User login
FDA approves biosimilar filgrastim
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Nivestym™ (filgrastim-aafi), a biosimilar to Neupogen (filgrastim).
Nivestym is approved to treat patients with nonmyeloid malignancies who are receiving myelosuppressive chemotherapy or undergoing bone marrow transplant, acute myeloid leukemia patients receiving induction or consolidation chemotherapy, patients undergoing autologous peripheral blood progenitor cell collection, and patients with severe chronic neutropenia.
The FDA’s approval of Nivestym was based on a review of evidence suggesting the drug is highly similar to Neupogen, according to Pfizer, the company developing Nivestym.
The full approved indication for Nivestym is as follows:
- To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever
- To reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy in patients with acute myeloid leukemia
- To reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg, febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant
- For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis
- For chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (eg, fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
For more details on Nivestym, see the full prescribing information.
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Nivestym™ (filgrastim-aafi), a biosimilar to Neupogen (filgrastim).
Nivestym is approved to treat patients with nonmyeloid malignancies who are receiving myelosuppressive chemotherapy or undergoing bone marrow transplant, acute myeloid leukemia patients receiving induction or consolidation chemotherapy, patients undergoing autologous peripheral blood progenitor cell collection, and patients with severe chronic neutropenia.
The FDA’s approval of Nivestym was based on a review of evidence suggesting the drug is highly similar to Neupogen, according to Pfizer, the company developing Nivestym.
The full approved indication for Nivestym is as follows:
- To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever
- To reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy in patients with acute myeloid leukemia
- To reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg, febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant
- For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis
- For chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (eg, fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
For more details on Nivestym, see the full prescribing information.
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Nivestym™ (filgrastim-aafi), a biosimilar to Neupogen (filgrastim).
Nivestym is approved to treat patients with nonmyeloid malignancies who are receiving myelosuppressive chemotherapy or undergoing bone marrow transplant, acute myeloid leukemia patients receiving induction or consolidation chemotherapy, patients undergoing autologous peripheral blood progenitor cell collection, and patients with severe chronic neutropenia.
The FDA’s approval of Nivestym was based on a review of evidence suggesting the drug is highly similar to Neupogen, according to Pfizer, the company developing Nivestym.
The full approved indication for Nivestym is as follows:
- To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever
- To reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy in patients with acute myeloid leukemia
- To reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg, febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant
- For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis
- For chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (eg, fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
For more details on Nivestym, see the full prescribing information.
Tool may reveal optimal time for SCT in MF
A new tool can help patients with myelofibrosis (MF) decide when to pursue stem cell transplant (SCT), according to the MPN Research Foundation.
The SCT Spectrum Transplant Timing tool (SSTT) is an online MF risk calculator.
It is designed to inform patients of their risk status in a timely manner so they can be proactive in speaking to their doctors about SCT.
“There is an optimal time to start SCT, simply because the odds of success soar in our favor when we start early rather than late in the game,” said Zhenya Senyak, editor of MPN Forum, an MF patient, and creator of the SSTT.
“The SCT Spectrum Timing Tool can help inform the timing of that decision.”
The SSTT incorporates the Dynamic International Prognostic Scoring System (DIPSS), asking patients about their age, white blood cell count, hemoglobin level, peripheral blood blast percentage, and constitutional symptoms.
Patients’ answers are used to create a color signal that indicates their current MF risk level. Accompanying information explains what the risk level means, provides median survival times for that level, and alerts patients of the relative urgency of SCT.
Patients can review these results with their hematologist to ensure accuracy and incorporate risk factors not measured by the SSTT.
The SSTT also includes resources to support SCT discussions between patients and hematologists.
Senyak created SSTT with help from the MPN SCT Transplantation Timing Taskforce, a group of 18 MPN and transplant specialists, patient advocates, and SCT survivors. The work was sponsored by the MPN Research Foundation.
“The decision of whether or not to pursue a stem cell transplant is incredibly fraught for any person,” said Ruben Mesa, MD, a member of the MPN SCT Transplantation Timing Taskforce.
“Our hope in assisting with this effort is to lend our knowledge and experience to create a guide to assist with patient-doctor communication around this very complicated issue.”
A new tool can help patients with myelofibrosis (MF) decide when to pursue stem cell transplant (SCT), according to the MPN Research Foundation.
The SCT Spectrum Transplant Timing tool (SSTT) is an online MF risk calculator.
It is designed to inform patients of their risk status in a timely manner so they can be proactive in speaking to their doctors about SCT.
“There is an optimal time to start SCT, simply because the odds of success soar in our favor when we start early rather than late in the game,” said Zhenya Senyak, editor of MPN Forum, an MF patient, and creator of the SSTT.
“The SCT Spectrum Timing Tool can help inform the timing of that decision.”
The SSTT incorporates the Dynamic International Prognostic Scoring System (DIPSS), asking patients about their age, white blood cell count, hemoglobin level, peripheral blood blast percentage, and constitutional symptoms.
Patients’ answers are used to create a color signal that indicates their current MF risk level. Accompanying information explains what the risk level means, provides median survival times for that level, and alerts patients of the relative urgency of SCT.
Patients can review these results with their hematologist to ensure accuracy and incorporate risk factors not measured by the SSTT.
The SSTT also includes resources to support SCT discussions between patients and hematologists.
Senyak created SSTT with help from the MPN SCT Transplantation Timing Taskforce, a group of 18 MPN and transplant specialists, patient advocates, and SCT survivors. The work was sponsored by the MPN Research Foundation.
“The decision of whether or not to pursue a stem cell transplant is incredibly fraught for any person,” said Ruben Mesa, MD, a member of the MPN SCT Transplantation Timing Taskforce.
“Our hope in assisting with this effort is to lend our knowledge and experience to create a guide to assist with patient-doctor communication around this very complicated issue.”
A new tool can help patients with myelofibrosis (MF) decide when to pursue stem cell transplant (SCT), according to the MPN Research Foundation.
The SCT Spectrum Transplant Timing tool (SSTT) is an online MF risk calculator.
It is designed to inform patients of their risk status in a timely manner so they can be proactive in speaking to their doctors about SCT.
“There is an optimal time to start SCT, simply because the odds of success soar in our favor when we start early rather than late in the game,” said Zhenya Senyak, editor of MPN Forum, an MF patient, and creator of the SSTT.
“The SCT Spectrum Timing Tool can help inform the timing of that decision.”
The SSTT incorporates the Dynamic International Prognostic Scoring System (DIPSS), asking patients about their age, white blood cell count, hemoglobin level, peripheral blood blast percentage, and constitutional symptoms.
Patients’ answers are used to create a color signal that indicates their current MF risk level. Accompanying information explains what the risk level means, provides median survival times for that level, and alerts patients of the relative urgency of SCT.
Patients can review these results with their hematologist to ensure accuracy and incorporate risk factors not measured by the SSTT.
The SSTT also includes resources to support SCT discussions between patients and hematologists.
Senyak created SSTT with help from the MPN SCT Transplantation Timing Taskforce, a group of 18 MPN and transplant specialists, patient advocates, and SCT survivors. The work was sponsored by the MPN Research Foundation.
“The decision of whether or not to pursue a stem cell transplant is incredibly fraught for any person,” said Ruben Mesa, MD, a member of the MPN SCT Transplantation Timing Taskforce.
“Our hope in assisting with this effort is to lend our knowledge and experience to create a guide to assist with patient-doctor communication around this very complicated issue.”
Diabetics have higher risk of hematologic, other cancers
A review of data from more than 19 million people indicates that diabetes significantly raises a person’s risk of developing cancer.
When researchers compared patients with diabetes and without, both male and female diabetics had an increased risk of leukemias and lymphomas as well as certain solid tumors.
Researchers also found that diabetes conferred a higher cancer risk for women than men, both for all cancers combined and for some specific cancers, including leukemia.
“The link between diabetes and the risk of developing cancer is now firmly established,” said Toshiaki Ohkuma, PhD, of The George Institute for Global Health at the University of New South Wales in Australia.
“We have also demonstrated, for the first time, that women with diabetes are more likely to develop any form of cancer and have a significantly higher chance of developing kidney, oral, and stomach cancers and leukemia.”
Dr Ohkuma and his colleagues reported these findings in Diabetologia.
The researchers conducted a systematic search in PubMed MEDLINE to identify reports on the links between diabetes and cancer. Additional reports were identified from the reference lists of the relevant studies.
Only those cohort studies providing relative risks (RRs) for the association between diabetes and cancer for both women and men were included. In total, 107 relevant articles were identified, along with 36 cohorts of individual participant data.
RRs for cancer were obtained for patients with diabetes (types 1 and 2 combined) versus those without diabetes, for both men and women. The women-to-men ratios of these relative risk ratios (RRRs) were then calculated to determine the excess risk in women if present.
Data on all-site cancer was available from 47 studies, involving 121 cohorts and 19,239,302 individuals.
Diabetics vs non-diabetics
Women with diabetes had a 27% higher risk of all-site cancer compared to women without diabetes (RR=1.27; 95% CI 1.21, 1.32; P<0.001).
For men, the risk of all-site cancer was 19% higher among those with diabetes than those without (RR=1.19; 95% CI 1.13, 1.25; P<0.001).
There were several hematologic malignancies for which diabetics had an increased risk, as shown in the following table.
| Cancer type | RR for women (99% CI) | RR for men (99% CI) |
| Lymphatic and hematopoietic tissue | 1.24 (1.05, 1.46)* | 1.21 (0.98, 1.48) |
| Leukemia | 1.53 (1.00, 2.33) | 1.22 (0.80, 1.85) |
| Myeloid leukemia | 0.83 (0.39, 1.76) | 1.12 (0.77, 1.62) |
| Acute myeloid leukemia | 1.33 (1.12, 1.57)* | 1.14 (0.56, 2.33) |
| Chronic myeloid leukemia | 1.67 (1.27, 2.20)* | 1.62 (1.32, 1.98)* |
| Lymphoid leukemia | 1.74 (0.31, 9.79) | 1.20 (0.86, 1.68) |
| Lymphoma | 2.31 (0.57, 9.30) | 1.80 (0.68, 4.75) |
| Non-Hodgkin lymphoma | 1.16 (1.02, 1.32)* | 1.20 (1.08, 1.34)* |
| Hodgkin lymphoma | 1.20 (0.61, 2.38) | 1.36 (1.05, 1.77)* |
| Multiple myeloma | 1.19 (0.97, 1.47) | 1.12 (0.90, 1.41) |
| *denotes statistical significance with a P value < 0.01 | ||
Sex comparison
Calculation of the women-to-men ratio revealed that women with diabetes had a 6% greater excess risk of all-site cancer compared to men with diabetes (RRR=1.06; 95% CI 1.03, 1.09; P<0.001).
