, radiation, and surgery.1 More than one-third of cancer survivors experience pain, and it is often ineffectively managed due to lack of training, fear of AEs, and addiction.20 The goals of pain management are to increase comfort and improve quality of life. Short-acting and long-acting opioids remain an important treatment for cancer pain. Drug selection should be guided by previous exposure and comorbidities.15 Opioid therapy AEs, such as constipation, sleep apnea, and hypogonadism, should be recognized and treated. Antidepressants and anticonvulsants, such as gabapentin and pregabalin, may be used to manage neuropathic pain. A multidisciplinary approach using pharmacologic therapy, psychosocial therapy, behavioral interventions, exercise, and physical therapy is recommended.15 Patients with refractory pain are treated with interventional approaches, such as a neuronal blockade.
Survivorship Resources
Survivorship resources were developed by various cancer societies, such as NCCN, ASCO, Livestrong, and the Children’s Oncology Group. These resources help providers develop and deliver a quality survivorship care program (Table 2).5 The NCCN provides a template to help create cancer treatment summaries; guidelines for follow-up care for different cancers; and information for patients on legal and employment issues, smoking cessation, nutrition, and weight loss. In addition, the NCCN provides tools to assess anthracyclineinduced cardiac toxicity, anxiety, depression, cognitive dysfunction, pain, sexual dysfunction, and fatigue.
The CAVHS has several resources to develop a survivor ship progr am: It offers complementary and alternative therapy (a multidisciplinary approach) to help veterans with chronic pain and psychological distress. This resource incorporates acupuncture, yoga, hypnotherapy, biofeedback, mind-body approaches, stress management, nutritional counseling, physical therapy, and other mental and behavioral health support.
In addition, VA is implementing a patient-centered care and cultural transformation program to help veterans establish a relationship with their health care providers.21 The patient-centered approach along with the complementary and alternative therapy program, mindfulness program, and telehealth exercise motivation program can be incorporated to develop a multidisciplinary survivorship program.
Survivorship Research
Survivorship is an emerging field, and there is need for research to improve the implementation of quality survivorship care. Cancer survivorship research encompasses the physical, psychosocial, and economic sequelae of cancer diagnosis and its treatment among both pediatric and adult survivors of cancer. It also includes issues related to health care delivery, access, and follow-up care.
Mayer and colleagues reported that there were 42 published studies of SCPs in adult cancer survivors.22 Eleven studies reported that SCP use was limited and that < 25% of oncology providers never used an SCP. Research also showed that oncologists who have had training in long-term AEs of cancer and those who have used the EHRs were more likely to use SCPs.23 An integrated review of studies of SCPs recommended areas of research for survivorship. Thus the quality and quantity of SCP research are limited, and there is more need for quality research in survivorship to endorse the effective use of SCPs.
Conclusion
Cancer patients experience various AEs of cancer treatment, psychosocial distress, and can be lost to follow-up. It is important for health care providers to recognize and screen for the complications and provide SCPs, which includes a treatment summary and follow-up care to communicate and coordinate quality cancer survivorship care. Providers can use tools and resources developed by various organizations to develop and implement SCPs. The CoC surveys and accredits the cancer programs for quality measures, and has developed a new standard to provide SCPs for all patients effective 2015.
The CAVHS has already adopted this standard and has developed a template to complete a treatment summary and follow-up care planning in the EHR. The cancer treatment summary is given to the patient and is available to the PCP for review. There is also a need for more quality research in survivorship.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
With advances in treatment, supportive care, and early diagnosis, the prevalence of cancer is increasing. An individual is considered a cancer survivor from the time of diagnosis to the end of his or her life.1 Although many patients with cancer are cured, they experience various short-term and long-term effects of cancer treatment, a high risk of recurrence and second cancer, anxiety, chronic pain, fatigue, depression, sexual dysfunction, and infertility.1
As of January 1, 2014, there were about 14.5 million cancer survivors in the U.S. The most common cancers in this population include prostate (43%), colon and rectal (9%), and melanoma (8%) in males; breast (41%), uterine corpus (8%), and colon and rectal (8%) in females.2 This estimate does not include noninvasive cancers, but does include bladder, basal cell, and squamous cell skin cancers. By January 1, 2024, the population of cancer survivors is predicted to increase to almost 19 million: 9.3 million males and 9.6 million females.1 Most of the cancer survivors (64%) were diagnosed 5 or more years ago, and 15% were diagnosed 20 or more years ago. Nearly half (46%) of cancer survivors are aged ≥ 70 years, and only 5% are aged < 40 years.2
Moye and colleagues reported that 524,052 (11%) of veterans treated in 2007 were cancer survivors.3 The most common types of cancers among these veterans were prostate, skin (nonmelanoma), and colorectal cancers. Compared with the general population of cancer survivors in the SEER database, veteran survivors were older.3 Because of the increasing prevalence of cancer survivors, greater attention is focused on long-term complications of cancer treatment. Recent studies have demonstrated that cancer survivors are less likely to receive general preventive care and care associated with noncancer-related medical conditions than are individuals without cancer.4
Survivorship Care Components
In 2005, the Institute of Medicine (IOM) published From Cancer Patient to Cancer Survivor: Lost to Transition.5 The report addresses 4 essential components of survivorship care: (1) prevention of recurrence, new cancers, and other late effects; (2) surveillance for cancer spread, recurrence, second cancers, and medical and psychosocial adverse events (AEs); (3) interventions for consequences of cancer and its treatment (medical problems, symptoms, psychological distress experienced by cancer survivors and their caregivers, and concerns related to employment, insurance, and disability); and (4) coordination between the specialist and primary care providers (PCPs) to ensure that all the survivors’ health needs are met.5
Cancer Treatment Summary
To ensure better transition, the IOM recommended that survivorship plans be made with a summary of treatment provided by the primary oncologist who treated the patient, to improve communication among all health care providers and between the providers and the patient.5 The summary should include the date of diagnosis; diagnostic tests; stage of diagnosis; a medical, surgical, and radiation treatment summary; and a detailed follow-up care plan. The IOM also recommends preventive practices to maintain health and well-being; information on legal protection regarding employment and health insurance; the availability of psychosocial services in the community; and screening for psychosocial distress in cancer survivors.3,6 However, studies have shown that gaps in adherence with IOM recommendations exist even in dedicated survivorship centers.7,8
The American Society of Clinical Oncology (ASCO) and organizations such as Livestrong also have developed templates for survivorship care plans (SCPs). But there has been limited success in implementing SCPs.7,8 The American College of Surgeons Commission on Cancer (CoC) Standard 3.3 is scheduled to be implemented in 2015.9 The standard is expected to require that a cancer care committee develop and implement a process to disseminate a comprehensive care summary and follow-up plan for cancer survivors.9 To address this need, ASCO has formed a joint work group for improving cancer survivorship and a new version of an SCP template. This group recommended including contact information for the oncology providers who administered the treatment; basic diagnostic and staging information; and information on surgery, radiation therapy, systemic therapy (both chemotherapy and biologic therapy), and ongoing significant toxicities, including dates (Table 1).10
The ASCO also developed a follow-up care plan that includes a surveillance plan to detect recurrence and late AEs; interventions to manage ongoing problems resulting from cancer and its treatment; and age- and sex-appropriate health care, including cancer screening and general health promotion. It also recommended that the follow-up care plan should include a schedule of clinic visits in a table format, surveillance care testing to detect recurrence and second primary cancers, such as early breast cancer screening for women who underwent chest radiation for Hodgkin lymphoma. The person responsible for ordering these screening test should be included in the follow-up care plan.10 In addition, ASCO developed a cancer survivorship compendium, which included not only tools and resources, but also different models for cancer survivorship care.11 In addition, ASCO developed a Quality Oncology Practice Initiative (QOPI) to assess the quality of survivorship programs.9
The Central Arkansas Veterans Healthcare System (CAVHS) adopted National Comprehensive Cancer Network (NCCN), ASCO, and National Cancer Institute (NCI) guidelines and developed a template for a cancer treatment summary that includes the cancer type; grade; staging; and date of diagnosis and duration of treatment, including chemotherapy, surgery, and radiation with a disease-specific follow-up care plan for each type of cancer. The treatment summary is a useful tool to communicate a patient’s treatment and disease status to PCPs and patients.
Models of Care
Eight models for delivering survivorship care have been developed by ASCO11:
- Oncologist Specialist Care: Care occurs as a continuation in the oncology setting
- Multidisciplinary Survivor Clinic: Different specialists provide care
- General Survivorship Clinic: Care is provided by a physician or advanced care provider (APN) and implemented at a cancer center or private practice
- Consultative Survivorship Clinic: Initial follow-up is provided in an oncology setting with an eventual transition to a PCP; patients may be directed to the cancer center for needed services
- Integrated Survivorship Clinic: Care is provided by a physician or APN, and the care is coordinated with the PCP and other specialists as needed
- Community Generalist Model: The PCP, APN, or internist within the community provides care
- Shared-Care of Survivor: Care is coordinated and provided by any combination of specialists, PCPs, and nurses and is patient directed
Depending on the patients and the setting, practices can adopt various models to deliver survivorship care.
At CAVHS, cancer survivors are followed by an oncologist for their yearly examinations. This model is an illness model rather than a wellness model. A multidisciplinary clinic model can be initiated at CAVHS with the help of palliative care; complementary and alternative medicine for pain management; psychologists, chaplain services, and social workers for distress management; and coordination of survivorship care.