The women-to-men ratios also showed significantly higher risks for female diabetics for:
- Kidney cancer—RRR=1.11 (99% CI 1.04, 1.18; P<0.001)
- Oral cancer—RRR=1.13 (99% CI 1.00, 1.28; P=0.009)
- Stomach cancer—RRR=1.14 (99% CI 1.07, 1.22; P<0.001)
- Leukemia—RRR=1.15 (99% CI 1.02, 1.28; P=0.002).
However, women had a significantly lower risk of liver cancer (RRR=0.88; 99% CI 0.79, 0.99; P=0.005).
There are several possible reasons for the excess cancer risk observed in women, according to study author Sanne Peters, PhD, of The George Institute for Global Health at the University of Oxford in the UK.
For example, on average, women are in the pre-diabetic state of impaired glucose tolerance 2 years longer than men.
“Historically, we know that women are often under-treated when they first present with symptoms of diabetes, are less likely to receive intensive care, and are not taking the same levels of medications as men,” Dr Peters said. “All of these could go some way into explaining why women are at greater risk of developing cancer, but, without more research, we can’t be certain.”
A review of data from more than 19 million people indicates that diabetes significantly raises a person’s risk of developing cancer.
When researchers compared patients with diabetes and without, both male and female diabetics had an increased risk of leukemias and lymphomas as well as certain solid tumors.
Researchers also found that diabetes conferred a higher cancer risk for women than men, both for all cancers combined and for some specific cancers, including leukemia.
“The link between diabetes and the risk of developing cancer is now firmly established,” said Toshiaki Ohkuma, PhD, of The George Institute for Global Health at the University of New South Wales in Australia.
“We have also demonstrated, for the first time, that women with diabetes are more likely to develop any form of cancer and have a significantly higher chance of developing kidney, oral, and stomach cancers and leukemia.”
Dr Ohkuma and his colleagues reported these findings in Diabetologia.
The researchers conducted a systematic search in PubMed MEDLINE to identify reports on the links between diabetes and cancer. Additional reports were identified from the reference lists of the relevant studies.
Only those cohort studies providing relative risks (RRs) for the association between diabetes and cancer for both women and men were included. In total, 107 relevant articles were identified, along with 36 cohorts of individual participant data.
RRs for cancer were obtained for patients with diabetes (types 1 and 2 combined) versus those without diabetes, for both men and women. The women-to-men ratios of these relative risk ratios (RRRs) were then calculated to determine the excess risk in women if present.
Data on all-site cancer was available from 47 studies, involving 121 cohorts and 19,239,302 individuals.
Diabetics vs non-diabetics
Women with diabetes had a 27% higher risk of all-site cancer compared to women without diabetes (RR=1.27; 95% CI 1.21, 1.32; P<0.001).
For men, the risk of all-site cancer was 19% higher among those with diabetes than those without (RR=1.19; 95% CI 1.13, 1.25; P<0.001).
There were several hematologic malignancies for which diabetics had an increased risk, as shown in the following table.
| Cancer type | RR for women (99% CI) | RR for men (99% CI) |
| Lymphatic and hematopoietic tissue | 1.24 (1.05, 1.46)* | 1.21 (0.98, 1.48) |
| Leukemia | 1.53 (1.00, 2.33) | 1.22 (0.80, 1.85) |
| Myeloid leukemia | 0.83 (0.39, 1.76) | 1.12 (0.77, 1.62) |
| Acute myeloid leukemia | 1.33 (1.12, 1.57)* | 1.14 (0.56, 2.33) |
| Chronic myeloid leukemia | 1.67 (1.27, 2.20)* | 1.62 (1.32, 1.98)* |
| Lymphoid leukemia | 1.74 (0.31, 9.79) | 1.20 (0.86, 1.68) |
| Lymphoma | 2.31 (0.57, 9.30) | 1.80 (0.68, 4.75) |
| Non-Hodgkin lymphoma | 1.16 (1.02, 1.32)* | 1.20 (1.08, 1.34)* |
| Hodgkin lymphoma | 1.20 (0.61, 2.38) | 1.36 (1.05, 1.77)* |
| Multiple myeloma | 1.19 (0.97, 1.47) | 1.12 (0.90, 1.41) |
| *denotes statistical significance with a P value < 0.01 | ||
Sex comparison
Calculation of the women-to-men ratio revealed that women with diabetes had a 6% greater excess risk of all-site cancer compared to men with diabetes (RRR=1.06; 95% CI 1.03, 1.09; P<0.001).
The women-to-men ratios also showed significantly higher risks for female diabetics for:
- Kidney cancer—RRR=1.11 (99% CI 1.04, 1.18; P<0.001)
- Oral cancer—RRR=1.13 (99% CI 1.00, 1.28; P=0.009)
- Stomach cancer—RRR=1.14 (99% CI 1.07, 1.22; P<0.001)
- Leukemia—RRR=1.15 (99% CI 1.02, 1.28; P=0.002).
However, women had a significantly lower risk of liver cancer (RRR=0.88; 99% CI 0.79, 0.99; P=0.005).
There are several possible reasons for the excess cancer risk observed in women, according to study author Sanne Peters, PhD, of The George Institute for Global Health at the University of Oxford in the UK.
For example, on average, women are in the pre-diabetic state of impaired glucose tolerance 2 years longer than men.
“Historically, we know that women are often under-treated when they first present with symptoms of diabetes, are less likely to receive intensive care, and are not taking the same levels of medications as men,” Dr Peters said. “All of these could go some way into explaining why women are at greater risk of developing cancer, but, without more research, we can’t be certain.”
A review of data from more than 19 million people indicates that diabetes significantly raises a person’s risk of developing cancer.
When researchers compared patients with diabetes and without, both male and female diabetics had an increased risk of leukemias and lymphomas as well as certain solid tumors.
Researchers also found that diabetes conferred a higher cancer risk for women than men, both for all cancers combined and for some specific cancers, including leukemia.
“The link between diabetes and the risk of developing cancer is now firmly established,” said Toshiaki Ohkuma, PhD, of The George Institute for Global Health at the University of New South Wales in Australia.
“We have also demonstrated, for the first time, that women with diabetes are more likely to develop any form of cancer and have a significantly higher chance of developing kidney, oral, and stomach cancers and leukemia.”
Dr Ohkuma and his colleagues reported these findings in Diabetologia.
The researchers conducted a systematic search in PubMed MEDLINE to identify reports on the links between diabetes and cancer. Additional reports were identified from the reference lists of the relevant studies.
Only those cohort studies providing relative risks (RRs) for the association between diabetes and cancer for both women and men were included. In total, 107 relevant articles were identified, along with 36 cohorts of individual participant data.
RRs for cancer were obtained for patients with diabetes (types 1 and 2 combined) versus those without diabetes, for both men and women. The women-to-men ratios of these relative risk ratios (RRRs) were then calculated to determine the excess risk in women if present.
Data on all-site cancer was available from 47 studies, involving 121 cohorts and 19,239,302 individuals.
Diabetics vs non-diabetics
Women with diabetes had a 27% higher risk of all-site cancer compared to women without diabetes (RR=1.27; 95% CI 1.21, 1.32; P<0.001).
For men, the risk of all-site cancer was 19% higher among those with diabetes than those without (RR=1.19; 95% CI 1.13, 1.25; P<0.001).
There were several hematologic malignancies for which diabetics had an increased risk, as shown in the following table.
| Cancer type | RR for women (99% CI) | RR for men (99% CI) |
| Lymphatic and hematopoietic tissue | 1.24 (1.05, 1.46)* | 1.21 (0.98, 1.48) |
| Leukemia | 1.53 (1.00, 2.33) | 1.22 (0.80, 1.85) |
| Myeloid leukemia | 0.83 (0.39, 1.76) | 1.12 (0.77, 1.62) |
| Acute myeloid leukemia | 1.33 (1.12, 1.57)* | 1.14 (0.56, 2.33) |
| Chronic myeloid leukemia | 1.67 (1.27, 2.20)* | 1.62 (1.32, 1.98)* |
| Lymphoid leukemia | 1.74 (0.31, 9.79) | 1.20 (0.86, 1.68) |
| Lymphoma | 2.31 (0.57, 9.30) | 1.80 (0.68, 4.75) |
| Non-Hodgkin lymphoma | 1.16 (1.02, 1.32)* | 1.20 (1.08, 1.34)* |
| Hodgkin lymphoma | 1.20 (0.61, 2.38) | 1.36 (1.05, 1.77)* |
| Multiple myeloma | 1.19 (0.97, 1.47) | 1.12 (0.90, 1.41) |
| *denotes statistical significance with a P value < 0.01 | ||
Sex comparison
Calculation of the women-to-men ratio revealed that women with diabetes had a 6% greater excess risk of all-site cancer compared to men with diabetes (RRR=1.06; 95% CI 1.03, 1.09; P<0.001).
The women-to-men ratios also showed significantly higher risks for female diabetics for:
- Kidney cancer—RRR=1.11 (99% CI 1.04, 1.18; P<0.001)
- Oral cancer—RRR=1.13 (99% CI 1.00, 1.28; P=0.009)
- Stomach cancer—RRR=1.14 (99% CI 1.07, 1.22; P<0.001)
- Leukemia—RRR=1.15 (99% CI 1.02, 1.28; P=0.002).