Implementation Barriers
There are many barriers to implementing SCPs, including time required by the providers to complete SCPs, inadequate reimbursement for the time and resources required to complete SCPs, challenges in coordinating care between survivors and providers, and lack of compatibility of the existing template with the electronic health record (EHR).7-10 A study regarding the barriers to implementation of SCPs, conducted at 14 NCI community cancer centers, demonstrated that the most common barrier is lack of personnel and time required to complete SCPs. The most widely used strategies was the use of a template with prespecified fields and delegating the completion of SCPs to one individual.12
Long-Term Complications for Survivors
Cancer survivors experience the physical and psychosocial effects of cancer treatment and have a very high risk of recurrence and second primary cancers. Common chronic AEs include fatigue, pain, neuropathy, infertility, sexual dysfunction, hypothyroidism, organ dysfunction, and urinary and bowel incontinence. In addition, patients also experience psychological AEs, including anxiety, depression, posttraumatic stress disorder, and sleep disturbances. Because of the AEs, cancer survivors have difficulty obtaining employment and insurance.1,5
Fatigue
Fatigue is the most common AE in cancer survivors.1 It may develop during treatment and persist for years. It is related to chemotherapy, radiation, surgical complications, depression, and insomnia.1 It is underrecognized and often untreated. It is important to assess and treat underlying comorbidities such as anemia, hypothyroidism, pain, depression and insomnia.13,14 Pharmacologic therapy with central nervous system stimulants and antidepressants have not shown any benefit.15 Studies on modafinil and armodafinil are ongoing.15 Exercise, treating underlying depression, sleep hygiene, behavioral and cognitive therapy, and yoga and mindfulness management of distress can help in treating fatigue.13 Meta-analyses showed that physical exercise helped reduce cancerrelated fatigue.15 A randomized controlled trial demonstrated that yoga led to significant improvement in fatigue in breast cancer survivors.16 Hence, it is important for providers to recognize and treat cancer-related fatigue and encourage patients to exercise.17
Psychological Adverse Effects
Cancer survivors also experience psychological AEs such as anxiety and depression because of the cancer diagnosis and the uncertainty of the outcome and the fear of relapse. Veterans may be at a higher risk of psychological AEs because of underlying mental illnesses. Counseling about the disease, psychotherapy interventions, and a mindfulness approach are recommended to treat anxiety and depression.14 The CAVHS cancer program has developed a mindfulness program as a multidisciplinary approach to manage psychological AEs.18
Sexual Dysfunction
Many patients may experience sexual dysfunction and infertility as a result of endocrine treatments, chemotherapy, radiation, and urologic and gynecologic surgeries.1 Over half of prostate cancer and breast cancer survivors report sexual dysfunction. Despite its high prevalence, sexual dysfunction often is not discussed with patients due to reluctance to discuss, lack of training, and lack of a standardized sexuality questionnaire.19 A brief sexual symptom checklist for women can be used as a primary screening tool. It is also recommended to screen for treatment-related infertility. Patients with sexual dysfunction should undergo screening for psychosocial problems such as anxiety, depression, and drug and alcohol use and treatment as these can contribute to sexual dysfunction.1
Vaginal lubricants are recommended to treat vaginal dryness. Vaginal estrogen creams are effective in patients with nonhormone-dependent gynecologic cancer without risk of breast cancer. The FDA approved the selective estrogen receptor modulator ospemifene for treating dyspareunia in postmenopausal women without risk of breast cancer.1 Oral phosphodiesterase-5 inhibitors, such as sildenafil, vacuum erection devices, penile prosthesis, and intracavernous injections, have shown to be effective in treating male erectile dysfunction (ED).19
Cardiovascular risk should be estimated in all patients with ED, because most of these patients will have common risk factors and need to be referred to a cardiologist before treating ED.1
Chronic Pain
Patients may also experience chronic pain as a late complication of chemotherapy, radiation, and surgery.1 More than one-third of cancer survivors experience pain, and it is often ineffectively managed due to lack of training, fear of AEs, and addiction.20 The goals of pain management are to increase comfort and improve quality of life. Short-acting and long-acting opioids remain an important treatment for cancer pain. Drug selection should be guided by previous exposure and comorbidities.15 Opioid therapy AEs, such as constipation, sleep apnea, and hypogonadism, should be recognized and treated. Antidepressants and anticonvulsants, such as gabapentin and pregabalin, may be used to manage neuropathic pain. A multidisciplinary approach using pharmacologic therapy, psychosocial therapy, behavioral interventions, exercise, and physical therapy is recommended.15 Patients with refractory pain are treated with interventional approaches, such as a neuronal blockade.
Survivorship Resources
Survivorship resources were developed by various cancer societies, such as NCCN, ASCO, Livestrong, and the Children’s Oncology Group. These resources help providers develop and deliver a quality survivorship care program (Table 2).5 The NCCN provides a template to help create cancer treatment summaries; guidelines for follow-up care for different cancers; and information for patients on legal and employment issues, smoking cessation, nutrition, and weight loss. In addition, the NCCN provides tools to assess anthracyclineinduced cardiac toxicity, anxiety, depression, cognitive dysfunction, pain, sexual dysfunction, and fatigue.
The CAVHS has several resources to develop a survivor ship progr am: It offers complementary and alternative therapy (a multidisciplinary approach) to help veterans with chronic pain and psychological distress. This resource incorporates acupuncture, yoga, hypnotherapy, biofeedback, mind-body approaches, stress management, nutritional counseling, physical therapy, and other mental and behavioral health support.
In addition, VA is implementing a patient-centered care and cultural transformation program to help veterans establish a relationship with their health care providers.21 The patient-centered approach along with the complementary and alternative therapy program, mindfulness program, and telehealth exercise motivation program can be incorporated to develop a multidisciplinary survivorship program.
Survivorship Research
Survivorship is an emerging field, and there is need for research to improve the implementation of quality survivorship care. Cancer survivorship research encompasses the physical, psychosocial, and economic sequelae of cancer diagnosis and its treatment among both pediatric and adult survivors of cancer. It also includes issues related to health care delivery, access, and follow-up care.
Mayer and colleagues reported that there were 42 published studies of SCPs in adult cancer survivors.22 Eleven studies reported that SCP use was limited and that < 25% of oncology providers never used an SCP. Research also showed that oncologists who have had training in long-term AEs of cancer and those who have used the EHRs were more likely to use SCPs.23 An integrated review of studies of SCPs recommended areas of research for survivorship. Thus the quality and quantity of SCP research are limited, and there is more need for quality research in survivorship to endorse the effective use of SCPs.
Conclusion
Cancer patients experience various AEs of cancer treatment, psychosocial distress, and can be lost to follow-up. It is important for health care providers to recognize and screen for the complications and provide SCPs, which includes a treatment summary and follow-up care to communicate and coordinate quality cancer survivorship care. Providers can use tools and resources developed by various organizations to develop and implement SCPs. The CoC surveys and accredits the cancer programs for quality measures, and has developed a new standard to provide SCPs for all patients effective 2015.
The CAVHS has already adopted this standard and has developed a template to complete a treatment summary and follow-up care planning in the EHR. The cancer treatment summary is given to the patient and is available to the PCP for review. There is also a need for more quality research in survivorship.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: survivorship. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. Updated February 27, 2015. Accessed July 20, 2015.
2. American Cancer Society. Cancer treatment and survivorship facts and figures 2014-2015. American Cancer Society Website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf. Accessed July 8, 2015.
3. Moye J, Schuster J, Latini D, Naik A. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
4. Snyder CF, Frick KD, Kantisiper ME, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27(7):1054-1061.
5. Hewitt M, Greenfield S, Stovall E, eds; Committee on Cancer Survivorship: Improving Care and Quality of Life, Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2005.
6. Adler NE, Page AEK, eds; Institute of Medicine (US) Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press; 2008. http://www.ncbi.nlm.nih.gov/books/NBK4015/. Accessed July 10, 2015.
7. Stricker CT, Jacobs LA, Risendal B, et al. Survivorship care planning after the institute of medicine recommendations: how are we faring? J Cancer Surviv. 2011;5(4):358-370.
8. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
9. American College of Surgeons Commission on Cancer. Cancer Program Standards 2012: Ensuring Patient-Centered Care.V1.2.1. Chicago, IL: American College of Surgeons;2012. https://www.facs.org/~/media/files/quality%20programs/cancer
/coc/programstandards2012.ashx. Accessed July 8, 2015.
10. Mayer DK, Nekhlyudov L, Snyder CF, Merill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
11. American Society of Clinical Oncology. ASCO cancer survivorship compendium. American Society of Clinical Oncology Website. http://www.asco.org/practice-research/asco-cancer-survivorship-compendium. Accessed July 10, 2015.
12. Forsythe LP, Alfano CM, Leach CR, Ganz PA, Stefanek ME, Rowland JH. Who provides psychosocial follow-up care for post-treatment cancer survivors? A survey of medical oncologists and primary care physicians. J Clin Oncol. 2012;30(23):2897-2905.
13. Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following
treatment. Clin J Oncol Nurs. 2014;18(suppl):38-58.
14. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for
adult cancer survivors: highlighing ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015;35:188-194.
15. Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30(30):3687-3696.
16. Cramp F, Daniel J. Exercise for the management of cancer–related fatigue in adults. Cochrane Database Syst Rev. 2008(2):CD006145.
17. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2011;118(15):3766-3775.
18. Mesidor M, Kunthur A, Mehta P, et al. Mindfulness and cancer. Paper presented at: Annual meeting of Association of VA Hematologists/Oncologists; October 2015; Washington, DC.
19. Goncalves P, Groninger H. Sexual dysfunction in cancer patients and survivors #293. J Palliat Med. 2015 [Epub ahead of print].
20. Pargeon KL, Hailey BJ. Barriers to effective pain management: a review of the literature. J Pain Symptom Manage. 1999;18(5):358-368.
21. Heneghan C. The future of veteran care? Health Care Journal of Little Rock. May/June. http://www.healthcarejournallr.com/the-journal/contents-index/features/567-integrative-medicine.html. Accessed July 10, 2015.
22. Mayer DK, Birken SA, Check DK, Chen RC. Summing it up: an integrated
review of studies of cancer survivorship care plans (2006-2013). Cancer. 2015;121(7):978-996.
23. Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol. 2014;32(15):1578-1585.
1. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: survivorship. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. Updated February 27, 2015. Accessed July 20, 2015.
2. American Cancer Society. Cancer treatment and survivorship facts and figures 2014-2015. American Cancer Society Website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf. Accessed July 8, 2015.
3. Moye J, Schuster J, Latini D, Naik A. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
4. Snyder CF, Frick KD, Kantisiper ME, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27(7):1054-1061.
5. Hewitt M, Greenfield S, Stovall E, eds; Committee on Cancer Survivorship: Improving Care and Quality of Life, Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2005.
6. Adler NE, Page AEK, eds; Institute of Medicine (US) Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press; 2008. http://www.ncbi.nlm.nih.gov/books/NBK4015/. Accessed July 10, 2015.
7. Stricker CT, Jacobs LA, Risendal B, et al. Survivorship care planning after the institute of medicine recommendations: how are we faring? J Cancer Surviv. 2011;5(4):358-370.
8. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
9. American College of Surgeons Commission on Cancer. Cancer Program Standards 2012: Ensuring Patient-Centered Care.V1.2.1. Chicago, IL: American College of Surgeons;2012. https://www.facs.org/~/media/files/quality%20programs/cancer
/coc/programstandards2012.ashx. Accessed July 8, 2015.
10. Mayer DK, Nekhlyudov L, Snyder CF, Merill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
11. American Society of Clinical Oncology. ASCO cancer survivorship compendium. American Society of Clinical Oncology Website. http://www.asco.org/practice-research/asco-cancer-survivorship-compendium. Accessed July 10, 2015.
12. Forsythe LP, Alfano CM, Leach CR, Ganz PA, Stefanek ME, Rowland JH. Who provides psychosocial follow-up care for post-treatment cancer survivors? A survey of medical oncologists and primary care physicians. J Clin Oncol. 2012;30(23):2897-2905.
13. Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following
treatment. Clin J Oncol Nurs. 2014;18(suppl):38-58.
14. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for
adult cancer survivors: highlighing ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015;35:188-194.
15. Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30(30):3687-3696.
16. Cramp F, Daniel J. Exercise for the management of cancer–related fatigue in adults. Cochrane Database Syst Rev. 2008(2):CD006145.
17. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2011;118(15):3766-3775.
18. Mesidor M, Kunthur A, Mehta P, et al. Mindfulness and cancer. Paper presented at: Annual meeting of Association of VA Hematologists/Oncologists; October 2015; Washington, DC.
19. Goncalves P, Groninger H. Sexual dysfunction in cancer patients and survivors #293. J Palliat Med. 2015 [Epub ahead of print].
20. Pargeon KL, Hailey BJ. Barriers to effective pain management: a review of the literature. J Pain Symptom Manage. 1999;18(5):358-368.
21. Heneghan C. The future of veteran care? Health Care Journal of Little Rock. May/June. http://www.healthcarejournallr.com/the-journal/contents-index/features/567-integrative-medicine.html. Accessed July 10, 2015.
22. Mayer DK, Birken SA, Check DK, Chen RC. Summing it up: an integrated
review of studies of cancer survivorship care plans (2006-2013). Cancer. 2015;121(7):978-996.
23. Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol. 2014;32(15):1578-1585.
Castleman Disease
Castleman disease (CD) is a rare nonclonal lymphoproliferative disorder, also known as angiofollicular lymph-node hyperplasia or giant node hyperplasia. It was first reported in 1954 and in 1956 described by Benjamin Castleman, MD, in a case series of localized mediastinal lymph-node hyperplasia.1 Unicentric Castleman (UCD) disease presents as a localized disease affecting a single lymph node/lymph node chain. Multicentric Castleman disease (MCD) is a more widespread or generalized disease (Table 1). About 4,000 to 6,000 new cases of CD are diagnosed per year of which about 20% to 25% cases are MCD. The estimated incidence rate for CD has recently been calculated as 21 to 25 per million person-years, or about 6,000 new cases annually.2
The clinical presentation of CD often overlaps with autoimmune, infectious, or other malignant diseases. The diagnosis is confirmed by a biopsy of the affected lymph-node tissue. Interleukin-6 (IL-6) and a viral analog of IL-6 play major role in the pathogenesis by stimulating a widespread inflammatory response that results in systemic manifestations. It is often associated with HIV and human herpesvirus-8 (HHV-8) infections. Castleman disease is histologically characterized into the hyaline vascular variant, the plasma-cell variant, and the mixed form. The plasmablastic variety is associated with HIV and HHV-8 infections. The prognosis ranges from good in UCD (91% overall survival [OS] at 5 y) to poor in MCD (65% OS at 5 y).3
Treatment options range from local surgical excision to systemic treatments. Newer therapies include monoclonal antibodies against both IL-6 and CD20 and a few other targets in the inflammatory cascade. This article discusses the updated approach to diagnosis and management of CD.
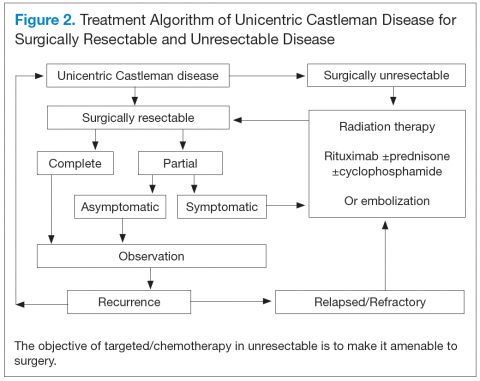
Unicentric Castleman Disease
Unicentric CD is more common than MCD, presents as a localized lymph node or chain involvement, and is generally diagnosed in the third or fourth decade of life but has been reported in children. The presenting symptoms of UCD vary by site. It presents as nontender lymphadenopathy when confined to peripheral lymph nodes, whereas respiratory symptoms or bowel obstruction may be seen with lymphadenopathy in the chest/mediastinum, neck, or abdomen. The systemic
symptoms, such as fever, night sweats, and weight loss, are uncommon.
Dysplastic follicular dendritic cells characterize UCD. Histologically, it is usually classified as hyaline vascular disease with the follicles comprising small lymphocytes
and dendritic cells forming concentric rings with prominent vascularity.4,5 No association with HIV or HHV-8 has been seen.
Unicentric CD is often amenable to resection, and a complete cure can be achieved.6 Partial resection may be attempted when complete resection is not possible. Radiation therapy is offered for unresectable disease.7 In patients who are not candidates for any intervention, close long-term follow-up is recommended unless patients are symptomatic, in which case systemic treatment should be considered.
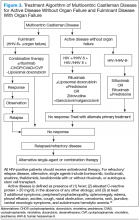
Multicentric Castleman Disease
The more widespread MCD is generally diagnosed in the fifth or sixth decade of life. It is more aggressive than UCD and presents a wide spectrum of symptoms and abnormal laboratory findings (Table 2).8
It is histologically classified into (a) plasmablastic or HHV-8 associated: It is often seen in patients with MCD infected with HIV, which can give rise to large B-cell lymphoma, known as HHV-8 plasmablastic lymphoma9; (b) plasmacytic variant has marked paracortical plasmacytosis with retained nodal architecture10; and (c) mixed MCD has abundant plasma cells with features similar to those of the hyaline-vascular variant.
Most patients with HIV-associated MCD are co-infected with HHV-8. The HHV-8 infection is also present in about 50% of HIV-negative cases.11 The incidence of HIVassociated MCD is increasing in the highly active antiretroviral therapy (HAART) era secondary to improved survival of patients infected with HIV.12 To diagnose active HIV MCD, the French Agence Nationale de Recherche sur le SIDA 117 CastlemaB trial group has described criteria based on the clinical symptoms, including fever, a raised serum C-reactive protein > 20 mg/L without any other cause, and 3 of 12 additional clinical findings described as peripheral lymphadenopathy, splenomegaly, ascites, edema, pleural effusion, cough, nasal obstruction, xerostomia, rash, central neurologic symptoms, jaundice, and autoimmune hemolytic anemia.13 The reported 2-year survival of patients who are HIV-negative is 97% compared with HIV-positive cases at 67%.14
Idiopathic MCD is diagnosed when there is no evidence of any underlying infectious, autoimmune, and neoplastic process.15
Patients with MCD are at an increased risk of developing non-Hodgkin and Hodgkin lymphoma, Kaposi sarcoma, primary effusion lymphoma, and follicular dendritic
cell sarcoma. POEMS (peripheral neuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome and paraneoplastic disease, such as paraneoplastic pemphigus myasthenia gravis, may be commonly diagnosed concurrently or sequentially with MCD.16-20
The disease course of MCD ranges from indolent to rapidly progressive, and its 5-year OS is about 65%. When associated with POEMS syndrome, the 5-year survival was estimated to be 90% with the osteosclerotic variant and 27% without osteosclerotic lesions.3 Treatment options for MCD include systemic chemotherapy, including antiviral therapy for HHV-8 positive and HAART for HIV positive and newer monoclonal antibody therapies targeting CD20 or IL-6.
Pathophysiology
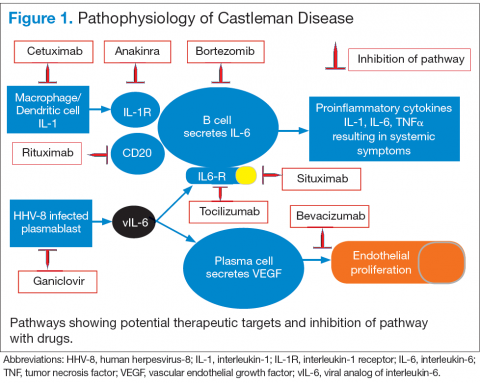
Interleukin-6 plays an important role for inflammation in both UCD and MCD (Figure 1). There is dysregulation and overproduction of IL-6, which further stimulates the production of acute-phase reactants, resulting in various systemic manifestations.15,21,22 There is increased expression of IL-1 and IL-6, upregulation of IL-6 secondary to interaction of IL-1 with nuclear factor-kappa B (NF-kappa B), thus stimulating B-cell proliferation. IL-6 binding to IL-6 receptor (IL6-R) results in downstream activation of transcription Janus kinases/signal transducers and activators of the transcription pathway. This promotes the transcription of genes encoding the acute-phase reactant proteins. Hence, interfering with IL-6 transduction by blocking downstream signals are potential therapeutic targets. The mitogen-activated protein kinase cascade, the rapidly accelerated fibrosarcoma kinases, and the overexpression of the endothelial growth factor receptor (EGFR), all contribute to disease pathogenesis by promoting increased B-cell proliferation and vascular EGFR mediated angiogenesis. 23,24
In HHV-8–associated MCD, the virus replicates within lymph node plasmablasts, causing increased production of viral IL-6 analog, human IL-6, and other proinflammatory proteins resulting in B-cell and plasma-cell proliferation, increased vascular endothelial growth factor secretion and angiogenesis.25,26 The HHV-8–infected plasmablasts are marked by variable expression of CD20, and therefore, anti-CD20 is also shown to be an effective treatment. The calmodulin/calcineurin nuclear factor assists in the proliferation of HHV-8, thereby making calcineurin another potential target for the antiviral proliferation.27
Staging
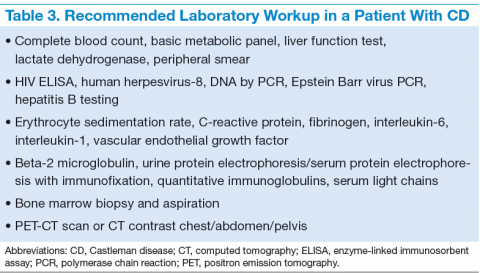
The treatment decisions and prognosis for patients with CD is based on the clinical and histologic staging. The initial workup includes but is not limited to routine laboratory evaluation, imaging, and HIV and HHV-8 testing (Table 3). Routine tests of the levels of cytokines are not recommended. Other relevant tests for known disease associations should be obtained when relevant.
Treatment
Better understanding of the disease process in CD has helped to identify potential therapeutic targets (Figures 2 and 3).