However, women had a significantly lower risk of liver cancer (RRR=0.88; 99% CI 0.79, 0.99; P=0.005).
There are several possible reasons for the excess cancer risk observed in women, according to study author Sanne Peters, PhD, of The George Institute for Global Health at the University of Oxford in the UK.
For example, on average, women are in the pre-diabetic state of impaired glucose tolerance 2 years longer than men.
“Historically, we know that women are often under-treated when they first present with symptoms of diabetes, are less likely to receive intensive care, and are not taking the same levels of medications as men,” Dr Peters said. “All of these could go some way into explaining why women are at greater risk of developing cancer, but, without more research, we can’t be certain.”
L-glutamine reduces complications of SCD
Results of a phase 3 trial showed that L-glutamine can reduce complications of sickle cell disease (SCD).
SCD patients who received pharmaceutical-grade L-glutamine (with or without hydroxyurea) had a reduction in sickle cell crises, hospitalizations, and acute chest syndrome (ACS) when compared to patients who received placebo (with or without hydroxyurea).
Gastrointestinal events and pain in the chest (noncardiac), back, and extremities were more frequent in L-glutamine recipients than controls.
Yutaka Niihara, MD, of Emmaus Medical, Inc., in Torrance, California, and his colleagues reported these results in NEJM. The research was sponsored by Emmaus Medical.
Dr Niihara noted that L-glutamine (Endari), which was approved by the US Food and Drug Administration last summer, is the first treatment approved to treat SCD in pediatric patients age 5 and older and the first SCD treatment approved for adults in nearly 20 years.
“Our hope in sharing the results [of the phase 3 trial] is to aid in increasing the awareness of sickle cell disease, a life-long hereditary blood disorder which commonly affects those of African descent, as well as those from Central and South America and people of Middle Eastern, Asian, Indian, and Mediterranean descent,” Dr Niihara said.
He and his colleagues enrolled 230 patients on the trial. One hundred and fifty-two were randomized to receive L-glutamine at 0.3 g/kg twice daily (maximum 30 g/day), and 78 were randomized to placebo (maltodextrin) at 0.3 g/kg twice daily for 48 weeks.
Baseline characteristics were similar between the treatment arms. Most patients had sickle cell anemia—89.5% (n=136) in the L-glutamine arm and 91% (n=71) in the placebo arm. Several patients had sickle B0 thalassemia—9.2% (n=14) and 9.0% (n=7), respectively. Two patients in the L-glutamine arm had B+ thalassemia, but 1 of these patients did not receive L-glutamine.
The median age was 19 (range, 5-57) in the L-glutamine arm and 17 (range, 5-58) in the placebo arm. There were more females than males in both arms—52% (n=79) and 57.7% (n=45), respectively.
Most patients received concomitant hydroxyurea—66.4% (n=101) in the L-glutamine arm and 66.7% (n=52) in the placebo arm.
Efficacy
Patients in the L-glutamine arm had 25% fewer pain crises than patients in the placebo arm. The median number of crises was 3 and 4, respectively (P=0.005).
The median time to a first crisis was 84 days in the L-glutamine arm and 54 days in the placebo arm (P=0.02). The median time to a second crisis was 212 days and 133 days, respectively (P=0.03).
The median number of hospitalizations was 2 in the L-glutamine arm and 3 in the placebo arm, which is a difference of 33% (P=0.005). The median cumulative number of days in the hospital was 6.5 and 11, respectively (P=0.02).
The incidence of ACS was significantly lower in the L-glutamine arm, with 8.6% of patients in that group having at least 1 episode of ACS and 23.1% of the placebo group having at least 1 episode (P=0.003).
Safety
The rate of adverse events (AEs) was 98% in the L-glutamine arm and 100% in the placebo arm. The rate of serious AEs was 78.2% and 87.1%, respectively.
AEs with a higher incidence in the L-glutamine arm (at least 5%) are listed in the table below.
| AE | L-glutamine (n=151) |
Placebo (n=78) |
| Constipation | 38 (25.2%) | 19 (24.4%) |
| Nausea | 34 (22.5%) | 13 (16.7%) |
| Headache | 32 (21.2%) | 14 (17.9%) |
| Pain in extremity | 24 (15.9%) | 6 (7.7%) |
| Vomiting | 22 (14.6%) | 10 (12.8%) |
| Chest pain (noncardiac) | 21 (13.9%) | 7 (9.0%) |
| Back pain | 20 (13.2%) | 5 (6.4%) |
| Upper abdominal pain | 16 (10.6%) | 6 (7.7%) |
| Diarrhea | 12 (7.9%) | 5 (6.4%) |
| Nasal congestion | 11 (7.3%) | 5 (6.4%) |
| Urinary tract infection | 10 (6.6%) | 3 (3.8%) |
| Fatigue | 9 (6.0%) | 1 (1.3%) |
| Tachycardia | 8 (5.3%) | 4 (5.1%) |
| Dizziness | 8 (5.3%) | 4 (5.1%) |
![]()
Results of a phase 3 trial showed that L-glutamine can reduce complications of sickle cell disease (SCD).
SCD patients who received pharmaceutical-grade L-glutamine (with or without hydroxyurea) had a reduction in sickle cell crises, hospitalizations, and acute chest syndrome (ACS) when compared to patients who received placebo (with or without hydroxyurea).
Gastrointestinal events and pain in the chest (noncardiac), back, and extremities were more frequent in L-glutamine recipients than controls.
Yutaka Niihara, MD, of Emmaus Medical, Inc., in Torrance, California, and his colleagues reported these results in NEJM. The research was sponsored by Emmaus Medical.
Dr Niihara noted that L-glutamine (Endari), which was approved by the US Food and Drug Administration last summer, is the first treatment approved to treat SCD in pediatric patients age 5 and older and the first SCD treatment approved for adults in nearly 20 years.
“Our hope in sharing the results [of the phase 3 trial] is to aid in increasing the awareness of sickle cell disease, a life-long hereditary blood disorder which commonly affects those of African descent, as well as those from Central and South America and people of Middle Eastern, Asian, Indian, and Mediterranean descent,” Dr Niihara said.
He and his colleagues enrolled 230 patients on the trial. One hundred and fifty-two were randomized to receive L-glutamine at 0.3 g/kg twice daily (maximum 30 g/day), and 78 were randomized to placebo (maltodextrin) at 0.3 g/kg twice daily for 48 weeks.
Baseline characteristics were similar between the treatment arms. Most patients had sickle cell anemia—89.5% (n=136) in the L-glutamine arm and 91% (n=71) in the placebo arm. Several patients had sickle B0 thalassemia—9.2% (n=14) and 9.0% (n=7), respectively. Two patients in the L-glutamine arm had B+ thalassemia, but 1 of these patients did not receive L-glutamine.
The median age was 19 (range, 5-57) in the L-glutamine arm and 17 (range, 5-58) in the placebo arm. There were more females than males in both arms—52% (n=79) and 57.7% (n=45), respectively.
Most patients received concomitant hydroxyurea—66.4% (n=101) in the L-glutamine arm and 66.7% (n=52) in the placebo arm.
Efficacy
Patients in the L-glutamine arm had 25% fewer pain crises than patients in the placebo arm. The median number of crises was 3 and 4, respectively (P=0.005).
The median time to a first crisis was 84 days in the L-glutamine arm and 54 days in the placebo arm (P=0.02). The median time to a second crisis was 212 days and 133 days, respectively (P=0.03).
The median number of hospitalizations was 2 in the L-glutamine arm and 3 in the placebo arm, which is a difference of 33% (P=0.005). The median cumulative number of days in the hospital was 6.5 and 11, respectively (P=0.02).
The incidence of ACS was significantly lower in the L-glutamine arm, with 8.6% of patients in that group having at least 1 episode of ACS and 23.1% of the placebo group having at least 1 episode (P=0.003).
Safety
The rate of adverse events (AEs) was 98% in the L-glutamine arm and 100% in the placebo arm. The rate of serious AEs was 78.2% and 87.1%, respectively.
AEs with a higher incidence in the L-glutamine arm (at least 5%) are listed in the table below.
| AE | L-glutamine (n=151) |
Placebo (n=78) |
| Constipation | 38 (25.2%) | 19 (24.4%) |
| Nausea | 34 (22.5%) | 13 (16.7%) |
| Headache | 32 (21.2%) | 14 (17.9%) |
| Pain in extremity | 24 (15.9%) | 6 (7.7%) |
| Vomiting | 22 (14.6%) | 10 (12.8%) |
| Chest pain (noncardiac) | 21 (13.9%) | 7 (9.0%) |
| Back pain | 20 (13.2%) | 5 (6.4%) |
| Upper abdominal pain | 16 (10.6%) | 6 (7.7%) |
| Diarrhea | 12 (7.9%) | 5 (6.4%) |
| Nasal congestion | 11 (7.3%) | 5 (6.4%) |
| Urinary tract infection | 10 (6.6%) | 3 (3.8%) |
| Fatigue | 9 (6.0%) | 1 (1.3%) |
| Tachycardia | 8 (5.3%) | 4 (5.1%) |
| Dizziness | 8 (5.3%) | 4 (5.1%) |
![]()
Results of a phase 3 trial showed that L-glutamine can reduce complications of sickle cell disease (SCD).
SCD patients who received pharmaceutical-grade L-glutamine (with or without hydroxyurea) had a reduction in sickle cell crises, hospitalizations, and acute chest syndrome (ACS) when compared to patients who received placebo (with or without hydroxyurea).
Gastrointestinal events and pain in the chest (noncardiac), back, and extremities were more frequent in L-glutamine recipients than controls.
Yutaka Niihara, MD, of Emmaus Medical, Inc., in Torrance, California, and his colleagues reported these results in NEJM. The research was sponsored by Emmaus Medical.
Dr Niihara noted that L-glutamine (Endari), which was approved by the US Food and Drug Administration last summer, is the first treatment approved to treat SCD in pediatric patients age 5 and older and the first SCD treatment approved for adults in nearly 20 years.
“Our hope in sharing the results [of the phase 3 trial] is to aid in increasing the awareness of sickle cell disease, a life-long hereditary blood disorder which commonly affects those of African descent, as well as those from Central and South America and people of Middle Eastern, Asian, Indian, and Mediterranean descent,” Dr Niihara said.
He and his colleagues enrolled 230 patients on the trial. One hundred and fifty-two were randomized to receive L-glutamine at 0.3 g/kg twice daily (maximum 30 g/day), and 78 were randomized to placebo (maltodextrin) at 0.3 g/kg twice daily for 48 weeks.
Baseline characteristics were similar between the treatment arms. Most patients had sickle cell anemia—89.5% (n=136) in the L-glutamine arm and 91% (n=71) in the placebo arm. Several patients had sickle B0 thalassemia—9.2% (n=14) and 9.0% (n=7), respectively. Two patients in the L-glutamine arm had B+ thalassemia, but 1 of these patients did not receive L-glutamine.
The median age was 19 (range, 5-57) in the L-glutamine arm and 17 (range, 5-58) in the placebo arm. There were more females than males in both arms—52% (n=79) and 57.7% (n=45), respectively.
Most patients received concomitant hydroxyurea—66.4% (n=101) in the L-glutamine arm and 66.7% (n=52) in the placebo arm.
Efficacy
Patients in the L-glutamine arm had 25% fewer pain crises than patients in the placebo arm. The median number of crises was 3 and 4, respectively (P=0.005).
The median time to a first crisis was 84 days in the L-glutamine arm and 54 days in the placebo arm (P=0.02). The median time to a second crisis was 212 days and 133 days, respectively (P=0.03).
The median number of hospitalizations was 2 in the L-glutamine arm and 3 in the placebo arm, which is a difference of 33% (P=0.005). The median cumulative number of days in the hospital was 6.5 and 11, respectively (P=0.02).
The incidence of ACS was significantly lower in the L-glutamine arm, with 8.6% of patients in that group having at least 1 episode of ACS and 23.1% of the placebo group having at least 1 episode (P=0.003).
Safety
The rate of adverse events (AEs) was 98% in the L-glutamine arm and 100% in the placebo arm. The rate of serious AEs was 78.2% and 87.1%, respectively.
AEs with a higher incidence in the L-glutamine arm (at least 5%) are listed in the table below.
| AE | L-glutamine (n=151) |
Placebo (n=78) |
| Constipation | 38 (25.2%) | 19 (24.4%) |
| Nausea | 34 (22.5%) | 13 (16.7%) |
| Headache | 32 (21.2%) | 14 (17.9%) |
| Pain in extremity | 24 (15.9%) | 6 (7.7%) |
| Vomiting | 22 (14.6%) | 10 (12.8%) |
| Chest pain (noncardiac) | 21 (13.9%) | 7 (9.0%) |
| Back pain | 20 (13.2%) | 5 (6.4%) |
| Upper abdominal pain | 16 (10.6%) | 6 (7.7%) |
| Diarrhea | 12 (7.9%) | 5 (6.4%) |
| Nasal congestion | 11 (7.3%) | 5 (6.4%) |
| Urinary tract infection | 10 (6.6%) | 3 (3.8%) |
| Fatigue | 9 (6.0%) | 1 (1.3%) |
| Tachycardia | 8 (5.3%) | 4 (5.1%) |
| Dizziness | 8 (5.3%) | 4 (5.1%) |
![]()
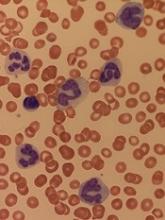
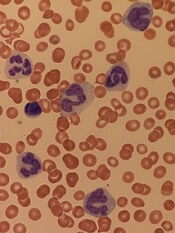
How ALL invades the CNS
Researchers believe they have solved the mystery of how acute lymphoblastic leukemia (ALL) infiltrates the central nervous system (CNS).
Experiments in mice suggested that ALL enters the CNS not by breaching the blood-brain barrier but by evading it.
The researchers said they found that expression of the laminin receptor α6 integrin, which is common in ALL, allows cells to use neural migratory pathways to invade the CNS.
“It’s a very unexpected way for cells to travel into the central nervous system,” said Dorothy Sipkins, MD, PhD, of Duke University in Durham, North Carolina.
She and her colleagues described the cells’ journey in Nature.
The researchers said they found that α6 integrin–laminin interactions mediate the migration of ALL cells toward the cerebrospinal fluid.
The team noted that α6 integrin is expressed in most cases of ALL, and laminin surrounds blood vessels that pass directly through the vertebrae to the meninges tissue that lines the spinal cord and brain.
Experiments indicated that ALL cells latch onto the laminin surrounding these blood vessels and travel down into the meninges region where cerebral spinal fluid circulates.
“Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” Dr Sipkins noted.
She and her colleagues found that treatment with a PI3Kδ inhibitor may be one way to do that.
The team tested the PI3Kδ inhibitor GS-649443 in a mouse model of CNS ALL (Nalm-6) and found the drug decreased α6 integrin expression on ALL cells.
Mice treated with the inhibitor had a 50% decrease in CNS disease burden compared to vehicle-treated controls. However, there was no significant difference between treated mice and controls when it came to bone marrow or splenic Nalm-6 disease burden or peripheral blood cell counts.
The researchers observed similar results in another model of CNS disease (RCH-ACV ALL).
The team also tested α6 integrin-neutralizing antibodies in Nalm-6-engrafted mice. There was no difference in peripheral disease burden between targeted and isotype control antibody-treated mice. However, anti-α6 integrin-treated mice had a reduction in cerebrospinal fluid blast counts.
This research was supported by the Duke Cancer Institute and Gilead Sciences, Inc., which provided the PI3Kδ inhibitor.
Researchers believe they have solved the mystery of how acute lymphoblastic leukemia (ALL) infiltrates the central nervous system (CNS).
Experiments in mice suggested that ALL enters the CNS not by breaching the blood-brain barrier but by evading it.
The researchers said they found that expression of the laminin receptor α6 integrin, which is common in ALL, allows cells to use neural migratory pathways to invade the CNS.
“It’s a very unexpected way for cells to travel into the central nervous system,” said Dorothy Sipkins, MD, PhD, of Duke University in Durham, North Carolina.
She and her colleagues described the cells’ journey in Nature.
The researchers said they found that α6 integrin–laminin interactions mediate the migration of ALL cells toward the cerebrospinal fluid.
The team noted that α6 integrin is expressed in most cases of ALL, and laminin surrounds blood vessels that pass directly through the vertebrae to the meninges tissue that lines the spinal cord and brain.
Experiments indicated that ALL cells latch onto the laminin surrounding these blood vessels and travel down into the meninges region where cerebral spinal fluid circulates.
“Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” Dr Sipkins noted.
She and her colleagues found that treatment with a PI3Kδ inhibitor may be one way to do that.
The team tested the PI3Kδ inhibitor GS-649443 in a mouse model of CNS ALL (Nalm-6) and found the drug decreased α6 integrin expression on ALL cells.
Mice treated with the inhibitor had a 50% decrease in CNS disease burden compared to vehicle-treated controls. However, there was no significant difference between treated mice and controls when it came to bone marrow or splenic Nalm-6 disease burden or peripheral blood cell counts.
The researchers observed similar results in another model of CNS disease (RCH-ACV ALL).
The team also tested α6 integrin-neutralizing antibodies in Nalm-6-engrafted mice. There was no difference in peripheral disease burden between targeted and isotype control antibody-treated mice. However, anti-α6 integrin-treated mice had a reduction in cerebrospinal fluid blast counts.
This research was supported by the Duke Cancer Institute and Gilead Sciences, Inc., which provided the PI3Kδ inhibitor.
Researchers believe they have solved the mystery of how acute lymphoblastic leukemia (ALL) infiltrates the central nervous system (CNS).
Experiments in mice suggested that ALL enters the CNS not by breaching the blood-brain barrier but by evading it.
The researchers said they found that expression of the laminin receptor α6 integrin, which is common in ALL, allows cells to use neural migratory pathways to invade the CNS.
“It’s a very unexpected way for cells to travel into the central nervous system,” said Dorothy Sipkins, MD, PhD, of Duke University in Durham, North Carolina.
She and her colleagues described the cells’ journey in Nature.
The researchers said they found that α6 integrin–laminin interactions mediate the migration of ALL cells toward the cerebrospinal fluid.
The team noted that α6 integrin is expressed in most cases of ALL, and laminin surrounds blood vessels that pass directly through the vertebrae to the meninges tissue that lines the spinal cord and brain.
Experiments indicated that ALL cells latch onto the laminin surrounding these blood vessels and travel down into the meninges region where cerebral spinal fluid circulates.
“Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” Dr Sipkins noted.
She and her colleagues found that treatment with a PI3Kδ inhibitor may be one way to do that.
The team tested the PI3Kδ inhibitor GS-649443 in a mouse model of CNS ALL (Nalm-6) and found the drug decreased α6 integrin expression on ALL cells.
Mice treated with the inhibitor had a 50% decrease in CNS disease burden compared to vehicle-treated controls. However, there was no significant difference between treated mice and controls when it came to bone marrow or splenic Nalm-6 disease burden or peripheral blood cell counts.
The researchers observed similar results in another model of CNS disease (RCH-ACV ALL).
The team also tested α6 integrin-neutralizing antibodies in Nalm-6-engrafted mice. There was no difference in peripheral disease burden between targeted and isotype control antibody-treated mice. However, anti-α6 integrin-treated mice had a reduction in cerebrospinal fluid blast counts.
This research was supported by the Duke Cancer Institute and Gilead Sciences, Inc., which provided the PI3Kδ inhibitor.
Study suggests dasatinib could treat AML, JMML
New research suggests dasatinib could treat certain patients with juvenile myelomonocytic leukemia (JMML) or acute myeloid leukemia (AML).
The study showed that TNK2 inhibition has a negative effect on PTPN11-mutant leukemias.
PTPN11-mutant JMML and AML cells were sensitive to treatment with dasatinib, which inhibits TNK2.
Dasatinib also induced hematologic remission in a patient with PTPN11-mutant JMML.