For UCD, surgery is the mainstay of treatment.4,28,29 In surgically unresectable cases, radiation therapy is helpful for local disease control. Alternatively, neoadjuvant
chemotherapy and rituximab are used. Corticosteroids are generally used to treat acute exacerbations and as adjuncts to chemotherapy.
For MCD, the treatment approach depends on the HIV and HHV-8 status of the patient. For patients with HHV-8 infection, both with and without HIV co-infection, antiviral agents, such as ganciclovir, foscarnet, or cidofovir, have shown in vitro activity against HHV-8 but with limited clinical success.30 In patients infected with HIV, the aim of treating with HAART is to control the disease, prevent opportunistic infections, and improve tolerance to chemotherapy.31-33 Rituximab with or without chemotherapy is the standard treatment approach. The additional chemotherapeutic agents are used depending on the presence or absence of organ failure. This approach has improved the OS in HIV-associated MCD.34,35 Treatment with HAART does not decrease the risk of relapse in HIV MCD; therefore, the role of rituximab and antiherpesvirus agents as maintenance therapy has been explored.36 In patients who fail to respond to or relapse rapidly following rituximab monotherapy, the use of either single-agent chemotherapy with or without rituximab or antiherpesvirus therapy with high-dose zidovudine and valganciclovir is recommended.37
The cytotoxic chemotherapy with single agents, such as etoposide, vinblastine, cyclophosphamide, cladribine, chlorambucil, and liposomal doxorubicin, has been used with limited success.22 The combination chemotherapy with cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP) or cyclophosphamide/vincristine/
prednisone (CVP) without rituximab has been shown to achieve durable remissions. Corticosteroids are usually administered as an initial adjunct to chemotherapy or for acute exacerbations. In patients with MCD, regardless of HIV status, the interferon therapy was found to achieve long-term remission.38,39 The interferon therapy
exerts antiviral effects via downregulation of the IL-6R and inhibition of HHV-8 replication. For patients in remission, maintenance therapy with oral valganciclovir is promising.40
Immunomodulators & Targeted Therapies
For unresectable UCD or MCD with organ failure or relapse, the use of alternativesingle-agent or combination chemotherapies with or without rituximab is recommended. Thalidomide has shown some success, probably secondary to disruption of IL-6 production.41 In cases of progression following second-line therapy, bortezomib, antiherpesvirus therapies, or IL-6–directed therapy with siltuximab or tocilizumab should be considered.
Rituximab is a monoclonal chimeric antibody that targets CD20 on B cells, thus leading to B-cell lymphodepletion via activating complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity. As monotherapy, it has been shown to achieve 2-year progression-free survival in 80% of patients.42 In patients with MCD who are HIV positive, rituximab with and without chemotherapy has shown improved overall and disease-free survival of 70% to 80% at 2 years.43
Siltuximab is a chimeric human-mouse monoclonal antibody to IL-6 that has been approved for treatment of patients with MCD who are both HIV negative and HHV-8 negative.44-46 Tocilizumab targets the IL-6R. The antibody has shown improvement in a study in HIVseronegative adults with MCD.47,48
Bortezomib is a proteasome inhibitor that inhibits the NF-kappa B pathway, which induces the expression of numerous proinflammatory proteins, including IL-6. It is recommended for relapsed or refractory disease.49,50
Anakinra is a recombinant IL-1R antagonist that blocks IL-1 effects and controls disease by decreasing IL-6 production.51
Conculsion
There has been significant progress in disease diagnosis and management as more information is available about the incidence, clinical presentation, and pathophysiology of CD. The understanding of the disease pathogenesis and biology has helped to discover multiple potential therapeutic targets. Successful treatment has been achieved through targeting HHV-8 replication, CD20, and IL-6 and anti– IL-6R antibodies. Although surgical resection continues to be the
standard of therapy for UCD, the management of MCD and relapsed or refractory disease continues to evolve. Exploration of various treatment strategies in different clinical presentations is warranted.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S.
Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information
for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to
patients.
Click here to read the digital edition.
1. Castleman B, Iverson L, Menendez VP. Localized mediastinal lymph-node hyperplasia
resembling thymoma. Cancer. 1956;9(4):822-830.
2. Munshi N, Mehra M, van de Velde H, Desai A, Potluri R, Vermeulen J. Use of a claims database to characterize and estimate the incidence rate for Castleman disease. Leuk Lymphoma. 2015;56(5):1252-1260.
3. Dispenzieri A, Armitage JO, Loe MJ, et al. The clinical spectrum of Castleman’s disease. Am J Hematol. 2012;87(11):997-1002.
4. Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29(3):670-683.
5. Cronin DM, Warnke RA. Castleman disease: an update on classification and the
spectrum of associated lesions. Adv Anat Pathol. 2009;16(4):236-246.
6. Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman’s disease: a systematic review of 404 published cases. Ann Surg. 2012;255(4):677-684.
7. Chronowski GM, Ha CS, Wilder RB, Cabanillas F, Manning J, Cox JD. Treatment of unicentric and multicentric Castleman disease and the role of radiotherapy. Cancer. 2001;92(3):670-676.
8. Herrada J, Cabanillas F, Rice L, Manning J, Pugh W. The clinical behavior of localized and multicentric Castleman disease. Ann Intern Med. 1998;128(8):657-662.
9. Dupin N, Diss TL, Kellam P, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95(4):1406-1412.
10. Ferry JA, Harris NL. Atlas of Lymphoid Hyperplasia and Lymphoma. Philadelphia, PA: W.B. Saunders; 1997.
11. Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86(4):1276-1280.
12. Powles T, Stebbing J, Bazeos A, et al. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman’s disease. Ann Oncol. 2009;20(4):775-779.
13. Gérard L, Bérezné A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman’s disease: ANRS 117 CastlemaB Trial. J Clin Oncol. 2007;25(22):3350-3356.
14. Casper C, Teltsch DY, Robinson D Jr, et al. Clinical characteristics and healthcare utilization of patients with multicentric Castleman disease. Br J Haematol. 2015;168(1):82-93.
15. Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123(19):2924-2933.
16. Larroche C, Cacoub P, Soulier J, et al. Castleman’s disease and lymphoma: report of eight cases in HIV-negative patients and literature review. Am J Hematol. 2002;69(2):119-126.
17. Dispenzieri A. POEMS syndrome: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(2):214-223.
18. Andhavarapu S, Jiang L. POEMS syndrome and Castleman disease. Blood. 2013;122(2):159.
19. Bélec L, Mohamed AS, Authier FJ, et al. Human herpesvirus 8 infection in patients with POEMS syndrome-associated multicentric Castleman’s disease. Blood. 1999;93(11):3643-3653.
20. Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcomaassociated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99(7):2331-2336.
21. Yoshizaki K, Matsuda T, Nishimoto N, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74(4):1360-1367.
22. El-Osta HE, Kurzrock R. Castleman’s disease: from basic mechanisms to molecular therapeutics. Oncologist. 2011;16(4):497-511.
23. Akula SM, Ford PW, Whitman AG, et al. B-Raf-dependent expression of vascular endothelial growth factor-A in Kaposi sarcoma-associated herpesvirus-infected human B cells. Blood. 2005;105(11):4516-4522.
24. Sun X, Chang KC, Abruzzo LV, Lai R, Younes A, Jones D. Epidermal growth factor receptor expression in follicular dendritic cells: a shared feature of follicular dendritic cell sarcoma and Castleman’s disease. Hum Pathol. 2003;34(9):835-840.
25. Adam N, Rabe B, Suthaus J, Grötzinger J, Rose-John S, Scheller J. Unraveling viral interleukin-6 binding to gp130 and activation of STAT-signaling pathways independently of the interleukin-6 receptor. J Virol. 2009;83(10):5117-5126.
26. Suda T, Katano H, Delsol G, et al. HHV-8 infection status of AIDS-unrelated and
AIDS-associated multicentric Castleman’s disease. Pathol Int. 2001;51(9):671-679.
27. Zoeteweij JP, Moses AV, Rinderknecht AS, et al. Targeted inhibition of calcineurin signaling blocks calcium-dependent reactivation of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97(8):2374-2380.
28. McCarty MJ, Vukelja SJ, Banks PM, Weiss RB. Angiofollicular lymph node hyperplasia
(Castleman’s disease). Cancer Treat Rev. 1995;21(4):291-310.
29. Bowne WB, Lewis JJ, Filippa DA, et al. The management of unicentric and multicentric Castleman’s disease: a report of 16 cases and a review of the literature. Cancer. 1999;85(3):706-717.
30. Reddy D, Mitsuyasu R. HIV-associated multicentric Castleman disease. Curr Opin Oncol. 2011;23(5):475-481.
31. Aaron L, Lidove O, Yousry C, Roudiere L, Dupont B, Viard JP. Human herpesvirus 8-positive Castleman disease in human immunodeficiency virus-infected patients: the impact of highly active antiretroviral therapy. Clin Infect Dis. 2002;35(7):880-882.
32. Sprinz E, Jeffman M, Liedke P, Putten A, Schwartsmann G. Successful treatment of AIDS-related Castleman’s disease following the administration of highly active antiretroviral therapy (HAART). Ann Oncol. 2004;15(2):356-358.
33. Lee SM, Edwards SG, Chilton DN, Ramsay A, Miller RF. Highly active antiretroviral therapy alone may be an effective treatment for HIV-associated multi-centric Castleman’s disease. Haematologica. 2010;95(11):1979-1981.
34. Bower M. How I treat HIV-associated multicentric Castleman disease. Blood. 2010;116(22):4415-4421.
35. Bower M, Newsom-Davis T, Naresh K, et al. Clinical features and outcome in HIVassociated
multicentric Castleman’s disease. J Clin Oncol. 2011;29(18):2481-2486.
36. Casper C, Nichols WG, Huang ML, Corey L, Wald A. Remission of HHV-8 and HIV-associated multicentric Castleman disease with ganciclovir treatment. Blood. 2004;103(5):1632-1634.
37. Uldrick TS, Polizzotto MN, Aleman K, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood. 2011;117(26):6977-6986.
38. Kumari P, Schechter GP, Saini N, Benator DA. Successful treatment of human immunodeficiency virus-related Castleman’s disease with interferon-alpha. Clin Infect Dis. 2000;31(2):602-604.
39. Nord JA, Karter D. Low dose interferon-alpha therapy for HIV-associated multicentric Castleman’s disease. Int J STD AIDS. 2003;14(1):61-62.