Investigators reported these results in Science Signaling.
Past research showed that mutations in PTPN11 result in excessive cell proliferation and drive tumor growth in some cases of JMML and AML.
In the current study, investigators analyzed PTPN11-mutated leukemia cells and found that PTPN11 is activated by TNK2.
The investigators said TNK2 phosphorylates PTPN11, which then dephosphorylates TNK2 in a negative feedback loop. They also found that coexpression of TNK2 and mutant PTPN11 results in “robust” MAPK pathway activation.
Inhibiting TNK2 with dasatinib blocked MAPK signaling and colony formation in vitro.
Additional experiments showed that PTPN11-mutant AML samples were significantly more sensitive to dasatinib than wild-type AML samples.
Investigators also tested dasatinib in a sample from a JMML patient carrying a PTPN11 G60R mutation.
This patient’s cells were 10 times more sensitive to dasatinib than the average sample from a cohort of 151 patients who had AML, acute lymphoblastic leukemia, myeloproliferative neoplasms, or chronic lymphocytic leukemia.
Because the JMML patient’s cells were so responsive to dasatinib, the investigators decided to administer the drug to the patient.
The patient achieved sustained hematologic remission with dasatinib, and this allowed him to receive a stem cell transplant using an unrelated cord blood donor. The patient had previously failed 2 transplants (with myeloablative conditioning) from a matched sibling donor.
The third transplant prolonged the patient’s life by a year, but he eventually died of relapsed disease.
The investigators said this case study and the in vitro results support further investigation into the efficacy of dasatinib and other TNK2 inhibitors in PTPN11-mutant leukemias.
New research suggests dasatinib could treat certain patients with juvenile myelomonocytic leukemia (JMML) or acute myeloid leukemia (AML).
The study showed that TNK2 inhibition has a negative effect on PTPN11-mutant leukemias.
PTPN11-mutant JMML and AML cells were sensitive to treatment with dasatinib, which inhibits TNK2.
Dasatinib also induced hematologic remission in a patient with PTPN11-mutant JMML.
Investigators reported these results in Science Signaling.
Past research showed that mutations in PTPN11 result in excessive cell proliferation and drive tumor growth in some cases of JMML and AML.
In the current study, investigators analyzed PTPN11-mutated leukemia cells and found that PTPN11 is activated by TNK2.
The investigators said TNK2 phosphorylates PTPN11, which then dephosphorylates TNK2 in a negative feedback loop. They also found that coexpression of TNK2 and mutant PTPN11 results in “robust” MAPK pathway activation.
Inhibiting TNK2 with dasatinib blocked MAPK signaling and colony formation in vitro.
Additional experiments showed that PTPN11-mutant AML samples were significantly more sensitive to dasatinib than wild-type AML samples.
Investigators also tested dasatinib in a sample from a JMML patient carrying a PTPN11 G60R mutation.
This patient’s cells were 10 times more sensitive to dasatinib than the average sample from a cohort of 151 patients who had AML, acute lymphoblastic leukemia, myeloproliferative neoplasms, or chronic lymphocytic leukemia.
Because the JMML patient’s cells were so responsive to dasatinib, the investigators decided to administer the drug to the patient.
The patient achieved sustained hematologic remission with dasatinib, and this allowed him to receive a stem cell transplant using an unrelated cord blood donor. The patient had previously failed 2 transplants (with myeloablative conditioning) from a matched sibling donor.
The third transplant prolonged the patient’s life by a year, but he eventually died of relapsed disease.
The investigators said this case study and the in vitro results support further investigation into the efficacy of dasatinib and other TNK2 inhibitors in PTPN11-mutant leukemias.
New research suggests dasatinib could treat certain patients with juvenile myelomonocytic leukemia (JMML) or acute myeloid leukemia (AML).
The study showed that TNK2 inhibition has a negative effect on PTPN11-mutant leukemias.
PTPN11-mutant JMML and AML cells were sensitive to treatment with dasatinib, which inhibits TNK2.
Dasatinib also induced hematologic remission in a patient with PTPN11-mutant JMML.
Investigators reported these results in Science Signaling.
Past research showed that mutations in PTPN11 result in excessive cell proliferation and drive tumor growth in some cases of JMML and AML.
In the current study, investigators analyzed PTPN11-mutated leukemia cells and found that PTPN11 is activated by TNK2.
The investigators said TNK2 phosphorylates PTPN11, which then dephosphorylates TNK2 in a negative feedback loop. They also found that coexpression of TNK2 and mutant PTPN11 results in “robust” MAPK pathway activation.
Inhibiting TNK2 with dasatinib blocked MAPK signaling and colony formation in vitro.
Additional experiments showed that PTPN11-mutant AML samples were significantly more sensitive to dasatinib than wild-type AML samples.
Investigators also tested dasatinib in a sample from a JMML patient carrying a PTPN11 G60R mutation.
This patient’s cells were 10 times more sensitive to dasatinib than the average sample from a cohort of 151 patients who had AML, acute lymphoblastic leukemia, myeloproliferative neoplasms, or chronic lymphocytic leukemia.
Because the JMML patient’s cells were so responsive to dasatinib, the investigators decided to administer the drug to the patient.
The patient achieved sustained hematologic remission with dasatinib, and this allowed him to receive a stem cell transplant using an unrelated cord blood donor. The patient had previously failed 2 transplants (with myeloablative conditioning) from a matched sibling donor.
The third transplant prolonged the patient’s life by a year, but he eventually died of relapsed disease.
The investigators said this case study and the in vitro results support further investigation into the efficacy of dasatinib and other TNK2 inhibitors in PTPN11-mutant leukemias.
FDA grants UCB product orphan designation
The US Food and Drug Administration (FDA) has granted orphan drug designation to NiCord for hematopoietic stem cell transplant.
NiCord is created by expanding and enriching a unit of umbilical cord blood (UCB).
The product consists of a CD133-positive fraction—which is cultured for 21 days with nicotinamide, thrombopoietin, IL-6, FLT-3 ligand, and stem cell factor—and a CD133-negative fraction that is provided at the time of transplant.
NiCord already has orphan drug designation from the FDA as a treatment for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma, and myelodysplastic syndromes (MDS).
The product also has breakthrough therapy designation from the FDA.
NiCord trials
Final results from a phase 1/2 study suggested that NiCord can be used as a stand-alone graft in patients with high-risk hematologic malignancies. The results were presented at the 2018 BMT Tandem Meetings in February.
The trial included 36 adolescents and adults with AML (n=17), ALL (n=9), MDS (n=7), chronic myeloid leukemia (CML, n=2), and Hodgkin lymphoma (n=1).
All patients received a single NiCord unit. Researchers compared engraftment results in the NiCord recipients to results in a cohort of 148 patients from the CIBMTR registry.
The registry patients underwent standard UCB transplants and had similar characteristics as the NiCord recipients. However, only 20% of the CIBMTR patients received a single UCB unit.
The median time to neutrophil engraftment was 11.5 days (range, 6-26) with NiCord and 21 days in the control cohort (P<0.001). The cumulative incidence of neutrophil engraftment was 94.4% and 89.7%, respectively.
The median time to platelet engraftment was 34 days (range, 25-96) with NiCord and 46 days in the controls (P<0.001). The cumulative incidence of platelet engraftment was 80.6% and 67.1%, respectively.
There was 1 case of primary graft failure among the NiCord recipients and 2 cases of secondary graft failure.
The estimated 2-year rate of non-relapse mortality in NiCord recipients was 23.8%, and the 2-year incidence of relapse was 33.2%.
The estimated disease-free survival was 49.1% at 1 year and 43.0% at 2 years. The overall survival was 51.2% at 1 year and 2 years.
At 100 days, the rate of grade 2-4 acute graft-vs-host disease (GVHD) was 44.0%, and the rate of grade 3-4 acute GVHD was 11.1%. The estimated 1-year rate of mild to severe chronic GVHD was 40.5%, and the 2-year rate of moderate to severe chronic GVHD was 9.8%.
These results prompted a phase 3 study of NiCord in patients with AML, ALL, CML, MDS, and lymphoma (NCT02730299). In this trial, researchers are comparing NiCord to standard single or double UCB transplant.
About orphan and breakthrough designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
Breakthrough designation entitles sponsors to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
The US Food and Drug Administration (FDA) has granted orphan drug designation to NiCord for hematopoietic stem cell transplant.
NiCord is created by expanding and enriching a unit of umbilical cord blood (UCB).
The product consists of a CD133-positive fraction—which is cultured for 21 days with nicotinamide, thrombopoietin, IL-6, FLT-3 ligand, and stem cell factor—and a CD133-negative fraction that is provided at the time of transplant.
NiCord already has orphan drug designation from the FDA as a treatment for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma, and myelodysplastic syndromes (MDS).
The product also has breakthrough therapy designation from the FDA.
NiCord trials
Final results from a phase 1/2 study suggested that NiCord can be used as a stand-alone graft in patients with high-risk hematologic malignancies. The results were presented at the 2018 BMT Tandem Meetings in February.
The trial included 36 adolescents and adults with AML (n=17), ALL (n=9), MDS (n=7), chronic myeloid leukemia (CML, n=2), and Hodgkin lymphoma (n=1).
All patients received a single NiCord unit. Researchers compared engraftment results in the NiCord recipients to results in a cohort of 148 patients from the CIBMTR registry.
The registry patients underwent standard UCB transplants and had similar characteristics as the NiCord recipients. However, only 20% of the CIBMTR patients received a single UCB unit.
The median time to neutrophil engraftment was 11.5 days (range, 6-26) with NiCord and 21 days in the control cohort (P<0.001). The cumulative incidence of neutrophil engraftment was 94.4% and 89.7%, respectively.
The median time to platelet engraftment was 34 days (range, 25-96) with NiCord and 46 days in the controls (P<0.001). The cumulative incidence of platelet engraftment was 80.6% and 67.1%, respectively.
There was 1 case of primary graft failure among the NiCord recipients and 2 cases of secondary graft failure.
The estimated 2-year rate of non-relapse mortality in NiCord recipients was 23.8%, and the 2-year incidence of relapse was 33.2%.