40. Oksenhendler E. HIV-associated multicentric Castleman disease. Curr Opin HIV AIDS. 2009;4(1):16-21.
41. Jung CP, Emmerich B, Goebel FD, Bogner JR. Successful treatment of a patient with HIV-associated multicentric Castleman disease (MCD) with thalidomide. Am J Hematol. 2004;75(3):176-177.
42. Ide M, Kawachi Y, Izumi Y, Kasagi K, Ogino T. Long-term remission in HIV negative patients with multicentric Castleman’s disease using rituximab. Eur J Haematol. 2006;76(2):119-123.
43. Marcelin AG, Aaron L, Mateus C, et al. Rituximab therapy for HIV-associated Castleman disease. Blood. 2003;102(8):2786-2788.
44. Van Rhee F, Fayad L, Voorhees P, et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. J Clin Oncol. 2010;28(23):3701-3708.
45. Wong RS, Casper C, Munshi N, et al. A multicenter, randomized, doubleblind, placebo-controlled study of the efficacy and safety of siltuximab, an antiinterleukin-6 monoclonal antibody, in patients with multicentric Castleman’s disease. Blood. 2013;122(21):505.
46. Van Rhee F, Casper C, Voorhees PM, et al. An open-label, phase 2, multicenter study of the safety of long-term treatment with siltuximab (an anti-interleukin-6 monoclonal antibody) in patients with multicentric Castleman’s disease. Blood. 2013;122(21):1806.
47. Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627-2632.
48. Müzes G, Sipos F, Csomor J, Sréter L. Successful tocilizumab treatment in a patient with human herpesvirus 8-positive and human immunodeficiency virusnegative multicentric Castleman’s disease of plasma cell type nonresponsive to rituximab-CVP therapy. APMIS. 2013;121(7):668-674.
49. Hess G, Wagner V, Kreft A, Heussel CP, Huber C. Effects of bortezomib on proinflammatory cytokine levels and transfusion dependency in a patient with multicentric Castleman disease. Br J Haematol. 2006;134(5):544-545.
50. Sobas MA, Alonso Vence N, Diaz Arias J, Bendaña Lopez A, Fraga Rodriguez M, Bello Lopez JL. Efficacy of bortezomib in refractory form of multicentric Castleman disease associated to poems syndrome (MCD-POEMS variant). Ann Hematol. 2010;89(2):217-219.
51. El-Osta H, Janku F, Kurzrock R. Successful treatment of Castleman’s disease with interleukin-1 receptor antagonist (Anakinra). Mol Cancer Ther. 2010;9(6):1485-1488.
Castleman disease (CD) is a rare nonclonal lymphoproliferative disorder, also known as angiofollicular lymph-node hyperplasia or giant node hyperplasia. It was first reported in 1954 and in 1956 described by Benjamin Castleman, MD, in a case series of localized mediastinal lymph-node hyperplasia.1 Unicentric Castleman (UCD) disease presents as a localized disease affecting a single lymph node/lymph node chain. Multicentric Castleman disease (MCD) is a more widespread or generalized disease (Table 1). About 4,000 to 6,000 new cases of CD are diagnosed per year of which about 20% to 25% cases are MCD. The estimated incidence rate for CD has recently been calculated as 21 to 25 per million person-years, or about 6,000 new cases annually.2
The clinical presentation of CD often overlaps with autoimmune, infectious, or other malignant diseases. The diagnosis is confirmed by a biopsy of the affected lymph-node tissue. Interleukin-6 (IL-6) and a viral analog of IL-6 play major role in the pathogenesis by stimulating a widespread inflammatory response that results in systemic manifestations. It is often associated with HIV and human herpesvirus-8 (HHV-8) infections. Castleman disease is histologically characterized into the hyaline vascular variant, the plasma-cell variant, and the mixed form. The plasmablastic variety is associated with HIV and HHV-8 infections. The prognosis ranges from good in UCD (91% overall survival [OS] at 5 y) to poor in MCD (65% OS at 5 y).3
Treatment options range from local surgical excision to systemic treatments. Newer therapies include monoclonal antibodies against both IL-6 and CD20 and a few other targets in the inflammatory cascade. This article discusses the updated approach to diagnosis and management of CD.
Unicentric Castleman Disease
Unicentric CD is more common than MCD, presents as a localized lymph node or chain involvement, and is generally diagnosed in the third or fourth decade of life but has been reported in children. The presenting symptoms of UCD vary by site. It presents as nontender lymphadenopathy when confined to peripheral lymph nodes, whereas respiratory symptoms or bowel obstruction may be seen with lymphadenopathy in the chest/mediastinum, neck, or abdomen. The systemic
symptoms, such as fever, night sweats, and weight loss, are uncommon.
Dysplastic follicular dendritic cells characterize UCD. Histologically, it is usually classified as hyaline vascular disease with the follicles comprising small lymphocytes
and dendritic cells forming concentric rings with prominent vascularity.4,5 No association with HIV or HHV-8 has been seen.
Unicentric CD is often amenable to resection, and a complete cure can be achieved.6 Partial resection may be attempted when complete resection is not possible. Radiation therapy is offered for unresectable disease.7 In patients who are not candidates for any intervention, close long-term follow-up is recommended unless patients are symptomatic, in which case systemic treatment should be considered.
Multicentric Castleman Disease
The more widespread MCD is generally diagnosed in the fifth or sixth decade of life. It is more aggressive than UCD and presents a wide spectrum of symptoms and abnormal laboratory findings (Table 2).8
It is histologically classified into (a) plasmablastic or HHV-8 associated: It is often seen in patients with MCD infected with HIV, which can give rise to large B-cell lymphoma, known as HHV-8 plasmablastic lymphoma9; (b) plasmacytic variant has marked paracortical plasmacytosis with retained nodal architecture10; and (c) mixed MCD has abundant plasma cells with features similar to those of the hyaline-vascular variant.
Most patients with HIV-associated MCD are co-infected with HHV-8. The HHV-8 infection is also present in about 50% of HIV-negative cases.11 The incidence of HIVassociated MCD is increasing in the highly active antiretroviral therapy (HAART) era secondary to improved survival of patients infected with HIV.12 To diagnose active HIV MCD, the French Agence Nationale de Recherche sur le SIDA 117 CastlemaB trial group has described criteria based on the clinical symptoms, including fever, a raised serum C-reactive protein > 20 mg/L without any other cause, and 3 of 12 additional clinical findings described as peripheral lymphadenopathy, splenomegaly, ascites, edema, pleural effusion, cough, nasal obstruction, xerostomia, rash, central neurologic symptoms, jaundice, and autoimmune hemolytic anemia.13 The reported 2-year survival of patients who are HIV-negative is 97% compared with HIV-positive cases at 67%.14
Idiopathic MCD is diagnosed when there is no evidence of any underlying infectious, autoimmune, and neoplastic process.15
Patients with MCD are at an increased risk of developing non-Hodgkin and Hodgkin lymphoma, Kaposi sarcoma, primary effusion lymphoma, and follicular dendritic
cell sarcoma. POEMS (peripheral neuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome and paraneoplastic disease, such as paraneoplastic pemphigus myasthenia gravis, may be commonly diagnosed concurrently or sequentially with MCD.16-20
The disease course of MCD ranges from indolent to rapidly progressive, and its 5-year OS is about 65%. When associated with POEMS syndrome, the 5-year survival was estimated to be 90% with the osteosclerotic variant and 27% without osteosclerotic lesions.3 Treatment options for MCD include systemic chemotherapy, including antiviral therapy for HHV-8 positive and HAART for HIV positive and newer monoclonal antibody therapies targeting CD20 or IL-6.
Pathophysiology
Interleukin-6 plays an important role for inflammation in both UCD and MCD (Figure 1). There is dysregulation and overproduction of IL-6, which further stimulates the production of acute-phase reactants, resulting in various systemic manifestations.15,21,22 There is increased expression of IL-1 and IL-6, upregulation of IL-6 secondary to interaction of IL-1 with nuclear factor-kappa B (NF-kappa B), thus stimulating B-cell proliferation. IL-6 binding to IL-6 receptor (IL6-R) results in downstream activation of transcription Janus kinases/signal transducers and activators of the transcription pathway. This promotes the transcription of genes encoding the acute-phase reactant proteins. Hence, interfering with IL-6 transduction by blocking downstream signals are potential therapeutic targets. The mitogen-activated protein kinase cascade, the rapidly accelerated fibrosarcoma kinases, and the overexpression of the endothelial growth factor receptor (EGFR), all contribute to disease pathogenesis by promoting increased B-cell proliferation and vascular EGFR mediated angiogenesis. 23,24
In HHV-8–associated MCD, the virus replicates within lymph node plasmablasts, causing increased production of viral IL-6 analog, human IL-6, and other proinflammatory proteins resulting in B-cell and plasma-cell proliferation, increased vascular endothelial growth factor secretion and angiogenesis.25,26 The HHV-8–infected plasmablasts are marked by variable expression of CD20, and therefore, anti-CD20 is also shown to be an effective treatment. The calmodulin/calcineurin nuclear factor assists in the proliferation of HHV-8, thereby making calcineurin another potential target for the antiviral proliferation.27
Staging
The treatment decisions and prognosis for patients with CD is based on the clinical and histologic staging. The initial workup includes but is not limited to routine laboratory evaluation, imaging, and HIV and HHV-8 testing (Table 3). Routine tests of the levels of cytokines are not recommended. Other relevant tests for known disease associations should be obtained when relevant.
Treatment
Better understanding of the disease process in CD has helped to identify potential therapeutic targets (Figures 2 and 3).
For UCD, surgery is the mainstay of treatment.4,28,29 In surgically unresectable cases, radiation therapy is helpful for local disease control. Alternatively, neoadjuvant
chemotherapy and rituximab are used. Corticosteroids are generally used to treat acute exacerbations and as adjuncts to chemotherapy.