The estimated disease-free survival was 49.1% at 1 year and 43.0% at 2 years. The overall survival was 51.2% at 1 year and 2 years.
At 100 days, the rate of grade 2-4 acute graft-vs-host disease (GVHD) was 44.0%, and the rate of grade 3-4 acute GVHD was 11.1%. The estimated 1-year rate of mild to severe chronic GVHD was 40.5%, and the 2-year rate of moderate to severe chronic GVHD was 9.8%.
These results prompted a phase 3 study of NiCord in patients with AML, ALL, CML, MDS, and lymphoma (NCT02730299). In this trial, researchers are comparing NiCord to standard single or double UCB transplant.
About orphan and breakthrough designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
Breakthrough designation entitles sponsors to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
The US Food and Drug Administration (FDA) has granted orphan drug designation to NiCord for hematopoietic stem cell transplant.
NiCord is created by expanding and enriching a unit of umbilical cord blood (UCB).
The product consists of a CD133-positive fraction—which is cultured for 21 days with nicotinamide, thrombopoietin, IL-6, FLT-3 ligand, and stem cell factor—and a CD133-negative fraction that is provided at the time of transplant.
NiCord already has orphan drug designation from the FDA as a treatment for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma, and myelodysplastic syndromes (MDS).
The product also has breakthrough therapy designation from the FDA.
NiCord trials
Final results from a phase 1/2 study suggested that NiCord can be used as a stand-alone graft in patients with high-risk hematologic malignancies. The results were presented at the 2018 BMT Tandem Meetings in February.
The trial included 36 adolescents and adults with AML (n=17), ALL (n=9), MDS (n=7), chronic myeloid leukemia (CML, n=2), and Hodgkin lymphoma (n=1).
All patients received a single NiCord unit. Researchers compared engraftment results in the NiCord recipients to results in a cohort of 148 patients from the CIBMTR registry.
The registry patients underwent standard UCB transplants and had similar characteristics as the NiCord recipients. However, only 20% of the CIBMTR patients received a single UCB unit.
The median time to neutrophil engraftment was 11.5 days (range, 6-26) with NiCord and 21 days in the control cohort (P<0.001). The cumulative incidence of neutrophil engraftment was 94.4% and 89.7%, respectively.
The median time to platelet engraftment was 34 days (range, 25-96) with NiCord and 46 days in the controls (P<0.001). The cumulative incidence of platelet engraftment was 80.6% and 67.1%, respectively.
There was 1 case of primary graft failure among the NiCord recipients and 2 cases of secondary graft failure.
The estimated 2-year rate of non-relapse mortality in NiCord recipients was 23.8%, and the 2-year incidence of relapse was 33.2%.
The estimated disease-free survival was 49.1% at 1 year and 43.0% at 2 years. The overall survival was 51.2% at 1 year and 2 years.
At 100 days, the rate of grade 2-4 acute graft-vs-host disease (GVHD) was 44.0%, and the rate of grade 3-4 acute GVHD was 11.1%. The estimated 1-year rate of mild to severe chronic GVHD was 40.5%, and the 2-year rate of moderate to severe chronic GVHD was 9.8%.
These results prompted a phase 3 study of NiCord in patients with AML, ALL, CML, MDS, and lymphoma (NCT02730299). In this trial, researchers are comparing NiCord to standard single or double UCB transplant.
About orphan and breakthrough designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
Breakthrough designation entitles sponsors to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Product receives orphan designation for use in HSCT
The US Food and Drug Administration (FDA) has granted orphan drug designation to dilanubicel (NLA101) for the reduction of morbidity and mortality associated with hematopoietic stem cell transplant (HSCT).
Dilanubicel is a universal-donor, ex-vivo-expanded hematopoietic stem and progenitor cell product.
It is intended to induce short-term hematopoiesis, which lasts until a patient’s immune system recovers.
However, dilanubicel may also produce long-term immunologic benefits and could potentially improve survival in HSCT recipients, according to Nohla Therapeutics, the company developing the product.
Dilanubicel is manufactured ahead of time, cryopreserved, and intended for immediate off-the-shelf use.
Phase 2 trials
The orphan drug designation for dilanubicel was supported by data from a phase 2, single-center study. Results from this study were presented in a poster at the 23rd Congress of European Hematology Association (EHA) in June.
The trial included 15 patients with hematologic malignancies who underwent a cord blood transplant. Conditioning consisted of fludarabine (75 mg/m2), cyclophosphamide (120 mg/kg), and total body irradiation (13.2 Gy).
Patients received unmanipulated cord blood unit(s), followed 4 hours later by dilanubicel infusion. Prophylaxis for graft-vs-host disease (GVHD) was cyclosporine/mycophenolate mofetil.
The researchers compared outcomes in the 15 dilanubicel recipients to outcomes in a concurrent control cohort of 50 patients treated with the same HSCT protocol, minus dilanubicel. There were no significant differences between the 2 cohorts with regard to baseline characteristics.
The time to neutrophil and platelet recovery were both significantly better in dilanubicel recipients than controls.
At day 100, the cumulative incidence of neutrophil recovery was 100% in dilanubicel recipients and 94% in controls (P=0.005). The median time to neutrophil recovery was 19 days (range, 9-31) and 25 days (range, 14-45), respectively.
The cumulative incidence of platelet recovery was 93% in dilanubicel recipients and 74% in controls (P=0.02). The median time to platelet recovery was 35 days (range, 21-86) and 48 days (range, 24-158), respectively.
At 100 days, there were no cases of grade 3-4 acute GVHD in dilanubicel recipients, but the incidence of grade 3-4 acute GVHD was 29% in the control group.
At 5 years, 27% of dilanubicel recipients had experienced chronic GVHD, compared to 38% of the control group.
There were no cases of transplant related mortality (TRM) in dilanubicel recipients, but the rate of TRM was 16% in the control group.
Two dilanubicel recipients (13%) relapsed post-transplant and subsequently died.
The 5-year disease-free survival rate was 87% in dilanubicel recipients and 66% in the control group. Overall survival rates were the same.
“Dilanubicel has shown encouraging initial activity as a novel cell therapy in patients with hematologic malignancies receiving a cord blood transplant,” said President and CEO of Nohla Therapeutics Katie Fanning.
“We believe the addition of dilanubicel has the potential to make a meaningful difference for these patients, and we look forward to having the top-line results from the fully enrolled, randomized, phase 2b trial later this year.”
The phase 2b trial (NCT01690520) has enrolled 160 patients with hematologic malignancies. The goal of the trial is to determine whether adding dilanubicel to standard donor cord blood transplant decreases the time to hematopoietic recovery, thereby reducing associated morbidities and mortality.
Another phase 2 trial, called LAUNCH (NCT03301597), is currently enrolling patients who have acute myeloid leukemia and chemotherapy-induced myelosuppression. The goals of this trial are to evaluate dilanubicel’s ability to reduce the rate of grade 3 or higher infections associated with chemotherapy-induced neutropenia and to identify the lowest effective cell dose of dilanubicel.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to dilanubicel (NLA101) for the reduction of morbidity and mortality associated with hematopoietic stem cell transplant (HSCT).
Dilanubicel is a universal-donor, ex-vivo-expanded hematopoietic stem and progenitor cell product.
It is intended to induce short-term hematopoiesis, which lasts until a patient’s immune system recovers.
However, dilanubicel may also produce long-term immunologic benefits and could potentially improve survival in HSCT recipients, according to Nohla Therapeutics, the company developing the product.
Dilanubicel is manufactured ahead of time, cryopreserved, and intended for immediate off-the-shelf use.
Phase 2 trials
The orphan drug designation for dilanubicel was supported by data from a phase 2, single-center study. Results from this study were presented in a poster at the 23rd Congress of European Hematology Association (EHA) in June.
The trial included 15 patients with hematologic malignancies who underwent a cord blood transplant. Conditioning consisted of fludarabine (75 mg/m2), cyclophosphamide (120 mg/kg), and total body irradiation (13.2 Gy).
Patients received unmanipulated cord blood unit(s), followed 4 hours later by dilanubicel infusion. Prophylaxis for graft-vs-host disease (GVHD) was cyclosporine/mycophenolate mofetil.
The researchers compared outcomes in the 15 dilanubicel recipients to outcomes in a concurrent control cohort of 50 patients treated with the same HSCT protocol, minus dilanubicel. There were no significant differences between the 2 cohorts with regard to baseline characteristics.
The time to neutrophil and platelet recovery were both significantly better in dilanubicel recipients than controls.
At day 100, the cumulative incidence of neutrophil recovery was 100% in dilanubicel recipients and 94% in controls (P=0.005). The median time to neutrophil recovery was 19 days (range, 9-31) and 25 days (range, 14-45), respectively.
The cumulative incidence of platelet recovery was 93% in dilanubicel recipients and 74% in controls (P=0.02). The median time to platelet recovery was 35 days (range, 21-86) and 48 days (range, 24-158), respectively.
At 100 days, there were no cases of grade 3-4 acute GVHD in dilanubicel recipients, but the incidence of grade 3-4 acute GVHD was 29% in the control group.
At 5 years, 27% of dilanubicel recipients had experienced chronic GVHD, compared to 38% of the control group.
There were no cases of transplant related mortality (TRM) in dilanubicel recipients, but the rate of TRM was 16% in the control group.
Two dilanubicel recipients (13%) relapsed post-transplant and subsequently died.
The 5-year disease-free survival rate was 87% in dilanubicel recipients and 66% in the control group. Overall survival rates were the same.
“Dilanubicel has shown encouraging initial activity as a novel cell therapy in patients with hematologic malignancies receiving a cord blood transplant,” said President and CEO of Nohla Therapeutics Katie Fanning.
“We believe the addition of dilanubicel has the potential to make a meaningful difference for these patients, and we look forward to having the top-line results from the fully enrolled, randomized, phase 2b trial later this year.”
The phase 2b trial (NCT01690520) has enrolled 160 patients with hematologic malignancies. The goal of the trial is to determine whether adding dilanubicel to standard donor cord blood transplant decreases the time to hematopoietic recovery, thereby reducing associated morbidities and mortality.