For MCD, the treatment approach depends on the HIV and HHV-8 status of the patient. For patients with HHV-8 infection, both with and without HIV co-infection, antiviral agents, such as ganciclovir, foscarnet, or cidofovir, have shown in vitro activity against HHV-8 but with limited clinical success.30 In patients infected with HIV, the aim of treating with HAART is to control the disease, prevent opportunistic infections, and improve tolerance to chemotherapy.31-33 Rituximab with or without chemotherapy is the standard treatment approach. The additional chemotherapeutic agents are used depending on the presence or absence of organ failure. This approach has improved the OS in HIV-associated MCD.34,35 Treatment with HAART does not decrease the risk of relapse in HIV MCD; therefore, the role of rituximab and antiherpesvirus agents as maintenance therapy has been explored.36 In patients who fail to respond to or relapse rapidly following rituximab monotherapy, the use of either single-agent chemotherapy with or without rituximab or antiherpesvirus therapy with high-dose zidovudine and valganciclovir is recommended.37
The cytotoxic chemotherapy with single agents, such as etoposide, vinblastine, cyclophosphamide, cladribine, chlorambucil, and liposomal doxorubicin, has been used with limited success.22 The combination chemotherapy with cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP) or cyclophosphamide/vincristine/
prednisone (CVP) without rituximab has been shown to achieve durable remissions. Corticosteroids are usually administered as an initial adjunct to chemotherapy or for acute exacerbations. In patients with MCD, regardless of HIV status, the interferon therapy was found to achieve long-term remission.38,39 The interferon therapy
exerts antiviral effects via downregulation of the IL-6R and inhibition of HHV-8 replication. For patients in remission, maintenance therapy with oral valganciclovir is promising.40
Immunomodulators & Targeted Therapies
For unresectable UCD or MCD with organ failure or relapse, the use of alternativesingle-agent or combination chemotherapies with or without rituximab is recommended. Thalidomide has shown some success, probably secondary to disruption of IL-6 production.41 In cases of progression following second-line therapy, bortezomib, antiherpesvirus therapies, or IL-6–directed therapy with siltuximab or tocilizumab should be considered.
Rituximab is a monoclonal chimeric antibody that targets CD20 on B cells, thus leading to B-cell lymphodepletion via activating complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity. As monotherapy, it has been shown to achieve 2-year progression-free survival in 80% of patients.42 In patients with MCD who are HIV positive, rituximab with and without chemotherapy has shown improved overall and disease-free survival of 70% to 80% at 2 years.43
Siltuximab is a chimeric human-mouse monoclonal antibody to IL-6 that has been approved for treatment of patients with MCD who are both HIV negative and HHV-8 negative.44-46 Tocilizumab targets the IL-6R. The antibody has shown improvement in a study in HIVseronegative adults with MCD.47,48
Bortezomib is a proteasome inhibitor that inhibits the NF-kappa B pathway, which induces the expression of numerous proinflammatory proteins, including IL-6. It is recommended for relapsed or refractory disease.49,50
Anakinra is a recombinant IL-1R antagonist that blocks IL-1 effects and controls disease by decreasing IL-6 production.51
Conculsion
There has been significant progress in disease diagnosis and management as more information is available about the incidence, clinical presentation, and pathophysiology of CD. The understanding of the disease pathogenesis and biology has helped to discover multiple potential therapeutic targets. Successful treatment has been achieved through targeting HHV-8 replication, CD20, and IL-6 and anti– IL-6R antibodies. Although surgical resection continues to be the
standard of therapy for UCD, the management of MCD and relapsed or refractory disease continues to evolve. Exploration of various treatment strategies in different clinical presentations is warranted.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S.
Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information
for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to
patients.
Click here to read the digital edition.
Castleman disease (CD) is a rare nonclonal lymphoproliferative disorder, also known as angiofollicular lymph-node hyperplasia or giant node hyperplasia. It was first reported in 1954 and in 1956 described by Benjamin Castleman, MD, in a case series of localized mediastinal lymph-node hyperplasia.1 Unicentric Castleman (UCD) disease presents as a localized disease affecting a single lymph node/lymph node chain. Multicentric Castleman disease (MCD) is a more widespread or generalized disease (Table 1). About 4,000 to 6,000 new cases of CD are diagnosed per year of which about 20% to 25% cases are MCD. The estimated incidence rate for CD has recently been calculated as 21 to 25 per million person-years, or about 6,000 new cases annually.2
The clinical presentation of CD often overlaps with autoimmune, infectious, or other malignant diseases. The diagnosis is confirmed by a biopsy of the affected lymph-node tissue. Interleukin-6 (IL-6) and a viral analog of IL-6 play major role in the pathogenesis by stimulating a widespread inflammatory response that results in systemic manifestations. It is often associated with HIV and human herpesvirus-8 (HHV-8) infections. Castleman disease is histologically characterized into the hyaline vascular variant, the plasma-cell variant, and the mixed form. The plasmablastic variety is associated with HIV and HHV-8 infections. The prognosis ranges from good in UCD (91% overall survival [OS] at 5 y) to poor in MCD (65% OS at 5 y).3
Treatment options range from local surgical excision to systemic treatments. Newer therapies include monoclonal antibodies against both IL-6 and CD20 and a few other targets in the inflammatory cascade. This article discusses the updated approach to diagnosis and management of CD.
Unicentric Castleman Disease
Unicentric CD is more common than MCD, presents as a localized lymph node or chain involvement, and is generally diagnosed in the third or fourth decade of life but has been reported in children. The presenting symptoms of UCD vary by site. It presents as nontender lymphadenopathy when confined to peripheral lymph nodes, whereas respiratory symptoms or bowel obstruction may be seen with lymphadenopathy in the chest/mediastinum, neck, or abdomen. The systemic
symptoms, such as fever, night sweats, and weight loss, are uncommon.
Dysplastic follicular dendritic cells characterize UCD. Histologically, it is usually classified as hyaline vascular disease with the follicles comprising small lymphocytes
and dendritic cells forming concentric rings with prominent vascularity.4,5 No association with HIV or HHV-8 has been seen.
Unicentric CD is often amenable to resection, and a complete cure can be achieved.6 Partial resection may be attempted when complete resection is not possible. Radiation therapy is offered for unresectable disease.7 In patients who are not candidates for any intervention, close long-term follow-up is recommended unless patients are symptomatic, in which case systemic treatment should be considered.
Multicentric Castleman Disease
The more widespread MCD is generally diagnosed in the fifth or sixth decade of life. It is more aggressive than UCD and presents a wide spectrum of symptoms and abnormal laboratory findings (Table 2).8
It is histologically classified into (a) plasmablastic or HHV-8 associated: It is often seen in patients with MCD infected with HIV, which can give rise to large B-cell lymphoma, known as HHV-8 plasmablastic lymphoma9; (b) plasmacytic variant has marked paracortical plasmacytosis with retained nodal architecture10; and (c) mixed MCD has abundant plasma cells with features similar to those of the hyaline-vascular variant.
Most patients with HIV-associated MCD are co-infected with HHV-8. The HHV-8 infection is also present in about 50% of HIV-negative cases.11 The incidence of HIVassociated MCD is increasing in the highly active antiretroviral therapy (HAART) era secondary to improved survival of patients infected with HIV.12 To diagnose active HIV MCD, the French Agence Nationale de Recherche sur le SIDA 117 CastlemaB trial group has described criteria based on the clinical symptoms, including fever, a raised serum C-reactive protein > 20 mg/L without any other cause, and 3 of 12 additional clinical findings described as peripheral lymphadenopathy, splenomegaly, ascites, edema, pleural effusion, cough, nasal obstruction, xerostomia, rash, central neurologic symptoms, jaundice, and autoimmune hemolytic anemia.13 The reported 2-year survival of patients who are HIV-negative is 97% compared with HIV-positive cases at 67%.14
Idiopathic MCD is diagnosed when there is no evidence of any underlying infectious, autoimmune, and neoplastic process.15
Patients with MCD are at an increased risk of developing non-Hodgkin and Hodgkin lymphoma, Kaposi sarcoma, primary effusion lymphoma, and follicular dendritic
cell sarcoma. POEMS (peripheral neuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome and paraneoplastic disease, such as paraneoplastic pemphigus myasthenia gravis, may be commonly diagnosed concurrently or sequentially with MCD.16-20
The disease course of MCD ranges from indolent to rapidly progressive, and its 5-year OS is about 65%. When associated with POEMS syndrome, the 5-year survival was estimated to be 90% with the osteosclerotic variant and 27% without osteosclerotic lesions.3 Treatment options for MCD include systemic chemotherapy, including antiviral therapy for HHV-8 positive and HAART for HIV positive and newer monoclonal antibody therapies targeting CD20 or IL-6.
Pathophysiology
Interleukin-6 plays an important role for inflammation in both UCD and MCD (Figure 1). There is dysregulation and overproduction of IL-6, which further stimulates the production of acute-phase reactants, resulting in various systemic manifestations.15,21,22 There is increased expression of IL-1 and IL-6, upregulation of IL-6 secondary to interaction of IL-1 with nuclear factor-kappa B (NF-kappa B), thus stimulating B-cell proliferation. IL-6 binding to IL-6 receptor (IL6-R) results in downstream activation of transcription Janus kinases/signal transducers and activators of the transcription pathway. This promotes the transcription of genes encoding the acute-phase reactant proteins. Hence, interfering with IL-6 transduction by blocking downstream signals are potential therapeutic targets. The mitogen-activated protein kinase cascade, the rapidly accelerated fibrosarcoma kinases, and the overexpression of the endothelial growth factor receptor (EGFR), all contribute to disease pathogenesis by promoting increased B-cell proliferation and vascular EGFR mediated angiogenesis. 23,24
In HHV-8–associated MCD, the virus replicates within lymph node plasmablasts, causing increased production of viral IL-6 analog, human IL-6, and other proinflammatory proteins resulting in B-cell and plasma-cell proliferation, increased vascular endothelial growth factor secretion and angiogenesis.25,26 The HHV-8–infected plasmablasts are marked by variable expression of CD20, and therefore, anti-CD20 is also shown to be an effective treatment. The calmodulin/calcineurin nuclear factor assists in the proliferation of HHV-8, thereby making calcineurin another potential target for the antiviral proliferation.27
Staging
The treatment decisions and prognosis for patients with CD is based on the clinical and histologic staging. The initial workup includes but is not limited to routine laboratory evaluation, imaging, and HIV and HHV-8 testing (Table 3). Routine tests of the levels of cytokines are not recommended. Other relevant tests for known disease associations should be obtained when relevant.
Treatment
Better understanding of the disease process in CD has helped to identify potential therapeutic targets (Figures 2 and 3).
For UCD, surgery is the mainstay of treatment.4,28,29 In surgically unresectable cases, radiation therapy is helpful for local disease control. Alternatively, neoadjuvant
chemotherapy and rituximab are used. Corticosteroids are generally used to treat acute exacerbations and as adjuncts to chemotherapy.
For MCD, the treatment approach depends on the HIV and HHV-8 status of the patient. For patients with HHV-8 infection, both with and without HIV co-infection, antiviral agents, such as ganciclovir, foscarnet, or cidofovir, have shown in vitro activity against HHV-8 but with limited clinical success.30 In patients infected with HIV, the aim of treating with HAART is to control the disease, prevent opportunistic infections, and improve tolerance to chemotherapy.31-33 Rituximab with or without chemotherapy is the standard treatment approach. The additional chemotherapeutic agents are used depending on the presence or absence of organ failure. This approach has improved the OS in HIV-associated MCD.34,35 Treatment with HAART does not decrease the risk of relapse in HIV MCD; therefore, the role of rituximab and antiherpesvirus agents as maintenance therapy has been explored.36 In patients who fail to respond to or relapse rapidly following rituximab monotherapy, the use of either single-agent chemotherapy with or without rituximab or antiherpesvirus therapy with high-dose zidovudine and valganciclovir is recommended.37
The cytotoxic chemotherapy with single agents, such as etoposide, vinblastine, cyclophosphamide, cladribine, chlorambucil, and liposomal doxorubicin, has been used with limited success.22 The combination chemotherapy with cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP) or cyclophosphamide/vincristine/
prednisone (CVP) without rituximab has been shown to achieve durable remissions. Corticosteroids are usually administered as an initial adjunct to chemotherapy or for acute exacerbations. In patients with MCD, regardless of HIV status, the interferon therapy was found to achieve long-term remission.38,39 The interferon therapy
exerts antiviral effects via downregulation of the IL-6R and inhibition of HHV-8 replication. For patients in remission, maintenance therapy with oral valganciclovir is promising.40
Immunomodulators & Targeted Therapies
For unresectable UCD or MCD with organ failure or relapse, the use of alternativesingle-agent or combination chemotherapies with or without rituximab is recommended. Thalidomide has shown some success, probably secondary to disruption of IL-6 production.41 In cases of progression following second-line therapy, bortezomib, antiherpesvirus therapies, or IL-6–directed therapy with siltuximab or tocilizumab should be considered.
Rituximab is a monoclonal chimeric antibody that targets CD20 on B cells, thus leading to B-cell lymphodepletion via activating complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity. As monotherapy, it has been shown to achieve 2-year progression-free survival in 80% of patients.42 In patients with MCD who are HIV positive, rituximab with and without chemotherapy has shown improved overall and disease-free survival of 70% to 80% at 2 years.43
Siltuximab is a chimeric human-mouse monoclonal antibody to IL-6 that has been approved for treatment of patients with MCD who are both HIV negative and HHV-8 negative.44-46 Tocilizumab targets the IL-6R. The antibody has shown improvement in a study in HIVseronegative adults with MCD.47,48
Bortezomib is a proteasome inhibitor that inhibits the NF-kappa B pathway, which induces the expression of numerous proinflammatory proteins, including IL-6. It is recommended for relapsed or refractory disease.49,50
Anakinra is a recombinant IL-1R antagonist that blocks IL-1 effects and controls disease by decreasing IL-6 production.51
Conculsion
There has been significant progress in disease diagnosis and management as more information is available about the incidence, clinical presentation, and pathophysiology of CD. The understanding of the disease pathogenesis and biology has helped to discover multiple potential therapeutic targets. Successful treatment has been achieved through targeting HHV-8 replication, CD20, and IL-6 and anti– IL-6R antibodies. Although surgical resection continues to be the
standard of therapy for UCD, the management of MCD and relapsed or refractory disease continues to evolve. Exploration of various treatment strategies in different clinical presentations is warranted.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S.
Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information
for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to
patients.
Click here to read the digital edition.
1. Castleman B, Iverson L, Menendez VP. Localized mediastinal lymph-node hyperplasia
resembling thymoma. Cancer. 1956;9(4):822-830.
2. Munshi N, Mehra M, van de Velde H, Desai A, Potluri R, Vermeulen J. Use of a claims database to characterize and estimate the incidence rate for Castleman disease. Leuk Lymphoma. 2015;56(5):1252-1260.
3. Dispenzieri A, Armitage JO, Loe MJ, et al. The clinical spectrum of Castleman’s disease. Am J Hematol. 2012;87(11):997-1002.
4. Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29(3):670-683.
5. Cronin DM, Warnke RA. Castleman disease: an update on classification and the
spectrum of associated lesions. Adv Anat Pathol. 2009;16(4):236-246.
6. Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman’s disease: a systematic review of 404 published cases. Ann Surg. 2012;255(4):677-684.
7. Chronowski GM, Ha CS, Wilder RB, Cabanillas F, Manning J, Cox JD. Treatment of unicentric and multicentric Castleman disease and the role of radiotherapy. Cancer. 2001;92(3):670-676.
8. Herrada J, Cabanillas F, Rice L, Manning J, Pugh W. The clinical behavior of localized and multicentric Castleman disease. Ann Intern Med. 1998;128(8):657-662.
9. Dupin N, Diss TL, Kellam P, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95(4):1406-1412.
10. Ferry JA, Harris NL. Atlas of Lymphoid Hyperplasia and Lymphoma. Philadelphia, PA: W.B. Saunders; 1997.
11. Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86(4):1276-1280.
12. Powles T, Stebbing J, Bazeos A, et al. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman’s disease. Ann Oncol. 2009;20(4):775-779.
13. Gérard L, Bérezné A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman’s disease: ANRS 117 CastlemaB Trial. J Clin Oncol. 2007;25(22):3350-3356.
14. Casper C, Teltsch DY, Robinson D Jr, et al. Clinical characteristics and healthcare utilization of patients with multicentric Castleman disease. Br J Haematol. 2015;168(1):82-93.
15. Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123(19):2924-2933.
16. Larroche C, Cacoub P, Soulier J, et al. Castleman’s disease and lymphoma: report of eight cases in HIV-negative patients and literature review. Am J Hematol. 2002;69(2):119-126.
17. Dispenzieri A. POEMS syndrome: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(2):214-223.
18. Andhavarapu S, Jiang L. POEMS syndrome and Castleman disease. Blood. 2013;122(2):159.
19. Bélec L, Mohamed AS, Authier FJ, et al. Human herpesvirus 8 infection in patients with POEMS syndrome-associated multicentric Castleman’s disease. Blood. 1999;93(11):3643-3653.
20. Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcomaassociated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99(7):2331-2336.
21. Yoshizaki K, Matsuda T, Nishimoto N, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74(4):1360-1367.
22. El-Osta HE, Kurzrock R. Castleman’s disease: from basic mechanisms to molecular therapeutics. Oncologist. 2011;16(4):497-511.
23. Akula SM, Ford PW, Whitman AG, et al. B-Raf-dependent expression of vascular endothelial growth factor-A in Kaposi sarcoma-associated herpesvirus-infected human B cells. Blood. 2005;105(11):4516-4522.
24. Sun X, Chang KC, Abruzzo LV, Lai R, Younes A, Jones D. Epidermal growth factor receptor expression in follicular dendritic cells: a shared feature of follicular dendritic cell sarcoma and Castleman’s disease. Hum Pathol. 2003;34(9):835-840.
25. Adam N, Rabe B, Suthaus J, Grötzinger J, Rose-John S, Scheller J. Unraveling viral interleukin-6 binding to gp130 and activation of STAT-signaling pathways independently of the interleukin-6 receptor. J Virol. 2009;83(10):5117-5126.
26. Suda T, Katano H, Delsol G, et al. HHV-8 infection status of AIDS-unrelated and
AIDS-associated multicentric Castleman’s disease. Pathol Int. 2001;51(9):671-679.
27. Zoeteweij JP, Moses AV, Rinderknecht AS, et al. Targeted inhibition of calcineurin signaling blocks calcium-dependent reactivation of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97(8):2374-2380.
28. McCarty MJ, Vukelja SJ, Banks PM, Weiss RB. Angiofollicular lymph node hyperplasia
(Castleman’s disease). Cancer Treat Rev. 1995;21(4):291-310.
29. Bowne WB, Lewis JJ, Filippa DA, et al. The management of unicentric and multicentric Castleman’s disease: a report of 16 cases and a review of the literature. Cancer. 1999;85(3):706-717.
30. Reddy D, Mitsuyasu R. HIV-associated multicentric Castleman disease. Curr Opin Oncol. 2011;23(5):475-481.
31. Aaron L, Lidove O, Yousry C, Roudiere L, Dupont B, Viard JP. Human herpesvirus 8-positive Castleman disease in human immunodeficiency virus-infected patients: the impact of highly active antiretroviral therapy. Clin Infect Dis. 2002;35(7):880-882.
32. Sprinz E, Jeffman M, Liedke P, Putten A, Schwartsmann G. Successful treatment of AIDS-related Castleman’s disease following the administration of highly active antiretroviral therapy (HAART). Ann Oncol. 2004;15(2):356-358.
33. Lee SM, Edwards SG, Chilton DN, Ramsay A, Miller RF. Highly active antiretroviral therapy alone may be an effective treatment for HIV-associated multi-centric Castleman’s disease. Haematologica. 2010;95(11):1979-1981.
34. Bower M. How I treat HIV-associated multicentric Castleman disease. Blood. 2010;116(22):4415-4421.
35. Bower M, Newsom-Davis T, Naresh K, et al. Clinical features and outcome in HIVassociated
multicentric Castleman’s disease. J Clin Oncol. 2011;29(18):2481-2486.
36. Casper C, Nichols WG, Huang ML, Corey L, Wald A. Remission of HHV-8 and HIV-associated multicentric Castleman disease with ganciclovir treatment. Blood. 2004;103(5):1632-1634.
37. Uldrick TS, Polizzotto MN, Aleman K, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood. 2011;117(26):6977-6986.
38. Kumari P, Schechter GP, Saini N, Benator DA. Successful treatment of human immunodeficiency virus-related Castleman’s disease with interferon-alpha. Clin Infect Dis. 2000;31(2):602-604.
39. Nord JA, Karter D. Low dose interferon-alpha therapy for HIV-associated multicentric Castleman’s disease. Int J STD AIDS. 2003;14(1):61-62.
40. Oksenhendler E. HIV-associated multicentric Castleman disease. Curr Opin HIV AIDS. 2009;4(1):16-21.
41. Jung CP, Emmerich B, Goebel FD, Bogner JR. Successful treatment of a patient with HIV-associated multicentric Castleman disease (MCD) with thalidomide. Am J Hematol. 2004;75(3):176-177.
42. Ide M, Kawachi Y, Izumi Y, Kasagi K, Ogino T. Long-term remission in HIV negative patients with multicentric Castleman’s disease using rituximab. Eur J Haematol. 2006;76(2):119-123.
43. Marcelin AG, Aaron L, Mateus C, et al. Rituximab therapy for HIV-associated Castleman disease. Blood. 2003;102(8):2786-2788.
44. Van Rhee F, Fayad L, Voorhees P, et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. J Clin Oncol. 2010;28(23):3701-3708.