Another phase 2 trial, called LAUNCH (NCT03301597), is currently enrolling patients who have acute myeloid leukemia and chemotherapy-induced myelosuppression. The goals of this trial are to evaluate dilanubicel’s ability to reduce the rate of grade 3 or higher infections associated with chemotherapy-induced neutropenia and to identify the lowest effective cell dose of dilanubicel.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to dilanubicel (NLA101) for the reduction of morbidity and mortality associated with hematopoietic stem cell transplant (HSCT).
Dilanubicel is a universal-donor, ex-vivo-expanded hematopoietic stem and progenitor cell product.
It is intended to induce short-term hematopoiesis, which lasts until a patient’s immune system recovers.
However, dilanubicel may also produce long-term immunologic benefits and could potentially improve survival in HSCT recipients, according to Nohla Therapeutics, the company developing the product.
Dilanubicel is manufactured ahead of time, cryopreserved, and intended for immediate off-the-shelf use.
Phase 2 trials
The orphan drug designation for dilanubicel was supported by data from a phase 2, single-center study. Results from this study were presented in a poster at the 23rd Congress of European Hematology Association (EHA) in June.
The trial included 15 patients with hematologic malignancies who underwent a cord blood transplant. Conditioning consisted of fludarabine (75 mg/m2), cyclophosphamide (120 mg/kg), and total body irradiation (13.2 Gy).
Patients received unmanipulated cord blood unit(s), followed 4 hours later by dilanubicel infusion. Prophylaxis for graft-vs-host disease (GVHD) was cyclosporine/mycophenolate mofetil.
The researchers compared outcomes in the 15 dilanubicel recipients to outcomes in a concurrent control cohort of 50 patients treated with the same HSCT protocol, minus dilanubicel. There were no significant differences between the 2 cohorts with regard to baseline characteristics.
The time to neutrophil and platelet recovery were both significantly better in dilanubicel recipients than controls.
At day 100, the cumulative incidence of neutrophil recovery was 100% in dilanubicel recipients and 94% in controls (P=0.005). The median time to neutrophil recovery was 19 days (range, 9-31) and 25 days (range, 14-45), respectively.
The cumulative incidence of platelet recovery was 93% in dilanubicel recipients and 74% in controls (P=0.02). The median time to platelet recovery was 35 days (range, 21-86) and 48 days (range, 24-158), respectively.
At 100 days, there were no cases of grade 3-4 acute GVHD in dilanubicel recipients, but the incidence of grade 3-4 acute GVHD was 29% in the control group.
At 5 years, 27% of dilanubicel recipients had experienced chronic GVHD, compared to 38% of the control group.
There were no cases of transplant related mortality (TRM) in dilanubicel recipients, but the rate of TRM was 16% in the control group.
Two dilanubicel recipients (13%) relapsed post-transplant and subsequently died.
The 5-year disease-free survival rate was 87% in dilanubicel recipients and 66% in the control group. Overall survival rates were the same.
“Dilanubicel has shown encouraging initial activity as a novel cell therapy in patients with hematologic malignancies receiving a cord blood transplant,” said President and CEO of Nohla Therapeutics Katie Fanning.
“We believe the addition of dilanubicel has the potential to make a meaningful difference for these patients, and we look forward to having the top-line results from the fully enrolled, randomized, phase 2b trial later this year.”
The phase 2b trial (NCT01690520) has enrolled 160 patients with hematologic malignancies. The goal of the trial is to determine whether adding dilanubicel to standard donor cord blood transplant decreases the time to hematopoietic recovery, thereby reducing associated morbidities and mortality.
Another phase 2 trial, called LAUNCH (NCT03301597), is currently enrolling patients who have acute myeloid leukemia and chemotherapy-induced myelosuppression. The goals of this trial are to evaluate dilanubicel’s ability to reduce the rate of grade 3 or higher infections associated with chemotherapy-induced neutropenia and to identify the lowest effective cell dose of dilanubicel.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
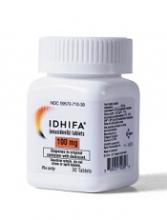
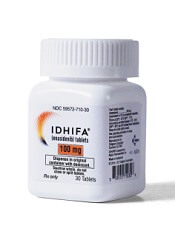
Explaining enasidenib resistance in AML
New research helps explain enasidenib resistance among patients with IDH2-mutant acute myeloid leukemia (AML).
Researchers found that leukemic cells stop responding to enasidenib when IDH2 clones develop additional mutations.
This may mean that enasidenib will have to be combined with other drugs to prevent AML relapse, the researchers said.
They reported their findings in Nature Medicine.
Previous research indicated that enasidenib prompts differentiation to induce responses in AML. In a phase 1/2 trial, enasidenib produced responses in about 40% of patients with relapsed/refractory, IDH2-mutated AML. However, most patients eventually relapsed.
“[T]he initial studies did not show which AML cells responded to enasidenib and started to differentiate again,” said Stéphane de Botton, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
“It was also unclear how the cells become resistant to therapy. We wanted to answer these questions.”
To do so, Dr de Botton and his colleagues analyzed sequential samples from 37 AML patients treated with enasidenib on the phase 1/2 trial. Thirty of these patients had initially responded to the drug.
“We used techniques to study genetic mutations on a cell-by-cell basis and reconstructed the ‘family tree’ of a patient’s AML,” said Lynn Quek, MD, of the University of Oxford in the UK.
“We then tracked changes in the family of AML cells as they responded to enasidenib and as patients lost response to the drug. This is the first time that anyone has done such a detailed study at a single-cell level.”
The researchers said they observed variable differentiation arrest in IDH2-mutant clones before enasidenib treatment.
Overall, treatment promoted hematopoietic differentiation from either terminal or ancestral mutant clones. However, enasidenib also promoted differentiation of nonmutant cells in a minority of patients.
When the researchers compared samples taken at diagnosis and relapse, they did not find second-site mutations in IDH2 at relapse.
The team said relapse was the result of clonal evolution or selection of terminal or ancestral clones, which suggests there are multiple pathways that could potentially be targeted to restore differentiation arrest.
“We have provided genetic proof that enasidenib was able to differentiate cancer cells so that some of their normal functions were restored, even though they still contained the IDH2 mutation,” said Virginie Penard-Lacronique, of Gustave Roussy.
“This is important because, unless we can track these clones, we don’t know whether the mature cells in a patient are coming from normal cells after all the cancer cells have been killed or from leukemic cells that are now able to mature. In this paper, we show that, in 4 out of 5 cases, the mature cells are coming from leukemic bone marrow cells that can be induced to differentiate by this new drug.”
The researchers said these results suggest enasidenib must be combined with other drugs to prevent AML relapse.
“Now that we have shown that [enasidenib] needs to be combined with other drugs to stop the cancer returning, we think that it’s important that the combination therapy should be given to AML patients early on in their disease,” Dr de Botton said. “International trials are now taking place comparing combinations of enasidenib and other drugs with the normal standard of care.”
New research helps explain enasidenib resistance among patients with IDH2-mutant acute myeloid leukemia (AML).
Researchers found that leukemic cells stop responding to enasidenib when IDH2 clones develop additional mutations.
This may mean that enasidenib will have to be combined with other drugs to prevent AML relapse, the researchers said.
They reported their findings in Nature Medicine.
Previous research indicated that enasidenib prompts differentiation to induce responses in AML. In a phase 1/2 trial, enasidenib produced responses in about 40% of patients with relapsed/refractory, IDH2-mutated AML. However, most patients eventually relapsed.
“[T]he initial studies did not show which AML cells responded to enasidenib and started to differentiate again,” said Stéphane de Botton, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
“It was also unclear how the cells become resistant to therapy. We wanted to answer these questions.”
To do so, Dr de Botton and his colleagues analyzed sequential samples from 37 AML patients treated with enasidenib on the phase 1/2 trial. Thirty of these patients had initially responded to the drug.
“We used techniques to study genetic mutations on a cell-by-cell basis and reconstructed the ‘family tree’ of a patient’s AML,” said Lynn Quek, MD, of the University of Oxford in the UK.
“We then tracked changes in the family of AML cells as they responded to enasidenib and as patients lost response to the drug. This is the first time that anyone has done such a detailed study at a single-cell level.”
The researchers said they observed variable differentiation arrest in IDH2-mutant clones before enasidenib treatment.
Overall, treatment promoted hematopoietic differentiation from either terminal or ancestral mutant clones. However, enasidenib also promoted differentiation of nonmutant cells in a minority of patients.
When the researchers compared samples taken at diagnosis and relapse, they did not find second-site mutations in IDH2 at relapse.
The team said relapse was the result of clonal evolution or selection of terminal or ancestral clones, which suggests there are multiple pathways that could potentially be targeted to restore differentiation arrest.
“We have provided genetic proof that enasidenib was able to differentiate cancer cells so that some of their normal functions were restored, even though they still contained the IDH2 mutation,” said Virginie Penard-Lacronique, of Gustave Roussy.
“This is important because, unless we can track these clones, we don’t know whether the mature cells in a patient are coming from normal cells after all the cancer cells have been killed or from leukemic cells that are now able to mature. In this paper, we show that, in 4 out of 5 cases, the mature cells are coming from leukemic bone marrow cells that can be induced to differentiate by this new drug.”
The researchers said these results suggest enasidenib must be combined with other drugs to prevent AML relapse.
“Now that we have shown that [enasidenib] needs to be combined with other drugs to stop the cancer returning, we think that it’s important that the combination therapy should be given to AML patients early on in their disease,” Dr de Botton said. “International trials are now taking place comparing combinations of enasidenib and other drugs with the normal standard of care.”
New research helps explain enasidenib resistance among patients with IDH2-mutant acute myeloid leukemia (AML).
Researchers found that leukemic cells stop responding to enasidenib when IDH2 clones develop additional mutations.
This may mean that enasidenib will have to be combined with other drugs to prevent AML relapse, the researchers said.
They reported their findings in Nature Medicine.