45. Wong RS, Casper C, Munshi N, et al. A multicenter, randomized, doubleblind, placebo-controlled study of the efficacy and safety of siltuximab, an antiinterleukin-6 monoclonal antibody, in patients with multicentric Castleman’s disease. Blood. 2013;122(21):505.
46. Van Rhee F, Casper C, Voorhees PM, et al. An open-label, phase 2, multicenter study of the safety of long-term treatment with siltuximab (an anti-interleukin-6 monoclonal antibody) in patients with multicentric Castleman’s disease. Blood. 2013;122(21):1806.
47. Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627-2632.
48. Müzes G, Sipos F, Csomor J, Sréter L. Successful tocilizumab treatment in a patient with human herpesvirus 8-positive and human immunodeficiency virusnegative multicentric Castleman’s disease of plasma cell type nonresponsive to rituximab-CVP therapy. APMIS. 2013;121(7):668-674.
49. Hess G, Wagner V, Kreft A, Heussel CP, Huber C. Effects of bortezomib on proinflammatory cytokine levels and transfusion dependency in a patient with multicentric Castleman disease. Br J Haematol. 2006;134(5):544-545.
50. Sobas MA, Alonso Vence N, Diaz Arias J, Bendaña Lopez A, Fraga Rodriguez M, Bello Lopez JL. Efficacy of bortezomib in refractory form of multicentric Castleman disease associated to poems syndrome (MCD-POEMS variant). Ann Hematol. 2010;89(2):217-219.
51. El-Osta H, Janku F, Kurzrock R. Successful treatment of Castleman’s disease with interleukin-1 receptor antagonist (Anakinra). Mol Cancer Ther. 2010;9(6):1485-1488.
1. Castleman B, Iverson L, Menendez VP. Localized mediastinal lymph-node hyperplasia
resembling thymoma. Cancer. 1956;9(4):822-830.
2. Munshi N, Mehra M, van de Velde H, Desai A, Potluri R, Vermeulen J. Use of a claims database to characterize and estimate the incidence rate for Castleman disease. Leuk Lymphoma. 2015;56(5):1252-1260.
3. Dispenzieri A, Armitage JO, Loe MJ, et al. The clinical spectrum of Castleman’s disease. Am J Hematol. 2012;87(11):997-1002.
4. Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29(3):670-683.
5. Cronin DM, Warnke RA. Castleman disease: an update on classification and the
spectrum of associated lesions. Adv Anat Pathol. 2009;16(4):236-246.
6. Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman’s disease: a systematic review of 404 published cases. Ann Surg. 2012;255(4):677-684.
7. Chronowski GM, Ha CS, Wilder RB, Cabanillas F, Manning J, Cox JD. Treatment of unicentric and multicentric Castleman disease and the role of radiotherapy. Cancer. 2001;92(3):670-676.
8. Herrada J, Cabanillas F, Rice L, Manning J, Pugh W. The clinical behavior of localized and multicentric Castleman disease. Ann Intern Med. 1998;128(8):657-662.
9. Dupin N, Diss TL, Kellam P, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95(4):1406-1412.
10. Ferry JA, Harris NL. Atlas of Lymphoid Hyperplasia and Lymphoma. Philadelphia, PA: W.B. Saunders; 1997.
11. Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86(4):1276-1280.
12. Powles T, Stebbing J, Bazeos A, et al. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman’s disease. Ann Oncol. 2009;20(4):775-779.
13. Gérard L, Bérezné A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman’s disease: ANRS 117 CastlemaB Trial. J Clin Oncol. 2007;25(22):3350-3356.
14. Casper C, Teltsch DY, Robinson D Jr, et al. Clinical characteristics and healthcare utilization of patients with multicentric Castleman disease. Br J Haematol. 2015;168(1):82-93.
15. Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123(19):2924-2933.
16. Larroche C, Cacoub P, Soulier J, et al. Castleman’s disease and lymphoma: report of eight cases in HIV-negative patients and literature review. Am J Hematol. 2002;69(2):119-126.
17. Dispenzieri A. POEMS syndrome: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(2):214-223.
18. Andhavarapu S, Jiang L. POEMS syndrome and Castleman disease. Blood. 2013;122(2):159.
19. Bélec L, Mohamed AS, Authier FJ, et al. Human herpesvirus 8 infection in patients with POEMS syndrome-associated multicentric Castleman’s disease. Blood. 1999;93(11):3643-3653.
20. Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcomaassociated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99(7):2331-2336.
21. Yoshizaki K, Matsuda T, Nishimoto N, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74(4):1360-1367.
22. El-Osta HE, Kurzrock R. Castleman’s disease: from basic mechanisms to molecular therapeutics. Oncologist. 2011;16(4):497-511.
23. Akula SM, Ford PW, Whitman AG, et al. B-Raf-dependent expression of vascular endothelial growth factor-A in Kaposi sarcoma-associated herpesvirus-infected human B cells. Blood. 2005;105(11):4516-4522.
24. Sun X, Chang KC, Abruzzo LV, Lai R, Younes A, Jones D. Epidermal growth factor receptor expression in follicular dendritic cells: a shared feature of follicular dendritic cell sarcoma and Castleman’s disease. Hum Pathol. 2003;34(9):835-840.
25. Adam N, Rabe B, Suthaus J, Grötzinger J, Rose-John S, Scheller J. Unraveling viral interleukin-6 binding to gp130 and activation of STAT-signaling pathways independently of the interleukin-6 receptor. J Virol. 2009;83(10):5117-5126.
26. Suda T, Katano H, Delsol G, et al. HHV-8 infection status of AIDS-unrelated and
AIDS-associated multicentric Castleman’s disease. Pathol Int. 2001;51(9):671-679.
27. Zoeteweij JP, Moses AV, Rinderknecht AS, et al. Targeted inhibition of calcineurin signaling blocks calcium-dependent reactivation of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97(8):2374-2380.
28. McCarty MJ, Vukelja SJ, Banks PM, Weiss RB. Angiofollicular lymph node hyperplasia
(Castleman’s disease). Cancer Treat Rev. 1995;21(4):291-310.
29. Bowne WB, Lewis JJ, Filippa DA, et al. The management of unicentric and multicentric Castleman’s disease: a report of 16 cases and a review of the literature. Cancer. 1999;85(3):706-717.
30. Reddy D, Mitsuyasu R. HIV-associated multicentric Castleman disease. Curr Opin Oncol. 2011;23(5):475-481.
31. Aaron L, Lidove O, Yousry C, Roudiere L, Dupont B, Viard JP. Human herpesvirus 8-positive Castleman disease in human immunodeficiency virus-infected patients: the impact of highly active antiretroviral therapy. Clin Infect Dis. 2002;35(7):880-882.
32. Sprinz E, Jeffman M, Liedke P, Putten A, Schwartsmann G. Successful treatment of AIDS-related Castleman’s disease following the administration of highly active antiretroviral therapy (HAART). Ann Oncol. 2004;15(2):356-358.
33. Lee SM, Edwards SG, Chilton DN, Ramsay A, Miller RF. Highly active antiretroviral therapy alone may be an effective treatment for HIV-associated multi-centric Castleman’s disease. Haematologica. 2010;95(11):1979-1981.
34. Bower M. How I treat HIV-associated multicentric Castleman disease. Blood. 2010;116(22):4415-4421.
35. Bower M, Newsom-Davis T, Naresh K, et al. Clinical features and outcome in HIVassociated
multicentric Castleman’s disease. J Clin Oncol. 2011;29(18):2481-2486.
36. Casper C, Nichols WG, Huang ML, Corey L, Wald A. Remission of HHV-8 and HIV-associated multicentric Castleman disease with ganciclovir treatment. Blood. 2004;103(5):1632-1634.
37. Uldrick TS, Polizzotto MN, Aleman K, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood. 2011;117(26):6977-6986.
38. Kumari P, Schechter GP, Saini N, Benator DA. Successful treatment of human immunodeficiency virus-related Castleman’s disease with interferon-alpha. Clin Infect Dis. 2000;31(2):602-604.
39. Nord JA, Karter D. Low dose interferon-alpha therapy for HIV-associated multicentric Castleman’s disease. Int J STD AIDS. 2003;14(1):61-62.
40. Oksenhendler E. HIV-associated multicentric Castleman disease. Curr Opin HIV AIDS. 2009;4(1):16-21.
41. Jung CP, Emmerich B, Goebel FD, Bogner JR. Successful treatment of a patient with HIV-associated multicentric Castleman disease (MCD) with thalidomide. Am J Hematol. 2004;75(3):176-177.
42. Ide M, Kawachi Y, Izumi Y, Kasagi K, Ogino T. Long-term remission in HIV negative patients with multicentric Castleman’s disease using rituximab. Eur J Haematol. 2006;76(2):119-123.
43. Marcelin AG, Aaron L, Mateus C, et al. Rituximab therapy for HIV-associated Castleman disease. Blood. 2003;102(8):2786-2788.
44. Van Rhee F, Fayad L, Voorhees P, et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. J Clin Oncol. 2010;28(23):3701-3708.
45. Wong RS, Casper C, Munshi N, et al. A multicenter, randomized, doubleblind, placebo-controlled study of the efficacy and safety of siltuximab, an antiinterleukin-6 monoclonal antibody, in patients with multicentric Castleman’s disease. Blood. 2013;122(21):505.
46. Van Rhee F, Casper C, Voorhees PM, et al. An open-label, phase 2, multicenter study of the safety of long-term treatment with siltuximab (an anti-interleukin-6 monoclonal antibody) in patients with multicentric Castleman’s disease. Blood. 2013;122(21):1806.
47. Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627-2632.
48. Müzes G, Sipos F, Csomor J, Sréter L. Successful tocilizumab treatment in a patient with human herpesvirus 8-positive and human immunodeficiency virusnegative multicentric Castleman’s disease of plasma cell type nonresponsive to rituximab-CVP therapy. APMIS. 2013;121(7):668-674.
49. Hess G, Wagner V, Kreft A, Heussel CP, Huber C. Effects of bortezomib on proinflammatory cytokine levels and transfusion dependency in a patient with multicentric Castleman disease. Br J Haematol. 2006;134(5):544-545.
50. Sobas MA, Alonso Vence N, Diaz Arias J, Bendaña Lopez A, Fraga Rodriguez M, Bello Lopez JL. Efficacy of bortezomib in refractory form of multicentric Castleman disease associated to poems syndrome (MCD-POEMS variant). Ann Hematol. 2010;89(2):217-219.
51. El-Osta H, Janku F, Kurzrock R. Successful treatment of Castleman’s disease with interleukin-1 receptor antagonist (Anakinra). Mol Cancer Ther. 2010;9(6):1485-1488.