Previous research indicated that enasidenib prompts differentiation to induce responses in AML. In a phase 1/2 trial, enasidenib produced responses in about 40% of patients with relapsed/refractory, IDH2-mutated AML. However, most patients eventually relapsed.
“[T]he initial studies did not show which AML cells responded to enasidenib and started to differentiate again,” said Stéphane de Botton, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
“It was also unclear how the cells become resistant to therapy. We wanted to answer these questions.”
To do so, Dr de Botton and his colleagues analyzed sequential samples from 37 AML patients treated with enasidenib on the phase 1/2 trial. Thirty of these patients had initially responded to the drug.
“We used techniques to study genetic mutations on a cell-by-cell basis and reconstructed the ‘family tree’ of a patient’s AML,” said Lynn Quek, MD, of the University of Oxford in the UK.
“We then tracked changes in the family of AML cells as they responded to enasidenib and as patients lost response to the drug. This is the first time that anyone has done such a detailed study at a single-cell level.”
The researchers said they observed variable differentiation arrest in IDH2-mutant clones before enasidenib treatment.
Overall, treatment promoted hematopoietic differentiation from either terminal or ancestral mutant clones. However, enasidenib also promoted differentiation of nonmutant cells in a minority of patients.
When the researchers compared samples taken at diagnosis and relapse, they did not find second-site mutations in IDH2 at relapse.
The team said relapse was the result of clonal evolution or selection of terminal or ancestral clones, which suggests there are multiple pathways that could potentially be targeted to restore differentiation arrest.
“We have provided genetic proof that enasidenib was able to differentiate cancer cells so that some of their normal functions were restored, even though they still contained the IDH2 mutation,” said Virginie Penard-Lacronique, of Gustave Roussy.
“This is important because, unless we can track these clones, we don’t know whether the mature cells in a patient are coming from normal cells after all the cancer cells have been killed or from leukemic cells that are now able to mature. In this paper, we show that, in 4 out of 5 cases, the mature cells are coming from leukemic bone marrow cells that can be induced to differentiate by this new drug.”
The researchers said these results suggest enasidenib must be combined with other drugs to prevent AML relapse.
“Now that we have shown that [enasidenib] needs to be combined with other drugs to stop the cancer returning, we think that it’s important that the combination therapy should be given to AML patients early on in their disease,” Dr de Botton said. “International trials are now taking place comparing combinations of enasidenib and other drugs with the normal standard of care.”
Investigators question utility of SFLCA
Researchers are questioning the clinical usefulness of the serum free light chain assay (SFLCA) for patients with monoclonal gammopathies.
The investigators found evidence suggesting that, about 25% of the time, SFLCA provides a negative κ/λ ratio in patients with lambda chain monoclonal gammopathies who have free homogenous lambda light chains detectable in their urine.
“If you have a lambda chain-associated lesion and you don’t do a urine study—just rely on the serum free light chain assay—about 1 out of 4 times, the assay will tell you that you don’t have anything when you actually do,” explained Won Sok Lee, MD, of the Medical College of Georgia at Augusta University.
“When you test the serum, we suggest you also test the urine whenever you suspect that somebody has a tumor of the plasma cells,” said Gurmukh Singh, MD, PhD, also of the Medical College of Georgia.
Drs Singh and Lee made this recommendation and detailed the supporting research in the Journal of Clinical Medicine Research.
The researchers evaluated results of serum and urine tests in 175 patients with monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM), or multiple/plasma cell myeloma (MM).
In addition to results of SFLCA, the investigators looked at results of serum protein electrophoresis, serum protein immunofixation electrophoresis, urine protein electrophoresis, and urine protein immunofixation electrophoresis.
This analysis revealed “systematic under-detection” of serum free lambda light chains by SFLCA as well as an under-detection of the lambda-dominant ratio.
The researchers integrated the results of this study with findings from an earlier study* and concluded that, as compared to kappa chain lesions:
- The excess false-negative rate of κ/λ ratio for lambda chain lesions in MGUS is 29%
- The excess false-negative rate of κ/λ ratio for lambda chain lesions in MM is 32%
- The excess false-negative rate of κ/λ ratio for lambda chain lesions in all neoplastic monoclonal gammopathies (MGUS, MM, and SMM) is approximately 30%.
The investigators said they believe that 5% of the 30% false-negative rate is a result of under-production of excess free lambda light chains, and about 25% could be due to under-detection of monoclonal lambda light chains by SFLCA.
“[A patient] may go undiagnosed because the serum free light chain test either is not picking up those abnormal proteins or the lambda lesions don’t make that many excess abnormal proteins,” Dr Singh said.
However, Drs Singh and Lee also said it’s possible that unknown factors, such as general over-production of polyclonal kappa light chains in tertiary care patients, may alter the κ/λ ratio.
*Singh, G. Serum Free Light Chain Assay and κ/λ Ratio: Performance in Patients With Monoclonal Gammopathy-High False Negative Rate for κ/λ Ratio. J Clin Med Res. 2017 Jan; 9(1): 46–57.
Researchers are questioning the clinical usefulness of the serum free light chain assay (SFLCA) for patients with monoclonal gammopathies.
The investigators found evidence suggesting that, about 25% of the time, SFLCA provides a negative κ/λ ratio in patients with lambda chain monoclonal gammopathies who have free homogenous lambda light chains detectable in their urine.
“If you have a lambda chain-associated lesion and you don’t do a urine study—just rely on the serum free light chain assay—about 1 out of 4 times, the assay will tell you that you don’t have anything when you actually do,” explained Won Sok Lee, MD, of the Medical College of Georgia at Augusta University.
“When you test the serum, we suggest you also test the urine whenever you suspect that somebody has a tumor of the plasma cells,” said Gurmukh Singh, MD, PhD, also of the Medical College of Georgia.
Drs Singh and Lee made this recommendation and detailed the supporting research in the Journal of Clinical Medicine Research.
The researchers evaluated results of serum and urine tests in 175 patients with monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM), or multiple/plasma cell myeloma (MM).
In addition to results of SFLCA, the investigators looked at results of serum protein electrophoresis, serum protein immunofixation electrophoresis, urine protein electrophoresis, and urine protein immunofixation electrophoresis.
This analysis revealed “systematic under-detection” of serum free lambda light chains by SFLCA as well as an under-detection of the lambda-dominant ratio.
The researchers integrated the results of this study with findings from an earlier study* and concluded that, as compared to kappa chain lesions:
- The excess false-negative rate of κ/λ ratio for lambda chain lesions in MGUS is 29%
- The excess false-negative rate of κ/λ ratio for lambda chain lesions in MM is 32%
- The excess false-negative rate of κ/λ ratio for lambda chain lesions in all neoplastic monoclonal gammopathies (MGUS, MM, and SMM) is approximately 30%.
The investigators said they believe that 5% of the 30% false-negative rate is a result of under-production of excess free lambda light chains, and about 25% could be due to under-detection of monoclonal lambda light chains by SFLCA.
“[A patient] may go undiagnosed because the serum free light chain test either is not picking up those abnormal proteins or the lambda lesions don’t make that many excess abnormal proteins,” Dr Singh said.
However, Drs Singh and Lee also said it’s possible that unknown factors, such as general over-production of polyclonal kappa light chains in tertiary care patients, may alter the κ/λ ratio.
*Singh, G. Serum Free Light Chain Assay and κ/λ Ratio: Performance in Patients With Monoclonal Gammopathy-High False Negative Rate for κ/λ Ratio. J Clin Med Res. 2017 Jan; 9(1): 46–57.
Researchers are questioning the clinical usefulness of the serum free light chain assay (SFLCA) for patients with monoclonal gammopathies.
The investigators found evidence suggesting that, about 25% of the time, SFLCA provides a negative κ/λ ratio in patients with lambda chain monoclonal gammopathies who have free homogenous lambda light chains detectable in their urine.
“If you have a lambda chain-associated lesion and you don’t do a urine study—just rely on the serum free light chain assay—about 1 out of 4 times, the assay will tell you that you don’t have anything when you actually do,” explained Won Sok Lee, MD, of the Medical College of Georgia at Augusta University.
“When you test the serum, we suggest you also test the urine whenever you suspect that somebody has a tumor of the plasma cells,” said Gurmukh Singh, MD, PhD, also of the Medical College of Georgia.
Drs Singh and Lee made this recommendation and detailed the supporting research in the Journal of Clinical Medicine Research.
The researchers evaluated results of serum and urine tests in 175 patients with monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM), or multiple/plasma cell myeloma (MM).
In addition to results of SFLCA, the investigators looked at results of serum protein electrophoresis, serum protein immunofixation electrophoresis, urine protein electrophoresis, and urine protein immunofixation electrophoresis.
This analysis revealed “systematic under-detection” of serum free lambda light chains by SFLCA as well as an under-detection of the lambda-dominant ratio.
The researchers integrated the results of this study with findings from an earlier study* and concluded that, as compared to kappa chain lesions:
- The excess false-negative rate of κ/λ ratio for lambda chain lesions in MGUS is 29%
- The excess false-negative rate of κ/λ ratio for lambda chain lesions in MM is 32%
- The excess false-negative rate of κ/λ ratio for lambda chain lesions in all neoplastic monoclonal gammopathies (MGUS, MM, and SMM) is approximately 30%.
The investigators said they believe that 5% of the 30% false-negative rate is a result of under-production of excess free lambda light chains, and about 25% could be due to under-detection of monoclonal lambda light chains by SFLCA.
“[A patient] may go undiagnosed because the serum free light chain test either is not picking up those abnormal proteins or the lambda lesions don’t make that many excess abnormal proteins,” Dr Singh said.
However, Drs Singh and Lee also said it’s possible that unknown factors, such as general over-production of polyclonal kappa light chains in tertiary care patients, may alter the κ/λ ratio.
*Singh, G. Serum Free Light Chain Assay and κ/λ Ratio: Performance in Patients With Monoclonal Gammopathy-High False Negative Rate for κ/λ Ratio. J Clin Med Res. 2017 Jan; 9(1): 46–57.